Methods and compositions for the treatment of metabolic disorders
a metabolic disorder and composition technology, applied in the field of metabolic disorders, can solve the problems of severe mental retardation, irreversible damage to the nervous system, and excess phenylalanine (phe) in the brain and plasma, and achieve the effect of lowering the concentration of ph
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Evaluation with 6R-Tetrahydrobiopterin
[0226]The following example provides guidance on the parameters to be used for the clinical evaluation BH4 in the therapeutic methods of the present invention. As discussed herein throughout, BH4 will be used in the treatment of HPA including HPA, mild phenylketonuria (PKU) and classic PKU. Clinical trials will be conducted which will provide an assessment of daily oral doses of BH4 for safety, pharmacokinetics, and initial response of both surrogate and defined clinical endpoints. The trial will be conducted for a minimum, but not necessarily limited to, 6 weeks to collect sufficient safety information for 30 evaluable patients.
[0227]The initial dose for the trials will vary from about 10 to about 20 mg / kg. In the event that this dose does not produce a reduction in excess plasma phenylalanine (Phe) levels in a patient, or produce a significant direct clinical benefit measured as an ability to increase daily oral Phe intake without inc...
example 2
Preparation of Stabilized Crystallized Form of BH4
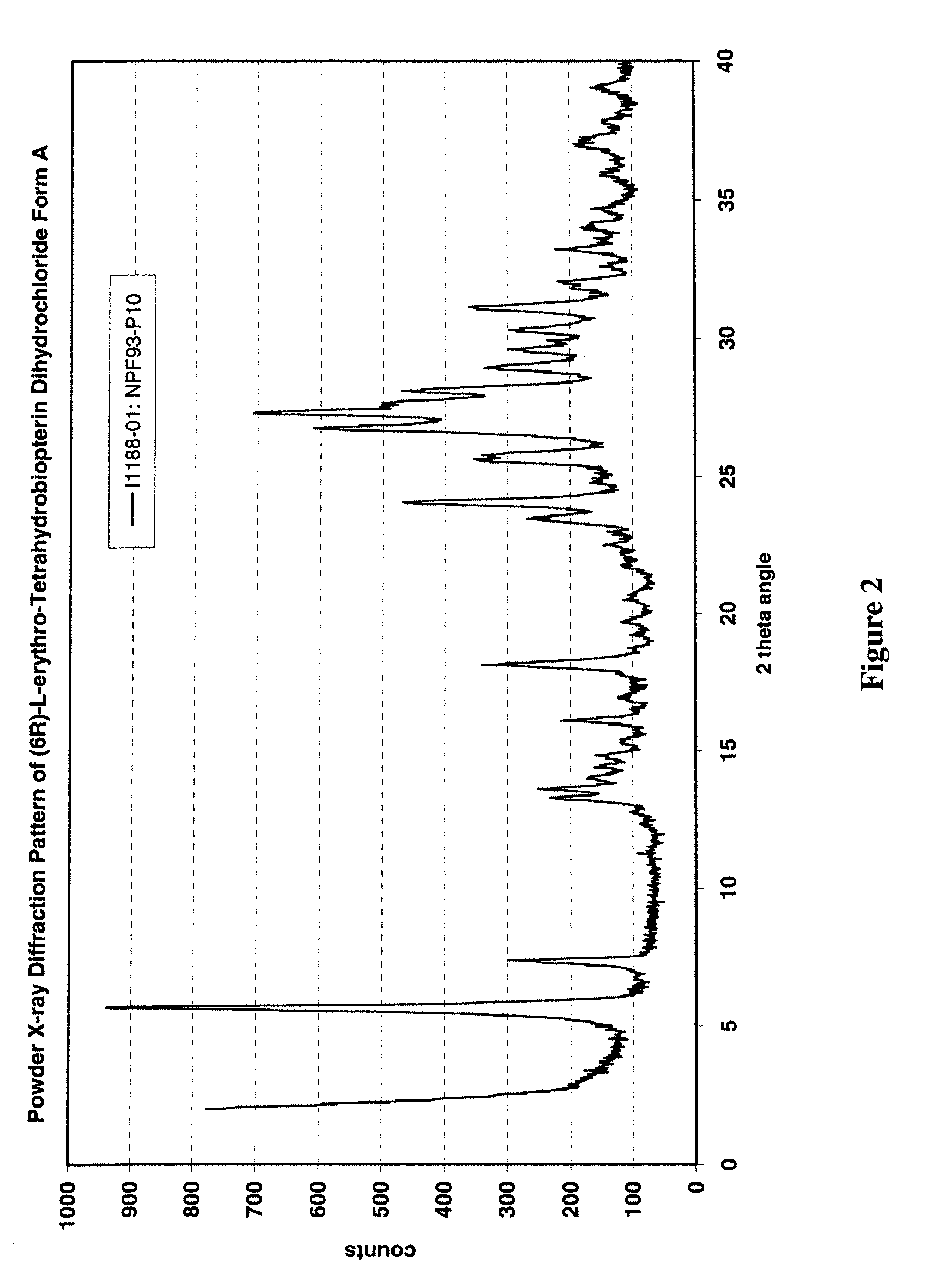
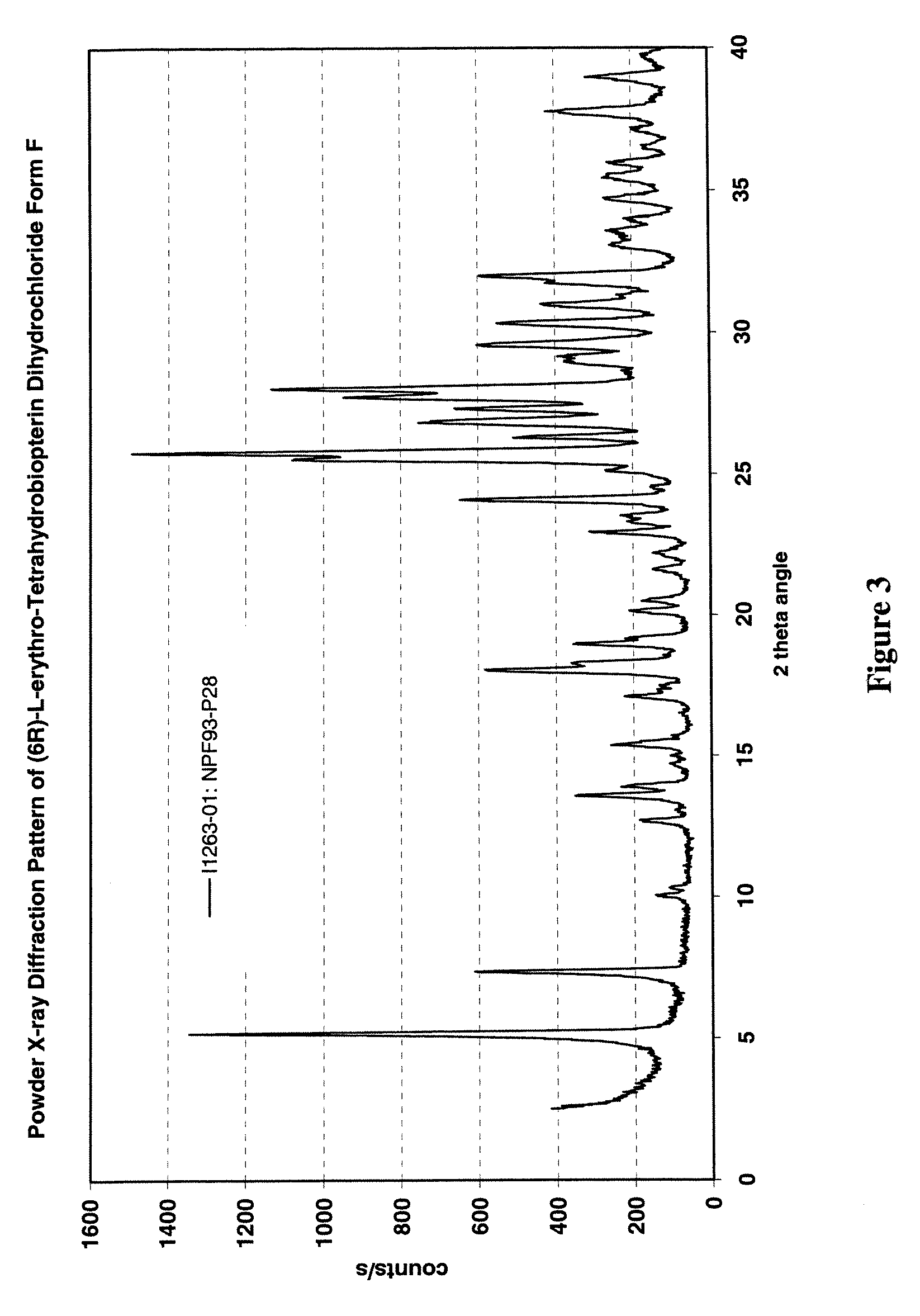
[0235]U.S. Provisional Patent Application Ser. No. 60 / 520,377, entitled “Polymorphs of (6R)-L-erythro-tetrahydrobiopterin dihydrochloride” filed on Nov. 17, 2003 in the name of Applicants Rudolf MOSER, of Schaffhausen, Switzerland and Viola GROEHN of Dachsen, Switzerland and assigned Merck-Eprova internal reference number 216, and U.S. patent application Ser. No. ______, entitled “Polymorphs of (6R)-L-erythro-tetrahydrobiopterin dihydrochloride” filed concurrently herewith on Nov. 17, 2004 in the name of Applicants Rudolf MOSER, of Schaffhausen, Switzerland and Viola GROEHN of Dachsen, Switzerland and assigned Merck-Eprova internal reference number 216 / US CIP (both of the Moser et al. applications are collectively referred to herein as the “Moser Applications” and both are incorporated herein by reference in their entireties. The examples of that specification describe X ray and Raman spectra studies to characterize the polymorphs of...
example 3
Administration of Tetrahydrobiopterin to Humans with Elevated Serum Phe Levels
[0244]An open label, single and multiple dose study was conducted in a total of 20 patients to demonstrate the safety and efficacy of tetrahydrobiopterin in humans with elevated blood levels of phenylalanine (>600 μmol / L). Criteria for inclusion in the study included (1) baseline blood Phe levels of >600 μmol / L, (2) age of at least 8 years. Criteria for exclusion from the study included (1) pregnancy or breastfeeding, (2) concurrent diseases or conditions that require medication or treatment, (3) concurrent treatment with any drug known to inhibit folate synthesis, and (4) treatment with any investigational drug within 30 days. Each of the patients also was identified as having a mutation in the phenylalanine hydroxylase (PAH) gene. Study subjects underwent baseline assessments, including medical history with assessment of phenylketonuria (PKU) or hyperphenylalaninemia (HPA) related signs and symptoms, phy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| dry weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com