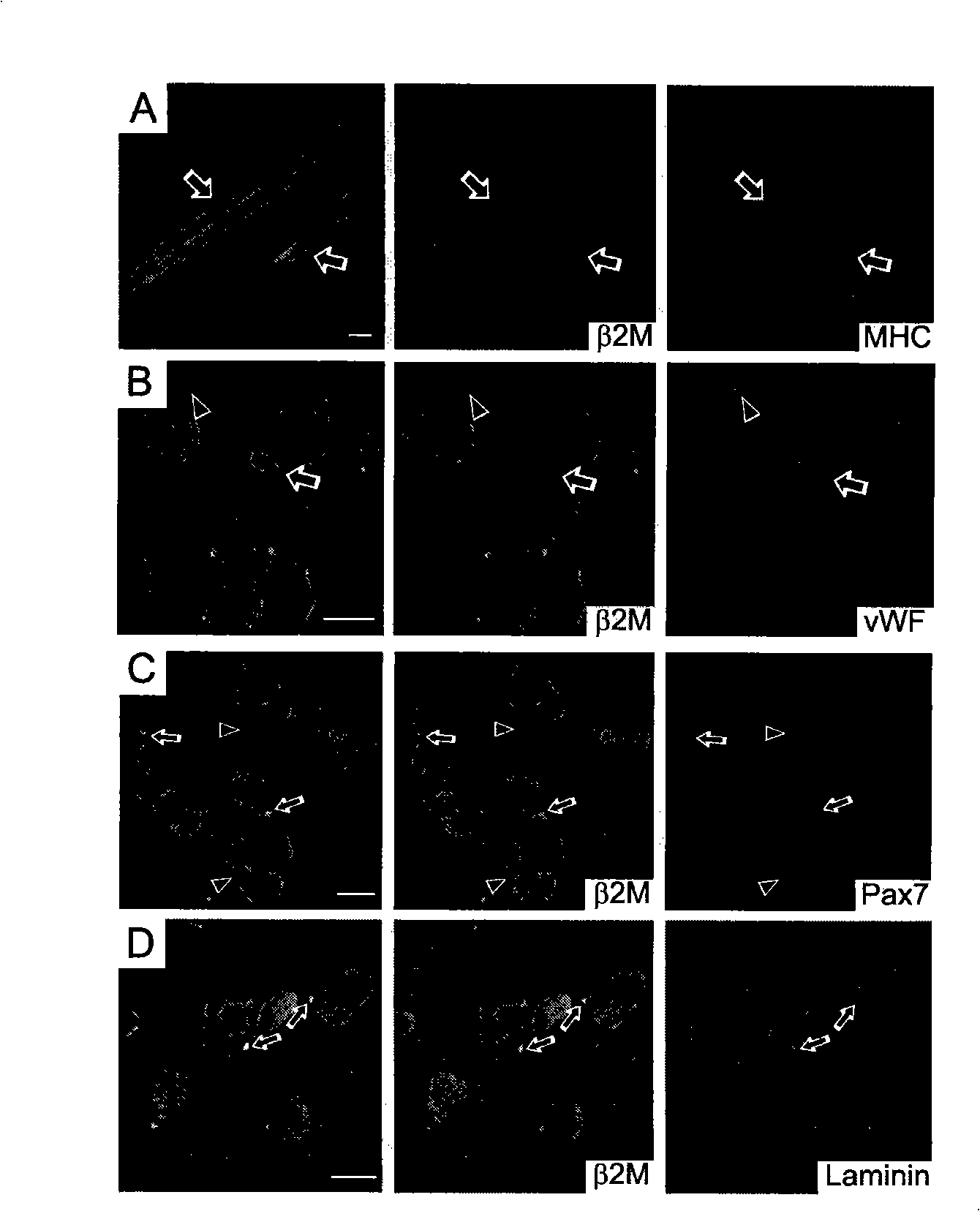
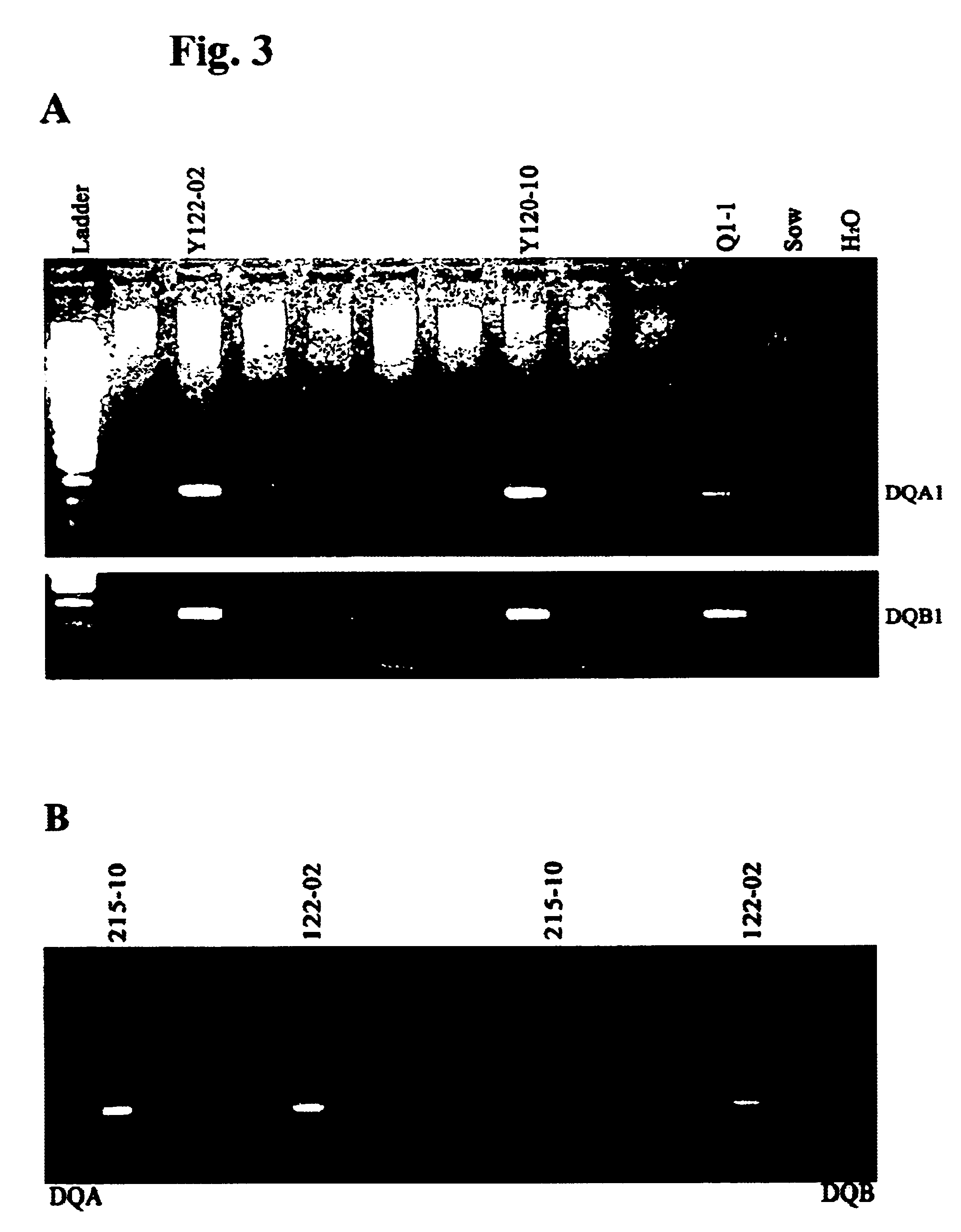
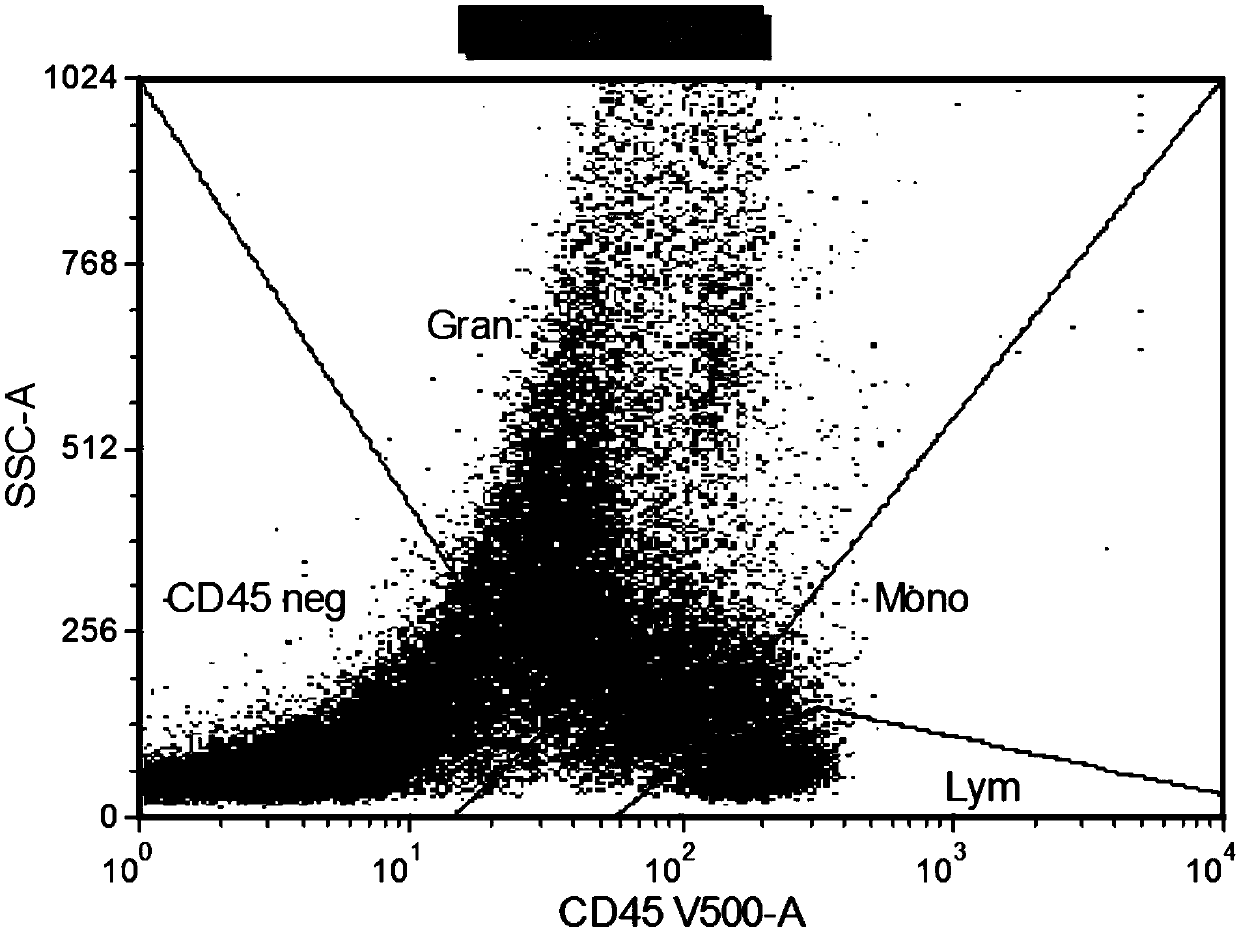
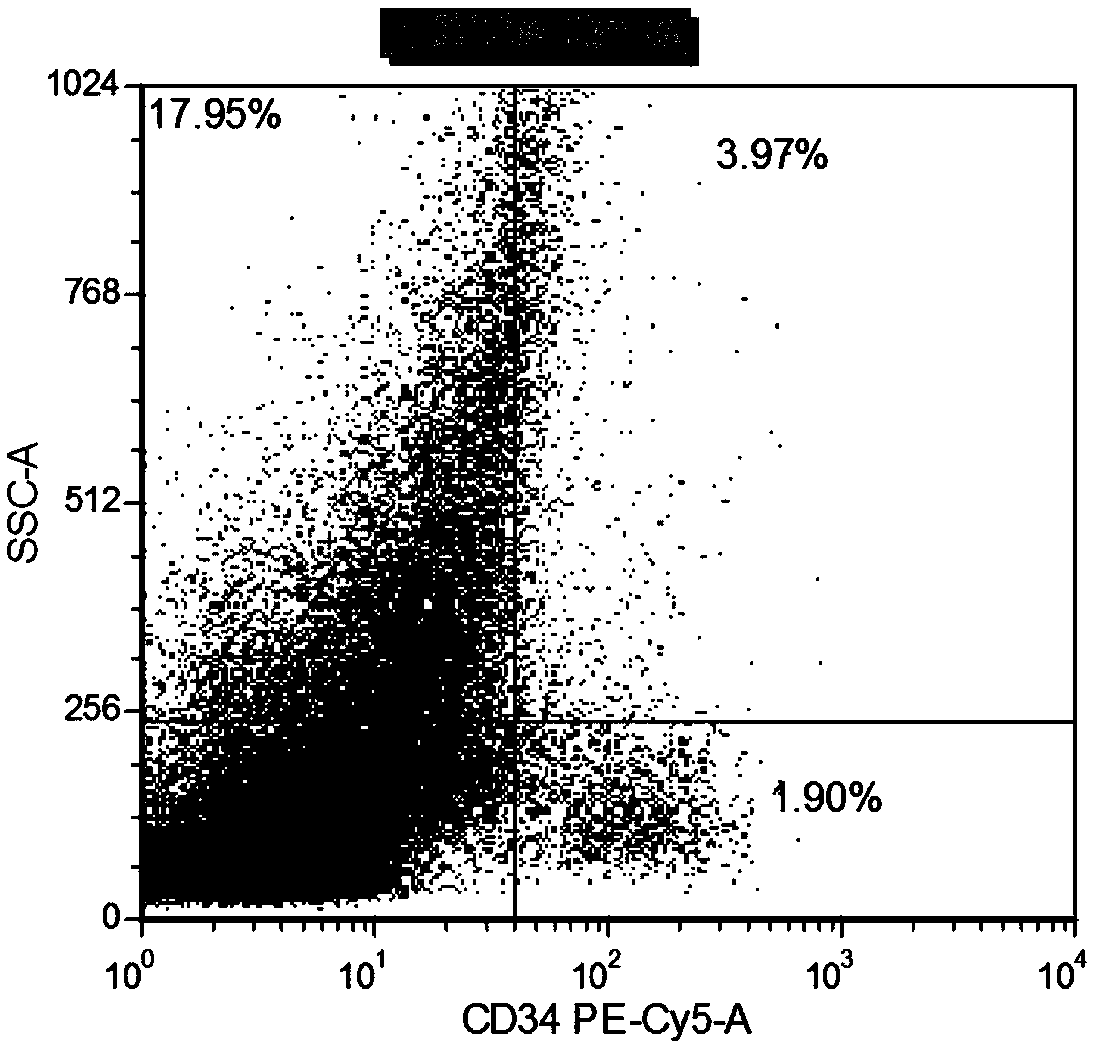
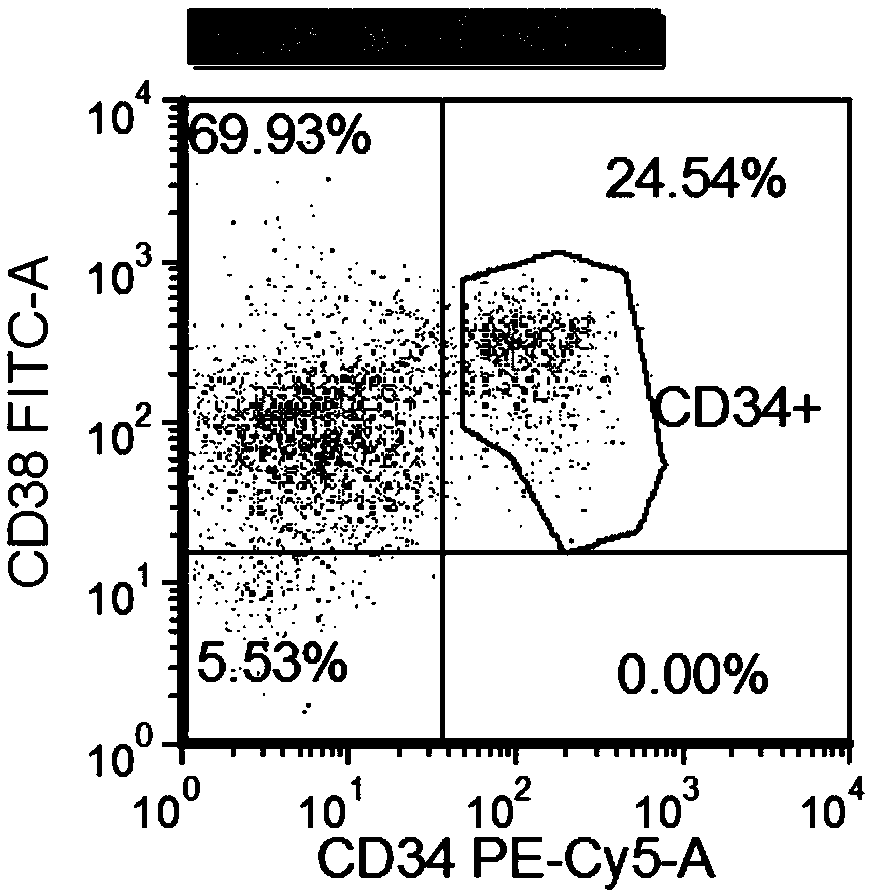
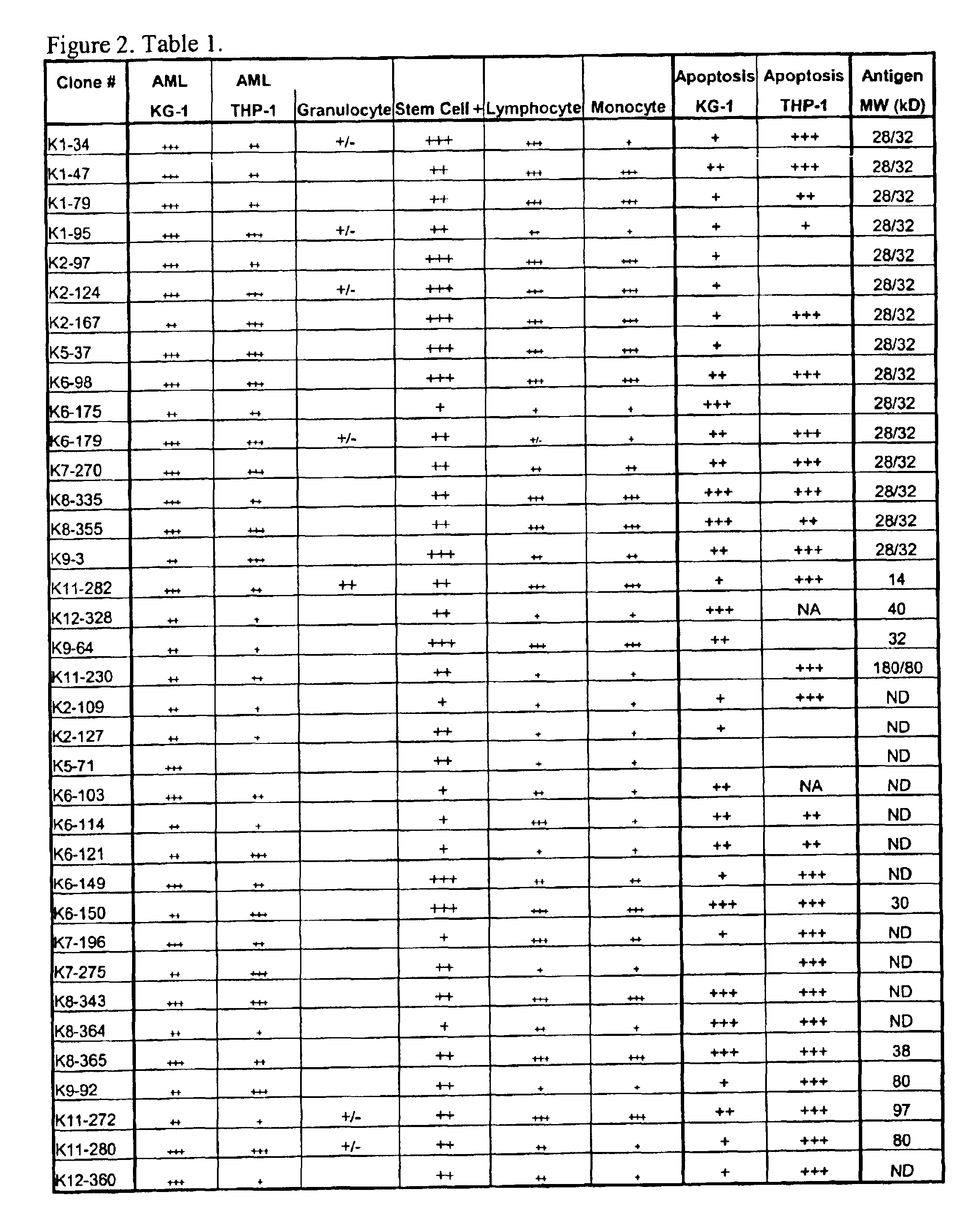
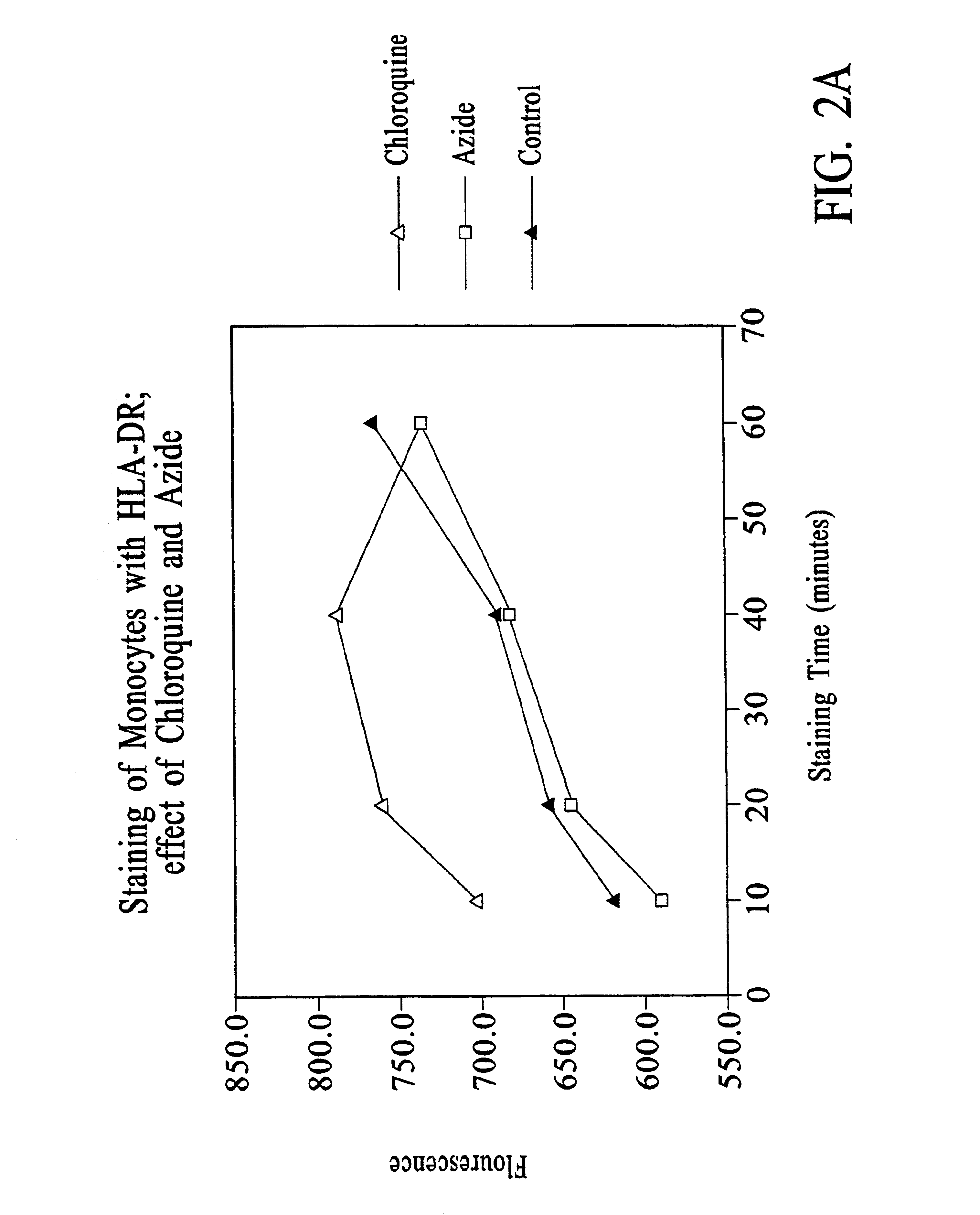
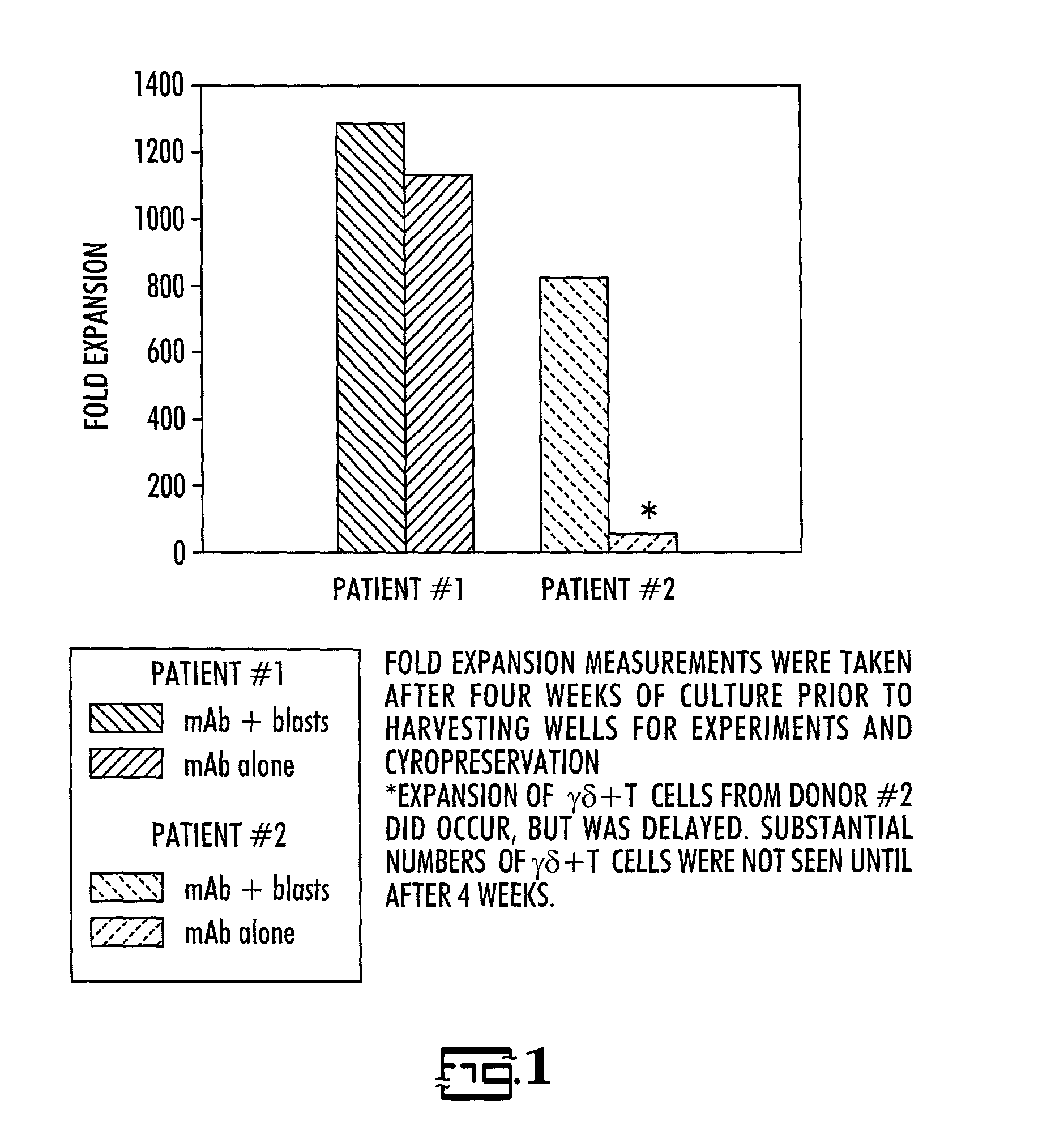
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Human leukocyte antigen DR" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HLA-DR is an MHC class II cell surface receptor encoded by the human leukocyte antigen complex on chromosome 6 region 6p21.31. The complex of HLA-DR (Human Leukocyte Antigen – antigen D Related) and its ligand, a peptide of 9 amino acids in length or longer, constitutes a ligand for the T-cell receptor (TCR).
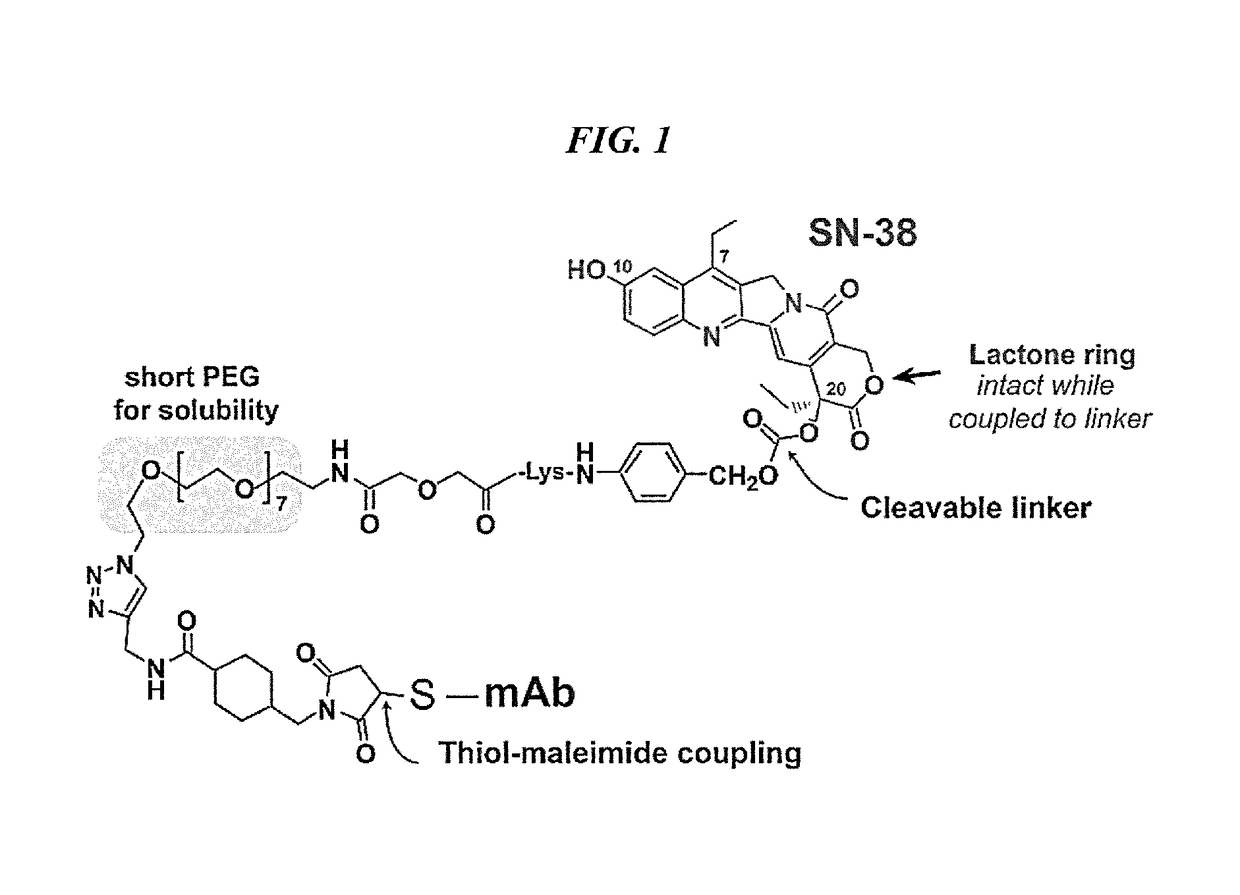
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
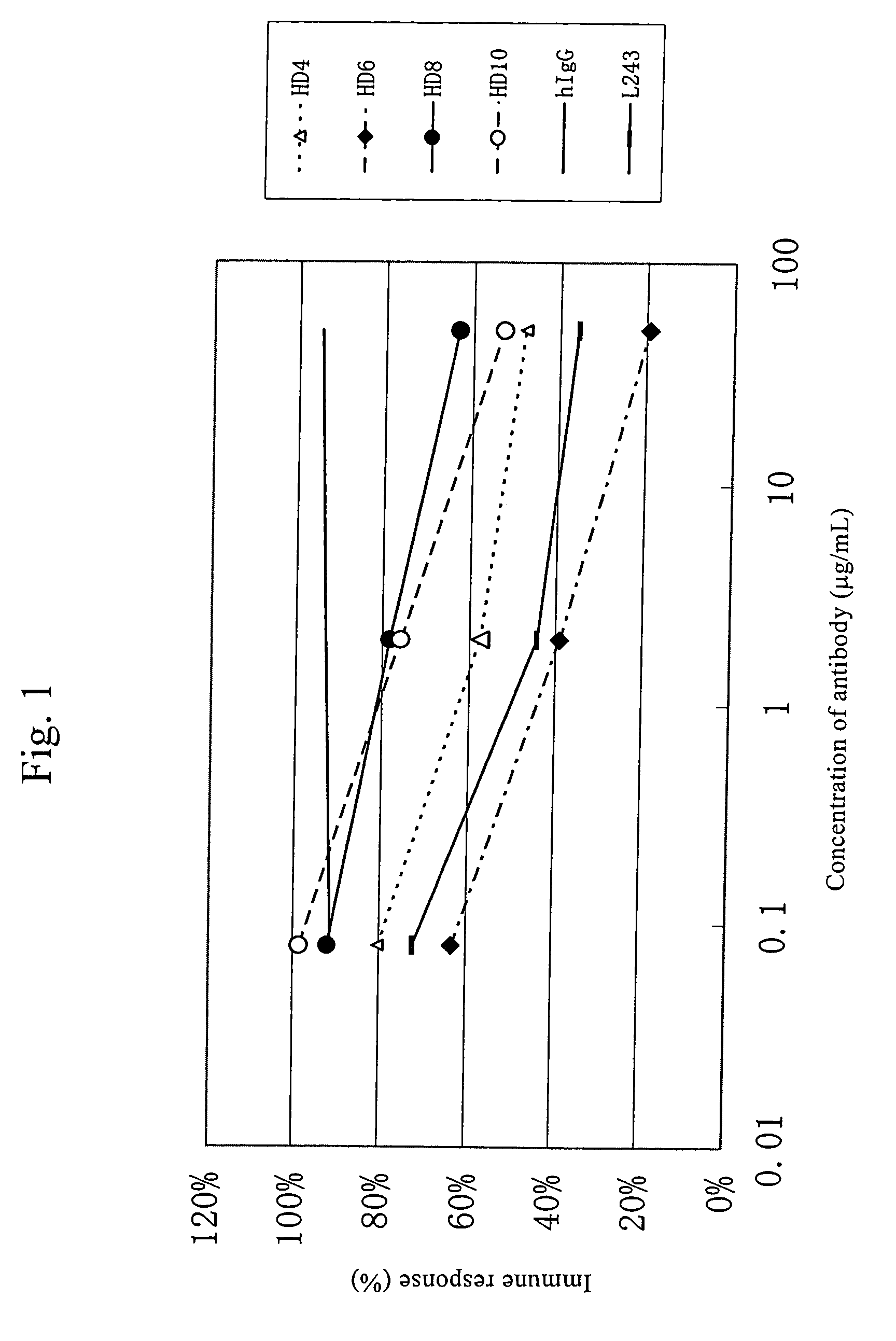
Anti-HLA-DR antibody
InactiveUS7262278B2Suppress lowering survival ratioSurvival ratioFungiBacteriaCancer cellMonoclonal antibody
This invention provides an anti-HLA-DR monoclonal antibody. This invention relates to an antibody binding to HLA-DR or a functional fragment thereof having (a) life-extending effects in nonhuman animals bearing HLA-DR-expressing cancer cells and (b) activity of suppressing immune responses lower than that of L243, or an antibody binding to HLA-DR or a functional fragment thereof exhibiting immunosuppressive activity equivalent to or higher than that of the mouse anti-HLA-DR monoclonal antibody L243 (ATCC HB-55).
Owner:KIRIN BREWERY CO LTD
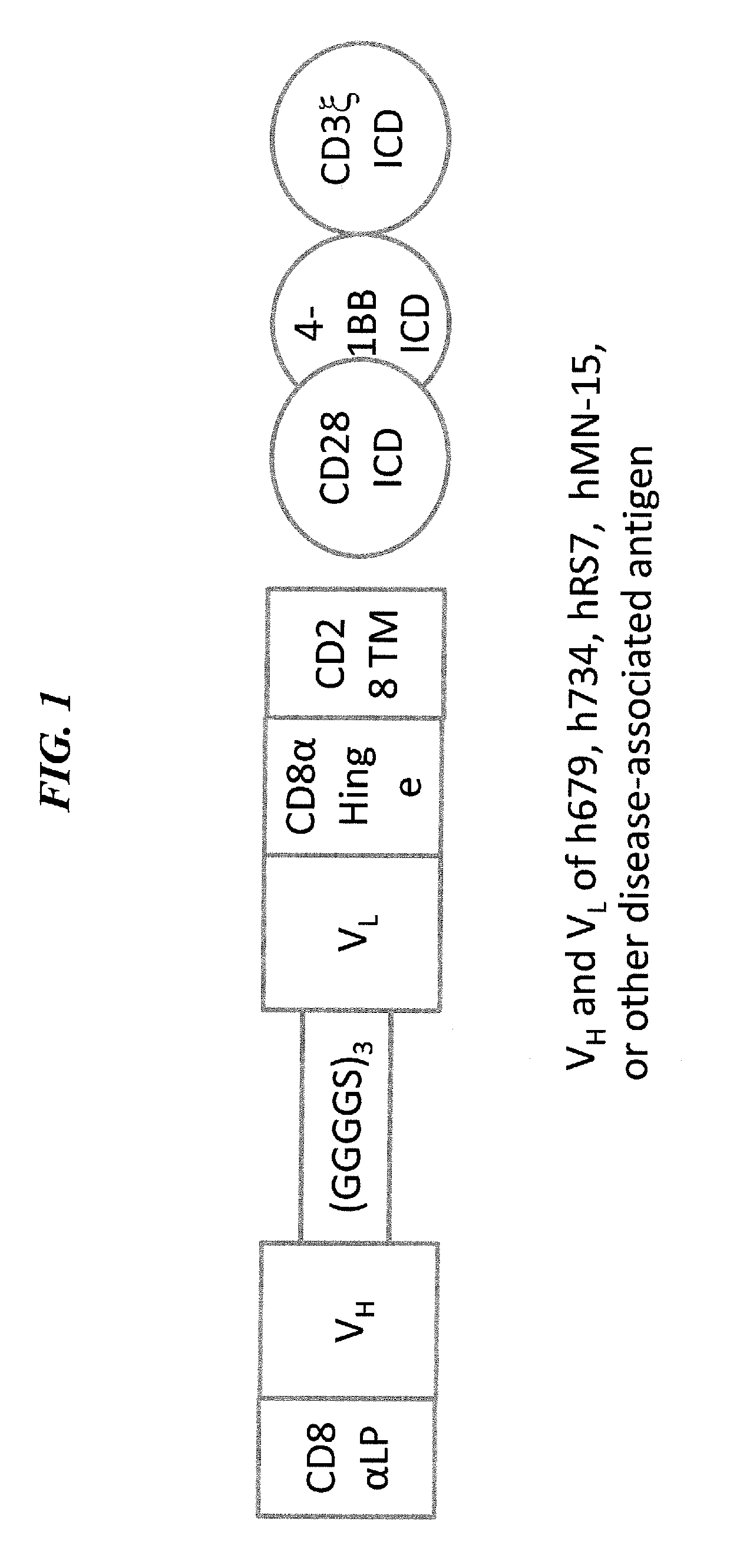
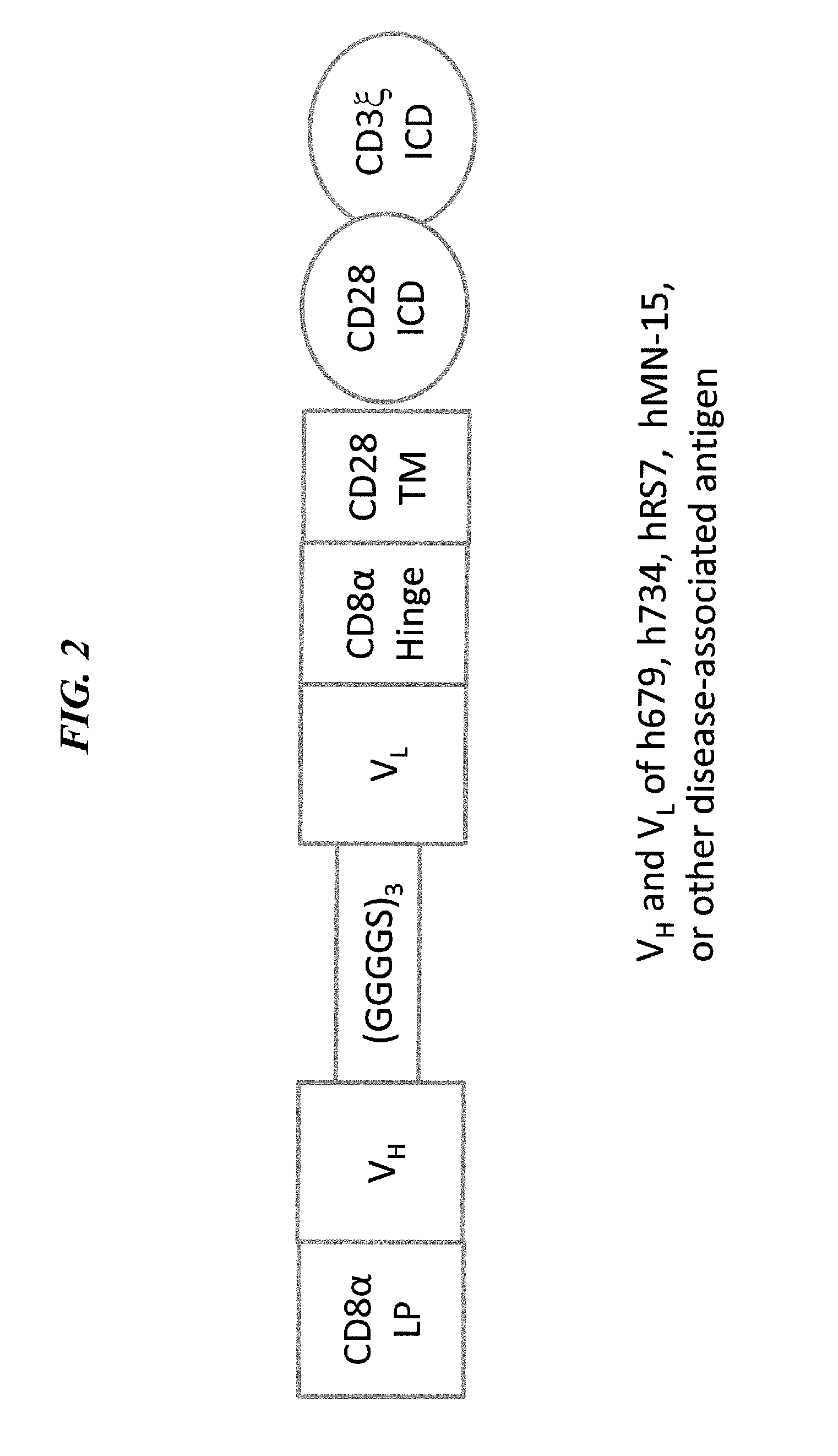
Disease therapy with chimeric antigen receptor (CAR) constructs and t cells (car-t) or nk cells (car-nk) expressing car constructs
InactiveUS20160361360A1Maintain self-toleranceModulate durationAntibacterial agentsPeptide/protein ingredientsAutoimmune conditionDebulking Procedure
The present invention concerns CAR, CAR-T and CAR-NK constructs, preferably comprising a scFv antibody fragment against a disease-associated antigen or a hapten. More preferably, the antigen is a TAA, such as Trop-2. The constructs may be administered to a subject with a disease, such as cancer, autoimmune disease, or immune dysfunction disease, to induce an immune response against disease-associated cells. Where the constructs bind to a hapten, the subject is first treated with a hapten-conjugated antibody that binds to a disease associated antigen. Therapy may be supplemented by other treatments, such as debulking procedures (e.g., surgery, chemotherapy, radiation therapy) or coadministration of other agents. More preferably, administration of the construct is preceded by predosing with an unconjugated antibody that binds to the same disease-associated antigen. Most preferably, an antibody against CD74 or HLA-DR is administered to reduce systemic immunotoxicity induced by the constructs.
Owner:IMMUNOMEDICS INC
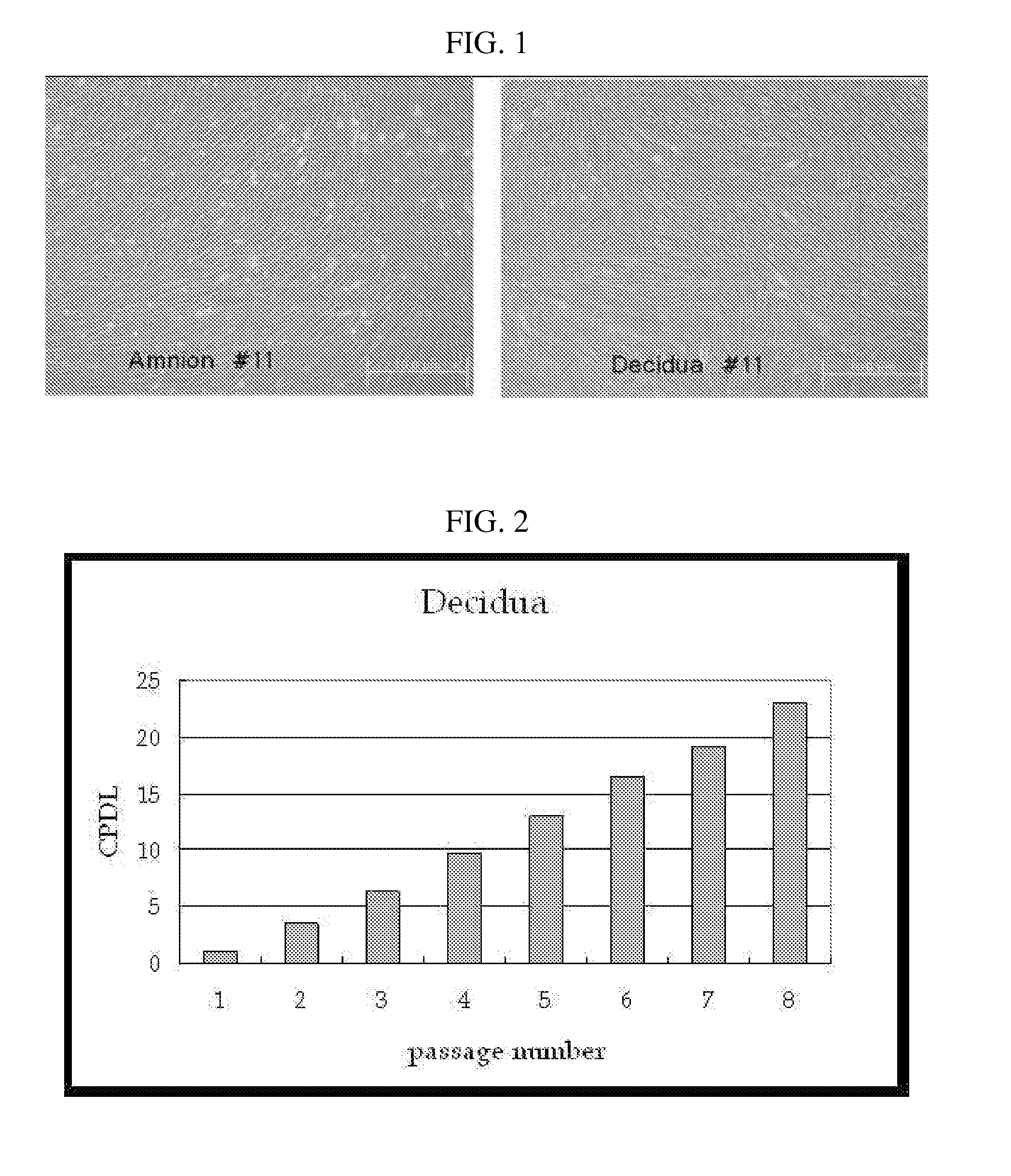
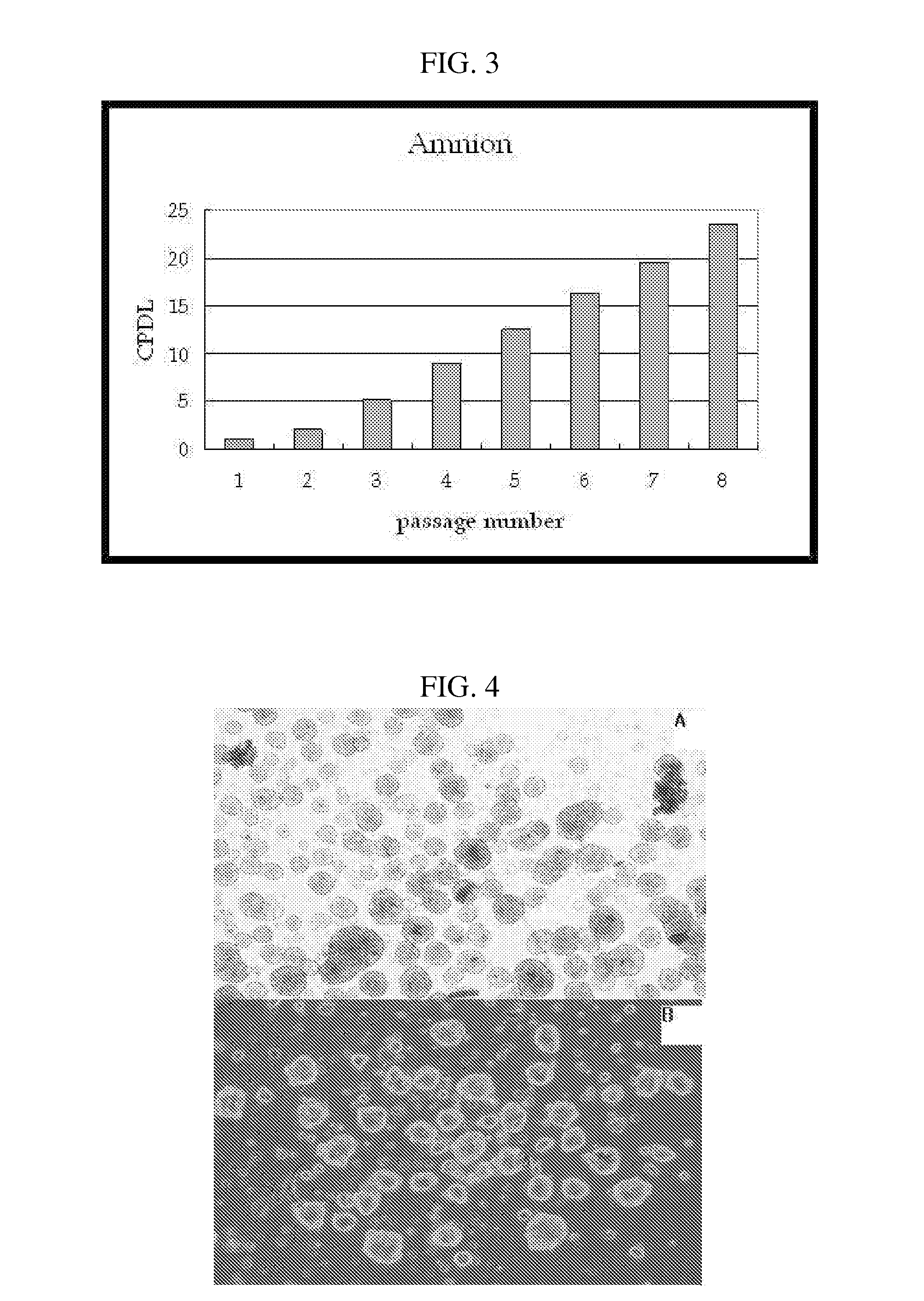
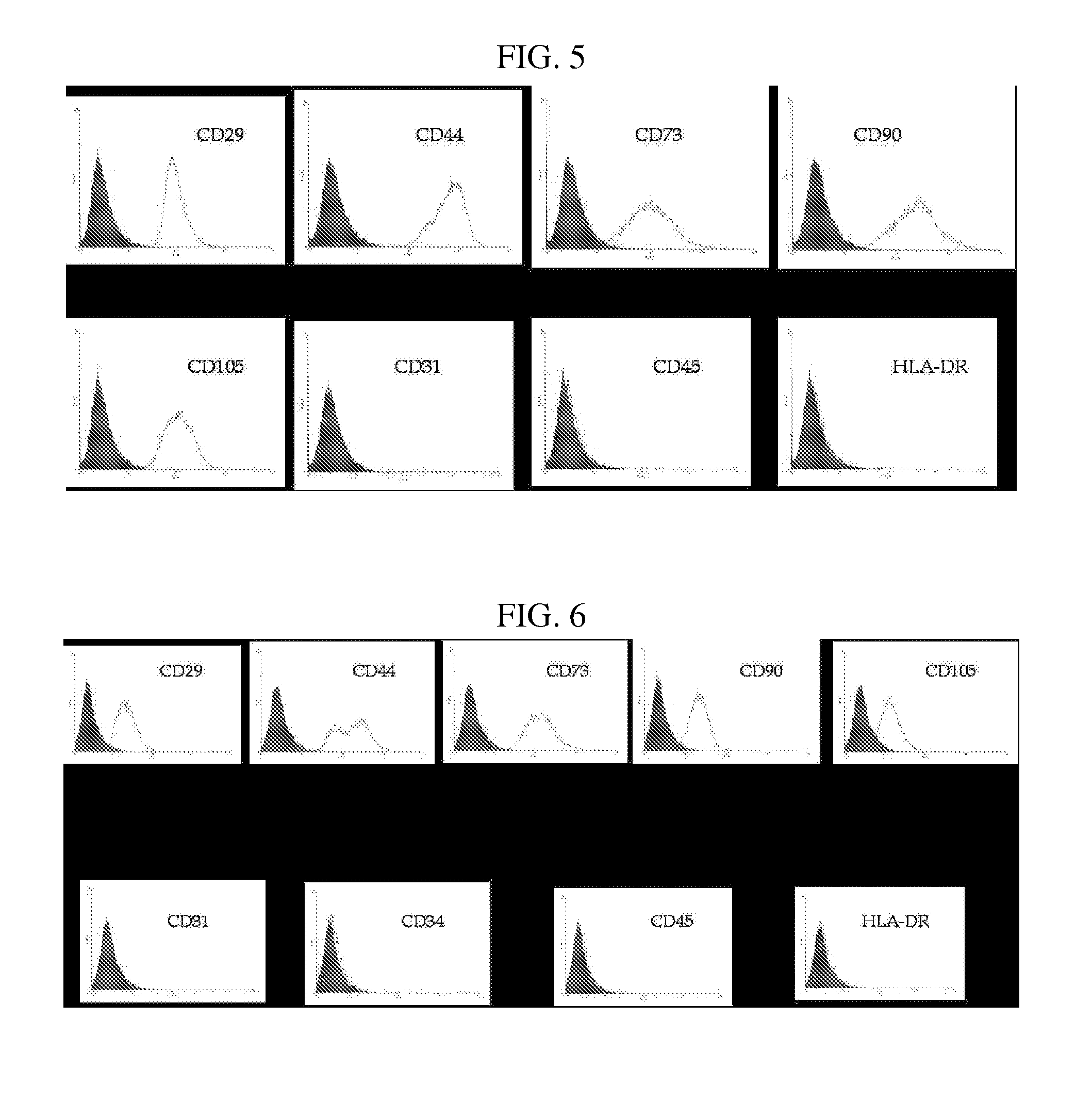
Multipotent stem cells derived from placenta tissue and cellular therapeutic agents comprising the same
InactiveUS20070243172A1Negative immunological responseBiocideArtificial cell constructsGerm layerDisease
The present invention relates to placenta tissue-derived multipotent stem cells and cell therapeutic agents containing the same. More specifically, to a method for producing placenta stem cells having the following characteristics, the method comprising culturing amnion, chorion, decidua or placenta tissue in a medium containing collagenase and bFGF and collecting the cultured cells: (a) showing a positive immunological response to CD29, CD44, CD73, CD90 and CD105, and showing a negative immunological response to CD31, CD34, CD45 and HLA-DR; (b) showing a positive immunological response to Oct4 and SSEA4; (c) growing attached to plastic, showing a round-shaped or spindle-shaped morphology, and forming spheres in an SFM medium so as to be able to be maintained in an undifferentiated state for a long period of time; and (d) having the ability to differentiate into mesoderm-, endoderm- and ectoderm-derived cells. Also the present invention relates to placenta stem cells obtained using the production method. The inventive multipotent stem cells have the ability to differentiate into muscle cells, vascular endothelial cells, osteogenic cells, nerve cells, satellite cells, fat cells, cartilage-forming cells, osteogenic cells, or insuline-secreting pancreatic β-cells, and thus are effective for the treatment of muscular diseases, osteoporosis, osteoarthritis, nervous diseases, diabetes and the like, and are useful for the formation of breast tissue.
Owner:RNL BIO
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140170063A1Overcome tumorImprove targetingHeavy metal active ingredientsOrganic active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 ROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of Immunoconjugates of Antibodies and SN-38 for Improved Efficacy and Decreased Toxicity
ActiveUS20140219914A1Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsRadioactive preparation carriersCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
Dosages of immunoconjugates of antibodies and SN-38 for improved efficacy and decreased toxicity
ActiveUS9028833B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsCD20Lymphatic Spread
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an antibody or antigen-binding antibody fragment. The antibody may bind to EGP-1 (TROP-2), CEACAM5, CEACAM6, CD74, CD19, CD20, CD22, CSAp, HLA-DR, AFP or MUC5ac and the immunoconjugate may be administered at a dosage of between 4 mg / kg and 24 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy.
Owner:IMMUNOMEDICS INC
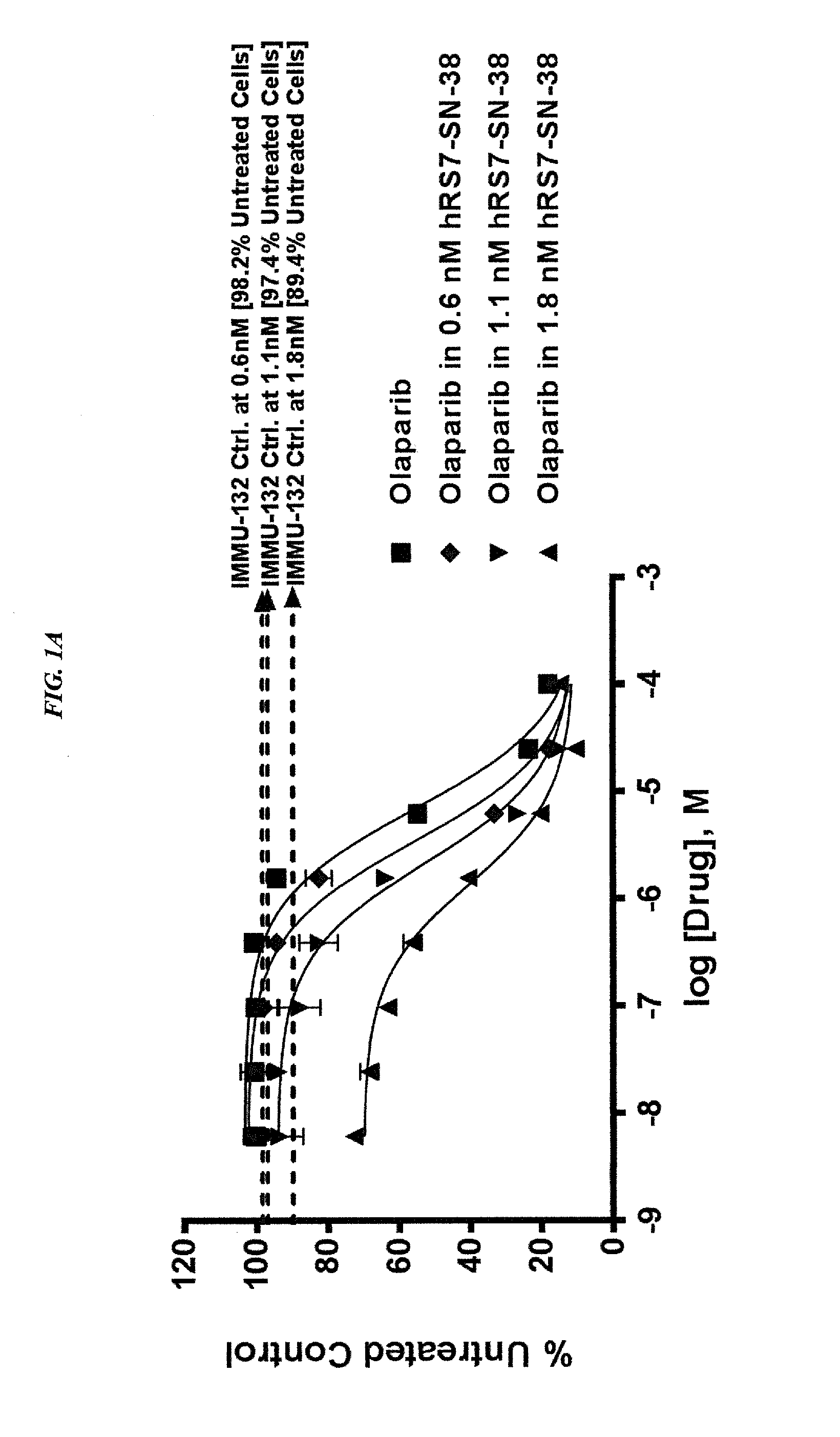
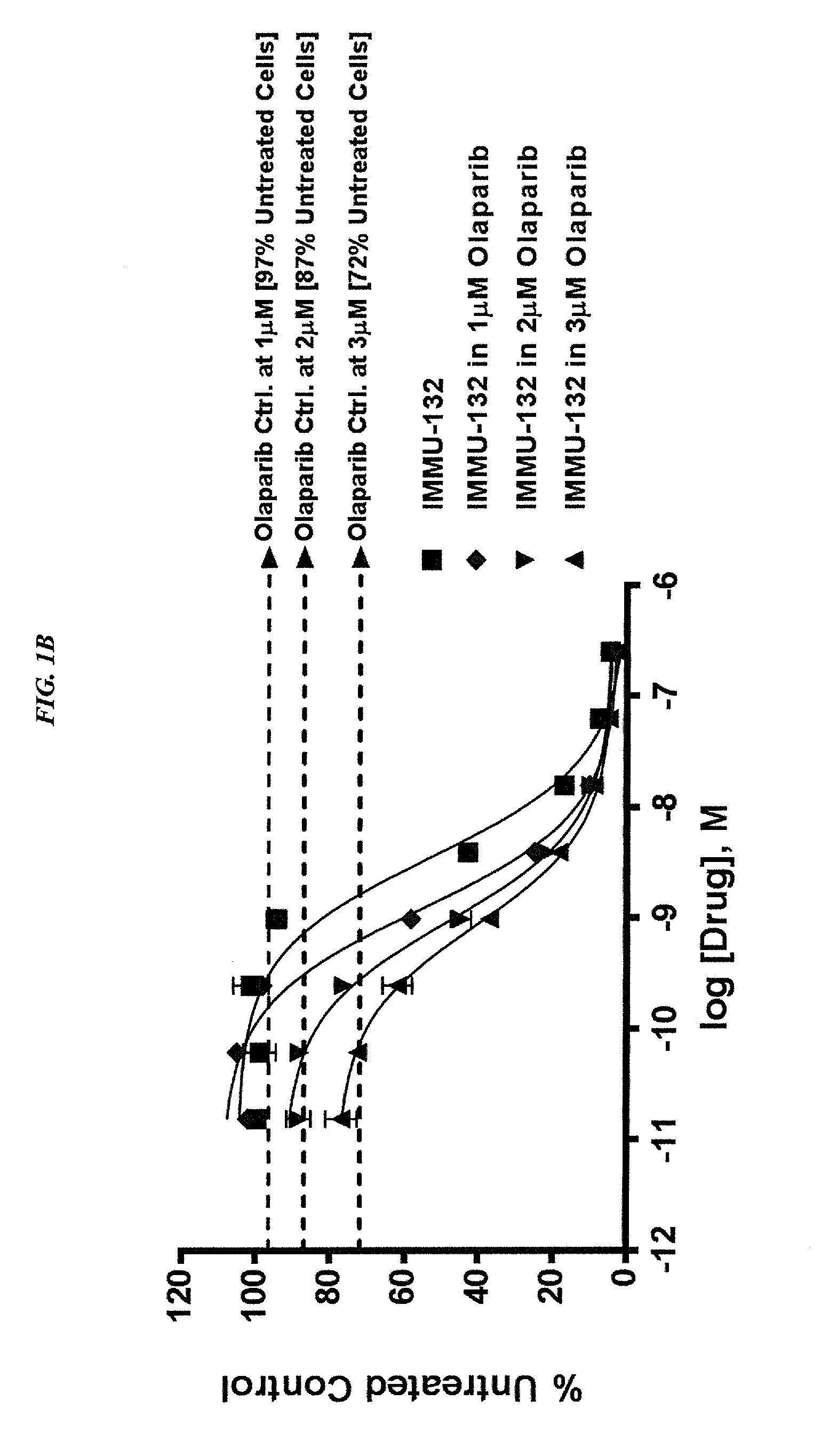
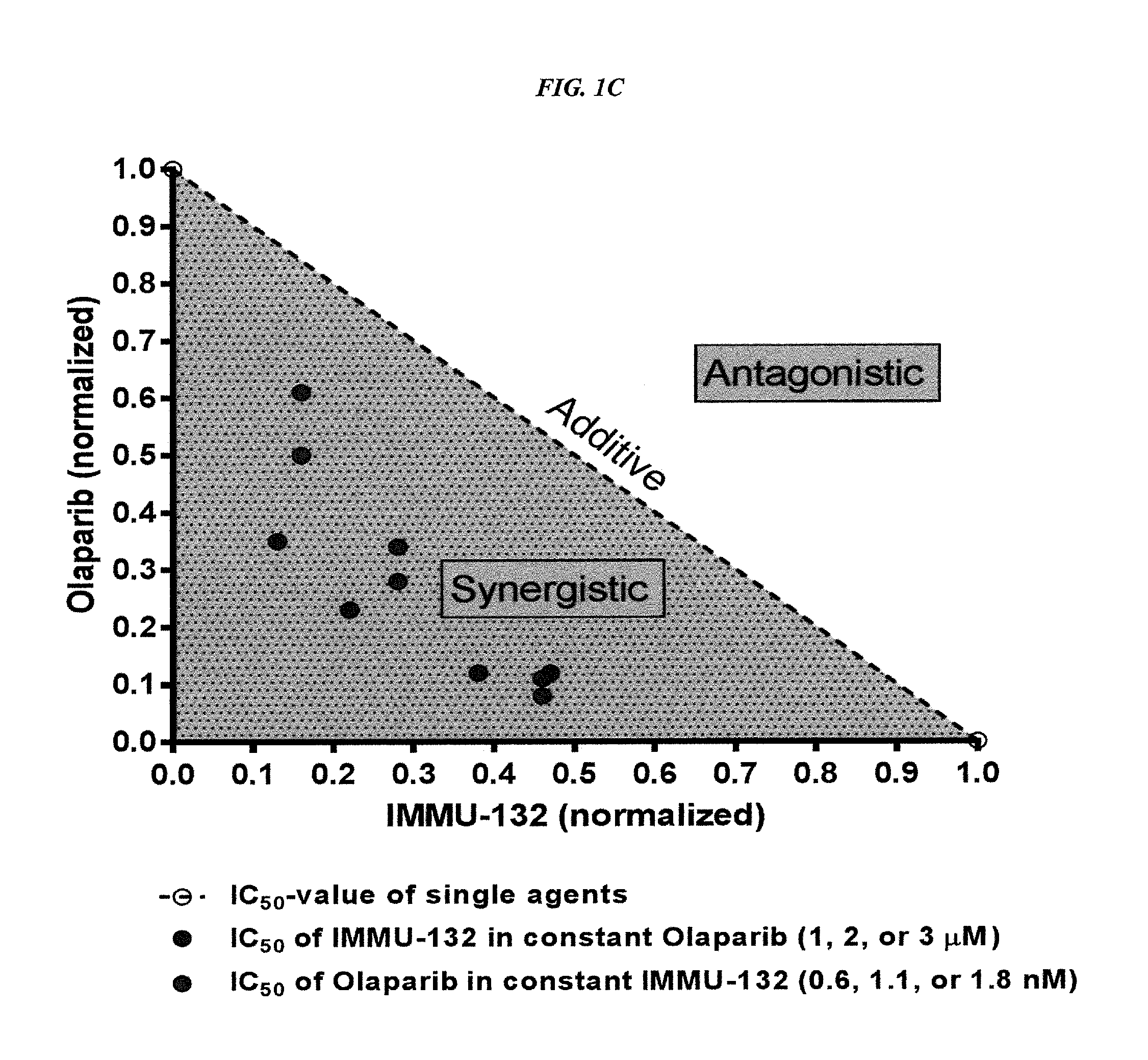
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS20160296633A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadTreatment effect
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Induction of immune response against desired determinants
InactiveUS20050049197A1Easily employedEasy to useAntibacterial agentsBiocideBinding peptideHuman leukocyte antigen DR
This invention provides HLA-DR binding peptides, called “pan DR binding peptides” that are recognized by a broad spectrum of DR molecules. In particular, the present invention provides compositions comprising nucleic acid segments that encode such pan DR binding peptides, which are useful for enhancing the immune response to a desired immunogen.
Owner:EPIMMUNE
Combining Anti-hla-dr or Anti-trop-2 antibodies with microtubule inhibitors, parp inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
InactiveUS20170274093A1Reducing certain severe side effectsReceive treatment wellHeavy metal active ingredientsOrganic active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
Method for isolated culture of human fat mesenchyma stem cell and special culture medium thereof
ActiveCN101314766AThe method of isolation and culture is simpleImprove efficiencySkeletal/connective tissue cellsAntigenMuscle injury
The invention discloses a method for separately culturing a human adipose mesenchymal stem cell and a dedicated culture medium thereof. The culture medium used for separately culturing the human adipose mesenchymal stem cell comprises an animal cell basic culture medium, fetal calf serum, an epidermal growth factor and a platelet-derived growth factor. The final concentration of the fetal calf serum is 1-200 mL / L, the final concentration of the epidermal growth factor is 1-100 ng / ml, and the final concentration of the platelet-derived growth factor is 1-100 ng / ml. The adipose mesenchymal stem cell of the invention has CD31-, CD34-, CD45- and HLA-DR-, as well as the phenotype of CD29+, CD44+, CD105+ and Flk-1+. The specificity cell surface marker and the relevant antihelion molecule of a skeletal muscle cell and a vascular endothelia cell can be expressed after inducement is performed in vitro. Muscle fiber, vascular endothelin and functional muscle satellite cells can be differentiated in a muscle injury model mouse body caused by medicine and the expression of dystrophin protein on the ducheme muscular dystrophy (DMD) model mouse (mdx) myolemma can be partially recovered, so as to release the pathological symptom of the model mouse.
Owner:微能生命科技集团有限公司
Kit and method for comprehensively evaluating functions of immune cells in human peripheral blood
The invention provides a kit and a method for comprehensively evaluating functions of immune cells in human peripheral blood. The kit for comprehensively evaluating the functions of the immune cells in the human peripheral blood comprises an anti-human CD3, CD4, CD8, CD19, CD21, CD24, CD25, CD27, CD28, CD38, CD56, CD57, CD94, CD127, CD45RA, CXCR3, CXCR5, CCR4, CCR6, CCR7, HLA-DR, PD-1, P30, P46, NKG2D, KIR (NKB1), gamma delta, v delta 2, IgD and IgM antibodies, wherein the antibodies carry fluorescein labels. The kit can be used for comprehensively evaluating the functions of the immune cellsin the human peripheral blood, and is convenient and safe to use.
Owner:东莞暨南大学研究院
Prediction of Responsiveness to Treatment with Immunomodulatory Therapeutics and Method of Monitoring Abscopal Effects During Such Treatment
Efficacy of a therapeutic to enhance antitumor immunity in a patient is predicted, where the therapeutic is one that targets an immunomodulatory leukocyte membrane protein (ILMP) to enhance immune activity. Peripheral blood sample from the patient is tested for levels of monocytes having specific cell surface markers (CD14+, HLA-DRlow) prior to treatment. Low levels of monocytes of this type (PBM14+HLA-DRlow) indicate a greater likelihood of therapeutic efficacy. In specific exemplary embodiments of the invention, the therapeutic is an antibody to CTLA4, such as ipilimumab or tremelimumab.
Owner:SLOAN KETTERING INST FOR CANCER RES
Isolated peptides which bind to MHC class II molecules, and uses thereof
InactiveUS6800730B1Tumor rejection antigen precursorsPeptide/protein ingredientsHuman leukocyte antigen DRCD4 antigen
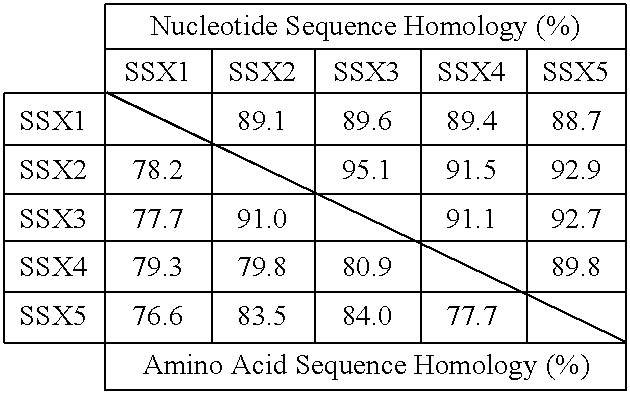
Peptides which have an amino acid sequence identical to sequences found in tumor rejection antigen precursors, such as NY-ESO-1, and SSX-2, are disclosed. These peptides bind to MHC-Class II molecules, such as HLA-DR molecules, and provoke proliferation of CD4+ cells.
Owner:LUDWIG INST FOR CANCER RES
Combining anti-HLA-DR or anti-Trop-2 antibodies with microtubule inhibitors, PARP inhibitors, bruton kinase inhibitors or phosphoinositide 3-kinase inhibitors significantly improves therapeutic outcome in cancer
ActiveUS9707302B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsHeavy metal active ingredientsLymphatic SpreadCombined Modality Therapy
The present invention relates to combination therapy with drugs, such as microtubule inhibitors, PARP inhibitors, Bruton kinase inhibitors or PI3K inhibitors, with antibodies or immunoconjugates against HLA-DR or Trop-2. Where immunoconjugates are used, they preferably incorporate SN-38 or pro-2PDOX. The immunoconjugate may be administered at a dosage of between 1 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8 or 10 mg / kg. The combination therapy can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the combination therapy has an additive effect on inhibiting tumor growth. Most preferably, the combination therapy has a synergistic effect on inhibiting tumor growth.
Owner:IMMUNOMEDICS INC
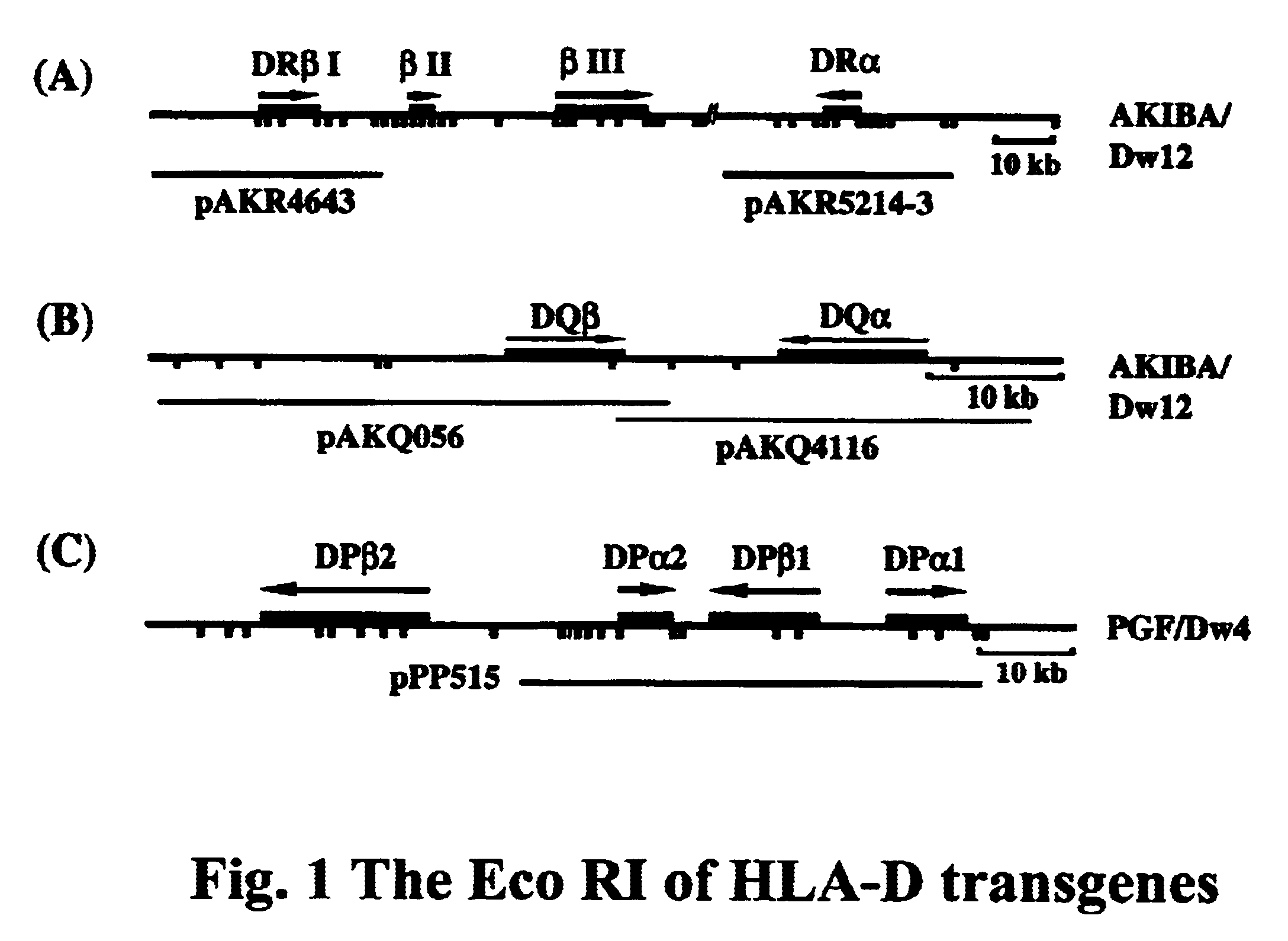
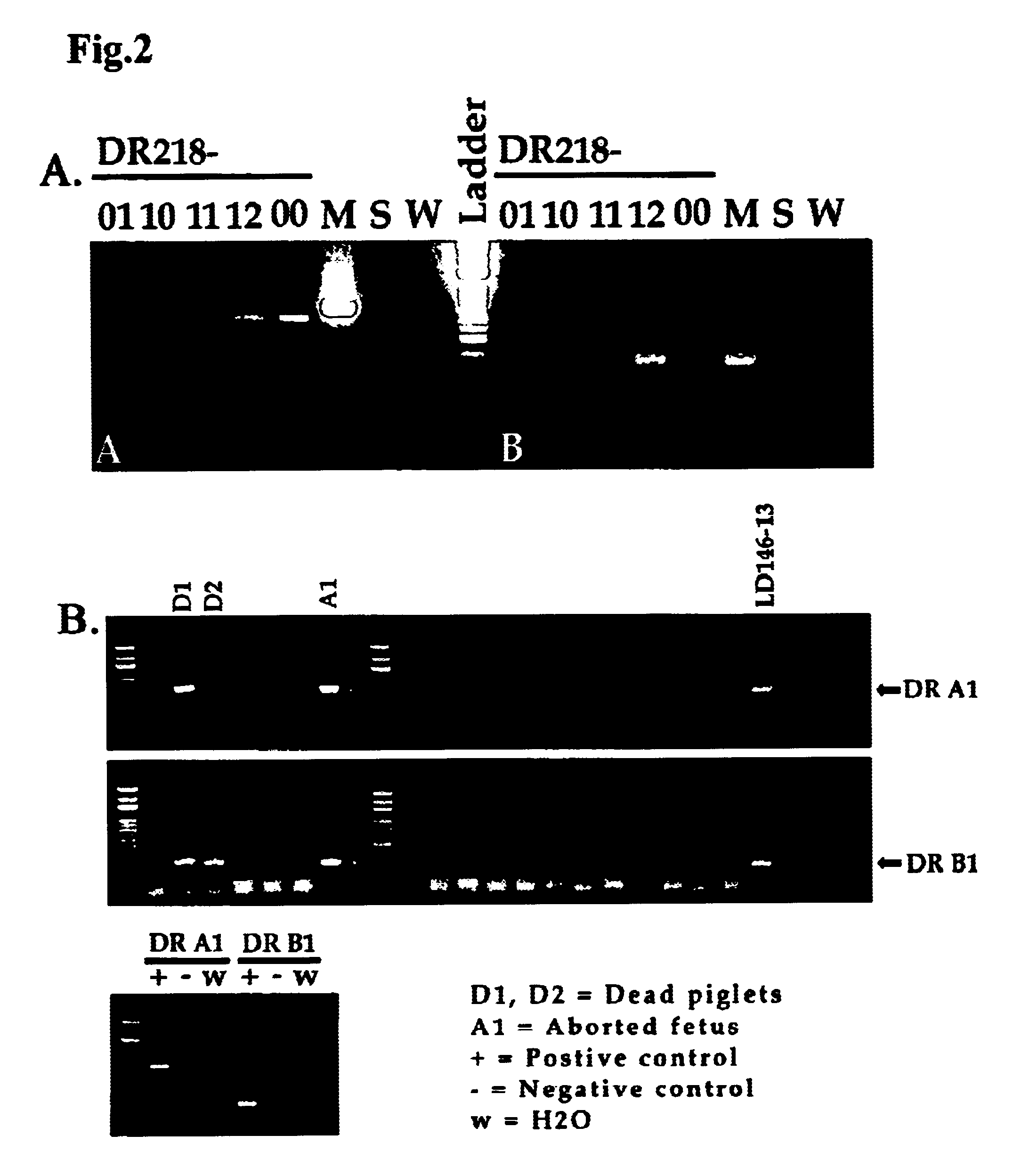
Transgenic swine having HLA-D gene, swine cells thereof and xenografts therefrom
InactiveUS6639122B1Decreased cellular responseReduced responseBiocideGenetic material ingredientsGraft tissueHuman leukocyte antigen DR
The invention provides a transgenic swine having a transgene encoding a human HLA-DQ or HLA-DR protein, and the swine cells thereof. The invention also provides a graft derived from the transgenic swine, in particular a graft-organ or a graft-tissue, which is useful for grafting into a human recipient.
Owner:ANIMAL TECH INST TAIWAN
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Methods of upscaling mesenchymal stromal cell production, compositions and kit thereof
InactiveUS20160002601A1Dead animal preservationSkeletal/connective tissue cellsHigh cellClinical grade
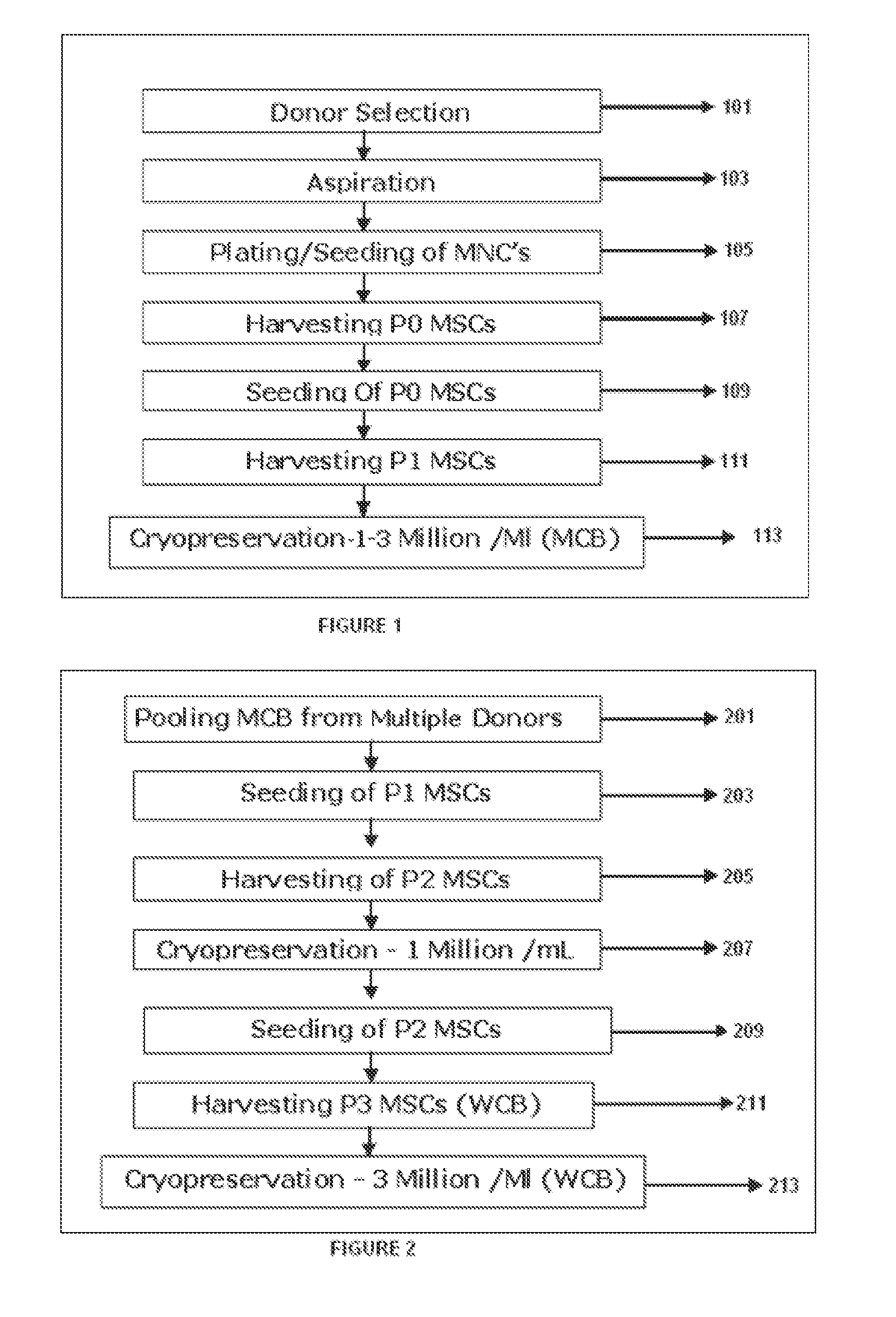
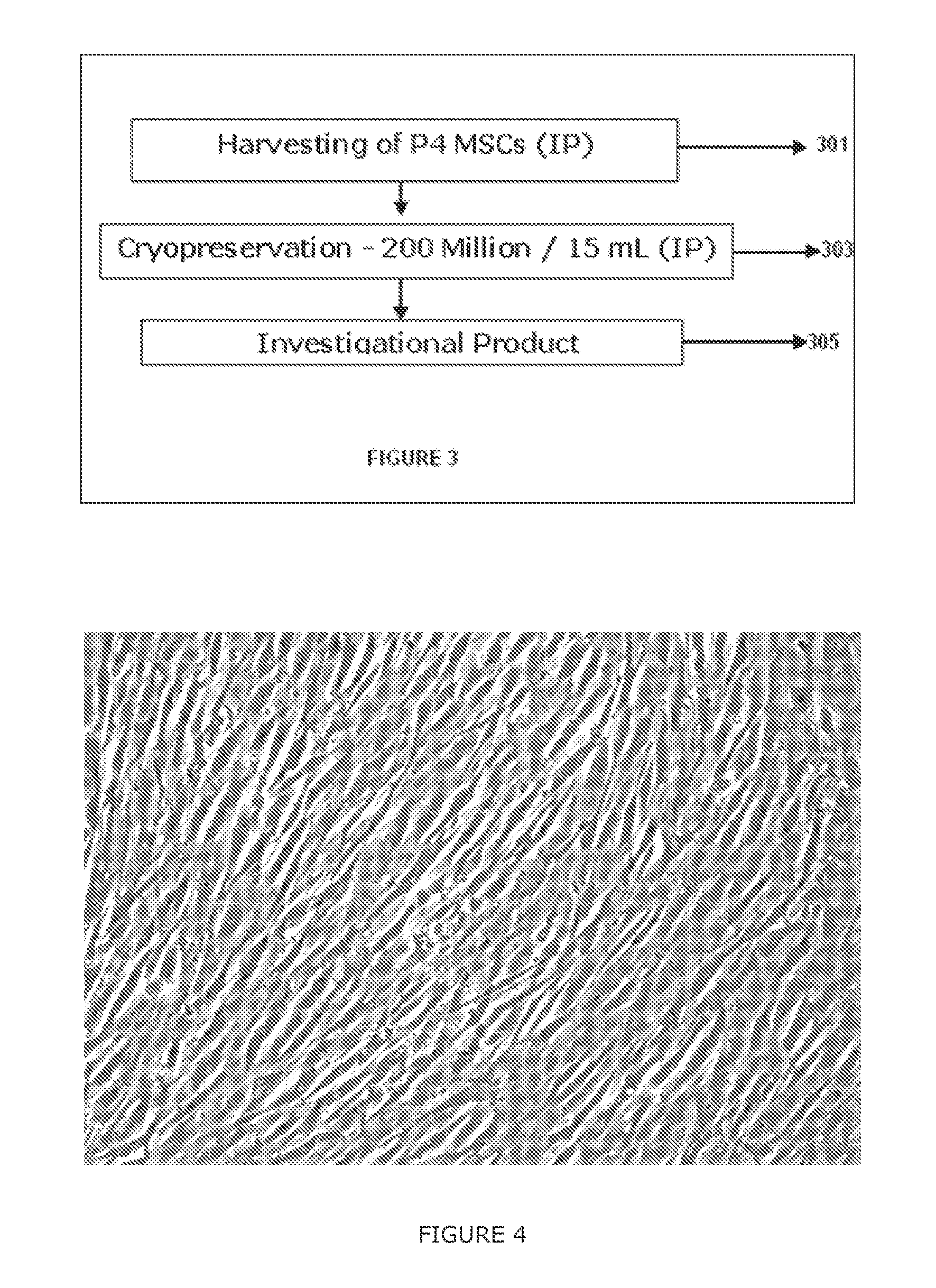
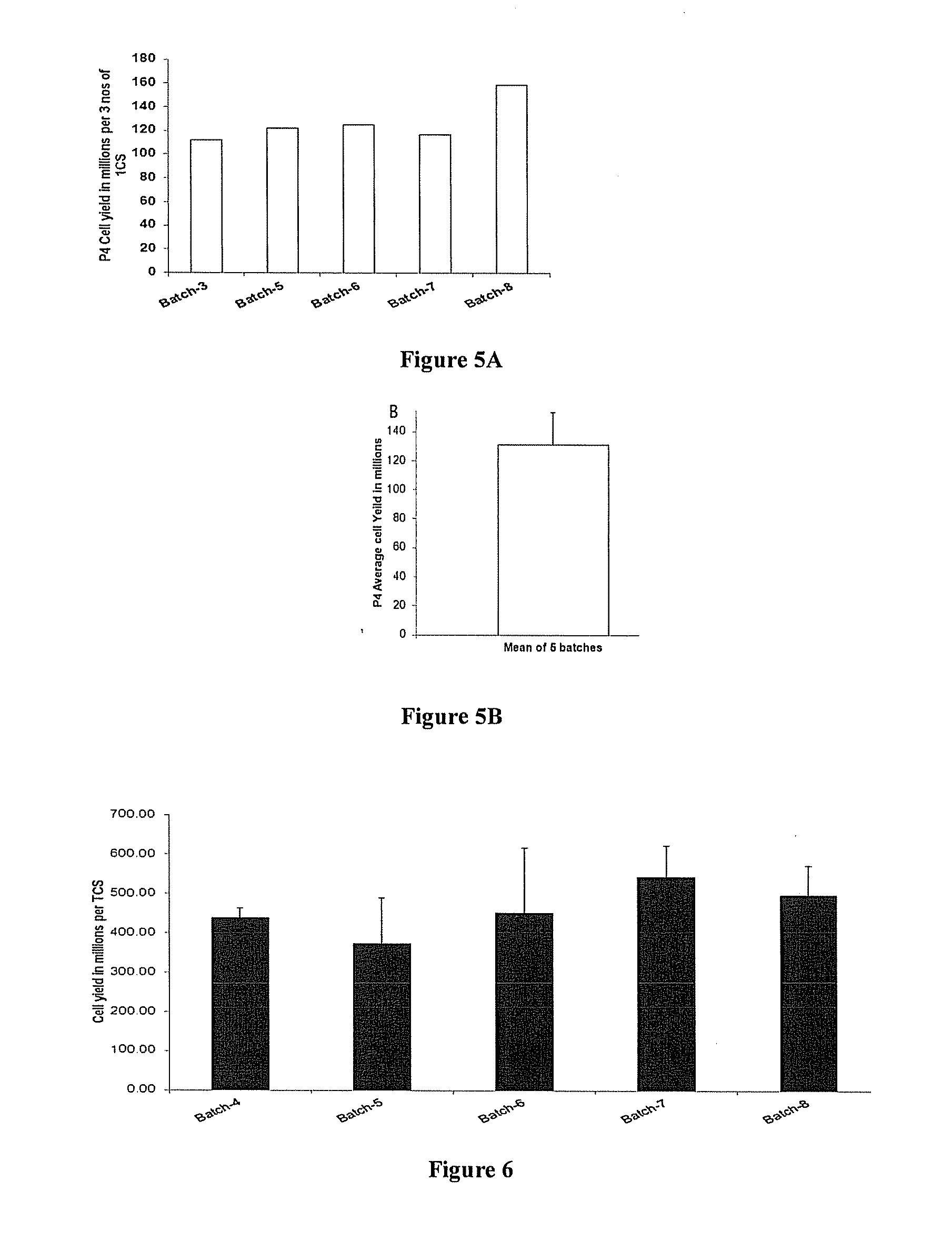
The present invention discloses a method of isolation, pooling and further culturing of Mesenchymal Stem cells (MSC) for clinical application. Present invention also discloses the method of establishing Master Cell bank, followed by Working Cell Bank from which the final therapeutic composition referred to as Investigational Product / Investigational Medicinal Product comprising of allogenic bone marrow-derived MSC is formulated for clinical applications. Present disclosure also discloses a robust manufacturing process for consistent production of clinical grade Mesenchymal Stromal cells (MSCs). The process enables production of highly viable potent cells. The process steps relating to preparation of media, cell seeding, harvesting are fine tuned to achieve consistency in cell yield, superior cell viability, purity, improved cell proliferation, high cell recovery, low HLA-DR expression, reduction in culture duration. The viability and purity of cells are further improved by optimized wash process without cell loss / cell stress. The disclosure further provides a method of cyrostoring MSCs at high cell density without affecting the viability of cells. It further provides economical means to store and transport at −80° C.
Owner:STEMPEUTICS RES PRIVATE
Anti-HLA-DA antibodies and the methods of using thereof
InactiveUS6894149B2Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsMelanomaHuman leukocyte antigen DR
This invention provides anti-HLA-DR antibodies and the methods of use thereof for the treatment of leukemia or lymphomas, or solid tumors such as ovarian cancer or melanoma.
Owner:ABBVIE BIOTHERAPEUTICS
Methods and reagents for quantitation of cell-surface molecule expression on peripheral blood cells
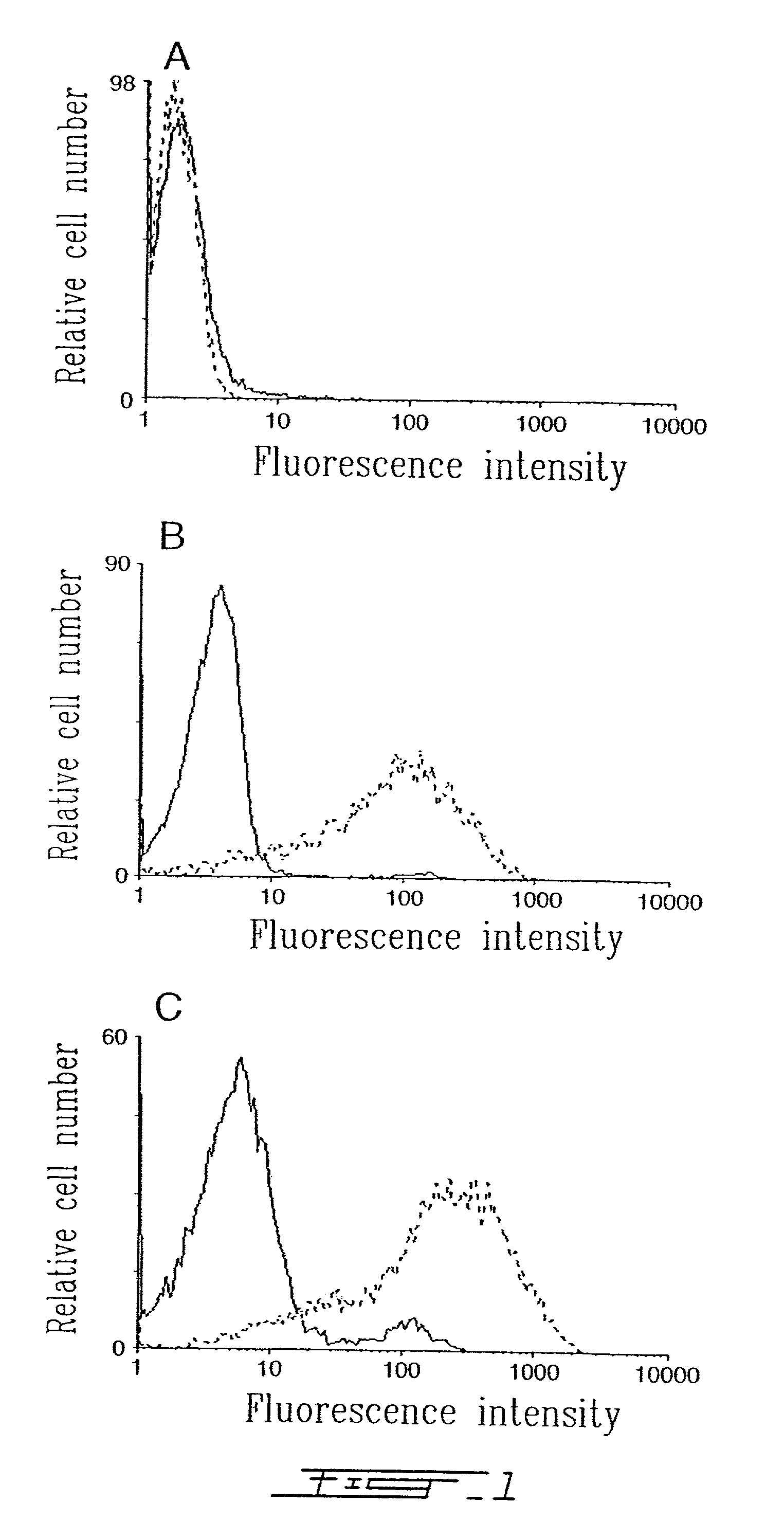
InactiveUS6951727B2Stable expressionRapid and reliable quantitative measurement of HLA-DREnzymologyImmunoglobulins against cell receptors/antigens/surface-determinantsImmunologic CompetenceLysosome
Improved methods, reagents, and kits for quantitation of HLA-DR and / or CD11b expression on peripheral blood cells are presented. Inclusion of a lysosomotropic amine, such as chloroquine, during staining stabilizes HLA-DR and CD11b expression. Use of a novel anti-CD14 conjugate, anti-CD14-PerCP / CY5.5, permits the ready discrimination of monocytes. The improved methods, reagents, and kits may be used to assess immune competence, and to direct and monitor immunostimulatory therapies in septic patients exhibiting monocyte deactivation.
Owner:BECTON DICKINSON & CO
In vitro activated gamma delta lymphocytes
Owner:PALMETTO HEALTH ALLIANCE
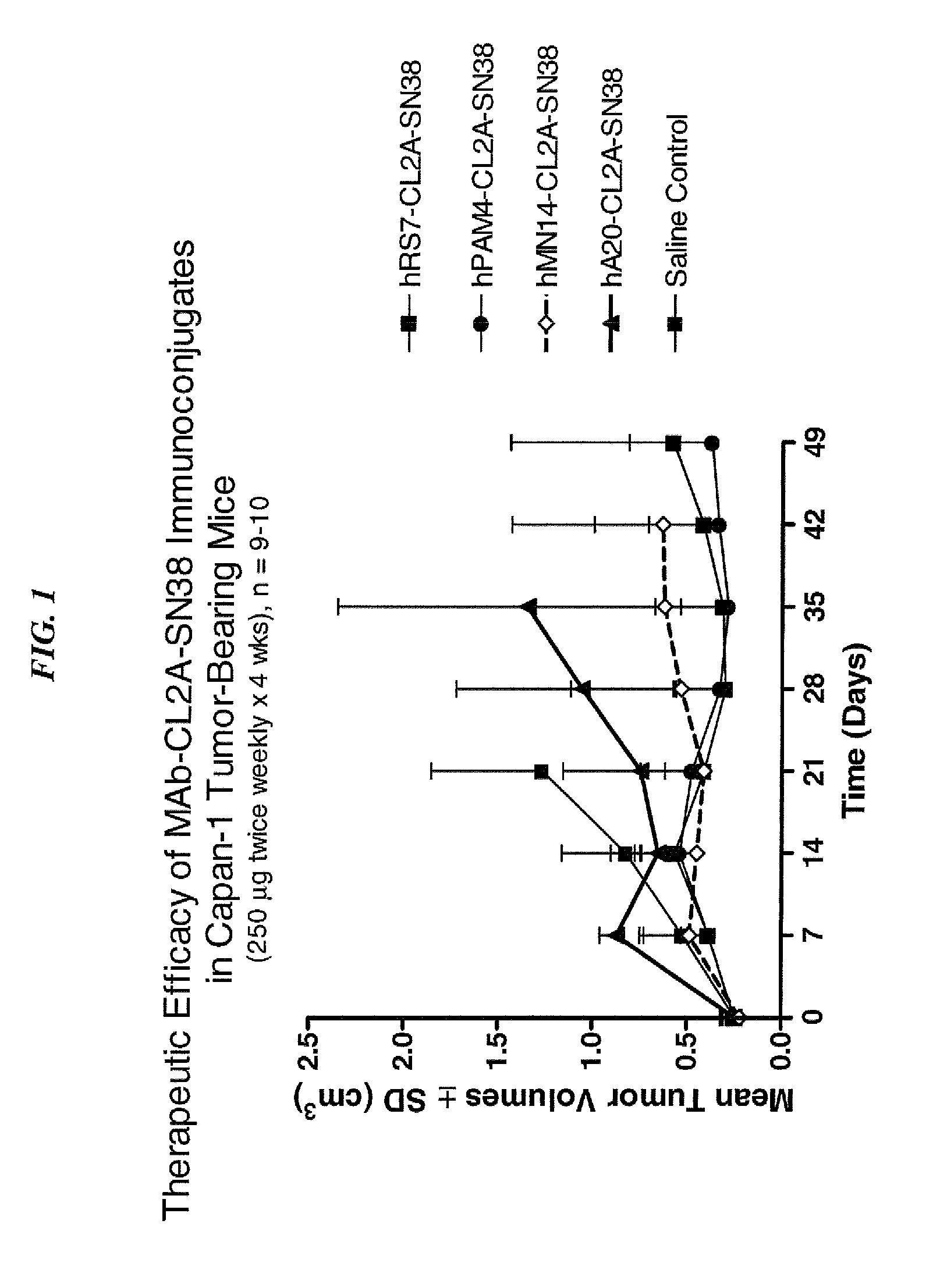
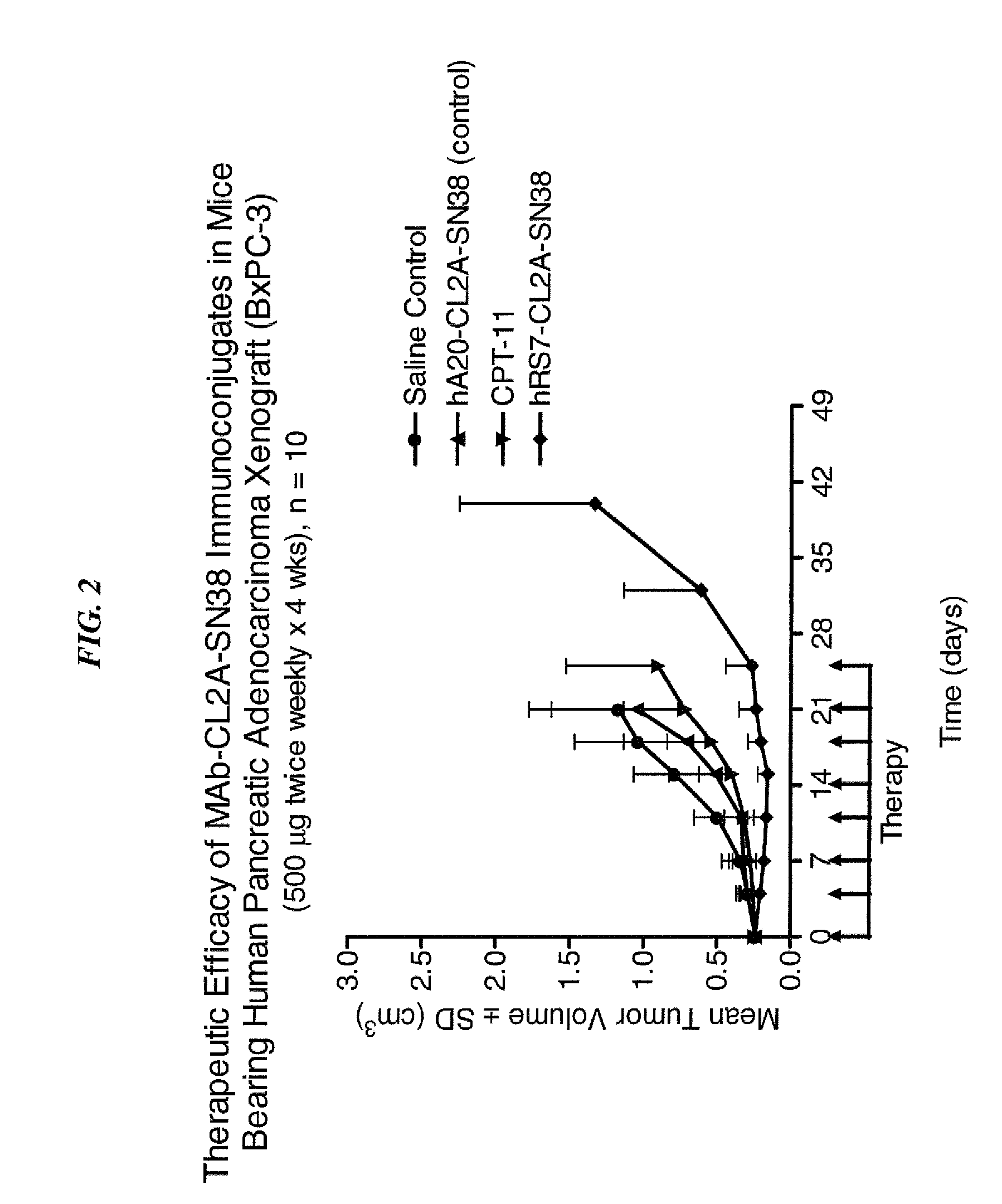
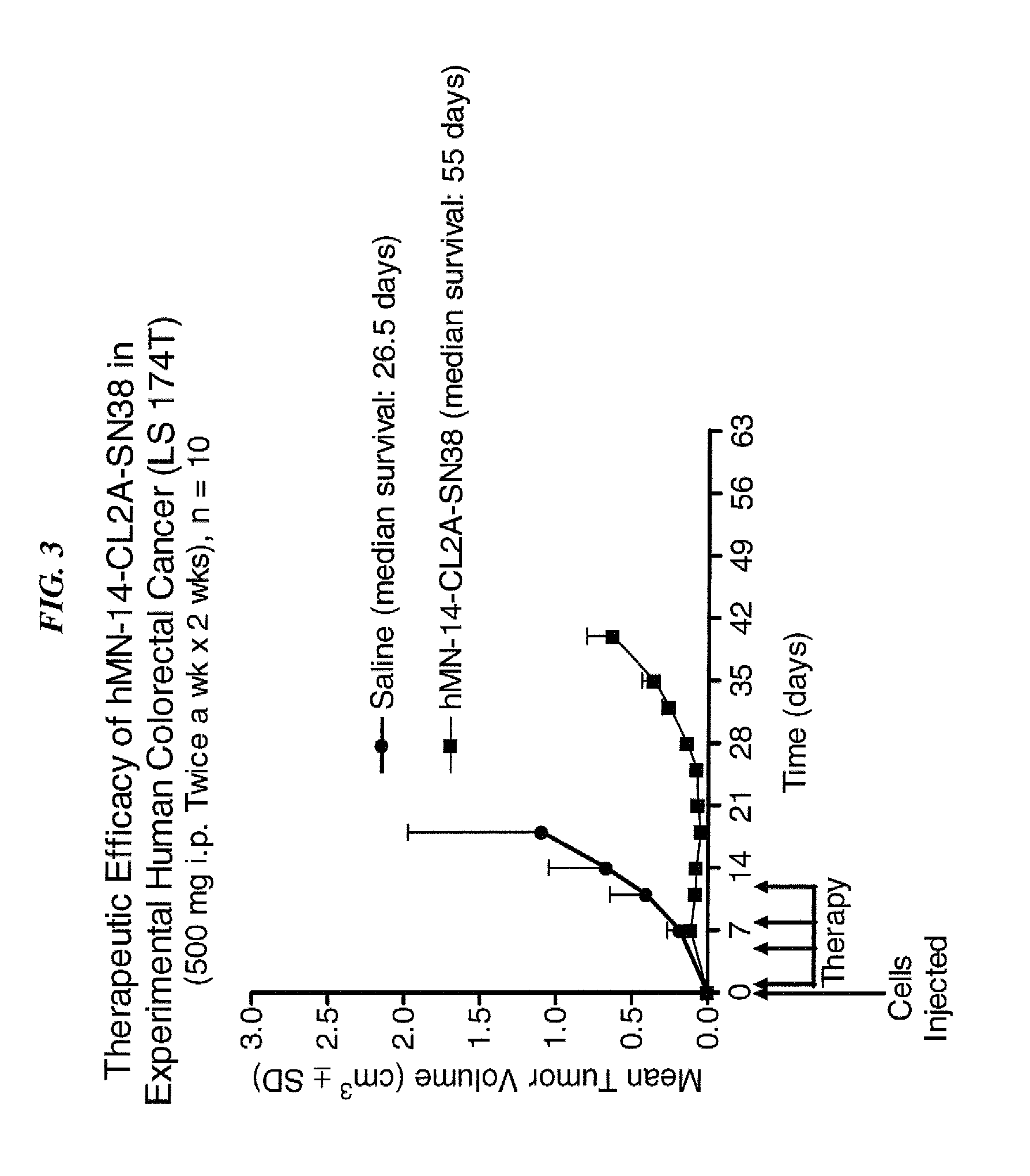
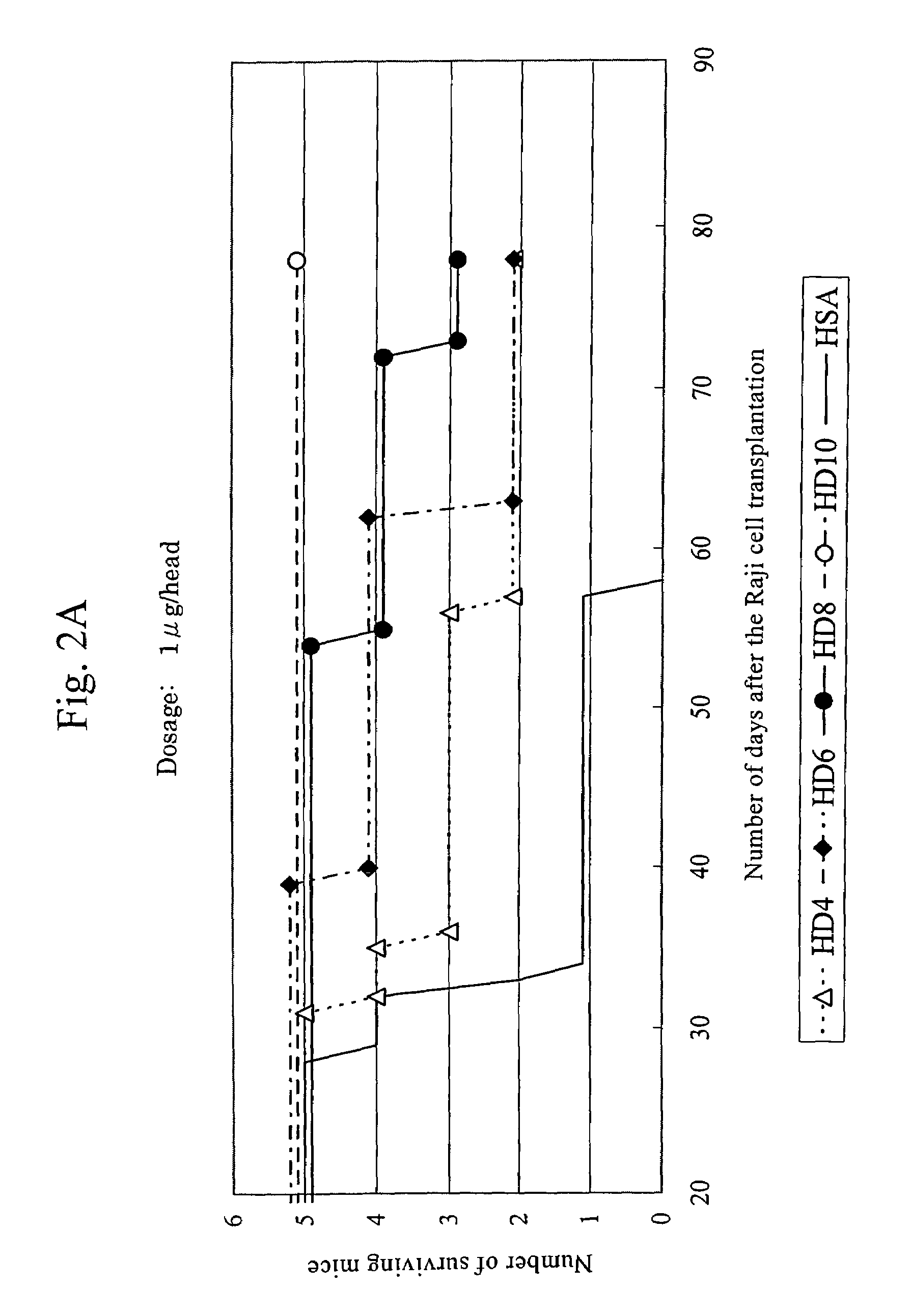
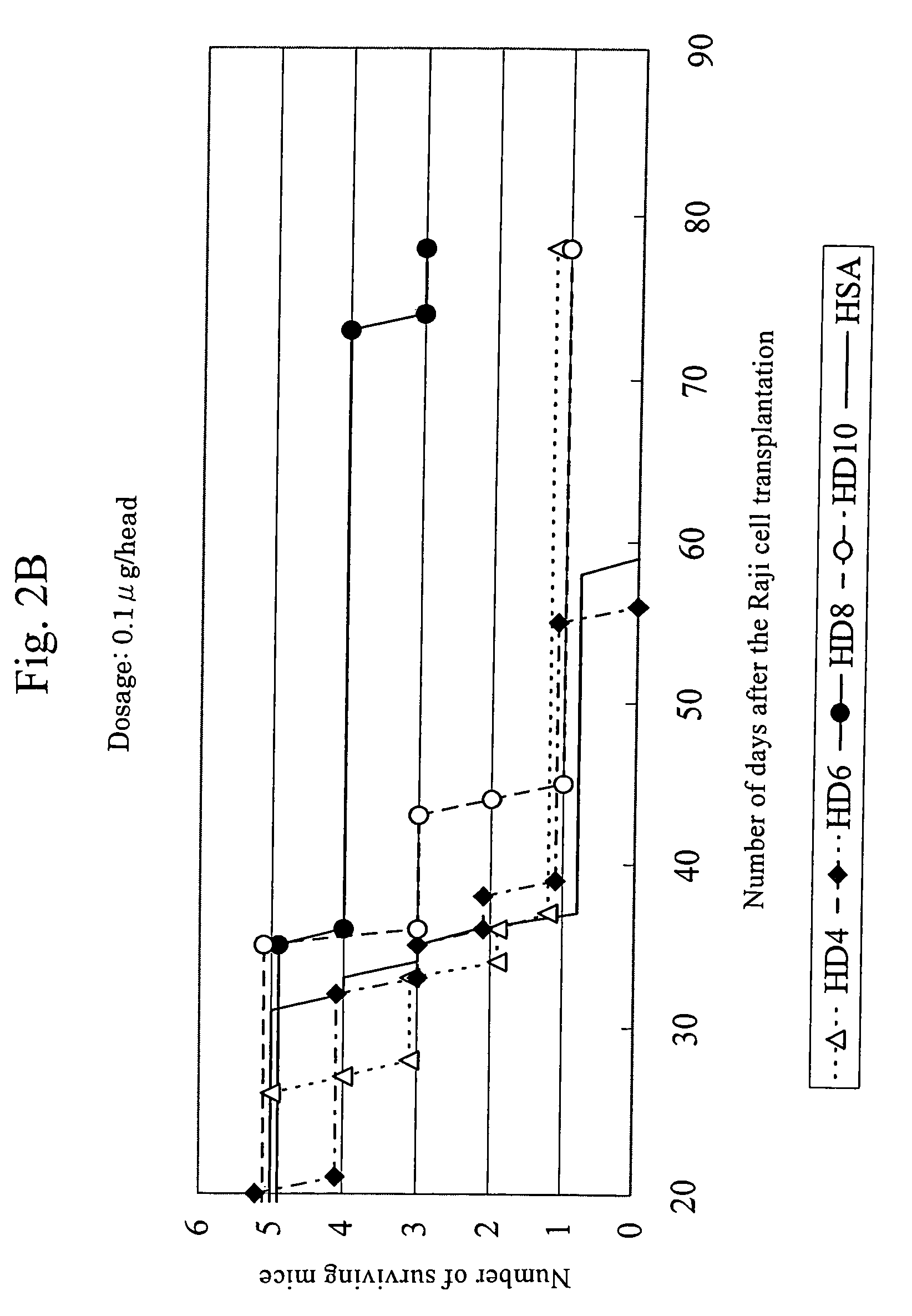
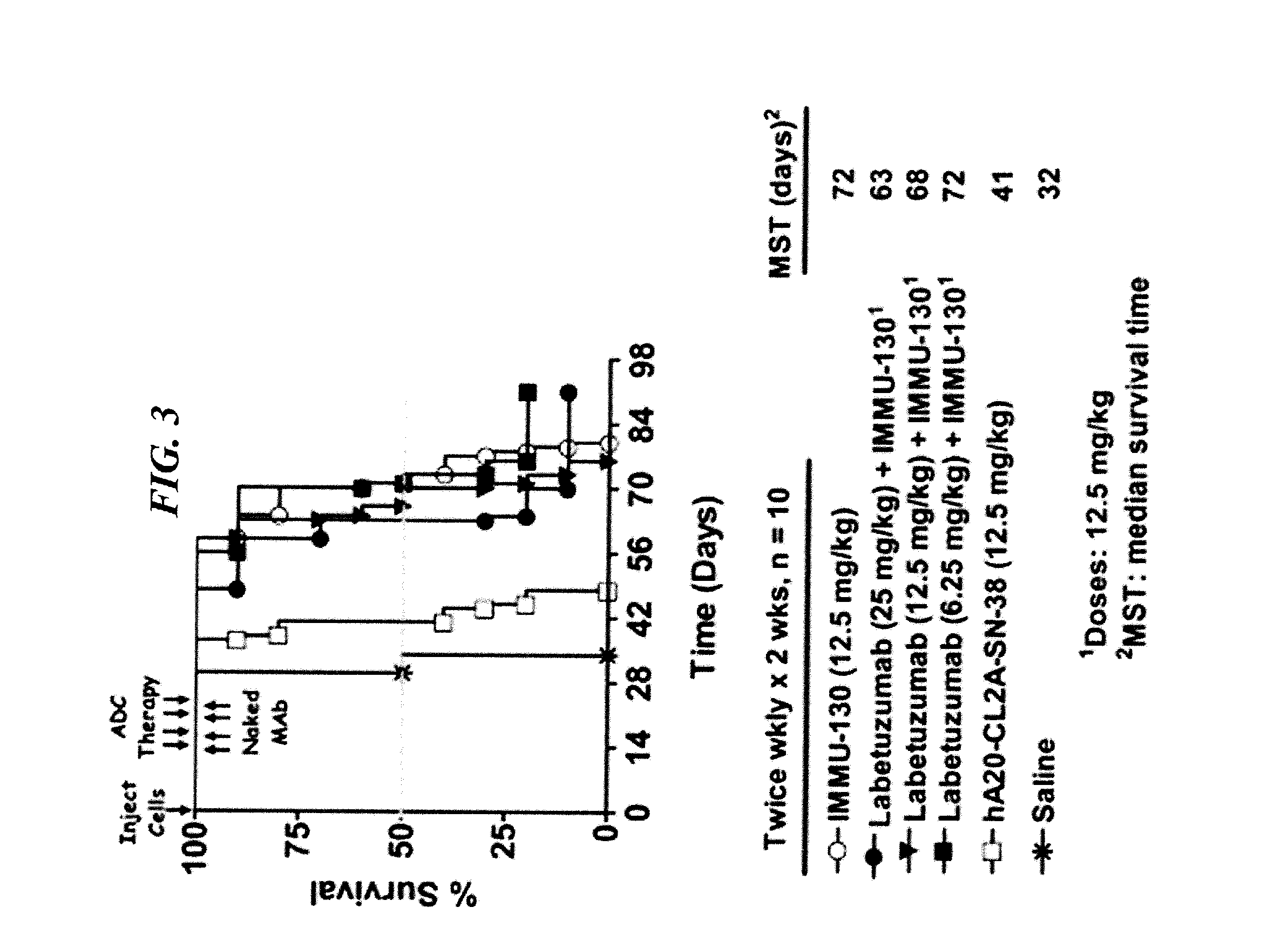
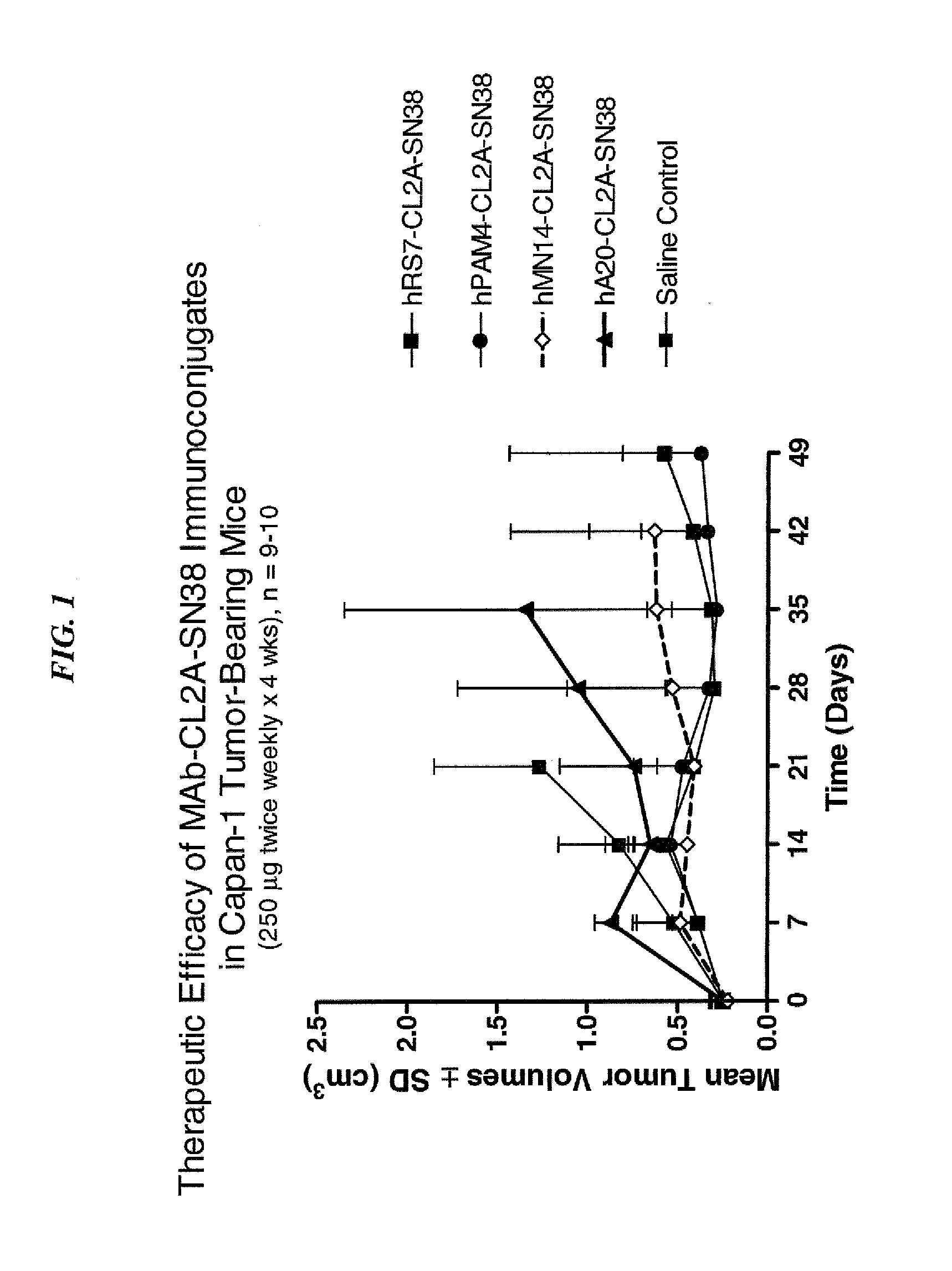
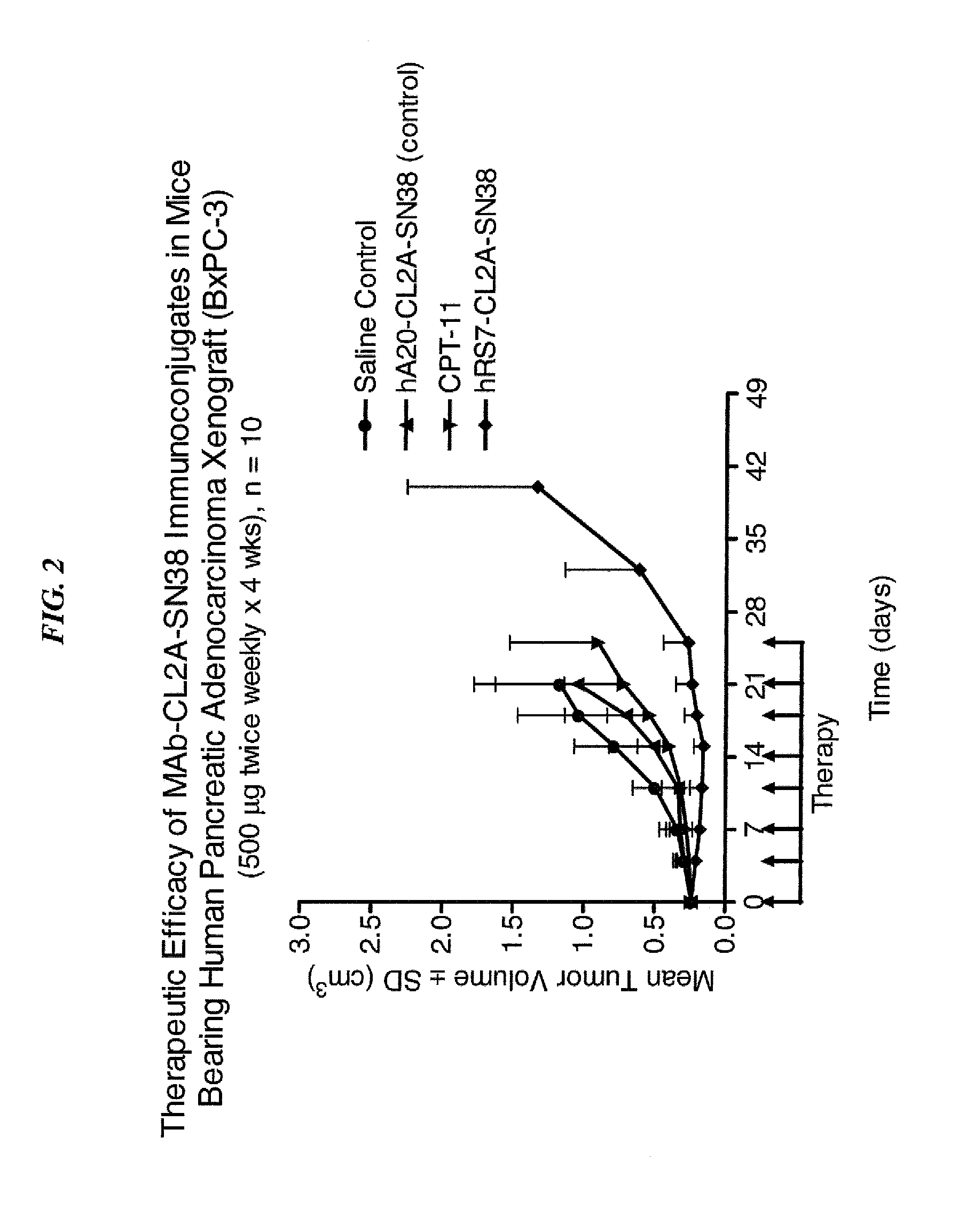
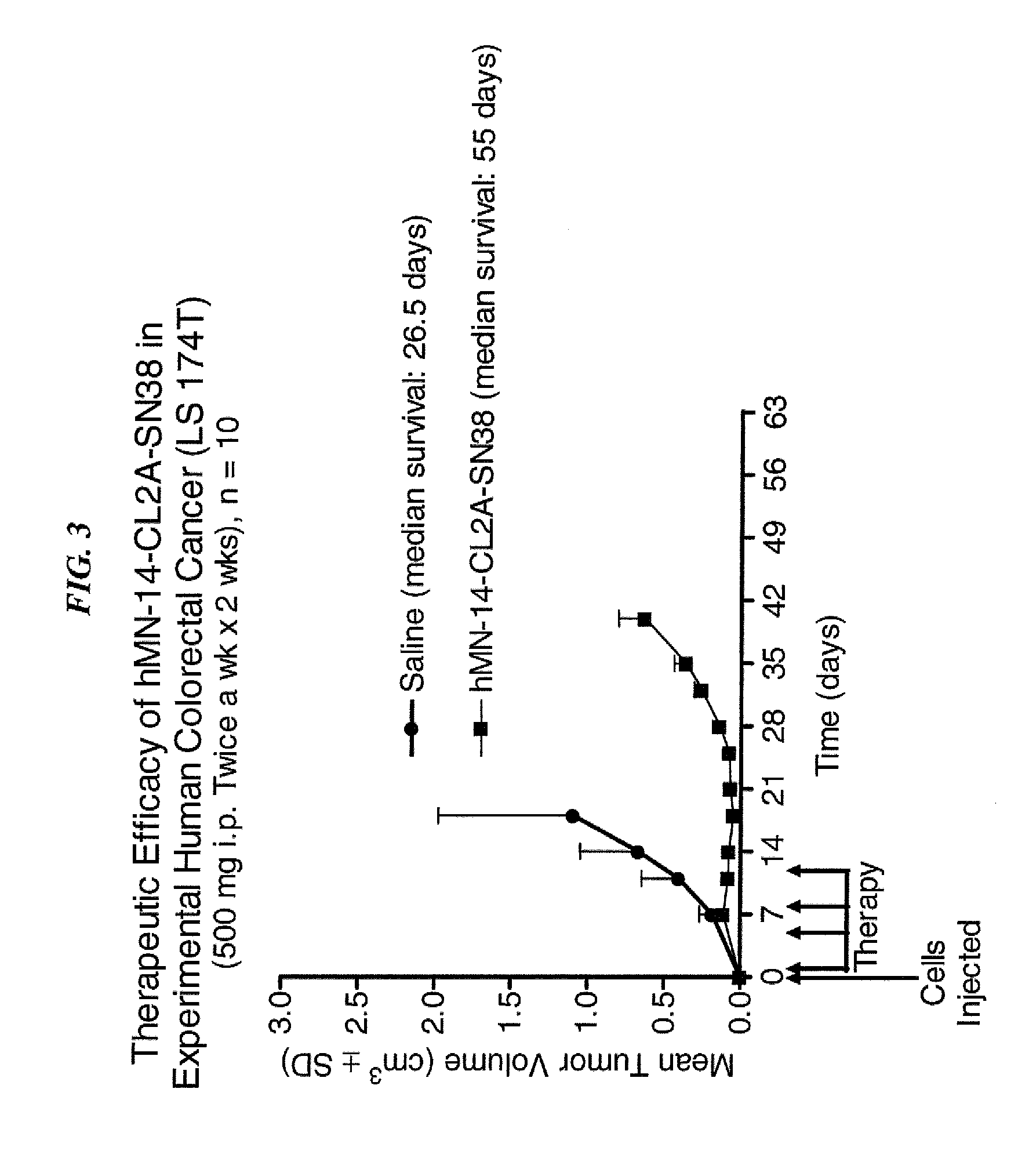
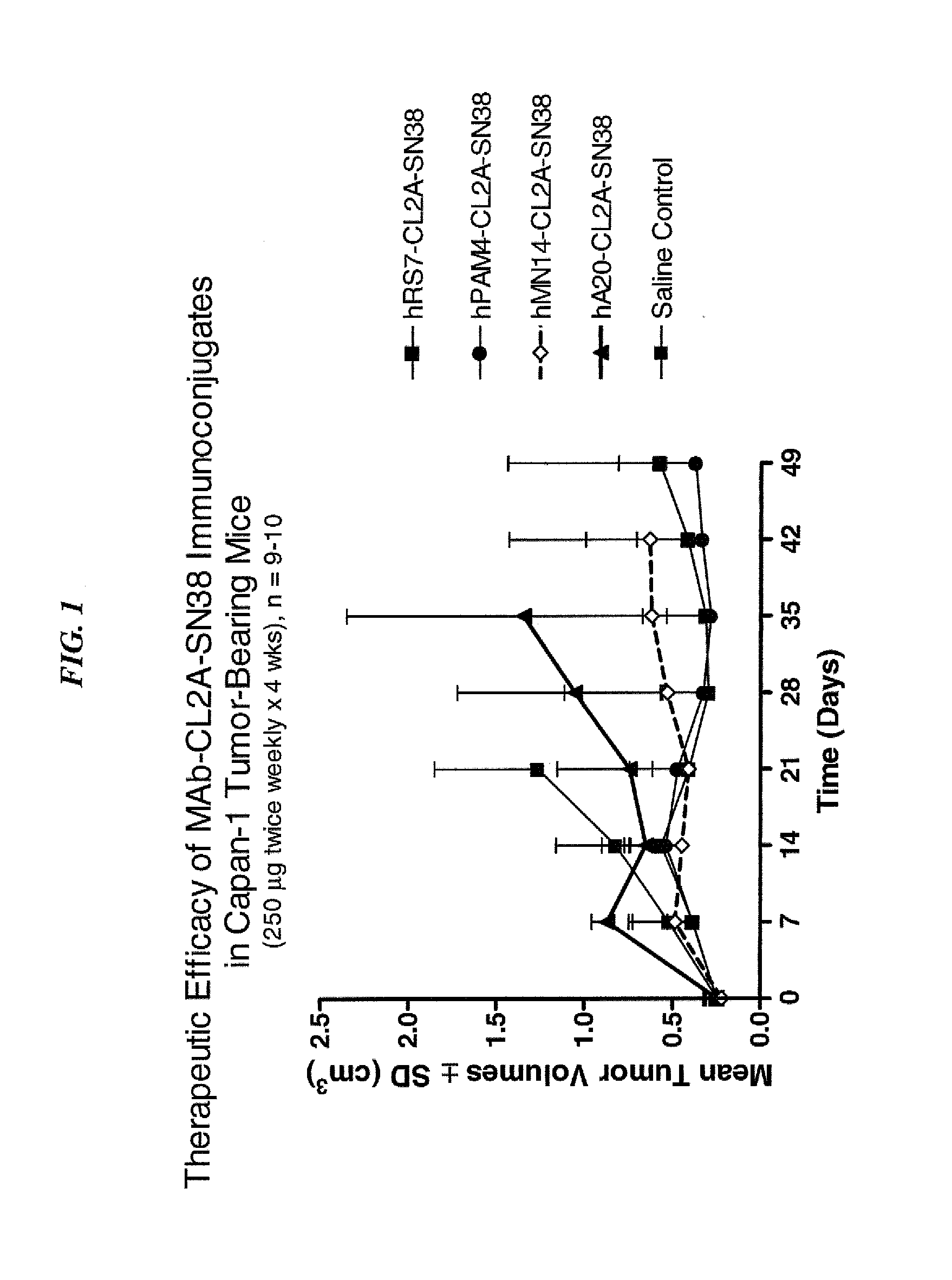
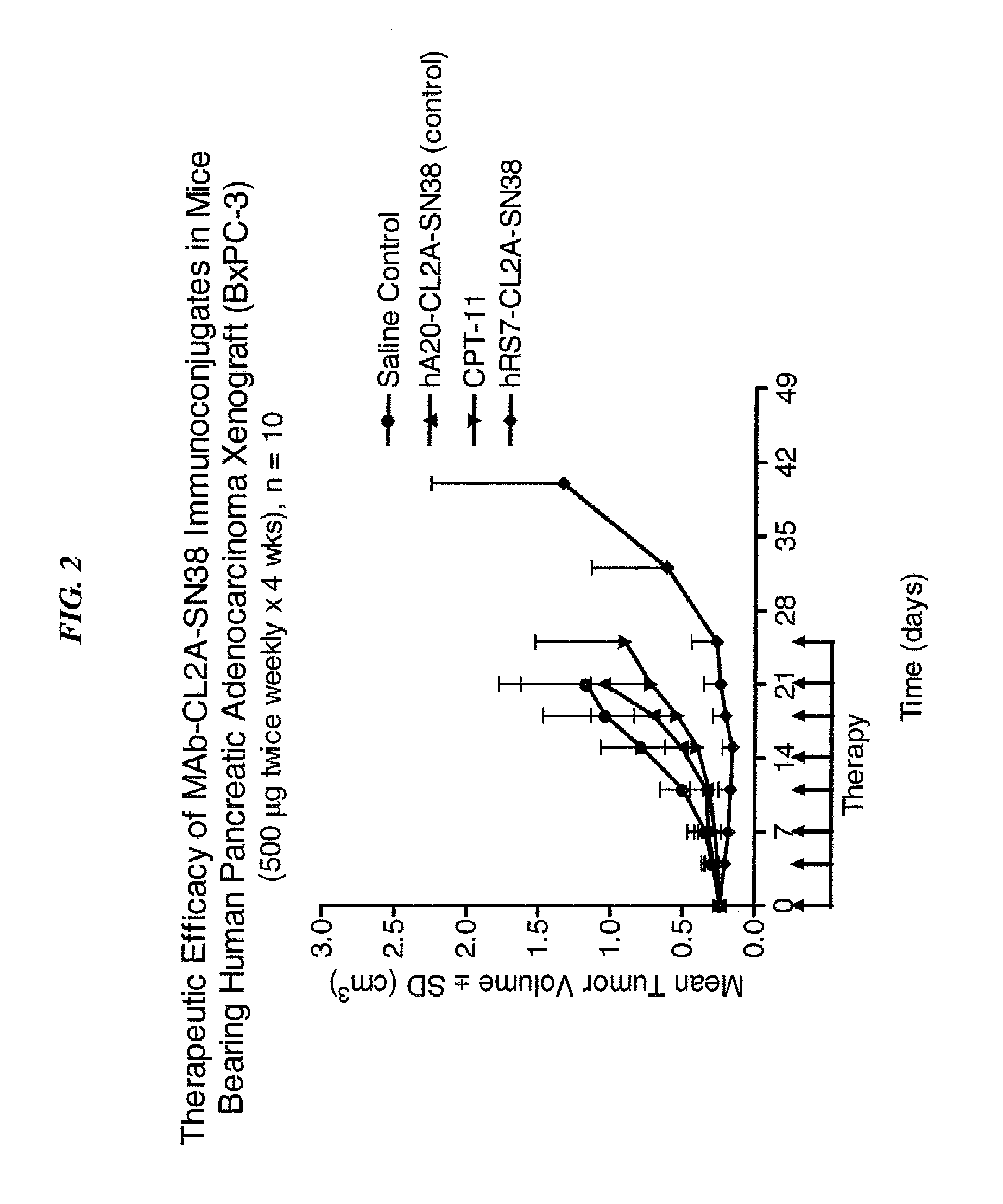
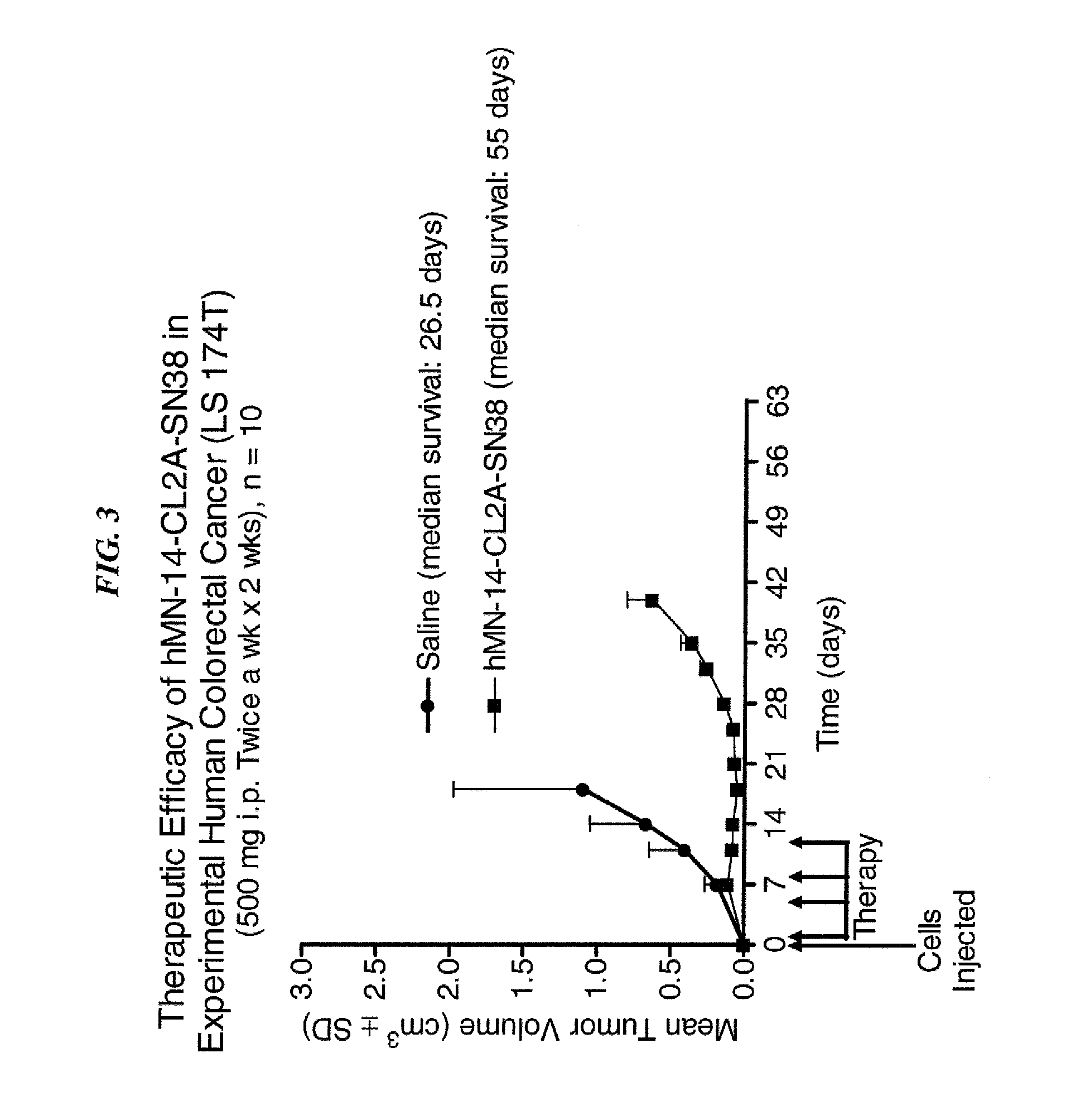
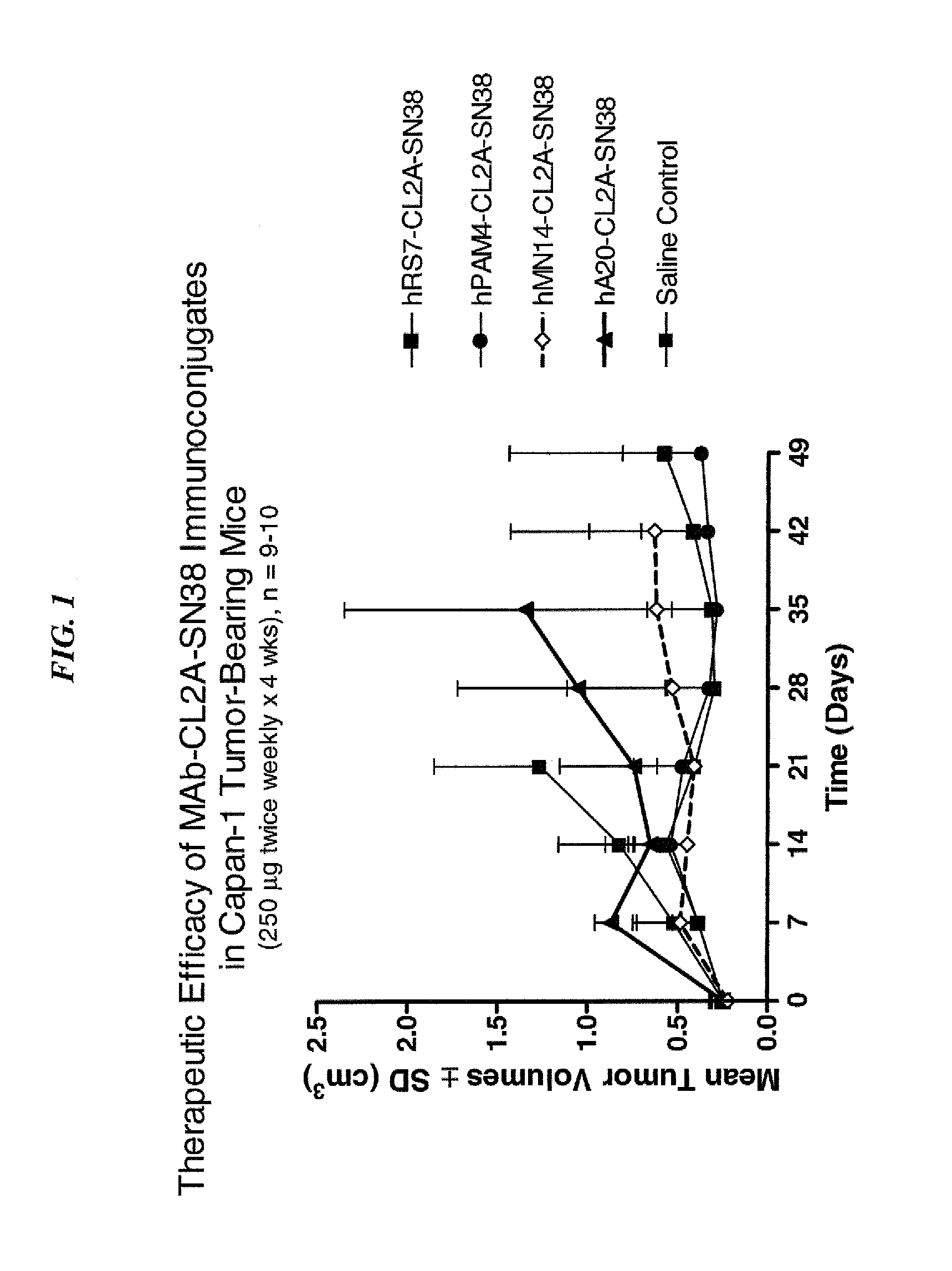
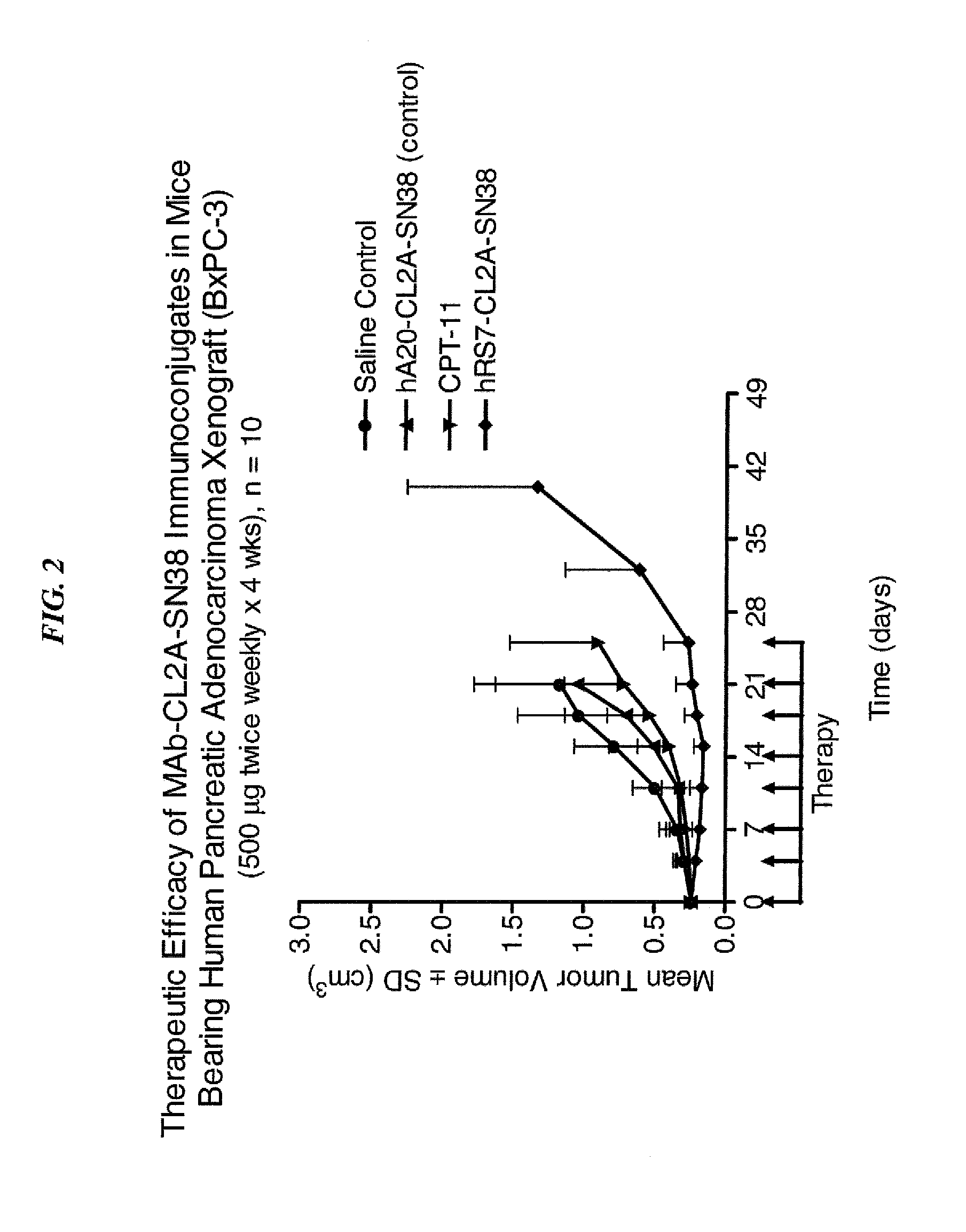
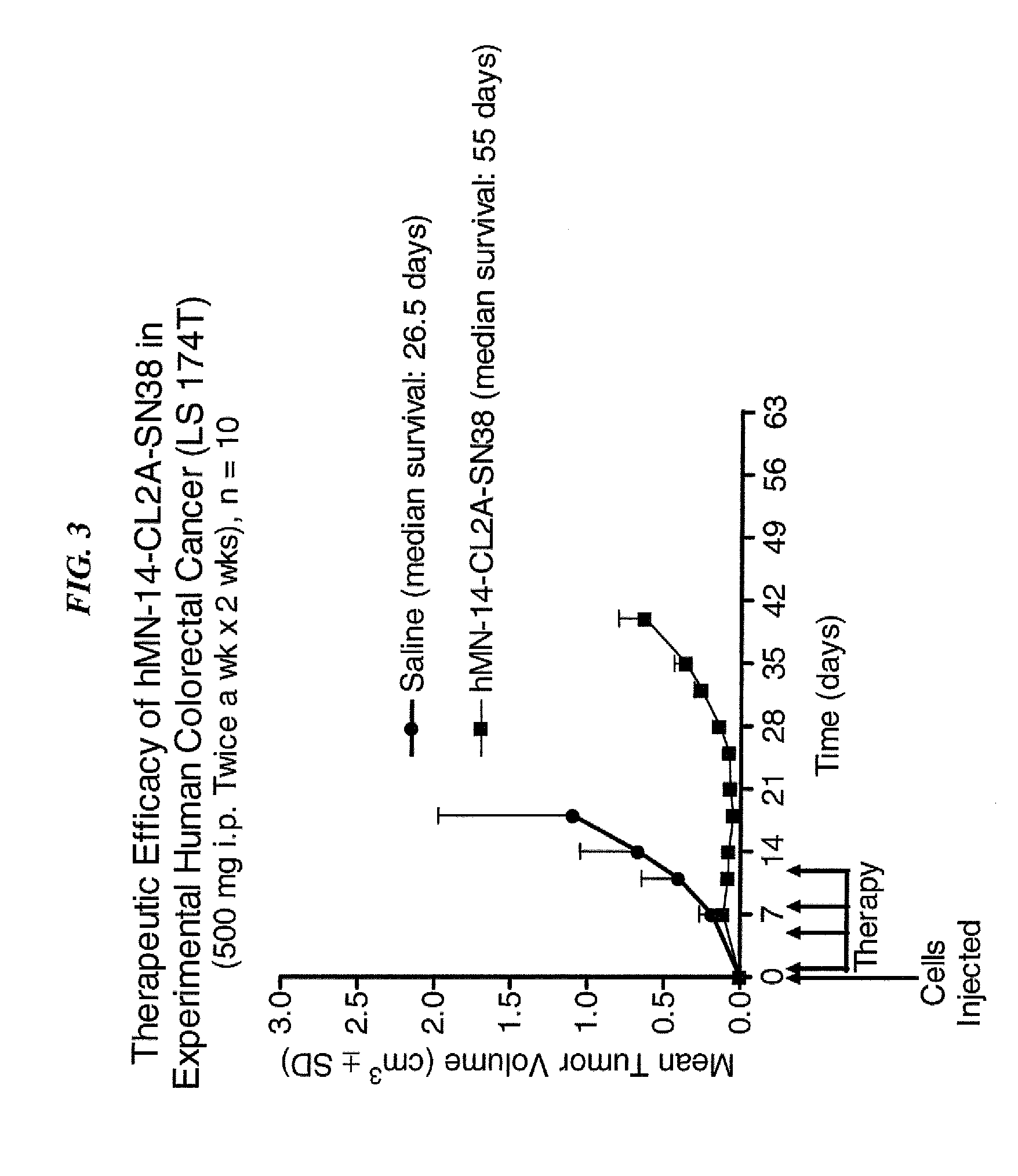
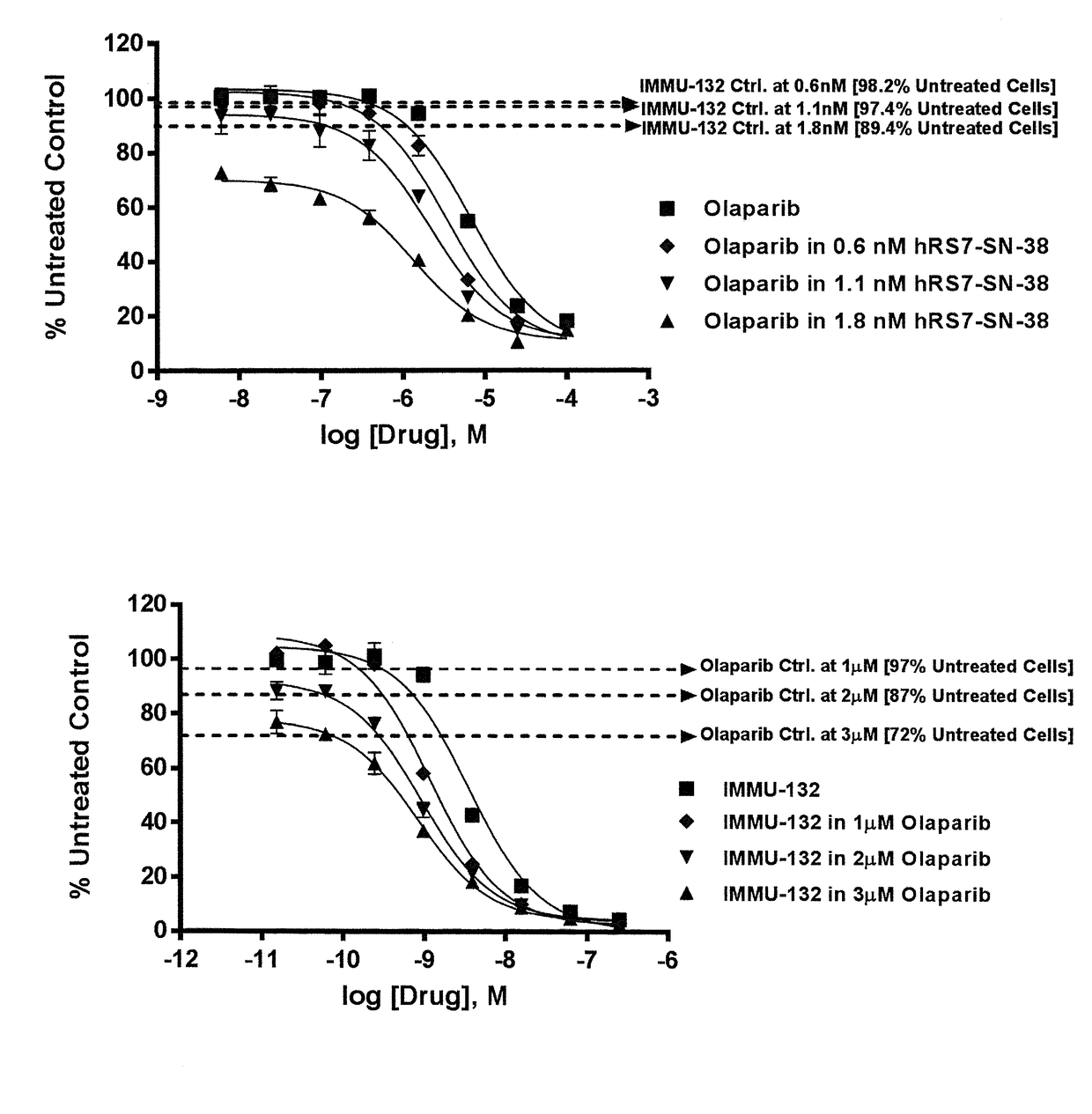
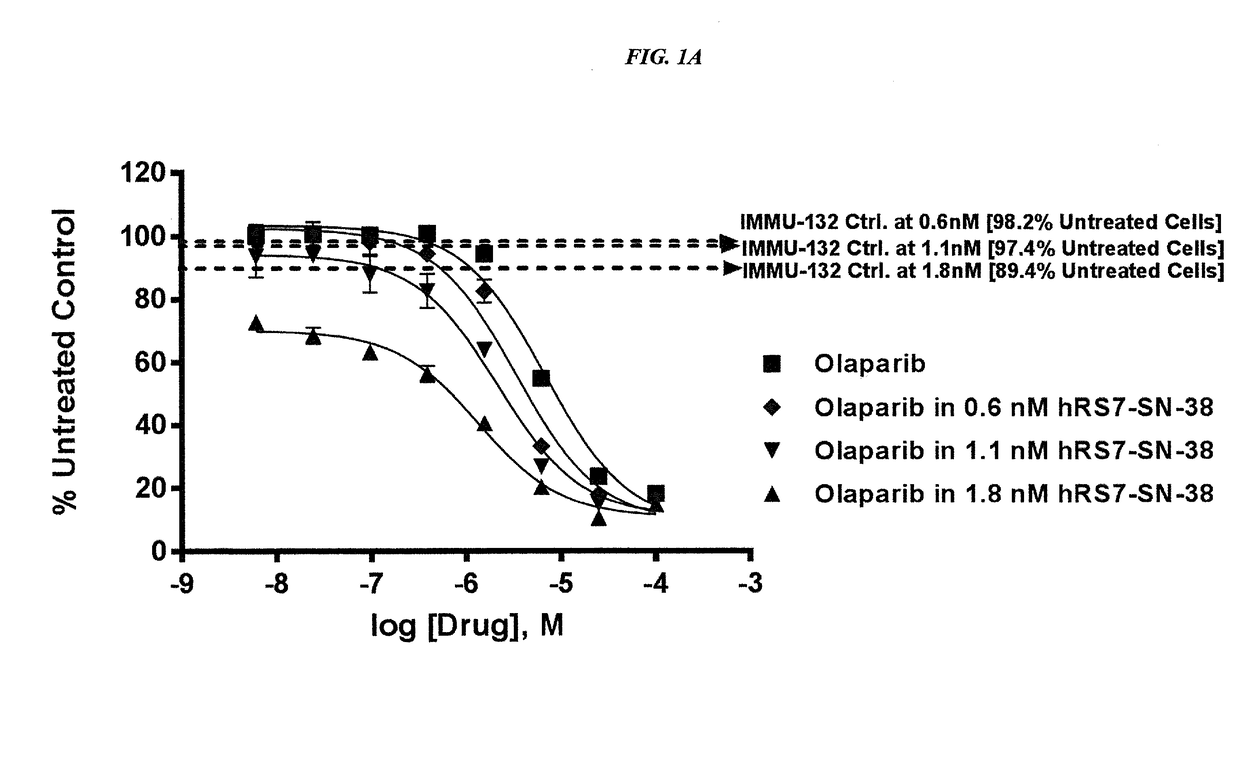
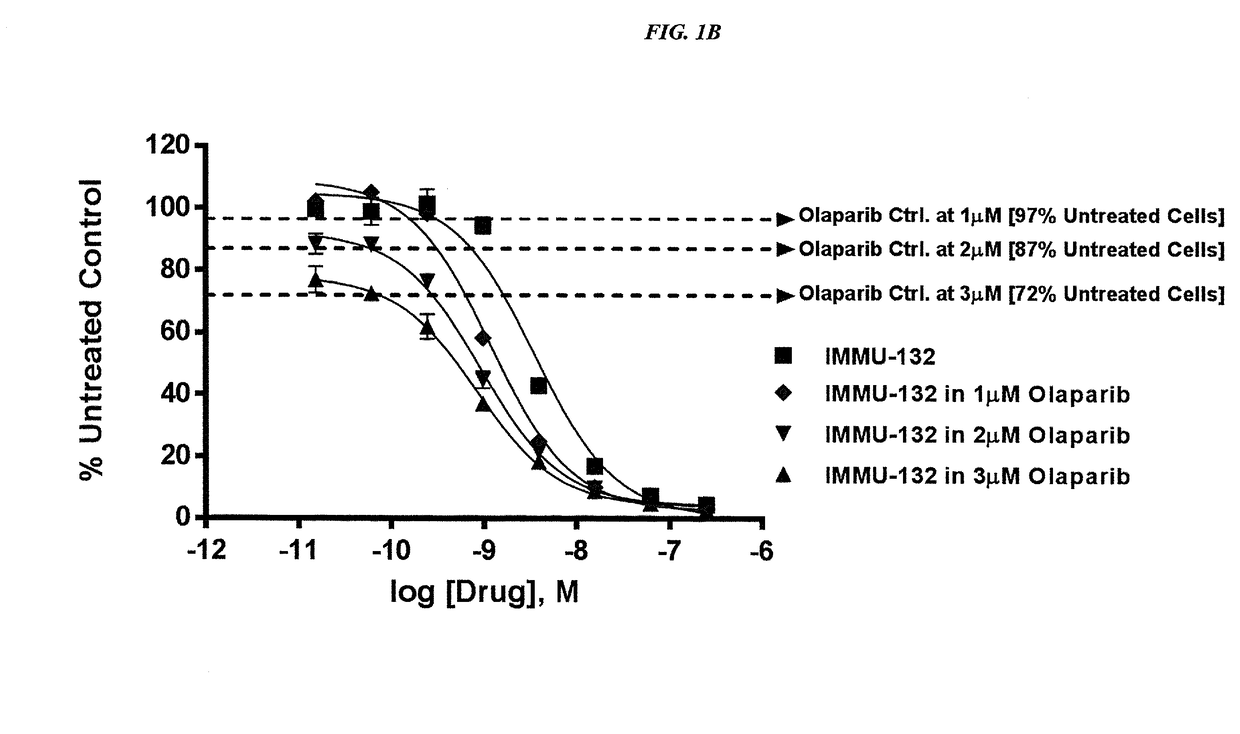
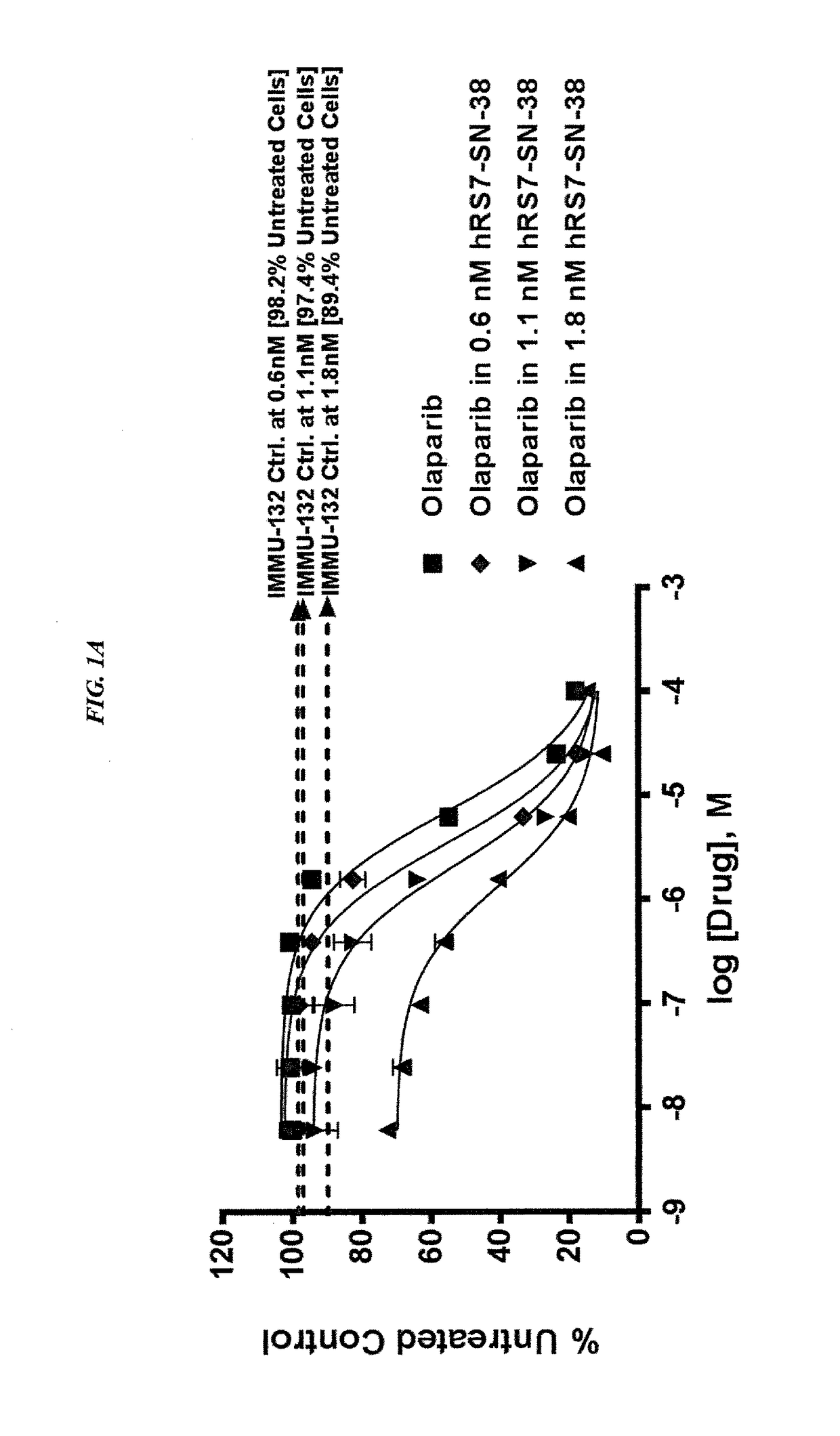
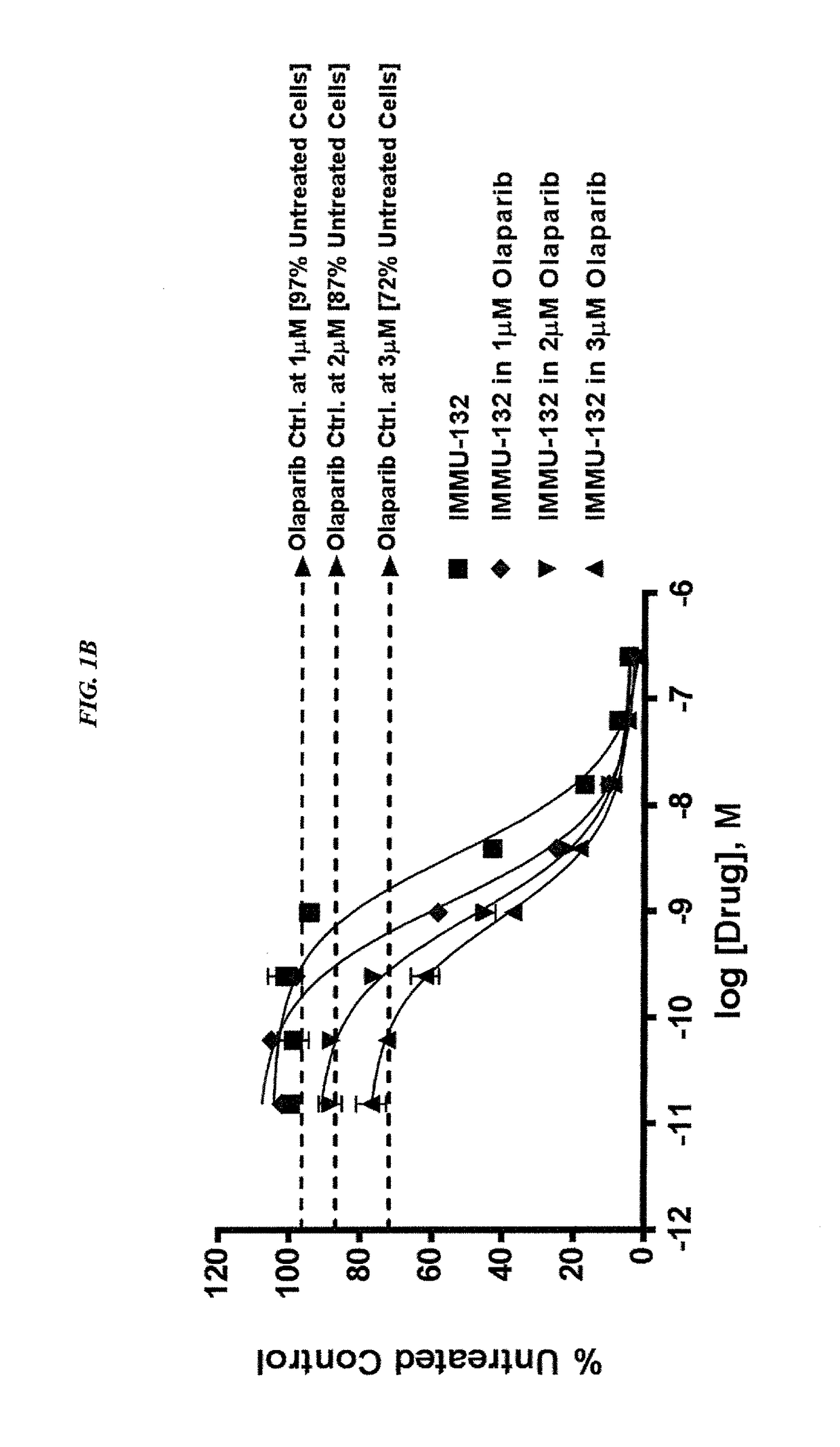
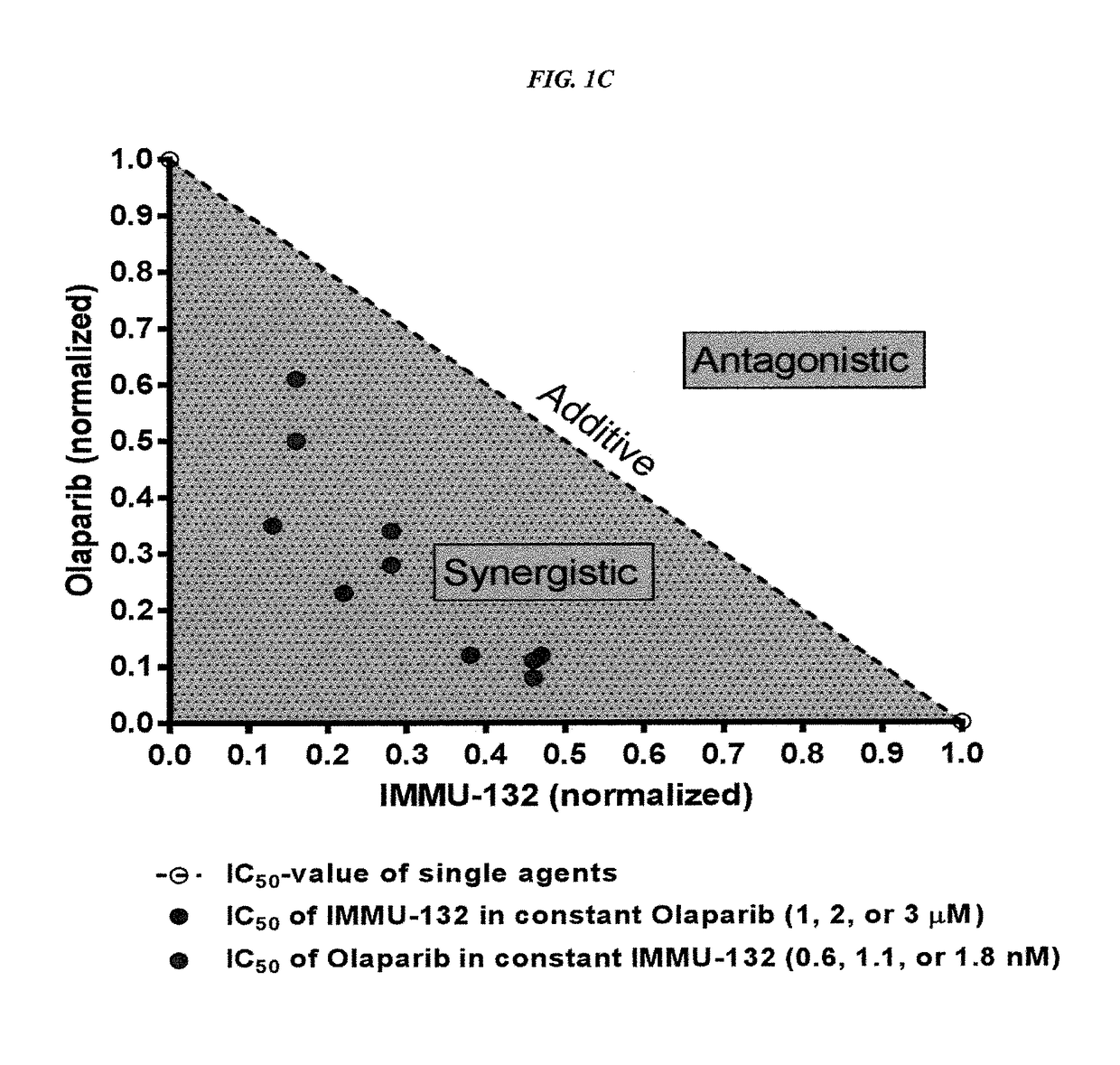
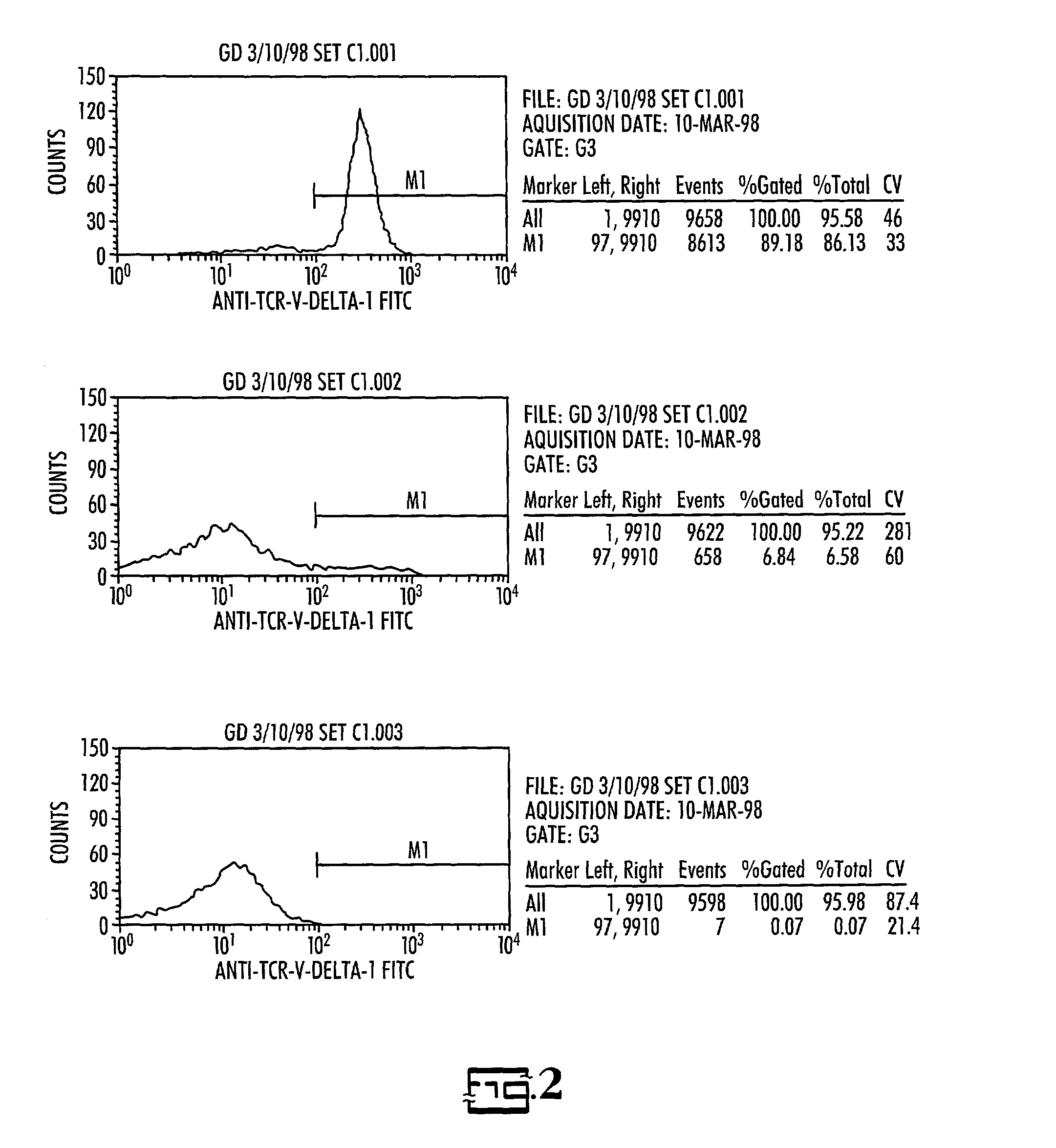
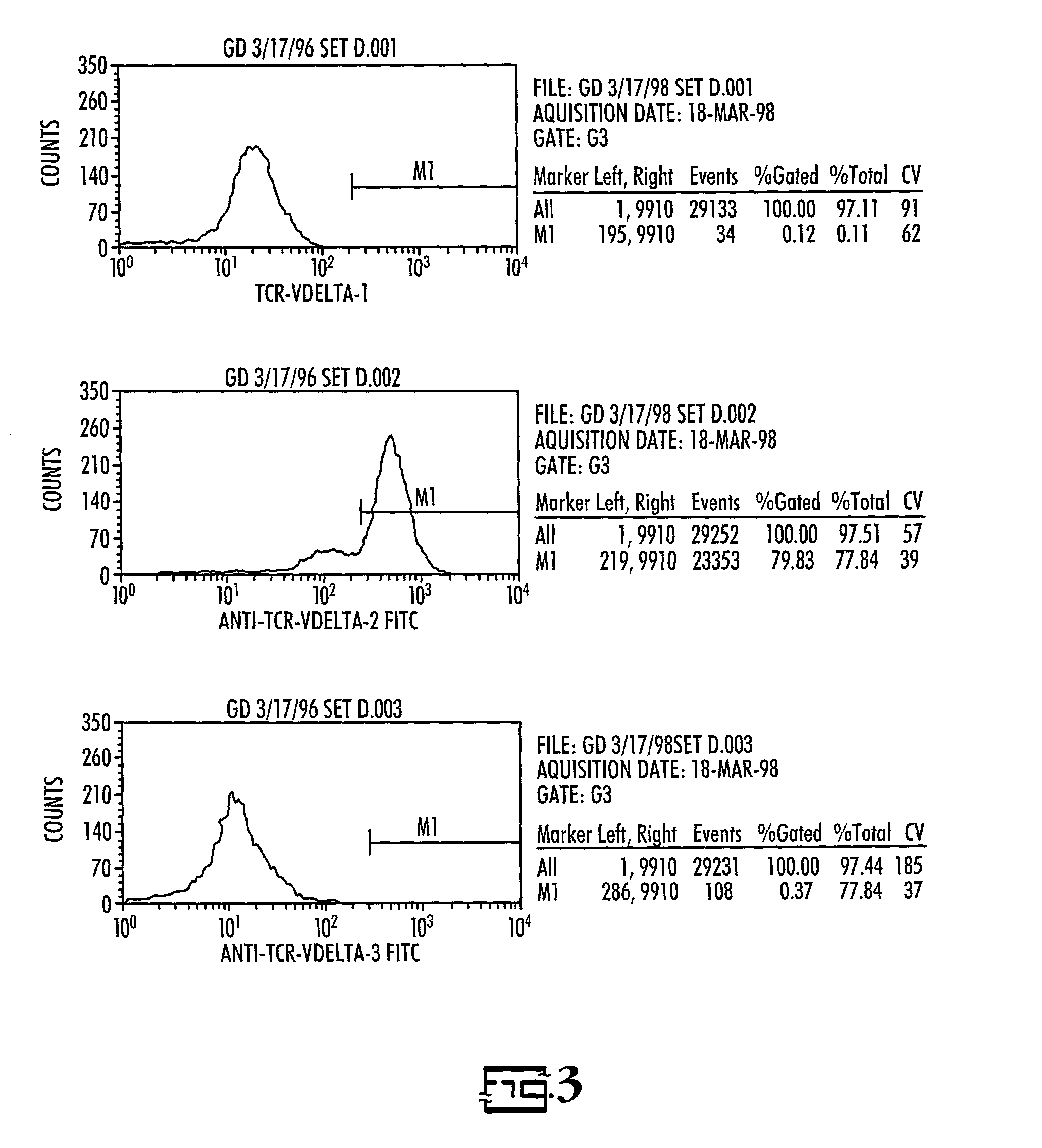
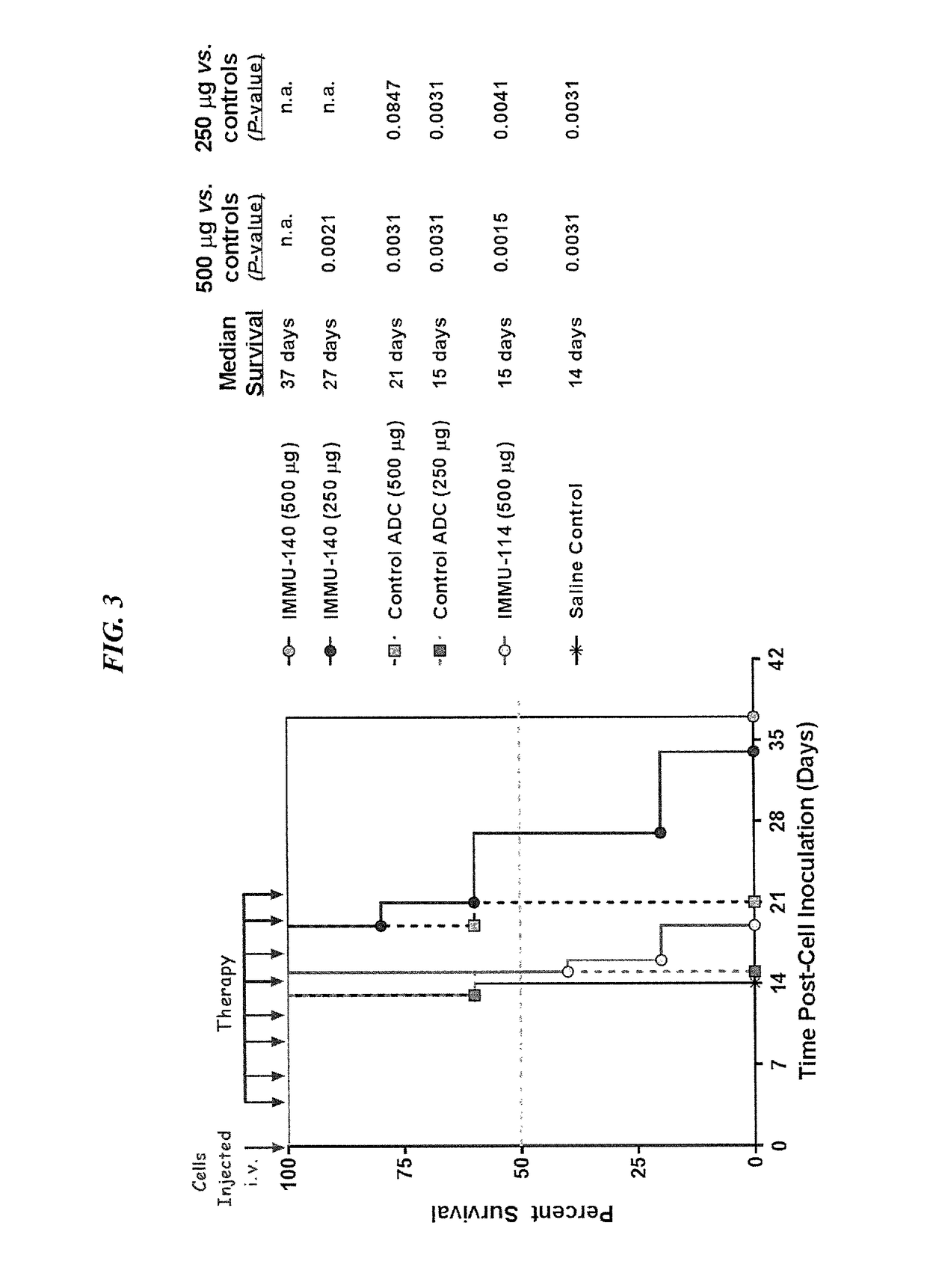
Efficacy of anti-HLA-DR antiboddy drug conjugate IMMU-140 (hL243-CL2A-SN-38) in HLA-DR positive cancers
ActiveUS10206918B2Reducing certain severe side effectsReceive treatment wellOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic immunoconjugates comprising SN-38 attached to an anti-HLA-DR antibody or antigen-binding antibody fragment. The immunoconjugate may be administered at a dosage of between 3 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, more preferably 8, 10 or 12 mg / kg. When administered at specified dosages and schedules, the immunoconjugate can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. The methods and compositions are particularly useful for treating AML, ALL or multiple myeloma.
Owner:IMMUNOMEDICS INC
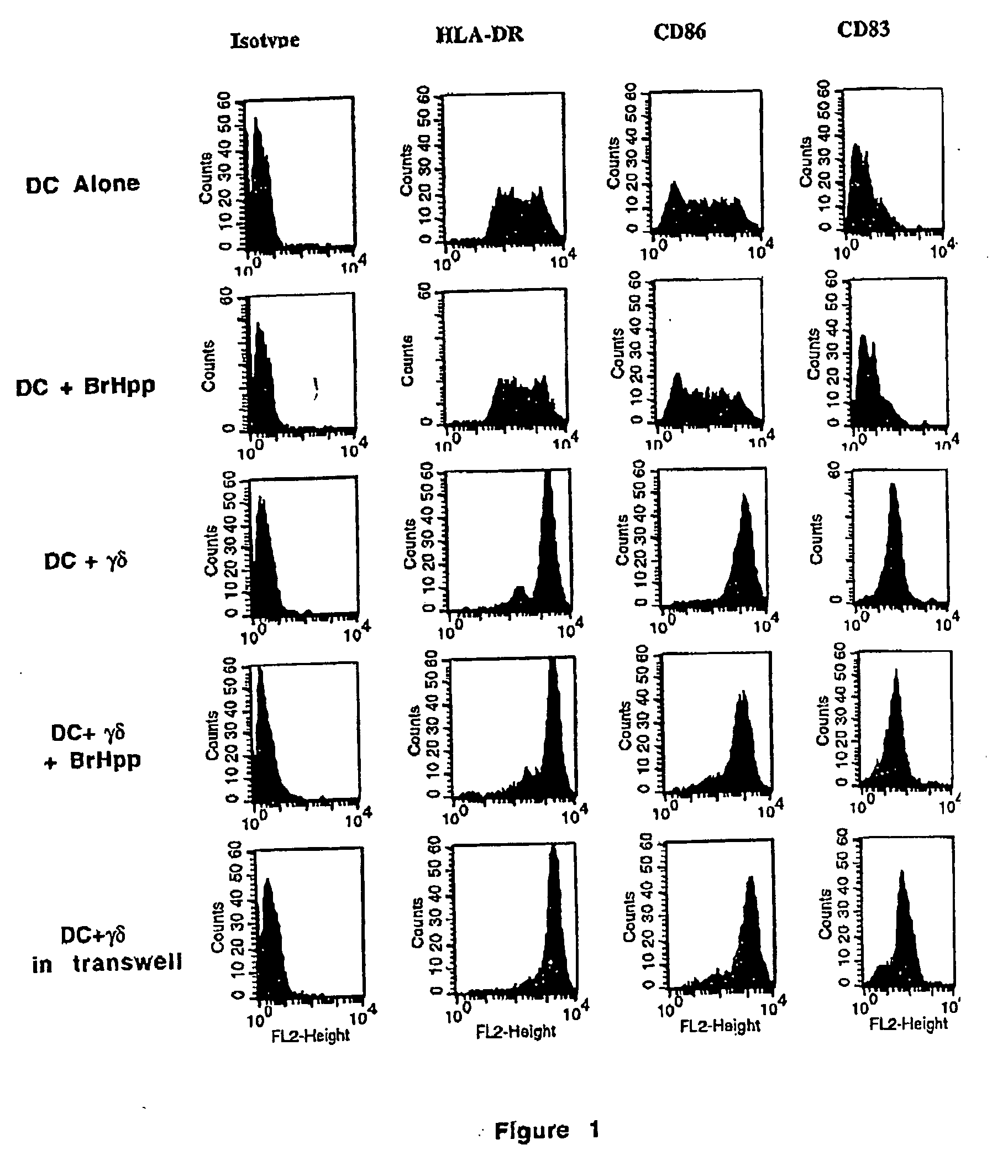
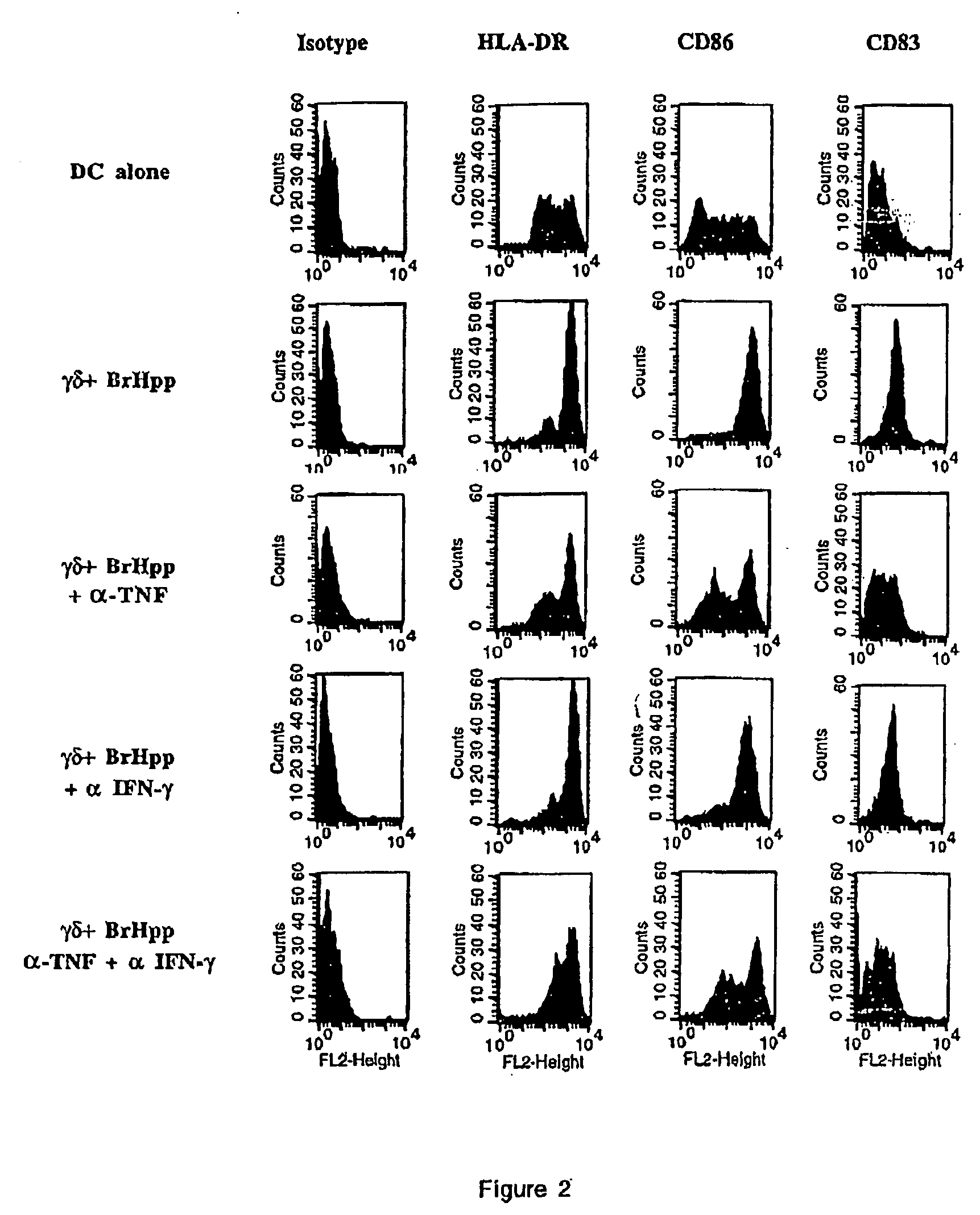
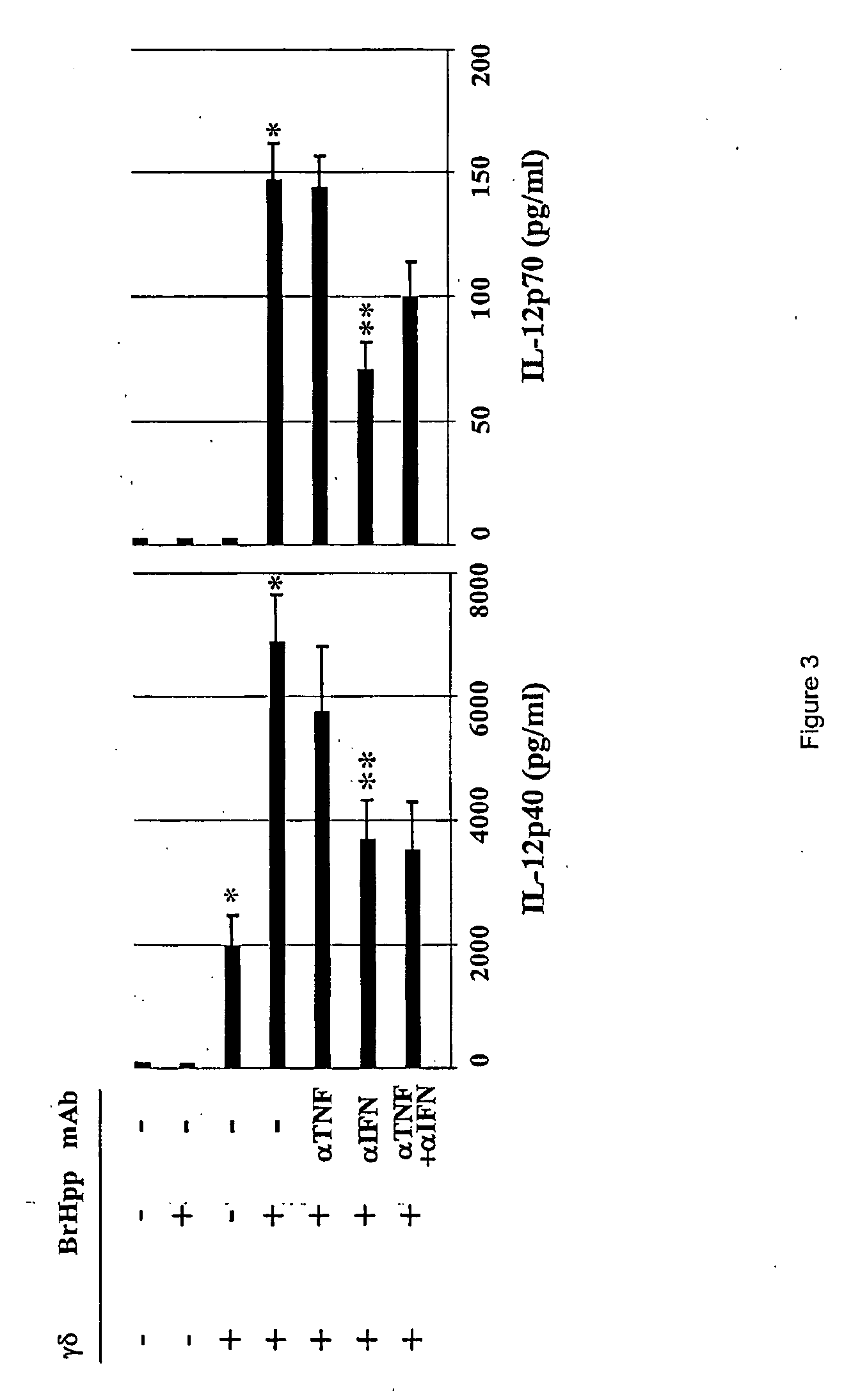
Generation and use of new types of dendritic cells
InactiveUS20050042751A1Eliminate effectiveEffective preventionArtificial cell constructsCancer antigen ingredientsCD86Human leukocyte antigen DR
Owner:INNATE PHARMA SA
Method and detection kit to judge whether solid tumors are suited for immunotherapy
ActiveCN108624650AIncreased objective response rateMicrobiological testing/measurementMaterial analysisPeripheral blood mononuclear cellCD16
The invention provides a detection kit to judge whether solid tumors are suited for immunotherapy. The detection kit includes detection reagents for detecting the indexes: tumor mutation load of peripheral blood circulating tumor DNA, HLA typing, HLA state of loss of heterozygosity, PD-L1 membrane expression positive tumor cell percentage, infiltration level of CD8+ immune cells, infiltration level of FOXP3+ immune cells, mRNA expression score of T-cell inflammation related genes, percentage of CD14+CD16-HLA-DR+ monocytes in peripheral blood mononuclear cells, and micro-satellite instability.The invention also provides, correspondingly, a method to judge whether solid tumors are suited for immunotherapy. The kit and method herein can be used to screen patients with solid tumors so as to provide significantly increased objective response rate and have a promising application prospect in terms of clinical screening of patients with solid tumors suited for immunotherapy.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Peptides associated with HLA-DR MHC class II molecule and involved in Rheumatoid arthritis
InactiveUS20090010943A1Improve usabilityInhibition of activationPeptide/protein ingredientsAntipyreticShared epitopeEpitope
Owner:LONDON HEALTH SCI CENT RES
Method for in vitro amplification of hemopoietic stem cells and precursor cells
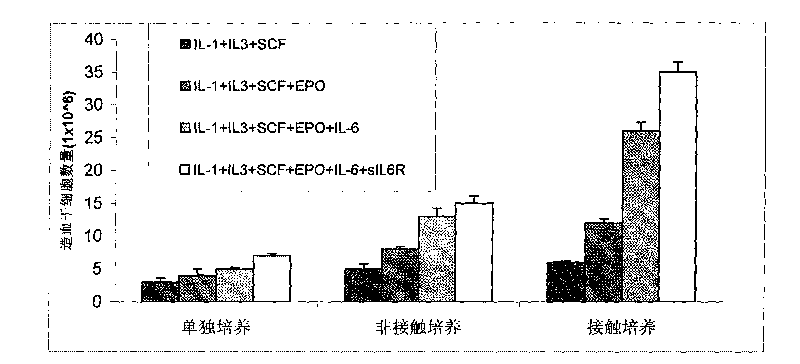
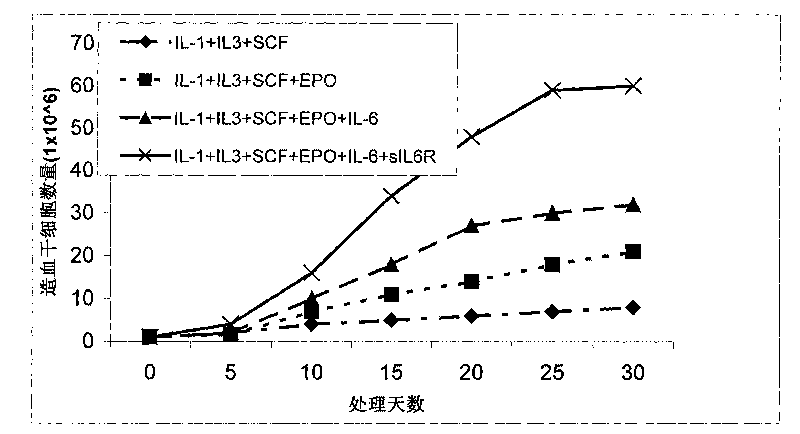
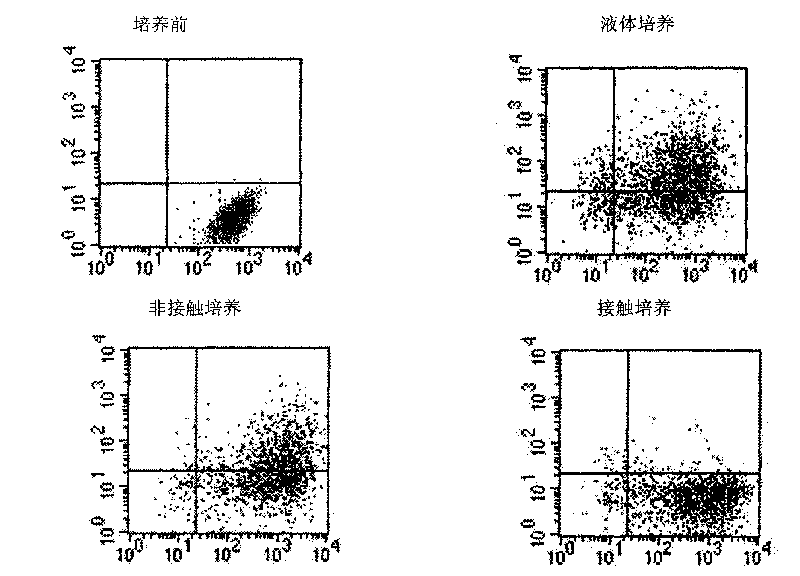
ActiveCN101735979ASymptoms improvedGood amplification effectSkeletal/connective tissue cellsBlood/immune system cellsDiseaseWhole body
The invention discloses a method for in vitro amplification of stem cells, in particular a method for amplifying the hemopoietic stem cells and the precursor cells by co-culturing hemopoietic stem cells and precursor cells, which are separated from human peripheral blood or marrow, and endothelial cells. The method comprises the following steps of: separating the CD34 positive marrow stem cells and precursor cells; making the stem cells and the precursor cells directly contacted with the endothelial cells for culture; adding at least one type of cytokine to amplify the stem cells and the precursor cells; and recognizing the stem cells and the precursor cells by the cell surface antigens, and selecting the stem cells and the precursor cells, which have positive CD34 negative CD38, negative HLA-DR, negative CD15, negative Lin, positive c-kit, and no less than 85 percent purity. The method has the advantages of having good cell amplification effect, obviously reducing the drawing amount of the marrow and peripheral blood of a patient, lowering the necessity of general anaesthesia, enforcing quick recovery of hemopoiesis, and shortening the time when the patient stays in the hospital. The method has a wide application prospect in disease treatment.
Owner:杭州中赢生物医疗科技有限公司
Methods and formulations for targeting infectious agents bearing host cell proteins
A formulation is disclosed for the treatment of diseases caused by an infectious agent which acquires host membranes protein during its life cycle. The formulation is a targeting pharmaceutical composition. It comprises a ligand capable of binding the host membrane proteins coupled to a lipid-comprising vesicle, which may comprise or not a drug effective in the treatment of the disease. Specific liposomes bearing anti-HLA-DR or anti-CD4 antibodies comprising or not antiviral drugs, namely anti-HIV drugs, are disclosed and claimed. A method of formulation as well as a method of using the formulation in the treatment of a disease are also disclosed.
Owner:INFECTIO RECHERCHE INC
Polypeptide composition for detecting immune antibody of rheumatoid arthritis in vitro
ActiveCN101819201AFacilitate in vitro detectionHigh affinityBiological testingHybrid peptidesArginineSide chain
The invention discloses a polypeptide composition for detecting an immune antibody of rheumatoid arthritis in vitro. At least one arginine side chain in various polypeptide sequences is modified to be electro-neutral or electro-negative amino acid. Cyclic polypeptide is produced by forming a disulfide bond with two non-adjacent cysteine side chain sulfhydryl groups in the sequence. The polypeptide not only can be combined with the autoimmune antibody of the rheumatoid arthritis, but also have high affinity to human leukocyte antigen DR (HLA-DR). Experiments prove that the specificity for the combination of the polypeptide and the autoimmune antibody of the rheumatoid arthritis is more than 90 percent and the flexibility for the detection of the autoimmune antibody of the rheumatoid arthritis is more than 75 percent. Compared with the similar products sold on the market, the polypeptide composition has obviously improved sensitivity so as to more contribute to detecting the immune antibody of the rheumatoid arthritis in vitro.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
Immune cell function evaluation kit and evaluation method for tumor patient
The invention provides an immune cell function evaluation kit and evaluation method for a tumor patient. The immune cell function evaluation kit for the tumor patient comprises an anti-human CD3 antibody, an anti-human CD4 antibody, an anti-human CD8 antibody, an anti-human CD25 antibody, an anti-human CD28 antibody, an anti-human CD38 antibody, an anti-human CD56 antibody, an anti-human CD57 antibody, an anti-human CD94 antibody, an anti-human CD127 antibody, an anti-human CD45RA antibody, an anti-human CCR7 antibody, an anti-human HLA-DR antibody, an anti-human PD-1 antibody, an anti-human NKP30 antibody, an anti-human NKP46 antibody, an anti-human NKG2D antibody, an anti-human KIR antibody, an anti-human gamma-sigma antibody and an anti-human Vsigma2 antibody. All the above antibodies carry fluorochrome labels. The kit can be used for comprehensively evaluating an immune cell function of the tumor patient and is convenient and safe to use.
Owner:东莞市暨科生物科技有限公司
Novel MHC II associated peptides
InactiveUS20050202009A1Tumor rejection antigen precursorsPeptide/protein ingredientsAbnormal tissue growthMHC class II
The present invention provides novel naturally-processed antigenic peptides which are candidate tumor antigens in melanoma and other tumors. These antigenic peptides are presented by human MHC class II HLA-DR molecules. They originate from the translation factor eIF-4A, the IFN-gamma-inducible protein p78, the cytoskeletal protein vimentin and the iron-binding surface protein melanotransferrin. The antigenic peptides of the present invention can be used as markers in diagnosis of the respective tumors and in therapy as anti-tumor vaccines.
Owner:F HOFFMANN LA ROCHE & CO AG
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com