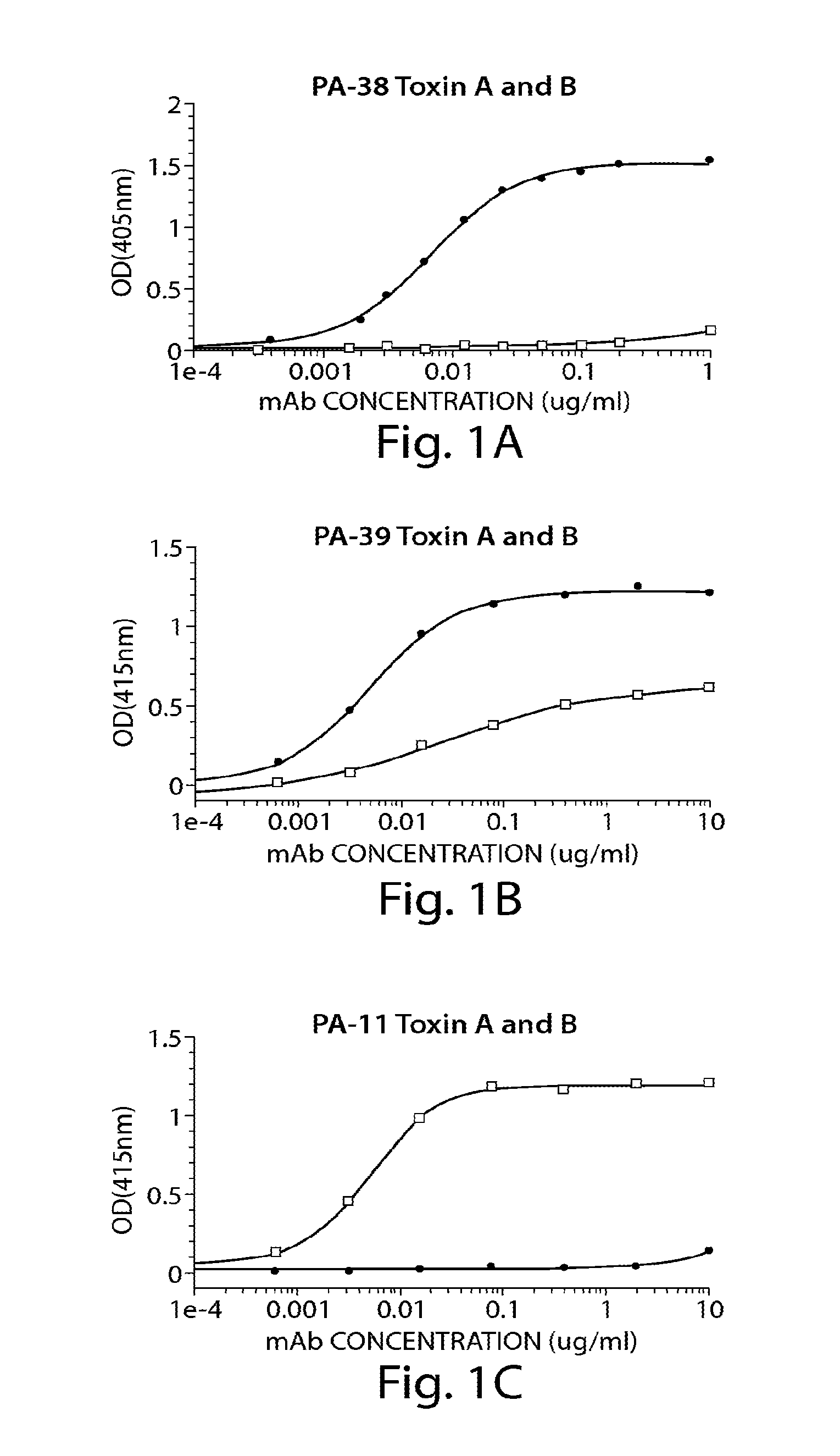
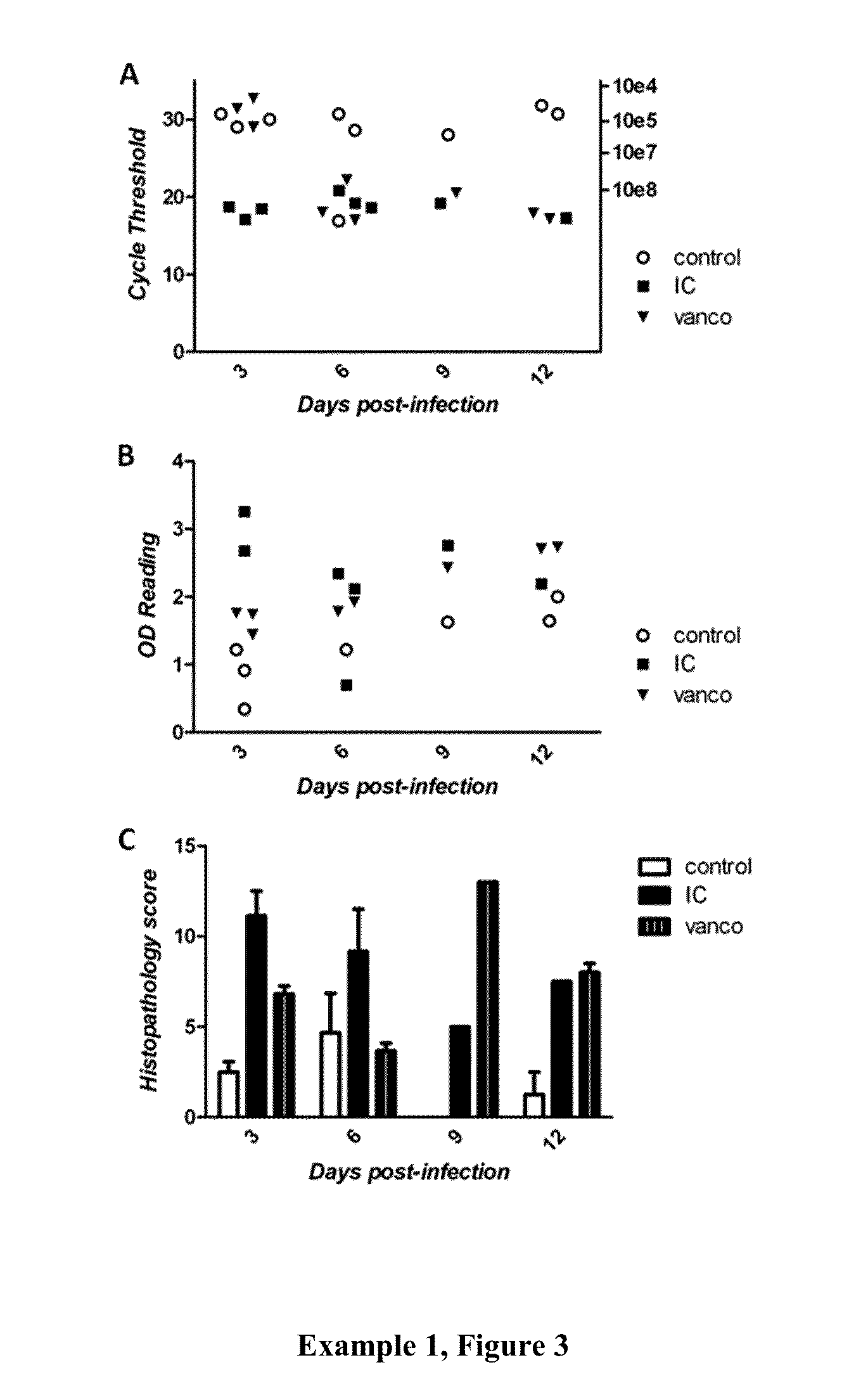
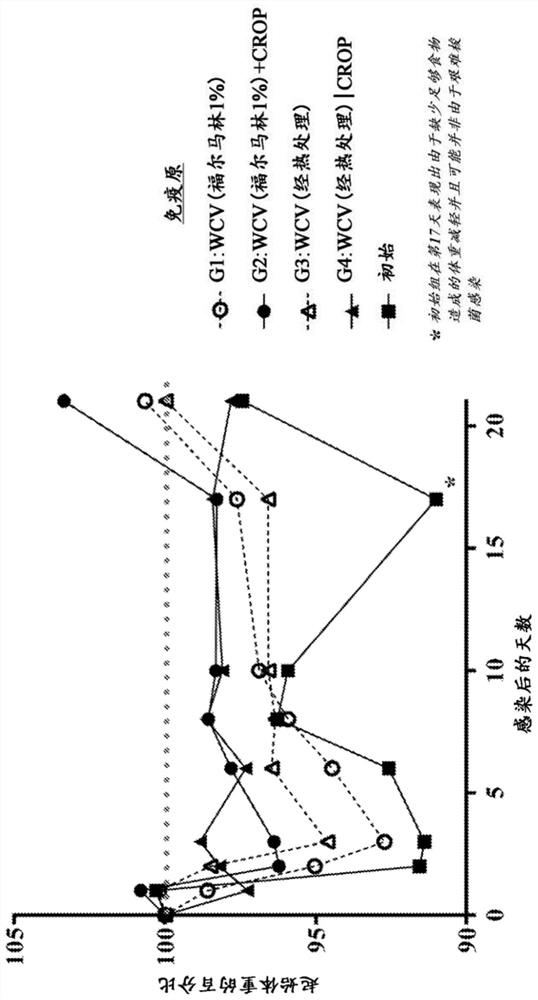
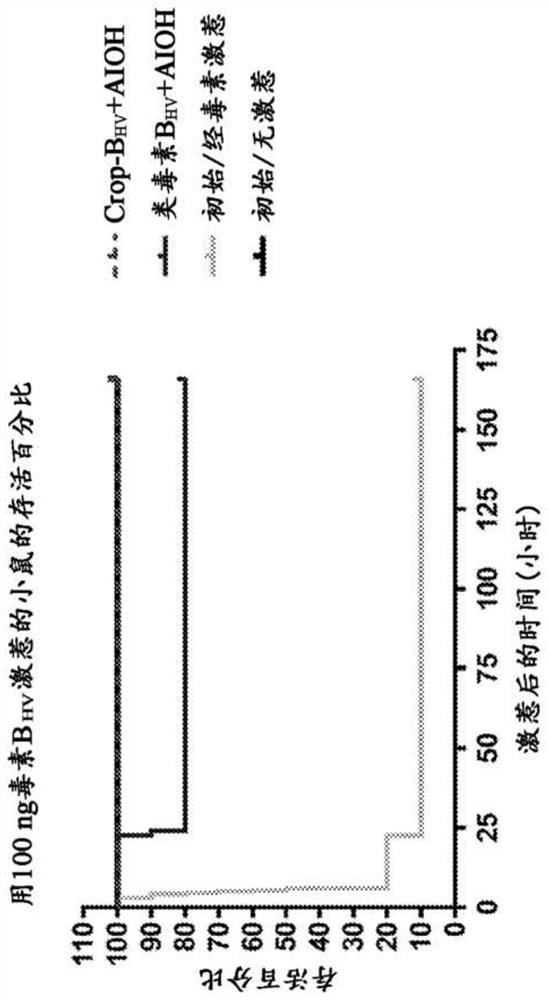
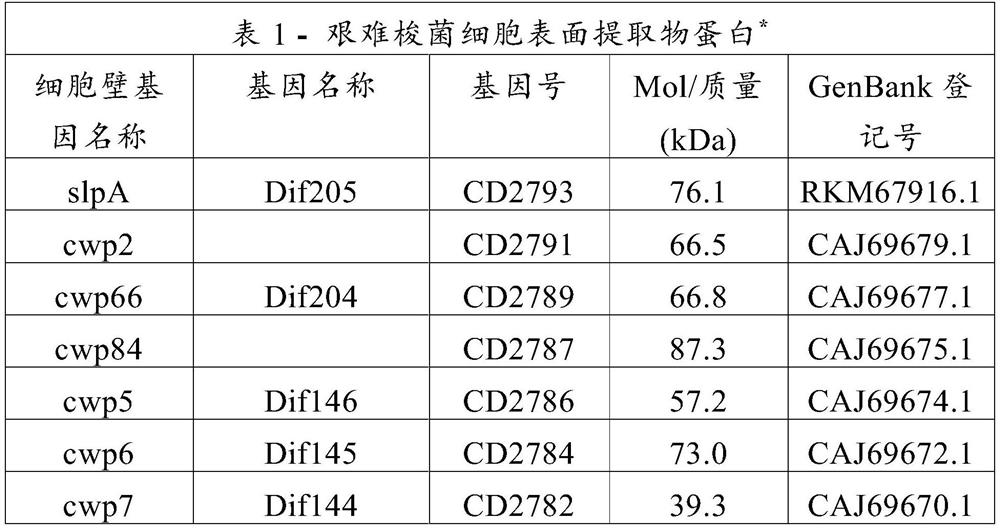
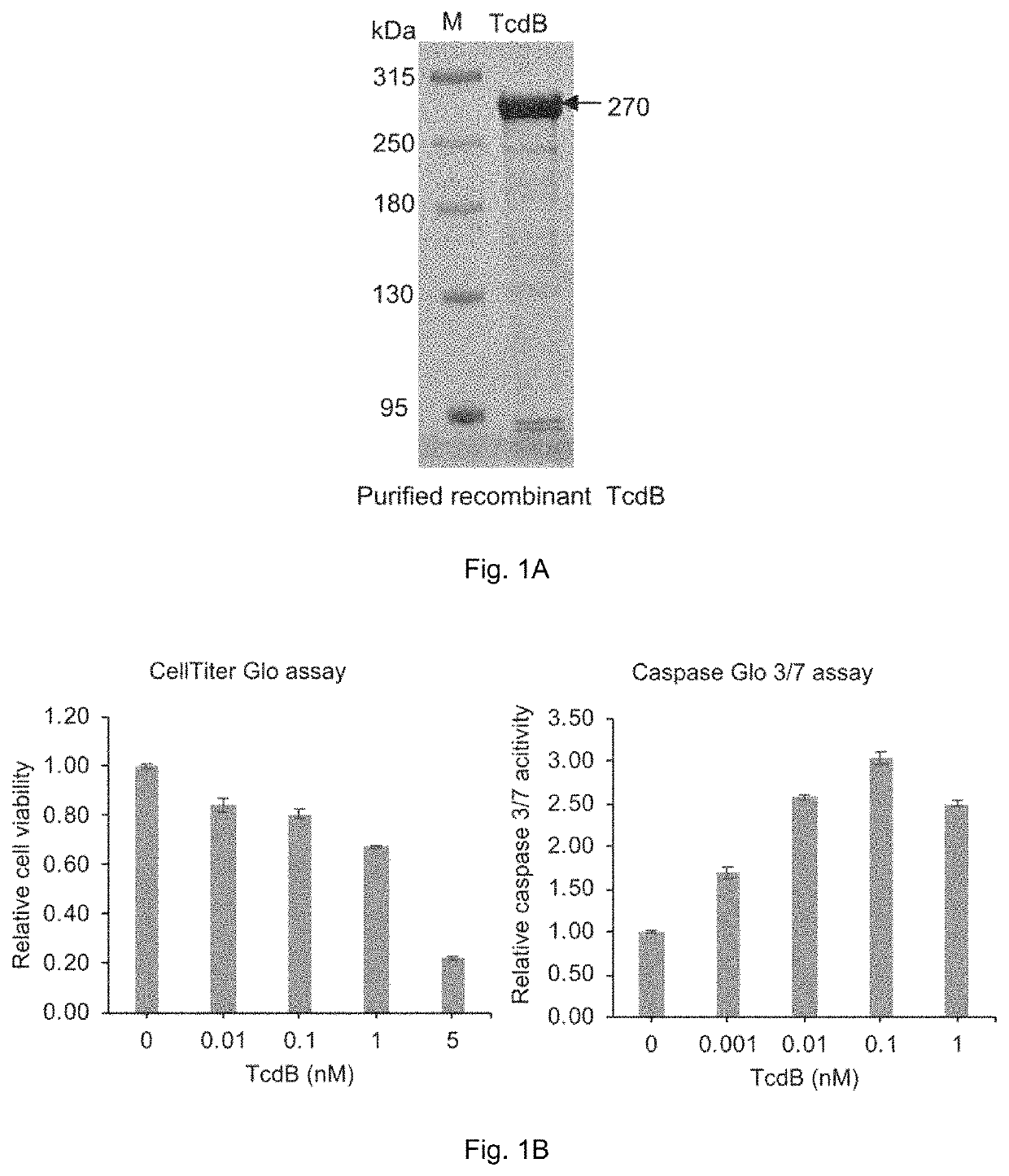
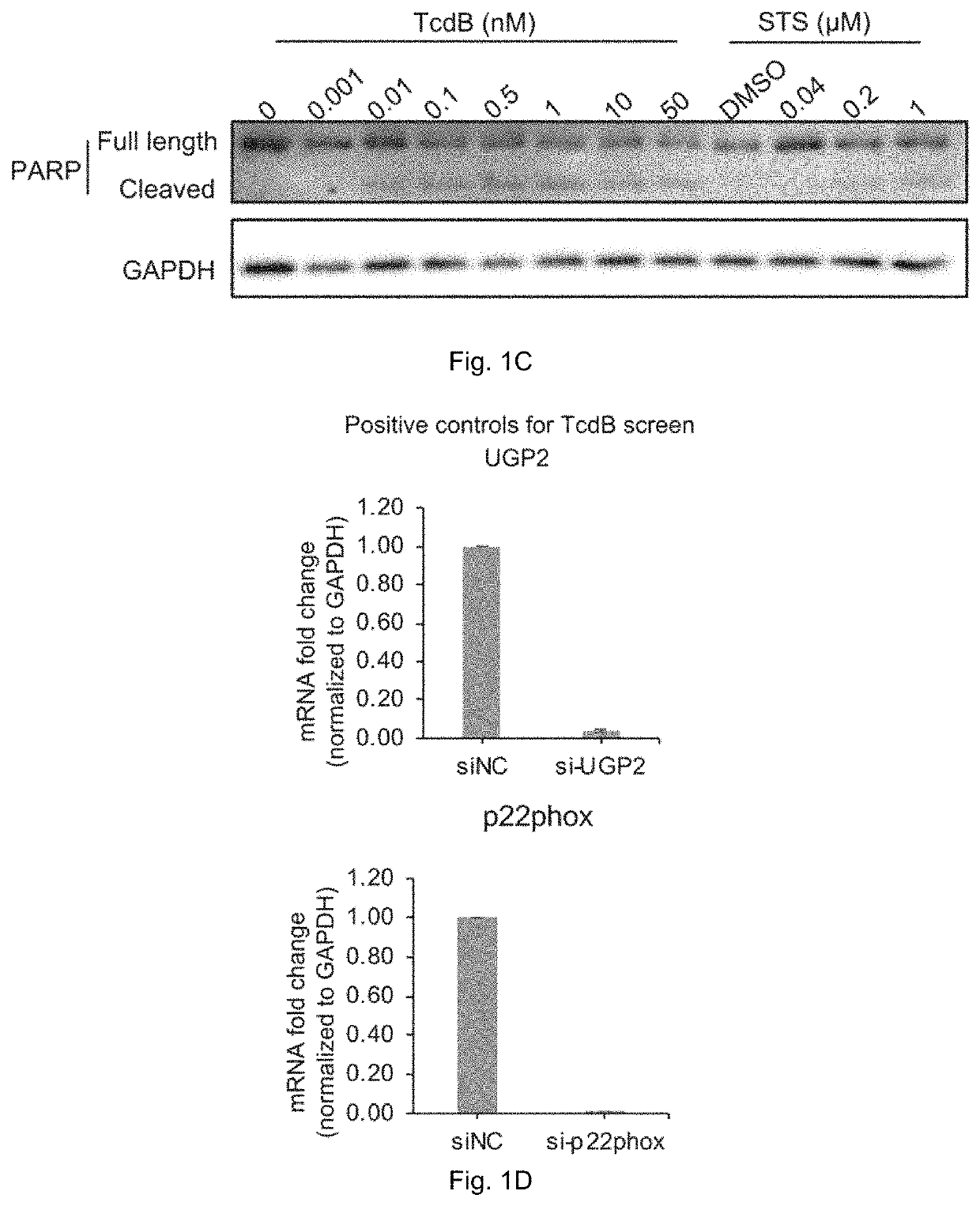
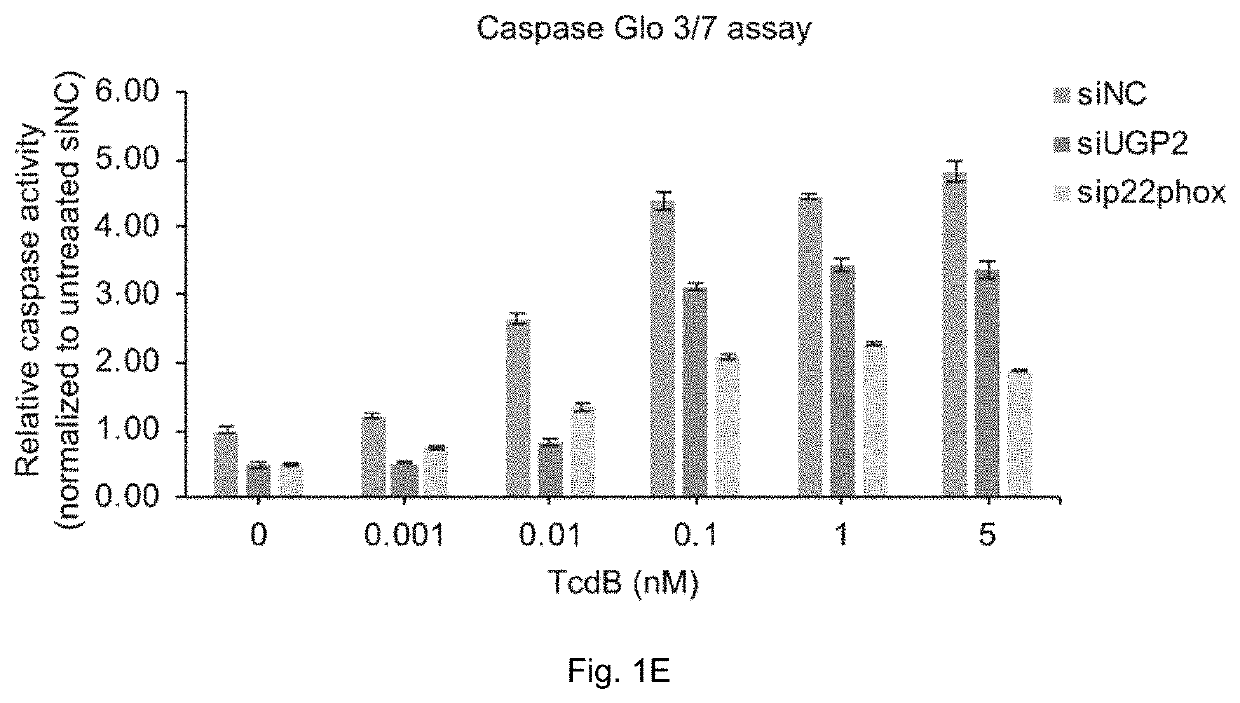
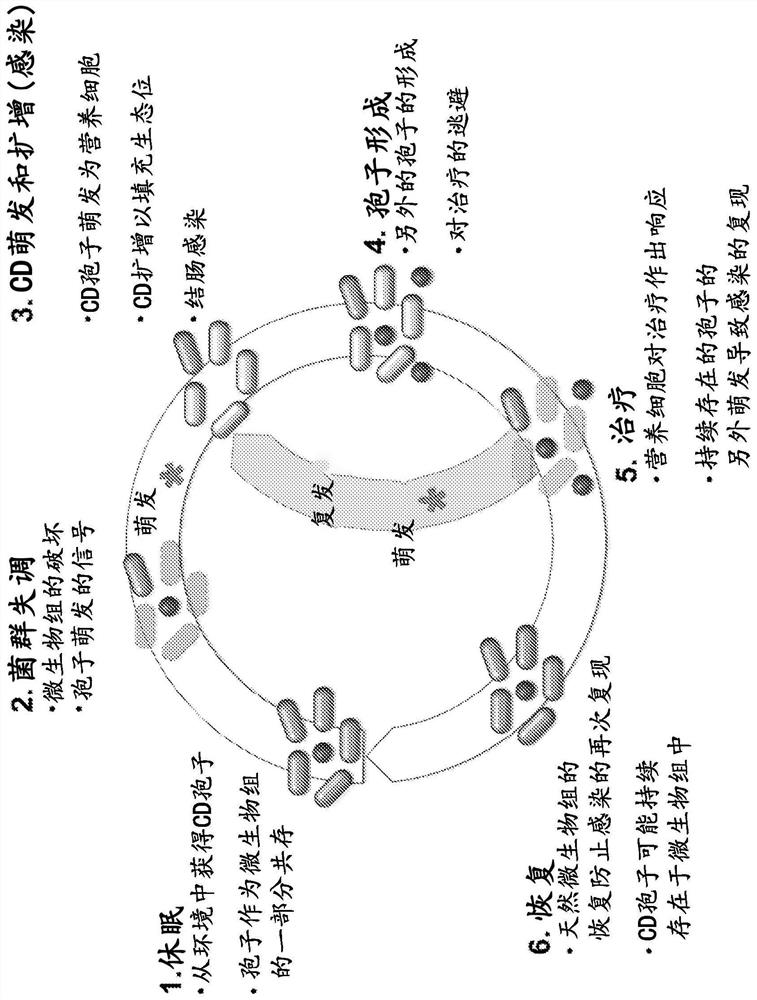
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Clostridium Infections" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
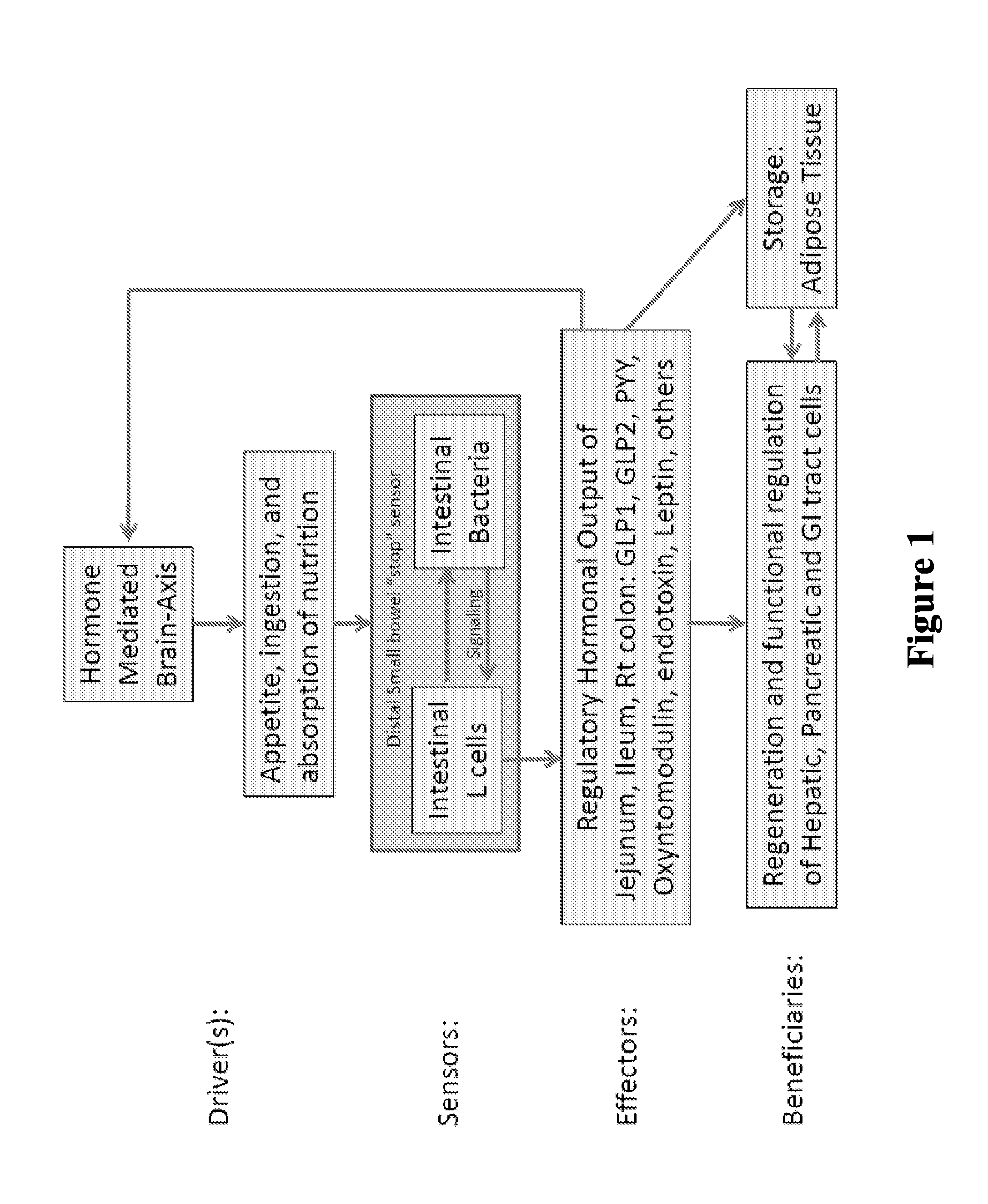
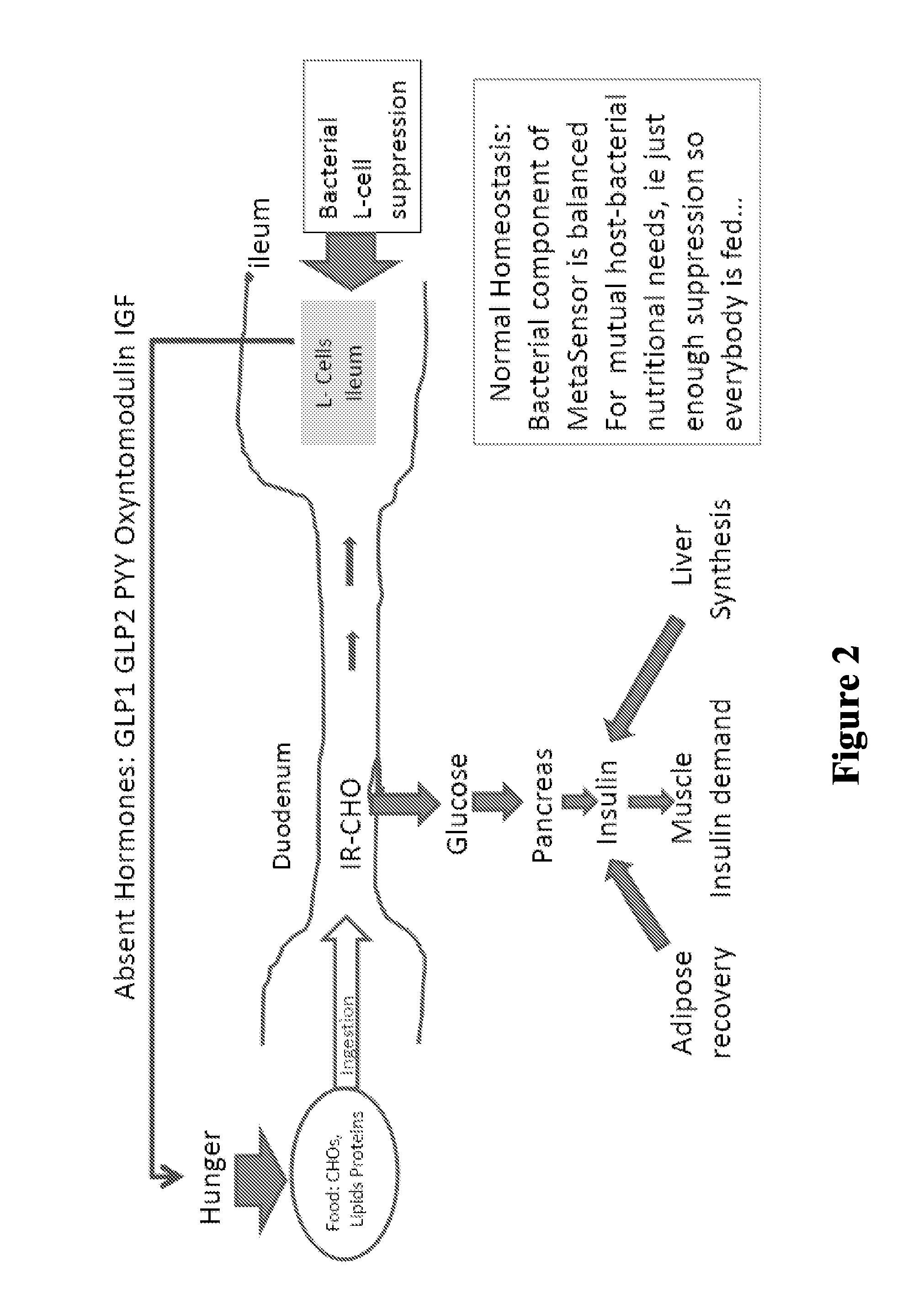
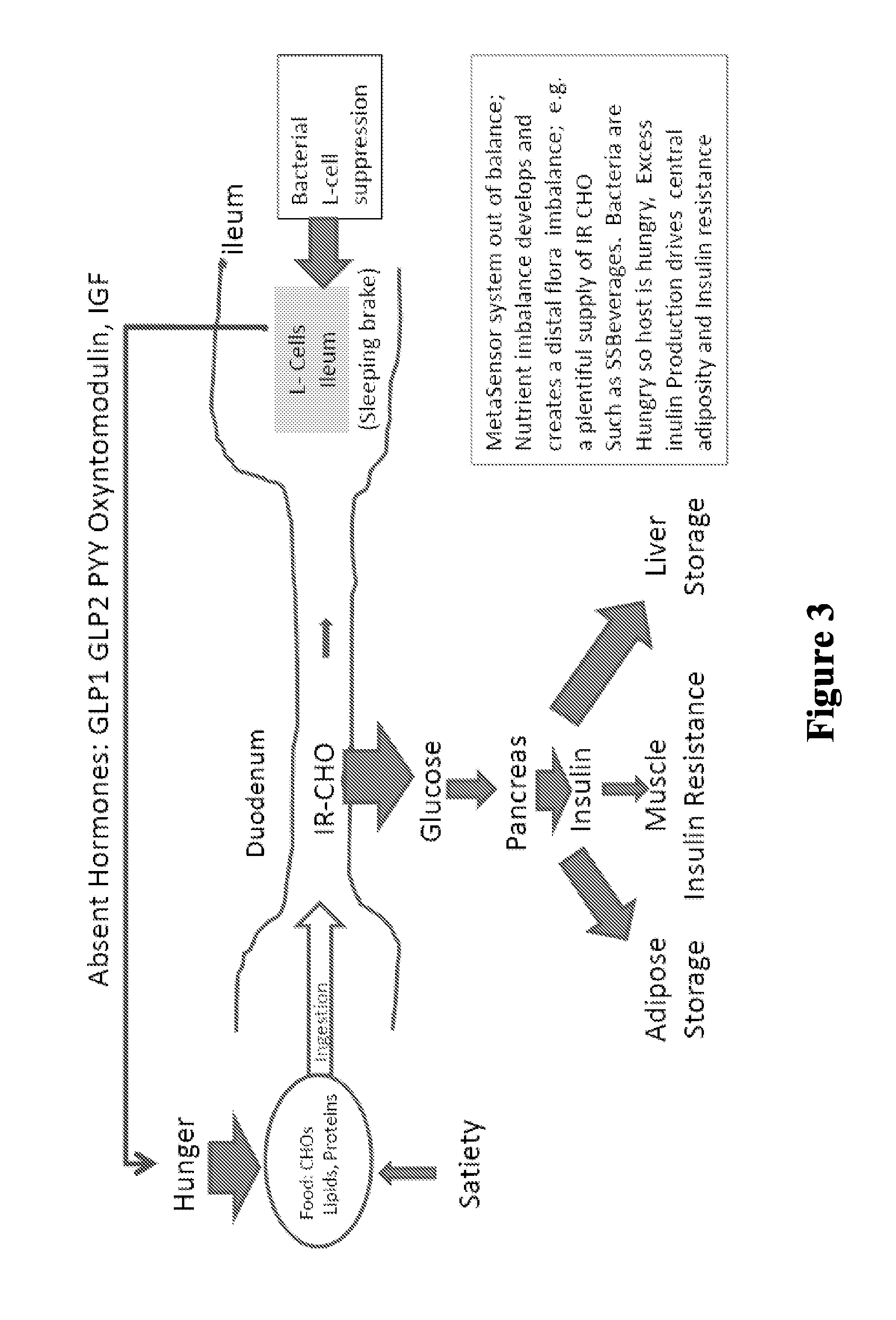
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
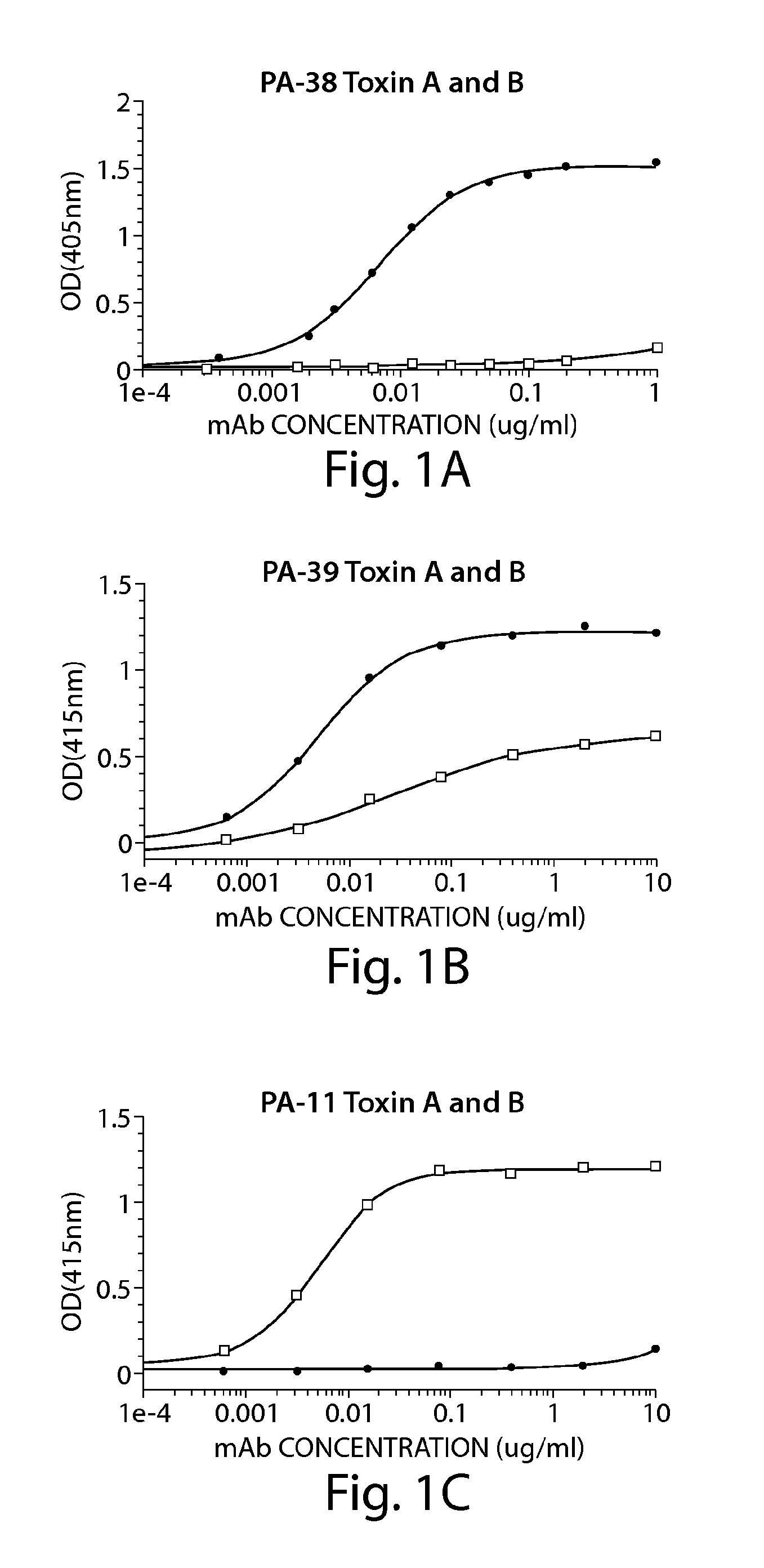
Antibodies for the treatment of Clostridium difficile-associated infection and disease
ActiveUS8986697B2Improve the quality of lifeImprove survivabilityAntibacterial agentsPeptide/protein ingredientsAntigenDisease
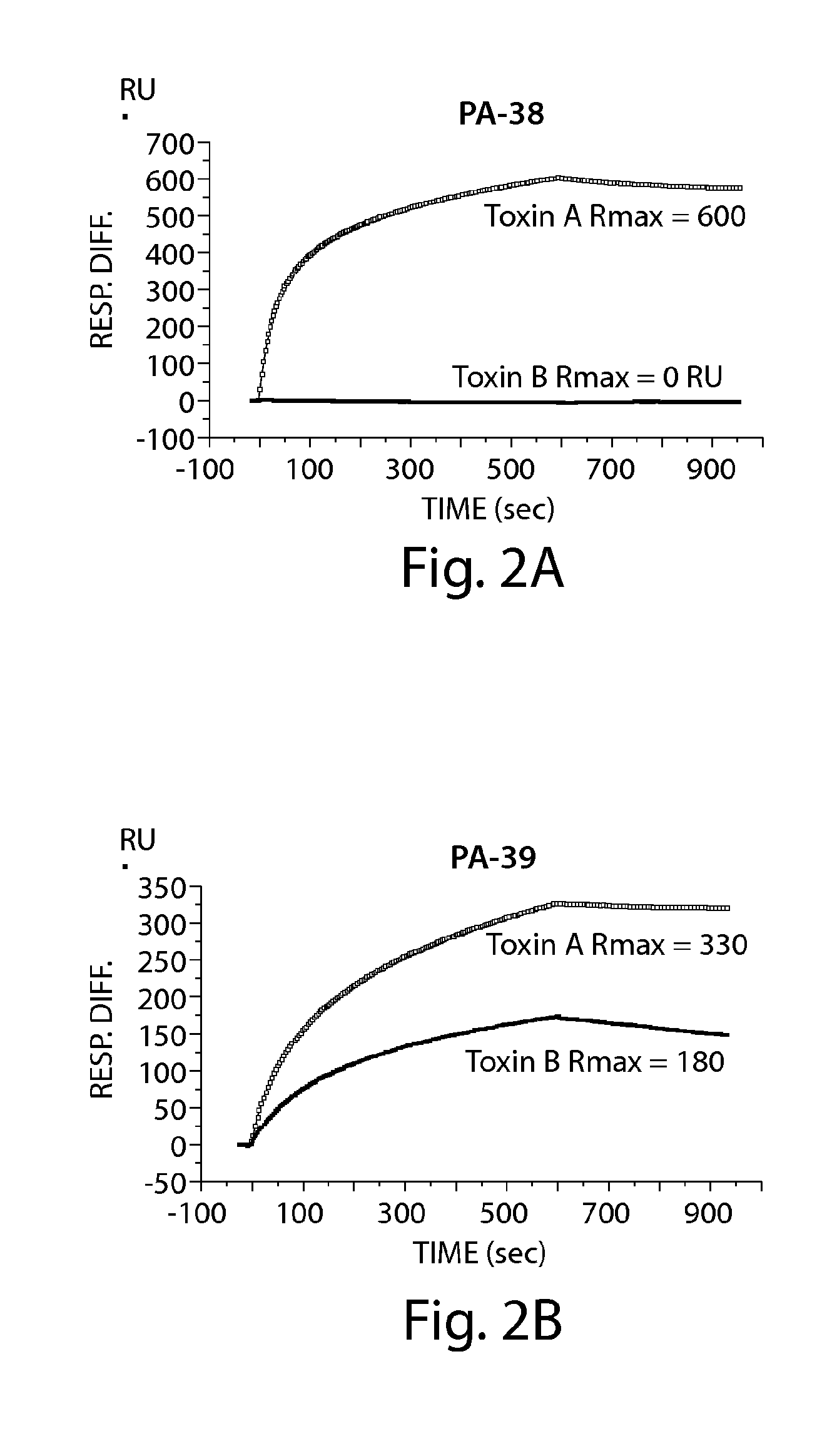
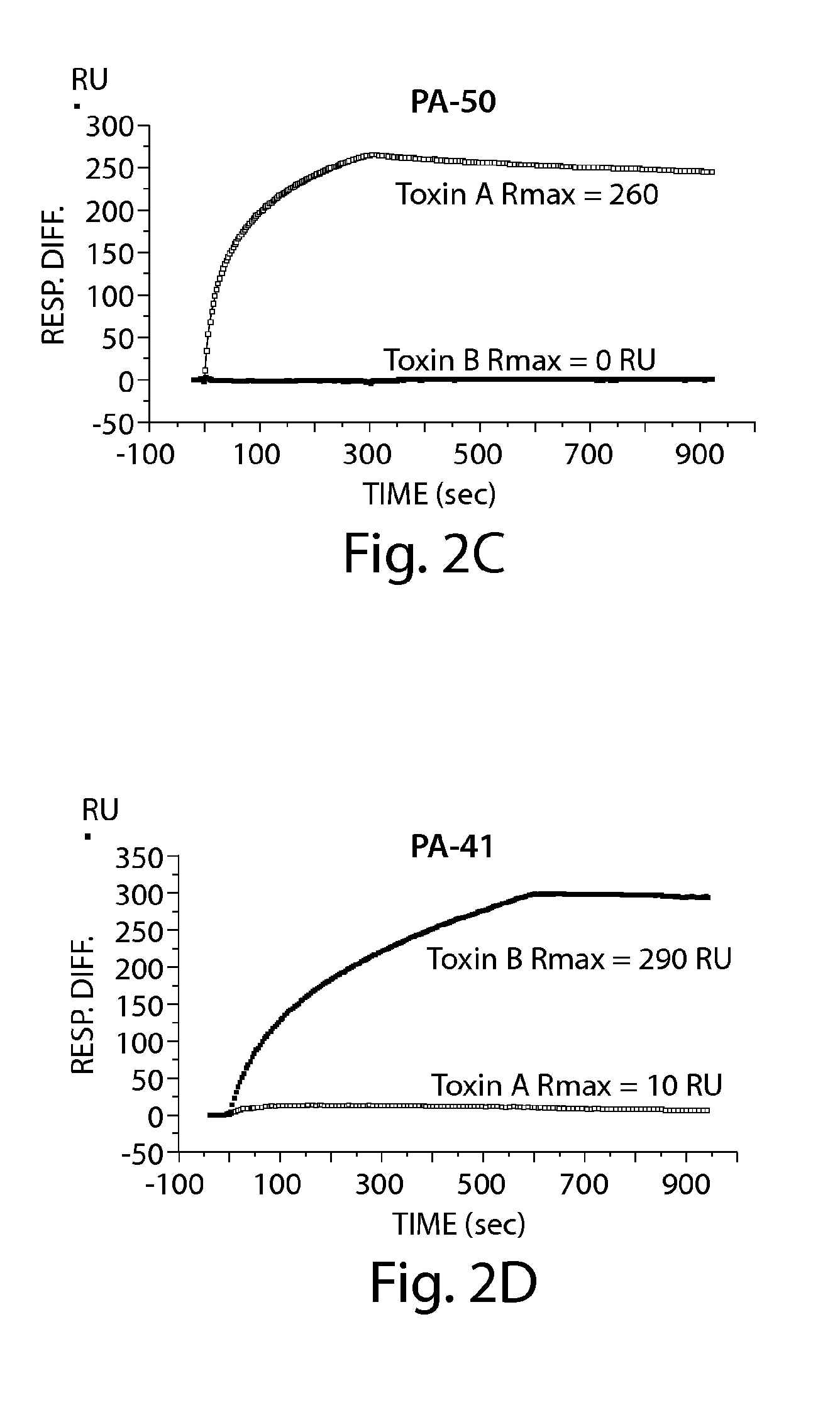
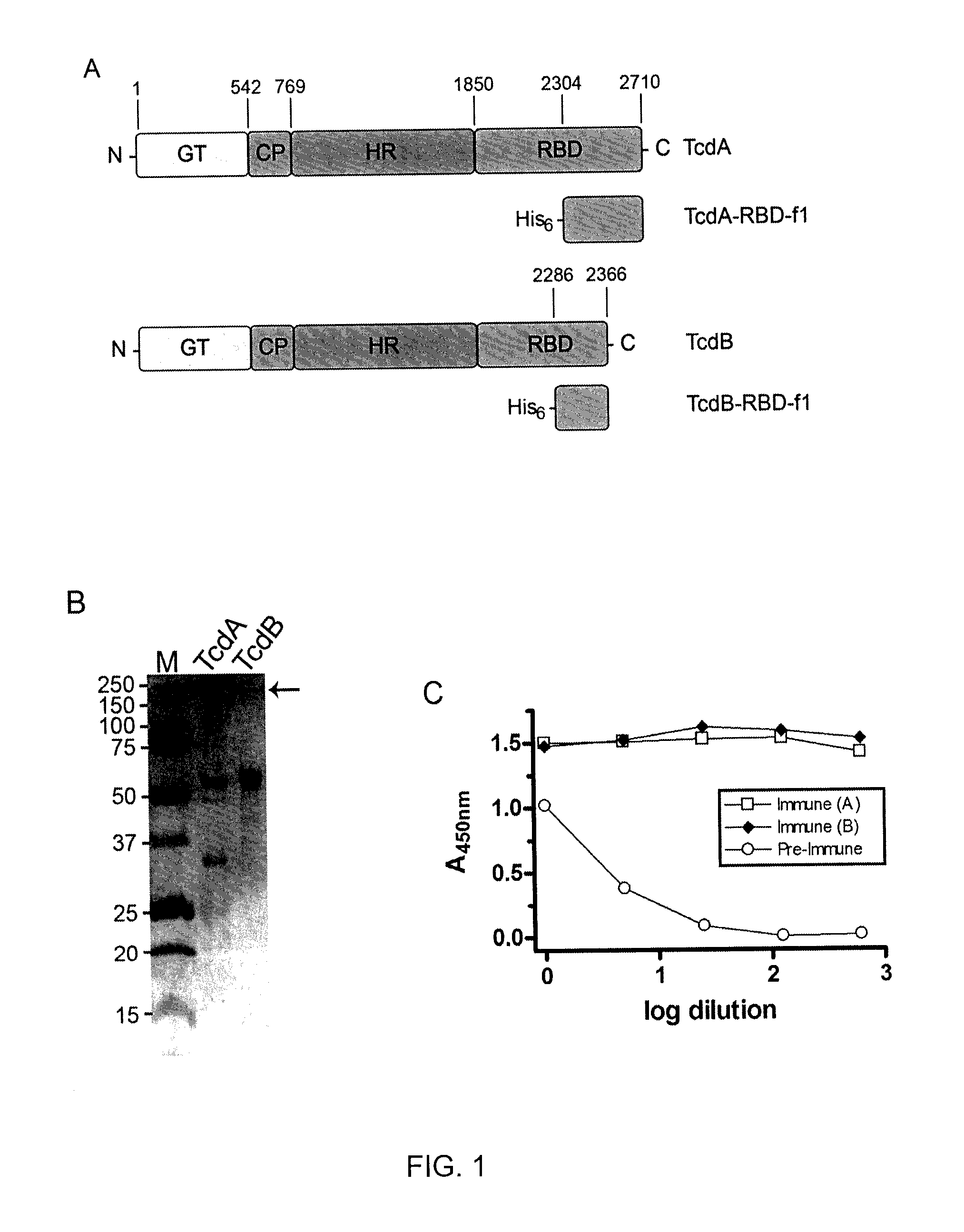
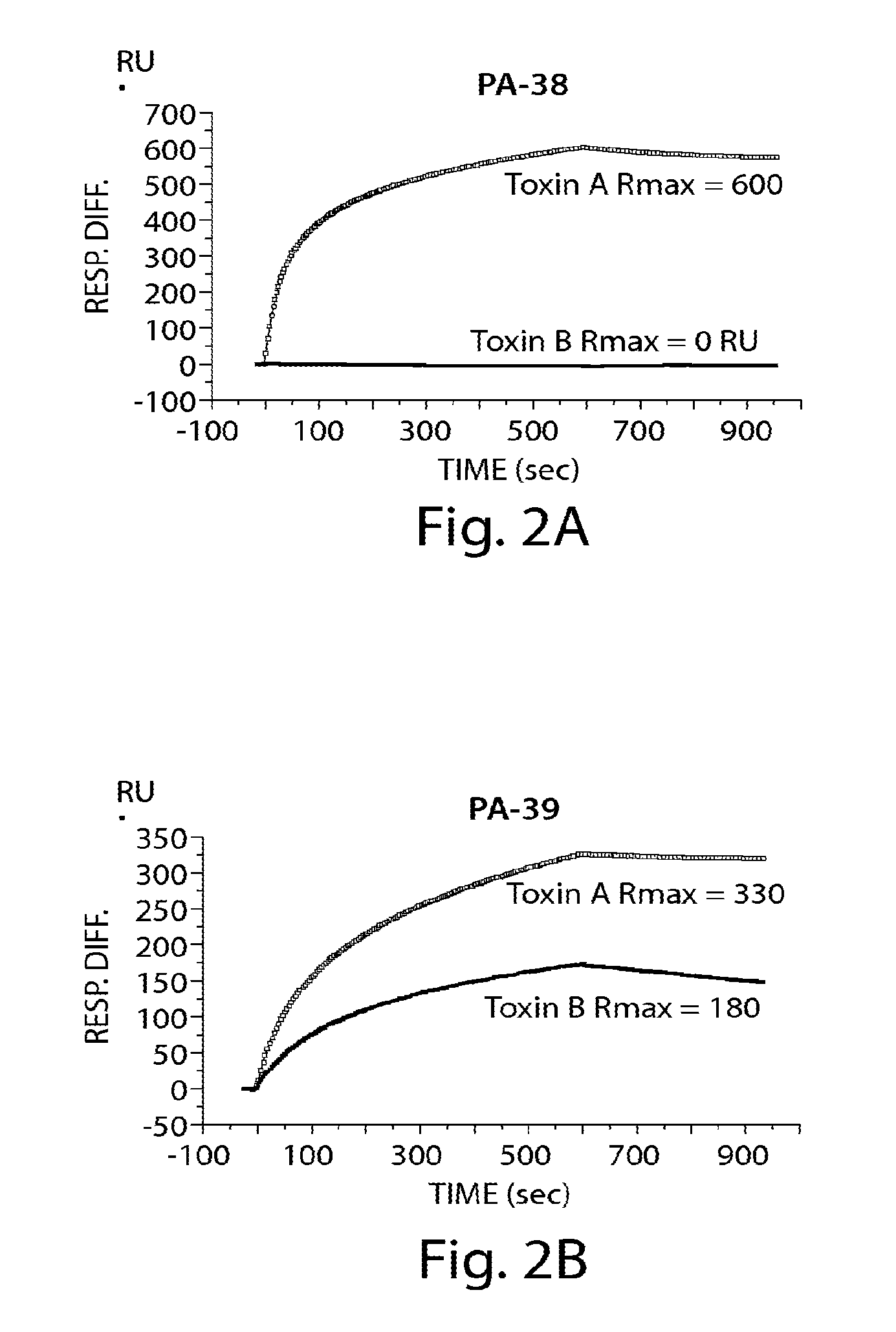
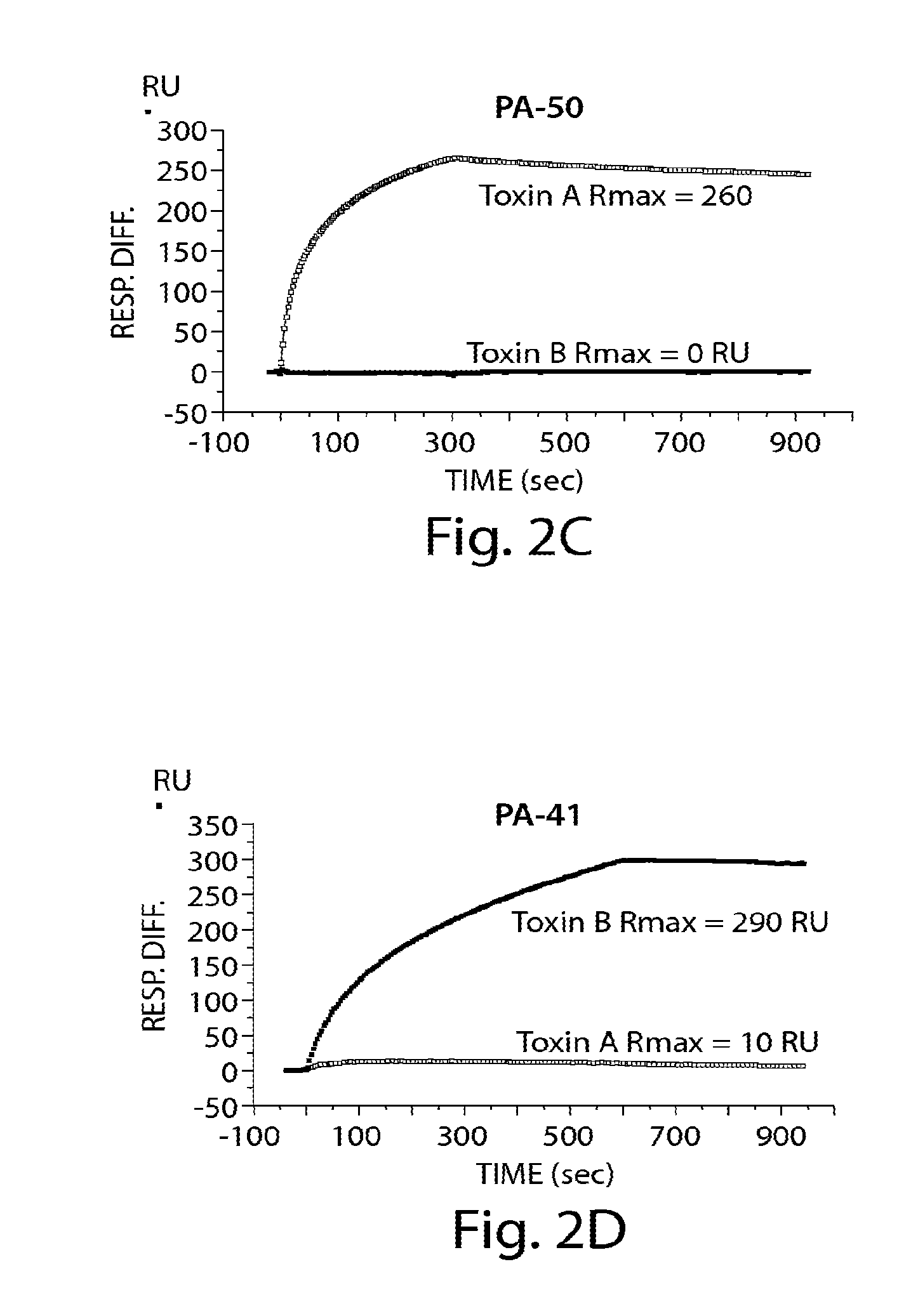
Provided herein are reagents, compositions, and therapies with which to treat Clostridium difficile infection and related disease conditions and pathologies, such as Clostridium difficile-associated diarrhea, resulting from infection by Clostridium difficile bacteria and the enterotoxins produced by these bacteria. In particular, antibodies or antigen-binding fragments thereof that bind specifically to toxin A and / or toxin B of C difficile and neutralize the activities of these toxins; compositions comprising such antibodies; and methods of using the antibodies and the compositions are provided.
Owner:PROGENICS PHARMA INC
Halogenated salicylanilides for treating clostridium infections
ActiveUS10463680B2Reduce probabilityEffective treatmentAntibacterial agentsOrganic active ingredientsDiseaseSalicylanilides
The present invention relates to halogenated salicylanilides, or pharmaceutically acceptable salts or esters thereof, for use in the treatment of an infection in a subject caused by Clostridium bacteria, particularly a C. difficile infection. The halogenated salicylanilides are expected to be useful in the treatment of C. difficile associated diseases including C. difficile associated diarrhea and C. difficile associated colitis.
Owner:UNION THERAPEUTICS AS
Methods and compositions for reducing clostridium difficile infection
InactiveUS20170087196A1Reduce riskReduce severityPeptide/protein ingredientsBacteria material medical ingredientsClostridial infectionClostridium difficile infections
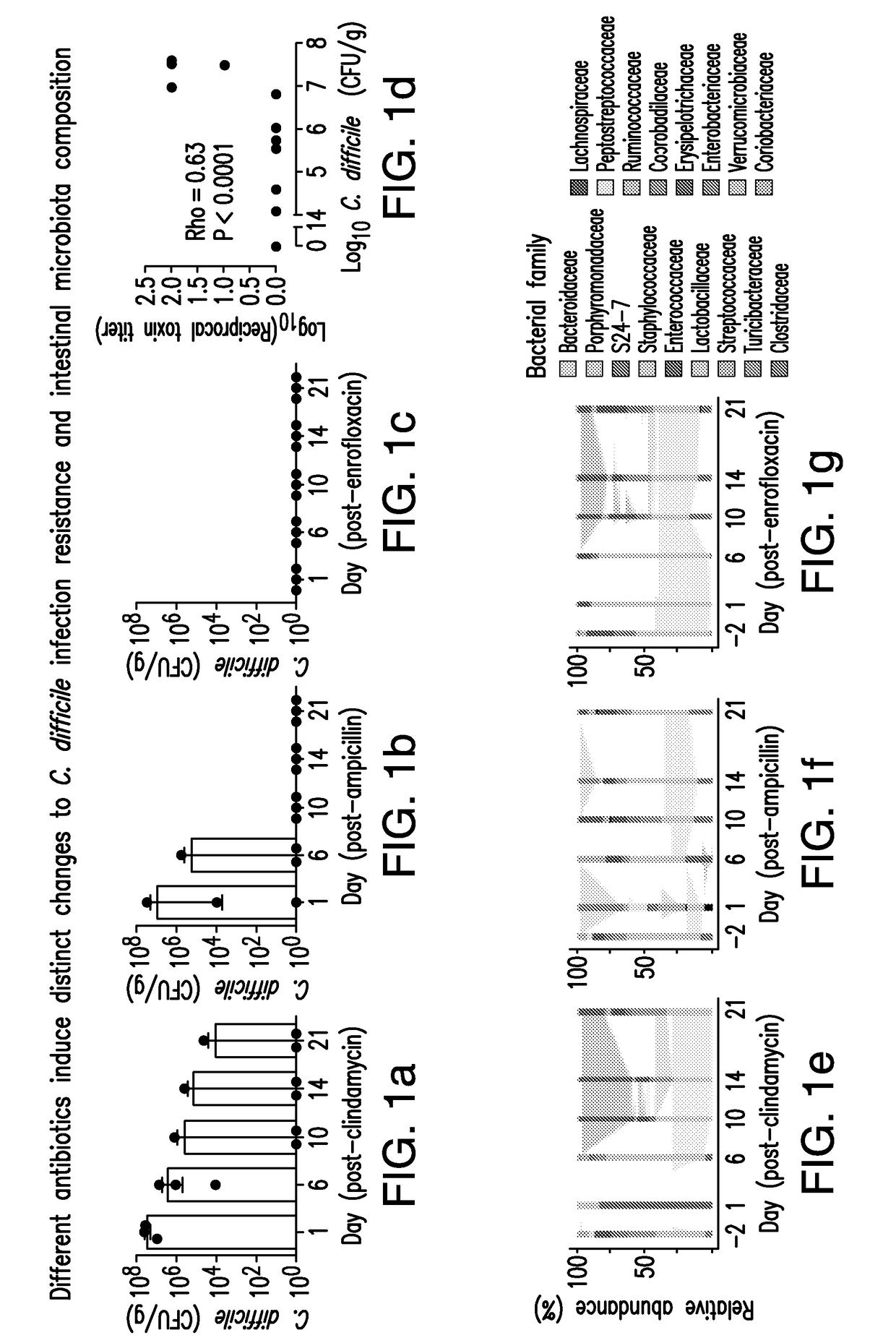
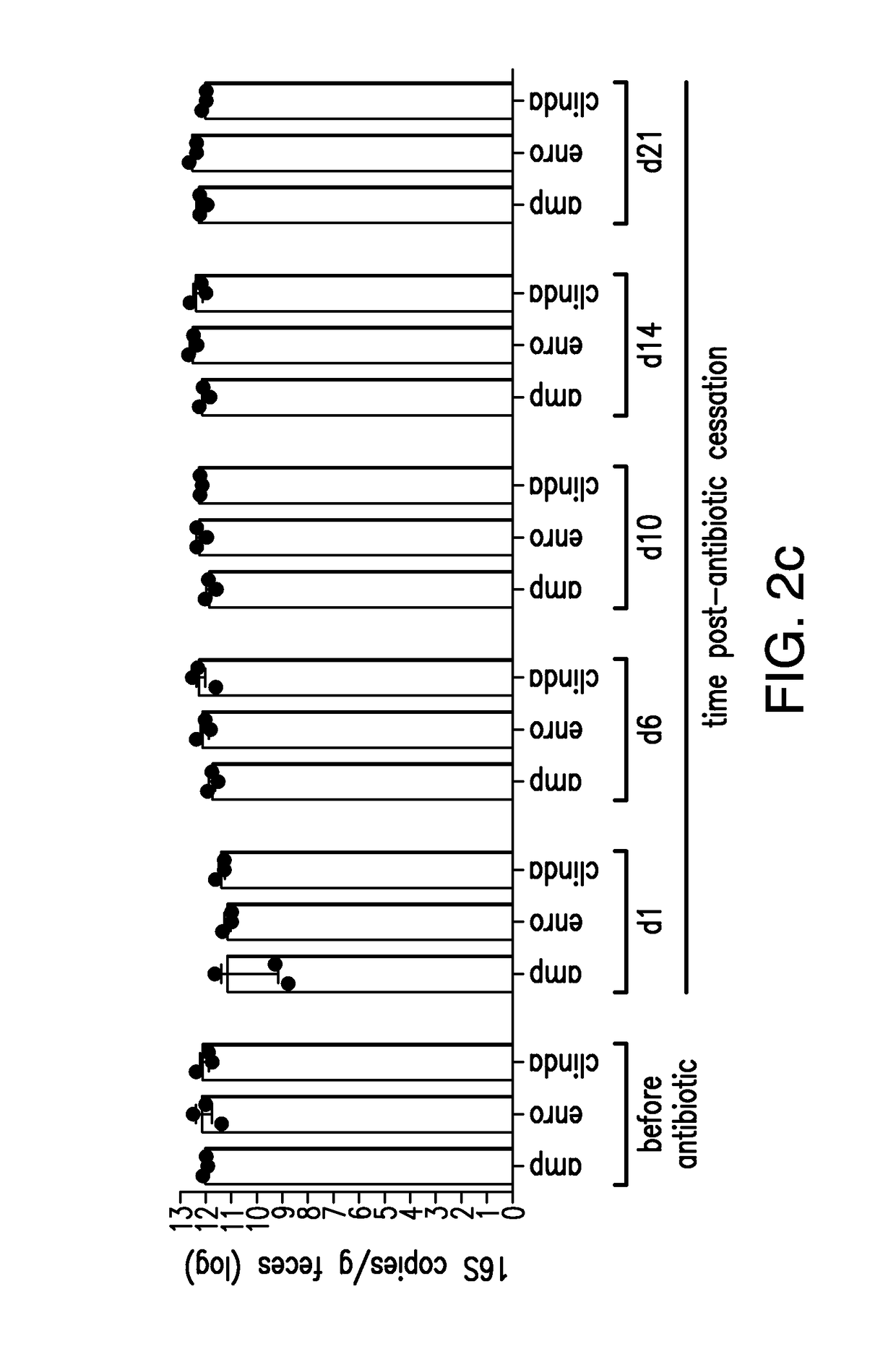
The present invention relates to methods and compositions for reducing the risk and severity of C. difficile infection. It is based, at least in part, on the discovery that a restricted fraction of the gut microbiota, including the bacterium Clostridium scindens, contributes substantially to resistance against C. difficile infection. Without being bound by any particular theory, it is believed that this is achieved through the biosynthesis of secondary bile acids.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Hybridoma cell strain secreting tetanus exotoxin monoclonal antibody, monoclonal antibody prepared by same, Fab antibody and application
InactiveCN102690789AGood effectHas the effect of neutralizing tetanus exotoxinAntibacterial agentsFungiClostridium tetaniTotal rna
The invention relates to a mouse hybridoma cell strain secreting a clostridium tetani bacillus exotoxin monoclonal antibody, wherein the strain has a preservation number of CCTCC (China Center for Type Culture Collection) NO. C201257. The invention further relates to a clostridium tetani bacillus exotoxin monoclonal antibody prepared by the mouse hybridoma cell strain; the invention further provides an Fab antibody, comprising a kappa chain and an Fd chain; the kappa chain and the Fd chain are obtained by amplifying from total RNA (ribonucleic acid) of the hybridoma cell strain. The invention further relates to a medicine for preventing or treating clostridium tetani bacillus infection, comprising the clostridium tetani bacillus exotoxin monoclonal antibody and / or the Fab antibody, and a pharmaceutically acceptable carrier. According to the invention, a monoclonal antibody for effectively neutralizing tetanus exotoxin is screened with natural tetanus exotoxin, and an Fab gene engineering antibody which is produced by large scale in vitro and which has toxin neutralizing effect is prepared with a gene engineering antibody technology on the basis of the monoclonal antibody.
Owner:ARMY MEDICAL UNIV +1
Clostridium difficile-specific antibodies and uses thereof
InactiveUS20130230537A1Improve heat resistanceReduced thermal stabilityAntibacterial agentsSugar derivativesClostridial toxinAntiendomysial antibodies
The present invention is directed to Clostridium difficile toxin-specific antibodies, compositions, and uses thereof. The anti-toxin antibodies may be specific for either TcdA or TcdB. The invention also includes methods of treating a Clostridium difficile infection, methods of capturing Clostridium difficile toxins, and methods of detecting Clostridium difficile toxins.
Owner:NAT RES COUNCIL OF CANADA
Compositions and methods for prophylaxis and therapy of clostridium difficile infection
InactiveUS20160250283A1Avoid infectionNone of approaches is suitableBacterial antigen ingredientsPeptide/protein ingredientsSporeClostridium difficile infections
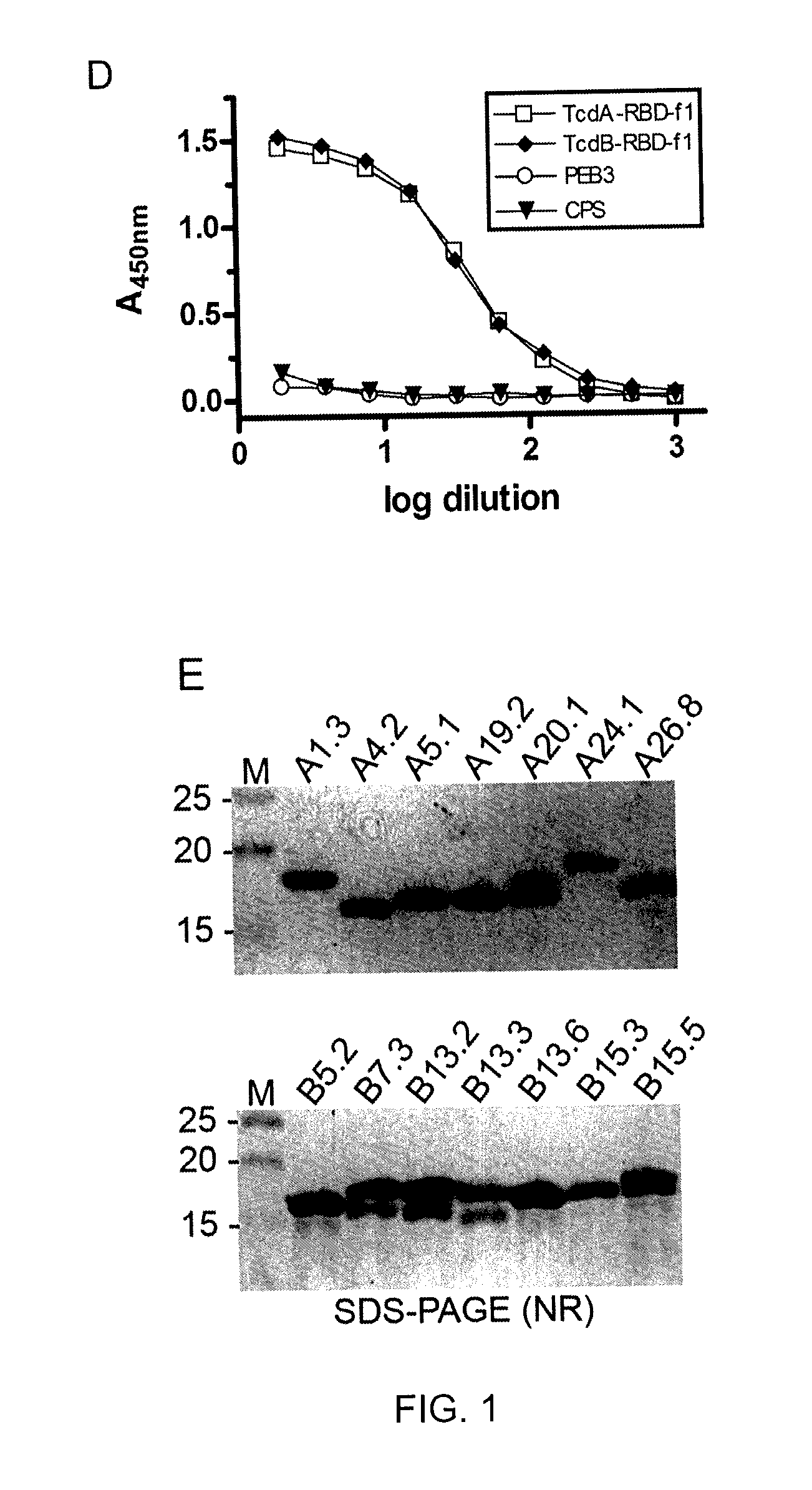
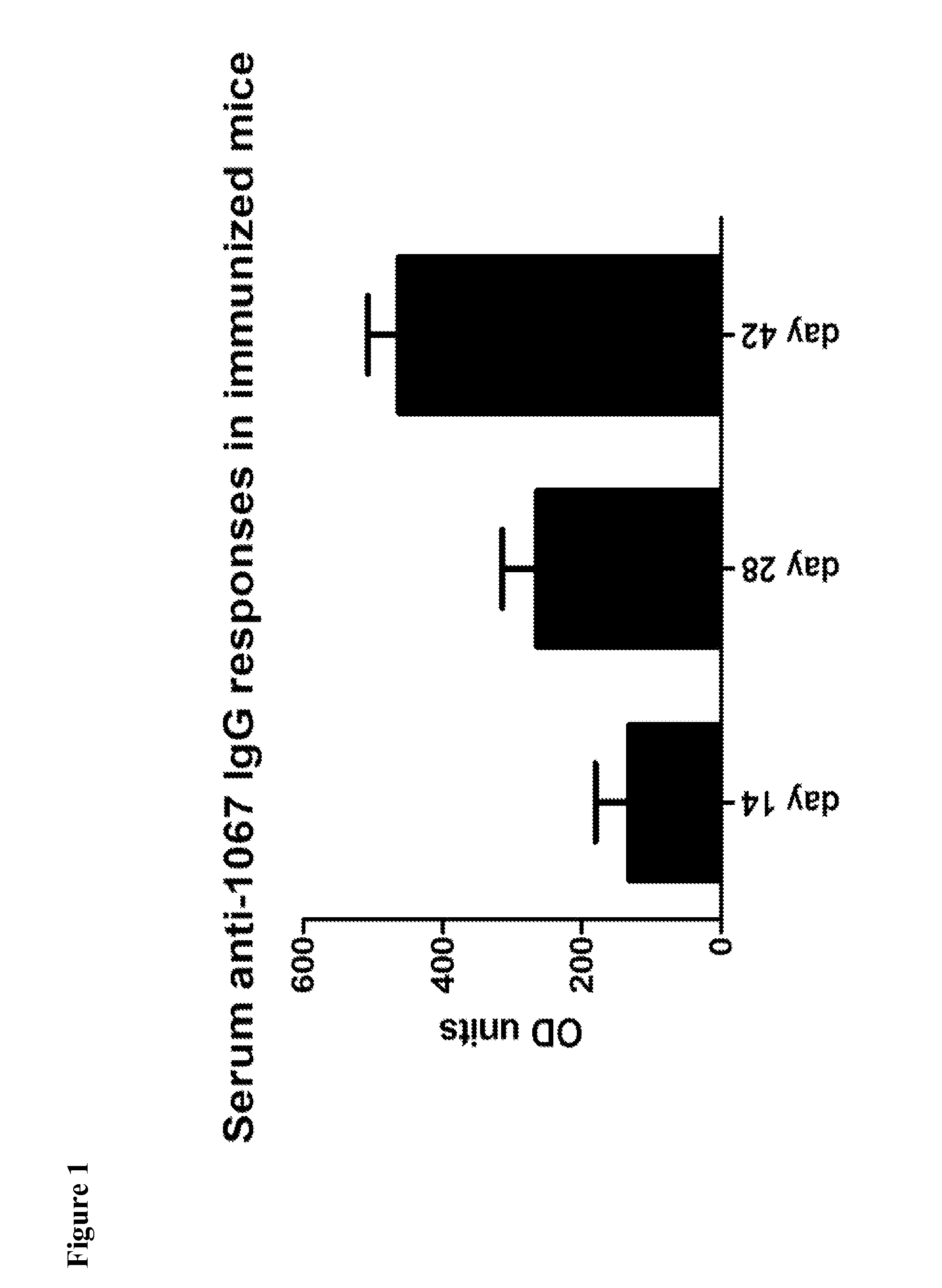
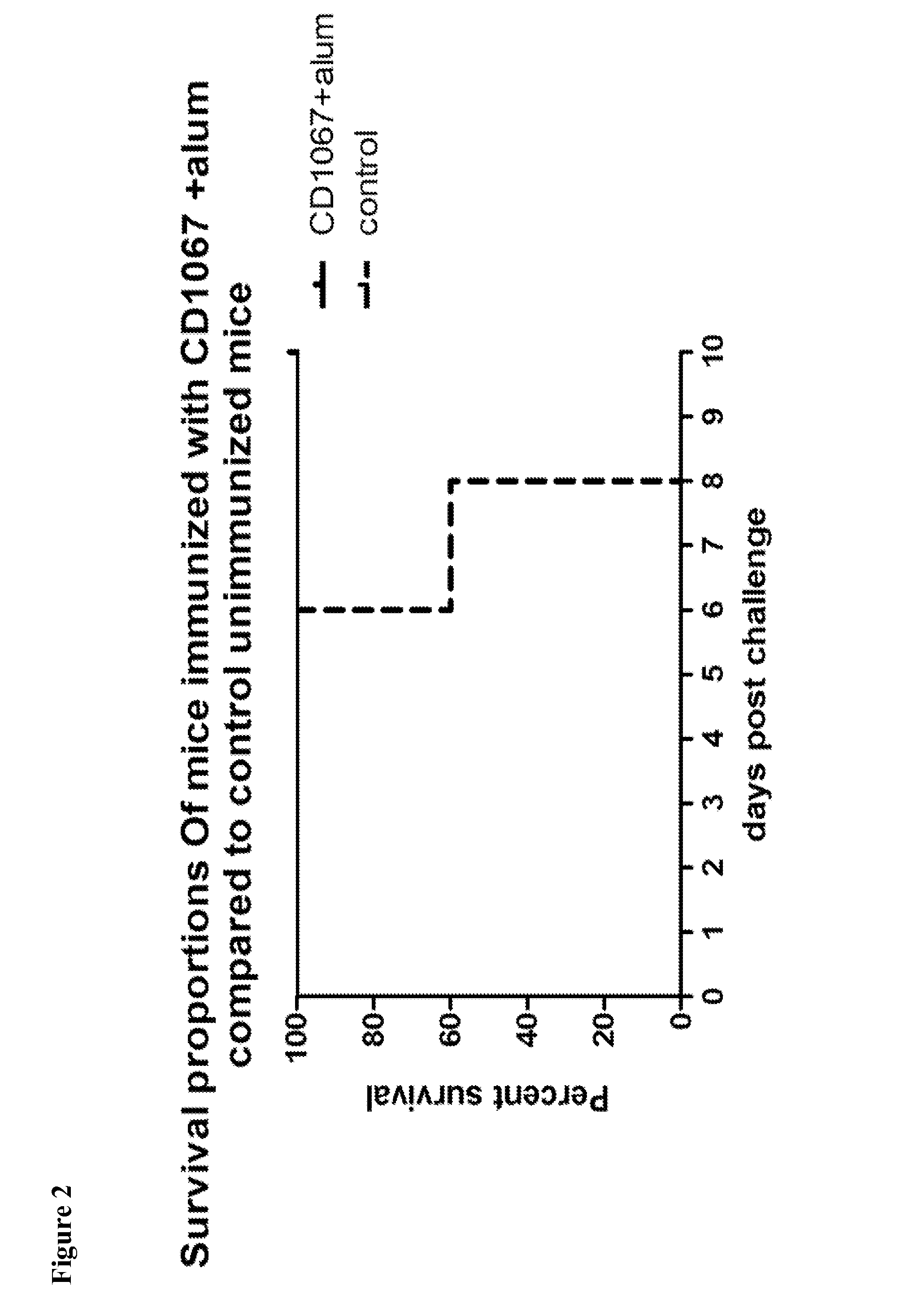
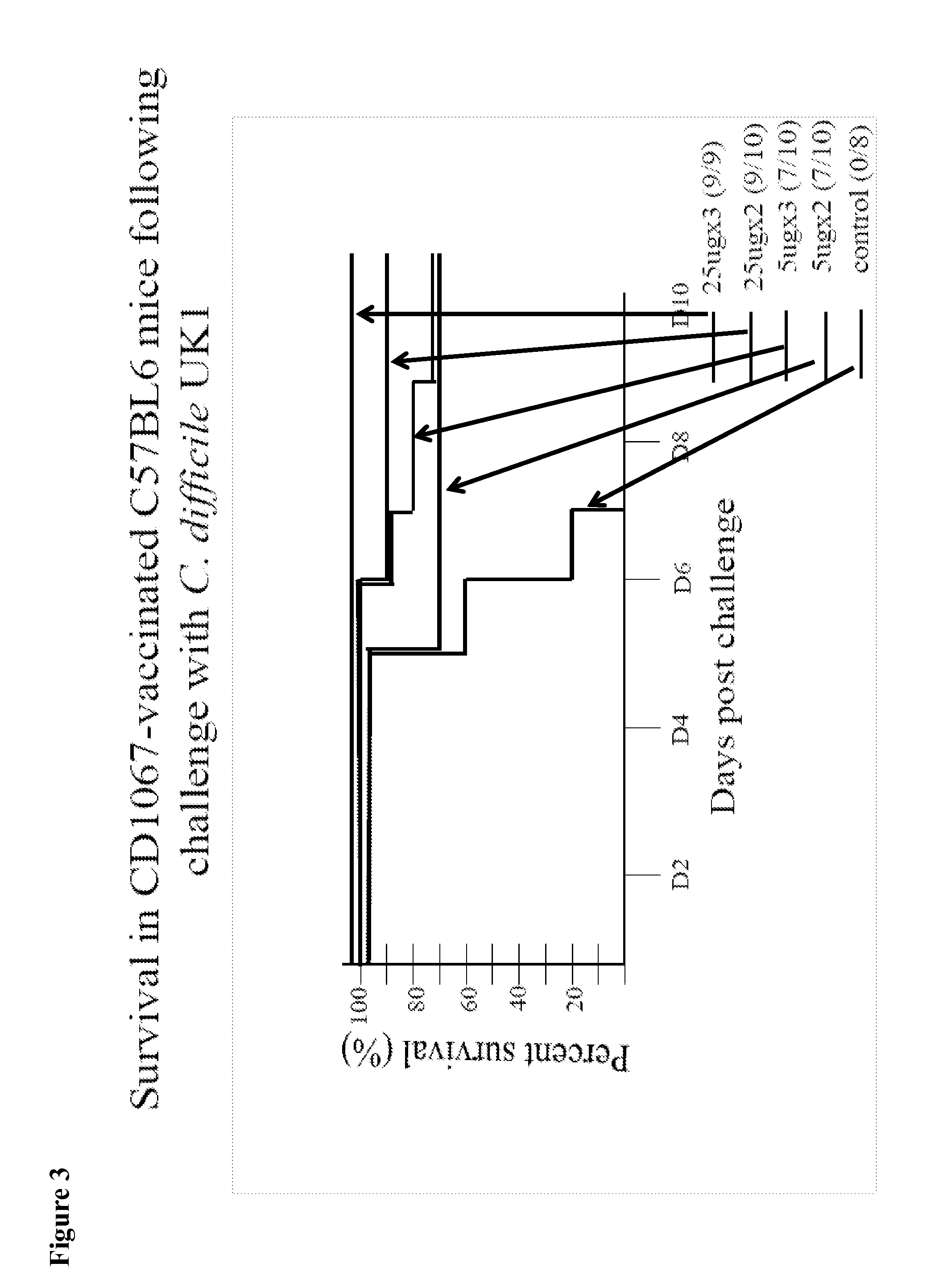
Provided are compositions and methods for prophylaxis and / or therapy of C. difficile infection, and for inhibiting dissemination of C. difficile spores. The compositions contain C. difficile proteins, including distinct proteins and fusions of C. difficile CD 1067, BclA1, SleC, CotA, Spl7, FliC, FliD, CD toxin A, CD toxin B, and combinations thereof. The methods include prophylaxis and / or therapy of C. difficile infection by administering to a subject in need a composition that includes the C. difficile protein(s).
Owner:THE ROCKEFELLER UNIV
Methods and compositions for reducing clostridium difficile infection
ActiveUS20190381113A1Lessen risk of and severityReduce riskPeptide/protein ingredientsBacteria material medical ingredientsClostridial infectionBacteroides
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Halogenated salicylanilides for treating clostridium infections
ActiveUS20180207179A1Useful in treatingReduce usageAntibacterial agentsOrganic active ingredientsBacteroidesSalicylanilides
The present invention relates to halogenated salicylanilides, or pharmaceutically acceptable salts or esters thereof, for use in the treatment of an infection in a subject caused by Clostridium bacteria, particularly a C. difficile infection. The halogenated salicylanilides are expected to be useful in the treatment of C. difficile associated diseases including C. difficile associated diarrhoea and C. difficile associated colitis.
Owner:UNION THERAPEUTICS AS
Tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof
ActiveCN108314730AAntibacterial agentsImmunoglobulins against bacteriaClostridial infectionClostridium tetani
The invention discloses a tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof. The antibody can be used for preventing, treating and diagnosing tetanus infection and / or treating one or more symptoms mediated by clostridium tetani infection. The invention further provides a method for producing an antibody capable of combining with tetanus toxin immunologically specifically.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
Methods and compositions for the treatment and/or prophylaxis of clostridium difficile associated disease
The present invention relates to methods and compositions for the treatment and / or prophylaxis of Clostridium difficile associated disease (CD AD). In particular, the invention relates to antibodies that bind to C. difficile antigens and are capable of inhibiting C. difficile infection, at least one symptom of C. difficile associated disease, shedding of C. difficile, and C. difficile associated mortality. The compositions of the present application comprise: mammalian or avian antibodies which bind to a C. difficile Toxin B; and mammalian or avian antibodies that bind to a C. difficile vegetative cell antigen and / or a C. difficile endospore antigen.
Owner:IMMURON
Recombinant alpha protein for inhibiting clostridium perfringens infection and preparation method and application thereof
InactiveCN106008684AHigh expressionOptimizing expression conditionsAntibacterial agentsBacterial antigen ingredientsSolubilityProtein tag
The invention discloses recombinant alpha protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant alpha protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.353 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant alpha protein enables animals to have a higher serum antibody level and resist attack of clostridium perfringens after the animals are immunized with the recombinant alpha protein. The recombinant alpha protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Antibodies for the treatment of clostridium difficile-associated infection and disease
InactiveUS20150175681A1Improve the quality of lifeImprove survivabilityAntibacterial agentsPeptide/protein ingredientsAntigenDisease
Provided herein are reagents, compositions, and therapies with which to treat Clostridium difficile infection and related disease conditions and pathologies, such as Clostridium difficile-associated diarrhea, resulting from infection by Clostridium difficile bacteria and the enterotoxins produced by these bacteria. In particular, antibodies or antigen-binding fragments thereof that bind specifically to toxin A and / or toxin B of C difficile and neutralize the activities of these toxins; compositions comprising such antibodies; and methods of using the antibodies and the compositions are provided.
Owner:PROGENICS PHARMA INC
Application of natural acid glycolipid molecules in neutralizing clostridium perfringens Epsilon toxin
ActiveCN110464729AReduce deathImprove survival rateAntibacterial agentsOrganic active ingredientsAntibiotic YTherapeutic effect
The invention relates to an application of natural acid glycolipid molecules in neutralizing clostridium perfringens Epsilon toxin, in particular, relates to the application in preparation of relateddrugs or reagents for prevention or treatment of clostridium perfringens infection in animals, and belongs to the biomedical field. The invention discloses one or more of natural acid glycolipid molecules sialic acid, ganglioside and sulfatide, wherein the natural acid glycolipid molecules have a good neutralization effect of Epsilon toxin, and have a good therapeutic effect on either animal-lethal clostridium perfringens infection or invasion and infection of Epsilon toxin. Three kinds of natural acid molecules sialic acid, ganglioside and glucosinolate have the advantages of simple molecularstructure, economic price, easily availability on the market and the like. The natural acid glycolipid molecules are expected to be used as substitute antibiotics or antibodies for prevention and treatment of clostridium perfringens infection, and have good application prospects in animal husbandry and veterinary.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI +1
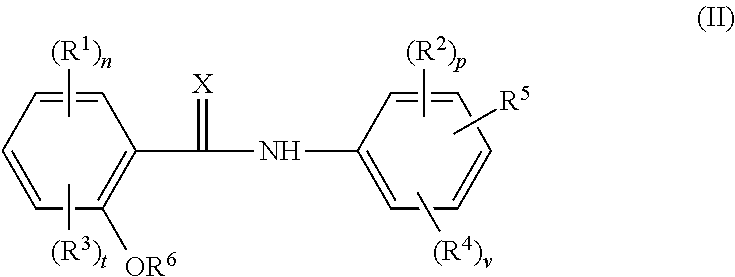
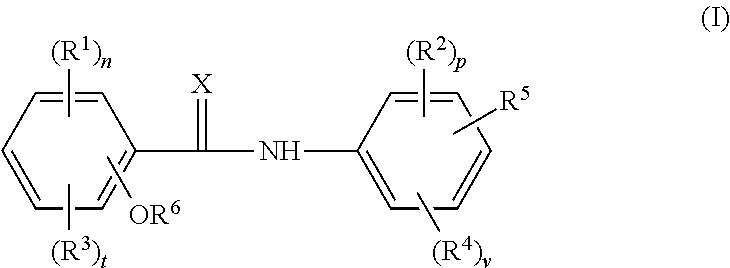
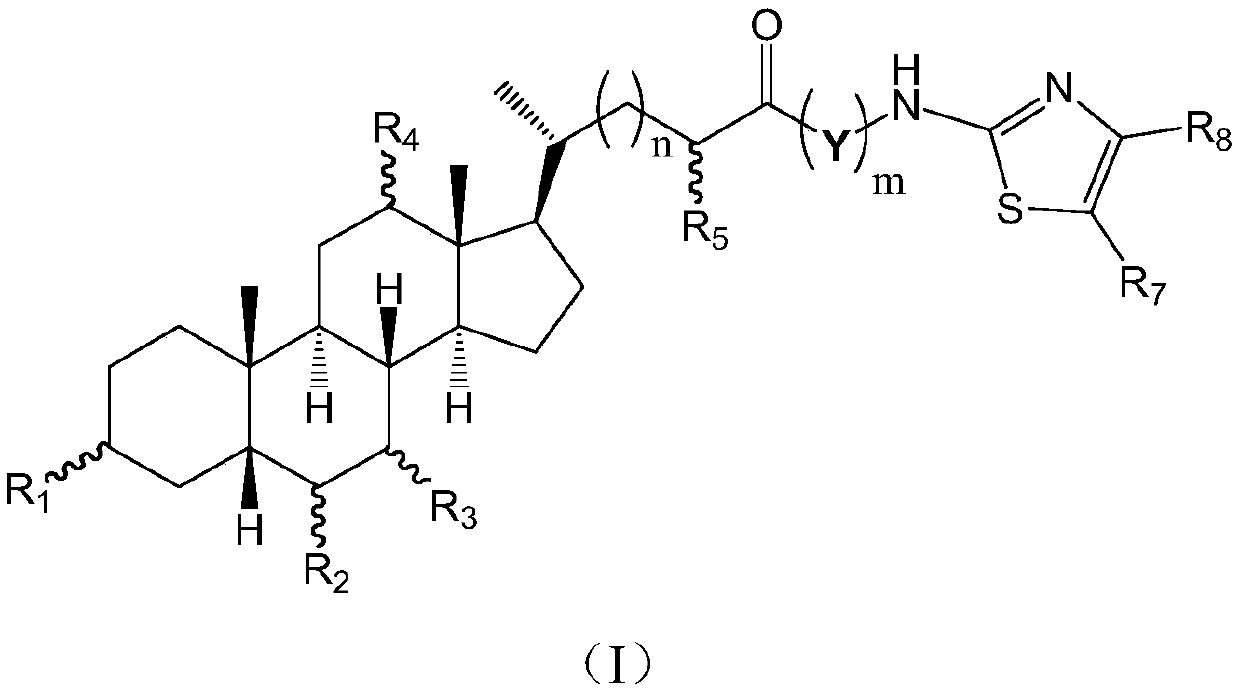
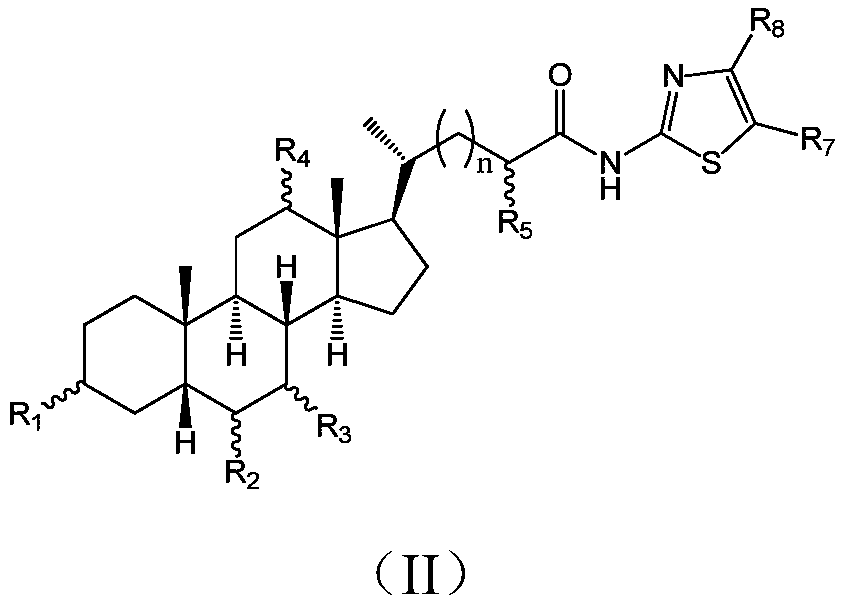
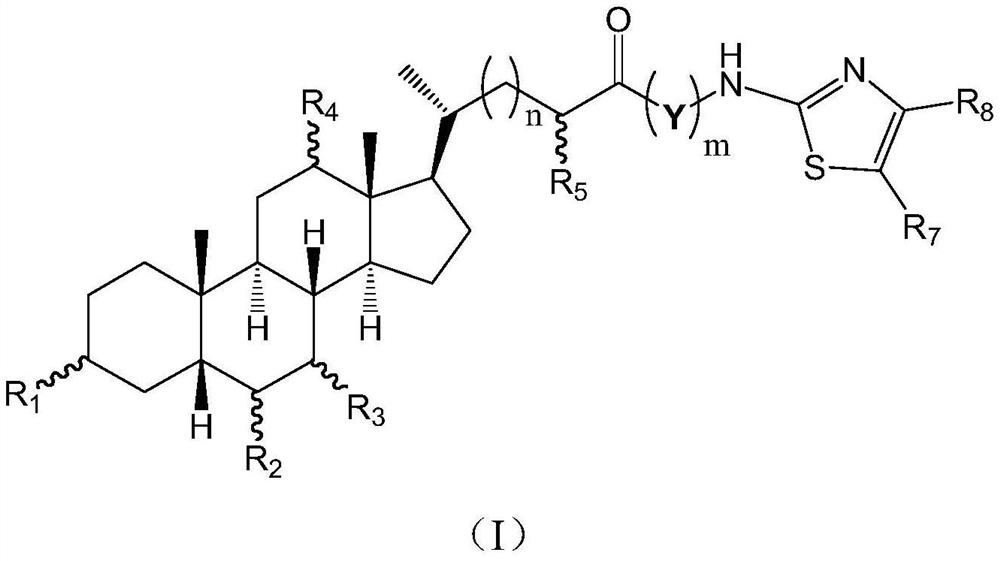
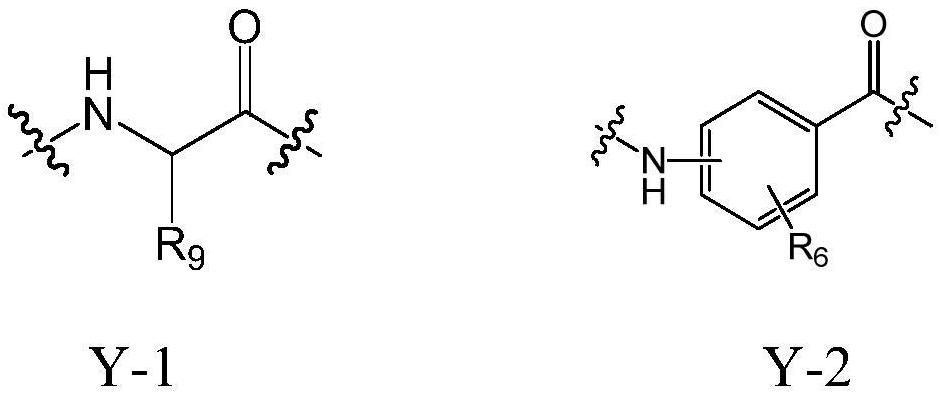
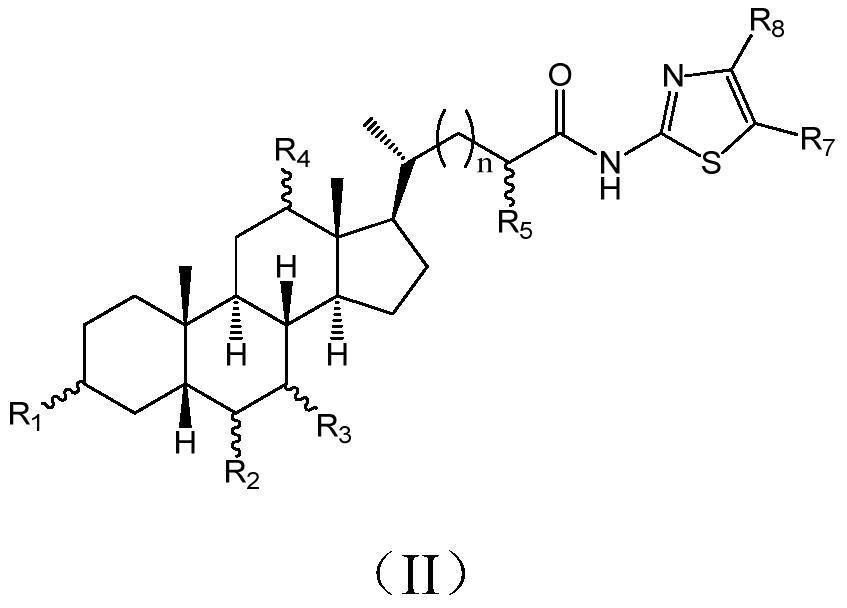
Cholic acid derivative with antibacterial activity and pharmaceutical composition thereof
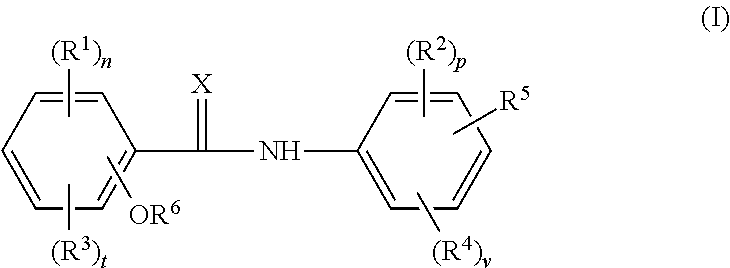
The invention provides a compound shown as formula (I), or a stereoisomer, or a salt, or a solvate, or a prodrug, or a metabolite thereof. The compound can effectively inhibit the growth of clostridium difficile thalli, has remarkable antibacterial activity, and has a very promising application prospect in preparing medicines for preventing and / or treating clostridium difficile infectious diseases, recurrence of clostridium difficile infectious diseases or complications of clostridium difficile infectious diseases.
Owner:CHENGDU BIOBEL BIOTECHNOLOGY CO LTD
Recombinant alpha protein for inhibiting Clostridium perfringens infection and its preparation method and application
InactiveCN106008684BHigh expressionOptimizing expression conditionsAntibacterial agentsBacterial antigen ingredientsProtein tagBacillus perfringens
The invention discloses recombinant alpha protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant alpha protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.353 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant alpha protein enables animals to have a higher serum antibody level and resist attack of clostridium perfringens after the animals are immunized with the recombinant alpha protein. The recombinant alpha protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Recombinant ε protein for inhibiting Clostridium perfringens infection and its preparation method and application
InactiveCN106008682BOptimizing expression conditionsHigh expressionAntibacterial agentsBacterial antigen ingredientsProtein tagBacillus perfringens
Owner:CHINA ANIMAL DISEASE CONTROL CENT
Preventive and/or therapeutic agent for clostridium difficile infection
PendingCN112823014APrevent and/or treat infectionAntibacterial agentsPharmaceutical delivery mechanismClostridium difficile infectionsGenus Enterococcus
Provided is a novel preventive and / or therapeutic agent for Clostridium difficile infection. It has been discovered that bacteria belonging to the genus Enterococcus are capable of preventing and / or curing Clostridium difficile infection. Disclosed herein are: an agent that is for preventing and / or curing Clostridium difficile infection and that contains a bacterium belonging to the genus Enterococcus; a medicinal product that is for preventing and / or curing Clostridium difficile infection and that contains a bacterium belonging to the genus Enterococcus; and a food product that is for preventing and / or curing Clostridium difficile infection and that contains a bacterium belonging to the genus Enterococcus.
Owner:NUTRI CO LTD
Compositions and methods for treating clostridium infection and preventing recurrence of infection
ActiveUS20150133366A1High IgG levelReduce productionBiocideSaccharide peptide ingredientsAntibiotic-associated diarrhoeaRegimen
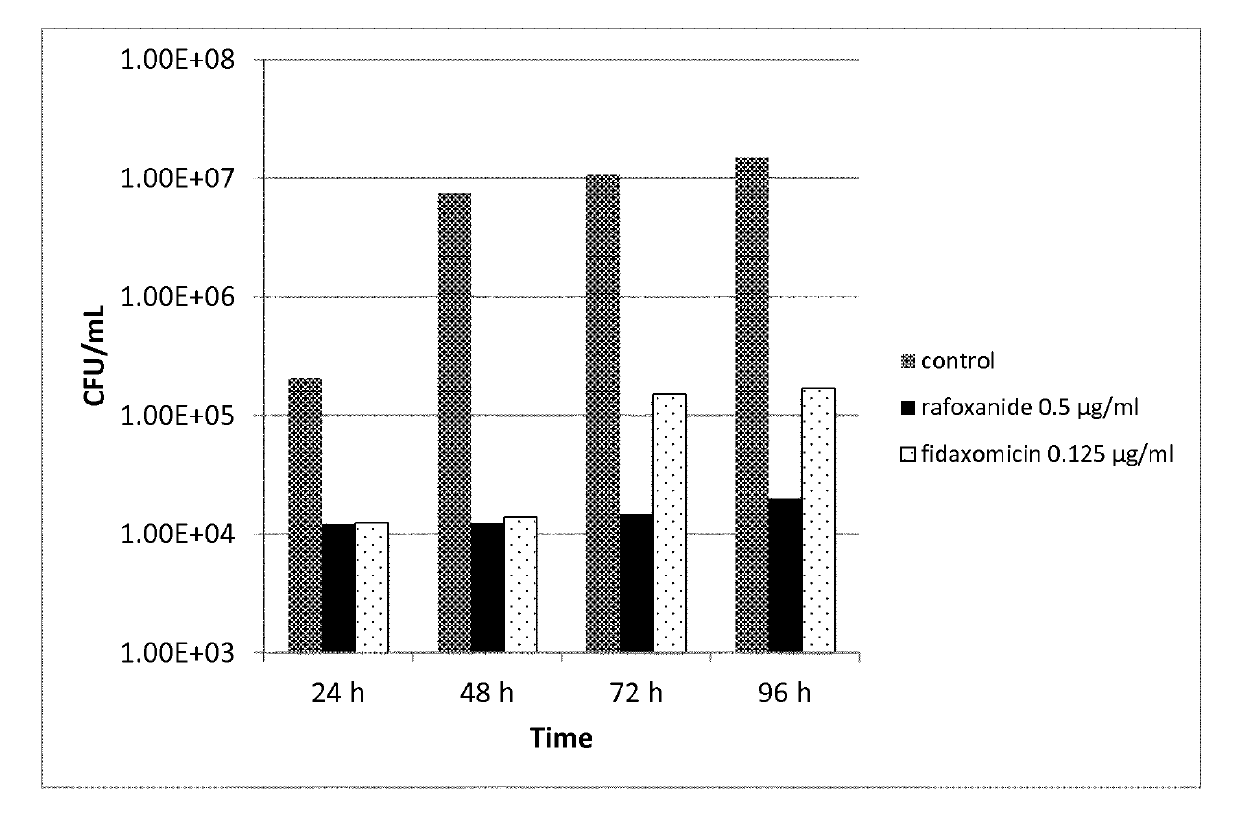
C. difficile infection (CDI) is the most common cause of antibiotic-associated diarrhea. Unfortunately, antibiotic therapy remains as the standard treatment for this antibiotic-induced disease and relapses are common. Antibiotic treatment typically is given for 10 to 14 days for initial or second episode of CDI. For recurrent episodes, more prolonged courses are recommended. It is disclosed herein that lower dose or shorter course of the antimicrobial treatment is sufficient to treat the disease and prevent recurrent disease by enabling a good immunologic response to infection, and perhaps also by better preserving normal flora, thus protecting against relapses or reinfection.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Clostridium difficile multi-component vaccine
PendingCN113329766AAntibacterial agentsBacterial antigen ingredientsClostridial toxinClostridium difficile (bacteria)
Immunogenic compositions for combating clostridium (C.) difficile infection are disclosed comprising an admixture of at least two components (a) and (b), where component (a) comprises inactivated cells of at least one strain of C. difficile, or or cell surface extracts (CSE) from one or more strains of C. difficile bacteria; and component (b) comprises at least one toxoid or a non-toxic, immunogenic polypeptide fragment of a C. difficile Toxin A or Toxin B. Administration of the immunogenic composition is effective to elicit an immune response in a subject immunized with said composition to produce antibodies reactive with at least one C. difficile strain and at least one C. difficile toxin.
Owner:MATRIVAX
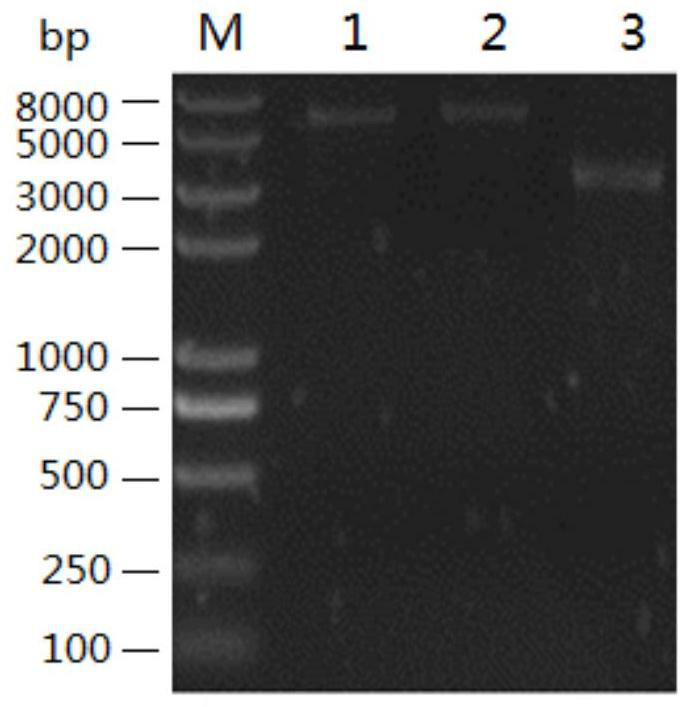
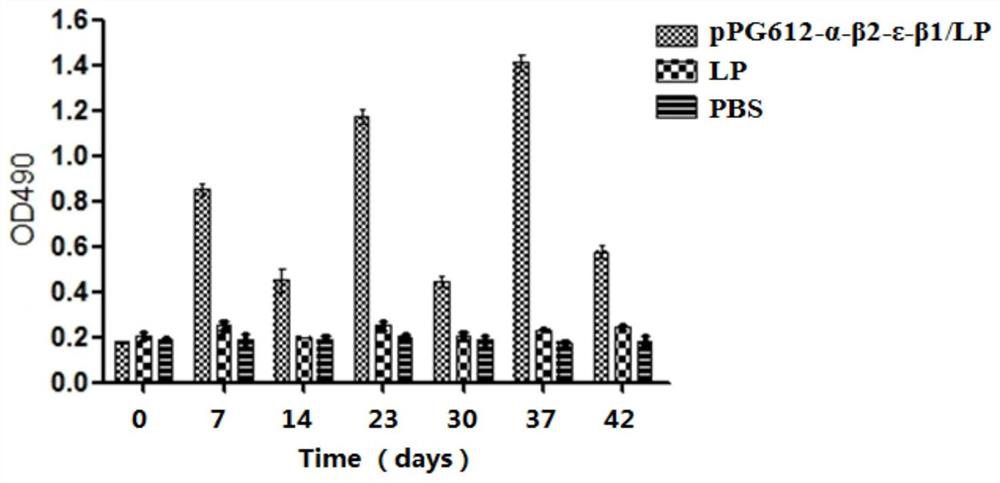
A recombinant lactobacillus that simultaneously expresses Clostridium perfringens α, β2, ε, β1 exotoxin and its construction method and application
ActiveCN106987548BAvoid infectionPreserve immunogenicityAntibacterial agentsBacterial antigen ingredientsImmunologic preparationBacillus perfringens
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
A bile acid derivative with antibacterial activity and its pharmaceutical composition
The present invention provides the compound represented by formula (I), or its stereoisomer, or its salt, or its solvate, or its prodrug, or its metabolite. This type of compound can effectively inhibit the growth of Clostridium difficile bacteria, and has significant antibacterial activity. It has a very good application prospect in the medicine of disease complications.
Owner:CHENGDU BIOBEL BIOTECHNOLOGY CO LTD
Methods for treating and preventing c. difficile infection
PendingUS20200281948A1Antibacterial agentsTetracycline active ingredientsClostridium difficile infectionsMicrobiology
Methods of treating or preventing a C. difficile infection and the associated pathological conditions related to C. difficile infection, are disclosed.
Owner:PARATEK PHARM INC
Method of treating Clostridium difficile infection or its associated symptoms
ActiveUS11318154B2Delay the onset of TcdBReduce activationAntibacterial agentsOrganic active ingredientsDiseaseCellular Cytotoxicity
A method of preventing or treating a subject suffering from Clostiridium difficile infection or its associated symptom includes administering a therapeutic effective amount of a HMGB1 inhibitor to the subject. A method of inhibiting toxin-induced cytotoxic effect in colon cells includes contacting the colon cells with an effective amount of a HMGB1 inhibitor.
Owner:CITY UNIVERSITY OF HONG KONG +1
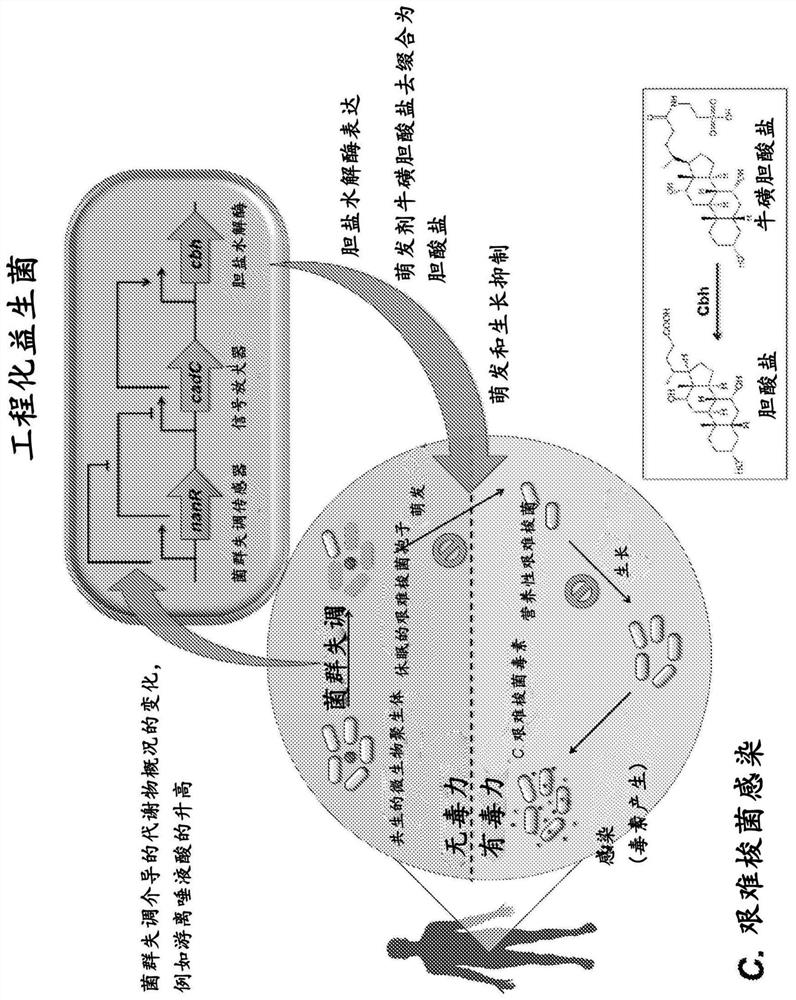
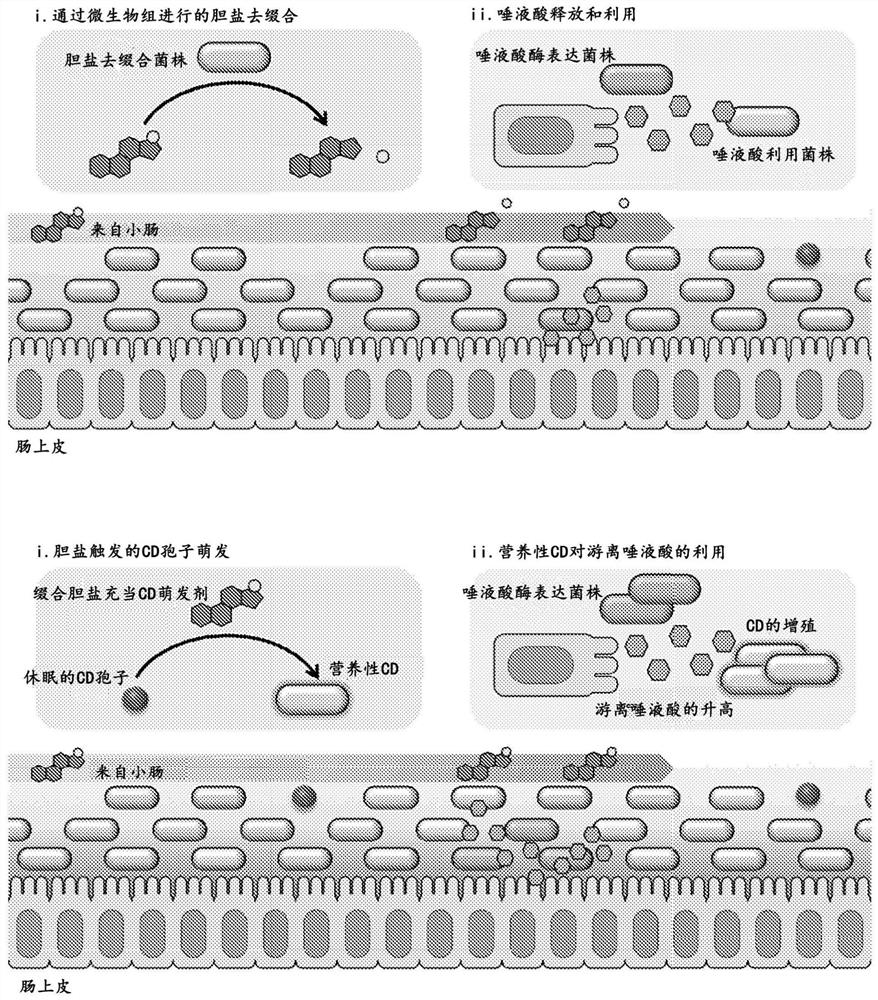
Engineered flora disorder sensing probiotics for clostridium difficile infection and recurrent infection management
PendingCN114269928AOvercome resistanceAntibacterial agentsBacteriaBile salt hydrolaseRecurrent infections
The present invention relates to methods of metabolic engineering of bacterial cells to produce bile salt hydrolases to inhibit germination of clostridium difficile endophytic spores and colonization within the human gastrointestinal tract. The bile salt hydrolase is operably linked to a sialic acid inducible promoter pNanA, wherein the pNanA is in turn controlled by a repressor nanoR. A recombinant bacterium expressing the bile salt hydrolase may be a probiotic strain to be used in the prevention or treatment of Clostridium difficile infection.
Owner:NAT UNIV OF SINGAPORE
Recombinant β2 protein for inhibiting Clostridium perfringens infection and its preparation method and application
InactiveCN106008683BHigh expressionResist attackAntibacterial agentsBacterial antigen ingredientsProtein tagBacillus perfringens
The invention discloses recombinant beta-2 protein for inhibiting clostridium perfringens infection and a preparation method and application thereof. The recombinant beta-2 protein is shown in (a) or (b) or (c), wherein the protein in (a) is composed of amino acid sequences shown as SEQ ID No.2; the protein in (b) is composed of amino acid sequences shown at the sites No.51-No.286 of SEQ ID No.2; the fusion protein in (c) is obtained by fusing protein tags at carboxyl terminals or / and amino terminals of the protein shown in (a) or (b). The recombinant beta-2 protein enables animals to have a higher serum antibody level and resist the attack of clostridium perfringens after the animals are immunized with the recombinant beta-2 protein. The recombinant beta-2 protein is good in solubility and easy to purify and can serve as a diagnostic antigen to be prepared into a monoclonal antibody or be used for further research on protein functions and conformation relations.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
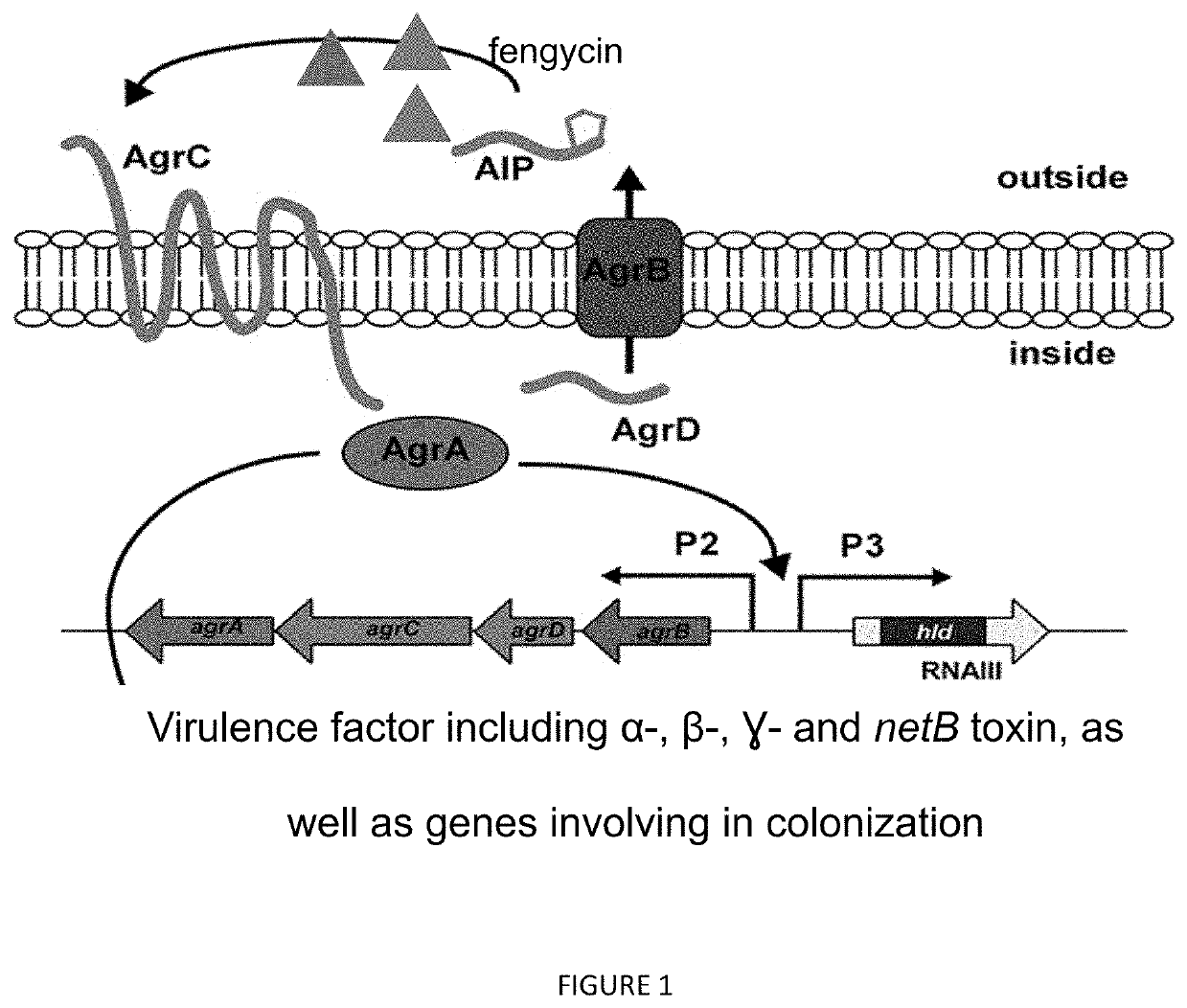
Method for controlling clostridium infection in animals
The present invention relates to compositions and methods of inhibiting quorum sensing processes of C. perfringens using fengycin. Fengycin effectively downregulates the expression of agrD and netB genes, resulting in reduced pathogenesis of C. perfringens. The composition may contain fengycin or a source of fengycin, such as Bacillus subtilis PB6.
Owner:KEMIN IND INC
Methods and materials for using biomarkers which predict susceptibility to clostridium difficile infection
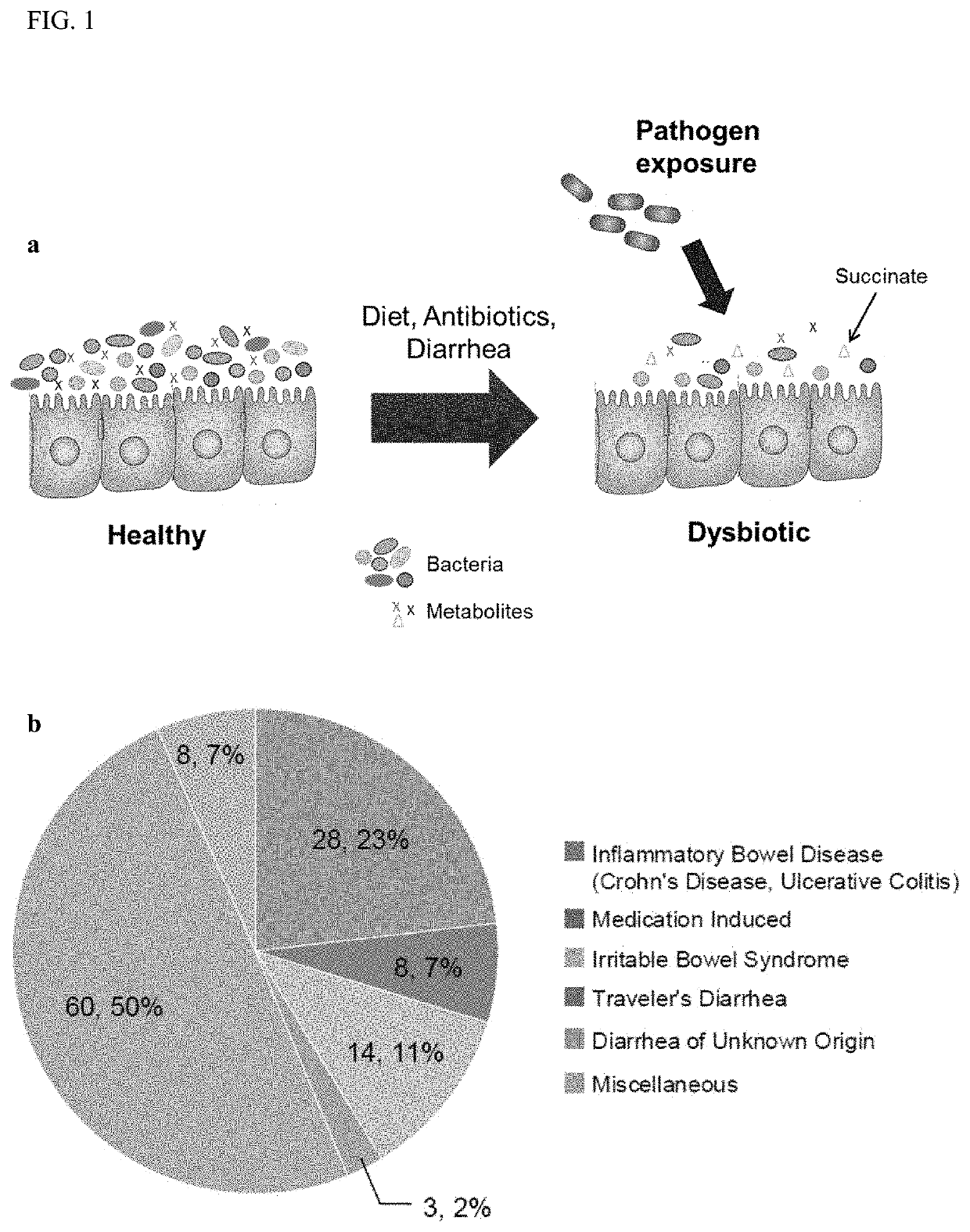
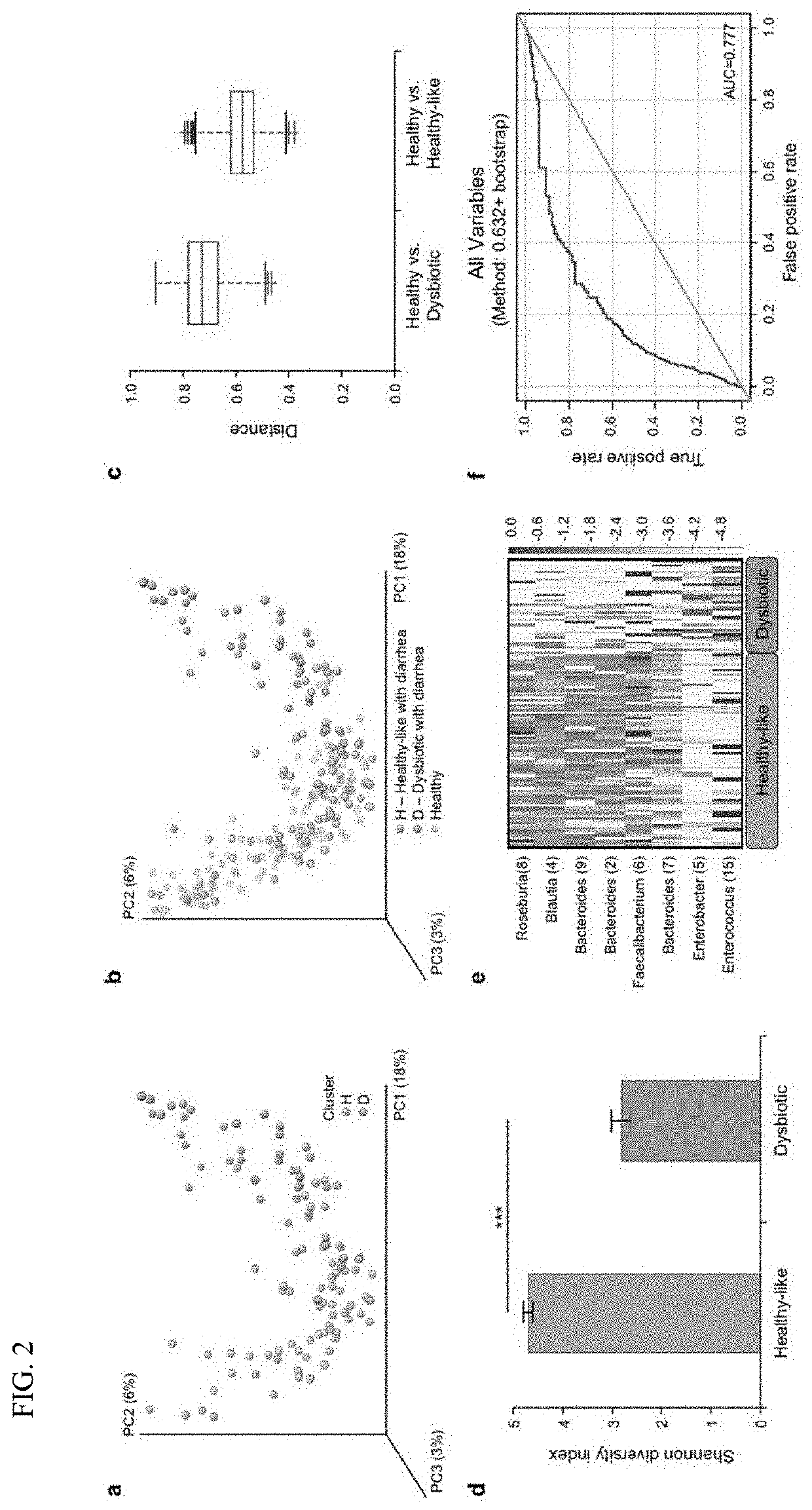
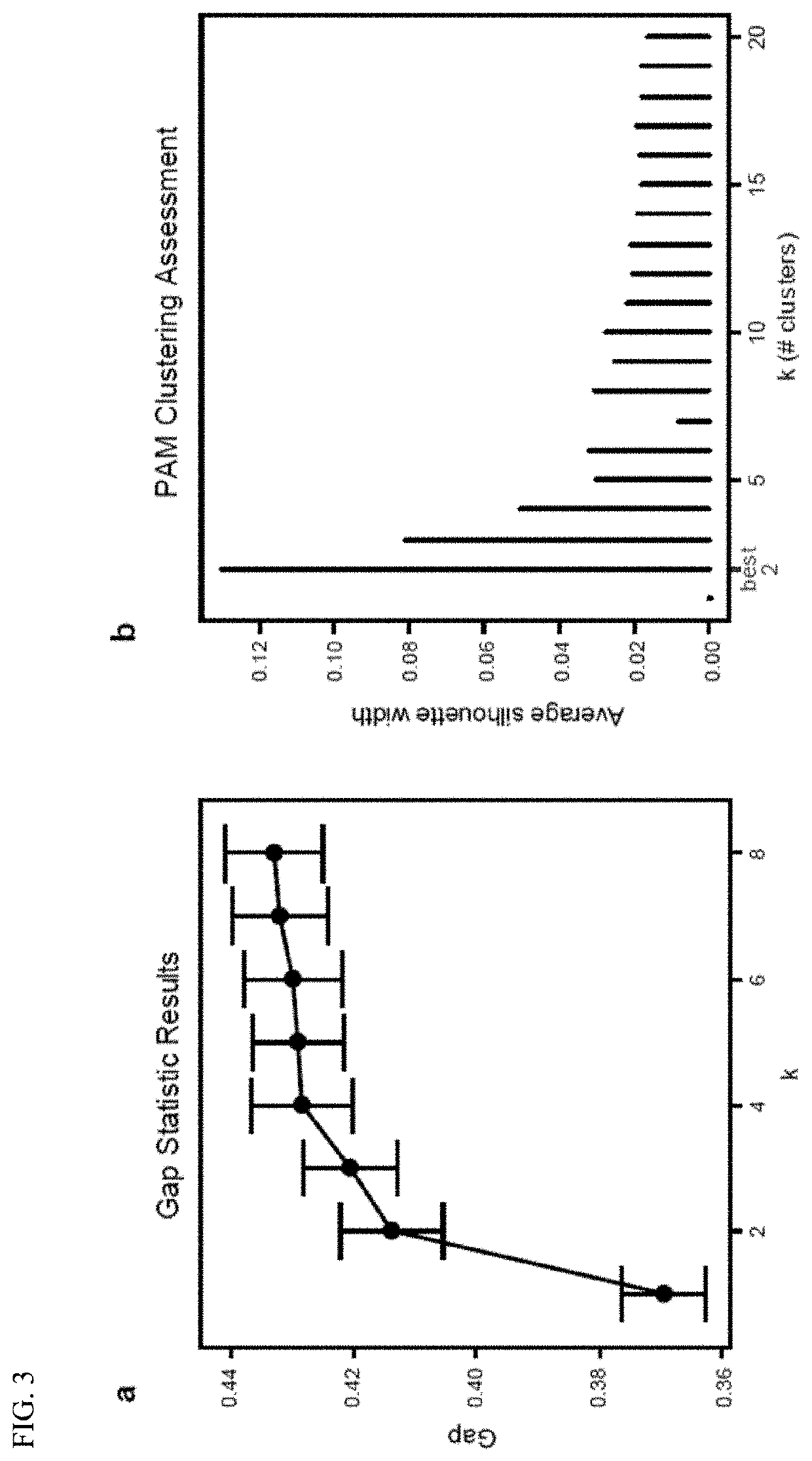
PendingUS20210128644A1Remodel the gut microenvironmentAntibacterial agentsMicrobiological testing/measurementClostridium difficile infectionsIntestinal microorganisms
This document relates to biomarkers of gut microbiota dysbiosis. Bacteria that are increased or decreased in gut microbiota dysbiosis can be used as biomarkers to predict dysbiosis in patients with diarrhea and / or to predict susceptibility to Clostridium difficile infection (CDI). In addition, provided herein are compositions including at least three bacteria that are decreased in gut microbiota dysbiosis which can be used, for example, to restore heathy gut microbiota (e.g., by probiotic or by fecal microbiota transplant) to treat CDI.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Compounds for the treatment of clostridium difficile infection
PendingUS20210340117A1Antibacterial agentsOrganic chemistryBiotechnologyClostridium difficile infections
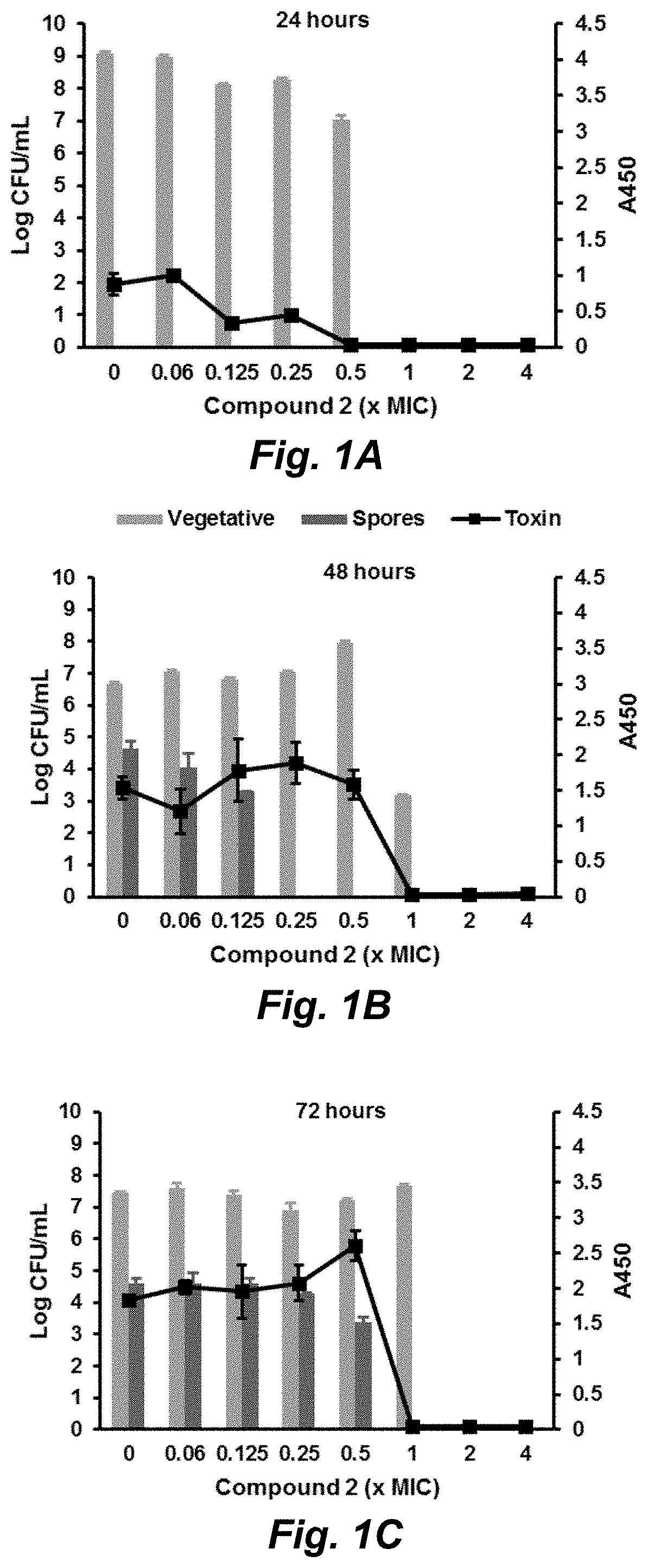
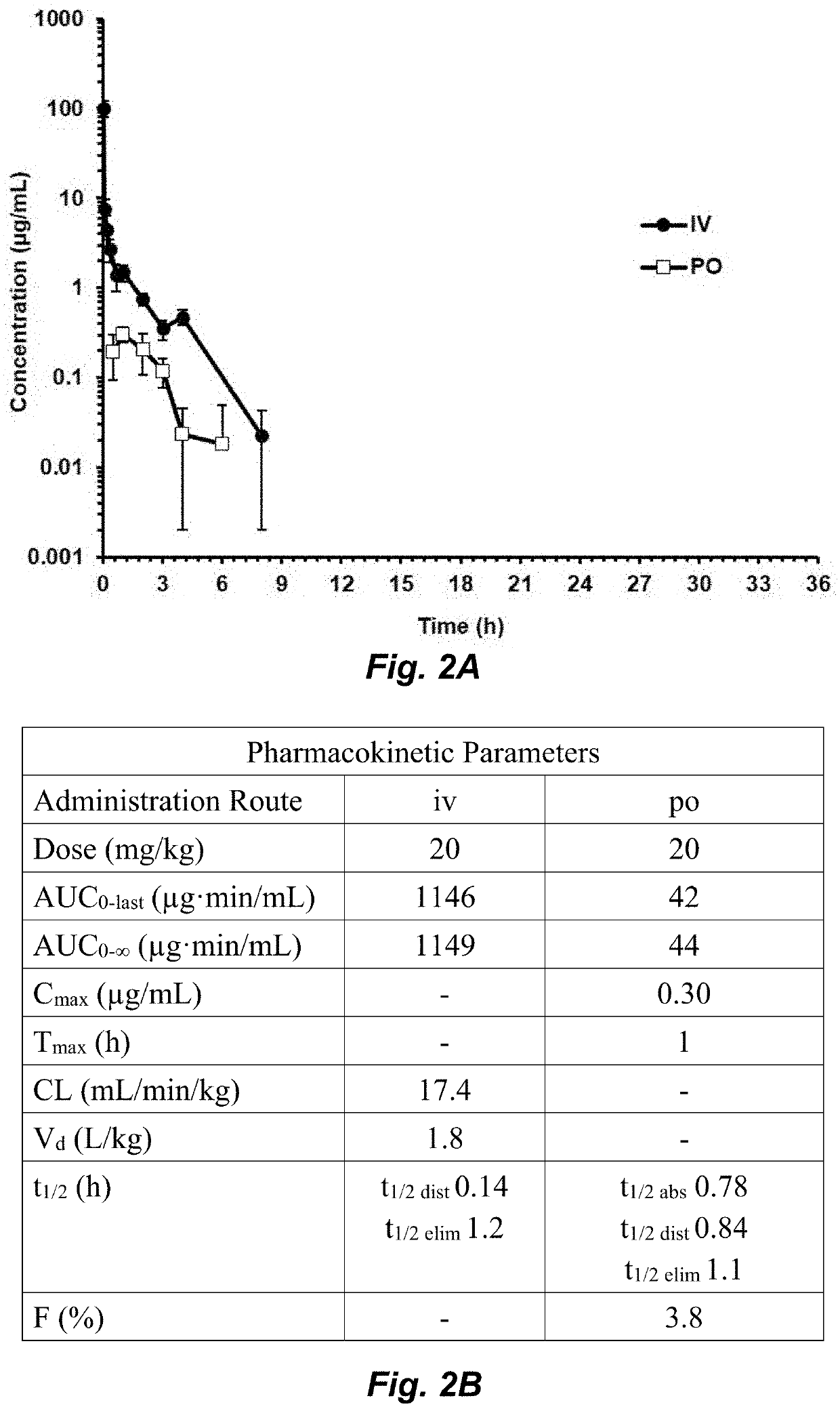
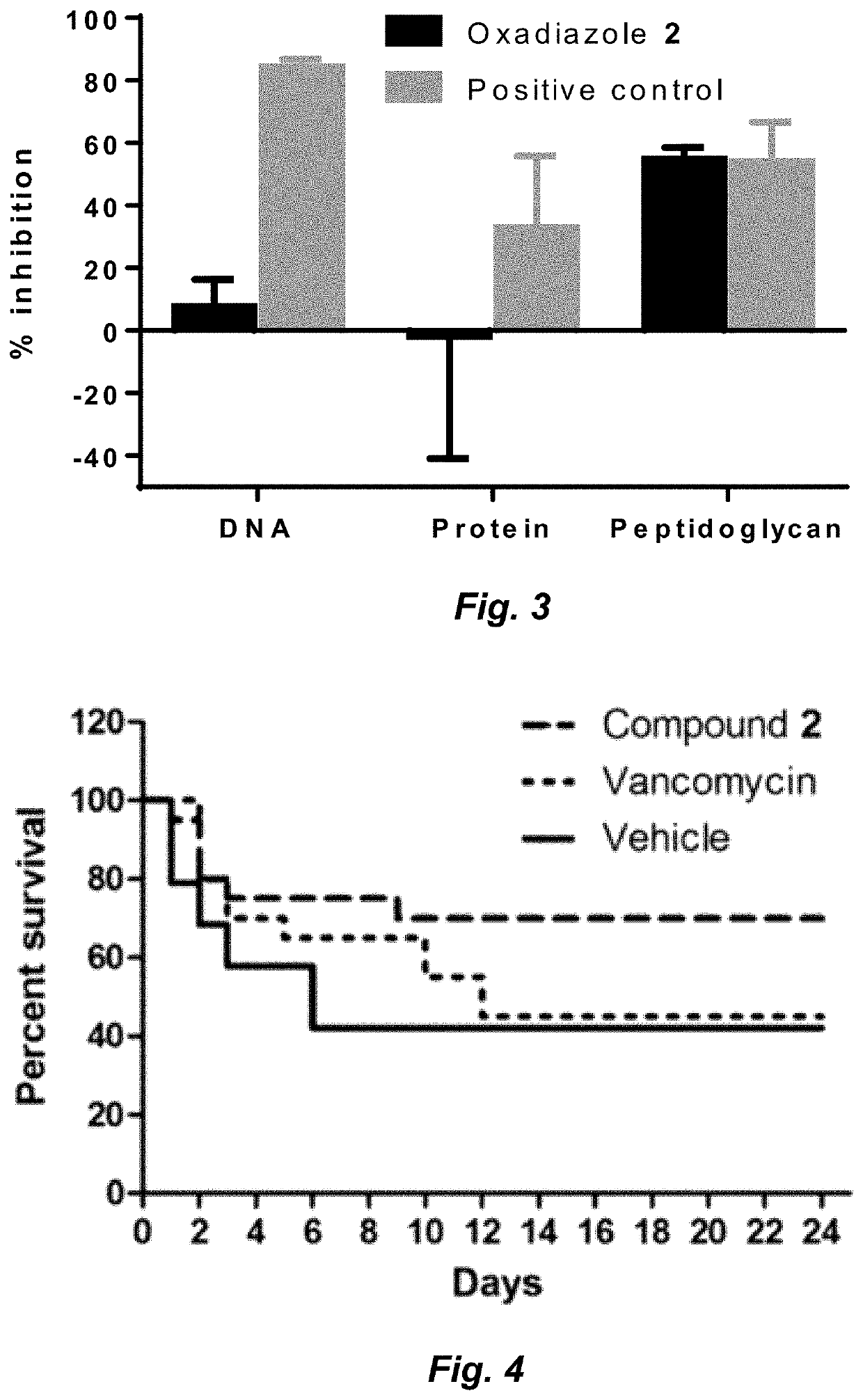
Clostridium difficile infection (CDI) is a public health threat that results in 14,000 annual deaths in the United States. Challenges involve the production of CDI spores that can remain dormant for years and the production of toxins that damage the gut. Current therapies for CDI include vancomycin and metronidazole, but neither inhibits spore or toxin production. Thus, recurrence of infection occurs in 25% of patients and there are no antibiotics that are effective for multiple recurrences. We describe oxadiazoles with activity against C. difficile, including the highly virulent NAP1 / 027 strain with increased production of toxins A and B, as well as the additional binary toxin. Oxadiazole 2 is poorly absorbed, thus advantageously achieving high concentrations in the gut. The compound targets peptidoglycan synthesis and inhibits vegetative cells, spores, and toxin production.
Owner:UNIV OF NOTRE DAME DU LAC
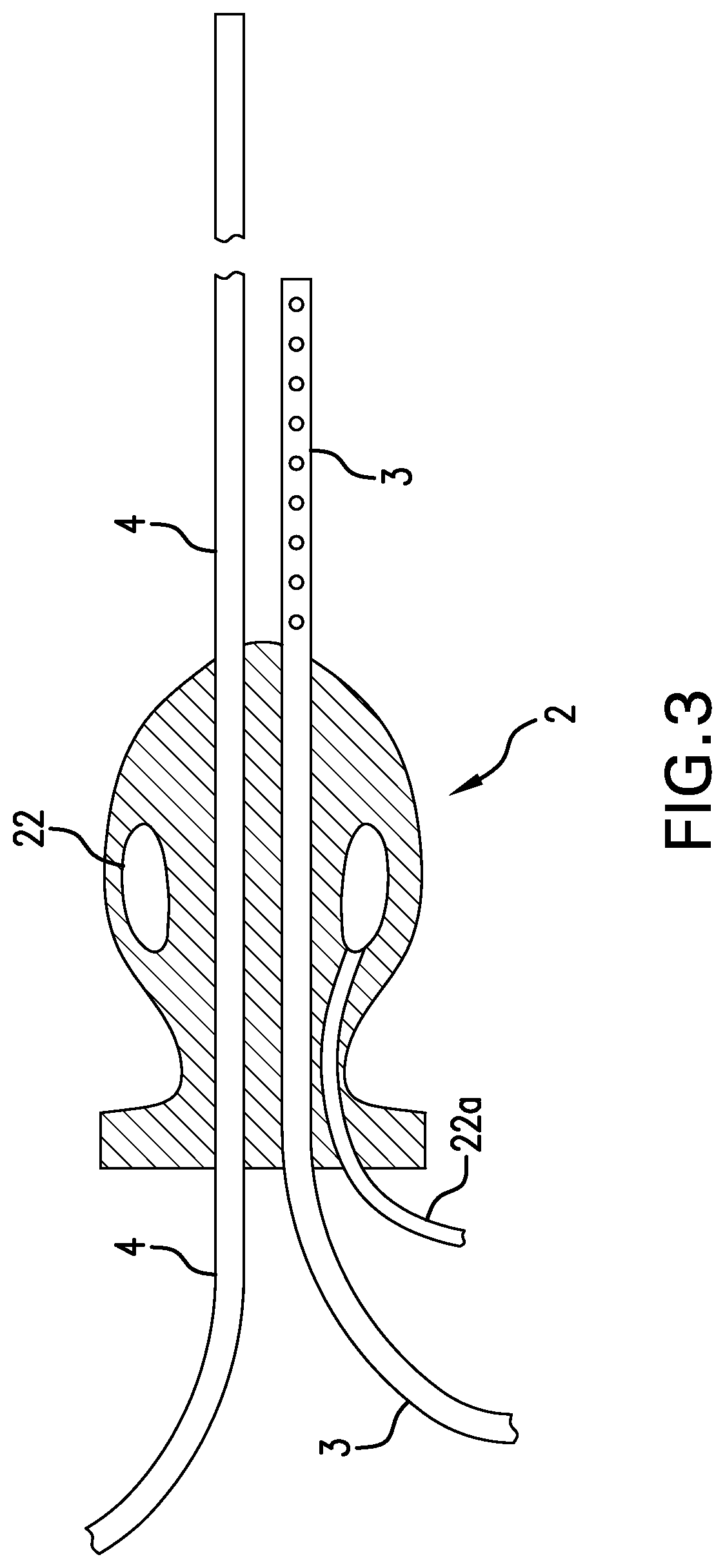
Luminal oxygen delivery and device for treating diseases and conditions associated with strict anaerobic microorganisms
PendingUS20220305218A1Increase diversityRaise oxygen partial pressureAnaesthesiaMedical devicesMicroorganismOxygen delivery
This disclosure relates to methods and apparatus for treating diseases and conditions of the colon and alimentary canal which are associated with obligate anaerobic microorganisms, and particularly, for treating Clostridioides difficile infection. The method and apparatus provide for continuous administration of intraluminal oxygen to the rectum, colon, and cecum to maintain oxygen in those environments at levels toxic to obligate anaerobic microorganisms such as Clostridioides difficile.
Owner:VON REUSNER JONATHAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com