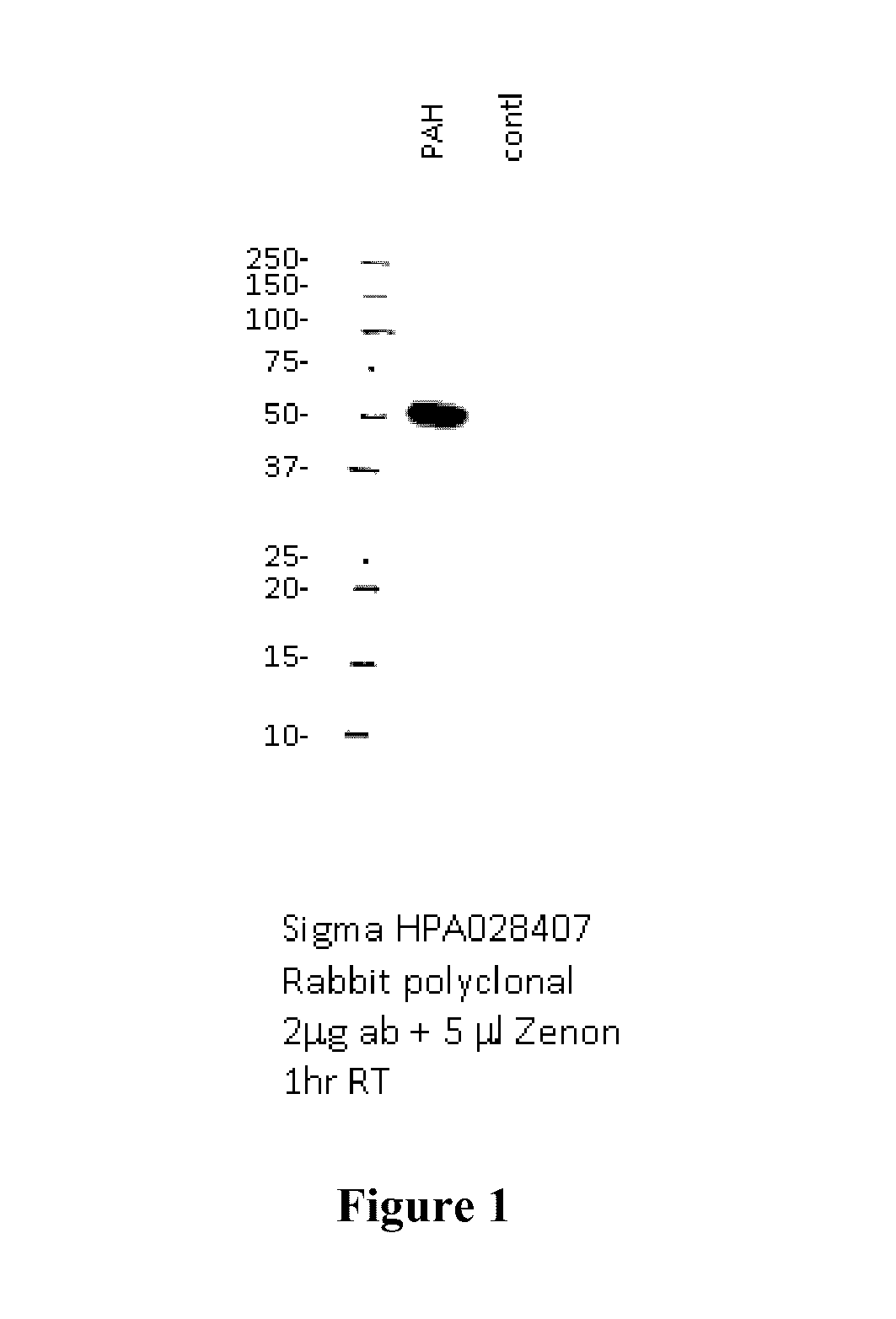
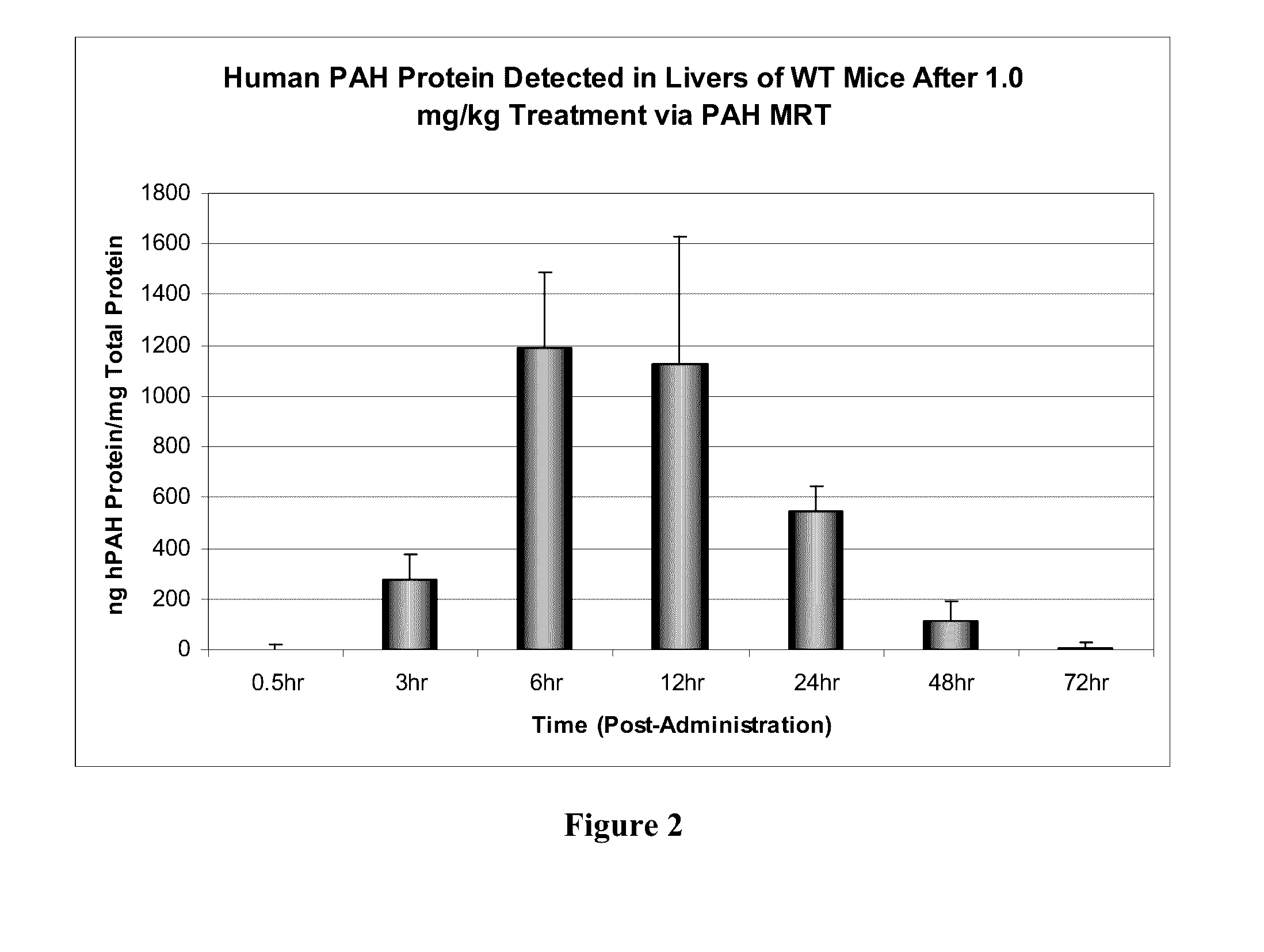
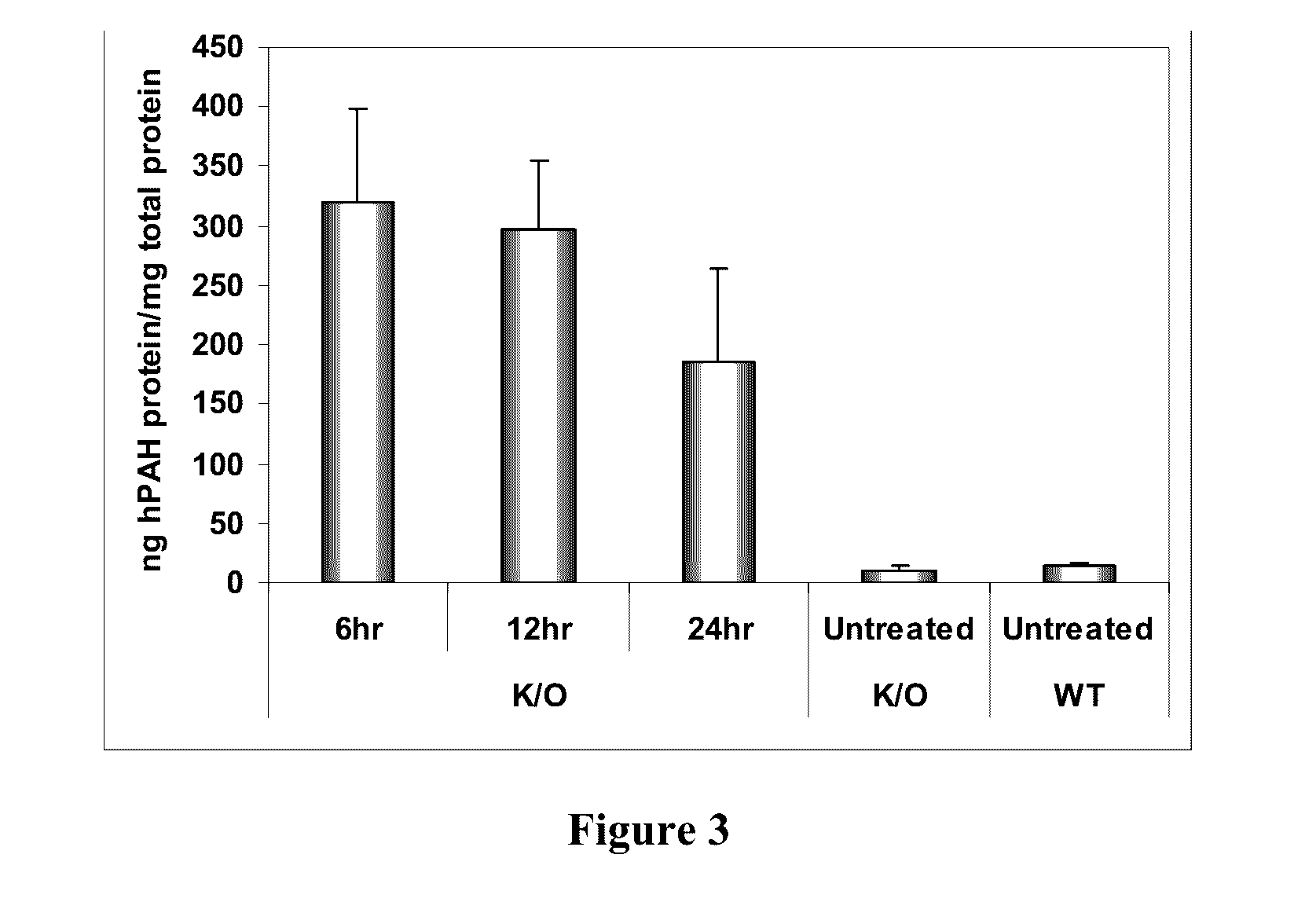
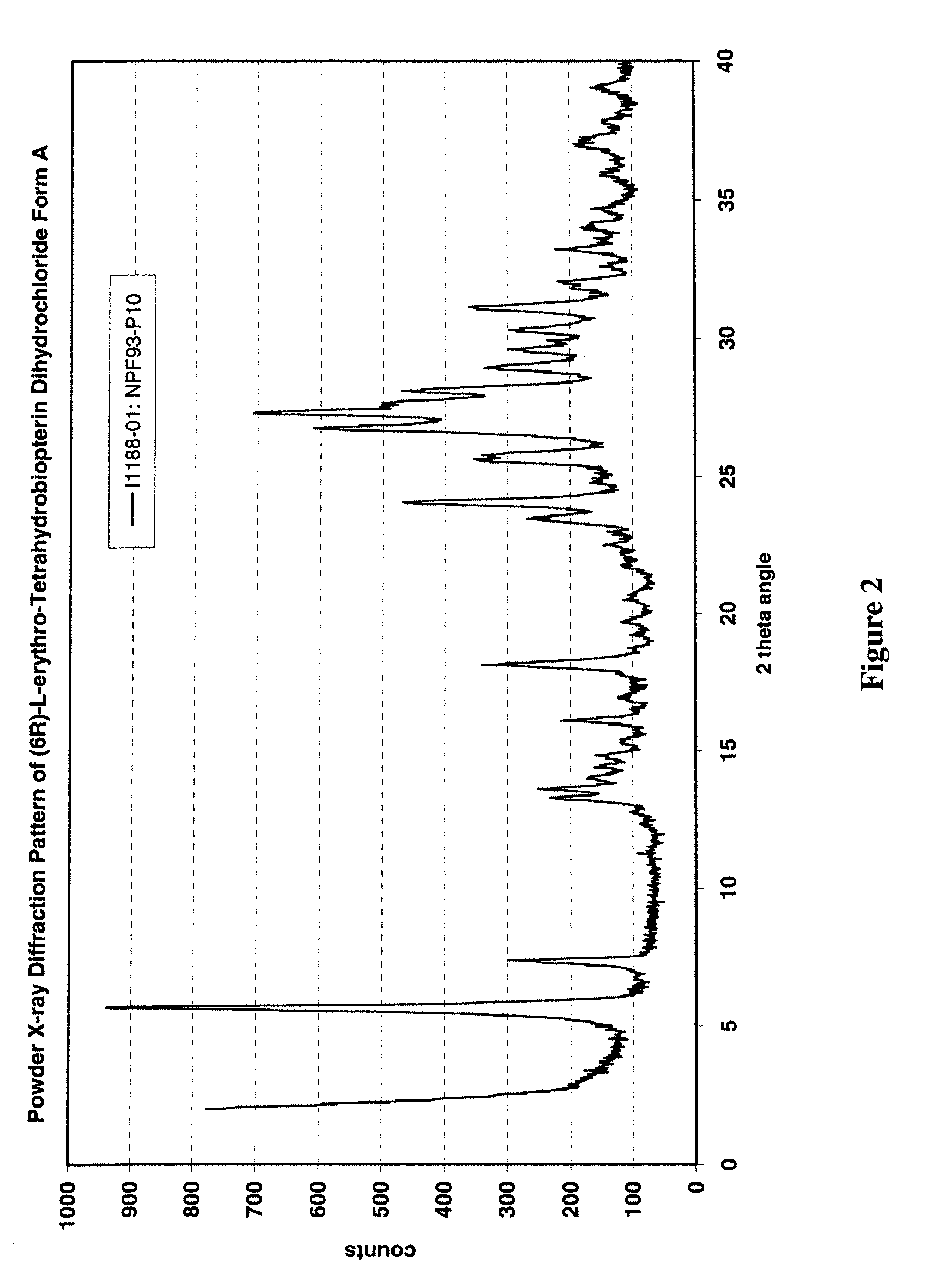
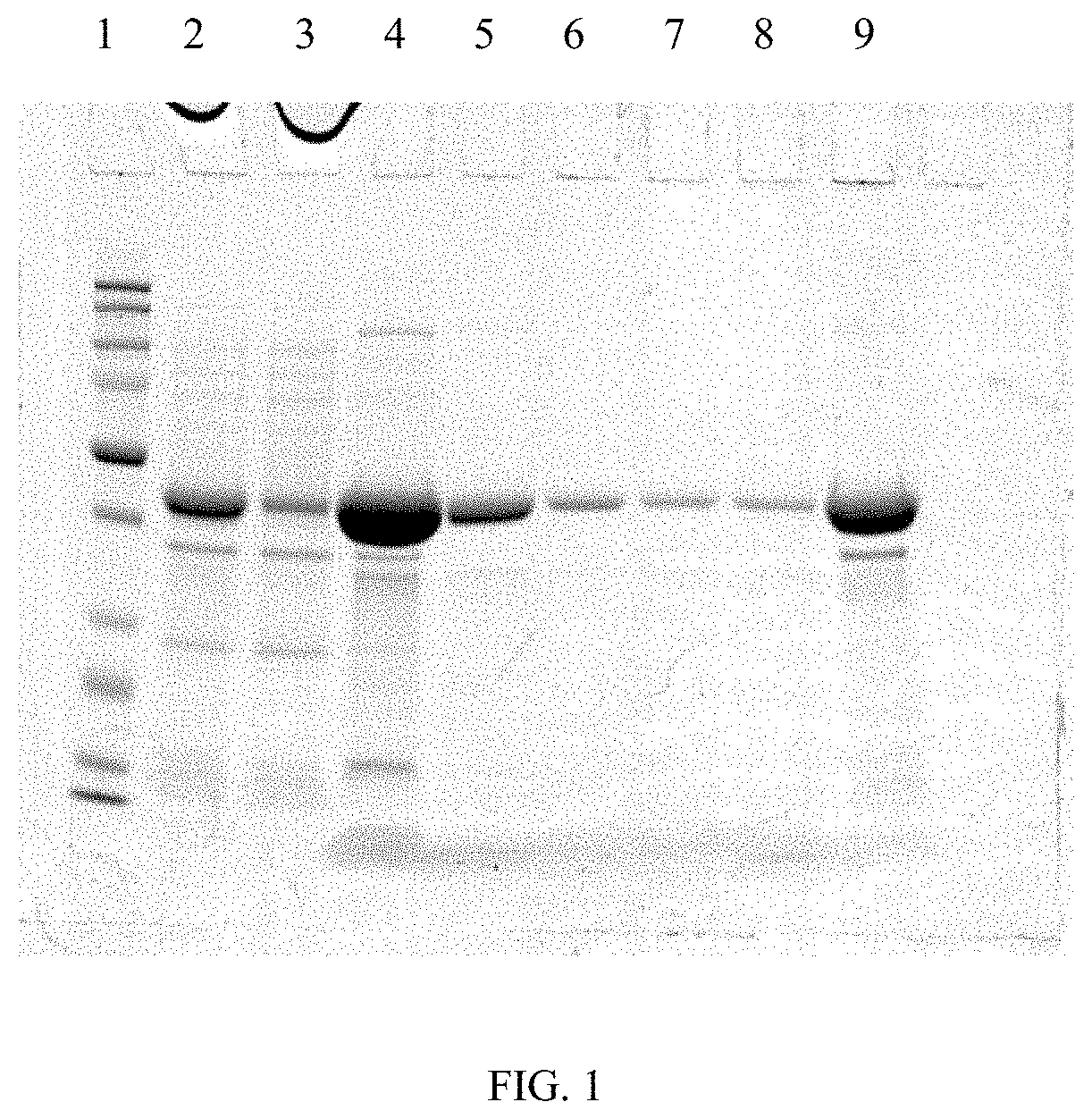
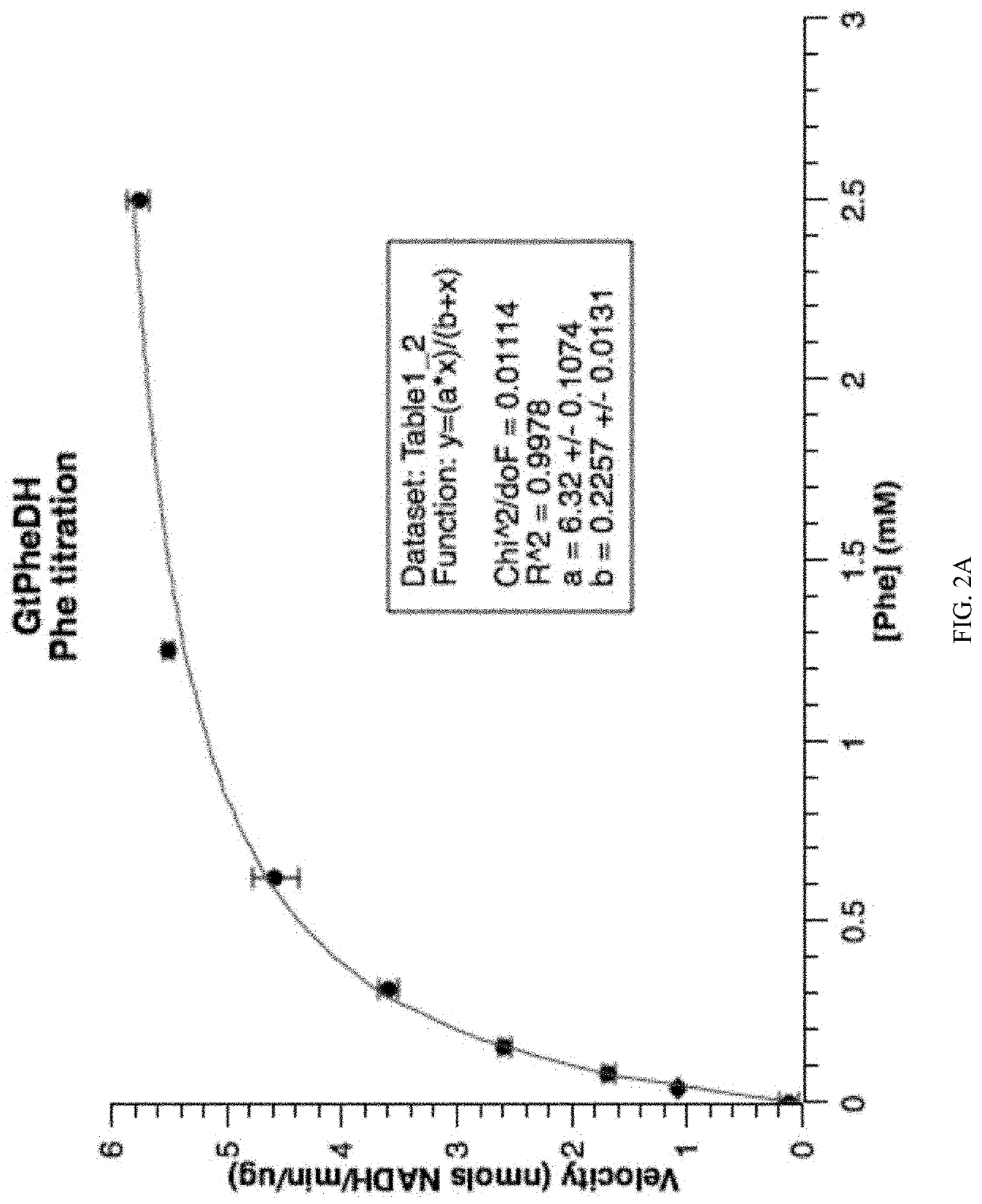
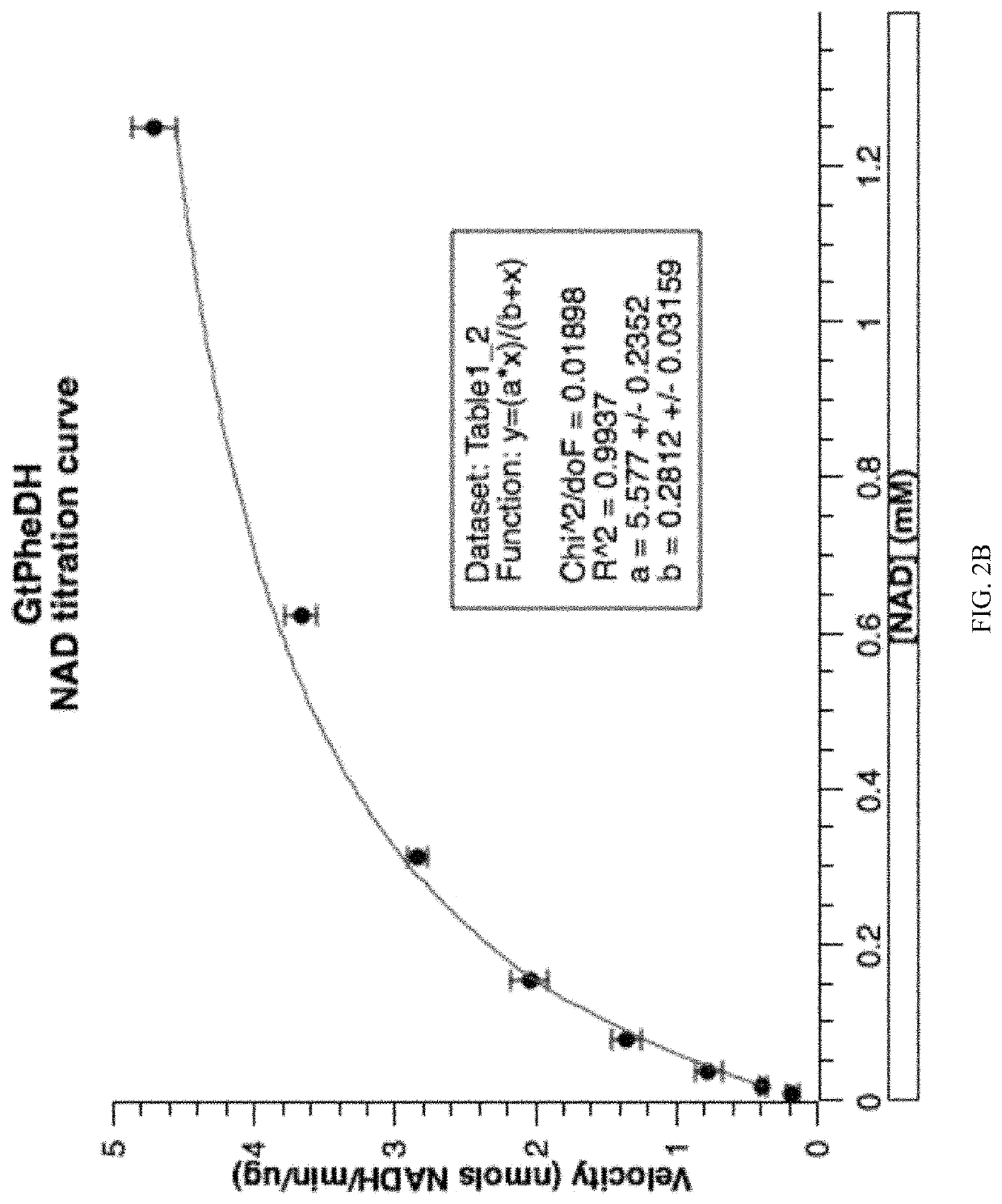
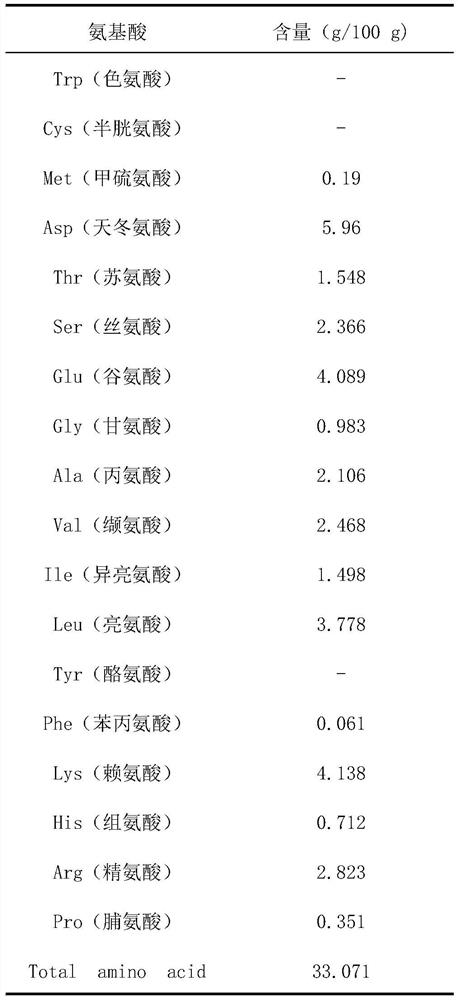
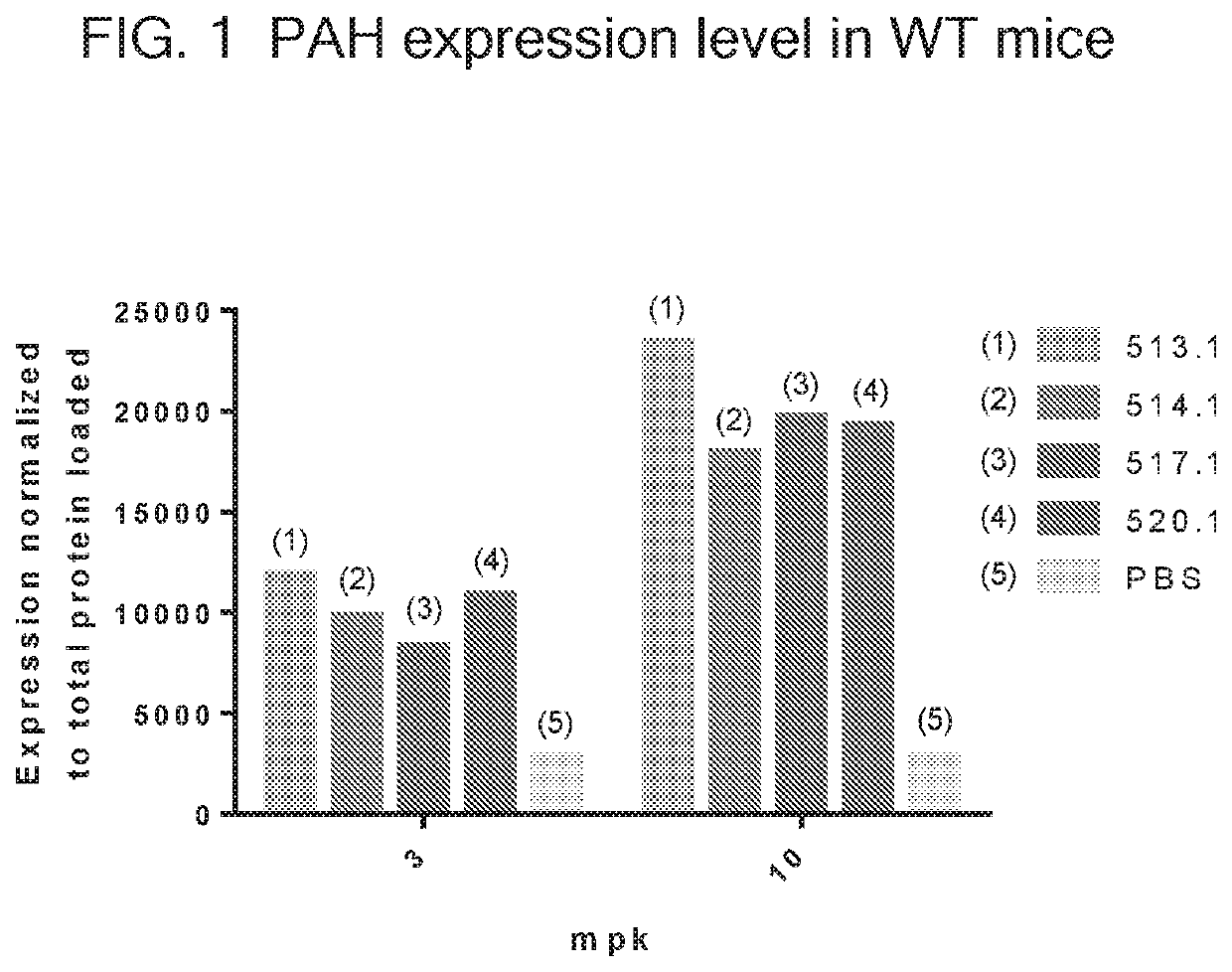
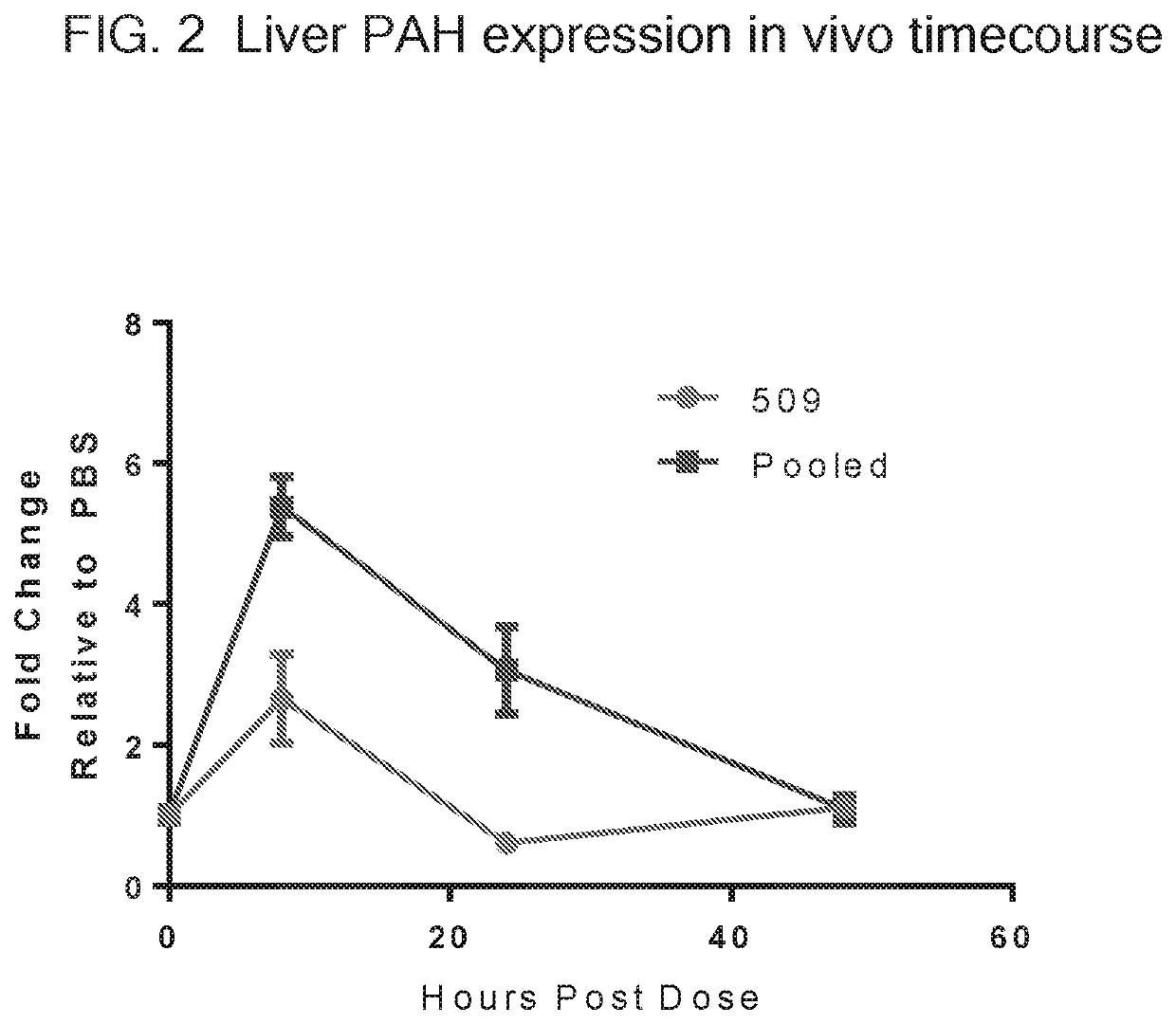
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Phenylketonurias" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genetic disorder in which the body lacks the enzyme necessary to metabolize phenylalanine
MRNA therapy for phenylketonuria
ActiveUS9522176B2Efficient productionEffective treatmentNervous disorderSugar derivativesLipofectaminePhenylalanine hydroxylase cofactor
The present invention provides, among other things, methods of treating phenylketonuria (PKU), including administering to a subject in need of treatment a composition comprising an mRNA encoding phenylalanine hydroxylase (PAH) at an effective dose and an administration interval such that at least one symptom or feature of PKU is reduced in intensity, severity, or frequency or has delayed in onset. In some embodiments, the mRNA is encapsulated in a liposome comprising one or more cationic lipids, one or more non-cationic lipids, one or more cholesterol-based lipids and one or more PEG-modified lipids.
Owner:TRANSLATE BIO INC
Methods and compositions for the treatment of metabolic disorders
InactiveUS20080090832A1Avoid high concentrationsBiocideMetabolism disorderMedicineCombination therapy
The present invention is directed to a novel methods and compositions for the therapeutic intervention in hyperphenylalaninemia. More specifically, the specification describes methods and compositions for treating various types of phenylketonurias using compositions comprising BH4. Combination therapies of BH4 and other therapeutic regimens are contemplated.
Owner:BIOMARIN PHARMA INC +1
Compositions of prokaryotic phenylalanine ammonia-lyase variants and methods of using compositions thereof
Provided herein are phenylalanine ammonia-lyase (PAL) variants produced by prokaryotes, wherein such prokaryotic PAL variant has a greater phenylalanine-converting activity and / or a reduced immunogenicity as compared to a wild-type PAL. Further provided are compositions of prokaryotic PAL and biologically active fragments, mutants, variants or analogs thereof, as well as methods for the production, purification, formulation, and use of such compositions for industrial and therapeutic purposes, [e.g., treating hyperphenylalaninemia, (including phenylketonuria)], and other disorders (including cancer).
Owner:BIOMARIN PHARMA INC
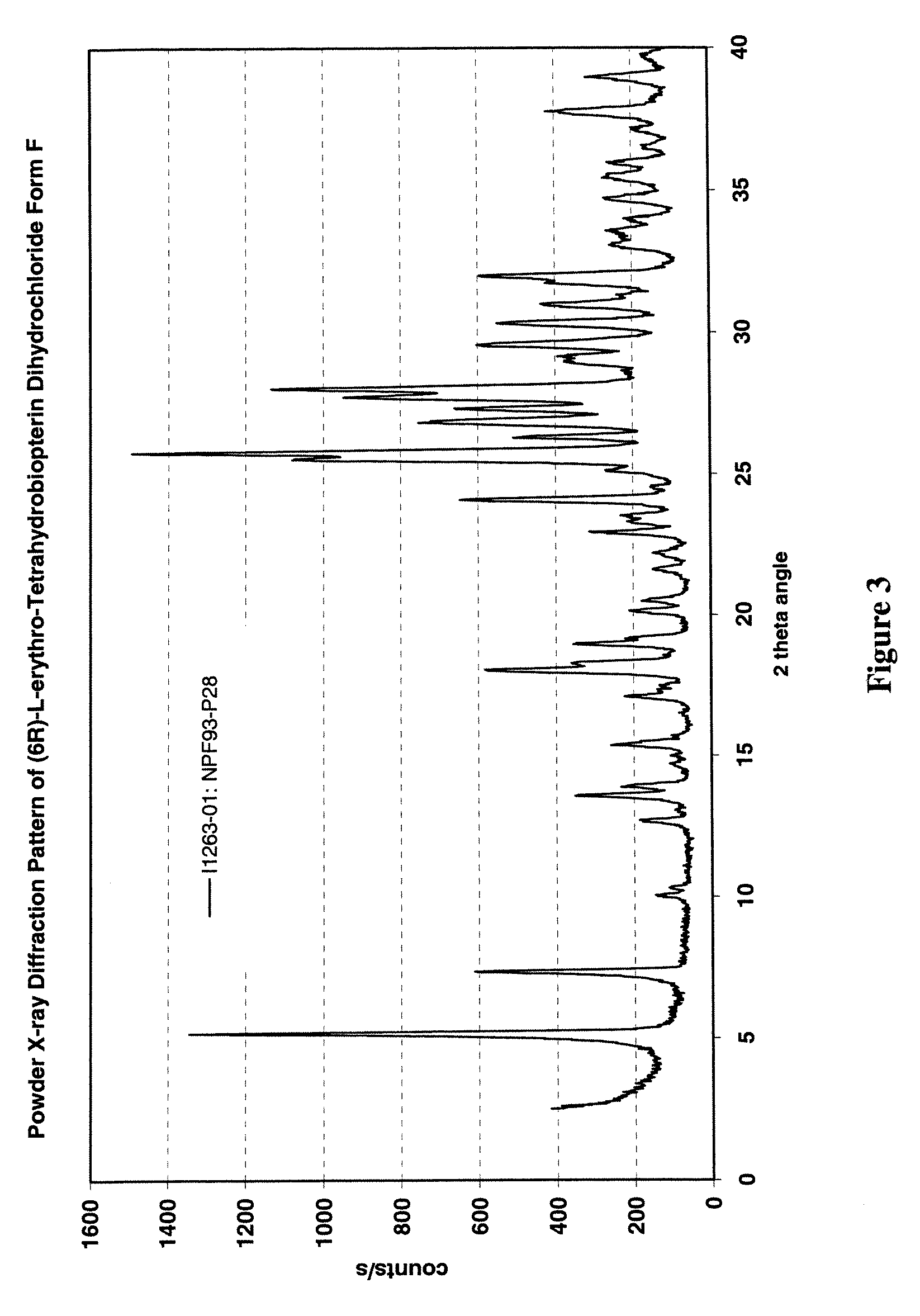
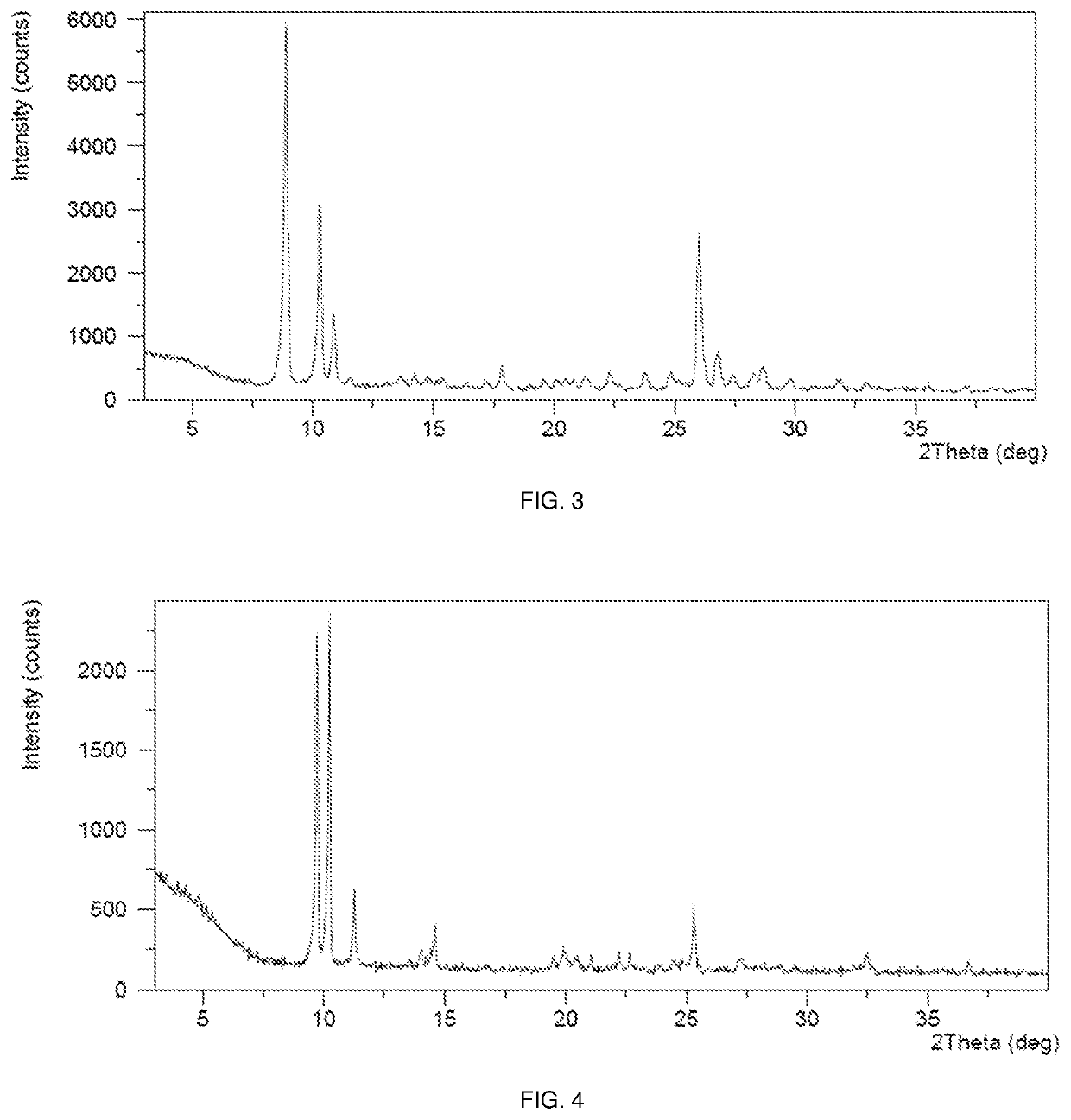
Polymorphs of sepiapterin and salts thereof
ActiveUS11072614B2Diminishment of extentImprove clinical outcomesOrganic active ingredientsNervous disorderDiseasePterin
Disclosed are crystalline forms of sepiapterin free base selected from polymorphs A, B, C, D, E, F, and G, and combinations thereof, as well as crystalline polymorphs of salts of sepiapterin. Also disclosed are pharmaceutical compositions containing one or more such polymorphs and methods for preparing such polymorphs. Sepiapterin is useful in the treatment of a number diseases associated with low cellular levels of BH4, for example, phenylketonuria.
Owner:PTC THERAPEUTICS MP INC
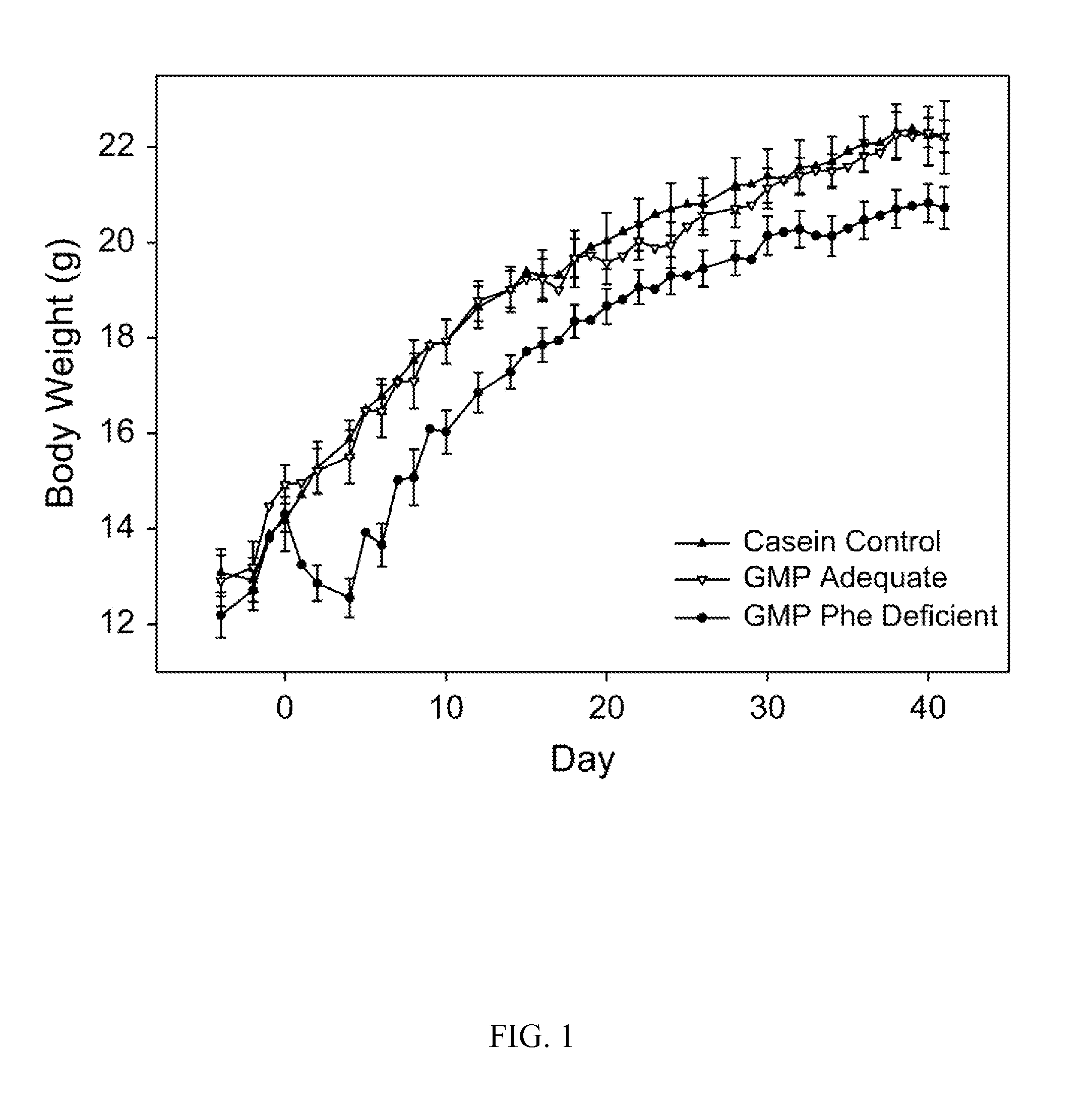
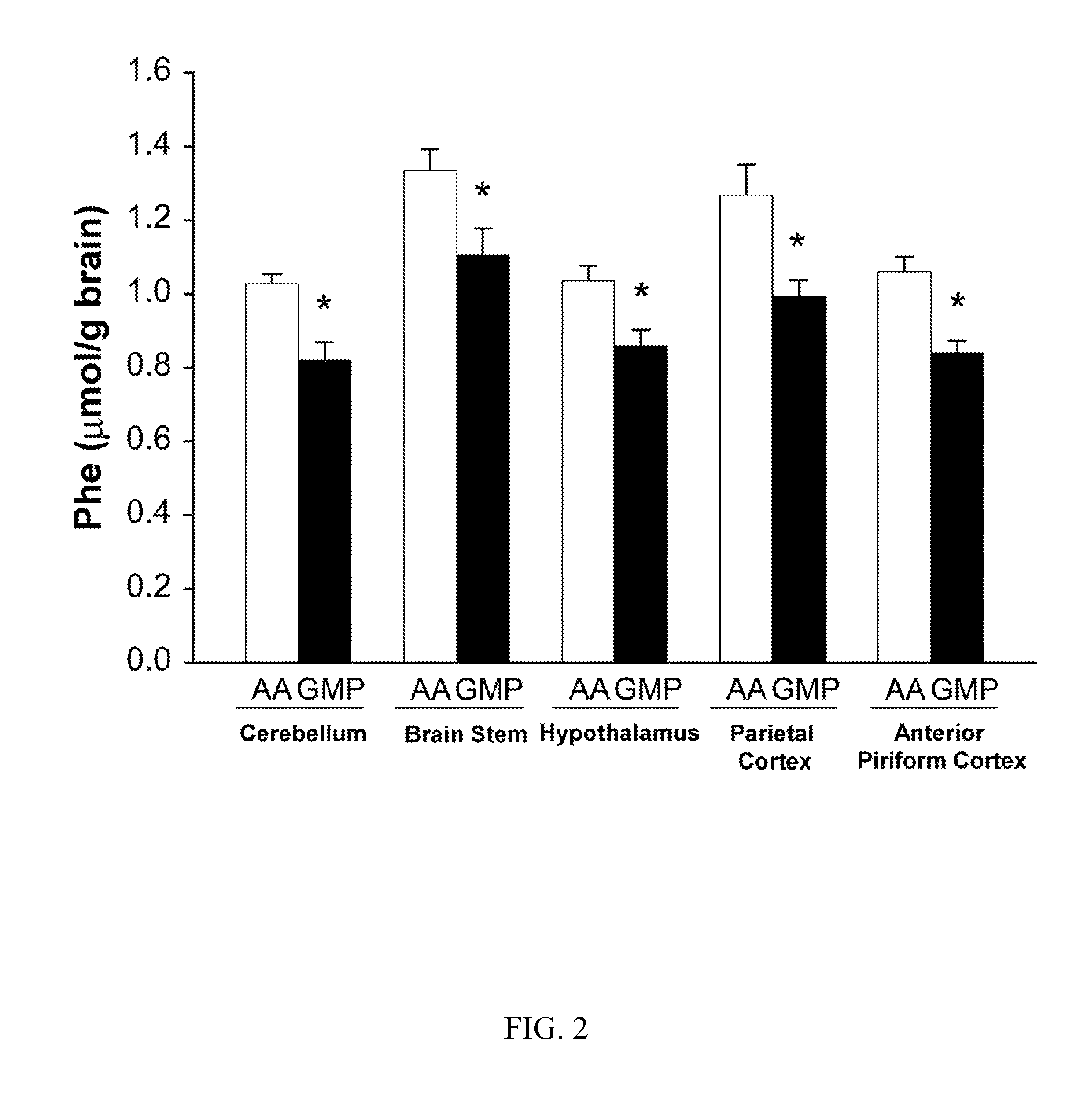
Glycomacropeptide medical foods for nutritional management of phenylketonuria and other metabolic disorders
InactiveUS20130196024A1Increase dietary compliance and quality of lifeNervous disorderFruit and vegetables preservationComplete proteinArginine
Medical foods containing glycomacroprotein and additional supplemented amounts of arginine, leucine, and optionally other amino acids, such as tyrosine, are disclosed. The medical foods can be used to provide the complete protein requirements for patients having metabolic disorders such as phenylketonuria.
Owner:WISCONSIN ALUMNI RES FOUND
Pharmaceutical compositions comprising sepiapterin and uses thereof
PendingUS20200061070A1Improve propertiesImprove bioavailabilityPowder deliveryOrganic active ingredientsDiseasePterin
The present invention features pharmaceutical compositions including sepiapterin, or a pharmaceutically acceptable salt and / or co-crystal thereof, and methods for the treatment of tetrahydrobiopterin-related disorders (e.g., tetrahydrobiopterin deficiency or phenylketonuria) with such compositions.
Owner:PTC THERAPEUTICS MP INC
Non-viral DNA vectors and uses thereof for expressing phenylalanine hydroxylase (PAH) therapeutics
The application describes ceDNA vectors having linear and continuous structure for delivery and expression of a transgene. ceDNA vectors comprise an expression cassette flanked by two ITR sequences, where the expression cassette encodes a transgene encoding PAH protein. Some ceDNA vectors further comprise cis-regulatory elements, including regulatory switches. Further provided herein are methods and cell lines for reliable gene expression of PAH protein in vitro, ex vivo and in vivo using the ceDNA vectors. Provided herein are method and compositions comprising ceDNA vectors useful for the expression of PAH protein in a cell, tissue or subject, and methods of treatment of diseases with said ceDNA vectors expressing PAH protein. Such PAH protein can be expressed for treating disease, e.g., Phenylketonuria (PKU).
Owner:GENERATION BIO CO
Phenylketonuria PAH gene detection kit
InactiveCN111172277AGuaranteed accuracyAvoid false negative resultsMicrobiological testing/measurementPrenatal diagnosisPhenylketonurias
The invention discloses a phenylketonuria PAH gene detection kit, and relates to the technical field of gene detection. The kit includes a reagent for detecting polymorphic sites; and the polymorphicsites at least include one of c.611A>G, c.728G>A, c.1068C>A, c.1238G>C, c.442-1G>A, c.1197A>T, c.721C>T and c.331C>T. The PAH gene detection kit provides the allele-specific scorpion primers and shared primers thereof of eight common mutation sites for detecting PAH genes, so that a joint detection platform can be constructed, synchronous joint detection can be realized, detection specificity canbe enhanced, costs can be reduced, and detection time can be shortened; and the kit has important significance on carrying out the screening, genetic counseling and prenatal diagnosis of people with phenylketonuria.
Owner:北京杰智慧创科技发展中心
Preparation method of biological polypeptide for phenylketonuria medicinal food, and use thereof
PendingCN110229858AMeet the restrictionsMeet food requirementsPeptide preparation methodsFermentationPhenylalaninePepsin
The present invention discloses a preparation method of a biological polypeptide for phenylketonuria medicinal food, and a use thereof, belongs to the field of biomedicines, and solves a problem thatseparation and purification of phenylalanine-free biological polypeptide are difficult. The preparation method comprises the following steps: one of whey powder, milk powder or fresh milk is selectedto be used as a preparation raw material; trypsin, pepsin and chymosin are selected to be combined as biological enzymes for preparing the biological polypeptide; the biological enzymes are added in the preparation raw material for enzymatic hydrolysis; and separation and purification of the target biological polypeptide are conducted, and a mass fraction of phenylalanine in the purified biological polypeptide is 0-0.15%. The amount of the phenylalanine in the biological polypeptide is controlled to be within 0.15%, avoiding a problem of difficulty in separation and purification caused by pursuing product purity and reducing industrial production costs; and the prepared biological polypeptide solves limitation requirements of patients with phenylketonuria for the phenylalanine and also ensures nutritional needs of the patients for amino acids and proteins.
Owner:兰州天禾生物催化技术有限公司
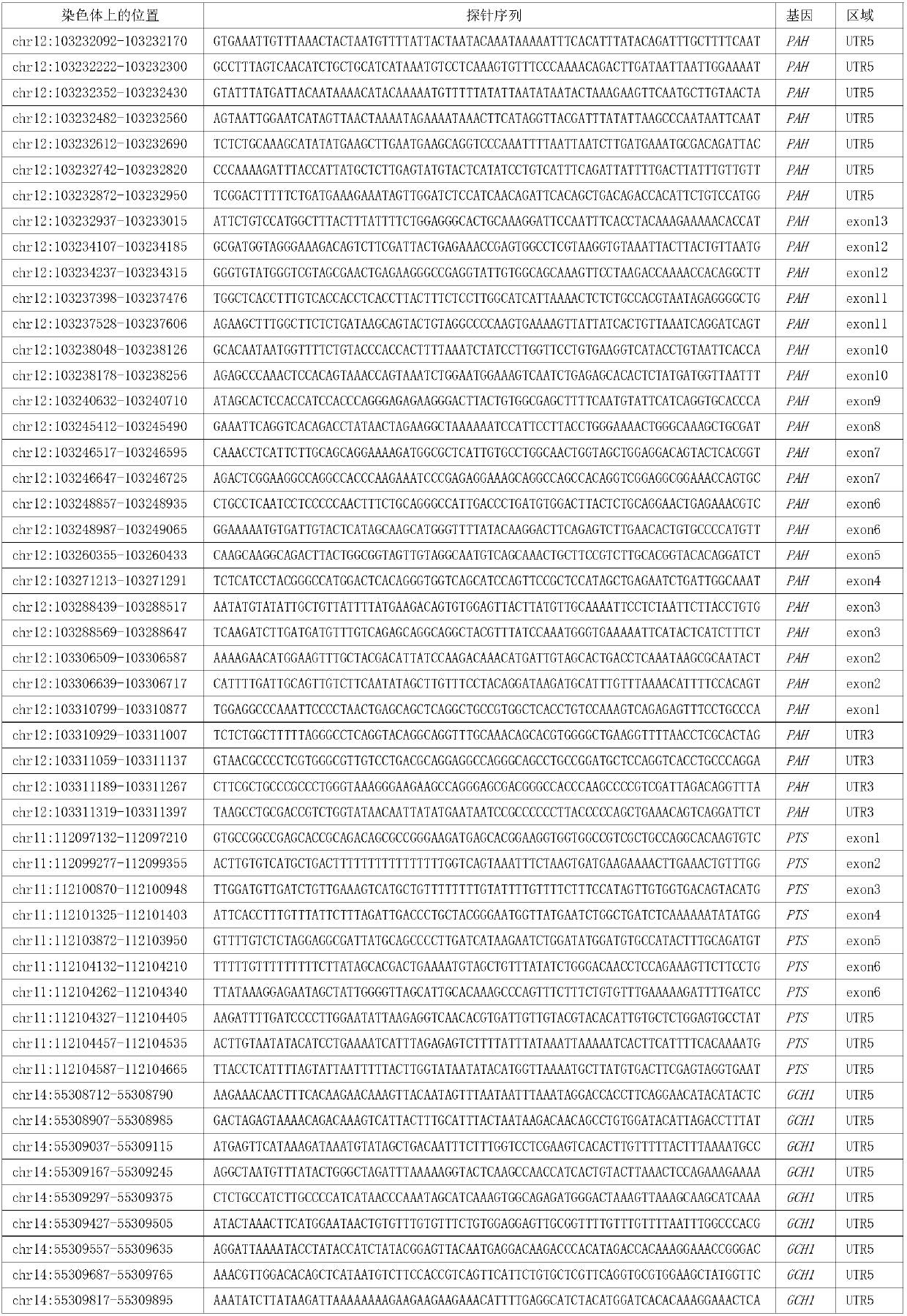
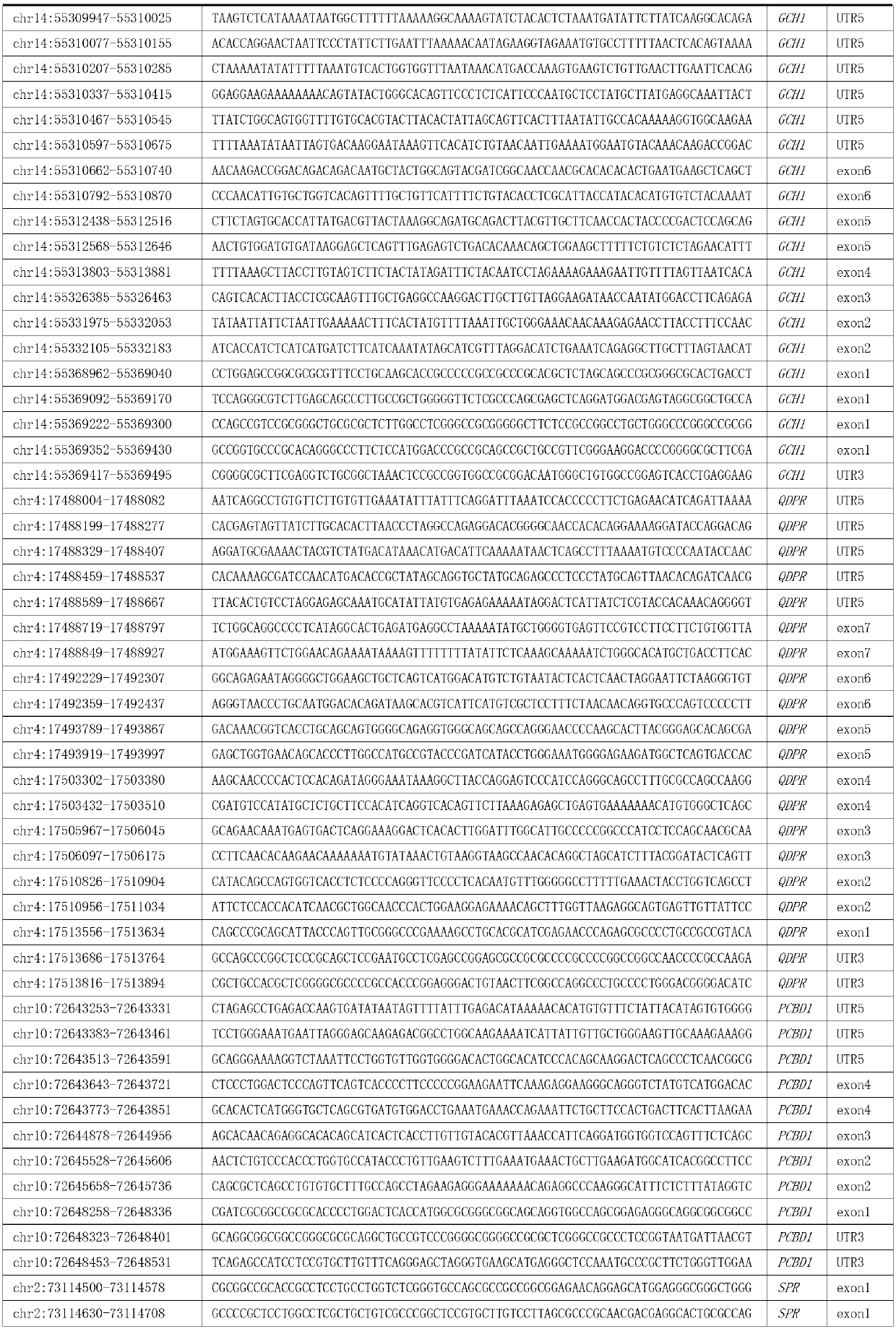
Reagent kit and special capture probe set thereof for detecting pathogenic genes related with phenylketonuria
ActiveCN111394446AFacilitate the discovery of disease-causing mutationsMicrobiological testing/measurementDNA/RNA fragmentationQDPRPhenylketonurias
The invention discloses a reagent kit and special capture probe set thereof for detecting pathogenic genes related with phenylketonuria. The capture probe set provided by the invention consists of probes shown as a sequence 1 to a sequence 108 in a sequence table. The reagent kit can detect 6 pathogenic genes related with phenylketonuria, including PAH, PTS, GCH1, QDPR, PCBD1 and SPR at the same time, can detect phenylketonuria of various point mutation types, and can detect the overall length of the genes of PAH, PTS, GCH1, QDPR, PCBD1 and SPR, finding of new pathogenic mutation is facilitated, and a new finding means is provided for heredity foundation of the phenylketonuria.
Owner:北京迈基诺基因科技股份有限公司
Preparation method of low-phenylalanine egg white protein peptide
ActiveCN112080542AImprove the effect of enzymatic hydrolysisIncrease contact areaHydrolysed protein ingredientsPeptide preparation methodsBiotechnologyPROTEIN S HEERLEN
The invention discloses a preparation method of low-phenylalanine egg white protein peptide, and belongs to the field of foods. The method comprises the following steps that after egg white protein powder is subjected to dissolving, heat treatment and shearing treatment, trypsin is combined with flavourzyme to carry out two-step protease enzymolysis on the egg white protein powder, an obtained enzymatic hydrolysate is centrifuged to remove insoluble substances, a collected supernatant is subjected to static adsorption by activated carbon to remove phenylalanine so as to obtain a low-phenylalanine egg white protein peptide mixed solution, and reverse osmosis concentration and drying are carried out so as to obtain low-phenylalanine egg white protein peptide powder. According to the invention, egg white protein is deeply hydrolyzed through a two-step enzyme method, and the hydrolysis degree reaches 50% or above; and the method provided by the invention can remove 98% or above of the phenylalanine in the egg white protein, the content of obtained polypeptide phenylalanine accounts for 0.11-0.19% of the equivalent weight of the protein, and the method has the potential of being used for preparing formula foods or health care products for special medical purposes of patients with phenylketonuria and the like.
Owner:JIANGNAN UNIV
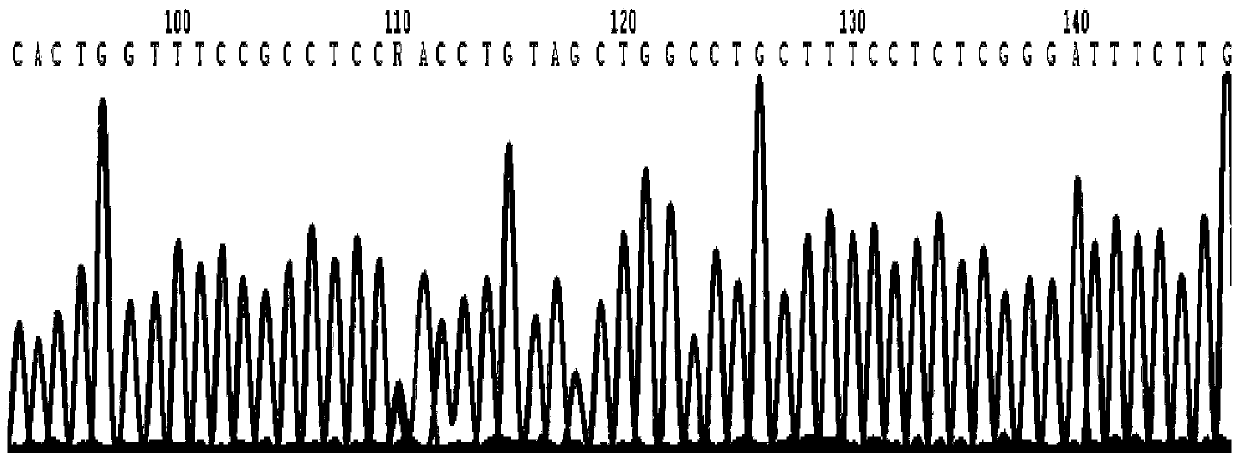
Primer, kit and method for detecting PAH gene mutation
PendingCN111057759AReduce usageGood amplification efficiencyMicrobiological testing/measurementDNA/RNA fragmentationMedicineExon
The invention discloses primers for detecting hotspot mutation of a phenylketonuria related gene PAH gene. The primers comprise primers for amplifying the sixth exon and the seventh exon of the PAH gene; by adopting a Sanger sequencing technology, the primers can be used for rapidly detecting mutation of the sixth exon and the seventh exon of the PAH gene in the body of a patient with phenylketonuria. The detection result completed by the kit is accurate, and the kit has important reference significance for rapid diagnosis of phenylketonuria patients.
Owner:北京艾迪康医学检验实验室有限公司 +1
Formula powder used for phenylketonuria patient, and preparation method for formula powder
PendingCN110693031AGuaranteed intakeMaintain metabolismFood ingredient functionsVegetable oilAmino acid supplement
The invention aims to disclose a formula powder used for a phenylketonuria patient, and a preparation method for the formula powder. The formula powder is prepared from the following ingredients in parts by weight: 60-90% of plant oil fatty powder, 8-20% of compound amino acid, 0-6.45% of Fos (Fructo oligosaccharide), 0-6.45% of Gos (galacto-oligosaccharide), 0.012-0.058% of nucleotide, 0.044-0.13% of vitamin complex and 1.195-4.717% of complex mineral substance, and does not contain phenylalanine. Compared with the prior art, fat, carbohydrates, amino acids, vitamins, mineral substances and the like are taken as a basic composition, a proportion is scientific, and the growth and development requirements of infants can be met. The plant oil fatty powder contains the fat and the carbohydrates, energy is provided for organisms, the compound amino acid supplements the growth and development requirements of a human body with necessary amino acids, in addition, phenylalanine is not added, the accumulation of alanine and a ketonic acid thereof is not generated, the intake of the amino acids of a patient is guaranteed, the normal metabolism of the amino acid of the organisms is maintained, and the purposes of the formula powder disclosed by the invention is realized.
Owner:杨国民
Formula powder for children with phenylketonuria and preparation method thereof
ActiveCN104012659BAdequate nutrient absorptionEnsure physiological needsMilk preparationBiotechnologyNutrition
Owner:中恩(天津)医药科技有限公司
Nutritional composition for the management of phenylketonuria and method of preparation
PendingUS20210235739A1Organic active ingredientsPeptide/protein ingredientsProtein profilingArginine
Nutritional formulation suitable to manage the diet for phenylketonuria, which comprises glycomacropeptide (GMP) as the primary source of proteins and additional complementary essential amino acids, to complete the required protein profile, supplied in a mixture comprising arginine, cystine, histidine, tryptophan, tyrosine and leucine.
Owner:SOC DES PROD NESTLE SA
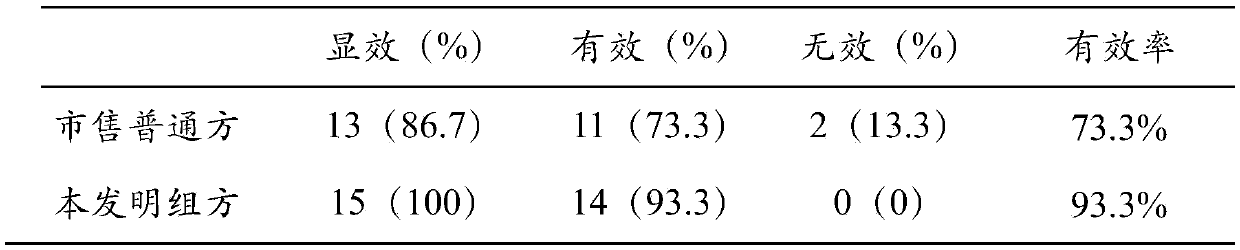
Application of composition of enterococcus and berberine in treatment of phenylketonuria
PendingCN113694087AReduce contentReduce accessOrganic active ingredientsMetabolism disorderBerberinePharmaceutical drug
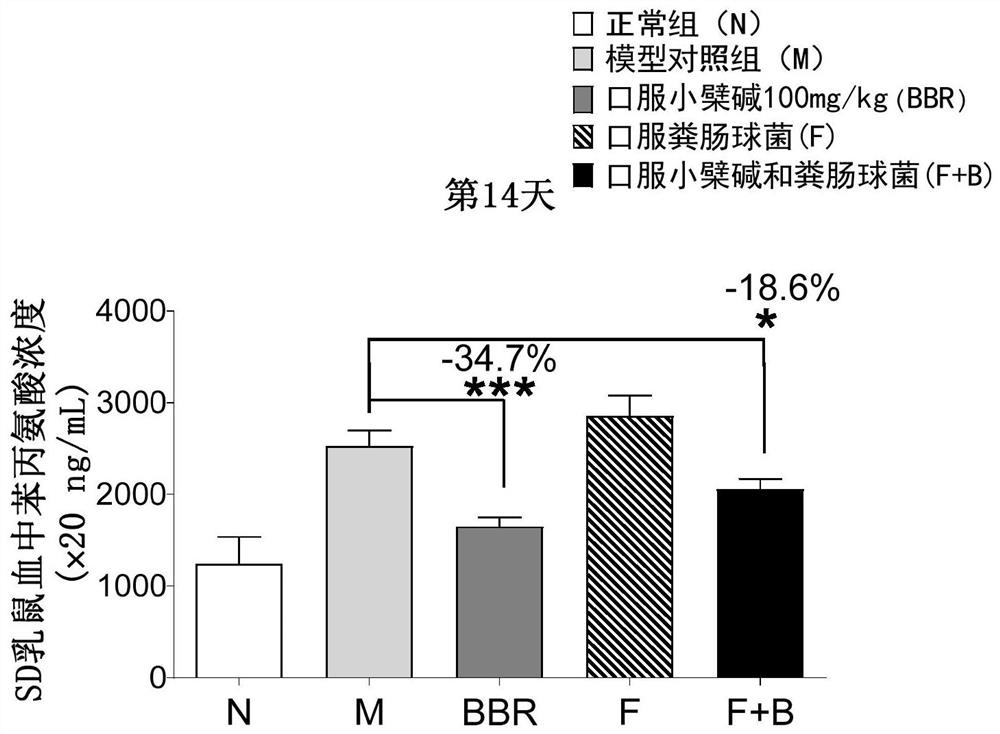
The invention relates to the technical field of medicines, and provides application of a composition of enterococcus and berberine or berberine salt in preparation of medicines for preventing and / or treating phenylketonuria. Specifically, the enterococcus can obviously metabolize phenylalanine in an in-vitro culture medium system; the enterococcus belongs to the phylum of Thyrium, Bacillus, Lactobacillus and Enterococcus family. Meanwhile, the combination of the enterococcus and the berberine can significantly treat the phenylketonuria, and the plasma phenylalanine of a suckling mouse with the phenylketonuria can be reduced by 34.2% after 4 weeks of treatment. Therefore, the combination of the enterococcus and the berberine has an outstanding treatment effect in preventing and / or treating the phenylketonuria.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Construction method and application of probiotic engineering strain for treating phenylketonuria
The invention provides a recombinant expression vector, the recombinant expression vector can be expressed in lactobacillus reuteri to generate phenylalanine deaminase, and the phenylalanine deaminase can decompose phenylalanine and metabolites thereof, which are ingested in the stomach and intestine, so that the phenylalanine and the metabolites thereof are prevented from being accumulated in a body, and the purpose of treating the phenylketonuria is achieved.
Owner:苏州优信合生技术有限公司
Method for reducing content of phenylalanine in product
InactiveCN114028839AAdsorption will notLarge exchange capacitySolid sorbent liquid separationFermentationActivated carbonMedicine
The invention discloses a method for reducing the content of phenylalanine in a product, and belongs to the field of food processing. Protein-containing products are used as raw materials, hydrolysate obtained through enzymolysis is adsorbed through macroporous resin, and the removal rate of phenylalanine in the protein hydrolysate after adsorption through macroporous non-polar adsorption resin can reach 15%-90% by adjusting the adsorption temperature, the pH value of adsorption liquid and the adsorption time. The resin adsorption specificity is high, the removal rate of phenylalanine in the obtained product is high, the defects that the adsorption range of traditional activated carbon is wide, nutrient substances are lost and the like are overcome through resin adsorption, the operation process is simple and convenient, and the method is suitable for industrial production; and the method has the potential of being used for preparing special medical purpose formula food or health care products for patients suffering from phenylketonuria and the like.
Owner:CHINA AGRI UNIV
Methods and compositions for treating phenylketonuria
ActiveUS10792339B2Lower Level RequirementsReducing phenylalanine levelCarbon-nitrogen lyasesOrganic active ingredientsPhenylalanine dehydrogenaseBiochemistry
The present invention provides compositions and methods of treating hyperphenylalaninemia (e.g., phenylketonuria) in a subject in need thereof comprising administering to the subject an effective amount of a phenylalanine dehydrogenase (PheDH) polypeptide. The present invention also provides pharmaceutical formulations comprising PheDH for lowering the phenylalanine concentration in the subject (e.g., in the intestines and / or blood).
Owner:CHILDRENS NAT MEDICAL CENT
A kind of preparation method of low-phenylalanine egg white protein peptide
ActiveCN112080542BImprove the effect of enzymatic hydrolysisIncrease contact areaHydrolysed protein ingredientsPeptide preparation methodsBiotechnologyPROTEIN S HEERLEN
The invention discloses a preparation method of low-phenylalanine egg white protein peptide, which belongs to the field of food. The method of the present invention comprises the following steps: after the egg white protein powder is dissolved, heat-treated and sheared, it is subjected to two-step proteolysis by using trypsin combined with flavor protease, and the obtained enzymolyzate is centrifuged to remove insoluble matter, The collected supernatant is statically adsorbed by activated carbon to remove phenylalanine to obtain a low-phenylalanine egg white protein peptide mixture, which is concentrated by reverse osmosis and dried to obtain a low-phenylalanine egg white protein peptide powder. The present invention deeply hydrolyzes egg white protein through a two-step enzymatic method, and the degree of hydrolysis reaches more than 50%; the method of the present invention can remove more than 98% of phenylalanine in egg white protein, and at the same time, the content of phenylalanine in the obtained polypeptide accounts for 50% of the protein equivalent. 0.11‑0.19%, which has the potential to be used to prepare special medical formula food or health products for patients with phenylketonuria.
Owner:JIANGNAN UNIV
Low-phenylalanine vegetable protein peptide and preparation method thereof
PendingCN114032272AFacilitate dissociationHigh purityPeptide preparation methodsFermentationBiotechnologyAlkaline protease
The invention discloses a low-phenylalanine vegetable protein peptide and a preparation method thereof, and belongs to the field of food. The content of phenylalanine in the low-phenylalanine plant protein peptide accounts for 0.13-0.19% of the protein equivalent, and the low-phenylalanine plant protein peptide has the potential of being used for preparing formula food or health care products with special medical purposes for patients suffering from phenylketonuria and the like. The method comprises the following steps: dissolving plant protein powder, carrying out two-step protease enzymolysis on the plant protein powder by using alkaline protease and flavourzyme, and centrifuging the obtained zymolyte to remove insoluble substances; enabling the collected supernate to be subjected to static adsorption by activated carbon to remove phenylalanine, and obtaining a low-phenylalanine vegetable protein peptide mixed solution; carrying out vacuum filtration to remove the activated carbon, and performing freeze-drying to obtain the low-phenylalanine vegetable protein peptide powder. The vegetable protein is hydrolyzed through a compound enzyme method, more than 96% of phenylalanine in the vegetable protein can be removed, and the preparation method of the low-phenylalanine protein peptide is effective.
Owner:CHINA AGRI UNIV
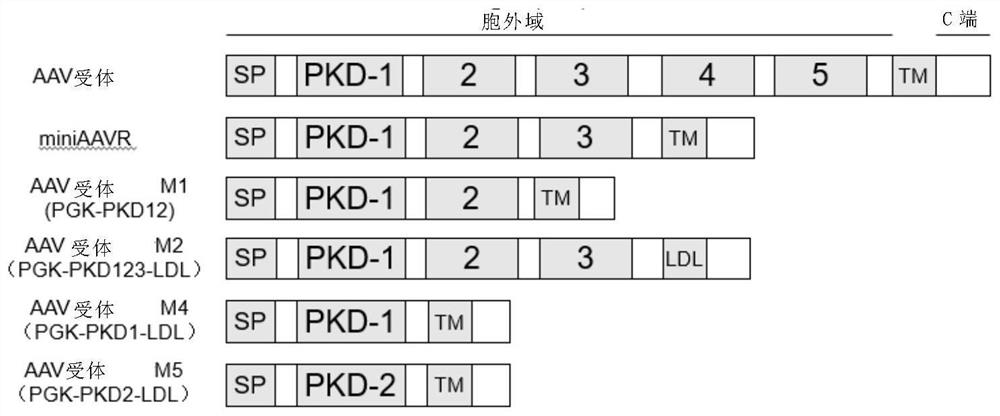
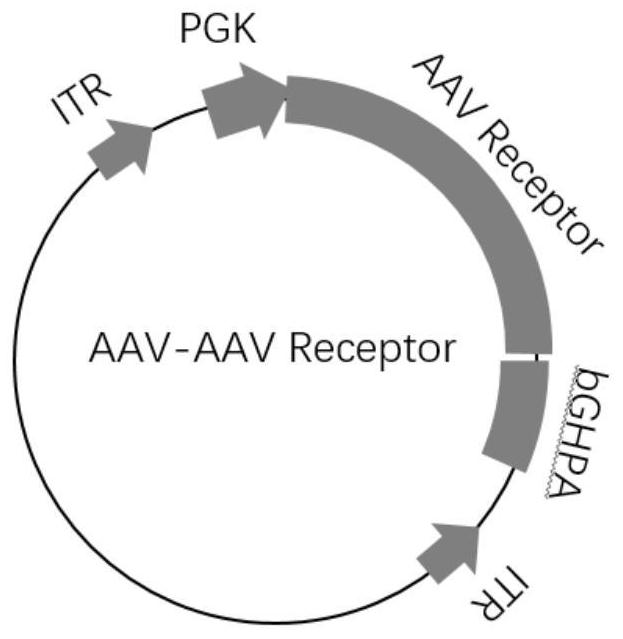
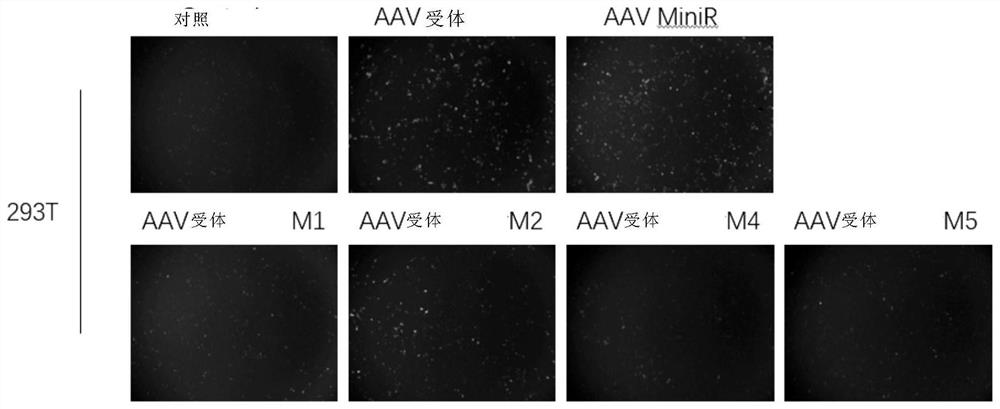
A method for improving the efficiency of gene therapy by overexpressing adeno-associated virus receptor
The present invention provides a method for improving gene therapy efficiency by overexpressing adeno-associated virus receptors, specifically, the present invention provides an infection enhancing element, the element has the following structure: Z1-Z2-Z3-Z4(I ), wherein Z1 is an optional signal peptide element; Z2 is a PKD‑1 domain, a PKD‑2 domain, or a PKD‑1‑PKD‑2 domain; Z3 is none or a PKD‑3 domain; Z4 is Transmembrane element; "‑" indicates the peptide bond connecting the above elements; the additional condition is that when Z3 is a PKD‑3 element, Z4 is not a transmembrane element of AAVR. The infection enhancing element of the present invention can significantly improve the infection efficiency of the AAV virus vector system, and also improve the gene editing efficiency, and can significantly reduce the blood phenylalanine content of mice with phenylketonuria, thereby treating phenylketonuria .
Owner:EAST CHINA NORMAL UNIV +1
Pharmaceutical compositions comprising sepiapterin and uses thereof
The present invention features pharmaceutical compositions including sepiapterin, or a pharmaceutically acceptable salt and / or co-crystal thereof, and methods for the treatment of tetrahydrobiopterin-related disorders (e.g., tetrahydrobiopterin deficiency or phenylketonuria) with such compositions.
Owner:PTC医疗MP公司
Application of engineering enterococcus faecalis in drugs for treating phenylketonuria
PendingCN113755516AReduce accessConvenient treatmentMetabolism disorderNucleic acid vectorPhenylalanine hydroxylase cofactorTherapeutic effect
The invention belongs to the technical field of biology, and provides an application of engineering enterococcus faecalis capable of expressing human phenylalanine hydroxylase in drugs for treating phenylketonuria, and plasmids containing the human-derived phenylalanine hydroxylase gene optimized according to enterococcus faecalis codons are introduced into enterococcus faecalis, so that the human phenylalanine hydroxylase is expressed in the intestinal tract. Experimental results show that: the human phenylalanine hydroxylase is successfully expressed in the enterococcus faecalis through a gene engineering technology; the engineering enterococcus faecalis can obviously metabolize phenylalanine in an in-vitro culture medium system; meanwhile, the phenylketonuria can be remarkably treated by orally taking the engineering enterococcus faecalis, and the phenylalanine in the plasma of a suckling mouse with phenylketonuria can be reduced by 46.8% after the engineering enterococcus faecalis is used for treatment for 4 weeks. It is shown that the engineering enterococcus faecalis has an outstanding treatment effect in prevention and / or treatment of phenylketonuria.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Triamterene or nolatrexed for use in the treatment of phenylketonuria
PendingUS20220133726A1Effect activity of in activityEffect of in stabilityOrganic active ingredientsMetabolism disorderPyridazinePharmaceutical medicine
Owner:SOM INNOVATION BIOTECH SA
Methods and compositions for treating phenylketonuria
ActiveUS8440182B2Carbon-nitrogen lyasesPeptide/protein ingredientsPhenylalanine hydroxylase cofactorSmall intestine
Provided herein are methods for treating a subject suffering from phenylketonuria by administering a phenylalanine hydroxylase (“PAH”) and / or a phenylalanine ammonia lyase (“PAL”) to the subject under conditions effective to deliver the phenylalanine 4-hydroxylase and / or PAL to the subject's small intestine. Also provided are methods for increasing the therapeutic activity of a phenylalanine 4-hydroxylase by thiolating the phenylalanine 4-hydroxylase. In addition, provided are oral dosage forms that include a phenylalanine 4-hydroxylase and / or a PAL and an enteric coating.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Engineering probiotics for treating phenylketonuria as well as construction method and application thereof
ActiveCN113969292AEfficient degradationReduce intakeBacteriaPeptide/protein ingredientsNucleotideProbiotic bacterium
The invention relates to the fields of medicine and biotechnology, in particular to engineering probiotics for treating phenylketonuria as well as a construction method and application thereof. The recombinant expression vector comprises a recombinant expression vector I and a recombinant expression vector II; the recombinant expression vector I is an expression vector containing a phenylalanine ammonia lyase gene PAL nucleotide sequence, a protein secretion processing domain coding gene prtP nucleotide sequence and a lactobacillus acidophilus constitutive promoter SL; the recombinant expression vector II is an expression vector containing a phenylalanine ammonia lyase gene PAL nucleotide sequence, a phenylalanine transfer pump gene pheP and a lactobacillus acidophilus constitutive promoter SL; the engineering probiotics capable of secreting phenylalanine ammonia lyase to the outside of the bacteria in gastrointestinal tracts are constructed for the first time, phenylalanine in digested food in the gastrointestinal tracts is effectively degraded, the phenylalanine intake of patients is reduced, and the engineering probiotics are used for treating PKU.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Method for improving recognition and/or working memory in hyperphenylalanimenia and phenylketonuria patients
The invention pertains to the use of a composition in the manufacture of a product or method for therapeutically improving, promoting, restoring or maintaining recognition and / or working memory in a subject suffering from PKU and / or in a subject suffering from or at increased risk of impaired recognition and / or working memory associated with PKU, said composition comprising therapeutic amounts of: (i) at least one ω-3 polyunsaturated fatty acid (LCPUFA) selected from the group consisting of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and docosapentaenoic acid (DPA); (ii) choline, a choline salt and / or choline ester; and (iii) at least one B vitamin selected from the group consisting of vitamin B6, vitamin B12 and vitamin B9, including functional equivalents thereof, preferably vitamin B6 and / or vitamin B12, and functional equivalents thereof. The invention also pertains to a composition for use in such use or method.
Owner:NV NUTRICIA
Therapeutics for phenylketonuria
PendingUS20200109375A1Ameliorating and preventing and treating diseaseLong half-lifeOrganic active ingredientsOxidoreductasesDiseasePhenylalanine hydroxylase cofactor
This invention provides a range of translatable polynucleotide and oligomer molecules for expressing a human phenylalanine hydroxylase (PAH), or a fragment thereof having PAH activity. The polynucleotide and oligomer molecules are expressible to provide the human PAH or a fragment thereof having PAH activity. The molecules can be used as active agents to express an active polypeptide or protein in cells or subjects. The agents can be used in methods for ameliorating, preventing, delaying onset, or treating a disease or condition associated with phenylketonuria, decreased metabolism of phenylalanine, or increased levels of phenylalanine in a subject.
Owner:ARCTURUS THERAPEUTICS
Nutritional composition for the treatment of metabolic diseases
PendingUS20210322353A1Reduce riskOrganic active ingredientsInorganic phosphorous active ingredientsDiseaseSulfur aminoacid
Owner:AJINOMOTO CAMBROOKE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com