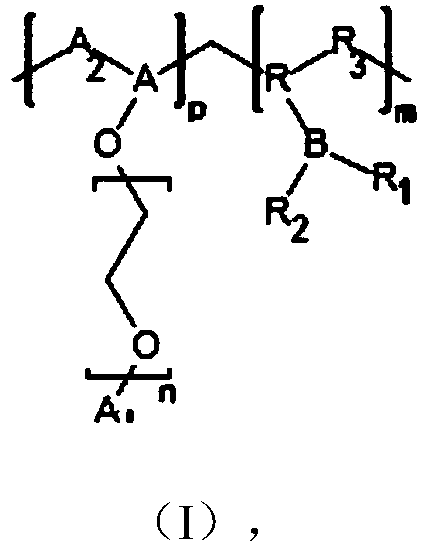
Solid polymer electrolyte containing boron-fluorine structure as well as preparation method and application thereof
A polymer and electrolyte technology, applied in solid electrolytes, non-aqueous electrolytes, electrolyte immobilization/gelation, etc., can solve the problems of low room temperature lithium ion conductivity, poor interface contact, and low conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
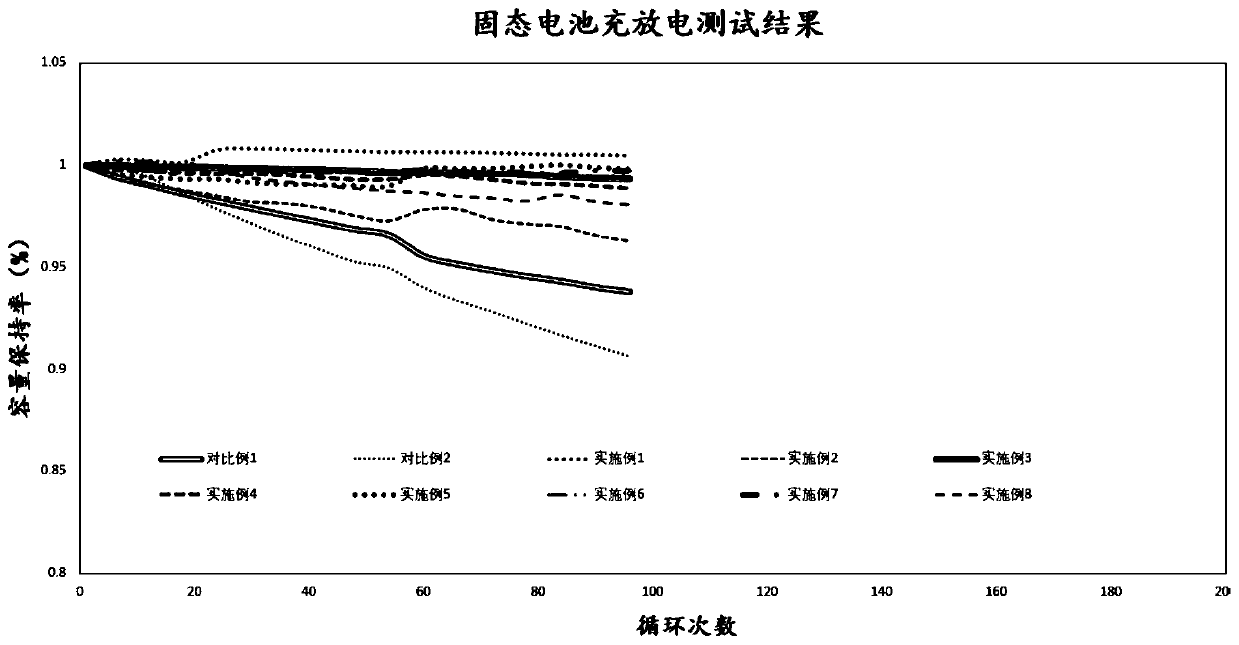
Examples
Embodiment 1
[0072] This embodiment discloses a method for preparing a solid polymer electrolyte, comprising the following steps:
[0073] S1: In parts by weight, 5 parts of Alpha-(trifluoromethyl)ethylene boronic acid, 5 parts of 1-(trifluoromethyl)ethylene hexyl borate, 60 parts of polyethylene glycol dimethacrylate, Add 30 parts of polyethylene glycol phenyl ether acrylate, 0.5 parts of polymethyl methacrylate, and 0.5 parts of polyacrylic acid into 150 parts of acetonitrile and 50 parts of N-methylpyrrolidone, and keep nitrogen or inert gas atmosphere at 200r / Stir at a speed of min for 200 min, then add 0.05 parts of azobisisobutyronitrile, then react at 50°C for 10 h, and obtain polymer system B after purification;
[0074]S2: In parts by weight, 40 parts of polymer system B, 2 parts of lithium oxalate difluoroborate, 1 part of lithium bistrifluoromethanesulfonimide, 2 parts of diboron trioxide doped lithium phosphate, 2 parts Lithium titanium phosphate, 1 part of nano-alumina, 1 pa...
Embodiment 2
[0080] This embodiment discloses a method for preparing a solid polymer electrolyte, comprising the following steps:
[0081] S1: In parts by weight, 20 parts of trans-2-[4-(trifluoromethyl)phenyl]vinylboronic acid, 70 parts of 1-trifluoromethylvinylboronic acid, 10 parts of polyethylene glycol Add methacrylate and 10 parts of polyethylene glycol methacrylate to 300 parts of toluene and 100 parts of N,N-dimethylformamide, keep nitrogen or inert gas atmosphere, and stir at 1000r / min for 100min, Then add 0.1 part of benzoyl peroxide, then react at 120°C for 3 hours, and obtain polymer system B after purification;
[0082] S2: In parts by weight, 70 parts of polymer system B, 15 parts of lithium tetrafluoroborate (LiBF 4 ), 10 parts of lithium 4,5-dicyano-2-trifluoromethylimidazolium (LiDTI), 5 parts of lithium bis(trifluoromethylsulfonyl)imide, 5 parts of lithium silicon phosphate, and 1 part of lanthanum titanate Lithium, add 100 parts of acetone, 200 parts of acetonitrile, u...
Embodiment 3
[0088] This embodiment discloses a method for preparing a solid polymer electrolyte, comprising the following steps:
[0089] S1: In parts by weight, 20 parts of 1-(4-fluorophenyl)vinylboronic acid pinacol ester, 5 parts of trans-2-(3-fluorophenyl)vinylboronic acid, 20 parts of polyethylene Diol methacrylic acid, 10 parts of polyethylene glycol methacrylate, 10 parts of triethylene glycol dimethacrylate, 3 parts of methyl methacrylate, 2 parts of acrylonitrile, 5 parts of polystyrene, add 200 100 parts of benzene and 100 parts of acetonitrile, keep nitrogen or inert gas atmosphere, stir at 800r / min for 80min, then add 0.5 parts of benzoyl tert-butyl peroxide, then react at 100°C for 20h, and obtain polymerization after purification object system B;
[0090] S2: In parts by weight, 80 parts of polymer system B, 20 parts of lithium bisfluorosulfonimide (LiFSI), 5 parts of lithium perchlorate (LiClO 4 ), 5 parts lithium hexafluorophosphate (LiPF 6 ), 5 parts lithium hexafluoro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com