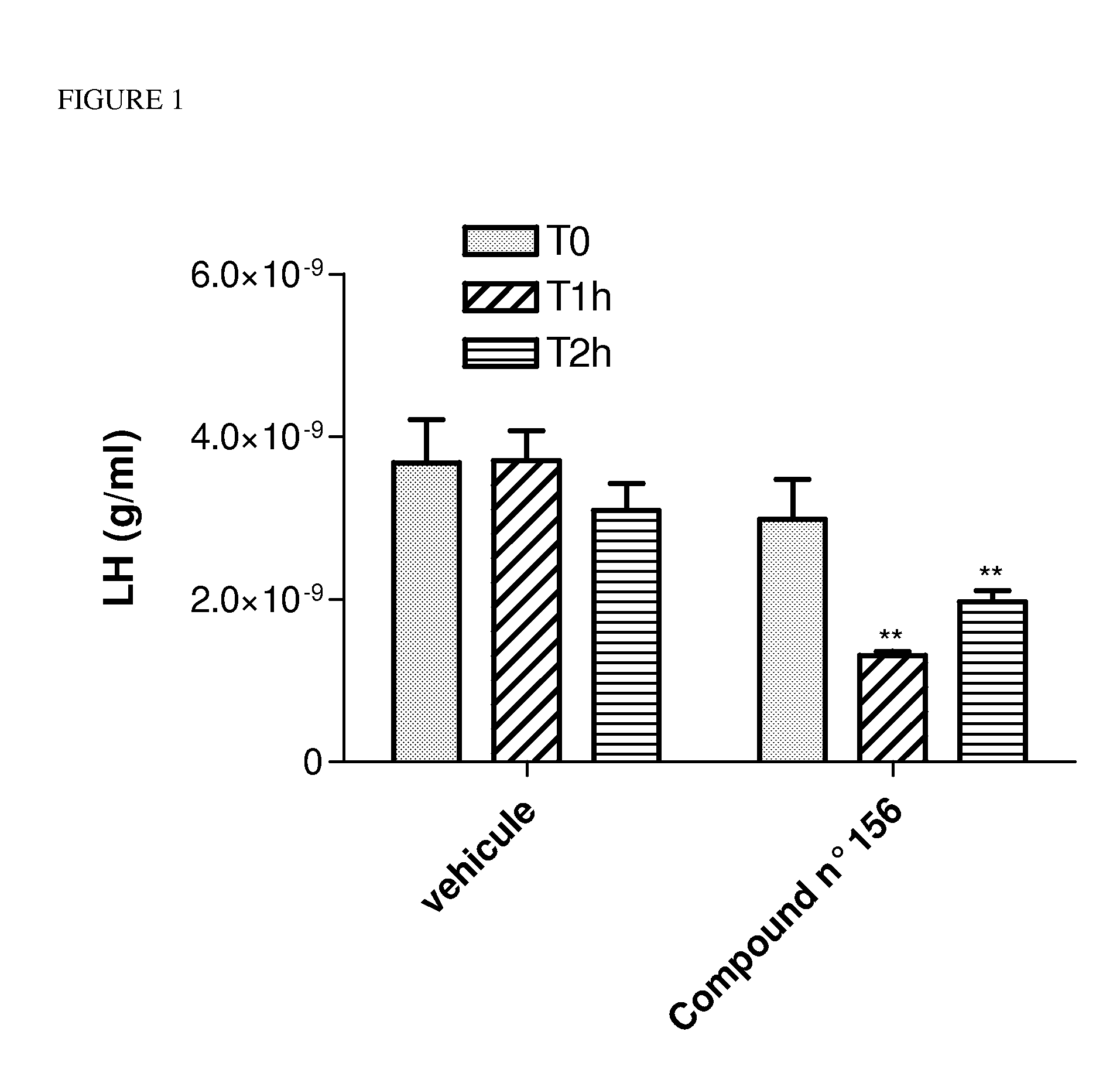
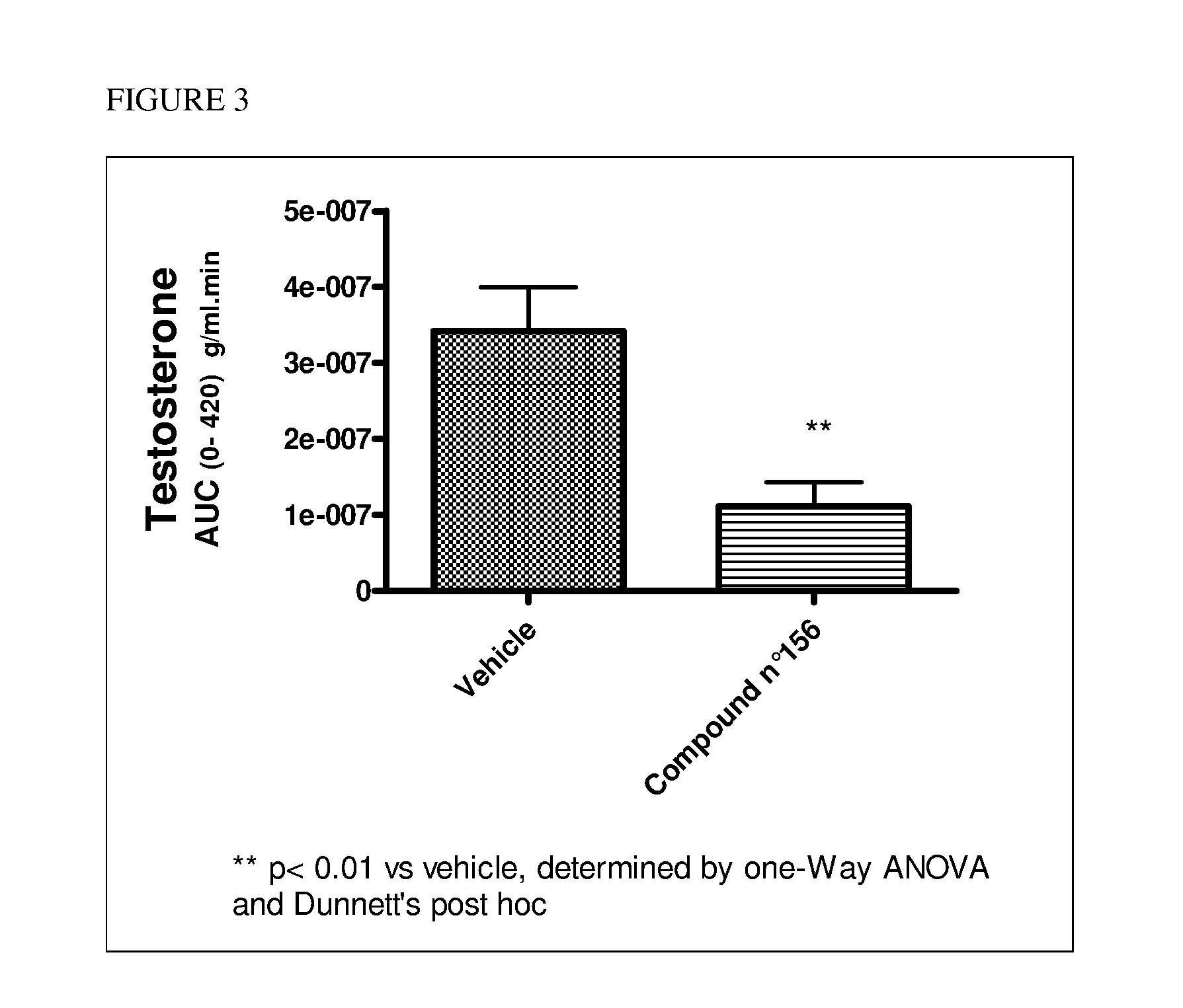
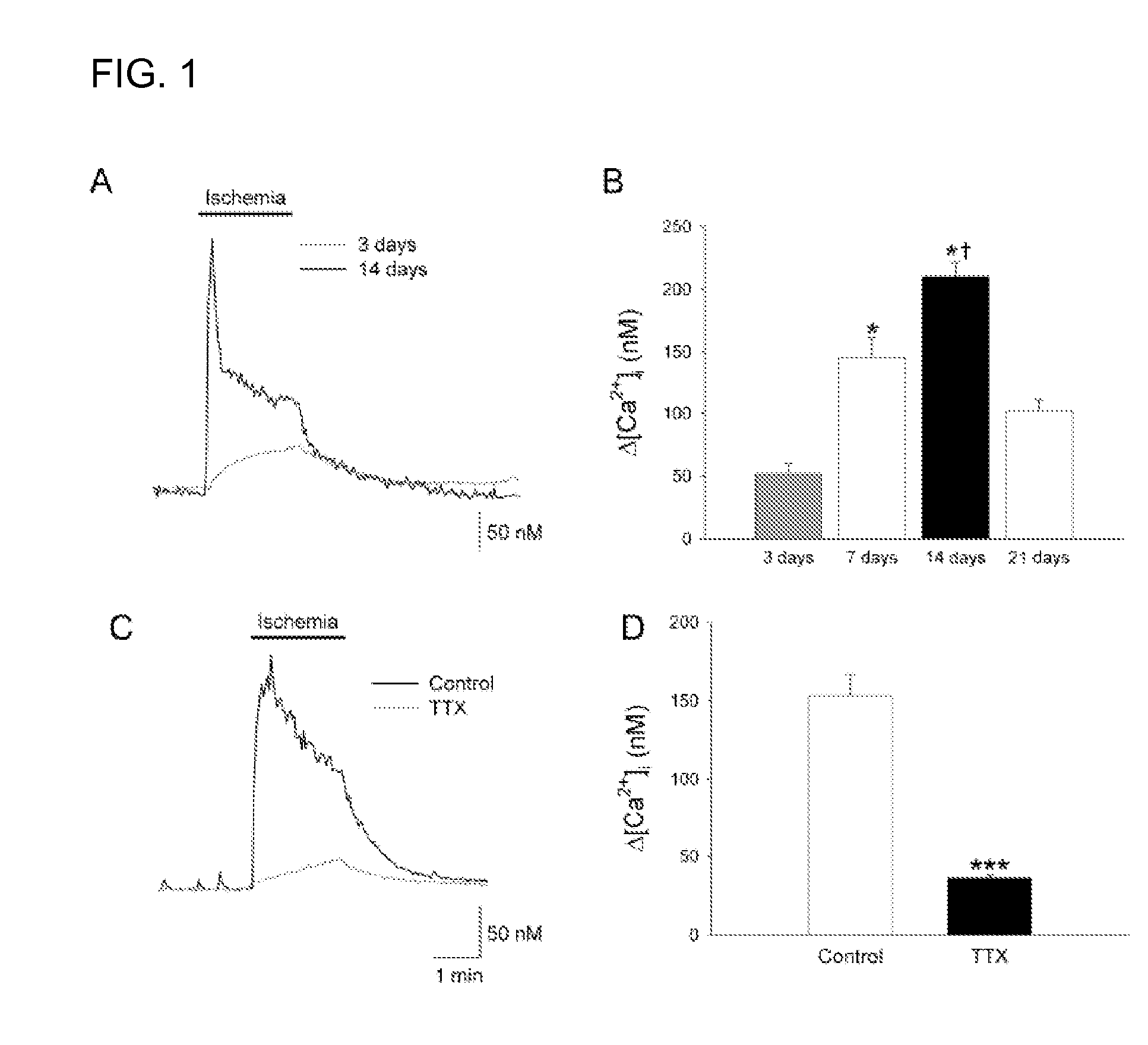
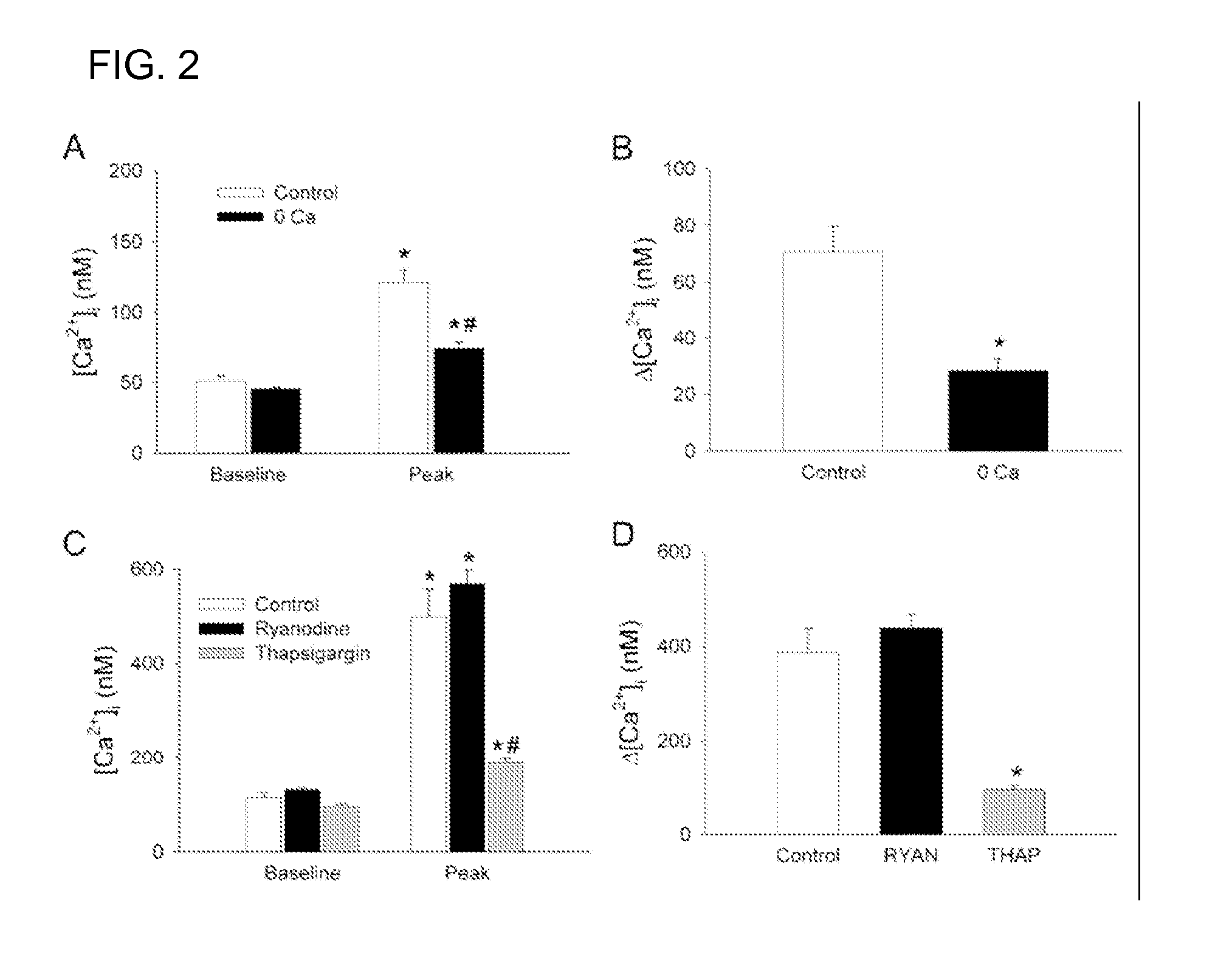
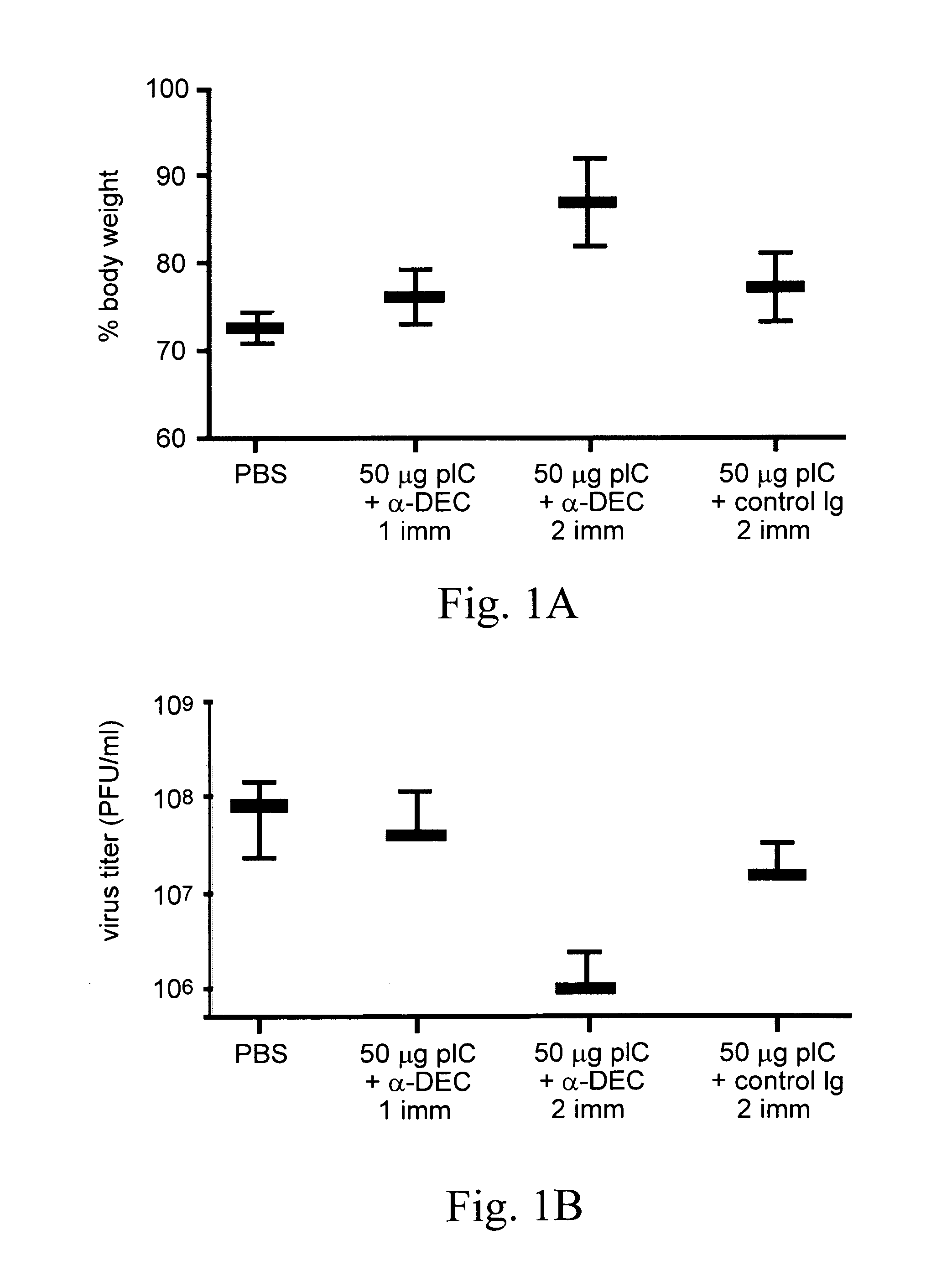
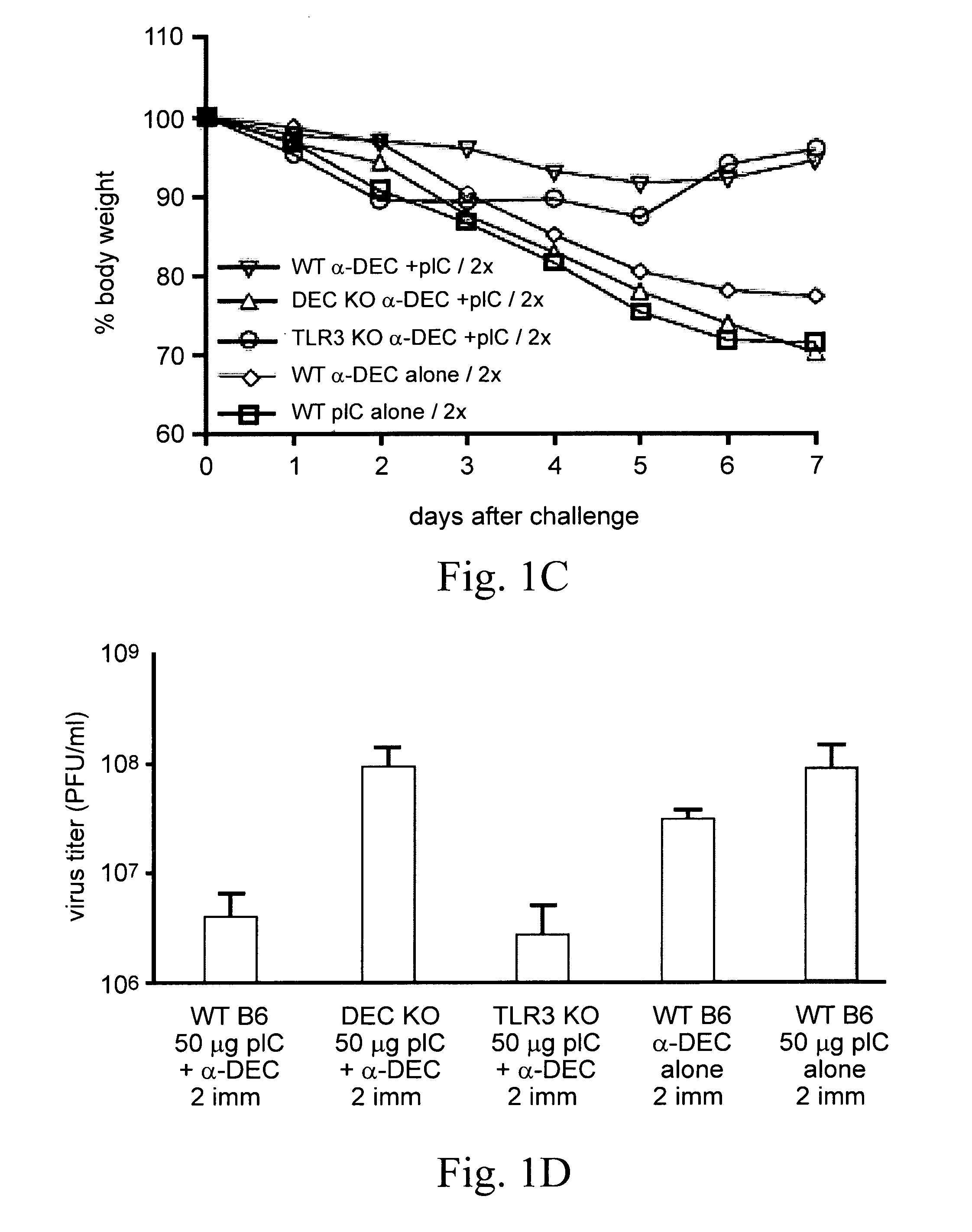
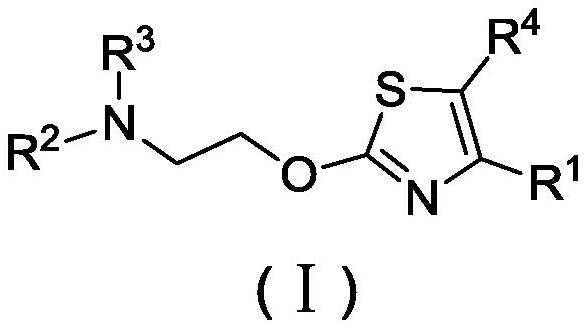
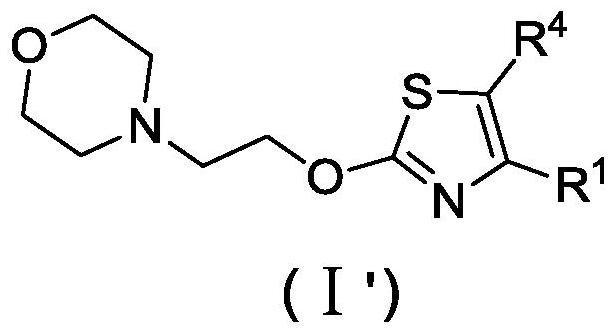
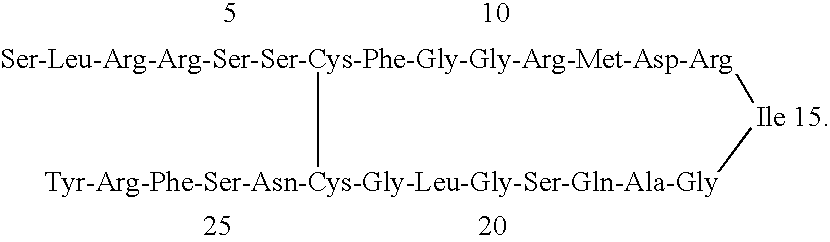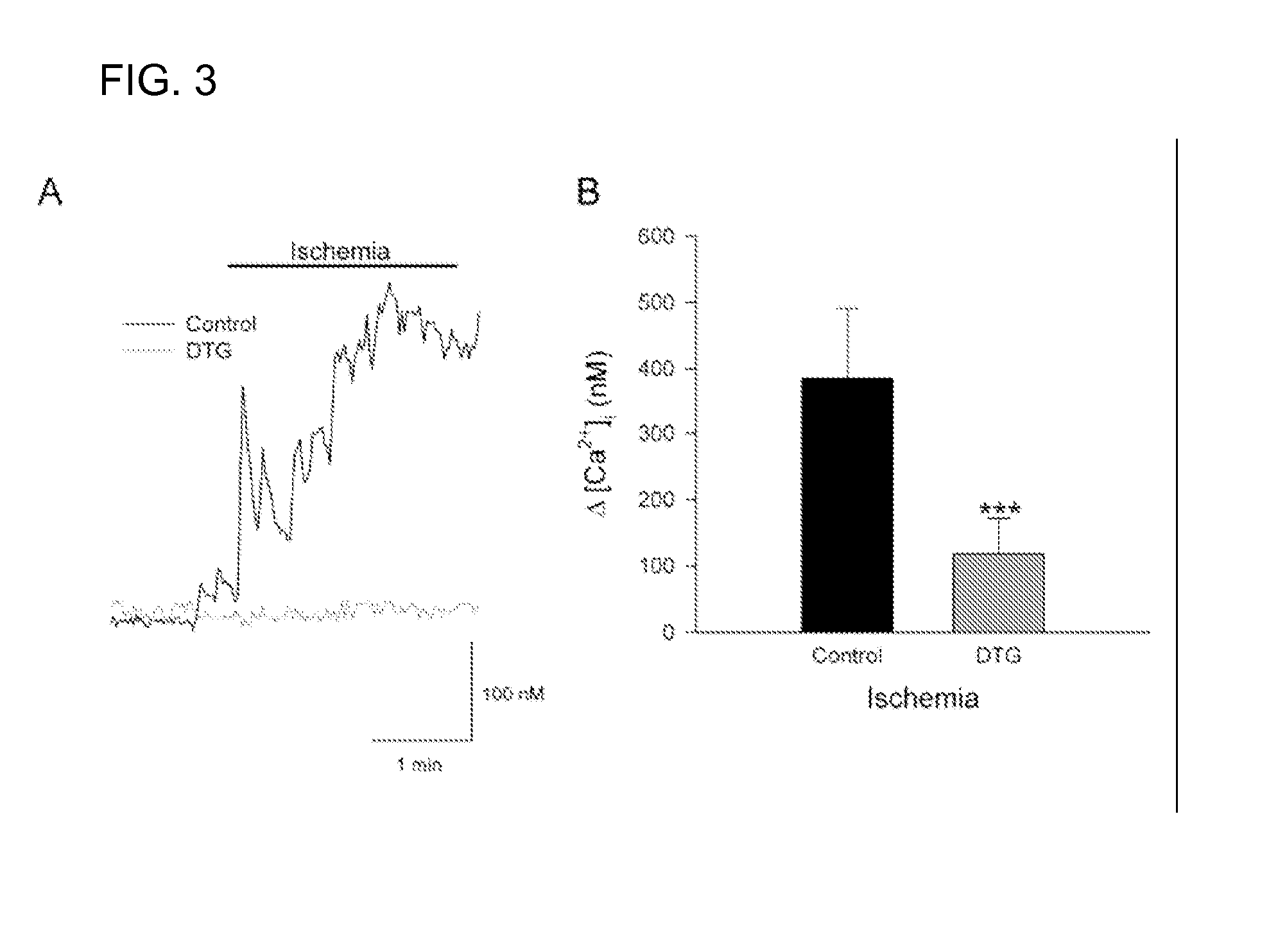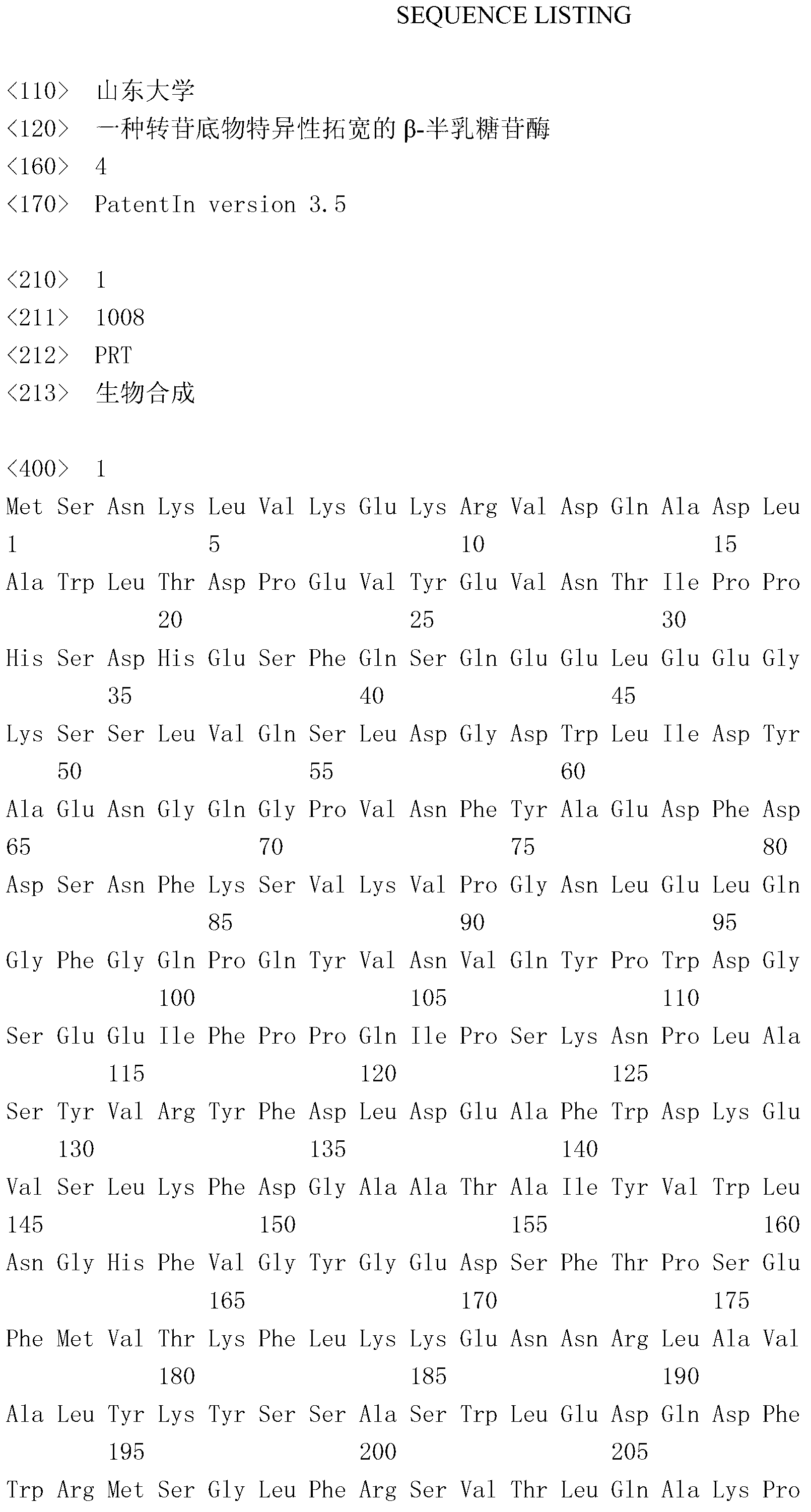Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Receptor selectivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment of conditions that present with low bone mass by continuous combination therapy with selective prostaglandin ep4 receptor agonists and an estrogen
InactiveUS20070191319A1High cure rateLong bone extension is enhancedBiocideSkeletal disorderPresent methodCarboxylic acid
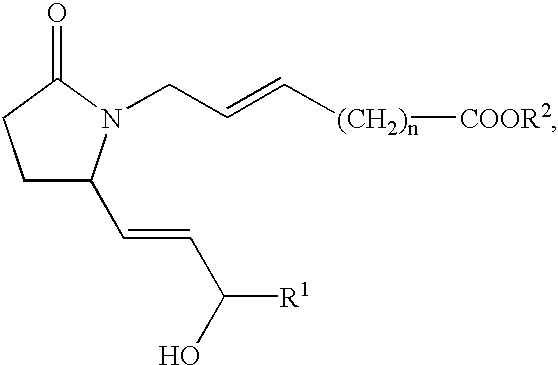
This invention is directed to methods for treating conditions which present with low bone mass in a patient in need thereof using continuous combination therapy with a synergistically effective combination of an EP4 receptor selective agonist or a pharmaceutically acceptable salt thereof, such as 5-(3-{2S-[3R-hydroxy-4-(3-trifluoromethyl-phenyl)-butyl]-5-oxo-pyrrolidin-1-yl}-propyl)-thiophene-2-carboxylic acid or a pharmaceutically acceptable salt thereof; and an estrogen or a pharmaceutically effective salt thereof, The present methods are useful for treating conditions that present with low bone mass including osteoporosis, osteotomy, osteoporotic fracture, childhood idiopathic bone loss, periodontitis and low bone mass and for enhancing bone healing following facial reconstruction, maxillary reconstruction or mandibular reconstruction, inducing vertebral synostosis, enhancing long bone extension, enhancing the healing rate of a bone graft or a long bone fracture or enhancing prosthetic ingrowth in a patient in need thereof.
Owner:PFIZER INC
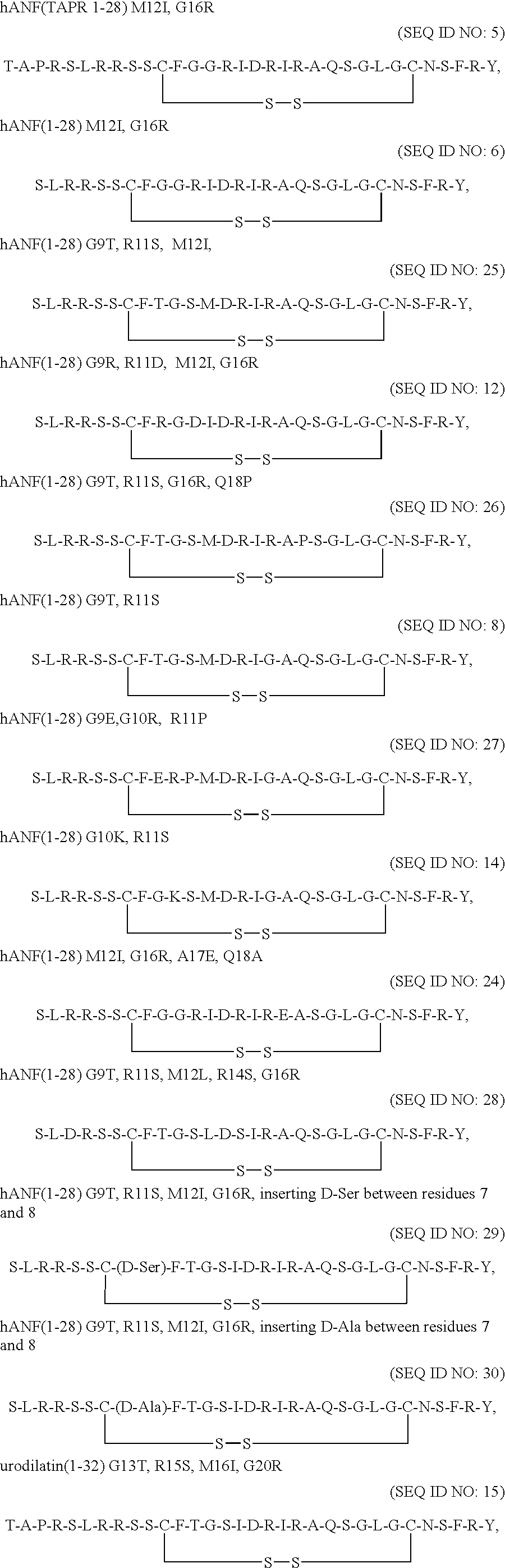
Receptor specific atrial natriuretic peptides
Human receptor selective atrial natriuretic factor variants containing various substitutions, especially G16R, show equal potency and binding affinity for the human A-receptor but have decreased affinity for the human clearance or C-receptor. These ANF variants have natriuretic, diuretic and vasorelaxant activity but have increased metabolic stability, making them suitable for treating congestive heart failure, acute kidney failure and renal hypertension.
Owner:GENENTECH INC
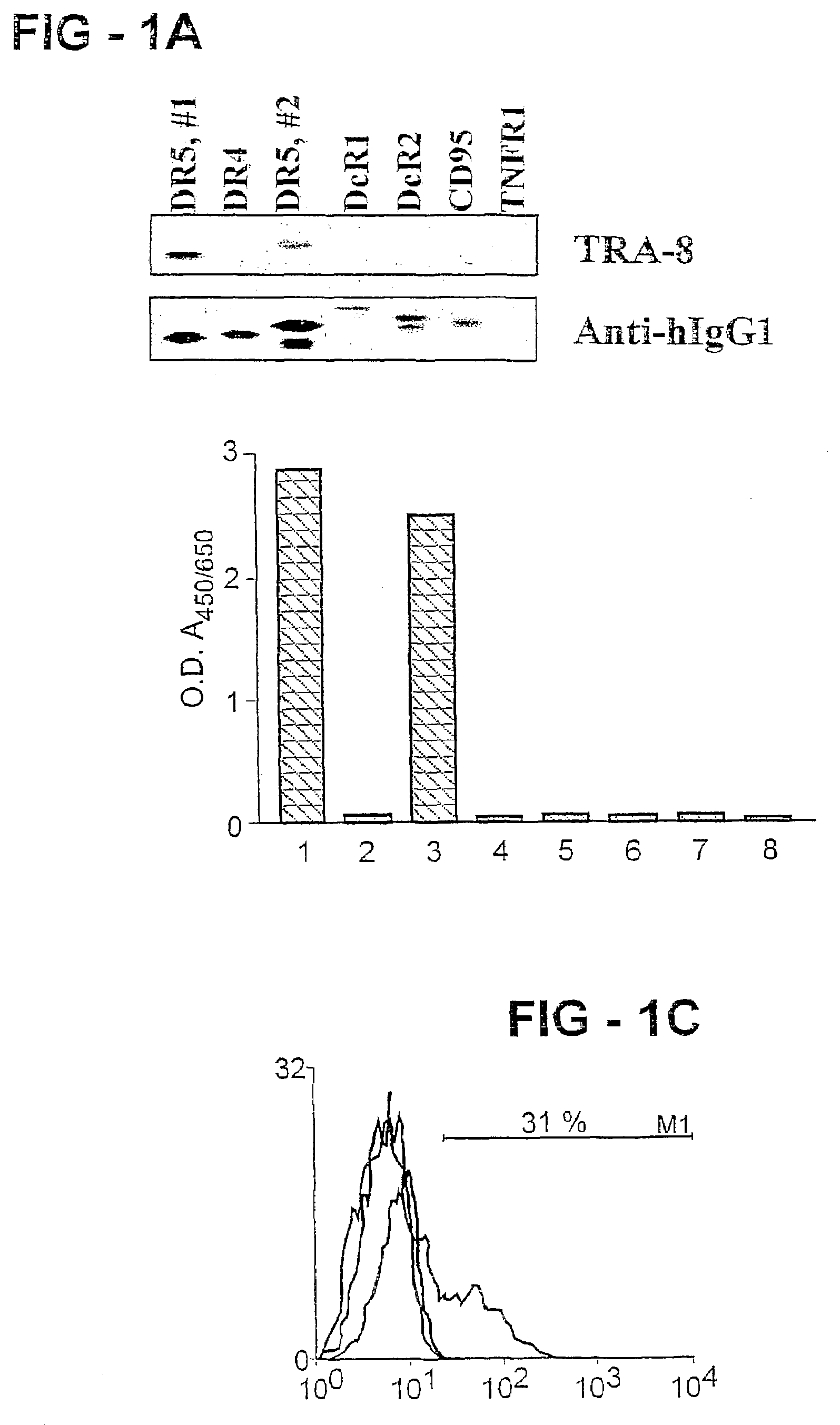
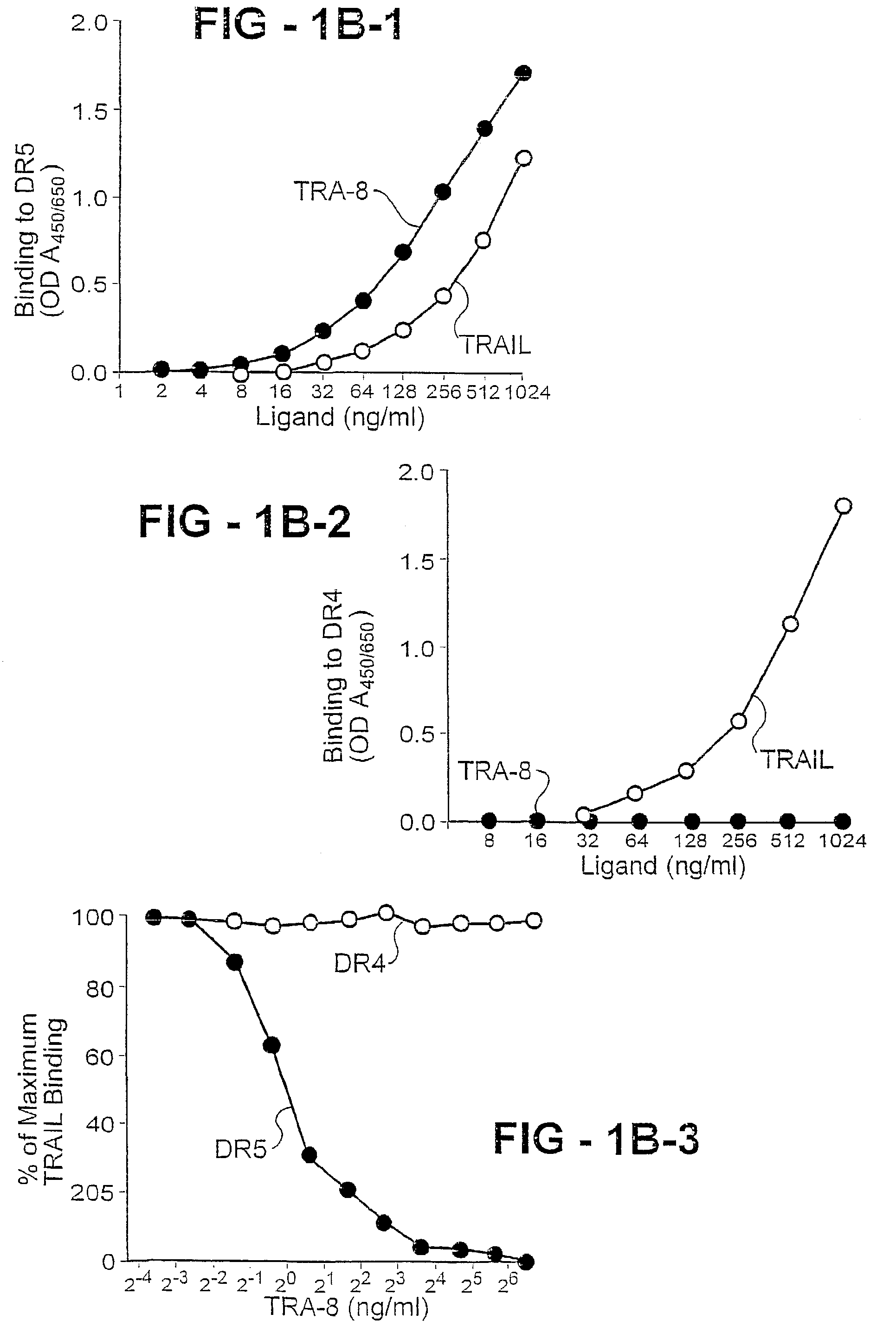
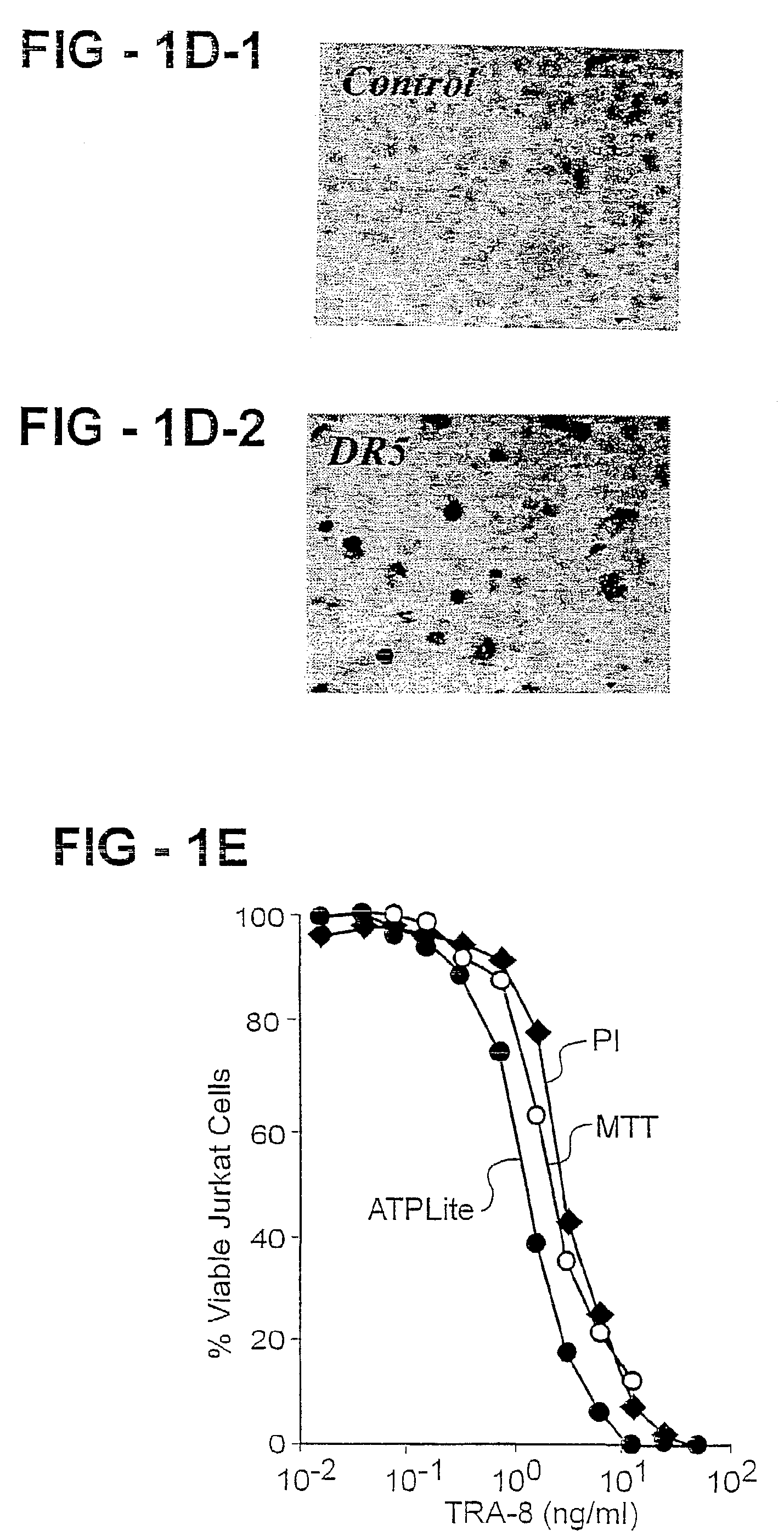
Antibody selective for a tumor necrosis factor-related apoptosis-inducing ligand receptor and uses thereof
An antibody of the invention interacts with human DR5 to produce agonistic or antagonistic effects downstream of the receptor including inhibition of cell proliferation and apoptosis. Nucleic acid sequences and amino acid sequences of anti-DR5 antibodies have been elucidated and vectors and cells containing and expressing these sequences have been generated. Methods and uses for the antibodies are detailed including treatment of apoptosis-related disease and treatment of dysregulated cell growth.
Owner:UAB RES FOUND
Receptor(SSTR4)-selective somatostatin analogs
Analogs of SRIF which are selective for SSTR4 in contrast to the other cloned SRIF receptors are useful in determining tissue and cellular expression of the receptor SSTR4 and its biological role in regulating tumor growth. SRIF analog peptides, such as des-AA1,2,4,5,12,13[Ala7]-SRIF; des-AA1,2,4,5,12,13[Aph7]-SRIF, des-AA1,2,4,5,12,13[Aph7]Cbm-SRIF; des-AA1,2,4,5,12,13[Tyr2,Ala7]-Cbm-SRIF, and des-AA1,2,4,5,12,13[Tyr7,CβMe-L-2Nal8]-SRIF, and counterparts incorporating D-Cys3 and / or D-Trp8 and / or Ala11, bind with high affinity to the cloned human receptor SSTR4 and activate the receptor, but they do not bind with significant affinity to human SSTR1, SSTR2, SSTR3 or SSTR5. By incorporating an iodinated tyrosine in position-2 in these SSTR4-selective SRIF analogs, a labeled compound useful in drug-screening methods is provided. Alternatively, for use in therapy, cytotoxins or highly radioactive elements can be N-terminally coupled or complexed thereto.
Owner:SALK INST FOR BIOLOGICAL STUDIES
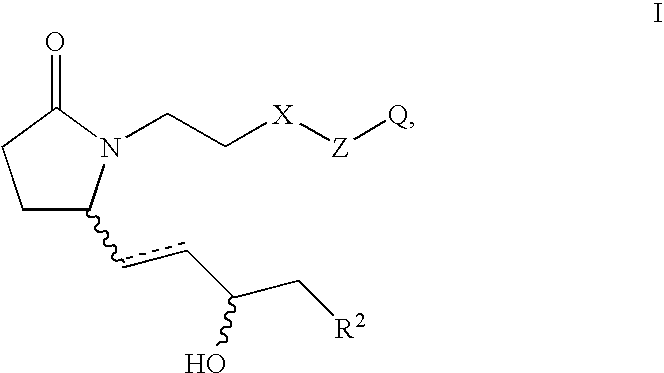
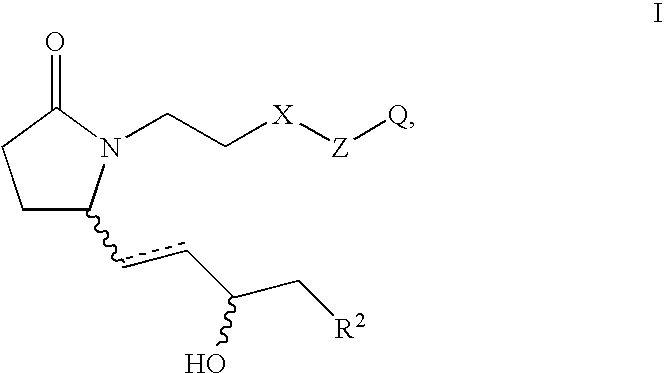
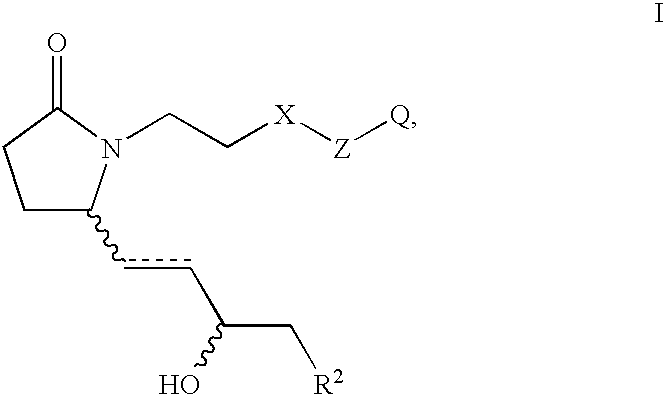
EP4 receptor selective agonists in the treatment of osteoporosis
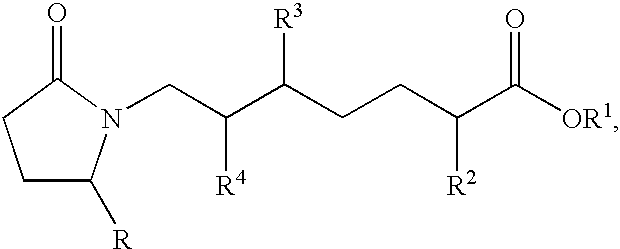
This invention is directed to EP4 receptor selective prostaglandin agonists of the Formula I, wherein R2, X, Z and Q are as defined in the specification. This invention is also directed to pharmaceutical compositions containing those compounds. This invention is also directed to methods of treating conditions which present with low bone mass, particularly osteoporosis, frailty, an osteoporotic fracture, a bone defect, childhood idiopathic bone loss, alveolar bone loss, mandibular bone loss, bone fracture, osteotomy, bone loss associated with periodontitis, or prosthetic ingrowth in a mammal comprising administering those compounds.
Owner:PFIZER INC
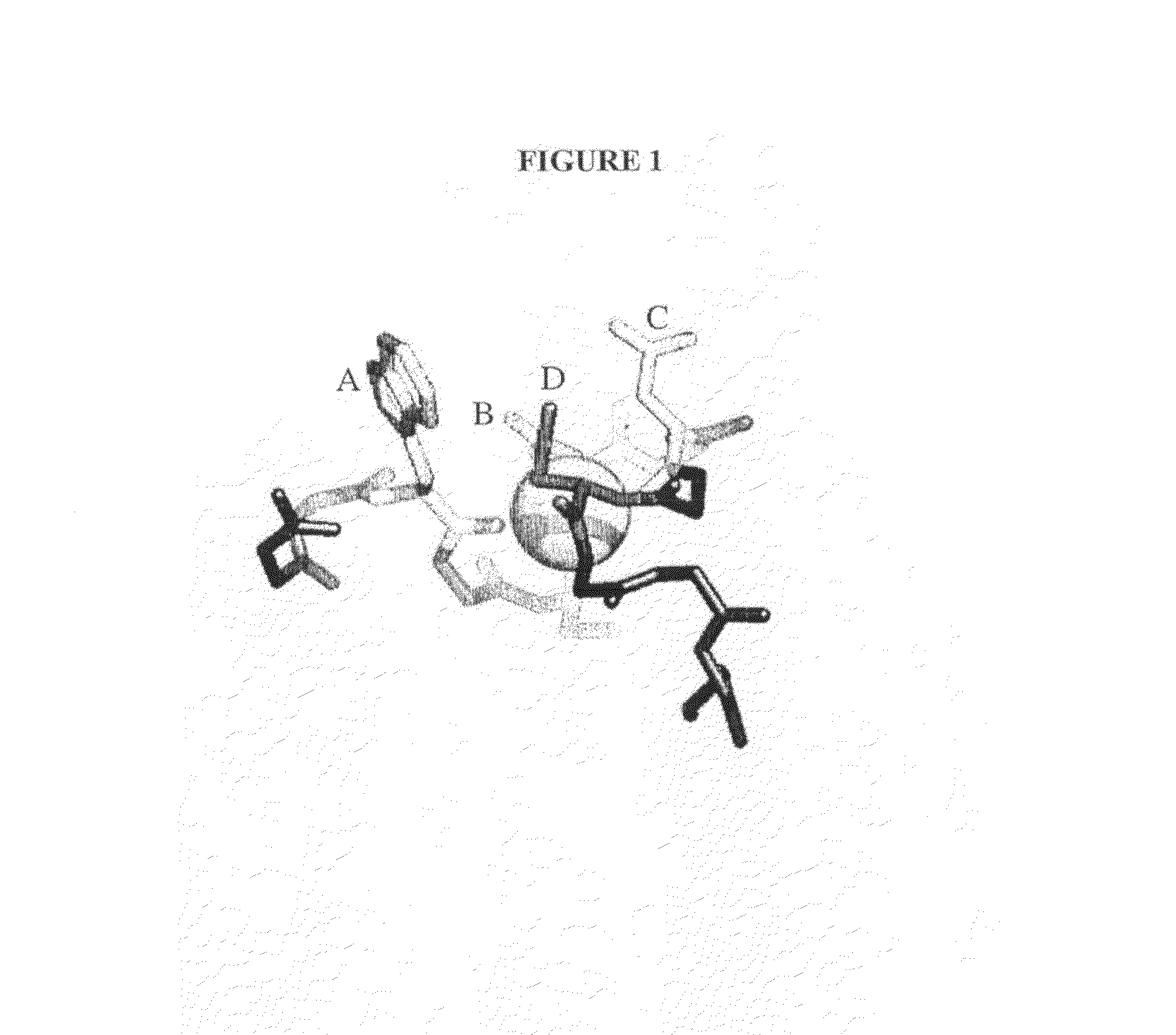
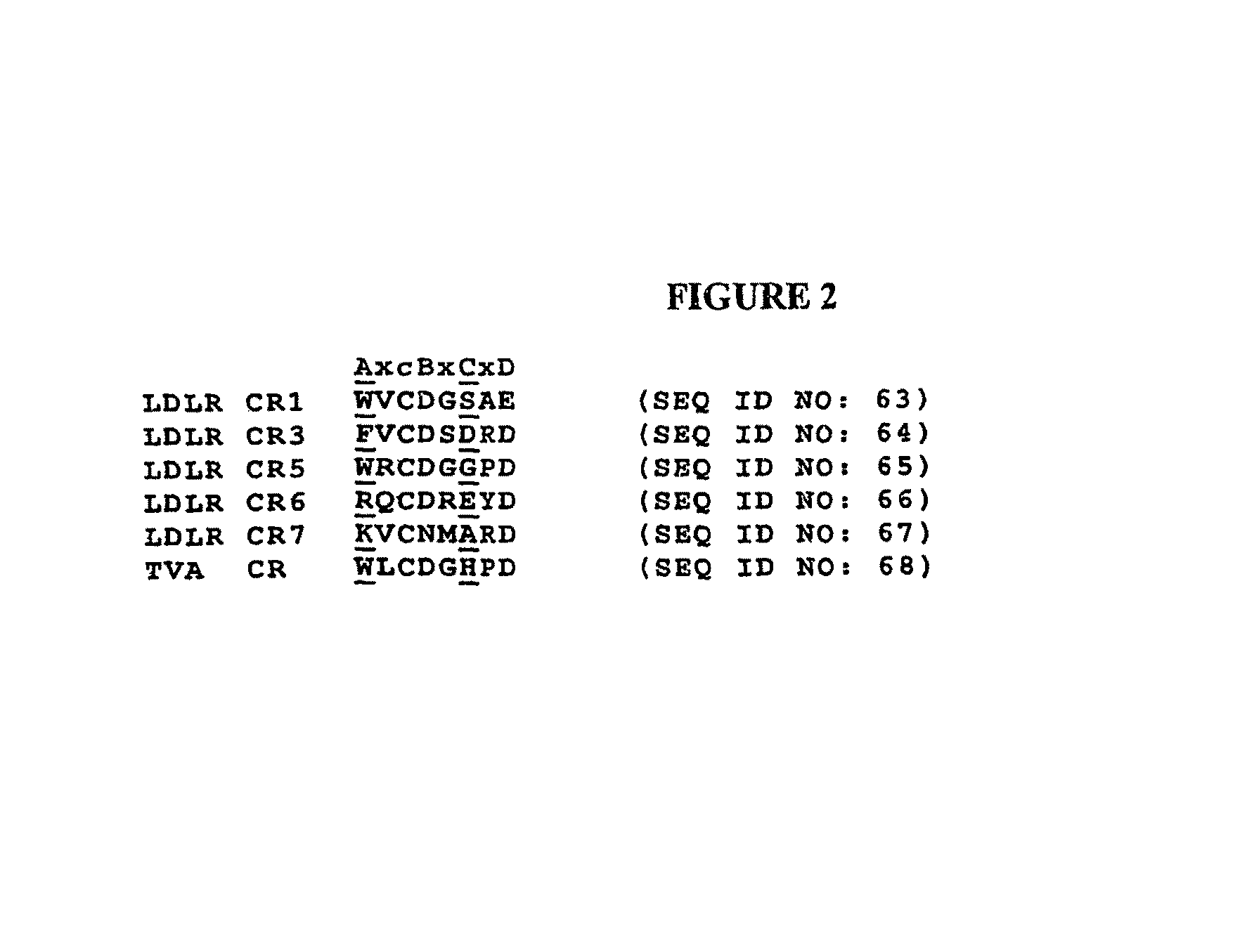
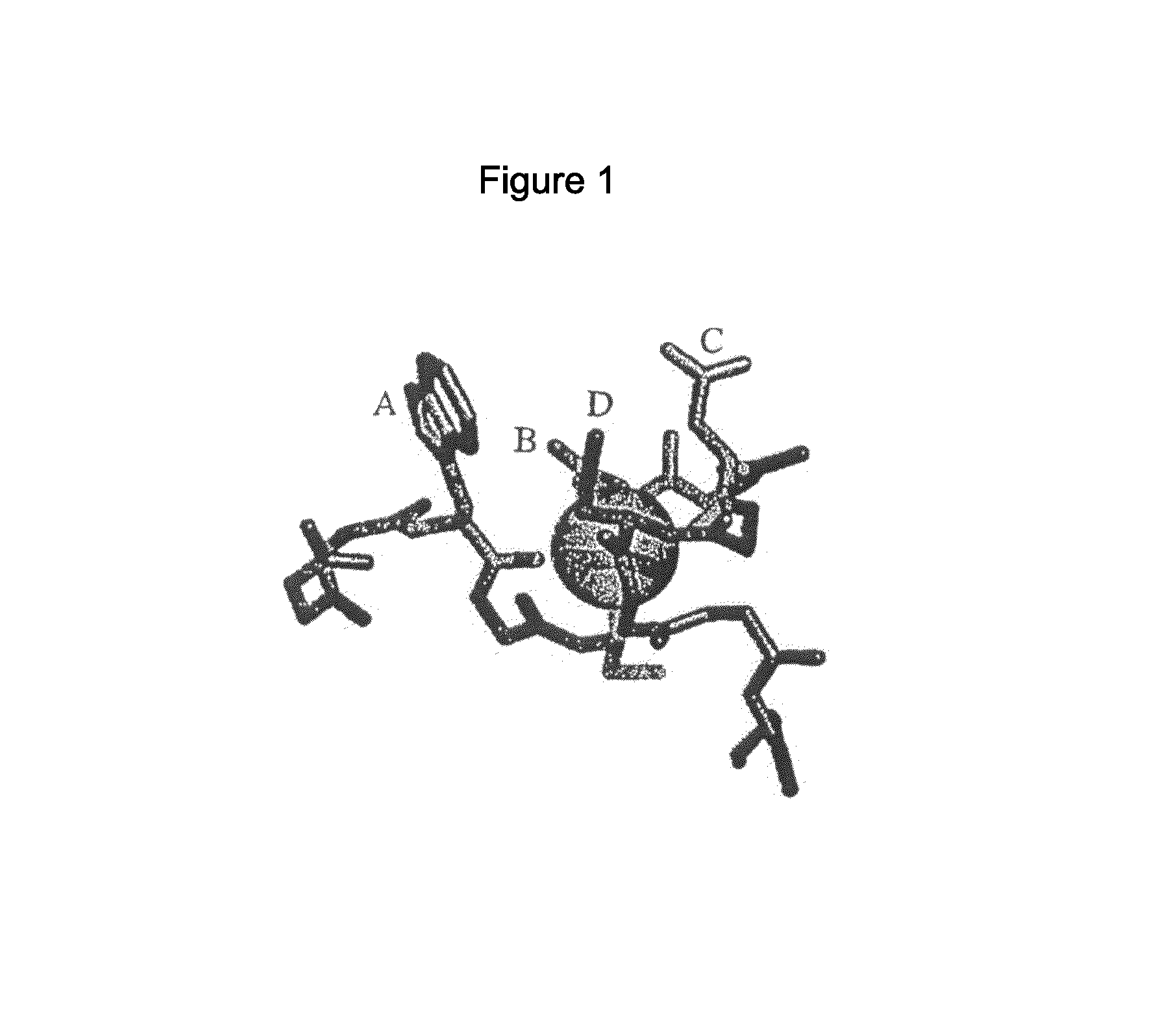
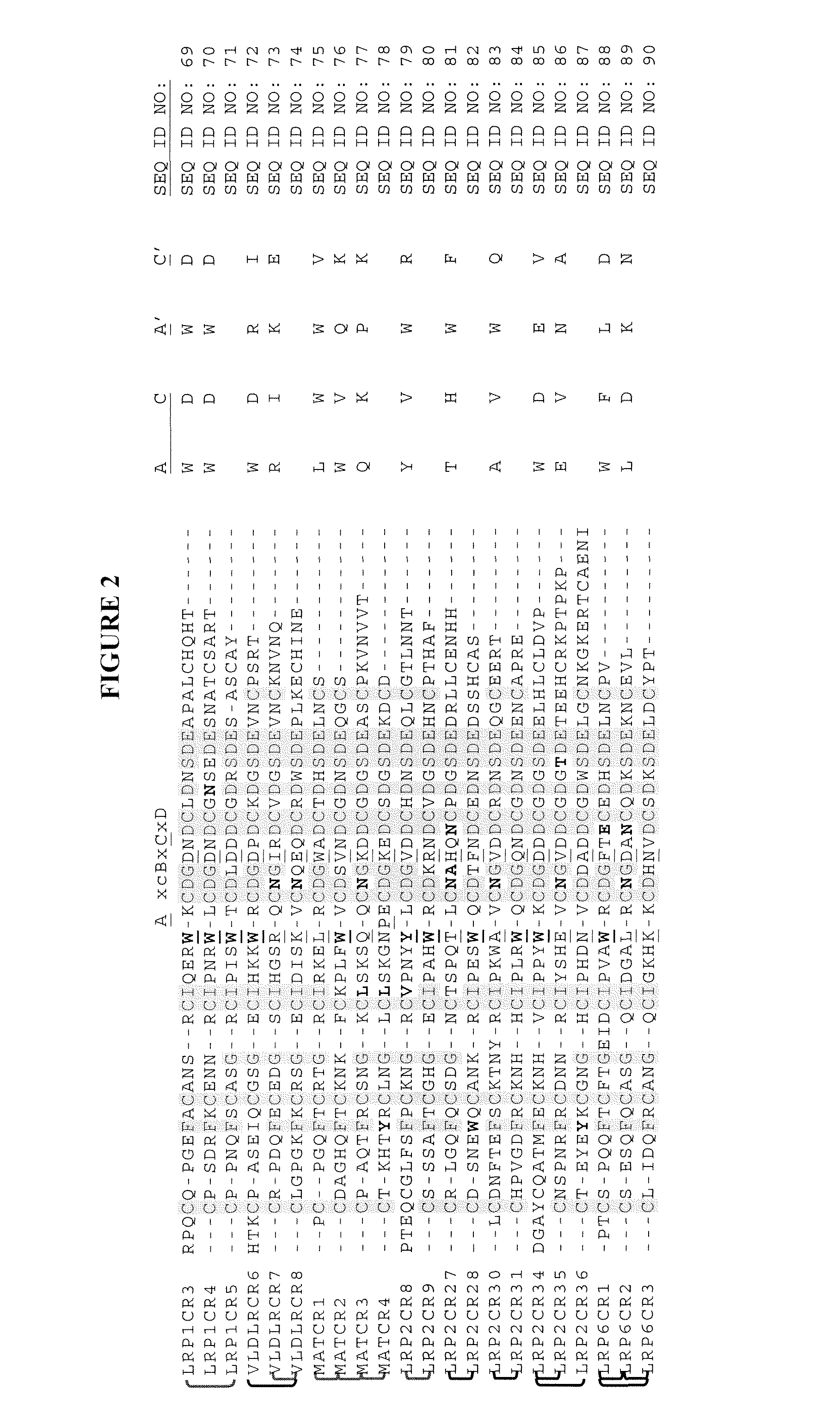
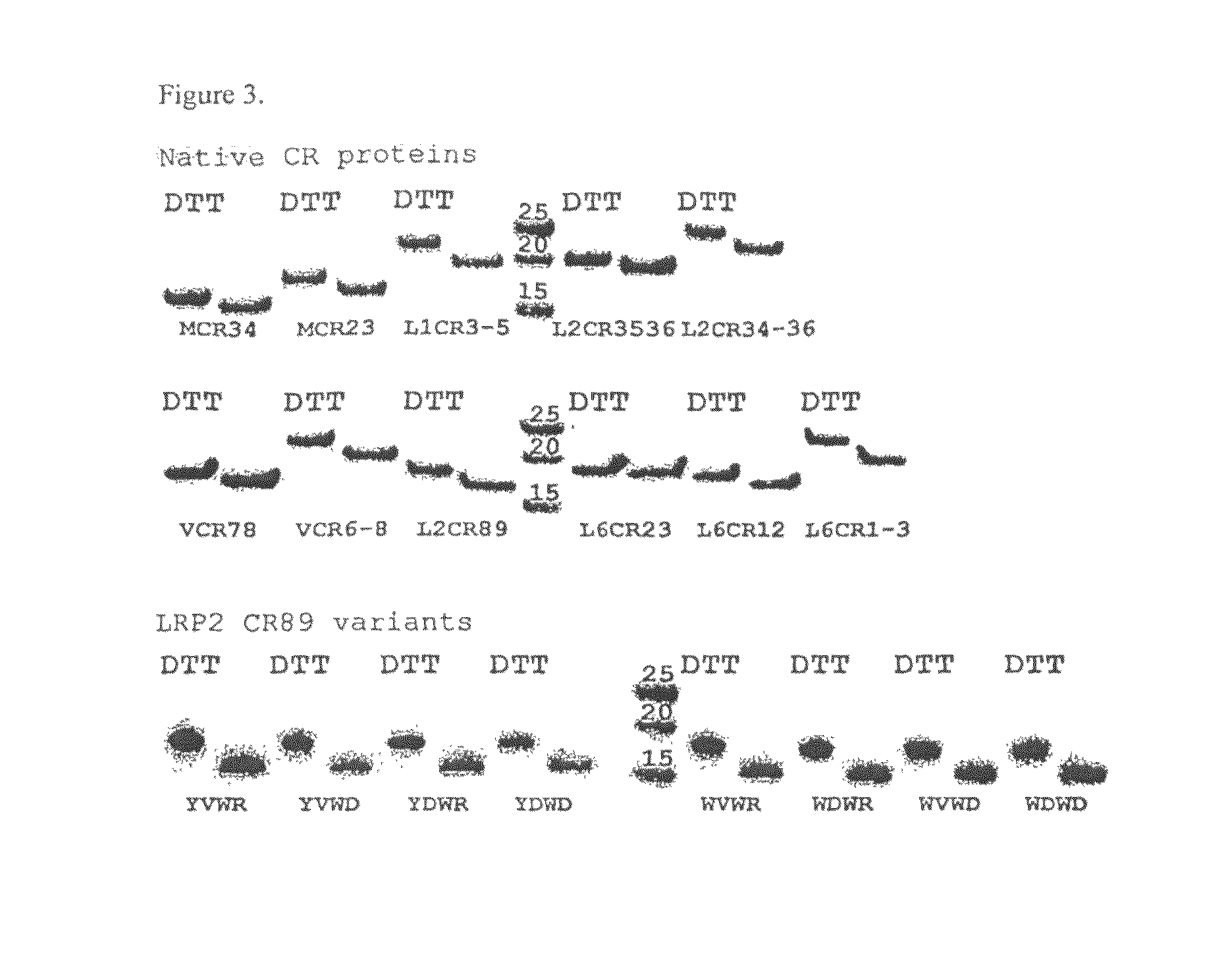
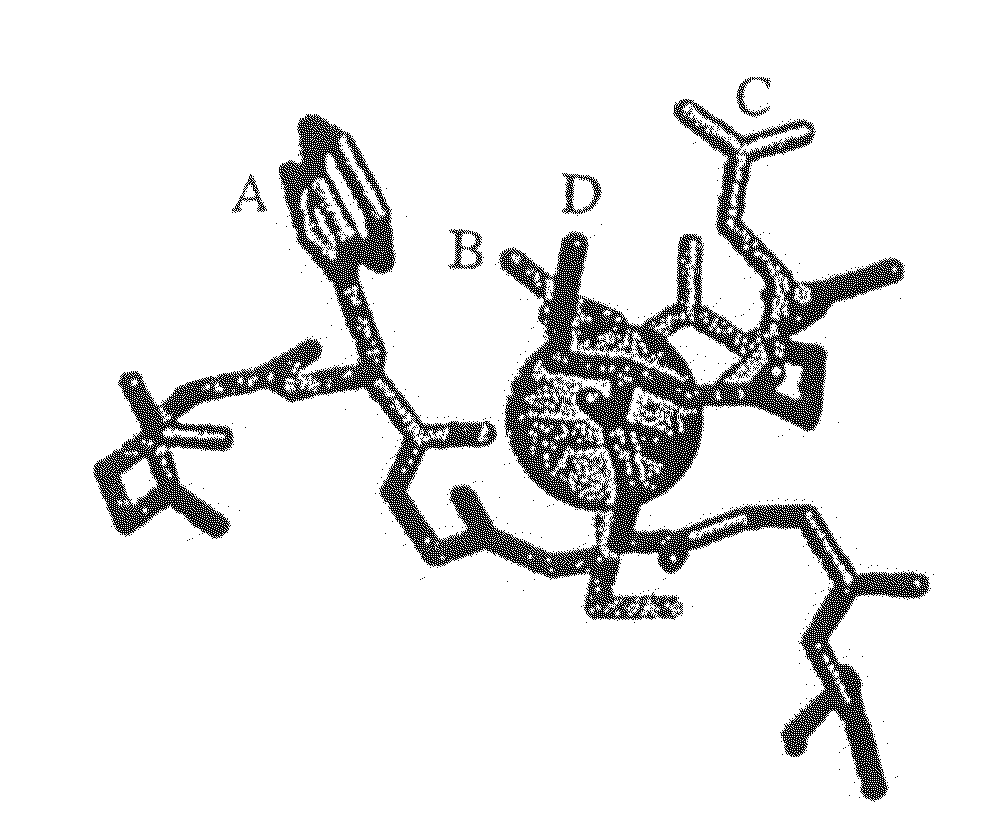
Compositions Comprising Receptor-Associated Protein (RAP) Variants Specific for LRP2 and Uses Thereof
InactiveUS20090269346A1Reducing metastatic effectReduce the impactCell receptors/surface-antigens/surface-determinantsSugar derivativesReceptor selectivityLRP2
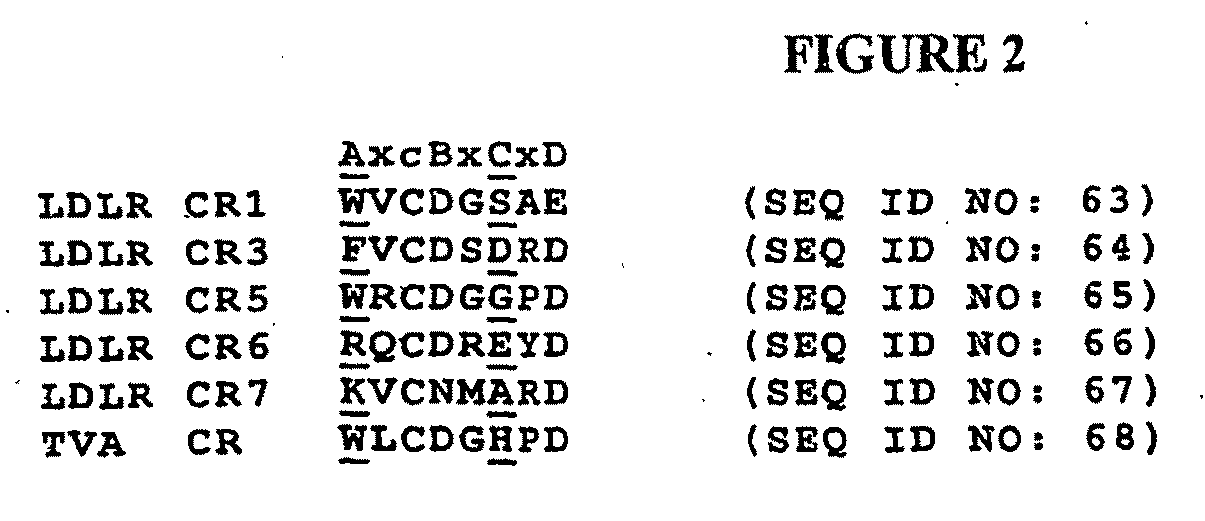
The present invention relates generally to receptor-selective variants of the low-density lipoprotein receptor-associated protein (RAP) and compositions thereof, methods of generating such variants and methods of using such receptor-selective RAP variant compositions for therapeutic purposes. The invention also relates to antibodies that bind to one or a small subset of CR-containing proteins.
Owner:HORIZON ORPHAN LLC
Complex external medicine for treating psoriasis
InactiveCN1528313ALess irritatingClear targetOrganic active ingredientsPeptide/protein ingredientsExternal applicationIrritation
The present invention relates to a compound medicine for external application for curing psoriasis. It is characterized by that in the matrix carrier of said compound medicine for external application are contained the following effective active components: (wt%) tazarotenje 0.025-0.1% and cortical hormone 0.01-0.2%. Its therapeutic effect is good, and it has no adverse reaction.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Treatment with Sigma Receptor Agonists Post-Stroke
InactiveUS20070123556A1Decrease in infarction areaHigh affinityBiocidePeptide/protein ingredientsHippocampal regionGlial fibrillary acidic protein
A method of post-stroke treatment at delayed timepoints with sigma receptor agonists. Sigma receptors are promising targets for neuroprotection following ischemia. One of the key components in the demise of neurons following ischemic injury is the disruption of intracellular calcium homeostasis. The sigma receptor agonist, DTG, was shown to depress [Ca2+]i elevations observed in response to ischemia induced by sodium azide and glucose deprivation. Two sigma receptor antagonists, metaphit and BD-1047, were shown to blunt the ability of DTG to inhibit ischemia-evoked increases in [Ca2+]i. DTG inhibition of ischemia-induced increases in [Ca2+]i was mimicked by the sigma-1 receptor-selective agonists, carbetapentane, (+)-pentazocine and PRE-084, but not by the sigma-2 selective agonist, ibogaine, showing that activation of sigma-1 receptors is responsible for the effects. Activation of sigma receptors can ameliorate [Ca2+]i dysregulation associated with ischemia in cortical neurons, providing neuroprotective properties. The effects of 1,3-di-o-tolyguanidine (DTG), a high affinity sigma receptor agonist, as a potential treatment for decreasing infarct area at delayed time points was further examined in rats. DTG treatment significantly reduced infarct area in both cortical / striatal and cortical / hippocampal regions by >80%, relative to control rats. These findings were confirmed by immunohistochemical experiments using the neuronal marker, mouse anti-neuronal nuclei monoclonal antibody (NeuN), which showed that application of DTG significantly increased the number of viable neurons in these regions. Furthermore, DTG blocked the inflammatory response evoked by MCAO, as indicated by decreases in the number of reactive astrocytes and activated microglia / macrophages detected by immunostaining for glial fibrillary acidic protein (GFAP) and binding of isolectin IB4, respectively. Thus, the sigma receptor-selective agonist, DTG, can enhance neuronal survival when administered 24 hr after an ischemic stroke. In addition, the efficacy of sigma receptors for stroke treatment at delayed time points is likely the result of combined neuroprotective and anti-inflammatory properties of these receptors.
Owner:UNIV OF SOUTH FLORIDA
FGF-2 variants having N-terminal deletions and increased receptor selectivity and uses thereof
The present invention relates to the design, manufacture and use of fibroblast growth factor (FGF) polypeptides having improved receptor specificity. In particular, the invention relates to isolated FGF2 and FGF4 polypeptides that include a truncated N-terminus and optionally N-terminal amino acid substitutions. The present invention provides polypeptides, nucleic acids encoding the polypeptides, compositions of the same and methods for use thereof.
Owner:HEPACORE LTD +1
Formulations and uses of retinoic acid receptor selective agonists
ActiveUS20130085166A1Low toxicityLess side effectsBiocideSenses disorderDiseaseRetinoic acid receptor
The invention provides retinoic acid receptor (RAR) selective agonists and formulations thereof for the treatment of disease or for inducing a medically beneficial effect.
Owner:QURETINO THERAPEUTICS
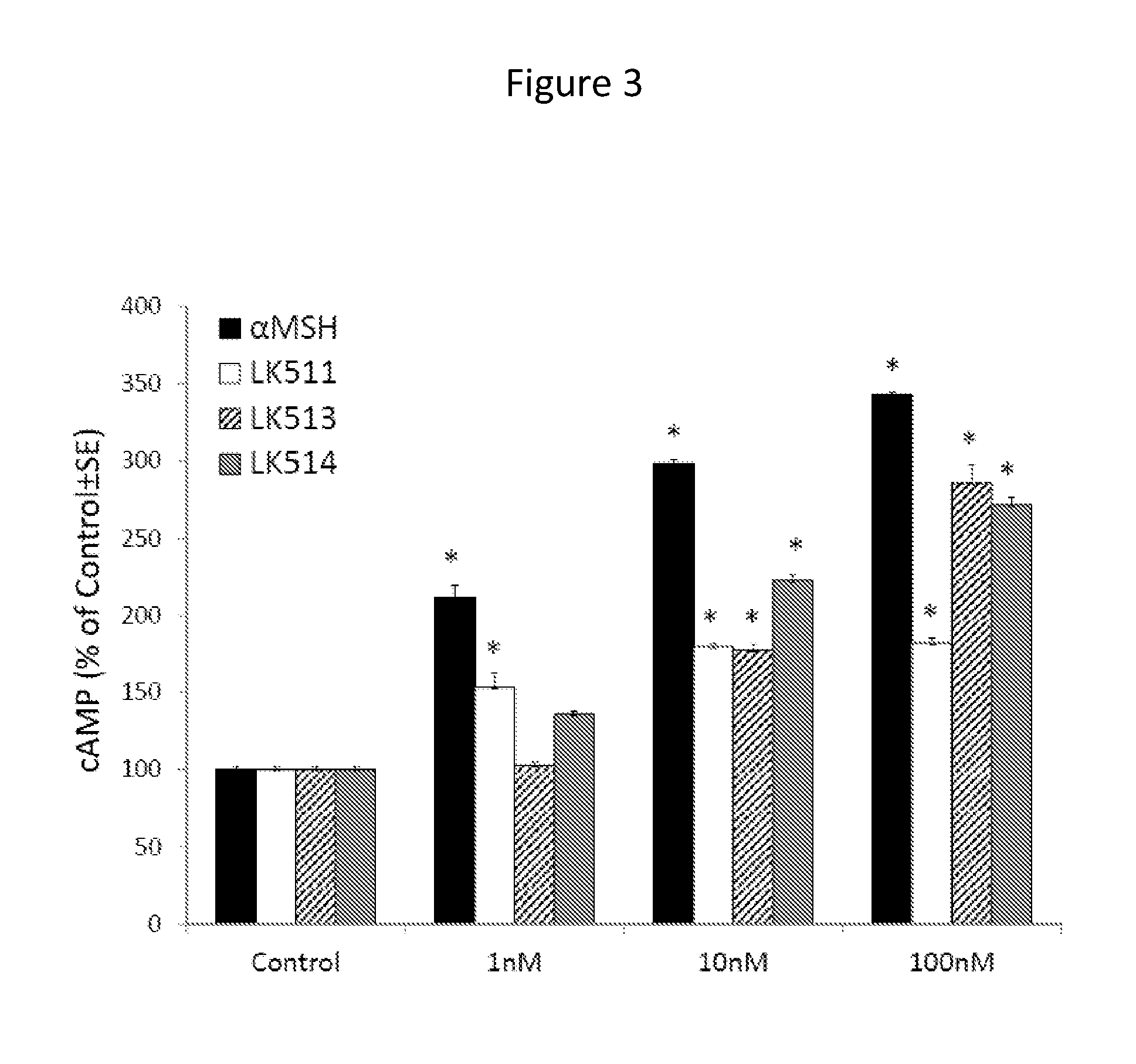
Skin care compositions and methods comprising selective agonists of melanocortin 1 receptor
Short tri- and tetrapeptides according to the following Formula I Ar(CH2)mX1—X2—CO—X3—X4—X5-(Trp)n-NX6R are potent, selective agonists of melanocortin 1 receptor (MC1R). Provided herein are skin care compositions including Formula I peptide agonists of MC1R and methods of regulating a skin condition of a mammal that include applying to a treatment surface of the body a safe and effective amount of a skin care composition including a Formula I peptide. The peptides, skin care compositions, and skin care methods described herein are useful in regulating a skin condition of a mammal associated with exposure ultraviolet (UV) radiation, including sunburn, UV sensitivity, photoaging, and skin pigmentation, particularly in the absence of sun exposure.
Owner:UNIVERSITY OF CINCINNATI
Compositions comprising receptor-associated protein (RAP) variants specific for LRP2 and uses thereof
Owner:HORIZON ORPHAN LLC
Compositions comprising receptor-associated protein (RAP) variants specific for CR-containing proteins and uses thereof
The present invention relates generally to receptor-selective variants of the low-density lipoprotein receptor-associated protein (RAP) and compositions thereof, methods of generating such variants and methods of using such receptor-selective RAP variant compositions for therapeutic purposes.
Owner:HORIZON ORPHAN LLC
Compositions Comprising Receptor-Associated Protein (RAP) Variants Specific for CR-Containing Proteins and Uses Thereof
InactiveUS20090281024A1Antibacterial agentsNervous disorderReceptor selectivityLow-density lipoprotein
The present invention relates generally to receptor-selective variants of the low-density lipoprotein receptor-associated protein (RAP) and compositions thereof, methods of generating such variants and methods of using such receptor-selective RAP variant compositions for therapeutic purposes.
Owner:HORIZON ORPHAN LLC
Piperazine (piperidine) cyclohexyl derivative and applications of piperazine (piperidine) cyclohexyl derivative in treatment of neuropsychiatric diseases
ActiveCN105367565AGood receptor selectivityImproving the effect of cognitive impairmentOrganic active ingredientsNervous disorderLow affinityAcute toxicity testing
The present invention discloses a piperazine (piperidine) cyclohexyl derivative and applications of the piperazine (piperidine) cyclohexyl derivative in treatment of neuropsychiatric diseases. According to the present invention, the pharmacological experiment results show that the piperazine (piperidine) cyclohexyl derivative provides high affinity with a dopamine D2 receptor, a dopamine D3 receptor, a serotonin 5-HT1A receptor and a serotonin 5-HT2A receptor, provides good D3 / D2 receptor selectivity and good 5-HT1A / 5-HT2A receptor selectivity, and provides low affinity with alpha receptor; the in vivo test results show that the preferred compound has characteristics of good anti-schizophrenia effect, good pharmacokinetic property, low side effect, low acute toxicity and high safety, and has the development value of being adopted as the novel efficient and low-toxic anti-neuropsychiatric disease; and the piperazine (piperidine) cyclohexyl derivative is a compound represented by the structural general formula (I), or a geometric isomer, an optical isomer, a salt, a hydrate or a solvate thereof. The formula (I) is defined in the specification.
Owner:SHANGHAI INST OF PHARMA IND +1
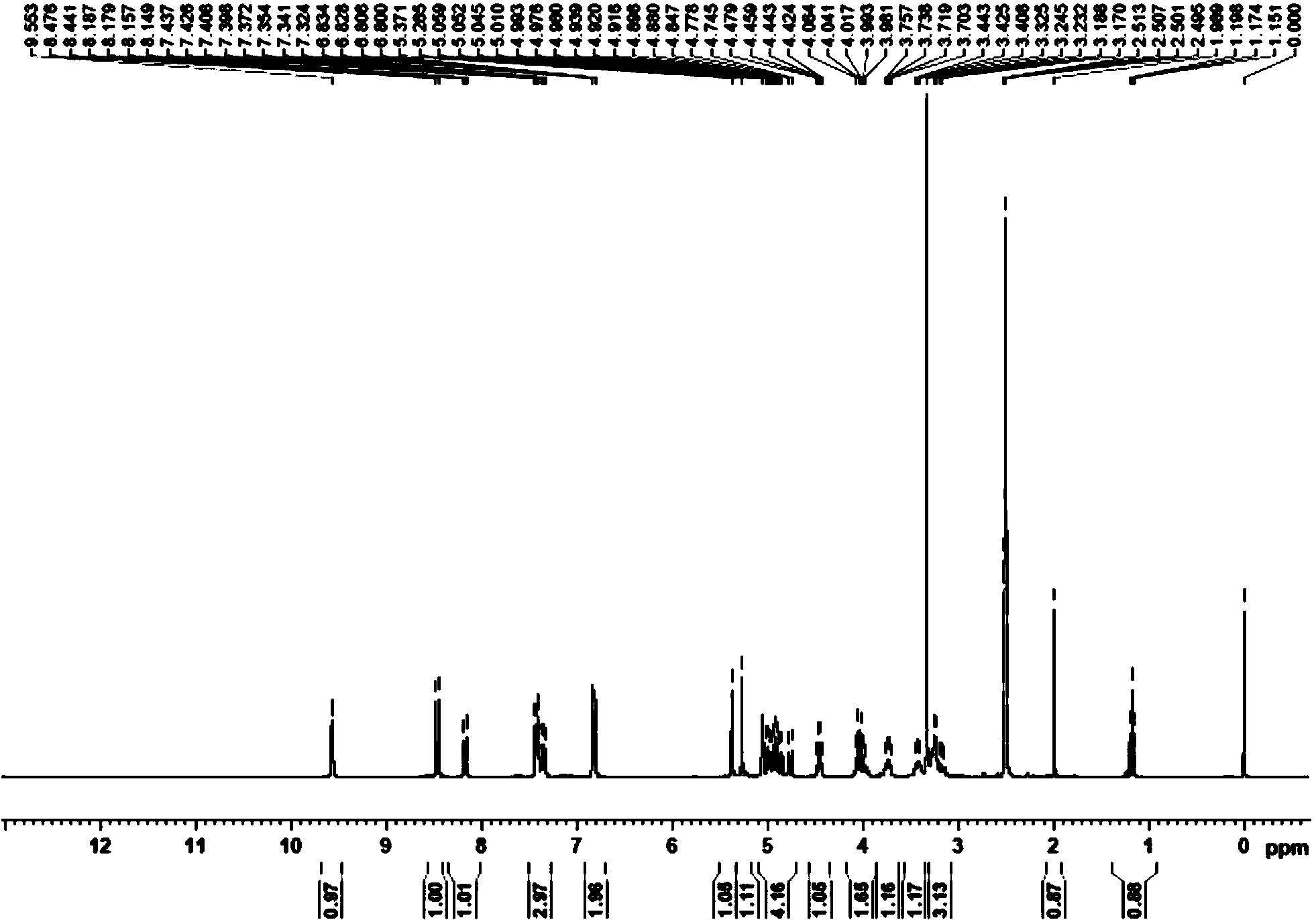
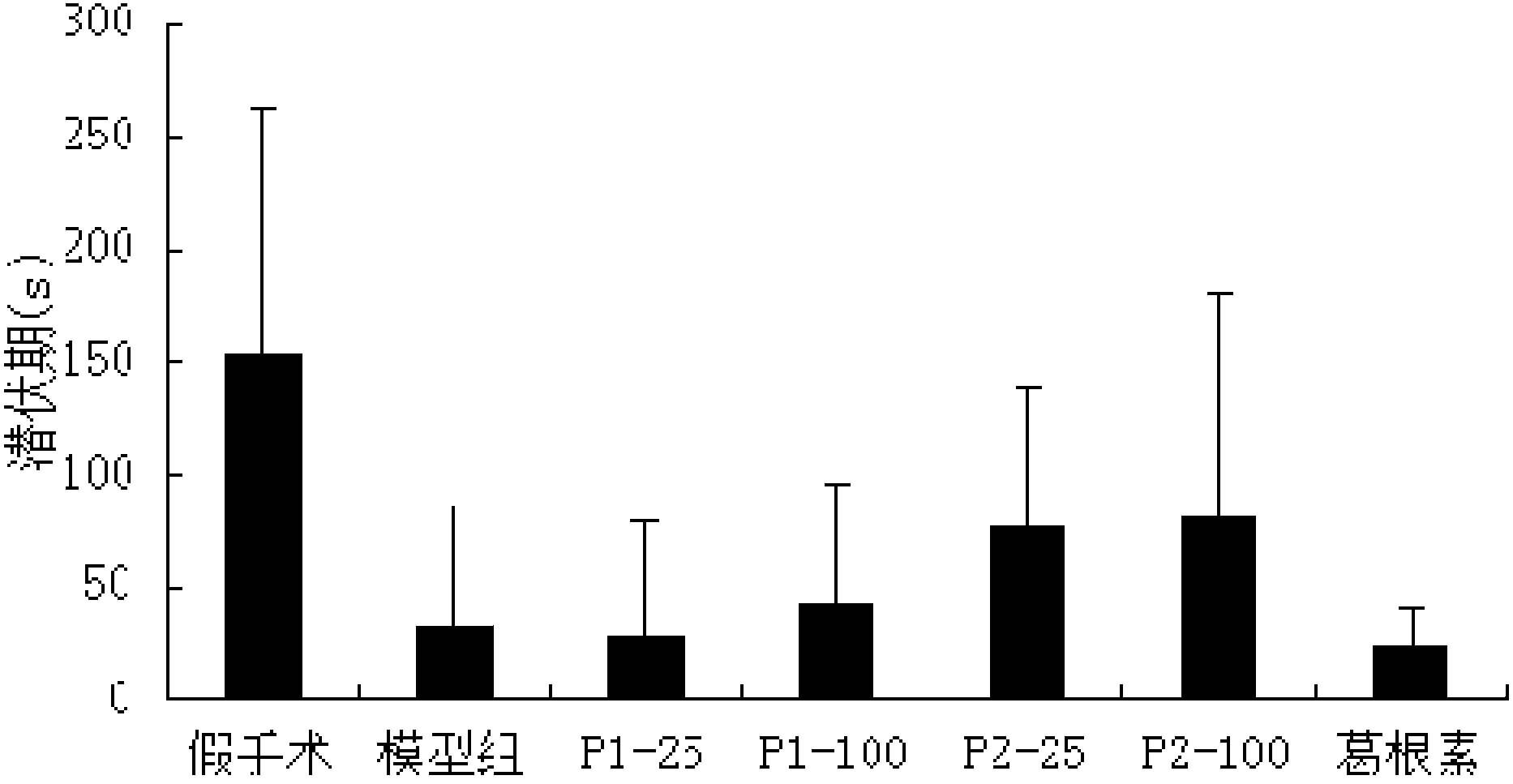
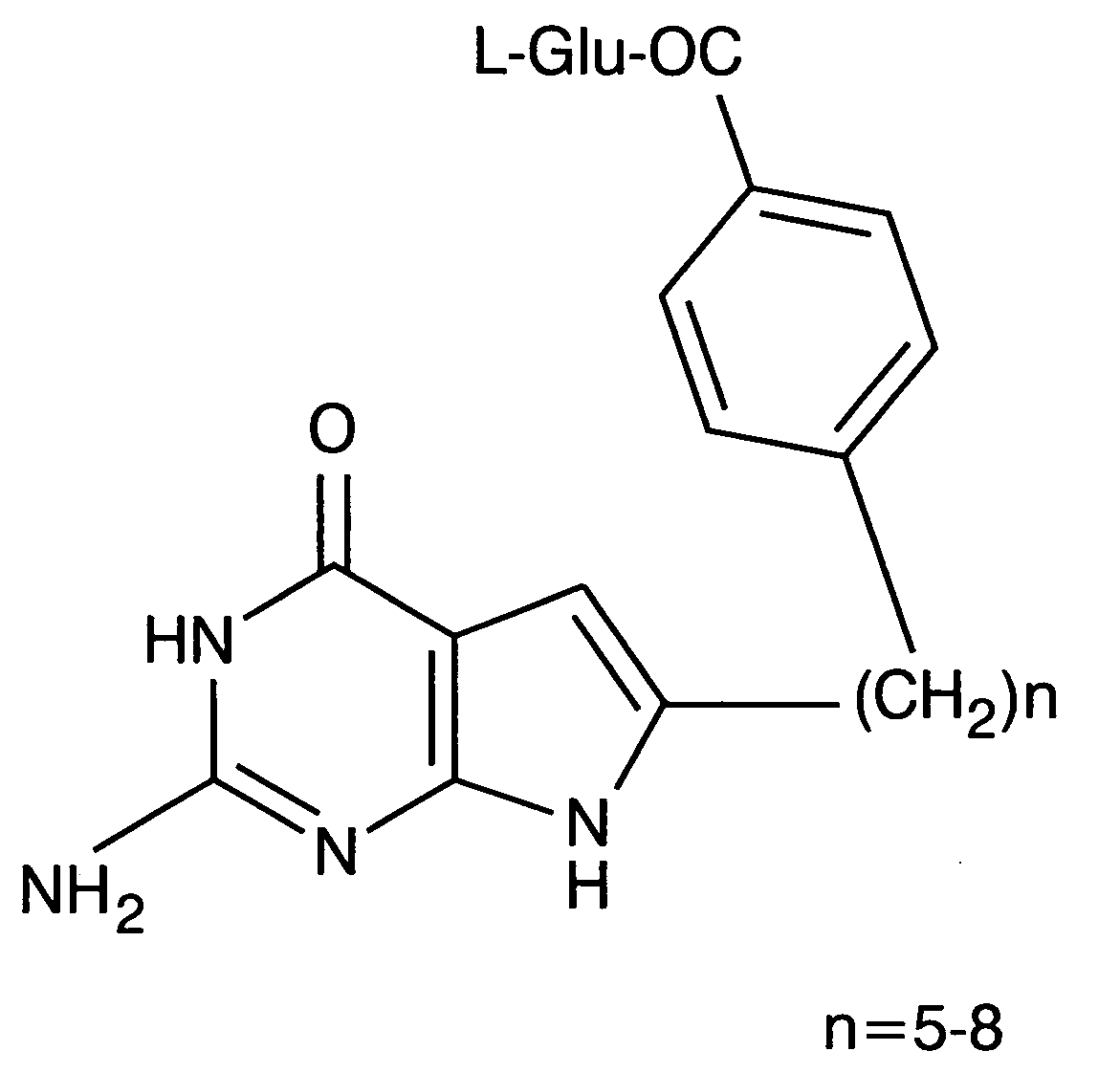
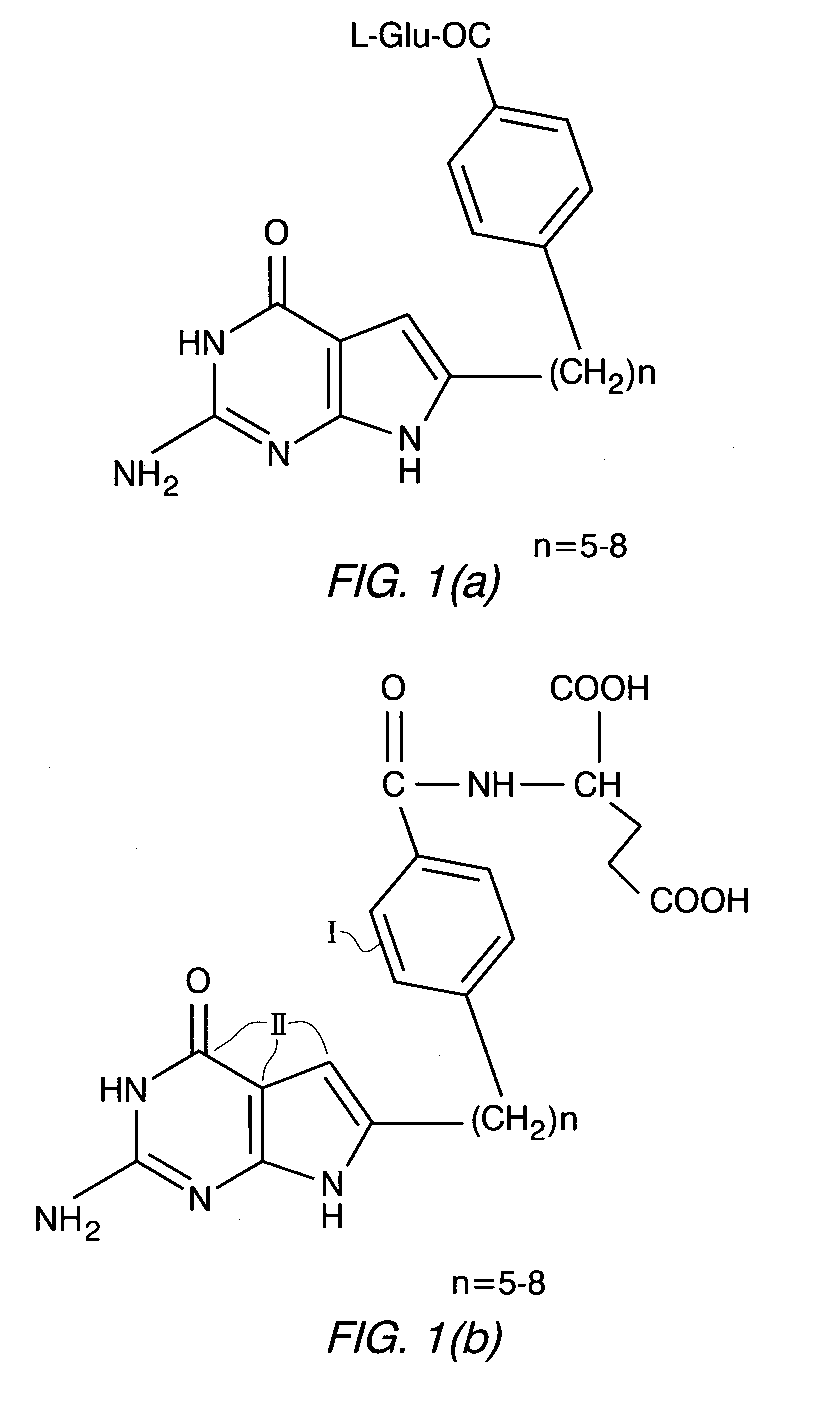
Puerarin derivative and synthetic method and application thereof
ActiveCN103382203AIncrease fat solubilityImprove fat solubility andOrganic active ingredientsNervous disorderChemical synthesisSolubility
A puerarin derivative and a synthetic method and an application thereof belong to the field of cerebrovascular diseases. Selectivity of a vascular dementia therapeutic target is improved by decorating puerarin. The puerarin derivative is indicated through a formula (1) which is as follows. By means of a chemical synthetic method, products are applied to treatment of vascular dementia. Fat solubility and water solubility of the puerarin are improved, receptor selectivity of the puerarin is improved, osmotic pressures inside and outside brain cells are balanced, cellular swelling caused by inflammatory injury is improved, a pharmacologic action of the puerarin itself of improving microcirculation and cholinergic nerve system functions is highlighted, and learning and memory disorders caused by damage caused by ischemia reperfusion are avoided.
Owner:HARBIN UNIV OF COMMERCE
Chemotherapeutic compounds for selectively targeting tumor cells with FR type receptors
A compound for treating cancer tumors, particularly ovarian cancer tumors, is described, where a fused cyclic pyrimidine having a cancer treating ability is effective to allow selective delivery to a cancerous tumor.
Owner:DUQUESNE UNIVERSITY
Formulations and uses of retinoic acid receptor selective agonists
The invention provides retinoic acid receptor (RAR) selective agonists and formulations thereof for the treatment of disease or for inducing a medically beneficial effect.
Owner:QURETINO THERAPEUTICS
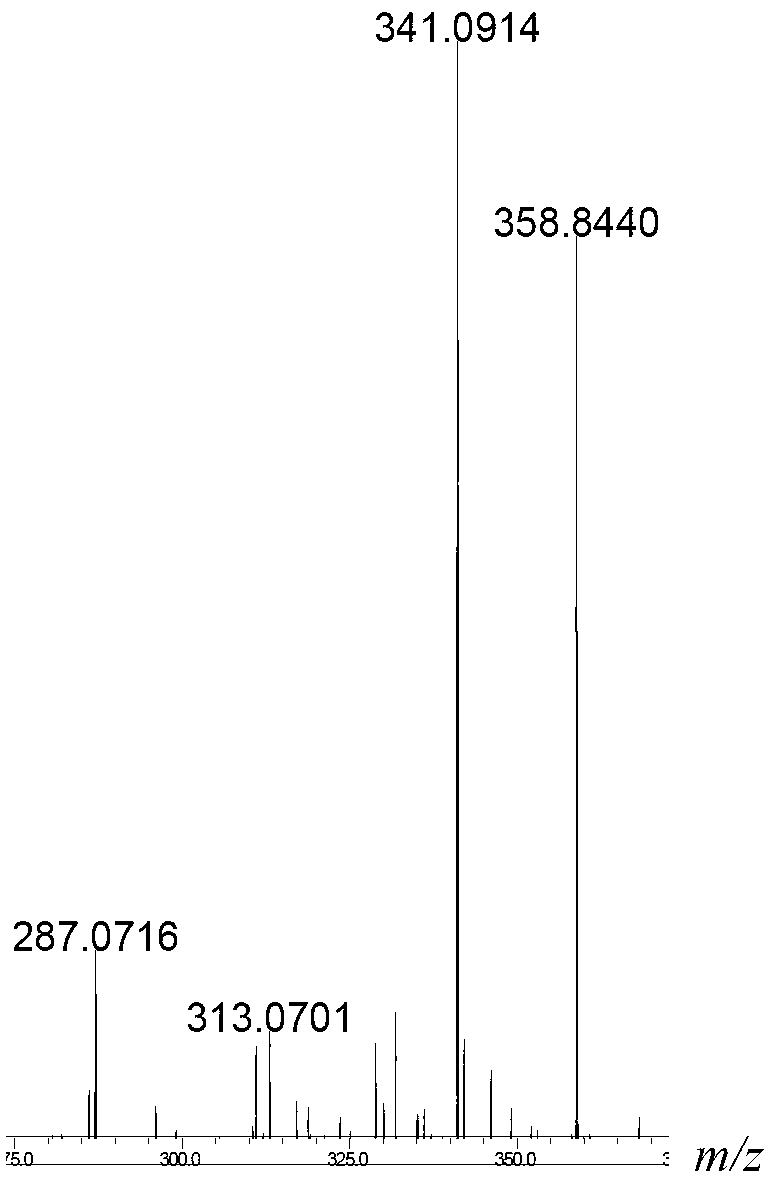
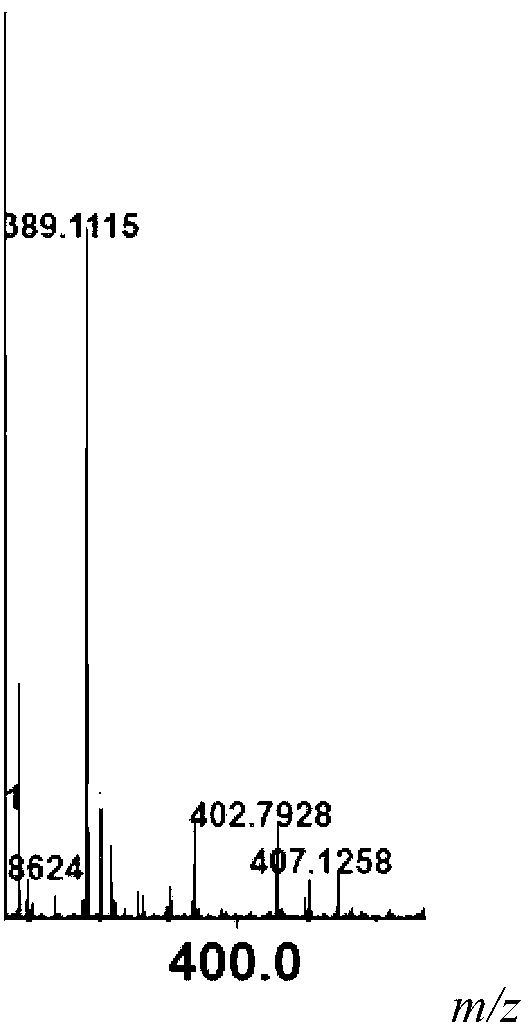
Beta-galactosidase with broadened nucleoside substrate specificity
The invention relates to beta-galactosidase with broadened nucleoside substrate specificity. An amino acid sequence of the beta-galactosidase with the broadened nucleoside substrate specificity is shown as SEQ ID No. 1 (sequence identifier number 1). The invention further provides a gene for encoding the beta-galactosidase with the broadened nucleoside substrate specificity. The beta-galactosidase with the broadened nucleoside substrate specificity has new receptor selectivity, can conduct transglycosylation on a phenolic compound which is difficult to glycosylate or cannot be glycosylated by the natural beta-galactosidase, can serve as a powerful tool to modify the phenolic compound and relevant drug molecules, and has a wide application prospect.
Owner:SHANDONG UNIV
Dopamine receptor ligands
Described herein are D4 receptor-selective compounds of the general formula: wherein: A and B are independently selected, optionally substituted, saturated or unsaturated 5- or 6-membered, homo- or heterocyclic rings; X1 is selected from CH2, O, NH, S, C=O, CH-OH, CH-N(C1-4alkyl)2, C=CHCl, C=CHCN, N-C1-4alkyl, N-acetyl, SO2 and SO; X2 - - - is selected from N=, CH2-, CH=, C(O)-, O-, and S-; R1 represents C1-4alkyl; Y is selected from CH and N; n is 0, 1 or 2; q is 1 or 2; R2 is C1-6alkyl optionally incorporating a heteroatom selected from N, O and S; D is cyclohexane or benzene; and E is a saturated or unsaturated 5- or 6-membered heterocycle incorporating 1, 2 or 3 heteroatoms selected from O, N, and S, wherein E is optionally substituted with 1 or 2 substituents selected from halogen, C1-4alkyl and halogen-substituted C1-4alkyl; and acid addition salts, solvates and hydrates thereof. Their use as ligands for dopamine receptor identification and in a drug screening program, and as pharmaceuticals to treat indications in which the D4 receptor is implicated, such as schizophrenia, is also described.
Owner:NPS PHARM INC
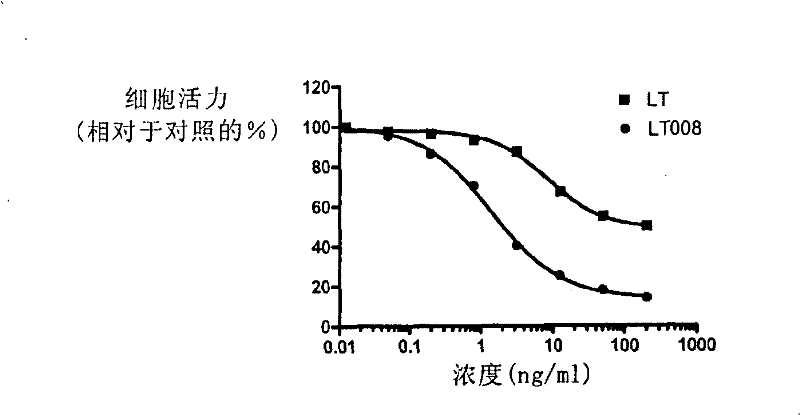
Receptor selectivity lymphotoxin derivates
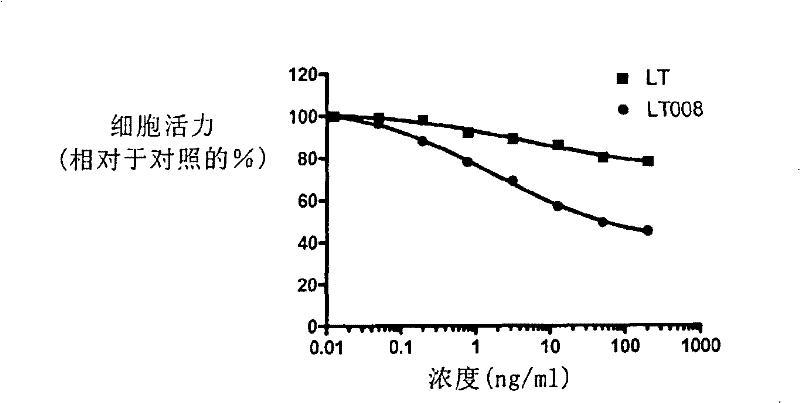
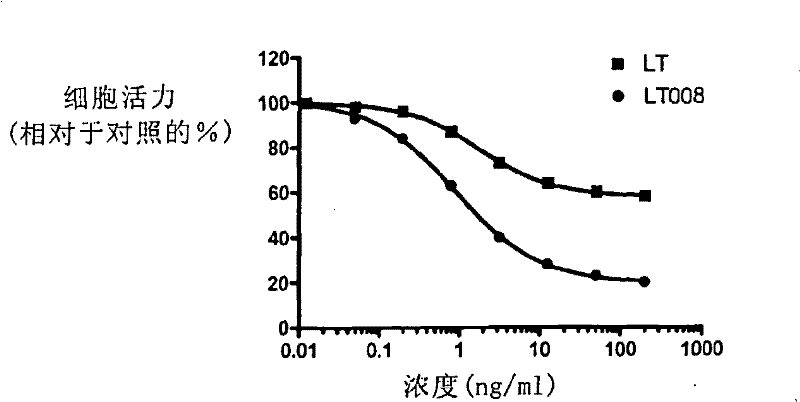
This invention discloses some receptor-selective lymphotoxin (LT) mutants and the ratio, the ability of these lymphotoxin mutants combining with the human TNFRI to that with the human TNFRII, is more than 10. The preparation method and the use of the above said LT mutants are also disclosed. In this invention, the LT mutants bind to the TNFRI selectively and that decreases the binding to the TNFRII in a large range, thereby the toxic effect which may be caused by the TNFRII will be reduced.
Owner:上海复旦张江生物医药股份有限公司 +1
Method for determining co-receptor selectivity of Human Immunodeficiency Virus-1
InactiveUS7638319B2Microbiological testing/measurementViruses/bacteriophagesNucleotideImmunodeficiency virus
Newly discovered structural characteristic of the gp120 V3 loop have resulted in a “rule” or algorithm, that is used in a method for determining whether a subject is infected with HIV-1 virus that expresses selectivity for CXCR4 or CCR5 chemokine receptors. A positively charged surface patch defined by V3 loop residues 11 and 24 or 25 at the base of the β-strands in the V3 loop and the homologous β2-β3 chemokine hairpin is responsible for CXCR4 receptor selection. Thus a method for detecting the presence of HIV-1 virus that is selective for X4-co-receptors in a subject infected with HIV-1 or suspected of being infected, from the amino acid sequence of at least a part of the HIV-1 gp120 V3 region peptide that includes residues 11, 24 and 25, or from the nucleotide sequence of a nucleic acid encoding said V3 region peptide, is disclosed.
Owner:NEW YORK UNIV
Selective agonist of toll-like receptor 3
InactiveUS20100310600A1Disease in the subject is reduced or eliminatedImprove morbidityAntibacterial agentsBiocideAgonistAntiproliferative Agents
A mismatched double-stranded ribonucleic acid, which is an agonist for Toll-like receptor 3 (TLR3), is used in vitro or in vivo as an antimicrobial agent, antiproliferative agent, and / or immunostimulant. Poly(l:C11-14U) is a more selective agonist of TLR3 as compared to poly(l:C) even though the both double-stranded RNA are structurally analogous.
Owner:CARTER WILLIAM A +1
Novel thiazole derivative or salt, isomer, preparation method and application thereof
ActiveCN112341404AHas an analgesic effectExtended half-lifeOrganic active ingredientsNervous disorderAntagonismEthylic acid
The invention discloses a novel thiazole derivative or salt, an isomer, a preparation method and application thereof. A compound shown as a formula (I) has a Sigma 1 receptor antagonism effect, and has strong in vitro affinity to sigma 1R in the aspect of in vitro receptor affinity. Meanwhile, in D2L radioactive ligand binding analysis and 5HT-1A receptor, CB1 receptor and CB2 receptor cAMP tests,the affinity to the receptors is poor, and high receptor selectivity is shown. Mouse acetic acid twisting tests show that the compound has an analgesic effect. Through rat oral administration pharmacokinetic experiments, it can be known that the compound is long in half-life period, low in clearance rate and large in AUClast; Cmax can be quickly reached after oral administration, and the compoundis extremely quick to absorb and suitable for medicine.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
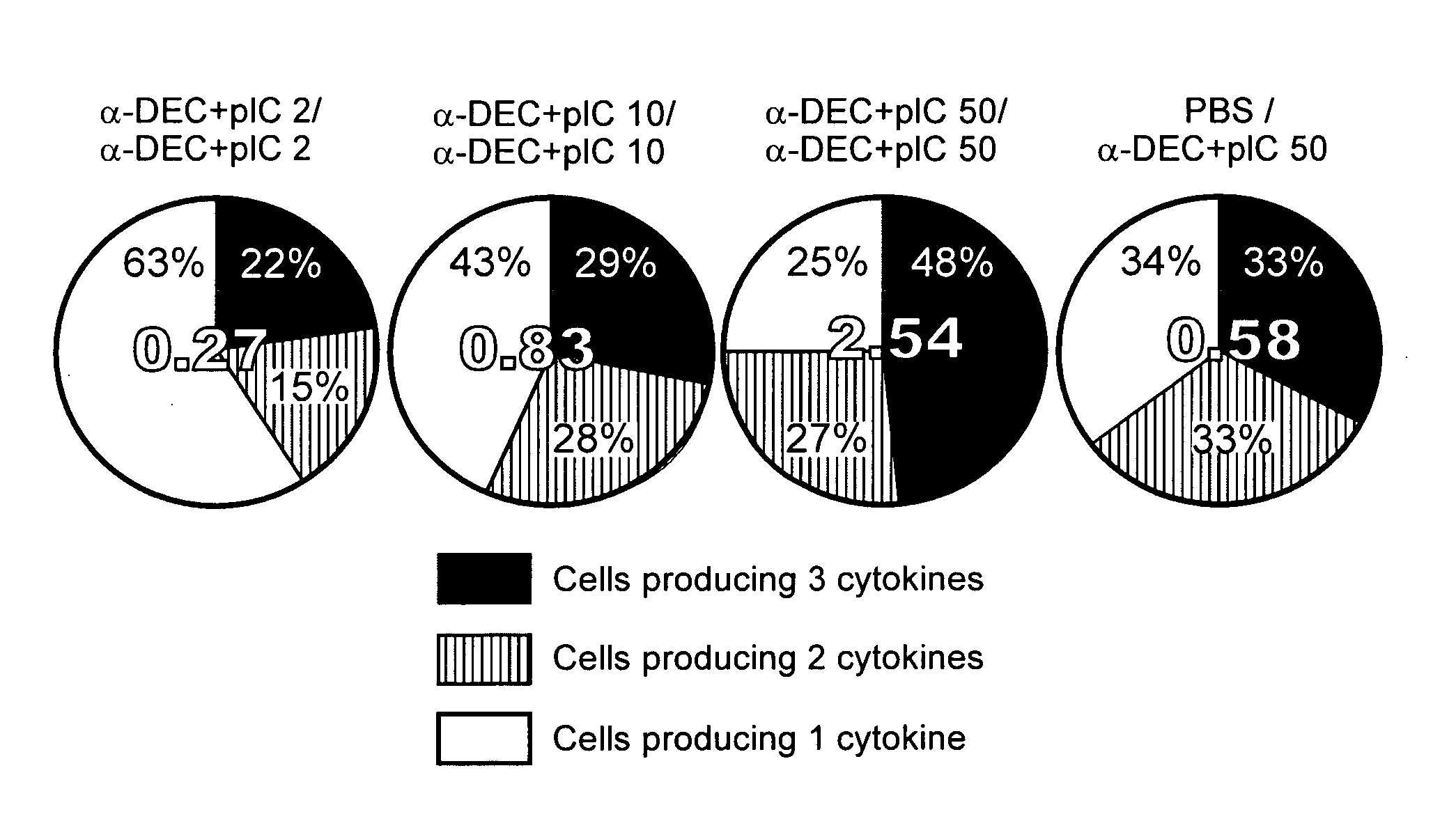
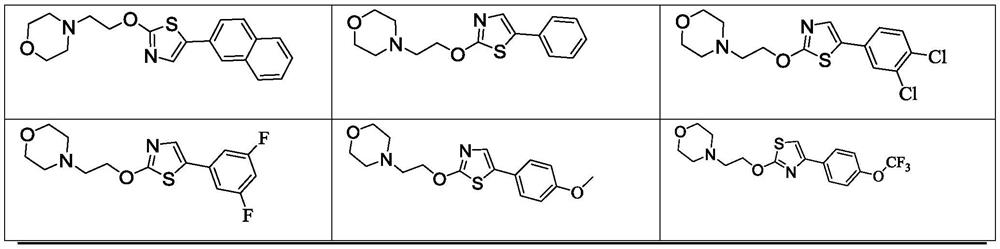
Immunotherapeutic tumor treatment method using an interleukin-2 receptor alpha, beta-selective agonist in combination with adoptive cell transfer therapy
PendingCN110366421APeptide/protein ingredientsPharmaceutical delivery mechanismReceptor selectivityTumor therapy
Provided are methods and compositions directed towards the treatment of an individual having cancer by providing adoptive cell transfer therapy and administering to the individual a long acting IL-2Ralpha beta-biased agonist.
Owner:NEKTAR THERAPEUTICS INC +1
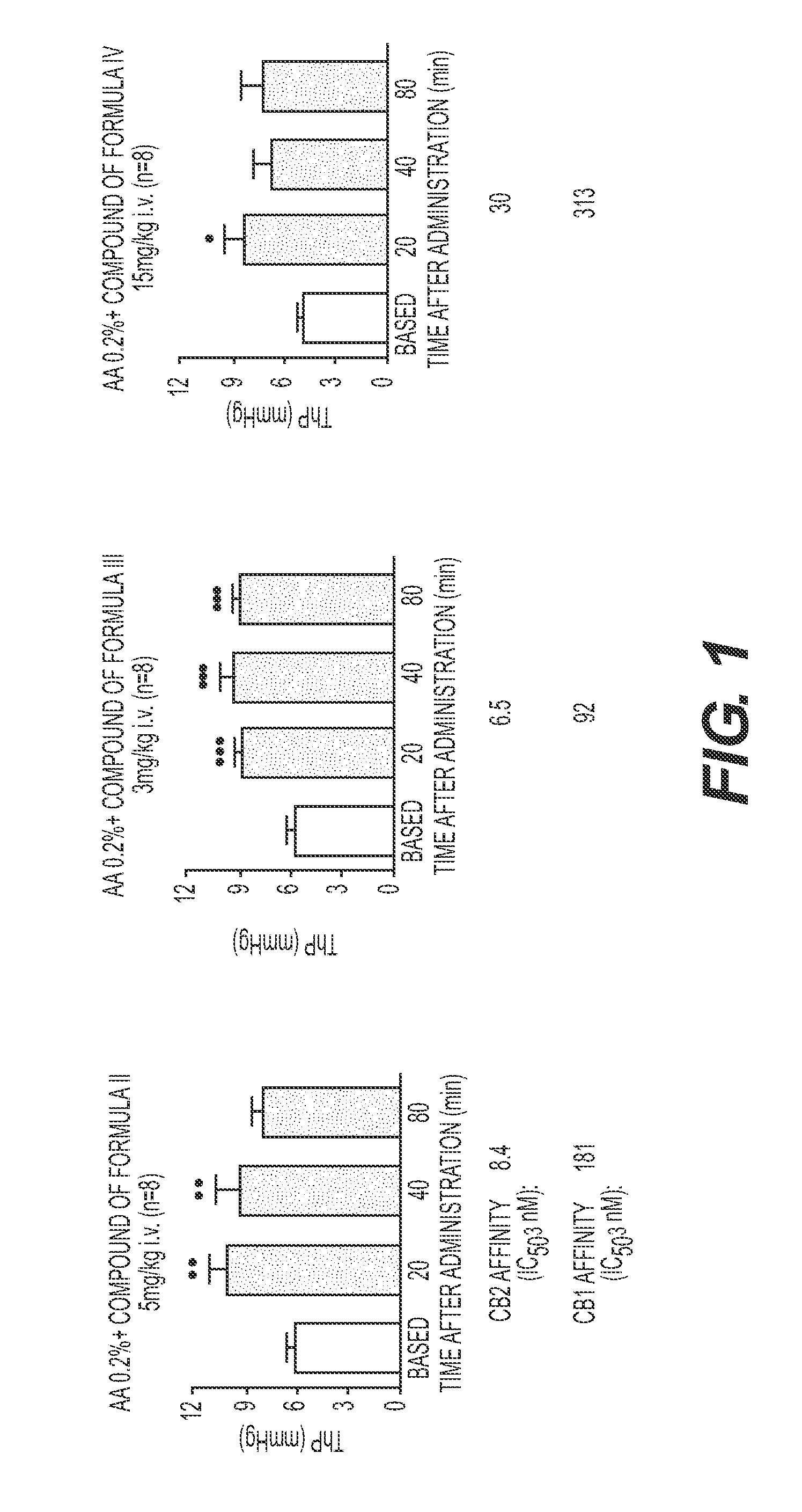
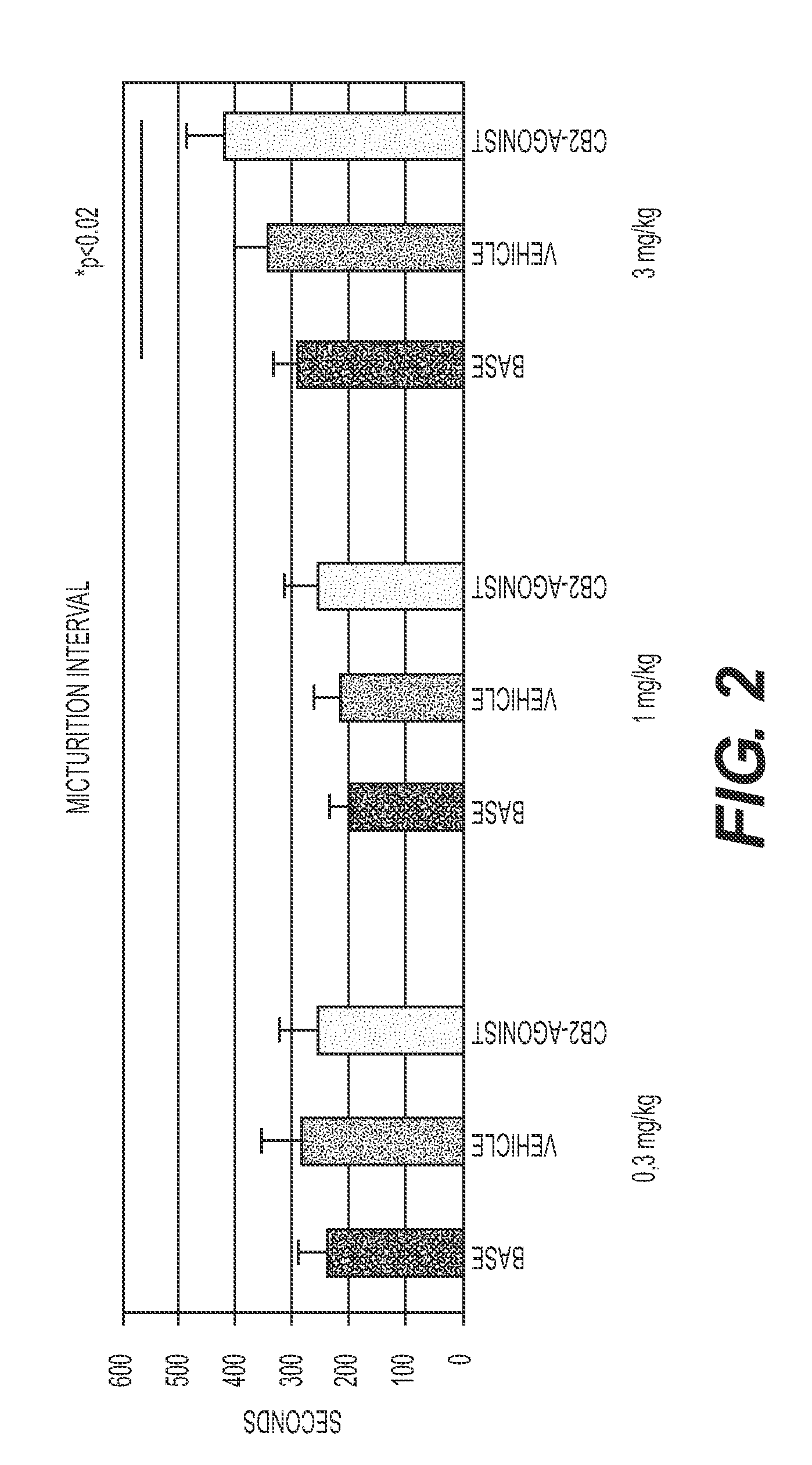
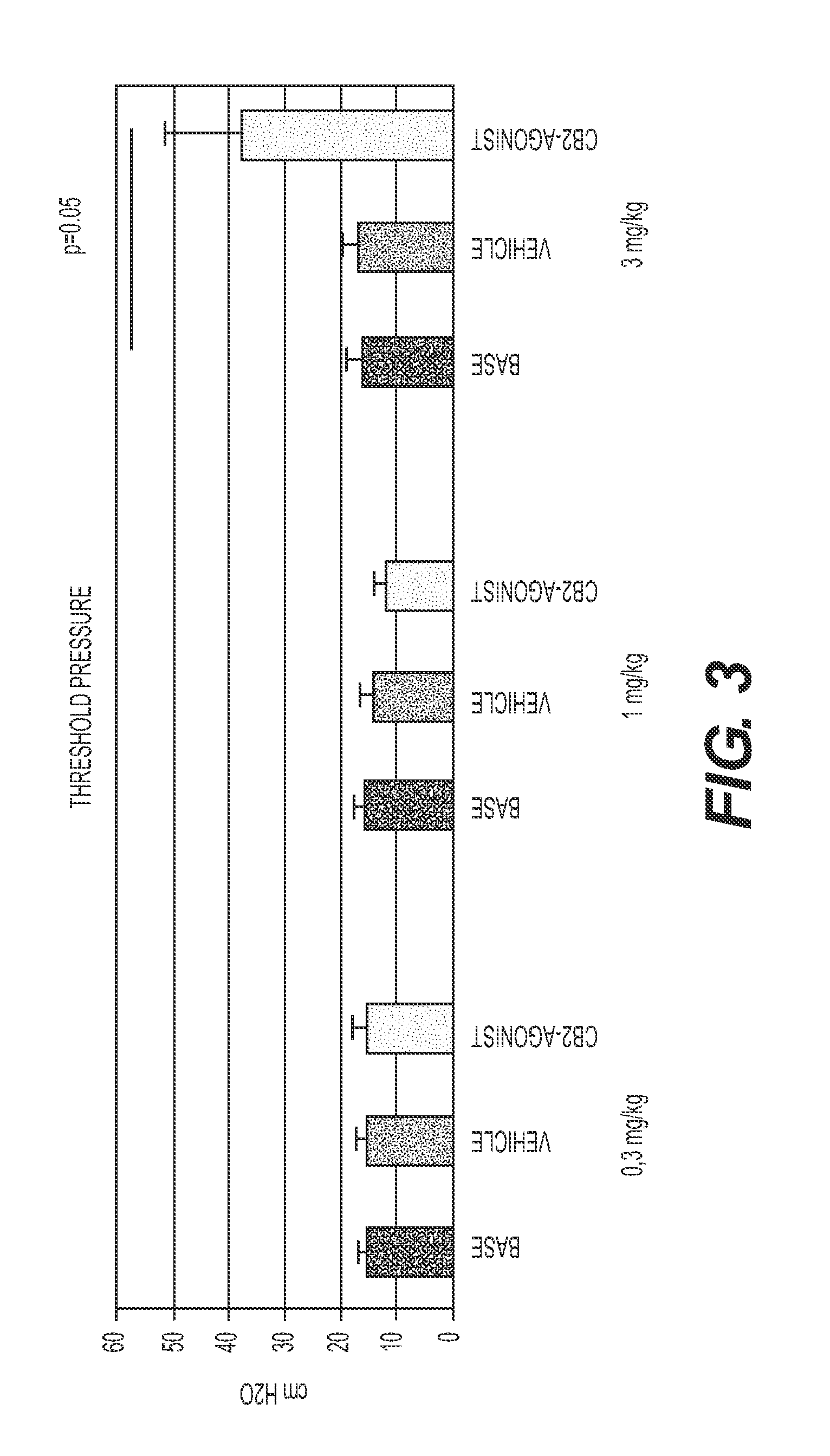
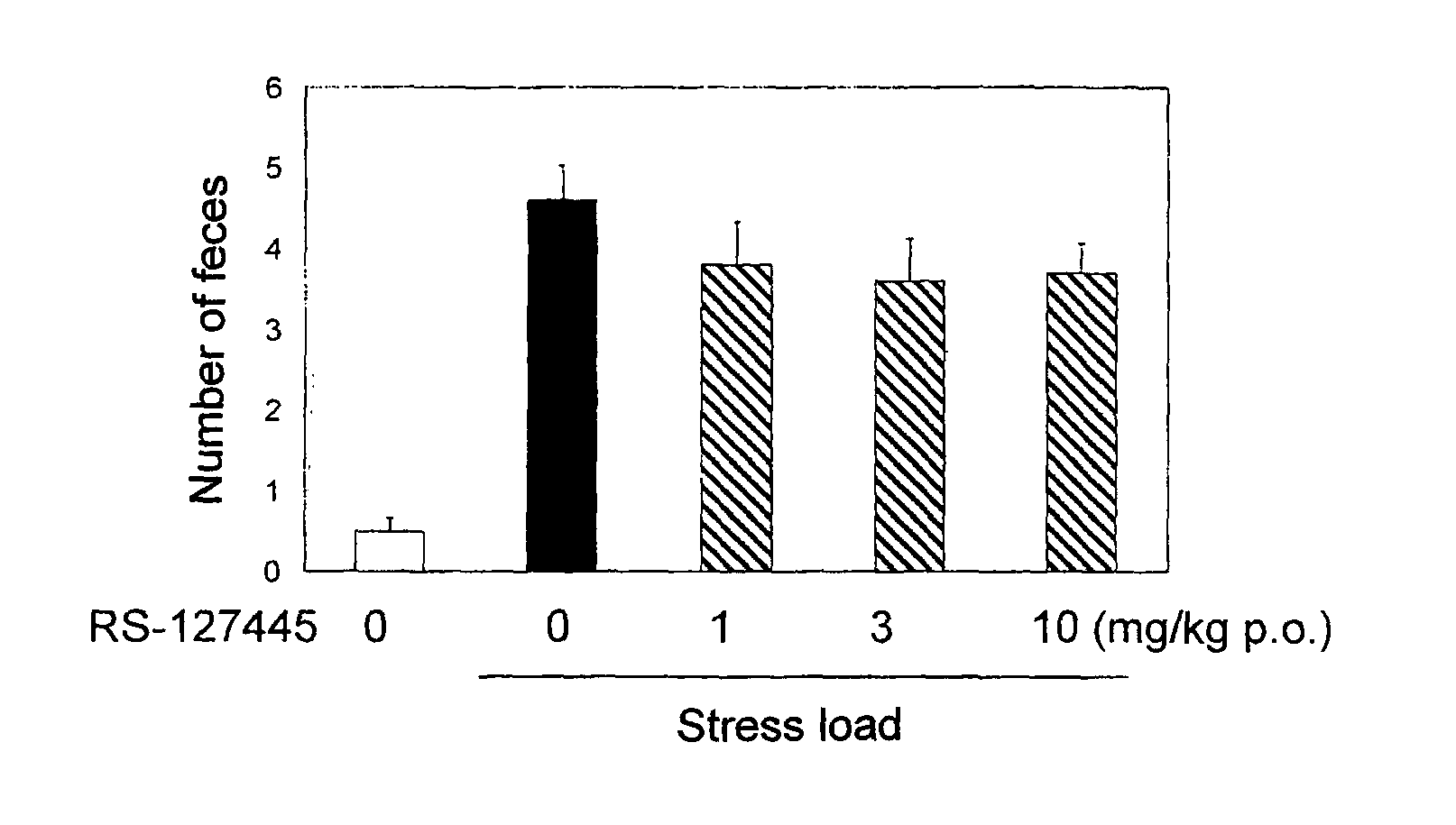
Treatment Of Lower Urinary Tract Dysfunction With CB2-Receptor-Selective Agonists
InactiveUS20090312414A1BiocideHydroxy compound active ingredientsReceptor selectivityOveractive bladder
A method is disclosed utilizing a cannabinoid receptor type 2-receptor-selective agonist for treating or preventing lower urinary tract dysfunction, including overactive bladder, lower urinary tract symptoms and detrusor overactivity.
Owner:WARNER CHILCOTT CO LLC
Acylguanidine derivative or salt thereof
InactiveUS8076348B2High antagonistic activityExcellent IBS treating effect and migraine preventing effectBiocideNervous disorderDisease5-HT4 receptor
Owner:ASTELLAS PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com