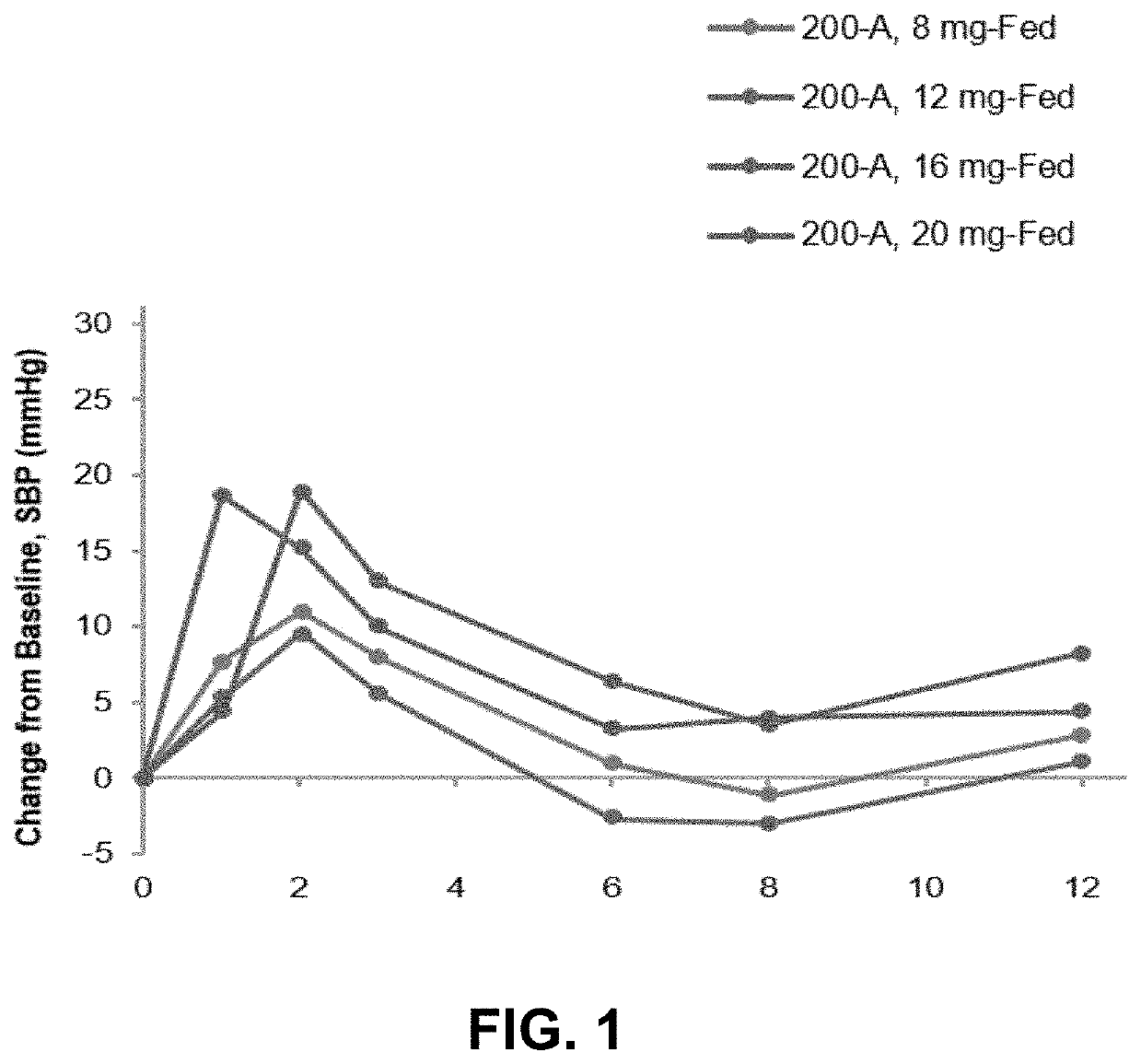
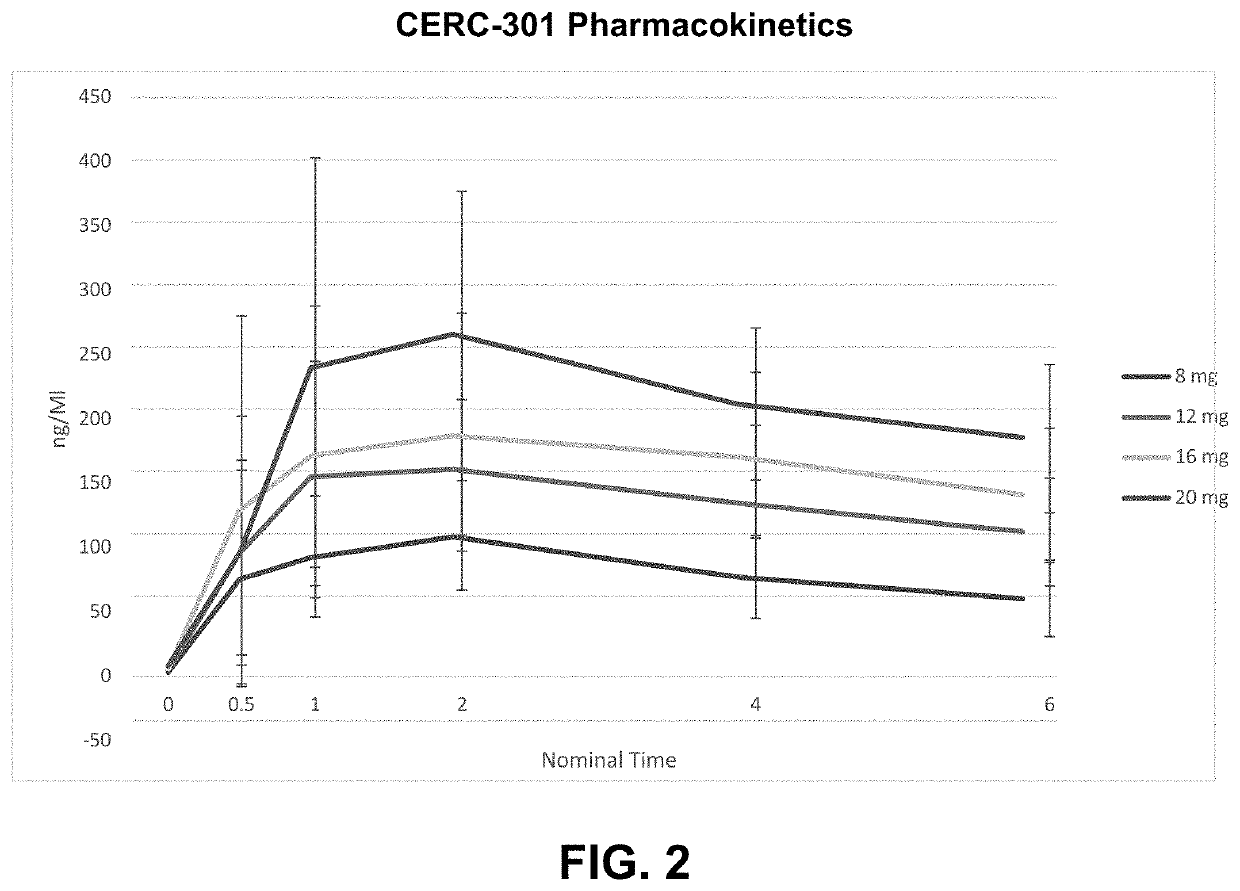
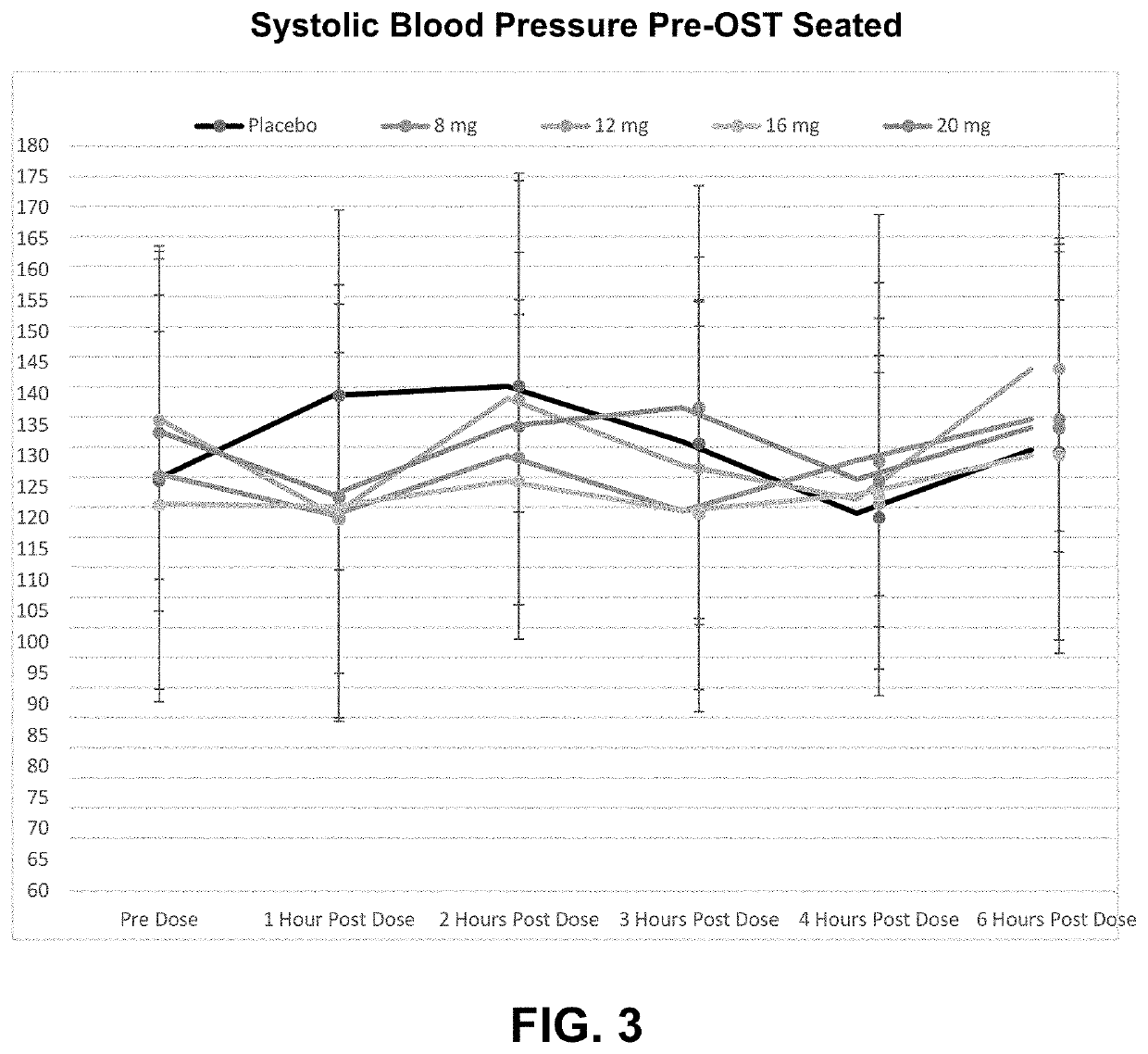
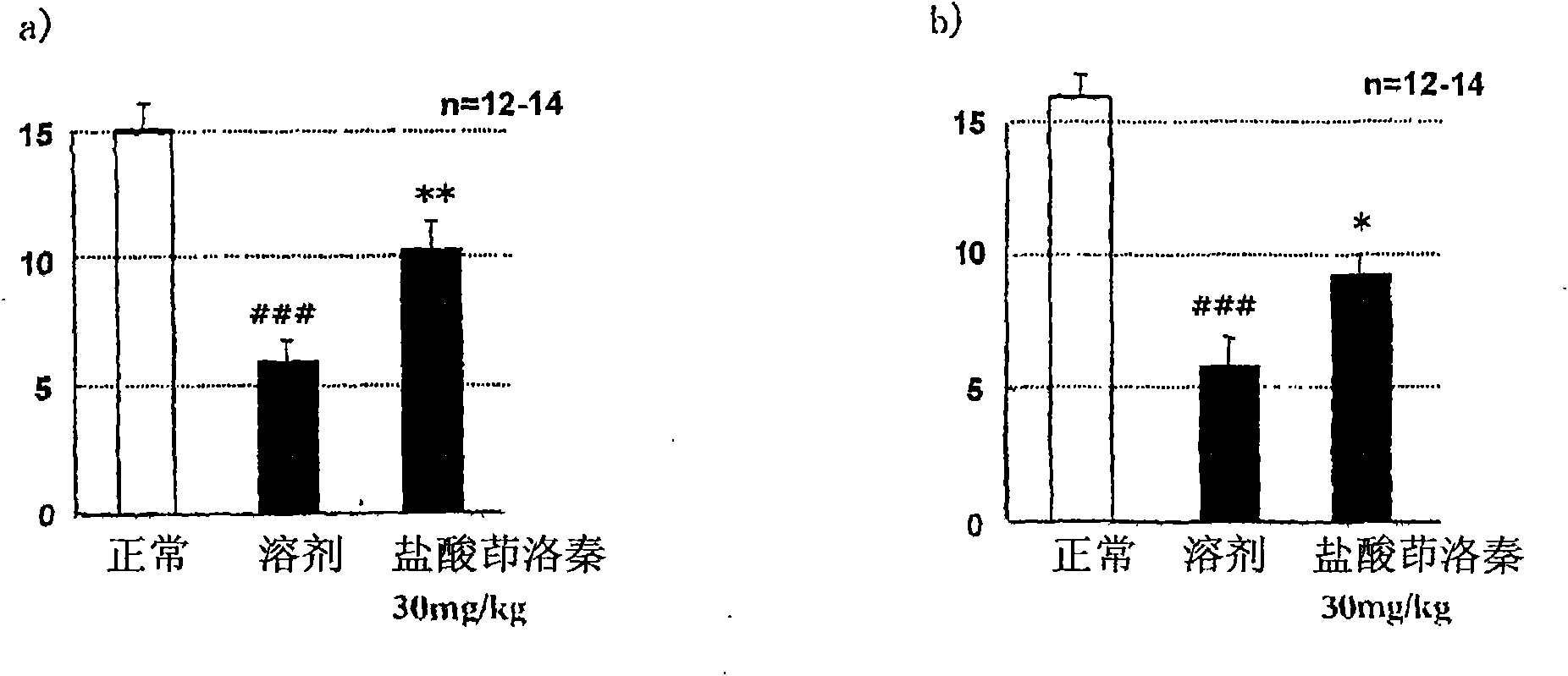
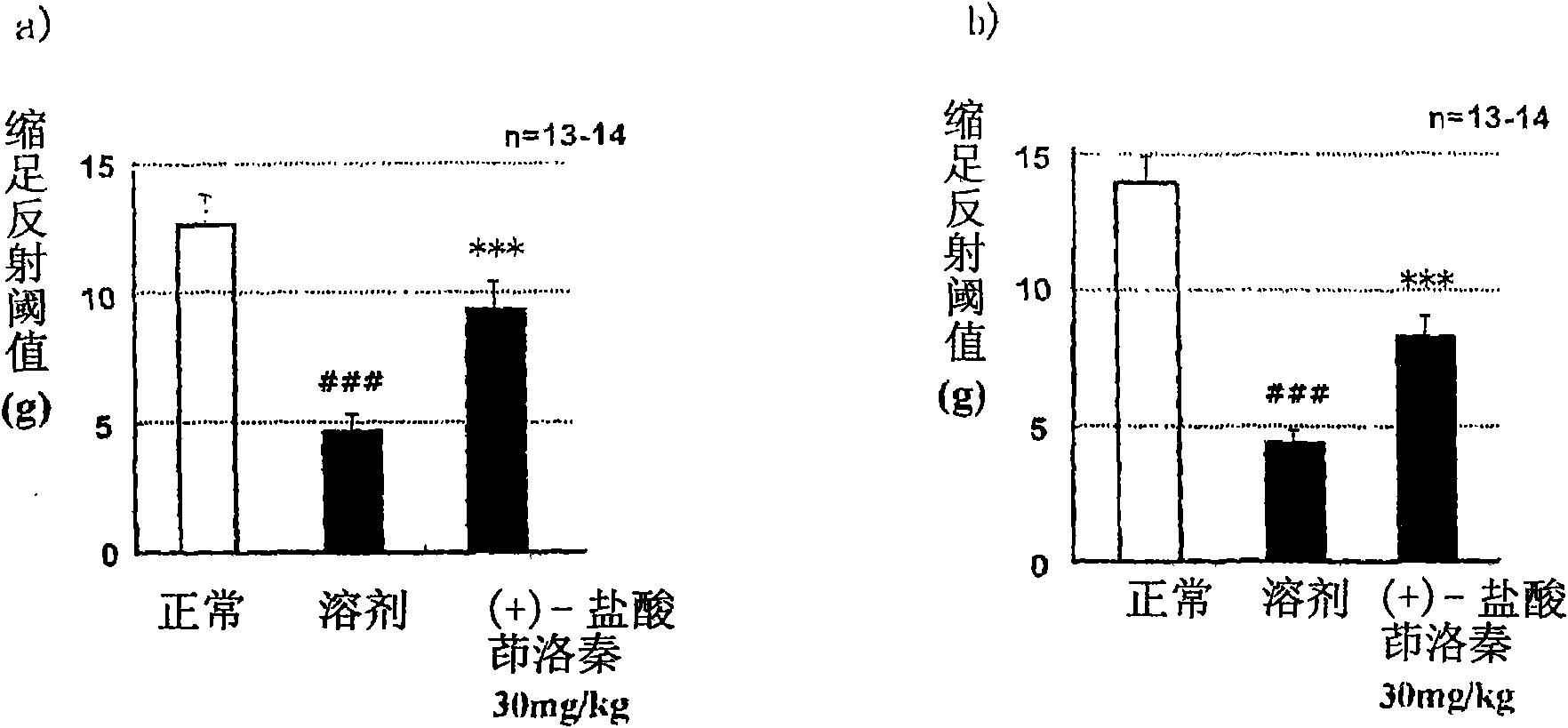
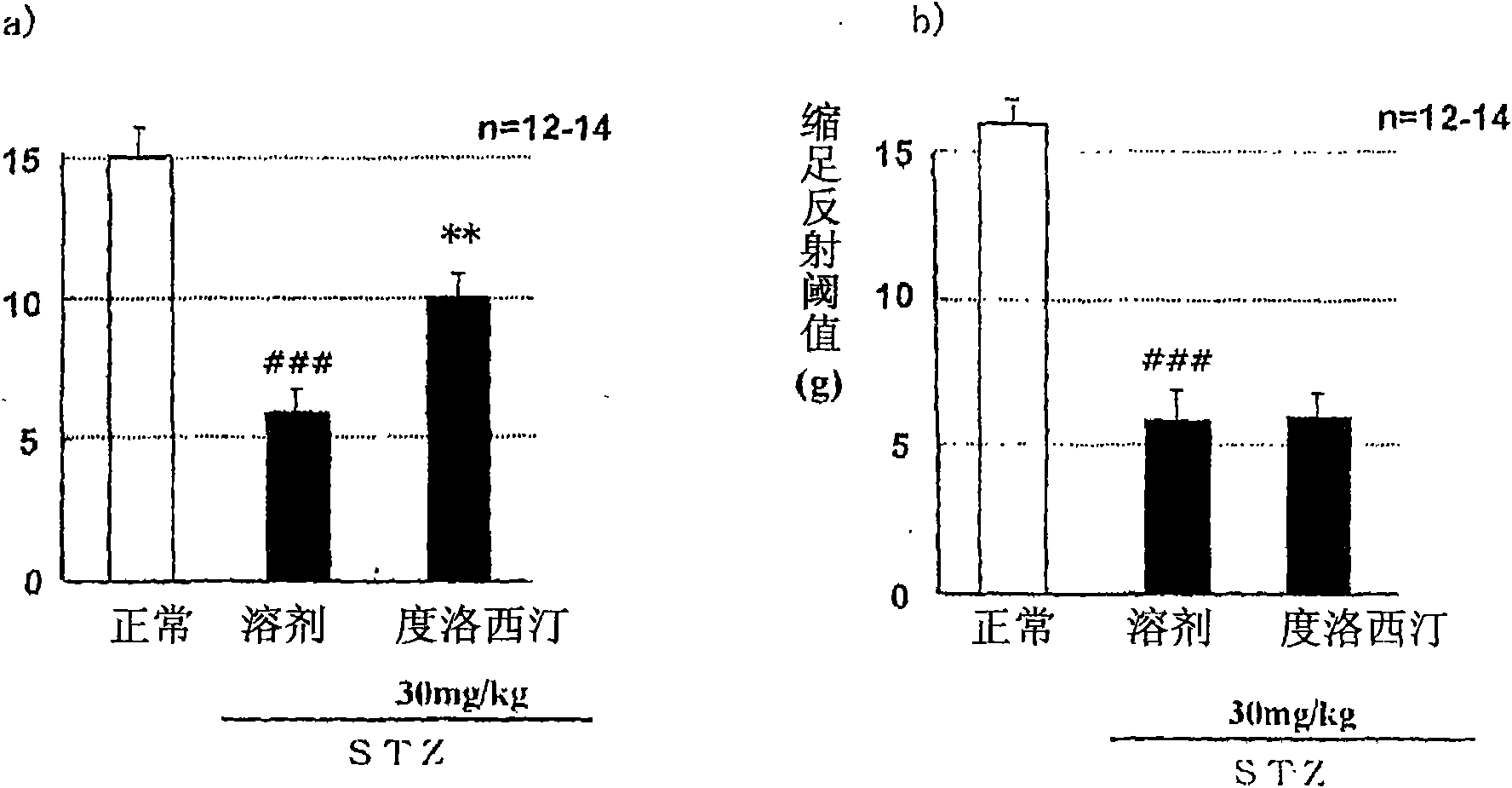
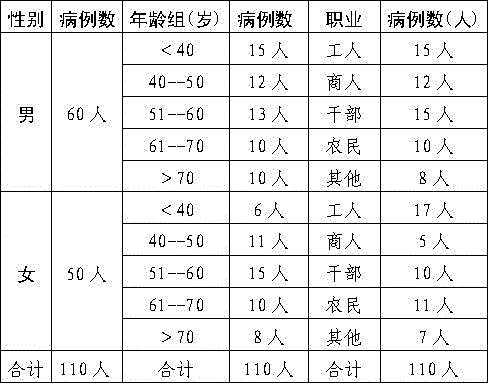
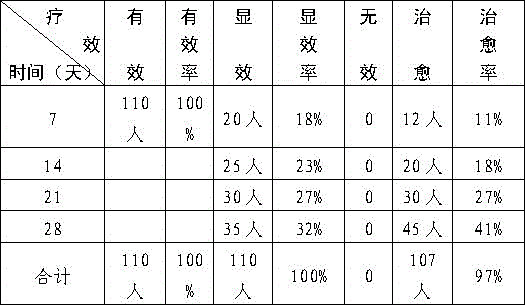
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Orthostatic BP" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
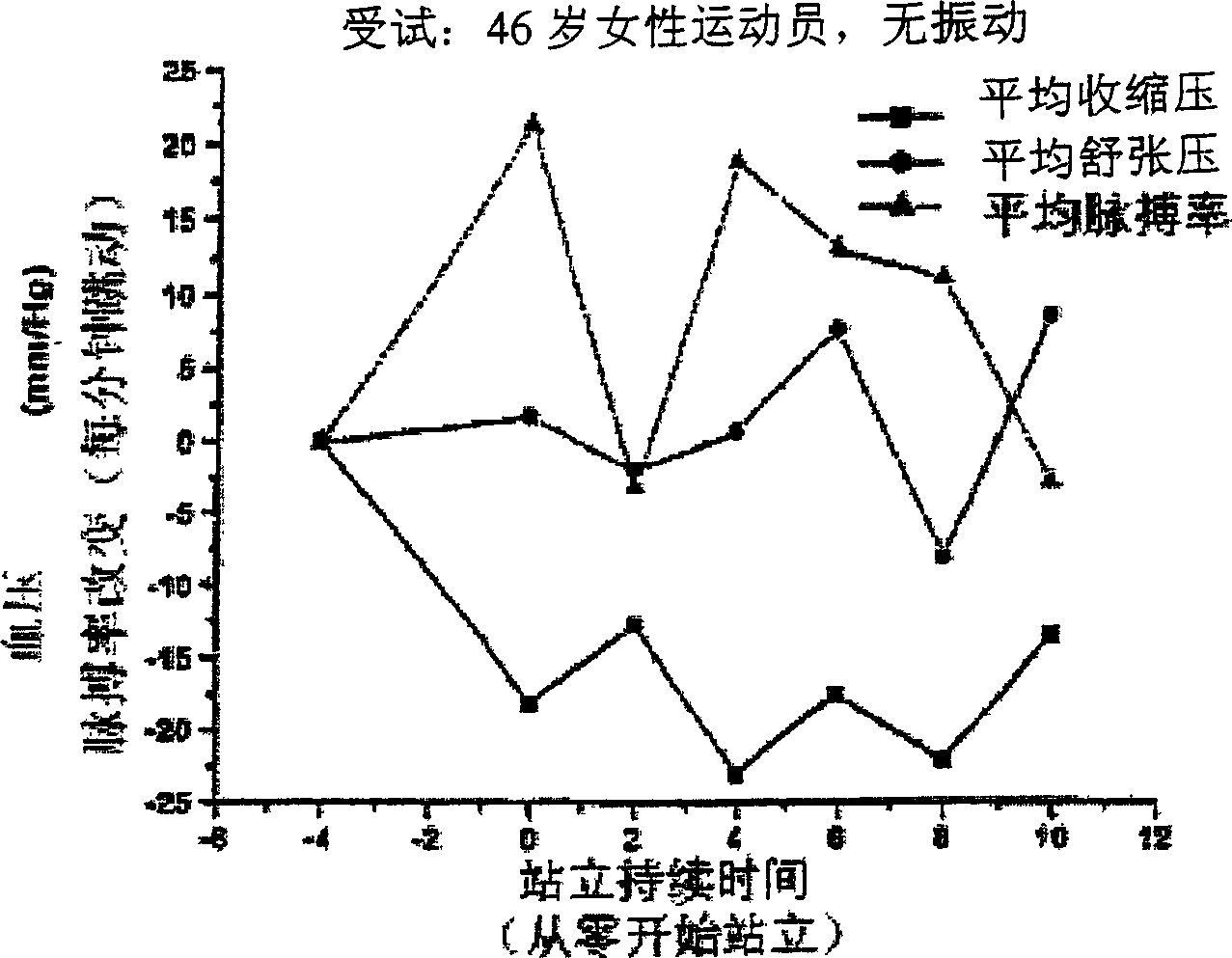
Orthostatic hypotension, also known as postural hypotension, occurs when a person's blood pressure falls when suddenly standing up from a lying or sitting position. It is defined as a fall in systolic blood pressure of at least 20 mm Hg or diastolic blood pressure of at least 10 mm Hg when a person assumes a standing position.
Modified release compositions of milnacipran
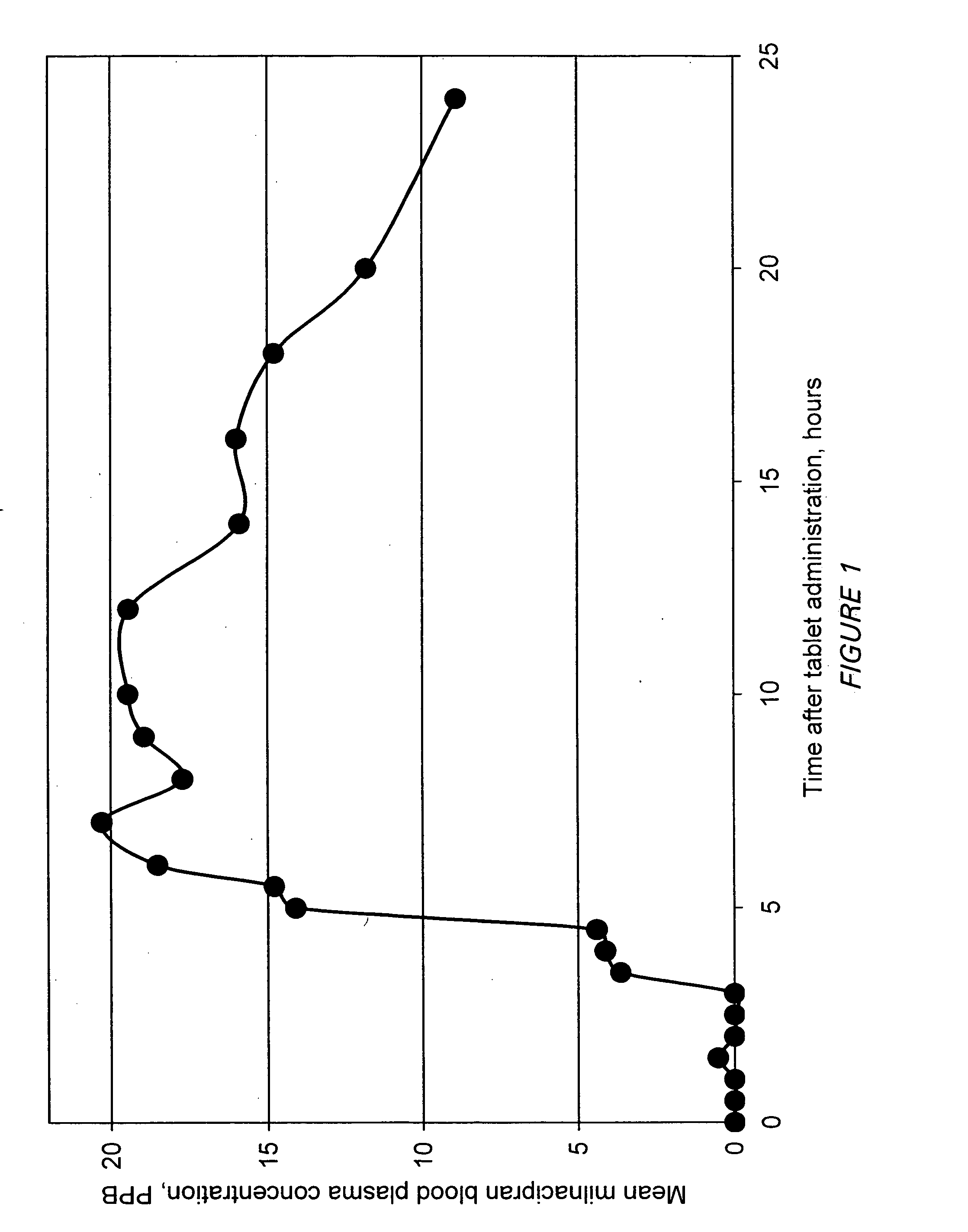
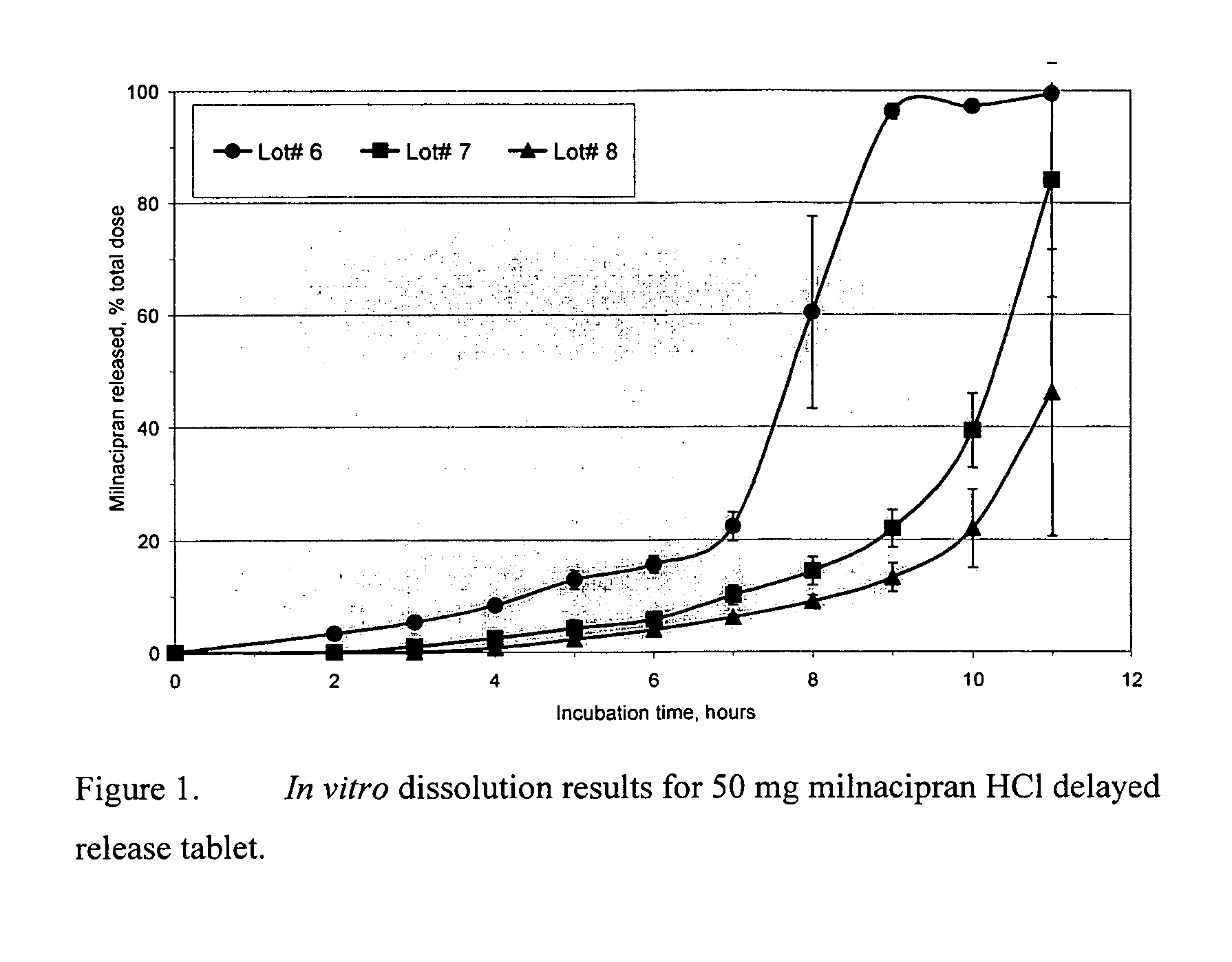
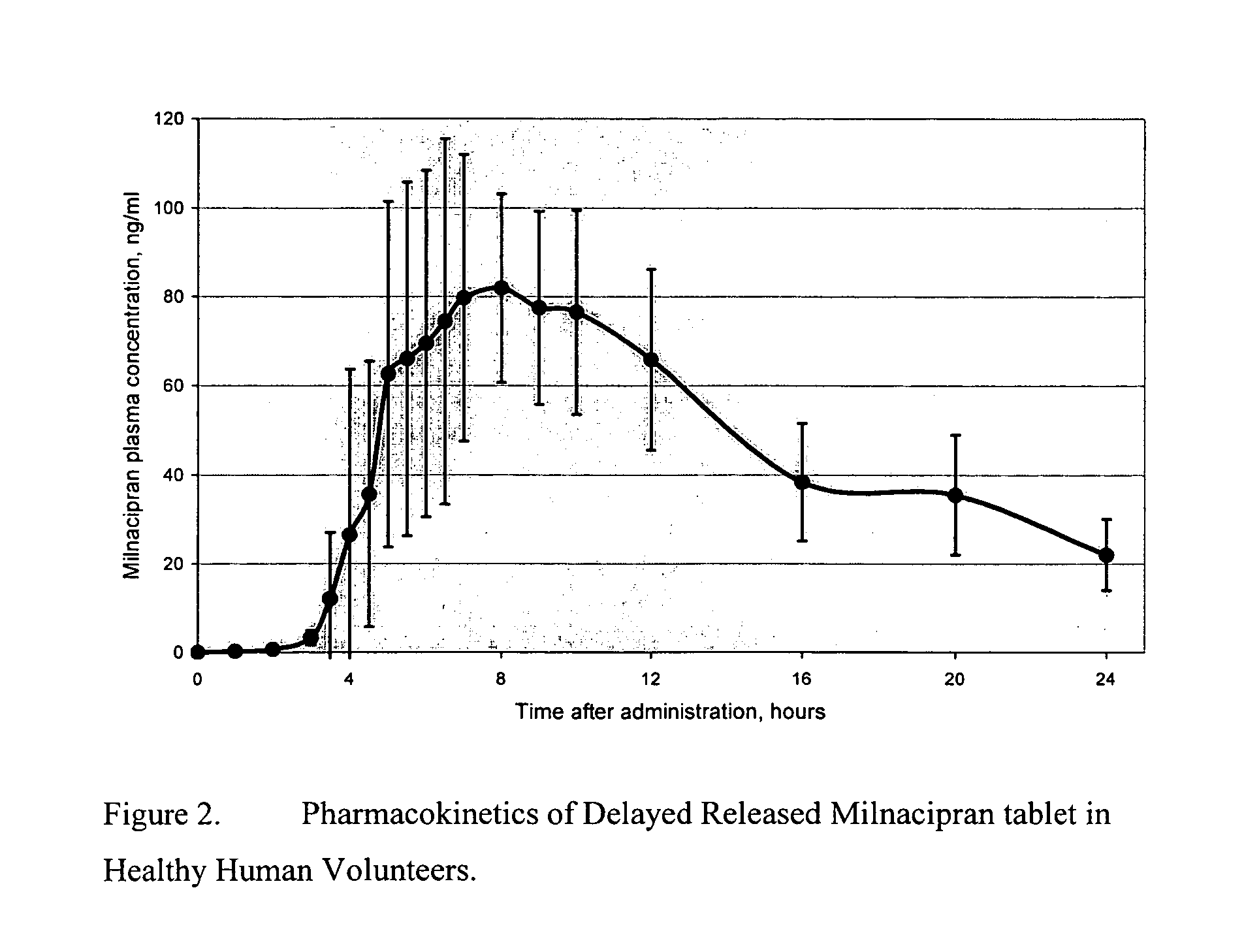
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Derivate von dihydroxyphenylalanin
The invention relates to dihydroxyphenylalanine derivatives, the production thereof, and pharmaceutical compositions containing said dihydroxyphenylalanine derivatives. The invention further relates to the use of said dihydroxyphenylalanine derivatives and pharmaceutical compositions for the treatment and prevention of movement disorders, neurodegenerative diseases, Alzheimer, Parkinson's disease, hemiatrophy hemiparkinsonism, Parkinson's syndrome, Lewy bodies disease, frontotemporal dementia, Lytico-Bodig disease (Parkinsonism-dementia-amyotrophic lateral sclerosis, striatonigral degeneration, Shy-Drager syndrome, sporadic olivopontocerebellar degeneration, progressive pallidal atrophy, progressive supranuclear palsy, Hallervorden-Spatz disease, Huntington's disease, X chromosome-linked dystonia (Morbus Lubag), mitochondrial cytopathy with striatal necrosis, neuroacanthocytosis, restless leg syndrome, Wilson's disease.
Owner:ELLNEUROXX LTD
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Controlled release pharmaceutical composition for oral use containing midodrine and/or active metabolite, desglymidodrine
InactiveUS7070803B2Increase surface areaEfficient processOrganic active ingredientsNanomedicineSide effectDesglymidodrine
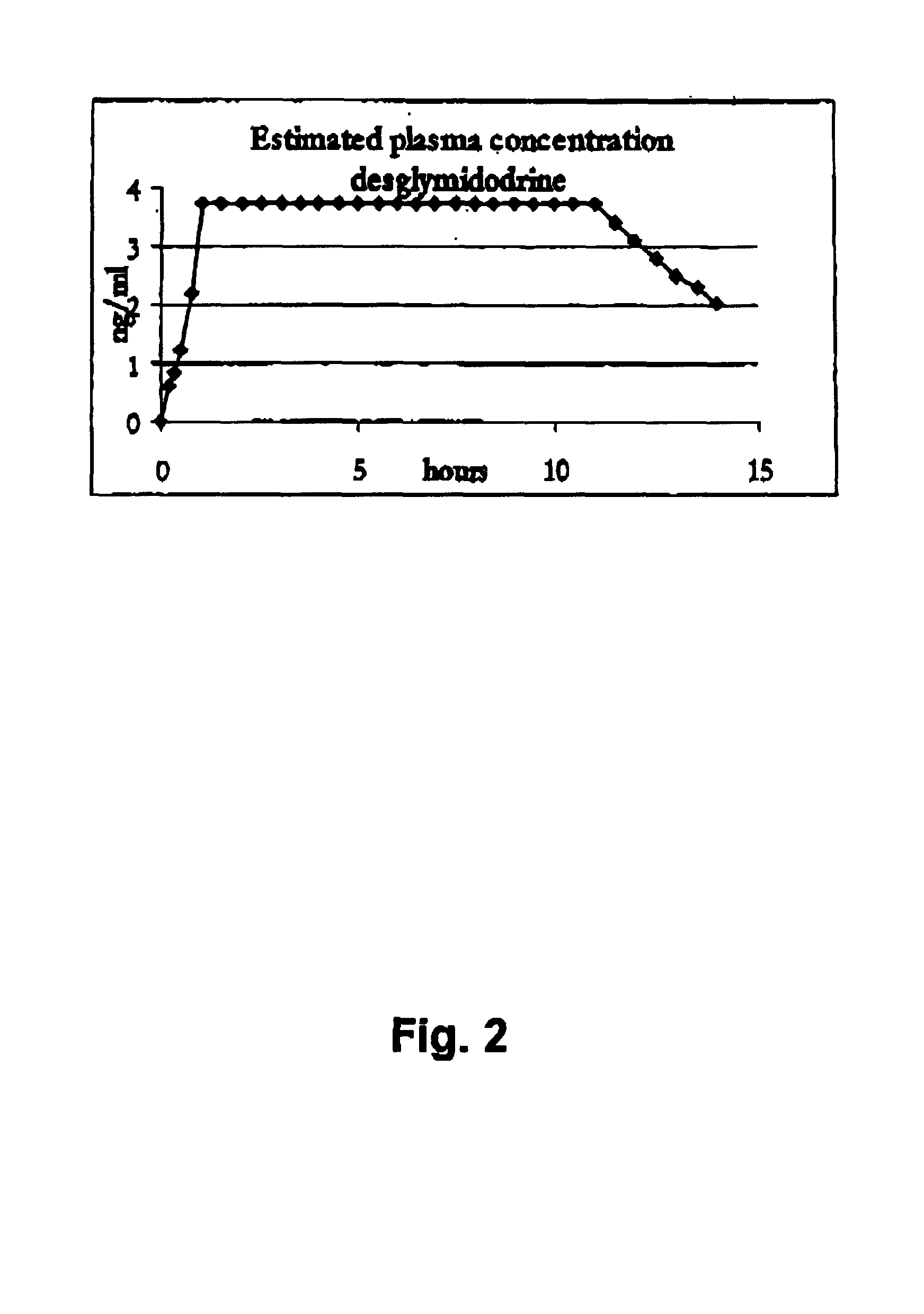
Novel controlled release pharmaceutical compositions for oral use containing midodrine and / or its active metabolite desglymidodrine. The novel compositions are designed to release midodrine and / or desglymidodrine after oral intake in a manner which enables absorption to take place in the gastrointestinal tract so that a relatively fast peak plasma concentration of the active metabolite desglymidodrine is obtained followed by a prolonged and relatively constant plasma concentration of desglymidodrine.The novel compositions may be designed for administration once or twice daily, i.e. a therapeutically effective concentration of desglymidodrine is maintained for a period of at least 10-16 hours followed by a wash out period of about 8-12 hours in order to avoid the well-known midodrine related side effect with respect to supine hypertension. The therapeutically effective concentration of desglymidodrine is regarded as a plasma concentration of desglymidodrine of at least about 3 ng / ml. A composition is designed to release midodrine and / or desglymidodrine in at least the following consecutive steps: i) an initial relatively fast release of midodrine and / or desglymidodrine (in order to obtain a relatively fast onset of action), ii) a steady release or a slower release than in step 1 of midodrine and / or desglymidodrine (in order to maintain a plasma concentration of desglymidodrine which is prolonged and relatively constant), iii) a second rise in release of midodrine and / or desglymidodrine (in order to take advantage of absorption from the colon, i.e. such a second rise release is designed to take place when the composition (or the disintegrated parts of the composition) reaches the colon; normally this is regarded to take about 8 hours after oral intake, and iv) a decline in release rate corresponding to that essentially all midodrine and / or desglymidodrine have been released from the composition.Also disclosed is a method for treating orthostatic hypotension and / or urinary incontinence, the method comprising administration to a patient in need thereof of an effective amount of midodrine and / or desglymidodrine in a composition according to the invention.
Owner:NYCOMED AUSTRIA
Triple drug therapy for the treatment of narcotic and alcohol withdrawal symptoms
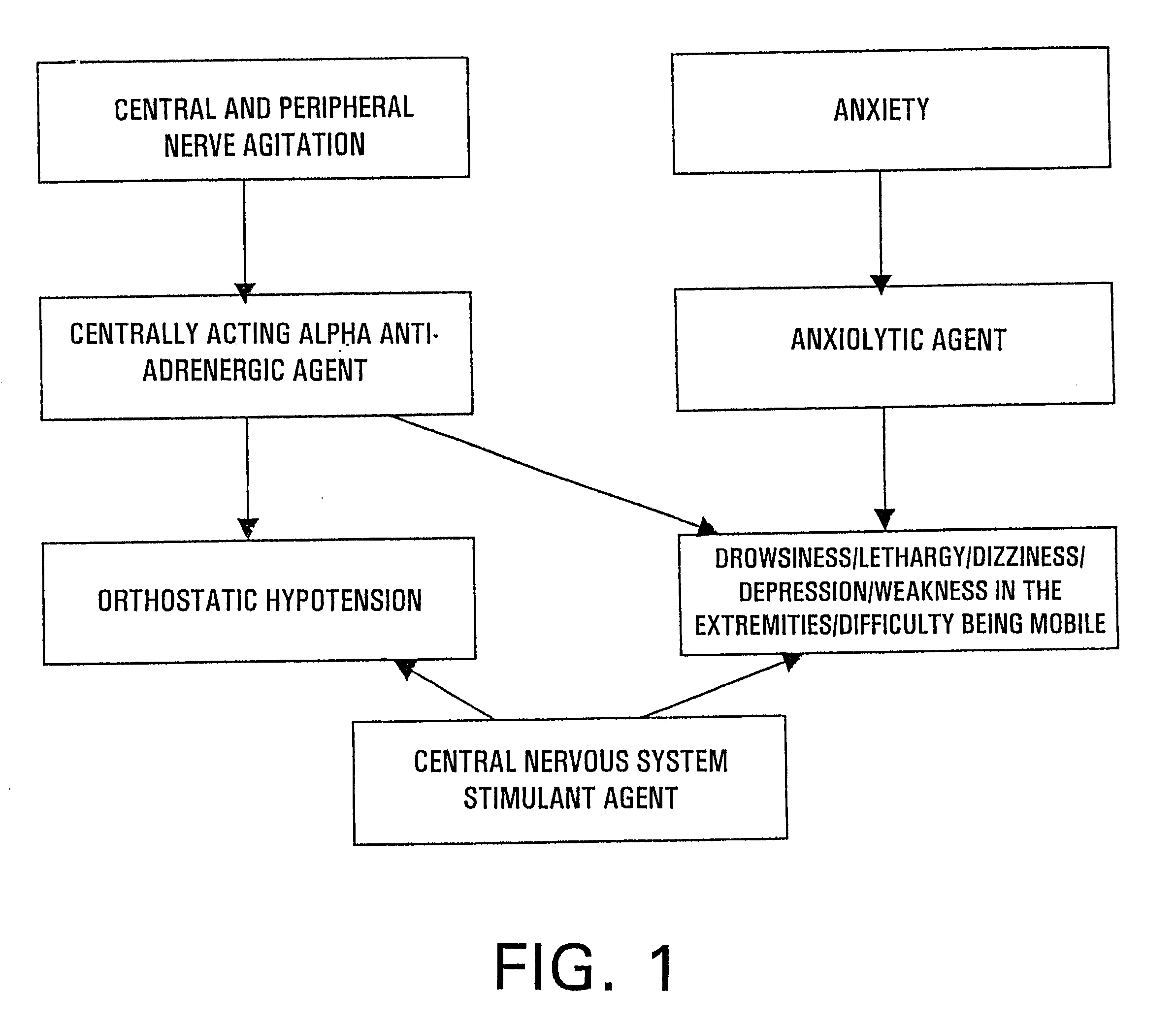
A triple drug, pharmaceutical kit, composition, and method of treatment containing a combination of effective amounts of at least one anxiolytic agent, at least one centrally acting alpha antiadrenergic agent, and at least one central nervous system stimulant for the reduction or prevention of alcohol and narcotic withdrawal side effects of dizziness, drowsiness, depression, lethargy, orthostatic hypotension, weakness in the extremities, and difficulty in being mobile, caused by therapeutic agents utilized for the treatment of alcohol or narcotic withdrawal symptoms in patients overcoming alcohol or narcotic addiction.
Owner:OCKERT DAVID M
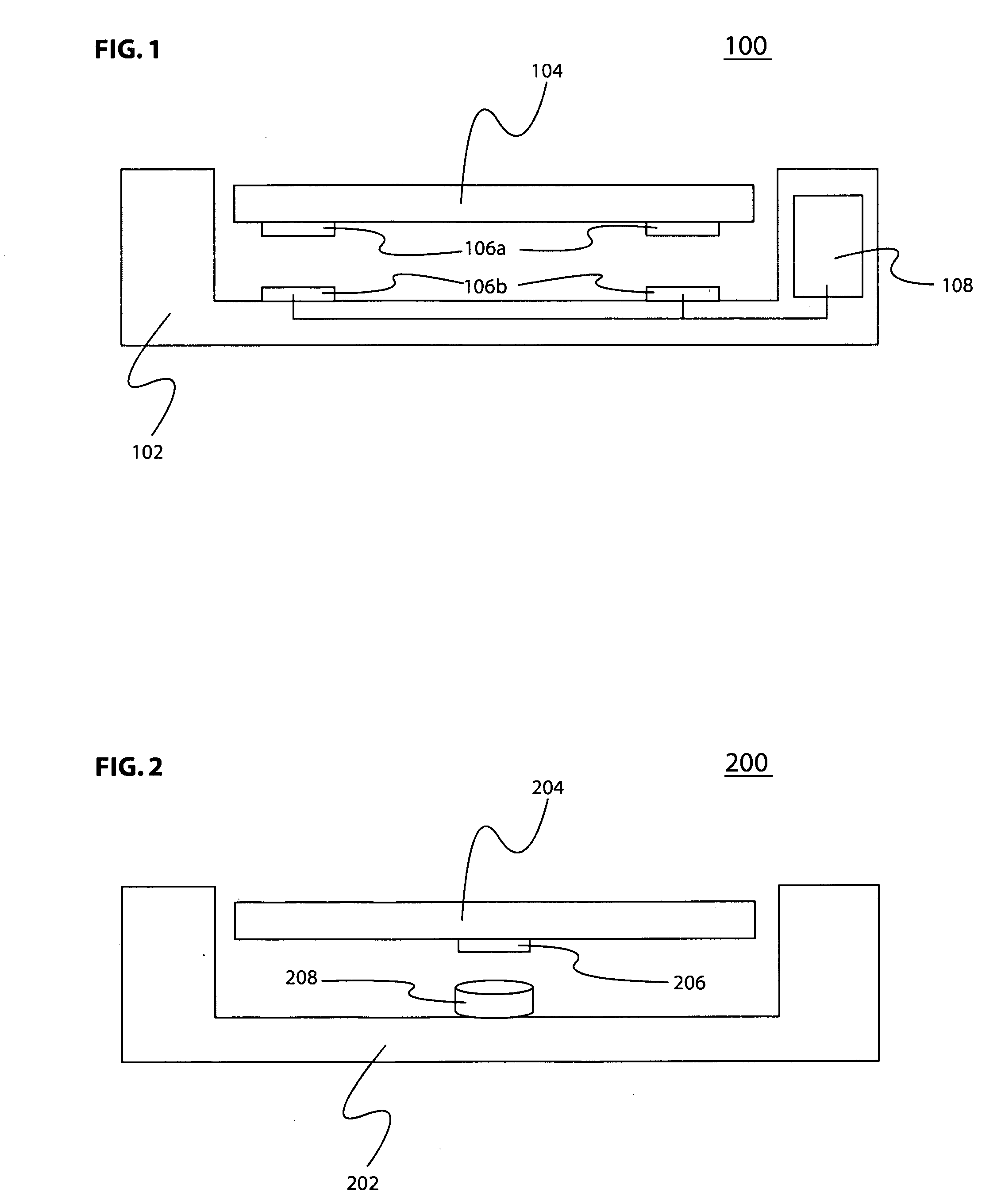
System and method for a low profile vibrating plate
InactiveUS20060241528A1Improve portabilityReduce power consumptionChiropractic devicesVibration massageDiseaseBone structure
A medical treatment system and method are provided for the treatment of tissue ailments, including weakened bone structures caused by fractures, osteoporosis, or other bone related ailments, and orthostatic hypotension, using a vibrating plate. The system and method use magnetic fields to provide vertical vibrational motion to a platform, thus allowing the system to have a lower profile.
Owner:AMERICAN MEDICAL INNOVATIONS LLC
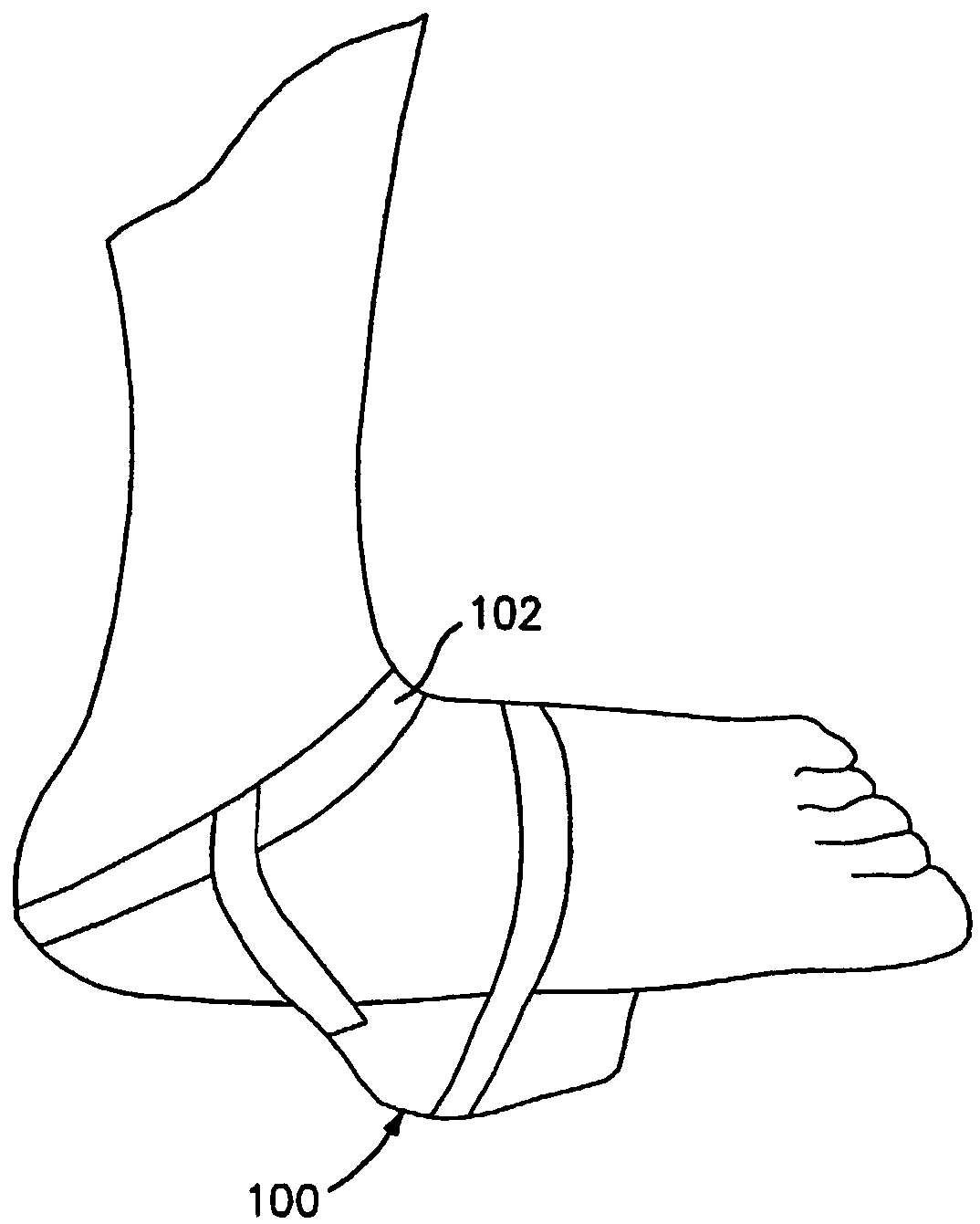
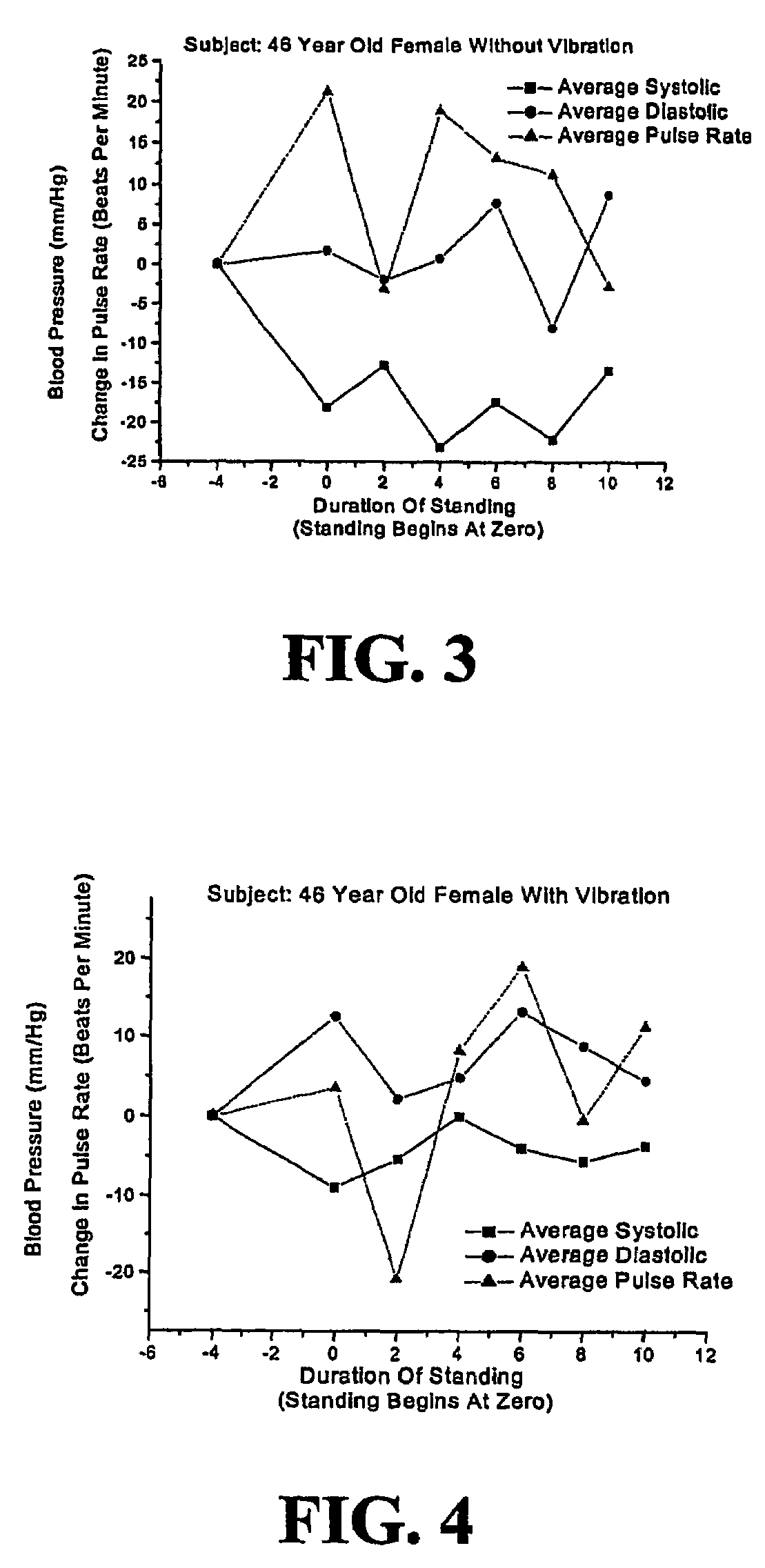
Non-invasive method and apparatus for treating orthostatic hypotension
InactiveUS7402144B2Reduce the impactIncrease blood flowElectrotherapyDiagnosticsPhysical medicine and rehabilitationNon invasive
A non-invasive method and apparatus are provided for treating orthostatic hypotension and for reducing the effects caused there from. An increase in blood and fluid flow in the lower extremities is achieved by vibrating the lower body of the individual at a frequency in the range of 10-120 Hz. The apparatus includes a strap for being secured to a body part of the individual's lower extremities, e.g., the sole of one's foot, and a displacement sensor which senses any movement of the body part. The displacement sensor continuously sends signals to a processor of the apparatus which indicate whether there was any movement of the body part. If the displacement sensor did not sense any substantial movement of the body part for a predetermined period of time the processor sends a signal to a vibrating mechanism. The signal activates the vibrating mechanism causing vibration of the body part for increasing blood and fluid flow in the lower extremities. The vibrating mechanism causes vibration for a predetermined period of time.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
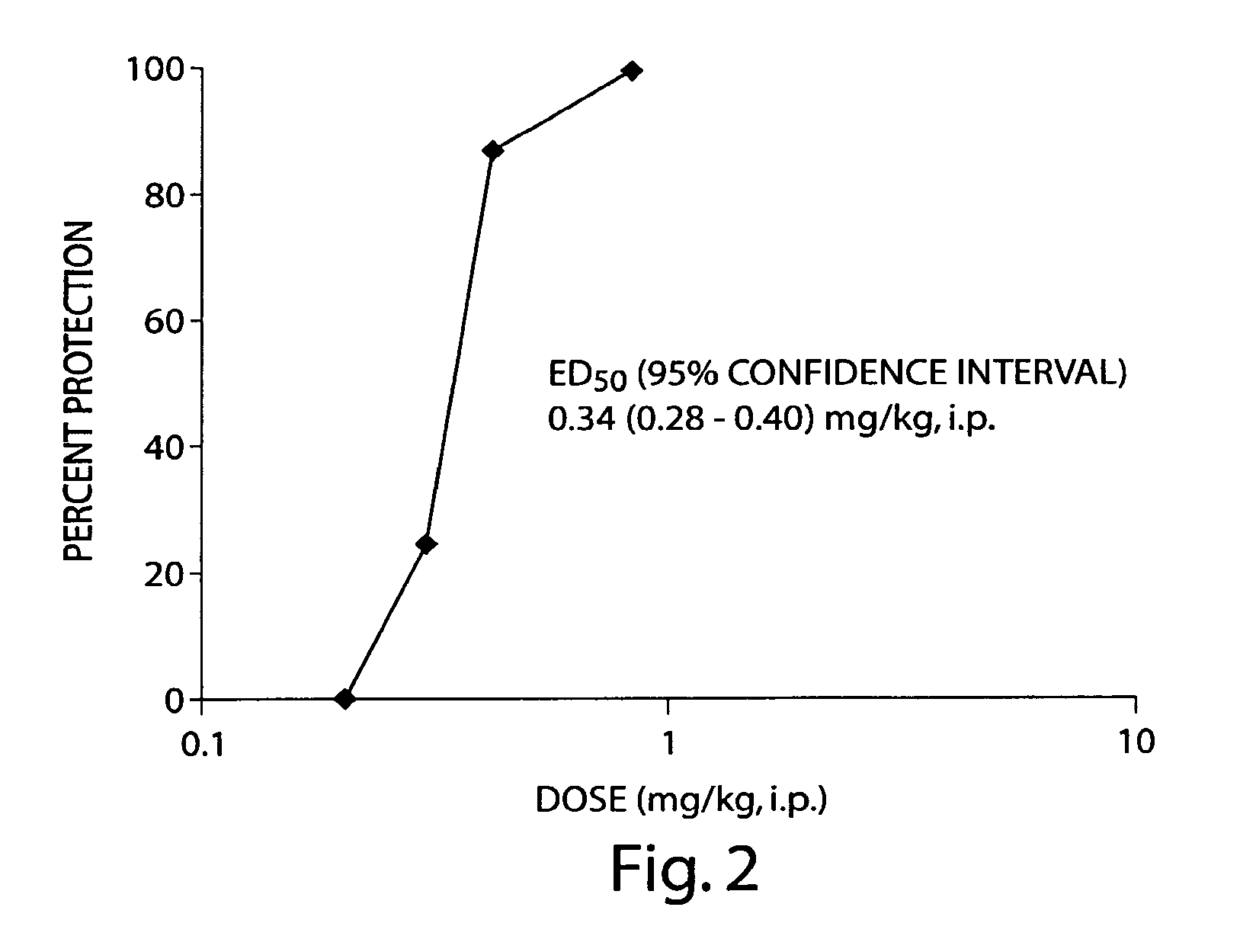
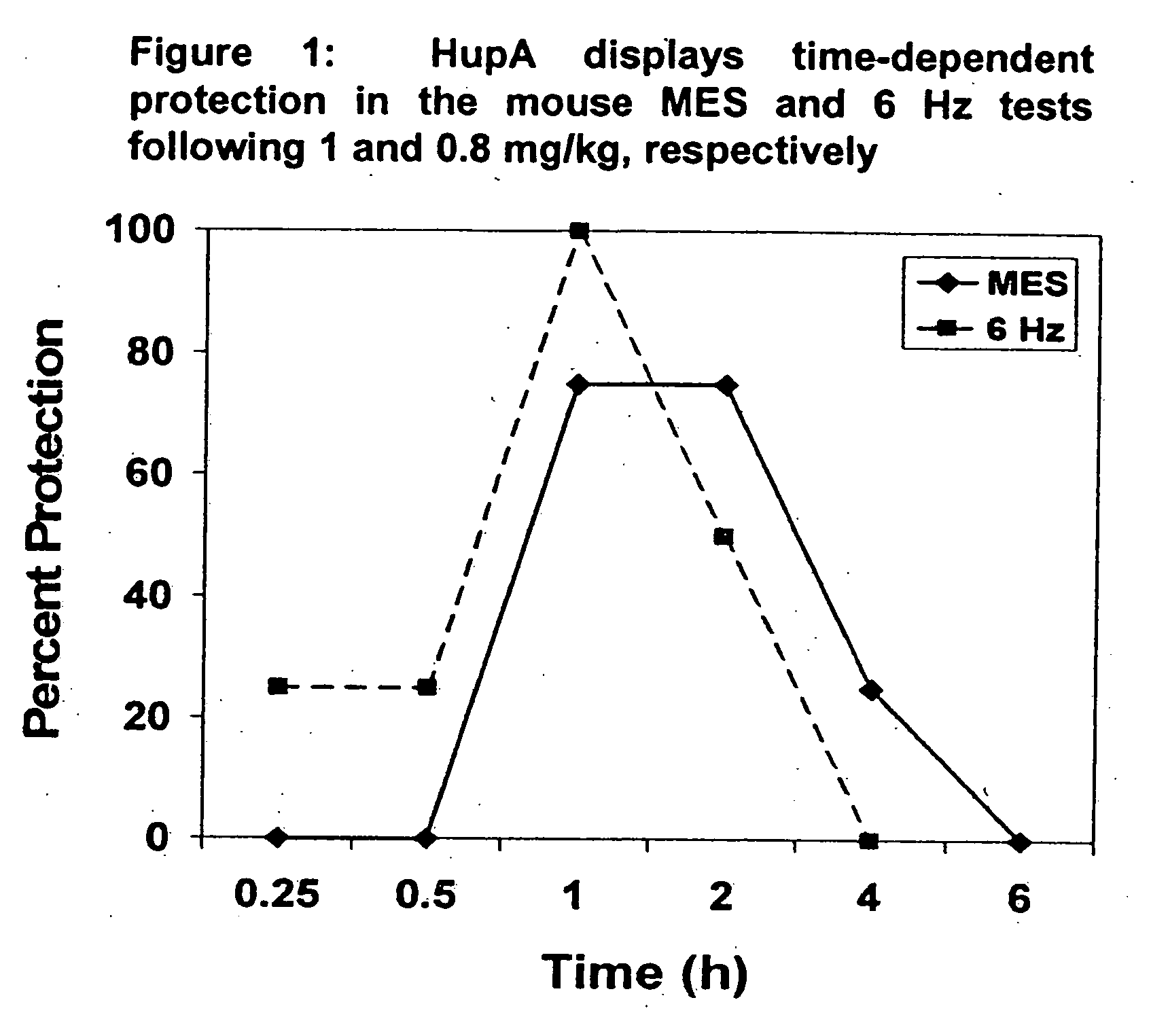
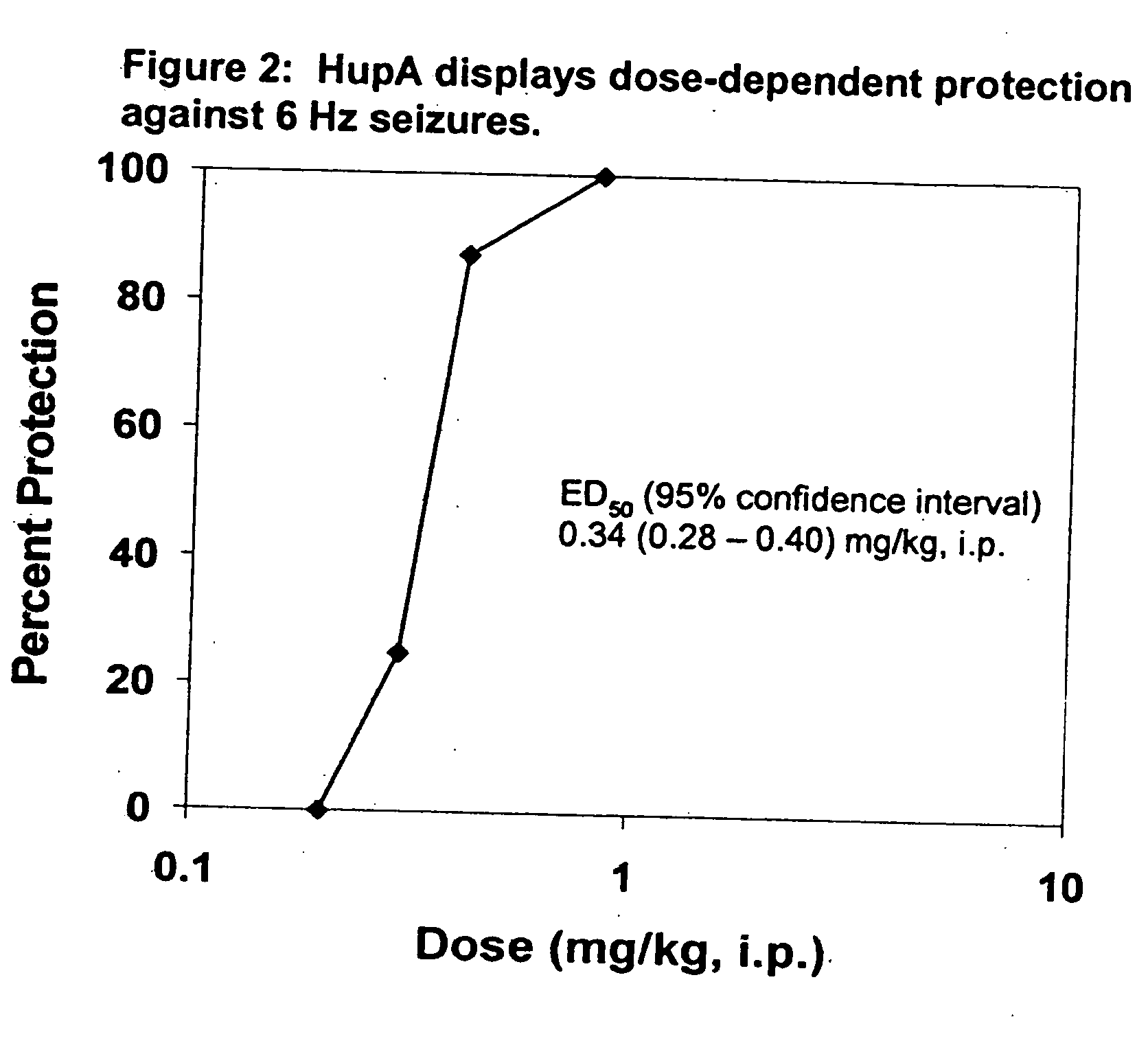
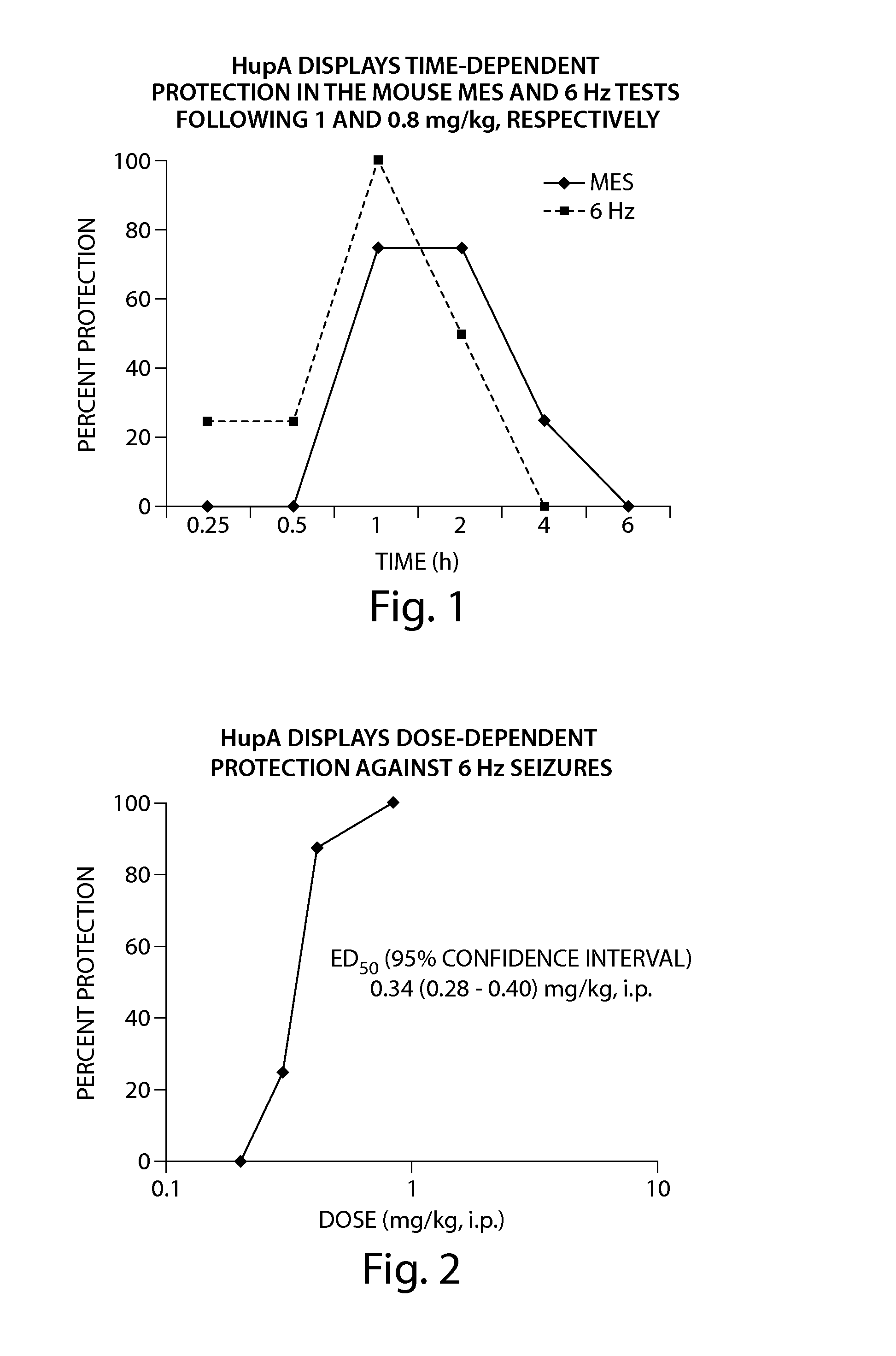
Use of huperzine for disorders
Methods and compositions containing huperzine are used to prevent and alleviate neuropathic pain. The invention is also directed to methods and compositions for using huperzine for the prevention and / or treatment of neuropathic pain and orthostatic hypotension.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Use of huperzine for disorders
Methods and compositions containing huperzine are used to alleviate pain and for the prevention and / or treatment of seizures and epilepsy. The invention is also directed to methods and compositions for using huperzine for the prevention and / or treatment of neuropathic pain and orthostatic hypotension.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Treatment Of Neurological Disorders Using Huperzine
InactiveUS20110224245A1Reduce perception of painReduce severityBiocideNervous disorderDiseaseNervous system
Methods and compositions containing huperzine for direct delivery to central nervous system (CNS) tissue are used to alleviate pain and for the prevention and / or treatment of seizures and epilepsy. The invention is also directed to methods and compositions for using huperzine for the prevention and / or treatment of neuropathic pain and orthostatic hypotension.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Use of huperzine for neuropathic pain
ActiveUS20060264454A1Alleviate and suppress and inhibit existing painAvoid painBiocideNervous disorderNeuropathic painOrthostatic BP
Methods and compositions containing huperzine are used to prevent and alleviate neuropathic pain. The invention is also directed to methods and compositions for using huperzine for the prevention and / or treatment of neuropathic pain and orthostatic hypotension.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Postural stability and incident functions in patients
InactiveUS20130197090A1Increase in severityBiocideOrganic active ingredientsActive agentDisease patient
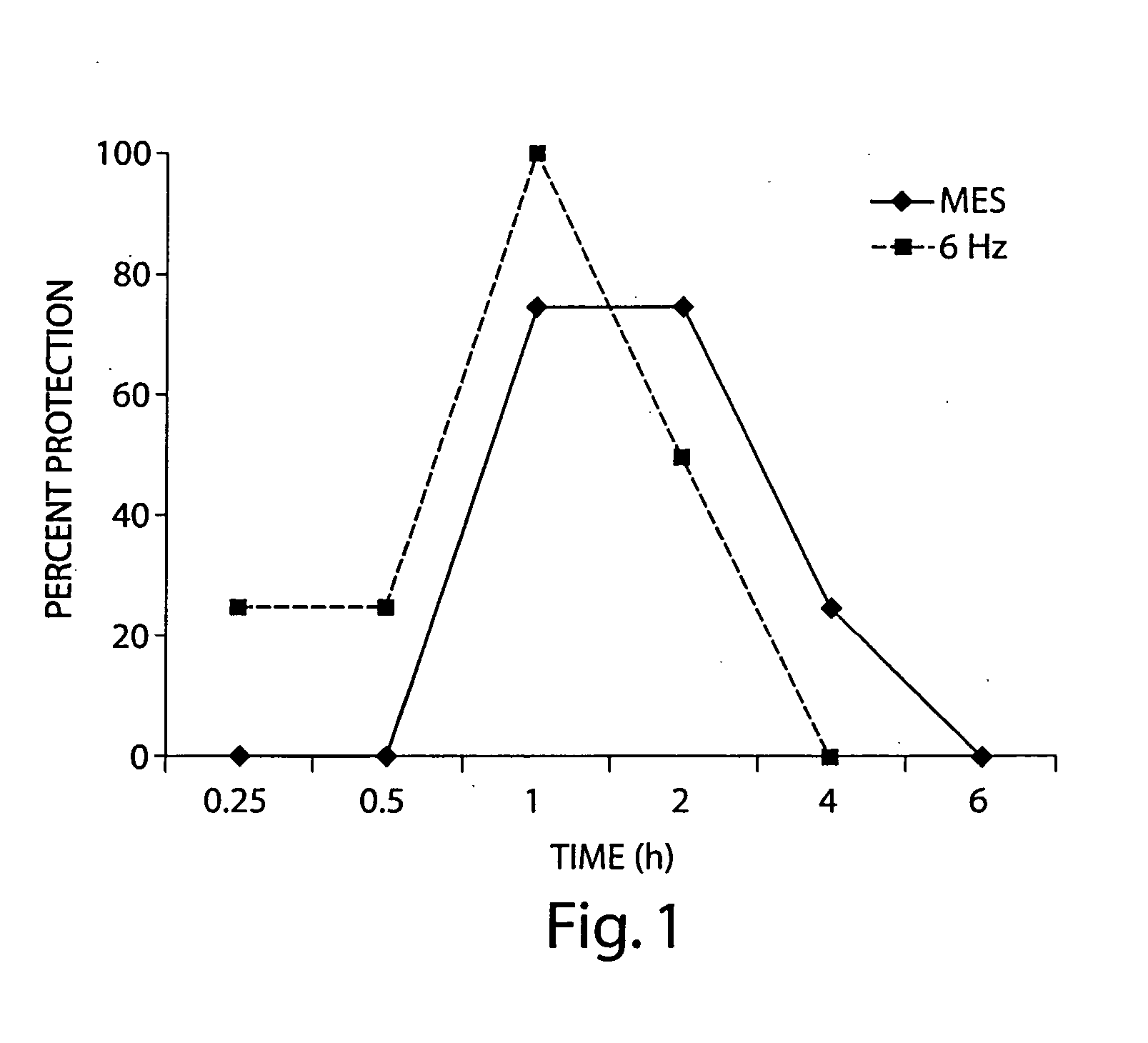
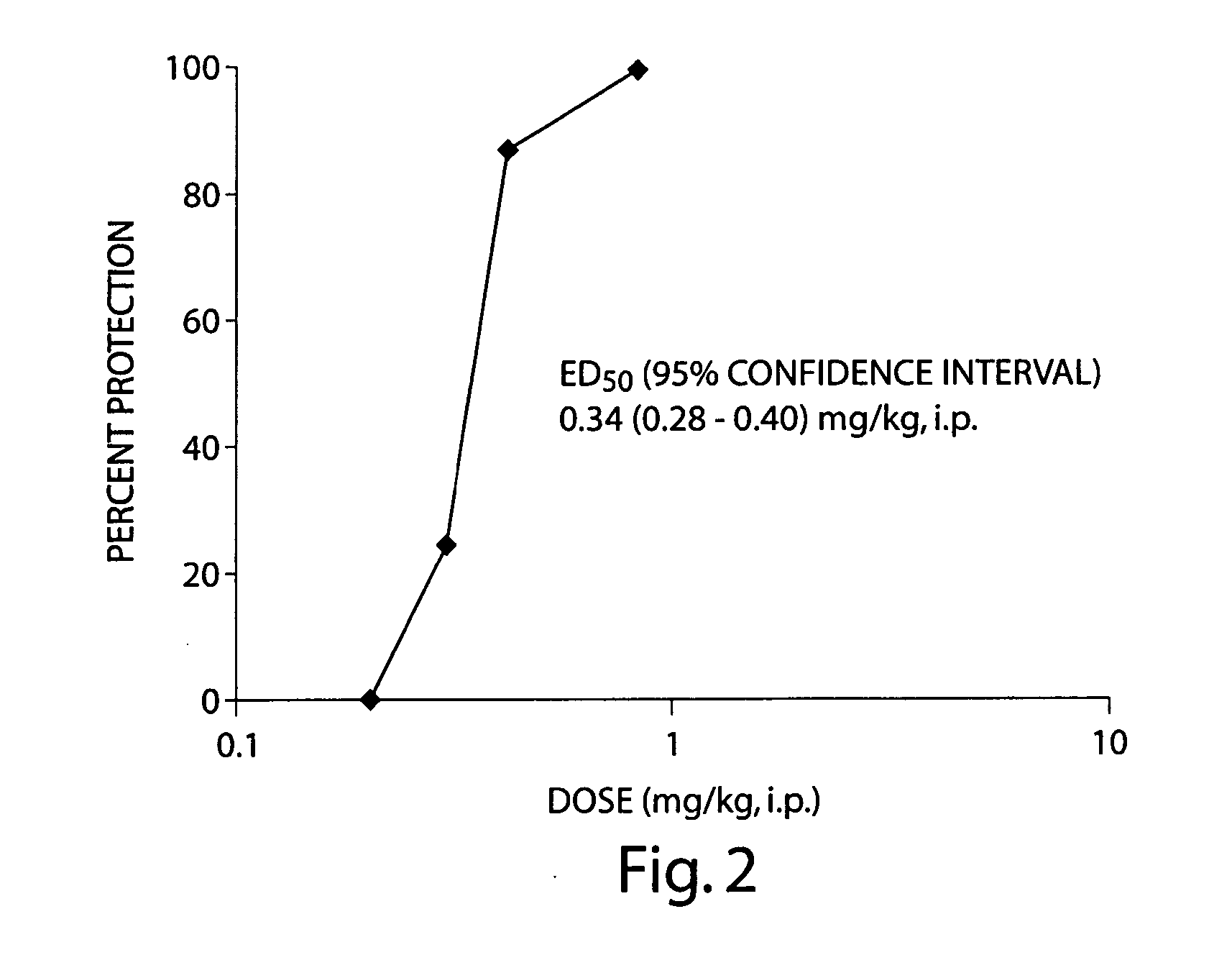
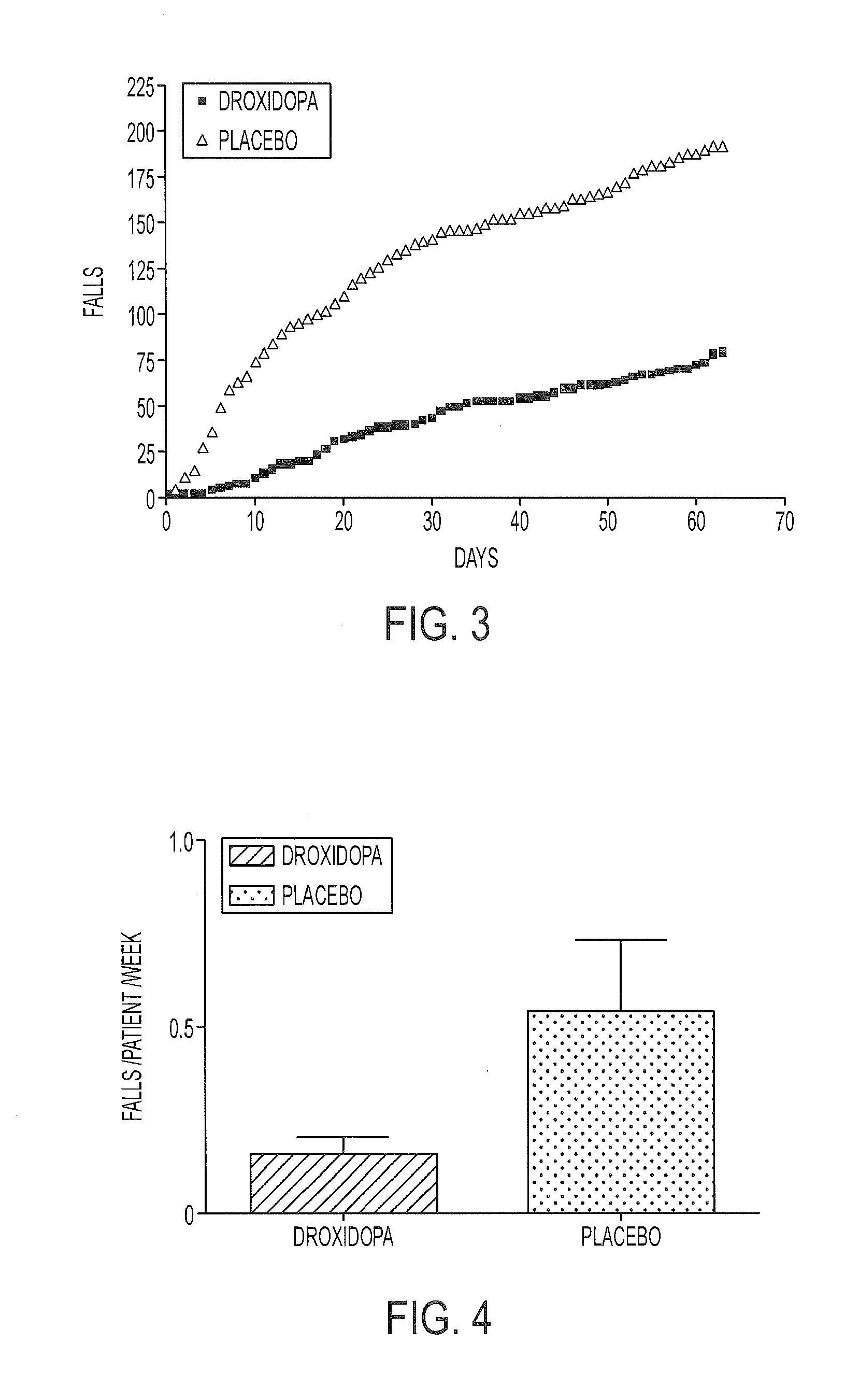
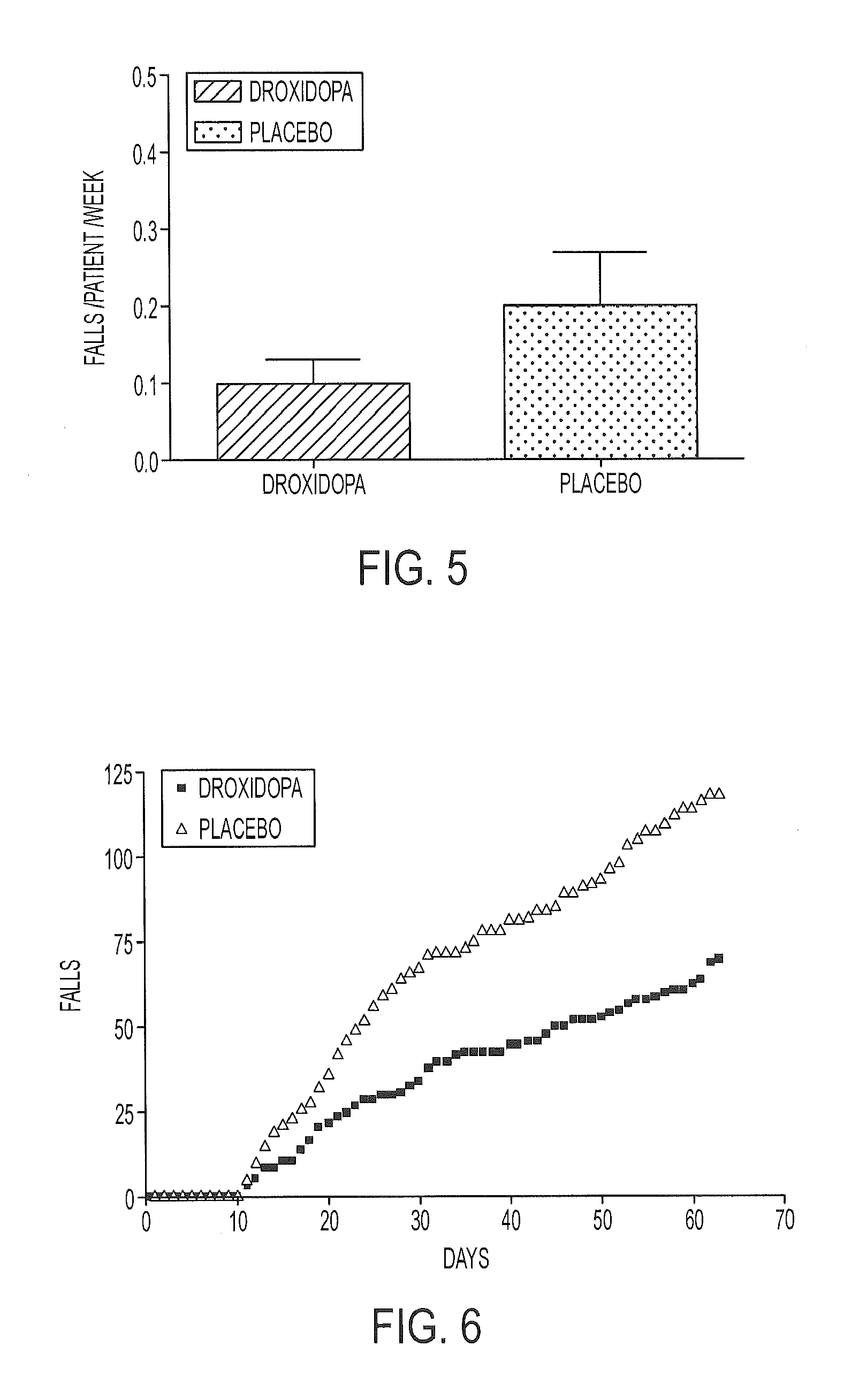
The present invention provides methods and systems for reducing falls in patients that are recurrent fallers. Specifically, the compositions, systems, and methods can relate to Parkinson's disease patients, particularly such patients that are suffering from neurogenic orthostatic hypotension. The compositions, systems, and methods comprise the use of droxidopa, optionally in combination with a further active agent. Administration of droxidopa has been found to reduce the mean number of falls per patient per week, as well as provide improvements in the patient's Hoehn and Yahr rating scale score, which is indicative of improvements in postural stability, and provide improvements in the patient's Unified Parkinson's disease Rating Scale score, which is indicative of improvements in the severity of motor and / or non-motor symptoms of Parkinson's disease.
Owner:CHELSEA THERAPEUTICS
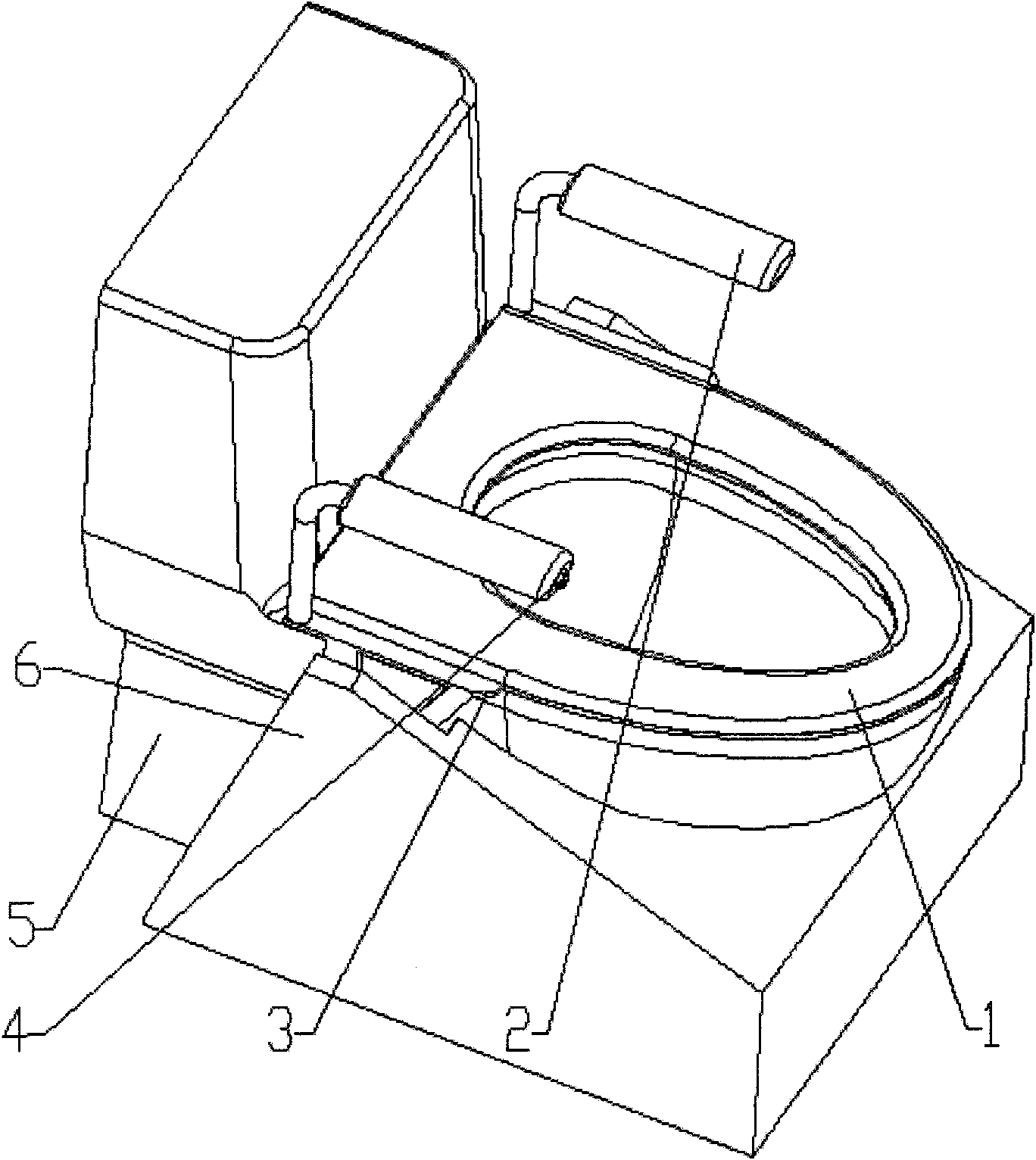
Pedestal pan ring capable of automatic lifting
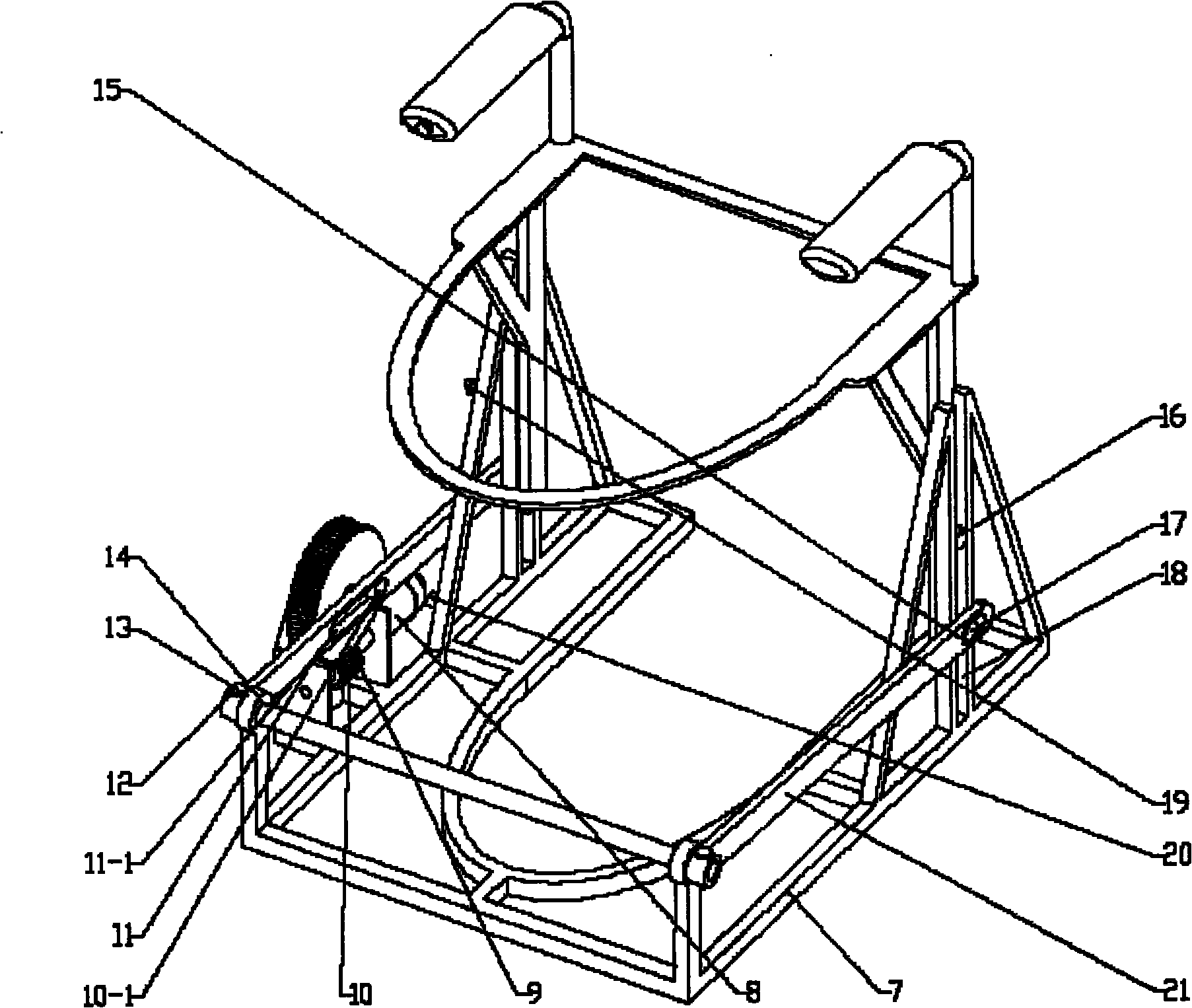
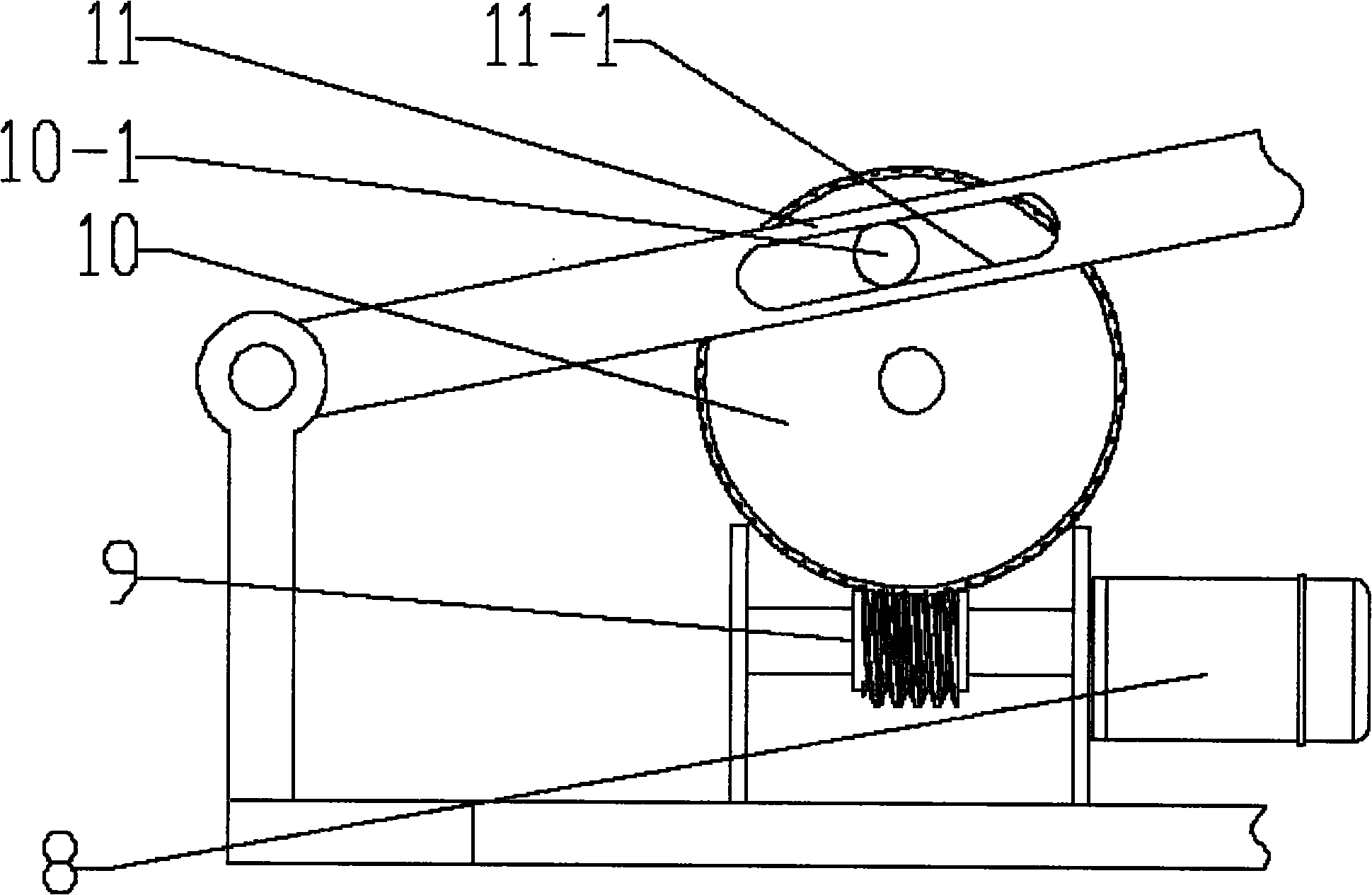
InactiveCN102133066ARelieves symptoms of stress numbnessAvoiding the danger of fallingBathroom coversPins and needlesGear wheel
The invention discloses a pedestal pan ring capable of automatic lifting, which is characterized in that the pedestal pan ring can lift automatically through a lifting platform. The lifting power supply is provided by a motor, a driving gear of the output end of the motor is a worm, and a driven gear engaged with the driving gear is a turbine; an eccentric cylindrical sliding block is fixedly arranged at the side wall of the turbine and extends into a slideway of a swinging rod, the front end of the swinging rod is pinned on one side of a shaft arranged at the upper front end of a stand, and the swinging rod is also symmetrically arranged at the opposite side of the shaft and pinned on the other side of the shaft; back ends of two swinging rods are both provided with symmetrical slideways, two cylindrical sliding blocks are respectively arranged upside and downside two sides of the lifting platform integrated with the pedestal pan ring into a whole, and the lower cylindrical sliding block extends into the slideway; the upper and the lower cylindrical sliding blocks at two sides are arranged in the slideway at the back of the stand and perpendicular to the stand in a penetrating way; and an upper travel switch and a lower travel switch are arranged between the areas where the swinging rods move on the stand. After defecation, the motor is started and then the pedestal pan ring can be lifted slowly, therefore, the difficulties of pins and needles and standing up caused by compression of leg nervus vascularis are quickly relieved and orthostatic hypotension dizziness is prevented.
Owner:王一川
Method to Reduce Slosh Energy Absorption and its Damaging Effects Through the Reduction of Inelastic Collisions in an Organism
PendingUS20180333159A1Reduce riskSymptoms improvedHydroxy compound active ingredientsTetracycline active ingredientsSIDS - Sudden infant death syndromeEnergy absorption
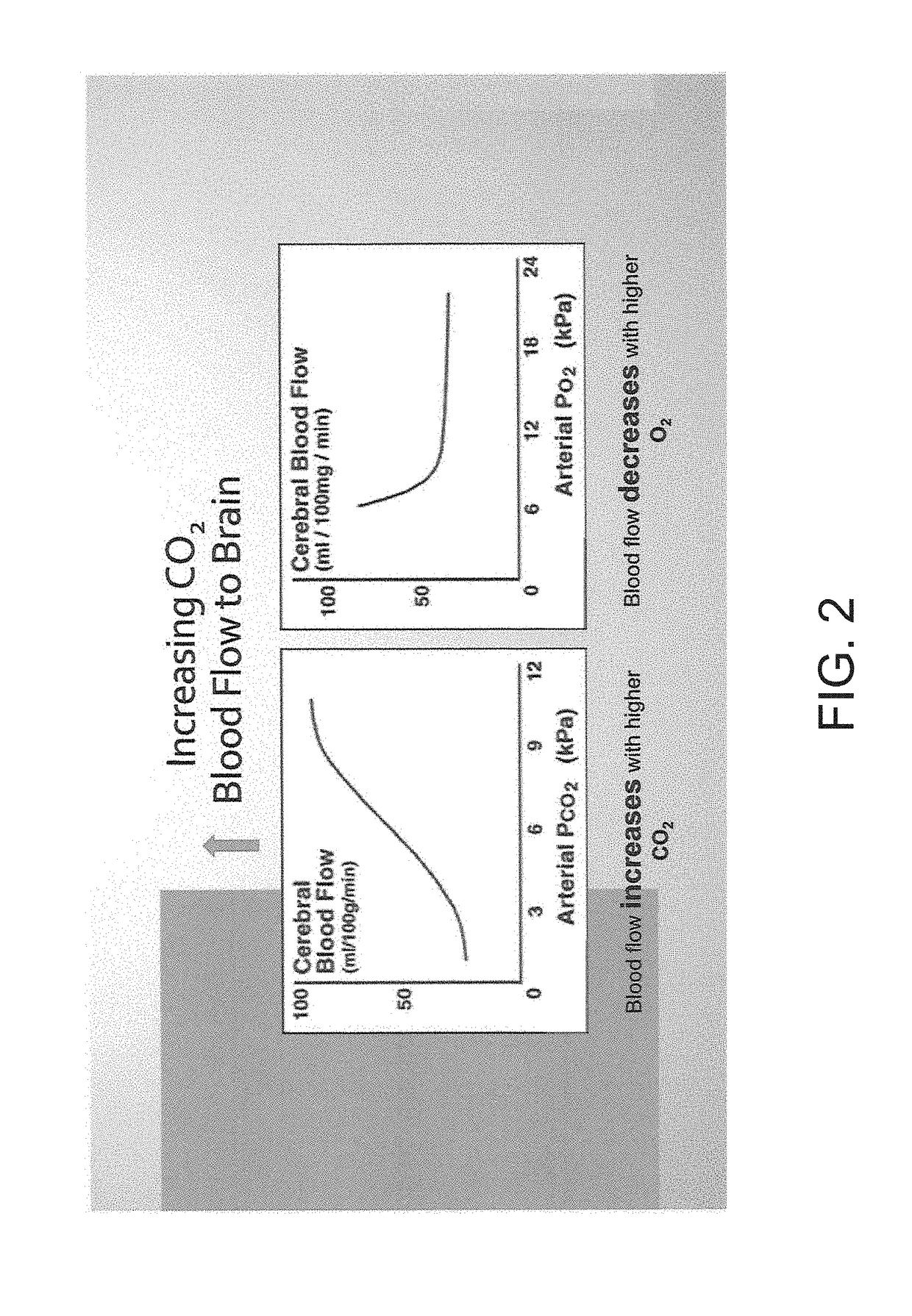
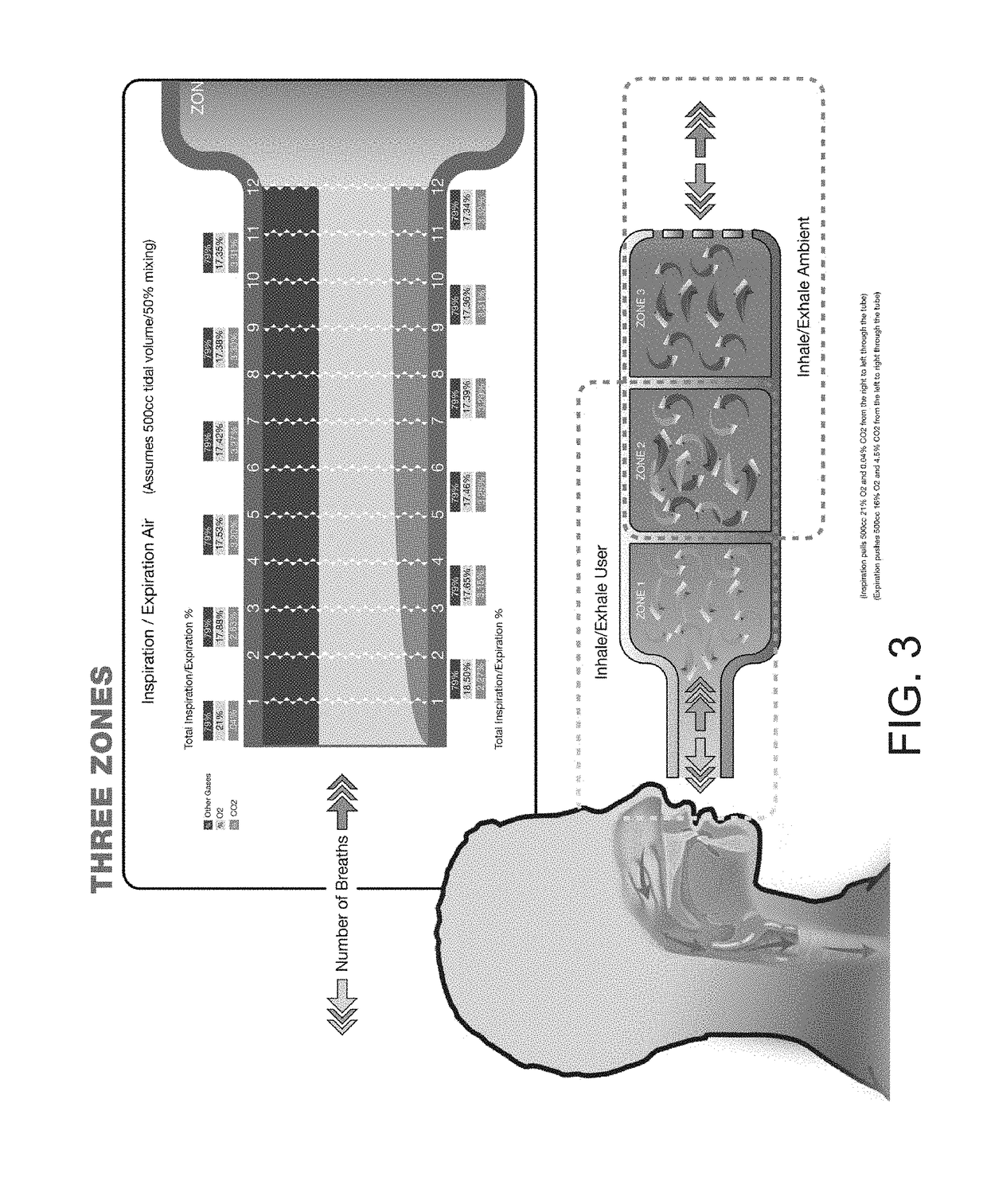
A method is provided for reducing the risk of sustaining a traumatic brain injury caused by a traumatic event that includes identifying a subject at risk of sustaining a traumatic brain injury, and then precisely increasing the partial pressure of carbon-dioxide (CO2) in the blood of the subject (pCO2). This method can be applied to raise the CO2 and pCO2 to improve orthostatic hypotension in conditions such as dysautonomias (like Positional Orthostatic Tachycardic Syndrome POTS) and to facilitate the drive to breathe in conditions like Central Sleep Apnea (CSA) and Sudden Infant Death Syndrome (SIDS). The pCO2 of the person is increased by placing a breathing apparatus over the mouth of the person through which the person must breath, wherein the breathing apparatus includes an enlarged dead space volume in which expired CO2 collects to be inhaled or re-breathed by the person on the next inhalation.
Owner:SMITH DAVID
Eucommia ulmoides oliver and corn peptide composition capable of reducing blood pressure and blood fat and preparation method thereof
ActiveCN109876135APrevent thrombosisLower blood fatHydrolysed protein ingredientsMetabolism disorderLiver and kidneySide effect
The invention discloses a eucommia ulmoides oliver leaf and corn peptide composition capable of reducing blood pressure and blood fat and relates to the field of blood pressure and blood fat reduction. The composition comprises an eucommia ulmoides oliver leaf extract, a dendrobium officinale extract and an active corn peptide. The eucommia ulmoides oliver leaf and corn peptide composition adoptsa traditional Chinese medicine formula, has the excellent effects of reducing blood pressure and blood fat, and can also effectively solve the accompanying symptoms of hypertension and hyperlipidemia,such as headache, dizziness, tinnitus, palpitation and insomnia, is nontoxic and free of side effects, does not affect the liver and kidney functions of the body and does not cause the liver and kidney burden. In addition, the eucommia ulmoides oliver leaf and corn peptide composition does not cause physical dependence on taking, and does not cause symptoms such as orthostatic hypotension for theaged. The invention also provides a preparation method of the eucommia ulmoides oliver leaf and corn peptide composition capable of reducing blood pressure and blood fat. The effective ingredients inthe raw materials can be fully extracted by adopting the preparation method, and the preparation method is simple and easy to operate.
Owner:陈东良 +1
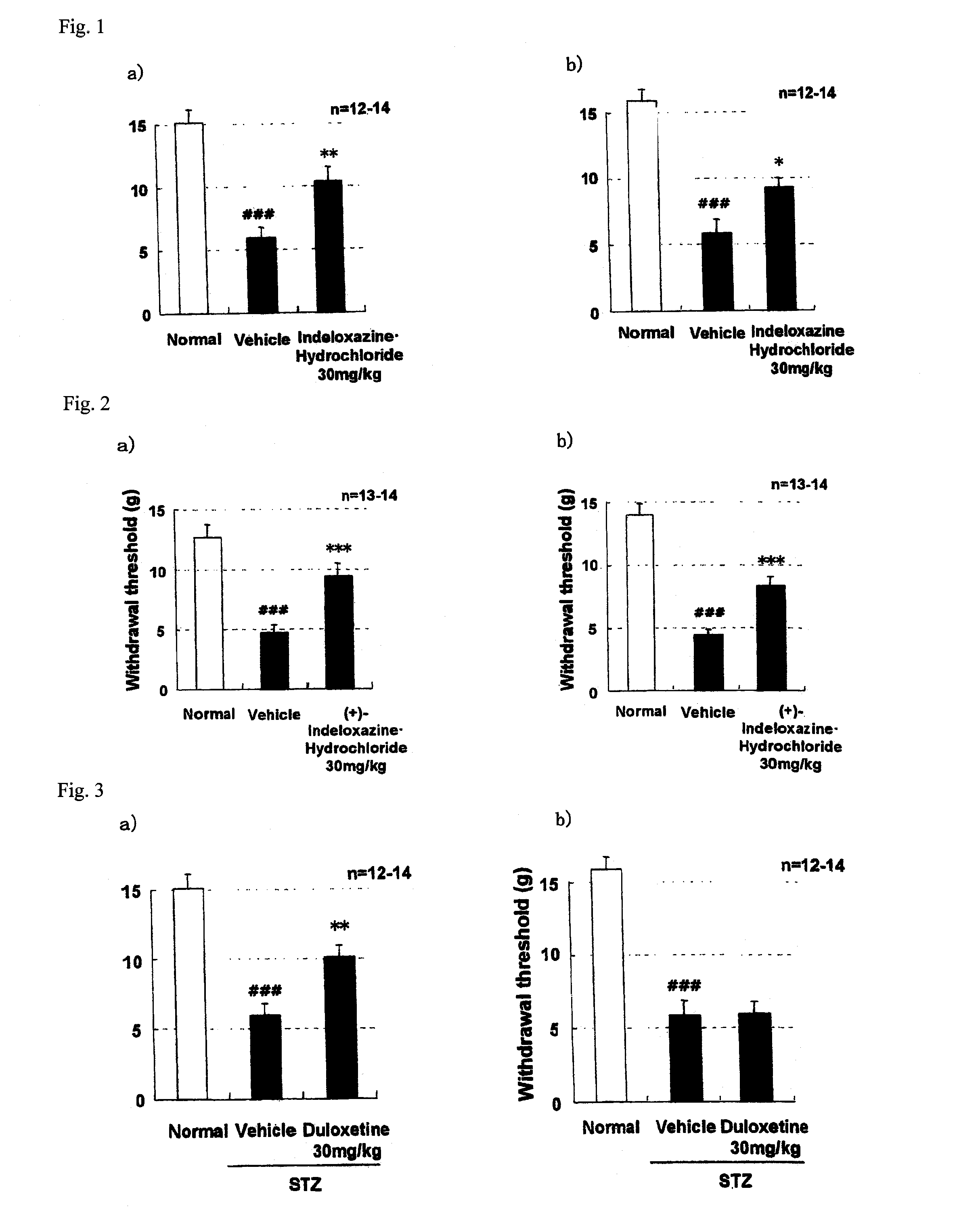
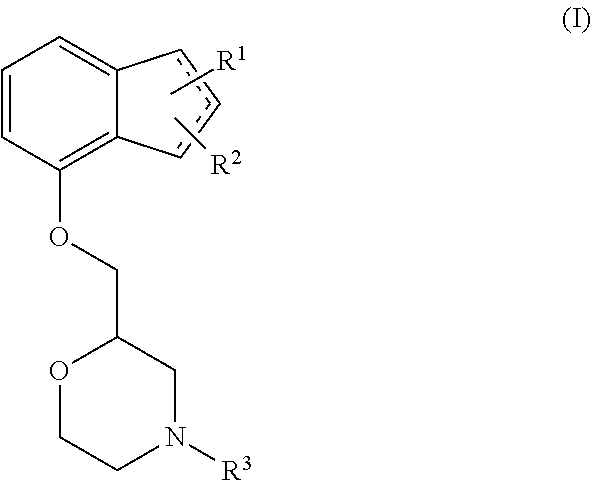
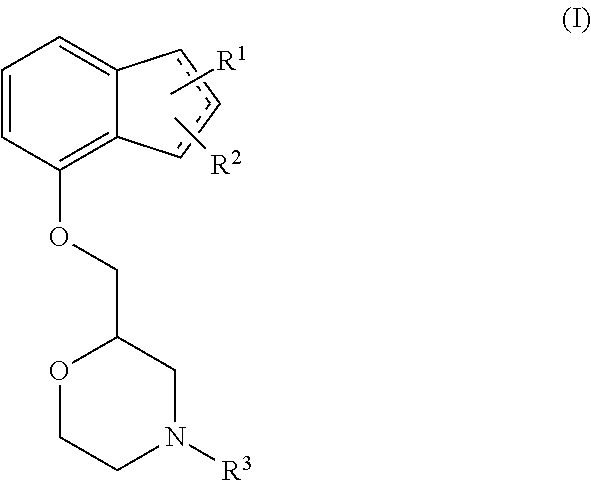
Novel preventive and/or therapeutic agent for diabetic neuropathy
InactiveUS20100113451A1Prevent goodExcellent therapeutic agentOrganic active ingredientsNervous disorderTherapeutic effectMotor neuropathy
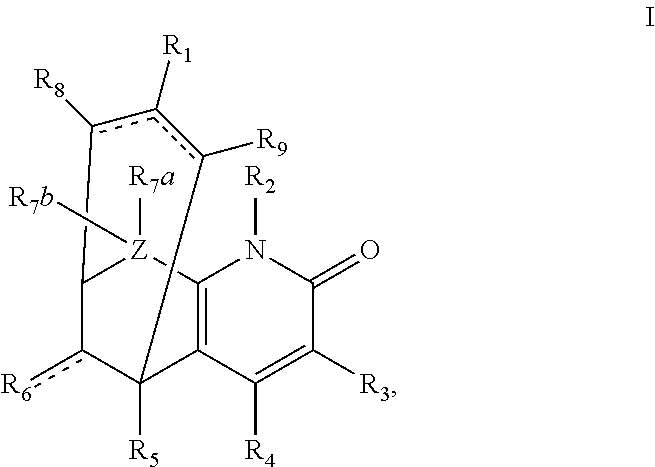
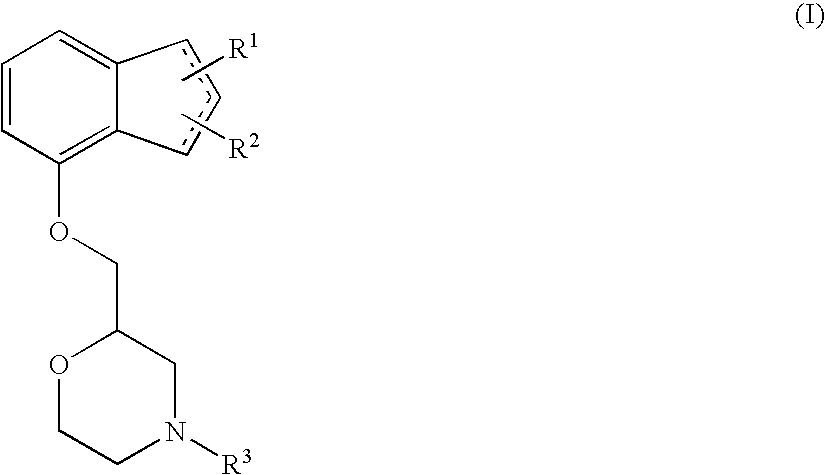
The present invention relates to an agent for preventing and / or treating diabetic neuropathy comprising a 2-[(substituted-inden-7-yloxy)methyl]morpholine of the formula (I) or a pharmaceutically acceptable salt thereof as an active ingredient. The invention is useful for providing an excellent agent for preventing and / or treating diabetic neuropathy, and particularly useful for providing an agent for preventing and / or treating diabetic motor neuropathy (such as muscle weakness disorder (such as muscle weakness disorder with inability to walk independently)), diabetic sensory neuropathy (such as paresthesia (such as vibration perception abnormality), allodynia, hypoesthesia (such as numbness of extremities or cold sensation), or pain), or diabetic autonomic neuropathy (such as stool abnormality (such as constipation or diarrhea), urination disorder, impotence, orthostatic hypotension, sudomotor dysfunction, abnormal heart rate variability, or delayed gastric emptying). Further, the invention is particularly useful for providing an agent for improving pathophysiology of diabetic neuropathy.
Owner:ASTELLAS PHARMA INC
Compositions and methods for the treatment of autonomic and other neurological disorders
InactiveUS20150210667A1Treat and prevent and ameliorate effectBiocideNervous disorderDiseaseEnantiomer
The invention relates to the compounds of formula I or its pharmaceutical acceptable salts, as well as polymorphs, solvates, enantiomers, stereoisomers and hydrates thereof. The pharmaceutical compositions comprising an effective amount of compounds of formula I, and methods for the treatment of autonomic and other neurological disorders may be formulated for oral, buccal, rectal, topical, transdermal, transmucosal, intravenous, parenteral administration, syrup, or injection. Such compositions may be used to treatment of neurogenic orthostatic hypotension (NOH), as well as NOH associated with multiple system atrophy (MSA), familial amyloid polyneuropathy (FAP), pure autonomic failure (PAF), and Parkinson's disease (PD), intradialytic hypotension (IDH) or hemodialysis-induced hypotension, hypotension associated with fibromyalgia syndrome (FMS) and chronic fatigue syndrome (CFS).
Owner:KANDULA MAHESH
Methods and materials for treating orthostatic hypotension or postural tachycardia syndrome
This document provides methods and materials related to treating orthostatic hypotension and / or postural tachycardia syndrome. For example, methods and materials for using a composition containing 3,4-diaminopyridine, 4-aminopyridine, or both to treat patients with orthostatic hypotension, postural tachycardia syndrome, or both orthostatic hypotension and postural tachycardia syndrome are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Method of preventing and/or treating diabetic neuropathy
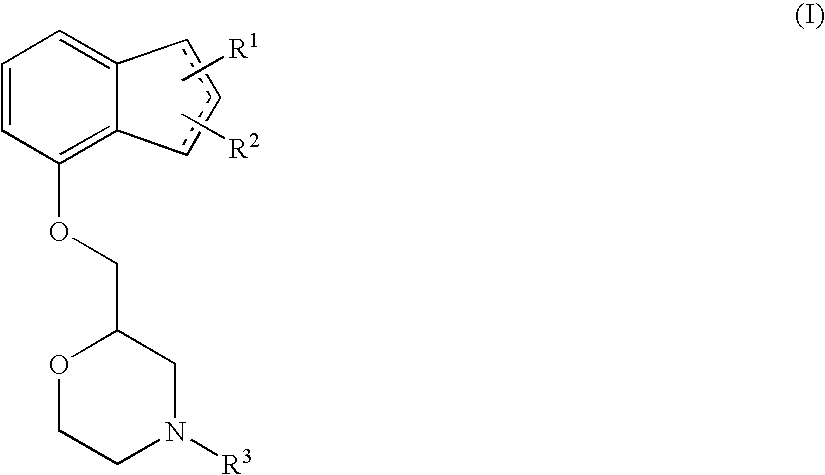
InactiveUS20130023535A1Excellent preventive and therapeutic agentImproving pathophysiologyOrganic active ingredientsNervous disorderTruncal muscle weaknessMotor neuropathy
The present invention relates to an agent for preventing and / or treating diabetic neuropathy comprising a 2-[(substituted-inden-7-yloxy)methyl]morpholine of the formula (I) or a pharmaceutically acceptable salt thereof as an active ingredient. The invention is useful for providing an excellent agent for preventing and / or treating diabetic neuropathy, and particularly useful for providing an agent for preventing and / or treating diabetic motor neuropathy (such as muscle weakness disorder (such as muscle weakness disorder with inability to walk independently)), diabetic sensory neuropathy (such as paresthesia (such as vibration perception abnormality), allodynia, hypoesthesia (such as numbness of extremities or cold sensation), or pain), or diabetic autonomic neuropathy (such as stool abnormality (such as constipation or diarrhea), urination disorder, impotence, orthostatic hypotension, sudomotor dysfunction, abnormal heart rate variability, or delayed gastric emptying). Further, the invention is particularly useful for providing an agent for improving pathophysiology of diabetic neuropathy.
Owner:ASTELLAS PHARMA INC
Pedestal pan ring capable of automatic lifting
InactiveCN102133066BRelieves symptoms of stress numbnessAvoiding the danger of fallingBathroom coversPins and needlesGear wheel
The invention discloses a pedestal pan ring capable of automatic lifting, which is characterized in that the pedestal pan ring can lift automatically through a lifting platform. The lifting power supply is provided by a motor, a driving gear of the output end of the motor is a worm, and a driven gear engaged with the driving gear is a turbine; an eccentric cylindrical sliding block is fixedly arranged at the side wall of the turbine and extends into a slideway of a swinging rod, the front end of the swinging rod is pinned on one side of a shaft arranged at the upper front end of a stand, and the swinging rod is also symmetrically arranged at the opposite side of the shaft and pinned on the other side of the shaft; back ends of two swinging rods are both provided with symmetrical slideways, two cylindrical sliding blocks are respectively arranged upside and downside two sides of the lifting platform integrated with the pedestal pan ring into a whole, and the lower cylindrical sliding block extends into the slideway; the upper and the lower cylindrical sliding blocks at two sides are arranged in the slideway at the back of the stand and perpendicular to the stand in a penetrating way; and an upper travel switch and a lower travel switch are arranged between the areas where the swinging rods move on the stand. After defecation, the motor is started and then the pedestal pan ring can be lifted slowly, therefore, the difficulties of pins and needles and standing up caused by compression of leg nervus vascularis are quickly relieved and orthostatic hypotension dizziness is prevented.
Owner:王一川
Methods for treating symptomatic orthostatic hypotension
PendingUS20220168301A1Long-term effective therapeutic treatmentImprove toleranceOrganic active ingredientsOrganic chemistryReceptor subunitAspartic acid
The present disclosure provides a method for treating symptomatic orthostatic hypotension using a potent selective antagonist of N-methyl-D-aspartate receptor subunit 2B (NMDA-GluN2B or NR2B).
Owner:CERECOR INC
Beta 2 adrenoceptor antagonists for treating orthostatic hypotension
InactiveUS20140357724A1Decrease activity of nerveSuppress nerve activityOrganic active ingredientsBiocideΒ2 adrenoceptorOrthostatic BP
Methods of treating orthostatic hypotension are disclosed. The methods include administering to a subject in need thereof an effective amount of a beta 2 (β2) adrenoceptor antagonist, and in particular, the specific β2 adrenoceptor antagonist, 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol.
Owner:INDIANA UNIV RES & TECH CORP
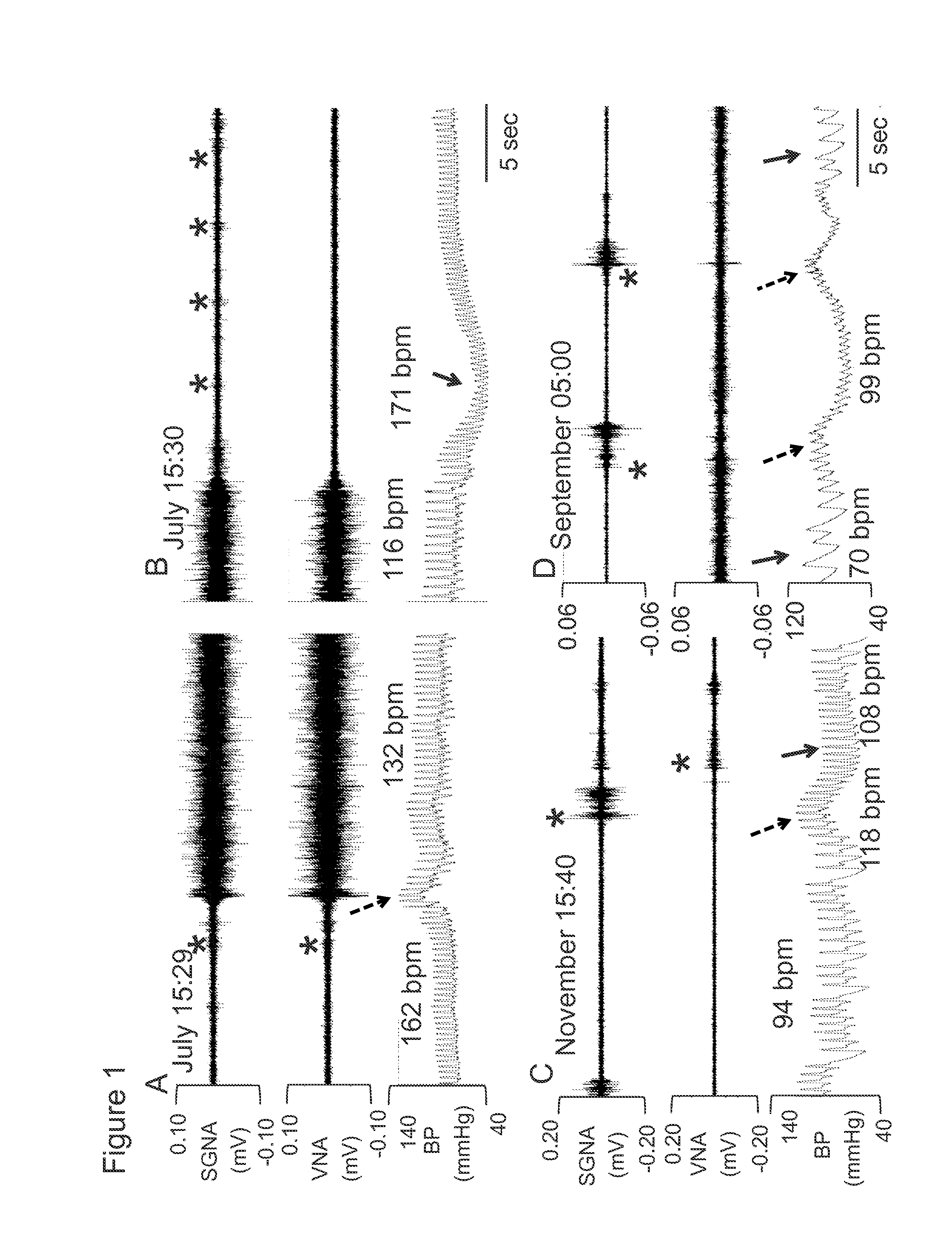
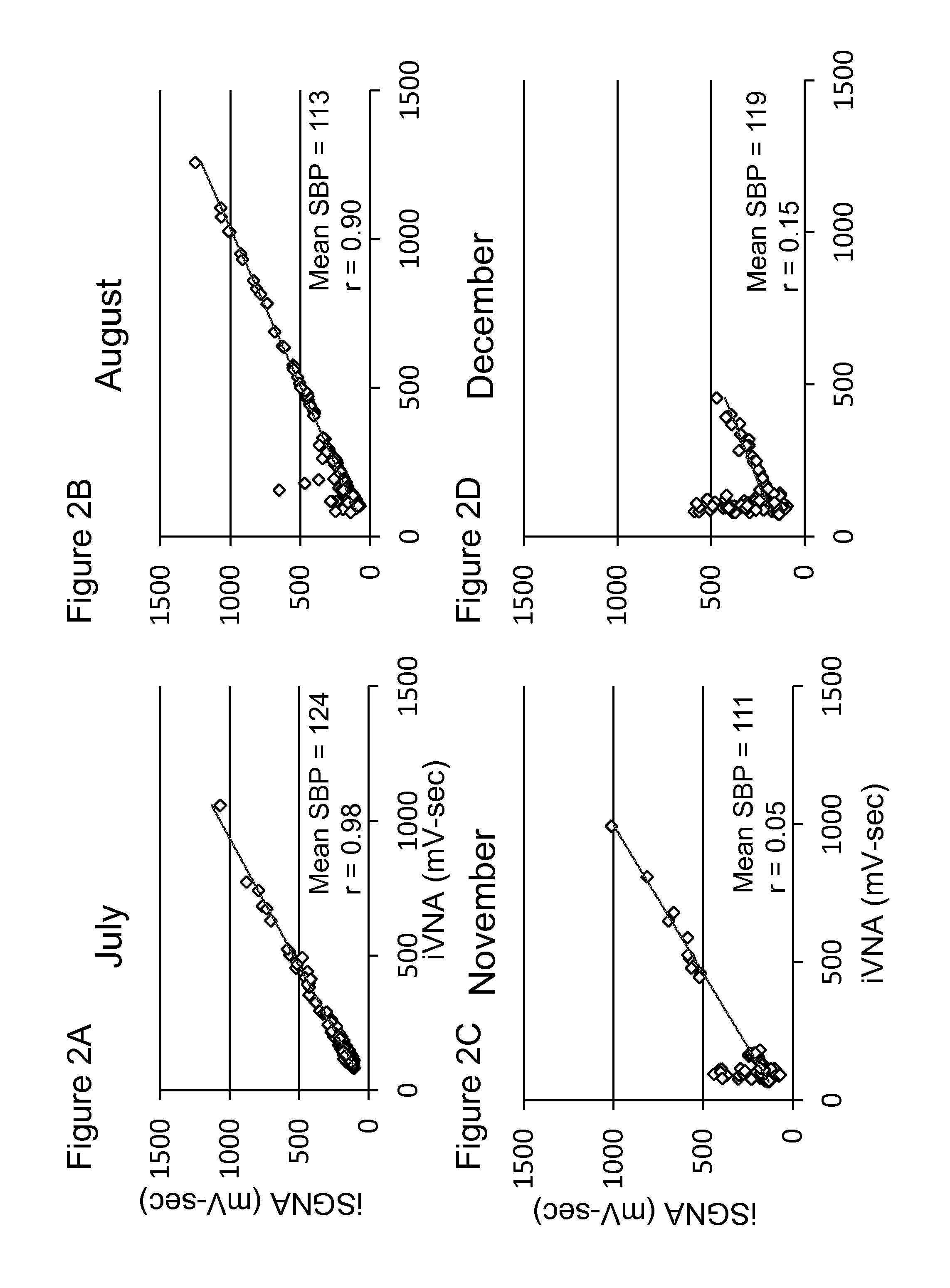
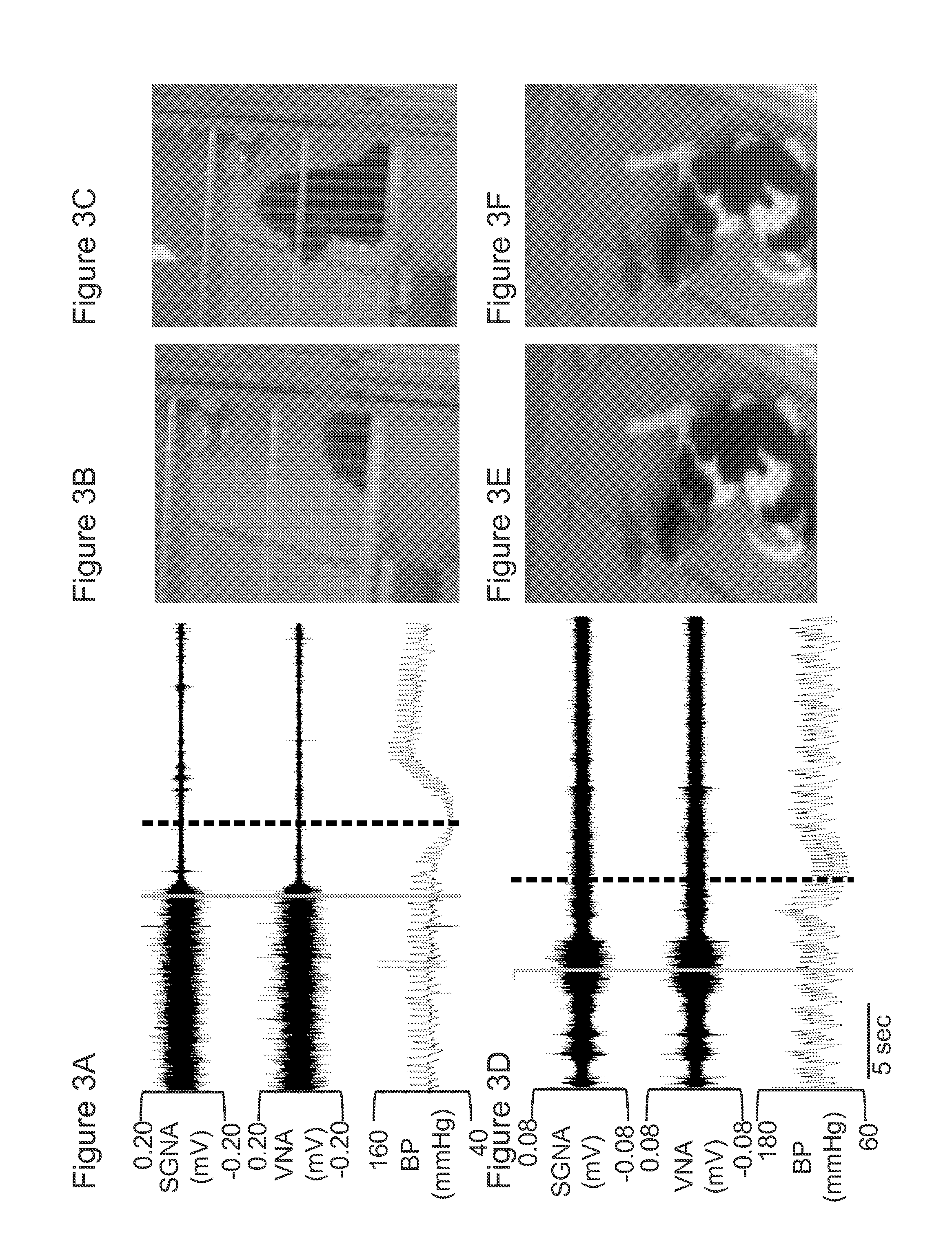
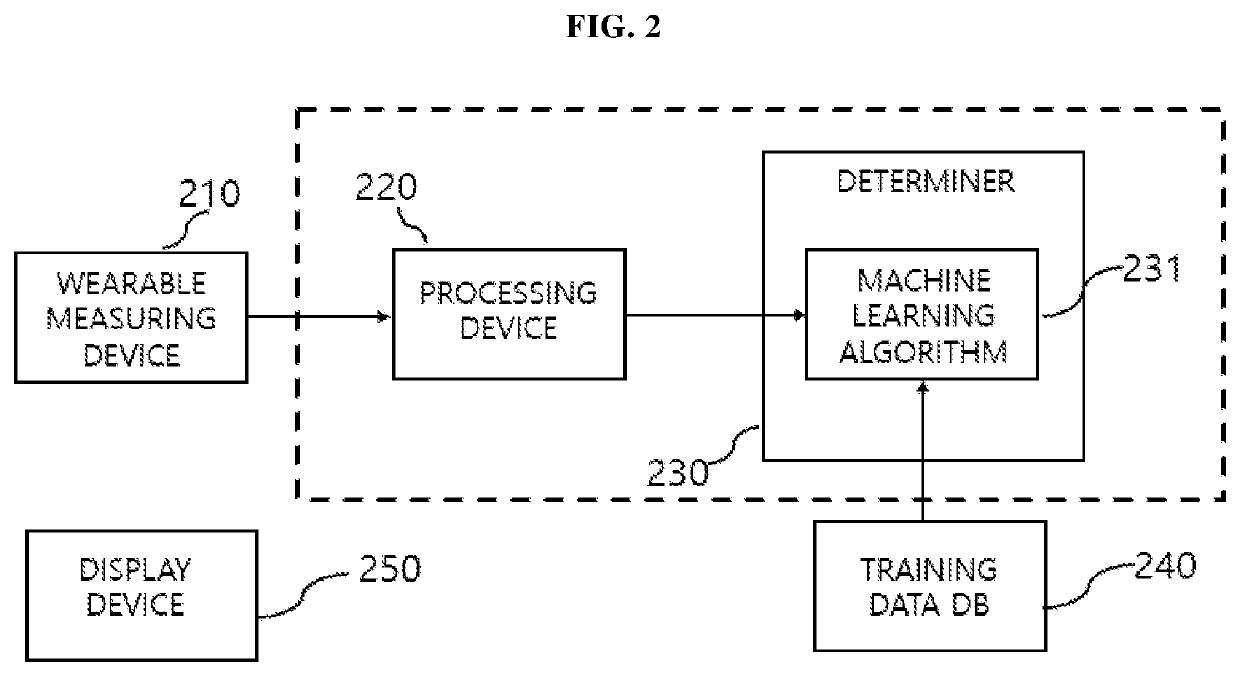
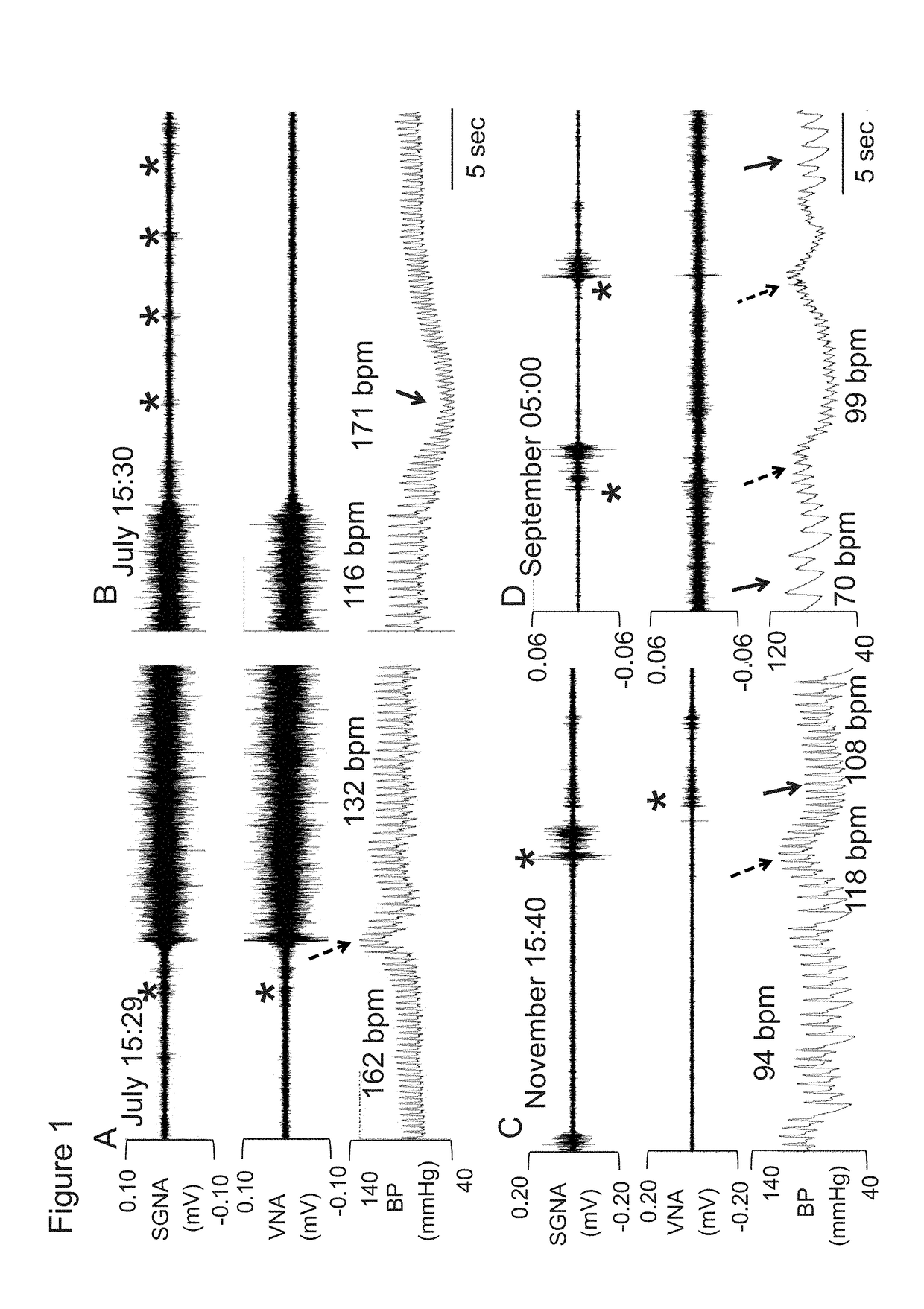
System and method for screening orthostatic hypotension by using heart rate-based machine learning algorithm, and wearable measurement device
PendingUS20220280046A1Accurate diagnosisHealth-index calculationEvaluation of blood vesselsLow blood pressuresPhysical therapy
An orthostatic hypotension screening system using a heart rate-based machine learning algorithm includes an input unit configured to receive a variable comprising at least one of a patient's age, blood pressure, an expiration (E)-inspiration (I) difference and an E:I ratio calculated from a heart rate, and a Valsalva ratio calculated according to a Valsalva method; and a determination unit configured to determine whether the patient has orthostatic hypotension according to a machine learning algorithm that is pre-trained based on the variable received through the input unit.
Owner:TOBEDTX
Novel prophylactic and/or therapeutic agent for diabetic neuropathy
Disclosed is an excellent prophylactic and / or therapeutic agent for diabetic neuropathy, which comprises a 2-¢(substituted-indene-7-yloxy)methyl!morpholine represented by the formula (I) or a pharmaceutically acceptable salt thereof as an active ingredient. The prophylactic and / or therapeutic agent is particularly useful as a prophylactic and / or therapeutic agent for diabetic motor nerve disorder(e.g., decrease in muscular strength (decrease in muscular strength to an extent of being unable to independently walk)), diabetic sensory nerve disorder (paresthetic (abnormal vibratory sensation), allodynia, reduced sensation (numbness in limbs, psychroesthesia) or pain), or diabetic autonomic nerve disorder (e.g., abnormal bowel habituation such as constipation and diarrhea, urinary dysfunction, impotence, orthostatic hypotension, dyshidrosis, abnormal heart rate variability, delayed gastric emptying). Also disclosed is an ameliorating gent for a disease condition of diabetic nerve disorder.
Owner:ASTELLAS PHARMA INC
Methods and materials for treating orthostatic hypotension or postural tachycardia syndrome
ActiveUS20110053989A1Reduce severityReduce frequencyBiocideAnimal repellantsOrthostatic tachycardiaMedicine
This document provides methods and materials related to treating orthostatic hypotension and / or postural tachycardia syndrome. For example, methods and materials for using a composition containing 3,4-diaminopyridine, 4-aminopyridine, or both to treat patients with orthostatic hypotension, postural tachycardia syndrome, or both orthostatic hypotension and postural tachycardia syndrome are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Traditional Chinese medicine composition for treating spleen-kidney deficiency type orthostatic hypotension
InactiveCN105535496AImprove clinical symptomsClinical symptoms improvedDigestive systemCardiovascular disorderDiseaseAralia elata
The invention discloses a traditional Chinese medicine composition for treating spleen-kidney deficiency type orthostatic hypotension. The traditional Chinese medicine composition is characterized by being prepared from the following medicinal materials in parts by weight: 15 parts of longan aril, 30 parts of polyalthia nemoralis, 20 parts of hibiscus cancellatus roots, 10 parts of streptopus obtusatus, 25 parts of lyonia ovalifolia, 12 parts of rhizome of variegated solomonseal, 8 parts of honey-fried licorice root, 12 parts of all-grass of Bulley Herminium, 20 parts of neofinetia falcate, 15 parts of flatstem milkvetch seeds, 60 parts of stellaria yunnanensis, 12 parts of drynaria quercifolia, 25 parts of aralia elata, 25 parts of dioscorea opposita and 15 parts of ramontchi. The traditional Chinese medicine composition disclosed by the invention can be used for treating spleen-kidney deficiency type orthostatic hypotension and is high in effective rate and reliable in treatment effect, and the disease does not reoccur after being treated.
Owner:杨君
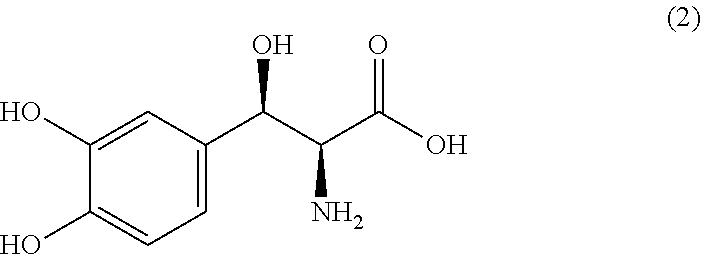
Process for producing threo-3-(3,4-dihydroxyphenyl)-l-serine
ActiveUS20120095256A1Improve production efficiencyLow priceOrganic compound preparationAmino-carboxyl compound preparationL serineMedicinal chemistry
The present invention provides a process for producing Droxidopa or a pharmaceutically acceptable salt thereof comprising a step of reacting threo-N-phthaloyl-3-(3,4-dihydroxyphenyl)-L-serine represented by the formula (1) with methylamine, whereby a process for producing threo-3-(3,4-dihydroxyphenyl)-L-serine (common name: Droxidopa), which is useful as an agent for treatment of peripheral orthostatic hypotension or an agent for treatment of Parkinson's disease, with high production efficiency and without requiring troublesome operations.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Non-invasive method and apparatus for treating orthostatic hypotension
InactiveCN1568169AReduce fatigueElectrotherapyChiropractic devicesPhysical medicine and rehabilitationNon invasive
A non-invasive method and apparatus are provided for treating orthostatic hypotension and for reducing the effects caused there from. An increase in blood and fluid flow in the lower extremities is achieved by vibrating the lower body of the individual at a frequency in the range of 10-120 Hz. The apparatus includes a strap for being secured to a body part of the individual's lower extremities, e.g., the sole of one's foot, and a displacement sensor which senses any movement of the body part. The displacement sensor continuously sends signals to a processor of the apparatus which indicate whether there was any movement of the body part. If the displacement sensor did not sense any substantial movement of the body part for a predetermined period of time the processor sends a signal to a vibrating mechanism. The signal activates the vibrating mechanism causing vibration of the body part for increasing blood and fluid flow in the lower extremities. The vibrating mechanism causes vibration for a predetermined period of time.
Owner:桑尼研究基金会
Beta 2 adrenoceptor antagonists for treating orthostatic hypotension
InactiveUS9974759B2Elevated sympathetic toneEffectively reduce BPOrganic active ingredientsΒ2 adrenoceptorsAdrenergic
Methods of treating orthostatic hypotension are disclosed. The methods include administering to a subject in need thereof an effective amount of a beta 2 (β2) adrenoceptor antagonist, and in particular, the specific β2 adrenoceptor antagonist, 3-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol.
Owner:INDIANA UNIV RES & TECH CORP
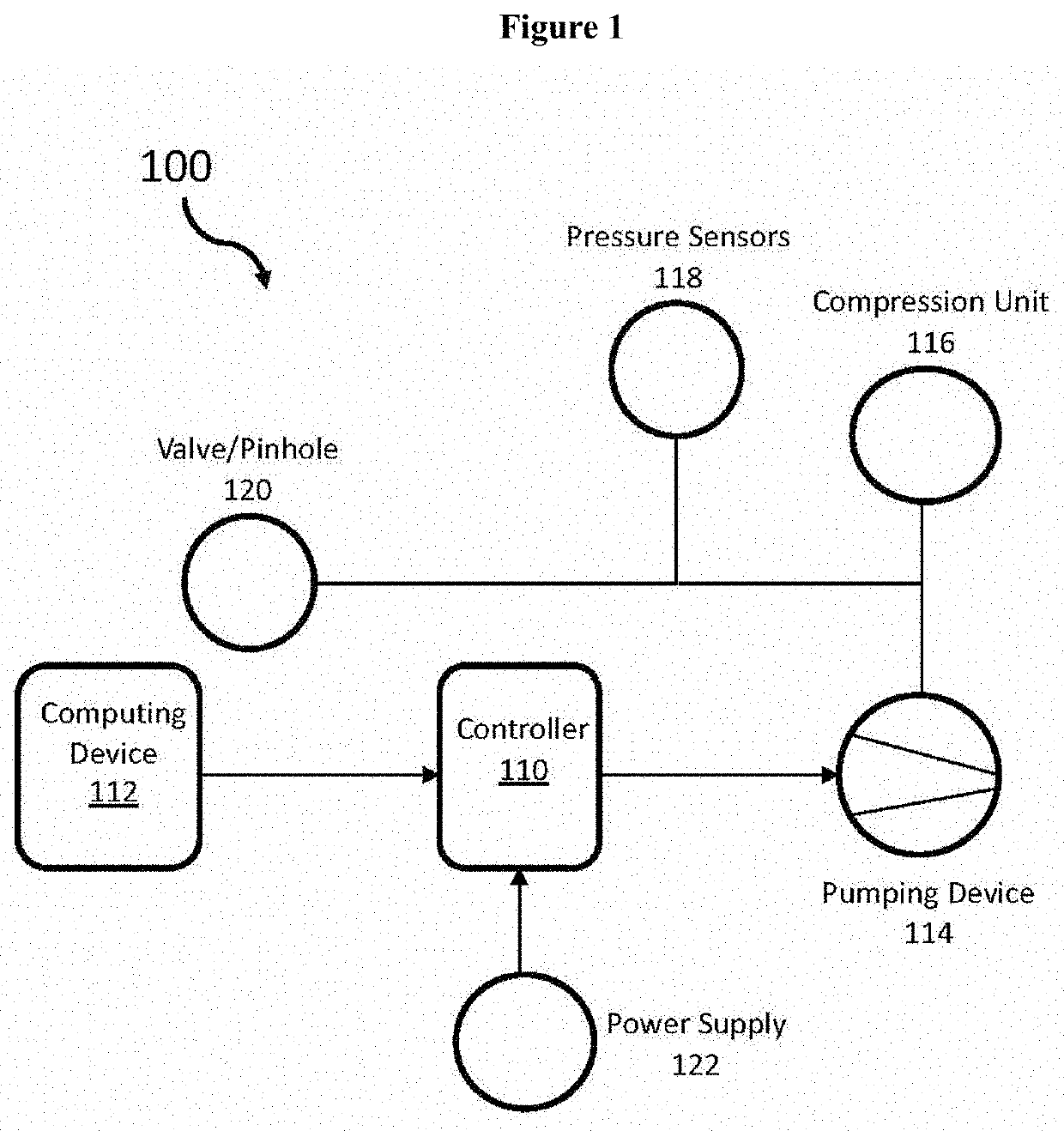
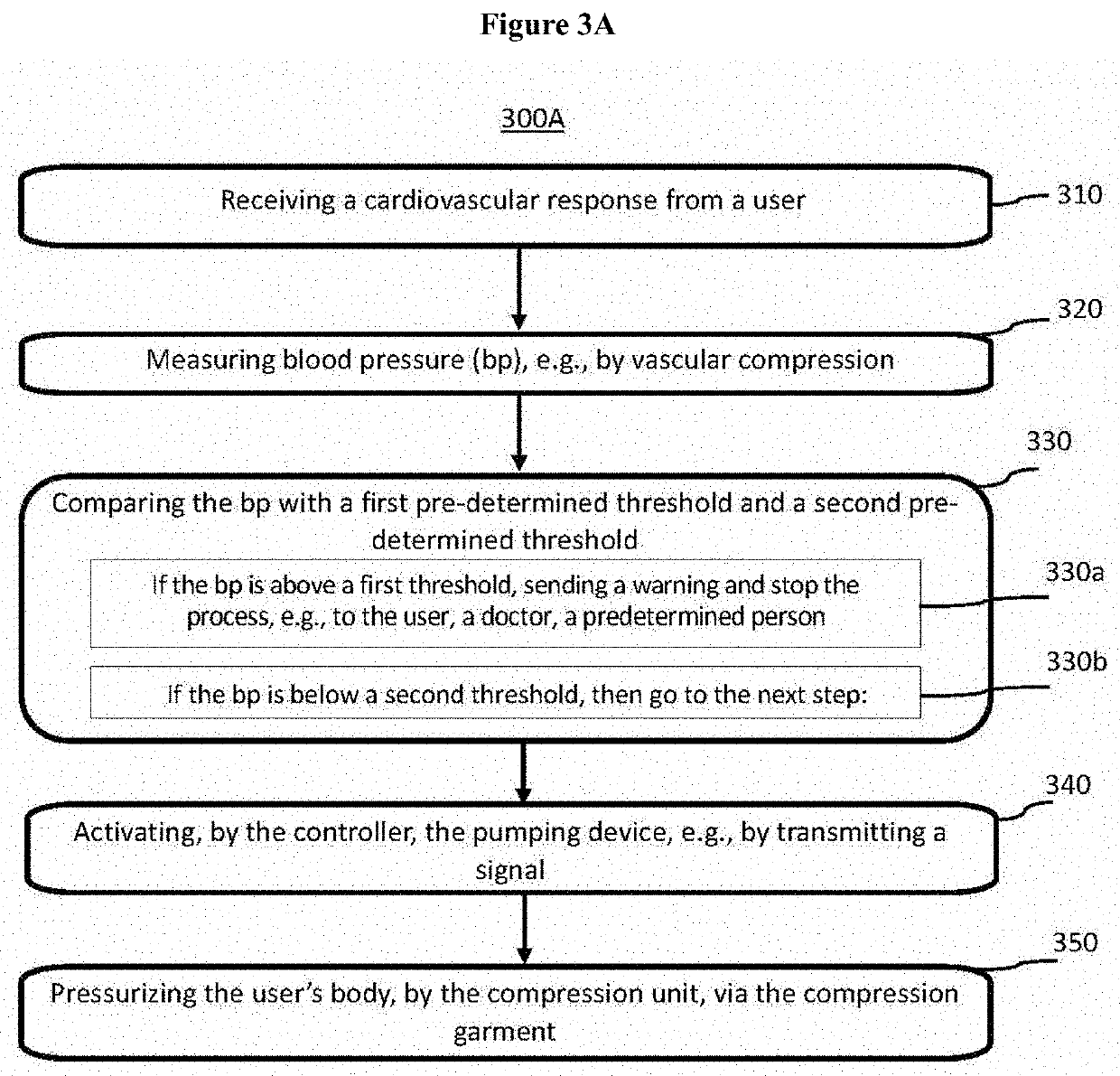
Dynamic compression garment and uses thereof
PendingUS20210106470A1Blood stagnation preventionMechanical/radiation/invasive therapiesBiometric dataVenous pressure
The subject matter presented herein provides an efficacious system and method for diagnosing orthostatic intolerance and for treating orthostatic hypotension. A dynamic compression garment that comprises a servo-controlled splanchnic venous compression with automated binder system is used to regulate blood flow, for instance, during orthostasis. The system includes a programmable controller and a computing device in communication with the controller, an inflator, one or more sensors, and a power supply. The system can collect a user's biometric data, which may be transmitted to the user, a physician in charge of the user, or a third-party. Moreover, the biometric data may be incorporated into a machine learning model that can be used to further program the controller.
Owner:SHAHIDI RAMIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com