Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Interleukin 1 receptor antagonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The interleukin-1 receptor antagonist (IL-1RA) is a protein that in humans is encoded by the IL1RN gene. IL-1RA was initially called the IL-1 inhibitor and was discovered separately in 1984 by two independent laboratories. IL-1RA is an agent that binds non-productively to the cell surface interleukin-1 receptor (IL-1R), the same receptor that binds interleukin 1 (IL-1), preventing IL-1 from sending a signal to that cell.
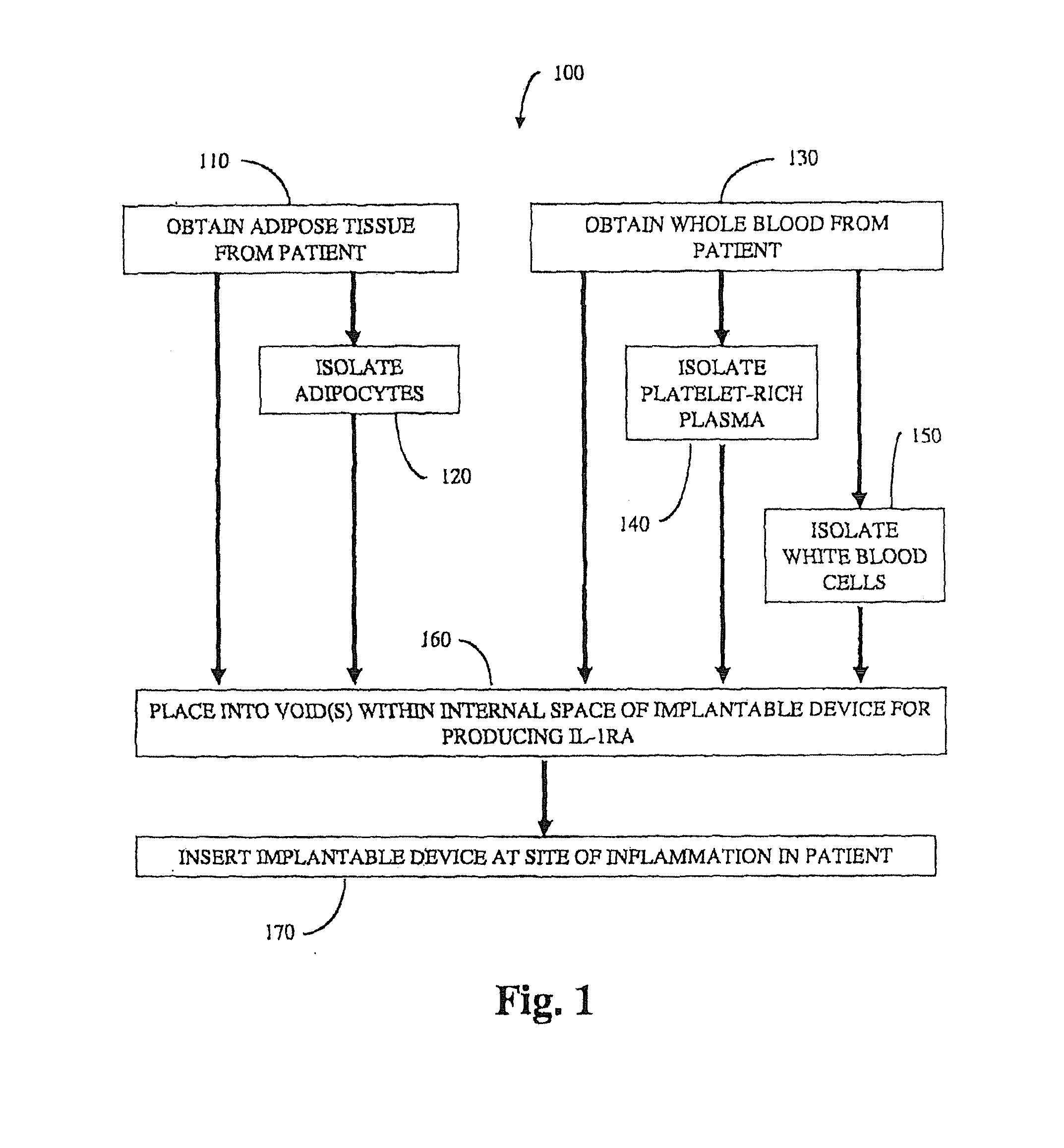
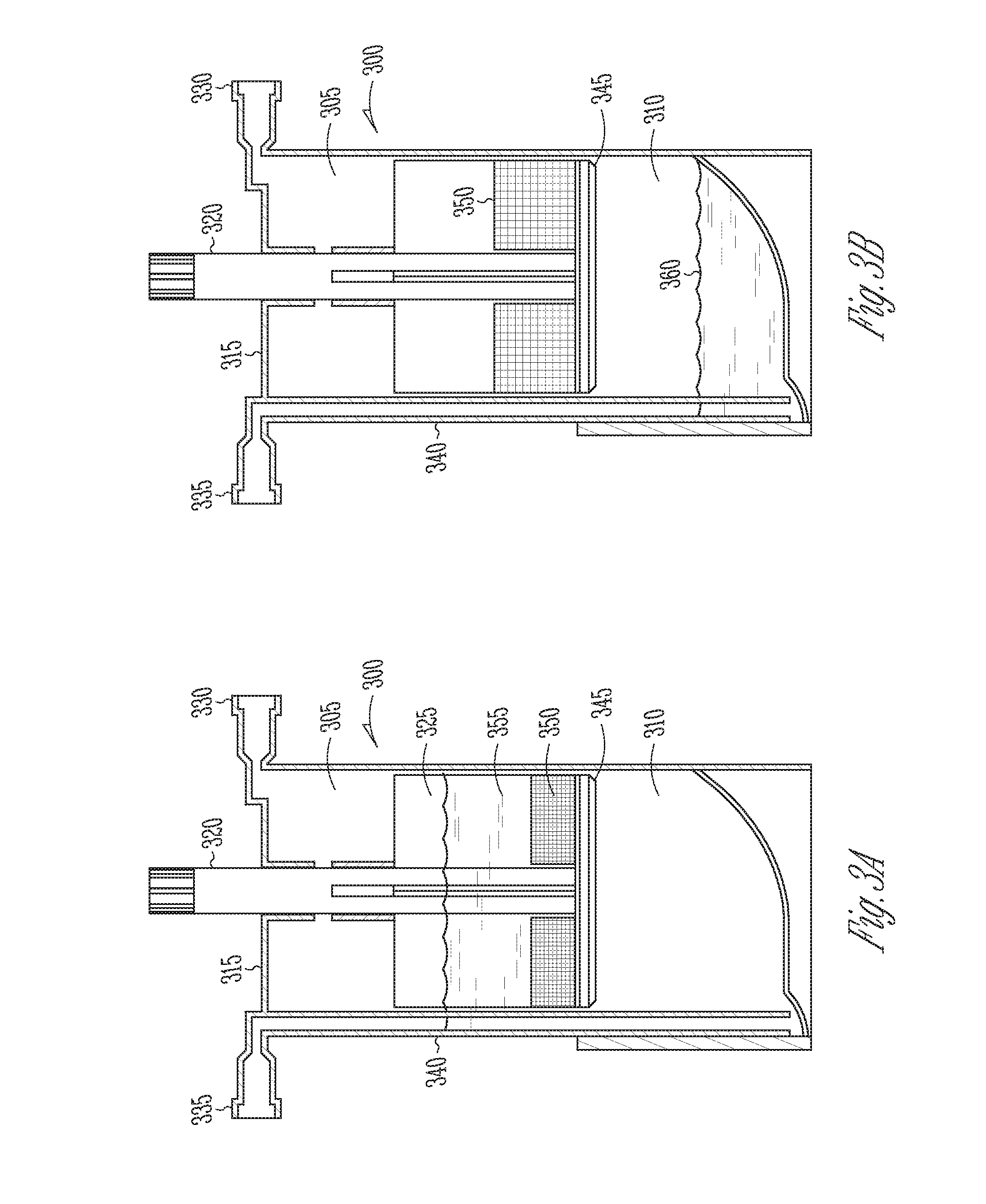
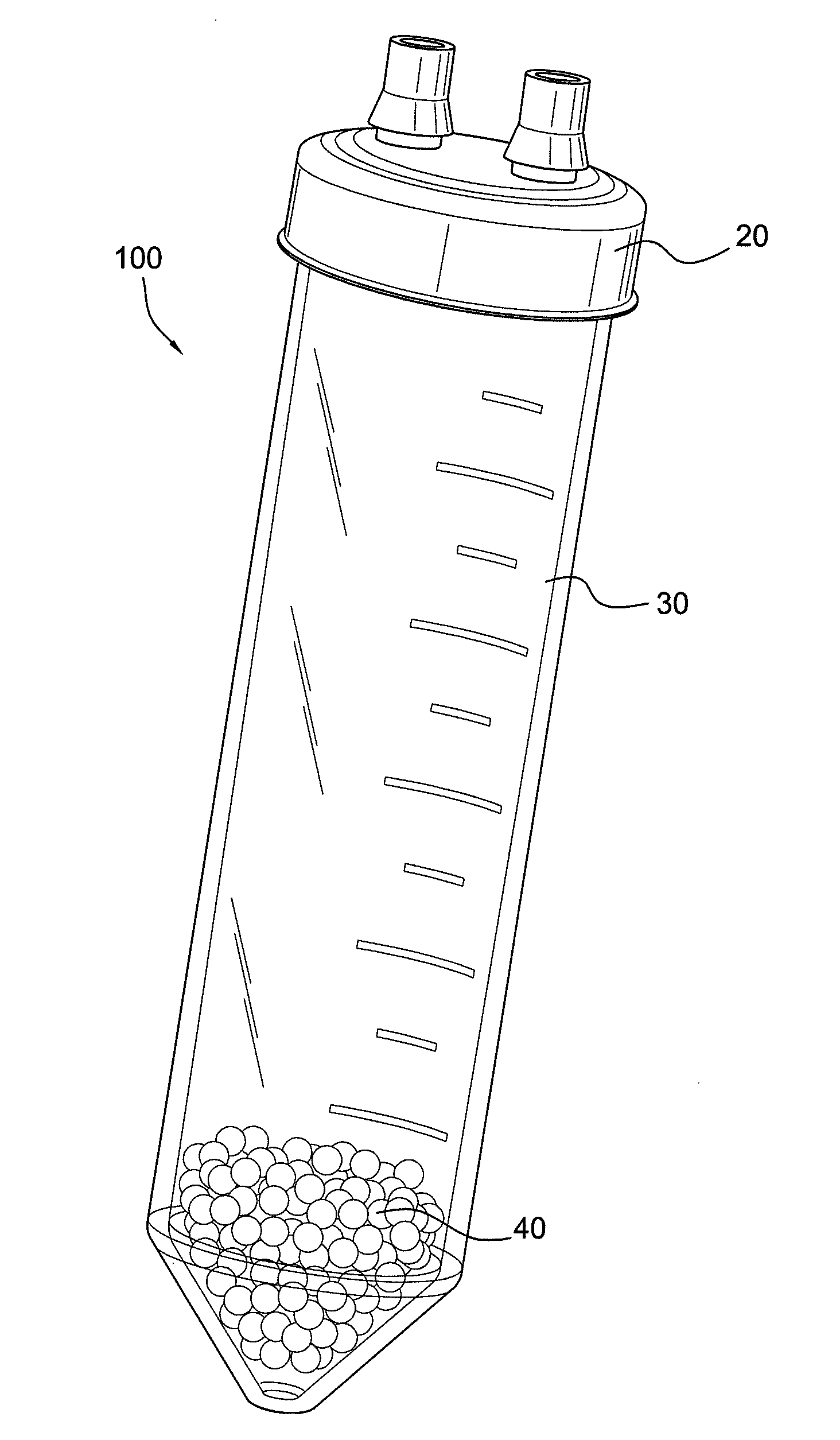
Methods and compositions for delivering interleukin-1 receptor antagonist
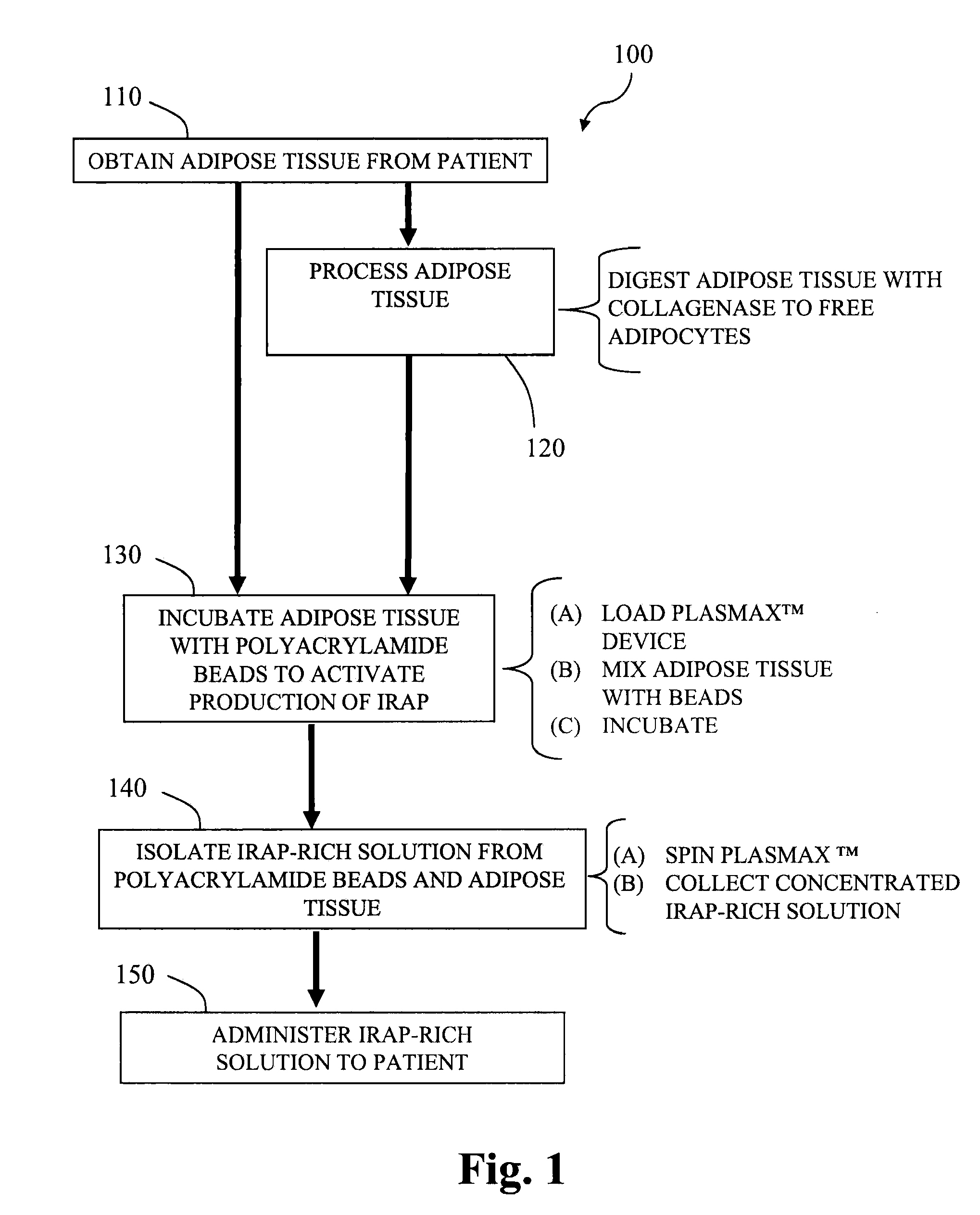
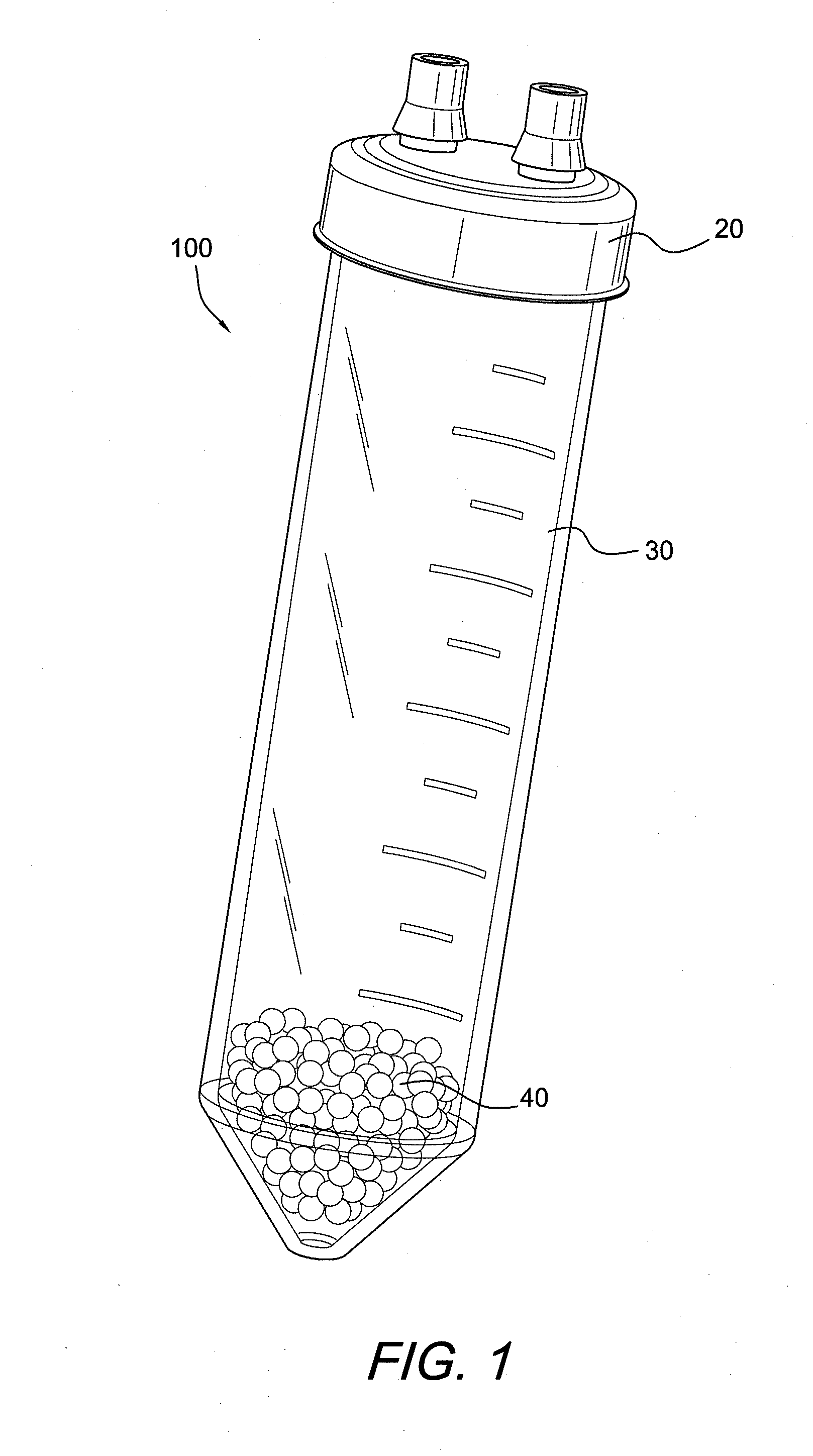
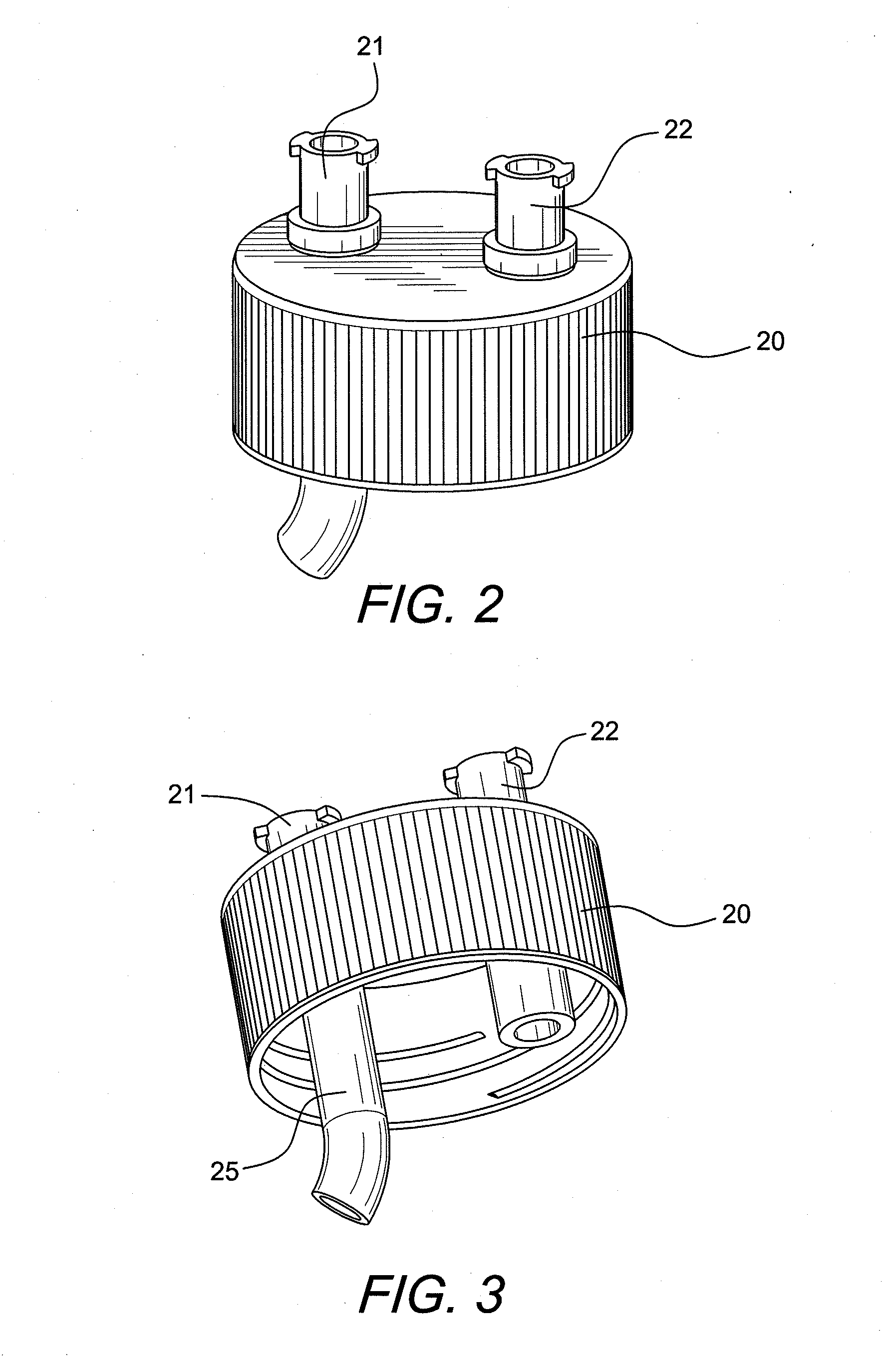
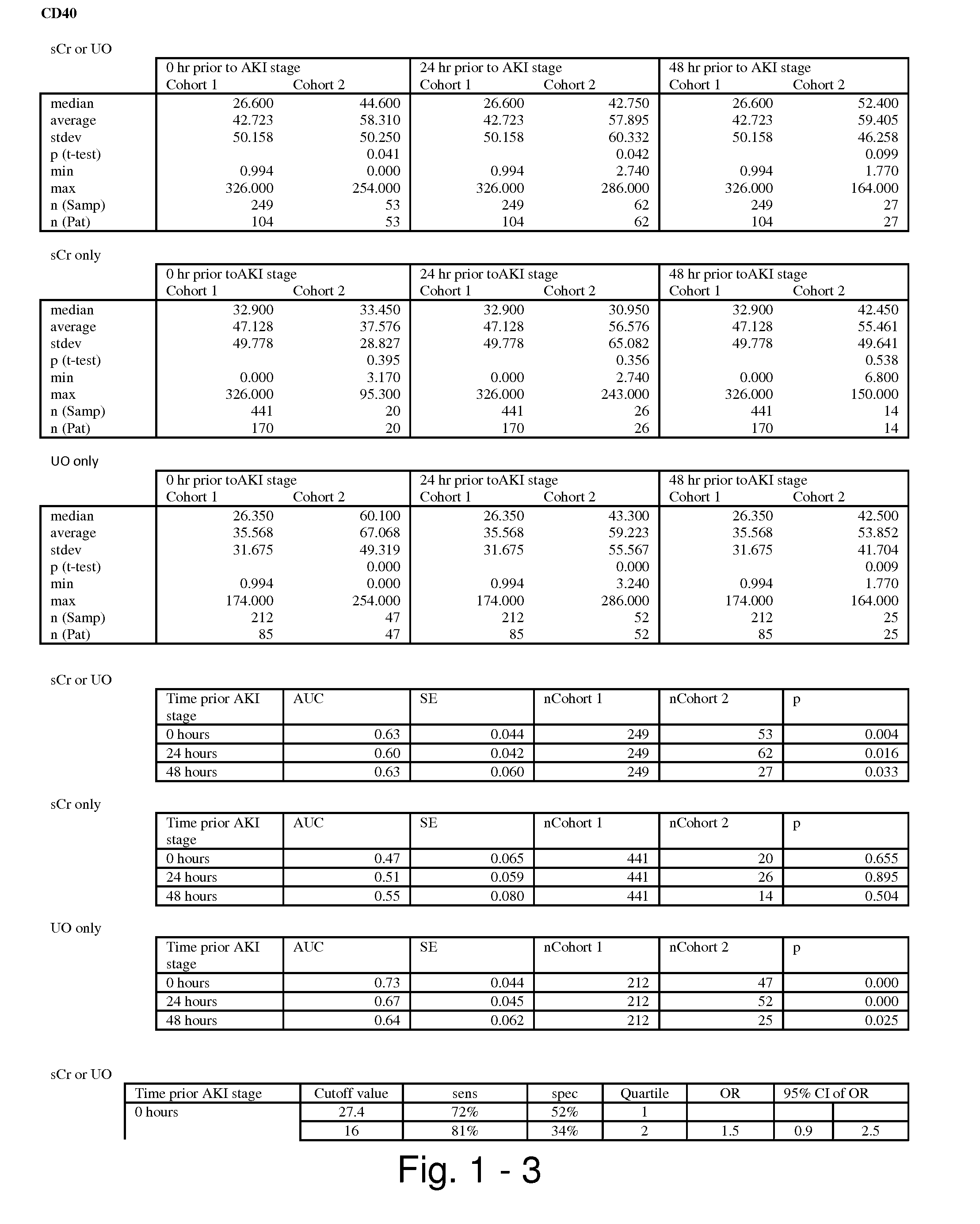
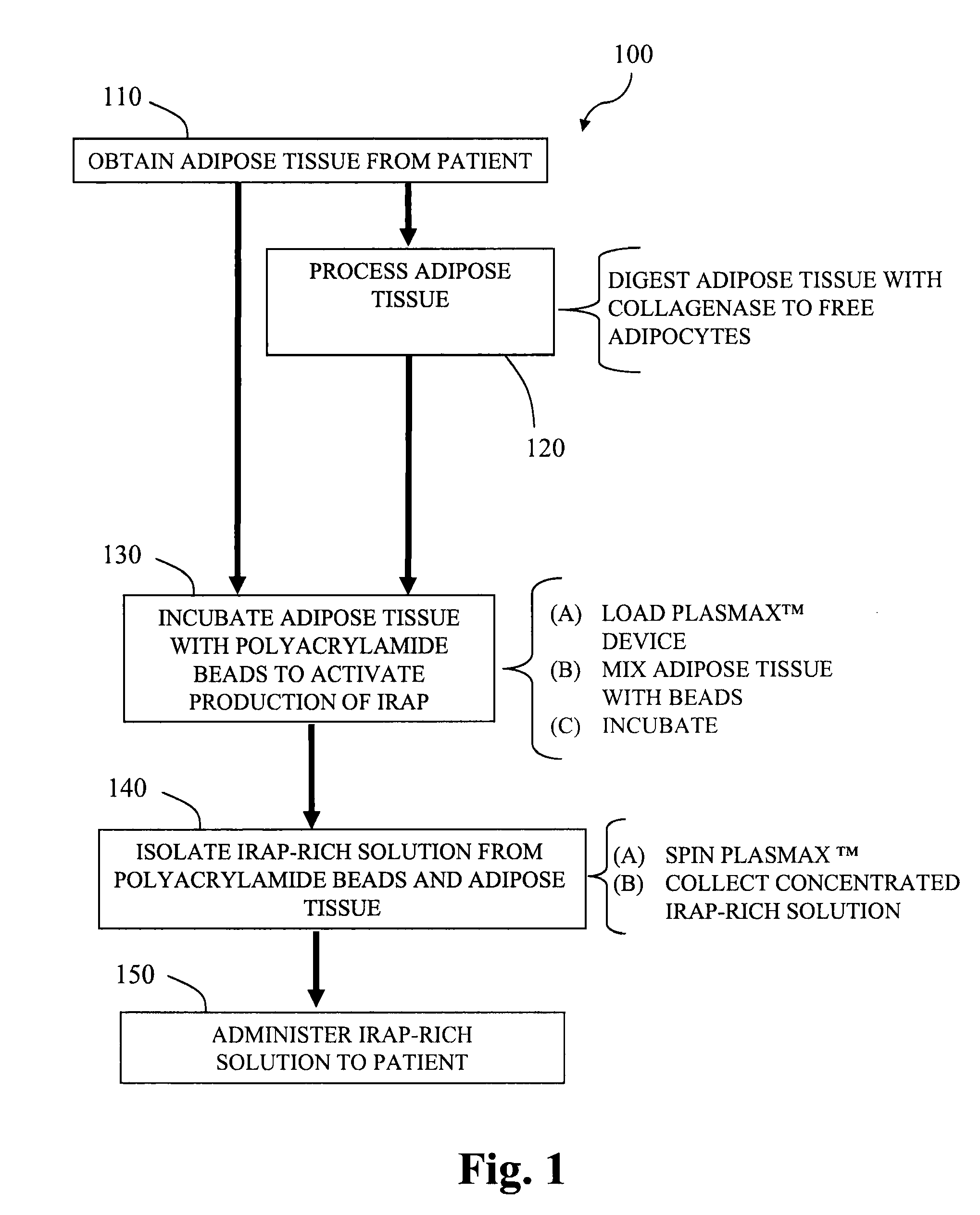
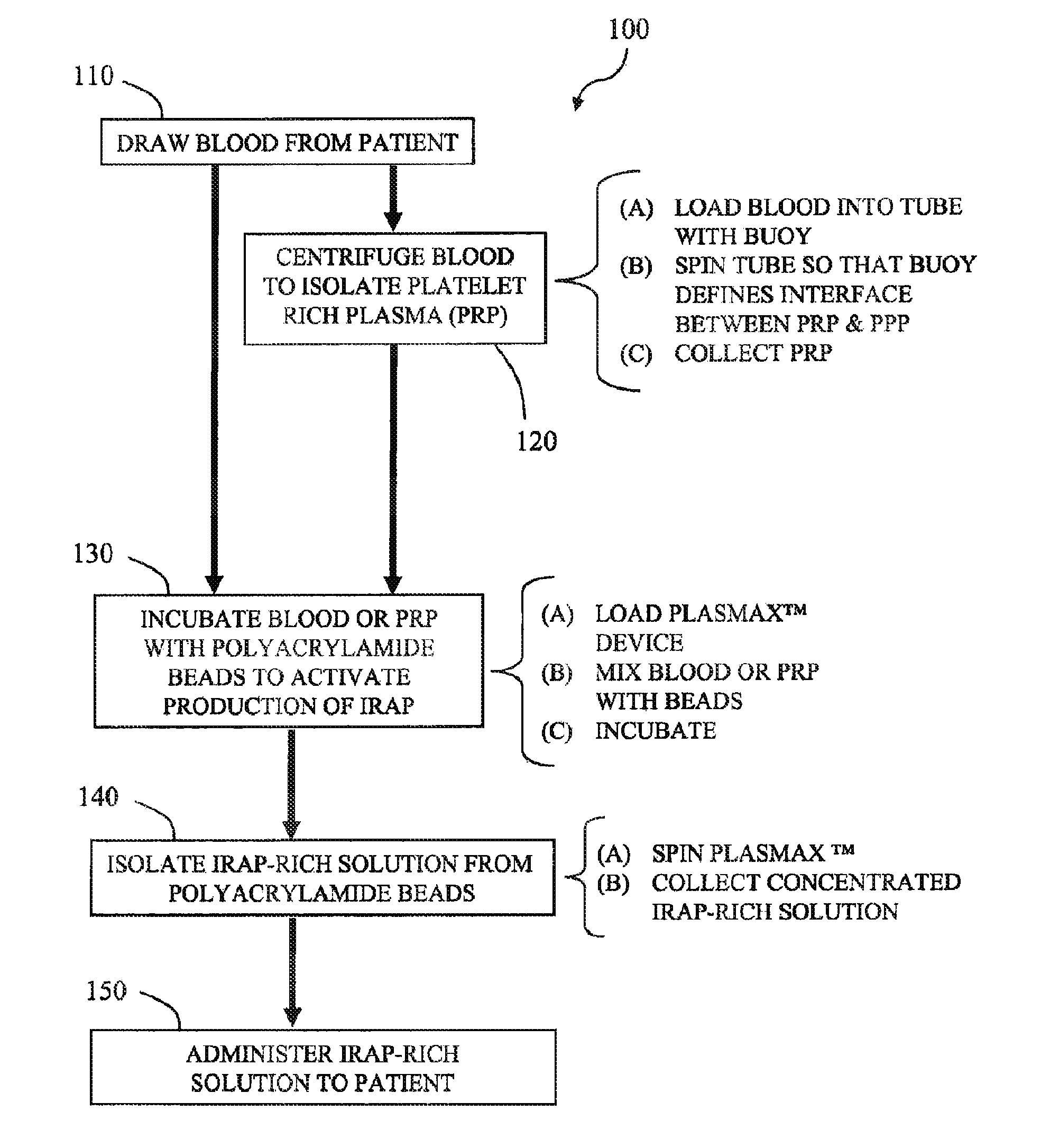
ActiveUS20100055087A1Contribute to degenerationIncrease productionPeptide/protein ingredientsAntipyreticInterleukin 1 Receptor Antagonist ProteinPolyacrylamide
Methods and compositions generating and using an interleukin-1 receptor antagonist (IL-1ra)-rich solution. Methods for generating and isolating interleukin-1 receptor antagonist include incubating adipose tissue and / or adipocytes with polyacrylamide beads to produce interleukin-1 receptor antagonist. The interleukin-1 receptor antagonist is isolated from the polyacrylamide beads to obtain the solution rich in interleukin-1 receptor antagonist. Methods for treating a site of inflammation in a patient include administering to the site of inflammation the solution rich in interleukin-1 receptor antagonist.
Owner:BIOMET MFG CORP
Interleukin-1 receptor antagonists, compositions, and methods of treatment
ActiveUS20060094663A1Synthetic is simpleInhibition of activationNervous disorderPeptide/protein ingredientsArthritisDrug biological activity
Peptides that are designed to inhibit the biological activity of the IL-1R type 1 receptor and inhibit IL-1R / IL-1RacP related cell signaling and biological activity are disclosed. Compositions comprising IL-1R antagonists of the present invention are useful in the treatment of IL-1 related diseases or conditions such as arthritis, rheumatoid arthritis, osteoarthritis, and inflammatory bowel disease as well as other chronic or acute inflammatory diseases. This invention also discloses an isolated compound having an IL-1R antagonist activity, said compound being selected from the group consisting of: a peptide comprising the amino acid sequence RYTPELX, wherein R, Y, T, P, E, L, refer to their corresponding amino acids, and X is selected from no amino acid and alanine (A); and a derivative of (a) wherein the derivative incorporates one, two or three amino acid modification selected from an amino acid addition, deletion or substitution in the RYTPEL portion of the peptide, and wherein said derivative maintains its antagonist IL-1R activity.
Owner:VALORISATION HSJ LLP
Nucleic acids encoding interleukin-1 inhibitors and processes for preparing interleukin-1 inhibitors
Compounds are disclosed having the general formula R1—X—R2, wherein R1 and R2 are biologically active groups, at least one of which is polypeptidic. X is a non-peptidic polymeric group. R1 and R2 may be the same or different. Preferred R1 and R2 groups are interleukin-1 receptor antagonist, 30 kDa TNF inhibitor, interleukin-2 receptors and CR1 and muteins thereof. Also included are site selectively modified interleukin-1 receptor antagonist and 30 kDa TNF inhibitor.
Owner:UNIV OF COLORADO THE REGENTS OF +1
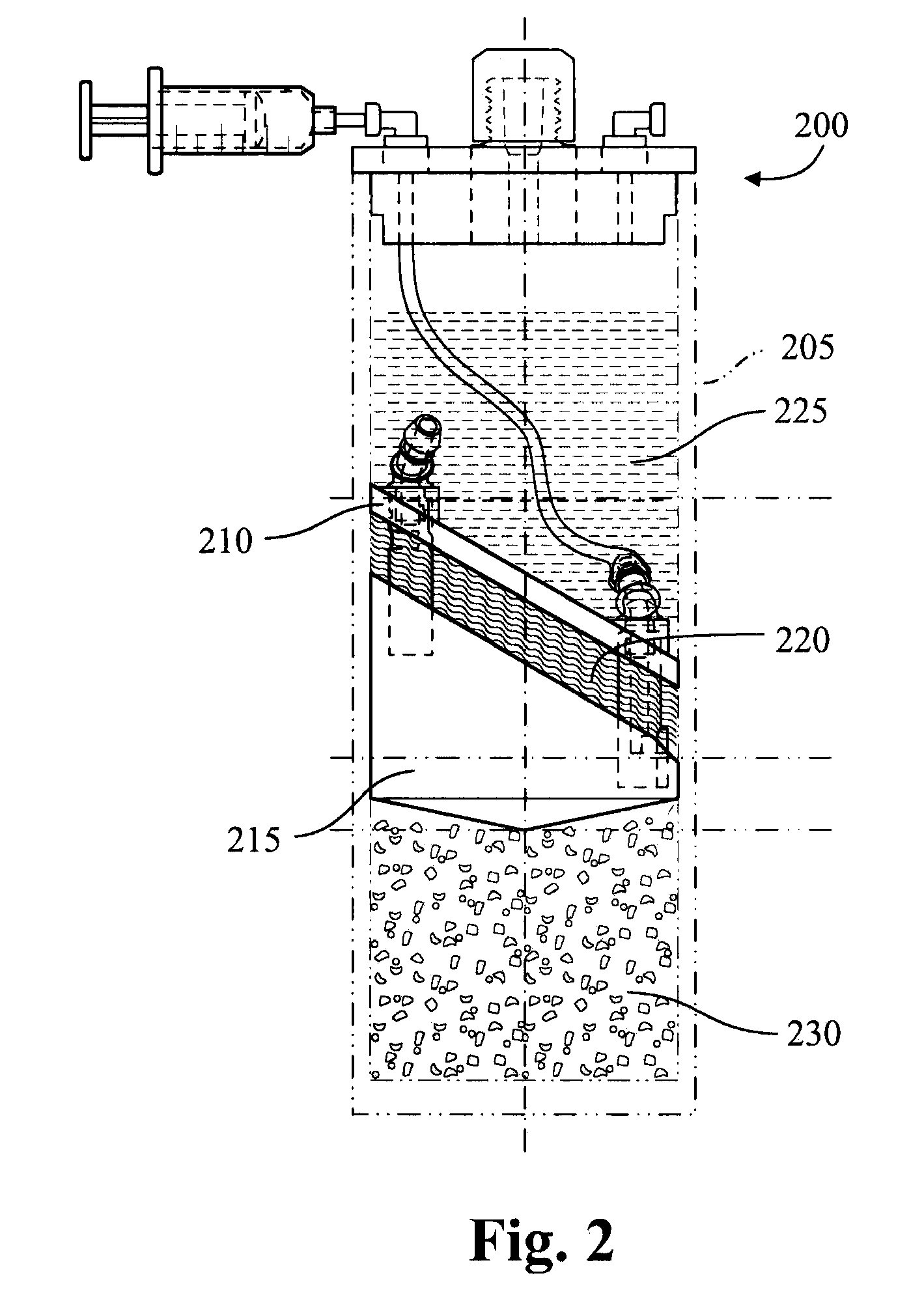
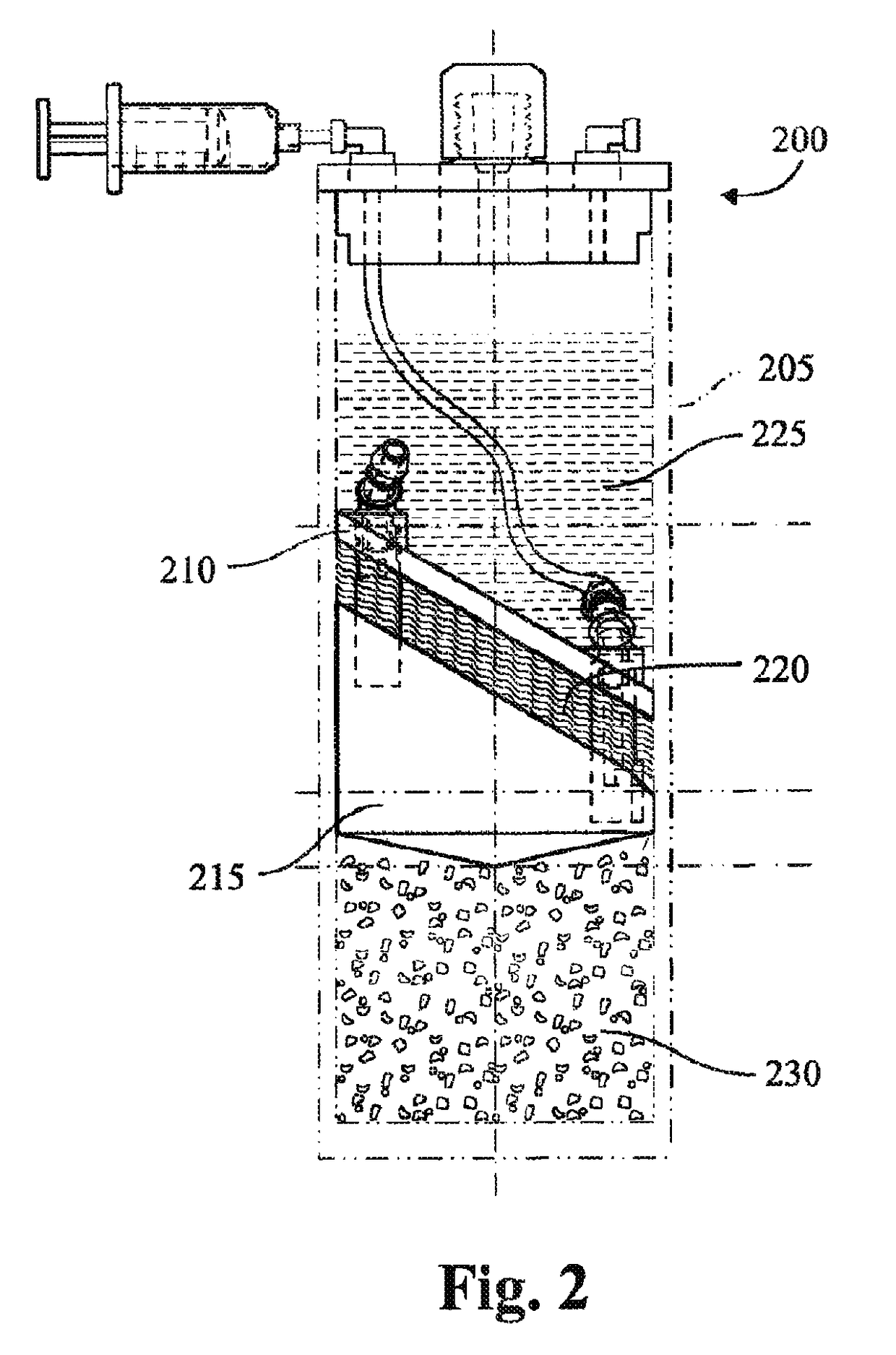
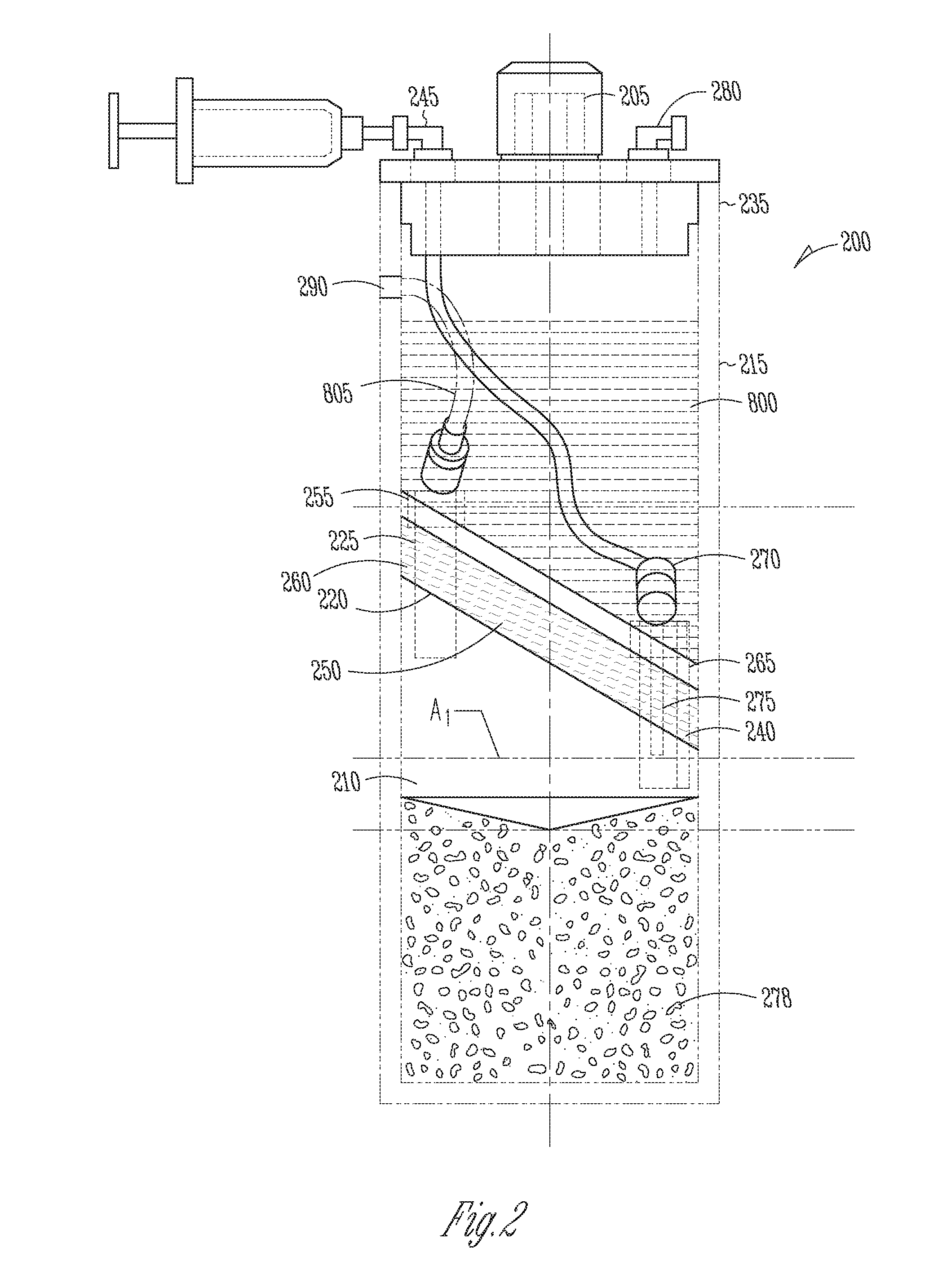
Cytokine concentration system
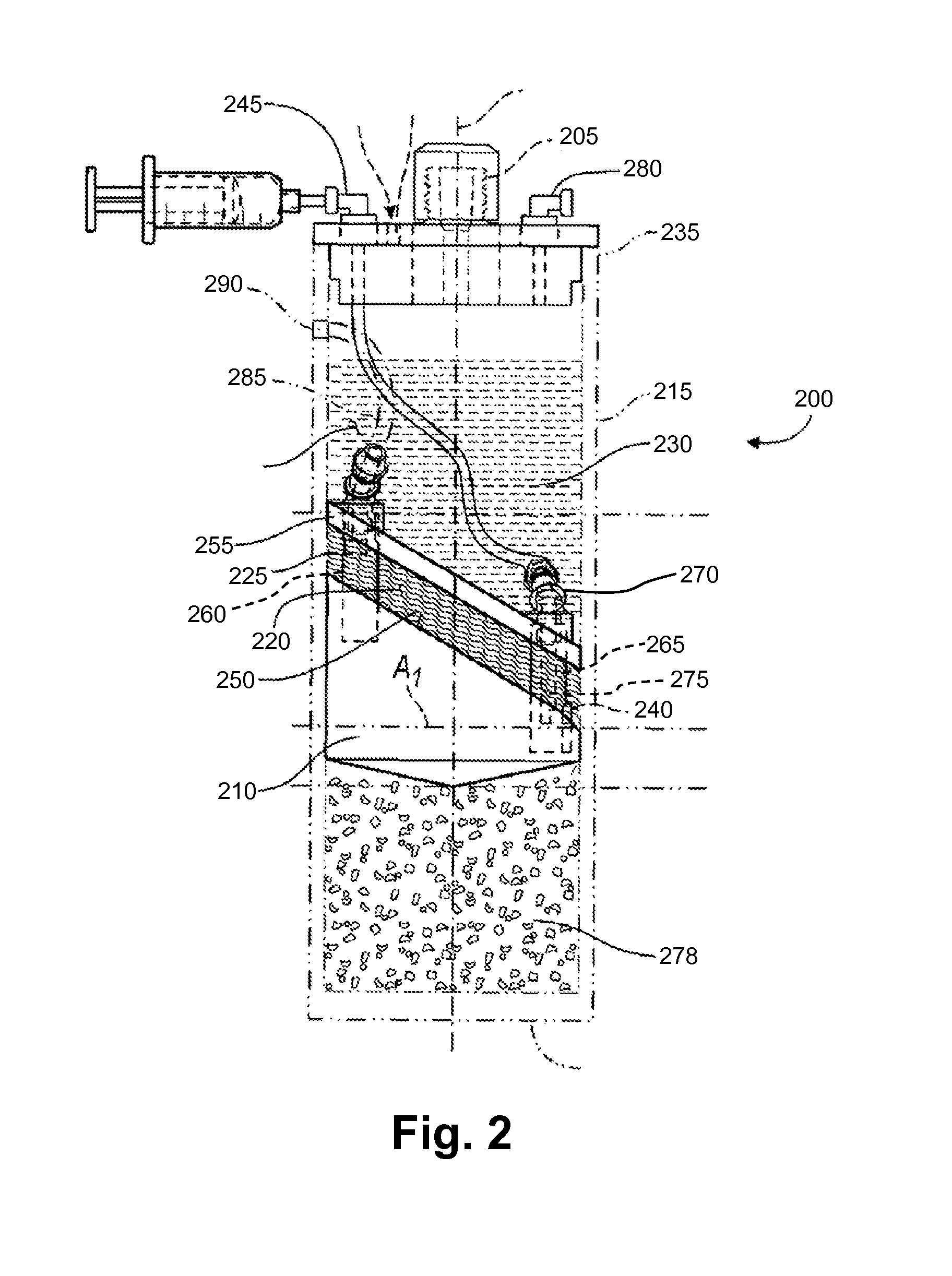
ActiveUS20100125236A1Avoid crackingOther blood circulation devicesDiagnosticsCentrifugationActive protein
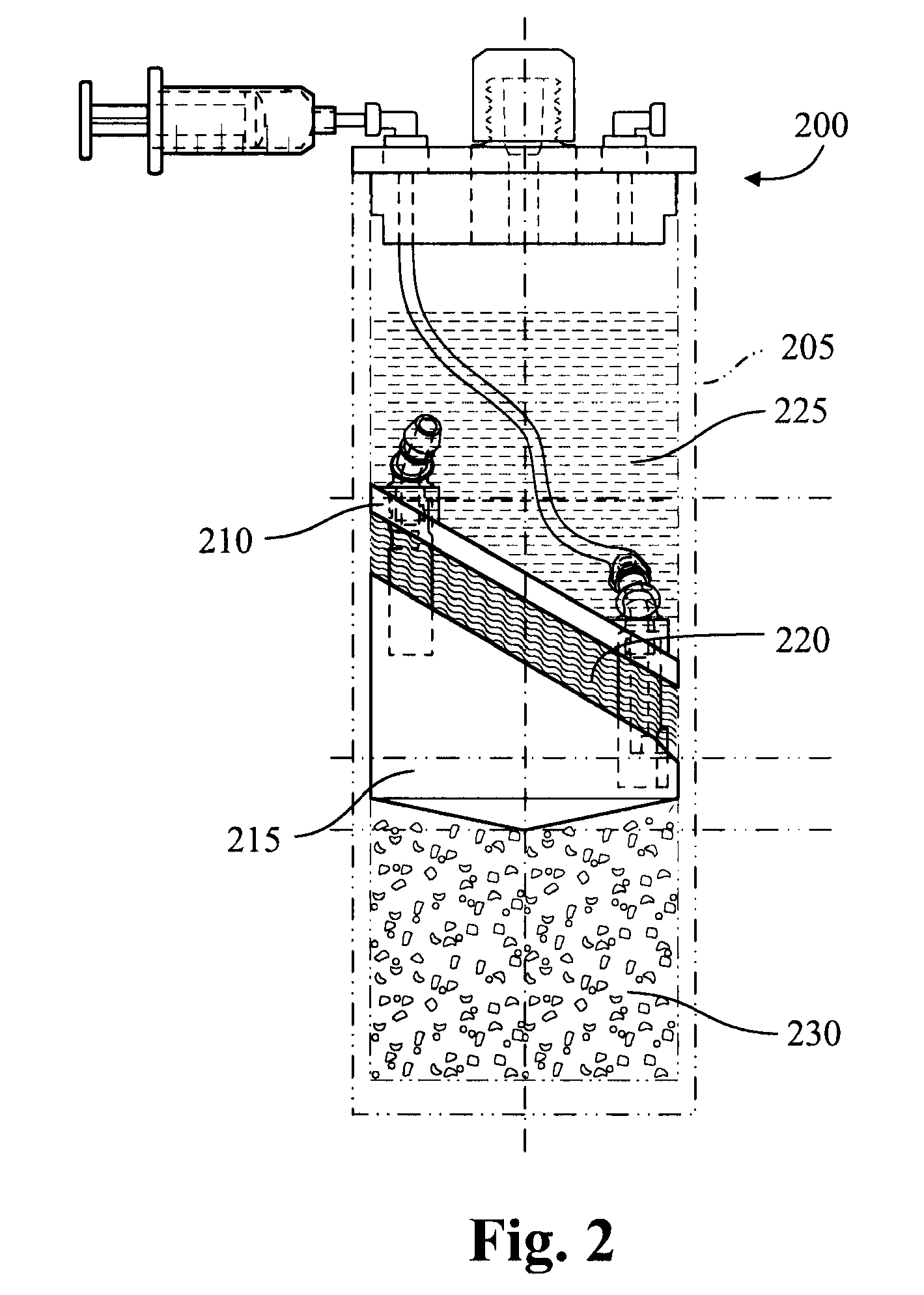
Apparatus and methods for producing interleukin-1 receptor antagonist and / or other prophylatically or therapeutically effective protein. Blood is obtained from a patient with a conventional syringe and then introduced into dual luer lock centrifuge tube. The dual luer lock centrifuge tube is provided with beads that are coated with a silanized coating. The container is then incubated and centrifuged. Subsequent to the incubation and the centrifugation, the serum containing autologous therapeutically active protein, such as IL-1Ra, in the container is withdrawn through the luer lock of the container, and injected back into the patient.
Owner:ARTHREX
Methods And Non-Immunogenic Compositions For Treating Inflammatory Disorders
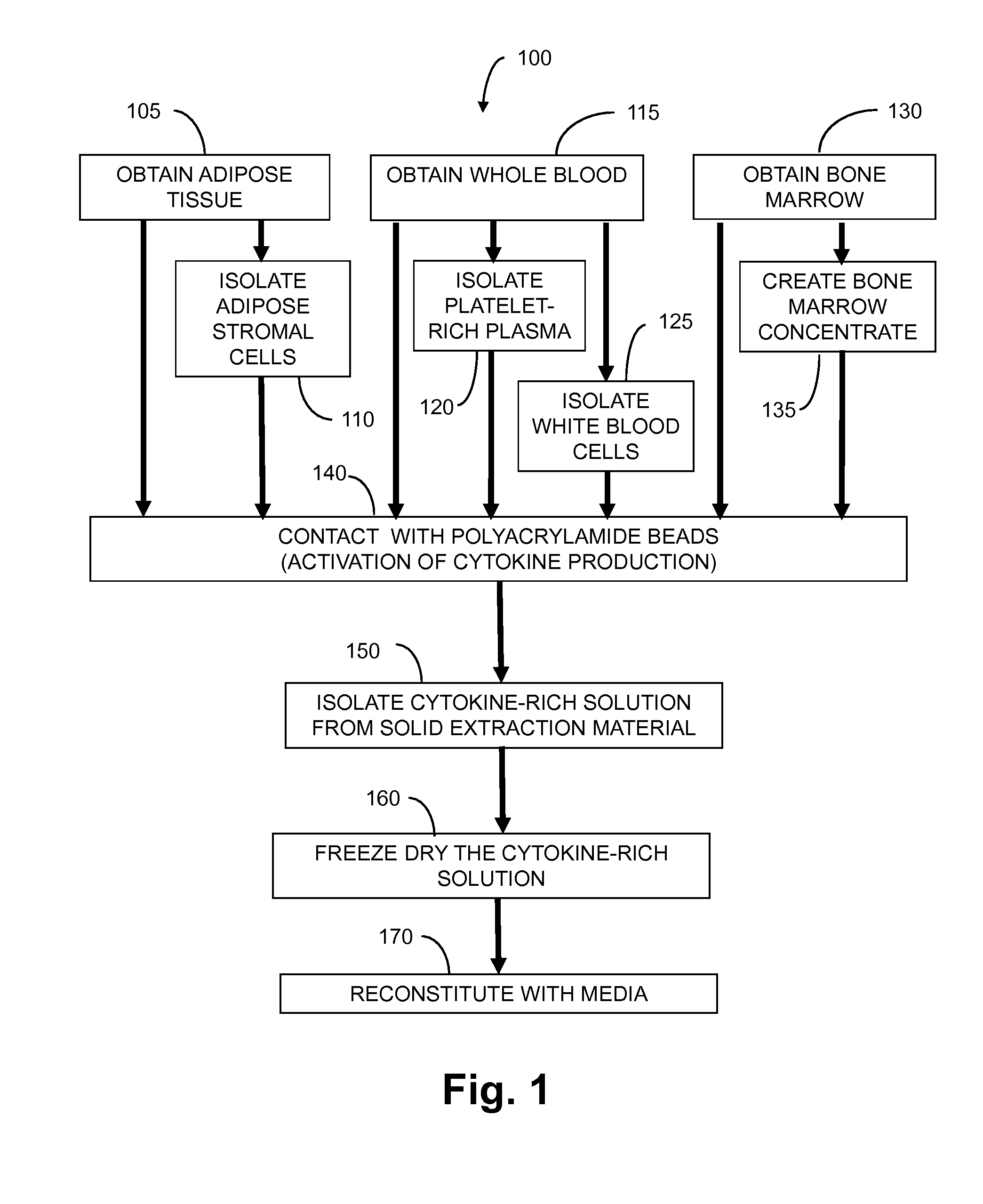
ActiveUS20140274895A1Peptide/protein ingredientsMammal material medical ingredientsWhite blood cellInflammatory disorder
Methods for making non-immunogenic anti-inflammatory cytokine compositions, comprising (a) obtaining a liquid comprising cytokine producing cells from a mammalian donor; (b) contacting the liquid with a solid extraction material to generate a composition rich in interleukin-1 receptor antagonist; and performing one or both of (i) removing cells from the composition and (ii) freezing the composition. The compositions comprise two or more of IL1-ra, sTNF-R1, sTNF-RII, IGF-I, EGF, HGF, PDGF-AB, PDGF-BB, VEGF, TGF-β1, and sIL-1RII, Compositions may also contain white blood cells and platelets.
Owner:BIOMET MFG CORP
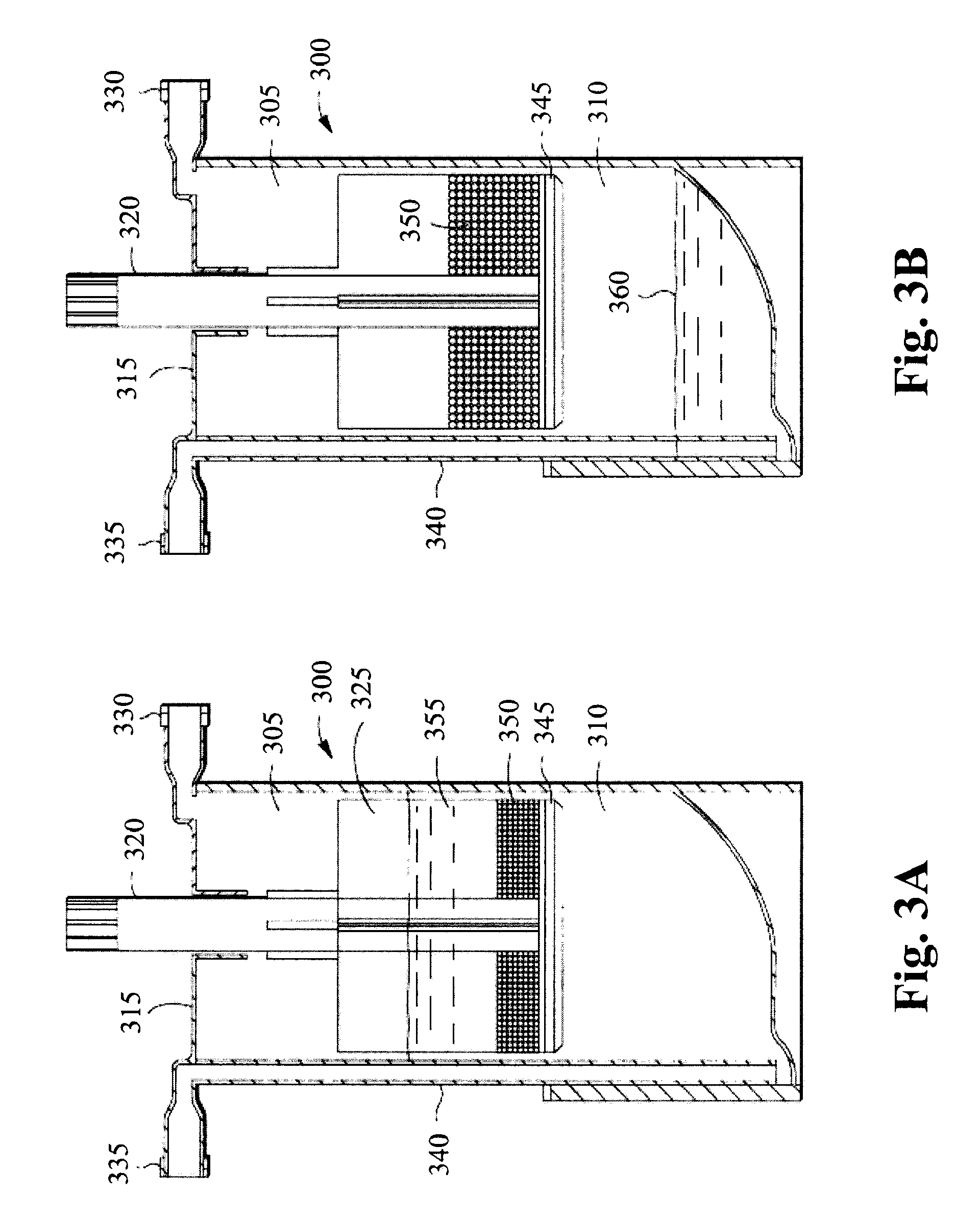
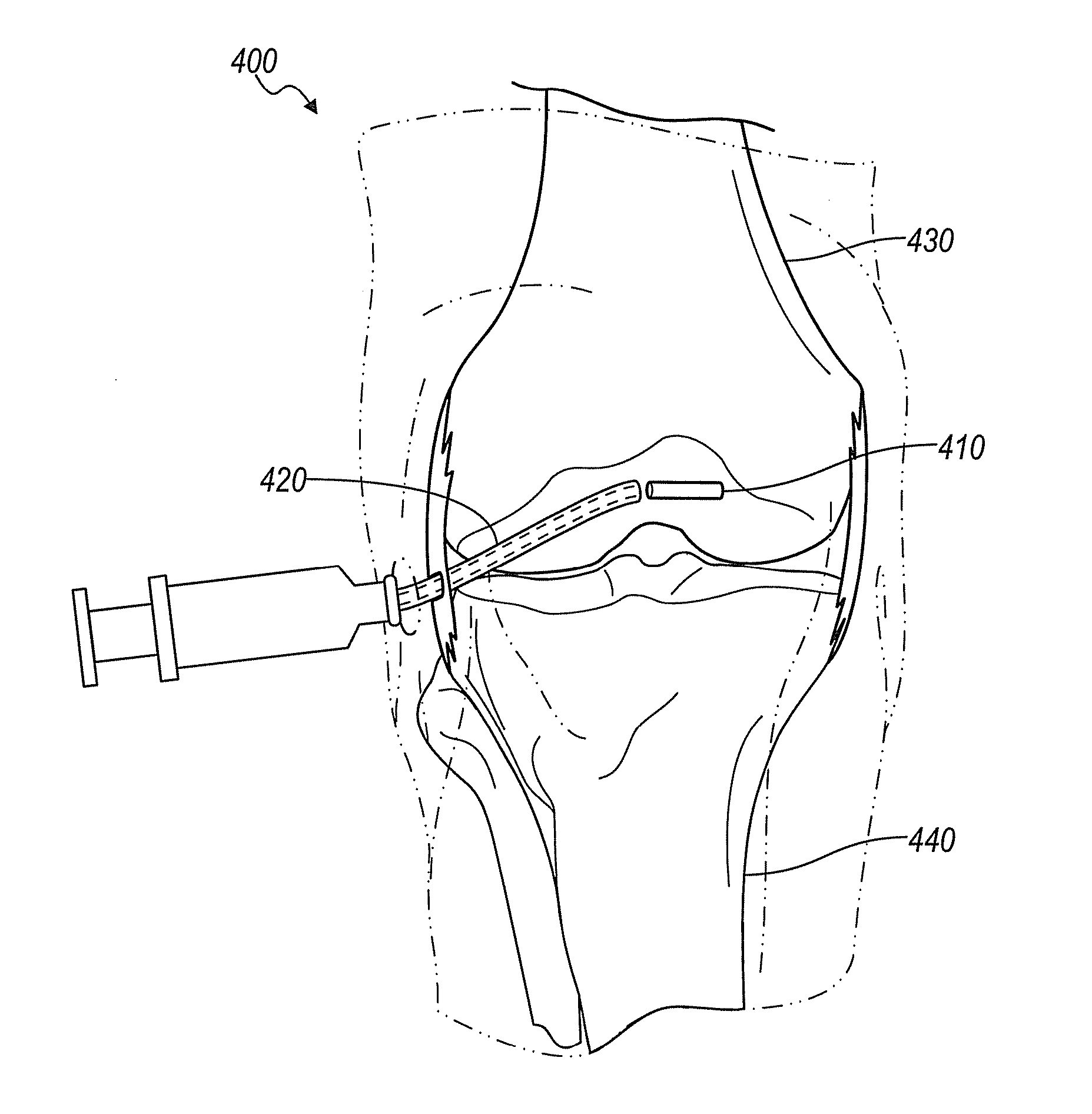
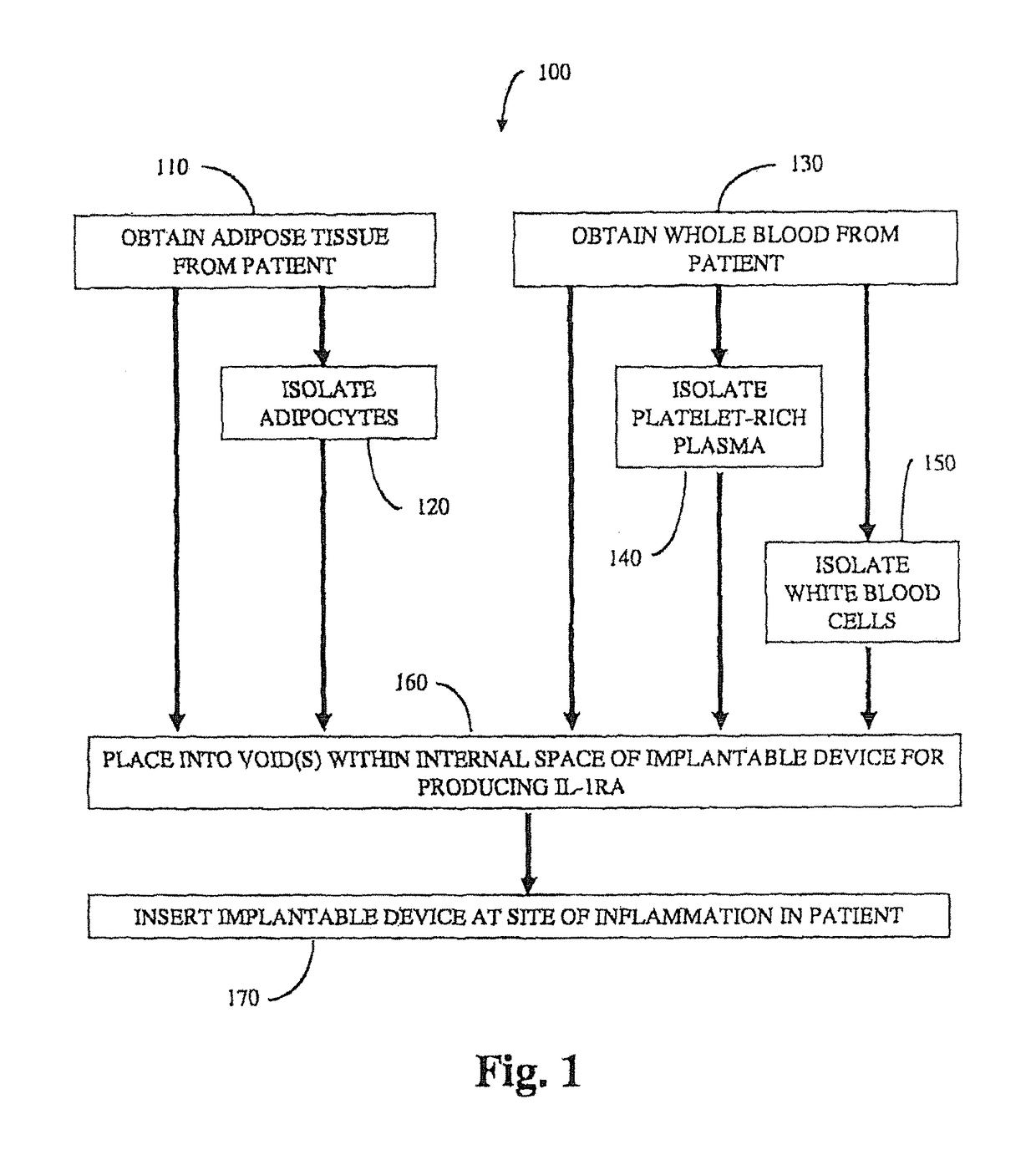
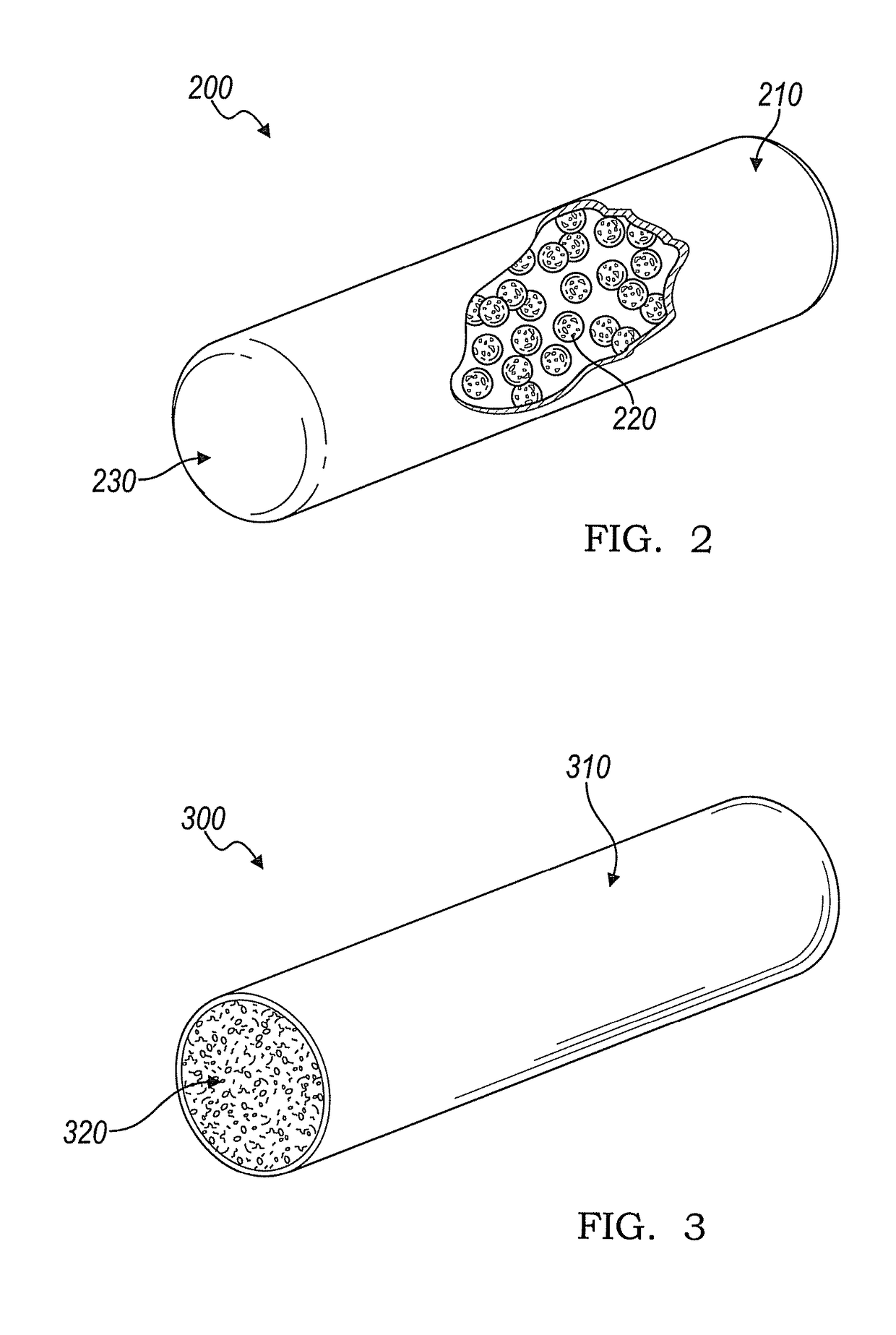
Implantable device for production of interleukin-1 receptor antagonist
ActiveUS20120172836A1Contribute to degenerationIncrease productionPeptide/protein ingredientsAntipyreticInterior spaceWhite blood cell
Treatments and devices for generating and using interleukin-1 receptor antagonist (IL-1ra). An implantable device is loaded with adipose tissue and / or white blood cells and inserted into an inflammation site in a patient to produce interleukin-1 receptor antagonist in vivo. The implantable device has an enclosed or substantially enclosed body that defines an internal space. At least a portion of the body comprises a first bioresorbable material and a second bioresorbable material is within the internal space along with one or more voids. The second bioresorbable material includes an activation surface to activate adipose tissue and / or white blood cells loaded into the device to produce IL-1ra.
Owner:BIOMET MFG CORP
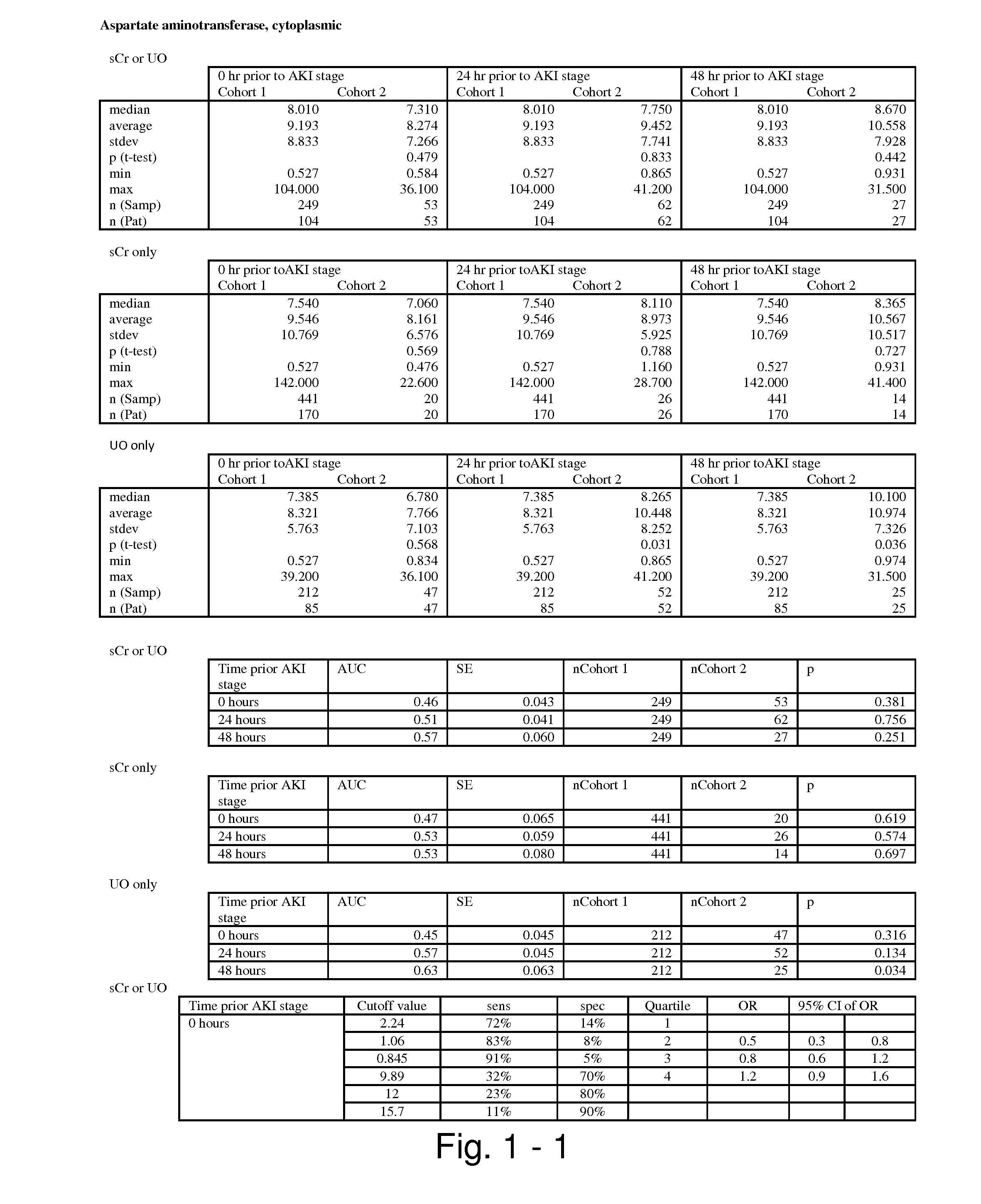
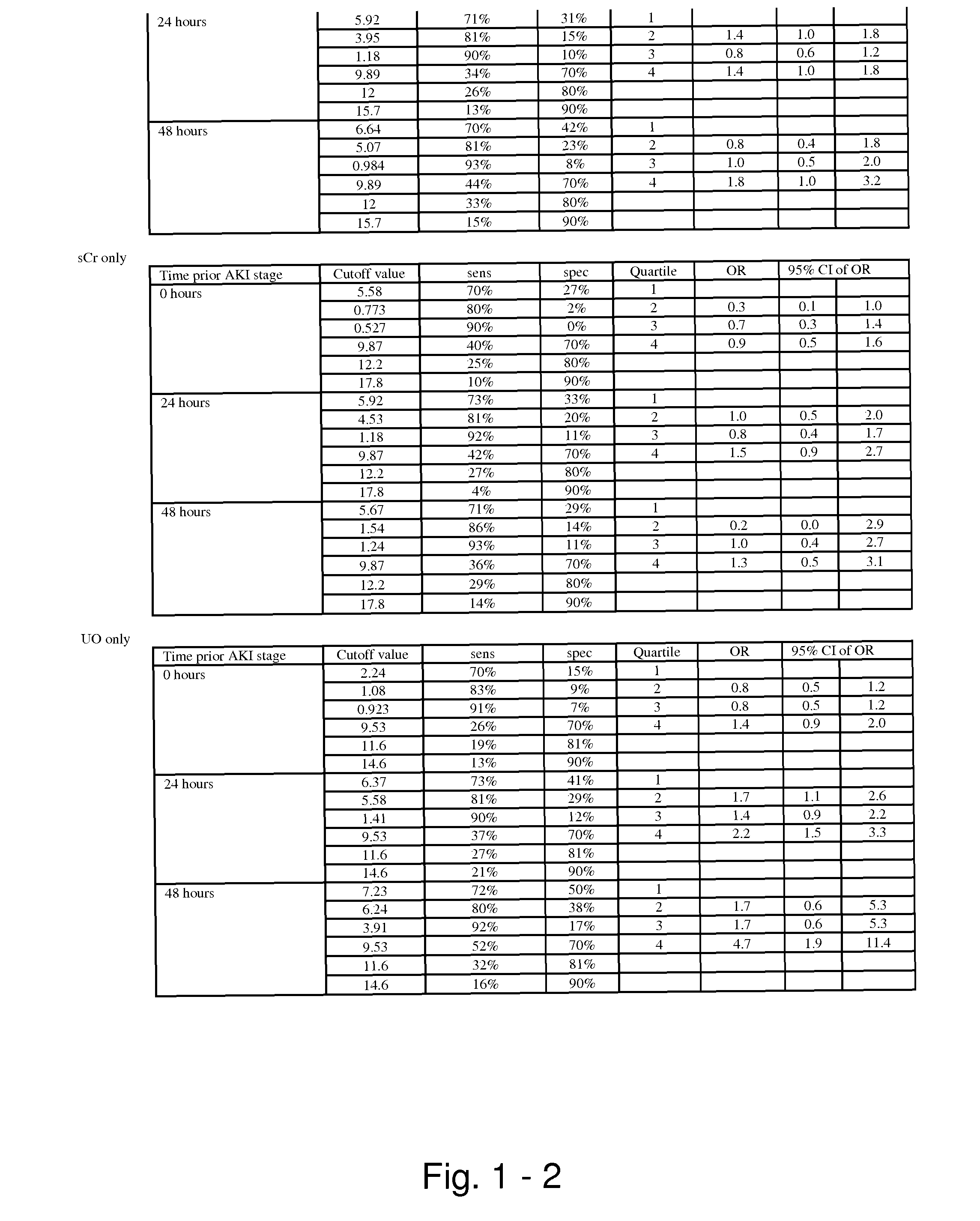
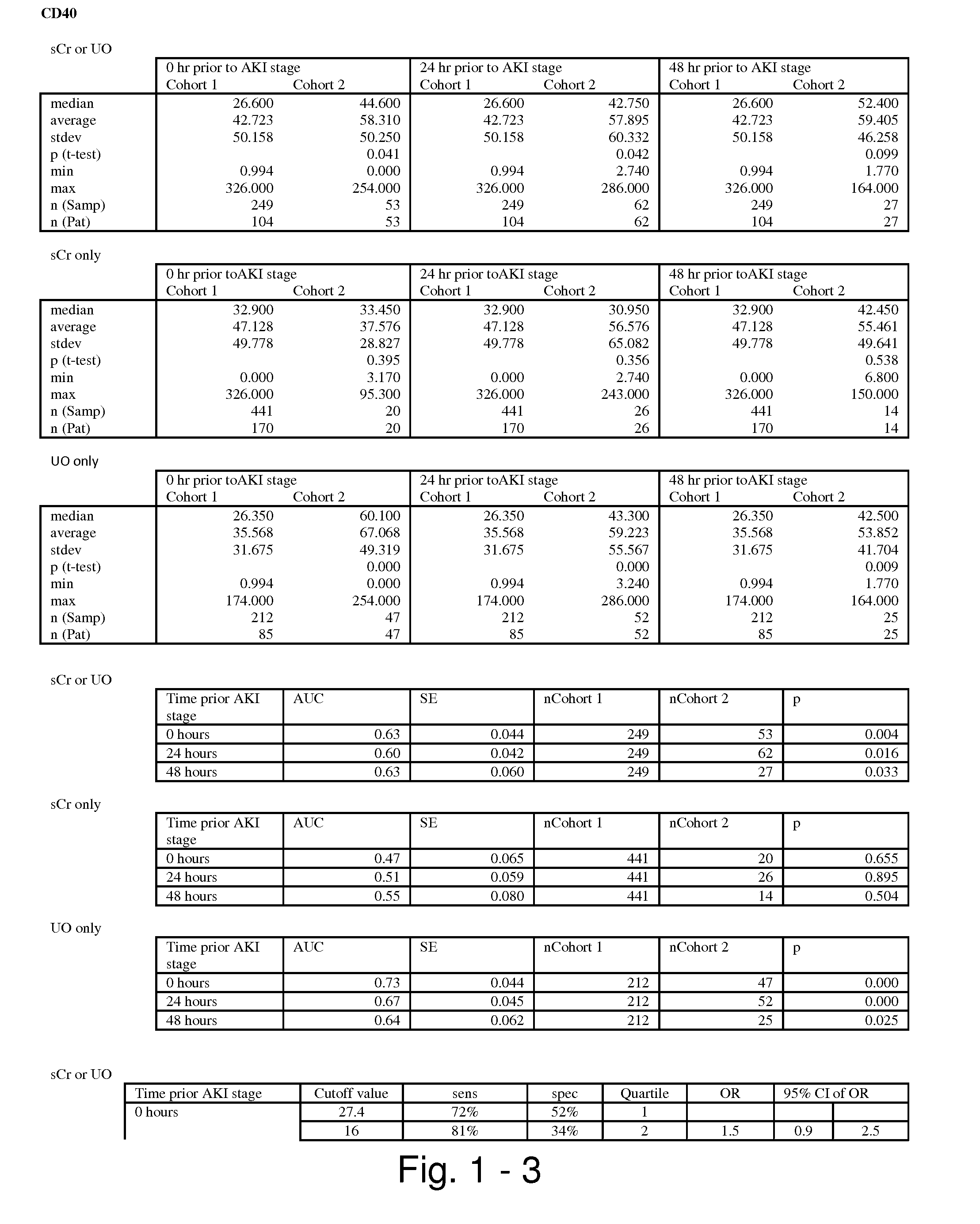
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
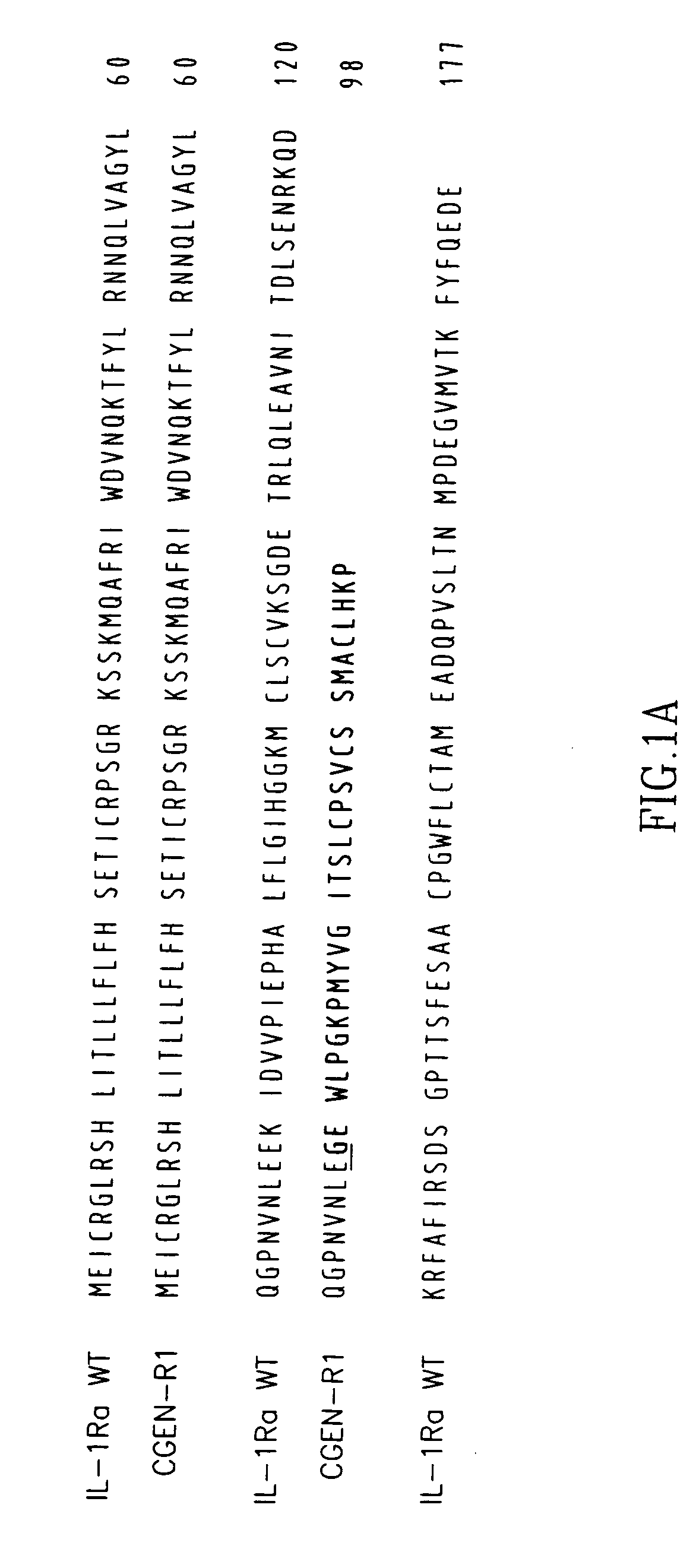
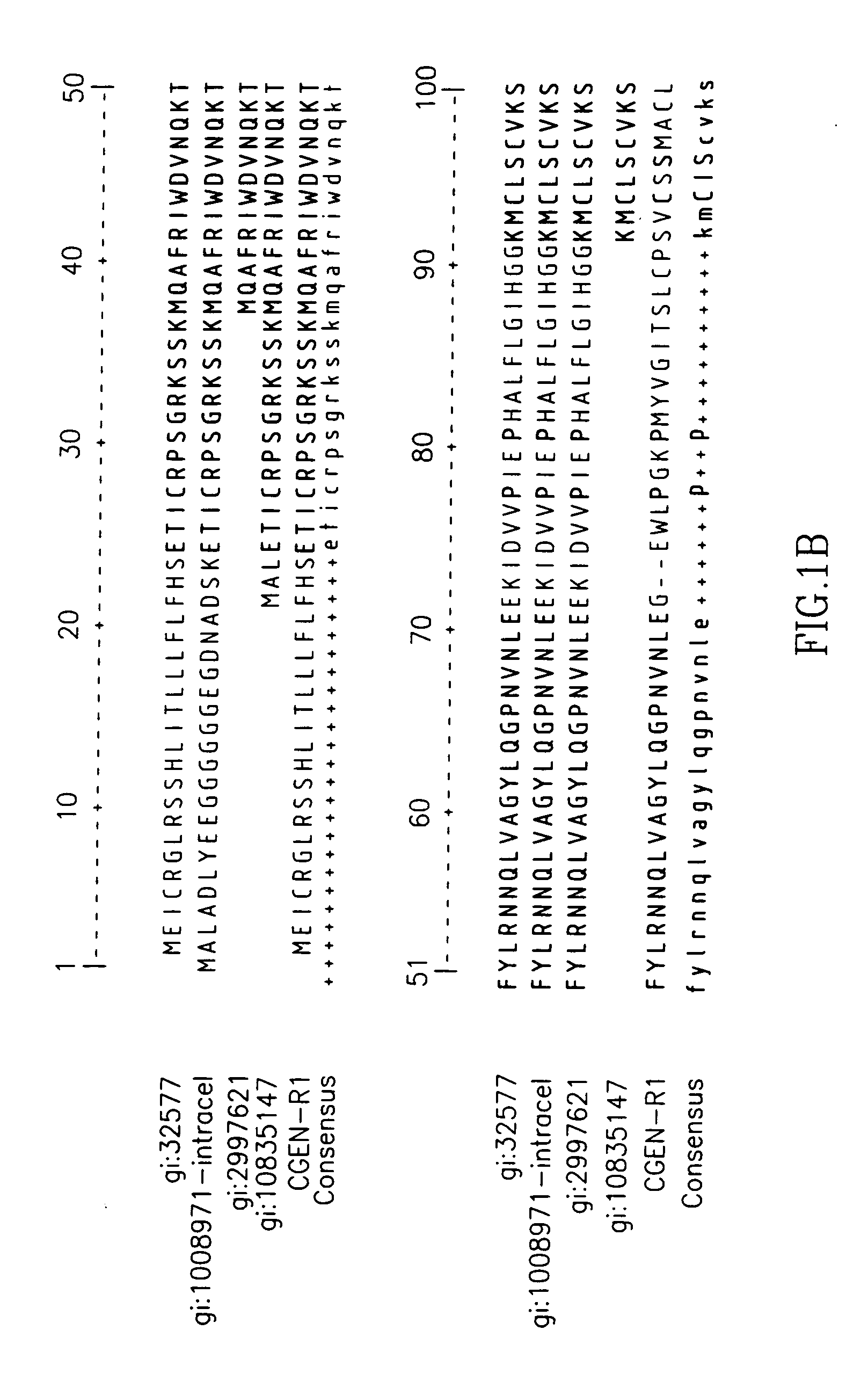
Interleukin-1 receptor antagonist and uses thereof
The present invention provides novel nucleic acids, the novel polypeptide sequences encoded by these nucleic acids and uses thereof. These novel polynucleotide and polypeptide sequences were determined to be a novel Interleukin-1 Receptor Antagonist.
Owner:NUVELO INC
Methods and compositions for delivering interleukin-1 receptor antagonist
ActiveUS8753690B2Contribute to degenerationIncrease productionPeptide/protein ingredientsAntipyreticInterleukin 1 Receptor Antagonist ProteinIL1 Inhibitor
Methods and compositions generating and using an interleukin-1 receptor antagonist (IL-1ra)-rich solution. Methods for generating and isolating interleukin-1 receptor antagonist include incubating adipose tissue and / or adipocytes with polyacrylamide beads to produce interleukin-1 receptor antagonist. The interleukin-1 receptor antagonist is isolated from the polyacrylamide beads to obtain the solution rich in interleukin-1 receptor antagonist. Methods for treating a site of inflammation in a patient include administering to the site of inflammation the solution rich in interleukin-1 receptor antagonist.
Owner:BIOMET MFG CORP
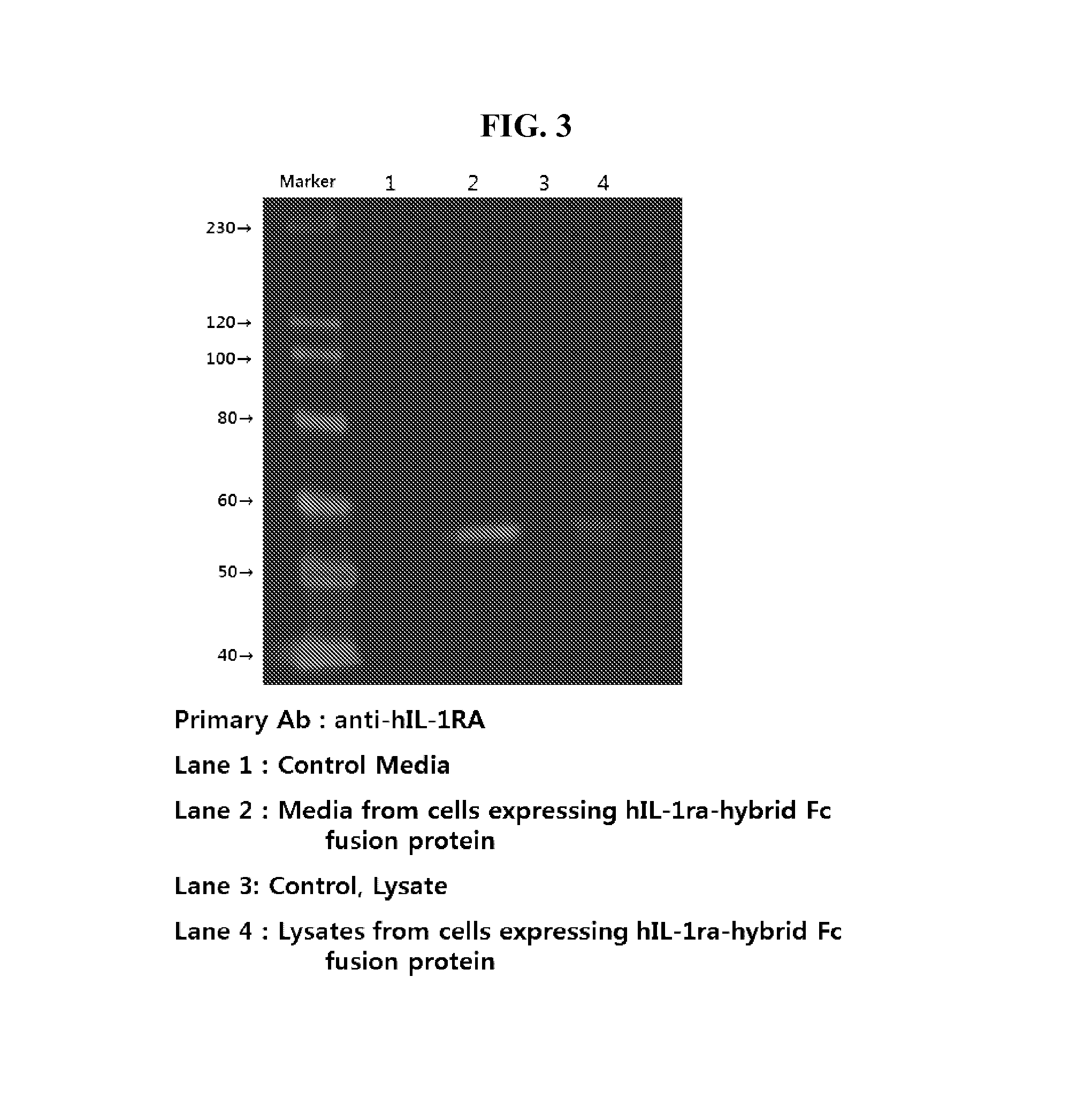
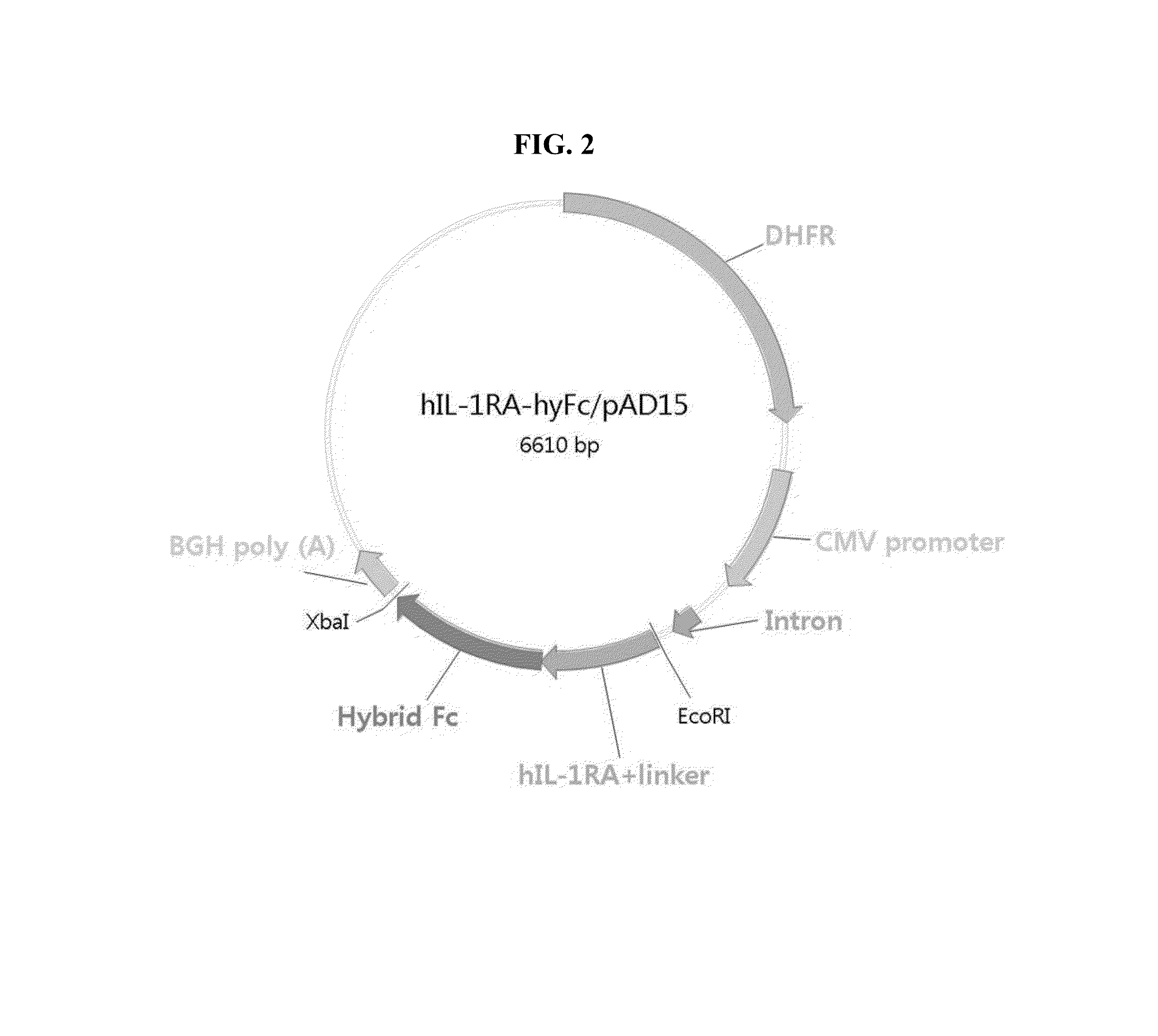
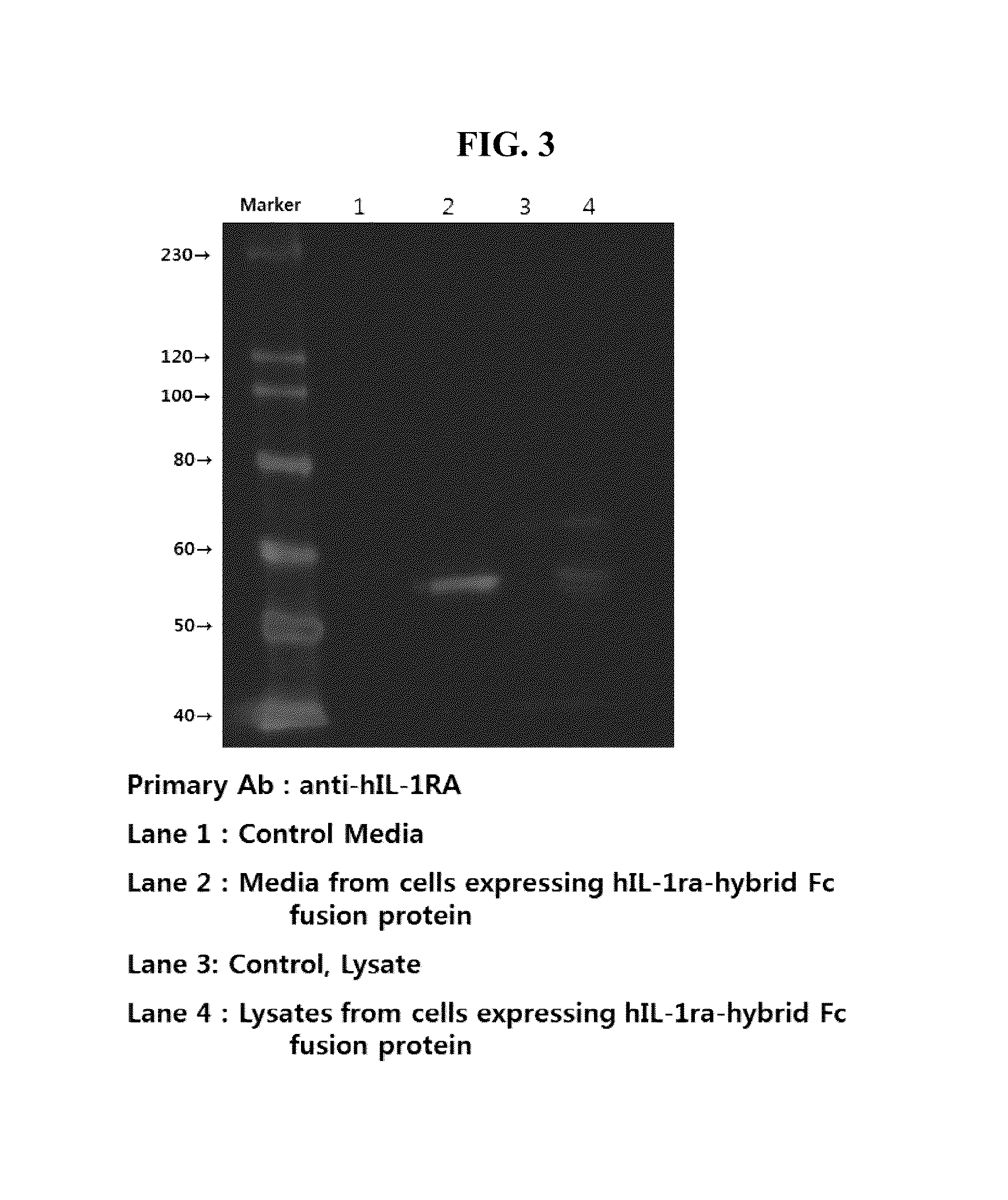
Human interleukin-1 receptor antagonist - hybrid fc fusion protein
ActiveUS20130217864A1Suppress immune responseLong period of timeSugar derivativesPeptide/protein ingredientsUlcerative colitisAutoimmune disease
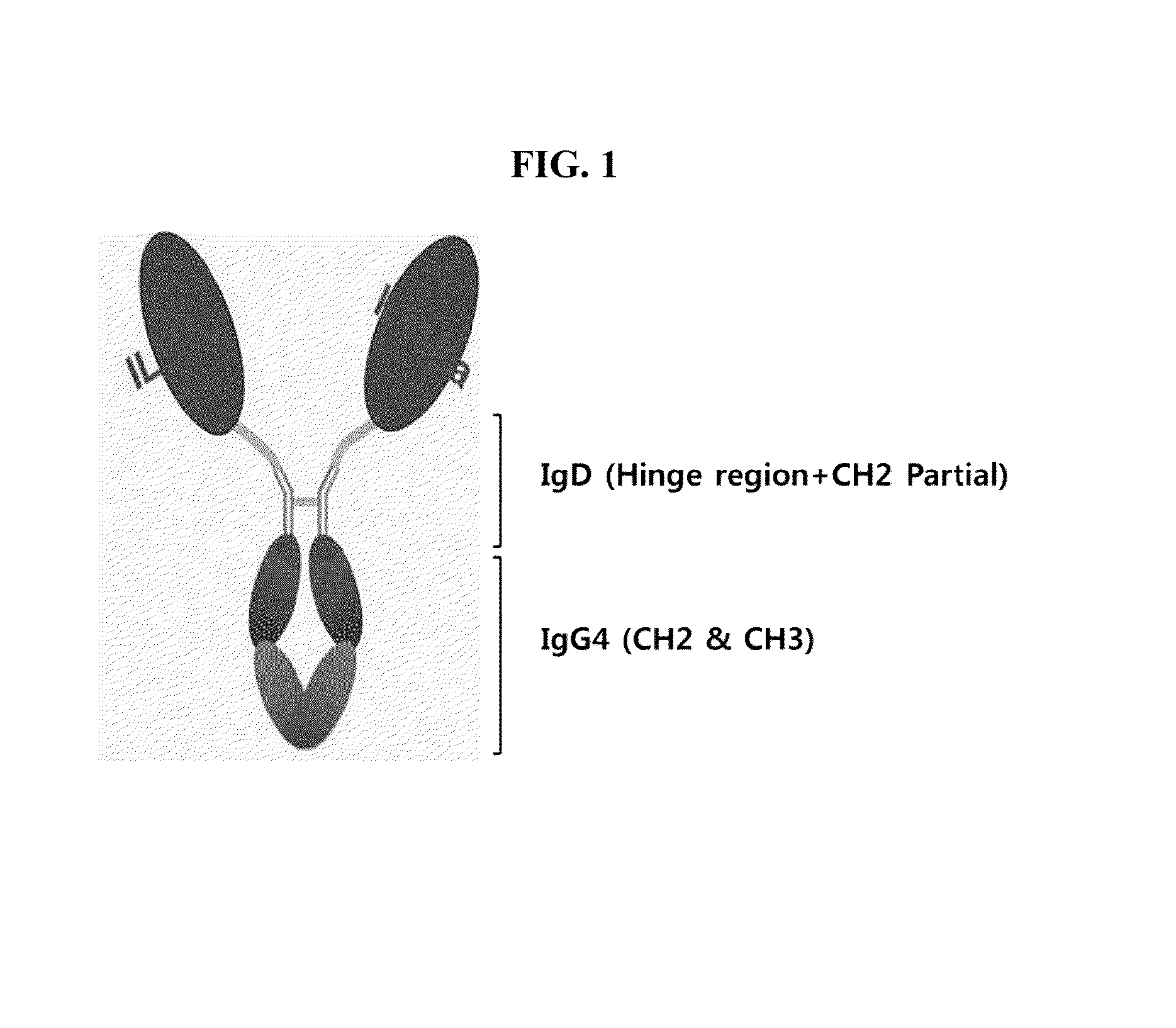
The present disclosure provides a fusion protein comprising IL-1 receptor antagonist fused to a hybrid Fc. Particularly the present disclosure relates to a fusion protein comprising IL-1 receptor antagonist fused to a human immunoglobulin hybrid Fc fragment. In one embodiment, the hybrid Fc fragment comprises IgD and IgG4. Also provided is a pharmaceutical composition comprising the present fusion protein, which are useful for treating autoimmune disease including rheumatoid arthritis, inflammatory bowel disease (Crohn's disease, ulcerative colitis), psoriasis and diabetes and the like. The present fusion protein with excellent efficacy and reduced side effects is qualified for clinical development as therapeutic antibodies to treat autoimmune disease.
Owner:GENEXINE CO LTD 20 +2
Human interleukin-1 receptor antagonist—hybrid Fc fusion protein
ActiveUS8883134B2Extended half-lifeLess toxicityPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectUlcerative colitis
The present disclosure provides a fusion protein comprising IL-1 receptor antagonist fused to a hybrid Fc. Particularly the present disclosure relates to a fusion protein comprising IL-1 receptor antagonist fused to a human immunoglobulin hybrid Fc fragment. In one embodiment, the hybrid Fc fragment comprises IgD and IgG4. Also provided is a pharmaceutical composition comprising the present fusion protein, which are useful for treating autoimmune disease including rheumatoid arthritis, inflammatory bowel disease (Crohn's disease, ulcerative colitis), psoriasis and diabetes and the like. The present fusion protein with excellent efficacy and reduced side effects is qualified for clinical development as therapeutic antibodies to treat autoimmune disease.
Owner:GENEXINE CO LTD 20 +2
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS8778615B2Easy to adaptMicrobiological testing/measurementAntibody ingredientsInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Methods and compositions for delivering interleukin-1 receptor antagonist
ActiveUS9701728B2Contribute to degenerationIncrease productionPeptide/protein ingredientsPharmaceutical delivery mechanismInterleukin 1 Receptor Antagonist ProteinIL1 Inhibitor
Methods and compositions generating and using an interleukin-1 receptor antagonist (IL-1ra)-rich solution. Methods for generating and isolating interleukin-1 receptor antagonist include incubating a liquid volume of white blood cells and platelets with polyacrylamide beads to produce interleukin-1 receptor antagonist. The interleukin-1 receptor antagonist is isolated from the polyacrylamide beads to obtain the solution rich in interleukin-1 receptor antagonist. Methods for treating a site of inflammation in a patient include administering to the site of inflammation the solution rich in interleukin-1 receptor antagonist.
Owner:BIOMET MFG CORP
Methods of treating pain using protein solutions
ActiveUS20160136245A1Low experience requirementPain reduction and preventionPeptide/protein ingredientsMammal material medical ingredientsProtein solutionInternal medicine
Methods and compositions for treating pain in mammalian subjects are provided. The methods include obtaining blood or a fraction of blood from the subject, measuring a therapeutic indicator in the blood or in the fraction blood, and administering an anti-inflammatory composition to the subject if the therapeutic indicator is equal to or above a threshold level. The anti-inflammatory composition comprises a protein solution including interleukin-1 receptor antagonist. The protein solution may be prepared from the blood or blood fraction obtained from the subject.
Owner:BIOMET BIOLOGICS
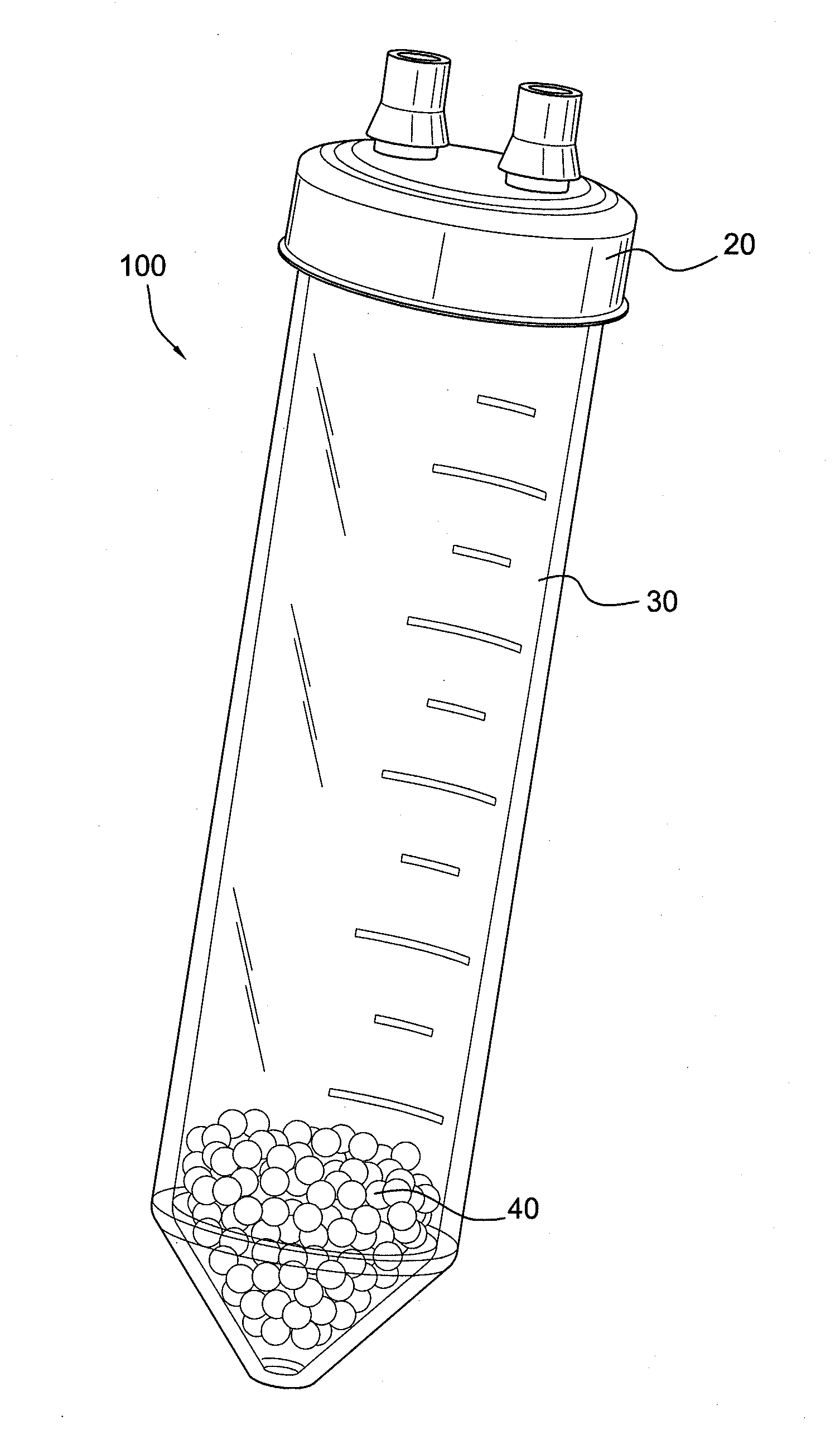
Cytokine concentration system
Apparatus and methods for producing interleukin-1 receptor antagonist and / or other prophylatically or therapeutically effective protein. Blood is obtained from a patient with a conventional syringe and then introduced into dual luer lock centrifuge tube. The dual luer lock centrifuge tube is provided with beads that are coated with a silanized coating. The container is then incubated and centrifuged. Subsequent to the incubation and the centrifugation, the serum containing autologous therapeutically active protein, such as IL-1Ra, in the container is withdrawn through the luer lock of the container, and injected back into the patient.
Owner:ARTHREX
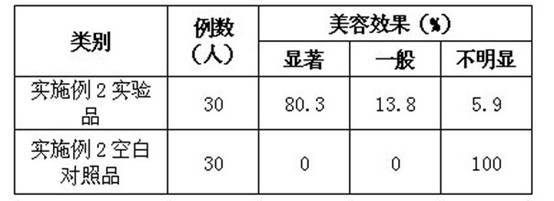
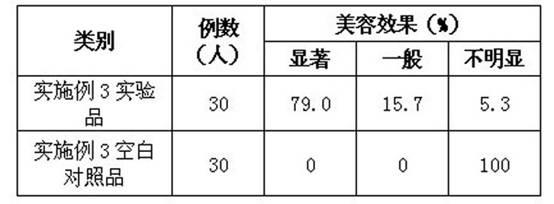
Method for preparing relaxing and face-cleansing cosmetics and application
ActiveCN102379838ACosmetic preparationsAntipyreticRecombinant Human Keratinocyte Growth FactorVitamin C
The invention relates to a method for preparing relaxing and face-cleansing cosmetics and application. The cosmetics comprises the following components in percentage by mass: 0.00001 to 0.001 percent of recombinant human keratinocyte growth factors, 0.00001 to 0.001 percent of recombinant human interleukin-1 receptor antagonist, 0.1 to 0.5 percent of low-molecular sodium heparin, 5 to 25 percent of stabilizer, 0.02 to 0.3 percent of hyaluronic acid, 0.5 to 8 percent of vitamin C sodium phosphate, 0.5 to 5 percent of methyl propanediol, 0.3 to 0.7 percent of 1,2 hexanediol, 0.5 to 3.5 percent of lactobacillus / mung bean fermentation liquor, 0.5 to 5 percent of dissolved protease and the balance of water. The relaxing and face-cleansing cosmetics are applied to sensitive skin, can be stored for a long time, and is suitable for long-term use.
Owner:广州泰润合投资有限公司
Methods and compositions for delivering interleukin-1 receptor antagonist
ActiveUS20160017010A1Contribute to degenerationIncrease productionBiocidePeptide/protein ingredientsInterleukin 1 Receptor Antagonist ProteinIL1 Inhibitor
Owner:BIOMET MFG CORP
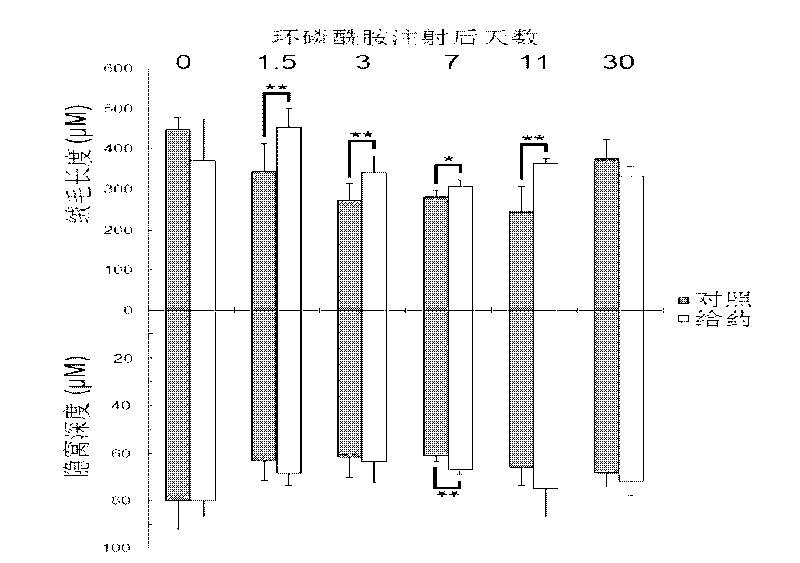
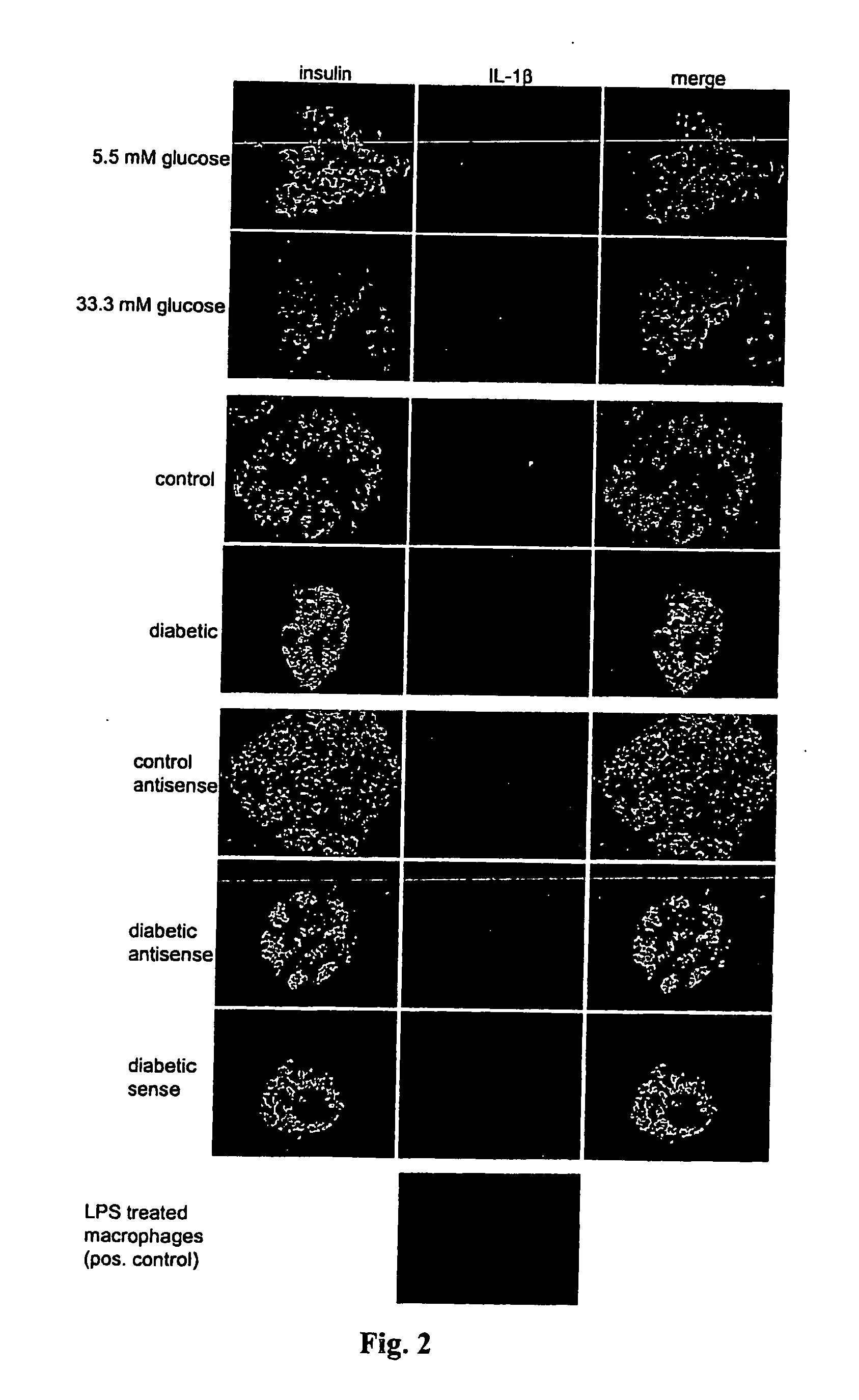
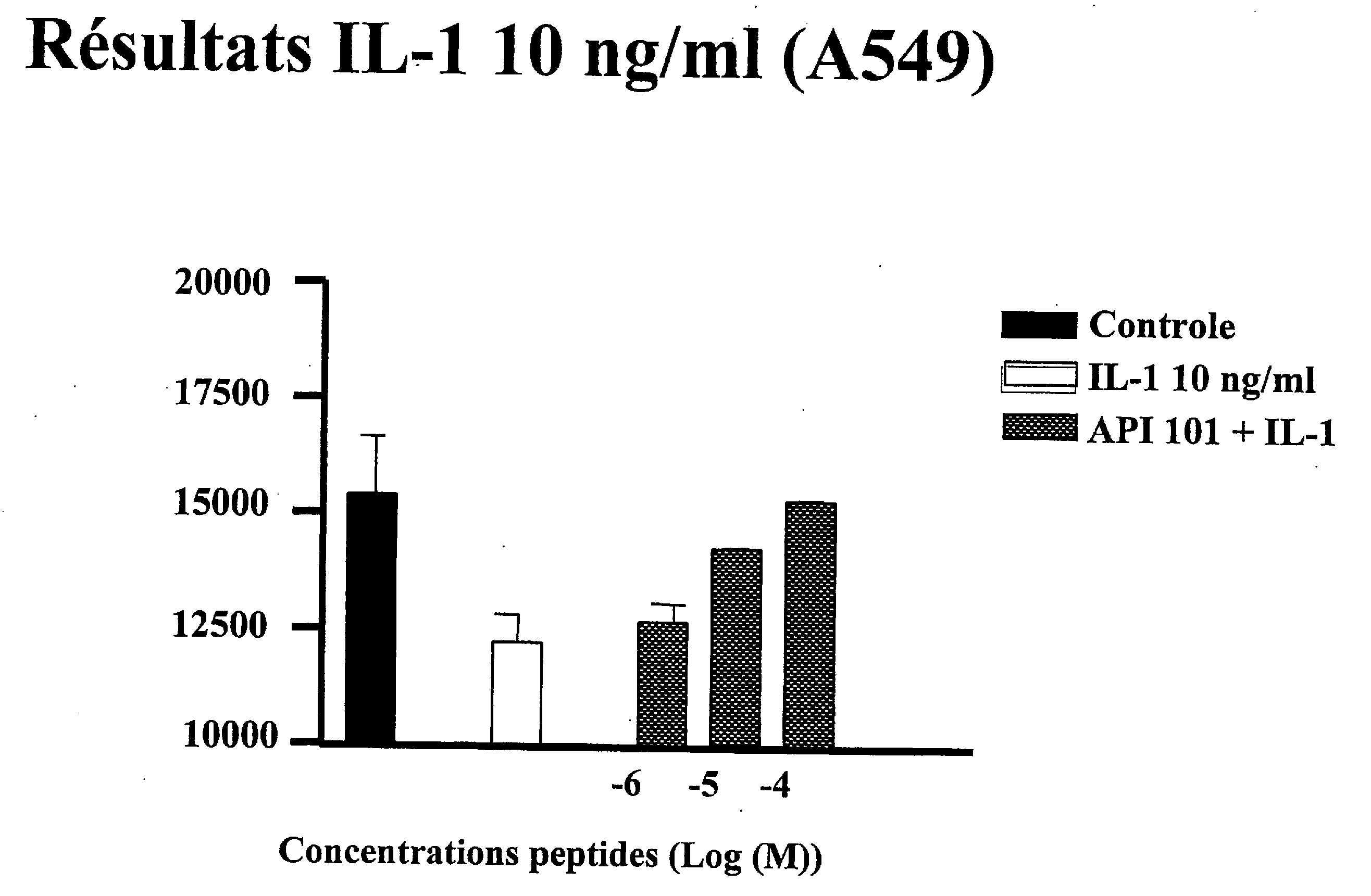
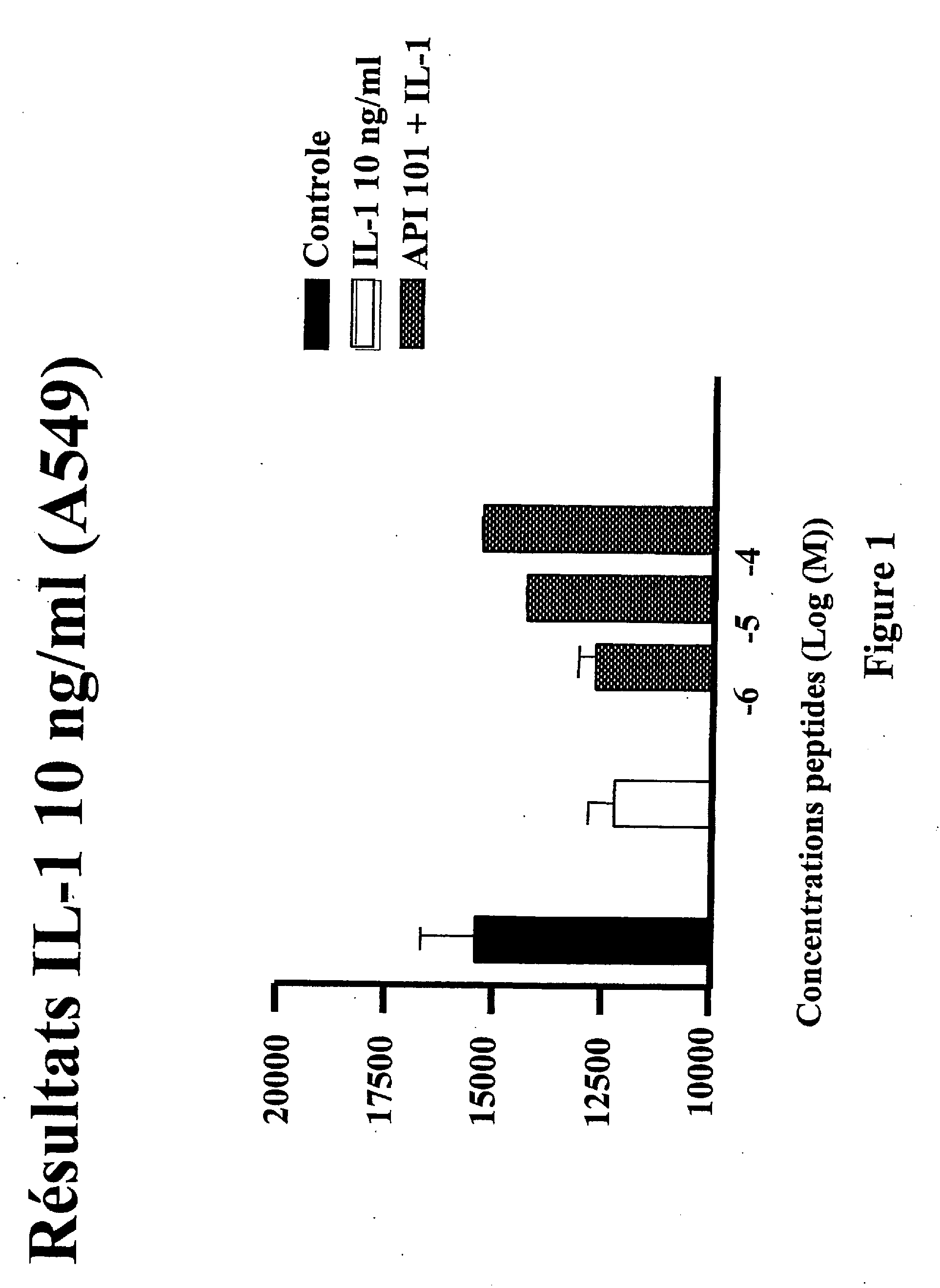
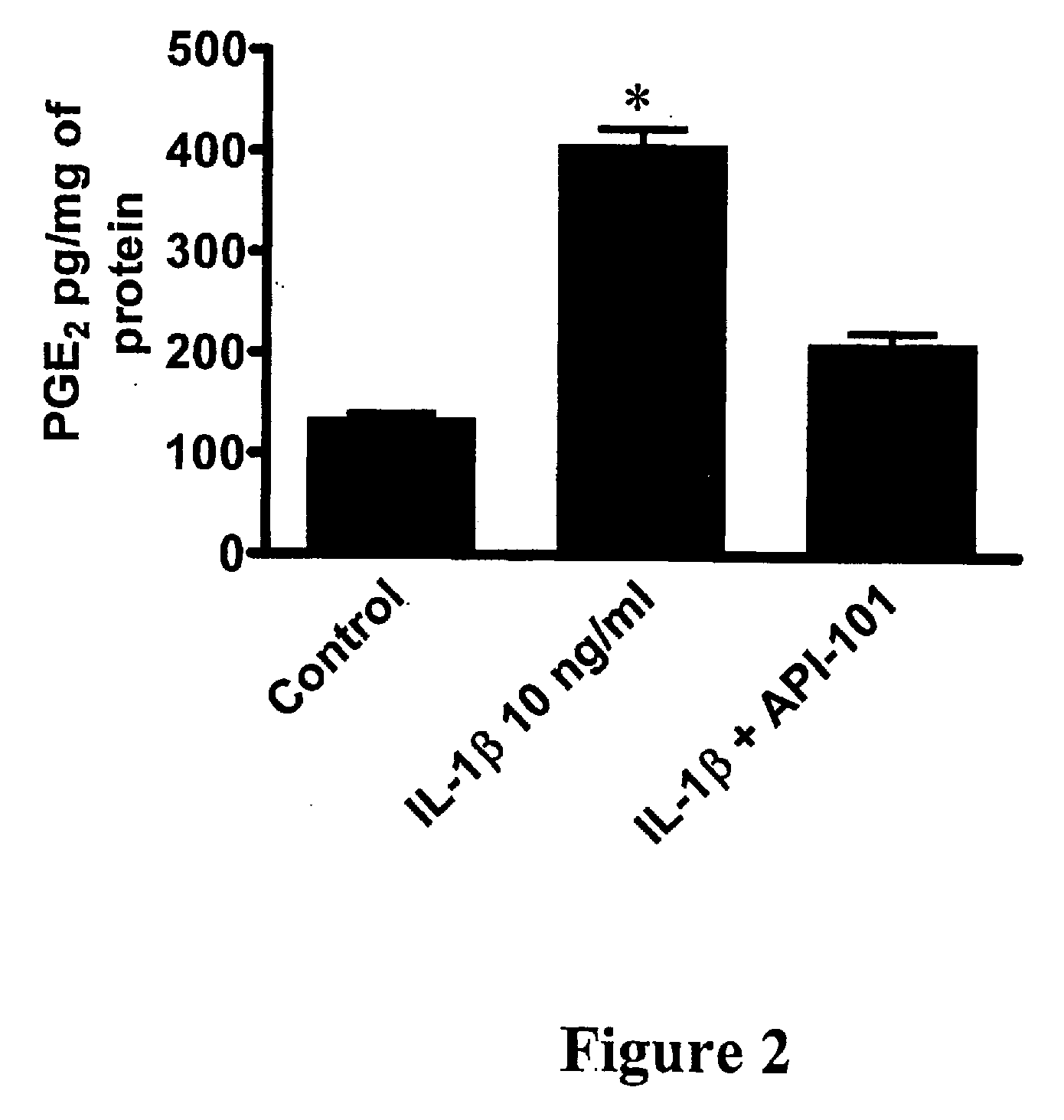
Use of an interleukin 1 receptor antagonist and/or pyrrolidinedithiocarbamate for the treatment or prophylaxis of type 2 diabetes
Substances that inhibit the action of the members of the IL-1β / NF-κB pathway can be used for protecting and preserving β-cell mass and function in prediabetic and diabetic type 2 patients. Specifically, the present invention relates to the use of an Interleukin 1 receptor antagonist (IL-1Ra) and / or pyrrolidinedithiocarbamate (PDTC) for the treatment or prophylaxis of type 2 diabetes, as well as a method for the treatment of type 2 diabetes.
Owner:UNIV ZURICH
Implantable device for production of interleukin-1 receptor antagonist
ActiveUS9763875B2Contribute to degenerationIncrease productionPeptide/protein ingredientsAntipyreticMedicineWhite blood cell
Owner:BIOMET MFG CORP
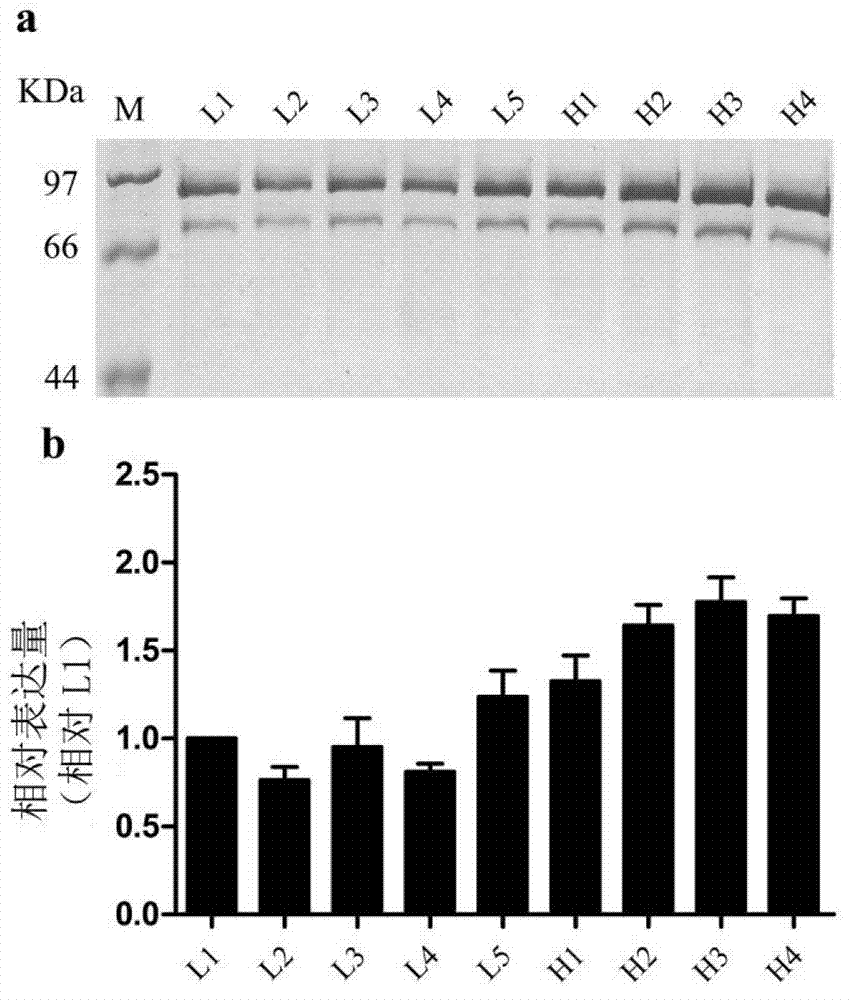
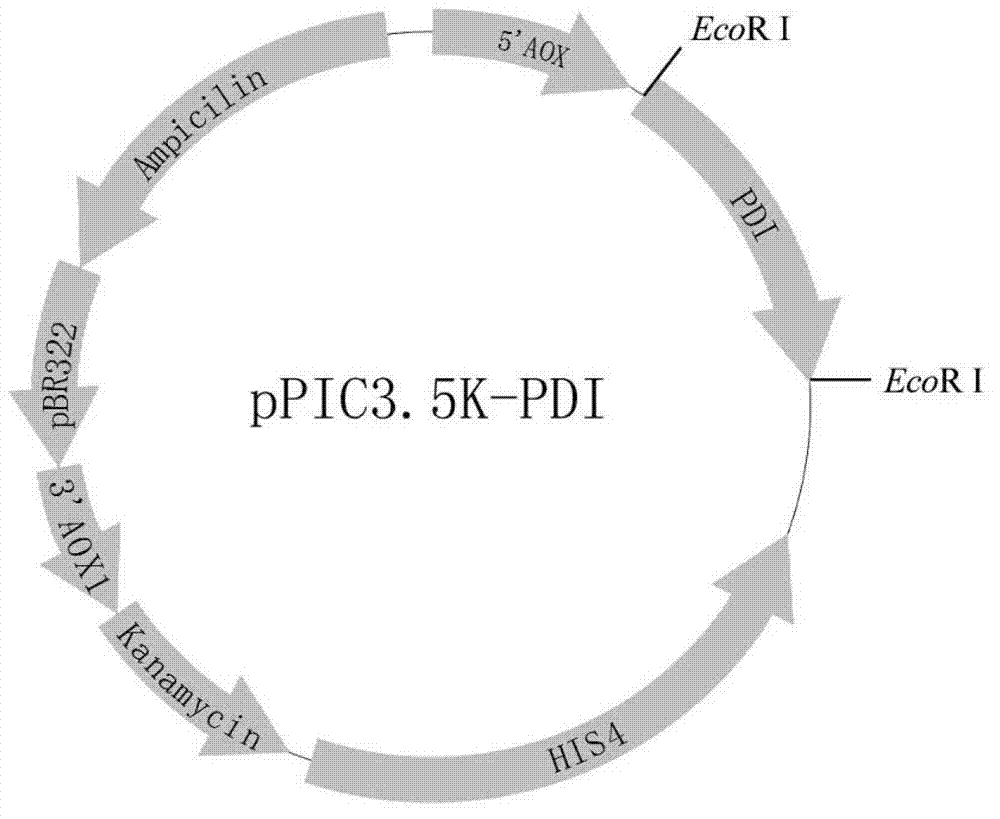
Expression system of fusion protein from human serum albumin and interleukin-1 receptor antagonist
ActiveCN102766648AEasy to separateLow immunogenicityFungiMicroorganism based processesPichia pastorisYeast
The invention discloses an expression system of fusion protein from human serum albumin and interleukin-1 receptor antagonist. The expression system comprises a host cell and an expression vector transferred into the same. The expression vector comprises a first expression vector with an inserted fusion protein gene and a second expression vector with an inserted protein disulfide isomerase gene.The fusion protein gene comprises the human serum albumin and the interleukin-1 receptor antagonist. The host cell is Pichia pastoris GS115. The co-expression host of PDI (protein disulfide isomerase) and IGH (immunoglobulin heavy) is established, and expression index of the IGH is evidently increased. The PDI is expressed intracellularly, the IGH secretory expression is subjected to extracellular secretory expression, and accordingly no newly generated other proteins occur when concentration of the IGH in collected medium supernate increases.
Owner:ZHEJIANG UNIV
Variants of interleukin-1 receptor antagonist: compositions and uses thereof
InactiveUS20050159590A1Inhibition of physiological activityInhibitory activityPeptide/protein ingredientsGenetic material ingredientsWhite blood cellIL1 Inhibitor
The present invention provides Interleukin-1 receptor antagonist splice variants, including isolated nucleic acids encoding these variants and the encoded amino acid sequences, as well as antibodies, antisense oligonucleotides, expression vectors and host cells that include these sequences. The present invention further discloses methods of using these sequences in the diagnosis, prognosis, treatment, and prevention of diseases and disorders mediated by Interleukin-1.
Owner:COMPUGEN
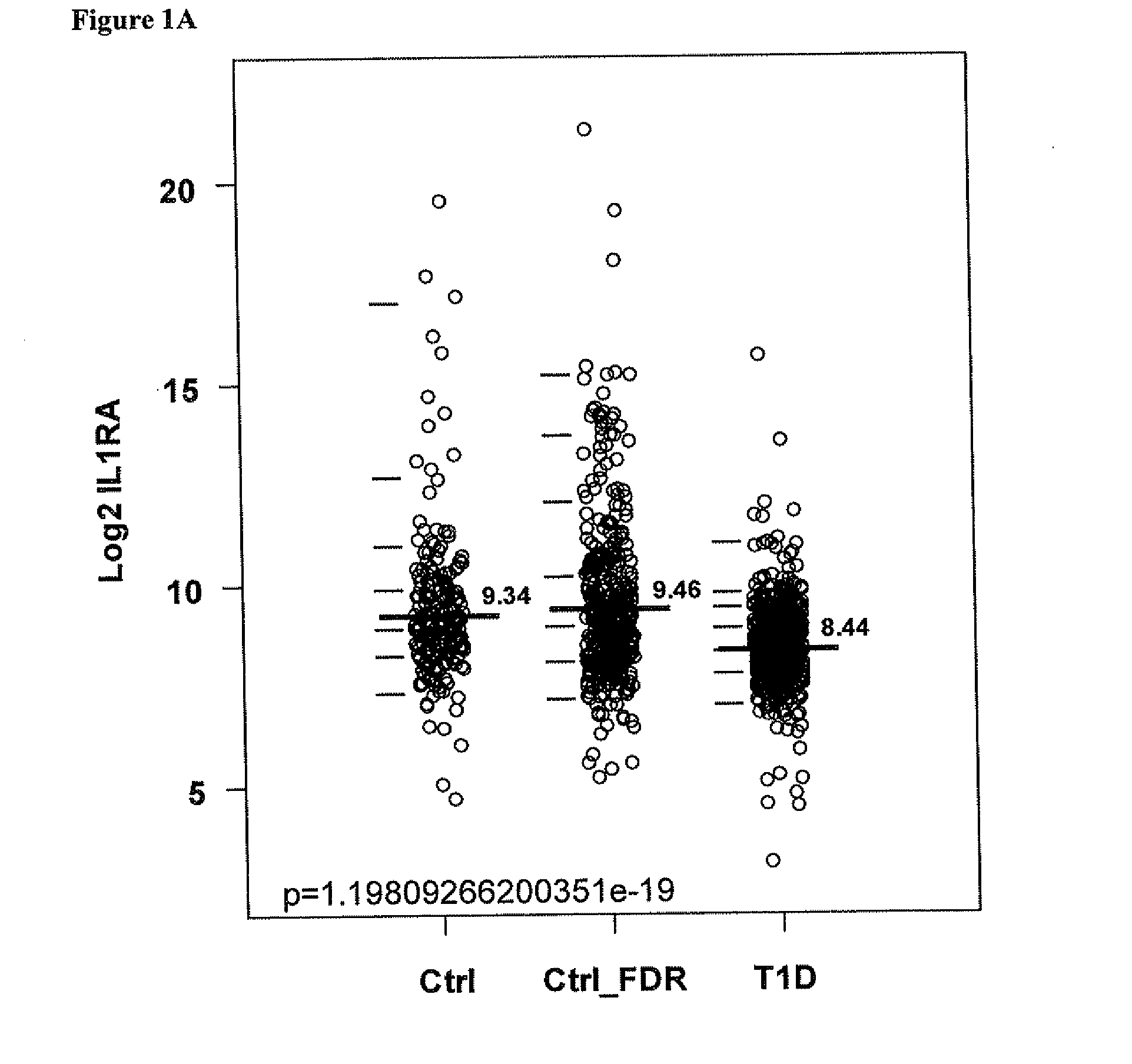
Biomarkers and therapeutic targets for type 1 diabetes
InactiveUS20120177660A1Lower Level RequirementsPeptide/protein ingredientsMetabolism disorderAutoimmune responsesWhite blood cell
Compositions and methods for determining a subject's risk of developing type 1 diabetes (T1D) and diabetic complications are provided. One embodiment provides a method involving measuring the levels of interleukin-1-receptor antagonist (IL-1ra) in a sample from the subject. In other embodiments, the method involves measuring the levels of MIP-1β, IL-8, MCP-1, MPO, SAA, IGFBP2, Adiponectin, or combinations thereof. Another embodiment provides preventing islet autoimmunity and T1D using agonist of IL-1ra, MIP-1β, IL-8, MCP-1, MPO, or a combination thereof. Another embodiment provides preventing islet autoimmunity, T1D and diabetic complications using antagonist of SAA, IGFBP2, Adiponectin, or a combination thereof.
Owner:GEORGIA HEALTH SCI UNIV RES INST
Application of interleukin-1 receptor antagonist and medicinal composition thereof
ActiveCN101690801APromote proliferationPrevent or treat injury diseasePeptide/protein ingredientsMetabolism disorderDiseaseDiarrhea
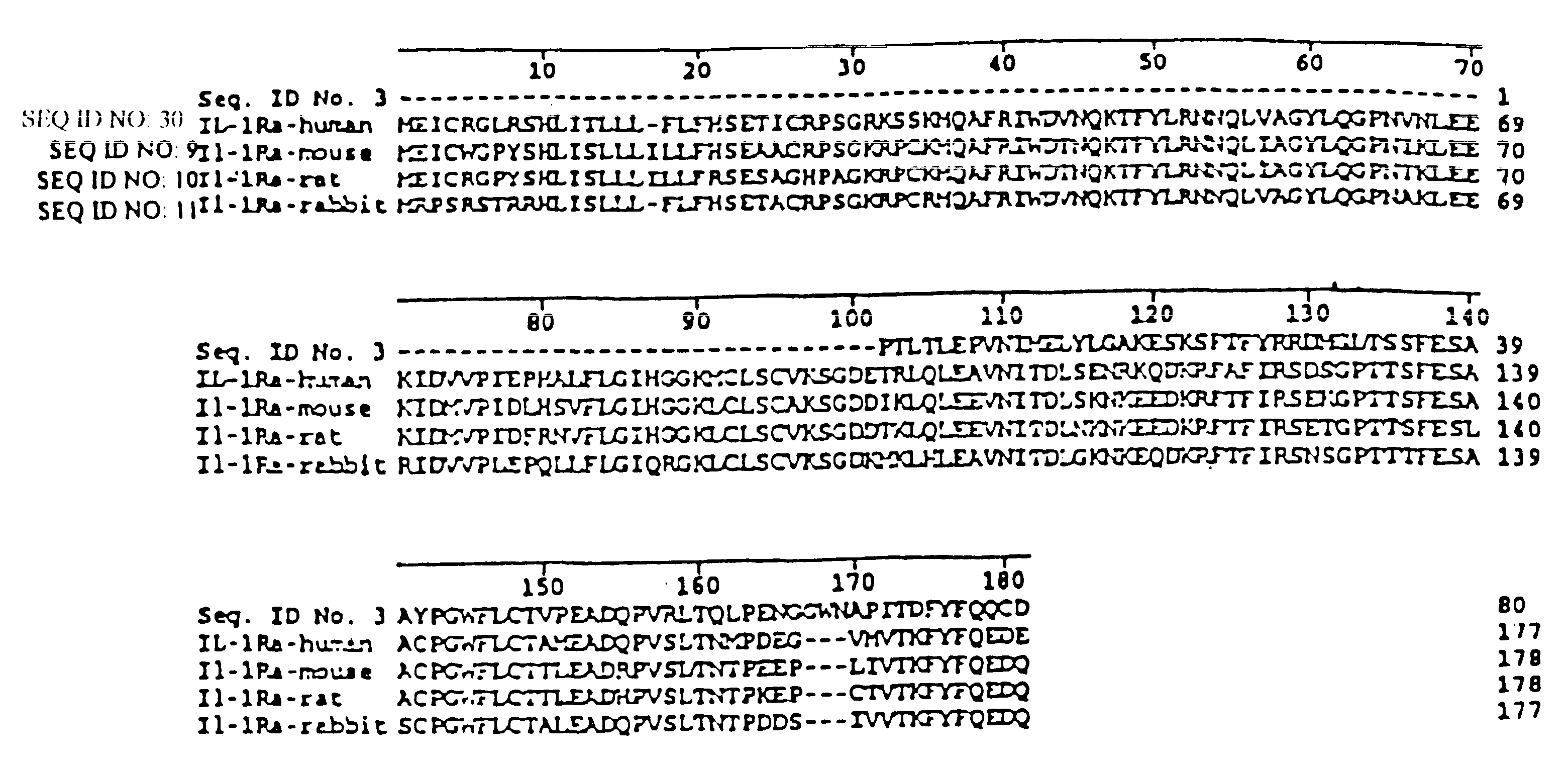
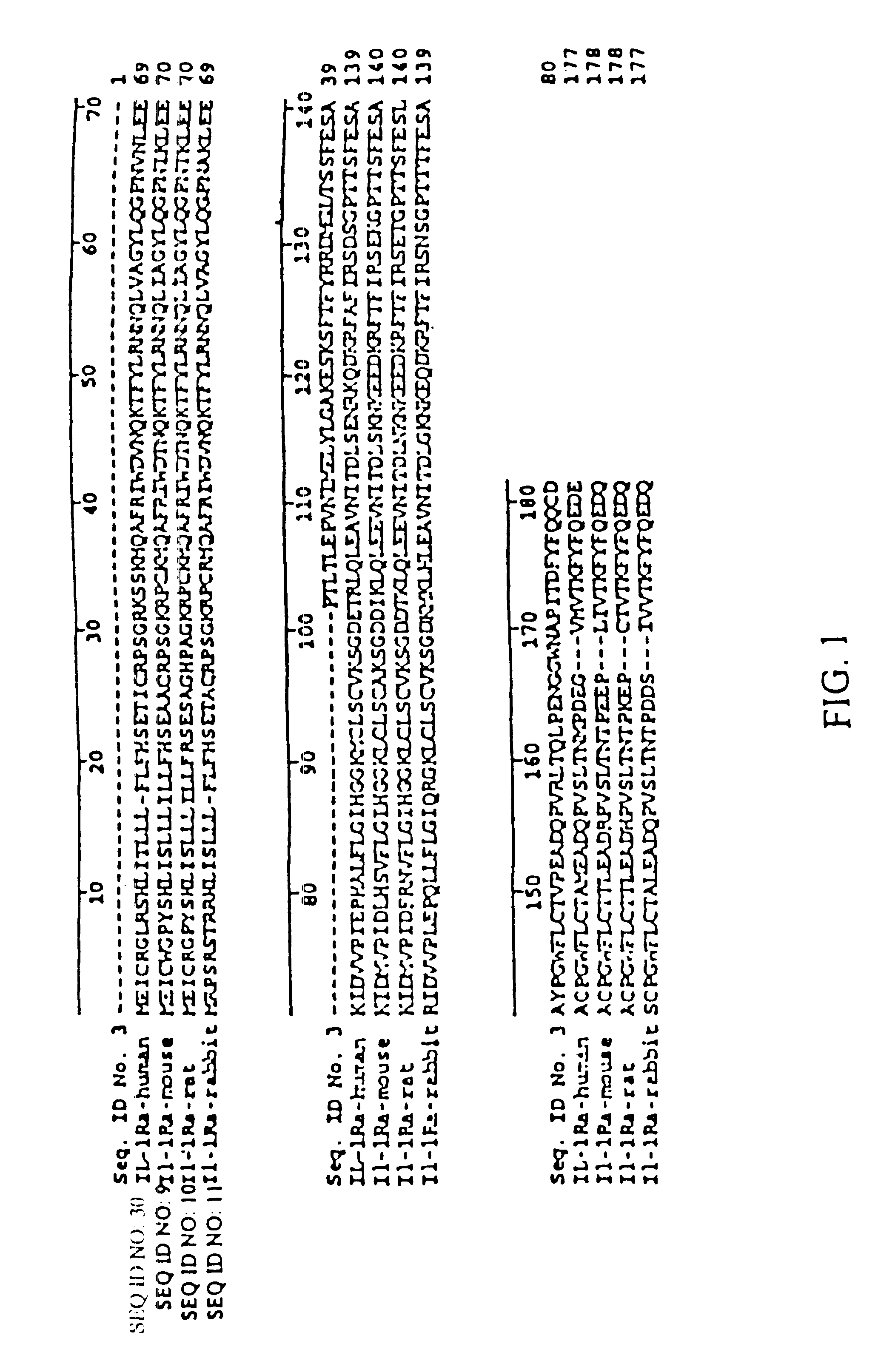
The invention discloses application of an interleukin-1 receptor antagonist in the field of biological medicine and a medicinal composition thereof. The invention discloses application of the following (a) or (b) protein in preparing a medicament for preventing or treating intestinal mucosal epithelial cell injury disease: (a) the protein of which an amino acid sequence is shown in SEQ ID NO: 1; and (b) the protein which has at least 70 percent of homology with the amino acid sequence in the (a) and has the effect of preventing or treating the intestinal mucosal epithelial cell injury disease. The proteins can effectively prevent or treat the intestinal mucosal epithelial cell injury disease, can reduce diarrhea and body weight loss, and reduce and improve the injury of chemotherapy on small intestines.
Owner:USYNOVA PHARM
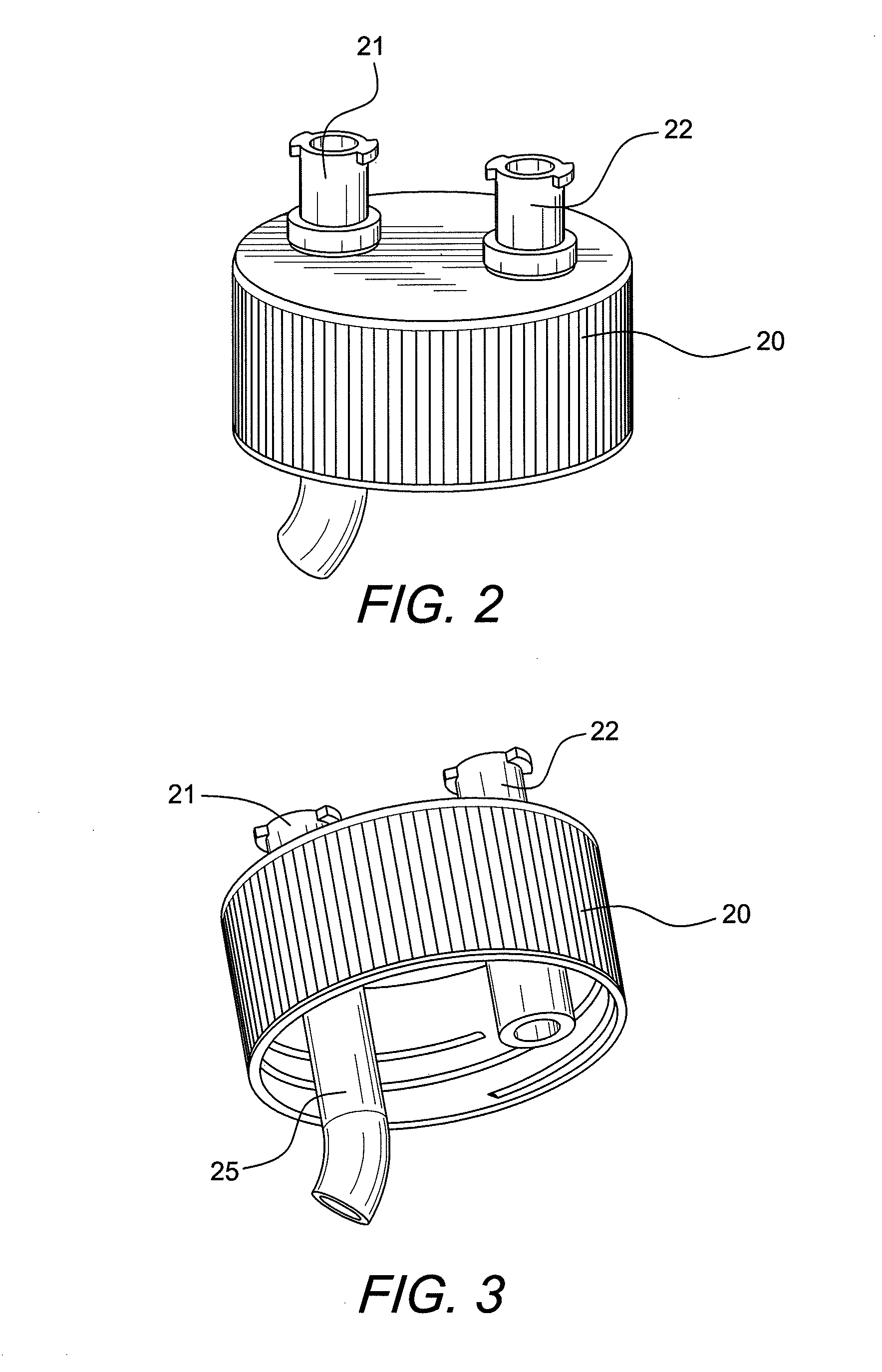
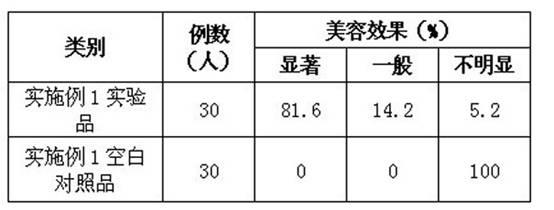
Fuel reforming device
Substances that inhibit the action of the members of the IL-1β / NF-κB pathway can be used for protecting and preserving β-cell mass and function in prediabetic and diabetic type 2 patients. Specifically, the present invention relates to the use of an Interleukin 1 receptor antagonist (IL-1Ra) and / or pyrrolidinedithiocarbamate (PDTC) for the treatment or prophylaxis of type 2 diabetes, as well as a method for the treatment of type 2 diabetes.
Owner:UNIV ZURICH
Interleukin-1 receptor antagonists, compositions, and methods of treatment
InactiveUS20100041609A1Synthetic is simpleInhibition of activationPeptide/protein ingredientsAntipyreticArthritisDrug biological activity
Peptides that are designed to inhibit the biological activity of the IL-1R type 1 receptor and inhibit IL-1R / IL-1RacP related cell signaling and biological activity are disclosed. Compositions comprising IL-1R antagonists of the present invention are useful in the treatment of IL-1 related diseases or conditions such as arthritis, rheumatoid arthritis, osteoarthritis, and inflammatory bowel disease as well as other chronic or acute inflammatory diseases. This invention also discloses an isolated compound having an IL-1R antagonist activity, said compound being selected from the group consisting of: a peptide comprising the amino acid sequence RYTPELX, wherein R, Y, T, P, E, L, refer to their corresponding amino acids, and X is selected from no amino acid and alanine (A); and a derivative of (a) wherein the derivative incorporates one, two or three amino acid modification selected from an amino acid addition, deletion or substitution in the RYTPEL portion of the peptide, and wherein said derivative maintains its antagonist IL-1R activity.
Owner:VALORISATION RECH SOC & COMMANDITE
Recombinant human interleukin-1 receptor antagonist in-situ gel
ActiveCN101912355ASenses disorderPeptide/protein ingredientsResidence timeInterleukin 1 receptor antagonist
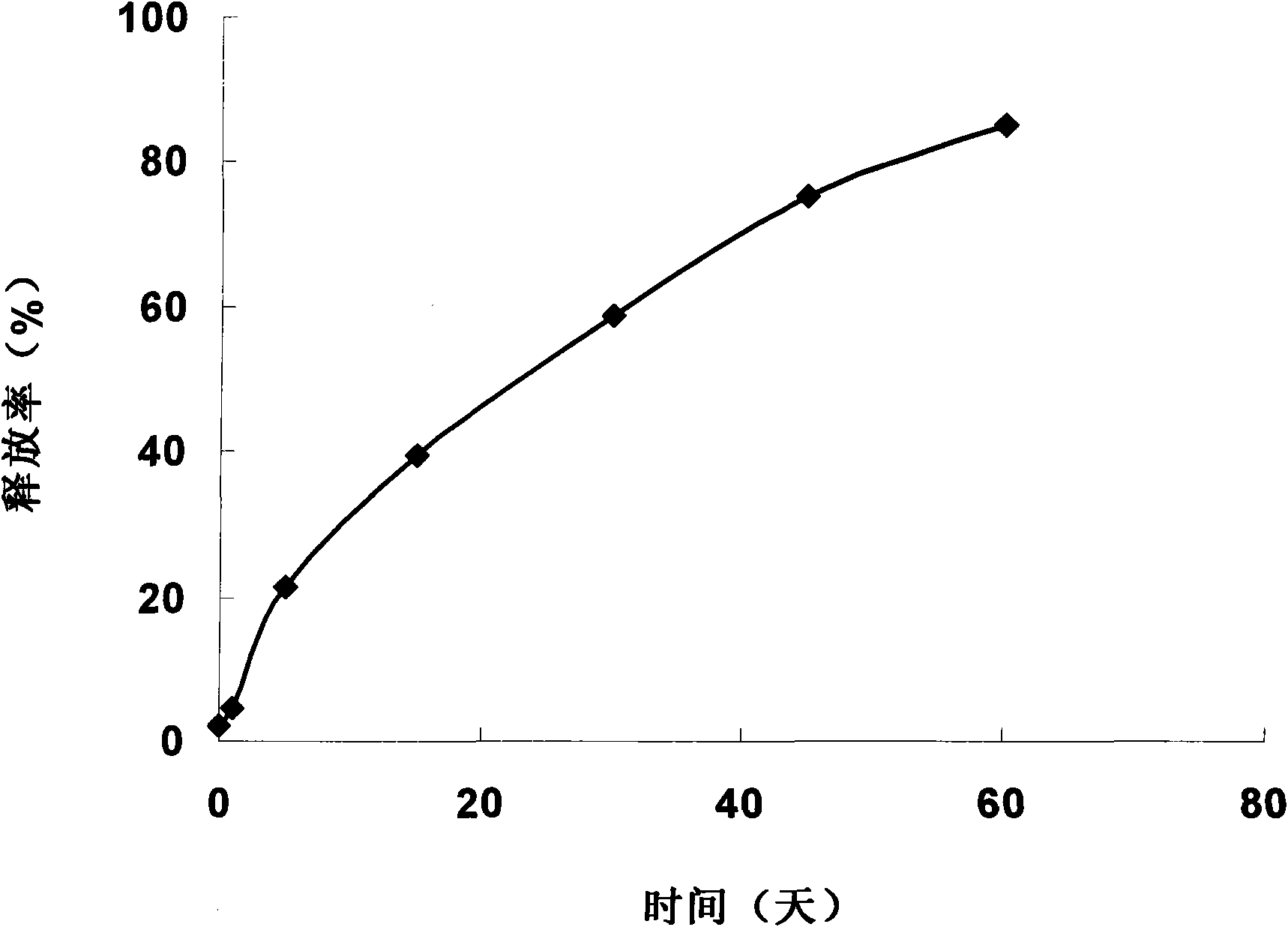
The invention discloses a recombinant human interleukin-1 receptor antagonist in-situ gel, which comprises a recombinant human interleukin-1 receptor antagonist and an in-situ gel material, wherein the in-situ gel material is one or more of a temperature-sensitive in-situ gel material, a pH-sensitive in-situ gel material and an ionic strength-sensitive in-situ gel material. The in-situ gel is flowable liquid at room temperature, has the advantages of easy access (injection, eye drop, nasal drop, spray, lung inhalation and the like), accurate dosage, and capacities of forming high-viscosity hardly flowing gel in short time when contacting tissues, prolonging the residence time in the tissues, increasing the medicament adsorption and fully exerting medicinal effects. The in-situ gel has the advantages of adhesiveness, slow release, and capacities of greatly improving the medicinal bioavailability and achieving possible sustained-release long-acting effect.
Owner:HAINAN ZHONGSEN BIOTECH CO LTD
Nucleic encoding interleukin-1 receptor antagonist-like proteins and uses thereof
The present invention provides novel Interleukin-1 Receptor Antagonist-Like (IL-1ra-L) polypeptides and nucleic acid molecules encoding the same. The invention also provides selective binding agents, vectors, host cells, and methods for producing IL-1ra-L polypeptides. The invention further provides pharmaceutical compositions and methods for the diagnosis, treatment, amelioration, and / or prevention of diseases, disorders, and conditions associated with IL-1ra-L polypeptides.
Owner:AMGEN INC
Functionally modified polypeptides and radiobiosynthesis
ActiveUS20180066298A1Stable to in vivo hydrolysisImprove in vivo stabilityIsotope introduction to peptides/proteinsLigasesIL1 InhibitorSerum albumin
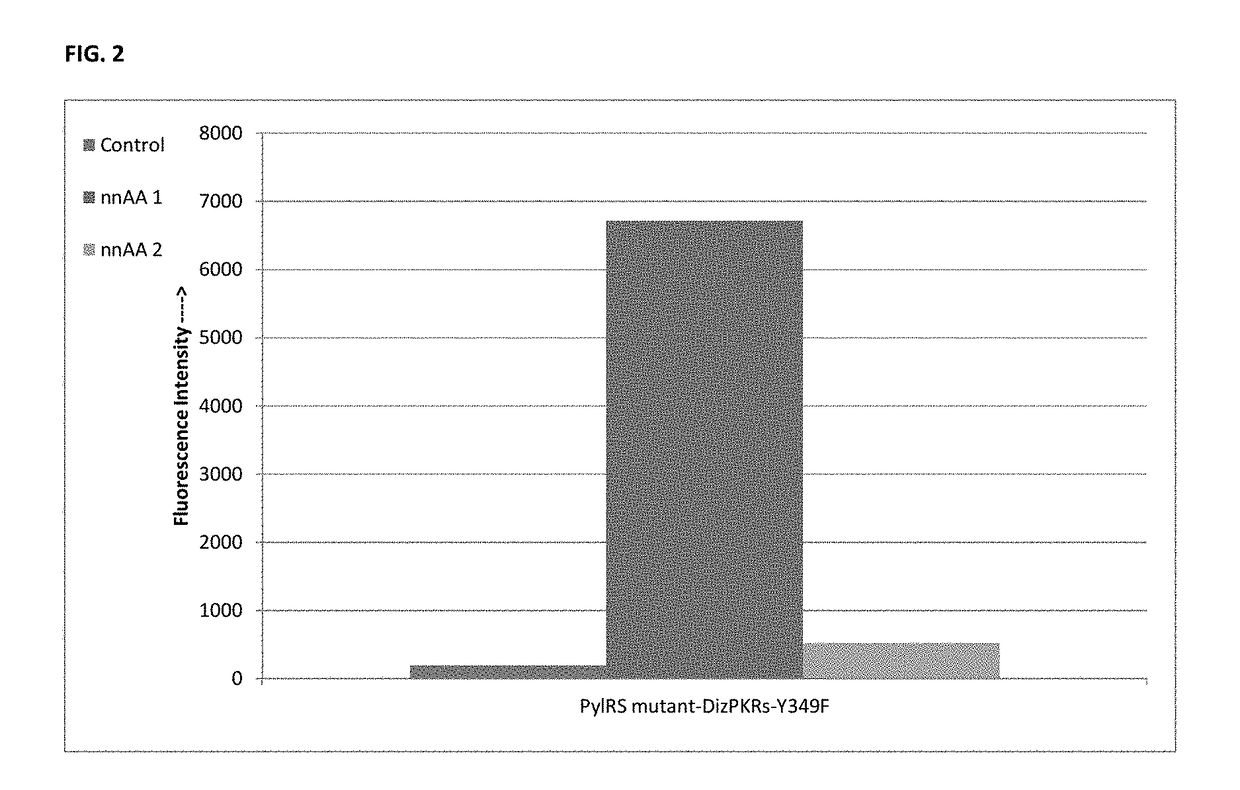
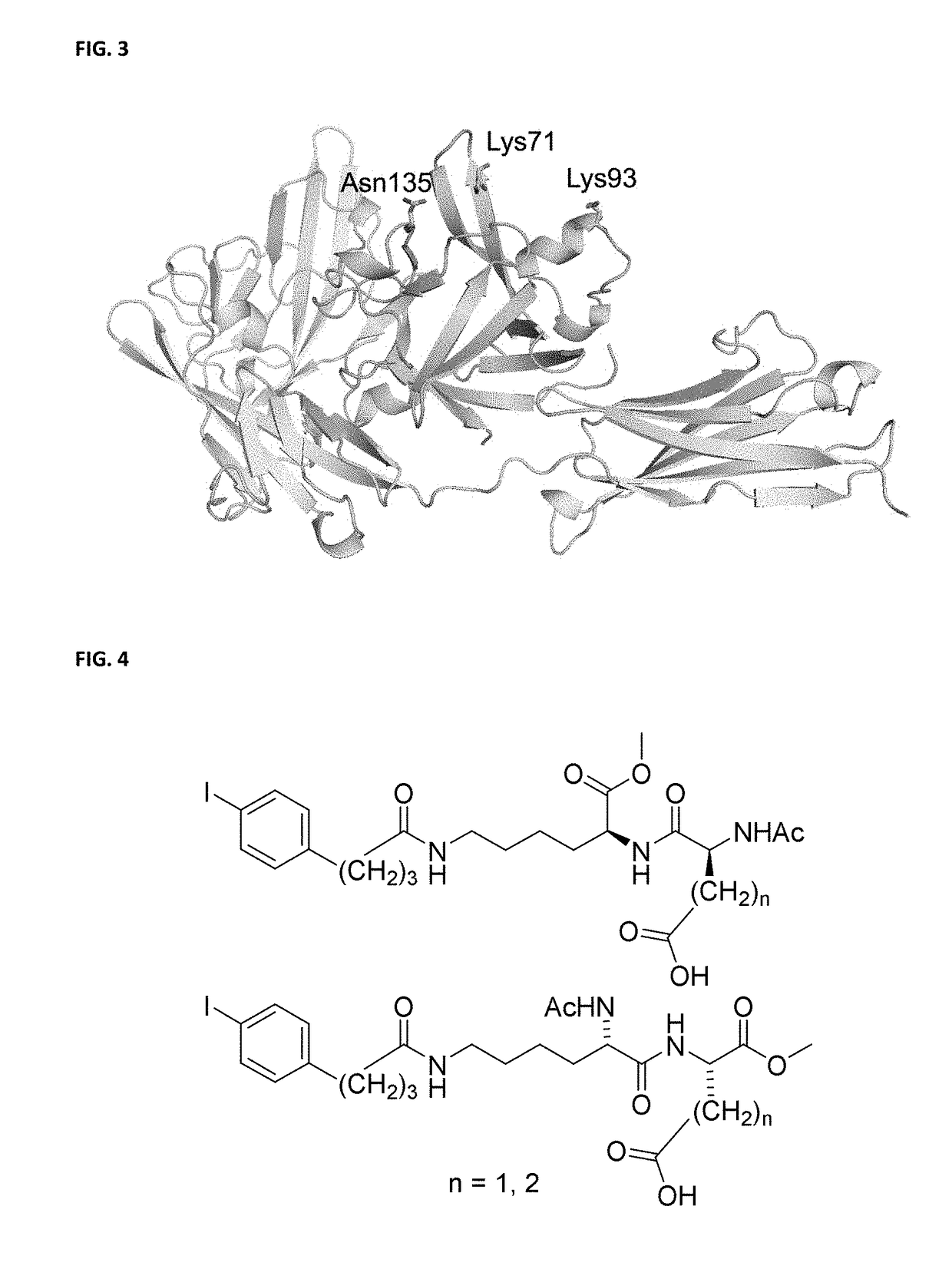
Provided herein are compositions and methods for generating polypeptides using non-natural amino acids (nnAAs) and genetic machinery, wherein the modified polypeptides, such as therapeutic polypeptides, bind to albumin, such as serum albumin. Methods of substituting a non-natural amino acid in a first polypeptide to obtain a modified polypeptide, the nnAA in some instances comprising an albumin targeting group, are disclosed, as are methods for making populations of such modified polypeptides. A therapeutic polypeptide, interleukin-1 receptor antagonist (IL-1RA) is exemplified using the disclosed methods.
Owner:IKARIA
Formula of medicament for treating non-infectious ocular inflammations, and inhibiting corneal neovascularization and anti-rejection reaction generated after corneal grafting
InactiveCN102210864AHigh activityEasy to storeSenses disorderPeptide/protein ingredientsMicrosphereRetention time
The invention discloses a formula of a medicament for treating non-infectious ocular inflammations, and inhibiting the corneal neovascularization and the anti-rejection reaction generated after corneal grafting. Through the selection of an isotonizing agent and a buffering agent and the addition of an antiseptic agent, a release microsphere preparation, a recombinant human interleukin-8 receptor antagonist and a chemokine-like factors 1 (CKLF1), the activity of a recombinant human interleukin-1 receptor antagonist is enhanced, and the retention time thereof is extended. In the invention, the problem that in the prior art, because of having a high environmental requirement on the outside, the existing recombinant human interleukin-1 receptor antagonist is easy to inactivate is solved; and through adding the recombinant human interleukin-8 receptor antagonist and the chemokine-like factors 1 (CKLF1) into the medicament, a synergistic effect is generated among the recombinant human interleukin-8 receptor antagonist, the chemokine-like factors 1 (CKLF1) and the recombinant human interleukin-1 receptor antagonist, thereby reducing the dosage of the medicament.
Owner:BEIJING DAZHOU HEKANG BIO TECH
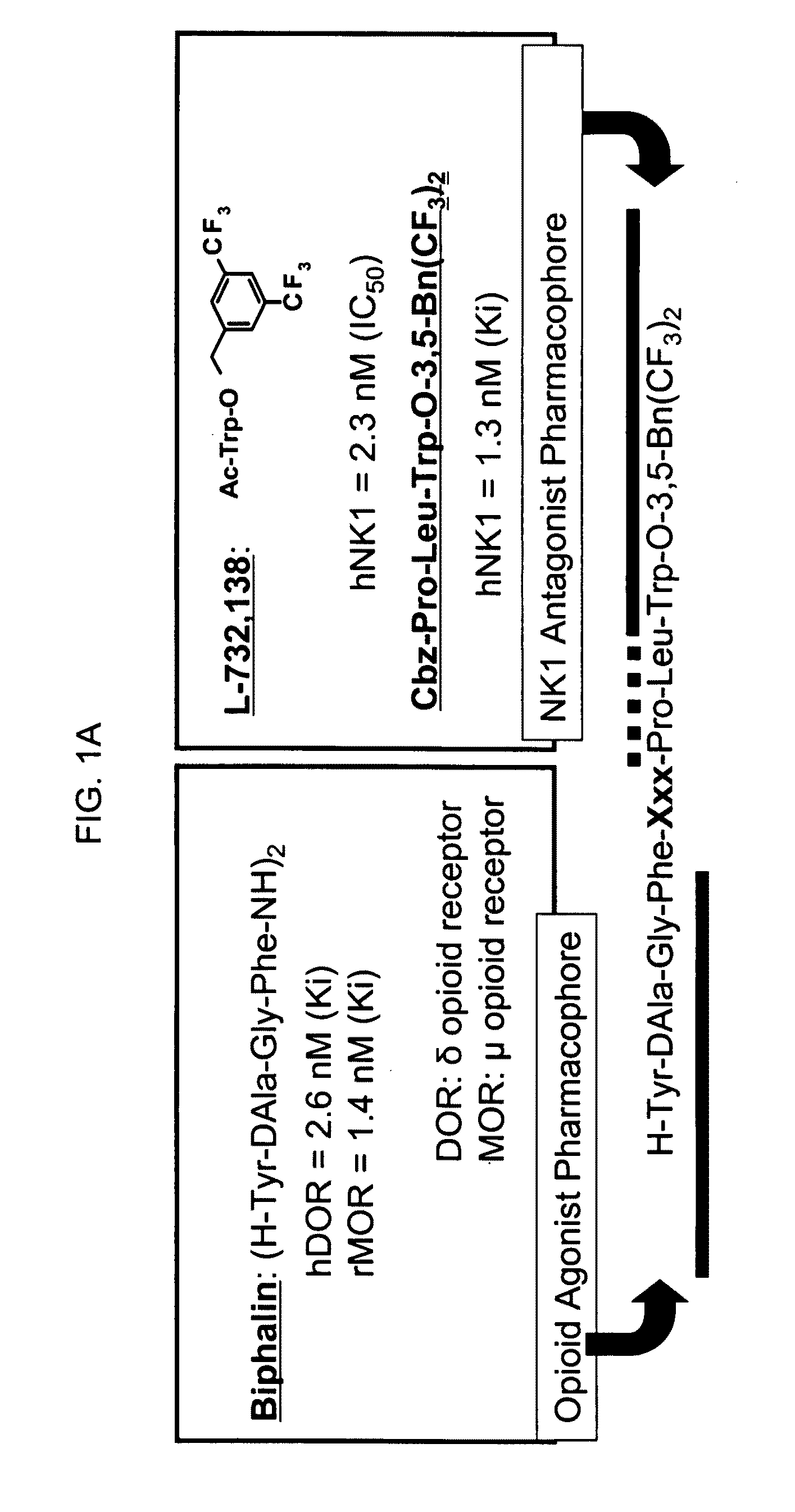
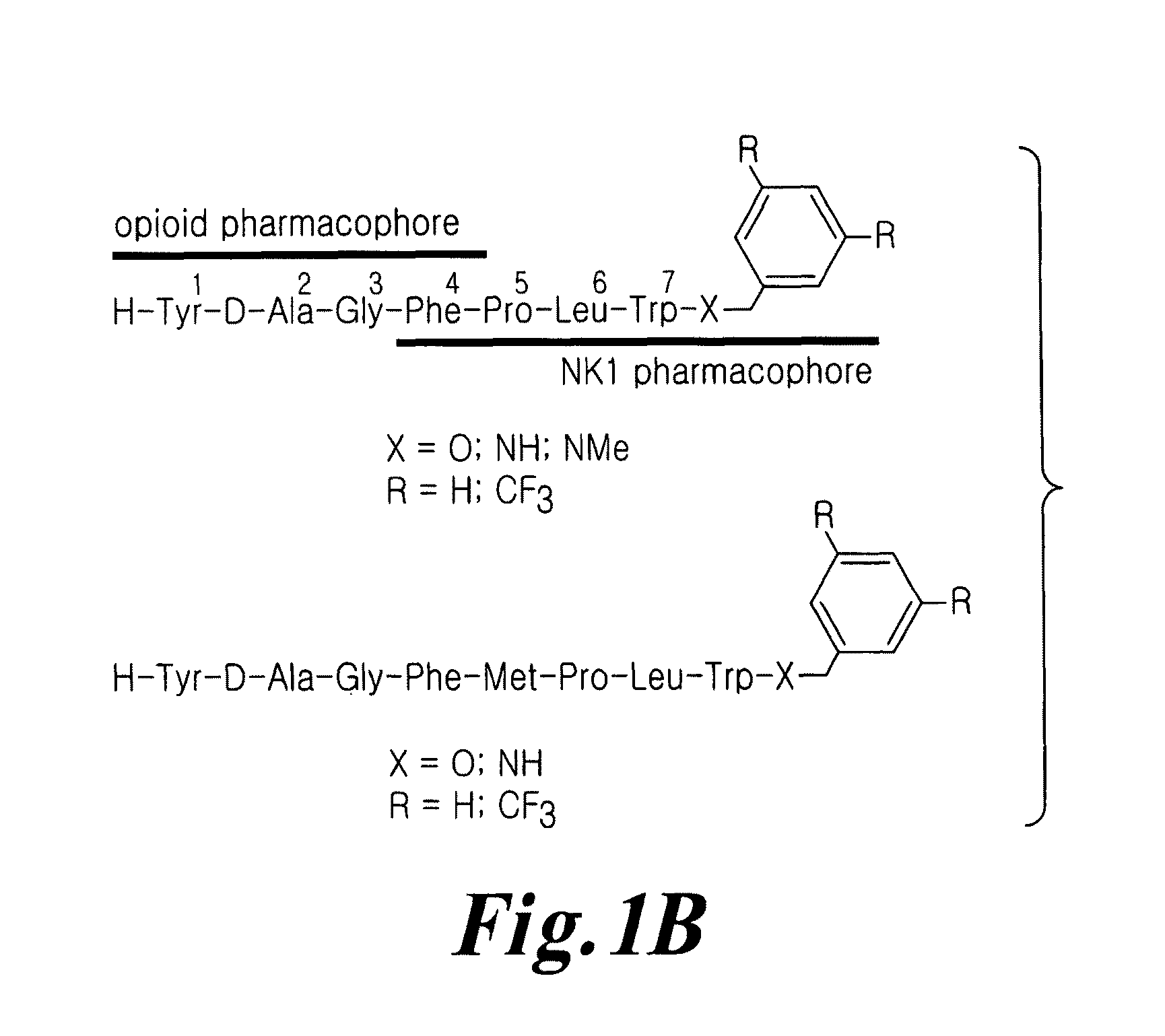
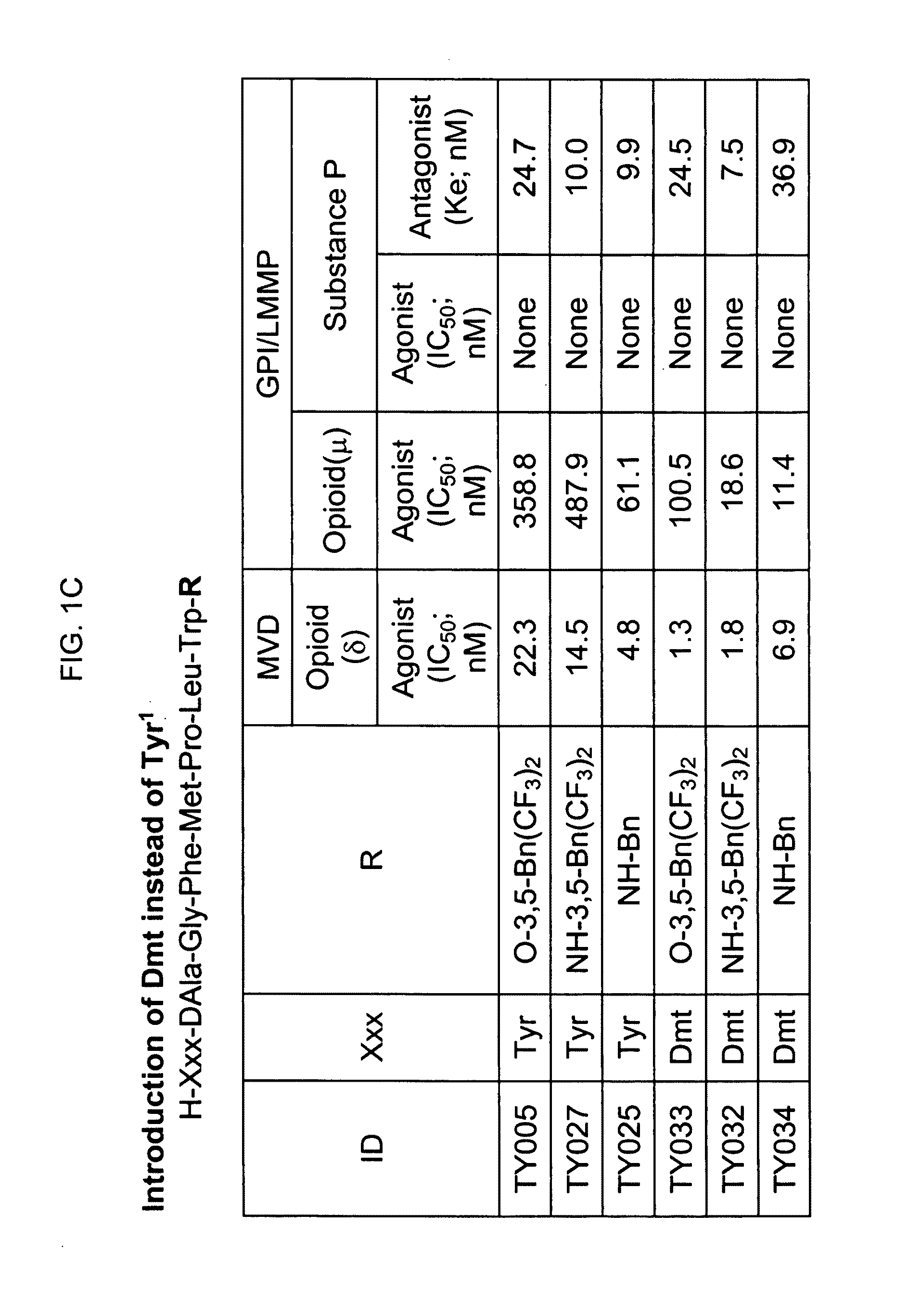
Bifunctional analgesic compounds for opioid receptor agonists and neurokinin-1 receptor antagonists
InactiveUS8026218B2Nervous disorderTripeptide ingredientsNeurokinin-1 Receptor AntagonistsΜ-opioid receptor
The present invention provides a novel chimeric compound comprising an agonist opioid receptor binding moiety at its N-terminus and an antagonist neurokinin-1 (NK1) receptor binding moiety at its C-terminus for producing analgesia, a pharmaceutical composition comprising the chimeric compound, a method of making the compound, and a method of treating pain using the novel chimeric compounds.
Owner:UNIV ARIZONA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com