Glucagon-like peptide-2 (GLP-2) analog, and preparation method and use thereof
A technology of GLP-2 and analogues, which is applied in the field of analogues of glucagon-like peptide-2 (GLP-2) and its preparation and application, and can solve the problem of limiting the use of GLP-2 and the low stability of endogenous DPPIV To achieve the effect of improving tolerance, improving chemical stability, and reducing the possibility of being degraded
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Example 1 GLP-2 Analogs
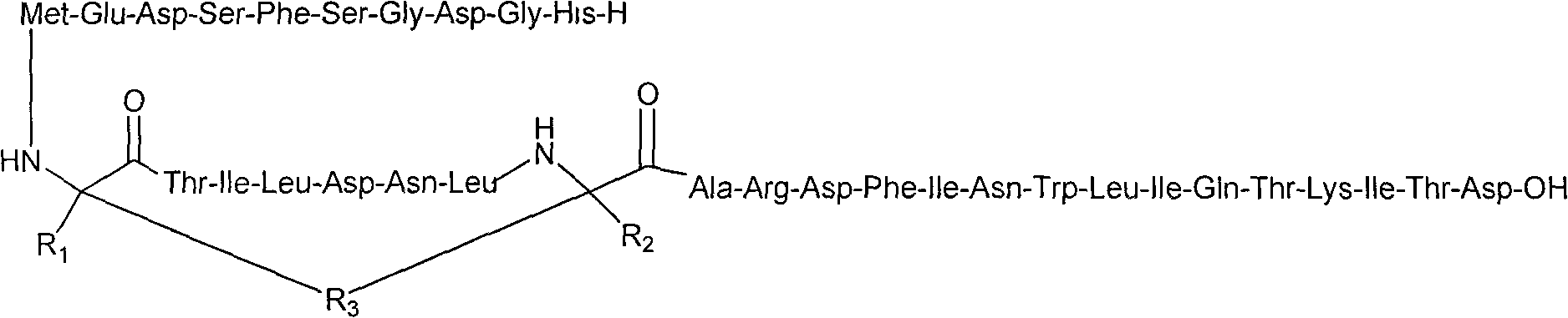
[0041] According to the conventional method of solid-phase synthesis, the amino acids with protective groups are attached to the resin one by one, and then the catalyst is used to catalyze the ring closure, and then the protective group is removed and the polypeptide is cut off from the resin to prepare the preferred GLP-2 similar to the following formula: thing:
[0042]
[0043] 1. Solid phase synthesis
[0044] Select 0.45g Fmoc-Asp(OtBu)-Wang resin (substitution degree: 0.33mmol / g) to prepare, soak the resin with DMF at room temperature for 60min, and arrange the amino acids with Fmoc protection in the synthesizer in order for activation connection , get as figure 1 The product of compound 2 in, the structure is:
[0045]
[0046] 2. Catalytic cyclization
[0047] The compound obtained in the above steps was washed twice with DCM (20ml) and DCE (20ml) respectively, then soaked with DCE (8ml) for 30min, and then Grubbs...
Embodiment 2
[0052] Example 2 Determination of resistance to dipeptidyl peptidase IV (DPP-IV)
[0053] To detect the DPP-IV resistance of rGLP-2 and the GLP-2 analog of Example 1, 2.5 μl of recombinant human DPP-IV (purchased from ProSpec) containing 0.125 mU was dissolved in 20 mM Tris-HCl buffer (pH 8) , 100mM NaCl, 1mM EDTA, 10% glycerin solution, add 50ul PBS solution (pH7.4) containing 0.2mg / ml peptide to be tested. Incubate in a circulating water bath at 37° C. for 24 hours, and add 50 ul of a PBS solution containing 4 mg / ml diprotinA (purchased from BIOPRC) to quench the incubation. Each sample was analyzed by reversed-phase (PR) HPLC: 90 ul of the quenched incubation mixture was injected into a Dynamax (available from Rainin) 300A, C5 (5 micron, 4.6*250 mm) column, and the sample was washed with acetonitrile containing 0.1% TFA Elution was performed with a linear gradient of modified water at a flow rate of 1 ml / min, the sample components were detected at 214 nm, and the degree of...
Embodiment 3
[0054] Example 3 Pharmacodynamic evaluation of GLP-2 analogues
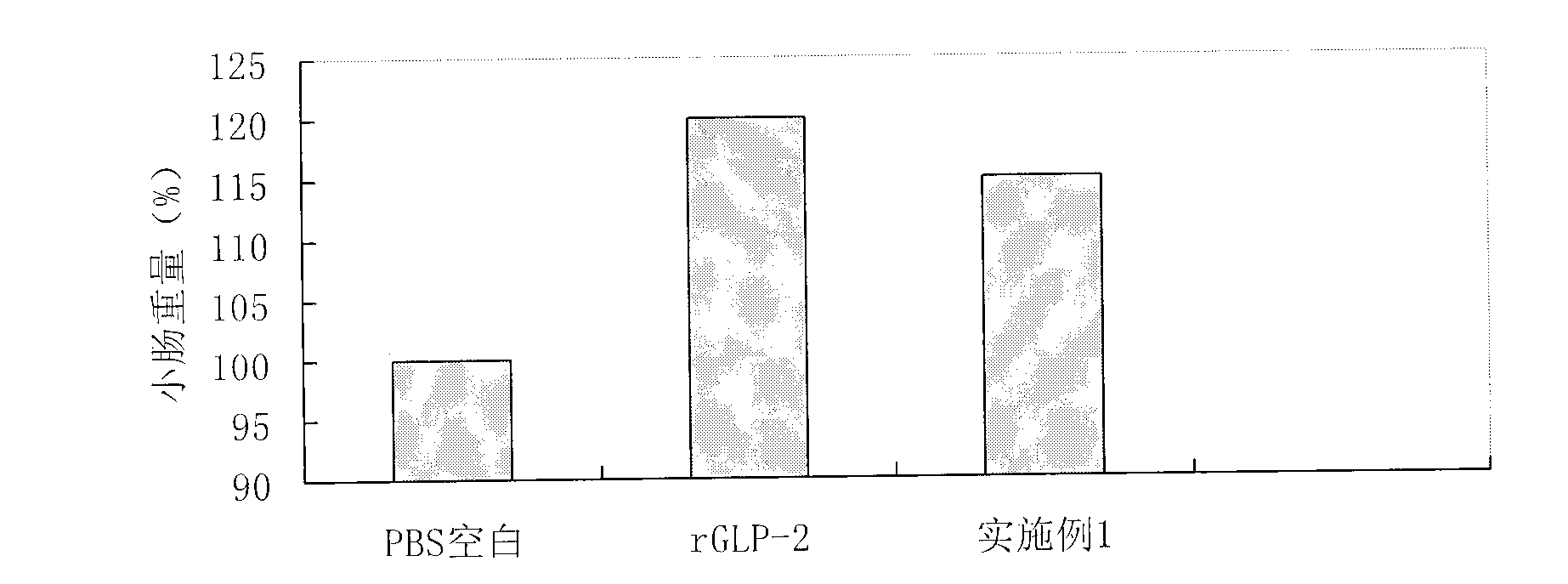
[0055] Select normal male SD rats (200+-20g), and randomly divide them into 3 groups, 6 rats / group, respectively PBS control group, rGLP-2 group, and Example 1 GLP-2 analog group. According to the administration of 0.5mg / kg / day, the abdomen was subcutaneously injected for 10 days, then sacrificed, the small intestine was taken, washed with normal saline, and weighed. like figure 2 The results showed that, compared with the PBS group, both the rGLP-2 group and the GLP-2 analogue group of Example 1 could significantly promote intestinal growth.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com