GLP-2 compounds, formulations, and uses thereof
A technology of GLP-2 and D33E-GLP-2, which is applied in the field of new human glucagon-like peptide-2 peptide and its derivatives, can solve the problems of high clearance rate and limited use, and achieve the purpose of reducing intestinal permeability, inhibiting Effects of gastric emptying and acid secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
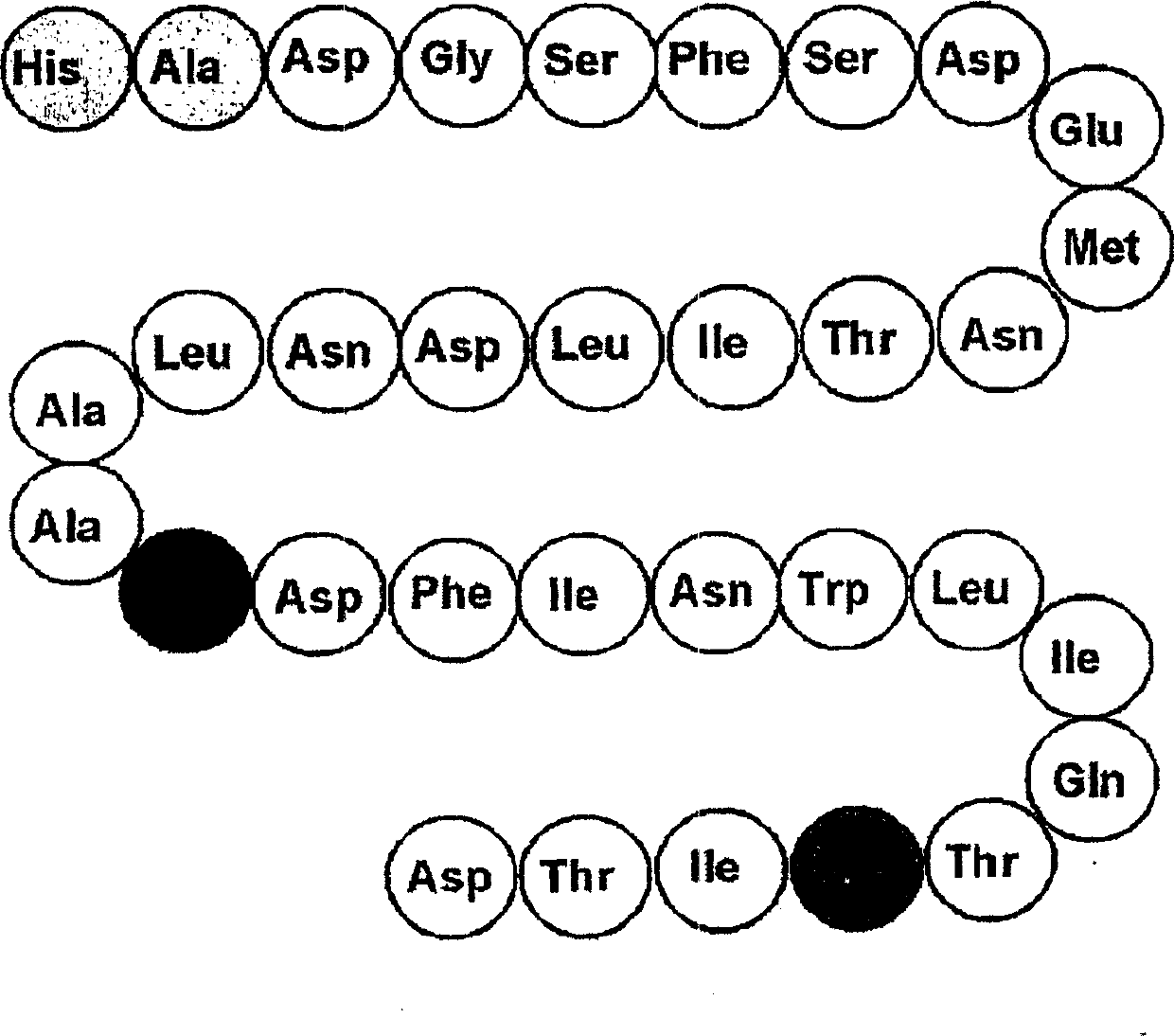
[0702] 1. GLP-2 peptide, which contains the amino acid sequence of formula I:
[0703] His-X2-X3-Gly-X5-Phe-X7-X8-X9-X10-X11-X12-X13-X14-X15-X16-X17-X18-Ala-Arg-X21-Phe-Ile-X24-Trp- Leu-Ile-X28-Thr-Arg-Ile-Thr-X33 (Formula I)
[0704] Or a fragment thereof; wherein X2 is Ala, Val or Gly; X3 is Asp or Glu; X5 is Ser or Lys; X7 is Ser or Lys; X8 is Asp, Glu or Lys; X9 is Asp, Glu or Lys; X10 is Met , Lys, Leu, Ile or Norleucine; X11 is Asn or Lys; X12 is Thr or Lys; X13 is Ile or Lys; X14 is Leu or Lys; X15 is Asp or Lys; X16 is Asn or Lys; X17 is Leu or Lys; X18 is Ala or Lys; X21 is Asp or Lys; X24 is Asn or Lys; X28 is Gln or Lys; X33 is Asp, Glu or Lys.
[0705] 2. The GLP-2 peptide according to embodiment 1, consisting of the following amino acid sequence
[0706]His-X2-X3-Gly-X5-Phe-X7-X8-X9-X10-X11-X12-X13-X14-X15-X16-X17-X18-Ala-X20-X21-Phe-Ile-X24-Trp- Leu-Ile-X28-Thr-Arg-Ile-Thr-X33
[0707] Or a fragment thereof; wherein X2 is Ala, Val or Gly; X3 is Asp or Glu; X5 is Ser ...
Embodiment 1
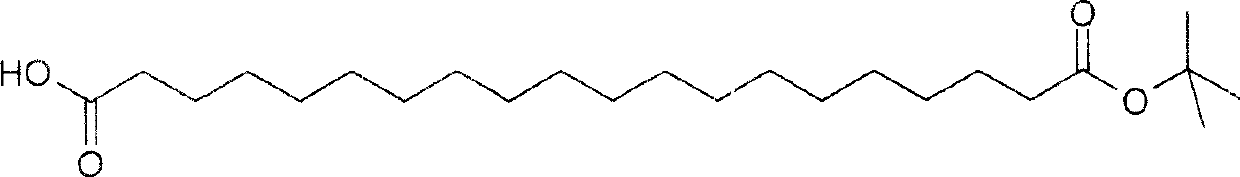
[1666] Preparation of derivatives of GLP-2 peptide analogs by peptide synthesis
[1667] The peptide bound to the resin with a fully protected group is acylated, and only the epsilon-amino group to be acylated on the peptide is deprotected. Use Fmoc chemistry to synthesize resin-bound peptides with appropriate protective groups, such as:
[1668] ↓Boc-[1-33, Lys(Dde)]-resin
[1669] ↓2% hydrazine / DMF treatment to remove Dde group
[1670] ↓Use Fmoc-Glu(γ-OH)-OBu t Acylation by HOAt / DIC / DIEA / NMP
[1671]↓ Piperidine treatment to remove Fmoc group
[1672] ↓Acylation by HOAt / DIC / DIEA / NMP with C16 acid
[1673] ↓Trifluoroacetic acid deprotection
[1674] ↓High performance liquid chromatography (HPLC) purification
[1675] ↓Freeze drying
[1676] ↓Analyze by liquid chromatography-mass spectrometry (LC-MS) and analytical high performance liquid chromatography
[1677] The length of the spacer and the fatty acid chain can be different. In the case of fixed acylation positions, three spa...
Embodiment 2
[1684] Synthesis of D3E / L17K((S)-4-carboxy-4-(hexadecanoylamino)butyryl) / K30R / D33E-GLP-2(1-33)
[1685] 2.a Synthesis of peptidyl resin with protective group
[1686] According to the Fmoc strategy, using Applied Biosystems' 0.25mmol scale 431A peptide synthesizer, using the FastMoc UV test protocol provided by the company, synthesizing a peptide-based resin with protective groups, that is, using HBTU(2-(1hydro-benzotriazole) -1-yl-1,1,3,3 tetramethylurea hexafluorophosphate) mediated coupling in NMP (N-methylpyrrolidone) and deprotection of Fmoc protecting group for UV detection. The synthetic raw material resin (400mg) is (4-((2′,4′-dimethoxyphenyl)-(Fmoc-Glu(OBut)-O-p-benzyloxybenzyl resin (Wang resin)) (Novabiochem, Bad Soden, Germany, product number: 04-12-2052), displacement capacity 0.53mmol / g.
[1687] The amino acid derivatives with protective groups used are Fmoc-Ala-OH, Fmoc-Arg(Pmc)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asp(OBut)-OH, Boc-His(Boc)- OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com