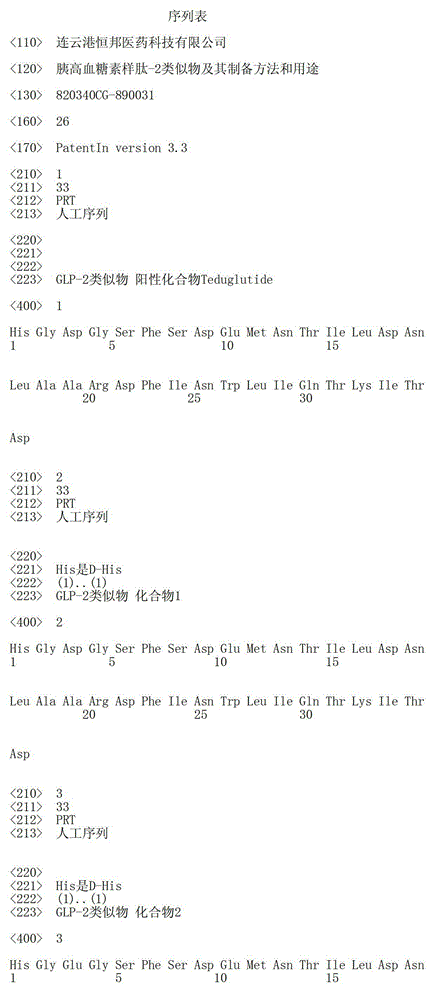Glucagon-like peptide-2 analogue, its preparation method and application
A technique for glucagon and analogs, applied in the field of glucagon-like peptide-2 analogs and their preparation and use, can solve the problems of inconclusive GLP-2 intestinal tumor and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
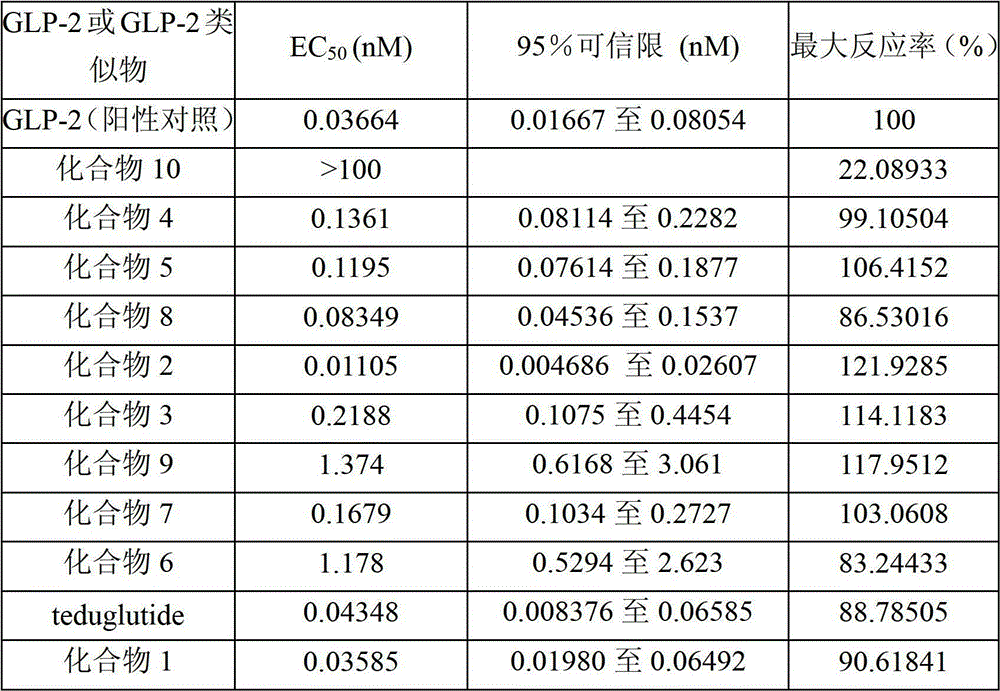
[0054] Example 1 Solid-phase synthesis of positive compound Teduglutide
[0055] The sequence of the positive compound Teduglutide is:
[0056] H-His-Gly-Asp-Gly-Ser-Phe-Ser-Asp-Glu-Met-Asn-Thr-Ile-Leu-Asp-Asn-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn- Trp-Leu-Ile-Gln-Thr-Lys-Ile-Thr-Asp-OH
[0057] (1) Drying and swelling of solid-phase synthetic resin
[0058] Weigh 40g (20mmol) of Fmoc-Asp(OtBu)-Wang resin (0.5mmol / g) dried in vacuum for 24 hours and place it in a 2L bubbling bottle, add 400mL of DMF swelling resin for 30 minutes, and remove the DMF solution;
[0059] (2) Fmoc-Asp(OtBu)-Wang resin to remove Fmoc protecting group
[0060] 200 mL of 20% piperidine / DMF solution was added to the bubbling bottle containing Fmoc-Asp(OtBu)-Wang resin, and the solution was drawn out after 5 minutes of reaction, and then 200 mL of 20% piperidine / DMF solution was added to react at room temperature for 20 minutes. After the reaction was completed, the resin was washed four times with 200 mL...
Embodiment 2
[0072] Example 2 Solid-phase synthesis of compound 1
[0073] The sequence of compound 1 is:
[0074] D-His-Gly-Asp-Gly-Ser-Phe-Ser-Asp-Glu-Met-Asn-Thr-Ile-Leu-Asp-Asn-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn- Trp-Leu-Ile-Gln-Thr-Lys-Ile-Thr-Asp-OH, that is, in the general formula (I), X1 is D-His, X2 is Gly, X3 is ASP, X10 is Met, and X11 is Asn , X15 is Asp, X16 is Asn, X24 is Asn, and X25 is Trp.
[0075] The preparation method is the same as in Example 1.
Embodiment 3
[0076] Example 3 Solid-phase synthesis of compound 2
[0077] The sequence of compound 2 is:
[0078]D-His-Gly-Glu-Gly-Ser-Phe-Ser-Asp-Glu-Met-Asn-Thr-Ile-Leu-Asp-Asn-Leu-Ala-Ala-Arg-Asp-Phe-Ile-Asn- Trp-Leu-Ile-Gln-Thr-Lys-Ile-Thr-Asp-OH, that is, in the general formula (I), X1 is D-His, X2 is Gly, X3 is Glu, X10 is Met, and X11 is Asn , X15 is Asp, X16 is Asn, X24 is Asn, and X25 is Trp.
[0079] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com