Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "WNT5A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protein Wnt-5a is a protein that in humans is encoded by the WNT5A gene.
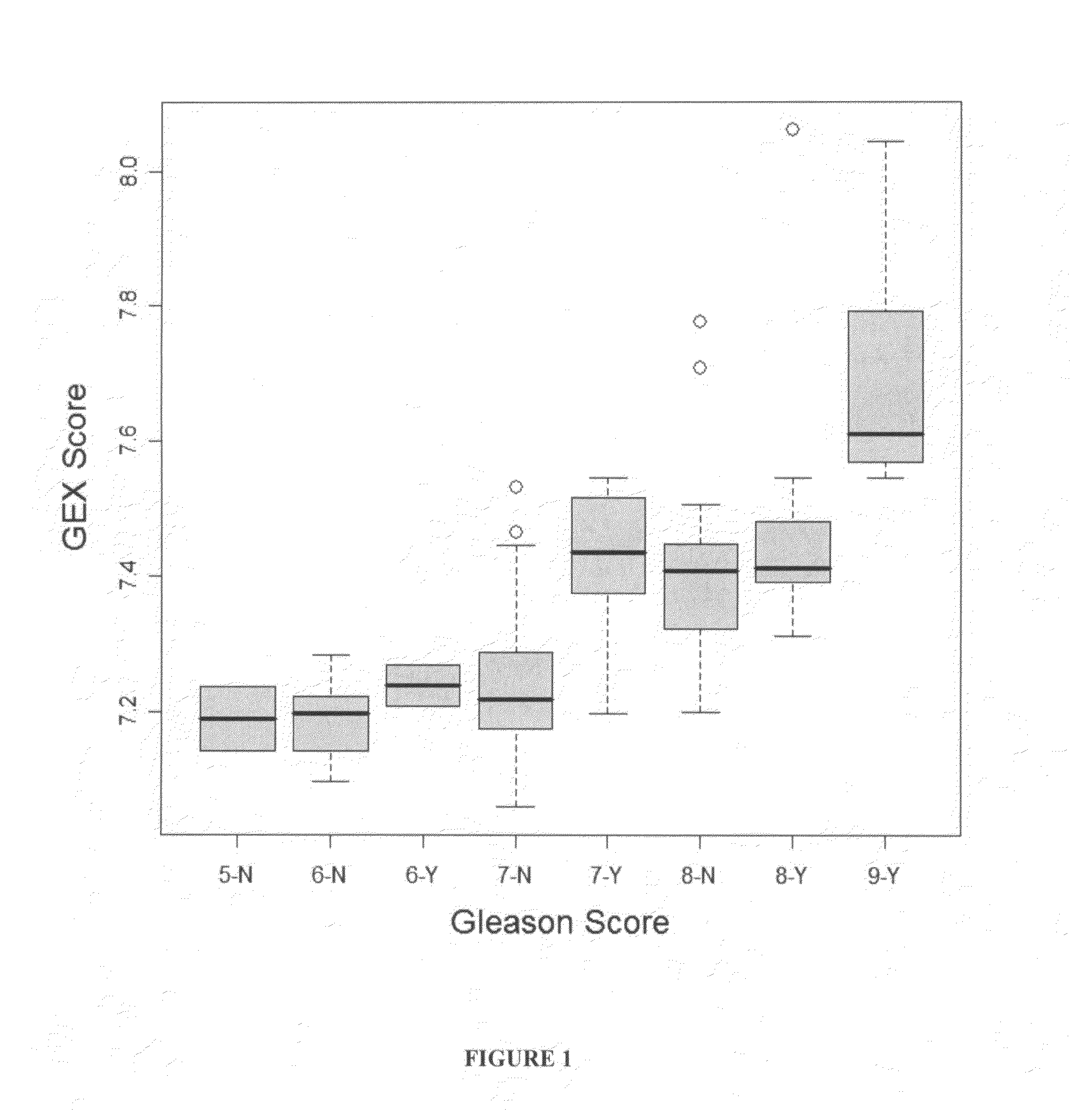
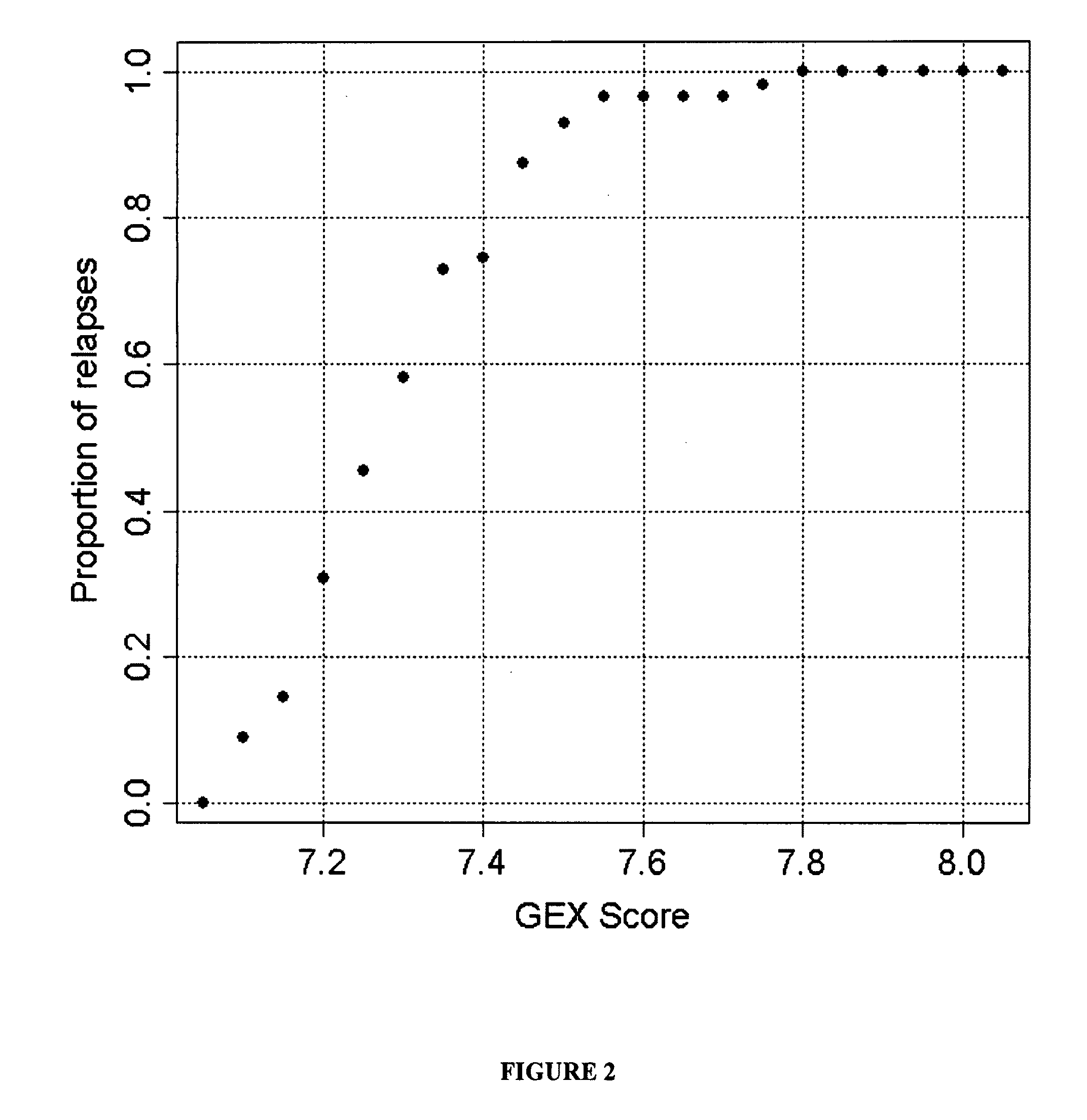
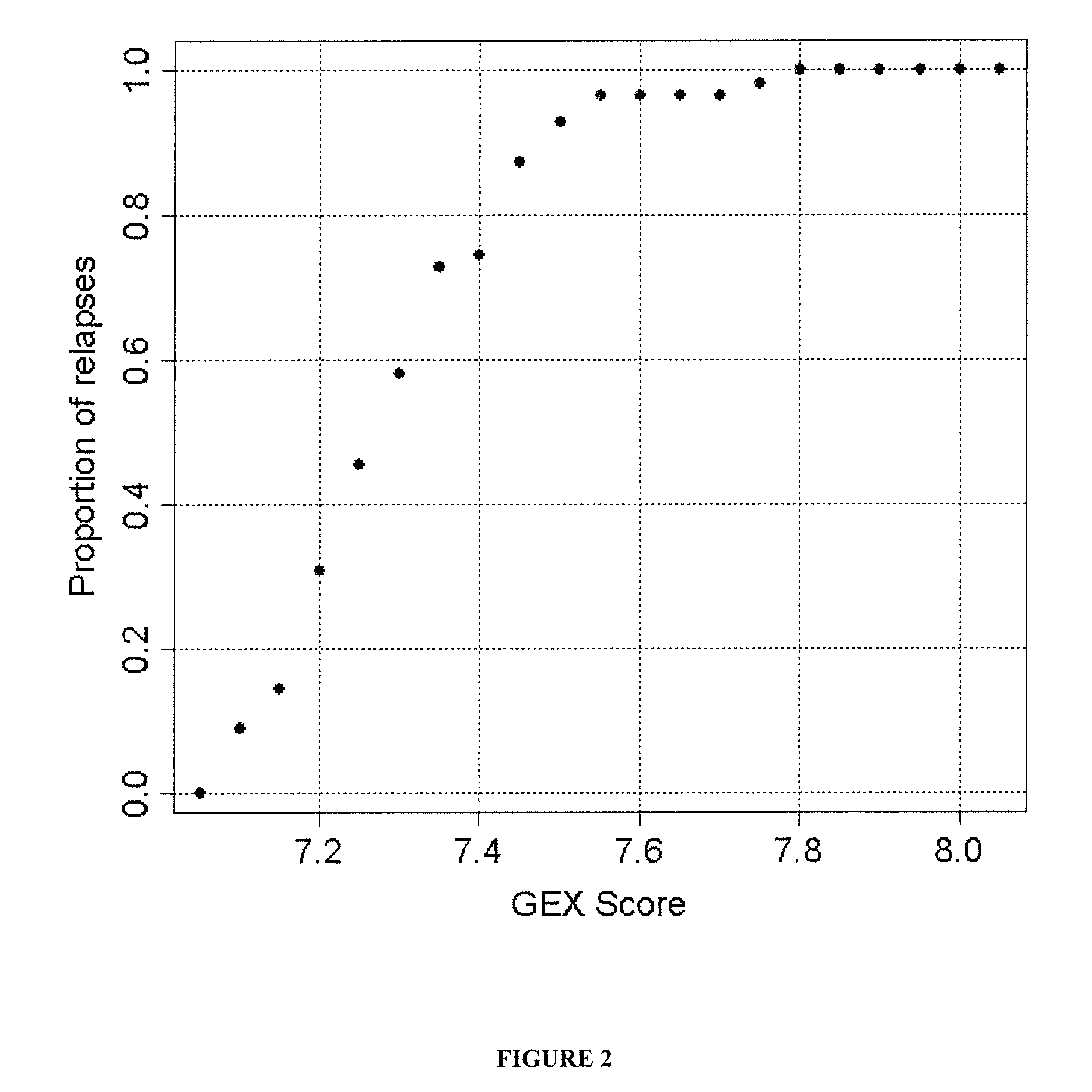
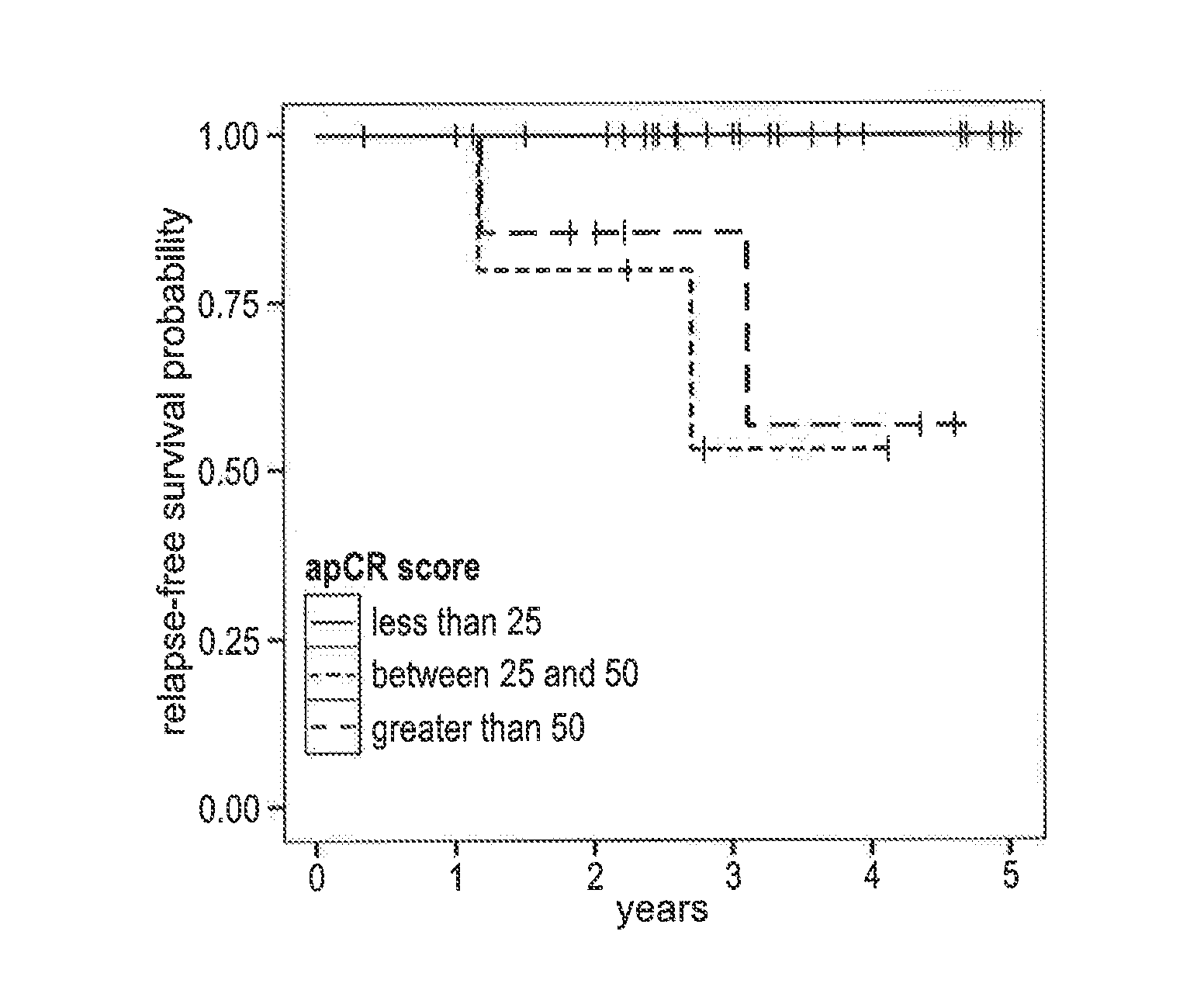
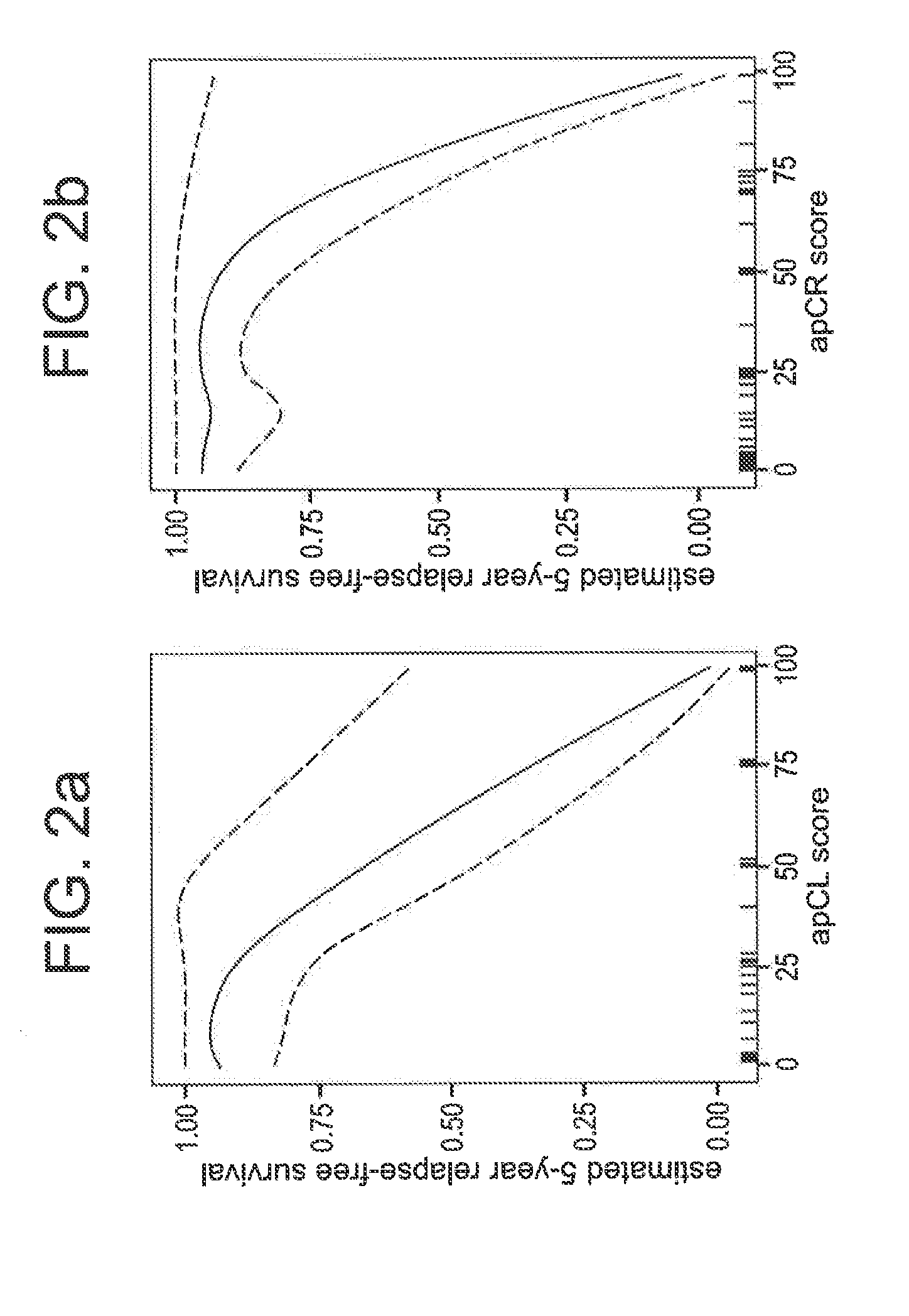
Gene expression profiles to predict relapse of prostate cancer
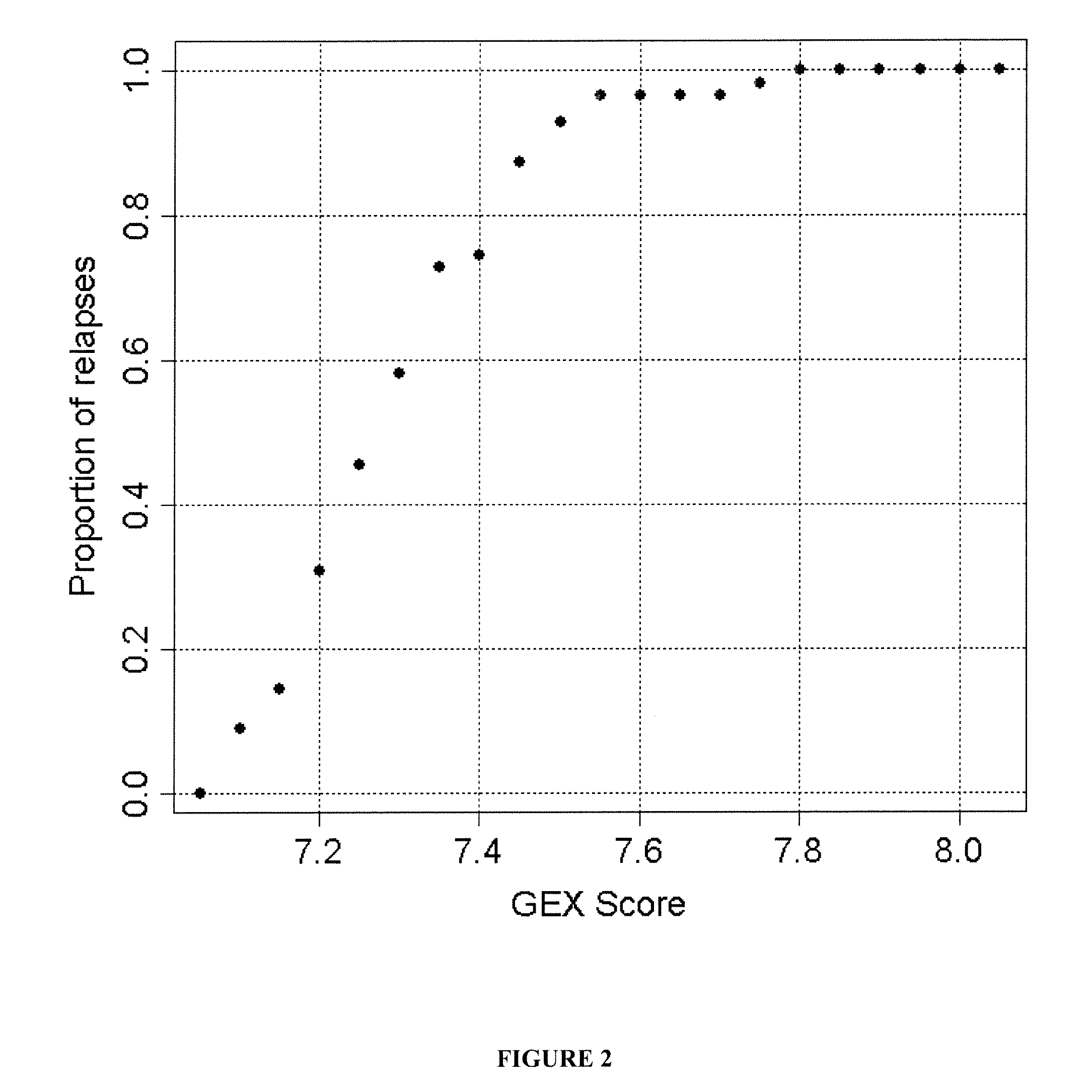
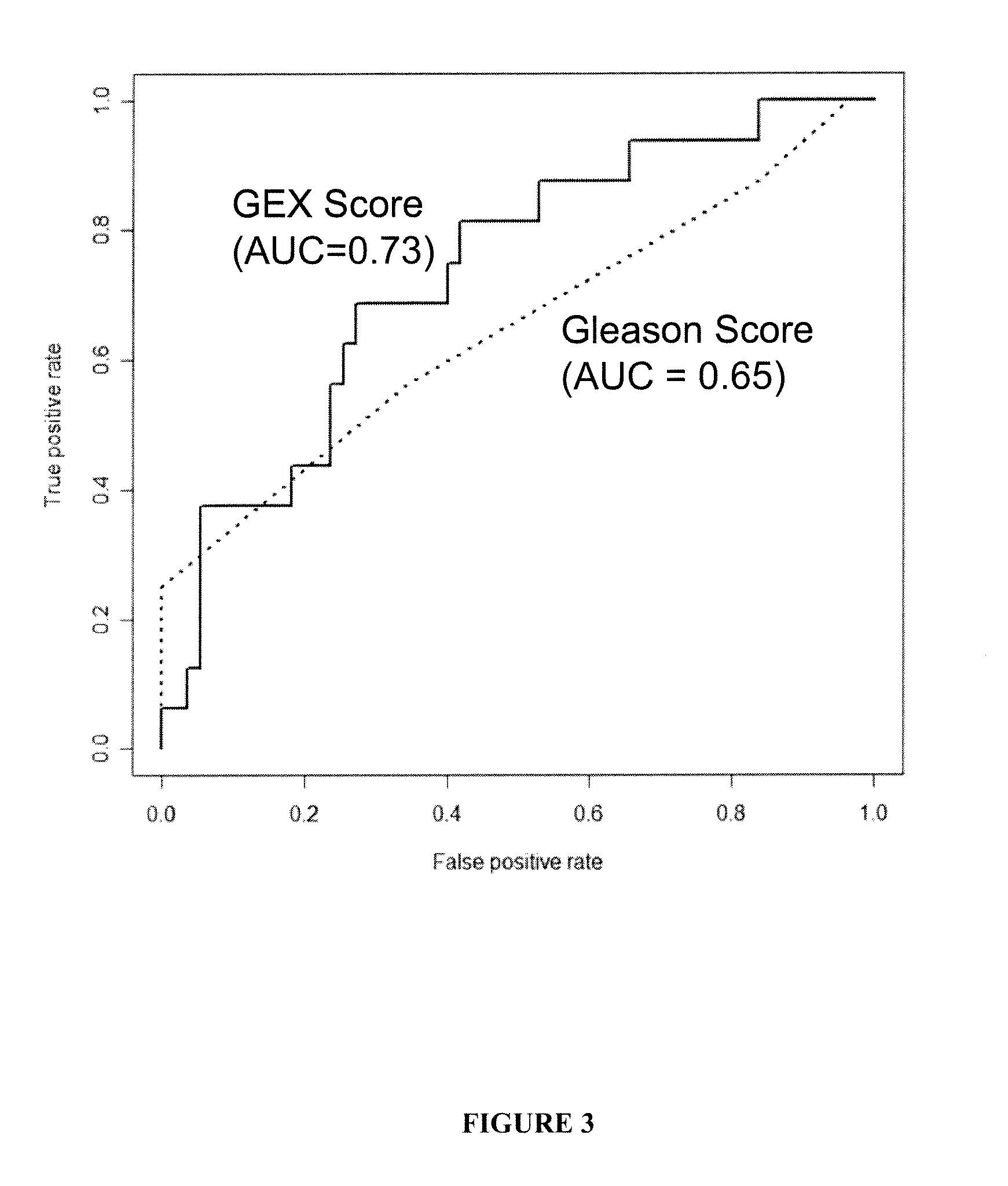
The present invention provides a method for preparing a reference model for cancer relapse prediction that provides higher resolution grading than Gleason score alone. The method encompasses obtaining from different individuals a plurality of prostate carcinoma tissue samples of known clinical outcome representing different Gleason scores; selecting a set of signature genes having an expression pattern that correlates positively or negatively in a statistically significant manner with the Gleason scores; independently deriving a prediction score that correlates gene expression of each individual signature gene with Gleason score for each signature gene in said plurality of prostate carcinoma tissue samples; deriving a prostate cancer gene expression (GEX) score that correlates gene expression of said set of signature genes with the Gleason score based on the combination of independently derived prediction scores in the plurality of prostate cancer tissue samples; and correlating said GEX score with the clinical outcome for each prostate carcinoma tissue sample. A set of signature genes is provided that encompasses all or a sub-combination of GI_2094528, KIP2, NRG1, NBL1, Prostein, CCNE2, CDC6, FBP1, HOXC6, MKI67, MYBL2, PTTG1, RAMP, UBE2C, Wnt5A, MEMD, AZGP1, CCK, MLCK, PPAP2B, and PROK1. Also provided a methods for predicting the probability of relapse of cancer in an individual and methods for deriving a prostate cancer gene expression (GEX) score for a prostate carcinoma tissue sample obtained from an individual.
Owner:ILLUMINA INC
Methods and Compounds for Lymphoma Cell Detection and Isolation
Compositions comprising a purified and / or isolated antibody, humanized antibodies, precipitates and anti-sera that specifically bind to or are otherwise directed against ROR1 protein. The compositions may be used for detecting ROR1 in a sample from a subject that is suspected or known to contain cancer cells. The ROR1 antibodies are especially useful in identifying and treating lymphomas and ademocarcinomas. Vaccines and related methods for protecting a subject against diseases that involve expression of ROR1 are also provided, as are human anti-sera effective in abrogating interactions between Wnt5a protein and ROR1 that contribute to the survival of certain cancer cells, such as CLL cells.
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for the treatment of malignant melanoma, breast, prostate, colon, papillary thyroid and pancreatic cancer
InactiveUS20100004304A1Mitigating and preventing complicationOrganic active ingredientsBiocideLymphatic SpreadGrowth cell
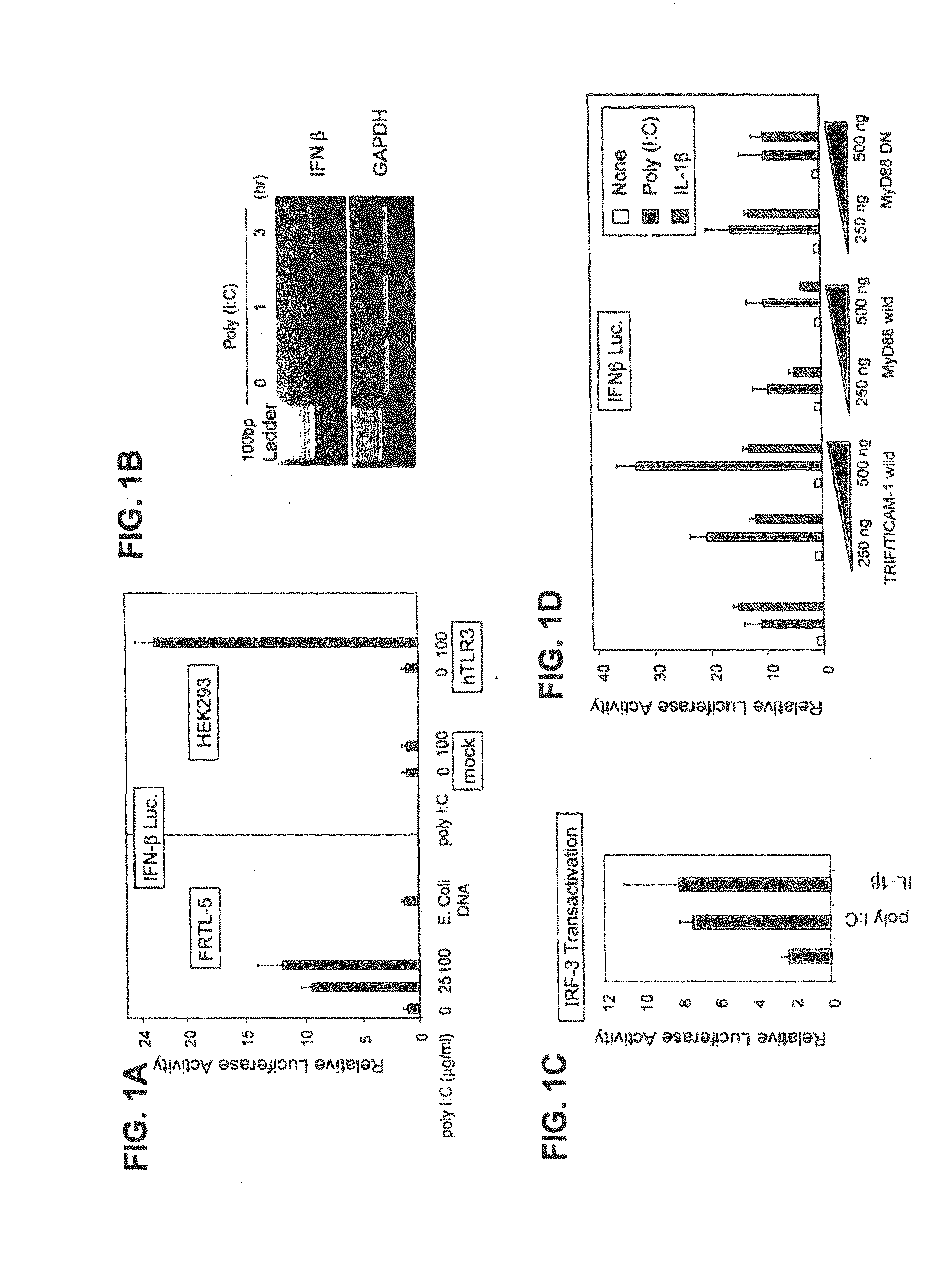
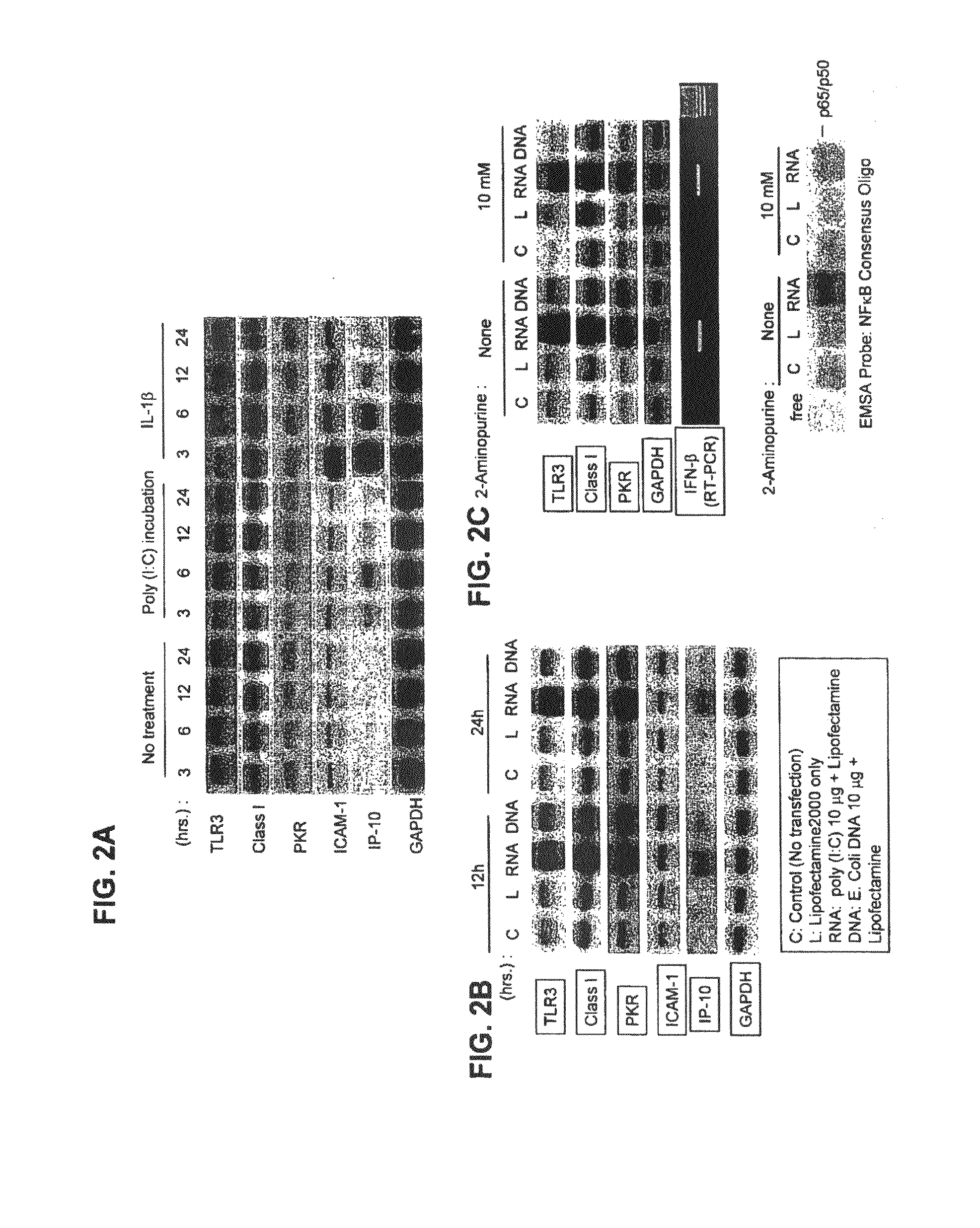
It is now recognized that chronic inflammation is an important risk factor for the development of cancer. The proinflammatory cytokine IL-6 is implicated in cancer because it is important for the activation of STAT, a key regulator of cancer growth, survival, metastasis, immune evasion and angiogenesis. Increased IL-6 and Stat-3 exists in vitro in pancreatic cancer, malignant melanoma, papillary thyroid cancer, breast cancer, colon cancer, and prostate cancer cells with high basal expression of Toll-like receptor 3 (TLR3) and Wnt5a. IL6 / STAT3 activation, mediated by overexpressed TLR3 signaling, appears important in the tumor growth process, it may increase Wnt5a signaling, and be associated with increased cellular growth and migration. Using a novel inhibitor of pathologic TLR3 signaling (5-phenylmethimazole [C10]) we have demonstrated decreases in these markers plus suppression of cell growth and migration in human pancreatic cancer, malignant melanoma, papillary thyroid cancer, breast cancer, colon cancer, and prostate cancer cells.
Owner:OHIO UNIV
Serum-free medium for mesenchymal stem cells as well as preparation method and applications of serum-free medium
ActiveCN106754670AReduced Pollution ChancesReduce allergensCulture processSkeletal/connective tissue cellsSerum free mediaLipid formation
Owner:湖南省生宝生物科技有限公司
Expression Profiles to Predict Relapse of Prostate Cancer
InactiveUS20110153534A1Microbiological testing/measurementDigital computer detailsReference modelImage resolution
The present invention provides a method for preparing a reference model for cancer relapse prediction that provides higher resolution grading than Gleason score alone. The method encompasses obtaining from different individuals a plurality of prostate carcinoma tissue samples of known clinical outcome representing different Gleason scores; selecting a set of signature genes having an expression pattern that correlates positively or negatively in a statistically significant manner with the Gleason scores; independently deriving a prediction score that correlates gene expression of each individual signature gene with Gleason score for each signature gene in said plurality of prostate carcinoma tissue samples; deriving a prostate cancer gene expression (GEX) score that correlates gene expression of said set of signature genes with the Gleason score based on the combination of independently derived prediction scores in the plurality of prostate cancer tissue samples; and correlating said GEX score with the clinical outcome for each prostate carcinoma tissue sample. A set of signature genes is provided that encompasses all or a sub-combination of GI_2094528, KIP2, NRG1, NBL1, Prostein, CCNE2, CDC6, FBP1, HOXC6, MKI67, MYBL2, PTTG1, RAMP, UBE2C, Wnt5A, MEMD, AZGP1, CCK, MLCK, PPAP2B, and PROK1. Also provided a methods for predicting the probability of relapse of cancer in an individual and methods for deriving a prostate cancer gene expression (GEX) score for a prostate carcinoma tissue sample obtained from an individual.
Owner:ILLUMINA INC
Expression profiles to predict relapse of prostate cancer
InactiveUS8110363B2Microbiological testing/measurementDigital computer detailsProstate cancerOncology
The present invention provides a method for preparing a reference model for cancer relapse prediction that provides higher resolution grading than Gleason score alone. The method encompasses obtaining from different individuals a plurality of prostate carcinoma tissue samples of known clinical outcome representing different Gleason scores; selecting a set of signature genes having an expression pattern that correlates positively or negatively in a statistically significant manner with the Gleason scores; independently deriving a prediction score that correlates gene expression of each individual signature gene with Gleason score for each signature gene in said plurality of prostate carcinoma tissue samples; deriving a prostate cancer gene expression (GEX) score that correlates gene expression of said set of signature genes with the Gleason score based on the combination of independently derived prediction scores in the plurality of prostate cancer tissue samples; and correlating said GEX score with the clinical outcome for each prostate carcinoma tissue sample. A set of signature genes is provided that encompasses all or a sub-combination of GI_2094528, KIP2, NRG1, NBL1, Prostein, CCNE2, CDC6, FBP1, HOXC6, MKI67, MYBL2, PTTG1, RAMP, UBE2C, Wnt5A, MEMD, AZGP1, CCK, MLCK, PPAP2B, and PROK1. Also provided a methods for predicting the probability of relapse of cancer in an individual and methods for deriving a prostate cancer gene expression (GEX) score for a prostate carcinoma tissue sample obtained from an individual.
Owner:ILLUMINA INC
Neurotherapeutic Nanoparticle Compositions and Devices
There are provided compositions and methods for treatment of neurodegeneative diseases and CNS injury. The compositions a pharmaceutically acceptable carrier solution; and a plurality of biodegradable nanoparticles, wherein the nanoparticles comprise a targeting moiety that is able to bind selectively to the surface of a neural stem cell and wherein the nanoparticles further comprise factors such as leukaemia inhibitory factor (LIF); XAV939 and / or one or more of : brain-derived neurotrophic factor (BDNF) or an agonist thereof; epidermal growth factor (EGF) or an agonist thereof; glial cell-derived neurotrophic factor (GDNF) or an agonist thereof; retinoic acid and derivatives thereof; ciliary neurotrophic factor (CTNF) or an agonist thereof; and Wnt5A. The biodegradable nanoparticles may deliver via controlled time release.
Owner:YALE UNIV
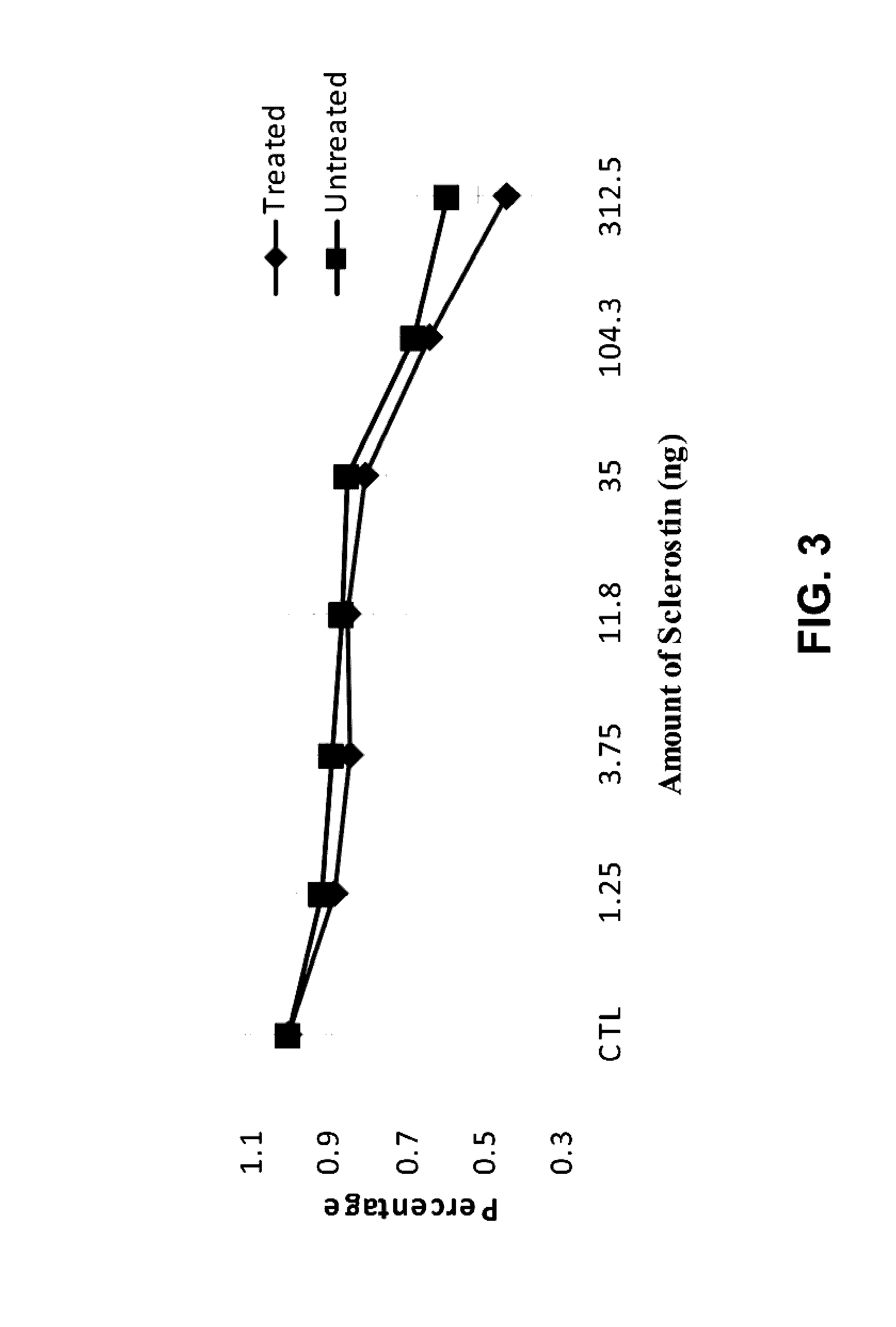
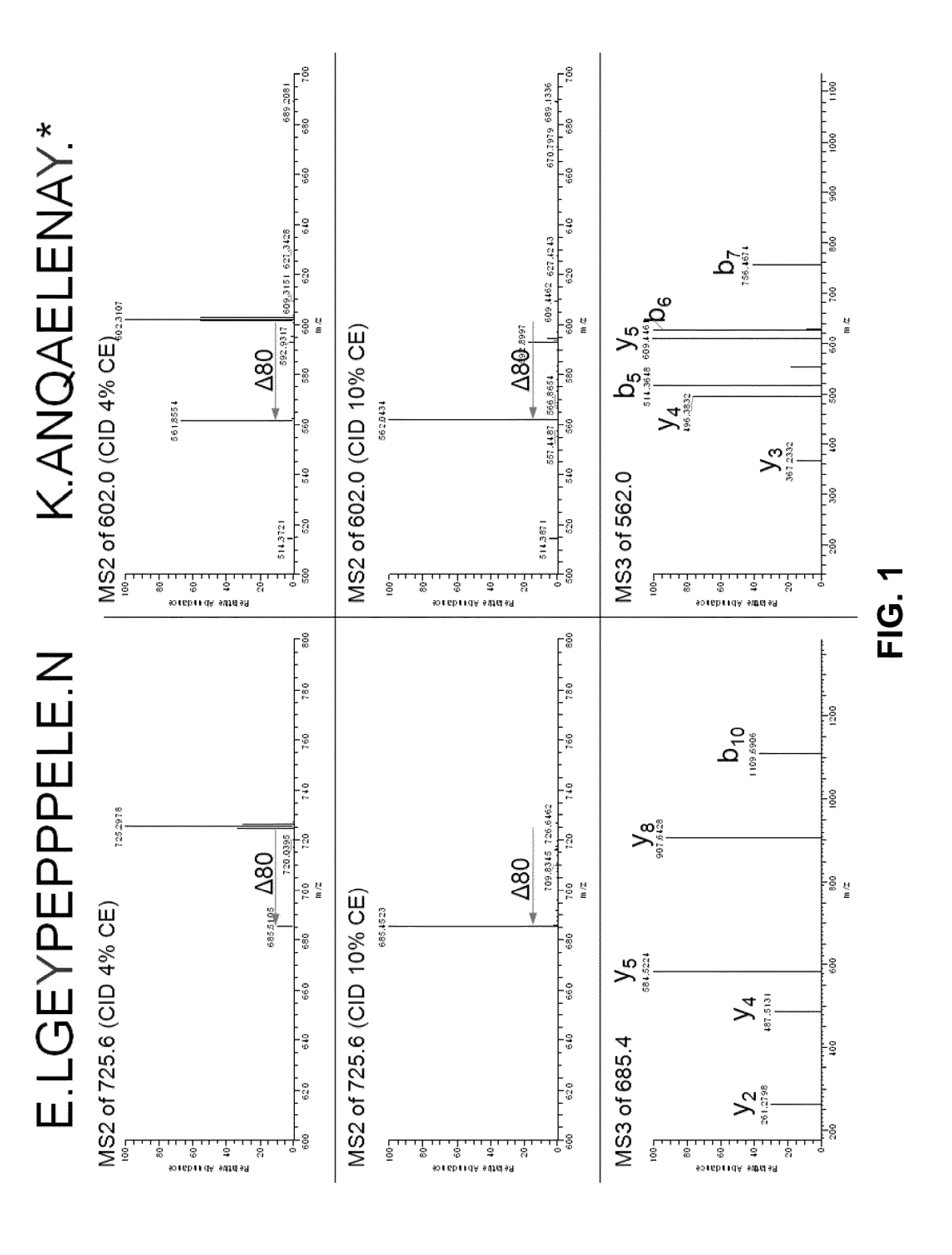
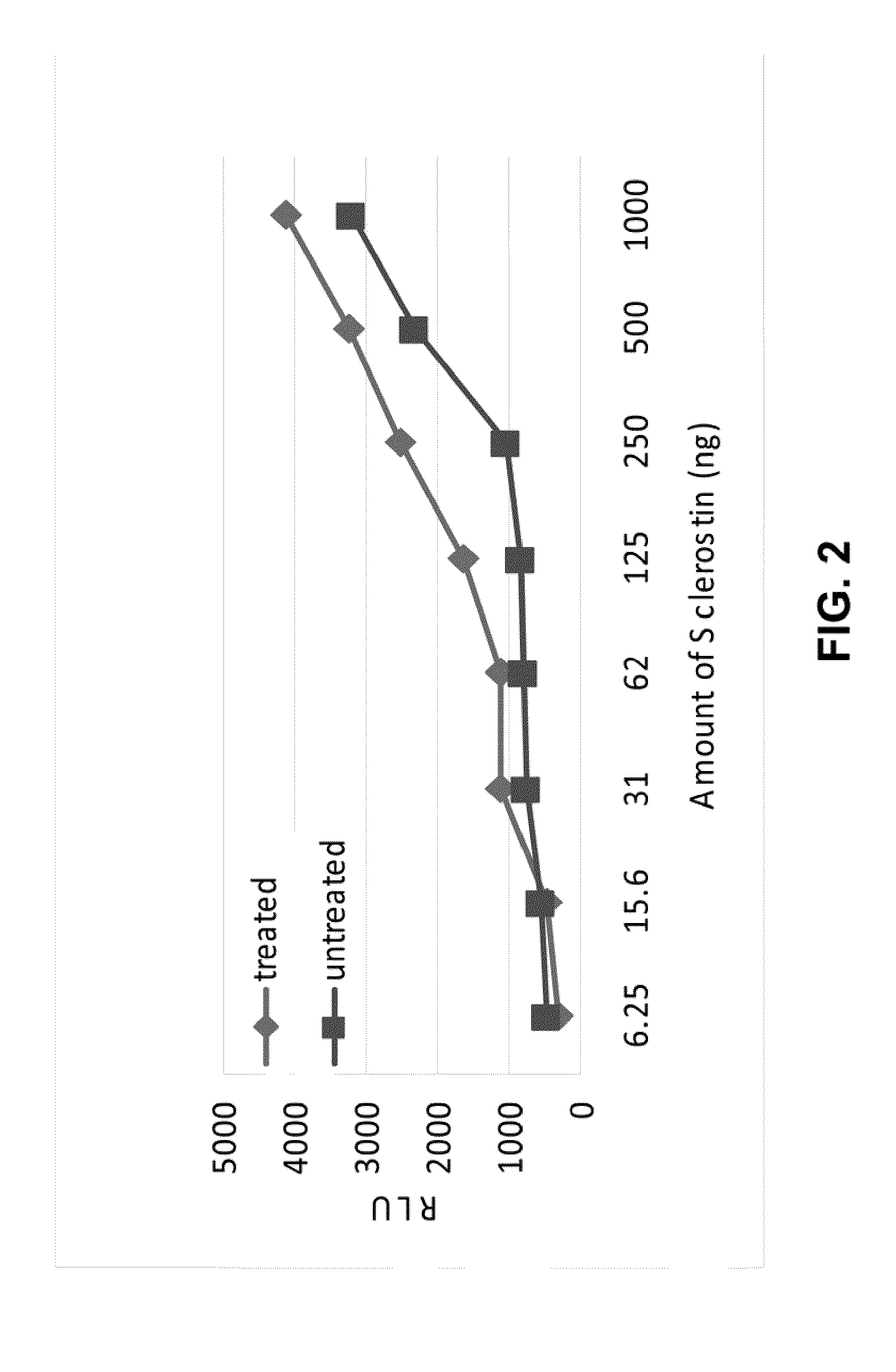
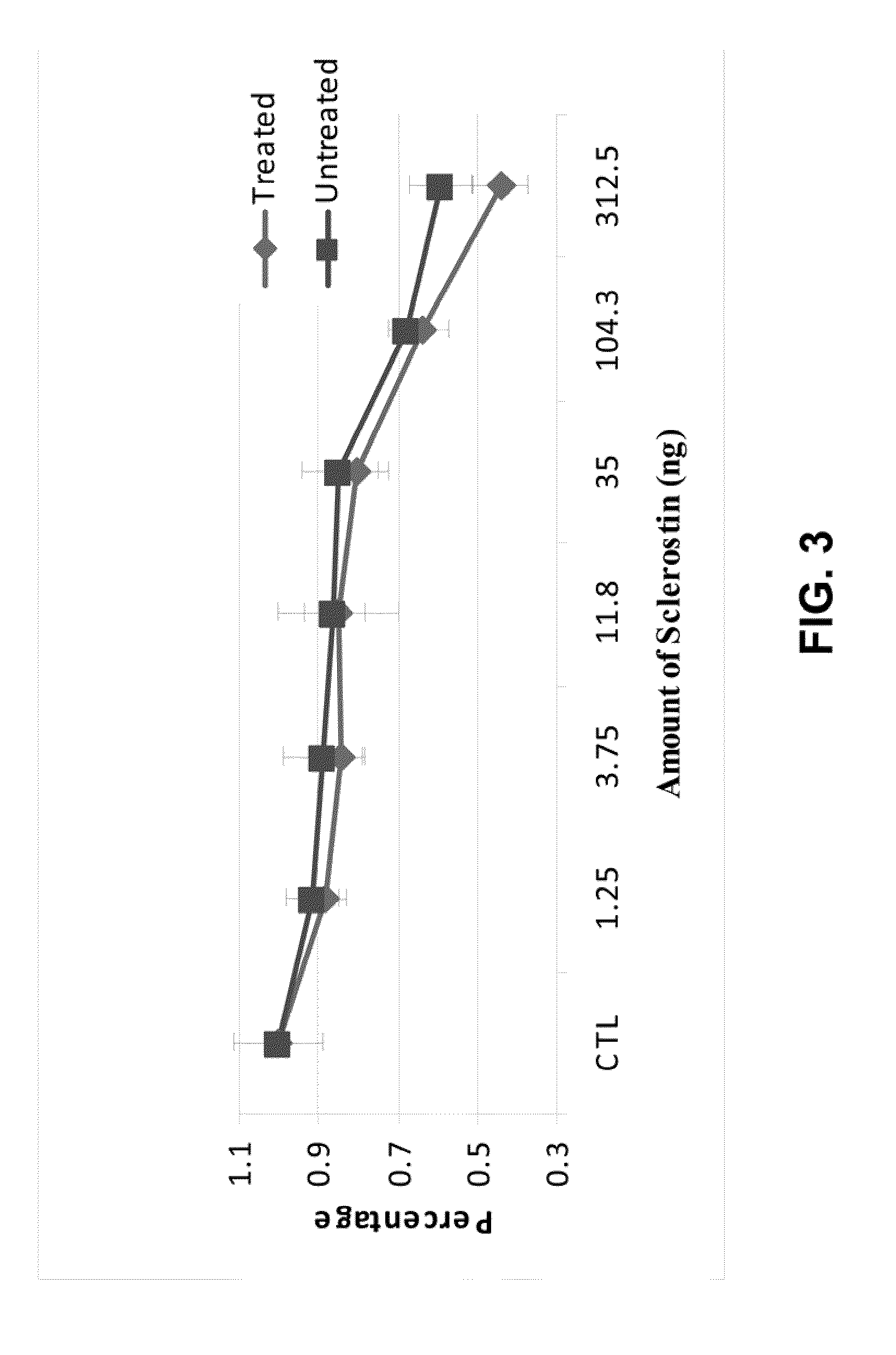
Antibodies specific for sulfated sclerostin
Provided is a protein comprising an antibody binding site that binds to a sulfated epitope of a Wnt pathway protein that is not Wnt5A, Wnt11, or Wnt3a. Also provided is a composition comprising an isolated and purified Wnt pathway protein, where the protein is sulfated but not glycosylated. Additionally provided is a preparation of a Wnt pathway protein comprising at least one sulfation site and at least one glycosylation site, where all of the Wnt pathway protein in the preparation is glycosylated but not sulfated. Further provided is a composition comprising a peptide less than 75 amino acids or amino acid analogs, the peptide consisting of a fragment of a Wnt pathway protein, wherein the fragment is sulfated. A modified Wnt pathway protein comprising a sulfation site that is not present in the native Wnt pathway protein is also provided. Also provided is a method of detecting or quantifying a sulfated Wnt pathway protein in a preparation. Additionally, a modified Wnt pathway protein lacking a sulfation site that is present in the native Wnt pathway protein is provided. Also provided is methods of treating a subject having a disease exacerbated by Wnt activation. Additionally, a method of treating a subject having a disease exacerbated by Wnt inhibition is provided.
Owner:ENZO BIOCHEM
Sulfation of wnt pathway proteins
Provided is a protein comprising an antibody binding site that binds to a sulfated epitope of a Wnt pathway protein that is not Wnt5A, Wnt11, or Wnt3a. Also provided is a composition comprising an isolated and purified Wnt pathway protein, where the protein is sulfated but not glycosylated. Additionally provided is a preparation of a Wnt pathway protein comprising at least one sulfation site and at least one glycosylation site, where all of the Wnt pathway protein in the preparation is glycosylated but not sulfated. Further provided is a composition comprising a peptide less than 75 amino acids or amino acid analogs, the peptide consisting of a fragment of a Wnt pathway protein, wherein the fragment is sulfated. A modified Wnt pathway protein comprising a sulfation site that is not present in the native Wnt pathway protein is also provided. Also provided is a method of detecting or quantifying a sulfated Wnt pathway protein in a preparation. Additionally, a modified Wnt pathway protein lacking a sulfation site that is present in the native Wnt pathway protein is provided. Also provided is methods of treating a subject having a disease exacerbated by Wnt activation. Additionally, a method of treating a subject having a disease exacerbated by Wnt inhibition is provided.
Owner:ENZO BIOCHEM
Diagnostic and therapeutic methods for corneal ectasia following refractive surgery, keratoconus or pellucid degeneration
The present invention relates to methods of diagnosis and treatment of corneal ectasia following refractive surgery, keratoconus or pellucid marginal degeneration in a subject by determining or modulating the level of expression of molecules associated with the Wnt signalling pathway. Marker molecules of the present invention include SFRP1, PITX2, LEF1, WNT16 and WNT5A.
Owner:THE UNIV OF SYDNEY
Methods and compositions for reducing growth, migration and invasiveness of brain cancer stem cells and improving survival of patients with brain tumors
ActiveUS20170368158A1Reduce tumor growthPromote migrationNervous disorderPeptide/protein ingredientsTumor therapyBrain stem tumor
The described invention relates to a pharmaceutical composition comprising a therapeutically effect amount of a therapeutic agent, wherein the therapeutic agent is effective (1) to reduce tumor growth, migration, invasion or a combination and (2) improve subject survival relative to a control. The described invention also relates to a method of treating a subject with a tumor, the method comprising: (1) providing a pharmaceutical composition; and (2) administering the pharmaceutical composition, wherein the composition comprises a therapeutically effective amount of a therapeutic agent which is effective to reduce tumor growth, migration, invasion or a combination. The method may further comprise preparing therapeutic agent and preparing the pharmaceutical composition. The therapeutic agents include but are not limited to Wnt5a derivative peptides or Wnt5a antagonists or Wnt5a blocking antibody. The tumor comprises a population of cancer stem cells.
Owner:HYPERSTEM
Modulators of ror1-ror2 binding
Provided herein are, inter alia, methods of identifying agents that are capable inhibiting the binding (e.g., coupling) between a ROR1 protein and a ROR2 protein. By interfering with ROR1-ROR2 coupling (binding) the agents identified using the methods provided herein inhibit non-canonical Wnt5a signaling. Thus, the agents identified by the methods provided herein may, inter alia, be useful for cancer diagnosis and therapy.
Owner:RGT UNIV OF CALIFORNIA
Hybridoma cell strain and anti-Wnt5a monoclonal antibody produced thereby as well as application thereof
InactiveCN103555668AStrong characteristicIncreased sensitivityImmunoglobulins against animals/humansTissue cultureWNT5ACell strain
The invention relates to a hybridoma cell strain and an anti-Wnt5a monoclonal antibody produced by the hybridoma cell strain as well as an application of the hybridoma cell strain. Particularly, based on molecular cloning and monoclonal antibody technology, hybridoma cell strain Wnt5a which can secrete the monoclonal antibody capable of specially recognizing wnt5a is screened. The monoclonal antibody secreted by the hybridoma cell strain Wnt5a can be used for clinically testing leukemia lesion in human bones and blood.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
A serum-free medium for mesenchymal stem cells and its preparation method and application
ActiveCN106754670BReduce the differenceClear ingredientsCulture processSkeletal/connective tissue cellsMesenchymal stem cellClinical research
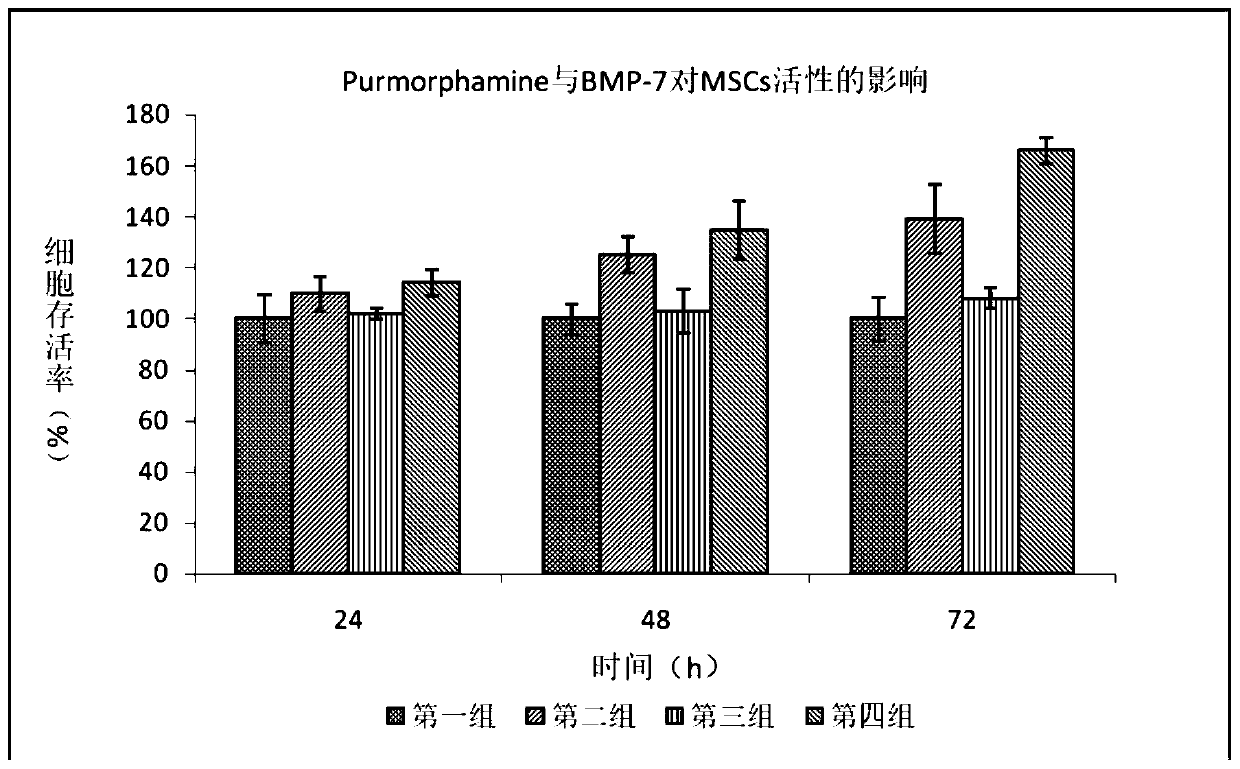
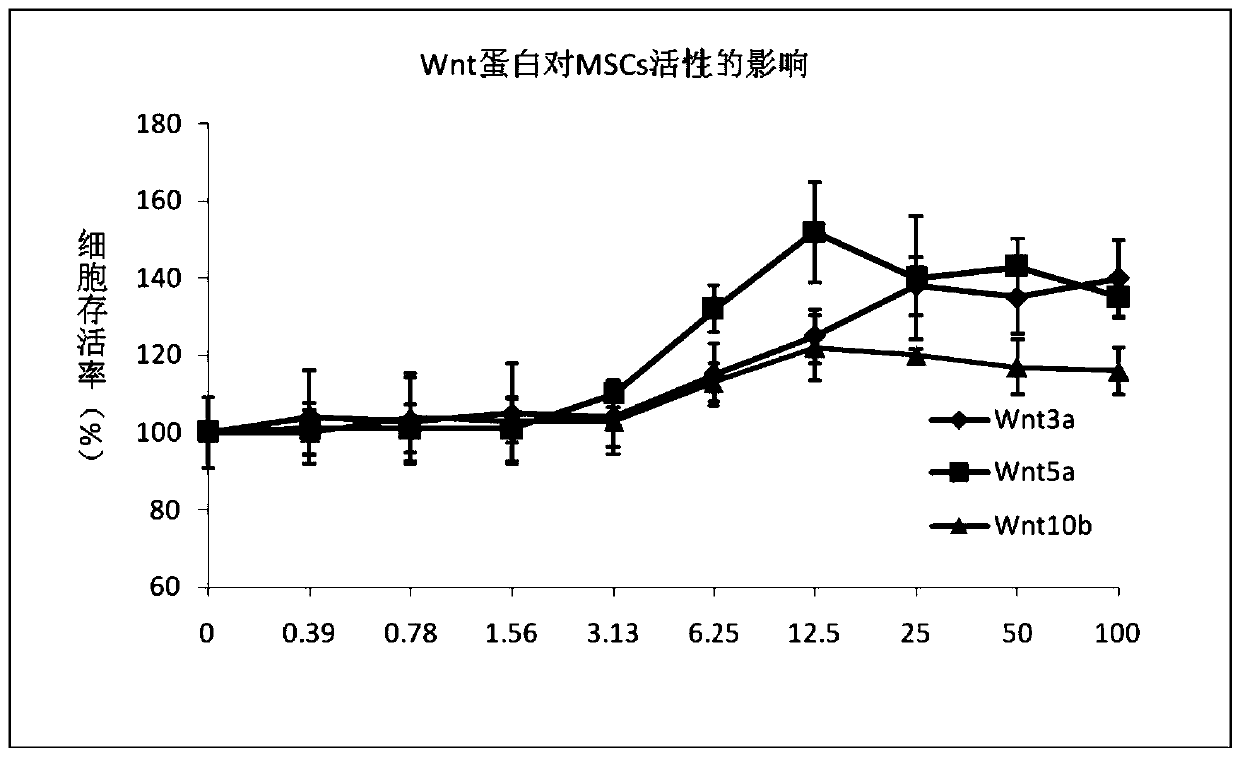
The invention discloses a serum-free medium for mesenchymal stem cells as well as a preparation method and applications of the serum-free medium. Cytokines in serum are replaced by adding EGF, PDGF, bFGF, beta-TGF, BMP-7 and wnt5a; lipids in serum are replaced by adding linoleic acid, linolenic acid and lecithin; through orthogonal design, the additives are compounded, and the good proliferation effect is obtained. In addition, the ingredients are definite, and the batch-to-batch differences are reduced; the potential risks of exogenous viruses and pathogenic factors are eliminated; the untoward effects of clinical research are reduced; and the application prospect is good.
Owner:湖南省生宝生物科技有限公司
Compounds and methods to measure metabolic function and restore normal metabolic function
InactiveUS20110274689A1High level of bindingExtended half-lifePeptide/protein ingredientsMetabolism disorderAntibody inhibitorNormal glucose level
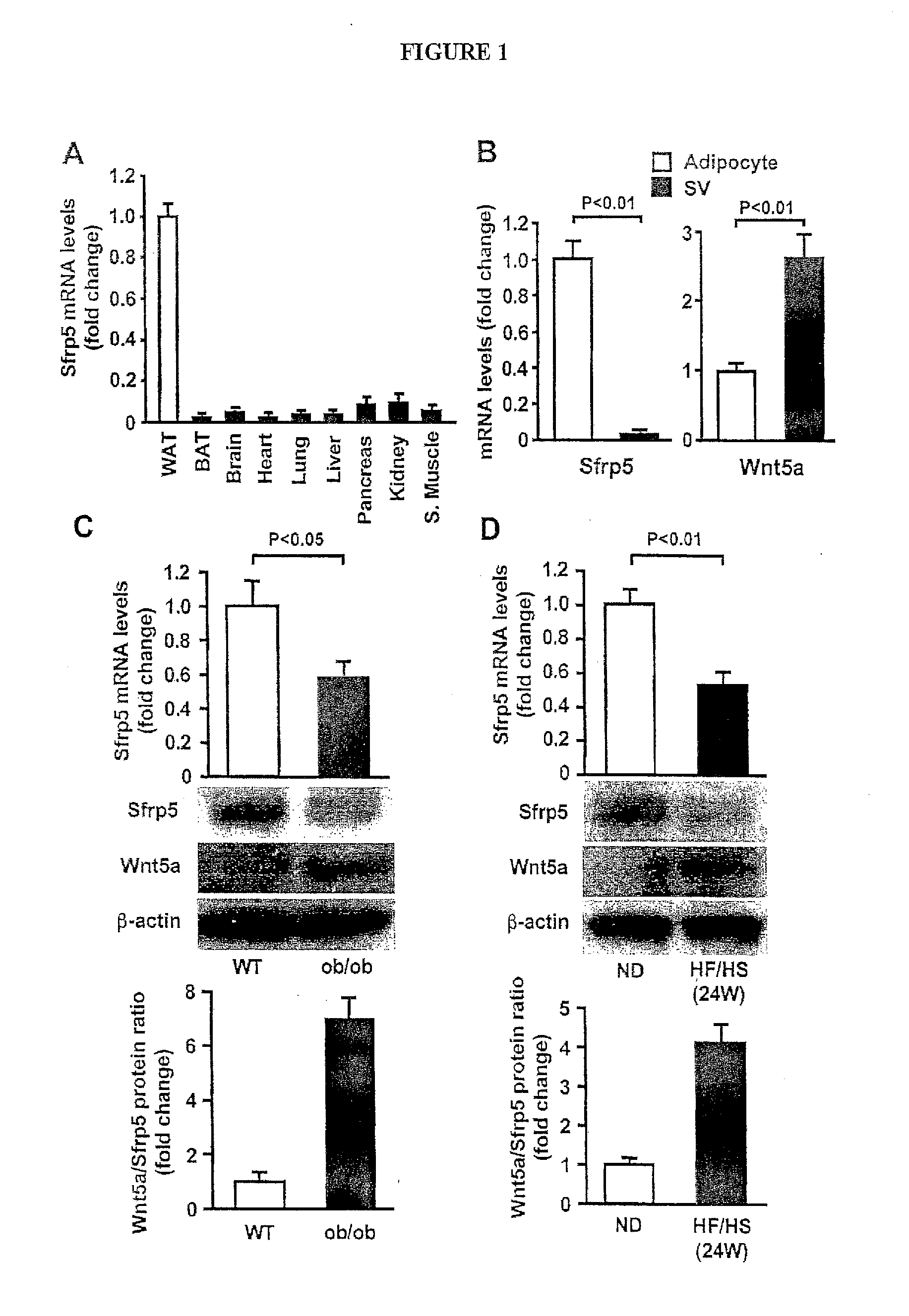
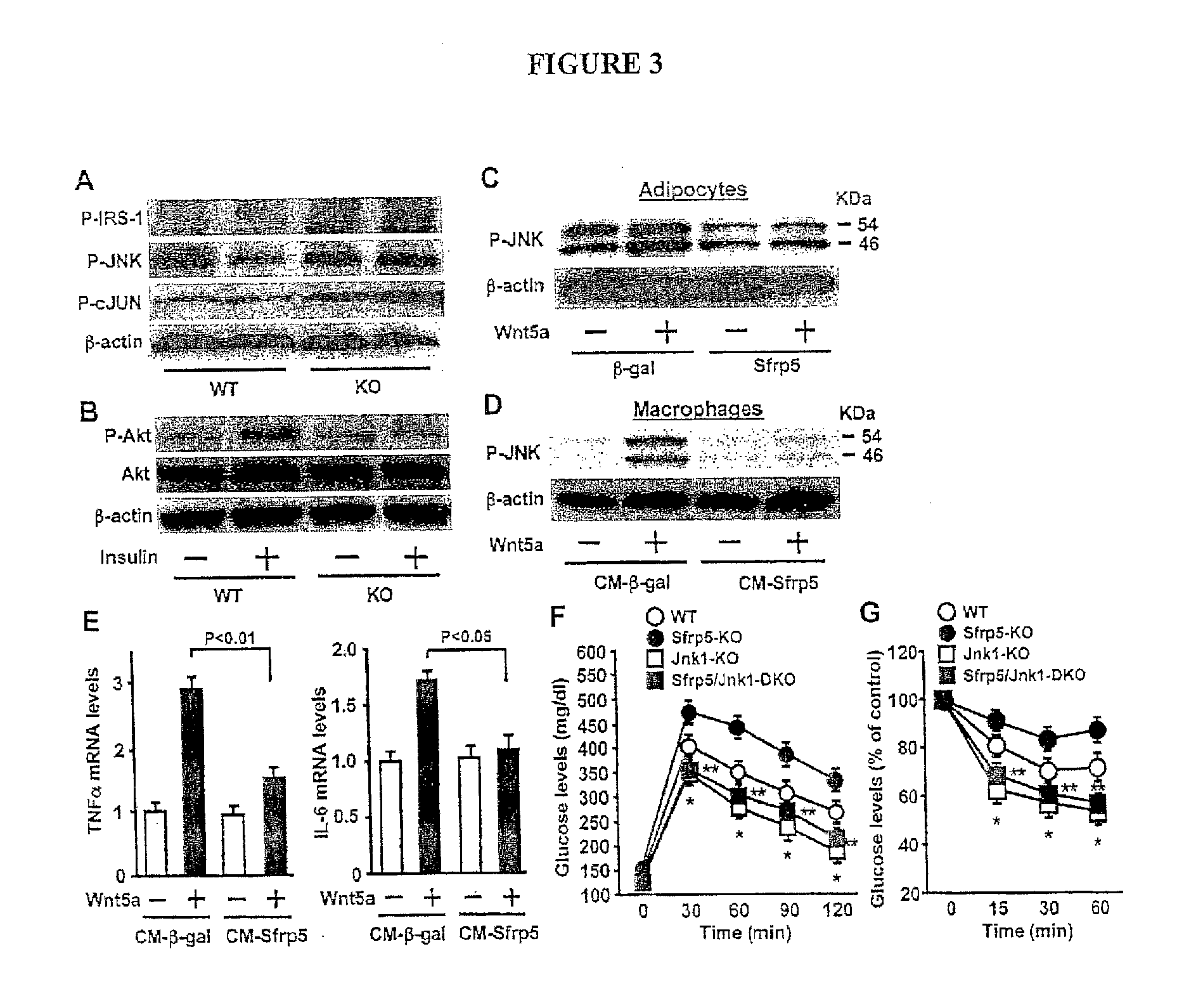
The invention relates to treatment to restore normal metabolic function, including but not limited to normal glucose levels. The invention in one embodiment contemplates methods of reducing elevated glucose levels in subjects with elevated glucose levels by administering a composition comprising at least a portion of human Sfrp5. The invention in one embodiment contemplates methods of reducing elevated glucose levels in subjects with elevated glucose levels by administering a composition comprising an inhibitor of Wnt5a, including but not limited to an antibody inhibitor.
Owner:WALSH KENNETH +1
Stratification of Left-Side and Right-Side Colon Cancer
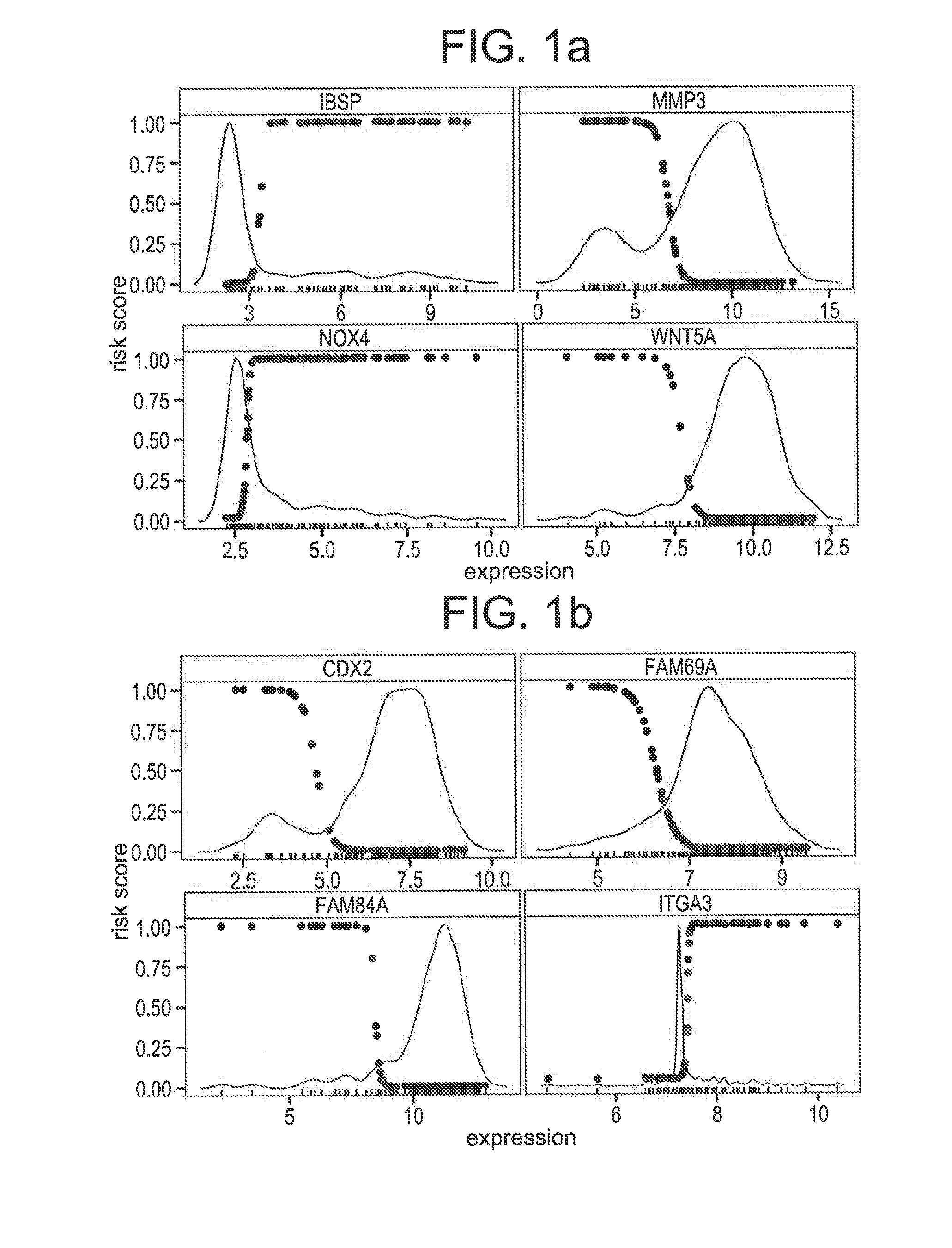
Compositions / methods for employing fresh-frozen or FFPE colon cancer tissue in left side colon cancer (LCC) and right-side colon cancer (RCC) disease patients for risk of relapse assessment / stratification is provided (3 strata and a 4 strata methodology). An RCC gene panel of 4 genes (FAM69A, CDX2, FAM84A, ITGA3), and 9 genes (FAM69A, CDX2, ITGA3, FAM84A, ITPRIP, RAB3B, SMAD3, PCSK5, MMP28), is provided. An LCC gene panel of 4 genes (NOX4, WNT5A, MMP3, IBSP), and a 9 genes (MMP3, WINT5A, NOX4, IBSP, SLC16A6, CYPIBI, TFAP2C, MATN3, ANKRD6), is provided. A microchip-based clinical tool, and a kit including a microchip, is presented. The invention also describes a computer-implemented method for assessing relative risk of relapse in LCC and / or RCC disease. An individual patient scoring method that presents a continuous stratification score useful in the post-surgical colon cancer management of LCC and / or RCC patient is also presented.
Owner:UNIV OF NOTRE DAME DU LAC
Molecular marker using Wnt5a gene as pig litter size character related detection
The invention belongs to the technical field of pig molecular markers screening, and particularly relates to a molecular marker using a Wnt5a gene as pig litter size character related detection. The molecular marker is obtained by cloning from the pig WNT5A gene. The basic group at the 213 site of a nucleotide sequence as shown in the SEQ ID NO: 1 has an A / G allele mutation, and the mutation causes the BcgI-RFLP polymorphism. The invention further discloses a screening method of the molecular marker and the application thereof in the pig assistant selection.
Owner:HUAZHONG AGRI UNIV
Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof
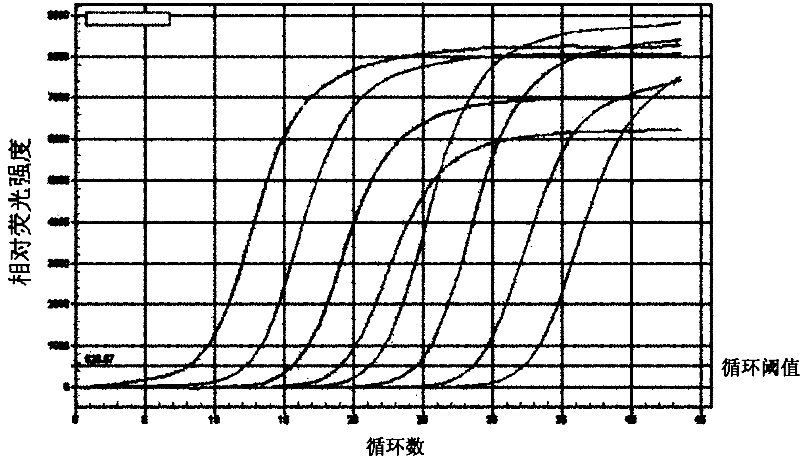
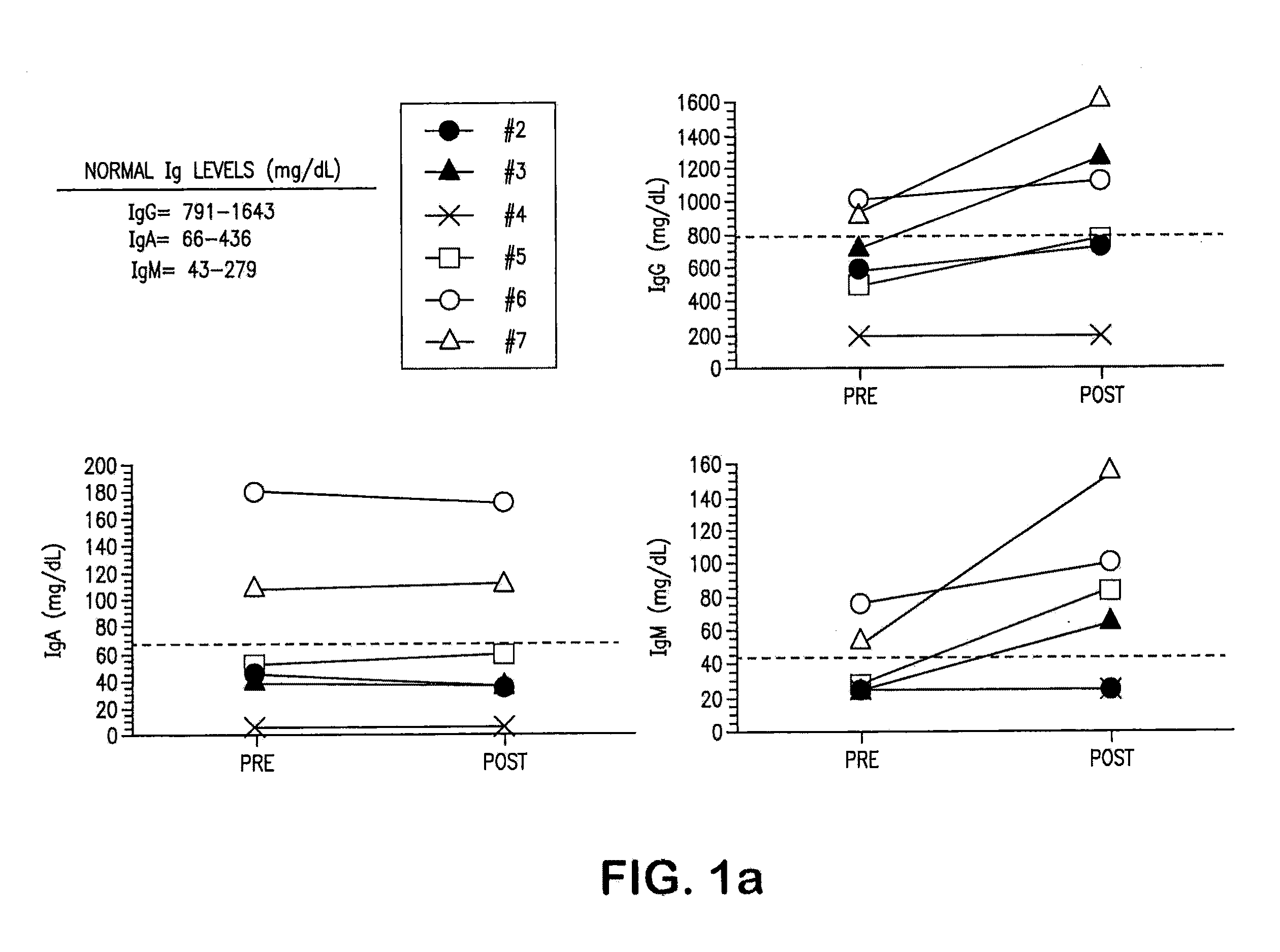
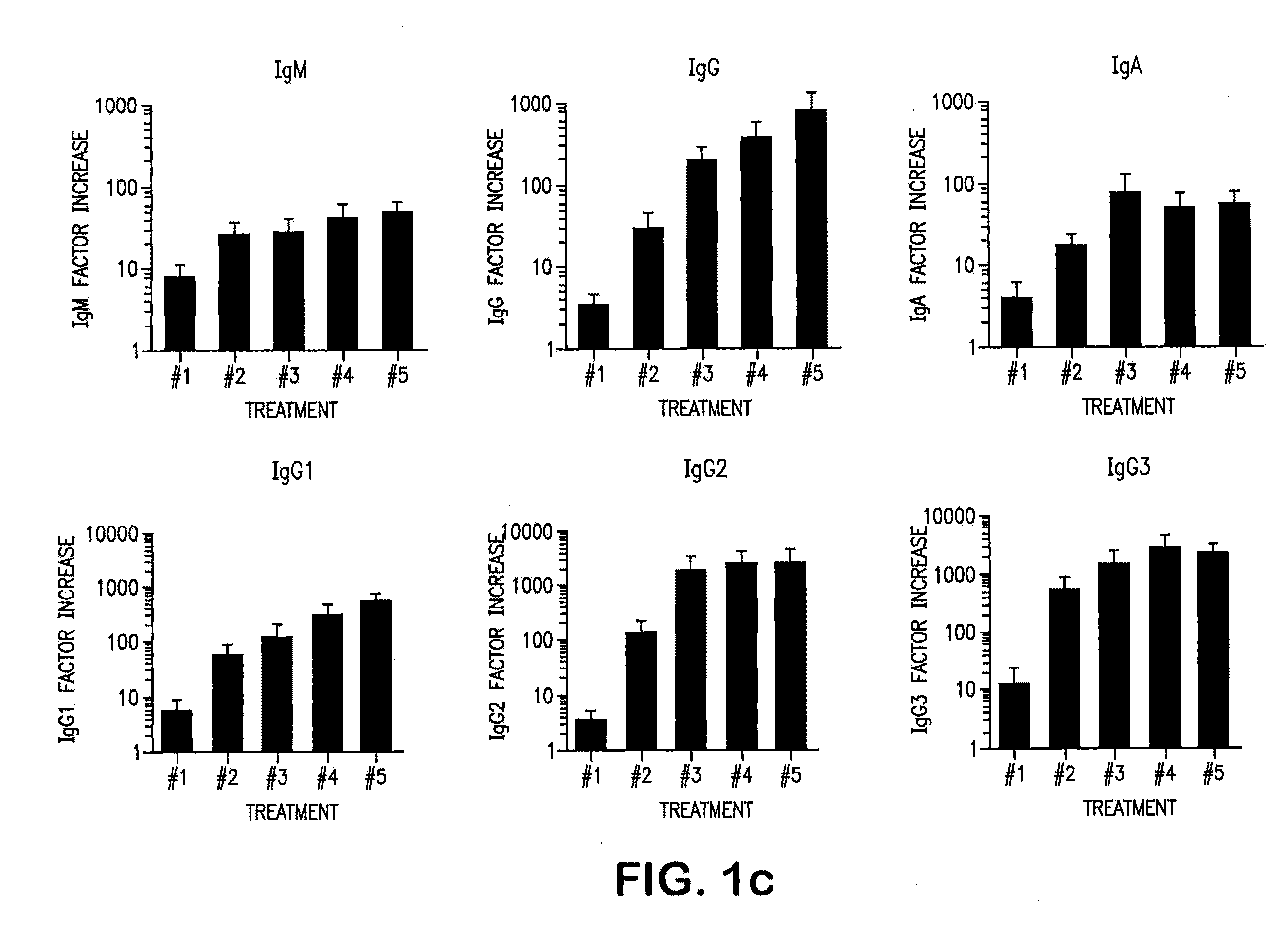
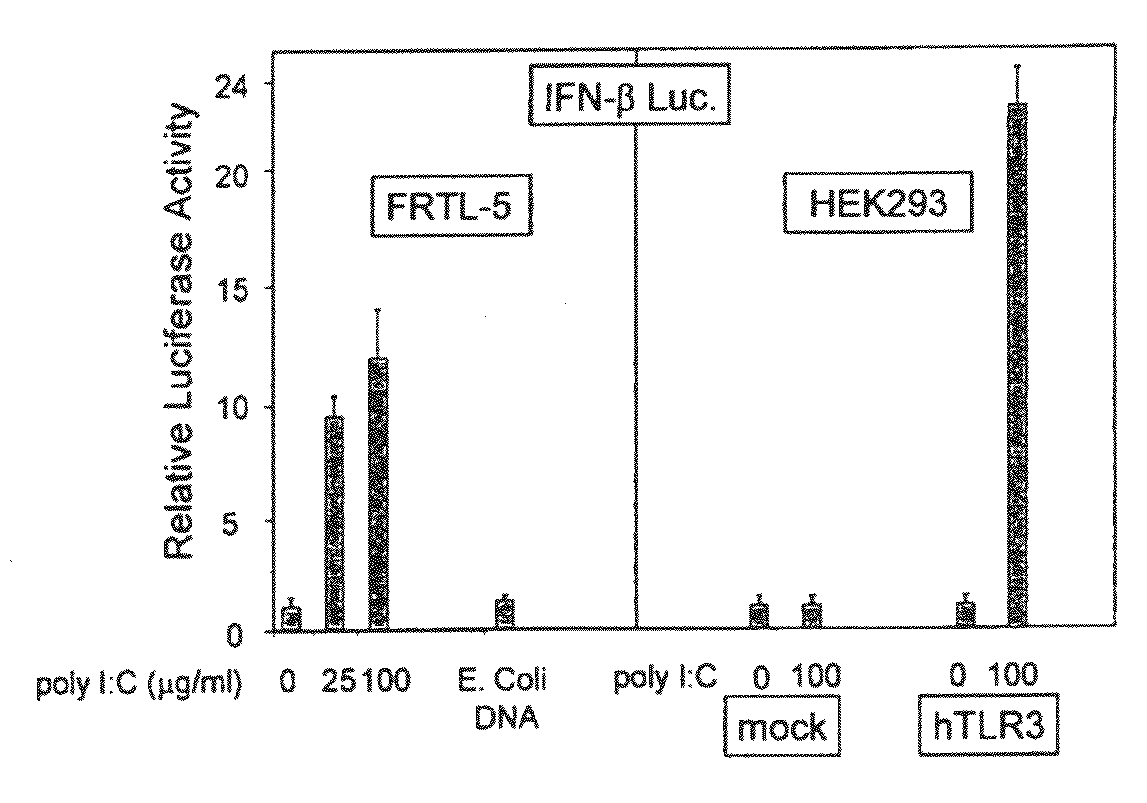
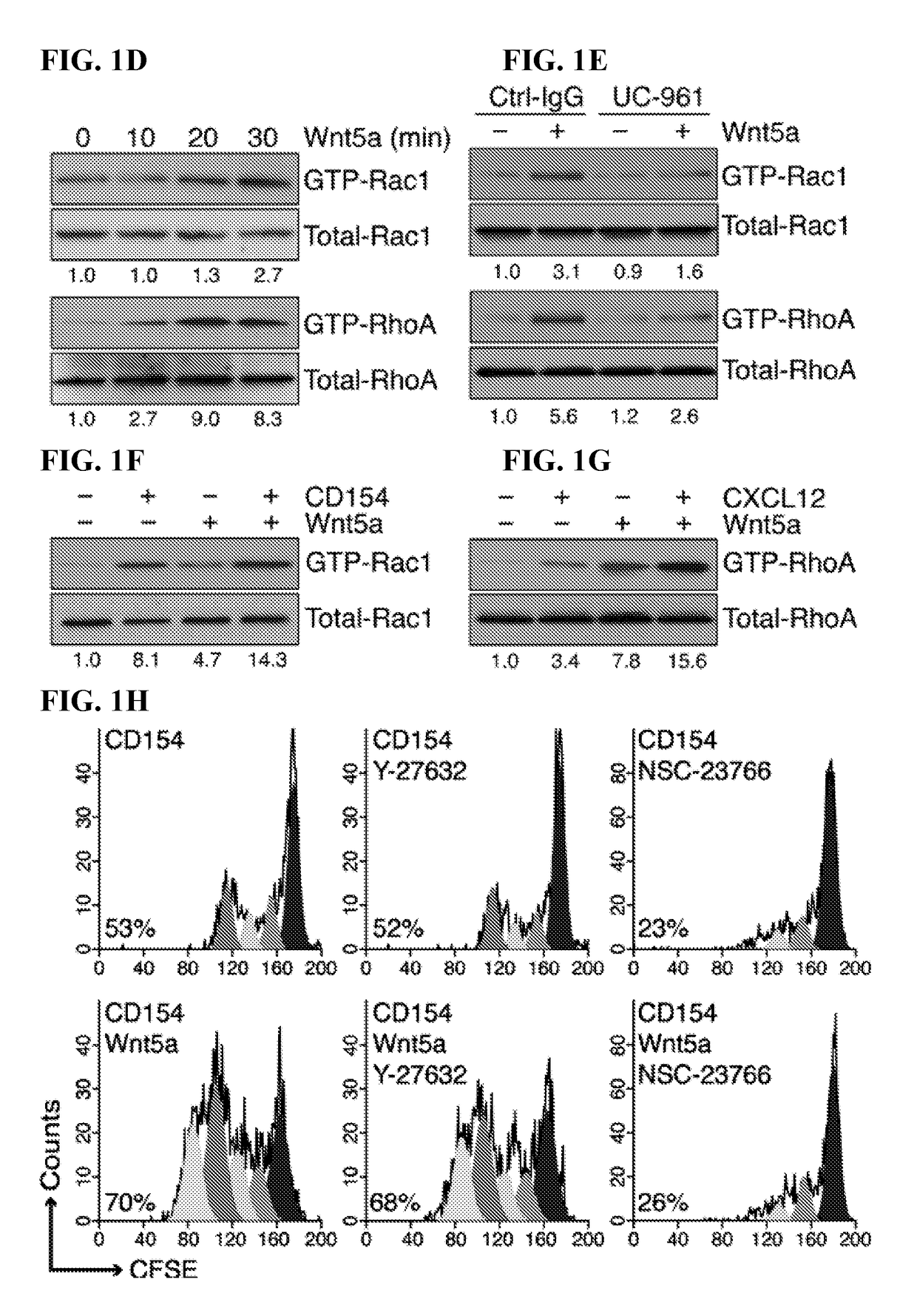
The invention provides an anti-human interleukin-6 (IL-6) receptor [beta]-chain monoclonal antibody. The cDNA and the amino sequence of a light-chain variable region at a Fab-fragment antigen-binding site of the antibody molecule are represented as the Seq ID No.1 and the Seq ID No.2, and the cDNA and the amino sequence of a heavy-chain variable region at the Fab-fragment antigen-binding site of the antibody molecule are represented as the Seq ID No.3 and the Seq ID No.4. The invention also provides a preparation method of the monoclonal antibody, and the applications of the monoclonal antibody in a drug for detecting and intervening in diagnosis and therapy of rheumatoid arthritis. In-vivo and in-vitro tests prove that the monoclonal antibody has a high affinity on positive cells of the IL-6 receptor [beta]-chain and can regulate and control the molecular mechanism of fibroblast-like synoviocytes IL-6 receptor [beta]-chain-RANKL-WNT5A signaling pathway of rheumatoid arthritis patients through antagonism, thereby achieving a result of alleviating immune function disorder and joint injury due to mediated bone metabolic imbalance of the rheumatoid arthritis patients.
Owner:SHANGHAI INST OF IMMUNOLOGY +3
Methods for inducing epithelial cancer cell senescence
InactiveUS20160354427A1Peptide/protein ingredientsMicrobiological testing/measurementTissue sampleWNT5A
Owner:INST FOR CANCER RES D B A THE RES INSTITUE OF FOX CHASE CANCER CENT
Peptides in combination with immune checkpoint inhibitors for use in treatment of cancer
PendingUS20220202901A1Reduce usageMinimize consequencesPeptide/protein ingredientsAntibody ingredientsWNT5AOncology
A WNT5A peptide or derivatives thereof in combination with one or more checkpoint inhibitors for use in treatment of cancer in a subject in need thereof. Furthermore, WNT5A peptides or derivatives thereof may be used in treatment of cancer in a subject, wherein the subject is responsive to immune checkpoint inhibitors.
Owner:WNTRES
Antibodies against human RYK and uses therefor
InactiveUS9862776B2Nervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsPhosphorylationAntigen Binding Fragment
The present invention relates to antibodies and antigen-binding fragments thereof that bind RYK, in particular human RYK and their use in regulating RYK-associated activities. Specifically there is provided an isolated monoclonal antibody or antigen-binding fragment or derivative thereof that specifically binds to the extracellular domain of human RYK, in particular, the antibody or antigen-binding fragment thereof, binds specifically to the WIF domain of human RYK. Preferably, the antibodies of the present invention modulate RYK-associated activity, which includes RYK mediated signal transduction activity and modulation of the interaction of Wnts with RYK and, preferably, modulate Wnt induced signaling. In particular, the antibodies inhibit the binding of Wnt5a and inhibit Wnt induced phosphorylation of Dishevelled (Dvl) 2 and / or Dvl3 proteins.
Owner:PETER MACCALLUM CANCER INST
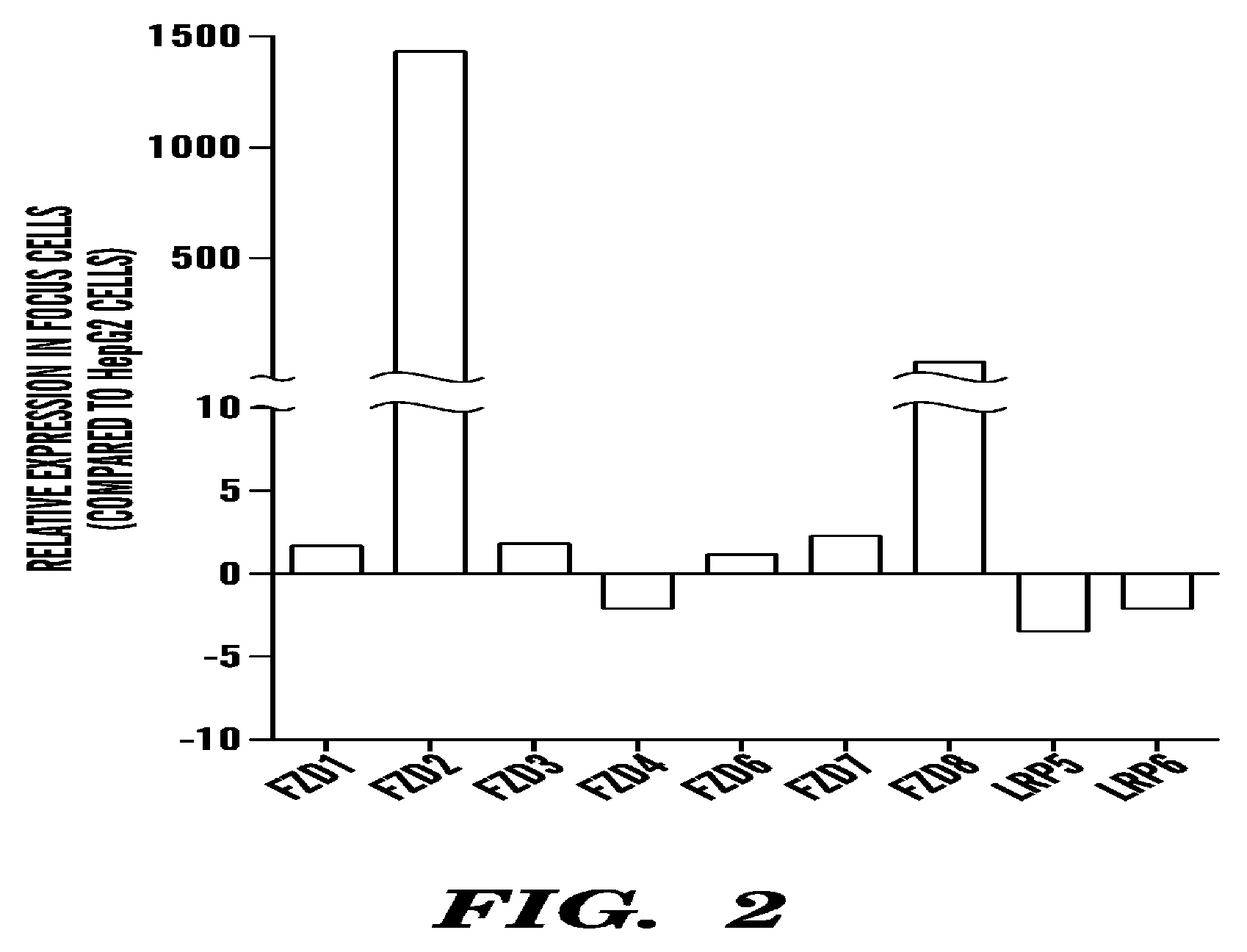
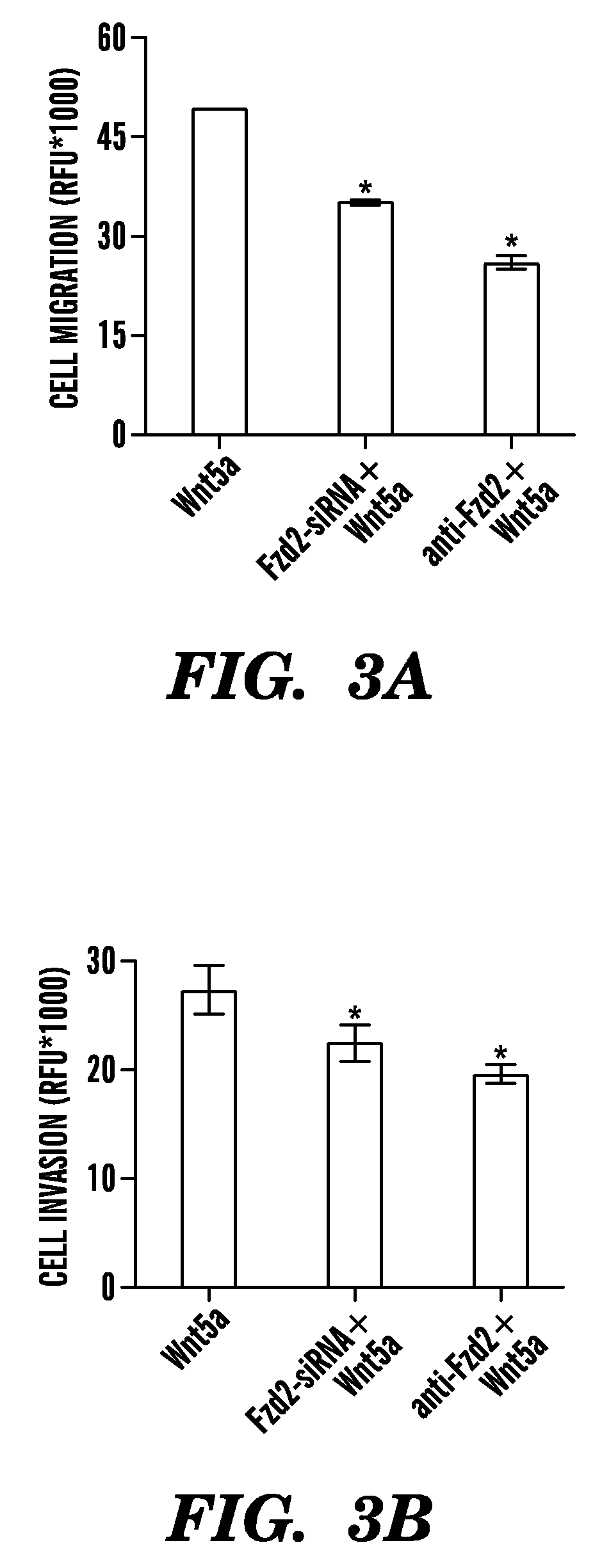
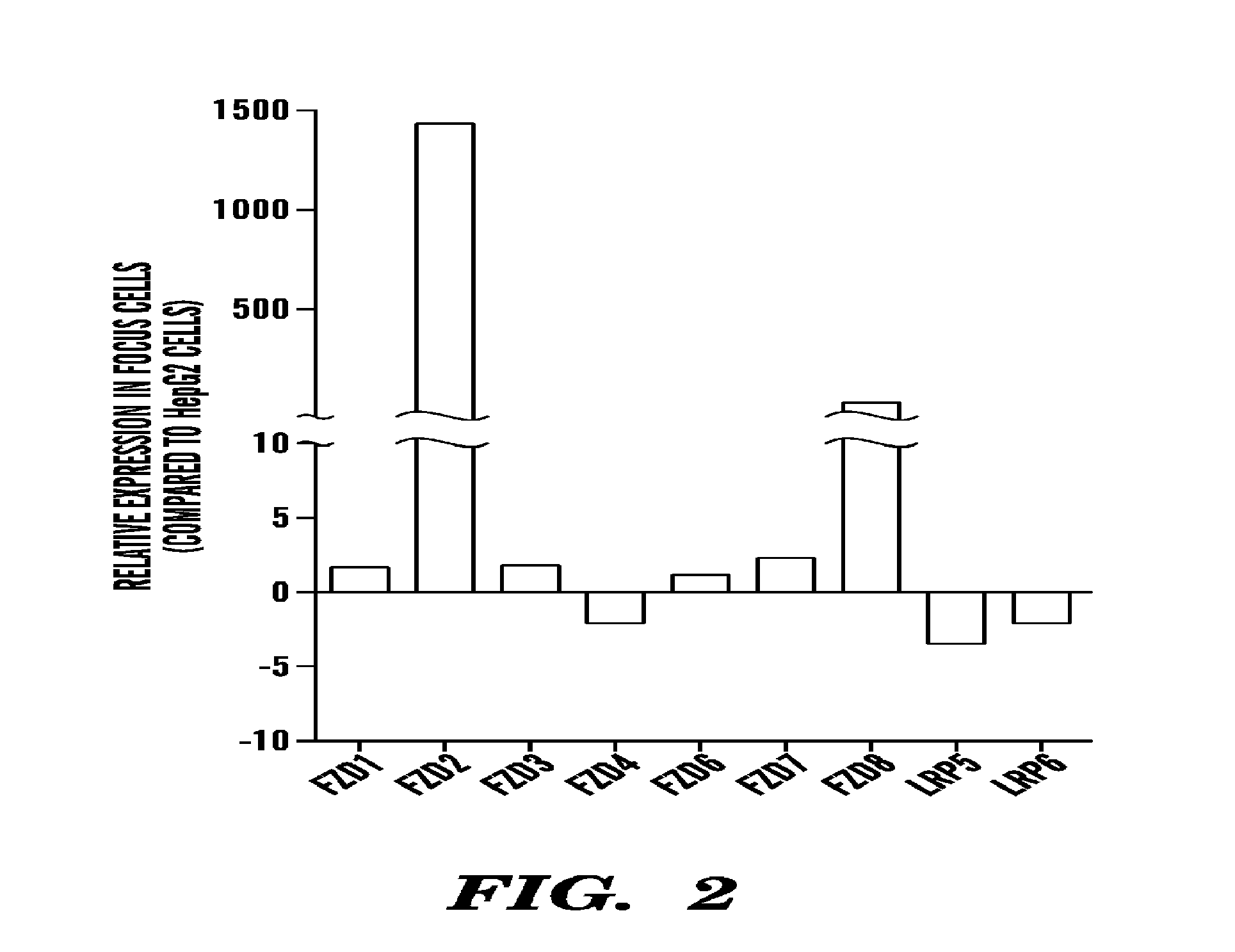
Frizzled 2 as a target for therapeutic antibodies in the treatment of cancer
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Wnt5a peptides in reduction of cancer stem cells
PendingCN111417403AImprove cancer treatmentEliminate side effectsOrganic active ingredientsPeptide/protein ingredientsCancer preventionWNT5A
Owner:ウィントリサーチアーベー
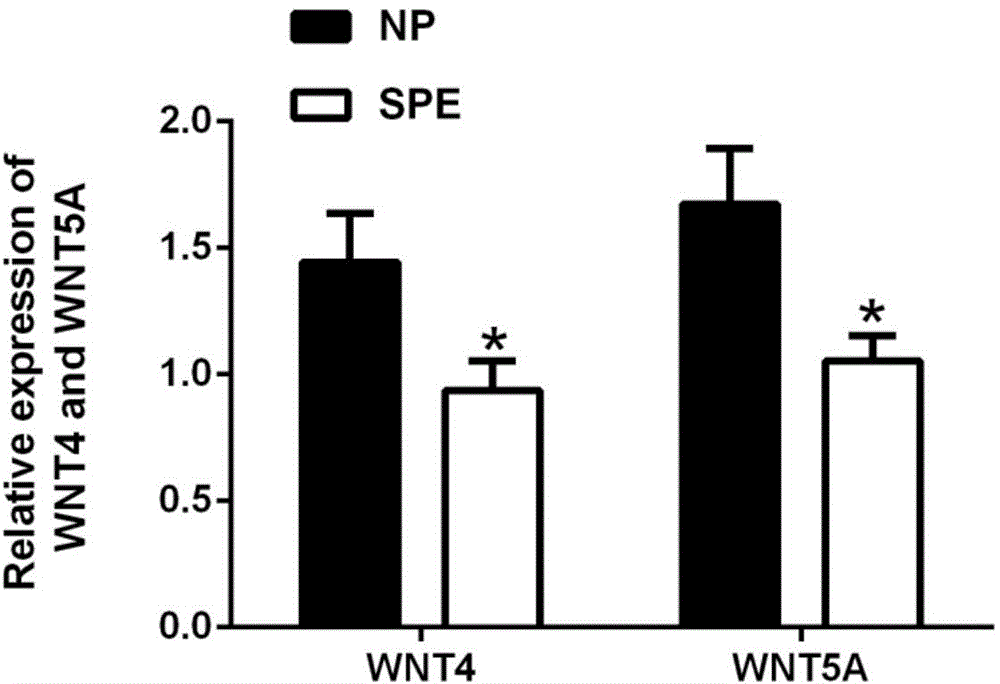
Markers and detection reagents for postpartum eclampsia diagnosis
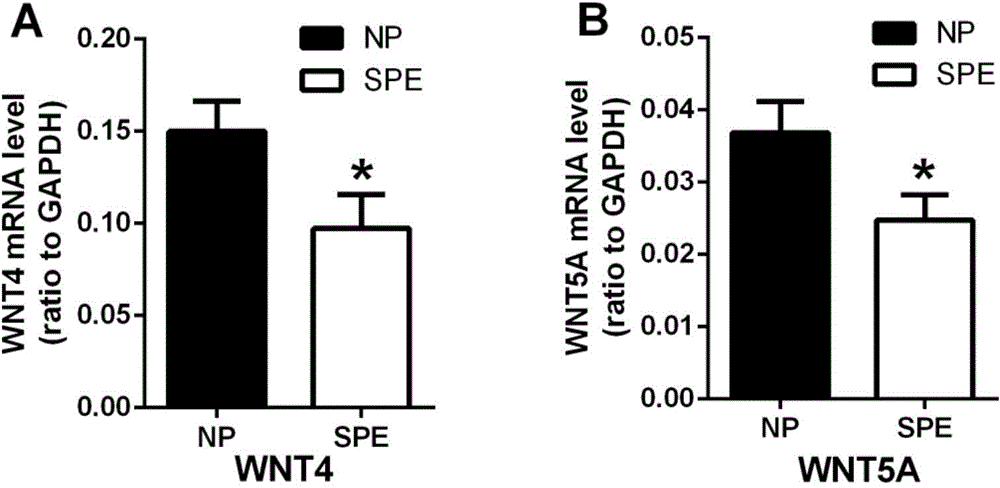
ActiveCN104714032BIncreased sensitivityStrong specificityDisease diagnosisBiological testingWNT5AWnt4 Protein
The invention discloses a marker for postpartum eclampsia diagnosis. The marker is one of WNT4 protein and WNT5A protein or a combination thereof. The invention also discloses a reagent for clinical diagnosis of postpartum eclampsia, wherein the reagent comprises one or both of a WNT4 antibody and a WNT5A antibody, an HRP rabbit anti-goat antibody and a beta-actin antibody. The detection reagent disclosed by the invention has the advantages of high sensitivity and high specificity and can be widely applied to preliminary investigation and health screening of postpartum eclampsia so as to provide reliable data to clinical diagnosis.
Owner:浙江佳米生物医药科技有限公司
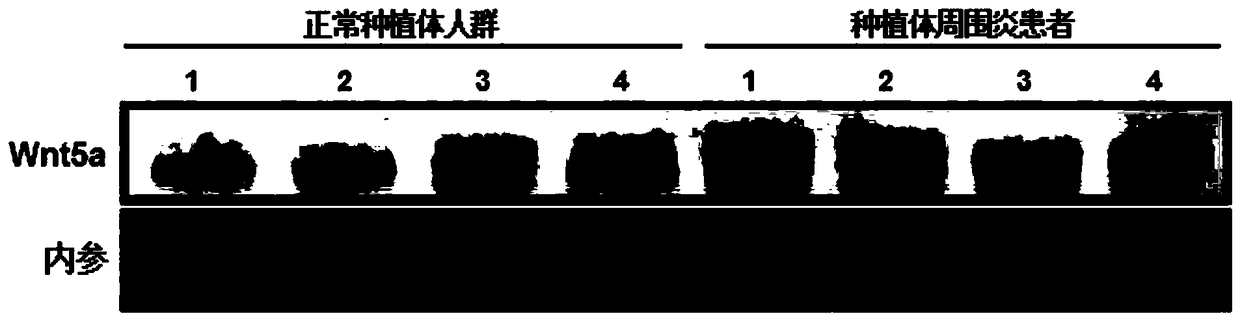
Application of Wnt5a as diagnosis marker of peri-implantitis
The invention belongs to the field of molecular diagnosis technology, and discloses application of Wnt5a as a diagnosis marker of peri-implantitis. According to the invention, it is provided that a risk of having the peri-implantitis by a subject can be judged or status of the peri-implantitis can be determined through detecting an expression level of Wnt5a which is in surrounding tissue of an implant or in fluid surrounding the implant. Compared with traditional diagnosis methods of the peri-implantitis, use of Wnt5a as the protein marker for diagnosis of the peri-implantitis has the significant advantages of timeliness, specificity, sensitivity and the like, and a patient can know a disease risk in an early stage of appearing of the peri-implantitis, and take appropriate preventive and therapeutic measures for a risk degree.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Peptides in combination with immune checkpoint inhibitors for use in treatment of cancer
PendingCN113939305APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsWNT5AOncology
A WNT5A peptide or derivatives thereof in combination with one or more checkpoint inhibitors for use in treatment of cancer in a subject in need thereof. Furthermore, WNT5A peptides or derivatives thereof may be used in treatment of cancer in a subject, wherein the subject is responsive to immune checkpoint inhibitors.
Owner:ウィントリサーチアーベー
Frizzled 2 as a target for therapeutic antibodies in the treatment of cancer
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Kit for diagnosing or detecting leukemia
InactiveCN101942519BMicrobiological testing/measurementFluorescence/phosphorescenceReverse transcriptaseFluorescence
The invention relates to a kit for diagnosing or detecting leukemia, which contains a lymphocytes separation medium, total RNA extracting solution, reverse transcription buffer solution, M-MLV reverse transcriptase, a reverse transcription primer Oligo(dT)18, an RNA enzyme inhibitor, DEPC water, quantitative PCR buffer solution, Taq DNA polymerase, dNTPs solution, a Wnt5a gene reference standard, an internal reference GAPDH gene reference standard, a primer and a fluorescent probe for detecting a Wnt5a gene and a primer and a fluorescent probe for detecting an internal reference GAPDH gene. The kit for diagnosing or detecting the leukemia has the advantages of simple preparation, convenient detection, rapidness, sensitivity, accurate quantification, high repetitiveness and specificity and suitability for clinical application.
Owner:ARMY MEDICAL UNIV
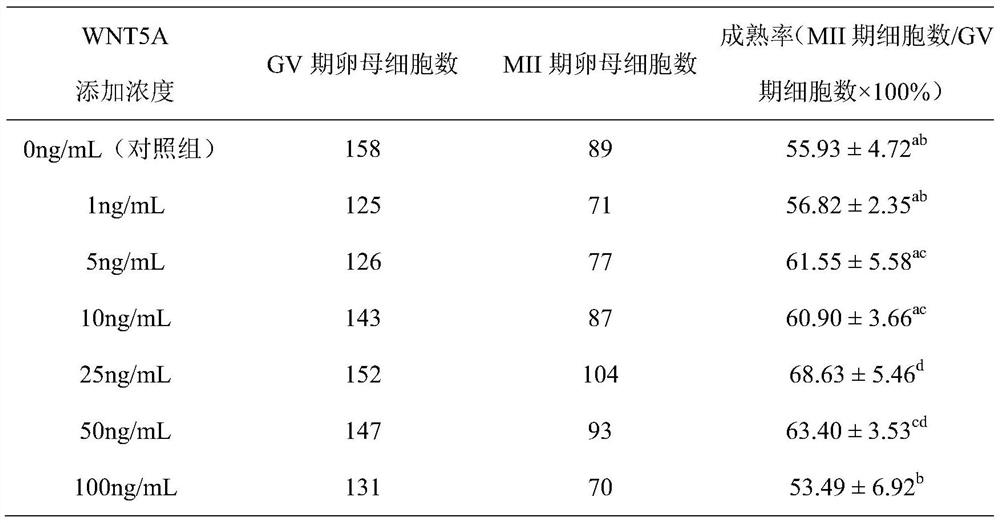
A culture medium for promoting porcine oocyte maturation in vitro
ActiveCN110951678BQuality improvementImprove maturity rateCulture processCell culture active agentsNuclear transferPancreatic hormone
The invention discloses a culture solution for promoting the in vitro maturation of pig oocytes. The culture solution is based on the M199 culture solution without HEPES, and is also added with porcine follicular fluid, L-cysteine, sodium pyruvate, Epidermal growth factor, insulin, gonadotropins, chorionic gonadotropin, and WNT5A cytokines. Using this culture medium to culture porcine oocytes in vitro can effectively improve the efficiency and quality of in vitro maturation of oocytes, and the mature oocytes thus obtained can be used for the production of pig in vitro fertilization and somatic cell nuclear transfer embryos, and the blastocysts develop Efficiency and quality have also been significantly improved.
Owner:HUAZHONG AGRI UNIV
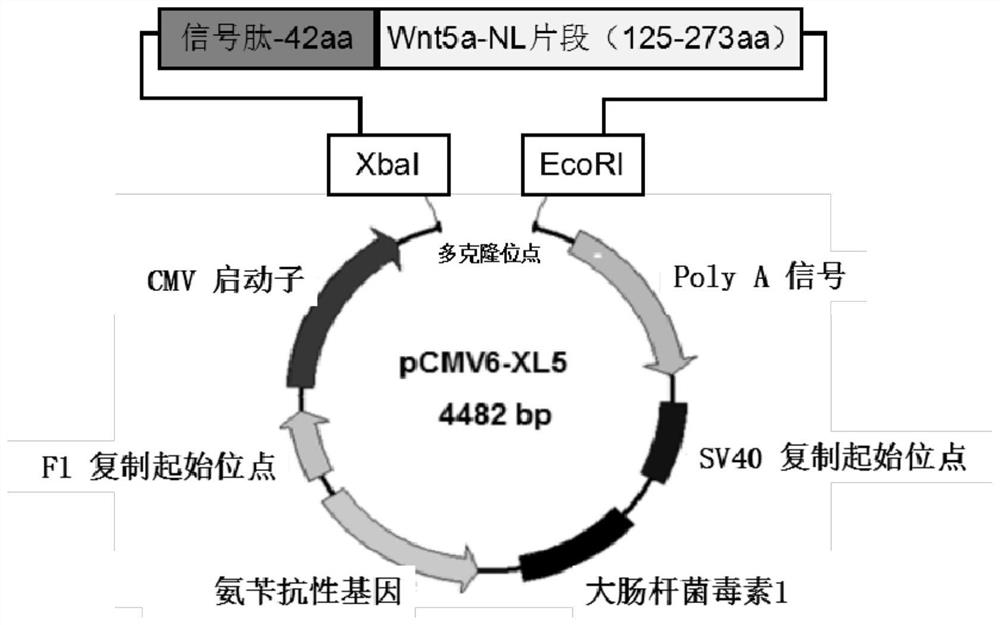
Human wnt5a-nl nucleic acid recombinant and its preparation method and application
InactiveCN108314721BGood treatment effectEasy to makePeptide/protein ingredientsAntipyreticDiseaseArthritis
The present invention provides a novel human Wnt5a-NL nucleic acid recombinant and its preparation method and application. The human Wnt5a-NL nucleic acid recombinant of the present invention is a recombinant plasmid obtained by constructing the human Wnt5a protein truncated gene encoding gene shown in SEQ ID NO: 1 on a eukaryotic expression vector. Experiments have shown that human Wnt5a-NL nucleic acid recombinant injection mice can inhibit inflammation and bone destruction, and can be developed for the treatment of rheumatoid arthritis and other diseases. The nucleic acid recombinant provided by the invention belongs to the therapeutic plasmid, which can provide a new treatment approach and idea for rheumatoid arthritis and other diseases. The nucleic acid recombinant has good therapeutic effect, simple preparation, low cost, good stability and convenient storage, and is suitable for large-scale production.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


























![Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9abd9ba-680e-41ac-9c38-21dd54d8667b/HDA0000600483710000011.PNG)
![Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9abd9ba-680e-41ac-9c38-21dd54d8667b/HDA0000600483710000012.PNG)
![Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof Anti-human interleukin-6 receptor [beta]-chain monoclonal antibody, preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a9abd9ba-680e-41ac-9c38-21dd54d8667b/HDA0000600483710000021.PNG)