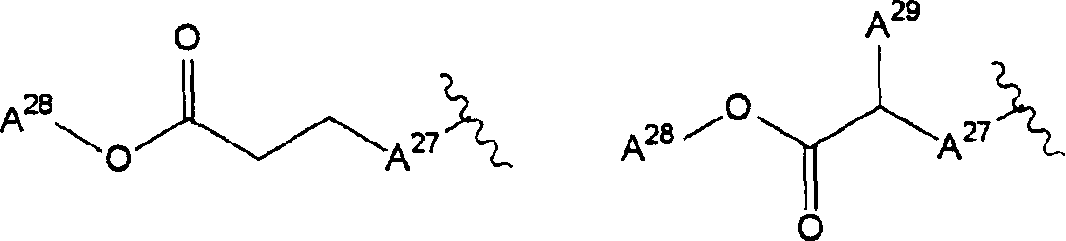Combination drug
A technology of pharmaceutical preparation and action, applied in the directions of pharmaceutical combination, pharmaceutical formulation, active ingredient of heterocyclic compounds, etc., can solve the problem of no report describing the concentration of GLP'2, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
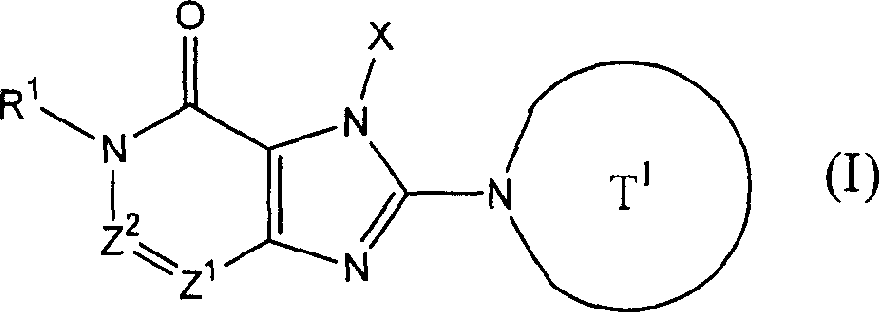
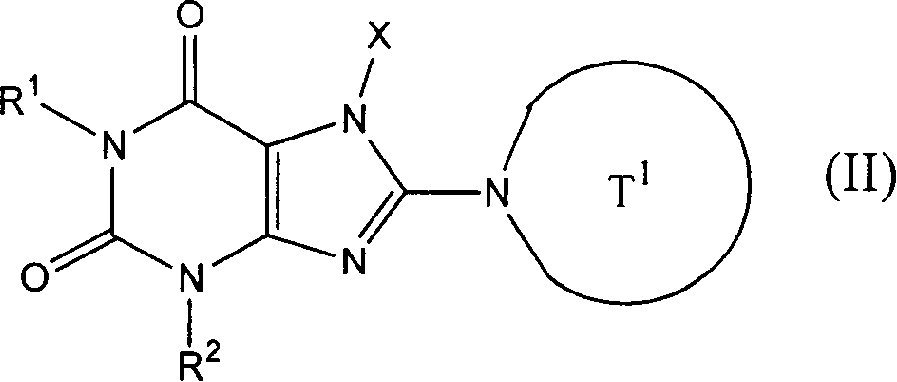
[0883] 4-[1-(2-Butynyl)-6-methyl-7-oxo-6,7-dihydro-1H-imidazo[4,5-d]pyridazin-2-yl]piperazine -1-tert-butyl carboxylate
[0884] (a) tert-butyl 5-methyl-4-oxo-4,5-dihydroimidazo[4,5-d]pyridazine-1-carboxylate
[0885] Will contain 1.0g of 5-methyl-3,5-dihydroimidazo[4,5-d]pyridazin-4-one, 16mg of 4-dimethylaminopyridine, 1.6g of di-tert-butyl dicarbonate A mixture of the ester and 5 ml of tetrahydrofuran was stirred overnight at room temperature. Then, a 0.5-ml tetrahydrofuran solution containing 300 mg of di-tert-butyl dicarbonate was added to the solution, and the resulting mixture was incubated in a room
[0886] Stir at warm temperature for 3 hours. 5 ml of t-butylmethyl ether was added to the reaction mixture, and the mixture was ice-cooled. The resulting crystals were collected by filtration to obtain 1.63 g of the title compound.
[0887] 1 H-NMR (CDCl 3 )δ1.72 (s, 9H) 3.93 (s, 3H) 8.38 (s, 1H) 8.54 (s, 1H)
[0888] (b) 2-Chloro-5-methyl-1,5-dihydroimidazo[4...
preparation Embodiment 2
[0898] tert-butyl 4-[7-(2-butynyl)-2,6-dichloro-7H-purin-8-yl]piperazine-1-carboxylate (a) 7-(2-butynyl)-3-methyl-3,7-dihydropurine-2,6-dione
[0899] Add 55.3ml of 1-bromo-2-butyne and 84.9g of anhydrous potassium carbonate to 100g of 3-methylxanthine [CAS No. 1076-22-8] and 1000ml of N,N-dimethyl in a mixture of formamides. The resulting mixture was stirred at room temperature for 18 hours. 1000 ml of water was added to the reaction solution, and the mixture was stirred at room temperature for 1 hr. The resulting white precipitate was collected by filtration. The white solid was washed with water and then tert-butyl methyl ether. Thus 112 g of the title compound are obtained.
[0900] 1 H-NMR (DMSO-d6) δ1.82 (t, J = 2.2Hz, 3H) 3.34 (s, 3H) 5.06 (q, J = 2.2Hz, 2H) 8.12 (s, 1H) 11.16 (br.s, 1H)
[0901] (b) 7-(2-butynyl)-8-chloro-3-methyl-3,7-dihydropurine-2,6-dione
[0902] 112g of 7-(2-butynyl)-3-methyl-3,7-dihydropurine-2,6-dione was dissolved in 2200ml of N,N...
Embodiment 1
[0912] [7-(2-Chlorophenyl)-1-methyl-6-oxo-8-(piperazin-1-yl)-6,7-dihydro-1H-purin-2-yloxy] Ethyl acetate trifluoroacetate (trifluoroacetate)
[0913] (a) [7-Benzyl-2,6-dioxo-1,2,6,7-tetrahydropurin-3-yl]methyl 2,2-dimethylpropionate
[0914] 8.66 g of 7-benzylxanthine was dissolved in 300 ml of N,N-dimethylformamide, and 1.57 g of sodium hydride and 7.7 ml of chloromethyl pivalate were added thereto. The resulting mixture was stirred overnight at room temperature. The reaction solution was diluted with ethyl acetate, and washed with water and 1N hydrochloric acid. The organic layer was dried over anhydrous magnesium sulfate, and then filtered. The solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography. Thus, 2.66 g of the title compound were obtained from the fraction eluted with hexane-ethyl acetate (1:1).
[0915] 1 H-NMR (CDCl 3 ) δ 1.18 (s, 9H) 5.45 (s, 2H) 6.06 (s, 2H) 7.34-7.39 (m, 5H) 7.58 (s, 1H) 8.18 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com