Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1097 results about "Blood vessel walls" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
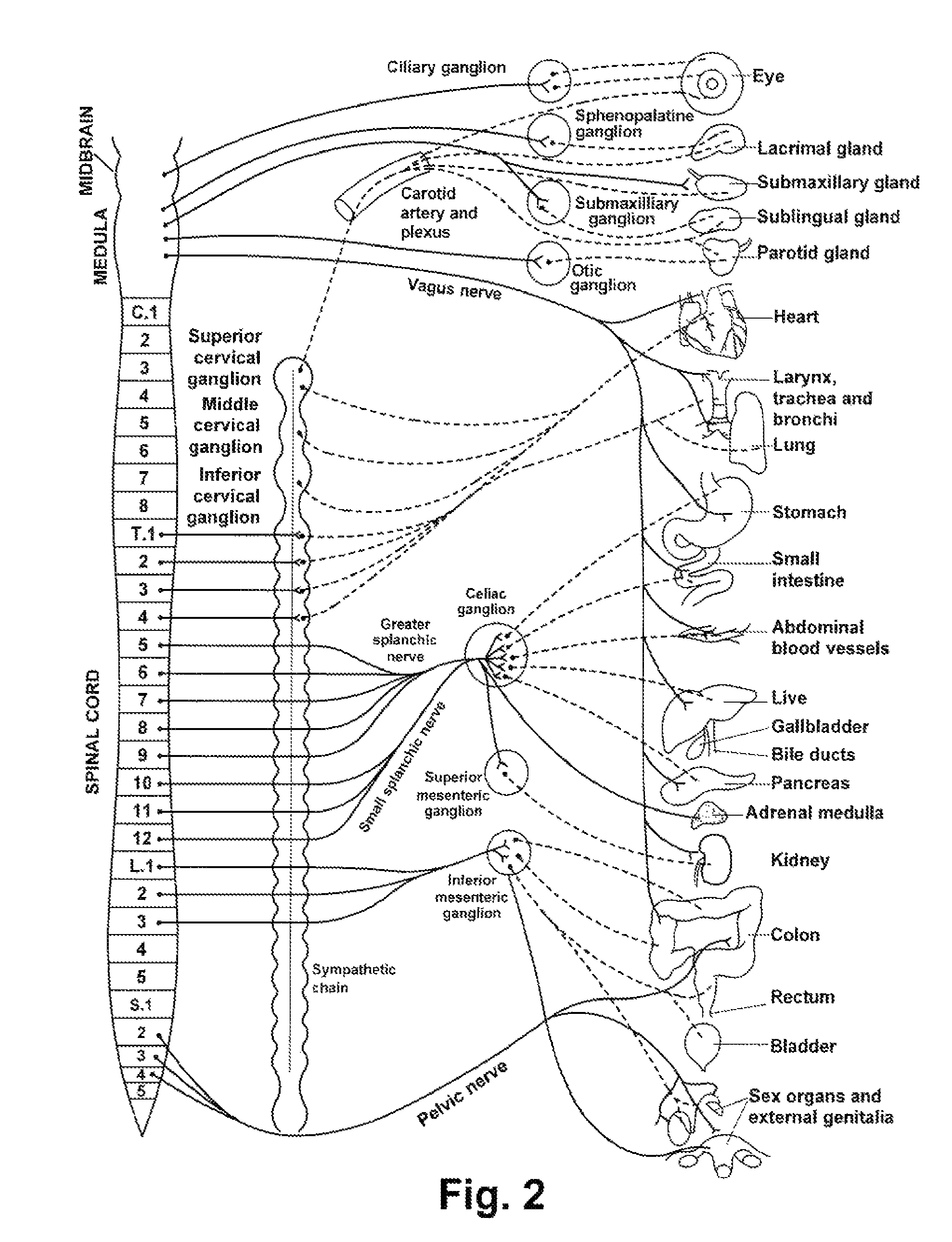
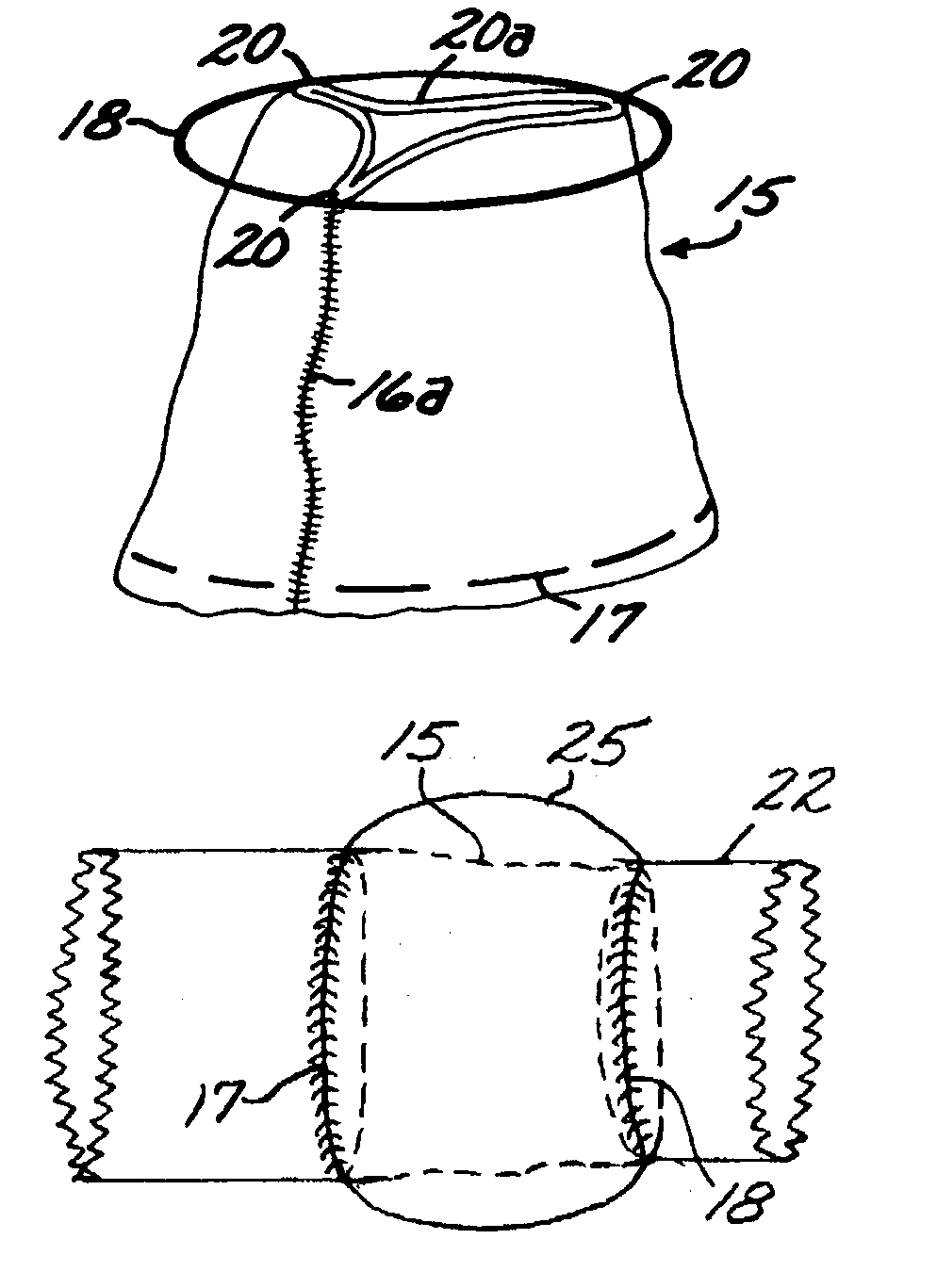
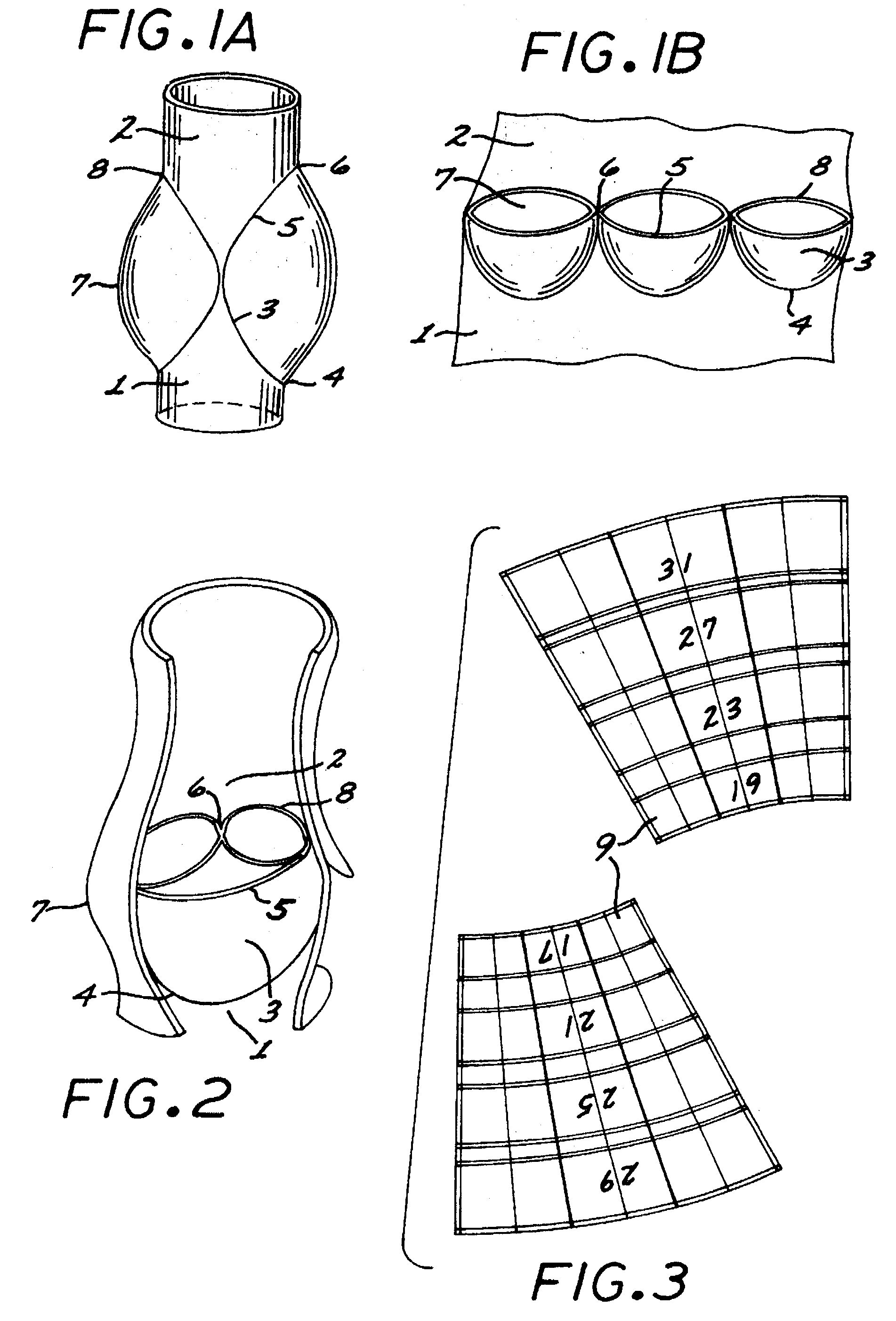
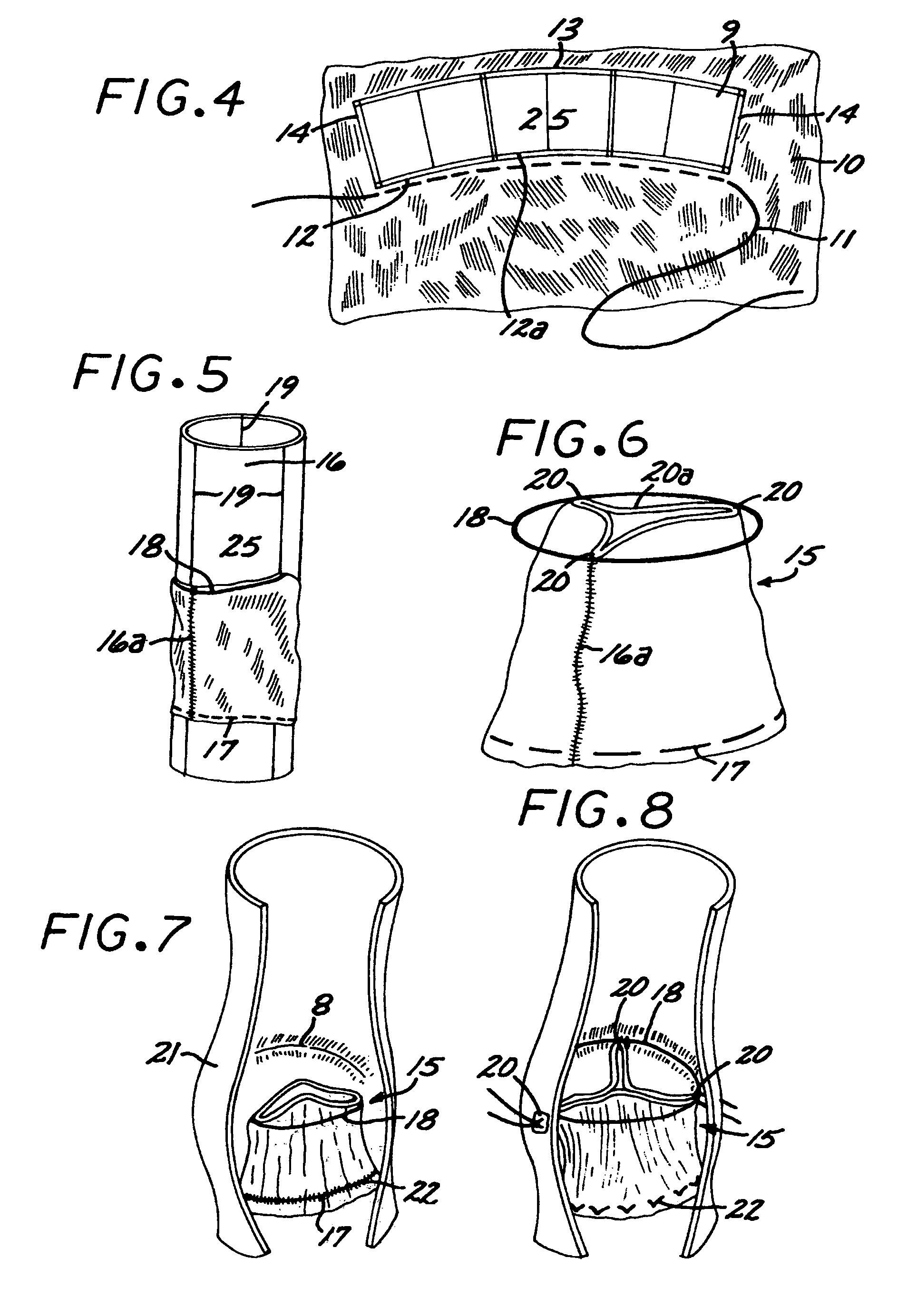
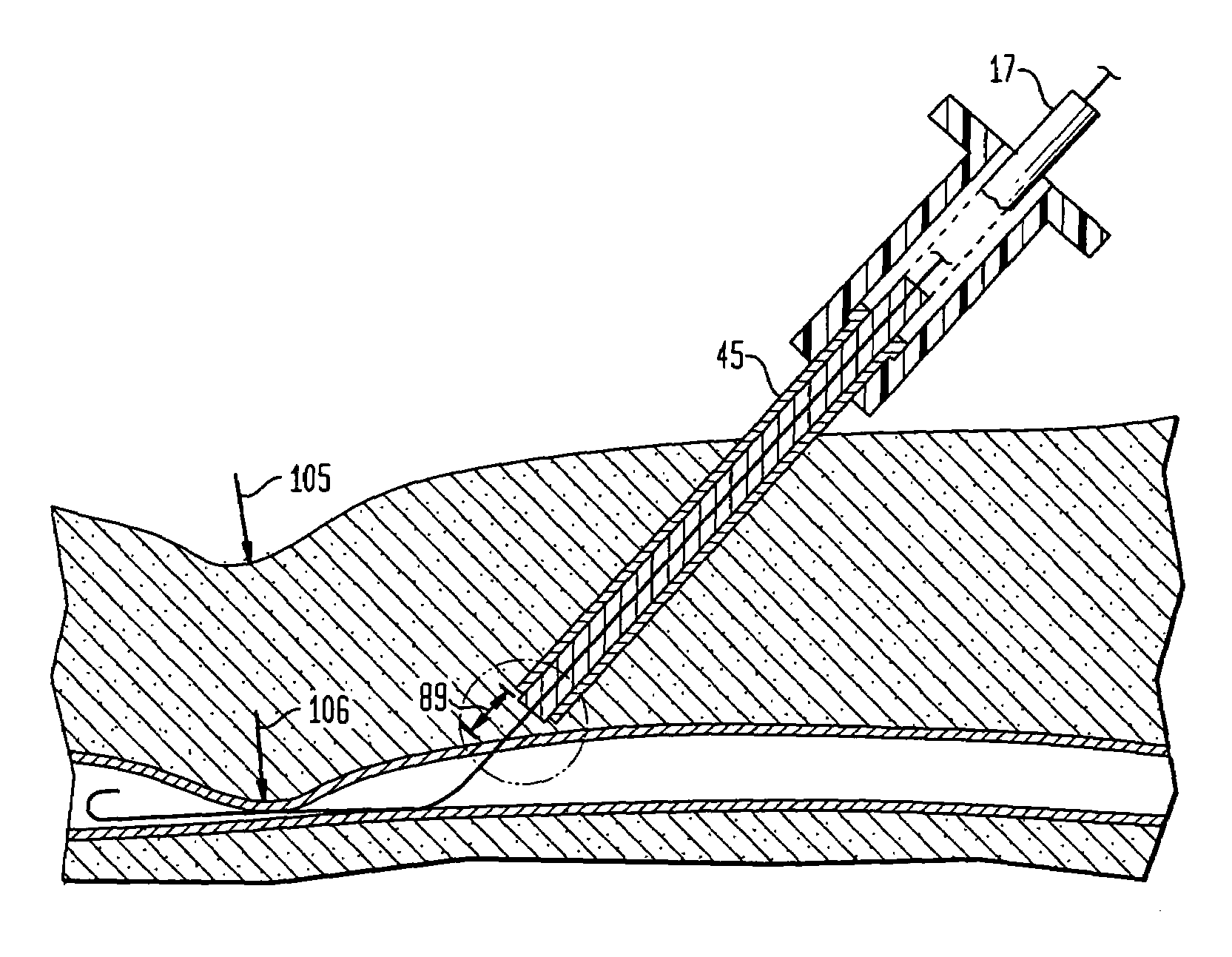
The human circulatory system is a closed circulatory system. The blood flows in closed vessels without coming in direct contact with the cells of the body. The walls of the blood vessels are made up of three layers: The blood flows in them due to the contraction and relaxation of these muscles.
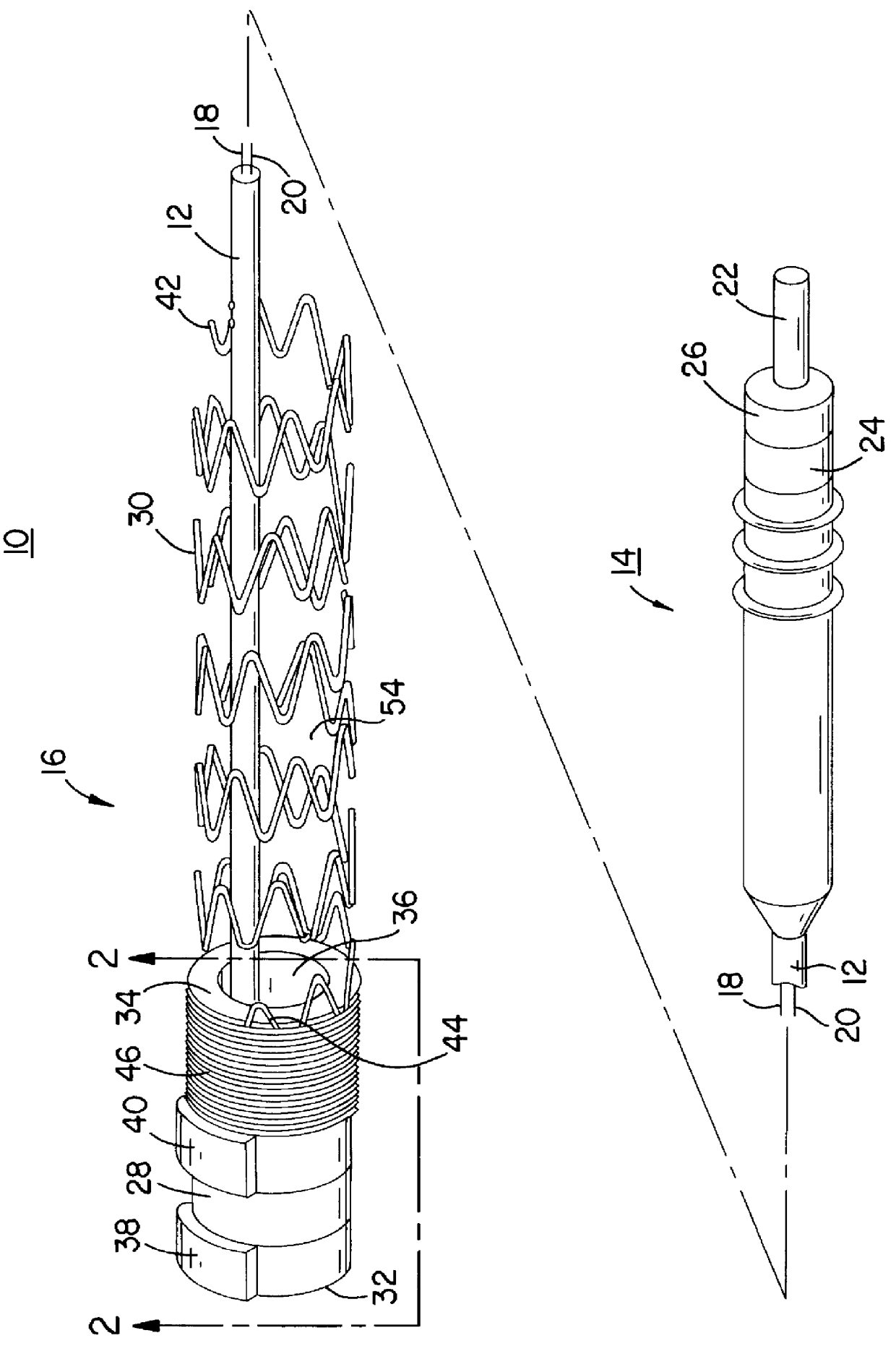
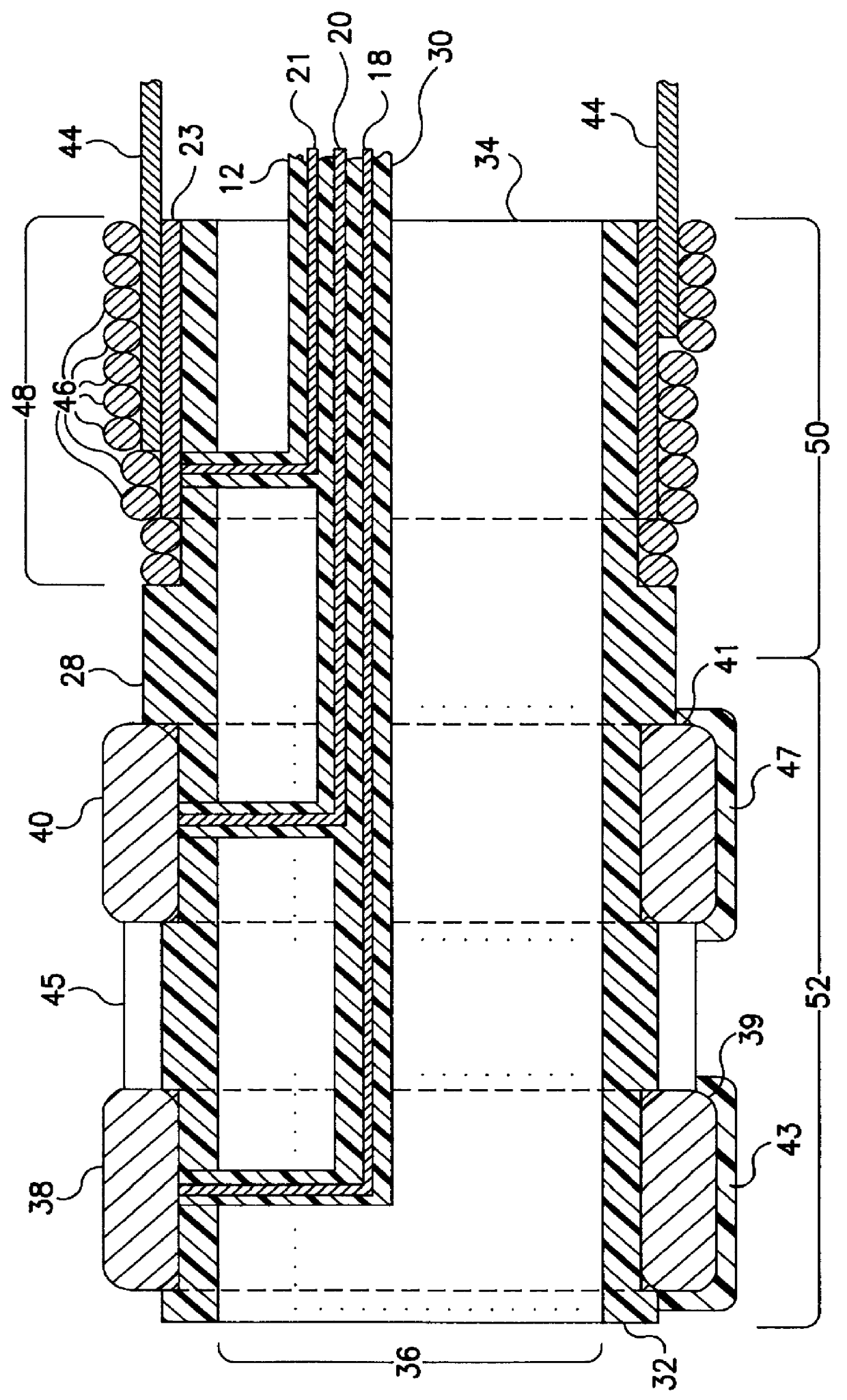
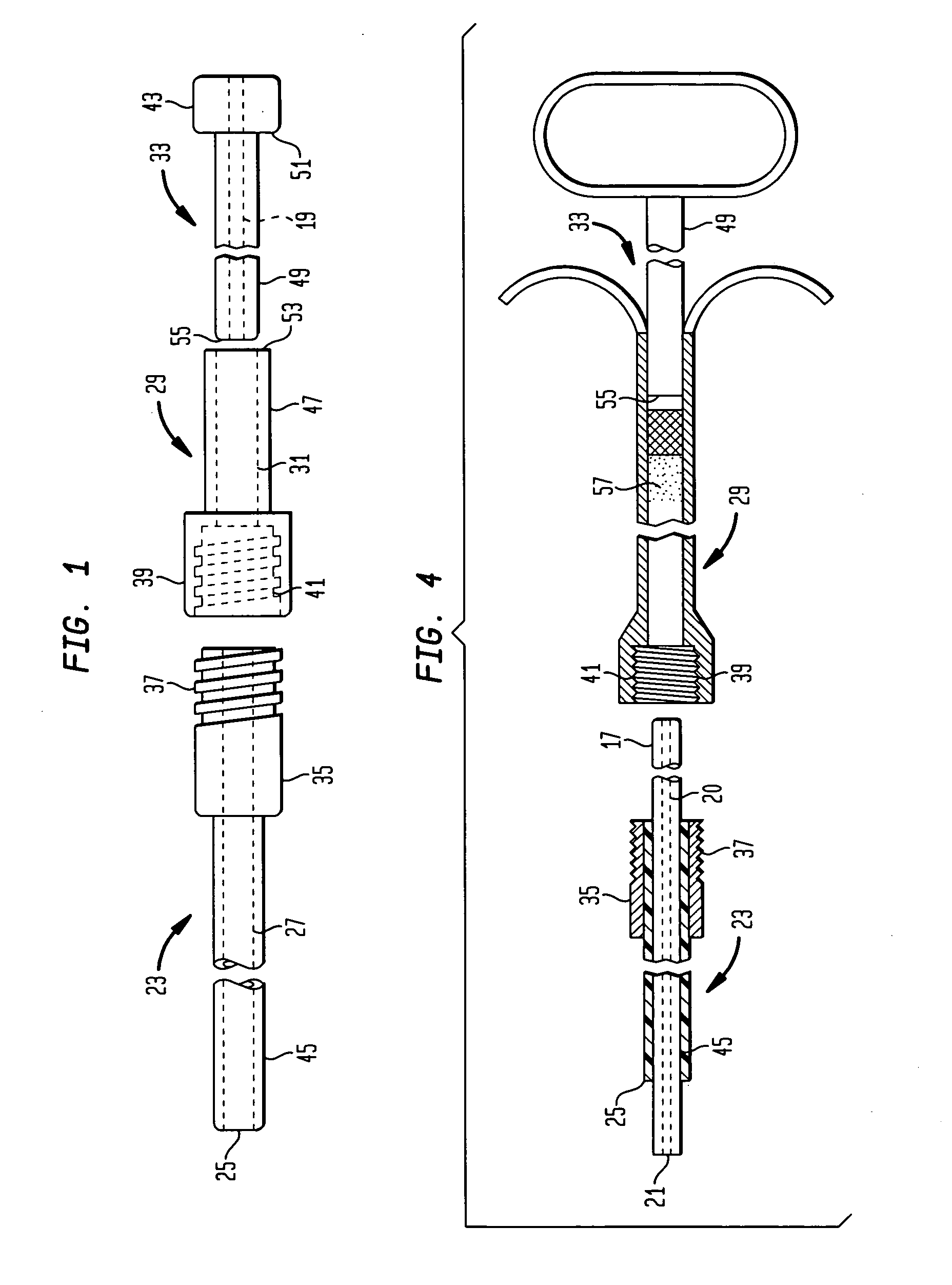
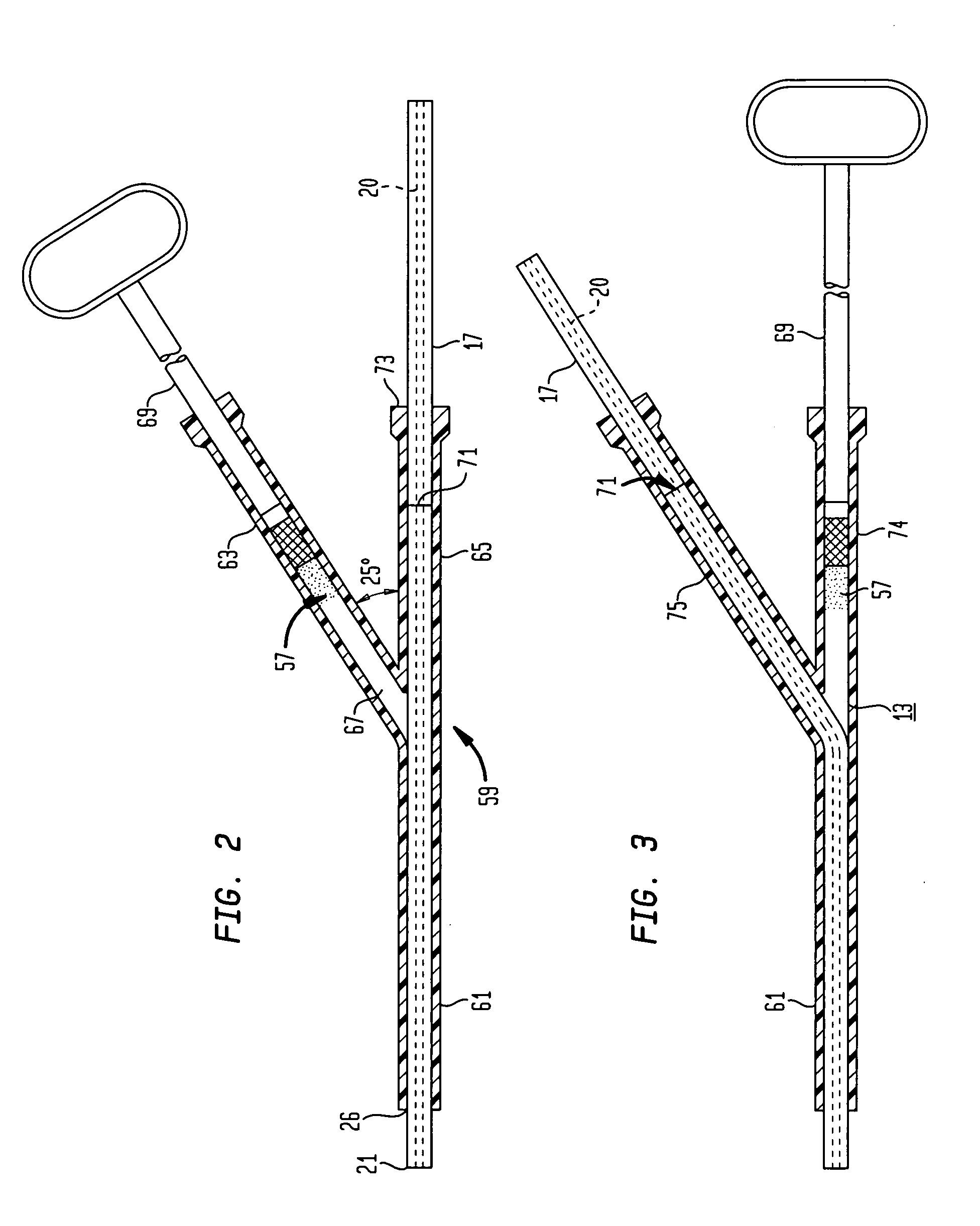
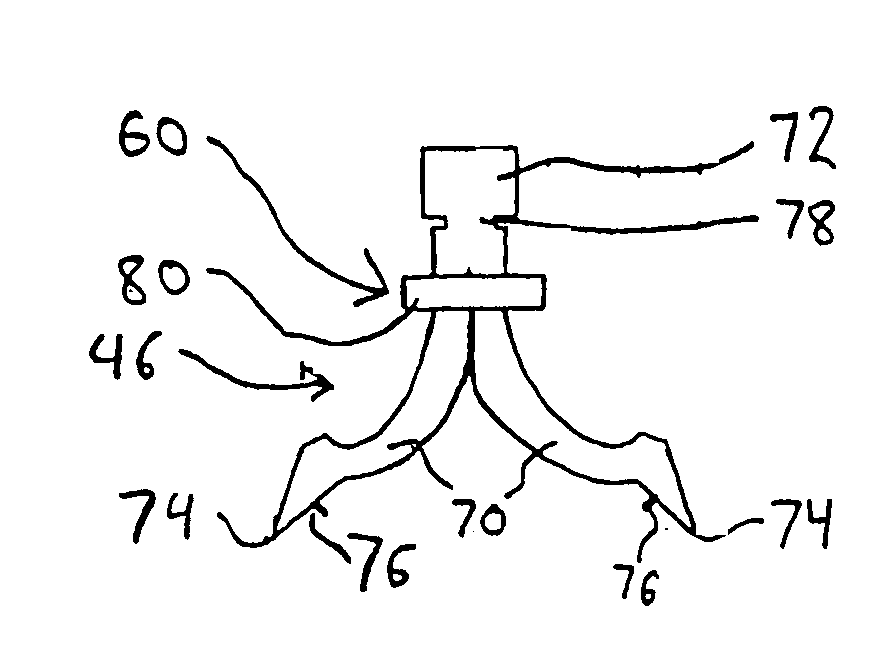
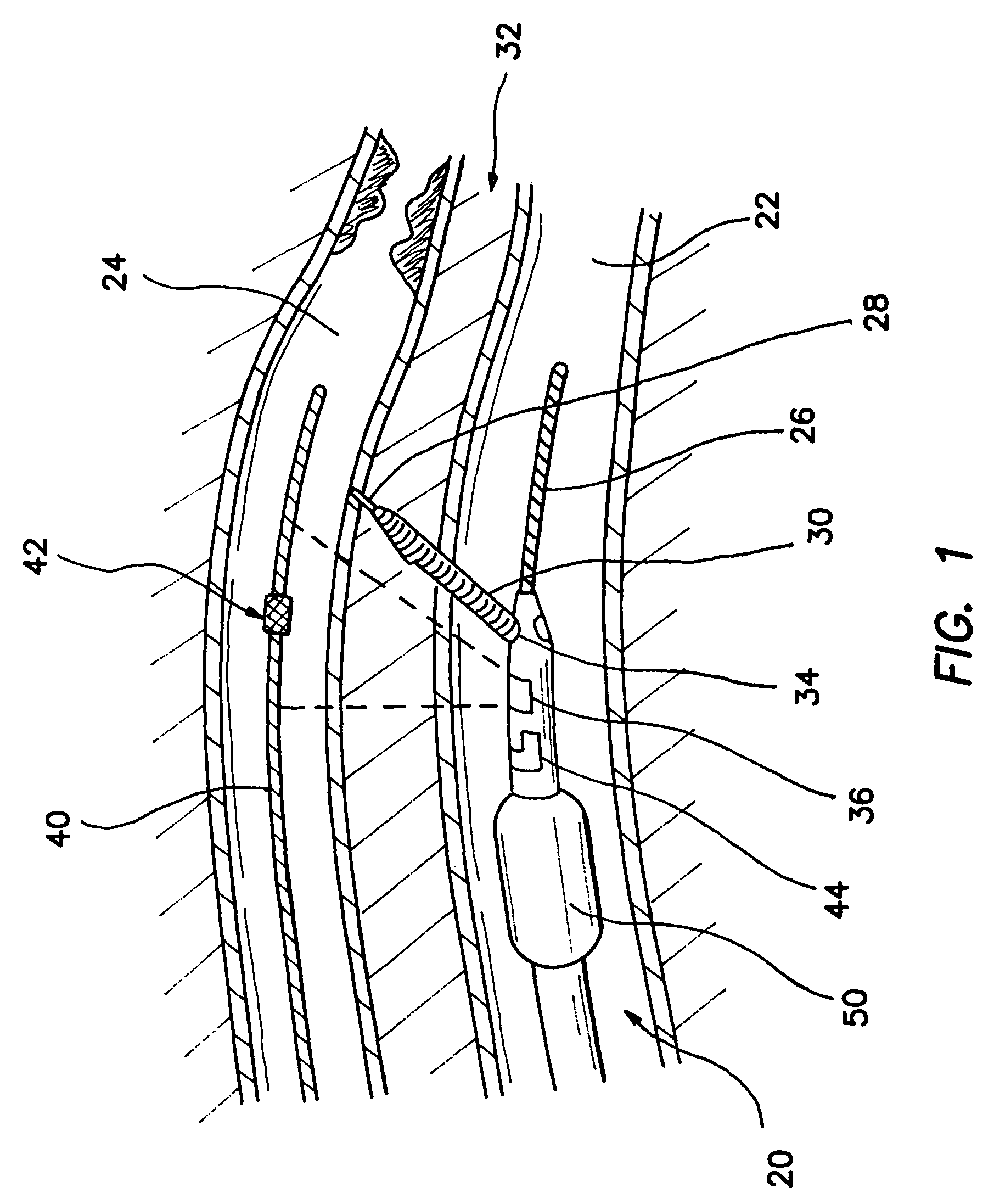
Apparatus and method for fixing electrodes in a blood vessel
InactiveUS6161029ASimple equipmentElectrocardiographyTransvascular endocardial electrodesBlood vessel wallsLeft Ventricles
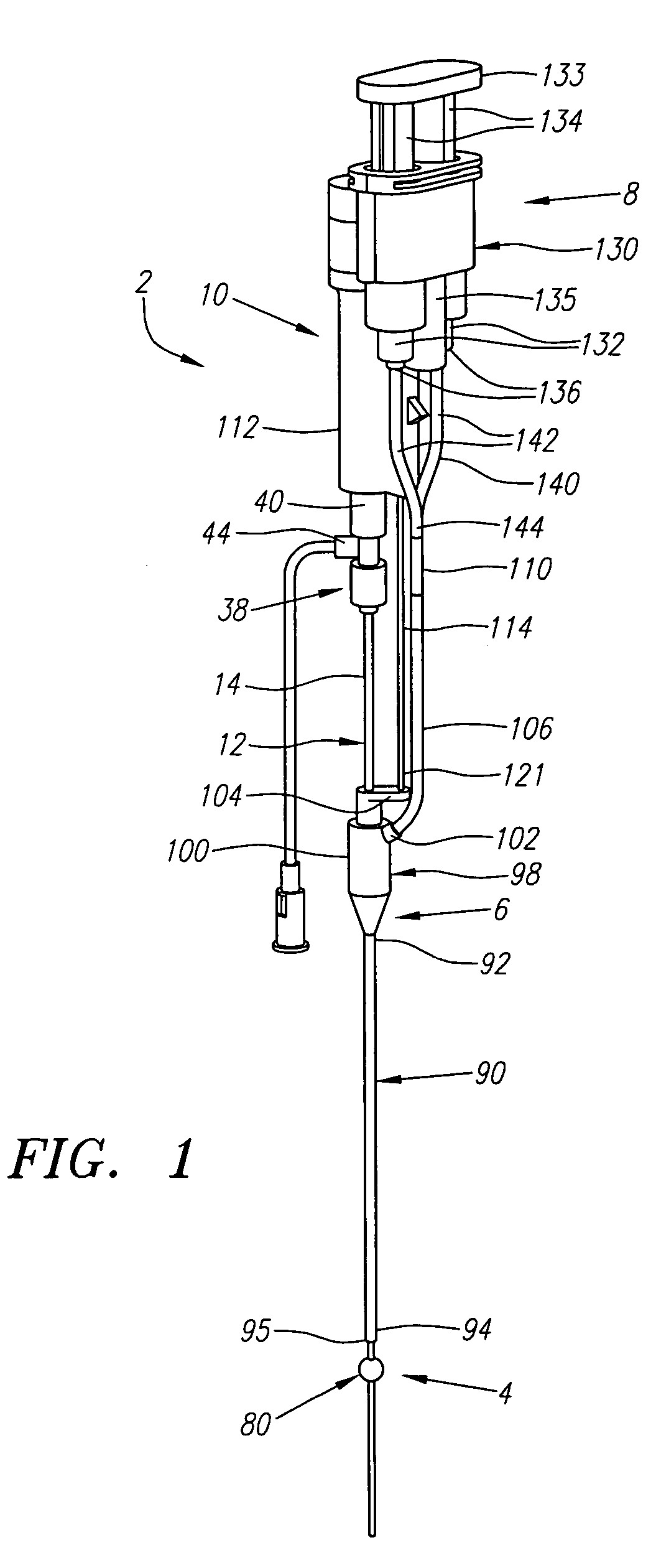
Endocardial implantable cardiac leads are disclosed for applying electrical stimulation to and / or sensing electrical activity of the heart at one or more distal electrode positioned at a cardiac implantation site within a cardiac vessel adjacent to and at a desired orientation to the left ventricle or atrium of the heart. The distal electrode(s) is supported by a tubular electrode support having a diameter large enough to bear against the blood vessel wall and a support lumen that allows blood to flow through it. A retention stent extends proximally from a distal stent end fixed to the tubular electrode support to a proximal stent end. After advancement to the cardiac implantation site employing a lead delivery mechanism, the retention stent is expandable from a collapsed stent state in which the outer diameter of the retention stent is less than the inner diameter of the vessel to an expanded stent state. The expanded stent is lodged against the blood vessel wall and inhibits movement of the stent and distal electrode support. The expanded stent lumen is aligned with the electrode support lumen for allowing blood to flow through the aligned electrode support lumen and expanded stent lumen. The retention stent may take any of the known forms that can be introduced in the collapsed stent state within an introducer lumen or mounted to an introducer and can either self-expand upon release in the blood vessel or be mechanically expanded within the blood vessel.
Owner:MEDTRONIC INC
Occlusion member and tensioner apparatus and methods of their use for sealing a vascular puncture
Apparatus for sealing a puncture includes an elongate occlusion member having a balloon attached to distal ends of telescoping inner and outer members. A housing on the proximal end of the outer member includes a piston coupled to the inner member and slidable within a chamber communicating with a fluid reservoir. A switch on the housing is actuated to direct fluid from the reservoir through the outer member into the balloon to expand the balloon and into the chamber to move the piston and pull the inner member, shortening the balloon as it expands. During use, the distal end of the occlusion member is introduced into a puncture communicating with a vessel until the collapsed balloon is disposed within the vessel. The balloon is expanded, and a tensioner is connected to the housing to apply a proximal force holding the balloon against the vessel wall to seal the puncture.
Owner:ACCESSCLOSURE
Clot capture systems and associated methods
ActiveUS20110125181A1Sufficient structureAvoid fragmentationSurgeryDilatorsBiomedical engineeringSurgery
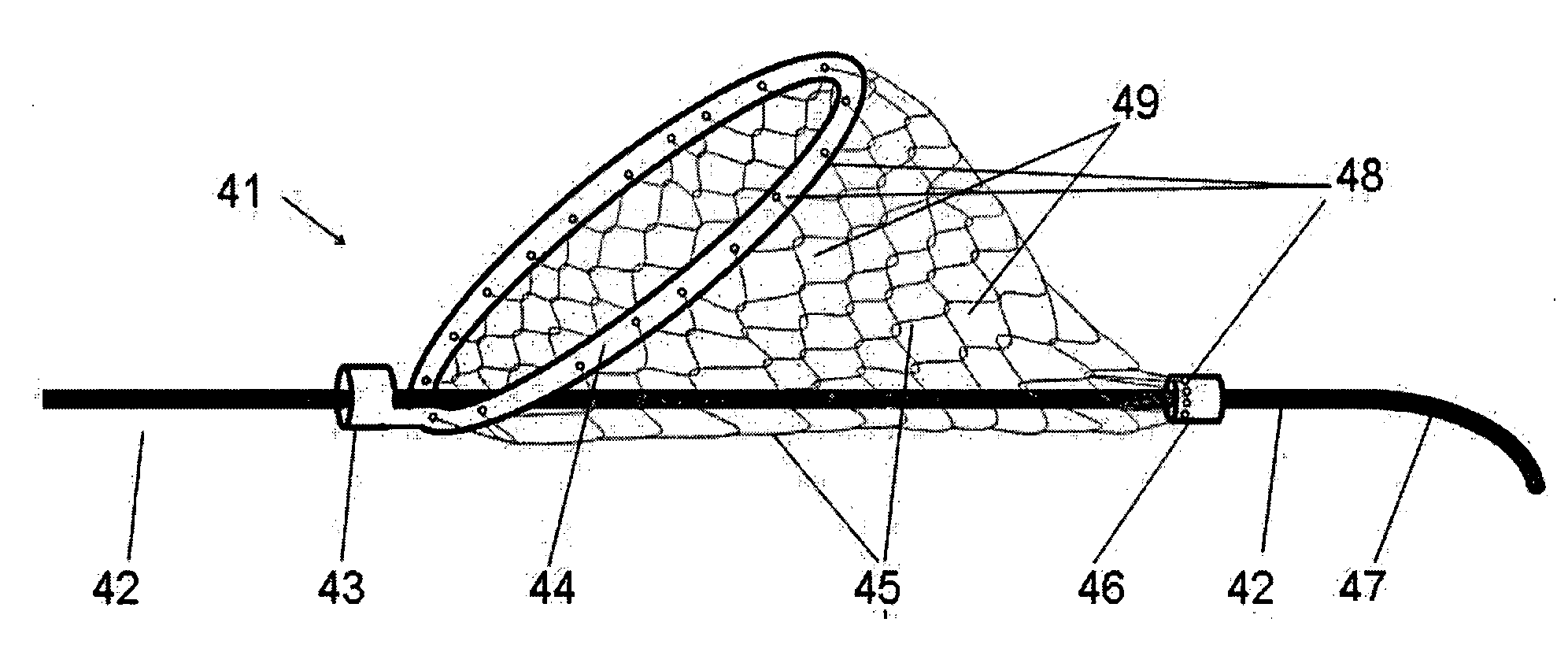
A clot capture system for disengaging a clot 2001 from a vessel wall 2002 and removing the clot 2001 from the vessel 202, comprises a clot capture device 2140 for placement on a distal side of a clot 2001. The clot capture device 2140 has a retracted delivery configuration and an expanded deployed configuration. The clot removal device has a proximal support frame 2012, and a distal fibre net 2130. The support frame 2012 has a retracted delivery configuration and an expanded deployed configuration. The proximal support frame 2012 in the expanded configuration defines a proximal inlet mouth for engaging a clot 2001 and a net 2130 for confining the clot 2001. An elongate member facilitates capture and / or withdrawal of a clot 2001 from a vessel 2002. The system also comprises a clot debonding device 2091 for placement on a proximal side of a clot 2001. The clot debonding device 2091 has a retracted delivery configuration and an expanded deployed configuration and comprises a clot engagement element 2112 which defines a distal abutment in the deployed configuration for urging a clot 2001 into the clot cap 2140.
Owner:NEURAVI
Method and apparatus for renal neuromodulation
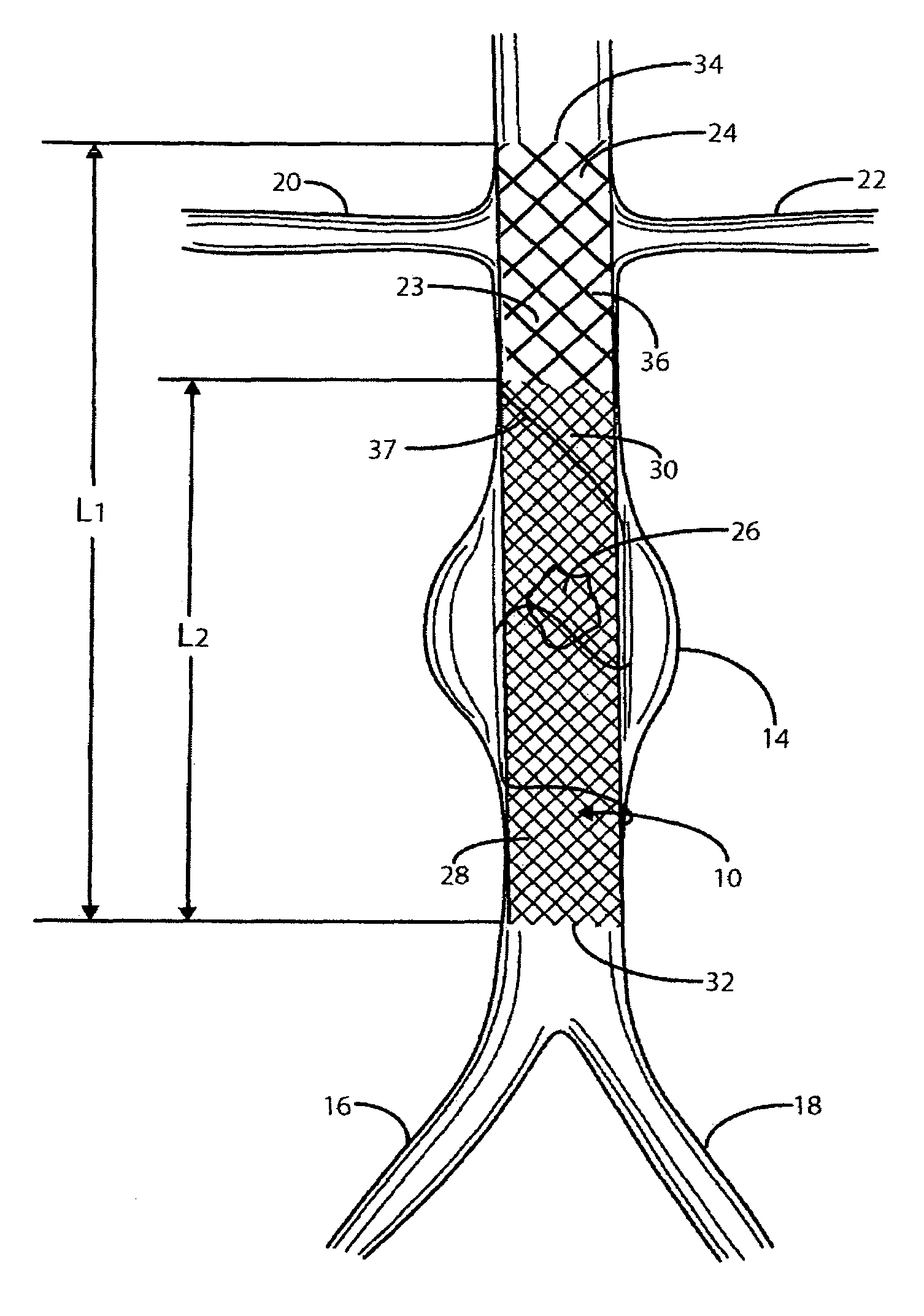
An apparatus for renal neuromodulation includes an expandable support member having a main body portion for engaging a wall of a blood vessel proximate a renal vasculature and at least one electrode connected with the main body portion. The at least one electrode is arranged to selectively deliver electric current to a desired location where modulation of the sympathetic nervous system is effective to alter renal function. The apparatus further includes an insulative material attached to at least a portion of the main body portion for isolating blood flow through the vessel from the electric current delivered by the at least one electrode.
Owner:THE CLEVELAND CLINIC FOUND
Method and apparatus for renal neuromodulation
An apparatus for renal neuromodulation includes an expandable support member having a main body portion for engaging a wall of a blood vessel proximate a renal vasculature and at least one electrode connected with the main body portion. The at least one electrode is arranged to selectively deliver electric current to a desired location where modulation of the sympathetic nervous system is effective to alter renal function. The apparatus further includes an insulative material attached to at least a portion of the main body portion for isolating blood flow through the vessel from the electric current delivered by the at least one electrode.
Owner:THE CLEVELAND CLINIC FOUND
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS20070168019A1Avoid interactionReduce overall outer diameterStentsCatheterVascular Skin TumorSaphenous veins
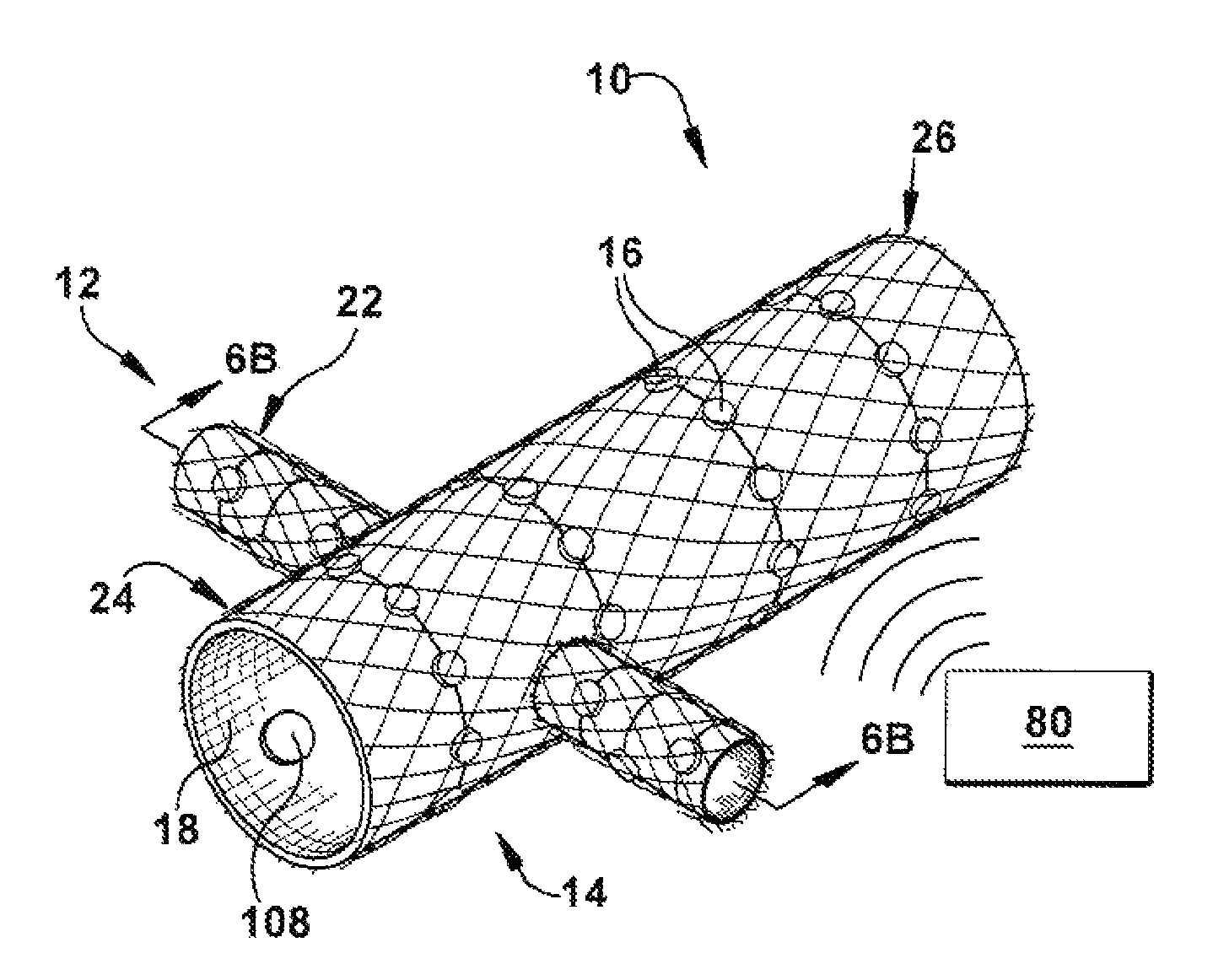
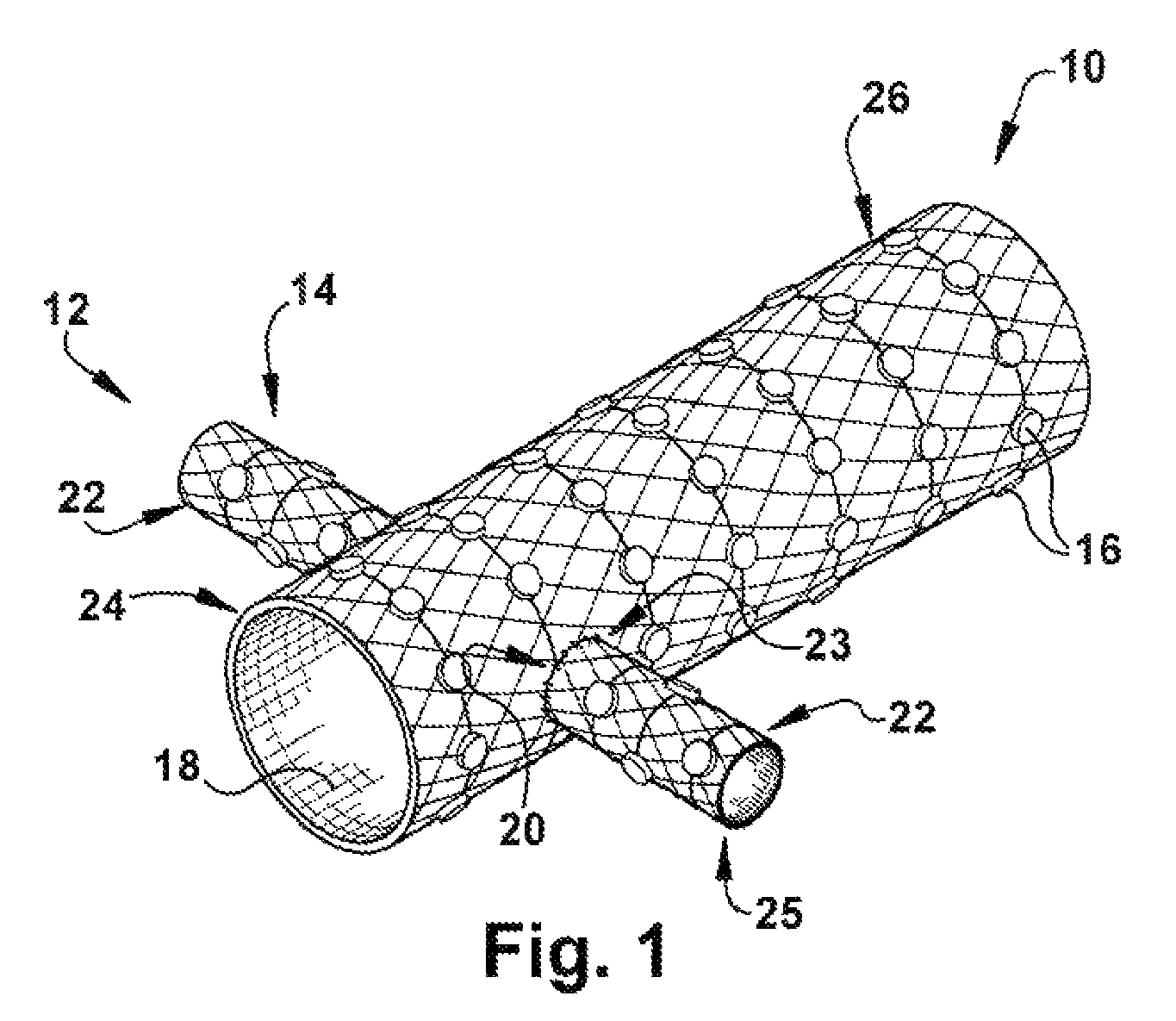
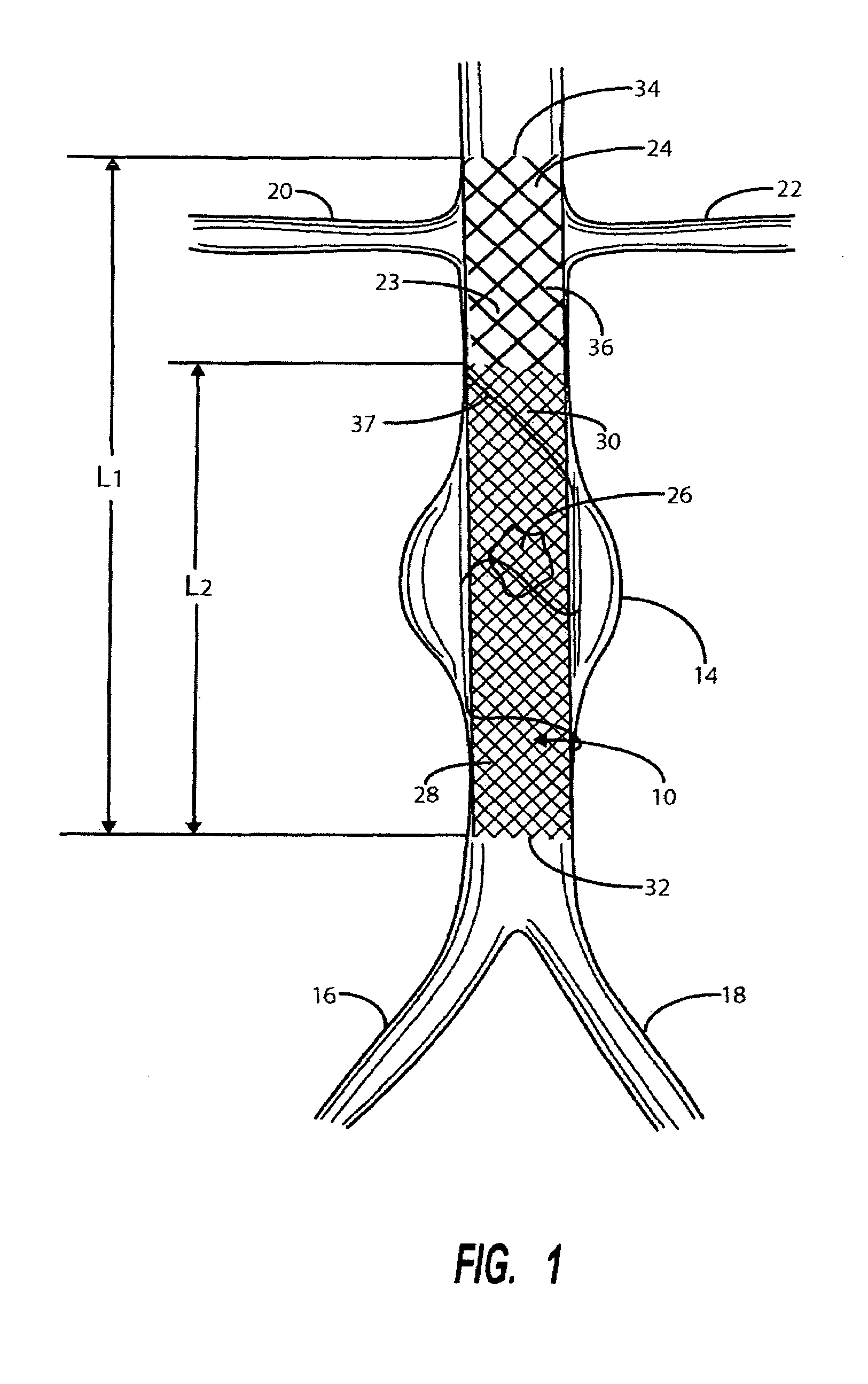
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Energy based devices and methods for treatment of anatomic tissue defects
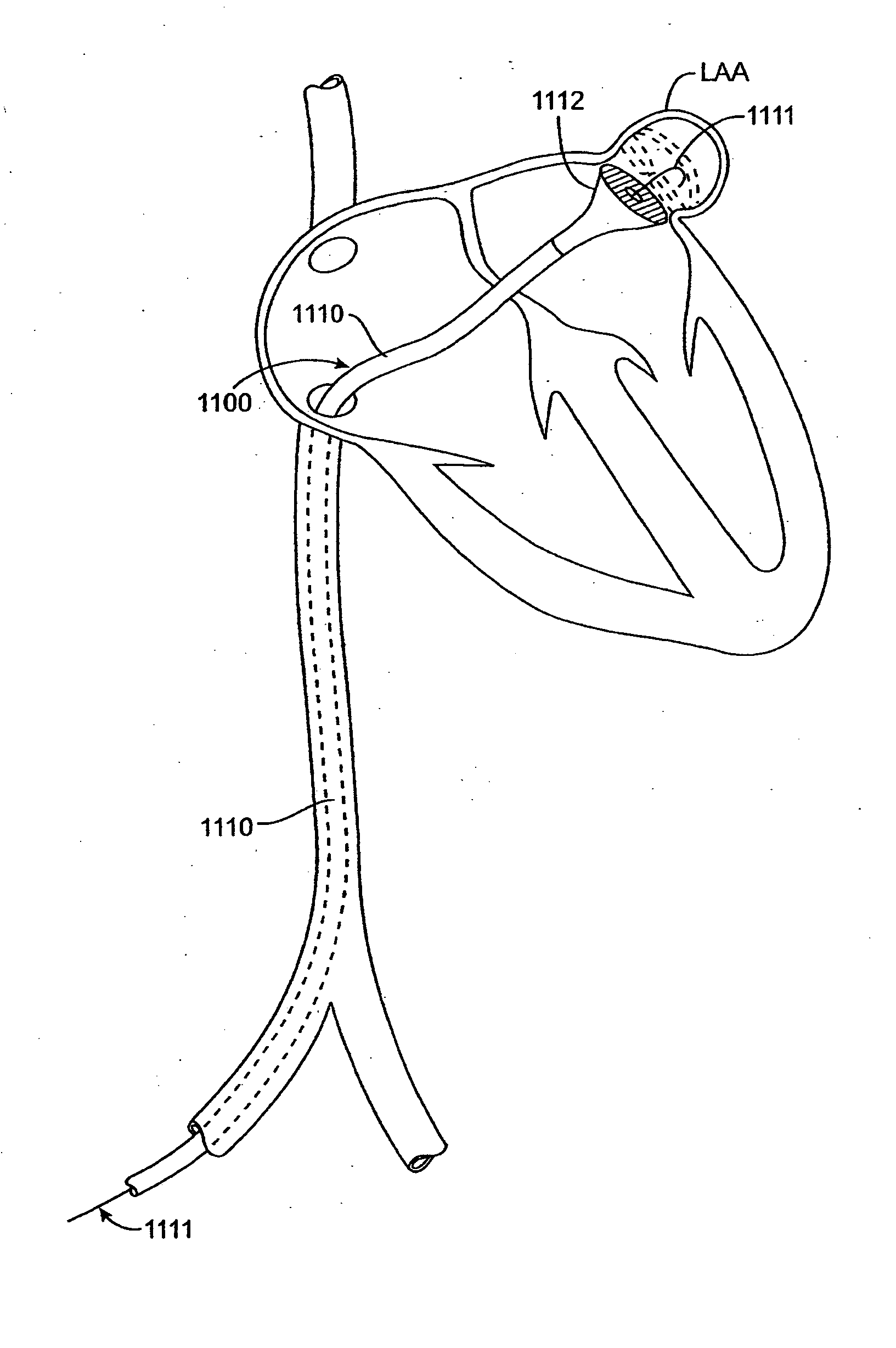
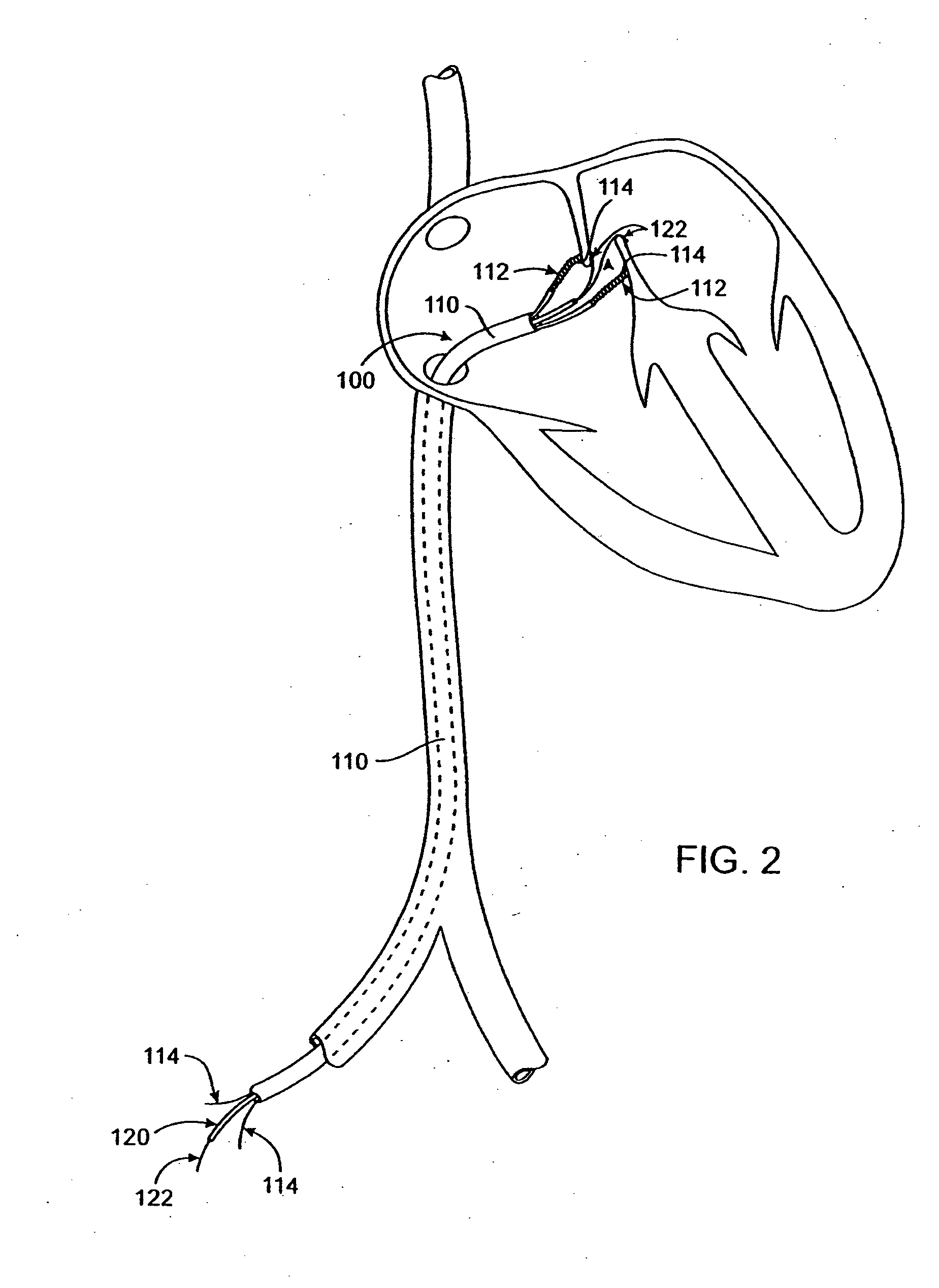
Methods and apparatus for treatment of anatomic defects in human tissues, such as patent foramen ovale (PFO), atrial or ventricular septal defects, left atrial appendage, patent ductus arteriosis, blood vessel wall defects and certain electrophysiological defects, involve positioning a distal end of an elongate catheter device at the site of the anatomic defect, engaging tissues at the site of the anatomic defect to bring the tissues together, and applying energy to the tissues with the catheter device to substantially close the anatomic defect acutely. Apparatus generally includes an elongate catheter having a proximal end and a distal end, a vacuum application member coupled with the distal end for engaging tissues at the site of the anatomic defect and applying vacuum to the tissues to bring them together, and at least one energy transmission member coupled with the vacuum application member for applying energy to tissues at the site of the anatomic defect to substantially close the defect acutely.
Owner:TERUMO KK
Apparatus and methods for facilitating hemostasis within a vascular puncture
ActiveUS20060047313A1Good hemostasisSuture equipmentsSurgical veterinaryBlood vesselMedical procedure
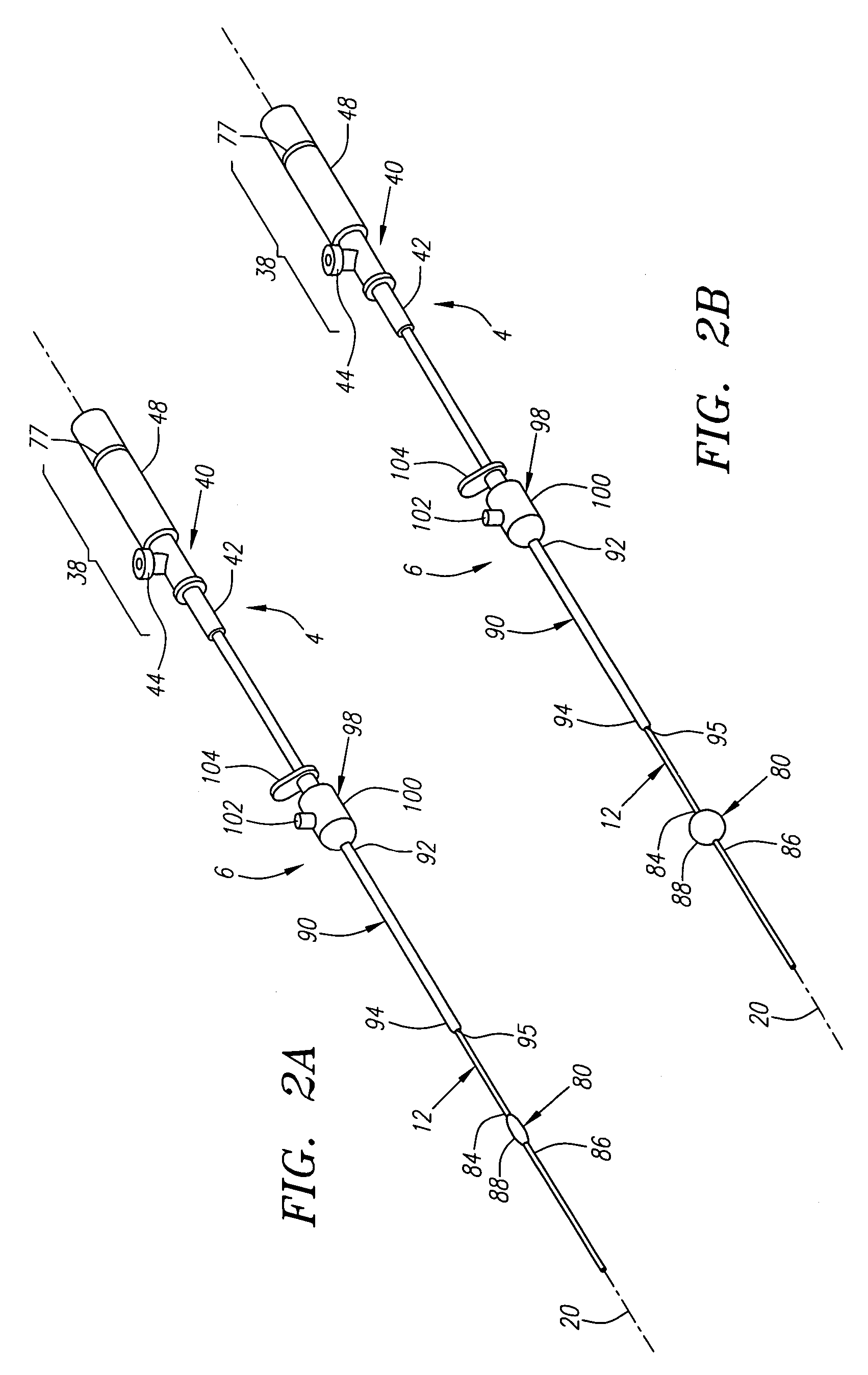
Apparatus for sealing a puncture communicating with a blood vessel includes a bioabsorbable sealing member secured to one end of a filament or other retaining member. The sealing member is delivered through the puncture into the vessel, and retracted against the wall of the vessel to provide temporary hemostasis. The sealing member is rapidly absorbed after exposure within the vessel, e.g., to an aqueous or heated physiological environment (e.g., exposure to blood or body temperature), immediately or shortly after completing a medical procedure via the puncture, e.g., within the time period that the patient is ambulatory optionally, extravascular sealing material is delivered into the puncture proximal to the sealing member. The retaining member and / or extravascular material may be bioabsorbable, being absorbed at a slower rate than the sealing member. Alternatively, the filament is removed from the puncture after hemostasis is established.
Owner:ACCESSCLOSURE
Sigmoid valve and method for its percutaneous implantation
Owner:THE INT HEART INST OF MONTANA FOUND
Device and method for sealing puncture wounds
A device is proposed for inserting hemostatic material through a tissue channel and against the outside wall of a blood vessel of a patient, wherein the blood vessel wall has a puncture therein adjacent the tissue channel. The device includes a charge of hemostatic material and a hollow sheath adapted to pass through the tissue channel, the sheath having a cross sectional profile larger than the puncture. The device places the hemostatic material in the hollow sheath and advances the hemostatic material through the sheath to the outside of the vessel wall around the puncture.
Owner:ST JUDE MEDICAL PUERTO RICO BV
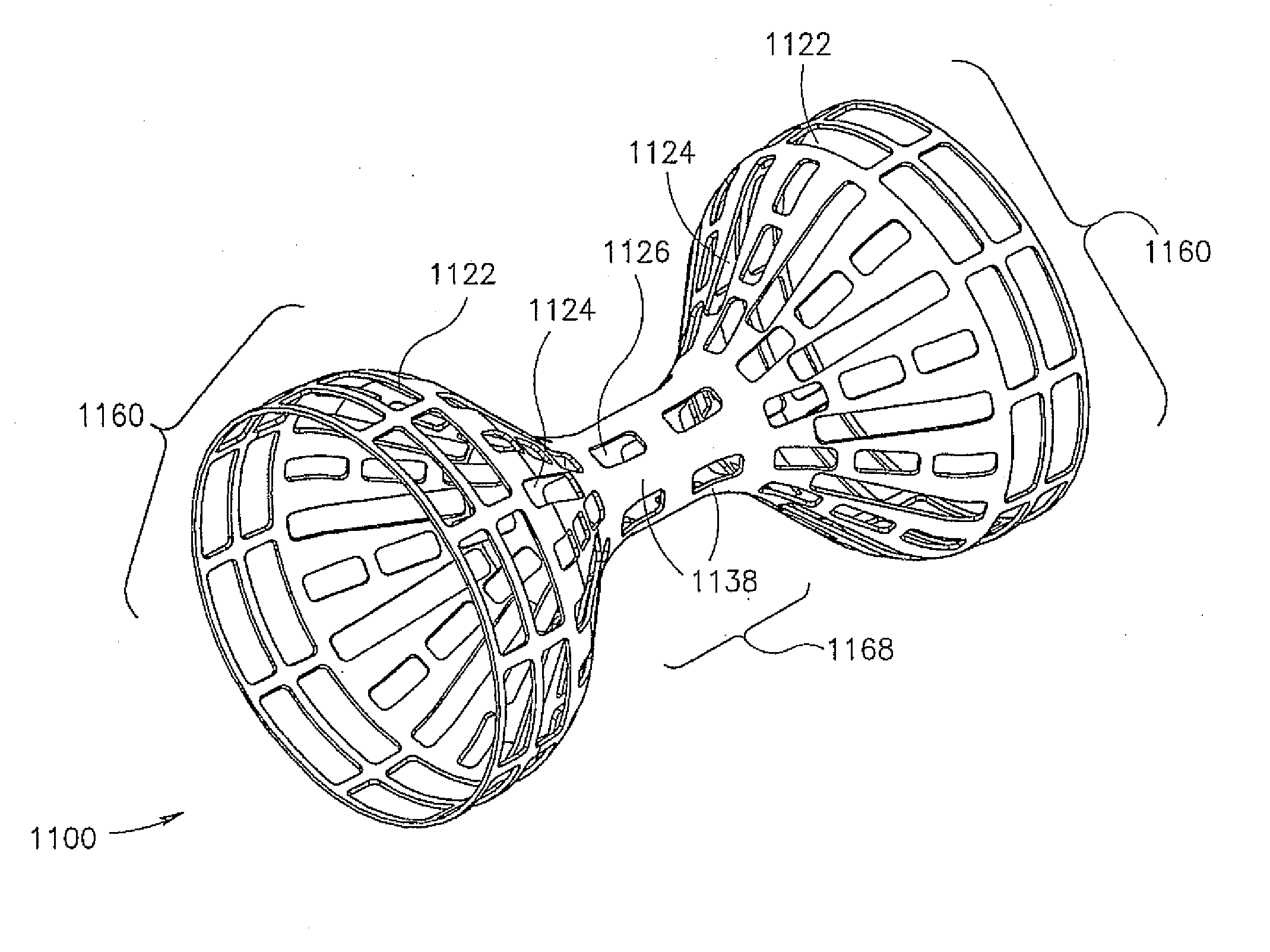
Flow reducing implant
InactiveUS20050055082A1Avoid rotational movementAvoid relative motionStentsDilatorsBiomedical engineeringBlood vessel
A flow reducing implant for reducing blood flow in a blood vessel having a cross sectional dimension, the flow reducing implant comprising a hollow element adapted for placement in the blood vessel defining a flow passage therethrough, said flow passage comprising at least two sections, one with a larger diameter and one with a smaller diameter, wherein said smaller diameter is smaller than a cross section of the blood vessel. A plurality of tabs anchor, generally parallel to the blood vessel wall, are provided in some embodiments of the invention.
Owner:NEOVASC MEDICAL LTD
Implant which is intended to be placed in a blood vessel
This implant comprises an endoprosthesis having an axis which can be spontaneously deployed in a radial manner between a compressed configuration and a dilated configuration. The implant comprises at least one radial runner which comprises a separation surface which extends radially with respect to an outer surface of the endoprosthesis and a member which can be deployed away from the axis. The runner is arranged so as to delimit a confinement housing which extends between the separation surface and the outer surface and a radial spacer which is defined by the separation surface and the deployable member. When the implant is retained in a state of radial compression, the maximum radial width of the housing is less than the maximum radial width of the spacer which is not equal to zero.
Owner:WL GORE & ASSOC INC
Flow reducing implant
A flow reducing implant for reducing blood flow in a blood vessel having a cross sectional dimension, the flow reducing implant comprising a hollow element adapted for placement in the blood vessel defining a flow passage therethrough, said flow passage comprising at least two sections, one with a larger diameter and one with a smaller diameter, wherein said smaller diameter is smaller than a cross section of the blood vessel. A plurality of tabs anchor, generally parallel to the blood vessel wall, are provided in some embodiments of the invention.
Owner:BIO IP VENTURES II LLC AS COLLATERAL AGENT
Methods, devices and systems for forming magnetic anastomoses
Methods, devices and systems for forming magnetic anastomoses between two blood vessels. A first anastomotic component is removably supported by the distal end of a delivery device for attachment to a first vessel. The delivery device also supports a second anastomotic component that has been secured to a second blood vessel. The device is operated to secure the first component to the first vessel, couple the second component to the first component, and then release the components to complete the anastomosis. A robotic anastomosis system includes several robotic instruments that may be positioned through ports in a patient, used to secure an anastomotic component to a vessel, and then used to magnetically couple the components. Delivery devices for deploying magnetic anastomotic components include an actuator that uses magnetic repulsion to move the components into engagement with the inner and outer surfaces of the vessel wall. The anastomotic components are secured to the vessel wall by magnetic force and in addition may be secured by mechanical attachment.
Owner:MEDTRONIC INC
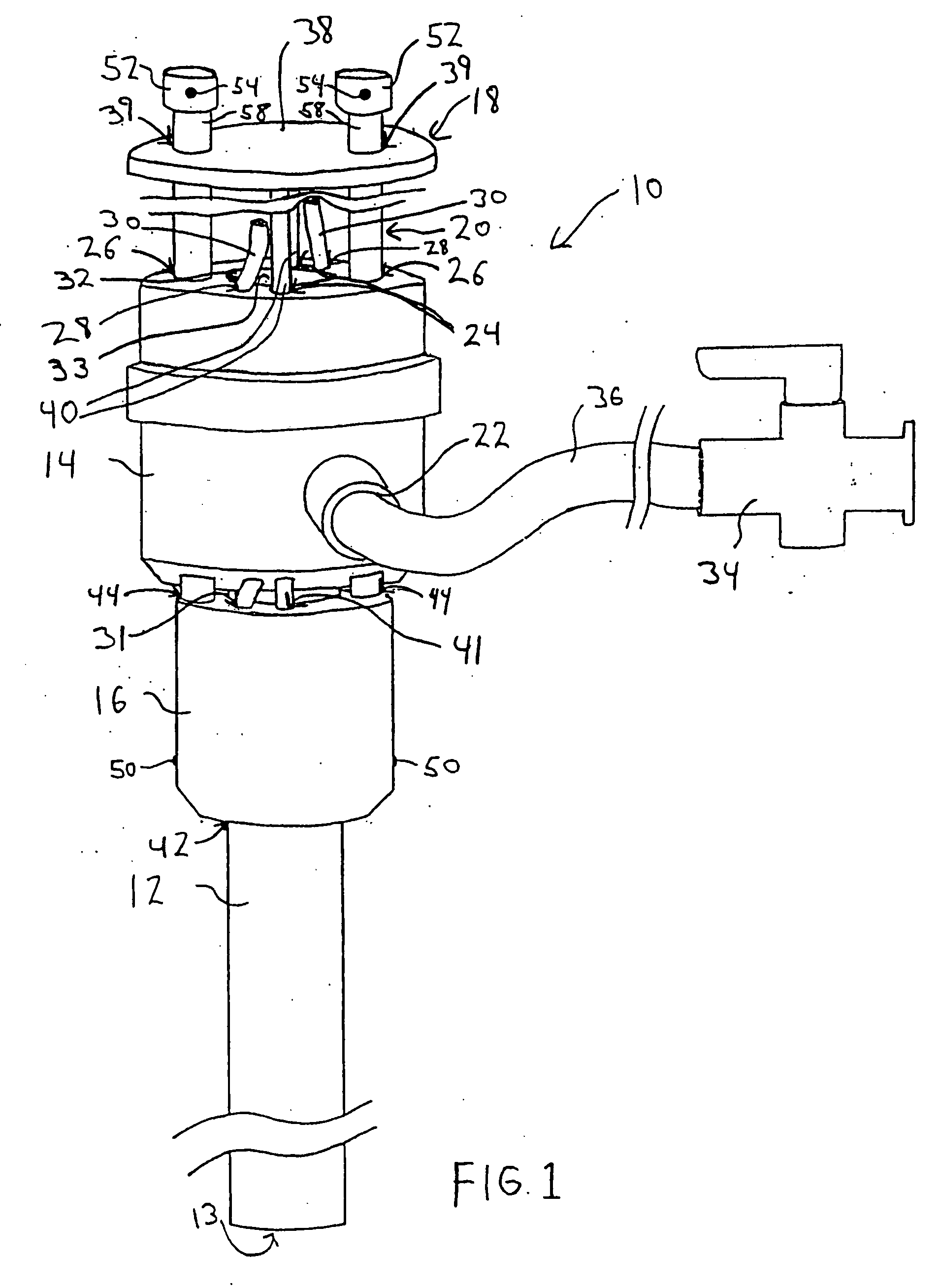
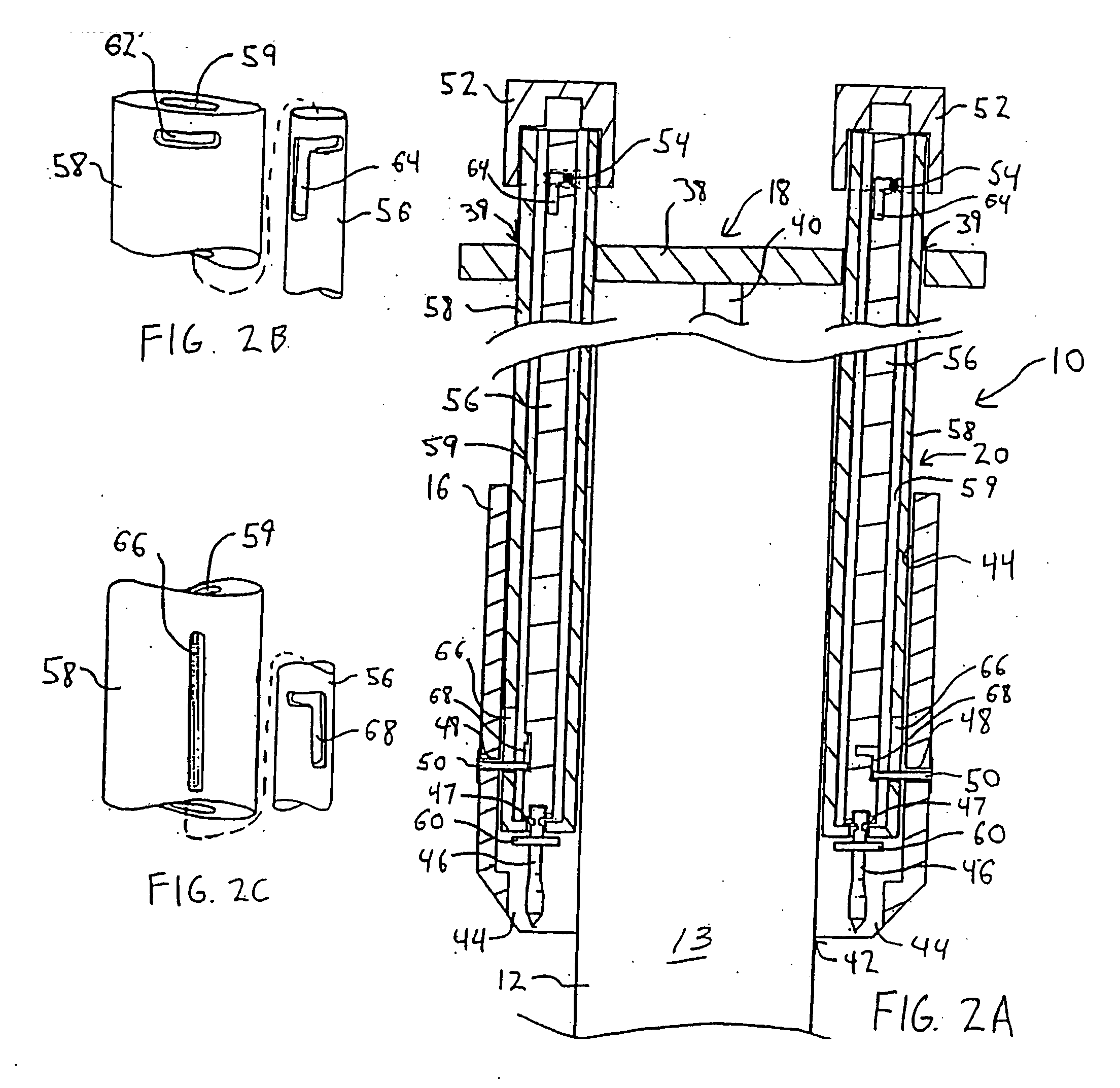
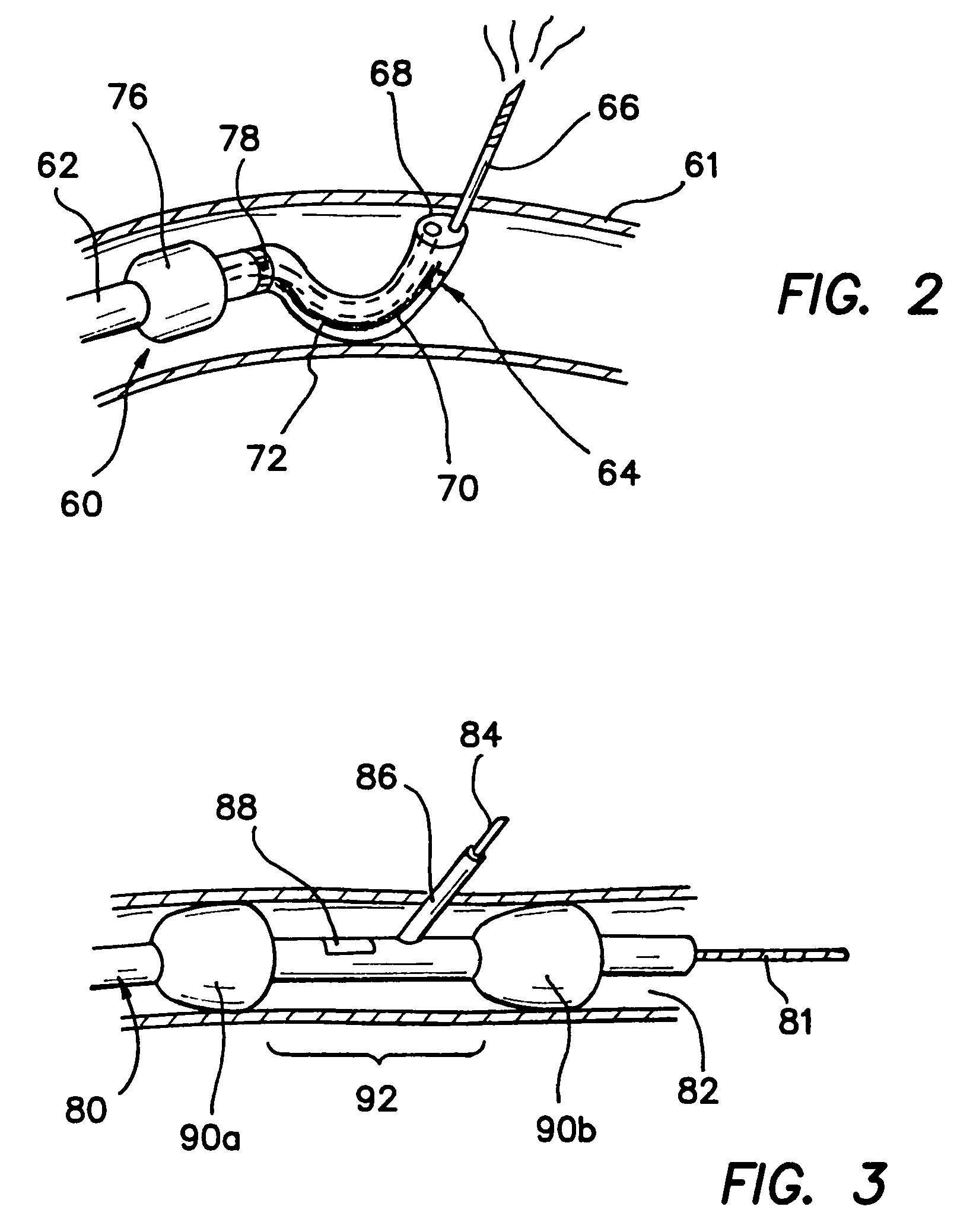
Vascular hemostasis device and deployment apparatus
A hemostasis device for percutaneously sealing a puncture in the wall of a blood vessel includes a rigid post, and a foot, a seal and a retaining member mounted on the rigid post. The hemostasis device may be deployed in the puncture so that the foot is positioned within the blood vessel. Tension is applied to the rigid post to hold the foot against the inside surface of the blood vessel. The retaining member is then pushed along the length of the rigid post, advancing the seal to a deployed state against the outside surface of the blood vessel. The puncture in the blood vessel is sandwiched between the foot and the seal in the deployed state. The rigid post, foot, seal and retaining member may all be formed from a resorbable polymeric material.
Owner:ST JUDE MEDICAL +1
Devices and methods for performing avascular anastomosis
InactiveUS6899718B2Efficient and reliable performanceEfficient executionStaplesNailsVascular anastomosisBlood vessel
Owner:HEARTPORT
Cryotherapy methods for treating vessel dissections and side branch occlusion
The present invention provides cryotherapy treatment of dissections in a blood vessel of a patient. The present invention further provides cryotherapy treatment of side branch occlusion in a bifurcated blood vessel. One method for treating potential or existing dissections in a blood vessel comprises cooling the blood vessel to a temperature and for a time sufficient to remodel the blood vessel such that dissections of the blood vessel are reduced. Another method for treating side branch occlusion in a bifurcated blood vessel, the bifurcated blood vessel having a side branch and a main branch, the main branch having plaque disposed thereon, comprises cooling an inner surface of the main branch to a temperature and for a time sufficient to inhibit plaque shift from the main branch into the side branch.
Owner:BOSTON SCI SCIMED INC
Replenishable stent and delivery system
InactiveUS20020077592A1Simple and inexpensive to produceSimple and inexpensive to and useStentsBalloon catheterGuide wiresBiomedical engineering
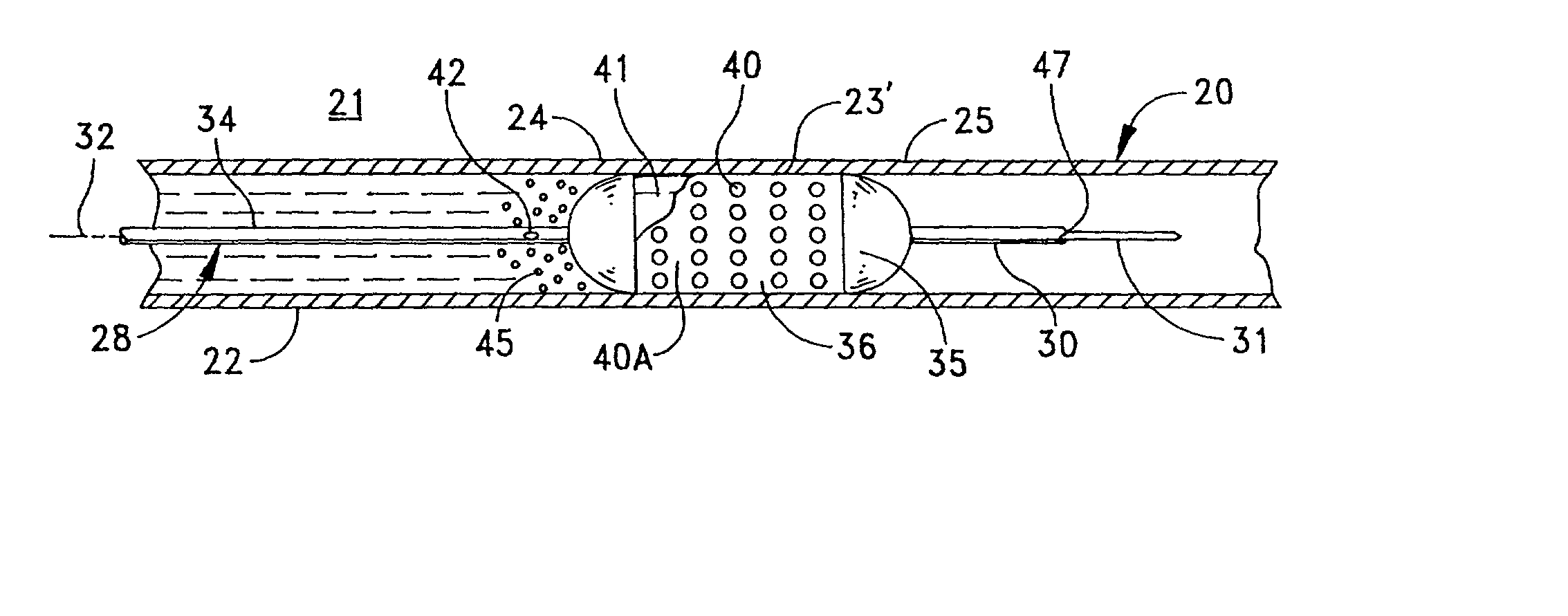
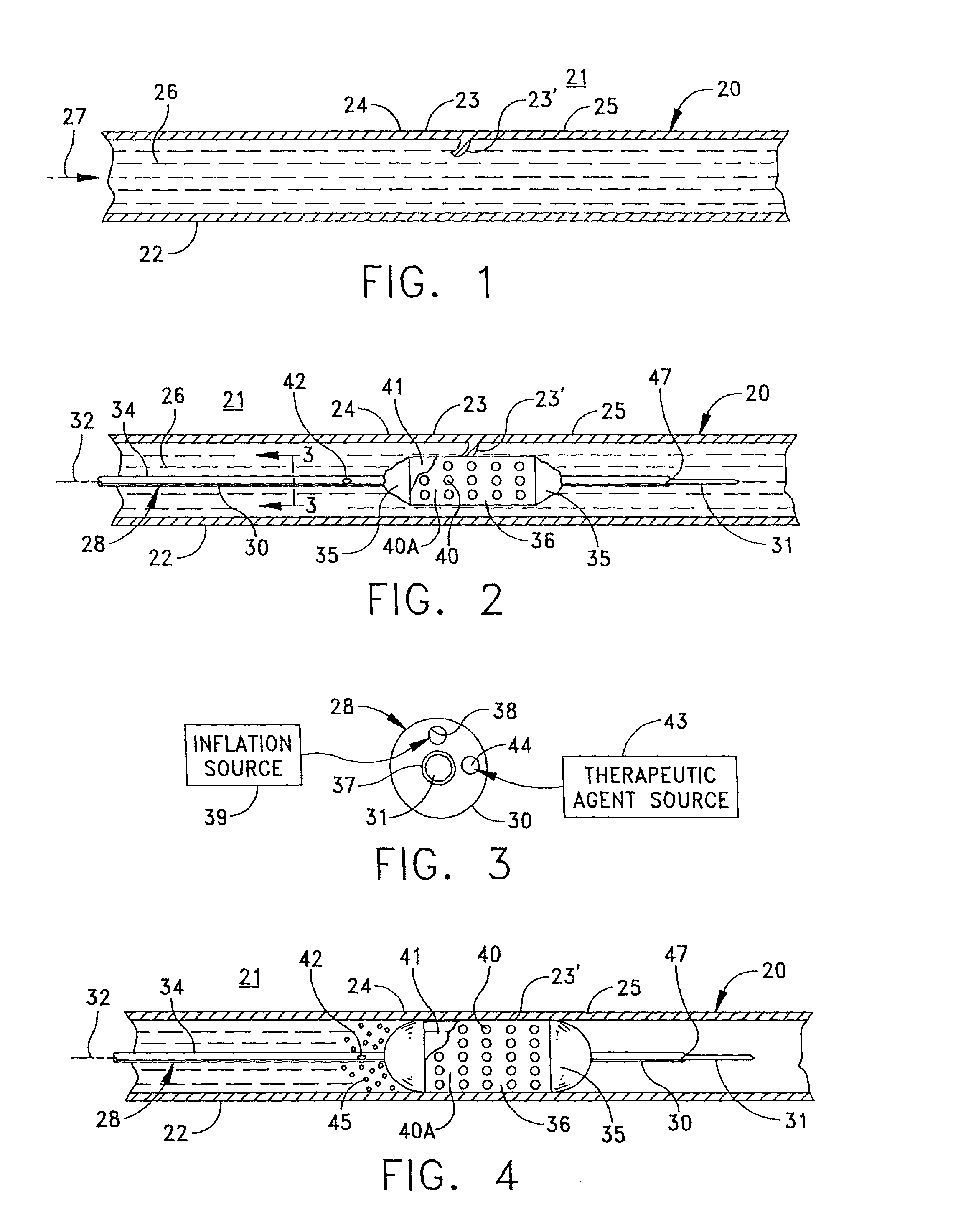
Apparatus and a method for treating an irregularity in a wall of a vessel of a patient defined by an irregular or afflicted wall portion with adjacent normal wall portions comprises a catheter having a distal end portion for being guided through the vessel to the site of the irregularity. A balloon associated with said distal end portion of the catheter for selective inflating to contact the walls of the vessel urges a stent carried by the distal end portion of the catheter in a constricted condition for passage through the vessel into an expanded form with the stent spanning the afflicted wall portion and contacting the adjacent wall portions. The catheter is formed with lumens for inflating the balloon, for receiving a guidewire for guiding the catheter through the vessel, and for connecting a port in the catheter proximate the afflicted wall portion to enable delivery of a therapeutic agent into the vessel to contact the stent and the irregular wall portion.
Owner:BOSTON SCI CORP
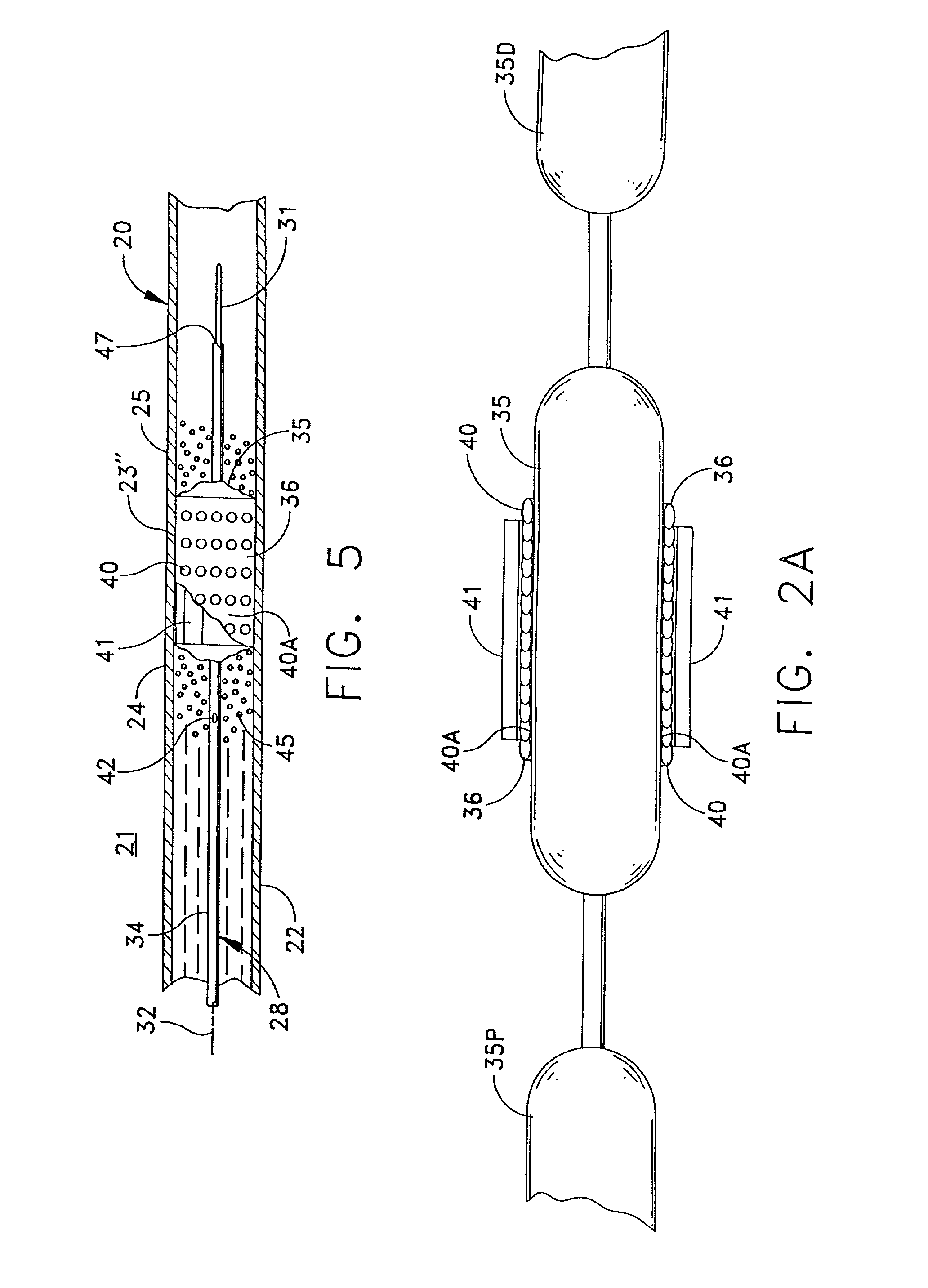
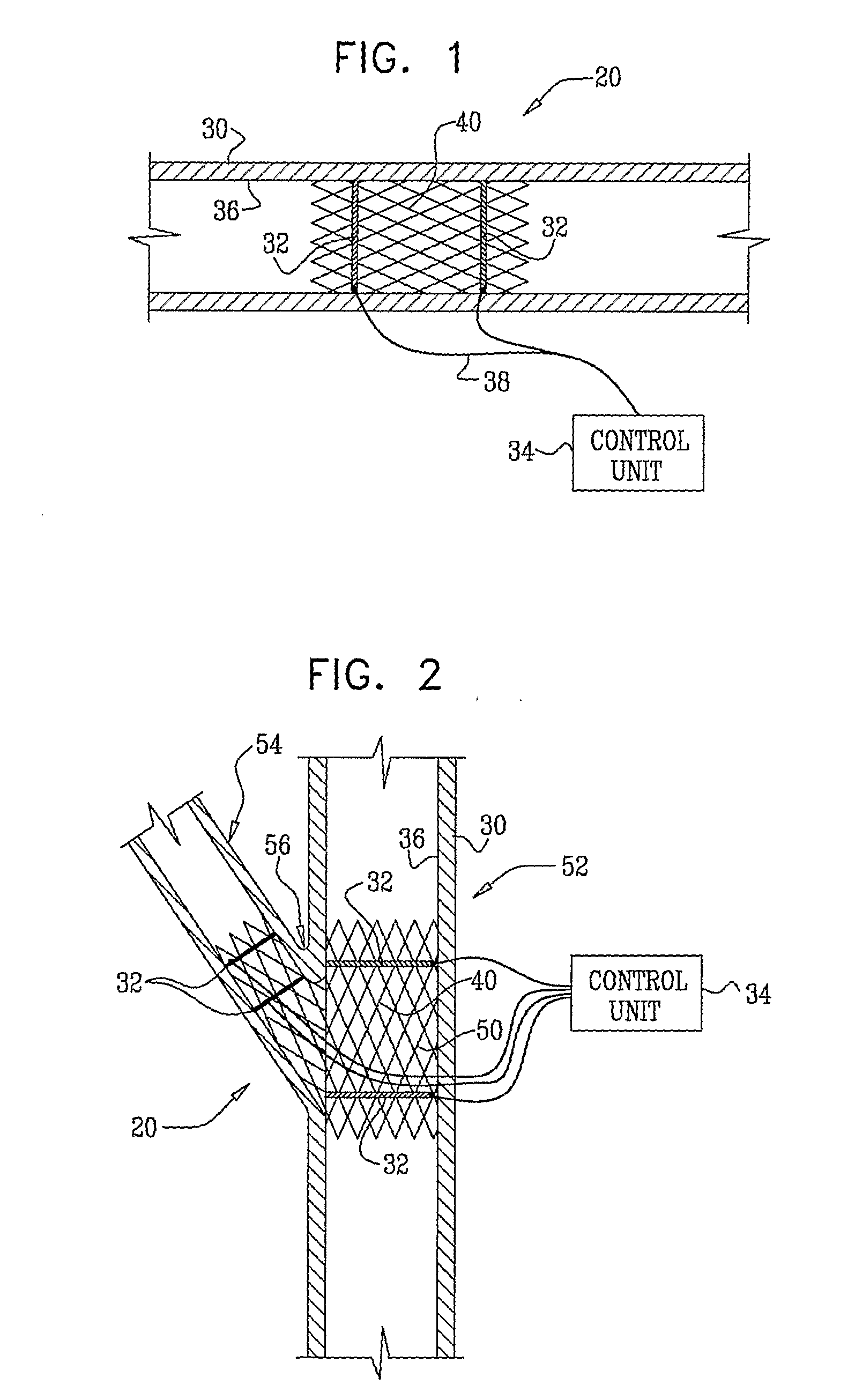
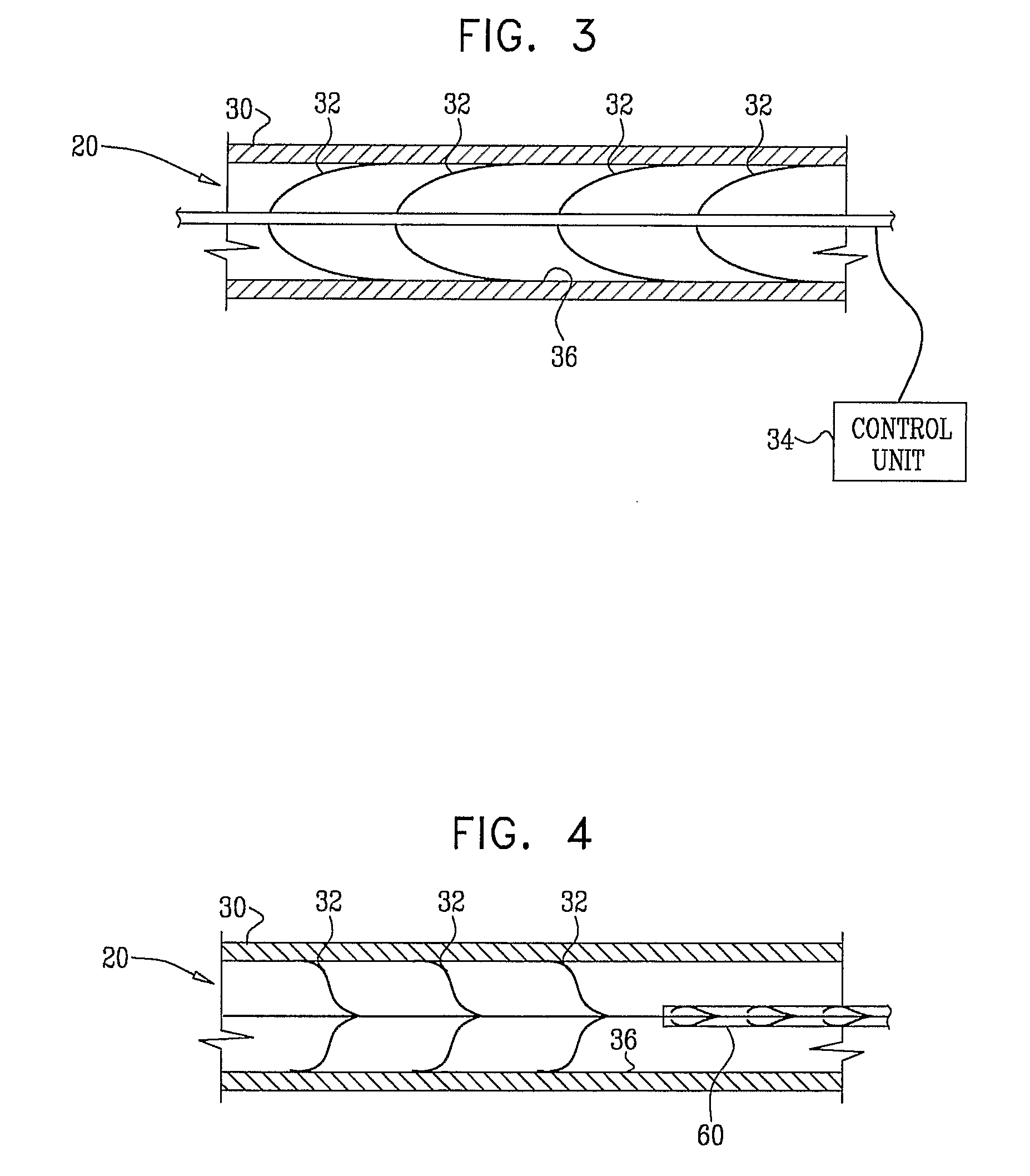
Electrical Stimulation of Blood Vessels
ActiveUS20080215117A1Increase heightReducing platelet aggregationStentsTransvascular endocardial electrodesNitric oxideSecretion
Apparatus (20) is provided, including a bifurcation stent (50) comprising one or more electrodes (32), the stent (50) configured to be placed in a primary passage (52) and a secondary passage (54) of a blood vessel (30), and a control unit (34), configured to drive the electrodes (32) to apply a signal to a wall (36) of the blood vessel (30), and to configure the signal to increase nitric oxide (NO) secretion by the wall (36). Other embodiments are also described.
Owner:ENOPACE BIOMEDICAL
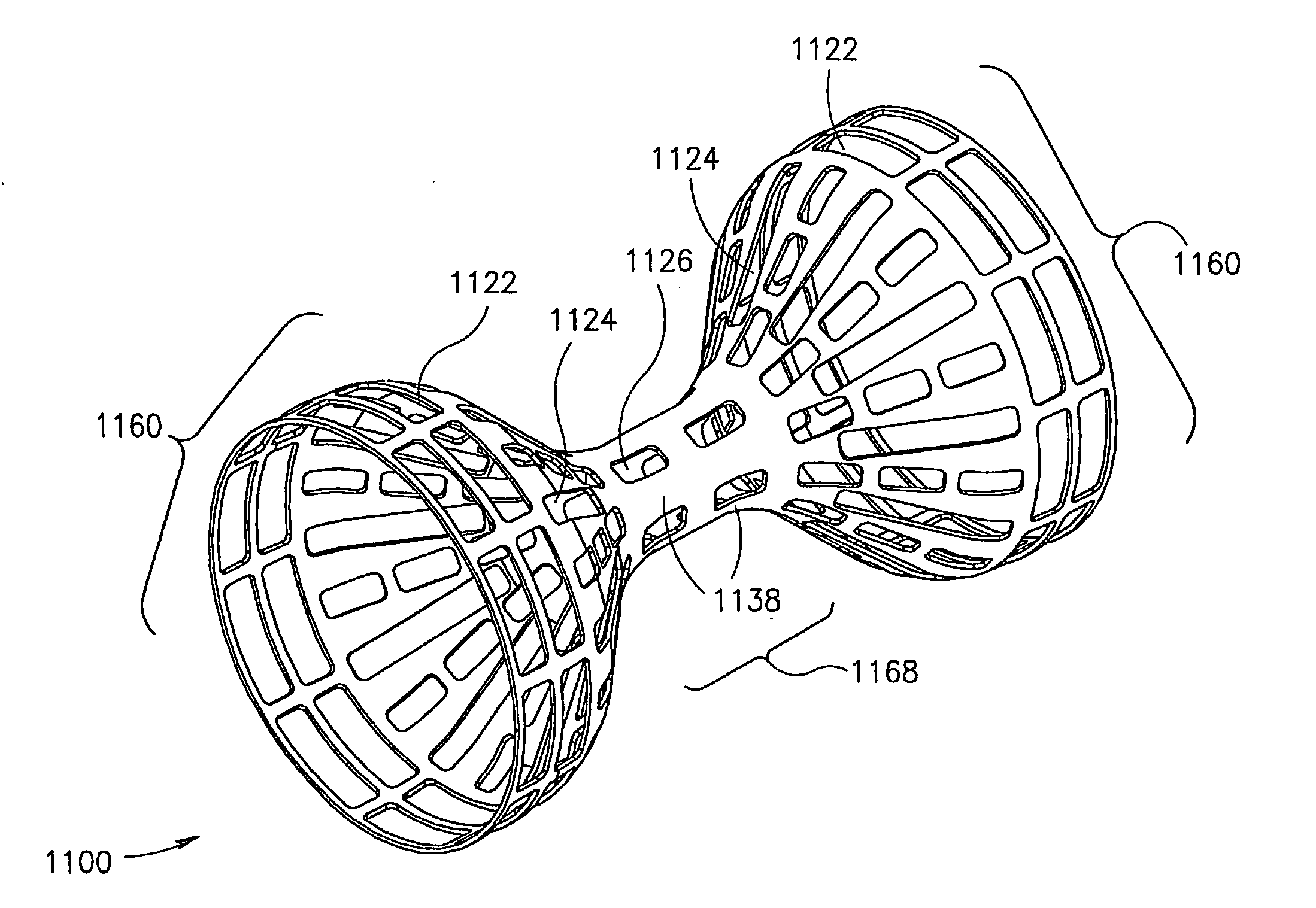
Devices and methods for treatment of vascular aneurysms
InactiveUS20060292206A1Easy to customizePromote cellular in-growthStentsBalloon catheterBlood flowReticular formation
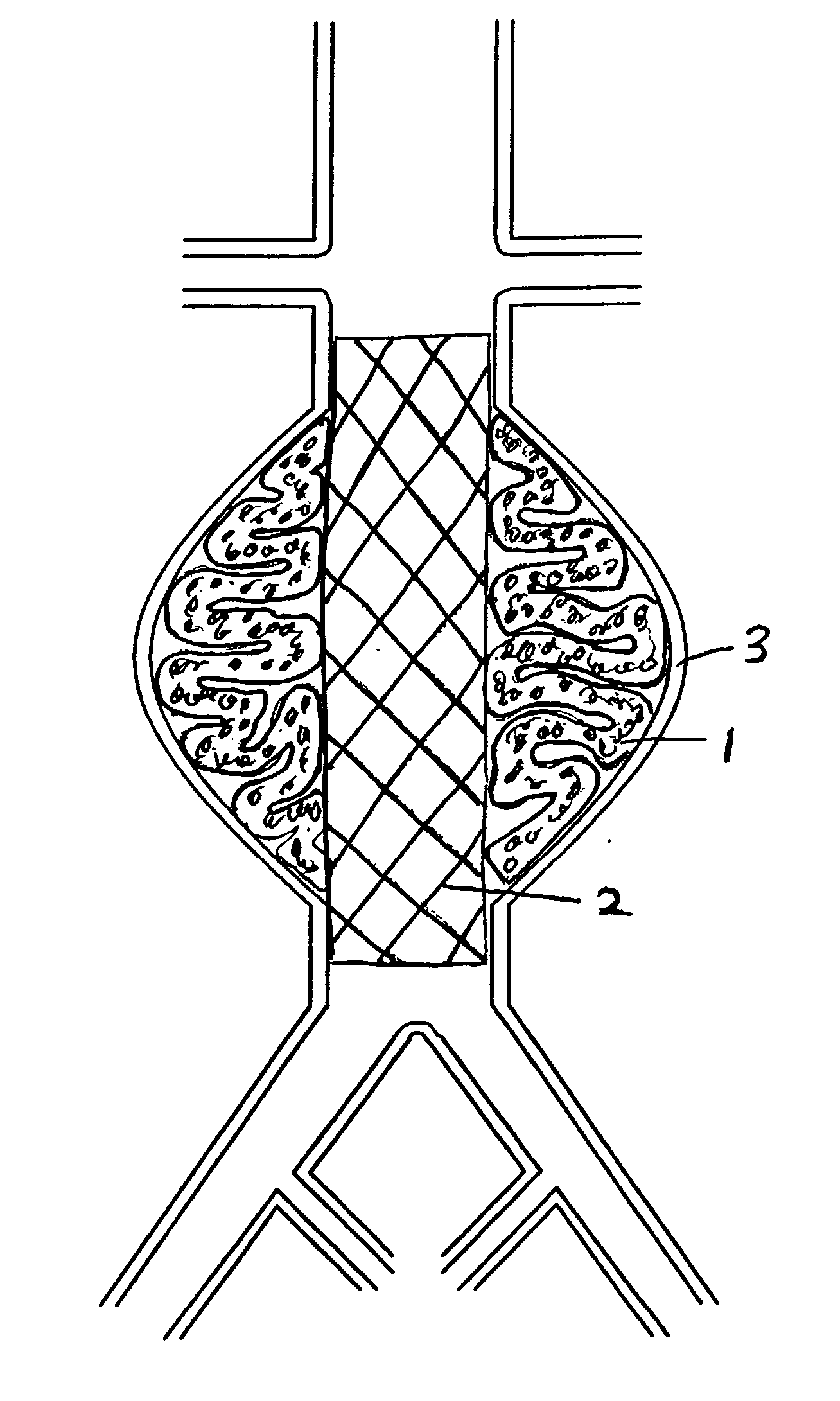
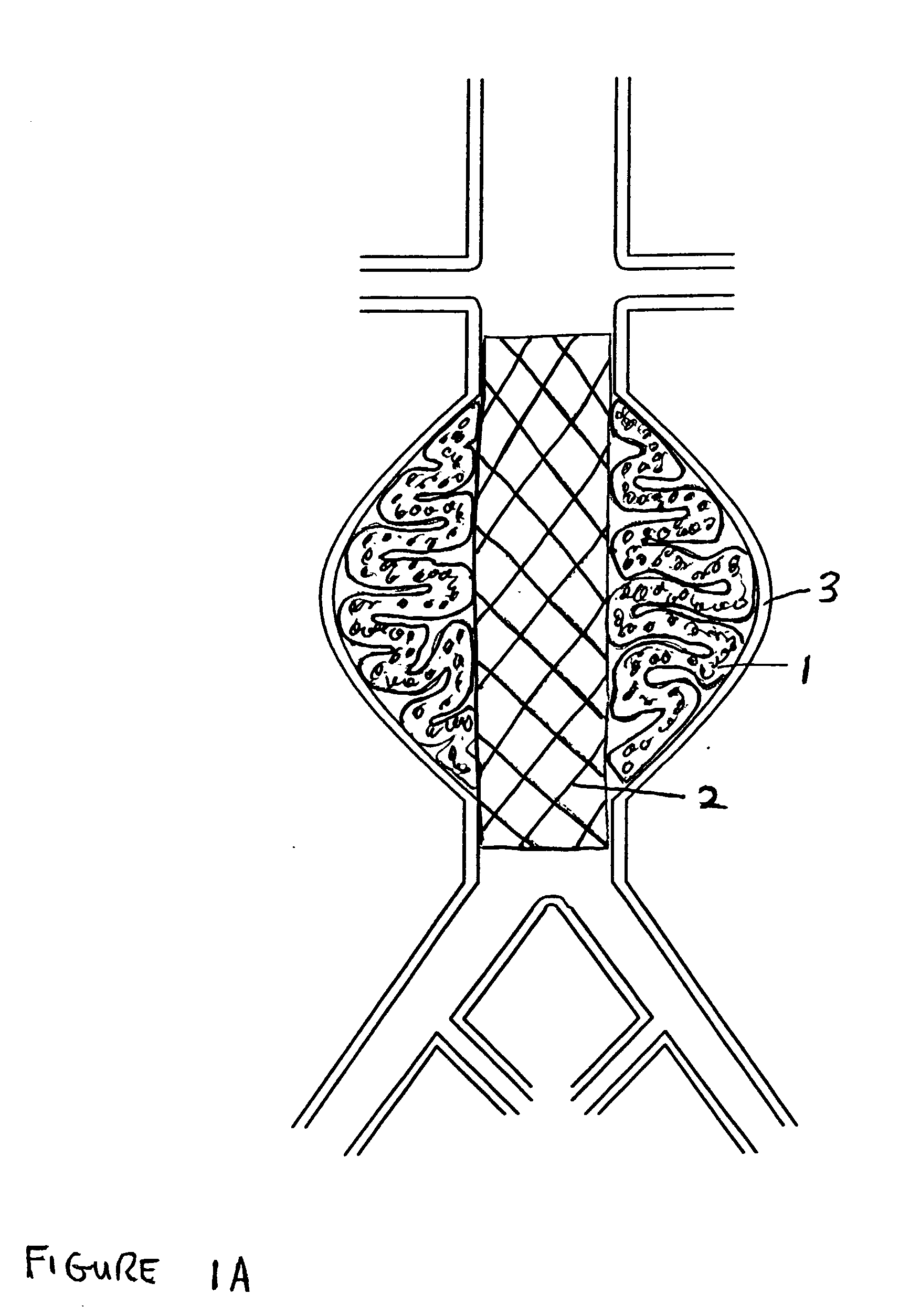
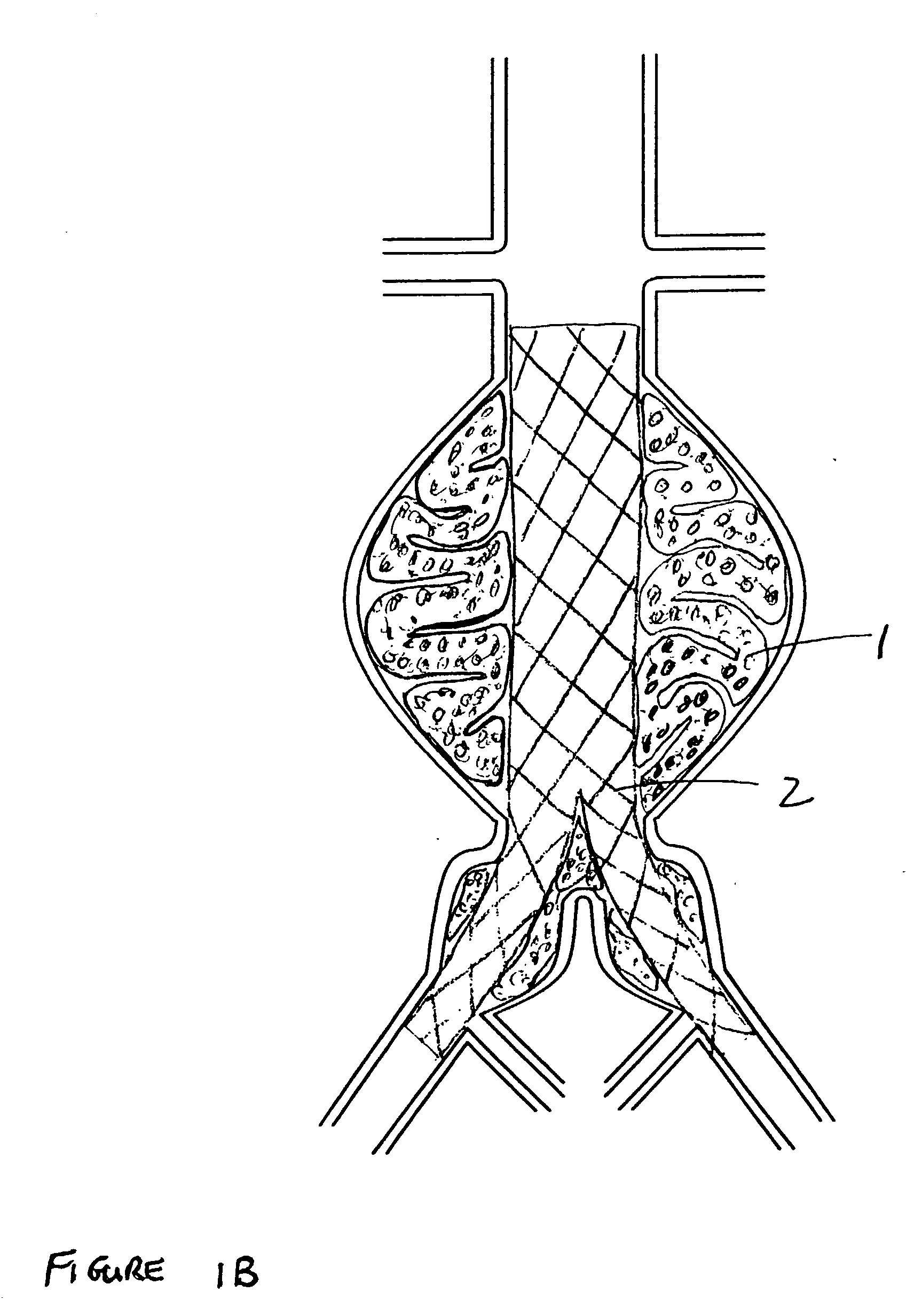
The present invention relates to devices and methods for the treatment of diseases in the vasculature, and more specifically, devices and methods for treatment of aneurysms found in blood vessels. In a first embodiment of the present invention, a two part prostheses, where one part is an expandable sponge structure and the other part is an expandable tubular mesh structure, is provided. In the first embodiment, the expandable sponge structure is intended to fill the aneurysm cavity to prevent further dilatation of the vessel wall by creating a buffer or barrier between the pressurized pulsating blood flow and the thinning vessel wall. In the first embodiment, the expandable tubular mesh structure is placed across the aneurysm, contacting the inner wall of healthy vessel proximal and distal to the aneurysm.
Owner:ENDOLOGIX LLC
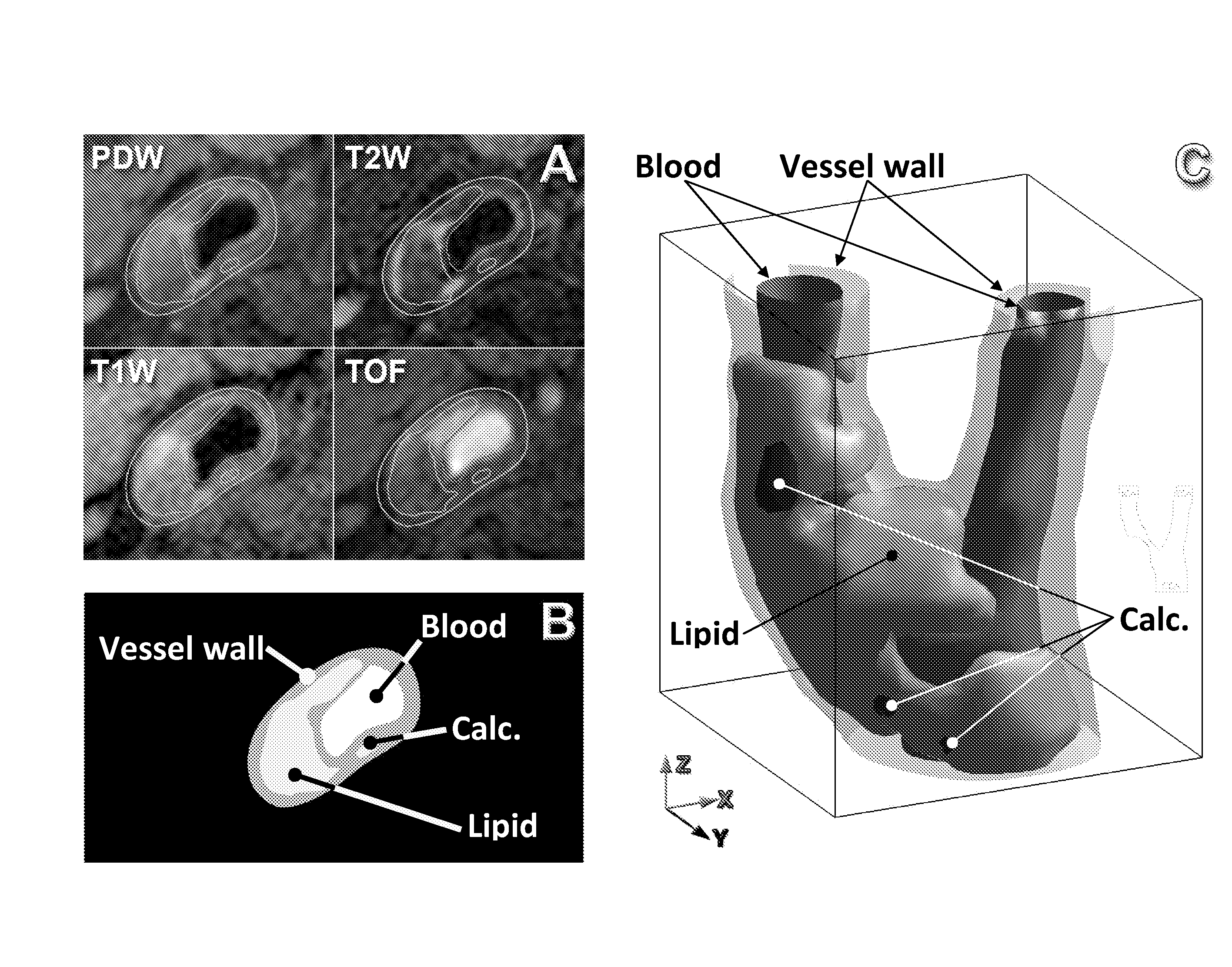
Method for calculating pressures in a fluid stream through a tube section, especially a blood vessel with atherosclerotic plaque
A method for calculating pressures in a fluid streaming through a tube section from an upstream end to a downstream end of the tube section, the method comprising scanning the tube section with a scanner and providing a plurality of 2D scanning images along the tube section with an inlet and at least two arms, by a computer program on the basis of the 2D images automatically N calculating a 3D image of the tube section by using interpolating between the 2D images, by a computer program performing a 2D sectional image cut through the 3D image, the image cut following the fluid stream, calculating in the sectional image cut a fluid pressure distribution along multiple locations inside the tube on the basis of given boundary conditions, the boundary conditions including fluid velocity or fluid pressure at the upstream end.
Owner:CABRA TECH
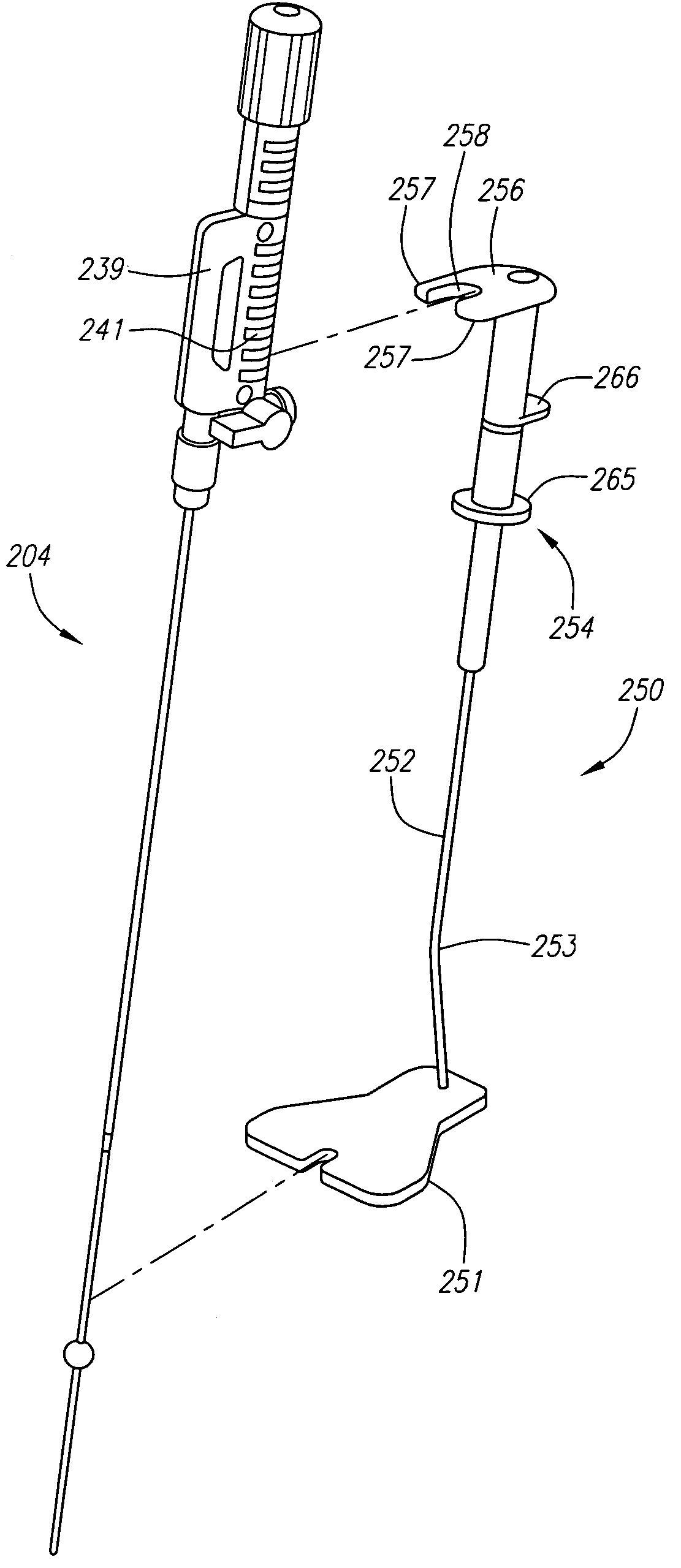
Delivery device for localized delivery of a therapeutic agent
InactiveUS20110137155A1Receive treatment wellImprove usabilityStentsBalloon catheterLesionBlood vessel
Therapeutic agent delivery devices and methods for delivering a therapeutic agent to a target location as well as methods for determining the location of a lesion on a vessel wall are disclosed. Various embodiments disclose an expandable member comprising a drug delivery matrix for selectively delivering a therapeutic agent to a lesion on a vessel wall. The drug delivery matrix may comprise one or more sensors and an electroactive polymer for releasing the therapeutic agent. Other embodiments disclose an expandable member comprising a plurality of radially-expanding flexible walls forming a plurality of channels for selectively delivering therapeutic agent to a target area adjacent one or more of the channels. Detecting a lesion may comprise using a plurality of Hall effect sensors disposed on a distal end of a catheter.
Owner:BOSTON SCI SCIMED INC
Vascular access closure system
InactiveUS7488340B2Easy to operateEasy to installSuture equipmentsSurgical veterinaryHand heldEngineering
A sealing device for sealing punctures in blood vessel walls including a flange connected to a flexible stem having an expansion portion in it. The flexible stem is adapted to be accommodated inside a delivery tube. The delivery tube further includes a hand-hold for ease of handling. The sealing device may further include a loader and a cutter. The flange and flexible stem are preferably constructed of a biodegradable material that has a tensile strength, rigidity, memory and other physical qualities similar to medical grade silicone. The resilient transverse expansion portion expands when deployed beyond the delivery tube in the tissue tract to create a frictional interface with the interior surface of the tissue tract to resist displacement of the flexible stem from a desired location in the tissue tract. The flexible stem also includes a flange at the distal end of the flexible stem to seal the puncture when the flexible stem is deployed.
Owner:TELEFLEX LIFE SCI LTD
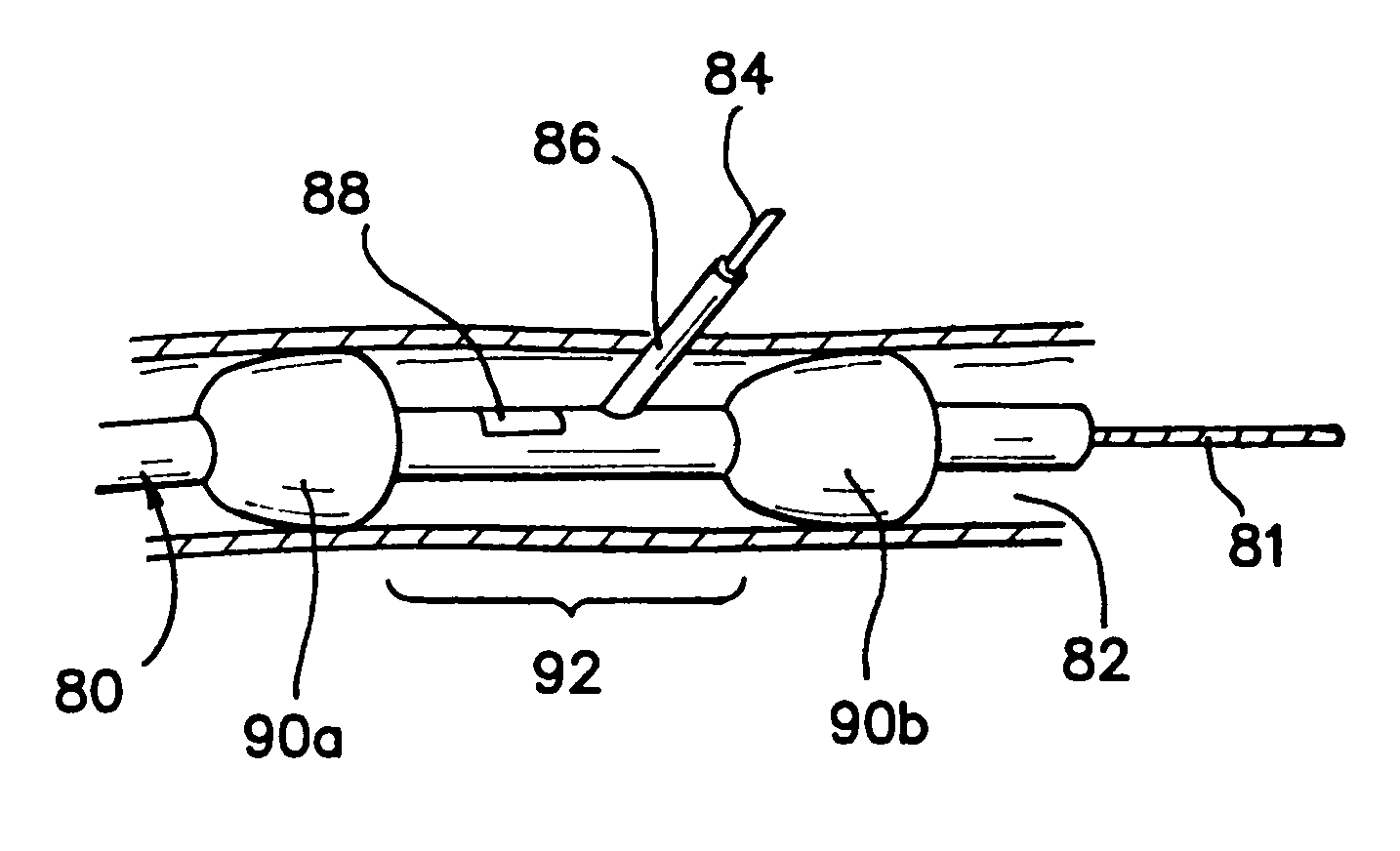
Vascular sheath with bioabsorbable puncture site closure apparatus and methods of use
InactiveUS20060195124A1Quickly and effectively achieve hemostasLow costGuide needlesSurgical needlesEngineeringBiomedical engineering
Apparatus and methods are provided for use in sealing a vascular puncture site. The invention comprises an introducer sheath with an integrated closure component. The closure component includes a fastener and an advanceable, deformable clip having a delivery configuration in which opposing sides do not contact one another, and a deployed configuration, in which the fastener causes opposing sides of the deformable clip to close towards one another. The clip is advanced along the sheath until it pierces opposing sides of a vessel wall at a puncture site. The clip is then deformed with the fastener to draw opposing sides of the puncture together, and the sheath is withdrawn to seal the wound. The clip and fastener preferably are bioabsorbable.
Owner:INTEGRATED VASCULAR SYST
Stabilized tissue penetrating catheters
InactiveUS7729738B2Accurate and reliable positioningAccurately penetratedUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsCoronary Revascularization ProcedureCoronary revascularization
A tissue penetrating catheter that is usable to advance a tissue penetrator from within a blood vessel, through the wall of the blood vessel to a target location. The catheter includes at least one stabilizing device thereon for stabilizing catheter prior to advancing the tissue penetrator. The tissue penetrator may extend through a lumen in the body of the catheter and project transversely through an exit port. The stabilizing device may be located closely adjacent to the exit port, or may surround the exit port. The stabilizing device may be one or more balloons, or other mechanical structure that is expandable into contact with the inner luminal wall of the blood vessel. Desirably, the exit port is forced into contact with the blood vessel wall to shorten the distance that the tissue penetrator projects from the catheter body to the target location. The catheter is particular useful for forming blood flow tracts between blood vessels, in particular in coronary revascularization procedures. Methods of utilizing such a catheter to bypass an arterial obstruction is also disclosed.
Owner:MEDTRONIC VASCULAR INC
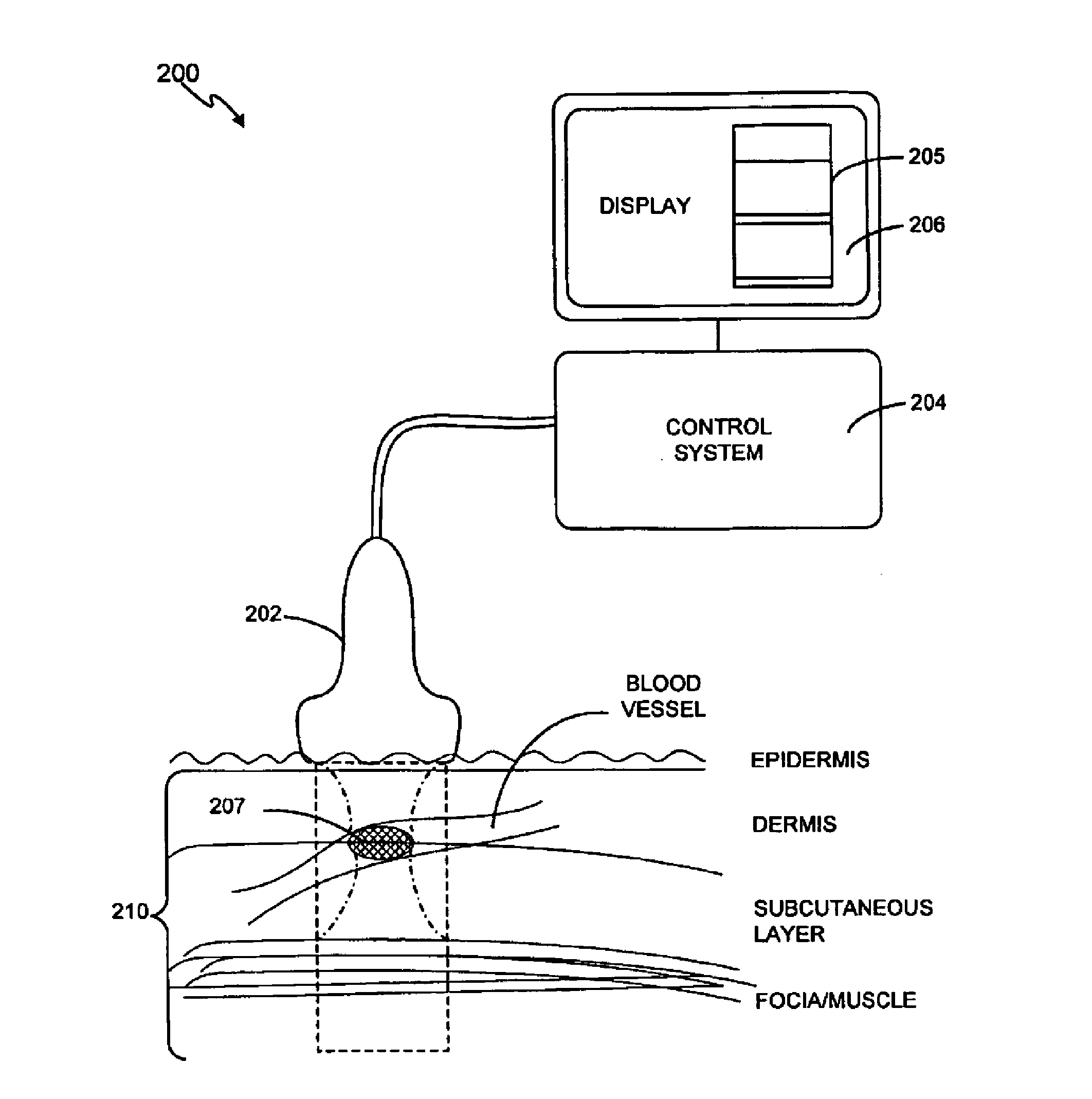
Noninvasive treatment of blood vessels
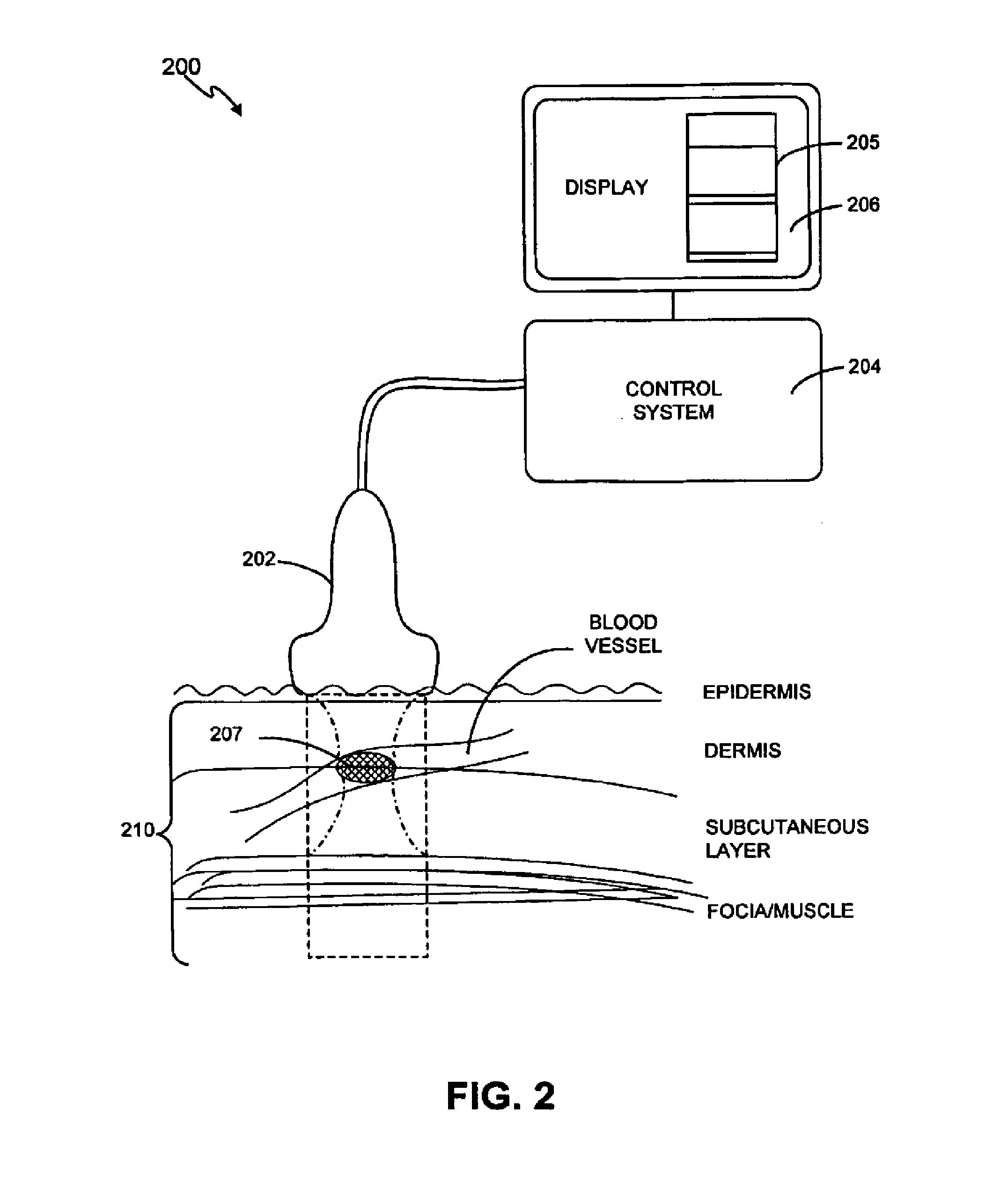
InactiveUS20120330197A1Good effectHighly focusedUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyVascular diseaseBlood vessel spasm
A non-invasive method and system for using ultrasound energy for the treatment of conditions resulting from vascular disorders is provided. In one embodiment, an image-treatment approach can be used to locate the blood vessel to be treated and then to ablate it non-invasively, while also monitoring the progress of the treatment. In another embodiment, a transducer is configured to deliver ultrasound energy to the regions of the superficial tissue (e.g., skin) such that the energy is deposited at the particular depth at which the vascular malformations are located below the skin surface. The ultrasound transducer can be driven at a number of different frequency regimes such that the depth and shape of energy concentration can match the region of treatment.
Owner:GUIDED THERAPY SYSTEMS LLC
Methods, materials and apparatus for deterring or preventing endoleaks following endovascular graft implantation
Methods and apparatus for treating or preventing endoleaks after an endovascular graft (e.g., a stent, tubular graft, stent-graft, coated stent, covered stent, intravascular flow modifier or other endovascular implant that affects, limits or prevents blood flow into a vascular defect such as an aneurysm, arterio-venous fistula, arterio-venous malformation, vessel wall perforation, etc.) has been implanted in the vasculature of a human or veterinary patient. An expansile polymeric material, such as a swellable polymer (e.g., a hydrogel), a flexible or elastomeric polymer foam (e.g. silicone, polyurethane, etc.) or a carrier member (e.g, a coil, filament, wire, etc) that carries a quantity of such expansile polymer is delivered into a perigraft space (i.e., space between the endovascular graft and the surrounding blood vessel wall) such that the polymeric material expands in situ to substantially fill the perigraft space or a portion thereof. The expansile polymeric material is delivered into he perigraft space through a catheter and / or cannula that is placed prior to, during or after the implantation of the endovascular graft. The invention includes an injector apparatus that is useable to deliver the expansile polymeric material through the wall of a previously implanted graft. After delivery into the perigraft space, the expanded polymeric material expands so as to fill all or an intended portion of the perigraft space in a manner that substantially prevents additional blood from leaking or flowing into such perigraft space. One type of blood-absorbing, porous, expansile polymeric material useable in this invention is a super-expansile hydrogel.
Owner:MICROVENTION INC
Vascular closure methods and apparatuses
InactiveUS20070049967A1Reduce shear forceReduce chanceSurgical veterinaryWound clampsVascular closure deviceVascular device
A vascular closure device comprising a retrievable sheath-delivered contractible, clip device with structural radial or terminal members with terminal and non-terminal hooks that engage the vessel wall. Unlike other vascular closure clips, this device is delivered on the outside rather than the inside of sheath. Closure of the tissue opening can be effected by the feet of the clip engaging the puncture, aperture, or wound edges and memory characteristics of the device cause a contraction of the members, bringing the members into apposition and the wound edges together, permitting immediate vascular closure and healing of the blood vessel. The device can be delivered and recovered by an intravascular sheath.
Owner:ABBOTT CARDIOVASCULAR
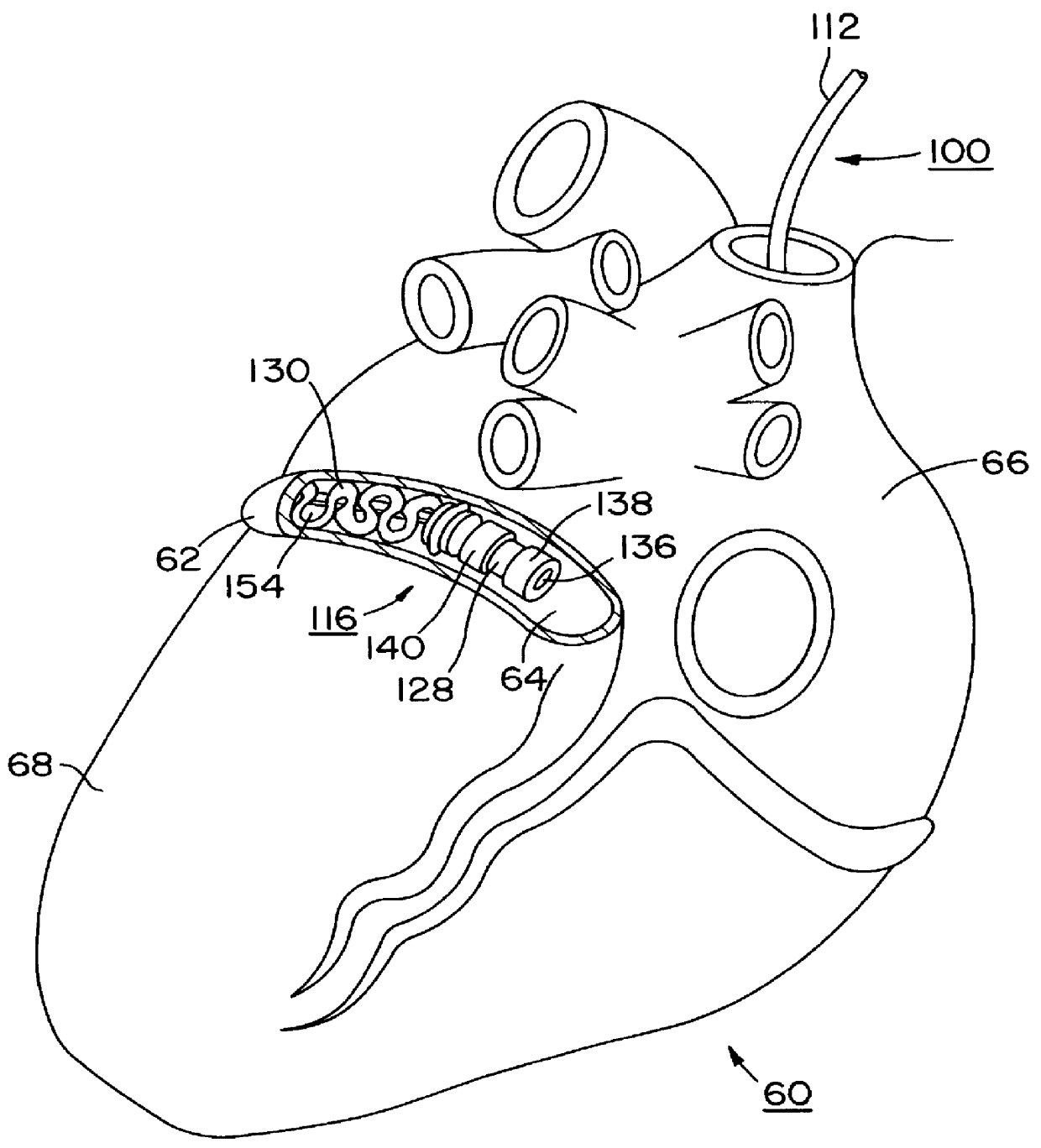
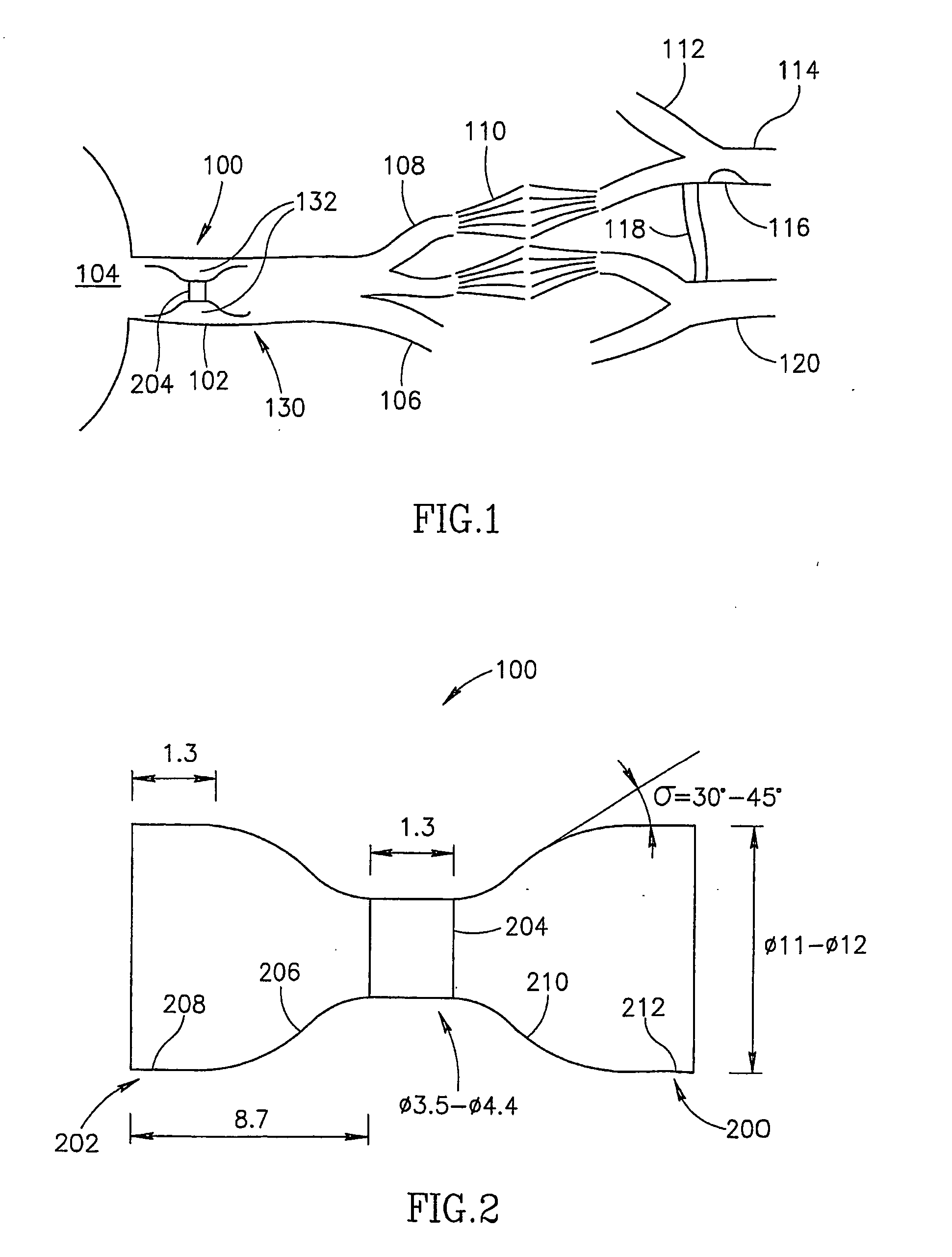
Permanent ventricular assist device for treating heart failure
The invention is a kit for a permanent ventricular assist device that can be permanently implanted into the circulatory system of a patient. The kit comprises one or more passive cores (Pcore), a stator, a power supply, and a controller unit. The inventors have realized that major open heart surgery, generally used for implementing a magnetic blood pump in the circulatory system, can be wholly avoided if the rotor and the stator are physically separated and are implanted respectively inside and around the blood vessel at the location of interest. Therefore the kit is characterized in that the one or more Pcores are configured to allow them to be implanted inside a blood vessel and the stator is configure to enable it to be placed outside of the blood vessel surrounding the Pcores. Also described are illustrative medical procedures for implanting the components of the kit at different locations in the body.
Owner:LEVITICUS CARDIO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com