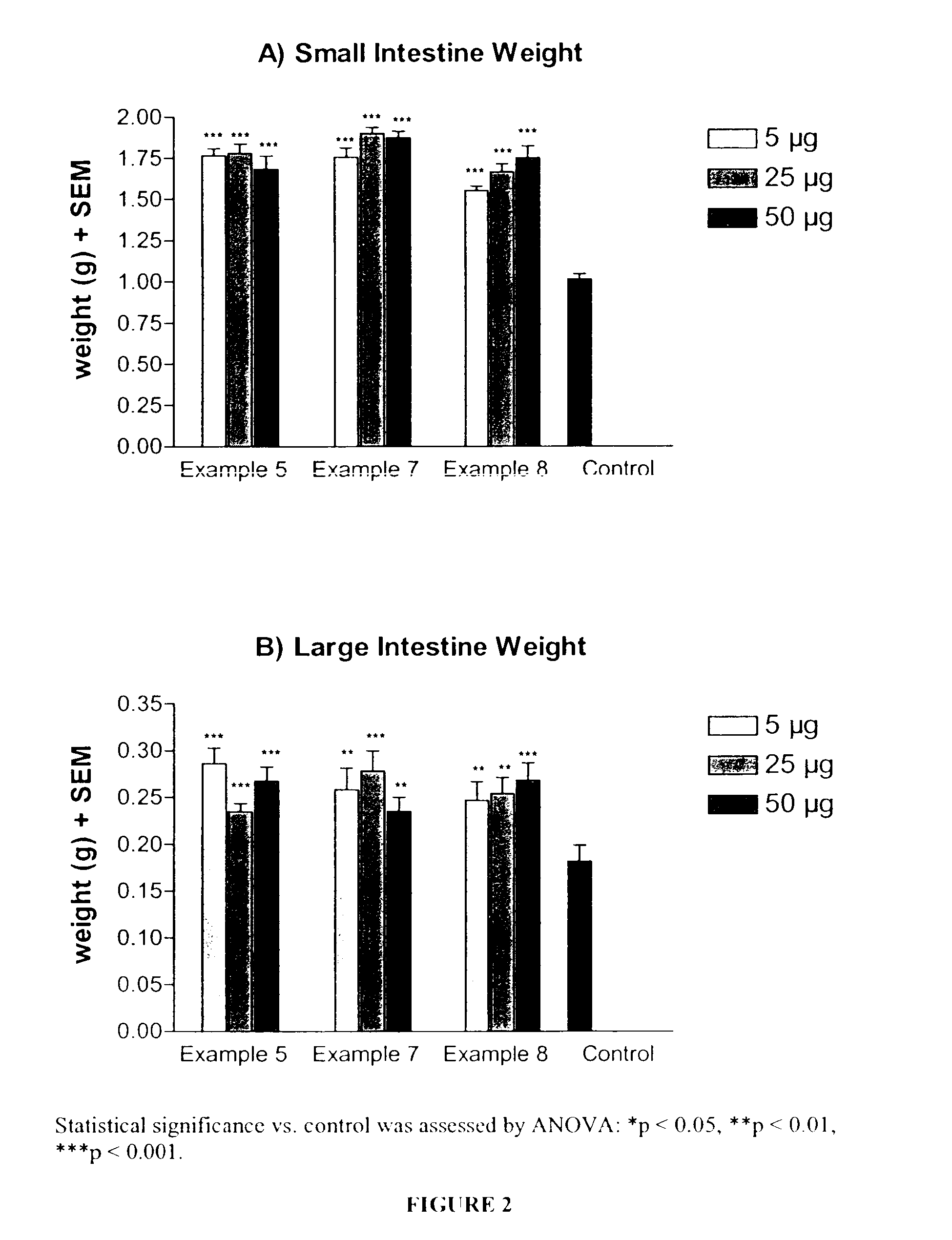
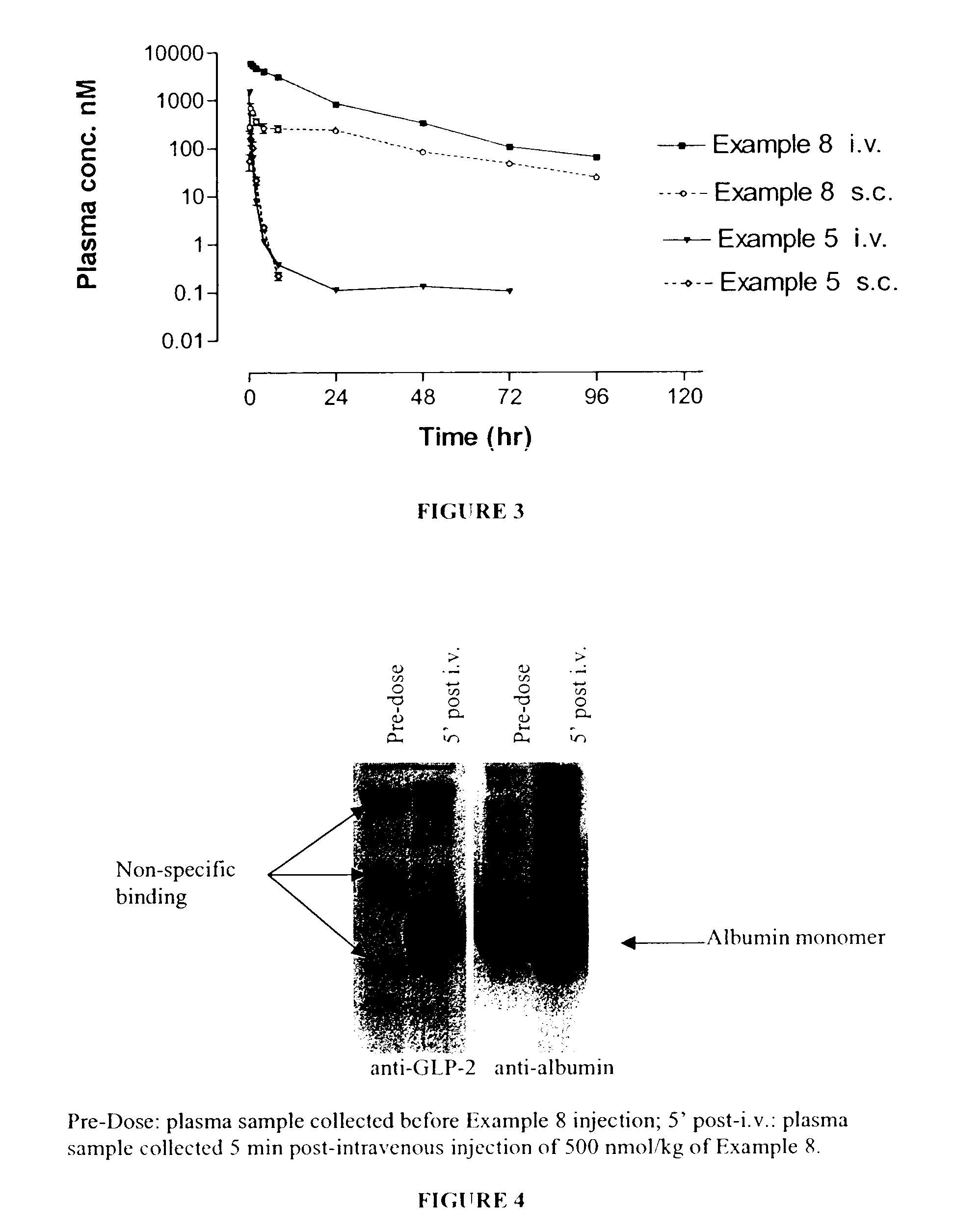
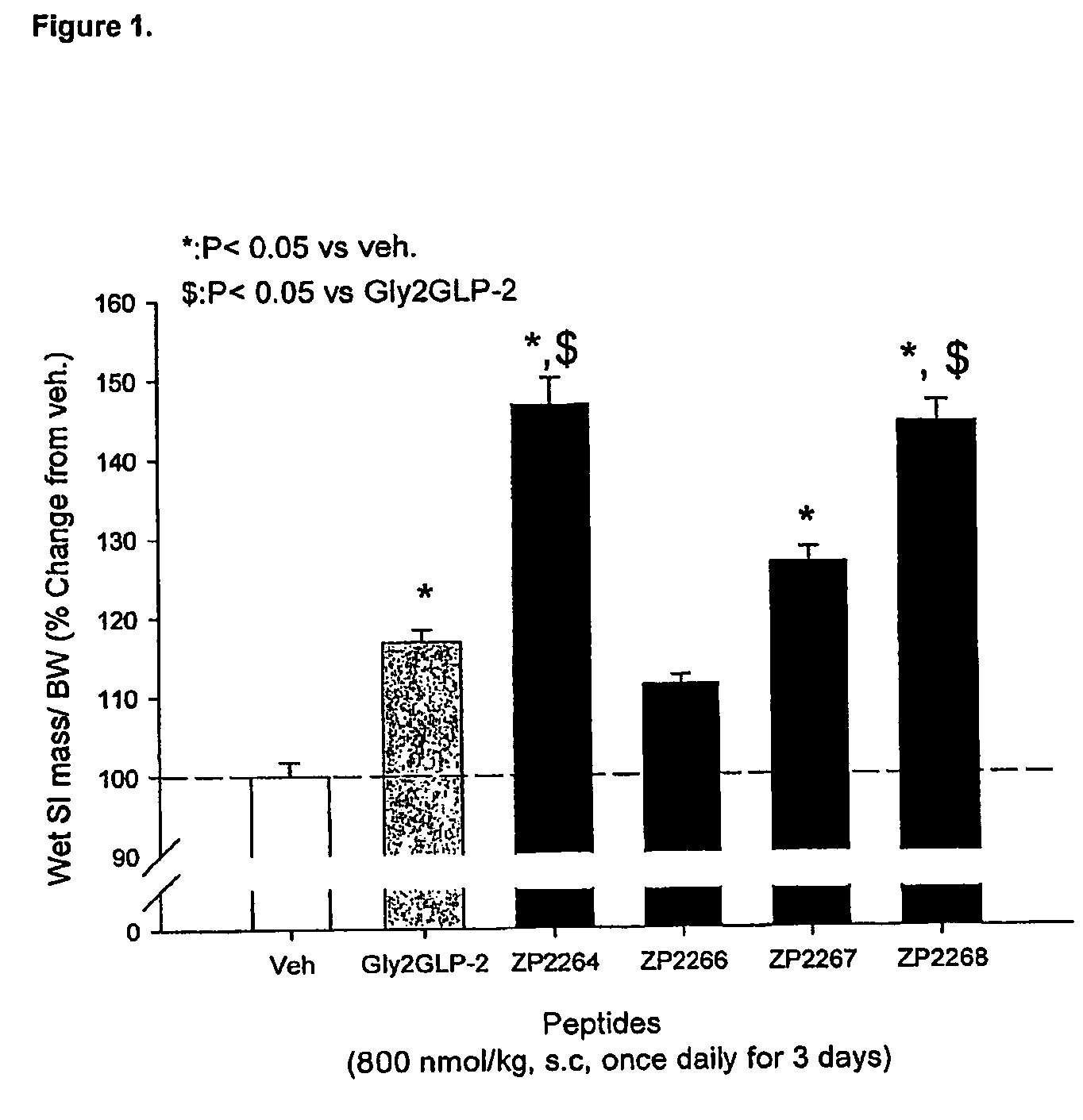
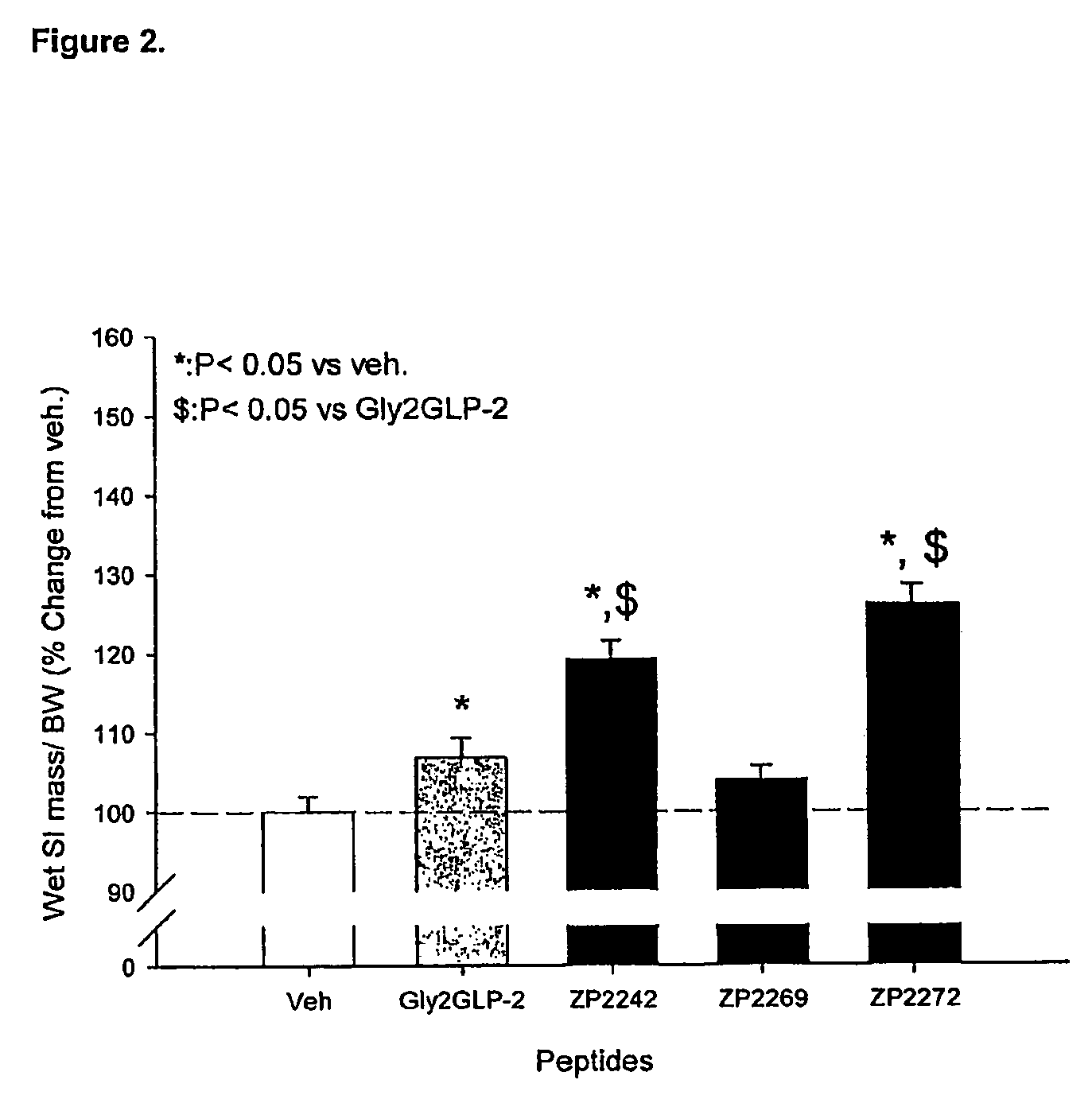
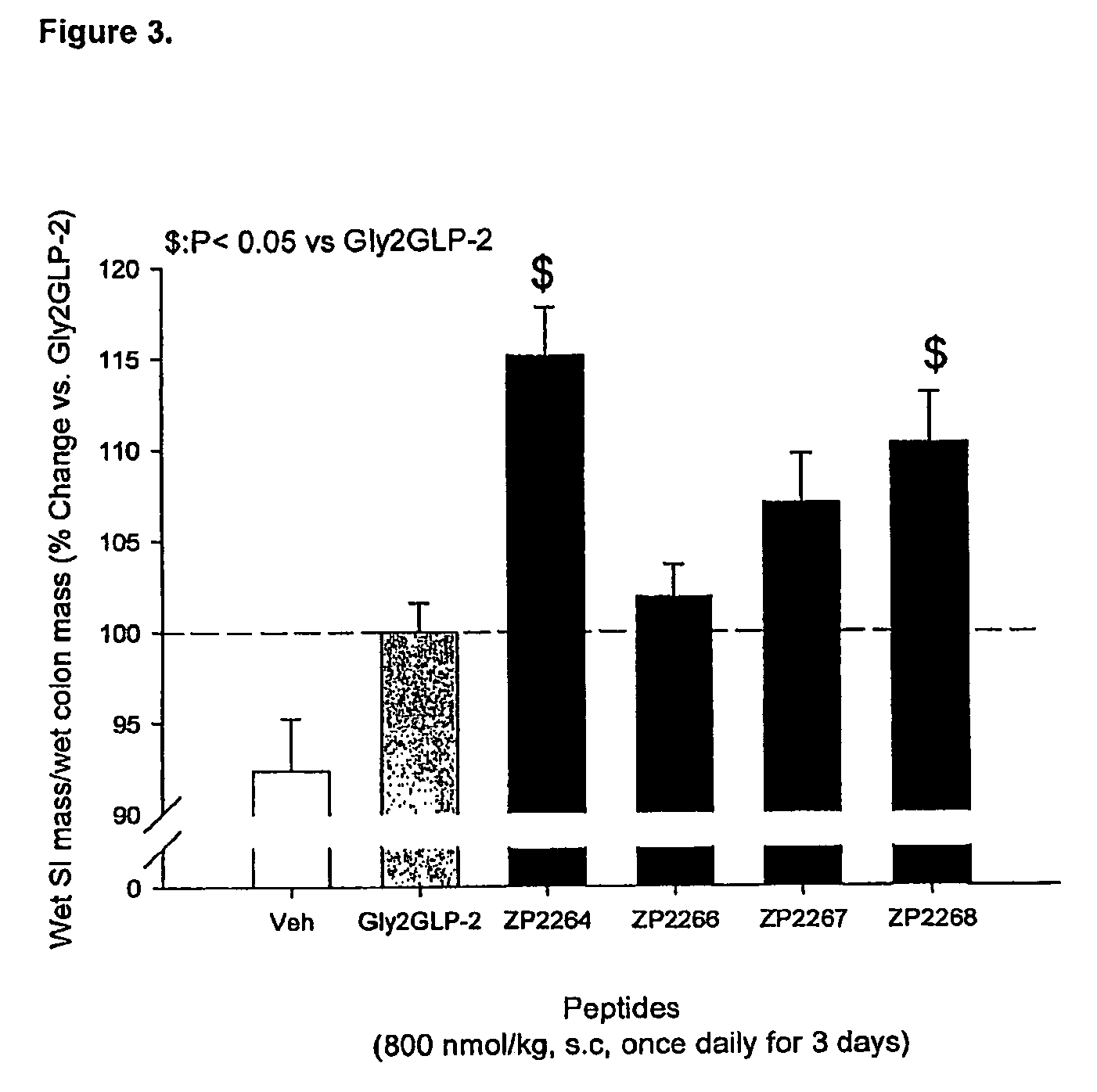
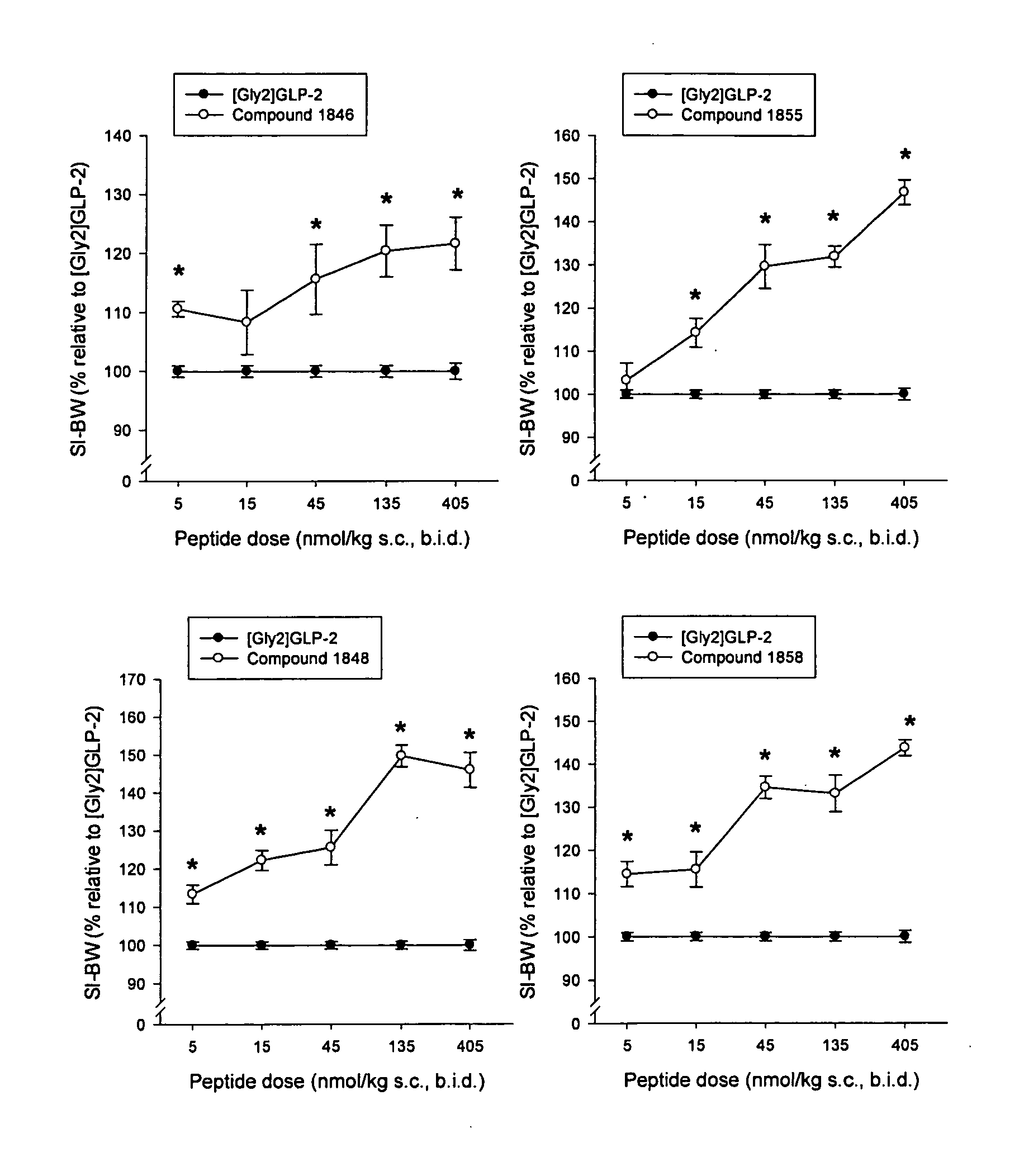
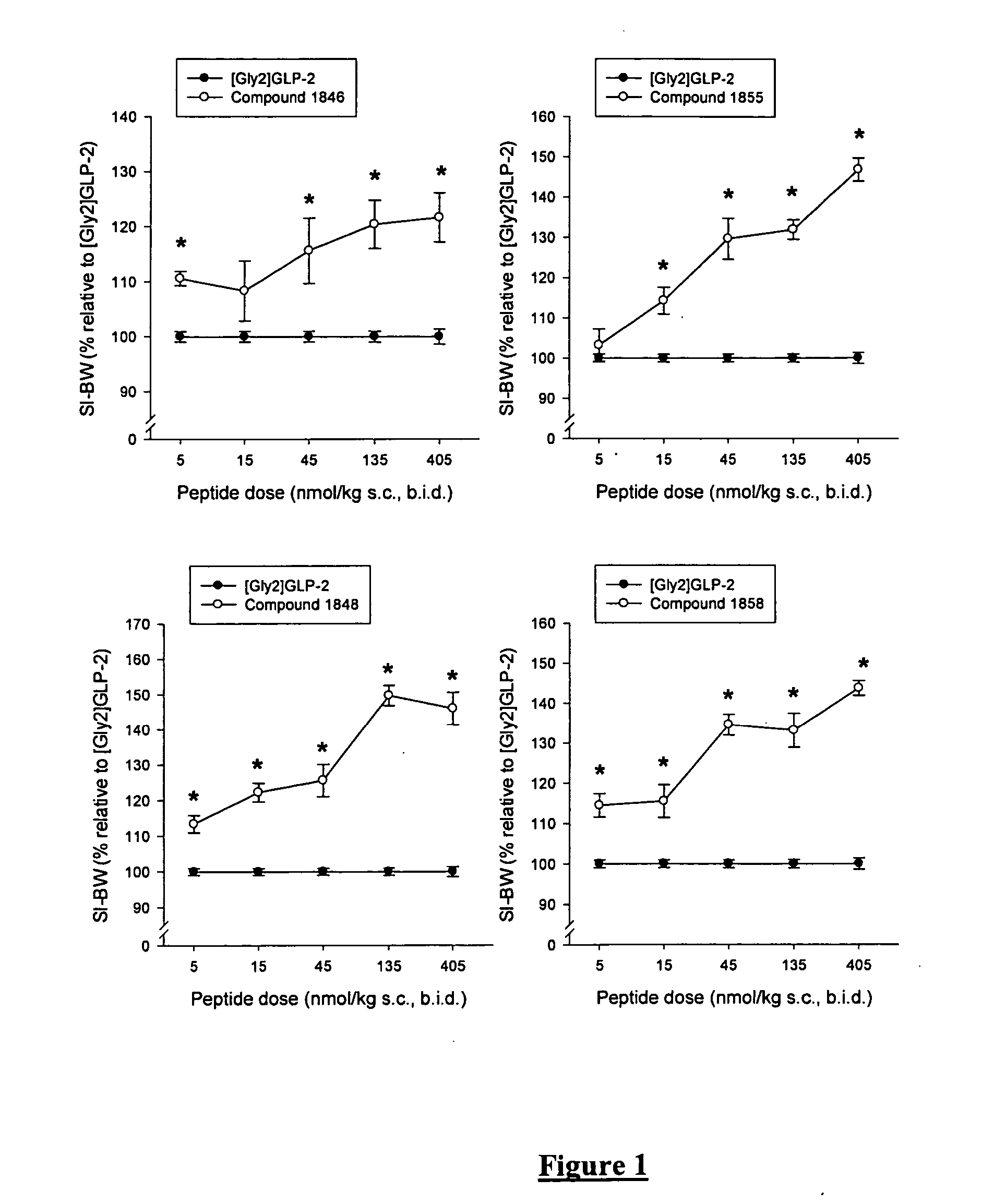
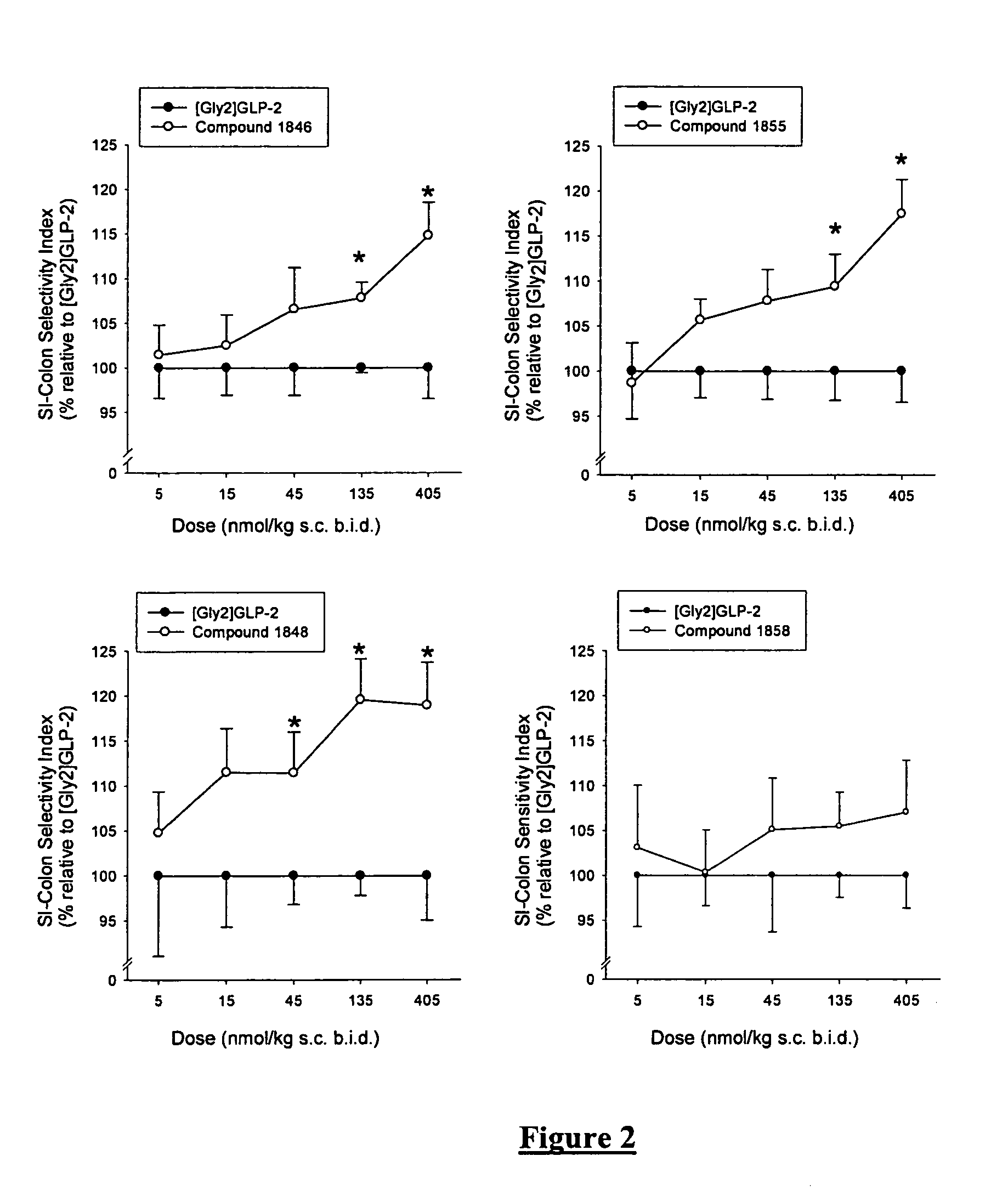
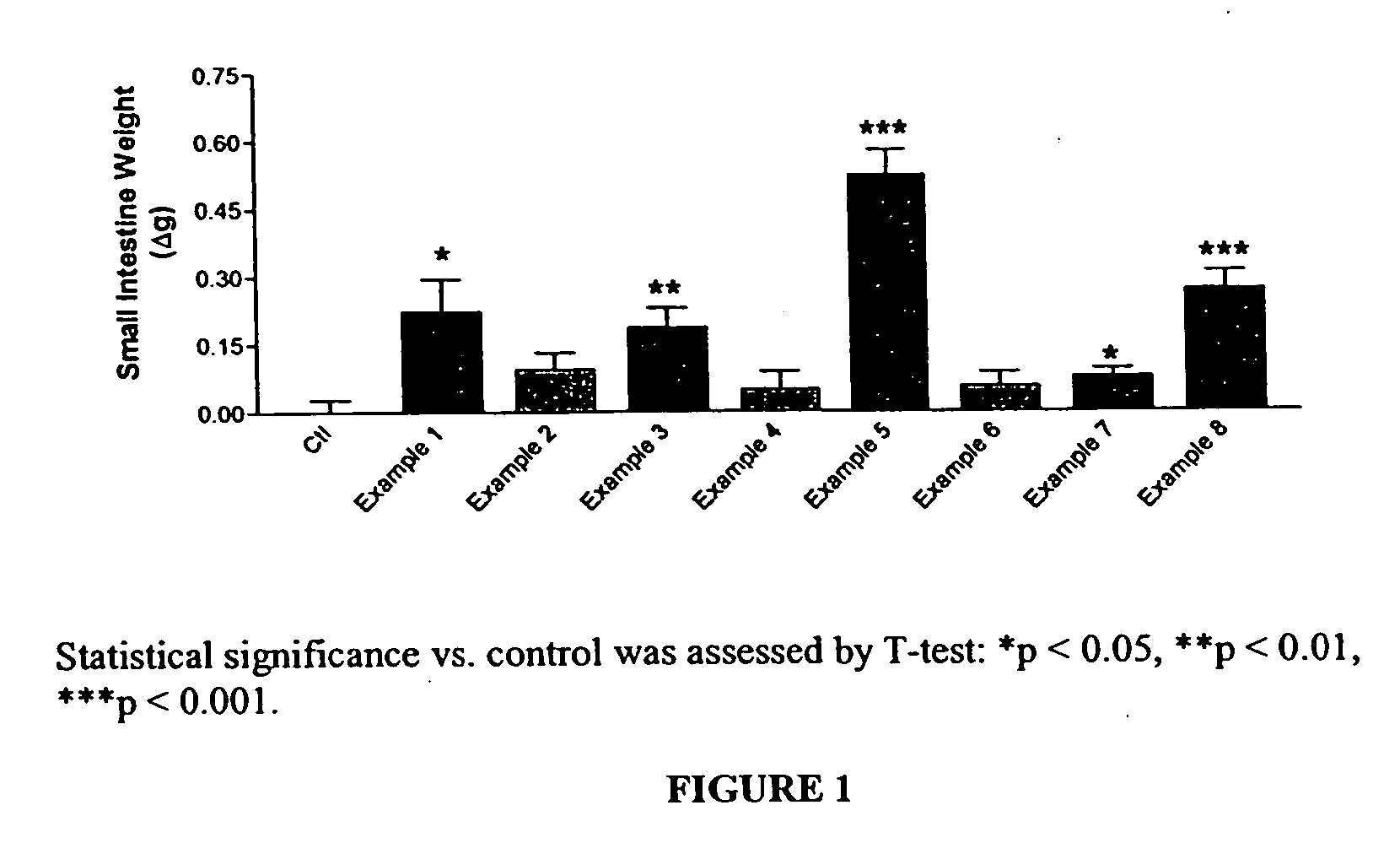
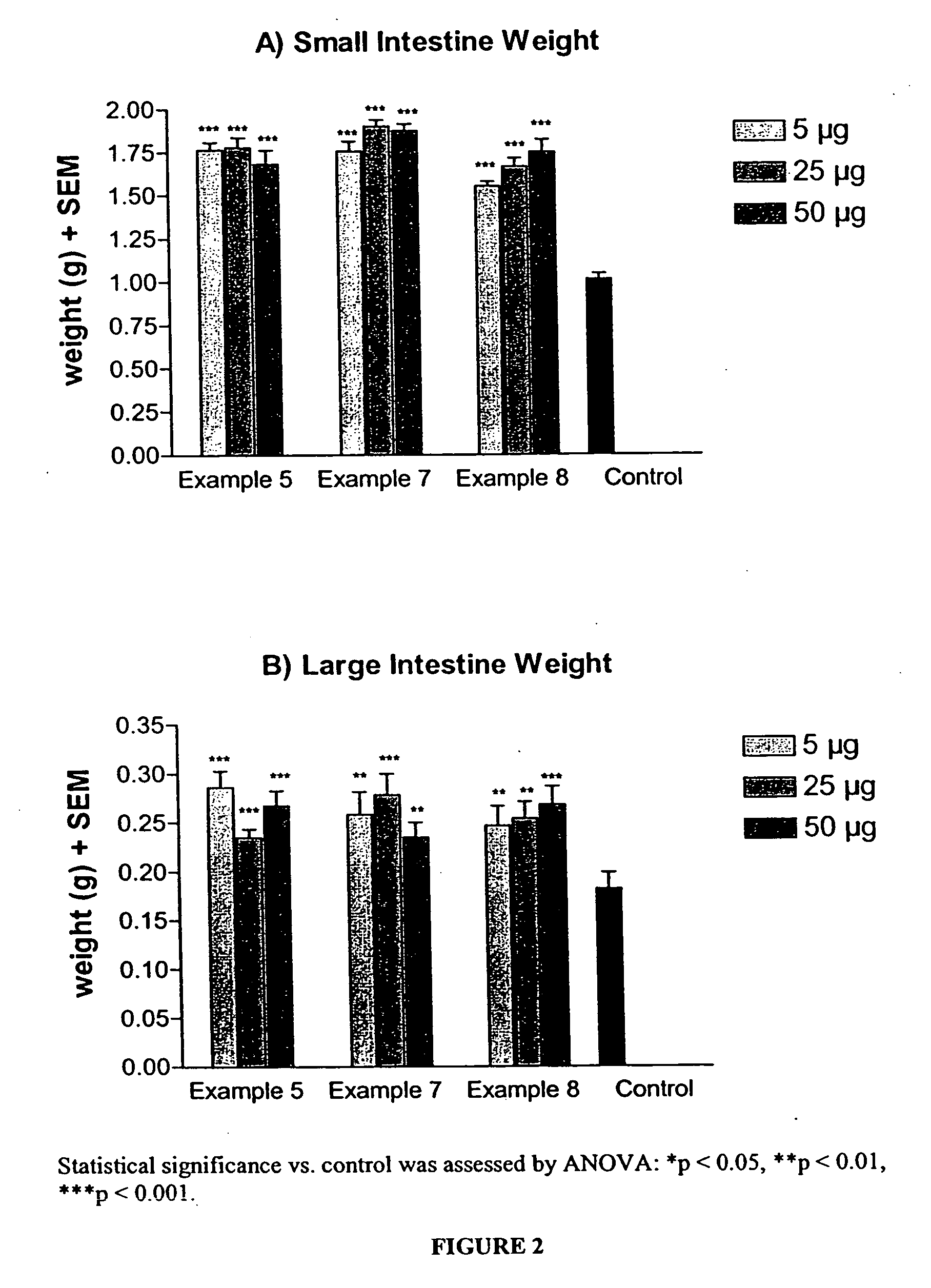
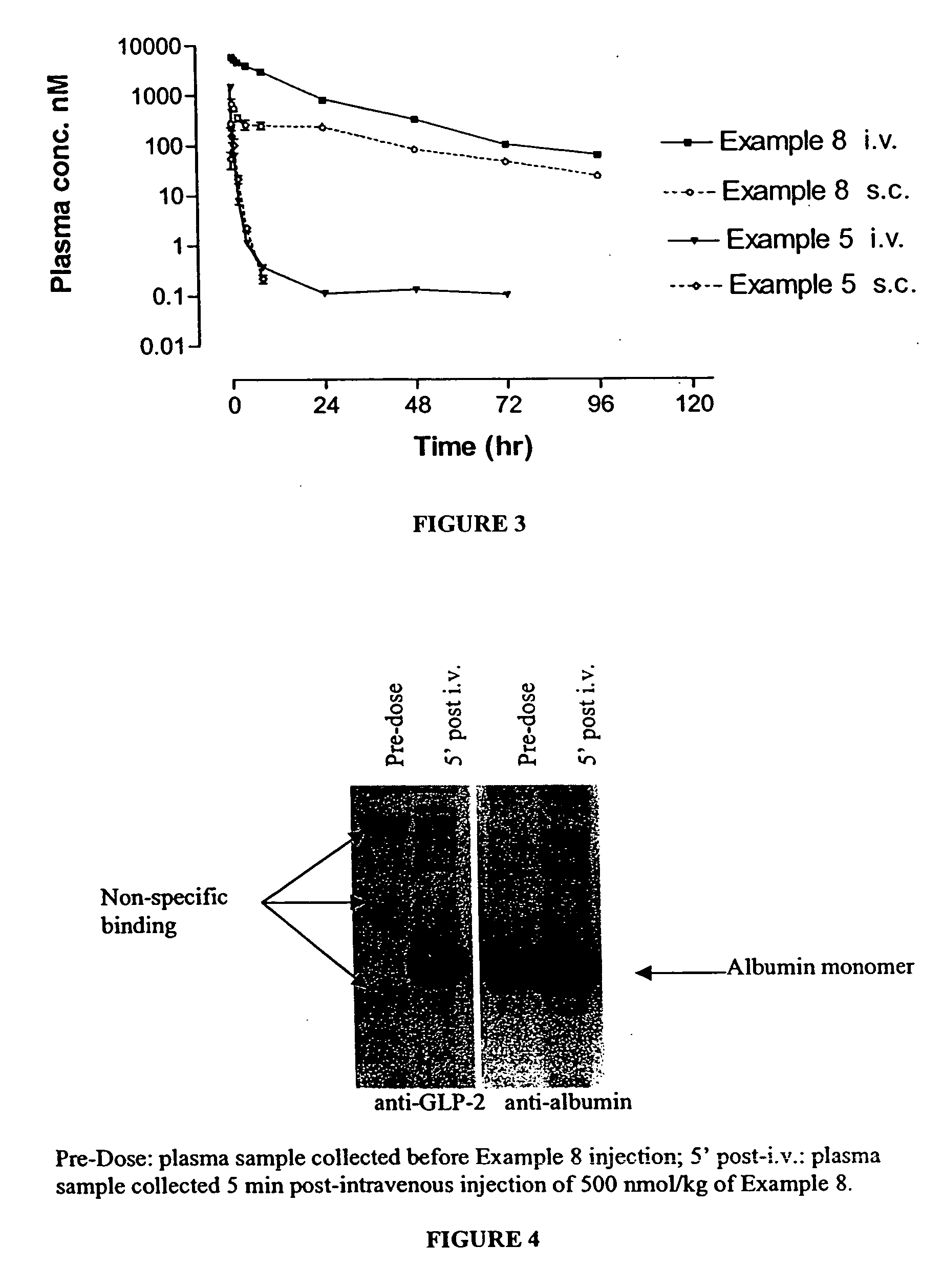
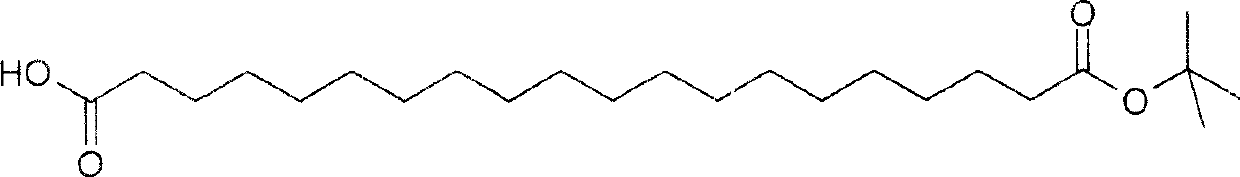
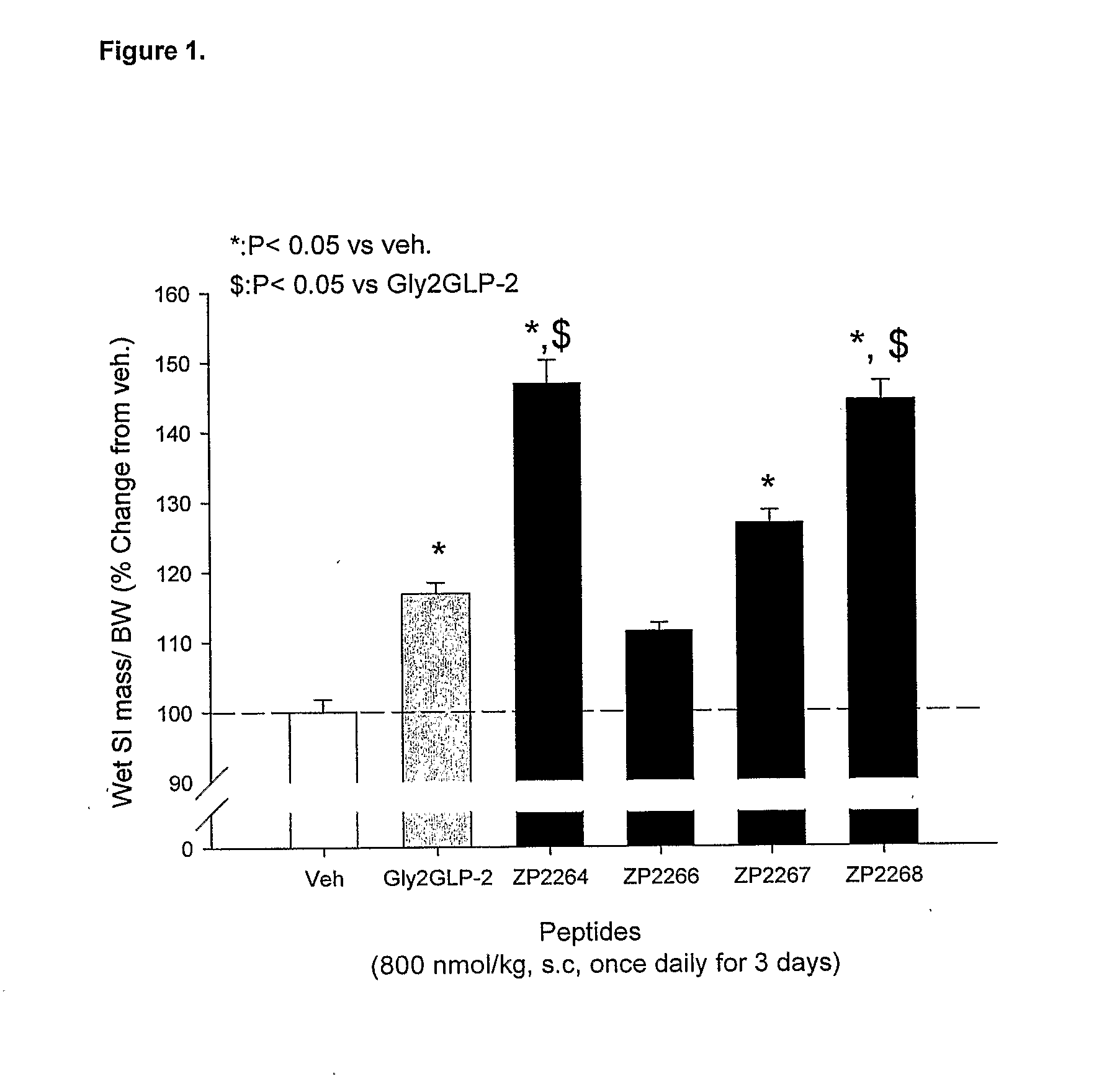
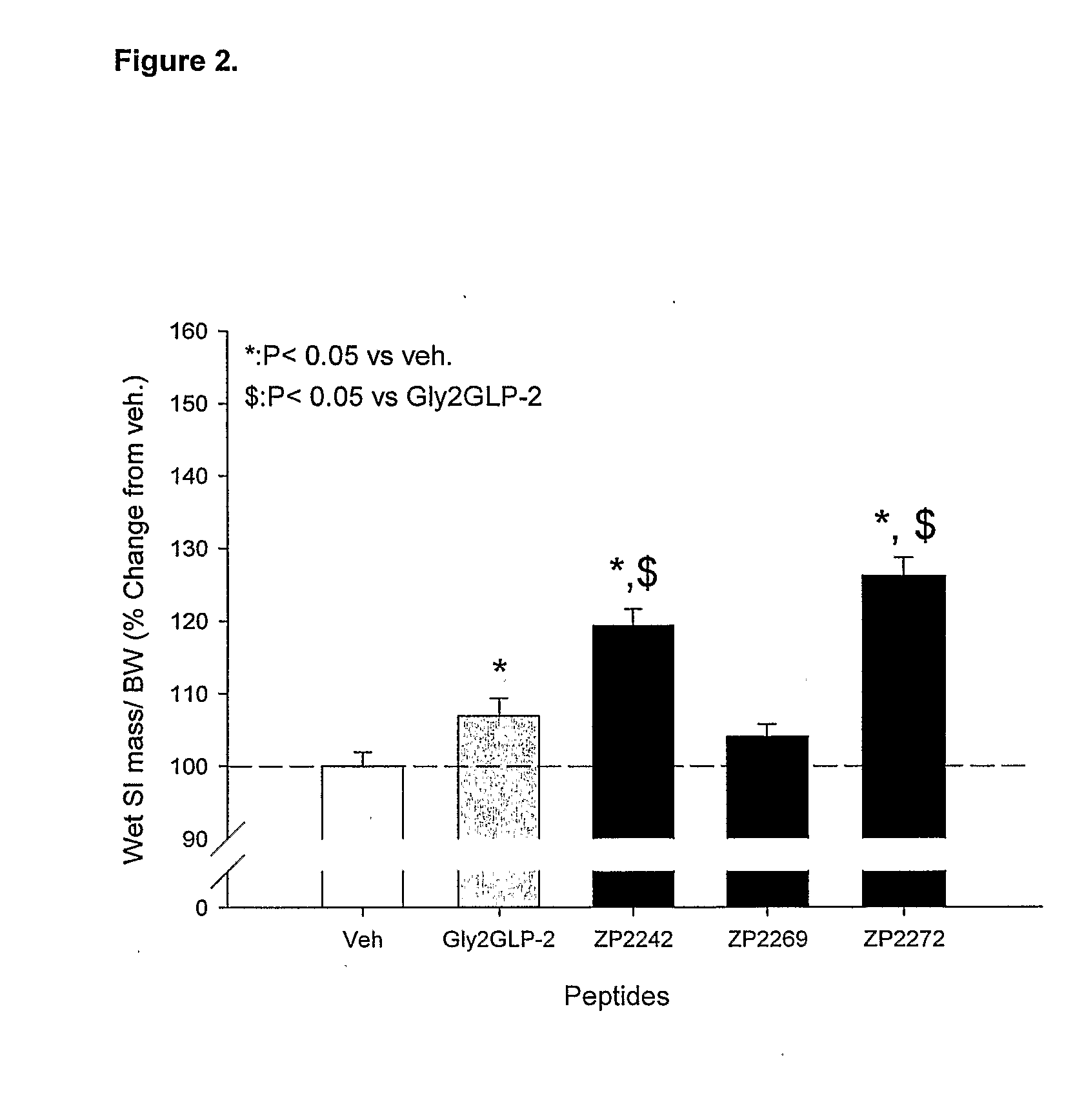
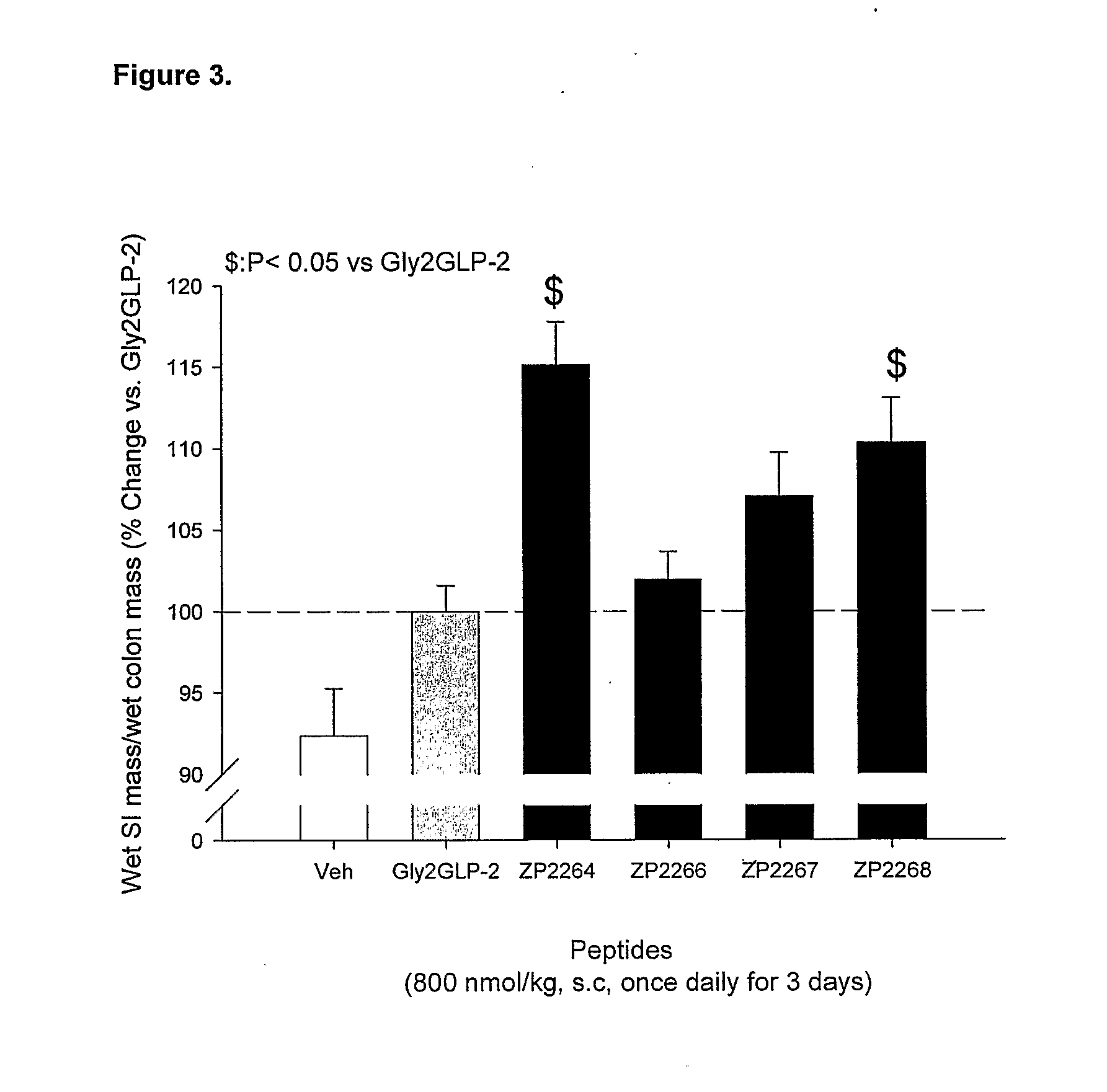
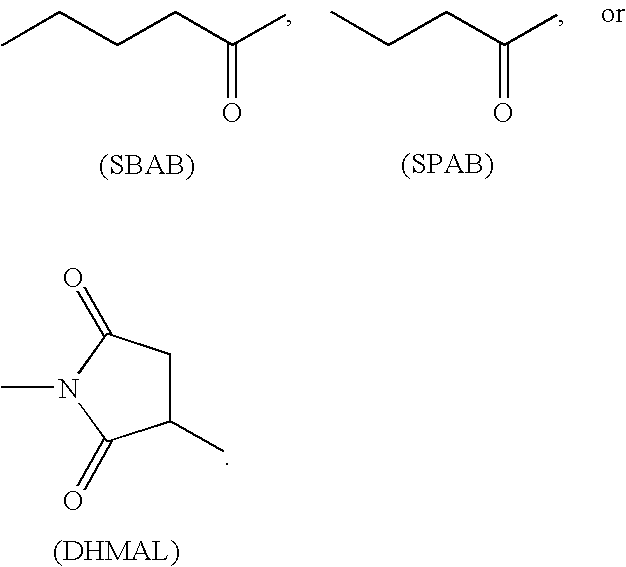
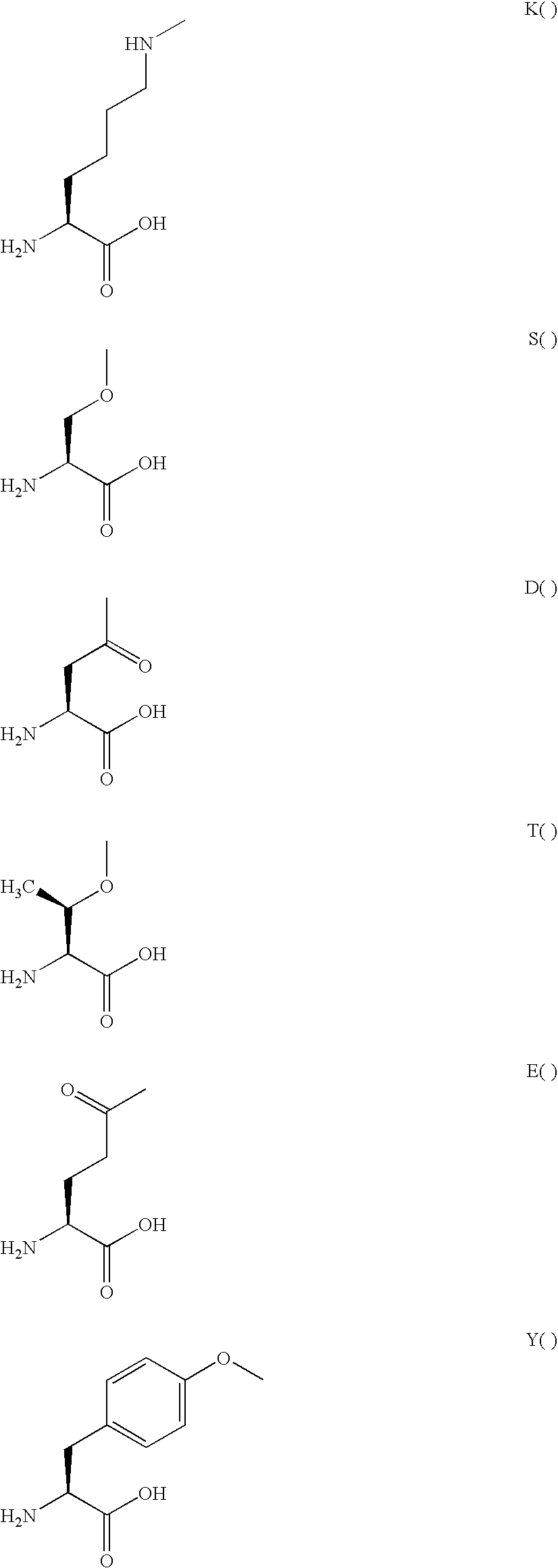
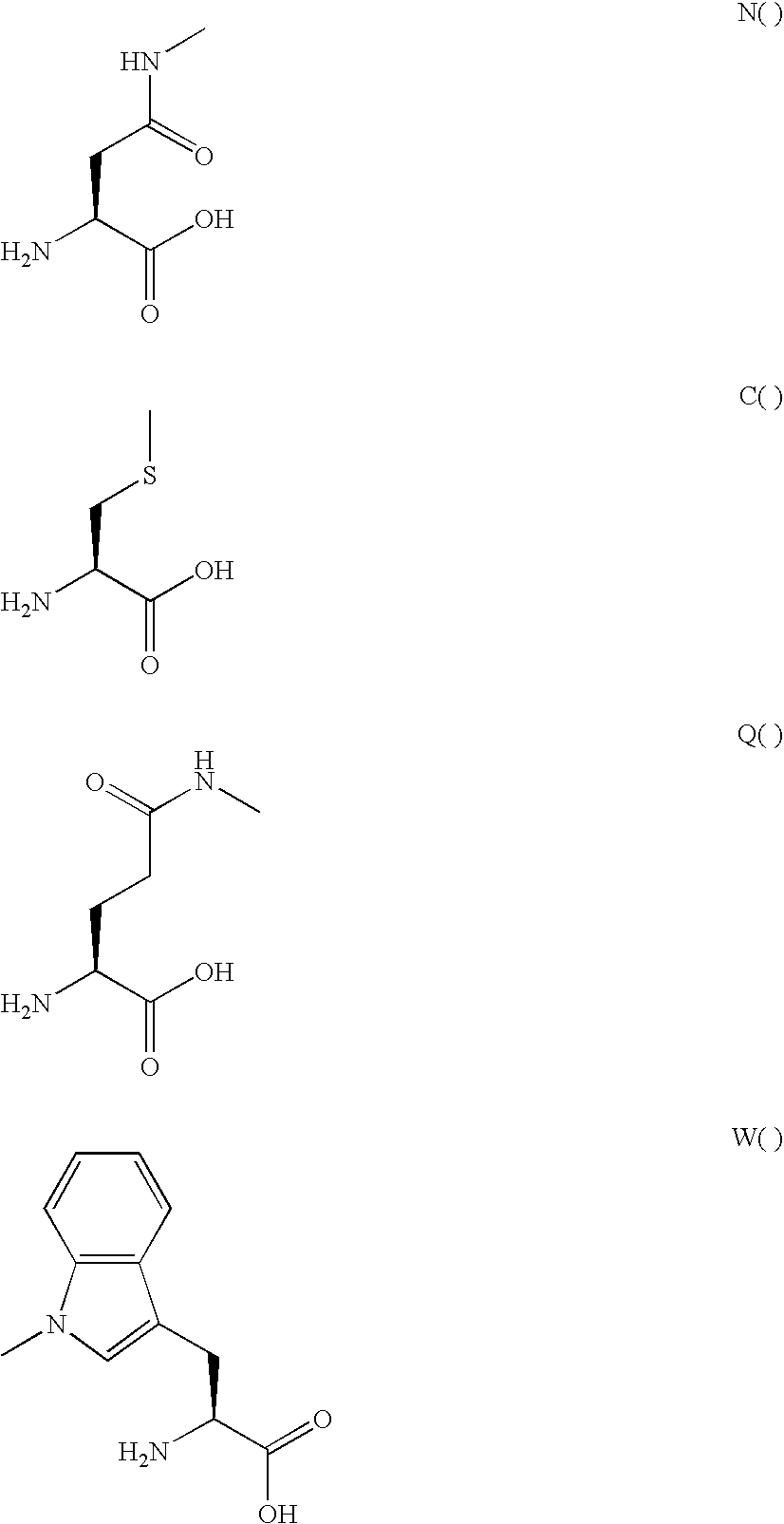
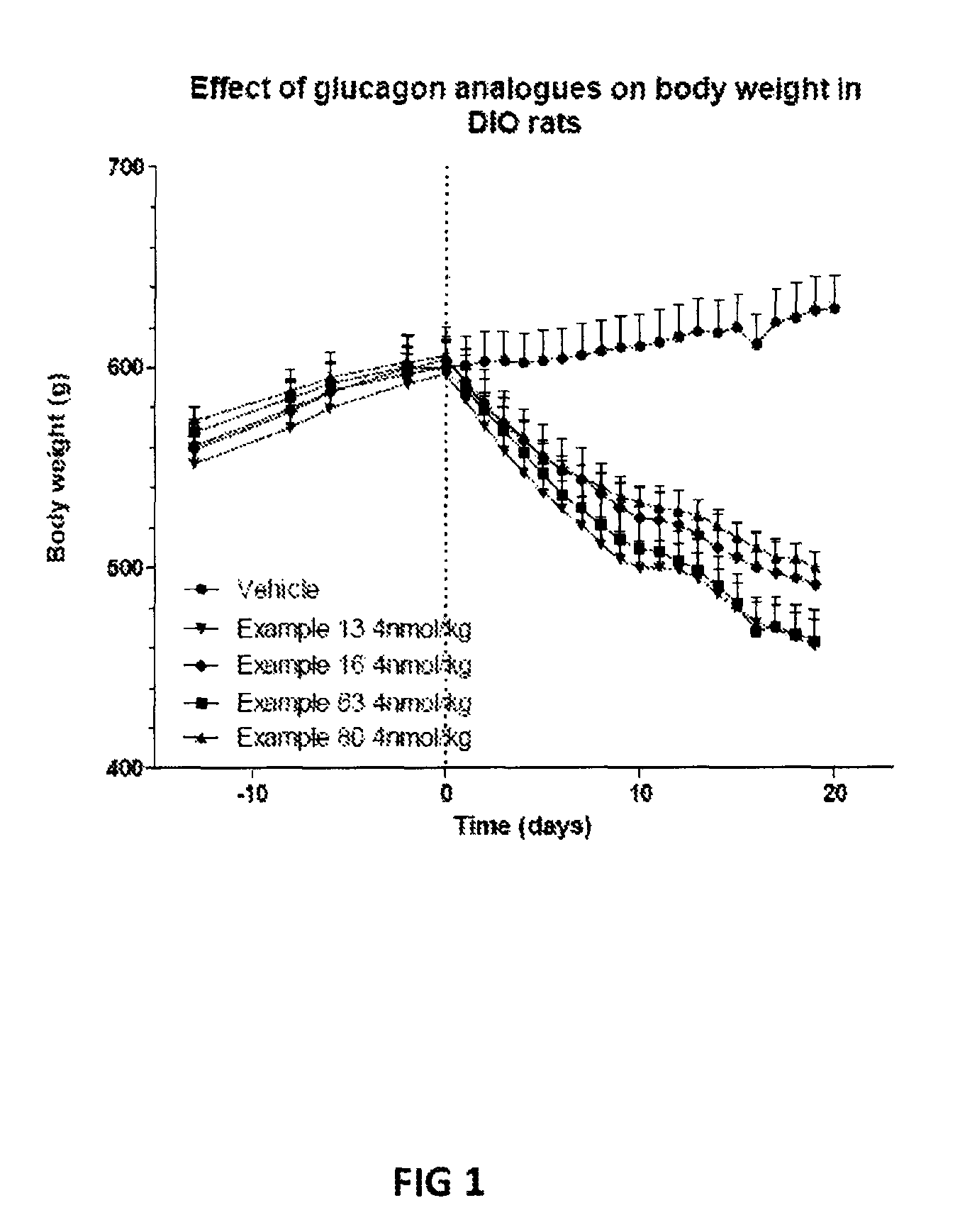
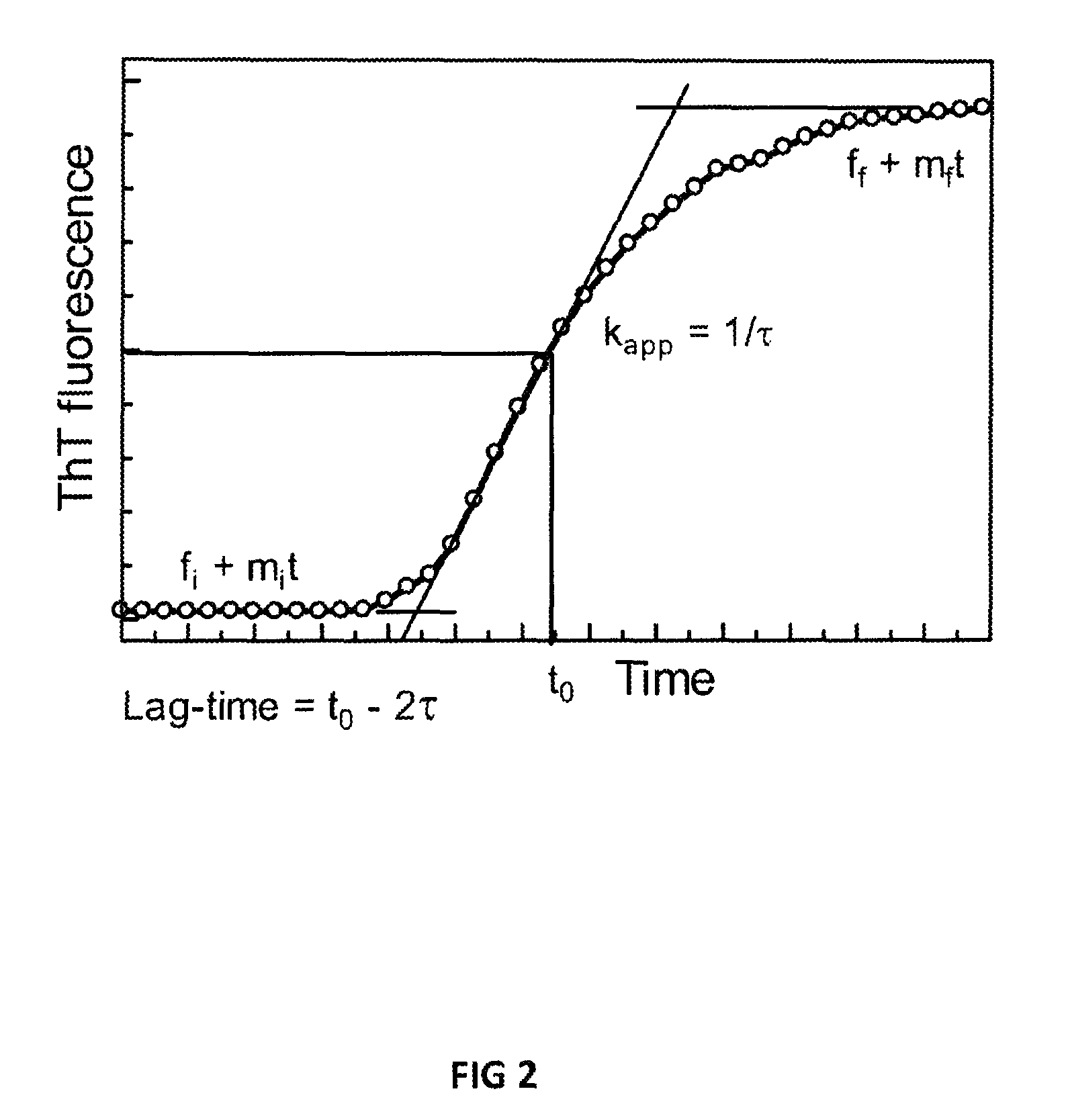
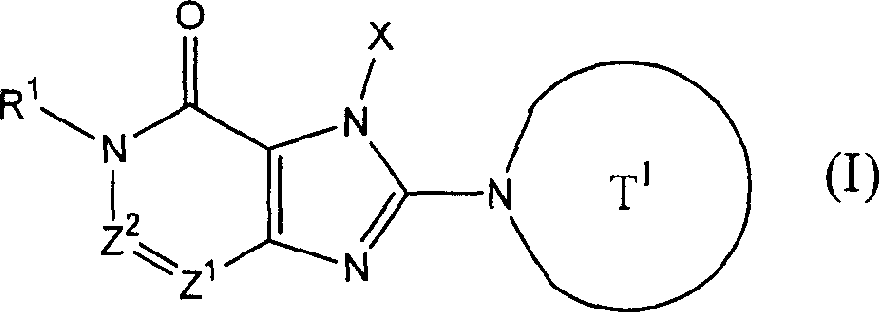
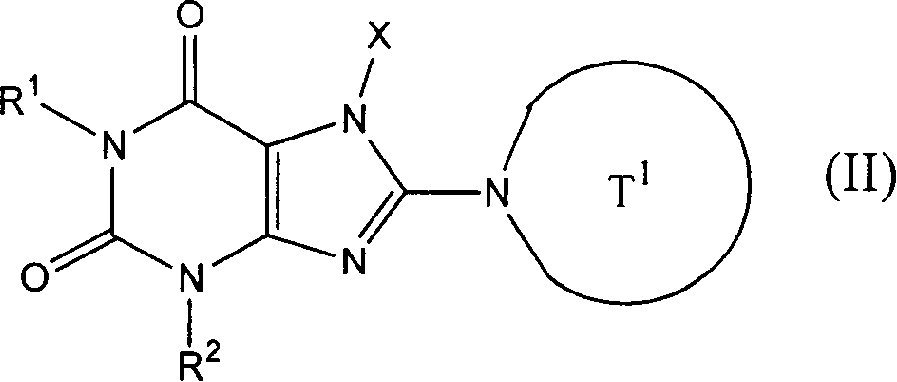
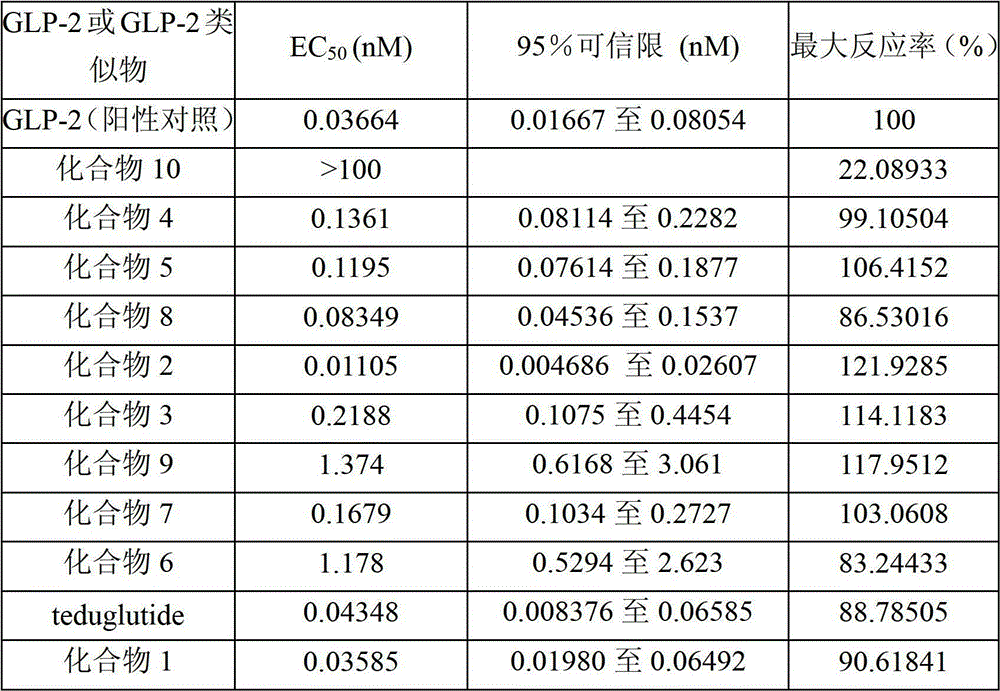
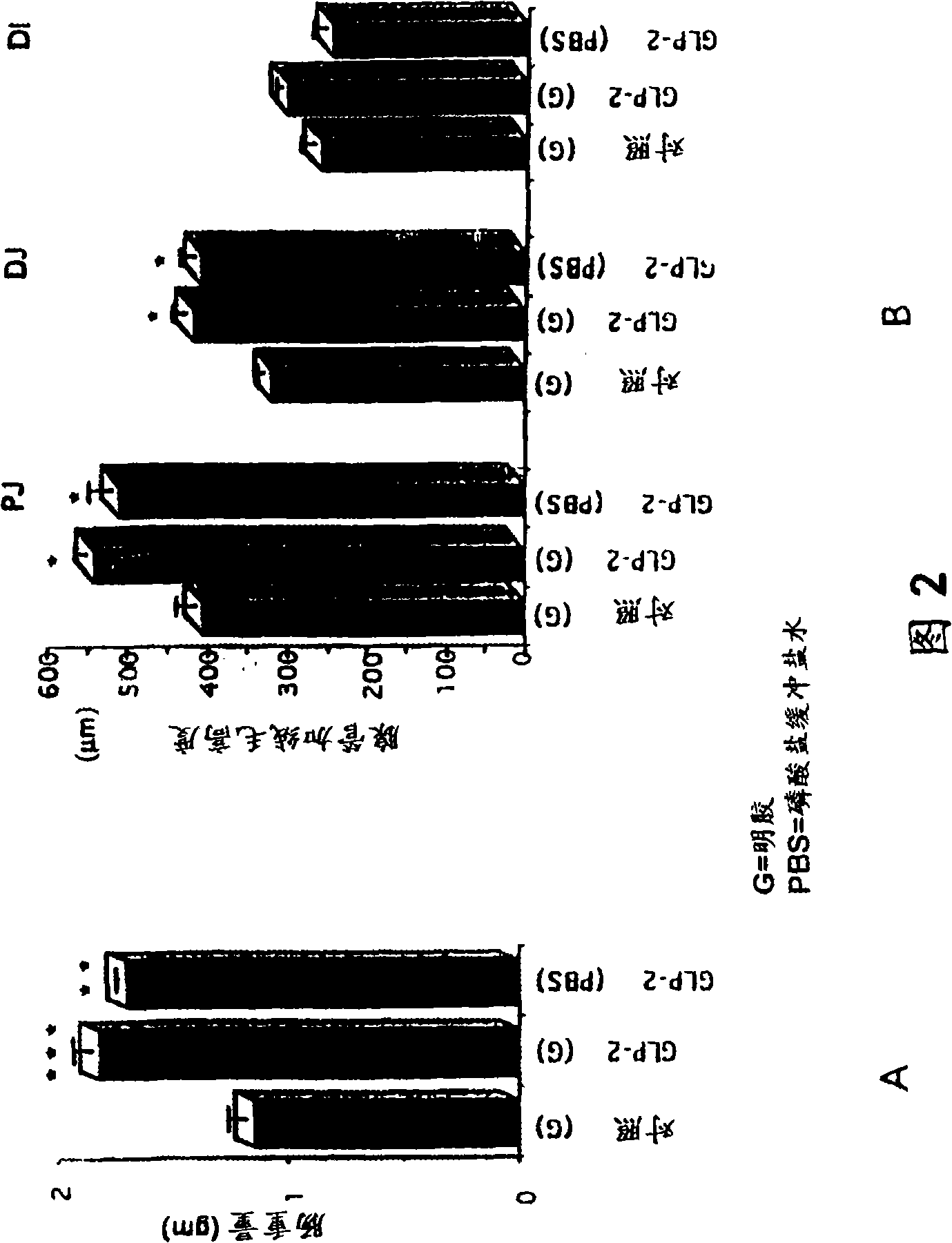
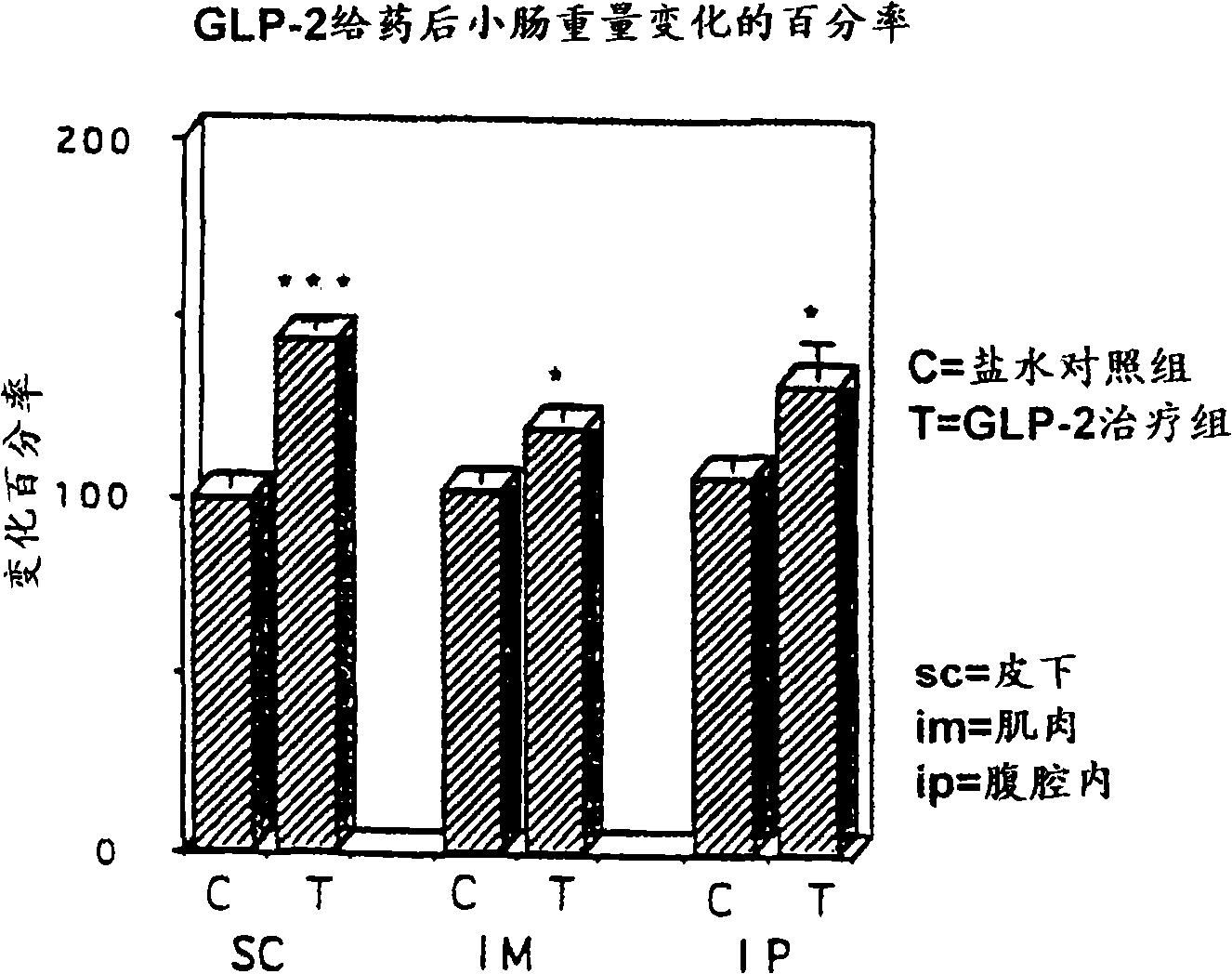
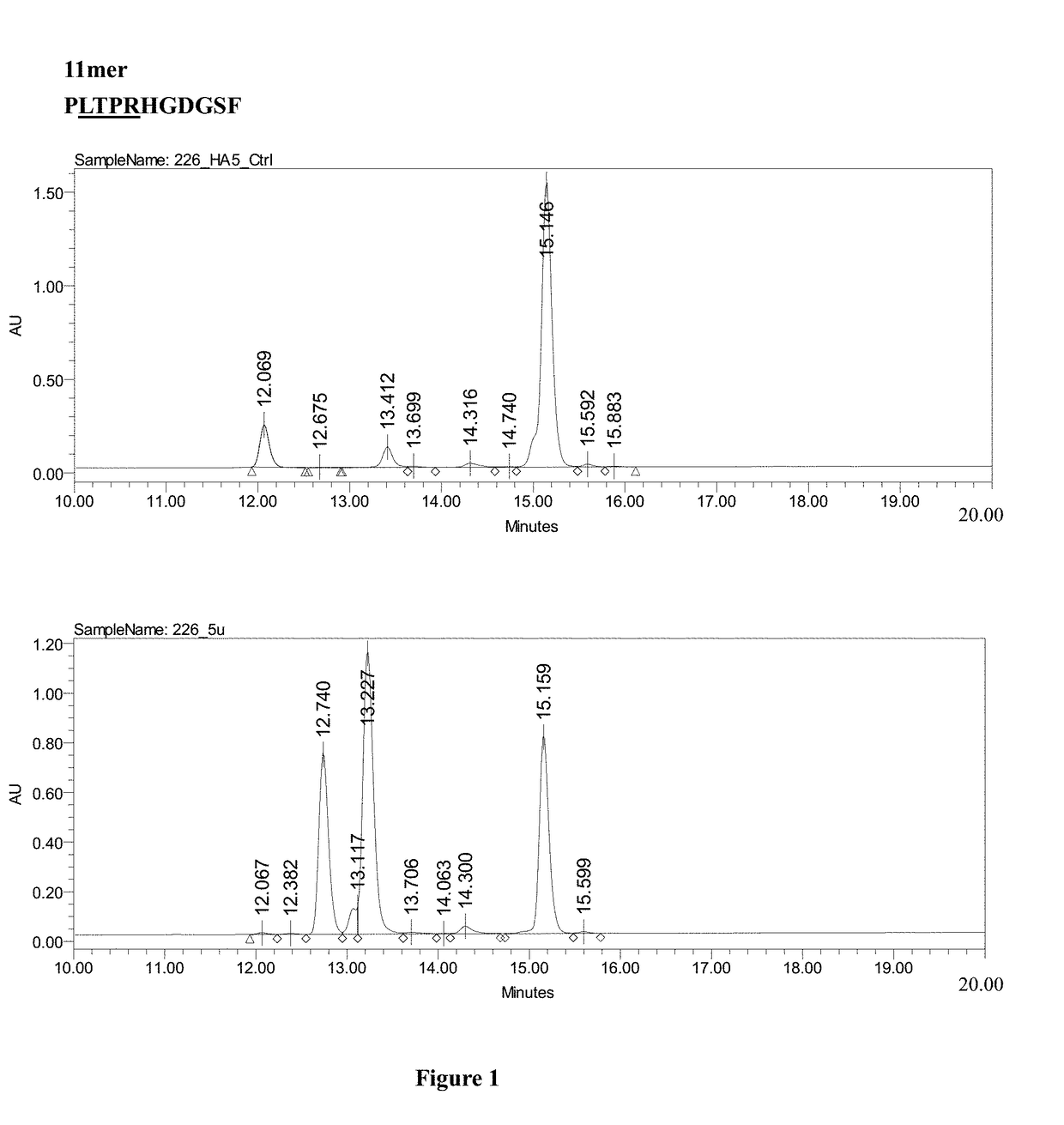
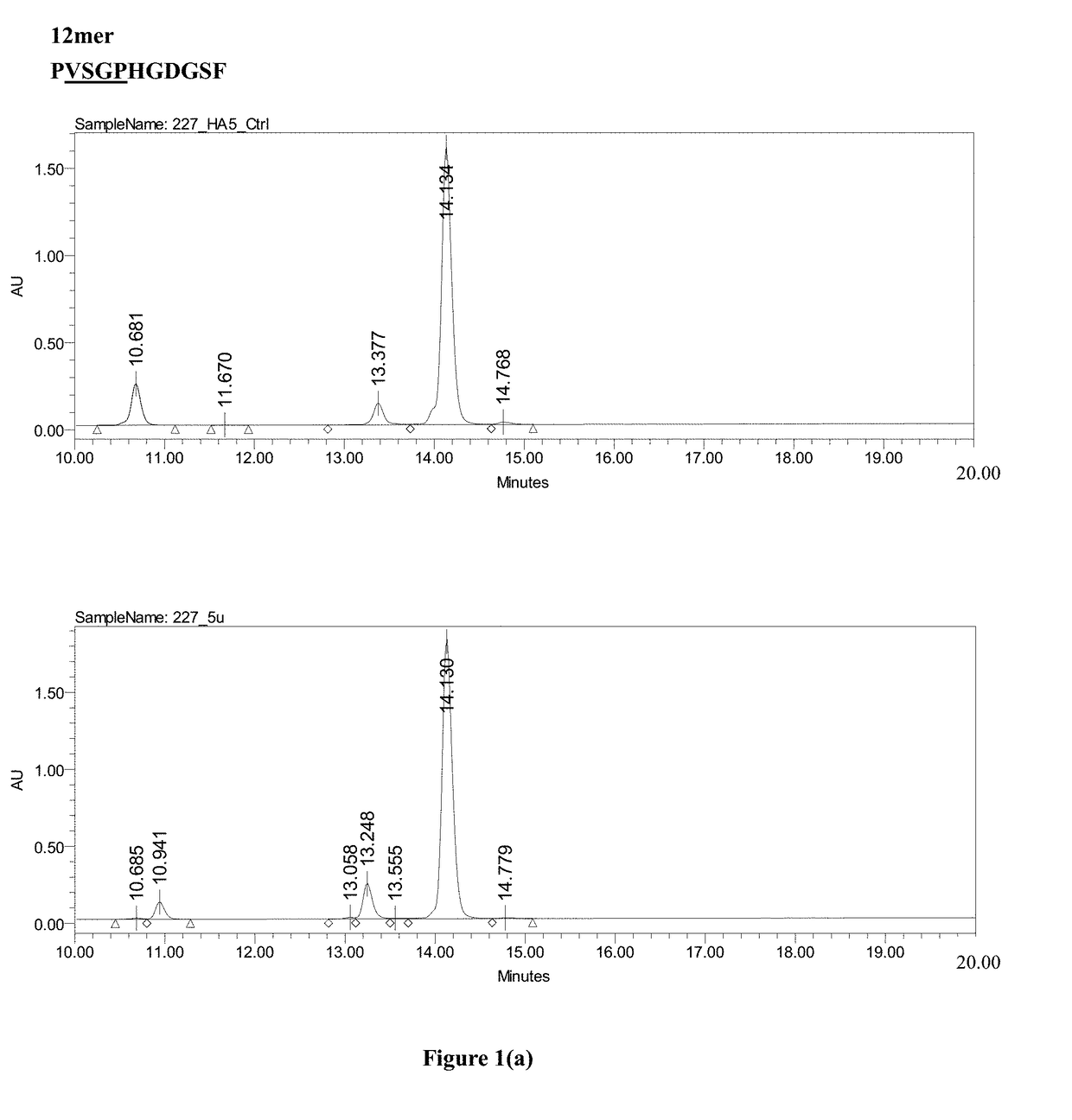
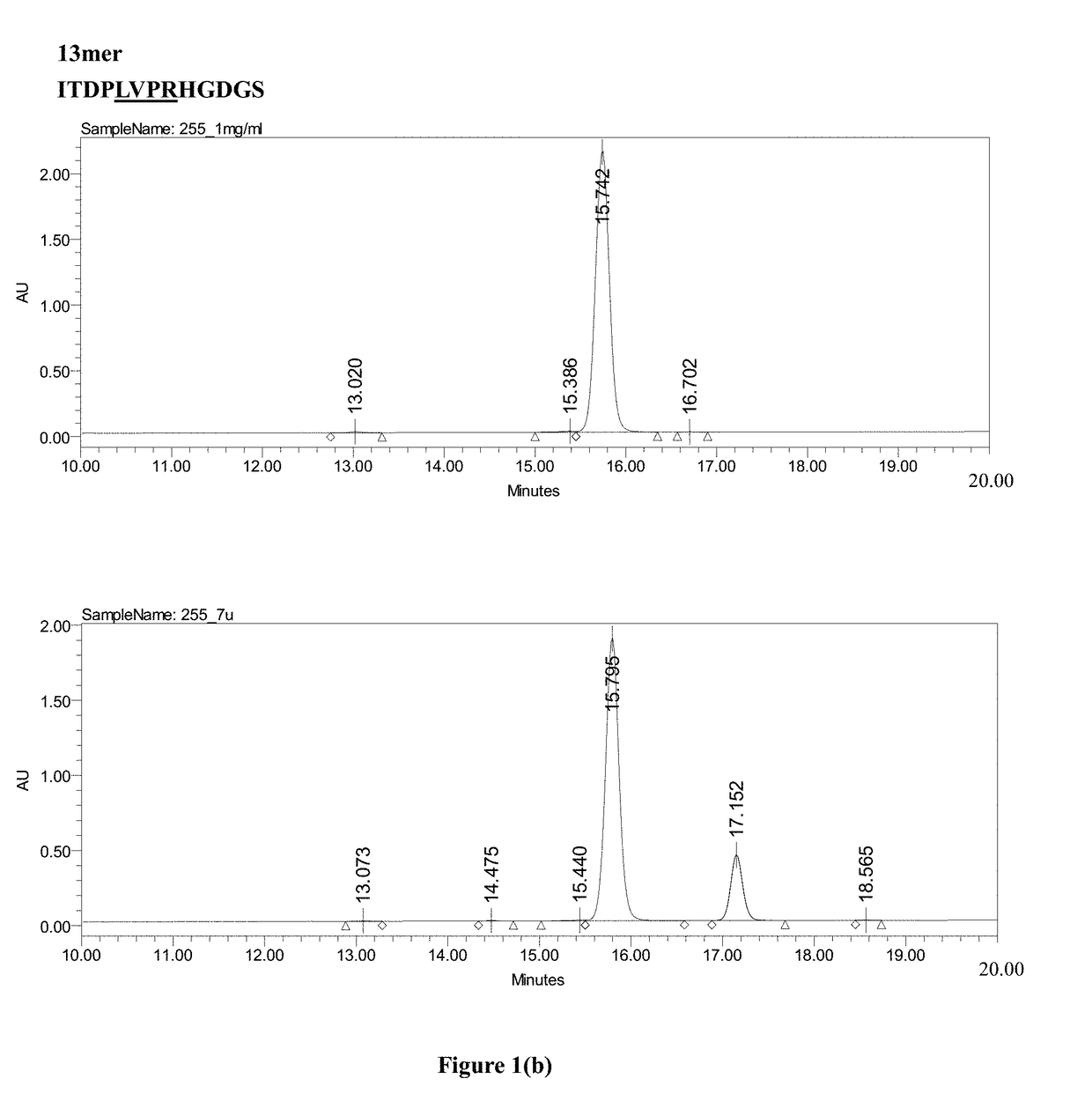
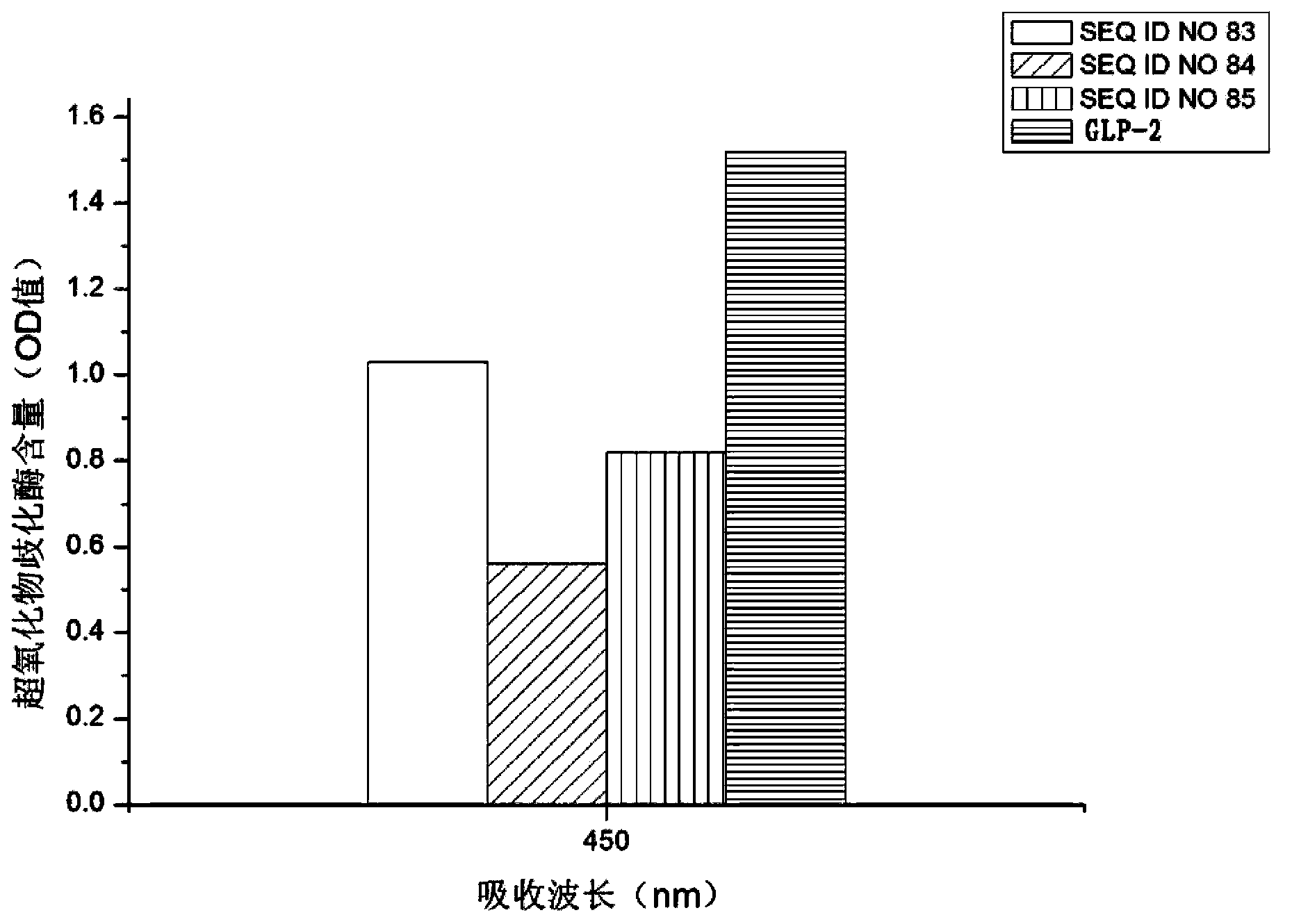
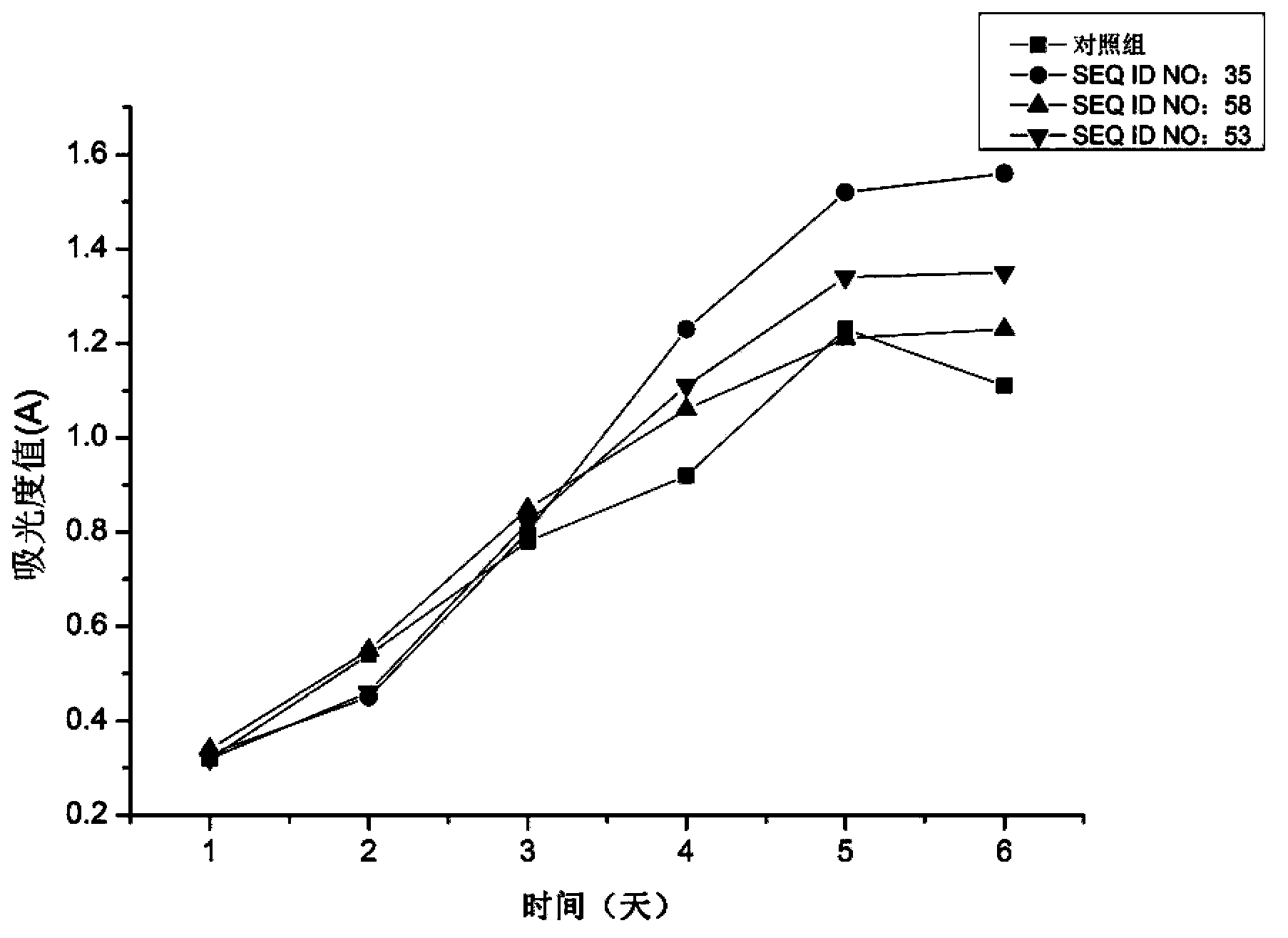
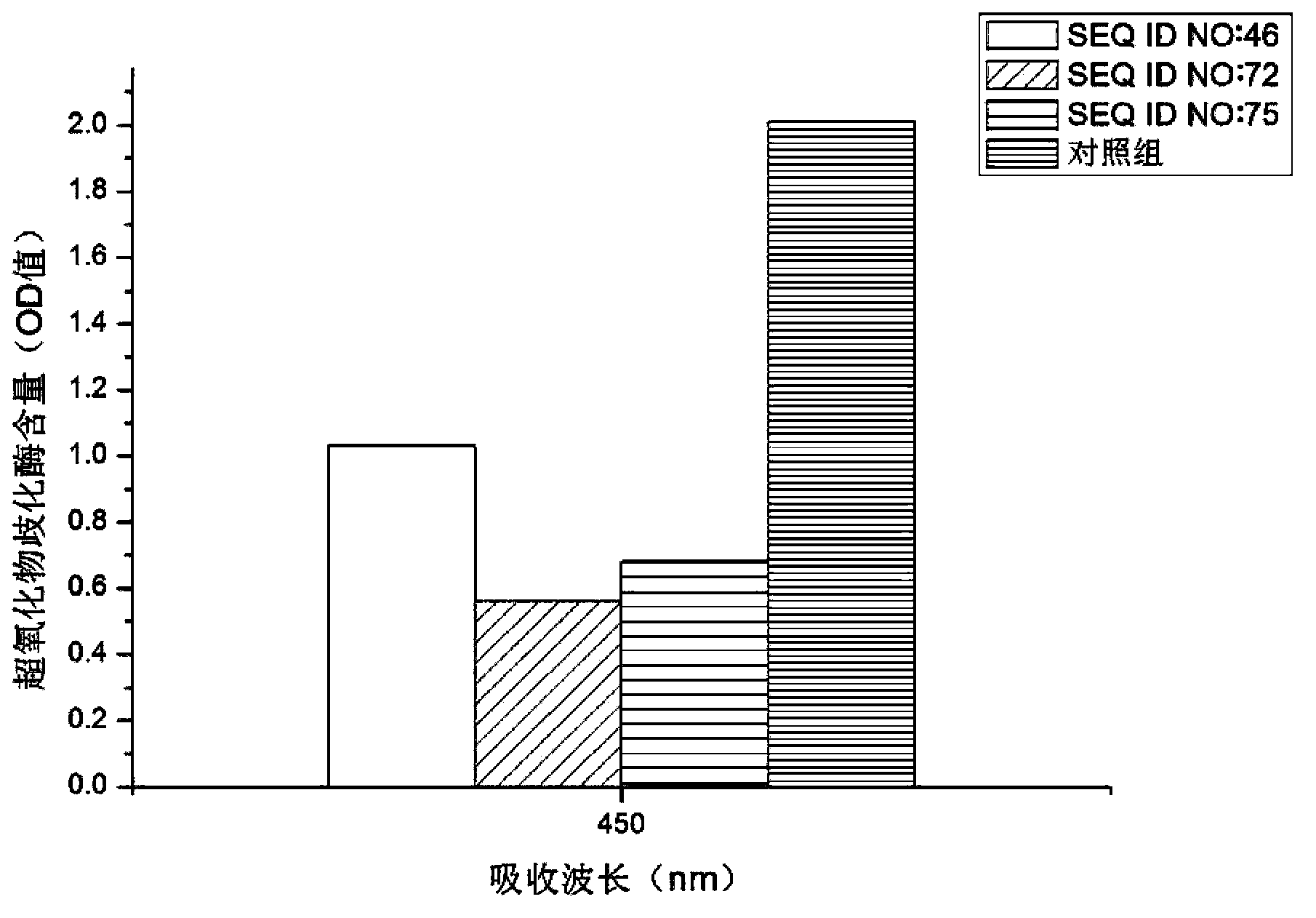
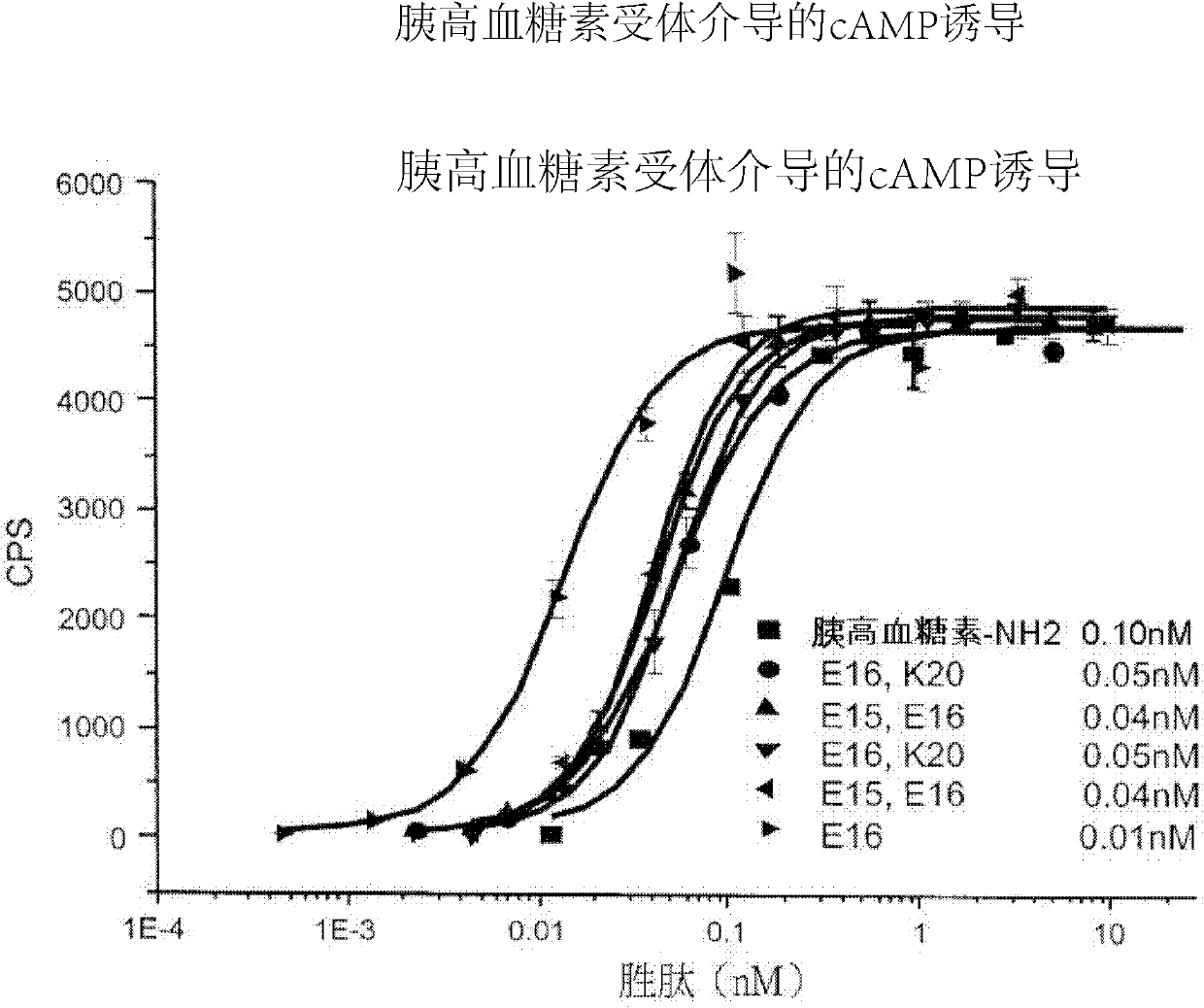
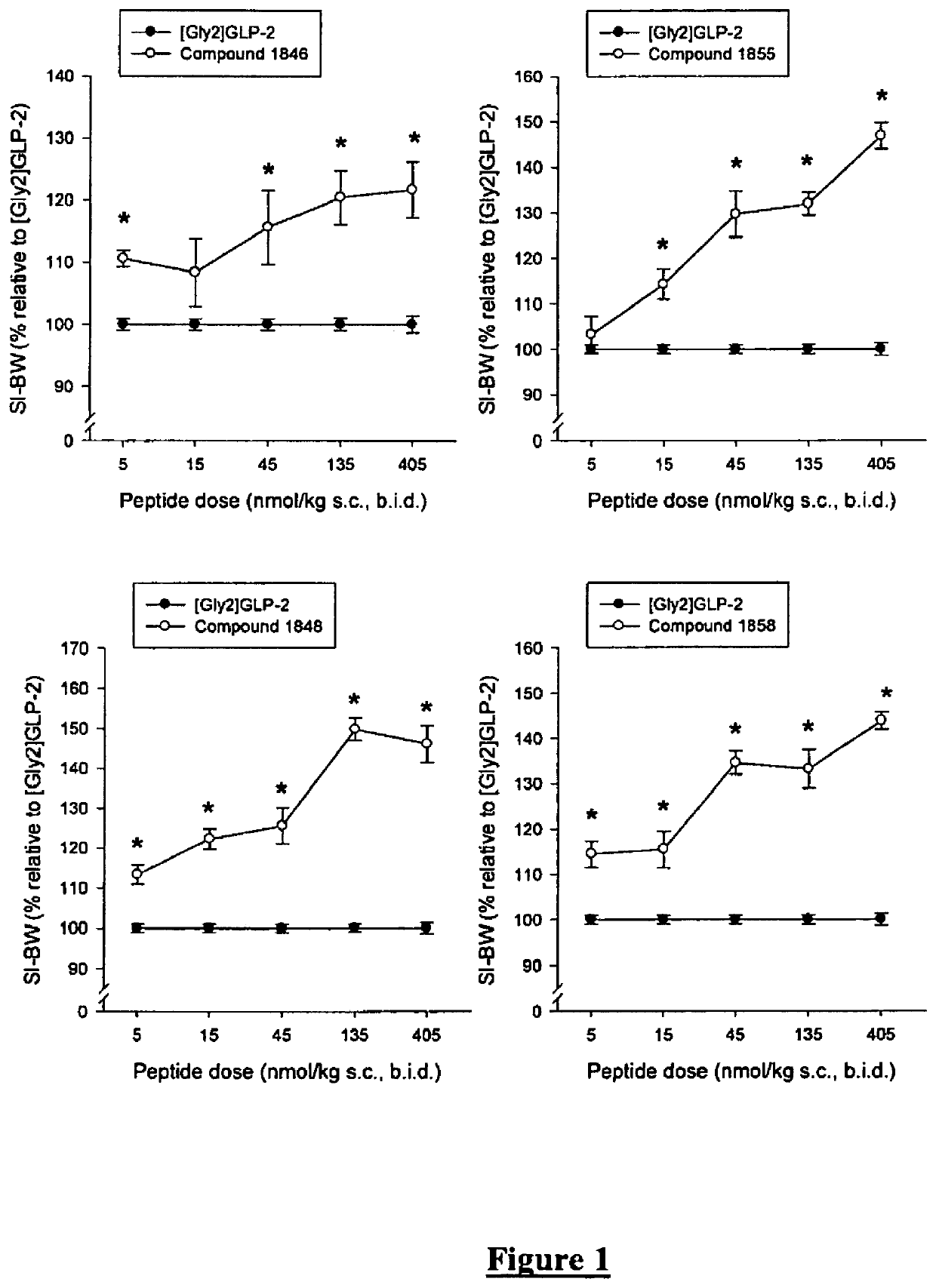
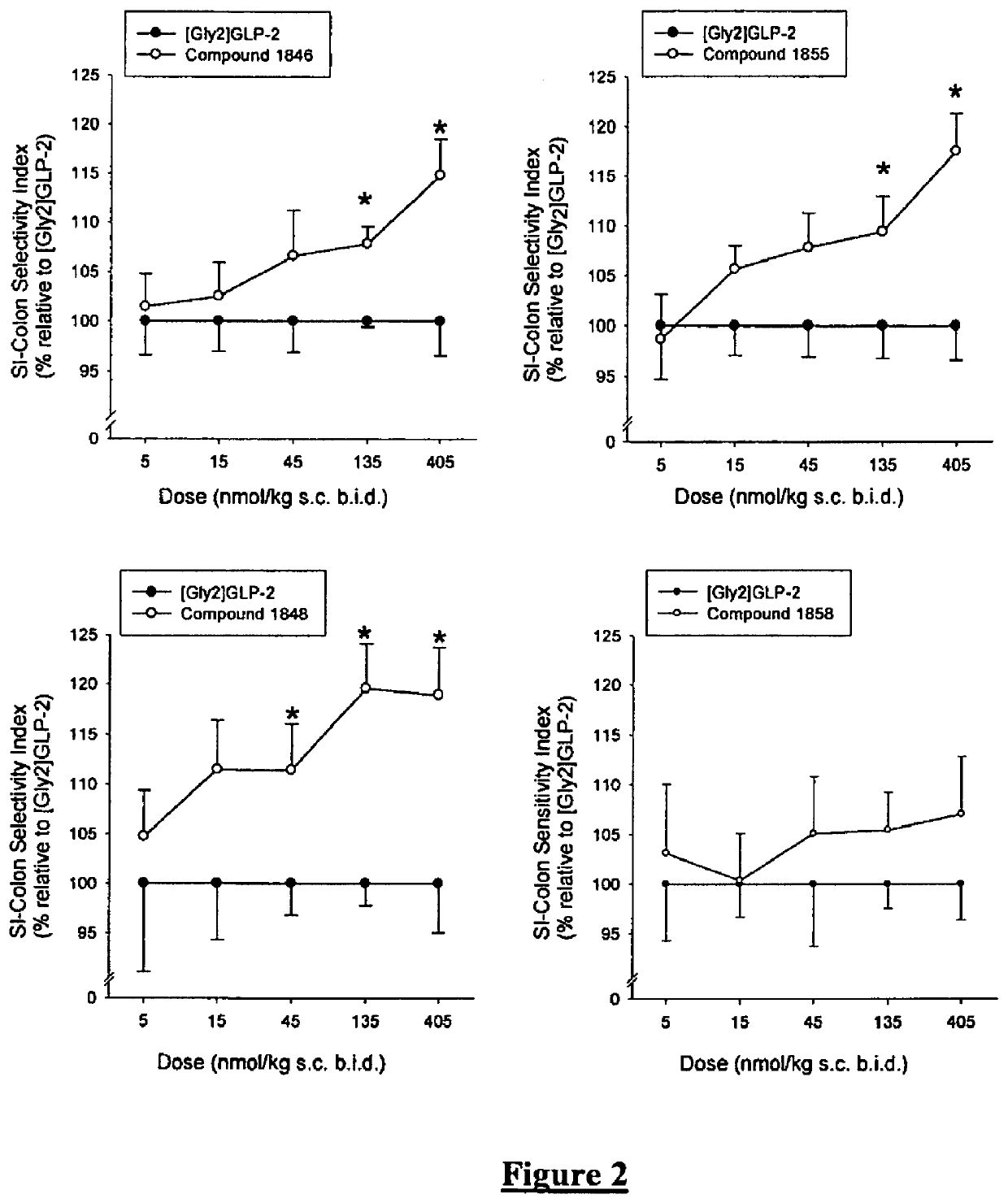
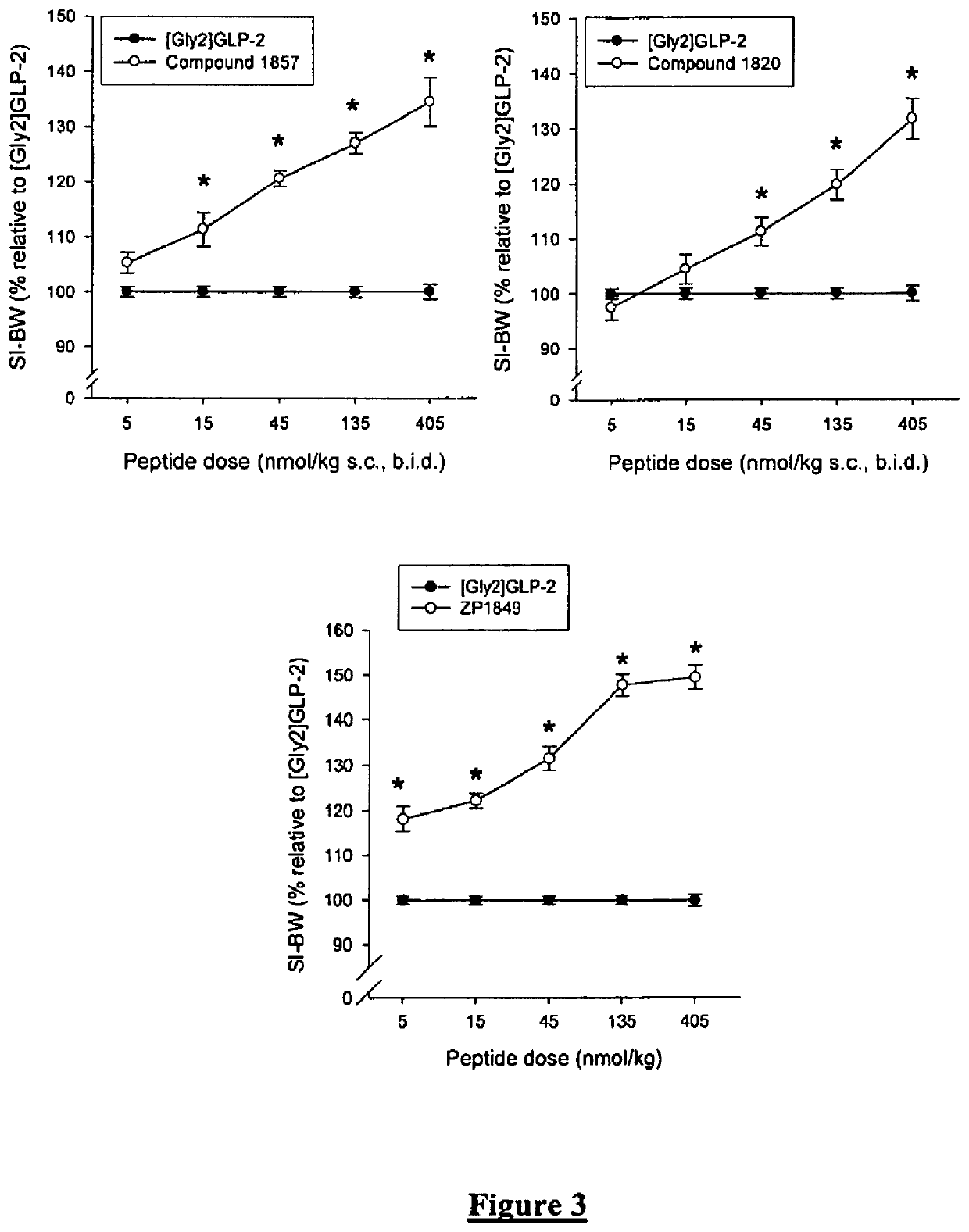
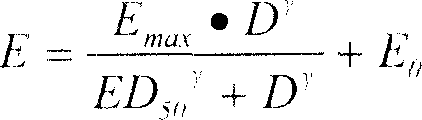Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Glucagon-like peptide-2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
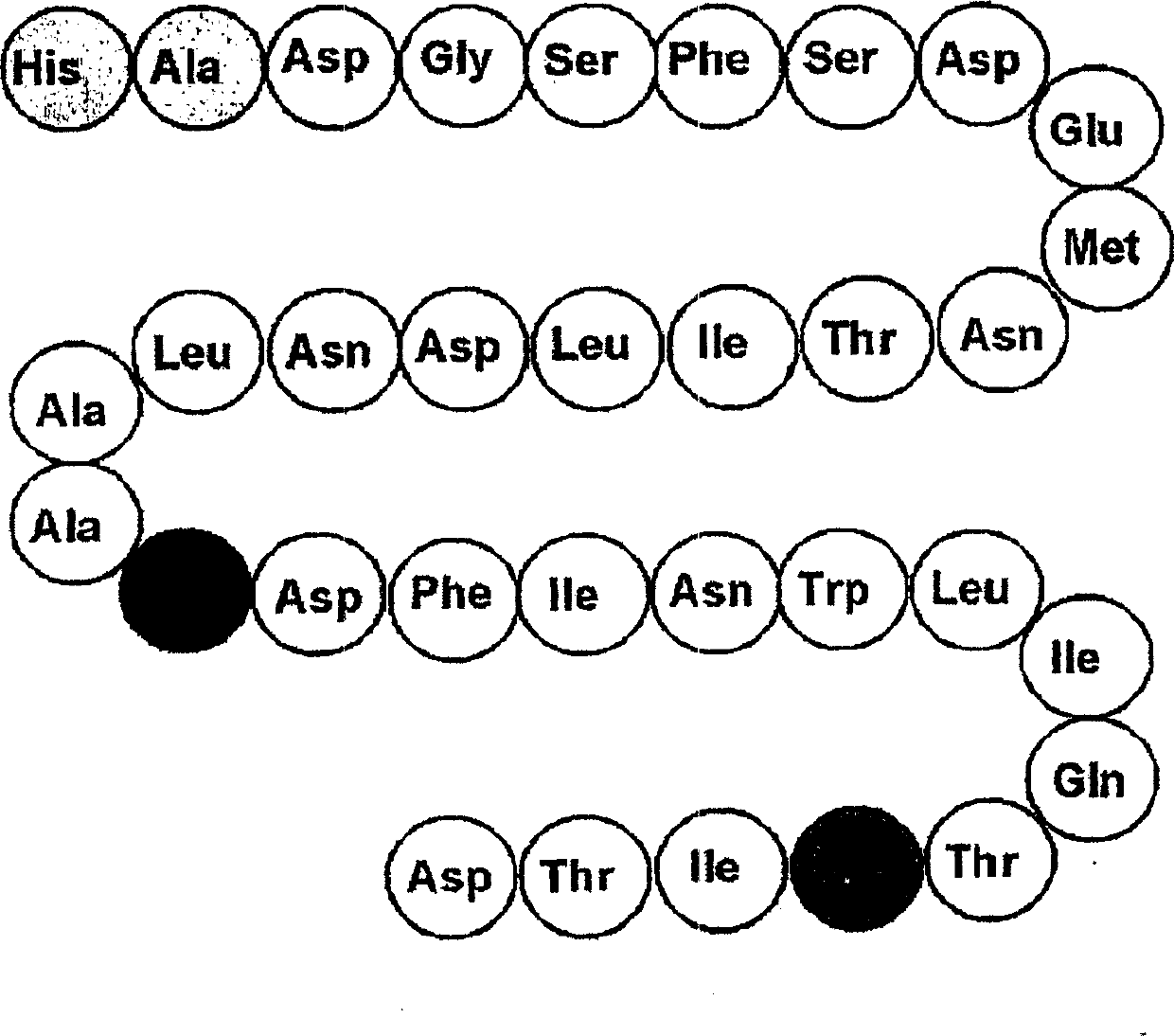
Glucagon-like peptide-2 (GLP-2) is a 33 amino acid peptide with the sequence HADGSFSDEMNTILDNLAARDFINWLIQTKITD (see Proteinogenic amino acid) in humans. GLP-2 is created by specific post-translational proteolytic cleavage of proglucagon in a process that also liberates the related glucagon-like peptide-1 (GLP-1). GLP-2 is produced by the intestinal endocrine L cell and by various neurons in the central nervous system. Intestinal GLP-2 is co-secreted along with GLP-1 upon nutrient ingestion.
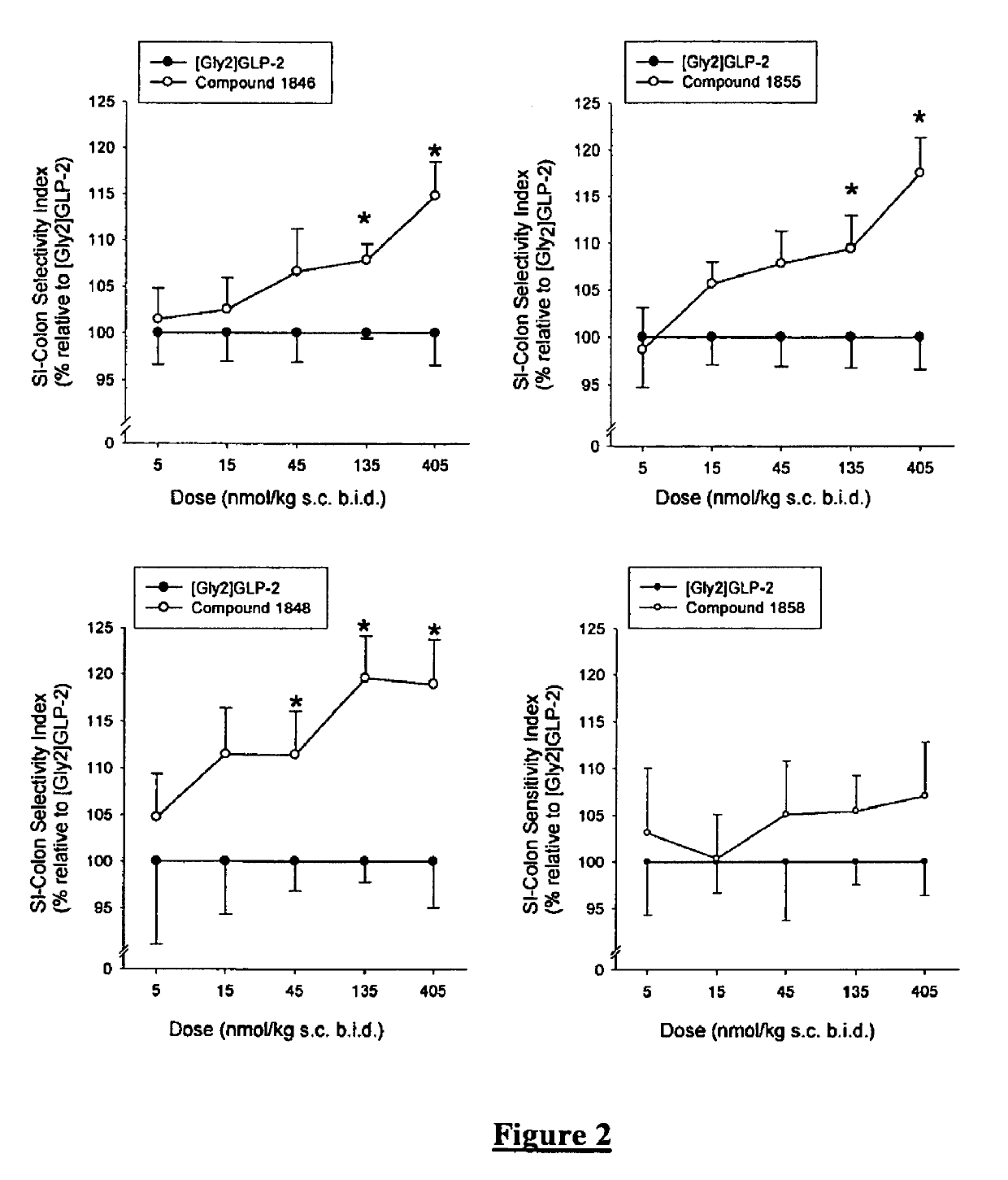
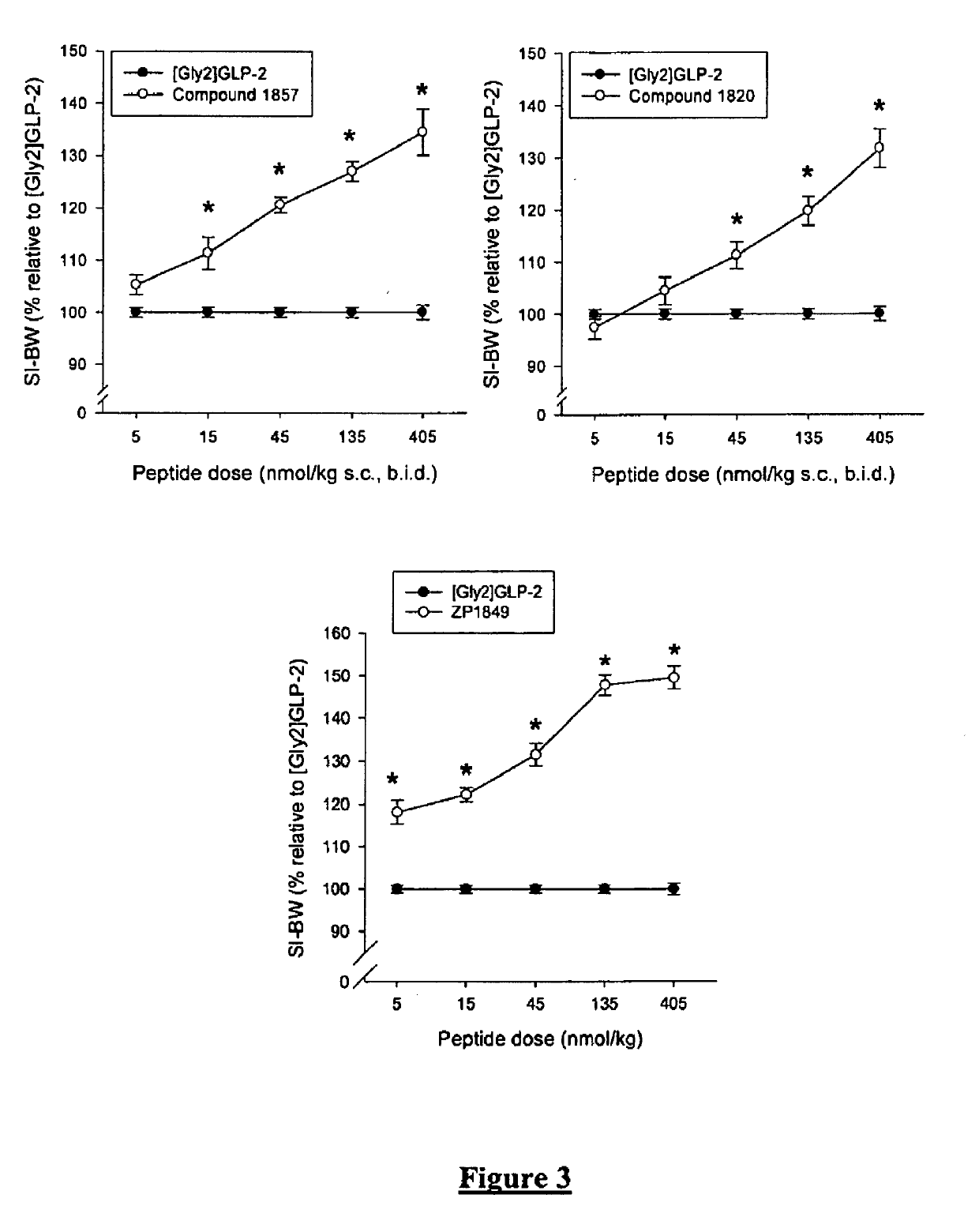
Glucagon-like-peptide-2 (GLP-2) analogues
ActiveUS20070117752A1Improve biological activityPreferential intestinal growth promoting activitySsRNA viruses negative-senseAntipyreticSide effectWild type
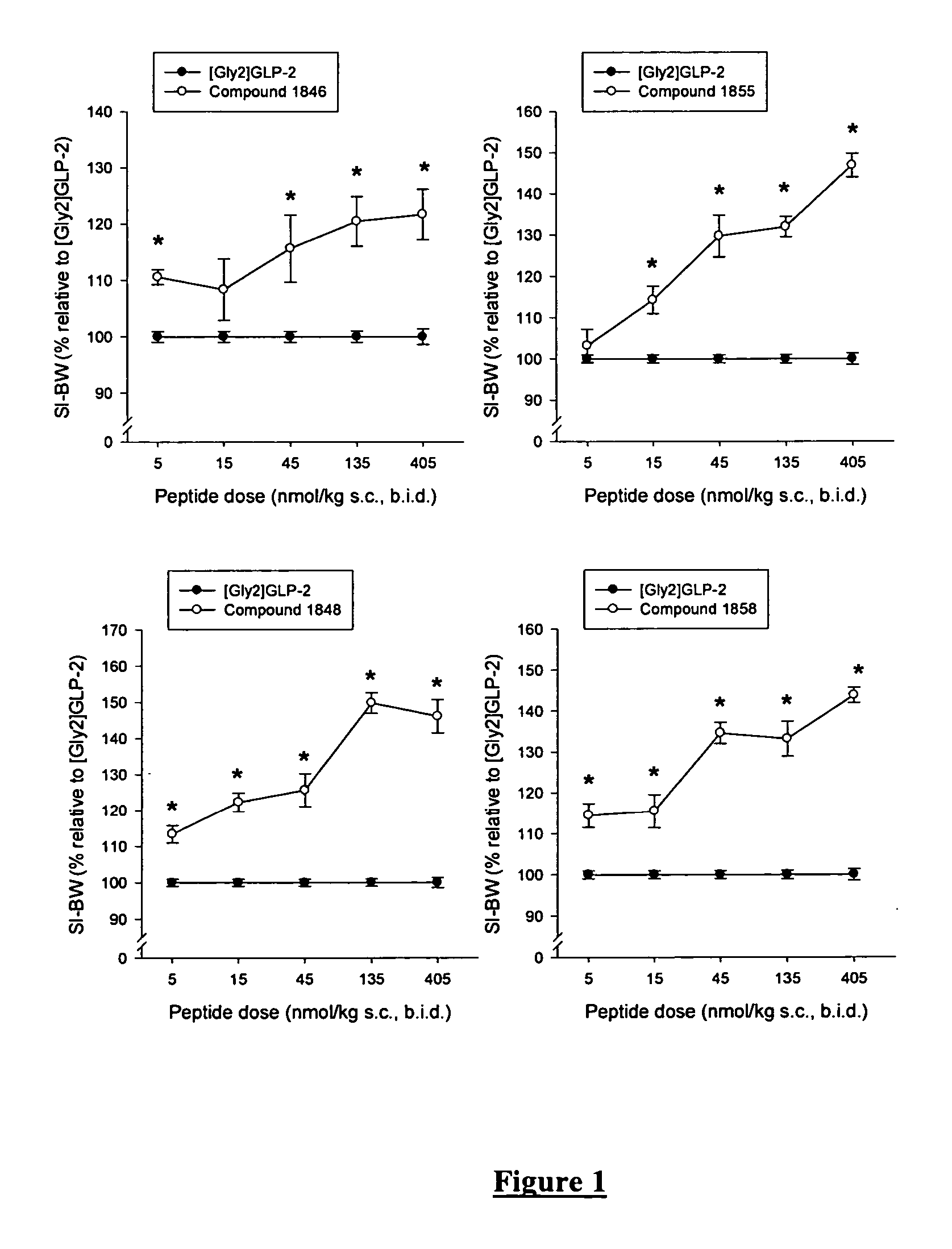
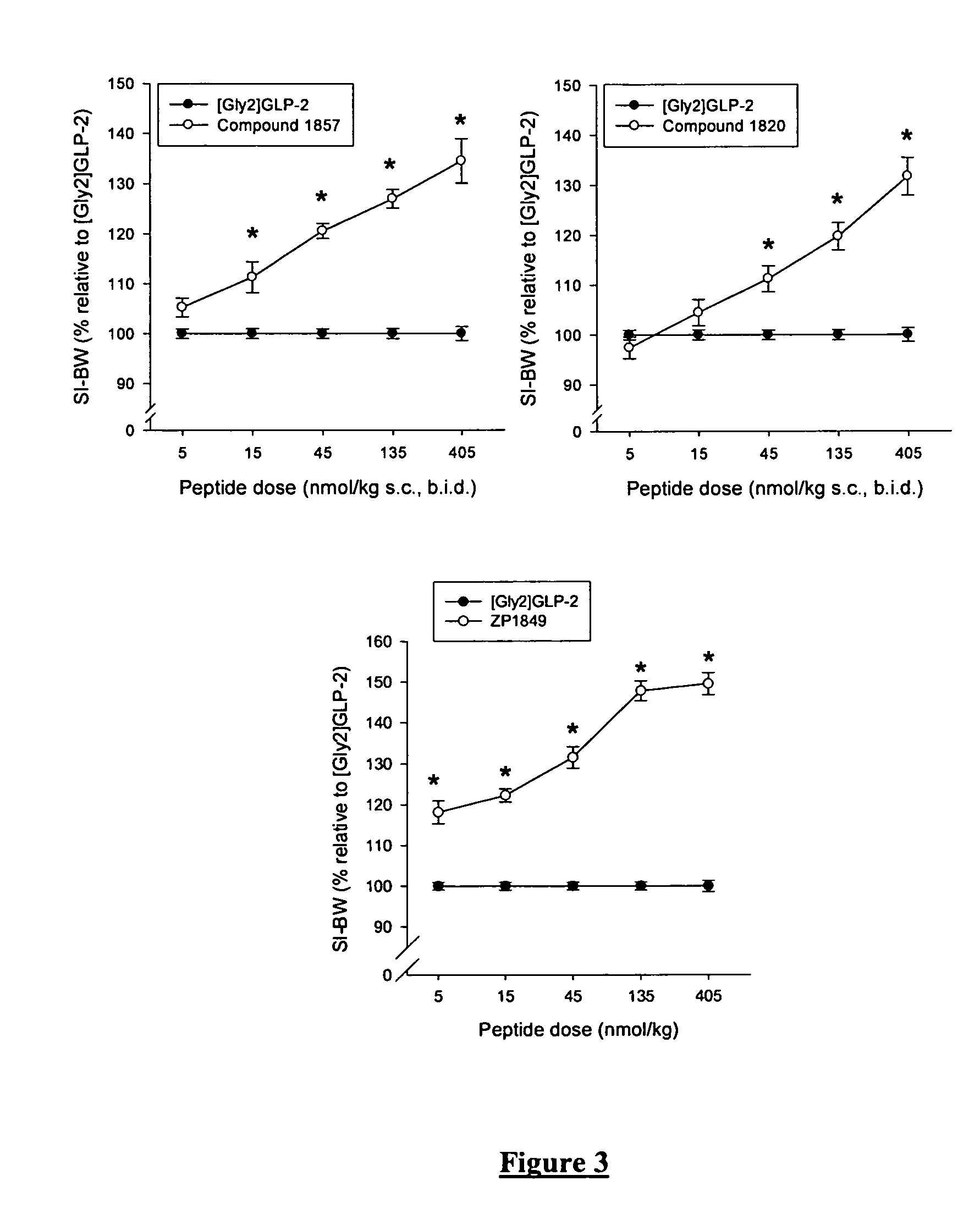
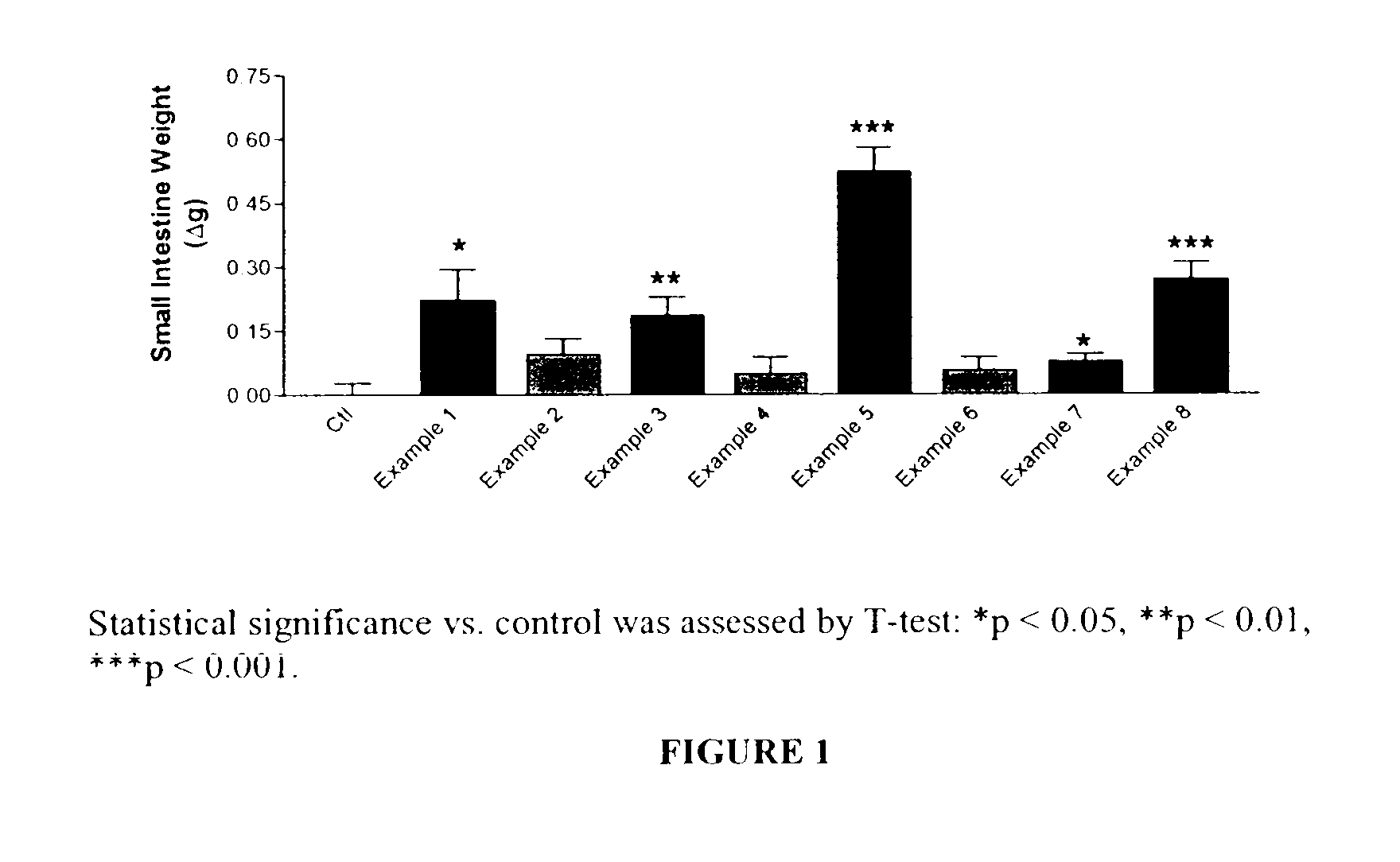
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g., as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy. Also disclosed are methods and kits for selecting a patient from populations suited for treatment with GLP-2 analogues.
Owner:ZEALAND PHARM AS
Long lasting glucagon-like peptide 2 (glp-2) for the treatment of gastrointestinal diseases and disorders
InactiveUS7112567B2Prevents undesirable cleavageHigh activityPeptide/protein ingredientsMetabolism disorderInflammatory Bowel DiseasesHalf-life
This invention relates to glucagon-like peptide 2 (GLP-2) derivatives. In particular this invention relates to GLP-2 peptide derivatives having an extended in vivo half-life, for the treatment or prevention of gastrointestinal disorders or diseases such as inflammatory bowel disease and other gastrointestinal functions, from any segment of the gastrointestinal tract, from the oesophagus to the anus.
Owner:CONJUCHEM
Selective glucagon-like-peptide-2 (GLP-2) analogues
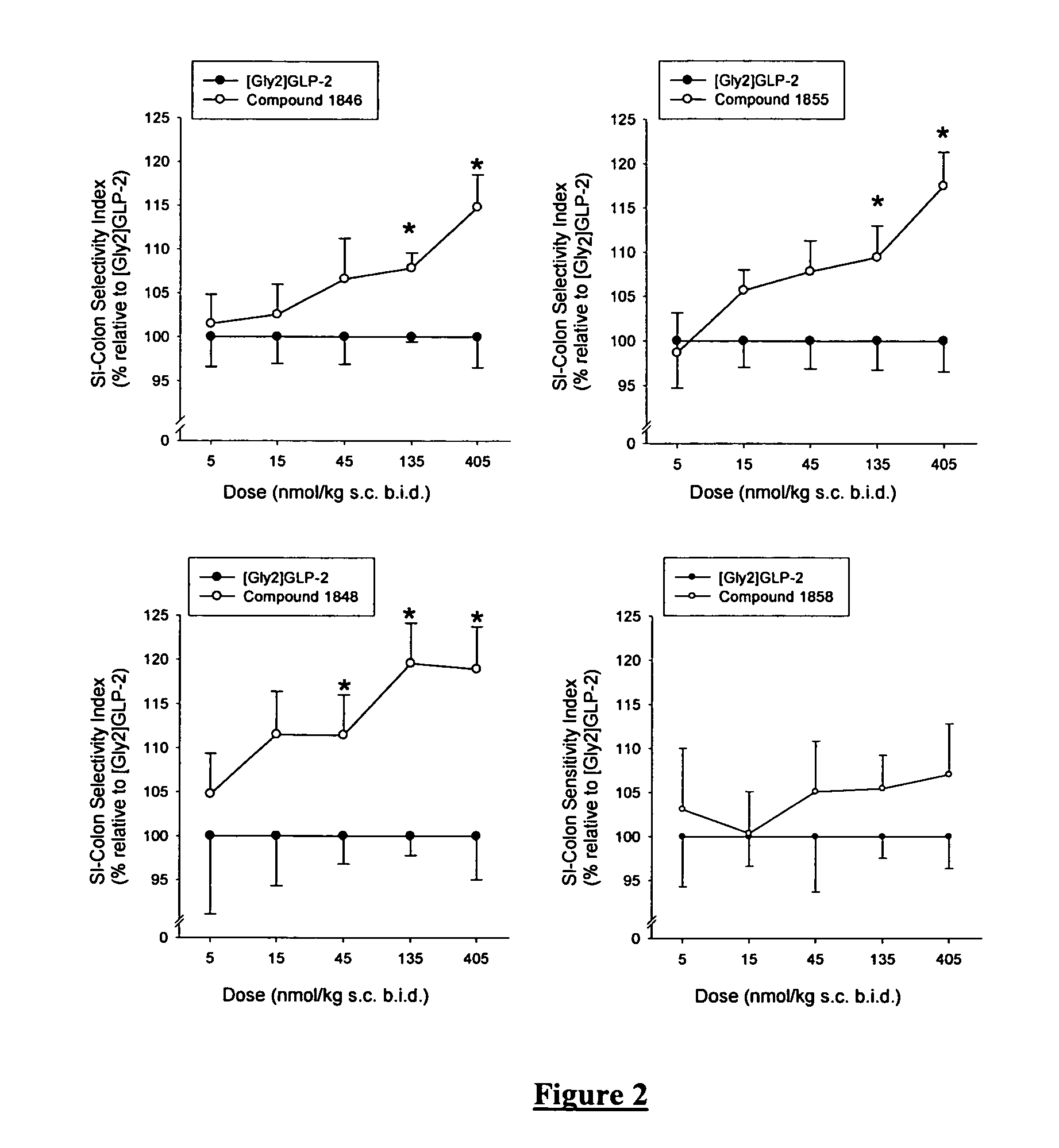
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to h[Gly2]GLP-2 and which may have the property of an increased small intestine / colon and stomach / colon selectivity. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 11, 16, 20, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 and one or more of positions 3, 5, 7, and 10, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy.
Owner:ZEALAND PHARM AS
Site-specific glp-2 conjugate using an immunoglobulin fragment
ActiveUS20140377290A1High binding affinityProlong half-life in vivoPeptide/protein ingredientsDigestive systemDiseaseHalf-life
The present invention relates to a glucagon-like peptide-2 (GLP-2) conjugate comprising native GLP-2 or its derivative and an immunoglobulin Fc fragment being covalently linked via a non-peptidyl polymer, wherein the native GLP-2 or its derivative has a thiol group introduced at its C-terminal end, and one end of the non-peptidyl polymer is linked to an amino acid residue of the GLP-2 other than the N-terminal amino group thereof; a method for preparing the GLP-2 conjugate; a pharmaceutical composition comprising the same; and a method for treating or preventing intestinal disease, intestinal injury, or gastrosia by using the same. Since the GLP-2 conjugate of the present invention has a remarkably increased binding affinity to a GLP-2 receptor, it shows a prolonged in vivo half-life and an improved in vivo durability and stability.
Owner:HANMI PHARMA
Glucagon-like-peptide-2 (GLP-2) analogues
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g. as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy.
Owner:ZEALAND PHARM AS
Long lasting glucagon-like peptide 2 (GLP-2) for the treatment of gastrointestinal diseases and disorders
InactiveUS20060217304A1Prevents undesirable cleavageHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsInflammatory Bowel DiseasesHalf-life
This invention relates to glucagon-like peptide 2 (GLP-2) derivatives. In particular, this invention relates to GLP-2 peptide derivatives having an extended in vivo half-life, for the treatment or prevention of gastrointestinal disorders or diseases such as inflammatory bowel disease and other gastrointestinal functions, from any segment of the gastrointestinal tract, from the oesophagus to the anus.
Owner:CONJUCHEM
GLP-2 compounds, formulations, and uses thereof
InactiveCN1705681ALow bone massPeptide/protein ingredientsGlucagonsNucleotideGlucagon-like peptide-2
The present invention relates to novel human glucagon-like peptide-2 (GLP-2) peptides and human glucagon-like peptide-2 derivatives which have a protracted profile of action as well as polynucleotide constructs encoding such peptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
Two series major vault protein of glucagon-like peptide-2 of people and preparation method thereof
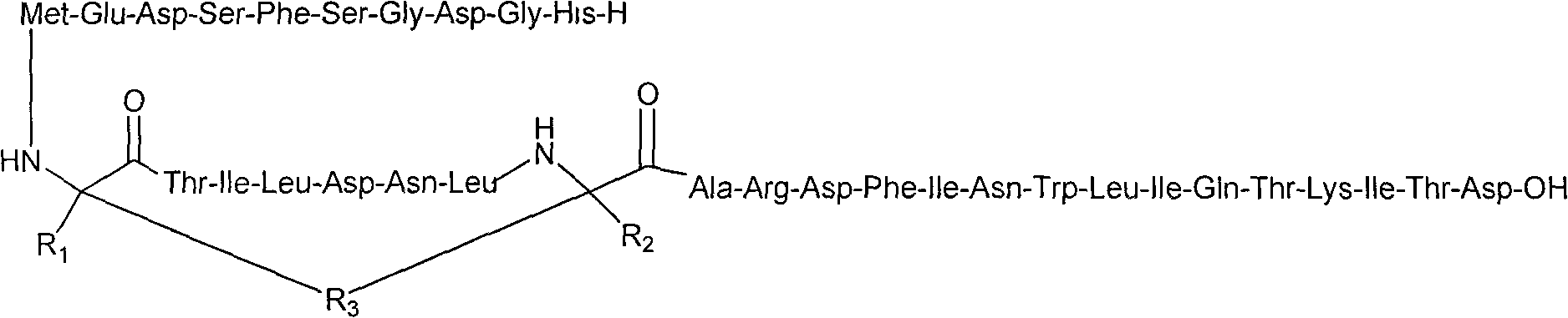
The invention belongs to the field of medical biotechnology, and relates to two series major vault protein of glucagon-like peptide-2 (GLP-2) of people and a preparation method of the two series major vault protein. A second place of an amino acid fragment of the glucagon-like peptide-2 of people is mutated, two segments are connected in series through connecting peptide, a dimmer structure of the GLP-2 is simulated, encoding gene of the two series major vault protein of the GLP-2 is obtained by a synthetic method, the encoding gene is cloned to a pET22b(+) pronucleus so as to express a vector, Escherichia coli BL21 is transformed, the encoding gene is expressed in the Escherichia coli efficiently, the purified reconstructed purpose protein is obtained through salt fractionation of ammonium sulfate in fractional and chromatography of anion exchange columns. The reconstructed purpose protein can stimulate a Caco-2 cell, multiplication of the Caco-2 cell can be promoted notably, the two series major vault protein of GLP-2 of people has natural biological activities, and therefore a foundation is established for further study and extensive use of the GLP-2 of people.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Selective glucagon-like-peptide-2 (glp-2) analogues
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to h[Gly2]GLP-2 and which may have the property of an increased small intestine / colon and stomach / colon selectivity. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions (11, 16, 20, 24) and / or (28) of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position (2) and one or more of positions (3, 5, 7), and (10), and / or a deletion of one or more of amino acids (31) to (33) and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy.
Owner:ZEALAND PHARM AS
GLP-2 derivatives
InactiveUS20060105948A1Reduce gapExtended half-lifeAntibacterial agentsSkeletal disorderGlucagon-like peptide-2Stereochemistry
Owner:NOVO NORDISK AS
Glucagon analogues
ActiveUS8541368B2Improve stabilityImproved pharmacokinetic propertiesNervous disorderPeptide/protein ingredientsAcute hyperglycaemiaDisease
The present invention relates to novel glucagon peptides, to the use of said glucagon peptides in therapy, to methods of treatment comprising administration of said glucagon peptides to patients in need thereof, and to the use of said glucagon peptides in the manufacture of medicaments. The glucagon peptides of the present invention are of particular interest in relation to the treatment of hyperglycemia, diabetes and obesity, as well as a variety of diseases or conditions associated with hyperglycemia, diabetes and obesity.
Owner:NOVO NORDISK AS
Glucagon-like-peptide-2 (glp-2) analogues
ActiveUS20150125431A1High activityPeptide/protein ingredientsAntipyreticMedicineGlucagon-like peptide-2
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to h[Gly2]GLP-2 and which may have the property of an altered GLP-1 activity, and their medical use. The analogues are particularly useful for the prophylaxis, treatment or ameliorating of the gastro-intestinal associated side effects of diabetes.
Owner:ZEALAND PHARM AS
Glucagon-like peptide-2 analogue, its preparation method and application
Owner:JIANGSU HANSOH PHARMA CO LTD
Glucagon-like peptide-2 poly(ethylene glycol) conjugate, and preparation method and application thereof
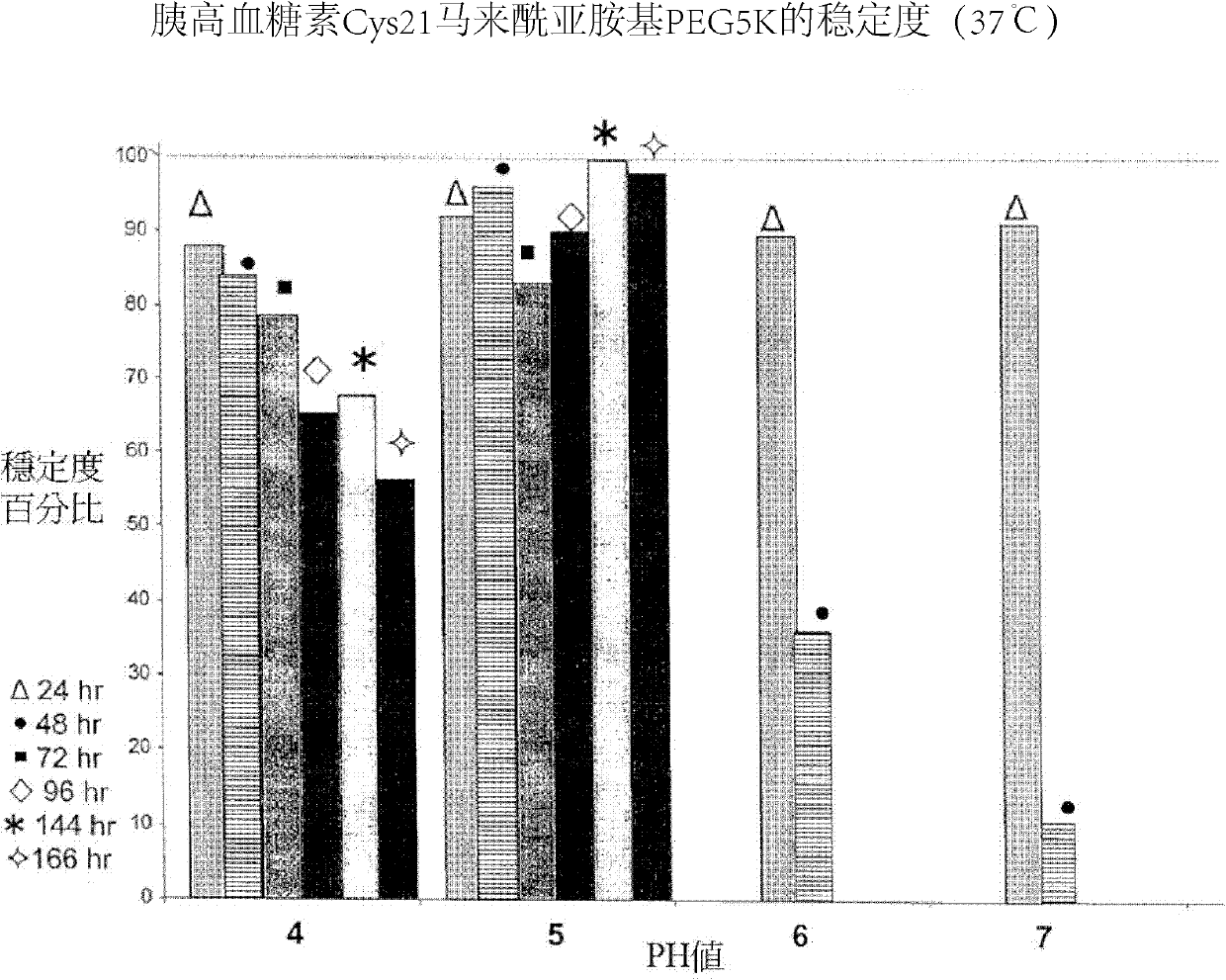
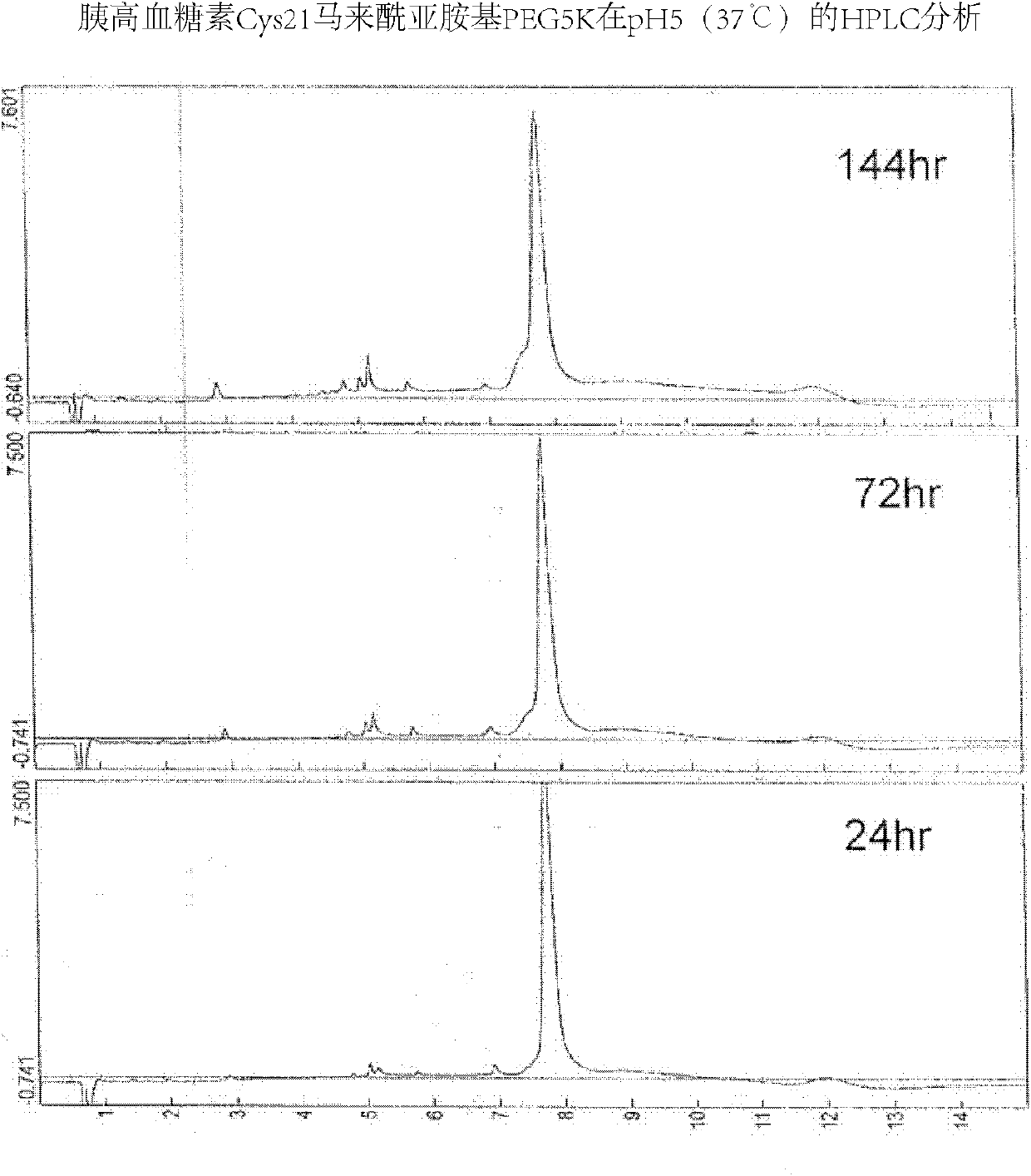
ActiveCN102212127AGood effectExtended half-lifePeptide/protein ingredientsDigestive systemUse medicationHalf-life
The invention provides a glucagon-like peptide-2 poly(ethylene glycol) conjugate, which is acquired by modifying glucagon-like peptide-2 with poly(ethylene glycol), wherein each glucagon-like peptide-2 covalently bonds with one poly(ethylene glycol) molecule. The invention also provides a preparation method and application of the glucagon-like peptide-2 poly(ethylene glycol) conjugate. According to the invention, a glucagon-like peptide-2 poly(ethylene glycol) conjugate with good efficacy and long half-life period is acquired by optimizing PEGylation reaction conditions; and if used clinically, the glucagon-like peptide-2 poly(ethylene glycol) conjugate is administered only 1-2 times a week, thus greatly reducing the pain of patients. Meanwhile, the recombinant production method of GLP-2 (glucagon-like peptide-2) provided by the invention is simple and safe, greatly lowers the cost in the preparation of the glucagon-like peptide-2 poly(ethylene glycol) conjugate, and has a good application prospect.
Owner:SHANGHAI JINGZE PHARMA CO LTD
Site-specific GLP-2 conjugate using an immunoglobulin fragment
ActiveUS9504757B2High binding affinityLong-lasting in vivo therapeutic efficacyPeptide/protein ingredientsDigestive systemDiseaseHalf-life
Provided are a glucagon-like peptide-2 (GLP-2) conjugate containing native GLP-2 or its derivative and an immunoglobulin Fc fragment being covalently linked via a non-peptidyl polymer, wherein the native GLP-2 or its derivative has a thiol group introduced at its C-terminal end, and one end of the non-peptidyl polymer is linked to an amino acid residue of the GLP-2 other than the N-terminal amino group thereof; a method for preparing the GLP-2 conjugate; a pharmaceutical composition comprising the same; and a method for treating or preventing intestinal disease, intestinal injury, or gastrosis by using the same. Since the GLP-2 conjugate of the present invention has a remarkably increased binding affinity to a GLP-2 receptor, it shows a prolonged in vivo half-life and an improved in vivo durability and stability.
Owner:HANMI PHARMA
Production of glucagon-like peptide 2
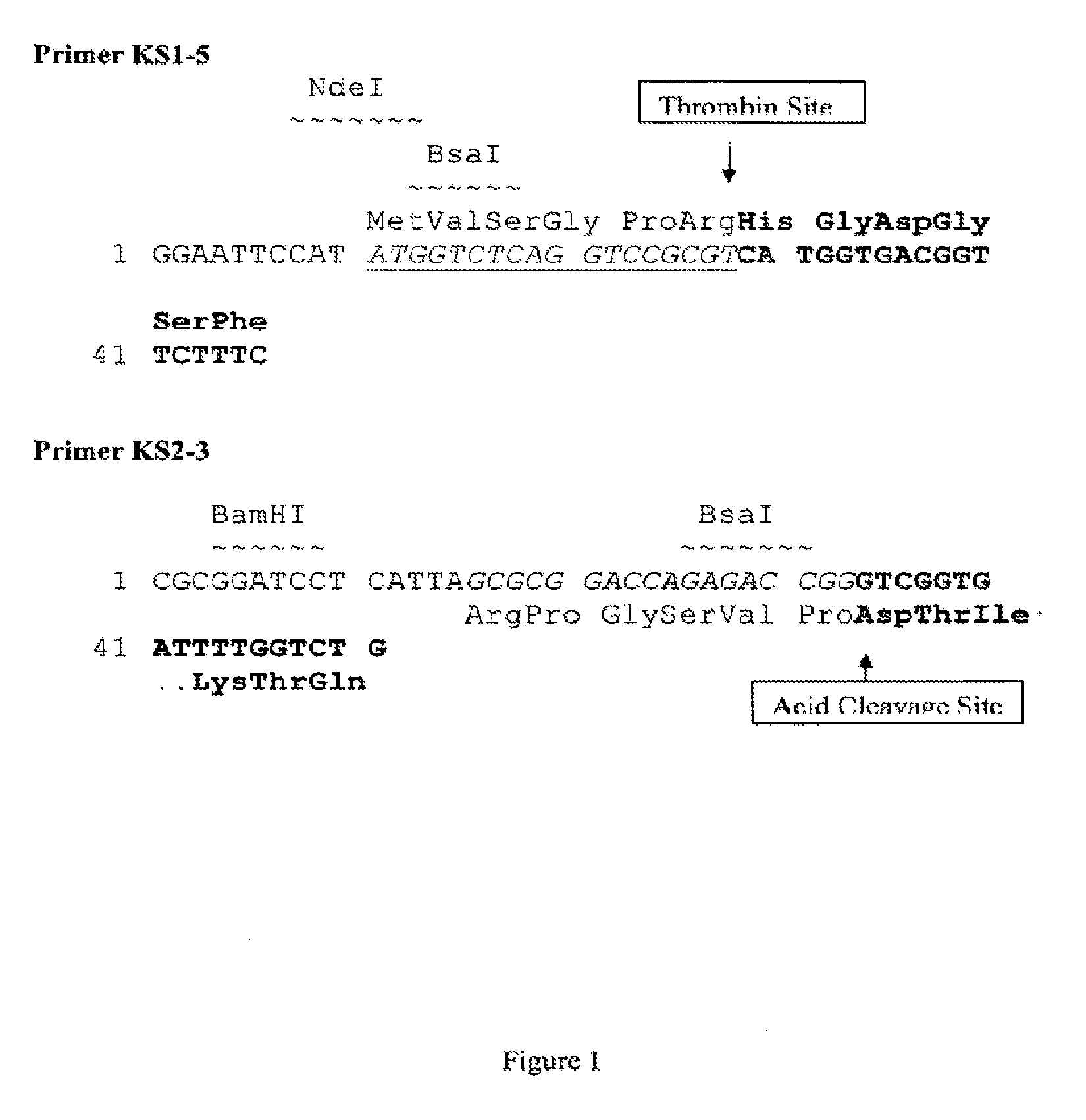
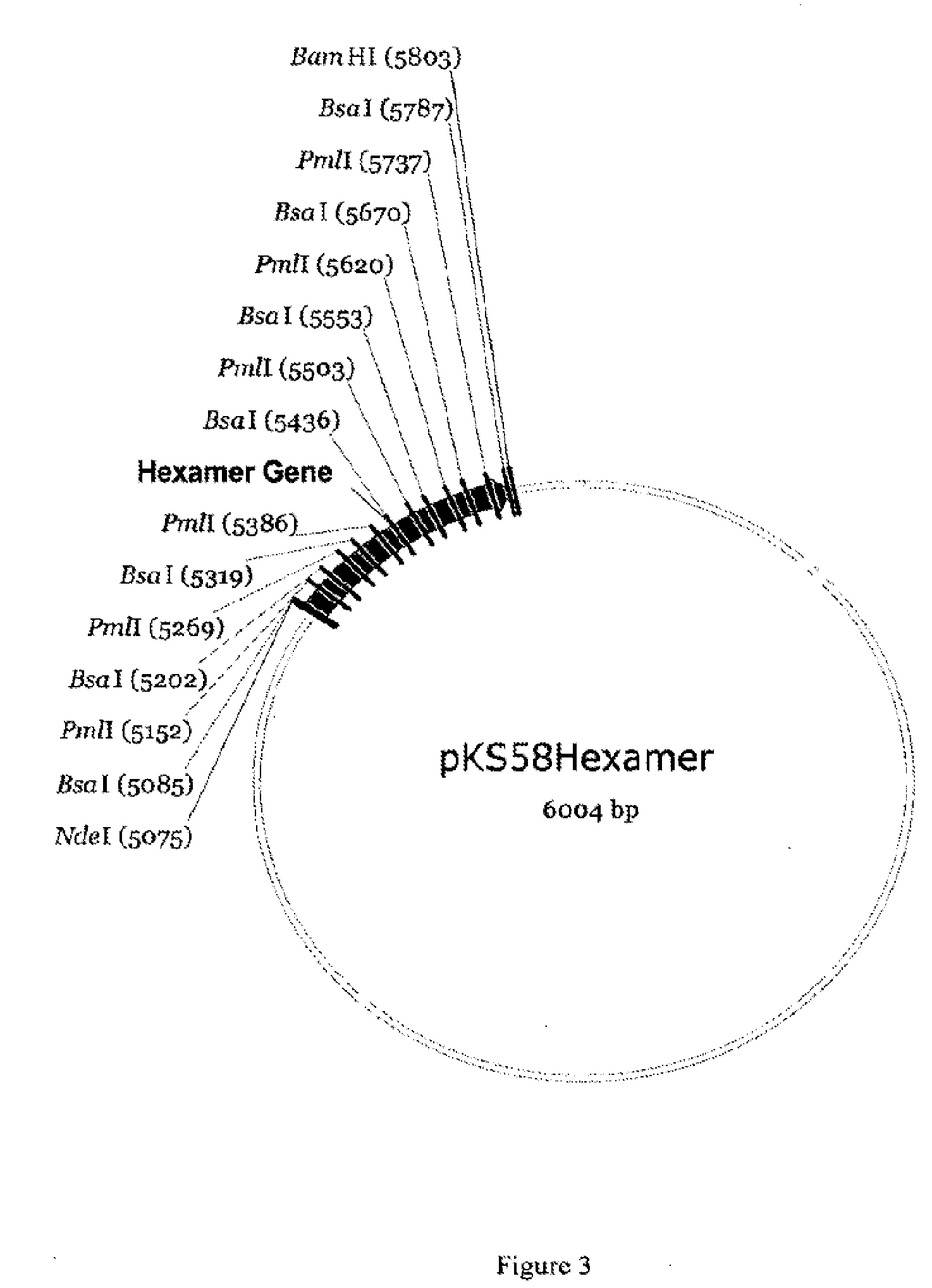
GLP-2 peptides and analogs thereof are produced in high yield and with desired, authentic termini by isolation from a GLP-2 peptide multimer in which at least two units of GLP-2 peptide are coupled through a linker that presents an N-terminal acid cleavage site and a C-terminal enzyme cleavage site. In a specific embodiment, [Gly2]hGLP-2 is produced from a multimeric precursor comprising 2-30 units thereof.
Owner:NPS PHARM INC
Glucagon-like peptide-2 and its therapeutic use
Glucagon-like peptide-2, a product of glucagon gene expression, and analogs of glucagon-like peptide-2, have been identified as gastrointestinal tissue growth factors. Their effects on the growth of small bowel and pancreatic islets are described. Their formulation as a pharmaceutical, and their therapeutic use in treating disorders of the bowel, are described.
Owner:1149336 ONTARIO
Intestinotrophic glucagon-like peptide-2 analogs
Glucagon-like peptide 2, a product of glucagon gene expression, and analogs of glucagon-like peptide 2, have been identified as gastrointestinal tissue growth factors. Their effects on the growth of small bowel and pancreatic islets are described. Their formulation as a pharmaceutical, and their therapeutic use in treating disorders of the bowel, are described.
Owner:1149336 ONTARIO
Method of preparing glucagon-like peptide-2 (glp-2) analog
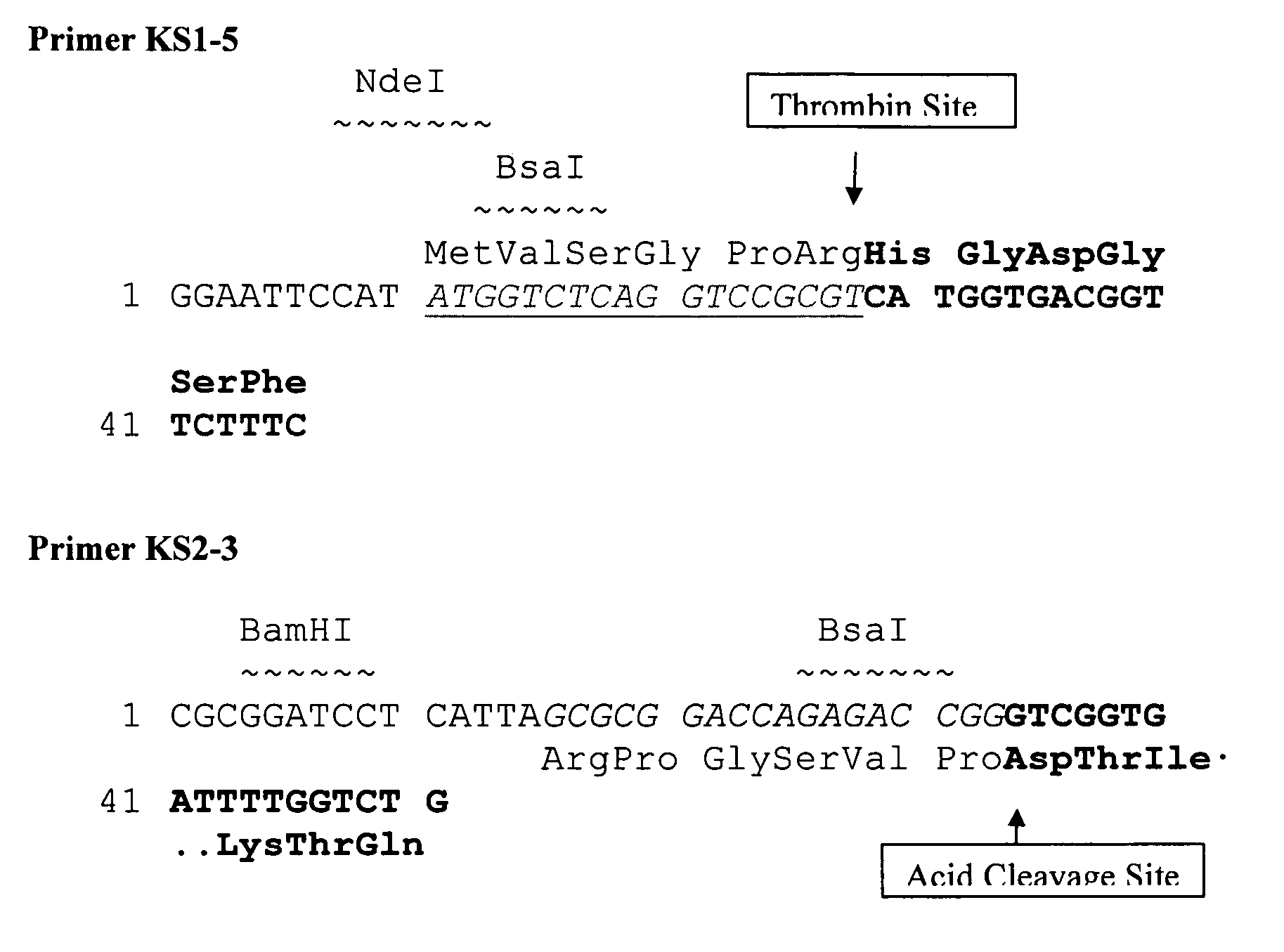
ActiveUS20180118800A1Quick purificationOvercomes drawbackFusions for enhanced expression stability/foldingFusion with protease siteThrombin activityNucleic acid sequencing
The present invention relates a method of preparing a Glucagon-like peptide 2 (GLP-2) analog by gene recombination. The present invention provides an expression vector, which includes: (a) a nucleic acid sequence encoding tag protein; (b) a nucleic acid sequence encoding Smt3 protein (SEQ ID NO: 1); and (c) a nucleic acid sequence encoding linker peptide (SEQ ID NO: 2) and GLP-2 analog. The fusion protein expressed by the expression vector can be cleaved by thrombin, wherein the cleaving position is at the linker peptide, and then the GLP-2 analog is produced.
Owner:CHUNGHWA CHEM SYNTHESIS & BIOTECH
Glucagon-like peptide-2 analog, and preparation method and application thereof
ActiveCN103910793AOvercoming the problem of short half-lifeExtended half-lifePeptide/protein ingredientsDigestive systemGlycineHalf-life
The invention provides a glucagon-like peptide-2 (GLP-2) analog, and a preparation method and an application thereof. The sequence of the GLP-2 analog is <1>HADX4SFSX8EMNTILDX16LAARDFIX24WLX27QTKITD<33>n1X1C n2X2 represented by SEQ ID NO:1, wherein X4 is glycine or cysteine; X8 is aspartic acid or cysteine; X16 is asparagine or cysteine; X24 is aspartic acid or cysteine; X27 is isoleucine or cysteine, only one of X4, X8, X16, X24 and X27 is cysteine, and cysteine is bonded with another cysteine through a disulfide bond; n1X1 represents n1 X1, and n1 is 1-30; and n2X2 represents n2 X2, and n2 is 0-30. The GLP-2 analog overcomes the short half life problem of GLP-2.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Glucagon-like-peptide-2 (glp-2) analogues
InactiveUS20190330295A1Sure easyHigh activitySsRNA viruses negative-sensePowder deliveryDiseaseSide effect
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g., as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy. Also disclosed are methods and kits for selecting a patient from populations suited for treatment with GLP-2 analogues.
Owner:ZEALAND PHARM AS
Construction method and application of recombinant lactobacillus acidophilus for expressing glucagon-like peptide-2
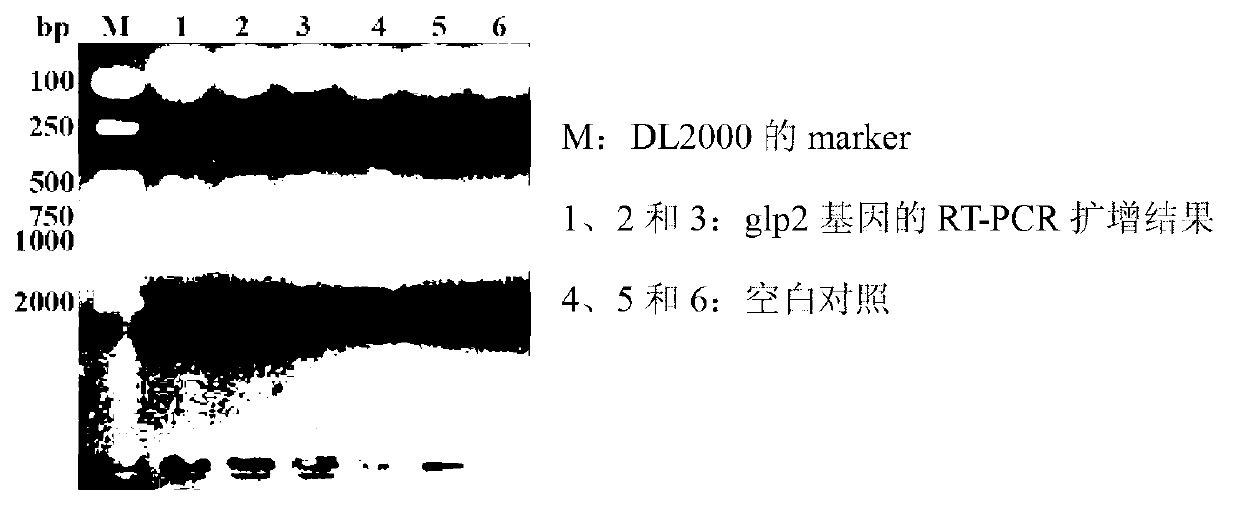
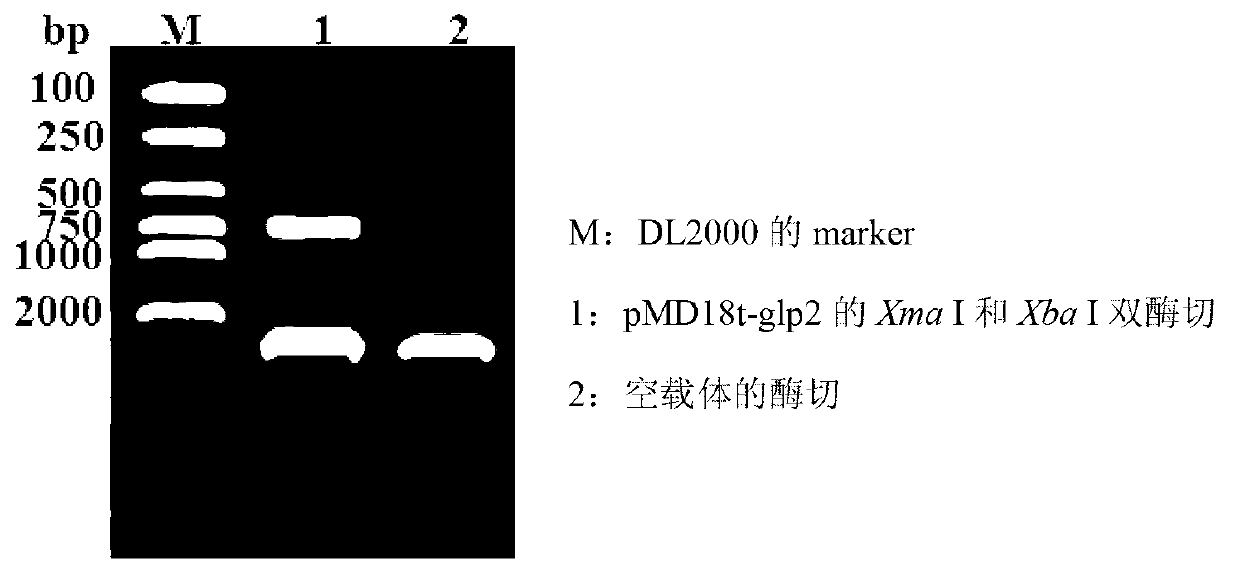
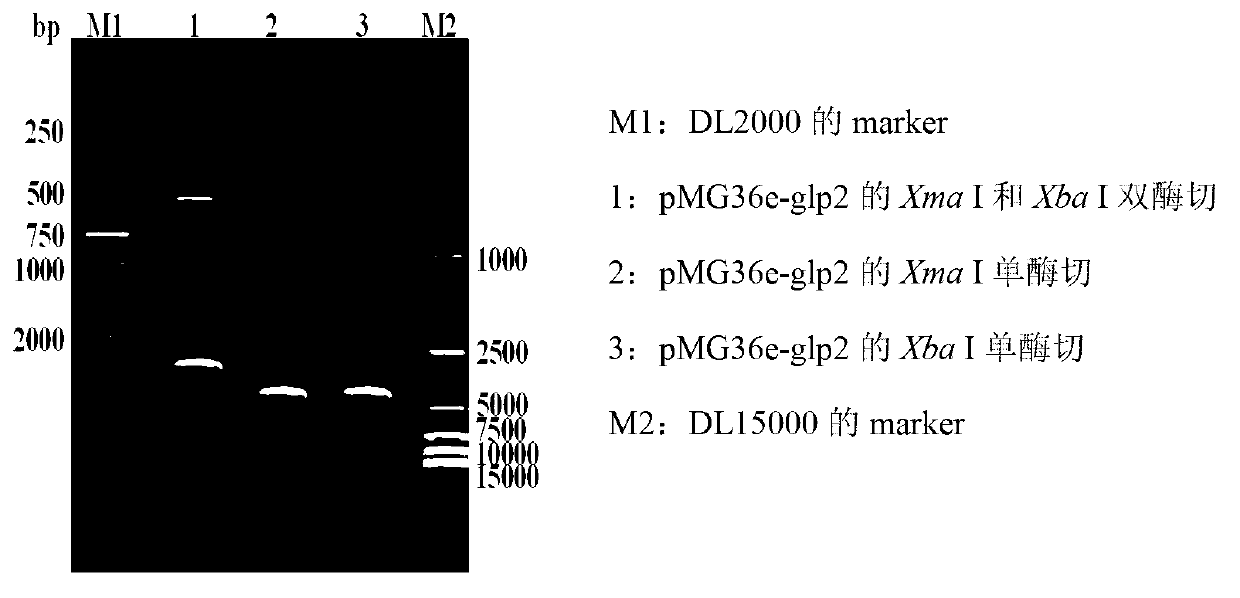
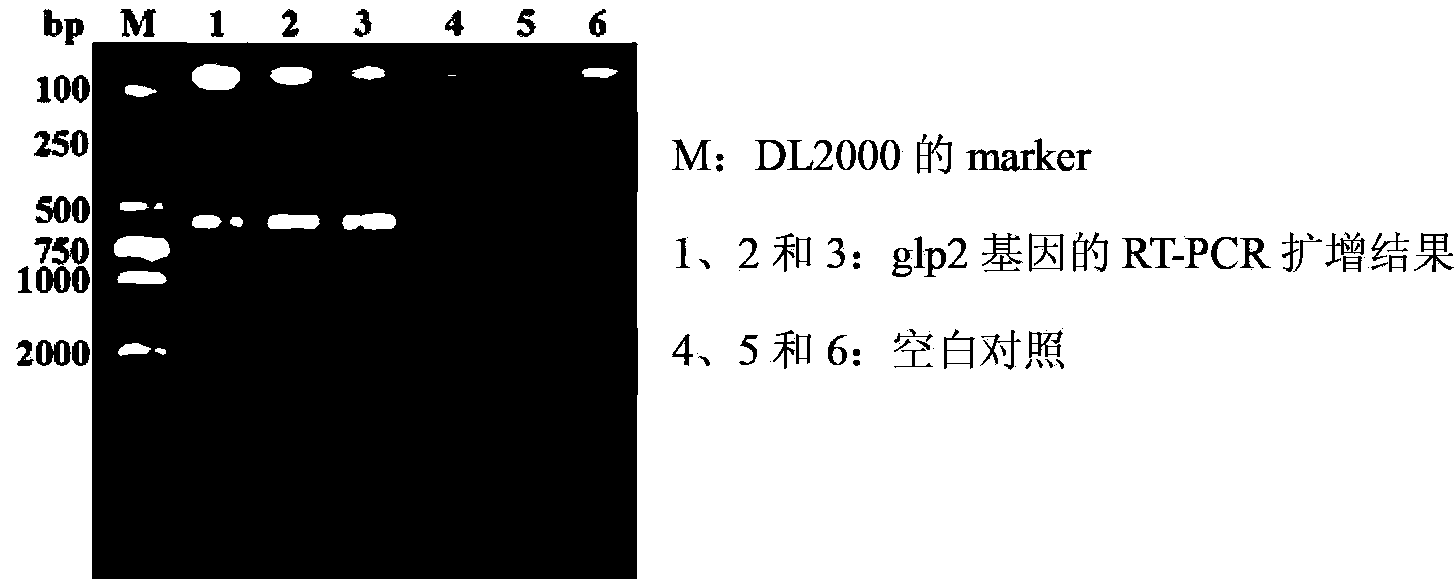
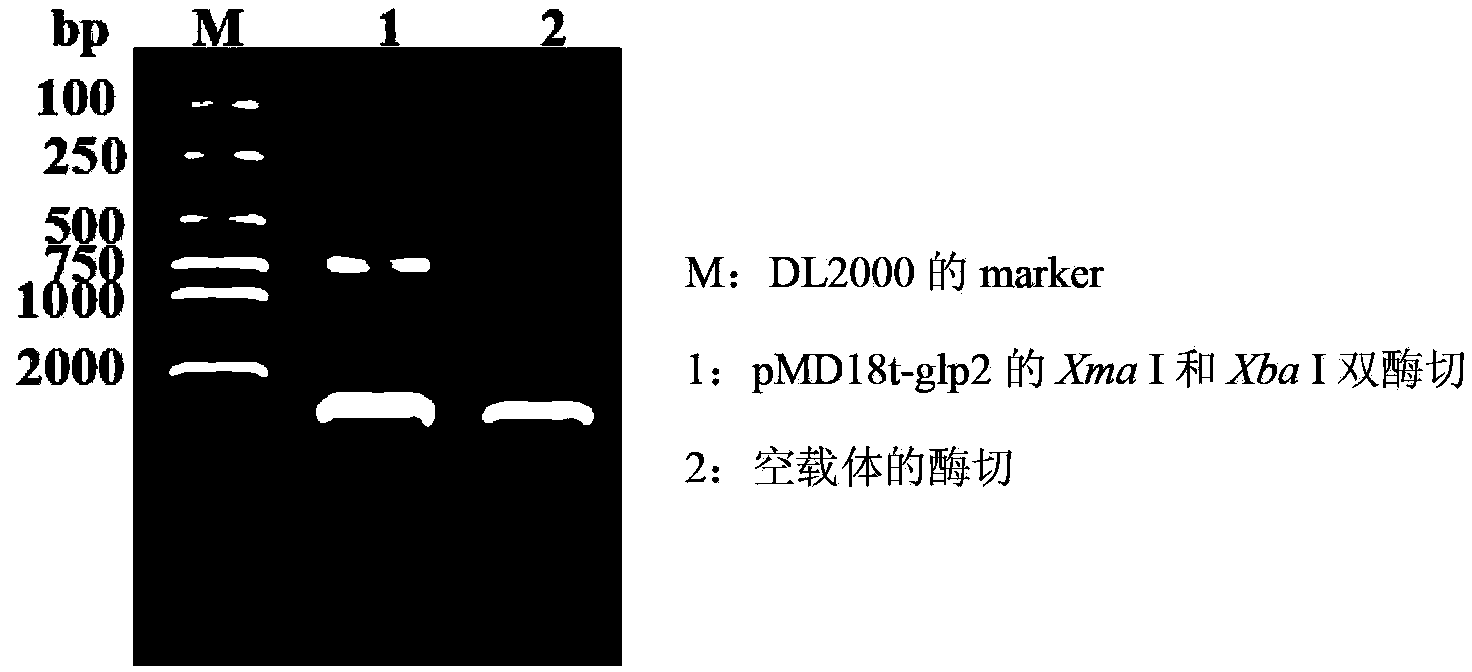
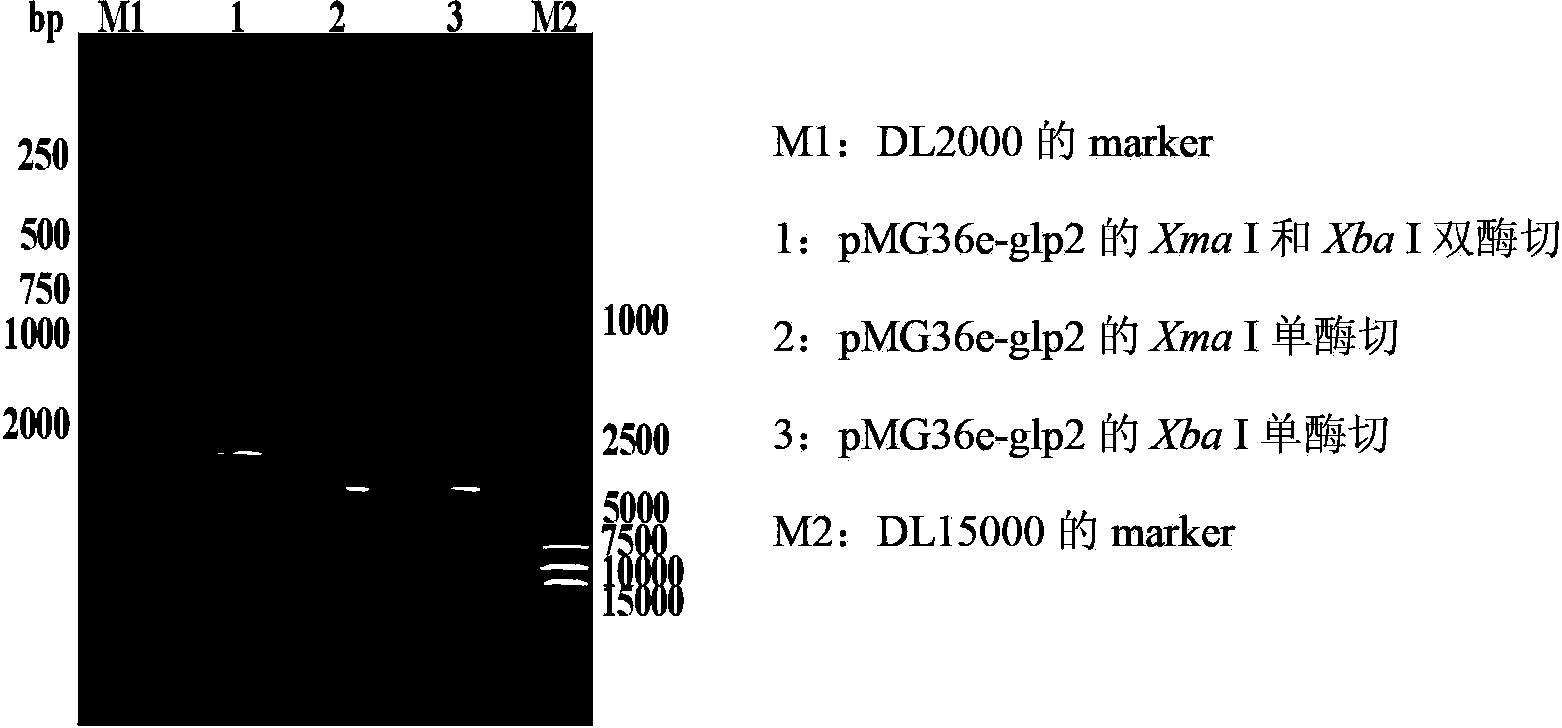
ActiveCN103103210APromote growthImprove intestinal environmentBacteriaAnimal feeding stuffRestriction enzyme digestionWestern blot
The invention discloses a construction method and application of recombinant lactobacillus acidophilus for expressing glucagon-like peptide-2. The construction method comprises the following steps: designing a primer containing specific restriction enzyme digestion site according to gene sequence and expression vector plasmid characteristic of disclosed glucagon-like peptide-2; by using small intestinal mucosa as a material, carrying out RT-PCR (reverse transcription-polymerase chain reaction) to obtain fragment containing glucagon-like peptide-2 gene; connecting the fragment with lactobacillus expression vector plasmid Pmg36e to obtain recombinant plasmid Pmg36e-glp2; transferring the recombinant plasmid in actobacillus acidophilus through an electrotransformation method to obtain recombinant lactobacillus acidophilus for expressing glucagon-like peptide-2; expressing under the induction of nisin; and analyzing and proving that the gene is correctly expressed through SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and Western blot. The recombinant lactobacillus acidophilus is used for preventing and treating diarrhea, improving the intestinal health of pig and improving the growth performance.
Owner:SHENZHEN JINXINNONG FEED
Production of glucagon like peptide 2 and analogs
GLP-2 peptides and analogs thereof are produced in high yield and with desired, authentic termini by isolation from a GLP-2 peptide multimer in which at least two units of GLP-2 peptide are coupled through a linker that presents an N-terminal acid cleavage site and a C-terminal enzyme cleavage site. In a specific embodiment, [Gly2]hGLP-2 is produced from a multimeric precursor comprising 2-30 units thereof.
Owner:NPS PHARM INC
Glucagon-like peptide-2 (GLP-2) analog, and preparation method and use thereof
ActiveCN102924589APeptide/protein ingredientsDigestive systemChemical structureGlucagon-like peptide-2
The invention relates to a new GLP-2 analog and a preparation method thereof. Compared with natural GLP-2, the GLP-2 analog has the advantages of improved chemical stability, and reservation of the pharmacological activities of the natural GLP-2. The GLP-2 analog has a chemical structure represented by a formula shown in the specification.
Owner:CHINESE PEPTIDE CO
Glucagon-like peptide-2 analogue dimer, preparation method and application thereof
ActiveCN103864917AOvercoming the problem of short half-lifeExtended half-lifePeptide/protein ingredientsDigestive systemDiseaseHalf-life
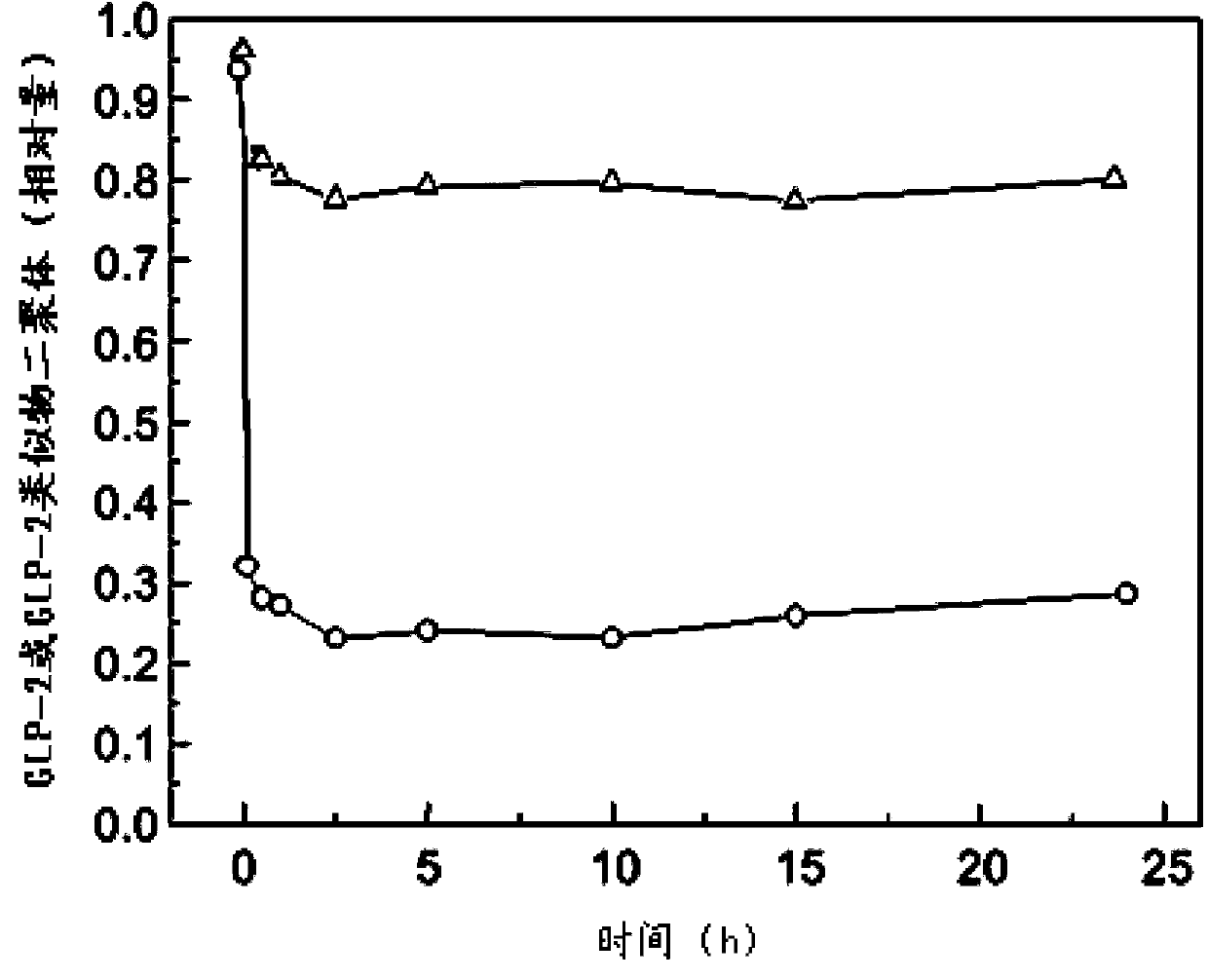
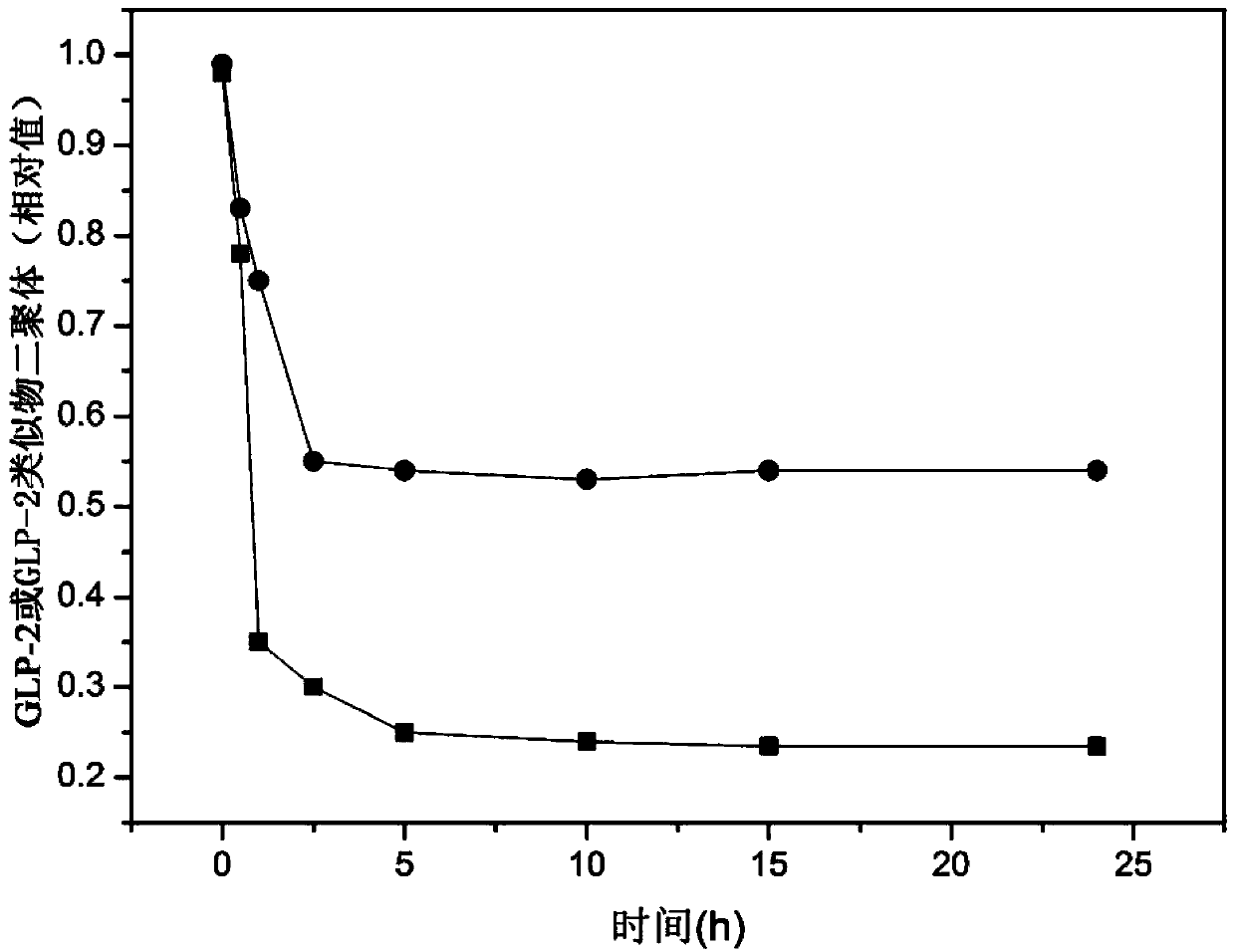
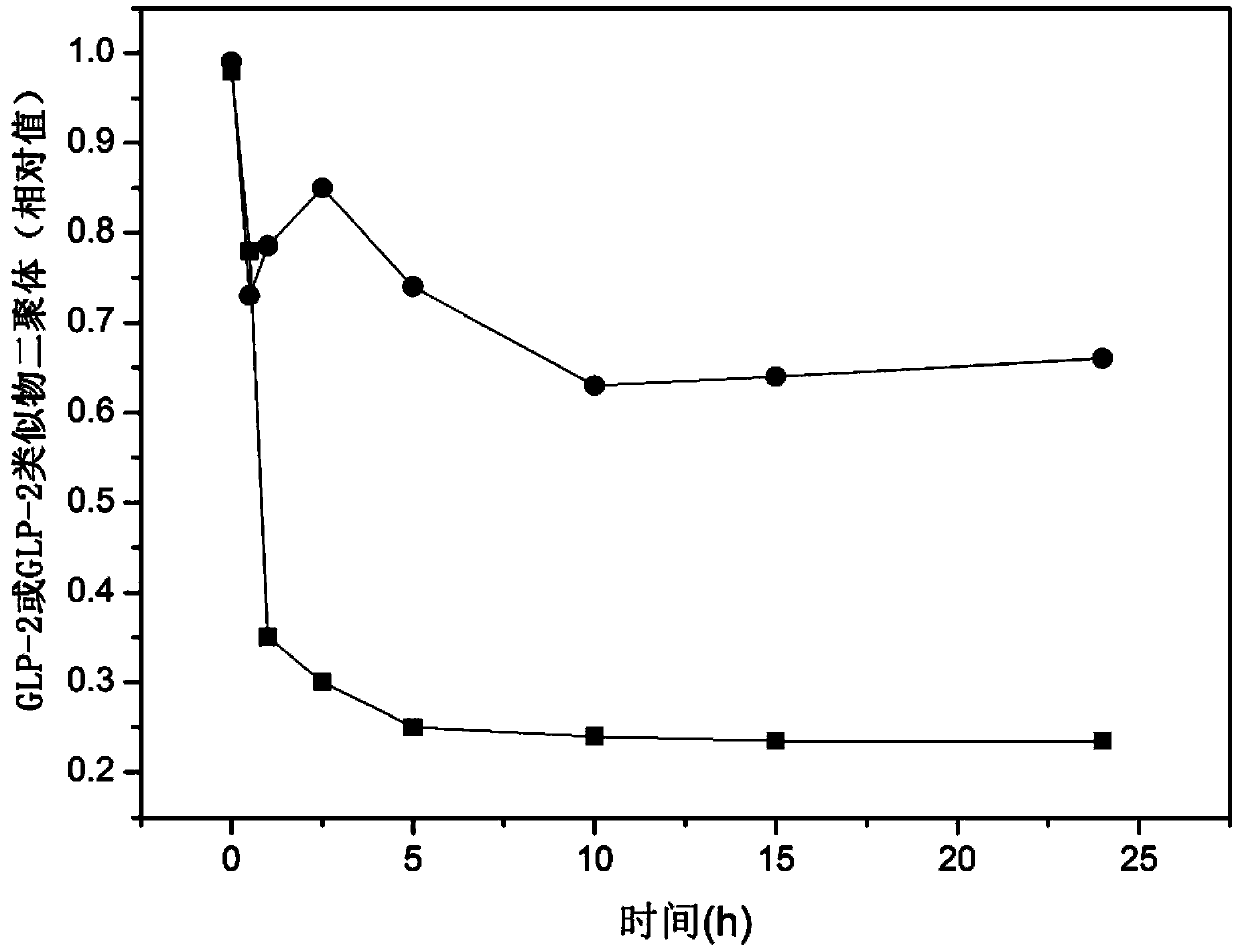
The present invention provides a glucagon-like peptide-2 analogue dimer, a preparation method and an application thereof, wherein two identical or different GLP-2 analogue monomers form a disulfide bond through cysteines on the monomers so as to prepare the dimer. The dimer preparation method comprises: adopting an Fmoc solid phase polypeptide synthesis method to synthesize a GLP-2 analogue monomer, and making the synthesized GLP-2 analogue monomers form a disulfide bond between the monomers. The application is an application of the GLP-2 analogue dimer in preparation of drugs for treatment of gastrointestinal related diseases. According to the present invention, with the GLP-2 analogue dimer formed from the GLP-2 analogue monomer, the problem of the short half-life of the GLP-2 is overcome, and the half-life of the GLP-2 analogue dimer can achieve more than 8-96 h in vivo, and is significantly prolonged compared with the half-life of the GLP-2 administered separately so as to substantially and easily achieve clinical promotion and application.
Owner:天津天诚新药评价有限公司
Production of glucagon like peptide 2 and analogs
GLP-2 peptides and analogs thereof are produced in high yield and with desired, authentic termini by isolation from a GLP-2 peptide multimer in which at least two units of GLP-2 peptide are coupled through a linker that presents an N-terminal acid cleavage site and a C-terminal enzyme cleavage site. In a specific embodiment, [Gly2]hGLP-2 is produced from a multimeric precursor comprising 2-30 units thereof.
Owner:NPS PHARM INC
Gip receptor-active glucagon compounds
Glucagon peptides with increased GIP activity are provided, optionally with GLP-I and / or glucagon activity. In some embodiments, C-terminally extended glucagon peptides comprising an amino acid sequence substantially similar to native glucagon are provided herein.
Owner:INDIANA UNIV RES & TECH CORP
Glucagon-like-peptide-2 (glp-2) analogues
PendingUS20210309708A1Preferential intestinal growth promoting activityImprove biological activitySsRNA viruses negative-sensePowder deliveryDiseaseSide effect
GLP-2 analogues are disclosed which comprise one of more substitutions as compared to [hGly2]GLP-2 and which improved biological activity in vivo and / or improved chemical stability, e.g., as assessed in in vitro stability assays. More particularly, preferred GLP-2 analogues disclosed herein comprise substitutions at one or more of positions 8, 16, 24 and / or 28 of the wild-type GLP-2 sequence, optionally in combination with further substitutions at position 2 (as mentioned in the introduction) and one or more of positions 3, 5, 7, 10 and 11, and / or a deletion of one or more of amino acids 31 to 33 and / or the addition of a N-terminal or C-terminal stabilizing peptide sequence. The analogues are particularly useful for the prophylaxis or treatment of stomach and bowel-related disorders and for ameliorating side effects of chemotherapy. Also disclosed are methods and kits for selecting a patient from populations suited for treatment with GLP-2 analogues.
Owner:ZEALAND PHARM AS
Construction method and application of recombinant lactobacillus acidophilus for expressing glucagon-like peptide-2
ActiveCN103103210BPromote growthImprove intestinal environmentBacteriaMicroorganism based processesRestriction enzyme digestionWestern blot
The invention discloses a construction method and application of recombinant lactobacillus acidophilus for expressing glucagon-like peptide-2. The construction method comprises the following steps: designing a primer containing specific restriction enzyme digestion site according to gene sequence and expression vector plasmid characteristic of disclosed glucagon-like peptide-2; by using small intestinal mucosa as a material, carrying out RT-PCR (reverse transcription-polymerase chain reaction) to obtain fragment containing glucagon-like peptide-2 gene; connecting the fragment with lactobacillus expression vector plasmid Pmg36e to obtain recombinant plasmid Pmg36e-glp2; transferring the recombinant plasmid in actobacillus acidophilus through an electrotransformation method to obtain recombinant lactobacillus acidophilus for expressing glucagon-like peptide-2; expressing under the induction of nisin; and analyzing and proving that the gene is correctly expressed through SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and Western blot. The recombinant lactobacillus acidophilus is used for preventing and treating diarrhea, improving the intestinal health of pig and improving the growth performance.
Owner:SHENZHEN JINXINNONG FEED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com