Application of tirofiban in preparation of medicine for treating human respiratory syncytial virus infection
A technology of tirofiban and syncytial virus, which is applied in the field of anti-viral infection, can solve problems such as the lack of vaccines on the market, and achieve the effects of good general condition, normal feed intake, and active spirit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
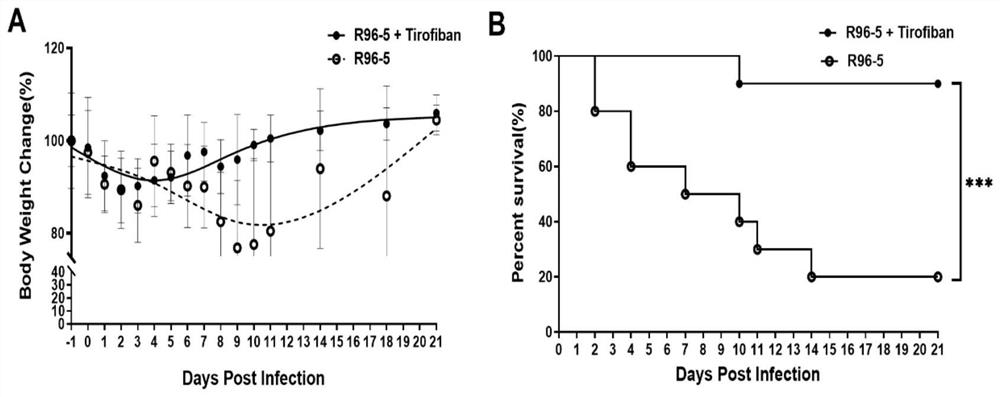
[0039] In vivo antiviral experiment: Mice were challenged 3 times with HRSV high-virulence strain R96-5 preserved in the Laboratory of Pathogen Biology, School of Basic Medicine, Guizhou Medical University, with a dose of 1 x 10 per challenge. 10 TCID 50 , at an interval of 12 hours, starting from the 1st day of the challenge, tirofiban was injected into the tail vein of mice at 50 μg / kg every day after the challenge to fight the virus infection, and injected continuously for 3 days; at the same time, a virus control group was set up. -5 mice were challenged 3 times with a dose of 1x 10 per challenge 10 TCID 50 , at an interval of 12 hours, starting from the first day of challenge, after daily challenge, the mice were injected with normal saline through the tail vein, and injected continuously for 3 days. The observation end point was 21 d after infection (the last challenge day was 0 d after infection).
[0040] During the observation period of 21 days after infection, we ...
Embodiment 2
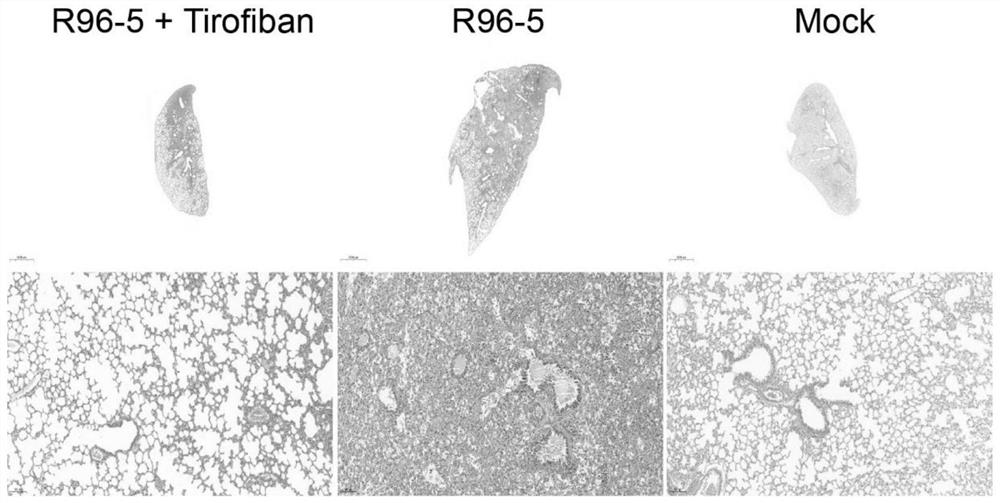
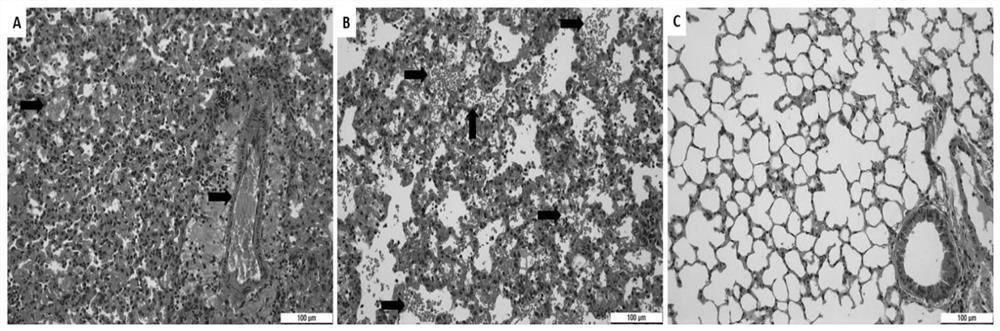
[0044] In the in vivo antiviral experiment of Example 1, mouse lungs were collected at different time points for pathological sections and H&E staining.
[0045] Mouse lung pathological sections, H&E staining results refer to figure 2 The results indicated that the local lung tissue of the tirofiban treatment group showed signs of mild to moderate viral pneumonia, and inflammatory cell infiltration was seen around small pulmonary blood vessels and bronchi, but most of the alveolar structures were intact, and no red blood cell leakage and thrombosis were found. . However, the HRSV infection group showed signs of severe viral pneumonia. A large number of inflammatory cells were infiltrated around small pulmonary blood vessels and bronchi, most of the alveolar structure was destroyed, and a large number of red blood cells leaked from small blood vessels to the alveolar space and pulmonary interstitium. Extensive thrombosis. figure 2 Middle Tirofiban: tirofiban; R96-5: HRSV hi...
Embodiment 3
[0050] In the in vivo antiviral experiment in Example 1, mouse lungs were collected at different time points for immunofluorescence staining experiments.
[0051] The expression level of HRSV F protein indicates the degree of virus infection, while the CD42b protein is a specific membrane protein of megakaryocytes and platelets. Figure 4 middle B and Figure 4 The results in middle G indicated that the degree of virus infection in the tirofiban treatment group was lower than that in the HRSV high strain R96-5 challenge group. Figure 4 Medium C and Figure 4 The results in middle H indicated that the number of virus platelets in the tirofiban treatment group was lower than that in the R96-5 challenge group, and the platelets of the mice in the HRSV high virus strain challenge group were distributed in alveolar tissue. image 3 The results in middle B indicate that the pulmonary vascular endothelial cells of the mice in the HRSV high strain challenge group were damaged, red ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com