Glucagon-like peptide-2 poly(ethylene glycol) conjugate, and preparation method and application thereof
A technology of glucagon and polyethylene glycol, which is applied in the field of glucagon-like peptide-2 polyethylene glycol conjugate and its preparation, can solve the influence of GLP-2 activity, the safety threat of research and development personnel, and the degree of substitution The problem of low molecular weight of glutide, to achieve the effect of alleviating pain, simple method and good efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
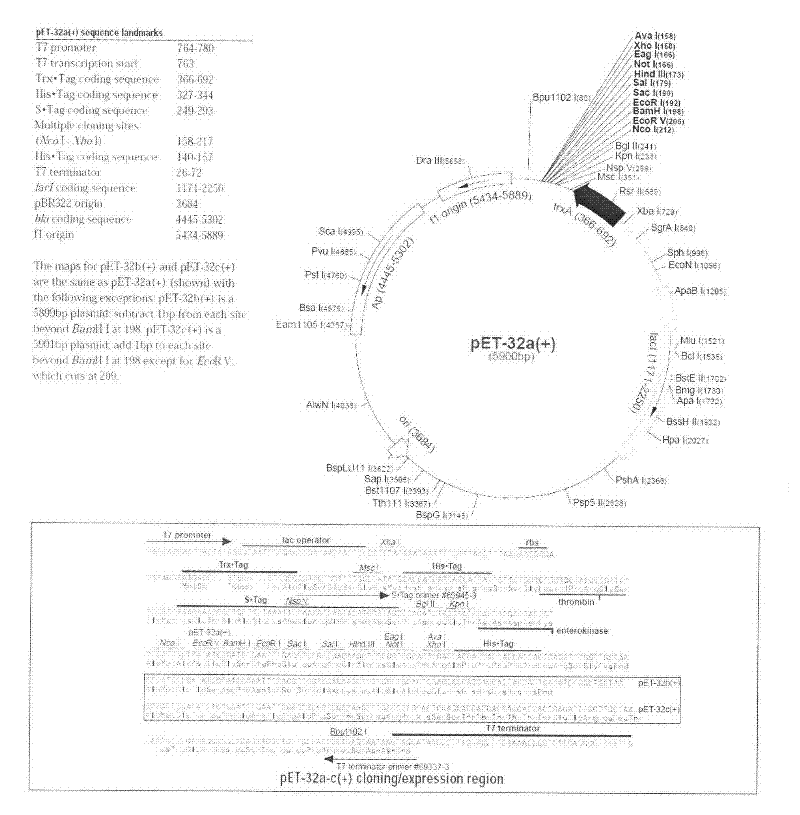
[0047] The recombinant production of embodiment 1GLP-2
[0048] (1) Construction of engineering strains
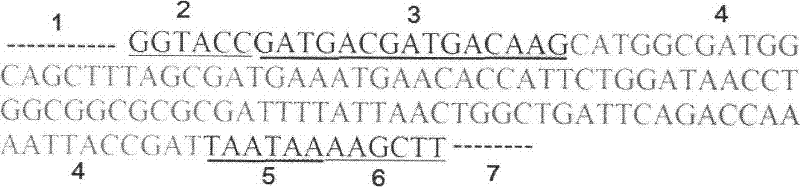
[0049] GLP-2, in which alanine-2 is replaced by glycine, contains 33 amino acids, and the amino acid sequence is shown in SEQ ID NO.1, which is His Gly Asp Gly Ser Phe Ser Asp Glu MET Asn Thr Ile Leu AspAsn Leu Ala Ala Arg Asp Phe Ile Asn Trp L
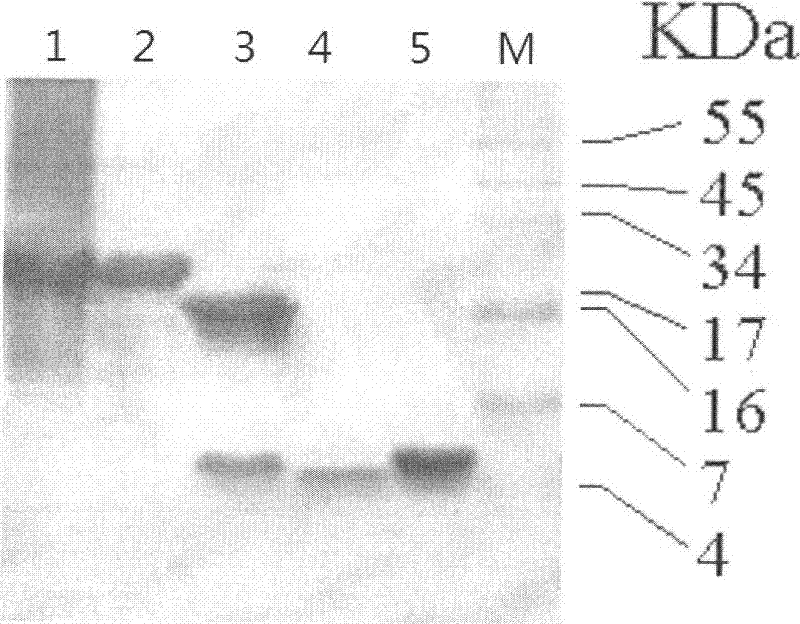
[0050] After the identified positive clone pET-GLP-2 plasmid was transformed into Escherichia coli BL21 (DE3), pick the transformants in 5 ml LB medium containing 100 μg / ml Amp (1% peptone, 0.5% yeast extract, 1% NaCl ) at 37°C for overnight culture, and transferred to 50ml LB medium containing 100μg / ml Amp at 1 / 100 volume and cultured at 37°C. When OD600 reached 0.5, IPTG was added to a final concentration of 0.5mM to induce expression, and samples were taken 4 hours later for SDS-PAGE electrophoresis. Compared with the uninduced control, the induced recombinants all have an expression band with an expected molecular weight of...
Embodiment 2
[0068] PEGylation parameter optimization experiment of embodiment 2GLP-2
[0069] According to relevant literature (such as J.Pharm.Res.1996 such as Kinstler, 13:996), the factor that influences the specificity reaction of PEG reagent to protein comprises the molar ratio of PEG reagent and protein, reaction time, reaction temperature, pH value, Molar concentration of protein, etc. Based on the literature, the relevant parameters were optimized:
[0070] 1) Effect of GLP-2 and mPEG-ALD20000 molar ratio and reaction time
[0071] Reaction mixture composition: 0.1M sodium acetate buffer (pH5.0), GLP-2 concentration 2mg / ml, 20mM catalyst sodium borohydride (NaBH3CN, Sigma), molar concentration ratio 1:10, 1:5, 1 : 2, 1: 1 (GLP-2: mPEG-ALD20000) was added with a modifier, and the mPEG2-ALD (mPEG2-propionaldehyde) reagent was purchased from Beijing Jiankai Technology Co., Ltd.
[0072] Gently shake the reaction at 25°C, and take samples at 1, 2, 5, 10, 15, and 20 hours. The reac...
Embodiment 3
[0083] The PEGylation of embodiment 3GLP-2 and the purification of product thereof
[0084] The GLP-2 of the present invention may be unmodified human glucagon-like peptide-2, or the amino acid sequence of the glucagon-like peptide-2 shown in SEQ ID NO.1, the glucagon-like peptide Alanine-2 of -2 was replaced by glycine. Meanwhile, the GLP-2 can be commercially available, or can be prepared by the method of Example 1.
[0085] The GLP-2 freeze-dried sample prepared in Example 1 was dissolved with 0.2M sodium acetate buffer solution with pH 5.0 to a final concentration of 2 mg / ml. The modifier was added at a molar concentration ratio of 1:2 (GLP-2:mPEG-ALD20000). The mPEG2-ALD (mPEG2-propionaldehyde) reagent was purchased from Beijing Jiankai Technology Co., Ltd. The catalyst sodium borohydride (NaBH3CN, Sigma) was added to a final concentration of 20 mM, stirred and dissolved, and reacted at 25° C. for 5 hours. The reaction was terminated by adjusting the pH to 2.0 with 2M ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com