Vesicles with inner and outer aqueous-phase gradient difference and preparation method and application thereof
A technology of gradient difference and vesicles, which is applied in pharmaceutical formulations, liposome delivery, drug delivery, etc., and can solve problems such as long time-consuming, low drug encapsulation rate, and limited drug loading range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Prescription HSPC 3g
[0095] CH 1g
[0096] PEG-CHS 1g
[0097] Hydration medium: 100ml of 300mmol citric acid solution (pH 4.00).
[0098] Preparation of blank liposomes: Weigh the prescribed amount of HSPC, CH (cholesterol), PEG-CHS (PEG molecular weight is 2000), and dissolve the membrane material with 10ml ethanol at 55°C to obtain the lipid phase; preheated to 55°C The hydration medium was injected into the lipid phase and incubated for 10 minutes to obtain the primary liposome product, which was then subjected to 20,000 psi high-pressure homogenization treatment to reduce the liposome particle size to 90nm, and passed through 0.8, 0.45, and 0.22 μm microporous membranes in sequence. That is, blank liposomes were obtained.
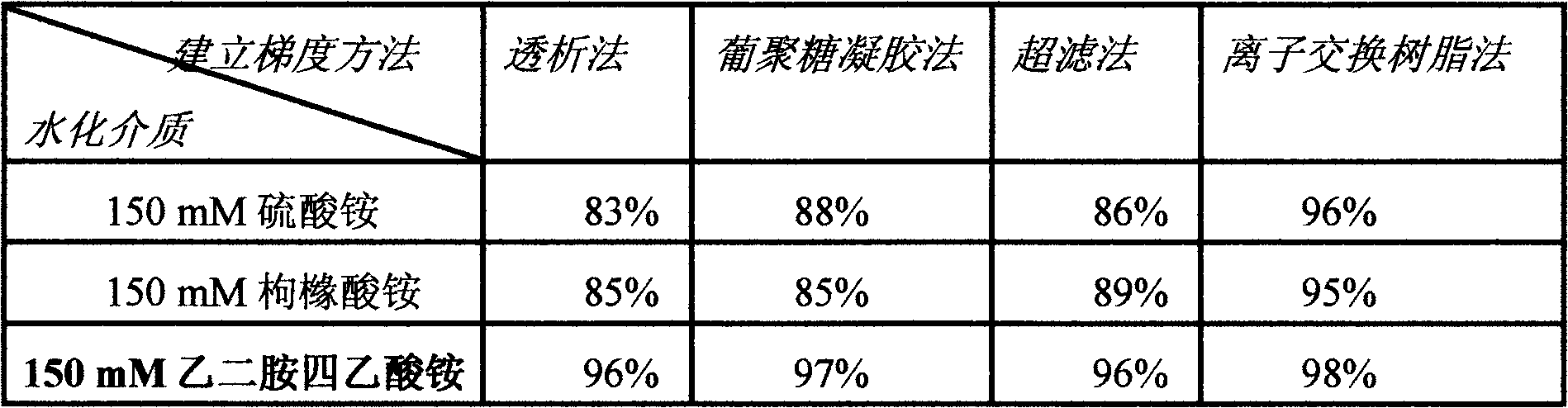
[0099] Establish gradient liposomes: Calculated according to the exchange capacity, 1.2ml (wet volume) anion resin (OH-) (717 type anion resin) can completely exchange 1ml of 300mmol / L citric acid solution. In this embodiment, 1 ml of blan...
Embodiment 2
[0104] Prescription DSPC 5g
[0105] 300mM citrate buffer 100ml
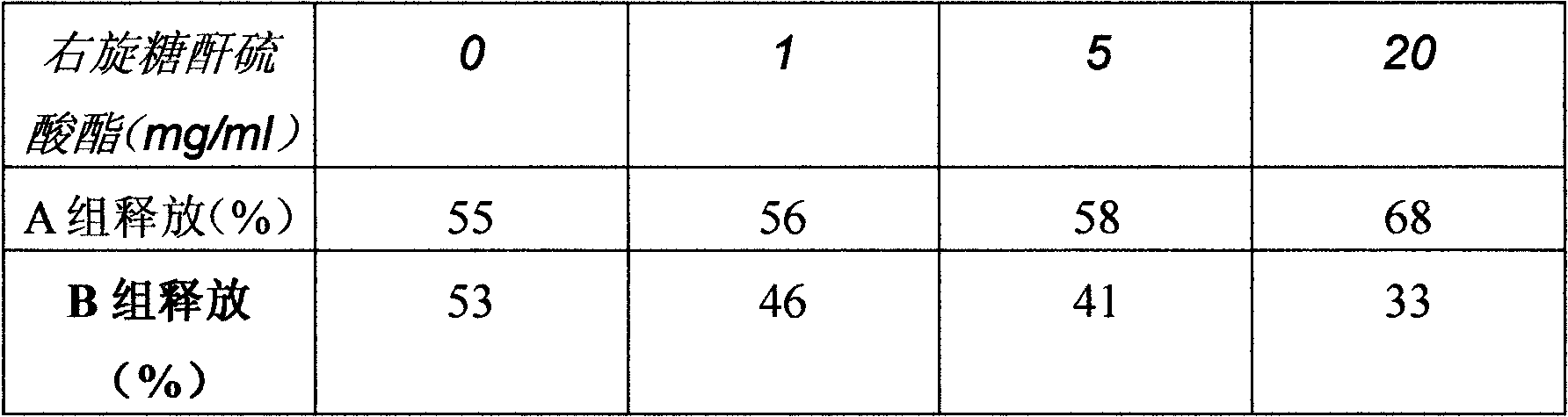
[0106] (1) Take an appropriate amount of chloroform to dissolve DSPC (distearoylphosphatidylcholine), and remove the chloroform by rotary evaporation to prepare a DSPC film. With 100ml 300mM citrate buffer as the hydration medium, the phospholipids are thinly hydrated to prepare DSPC large unilamellar liposomes (about 610nm), using sodium hydroxide solution (conventional method) or hydroxide type anion exchange resin (OH -) Adjust the pH of the external aqueous phase to 7 to establish a pH gradient. The liposomes whose gradient was established by sodium hydroxide solution was marked as A, and the liposomes whose gradient was established by hydroxide type anion exchange resin was marked as B. Microscopic observation revealed that the particle size of B was larger than that of A, and the PSS (Particle Sizing Systems) measurement results showed that the particle sizes of A and B were 596nm and 681nm, respectively...
Embodiment 3
[0110] Prescription: DSPC 3g
[0111] DSPE-PEG 2000 1g
[0112] Hydration medium: 200mM EDTA ammonium salt and 10mg / ml ammonium hyaluronate solution 100ml.
[0113] Prepare blank liposomes: weigh the prescribed amount of DSPC, DSPE-PEG 2000 , 55°C, dissolve the membrane material with 10ml ethanol to obtain the lipid phase; inject the hydration medium preheated to 55°C into the lipid phase, and incubate for 10 minutes to obtain the primary liposome, and then undergo 20,000psi high-pressure homogenization treatment, Reduce the particle size of liposomes to 100 nm, and pass through 0.8, 0.45, and 0.22 μm microporous membranes in turn to obtain blank liposomes.
[0114] Build gradient liposomes:
[0115] A mixed ion exchanger consisting of three ion exchangers: 331 weakly basic epoxy anion exchange resin, 122 weakly acidic phenolic cation exchange resin and DEAE-cellulose. The wet visual volume ratio of 331 weakly basic epoxy anion exchange resin and 122 weak acid phenoli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com