Serum-free medium for mesenchymal stem cells as well as preparation method and applications of serum-free medium
A serum-free medium and mesenchymal stem cell technology, applied in primary culture and large-scale subculture, the formula and preparation of new mesenchymal stem cell serum-free medium, can solve the difficulties of standardization of stem cell production, stem cell toxicity, Serum is expensive and other problems, to achieve the effects of standardization and continuous production, reducing the chance of contamination, and reliable experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the preferred version of the present invention is as follows:
[0072] The final concentration of each component in the basal medium after mixing the basal medium with concentrated supplements:
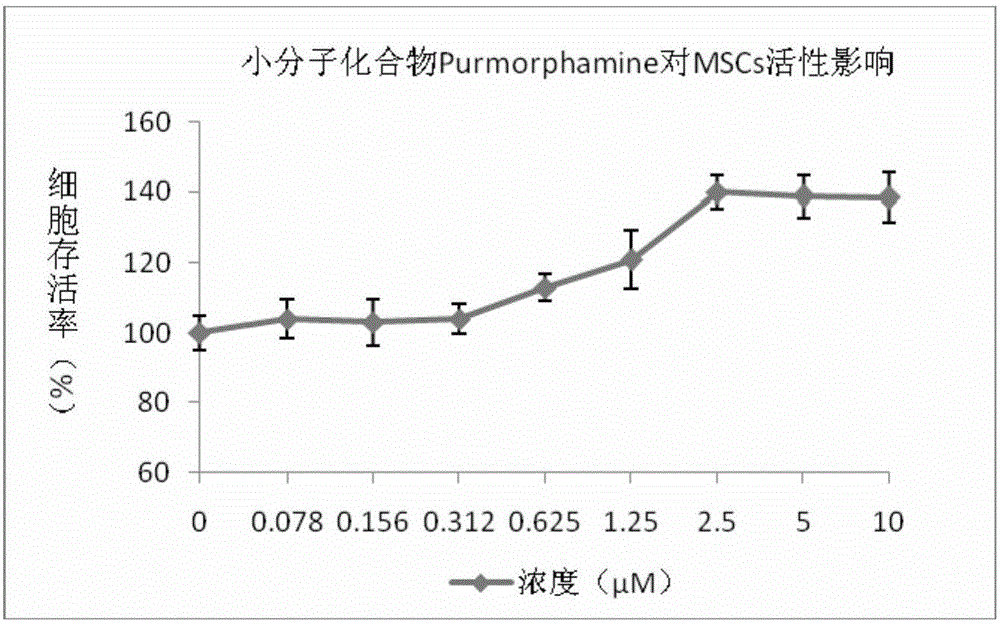
[0073] DMEM-LG medium powder 10g / L; Purmorphamine 2.5μM; Insulin 10.0mg / L; Transferrin 0.60mg / L; Ethanolamine 0.4mg / L; L-ascorbic acid-2-phosphate 15μmol / L; ×10 -5 mol / L; L-glutamine 150mg / L; L-alanyl-L-glutamine 50mg / L; sodium selenite 2.5mg / L; sodium pyruvate 150mg / L; ferrous sulfate 0.8mg / L L; zinc chloride 0.4mg / L; dexamethasone 1.0nmol / L; sodium bicarbonate 2000mg / L; 4-hydroxyethylpiperazineethanesulfonic acid 1000mg / L;
[0074] Concentrate Additive Components and Concentrations Prepared Before Mixing:
[0075] Human serum albumin 20g / L; fibronectin 10mg / L; α-antitrypsin 5mg / L; linoleic acid 5mg / L; linolenic acid 5mg / L; lecithin 2mg / L; BMP-7 5μg / L ; Wnt5a 125 μg / L; EGF 50 μg / L; bFGF 100 μg / L; PDGF 10 μg / L; TGF-β 2 μg / L.
Embodiment 2
[0076] Embodiment 2: The experiment is divided into three groups, which are respectively three kinds of culture media, as follows:
[0077] A culture medium: the serum-free medium for mesenchymal stem cells in the present invention is composed of basal medium and concentrated additives mixed in a volume ratio of 9:1;
[0078] B medium: commercial serum-free medium, StemPro MSC SFMCTS human mesenchymal stem cell serum-free medium from Life technologies;
[0079] C medium: commercial medium with serum, MEM+10% fetal bovine serum.
[0080] In this example, the cultivation of primary cells of human umbilical cord mesenchymal stem cells by three culture media was compared. Take the umbilical cord tissue, sterilize it with 75% alcohol, remove the arteries, veins and amniotic membrane under aseptic conditions, tear out Huatong glue, and cut it into pieces to about 1-3mm 3 Use a pipette to absorb the Huatong glue block, and divide it into three 75cm mediums respectively equipped wit...
Embodiment 3
[0084] Example 3: Primary umbilical cord mesenchymal stem cells were cultured using the serum-free A medium, B medium, and C medium of the present invention to detect their effects on cell phenotype. The primary umbilical cord mesenchymal stem cells cultured in three kinds of media were harvested, washed twice with normal saline, the supernatant was discarded, and the cell concentration was adjusted to 2×10 6 pieces / ml. Each sample was labeled with fluorescent antibodies. The labeled antibodies included CD13, CD14, CD19, CD29, CD31, CD34, CD44, CD73, CD90, CD105, HLA-ABC, and HLA-DR. Isotype antibodies were used as controls. Add 5 μl of the corresponding fluorescent antibody to each tube, and then add 100 μl of umbilical cord mesenchymal stem cells to each tube, shake well with a vortex shaker, and incubate at 4°C in the dark for 30 min. Add 3ml of normal saline to each tube, wash, centrifuge at 1200rpm for 5min and discard the supernatant; add 2ml of PBS to each tube, centri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com