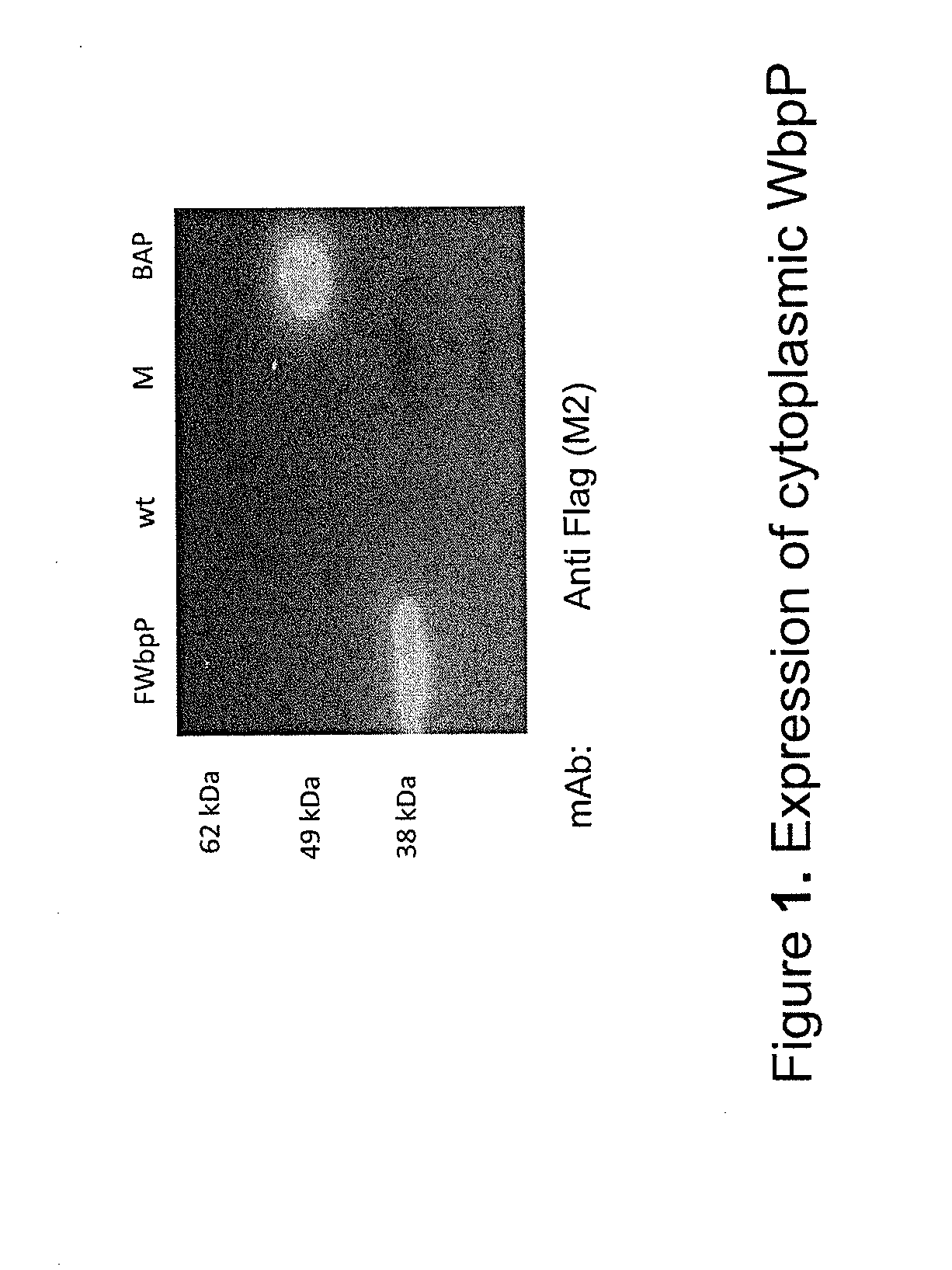
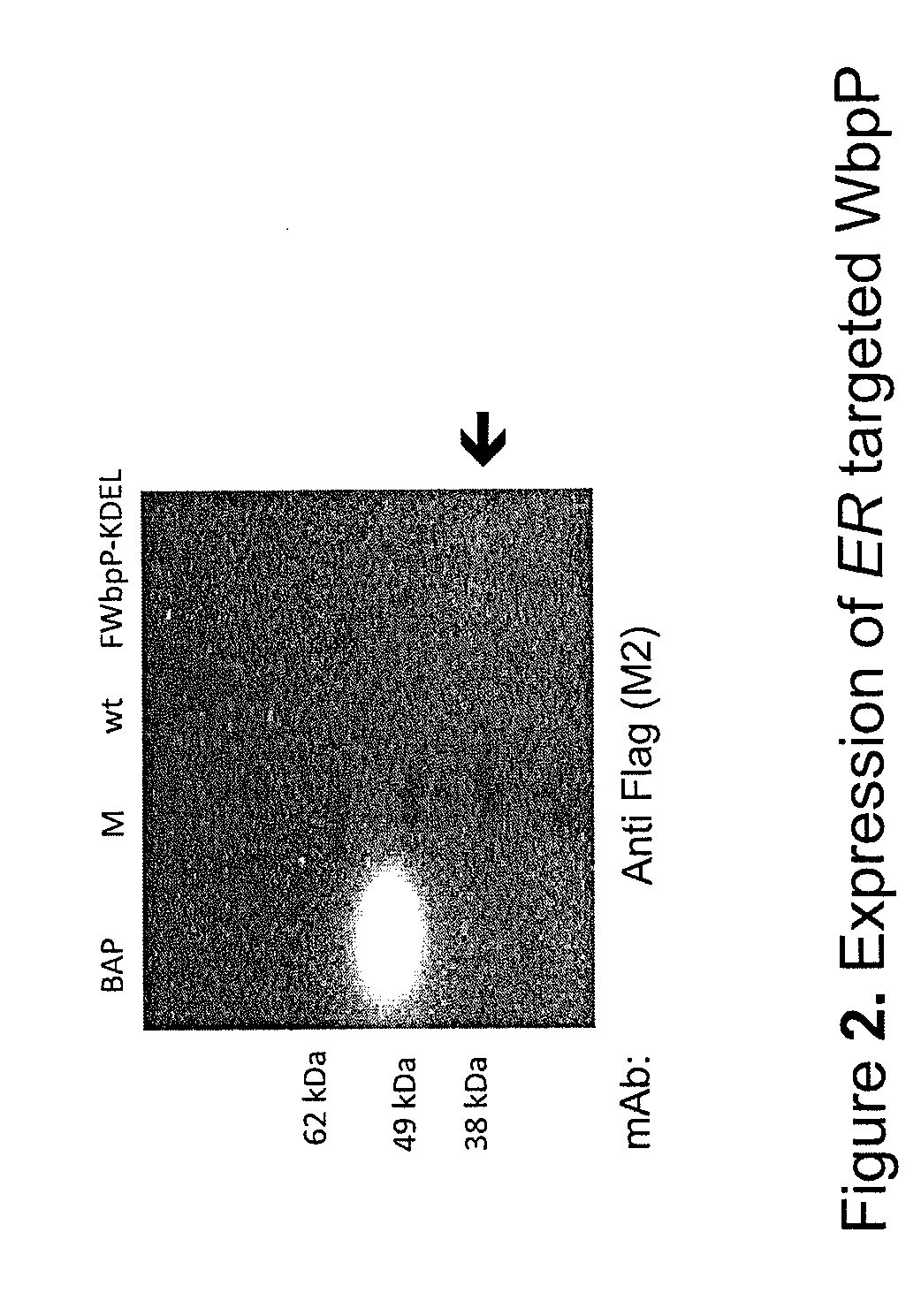
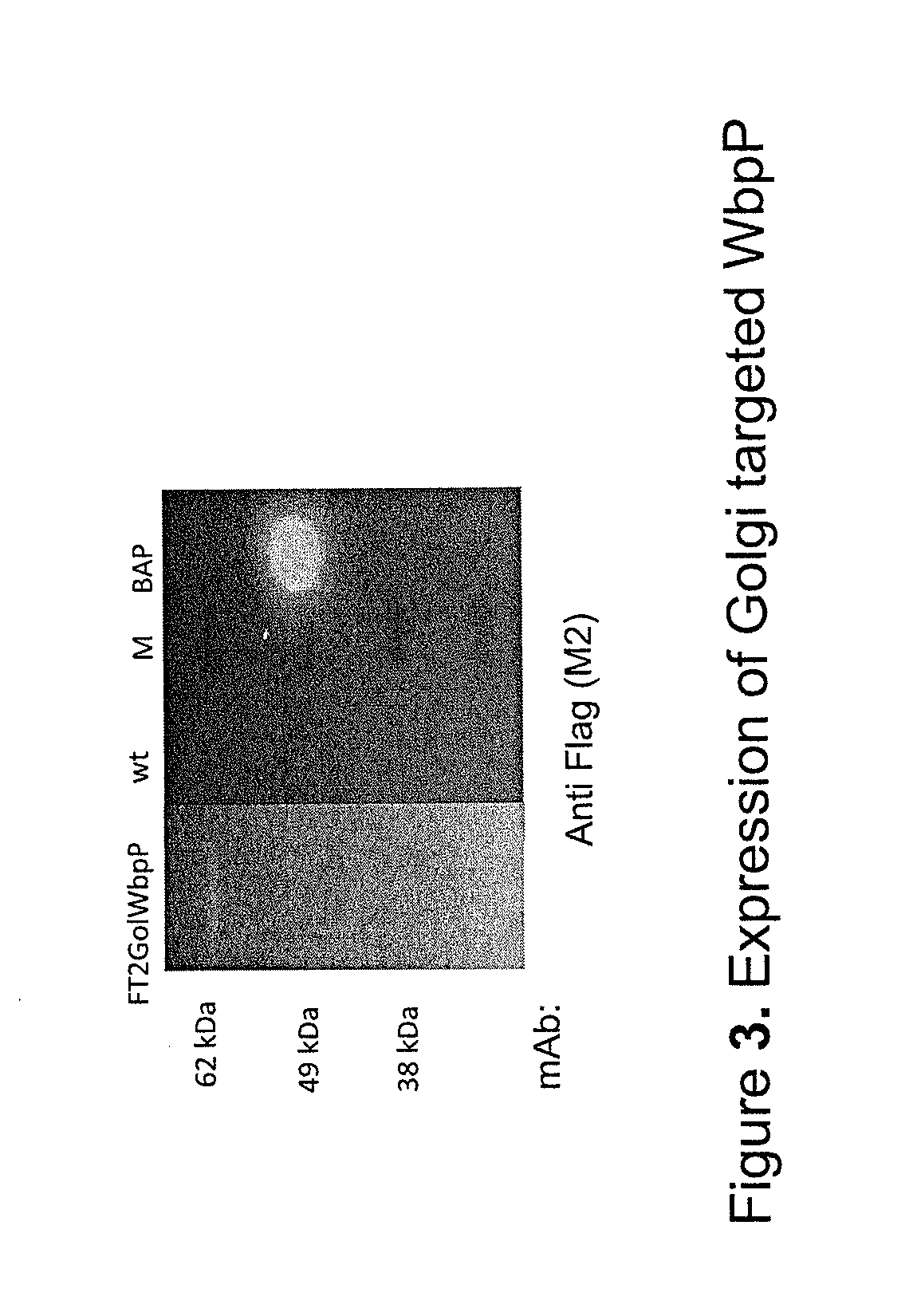
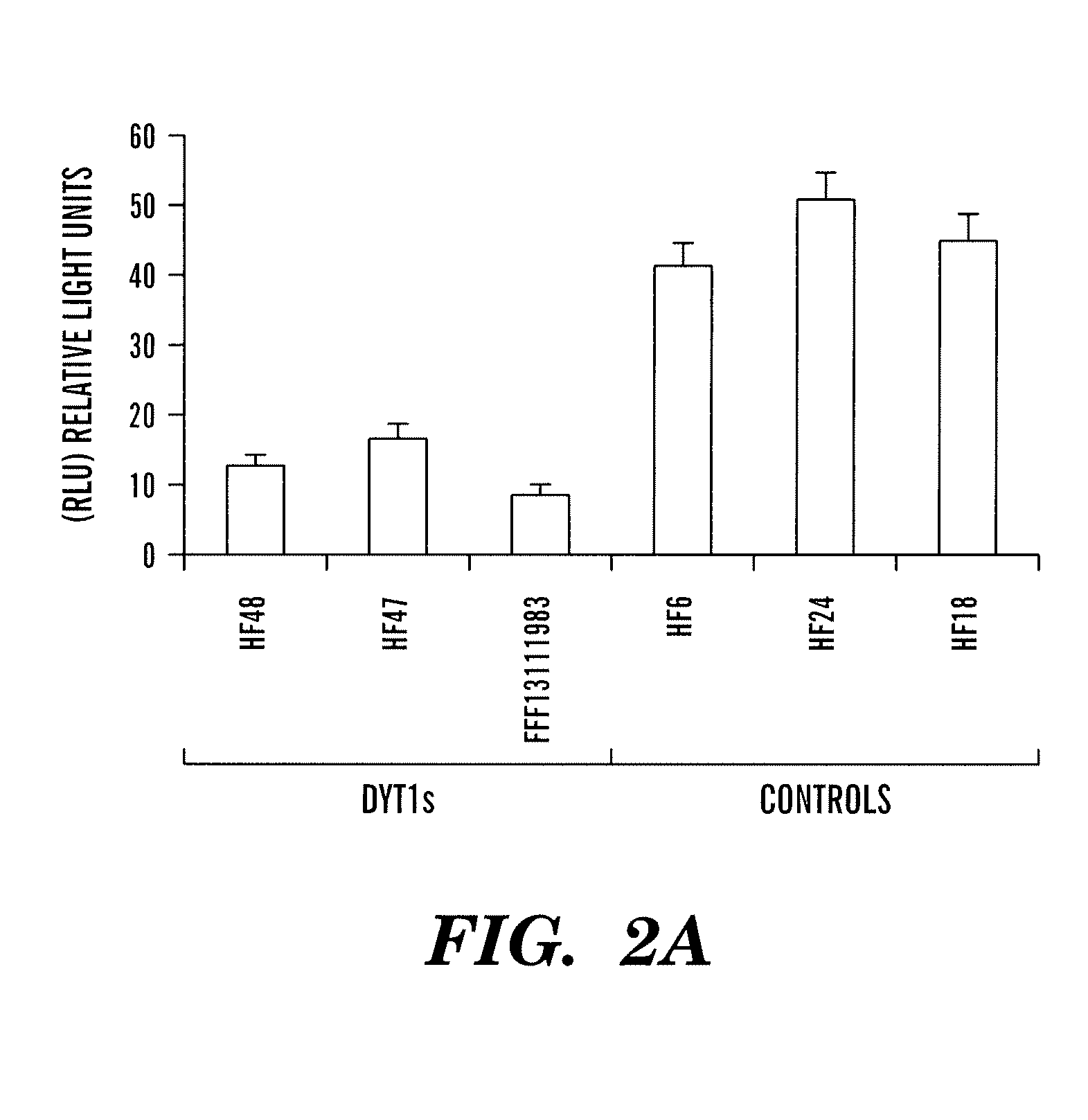
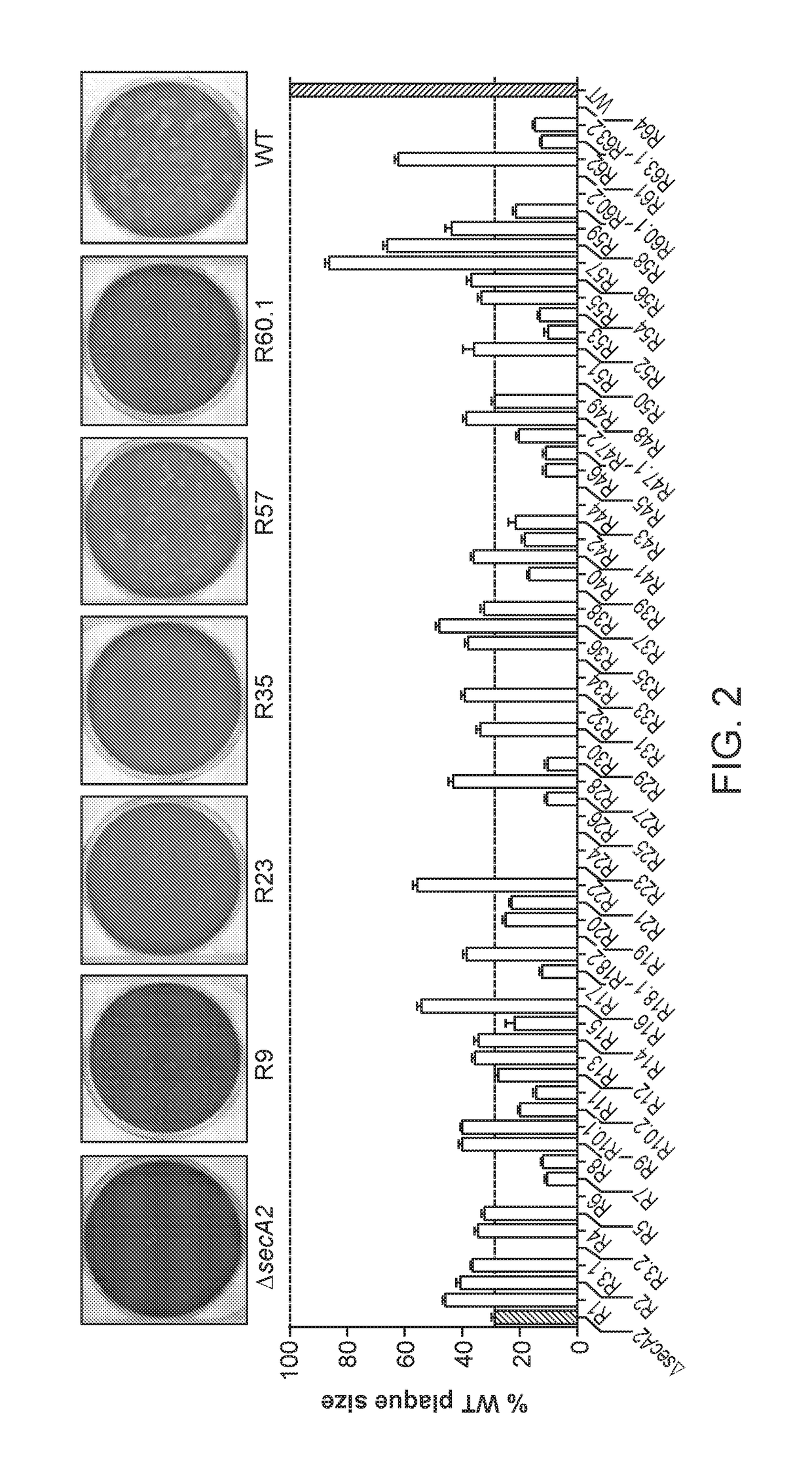
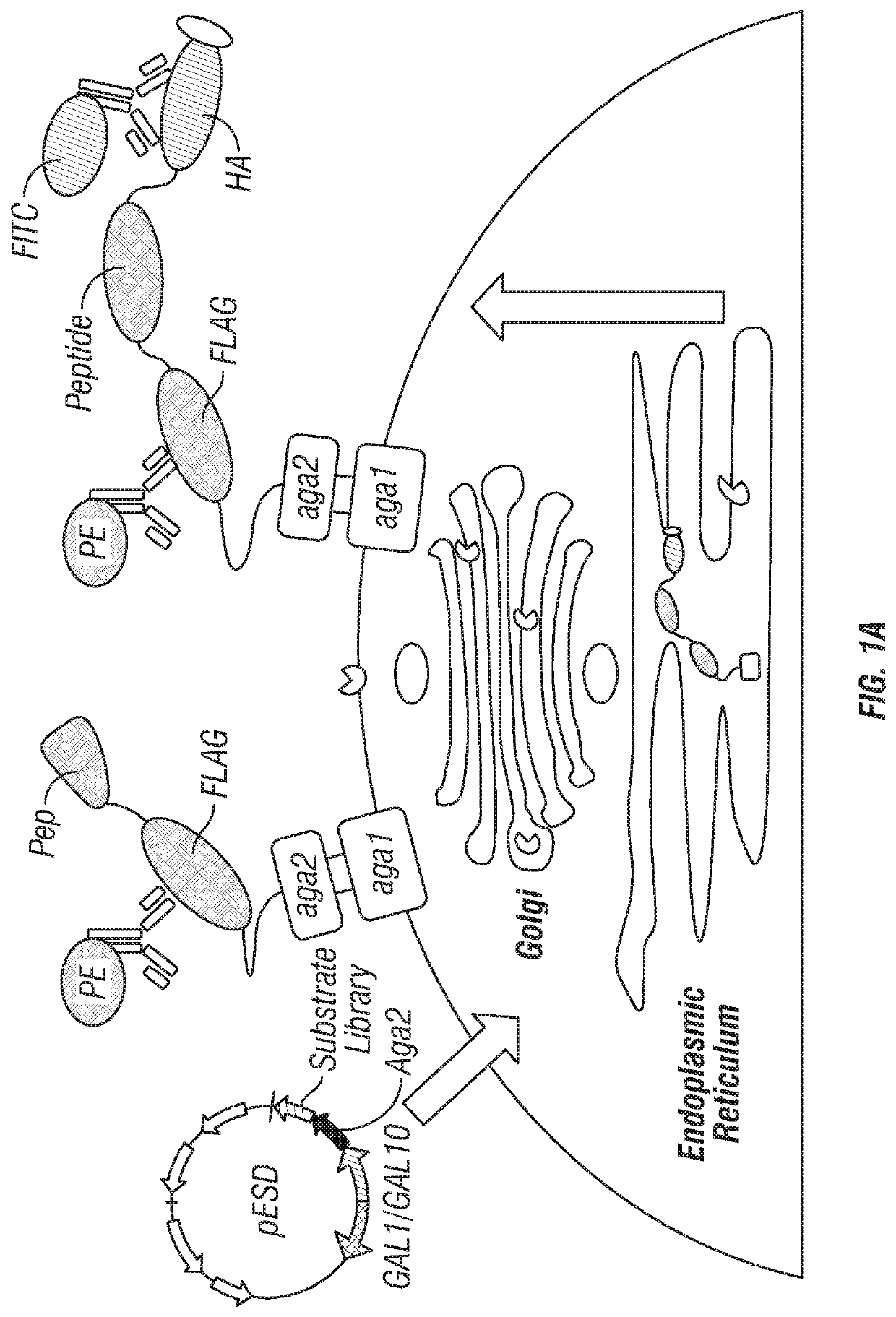
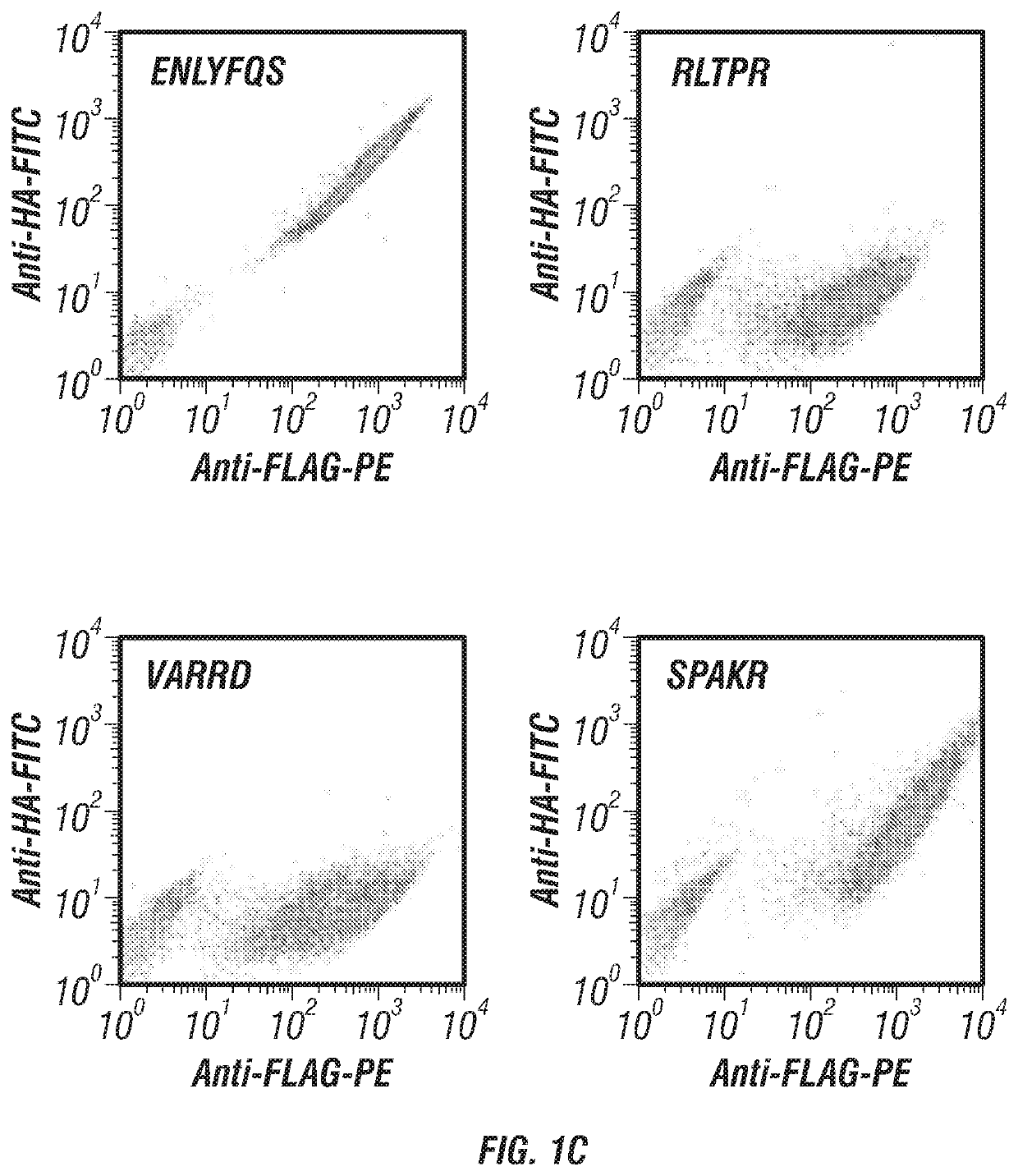
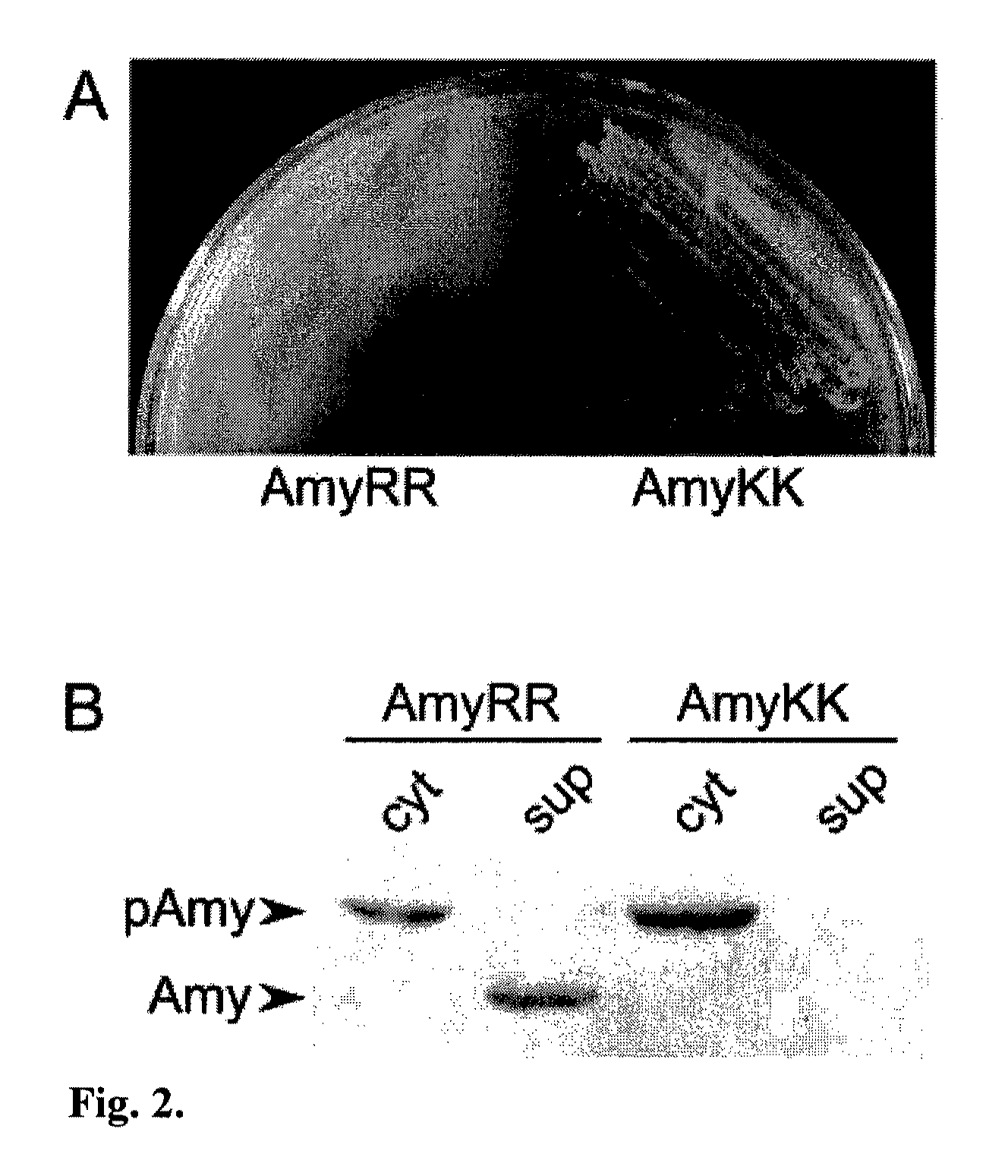
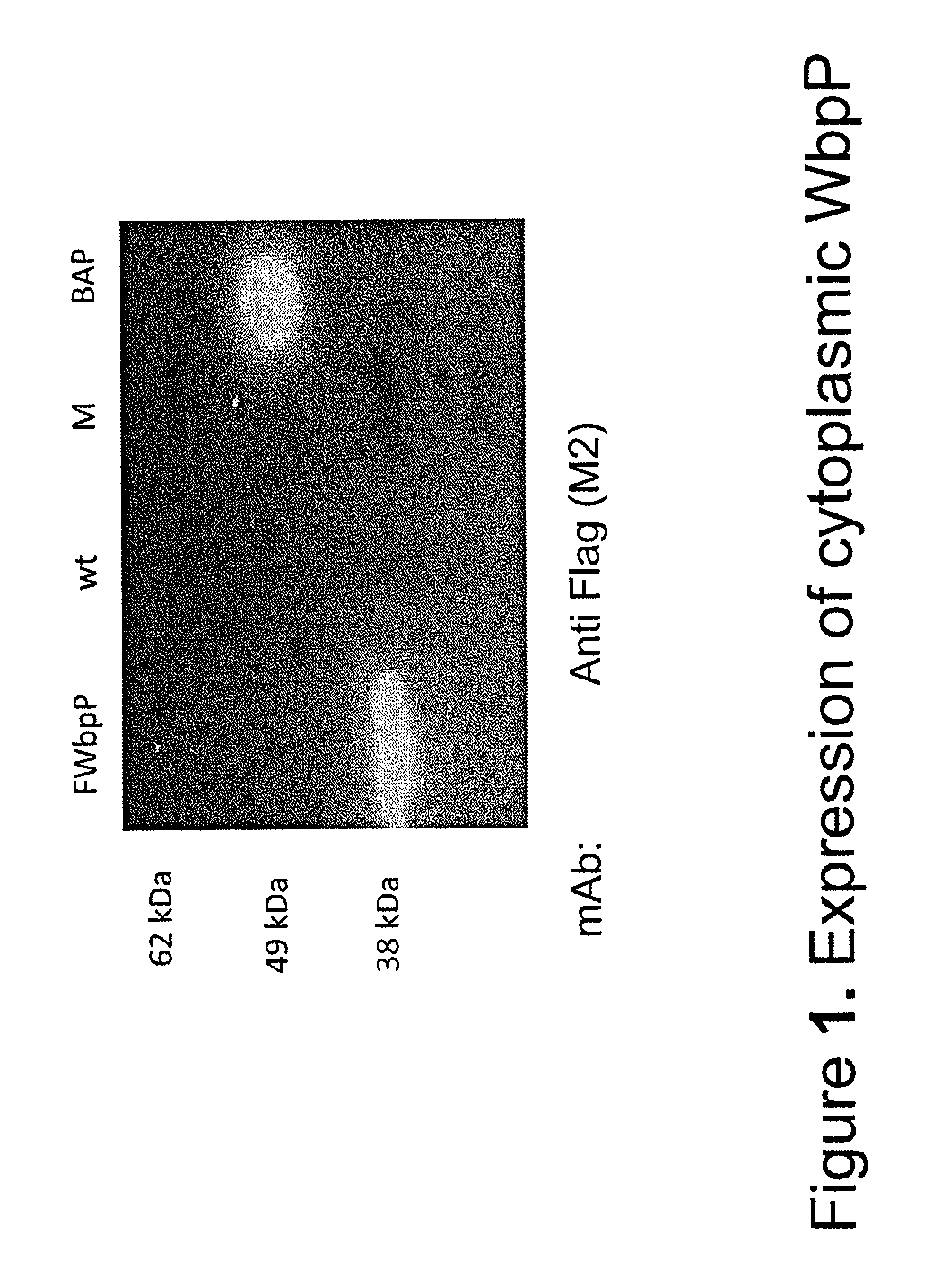
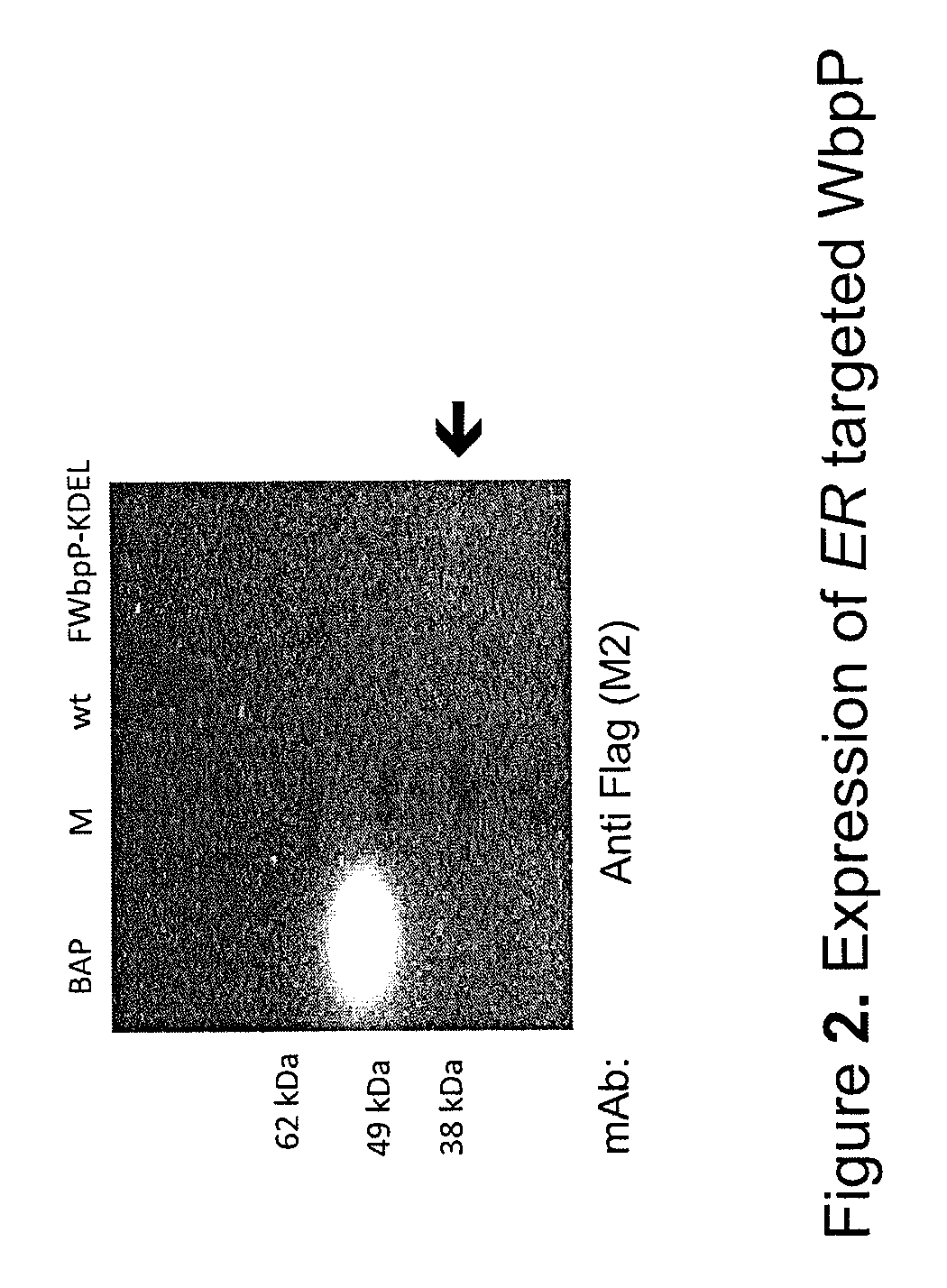
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Secretory pathway" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The secretory pathway is a series of steps a cell uses to move proteins out of the cell; a process known as secretion. The path of a protein destined for secretion has its origins in the rough endoplasmic reticulum, a membrane-bound compartment in the cell. The protein then proceeds through the many compartments of the Golgi apparatus and finally ends up in a vesicle that transiently fuses at the cell plasma membrane via permanent plasma membrane structures called porosomes, depositing the proteins outside of the cell. At each step along the way there are crucial factors that determine how and if the protein will proceed. Some of these factors include regulation of transportation, selection of particular proteins, the mechanics of proceeding to the next step, and modifications that can occur to the protein along the way. All of these factors contribute to how a protein arrives outside of a cell after being synthesized.
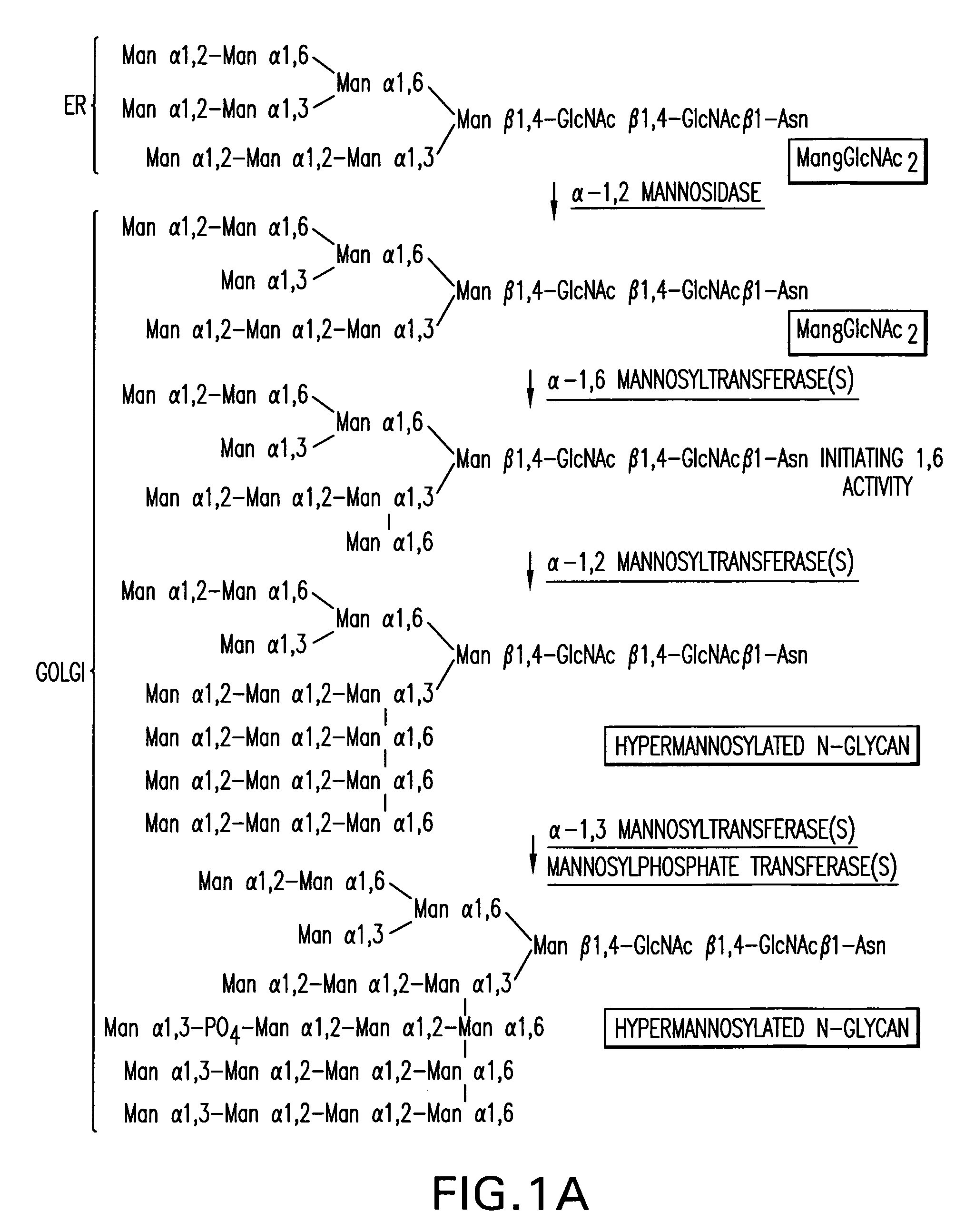
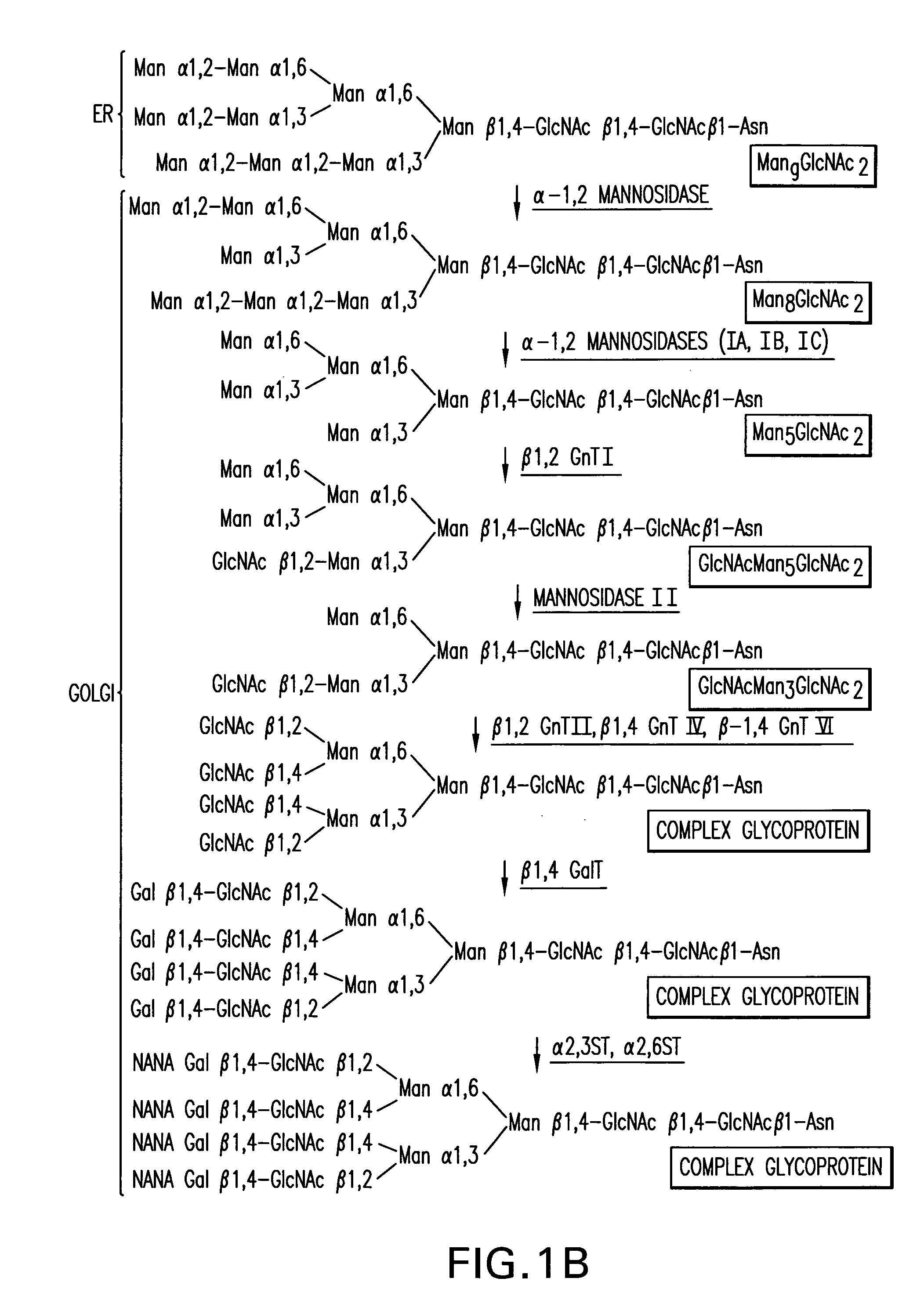
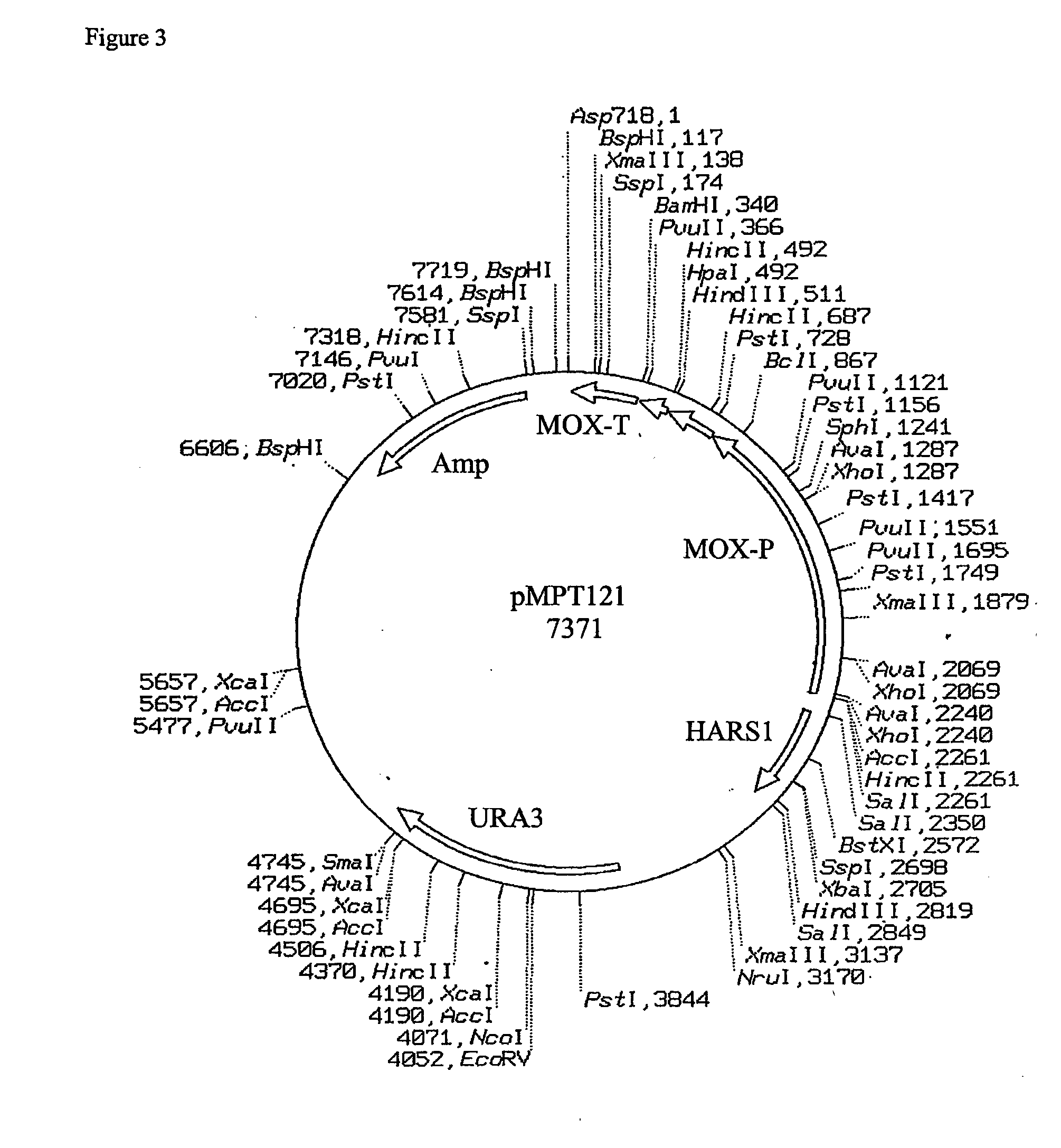
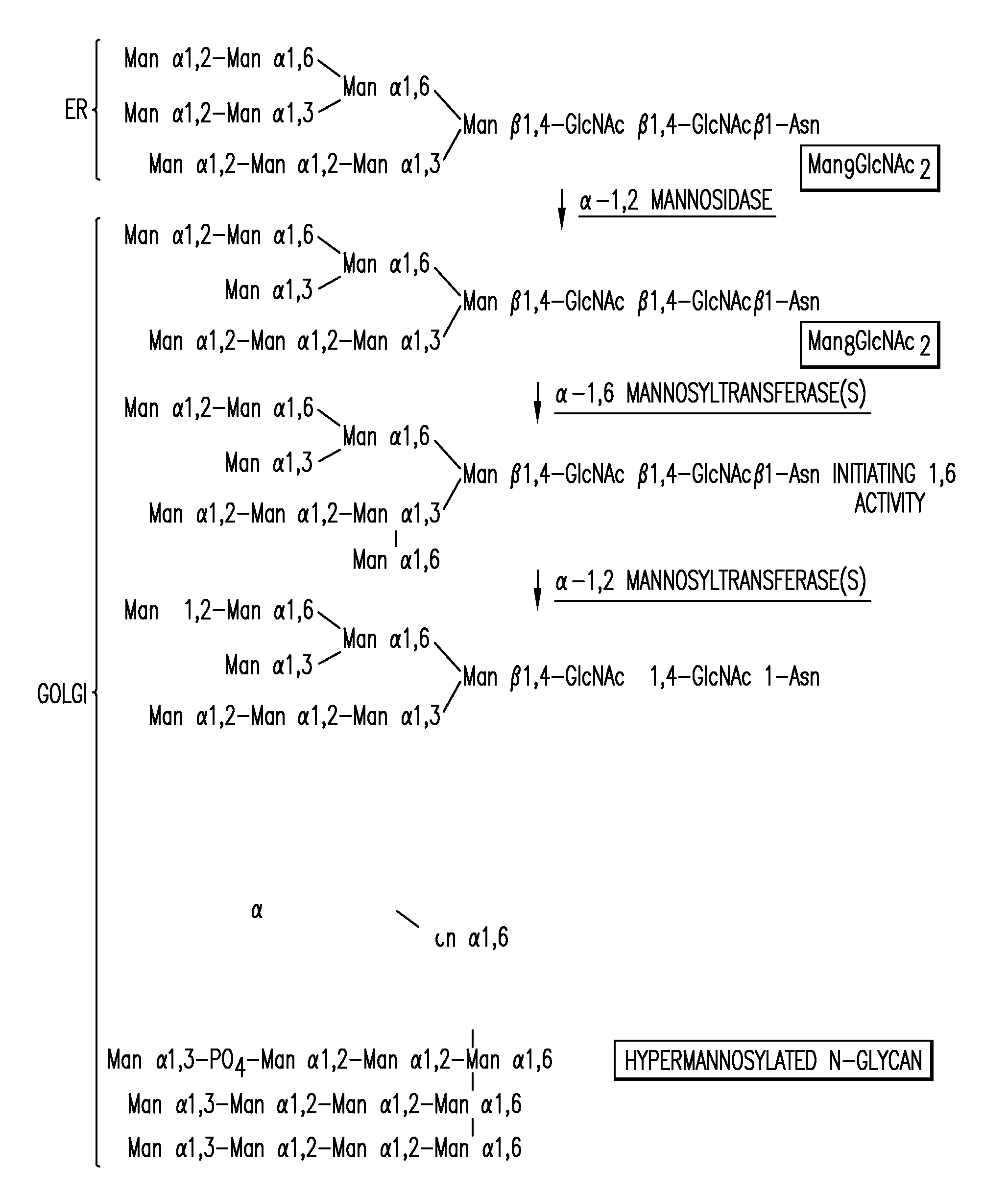
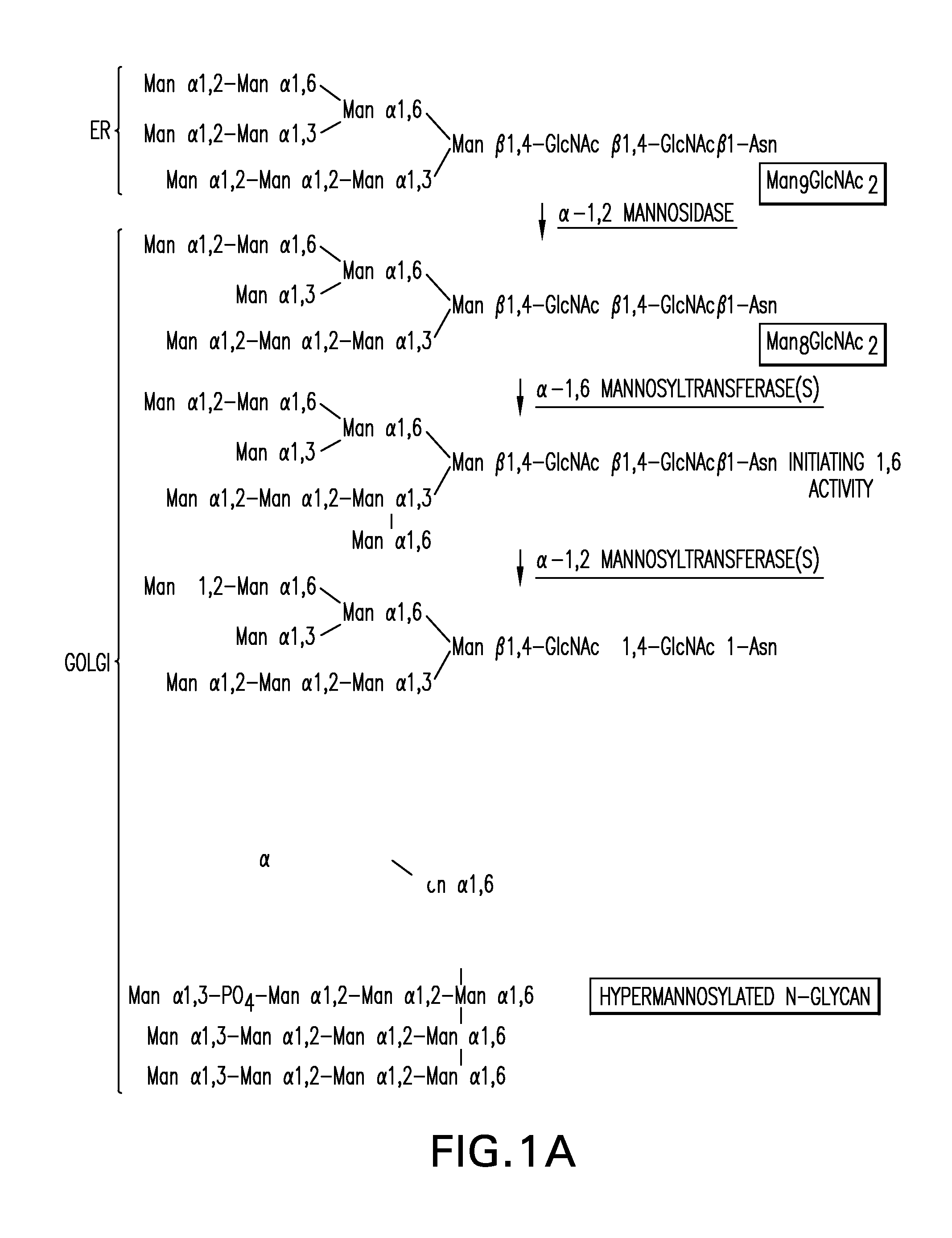
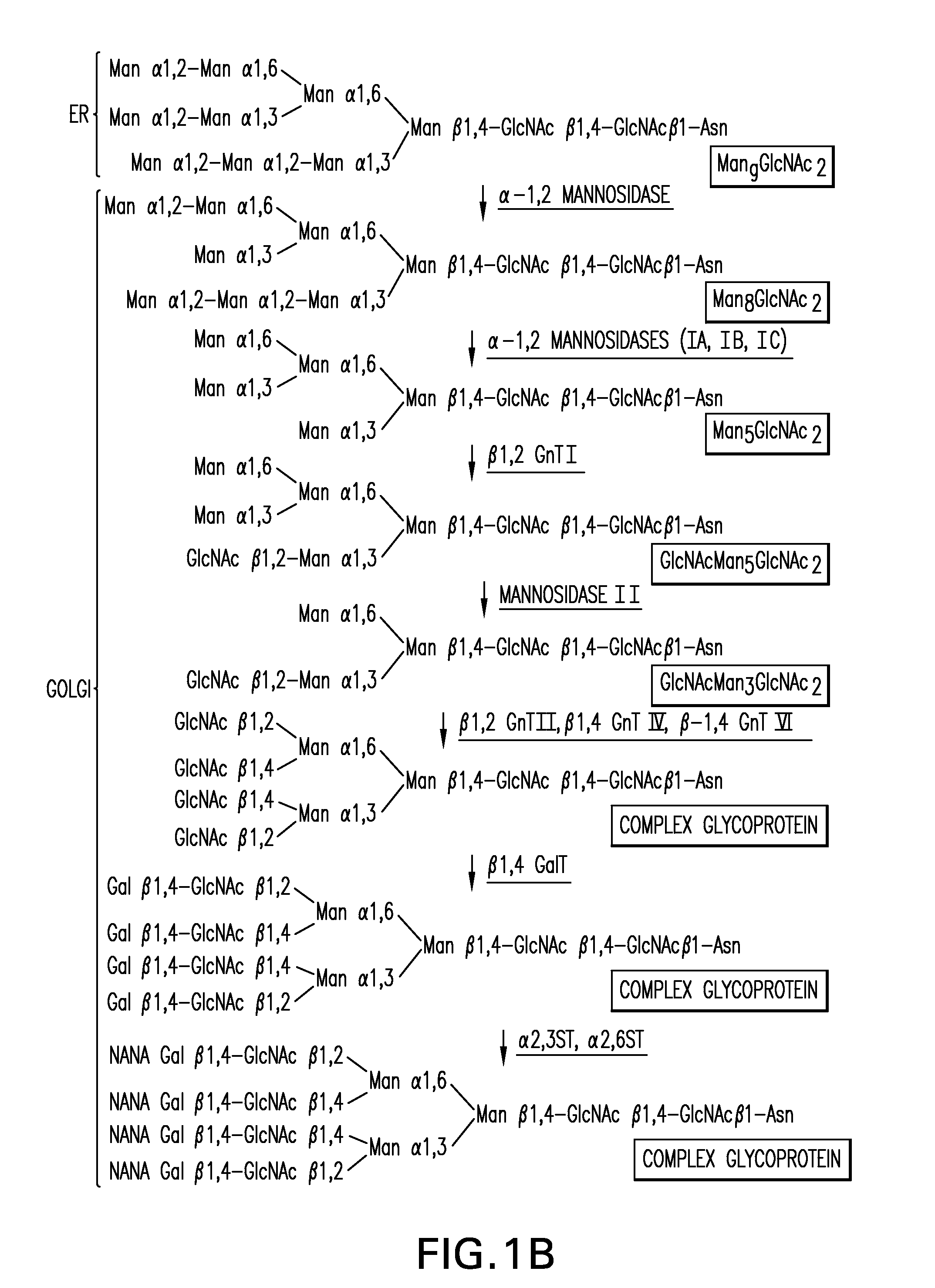
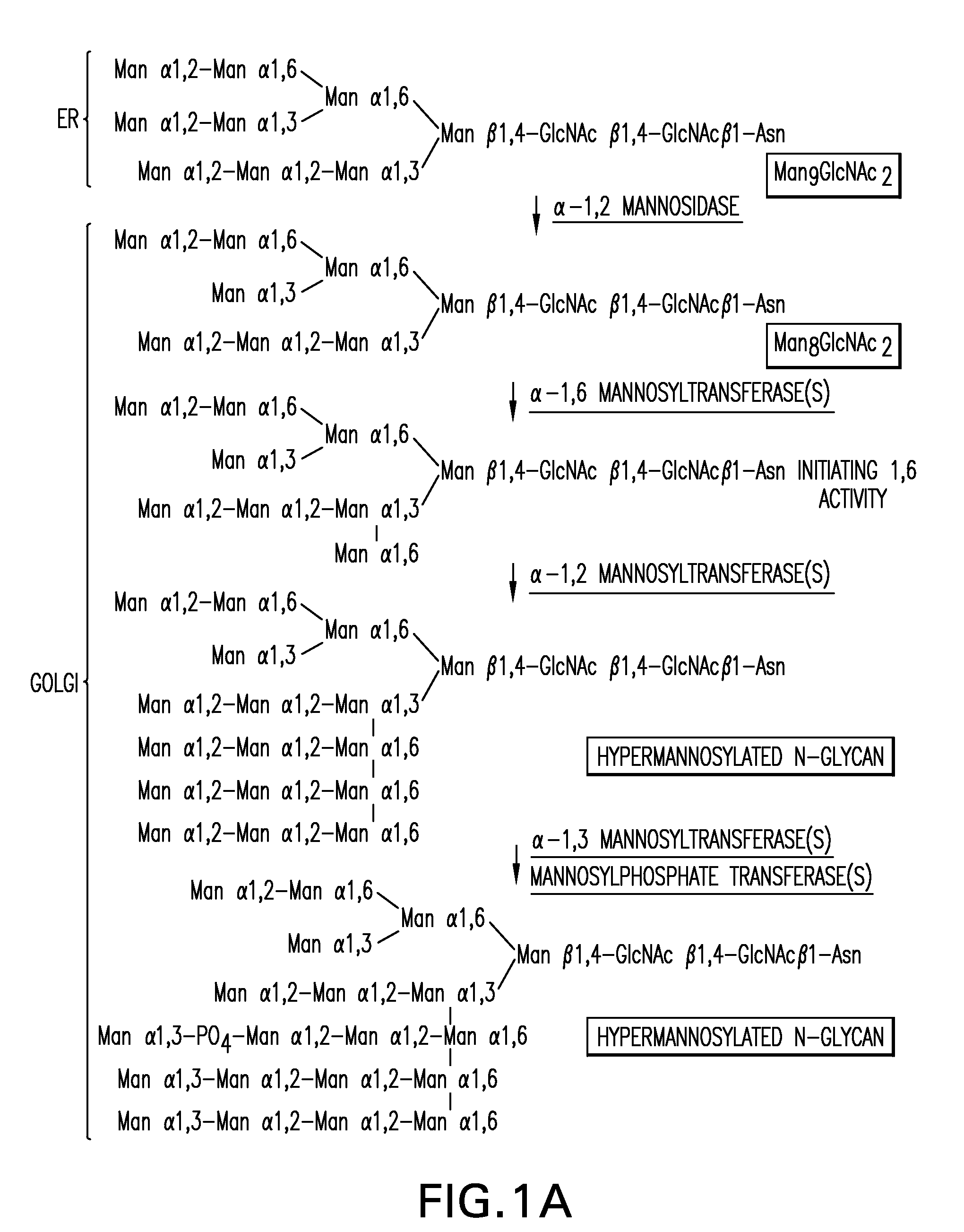
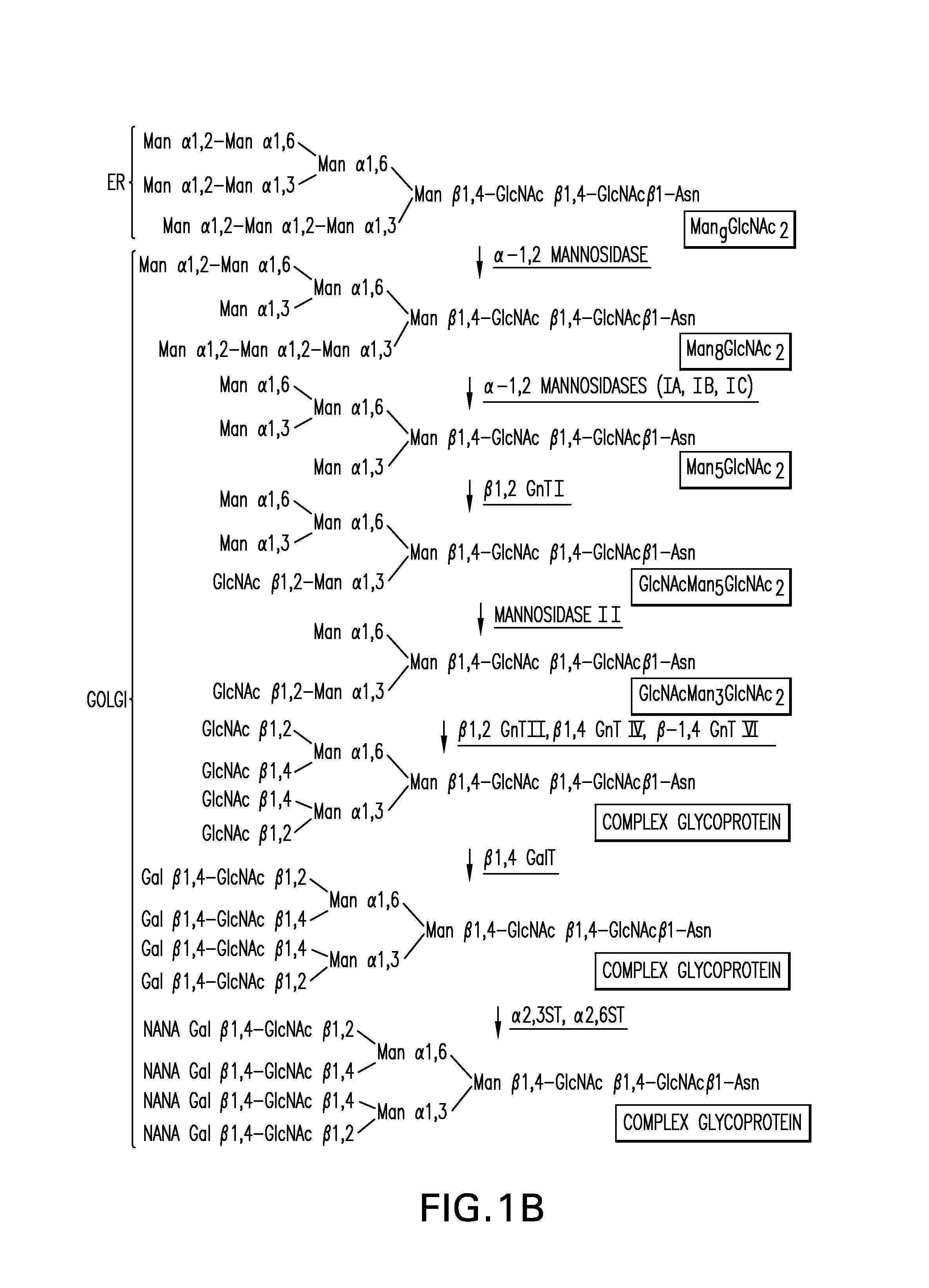
Expression of class 2 mannosidase and class III mannosidase in lower eukaryotic cells
A method for producing human-like glycoproteins by expressing a Class 2 α-mannosidase having a substrate specificity for Manα1,3 and Manα1,6 glycosidic linkages in a lower eukaryote is disclosed. Hydrolysis of these linkages on oligosaccharides produces substrates for further N-glycan processing in the secretory pathway.
Owner:GLYCOFI
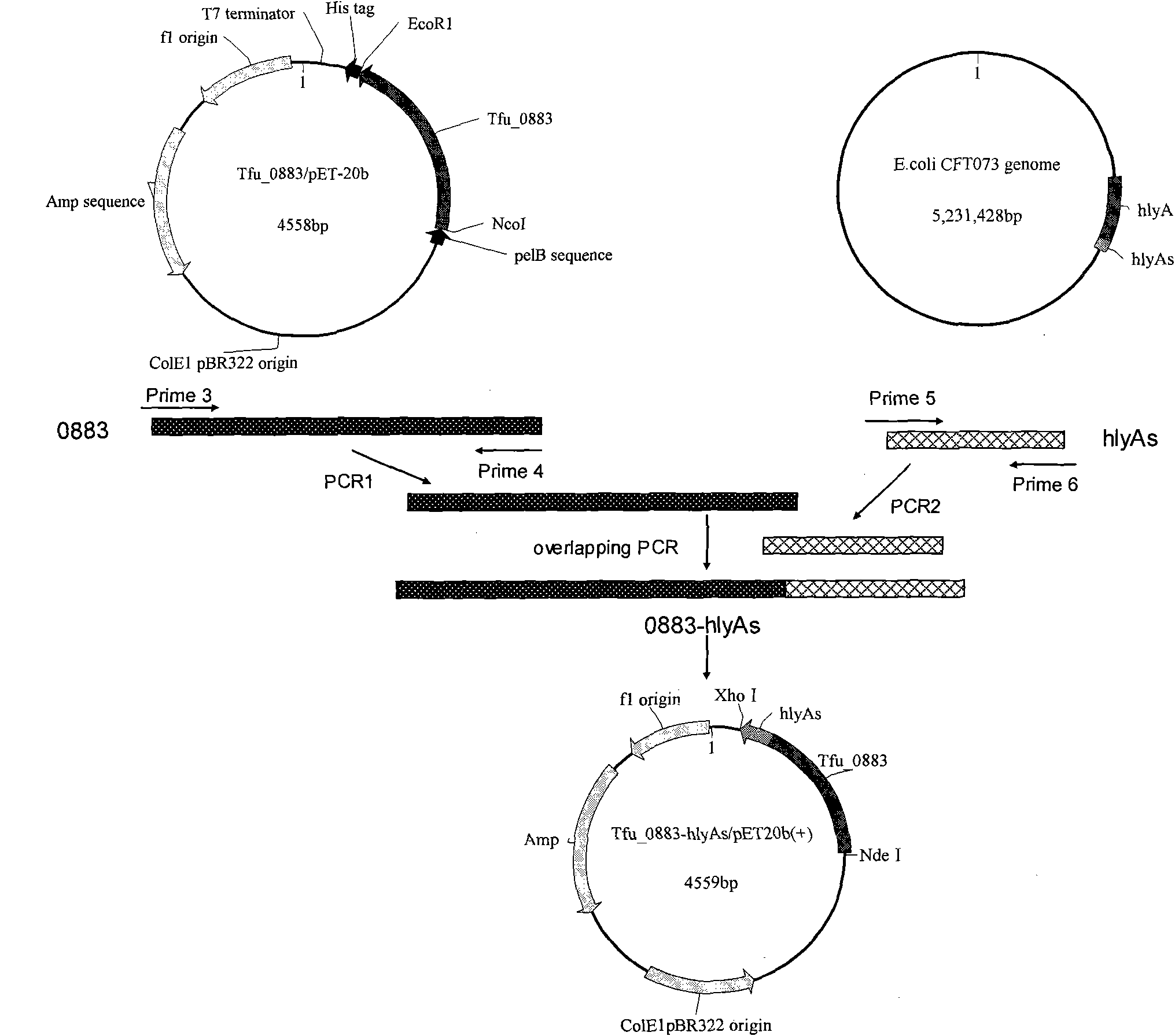
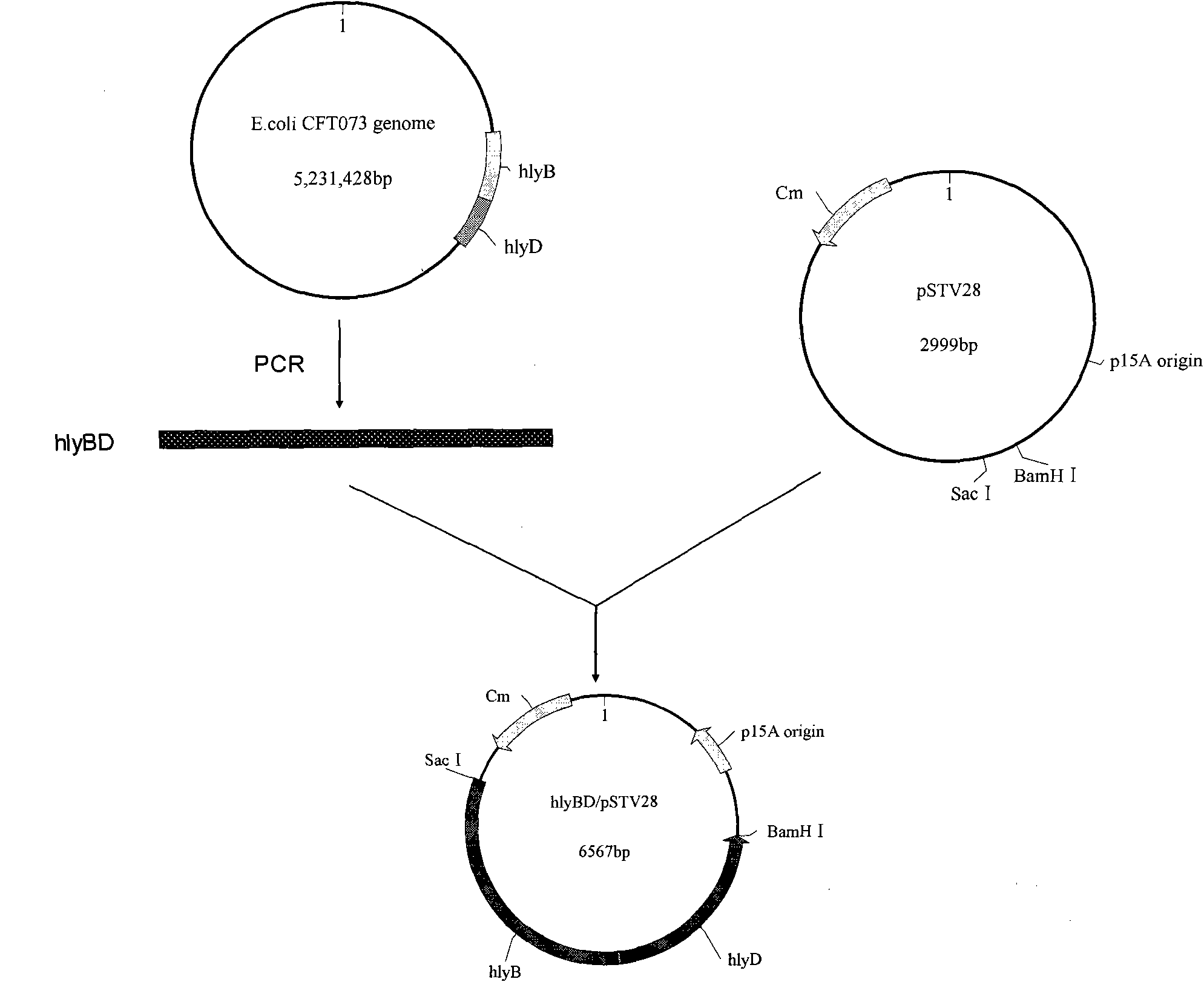
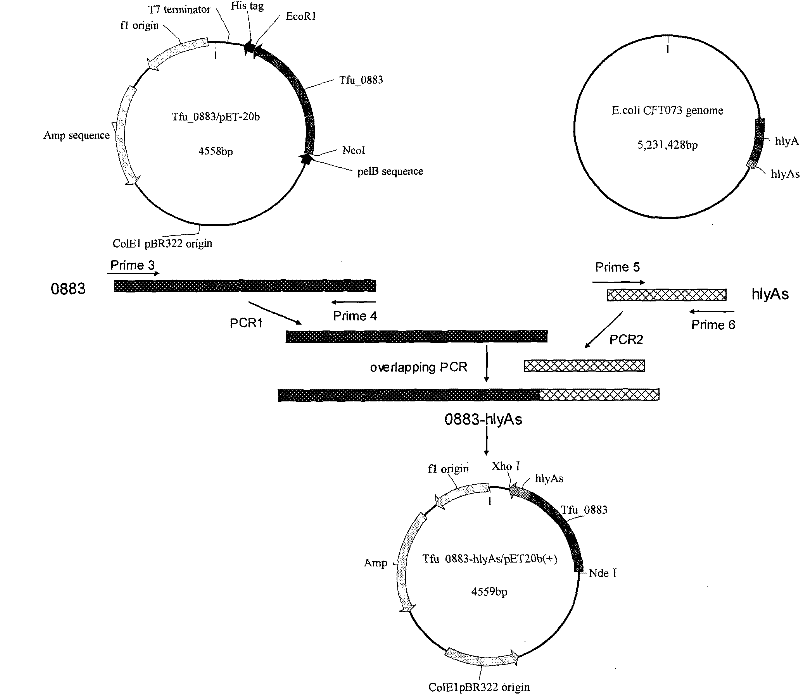
Genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase and method for constructing same
ActiveCN101792729AIncrease productionReduce pollutionBacteriaHydrolasesEscherichia coliBiotechnology
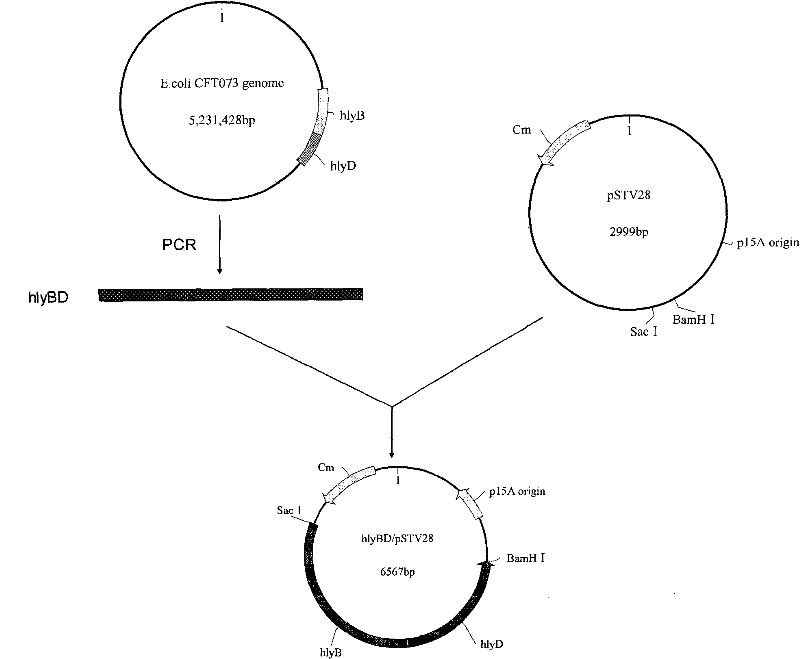
The invention relates to genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase and a method for constructing the same, belonging to the field of the bioengineering technology. The genetically engineered bacteria are escherichia coli BL21 (DE3) which carry two recombinant plasmids, and the recombinant plasmids are respectively plasmid pSTV28 carrying the specific genes in the Alpha-hemolysin A (hly A) pathway and plasmid pET20b(+) containing the cutinase-hly As genes. The method for constructing the genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase comprises the following steps: constructing two key recombinant plasmids and transforming the constructed recombinant plasmids in the escherichia coli BL21 (DE3) to obtain the genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase. The cutinase is produced by using the genetically engineered bacteria through culturing liquid and inducing and expressing the cutinase. The cutinase Tfu_0883 for thermophilic monospore bacteria of Thermobifida Fusca WSH03-11 is used as the report protein. The shaking flask fermentation shows that the extracellular output of the cutinase is 306U / mL which is 1.7 times of the output of the cutinase which adopts the II-type secreting pathway in the preliminary working process in the research laboratory. The cutinase is secreted and expressed efficiently.
Owner:JIANGNAN UNIV
Conductance of Improperly Folded Proteins Through the Secretory Pathway And Related Methods For Treating Disease
InactiveUS20080025921A1Improve breathabilityReduce concentrationHalogenated hydrocarbon active ingredientsBiocideReticulum cellMedicine
This invention provides the methodology and agents for treating any disease or clinical condition which is at least partly the result of endoplasmic reticulum-associated retention of proteins. Thus, the methods and agents of the present invention provide for the release of normally retained proteins from the endoplasmic reticulum. The present invention is particularly useful for treating any disease or clinical condition which is at least partly the result of endoplasmic reticulum-associated retention or degradation of mis-assembled or mis-folded proteins.
Owner:N FOLD LLC
Non-classical secretory protein and application thereof to protein secretory expression
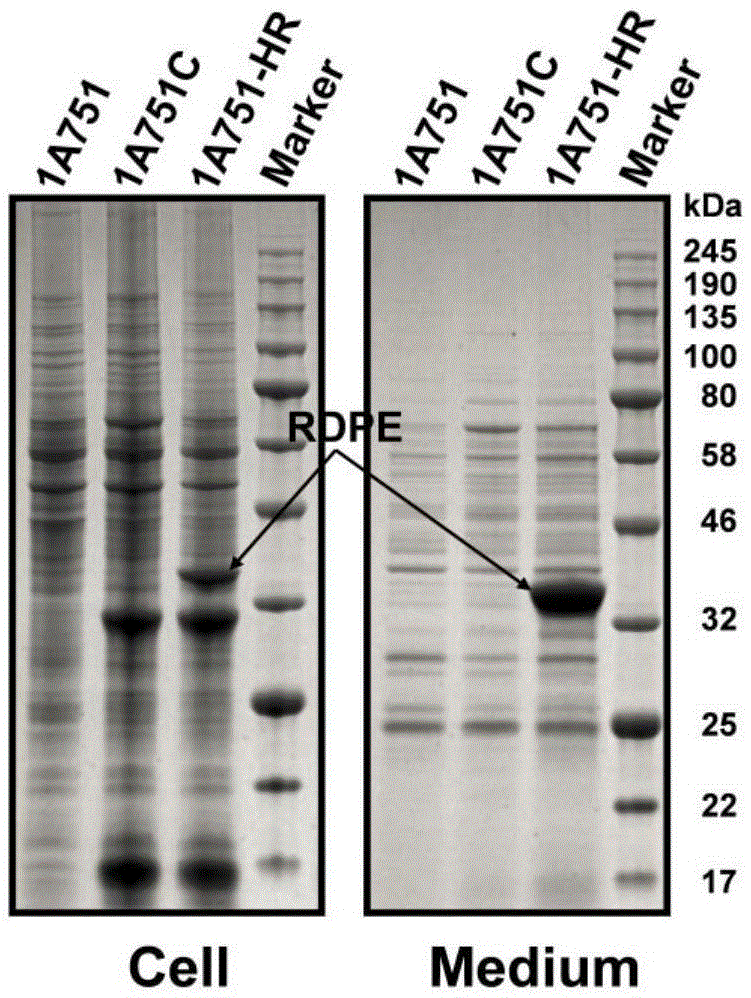
The invention relates to heterologous protein secreted in bacillus subtilis in a non-classical path and application thereof to protein secretory expression. Carrying no signal peptide or secretory signals, D-allulose3-epimerase RDPE from Ruminococcus sp.5-139B-FAA can be secreted out of cells in quantity; it is proved through experiments that RDPE is not secreted in an Sec or Tat path or leaked out of the cells due to cell autolysis and lysis. Therefore RDPE is non-classical secretory protein in the bacillus subtilis. RDPE is respectively fused with 18 kinds of target protein, an attempt is made to use RDPE as export signals for achieving secretory expression of the target protein, 16 kinds of fused protein are obviously expressed in the cells, 9 kinds of fused protein are successfully secreted out of the cells, and the secretion level of two kinds of fused protein accounts for more than 50% of the corresponding total protein. Therefore, a new strategy for achieving protein secretory expression in the bacillus subtilis is developed.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Heterologous protein production using the twin arginine translocation pathway
InactiveUS7447595B1Produce and purifyEfficient secretionTripeptide ingredientsAnalogue computers for chemical processesBacteroidesHeterologous
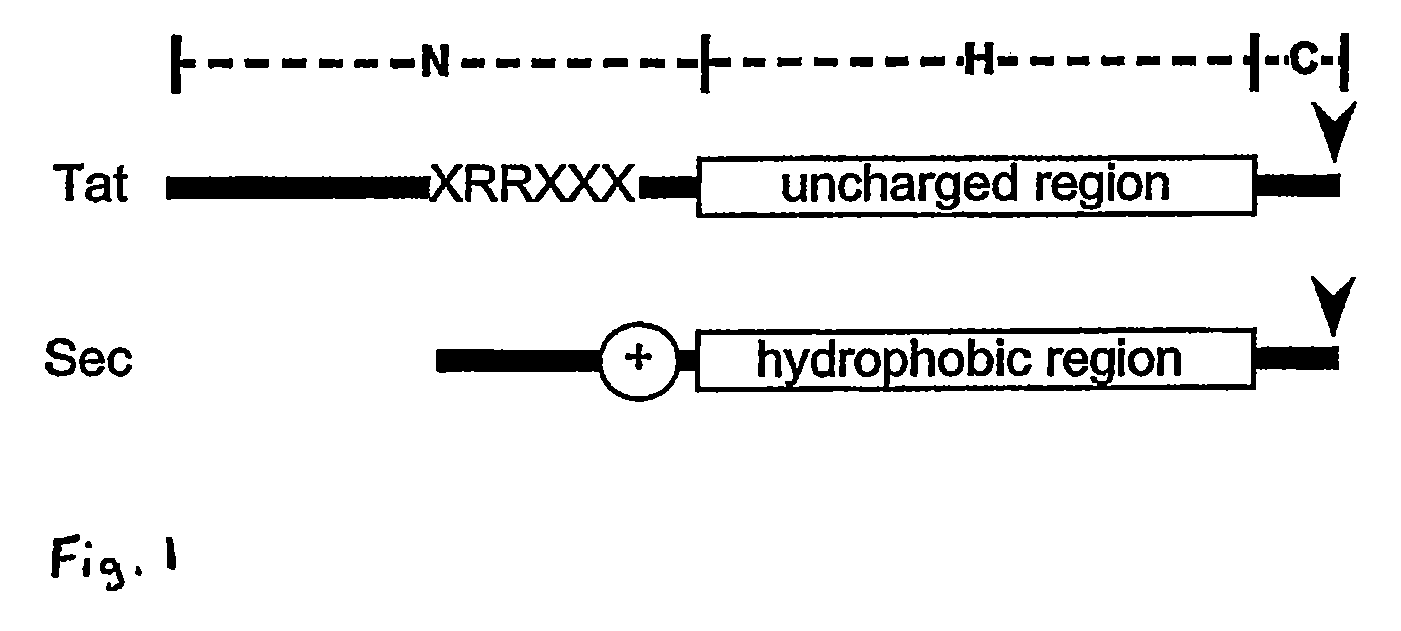
Provided are means for evaluating and identifying putative substrates of the twin arginine translocation (Tat) secretory pathway in Streptomyces and other bacterial species. Also provided, therefore, are simple ways to express, secrete and purify correctly folded heterologous proteins on a large scale using host microorganisms, such as, Streptomyces and the Tat pathway therein. Many of the thus-produced proteins are of significant therapeutic value in the pharmaceutical and biochemical industries, particularly when they can be secreted from the host in fully-folded active form. Accordingly, there are further provided the heterologous proteins produced by the Tat secretion pathway using the foregoing methods, and the computer algorithm used to identify the Tat signal sequence and putative substrates.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Angiogenesis inhibitor comprising meteorin as an active ingredient
ActiveUS7960345B2Induce functional maturity of the blood-brain barrierInhibit angiogenesisSenses disorderNervous disorderEmbryonic StageEmbryo
The present invention relates to an angiogenesis inhibitor comprising meteorin as an active ingredient that is highly expressed in astrocytes of the brain and retina in the late embryonic stage and after the birth of a mouse. It is in particular highly detected in astrocyte endfeet surrounding blood vessels and promotes the expression of thrombospondin-1 / -2 (TSP-1 / -2) via autocrine pathway and thus inhibits angiogenesis. The meteorin of the present invention can be effectively used for pharmaceutical compositions and health foods that prevent vascular diseases by inhibiting angiogenesis.
Owner:SEOUL NAT UNIV R&DB FOUND
Method for efficient secretory expression of foreign proteins by use of bacillus
InactiveCN107287229AOvercome the shortcomings of instability, inducible expression and low expression levelEasy to separateMicroorganism based processesFermentationBiotechnologyProtein target
The invention belongs to the field of biotechnology and particularly relates to a method for efficient secretory expression of foreign proteins by use of bacillus. The method is characterized in that target proteins are accumulated intracellularly in quantity and secreted largely into fermentation liquid. The method does not rely on signal peptide or feature structure and is essentially different from existing Sec way, Tat way, Com system and ABC transfer way. When any target proteins are expressed intracellularly in quantity, the target proteins can be secreted extracellularly in quantity through the way, and the maximum secretion quantity can reach a g / L level. The characteristics of small secretion quantity and low universality of existing secretion way are overcome, the target proteins can be secreted into the fermentation liquid non-selectively, and the difficulty of later separation and purification is reduced.
Owner:CHENGDU MYTECH BIOTECH +1
Methods for generating engineered enzymes
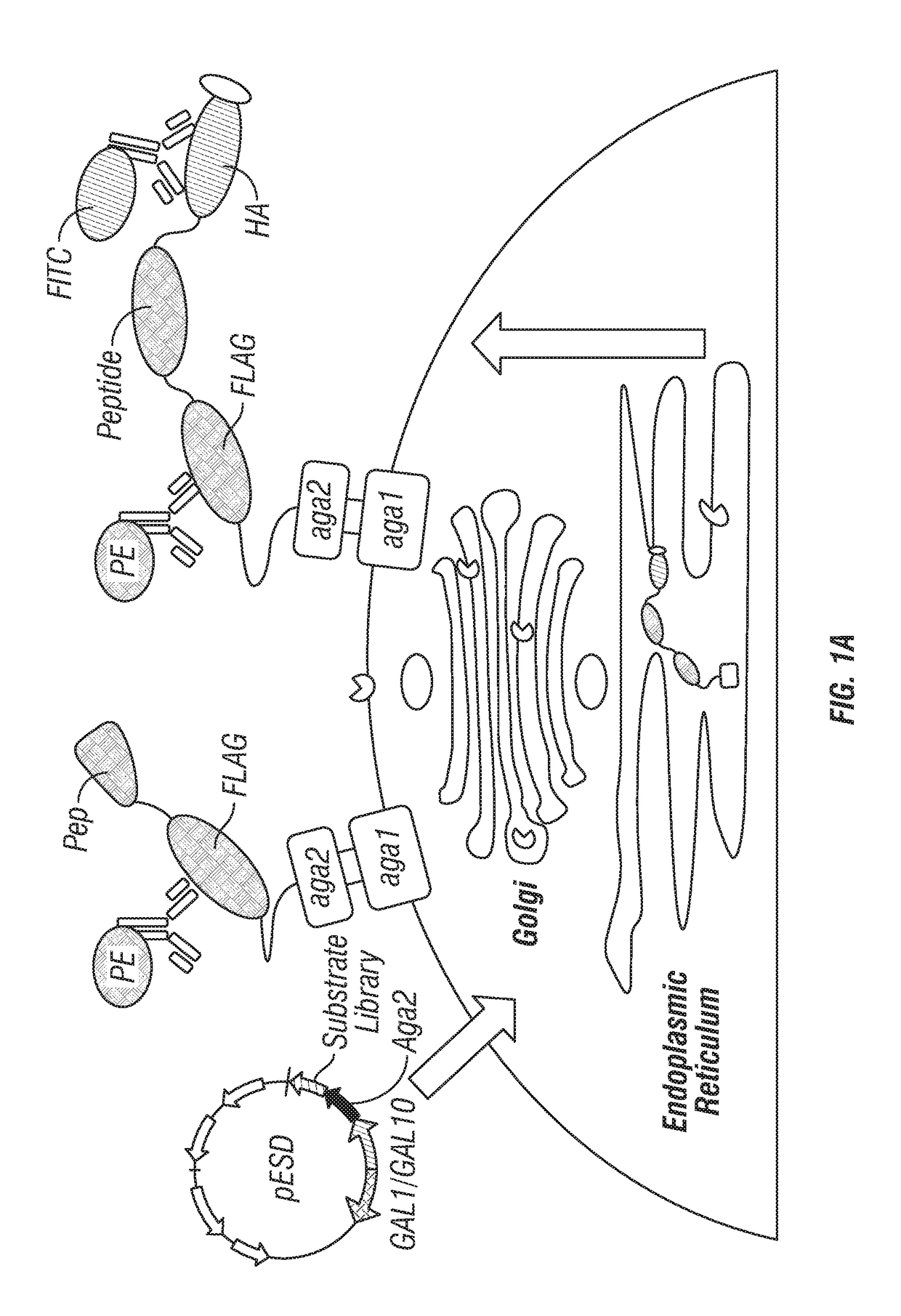
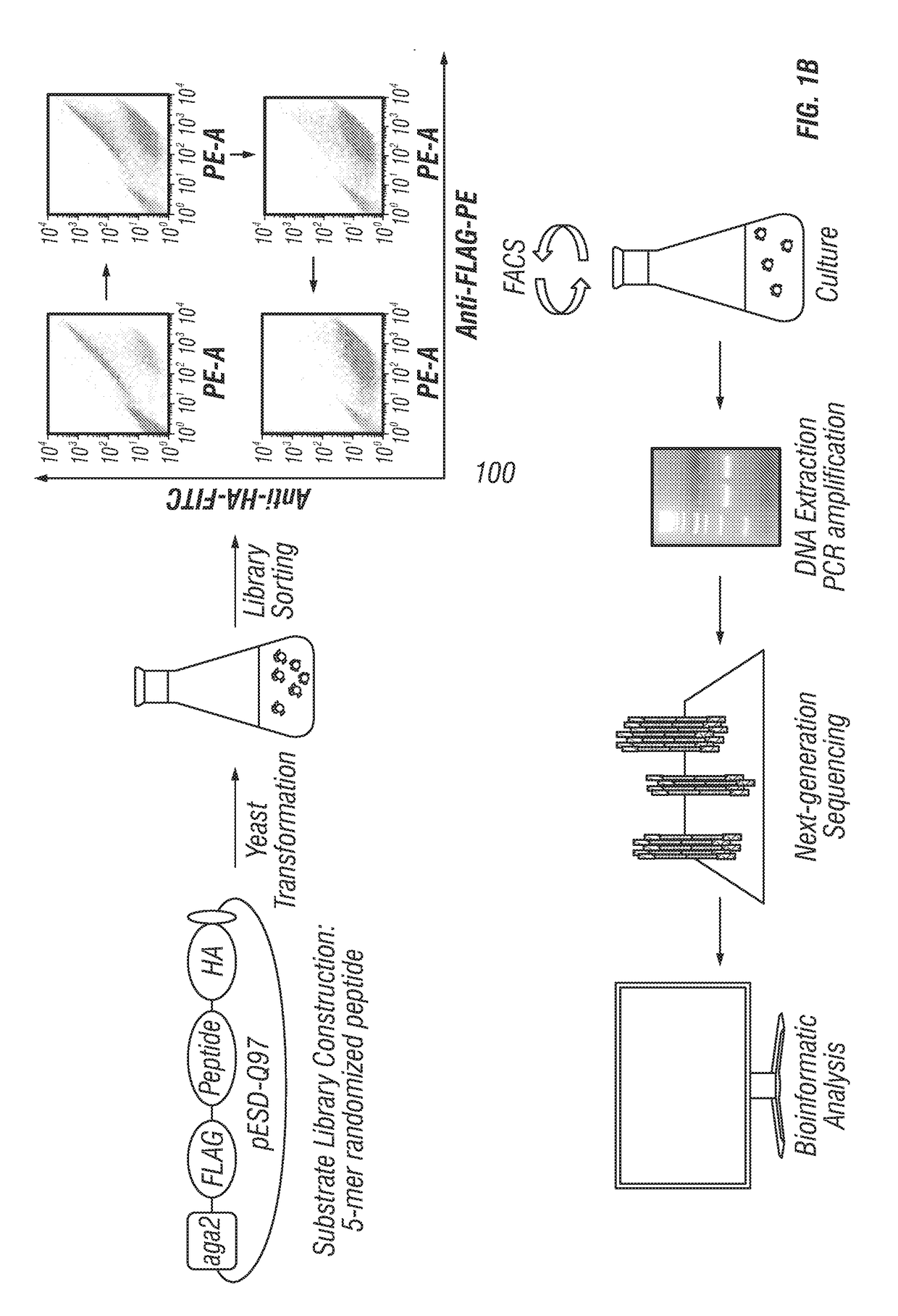
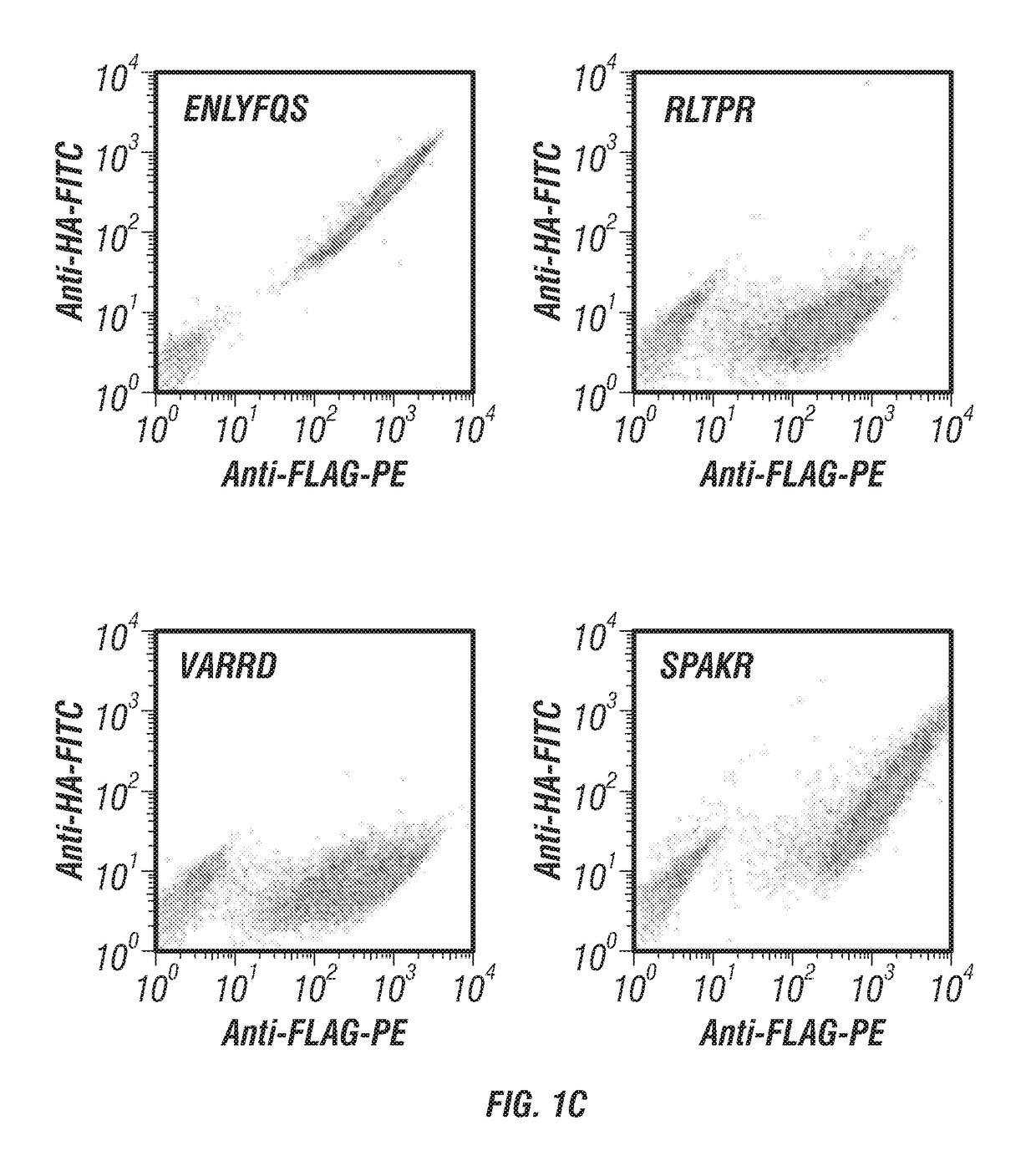
ActiveUS20170233781A1Accurate identificationPrevent unwanted cleavageMicrobiological testing/measurementTransferasesYeastProteinase activity
Provided are improved methods for identifying the substrate recognition specificity or activity of a protease, convertase (sortase), or kinase. In some embodiments, methods are provided for identifying the endogenous protease or convertase cleaving patterns (e.g., “cleaveOme”) inside the secretory pathway of a living cell. Select embodiments involve aspects of yeast endoplasmic reticulum sequestration screening and next generation sequencing. Methods of producing polypeptides in Kex2 knockout yeast are also provided.
Owner:RES DEVMENT FOUND
Methods for glyco-engineering plant cells for controlled human o-glycosylation
This invention discloses the development of a novel platform for recombinant production of bioactive glycoproteins and cancer specific vaccines in plants. Plants and plant cell cultures have been humanized with respect to human mucin-type protein O-glycosylation. A panel of plant cell factories for production of recombinant glycoproteins with designed human O-glycosylation, including an improved cancer vaccine candidate, has been developed. The platform provides basis for i) production of an essentially unlimited array of O-glycosylated human glycoprotein therapeutics, such as human interferon α2B and podoplanin, and ii) for further engineering of additional cancer specific O-glycans on glycoproteins of therapeutical value. Currently, mammalian cells are required for human O-glycosylation, but plants offer a unique cell platform for engineering O-glycosylation since they do not perform human type O-glycosylation. Introduction of O-glycosylation into plant cells requires i) that wild-type plant cells do not modify the target peptide substrates and ii) that the appropriate enzymes and substrates are introduced into of plant cells such that O-glycosylation in the secretory pathway proceed and the glycosylated peptide substrates are preferentially exported to the exterior of the cell or accumulated in the cell. In this invention i) the integrity of transiently and stably expressed ‘mucin’ type target peptides in plants cells has been determined and ii) mucin-type O-glycosylation has been established in plants by transient and stable introduction of a Pseudomonas aeruginosa C4-epimerase, the human polypeptide GalNAc-transferases T2 and T4 (GalNAc-T2 and T4) and various human target peptides or proteins. In the present invention GalNAc-T2 and -T4 have been used to produce a Tn cancer glycoform of MUC1.
Owner:YANG ZHANG +7
A non-canonical secretory protein and its application in protein secretory expression
The invention relates to heterologous protein secreted in bacillus subtilis in a non-classical path and application thereof to protein secretory expression. Carrying no signal peptide or secretory signals, D-allulose3-epimerase RDPE from Ruminococcus sp.5-139B-FAA can be secreted out of cells in quantity; it is proved through experiments that RDPE is not secreted in an Sec or Tat path or leaked out of the cells due to cell autolysis and lysis. Therefore RDPE is non-classical secretory protein in the bacillus subtilis. RDPE is respectively fused with 18 kinds of target protein, an attempt is made to use RDPE as export signals for achieving secretory expression of the target protein, 16 kinds of fused protein are obviously expressed in the cells, 9 kinds of fused protein are successfully secreted out of the cells, and the secretion level of two kinds of fused protein accounts for more than 50% of the corresponding total protein. Therefore, a new strategy for achieving protein secretory expression in the bacillus subtilis is developed.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Secreted Luciferase Fluorescent Protein Conjugate Nucleic Acid Construct and Uses Thereof
InactiveUS20100035287A1Easy to detectThe process is convenient and fastVirusesSugar derivativesProtein insertionFluorescence
The present invention relates generally to methods to monitor the transport of proteins through the secretory pathway, and methods to monitor ER stress. In particular, the present invention relates to methods to monitor, in real-time, the processing of protein through the secretory pathway, which can be monitored both at a subcellular level by florescence visualization and quantitatively by detecting the secreted luciferase reporter protein. The present invention also relates to methods to assess biological processes in cells, in particular the secretory pathway and ER stress, as well as methods to identify agents which augment or inhibit the secretory pathway and / or ER stress. The present invention also relates to compositions and nucleic constructs encoding a secreted luciferase-fluorescent protein conjugate for methods to monitor protein trafficking in the cell by simultaneous detection of fluorescence and luciferase secretion.
Owner:THE GENERAL HOSPITAL CORP
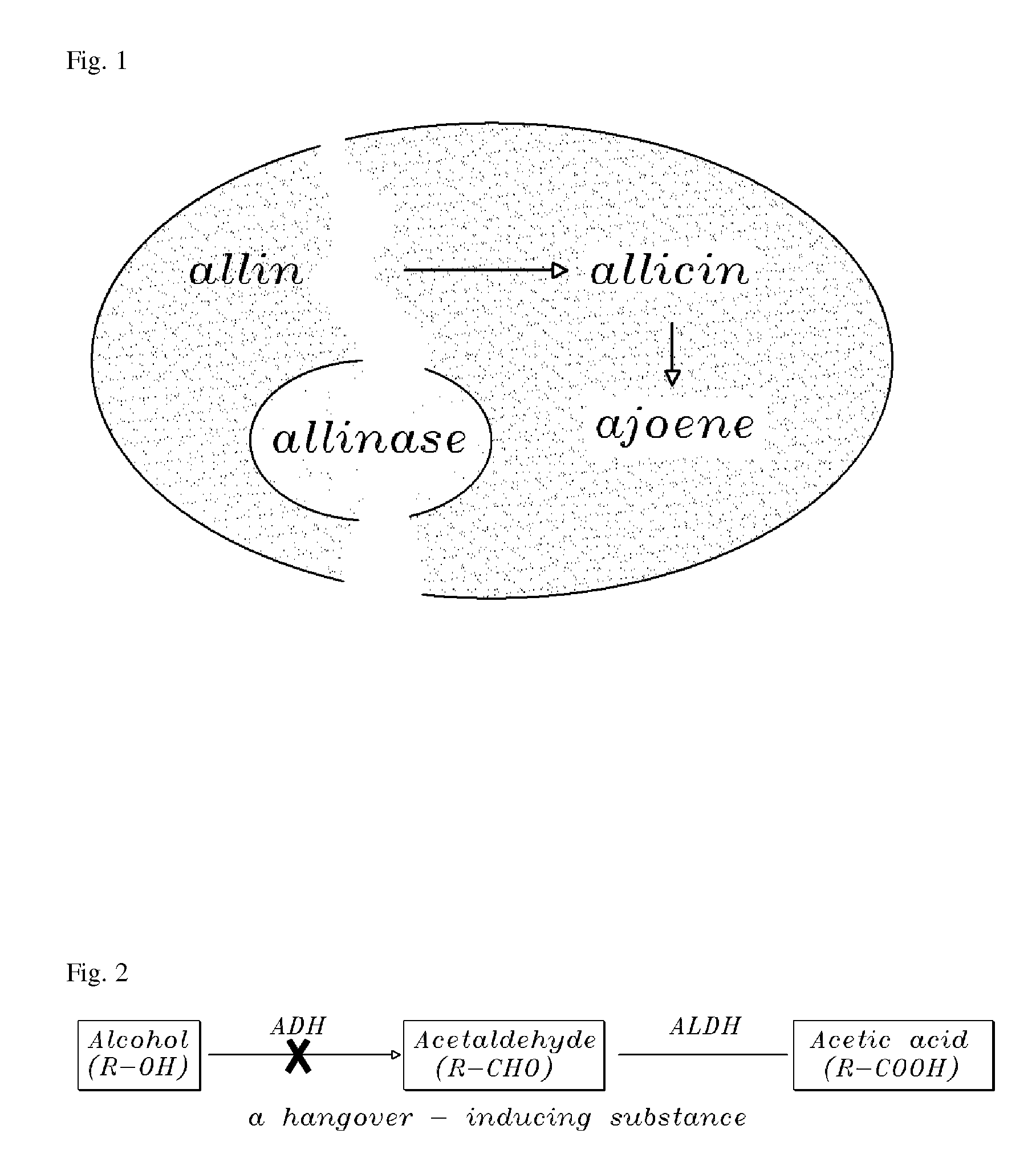
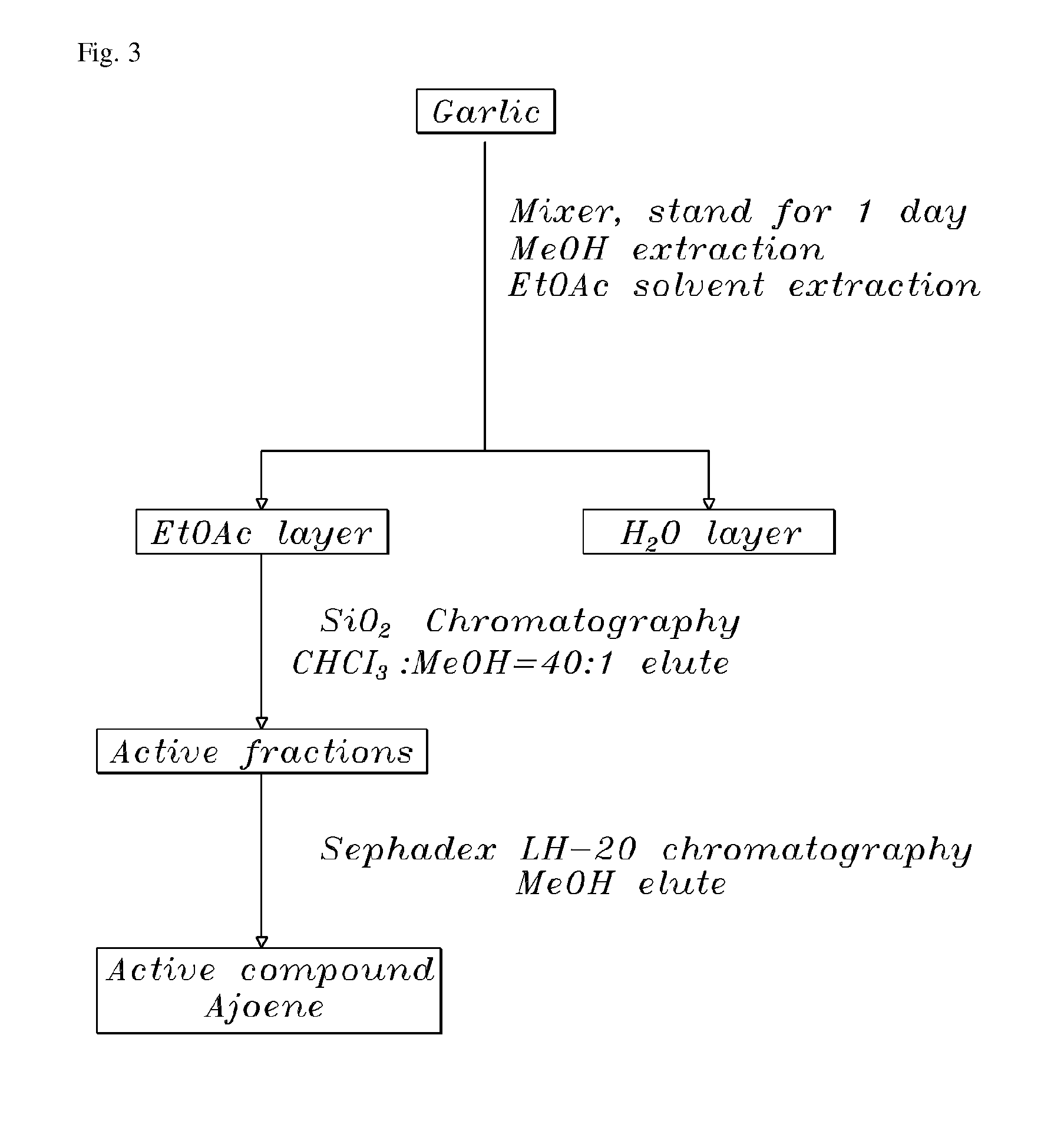
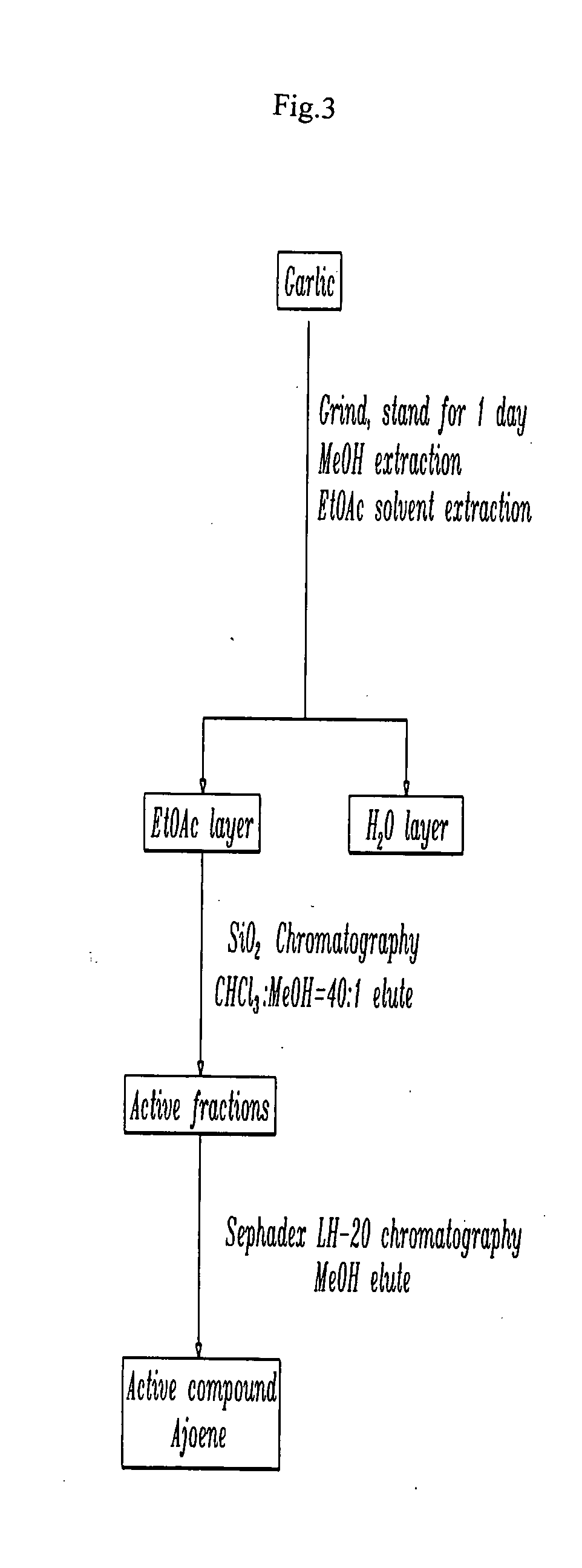
Method of using ajoene as alcohol dehydrogenase inhibitor, composition for removing hangover comprising ajoene and method of preparing the same
InactiveUS20080102142A1Suppress generationBiocideSulfur/selenium/tellurium active ingredientsEthanol dehydrogenaseNatural substance
The present invention provides a composition for removing a hangover. In particular, the present invention discloses a method of using ajoene as an alcohol dehydrogenase inhibitor based on the fact that ajoene strongly inhibits alcohol dehydrogenase (ADH) activity. A composition for removing a hangover comprising ajoene suppresses the over-production of acetaldehyde during alcohol metabolism to maintain acetaldehyde concentration in blood at a normal level that can be degraded in the body and eliminated therefrom through normal metabolic and secretory pathways to thereby prevent the hangover after consumption of alcohol, and a method of preparing the same, are also provided. The present invention also provides a novel use of ajoene, which is a natural substance obtained by purification of garlic, and a composition for removing a hangover comprising ajoene, which is harmless to the body and strongly inhibits ADH activity, thereby showing very excellent relieving effect on the hangover.
Owner:SAMSUNG ELECTRONICS CO LTD
Application of human amniotic epithelial cell secretory factor in preparation of medicine for ovarian function restoration
InactiveCN109528772AIncrease the number ofImprove developmentCell dissociation methodsMammal material medical ingredientsIntraperitoneal routeGranular cell
The invention relates to an application of a human amniotic epithelial cell secretory factor in preparation of a medicine for ovarian function restoration. The human amniotic epithelial cell secretoryfactor is concentrated and then is injected to a chemotherapically-damaged mouse through intraperitoneal injection; it is found that the secretory factor can significantly increase the number of follicles in a damaged ovary, improve expression of genes related to development of the ovary, and promote regeneration of blood vessels. It is proved that the human amniotic epithelial cell can be directly differentiated into granular cells participating in the ovarian function restoration process, and a paracrine pathway also contributes important function to the process. The human amniotic epithelial cell secretory factor can be used for preparing the medicine for ovarian function restoration.
Owner:THE INT PEACE MATERNITY & CHILD HEALTH HOSPITAL OF CHINA WELFARE INST
Method of using ajoene as alcohol dehydrogenase inhibitor, composition for removing hangover comprising ajoene and method of preparing the same
InactiveUS20070031520A1Environmentally friendlySuppress generationBiocideSulfur/selenium/tellurium active ingredientsEthanol dehydrogenaseNatural substance
The present invention provides a composition for removing a hangover. In particular, the present invention discloses a method of using ajoene as an alcohol dehydrogenase inhibitor based on the fact that ajoene strongly inhibits alcohol dehydrogenase (ADH) activity. A composition for removing a hangover comprising ajoene suppresses the over-production of acetaldehyde during alcohol metabolism to maintain acetaldehyde concentration in blood at a normal level that can be degraded in the body and eliminated therefrom through normal metabolic and secretory pathways to thereby prevent the hangover after consumption of alcohol, and a method of preparing the same, are also provided. The present invention also provides a novel use of ajoene, which is a natural substance obtained by purification of garlic, and a composition for removing a hangover comprising ajoene, which is harmless to the body and strongly inhibits ADH activity, thereby showing very excellent relieving effect on the hangover.
Owner:SAMSUNG ELECTRONICS CO LTD
Signal peptide and application thereof in production of L-arginine recombinant bacterium by means of starch
The invention discloses a signal peptide and application thereof in production of an L-arginine recombinant bacterium by means of starch, and belongs to the fields of gene engineering and enzyme engineering. Alpha-amylase which comes from Streptomyces kathirae CCTCC M2012432 and does not carry with a self signal peptide is fused with 12 signal peptides of two main secretory pathways, namely, Sec and Tat which come from different microorganisms and then expressed in a high-yield L-arginine strain, and the signal peptide SEQ ID No.1 which can efficiently secrete the alpha-amylase is screened. It is reported for the first time that recombination corynebacterium crenatum CGMCC No.0890 / pMSPamy which is mediated to secrete the alpha-amylase can produce L-arginine through fermentation with cheap starch, and the yield of a 5-L fermentor is 48 g / L under the optimal carbon source adding condition.
Owner:JIANGNAN UNIV
Yeast protein expression secretion system
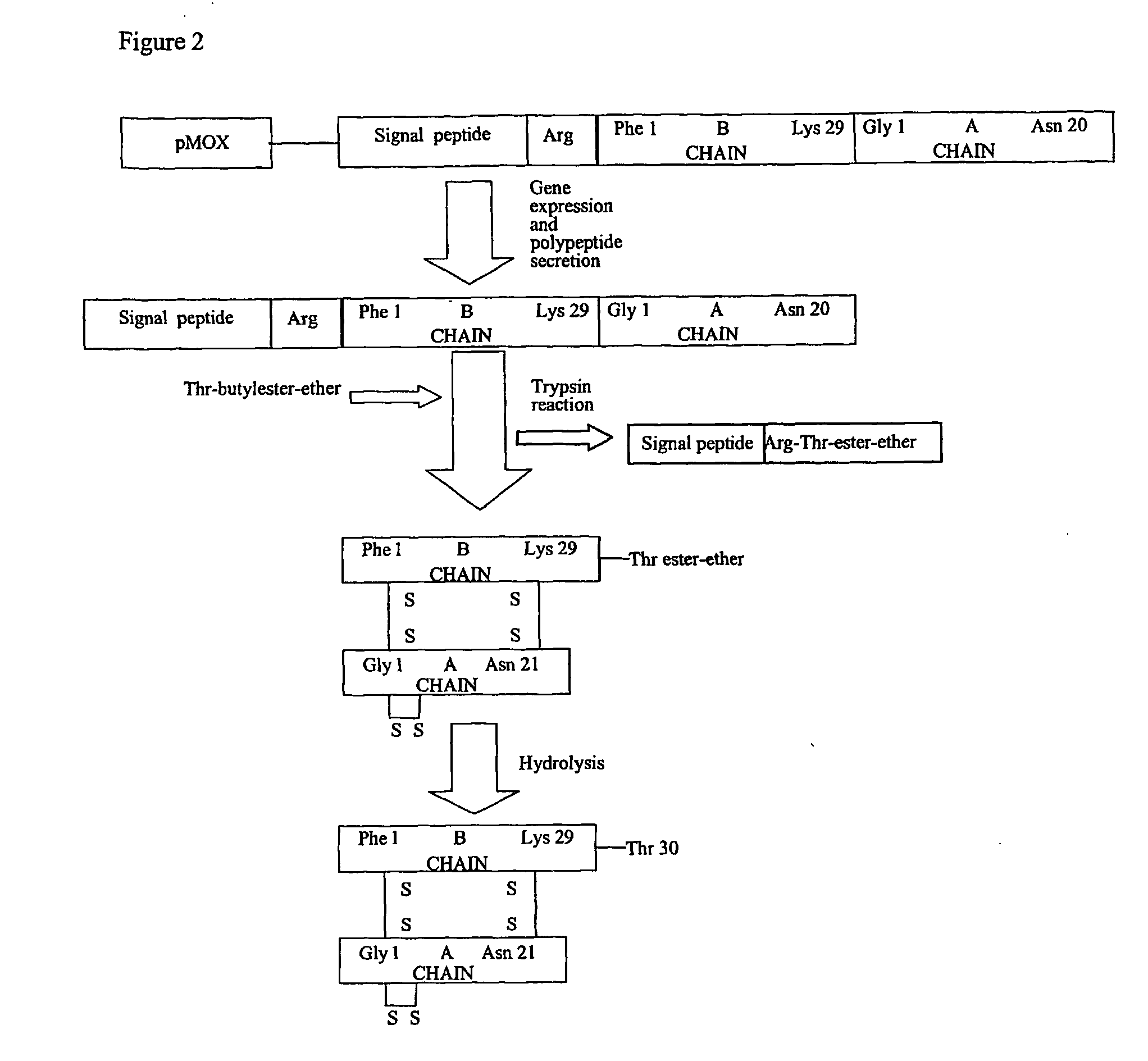
This invention discloses novel prepro-insulin polypeptides. The polypeptides consist of an N-terminal region, derived from N-terminal regions of secretory proteins, and a downstream insulin polypeptide region. The N-terminal region directs the polypeptides efficiently into the secretory pathway of yeasts. Modifications at the N-terminal region, just adjacent to the insulin polypeptide region, further increase the efficiency of secretion and improves the final yield of secreted insulin. The patent also discloses expression systems for the expression of said polypeptides under the regulation of yeast derived alcohol inducible promoters. Thus a combination of such promoters and precursors with the said N-terminal regions appear to function as very high yielding expression systems in yeasts.
Owner:WOCKHARDT LTD
Expression of Class 2 Mannosidase and Class III Mannosidase in Lower Eukaryotic Cells
A method for producing human-like glycoproteins by expressing a Class 2 α-mannosidase having a substrate specificity for Manα1,3 and Manα1,6 glycosidic linkages in a lower eukaryote is disclosed. Hydrolysis of these linkages on oligosaccharides produces substrates for further N-glycan processing in the secretory pathway.
Owner:GLYCOFI
Assay for compounds affecting invertebrate cell secretory pathways
The present invention provides assay methods for identifying agents that affect a secretory pathway in an invertebrate pest cell. The methods are useful for identifying agents that modulate a secretory pathway in an invertebrate pest cell, and that are therefore useful as pesticidal agents. The invention further provides methods for identifying secretory pathway proteins in an invertebrate pest cell, which secretory pathway proteins serve as targets for pesticides. The method further provides pesticidal agents identified using the assay methods of the instant invention.
Owner:BAYER CROPSCIENCE AG +1
Expression of Class 2 Mannosidase and Class III Mannosidase in Lower Eukaryotic Cells
A method for producing human-like glycoproteins by expressing a Class 2 α-mannosidase having a substrate specificity for Manα1,3 and Manα1,6 glycosidic linkages in a lower eukaryote is disclosed. Hydrolysis of these linkages on oligosaccharides produces substrates for further N-glycan processing in the secretory pathway.
Owner:GLYCOFI
Expression of Class 2 mannosidase and Class III mannosidase in lower eukaryotic cells
A method for producing human-like glycoproteins by expressing a Class 2 α-mannosidase having a substrate specificity for Manα1,3 and Manα1,6 glycosidic linkages in a lower eukaryote is disclosed. Hydrolysis of these linkages on oligosaccharides produces substrates for further N-glycan processing in the secretory pathway.
Owner:GLYCOFI
Screens and assays for agents useful in controlling parasitic nematodes
InactiveUS7064243B2Reduce expressionReduce processing stepsPeptide/protein ingredientsGenetic engineeringBiotechnologyAssay
The invention provides a method of identifying anti-nematode compounds and further provides transgenic nematodes that may be used to practice the method. In particular, the invention provides a screen for compounds that inhibit a nematode secretion pathway e.g, compounds that inhibit the secretion of proteins by nematodes. The transgenic nematodes express reporters for nematode secreted proteins. In preferred embodiments of the invention the screen is performed using C. elegans, i.e., certain embodiments of the invention utilize C. elegans and C. elegans secretory pathways as a model system for parasitic nematodes and parasitic nematode secretion pathways. The invention also provides pharmaceutical compositions that may be used in the treatment and prevention of nematode infection in humans and animals and anti-nematode agents that may be used to protect plants from plant-parasitic nematodes. In addition, the invention provides a genetic screen for identifying additional targets for anti-nematode compounds.
Owner:CAMBRIA PHARMA
Conductance of improperly folded proteins through the secretory pathway and related methods for treating disease
InactiveUS8058314B2Reduced activityReduce expressionHalogenated hydrocarbon active ingredientsBiocideDiseaseMedicine
This invention provides the methodology and agents for treating any disease or clinical condition which is at least partly the result of endoplasmic reticulum-associated retention of proteins. Thus, the methods and agents of the present invention provide for the release of normally retained proteins from the endoplasmic reticulum. The present invention is particularly useful for treating any disease or clinical condition which is at least partly the result of endoplasmic reticulum-associated retention or degradation of mis-assembled or mis-folded proteins.
Owner:N FOLD LLC
General secretory pathway (GSP) mutant listeria spp., and methods for making and using the same
General secretory pathway (GSP) mutant Listeria bacteria are provided. Aspects of the bacteria include the presence of a GSP mutation, e.g., a SecY and / or SecA mutation. Also provided are methods of making and using the Listeria bacteria comprising a GSP mutation as vectors and vaccines expressing a heterologous nucleic acid.
Owner:RGT UNIV OF CALIFORNIA
Methods for generating engineered enzymes
ActiveUS10900059B2Accurate identificationPrevent unwanted cleavageMicrobiological testing/measurementBiological material analysisYeastReticulum cell
Provided are improved methods for identifying the substrate recognition specificity or activity of a protease, convertase (sortase), or kinase. In some embodiments, methods are provided for identifying the endogenous protease or convertase cleaving patterns (e.g., “cleaveOme”) inside the secretory pathway of a living cell. Select embodiments involve aspects of yeast endoplasmic reticulum sequestration screening and next generation sequencing. Methods of producing polypeptides in Kex2 knockout yeast are also provided.
Owner:RES DEVMENT FOUND
Genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase and method for constructing same
ActiveCN101792729BIncrease productionReduce pollutionBacteriaHydrolasesBiotechnologyEscherichia coli
The invention relates to genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase and a method for constructing the same, belonging to the field of the bioengineering technology. The genetically engineered bacteria are escherichia coli BL21 (DE3) which carry two recombinant plasmids, and the recombinant plasmids are respectively plasmid pSTV28 carrying the specific genes in the Alpha-hemolysin A (hly A) pathway and plasmid pET20b(+) containing the cutinase-hly As genes. The method for constructing the genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase comprises the following steps: constructing two key recombinant plasmids and transforming the constructed recombinant plasmids in the escherichia coli BL21 (DE3) to obtain the genetically engineered bacteria for efficiently secreting, expressing and reconstructing cutinase. The cutinase is produced by using the genetically engineered bacteria through culturing liquid and inducing and expressing the cutinase. The cutinase Tfu_0883 for thermophilic monospore bacteria of Thermobifida Fusca WSH03-11 is used as the report protein. The shaking flask fermentation shows that the extracellular output of the cutinase is 306U / mL which is 1.7 times of the output of the cutinase which adopts the II-type secreting pathway in the preliminary working process in the research laboratory. The cutinase is secreted and expressed efficiently.
Owner:JIANGNAN UNIV
Method for enhancing glucose oxidase secretion through transforming folding and secreting pathway of protein
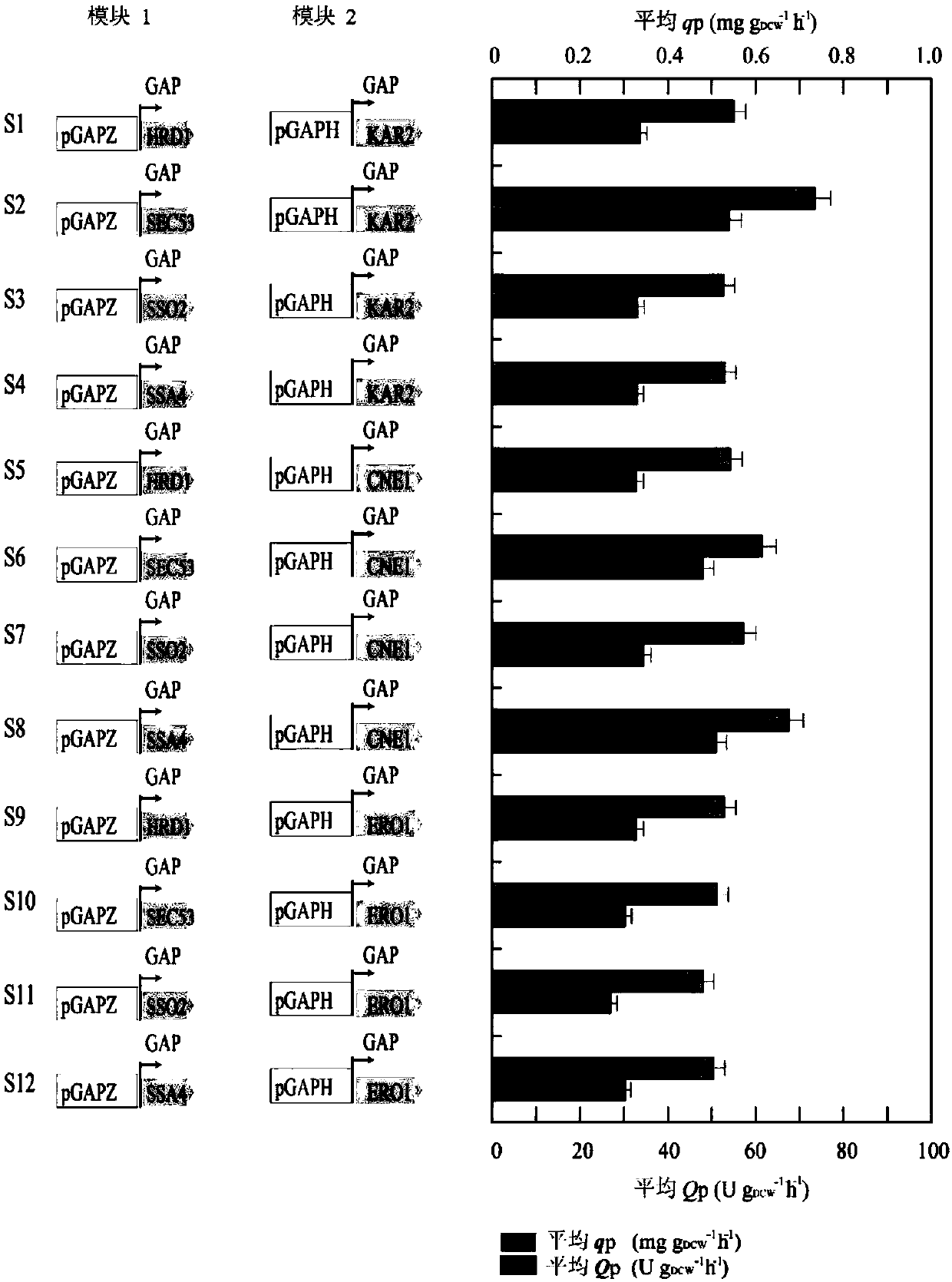
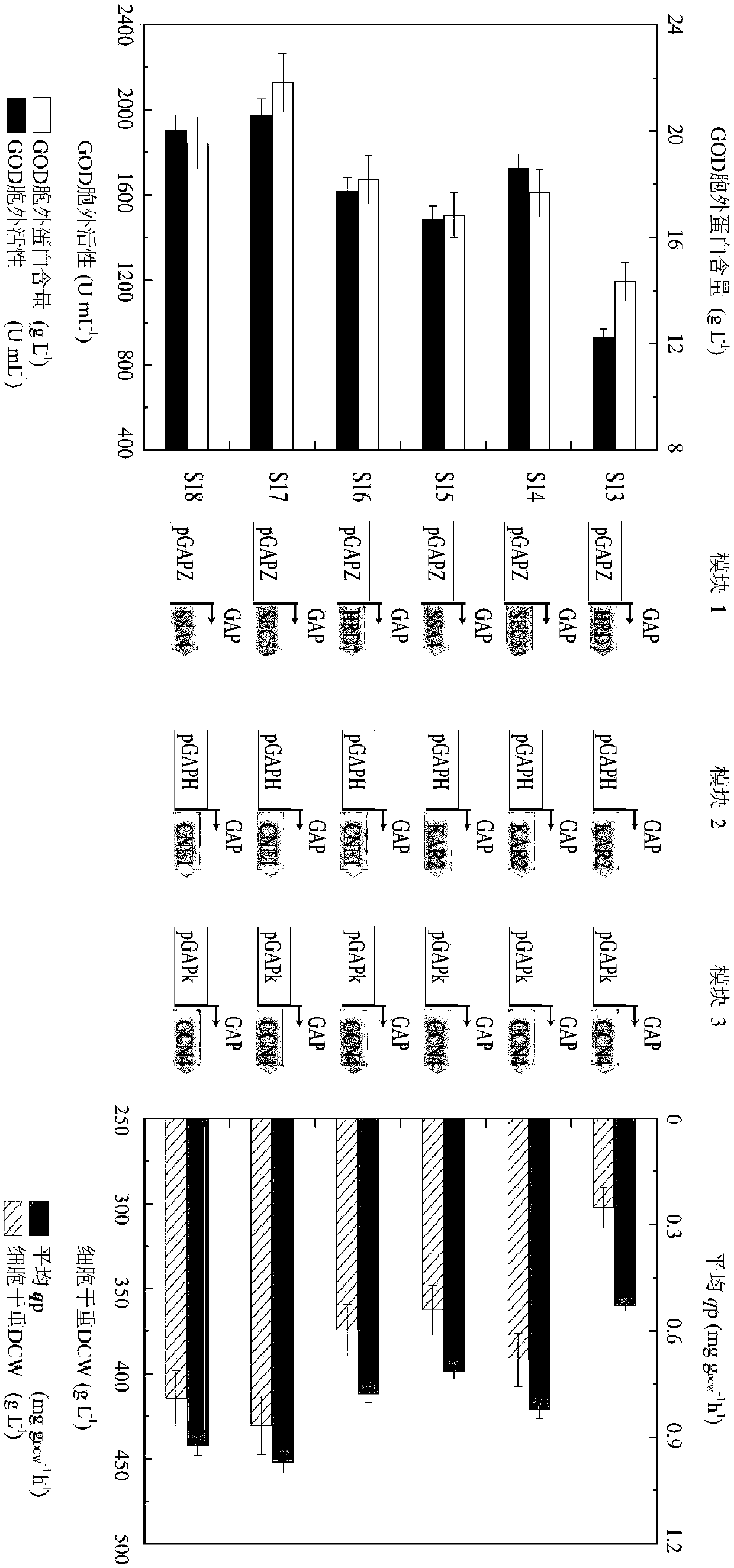
The invention discloses a method for enhancing the glucose oxidase secretion by transforming folding and secreting pathway of a protein, and belongs to the technical field of gene engineering. KAR2, CNE1, ERO1, SSA4, SSO2, SEC53, BMH2, HRD1, UBC1, GCN4 and other genes in Pichia pastoris are respectively cloned and linked into pGAPZ series derived plasmids of a Pichia pastoris expression vector bya gene recombination technology, the obtained plasmids and GOD was co-expressed and transferred into Pichia pastoris GS115 strains, the effect of the strain is investigated, modular combination optimization is carried out, and the strains are screened and identified to obtain a strain having a higher glucose oxidase secretion ability than the original strains. The enzyme activity of the glucose oxidase expressed by the strain in a 3 L fermentation tank is 1972.9 U / mL. The method lays a good foundation for the large-scale production of the glucose oxidase.
Owner:JIANGNAN UNIV
Method for production of a compound in a eukaryotic cell
InactiveUS8129143B2Polypeptide with localisation/targeting motifFungiCellular secretionPeroxisome localization
Owner:DSM IP ASSETS BV
Heterologous Protein Production Using The Twin Arginine Translocation Pathway
InactiveUS20090215989A1Produce and purifyEfficient secretionMicrobiological testing/measurementDepsipeptidesBacteroidesArginine
Provided are means for evaluating and identifying putative substrates of the twin arginine translocation (Tat) secretory pathway in Streptomyces and other bacterial species. Also provided, therefore, are simple ways to express, secrete and purify correctly folded heterologous proteins on a large scale using host microorganisms, such as, Streptomyces and the Tat pathway therein. Many of the thus-produced proteins are of significant therapeutic value in the pharmaceutical and biochemical industries, particularly when they can be secreted from the host in fully-folded active form. Accordingly, there are further provided the heterologous proteins produced by the Tat secretion pathway using the foregoing methods, and the computer algorithm used to identify the Tat signal sequence and putative substrates.
Owner:POHLSCHRODER MECHTILD +4
Methods for glyco-engineering plant cells for controlled human O-glycosylation
This invention discloses the development of a novel platform for recombinant production of bioactive glycoproteins and cancer specific vaccines in plants. Plants and plant cell cultures have been humanized with respect to human mucin-type protein O-glycosylation. A panel of plant cell factories for production of recombinant glycoproteins with designed human O-glycosylation, including an improved cancer vaccine candidate, has been developed. The platform provides basis for i) production of an essentially unlimited array of O-glycosylated human glycoprotein therapeutics, such as human interferon α2B and podoplanin, and ii) for further engineering of additional cancer specific O-glycans on glycoproteins of therapeutical value. Currently, mammalian cells are required for human O-glycosylation, but plants offer a unique cell platform for engineering O-glycosylation since they do not perform human type O-glycosylation. Introduction of O-glycosylation into plant cells requires i) that wild-type plant cells do not modify the target peptide substrates and ii) that the appropriate enzymes and substrates are introduced into of plant cells such that O-glycosylation in the secretory pathway proceed and the glycosylated peptide substrates are preferentially exported to the exterior of the cell or accumulated in the cell. In this invention i) the integrity of transiently and stably expressed ‘mucin’ type target peptides in plants cells has been determined and ii) mucin-type O-glycosylation has been established in plants by transient and stable introduction of a Pseudomonas aeruginosa C4-epimerase, the human polypeptide GalNAc-transferases T2 and T4 (GalNAc-T2 and T4) and various human target peptides or proteins. In the present invention GalNAc-T2 and -T4 have been used to produce a Tn cancer glycoform of MUC1.
Owner:YANG ZHANG +7
Modified growth hormone
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com