Production of glucagon like peptide 2 and analogs
a technology of glucagon and peptide, which is applied in the field of protein production, can solve the problems of limited product yield and quality in the prior art production system, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Gene Construction
[0043]To construct multimers of [Gly2]hGLP-2 gene, a [Gly2]hGLP-2 gene, as shown below, was first amplified by PCR using a plasmid, pG3M, which carried a codon optimized [Gly2]hGLP-2 gene and was re-named as pEW3G.
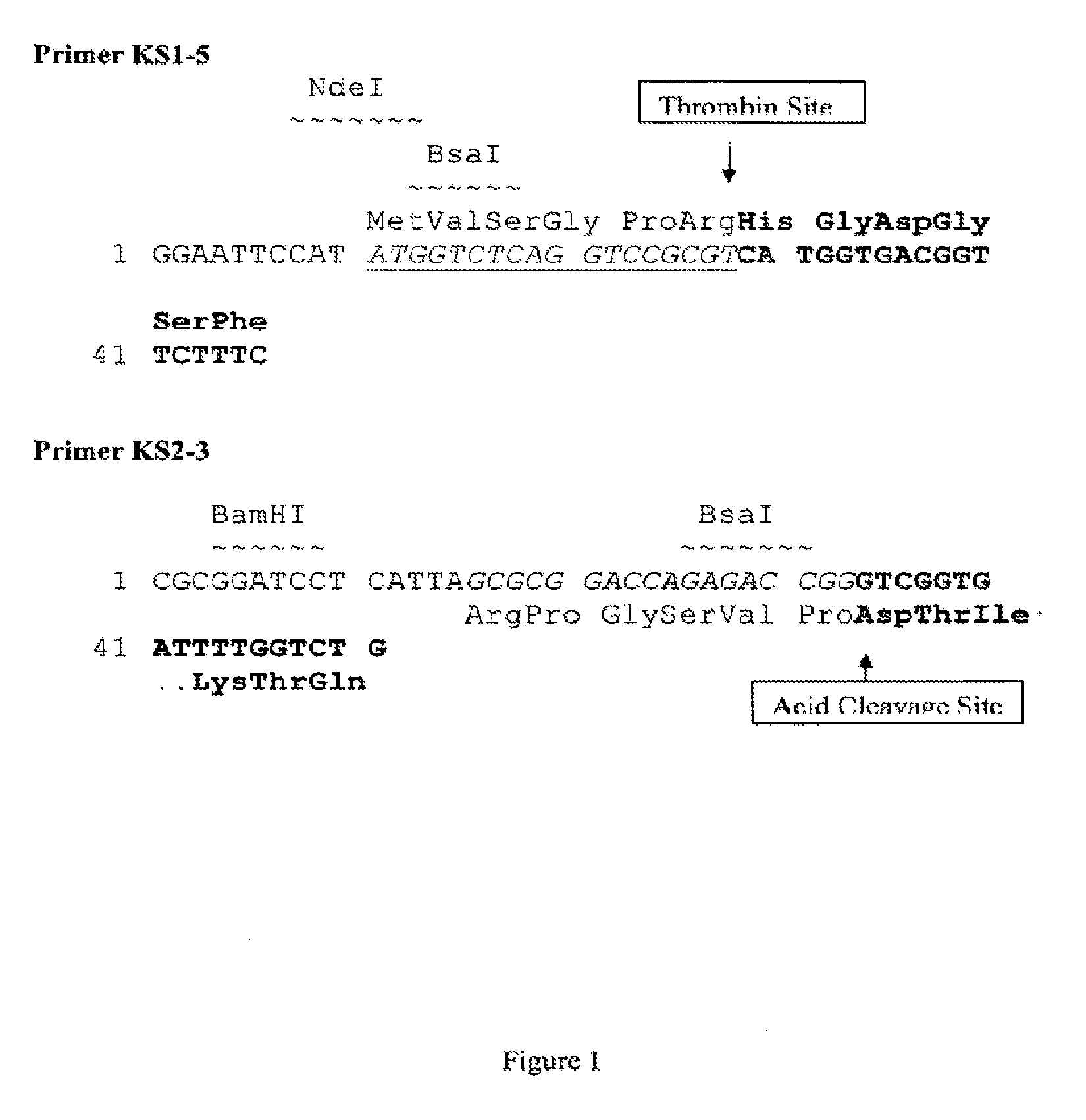
[0044]As shown in FIG. 1, the forward PCR primer sequence (Primer KS1-5) contained NdeI and Bsal endonuclease recognition sites, and thrombin cleavage site, which are followed by 18 nucleotides encoding the first six amino acids of [Gly2]hGLP-2.
[0045]The reverse PCR primer (Primer KS2-3) contained BamHI and Bsal endonuclease recognition sites, acid cleavage site, and an 18 nucleotide sequence, which encode the last six amino acid residues of [Gly2]hGLP-2.
[0046]For PCR reaction, the lower PCR reaction mixture was first prepared in a PCR tube. The lower mixture contained 41 μL of water, 5 of 10×TsgPlus buffer, 2 μL of deoxynucleotide mixture (2.5 mM each), 1 μL of primer KS 1-5 (100 pM), and 1 μL of primer KS2-3 (100 pM). To the lower mixture, a piece of Amp...
example 2
Vector Construction
[0057]To construct multimers of [Gly2]hGLP-2 gene, pKS35 was digested with Bsal at 50° C. for 2.5 hour and separated on 1.5% agarose gel. The larger DNA band (the vector portion) was cut out of the gel and DNA was extracted from the gel piece using QIA quick gel extraction kit. The smaller DNA band (the insert, approximately 110 bp) was cut out of the gel and the DNA was extracted using QIA ExII. The large vector portion was further treated with calf intestine alkaline phosphatase (CIP) to minimize self-ligation of the vector and purified by QIAPCR purification kit.
[0058]The CIP-treated vector DNA and the smaller insert DNA were mixed and ligated using Quick T4 ligase. The ligation mixture was used to transform DH5α, as described above, and then the bacteria cells were plated 2×Yeast Extract (2×YE) agar plates containing kanamycin (30 μg / mL).
[0059]To examine the number of [Gly2]hGLP-2 gene units present on plasmid in each transformant, the inserts were directly am...
example 3
Transformation and Culturing
[0065]The [Gly2]hGLP-2 multimer constructs were cloned into a plasmid pET29a in such a way that they were expressed under the control of phage T7 promoter. E. coli RNA polymerase cannot recognize the T7 promoter. T7 RNA polymerase is required for the transcription from T7 promoter. E. coli strain, BLR(DE3), carries a phage T7 RNA polymerase gene on its chromosome. Moreover, recA gene in BLR(DE3) is inactivated so that the chance of losing [Gly2]hGLP-2 gene units in the multimer constructs by homologous recombination is minimal in this strain. Both DH5α. and BLR(DE3) strains are available commercially, as is the T7 system used herein.
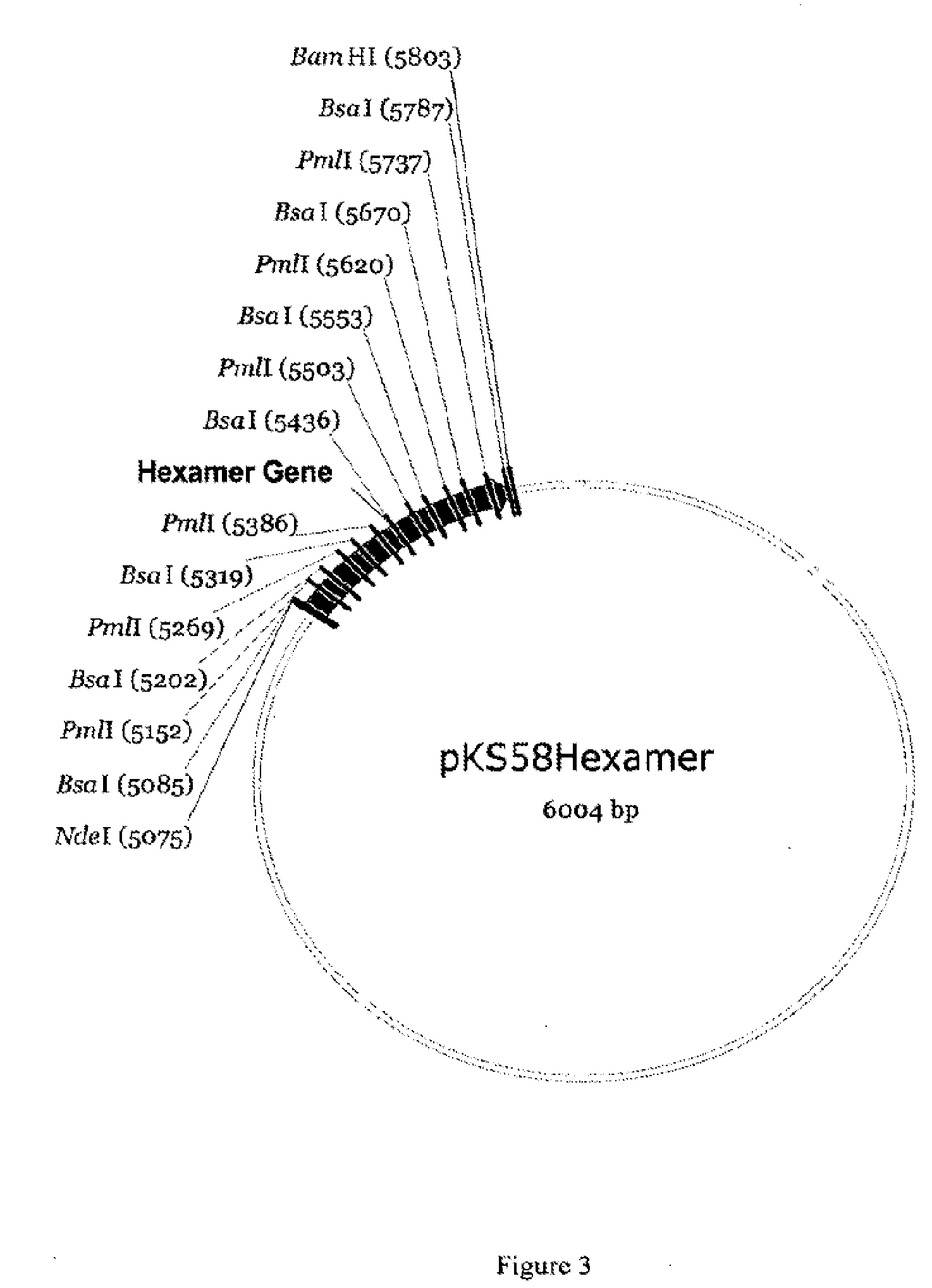
[0066]The pET29a carrying a hexamer construct of [Gly2]hGLP-2 was designated as pKS58 and isolated from the transformant cells using Qiagen Plasmid Midi Prep kit. The frozen competent cells of BLR(DE3) (20 μL) were thawed, mixed with 1 μL of pKS58, kept on ice for 5 minutes, heat-shocked at 42° C. for 30 sec, and then kept on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com