Markers of immune-related adverse events and applications thereof
An adverse reaction, immune-related technology, applied in the field of biomedicine to achieve the effect of improving the prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1 Experimental method
[0031] (1.1) Data Analysis of FAERS Personal Safety Report
[0032] This invention is obtained from FAERS (https: / / www.fda.gov / drugs / questions-and-answers-fdas-adverse-event-
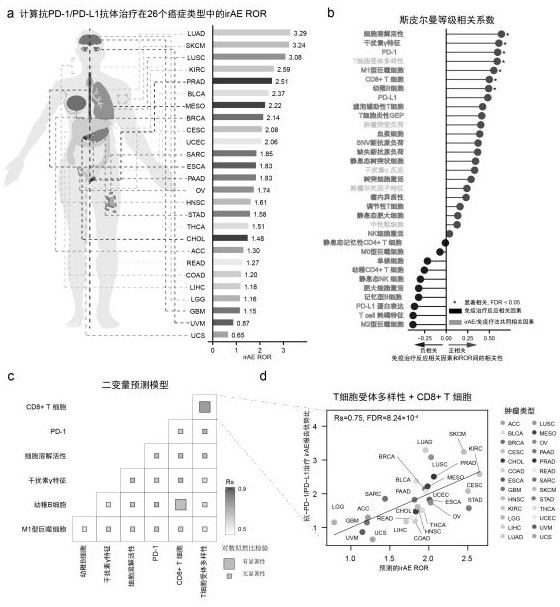
[0033] reporting-system-faers / fda-adverse-event-reporting-system-faers-public-dashboard) to obtain personal security reports from July 1, 2014 to June 30, 2019. Only patients treated with anti-PD-1 antibodies (nivolumab, pembrolizumab, similumab) and anti-PD-L1 (atezolizumab, avelumab, durvalumab) were collected Adverse reaction reports were reported, and patients who also received anti-CTLA-4 antibody therapy (ipilimumab, tremelimumab) were excluded. Use the peer-reviewed irAE management manual as a standard to define irAEs. An unbalanced analysis was adopted, and the entire database was used as a comparison sample to calculate the ROR of irAE risk. Patients who developed any type of irAE were included in the irAE group.
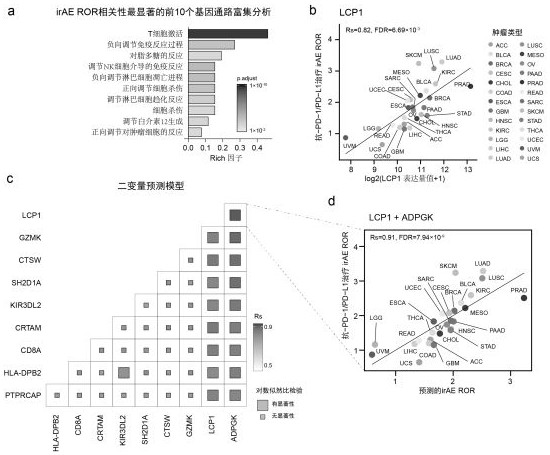
[0034] (1.2) Analysis of TCGA database and i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com