Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
125 results about "AdverseEvent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
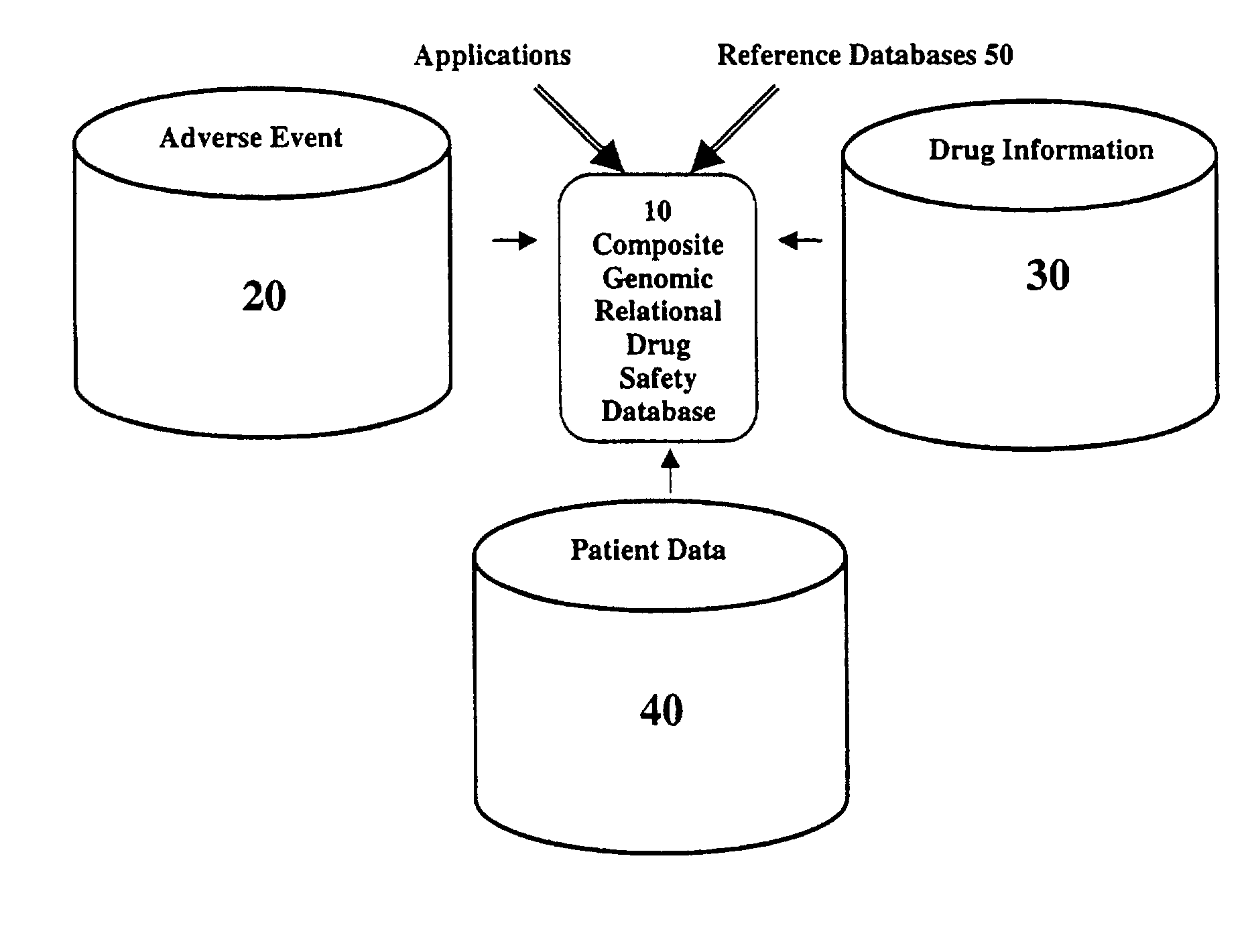
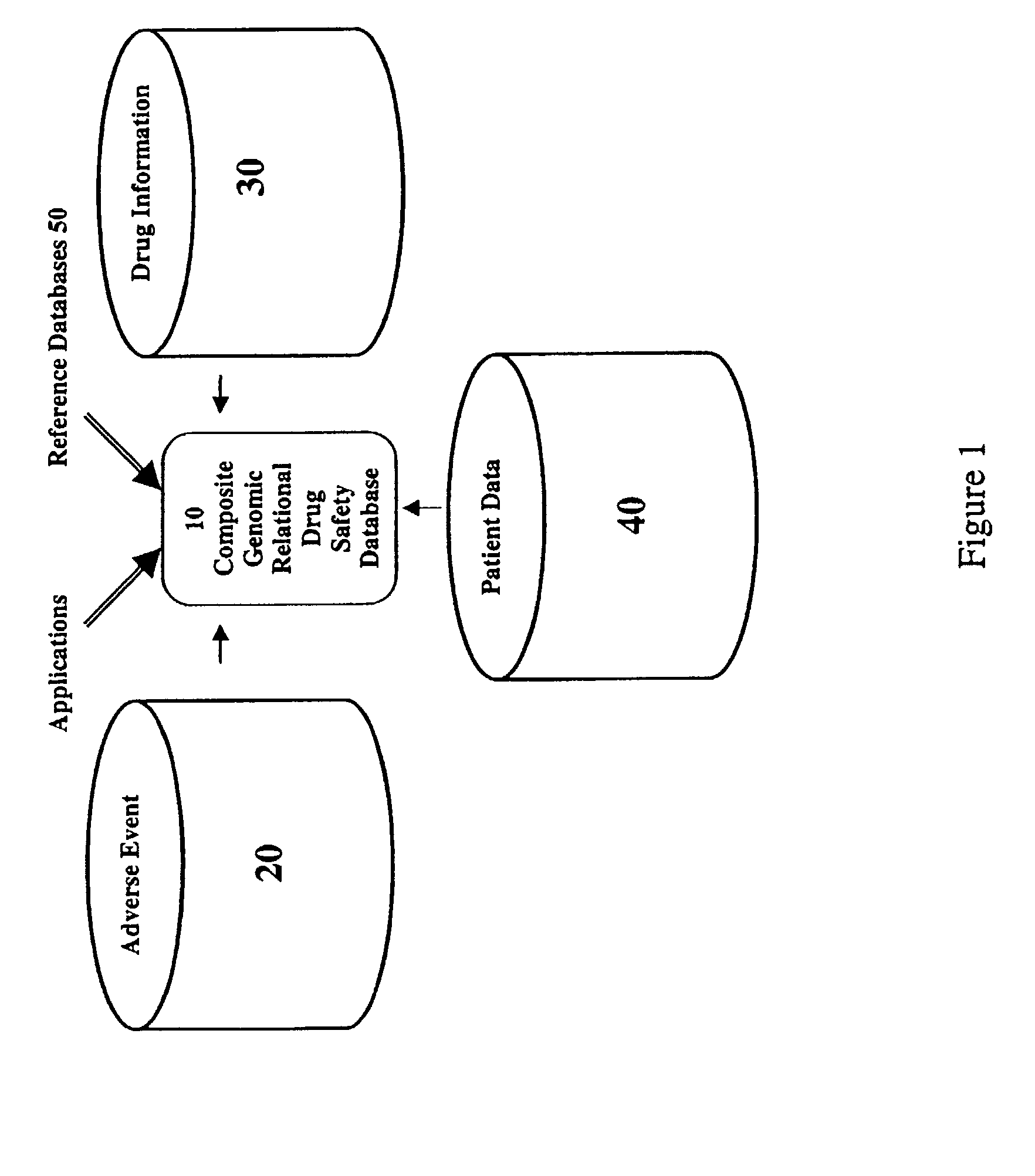
Method and system for the analysis and association of patient-specific and population-based genomic data with drug safety adverse event data
ActiveUS7461006B2Risk of adverse drug reactionIncreased and decreased chanceBiostatisticsComputer-assisted medical data acquisitionPatient characteristicsGenomic DNA
A method for assessing and analyzing one or more drugs, adverse effects and associated risks, and patient characteristics resulting from the use of at least drug of interest is disclosed. The method comprises the steps of selecting one or more cases for analysis, said cases describing the behavior between at least one drug of interest and a patient genotype; profiling statistically derived values from multiple cases related to the safety of the at least one drug, wherein at least one filter is employed for deriving said values; at least one data mining engine; and an output device for displaying the analytic results from the data mining engine. A system for performing the method is likewise disclosed.
Owner:DRUGLOGIC
Prophylaxis and treatment of enterocolitis associated with Anti-ctla-4 antibody therapy
InactiveUS20070243184A1Reduce inflammationReduce morbidityOrganic active ingredientsGenetic material ingredientsEnterocolitisImmunotherapy
The present invention provides methods for reducing the incidence of adverse events related to immunotherapy. More specifically, the present invention provides methods for reducing the incidence of enterocolitis associated with anti-CTLA-4 antibody immunotherapy.
Owner:CEDARS SINAI MEDICAL CENT +2
System and method for adverse event detection or severity estimation from surgical data
ActiveUS20200265273A1Easy to navigateEasy to handleImage enhancementImage analysisIntensive care medicineReoperative surgery
Embodiments described herein may provide devices, systems, methods, and / or computer readable medium for adverse event detection and severity estimation in surgical videos. The system can train multiple models for adverse detection and severity estimation. The system can load selected models for real-time adverse event detection and severity estimation.
Owner:SURGICAL SAFETY TECH INC
Methods of treating term and near-term neonates having hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension
InactiveUS20100330193A1Reduce riskReduces occurrenceOrganic active ingredientsInorganic active ingredientsCardiac echoInhalation
The invention relates methods of reducing the risk or preventing the occurrence of an adverse event (AE) or a serious adverse event (SAE) associated with a medical treatment comprising inhalation of nitric oxide.
Owner:IKARIA HLDG
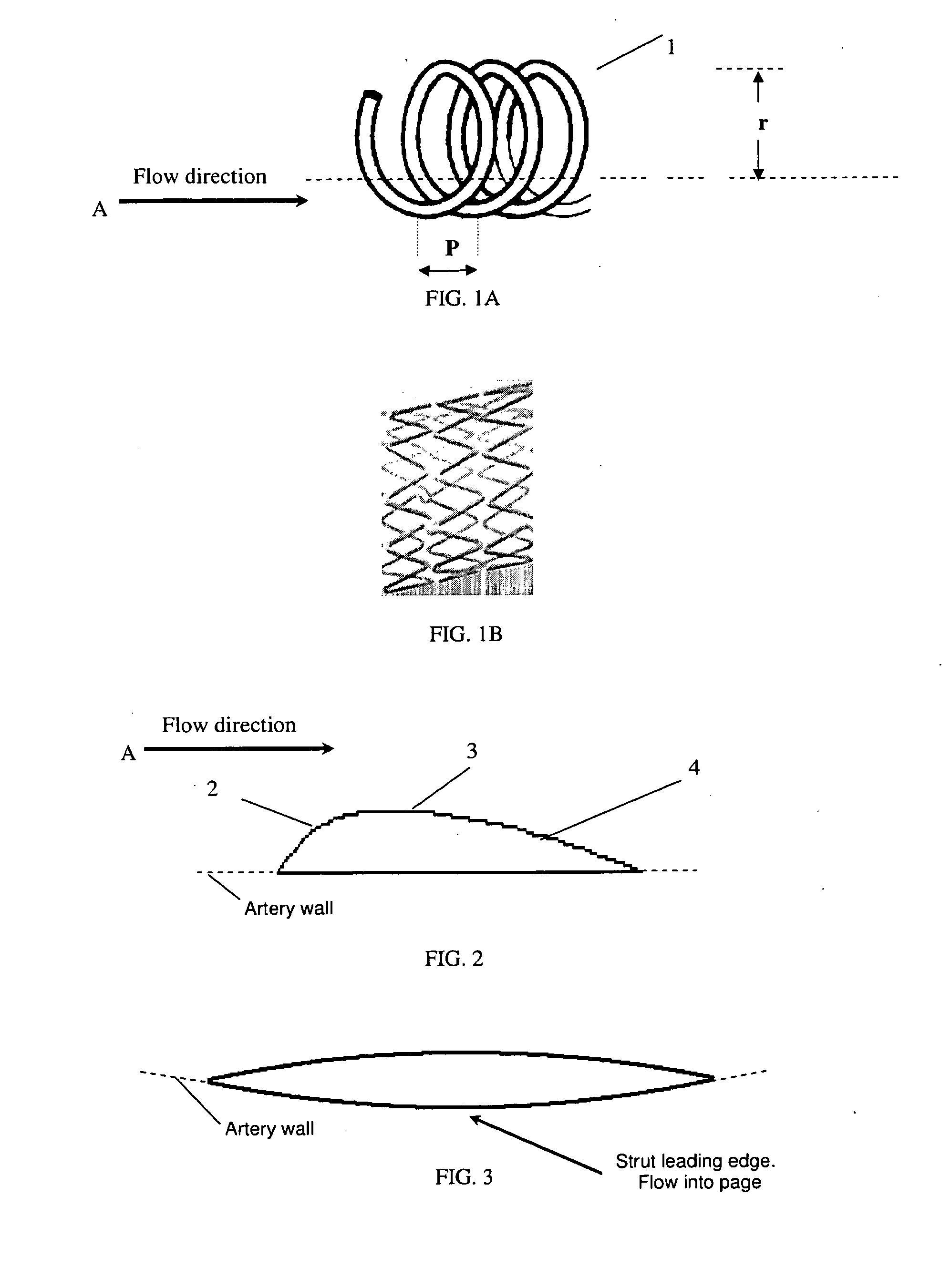
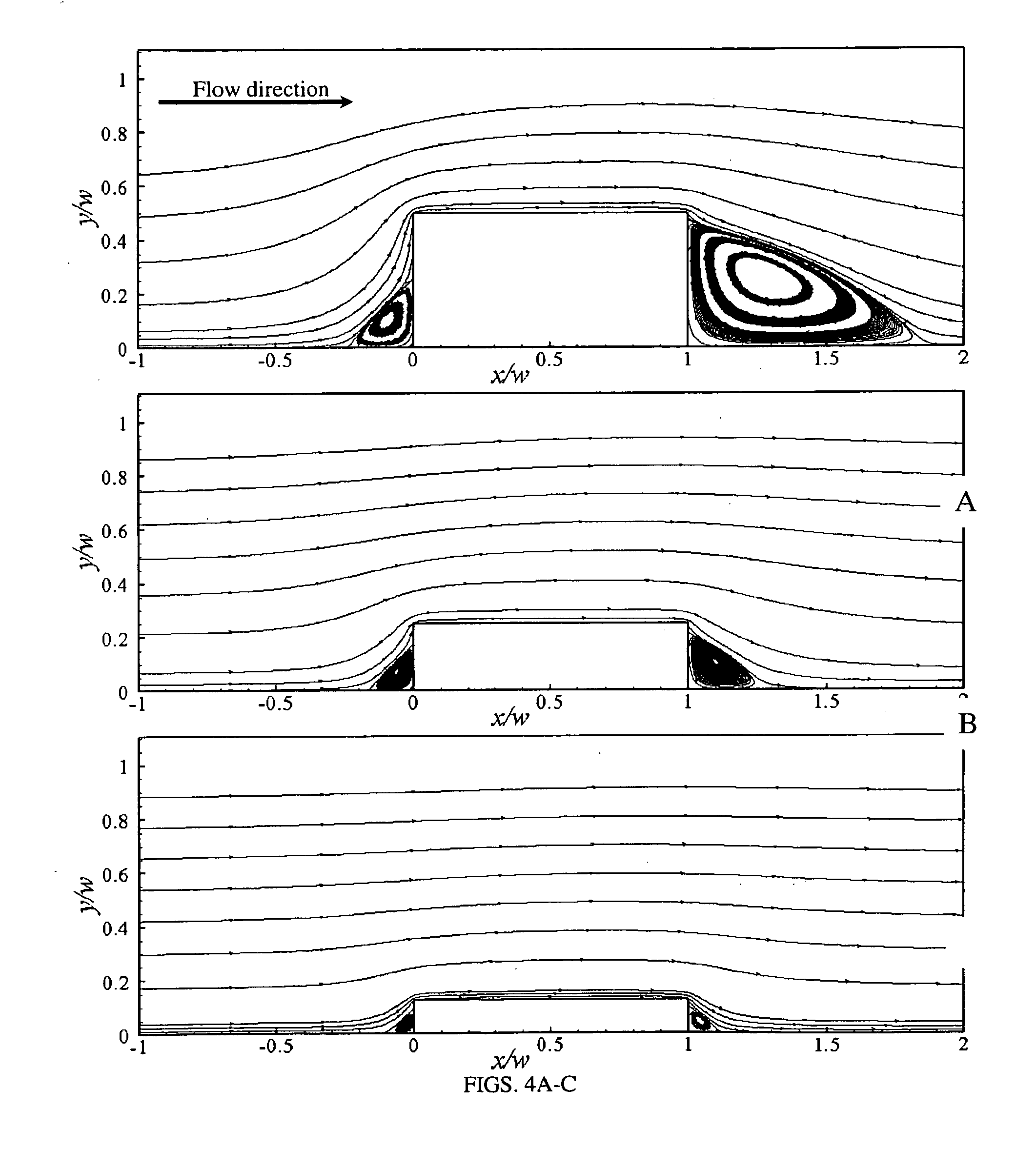
Vascular stent design
InactiveUS20110276123A1Minimize and eliminate local flow disturbanceEnhanced inhibitory effectStentsBlood vesselsRestenosisThrombus
This invention is directed to the design of radially expandable vascular stents to optimize hemodynamic flow characteristics that are favorable for the inhibition of stent-associated thrombosis, inflammation, and restenosis (neointimal formation) and that will reduce the risk of adverse events post-deployment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
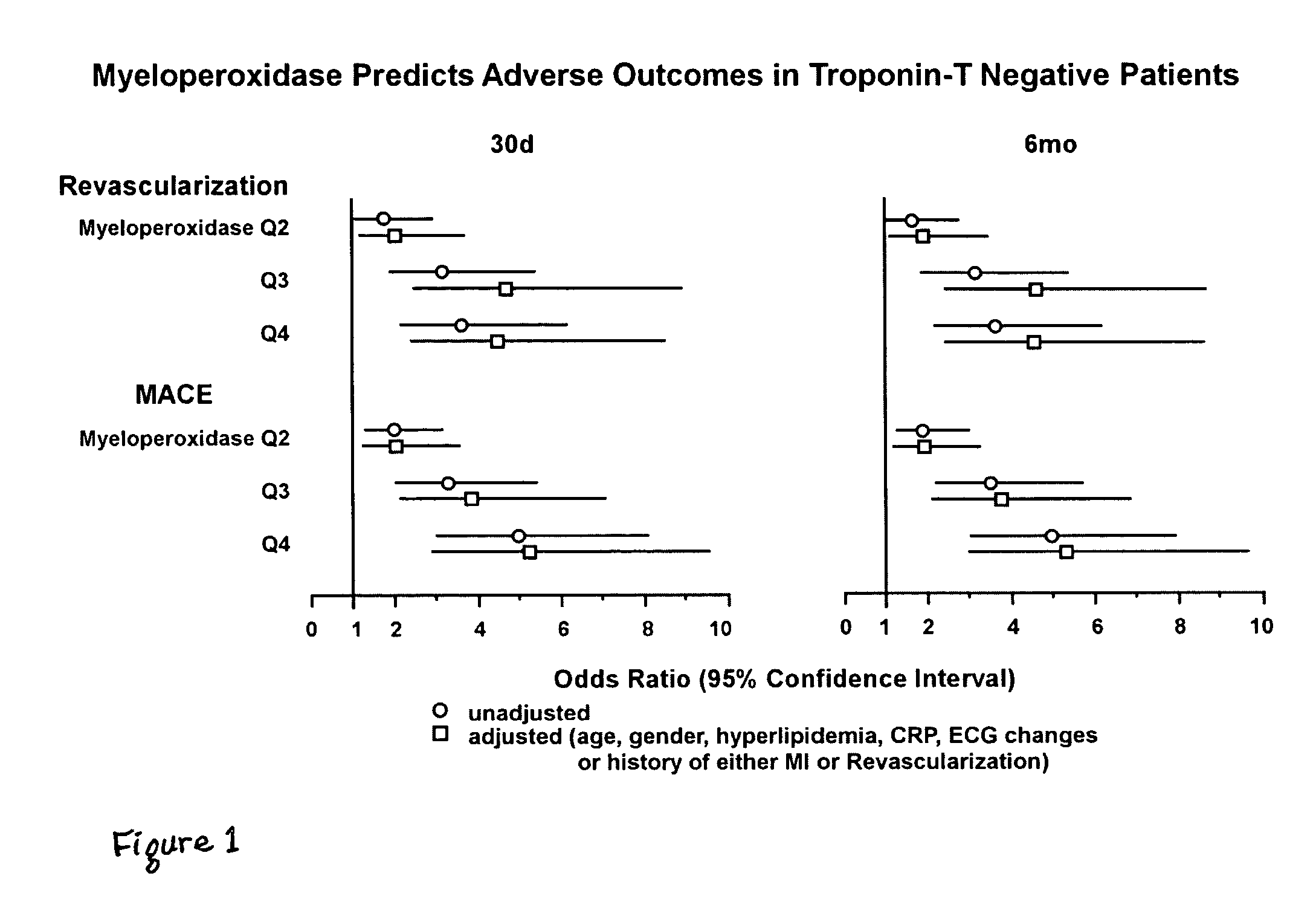
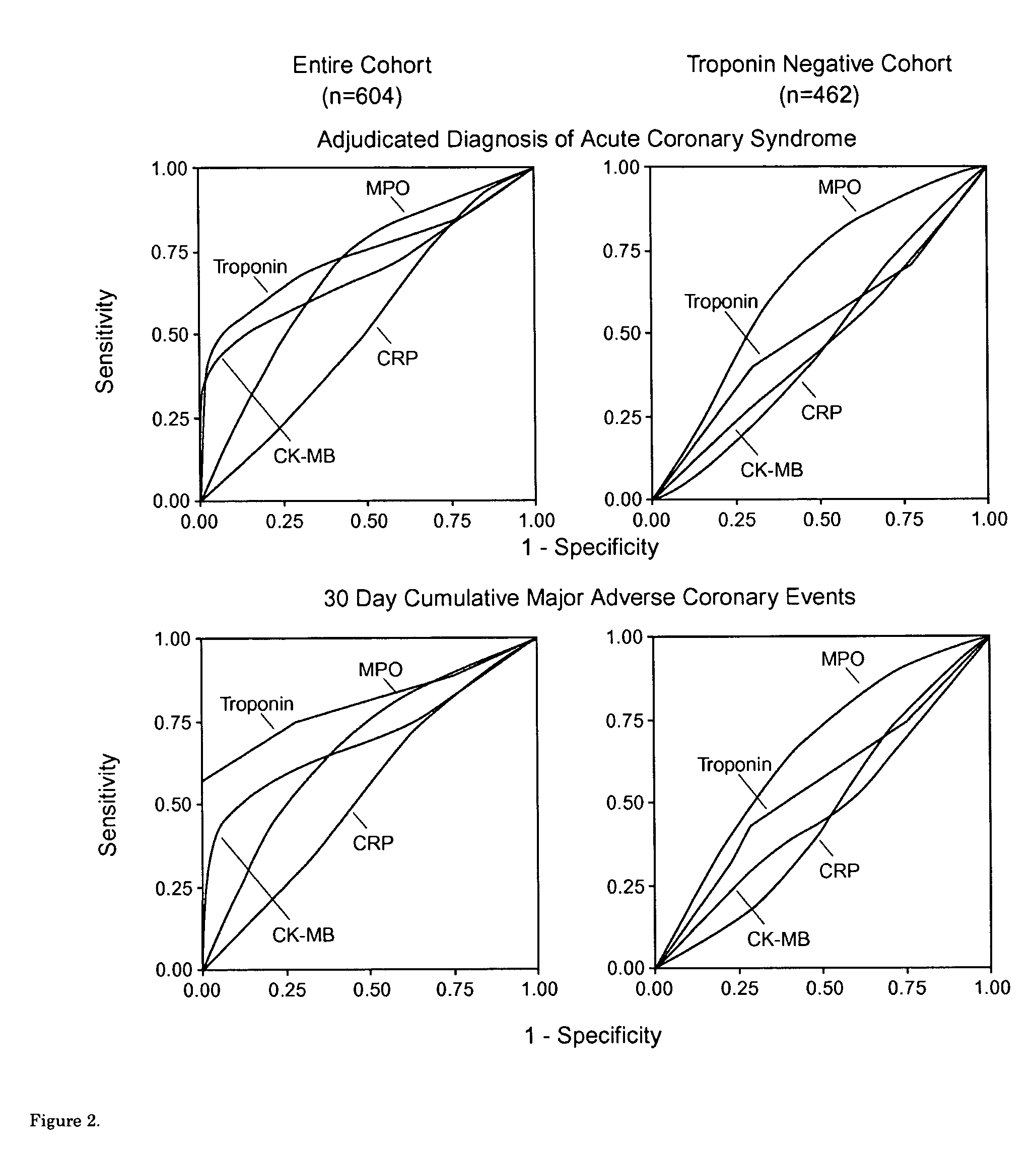
Assessing the risk of a major adverse cardiac event in patients with chest pain
InactiveUS7459286B1Milk preparationMicrobiological testing/measurementPopulationEmergency department
Methods for characterizing the near term risk of experiencing a major adverse cardiac event in a patient presenting with chest pain are provided. In one embodiment the method comprises determining the level of myeloperoxidase (MPO) activity in a bodily sample obtained from the patient. In another embodiment, the method comprises determining the level of MPO mass in a bodily sample obtained from the patient. In another embodiment, the method comprises determining the level of one or more select MPO-generated oxidation products in a bodily sample obtained from the patient. The select MPO-generated oxidation products are dityrosine, nitrotyrosine, chlorotyrosine, methionine sulphoxide or an MPO-generated lipid peroxidation product. Levels of MPO activity, MPO mass, or the select MPO-generated oxidation product in bodily samples from the test subject are then compared to a control value that is derived from measurements of MPO activity, MPO mass, or the select MPO-generated oxidation product in comparable bodily samples obtained from control population. Such comparison can also be used to determine treatment of the patient immediately at presentation in the emergency room.
Owner:THE CLEVELAND CLINIC FOUND
Method of providing pirfenidone therapy to a patient
The invention relates to methods for decreasing adverse events associated with pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) therapy. The invention discloses an optimized dose escalation scheme that results in the patient having increased tolerance to adverse events associated with the administration of pirfenidone. The invention also discloses a starter pack that may be used in conjunction with the dose escalation scheme.
Owner:INTERMUNE INC
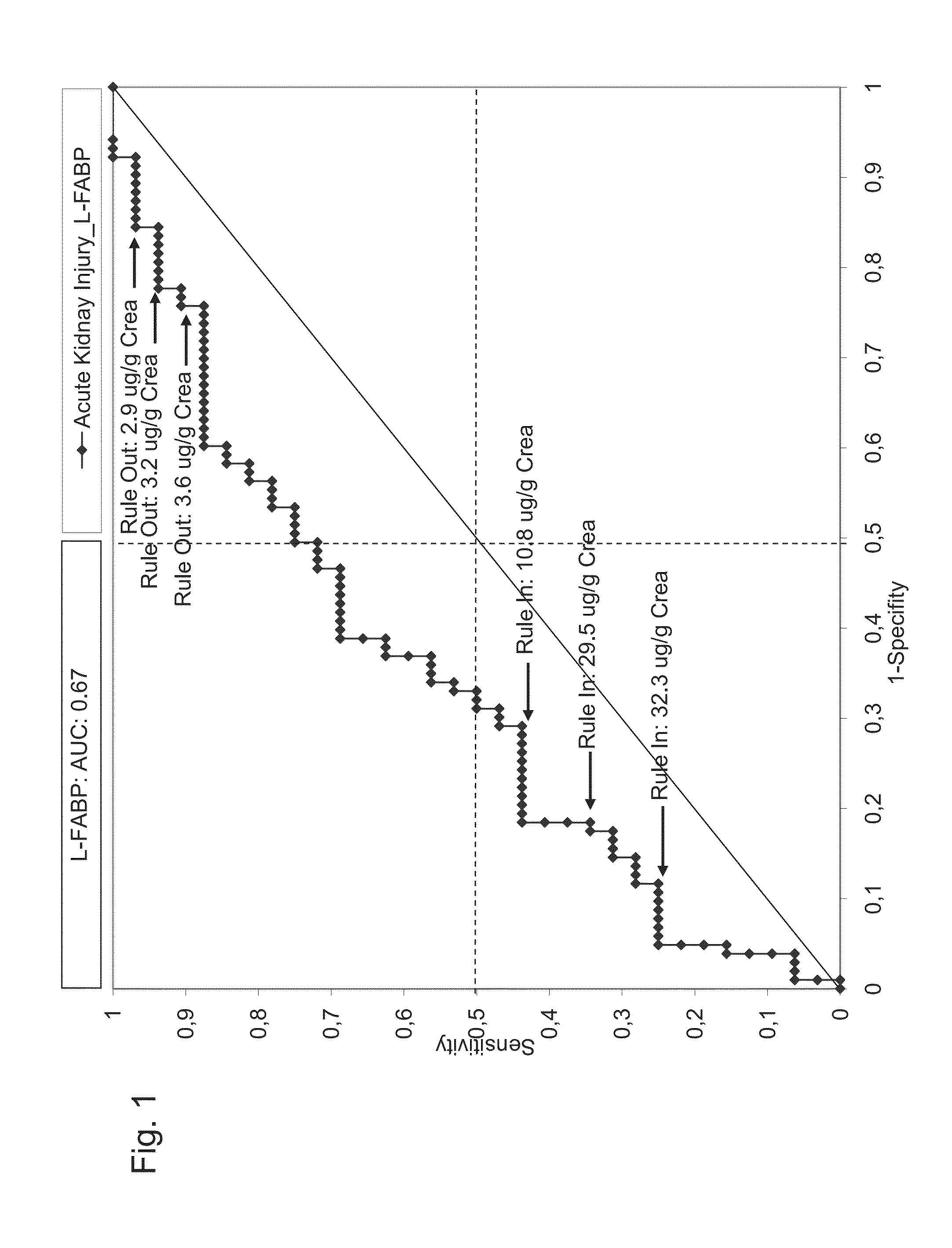
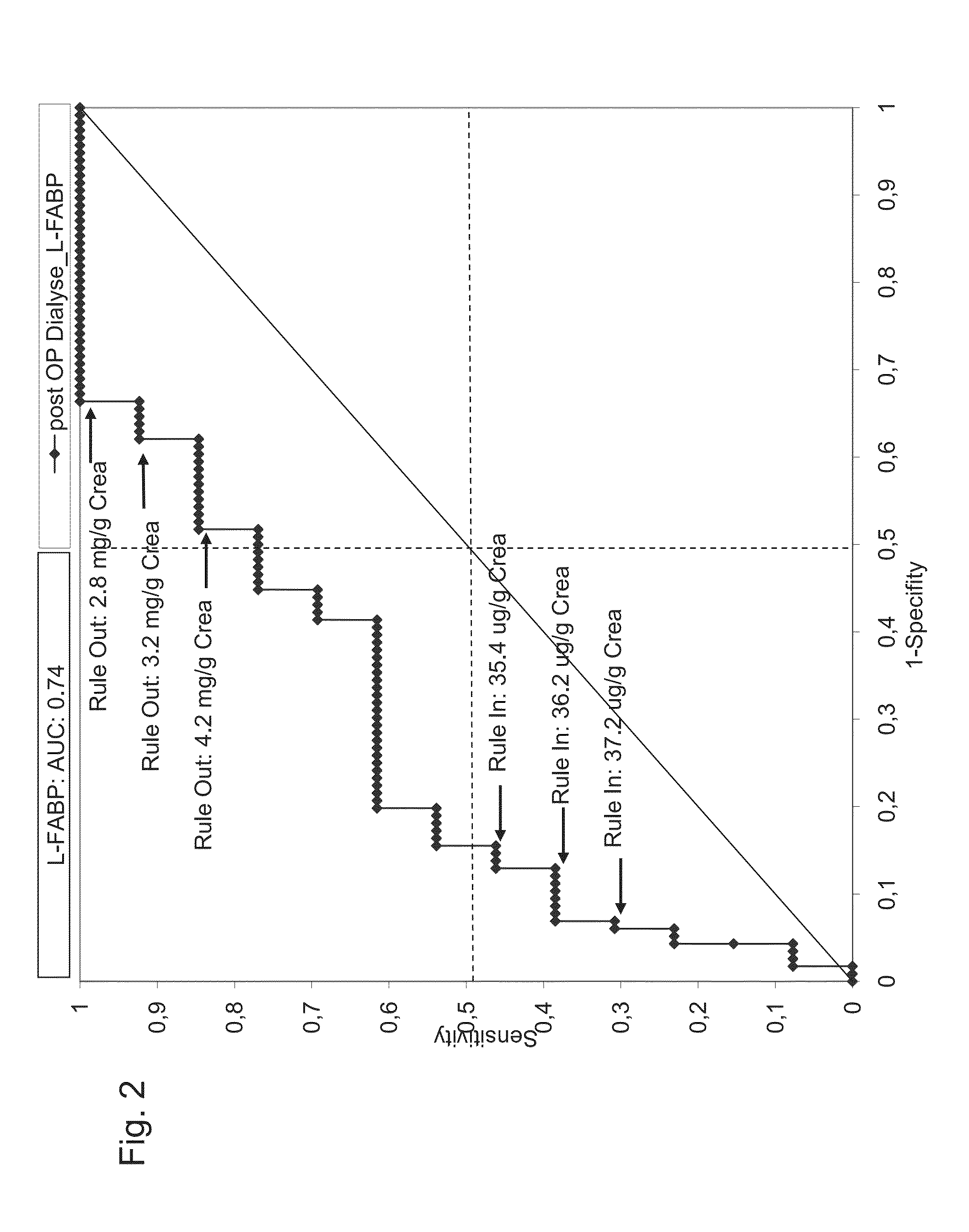
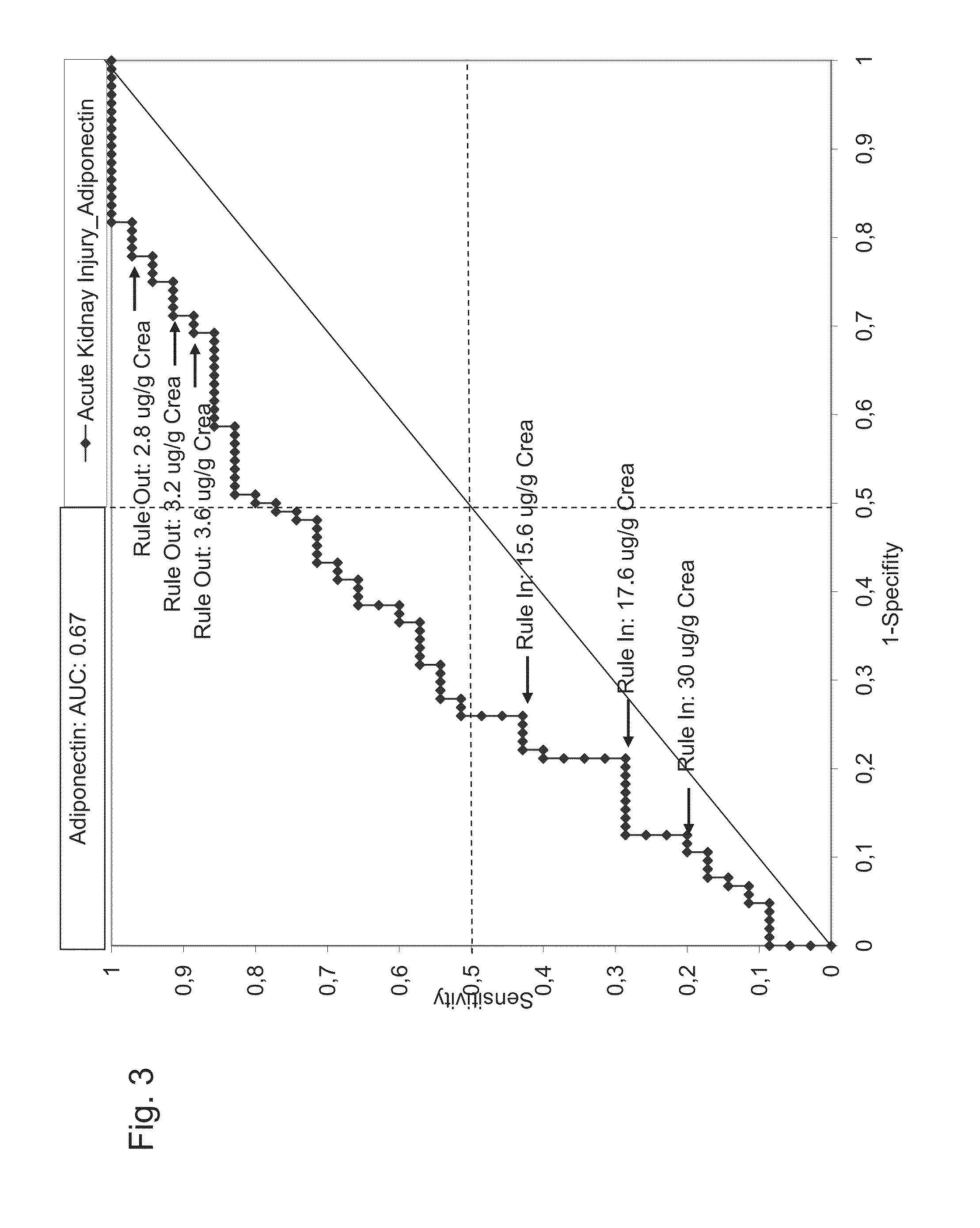
Prediction and recognition of acute kidney injury after surgery
Systems, kits, and methods for predicting the risk of an adverse event related to acute kidney injury AKI as a consequence of a surgical intervention in a subject. Embodiments of the system and methods include means and steps for determining an amount of liver-type fatty acid binding protein (L-FABP) in a sample, such as a urine-sample of a subject; comparing the amounts of the L-FABP with a reference amount, and predicting the risk of an adverse event related to acute kidney injury as a consequence of surgical intervention in the subject.
Owner:CMIC HLDG
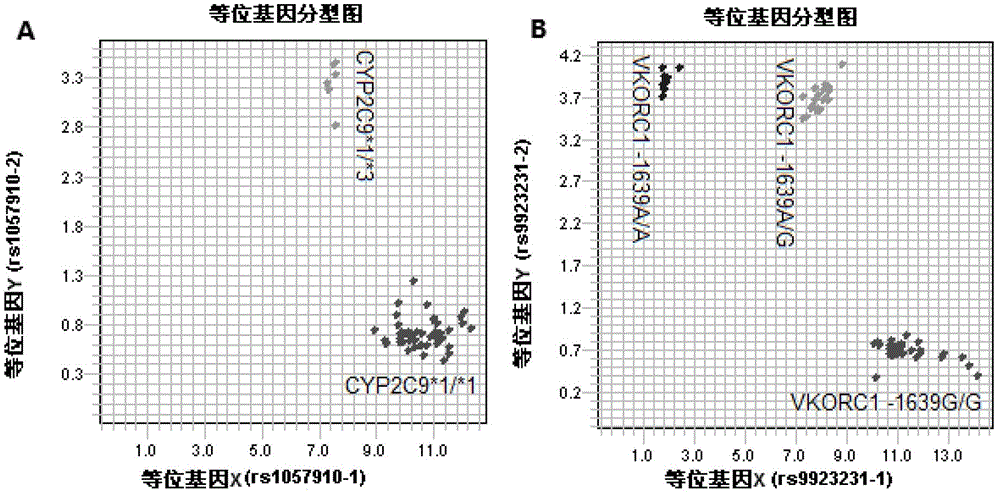
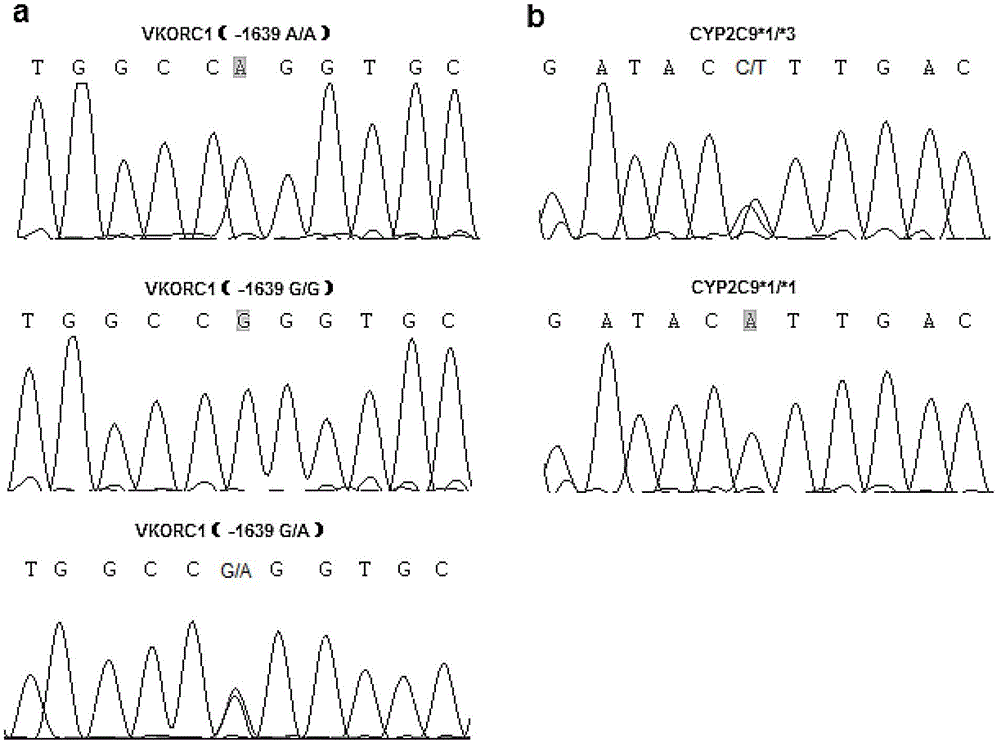
Kit for rapidly detecting warfarin individualized medication related gene SNP sites, and its detection method
The invention relates to a kit for rapidly detecting warfarin individualized medication related gene SNP sites, and its detection method, and belongs to a genetic detection technology in the clinic detection technique of the biomedical field. The genetic typing of two polymorphic sites comprising CYP2C9*3(1061A / C) and VKORC1(-1639G / A) is rapidly and accurately carried out through human peripheral blood DNA drawing, specific PCR amplification by a Taq man probe and fluorescence signal analysis. The invention provides primers, probes and kits which are used for detecting the polymorphic sites, a use of the primers and the probes in the preparation of the kits, and a use of the determination of the warfarin application amount of a patient according to the detection results of the polymorphic sites. The determination use can effectively reduce the generation of the warfarin application amount related adverse events and prevent thrombotic diseases. The detection method can be used for the auxiliary diagnosis and treatment of various patients needing warfarin clinically.
Owner:丁虎 +1
Predictive Biomarkers for CTLA-4 Blockade Therapy and for PD-1 Blockade Therapy
Biomarkers are described for predicting the efficacy, risk of relapse, risk of an immune related adverse event (irAE), or combination thereof for a CTLA-4 blockade treatment, such as ipilimumab, in a subject with melanoma. Biomarkers are also described for predicting the efficacy and clinical benefit for a PD-1 blockade treatment, such as a PD-1 blocking antibody, in a subject with melanoma.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
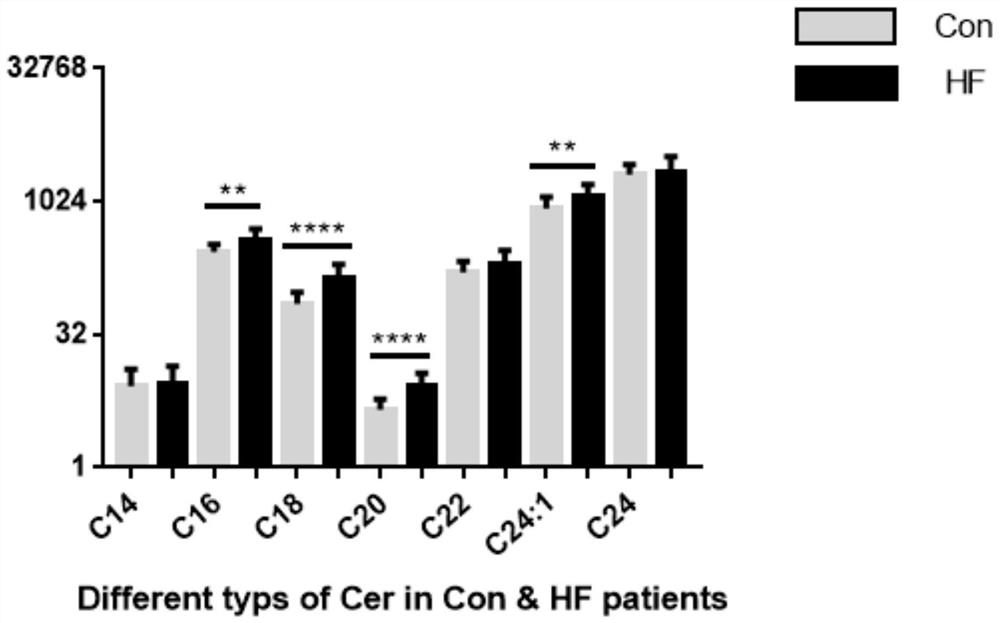
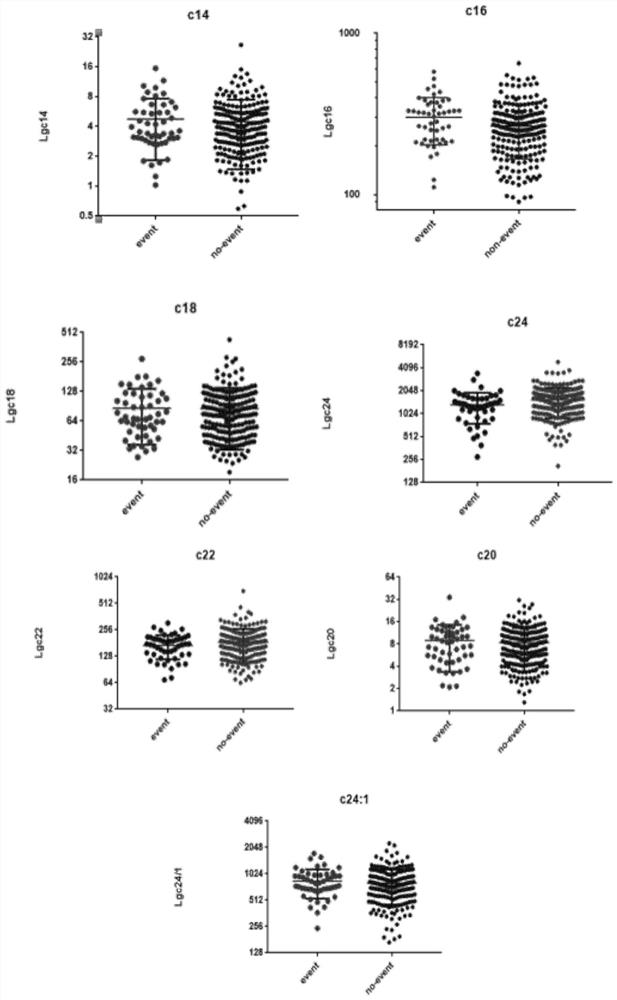
Application of ceramide in preparation of kit for evaluating adverse event risks of heart failure patients
The invention discloses application of a product for detecting ceramide in preparation of a kit. The application of the kit is at least one of the following (a1)-(a3): (a1) screening or assisting in diagnosing heart failure patients; (a2) evaluating or assisting in evaluating the prognosis risk of heart failure patients; and (a3) evaluating or assisting in evaluating the adverse event risk of heart failure patients. Research results show that the ratio of plasma ceramide cer (d18: 1 / 16: 0) to cer (d18: 1 / 24: 0) is an important predictive factor for the occurrence of adverse events of heart failure in patients with heart failure at the final stage, and is independent of currently used lipid markers, which facilitates the identification of high risk patients requiring more positive therapeutic interventions.
Owner:BEIJING INST OF HEART LUNG & BLOOD VESSEL DISEASES
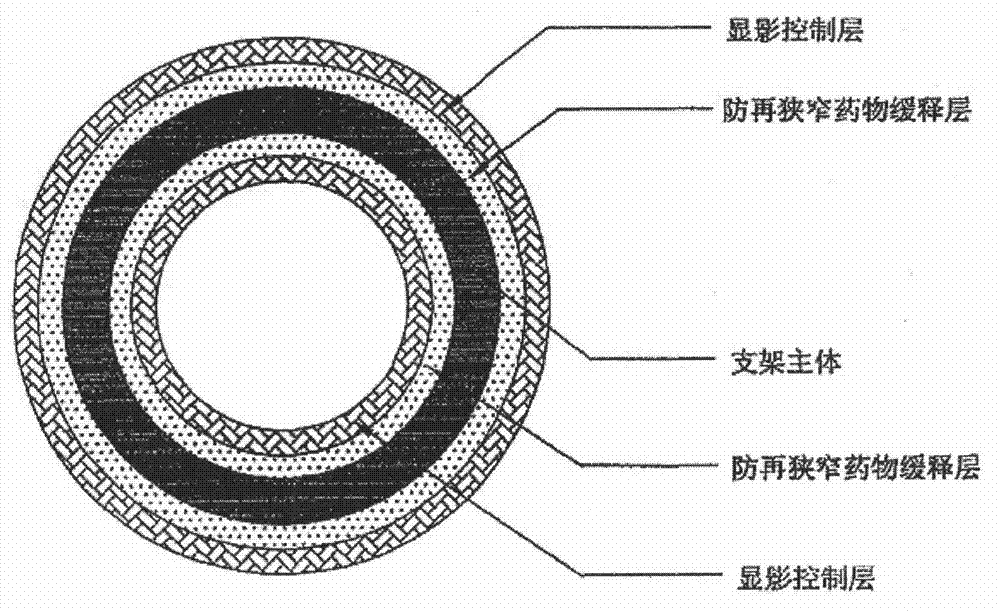
Biodegradable bracket with multiple drugs
The invention provides a biodegradable bracket with multiple drugs, belongs to the field of medical equipment and relates to a biodegradable bracket with a single coating. Besides support function, the bracket further comprises the single coating which contains anti-proliferative drugs, anti-inflammation and anti-immune drugs, drugs for promoting endothelial cells to grow and a developing agent. The bracket main body is made of biodegradable polymers of which mechanical strength is high. After implanted on a pathological position, the bracket has strong support force, so that action of supporting the pathological vessels in short time can be achieved. Because of the developing agent, doctors can accurately obverse the position and the expansion consistency of the bracket during operation, and displacement or other adverse events can be prevented; the anti-proliferative drugs can prevent the vessels from becoming narrow in short time after the bracket is implanted; and the drugs for promoting the endothelial cells to grow can promote the endothelial cells to grow on the surface of the bracket and can cover the bracket in the endothelium of the vessels, so that embolism phenomenon caused by degradation fragments of the bracket dropping in blood can be prevented.
Owner:深圳市昕力医疗设备开发有限公司
Risk evaluation and management strategy involving patient follow-ups relating to the use or discontinuation of a complement inhibitor
ActiveUS20160140298A1Reduce morbidityData processing applicationsDrug and medicationsComplement InhibitorsMedicine
This invention provides, inter alia, a complement-inhibitor-based treatment plan coupled with a risk evaluation and management strategy (“REMS”) and a safety support program (“SSP”) for reinforcing the REMS. The REMS and SPP are implemented using one or more computer devices with software tools programmed to enforce conditions of the REMS and / or prompt follow-ups by registered nurses enrolled in the SSP. The software tool(s) determines whether a prescriber requesting the complement inhibitor has agreed to abide by the REMS, and can prompt a provider of the complement inhibitor to provide updated educational materials to the prescriber at predetermined times or intervals, to monitor the prescriber for compliance with the REMS, and / or to monitor patients for signs of adverse events. Using exemplary embodiments described herein, a risk of adverse events (especially, but not limited to, meningococcal infections) can be managed and an incidence of the adverse events can be reduced.
Owner:ALEXION PHARMA INC
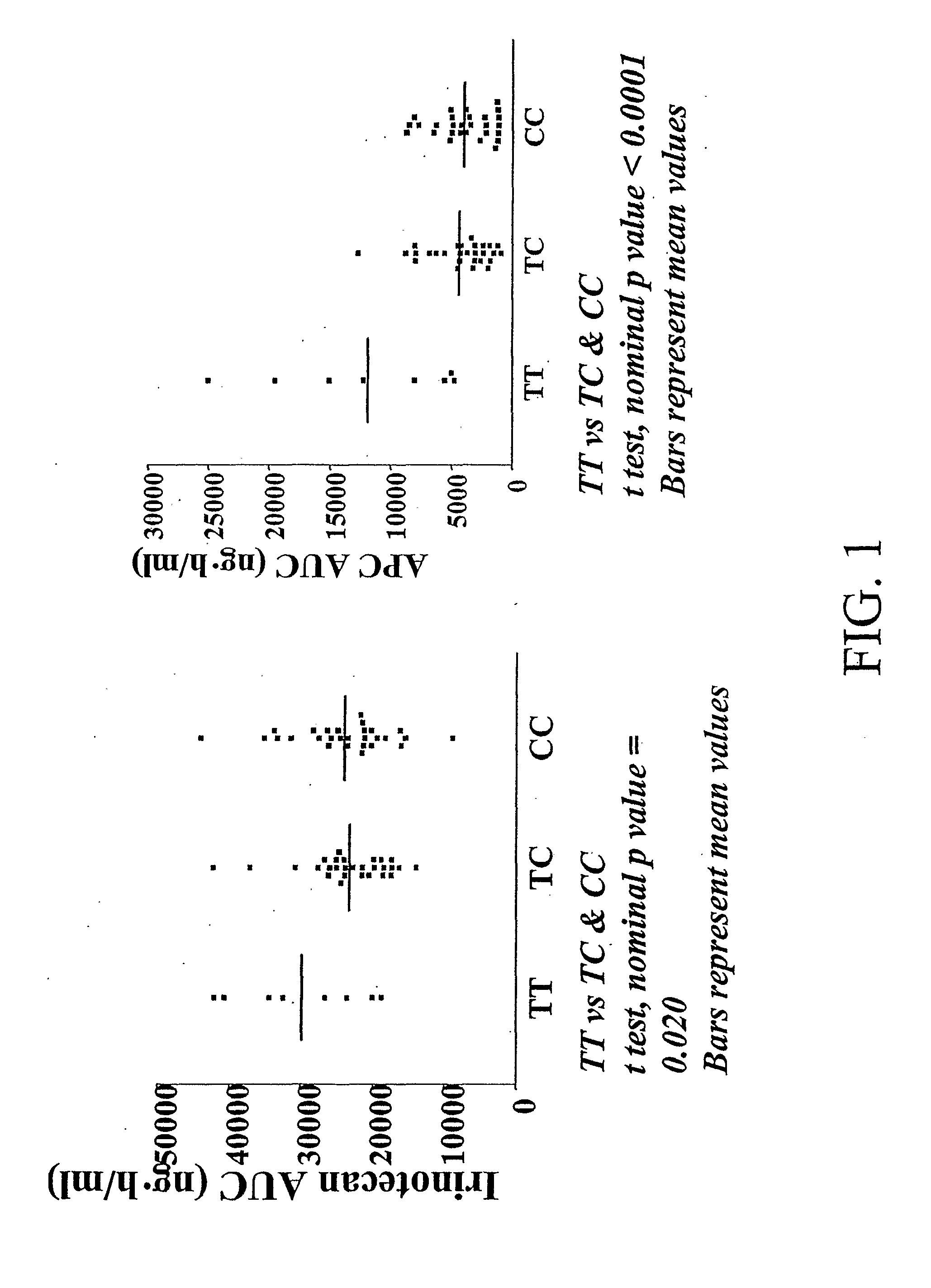
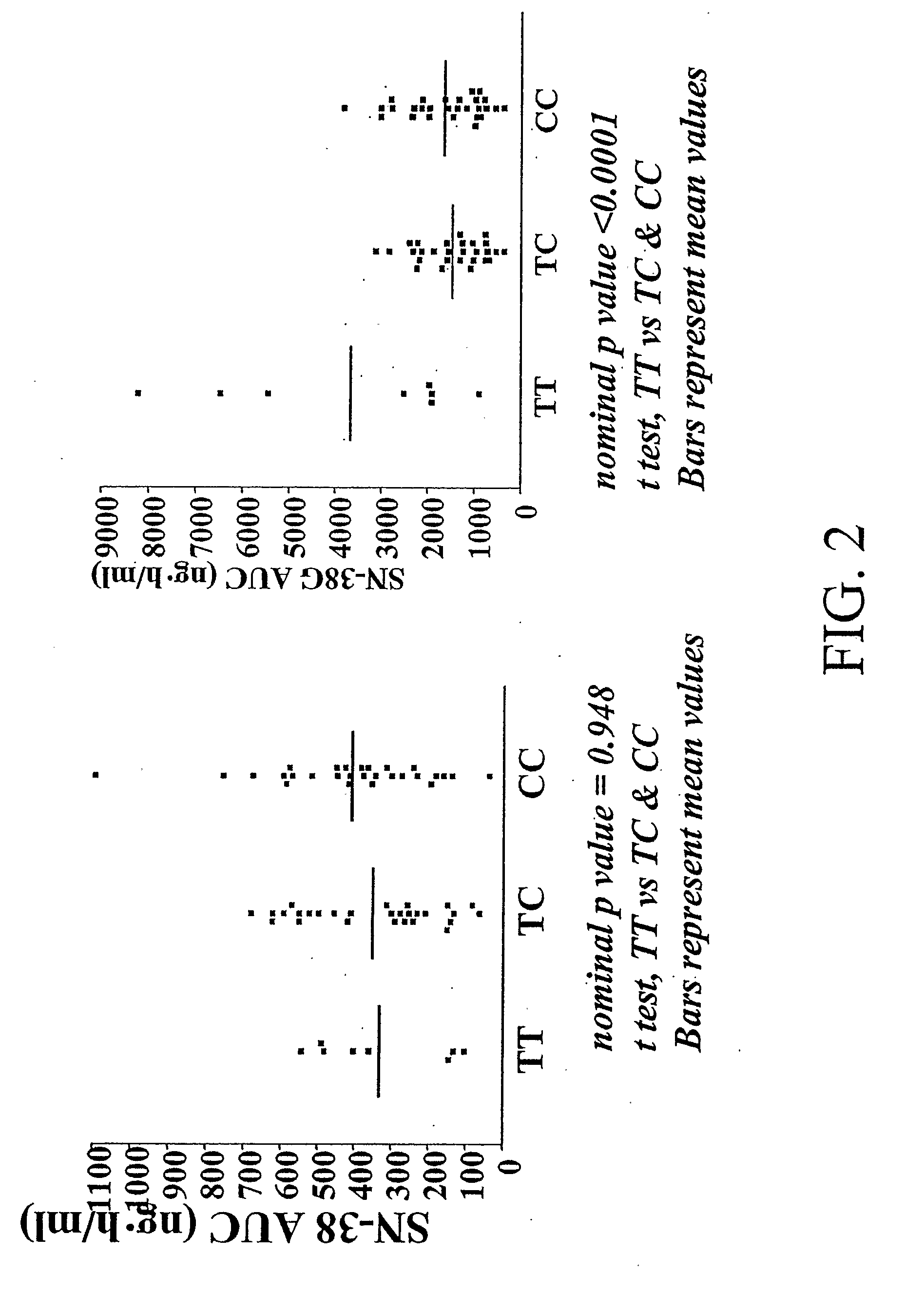
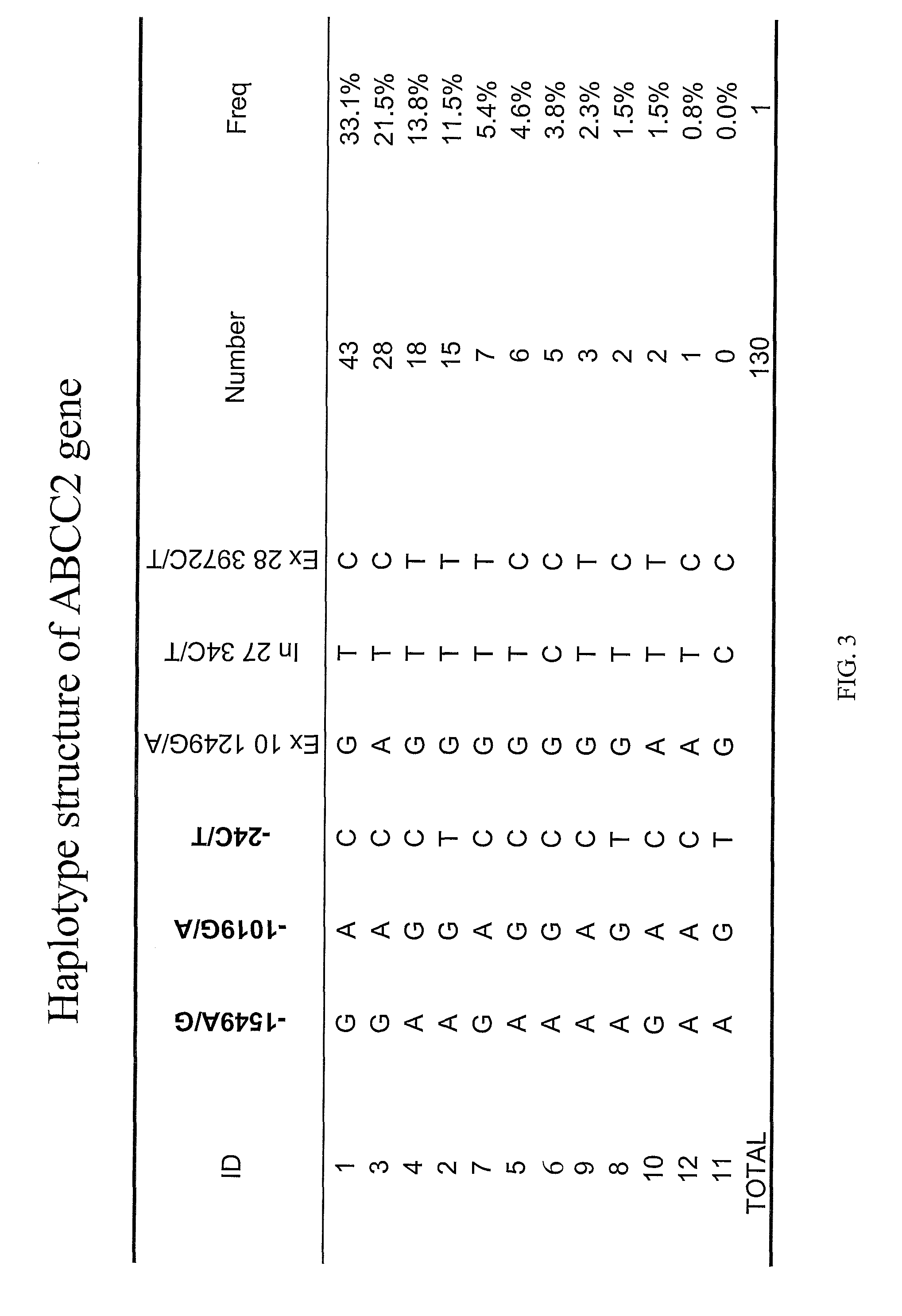
Methods and compositions relating to pharmacogenetics of different gene variants in the context of irinotecan-based therapies
InactiveUS20090247475A1Reduced cytotoxic activityIncrease and reduce chance of responseBiocideMicrobiological testing/measurementPharmacogeneticsSLCO1B1
The present invention is directed to methods and compositions for determining the presence or absence of polymorphisms within an ABCC2, UGT1A1, and / or SLCO1B1 gene and correlating these polymorphisms with activity levels of their gene products and making evaluations regarding the effect on their substrates, particularly those substrates that are drugs. In addition, there are methods and compositions of evaluating the risk of an individual for developing toxicity or adverse event(s) to an ABCC2, UGT1A1, and / or SLCO1B1 substrate. In some embodiments, the invention concerns methods and compositions for determining the presence or absence of ABCC2 3972C>T variant and predicting or anticipating the level of activity of ABCC2 and determining dosages of an ABCC2 drug substrate, such as irinotecan, in a patient. Such methods and compositions can be used to evaluate whether irinotecan-based therapy, or therapy involving other ABCC2 substrates, may pose toxicity problems if given to a particular patient or predicting their efficacy. Alterations in suggested therapy may ensue based on genotyping results.
Owner:UNIVERSITY OF CHICAGO +1
Rotator cuff balloon
PendingCN111096824AReduce foreign body sensationReduce dislocationLigamentsJoint implantsCoronal planeProsthesis
The invention discloses a rotator cuff balloon. The rotator cuff balloon comprises a limiting structure and a protection structure connected to the limiting structure. The limiting structure has an arc along the coronal plane. The prosthesis conforms to the physiological structure of the shoulder joint of the human body, is limited to the subacromial space, and can reduce adverse events such as foreign body sensation, dislocation and functional failure of a patient. The prosthesis conforms to the physiological structure of the shoulder joint of the human body, is limited to the subacromial space, and can reduce adverse events such as foreign body sensation, dislocation and functional failure of a patient.
Owner:SHANGHAI ENDOPHIX CO LTD
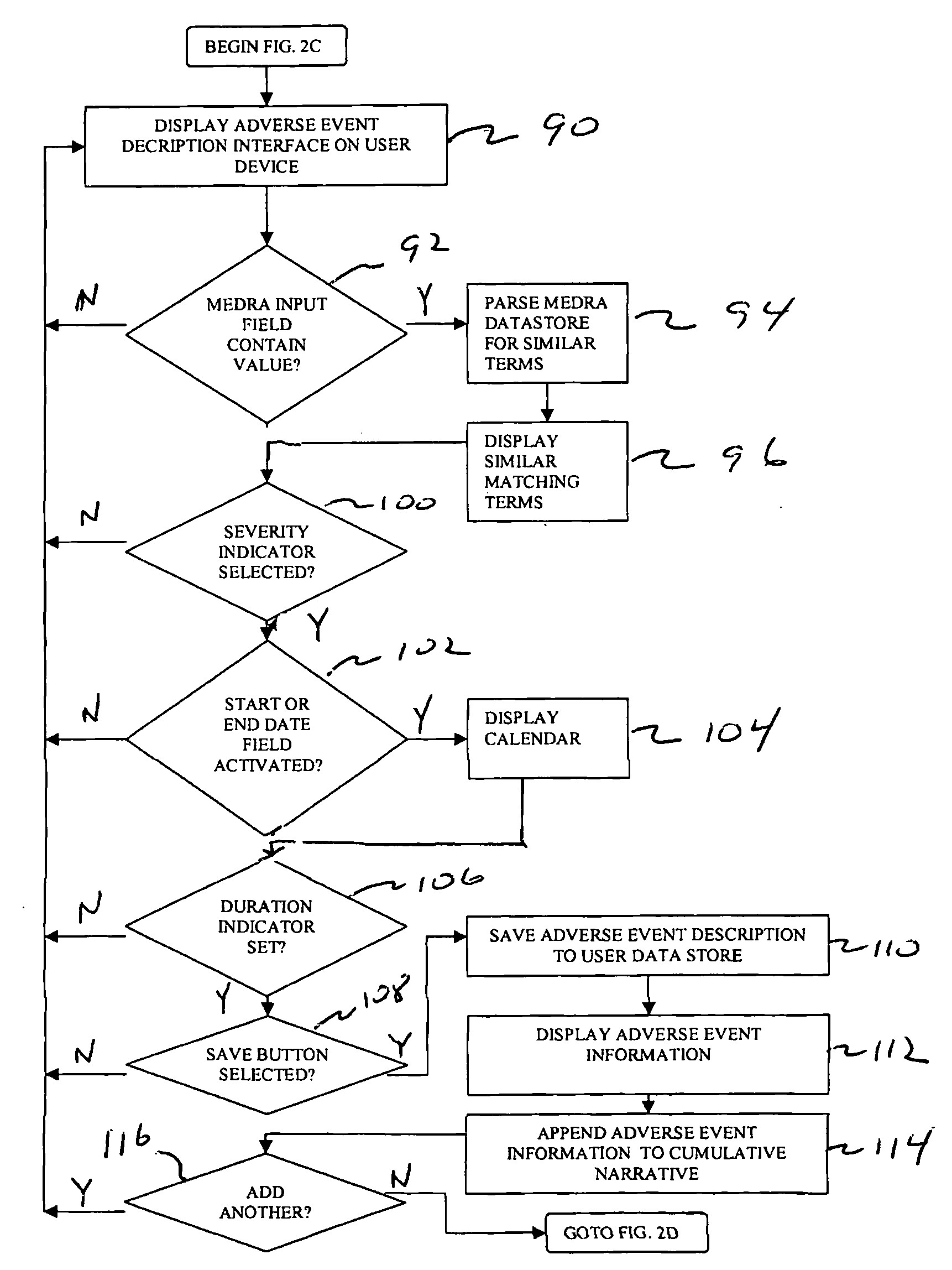
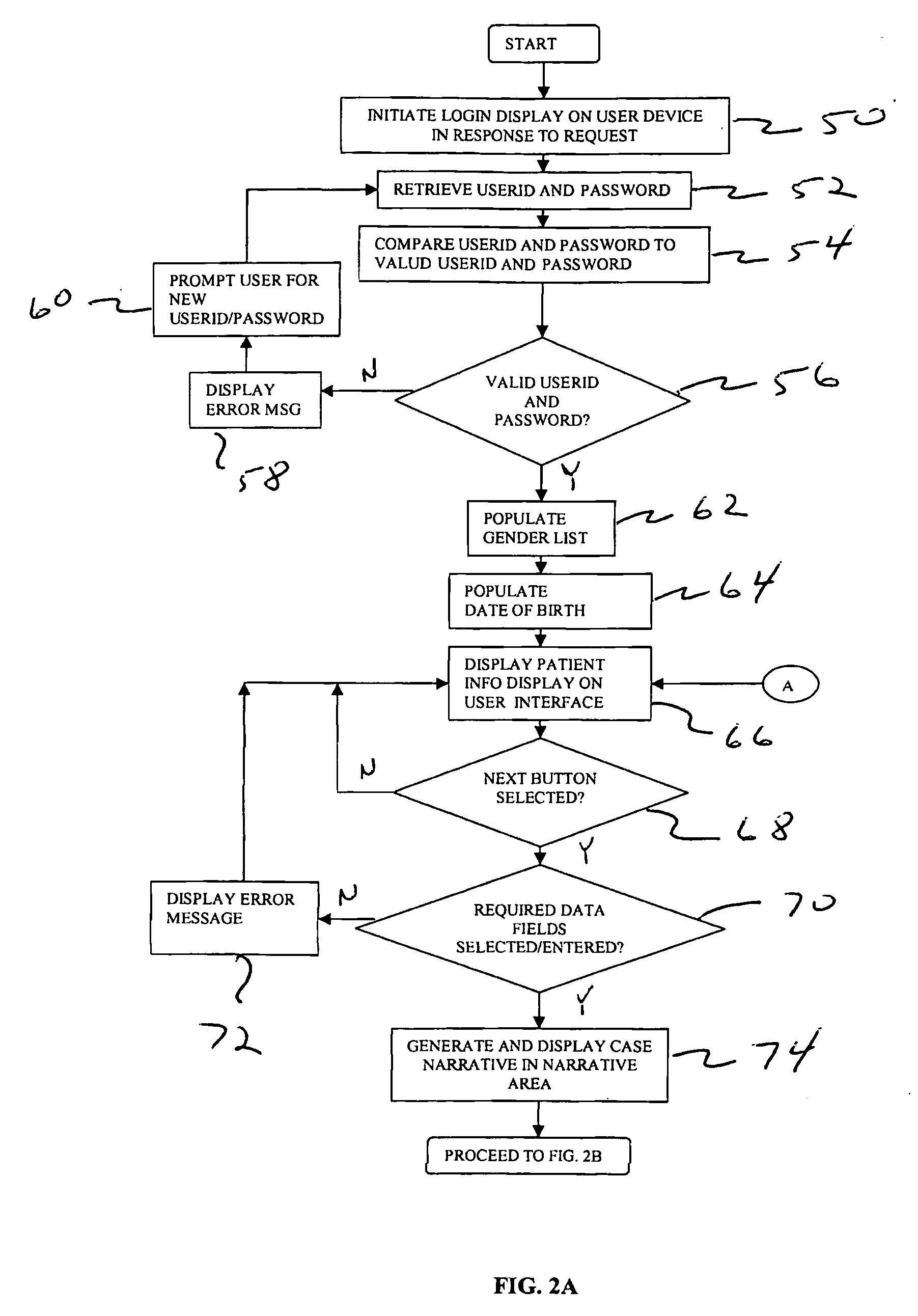
System and techniques for reporting adverse effects
InactiveUS20090319299A1Easy to processImprove accuracyData processing applicationsMedical report generationMedicineMedical terminology
Techniques for collecting and reporting adverse events associated with a patient are disclosed. The techniques include providing a user interface to identify drug and medical conditions associated with the patient using standardized drug and medical terminology. The techniques also include generating and displaying a cumulative narrative of user selections on the interface. The cumulative narrative can be directly transmitted to one or more companies and / or and regulatory agencies.
Owner:MEDIDATA SOLUTIONS
Methods of treating term and near-term neonates having hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension
ActiveUS20100330207A1Reduce riskIncrease riskNitrogen compoundsInorganic active ingredientsAcute respiratory failureNitric oxide
The invention relates methods of reducing the risk or preventing the occurrence of an adverse event (AE) or a serious adverse event (SAE) associated with a medical treatment comprising inhalation of nitric oxide.
Owner:MALLINCKRODT HOSPITAL PRODUCTS IP LTD
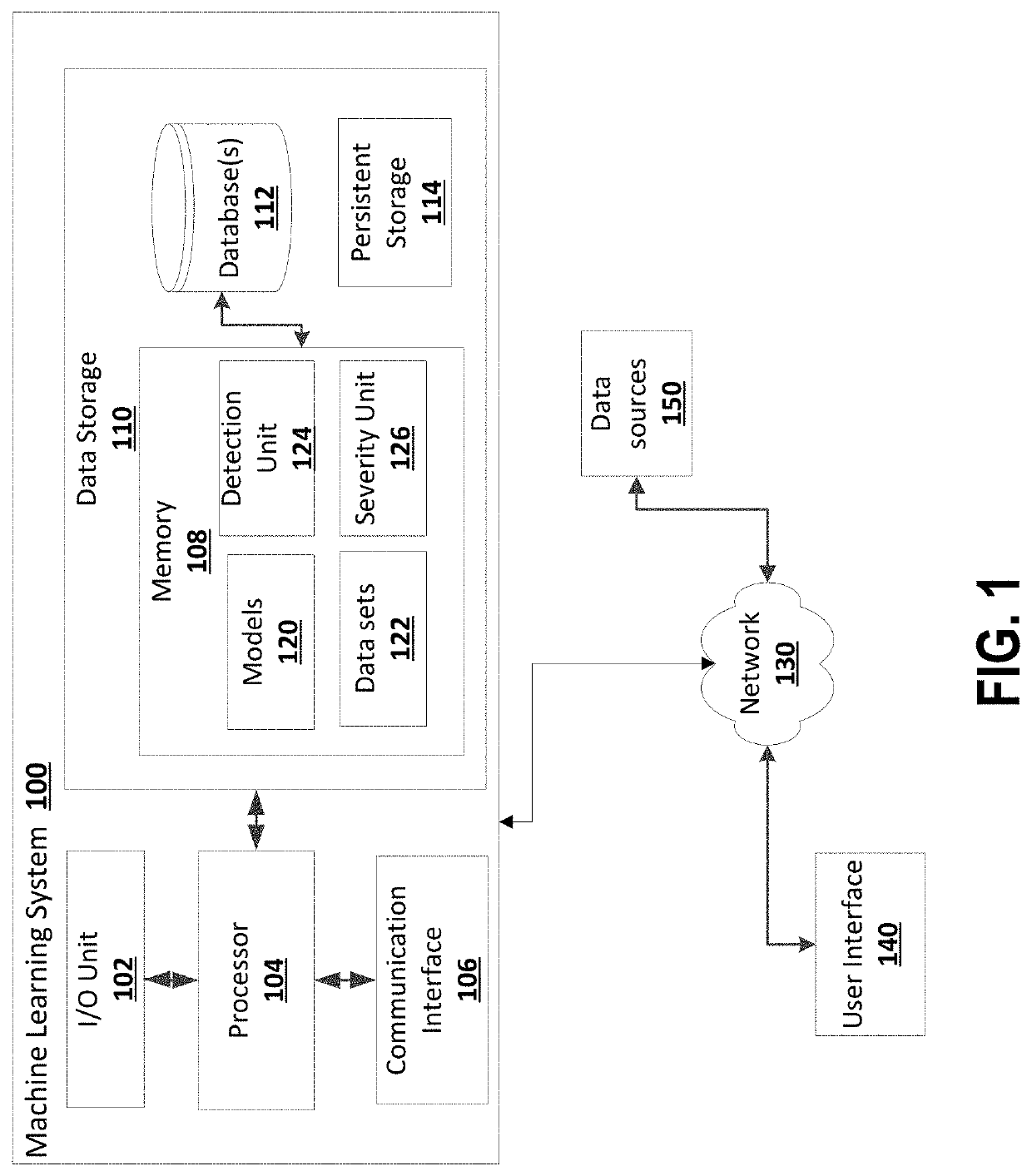
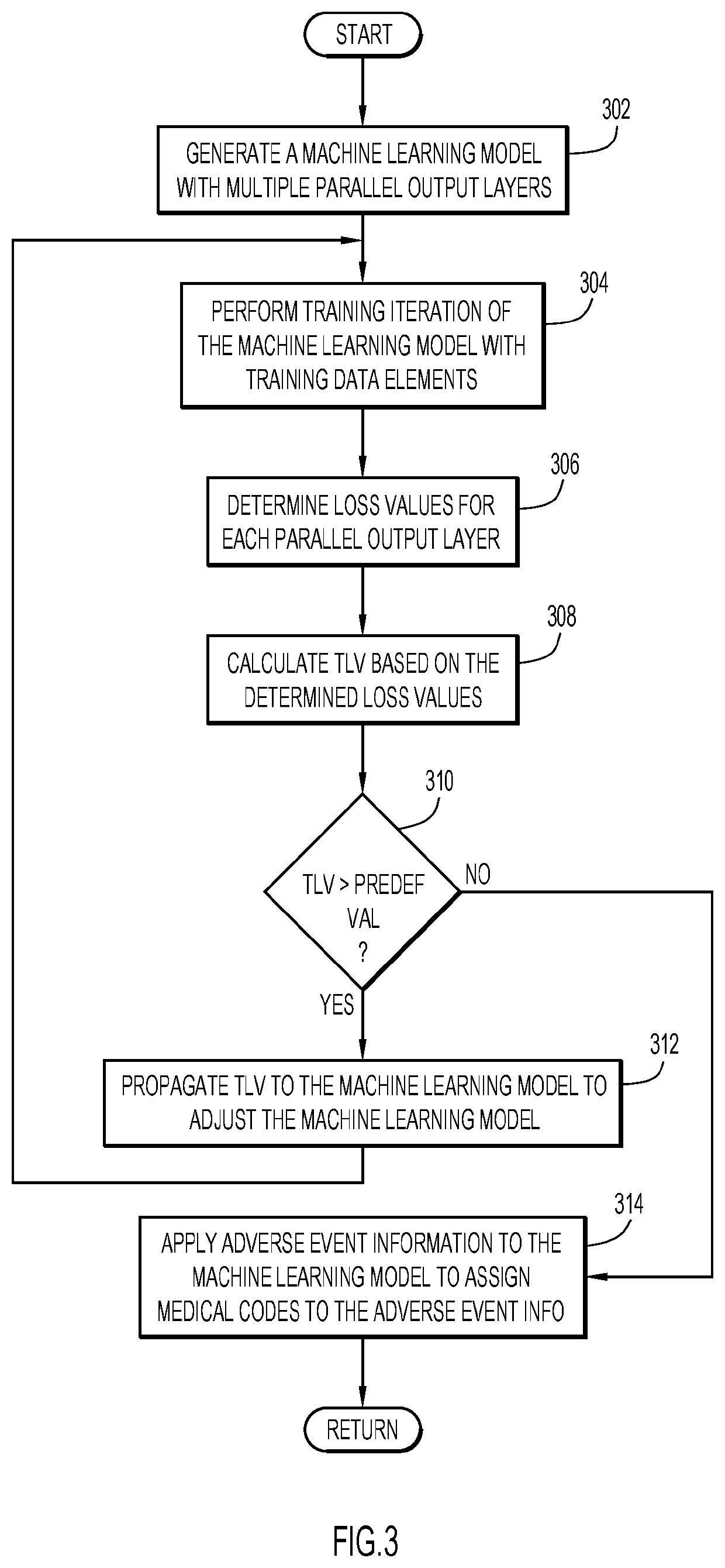
Architecture for machine learning model to leverage hierarchical semantics between medical concepts in dictionaries
A method, a system, and a computer program product are provided. A machine learning model is generated to process adverse event information and produce multiple corresponding medical codes associated with the adverse event information, wherein the multiple medical codes are semantically and hierarchically related in a medical taxonomy. The machine learning model includes multiple parallel output layers, each of which is associated with a corresponding medical code. The machine learning model is trained with training data elements, each of which includes adverse event information mapped to respective multiple medical codes, wherein results from each of the output layers adjusts the machine learning model. After completing the training, information pertaining to an adverse event is applied to the machine learning model to determine the corresponding multiple medical codes within the medical taxonomy.
Owner:MERATIVE US LP
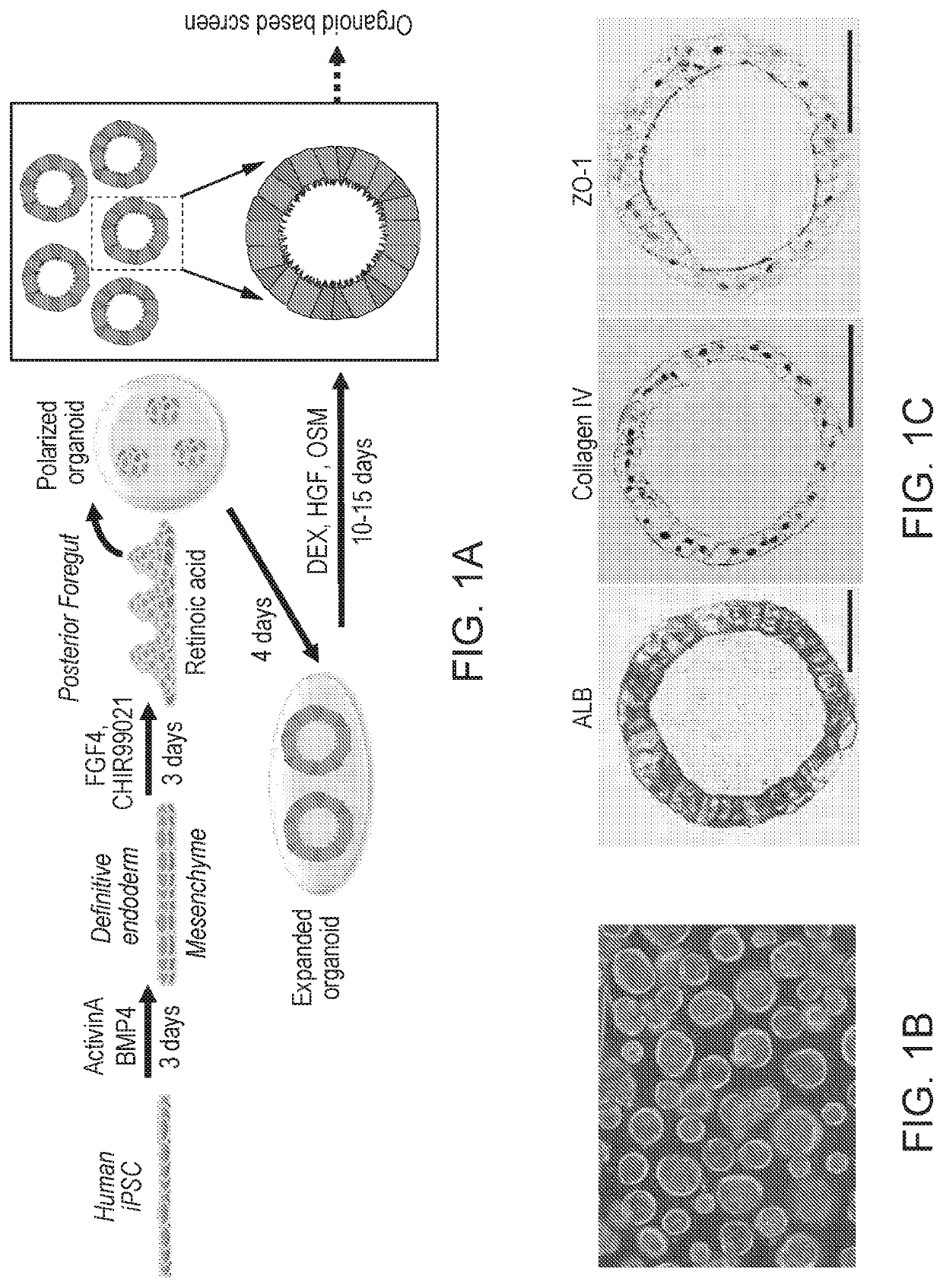
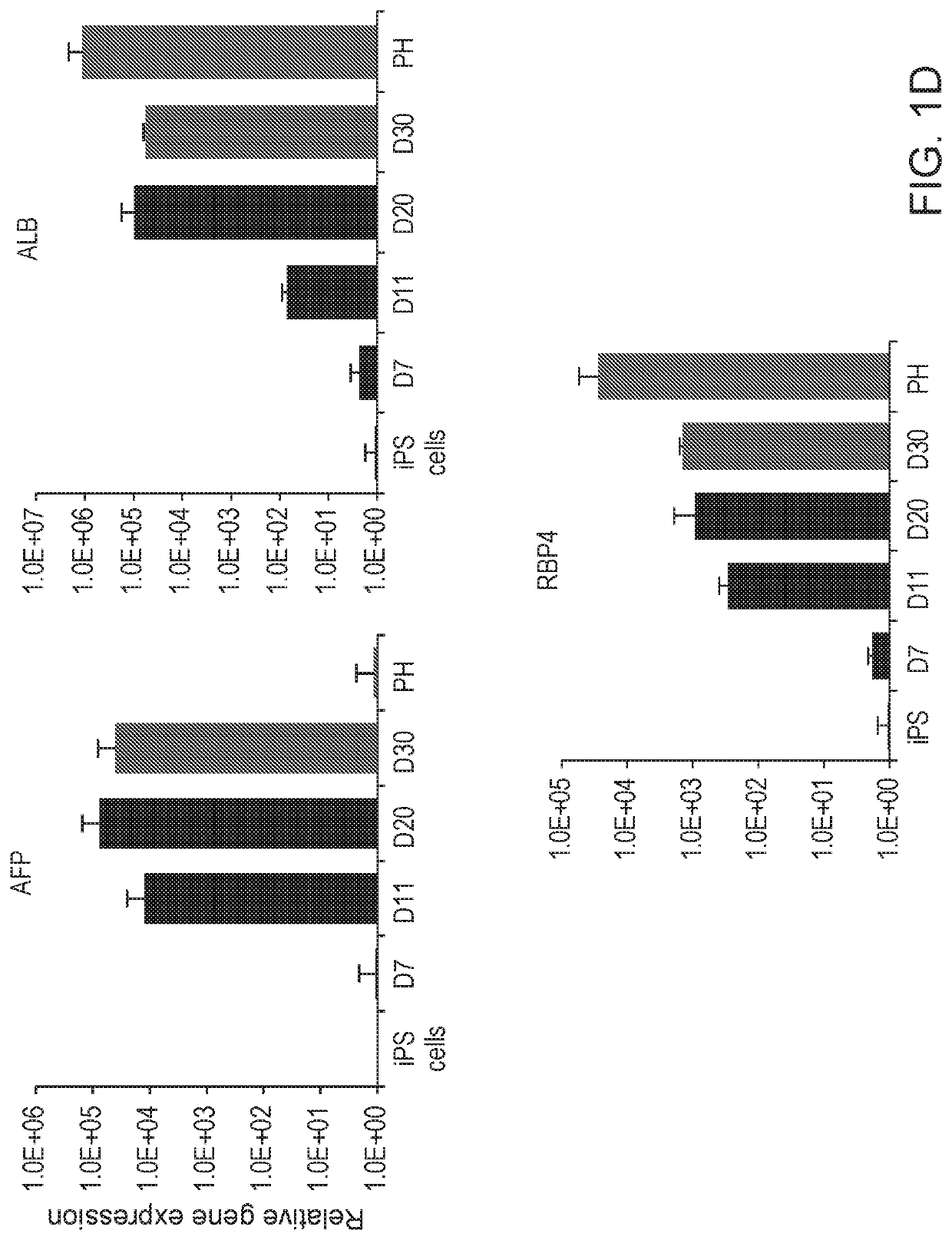
Liver organoid compositions and methods of making and using same
Disclosed are methods of inducing formation of a liver organoid from precursor cells, such as iPSC cells. The disclosed liver organoids may be used for screening for a serious adverse event (SAE), such as liver failure and / or drug induced liver injury (DILI), and / or drug toxicity. The disclosed liver organoids may also be used to treat an individual having liver damage, or for identifying a preferred therapeutic agent.
Owner:JAPAN SCI & TECH CORP
Methods for predicting response to treatment
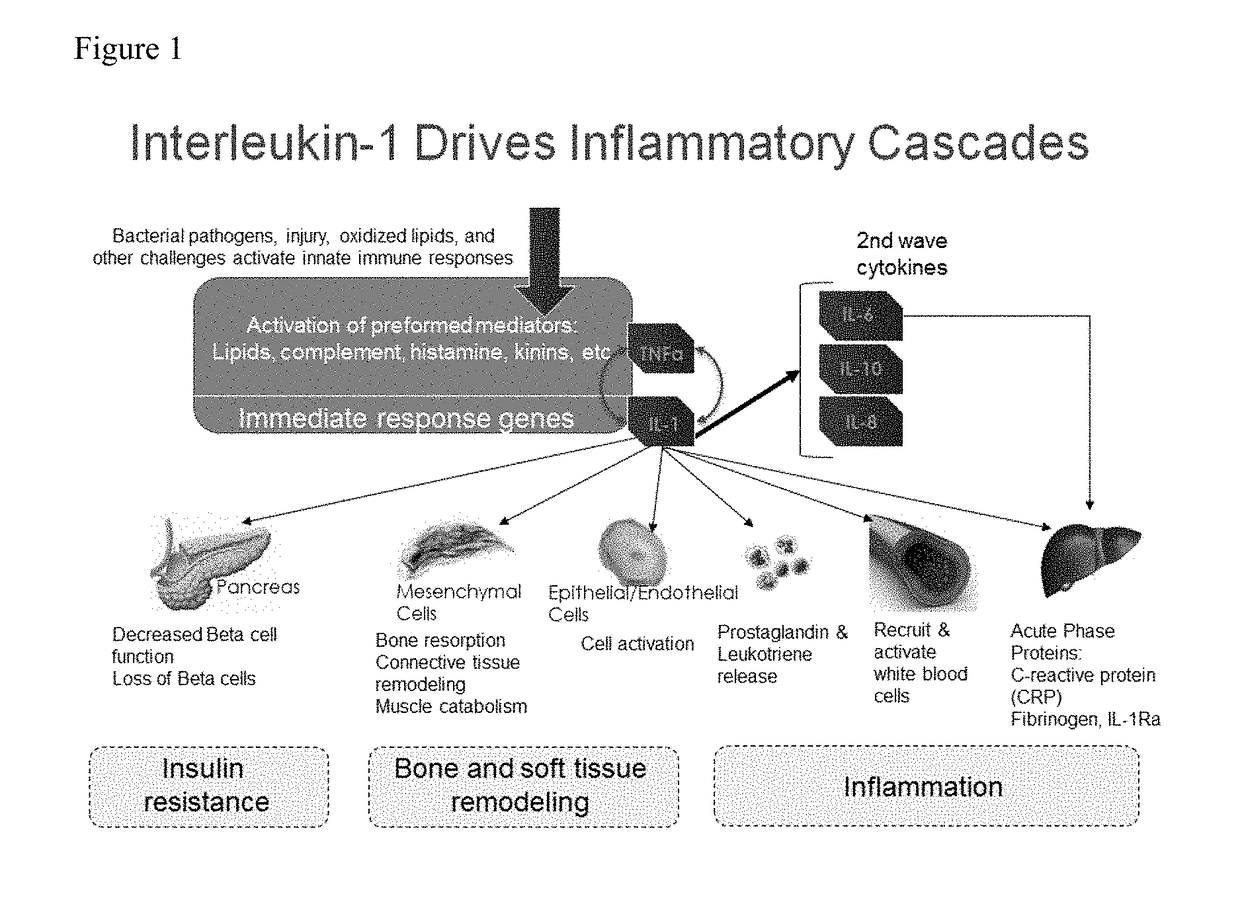
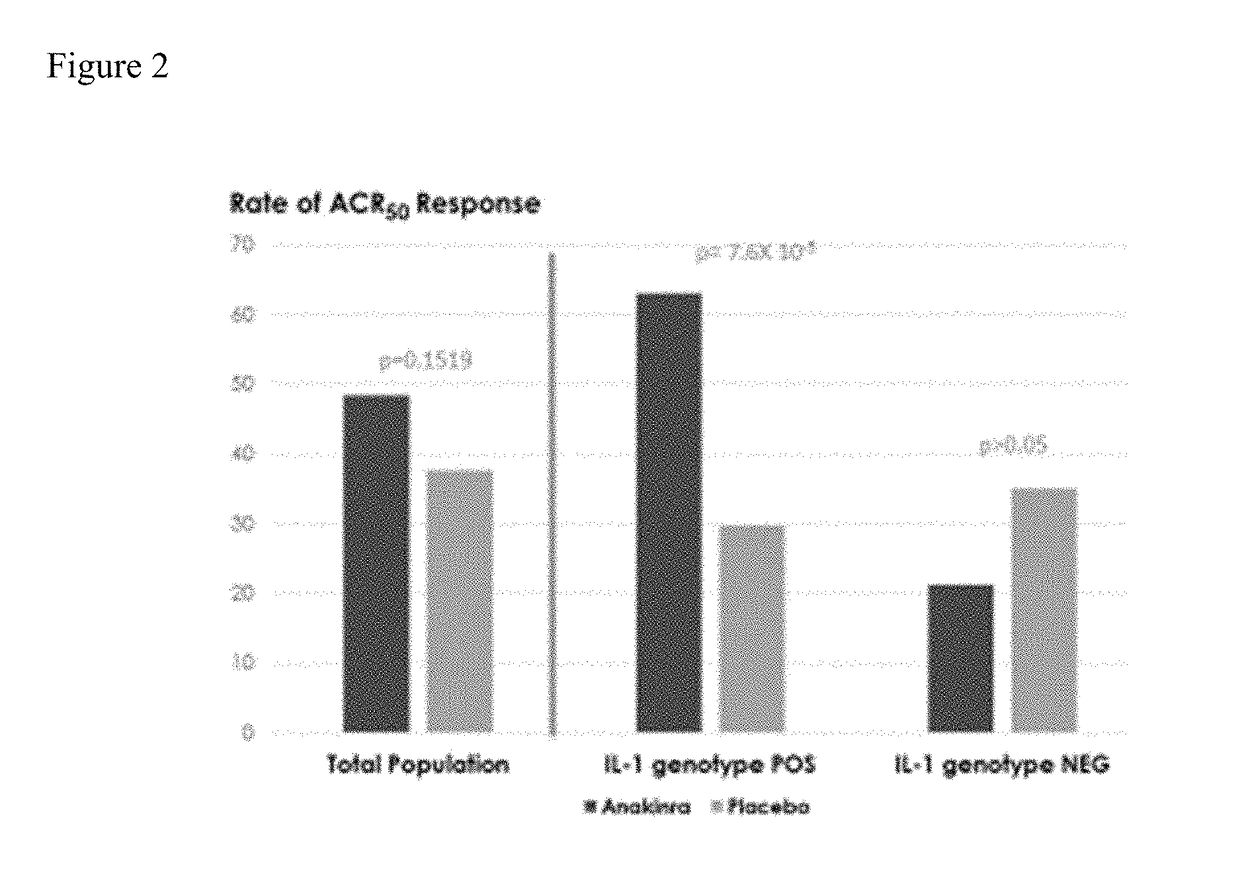
ActiveUS20170198350A1Reduce inflammationReduce drug toxicitySenses disorderPeptide/protein ingredientsHigh dosesAntiinflammatory drug
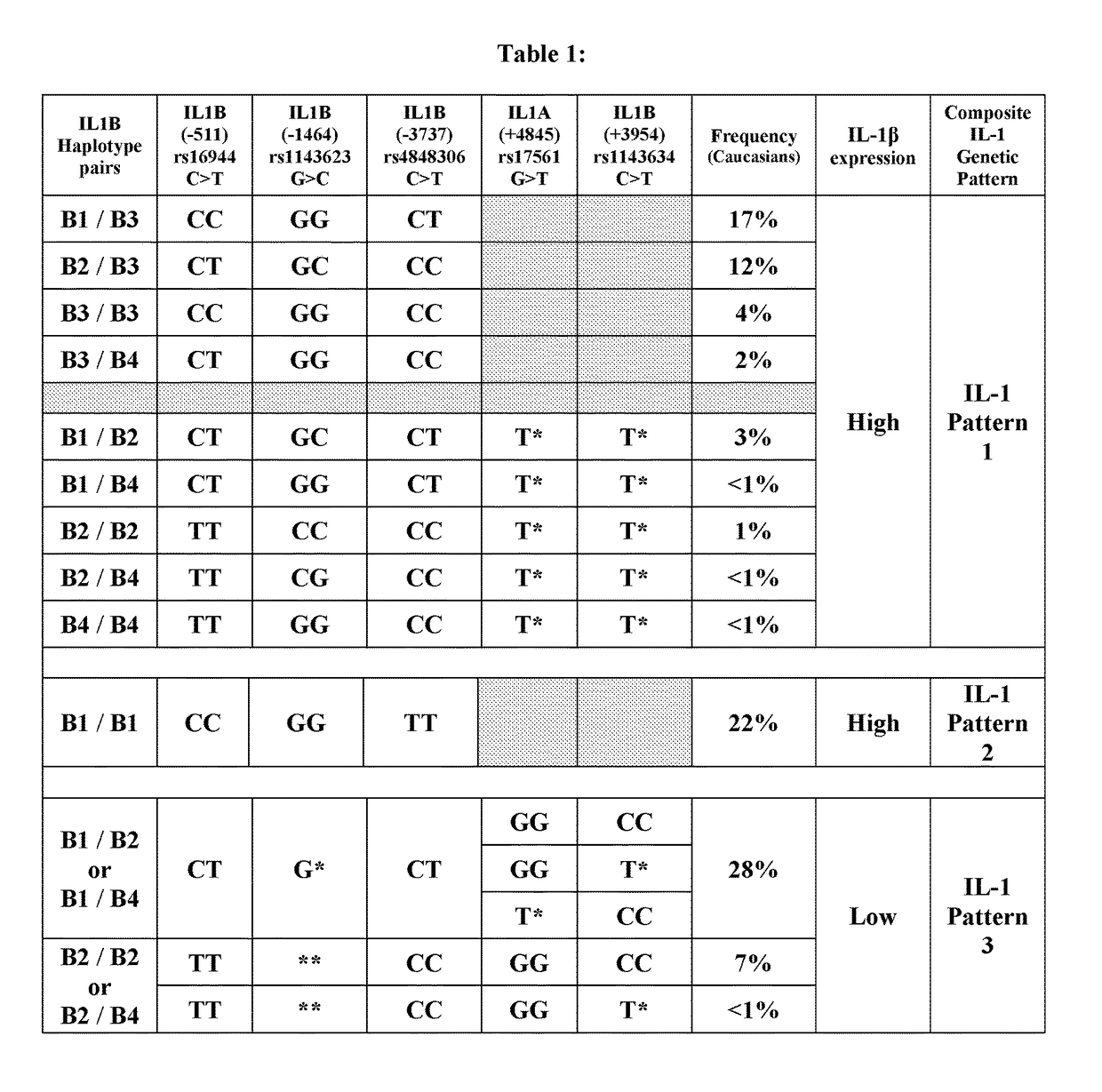
The present invention provides stratification methods and kits to identify whether an individual having an inflammation-related disorder has genotype-specific differential expression of IL-1, i.e., is a “high” or “low” producer of IL-1, such that s / he can be provided an appropriate anti-inflammatory drug and at an appropriate dose. The stratification of high or low IL-1 producers is used to guide assignment of different drug doses and therefore reduce drug toxicity and adverse events in an individual who, without stratification, may be prescribed a higher dose than needed to manage the primary indication. The use of IL-1 stratification of drug dosing would allow a better clinical benefit-risk appraisal in an individual patient.
Owner:SITOKINE LTD
Materials and methods for differential treatment of cancer
InactiveUS20150044224A1Treating and delaying onset and relapsePeptide librariesNucleotide librariesEfficacyOncology
The present invention concerns differential therapeutic treatment of cancer patients based on prognostic antigen / antibody profiles used for predicting (prognosticating) a clinical response (efficacy) and / or adverse event to an immunotherapy for treatment of a malignancy in a subject, and for treating or delaying the onset or relapse of a malignancy in a subject.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC +1
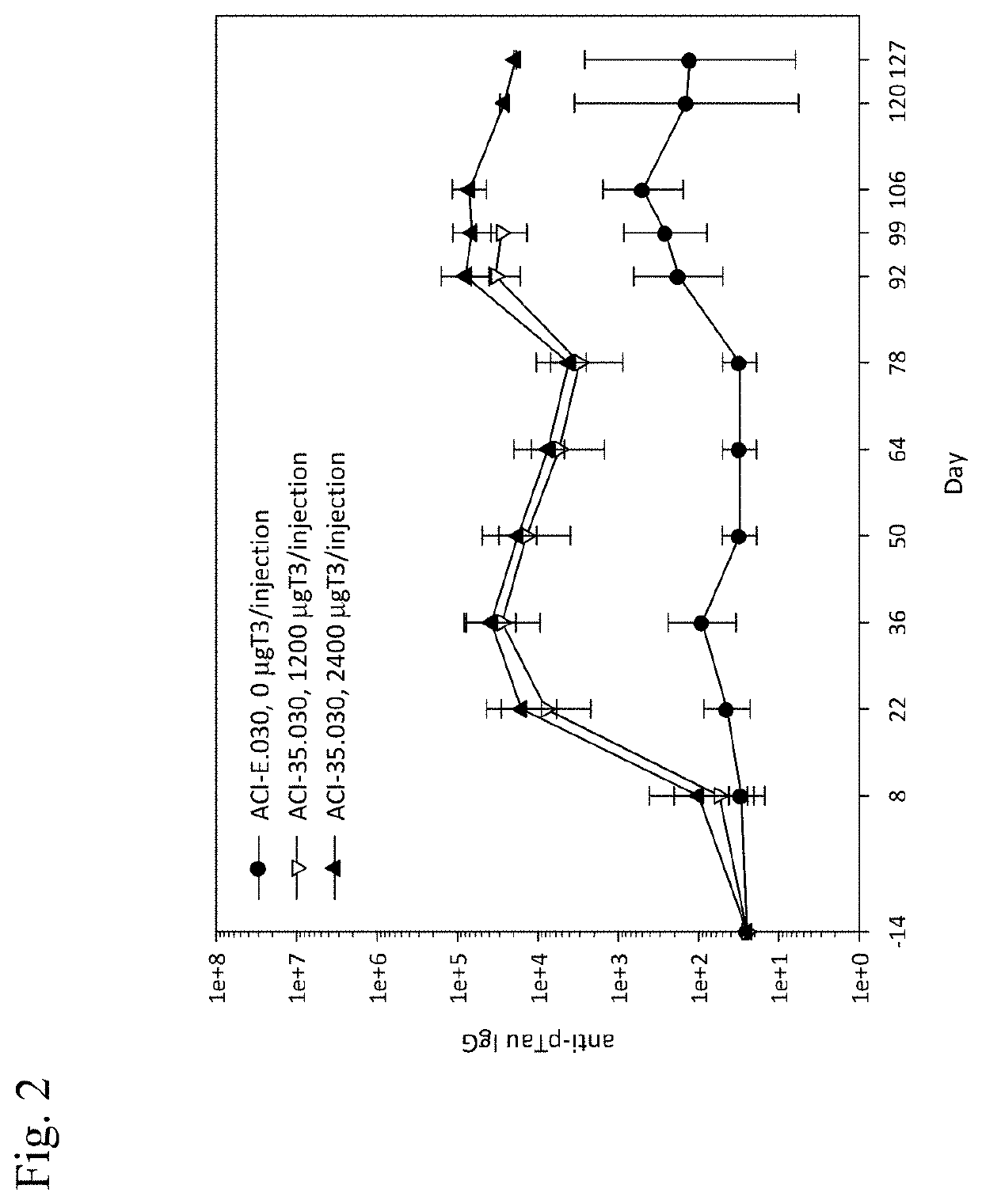
Method of safe administration of phosphorylated tau peptide vaccine
ActiveUS20200253873A1Improve toleranceNervous disorderNervous system antigen ingredientsLipofectamineAntiendomysial antibodies
Methods for inducing anti-phosphorylated Tau antibodies without inducing a severe adverse event in humans are described. The methods include administering to the subject an effective amount of liposomes including a toll-like receptor 4 agonist and a Tau phosphopeptide presented on the surface of the liposome.
Owner:JANSSEN PHARMA INC +1
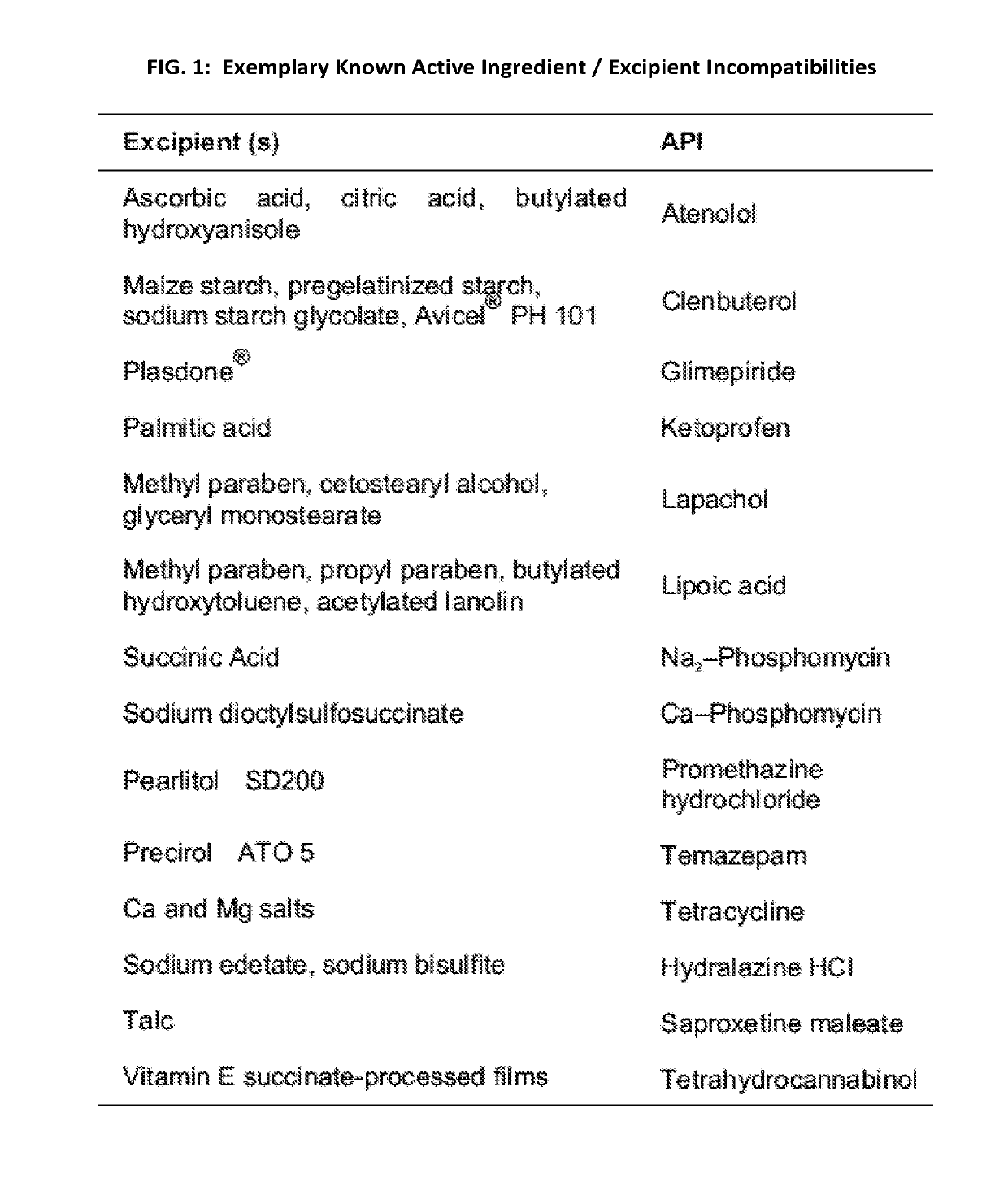
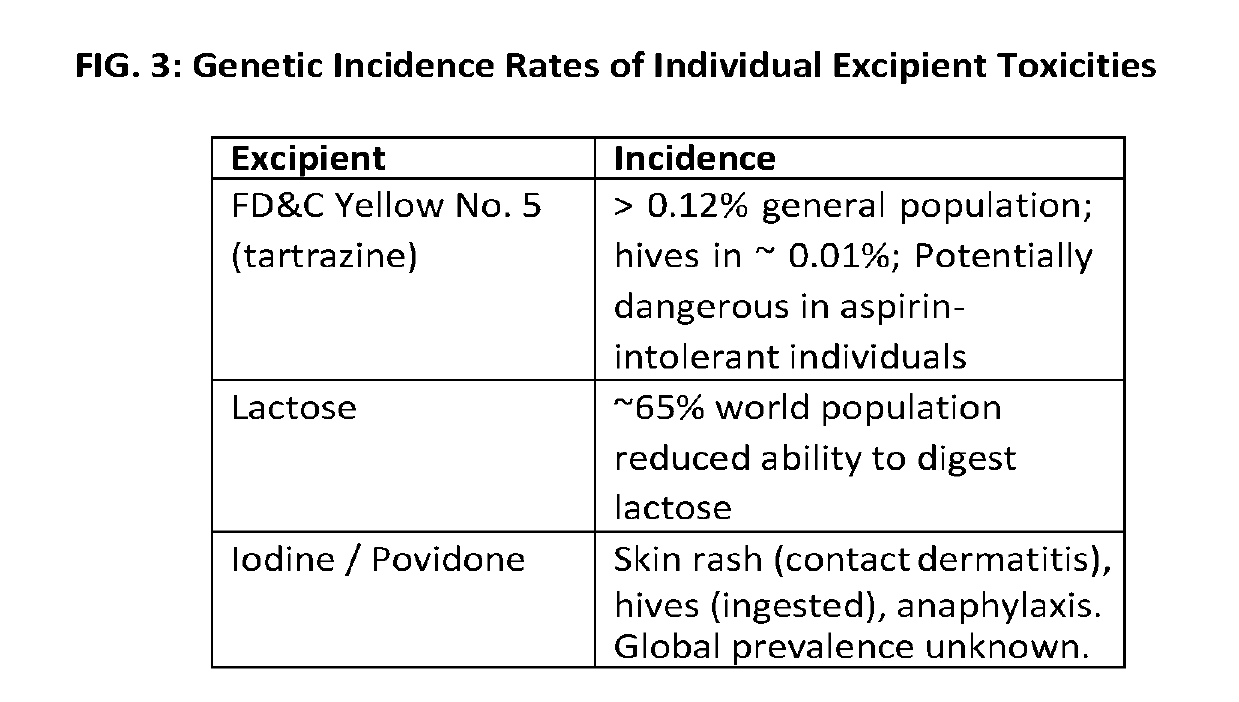
Methods of attenuating drug excipient cross reactivity
InactiveUS20190154648A1Pharmaceutical delivery mechanismTesting medicinal preparationsChemical compositionPharmaceutical drug
The present application relates generally to novel pharmaceutical drug formulations comprising modified chemical compositions having reduced capacity for excipient mediated cross reactivity. Methods for their use and methods for identifying excipient related incompatibilities and / or adverse events are also disclosed.
Owner:CHESAPEAKE THERAPEUTICS LLC
Methods for managing adverse events in patient populations requiring transfusion
ActiveUS20200276234A1Reduce riskReduced stored bloodOther blood circulation devicesAntipyreticPhysical medicine and rehabilitationPharmacy medicine
Owner:HEMANEXT INC
Individual and cohort pharmacological phenotype prediction platform
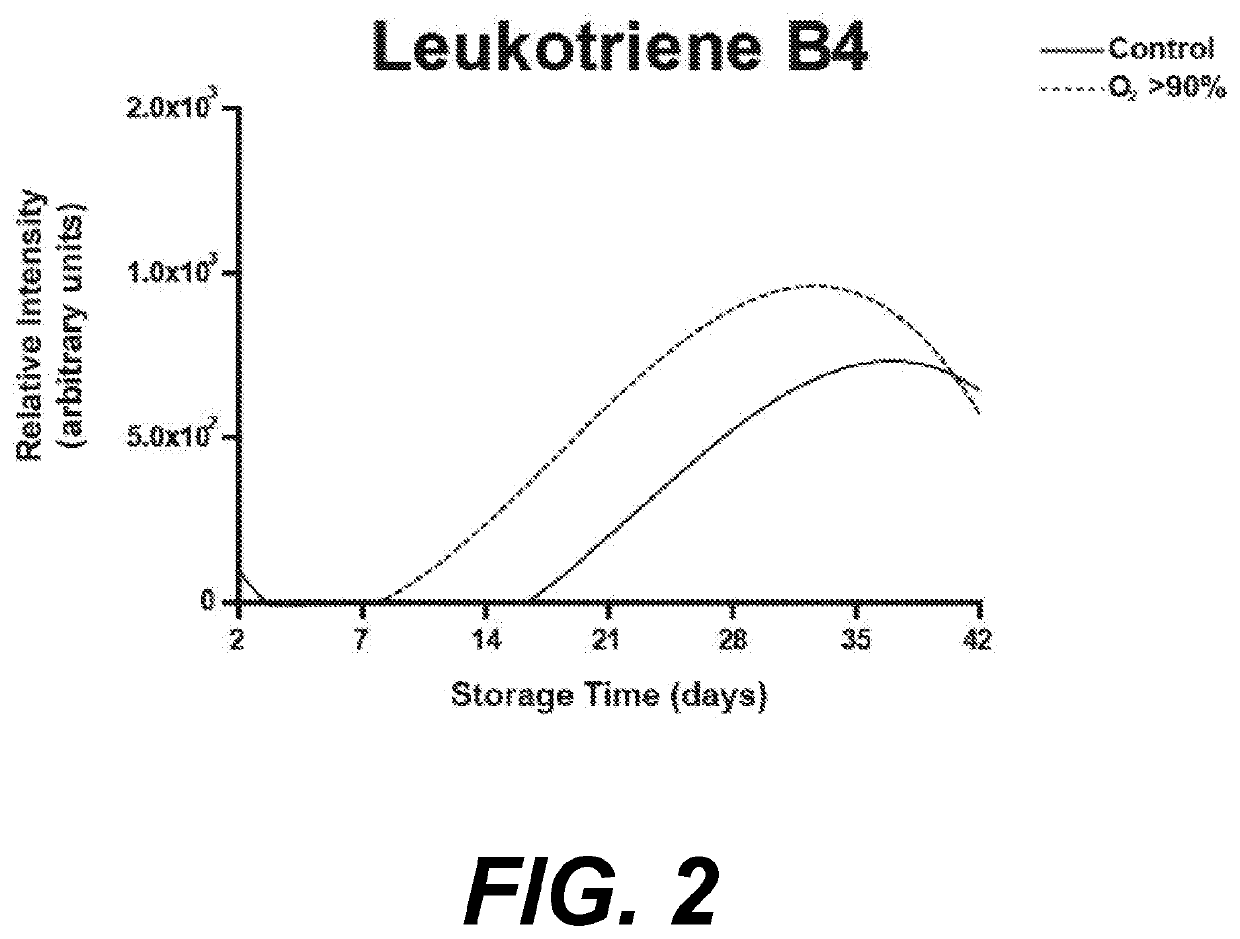
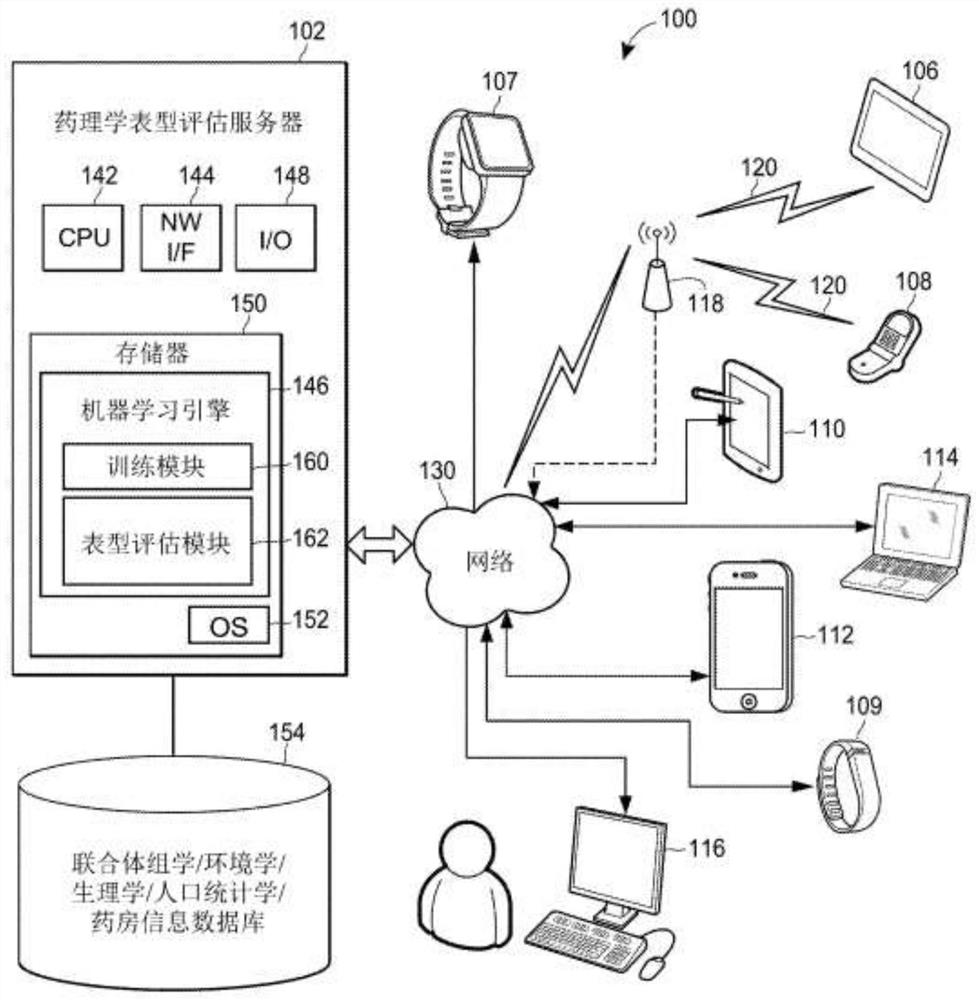
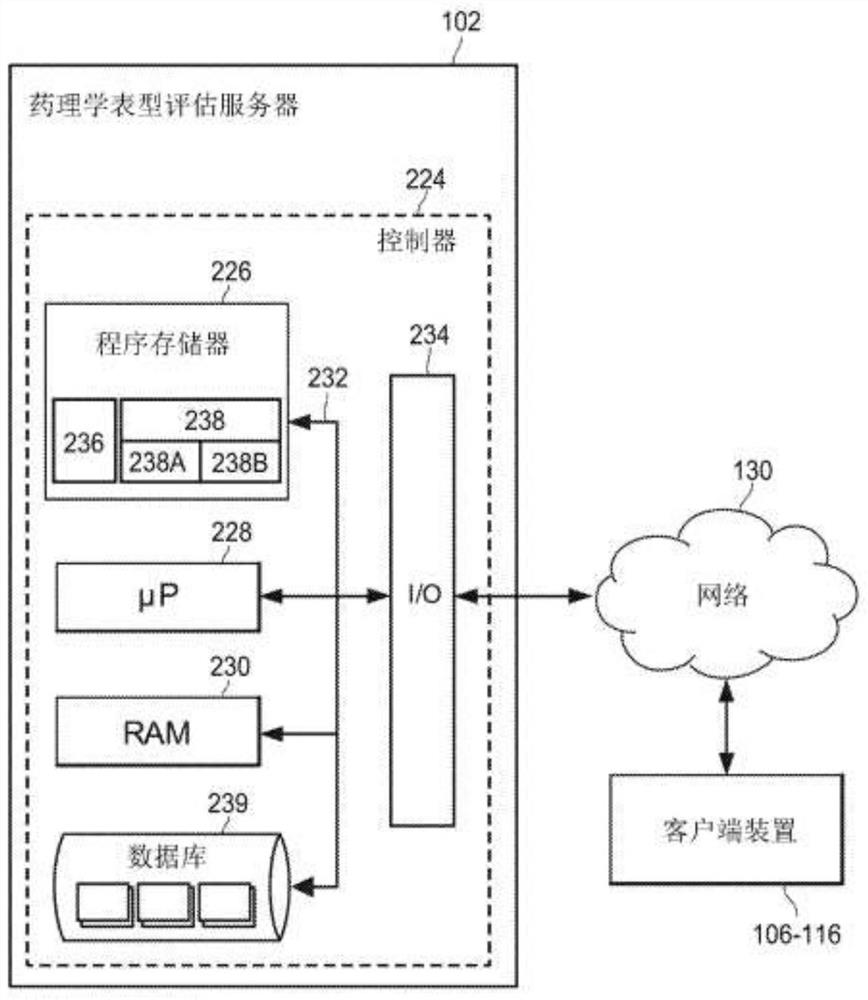
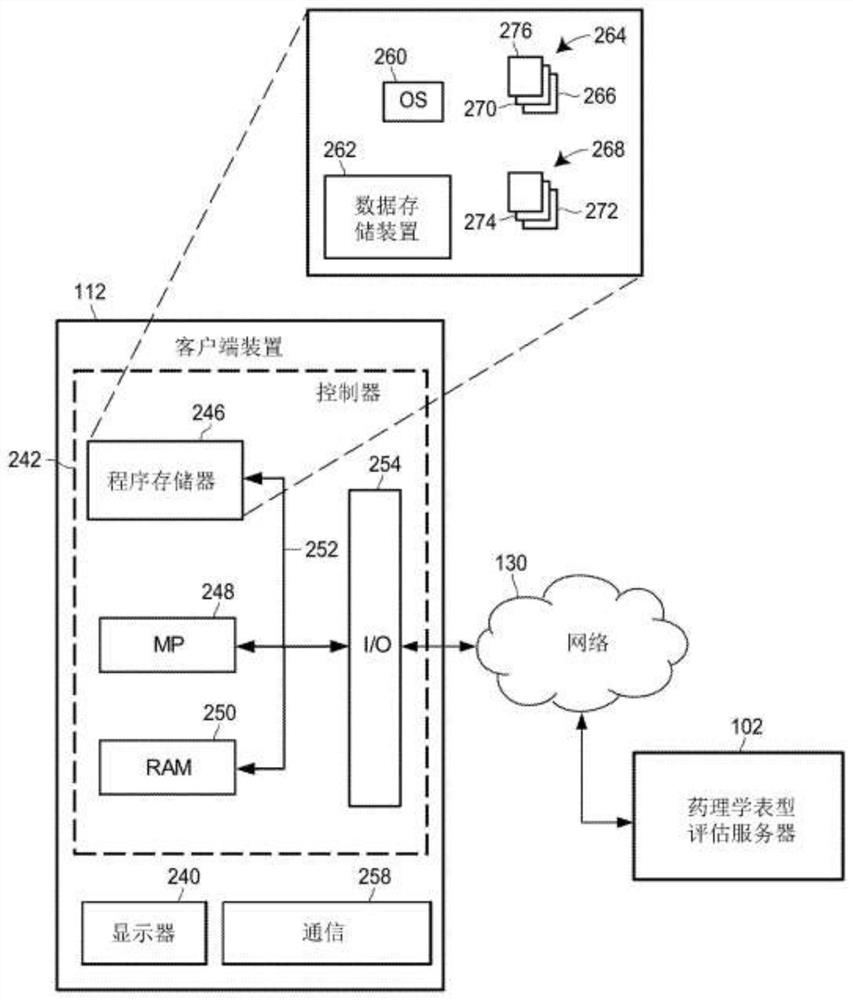
PendingCN111742370AIdentification is accurate and validMedical data miningHealth-index calculationSubstance abuserIdiopathic disease
For patients who exhibit or may exhibit primary or comorbid disease, pharmacological phenotypes may be predicted through the collection of panomic, physiomic, environmental, sociomic, demographic, andoutcome phenotype data over a period of time. A machine learning engine may generate a statistical model based on training data from training patients to predict pharmacological phenotypes, includingdrug response and dosing, drug adverse events, disease and comorbid disease risk, drug-gene, drug-drug, and polypharmacy interactions. Then the model may be applied to data for new patients to predict their pharmacological phenotypes, and enable decision making in clinical and research contexts, including drug selection and dosage, changes in drug regimens, polypharmacy optimization, monitoring,etc., to benefit from additional predictive power, resulting in adverse event and substance abuse avoidance, improved drug response, better patient outcomes, lower treatment costs, public health benefits, and increases in the effectiveness of research in pharmacology and other biomedical fields.
Owner:RGT UNIV OF MICHIGAN
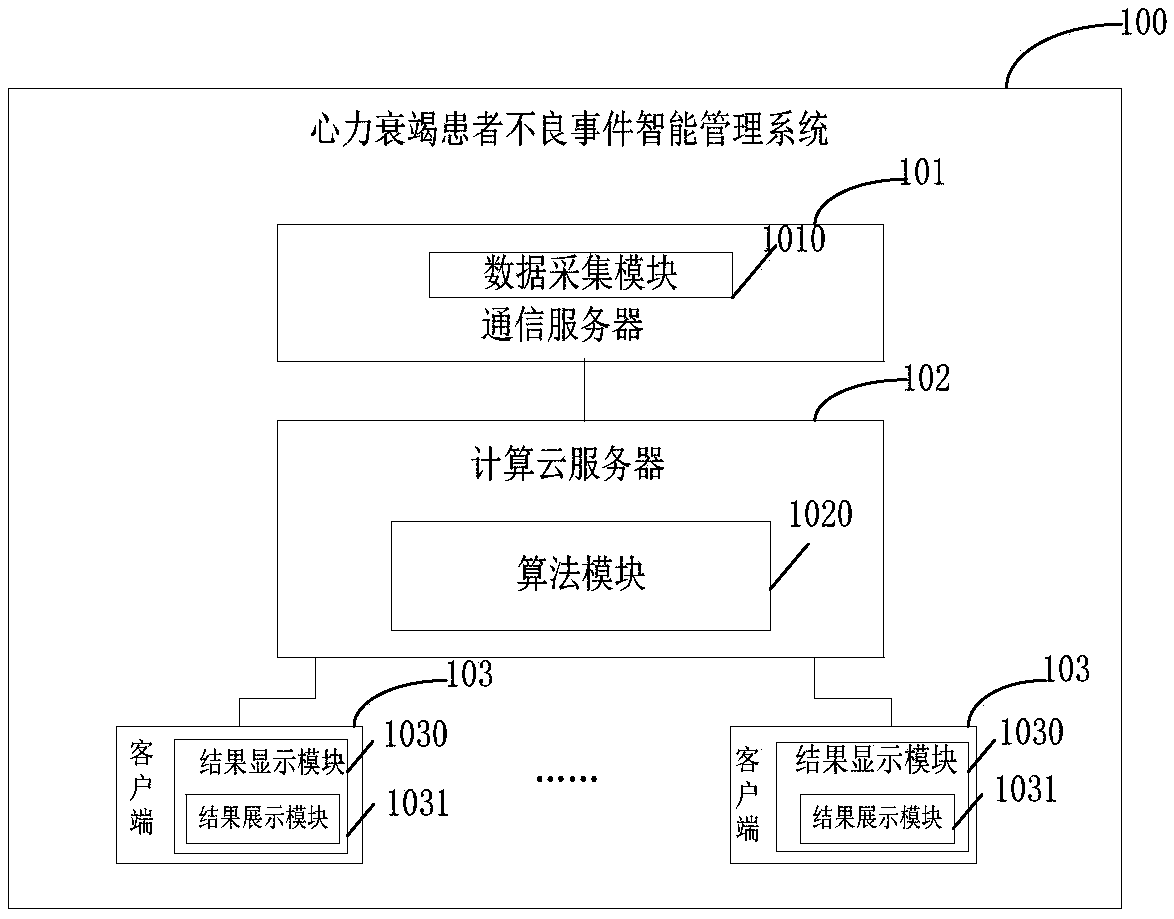
Intelligent management system and method for adverse events involving patients with heart failure
ActiveCN109192312AImprove the intelligent management process and levelImprove the level ofHealth-index calculationCommunications serverData input
The invention relates to an intelligent management system and method for adverse events involving patients with heart failure and belongs to the field of medical data management. The system comprisesa communication server, a computing cloud server, and one or more clients connected to the computing cloud server; the communication server comprises a data collection module for collecting the data of the patients with heart failure; the computing cloud server comprises an algorithm module which is used for modeling the data of the patient through using a DCC algorithm so as to obtain the analysis result of an adverse event model; and the result display module of the client is used for result output or data input and comprises a result demonstration module used for analysis result output or score input. With the intelligent management system and method of the invention adopted, a defect that an existing system and method fail to manage the adverse events involving patients with heart failure can be eliminated, and the standardization and improvement of the process and level of the intelligent management of the patients with heart failure can be benefitted.
Owner:GENERAL HOSPITAL OF PLA
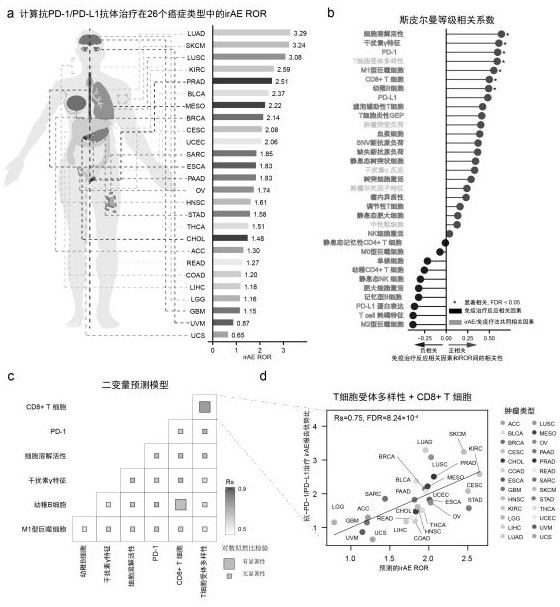
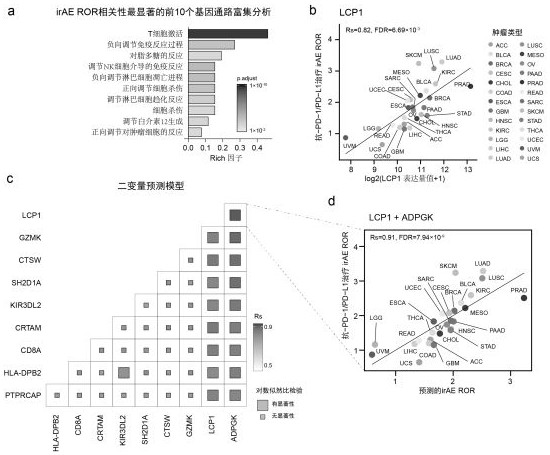
Markers of immune-related adverse events and applications thereof
ActiveCN111733253AImproved prognosisMicrobiological testing/measurementBiological material analysisAntiendomysial antibodiesAntibody therapy
The invention relates to markers of immune-related adverse events and applications thereof. The markers include LCP1 and / or ADPGK. Through the adoption of the biomarkers of the immune-related adverseevents and applications thereof, a kit and method for performing early prediction and active intervention on the immune-related adverse events caused by anti-PD-1 / PD-L1 antibody therapy can be provided, and therefore, the prognostic effects of patients can be enhanced.
Owner:BEIJING XINNUO WEIKANG TECH CO LTD
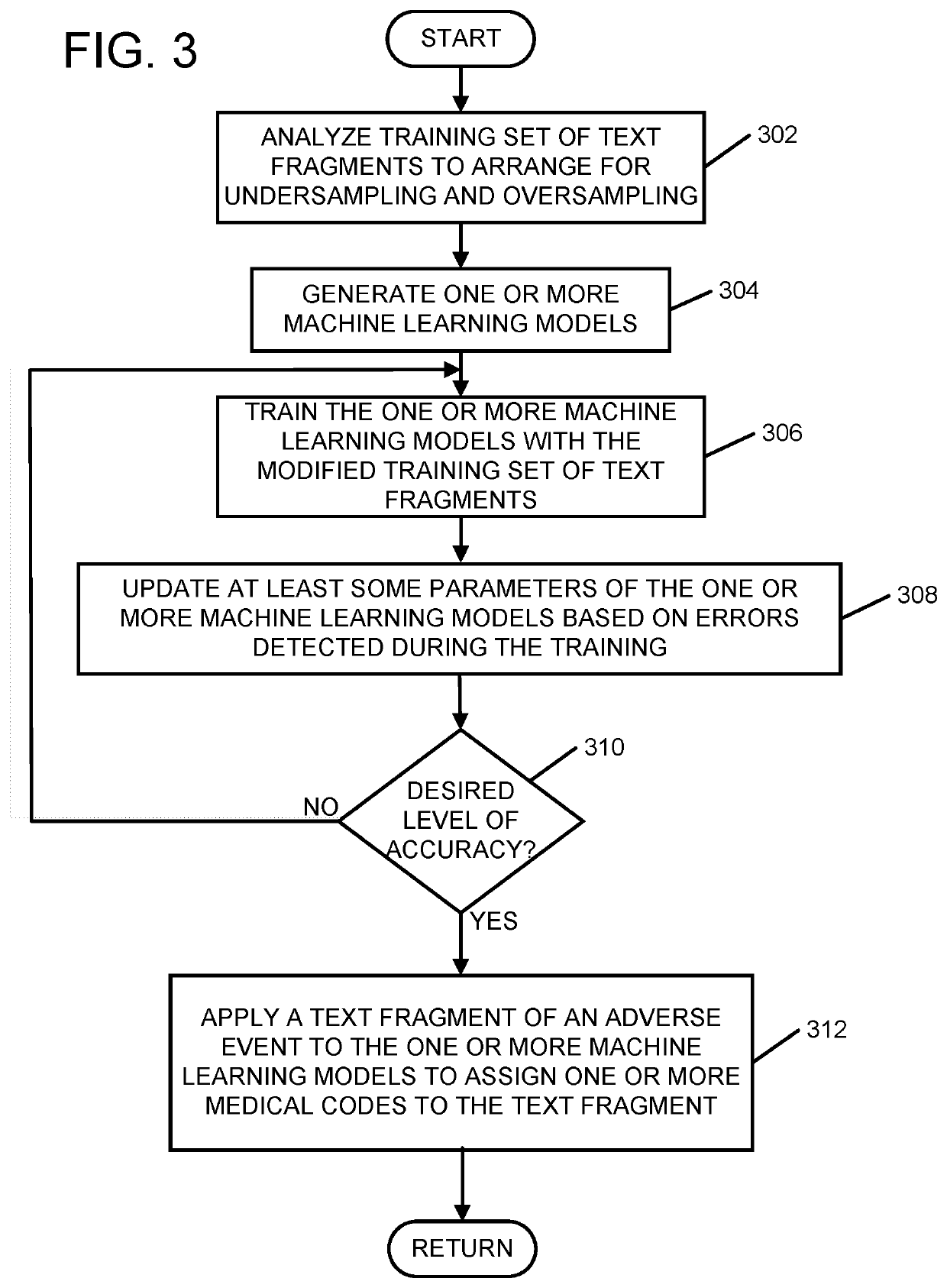
Automatic classification of adverse event text fragments
A method, a system, and a computer program product are provided. A training set of adverse event text fragments assigned to medical codes is analyzed to determine first text fragments having frequently occurring medical code assignments and second text fragments having infrequently occurring medical code assignments. The training set is modified to undersample the first text fragments and to oversample the second text fragments such that the text fragments of the modified training set correspond to a substantially uniform assignment of the medical codes. At least one machine learning model is generated and trained with the modified training set. Some parameters of the at least one machine learning model are updated based on errors detected during the training. After completing the training, an adverse event text fragment is applied to the at least one machine learning model to assign at least one medical code.
Owner:IBM CORP
In Vitro Cell-based Methods for Biological Validation and Pharmacological Screening of Chemical Entities and Biologicals
This patent describes a novel in vitro cell-based method for biological validation and pharmacological screening of drugs, new chemical entities (NCEs) and biologics, which is predictive of in vivo testing for efficacy and adverse events in patients, as occurs in clinical trials. The same method can be used to create an in vitro cell-based assay to identify the ‘right marketed medication for the right patient’ (personalized medicine), and to identify responders and non-responders in ongoing clinical trials with NCEs.
Owner:MOHANLAL RAMON W
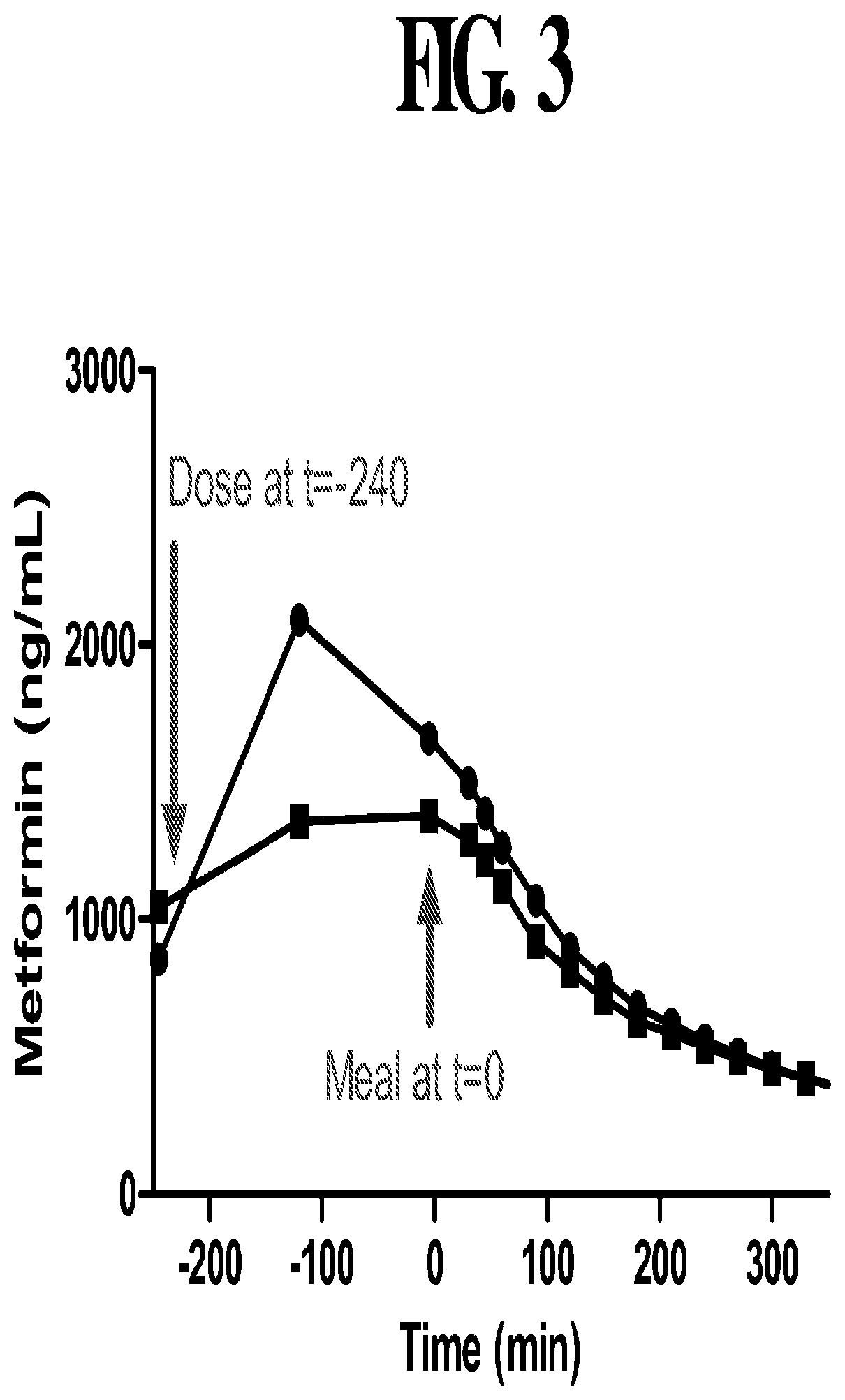
Compositions and methods for treating metabolic disorders
PendingUS20200093750A1Increased riskOrganic active ingredientsMetabolism disorderMetabolic disorderBiguanide
Compositions and methods for improving the pharmacokinetics and reducing the risk of adverse events resulting from biguanide compound administration are provided, comprising administering delayed release formulations of such compounds having a lag phase release.
Owner:ANJI PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com