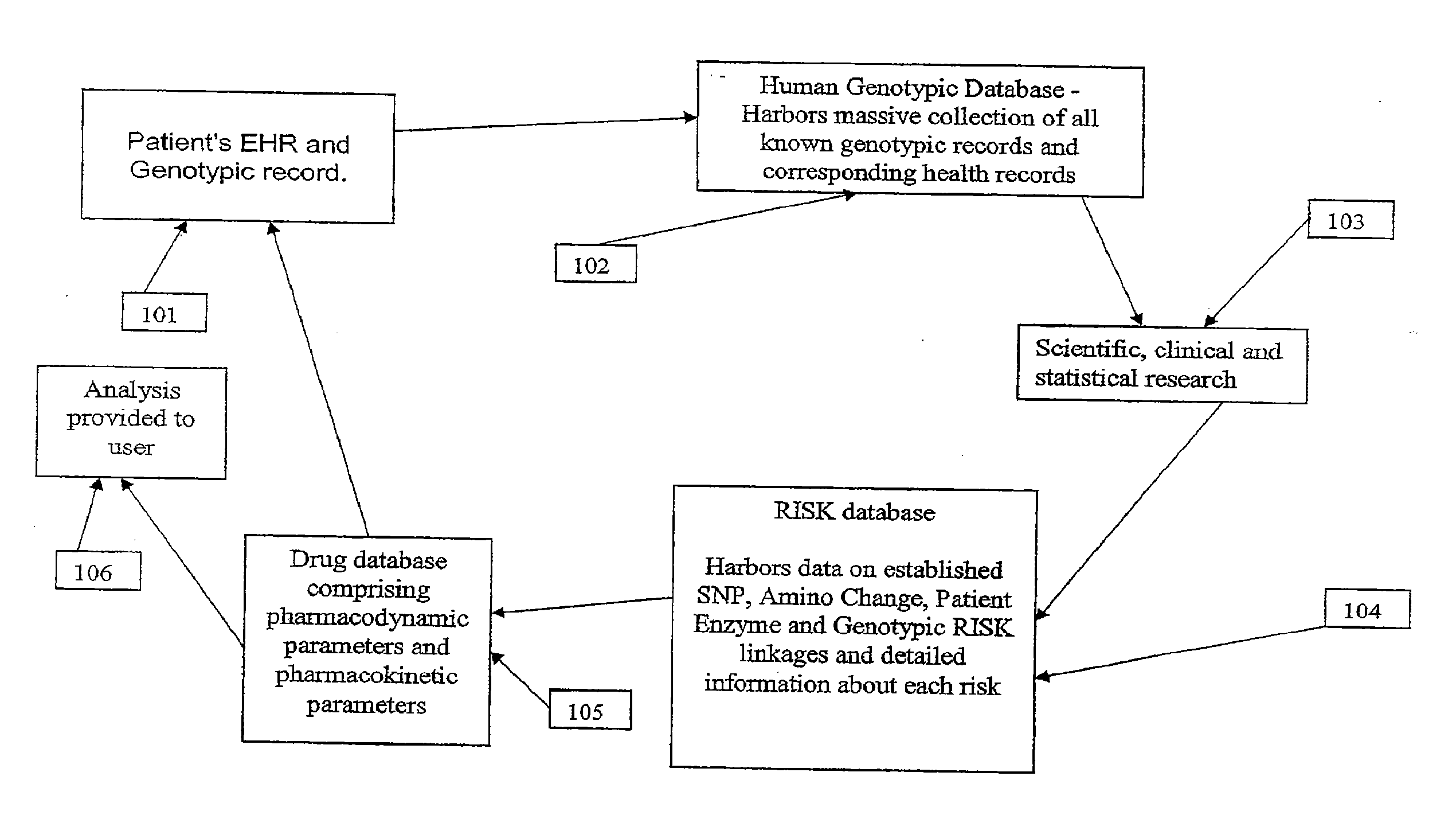
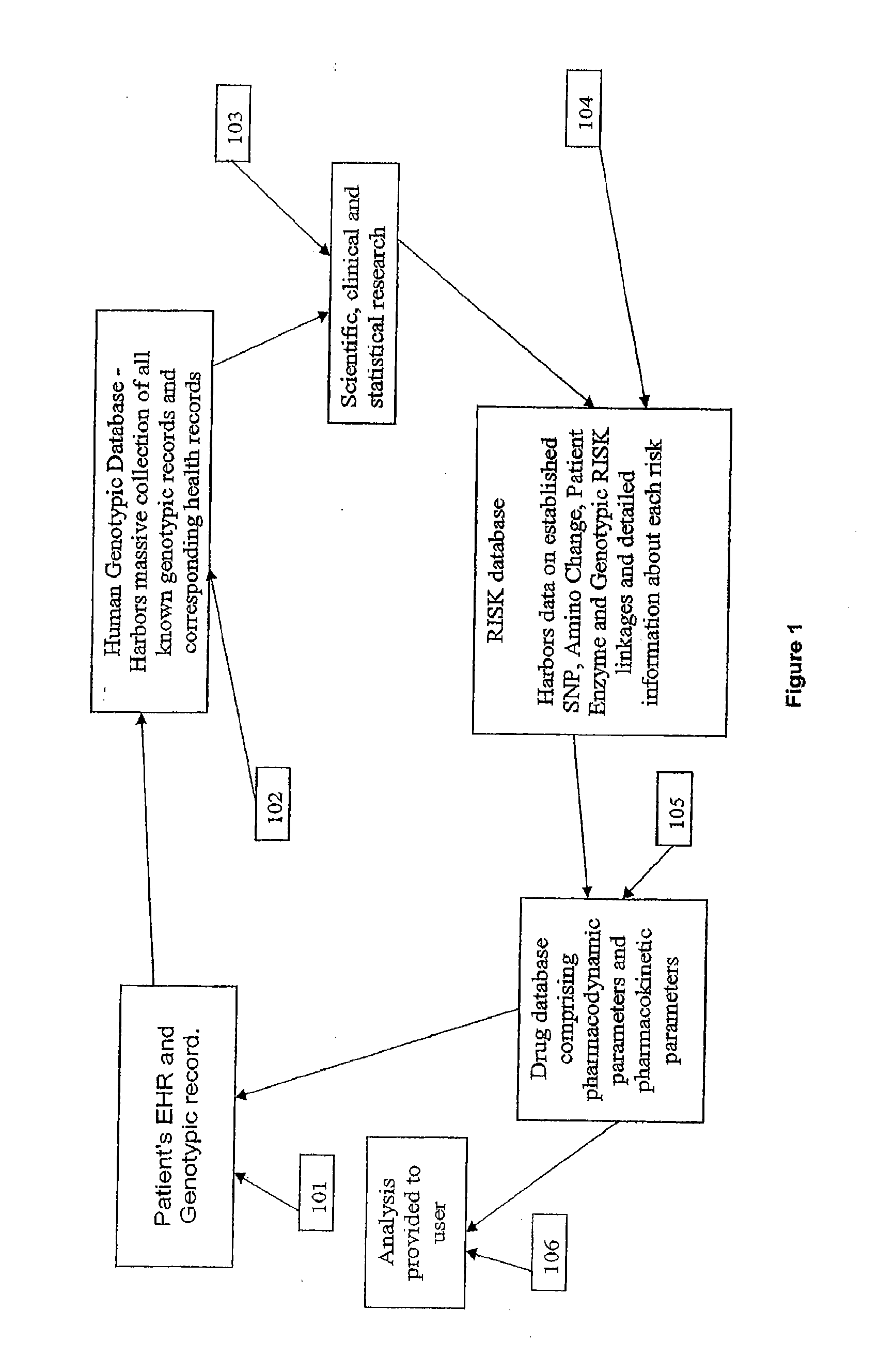
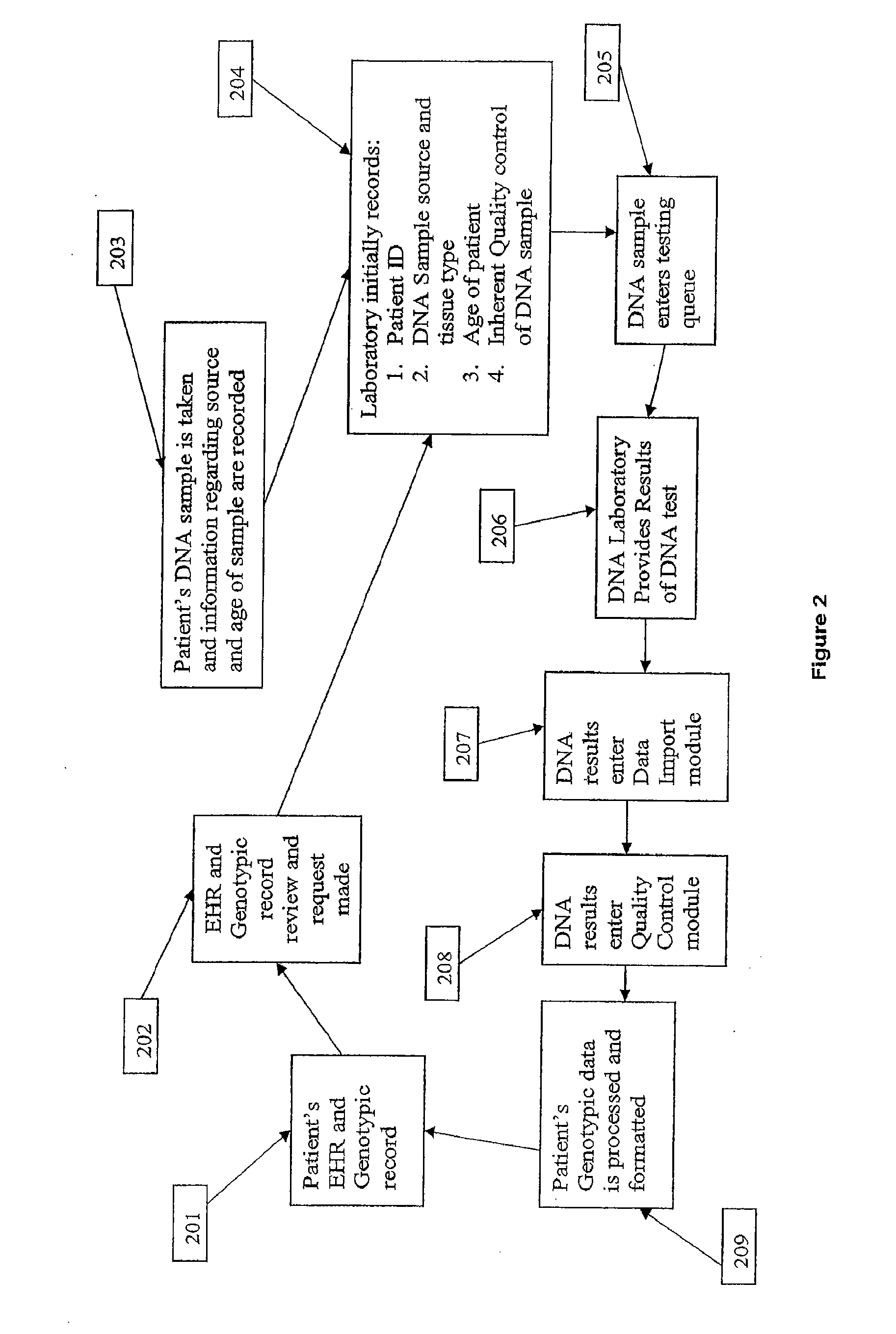
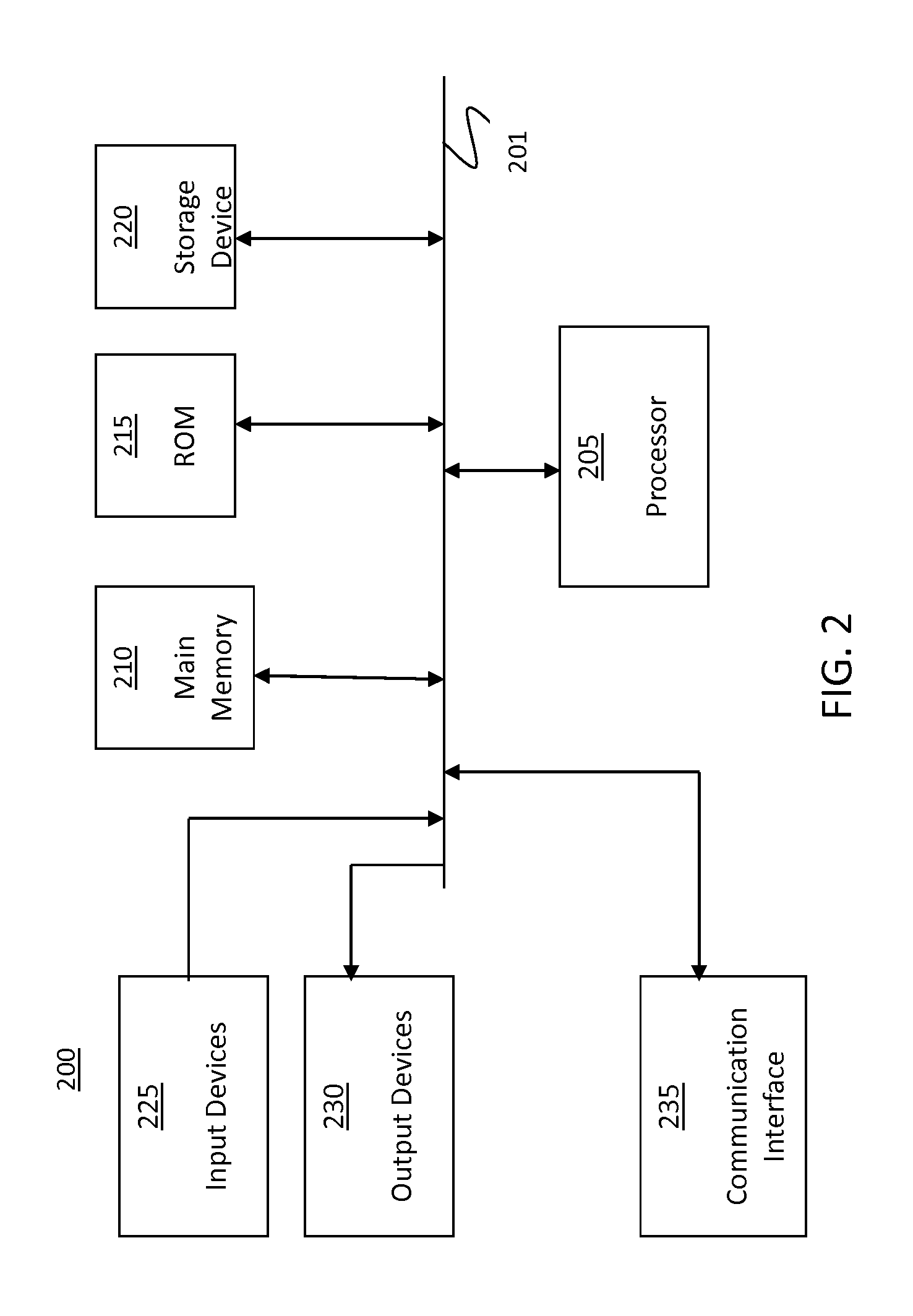
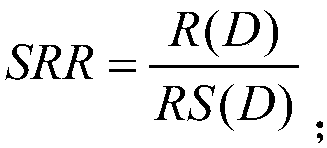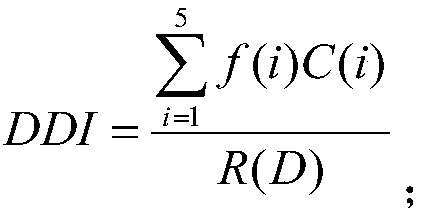Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
319 results about "ADR - Adverse drug reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
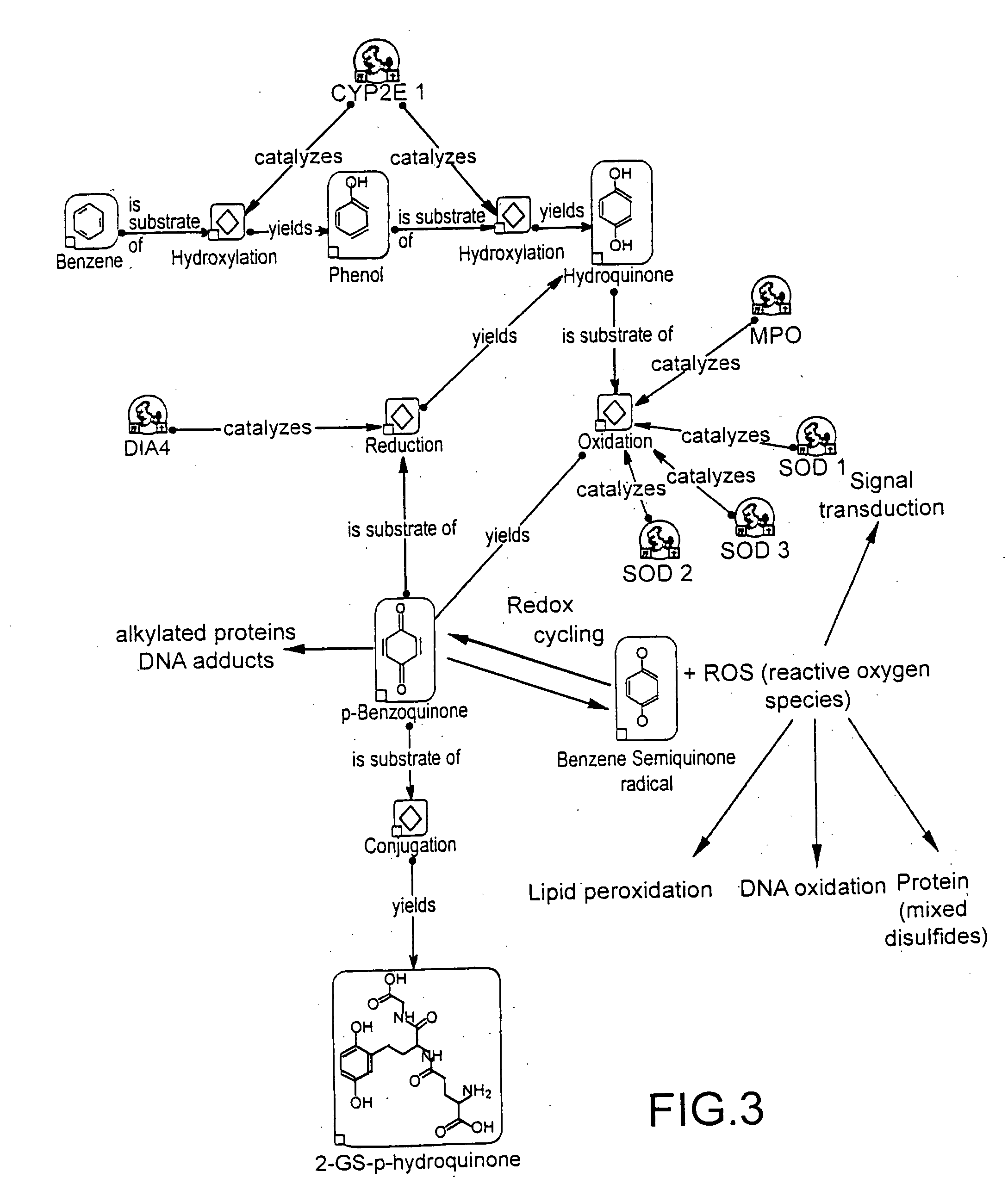
An adverse drug reaction (ADR) is an injury caused by taking a medication. ADRs may occur following a single dose or prolonged administration of a drug or result from the combination of two or more drugs.
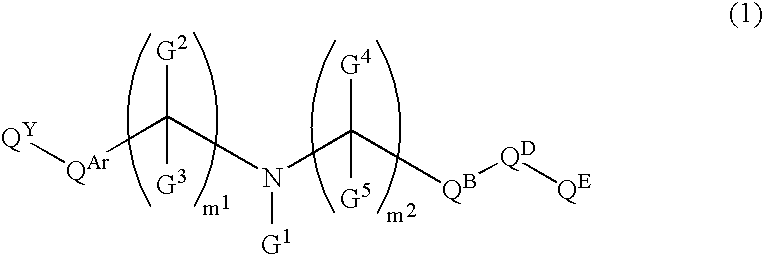
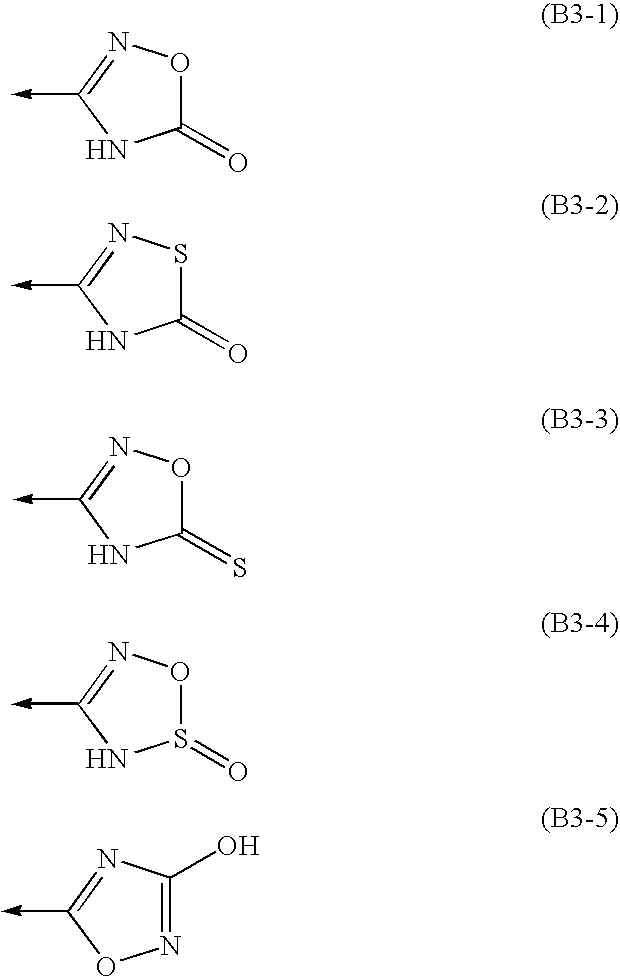
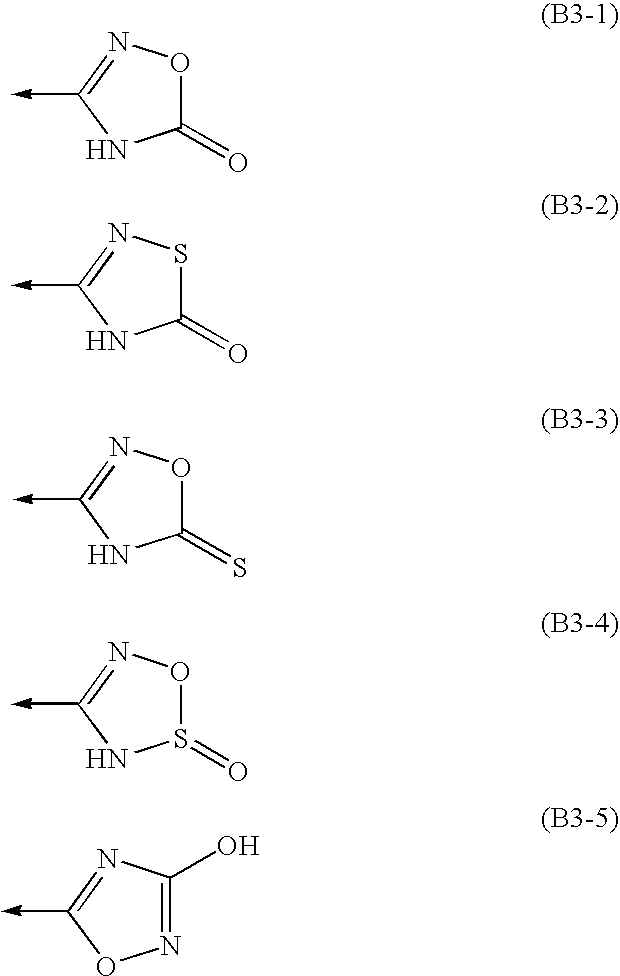
Amine Compounds
InactiveUS20080200535A1Potent immunosuppressive actionBiocideSenses disorderUveitisAutoimmune disease
There is provided a compound exhibiting an activity of suppressing immune response with reduced adverse drug reactions, which compound is useful in the chemotherapy for preventing or treating, for example, a wide range of various autoimmune diseases including systemic erythematodes, chronic rheumatoid arthritis, Type I diabetes, inflammatory bowel disease, biliary cirrhosis, uveitis, multiple sclerosis or other disorders, or chronic inflammatory diseases, or cancers, lymphoma or leukemia, or resistance to organ or tissue transplantation or rejection against transplantation.Novel amine compounds having an S1P1 / Edg1 receptor agonist effect, possible stereoisomers or racemic bodies of the compounds, or pharmacologically acceptable salts, hydrates or solvates of the compound, the stereoisomers or the racemic bodies, or prodrugs of the compounds, the stereoisomers, the racemic bodies, the salts, the hydrates or the solvates, are provided.
Owner:ASAHI KASEI PHARMA
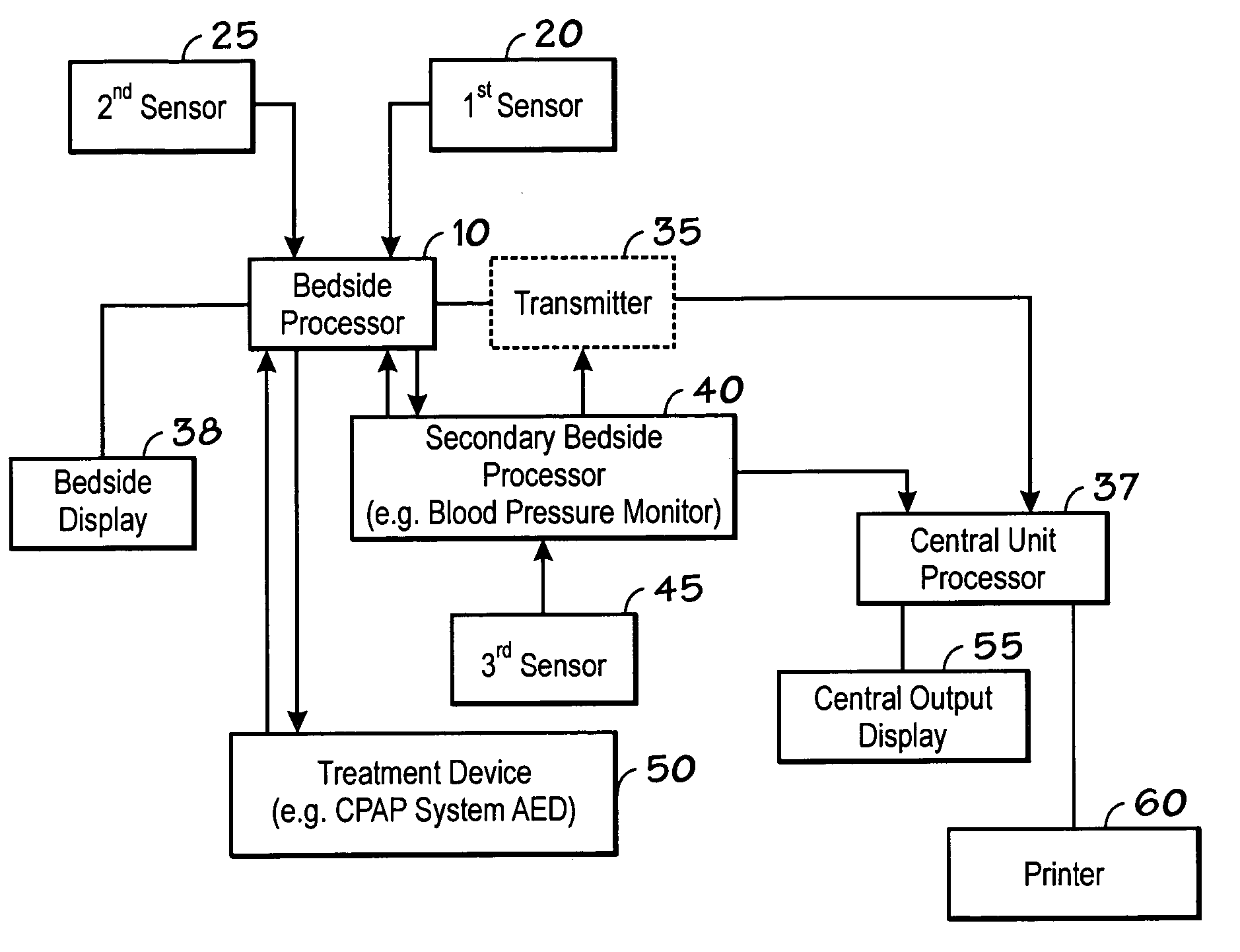
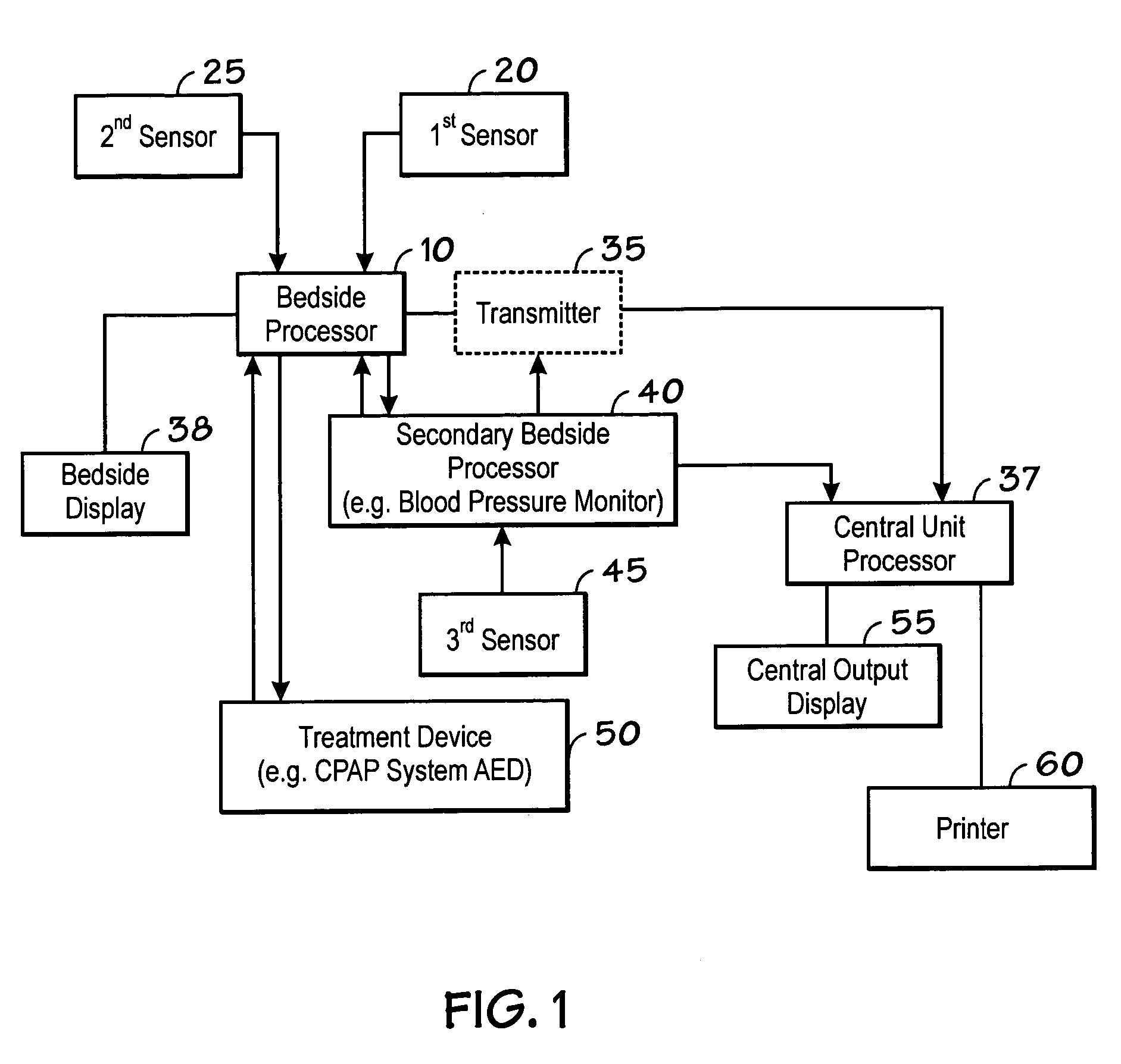
Centralized hospital monitoring system for automatically detecting upper airway instability and for preventing and aborting adverse drug reactions
InactiveUS20060195041A1Improve matchImprove instabilityDrug and medicationsRespiratory organ evaluationInstabilityDisplay device
A system and method for the automatic diagnosis of obstructive sleep apnea in a centralized hospital critical care monitoring system for the monitoring of a plurality of patients in at least one of a critical care, step down, and cardiac ward by telemetry. The system includes a central processor having a display, and a plurality of telemetry unit for mounting with patients, each of the telemetry units has a plurality of sensors for connection with each patient, the telemetry unit is capable of the transmission of multiple signals derived from the sensors to the central processor, in one preferred embodiment the method comprising steps of programming the system to analyze the signals and to automatically identify the presence and severity of obstructive sleep apnea and to provide an indication of the identification.
Owner:LYNN LAWRENCE A +1
Risk assessment for adverse drug reactions
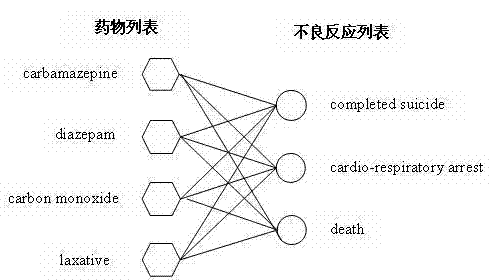
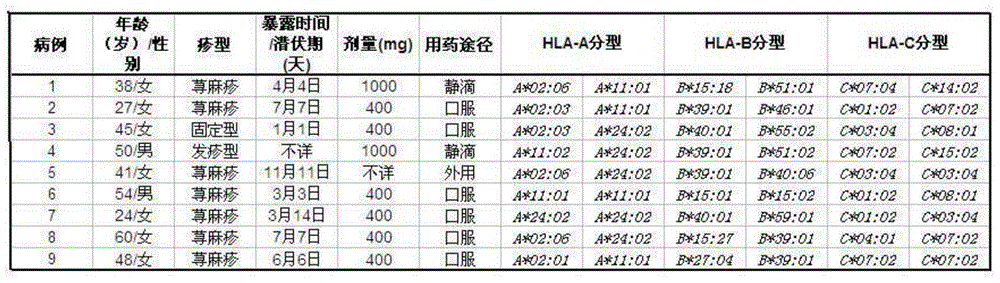
The present invention provides a method of predicting the risk of a patient for developing adverse drug reactions, particularly SJS or TEN. It was discovered that an HLA-B allele, HLA-B* 1502, is associated with SJS / TEN that is induced by a variety of drugs. The correlation with HLA-B* 1502 is most significant for carbamazepine-induced SJS / TEN, wherein all the patients tested have the HLA-B* 1502 allele. In addition, another HLA-B allele, HLA-B*5801, is particularly associated with SJS / TEN induced by allopurinol. Milder cutaneous reactions, such as maculopapular rash, erythema multiforme (EM), urticaria, and fixed drug eruption, are particularly associated with a third allele, HLA-B *4601. For any of the alleles, genetic markers (e.g., HLA markers, microsatellite, or single nucleotide polymorphism markers) located between DRB1 and HLA-A region of the specific HLA-B haplotype can also be used for the test.
Owner:ACAD SINIC
Drug composition for blood sugar control
InactiveUS20050267195A1Improve blood sugar controlInhibition is effectiveBiocideSenses disorderAcute hyperglycaemiaDisease
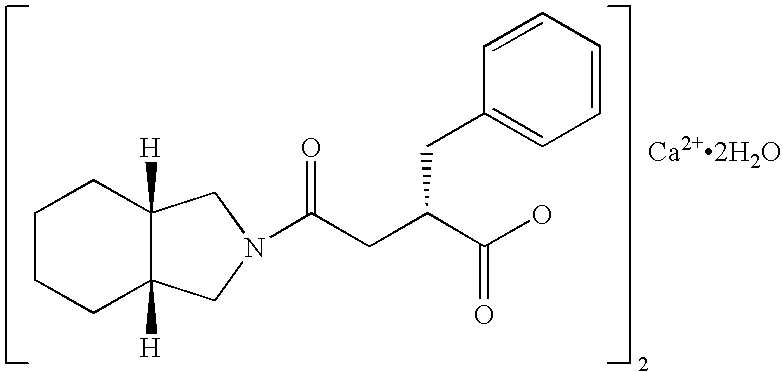
The present invention provides pharmaceutical compositions which can achieve good state of glycemic control and correct postprandial hyperglycemia and early morning fasting hyperglycemia. The present pharmaceutical composition is for administration before meal to control blood glucose, which comprises 5 to 45 mg, as a single dose, of mitiglinide or a pharmaceutically acceptable salt thereof, or a hydrate thereof (for example, mitiglinide calcium salt hydrate). And said compositions are extremely useful for prevention or treatment of, for example, type II diabetes, because the frequency of adverse drug reactions such as hypoglycemic symptoms and gastrointestinal disorders is low.
Owner:KISSEI PHARMA
Individual drug safety
InactiveUS20050037366A1Microbiological testing/measurementBiostatisticsProtein profilingActivity profiling
The invention provides means to determine the predisposition of individuals to adverse drug reactions (ADRs). The methods are based on genotyping or parallelized enzyme and protein profiling or both. Parallelized enzyme activity profiling can be used for drug screening and development. As examples of the invention we show the prediction of adverse drug reactions of pulmonary hypertension patients by identifying genes and alleles linked to known ADRs and liver enzyme reaction profiling with ADR correlation.
Owner:THERASTRAT
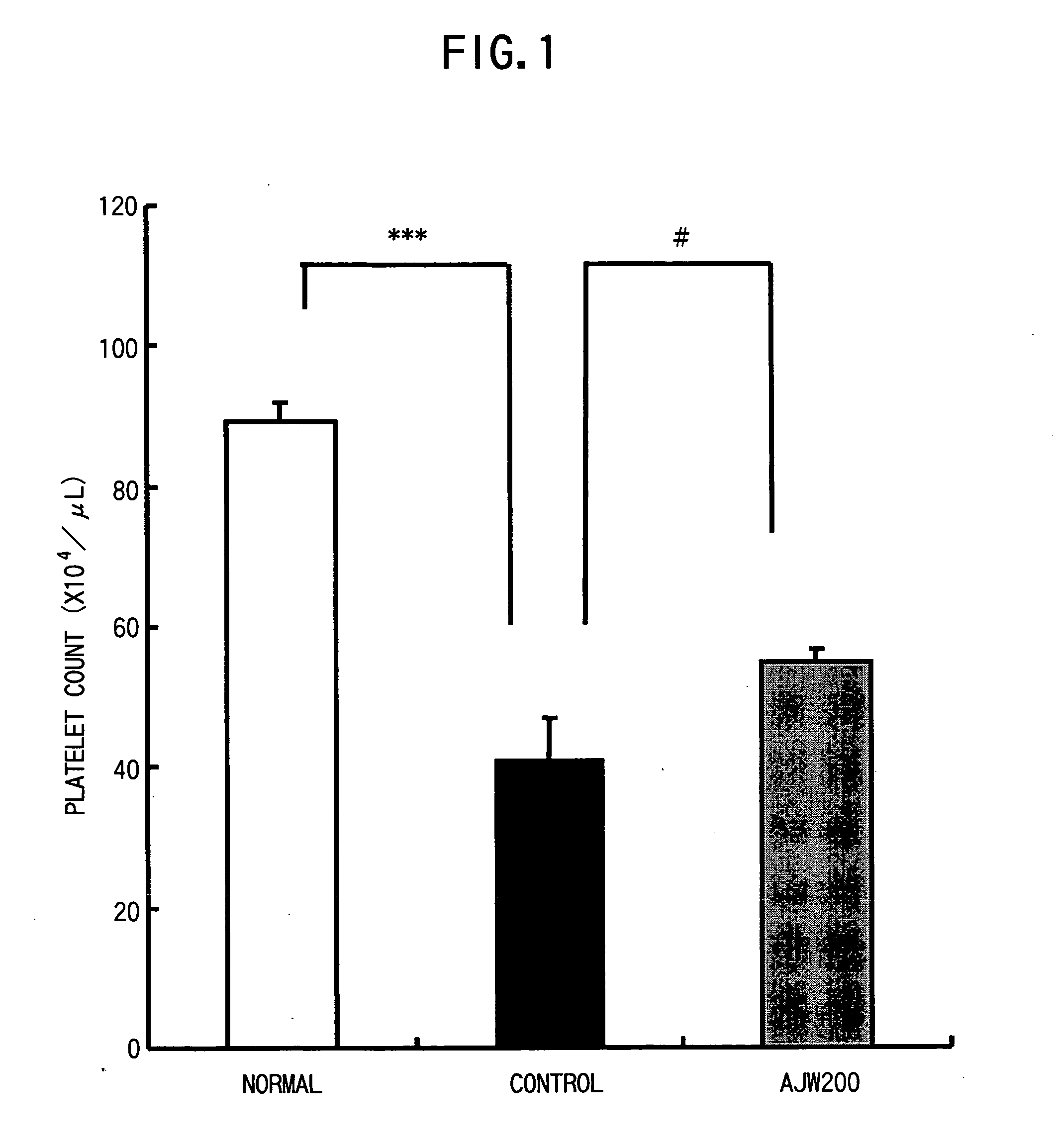
Pharmaceutical composition for the treatment of thrombocytopenia
InactiveUS20050136056A1Shorten the counting processImprove bindingImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsGlycoprotein IbFactor VIII vWF
The present invention provides a drug for the treatment of thrombocytopenia caused by hepatic failure, preferably a drug with few adverse drug reactions. A substance that inhibits binding between glycoprotein Ib (GPIb) and von Willebrand factor (vWF), for example, anti-GPIb antibody or anti-vWF antibody that inhibits binding between GPIb and vWF is an active ingredient of the drug for the treatment of thrombocytopenia.
Owner:AJINOMOTO CO INC
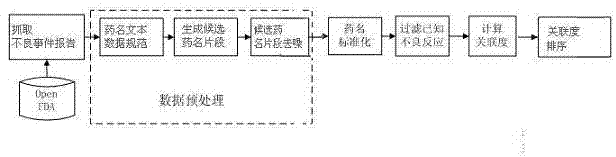
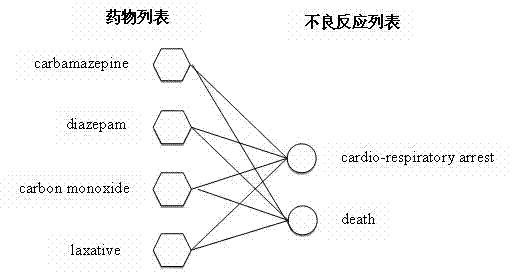
Method for mining potential adverse drug reaction data from big data
ActiveCN104765947AAdverse reactions are not limitedDiscover security risksSpecial data processing applicationsData setMedicine
The invention provides a method for mining potential adverse drug reaction data from big data. The method comprises the steps of A, collecting adverse drug reaction reports; B, preprocessing data in the adverse drug reaction reports of the adverse drug reaction event data set; C, standardizing the drug name; D, filtering known adverse reaction; E, calculating degrees of association; F, sequencing the degrees of association. The method is applied to the work of mining potential adverse drug reaction and the work is not limited to the category of drugs; the potential risk of marked drugs can be effectively found out, and the method is of important significance on increasing the health level of a user.
Owner:DALIAN UNIV OF TECH
System and method to produce and validate weighted relations between drug and adverse drug reactions
A system to produce validated weighted relations between drugs and adverse drug reactions (ADRs). At least one processor to monitor social media for links between drugs and ADRs; extract a relation between a drug and an ADR using named entity recognition to provide a weighted relation between the drug and the ADR, the weight based on confidence of the link between the drug and the ADR in social media. The at least one processor to use domain knowledge of ontologies of drug names and / or ADRs to refine the weighted social media relation; quantify the weighted social media relation by using drugs and ADR links extracted from research publications and / or from clinical trial reports and provide a research weight for the relation; and / or quantify the weighted social media relation by using internet search engine and searching for the drug and the ADR, numbers of hits quantify internet weight for the relation.
Owner:FUJITSU LTD
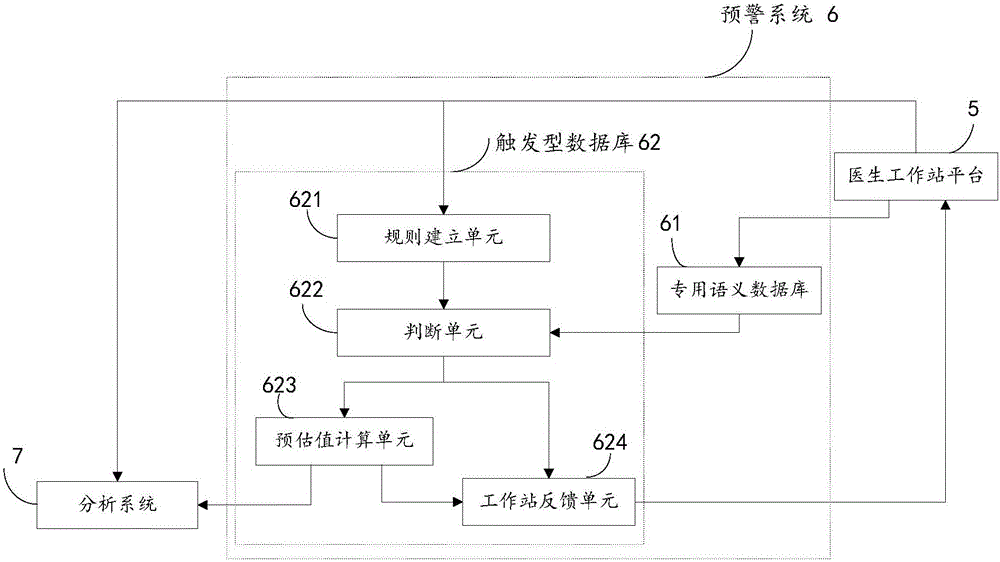
Adverse drug reaction early warning and analyzing system and method
InactiveCN105760698AImprove recognition rateImprove reporting rateMedical automated diagnosisComputer-assisted medicine prescription/deliveryMedical recordEarly warning system
The invention discloses an adverse drug reaction early warning and analyzing system and method. The adverse drug reaction early warning and analyzing system comprises a doctor workstation platform, an early warning system and an analyzing system. The adverse drug reaction early warning and analyzing method comprises the following steps: sending medical history information to the early warning system through the doctor workstation platform; judging whether data according with ADR (Adverse Drug Reaction) trigger rules exist in the medical history information or not through the early warning system, if the data exist, calculating an ADR estimate value and sending to the analyzing system through the early warning system, calculating the ADR occurrence rate and feeding back to the early warning system through the analyzing system, feeding back the ADR occurrence rate to the doctor workstation platform through the early warning system, modifying medication orders or sending medication use reasons to the early warning system and the analyzing system through the doctor workstation platform; and if the data do not exist, feeding back the prompt of executing the medical orders to the doctor workstation platform through the early warning system. The adverse drug reaction early warning and analyzing system and method which are disclosed by the invention have the advantages that in the process of transmitting the medication orders, the reasonability of the medication orders can be judged by means of computers; medical workers are prompted for possible medication use risks; the hospital-wide medical orders are automatically monitored; and the medication use safety is increased.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Genetic diagnosis for QT prolongation related adverse drug reactions
InactiveUS7179597B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMedicineIncreased risk
The specification is directed to a method of diagnosing whether a subject is predisposed to an adverse reaction to one or more pharmaceutical agents which may induce a prolonged QT interval or acquired LQTS in that individual. The diagnosis is genetic analysis of at least two polymorphisms or mutations which the individual may have, which are associated with an increased risk for prolonged QT intervals or Torsades de Pointes (TdP). Genetic screening for determining the predisposition of prolonged QT intervals induced by a pharmaceutical agent is performed by identifying genetic polymorphisms or mutations located in at least two classes of genes, wherein the genes are (1) LQT genes, (2) altered sensitivity genes (e.g., MiRP1) or (3) increased exposure genes (e.g., MDR genes or P450 cytochrome genes). The specification is also directed to compositions and kits for determining such predispositions to adverse drug reactions.
Owner:WOOSLEY RAYMOND L
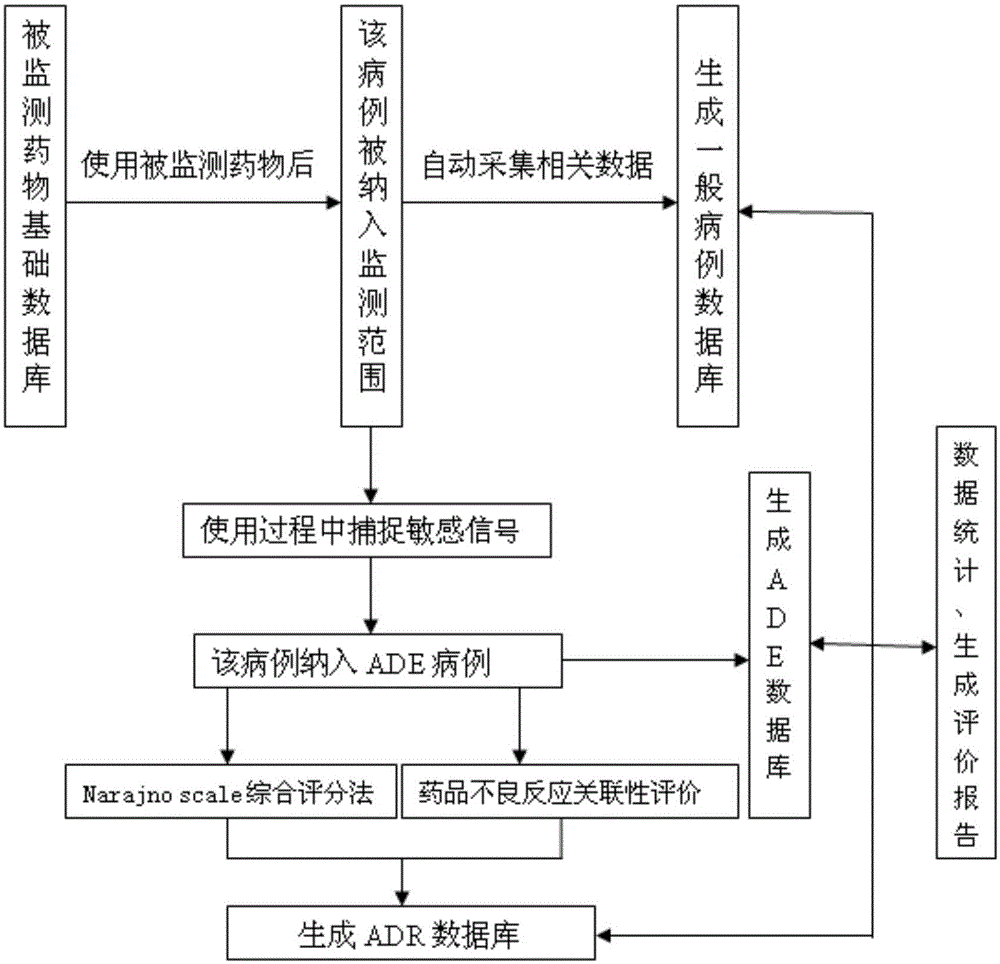
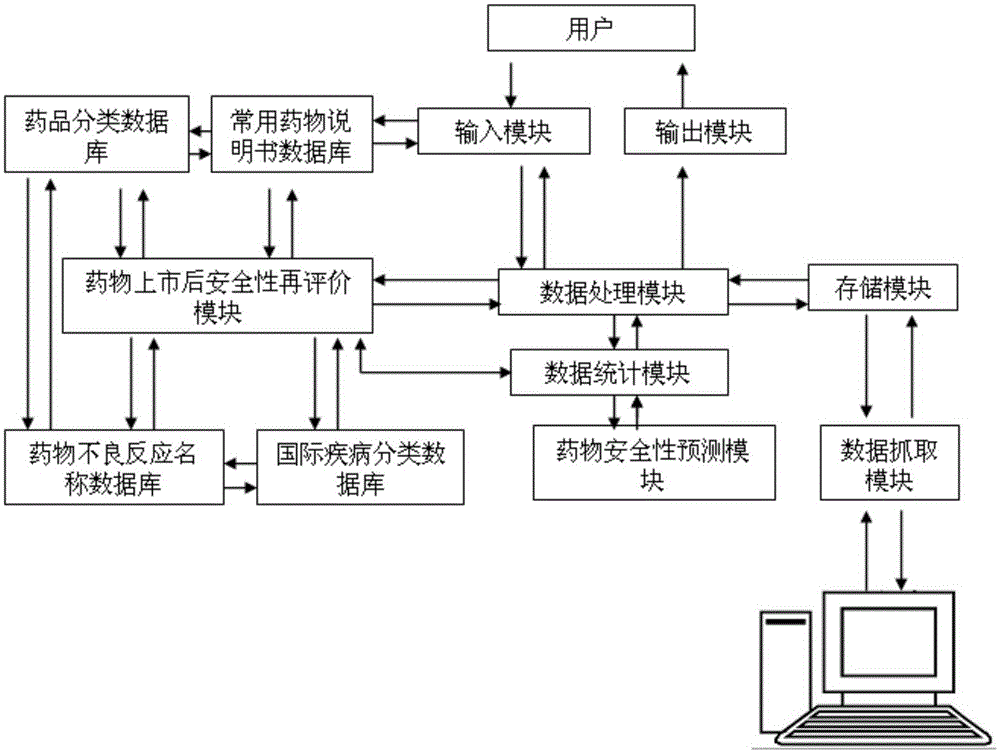
Method and system for reevaluating safety of drug after appearance on market
InactiveCN105139083APrevent serious adverse events/adverse reactionsSave human effortForecastingVisibilityDrug Databases
The invention discloses a method and a system for reevaluating the safety of a drug after appearance on the market. The method comprises the following steps of: determining a studied drug; setting a sensitive signal; determining a case collection quantity; acquiring data and establishing a database; processing the data and carrying out data statistics; establishing a logistic model and determining associated risk factors; and predicting the safety for applying the drug to a patient. The system comprises: an input module; an output module; a storage module; a commonly-used drug database; an international disease classification standard database; a drug classification database; an adverse drug reaction term set database; a signal capture module; a data processing and statistic module; and a safety reevaluating module and a safety predicting module for the drug after appearance on the market. The method and the system can provide a basis for clinical rational drug administration and risk management after the drug appears on the market, can provide a scientific and reasonable analysis platform for establishing the safety evaluation after the drug appears on the market, and are reliable in principle, timely and accurate in data capture, flexible and convenient in application operation, and good in visibility.
Owner:石庆平
Method for patient genotyping
The present invention is a system and method for utilizing human genetic and genomic information to guide prescription dispensing and improved drug safety in a pharmacy setting. The system and method of the present invention utilizes a dedicated information management system and software to utilize patient-specific genetic information to screen for increased risk of adverse drug reactions and therapeutic responses at the time of drug dispensing.
Owner:KANE MICHAEL D +3
Single nucleotide polymorphisms sensitively predicting adverse drug reactions (adr) and drug efficacy
InactiveUS20070128597A1Reducing primaryReducing secondary riskGenetic material ingredientsDisease diagnosisNucleotideEfficacy
Single Nucleotide Polymorphisms sensitively predicting Advserse Drug Reactions (ADR) and Drug Efficacy Abs tract. The invention provides diagnostic methods and kits including oligo and / or polynucleotides or derivatives, including as well antibodies determining whether a human subject is at risk of getting adverse drug reaction after statin therapy or whether the human subject is a high or low responder or a good a or bad metabolizer of statins. The invention provides further diagnostic methods and kits including antibodies determining whether a human subject is at risk for a cardiovascular disease. Still further the invention provides polymorphic sequences and other genes. The present invention further relates to isolated polynucleotides encoding a phenotype associated (PA) gene polypeptide useful in methods to identify therapeutic agents and useful for preparation of a medicament to treat cardiovascular disease or influence drug response, the polynucleotide is selected from the group comprising: SEQ ID 1-168 with allelic variation as indicated in the sequences section contained in a functional surrounding like full length cDNA for PA gene polypeptide and with or without the PA gene promoter sequence.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS GMBH
Method and system for registration, identifying and processing of drug specific data
InactiveUS20020095261A1Increase probabilityEasy to useBiostatisticsProteomicsDrug specific IgEScreening method
The invention relates to a method for registration, identifying and processing of drug specific data and for making drugs available to individual patients without severe adverse drug reactions. The invention relates also to a system for carrying out such a method. According to the present invention, the system comprises a master database correlating patterns of gene expression and genetic polymorphisms with drug-induced i.e. drug-related adverse effects and drug structure. The system also comprises a predictive data-tool. In this tool the structural and genetic fingerprints predictive for adverse effects in individual patients due to treatment with a selected drug are stored. The method according the invention is characterized in that the master database being coupled to the database of the predictive data-tool in such a way that a user of the system can develop and carry out different screening approaches either to verify the sociability of drugs for a specific selected category of patients or to search a specific drug for a selected category of patients which do not have adverse drug reactions or to make risk-analyses.
Owner:THERASTRAT
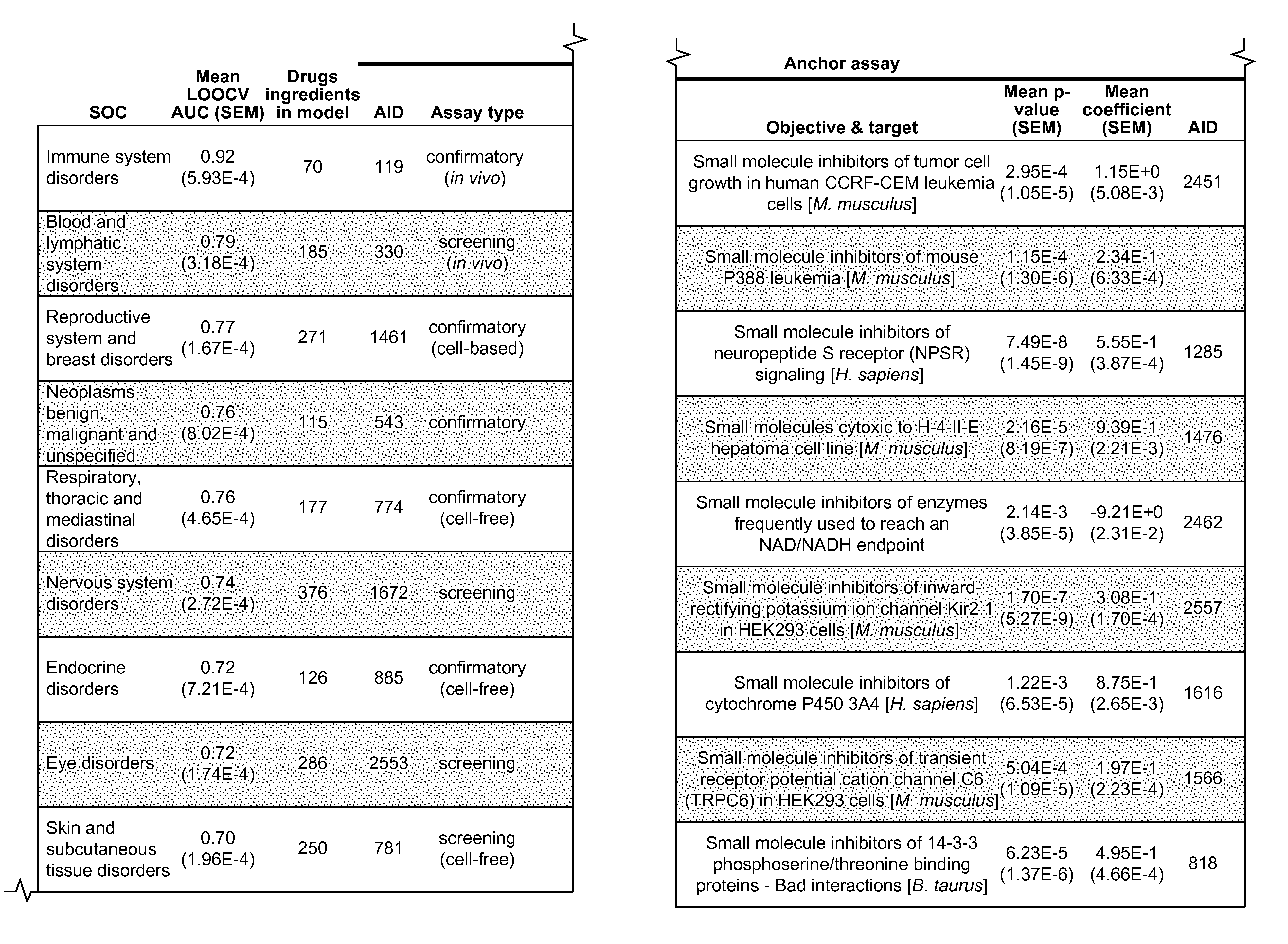
Method and System for Predicting Adverse Drug Reactions Using BioAssay Data
An embodiment of the present invention uses logistic regression models that correlate post-marketing ADRs with screening data from the PubChem BioAssay database. These models of the present invention analyze ADRs at the level of organ systems, the System Organ Classes (SOCs). In testing to evaluate an embodiment of the present invention, nine of 19 SOCs under consideration were found to be significantly correlated with pre-clinical screening data. For six of eight established drugs for which SOC-specific adversities could be retropredicted, prior knowledge was found that support these predictions. SOC-specific adversities were then predicted for three unapproved or recently introduced drugs.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Adverse drug reaction (ADR) report quality evaluation system and ADR report quality evaluation method
InactiveCN103646185AImprove abilitiesQuality improvementSpecial data processing applicationsData informationQuality assessment
The invention discloses an adverse drug reaction (ADR) report quality evaluation system and an ADR report quality evaluation method. The ADR report quality evaluation system includes a standard database module for recording standard data information, an evaluation rule management module for the settings of evaluation items, a report form sampling module for data sampling, a report form quality evaluation module for automatic report form quality evaluation according to the set rules, and a report form examination and verification quality evaluation module for setting the deadline for the report form monitoring, examination and verification. The ADR report quality evaluation system and the ADR report quality evaluation method provided by the invention have the advantages that manpower can be freed from cumbersome operations and concentrated on the content quality evaluation of report forms with more time and energy; the improvements of evaluation capability and quality are strongly supported through auxiliary functions such as standard database identification, intelligent evaluation and process recording, so as to achieve a higher report form quality evaluation capability; the guidance on the form filling and submission is provided with an objective analysis basis.
Owner:顾德国
Anaphylatoxins for detecting clinical conditions
InactiveUS20090226374A1Compounds screening/testingMicrobiological testing/measurementVasculitisBasophilia
Non-allergic hypersensitivity reactions can be observed in a sample of cells from a subject in response to anaphylatoxins. Accordingly, methods are provided for detecting clinical conditions such as cellular hyper-reactivity, non-allergic hypersensitivity, asthma, inflammation, chronic or acute infection, bacterial infection, viral infection, parasite infection, adverse drug reaction, organ rejection, vasculitis, mastocytosis, eosinophilia, basophilia, leukemia, and / or C3a or C5a receptor defects in a subject. Also provided are kits for detecting such clinical conditions in a subject.
Owner:HEALTH AIRE
Web-based computer-aided method and system for providing personalized recommendations about drug use, and a computer-readable medium
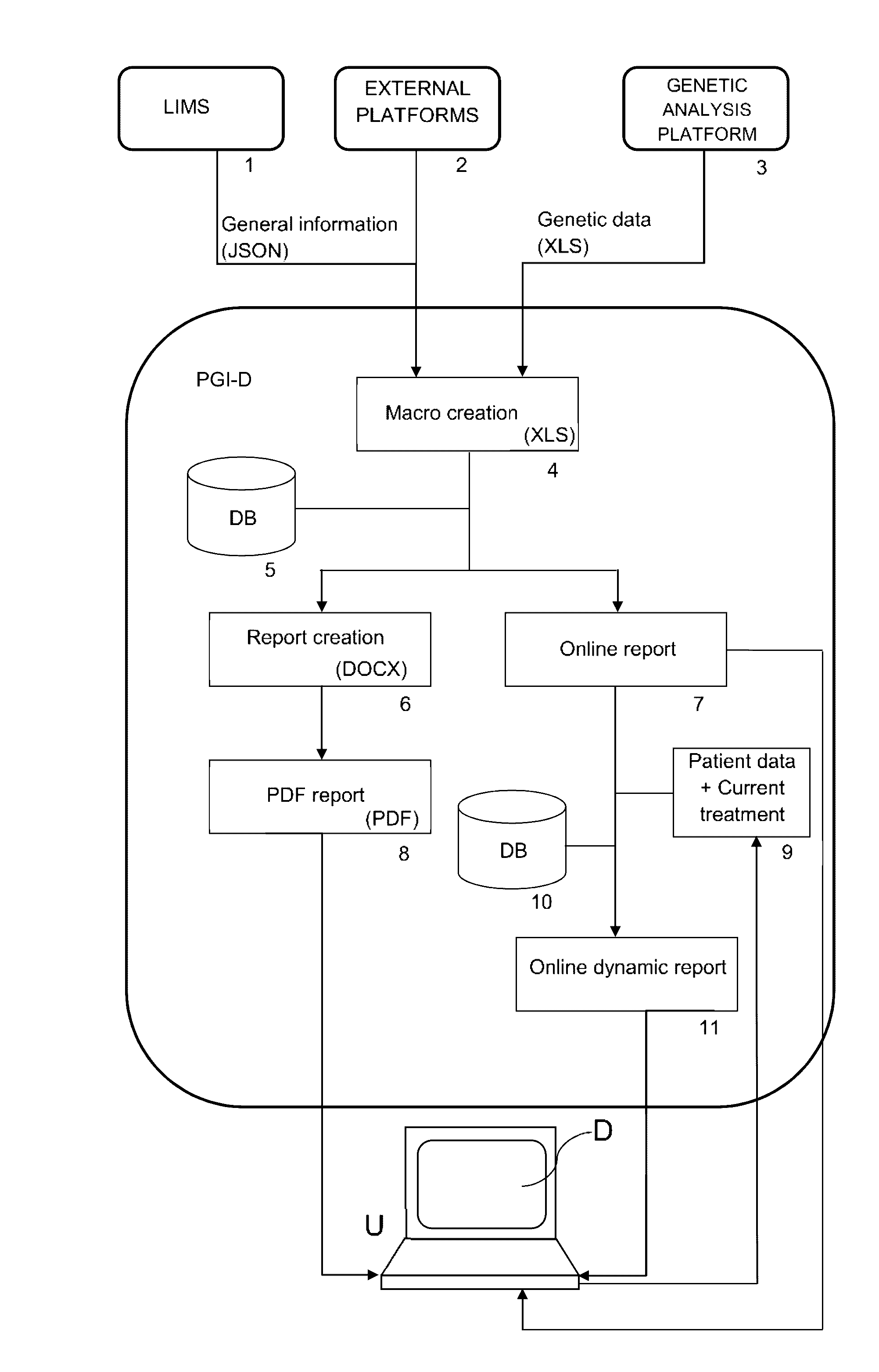
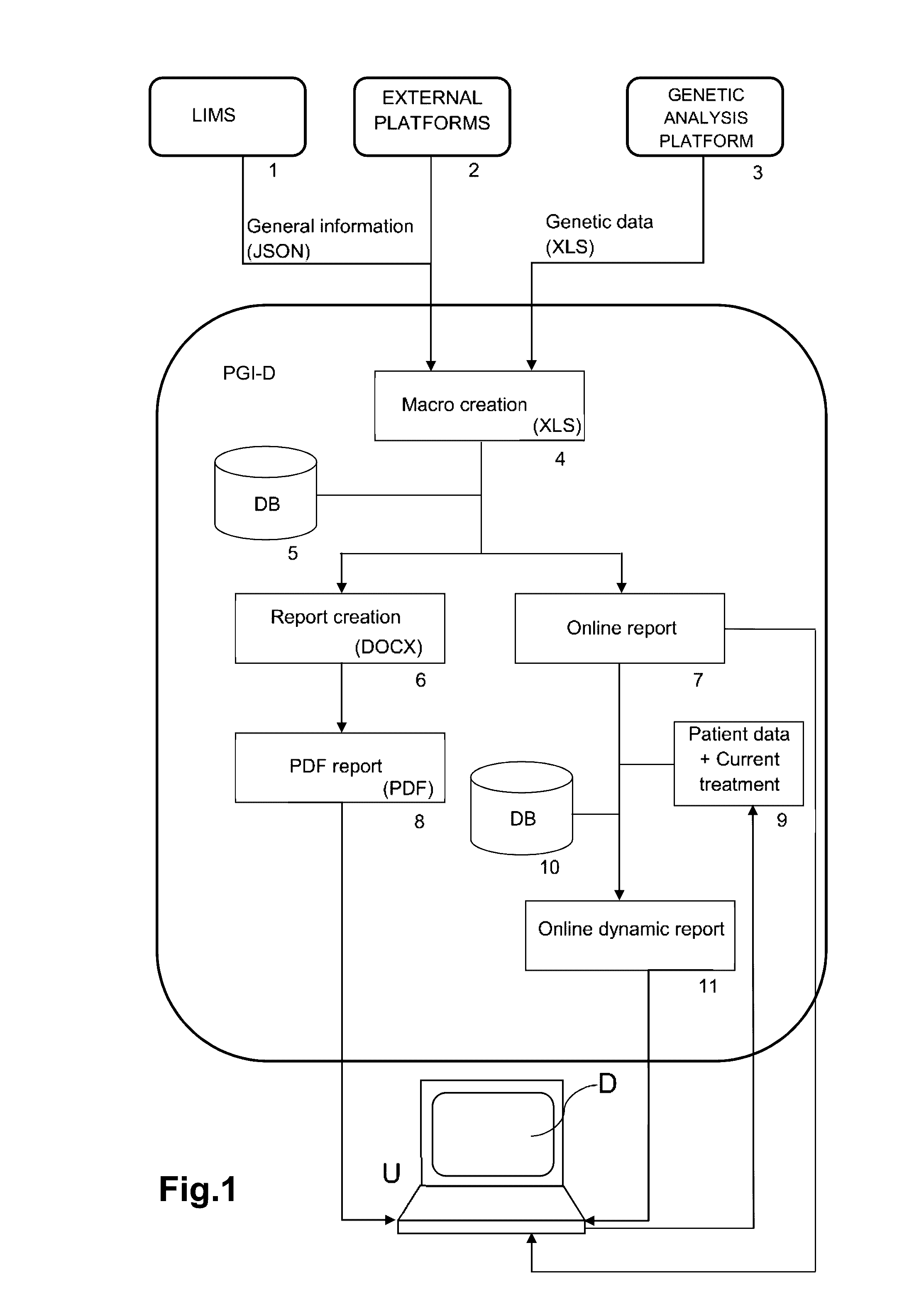
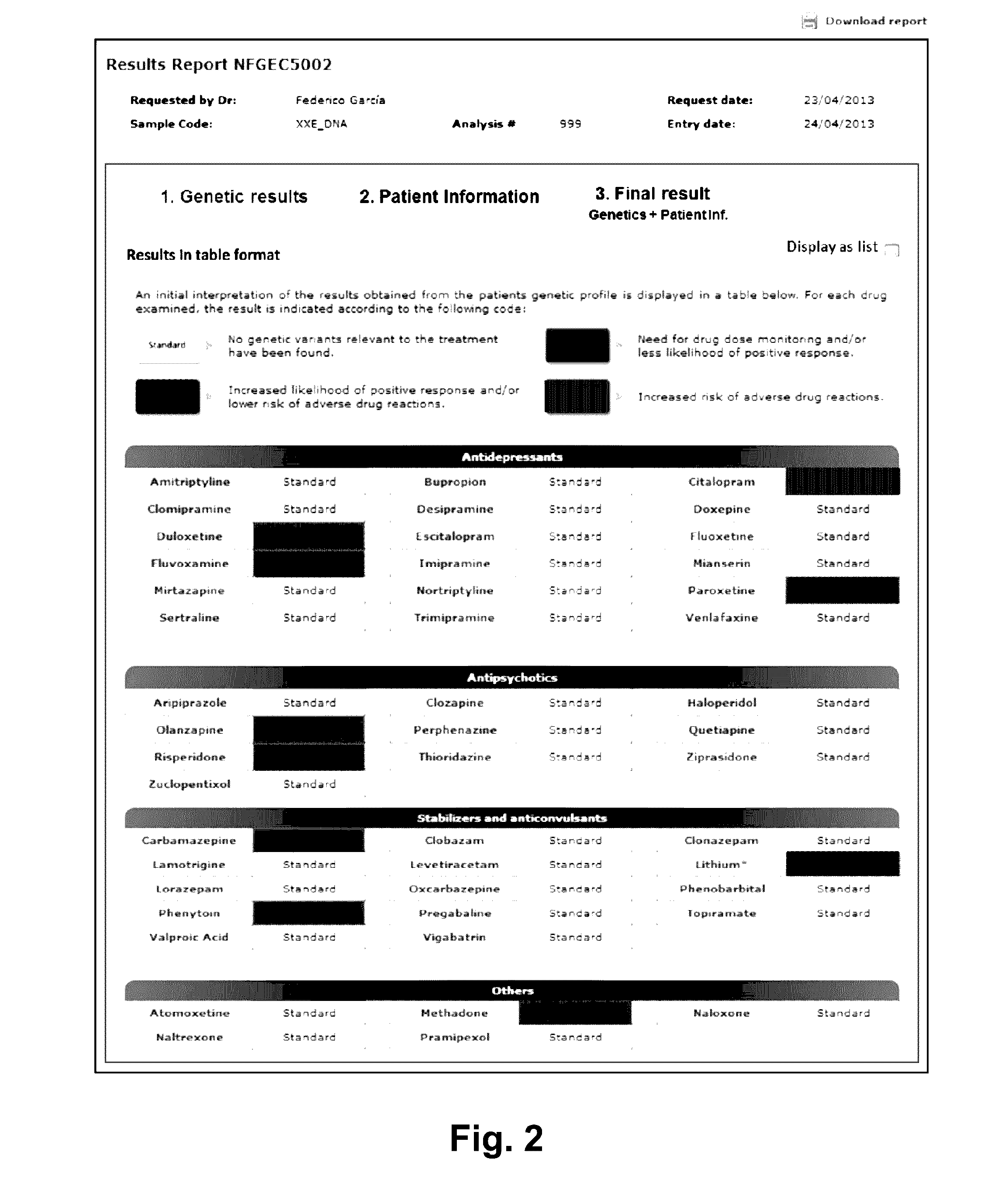
ActiveUS20160314251A1Quality improvementReduce usageChemical property predictionDrug and medicationsPersonalizationComputer aid
The present invention relates to a web-based computer-aided method and a system for providing personalized recommendations about drug use, based on pharmacogenetic information regarding genes and genetic variants associated to metabolism and genes and genetic variants which are not associated to metabolism, and which comprises automatically generating and displaying, by means of a graphical user interface (GUI) of a dynamic webpage, the personalized recommendations highlighting the ones associated to the highest adverse drug reactions.The present invention also relates to a computer-readable medium which contains program instructions for a computer to perform the method for providing personalized recommendations about drug use of the invention.The present invention also relates to a web-based computer-aided method and a system for generating a dynamic webpage, and a further computer-readable medium which contains program instructions for a computer to perform the method for generating a dynamic webpage.
Owner:BIOTICS
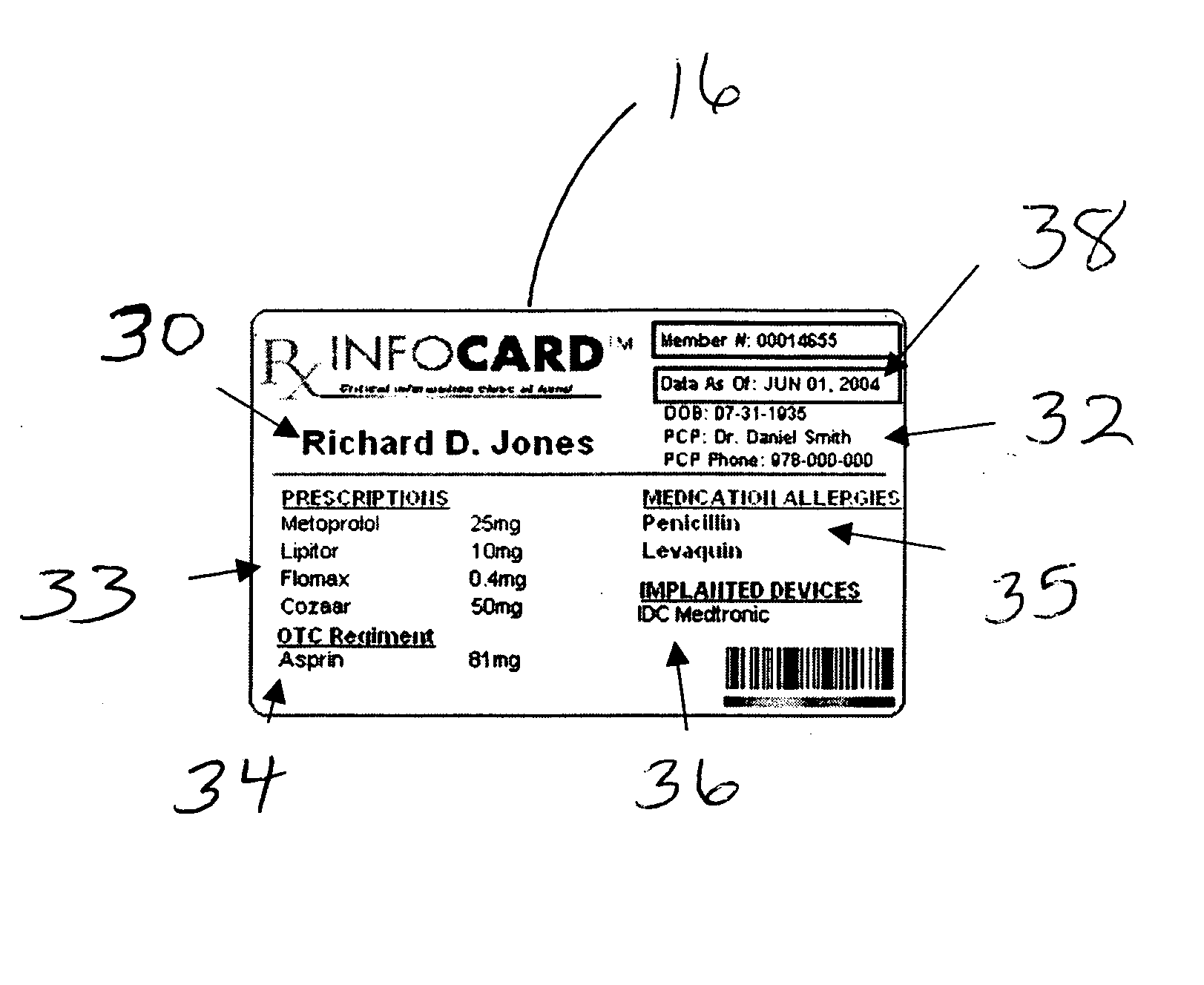
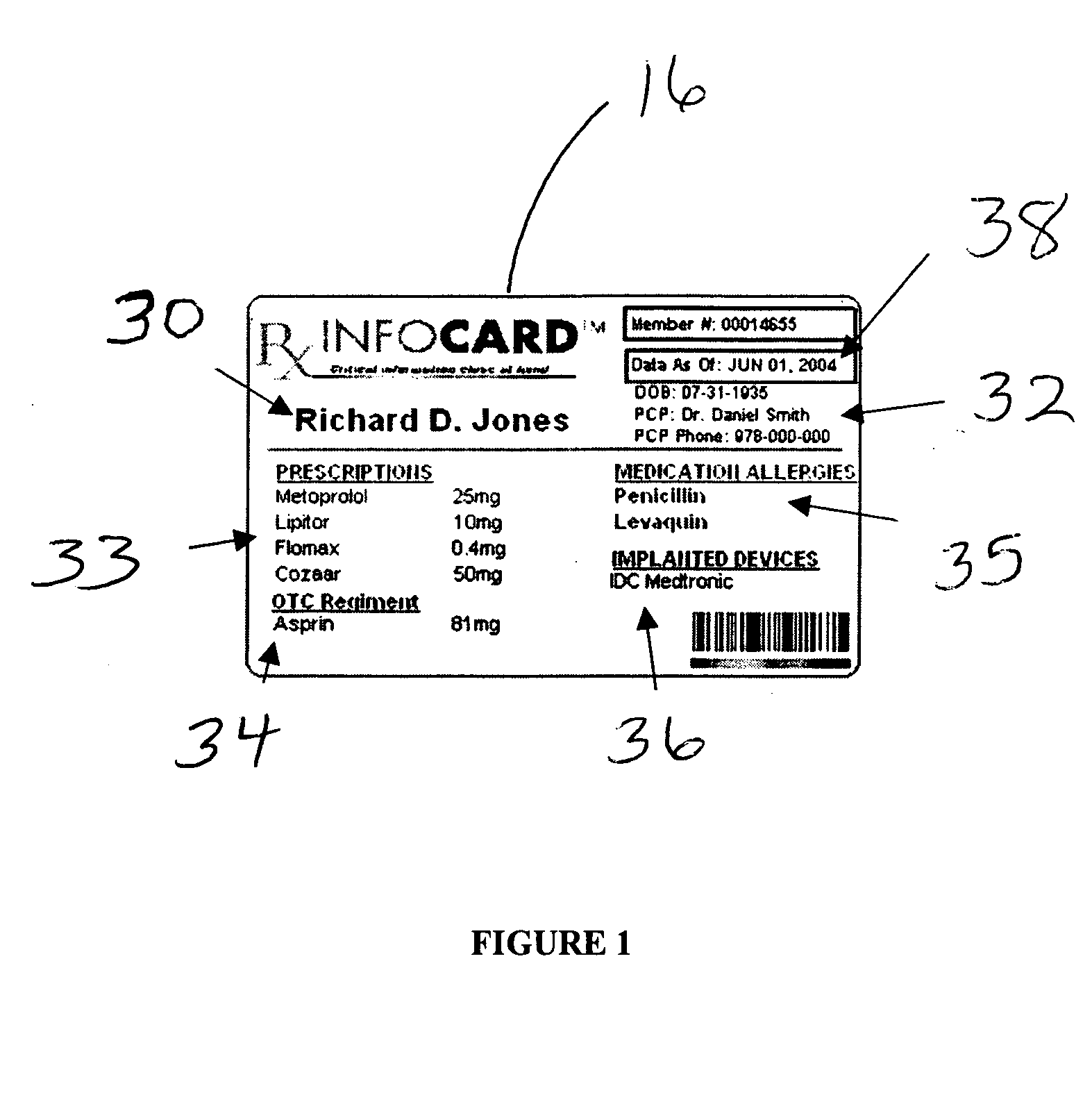
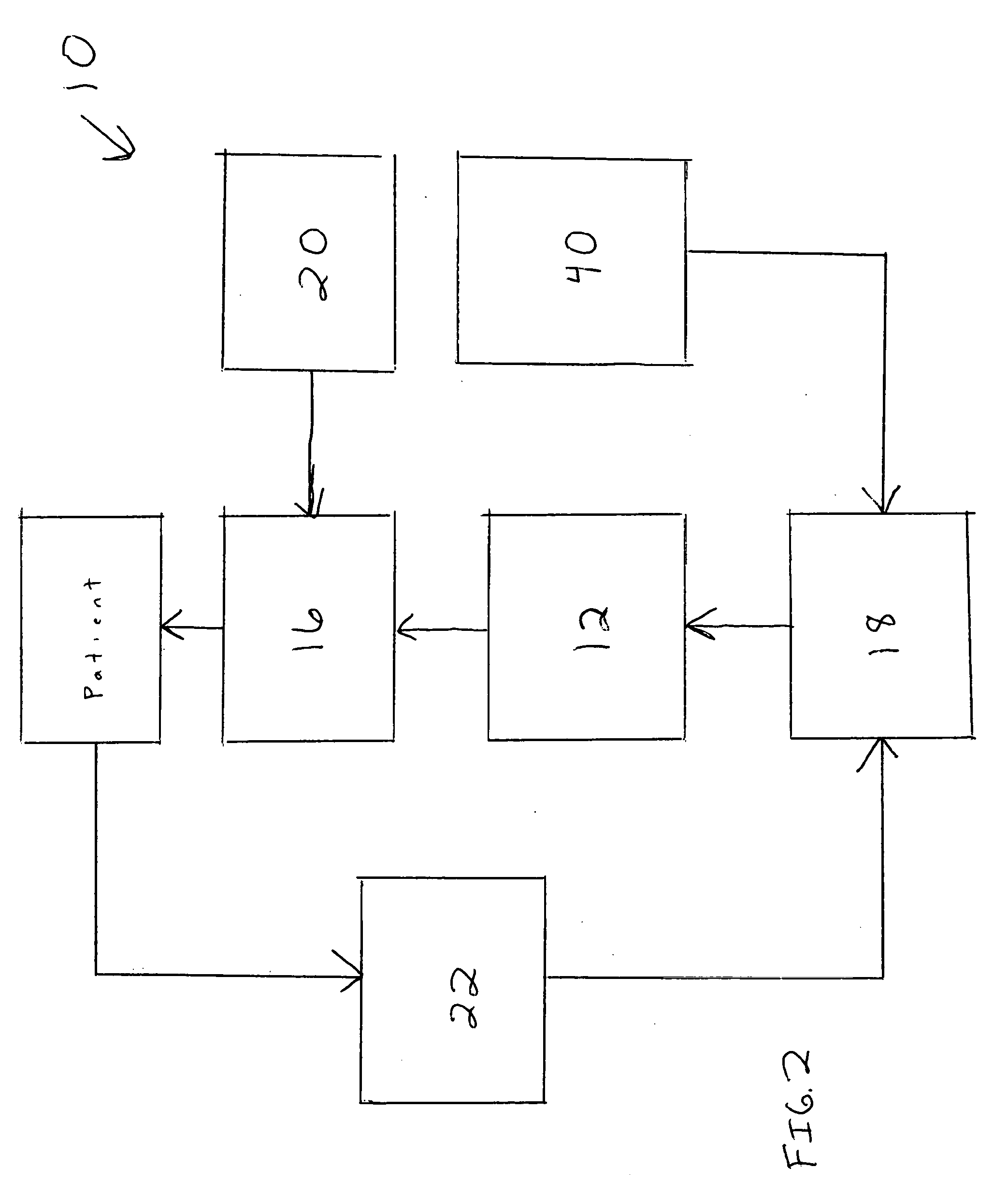
Medical information card and system and method for using same
InactiveUS20060173718A1Avoid reactionEfficient and effective communicationDrug and medicationsDiagnostic recording/measuringInformation CardComputer science
A system for ensuring that a person receives individual-specific health care and avoiding adverse drug reactions, generally comprises: an information card on which critical need medical information, specific to said person, is be stored, and a database that can be electronically accessed by the person so that the person can update their own information stored on the card.
Owner:MURPHY DANIEL J
Human leukocyte antigen gene detection kit and application thereof
PendingCN106282328AContribute to the establishment of a method for screening drug eruptions caused by metronidazoleTo establish a method for screening metronidazole-induced drug eruptionMicrobiological testing/measurementMetaboliteWhite blood cell
The invention belongs to the fields of biomedicine and reagent detection, and relates to a human leukocyte antigen gene detection kit and application thereof in screening metronidazole-induced adverse drug reactions of skin. The kit contains a reagent for detecting human leukocyte antigen gene HLA-B*39:01 nucleic acid or protein, and amplification primers or a labeled probe of the human leukocyte antigen gene or a specific antibody containing the human leukocyte antigen. The HLA-B*39:01 gene can be used as a labeled gene for predicting metronidazole-induced epispasis. The detection kit can be used for further preparing or evaluating a metronidazole-induced epispasis screening kit, thereby providing valuable reference data for instructing clinical medication, and reducing the possibility of metronidazole-induced epispasis; or the detection kit can also be used for preparing or evaluating therapeutic drugs and especially targeted drugs for metronidazole-induced epispasis, thereby preventing the interactions between the drugs and metronidazole or metabolites thereof in disease development.
Owner:FUDAN UNIV +1
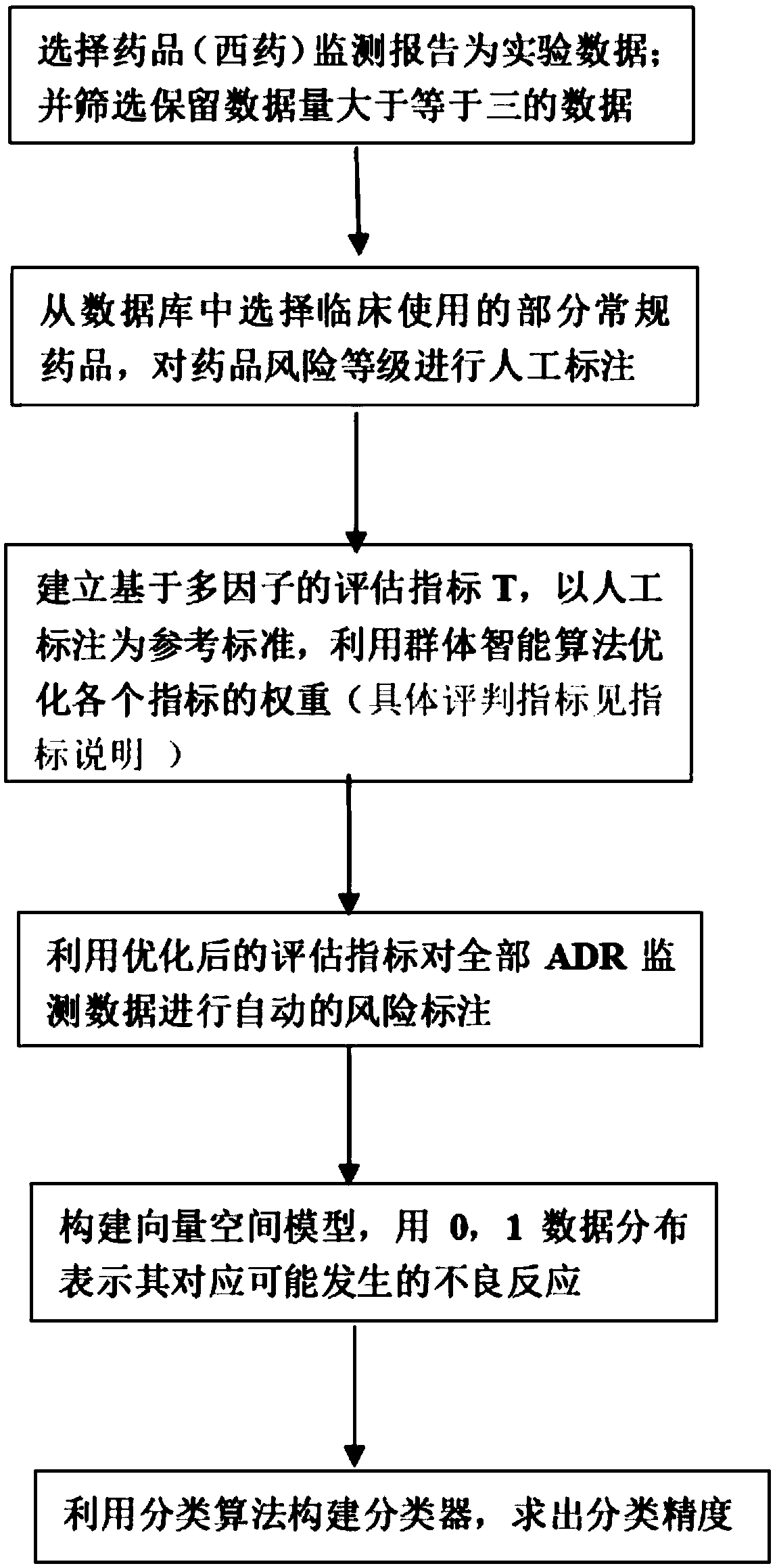
Machine learning-based drug risk ranking evaluation method
The invention discloses a machine learning-based drug risk ranking evaluation method. According to the method, on the basis of western drug report data in the adverse drug reactions (ADR) of Chinese drugs, a machine learning algorithm is utilized to study the problem of drug risk ranking; three main indicators, namely, a severity report rate, an ADR injury index and an ADR coverage rate, are usedas ranking standards; a support vector machine-based classification algorithm is used to perform risk ranking evaluation on the adverse drug reactions of Western drugs; and the drugs are classified into five safety ranks according to the risks of the adverse reactions. The method of the invention is of great reference significant for the evaluation of the risks of adverse drug reactions.
Owner:NANJING UNIV OF POSTS & TELECOMM
Active monitoring method and system for adverse drug reactions
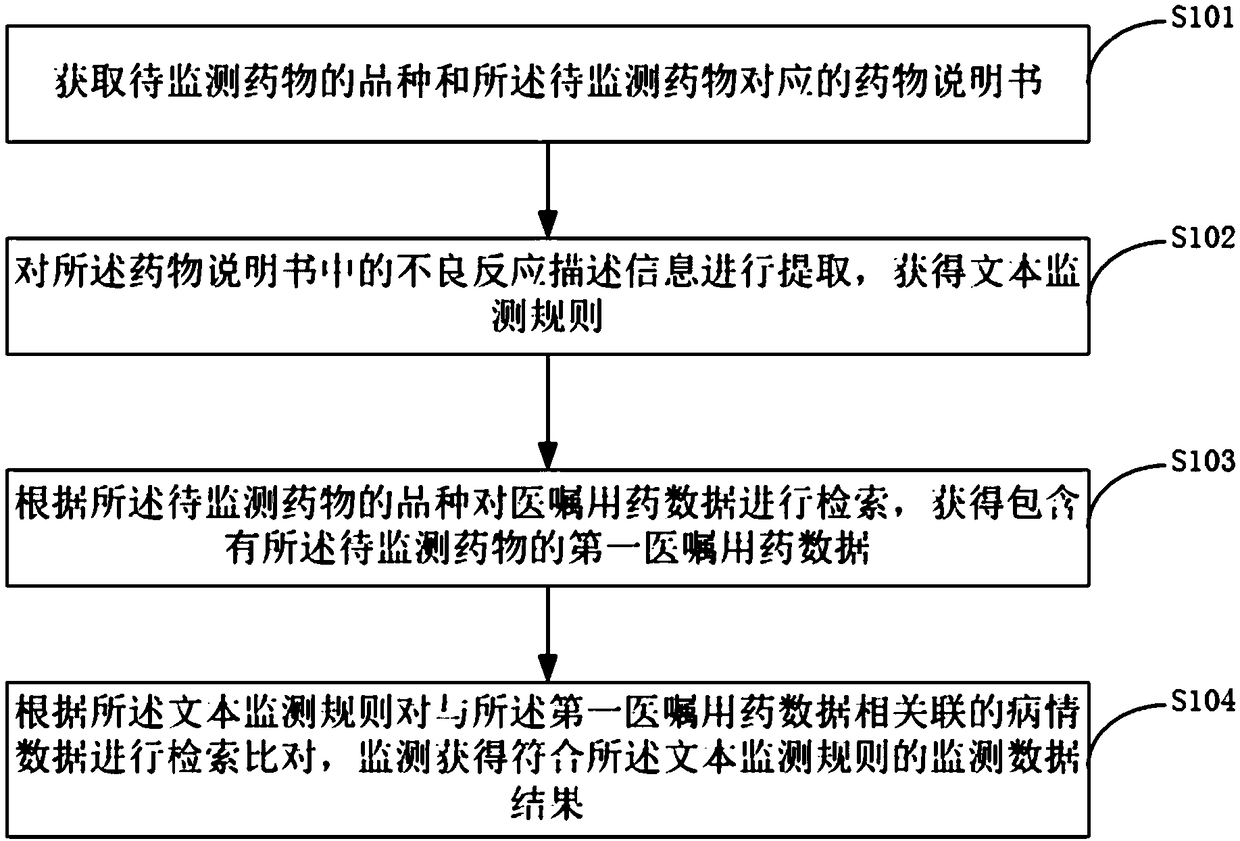
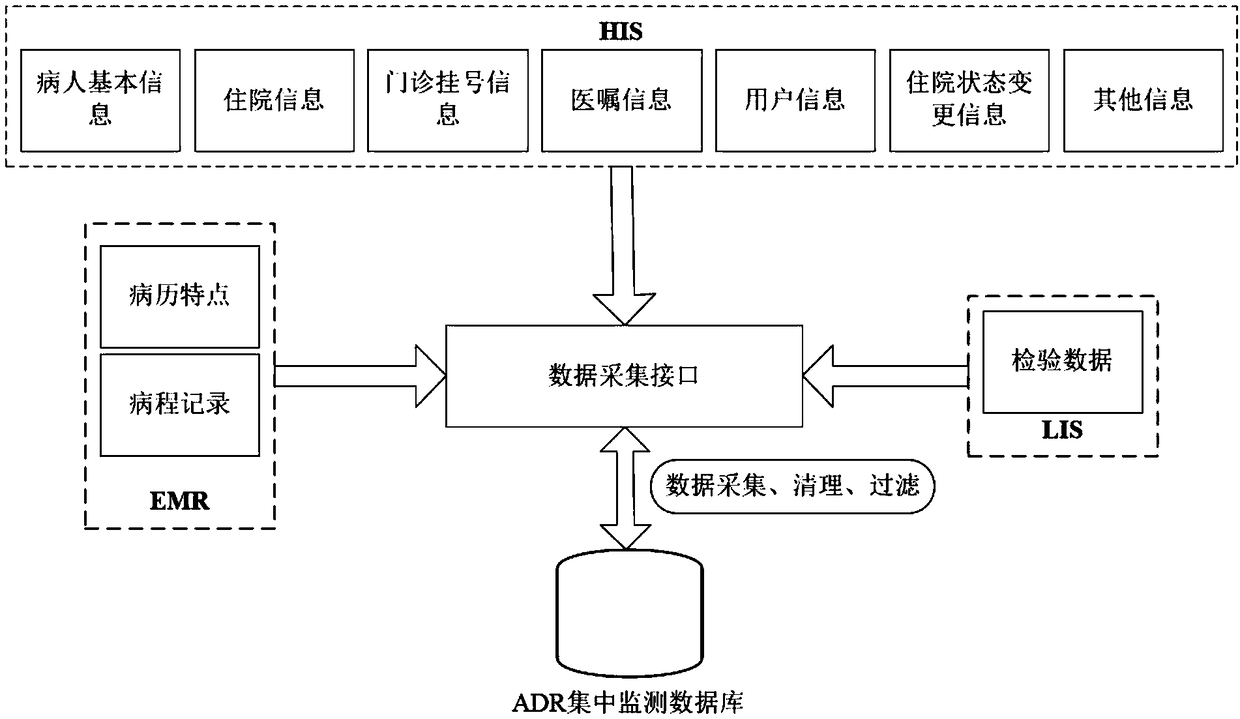
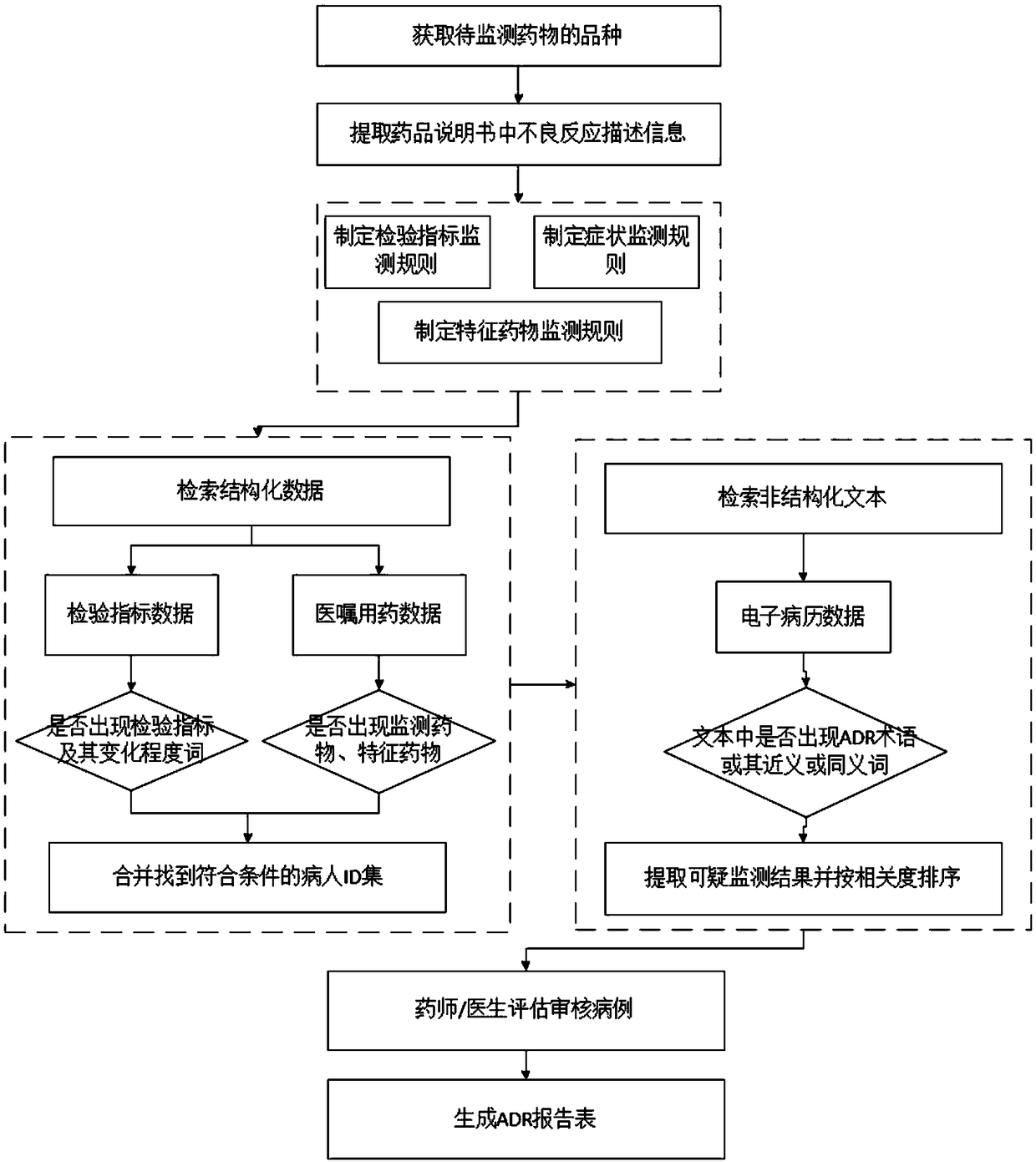
ActiveCN109346145ARealize monitoringImprove work efficiencyDrug and medicationsText database queryingDiseaseActive monitoring
The invention discloses an active monitoring method and system for adverse drug reactions. Through obtaining varieties of to-be-monitored drugs and drug specifications thereof and performing retrievalin medical drug data according to the to-be-monitored drugs, disease data corresponding to the to-be-monitored drugs contained in medical drugs are screened out, according to the adverse reaction description information of the drug instructions, text monitoring rules are established for the disease data, the text monitoring rules are utilized to monitor whether suspicious results with adverse reactions exist in the disease data, signs of possible adverse reactions in the drug use course of patients and conditions of the suspected adverse reaction are obtained through monitoring, moreover, thedrugs are monitored while hospitals can be facilitated to carry out targeted diagnosis and treatment for the patients in the follow-up process, and thereby work efficiency is improved.
Owner:国家食品药品监督管理总局药品评价中心 +1
Personalized adverse drug reaction prediction method, system and device and medium
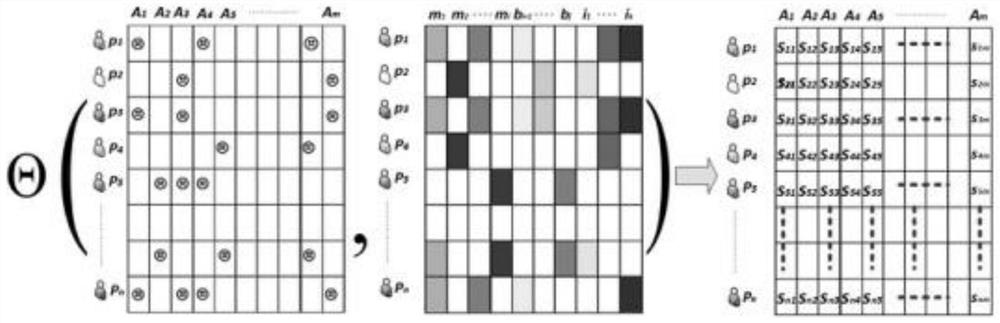
ActiveCN111863281AShorten the clinical trial cycleThe value of good practical applicationDrug referencesDrug adverse reactionsMedicine
The invention provides a personalized adverse drug reaction prediction method, system and device and a medium, and belongs to the technical field of biomedicine. The invention provides a multi-task learning model (KEMULA) based on multi-kernel function learning so as to replace traditional learning methods of universal application and complete individuation. More specifically, the model calculatesand ranks the risks of ADR development of patients by learning a constrained personalized ADR ranking function by assuming a sharing function of the model. This function is referred to as a personalized ADR ranking function, which is a linear combination of several scoring functions that calculate the risk of developing related ADR of patients. In addition, the model is also combined with Laplacian regularization to ensure that variable information trained by personADRank functions of similar patients is close, so that the causal relationship (true positive) of the model to the association between a given patient and the corresponding ADR can be improved. Therefore, the method has good practical application value.
Owner:SHANDONG UNIV
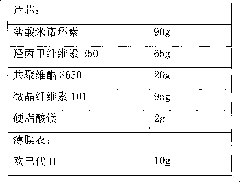
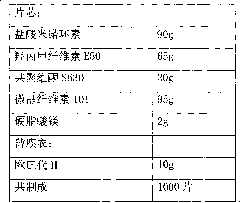
Minocycline hydrochloride sustained release tablet and preparation method thereof
InactiveCN101822650AReduce peak and valley fluctuationsEffectively control adverse reactionsAntibacterial agentsTetracycline active ingredientsSustained Release TabletBlood concentration
The invention relates to a minocycline hydrochloride sustained release tablet and a preparation method thereof. In the technical scheme, the minocycline hydrochloride sustained release tablet is composed of a tablet core and a film coat, the tablet core is composed of minocycline hydrochloride, a sustained release material, a bonding agent, a filling agent and a lubricating agent, the film coat is composed of a film forming material, in the preparation method, the granule is manufactured by means of the high shear wet method and obtained through the drying of a boiling bed, the temperature of the material dried by a fluidized bed is controlled to be 25-35 DEG C, and the moisture content of the granule is controlled to be 2.0-4.0 percent. The invention belongs to the technical field of pharmaceutical preparation, which aims to provide an available preparation process for the minocycline hydrochloride sustained release tablet and greatly reduce the relevant materials introduced druing the preparation by controlling the key parameters in the preparation process. The preparation method of the minocycline hydrochloride sustained release tablet and the method for controlling of the relevant materials aim to develop a sustained release formulation of minocycline hydrochloride, and various specifications can be issued based on the body weight of a patient, thereby reducing the peak valley fluctuation of the blood concentration, effectively controlling the adverse drug reaction, and improving the drug bioavailability.
Owner:HUZHOU R & D CENT FOR NUTRITION & HEALTH SHANGHAI INST FOR BIOLOGICAL SCI CHINESE ACADEMY OF SCI +1
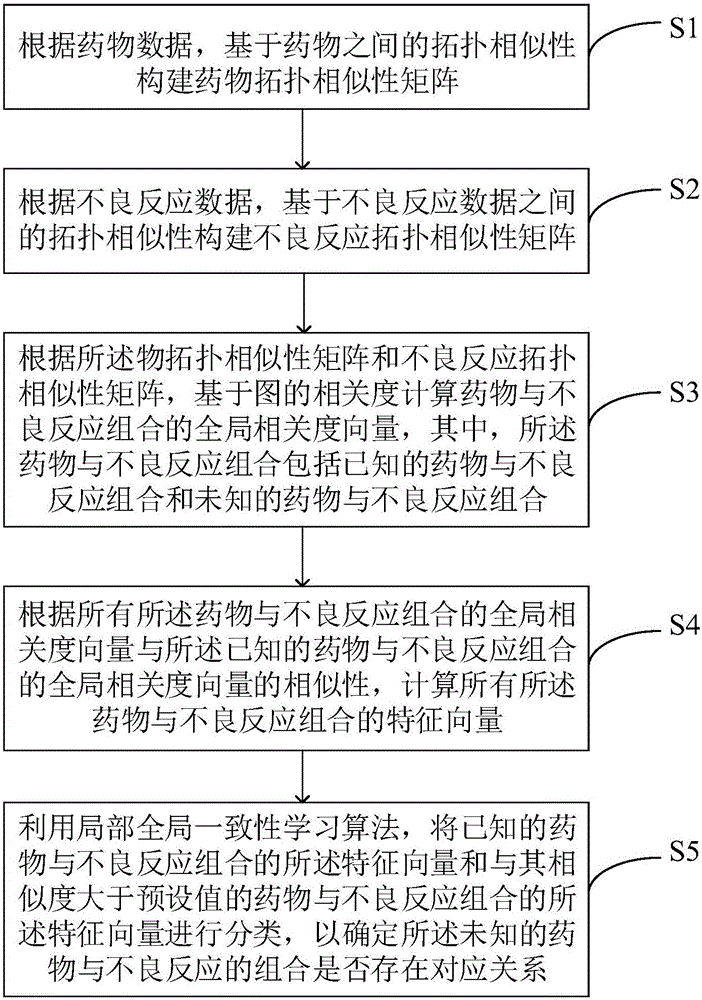
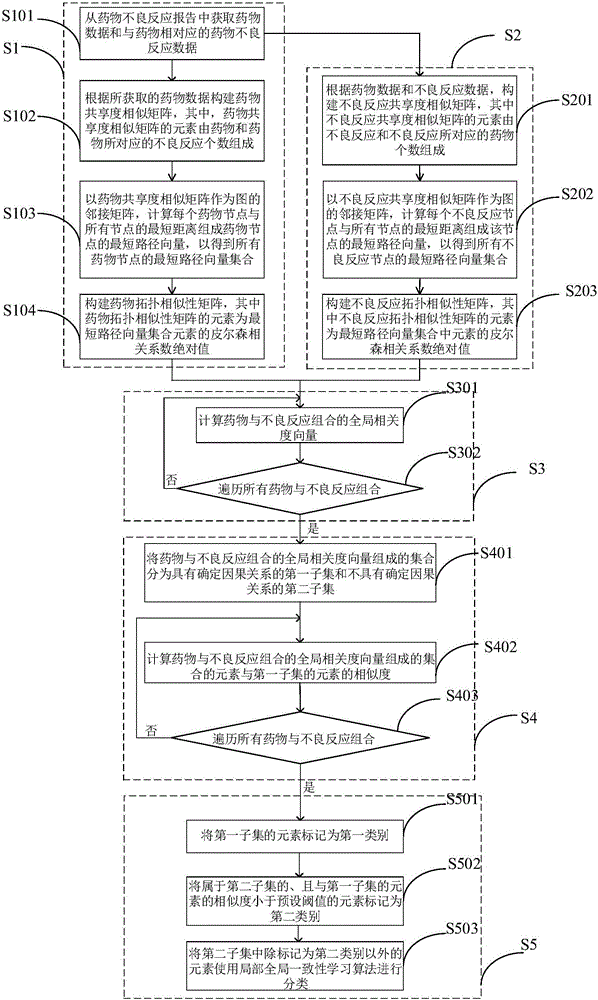
Adverse drug reaction mining method and system
InactiveCN106055879AReduce the risk of adverse reactionsMedical data miningSpecial data processing applicationsFeature vectorMedicine
The present invention discloses an adverse drug reaction mining method and system. According to the method, a drug topology similarity matrix and an adverse reaction topology similarity matrix are constructed, global correlation degree vectors of drug and adverse reaction combinations are calculated according to the drug topology similarity matrix and the adverse reaction topology similarity matrix, all characteristic vectors of the drug and adverse reaction combinations are calculated according to similarity of the global correlation degree vectors, by utilization of a learning algorithm with local and global consistency, characteristic vectors of known drug and adverse reaction combinations and characteristic vectors of drug and adverse reaction combinations having the similarity greater than a preset value are classified so as to determine whether unknown drug and adverse reaction combinations have corresponding relationships, so that risks of clinical adverse drug reaction are reduced.
Owner:BEIJING QUALITY & ZEAL INFORMATION TECH CO LTD
Detection of adverse reactions to medication using a communications network
A method for detecting an adverse drug event in a patient. The method includes reading an adverse drug reaction (ADR) description of the patient, reading identifiers for medications currently taken by the patient, and accessing ADR records corresponding to the identifiers. Each record comprises an identifier for a medication, an ADR description for the medication and a risk profile for the medication. Next, the method compares the ADR description of the patient with ADR descriptions in the ADR records, identifies an ADR record with a matching ADR description and calculates a level of risk that a medication of the ADR record is causing the ADR description of the patient. Finally, the method transmits an identifier and a risk profile for each medication of the ADR record, and a notice indicating that each medication of the ADR record may be causing the ADR description of the patient.
Owner:LUCCHINO RONALD
Biomarkers for Drug-Induced Liver Injury
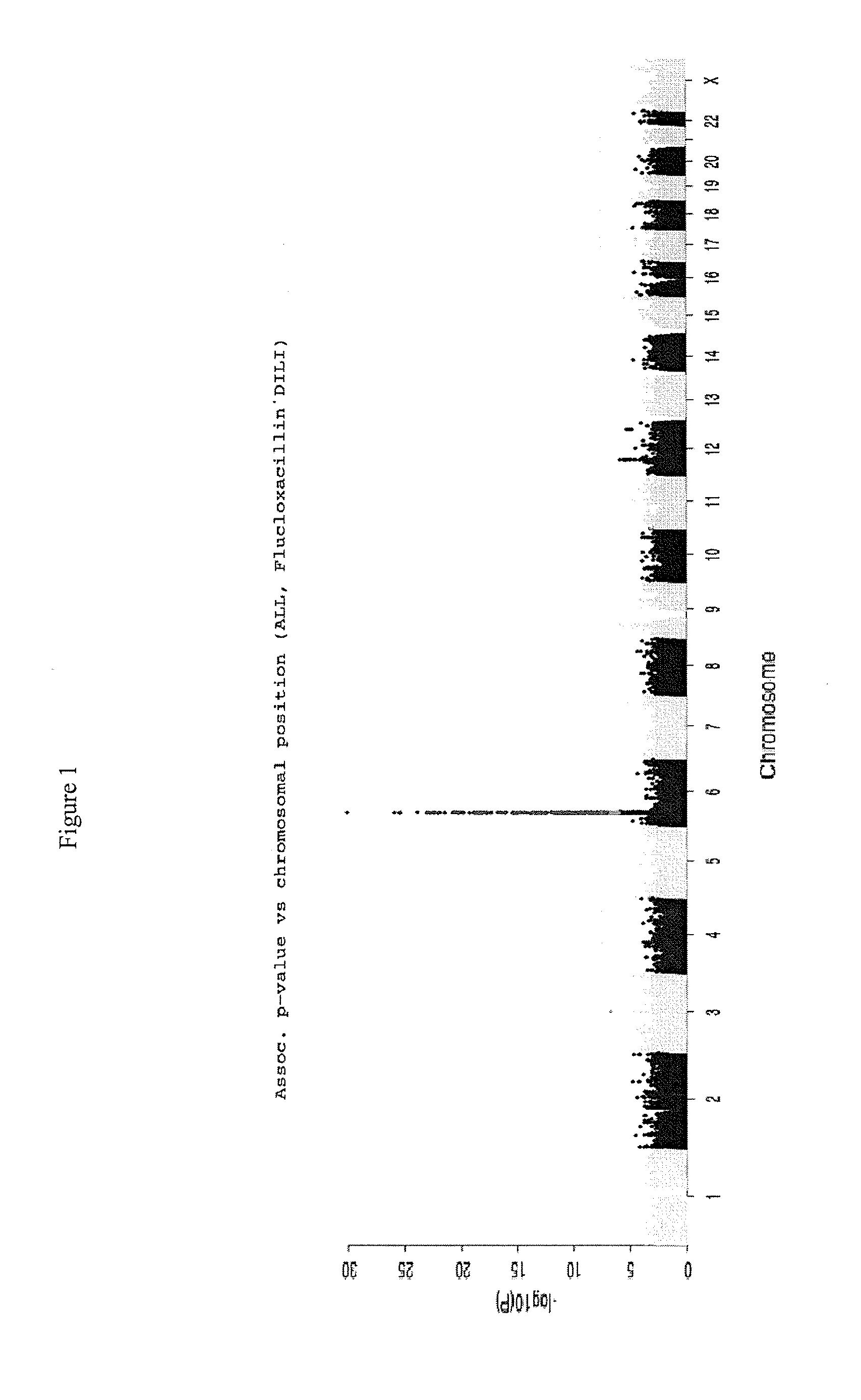
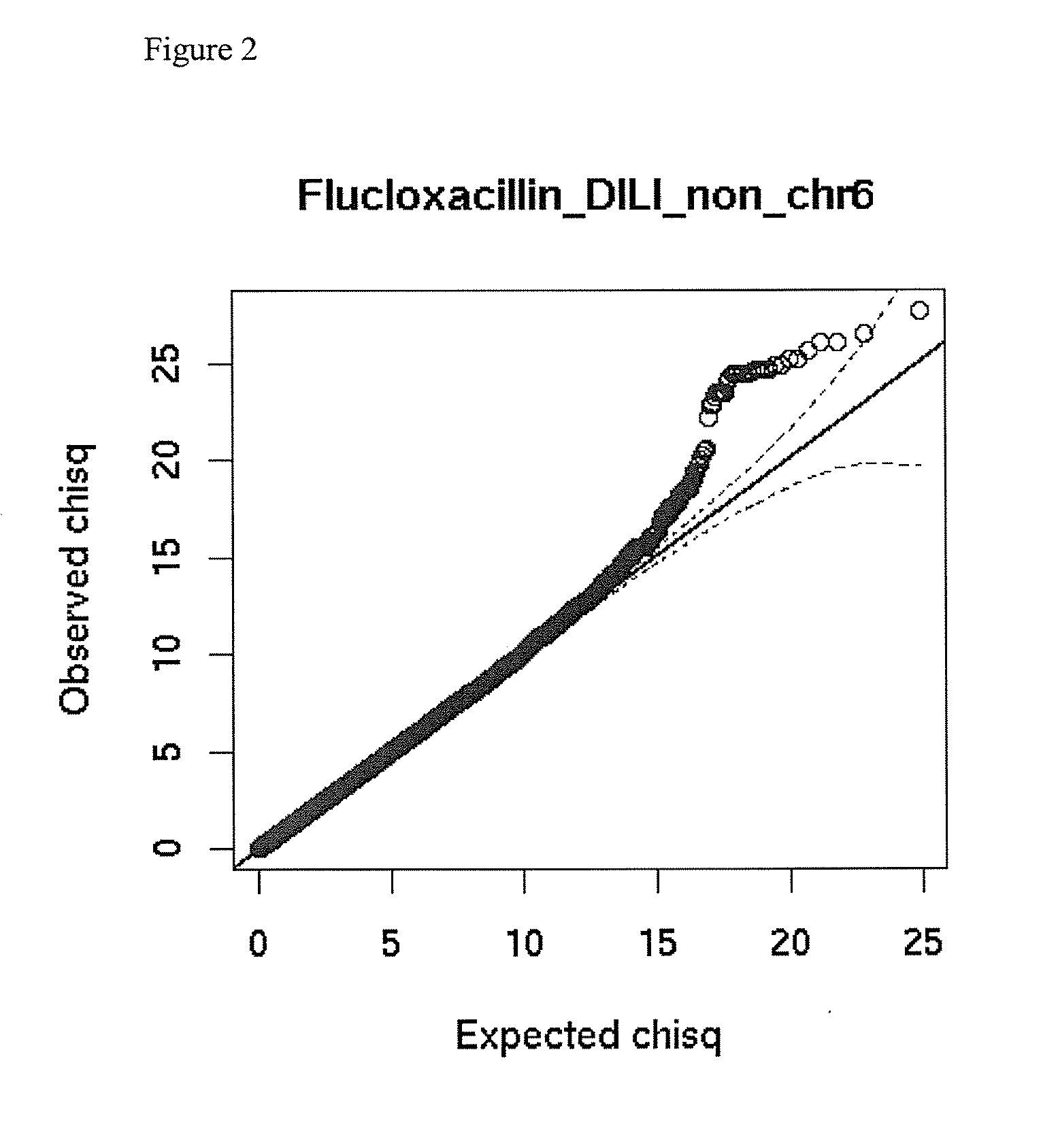
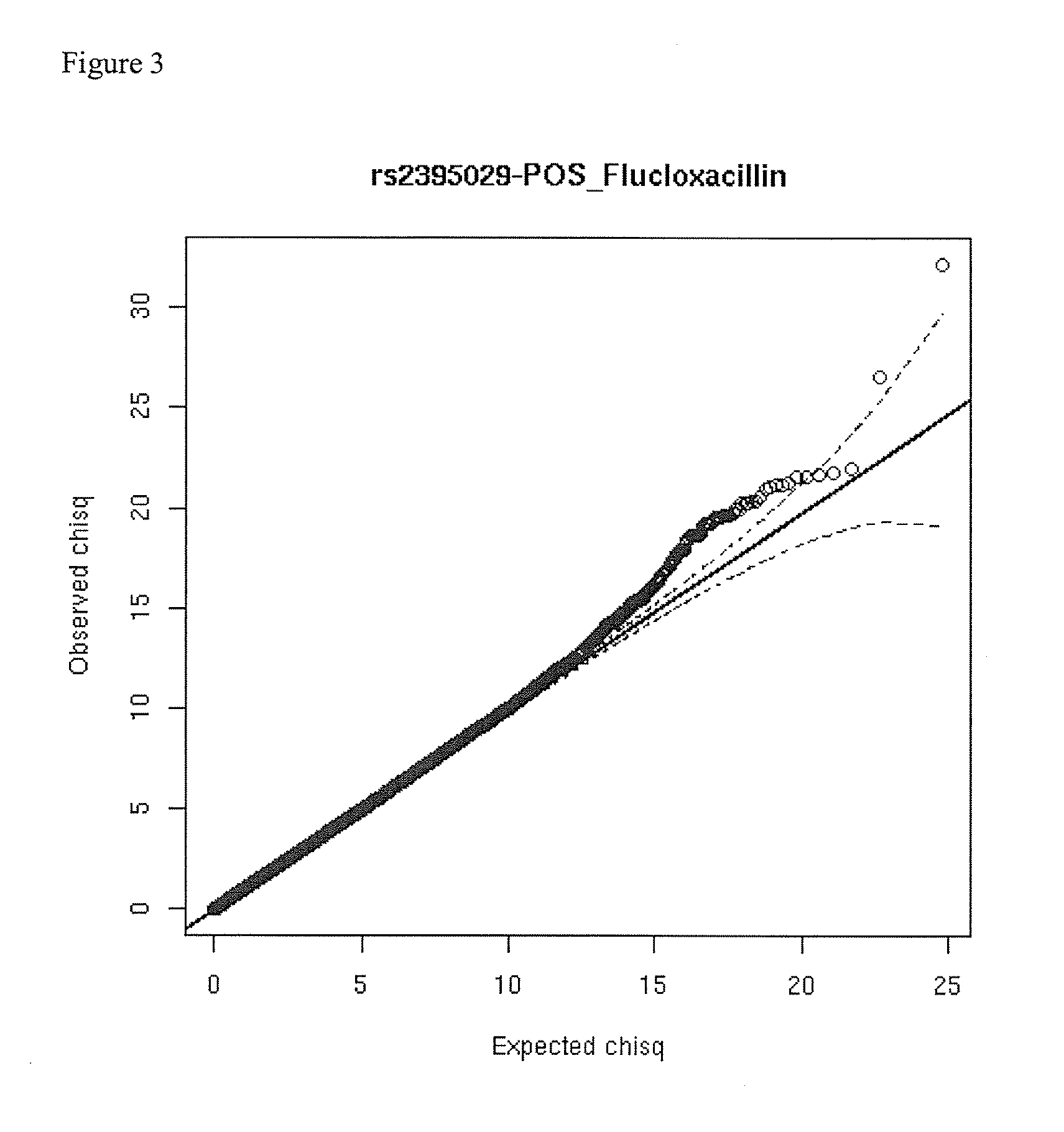
The present invention provides a method for predicting the risk of a patient for developing adverse drug reactions, particularly Drug-Induced Liver Injury (DILI) or hepatotoxicity. The invention also provides a method of identifying a subject afflicted with, or at risk of, developing DILI. In some aspects, the methods comprise analyzing at least one genetic marker, wherein the presence of the at least one genetic marker indicates that the subject is afflicted with, or at risk of, developing DILI.
Owner:FLORATOS ARIS +3
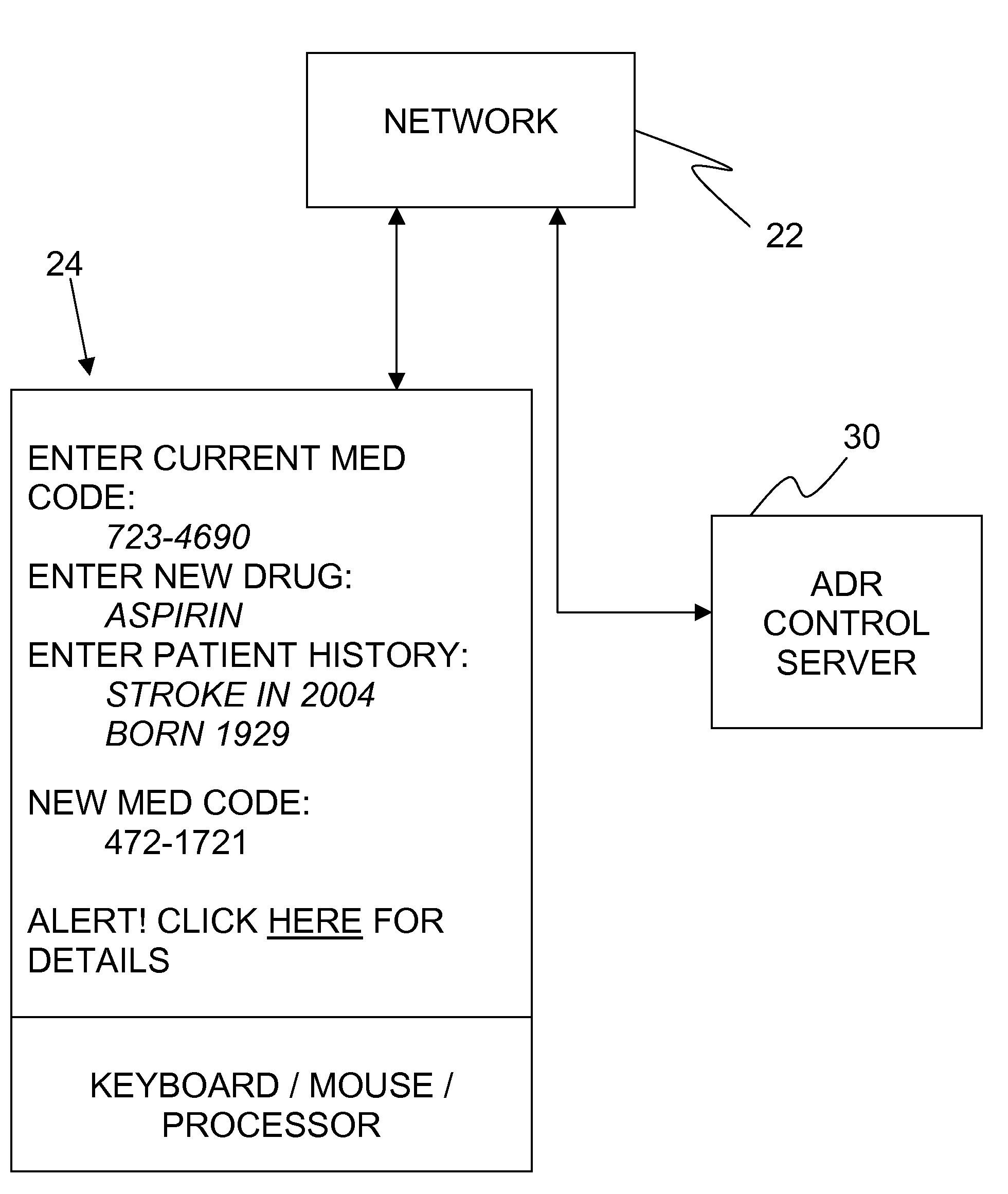
Adverse drug reaction reduction
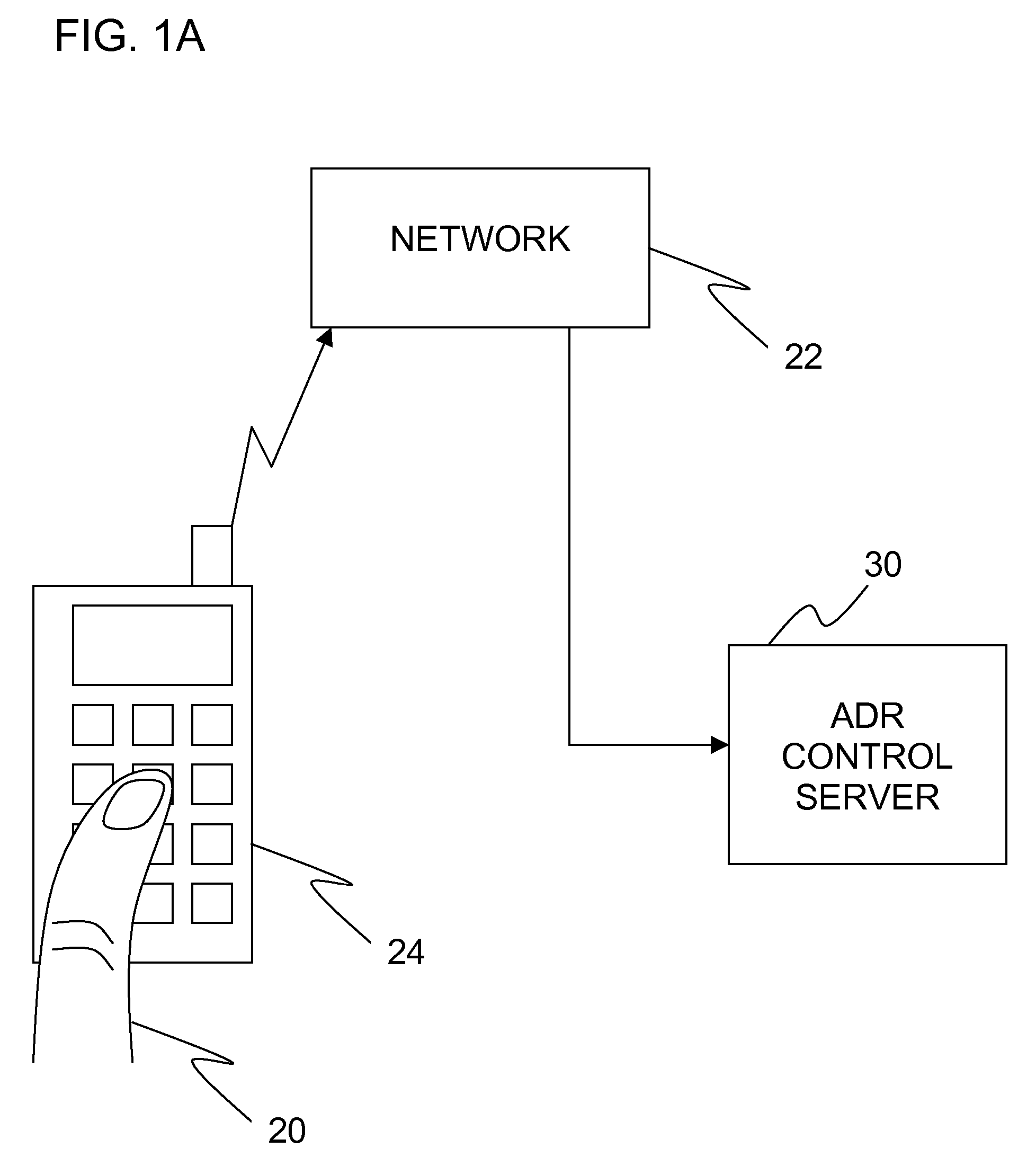
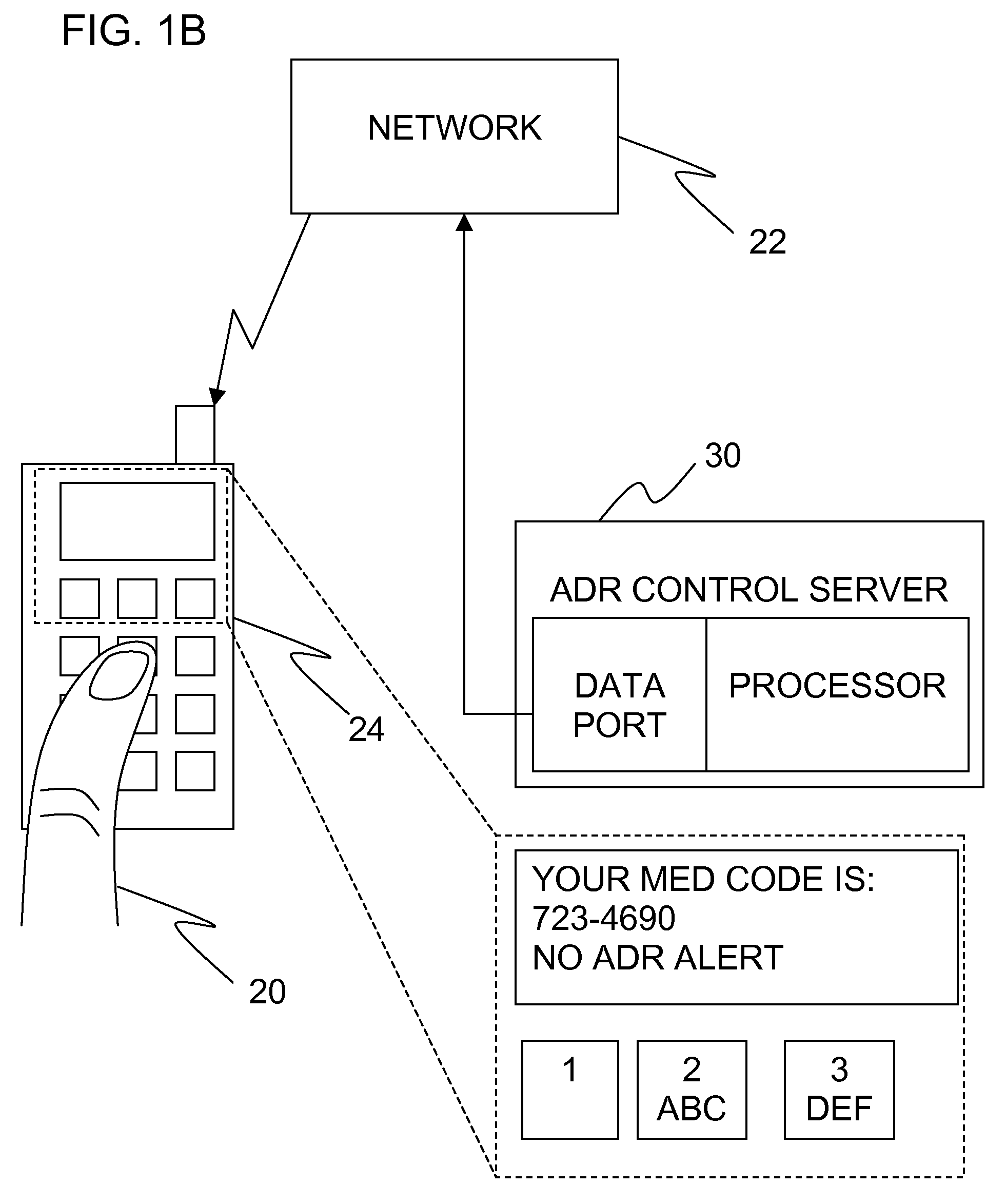
Apparatus is provided, including an adverse drug reaction (ADR) control server (3) including a processor and a data port. The server is configured to receive from a user (20), via the data port, in a first interaction with the user, a list of one or more current medications, and to send to the user a first medication code responsively to the list. In a second interaction with the user, the server receives the first medication code from the user and an indication of a new medication. The server accesses, by the processor, the list of one or more current medications based on the received first medication code, and generates an adverse drug reaction (ADR) warning based on the list of one or more current medications and the new medication. The server sends the user a new medication code based on the list of one or more current medications and the new medication. Other embodiments are also described.
Owner:MIRIK MEDICAL
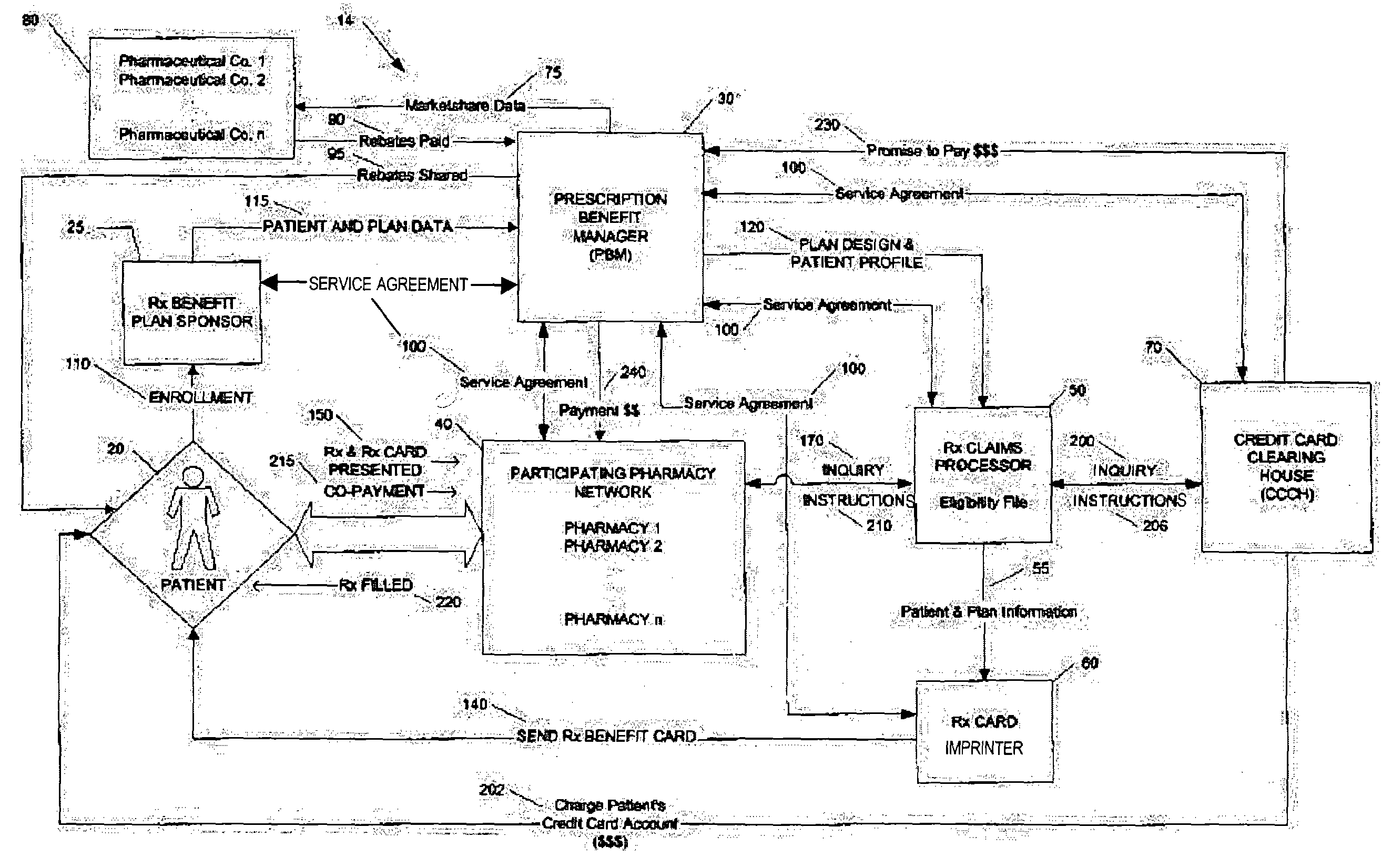
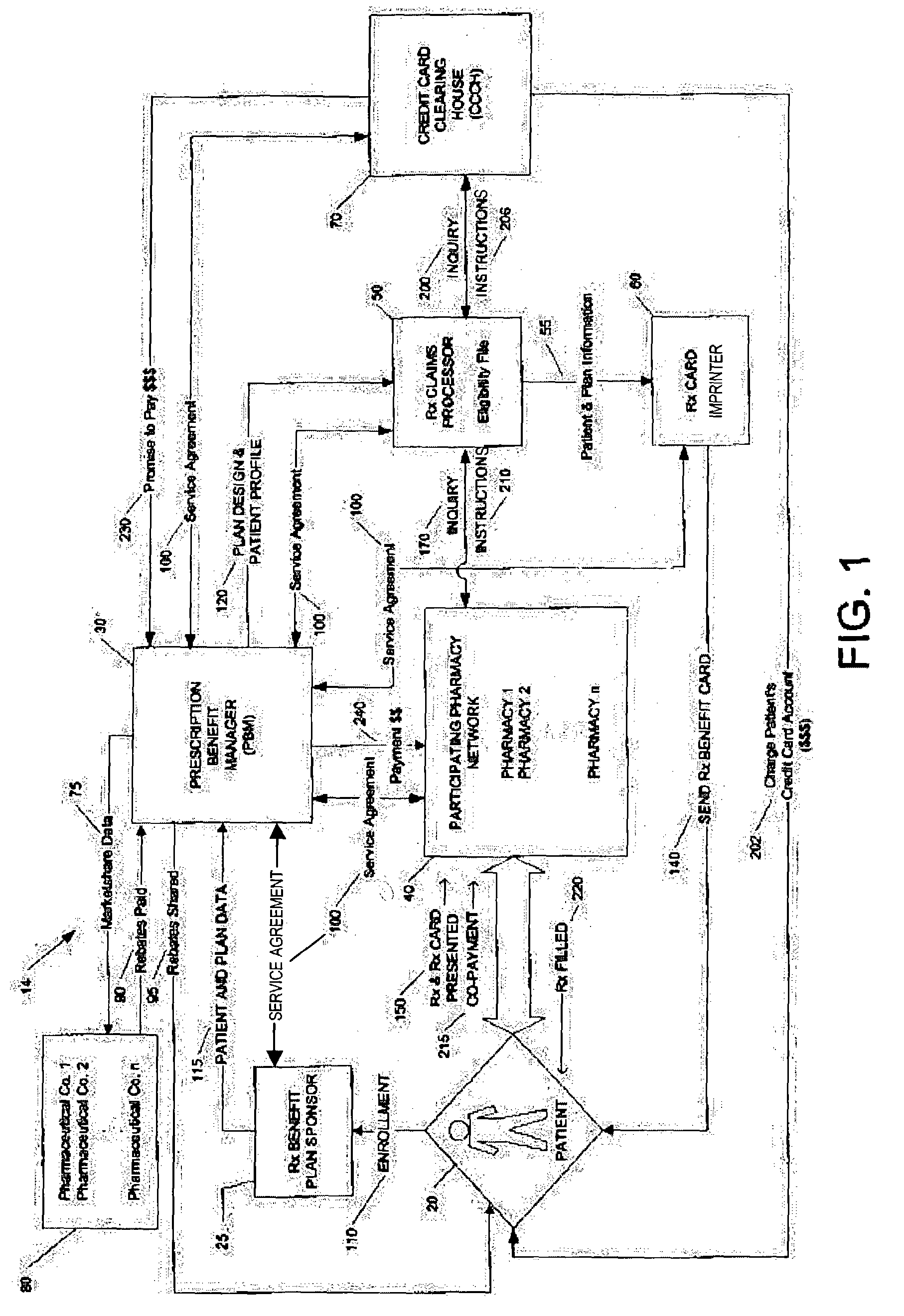
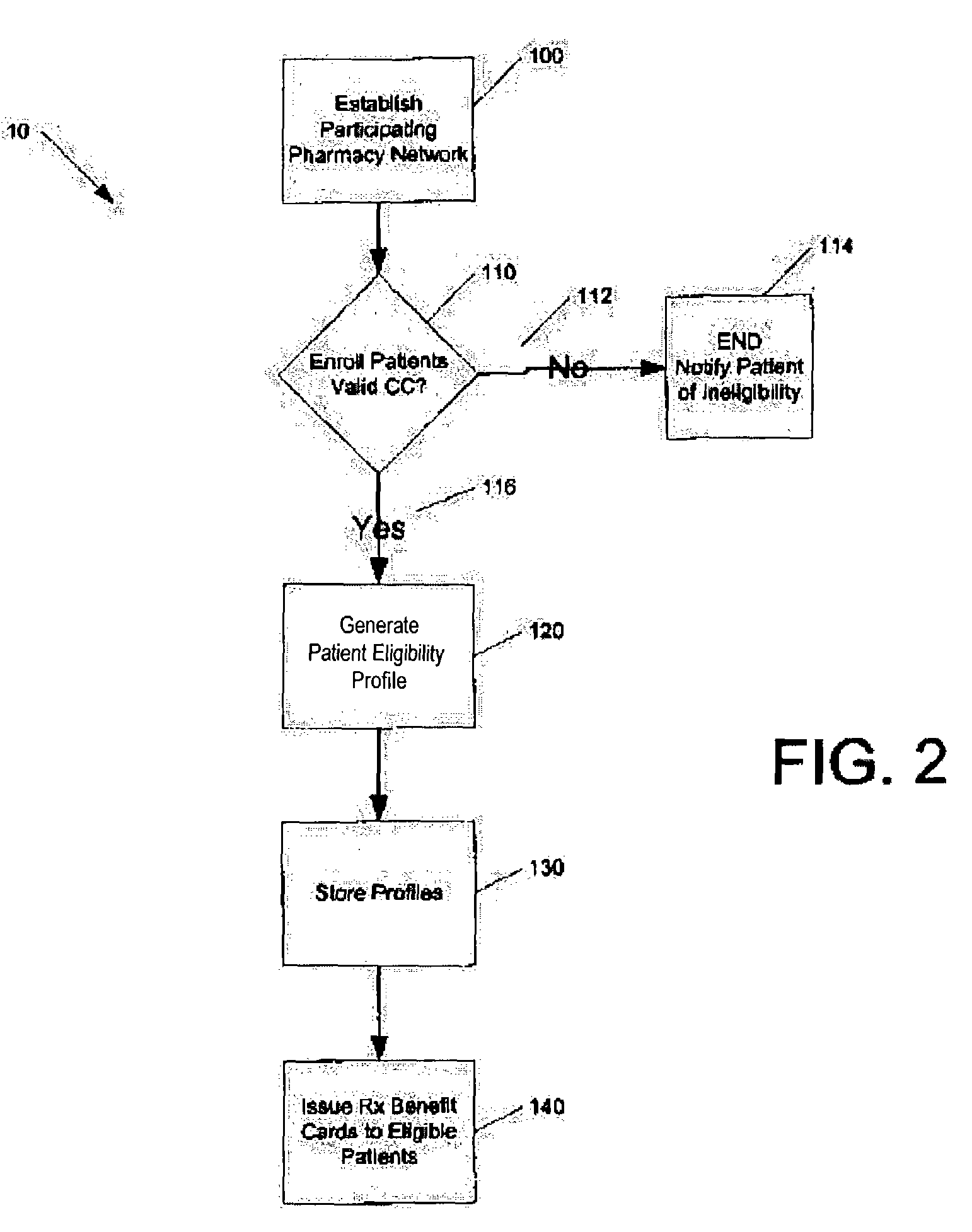
Method for conducting prescription drug co-payment plans
InactiveUS7657437B2Low costHigh net amountDrug and medicationsDiagnostic recording/measuringCredit cardPharmacy
A credit card-based prescription benefits plan utilizes an electronic means for telecommunication to rapidly adjudicate prescription claims. The adjudication process includes a third-party claims processor interposed the pharmacy and the patient's credit card clearinghouse to ensure that the subscriber receives all benefits available under the plan. The third-party claims processor may also provide patient counseling and advocacy by performing patient-specific drug regimen reviews to check for potential adverse drug reactions and drug interactions.
Owner:OMNICARE
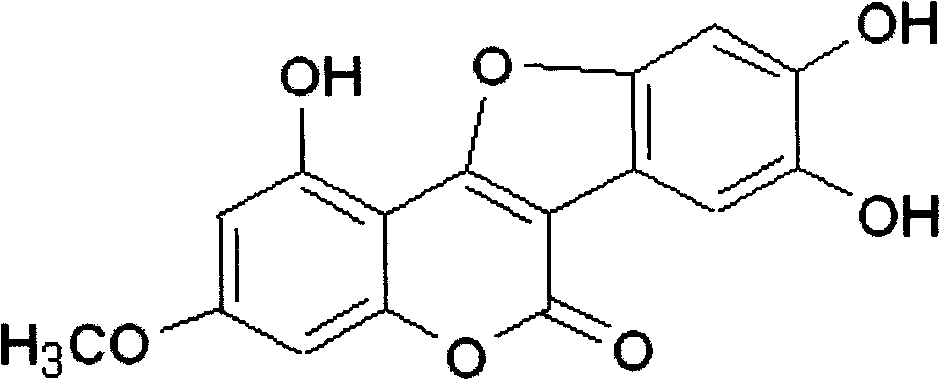
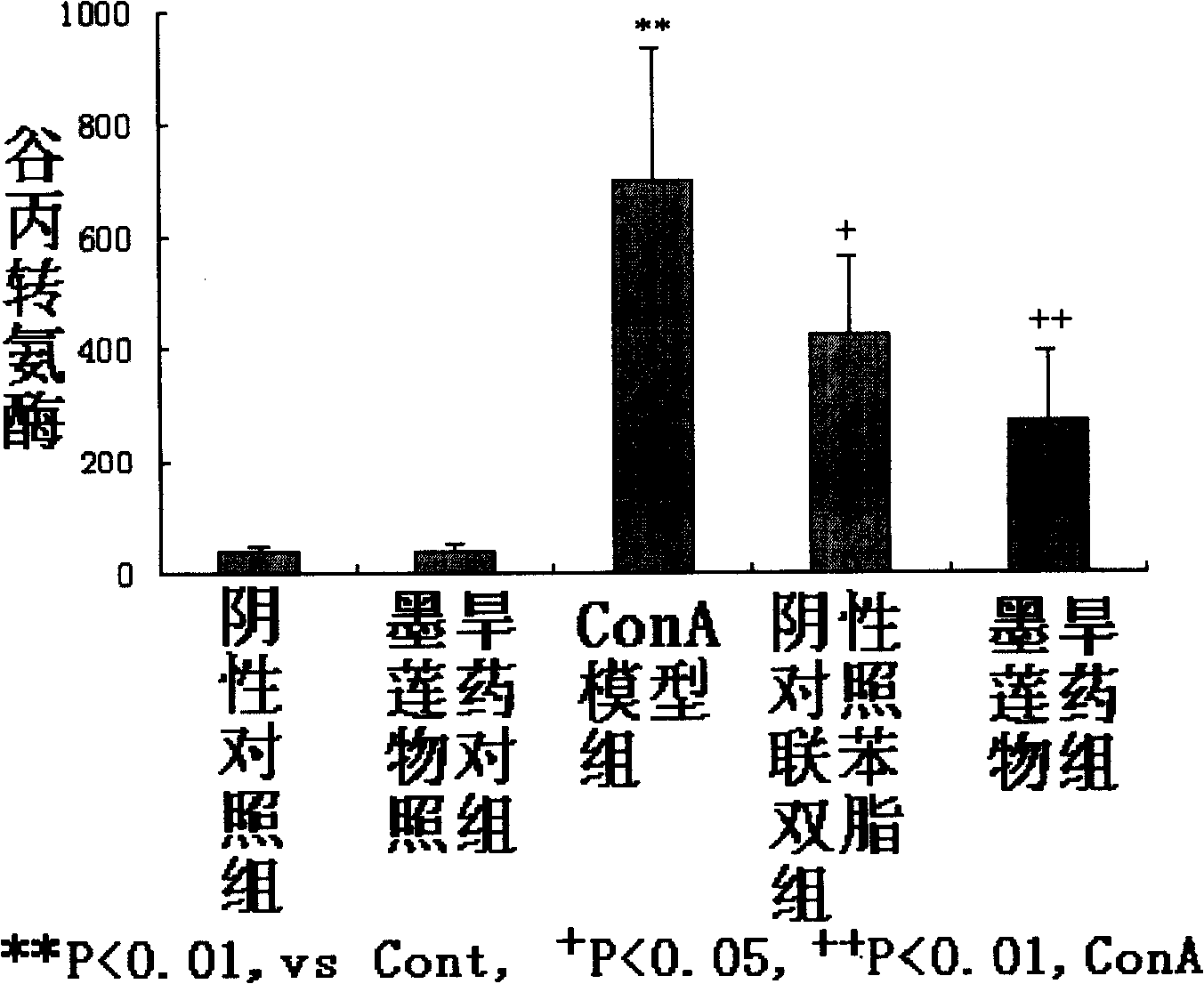
Pharmaceutical use of wedelolactone and its derivative
InactiveCN101259124APrevent proliferationNo inhibitionOrganic active ingredientsDigestive systemDiseaseWedelolactone
The invention relates to medical applications of wedelolactone and derivatives thereof, in particular to the applications in preparing medicines for treating T-cell mediated immunologic injury diseases (chronic hepatitis or autoimmune hepatitis). Traditional Chinese Medicine eclipta has a function of liver protection; wedelolactone is prepared after the active ingredient of eclipta is traced and separated, so the pharmaceutical function is proved to be produced by wedelolactone; the structure of wedelolactone is determined through the spectrum properties and physicochemical properties. Wedelolactone is provided with biological activities: high selective inhibition to ConA stimulated T lymphocyte proliferation, no inhibition to normal peripheral blood lymphocyte (NPBL), and no cytotoxicity; wedelolactone is the active ingredient of Traditional Chinese Medicine with liver protection function; the method for preparing a novel medicine from the active ingredient of eclipta, wedelolactone, has the advantages of discarding the dross and selecting the essential, clear medicinal ingredients, definite function, safety and effectiveness, controllable quality, reducing the adverse drug reaction, and can be suitable for mass production.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com