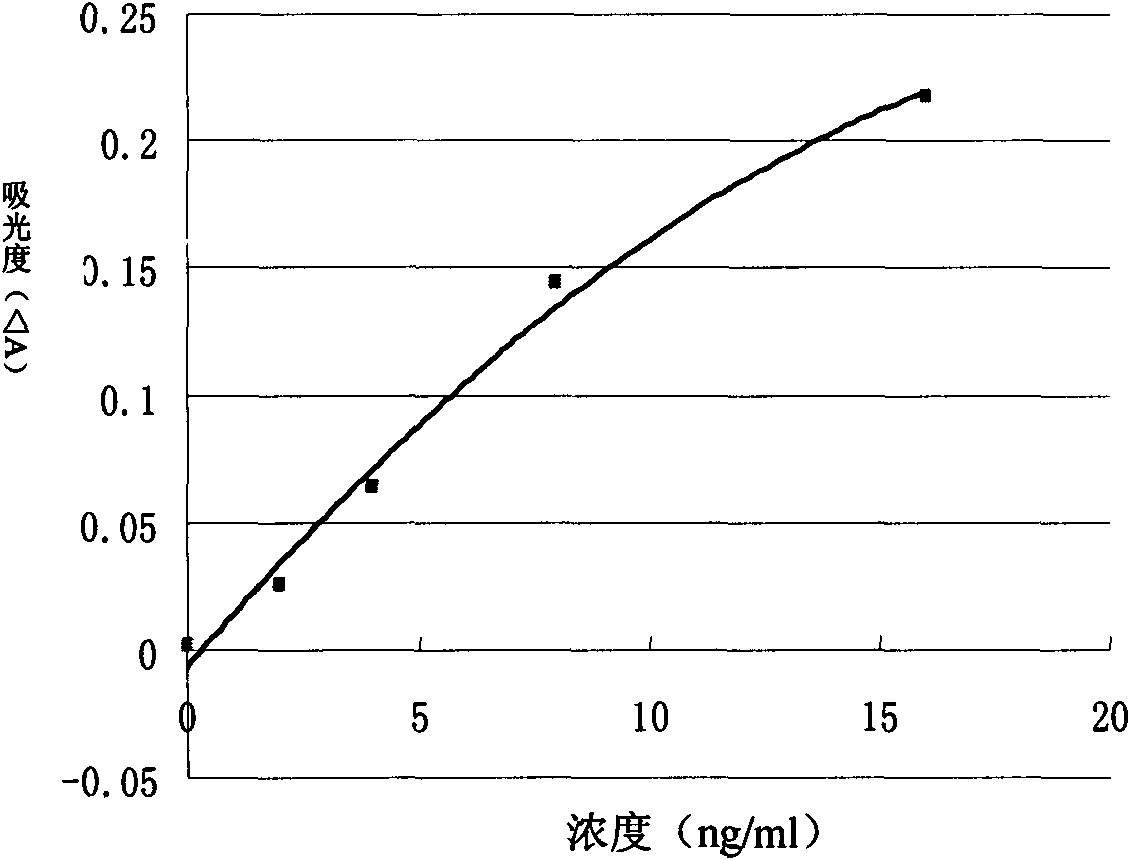
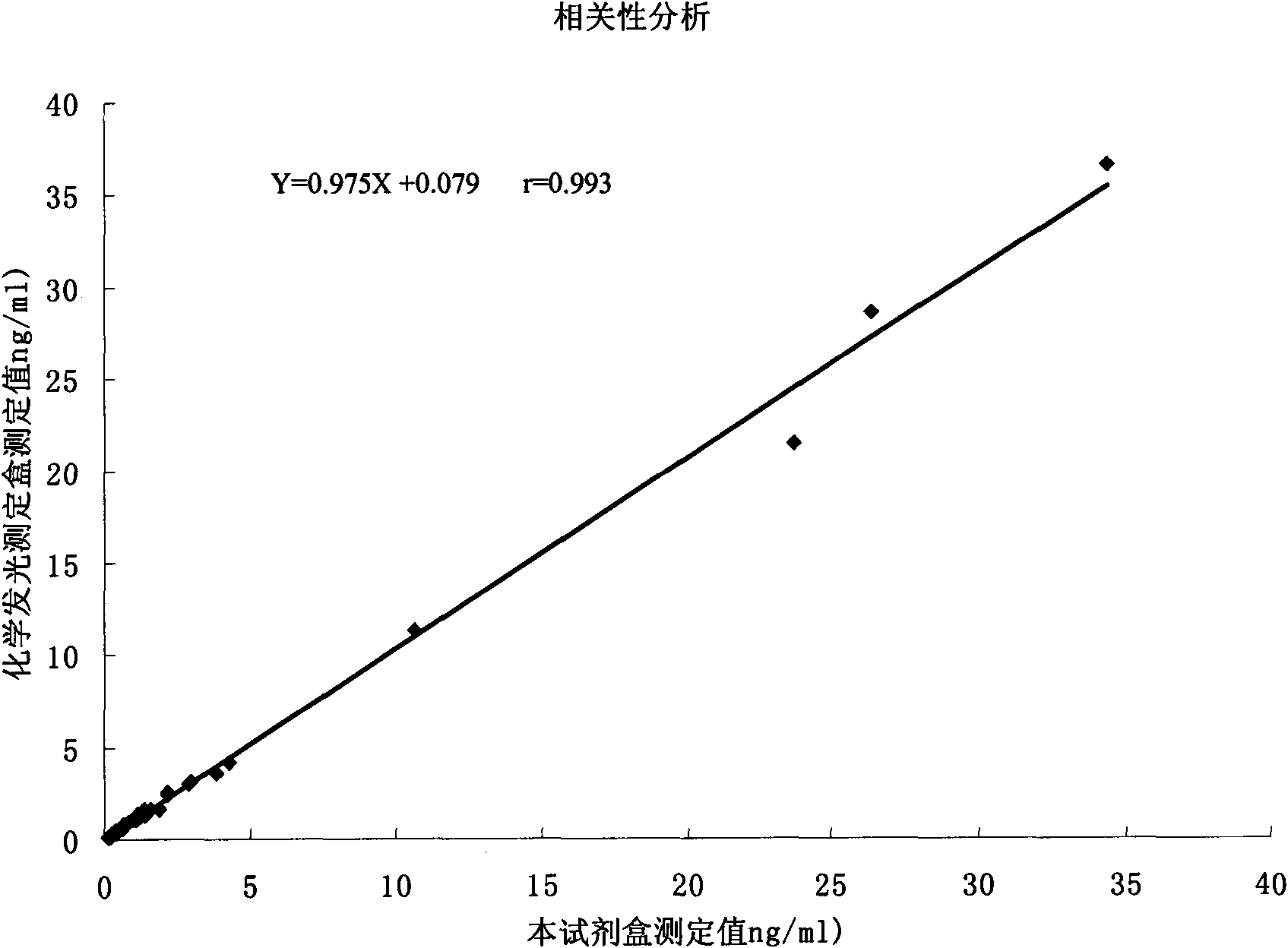
Troponin-T determination kit
A kit and reagent technology are applied in the field of kits for determining the content of troponin in human serum, which can solve the problems of environmental pollution, radioactive radiation, long detection time, high cost and the like, and achieve high detection sensitivity, simple operation and stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example
[0032] The invention discloses a kit for detecting the content of TNT, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications that are obvious to those skilled in the art are deemed to be included in the present invention. The products and applications of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the methods and applications described herein without departing from the content, spirit and scope of the present invention. The technology of the present invention.
[0033] The present invention will be further described below with specific examples.
[0034] One, TNT reagent R2 is the preparation of anti-human TNT antibody latex reagent
[0035] 1: Add 2g of carboxylated latex with a pore...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com