Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Significantly effective" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
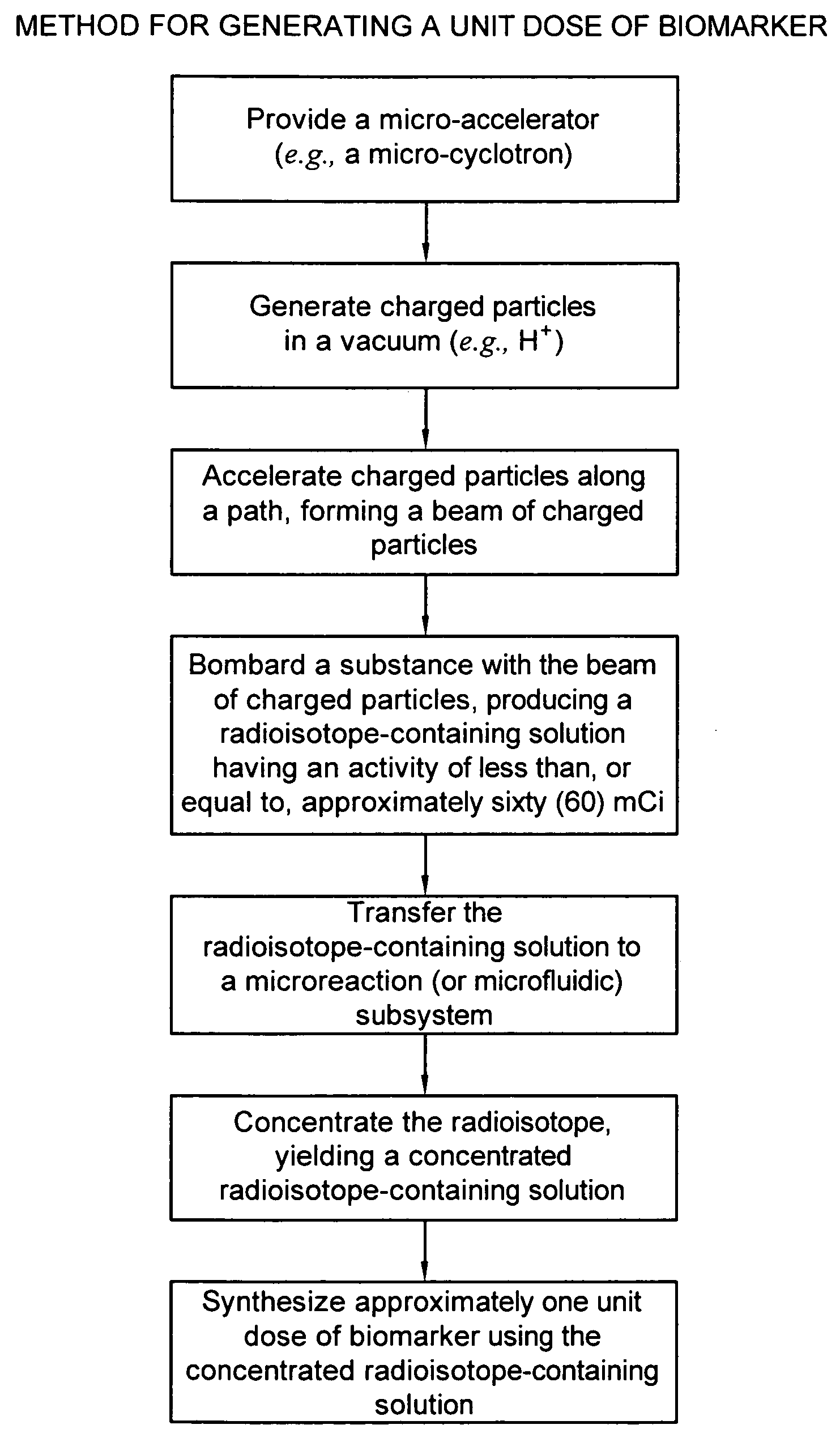
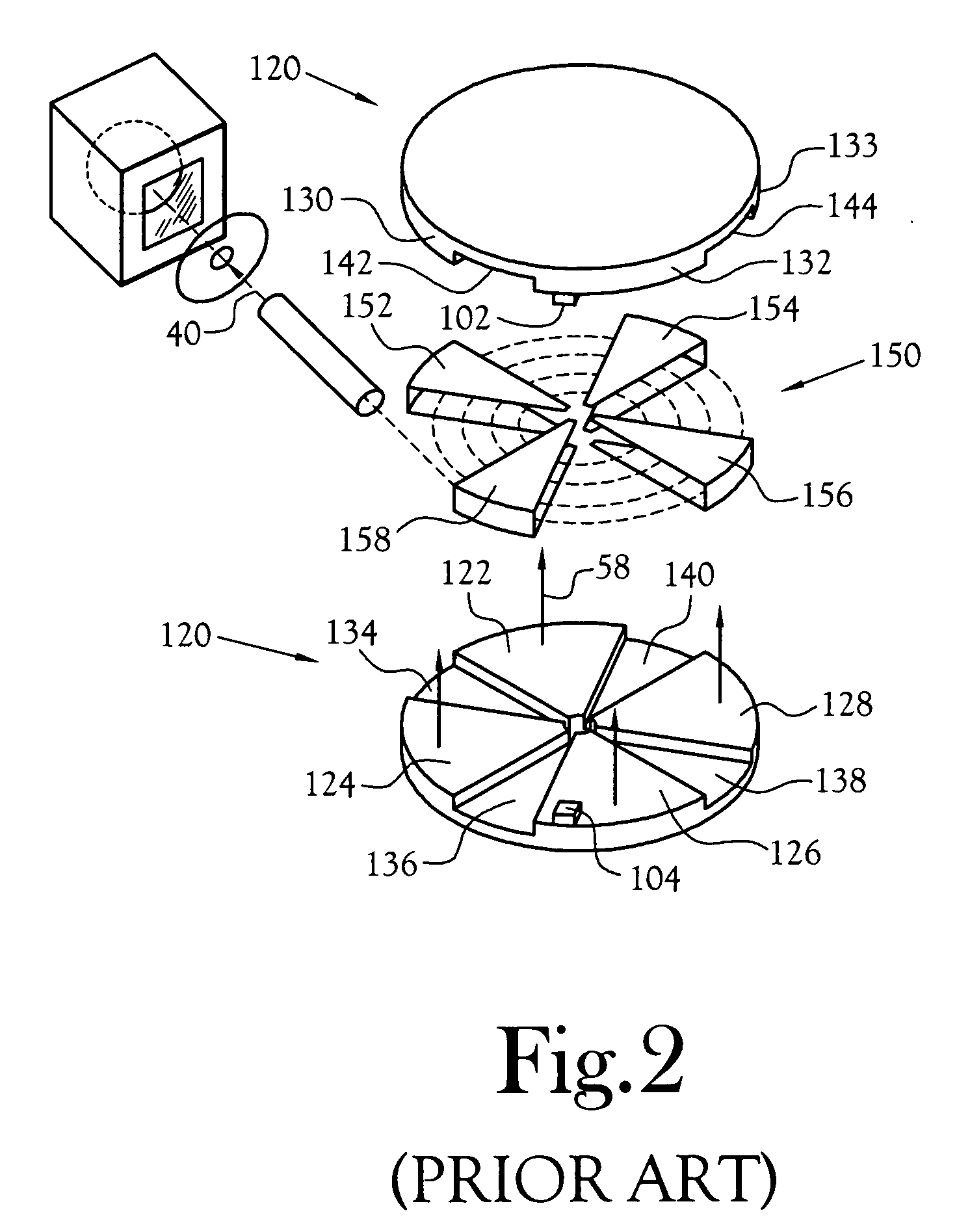
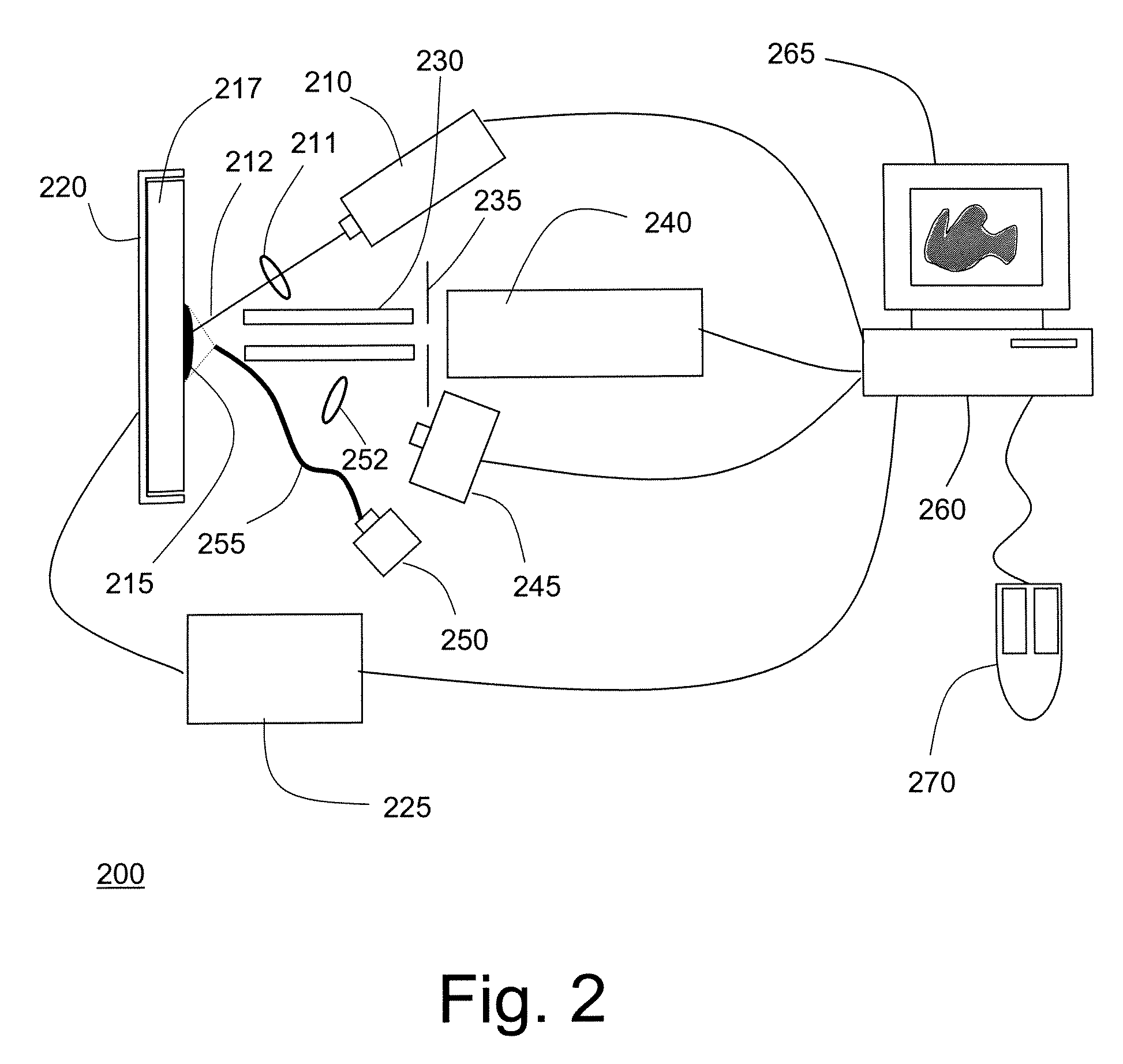
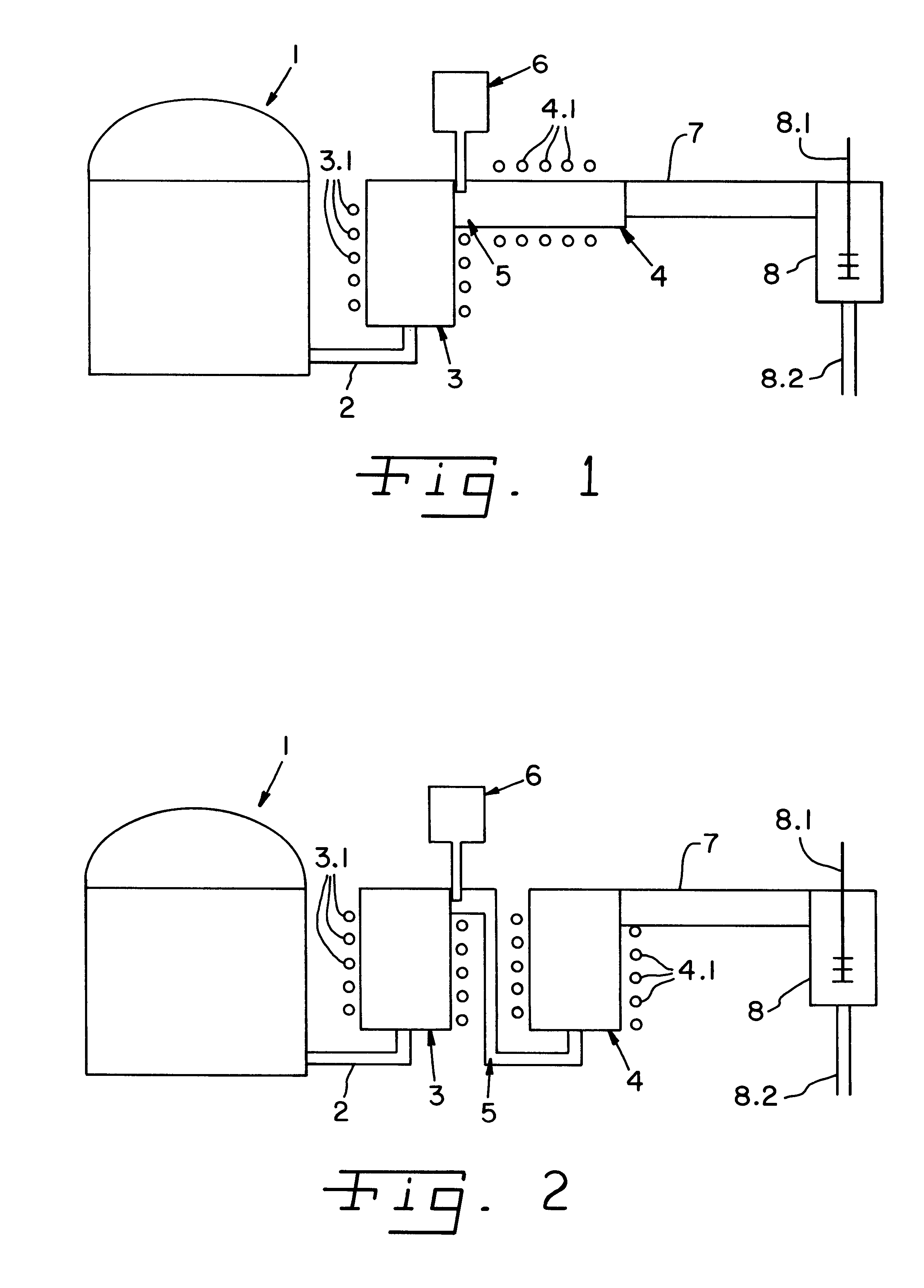
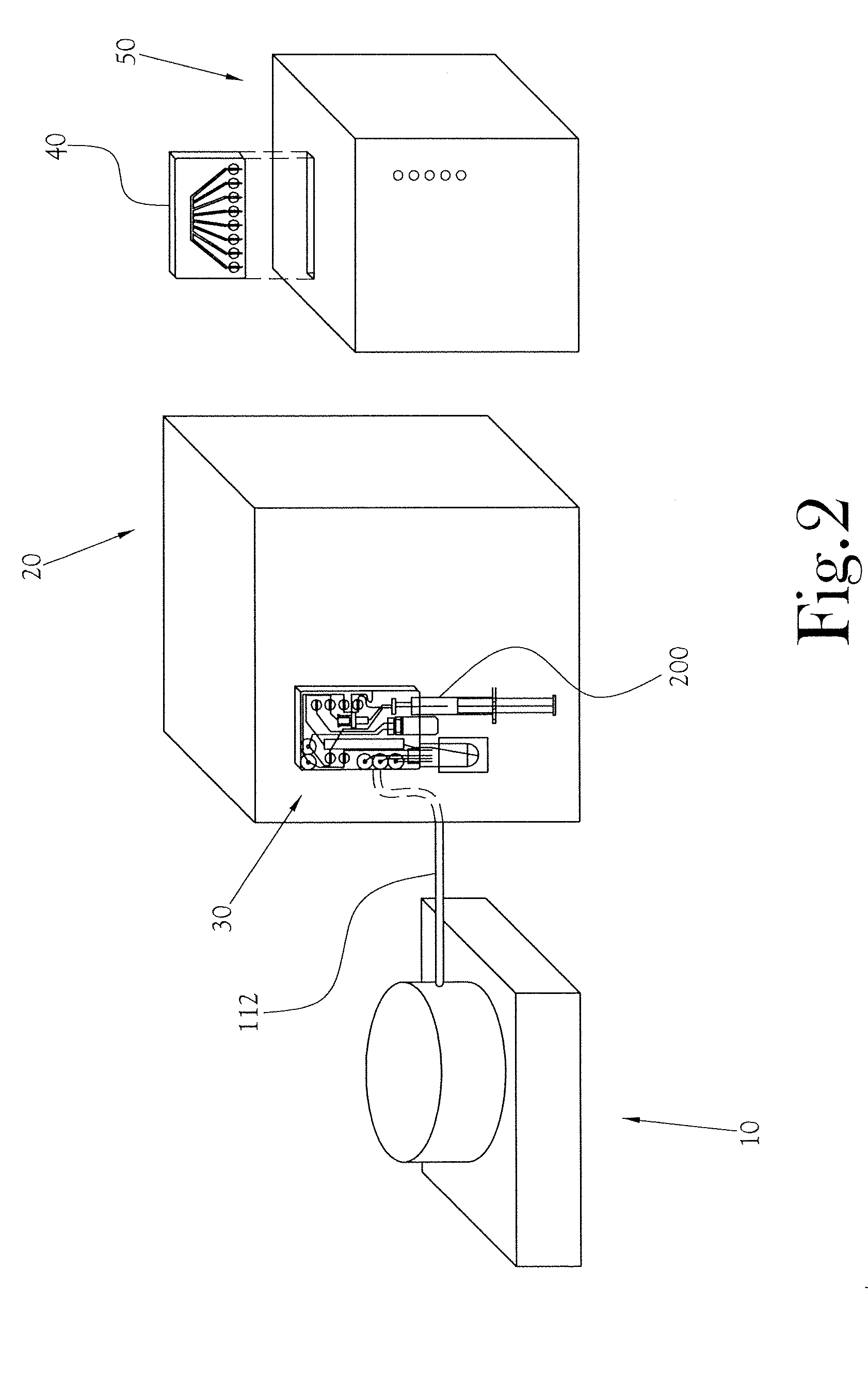
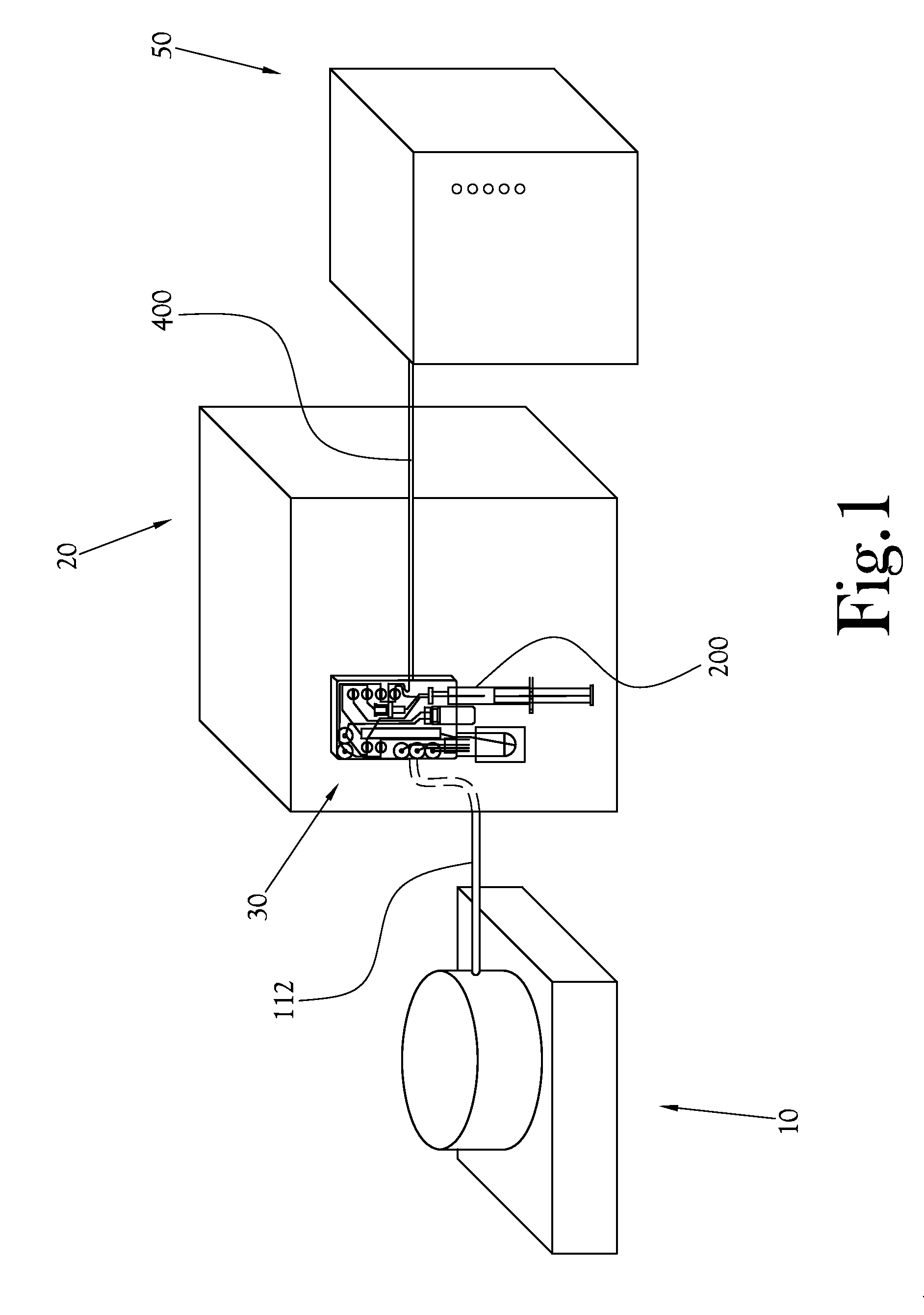
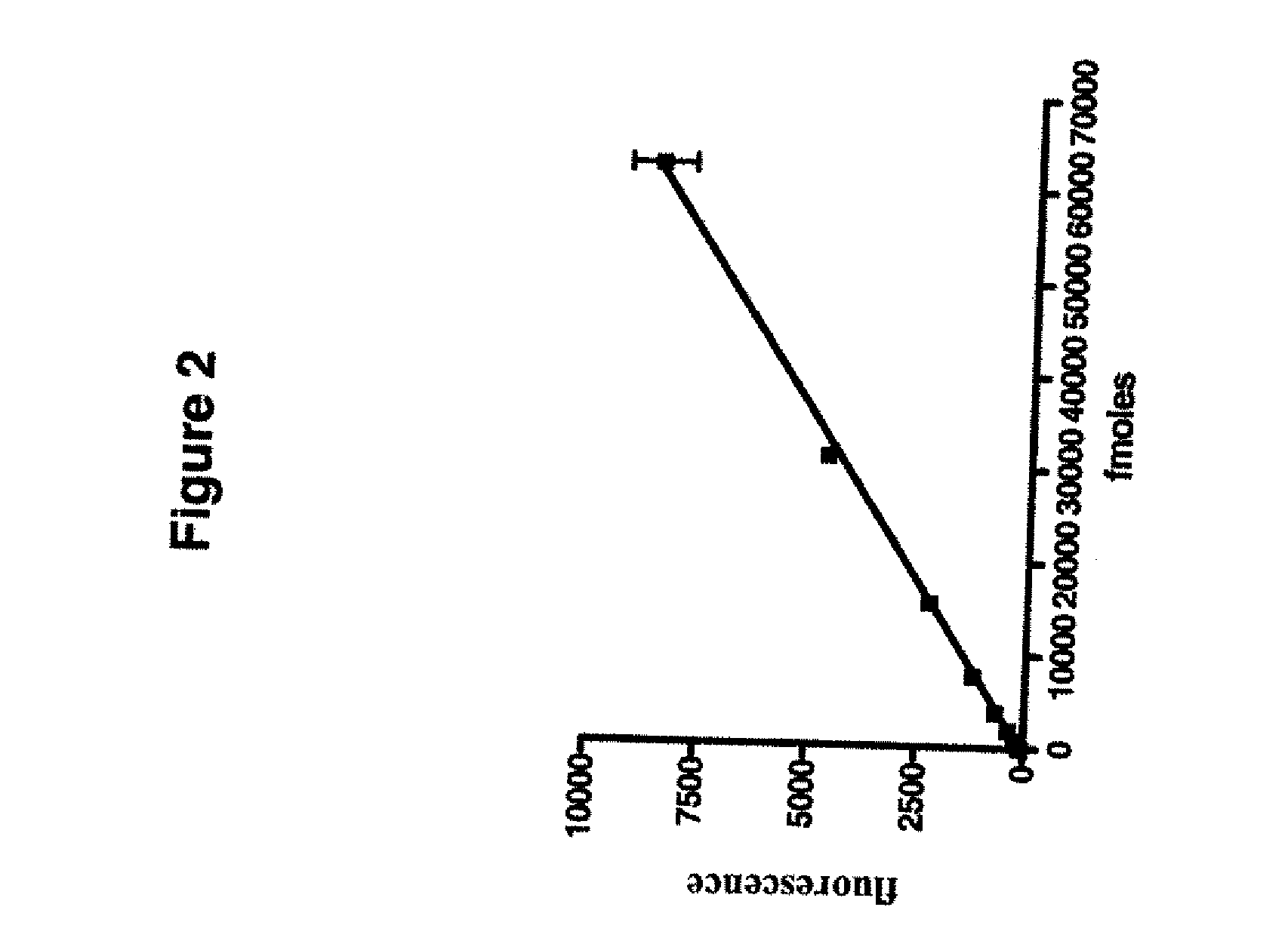
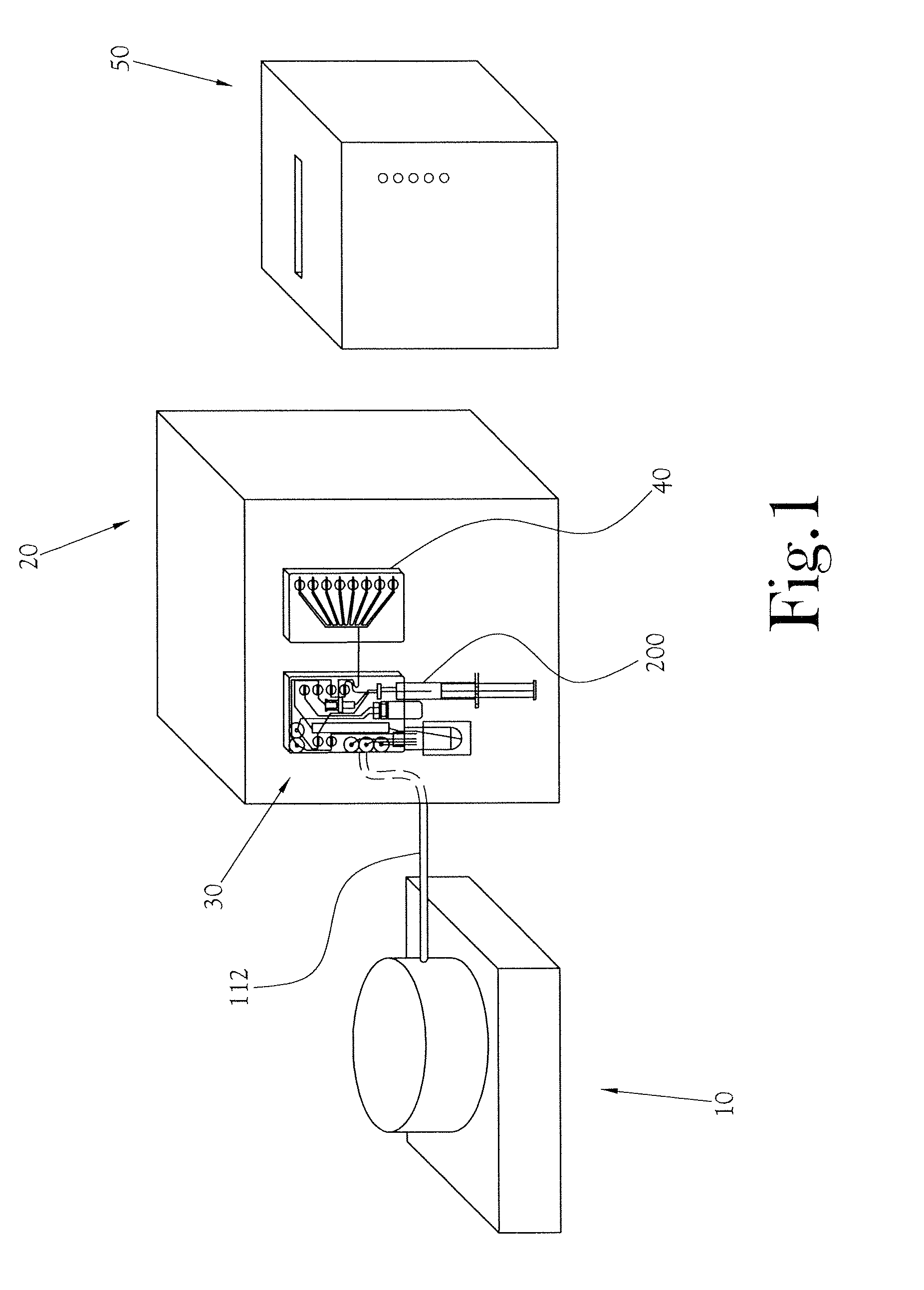
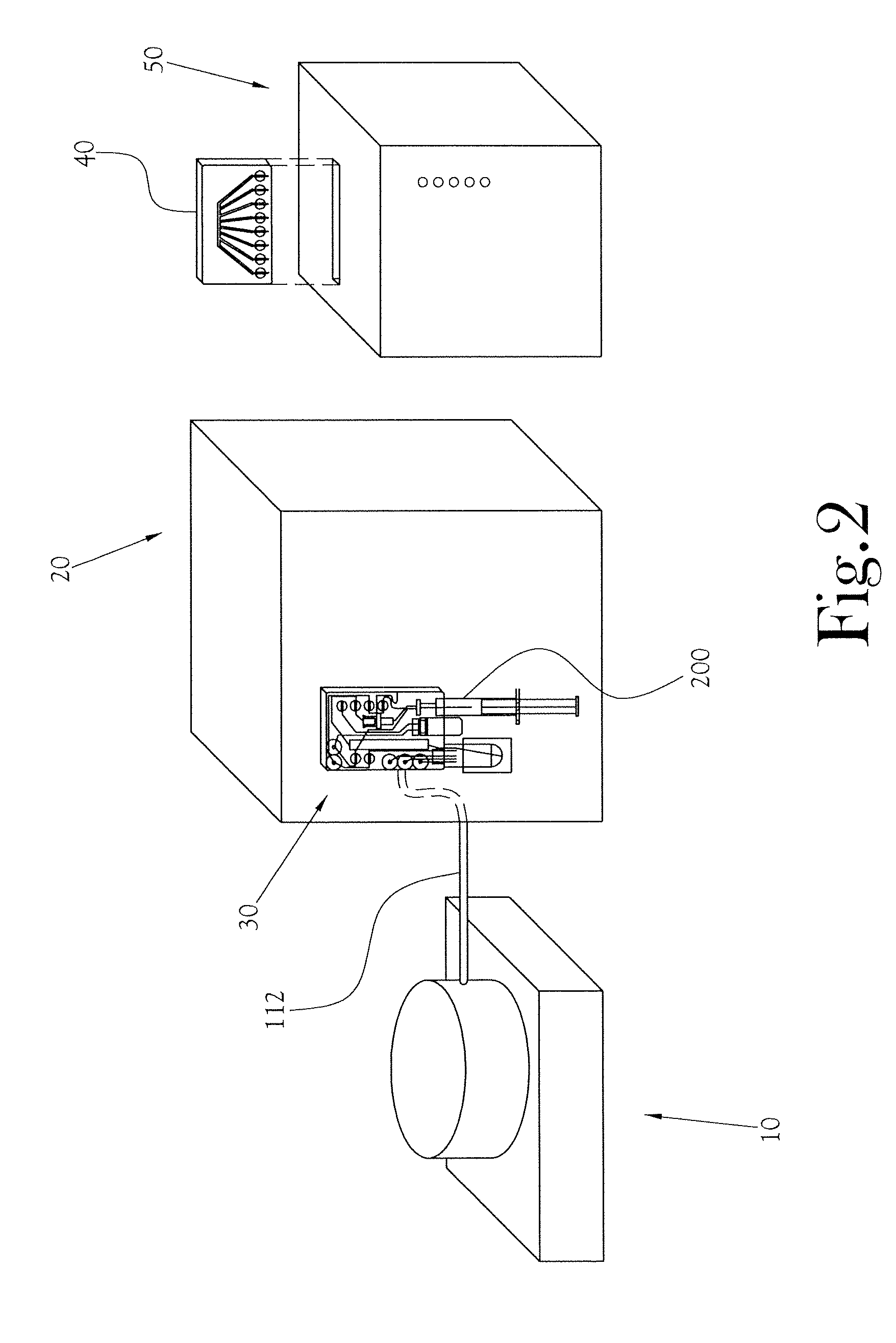
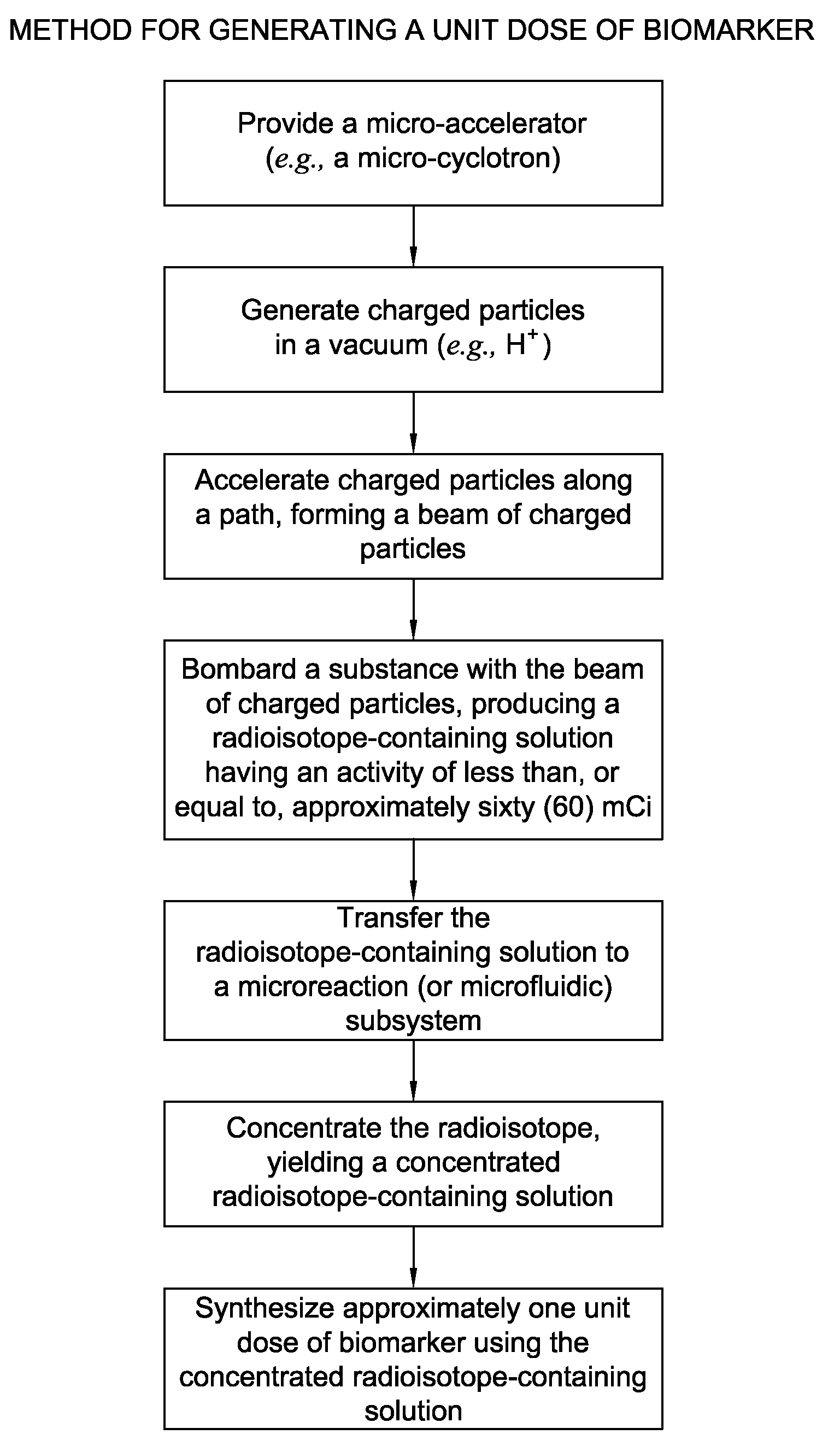
Biomarker generator system
ActiveUS7476883B2Efficient dosingEfficient productionMaterial analysis using wave/particle radiationIsotope delivery systemsChemical synthesisMicroreactor
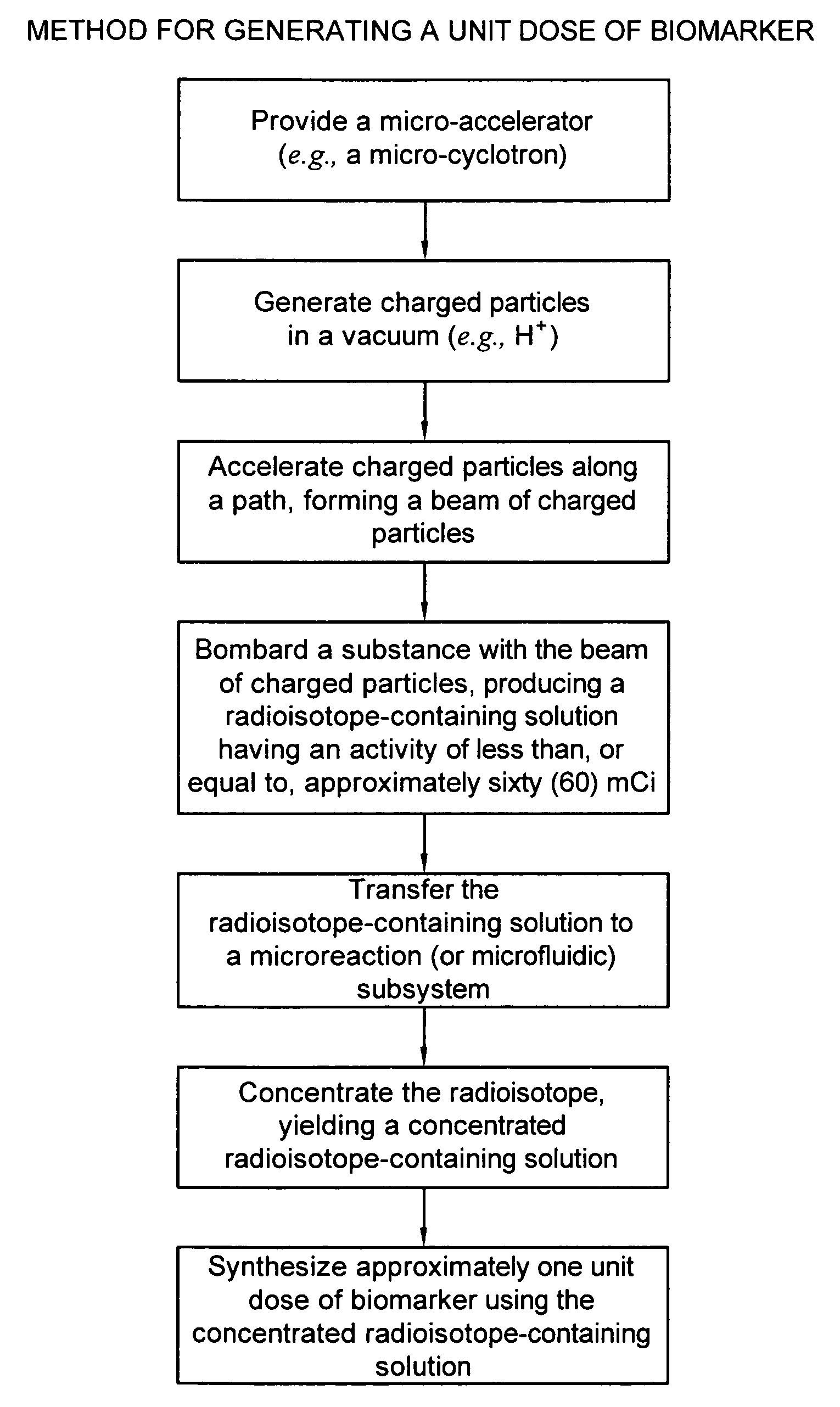
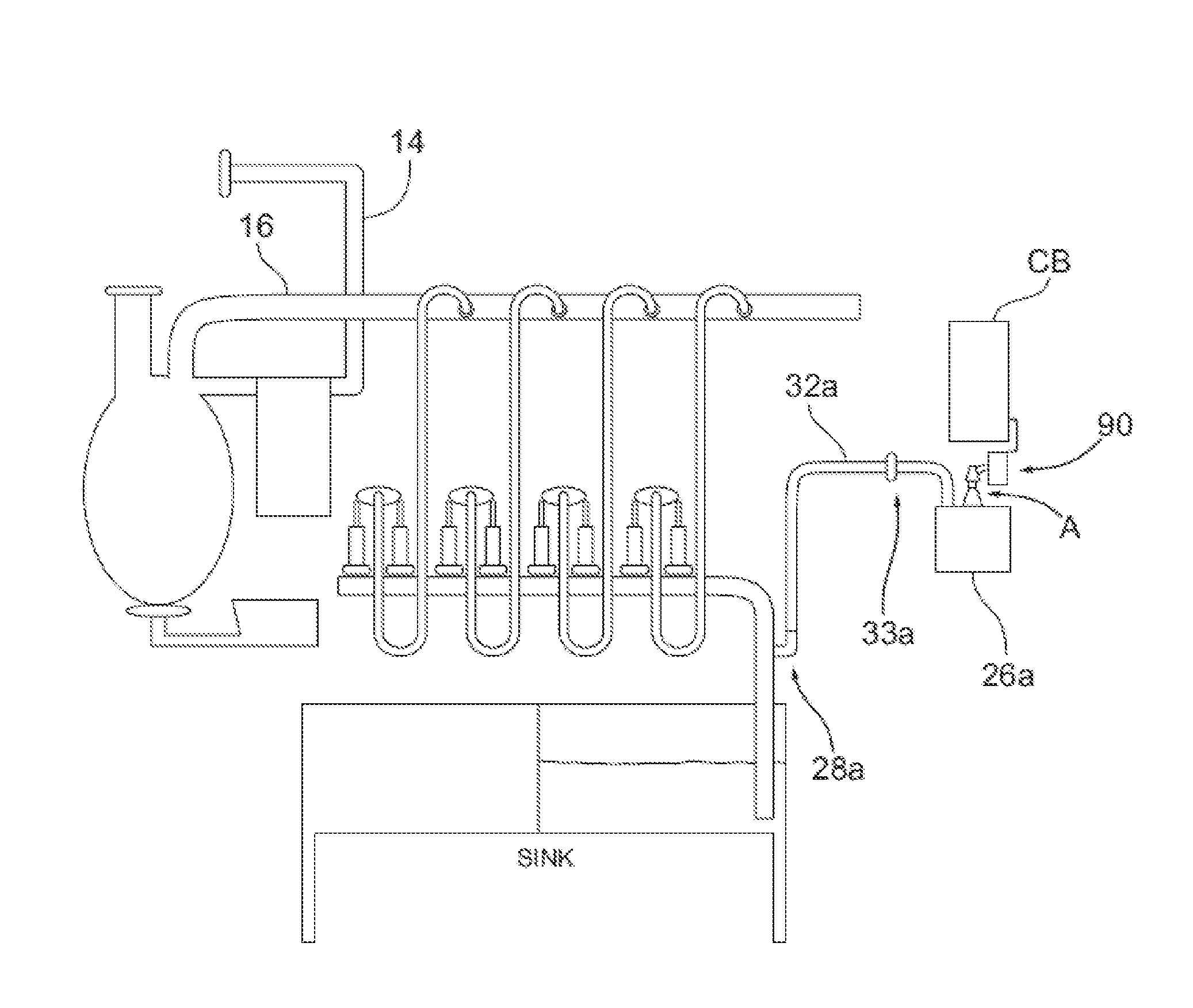
A biomarker generator system for producing approximately one (1) unit dose of a biomarker. The biomarker generator system includes a small, low-power particle accelerator (“micro-accelerator”) and a radiochemical synthesis subsystem having at least one microreactor and / or microfluidic chip. The micro-accelerator is provided for producing approximately one (1) unit dose of a radioactive substance, such as a substance that emits positrons. The radiochemical synthesis subsystem is provided for receiving the radioactive substance, for receiving at least one reagent, and for synthesizing the approximately one (1) unit dose of a biomarker.
Owner:BEST ABT INC
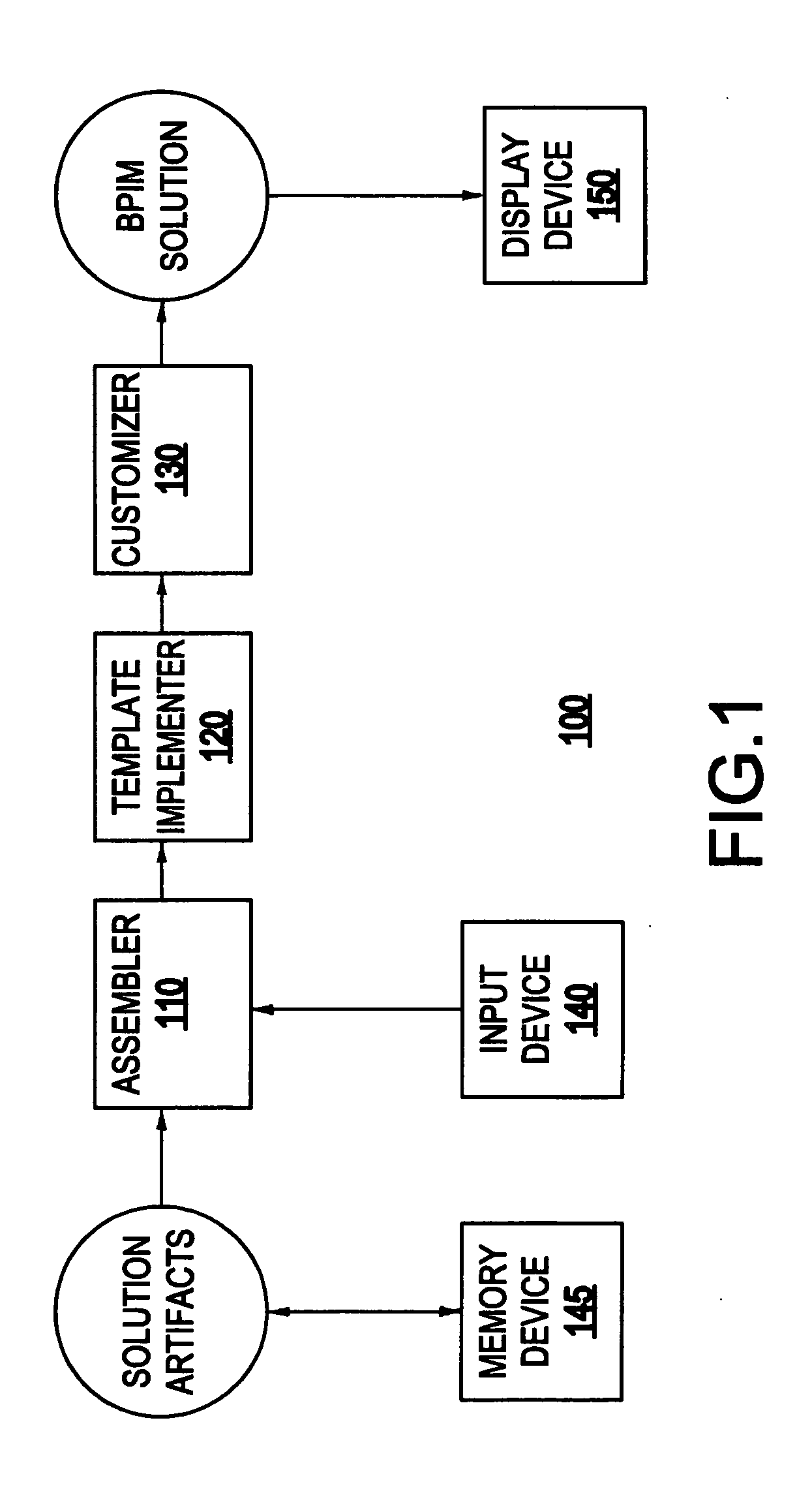
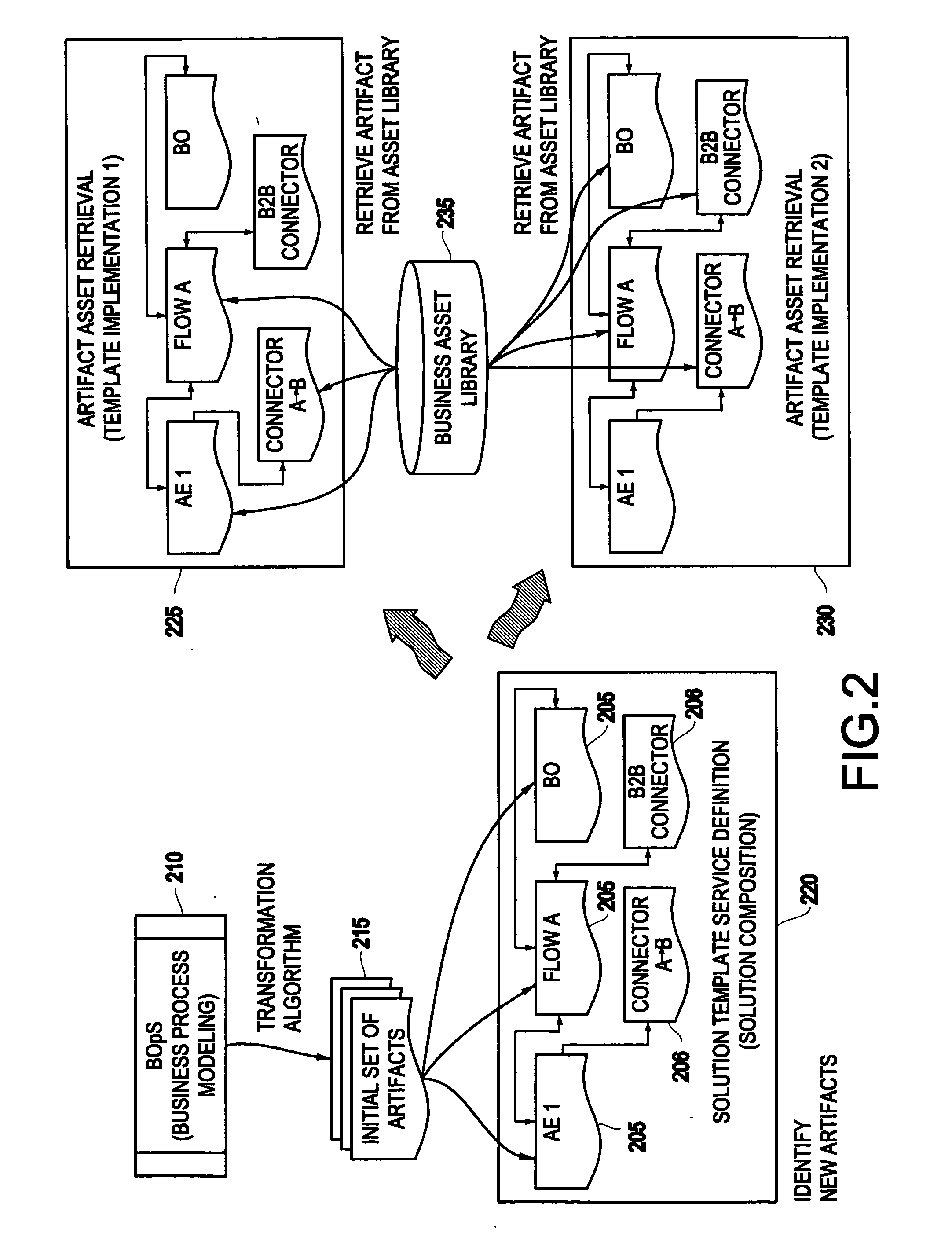
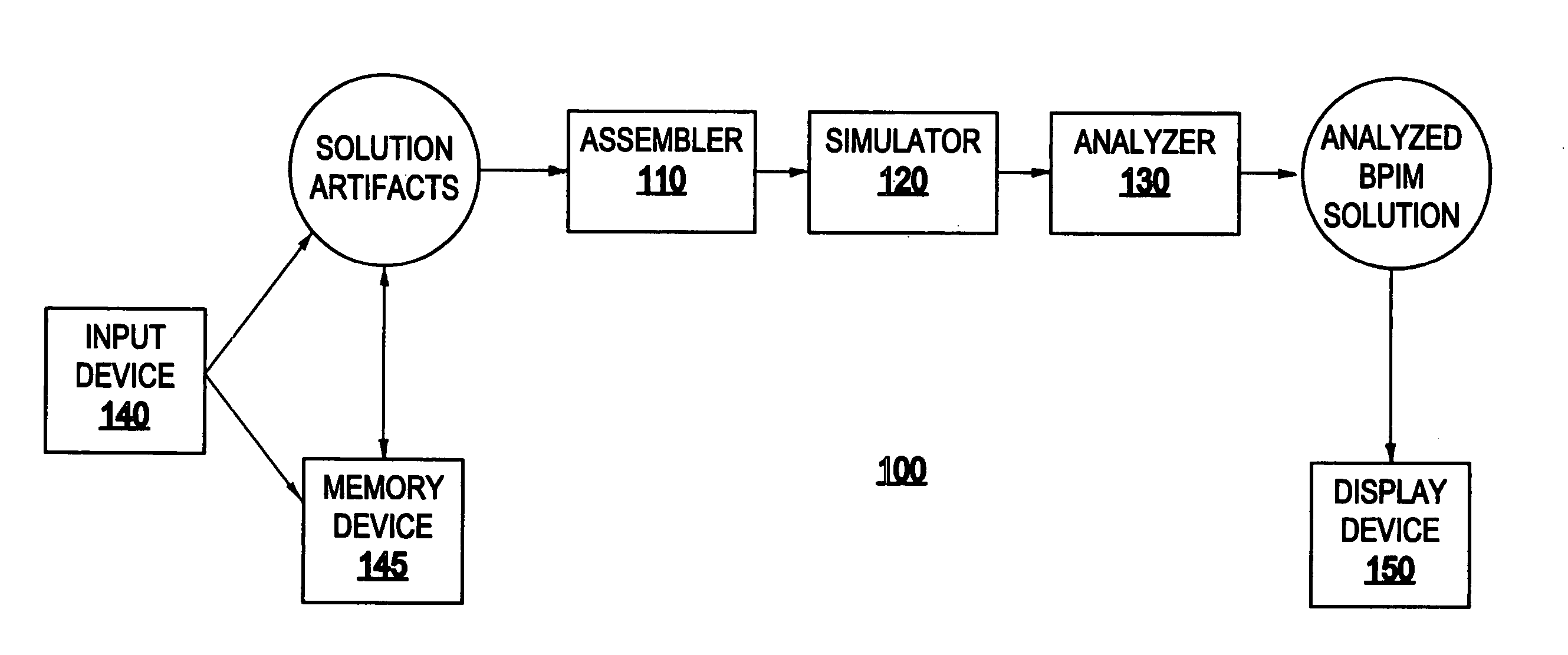
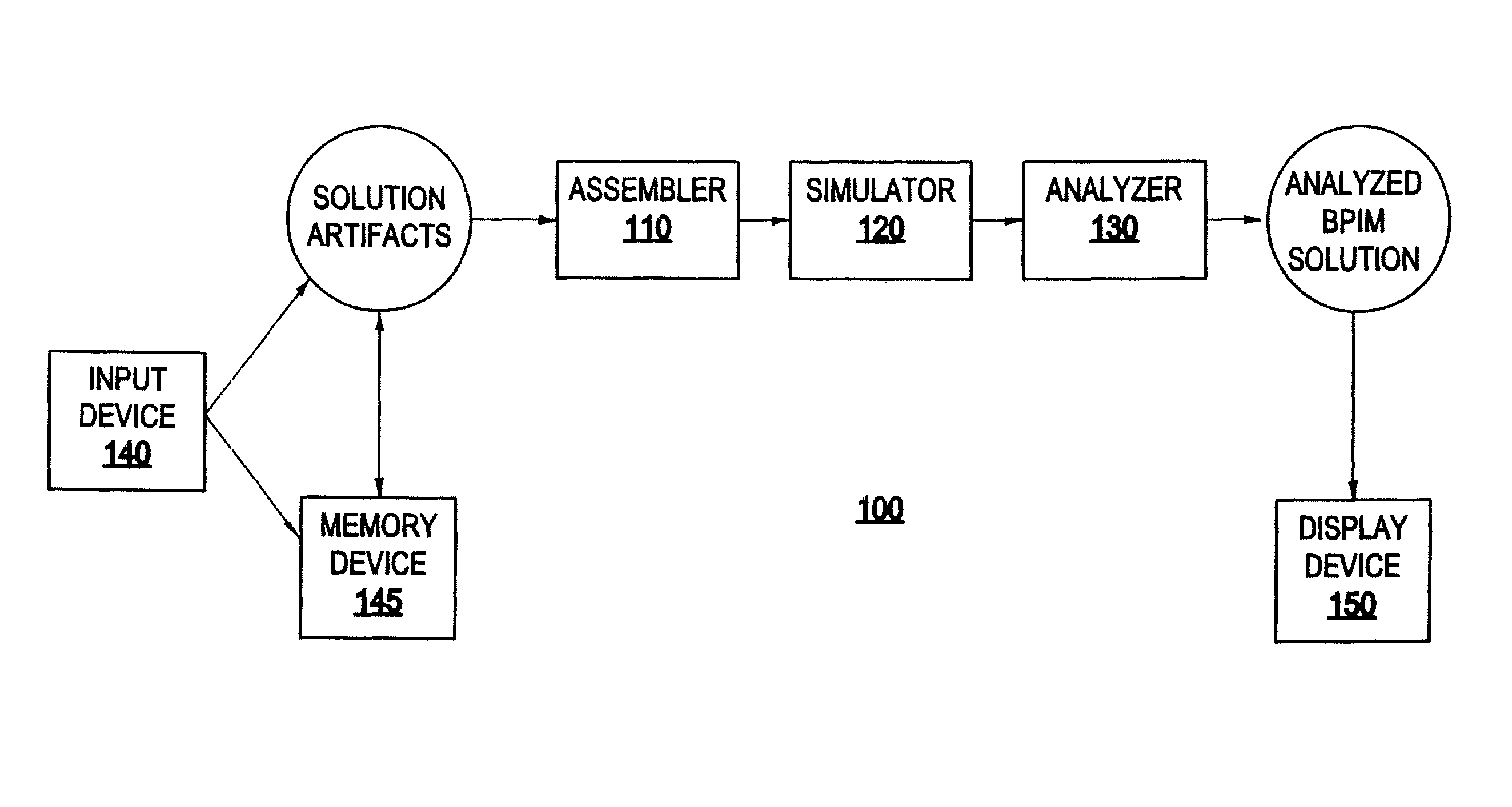
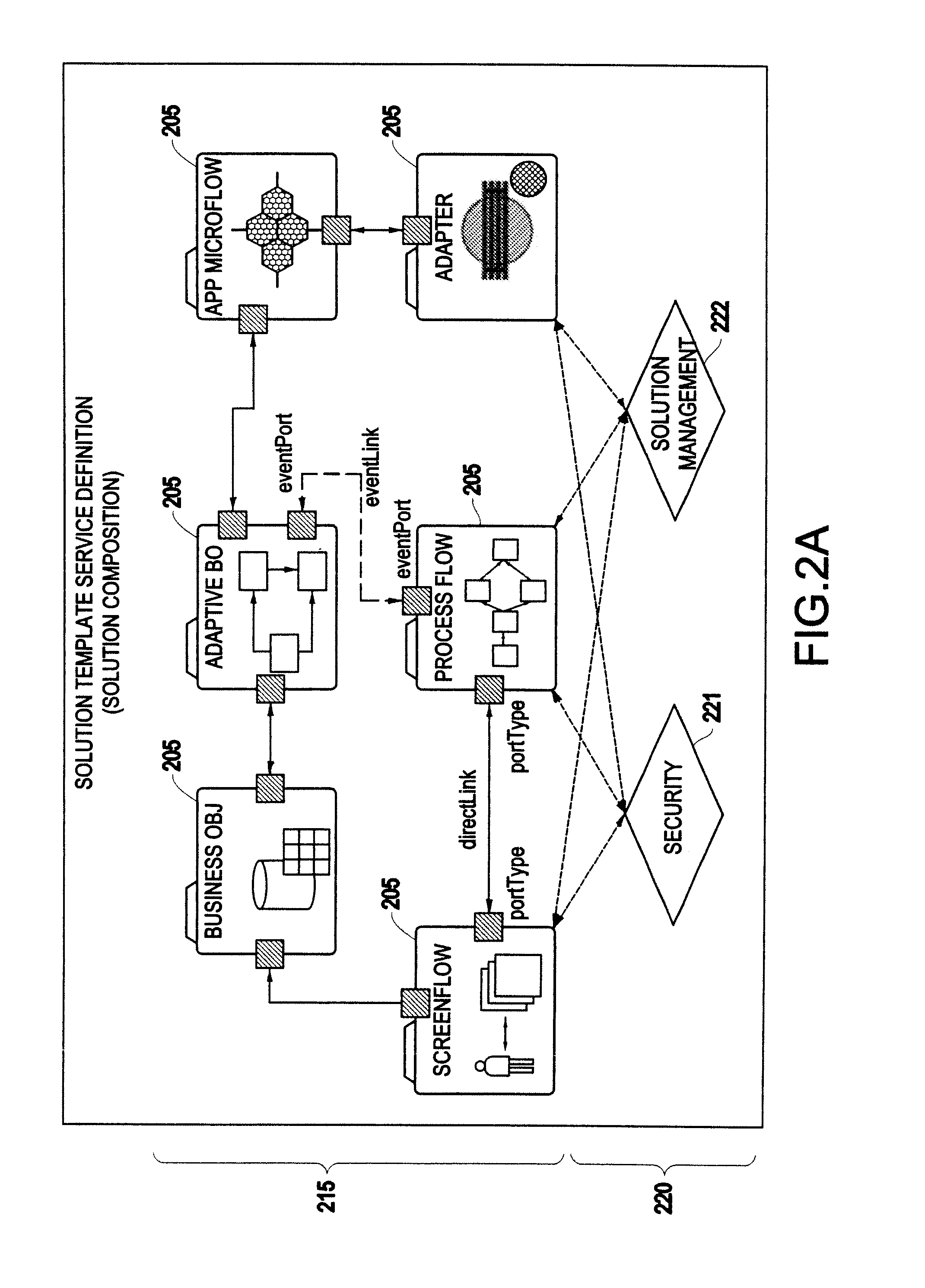
System and method for generating a business process integration and management (BPIM) solution
InactiveUS20050080640A1Significantly effectiveResourcesSpecial data processing applicationsComputer scienceBusiness process integration
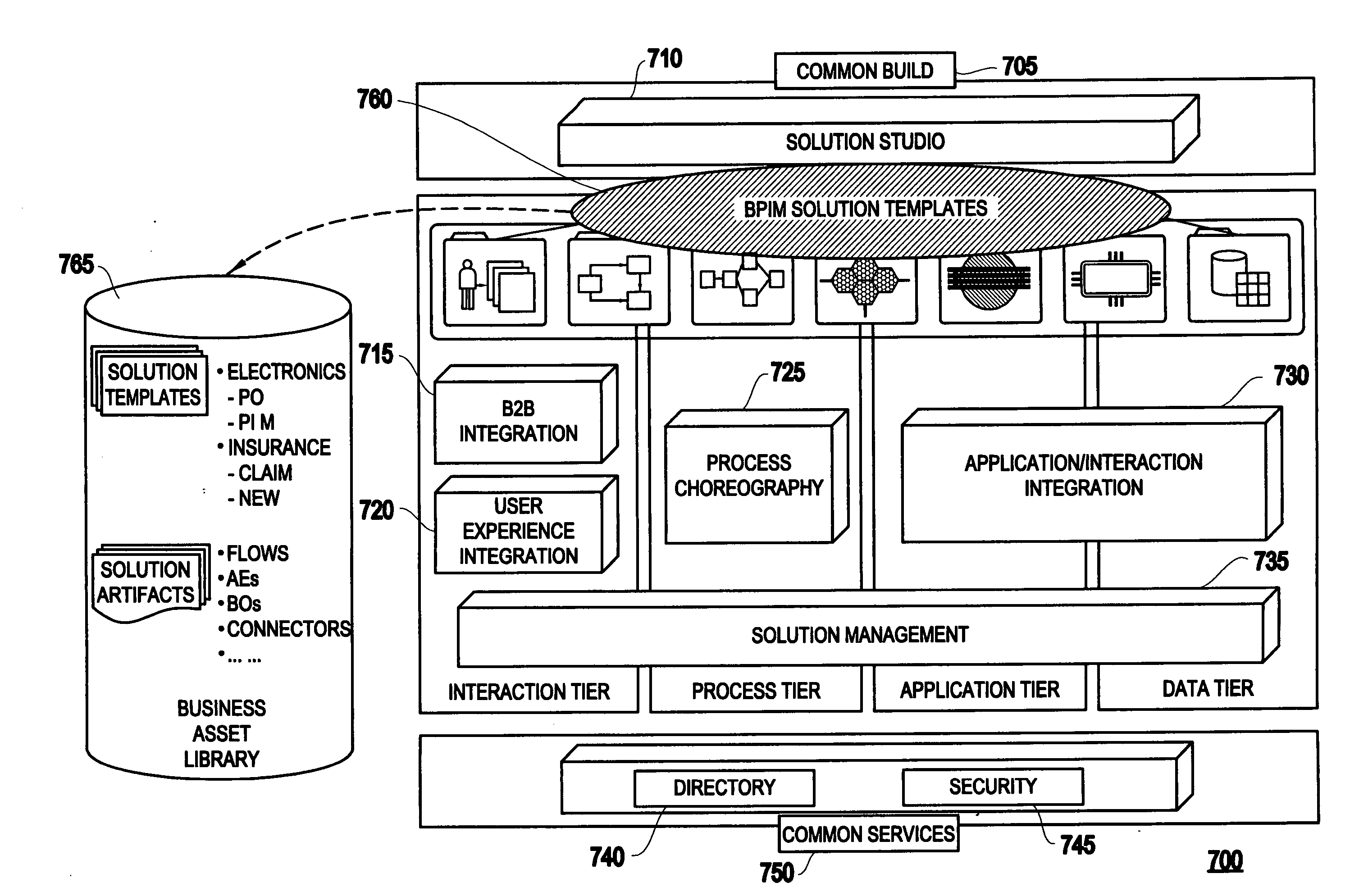
A system for generating a business process integration and management (BPIM) solution includes an assembler which assembles a plurality of solution artifacts to form a platform-independent solution template, a template implementer which implements the platform-independent solution template to form a template implementation, and a customizer which customizes the template implementation to generate a BPIM solution.
Owner:IBM CORP
Biomarker generator system
ActiveUS20080067413A1Efficient dosingEfficient productionIsotope delivery systemsMaterial analysis using wave/particle radiationMicroreactorParticle accelerator
A biomarker generator system for producing approximately one (1) unit dose of a biomarker. The biomarker generator system includes a small, low-power particle accelerator (“micro-accelerator”) and a radiochemical synthesis subsystem having at least one microreactor and / or microfluidic chip. The micro-accelerator is provided for producing approximately one (1) unit dose of a radioactive substance, such as a substance that emits positrons. The radiochemical synthesis subsystem is provided for receiving the radioactive substance, for receiving at least one reagent, and for synthesizing the approximately one (1) unit dose of a biomarker.
Owner:BEST ABT INC
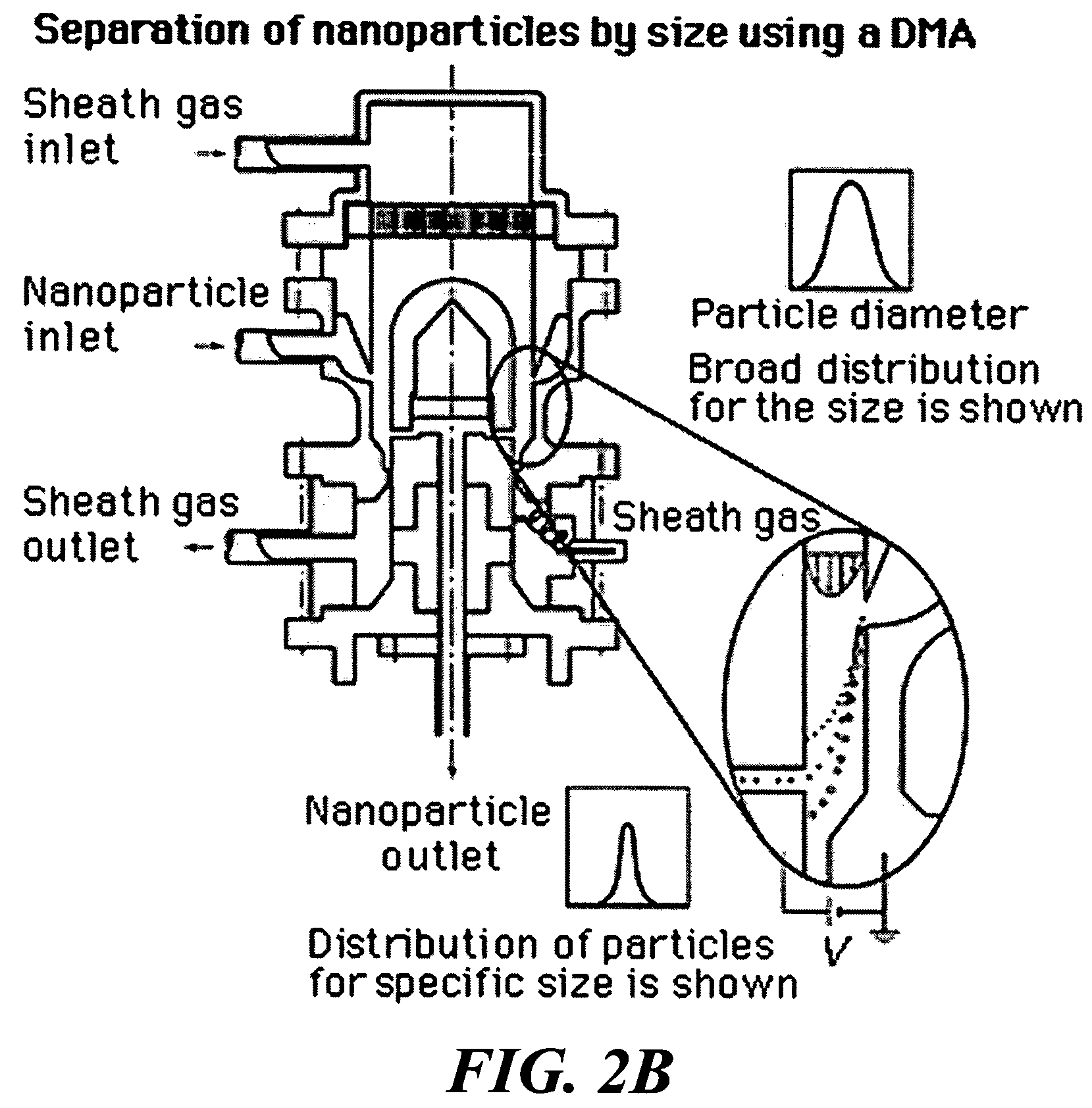
Reduction of scan time in imaging mass spectrometry
InactiveUS20070141718A1Raise the possibilityBig spaceImaging particle spectrometryBiological testingImage resolutionMass Spectrometry-Mass Spectrometry
Techniques are disclosed for reducing scan times in mass spectral tissue imaging studies. According to a first technique, a tissue imaging boundary is defined that closely approximates the edges of a tissue sample. According to a second technique, a low-resolution scan is performed to identify one or more areas of interest within the tissue sample, and the identified areas of interest are subsequently scanned at higher resolution.
Owner:THERMO FINNIGAN
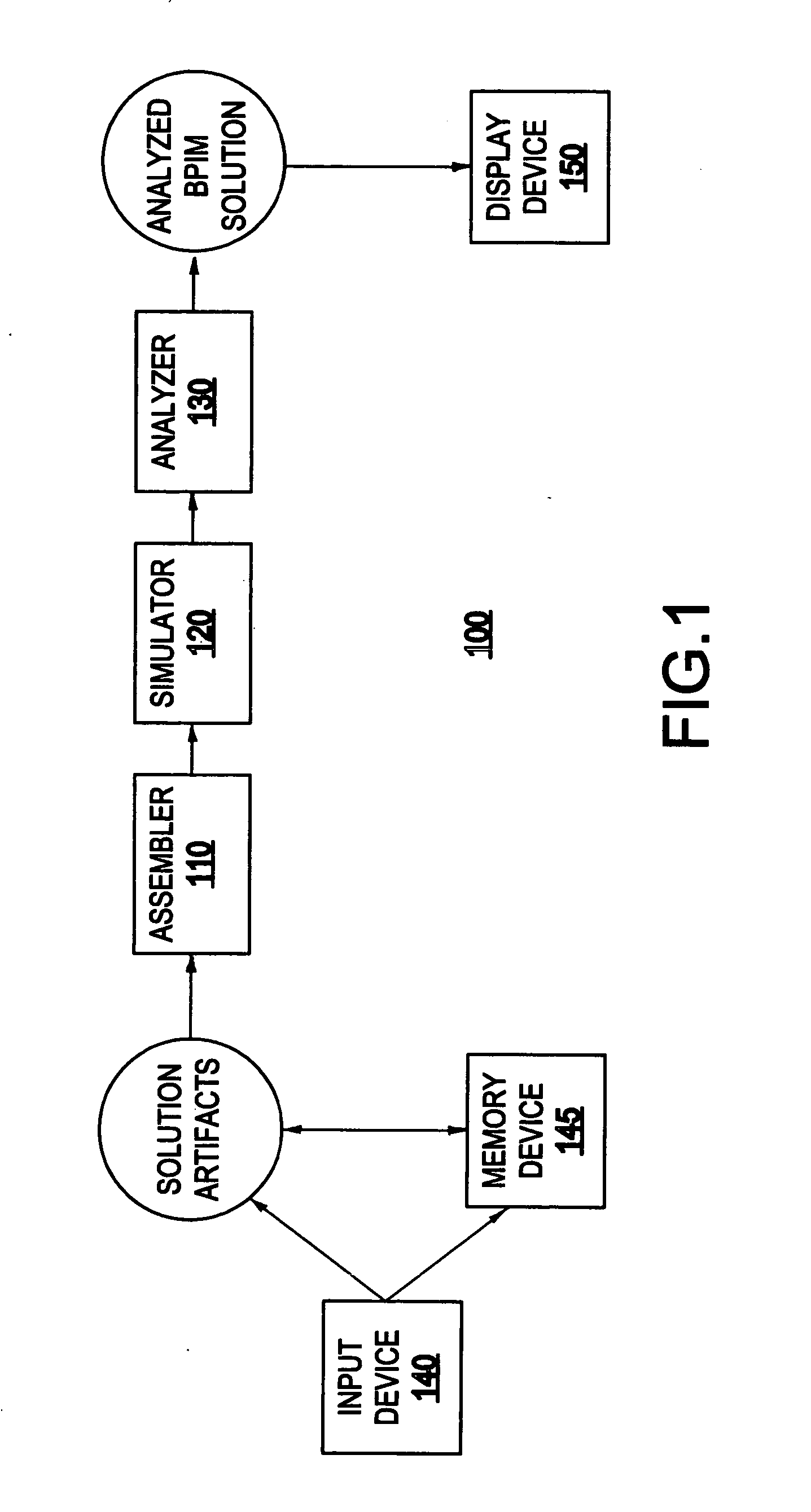
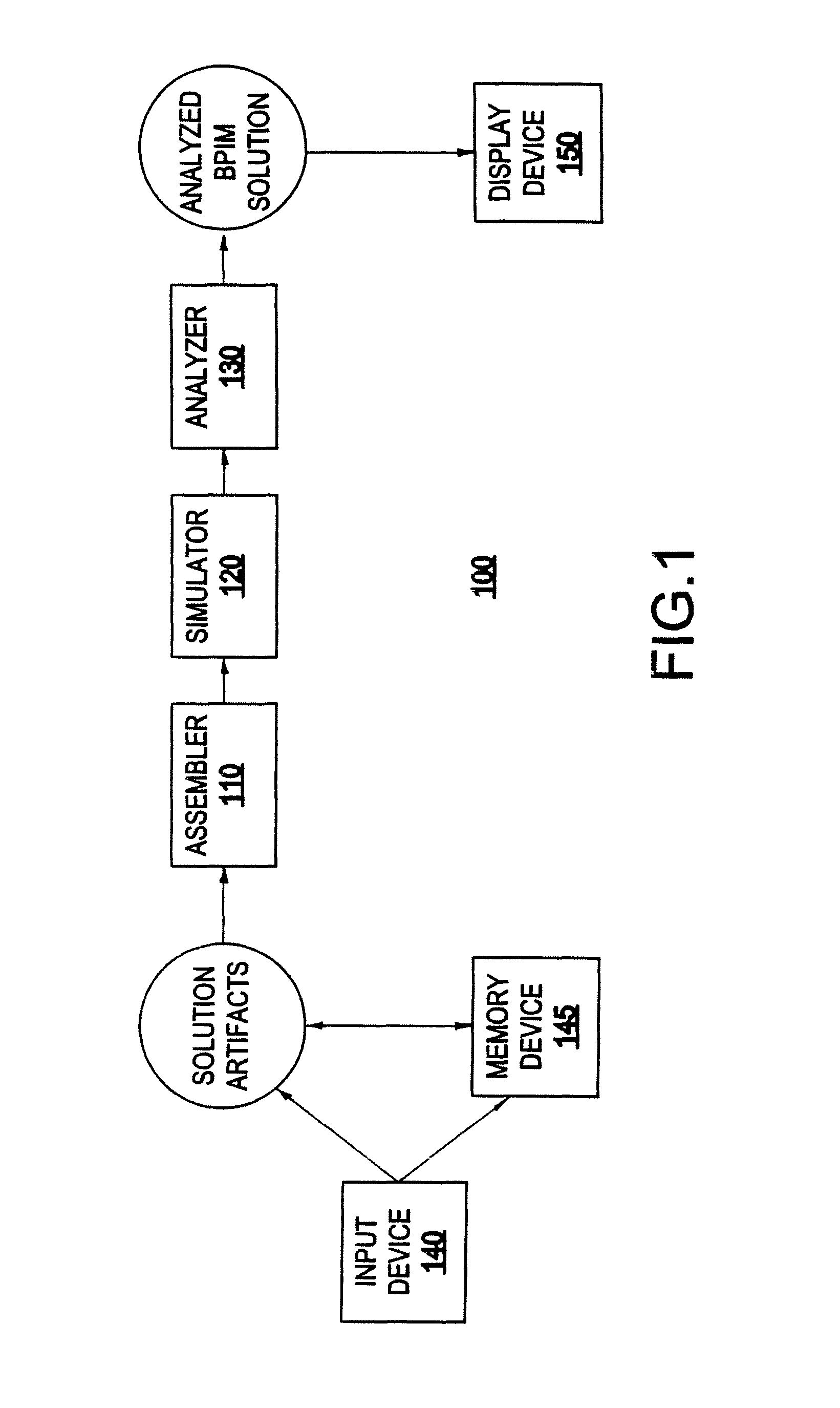
System and method for analyzing a business process integration and management (BPIM) solution
InactiveUS20050080609A1Efficient and effectiveSignificantly effectiveForecastingProgram controlProcess integrationBusiness process integration
A system for analyzing a business process integration and management (BPIM) solution includes an assembler which assembles a plurality of solution artifacts to form a platform independent solution template, a simulator which simulates an execution of a BPIM solution based on the platform independent solution template, and an analyzer for analyzing a performance of the BPIM solution.
Owner:IBM CORP
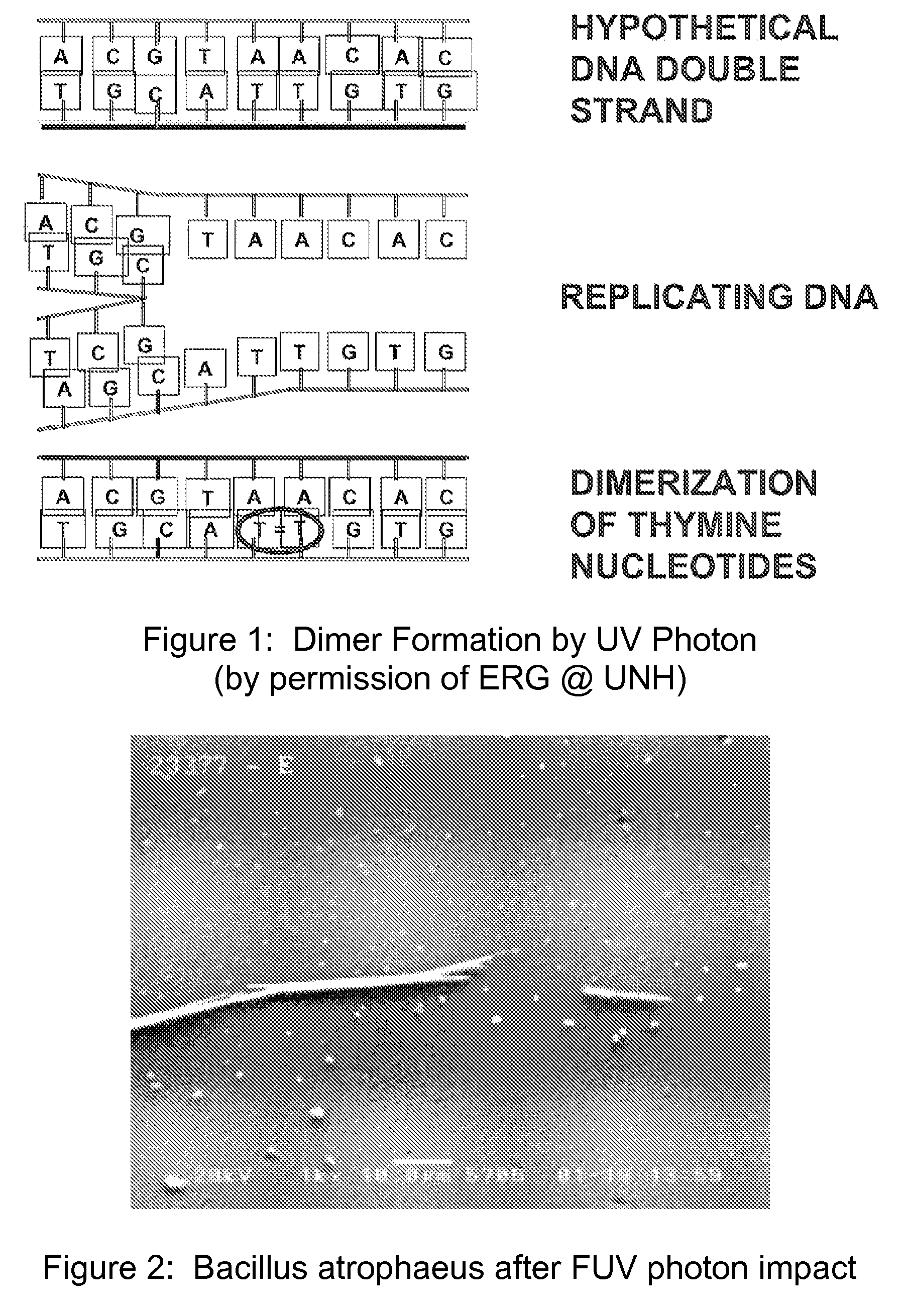
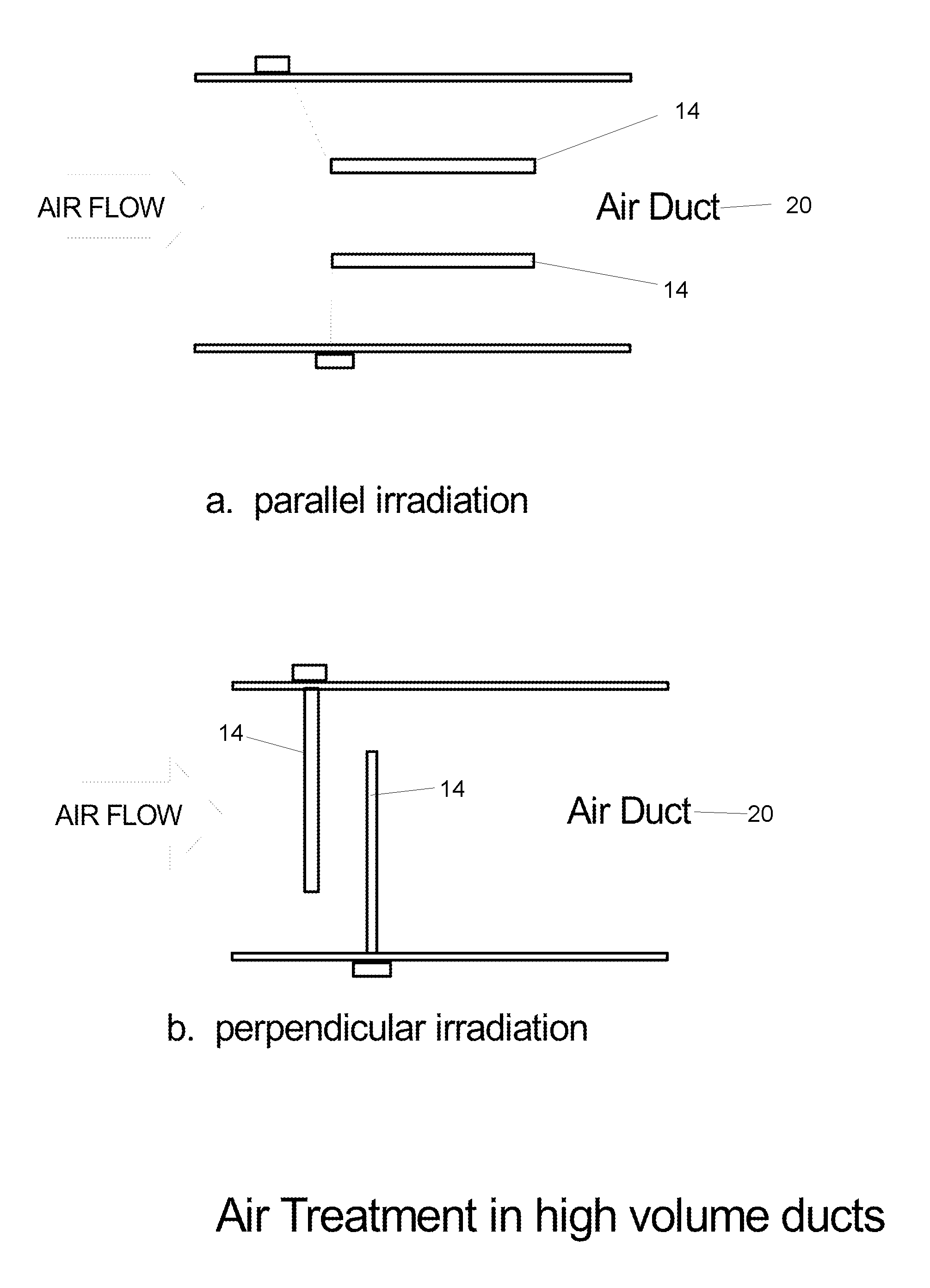
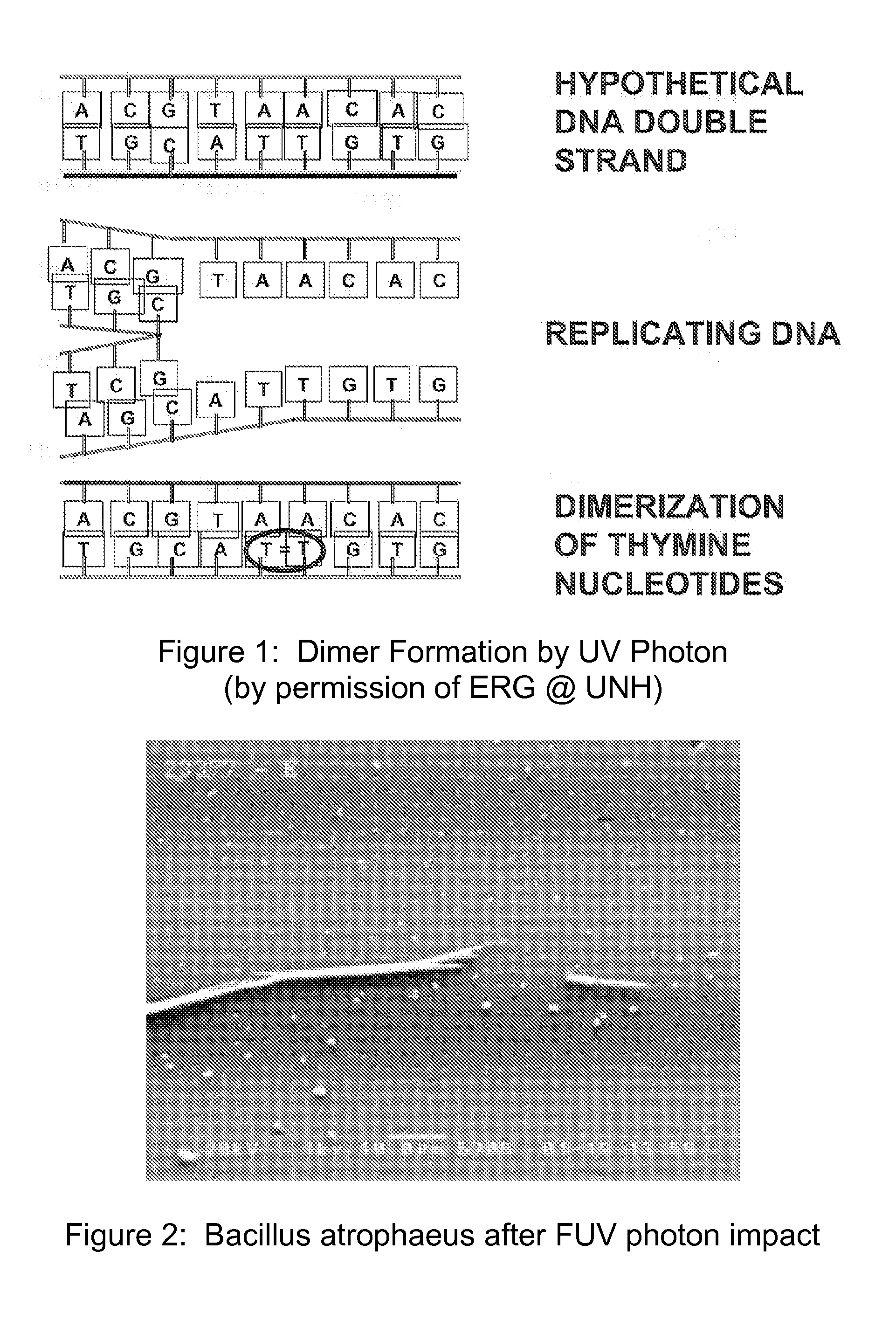
Method and apparatus for producing a high level of disinfection in air and surfaces
ActiveUS8481985B2Significantly effectiveOptical radiation measurementWater/sewage treatment by irradiationSporeSpectral width
This specification relates to an improved method, process and apparatus for disinfecting and sterilizing all types of surfaces and indoor air and room air contaminated with microorganisms. The improved apparatus consists of a multi-wavelength narrow spectral width UV source that is more effective than mercury based 254 nm germicidal lamps for destroying the DNA and outer shell or membrane of virus, bacteria, spores and cists.
Owner:CASALE RICHARD
Reduction of scan time in imaging mass spectrometry
ActiveUS7655476B2Raise the possibilityBig spaceCharacter and pattern recognitionImaging particle spectrometryImage resolutionTissue sample
Techniques are disclosed for reducing scan times in mass spectral tissue imaging studies. According to a first technique, a tissue imaging boundary is defined that closely approximates the edges of a tissue sample. According to a second technique, a low-resolution scan is performed to identify one or more areas of interest within the tissue sample, and the identified areas of interest are subsequently scanned at higher resolution.
Owner:THERMO FINNIGAN
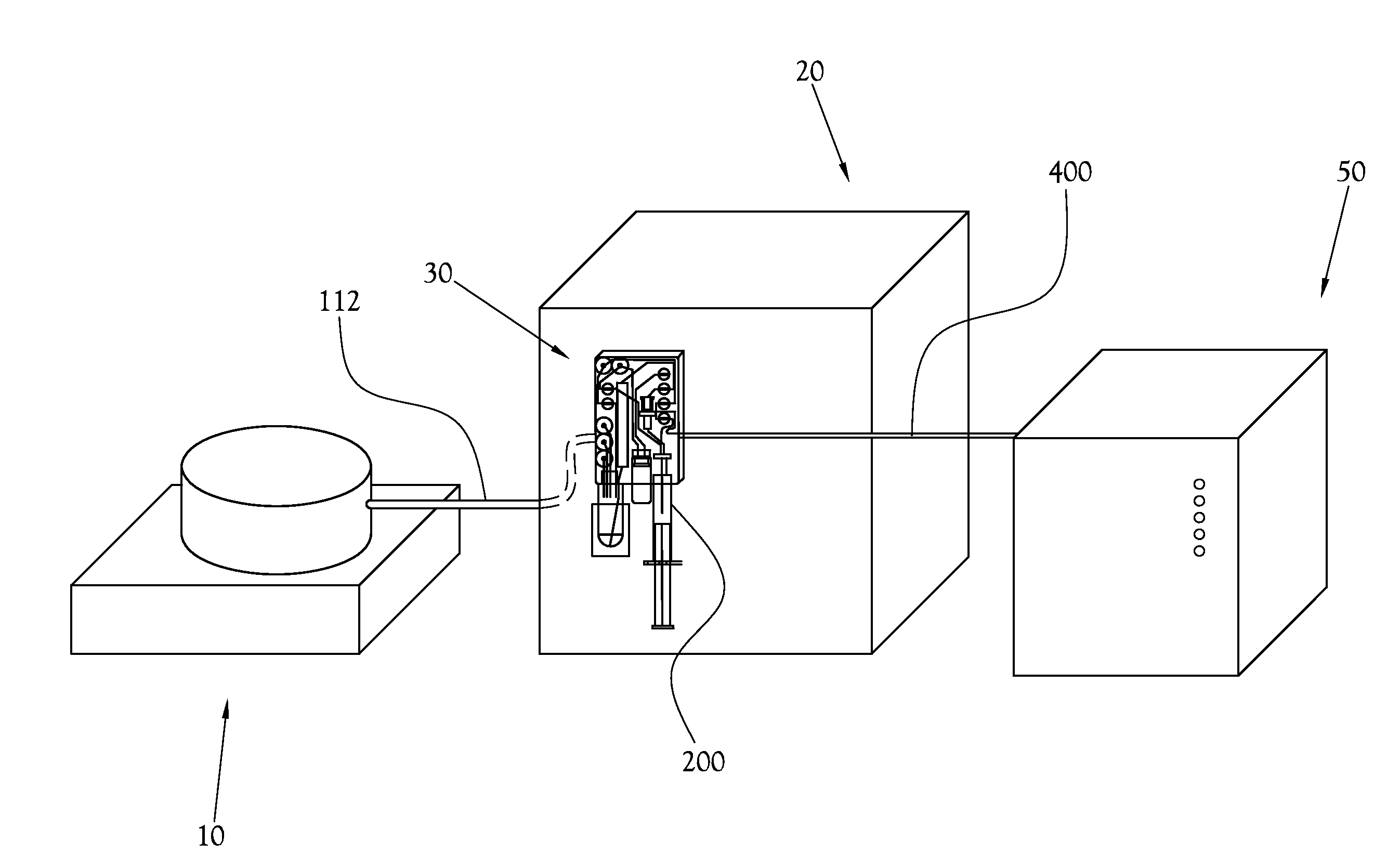
Low-Volume Biomarker Generator
ActiveUS20090218520A1Reduce size and power requirement and weightEfficient dosingIsotope delivery systemsRadiation applicationsChemical synthesisEngineering
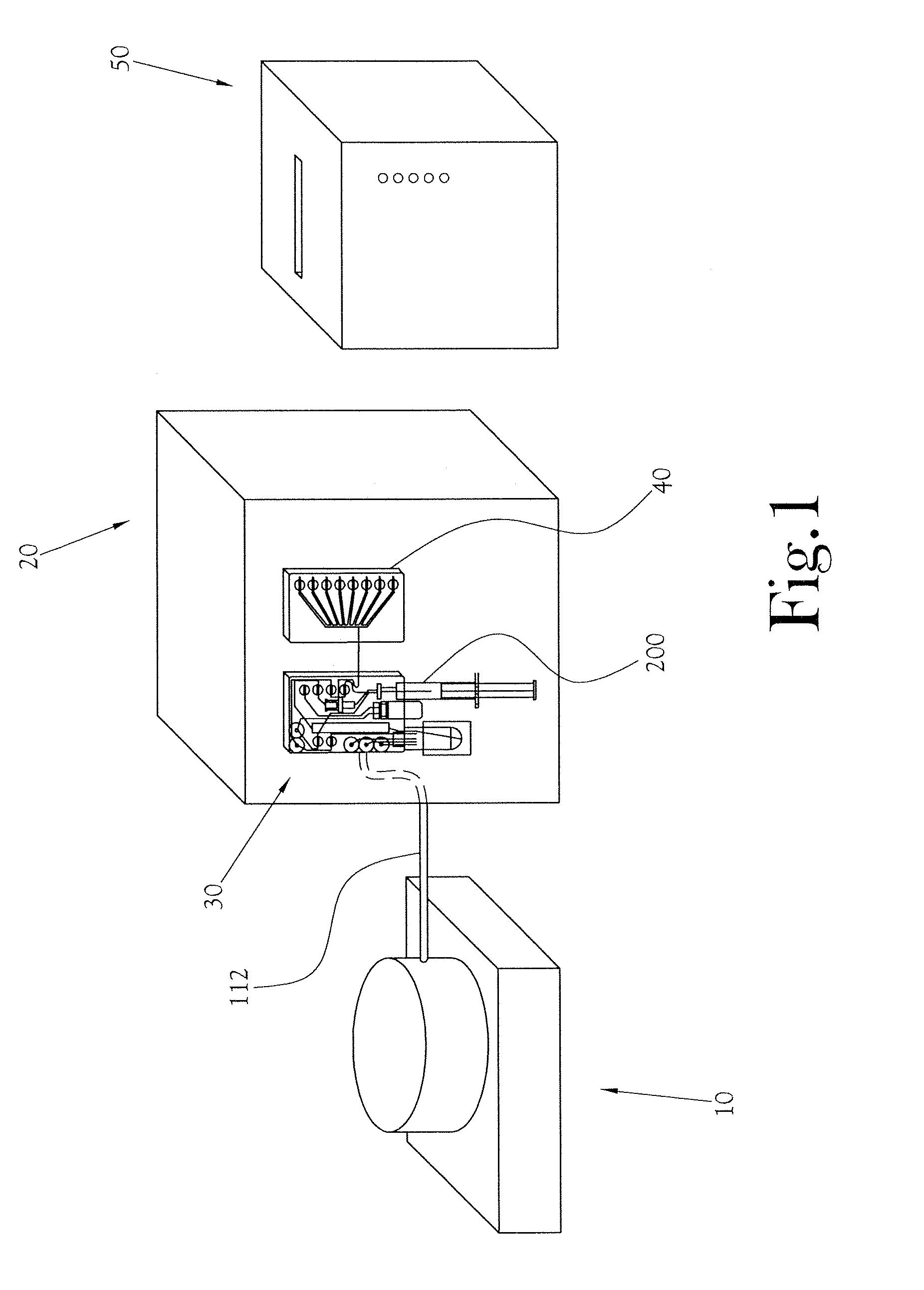
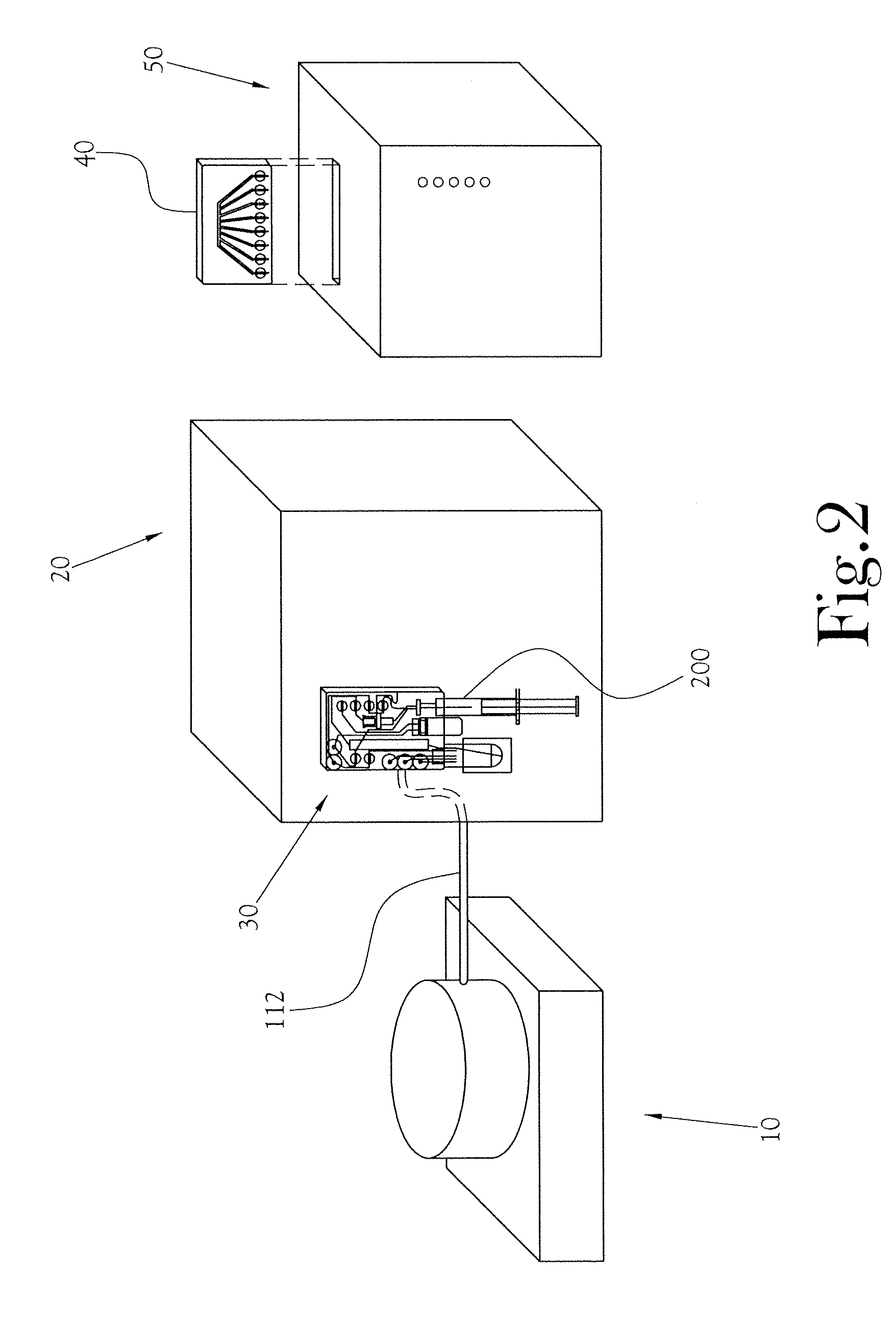
A low-volume biomarker generator for producing ultra-short lived radiopharmaceuticals. The low-volume biomarker generator system includes a low-power cyclotron and a radiochemical synthesis system. The cyclotron of the low-volume biomarker generator is optimized for producing radioisotopes useful in synthesizing radiopharmaceuticals in small quantities down to approximately one (1) unit dose. The cyclotron incorporates permanent magnets in place of electromagnets and / or an improved rf system to reduce the size, power requirements, and weight of the cyclotron. The radiochemical synthesis system of the low-volume biomarker is a small volume system optimized for synthesizing the radiopharmaceutical in small quantities of approximately one (1) unit dose.
Owner:BEST ABT INC
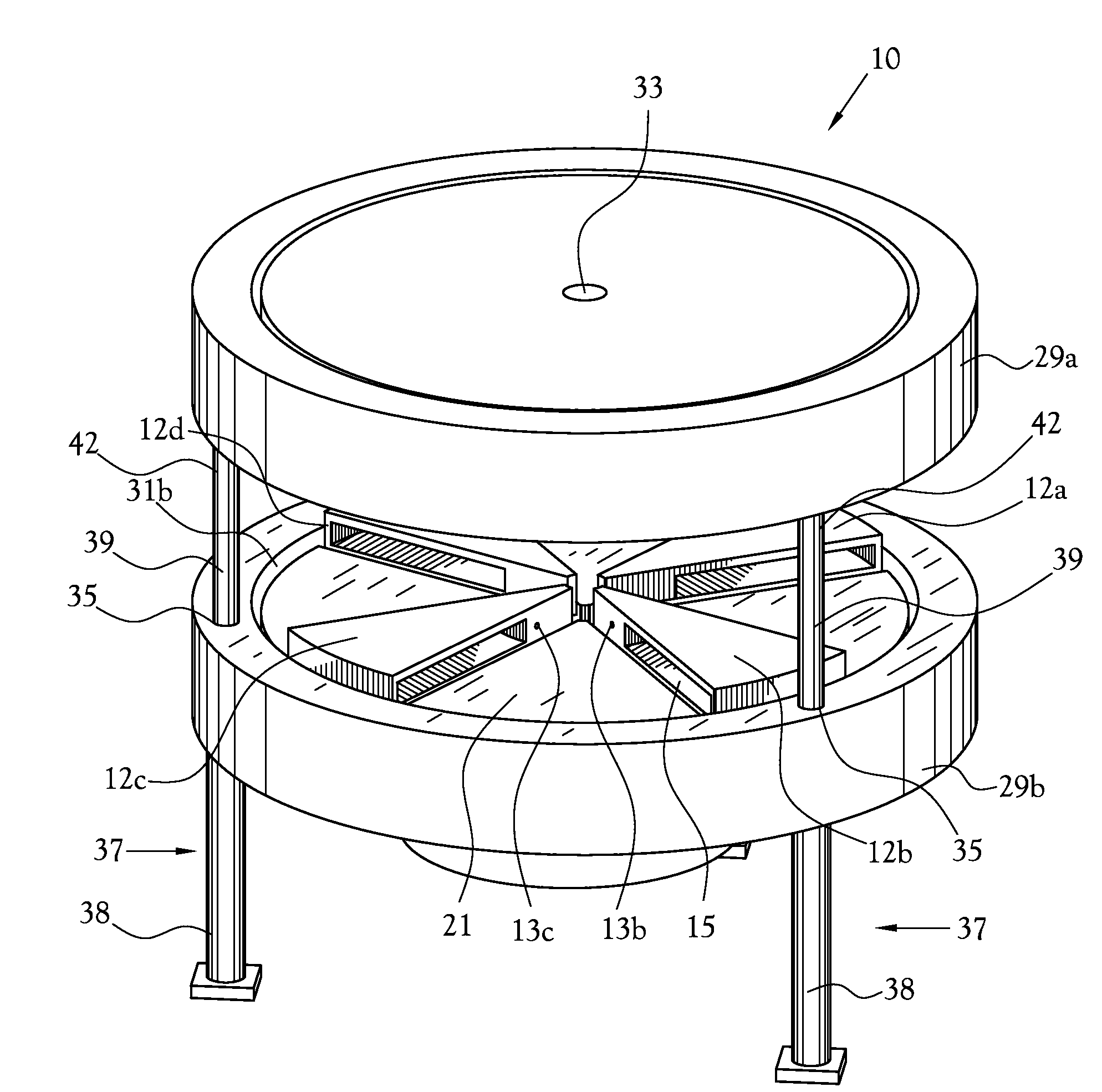
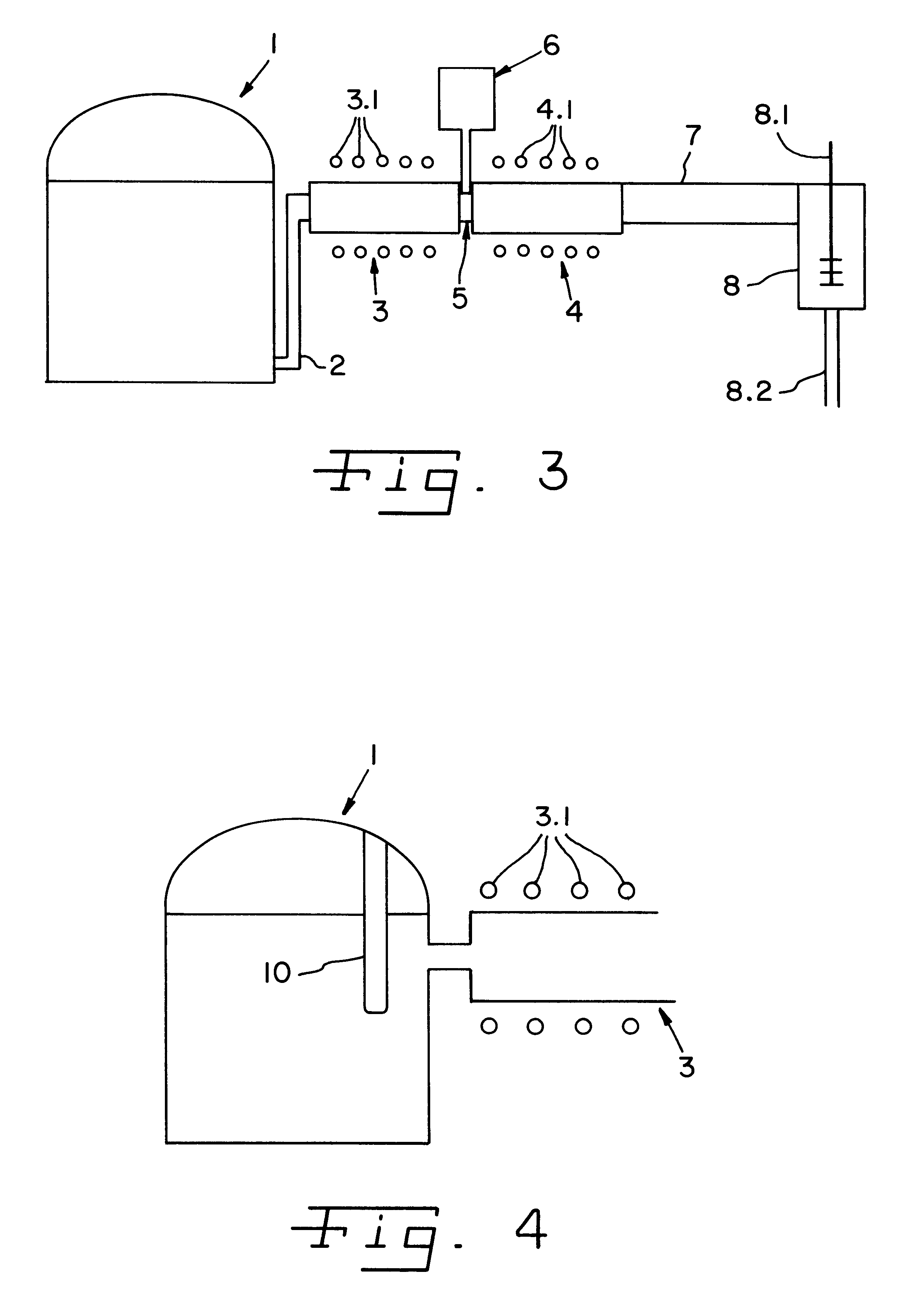
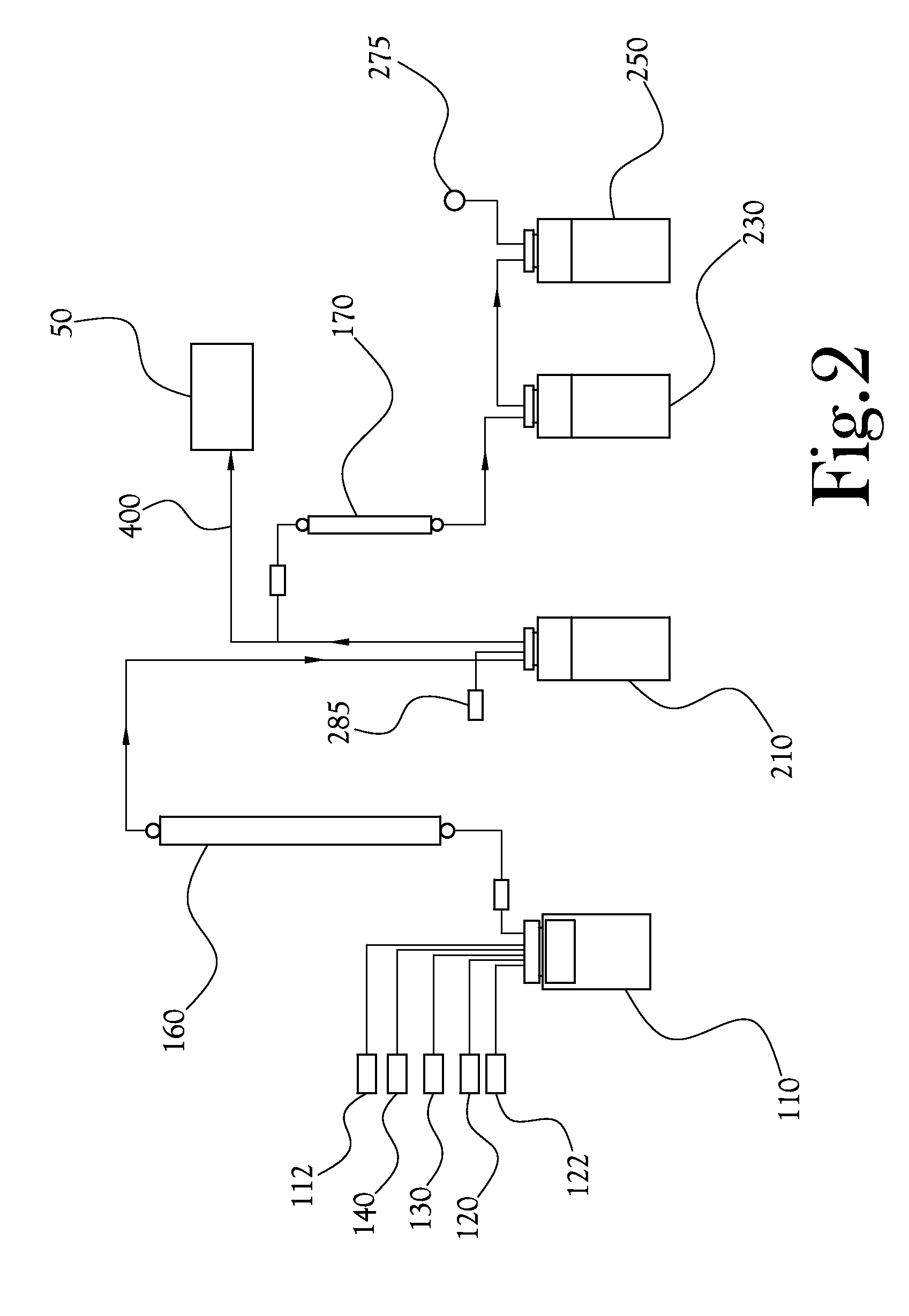
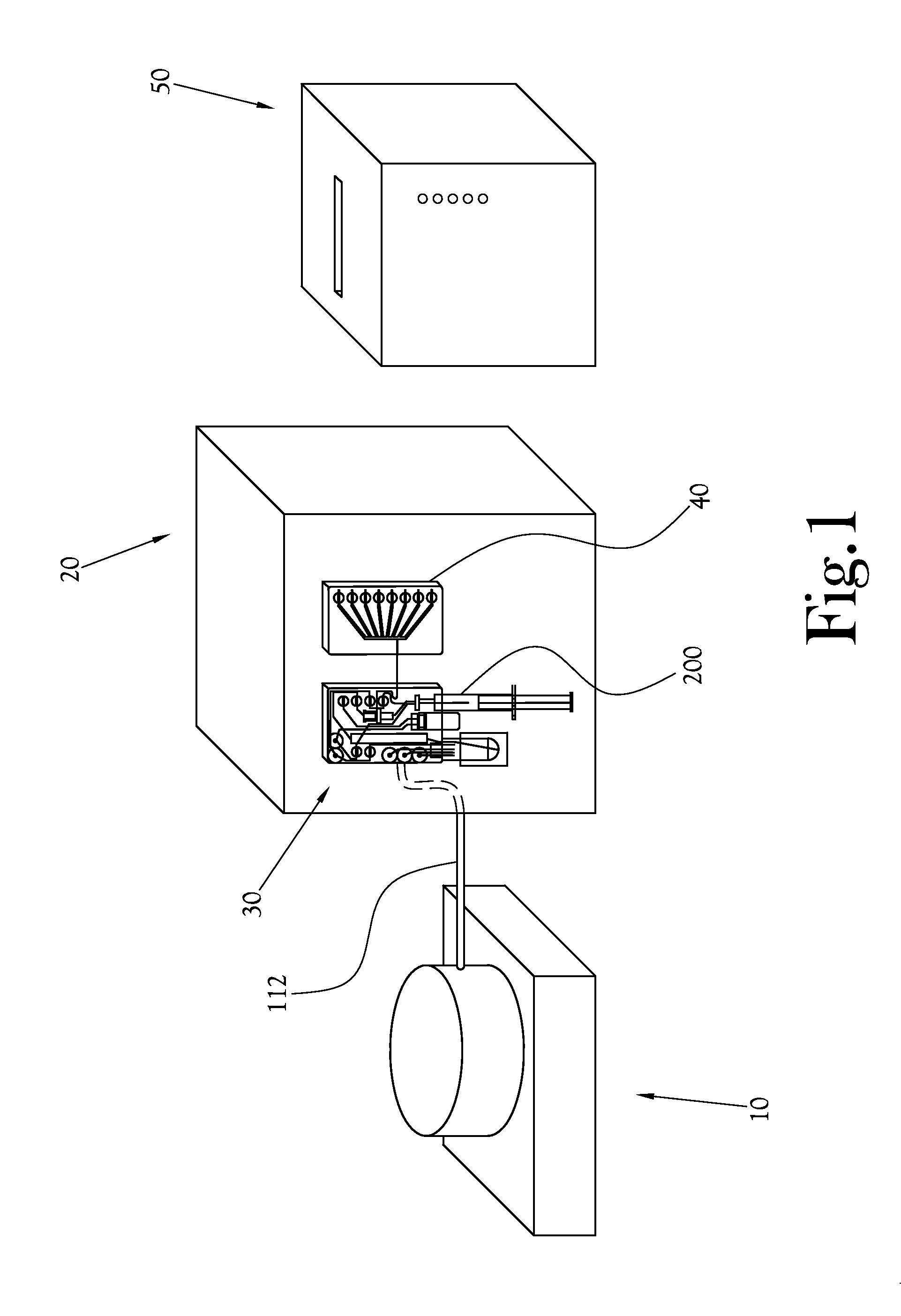
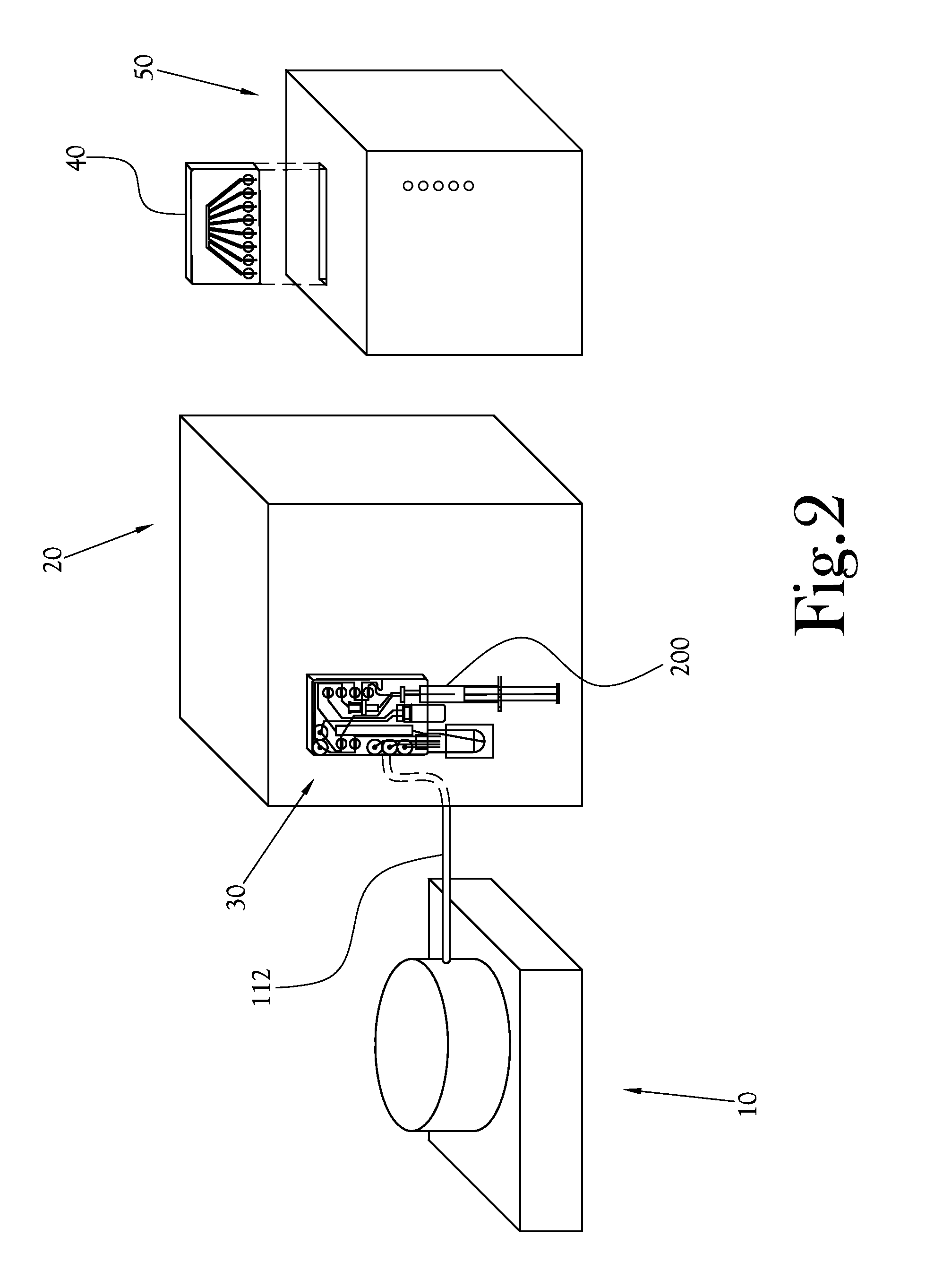
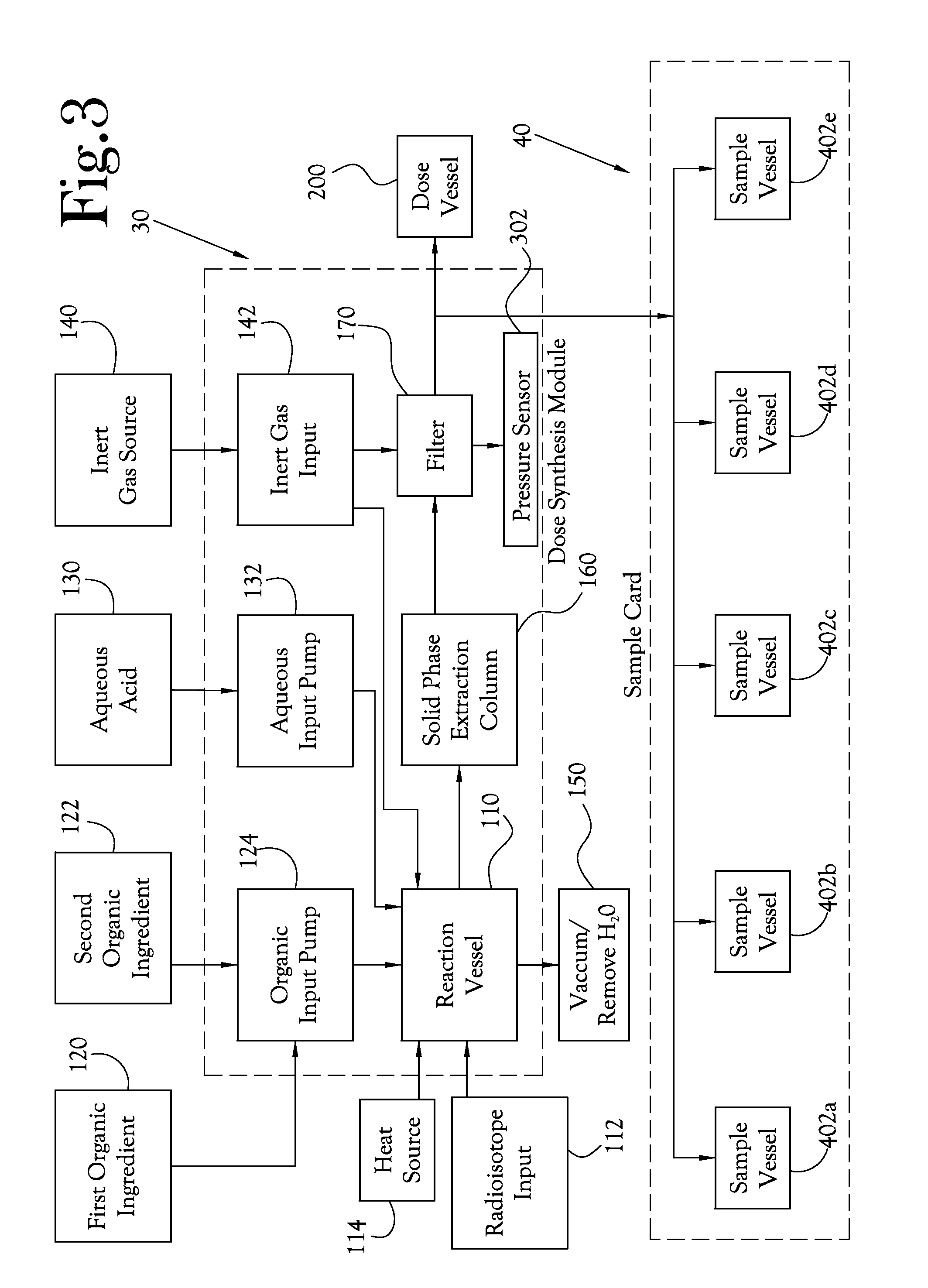
Dose Synthesis Mosule for Biomaker Generator System
ActiveUS20110070160A1Reduce in quantityLess stringent infrastructure requirementProcess control/regulationChemical/physical/physico-chemical microreactorsDose ReducedIsotope
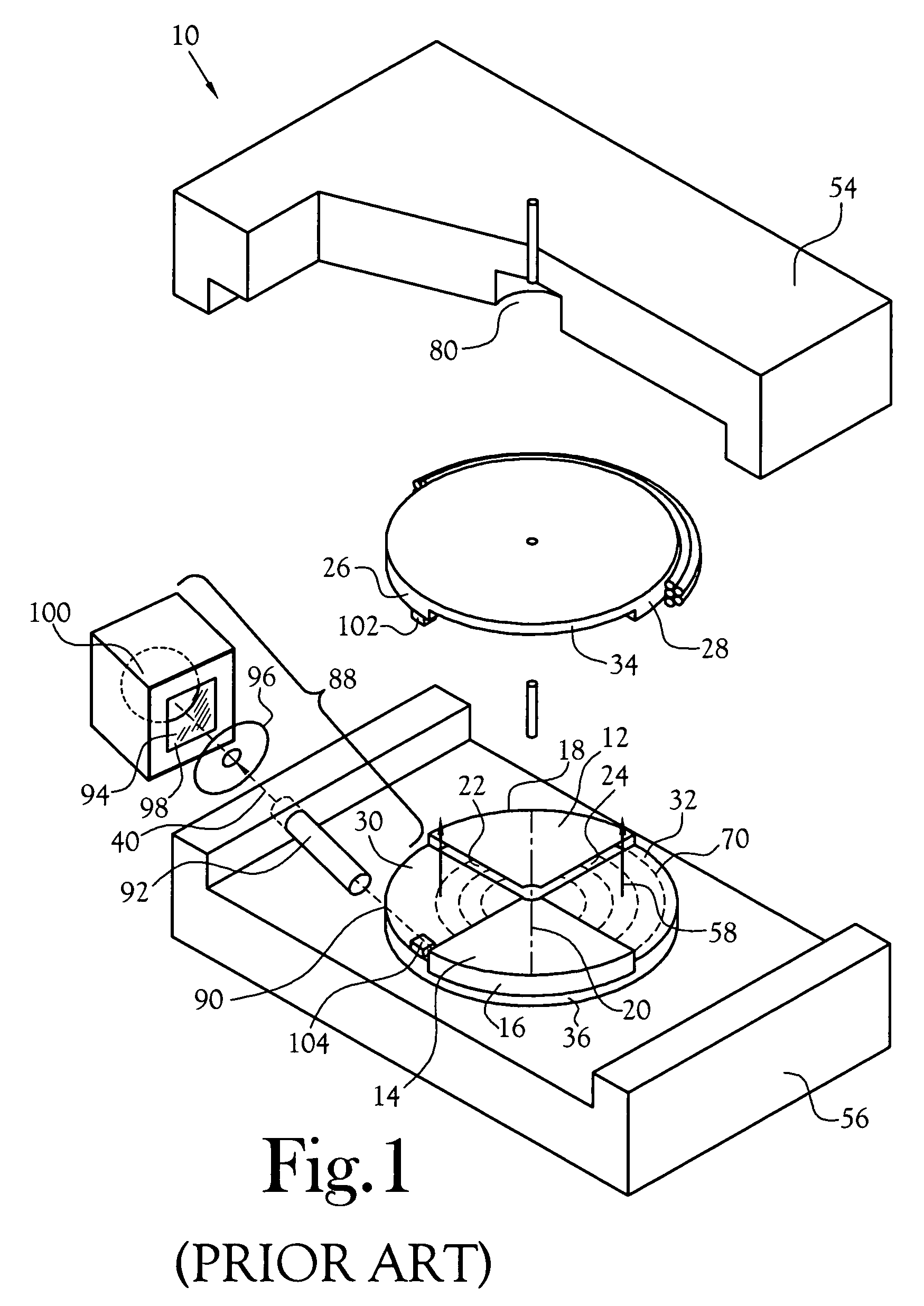
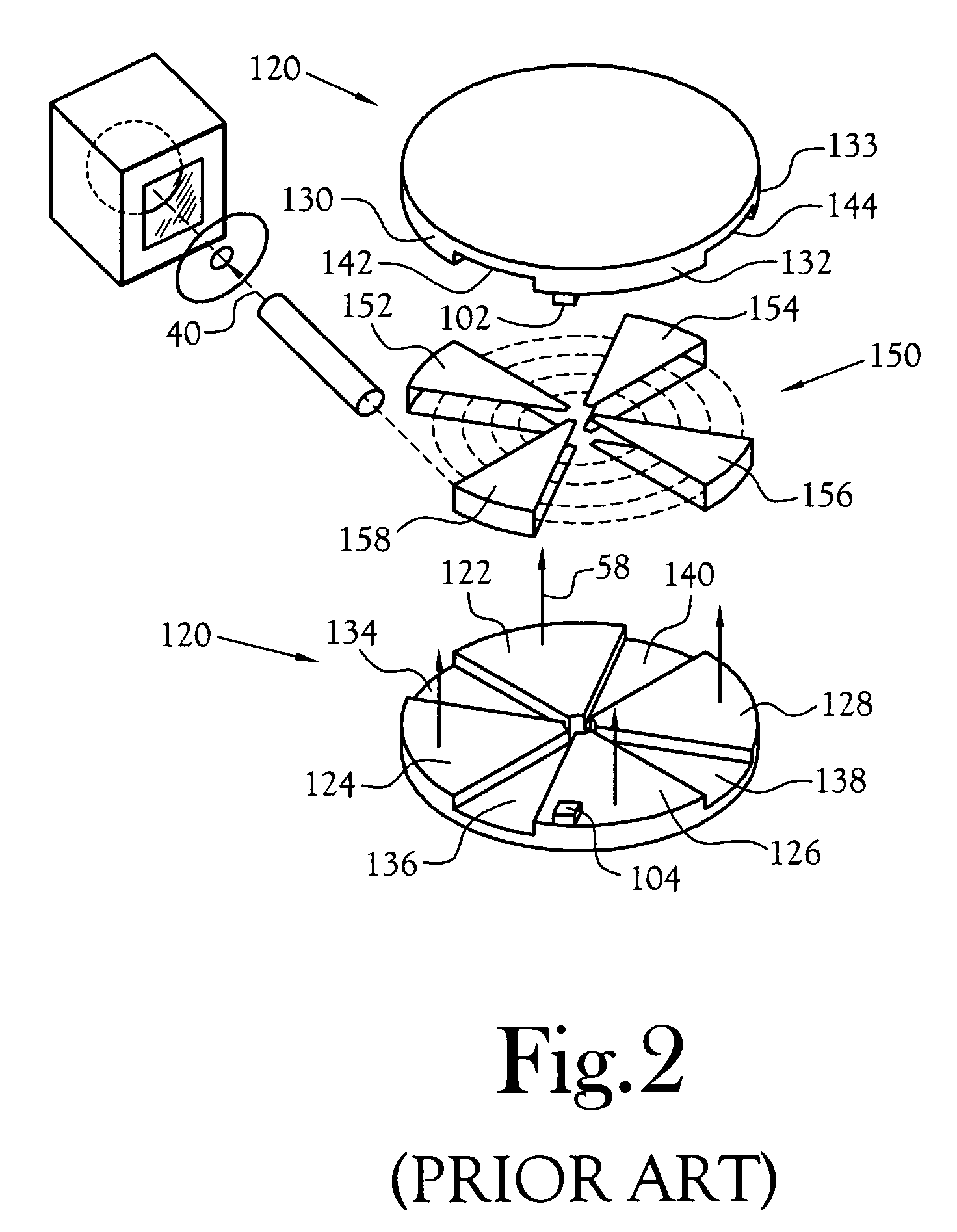
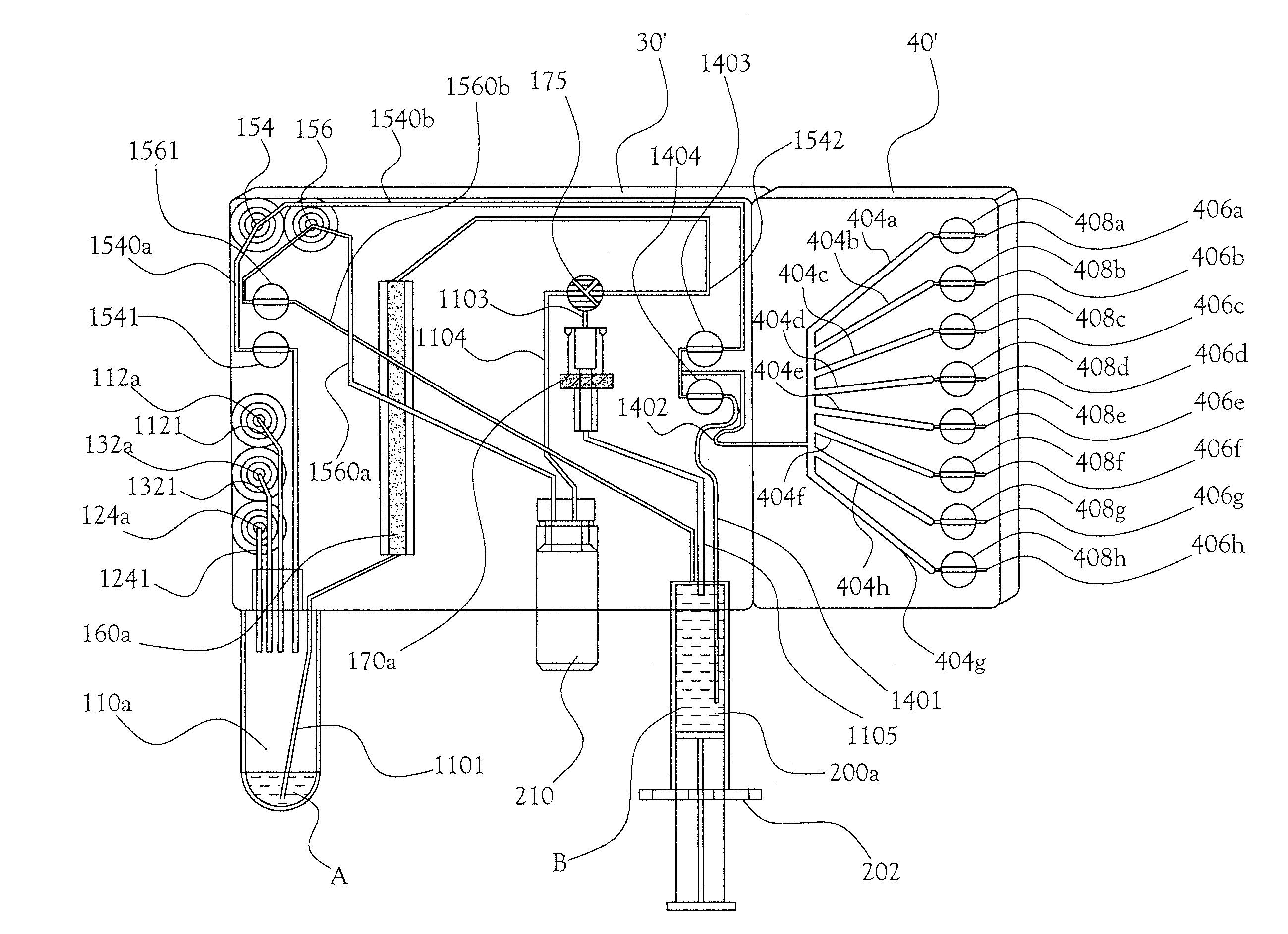
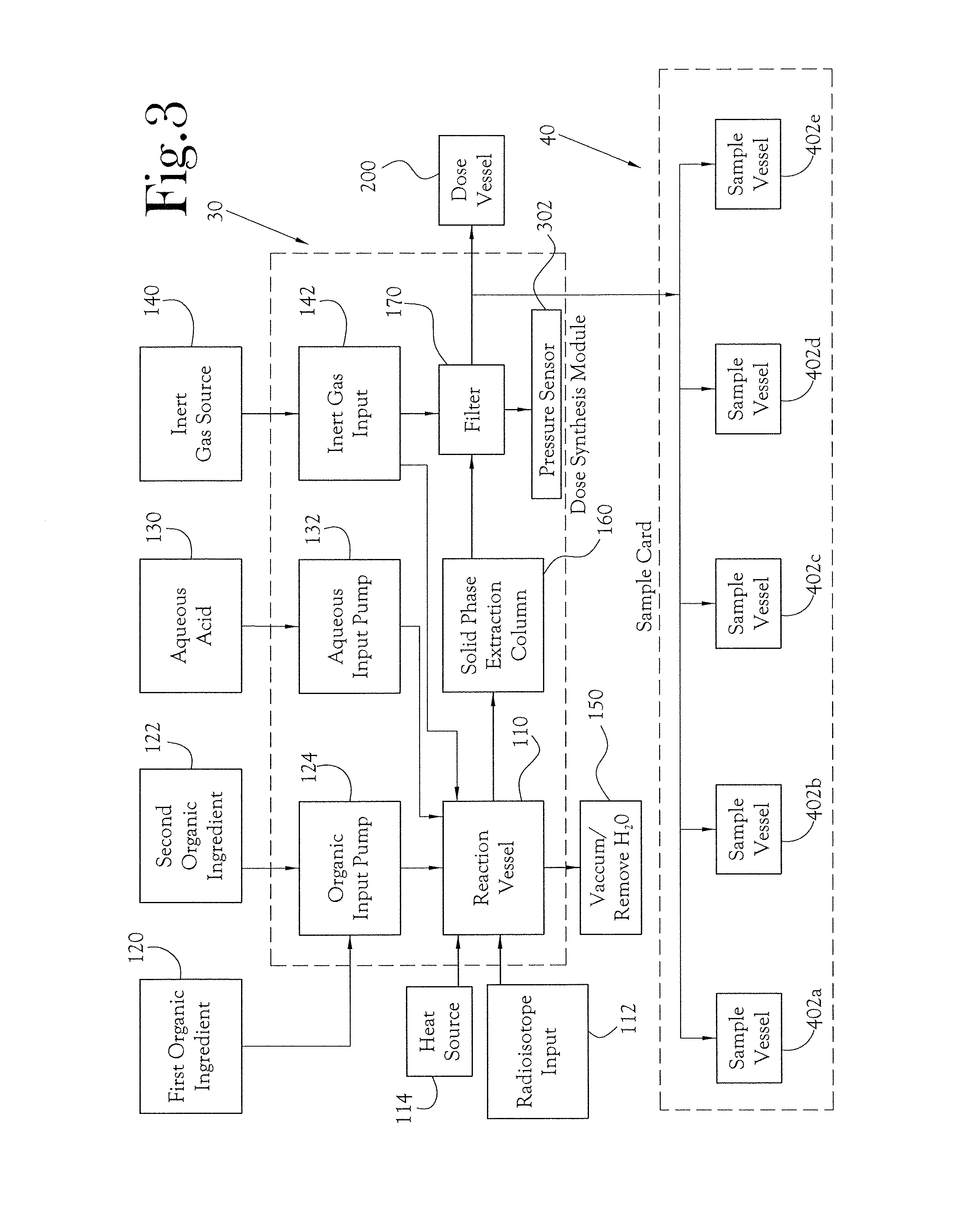
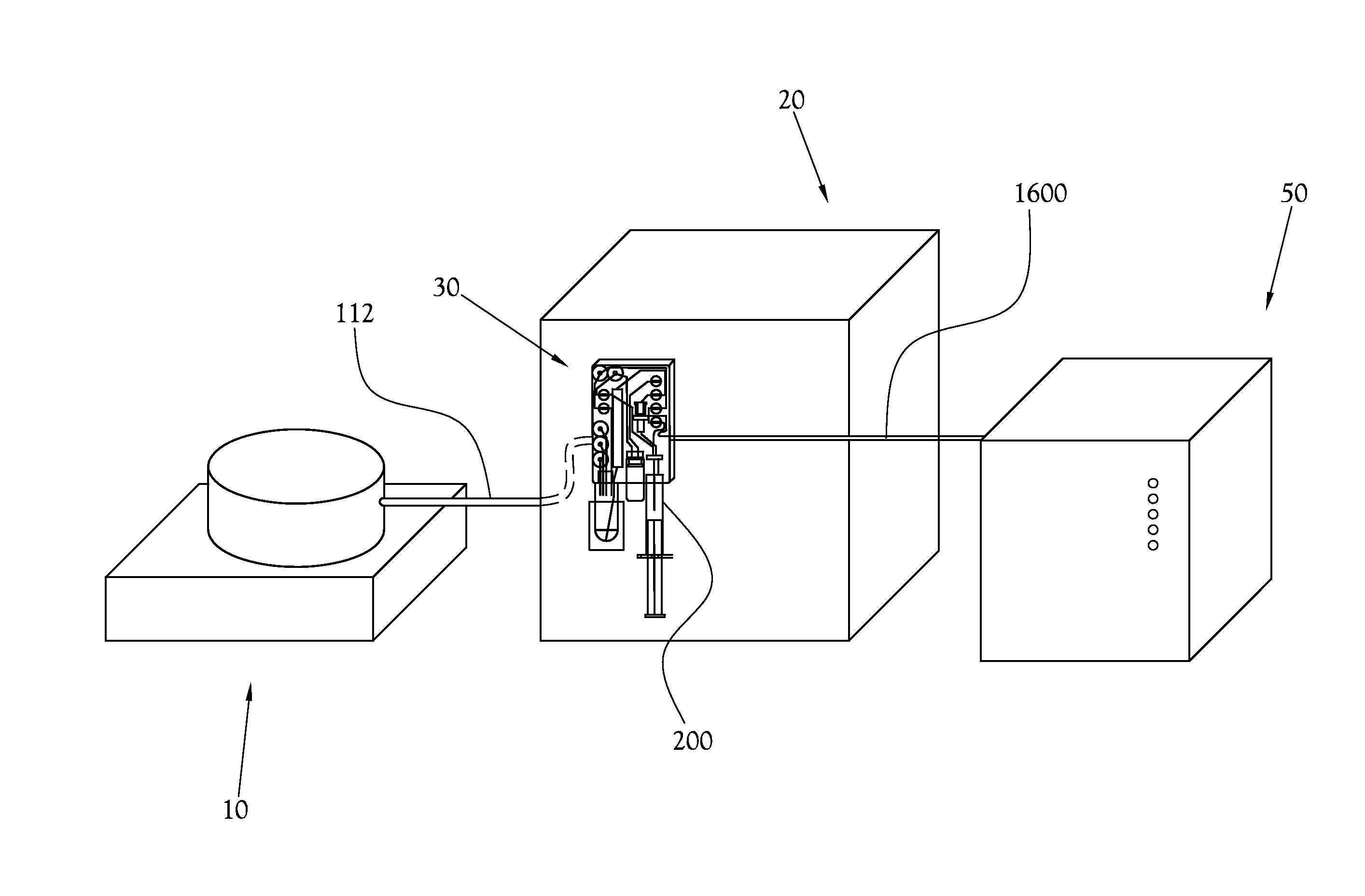
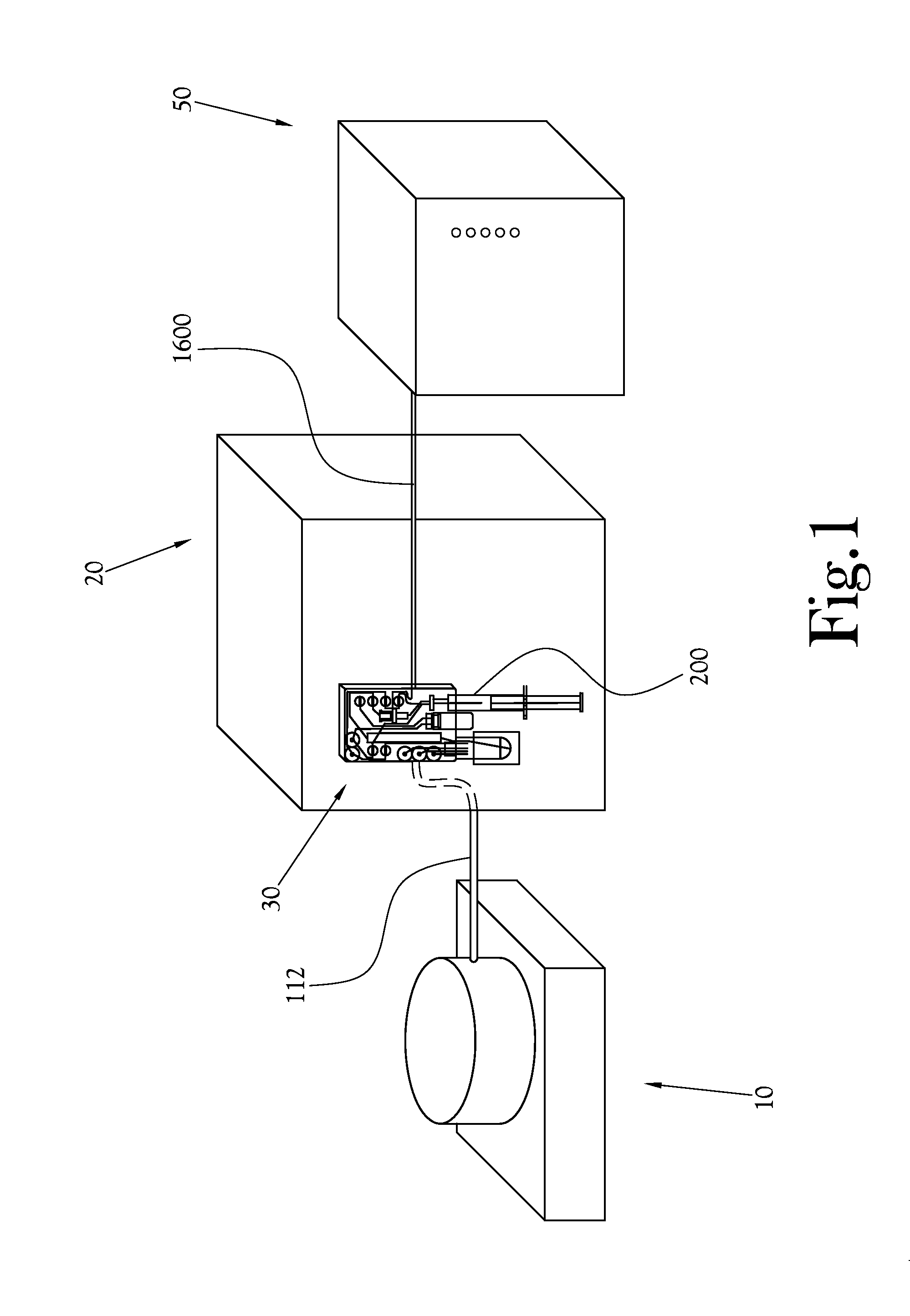
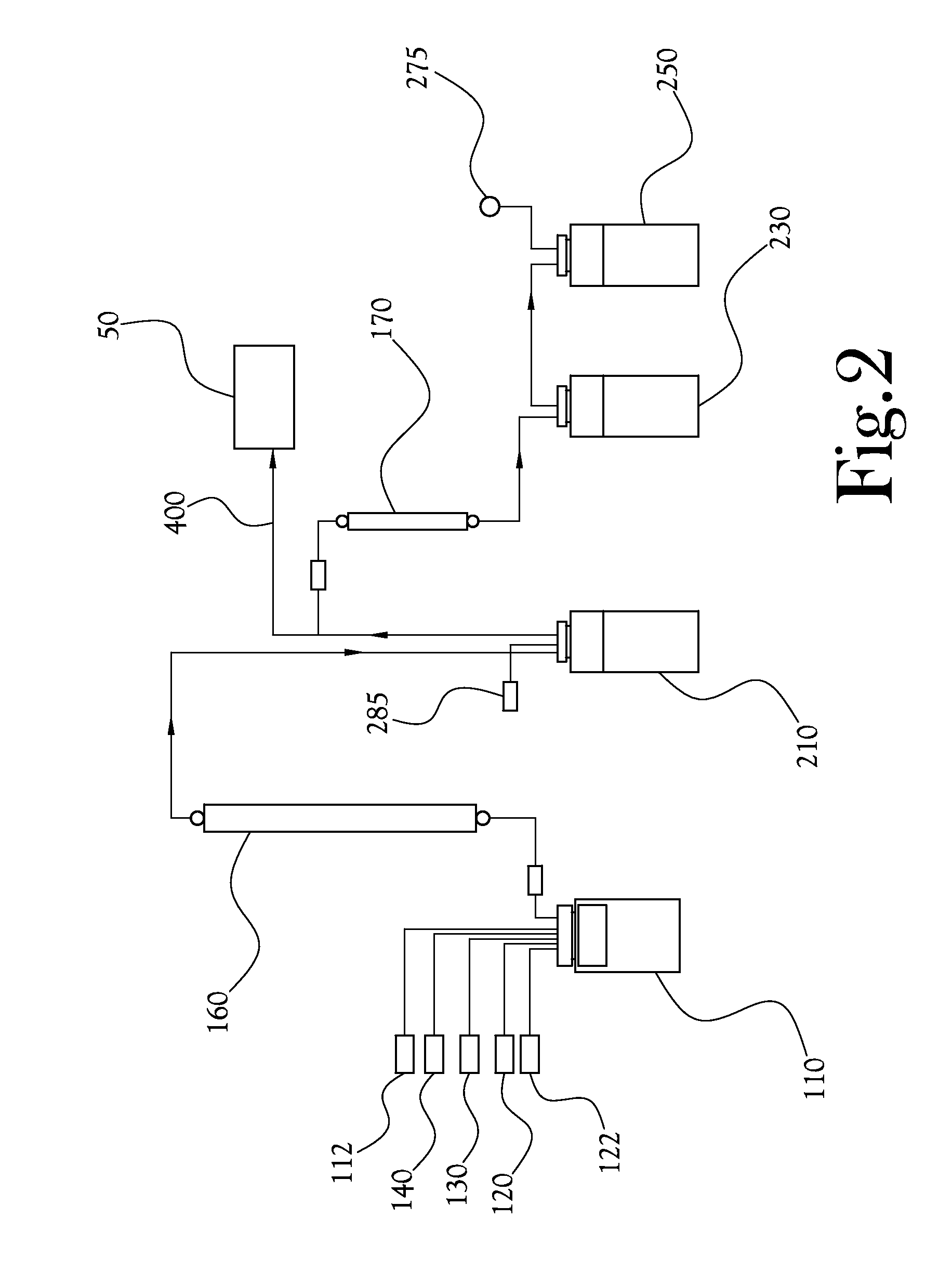
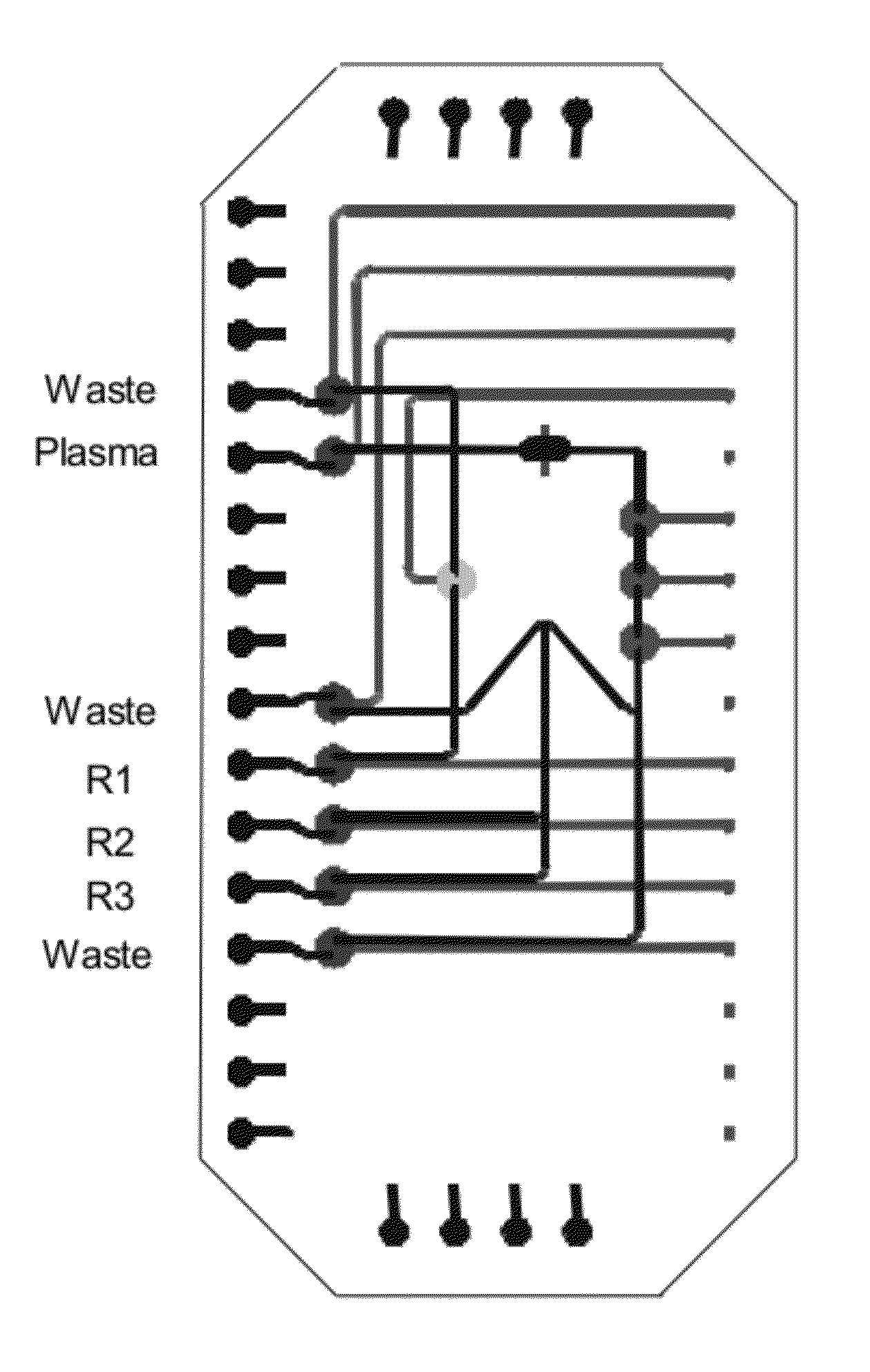
A microfluidic radiopharmaceutical production system and process for synthesizing per run approximately, but not less than, one (1) unit dose of a radiopharmaceutical biomarker for use in positron emission tomography (PET). The radiopharmaceutical production system includes a reaction vessel that receives a radioisotope from an accelerator or other radioisotope generator. Organic and aqueous reagents are introduced into the reaction vessel, and the mixture is heated to synthesize a solution of a pre-selected radiopharmaceutical. The radiopharmaceutical solution is purified by passing the solution through a solid phase extraction column and a filter. The synthesis process produces per run a quantity of radiopharmaceutical approximately equal to, but not less than, one (1) unit dose of a radiopharmaceutical, reducing waste and allowing for the production of radiopharmaceutical on an as-needed basis. The synthesis process allows for the production of biomarker radiopharmaceuticals on site and close to the location where the unit dose will be administered to the patient. On-site, as-needed production of radiopharmaceuticals in small doses reduces the time between the synthesis of the radiopharmaceutical and the administration of that radiopharmaceutical, thereby minimizing the loss of active isotopes through decay and allowing the production of lesser amounts of radioisotopes overall.
Owner:BEST ABT INC
Device and method of plaining glasses or glass-ceramics
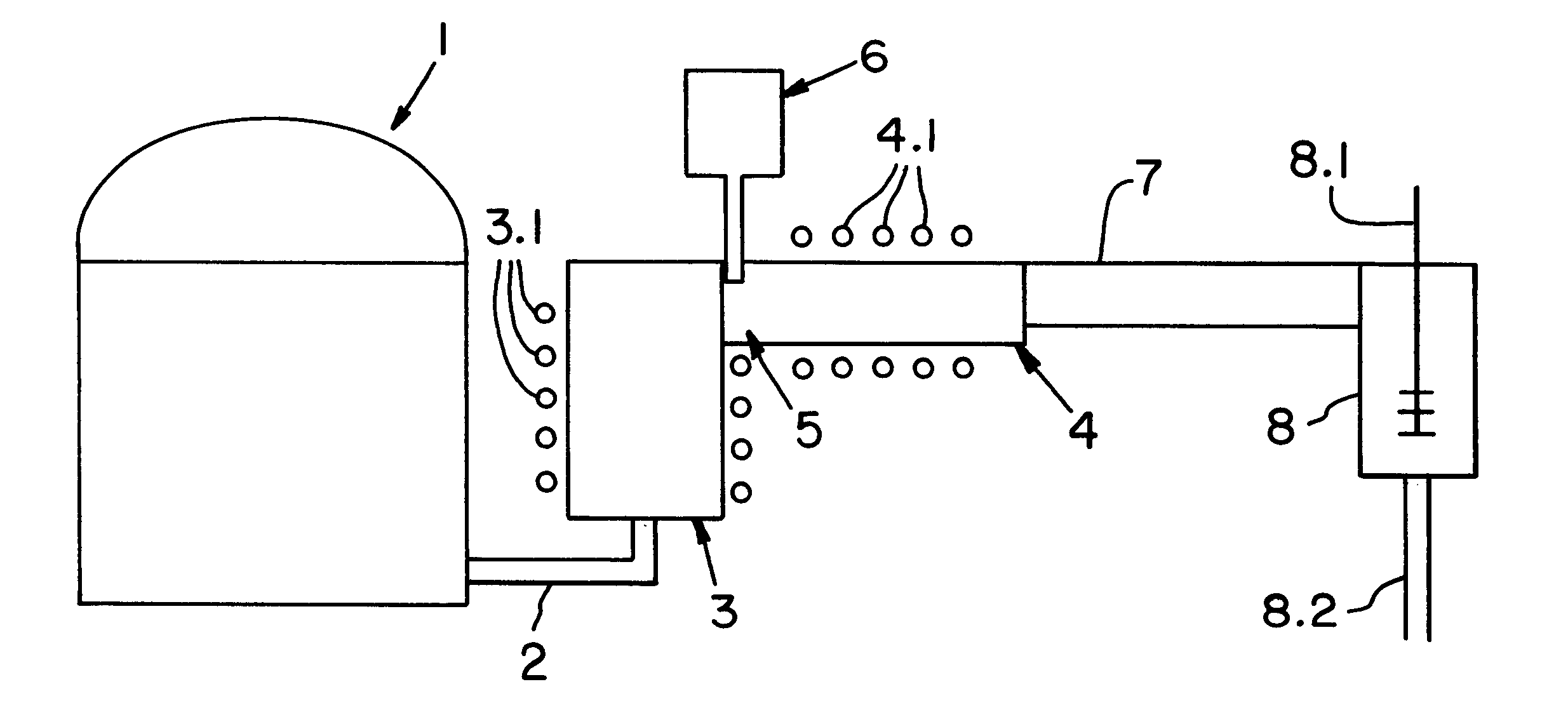
InactiveUS6588234B1Significantly effectiveImprove glass qualityGlass furnace apparatusGlass drawing apparatusEngineeringGlass-ceramic
A device and method for the plaining of glasses or glass-ceramics. The device is provided with a melting vat, at least two plaining containers serially connected after the outlet of the melting vat, and at least one of the plaining containers is built in accordance with the skull principle from a plurality of metal tubes comprising a cooling agent connection and a high-frequency device for inductively coupling high-frequency energy into the contents of the plaining container.
Owner:SCHOTT AG
Quality Control Module for Biomarker Generator System
InactiveUS20110070158A1Reduce in quantityLess stringent infrastructure requirementMaterial analysis by observing effect on chemical indicatorComponent separationQuality controlEngineering
A sample card and automated quality control module for a radiopharmaceutical synthesis system for conducting quality control tests on approximately one (1) unit dose of a radiopharmaceutical biomarker for use in positron emission tomography. The sample card and quality control module allow operators to conduct quality control tests in reduced time using micro-scale test samples from the radiopharmaceutical solution. The sample card works in conjunction with a microfluidic radiopharmaceutical synthesis system to collect samples of radiopharmaceutical solution on the scale of 5-20 microliters per sample. The sample card then interacts with the quality control module to feed the samples into a number of test vessels, where the samples undergo a number of automated quality control tests.
Owner:ABT MOLECULAR IMAGAING
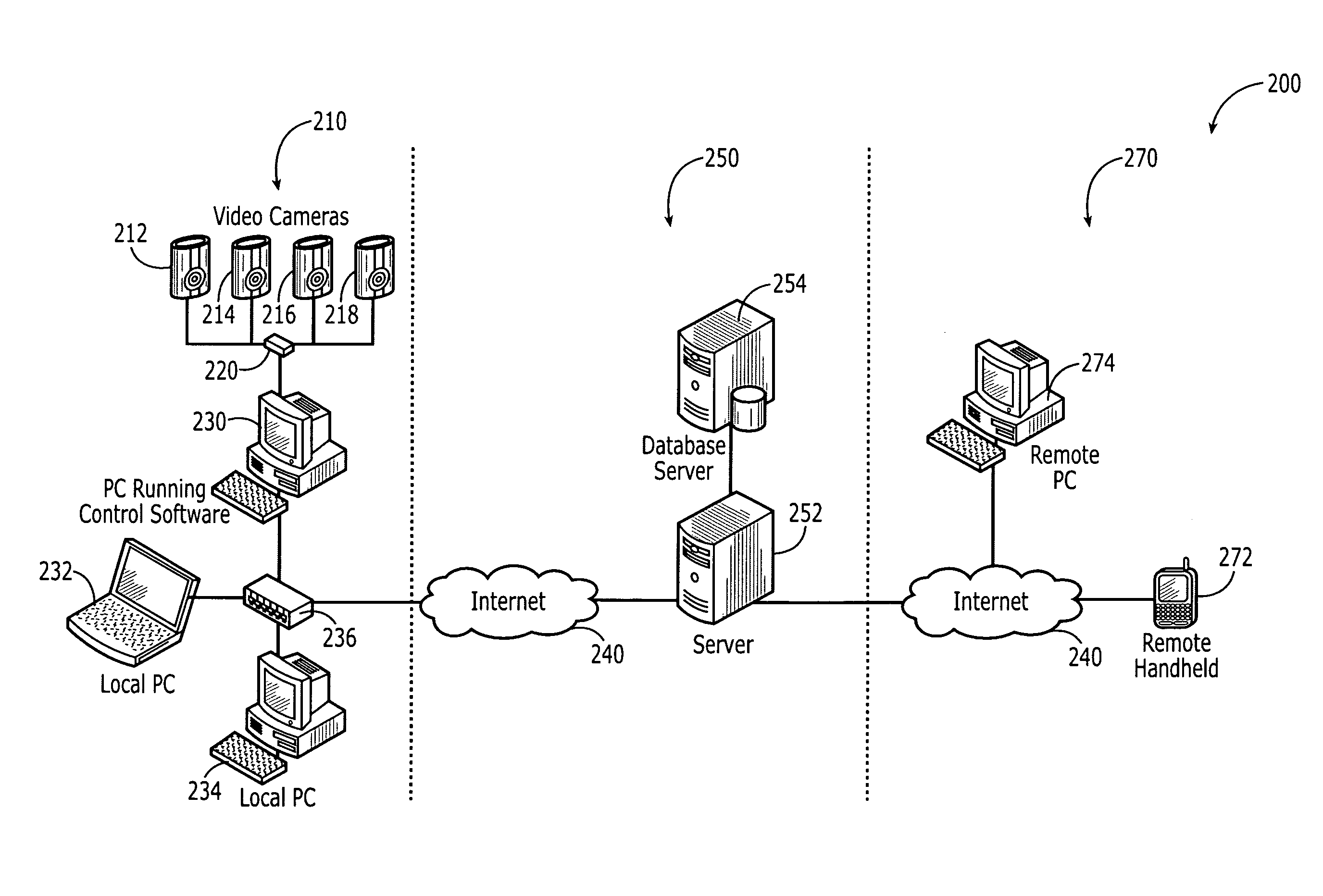
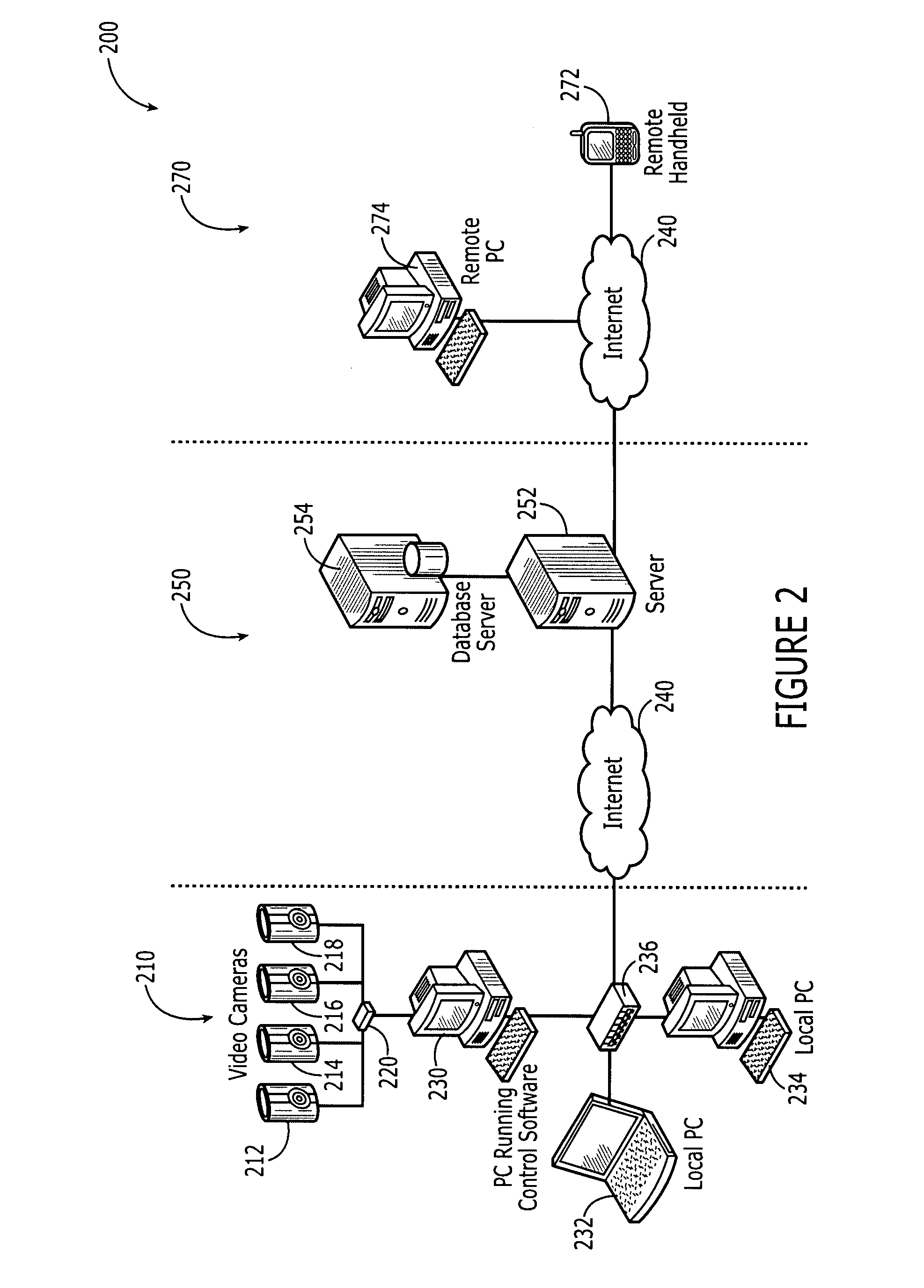
Remote video monitoring systems utilizing outbound limited communication protocols
InactiveUS20080122932A1Enhanced couplingLess bandwidthTelevision system detailsUnauthorised/fraudulent call preventionVideo monitoringTransport system
One embodiment of the present invention relates to a computer based remote video monitoring system including a set of video input sources, a control module, and a client module. The video input sources are coupled to the control module via a local data transmission system such as a local area network. The client module is indirectly data coupled to the control module through an intermediary data server via a global data transmission system such as the Internet. The indirect data coupling between the client module and the control module is limited to an outbound data limited communication protocol such as an instant messaging protocol specifically including the messaging and presence protocol XMPP. The indirect data coupling enables the client module to perform various video monitoring system management related functions including controlling the control module so as to effect the video data signals produced by the video input sources.
Owner:WILIFE
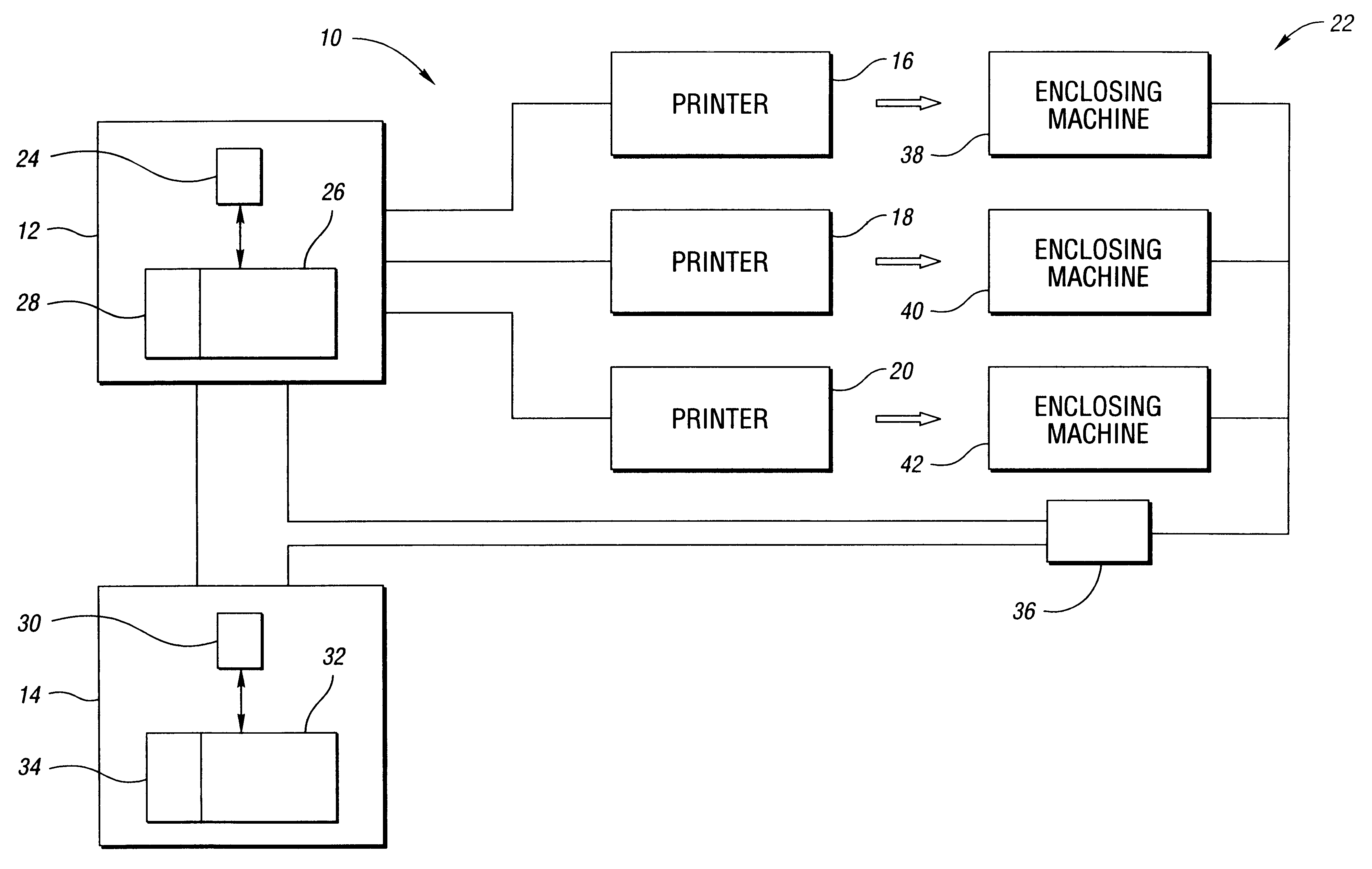
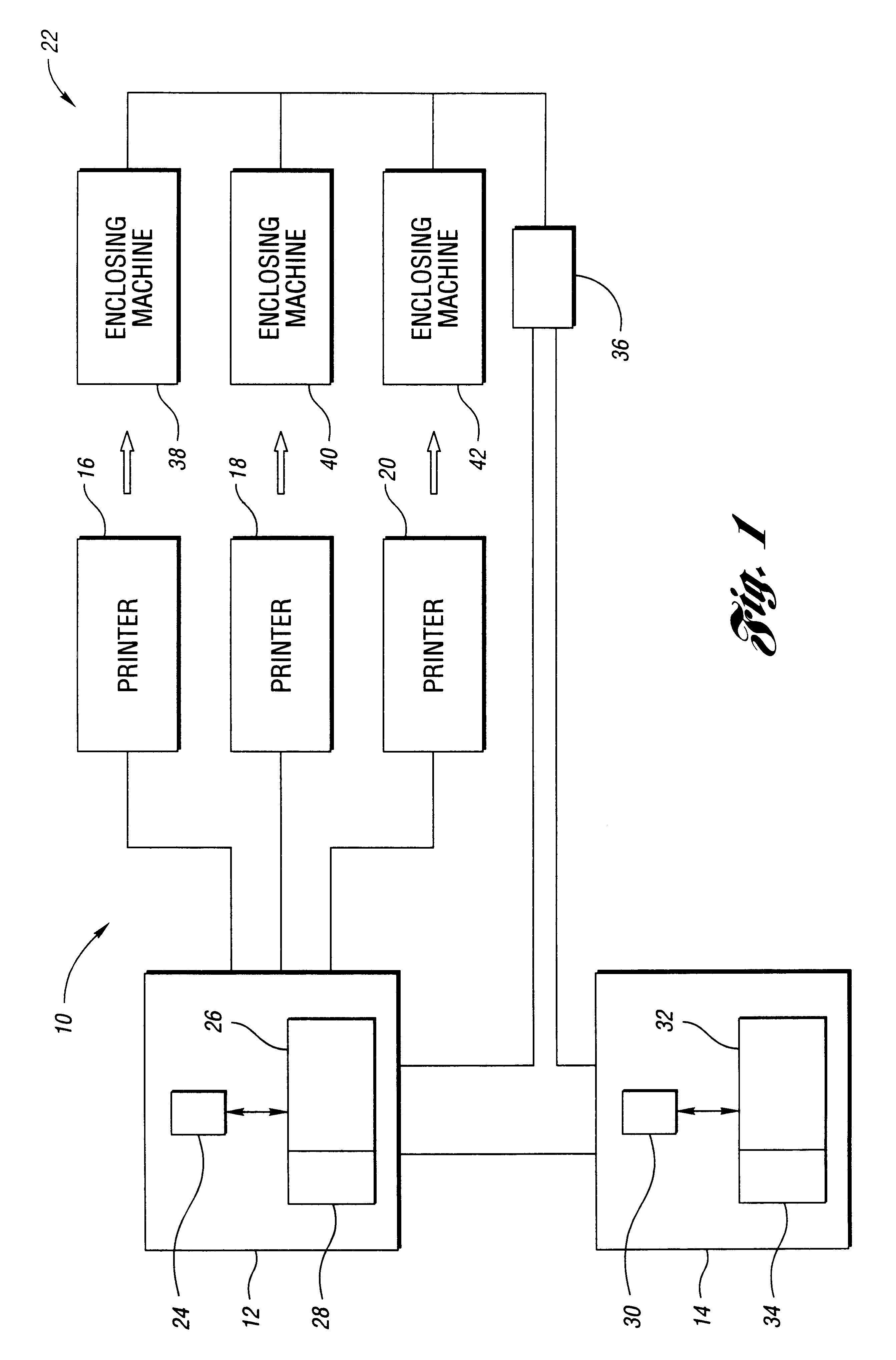
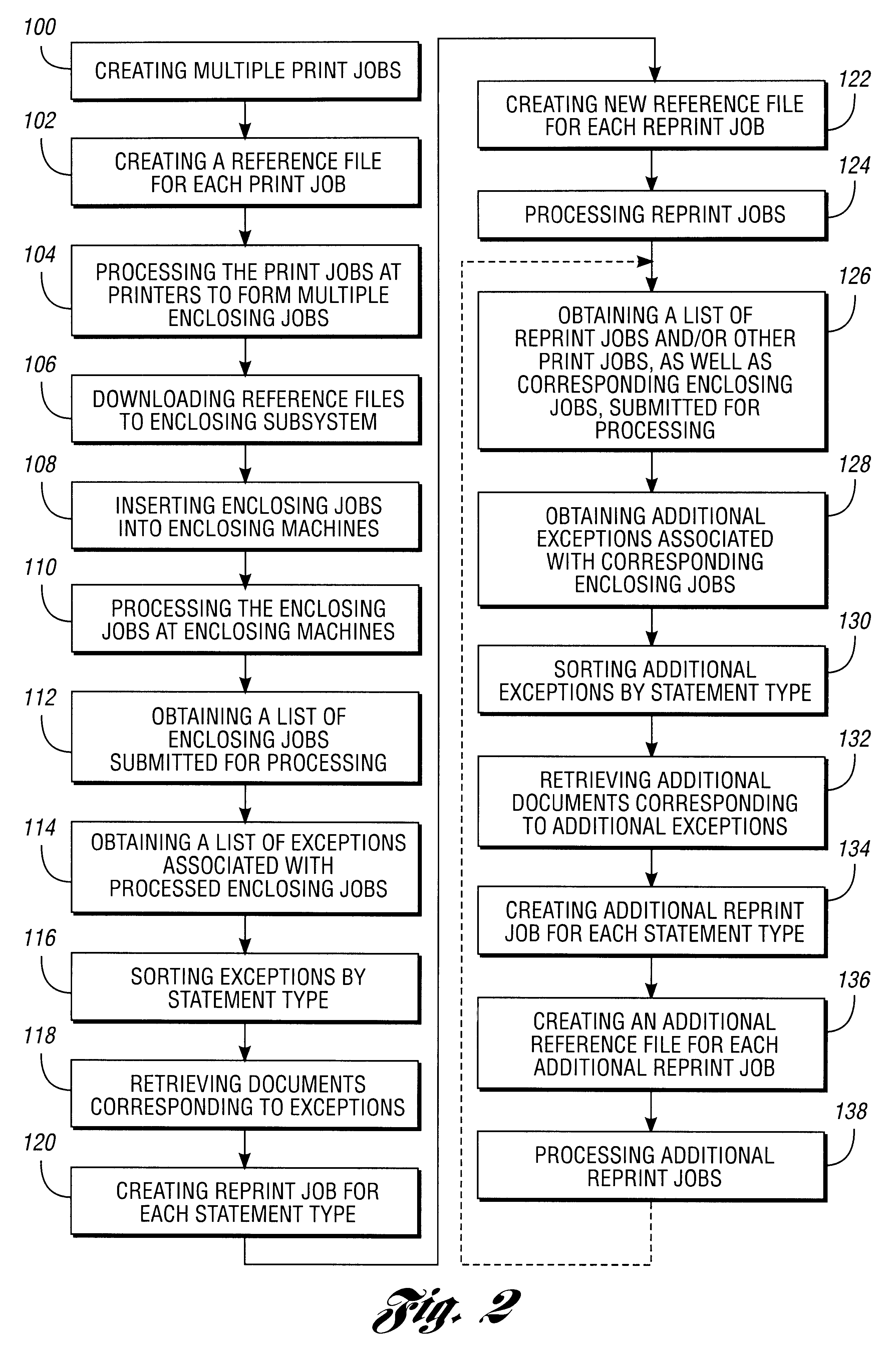
Document reprint method and system
InactiveUS6747749B1Significantly effectiveLess-costly to implementDigitally marking record carriersDigital computer detailsDocumentationReprint
A method for reprinting a plurality of documents includes obtaining exceptions associated with multiple processed enclosing jobs. The method further includes creating a reprint job that includes documents corresponding to a portion of the exceptions, wherein the portion of the exceptions is associated with at least two of the processed enclosing jobs. A system for reprinting a plurality of documents is also disclosed.
Owner:QWEST
Radiopharmaceutical Production System and Quality Control System Utilizing High Performance Liquid Chromatography
InactiveUS20130130309A1Minimal impactPer dose costBioreactor/fermenter combinationsBiological substance pretreatmentsRadiopharmaceutical CompoundQuality control system
HPLC-based quality control systems to perform quality control testing on a radiopharmaceutical solution shortly after synthesis. An HPLC-based quality control system makes efficient use of sample volume and is compatible with a variety of radioisotopes and radiopharmaceutical compounds. In several embodiments, the automated nature of an HPLC-based quality control system allows for quality control tests to be conducted quickly and with minimal impact on user workflow. When used as part of an integrated PET biomarker radiopharmaceutical production system, the present general inventive concept permits a manufacturer to produce product and conduct quality control tests with lower per dose costs.
Owner:BEST ABT INC
Method and Apparatus for Producing a High Level of Disinfection in Air and Surfaces
ActiveUS20110272595A1Significantly effectiveOptical radiation measurementWater/sewage treatment by irradiationSporeSpectral width
This specification relates to an improved method, process and apparatus for disinfecting and sterilizing all types of surfaces and indoor air and room air contaminated with microorganisms. The improved apparatus consists of a multi-wavelength narrow spectral width UV source that is more effective than mercury based 254 nm germicidal lamps for destroying the DNA and outer shell or membrane of virus, bacteria, spores and cists.
Owner:CASALE RICHARD
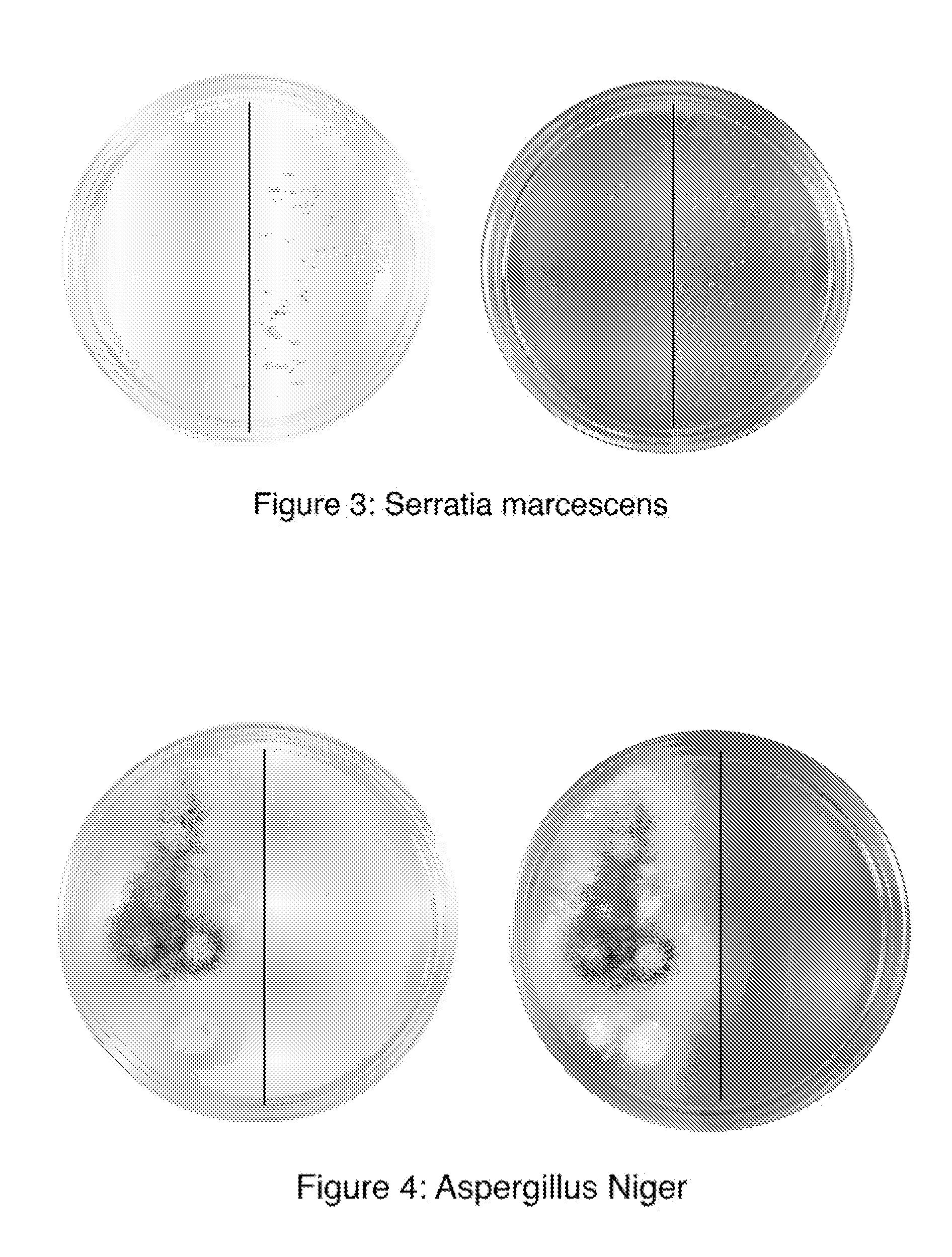
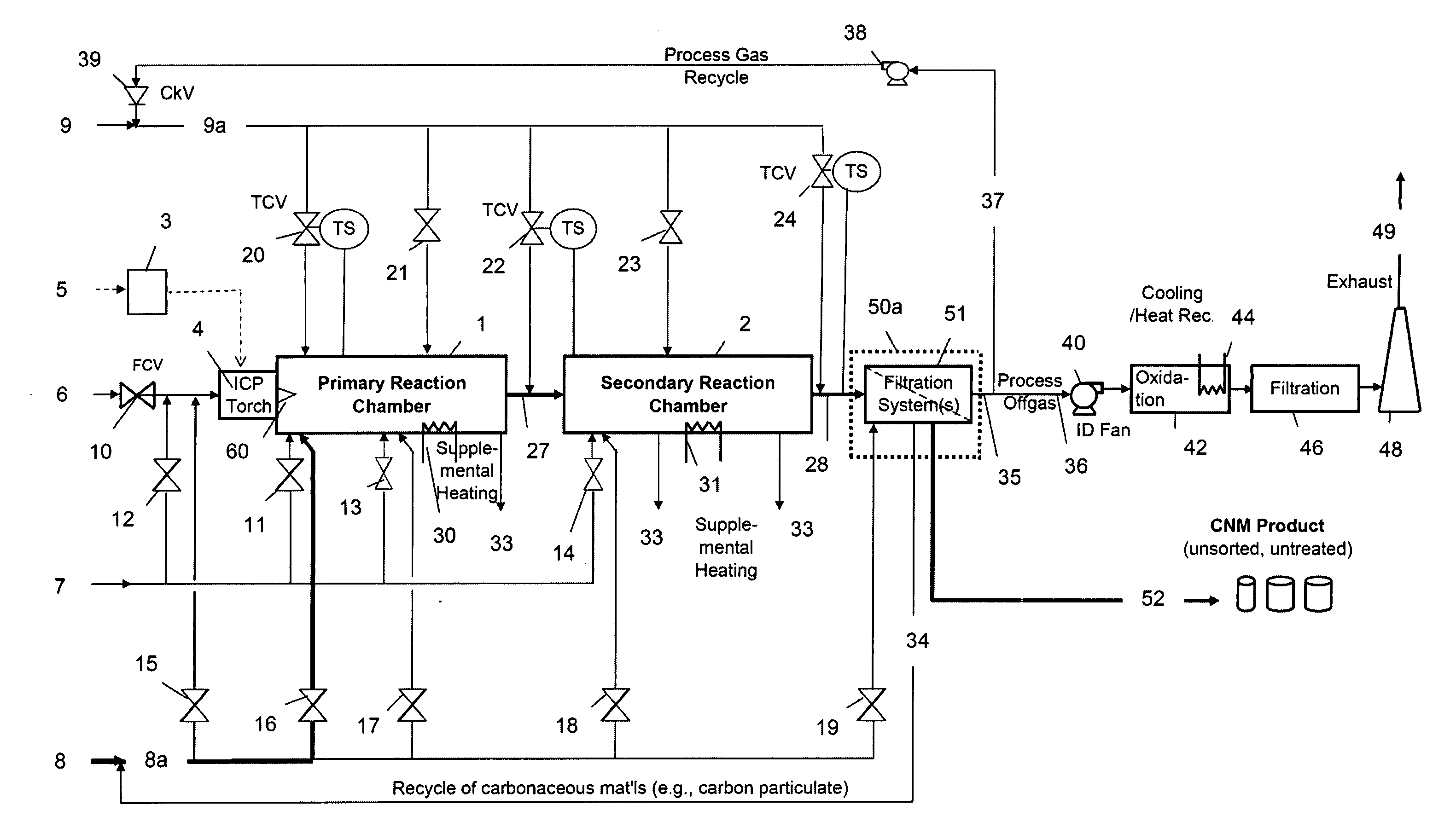
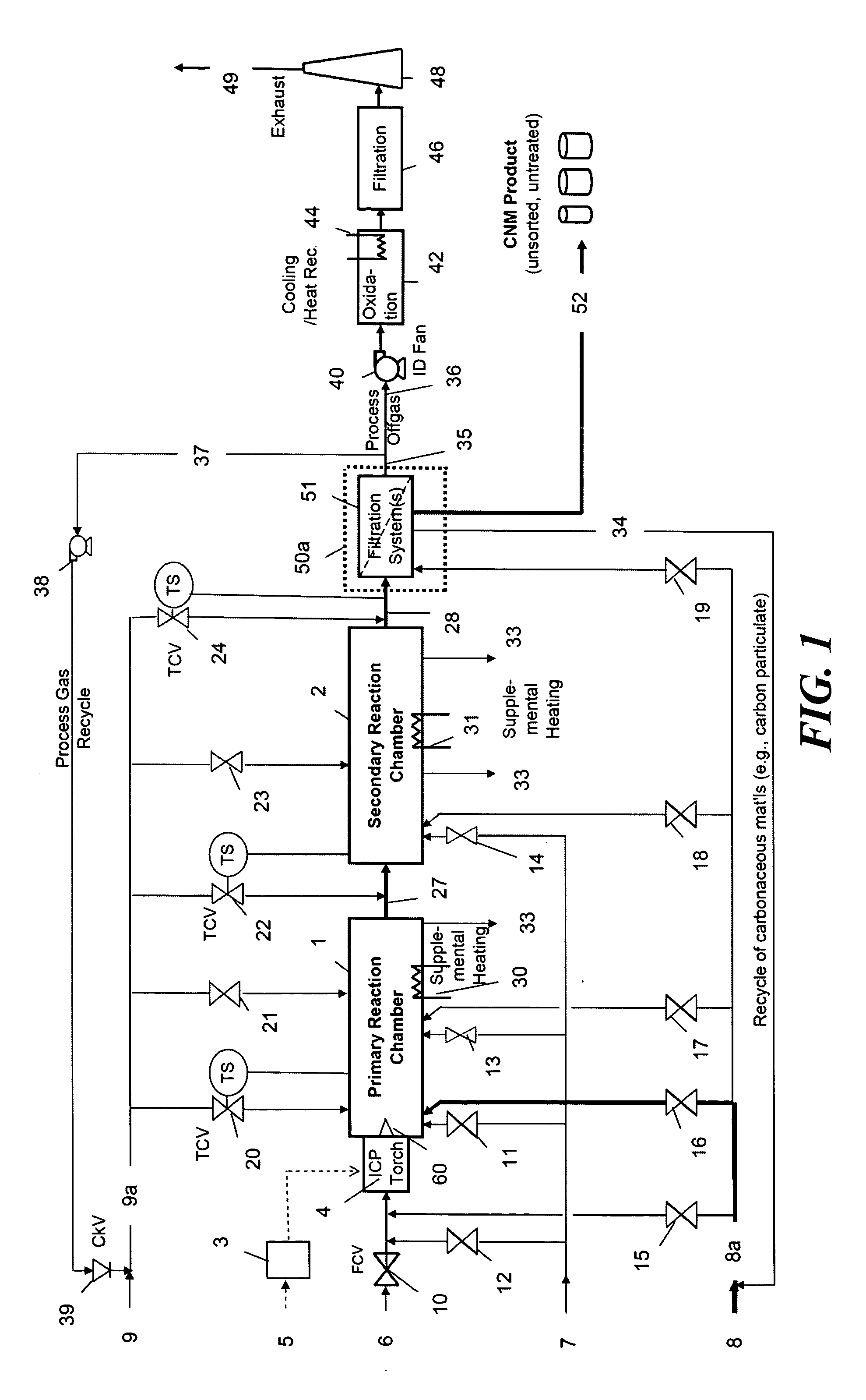
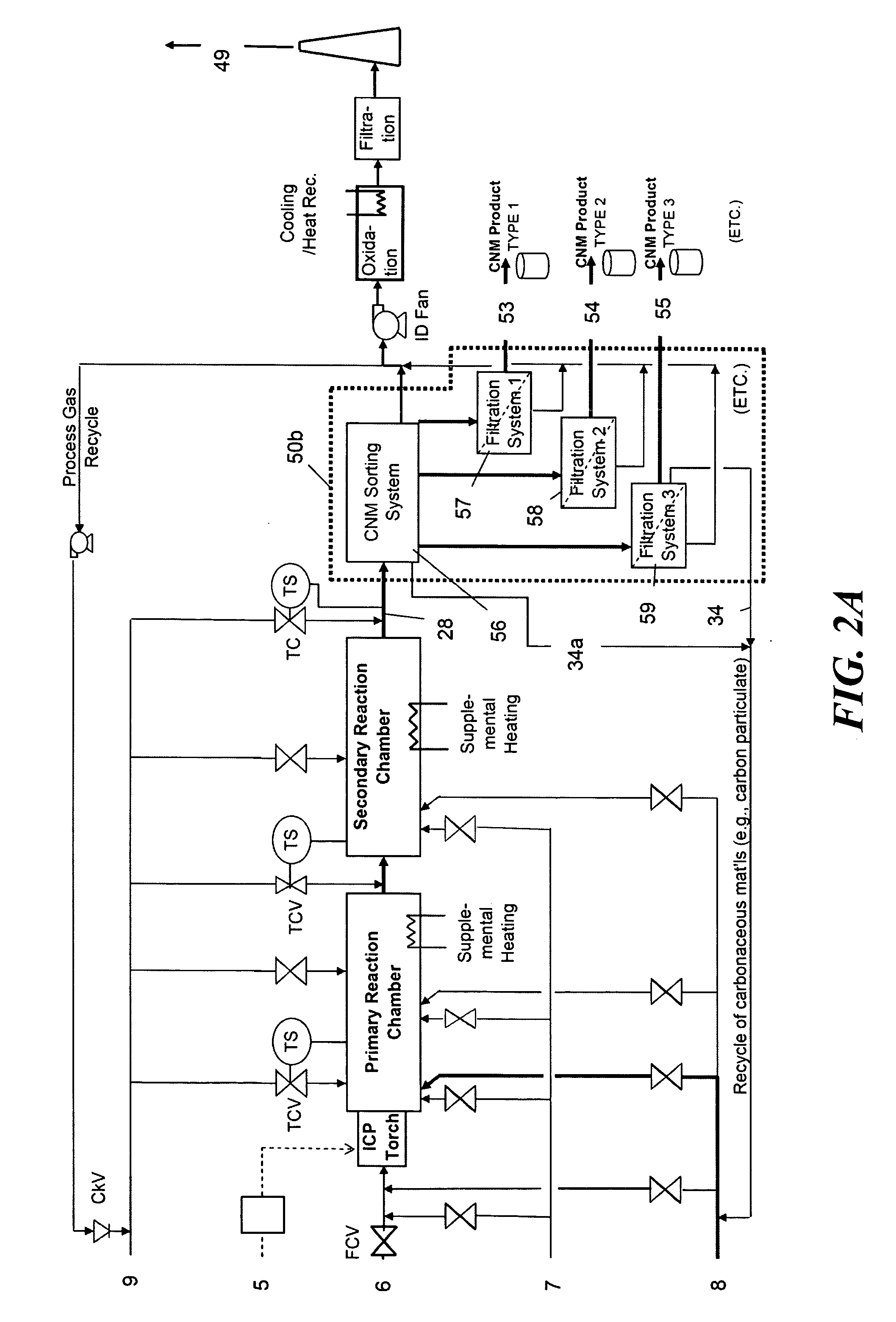
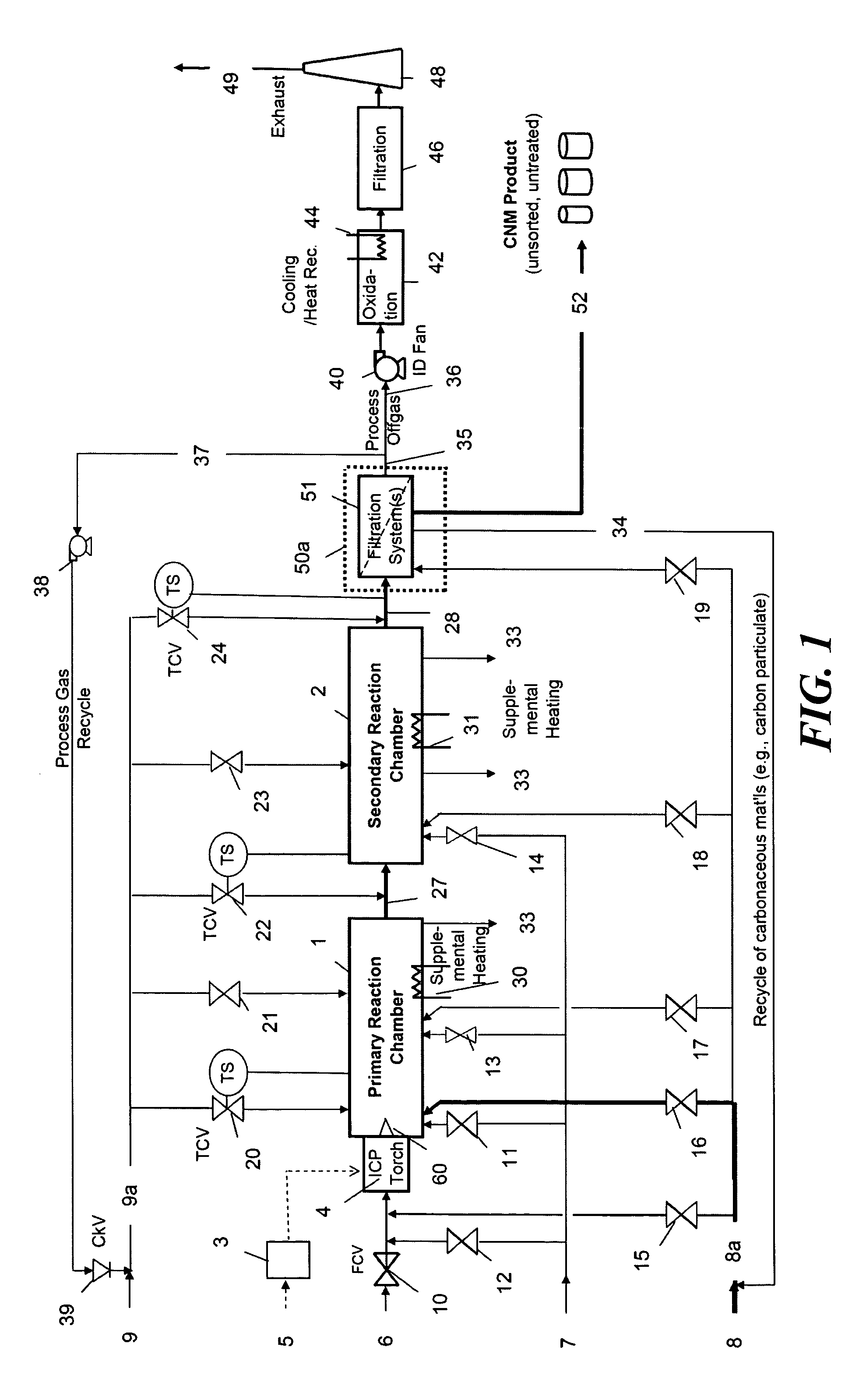
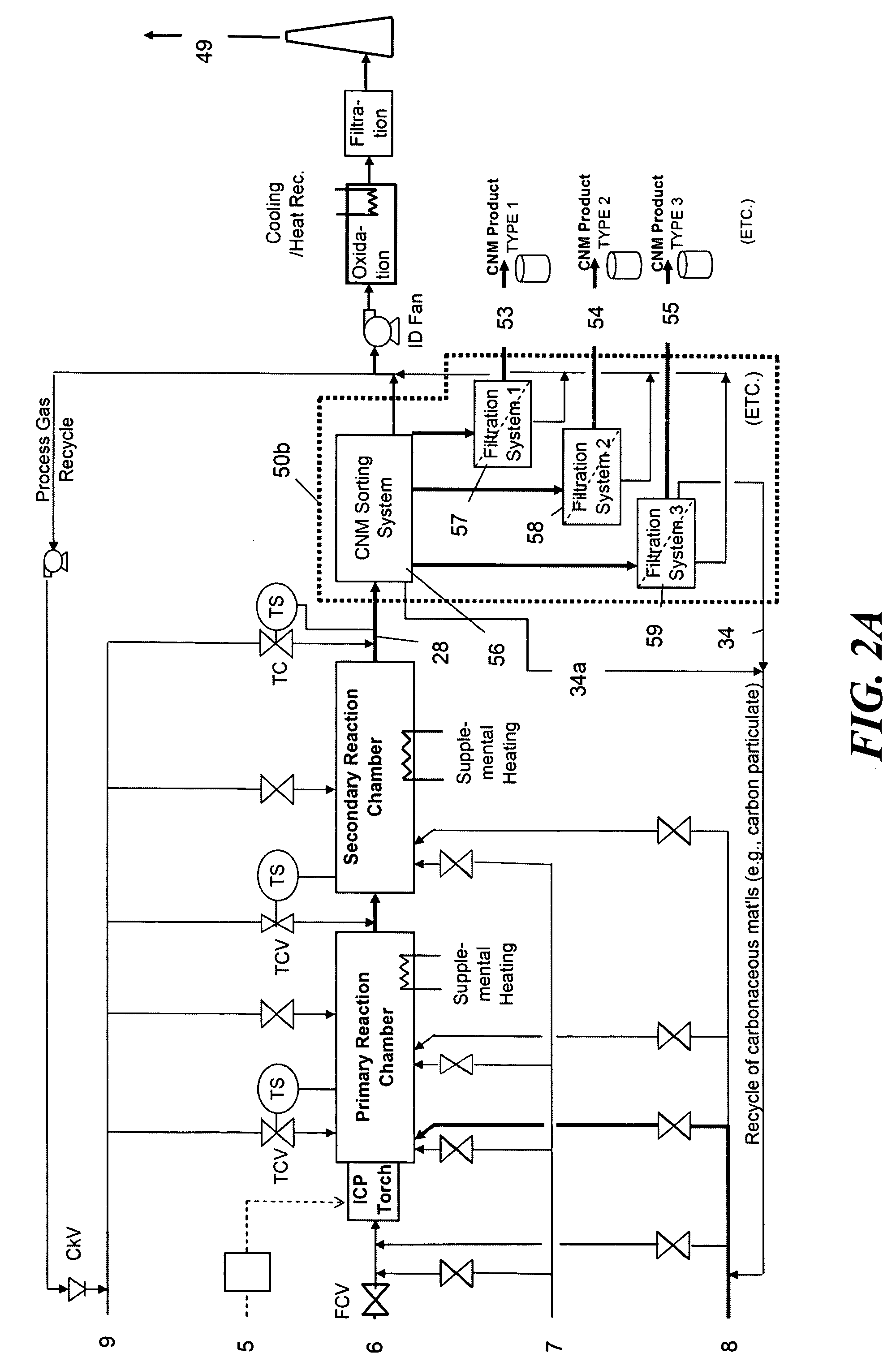
Continuous production of carbon nanomaterials using a high temperature inductively coupled plasma
InactiveUS20090099004A1Significantly effectiveEffective filteringMaterial nanotechnologyOther chemical processesIndustrial scaleReaction chamber
High-power inductively coupled plasma technology is used for thermal cracking and vaporization of continuously fed carbonaceous materials into elemental carbon, for reaction with separate and continuously fed metal catalysts inside a gas-phase high-temperature reactor system operating at or slightly below atmospheric pressures. In one particularly preferred embodiment, in-flight growth of carbon nanomaterials is initiated, continued, and controlled at high flow rates, enabling continuous collection and product removal via gas / solid filtration and separation methods, and / or liquid spray filtration and solid collection methods suitable for producing industrial-scale production quantities. In another embodiment, the reaction chamber and / or filtration / separation media include non-catalytic or catalytic metals to simultaneously or separately induce on-substrate synthesis and growth of carbon nanomaterials. The on-substrate grown carbon nanomaterials are produced in secondary chambers that are selectively isolated for periodic removal of the product.
Owner:SEQUOYAH FINANCE ONE
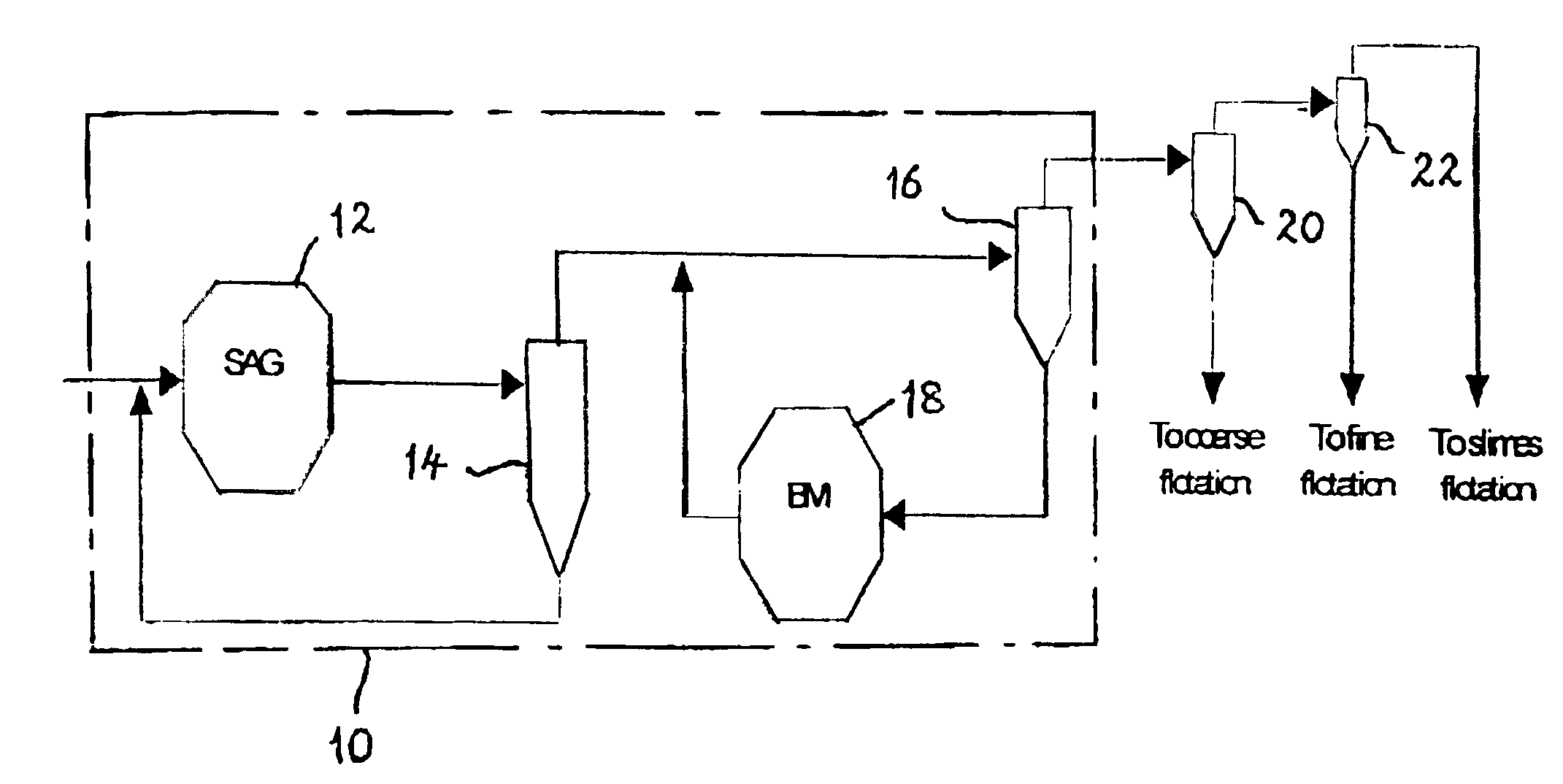
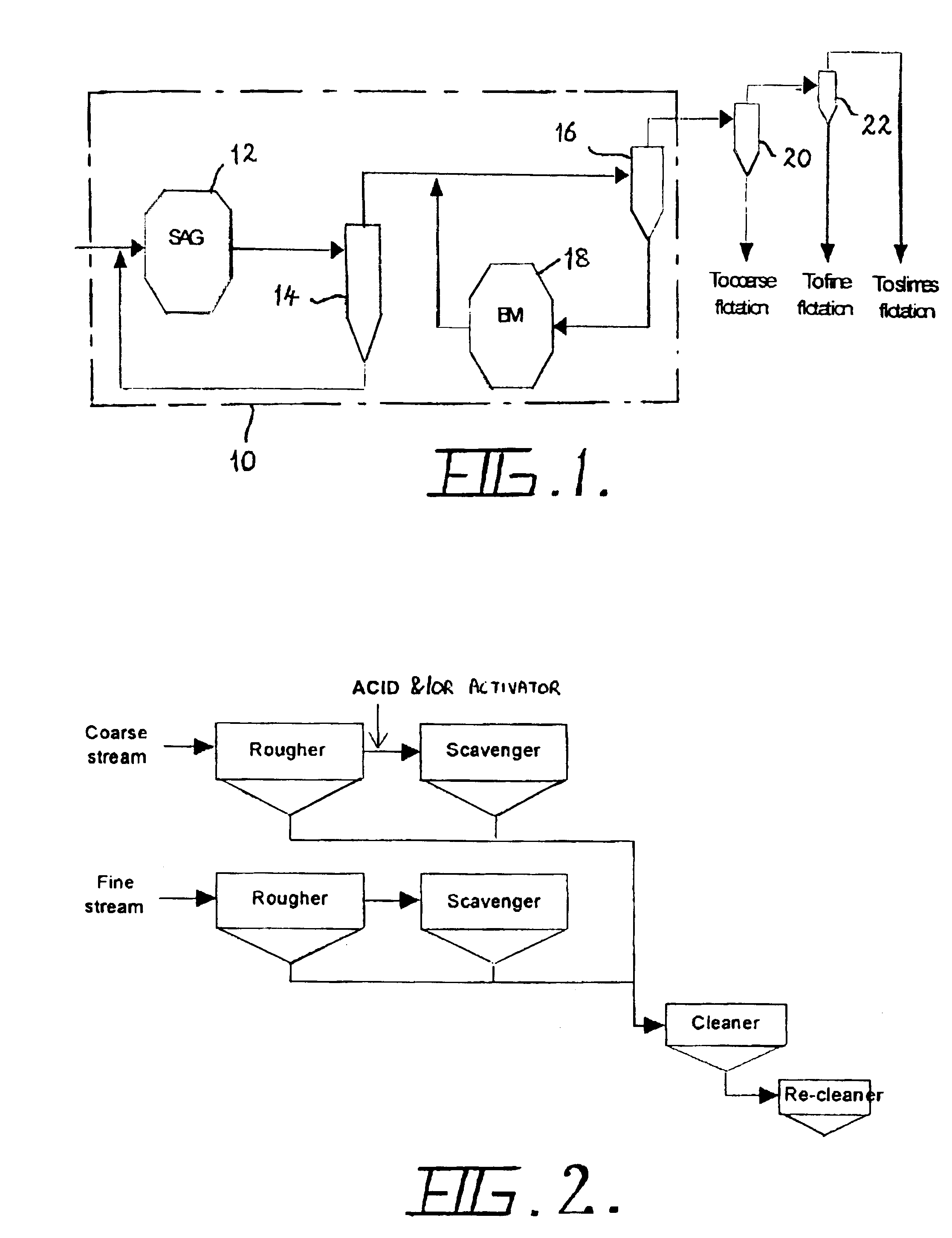
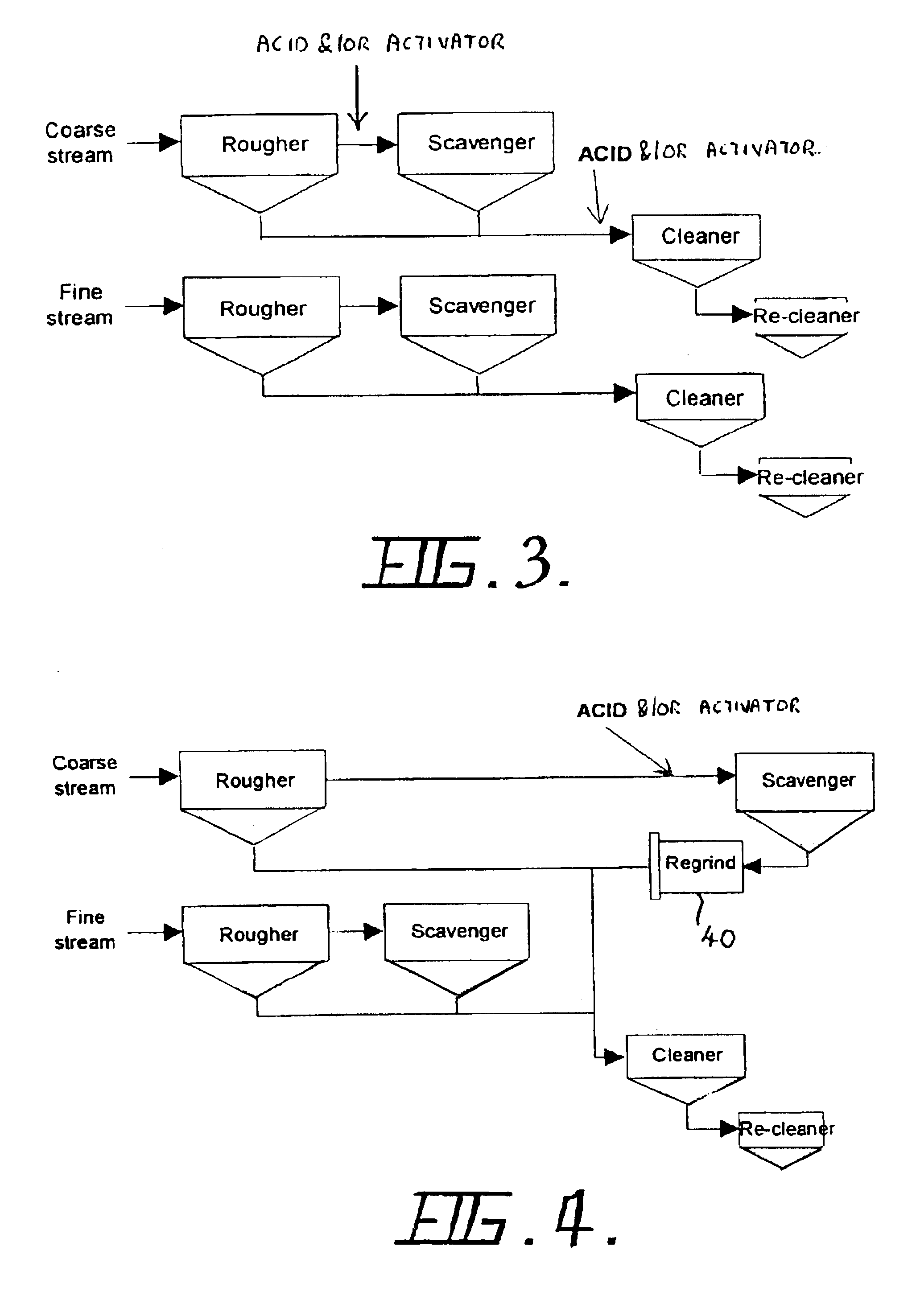
Flotation of sulphide minerals
InactiveUS6945407B2Delayed recoveryReduce dispersionTobacco preparationGold compoundsSulfide mineralsMaceral
The present invention relates generally to a process and an apparatus for flotation of sulphide minerals, such as sulphide minerals hosted in ores rich in magnesium minerals. The process involves grinding of the nickel ore rich in magnesium minerals and thereafter separation of the ground material into a coarse and fine stream of particles coarser than about 30 microns and finer than about 30 microns, respectively. Optionally, the fines stream may be further separated into a slimes fraction. The coarse and fine flotation streams are then fit to separate parallel flotation circuits. Acid and / or activator is added during flotation of the coarse stream only. Significantly improved recoveries and grades were obtained with reduced acid consumption.
Owner:BHP BILLITON SSM INDONESIA HLDG PTY LTD
System and method for analyzing a business process integration and management (BPIM) solution
InactiveUS7890309B2Significantly effectiveSignificantly efficientForecastingProgram controlProcess integrationBusiness process integration
Owner:INT BUSINESS MASCH CORP
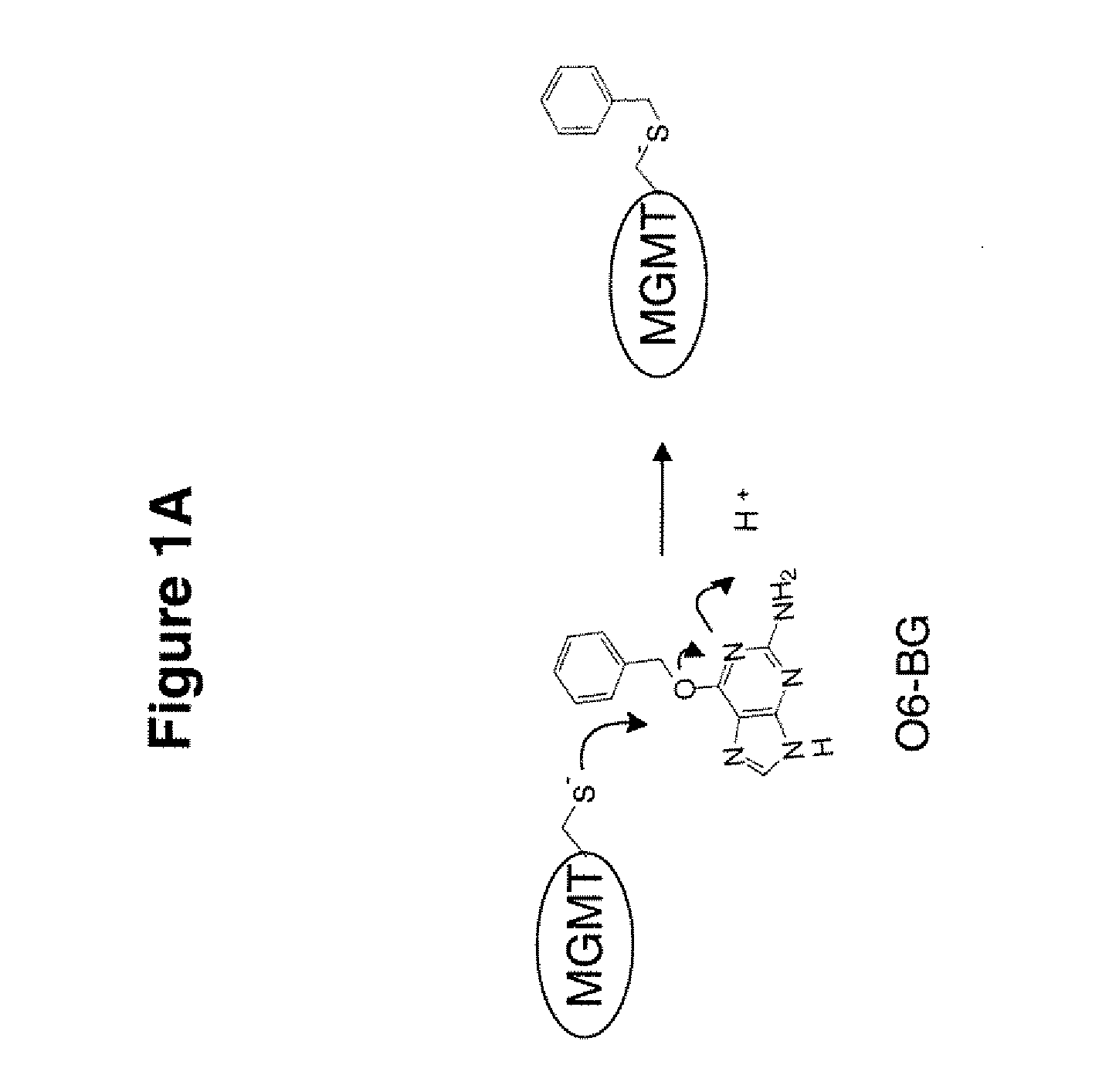
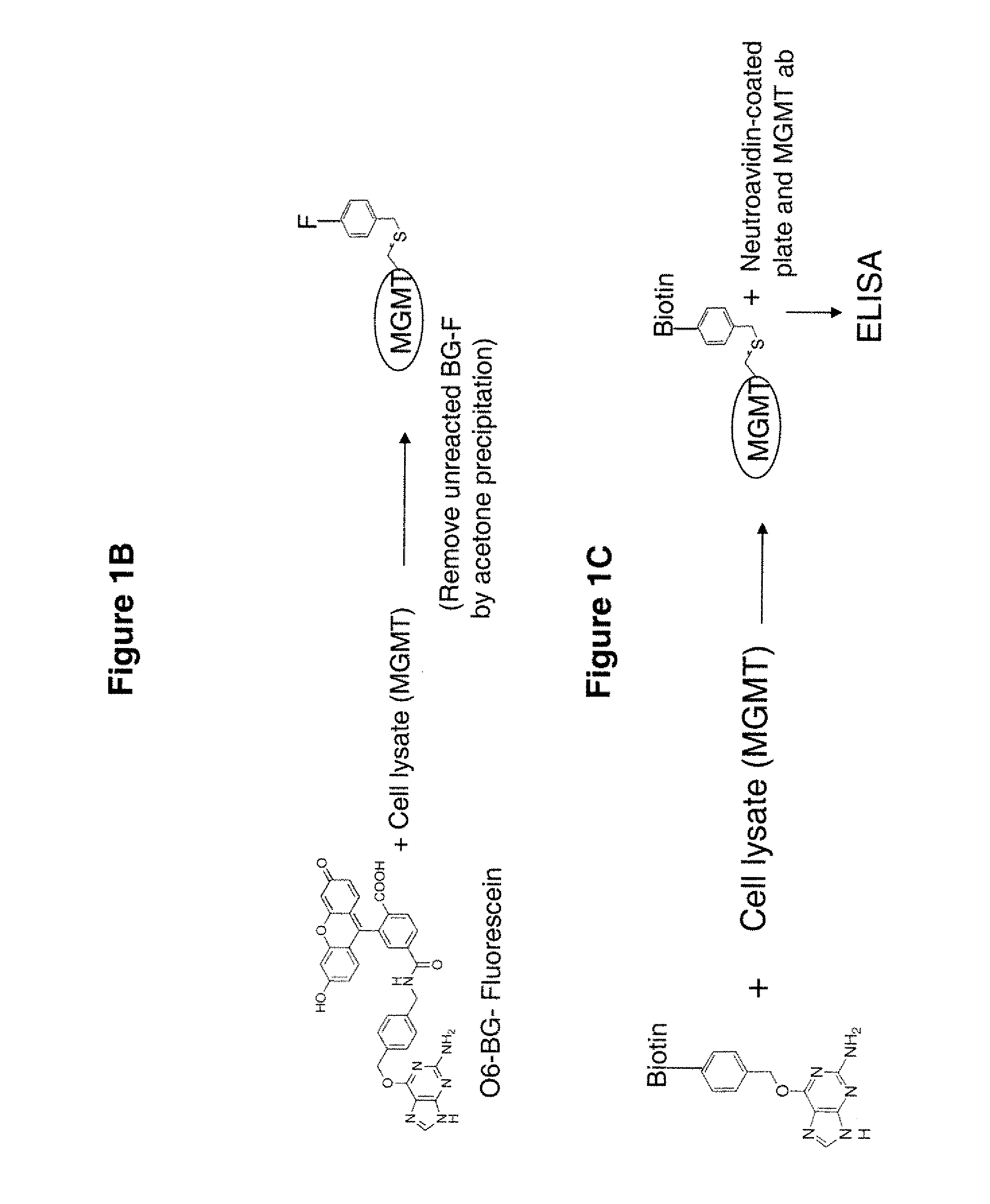
Development of a novel assay for mgmt (methyl guanine methyl transferase)
InactiveUS20070264672A1Shorten the timeLower potentialBioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceRepair enzyme
The present invention provides improved methods for assessing the level of MGMT activity in a variety of biological preparations. MGMT, a DNA repair enzyme, can reduce the chemotherapeutic efficacy of alkylating agents by repairing the damage that alkylating agents do to tumor cell DNA. The methods of the present invention can be used, inter alia, to measure MGMT levels and to thereby predict the clinical response to alkylating agents. The present invention includes three preferred assays for assessment of MGMT activity: (1) the immunoassay technique, (2) the labeled O6—BG technique, and (3) the fluorescence polarization technique. Kits useful for the performance of such assays are also provided.
Owner:SCHERING CORP
Dose Synthesis Card for Use with Automated Biomarker Production System
ActiveUS20150157743A1Reduce in quantityLess stringent infrastructure requirementChemical/physical/physico-chemical microreactorsRadioactive preparation carriersDose ReducedIsotope
Microfluidic radiopharmaceutical production system and process for synthesizing per run approximately, but not less than, ten (10) unit doses of radiopharmaceutical biomarker for use in positron emission tomography (PET). A radioisotope from an accelerator or other radioisotope generator is introduced into a reaction vessel, along with organic and aqueous reagents, and the mixture heated to synthesize a solution of a pre-selected radiopharmaceutical. The solution is purified by passing through a combination of solid phase extraction purification components, trap and release components, and a filter. The synthesis process reduces waste and allows for production of biomarker radiopharmaceuticals on site and close to the location where the unit dose will be administered to the patient. On-site, as-needed production of radiopharmaceuticals in small doses reduces the time between synthesis of the radiopharmaceutical and administration of that radiopharmaceutical, minimizing loss of active isotopes through decay and allowing production of lesser amounts of radioisotopes overall.
Owner:BEST ABT INC
Integrated military mobile work information management system
InactiveUS20180096309A1Improve network securityIncrease information dominanceDatabase queryingParticular environment based servicesGraphicsDocumentation procedure
Owner:SEVERN PACIFIC INC
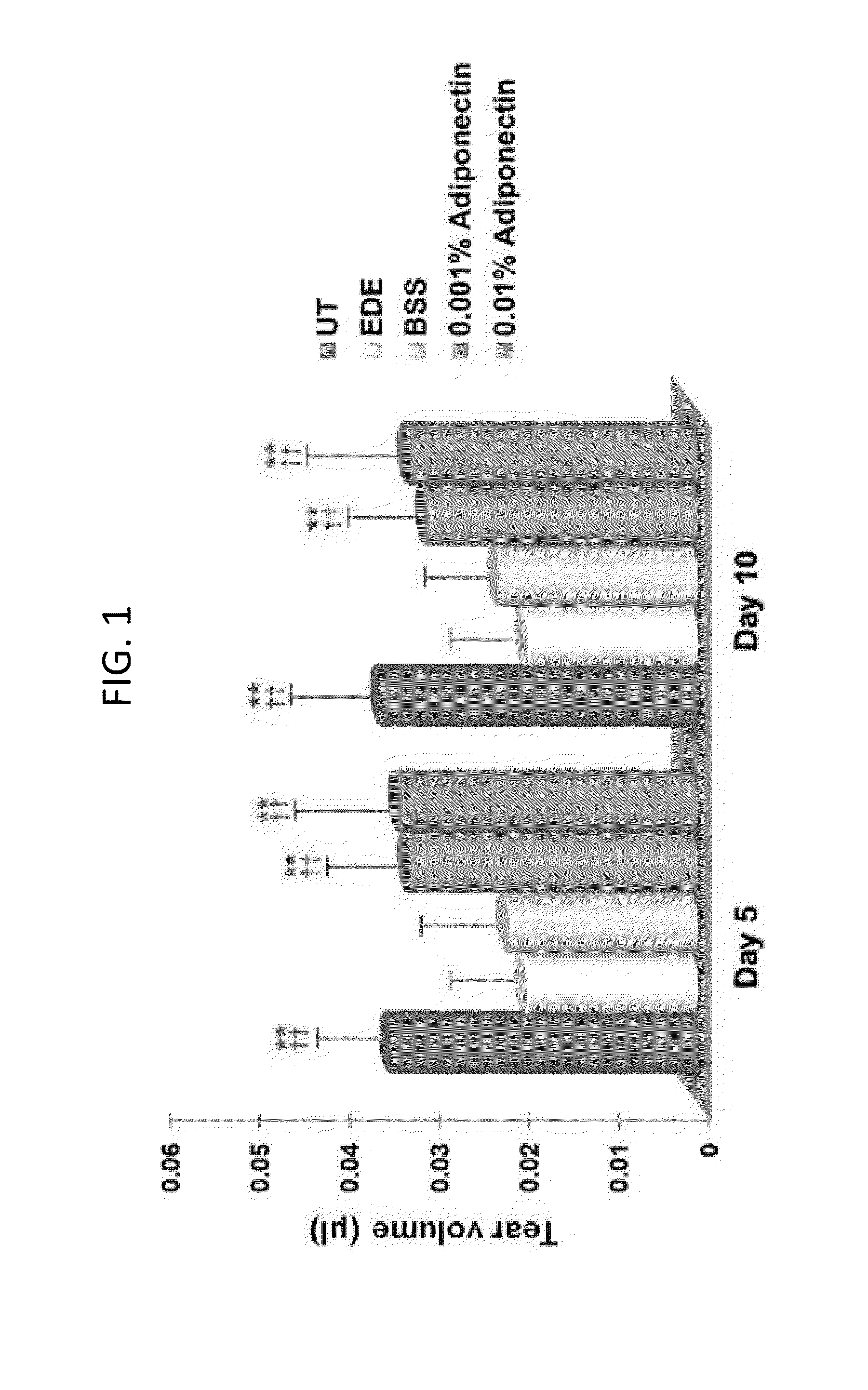
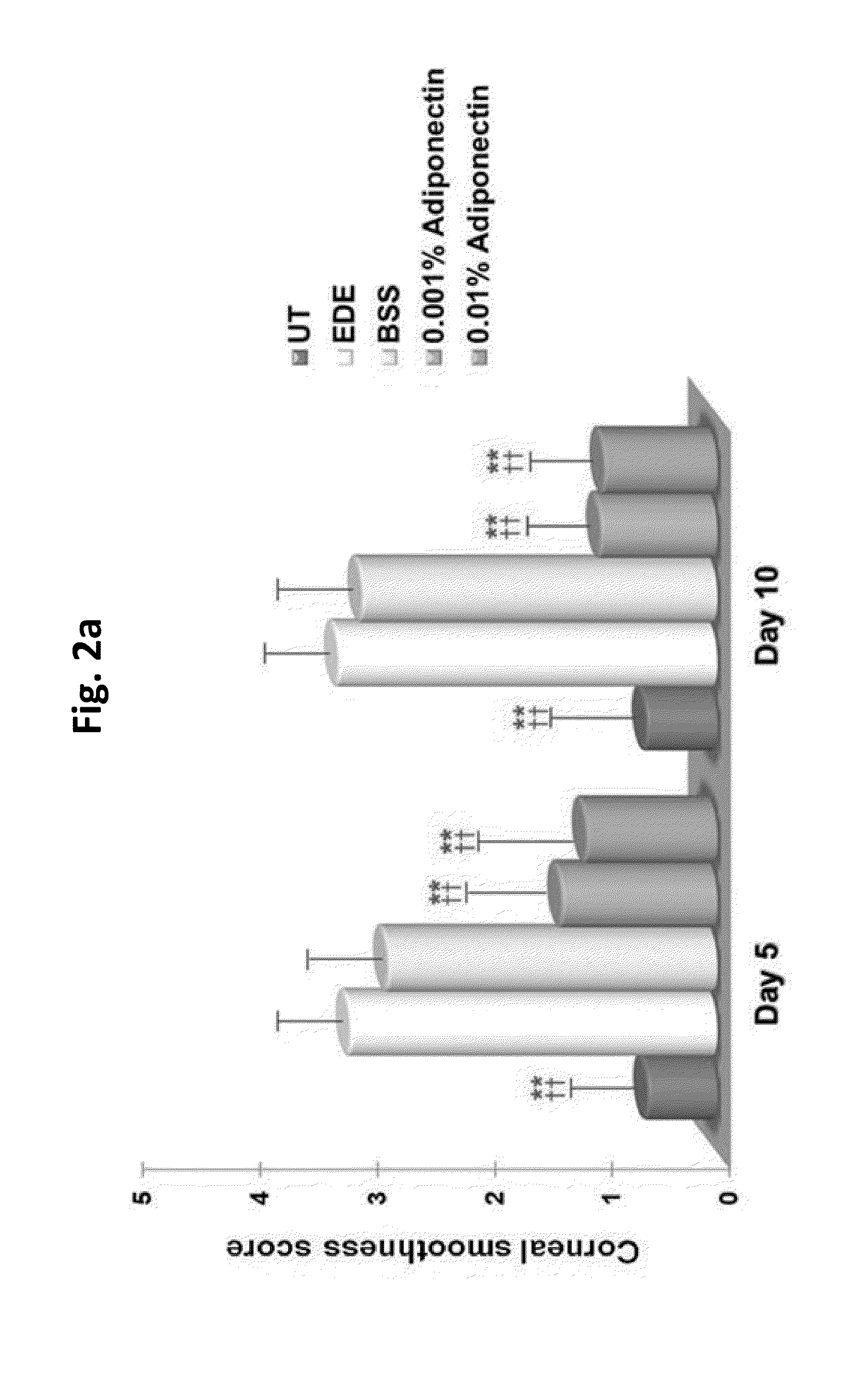
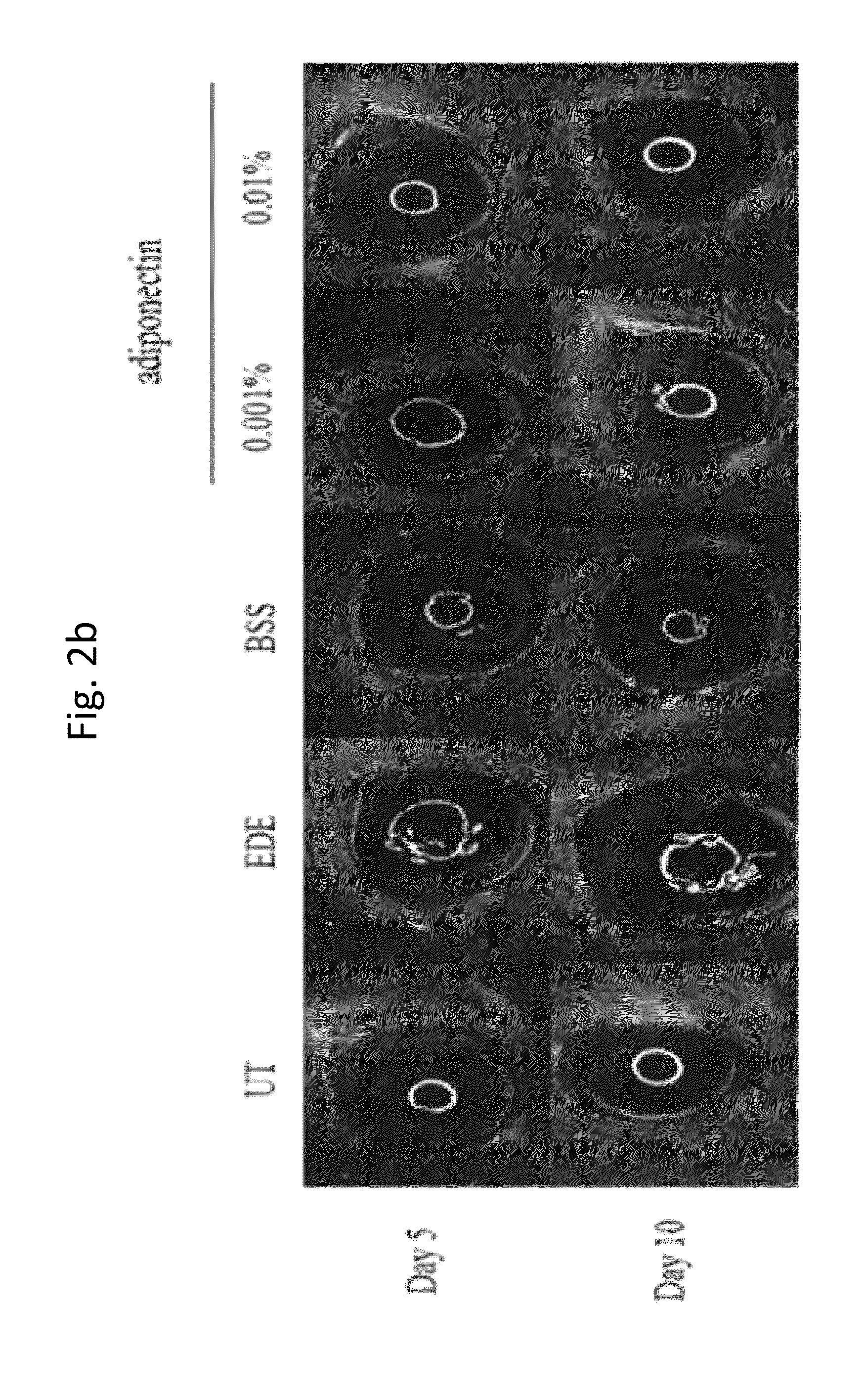
Methods for preventing or treating eye diseases using adiponectin
ActiveUS8815795B2Significantly effectivePromote productionHormone peptidesSenses disorderDiseaseSide effect
A composition for preventing or treating an eye disease includes adiponectin as an active ingredient. Adiponectin as an active ingredient is eventually revealed to show prevention or therapeutic efficacies for eye diseases such as dry eye (syndrome), inflammatory eye disease and side effects due to the use of contact lenses by promoting tear secretion, alleviating ocular surface irregularities, decreasing inflammatory cytokines on the ocular surface and lacrimal gland, and increasing conjunctival goblet cell density. In addition, the composition having eye contact lubrication effects may be used as cleaners or lubricants for preventing non-bacterial inflammation due to wearing contact lenses.
Owner:IND FOUND OF CHONNAM NAT UNIV
Dose synthesis module for biomarker generator system
ActiveUS8333952B2Reduce in quantityLess stringent infrastructure requirementProcess control/regulationIn-vivo radioactive preparationsDose ReducedIsotope
A microfluidic radiopharmaceutical production system and process for synthesizing per run approximately, but not less than, one (1) unit dose of a radiopharmaceutical biomarker for use in positron emission tomography (PET). The radiopharmaceutical production system includes a reaction vessel that receives a radioisotope from an accelerator or other radioisotope generator. Organic and aqueous reagents are introduced into the reaction vessel, and the mixture is heated to synthesize a solution of a pre-selected radiopharmaceutical. The radiopharmaceutical solution is purified by passing the solution through a solid phase extraction column and a filter. The synthesis process produces per run a quantity of radiopharmaceutical approximately equal to, but not less than, one (1) unit dose of a radiopharmaceutical, reducing waste and allowing for the production of radiopharmaceutical on an as-needed basis. The synthesis process allows for the production of biomarker radiopharmaceuticals on site and close to the location where the unit dose will be administered to the patient. On-site, as-needed production of radiopharmaceuticals in small doses reduces the time between the synthesis of the radiopharmaceutical and the administration of that radiopharmaceutical, thereby minimizing the loss of active isotopes through decay and allowing the production of lesser amounts of radioisotopes overall.
Owner:BEST ABT INC
Automated Quality Control System for Radiopharmaceuticals
InactiveUS20150160171A1Minimal impactLow costIn-vivo radioactive preparationsComponent separationRadiopharmaceutical CompoundControl system
An automated HPLC-based quality control system to perform quality control testing on a radiopharmaceutical solution shortly after synthesis. An automated HPLC-based quality control system makes efficient use of sample volume and is compatible with a variety of radioisotopes and radiopharmaceutical compounds. In several embodiments, the automated nature of an automated HPLC-based quality control system allows for quality control tests to be conducted quickly and with minimal impact on user workflow. When used as part of an integrated PET biomarker radiopharmaceutical production system, the present general inventive concept permits a manufacturer to produce product and conduct quality control tests with lower per dose costs and shorter testing times.
Owner:BEST ABT INC
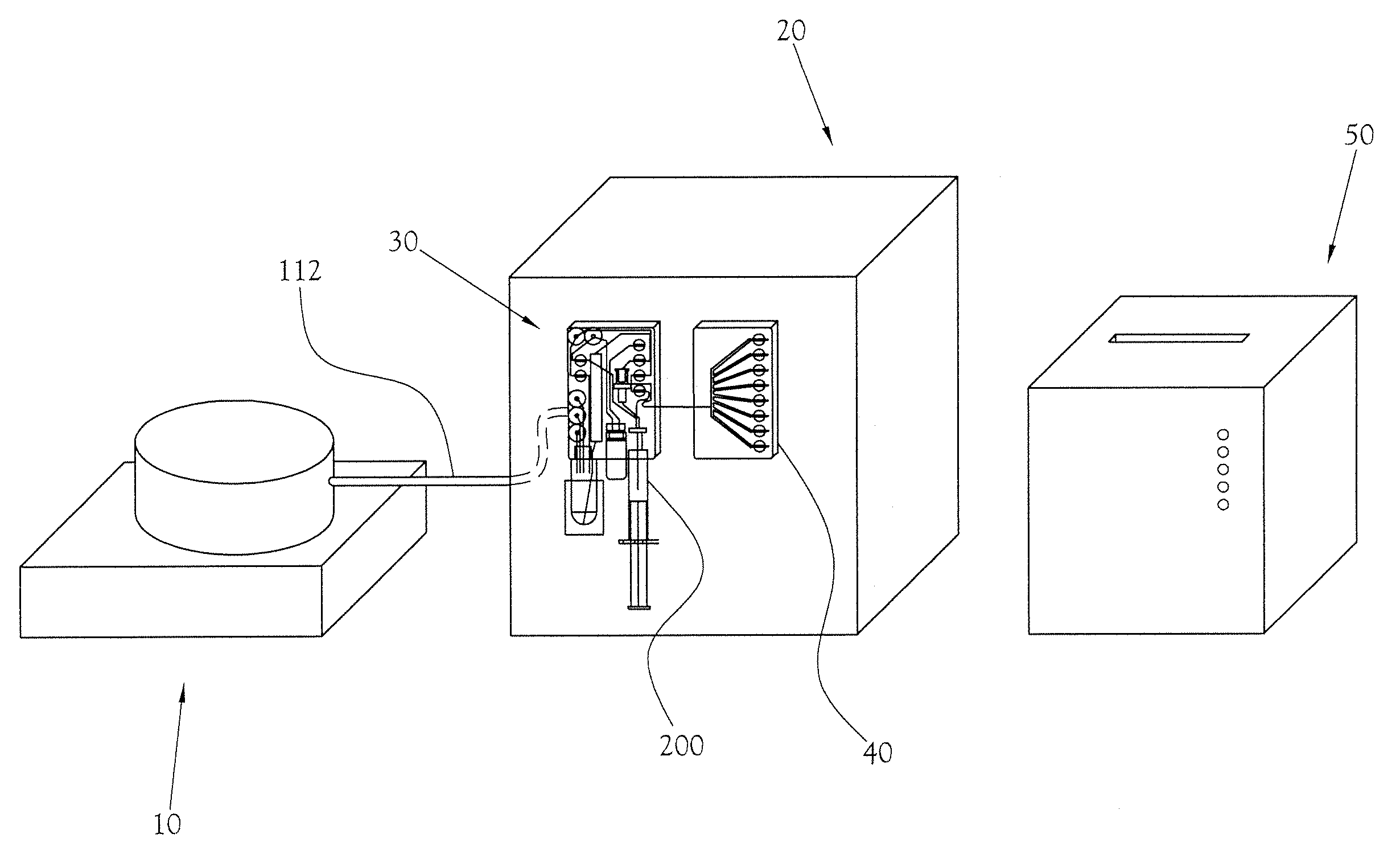
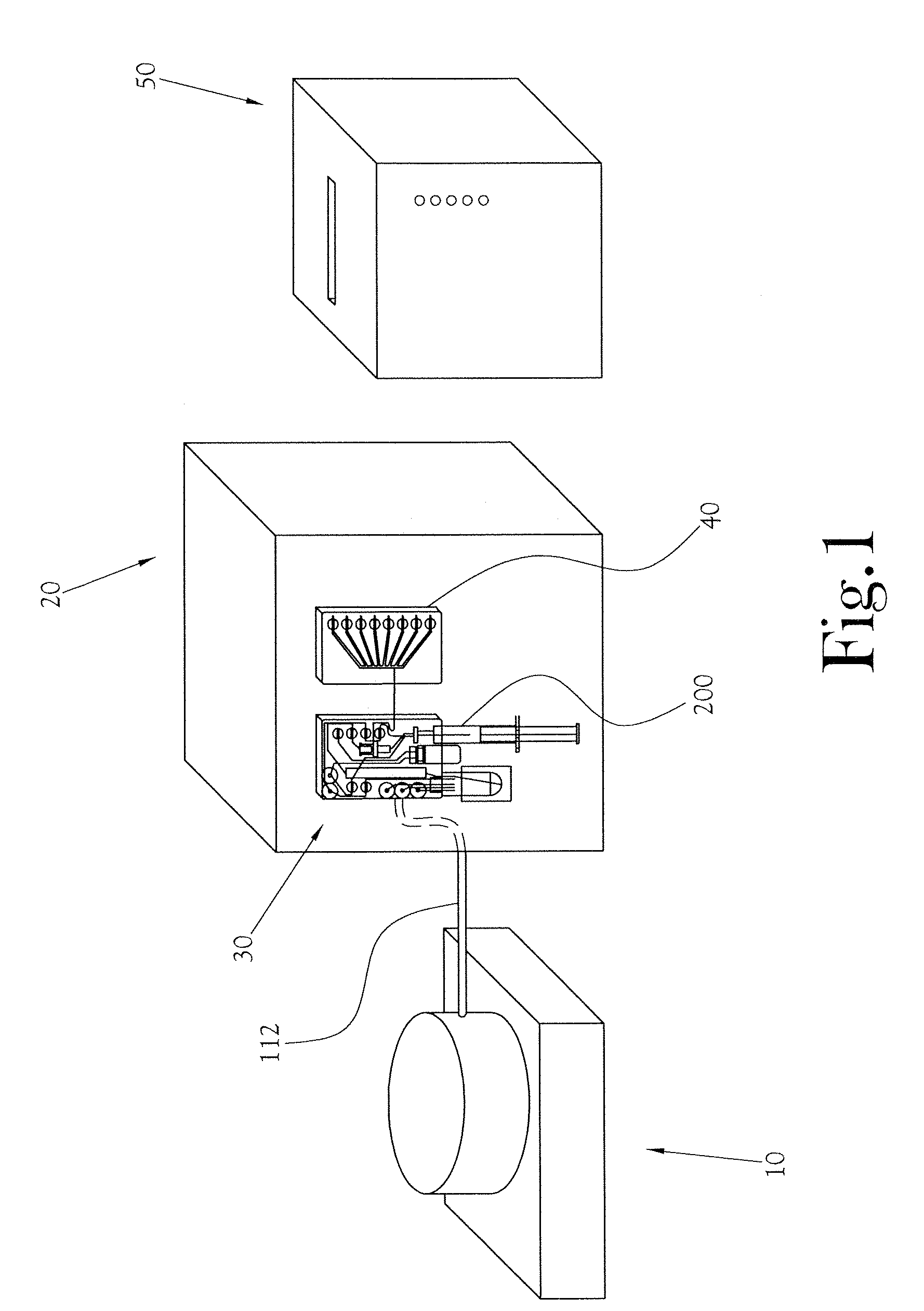
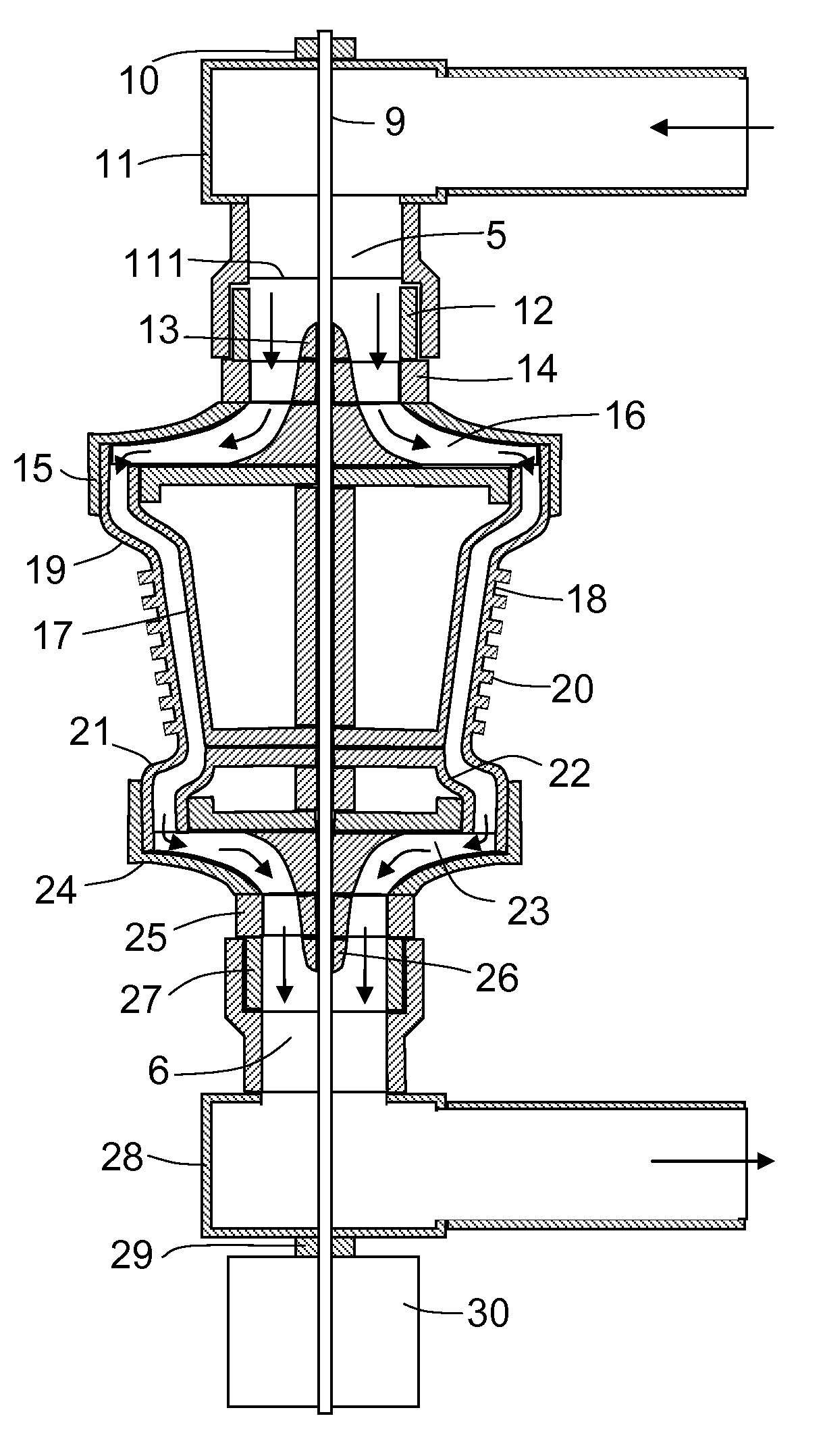
Microfluidic mixer
InactiveUS20120257470A1Rapid and efficient mixingPromote sportsSemi-permeable membranesShaking/oscillating/vibrating mixersEngineering
Provided is a microfluidic mixing system comprising a loop system for transferring one or more fluids, wherein the loop system comprises a plurality of sub loops, each sub loop formed from one or more common channels shared with at least one other sub loop and completed by an outer channel portion that is not shared by any other sub loop, and wherein the outer channel portion of each sub loop comprises one or more valves such that the sub loop is capable of isolation from all other sub loops and each common channel comprises one or more valves such that the common channel is capable of isolation from the remainder of the loop system, and wherein one or more sub loops in the system comprise valves that are configured to enable peristaltic mixing.
Owner:SCI TECH CORP
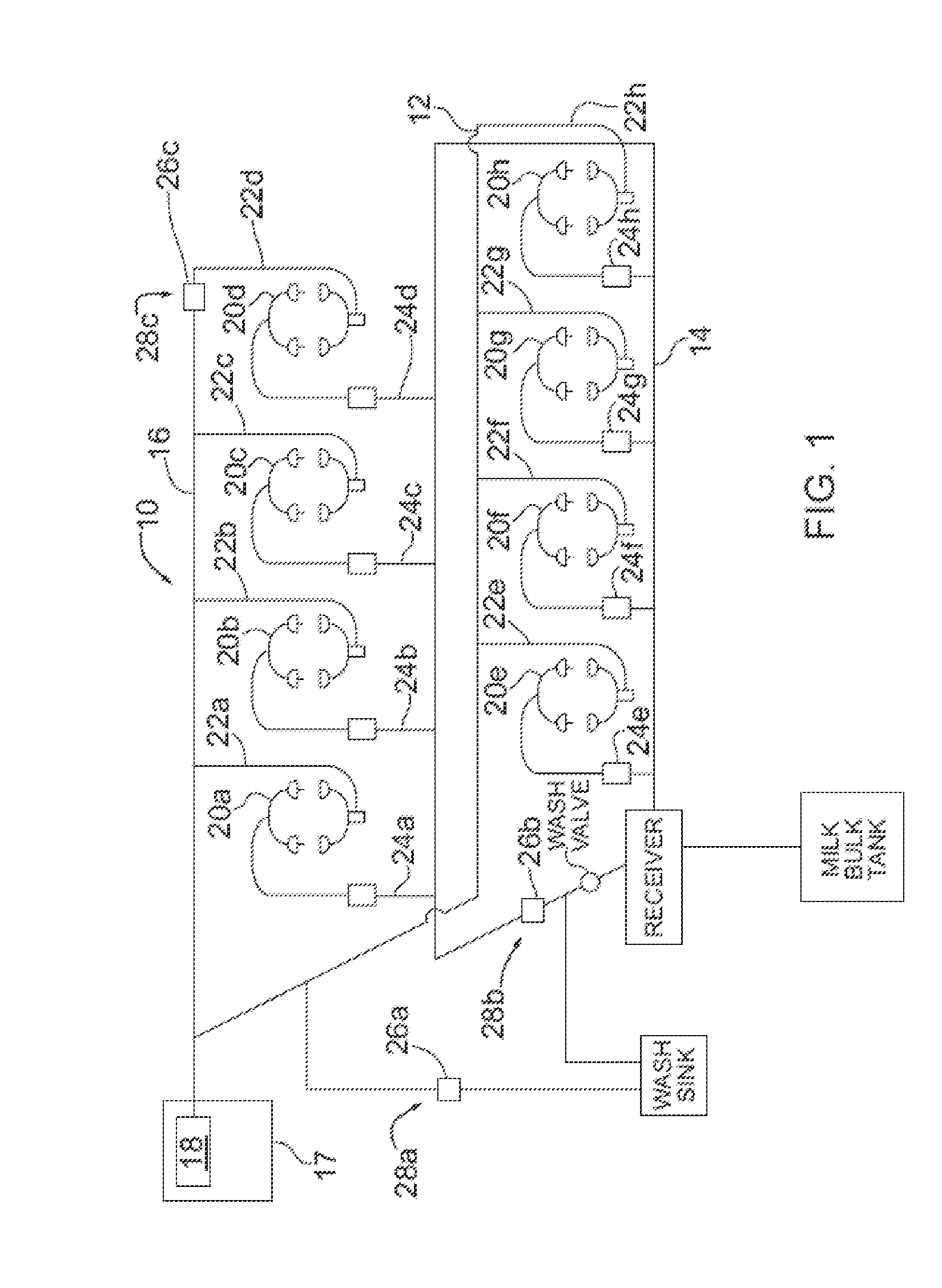
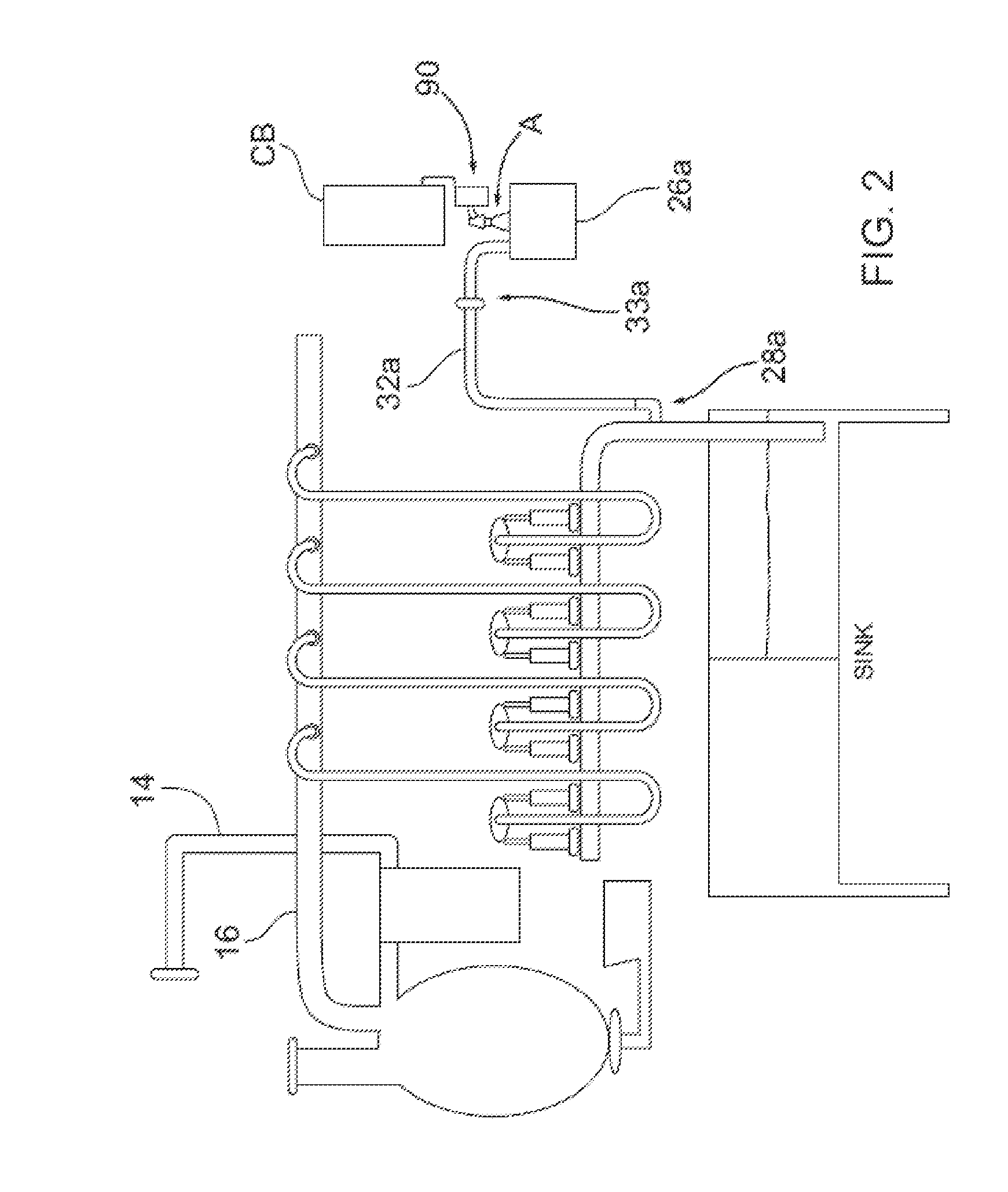
Dairy farm fluid line treatment
A fluid line treatment kit for a dairy farm milking system having a vacuum subsystem for imparting vacuum within a fluid line is provided. The fluid line treatment kit includes at least one ozone gas source; a conduit associated with each ozone gas source configured to convey ozone gas to within the fluid line at a respective location; and a control system configured to trigger each ozone gas source to produce ozone gas while the vacuum subsystem is actuated. Vacuum imparted by the vacuum subsystem in the fluid line draws ozone gas via the at least one conduit into and through the fluid line.
Owner:2178450 ONTARIO
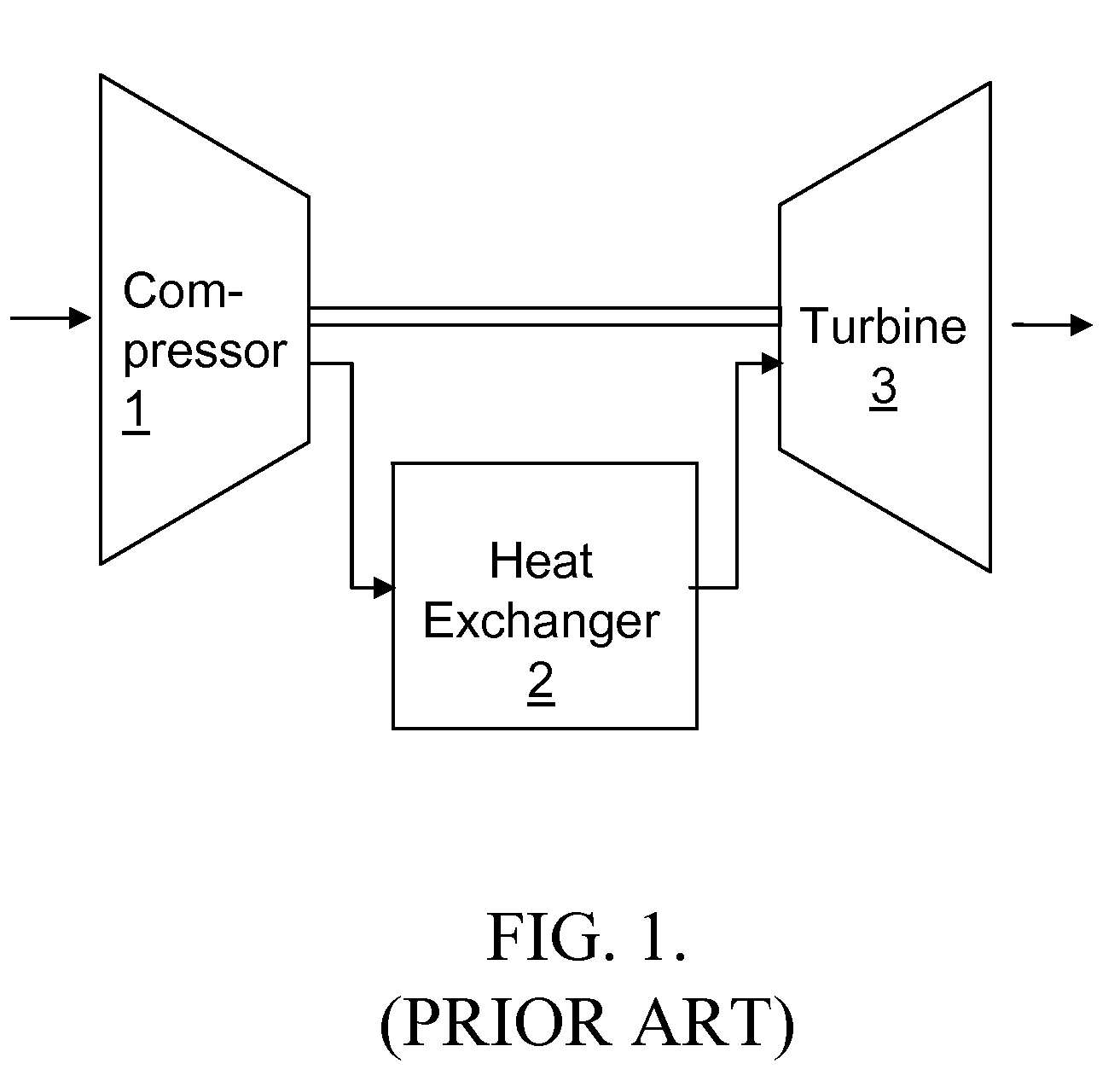
Centrifugal Air Cycle Air Conditioner
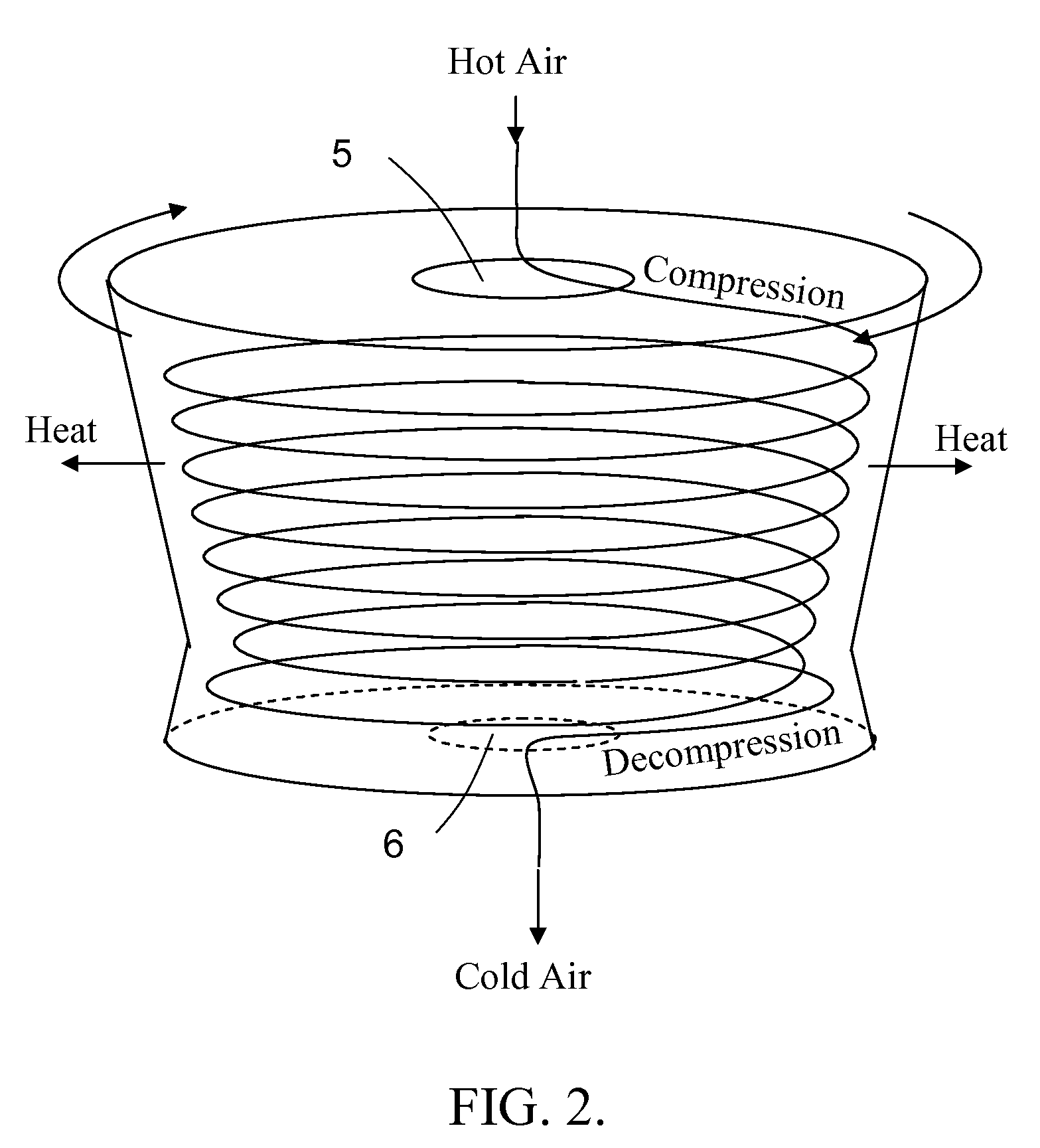
InactiveUS20130000328A1Thin layerMaximize heat transferDomestic cooling apparatusCompression machinesCold airAir cycle
The air conditioning system uses an air cycle thermodynamic process. The system comprises a centrifuge. This centrifuge includes at one of its ends, an axial inlet that funnels air into a centrifugal compressor rotating in unison with the centrifuge. The air is compressed, adiabatically heated and directed to a heat exchanger mounted in the rim of, and rotating with the centrifuge. The air is accelerated with respect the centrifuge by varying the centrifuge radius or by using forward leaning impeller blades. The air is cooled by the heat exchanger and directed to an expander also rotating with the centrifuge. The air is expanded and adiabatically further cooled. The cold air exits the centrifuge through an axial outlet located at the second end of the centrifuge.
Owner:LEVY GEORGE SAMUEL
Continuous production of carbon nanomaterials using a high temperature inductively coupled plasma
InactiveUS7666381B2Reduce the possibilityIncrease gas velocityMaterial nanotechnologyOther chemical processesGas phaseFiltration
High-power inductively coupled plasma technology is used for thermal cracking and vaporization of continuously fed carbonaceous materials into elemental carbon, for reaction with separate and continuously fed metal catalysts inside a gas-phase high-temperature reactor system operating at or slightly below atmospheric pressures. In one particularly preferred embodiment, in-flight growth of carbon nanomaterials is initiated, continued, and controlled at high flow rates, enabling continuous collection and product removal via gas / solid filtration and separation methods, and / or liquid spray filtration and solid collection methods suitable for producing industrial-scale production quantities. In another embodiment, the reaction chamber and / or filtration / separation media include non-catalytic or catalytic metals to simultaneously or separately induce on-substrate synthesis and growth of carbon nanomaterials. The on-substrate grown carbon nanomaterials are produced in secondary chambers that are selectively isolated for periodic removal of the product.
Owner:SEQUOYAH FINANCE ONE
Low-volume biomarker generator
ActiveUS7884340B2Reduce size and power requirement and weightEfficient dosingIsotope delivery systemsConversion outside reactor/acceleratorsChemical synthesisEngineering
Owner:BEST ABT INC
Method and Apparatus for Retaining Elevated Objects
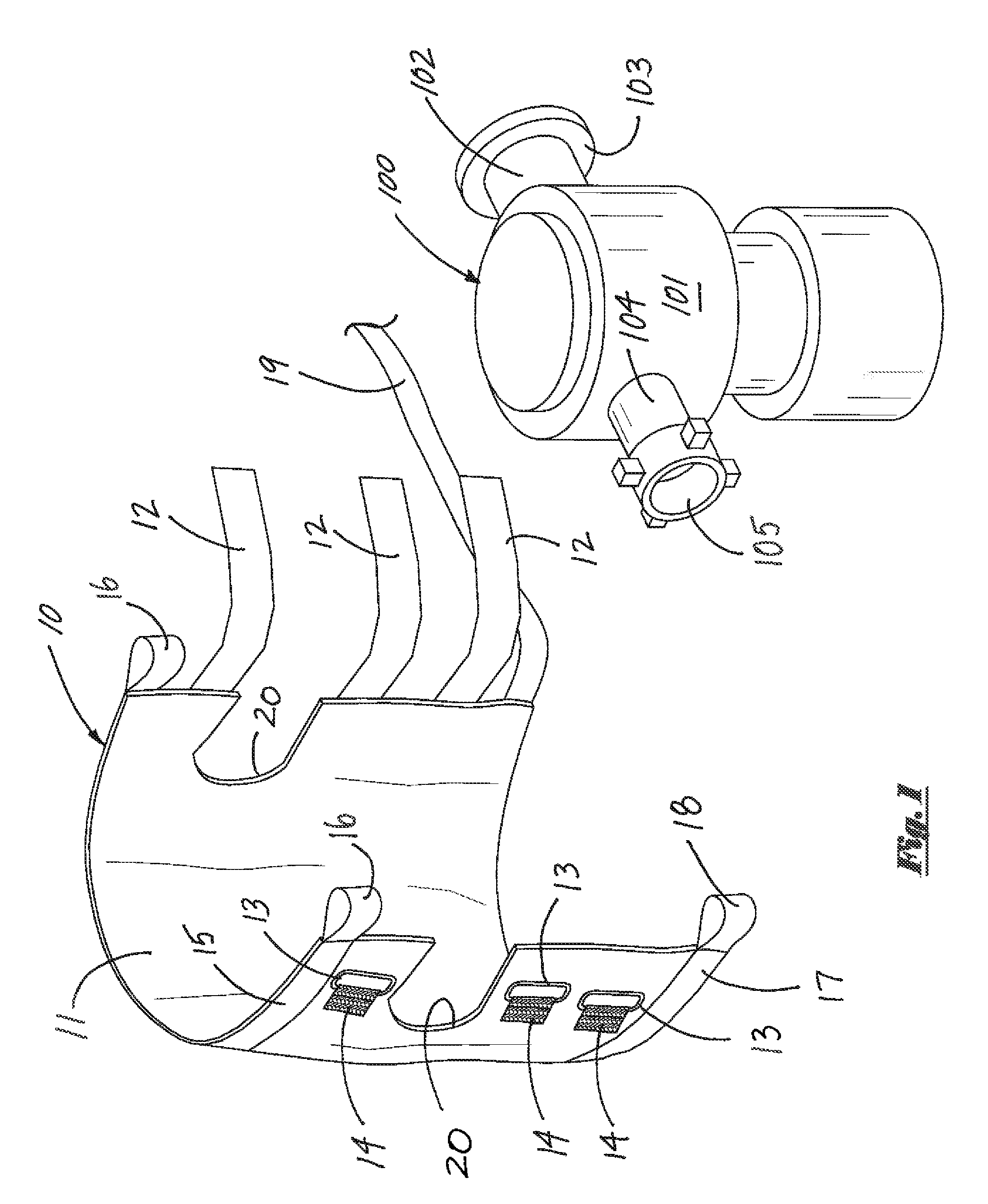
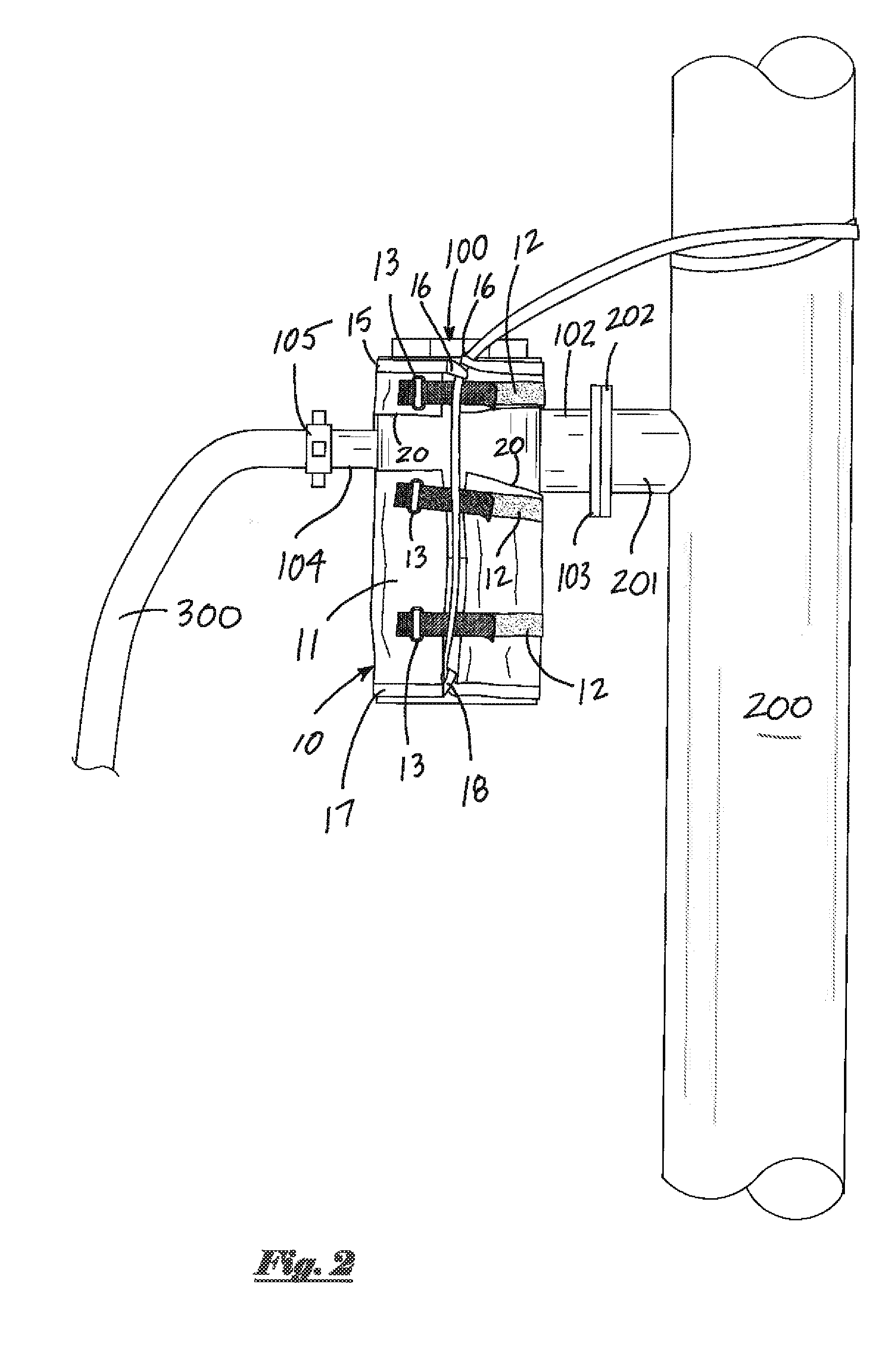
ActiveUS20140306069A1Quickly and efficiently secured/tightenedEffective protectionPipe supportsPipe elementsEngineeringMechanical engineering
An apparatus for retaining and securing elevated objects such as cement valves. A harness member is wrapped around the outer surface of an elevated object, and adjustable straps are tightened to secure the harness member in place. An elongate sling member, attached the harness member, is securely attached to a cement head assembly or other nearby support structure. The apparatus provides positive retention should an elevated object become detached, thereby preventing the object from falling and injuring personnel or damaging property.
Owner:FRANKS INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com