Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Human respiratory syncytial virus A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
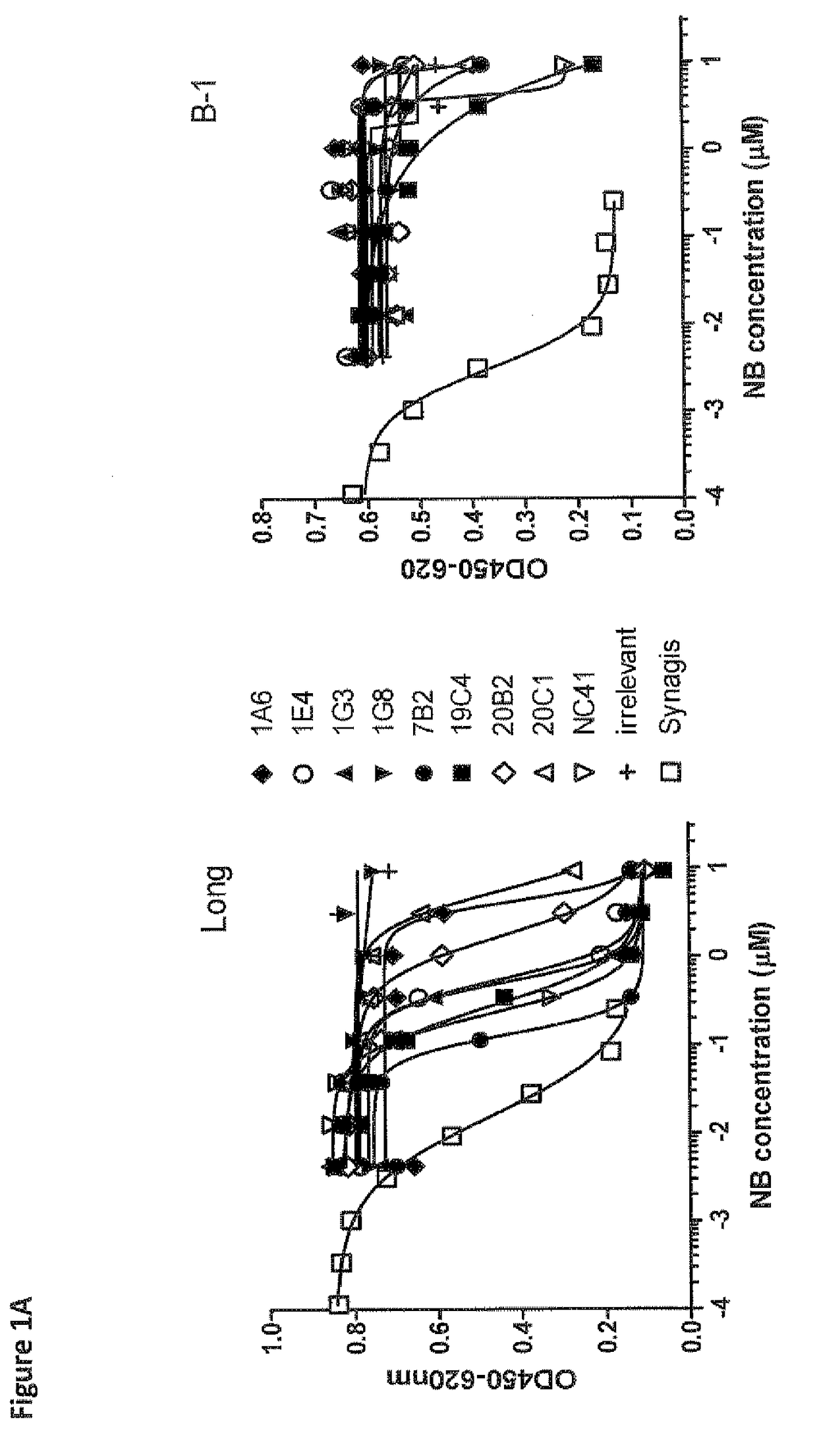
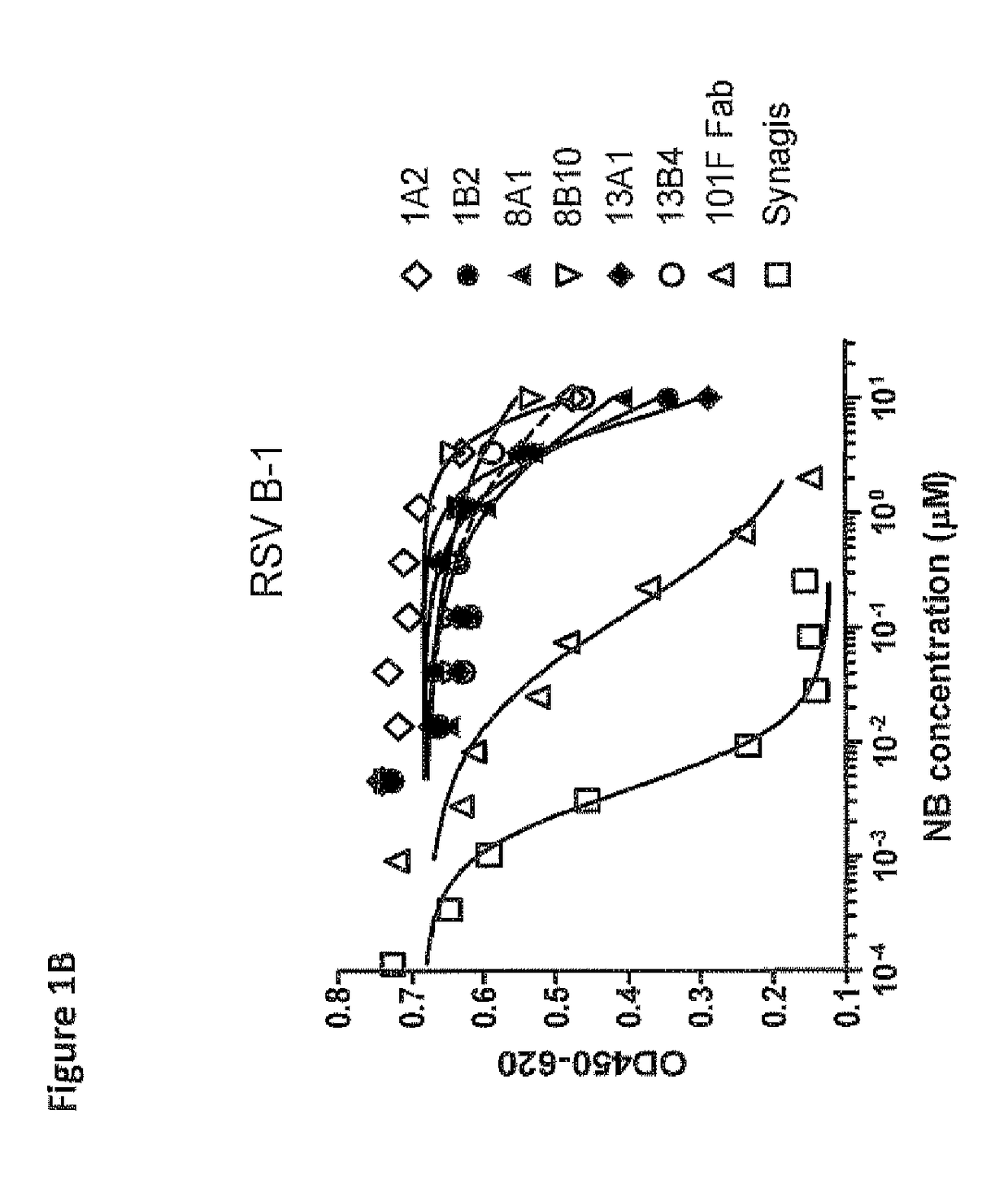
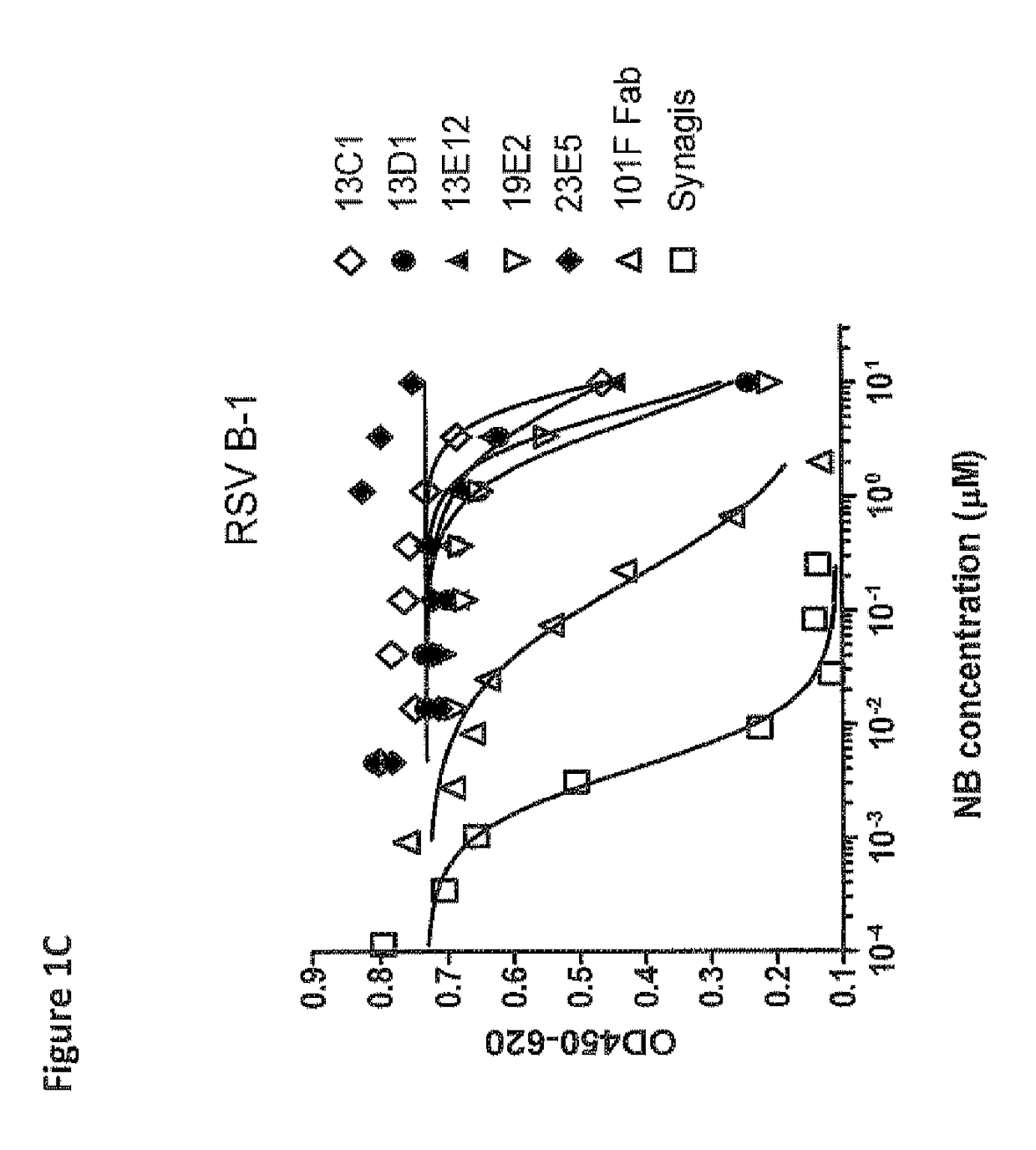
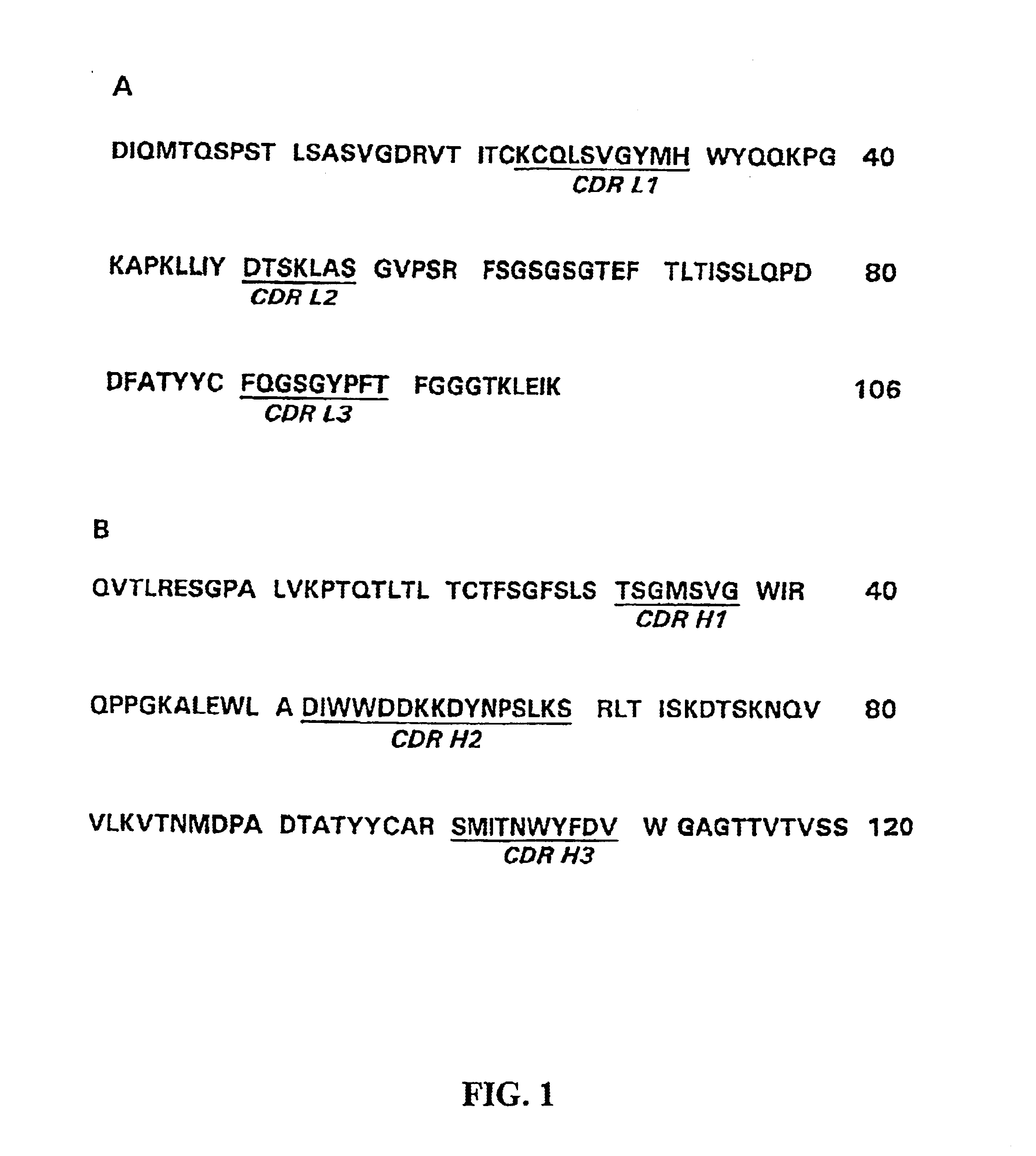
Anti-RSV antibodies
InactiveUS6818216B2Reduce dosing frequencyReduce dosageAnimal cellsSugar derivativesSerum igeAntibody fragments
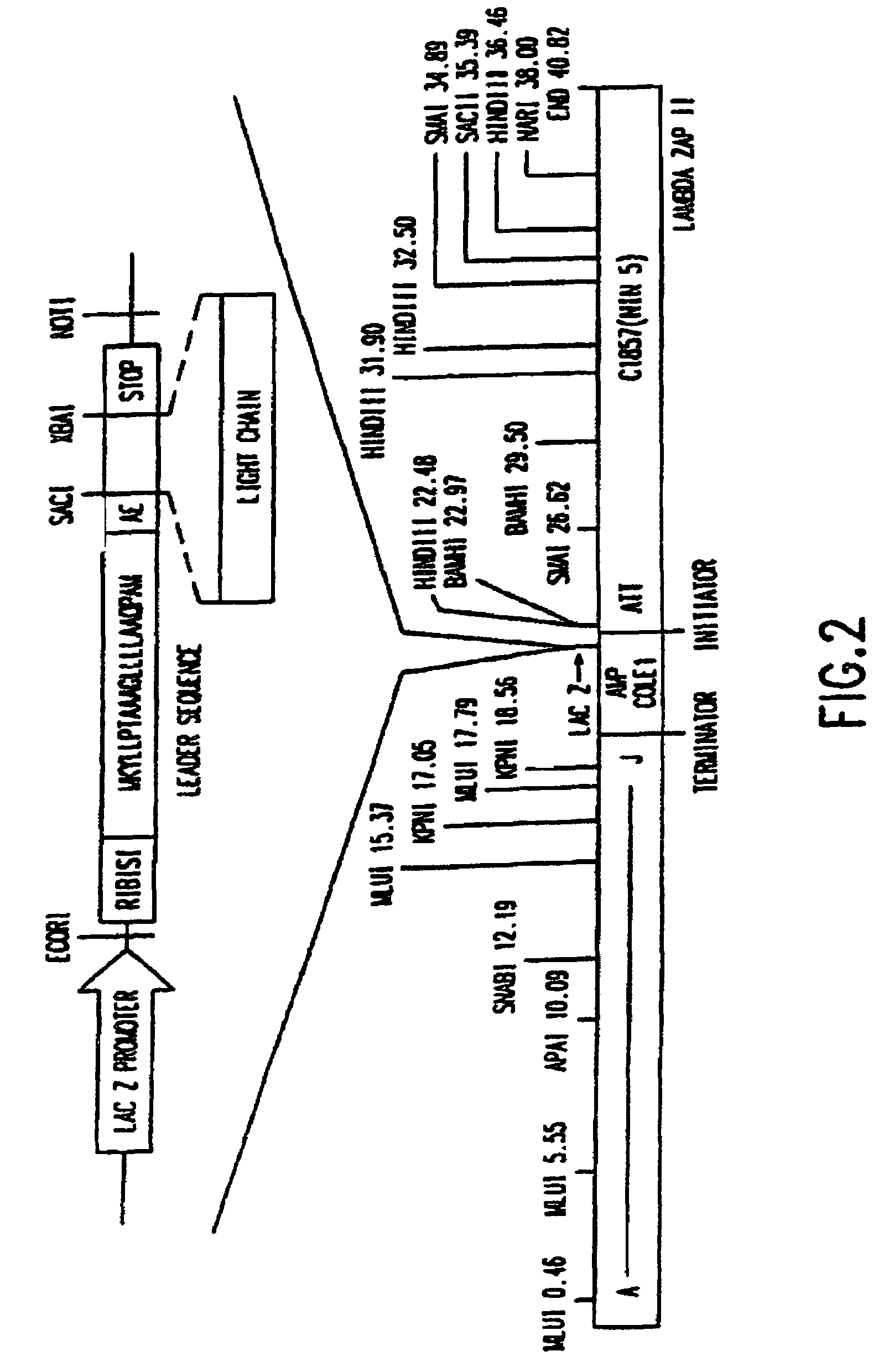
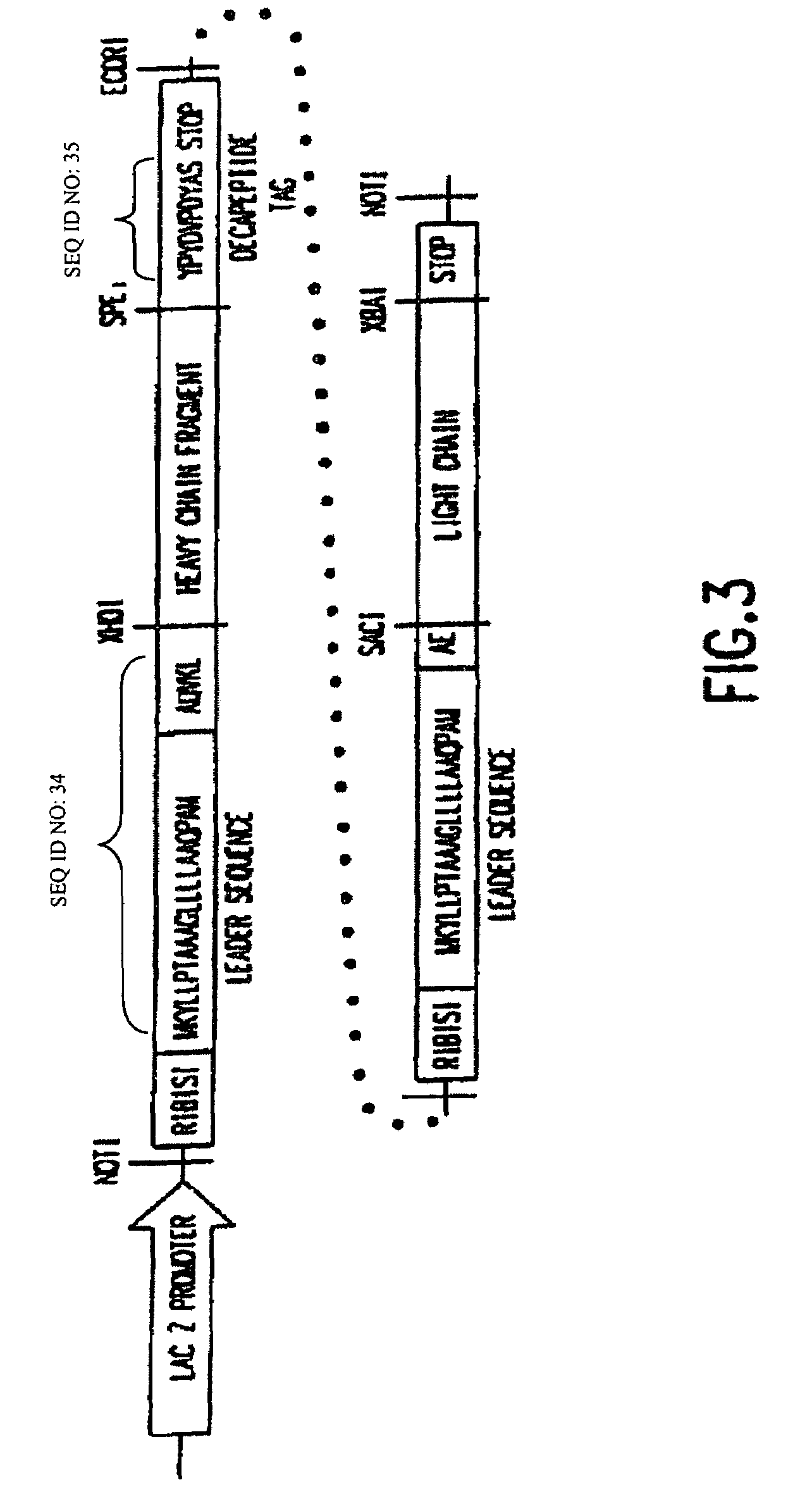
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Human neutralizing monoclonal antibodies to respiratory syncytial virus
InactiveUS7364737B2Reduce decreaseReduce the amount requiredPeptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunotherapyVirus
Human monoclonal antibodies and fragments thereof which bind, neutralize and provide passive immunotherapy to respiratory syncytial virus (RSV) antigenic subgroups A and B are disclosed. Also disclosed are diagnostic and immunotherapeutic methods of using the monoclonal antibodies as well as cell line producing the monoclonal antibodies.
Owner:THE SCRIPPS RES INST
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS20020177126A1Reduce dosageLess frequent administrationAnimal cellsSugar derivativesSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection.
Owner:MEDIMMUNE LLC
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS20050147616A1Reduce frequencyReduce dosageAnimal cellsSugar derivativesAntibody fragmentsRSV Infections
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection
Owner:MEDIMMUNE LLC
Antibodies against human respiratory syncytial virus (RSV) and methods of use
InactiveCN102656189AOrganic active ingredientsPeptide/protein ingredientsDiseaseAntigen Binding Fragment
Provided herein are antibodies or antigen-binding fragments thereof that immunospecifically bind to the fusion (F) protein of Respiratory Syncytial Virus (RSV). Also provided are methods for of prevention, treatment and diagnosis of viral infection and / or the treatment of one more symptoms of RSV-mediated disease. Methods of generating antibodies that immunospecifically bind RSV F protein also are provided.
Owner:JANSSEN VACCINES & PREVENTION BV
Methods of administering/dosing anti-RSV antibodies for prophylaxis and treatment
InactiveUS20050002926A1Reduce dosing frequencyReduce dosageImmunoglobulins against virusesAntibody ingredientsSerum igeAntibody fragments
The present invention encompasses novel antibodies and fragments thereof which immunospecifically bind to one or more RSV antigens and compositions comprising said antibodies and antibody fragments. The present invention encompasses methods preventing respiratory syncytial virus (RSV) infection in a human, comprising administering to said human a prophylactically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention also encompasses methods for treating or ameliorating symptoms associated with a RSV infection in a human, comprising administering to said human a therapeutically effective amount of one or more antibodies or fragments thereof that immunospecifically bind to one or more RSV antigens, wherein a certain serum titer of said antibodies or antibody fragments is achieved in said human subject. The present invention further encompasses compositions comprising antibodies or fragments thereof that immunospecifically bind to a RSV antigen, and methods using said compositions for detection or diagnosis a RSV infection
Owner:MEDIMMUNE LLC
Pyrazolo[1,5-a]pyrimidines for antiviral treatment
The invention provides compounds of Formula I or Formula II:or a pharmaceutically acceptable salt or ester, thereof, as described herein. The compounds and compositions thereof are useful for treating Pneumovirinae virus infections. The compounds, compositions, and methods provided are particularly useful for the treatment of Human respiratory syncytial virus infections.
Owner:GILEAD SCI INC
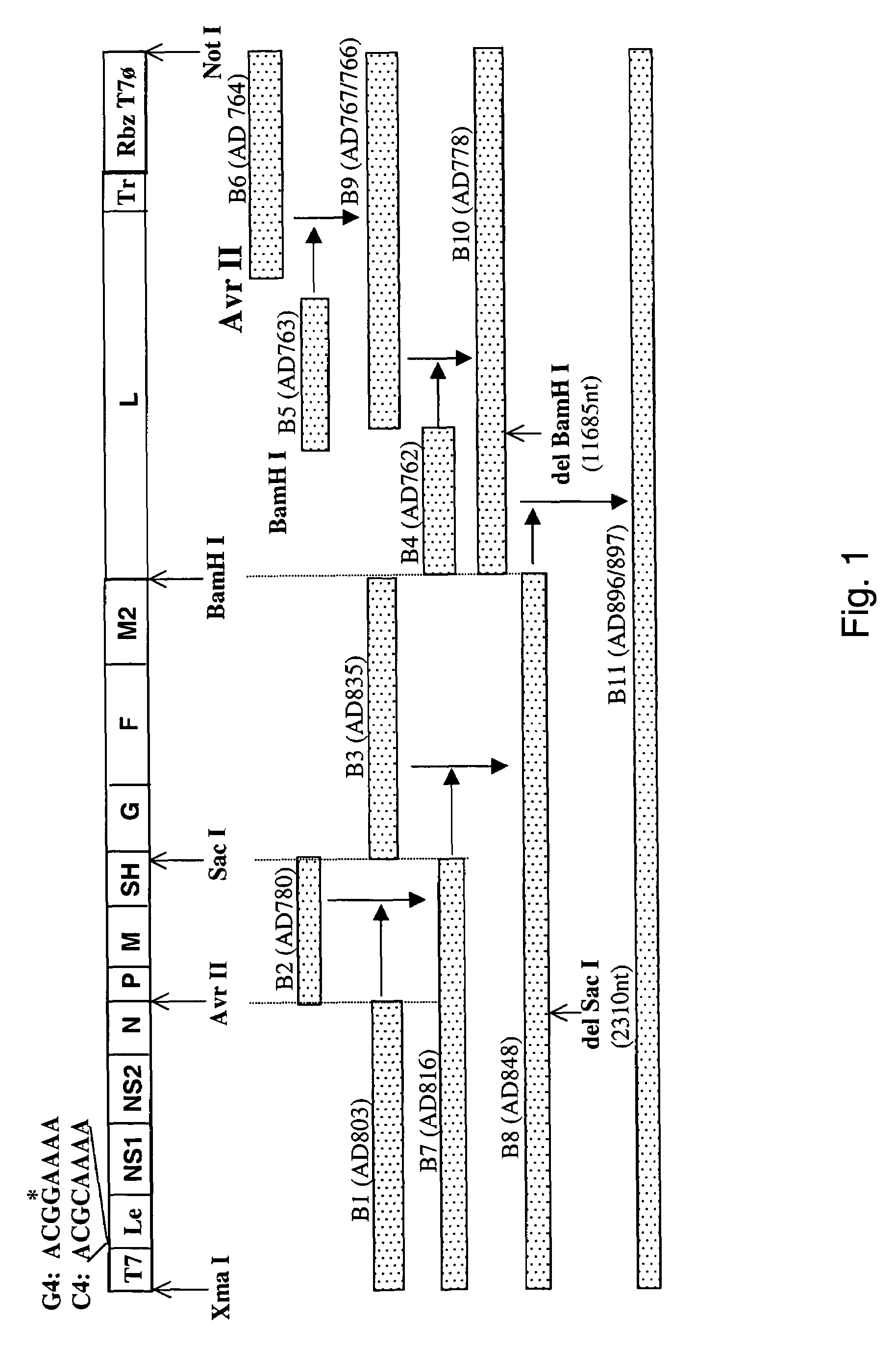
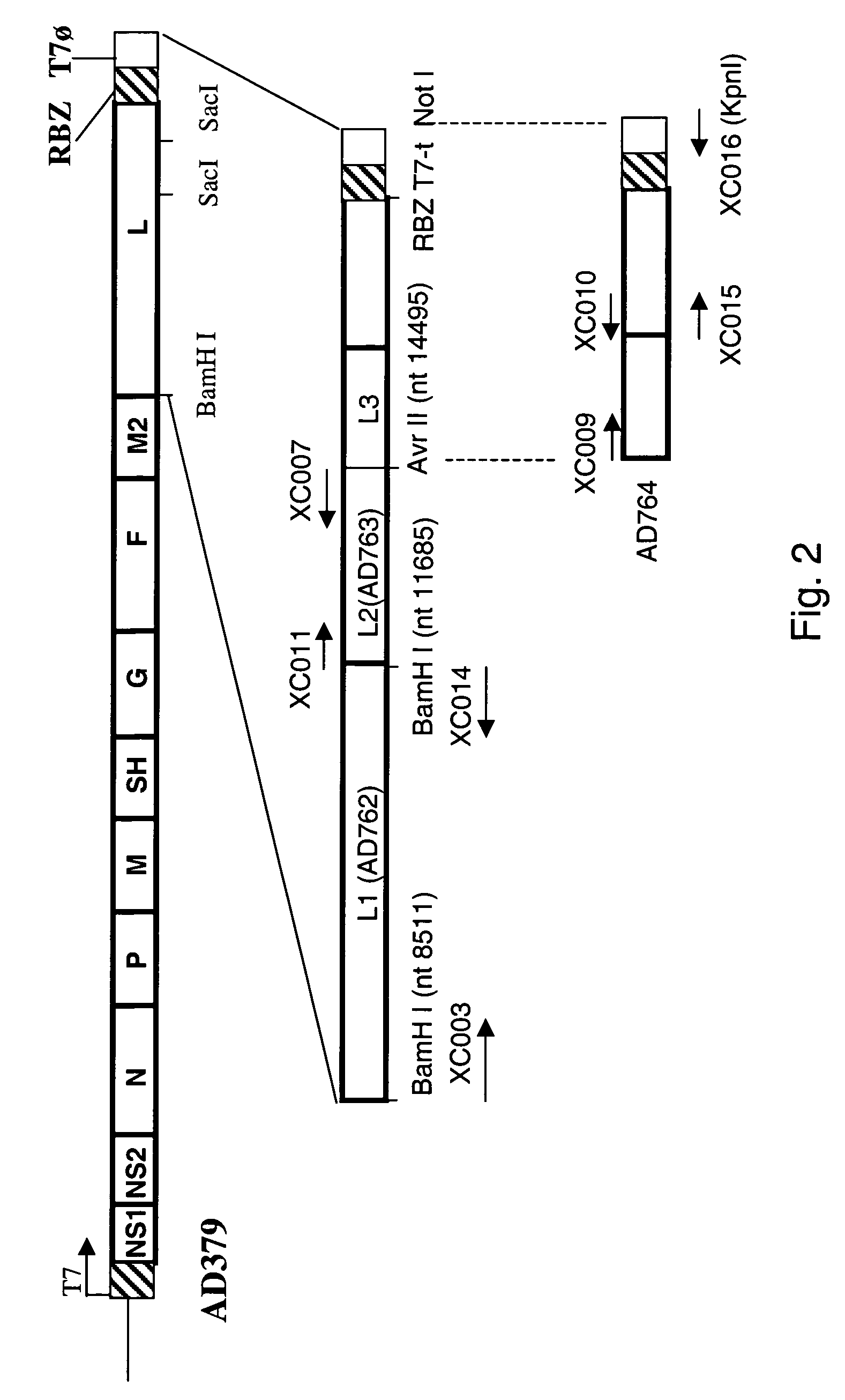
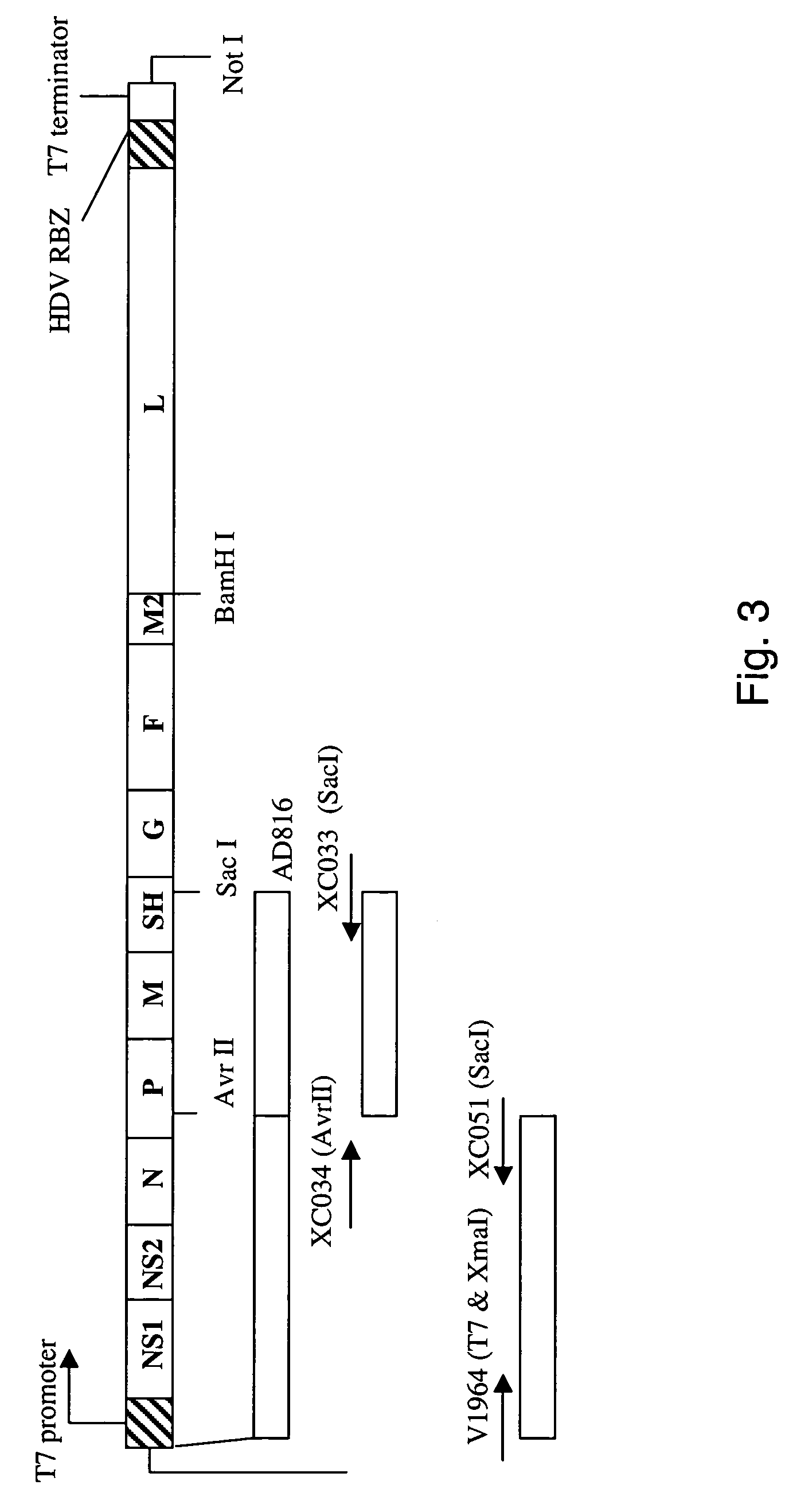
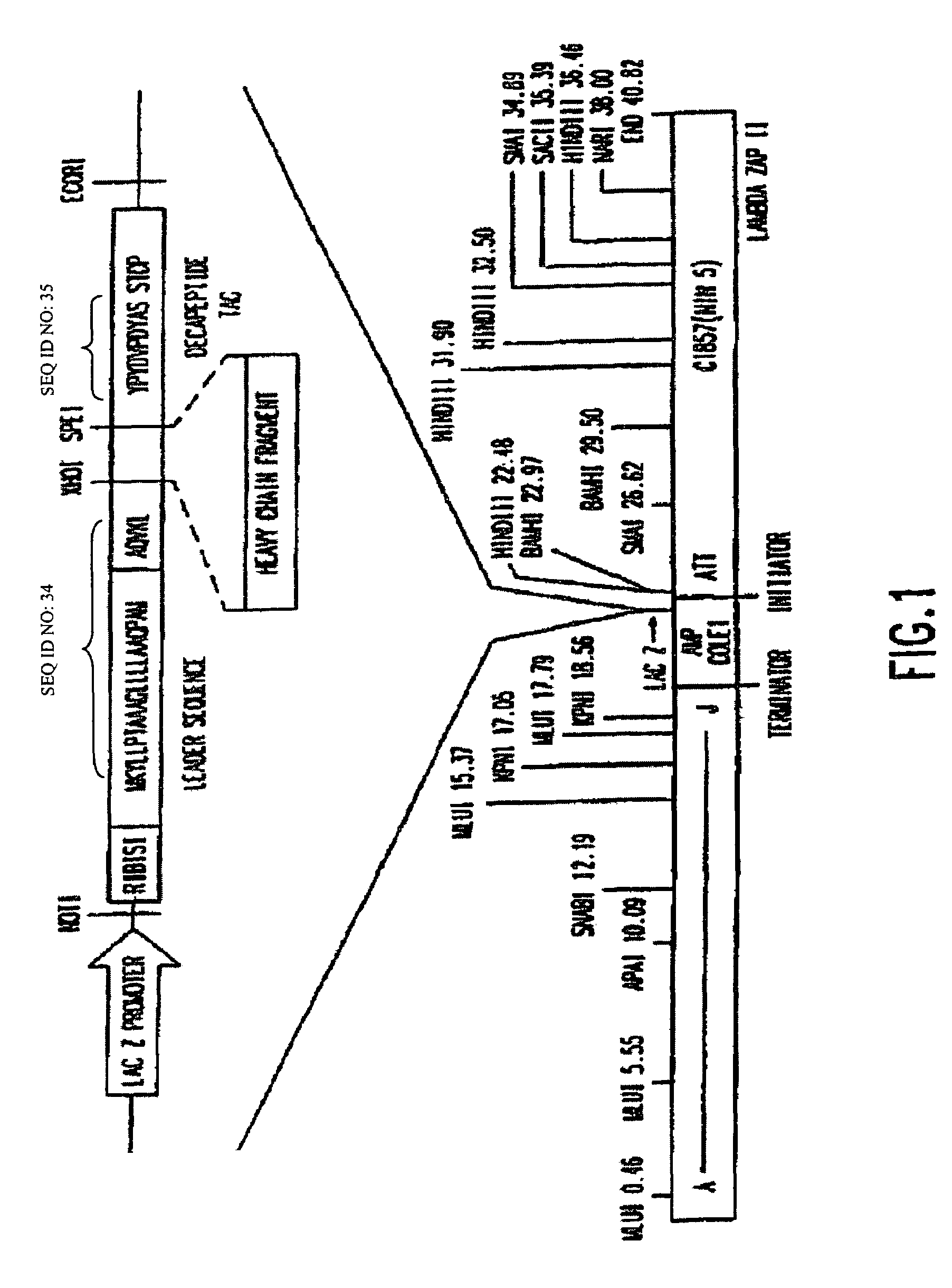
Nucleic acids encoding respiratory syncytial virus subgroup B strain 9320
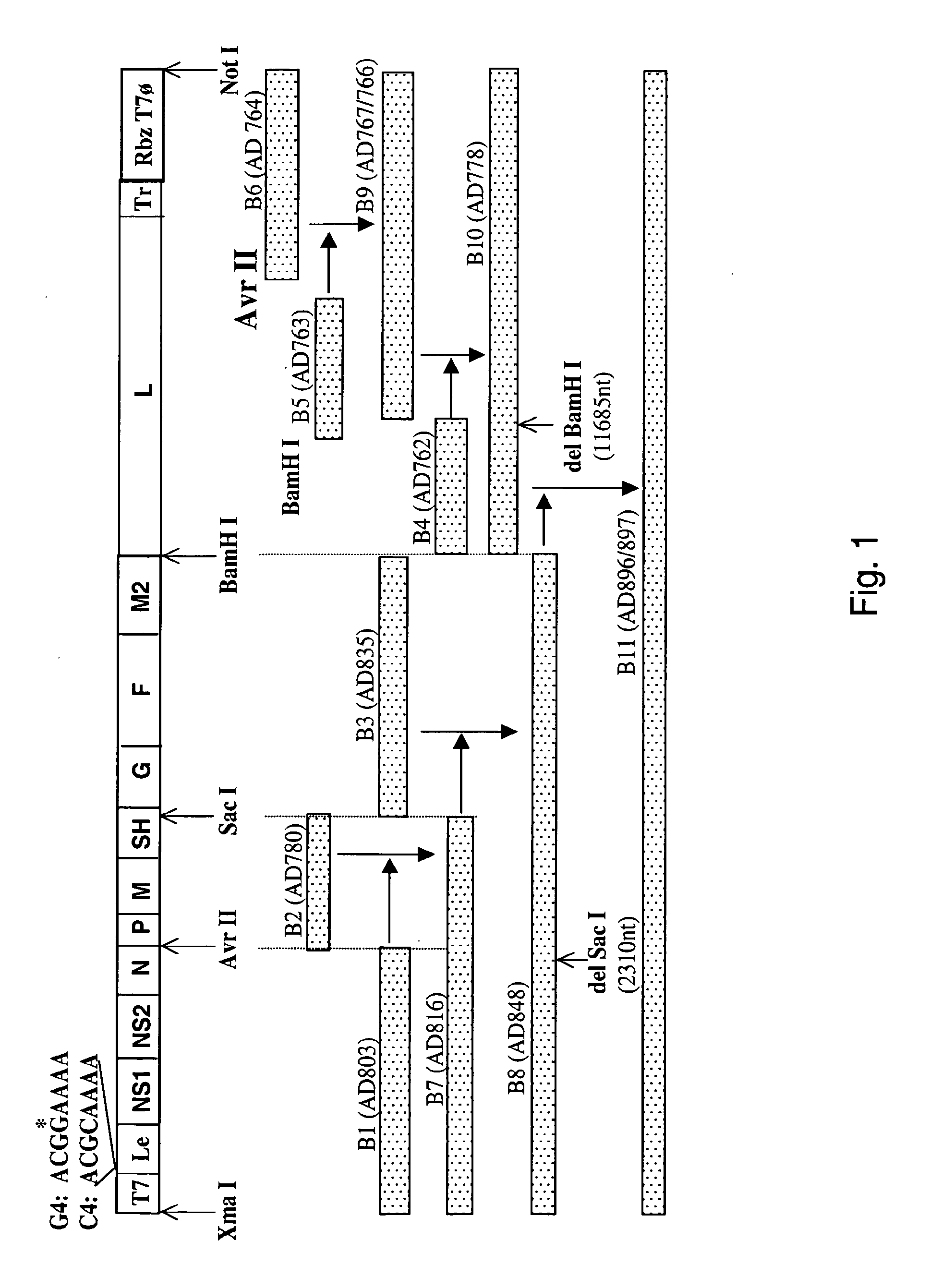
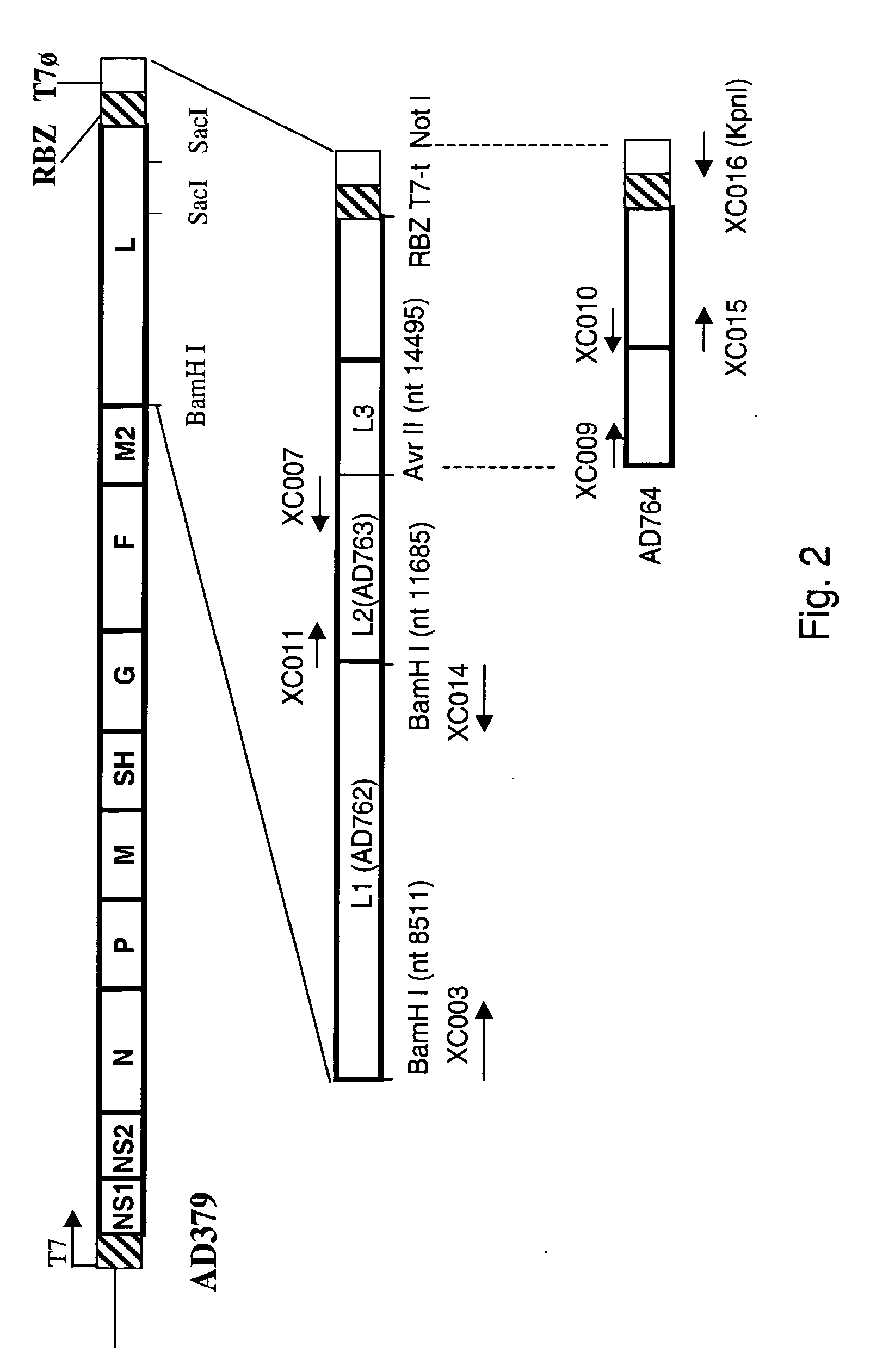
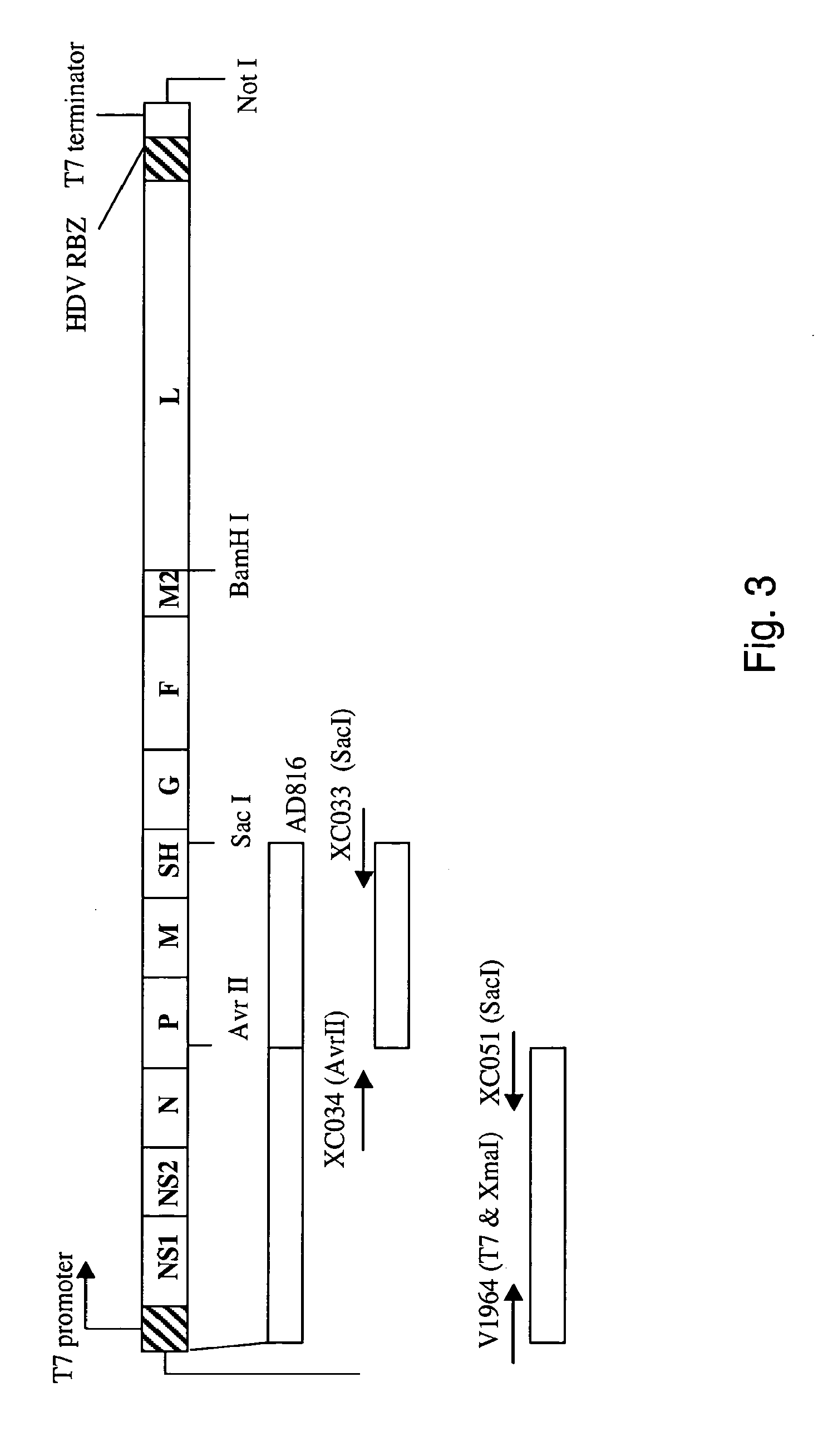
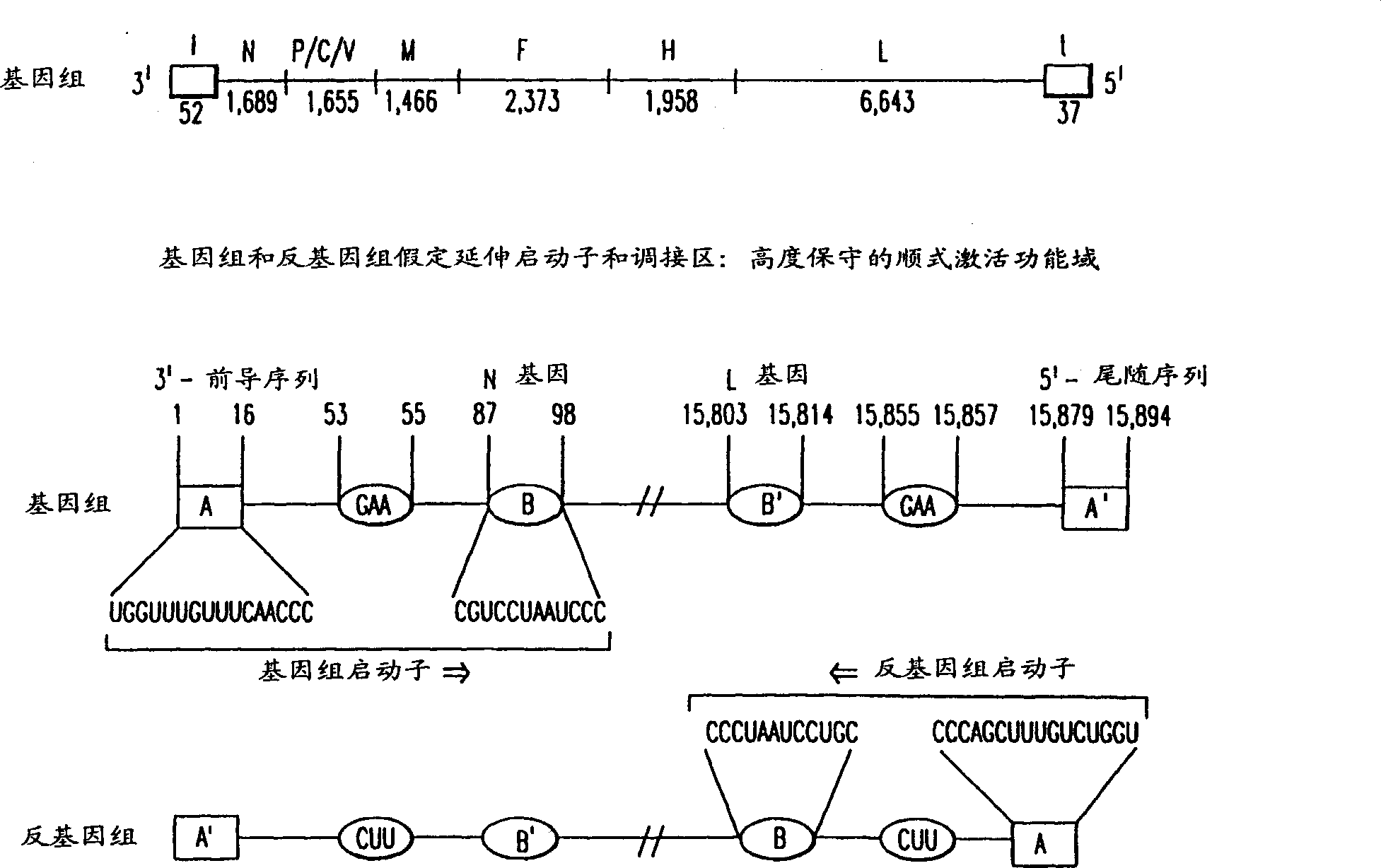
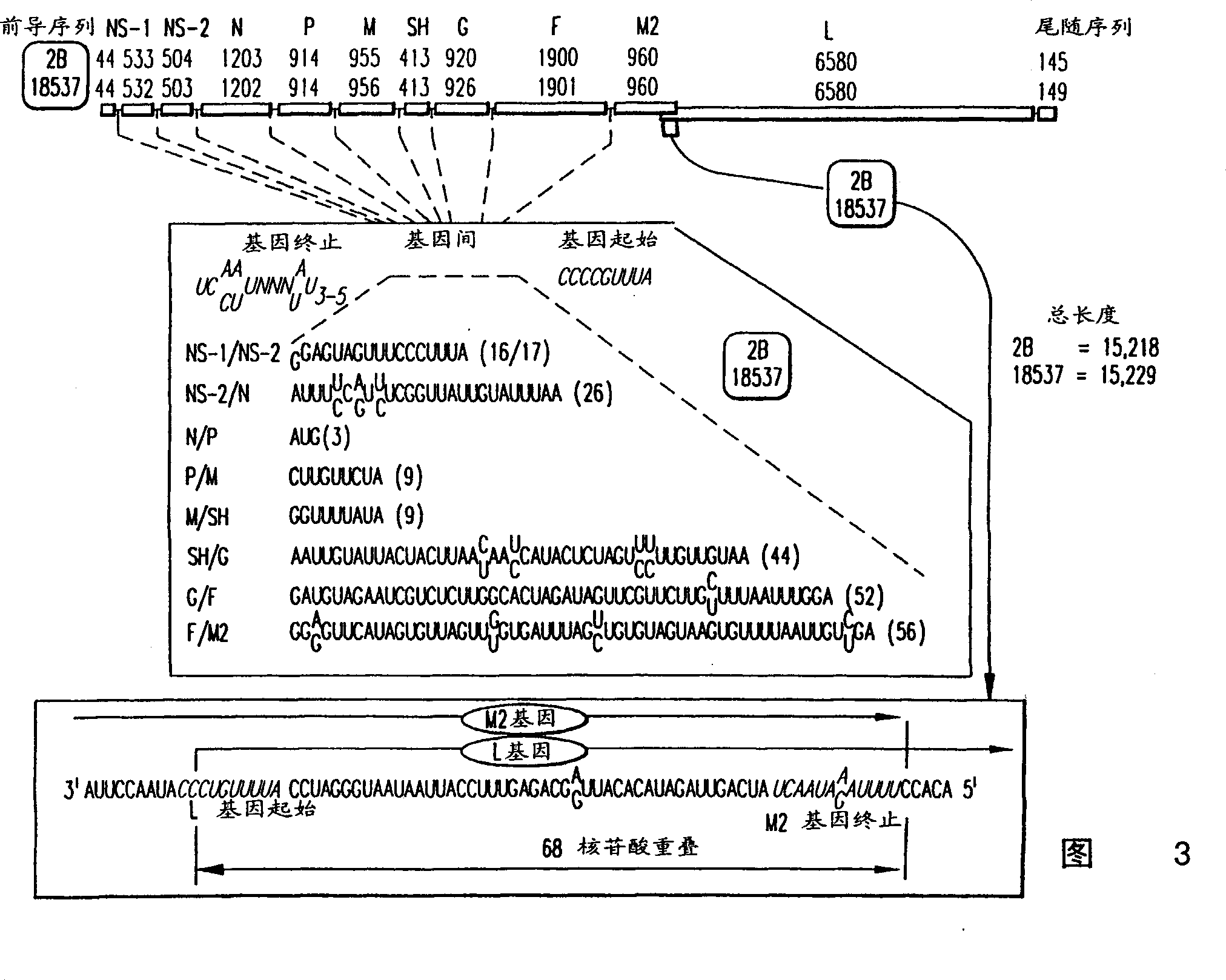
ActiveUS7572904B2SsRNA viruses negative-senseVirus peptidesAttachment proteinRespiratory syncytial virus (RSV)
The complete polynucleotide sequence of the human respiratory syncytial virus subgroup B strain 9320 genome is provided. Proteins encoded by this polynucleotide sequence are also provided. Isolated or recombinant RSV (e.g., attenuated recombinant RSV), nucleic acids, and polypeptides, e.g., comprising mutations in the attachment protein G, are also provided, as are immunogenic compositions comprising such isolated or recombinant RSV, nucleic acids, and polypeptides. Related methods are also described.
Owner:MEDIMMUNE LLC
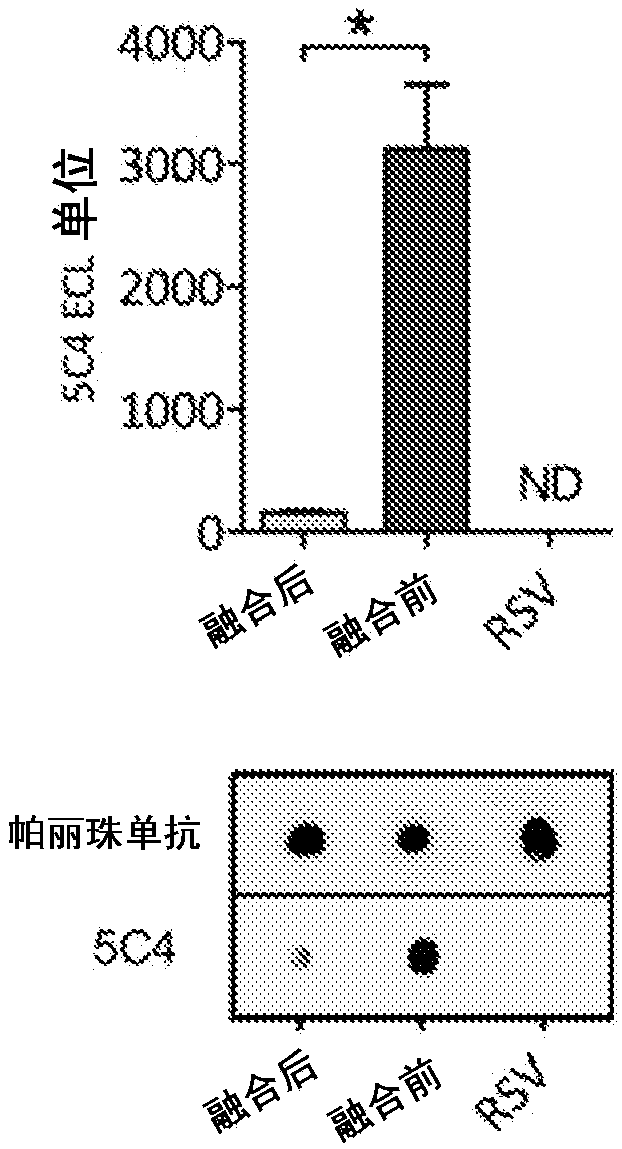
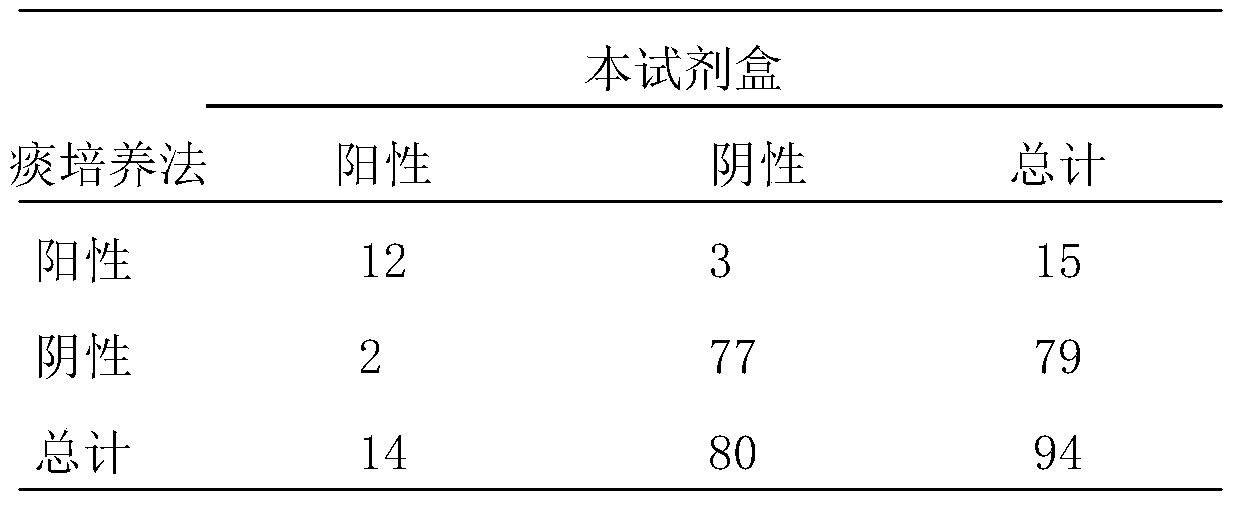
Fluorescence quantitative PCR reagent kit and detection method for human respiratory syncytial virus
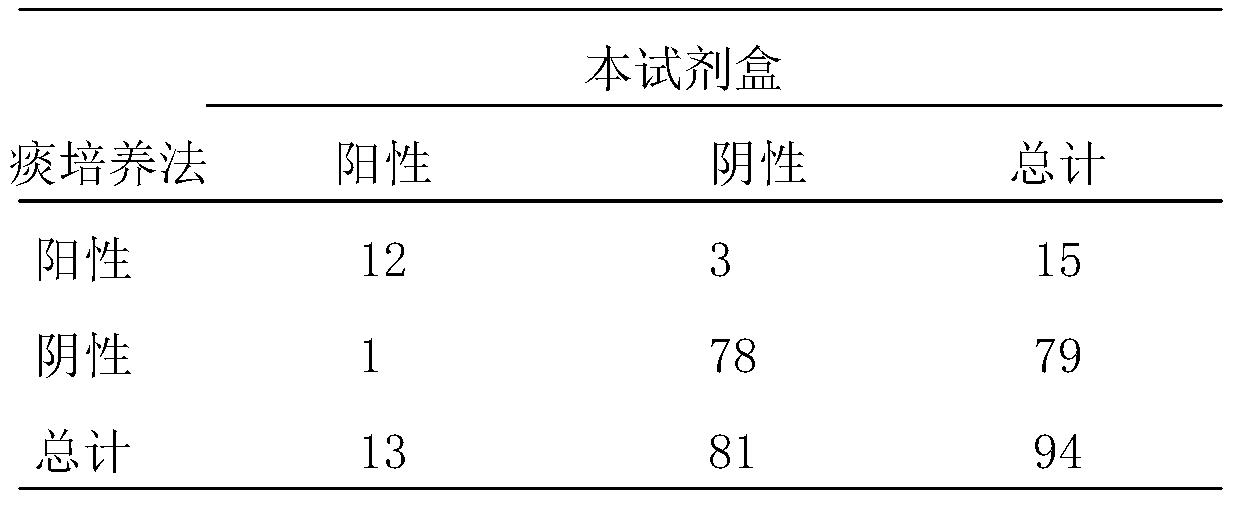
InactiveCN1544656AAvoid subjectivityStrong specificityMicrobiological testing/measurementBiological testingRNA extractionFluorescence
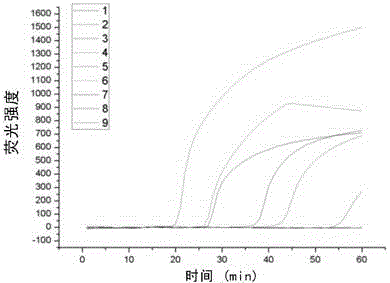
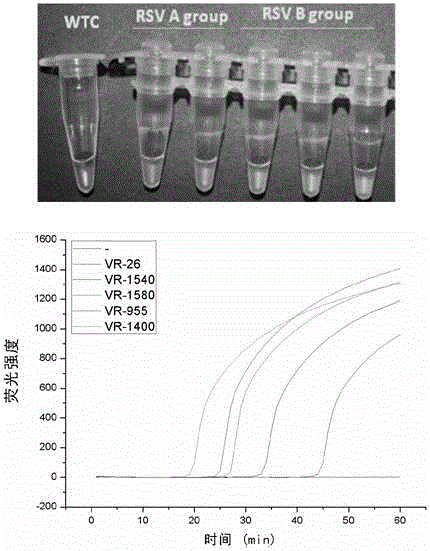
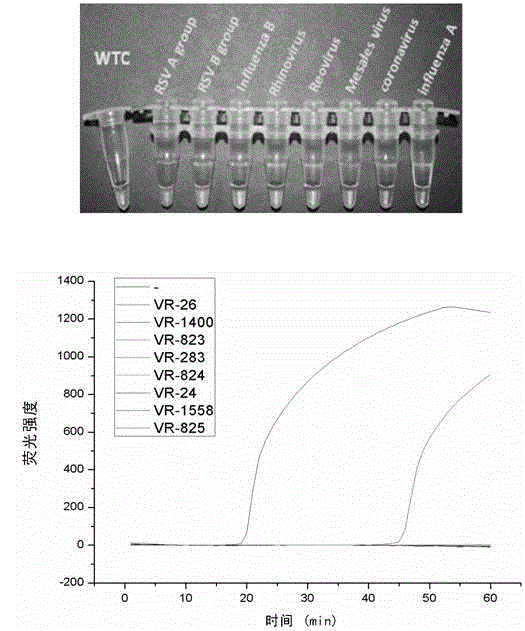
The invention discloses a Human respiratory syncytial virus (HRSV) fluorescent quantitative PCR reagent box and detecting method, the reagent box including RNA extract, reverse transcription enzyme, RNA enzyme inhibitor, reverse transcription reacting solution, standard positive template, Taq DNA polyase, fluorescent quantitative reacting solution and standard negative reference and control solution. Using the reagent box to firstly extract virus total RNA from the sample to be detected to make reverse transcription into cDNA, where cDNA and the standard positive template act as fluorescent quantitative PCR, and calculating the initial HRSV concentration by the software in fluorescent quantitative PCR apparatus. It has fast detection, convenience and safety of operation, time saving and high efficiency and can implement early diagnosis and effective prevention of HRSV.
Owner:WUHAN UNIV +1
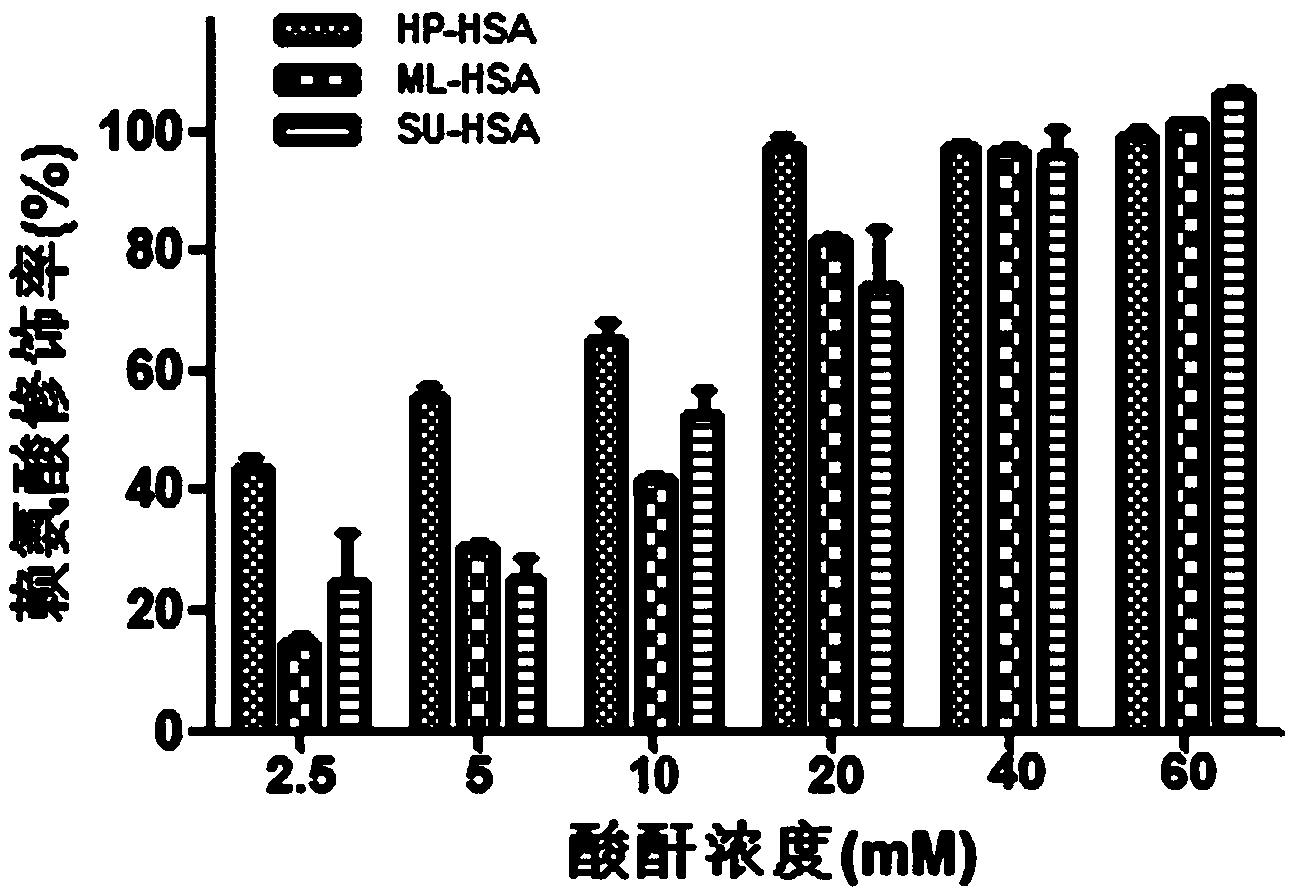
Biological preparation for preventing and controlling human respiratory syncytial virus infection and preparation method
ActiveCN104353063AImprove stabilityLow costPeptide/protein ingredientsAntiviralsHuman respiratory virusViral infection
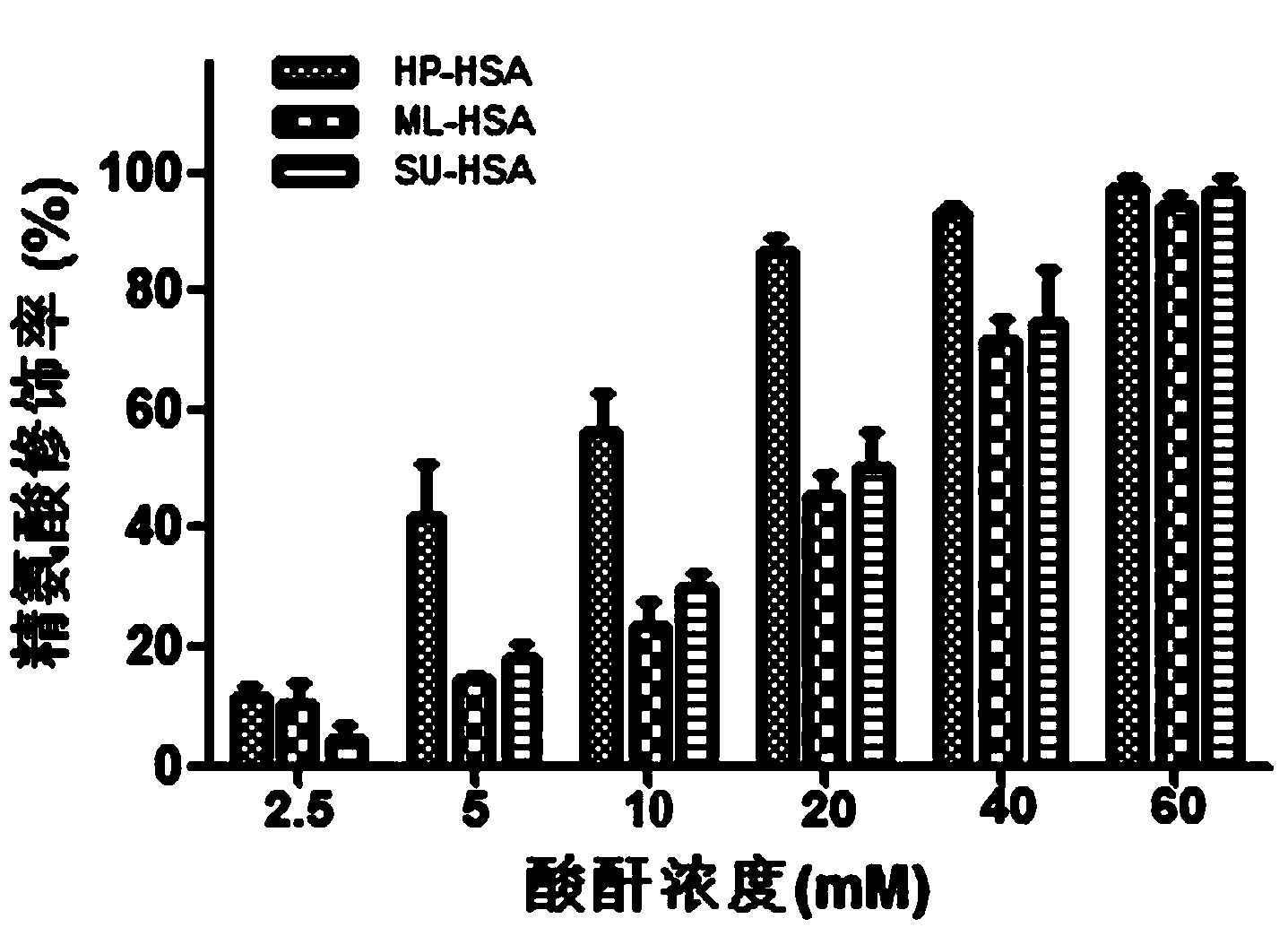
The invention relates to a novel antiviral biological preparation in the technical field of medicines, in particular to a biological preparation for preventing and controlling human respiratory syncytial virus infection and a preparation method. The biological preparation adopts globulin virus entry or fusion inhibitors. The biological preparation is applied to the first stage that virus invades target cells, and blocks the infection of virus to the cells, so as to achieve the effect of preventing and controlling the virus. The biological preparation adopts active anhydride to modify separated and purified amino acid with positive charge on the surface, thereby having the function of preventing the human RSV (respiratory syncytial virus) from entering and infecting the target cells.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Human respiratory syncytial virus vaccine
ActiveUS9492525B2Improve immunityPromotes the normal antigen conformationSsRNA viruses negative-senseOrganic active ingredientsHuman respiratory virusHIV vaccine
Owner:NANOBIO CORP
Anti-human respiratory syncytial virus (RSV) antibodies and methods of use
ActiveCN103097412ASsRNA viruses negative-senseOrganic active ingredientsDiseaseAntigen Binding Fragment
Provided herein are antibodies or antigen-binding fragments thereof that immunospecifically bind to the fusion (F) protein of Respiratory Syncytial Virus (RSV). Also provided are methods for of prevention, treatment and diagnosis of viral infection and / or the treatment of one more symptoms of RSV-mediated disease. Methods of generating antibodies that immunospecifically bind RSV F protein also are provided.
Owner:JANSSEN VACCINES & PREVENTION BV
Loop-mediated isothermal amplification based human respiratory syncytial virus detection kit
InactiveCN104419713AStrong specificityGood reproducibilityMicrobiological testing/measurementFermentationHuman respiratory virusLoop-mediated isothermal amplification
The invention relates to a loop-mediated isothermal amplification based human respiratory syncytial virus detection kit. The inventor finds a loop-mediated isothermal amplification primer having excellent specificity to RSV after comparison and screening; the primer is prevented from specific amplification for nucleic acids other than nucleic acids containing the RSV. The loop-mediated isothermal amplification based human RSV detection kit can be well applied to identifying the ingredients of the RSV, and has excellent reproducibility and sensitivity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Nucleic Acids Encoding Respiratory Syncytial Virus Subgroup B strain 9320
ActiveUS20090285853A1SsRNA viruses negative-senseVirus peptidesAttachment proteinRespiratory syncytial virus (RSV)
The complete polynucleotide sequence of the human respiratory syncytial virus subgroup B strain 9320 genome is provided. Proteins encoded by this polynucleotide sequence are also provided. Isolated or recombinant RSV (e.g., attenuated recombinant RSV), nucleic acids, and polypeptides, e.g., comprising mutations in the attachment protein G, are also provided, as are immunogenic compositions comprising such isolated or recombinant RSV, nucleic acids, and polypeptides. Related methods are also described.
Owner:MEDIMMUNE LLC
Anti-human respiratory syncytial virus N protein antibodies and immunochromatographic kit using the same
ActiveCN105753981AHigh potencyReduce manufacturing costImmunoglobulins against virusesMaterial analysisLinear epitopeHuman respiratory virus
The present invention relates to anti-human respiratory syncytial virus N protein antibodies and immunochromatographic kit for detection of human respiratory syncytial virus by using the same. The anti-human respiratory syncytial virus N protein antibodies separately recognize two linear epitopes consisting of No.21-34 amino acids and No.226-239 of human respiratory syncytial virus N protein; the human respiratory syncytial virus N protein has sequence number of AAB59852.1 in GenBank; amino acid sequence of sites No.21-34 and No.226-239 of the human respiratory syncytial virus N protein are respectively SKYTIQRSTGDSID and FGIAQSSTRGGSRV. The two kinds of rabbit anti-human respiratory syncytial virus N protein antibodies have the characteristics of good specificity, high purity, high titer and low preparation cost.
Owner:HUBEI UNIV OF TECH +1
Amino acid sequences directed against human respiratory syncytial virus (HRSV) and polypeptides comprising the same for the prevention and/or treatment of respiratory tract infections
ActiveUS9644022B2Low costImprove stabilityLiquid surface applicatorsPowdered material dispensingWAS PROTEINHuman respiratory virus
Amino acid sequences are provided that are directed against / and or that can specifically bind protein F of hRSV, as well as to compounds or constructs, and in particular proteins and polypeptides, that comprise or essentially consist of one or more such amino acid sequences. The amino acid sequences, polypeptides and therapeutic compounds and compositions provided by the invention show an improved stability, less immunogenicity and / or improved affinity and / or avidity for protein F of hRSV. The invention also relates to the uses of such amino acid sequences, polypeptides, compounds or constructs for prophylactic and / or therapeutic purposes.
Owner:ABLYNX NV
Vaccine candidates for human respiratory syncytial virus (RSV) having attenuated phenotypes
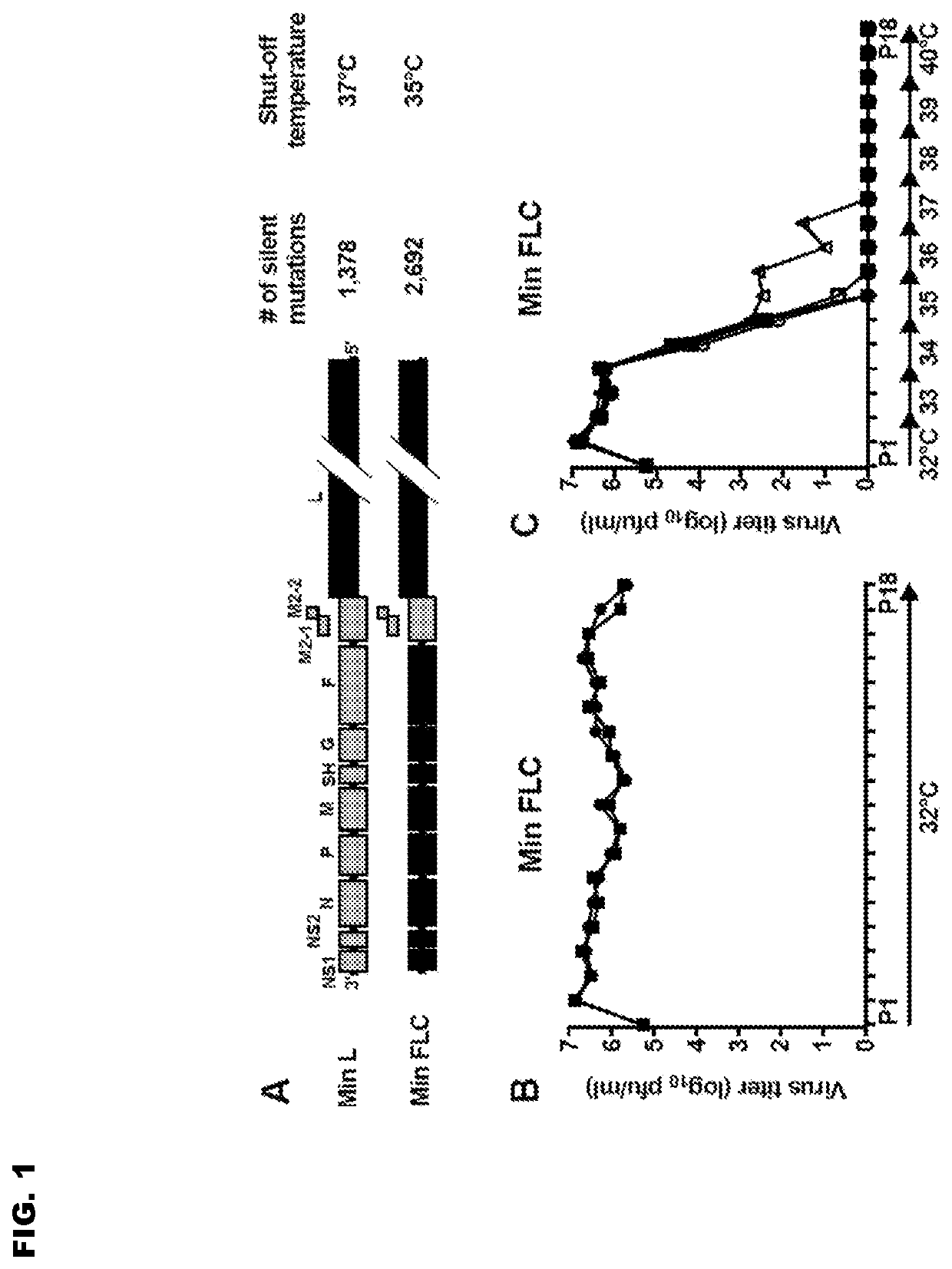
ActiveUS20190233476A1Suitable for useSsRNA viruses negative-senseVirus peptidesUltrasound attenuationWild type
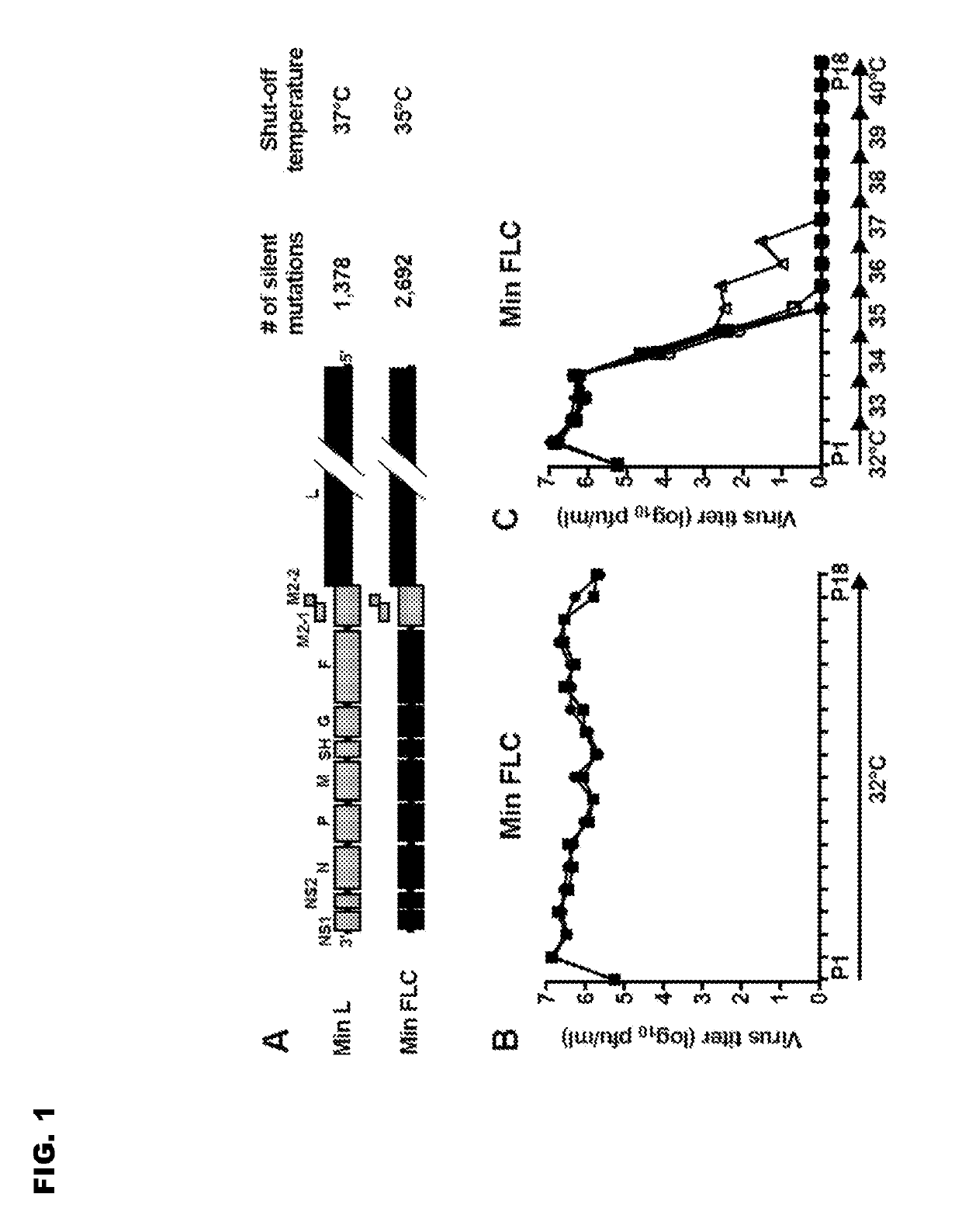
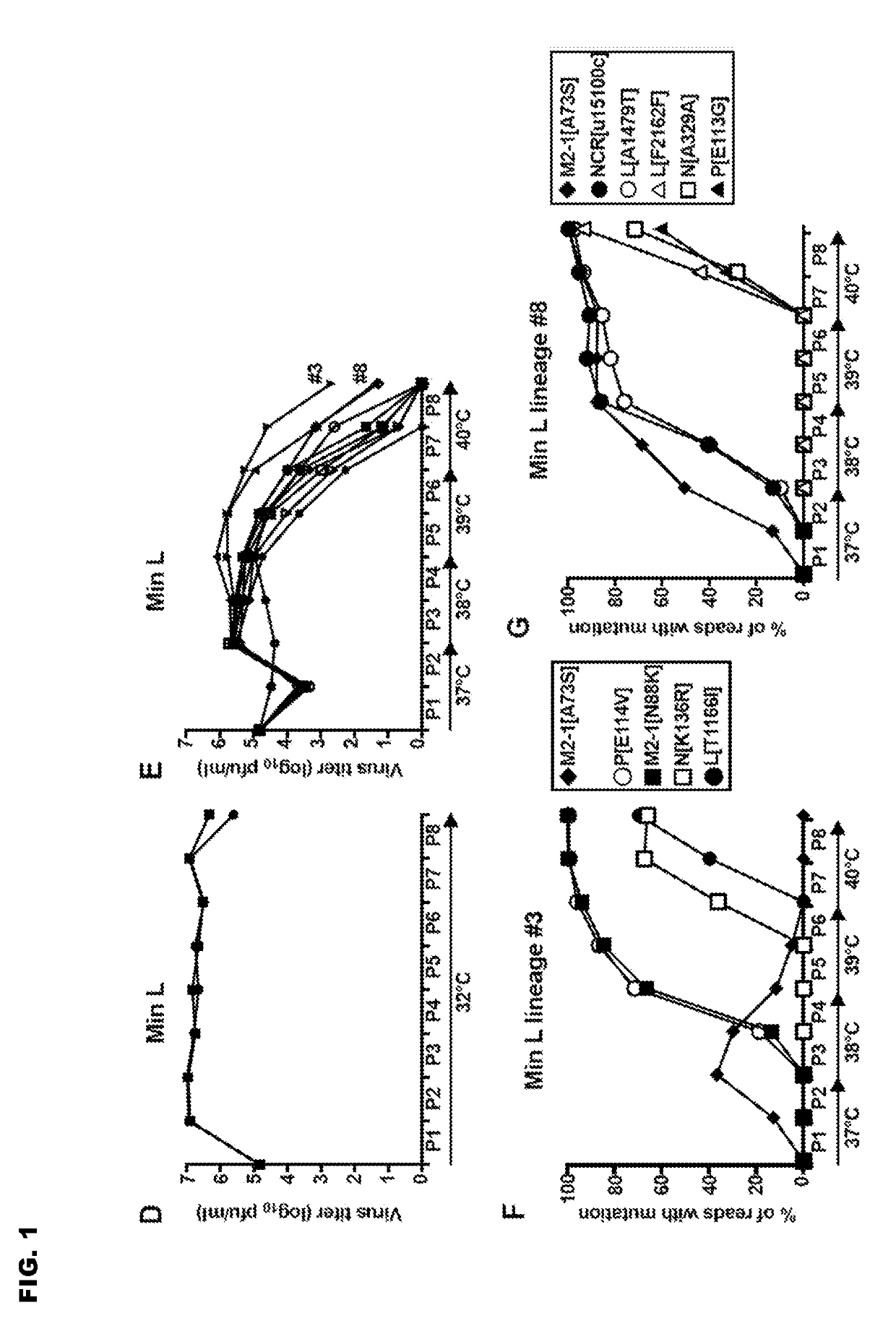
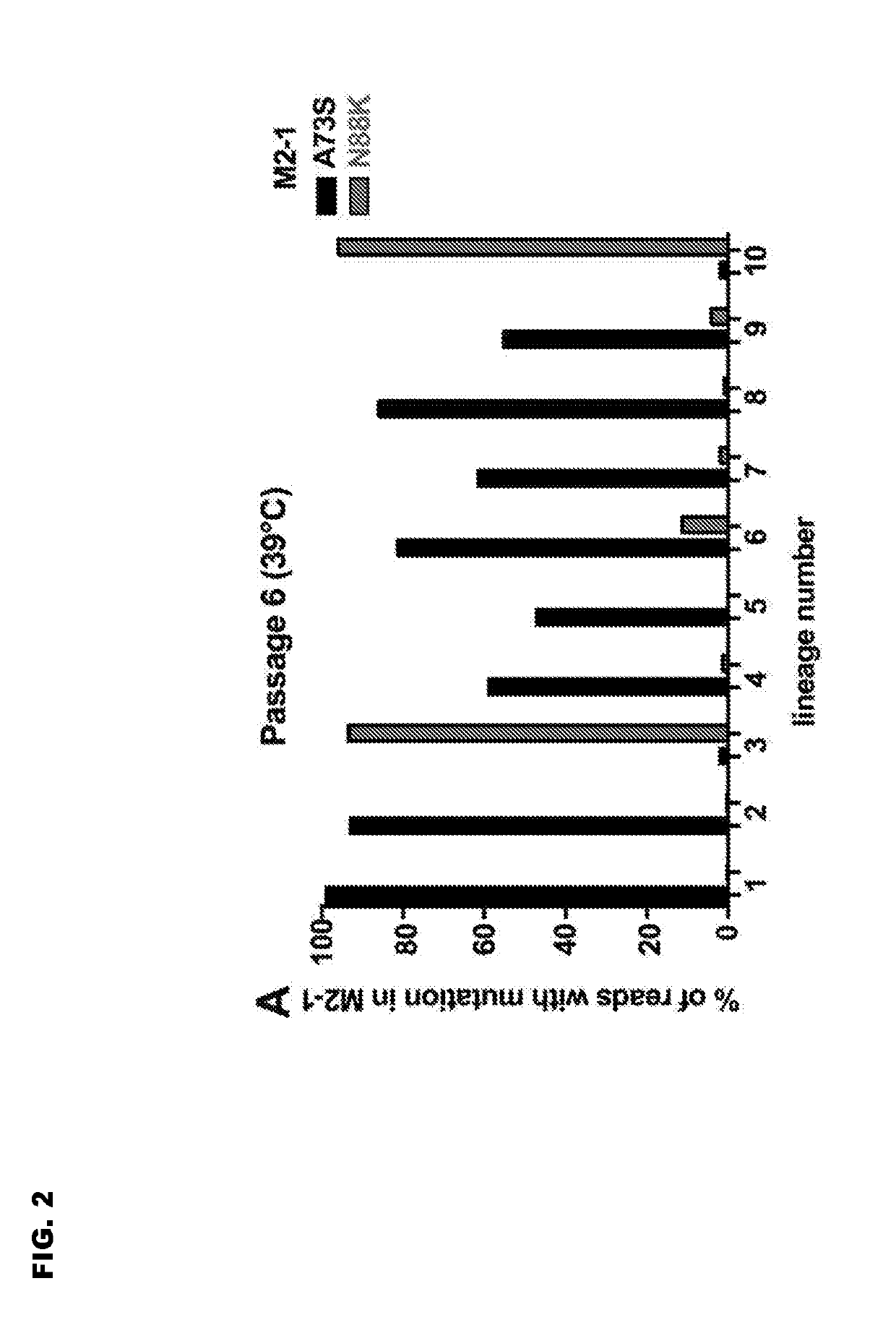
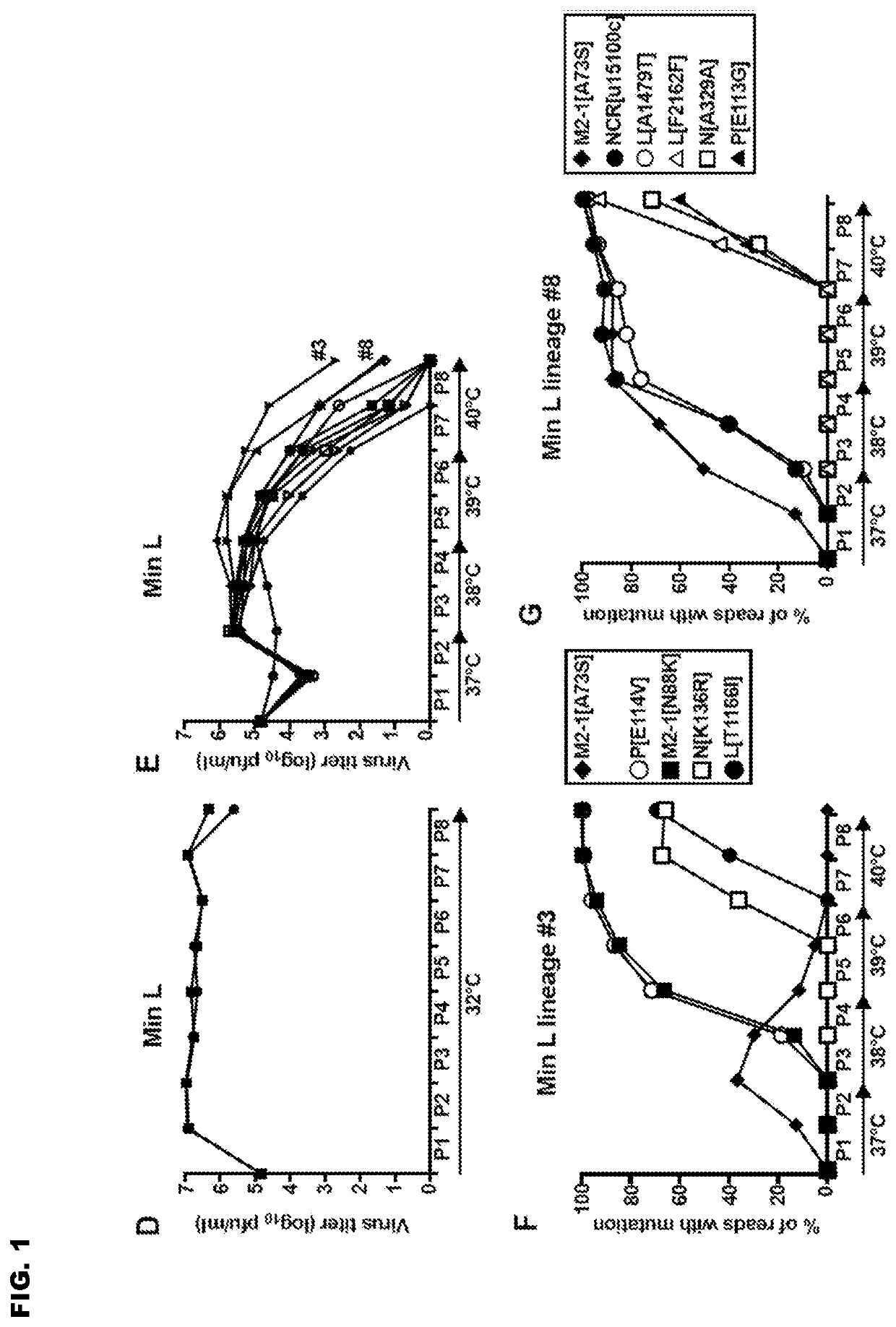
Reported herein are presumptively de-attenuating mutations that are useful, either individually or in combinations that may include other known mutations, in producing recombinant strains of human respiratory syncytial virus (RSV) exhibiting attenuation phenotypes. Also described herein is a novel RSV construct, Min_L-NPM2-1(N88K)L, which exhibits an attenuated phenotype, is stable and is as immunogenic as wild type RSV. The recombinant RSV strains described here are suitable for use as live-attenuated RSV vaccines. Exemplary vaccine candidates are described. Also provided are polynucleotide sequences capable of encoding the described viruses, as well as methods for producing and using the viruses.
Owner:CODAGENIX INC +1
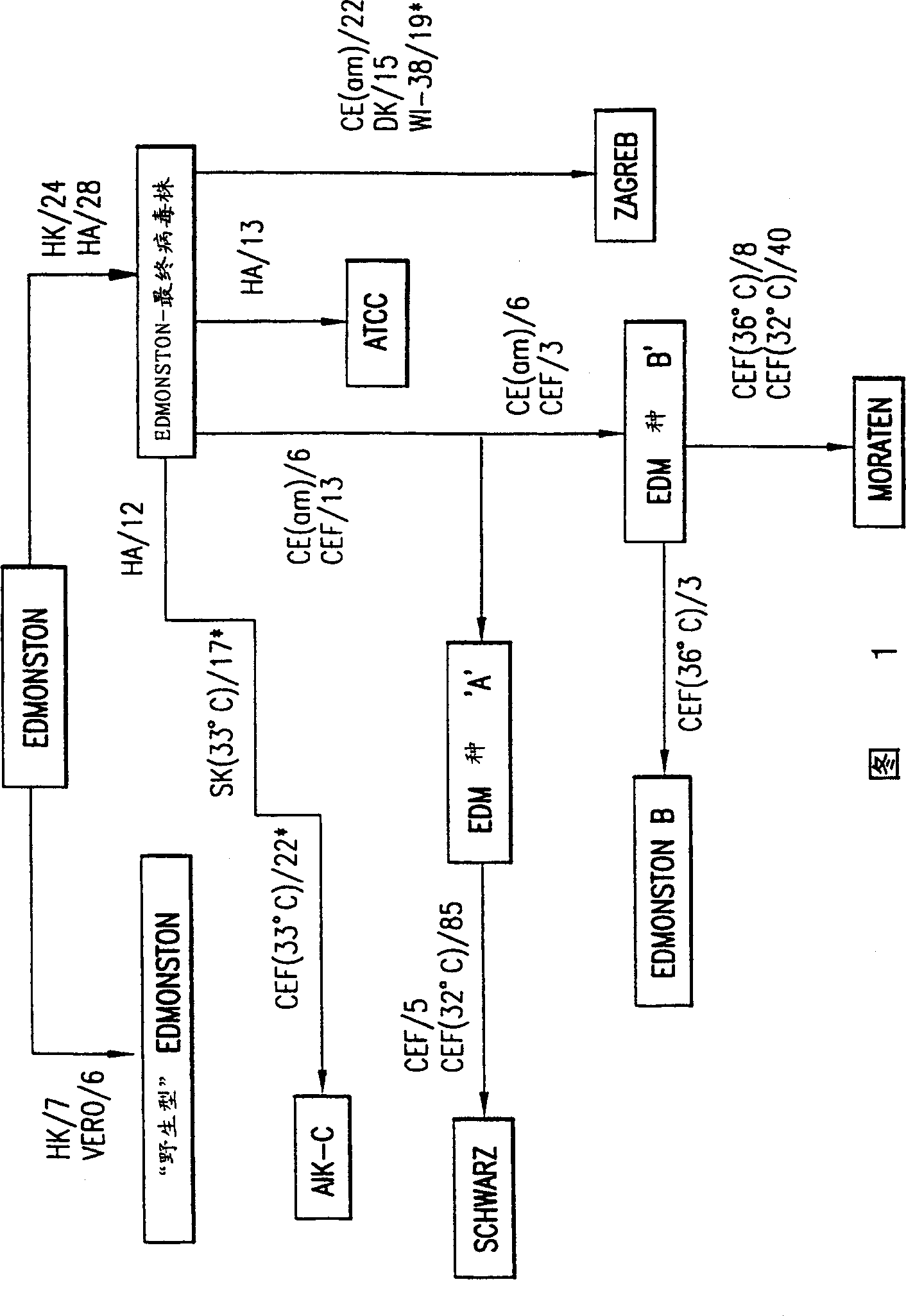
Mutations responsible for attenuation in measles virus or human respiratory syncytial virus subgroup B
InactiveCN1294628ASsRNA viruses negative-senseVirus peptidesHuman respiratory virusAnti-Measles Virus
Isolated, recombinantly-generated, attenuated measles viruses and respiratory syncytial subgroup B viruses having defined attenuating mutations are described. Vaccines are formulated comprising such viruses and a physiologically acceptable carrier. The vaccines are used for immunizing an individual to induce protection against measles virus or respiratory syncytial subgroup B virus.
Owner:WYETH HOLDINGS CORP
Mhc class II haplotype specific immunodominancy of peptides derived from rsv fusion (f) and attachement (g) proteins
InactiveUS20050249744A1SsRNA viruses negative-senseViral antigen ingredientsVaccinationAttachment protein
The present invention relates immunodominant peptides derived from human respiratory syncytial virus (H-RSV) that may be used in ex vivo diagnosis of immune responses to H-RSV The immunodominant peptides are derived from the H-RSV Fusion (F) and Attachment (G) proteins and are capable of inducing an antigen specific CD4+ T cell response ex vivo in a MHC class 11 haplotype restricted manner. The immunodominant H-RSV-derived peptides may further be used in methods for vaccination against H-RSV, preferably in a MHC class 11 haplotype specific manner.
Owner:DE STAAT DER NEDERLANDEN VERT DOOR DE MINIST VAN VWS
Human respiratory syncytial virus (HRSV) virus-like particles (VLPS) based vaccine
ActiveCN108348593ASsRNA viruses negative-senseViral antigen ingredientsVirus-like particleHuman respiratory virus
Described herein are virus-like particles (VLPs) that display on their surfaces antigenic paramyxovirus (e.g., RSV and / or MPV) proteins. Also described are methods of making and using these VLPs.
Owner:TECHNOVAX
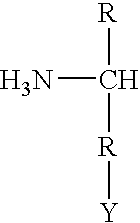
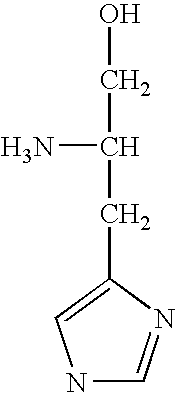
Cloning, overexpression and therapeutic use of bioactive histidine ammonia lyase
Owner:UNIVERSITY OF SOUTH CAROLINA
Anti-human respiratory syncytial virus n protein antibody and immunochromatography kit using the antibody
ActiveCN105753981BHigh potencyReduce manufacturing costImmunoglobulins against virusesMaterial analysisLinear epitopeHuman respiratory virus
The present invention relates to anti-human respiratory syncytial virus N protein antibodies and immunochromatographic kit for detection of human respiratory syncytial virus by using the same. The anti-human respiratory syncytial virus N protein antibodies separately recognize two linear epitopes consisting of No.21-34 amino acids and No.226-239 of human respiratory syncytial virus N protein; the human respiratory syncytial virus N protein has sequence number of AAB59852.1 in GenBank; amino acid sequence of sites No.21-34 and No.226-239 of the human respiratory syncytial virus N protein are respectively SKYTIQRSTGDSID and FGIAQSSTRGGSRV. The two kinds of rabbit anti-human respiratory syncytial virus N protein antibodies have the characteristics of good specificity, high purity, high titer and low preparation cost.
Owner:HUBEI UNIV OF TECH +1
Live attenuated recombinant hmpv with mutations in pdz motifs of m2-2 protein, vaccine containing and use thereof
ActiveUS20190192592A1SsRNA viruses negative-senseViral antigen ingredientsPneumoviridaeImmunogenicity
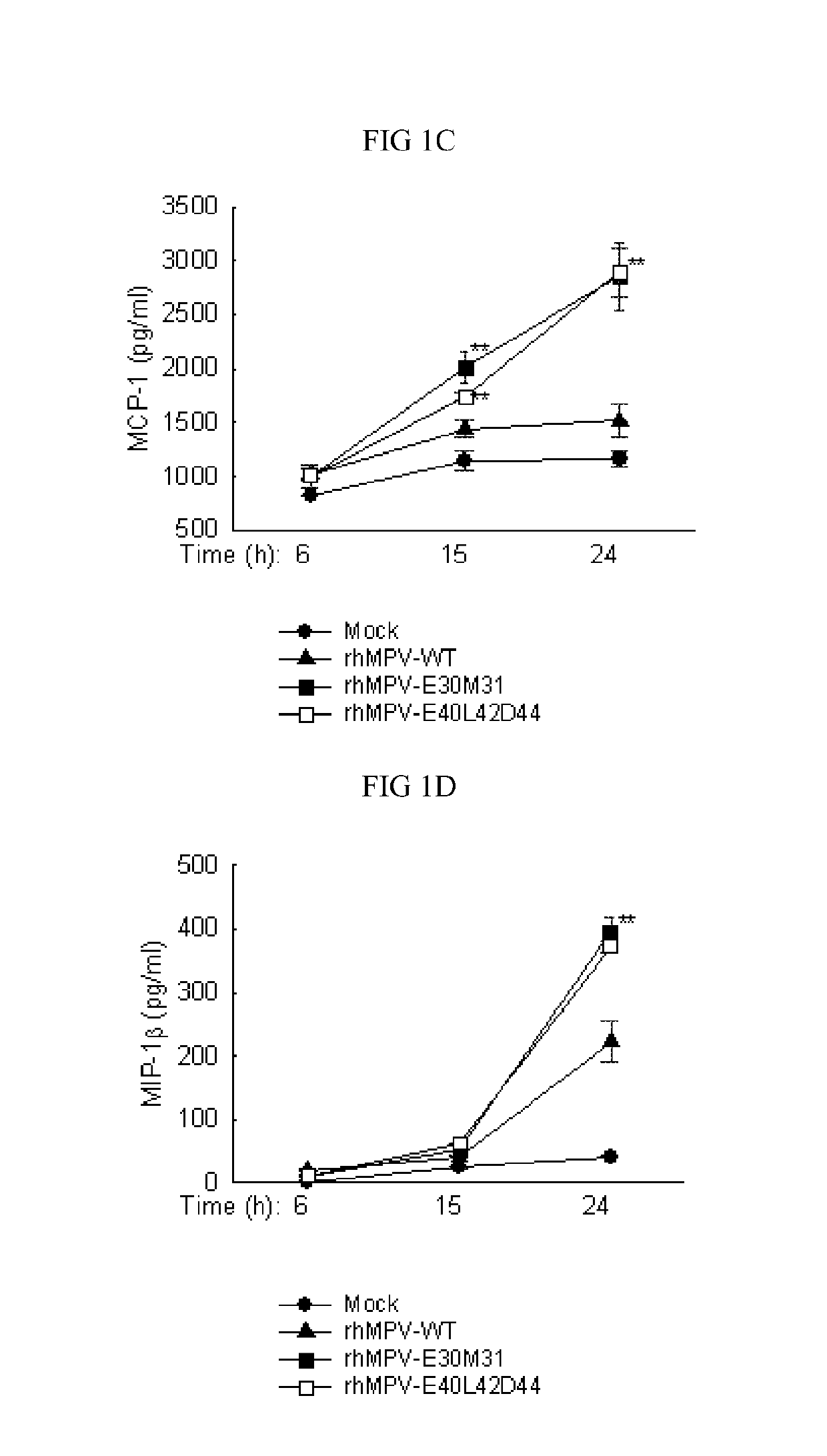
The present application generally relates to the development of live attenuated Pneumoviridae strains suitable for use as a vaccine. Particularly, human metapneumovirus (hMPV) ΔM2-2 strains (rhMPV-E30M31 and rhMPV-E40L42D44) containing point mutations in a PDZ motif of M2-2, which results in a strain that is both attenuated and immunogenic and, notably, maintains the function of F and G proteins. These live attenuated hMPV strains should be suitable for use in a vaccine capable of providing protection against respiratory infection elicited by hMPV. Additionally, human respiratory syncytial virus (hRSV) strains containing point mutations in a PDZ motif of M2-2 should also be suitable for use as a vaccine capable of providing protection against respiratory infection elicited by hRSV. These Pneumoviridae strains should be useful in vaccines for use in humans and animals, e.g., companion animals and livestock, in treating or providing immunoprotection against respiratory infections.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
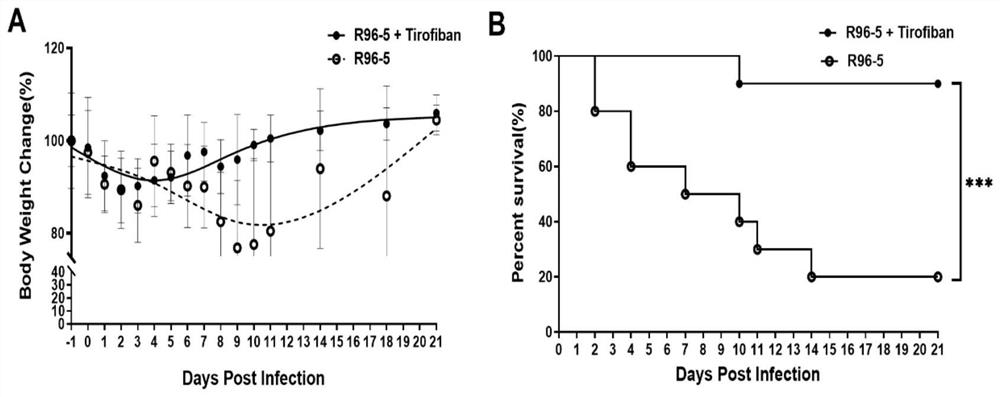
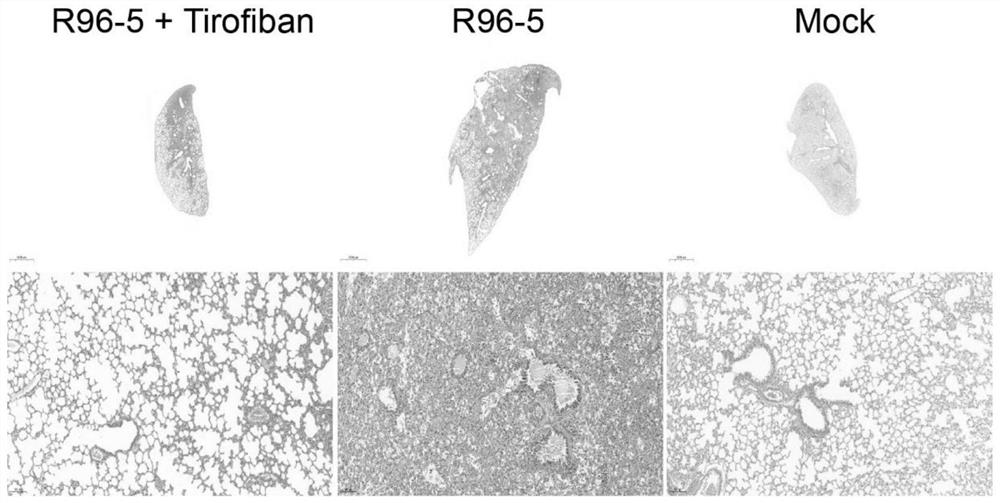
Application of tirofiban in preparation of medicine for treating human respiratory syncytial virus infection
ActiveCN114796215AGood treatment effectGood in vivo anti-human respiratory syncytial virus effectOrganic active ingredientsAntipyreticRespiratory syncytial virus (RSV)Antithrombotic
The invention discloses application of tirofiban in preparation of a medicine for treating human respiratory syncytial virus infection, and relates to the technical field of virus infection resistance. The invention provides favorable drug support for clinically treating human respiratory syncytial virus infection, enriches the drug types of clinical drugs for intervening human respiratory syncytial virus infection, and broadens the antiviral types of tirofiban. In addition, tirofiban as a clinical medicine has good safety and effectiveness, so that the inventor has a good safe medication basis when tirofiban is used for treating human respiratory syncytial virus infection. The invention lays a foundation for further developing medicines with antithrombotic ability into anti-infective medicines. The invention fills the blank of medicines for treating human respiratory syncytial virus, especially pediatric medicines, and has great social value.
Owner:GUIZHOU MEDICAL UNIV
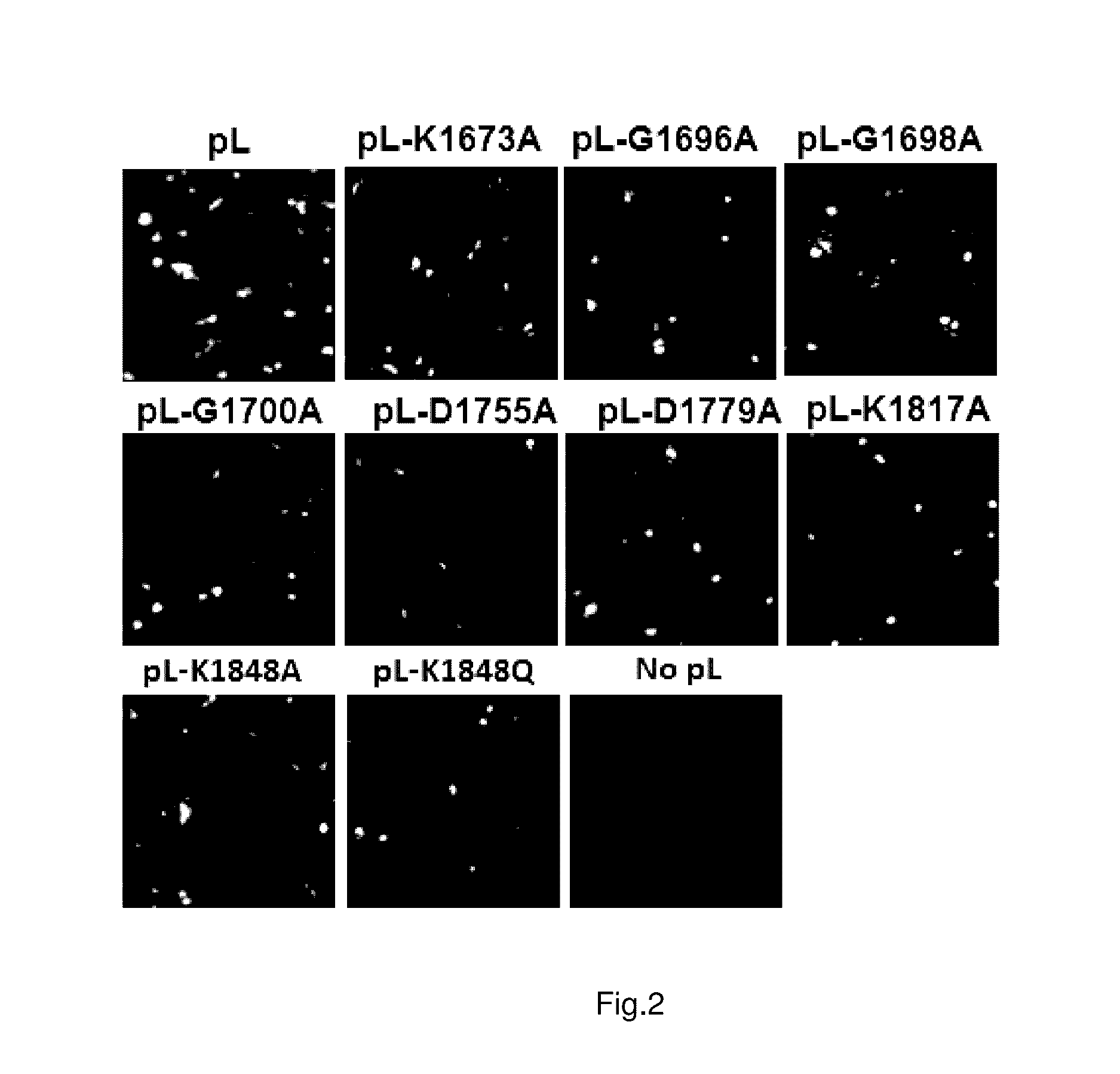
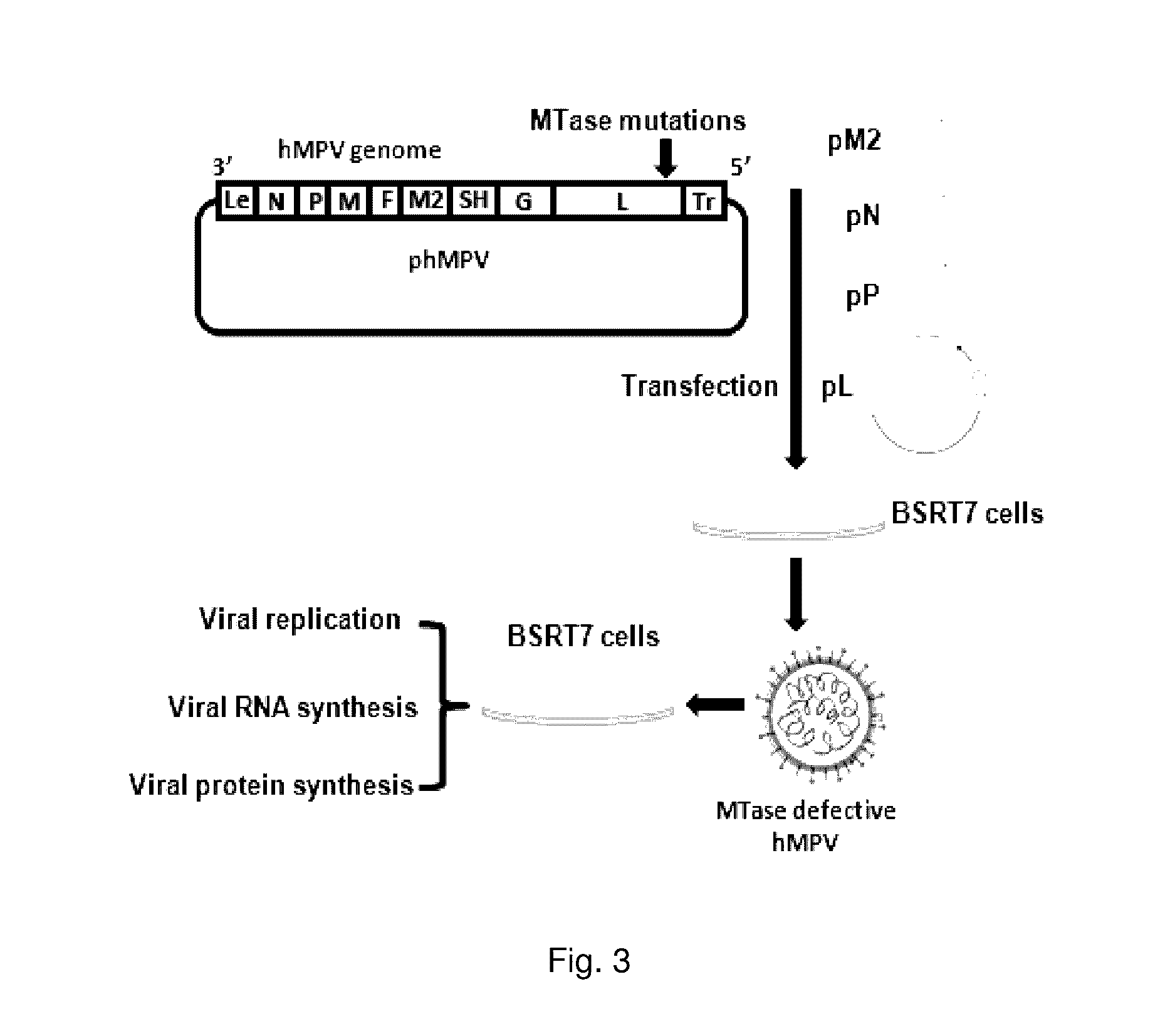
Paramyxovirus immunogens and related materials and methods
ActiveUS9572876B2SsRNA viruses negative-sensePeptide/protein ingredientsHuman Parainfluenza VirusHuman respiratory virus
The present invention includes methyltransferase (MTase)-defective recombinant viruses as live vaccine candidates for human metapneumovirus (hMPV), human respiratory syncytial virus (hRSV), and human parainfluenza virus type 3 (PIV3). Here the inventors provide the technical description for generating MTase-defective paramyxoviruses useful as immunogens, as well as related materials and methods.
Owner:OHIO STATE INNOVATION FOUND
PCR (Polymerase Chain Reaction) primer group and kit for jointly detecting various respiratory viruses
PendingCN114262759AEasy to operateAccurate detectionMicrobiological testing/measurementMicroorganism based processesEnterovirusMultiplex
The invention relates to the technical field of biology, in particular to a PCR (Polymerase Chain Reaction) primer group and a kit for jointly detecting various respiratory viruses. The PCR primer group comprises an influenza A virus detection primer pair, an influenza A virus H1N1 detection primer pair, an influenza A virus H3N2 detection primer pair, an influenza B virus detection primer pair, a human respiratory syncytial virus detection primer pair, a human metapneumovirus detection primer pair and a rhinovirus / enterovirus detection primer pair. The kit comprises a multiplex RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reactant, and the multiplex RT-PCR reactant comprises the PCR primer group. The primer group and the kit disclosed by the invention can be used for jointly detecting and identifying seven respiratory viruses, the method is simple to operate, accurate in detection, high in sensitivity and high in specificity, the detection time is remarkably shortened, and a detection result can be obtained within 2-3 hours.
Owner:重庆巴斯德生物医药科技有限公司
Monoclonal antibodies specifically for the antigen P of the human respiratory syncytial virus, produced and secreted by the cells hybridomas, useful for detection and diagnostic of the infection caused by RSV
ActiveUS10858419B2High detection sensitivityEfficient captureSsRNA viruses negative-senseSugar derivativesHeavy chainHuman respiratory virus
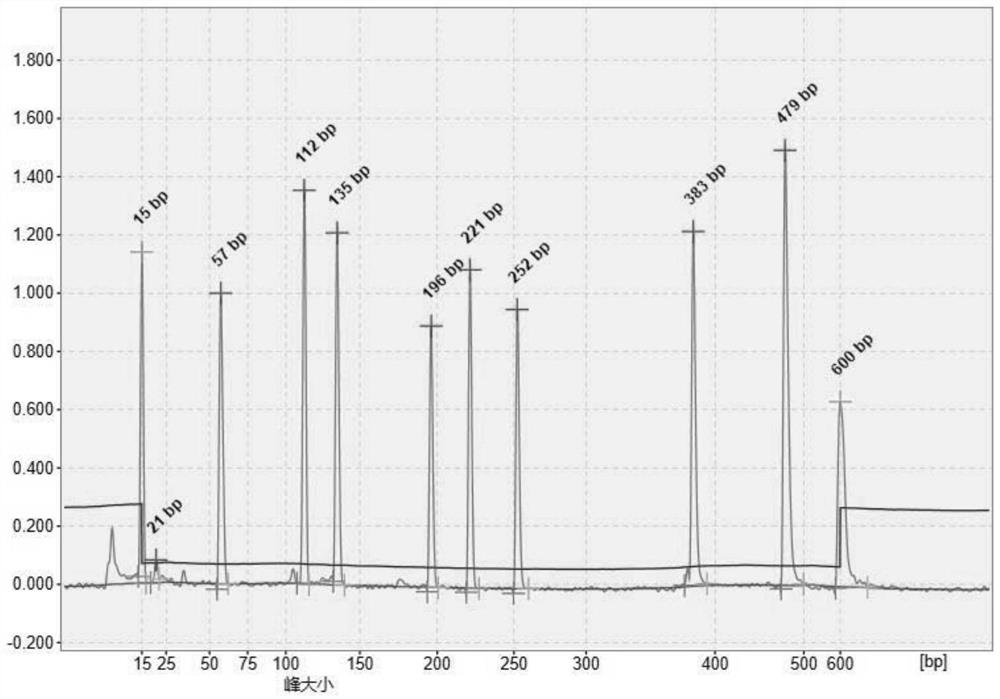
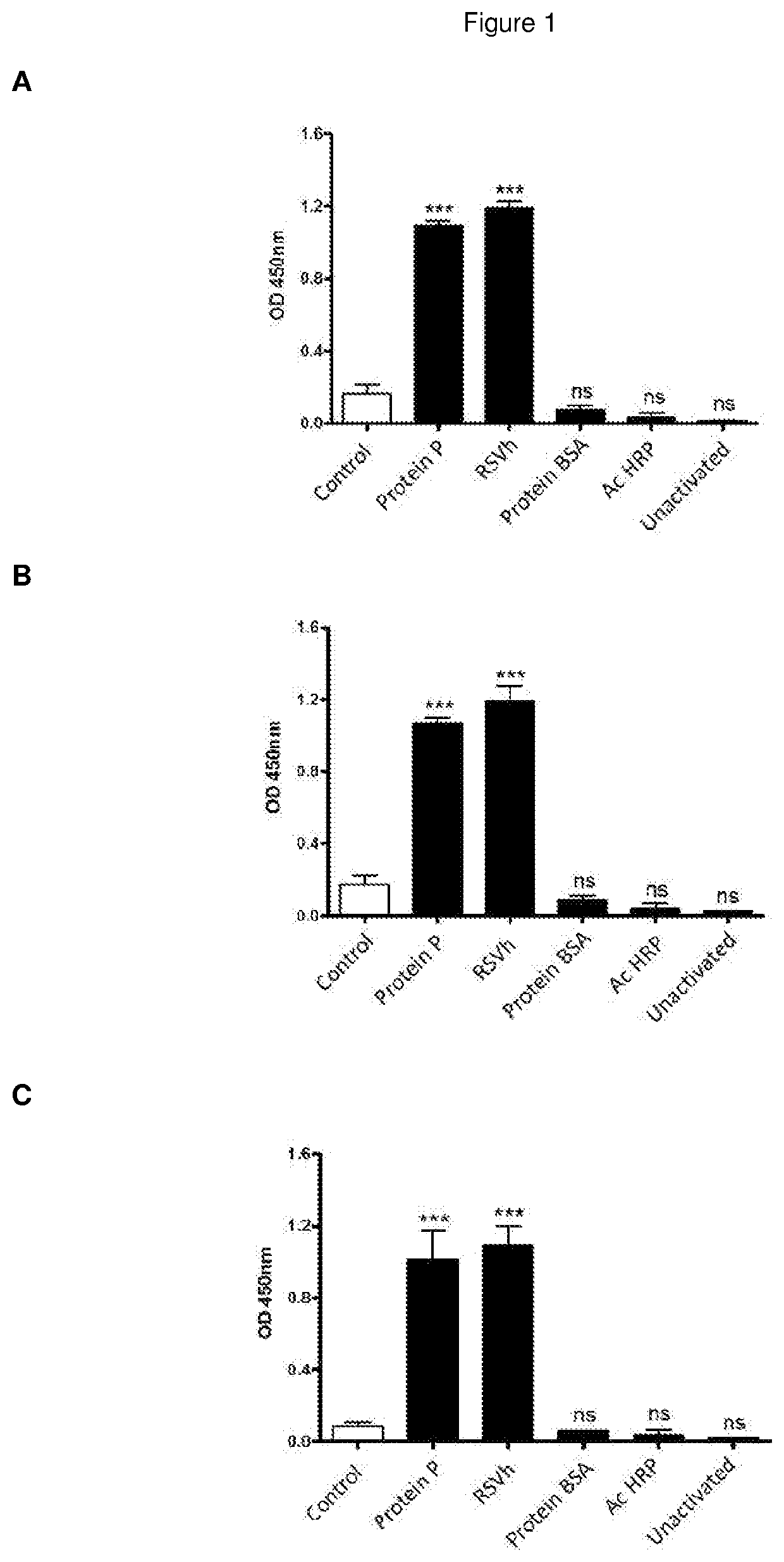
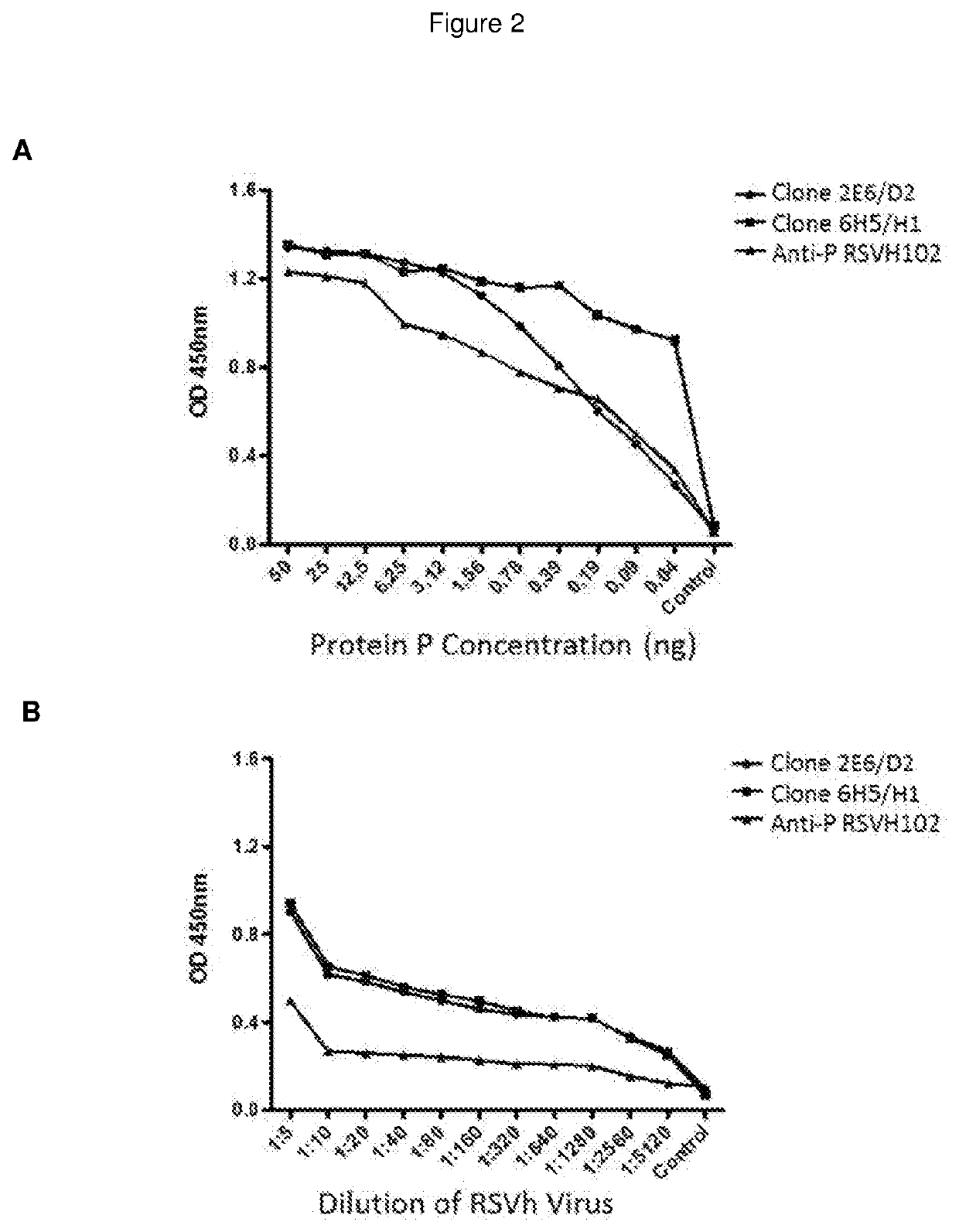
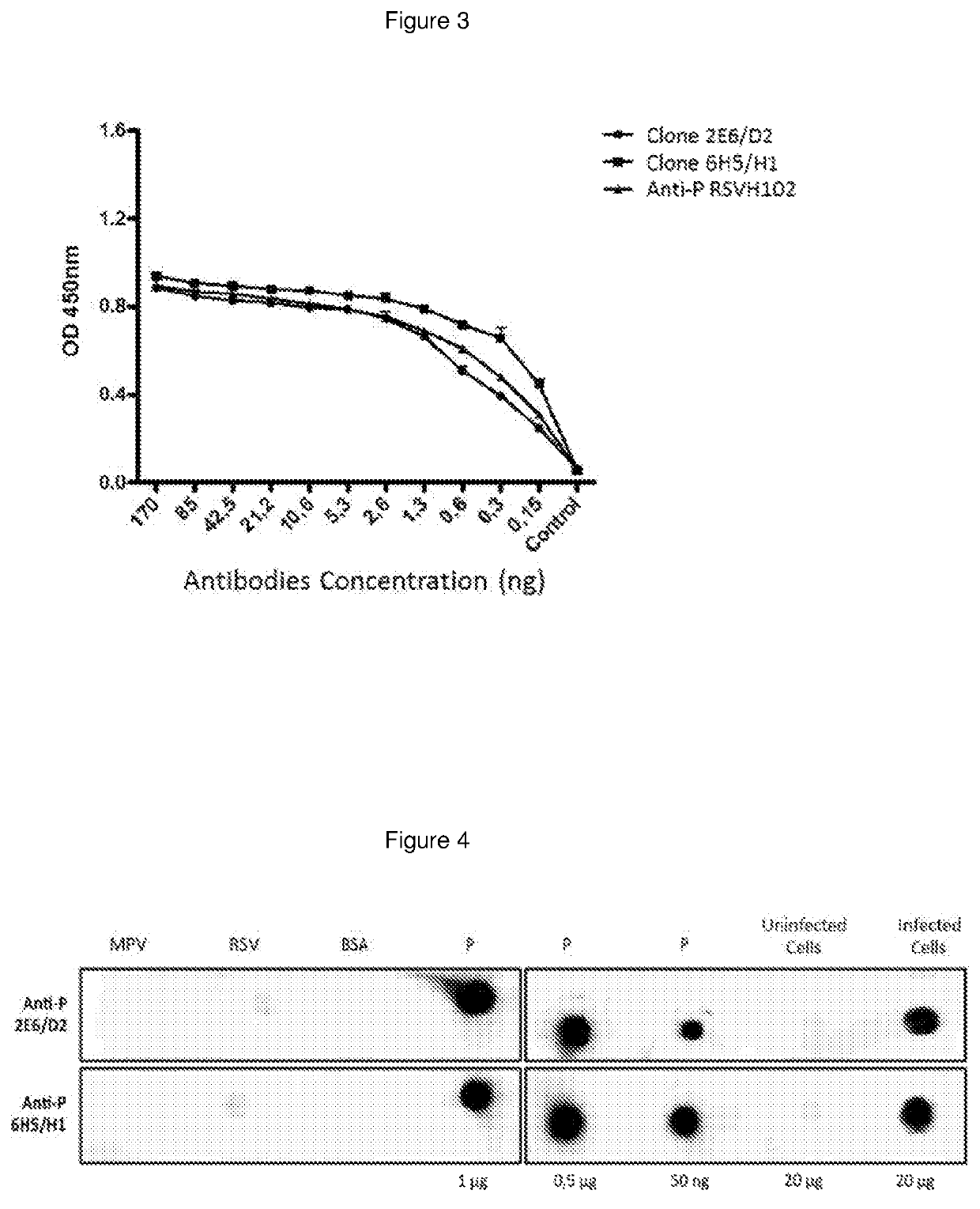
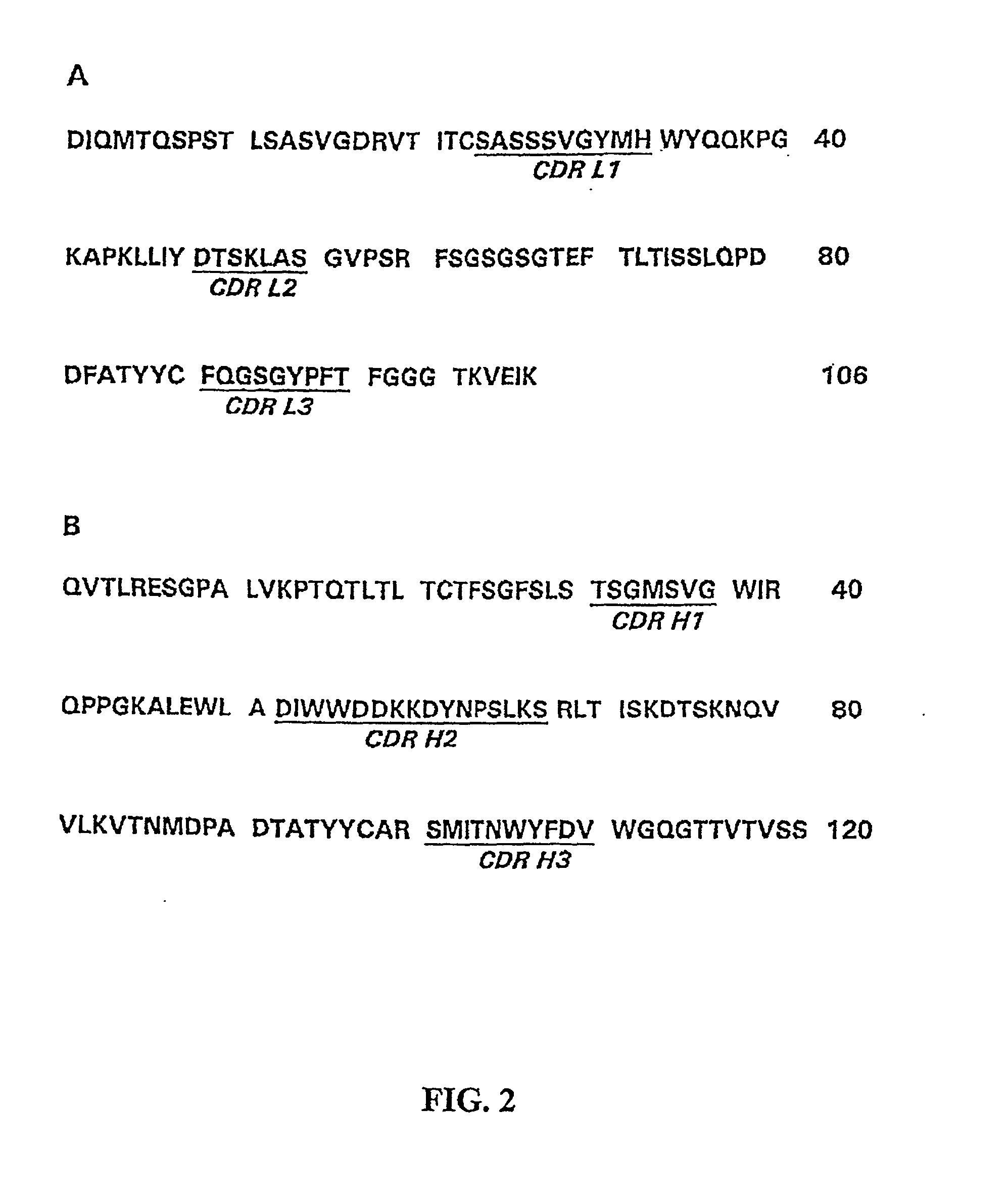
The present invention refers to monoclonal antibodies or fragments thereof which are binding to the protein P of the human Respiratory Syncytial Virus (RSV) which comprise a variable region of the heavy chain which has a sequence with at least a 90%, 95% or 99% of identity with the SEQ ID No: 1 or SEQ ID 5 or a variable region of the light chain which has a sequence with at least a 90%, 95% or 99% of identity with the SEQ ID No:2 or SEQ ID No: 6. The invention provides also diagnostic methods ex vivo or in vitro for detection of the viral antigen P of RSV, in which are used the monoclonal antibodies produced and secreted by the hybridomas 2E6 / D2 and 6H5 / H1. The invention can be used in detection for RSV kits, comprising the antibodies produced by the mentioned hybridomas.
Owner:PONTIFISIA UNIVERSIDAD KATOLIKA DE CHILE
Respiratory syncytial virus vaccine and preparation method thereof
InactiveCN103182079ALess side effectsFast immune responseAntiviralsAntibody medical ingredientsForeign proteinAdjuvant
The invention discloses a respiratory syncytial virus vaccine and a preparation method thereof. The vaccine contains membrane proteins obtained by cracking of whole virus particles of respiratory syncytial virus, wherein the respiratory syncytial virus vaccine contains 100-200 Mug of viral proteins per dose and 0.5 mg / dose of aluminum phosphate adjuvant. Compared with the existing viral purified stock solution, the respiratory syncytial virus vaccine for human, disclosed by the invention, has a greatly-reduced content of foreign proteins, is better in immunogenicity and potency, is safer in use, and is more suitable for large-scale production.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Pharmaceutical composition for treating and preventing respiratory tract pathogen infection and application
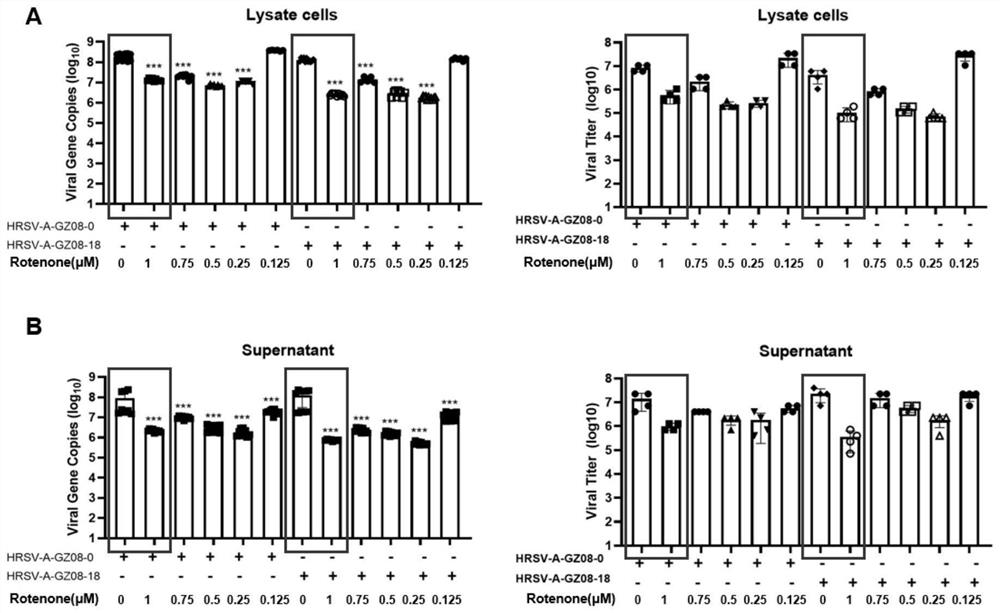
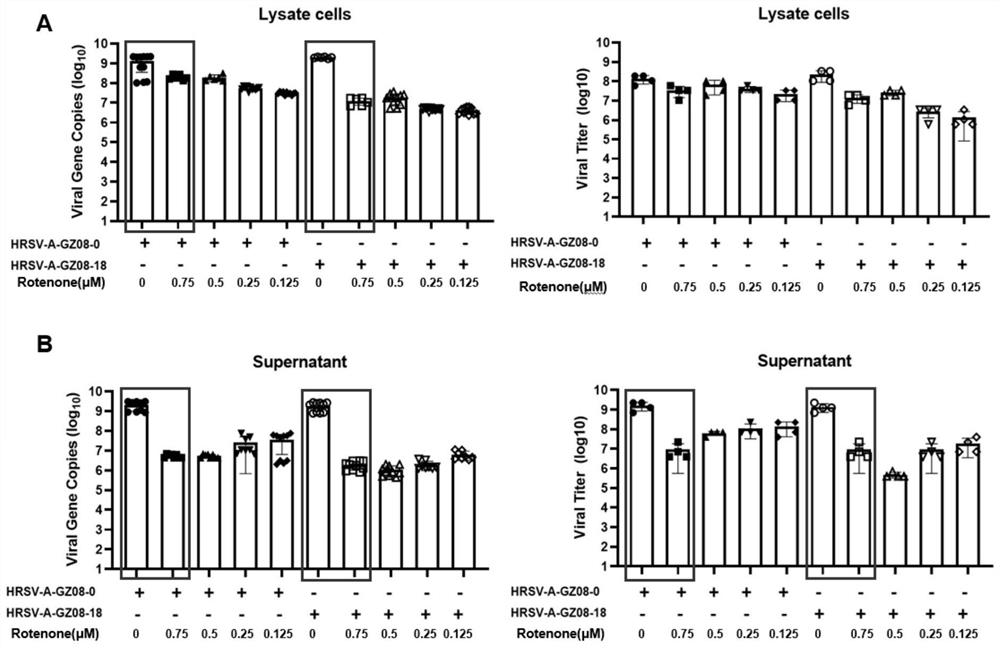
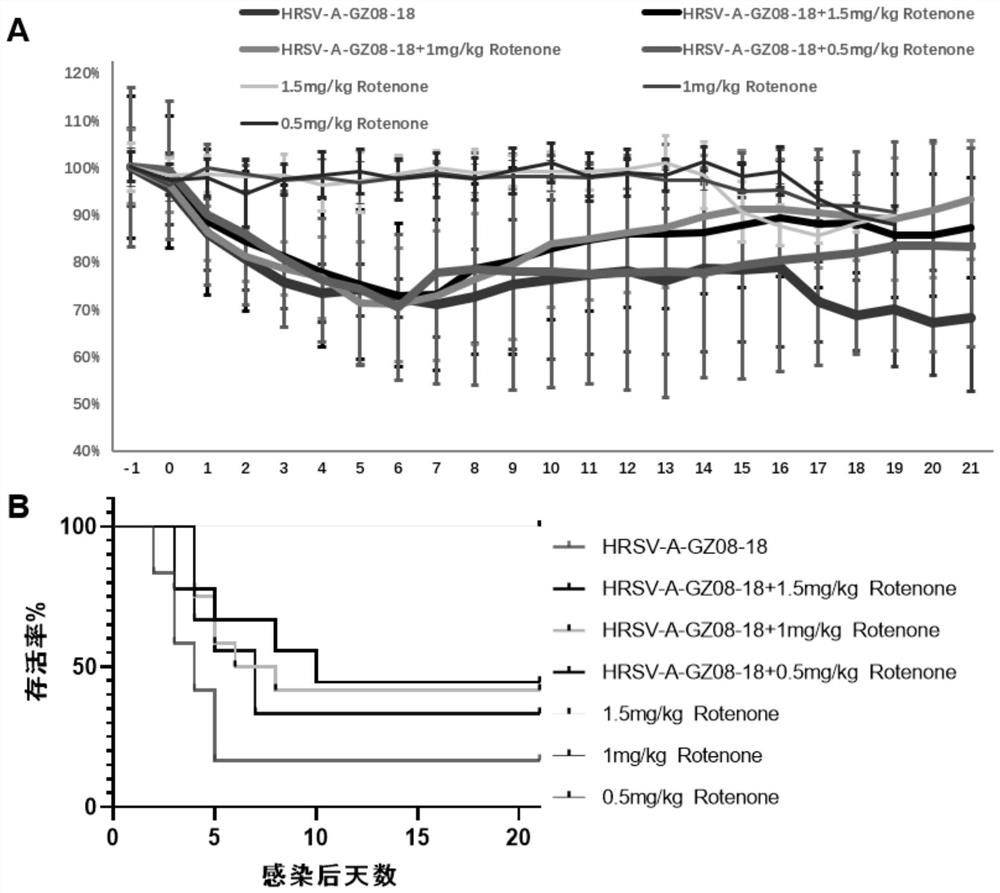
PendingCN114796189AReduced viral titerImprove survival rateAntibacterial agentsOrganic active ingredientsDiseaseRespiratory tract disease
The invention discloses a pharmaceutical composition for treating and preventing respiratory tract pathogen infection and application, and relates to the technical field of respiratory tract diseases. The inventor finds new application of rotenone. In-vitro and in-vivo experiments prove that the rotenone can reduce the virus titer of human laryngocarcinoma epithelial cells infected by human respiratory syncytial virus (HRSV), improve the survival rate of animals and promote the weight of the infected animals to be gradually increased from low level. Tests prove that the rotenone has a good effect of resisting respiratory pathogens (especially respiratory infection viruses), has a good application prospect when being used for treating and / or preventing respiratory pathogen infection, and can be developed into corresponding drugs for treating or preventing respiratory pathogen infection. And good news is brought to pneumonia patients such as viral pneumonia.
Owner:GUIZHOU MEDICAL UNIV
Vaccine candidates for human respiratory syncytial virus (RSV) having attenuated phenotypes
ActiveUS10808012B2SsRNA viruses negative-senseViral antigen ingredientsWild typeHuman respiratory virus
Reported herein are presumptively de-attenuating mutations that are useful, either individually or in combinations that may include other known mutations, in producing recombinant strains of human respiratory syncytial virus (RSV) exhibiting attenuation phenotypes. Also described herein is a novel RSV construct, Min_L-NPM2-1(N88K)L, which exhibits an attenuated phenotype, is stable and is as immunogenic as wild type RSV. The recombinant RSV strains described here are suitable for use as live-attenuated RSV vaccines. Exemplary vaccine candidates are described. Also provided are polynucleotide sequences capable of encoding the described viruses, as well as methods for producing and using the viruses.
Owner:CODAGENIX INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65ebc2a7-5baa-4fc2-ae18-dcd04adfaafa/US08486938-20130716-C00001.png)
![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65ebc2a7-5baa-4fc2-ae18-dcd04adfaafa/US08486938-20130716-C00002.png)
![Pyrazolo[1,5-a]pyrimidines for antiviral treatment Pyrazolo[1,5-a]pyrimidines for antiviral treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/65ebc2a7-5baa-4fc2-ae18-dcd04adfaafa/US08486938-20130716-C00003.png)