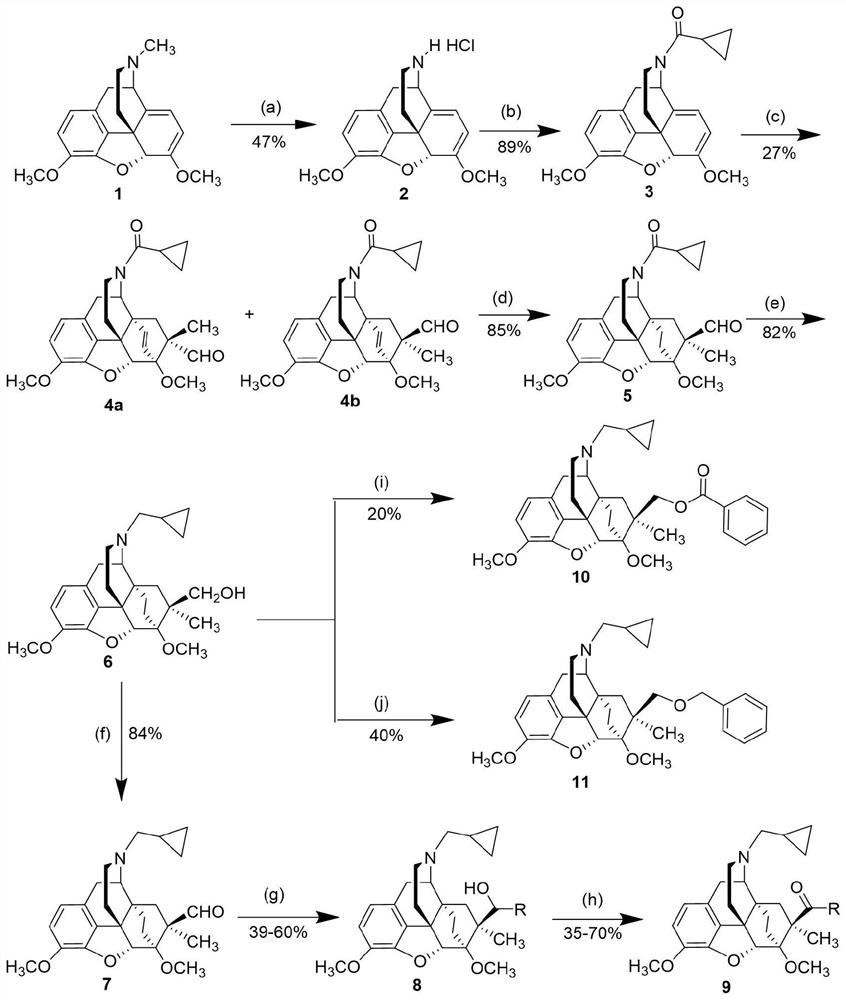
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Morphine derivatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
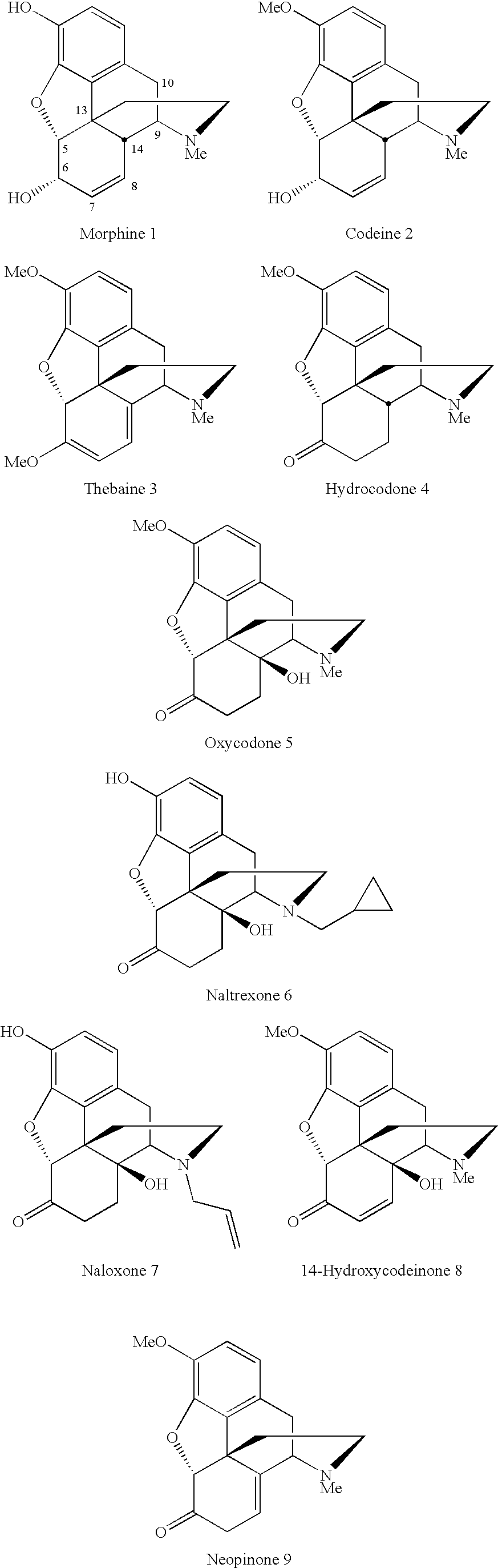
The most notorious derivative of morphine is heroin. It is synthesised by acetylation of the two hydroxyl groups of morphine with acetyl chloride, hence its other names, diacetylmorphine or diamorphine.
Effervescent granules and methods for their preparation
InactiveUS6649186B1Improve stabilityParticular regionPowder deliveryNervous disorderHYDROMORPHONE HYDROCHLORIDEAlpha adrenergic blockade
Disclosed here are effervescent granules having a controllable rate of effervescence. In some embodiments, the such granules comprise an acidic agent, an alkaline agent, a pharmacologically active agent, hot-melt extrudable binder capable of forming a eutectic mixture with the acidic agent and, optionally, a plasticizer. The effervescent granules are made by a hot-melt extrusion process. The present invention also provides a thermal heat process for preparing a pharmacologically active agent containing effervescent granule. In certain aspects, the granules contain pharmacologically active agents such as narcotics, antidiarrheal agents, antiviral agents, anxiolytic agents, a cholesterol lowering agent, an alpha adrenergic blocking agent, a phenanthrene derivative. By way of example, some of the narcotics that may be included in the granules and in the process of preparing the granules include, by way of example: phenanthrene derivatives (e.g., morphine sulfate), and morphine derivatives (e.g., hydromorphone hydrochloride).
Owner:ETHYPHARM SA
Effervescent granules and methods for their preparation
InactiveUS6488961B1Improve stabilityParticular regionPowder deliveryBiocideAnesthetic AgentHYDROMORPHONE HYDROCHLORIDE
Disclosed here are effervescent granules having a controllable rate of effervescence. In some embodiments, the such granules comprise an acidic agent, an alkaline agent, a pharmacologically active agent, hot-melt extrudable binder capable of forming a eutectic mixture with the acidic agent and, optionally, a plasticizer. The effervescent granules are made by a hot-melt extrusion process. The present invention also provides a thermal heat process for preparing a pharmacologically active agent containing effervescent granule. In certain aspects, the granules contain pharmacologically active agents such as narcotics, antidiarrheal agents, antiviral agents, anxiolytic agents, a cholesterol lowering agent, an alpha adrenergic blocking agent, a phenanthrene derivative. By way of example, some of the narcotics that may be included in the granules and in the process of preparing the granules include, by way of example: phenanthrene derivatives (e.g., morphine sulfate), and morphine derivatives (e.g., hydromorphone hydrochloride).
Owner:ETHYPHARM SA
Dosage form containing a morphine derivative and another drug
InactiveUS20050232987A1Extended maintenance periodBiocidePill deliveryPharmaceutical drugBlood plasma
A pharmaceutical dosage form which comprises a first drug which comprises at least one morphine derivative with antitussive activity and at least one second drug. The dosage form provides a plasma concentration within the therapeutic range of the at least one second drug over a period which is coextensive with at least about 70% of the period over which the dosage form provides a plasma concentration within the therapeutic range of the first drug. This abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Morphine derivatives with analgesic activity
PCT No. PCT / GB96 / 03193 Sec. 371 Date Dec. 8, 1998 Sec. 102(e) Date Dec. 8, 1998 PCT Filed Dec. 20, 1996 PCT Pub. No. WO97 / 22606 PCT Pub. Date Jun. 26, 1997Morphine derivatives of formula (I), process for perparing them and their use and use as analgesics.
Owner:GENES LTD
Conversion of thebaine to morphine derivatives
The present invention provides methods for the conversion of thebaine to a morphine derivative, such as hydrocodone. Novel ketal intermediates of the conversion are provided. A one-pot procedure for the conversion comprises treating thebaine with an acid in the presence of a metal catalyst.
Owner:BROCK UNIVERSITY
Conversion of thebaine to morphine derivatives
The present invention provides methods for the conversion of thebaine to a morphine derivative, such as hydrocodone. Novel ketal intermediates of the conversion are provided. A one-pot procedure for the conversion comprises treating thebaine with an acid in the presence of a metal catalyst.
Owner:BROCK UNIVERSITY
Preparation process for gas phase corosion inhibitor of morpholine adrivative
The invention is a morphine derivative gas-phase corrosion inhibitor preparing method, including the steps: mixing morphine with secondary amine, dropping in formaldehyde or aldehyde solution, making them uniformly mix and react, making acid curing reaction on the mixed solution and fatty acid / benzene carbonic acid, stirring, filtering and obtaining the object product. The raw materials such as morphine, secondary amine, formaldehyde / aldehyde, fatty acid and benzene carbonic acid are all easy to obtain and cheap, and suitable for large-scale industrialized production. The preparing course has only three steps and is relative simple, all the reactions can be completed on routine conditions, and the requirements for devices are low, thus further reducing the preparation cost. In addition, all the anticorrosive experiments show that the object product has excellent corrosion inhibiting property.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
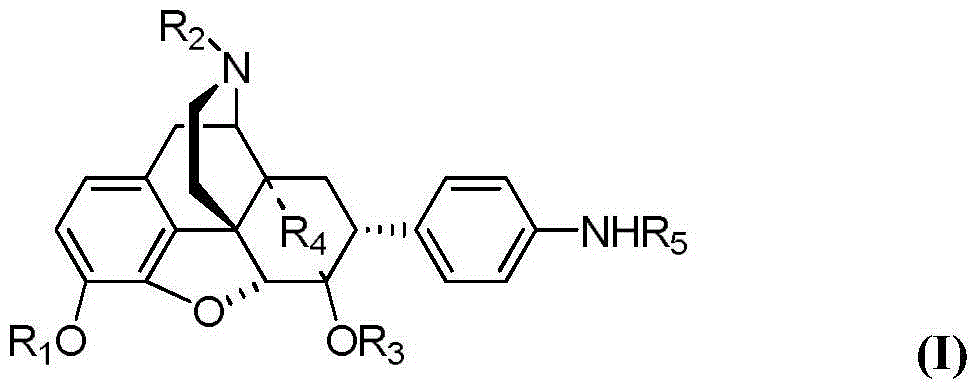
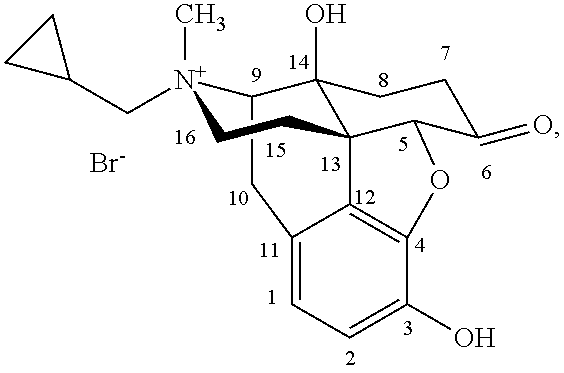
Morphinan derivative
A morphinan derivative represented by the following general formula (I):(in the formula, R1 represents hydrogen, C1-6 alkyl, cycloalkylalkyl (the cycloalkyl moiety has 3 to 6 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms), aralkyl (the aryl moiety has 6 to 10 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms) and the like,R2 represents hydrogen, C1-6 alkyl, C3-6 cycloalkyl, C6-10 aryl, aralkyl (the aryl moiety has 6 to 10 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms) and the like,R3, R4 and R5 represent hydrogen, hydroxy, carbamoyl, C1-6 alkoxy, C6-10 aryloxy and the like,R6a, R6b, R7, R8, R9, and R10 represent hydrogen and the like,X represents O or CH2, andY represents C═O, SO2, an atomic bond and the like), or an acid addition salt thereof is used as an analgesic.
Owner:NIPPON CHEMIPHAR CO LTD
Morphinan derivative
A morphinan derivative represented by the following general formula (I)(in the formula, R1 represents hydrogen, C1-6 alkyl, cycloalkylalkyl (the cycloalkyl moiety has 3 to 6 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms), aralkyl (the aryl moiety has 6 to 10 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms) and the like,R2 represents hydrogen, C1-6 alkyl, C3-6 cycloalkyl, C6-10 aryl, aralkyl (the aryl moiety has 6 to 10 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms) and the like,R3, R4 and R5 represent hydrogen, hydroxy, carbamoyl, C1-6 alkoxy, C6-10 aryloxy and the like,R6a, R6b, R7, R8, R9, and R10 represent hydrogen and the like,X represents O or CH2, andY represents C═O, SO2, an atomic bond and the like), or an acid addition salt thereof is used as an analgesic.
Owner:NIPPON CHEMIPHAR CO LTD
Intermediate as well as preparation method and application thereof
PendingCN113845478AEfficient conversionHigh yieldOrganic chemistryBulk chemical productionMorphinansPharmaceutical Substances
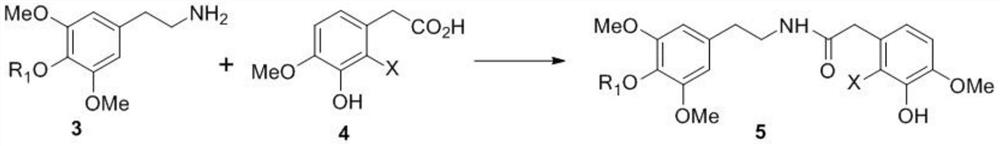
The invention relates to the field of medicine synthesis, and discloses an intermediate or application of a preparation method of the intermediate in total synthesis of morphine derivatives. The asymmetric hydrogenation reaction catalyzed by transition metal and the intramolecular Heck reaction in the process of preparing the intermediate are used as key reactions of total synthesis, and efficient preparation of the morphine derivative, especially representative oxycodone, is realized; wherein the total synthesis method has the advantages of excellent chemical and enantioselectivity, high synthesis efficiency, simple operation and large-scale amplification; meanwhile, the prepared intermediate product (compound 13) can be used for preparing other various morphine derivatives only through simple modification and conversion, and the method has important practical significance in changing the current single industrial production mode of depending on artificial planting, extraction and semi-synthesis of morphine derivative drugs of poppy and guaranteeing supply of morphine drugs.
Owner:SICHUAN UNIV
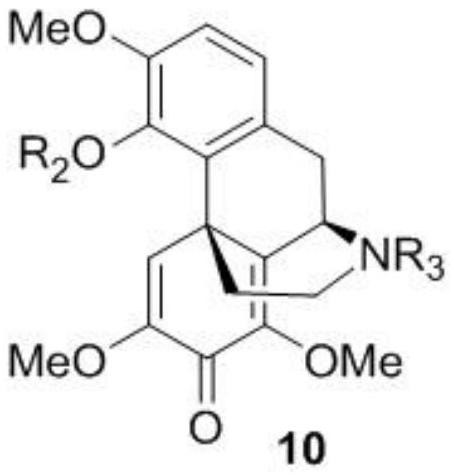
Fused ring morphines derivative, and preparation method therefor and application thereof
The invention belongs to the field of pharmacy, and relates to a fused ring morphines derivative, and a preparation method therefor and application thereof. The invention particularly relates to 7 alpha-(4'-substituted amino-phenyl)-6 alpha, 14 alpha-endo-ethylidene (alkenyl)-tetrahydrothebaine series derivatives of a general formula (I) and pharmaceutically acceptable salts. The derivatives of the general formula (I) are prepared by reaction of thebaine and substituted styrenes reagents and a series of conversion, and 7 alpha-(4'-benzamido-phenyl)-6 alpha, 14 alpha-endo-ethylidene (alkenyl)-tetrahydrothebaine is a typical high-selectivity k ligand, and can be used for preparing drugs for stopping pain, resisting depression, resisting opiate addiction, relieving itching and the like. The formula is as shown in the specification.
Owner:FUDAN UNIV
Deuterated morphine derivatives
ActiveUS20160052931A1Broad utilityReduce riskHeavy metal active ingredientsBiocideNormorphineΜ-opioid receptor
The invention relates to new morphine derivatives deuterated at the 7,8-position of the morphine ring, furthermore to a process for the preparation thereof, and to pharmaceutical compositions comprising them. The new deuterated morphine derivatives show high and selective μ-opioid receptor binding activity leading to the benefit of higher analgesic activity at lower dosages inducing thereby reduced adverse effects compared to the hydrogenated derivatives. The compounds of the invention are useful for example in the treatment of pain or can be used as antitussive agents with a reduced risk of the possibility of drug abuse.
Owner:SZEGEDI TUDOMANYEGYETEM
Synthetic method of morphine derivative and applications
ActiveCN107964030AQuality improvementSugar derivativesComponent separationResearch qualityMedicinal chemistry
The invention discloses a synthetic method of a morphine derivative. The method employs morphine as an initial raw material, and selective protection and glycosylation are carried out in order to obtain a target product. The HPLC purity of M3G obtained by the synthetic method reaches 99.8%, and the M3G can be used as a reference substance or a standard substance for researching quality of relatedproducts.
Owner:YICHANG HUMANWELL PHARMA
Deuterated morphine derivatives
ActiveUS9447108B2Wide range of usesHigh and selective μ-opioid receptor activityOrganic active ingredientsNervous disorderΜ-opioid receptorSubstance abuse
The invention relates to new morphine derivatives deuterated at the 7,8-position of the morphine ring, furthermore to a process for the preparation thereof, and to pharmaceutical compositions comprising them. The new deuterated morphine derivatives show high and selective μ-opioid receptor binding activity leading to the benefit of higher analgesic activity at lower dosages inducing thereby reduced adverse effects compared to the hydrogenated derivatives. The compounds of the invention are useful for example in the treatment of pain or can be used as antitussive agents with a reduced risk of the possibility of drug abuse.
Owner:SZEGEDI TUDOMANYEGYETEM
Morphinan derivatives and preparation methods thereof
InactiveUS8563721B2Simple processDifficult to purifySilicon organic compoundsMorphinanMorphinan derivatives
Owner:CHONGQING PHARMA RES INST
Morphinan derivative
ActiveUS20180057493A1Good effectSuppression of gastrointestinal motilityOrganic active ingredientsSenses disorderHydrogenHeteroatom
A morphinan derivative represented by the following general formula (I):(wherein R1 represents hydrogen, C1-10 alkyl, cycloalkylalkyl where the cycloalkyl moiety has 3 to 6 carbon atoms, and the alkylene moiety has 1 to 5 carbon atoms, etc.,R2 represents heterocyclic ring containing 1 to 4 heteroatoms selected from N, O and S and at least one carbon atom as ring-constituting atoms, containing at least one set of adjacent ring-constituting atoms bound by a double bond, and further substituted with at least one oxo group,Y binds to a carbon atom as a ring-constituting atom of R2,R3, R4, and R5 represent hydrogen; hydroxy, etc.,R6a and R6b represent hydrogen, etc.,R7 and R8 represent hydrogen, etc.,R9 and R10, which are the same or different, represent hydrogen, etc.,X represents O or CH2, andY represents C(═O)),a tautomer or stereoisomer of the compound, or a pharmaceutically acceptable salt thereof, or a solvate thereof is used as an anxiolytic drug, antidepressant, etc.
Owner:NIPPON CHEMIPHAR CO LTD
Novel morphine derivatives
The present invention relates to novel morphine-6-glucuronide derivatives, to pharmaceutical compositions containing them and to uses thereof. Said derivatives have the following structures, where the group Pcntite (A), except substituents X, is called MR36G-NR1 R2-S-, R1 = saturated or unsaturated, straight- or branched-chain C1-C10 alkyl, the alkyl chain being optionally interrupted by one or more heteroatoms selected from O, S and N, R2 = H, saturated or unsaturated, straight- or branched-chain C1-C5 alkyl, or an aryl, heteroaryl or (C1-C5) alkylaryl group, R3 = Y(C=Z)R or YR, Y and Z independently = O or S, R = saturated or unsaturated, straight- or branched-chain C1-C6 alkyl with the proviso that R3 is not -O-CH3, X = H, an -S-R4-W group, or a MR,6G-NR I R2-S- group, with R4 = saturated or unsaturated, straight- or branched-chain C1 -C8 alkyl which can include amide, ester or ether bonds and W is either a d-receptor antagonist, or a K-receptor antagonist and a pharmaceutically acceptable salt thereof.
Owner:NEORPHYS
Novel morphine derivatives
The present invention relates to novel morphine-6-glucuronide derivatives, to pharmaceutical compositions containing them and to uses thereof. Said derivatives have the following structures. The present invention relates to novel morphine-6-glucuronide derivatives, to pharmaceutical compositions containing them and to uses thereof. Said derivatives have the following structures, where the group Pcntite (A), except substituents X, is called MR36G-NR1 R2-S-, R1 = saturated or unsaturated, straight- or branched-chain C1-C10 alkyl, the alkyl chain being optionally interrupted by one or mogroup Pcntite (A), except substituents X, is called MR36G-NR1 R2-S-, R1 = saturated or unsaturated, straight- or branched-chain C1-C10 alkyl, the alkyl chain being optionally interrupted by one or more heteroatoms selected from O, S and N, R2 = H, saturated or unsaturated, straight- or branched-chain C1-C5 alkyl, or an aryl, heteroaryl or (C1-C5) alkylaryl group, R3 = Y(C=Z)R or YR, Y and Z indepre heteroatoms selected from O, S and N, R2 = H, saturated or unsaturated, straight- or branched-chain C1-C5 alkyl, or an aryl, heteroaryl or (C1-C5) alkylaryl group, R3 = Y(C=Z)R or YR, Y and Z independently = O or S, R = saturated or unsaturated, straight- or branched-chain C1-C6 alkyl with the proviso that R3 is not -O-CH3, X = H, an -S-R4-W group, or a MR,6G-NR I R2-S- group, with R4 = saturatendently = O or S, R = saturated or unsaturated, straight- or branched-chain C1-C6 alkyl with the proviso that R3 is not -O-CH3, X = H, an -S-R4-W group, or a MR,6G-NR I R2-S- group, with R4 = saturated or unsaturated, straight- or branched-chain C1 -C8 alkyl which can include amide, ester or ether bonds and W is either a d-receptor antagonist, or a K-receptor antagonist and a pharmaceutically saturated or unsaturated, straight- or branched-chain C1 -C8 alkyl which can include amide, ester or ether bonds and W is either a d-receptor antagonist, or a K-receptor antagonist and a pharmaceutically acceptable salt thereof.
Owner:NEORPHYS
A kind of fused ring morphine derivative and its preparation method and application
The invention belongs to the field of pharmacy, and relates to condensed ring morphine derivatives, a preparation method and application thereof. It specifically relates to 7α-(4'-substituted amino-phenyl)-6α, 14α-endo-ethenide (en)yl-tetrahydrothebaine series derivatives of general formula (I) and pharmaceutically acceptable salts ; The derivative of the general formula (I) is prepared by reacting thebaine with a substituted styrene reagent and through a series of conversion methods, wherein 7α-(4'-benzamido-phenyl)-6α, 14α-endo ‑Ethylidene‑tetrahydrothebaine is a typical highly selective κ ligand, which can be used to prepare drugs such as analgesia, antidepression, opioid addiction, and antipruritic.
Owner:FUDAN UNIV
7alpha-methyl fused ring morphine derivative as well as preparation method and application thereof
PendingCN114195798AAffinity can be measuredMeasurable selectivityNervous disorderOrganic chemistryMorphinansReceptor
The invention belongs to the field of pharmacy, and relates to a 7alpha-methyl fused ring morphine compound with a formula I and a formula II as well as a preparation method and an application of the 7alpha-methyl fused ring morphine compound. The compounds are prepared through a reaction of thebaine and a substituted styrene reagent and through a series of conversion methods, have opioid receptor binding activity, and multiple compounds including SLL-604, SLL-627 and SLL-631 are selective kappa receptor ligands or mu / kappa dual ligands. The method can be applied to research and development in the treatment fields of pain easing, depression resisting, opioid drug addiction withdrawal, itching relieving and the like.
Owner:FUDAN UNIV
Synthesis method of morphine derivative buprenorphine
PendingCN113956263AHigh chemistryHigh yieldOrganic chemistry methodsBulk chemical productionGrignard reagentTert butyl
The invention belongs to the field of synthesis of chemical drugs, and particularly relates to a novel synthesis method of a morphine derivative buprenorphine. Based on a possible biogenic pathway of morphine derivatives, a thibaine analogue intermediate is synthesized by taking an intramolecular oxidation dearomatization Heck reaction as a key reaction through a biomimetic synthesis strategy, and by starting from the intermediate through a Diels-Alder reaction, catalytic hydrogenation, addition with a tert-butyl Grignard reagent, removal of a secondary amine protecting group, introduction of cyclopropyl methyl, and removal of methyl and the like, efficient synthesis of the morphine derivative buprenorphine is realized. Compared with the existing route, the synthesis route has the advantages of few steps, high total yield and obvious reduction of the production cost.
Owner:SICHUAN UNIV
Improved process for making apomorphine and apocodeine
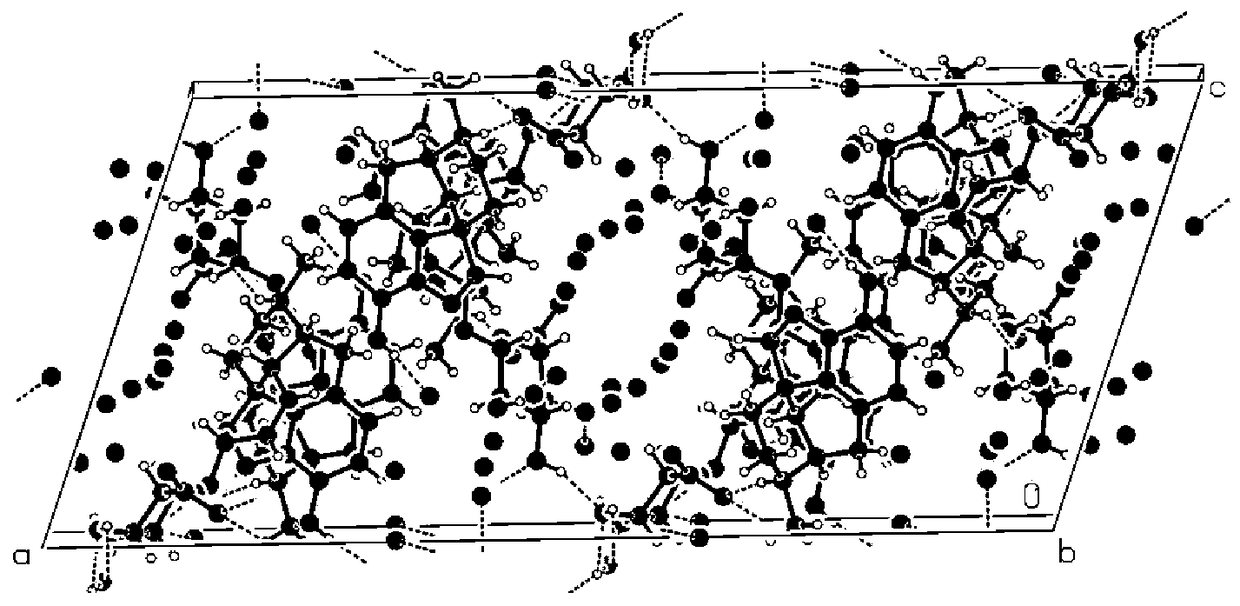
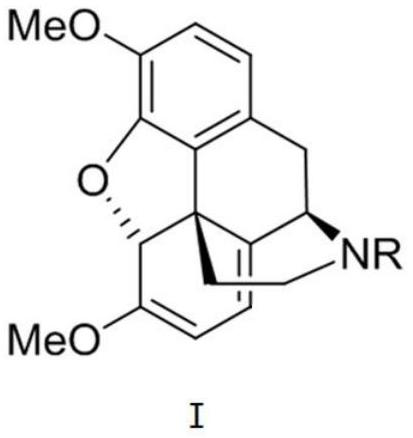
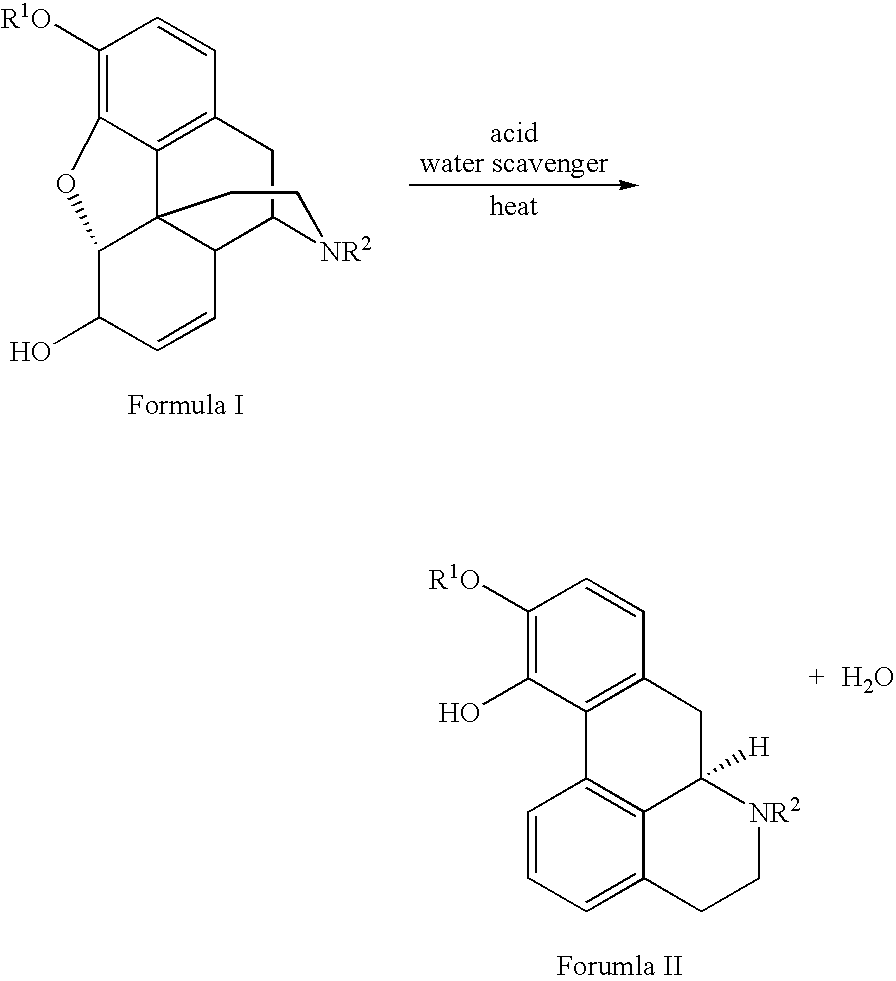
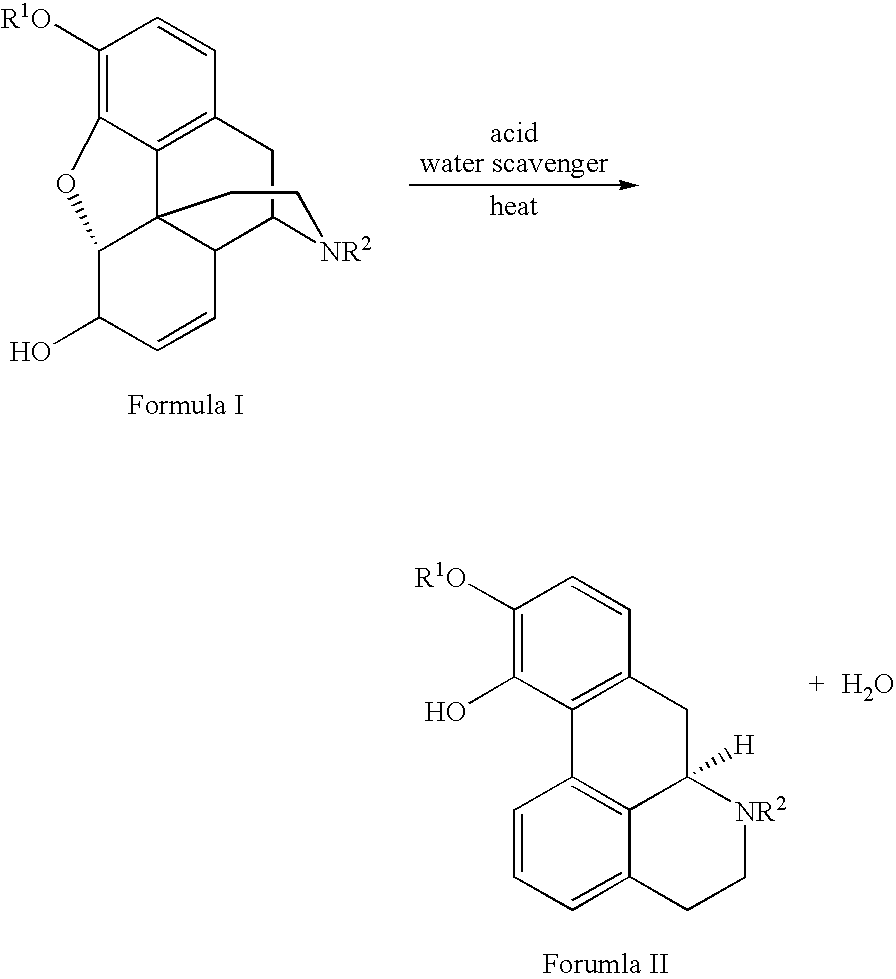
Provided is an improved and convenient process for the synthesis of aporphines, such as apomorphine and apocodeine, by the rearrangement of the corresponding morphine or codeine derivatives. The use of a suitable water scavenger in an acid catalyzed rearrangement of the morphine derivatives unexpectedly results in a reaction temperature convenient for plant operation without sacrificing product. The method of the present invention also alleviates the cumbersome operations that were employed in the prior art to eliminate water from the reaction mixture at the elevated temperatures. This process is adaptable for the general preparation of other aporphines from the corresponding morphine congeners.
Owner:MALLINCKRODT INC
Preparation process for gas phase corosion inhibitor of morpholine adrivative
The invention is a morphine derivative gas-phase corrosion inhibitor preparing method, including the steps: mixing morphine with secondary amine, dropping in formaldehyde or aldehyde solution, making them uniformly mix and react, making acid curing reaction on the mixed solution and fatty acid / benzene carbonic acid, stirring, filtering and obtaining the object product. The raw materials such as morphine, secondary amine, formaldehyde / aldehyde, fatty acid and benzene carbonic acid are all easy to obtain and cheap, and suitable for large-scale industrialized production. The preparing course has only three steps and is relative simple, all the reactions can be completed on routine conditions, and the requirements for devices are low, thus further reducing the preparation cost. In addition, all the anticorrosive experiments show that the object product has excellent corrosion inhibiting property.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
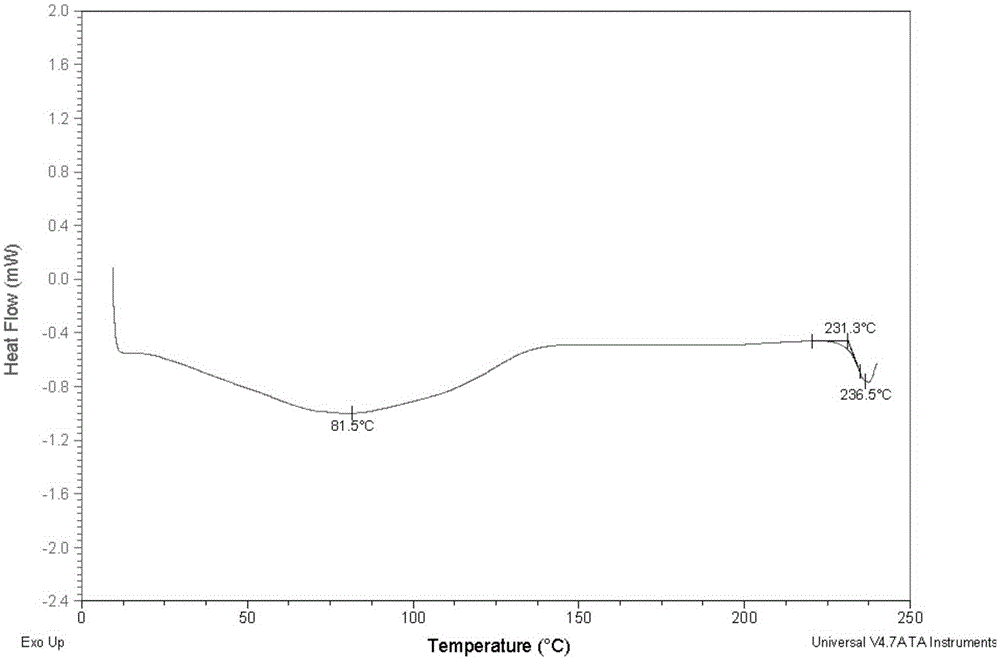
Morphine derivative crystal form III, preparation method, and application thereof
ActiveCN106554377AImprove bioavailabilityHigh purityOrganic active ingredientsSugar derivativesX-rayBioavailability
The invention provides a morphine derivative crystal form III represented as the formula (1). Through Cu-K[alpha] radiation, the crystal form has diffraction peaks in an X-ray powder diffraction diagram with (2[theta] + / - 0.5) being 9.36, 13.24, 14.74, 15.22, 16.22, 17.50, 19.90, 20.92 and 22.98 degrees. In addition, the invention also discloses a preparation method of the crystal form, a medicine composition of the crystal form and an application thereof. The crystal form III, compared with the crystal forms in the prior art, is quick to dissolve, has high bioavailability and is suitable for industrialization.
Owner:YICHANG HUMANWELL PHARMA
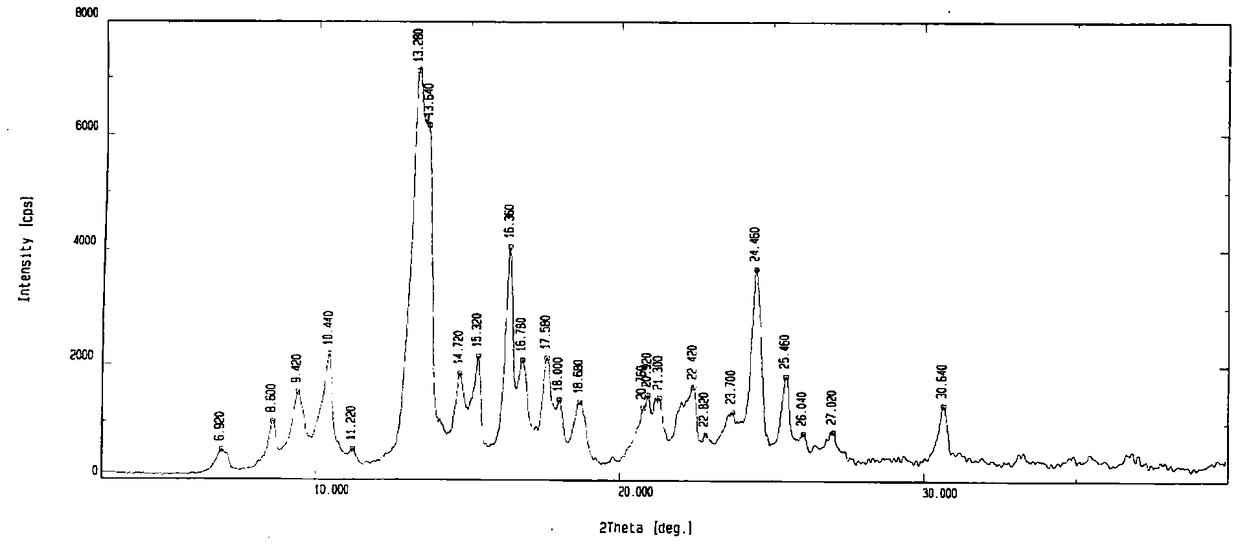
Morphine derivative crystal form I and its preparation method and use
ActiveCN106554357BHigh solubility and bioavailabilityHigh purityOrganic active ingredientsAntipyreticBioavailabilityPowder diffraction
The invention provides a morphine derivative crystal form I as shown in a formula 1. By using CuK alpha to radiate the morphine derivative crystal form I, an X powder diffraction pattern has diffraction peaks at the (2theta + / -0.5) DEG of 9.42, 10.44, 13.28, 14.72, 15.32, 16.36, 24.46 and 25.46. In addition, the invention also discloses a preparation method of the crystal form, a pharmaceutical composition and application thereof. Compared with the known crystal form, the crystal form I of the application has the characteristics of high dissolving speed, high bioavailability and suitability for industrialization.
Owner:YICHANG HUMANWELL PHARMA
A kind of synthetic method and application of morphine derivative
ActiveCN107964030BQuality improvementSugar derivativesComponent separationPerylene derivativesOrganic chemistry
The invention discloses a synthetic method of a morphine derivative. The method employs morphine as an initial raw material, and selective protection and glycosylation are carried out in order to obtain a target product. The HPLC purity of M3G obtained by the synthetic method reaches 99.8%, and the M3G can be used as a reference substance or a standard substance for researching quality of relatedproducts.
Owner:YICHANG HUMANWELL PHARMA
Novel intermediate as well as preparation method and application thereof
ActiveCN114502560AEfficient conversionHigh chemistryOrganic chemistry methodsBulk chemical productionBiomimetic synthesisDrugs synthesis
The invention relates to the field of drug synthesis, in particular to a novel intermediate as well as a preparation method and application thereof, the structural formula of the novel intermediate is as shown in formula I, and in the formula, R is a secondary amine protecting group. Based on a possible biogenic pathway of the morphine derivative, through a biomimetic synthesis strategy, an asymmetric transfer hydrogenation reaction and an intramolecular oxidation dearomatization Heck reaction in a process of preparing the intermediate are taken as key reactions of total synthesis, and efficient synthesis of the morphine derivative is realized. The novel intermediate provided by the invention is used for synthesizing the morphine derivative, and has the characteristics of obviously reducing the synthesis steps, improving the yield, reducing the emission of three wastes and saving the production cost.
Owner:SICHUAN UNIV
Process for Making Apomorphine and Apocodeine
There is provided an improved and convenient process for the synthesis of aporphines, such as apomorphine and apocodeine, by the rearrangement of the corresponding morphine or codeine derivatives. The use of a suitable water scavenger in an acid catalyzed rearrangement of the morphine derivatives unexpectedly results in a reaction temperature convenient for plant operation without sacrificing product. The method of the present invention also alleviates the cumbersome operations that were employed in the prior art to eliminate water from the reaction mixture at the elevated temperatures. This process is adaptable for the general preparation of other aporphines from the corresponding morphine congeners.
Owner:MALLINCKRODT INC
A Stereoselective Synthesis Method of 6β-Hydroxy-7,8-Dihydro-morphine Derivatives
The invention discloses a stereoselective synthesis method of 6β-hydroxyl-7,8-dihydro-morphine derivatives. The synthesis method comprises the following steps: (i) the compound of formula VI is in a hydrophilic organic solvent, In the presence of a catalytic amount of C1-C4 alkanoic acid, it is reduced to a compound of formula VII by sodium borohydride; (ii) the compound of formula VII obtained in step (i) is separated to obtain a compound of formula VIII; here, in formula VI, formula VII and VIII For the definition of substituents, please refer to the specification.
Owner:YICHANG HUMANWELL PHARMA
A kind of stereoselective synthesis method of 6β-hydroxymorphine derivative
ActiveCN110305142BImproving the quality of medicines and formulationsPrecise positioningSugar derivativesSugar derivatives preparationOrganic solventAklanonic acid
The invention discloses a stereoselective synthesis method of 6β-hydroxymorphine derivatives. The synthesis method comprises the following steps: (i) the compound of formula VI is present in a hydrophilic organic solvent in the presence of a catalytic amount of C1-C4 alkanoic acid Next, it is reduced to the compound of formula VII by sodium borohydride; (ii) the compound of formula VII obtained in step (i) is separated to obtain the compound of formula VIII; here, the definitions of substituents in formula VI, formula VII and VIII are detailed in the instructions.
Owner:YICHANG HUMANWELL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com