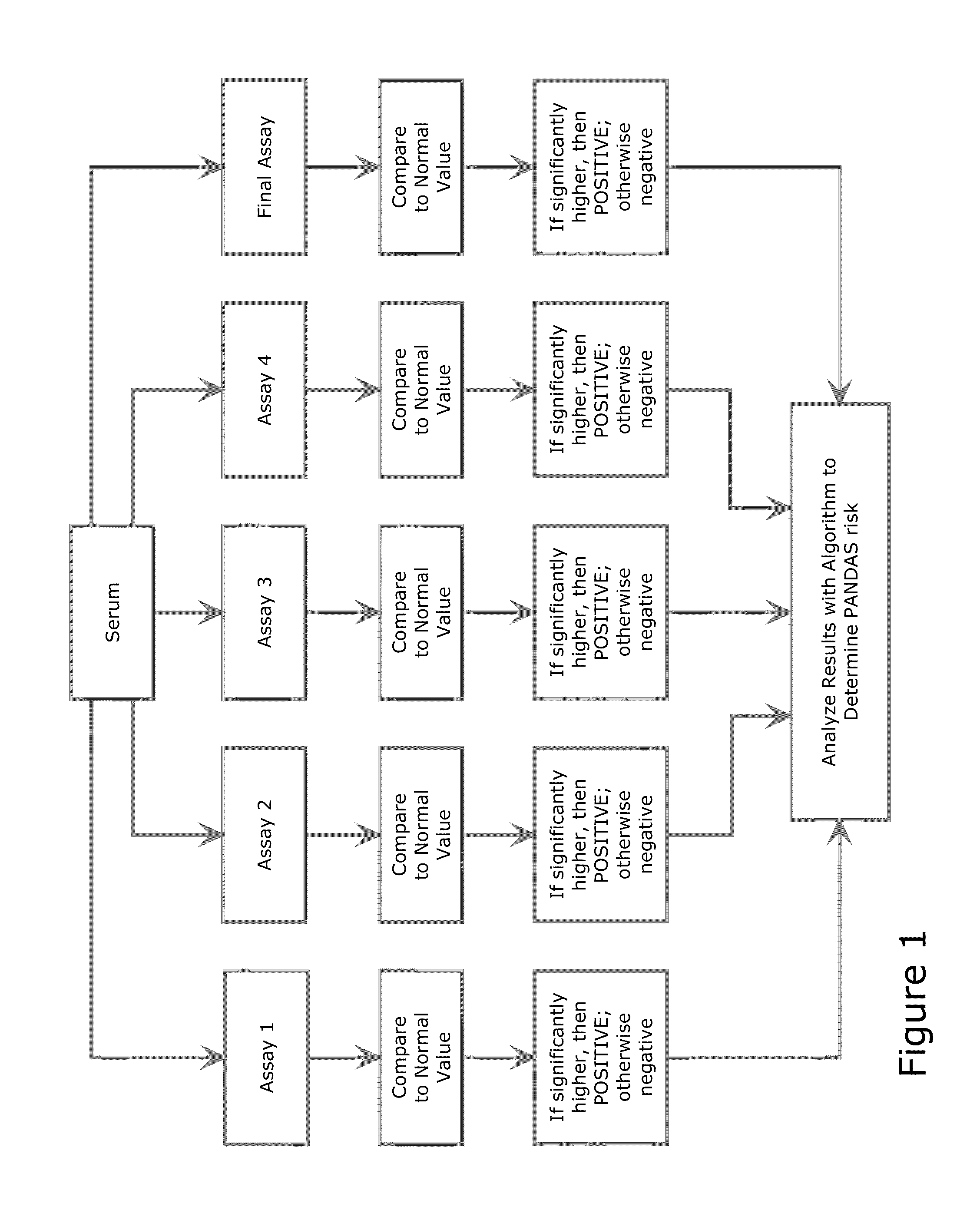
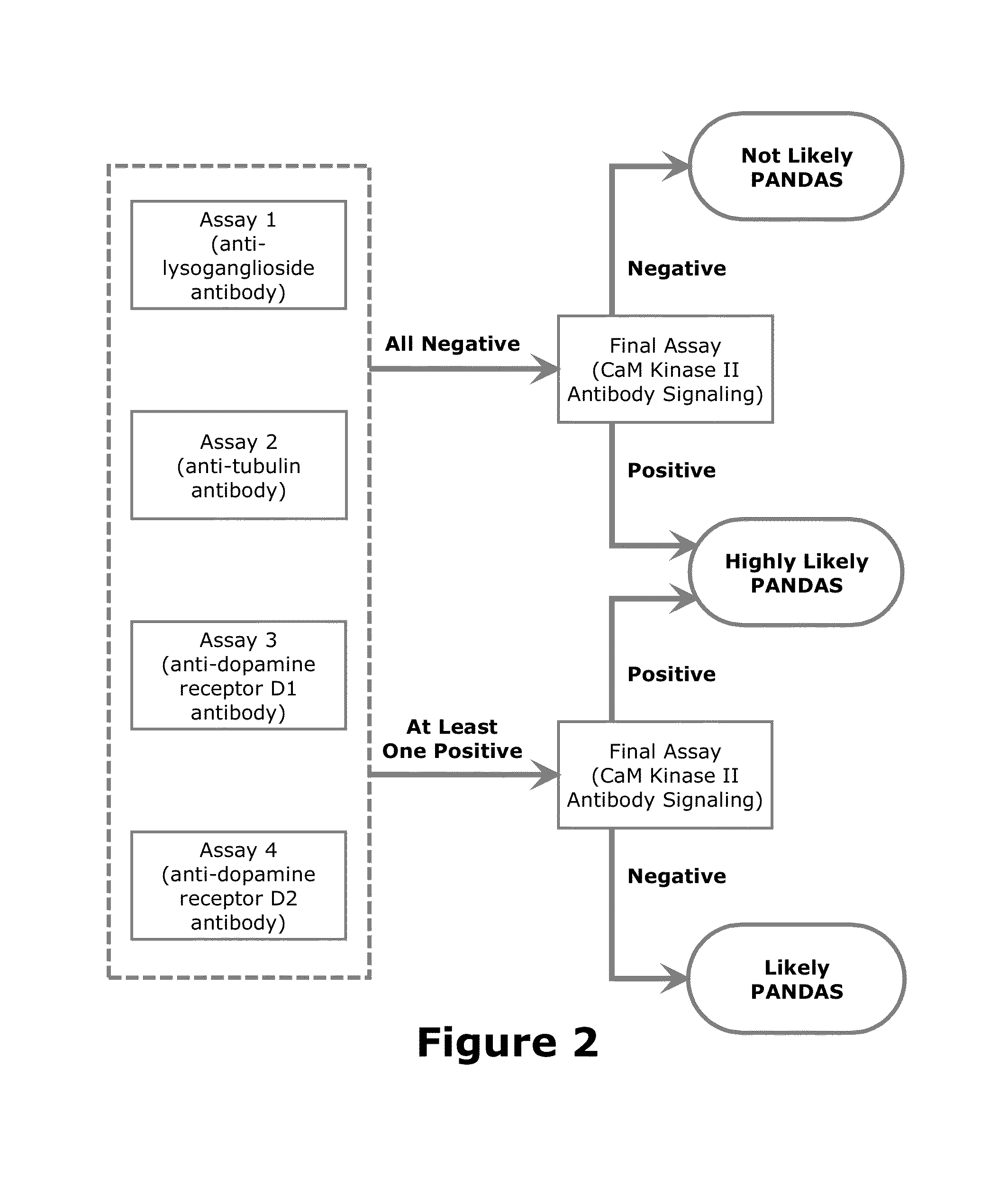
Diagnostic Method for Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococci Infection (PANDAS)
a neuropsychiatric syndrome and autoimmune neuropsychiatry technology, applied in the field of pandas, can solve the problems of inconvenient and accurate diagnosis, difficulty in agreeing on standard nomenclature for such conditions, and children that used to be comforted by hugs are now inconsolable, so as to achieve convenient and accurate diagnosis, reliably diagnose pans and/or pandas, and effective and timely
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Anti-Lysoganglioside Antibody Titer Protocol
[0109]The first assay is used to measure human serum IgG titers against lysoganglioside using the below described ELISA method. The antibodies are detected by coating microtiter plate wells with specific antigens, incubating the plates with serial dilutions of human sera, and washing away unbound antibodies. A secondary antibody conjugated to alkaline phosphatase is added to the plate and, after incubation, the excess conjugate is washed away. A color-developing substrate solution is then added. The amount of color developed is directly proportional to the amount of antibody in the sample. As always, when working with human fluids, biosafety practices must be followed.
[0110]The following materials were used:
MaterialDetailsLysoganglioside GM1from bovine brain (Sigma-Aldrich - G5660); store at −20° C.96-Well ELISA platesImmulon IV flat bottom plates (Thermo Scientific)Multichannel pipetFisherbrandPlastic wrapSingle channel pipettersGibsonDis...
example 2
Anti-Tubulin Antibody Titer Protocol
[0125]The second assay is used to measure human serum IgG titers against tubulin using the below described ELISA method. The antibodies are detected by coating microtiter plate wells with specific antigens, incubating the plates with serial dilutions of human sera, and washing away unbound antibodies. A secondary antibody conjugated to alkaline phosphatase is added to the plate and, after incubation, the excess conjugate is washed away. A color-developing substrate solution is then added. The amount of color developed is directly proportional to the amount of antibody in the sample. As always, when working with human fluids, biosafety practices must be followed.
[0126]The following materials were used:
MaterialDetailsTubulinfrom bovine brain (MP Biomedicals - 08771121); store lyophilized powder at 4° C.,once reconstituted with included PIPES buffer store at −80° C.96-Well ELISA platesImmulon IV flat bottom plates (Thermo Scientific)Multichannel pipe...
example 3
Anti-Dopamine D1 Receptor Antibody Titer Protocol
[0141]The third assay is used to measure human serum IgG titers against dopamine D1 receptors using the below described ELISA method. The antibodies are detected by coating microtiter plate wells with specific antigens, incubating the plates with serial dilutions of human sera, and washing away unbound antibodies. A secondary antibody conjugated to alkaline phosphatase is added to the plate and, after incubation, the excess conjugate is washed away. A color-developing substrate solution is then added. The amount of color developed is directly proportional to the amount of antibody in the sample. As always, when working with human fluids, biosafety practices must be followed.
[0142]The following materials were used:
MaterialDetailsDopamine D1 receptor400 μl frozen aliquot, membranes from cells (Membrane Target Systems,Perkin Elmer 6110513400UA); at −80° C.96-Well ELISA platesImmulon IV flat bottom plates (Thermo Scientific)Plastic wrapMu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com