Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "Complete monitoring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
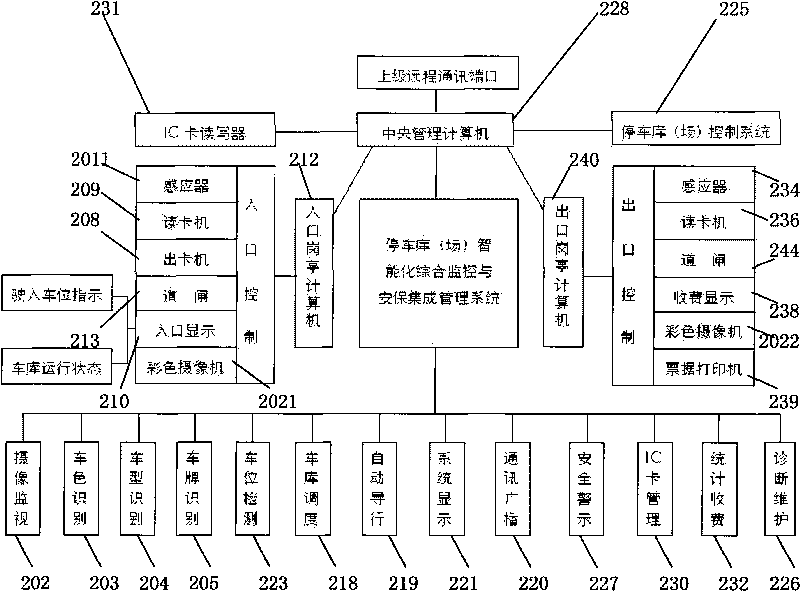
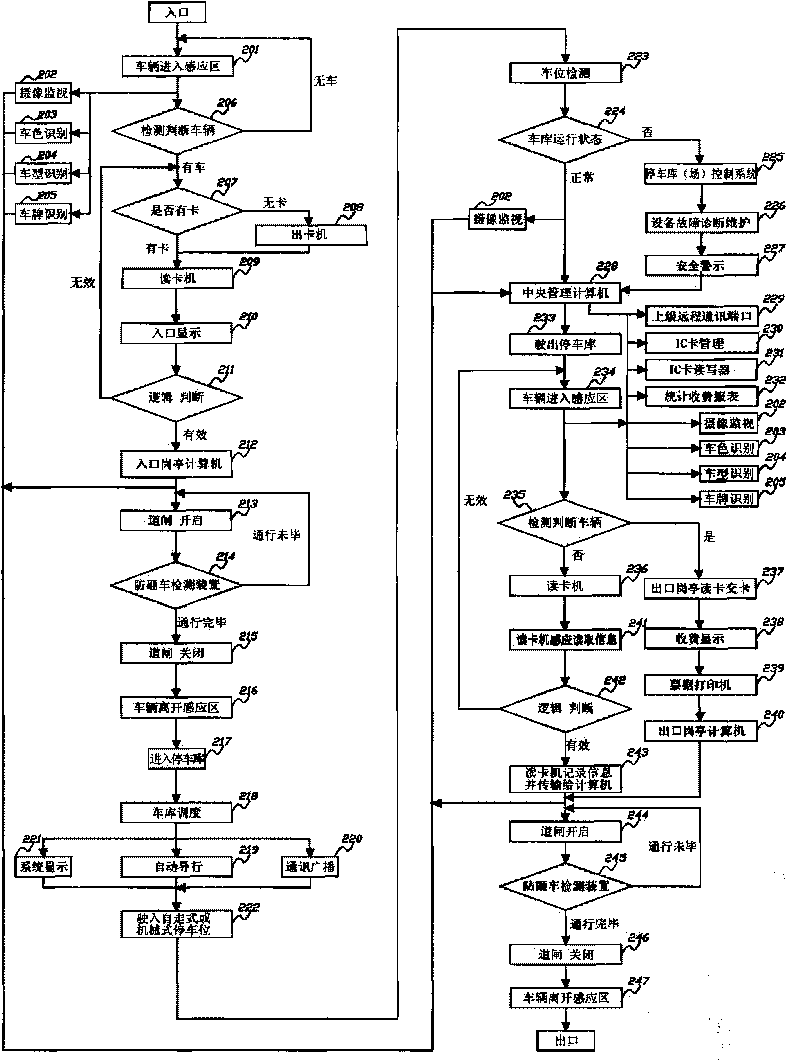
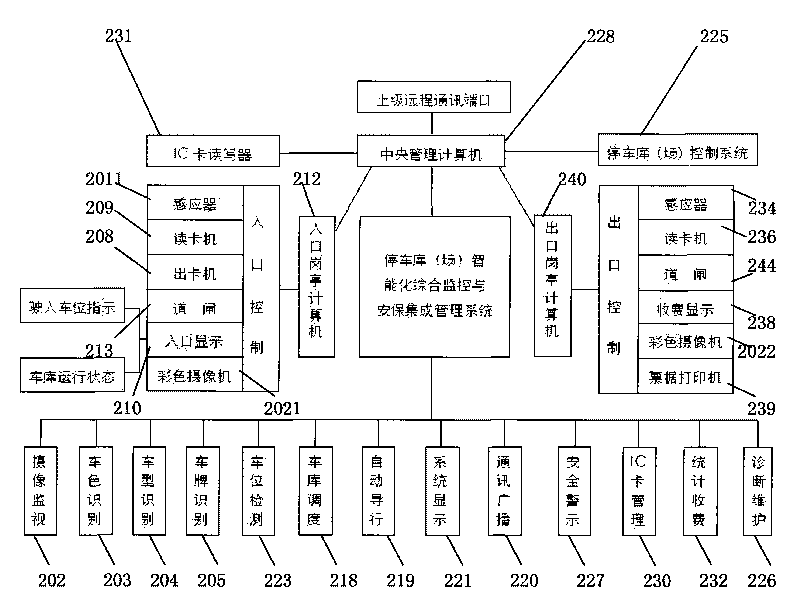
Intelligent comprehensive monitoring and security integrated management system for parking garage
ActiveCN101739841AComplete monitoringPracticalTicket-issuing apparatusIndication of parksing free spacesIntegrated monitoringEconomic benefits
The invention provides an intelligent comprehensive monitoring and security integrated management system for a parking garage. The system solves the comprehensive intelligent management problems of related automatic monitoring, automatic detection, display, different languages and voices, communication, dispatching, guide, digital quantity identification, security, forbidding and passing, charging, statistics, self diagnosis and maintenance and the like of various self-propelled and automatic three-dimensional mechanical parking garages. The system has significant meanings for improving the monitoring, security and management levels of various parking garages, promoting static traffic modernization construction and increasing the social and economic benefits of the parking garages.
Owner:上海久银车库工程有限公司
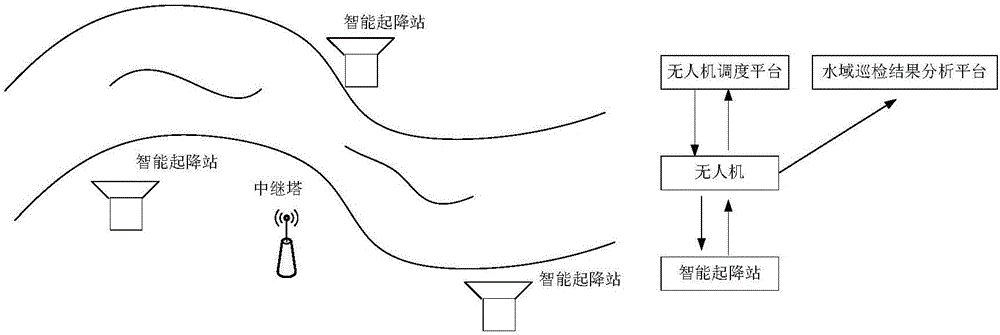
Water area automatic routing inspection system and method based on unmanned aerial vehicle
ActiveCN106774427AResolve lagComplete monitoringPosition/course control in three dimensionsAutomatic routingUncrewed vehicle
The invention discloses a water area automatic routing inspection system and method based on an unmanned aerial vehicle. The system partially comprises a ground control center, an intelligent take-off and landing station and the unmanned aerial vehicle, wherein the ground control center comprises an unmanned aerial vehicle dispatching platform and a water area routing inspection result analysis platform; the unmanned aerial vehicle dispatching platform is used for selecting vehicle types of the unmanned aerial vehicle; the water area routing inspection result analysis platform receives and stores the water area routing inspection data and analyzes the water area features through water area routing inspection data; the intelligent take-off and landing station comprises a data transmission module and an autonomous cruising module; the data transmission module is used for receiving data sent by the unmanned aerial vehicle in real time, and sending the data to the ground control center; the autonomous cruising module is used for using a non-manual method for changing a power supply battery of the unmanned aerial vehicle in the process of executing the water area routing inspection task by the unmanned aerial vehicle; the unmanned aerial vehicle cruising function is executed.
Owner:SHANDONG UNIV +1
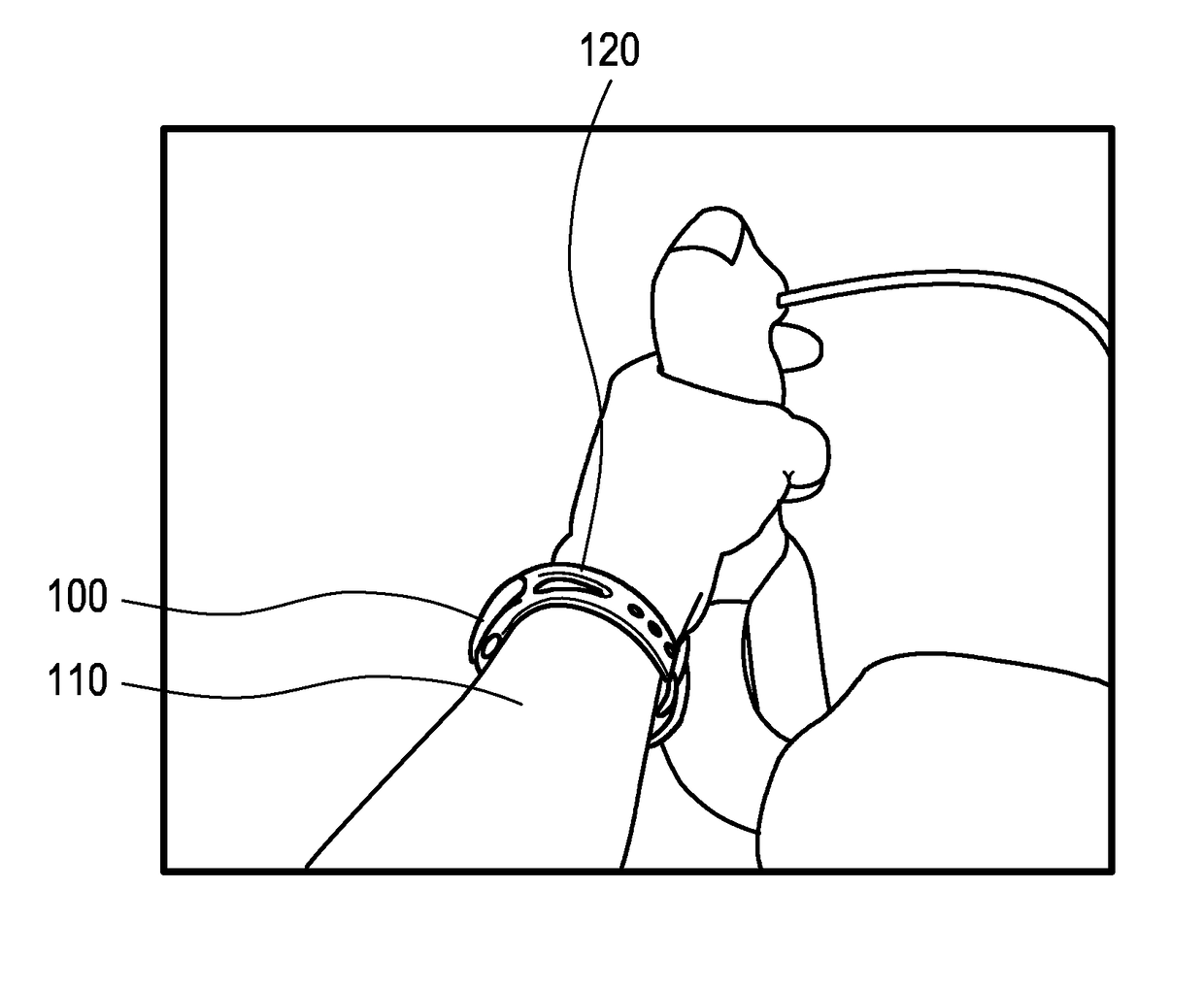
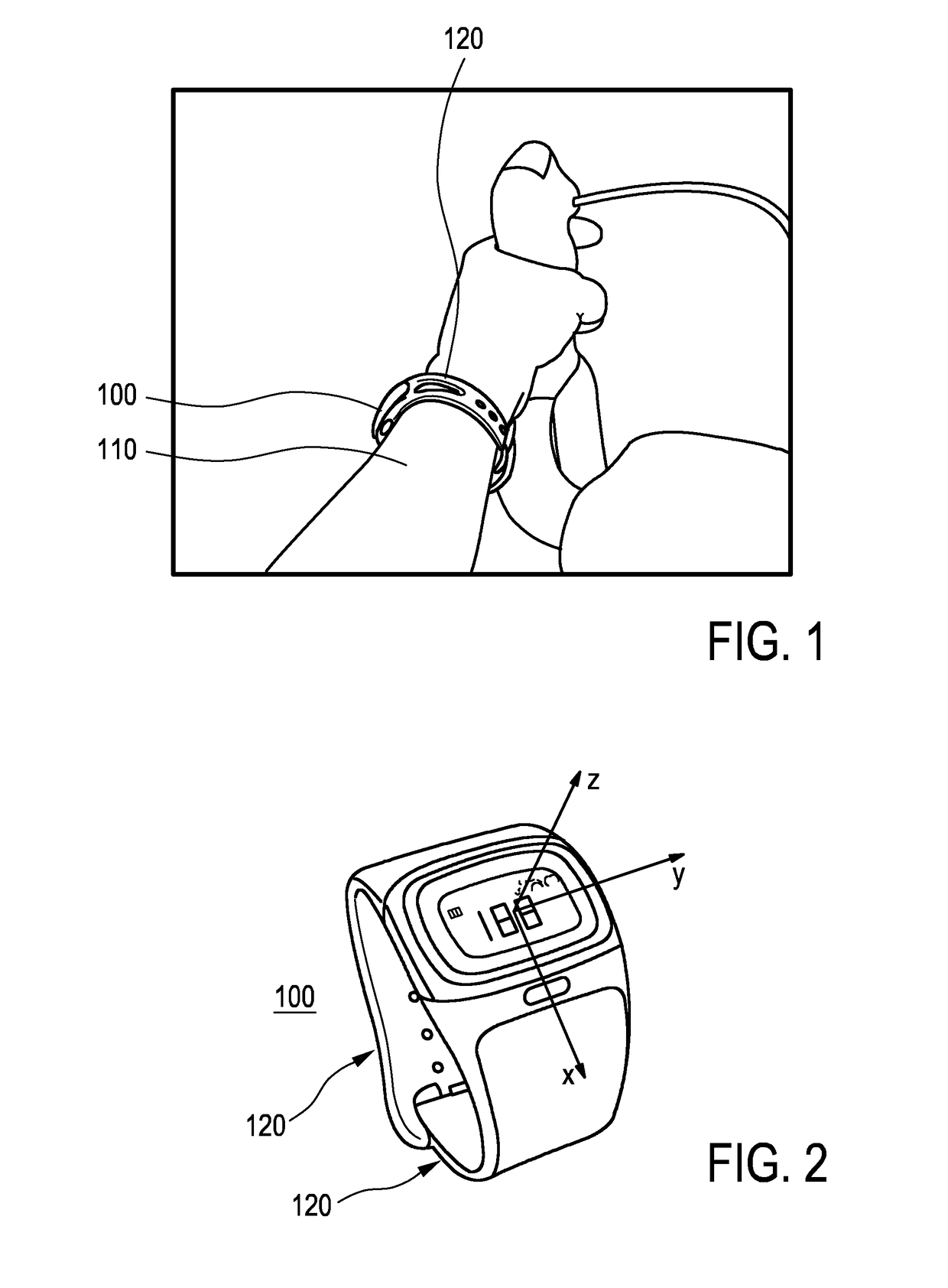
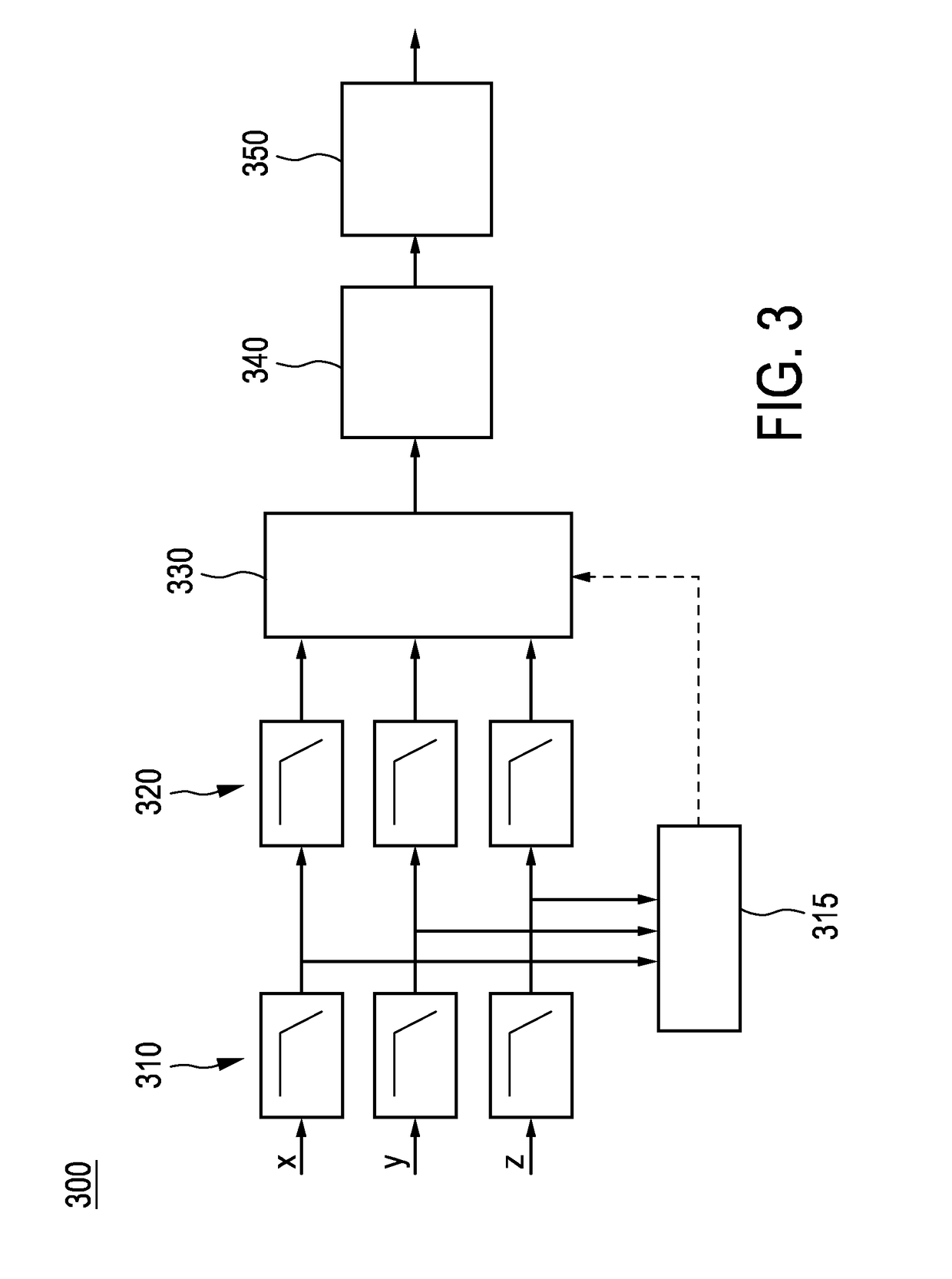
Device for measuring a cycling cadence
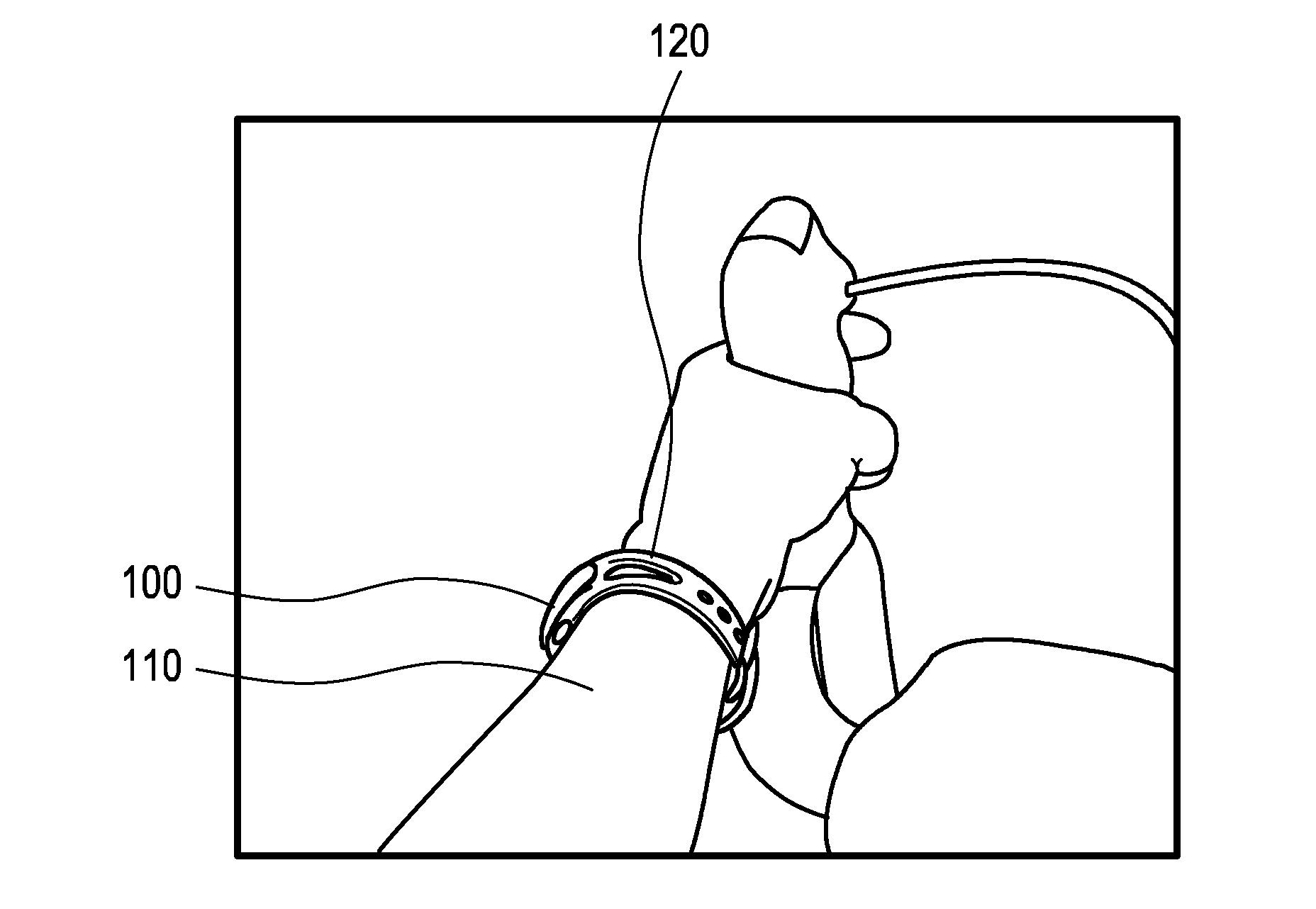
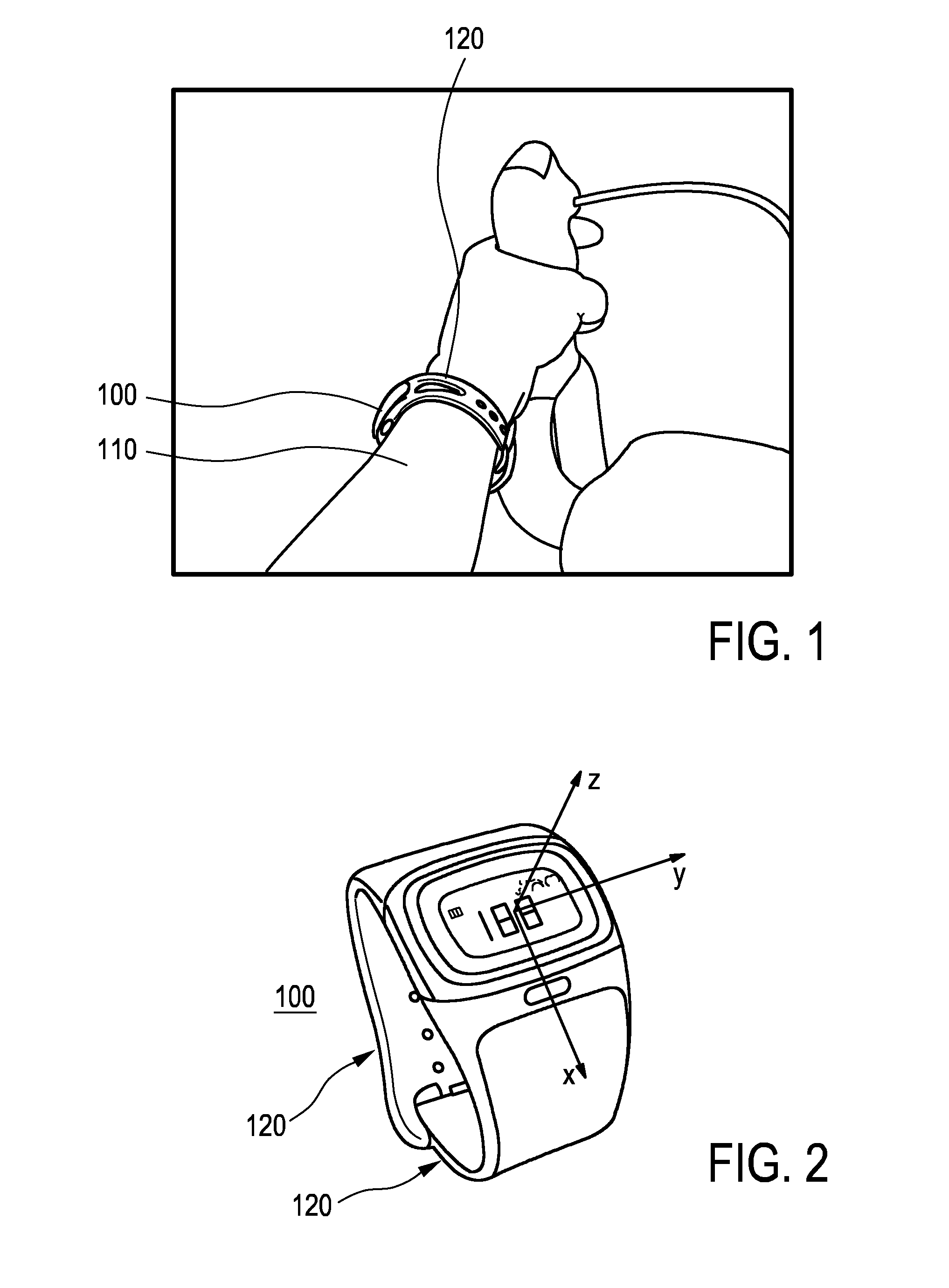
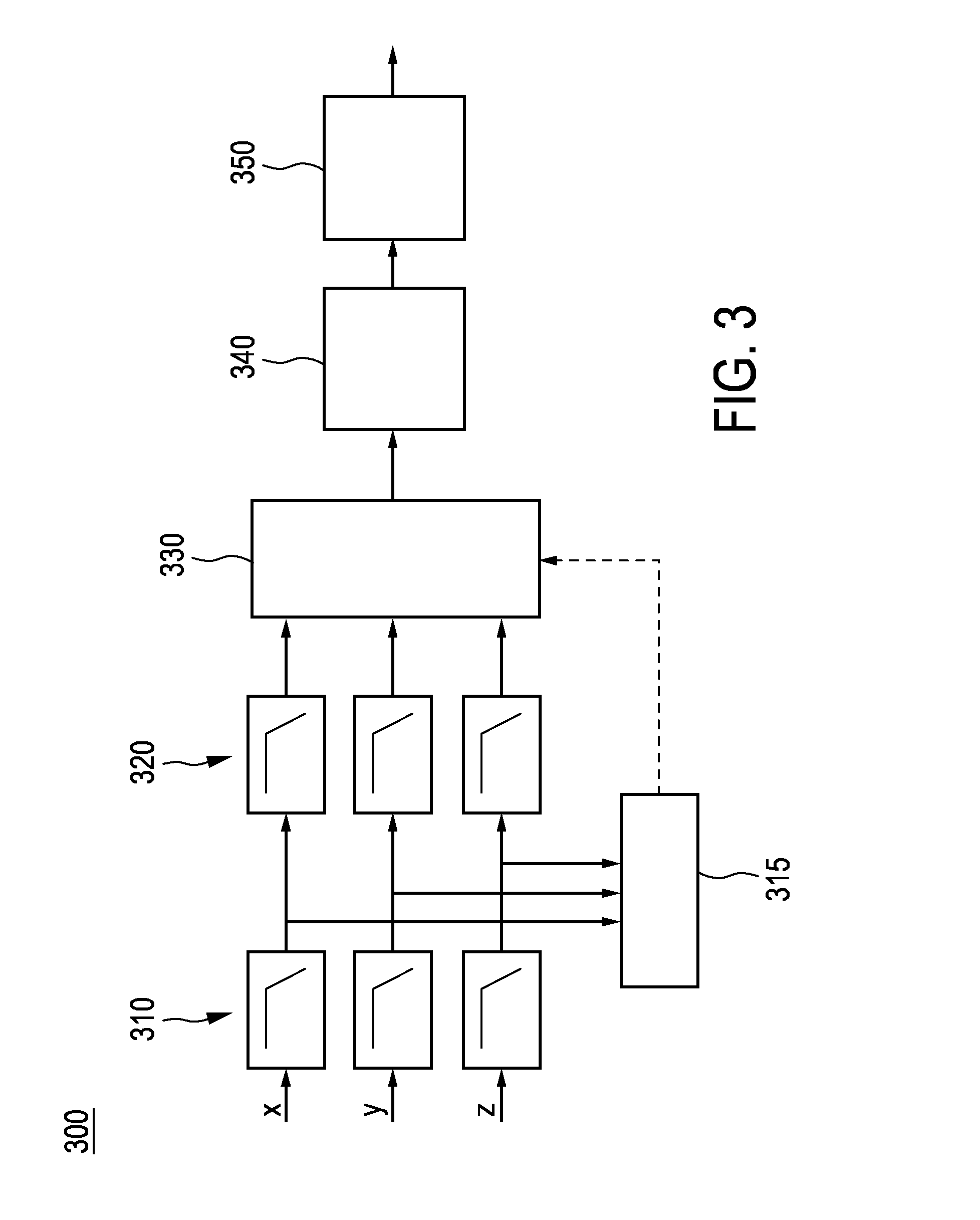
ActiveUS20170007166A1Easy to analyzeComplete monitoringCycle equipmentsSensorsAccelerometerEngineering
The present invention relates to a device (100) for measuring a cycling cadence, a method (500) of operating a device (100) for measuring a cycling cadence, and a cycling cadence computer program. The device (100) comprises a motion sensor (such as, e.g., an accelerometer) for detecting a movement of the device (100) and for generating a motion signal (x, y, z) corresponding to the movement; a cadence determination unit (300) for determining cycling cadence based on the motion signal (x, y, z). The device (100) can be worn on the cyclist's wrist or arm (110). The motion sensor in the device is able to pick up the tiny movements of the arm or wrist that correspond to the cadence. Optionally, an algorithm is applied that can derive the cadence from a noisy signal.
Owner:KONINKLJIJKE PHILIPS NV
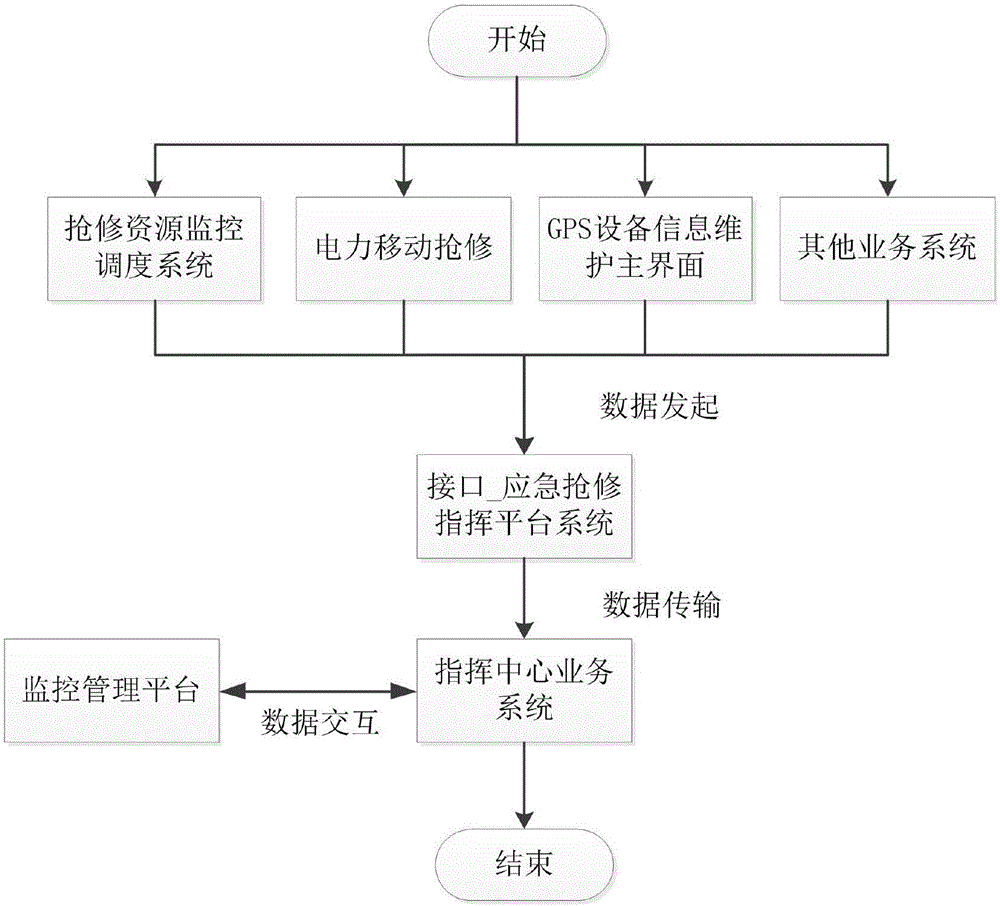
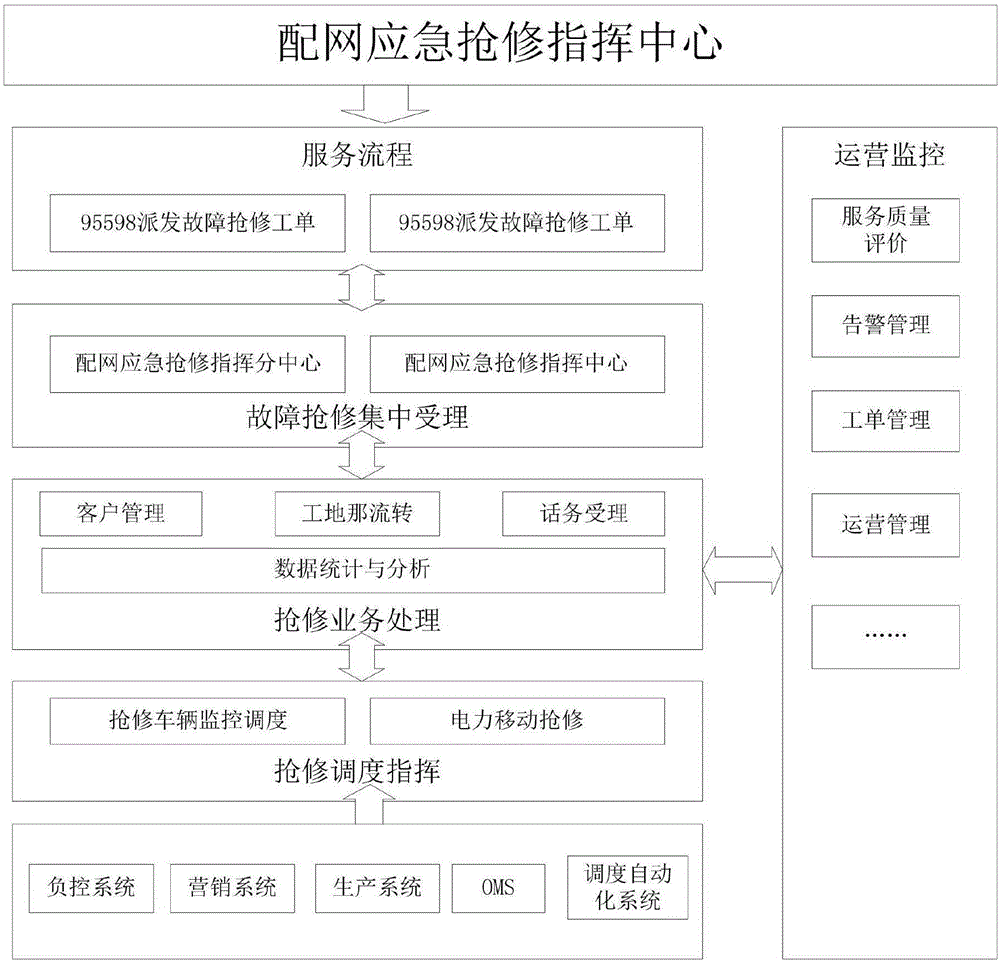
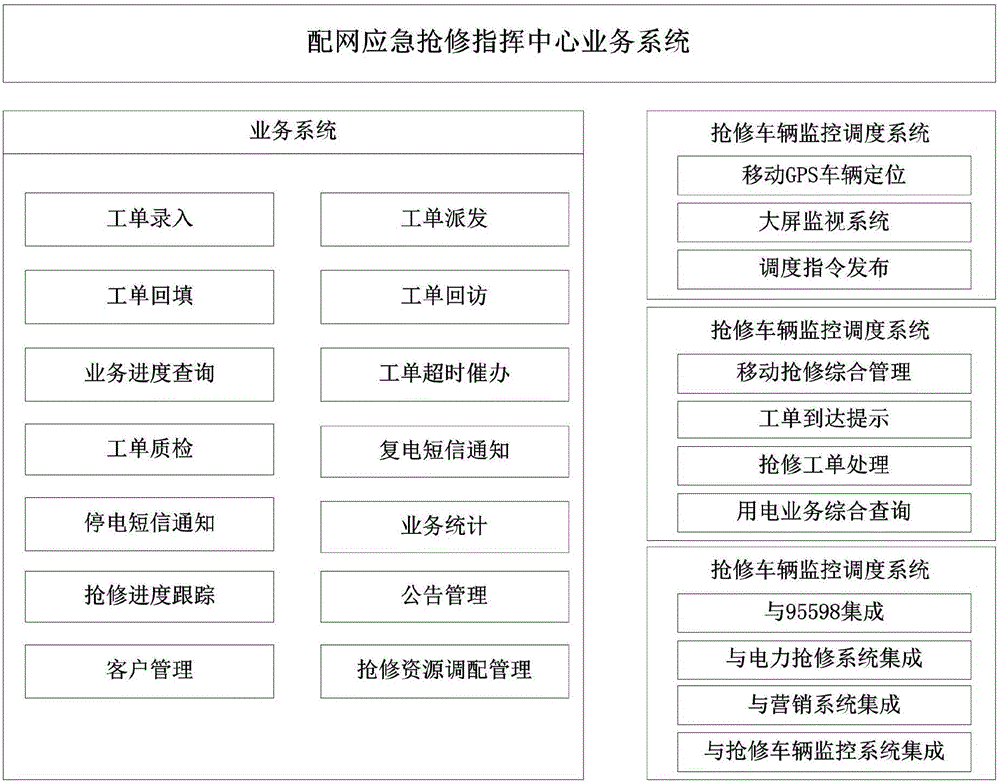
Emergency repair command platform for distribution network
The invention discloses an emergency repair command platform for a distribution network. The emergency repair command platform is composed of a command center business system, a monitoring and management platform, power mobile repair, a repair resource monitoring and dispatching system, a GPS device information maintenance main interface, and an interface_emergency repair command platform system. The emergency repair command platform has the functions of improving the management level of the fault repair, through the idea of "technological innovation plus management innovation", centralizing enterprise advantageous resources, setting up an emergency service center of the distribution network, accepting power failure repair businesses in a unified manner, realizing the intelligentized command of the power failure, mobile repair and active customer service, and improving the overall management level of fault repair and repair efficiency, so as to achieve the production goals of reducing the outage cost of users, reducing the energy-selling loss of power grid enterprises, and improving the power supply reliability of the power grid.
Owner:海南电网有限责任公司 +1
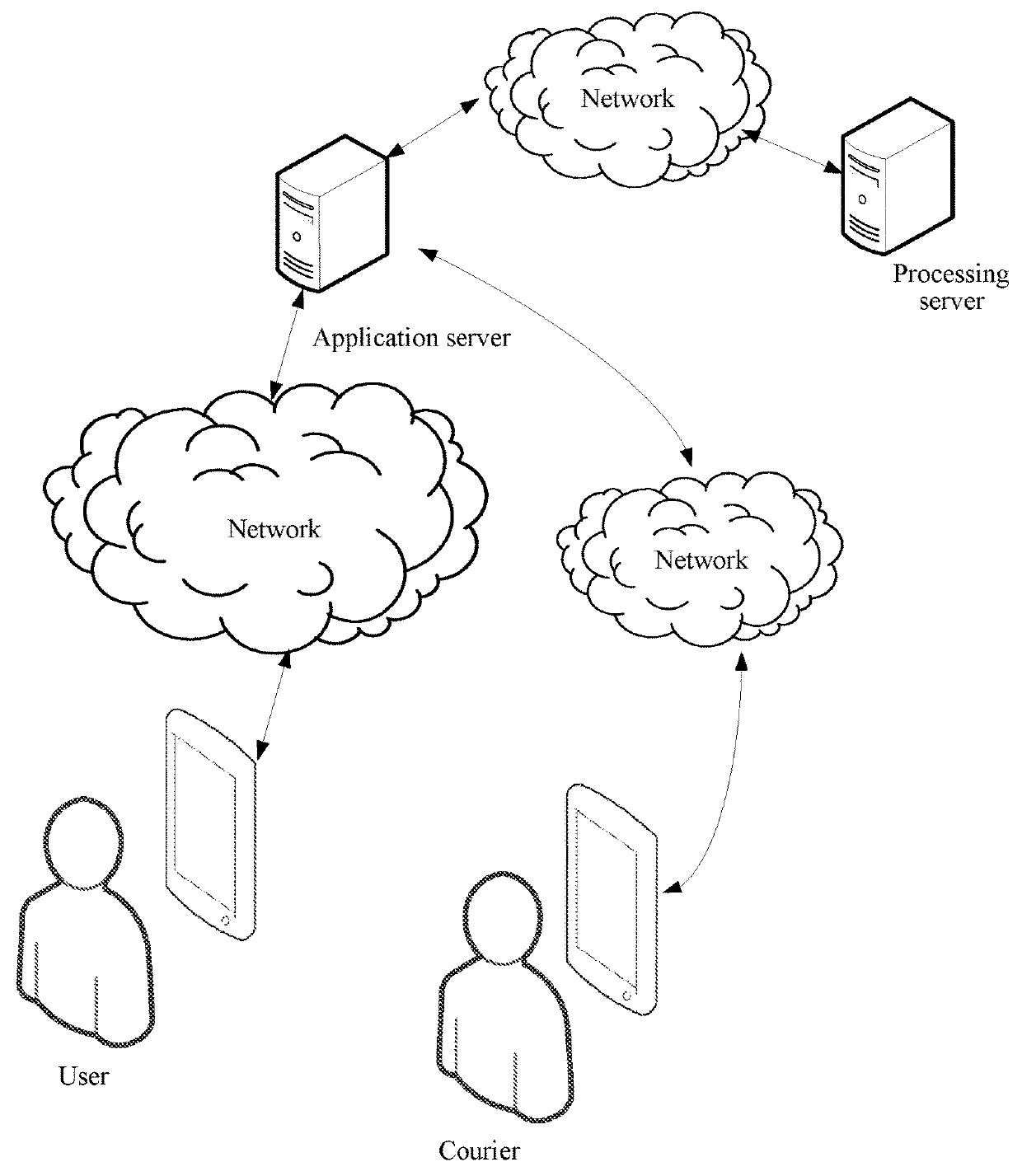
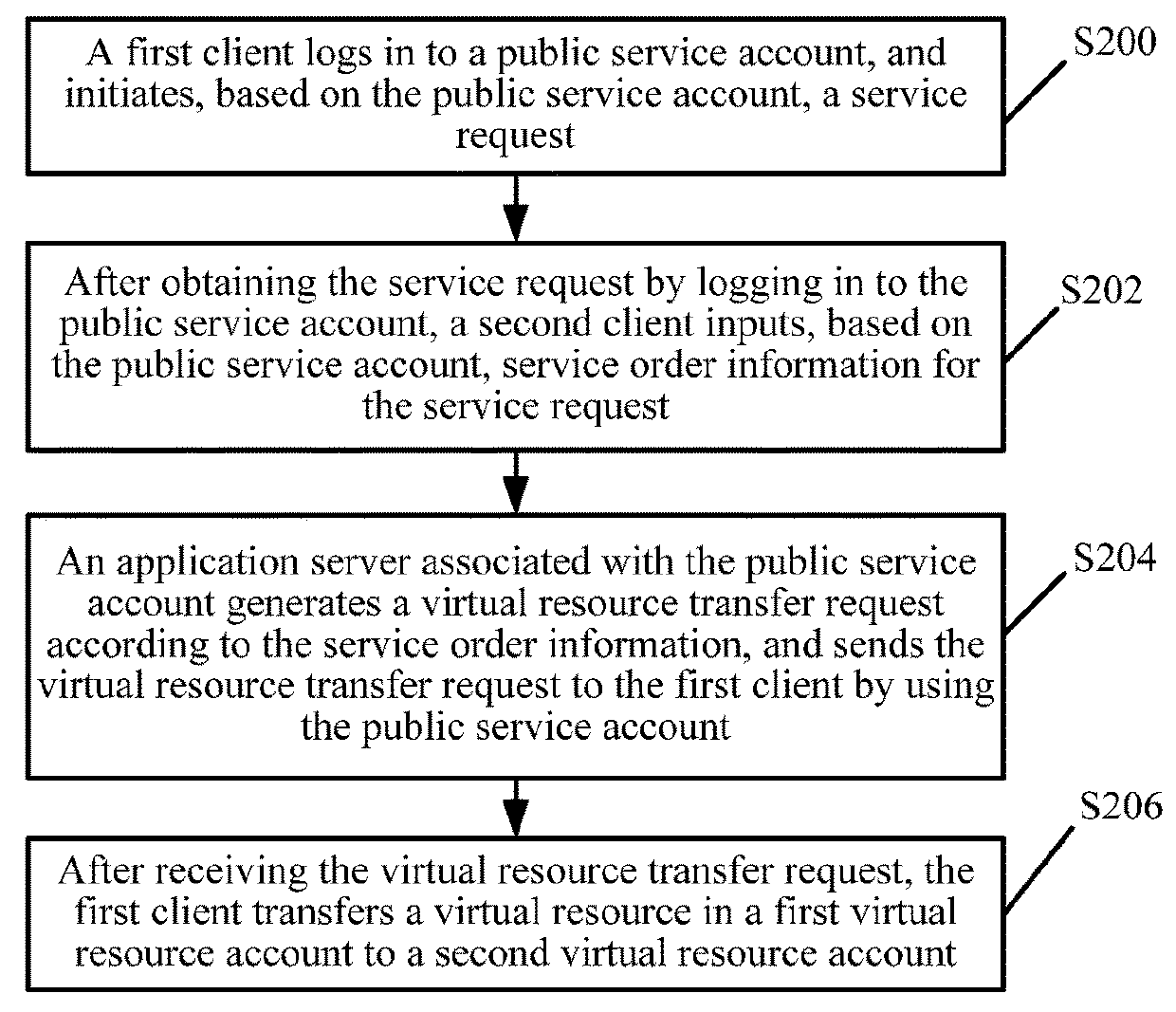
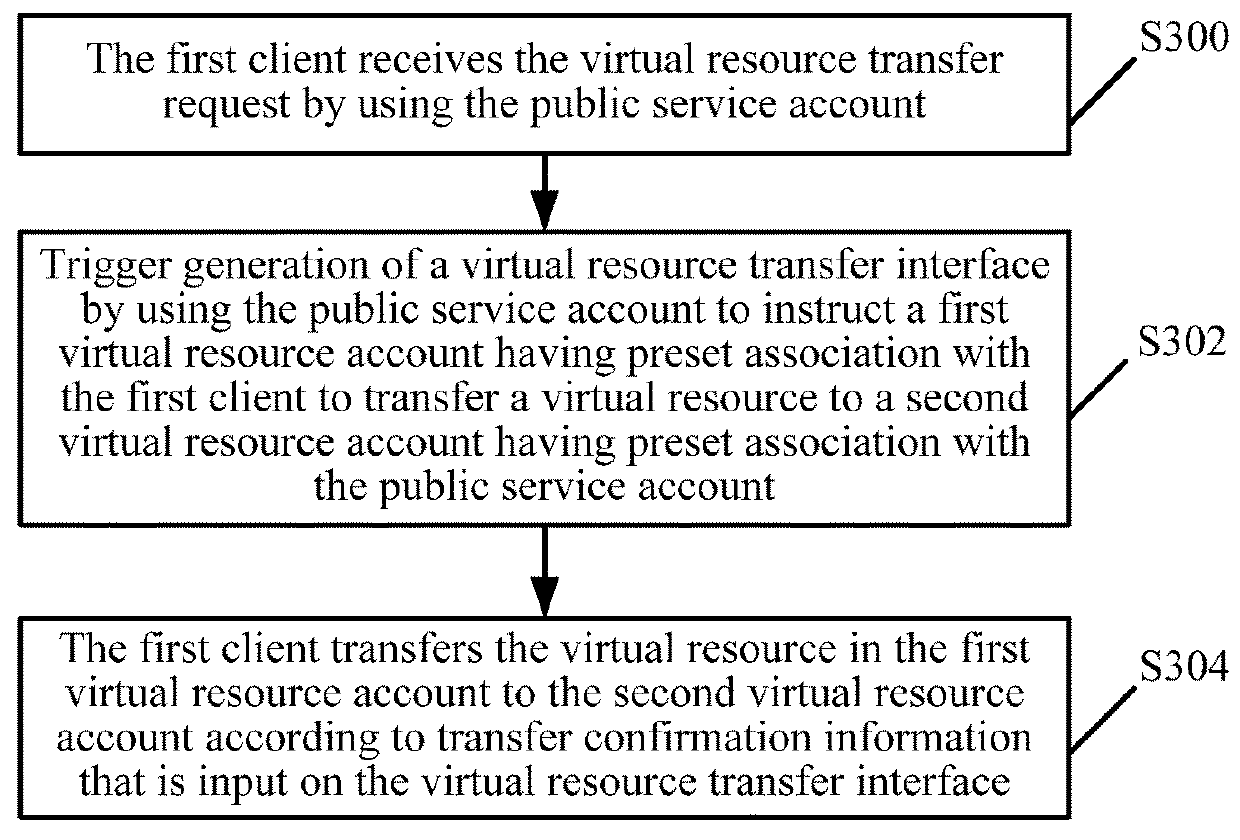
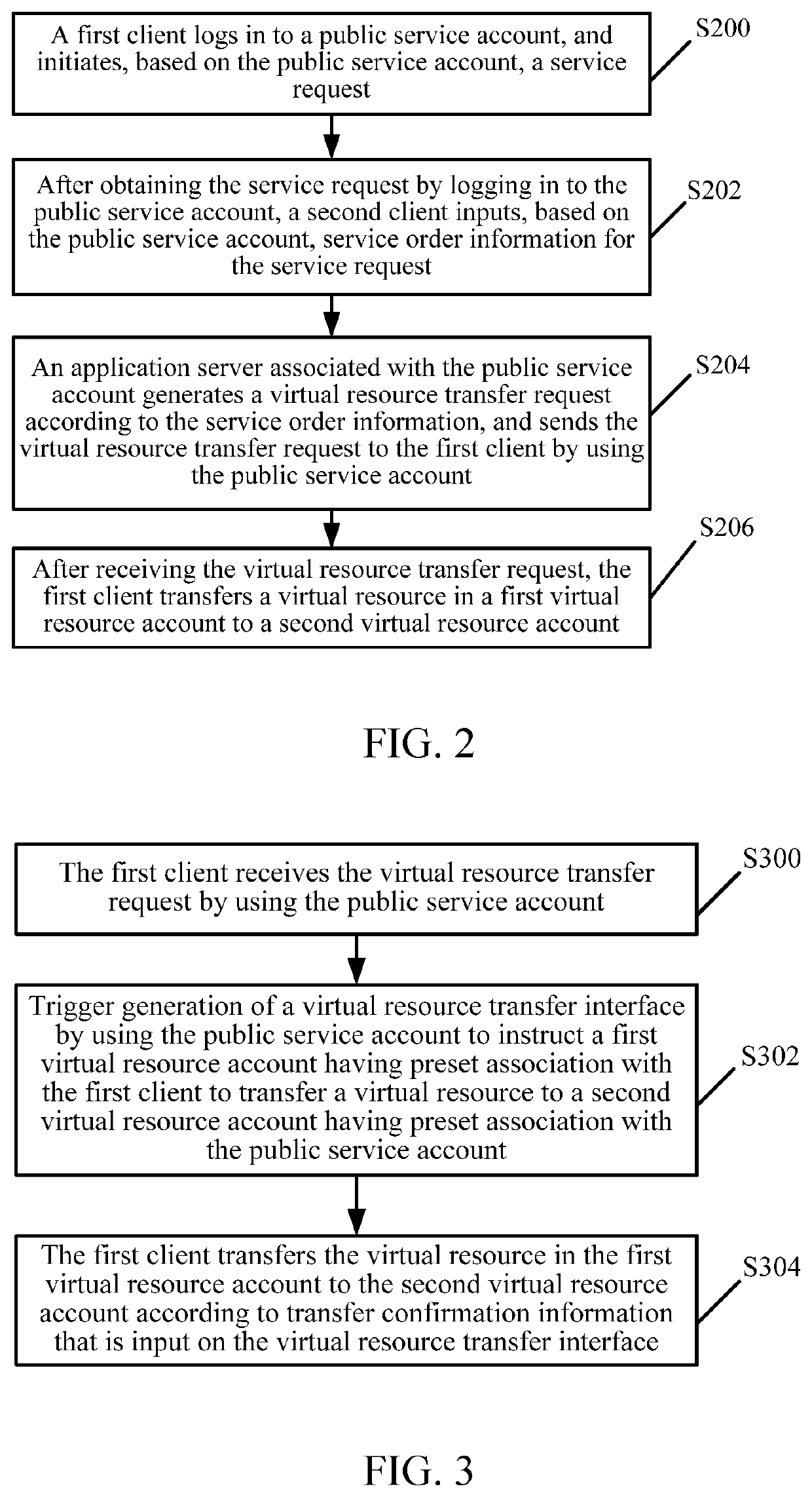
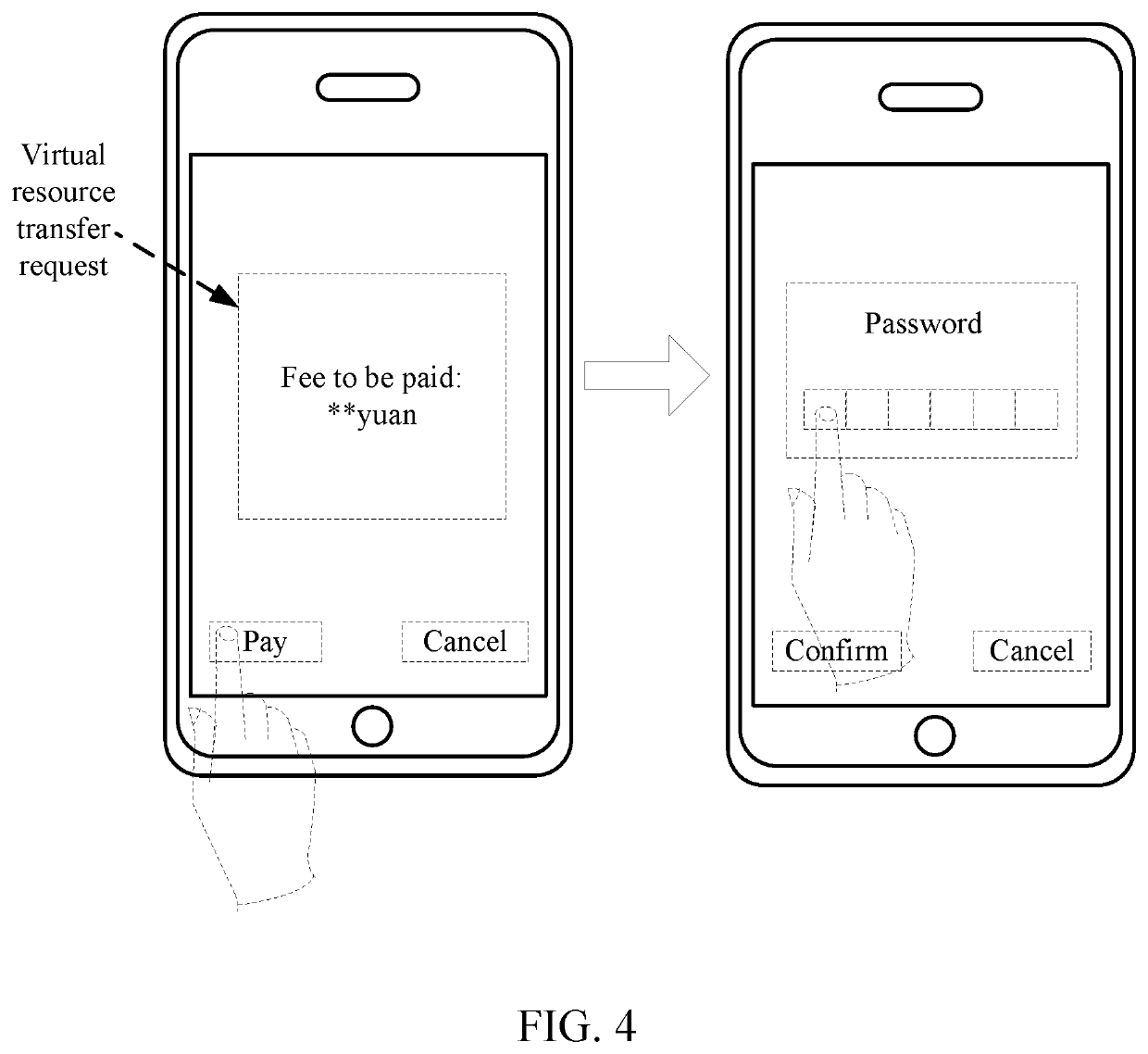
Virtual resource transfer method, client device, application server, and system
ActiveUS20180096416A1Complete monitoringFulfil requirementsResource allocationPayment protocolsApplication serverResource transfer
The present disclosure discloses a virtual resource transfer method, including: responding to a first login request from a first client for logging into a public service account; receiving a service request initiated by the first client; responding to a second login request from a second client for logging into the public service account; sending information about the service request of the first client to the second client; receiving service order information for the service request from the second client; generating, by an application server associated with the public service account, a virtual resource transfer request according to the service order information; sending the virtual resource transfer request to the first client by using the public service account, the virtual resource transfer request being configured for transferring, from the first client, a virtual resource in a first virtual resource account to a second virtual resource account.
Owner:TENCENT TECH (SHENZHEN) CO LTD
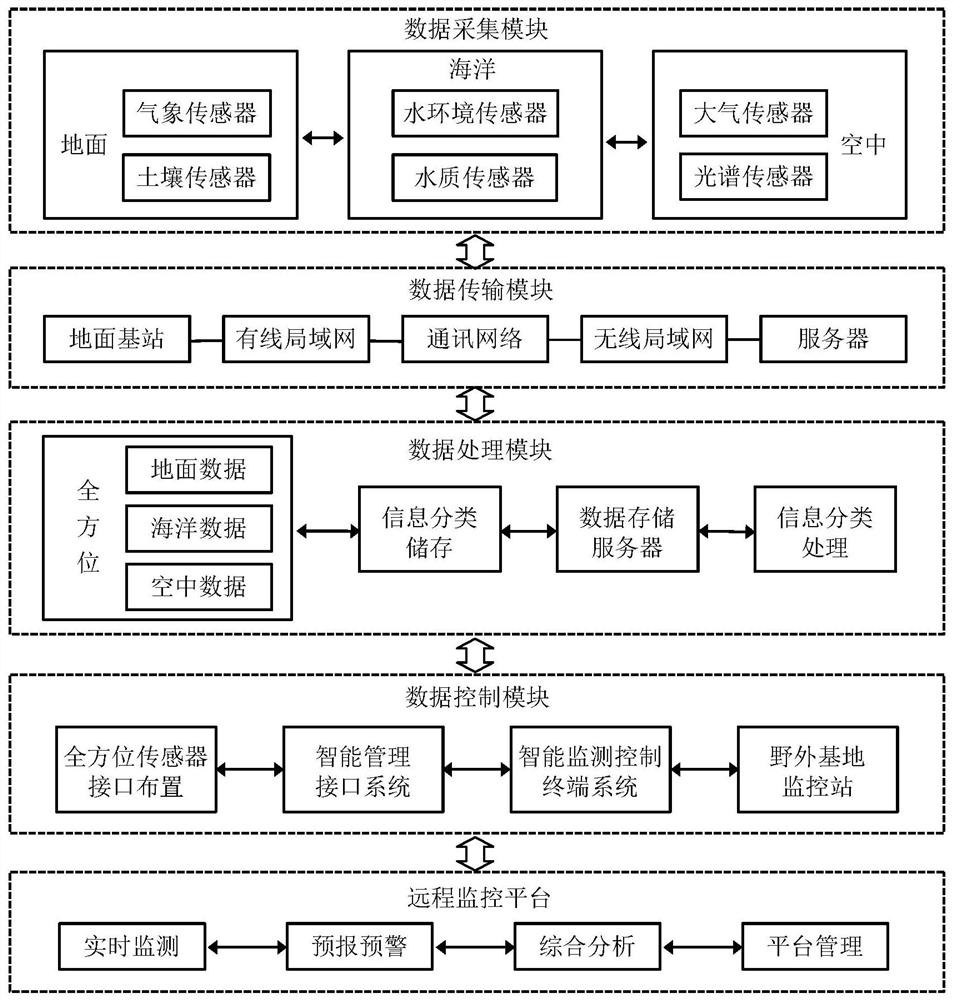
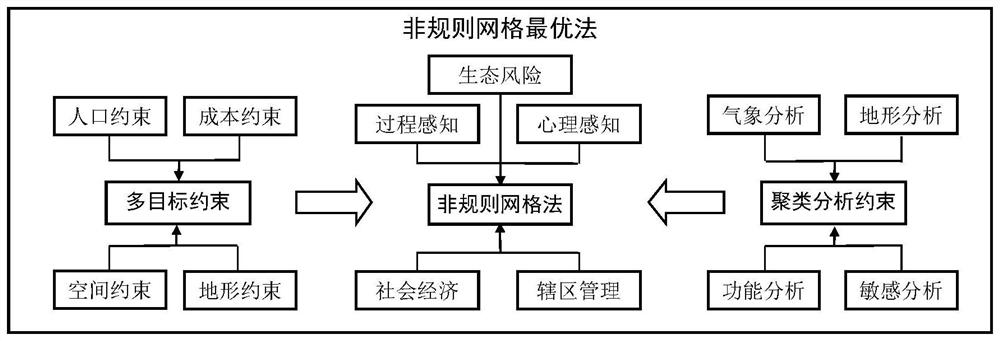
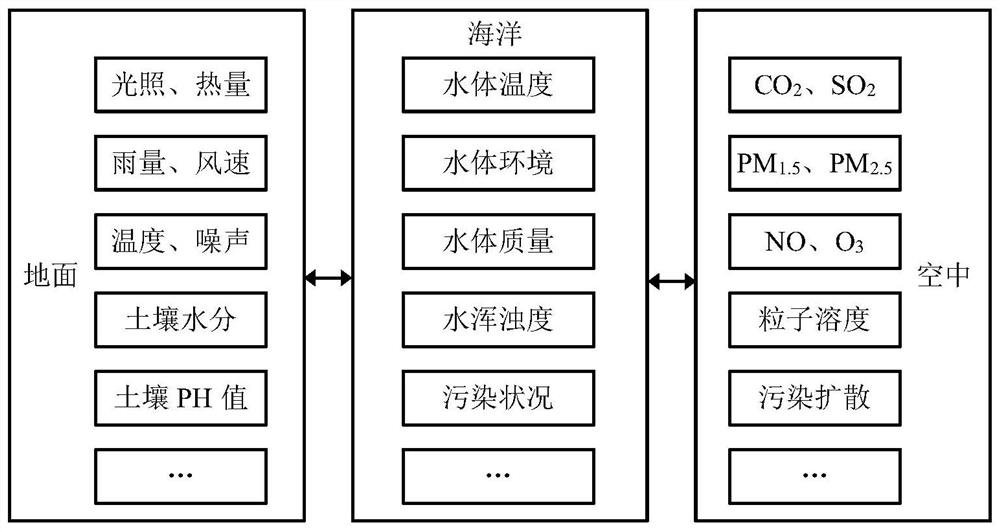
Ecological environment Internet of Things monitoring method and system based on landscape ecology
PendingCN111854847AComplete monitoringMonitoring SystemMeasurement devicesTransmissionEnvironmental resource managementEcological environment
The invention relates to an ecological environment Internet of Things monitoring method and system based on landscape ecology. The method comprises the steps of collecting ecological environment monitoring element data, determining natural elements and ecological environment monitoring elements in the aspect of physical perception on the basis of the content of a scene sensation ecology theory, arranging monitoring point locations by adopting an irregular grid optimization method, and finally arranging corresponding sensors on the monitoring point locations; conducting data transmission, specifically, transmitting the ecological environment monitoring element data acquired by the sensors to a data storage server of a field base monitoring station through a data transmission network; conducting data processing, specifically, completely performing classified storage and classified processing on information of various types of data according to a unified layout; carrying out data controlby designing omnibearing sensor interface arrangement, an intelligent management interface system, an intelligent monitoring control terminal system and a field base monitoring station; and carrying out real-time monitoring, forecasting and early warning and comprehensive analysis on the ecological environment on a remote monitoring platform.
Owner:INST OF URBAN ENVIRONMENT CHINESE ACAD OF SCI +1
A method and system for data quality management
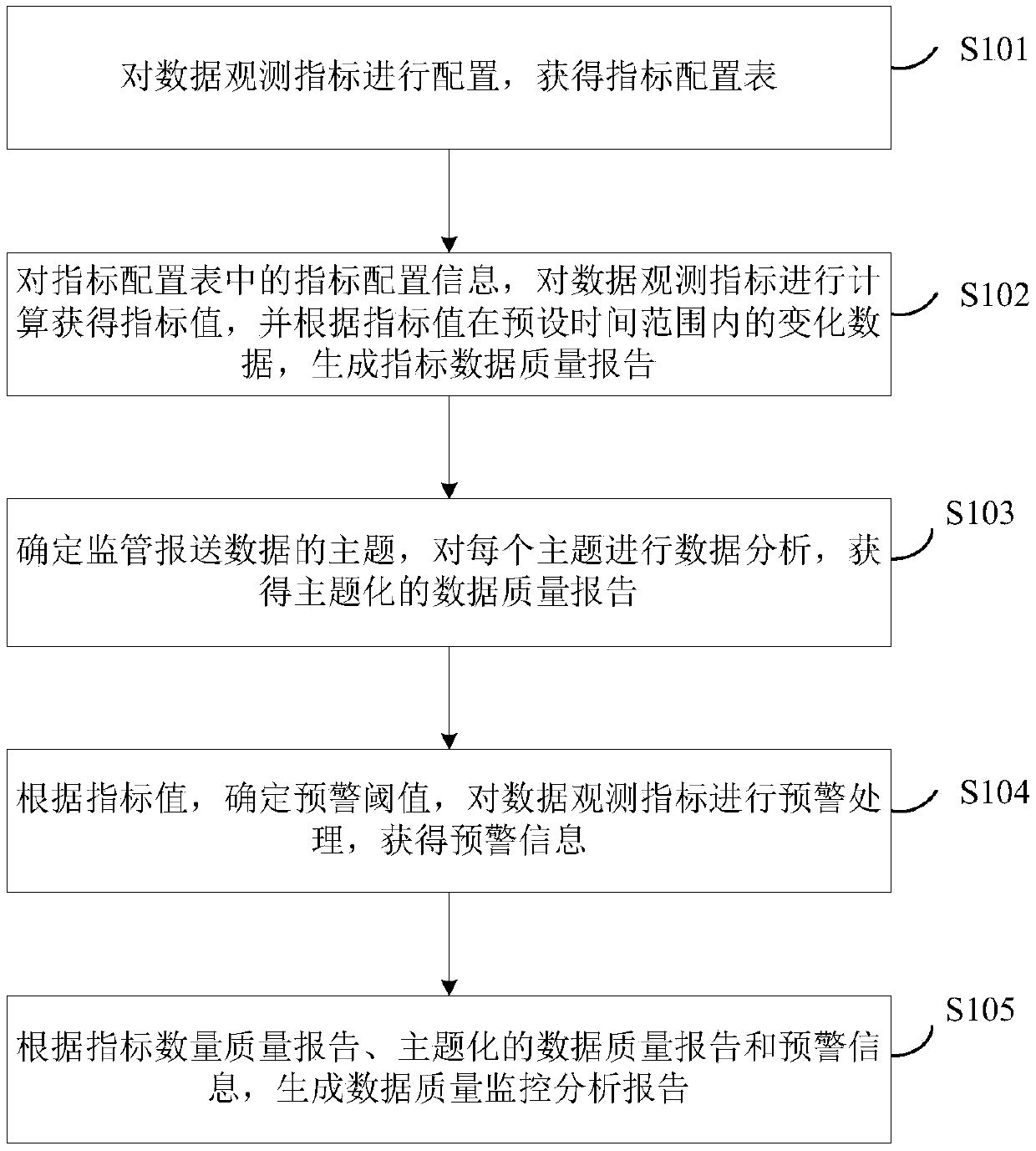
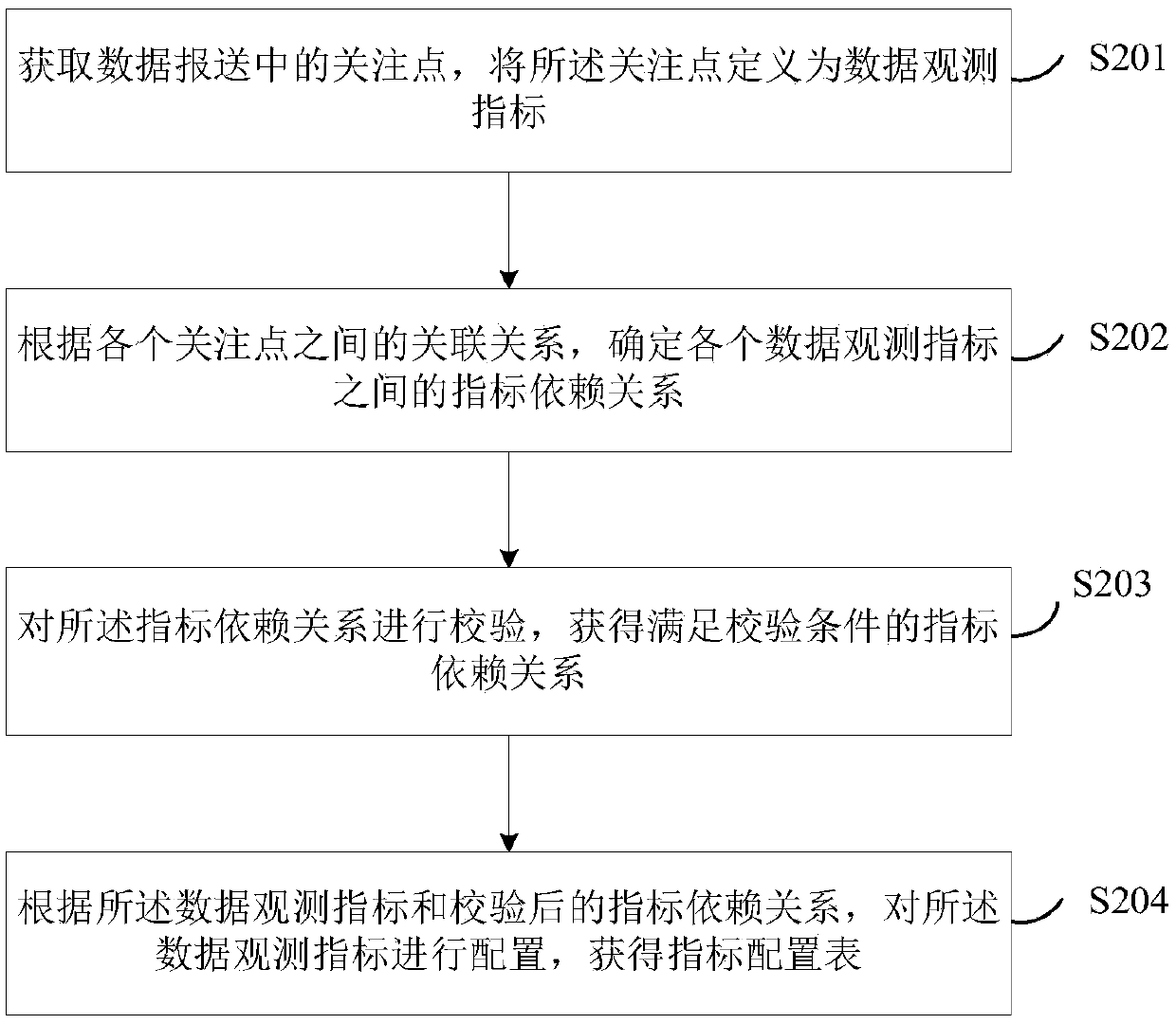
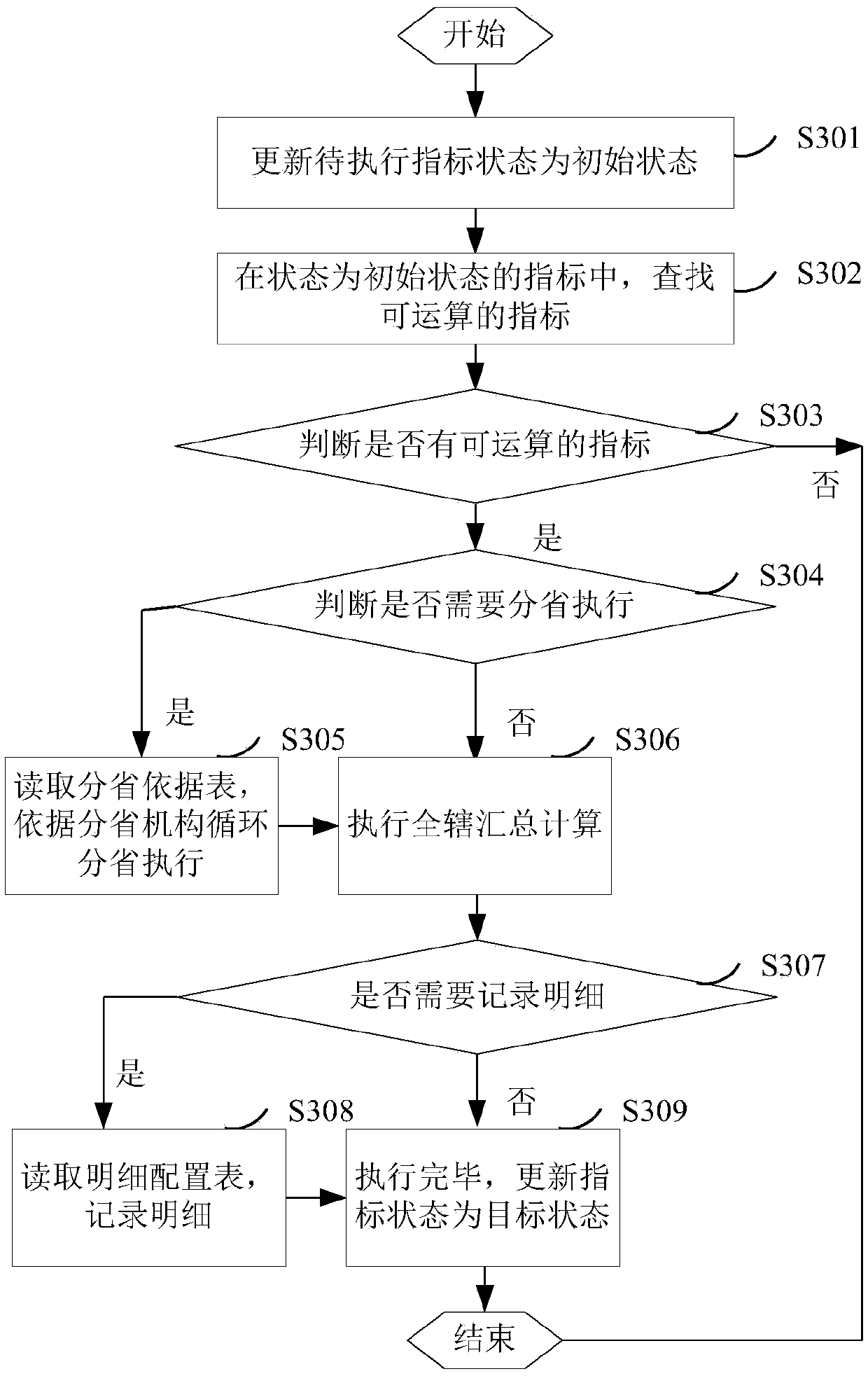
ActiveCN109522318AComplete monitoringMonitor complete and accurateDatabase queryingFinanceTime rangeData profiling
The invention discloses a method and system for data quality management. The method comprises the following steps: configuring a data observation index to obtain an index configuration table, wherein,the data observation index represents a point of interest in data submission; calculating The index configuration information in the index configuration table to obtain the index value by observing the data, and generating the index data quality report according to the change data of the index value in the preset time range. Identifying topics for regulatory reporting data, conduct data analysison each topic, and obtain thematic data quality reports; According to the index value, the early warning threshold is determined, and obtaining the early warning information hrough the early warning processing of the data observation index. Generating data quality monitoring and analysis reports based on indicator quantity and quality reports, thematic data quality reports and early warning information. The invention realizes the improvement of the data transmission quality and the accuracy of monitoring the data quality.
Owner:BANK OF CHINA
Safety device, closing device and evaluation unit
ActiveUS20130106601A1Complete monitoringAvoid accidental collisionDoor/window protective devicesFrequency-division multiplex detailsSignal onEngineering
A safety device for safeguarding a movable, guided movement element against undesired collisions with an object situated on a movement path of the movement element, said device comprising at least two sensors for detecting the object and the movement element and for outputting signals depending on the detection, and also having an evaluation unit for evaluating signals of the sensors and for generating a switch-off signal on the basis of the evaluation. For improved recognition of a risk of collision, the evaluation unit is designed to acquire from the at least two sensors a currently detected state vector from a set of state vectors which unambiguously comprise all possible combinations of the signals of the sensors, and to generate the switch-off signal in the case of predetermined state vectors.
Owner:CEDES AG
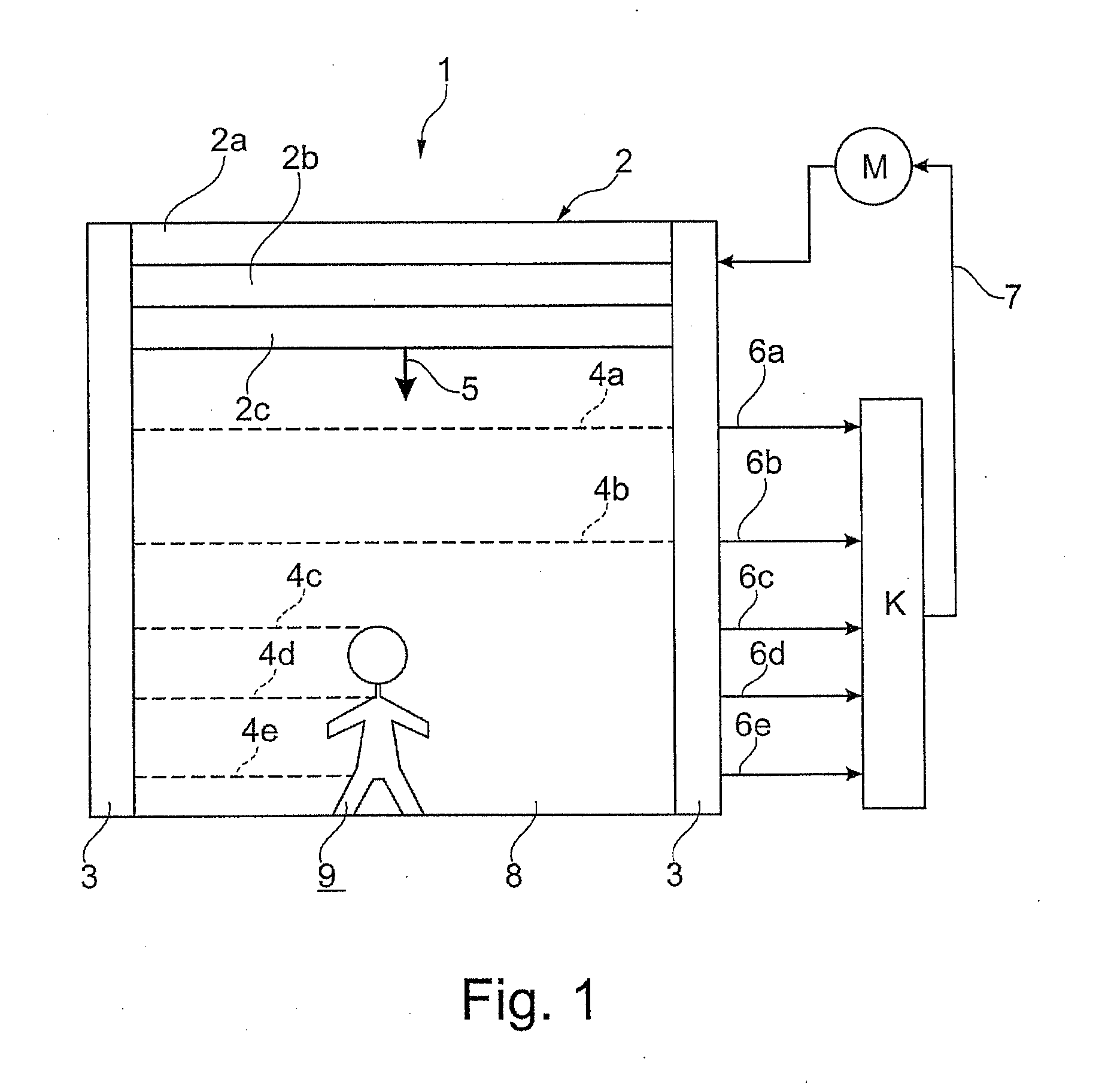
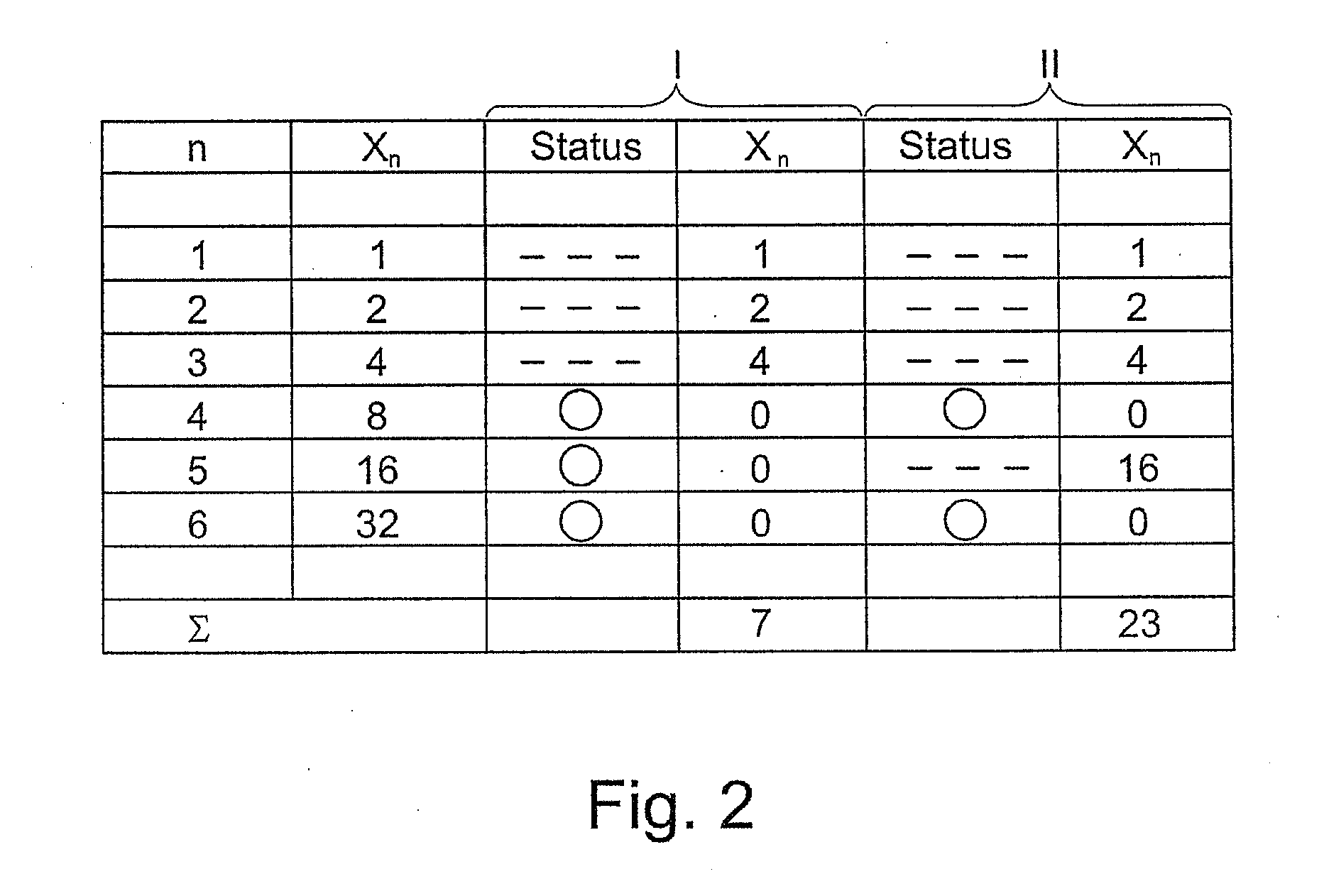
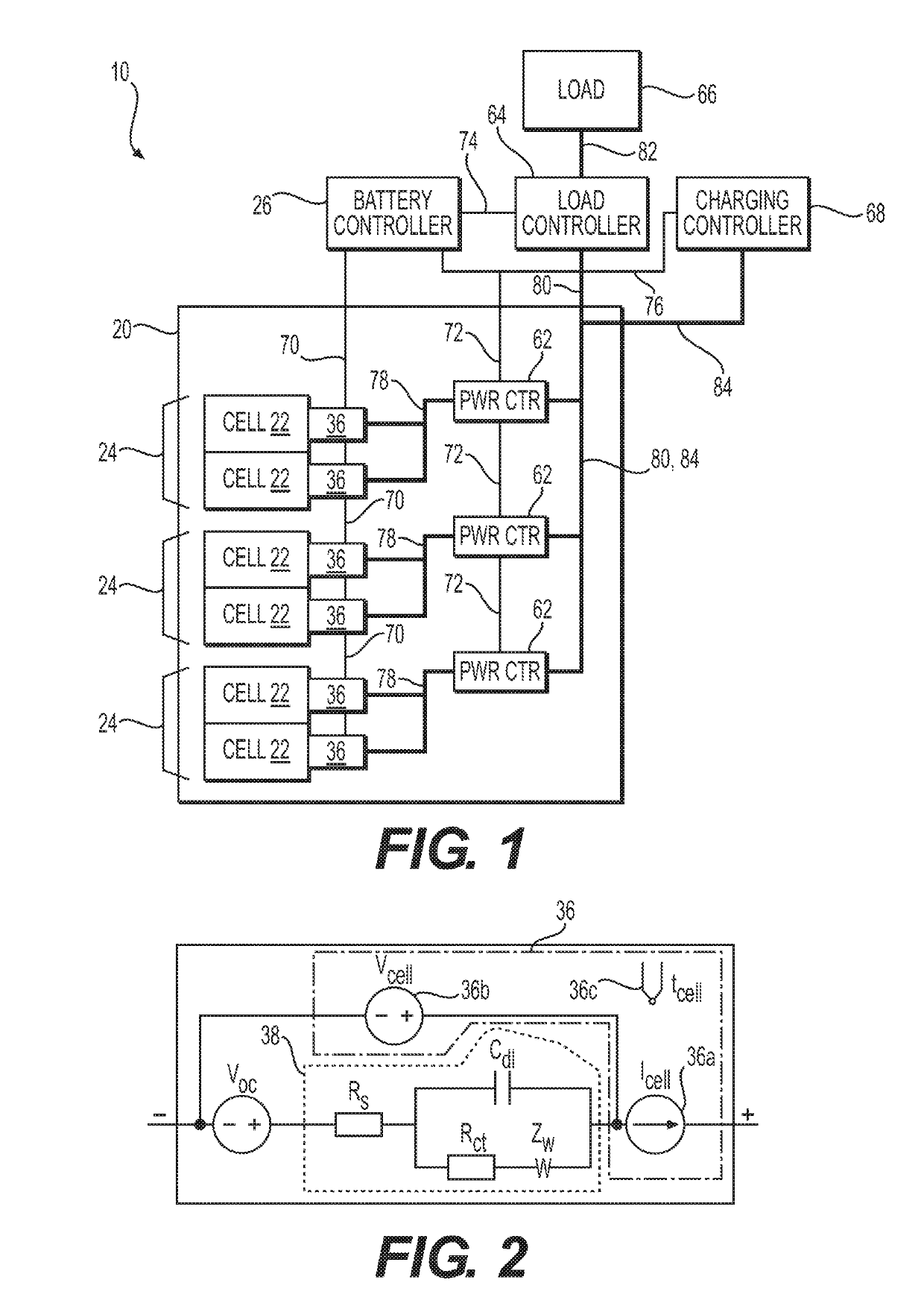
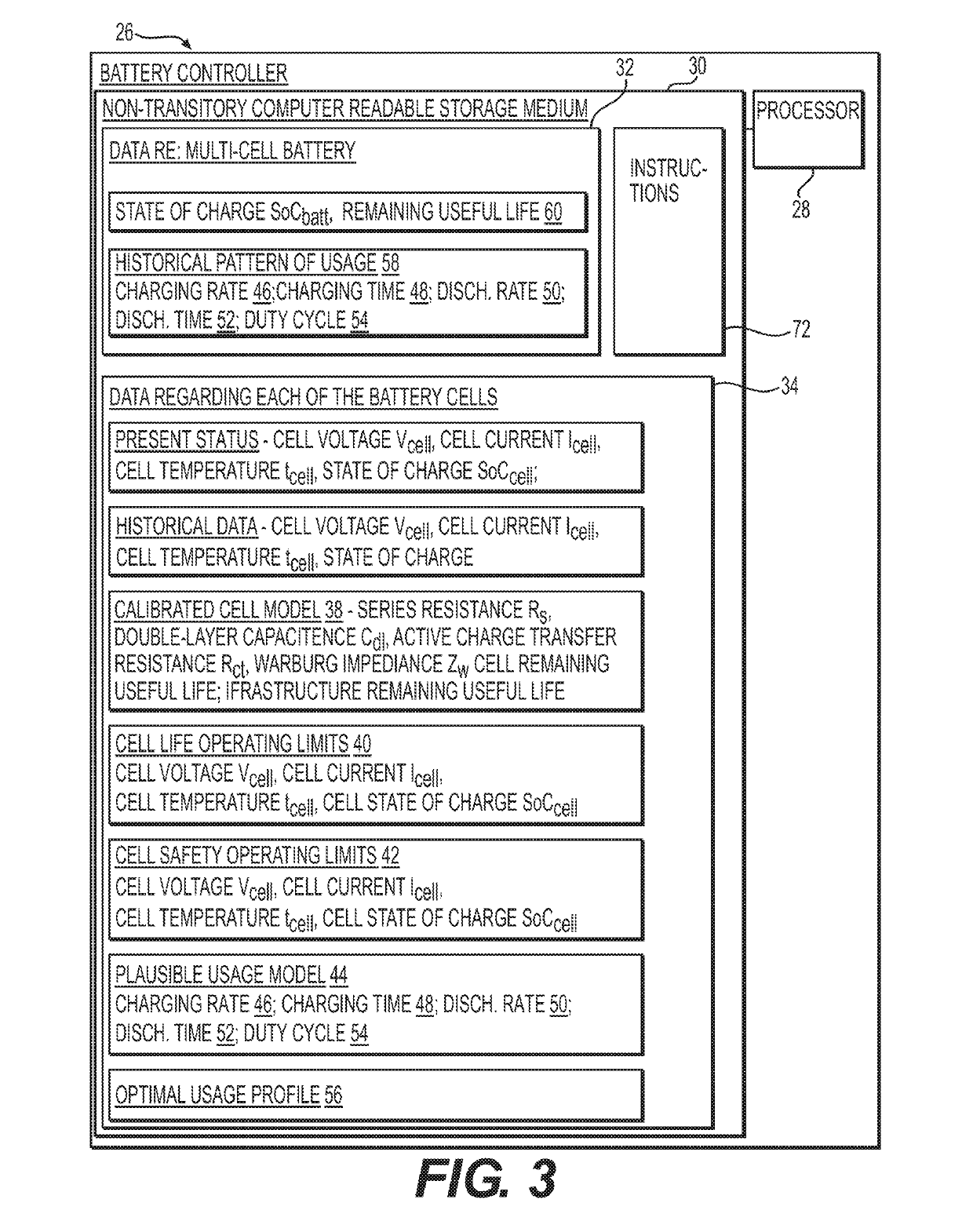
High coverage battery usage monitor
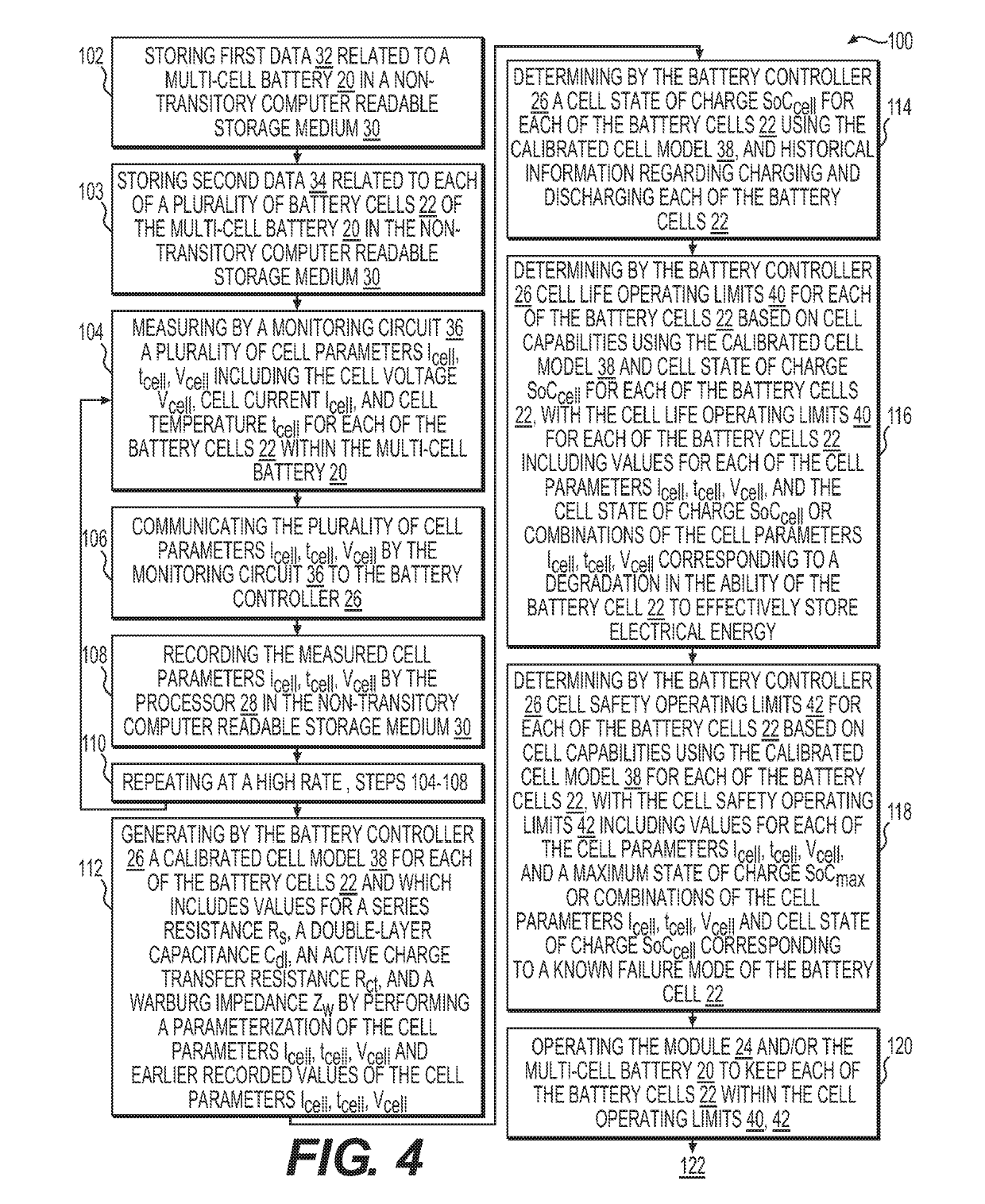
InactiveUS20190111800A1Improve operation and diagnostics and prognosticComplete monitoringElectrical testingCells structural combinationSimulationCell parameter
A method and system for monitoring and controlling a multi-cell battery including a plurality of battery cells uses a battery controller having a processor and a non-transitory computer readable storage medium. A monitoring circuit including current, voltage, and temperature sensors measures a plurality of cell parameters for each of the battery cells, which are communicated to the battery controller. The battery controller performs a parameterization of the cell parameters and past values of the cell parameters to generate a calibrated cell model for each of the battery cells. An optimal usage profile is determined for each of the battery cells as an optimized compromise of cell operating limits between different battery cells within the multi-cell battery, and then causes each of the battery cells to be operated according to the corresponding optimal usage profile. Calculating and reporting of the remaining useful life of the battery is also provided.
Owner:NEAPCO INTPROP HLDG LLC
3D lighting monitoring method, 3D lighting monitoring device and 3D lighting monitoring system
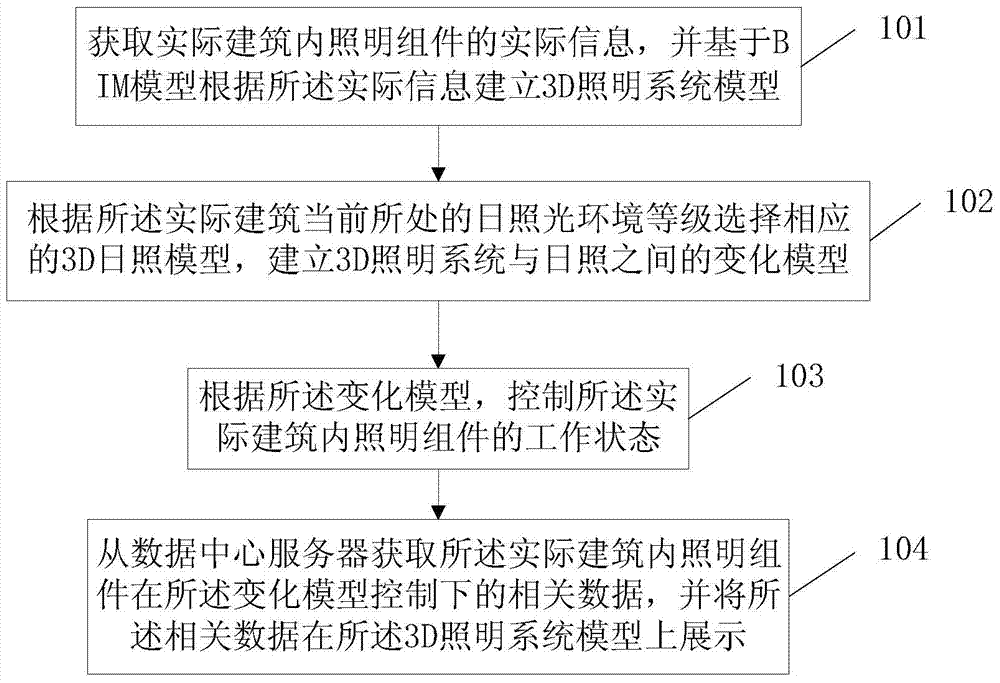
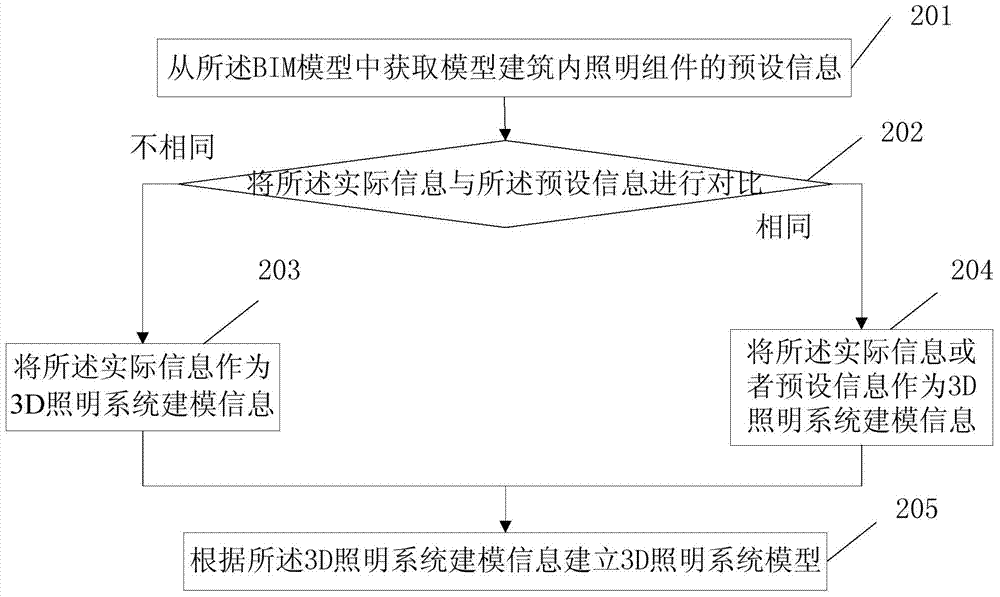
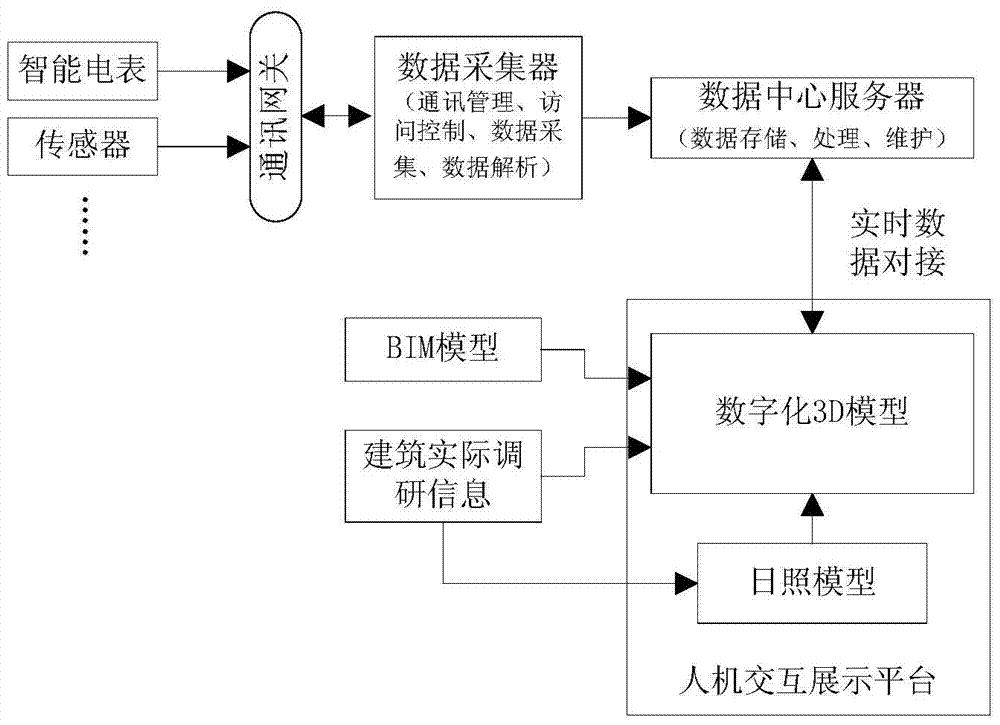
ActiveCN104768276AMonitoring is intuitiveComplete monitoringElectric light circuit arrangementSimulationMonitoring system
The invention discloses a 3D lighting monitoring method, a 3D lighting monitoring device and a 3D lighting monitoring system, and relates to the technical field of intelligent buildings. The operation state of lighting can be monitored, analyzed and managed more visually, comprehensively and completely. Moreover, lighting control can be realized by combining lighting operation state monitoring, analyzing and managing with a sunlight model, and energy saving can be achieved. The method of the invention mainly comprises the following steps: acquiring actual information of a lighting assembly in an actual building, and establishing a 3D lighting system model according to the actual information based on BIM (building information modeling); selecting a corresponding 3D sunlight model according to the level of a sunlight environment where the actual building is located currently, and establishing a change model between the 3D lighting system model and sunlight; controlling the working state of the lighting assembly in the actual building according to the change model; and acquiring related data of the lighting assembly in the actual building under the control of the change model from a data center server, and displaying the related data on the 3D lighting system model. The method of the invention is mainly used in the process of 3D lighting monitoring.
Owner:BEIJING PERSAGY ENERGY SAVING TECH
Communication energy storage power supply system
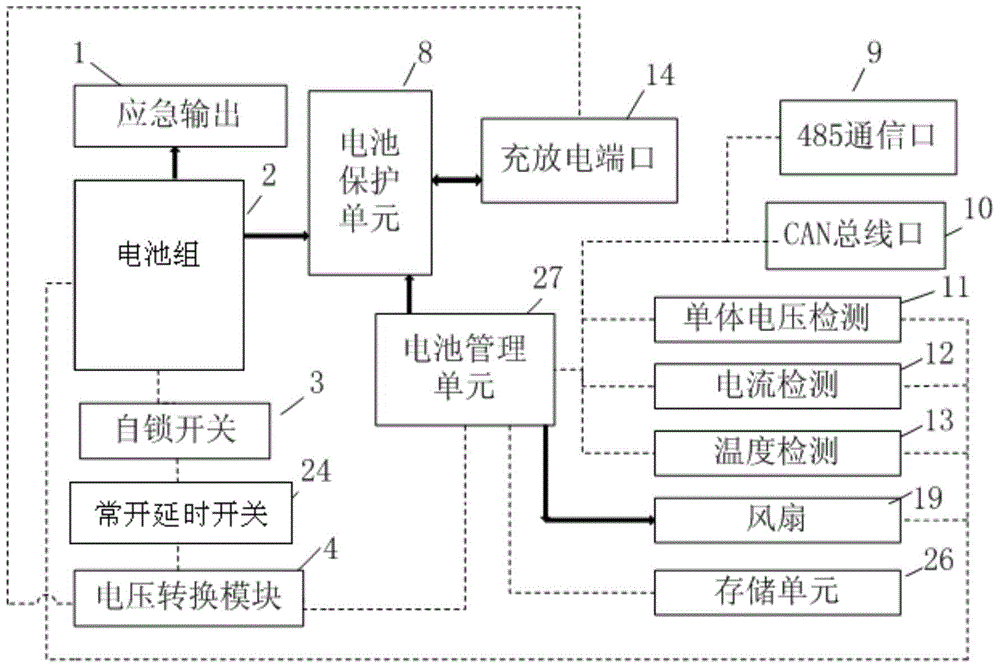
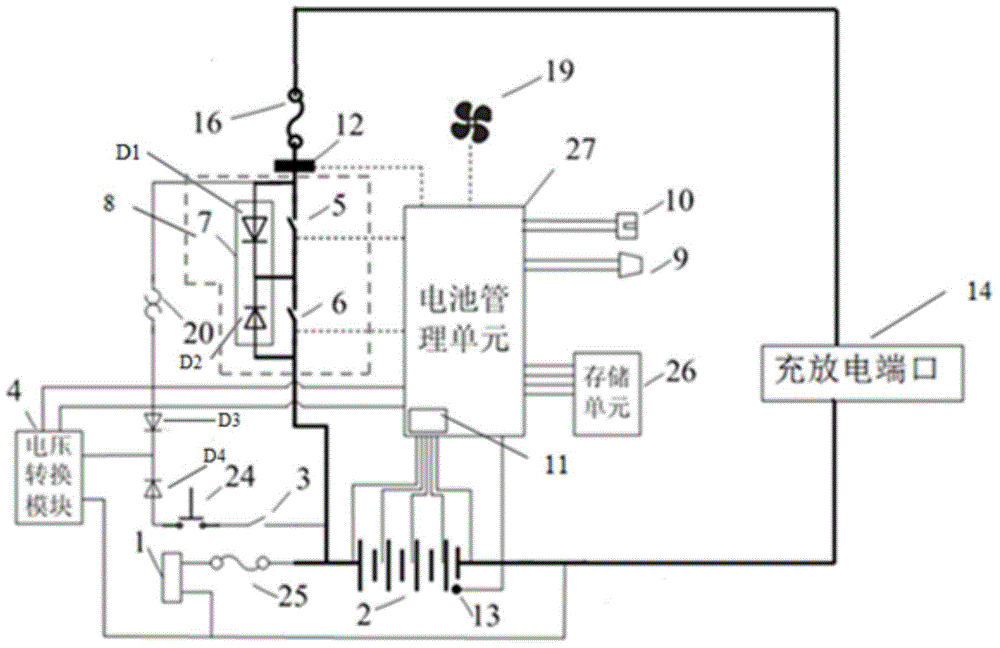
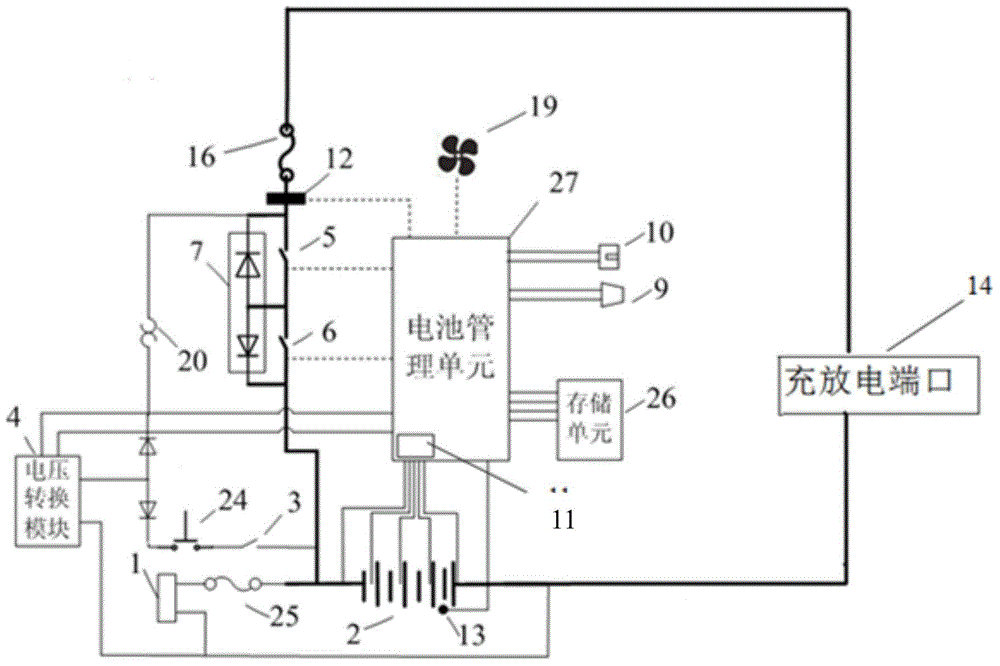
ActiveCN104882936ALess investmentAvoid single point of failure problemsBatteries circuit arrangementsElectric powerData centerThe Internet
The invention relates to a communication energy storage power supply system, and especially relates to a direct-current energy storage power supply system for Internet data centers and communication base stations. The invention discloses an intelligent control communication power supply system comprising a battery pack, a charge and discharge port, a battery protection unit, a voltage conversion module, a battery management unit, a normally open delay switch, and a self-locking switch. The communication energy storage power supply system of the invention has two different system activation modes and an intelligent battery management system.
Owner:李相哲
Device for measuring a cycling cadence
The present invention relates to a device (100) for measuring a cycling cadence, a method (500) of operating a device (100) for measuring a cycling cadence, and a cycling cadence computer program. The device (100) comprises a motion sensor (such as, e.g., an accelerometer) for detecting a movement of the device (100) and for generating a motion signal (x, y, z) corresponding to the movement; a cadence determination unit (300) for determining cycling cadence based on the motion signal (x, y, z). The device (100) can be worn on the cyclist's wrist or arm (110). The motion sensor in the device is able to pick up the tiny movements of the arm or wrist that correspond to the cadence. Optionally, an algorithm is applied that can derive the cadence from a noisy signal.
Owner:KONINKLJIJKE PHILIPS NV
Safety device, closing device and evaluation unit
ActiveUS8988213B2Avoid accidental collisionComplete monitoringDoor/window protective devicesFrequency-division multiplex detailsSignal onEngineering
A safety device for safeguarding a movable, guided movement element against undesired collisions with an object situated on a movement path of the movement element, said device comprising at least two sensors for detecting the object and the movement element and for outputting signals depending on the detection, and also having an evaluation unit for evaluating signals of the sensors and for generating a switch-off signal on the basis of the evaluation. For improved recognition of a risk of collision, the evaluation unit is designed to acquire from the at least two sensors a currently detected state vector from a set of state vectors which unambiguously comprise all possible combinations of the signals of the sensors, and to generate the switch-off signal in the case of predetermined state vectors.
Owner:CEDES AG
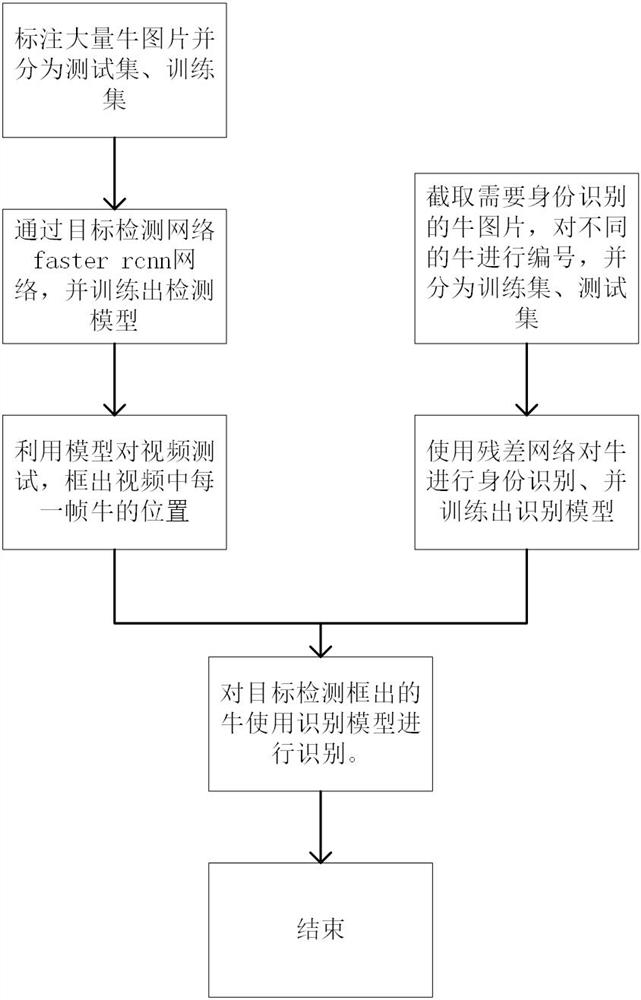
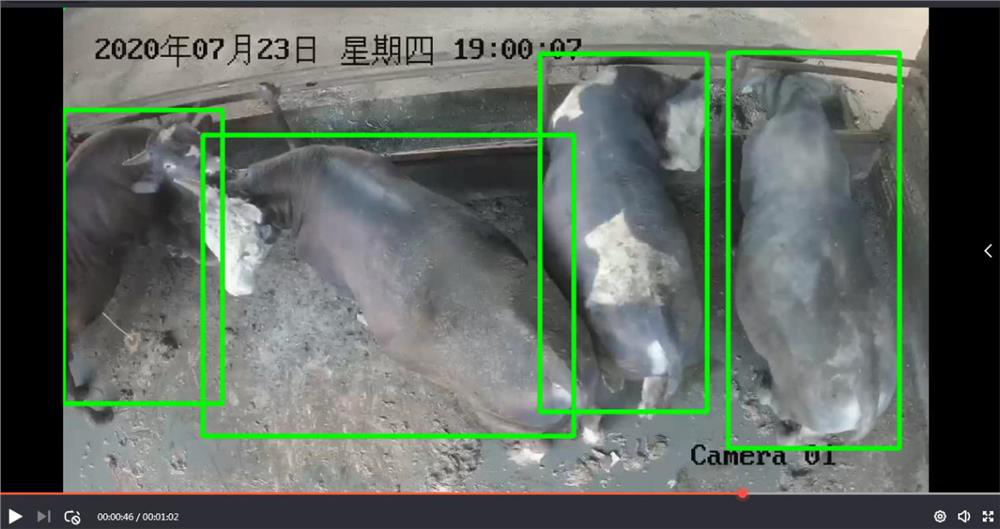
Intelligent cattle farm monitoring and recognition method and device based on deep learning
InactiveCN112101333AStatistically accurateComplete monitoringCharacter and pattern recognitionNeural architecturesVideo monitoringAnimal science
The invention relates to an intelligent cattle farm monitoring and recognition method and device based on deep learning, and the method comprises the following steps: 1) collecting cattle flock pictures of a cattle farm through video monitoring equipment; 2) marking the cattle in the cattle farm cattle flock picture to form a training set; 3) inputting the training set in the step 2) into a targetdetection network master rcnn, and training to obtain a cattle group target detection model; 4) selecting the position of each frame of cattle in the video; 5) numbering different cows to form a training set; 6) training to obtain a cattle identification model; and 7) carrying out identity identification, and visualizing an identification result to the back of the cattle. According to the invention, the number of cattle groups in a cattle farm can be accurately counted in real time; and identity training is carried out on the box-selected cattle back by using a residual network to successfully confirm the identity of the numbered cattle. Therefore, the monitoring and identification method provided by the invention can perform complete monitoring and identity identification on the cattle flock in the cattle farm.
Owner:四川圣点世纪科技有限公司
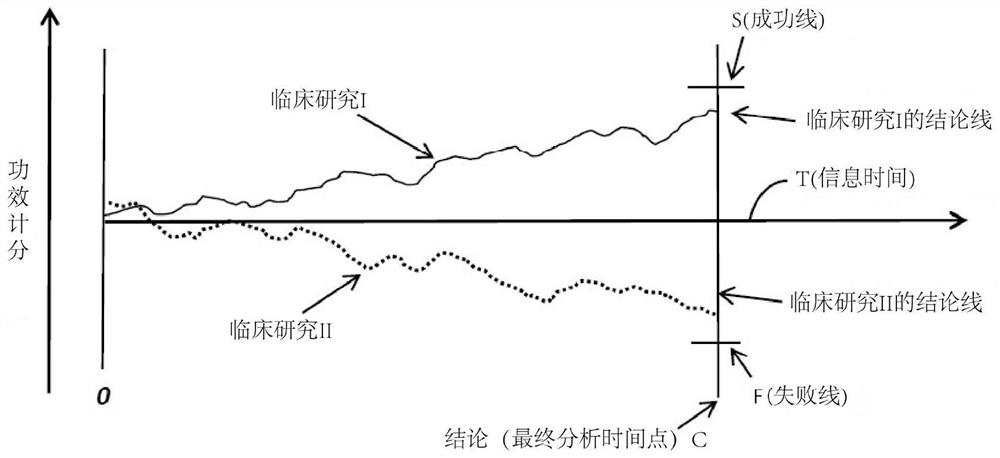
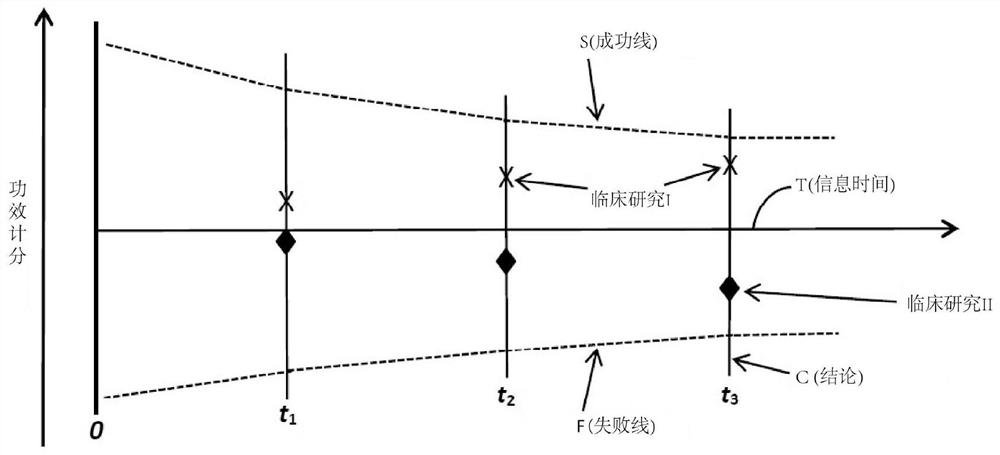
Systems, methods and processes for dynamic data monitoring and real-time optimization of ongoing clinical research trials
PendingCN112840314AComplete monitoringDigital data processing detailsInput/output processes for data processingConfidence intervalClinical trial
A method and process which dynamically monitors data from an on-going randomized clinical trial associated with a drug, device, or treatment automatically and continuously unblinds the study data without human involvement. In one embodiment, a complete trace of statistical parameters such as treatment effect, trend ratio, maximum trend ratio, mean trend ratio, minimum sample size ratio, confidence interval and conditional power are calculated continuously at all points along the information time. In one embodiment, a method early concludes a decision, i.e., futile, promising, sample size re-estimate, for an on-going clinical trial. In one embodiment, exact type I error rate control, median unbiased estimate of treatment effect, and exact two-sided confidence interval can be continuously calculated.
Owner:BRIGHT CLINICAL RES LTD
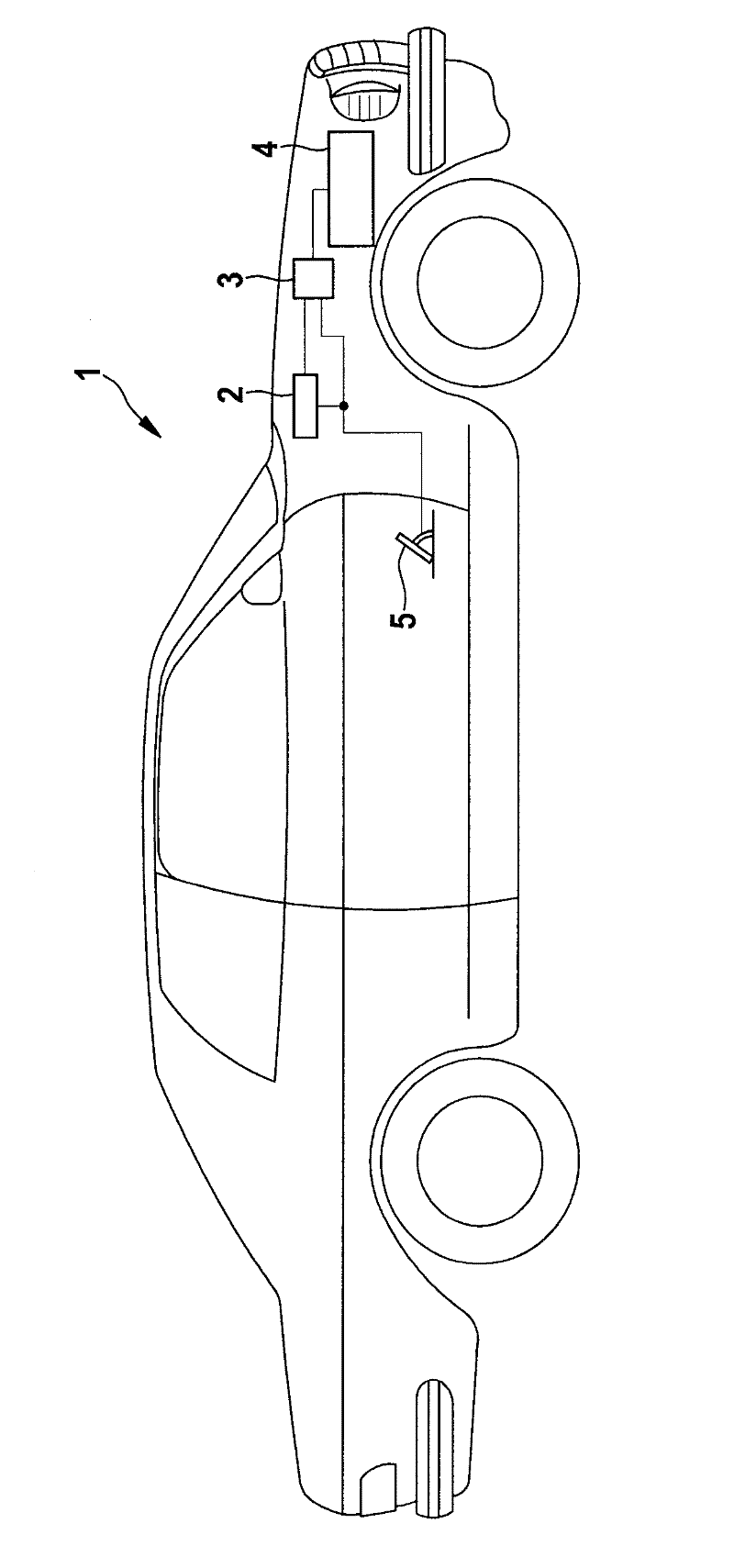
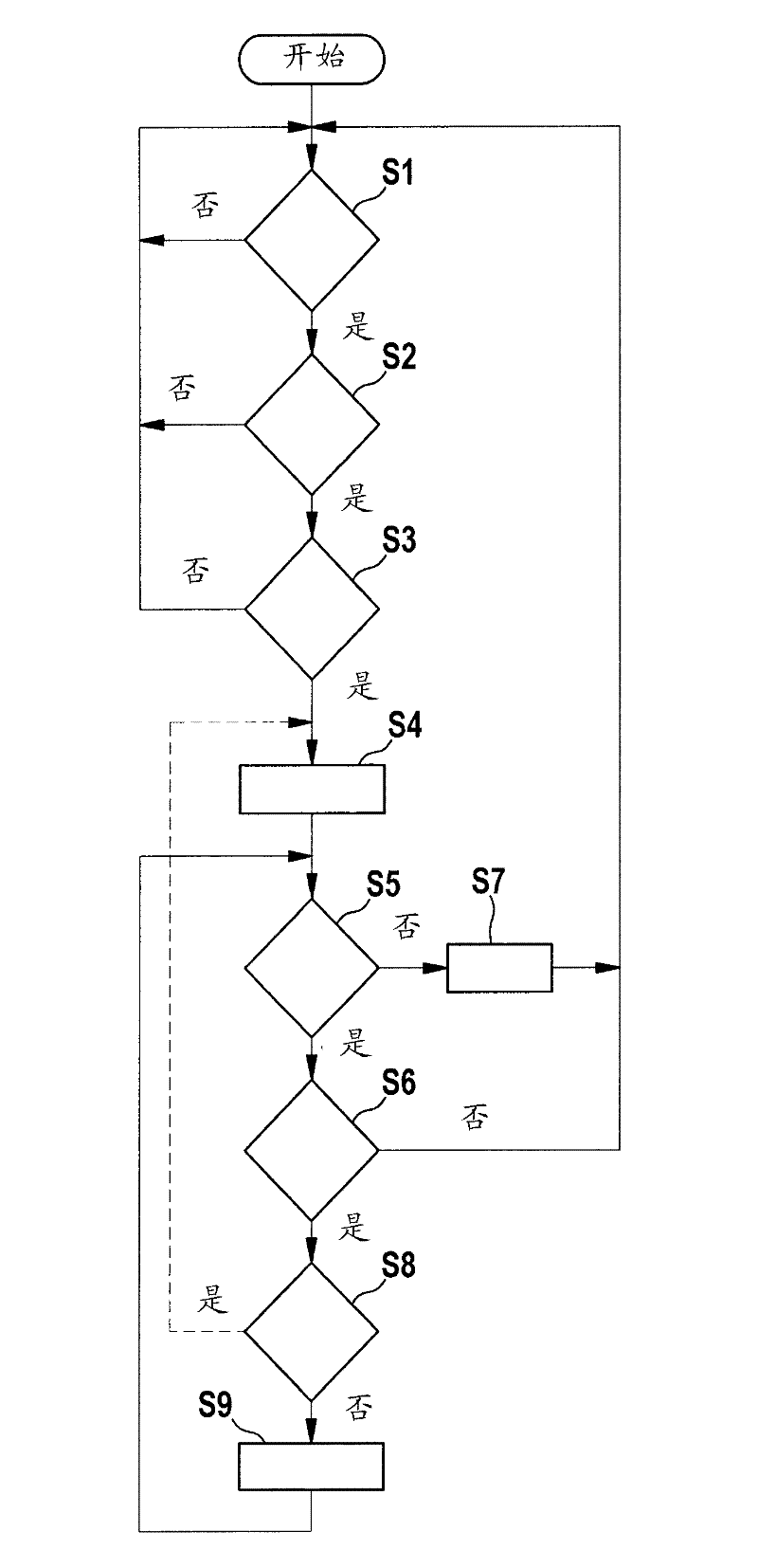
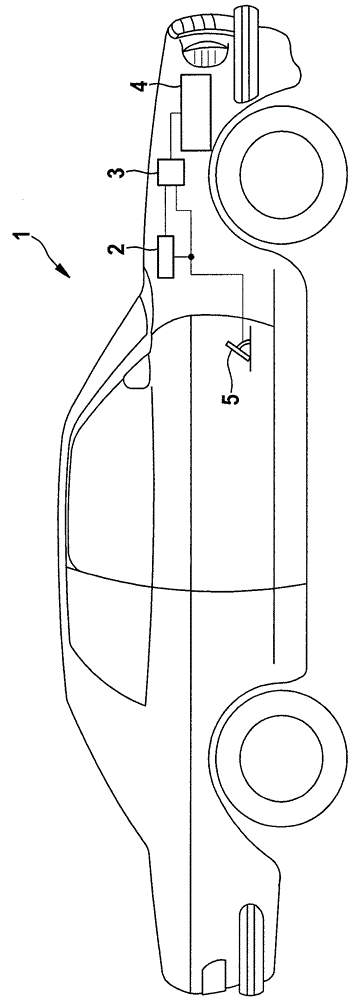
Method and apparatus for monitoring the driving apparatus of a driving system of a vehicle
ActiveCN102529968ASave complex calculationsComplete monitoringElectrical controlRoad transportEngineeringTime based
The invention provides a method and apparatus for monitoring the driving apparatus of a driving system of a vehicle. The method for monitoring the defects of the driving system of a vehicle (1) comprises the following steps: confirming whether the vehicle is in inertia operation because the driving apparatus of a vehicle is not supposed to provide driving torque in the inertia operation; if the inertia operation is confirmed to be underway, calculating the velocity threshold changing curve in term of time based on the current speed of the vehicle; and determining the defects if the current vehicle speed exceeds the predetermined threshold based on the velocity threshold changing curve in the process having inertia operation.
Owner:ROBERT BOSCH GMBH
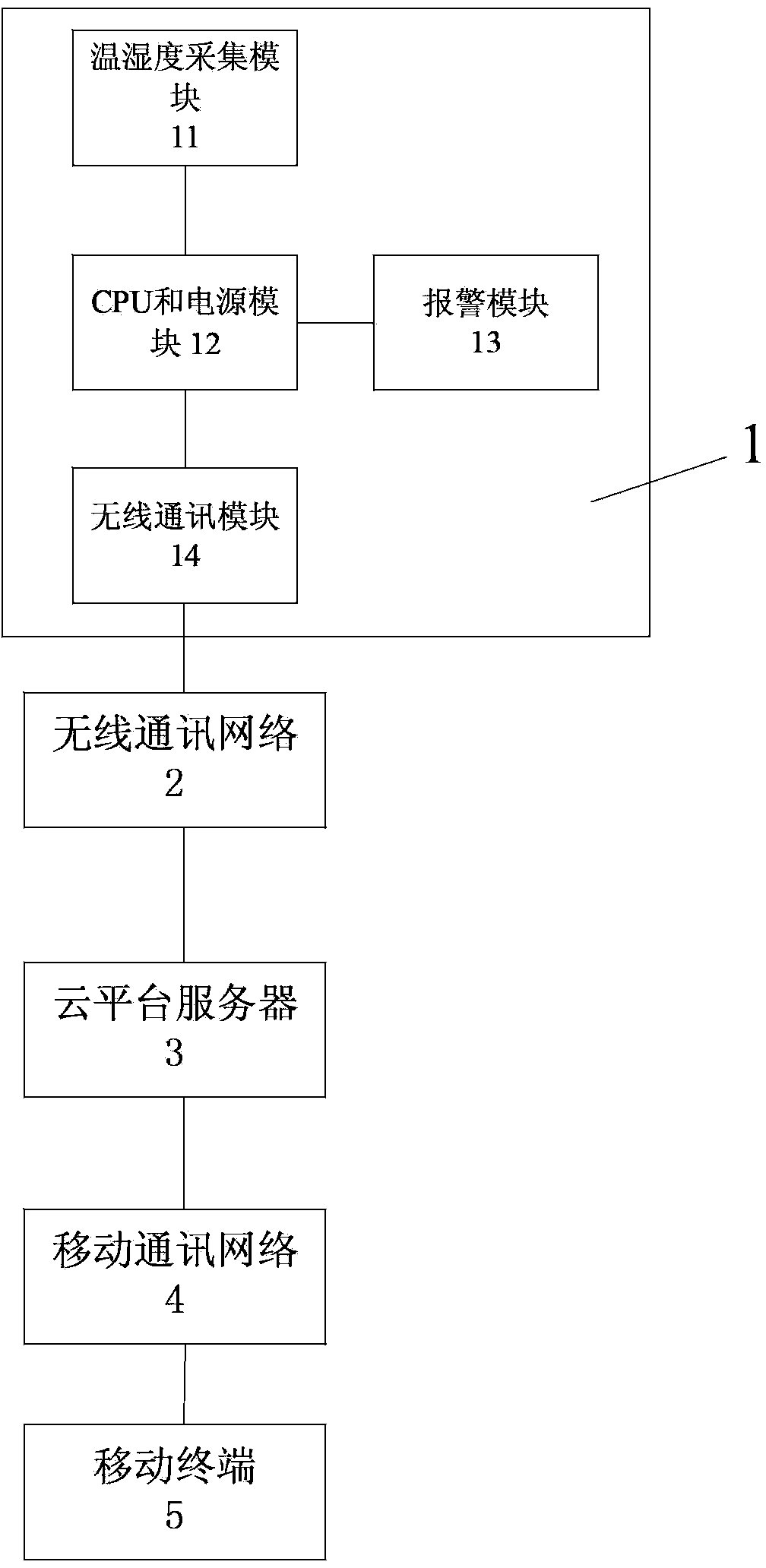
Real-time temperature and humidity monitoring and alarm method for fixed cold-chain equipment on basis of wireless communication
The invention relates to a real-time temperature and humidity monitoring and alarm method for fixed cold-chain equipment on the basis of wireless communication. The real-time temperature and humidity monitoring and alarm method includes steps that 1), a temperature and humidity acquisition module acquires temperature and humidity information of the fixed cold-chain equipment in real time and transmits the temperature and humidity information to a CPU (central processing unit); 2), the CPU receives the temperature and humidity information of the fixed cold-chain equipment, stores the temperature and humidity information locally and regularly transmits the temperature and humidity information to a cloud platform server via a wireless communication network, the temperature and humidity information is compared to set threshold values, a local alarm is raised if the temperature and humidity information exceeds the set threshold values, and alarm information is transmitted to the cloud platform server via the wireless communication network; 3), the cloud platform server transmits the alarm information to a user in real time via a mobile communication network by various information approaches, and the user can query the temperature and humidity information of the fixed cold-chain equipment via the cloud platform server. Compared with the prior art, the real-time temperature and humidity monitoring and alarm method has the advantages of wide monitoring range, high reliability, good safety and the like.
Owner:NEWLAND DIGITAL TECH CO LTD
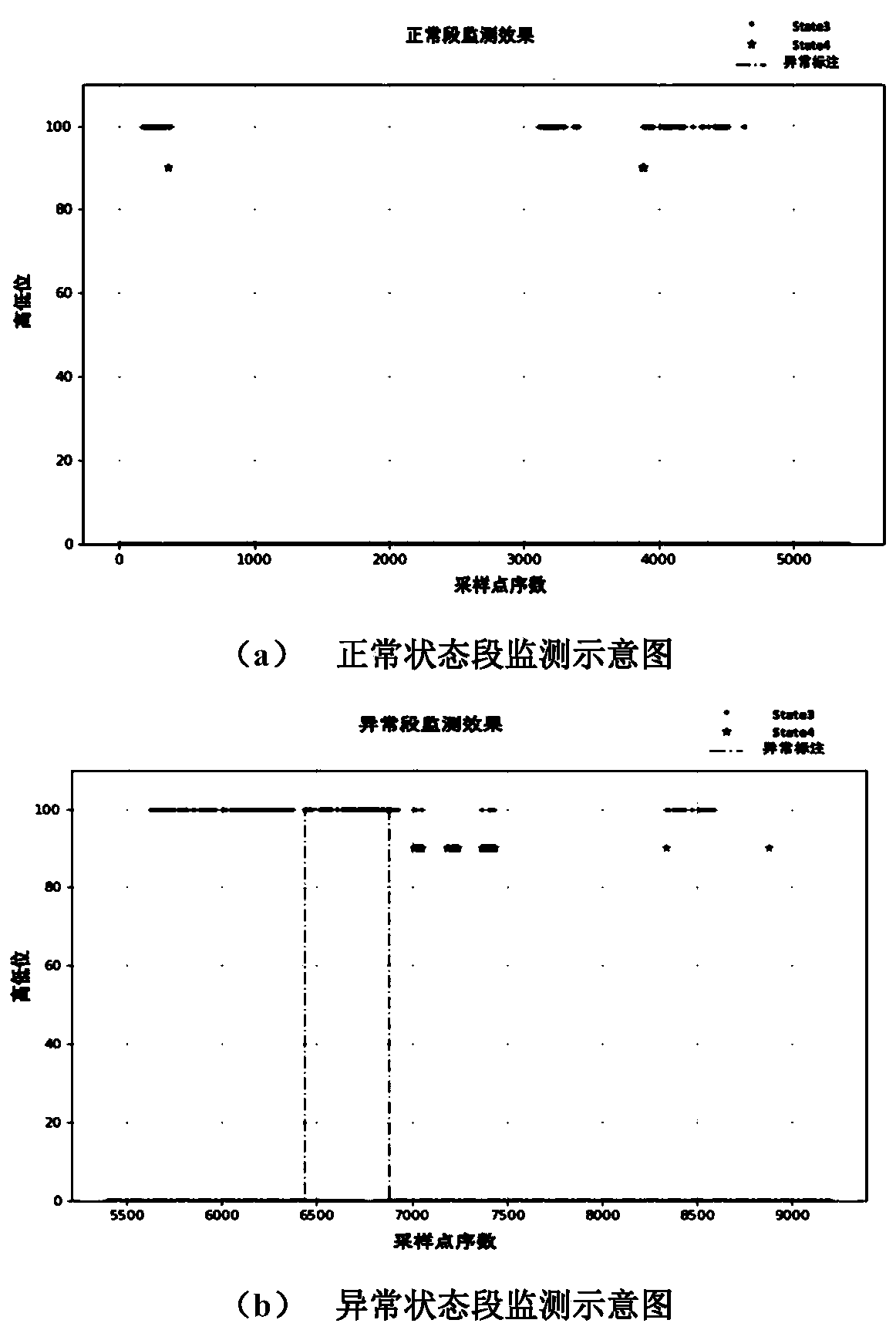
Fan blade icing abnormity monitoring method based on fine-grained wind power generation state division
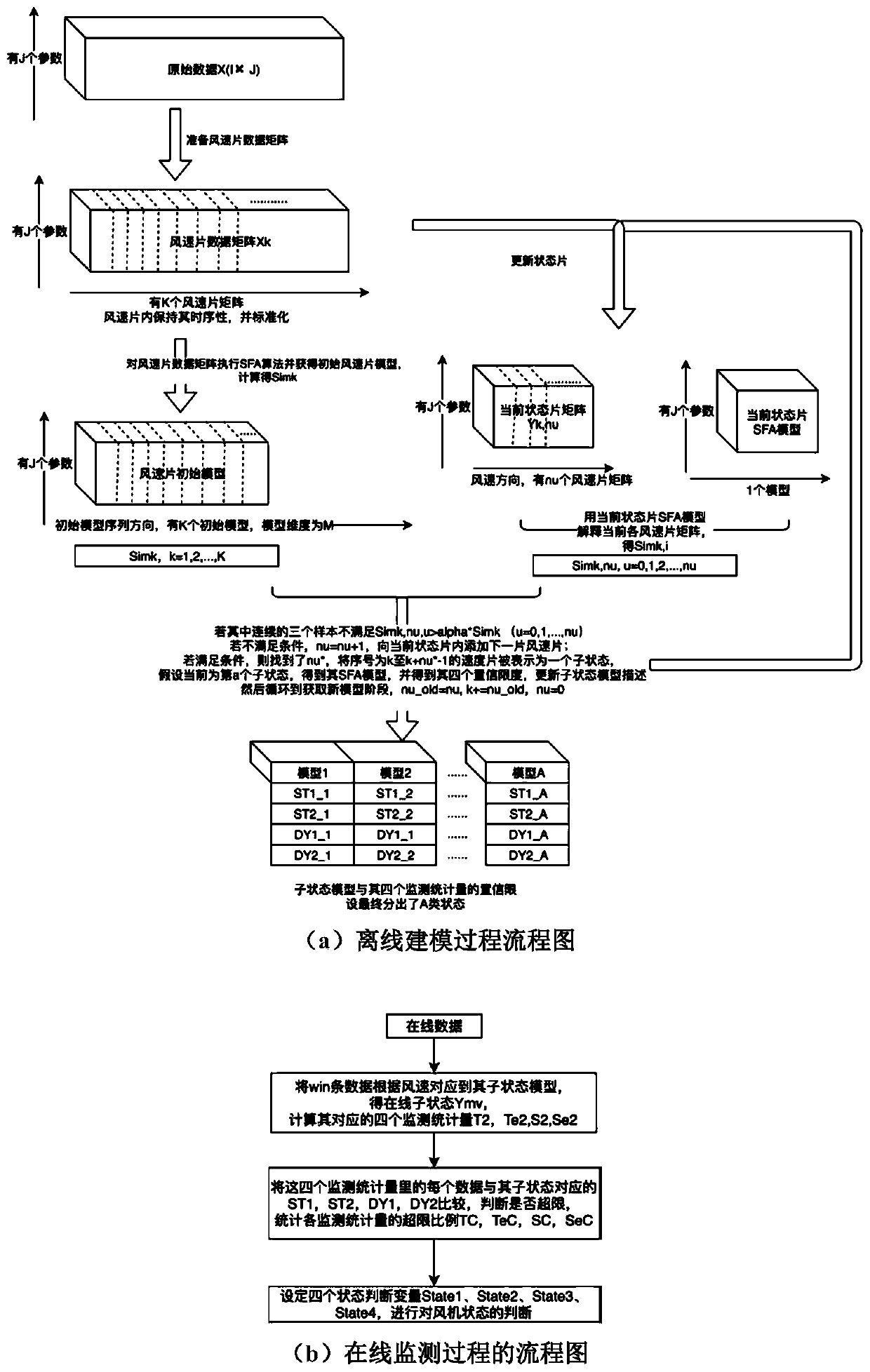
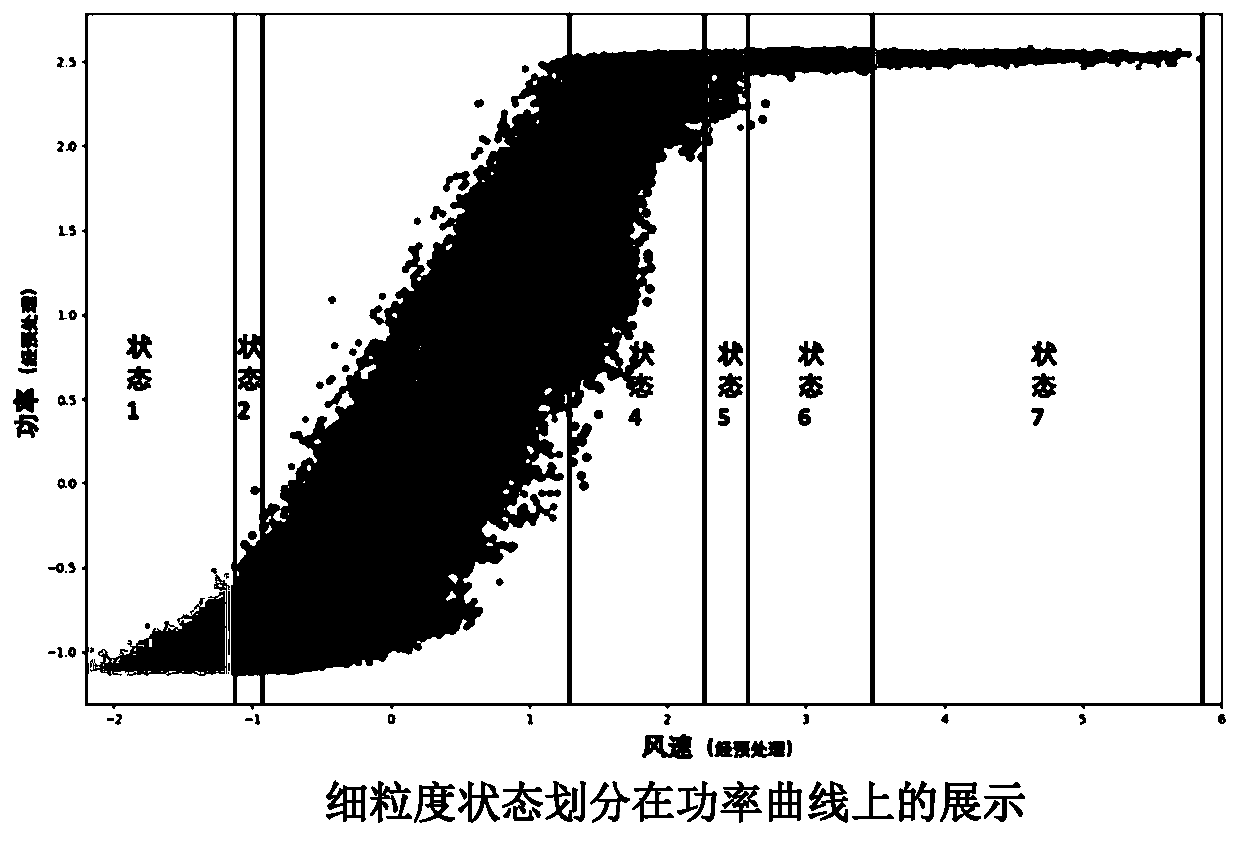
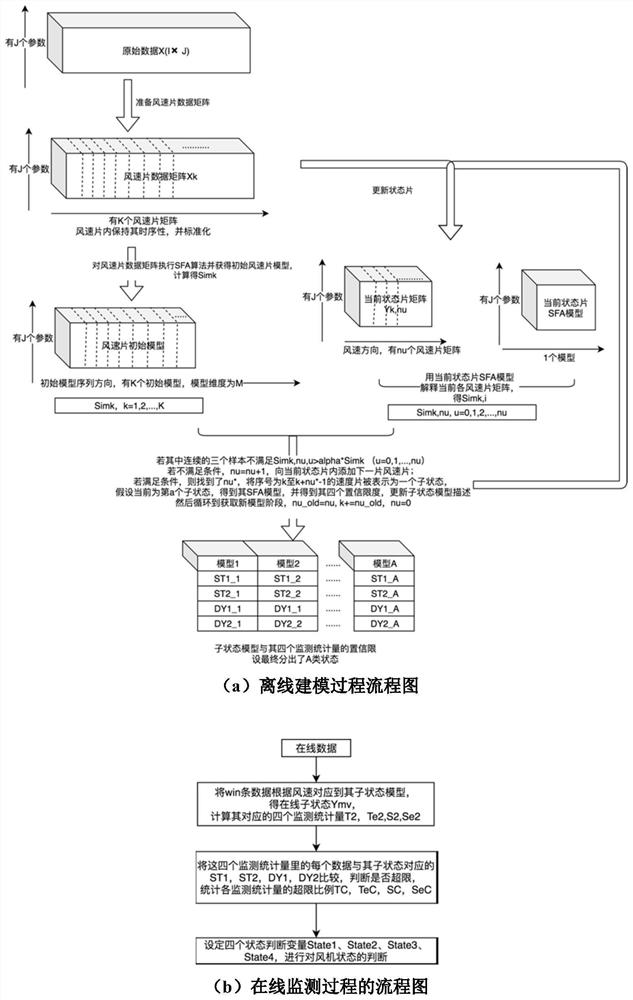
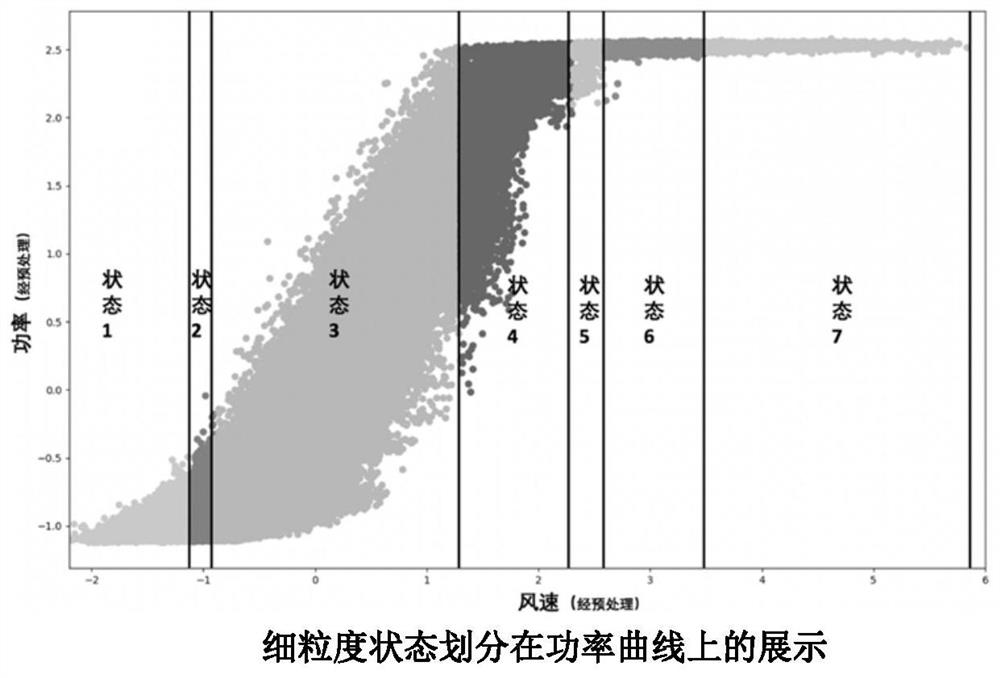
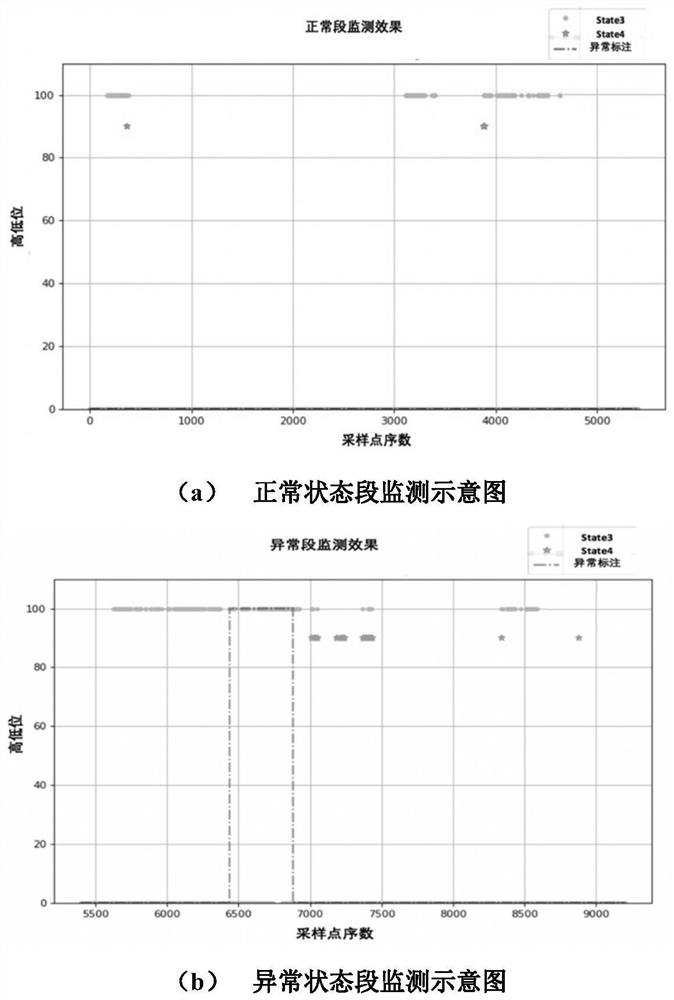
ActiveCN110222393AImprove utilization efficiencyComplete monitoringDesign optimisation/simulationComplex mathematical operationsEngineeringFan blade
The invention discloses a fan blade icing abnormity detection method based on fine-grained wind power generation state division. The fine-grained state division modeling method based on slow feature extraction is provided in a targeted manner after the segmentation characteristic of each variable relationship during variable-working-condition operation of a fan is revealed. Aiming at the dynamic change characteristic of wind power generation, the invention provides a dynamic and static collaborative monitoring method. Data collected by the wind field SCADA system are utilized, the monitoring model of each sub-state is established for the fan by using the method, and the effect of detecting the abnormal output of the fan by using the method provided by the invention is verified offline. Thedynamic characteristic of data when the wind turbine runs is fully utilized, the detection effect is effectively improved, timely diagnosis and processing of the icing condition of the blades by windfield maintenance personnel are facilitated, and therefore normal and stable running of the wind turbine generator set is guaranteed, and meanwhile the safety guarantee coefficient of personnel and property is improved.
Owner:ZHEJIANG UNIV
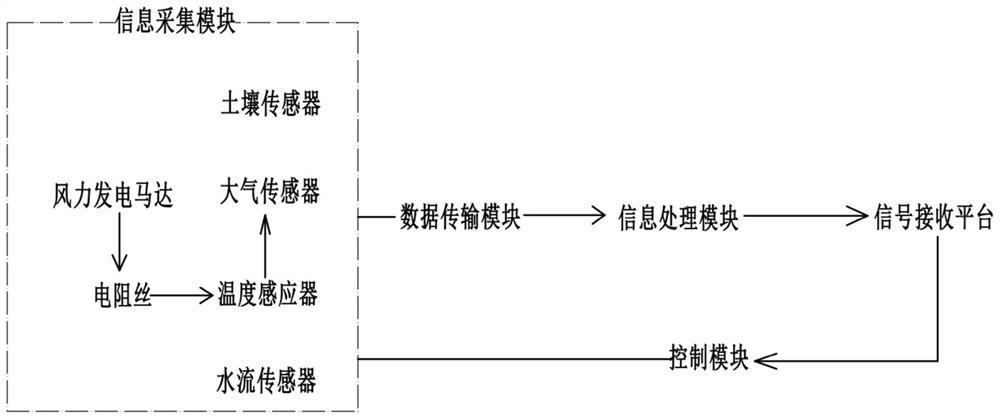
System for monitoring quality evaluation of ecological environment
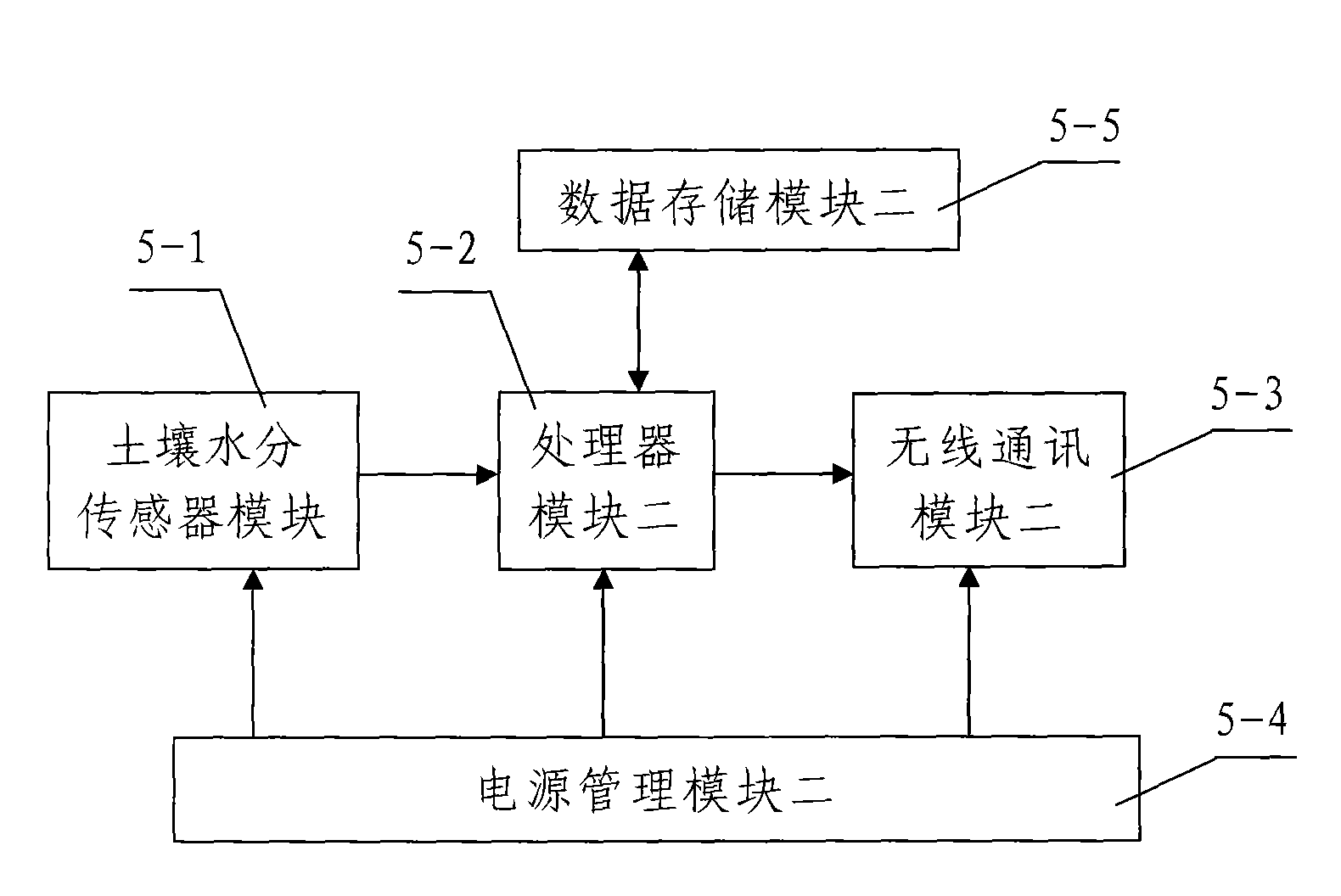
PendingCN113032971AReduce the impact of errorsAchieve captureGeneral water supply conservationForecastingParticulatesInformation processing
The invention discloses a system for monitoring quality evaluation of an ecological environment in the field of environment monitoring. The system comprises an information acquisition module, a data transmission module, an information processing module, a control module and a signal receiving platform, The information acquisition module comprises a soil sensor for acquiring soil information, an atmosphere sensor for acquiring atmosphere information and a water flow sensor for acquiring water quality, the atmosphere sensor comprises a particulate matter sensor and a wind power generation motor, the wind power generation motor is connected with a resistance wire, a temperature sensor is mounted on one side of the resistance wire, and a temperature sensor is mounted on the other side of the resistance wire; And the temperature sensor is connected in parallel with the particulate matter sensor, so that data of the temperature sensor and the particulate matter sensor are synchronously transmitted to the information processing module. According to the technical scheme, heat generated by the resistance wire is captured and then is synchronously transmitted with a signal of the particulate matter sensor, so that the wind power is captured and sensed by the heating temperature of the resistance wire, and wind power data is provided to assist correction and particulate matter content measurement.
Owner:广西壮族自治区生态环境监测中心 +1
Lawn environment parameter data acquisition system based on lawn lamps
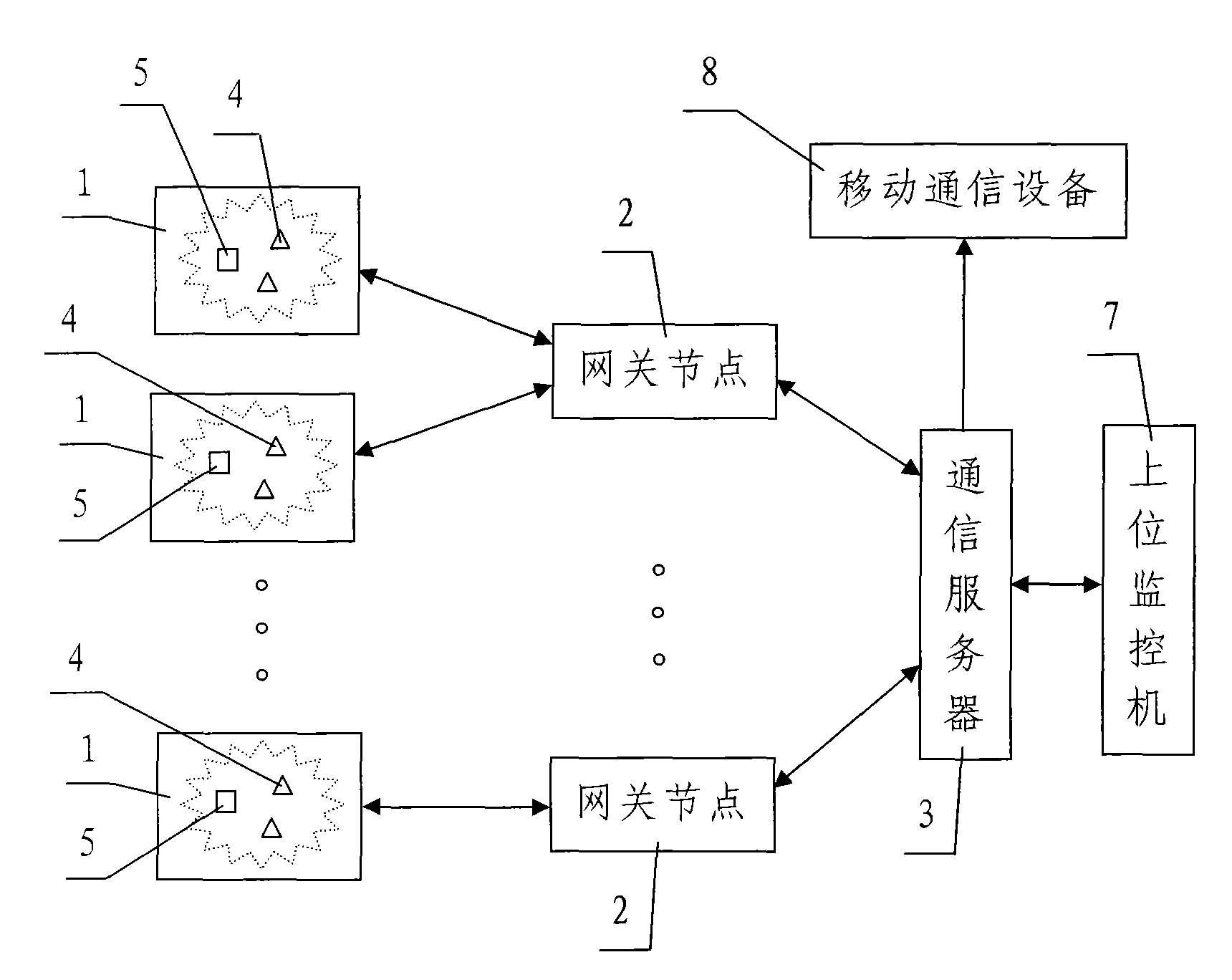
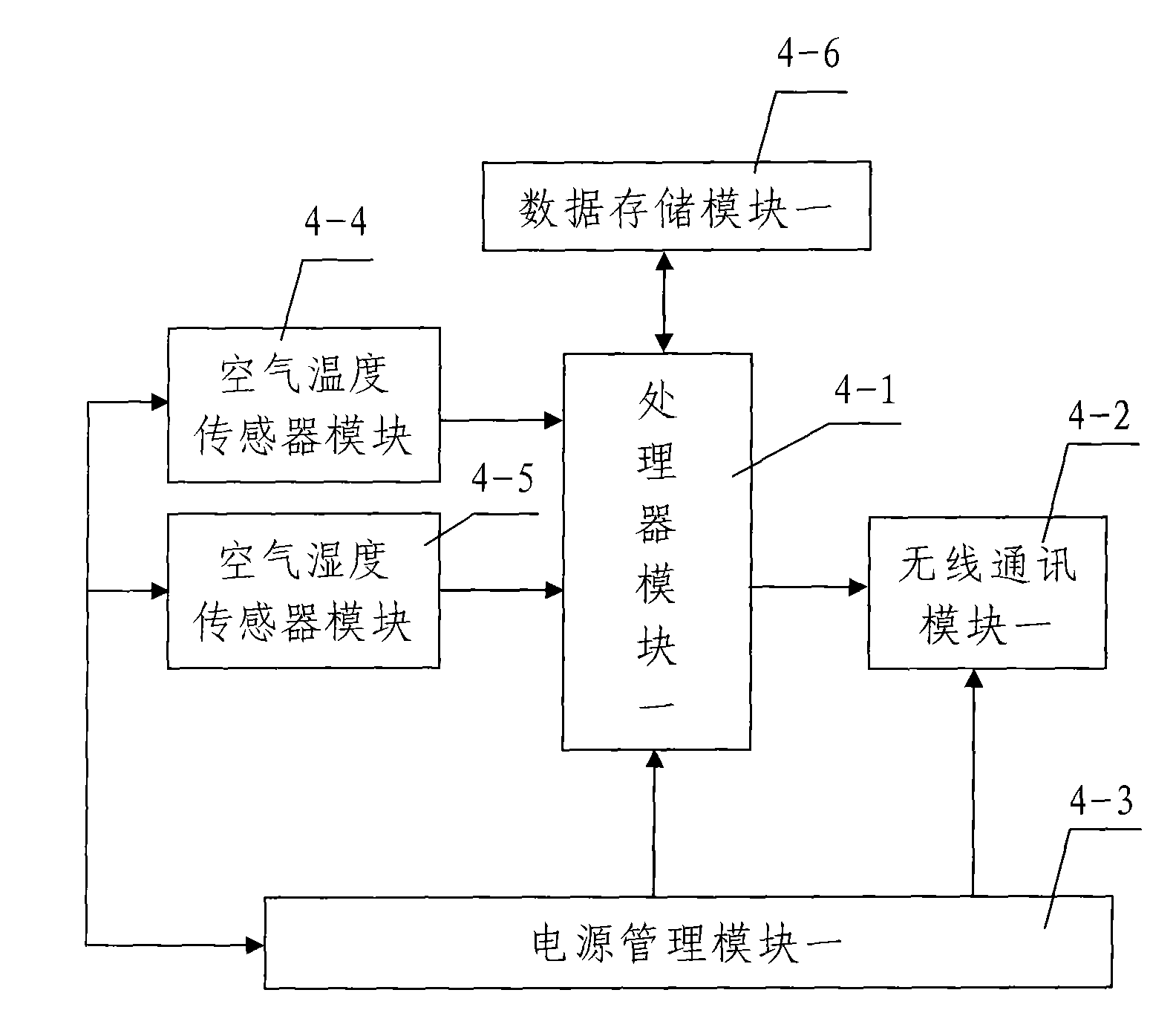
InactiveCN102054343AReasonable designLow costTransmission systemsNetwork topologiesWireless mesh networkSoil moisture sensor
The invention discloses a lawn environment parameter data acquisition system based on lawn lamps. The system comprises a plurality of wireless sensor networks comprising a plurality of wireless sensor nodes arranged in a monitored lawn, gateway nodes, a communication server connected with the gateway nodes and an upper monitor device connected with the communication server, wherein, the plurality of wireless sensor networks are divided into different areas which are respectively arranged in a plurality of power supply areas in the monitored lawn, a lawn lamp is correspondingly arranged in each power supply area, the wires sensor nodes comprise a plurality of air humiture sensor nodes and a plurality of soil moisture sensor nodes, and a plurality of wireless sensor nodes in the wireless sensor network in each power supply area are powered by the power supply circuit of the lawn lamp in the power supply area. The lawn environment parameter data acquisition system has the advantages of reasonable design, low cost, convenient power supply, good monitoring effect, and quick, accurate and comprehensive data acquisition, is convenient and easy to operate, and can achieve the purpose of completely and effectively monitoring the entire lawn.
Owner:XIAN CENTN TECH
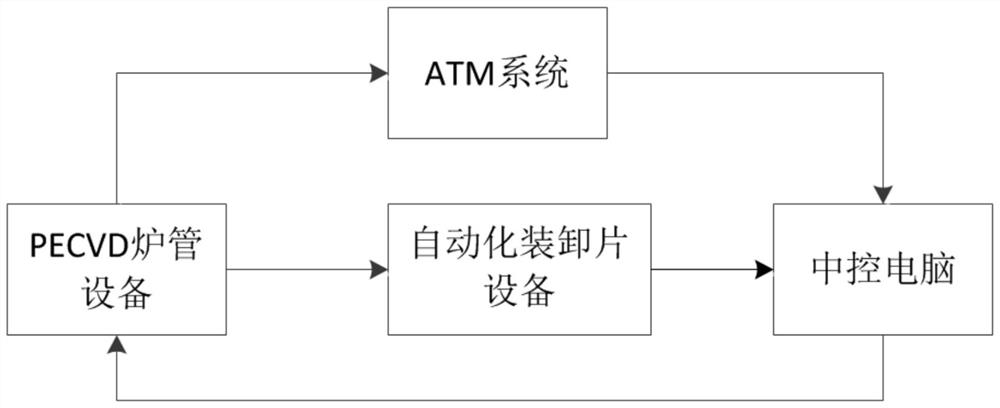
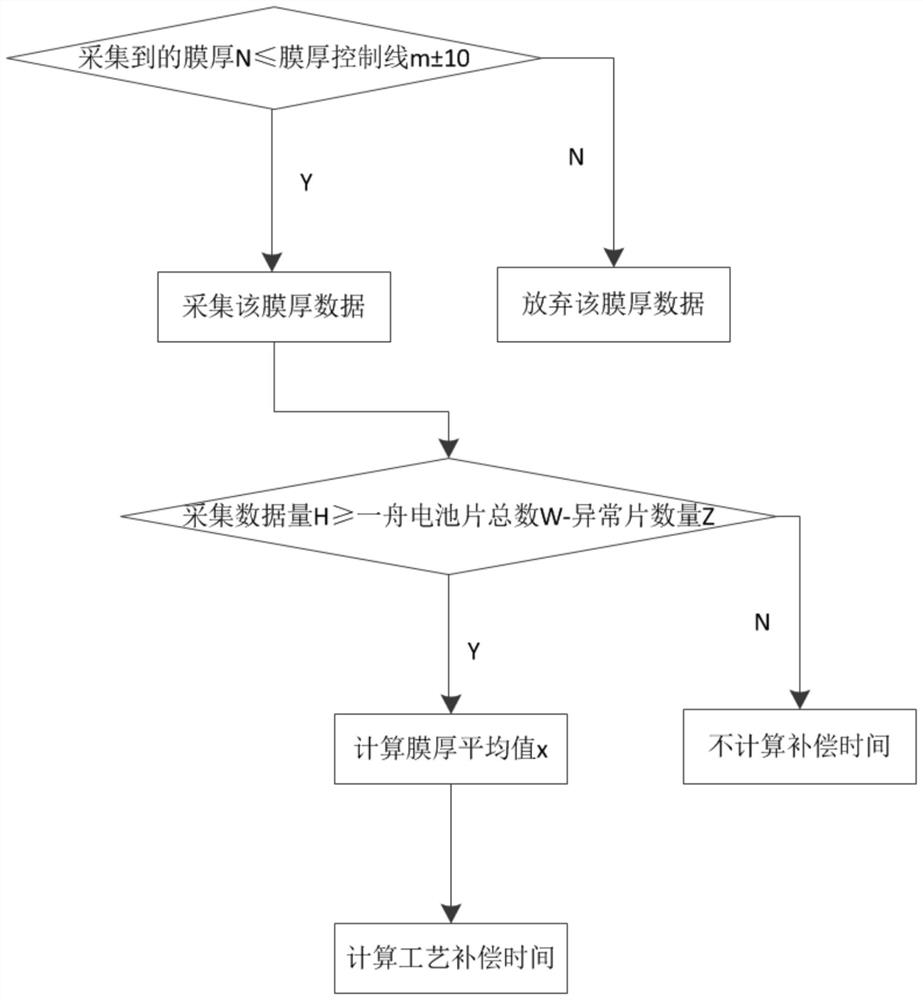
PECVD film thickness automatic statistical compensation system
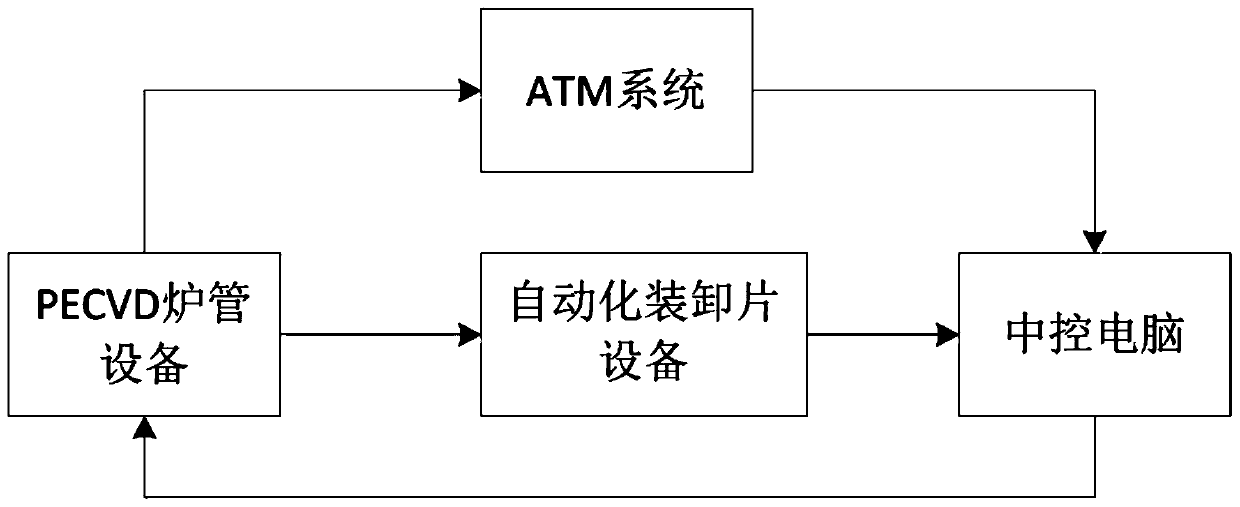
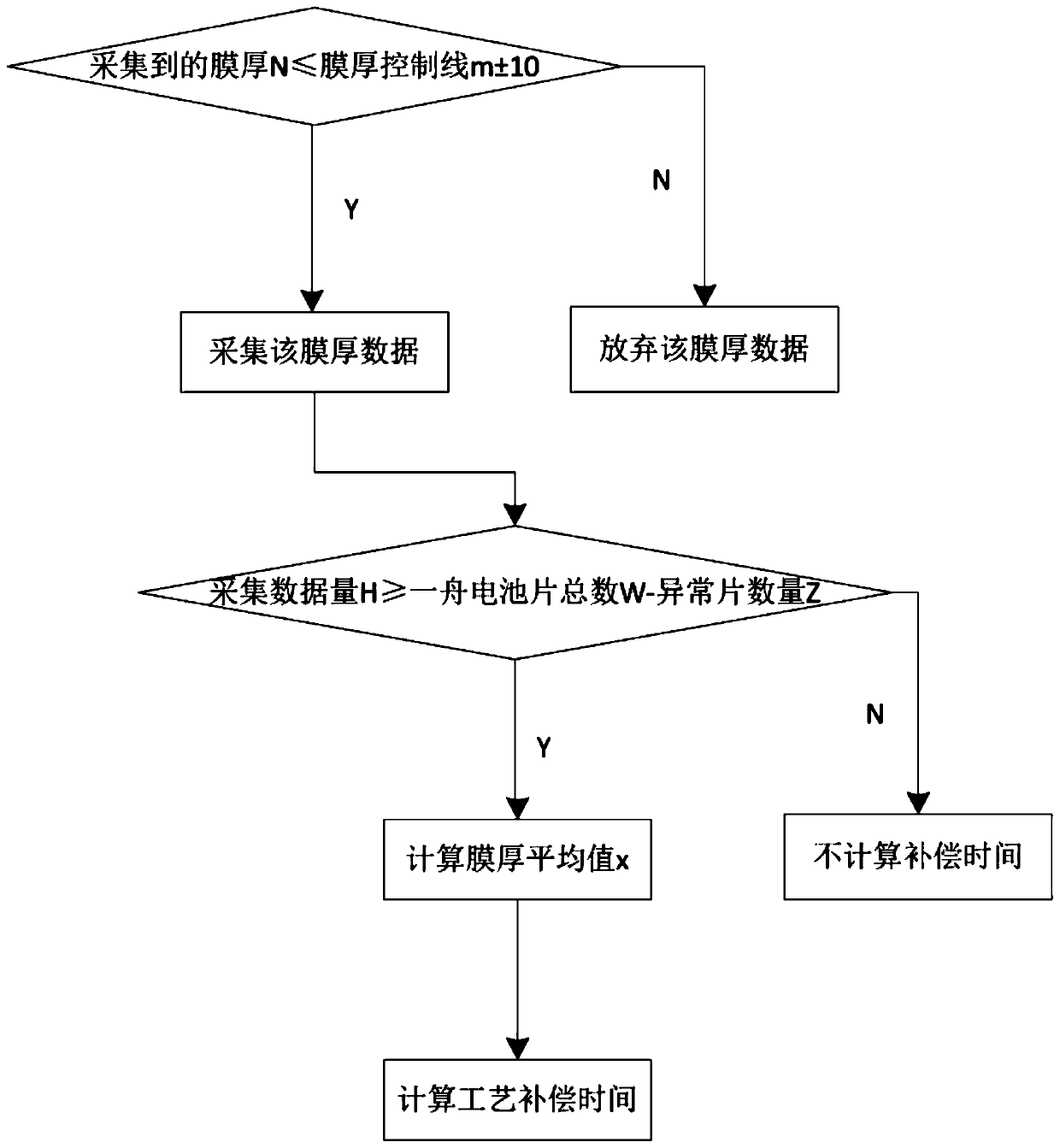
ActiveCN109817555AComplete monitoring of complete film thickness changesComplete complete monitoringFinal product manufactureSemiconductor/solid-state device manufacturingEngineeringSilicon solar cell
The invention discloses a PECVD film thickness automatic statistical compensation system, belongs to the field of silicon solar cell manufacturing, and aims to solve the problem of high reject ratio of products caused by difficulty in timely and effectively monitoring the film thickness in a PECVD (Plasma Enhanced Chemical Vapor Deposition) process in the prior art. The PECVD film thickness automatic statistical compensation system comprises PECVD furnace tube equipment and automatic sheet loading and unloading equipment, and is characterized by further comprising an ATM system and a centralcontrol computer, wherein the ATM system is used for acquiring solar cell film thickness data transmitted between the PECVD furnace tube equipment and the automatic sheet loading and unloading equipment and transmitting the acquired film thickness data to the central control computer; and the central control computer is used for calculating the compensation time required by a solar cell coating process in the PECVD furnace tube equipment and sending the time to the PECVD furnace tube equipment for process compensation. According to the invention, the film thickness data is measured in time, the complete monitoring of the change of the film thickness of the solar cell is completed, the process time can be automatically adjusted, and the overall finished product quality of the solar cell isimproved.
Owner:TONGWEI SOLAR ENERGY CHENGDU CO LID
Virtual resource transfer method, client device, application server, and system
ActiveUS11182844B2Complete monitoringFulfil requirementsResource allocationBuying/selling/leasing transactionsApplication serverResource transfer
The present disclosure discloses a virtual resource transfer method, including: responding to a first login request from a first client for logging into a public service account; receiving a service request initiated by the first client; responding to a second login request from a second client for logging into the public service account; sending information about the service request of the first client to the second client; receiving service order information for the service request from the second client; generating, by an application server associated with the public service account, a virtual resource transfer request according to the service order information; sending the virtual resource transfer request to the first client by using the public service account, the virtual resource transfer request being configured for transferring, from the first client, a virtual resource in a first virtual resource account to a second virtual resource account.
Owner:TENCENT TECH (SHENZHEN) CO LTD
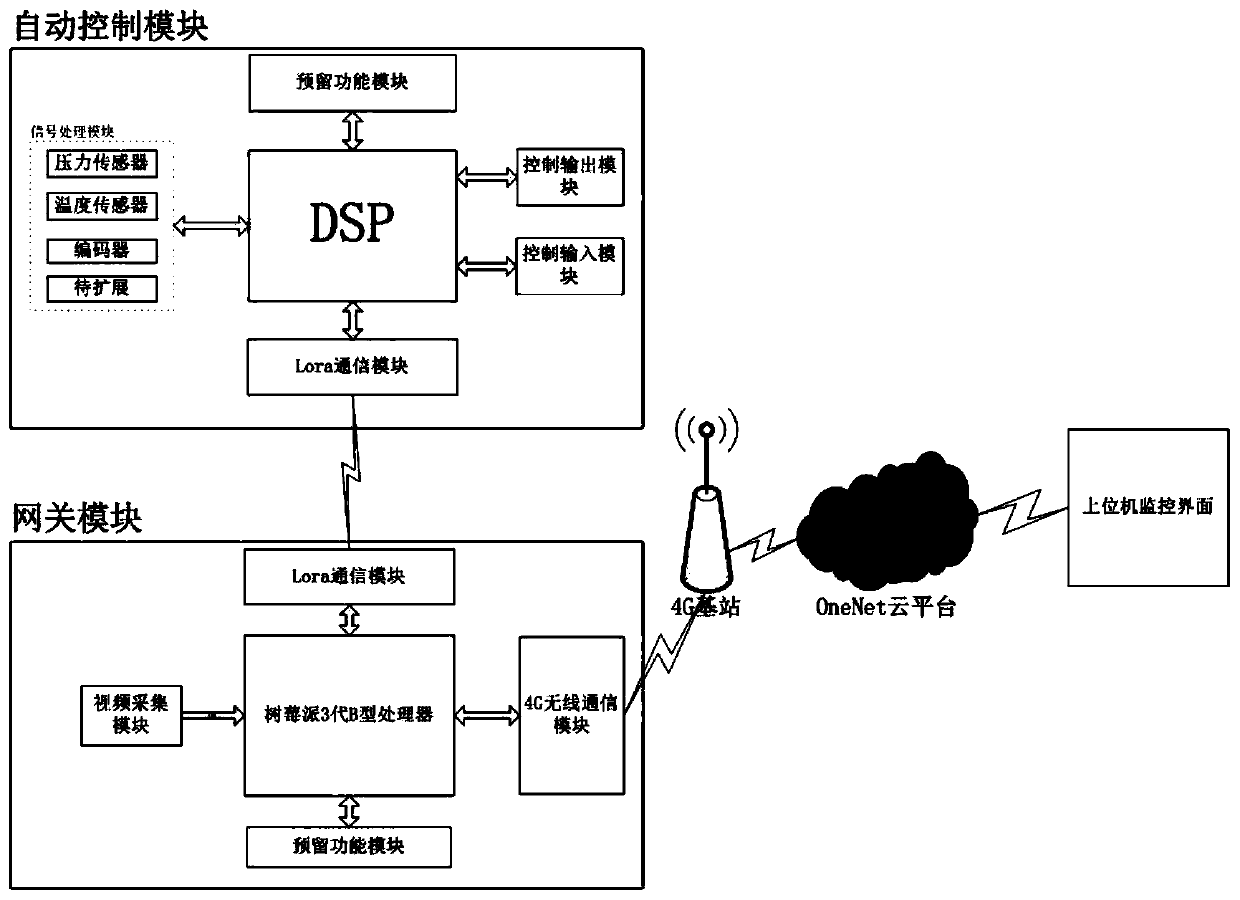
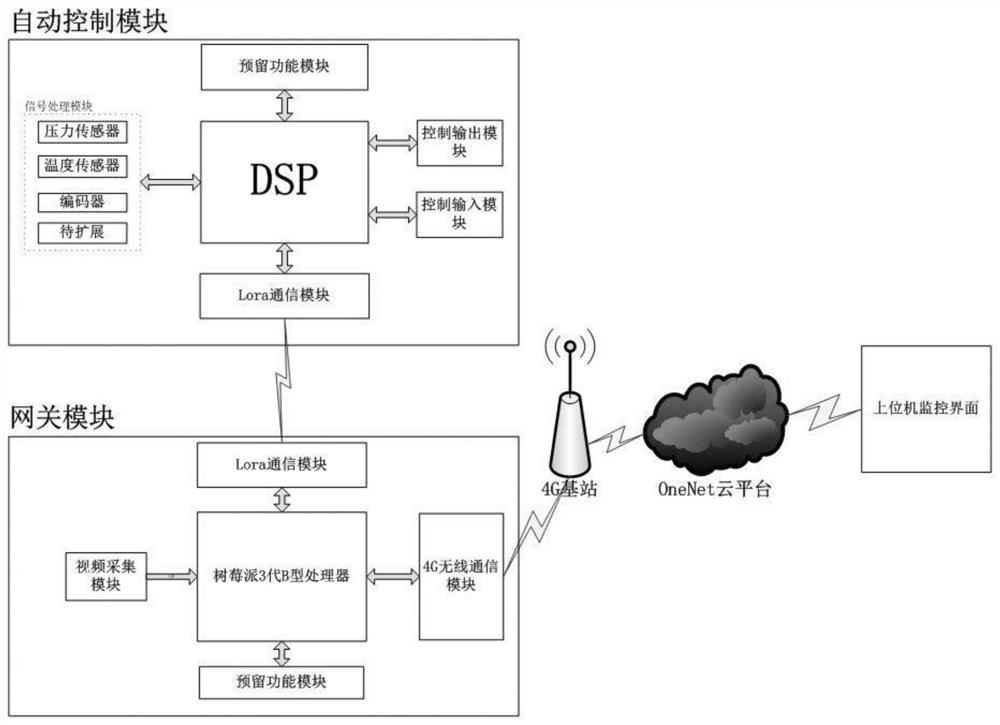
Ship engine monitoring system
ActiveCN110242427AWith signal processing functionEasy to controlEngine controllersMachines/enginesAutomatic controlMonitoring system
A ship engine monitoring system is provided. The invention relates to the ship engine monitoring system. Two-way signal transmission is achieved between a DSP, and a reserved functional module, a signal processing module, an Lora communication module I, a control input module and a control output module; a signal is transmitted wirelessly between the Lora communication module I and an Lora communication module II; after the Lora communication module II receives the signal, the signal is transmitted to a Raspberry Pi 3-generation B type processor; the Raspberry Pi 3-generation B type processor also transmits the signal to the Lora communication module II; the Raspberry Pi 3-generation B type processor further receives a signal of a video processing module; and two-way signal transmission is achieved between the Raspberry Pi 3-generation B type processor, and a 4G wireless communication module and the reserved functional module. The ship engine monitoring system achieves automatic control and parameter monitoring on ship engines.
Owner:JILIN UNIV
An Automatic Statistical Compensation System for PECVD Film Thickness
ActiveCN109817555BComplete monitoring of complete film thickness changesComplete complete monitoringFinal product manufactureSemiconductor/solid-state device manufacturingPhysical chemistryEngineering
The invention discloses an automatic statistical compensation system for PECVD film thickness, which belongs to the field of silicon solar cell manufacturing. It includes PECVD furnace tube equipment and automatic loading and unloading equipment, and is characterized in that it also includes an ATM system and a central control computer; the ATM system is used to collect solar cell film thickness data transported between PECVD furnace tube equipment and automatic loading and unloading equipment, And transmit the collected film thickness data to the central control computer; the central control computer is used to calculate the compensation time required for the solar cell coating process in the PECVD furnace tube equipment and send the time to the PECVD furnace tube equipment for the process compensate. The invention completes the complete monitoring of the change of the film thickness of the battery sheet by timely measuring the film thickness data, and can automatically adjust the process time, thereby improving the overall finished product quality of the solar battery sheet.
Owner:TONGWEI SOLAR ENERGY (CHENGDU) CO LID
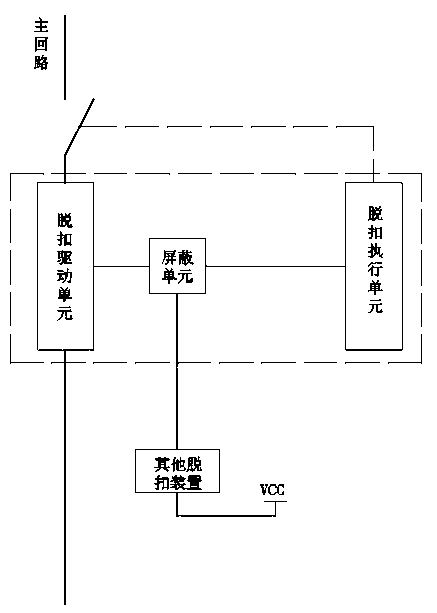
Tripping device, breaker and defencive function extension method of breaker
ActiveCN102201302BEnsure safetyComplete monitoringProtective switch operating/release mechanismsControl theoryCircuit breaker
The invention discloses a tripping device applied to a low-voltage circuit breaker. The tripping device comprises a first tripping executing unit and a first tripping driving unit. The first tripping driving unit is electrically connected with the first tripping executing unit; and a circuit formed by the first tripping driving unit and the first tripping executing unit is connected with a shielding unit, and the shielding unit can be controlled by an external signal to make the circuit be switched off or switched on. The invention further discloses a circuit breaker comprising the tripping device disclosed by the invention and a protection function expansion method of the circuit breaker. Compared with the prior art, the invention can realize multiple protection without interference one another.
Owner:CHANGSHU SWITCHGEAR MFG CO LTD (FORMER CHANGSHU SWITCHGEAR PLANT)
Solar campus track data collection intelligent brick
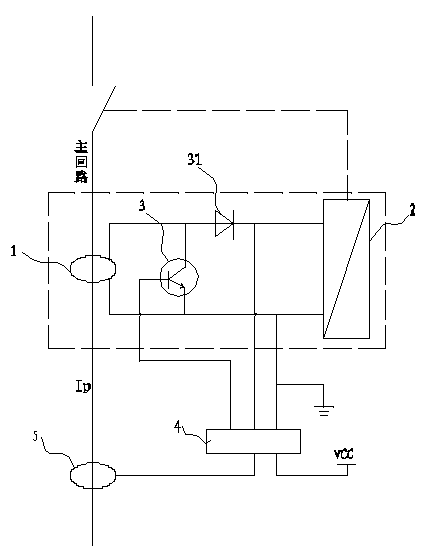
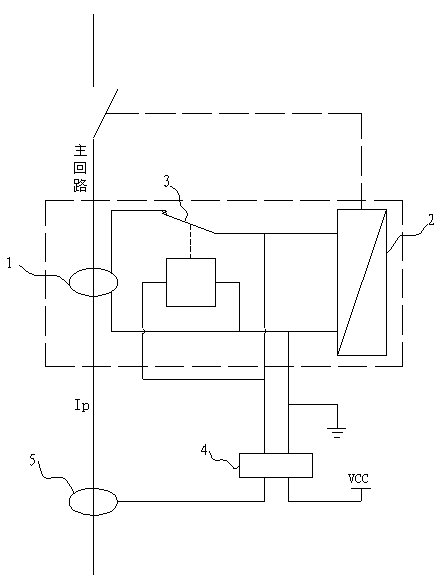
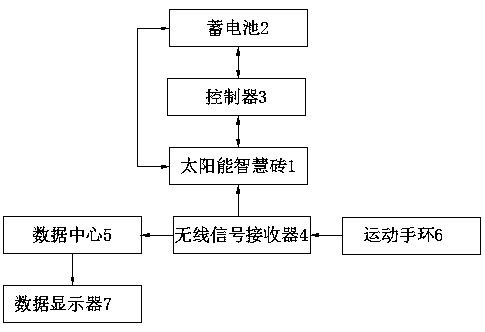
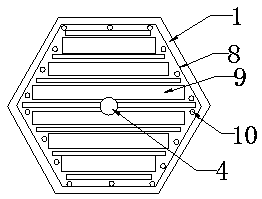
PendingCN107899232ASolve the problem of inaccurate monitoring resultsComplete monitoringSki bindingsBatteries circuit arrangementsElectric energyCollections data
The invention discloses a solar campus track data collection intelligent brick, which comprises a solar intelligent brick and an exercise wristband, wherein the solar intelligent brick can generate powder and store the electric energy in a storage battery which is connected with the solar intelligent brick through a wire and deep buried in a track, a controller is further connected between the storage battery and the solar intelligent brick through wires, a wireless signal receiver arranged in the solar intelligent brick can receive wireless signals emitted by the exercise wristband worn on the wrist of a student and send the wireless signals to a data center in a wireless transmission mode, and the data center can send the information to a data displayer in a wireless sending mode. The solar campus track data collection intelligent brick disclosed by the invention can absorb solar energy to generate power through the solar intelligent brick and can provide a landscape brightening andindicating functions at night; meanwhile, when the student wears the exercise wristband, running grades of the student can be recorded, the physical fitness of the student can be analyzed, the physical function operation situation can be completely monitored, no labor is wasted, and results are accurate.
Owner:地洲新能源科技(上海)有限公司
Method and device for monitoring a drive of a drive system of a motor vehicle
ActiveCN102529968BSave complex calculationsComplete monitoringElectrical controlRoad transportDrive motorLinear distance
The method involves determining whether a motor car (1) is in an overrun condition, where a drive of the motor car does not provide drive torque in the overrun condition. A temporal speed threshold value characteristic is determined based on a momentary vehicle speed and a preset detention of the motor car on a linear distance when the overrun condition is determined. An error is determined when the momentary vehicle speed present during the overrun condition exceeds a threshold value, which is predetermined by the threshold value characteristic. Independent claims are also included for the following: (1) a monitoring device for monitoring a drive system of a vehicle (2) a drive system of a vehicle comprising a drive motor (3) a computer program product comprising instructions for performing a method for monitoring a drive system of a vehicle on errors.
Owner:ROBERT BOSCH GMBH
A ship engine monitoring system
ActiveCN110242427BWith signal processing functionEasy to controlEngine controllersMachines/enginesWireless transmissionAutomatic control
A ship engine monitoring system is provided. The invention relates to the ship engine monitoring system. Two-way signal transmission is achieved between a DSP, and a reserved functional module, a signal processing module, an Lora communication module I, a control input module and a control output module; a signal is transmitted wirelessly between the Lora communication module I and an Lora communication module II; after the Lora communication module II receives the signal, the signal is transmitted to a Raspberry Pi 3-generation B type processor; the Raspberry Pi 3-generation B type processor also transmits the signal to the Lora communication module II; the Raspberry Pi 3-generation B type processor further receives a signal of a video processing module; and two-way signal transmission is achieved between the Raspberry Pi 3-generation B type processor, and a 4G wireless communication module and the reserved functional module. The ship engine monitoring system achieves automatic control and parameter monitoring on ship engines.
Owner:JILIN UNIV
A method for processing terminal application behavior reflection
ActiveCN110362301BComplete monitoringFlexible monitoringModel driven codeCode compilationAlgorithmTheoretical computer science
The present invention discloses a processing method for terminal application behavior reflection. Through the behavior interpreter, a complete, accurate and detailed self-statement of application behavior is generated, that is, the runtime model of terminal application application behavior, which overcomes the dynamic and multiple problems of the prior art. Insufficient in the variable and difficult-to-control application runtime, to realize the flexible and complete monitoring of the application behavior of the terminal application, and then based on the generated runtime model, define the operations on the runtime model and the influence of model fragments in the heap and stack areas Equivalence, realizes the decomposition of complex application behavior models, operable model fragments, and based on the decomposed model fragments, establishes causal associations between behavior models, application states, and application codes, and implements the application of terminal applications when they are running. Instruction-level control of behavior.
Owner:PEKING UNIV
An abnormal monitoring method for fan blade icing based on fine-grained wind power generation state division
ActiveCN110222393BImprove utilization efficiencyComplete monitoringDesign optimisation/simulationComplex mathematical operationsFan bladeReliability engineering
The invention discloses a method for detecting abnormal icing of fan blades based on fine-grained wind power generation status division. After revealing the segmental characteristics of the relationship between variables in the variable operating conditions of the wind turbine, a fine-grained state partition modeling method based on slow feature extraction is proposed. Aiming at the dynamic characteristics of wind power generation, a dynamic and static monitoring method is proposed. Using the data collected by the SCADA system of the wind farm, the above method is used to establish the monitoring model of each sub-state of the wind turbine, and the effect of the method proposed in this paper to detect the abnormal output of the wind turbine is verified offline. It makes full use of the dynamic characteristics of the data when the wind turbine is running, effectively improves the detection effect, and helps the wind farm maintenance personnel to diagnose and deal with the blade icing in a timely manner, thus ensuring the normal and stable operation of the wind turbine. The safety factor of personnel and property has been improved.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com