Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67 results about "Trypanosoma sp" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
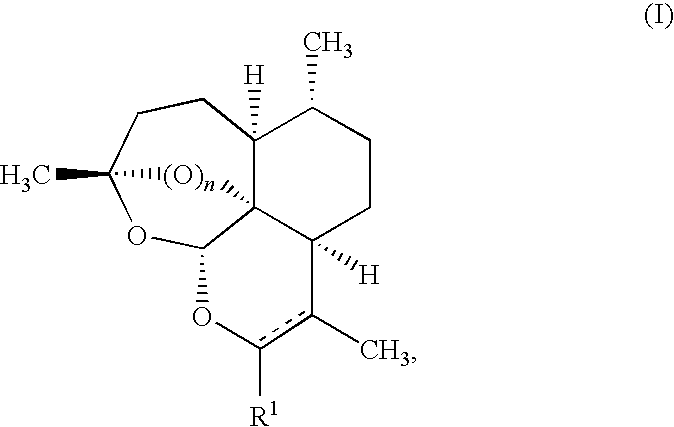
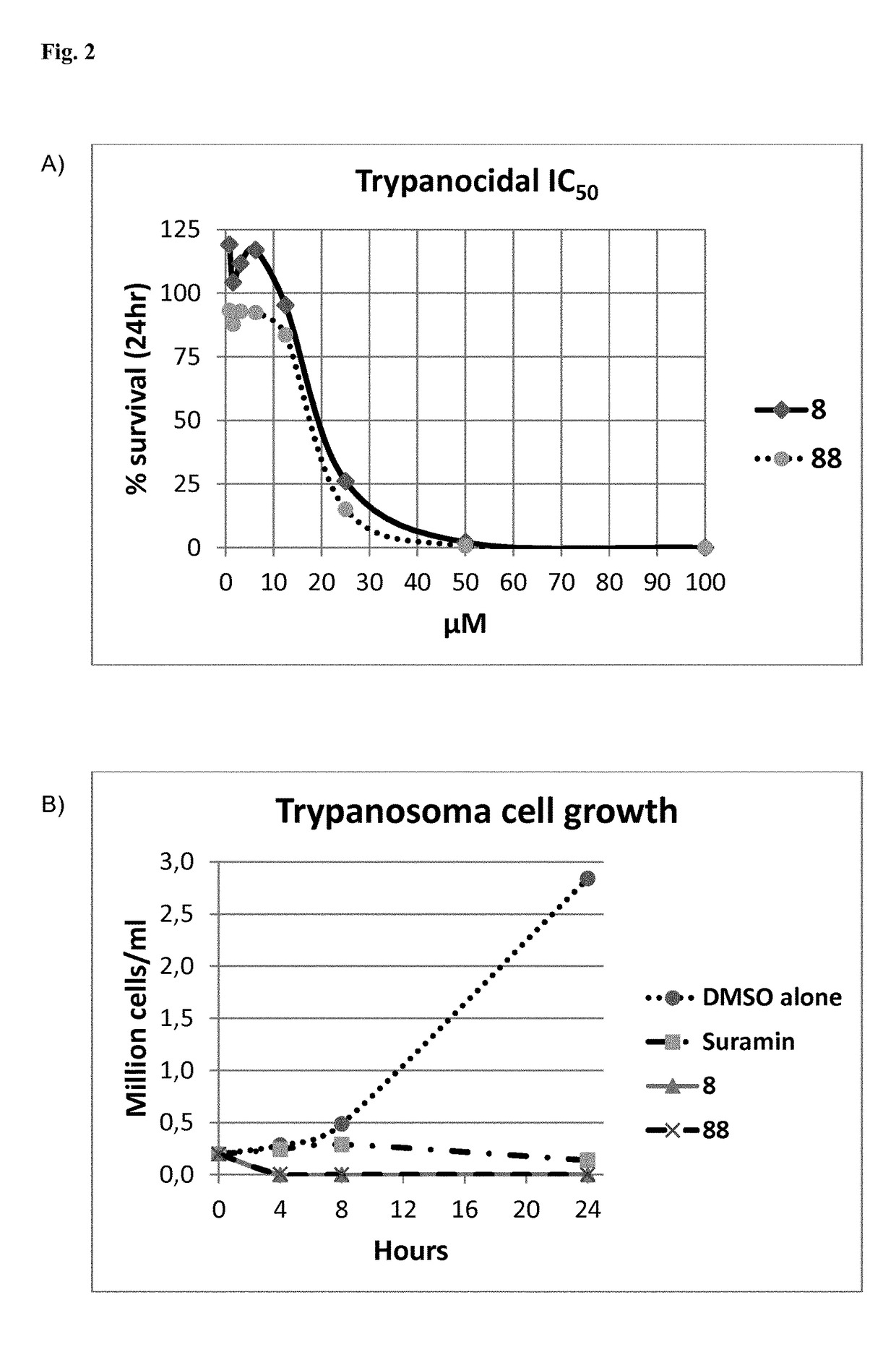
Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
A novel crystalline form of (S)—N-(5-((R)-2-(2,5-difluorophenyl)pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide, pharmaceutical compositions containing said crystalline form and the use of said crystalline form in the treatment of pain, cancer, inflammation, neurodegenerative disease or Trypanosoma cruzi infection are disclosed. In some embodiments, the novel crystalline form comprises a stable polymorph of (S)—N-(5-((R)-2-(2,5-difluorophenyl)pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate. The present invention is further directed to a process for the preparation of the novel crystalline form.
Owner:ARRAY BIOPHARMA
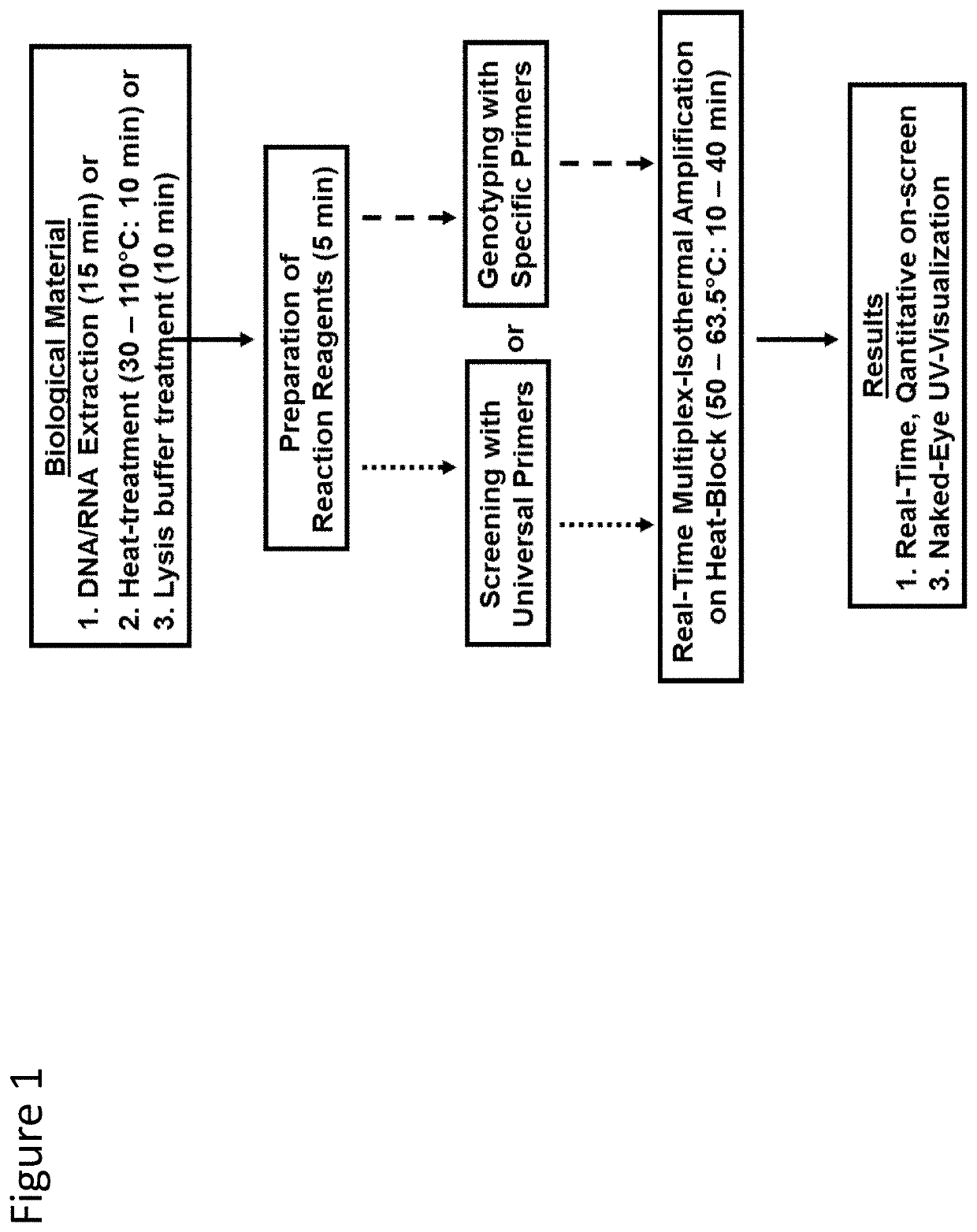
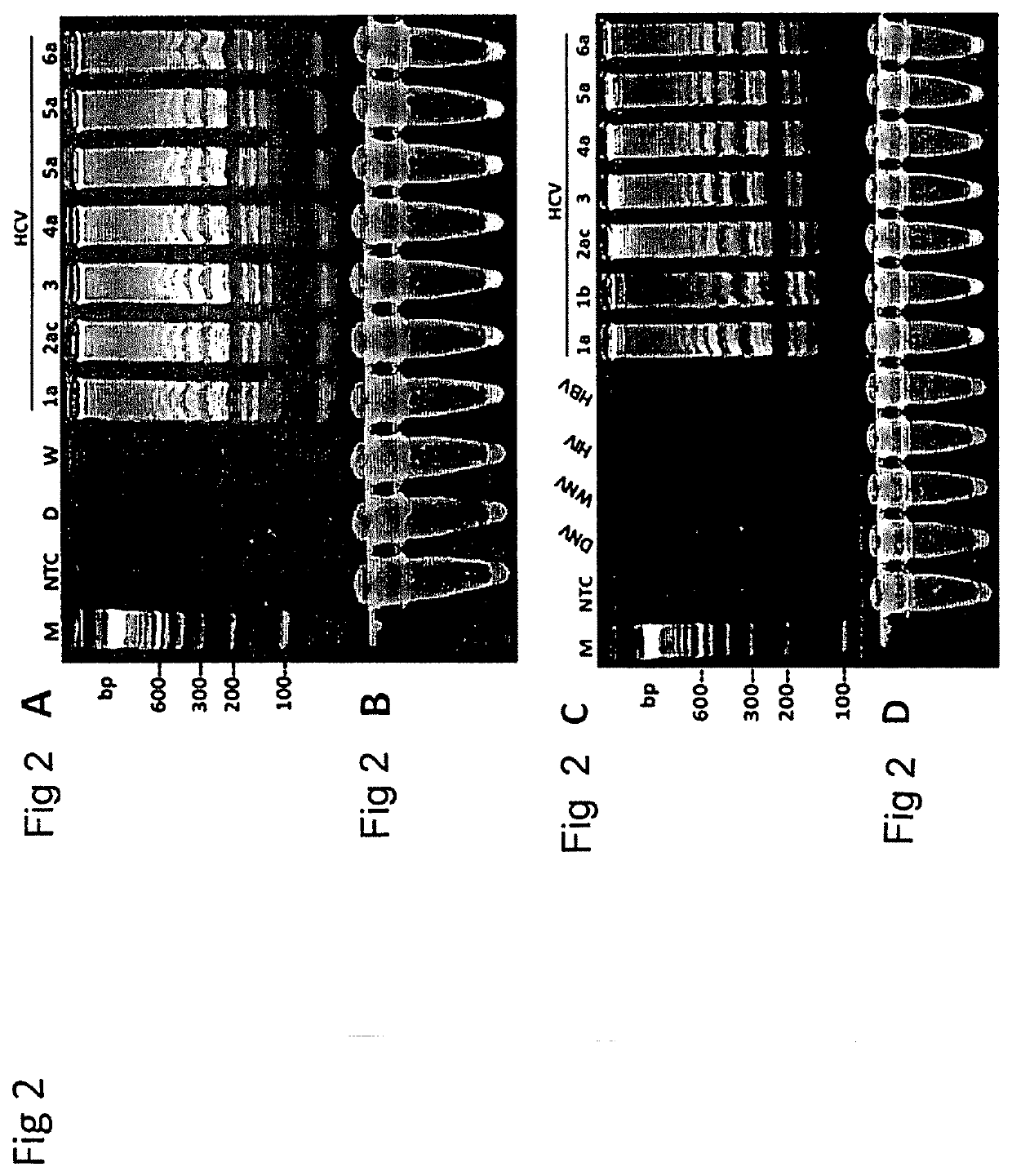
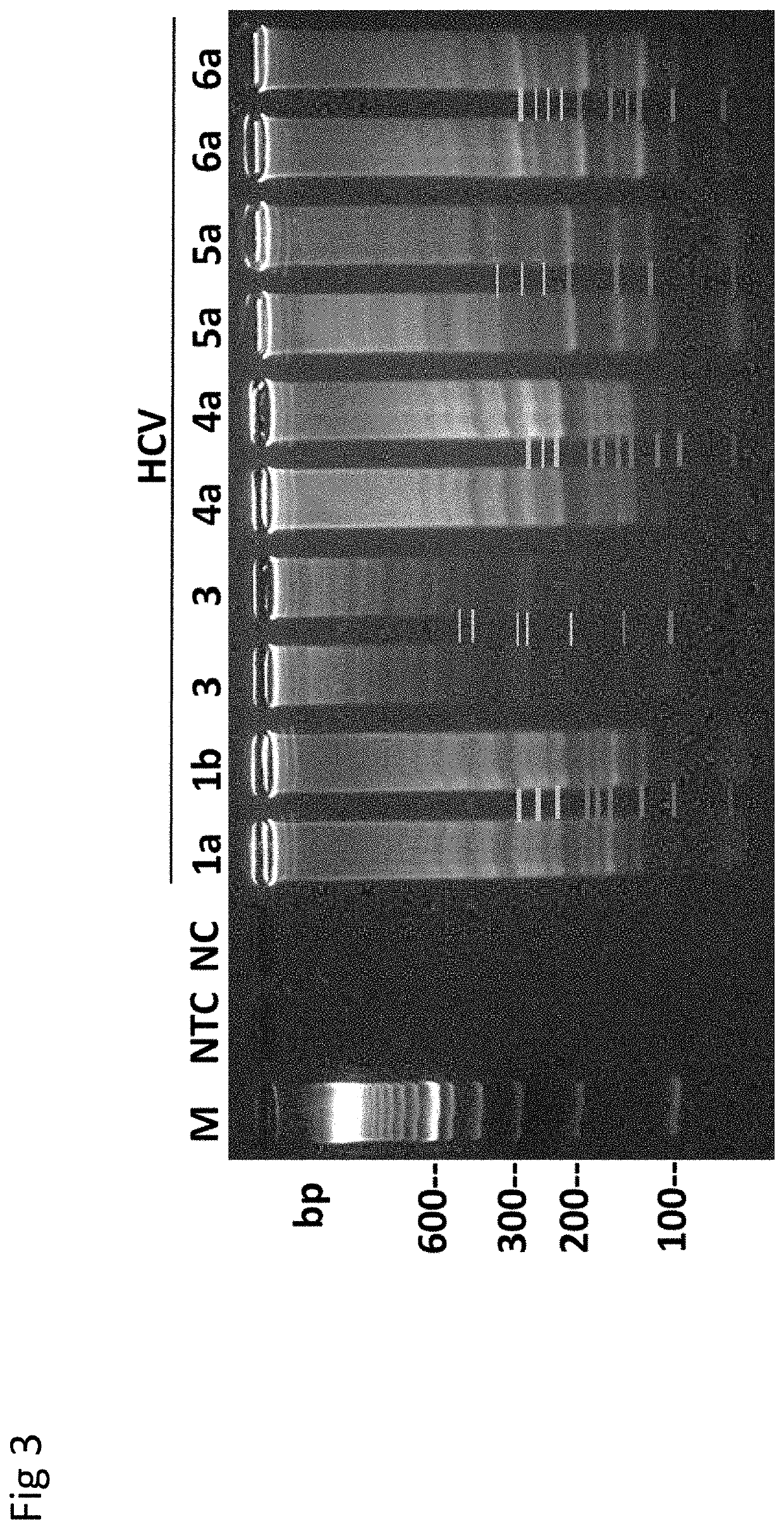
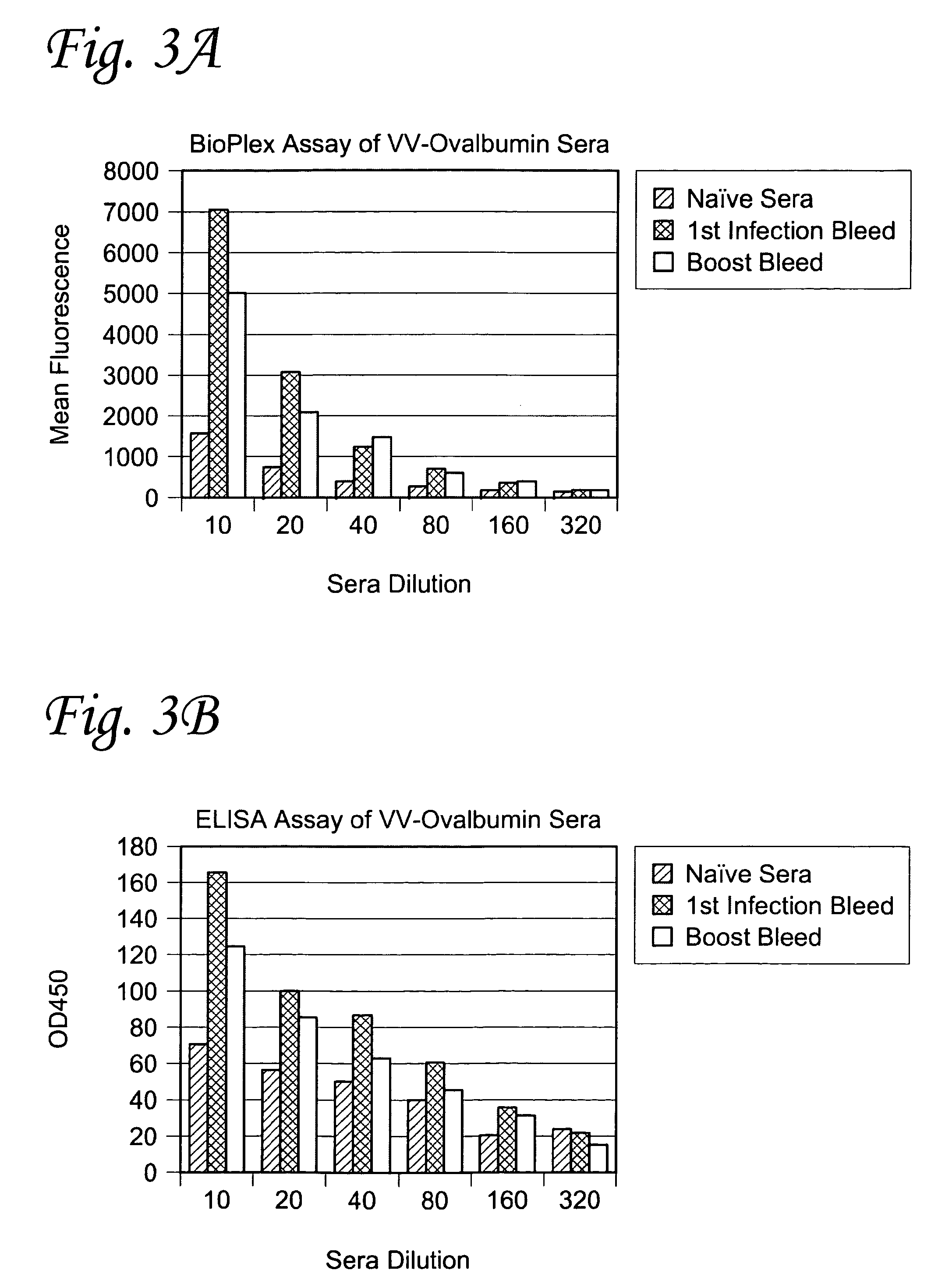
Methods for real-time multiplex isothermal detection and identification of bacterial, viral, and protozoan nucleic acids
ActiveUS20200048722A1Flexible processFacilitate strand displacementMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaLoop-mediated isothermal amplification
Herein disclosed are rapid real-time isothermal multiplex methods of detecting, identifying and quantifying bacterial, viral, and protozoan nucleic acids in a sample. These include contacting the sample with two or more sets of pathogen-specific reverse transcription loop-mediated isothermal amplification primers and novel oligofluorophores specific for the target bacterial, viral, and parasitic nucleic acids of interest such as human immunodeficiency virus, Ebola virus, Marburg virus, Yellow fever virus, hepatitis-B virus, Lassa fever virus, Plasmodium, hepatitis-C virus, hepatitis-E virus, dengue virus, Chikungunya virus, Japanese Encephalitis virus, Middle Eastern Respiratory Syndrome Corona virus, Mycobacterium, West Nile virus, Cytomegalovirus, Parvovirus, Leishmania, Trypanosoma, and Zika virus nucleic acids, under conditions sufficient to produce detectable real-time amplification signals in about 10 to 40 minutes. The amplification signals are produced by pathogen-specific fluorogenic labels included in one or more of the primers. Also, novel reaction and sample lysis buffers, primers, and kits for rapid multiplex detection, quantification, and identification of bacterial, viral, and protozoan nucleic acids by real-time isothermal amplification are herein disclosed.
Owner:NYAN DOUGBEH CHRIS
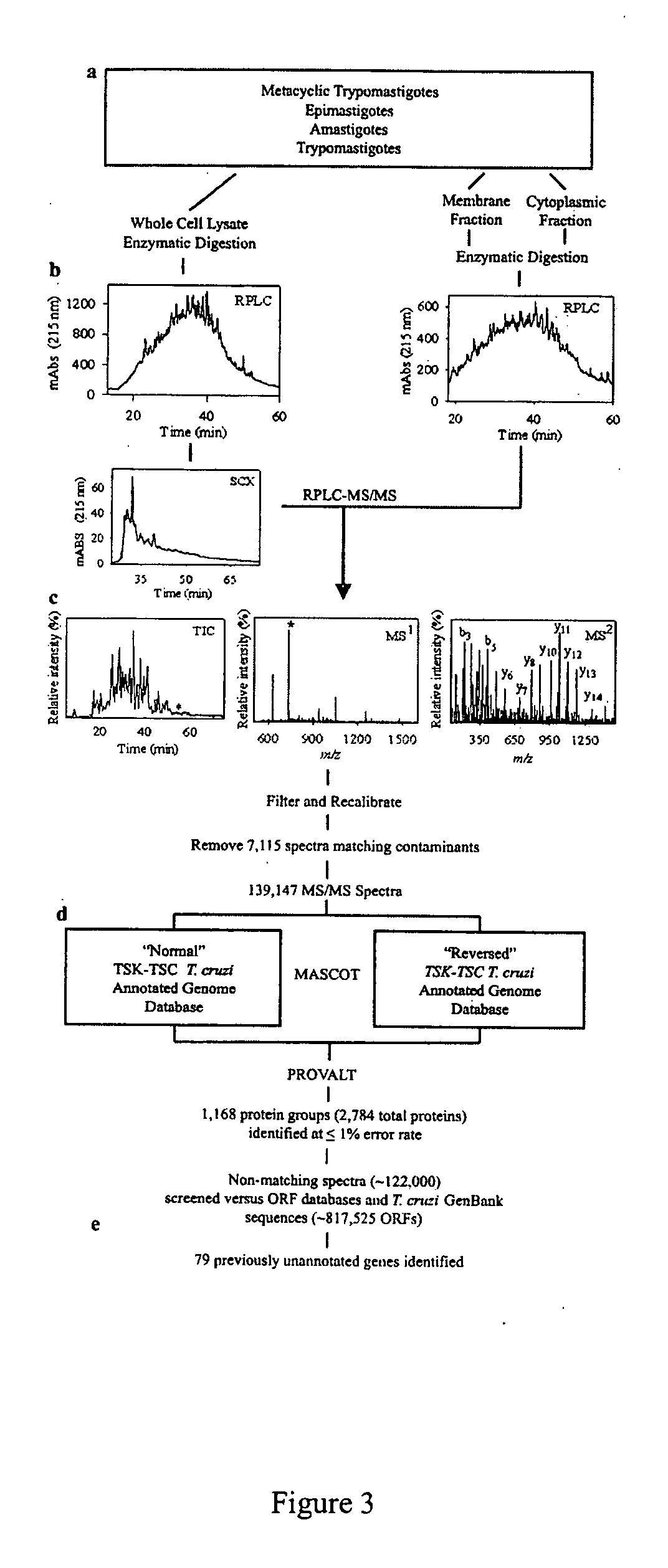
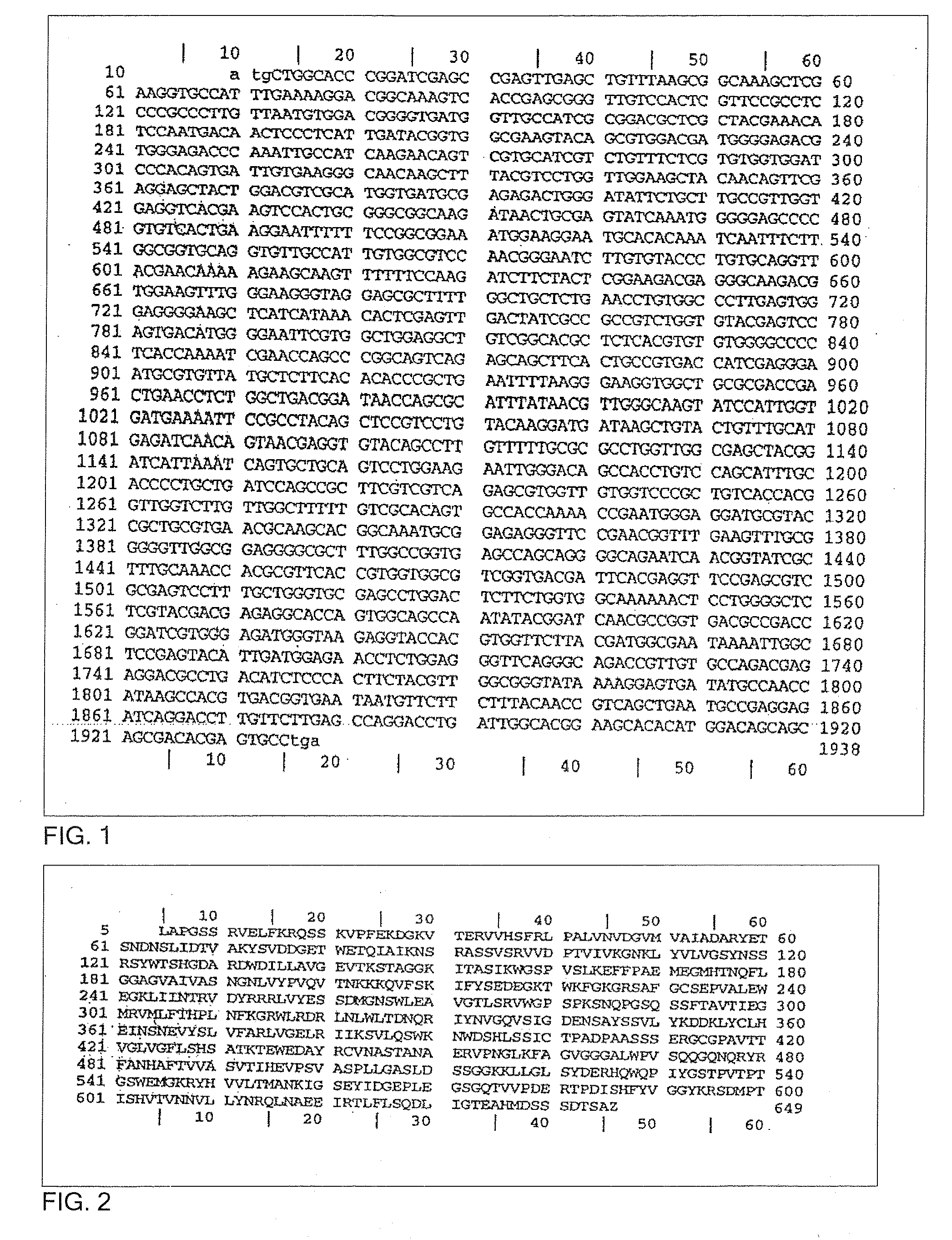
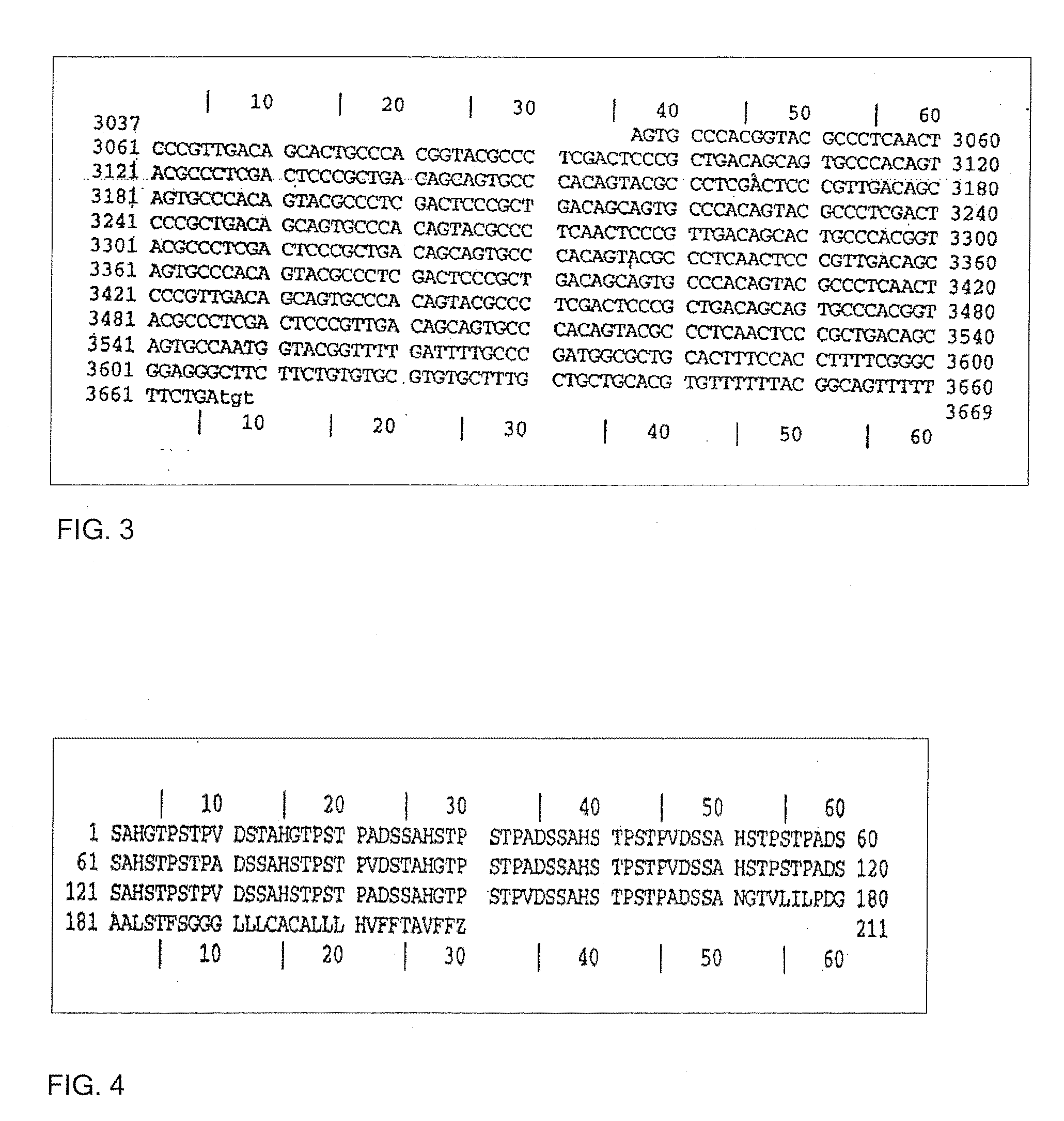
Trypanosoma cruzi proteome compositions and methods
Molecular targets are identified in T. cruzi suitable for use in diagnosis of Chagas disease, drug development, and vaccines, including live vaccines.
Owner:GEORGIA RESERACH FOUND INC UNIV OF
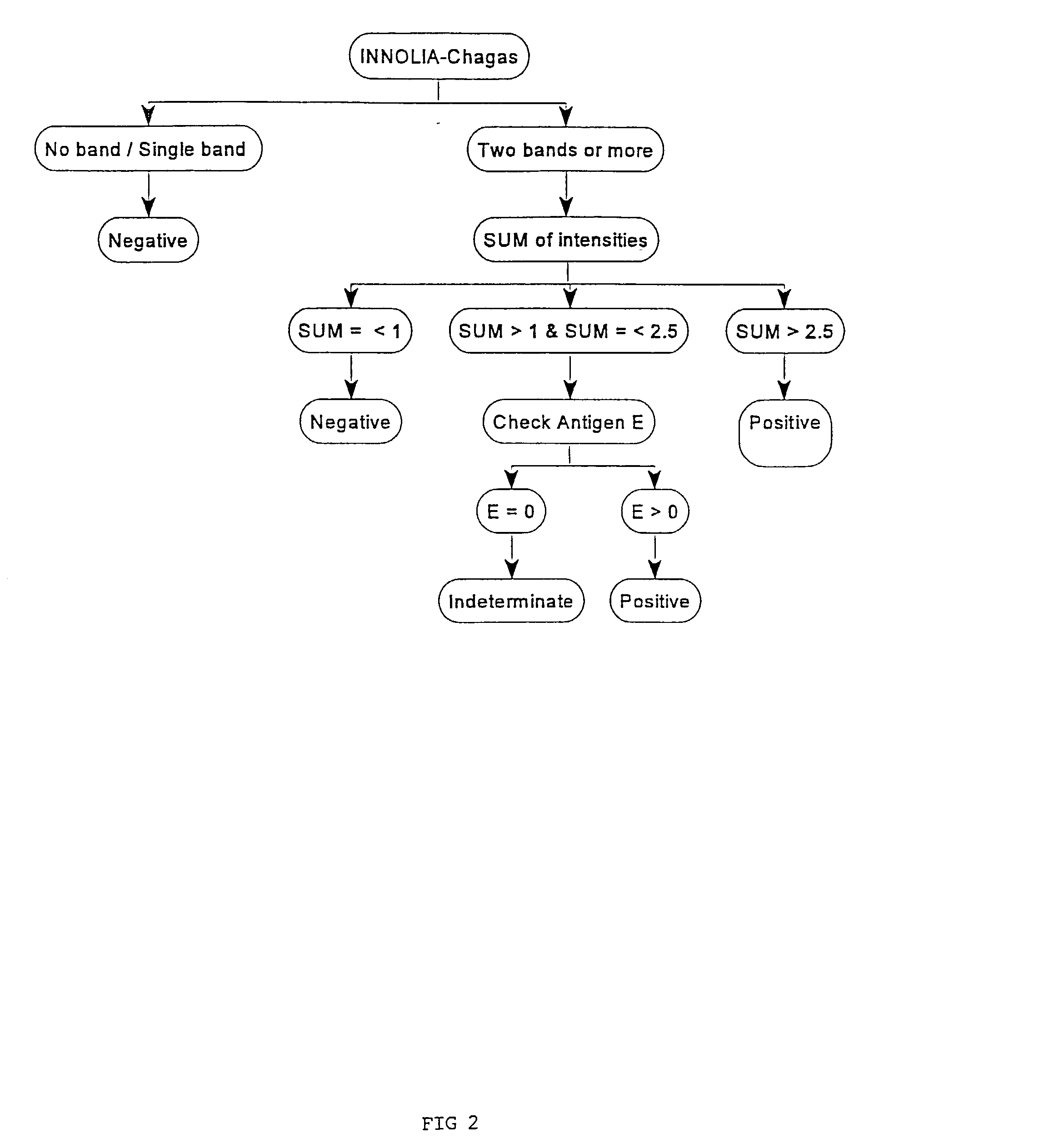
Antigens and immunoassays for diagnosing Chagas' disease
Transfusion of contaminated blood has become the major route of transmission for Chagas' disease. Current screening tests are insensitive and yield conflicting results, while confirmatory assays do not exist. The present invention relates to antigens and their use for serological diagnosis of Chagas' disease. More specifically, the present invention concerns assays which are able to reliably and accurately detect the presence of antibodies to various specific antigens of Trypanosoma cruzi in a highly sensitive and specific manner.
Owner:INNOGENETICS NV
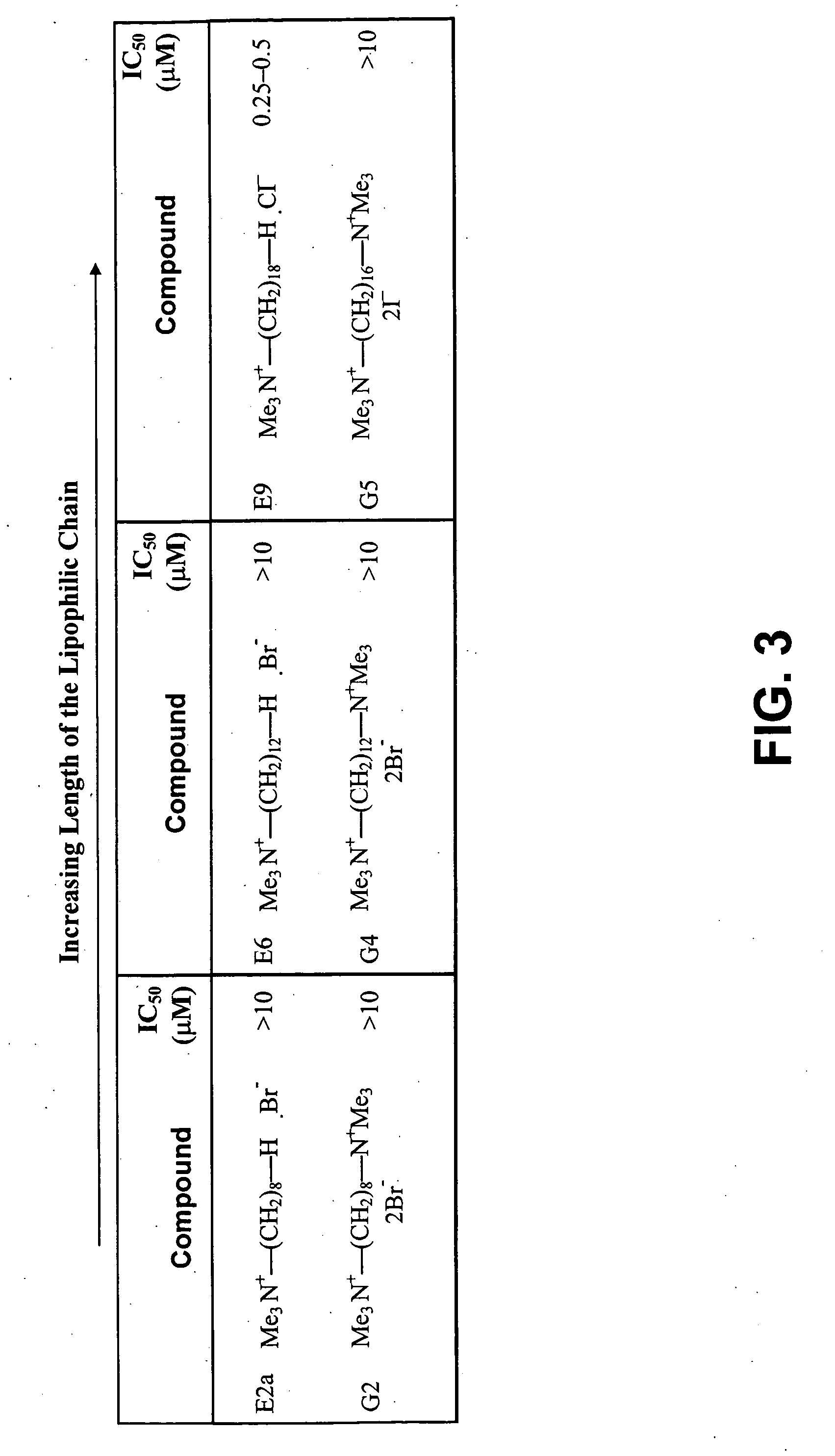
Alkylammonium compounds as antifungal and antitrypanosomal agents
InactiveUS20060025458A1Reduce deliveryAlter hydrophilicityBiocideAmine active ingredientsAntifungalLeishmania major
The use of alkyl quaternary ammonium compounds including certain choline analogs for treating or preventing fungal and trypanosomal (e.g., Leishmaniasis) infections is described. These compounds, characterized as mono- and bis-alkyl ammonium compounds, were demonstrated to be highly effective in inhibiting growth of Candida albicans, Saccharomyces cerevisiae and Leishmania major. Quaternary ammonium compounds were previously known as effective antimalarial compounds in vivo but not recognized as antifungals or as anti-trypanosomals (e.g., anti-Leishmanials).
Owner:UNIV OF CONNECTICUT
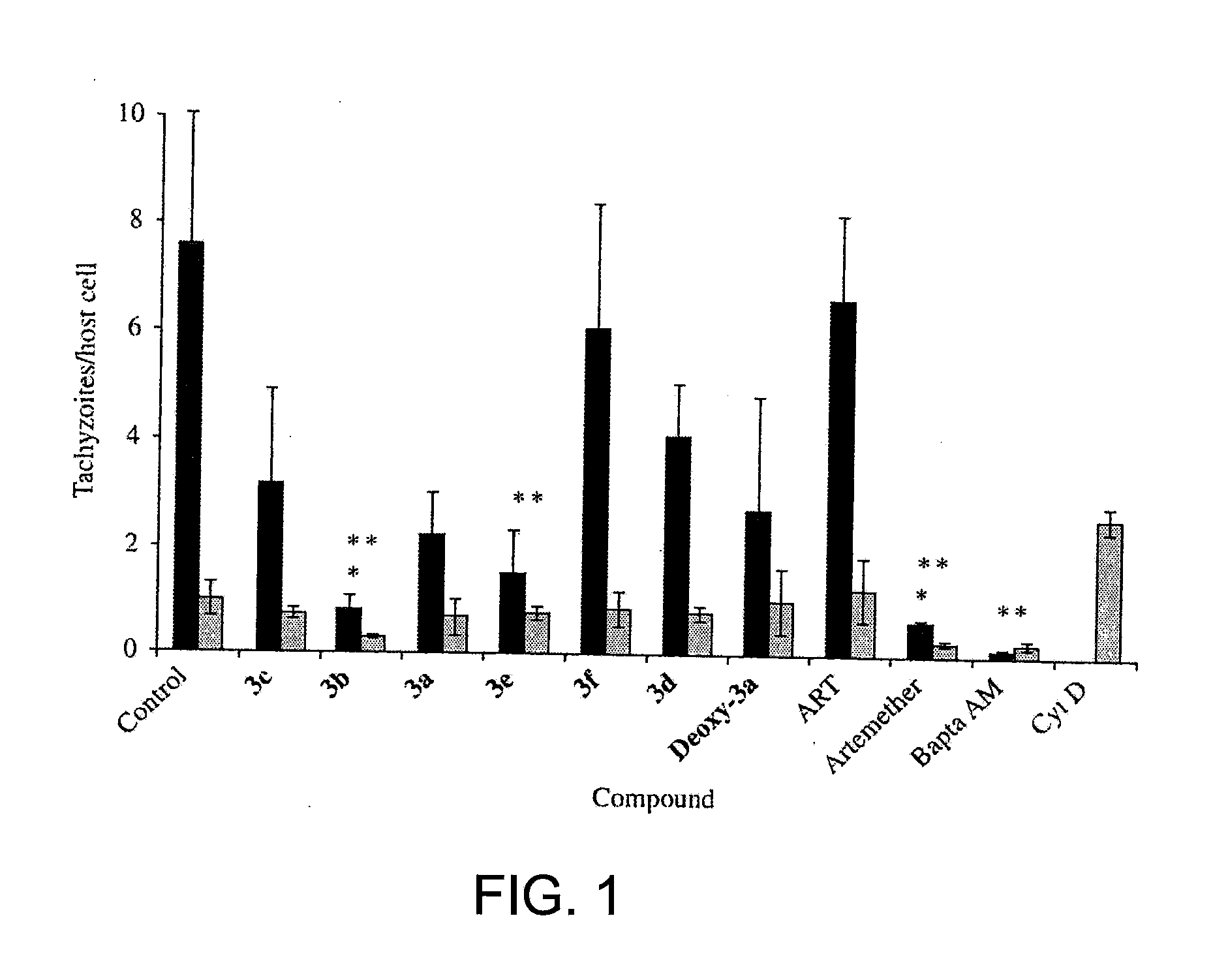
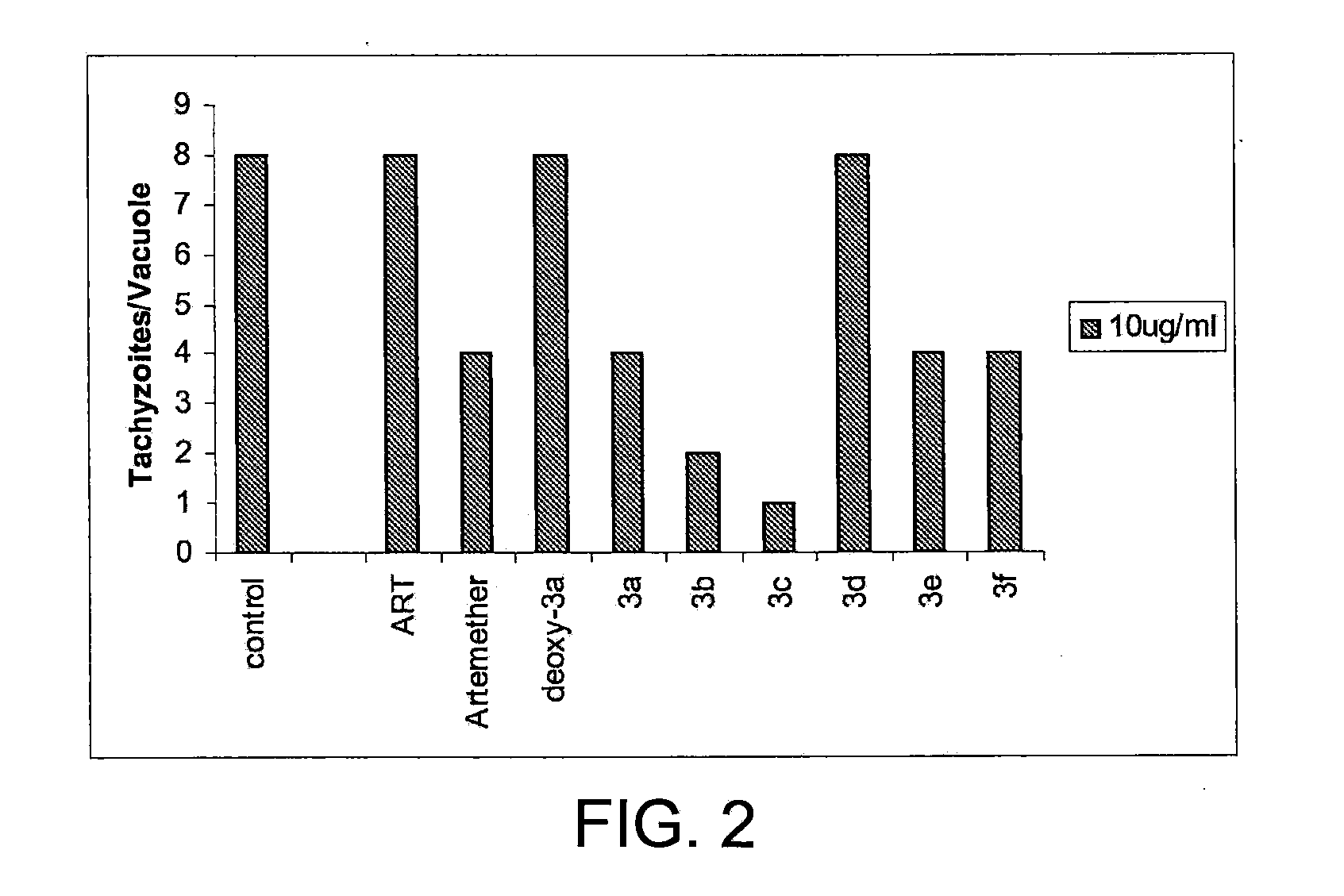
Artemisinin Derivatives
This disclosure provides improved derivatives of artemisinin; pharamaceutical compositions containing these compounds; methods for preparing these compounds and compositions; methods of using these compounds and compositions for preventing, controlling or treating infectious diseases including but not limited to parasitic infectious diseases such as T. gondii infection, trypanosome parasite infection, plasmodia parasite infection, and cryptosporidium parasite infection; methods for preventing, controlling or treating toxoplasma infection; and methods for treating psychiatric disorders associated with toxoplasma infection including but not limited to schizophrenia using the disclosed compounds and compositions alone or in combination with one or more antipsychotic drugs.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
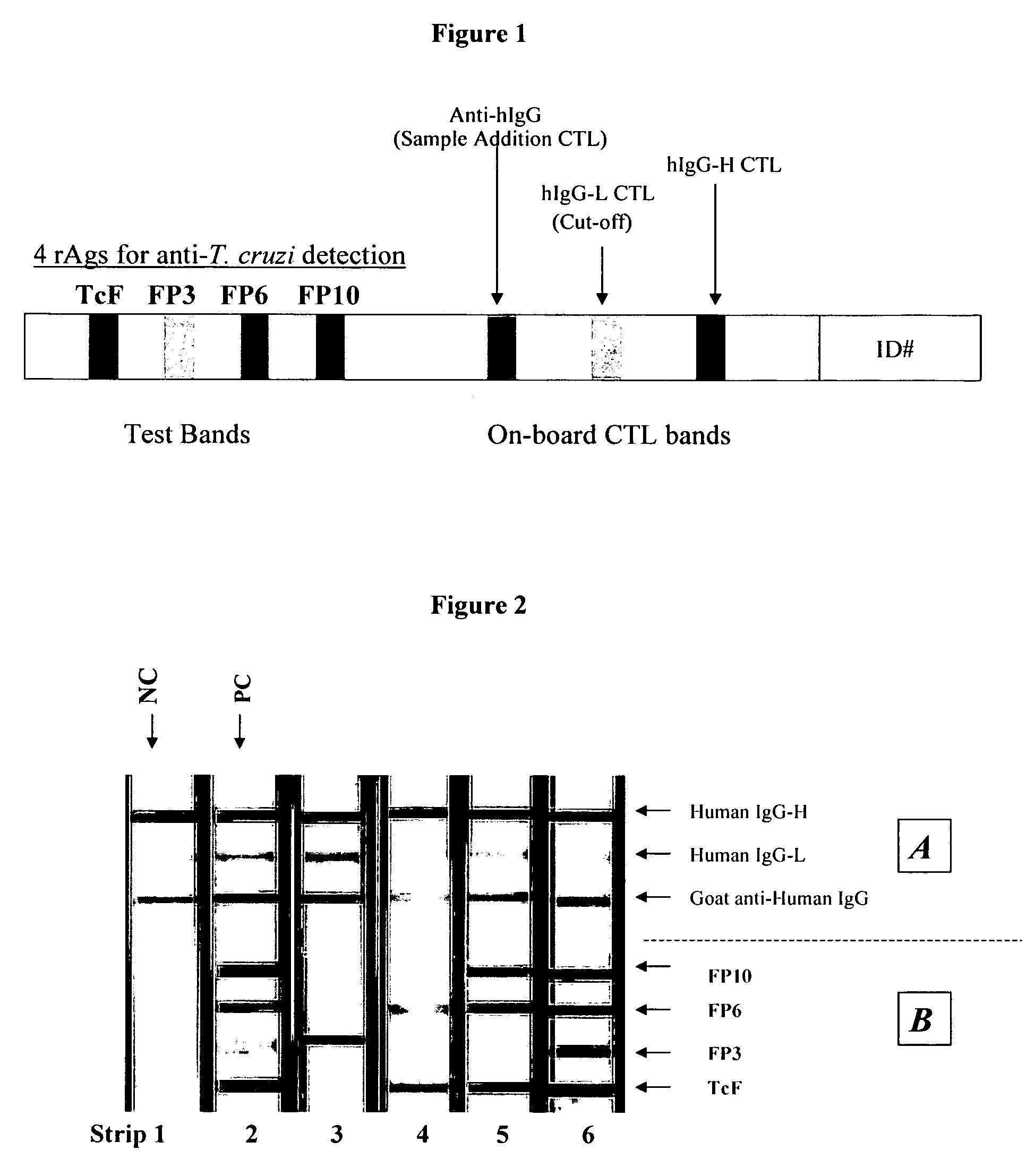
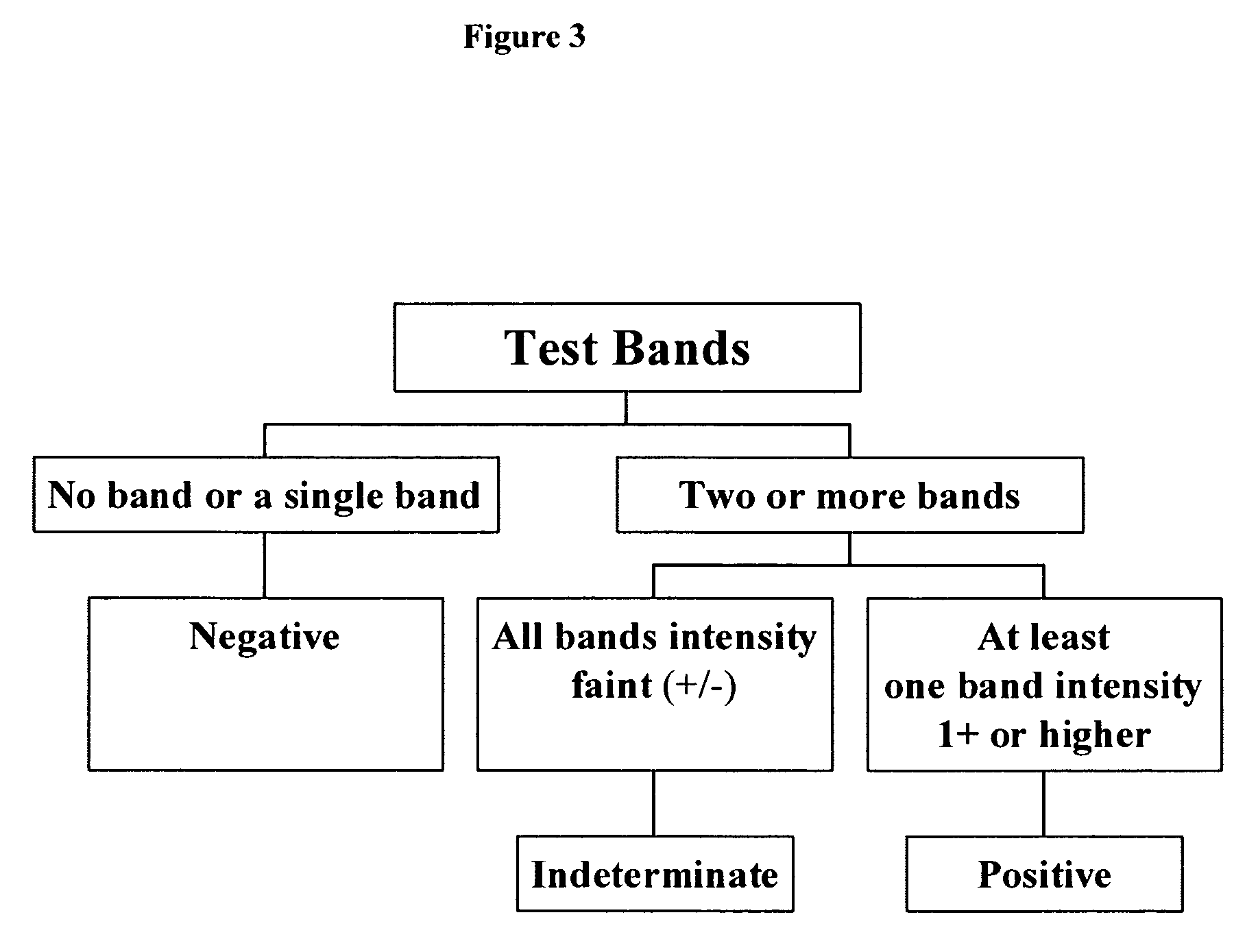
Diagnostic Assay For Trypanosoma Cruzi Infection
ActiveUS20080019995A1Reduce constructionEase of productionProtozoa antigen ingredientsDrug compositionsInfection diagnosisAssay
A sensitive, multicomponent diagnostic test for infection with T. cruzi, the causative agent of Chagas disease, including methods of making and methods of use. Also provided is a method for screening T. cruzi polypeptides to identify antigenic polypeptides for inclusion as components of the diagnostic test, as well as compositions containing antigenic T. cruzi polypeptides.
Owner:UNIV OF GEORGIA RES FOUND INC
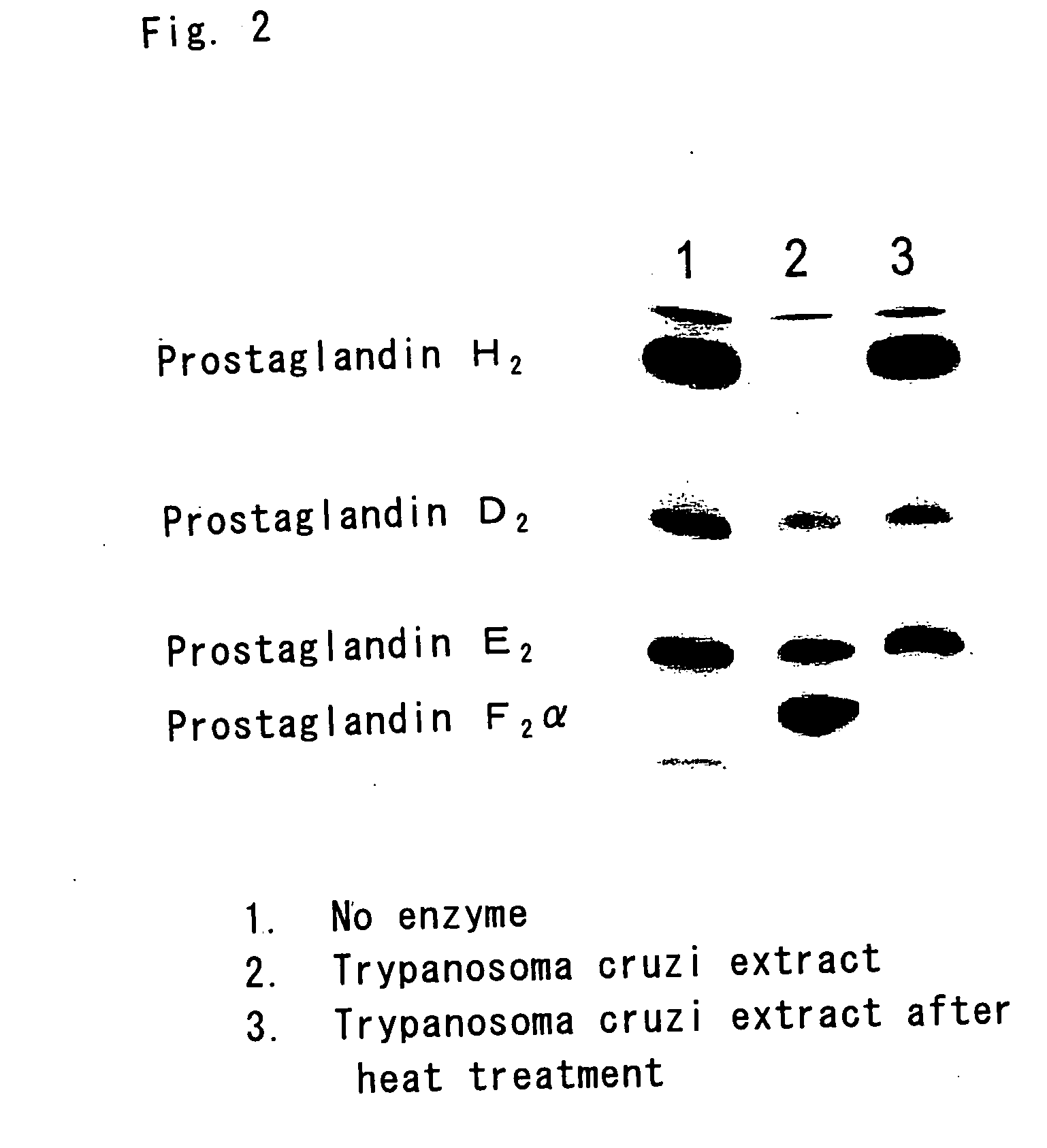
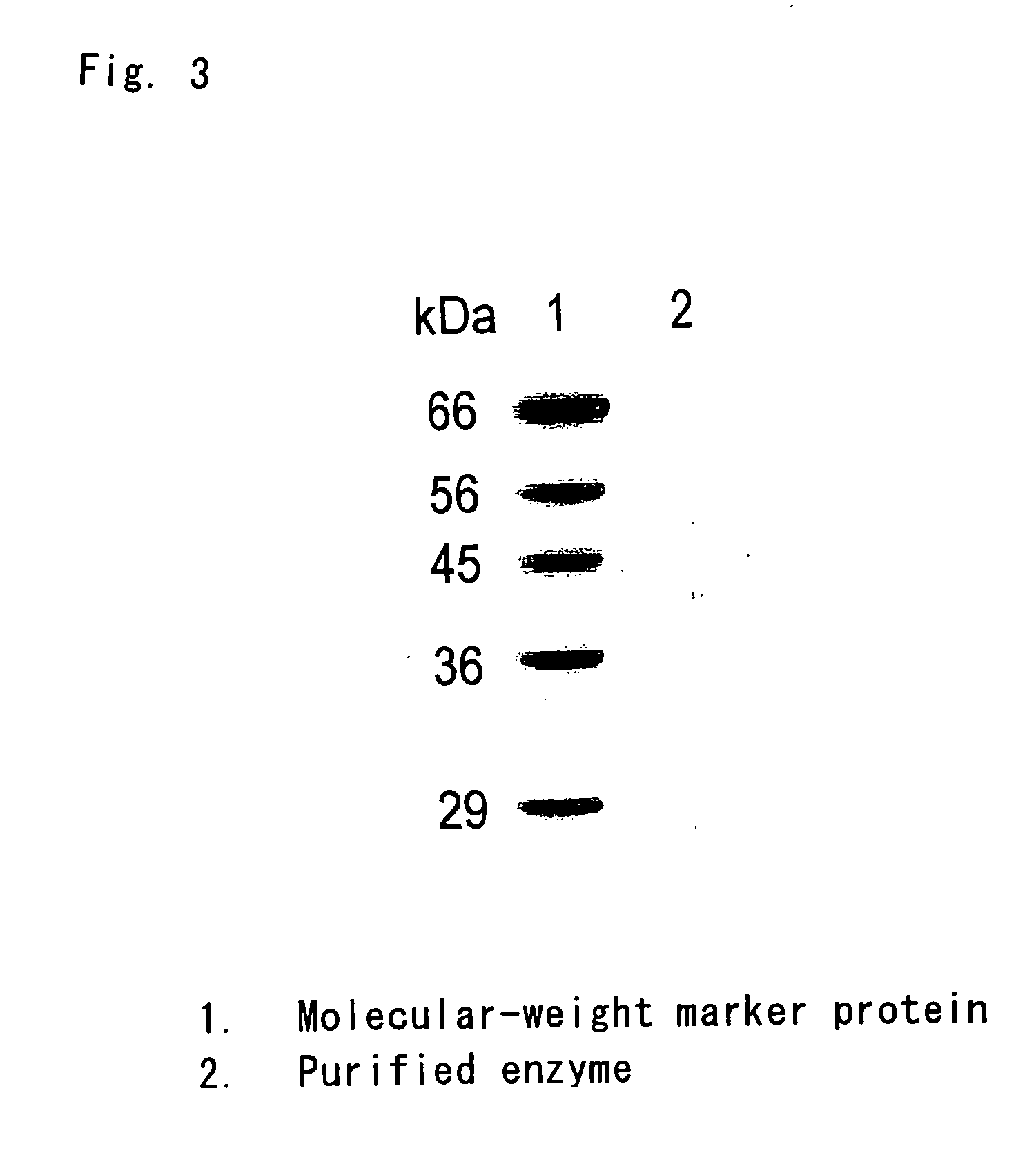
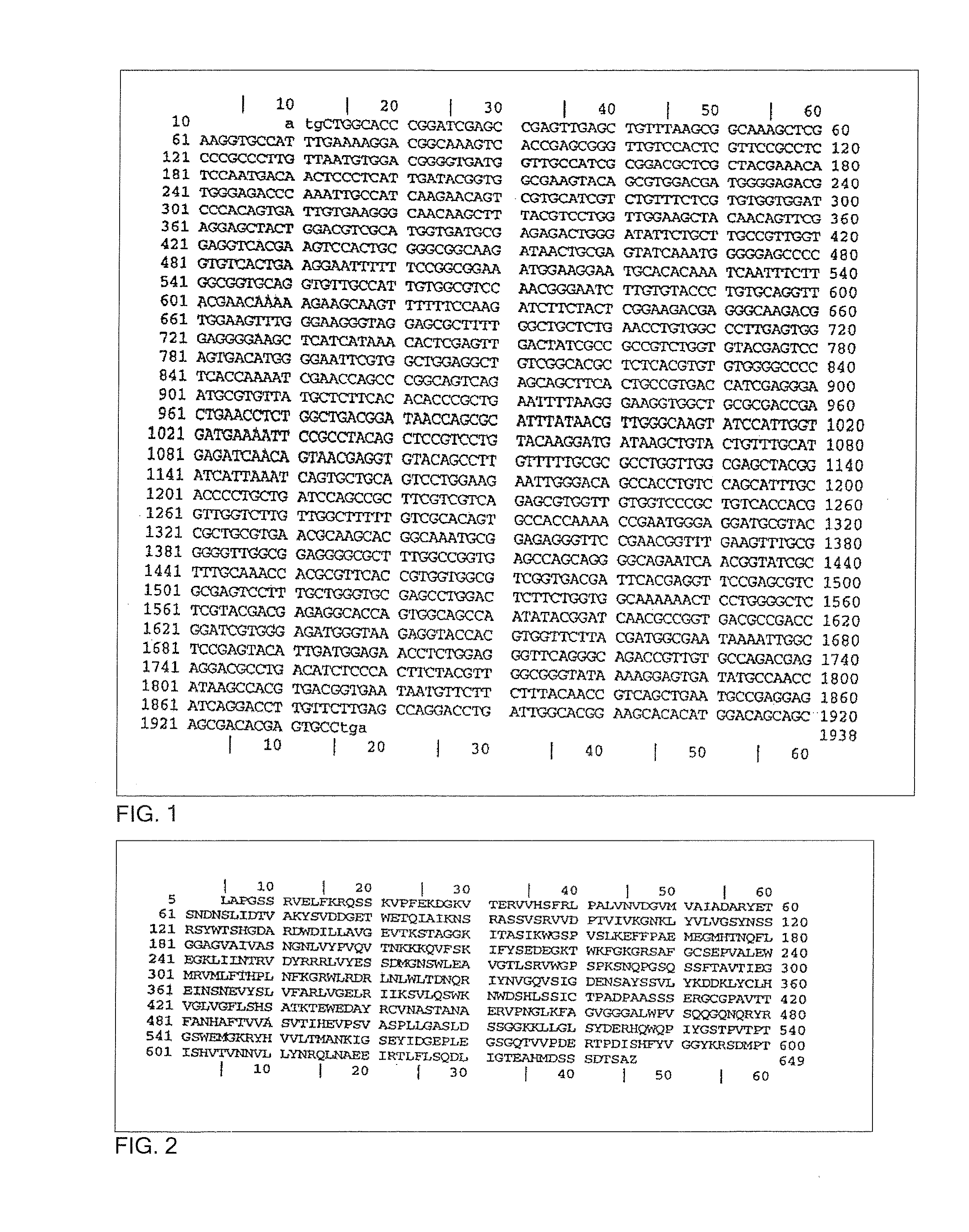
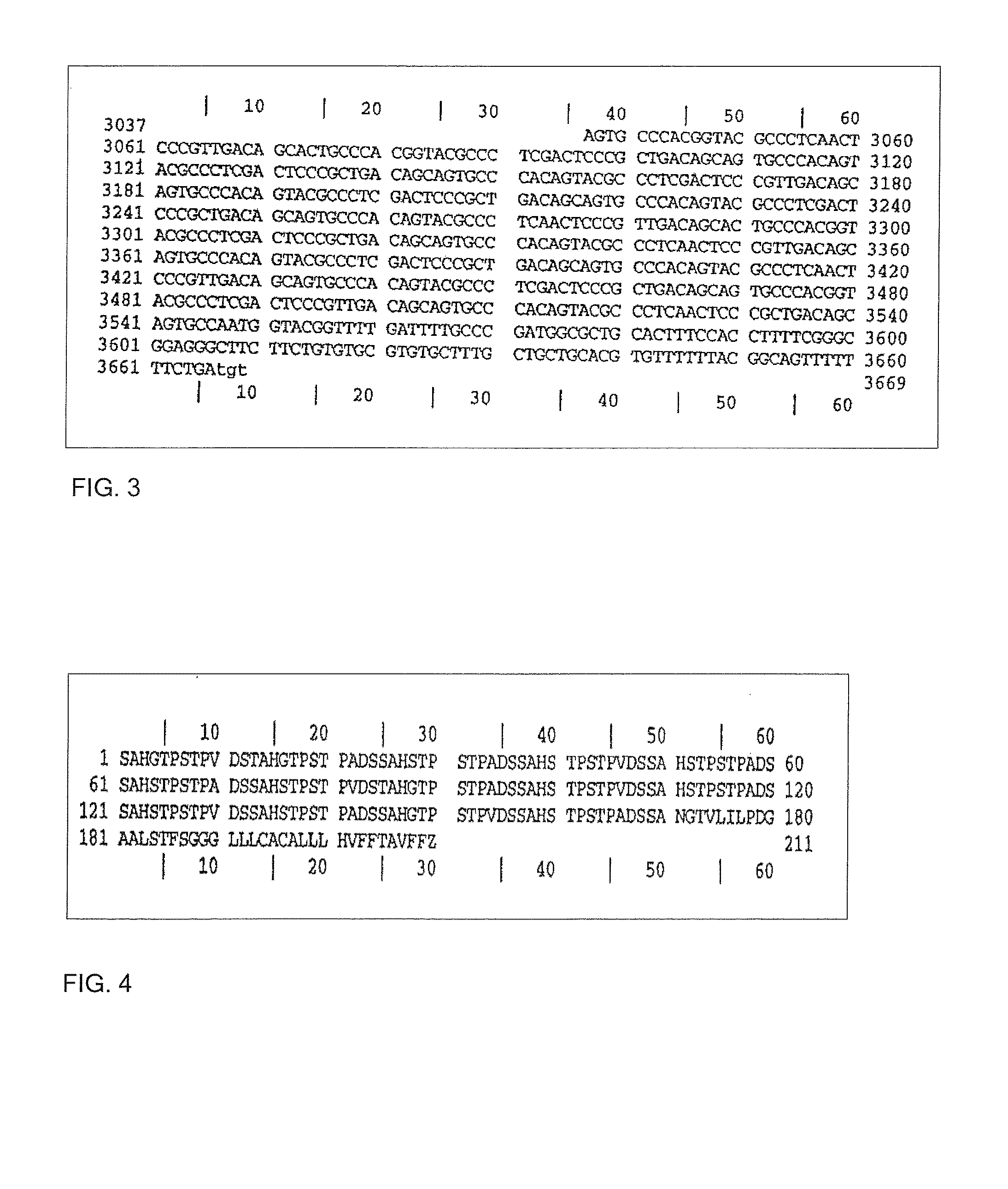
Flavin protein of trypanosoma cruzi, method of screening vermicide with the use of the same and diagnostic
InactiveUS20060275329A1Promote decompositionHighly specific and simple methodCompound screeningApoptosis detectionFlavoproteinTrypanocidal Drugs
It is intended to provide a method of diagnosing infection with Chagas disease by screening a trypanocidal drugs for Trypanosoma cruzi which is the pathogen of Chagas disease. Using a flavin protein TcOYE specific to Trypanosoma cruzi, a trypanocidal drugs effective against Trypanosoma cruzi is screened. Using the gene sequence of TcOYE and an antibody therefor, infection with Trypanosoma cruzi is diagnosed.
Owner:OSAKA BIOSCI INST +1
Multicomponent or monocomponent vaccine to be used against Chagas disease, pharmaceutical compositions containing them, procedure for the obtention of immunogen of said vaccines, and nucleic acid used in said procedure
InactiveUS8900598B2Stimulate immune responseEliminates itAntibacterial agentsPeptide/protein ingredientsChagas diseaseTrypanosomiasis
A vaccine against the Chagas disease, capable of stimulating the immune response against the trans-sialidase virulence factor of the Trypanosoma cruzi parasite, which is a multicomponent vaccine comprising: (a) an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of thereof and (b) one or more polynucleotides including the regions codifying one or more immunogenic polypeptides, or a monocomponent vaccine comprising at least one component selected among an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of them and a group of polynucleotides including the regions codifying one or more immunogenic polypeptides derived from Trypanosoma cruzi and pharmaceutical compositions containing said multicomponent and monocomponent vaccines, the procedures for obtaining the immunogen portion of said vaccines and the nucleic acid used in the procedure.
Owner:DE BAEREMAECKER BARROS CARLOS
Anti-trypanosomiasis vaccines and diagnostics
The present invention has as an object a novel genetic material coding for trans-sialidase-like proteins of African trypanosomes, and relates to the use of said genes and proteins in vaccines, therapeutics and diagnostics. The present invention also relates to the immunization of human and / or nonhuman animals against trypanosomosis.
Owner:UNIV BORDEAUX SEGALEN +1
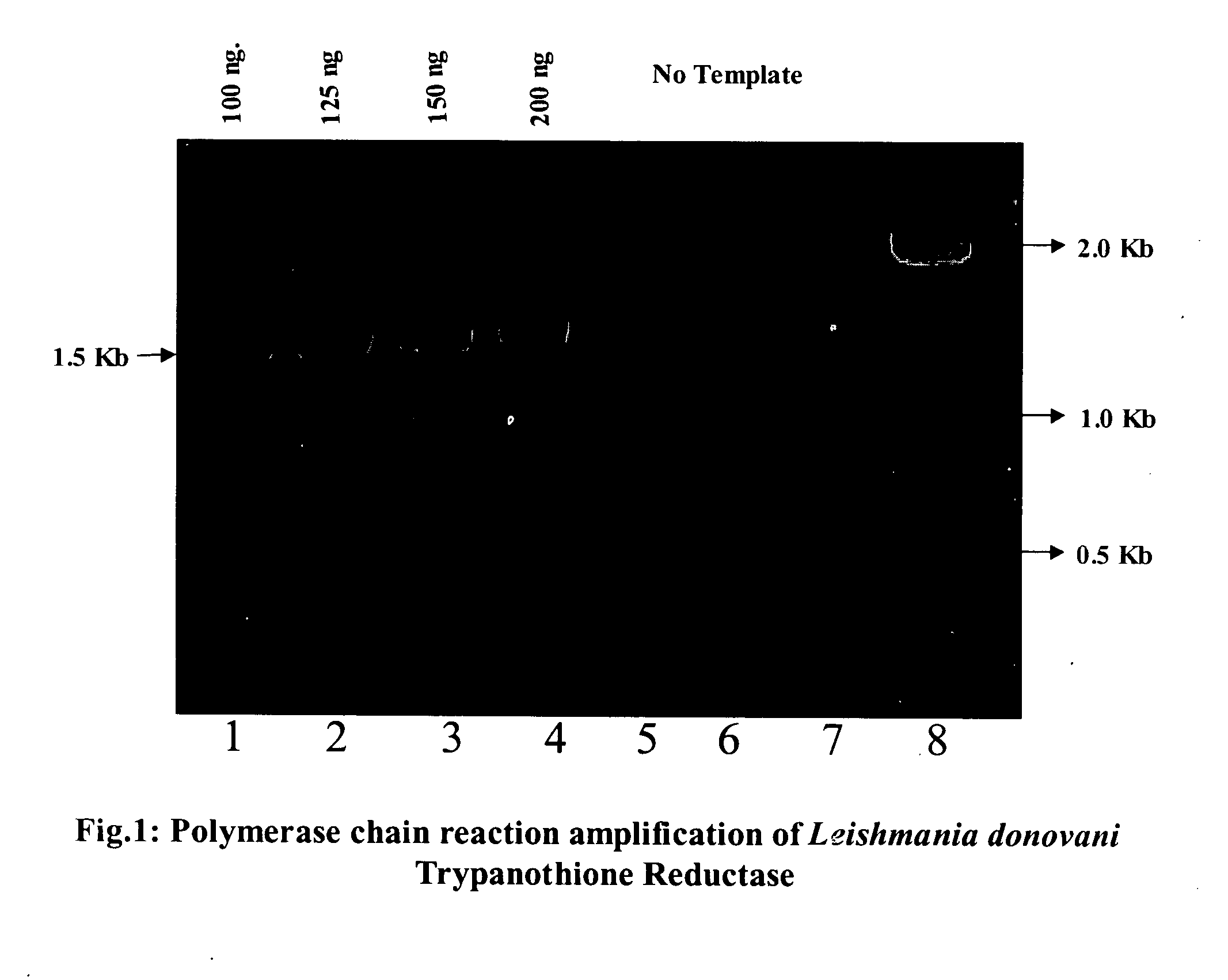
Heterologus expression of trypanothione reductase from Leishmania donovani in a prokaryotic system
InactiveUS20060110791A1High yieldCheapest and easy for large-scale yieldSugar derivativesBacteriaHeterologousActive enzyme
The present invention relates to a process for heterologous expression and large-scale production of functionally active enzyme trypanothione reductase of Leishmania donovani in prokaryotic system.
Owner:COUNCIL OF SCI & IND RES
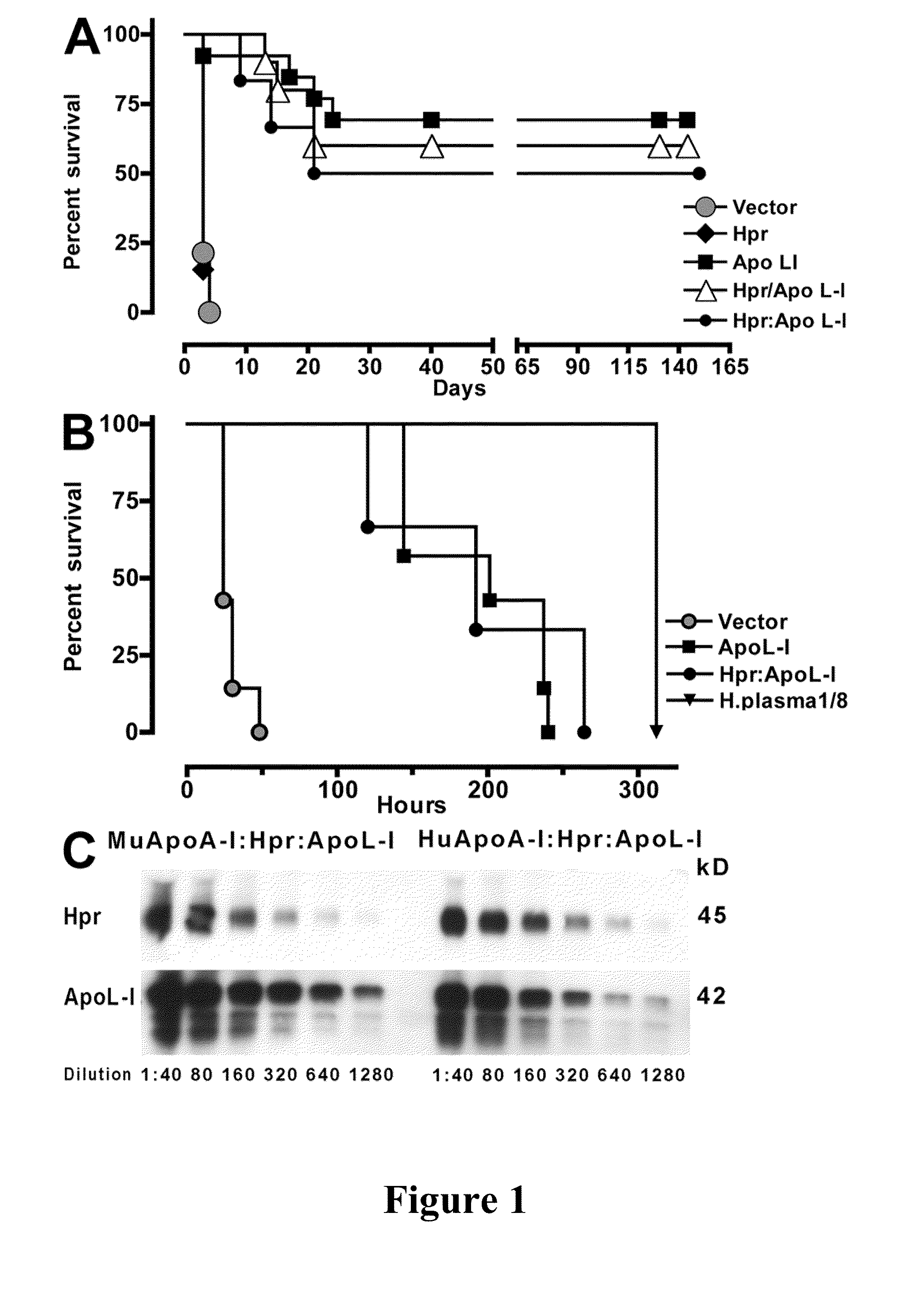
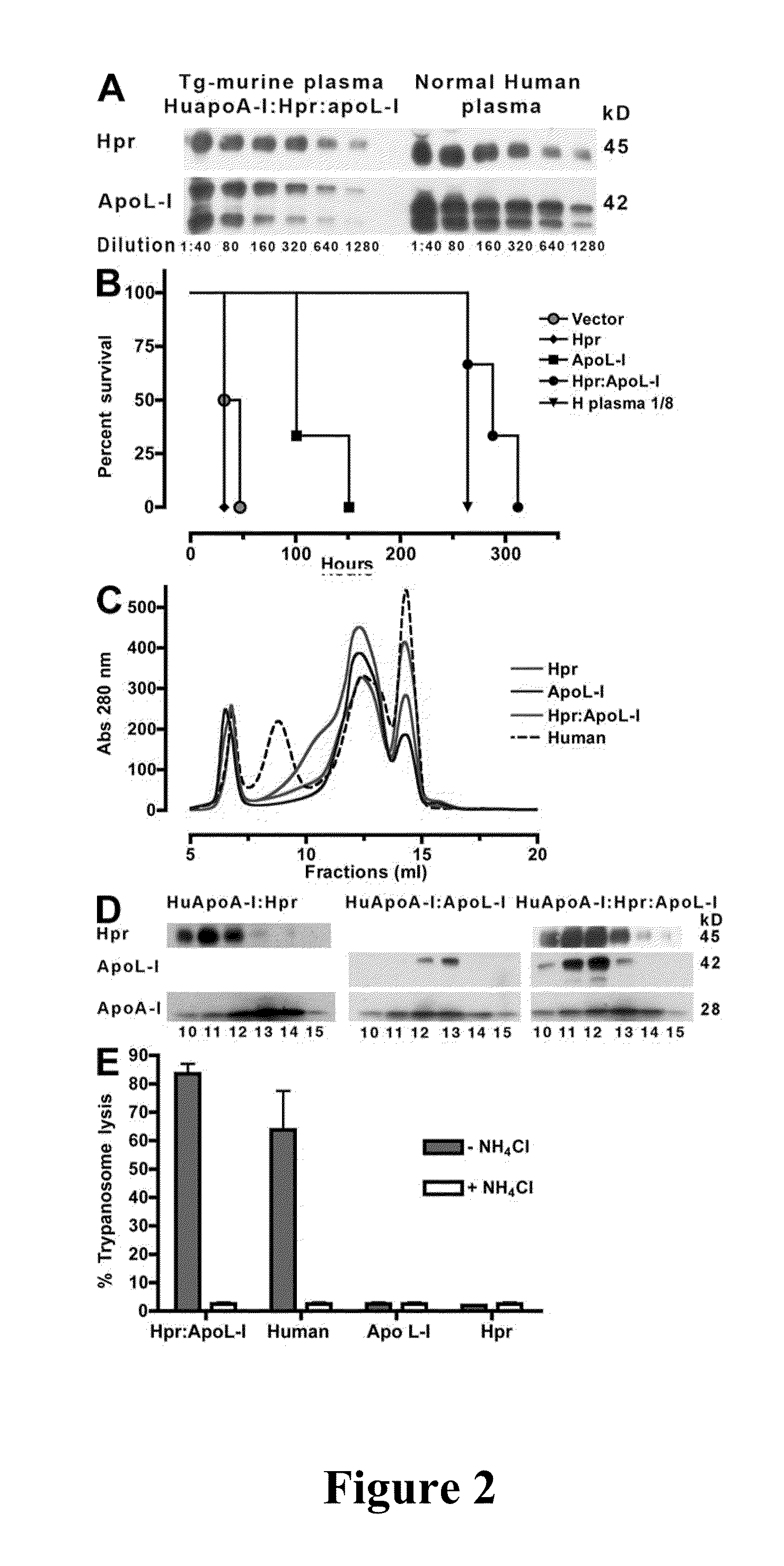
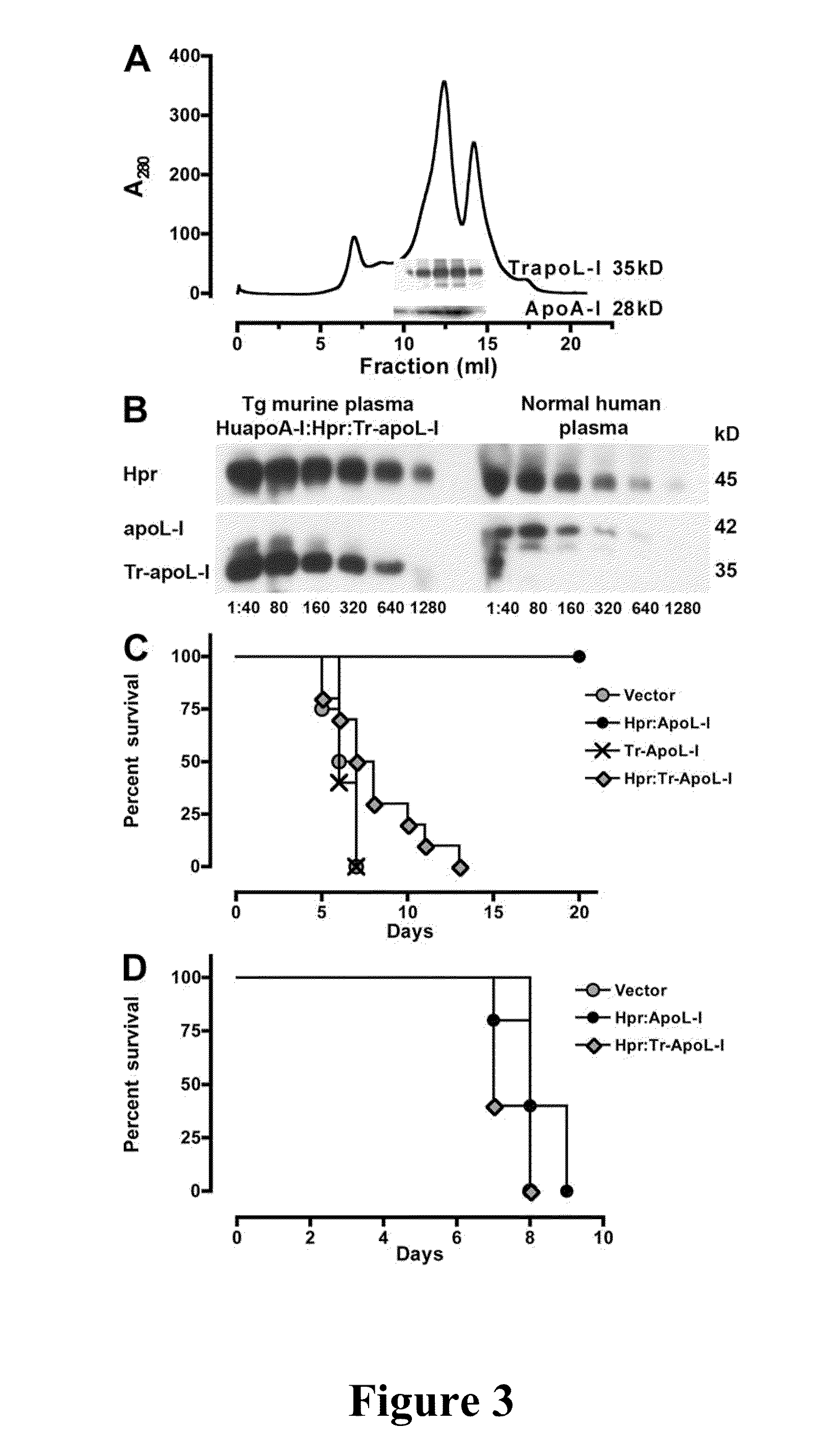
Trypanosome resistant non-human transgenic animal
InactiveUS20110030078A1Reduce mortalityReduce riskApolipeptidesGenetic engineeringTransgenePlant Germ Cells
The present invention is directed to a Trypanosome-resistant, non-human transgenic animal whose somatic and germ cells comprise a nucleic acid which encodes an apolipoprotein L-I polypeptide (apoL-I). The apoL-I protein has the amino acid sequence selected from the group consisting of SEQ ID NO: 1, SEQ ID NO: 3, SEQ ID NO: 4, and SEQ ID NO: 5. The first nucleic acid transgene is operatively associated with at least one expression regulatory sequence. Methods of producing and raising such transgenic animals as well as transgenic eggs and sperm are also disclosed.
Owner:NEW YORK UNIVERSITY
Multicomponent or monocomponent vaccine to be used against chagas disease, pharmaceutical compositions containing them, procedure for the obtention of immunogen of said vaccines, and nucleic acid used in said procedure
InactiveUS20100297186A1Stimulate immune responseReduces clinical consequenceAntibacterial agentsProtozoa antigen ingredientsChagas diseaseTrypanosomiasis
A vaccine against the Chagas disease, capable of stimulating the immune response against the trans-sialidase virulence factor of the Trypanosoma Cruzi parasite, which is a multicomponent vaccine comprising: (a) an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of thereof and (b) one or more polynucleotides including the regions that codifying one or more immunogenic polypeptides, or a monocomponent comprising at least one component selected among an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of them and a group of polynucleotides including the regions codifying one or more immunogenic polypeptides derived from Trypanosoma Cruzi and pharmaceutical compositions containing said multicomponent and monocomponent vaccines, the procedures for obtaining the immunogen portion of said vaccines and the nucleic acid used in the procedure.
Owner:DE BAEREMAECKER BARROS CARLOS
Diagnostic assay for trypanosoma cruzi infection
A sensitive, multicomponent diagnostic test for infection with T. cruzi, the causative agent of Chagas disease, including methods of making and methods of use. Also provided is a method for screening T. cruzi polypeptides to identify antigenic polypeptides for inclusion as components of the diagnostic test, as well as compositions containing antigenic T. cruzi polypeptides.
Owner:UNIV OF GEORGIA RES FOUND INC
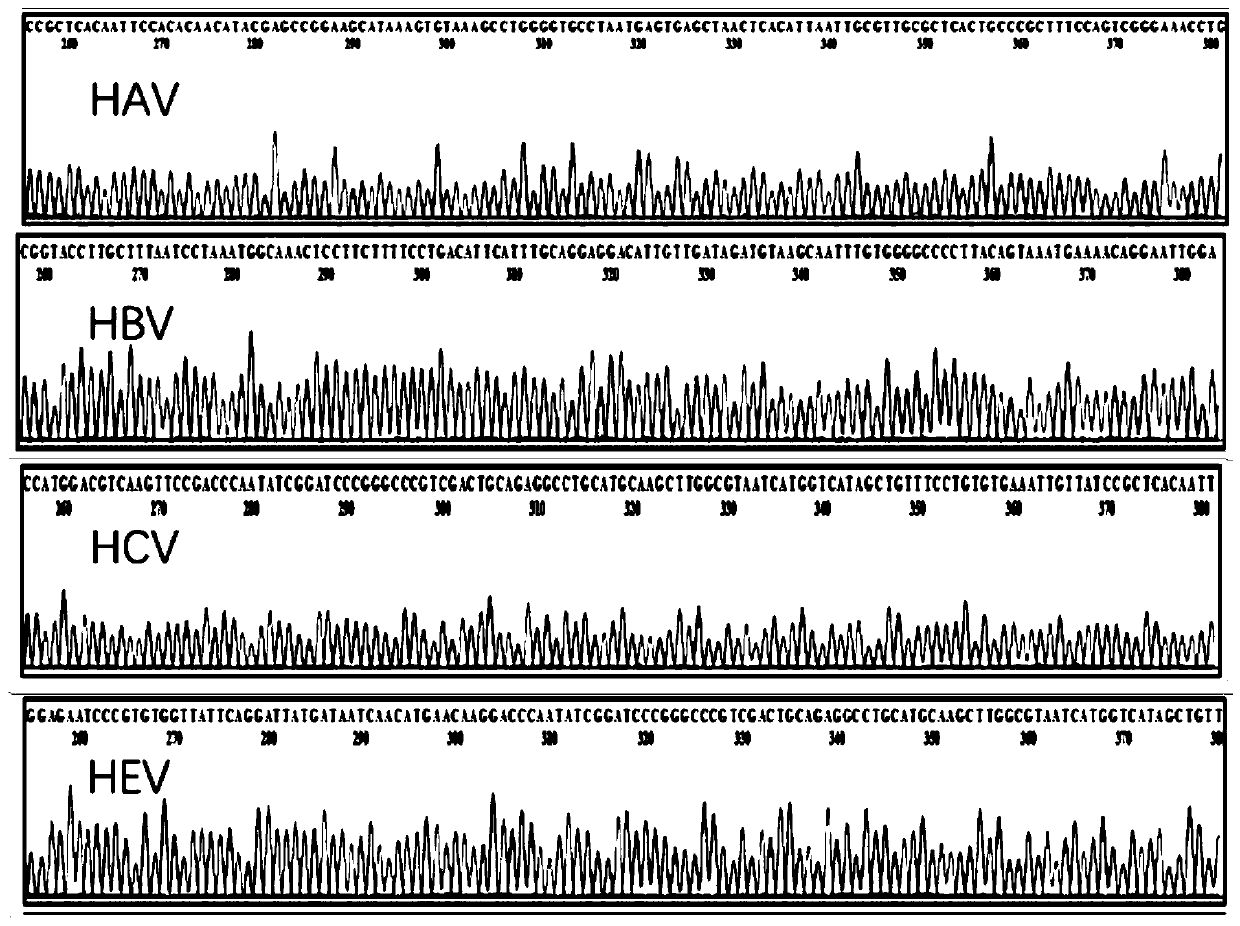
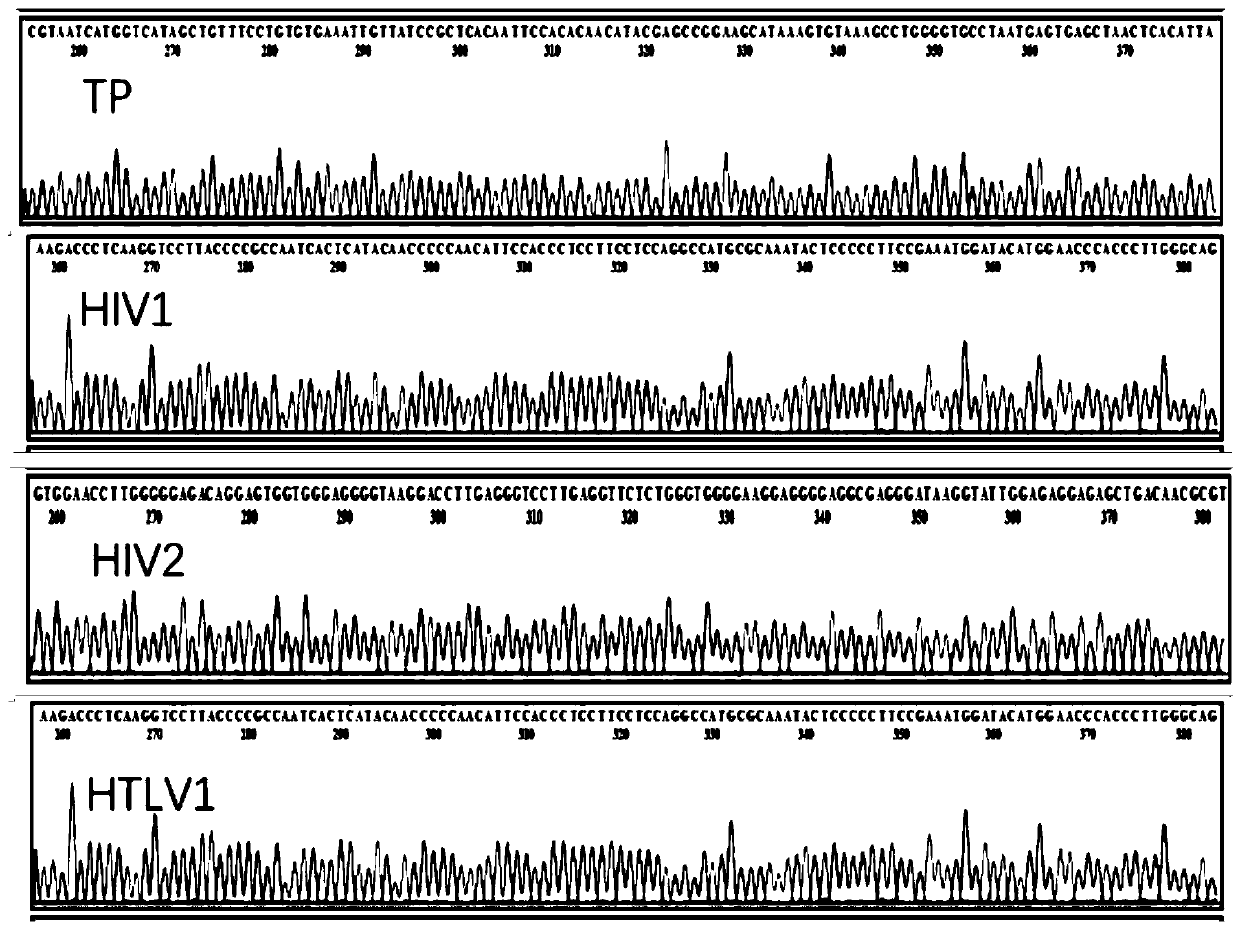
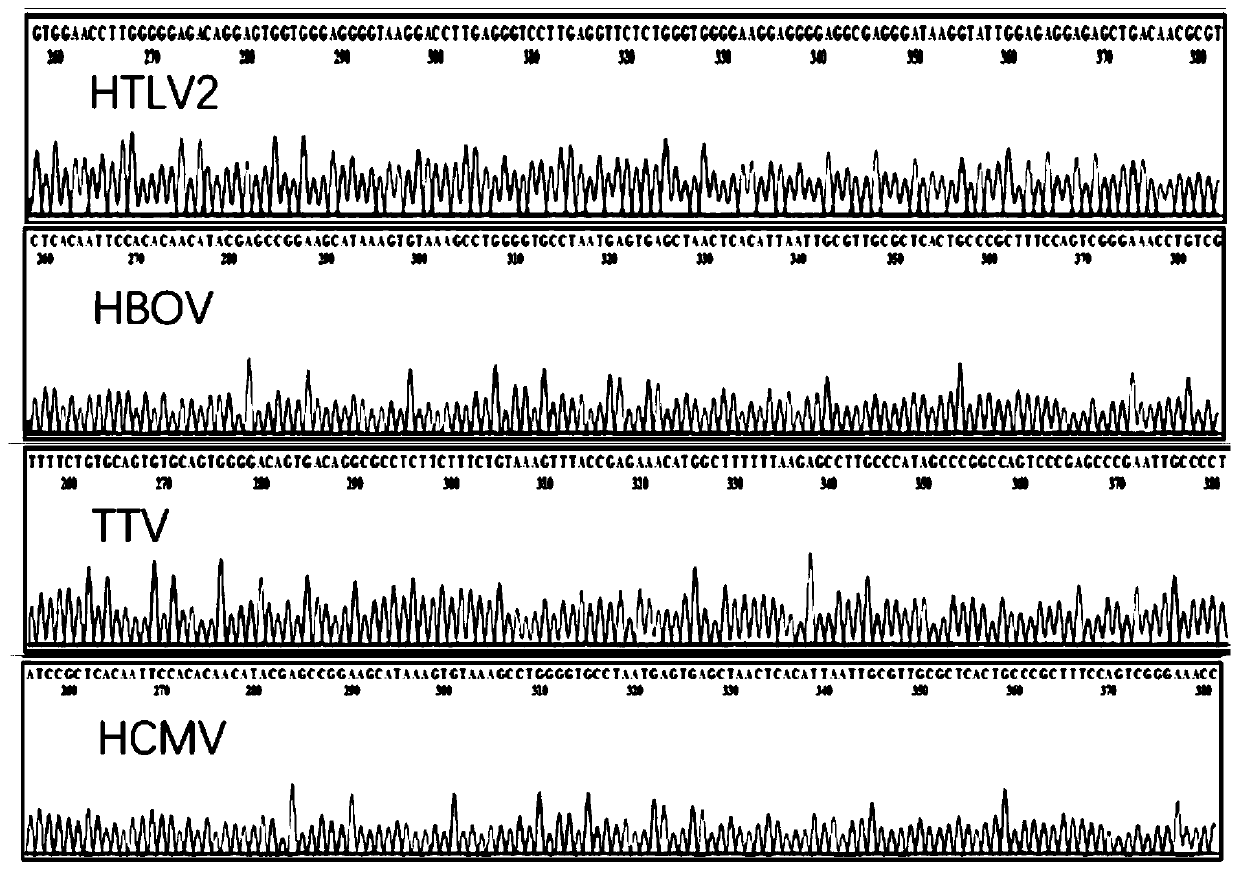
Reagent combination and kit for detecting blood transfusion transmitted pathogens and application
PendingCN111286557ARich varietyFull rangeMicrobiological testing/measurementMicroorganism based processesWhole blood productBlood product
The invention discloses application of a reagent combination for detecting pathogens in preparation of a kit for detecting the quality of a blood product. The pathogens are selected from a pathogen group consisting of HAV, HBV, HCV, HEV, TP, HIV1, HIV2, HTLV1, HTLV2, HBOV, TTV, HCMV, B19, DENV, ZIKV, WNV, babesia, trypanosoma krusei and plasmodium, and belongs to the field of medical examination.The invention further discloses a corresponding kit and a method for detecting the quality of the blood product. By means of the kit, the lowest detection limit of pathogens is 1.0 * 10 < 3 > copies / mL, the kit has good specificity and repeatability, and an important technology is provided for screening and evaluating blood transfusion transmitted pathogens.
Owner:山西国际旅行卫生保健中心
Methods for the detection and diagnosis of Trypanosoma cruzi infection
ActiveUS7749717B2Bioreactor/fermenter combinationsBiological substance pretreatmentsBiomedical engineeringVirology
Owner:ABBOTT LAB INC
Combined sustained release agent and preparation thereof for preventing and treating livestock helminth, acarid and ectosarc disease
InactiveCN1543941AHigh inclusion rateBroad spectrum repellentPowder deliveryClimate change adaptationSolventBeta-Cyclodextrins
The invention discloses a combination slow release preparation for prevention and cure of livestock helminth, acarid and protozoon diseases, and the process for preparation comprising the steps of, using closantel and beta-cyclodextrin as the principal raw materials, carrying out rationalized proportioning and certain technological conditions, dissolving the closantel in solvent, charging distilled water into the beta-cyclodextrin, grinding till pasty state, mixing the two, drying and disintegrating.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Pyrazolopyridine derivatives and their use in therapy
The present invention relates to pyrazolopyridine derivatives, which are useful as medicaments, pharmaceutical compositions comprising one or more of the pyrazolopyridine derivatives, and the use of one or more of the pyrazolopyridine derivatives in methods for treating and / or preventing a disease caused or mediated by a parasite of the family Trypanosomatidae.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT
Live antenuated parasite vaccine
The present invention relates inter alia to attenuated live parasites of the phylum Apicomplexa and the family of Trypanosomatdae and to the use of such attenuated live parasites in a vaccine and in the manufacturing of such a vaccine. Furthermore, the present invention relates to vaccines comprising such attenuated live parasites and to methods for the production of such vaccines. Finally, the invention relates to specific tet-repressor fusion proteins and to attenuated live parasites according to the invention comprising such tet-repressor fusion proteins.
Owner:INTERVET INT BV
Nitroimidazole Compounds
InactiveUS20080275035A1Maintain good propertiesAntibacterial agentsOrganic active ingredientsNitroimidazoleCombinatorial chemistry
The present invention relates to certain nitroimidazole compounds, which have interesting pharmaceutical properties. In particular, the compounds are useful in the treatment and / or prevention of infections such as those caused by Mycobacterium tuberculosis, Trypanosoma cruzi or Leishmania donovani. The invention also relates to pharmaceutical compositions containing the compounds, as well as processes for their preparation.
Owner:JIRICEK JAN +4
Combined sustained release agent and preparation thereof for preventing and treating livestock helminth, acarid and ectosarc disease
InactiveCN1232248CHigh inclusion rateBroad spectrum repellentPowder deliveryClimate change adaptationSolventBeta-Cyclodextrins
The invention discloses a combination slow release preparation for prevention and cure of livestock helminth, acarid and protozoon diseases, and the process for preparation comprising the steps of, using closantel and beta-cyclodextrin as the principal raw materials, carrying out rationalized proportioning and certain technological conditions, dissolving the closantel in solvent, charging distilled water into the beta-cyclodextrin, grinding till pasty state, mixing the two, drying and disintegrating.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
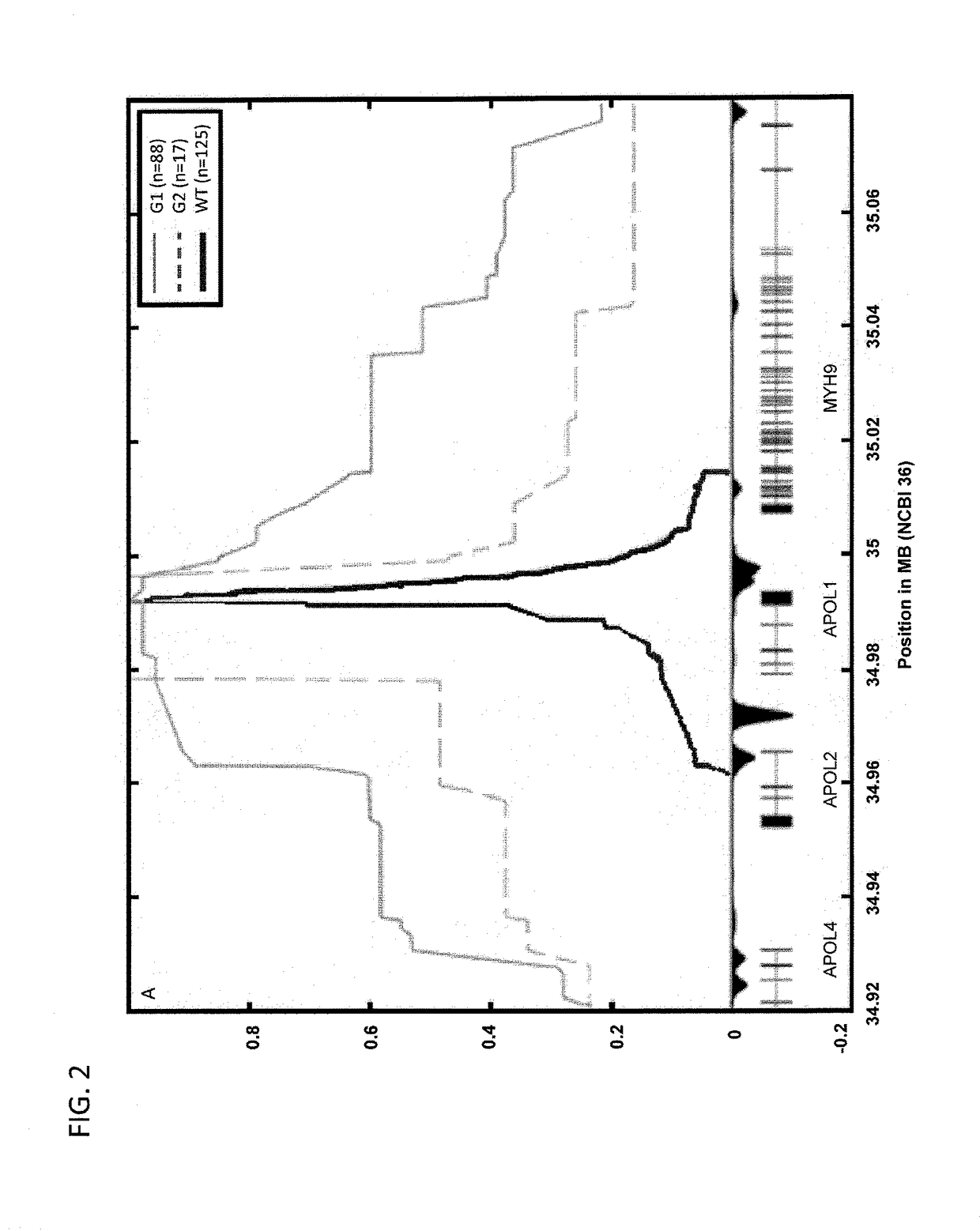
Methods of predicting predisposition to or risk of kidney disease
ActiveUS9828637B2Raise the possibilityMicrobiological testing/measurementLibrary screeningNephrosisNephropathy
Methods are disclosed herein for detecting a genetic predisposition to focal segmental glomerulosclerosis (FSGS) or hypertensive end-stage kidney disease (ESKD) or both in a human subject, e.g., by detecting the presence of at least one single nucleotide polymorphism (SNP) in an APOL1 gene, such as the C-terminal exon of an APOL1 gene. In a further embodiment, methods are disclosed for detecting resistance of a subject to a disease associated with Trypanosoma infection, e.g., by detecting at least one single nucleotide polymorphism (SNP) in an APOL1 gene, such as the C-terminal exon of an APOL1 gene. Also disclosed are methods for treating a subject infected with T. brucei. The methods include administering a therapeutically effective amount of an APOL1 protein including a S342G substitution, an I384M substitution, and / or a deletion of N388 and Y389 to the subject.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Therapeutic Compounds for Protozoal and Microbial Infections and Cancer
The compounds of the invention exhibit antiprotozoal, antimicrobial, and anticancer properties that are useful for the treatment or prevention of infections or cancer in a patient (e.g., a human). For example, the compounds and methods described herein can be used for the treatment or prevention of protozoal infections such as leishmaniasis, malaria, and trypanosoma infections, bacterial infections such as S. aureus and C. albicans, and cancers such as breast, colon, lung, or prostate cancer. The invention further provides methods of synthesizing such compounds as well as kits useful for administering the compounds.
Owner:UNIVERSITY OF MONTANA
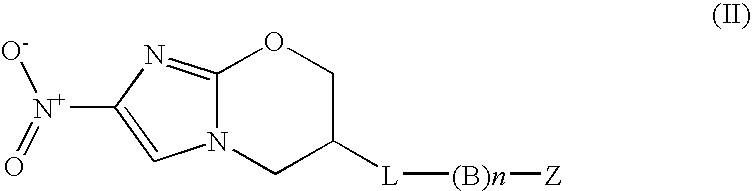
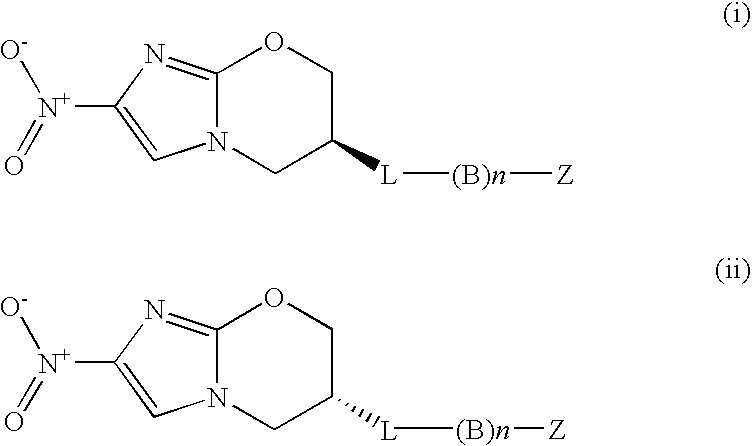
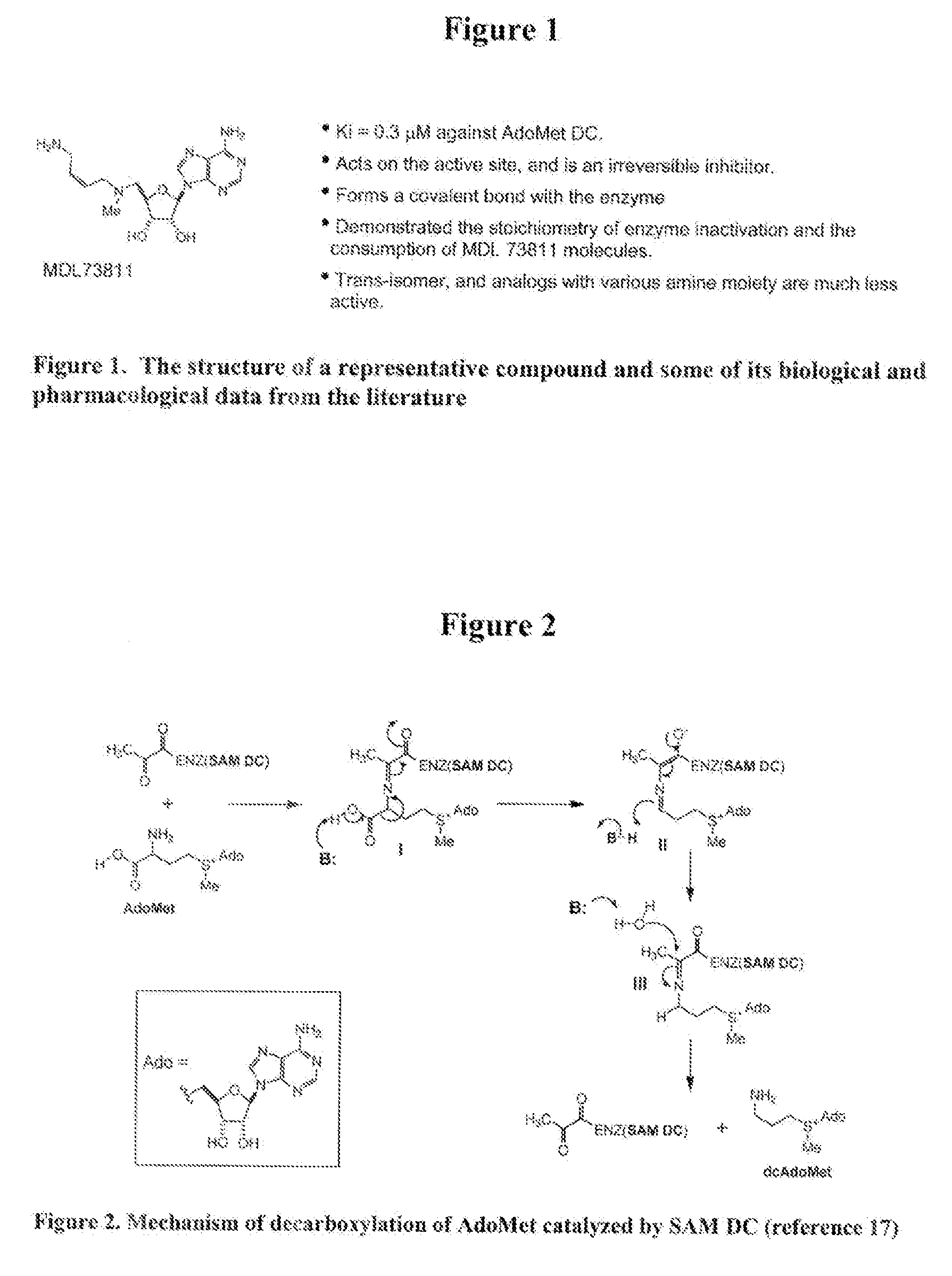
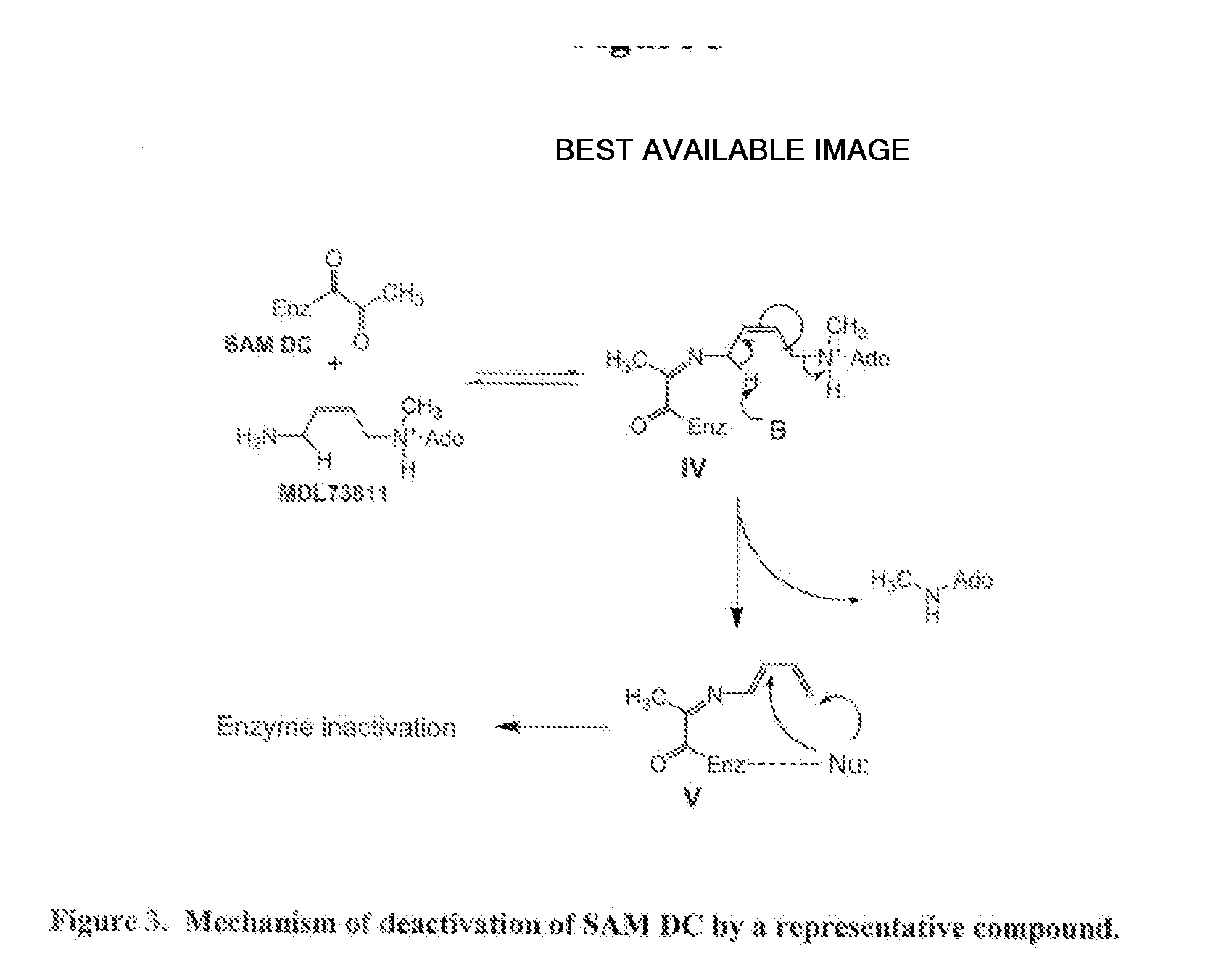
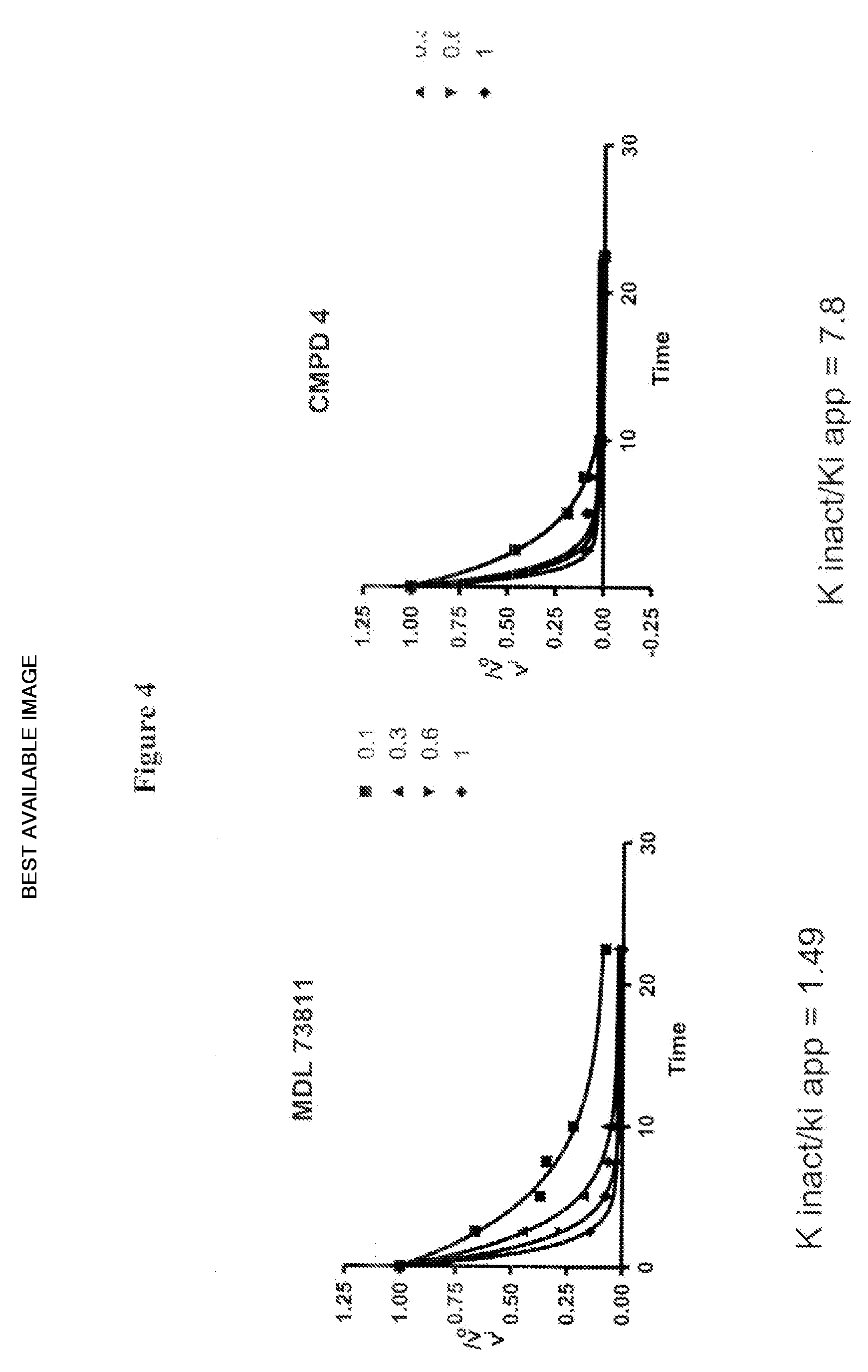
Inhibitors of s-adenosyl-l-methionine decarboxylase
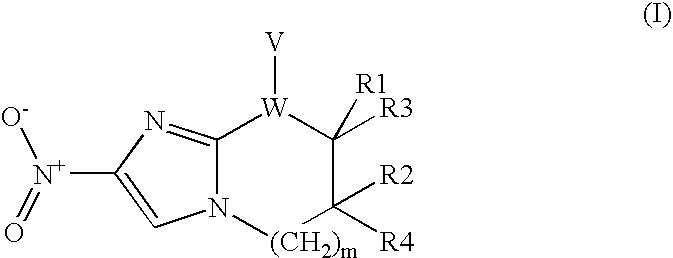
Novel mechanism-based inhibitors of S-adenosyl-L-methionine decarboxylase are provided. These compounds of formula (1) inhibit the life cycle of trypanosomes, and are useful to treat subjects infected with African trypanosomes. The invention includes pharmaceutical compositions and methods of using the compounds of formula (1).
Owner:GENZYME CORP
Specific primers, probe, kit and chip for Trypanosoma gene detection
PendingCN110964843AEasy diagnosisFacilitate Disease SurveillanceMicrobiological testing/measurementDNA/RNA fragmentationTrypanosoma brucei gambienseTrypanosoma gambiense
The invention provides specific primers for the detection of Trypanosoma cruzi with the sequences as shown in SEQ ID No.1 and SEQ ID No.2, provides specific primers for the detection of Trypanosoma brucei with the sequences as shown in SEQ ID No.3 and SEQ ID No.4, provides specific primers for the detection of Trypanosoma brucei rhodesiense with the sequences as shown in SEQ ID No.5 and SEQ ID No.6, provides specific primers for the detection of Trypanosoma brucei gambiense with the sequences as shown in SEQ ID No.7 and SEQ ID No.8, provides a probe for the detection of Trypanosoma with the sequences as shown in SEQ ID No.9 to 26, and also provides a kit and a chip for the detection of Trypanosoma. In the invention, differential diagnosis for Trypanosoma infection can be specifically carried out.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Trypanosoma cruzi antigen, gene encoding therefor and methods of detecting and treating chagas disease
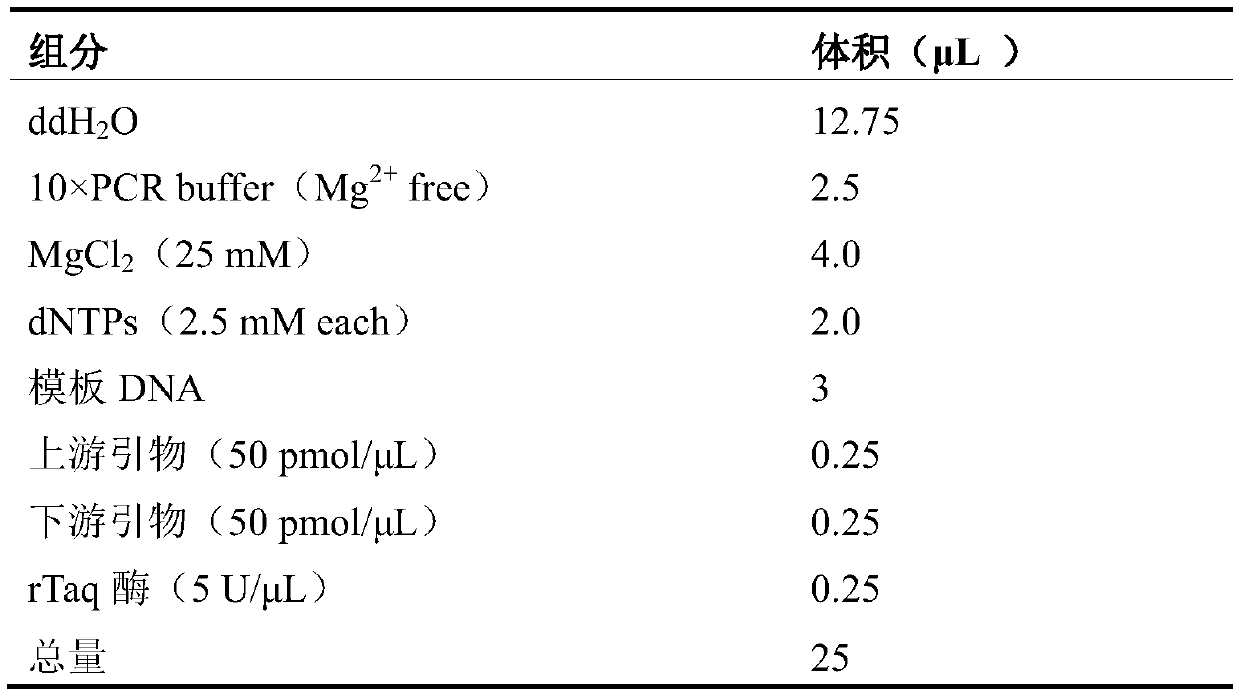
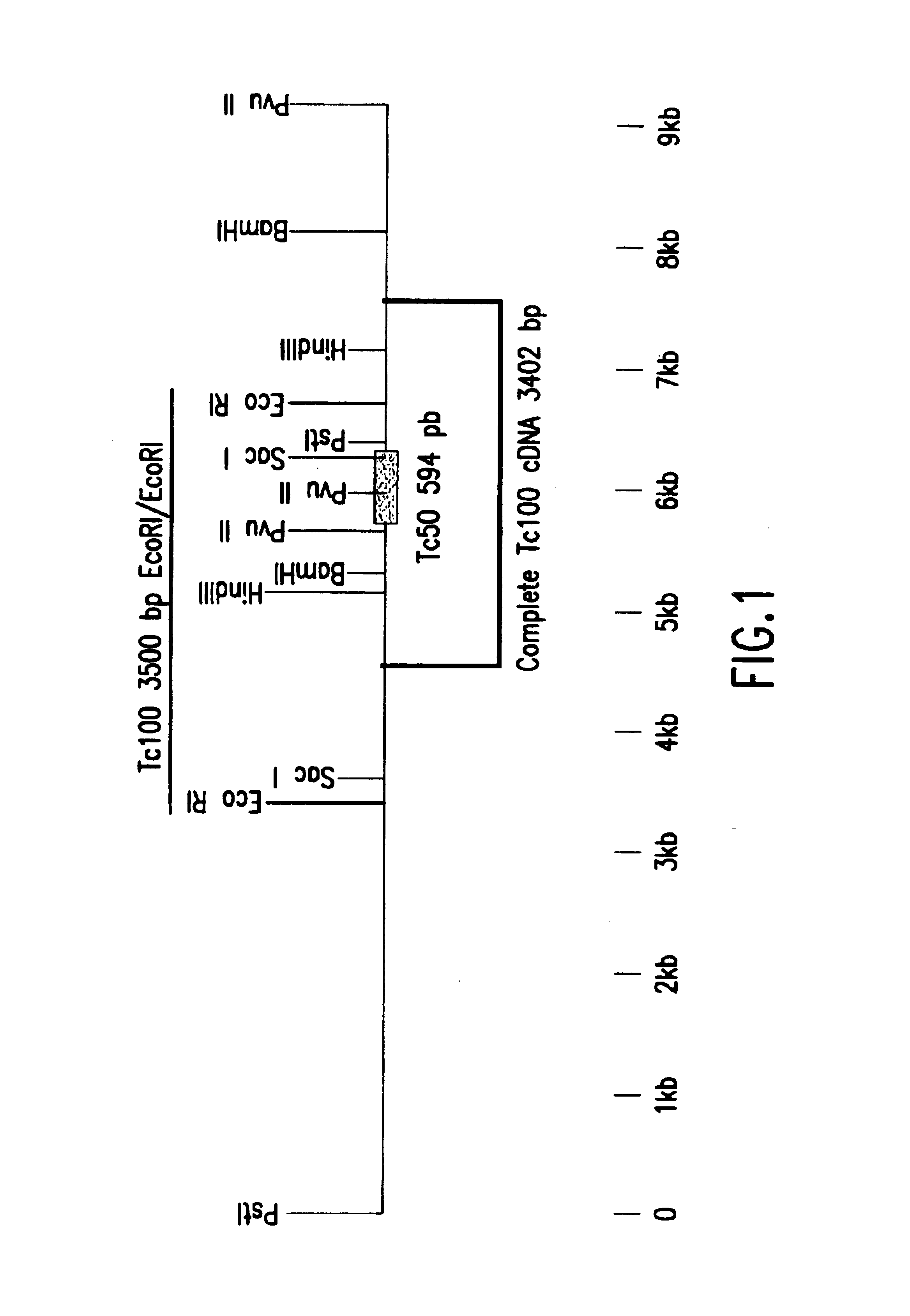
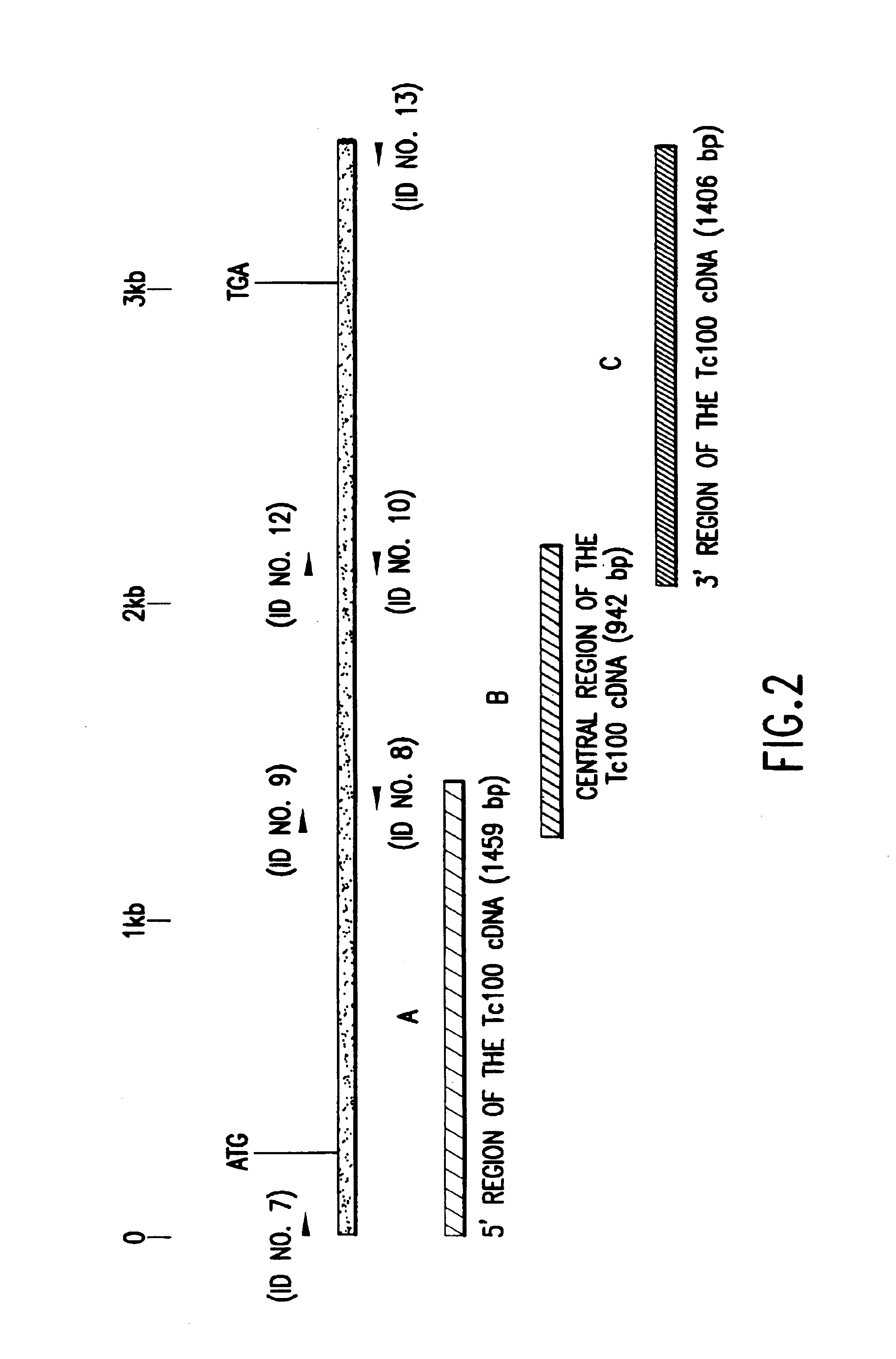
InactiveUS6933110B1Improve presentationImprove stabilitySugar derivativesMicrobiological testing/measurementAntigenTrypanosoma antigen
The nucleotide sequence of Tc100, a gene encoding PTc100, a new Trypanosoma antigen, and the amino acid sequence of PTc100 are described. Tc100 and PTc100, or fragments thereof, modified or otherwise, can be used directly or indirectly for the detection of Trypanosoma cruzi, or for the monitoring of the infection generated by Trypanosoma cruzi, in man or in animals.
Owner:BIOMERIEUX SA
Anti-trypanosomiasis vaccines and diagnostics
The present invention relates to a novel genetic material coding for trans-sialidase-like proteins belonging to the African trypanosomes, and relates to the use of said genes and proteins for vaccinal, therapeutic and diagnostic purposes. The present invention also relates to the immunization of humans and / or non-human animals against trypanosomiasis.
Owner:波尔多第二大学 +1
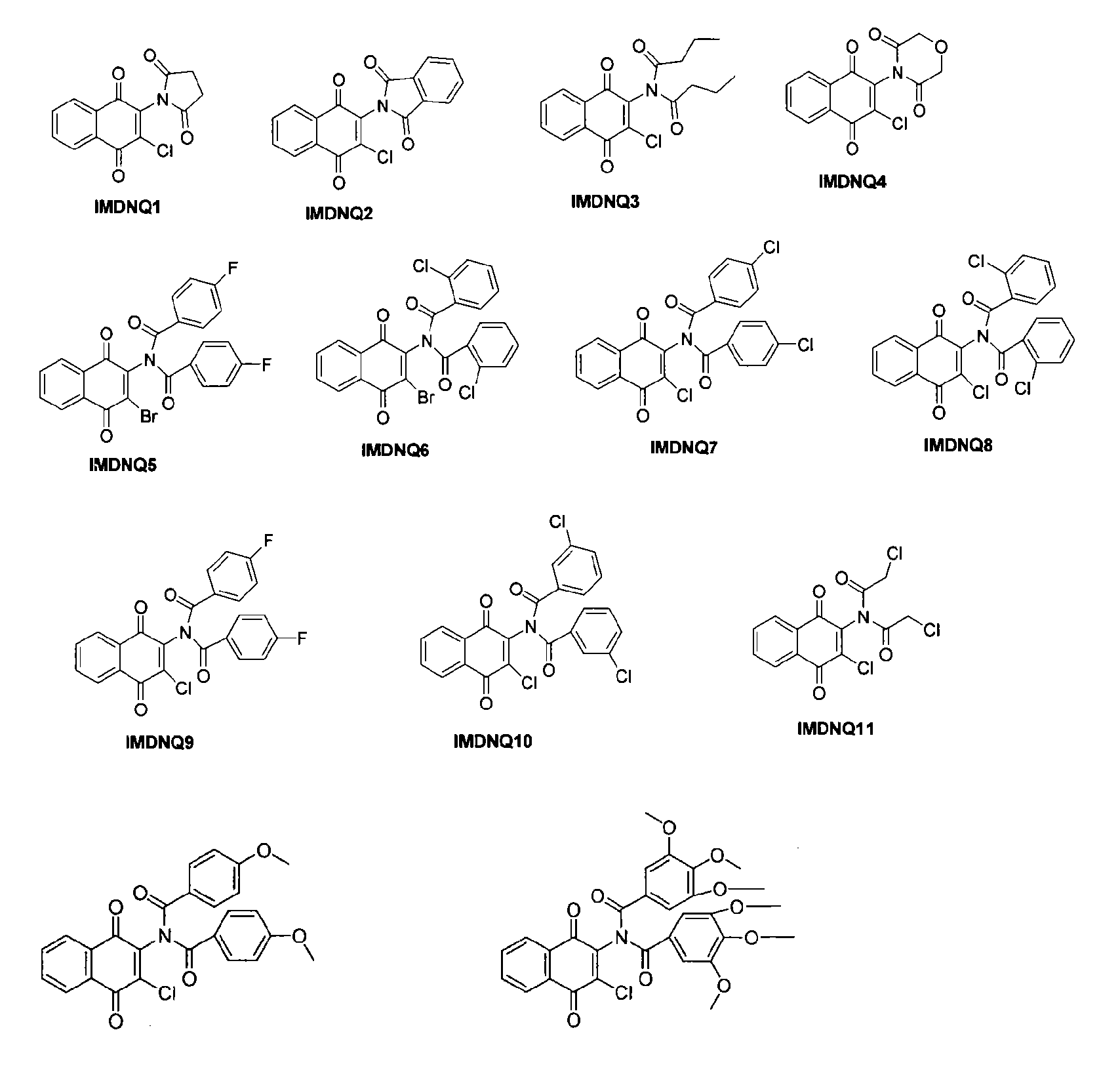
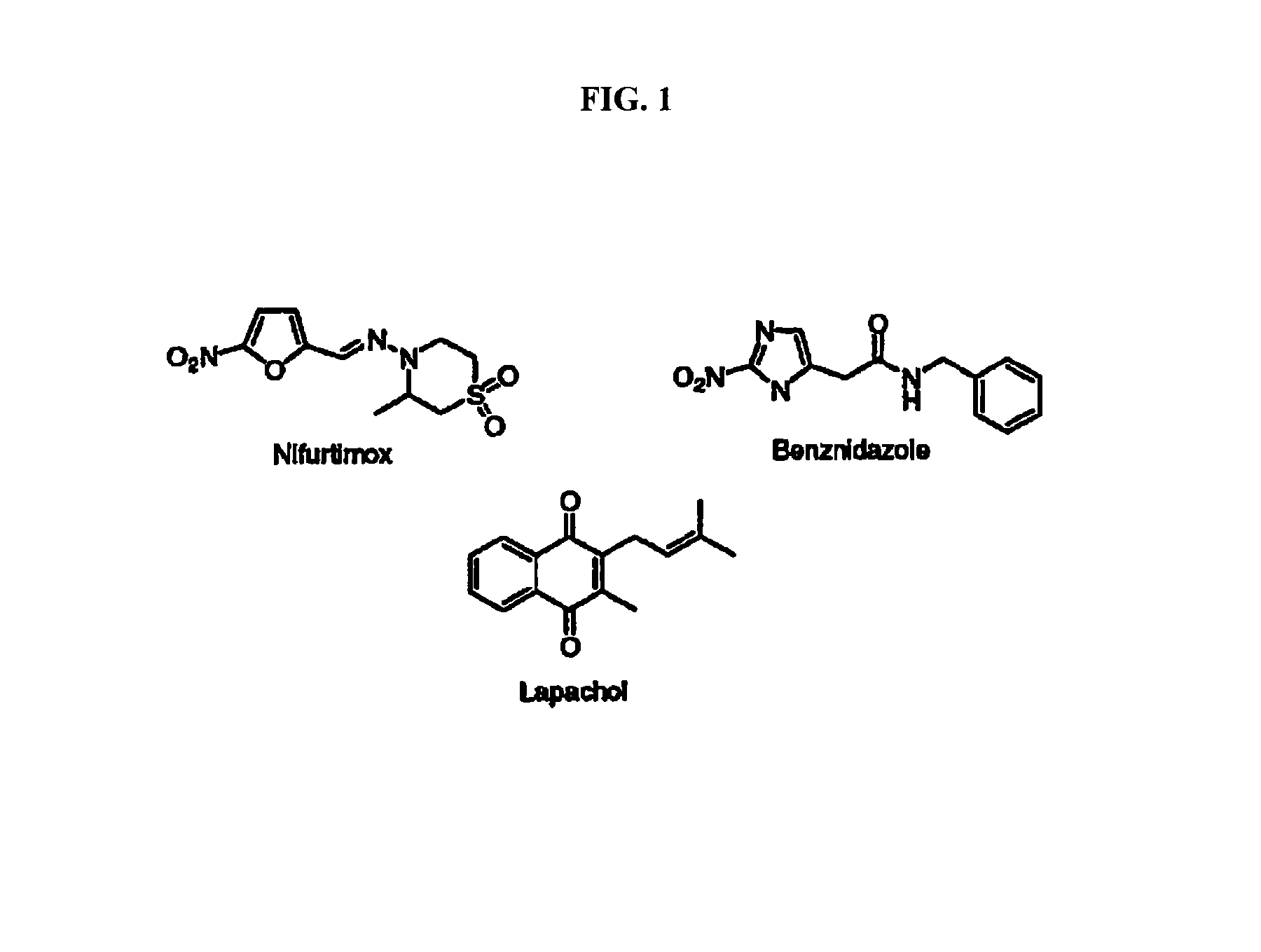
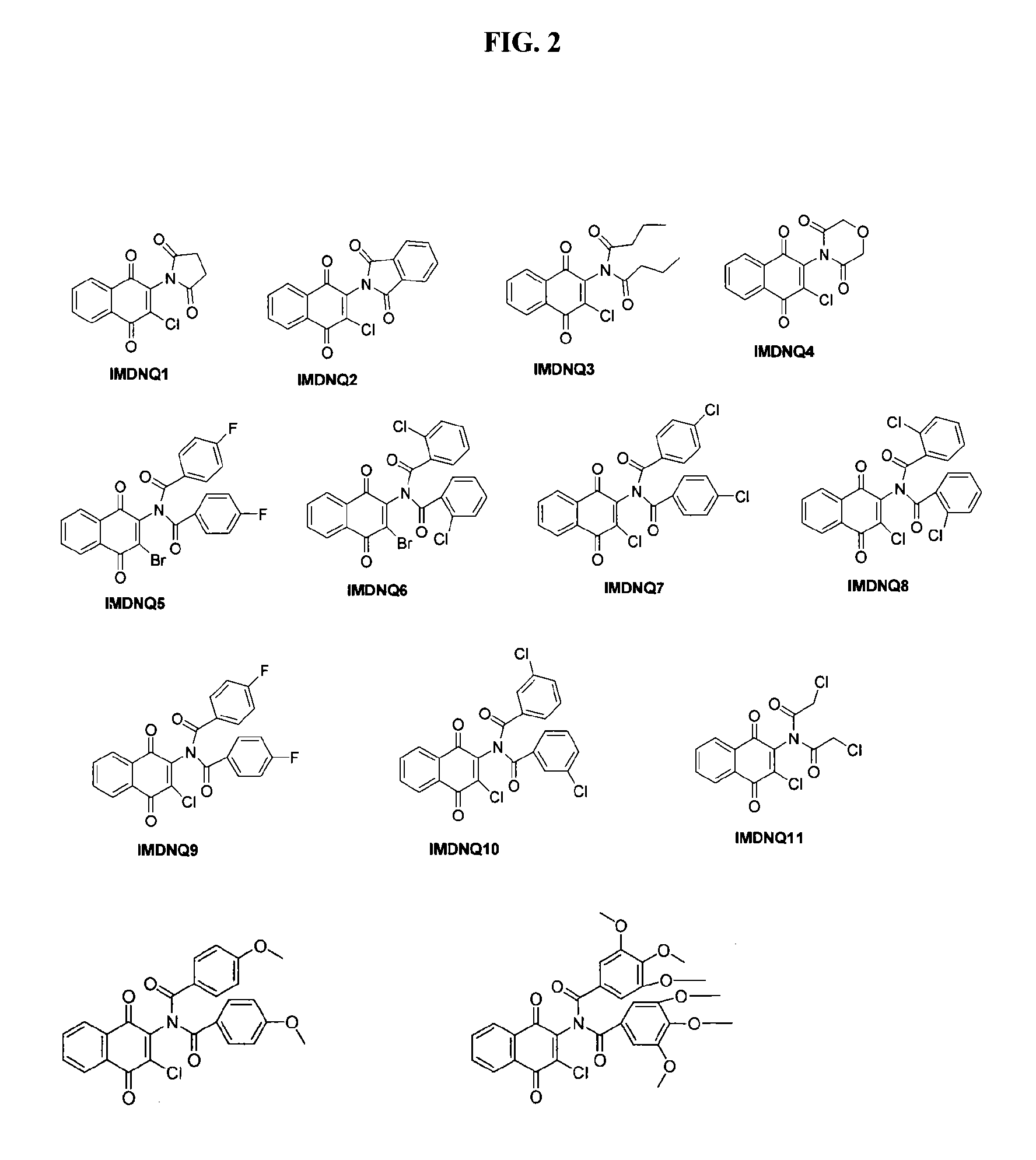
Method for inhibiting Trypanosoma cruzi
Methods are provided to inhibit proliferation of Trypanosoma cruzi with imido-substituted 1,4-naphthoquinones, including novel compounds. Administering an imido-substituted 1,4-naphthoquinone can be used to provide prophylaxis or treatment to a patient in need of treatment against Chagas disease.
Owner:HOWARD UNIVERSITY
Mucin-associated surface protein as vaccine against Chagas disease
Use of synthetic peptides derived from Trypanosoma cruzi antigens and their use in vaccination against trypomastigote infection and Chagas disease. T. cruzi uses several surface proteins to invade the host. In their role of protection, the surface protients ensure the targeting and invasion of specific cells or tissues. A conserved region in the family of mucin-associated surface proteins (MASP) was used to analyze the expression of MASP at different points of invasion and proved to be important for host cell invasion, thus suggesting MASP as a candidate for vaccine development. A synthetic peptide, MASPsyn, was studied and showed efficacy in stimulating antibody and cytokine production necessary for resistance against the parasite.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
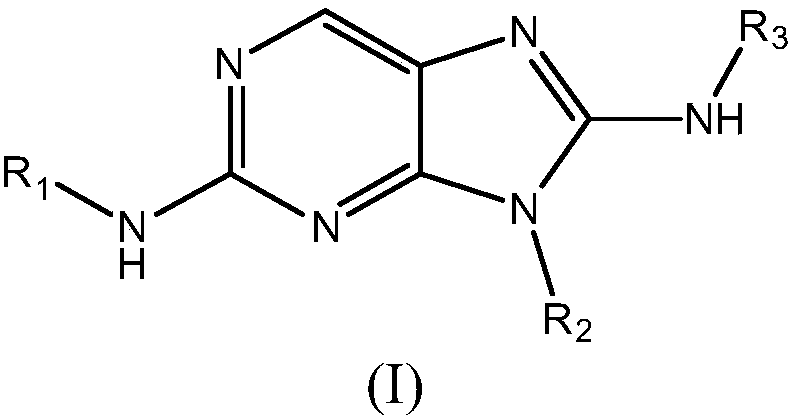
Animal and human Anti-trypanosomonal and Anti-leishmania agents
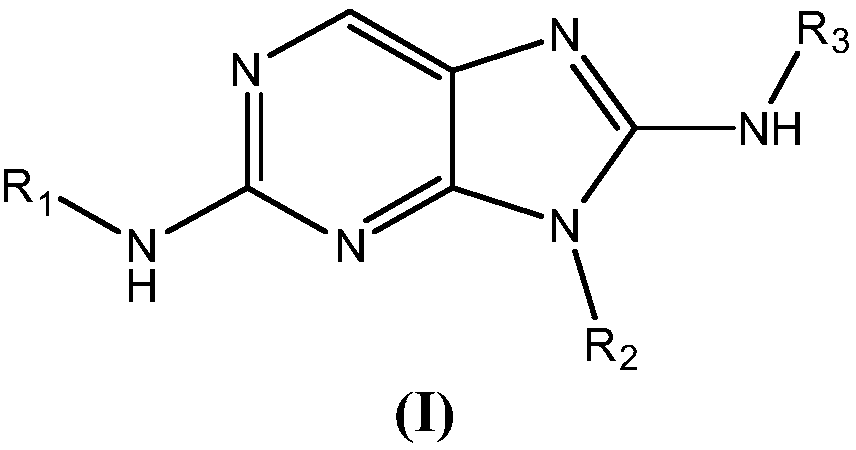
Provided herein are Aminopurine compounds of Formula (I) or pharmaceutically acceptable salts, tautomers, isotopologues, or stereoisomers thereof, wherein R1, R2, and R3 are as defined herein, compositions comprising an effective amount of an Aminopurine Compound, and methods for treating or preventing animal and human protozoal infections.
Owner:CELGENE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/188b02b2-acff-4d2c-ba88-4c922a659078/US20160137654A1-20160519-D00001.PNG)
![Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/188b02b2-acff-4d2c-ba88-4c922a659078/US20160137654A1-20160519-D00002.PNG)
![Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/188b02b2-acff-4d2c-ba88-4c922a659078/US20160137654A1-20160519-D00003.PNG)