Test strip and test card for fluorescence immunochromatography of myeloperoxidase
A technology of fluorescence immunochromatography and myeloperoxidase, which is applied in the direction of analyzing materials, measuring devices, instruments, etc., can solve parameters such as unrecorded detection linear range and sensitivity, undisclosed test strip linear range, sensitivity and other parameters To achieve reliable diagnosis results, improve clinical diagnosis efficiency, and lower configuration requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Prepare the MPO antibody-fluorescence immunochromatography quantitative detection test strip as follows:
[0039] a. Prepare the MPO antibody-fluorescent microsphere complex by EDC method:
[0040] Take 15ul fluorescent microspheres and add them to 0.6ml 0.05mol / l pH8.0 borate buffer solution, vortex and shake to mix; add 40ul 4mg / ml EDC, shake at room temperature for 30min; in the activated microsphere solution, add 125 μg of MPO-labeled antibody was coupled for 90 minutes; the coupled immune microsphere complex was reconstituted with a protective solution, the composition of which was 0.05M pH8.0 Tris-HCl buffer, 0.5% T-20 , 0.2% BSA, 0.5% casein, 4% trehalose and 0.05% NaN 3 , and then use blocking solution (containing 10% BSA, 0.05% NaN 3 ) to block for 2 hours to obtain the MPO antibody-fluorescent microsphere complex.
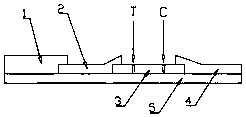
[0041] b. Prepare assembled test strips:
[0042] (1) Immerse the glass fiber member in the treatment solution, which includes Tris–HCl buffer...
Embodiment 2
[0049] Embodiment 2: Reaction condition optimization
[0050] Optimization of EDC amount:
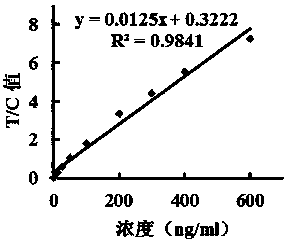
[0051] Take 15ul fluorescent microspheres and add them to 0.6ml 0.05mol / l pH8.0 borate buffer solution, vortex and oscillate to mix; add 4mg / ml EDC 10, 20, 40, 60, 80ul in turn, shake at room temperature for 30min, press The method of embodiment 1 prepares 5 groups of test paper strips, add sample and detect respectively with linear standard substance, fit and obtain standard equation, detect linear range and R 2 value, the results are shown in Table 1:
[0052] Table 1
[0053]
[0054] As shown in Table 1, under the condition of adding 160 μg of EDC, the linearity is the best, the best linear range is 3.125~600 ng / ml, R 2 =0.9803.
[0055] Optimization of coupling protein amount and coupling time:
[0056] Add 75, 100, 125, and 150 μg of MPO-labeled antibody in sequence to the activated microsphere solution, and couple for 30 min, 60 min, 90 min, and 120 min, respectively. Pr...
Embodiment 3
[0069] Stability detection is carried out to the test strip prepared by the embodiment of the present invention 1:
[0070] The test strips prepared in Example 1 were stored at 37°C and 4°C respectively, and by using linear standards to detect the test strips stored at 37°C and 4°C, the linearity of each condition R 2 The results of the changes are shown in Table 3.
[0071] table 3
[0072]
[0073] As can be seen from Table 3, the test strips of the present invention can store 180 antennas in R under 4°C. 2Both can reach more than 0.97, and the stability is good. When the test strip is stored at 37°C for 10 days, R 2 As the value decreases to 0.96, the detection error increases. Storing at 37°C for 1 day is close to the effect of storing at 4°C for about 45 days. It is inferred that the test strips can be stored at 4°C for at least 8 months.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com