Devices and methods for treatment of vascular aneurysms
a technology for vascular aneurysms and devices, applied in blood vessels, prostheses, bandages, etc., can solve the problems of high acute success rate of these devices, sponge filler acts like a graft, and has unparalleled durability, so as to prevent the migration of the graft and promote cellular ingrowth. , the effect of minimizing the volume of material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
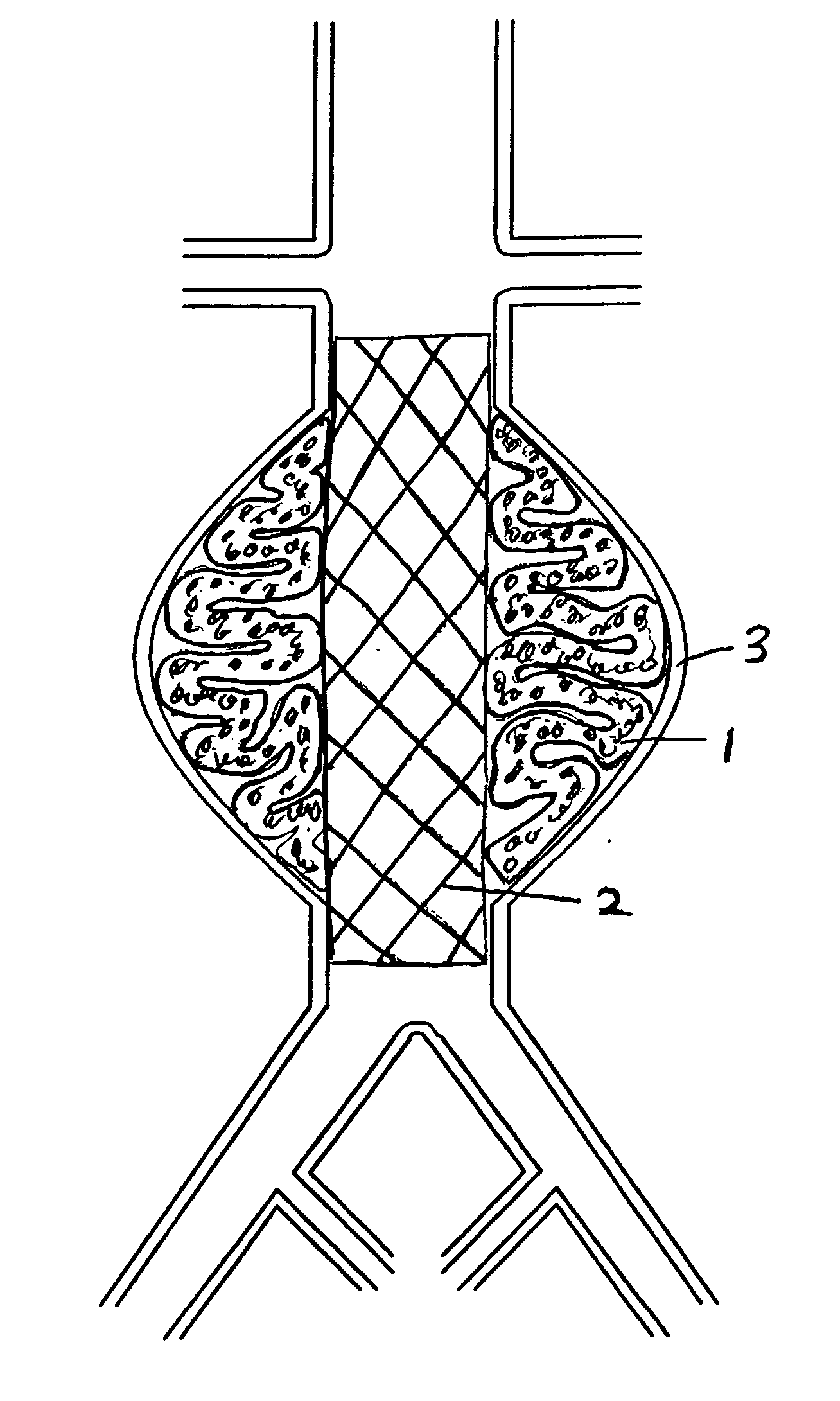
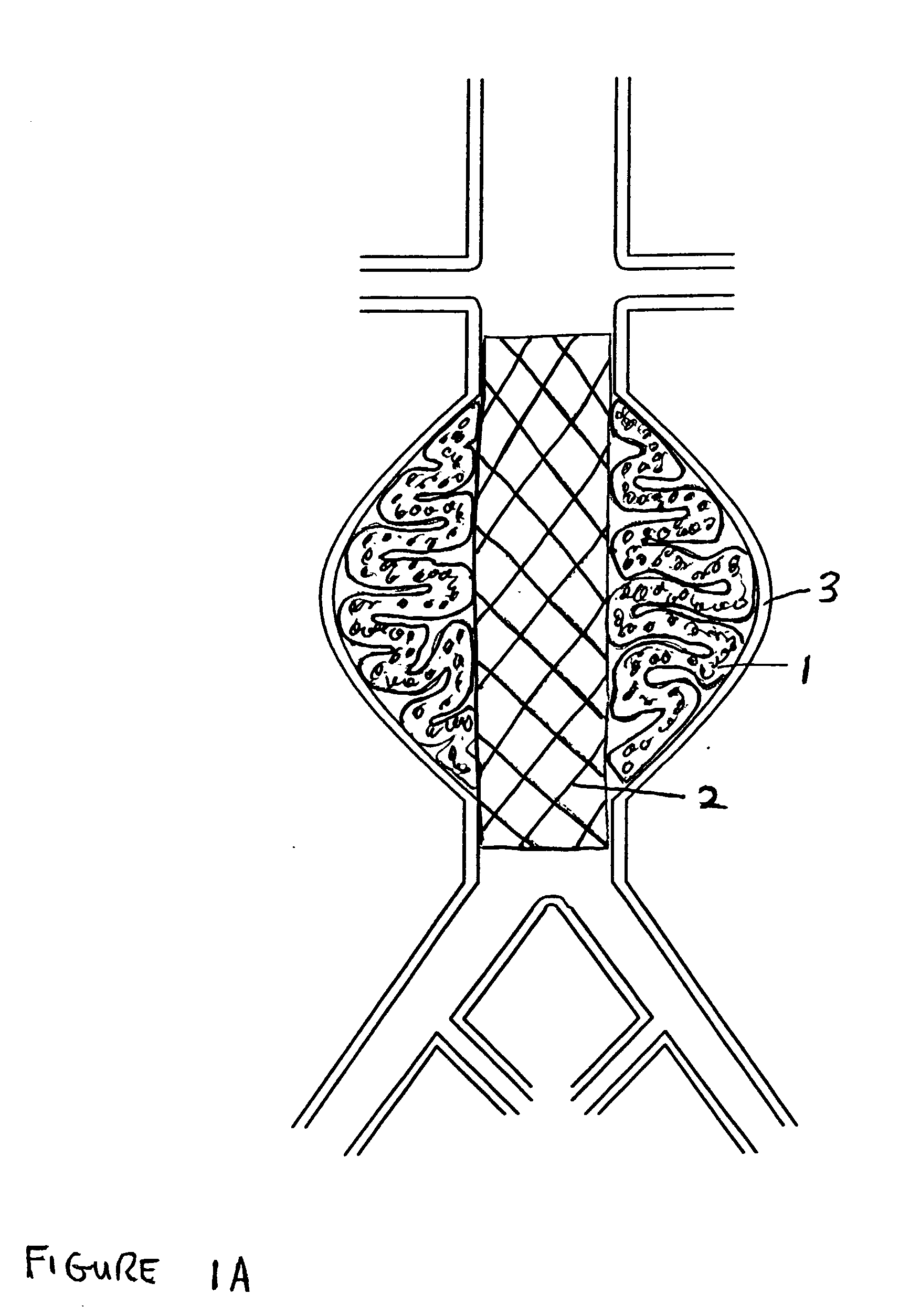
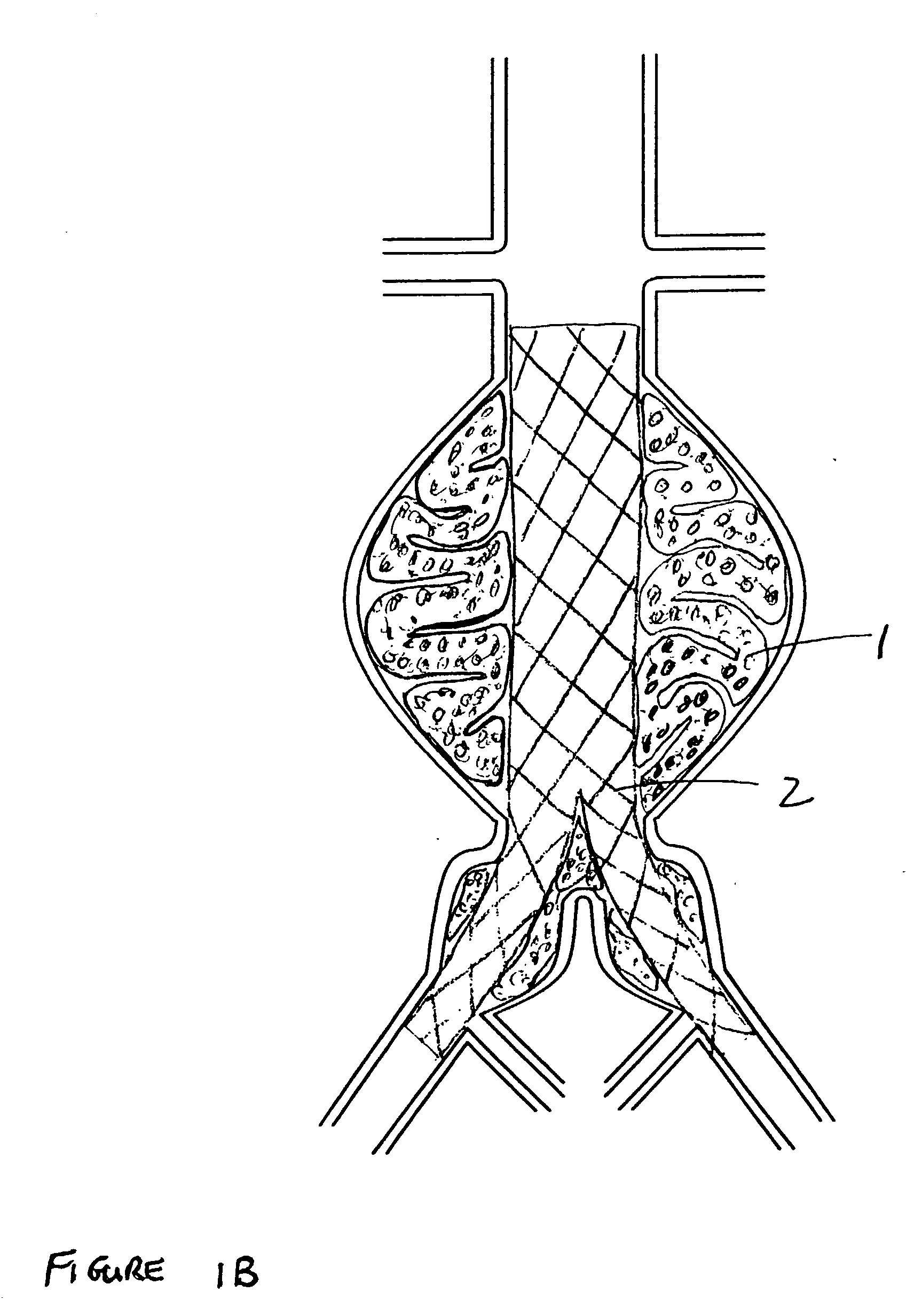
[0029]FIG. 1A shows the two-part prosthesis comprising of an expandable sponge structure 1 and an expandable tubular mesh structure 2 placed in an abdominal aortic aneurysm 3 located in the infra-renal aorta not involving the iliac arteries. FIG. 1B shows a bifurcated version of the expandable tubular mesh structure 2 and the expandable sponge structure 1 in an abdominal aortic aneurysm located in the infra-renal aorta and involving both iliac arteries. FIG. 1C shows an expandable tubular mesh structure 2 placed across an aneurysm commonly found in cerebral arteries and the expandable sponge structure 1 filling up the aneurysm. The expandable sponge structure 1 is placed through the expandable tubular mesh structure 2 into the aneurysm, filling up the aneurysmal sac which provides a barrier between the thin fragile wall of the aneurysm and the pressurized pulsating blood. The tubular mesh structure 2 keeps the expanded sponge 1 within the confines of the aneurysm and away from the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com