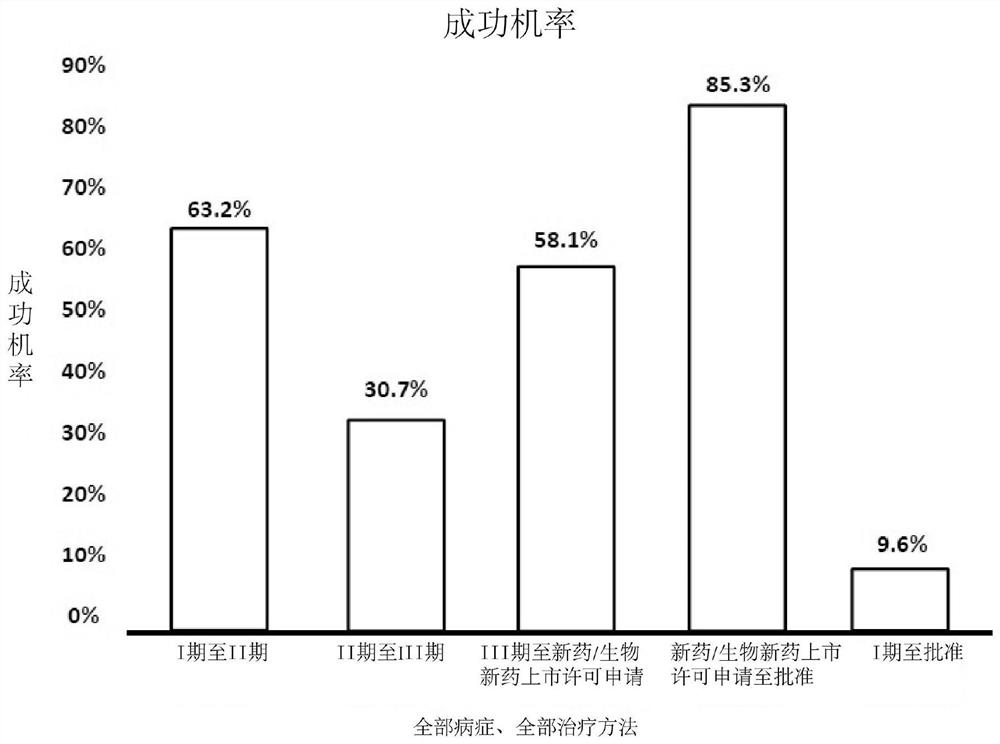
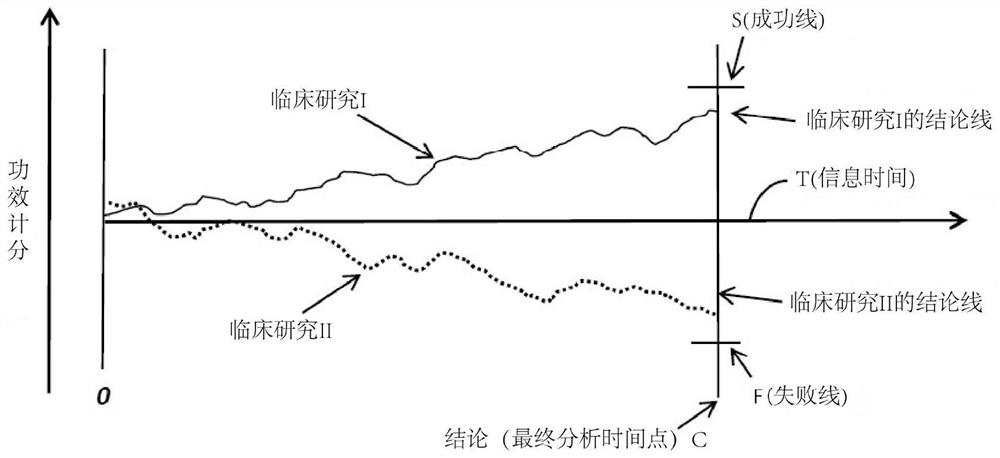
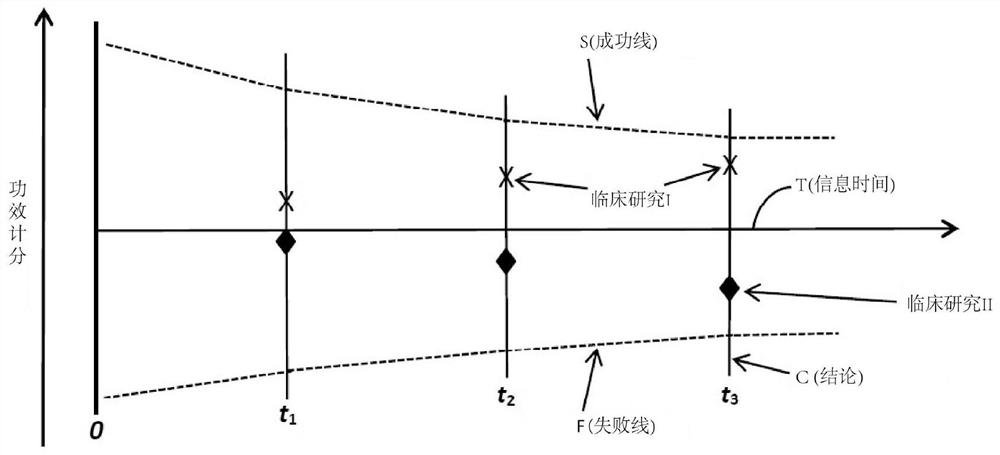
Systems, methods and processes for dynamic data monitoring and real-time optimization of ongoing clinical research trials
A technology for clinical trials and dynamic monitoring, applied in the input/output process of data processing, electronic clinical trials, electronic digital data processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] initial design
[0215] Assuming that θ is the experimental treatment effect, depending on the type of research data, its value may be the difference in means, odds ratio, risk ratio, etc. In the initial design of the experiment, the number of samples in each group is N 0 , the significance level is α and the expected statistical test power, the hypothesis test is carried out, the null hypothesis is that the treatment is ineffective, and the opposite hypothesis is that the treatment is effective (H 0 :θ=0versusH A :θ>0). Considering that the experiment is randomly assigned and the main indicators obey the assumption of normal distribution, the effect of the experimental group is X E subject to mean value μ E , the variance is The normal distribution of , then the effect of the control group is The experimental power is the difference between the two means θ = μ E -μ C . Estimates for other indicators can be obtained using the assumption of approaching norma...
Embodiment 2
[0244] Considering DAD / DDM of SSR: When the sample size is re-estimated
[0245] Condition test power is being calculated Useful at times, but not very useful in determining the timing of an SSR at an interim analysis. when Approaches , the equation (1) in by Bringing in, that is, when the cumulative number of people is as expected as the number of samples, there are two probabilities for the conditional test power, one is close to 0 (when close to C, but less than C), or close to 1 (when Approaching C, but greater than C)). When deciding on an SSR, Stability also needs to be considered. because when when increasing will be more stable. When the observed value equal When, the test verification force can be provided as additional information for , and when It will be more stable as it increases. However, if an adjustment is required, the later the SSR is performed, the less willing and feasible it is to adjust the sample size. Because "operating ...
Embodiment 3
[0266] DAD / DDM Considering Early Efficacy and Type I Error Rate Control
[0267] DAD / DDM is a method based on the seminal theory proposed by Lan, Rosenberger and Lachin (1993), aiming at the use of continuous monitoring in the early stage of the trial, and then seeing significant efficacy. DAD / DDM use alpha continuous cost function 0 will reject the null hypothesis.
[0268] Perform SSR after early efficacy monitoring with cohort sequence boundaries in the design and a final boundary value of C g , the second part of Example 1 discusses the formula for adjusting the final test threshold. For DAD / DDM with continuous monitoring, C g is 2.24.
[0269] On the other hand, if after performing SSR (either or CP mTR ) for continuous monitoring of efficacy, then the above alpha costs the z of the function α(t) 1-α / 2 The quantile should be adjusted to C of equation (3) 1 . Therefore, the bounds of the Z value will be adjusted to The information score t will be based on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com