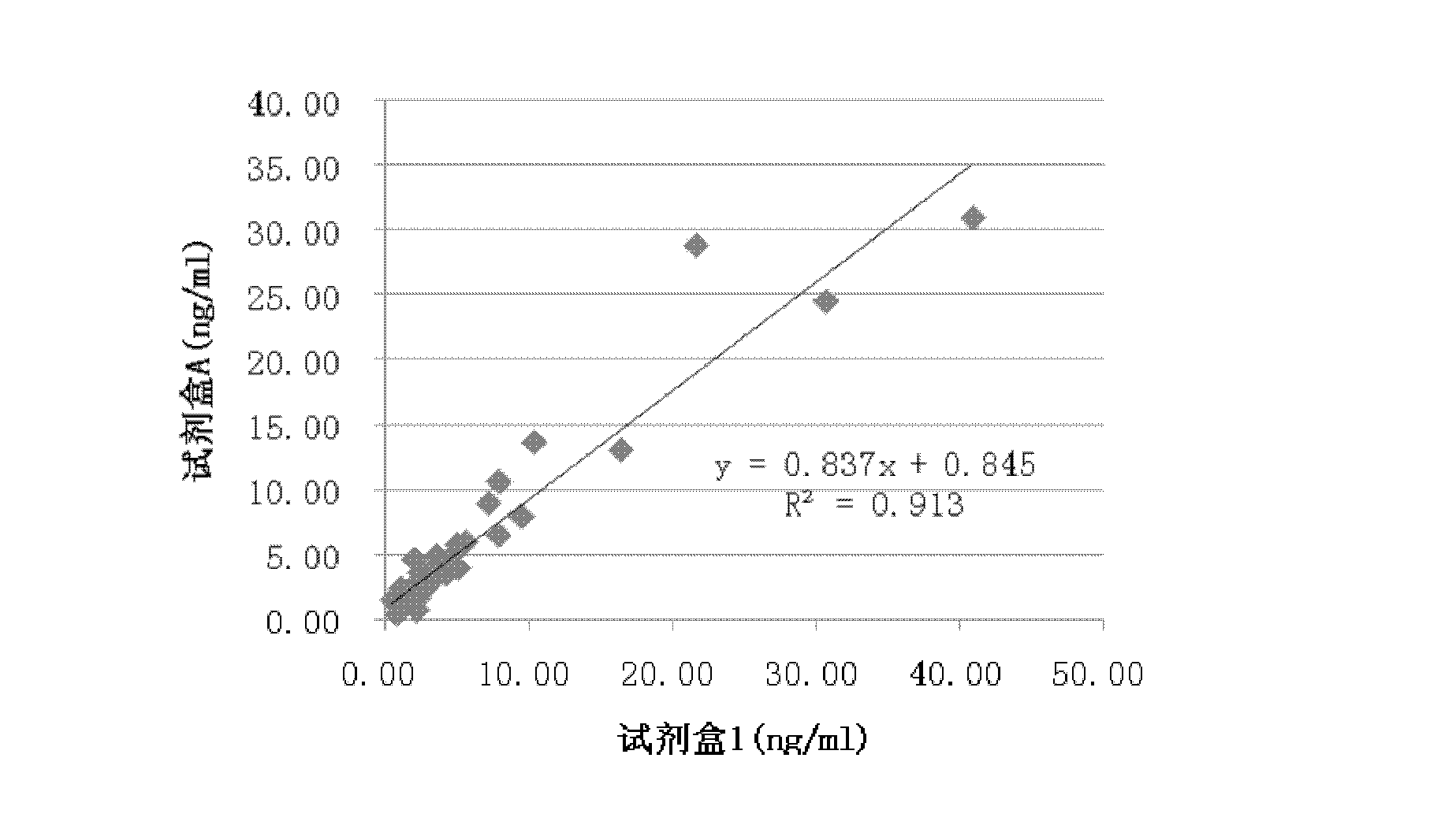
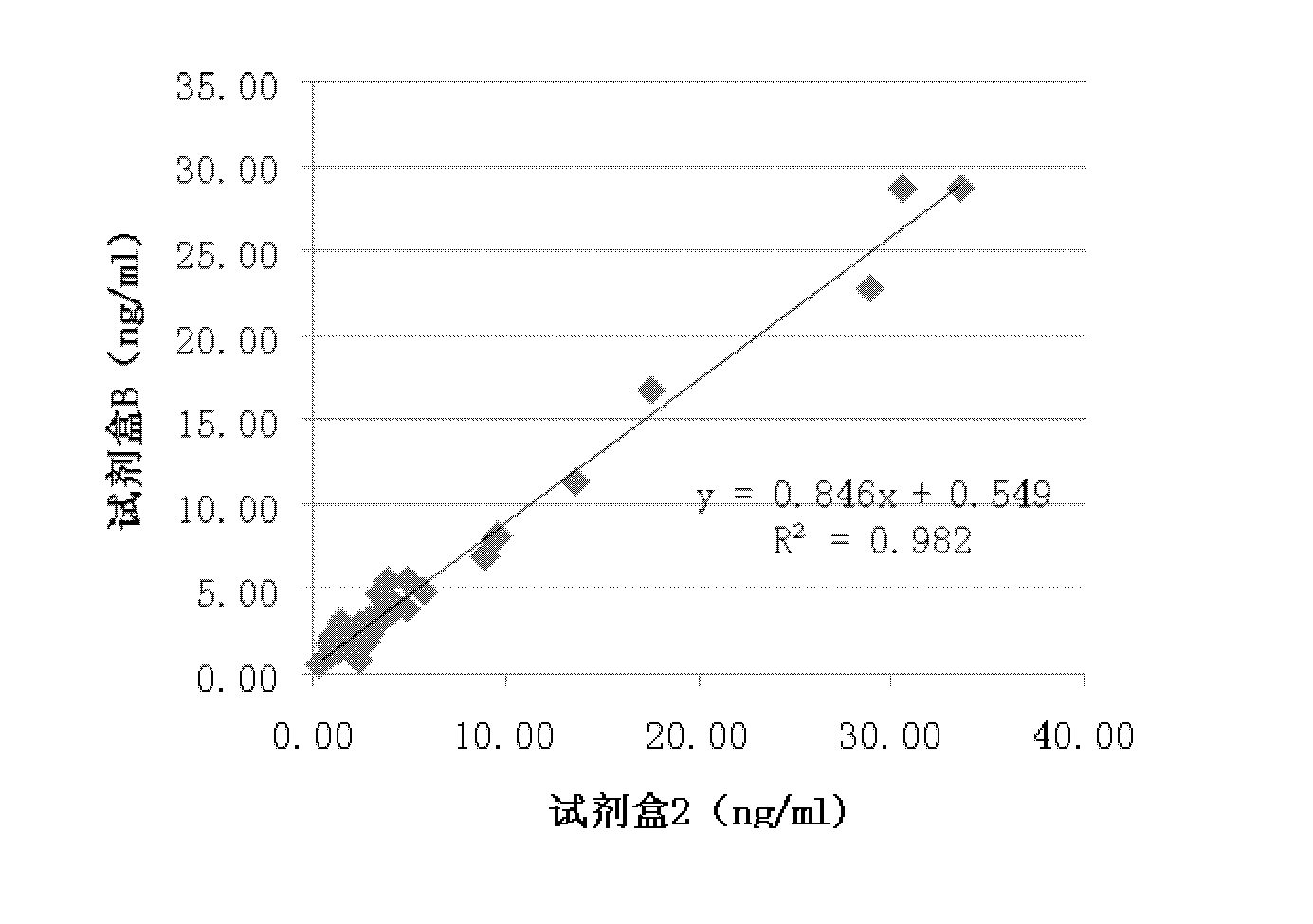
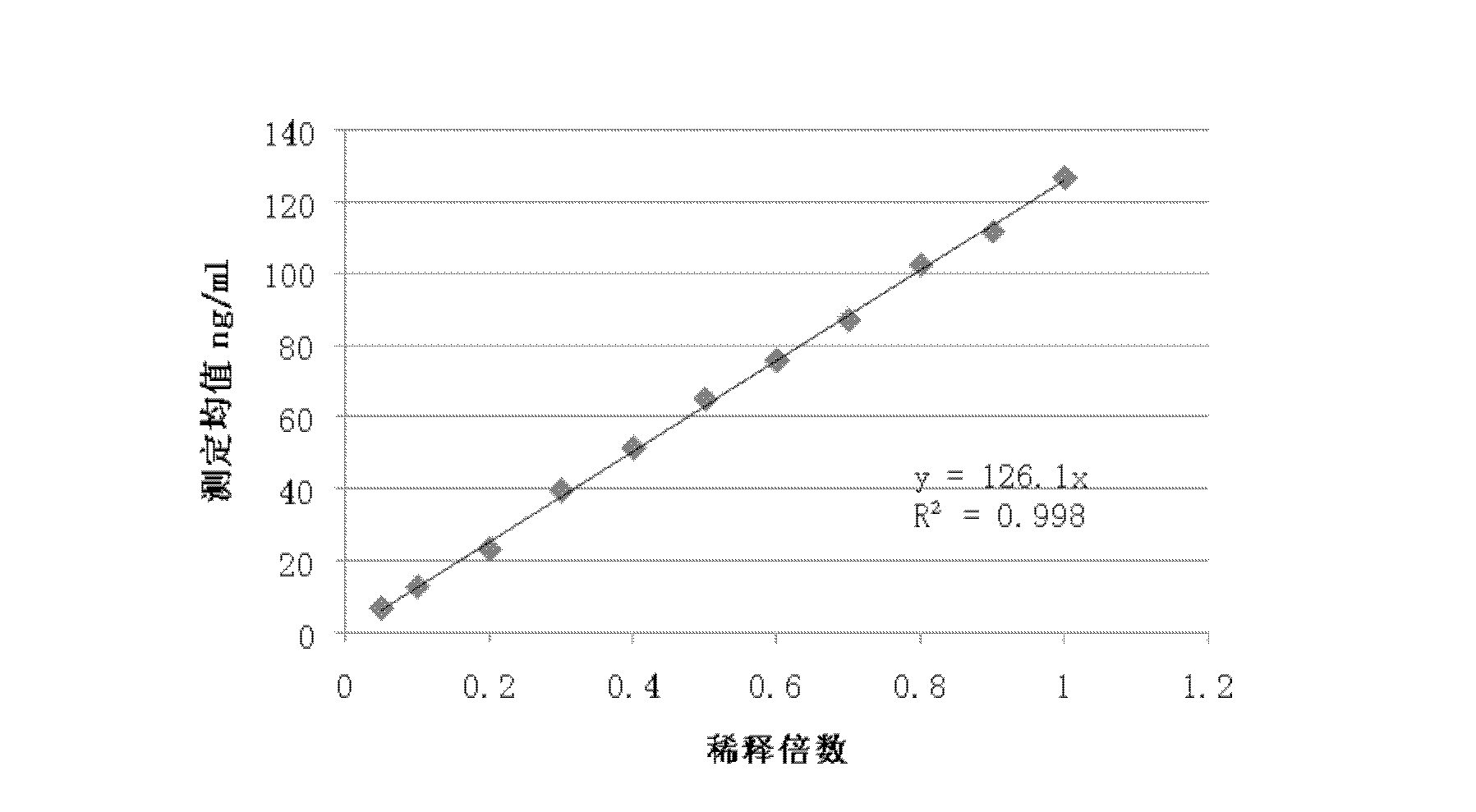
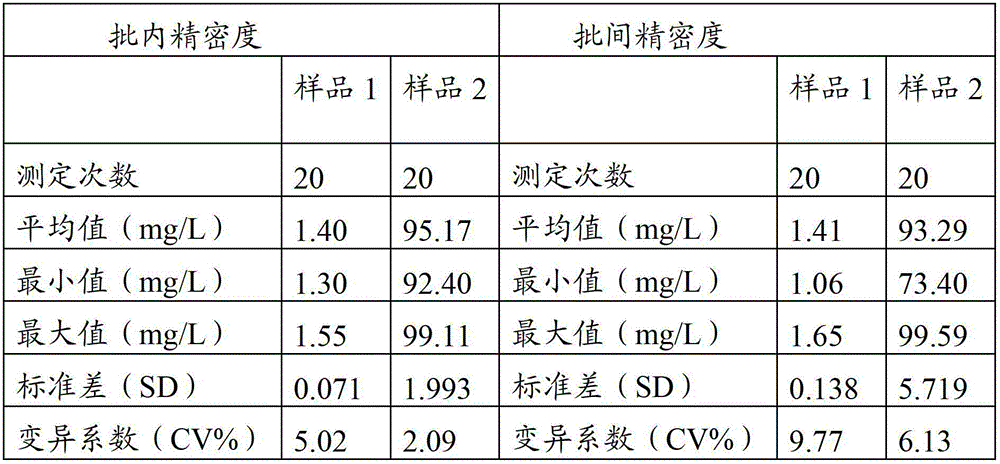
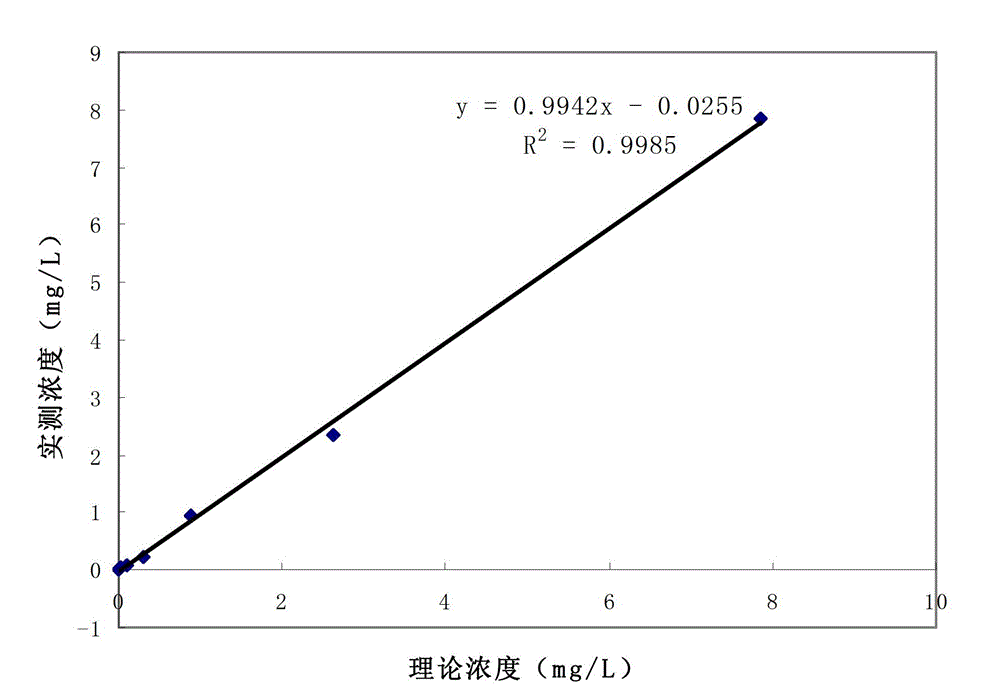
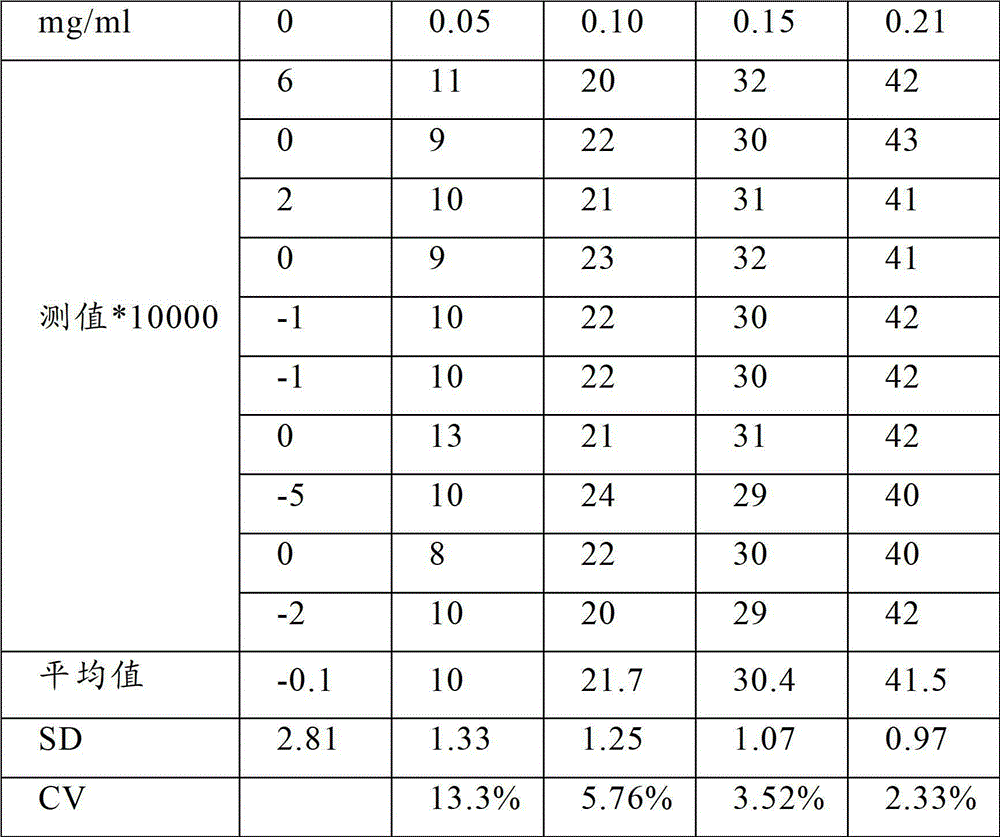
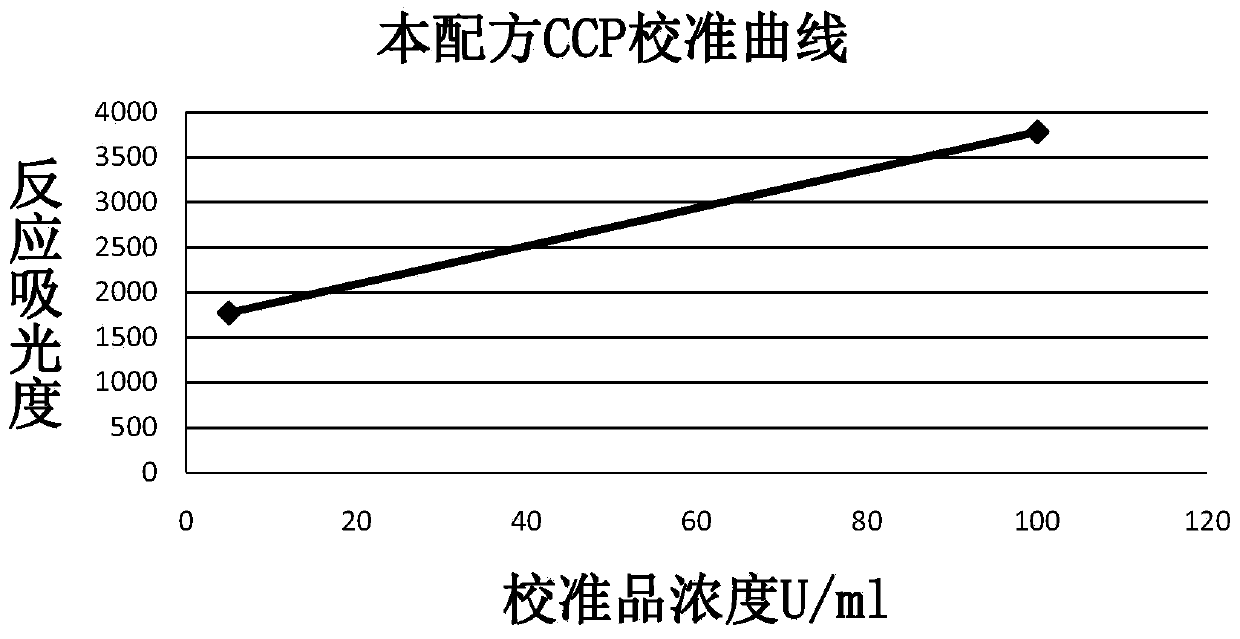
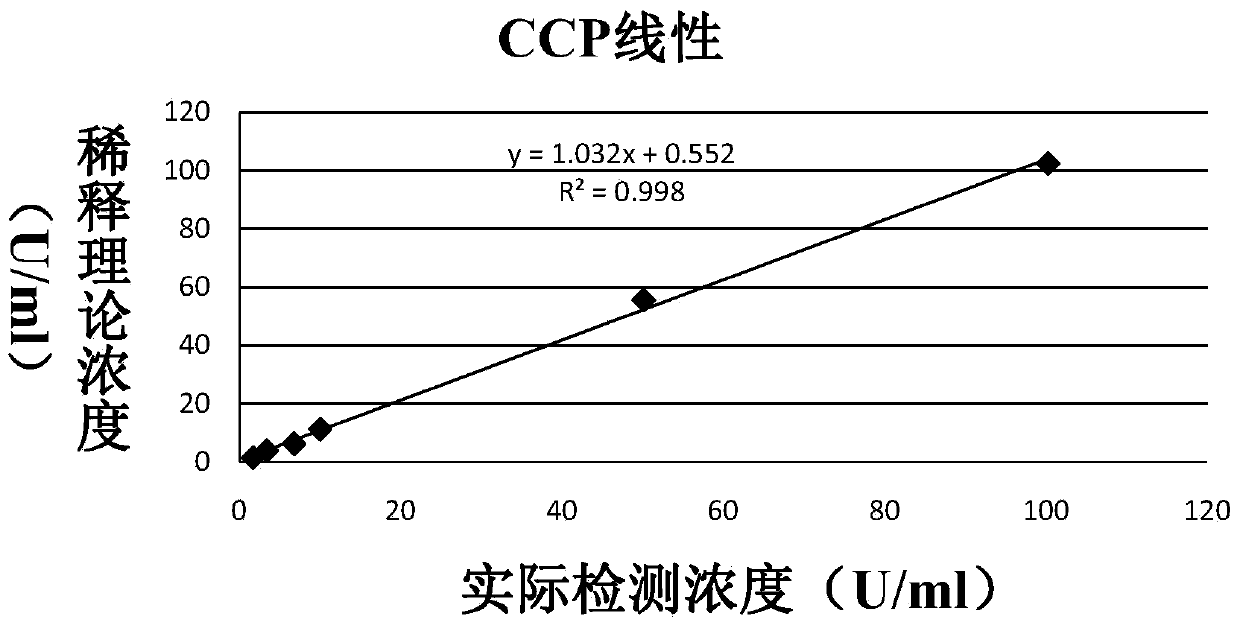
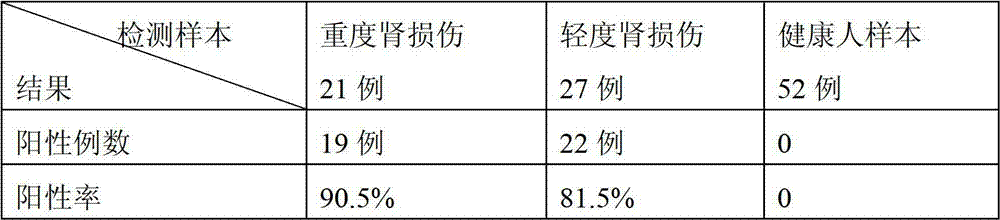
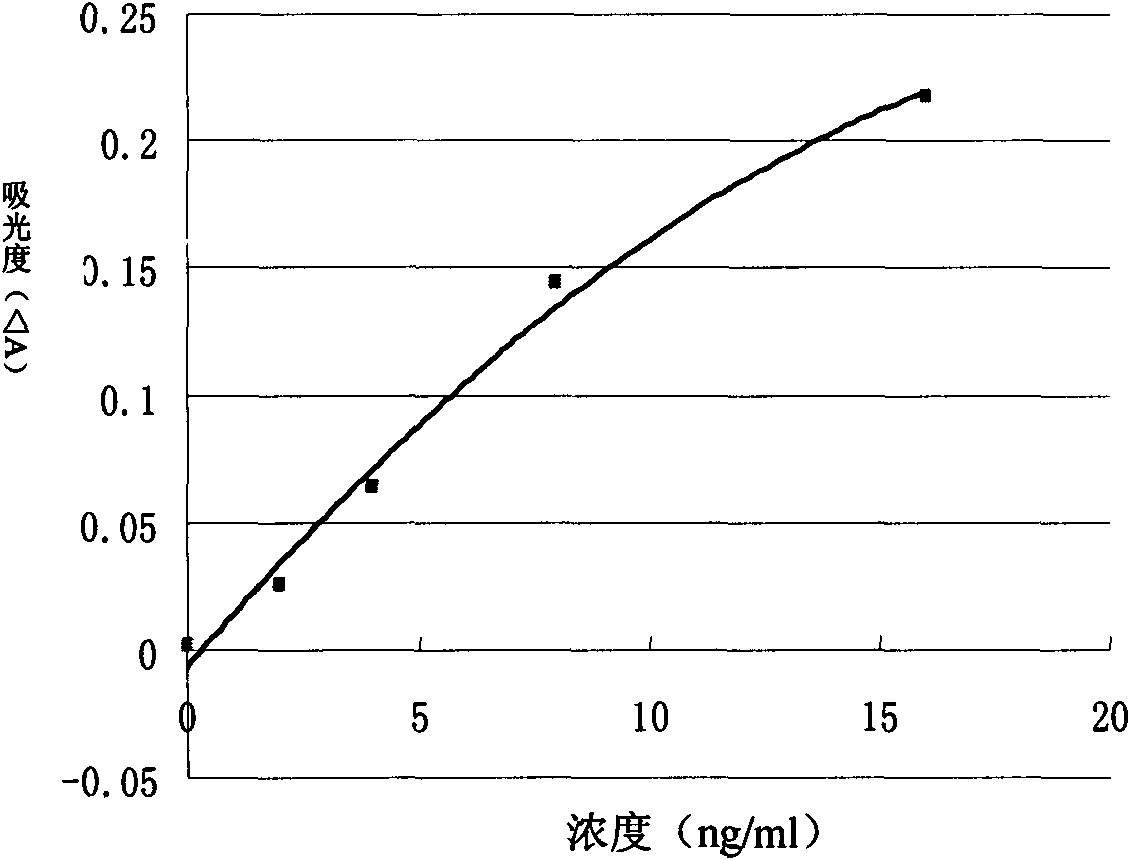
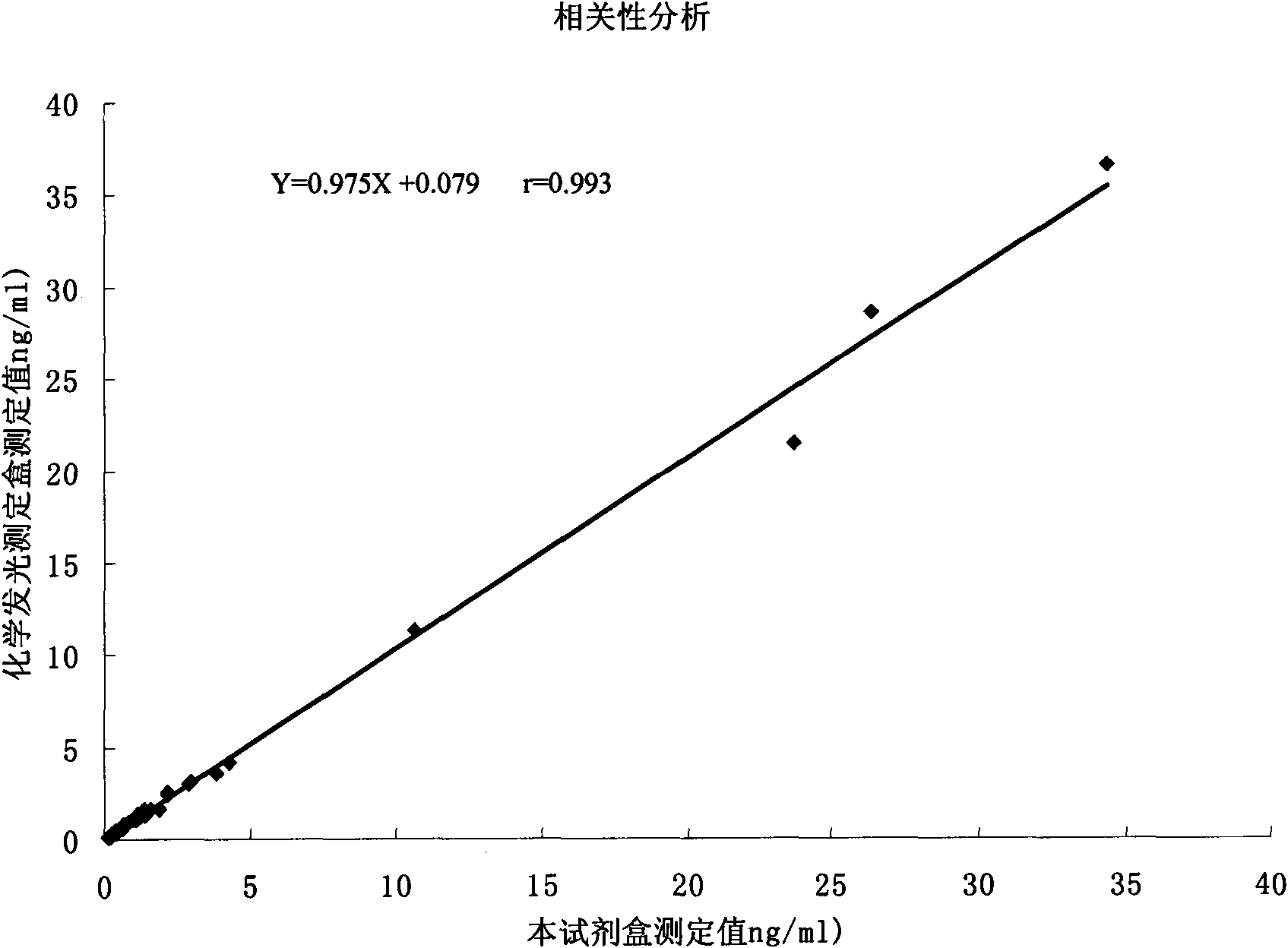
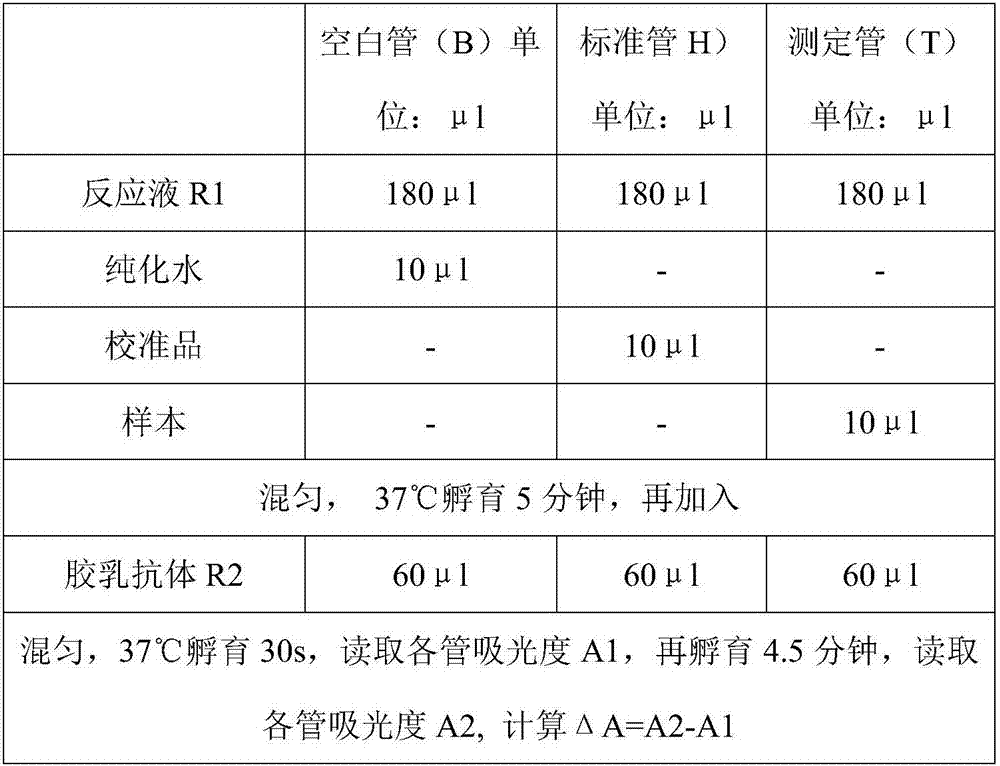
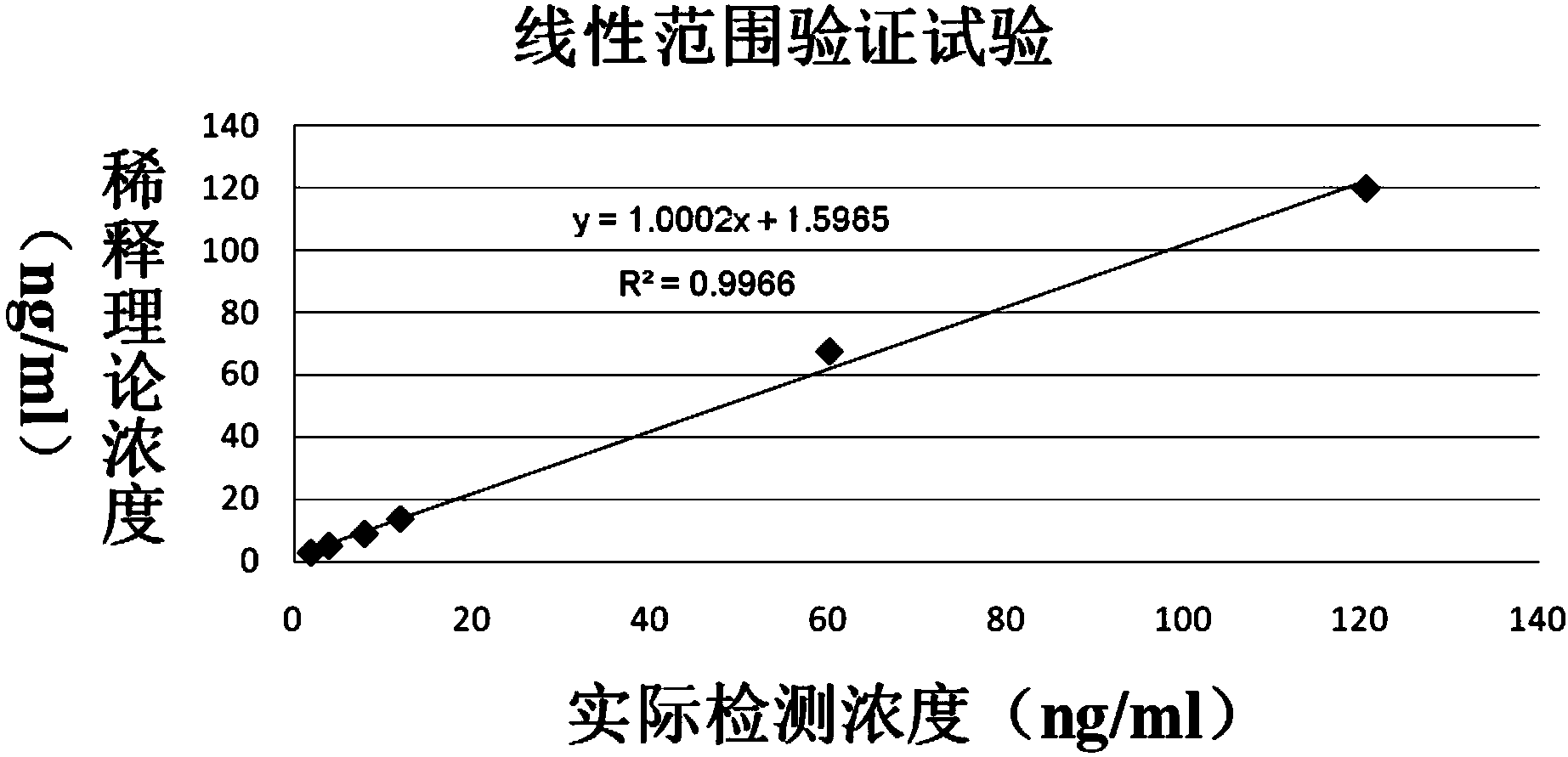
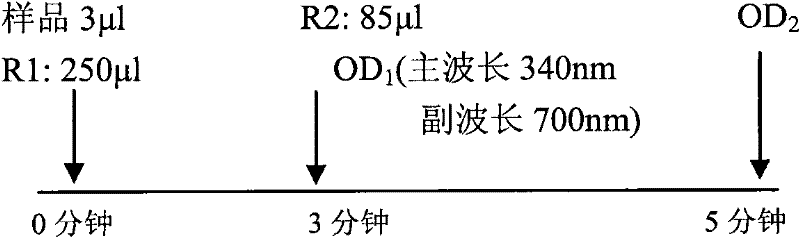
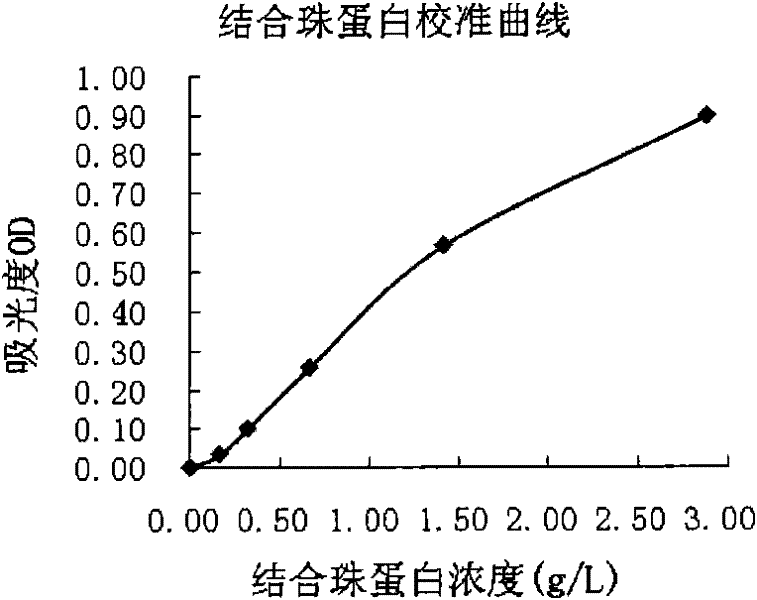
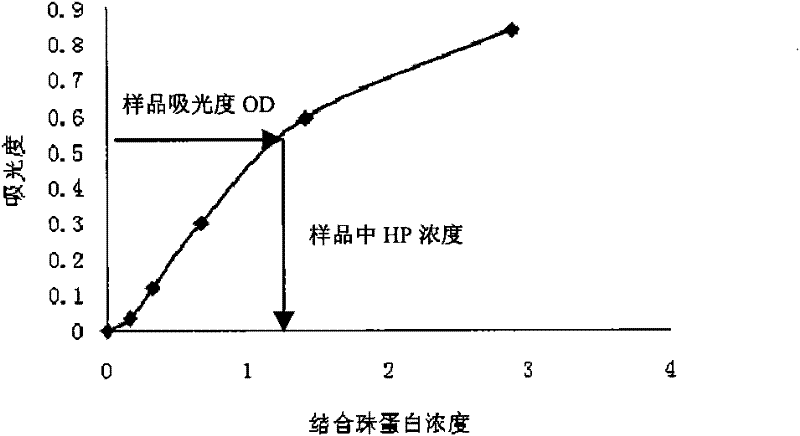
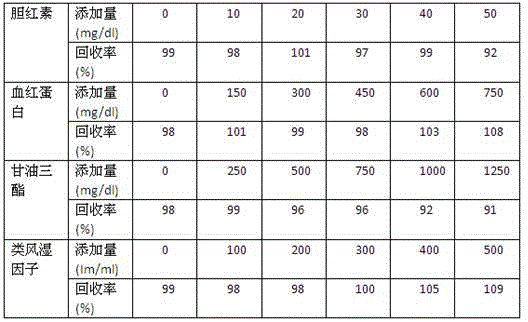
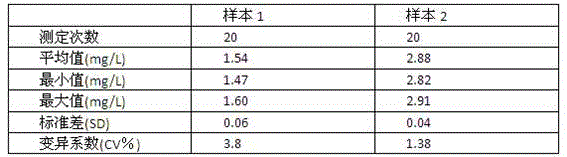
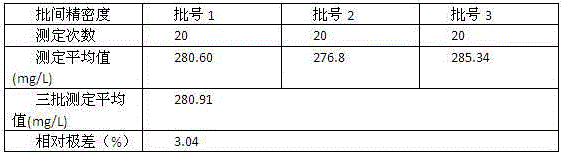
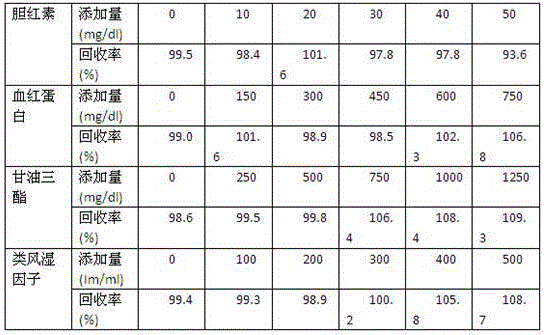
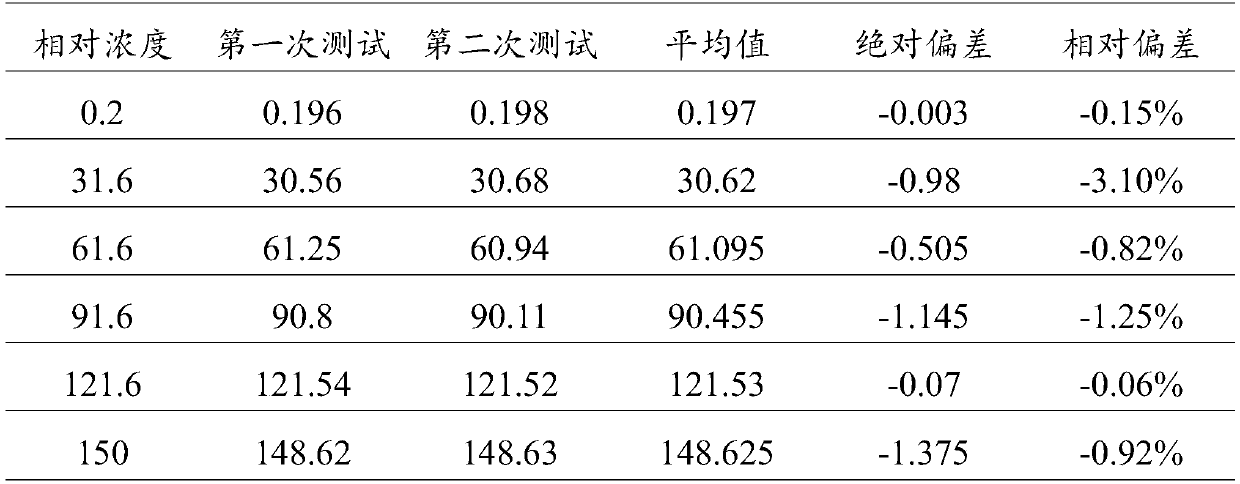
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Immunoturbidimetric Assays" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
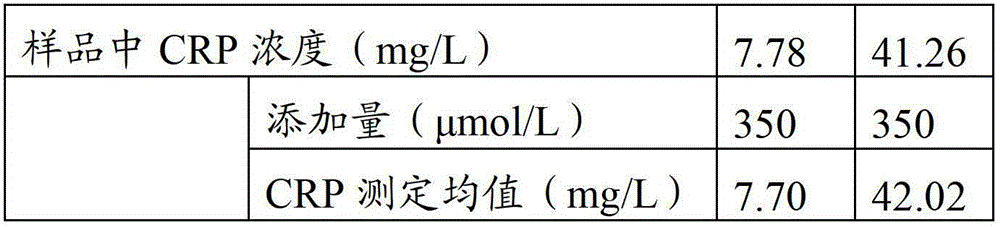
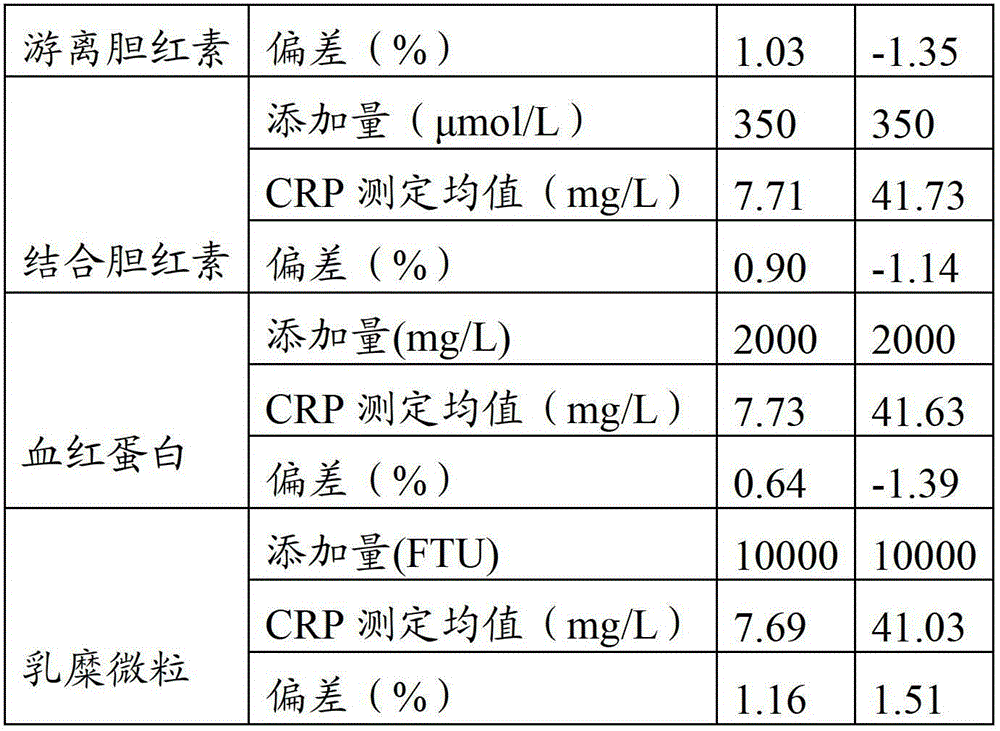
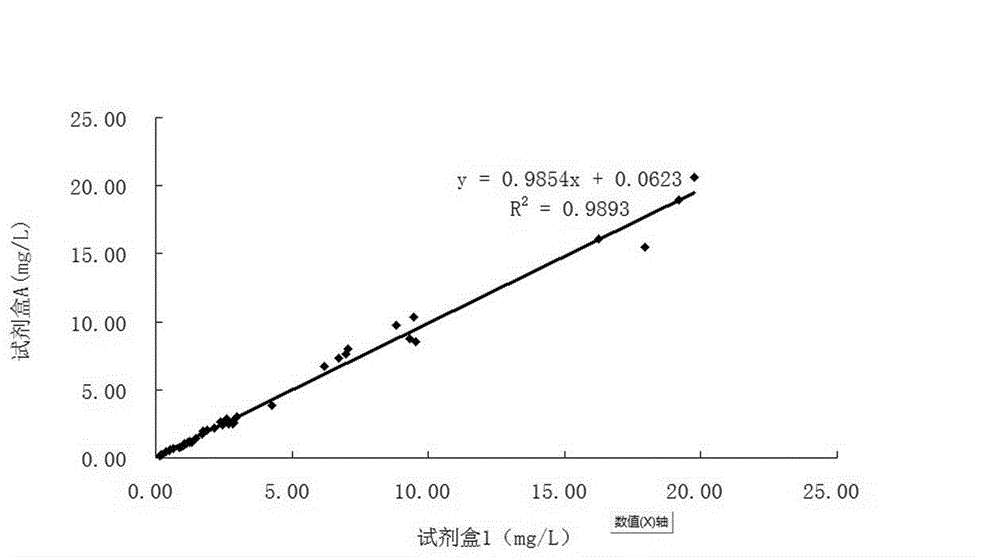
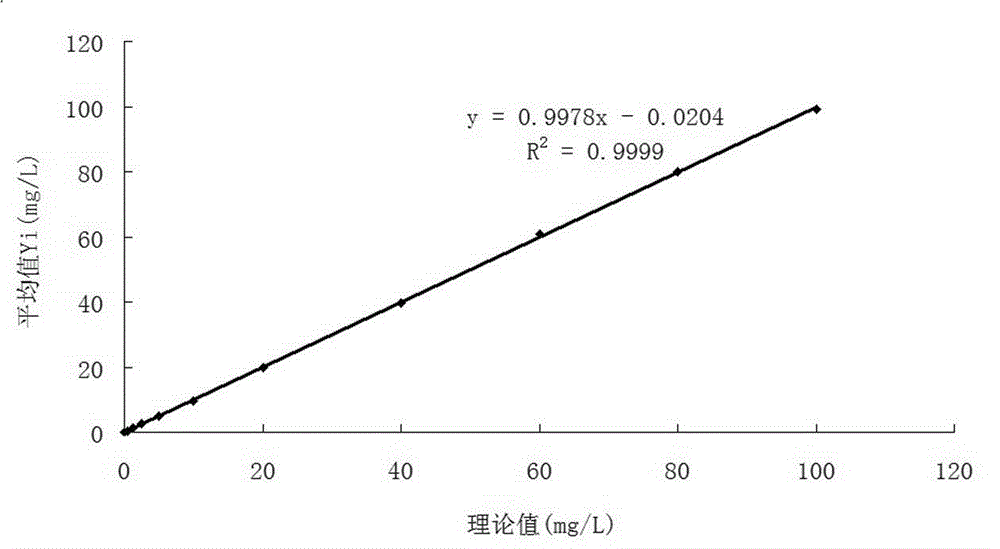
An immunoturbidimetric assay (or particle-enhanced turbidimetric immunoassay (PETIA)) for measuring Calprotectin is the fastest solution in the market with the shortest time to first result regardless of using sample batches or random access.
Kit for determining heart-type fatty acid binding protein in serum or urine by latex enhanced turbidimetric immunoassay
ActiveCN102628864AStrong specificityImprove accuracyColor/spectral properties measurementsBiological testingLatex particlePHA granule
The invention relates to a kit for determining heart-type fatty acid binding protein in serum or urine by latex enhanced turbidimetric immunoassay. Specifically, the kit for determining the heart-type fatty acid binding protein comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 contains a reaction promoter, an antiseptic, a surfactant, a stabilizing agent, an electrolyte and a buffer; the reagent R2 contains latex particles with binding of anti-heart-type fatty acid binding protein monoclonal antibody and polyclonal antibody, an antiseptic, a surfactant, a stabilizing agent, an electrolyte and a buffer; and the calibrator contains an antiseptic, an electrolyte, a stabilizing agent, a heart-type fatty acid binding protein pure product and a buffer. By the complex coating method of latex particles with the monoclonal antibody and the polyclonal antibody, high sensitivity and wide linear range of the kit are guaranteed. Simultaneously, the kit also has advantages of high accuracy, good repeatability, strong singularity, easy operation and the like, and is applicable to an automatic biochemical analyzer which is commonly used in clinic.
Owner:BEIJING STRONG BIOTECH INC
Full-scale C-reactive protein (CRP) colloidal gold immunoturbidimetric assay kit
The invention provides a full-scale CRP colloidal gold immunoturbidimetric assay kit. A quantitative assay purpose is reached through enhancing the turbidity by the colloidal gold. The method used by the kit provided by the invention solves a problem that the generation of a precipitate after a latex reinforced immunoturbidimetric reaction goes against biochemical instrument cleaning. The kit provided in the invention comprises a reagent R1 and a reagent R2, wherein the reagent R2 is a proper buffer solution; and the reagent R2 is a buffer solution of the colloidal gold combined with an antihuman CRP antibody. The kit has the characteristics of high sensitivity, strong specificity and good stability, can be used for assaying the content of the CRP in serum or blood plasma, and is suitable for clinical fully-automatic biochemical analyzers. The CRP assay sensitivity of the kit can reach 0.01mg / L, and the upper CRP assay limitation of the kit is 500mg / L.
Owner:重庆沃康生物科技有限公司
Kit (Latex-enhanced immunoturbidimetry) for detecting content of glycocholic acid in blood serum
ActiveCN102944673AHigh sensitivityAccurate automated detectionMaterial analysis by observing effect on chemical indicatorLatex particleSurface-active agents
The invention relates to a kit of latex-enhanced immunoturbidimetry for detecting content of glycocholic acid in blood serum. Specifically, the provided glycocholic acid detection kit comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 comprises a reaction promotion agent, a preservative, a surface active agent, a stabilizer, an electrolyte and a buffer solution; the reagent R2 comprises latex particles combined with a glycocholic acid antibody, a preservative, a surface active agent, a stabilizer, an electrolyte and a buffer solution; and the calibrator comprises a preservative, an electrolyte, a stabilizer, glycocholic acid pure products and a buffer solution. The kit for detecting the content of the glycocholic acid in the blood serum, disclosed by the invention, ensures the high sensitivity and wide linear range of a kit by utilizing a method of coating the latex particles by utilizing polyclonal antibodies, also has the advantages of high accuracy, good repeatability, strong specificity, simplicity in operation and the like, and can be applied to a clinical general full automatic biochemical analyzer.
Owner:CO HEALTH BEIJING LAB
Gastric cancer detecting method, reagent and gastric cancer detecting kit
InactiveCN104655843AImprove stabilityImprove anti-interference abilityMaterial analysisAntigenLatex particle
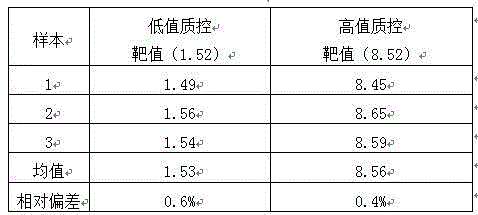
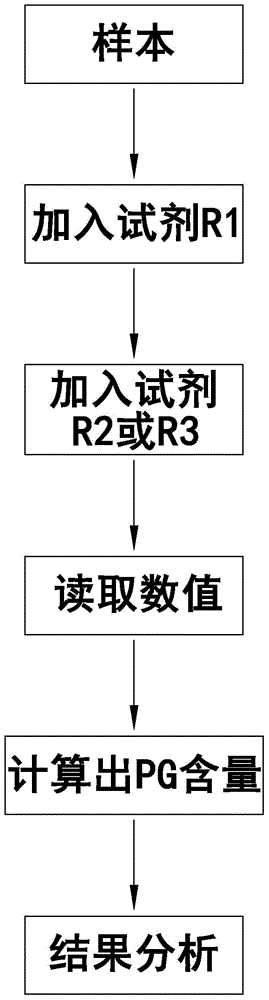
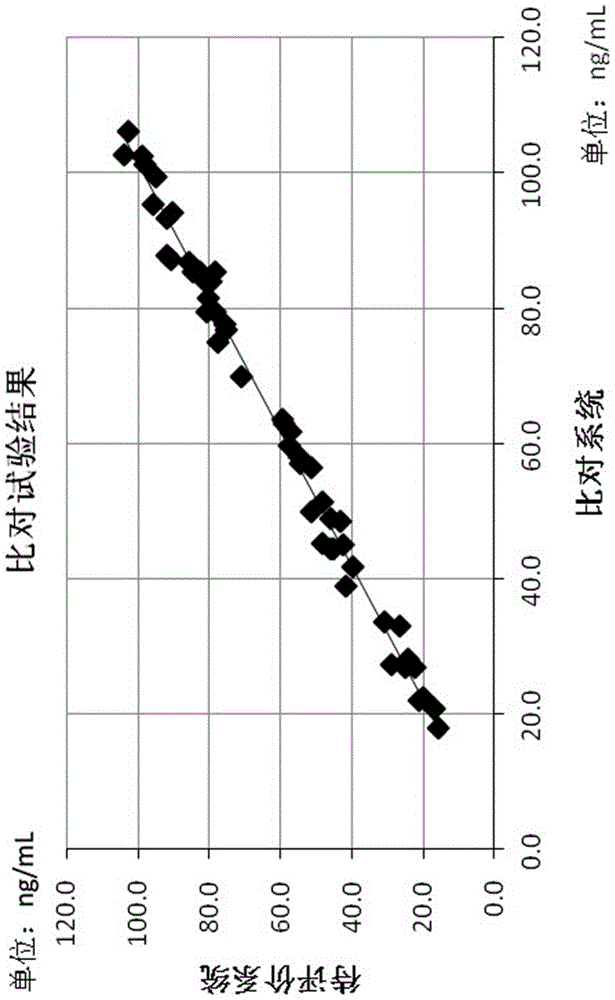
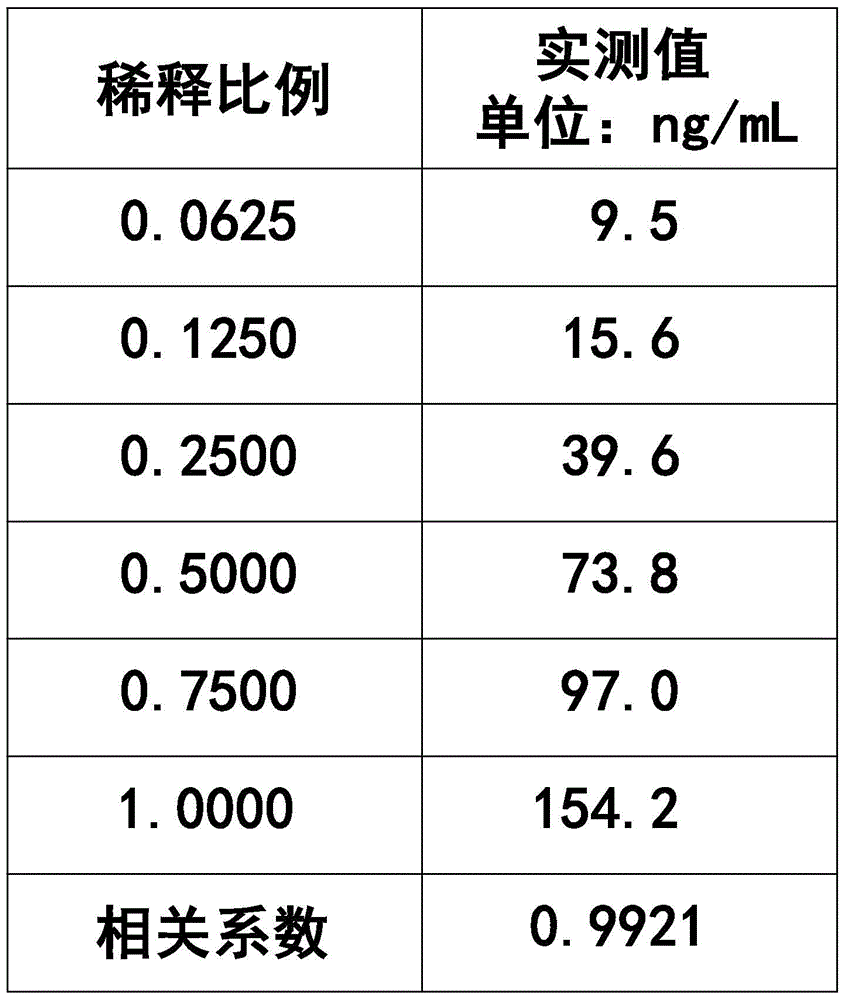
The invention provides a gastric cancer detecting method, a reagent and a gastric cancer detecting kit. The kit comprises a reagent R1 and a reagent R2 or R3; in vitro, the serum of a tested body is mixed with the reagent R1, and the reagent R2 or R3 is added after several minutes; latex particles included in the reagent R2 or R3 are coated with PG antibodies and can be combined with the PG antigen in a sample to form an insoluble complex; based on a latex-enhanced immunoturbidimetry principle, the absorbancy tested by a biochemical analyzer is compared with that of a calibration product, so that the content level of PG is calculated; compared with the content level of the serum PG of healthy people, the content level of PG shows that a health condition of the stomach of the tested human body is initially judged. By comparing a test result obtained by using the kit with that obtained by using an imported kit A, the kit has the advantages that the extremely-high correlation is achieved, and the correlation coefficient is more than or equal to 0.9750. The kit provided by the invention can be used for detecting the gastric cancer, and has stable and reliable detection results; moreover, the use cost can be reduced.
Owner:PUREBIO LAB NINGBO
Kit for performing retinol binding protein detection by using latex turbidimetry
InactiveCN102944679AHigh detection sensitivityGuaranteed SensitivityColor/spectral properties measurementsHydrogenTurbidimetry
The invention relates to the technical field of biotechnology, and particularly discloses a kit for detecting retinol binding protein content by using latex immunoturbidimetry. The kit comprises a reagent R1, a reagent R2 and a standard product, wherein the reagent R1 is buffer solution with pH (Potential of Hydrogen) value of 6-9; the reagent R2 is latex reagent coated by anti-retinol binding protein double antibodies; and the standard product is retinol binding protein solution with pH value of 5-8. According to the kit, the retinol binding protein content in a sample can be detected by using the latex immunoturbidimetry; the sensitivity is high and can reach 0.042 mg / L; and the kit has the advantages of high stability, easiness and quickness in operation, high specificity, low probability of interference, accurate quantification and broad application prospect.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
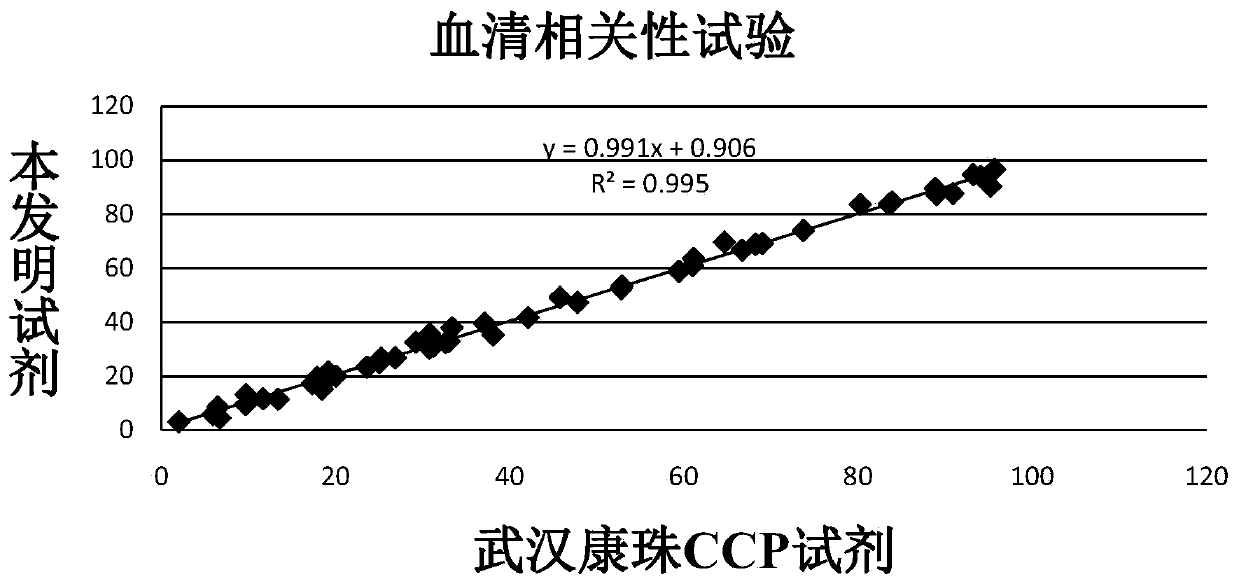
Anti-cyclic citrullinated peptide (CCP) antibody detection kit
ActiveCN104198725AHigh refractive indexHigh chemical inertnessBiological testingFluorescence/phosphorescenceAnti ccp antibodiesMicrosphere
The invention discloses an anti-cyclic citrullinated peptide (CCP) antibody detection kit. The kit comprises a reagent R1, a reagent R2, a standard substance and a standard diluent, wherein the reagent R1 is prepared by washing CCP antigen latex particles by 50mM Tris buffer solution containing 20-500mmol / L first stabilizer and 0.1-1% preservative and then dispersing; and CCP antigens and polystyrene latex microspheres are subjected to chemical crosslinking and then are closed, so that the CCP antigen latex particles are formed. For the kit, the latex-enhanced immunoturbidimetry is adopted for detecting the content of an anti-CCP antibody, the kit has the high sensitivity, the high stability and the high detection speed (the time from determination to result acquisition is only within 5-10min), can detect mass samples on a conventional biochemical analyzer and can greatly improve the detection efficiency.
Owner:上海睿康生物科技有限公司
CRP latex-reinforced immunonephelometry reagent, its kit and use of kit
ActiveCN104049085AMaterial analysis by observing effect on chemical indicatorBiological testingInorganic saltsInfection diagnosis
The invention provides a CRP latex-reinforced immunonephelometry reagent. The CRP latex-reinforced immunonephelometry reagent comprises CRP antibody-labeled latex particles, a buffer, a surfactant, an inorganic salt, a stabilizing agent, a suspending assistant, an excipient and an antiseptic. The invention also provides a kit for single-reagent CRP latex-reinforced immunonephelometry and a use thereof. The CRP latex-reinforced immunonephelometry reagent has simple composition, can be used simply and conveniently, has a fast reaction rate, high sensitivity and good repeatability, can be operated by a simple apparatus, has a wide apparatus adaptation range, has a low cost, can be widely used in large, middle and small hospitals and is suitable for clinical fast infection diagnosis and infection prognosis evaluation.
Owner:SUZHOU DIAGVITA BIOTECH
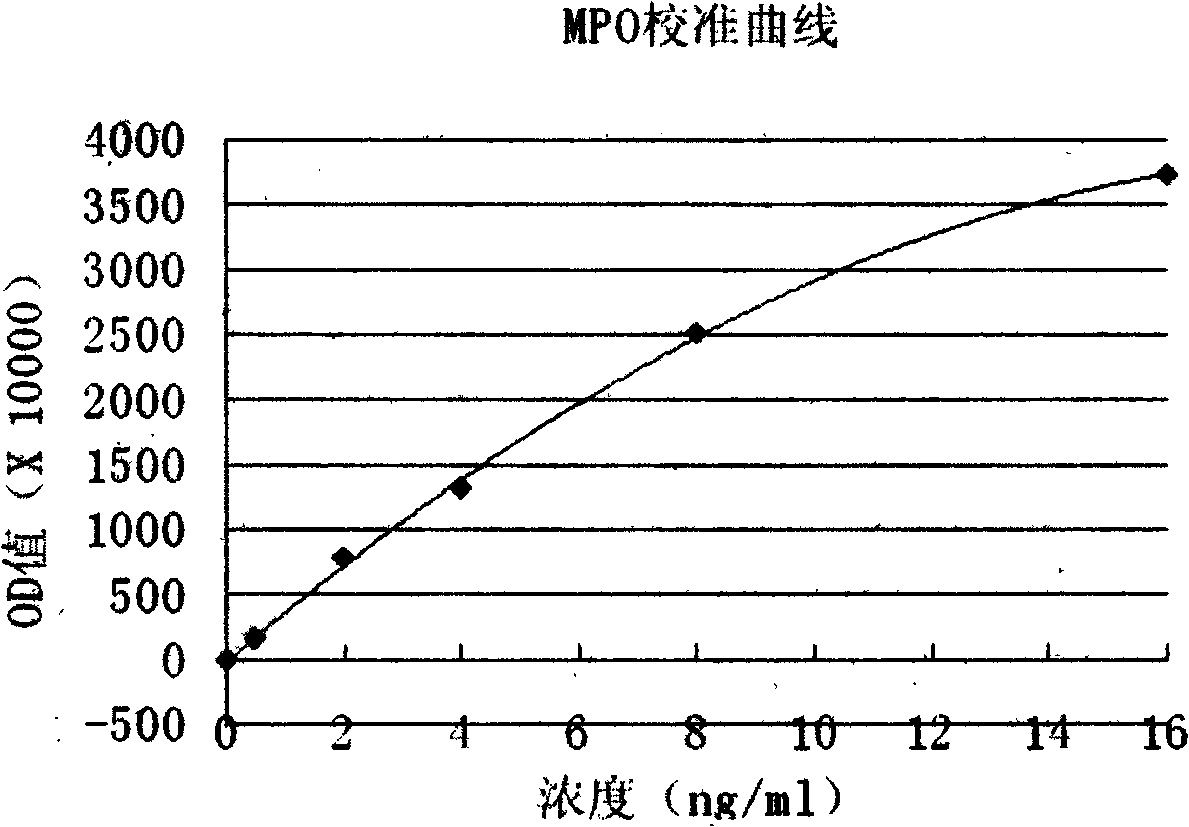
Myeloperoxidase (MPO) determination kit (by using latex enhanced turbidimetric immunoassay)
ActiveCN102680676AHigh detection sensitivityEasy to operateMaterial analysisImmunonephelometric AssaysMicrosphere
The invention relates to a kit for determining myeloperoxidase (MPO) content in serum. The invention aims to solve the technical problem to overcome the defects in the background art and provides a kit for determining myeloperoxidase content by enhanced turbidimetric immunoassay, which has the advantages of no need of dilution of a sample, simplicity in operation, high accuracy, good repeatability and suitability and is applied to various full-automatic biochemical analyzers and various special protein instruments. The invention adopts a technical scheme that the kit for determining the myeloperoxidase content by enhanced turbidimetric immunoassay comprises the following components: a, a reagent R1 which comprises buffer solution, a preservative, an accelerating agent, inorganic salt, a surface active agenet and the balance of purified water; b, a reagent R2 which comprises buffer agent, antibody combined with anti-human myeloperoxidase, a preservative, wherein the diameter of latex microspheres is 60-150nm; c, a reference calibration material which comprises buffer solution, a stabilizing agent, a preservative, a recombinant human myeloperoxidase pure product in a certain amount determined by concentration requirement and the balance of purified water. By the reagent combination, the calibration curve of MPO content is established, thereby achieving rapid determination of the MPO content in the serum on the full-automatic biochemical analyzer or the special protein instrument.
Owner:NANJING NORMAN BIOLOGICAL TECH
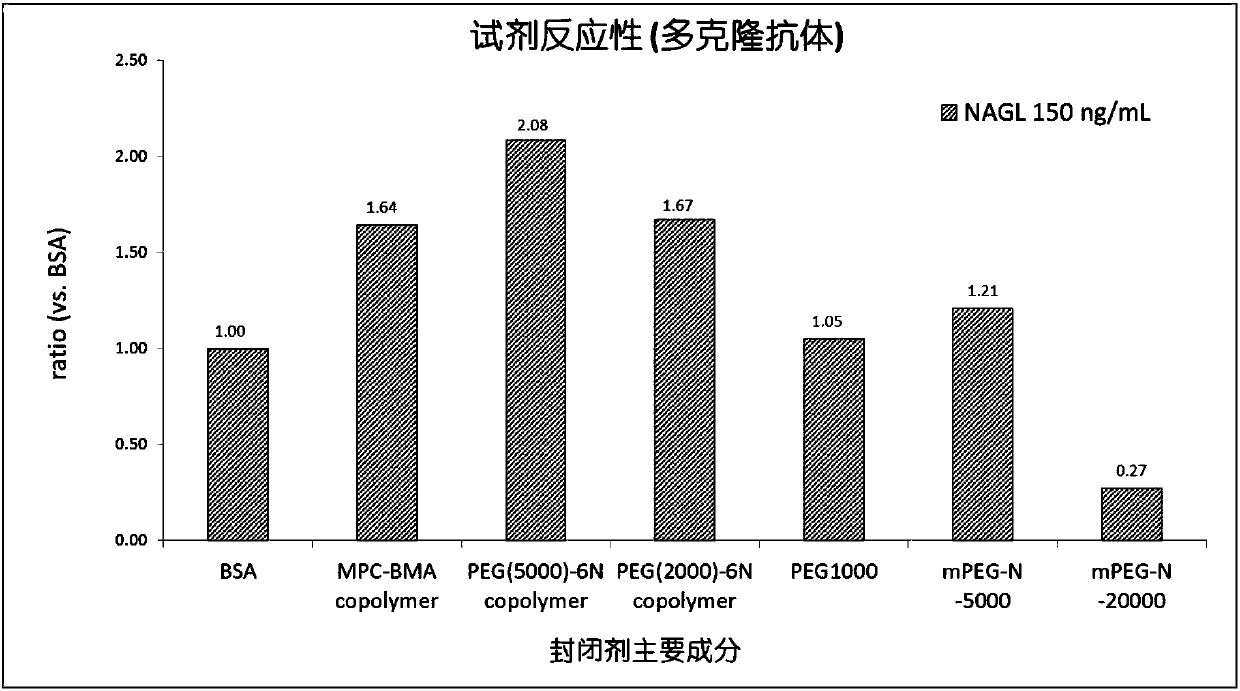
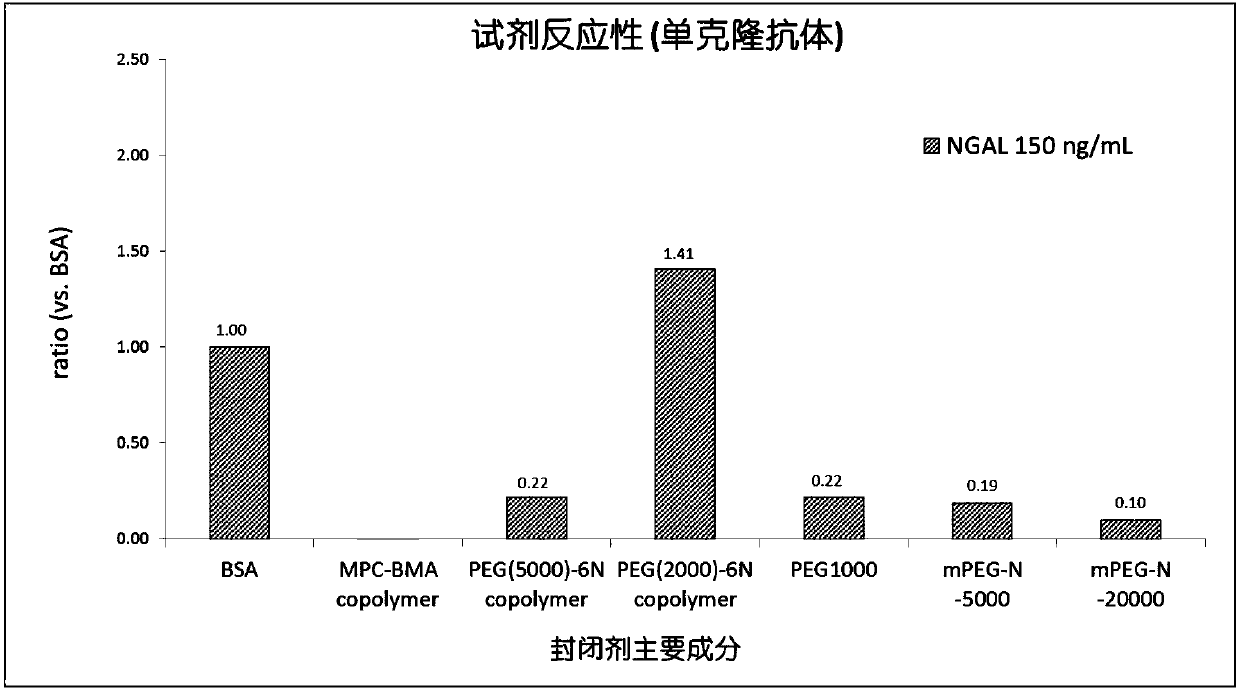
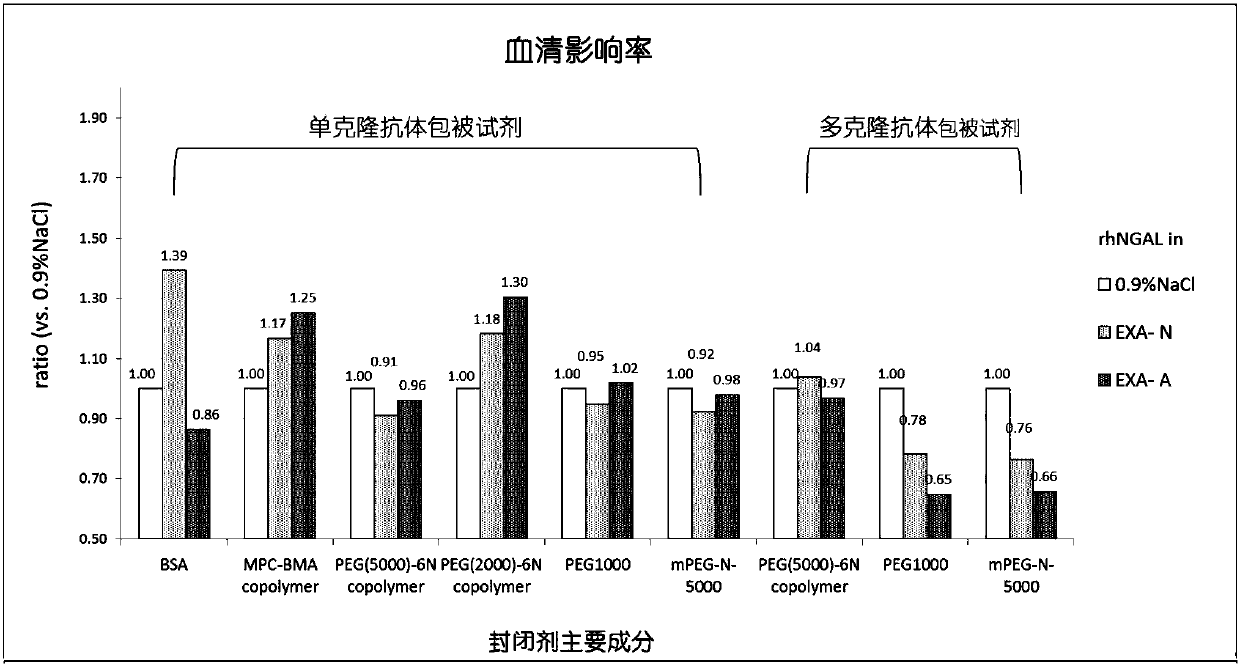
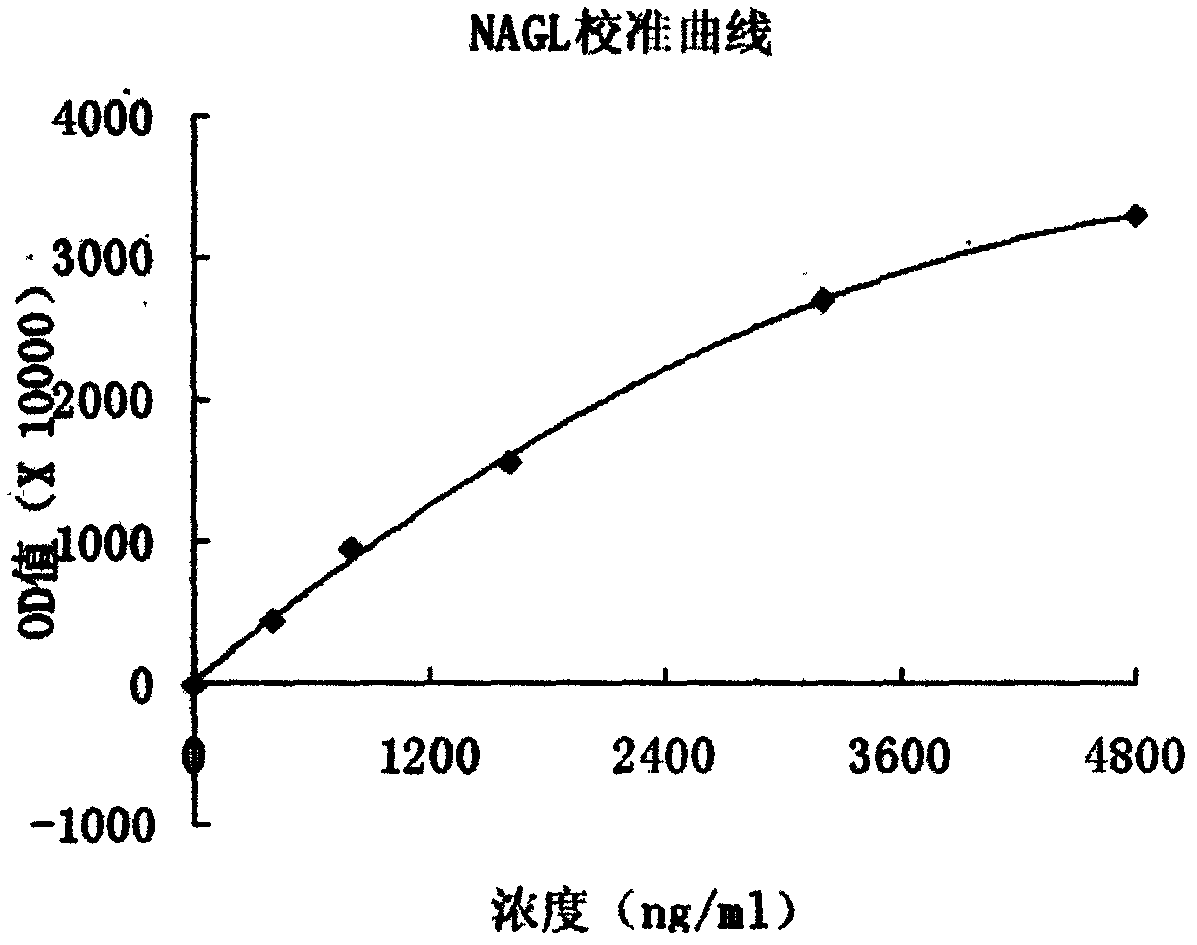
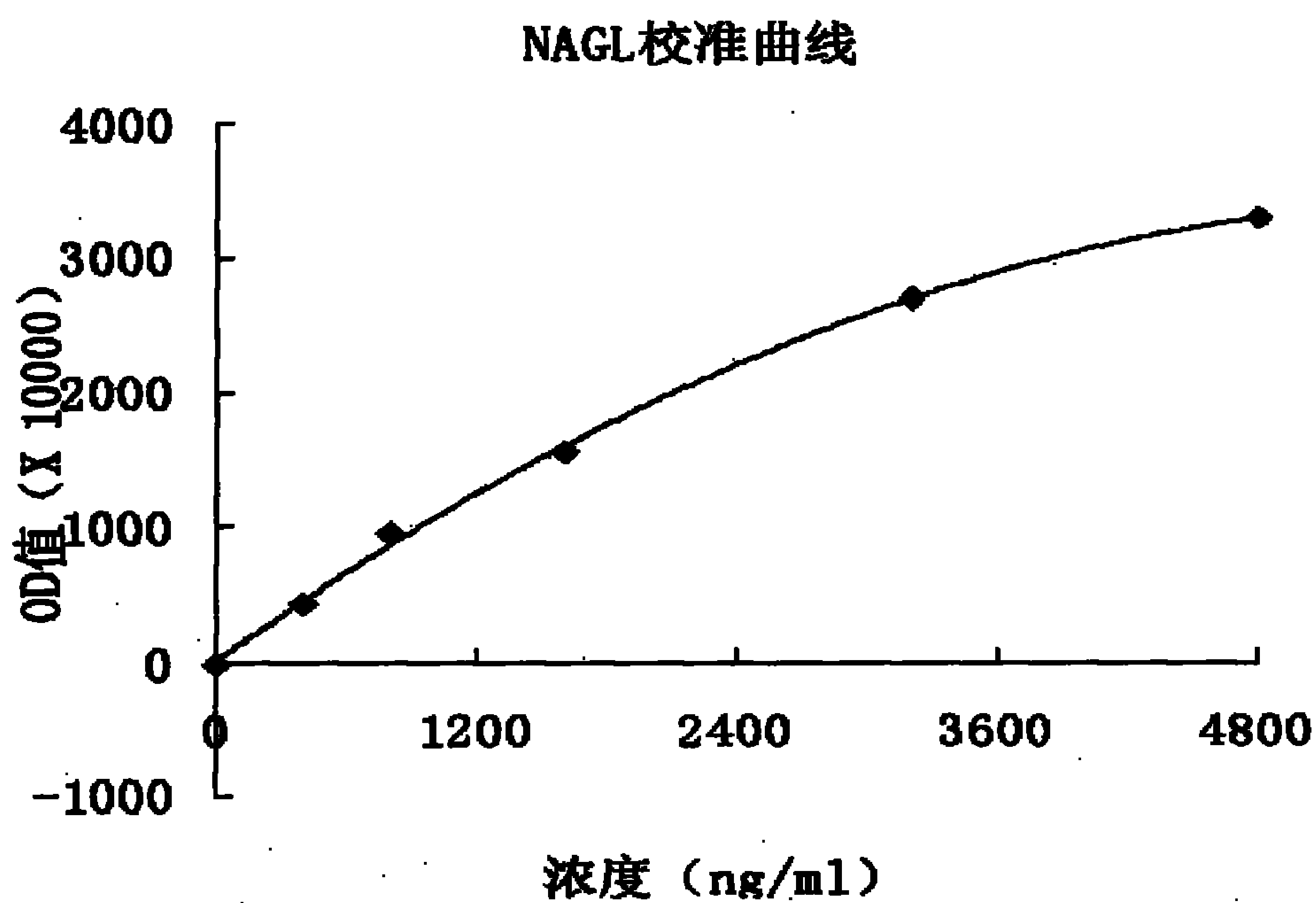
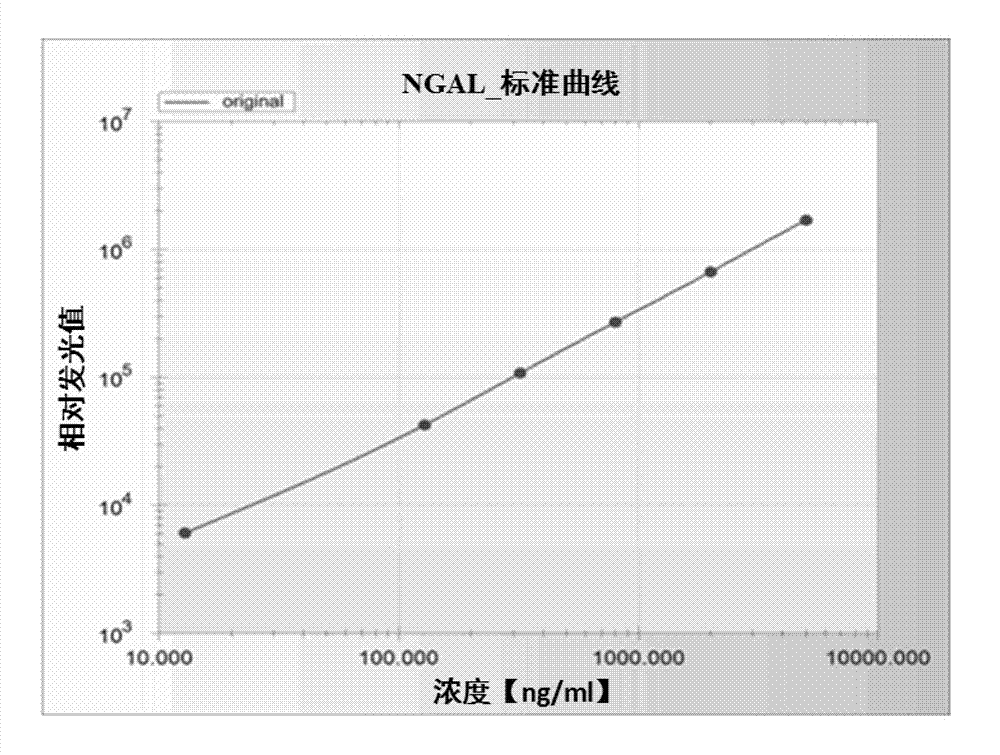
Latex-enhanced immunoturbidimetric assay kit for NGAL, and preparation method thereof
InactiveCN107942069AGood for three-dimensional structureImprove stabilityDisease diagnosisBiological testingGelatinasesFluorescence
The invention discloses a latex-enhanced immunoturbidimetric assay kit for neutropil gelatinase-associated lipocalin, and a preparation method thereof. Polyethylene glycol hexamine is selected as theblocking agent of an antibody binding latex particle. The kit used for latex-enhanced immunoturbidimetric assay of the NGAL has the advantages of high detection specificity, high sensitivity and goodstability, and can efficiently detect the NGAL content in urine, plasma and serum, and the detection result is well associated with fluorescence immunochromatography and enzyme linked immunosorbent assay.
Owner:捷和泰(北京)生物科技有限公司
Neutrophil gelatinase-associated lipocalin (NGAL) assay kit (latex-enhanced immunoturbidimetry)
ActiveCN102680698AHigh detection sensitivityEasy to operateBiological testingMicrosphereImmunoturbidimetry
The invention relates to a kit for assaying NGAL in serum, and provides an NGAL assay kit suitable for a full-automatic biochemical analyzer and a special protein analyzer. The technical scheme includes that the NGAL assay kit comprises reagent R1, reagent R2 and a calibrator, wherein the reagent R1 is composed of a buffer solution, an accelerator, a surfactant and the balance purified water; the reagent R2 is composed of a buffer solution and latex microspheres combined with anti-human NGAL antibodies; and the calibrator is composed of a buffer solution, a stabilizer, a preservative, a certain amount of pure recombinant human NGAL required by concentration, and the balance purified water. The NGAL content in the serum can be quickly assayed through the reagent combination.
Owner:NANJING NORMAN BIOLOGICAL TECH
Human cystatin C chemiluminescence quantitative detection method
InactiveCN103018465ANo pre-dilution requiredImprove accuracyChemiluminescene/bioluminescenceBiological testingDiseaseImmunonephelometric Assays
The invention provides a human cystatin C quantitative detection method. According to the method, a magnetic micro-particle separation technology and an enzymatic chemiluminescence technology are combined, the principle of a double-antibody sandwich one-step reaction method is adopted, and the method can be used for content determination of human cystatin C in serum, plasma and urine samples and assistance in diagnosis and treatment of various renal dysfunction diseases. The sensitivity of the method is not more than 0.01mu g / L, the quantitative detection range is 0.05-8mu g / L, the precision in analysis is less than 5%, the detection time is 10 minutes, the recovery rate of the added serum and urine is 90-115%, and the correlation analysis R2 with a reagent serum value detected by a latex-enhanced immunoturbidimetric method is 0.9862.
Owner:BEIJING LEADMAN BIOCHEM
Neutrophil gelatinase associated lipocalin (NGAL) chemiluminescence detection kit
InactiveCN102967714AOvercome the greater influence of blood lipid concentrationHigh sensitivityChemiluminescene/bioluminescenceBiological testingElisa methodBiology
The invention discloses a neutrophil gelatinase associated lipocalin (NGAL) chemiluminescence detection kit. The kit comprises the components of a standard substance of NGAL, an NGAL monoclonal antibody labeled by horseradish peroxidase, magnetic particles coated by the NGAL monoclonal antibody, a white non-transparent microwell plate, washing liquid, and chemiluminescence substrates A and B. The kit provided by the invention can be used for detecting the content of the NGAL in blood serum of a patient, and has important guiding significance to the detections on premature kidney diseases and injures. Compared with the conventional ELISA (Enzyme Linked Immunosorbent Assay) method, the NGAL chemiluminescence detection kit provided by the invention has the characteristics of being high in sensitivity and good in specificity and overcoming the large influence of immunoturbidimetry by blood fat concentration, and has the advantages of high stability, reliability and accuracy, security and environment friendliness, simplicity and convenience in operation and the like.
Owner:天津市协和医药科技集团有限公司
Troponin-T determination kit
ActiveCN102841206AHigh detection sensitivityEasy to operateMaterial analysis by observing effect on chemical indicatorBiological testingMicrosphereFully automatic
The invention relates to a kit for determining troponin-T (TNT) content of serum, aims to overcome the deficiencies of the above background technology, and provides a kit for detecting troponin-T by an immunoturbidimetry method, wherein the kit does not require sample dilution, has simple operation, high accuracy and good repeatability, and is suitable for various types of fully automatic biochemical analyzers and special protein instruments. A technical scheme is as below: the kit comprises: A. a reagent R1 containing a buffer solution, a preservative, an accelerating agent, inorganic salts, a surfactant and the balance of purified water; b. a reagent R2 containing a buffer solution, latex microspheres with diameter of 60-150 nm combined with anti-human troponin antibodies and an antiseptic; and c. a reference calibrator containing a buffer solution, a stabilizer, a preservative, a certain amount of recombinant human protein troponin-T pure product determined by concentration requirements and the balance of purified water.
Owner:NANJING NORMAN BIOLOGICAL TECH
KL-6 determination reagent and preparation method thereof
InactiveCN106841597ASimple and fast operationImprove accuracyMaterial analysisMicrosphereFully automatic
The invention discloses a KL-6 determination reagent. The KL-6 determination reagent comprises a pretreatment reagent, an antibody latex reagent and an adjusting reagent, wherein the pretreatment reagent 7.2-7.6 in pH value comprises a buffer agent, a signal enhancing agent and a preservative, the antibody latex reagent comprises a buffer agent, latex microspheres and a stabilizing agent, the latex microspheres 100-400 nm in diameter combine anti-human KL-6 antibodies, and the adjusting agent comprises quantitative KL-6. The invention further discloses a preparation method of the KL-6 determination reagent. The KL-6 determination reagent and the preparation method thereof have the advantages that through combination of the anti-human KL-6 antibodies and the latex microspheres, the content of KL-6 in human serum can be determined through a latex-enhanced turbidimetric immunoassay; the KL-6 determination reagent is simple and convenient to operate, high in accuracy degree and repeatability and suitable for high-throughput test, can be used on a fully-automatic biochemical analyzer and well conforms to results determined by an enzyme-linked immunosorbent assay method.
Owner:苏州普瑞斯生物科技有限公司
Kit for determining Lp-PLA2 based on latex particle-enhanced turbidimetric immunoassay and preparation method of kit
InactiveCN108008130AWide linear rangeImprove accuracyDisease diagnosisImmunonephelometric AssaysMicrosphere
The invention provides a kit for determining Lp-PLA2 based on latex particle-enhanced turbidimetric immunoassay and a preparation method of the kit. The kit comprises reaction liquid and a latex antibody reagent, wherein the latex antibody reagent comprises at least two latex antibodies formed by coupling monoclonal antibodies with latex microspheres. The preparation method comprises the followingsteps: screening out Lp-PLA2 monoclonal antibodies with higher specificity, and mixing multiple monoclonal antibodies instead of polyclonal antibodies to carry out latex coupling to obtain the latexantibodies; then preparing the reaction liquid; finally, forming the kit by using the latex antibodies and the reaction liquid, wherein the kit is used for determining the content of the Lp-PLA2. By using the kit prepared by the preparation method provided by the invention, sensitivity and specificity of determining the Lp-PLA2 by the latex particle-enhanced turbidimetric immunoassay can be simultaneously improved; further, the kit has the advantages of wider linear range, higher accuracy, extremely-high economic value and broad market application prospect.
Owner:海格德生物科技(深圳)有限公司
NGAL latex immunoturbidimetric detection kit and preparation method thereof
ActiveCN109738626AGuaranteed responsivenessImprove anti-interference abilityBiological testingImmunonephelometric AssaysMicrosphere
The present invention relates to an NGAL latex immunoturbidimetric detection kit and a preparation method thereof. The kit comprises a reagent R1, a reagent R2, a calibration product, and a quality control product. The reagent R1 comprises a buffer solution, an anti-interference agent, a sensitizer, an electrolyte and a preservative, and the reagent R2 comprises latex microspheres with an NGAL antibody, a buffer solution, a stabilizer and a preservative. Compared with the prior art, according to the technical scheme of the present invention, by using the anti-interference agent in the R1 reagent, the anti-interference ability can be significantly improved, and the applicable population of the neutrophil gelatinase-associated lipocalin assay kit is expanded; and the kit detection provided by the present invention has high sensitivity, high specificity, and good stability of the kit, the NGAL content in urine, plasma and serum can be efficiently detected, and the detection results can bewell correlated with imported reagents.
Owner:SHANGHAI FOSUN LONG MARCH MEDICAL SCI CO LTD
Heart-type fatty acid binding protein detection kit and making method thereof
ActiveCN104215772ASpeed up the immune responseShorten detection timeBiological testingImmunonephelometric AssaysSolvent
The invention relates to a heart-type fatty acid binding protein (H-FABP) detection kit and a making method thereof. The kit comprises a reagent R1, a reagent R2 and a standard substance; the reagent R1 comprises a solvent and solvates, the solvent is a buffer solution with the pH value of 7.0-7.6, and the solvates comprise a surfactant, a chelating agent, an accelerator, an antiseptic and purified water; the reagent R2 is an anti-human H-FABP antibody latex reagent; and the standard substance is a recombinant protein containing a quantitative amount of the H-FABP. The problems of bad anti-interference capability, low specificity, low reagent stability and narrow detection linearity range of latex enhanced immunoturbidimetery detection kits in the present market are solved. The kit disclosed in the invention has the characteristics of simple operation, high accuracy, good repeatability, high sensitivity and the like, can be used on a fully-automatic biochemical analyzer, a special protein analyzer or a spectrophotometer, and has a wide application prospect.
Owner:上海睿康生物科技有限公司
Method for detecting hoptoglobin in serum and detecting kit thereof
InactiveCN102128935AThe detection method is simpleDetection contentBiological testingSerum igeSerum samples
The invention provides a method for simply and quickly detecting hoptoglobin in serum, namely an immunoturbidimetry, and a detecting kit thereof. The method is a detecting method designed by using the principle that the hoptoglobin is a human protein, has antigenicity and generates cohesion after combining with an antigen antibody. The hoptoglobin in the serum sample is combined with adequate specific antibody in the agent to form an insoluble immune complex; the change of the absorbance of the reaction solution is relevant to the content of the hoptoglobin in the serum sample. The method for detecting the hoptoglobin in the serum and the detecting kit in the invention have the advantages that: the serum sample is not subjected with special treatment, and can be ordinarily detected on an automatic biochemistry analyzer; the result directly reflects the true protein content of the hoptoglobin in the serum sample; therefore, the invention is used for clinic detection.
Owner:BIOSINO BIO TECH & SCI
Beta 2-microglobulin detection kit
InactiveCN105606822AEasy to detectHigh sensitivityBiological testingEmergency treatmentBeta-2 microglobulin
The invention discloses a beta 2-microglobulin detection kit which comprises a reagent R1 and a reagent R2, and the reagent R1 is a buffer solution; the reagent R2 is a mixture of beta 2-microglobulin antibody sensitization polystyrene latex particles and a buffer solution. The detection kit has the advantages that the detection is simple and rapid, the sensitivity is high, the accuracy is good, the disturbance resistance is high and the production cost is low. An adopted beta 2-microglobulin detection method is latex enhanced immunoturbidimetry, the beta 2-microglobulin detection is more economical, convenient and rapid with the method, and the beta 2-microglobulin detection kit is suitable for automatic biochemistry analyzers in vast majority of hospitals, and particularly suitable for realizing rapid quantitative detection on emergency treatment.
Owner:宁波天康生物科技有限公司
Kit for testing lipoprotein a(Lp(a))
InactiveCN105675891AEasy to detectHigh sensitivityBiological material analysisBiological testingEmergency treatmentBuffer solution
The invention discloses a kit for testing lipoprotein a(Lp(a)). The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 is a buffer solution, and the reagent R2 is a mixture of lipoprotein a(Lp(a)) antibody sensitized polystyrene latex particles and the buffer solution. The kit has the advantages of simplicity and rapidness in detection, high sensitivity, good accuracy, high anti-interference capacity and low production cost; a method for detecting the lipoprotein a(Lp(a)) is latex enhanced immunoturbidimetry, with the adoption of the method, lipoprotein a(Lp(a)) can be detected more economically, more conveniently and more quickly, and the kit is suitable for automatic biochemical analyzers in most hospitals, and in particular, quick and quantitative detection can be realized during emergency treatment.
Owner:宁波天康生物科技有限公司
Method for improving sensitivity of latex enhanced turbidimetric immunoassay
ActiveCN104360056ALow costReduce concentrationColor/spectral properties measurementsLatex particleAbsorbance
The invention relates to the field of in-vitro diagnosing reagents, and particularly relates to a method for improving the sensitivity of latex enhanced turbidimetric immunoassay. The method comprises the following steps: (1) selecting latex particles with large diameters to react with an antibody, and carrying out antibody coating; (2) washing and sealing the coated latex, dispersing the latex in a buffer solution to prepare a reagent 2 of a latex enhanced turbidimetric immunoassay kit; (3) preparing a reagent 1 of the latex enhanced turbidimetric immunoassay kit; (4) setting parameters on a full-automatic biochemical analyzer; (5) operating the biochemical analyzer to calibrate a sample with known concentration and detect an absorbance change value of the sample to be detected, and calculating the content of to-be-detected object in the sample according to the calibration curve. The method for improving the sensitivity of latex enhanced turbidimetric immunoassay is capable of greatly improving the analyzing sensitivity and precision of a reagent without increasing the detection cost, and has an excellent application prospect.
Owner:ZYBIO INC
Latex enhanced turbidimetric immunoassay kit for detection of lipoprotein (a) and preparation method thereof
InactiveCN107607729AAmplification reactionMake up for the shortcomings of insufficient sensitivityBiological testingAlkylphenolPolyethylene glycol
The invention discloses a latex enhanced turbidimetric immunoassay kit for detection of lipoprotein (a). The kit comprises reagents R1 and R2. The reagent R1 contains a Tris buffer solution, sodium chloride, polyethylene glycol and alkylphenol polyoxyethylene. The reagent R2 contains a Tris buffer solution, anti-human lipoprotein (a) antibody coated latex, EDTA, trehalose and alkylphenol polyoxyethylene. By proportionally mixing a sample to be detected and the reagent for a reaction, detecting absorbance change rate at dominant wavelength of 600 nm through a fully automatic biochemical analyzer and referring to absorbance change rate of a calibrator, concentration of lipoprotein (a) in the sample is calculated. The detection method of lipoprotein (a) in the invention is a latex enhanced turbidimetric immunoassay method. The kit of the method has advantages of high detection sensitivity, high accuracy, good stability, strong anti-interference performance and the like.
Owner:QINGDAO BIOMEDICAL TECH
Method for detecting lipoprotein (a) by latex oriented coupling technology
InactiveCN106501505AIncrease profitConnection direction controllableMaterial analysisAntigenMicrosphere
The invention provides a method for detecting lipoprotein (a) by a latex oriented coupling technology. The principle of the detection method is based on latex-enhanced immunoturbidimetry, and the detection method is characterized by comprising the following steps: separately activating amino polystyrene latex microspheres of two particle sizes with a N-hydroxy succinimide / maleimide heterobifunctional crosslinking agent by use of an antibody oriented coupling technology; reducing a monoclonal antibody against human lipoprotein (a) by a reducing method; performing oriented coupling of the activated latex microspheres of two particle sizes respectively with the reduced monoclonal antibody against human lipoprotein (a) to form latex particles against human lipoprotein (a); connecting the antibody with the microspheres through Fc area, and outward extending the Fab area to realize efficient combination with the sample antigen; and at a constant temperature of 37 DEG C and under a wavelength of 600nm, measuring absorbance and calculating the content of sample lipoprotein (a). Compared with the traditional method, the detection method has the advantages of a high antibody utilization rate, high sensitivity and good linearity, can effectively control the batch difference, and deserves further promotion and application.
Owner:王贤俊
Troponin I detection reagent with high sensitivity through latex enhanced turbidimetric Immunoassay
InactiveCN105628930AImprove buffering effectGuaranteed cushioning effectDisease diagnosisBiological testingImmunonephelometric AssaysLatex particle
The invention relates to the technical field of troponin I detection, in particular to a troponin I detection reagent with high sensitivity through latex enhanced turbidimetric Immunoassay. The reagent comprises the following main components: a buffer solution, zinc chloride, Thesit, EMULGEN-A90, and a nitriloriacetic acid (NTA) preservative; a reagent R2 comprises the following main components: a buffer solution, triton-308, bovine serum albumin (BSA), a preservative, TnI antibody-coated latex particles and the like. The three surfactants, namely Thesit, EMULGEN-A90 and triton-308 are added, and the latex particles with appropriate particle sizes are selected, so that the reaction sensitivity is greatly improved, and the reagent is simple in product configuration, low in cost and very suitable for clinical expansion in large scale.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Human urine immunoglobulin G detection kit based on latex-enhanced immunoturbidimetry
InactiveCN109633172AImprove stabilityGood repeatabilityBiological testingHuman immunoglobulinsMicrosphere
The invention, which belongs to the technical field of medical in-vitro diagnostics, relates to a human urine immunoglobulin G detection kit based on latex-enhanced immunoturbidimetry, thereby solvinga problem of providing a human urine immunoglobulin G content detection kit having advantages of wide detection linear range, high detection accuracy, and high detection stability. The kit comprisesan R1 reagent, an R2 reagent and a calibrator. The R1 reagent includes a buffer solution, NaCl and a preservative. The R2 reagent includes a buffer solution, an antibody-coupling latex microsphere, aprotective agent and a preservative; and the antibody is the goat anti-human immunoglobulin polyclonal antibody or a rabbit anti-human immunoglobulin polyclonal antibody. The calibrator is formed by aplurality of solution; each solution includes human immunoglobulin G, a buffer solution and a preservative; and the concentrations of the human immunoglobulin G in different solutions are different.The kit has advantages of wide detection linear range, high detection accuracy, and high detection stability.
Owner:DIRUI MEDICAL TECH CO LTD
Combined orientation agents optimized latex coupled antibody detection method of prealbumin (PA)
InactiveCN106526198AIncrease profitImprove the coupling effectBiological testingMicrosphereImmunonephelometric Assays
The invention provides a combined orientation agents optimized latex coupled antibody detection method of prealbumin (PA). The method is based on a latex enhanced turbidimetric immunoassay method, and is characterized in that combined orientation agents are utilized: an orientation agent 1 and an orientation agent 2. Firstly, the orientation agent 1 is combined with a PA antibody to form a PA antibody compound; the orientation agent 2 is then combined with the PA antibody compound; and finally, the compound is respectively coupled with carboxyl latex microspheres of two different particle sizes to form PA latex particles. By the method, the formed reagent has high sensitivity and good precision and linearity, is simple to prepare and is worthy of further promotion and application.
Owner:王贤俊
Recombinant adiponectin antigen, antibody and adiponectin nano latex-enhanced turbidimetric immunoassay kit
The invention discloses a recombinant adiponectin antigen. A preparation method of the recombinant adiponectin antigen includes the steps of a, culturing a high-expression recombinant human full-length adiponectin protein cell strain in a high-glucose DMEM culture medium under oxygen input quantity of 0.25-0.75L / minute, carbon dioxide quantity of 5-10%, bovine serum quantity of 5-10% and rotationspeed of 100-200rpm to allow the cell number of the cell strain to reach 1-3*10<7>; b, replacing the culture medium with a serum-free culture medium, adding 0.5% of vitamin C, and allowing the cell strain to keep the cell number of 1-3*10<7> and continuously express adiponectin for 4-10 days. The invention further discloses an adiponectin antibody, adiponectin antibody nano latex particles and a kit containing the antigen and the antibody nano latex particles. The kit using the nano latex-enhanced turbidimetric immunoassay to detect adiponectin is high in accuracy, real and reliable in data and good in predicting effect on type 2 diabetes and pre-diabetes.
Owner:广东英诺生物科技有限公司
Sample treatment fluid for latex immunoturbidimetry detection
The invention relates to sample treatment fluid for latex immunoturbidimetry detection, which comprises triglycercide ethoxylate, a nonionic surfactant, glucan (20 thousands) and a buffer. The invention solves the problem that latex immunoturbidimetry can not be adopted for detection because the sample treatment fluid in the traditional reagent has poor effect of eliminating high-degree chyle or turbidity phenomenon of a sample.
Owner:南京卡博生物科技有限公司
Detection reagent for human CK18 protein and preparation method of reagent
The invention relates to a detection reagent for CK18 and a preparation method, in particular to a reagent for detecting the CK18 by adopting latex-enhanced immunoturbidimetry and a preparation method. The reagent includes a reagent R1, a reagent R2 and a calibration product, wherein the reagent R1 contains a reaction accelerant, a preservative, a surfactant, a stabilizer, an electrolyte and a buffer solution; the reagent R2 contains latex particles combined with anti-CK18 monoclonal antibodies and polyclonal antibodies, a preservative, a surfactant, a stabilizer, an electrolyte and a buffer solution; the calibration product contains a preservative, an electrolyte, a stabilizer, a CK18 pure product and a buffer solution. According to the detection reagent for the CK18 and the preparation method, by adopting the method of using the monoclonal antibodies and the polyclonal antibodies to be combined with and coat the latex particles, the high sensitivity and wide linearity range of a reagent kit are ensured, and meanwhile the detection reagent for the CK18 and the preparation method also have the advantages of high accuracy, good repeatability, high specificity, simple operation and the like, and can be applied to a clinical commonly-used full-automatic biochemical analyzer.
Owner:苏州和锐生物科技有限公司
Method for improving the accuracy of immunoturbidimetry
PendingCN109541201AHigh sensitivityHigh precisionMaterial analysis by observing effect on chemical indicatorAntigenHemolysis
The invention discloses a method for improving the accuracy of immunoturbidimetry. The method uses magnetic beads to couple antibodies, and uses a magnetic field to quickly separate, thereby shortening the coupling time, avoiding an agglomeration phenomenon caused by centrifugation, and improving the coupling efficiency. The method uses the magnetic beads with one or more particle sizes for coupling, and preferably uses magnetic beads with multi-particle size for coupling since the multi-particle size coupling has higher detection sensitivity and specificity relative to the single particle size. The immunoturbidimetric method is highly susceptible to hemolysis and fatty blood, resulting in inaccurate results. In the invention, the inaccurate results due to sample factors are avoided by applying a magnetic field for rapid separation, discarding the supernatant, adding the buffer to resuspend the complex, and then testing after a sample is reacted with the magnetic bead-antibody complex(after the antibody successfully captures the antigen).
Owner:海格德生物科技(深圳)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com