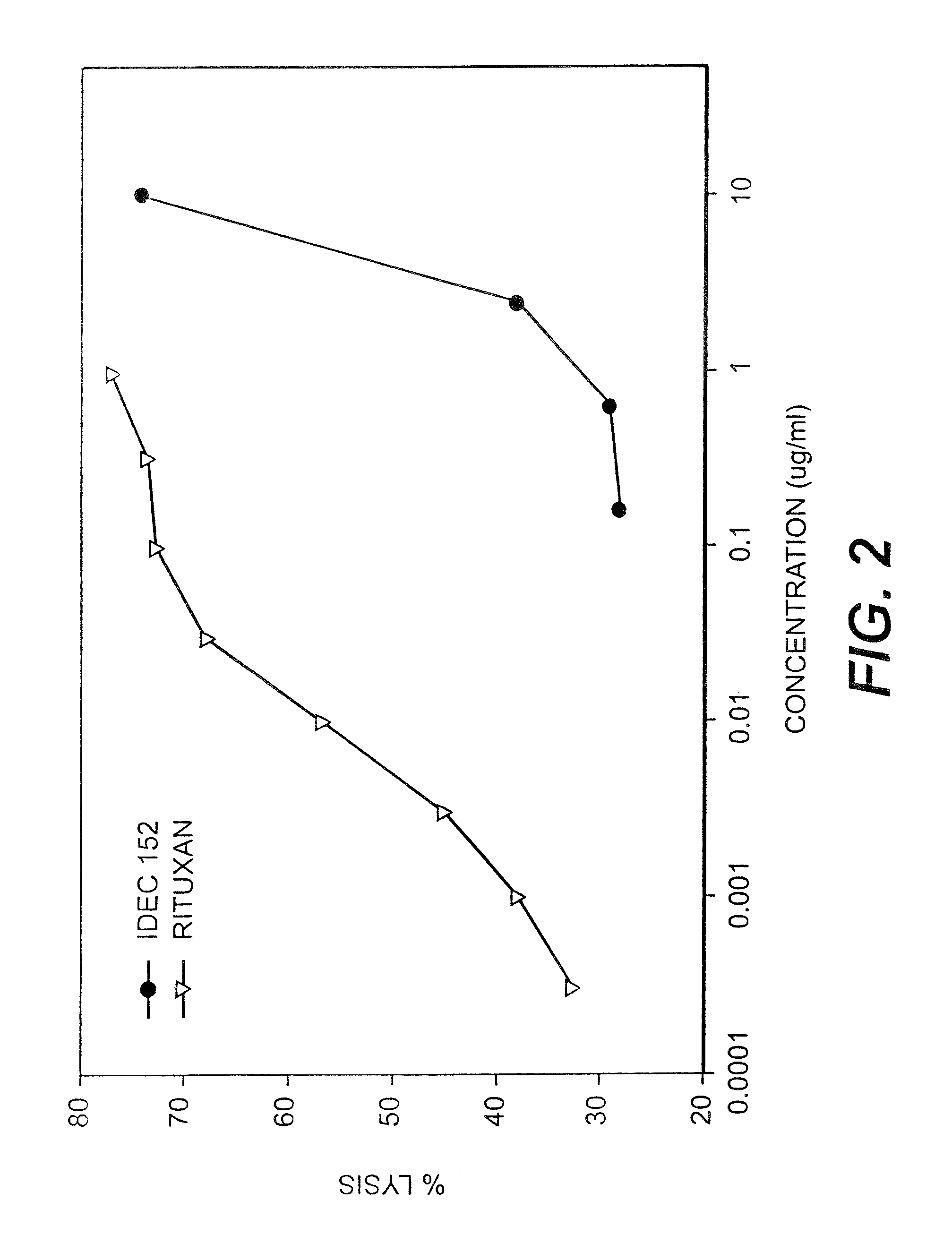
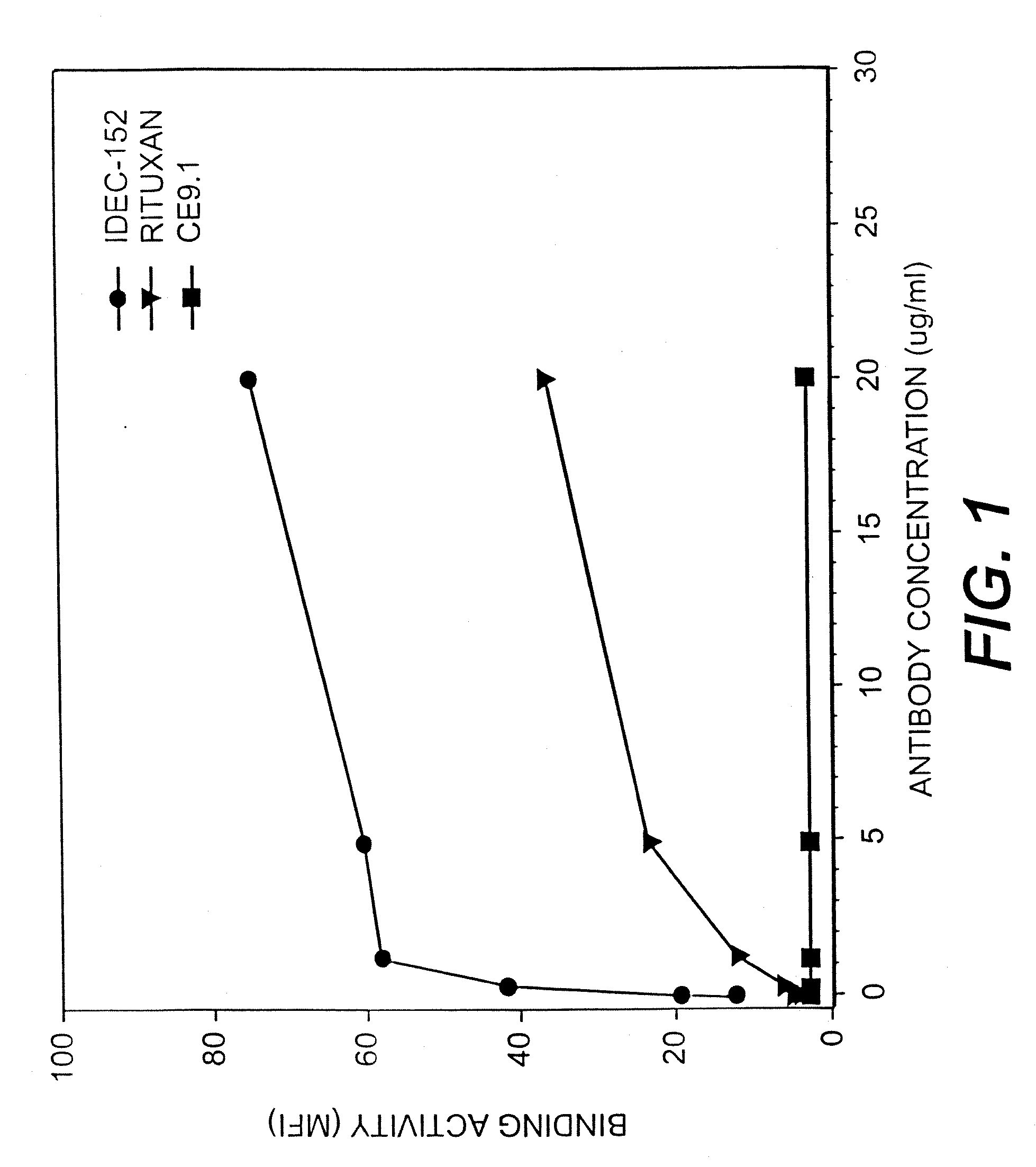
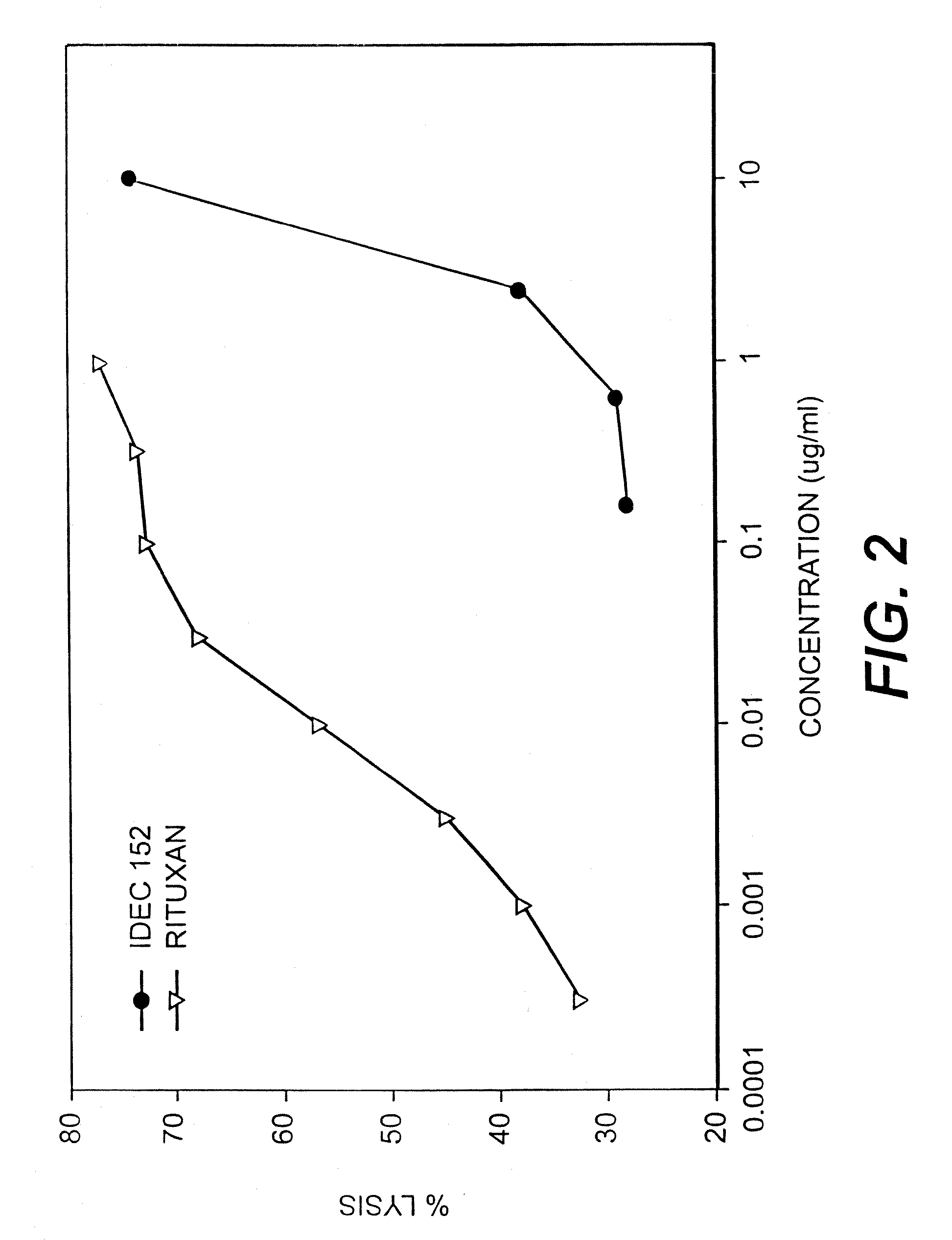
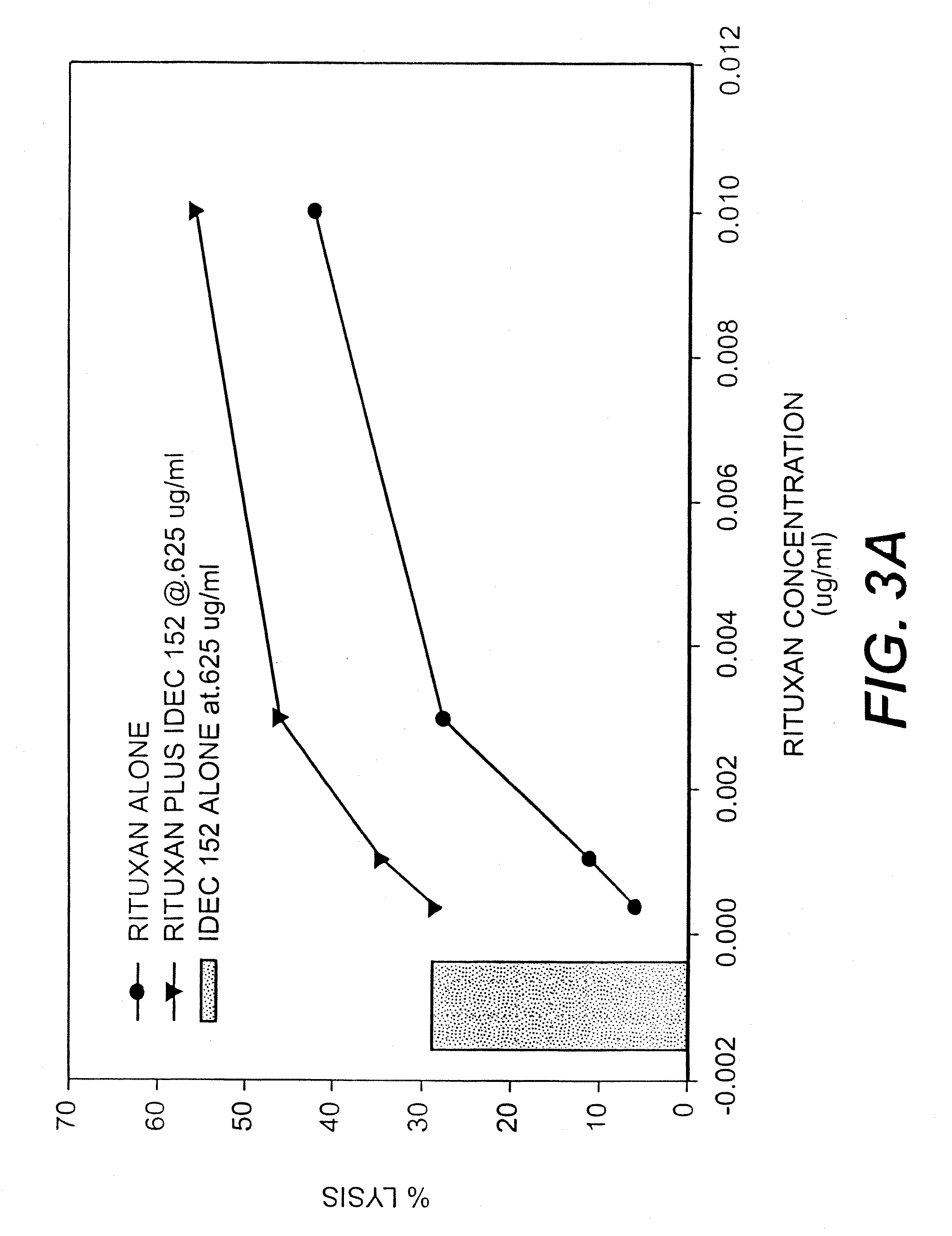
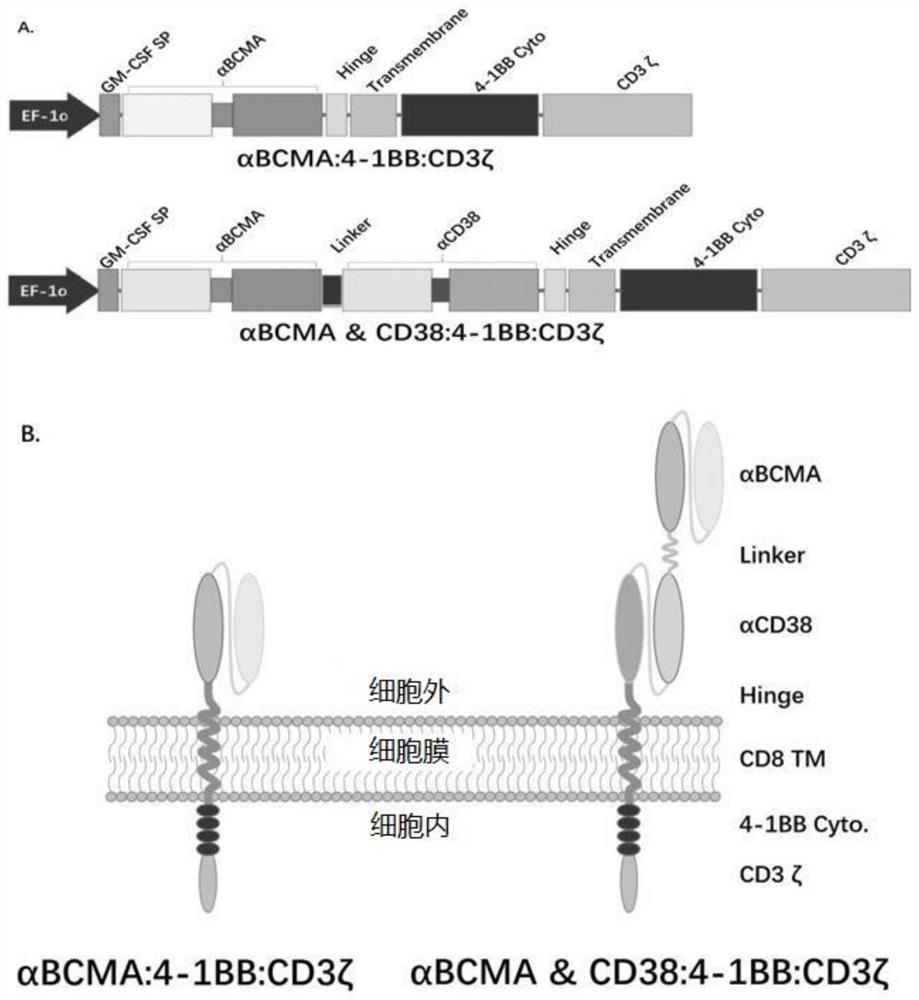
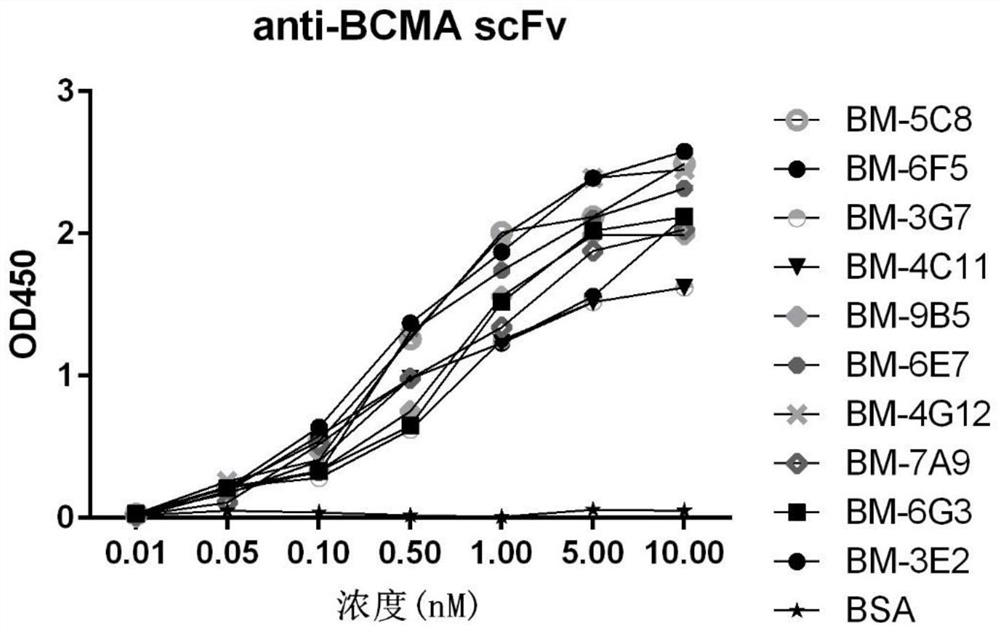
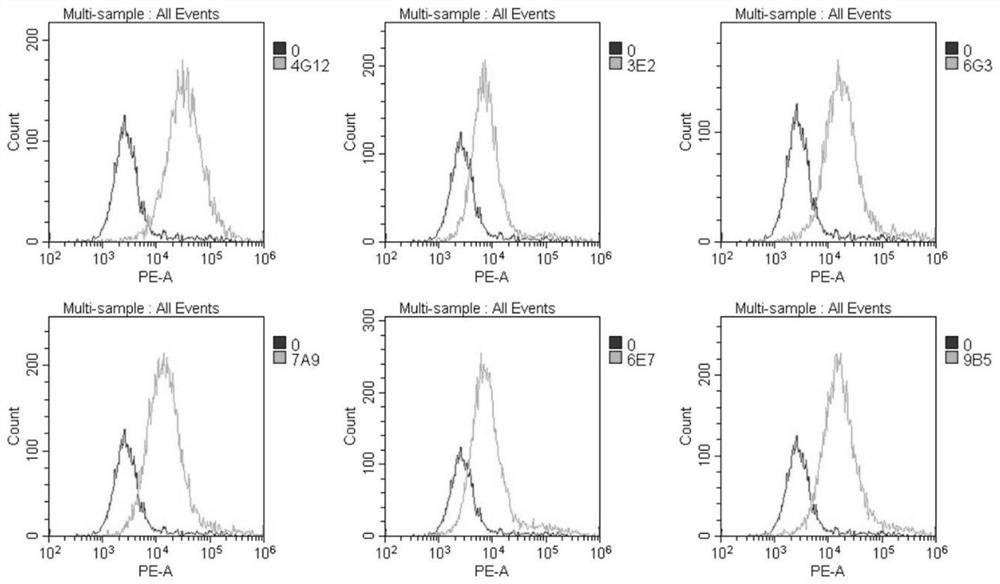
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "CD23" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
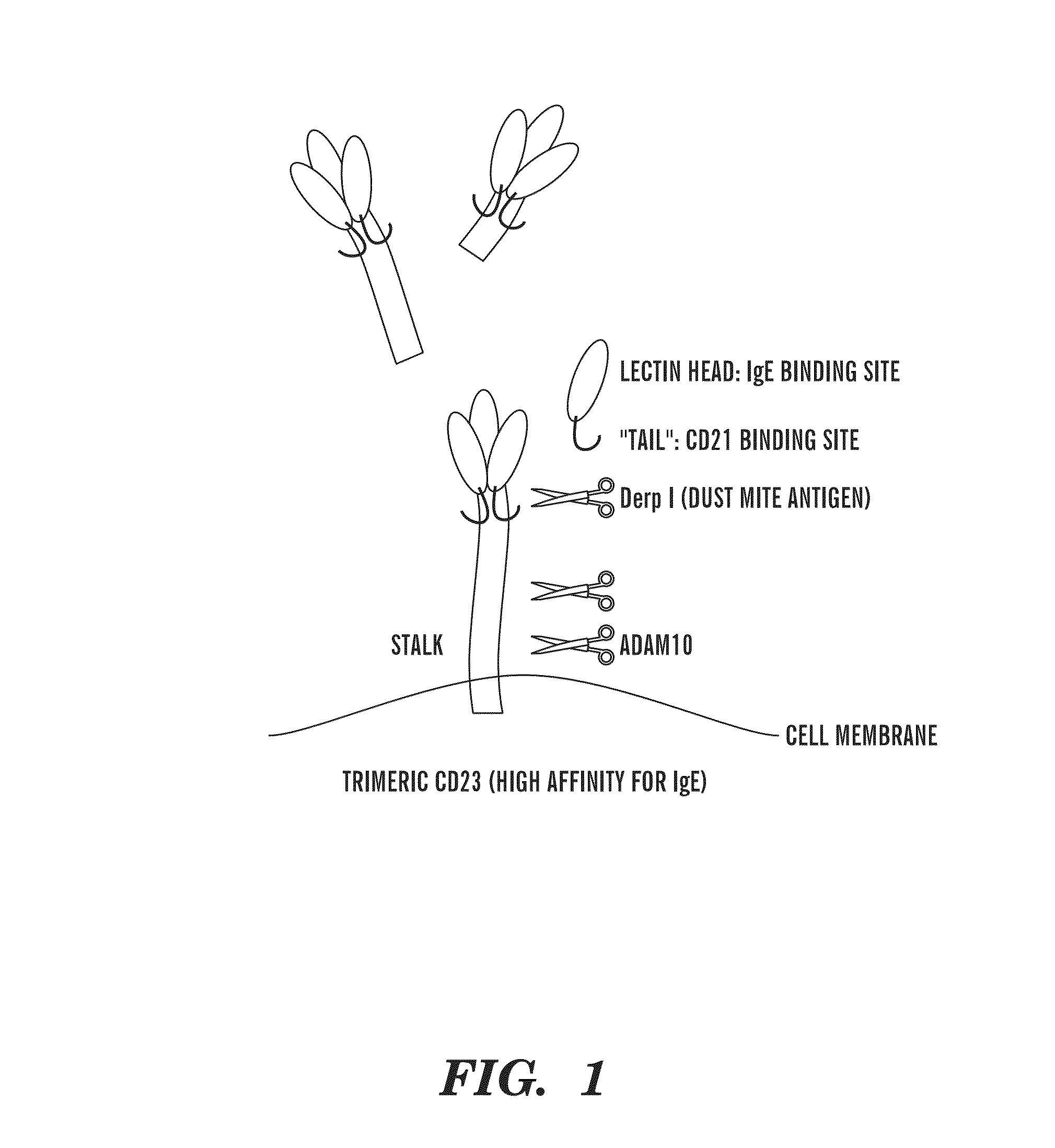
CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 is a C-type lectin. It is found on mature B cells, activated macrophages, eosinophils, follicular dendritic cells, and platelets.
Immunoregulatory antibodies and uses thereof
InactiveUS20030103971A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
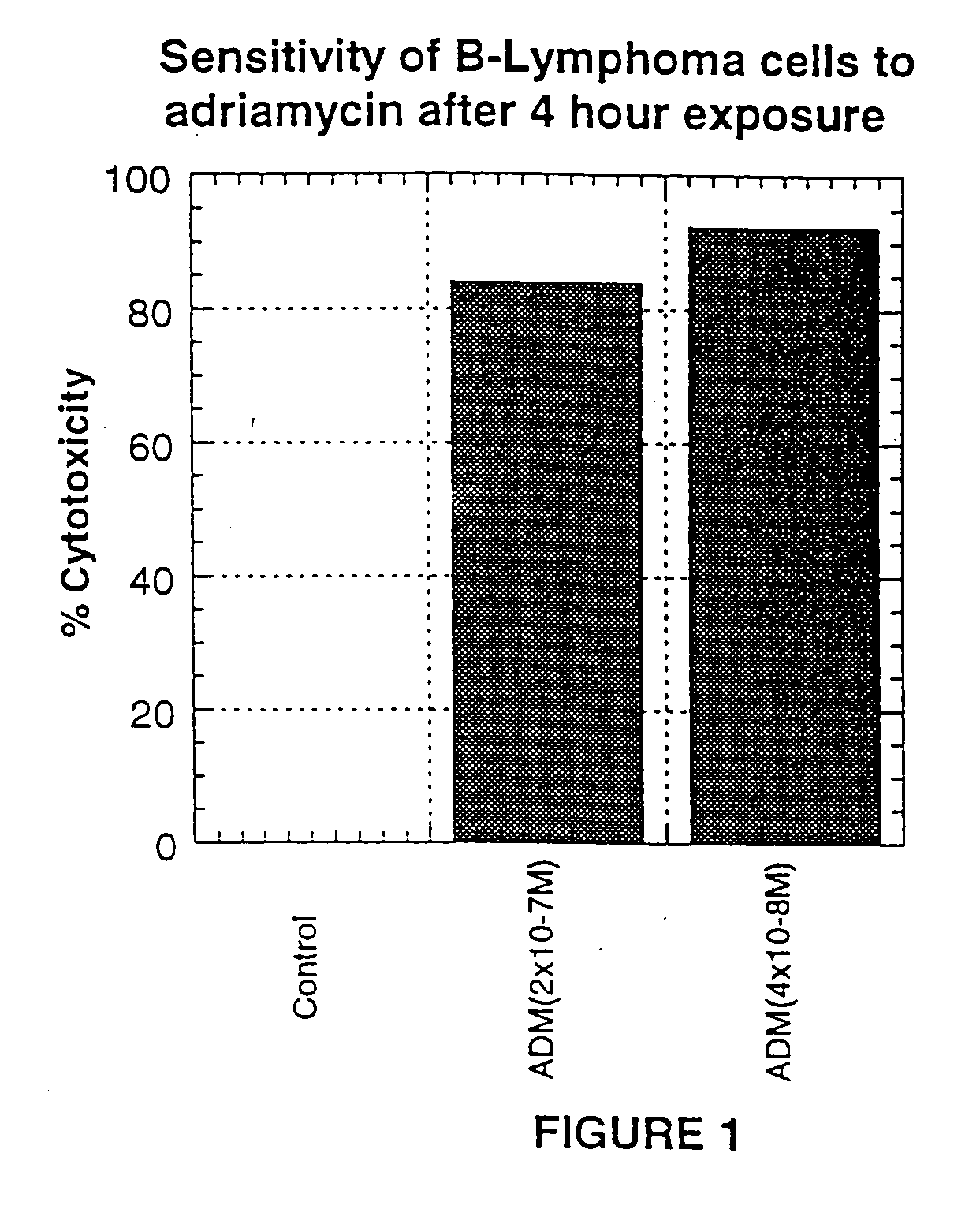
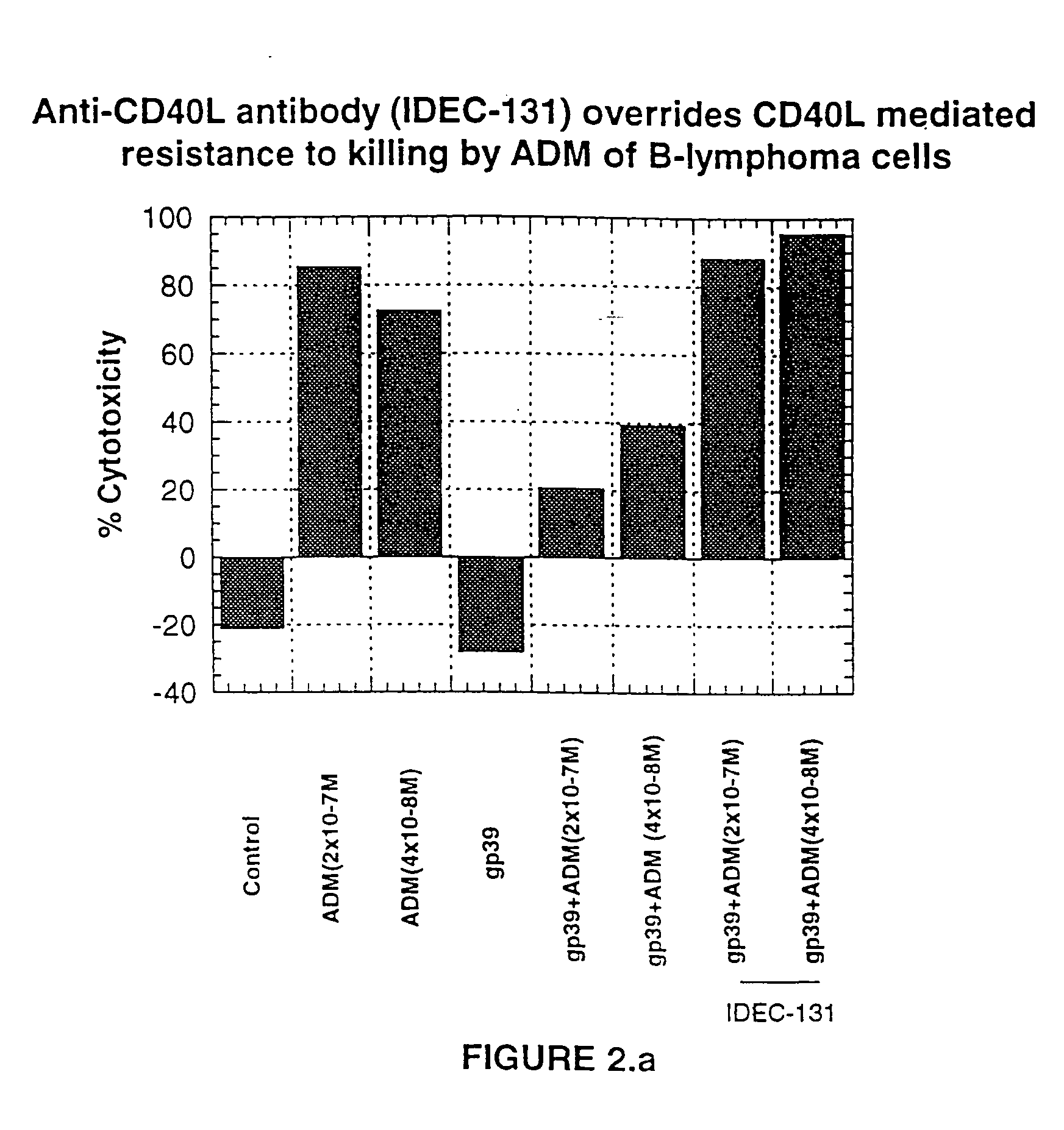
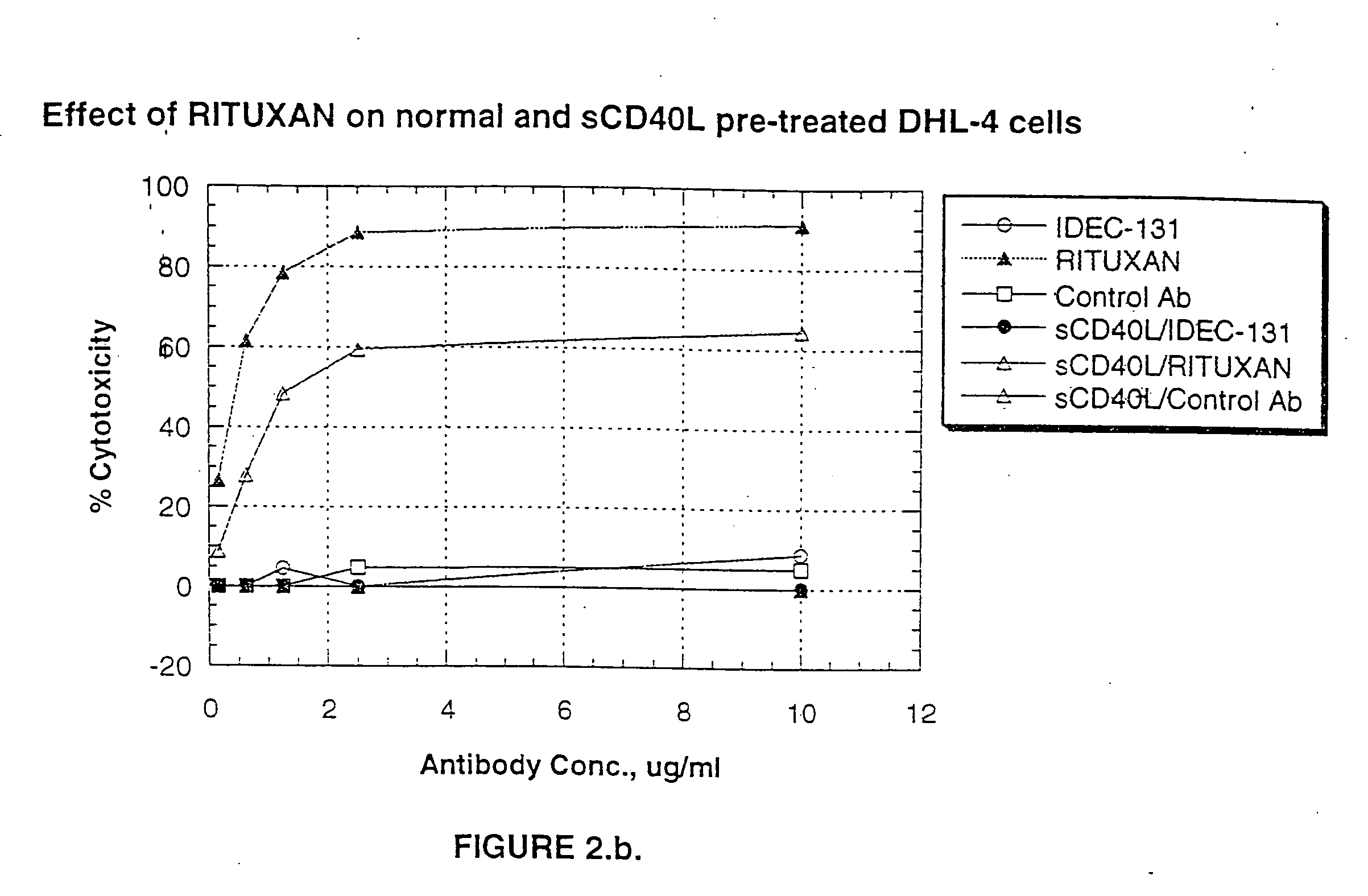
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
Cd23 binding molecules and methods of use thereof
InactiveUS20090155255A1Improve efficacyImprove stabilityAnimal cellsSugar derivativesDiseaseBiochemistry
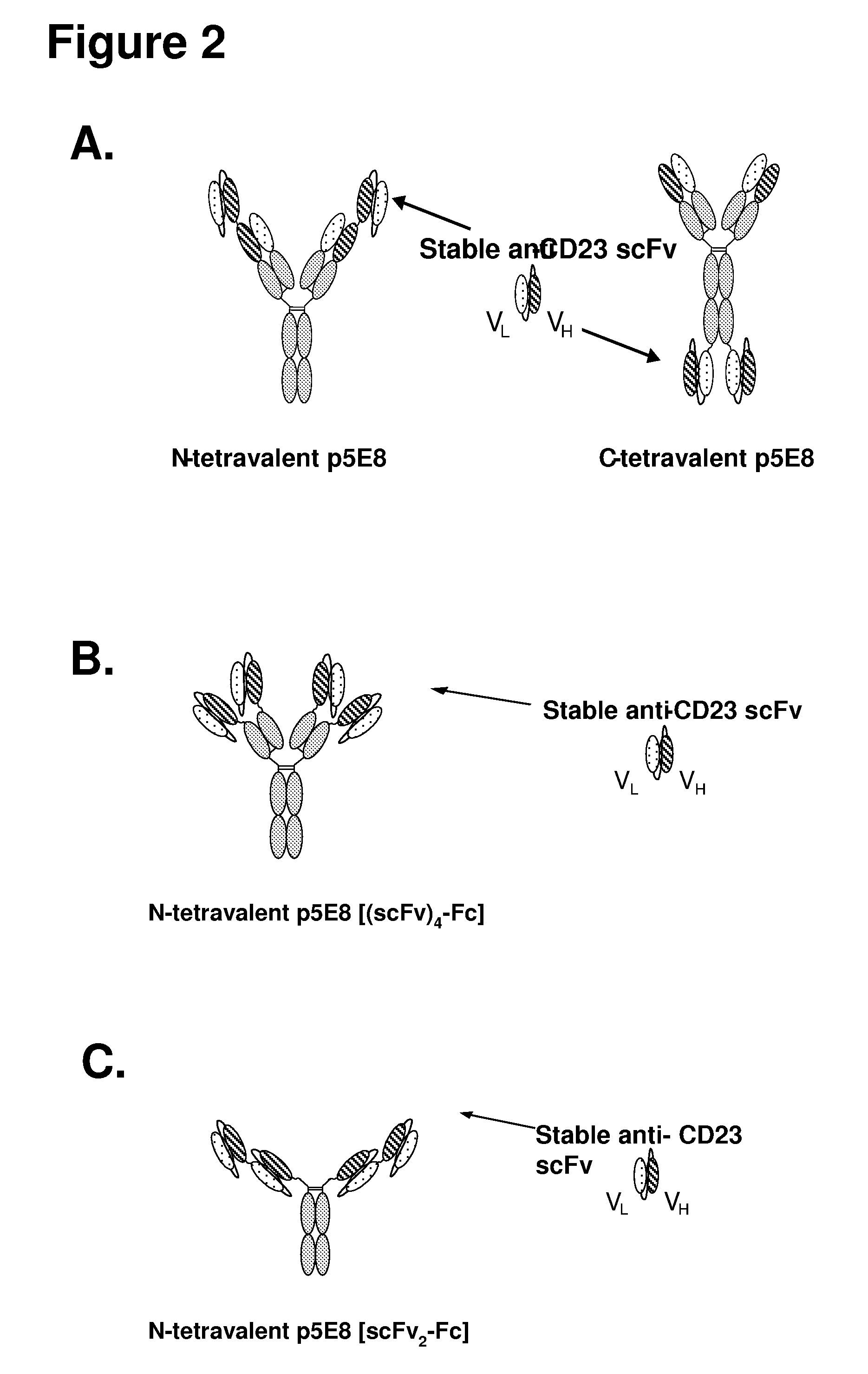
The invention is based, at least in part, on the development of multivalent and stabilized forms of CD23 binding molecules and methods of use thereof for the treatment of immune cell disorders, including leukemias or lymphomas such as CLL.
Owner:BIOGEN IDEC MA INC
Treatment of B cell malignancies using combination of B cell depleting antibody and immune modulating antibody related applications
InactiveUS20050123540A1Radioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:IDEC PHARM CORP +1
Immunoregulatory Antibodies and Uses Thereof
InactiveUS20070009519A1Convenient treatmentIn-vivo radioactive preparationsPharmaceutical containersCD37Combination therapy
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Use of CD23 antagonists for the treatment of neoplastic disorders
InactiveUS20060171950A1Reduces and inhibits and controls growth of neoplasticEfficient administrationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCD23
Methods and kits for the treatment of neoplastic disorders comprising the use of a CD23 antagonist are provided. The CD23 antagonist may be used alone or in combination with chemotherapeutic agents. In particularly preferred embodiments the CD23 antagonists may be used to treat B cell chronic lymphocytic leukemia (B-CLL).
Owner:BIOGEN IDEC MA INC
Immunoregulatory antibodies and uses thereof
InactiveUS20080227198A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Use of CD23 Antagonists for the Treatment of Neoplastic Disorders
InactiveUS20060286101A1Efficient administrationImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCD23
Methods and kits for the treatment of neoplastic disorders comprising the use of a CD23 antagonist are provided. The CD23 antagonist may be used alone or in combination with chemotherapeutic agents. In particularly preferred embodiments the CD23 antagonists may be used to treat B cell chronic lymphocytic leukemia (B-CLL).
Owner:BIOGEN MA INC
Immunoregulatory antibodies and uses thereof
InactiveUS20080226626A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD37CD23
A combination antibody therapy for treating B cell malignancies using an immunoregulatory antibody, especially an anti-B7, anti-CD23, or anti-CD40L antibody and a B cell depleting antibody, especially anti-CD19, anti-CD20, anti-CD22 or anti-CD37 antibody is provided. Preferably, the combination therapy will comprise anti-B7 and anti-CD20 antibody administration.
Owner:BIOGEN INC
Use of CD23 Antagonists for the Treatment of Neoplastic Disorders
InactiveUS20060286100A1Efficient administrationImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCD23
Methods and kits for the treatment of neoplastic disorders comprising the use of a CD23 antagonist are provided. The CD23 antagonist may be used alone or in combination with chemotherapeutic agents. In particularly preferred embodiments the CD23 antagonists may be used to treat B cell chronic lymphocytic leukemia (B-CLL).
Owner:BIOGEN MA INC
Duplex-specific chimeric antigen receptor molecule and application thereof to tumor therapy
ActiveCN109503716AIncreased persistenceImprove targetingPeptide/protein ingredientsMammal material medical ingredientsSequence signalCD20
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
Compositions and methods to treat asthma
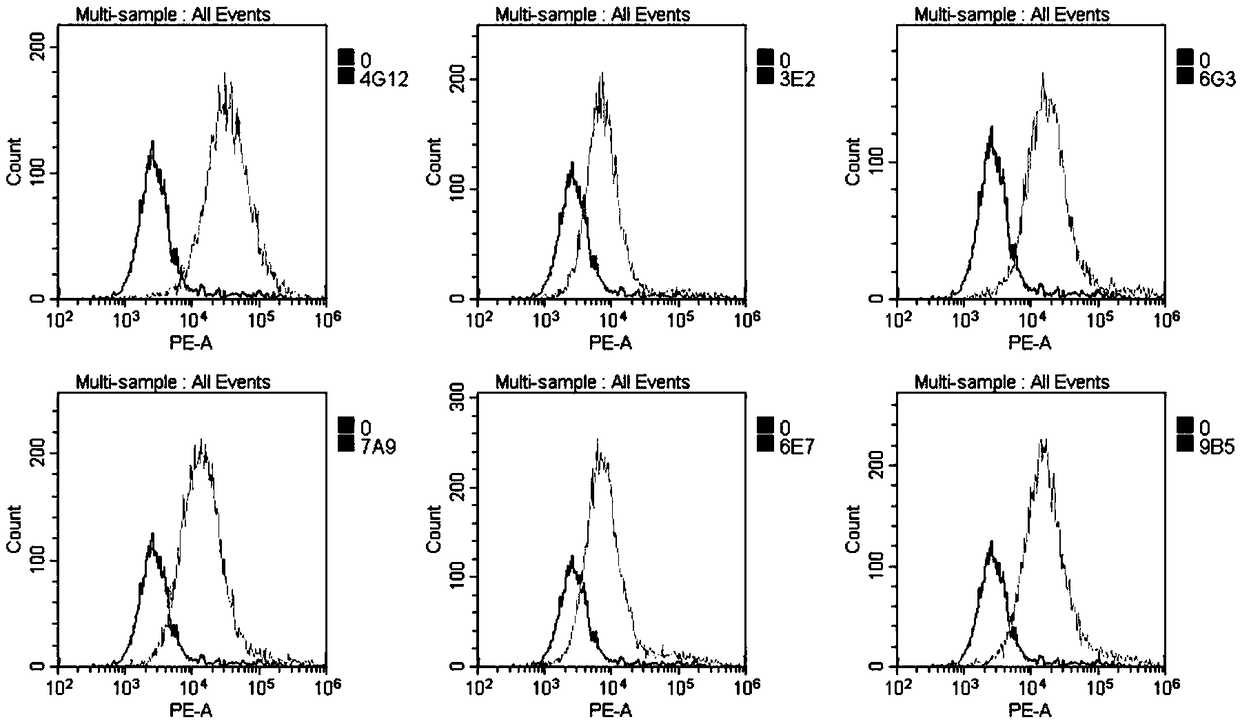
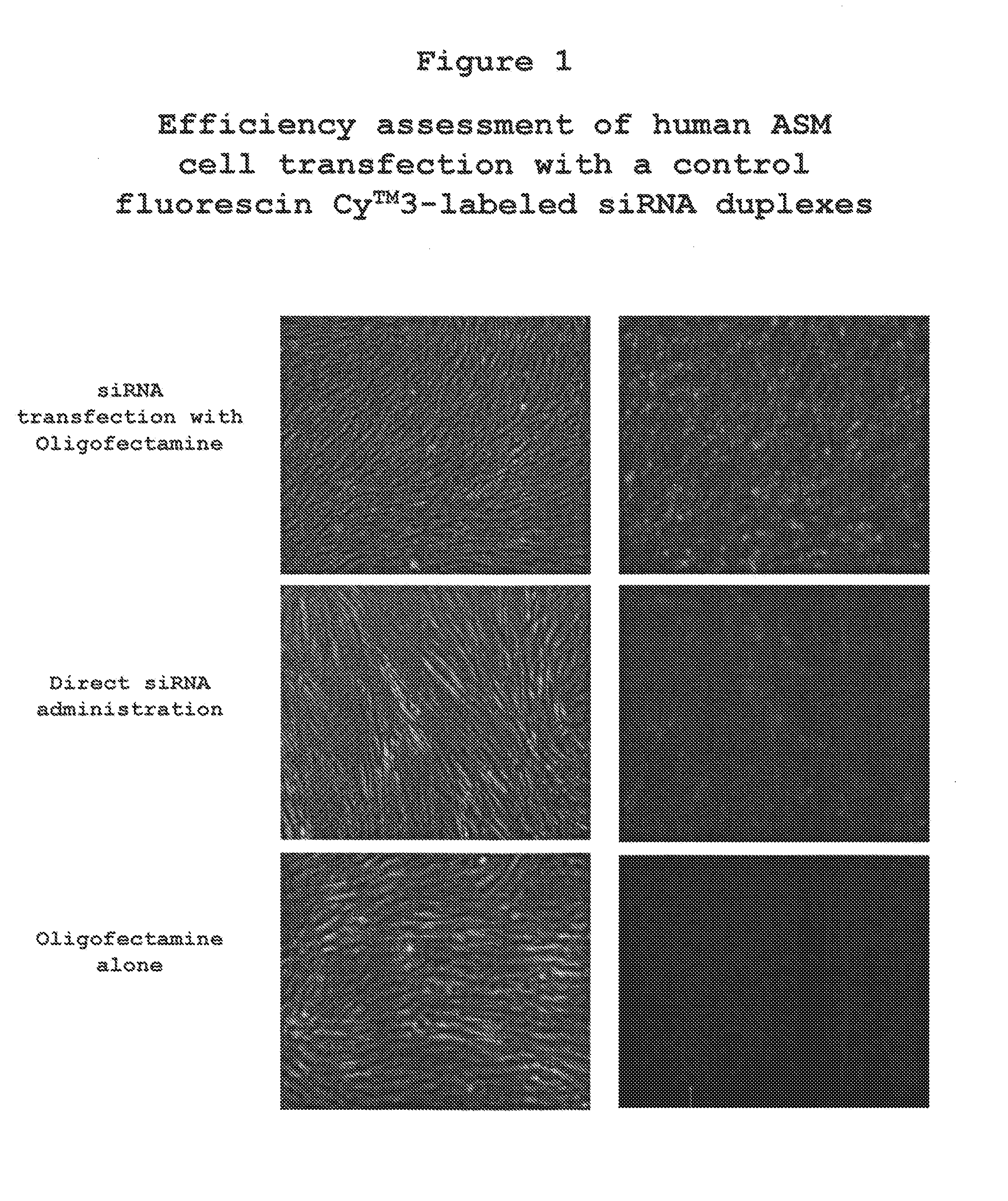
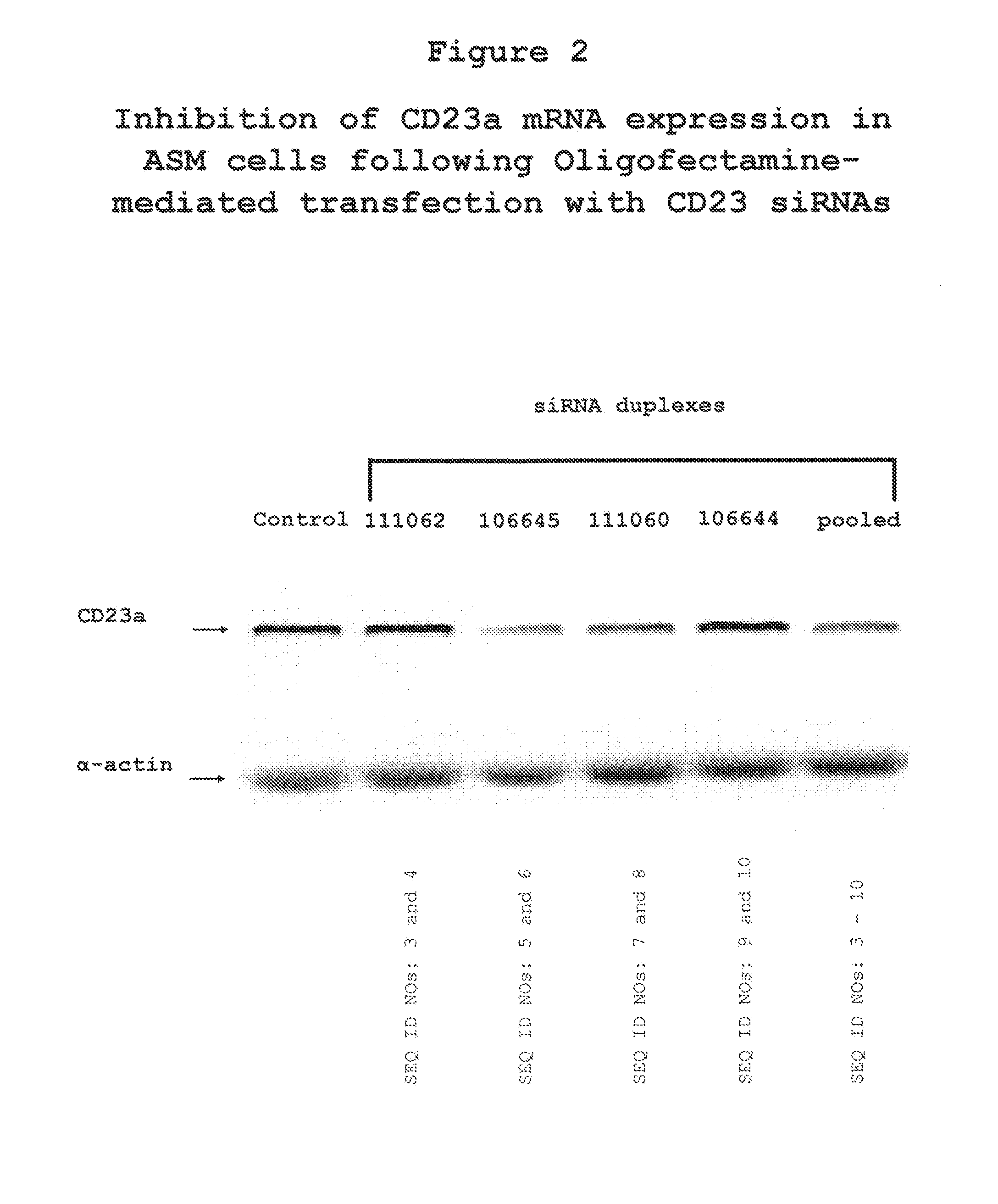
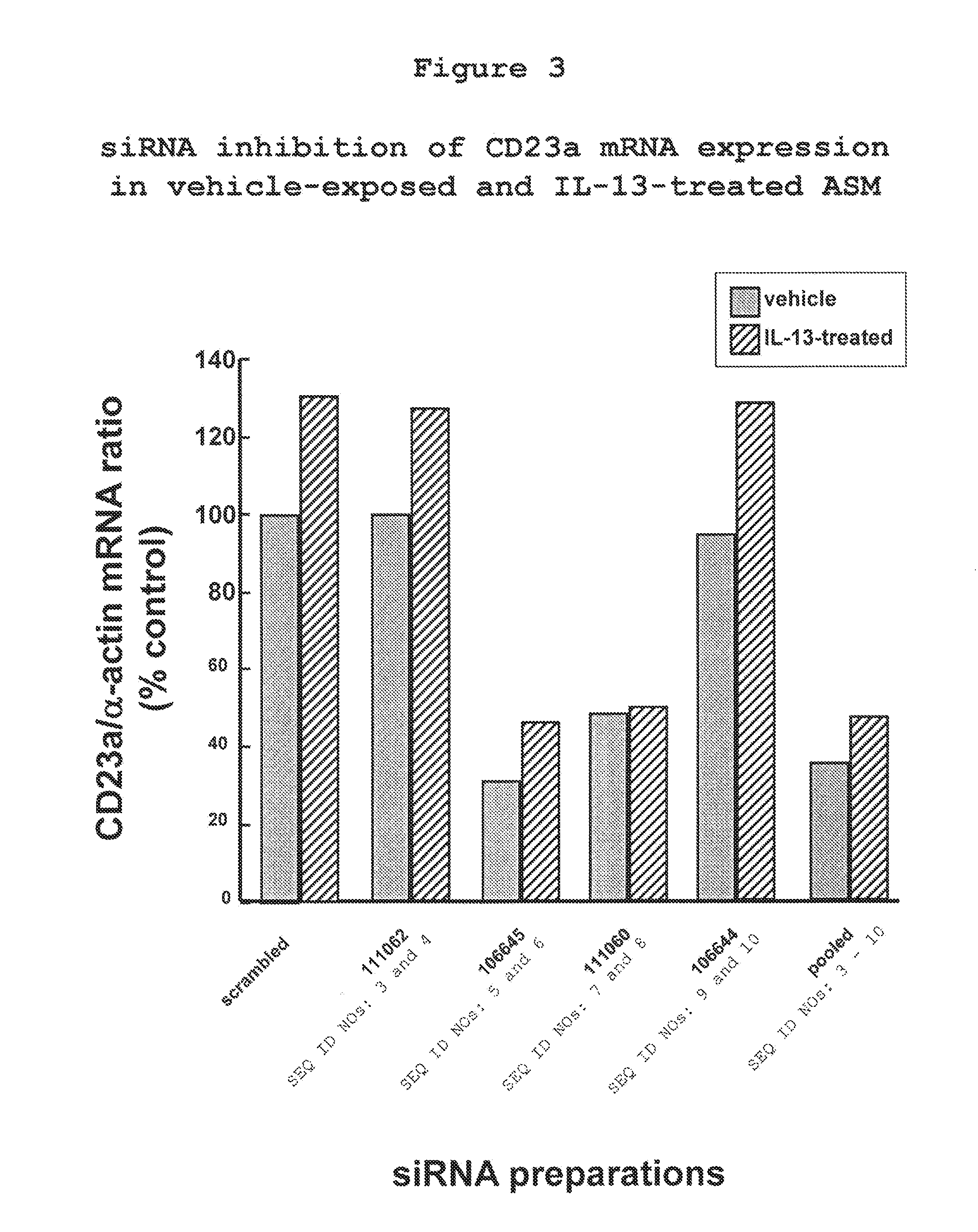
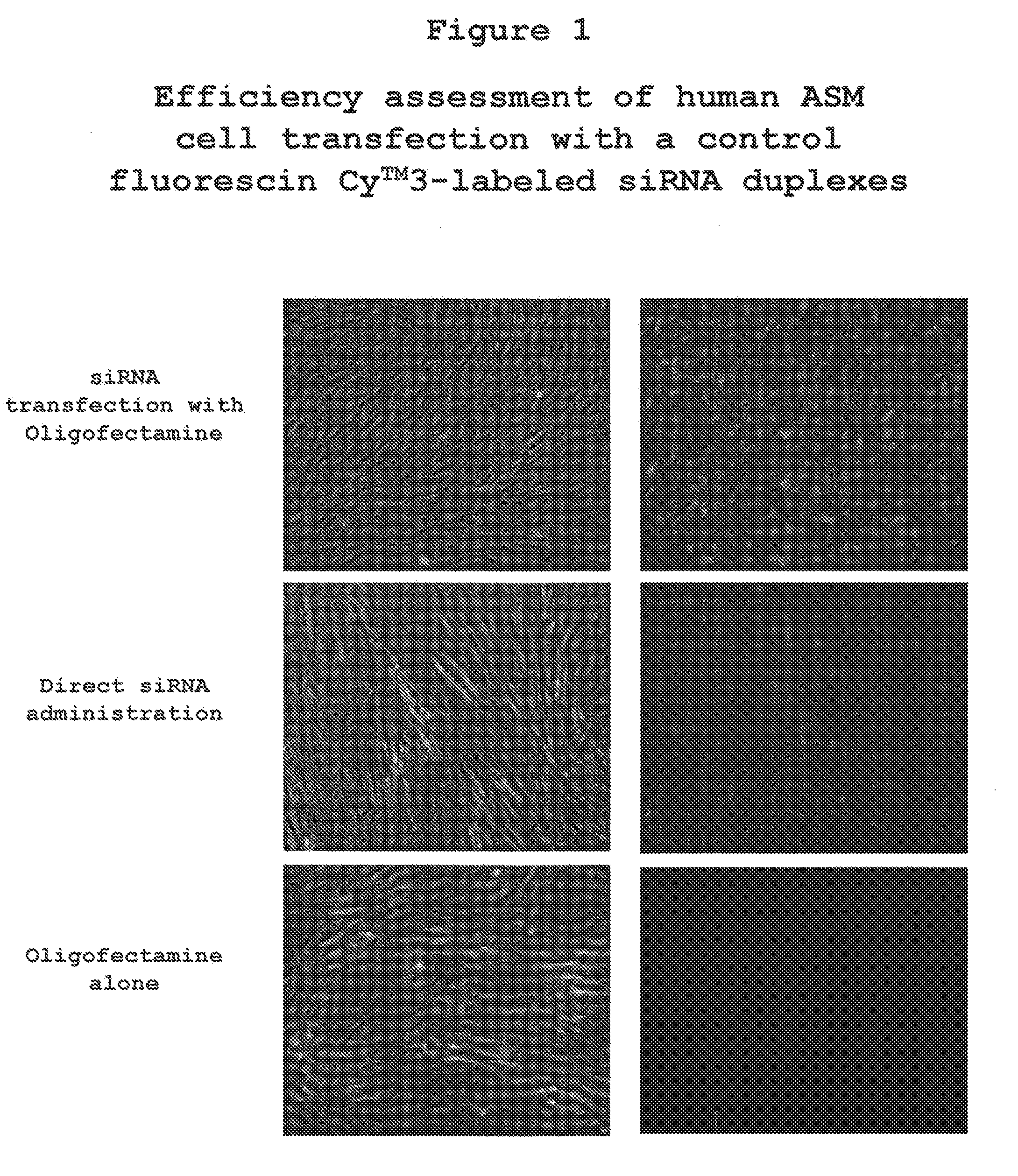
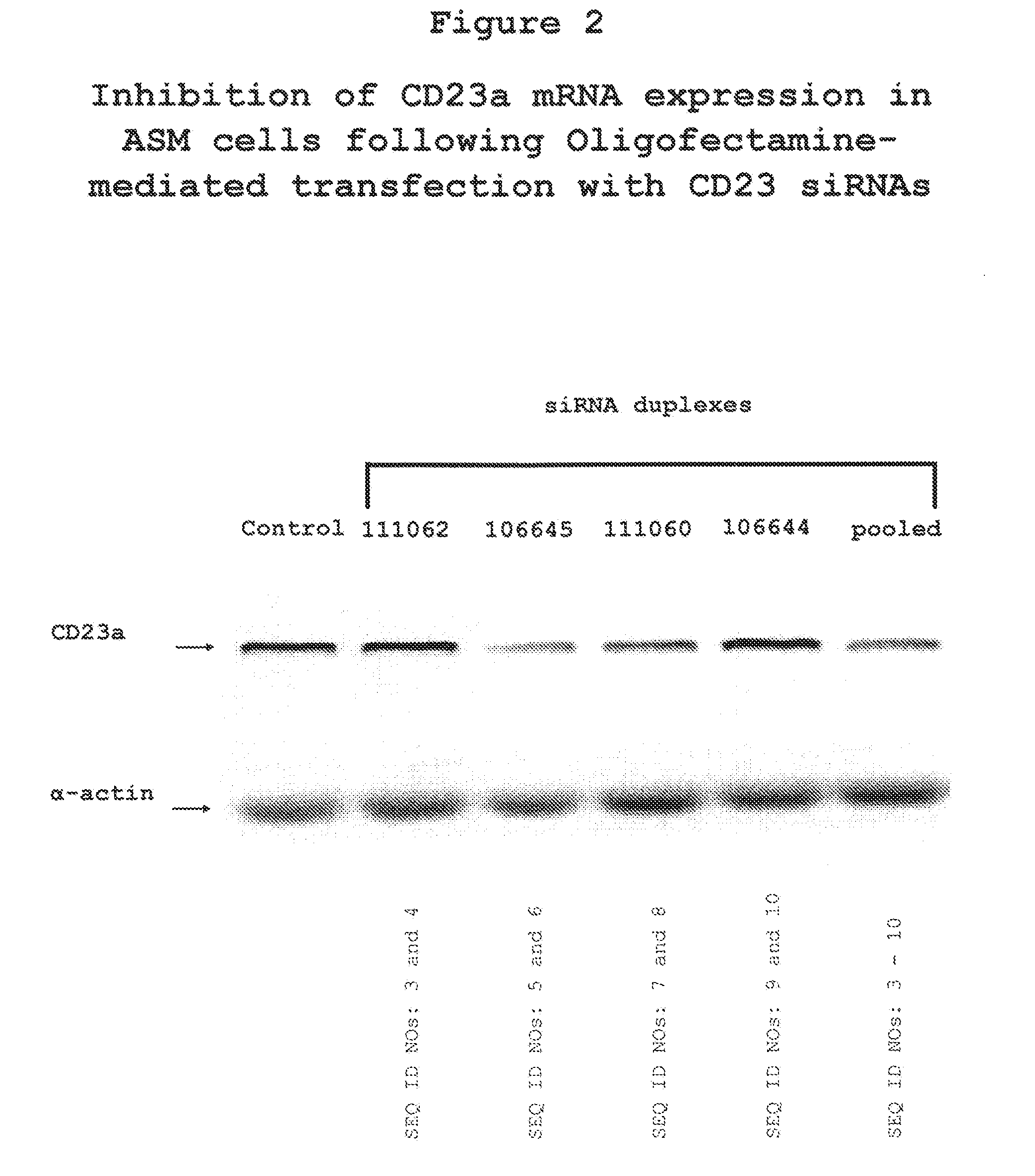
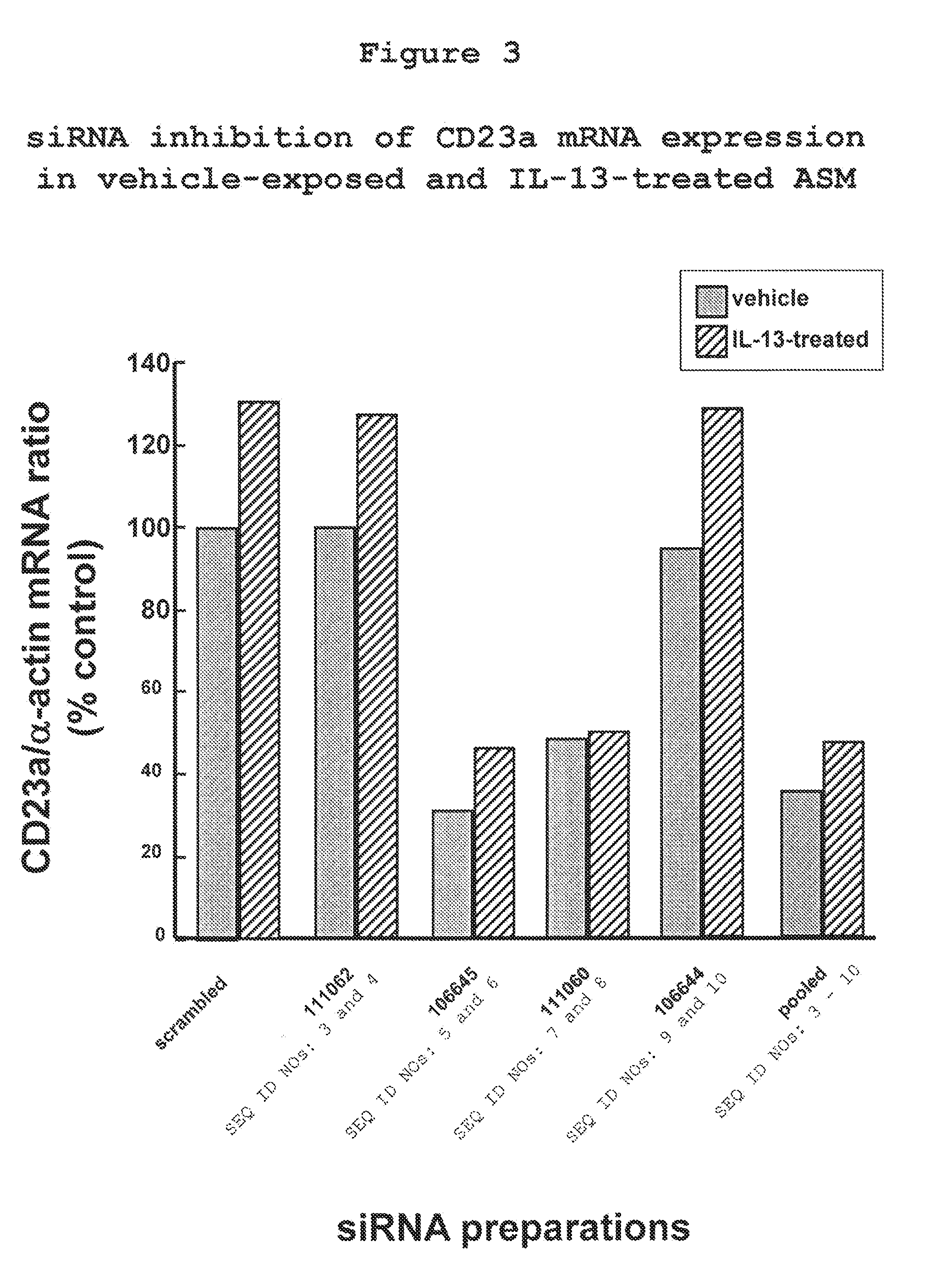
The present invention provides compositions and methods for the treatment of asthma. The compositions can be, for example, siRNA directed to CD23. The invention also provides a method of treating asthma with a formulation for in vivo delivery of a CD23 siRNA to inhibit IgE binding in a patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
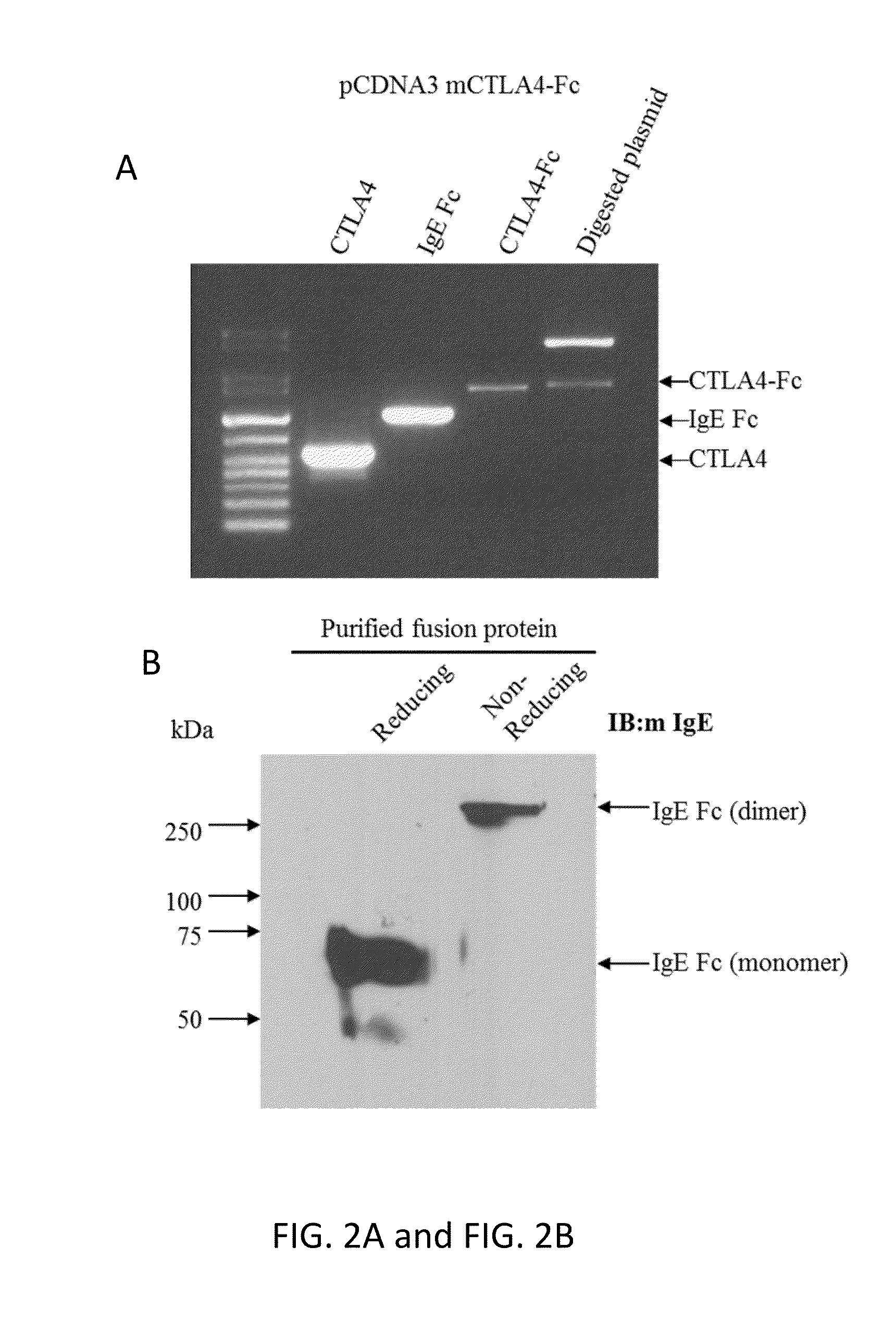
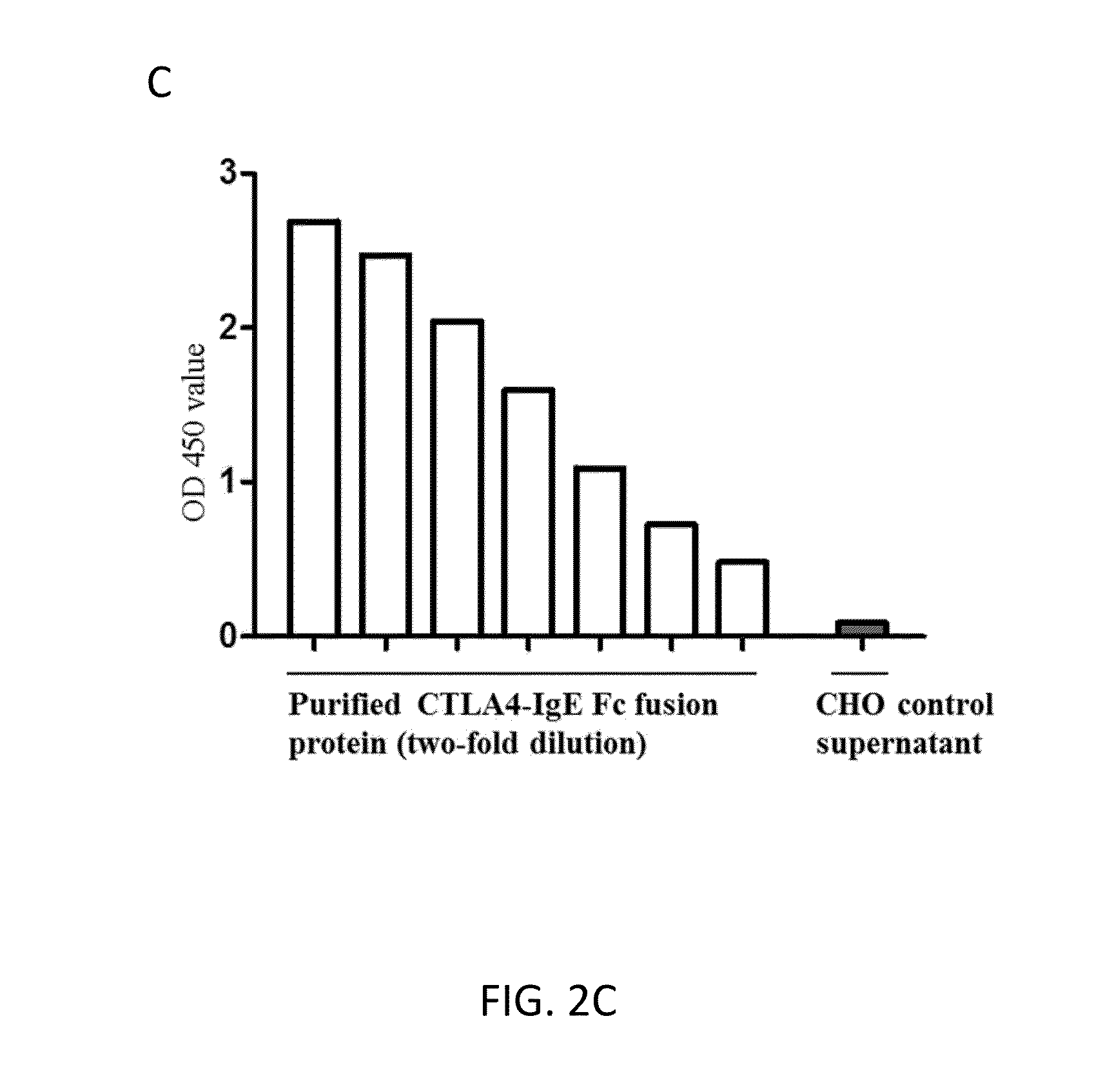
Fc Coupled Compositions and Methods of Their Use
InactiveUS20150004161A1Lower immune responseImmunoglobulin superfamilyPeptide/protein ingredientsAntigenImmunotherapeutic agent
Disclosed are compositions comprising an Fc portion of IgE coupled to an agent. For example, disclosed are compositions comprising an Fc portion of IgE coupled to an antigen or immunotherapeutic. These compositions can be used as a vaccine or an immunotherapeutic. Thus, these compositions can modulate the immune system by both increasing and decreasing the immune response. The Fc portion of IgE can bind to CD23 and transport the antigen or immunotherapeutic across airway epithelial cells. Disclosed are methods of treating airway inflammation with compositions comprising an Fc portion of IgE coupled to an antigen or immunotherapeutic.
Owner:UNIV OF MARYLAND
Compositions and Methods to Treat Asthma
The present invention provides compositions and methods for the treatment of asthma. The compositions can be, for example, siRNA directed to CD23. The invention also provides a method of treating asthma with a formulation for in vivo delivery of a CD23 siRNA to inhibit IgE binding in a patient.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Use of CD 23 antagonists for treatment of neoplastic disorders
The present invention provides methods and kits for treating neoplastic diseases, including the use of CD23 antagonists. The CD23 antagonists can be used alone or in combination with chemotherapeutic agents. In a particularly preferred embodiment, the CD23 antagonist may be used in the treatment of B-cell chronic lymphocytic leukemia (B-CLL).
Owner:IDEC PHARM CORP
Use of cd23 antagonists for the treatment of neoplastic disorders
InactiveUS20080171056A1Efficient administrationImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseB cell
Methods and kits for the treatment of neoplastic disorders comprising the use of a CD23 antagonist are provided. The CD23 antagonist may be used alone or in combination with chemotherapeutic agents. In particularly preferred embodiments the CD23 antagonists may be used to treat B cell chronic lymphocytic leukemia (B-CLL).
Owner:BIOGEN MA INC
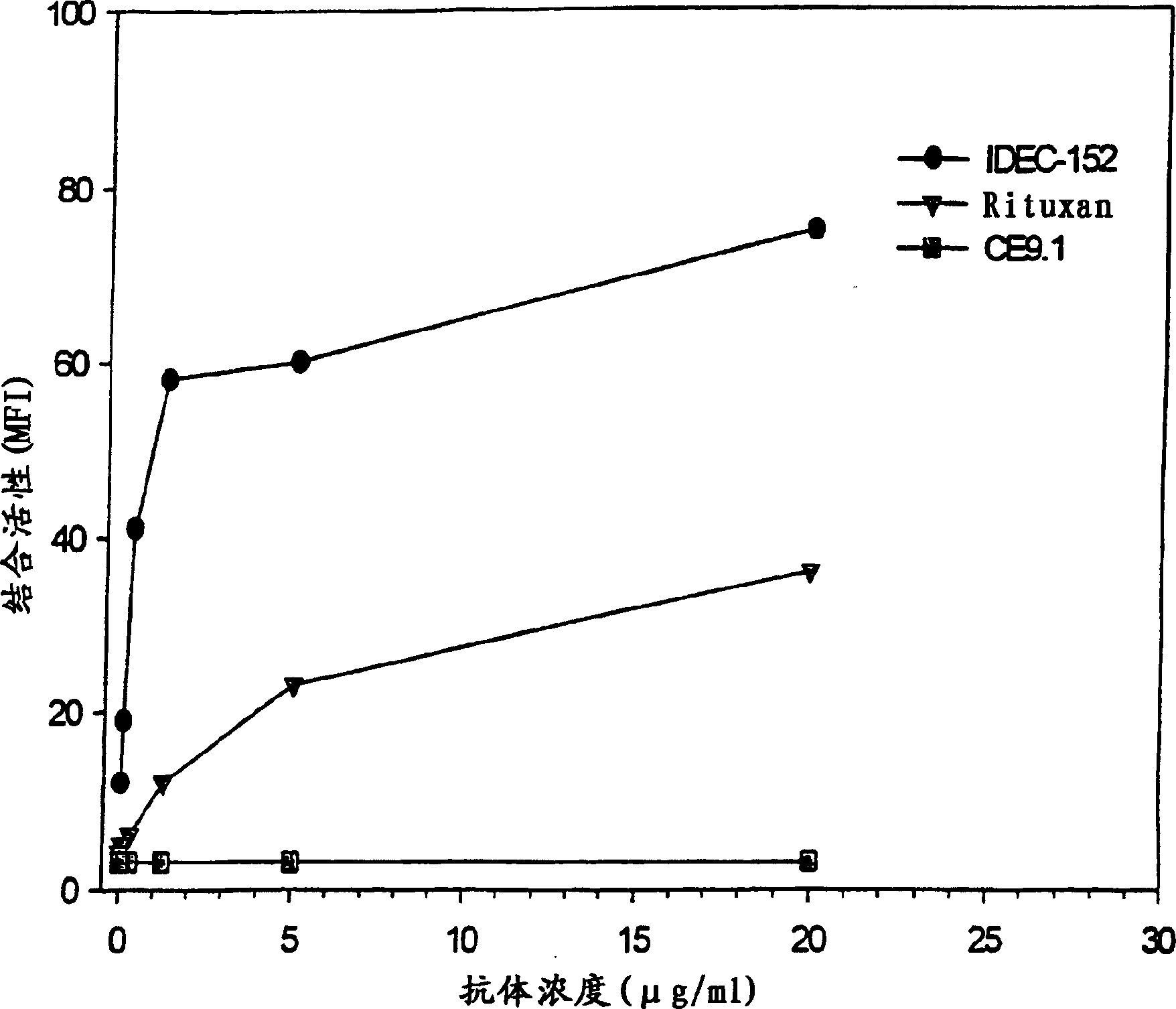
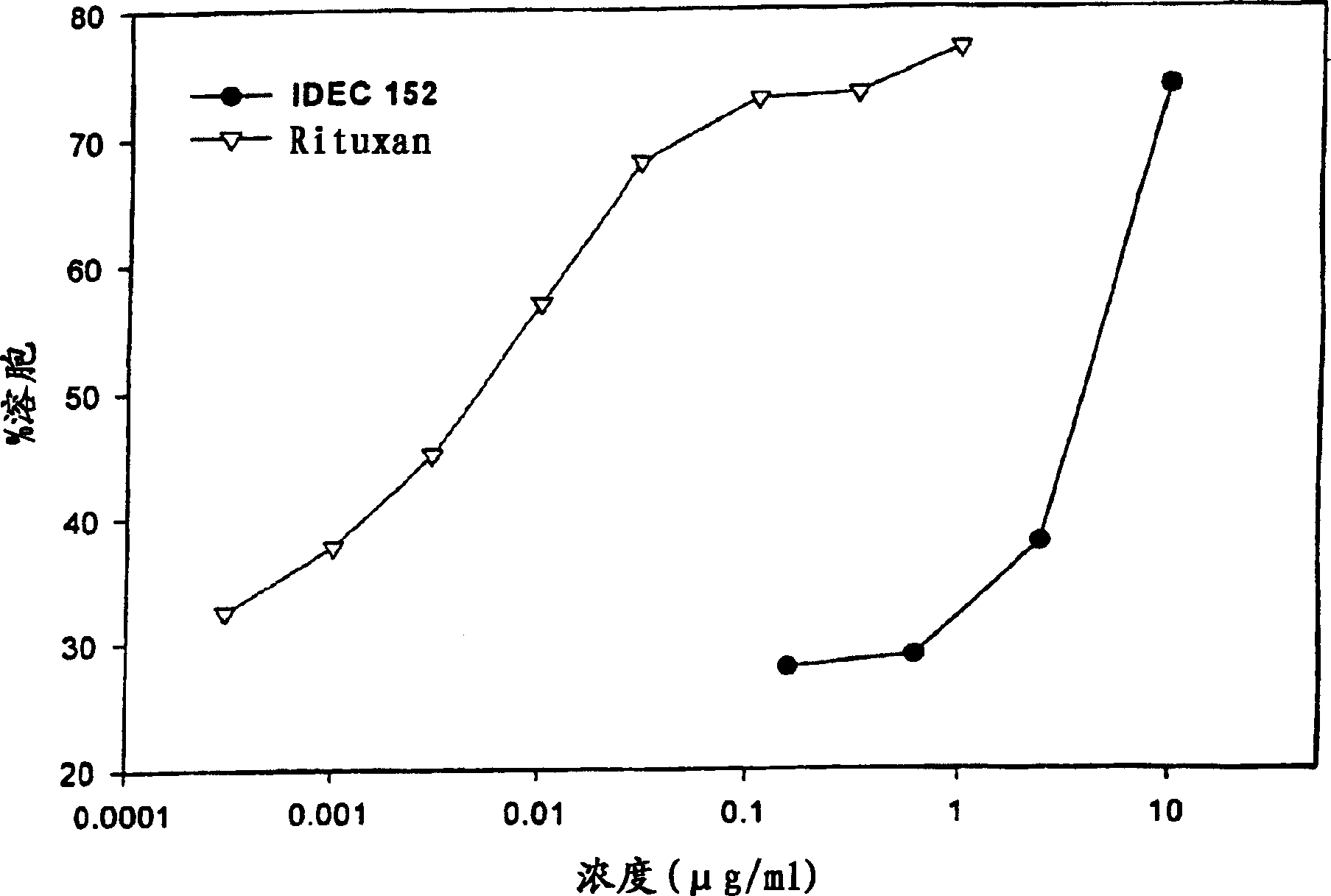
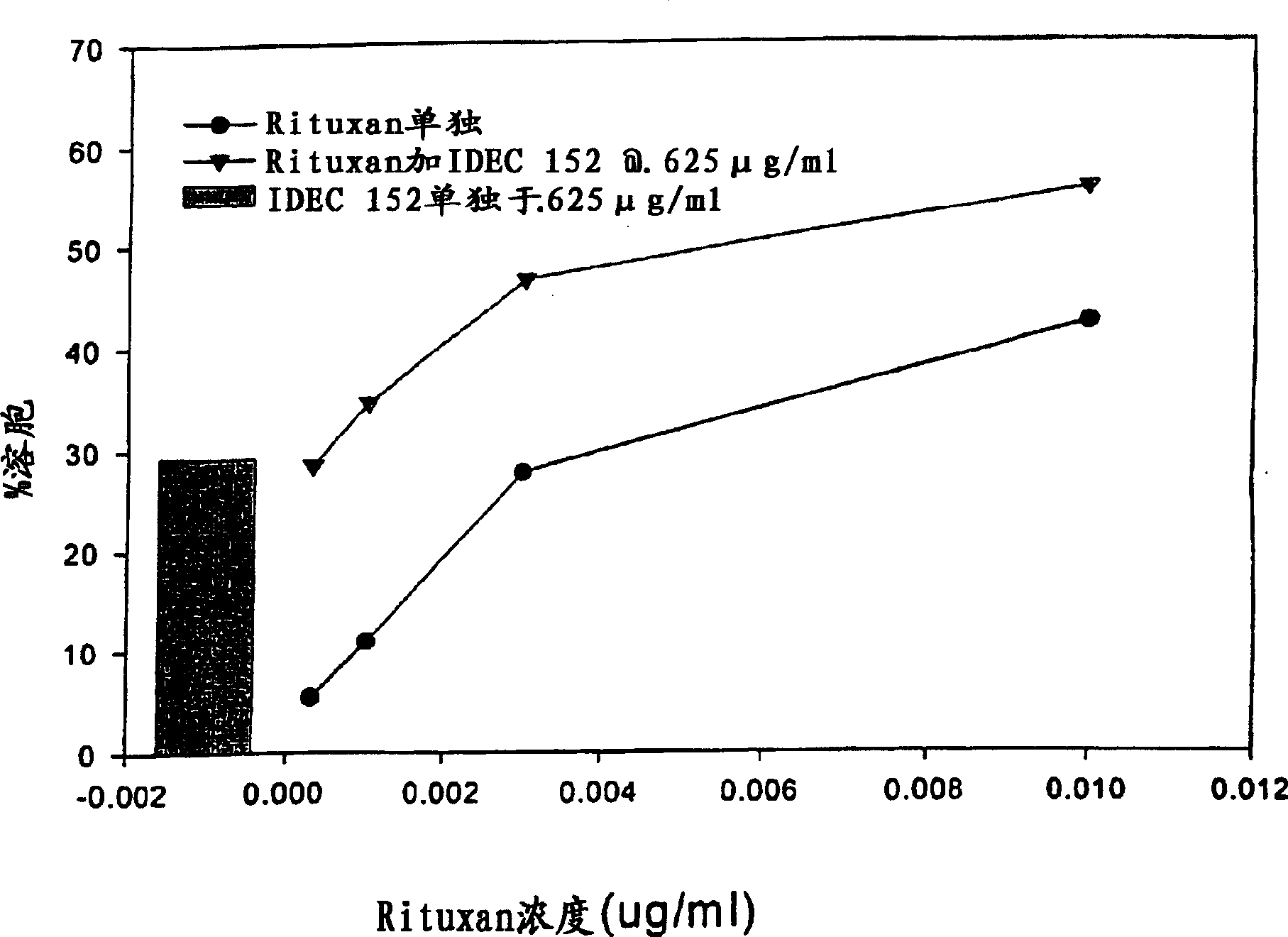
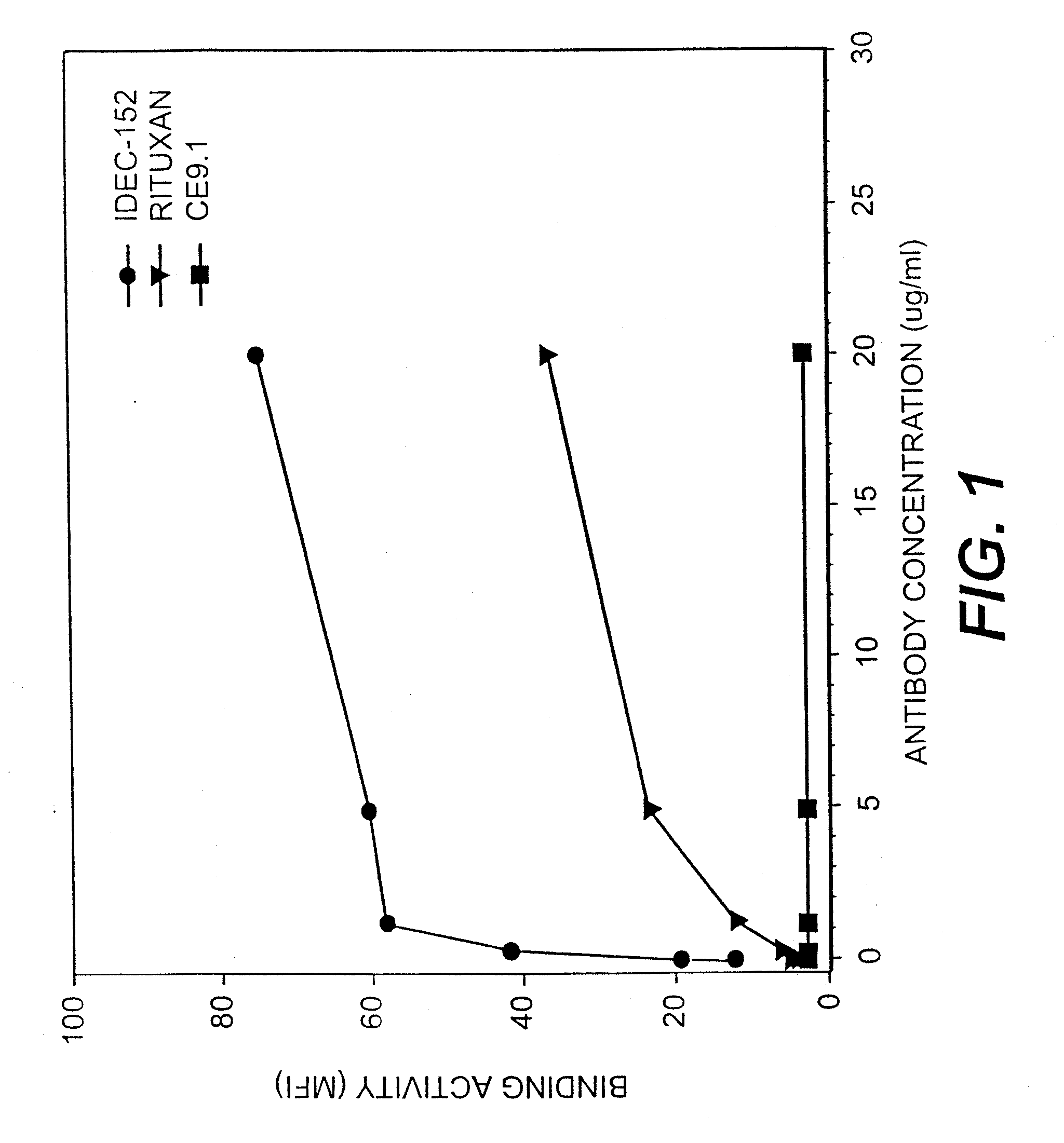
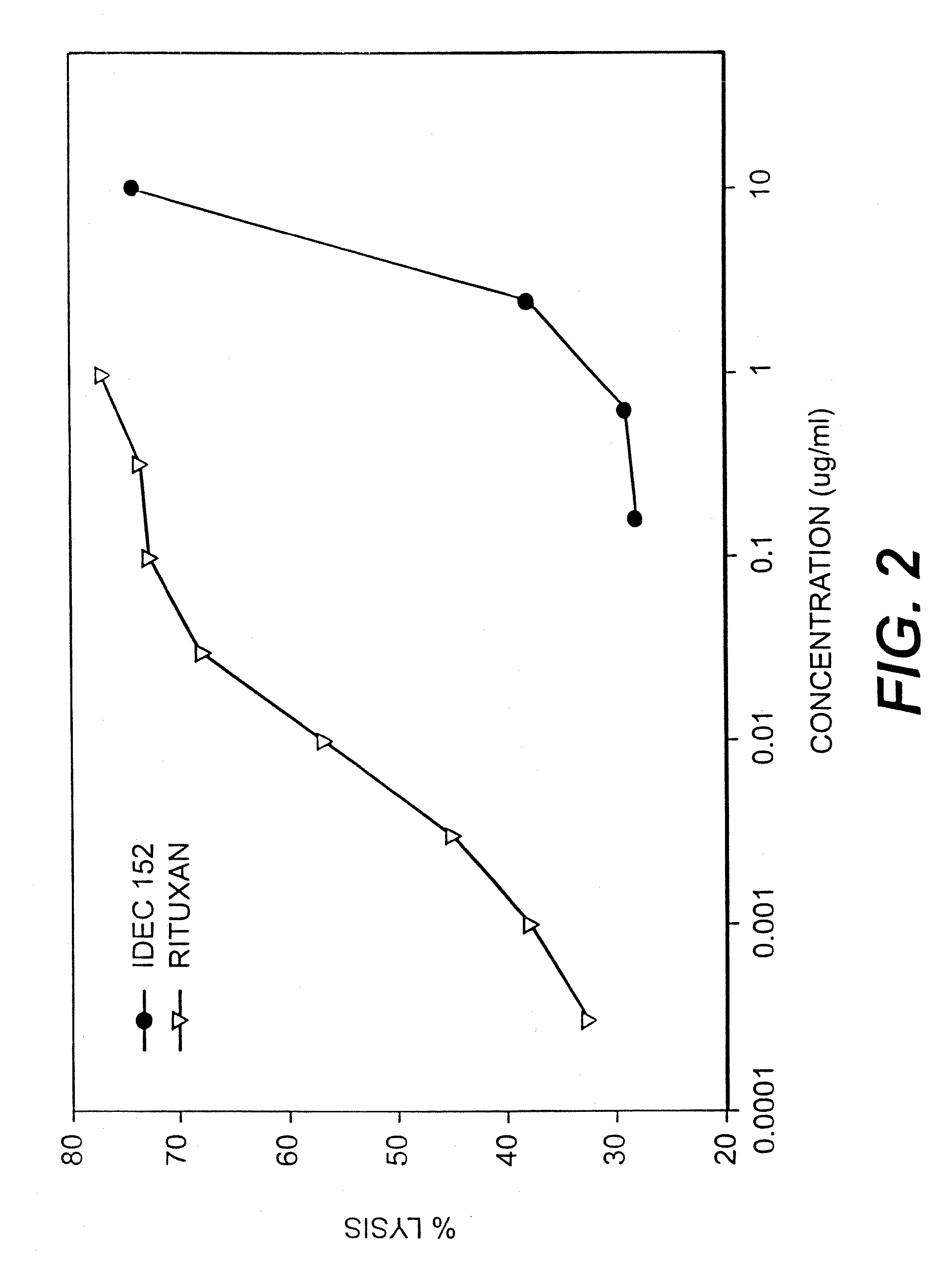
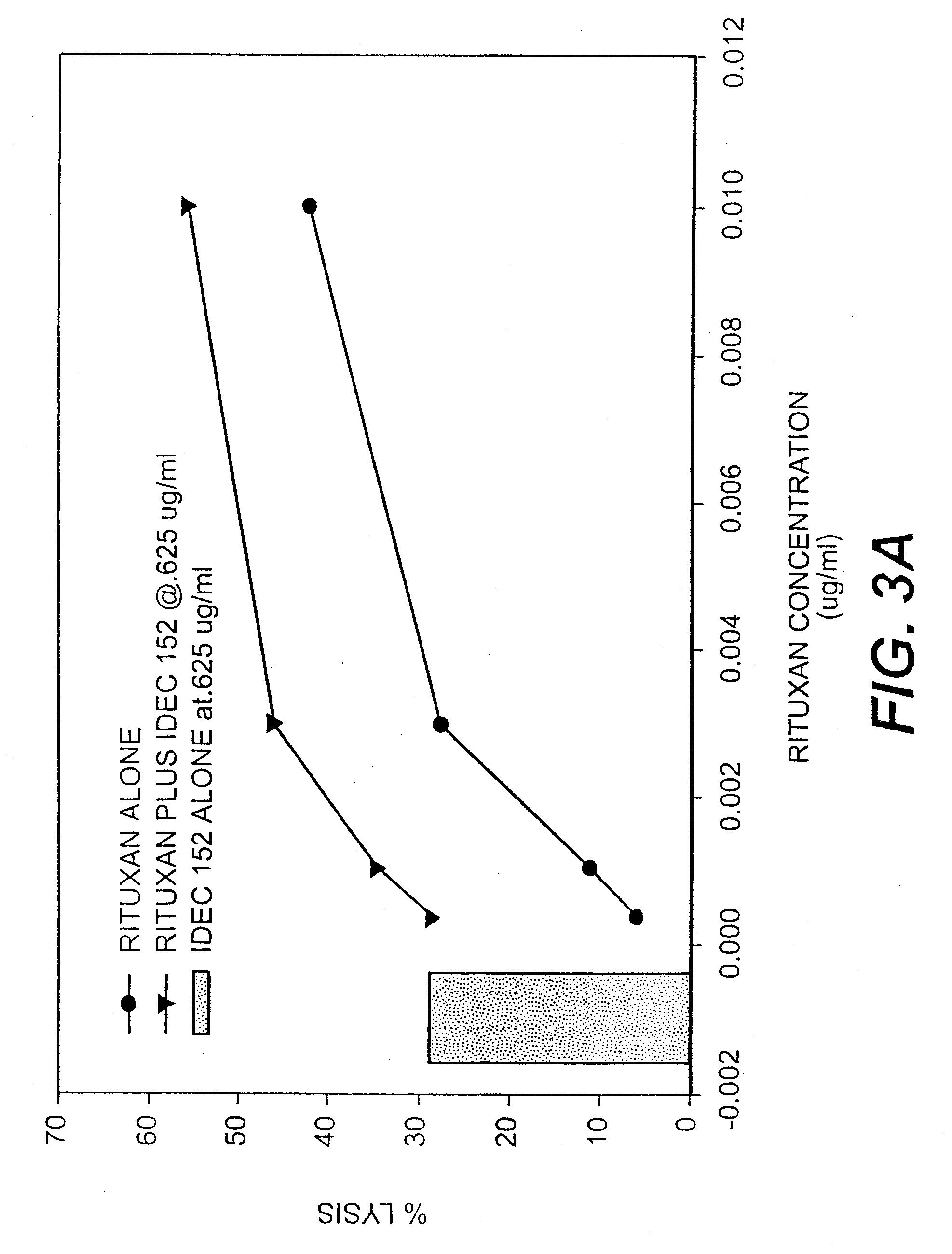
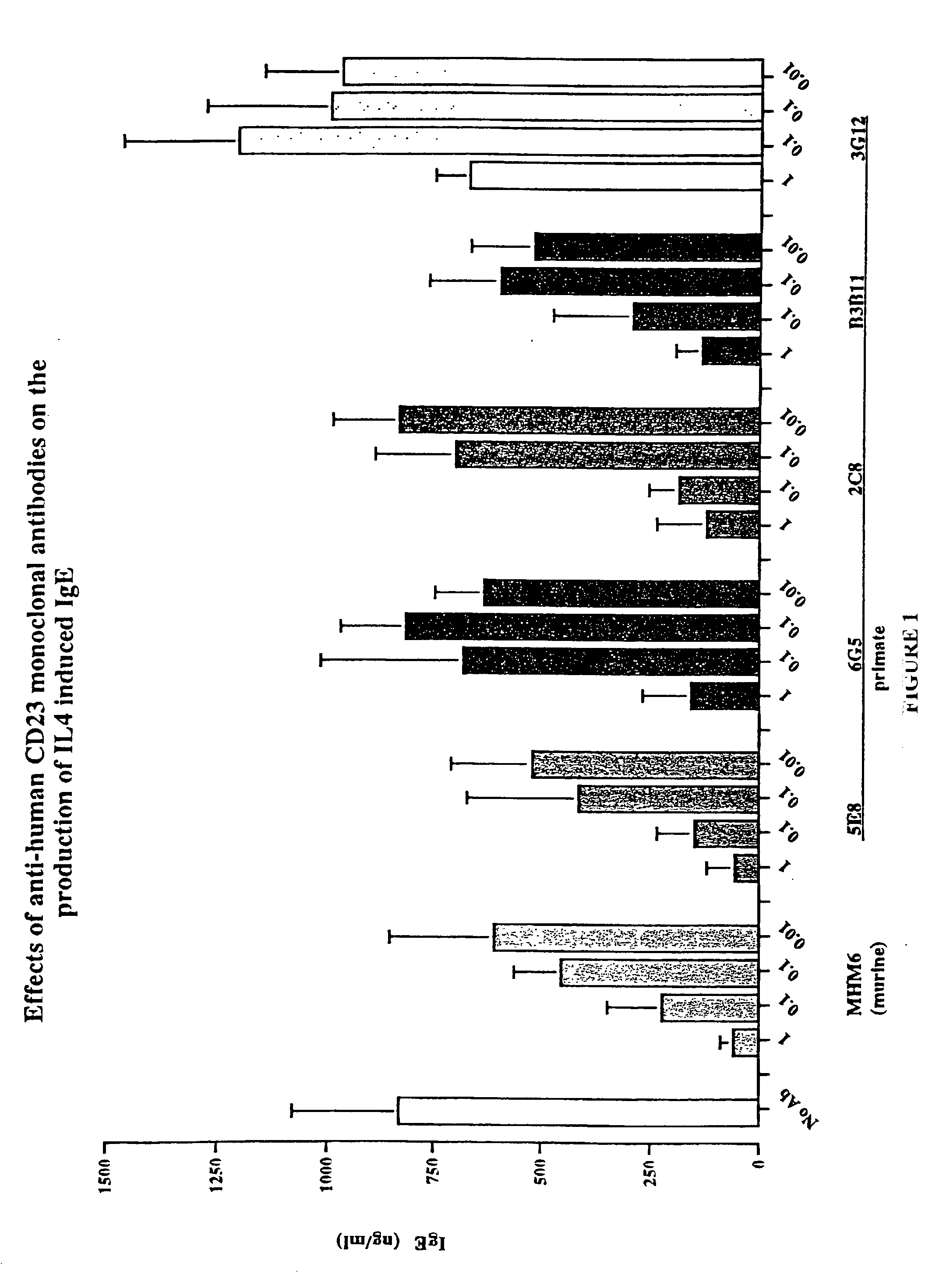
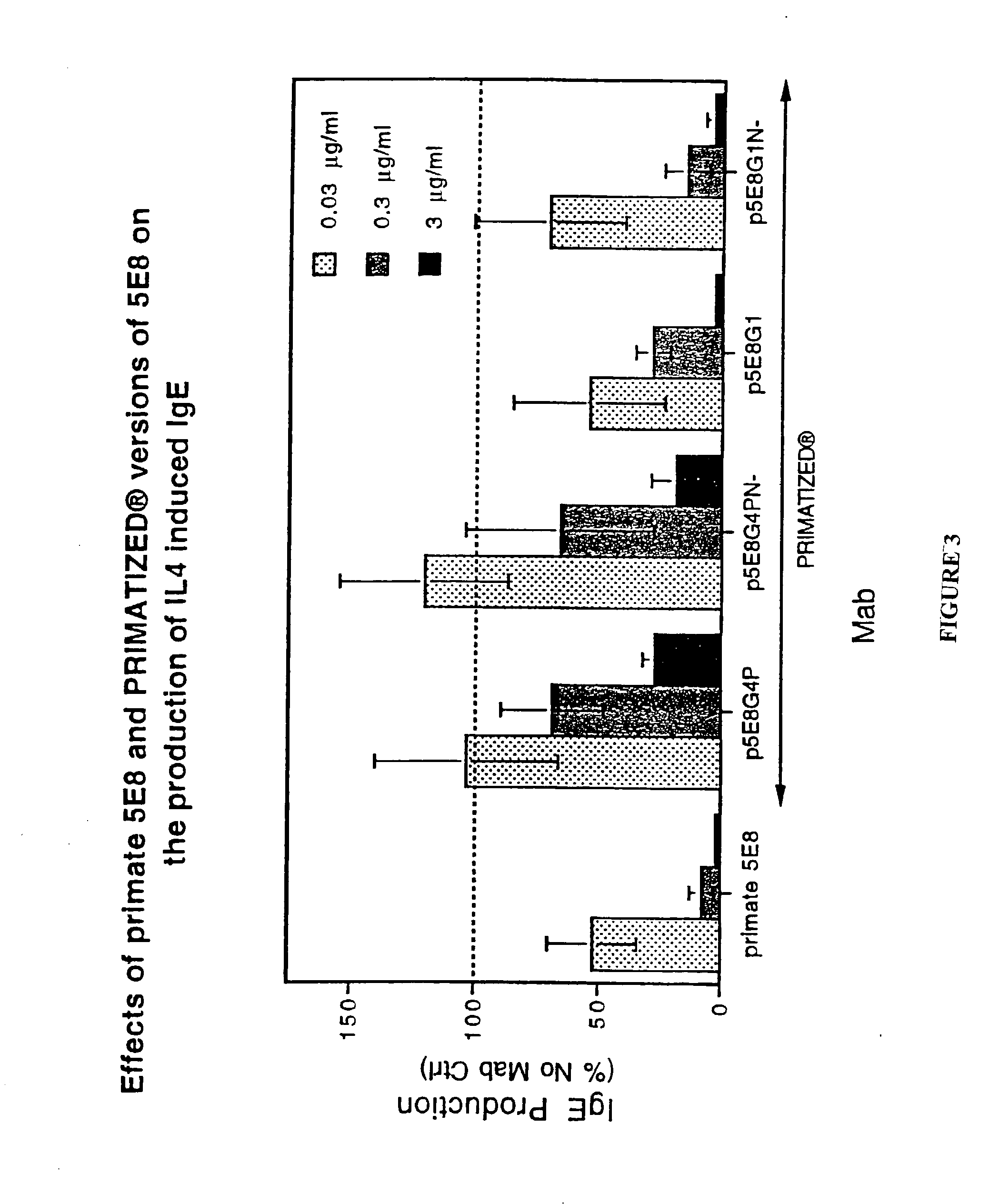
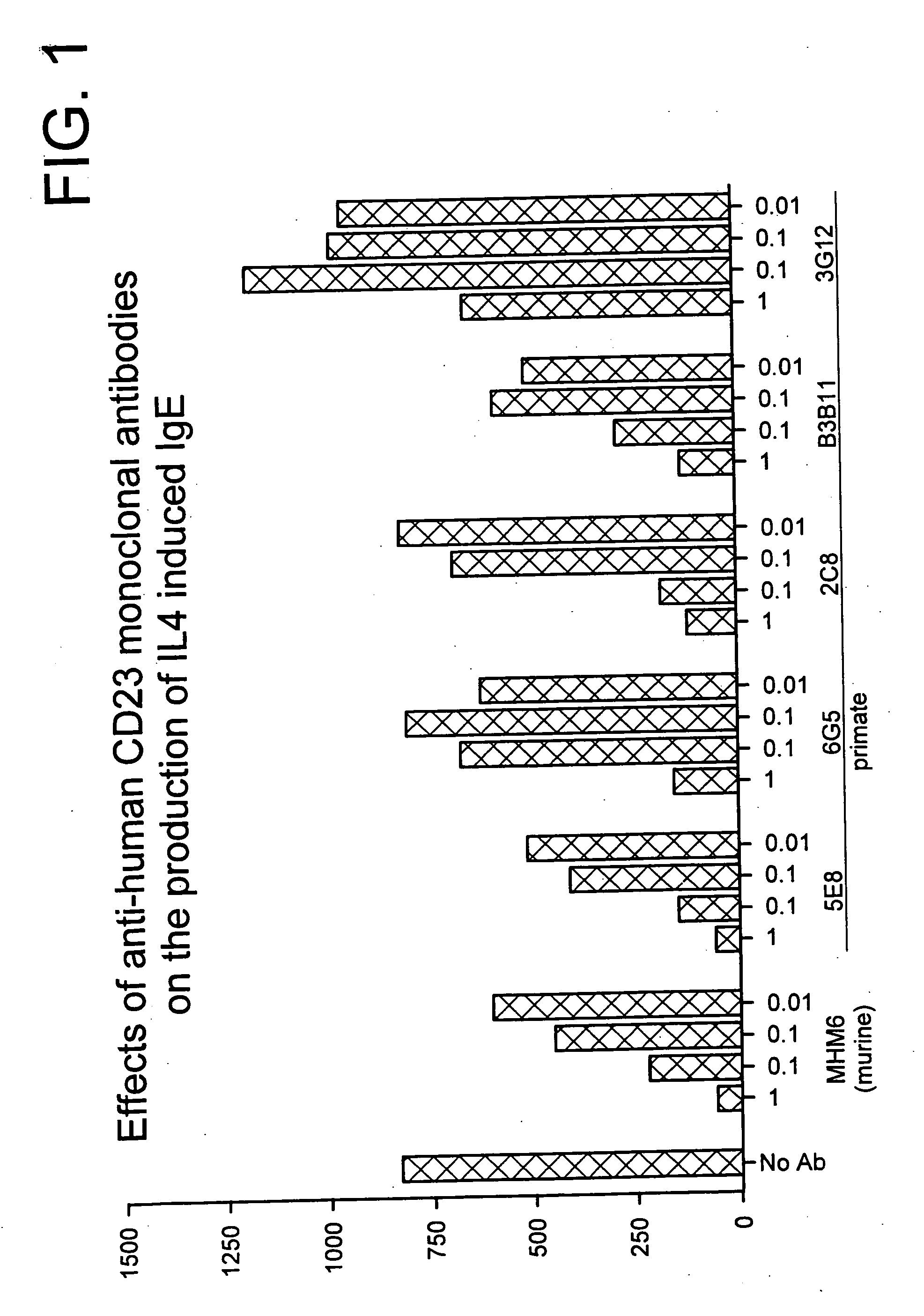
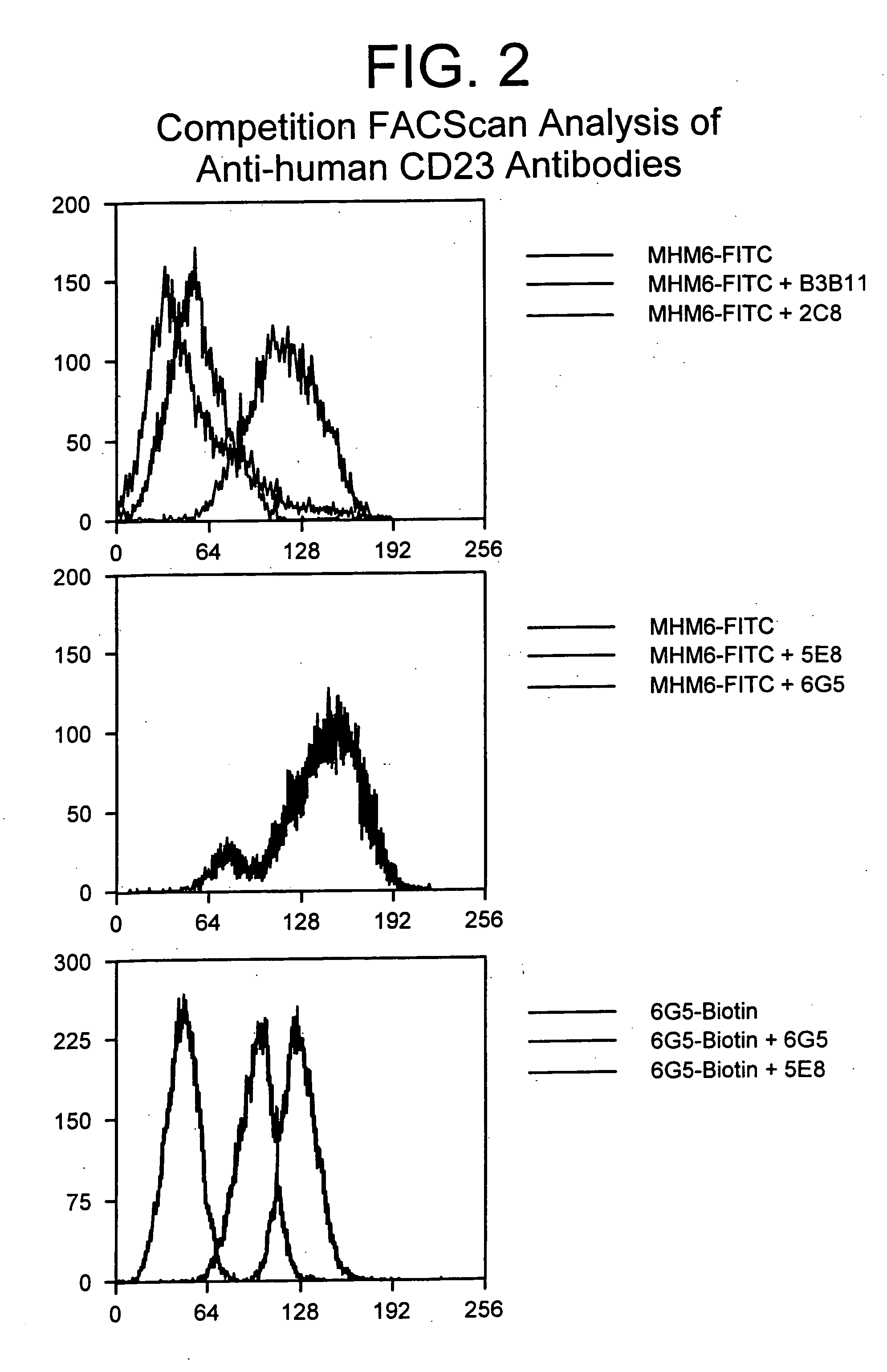
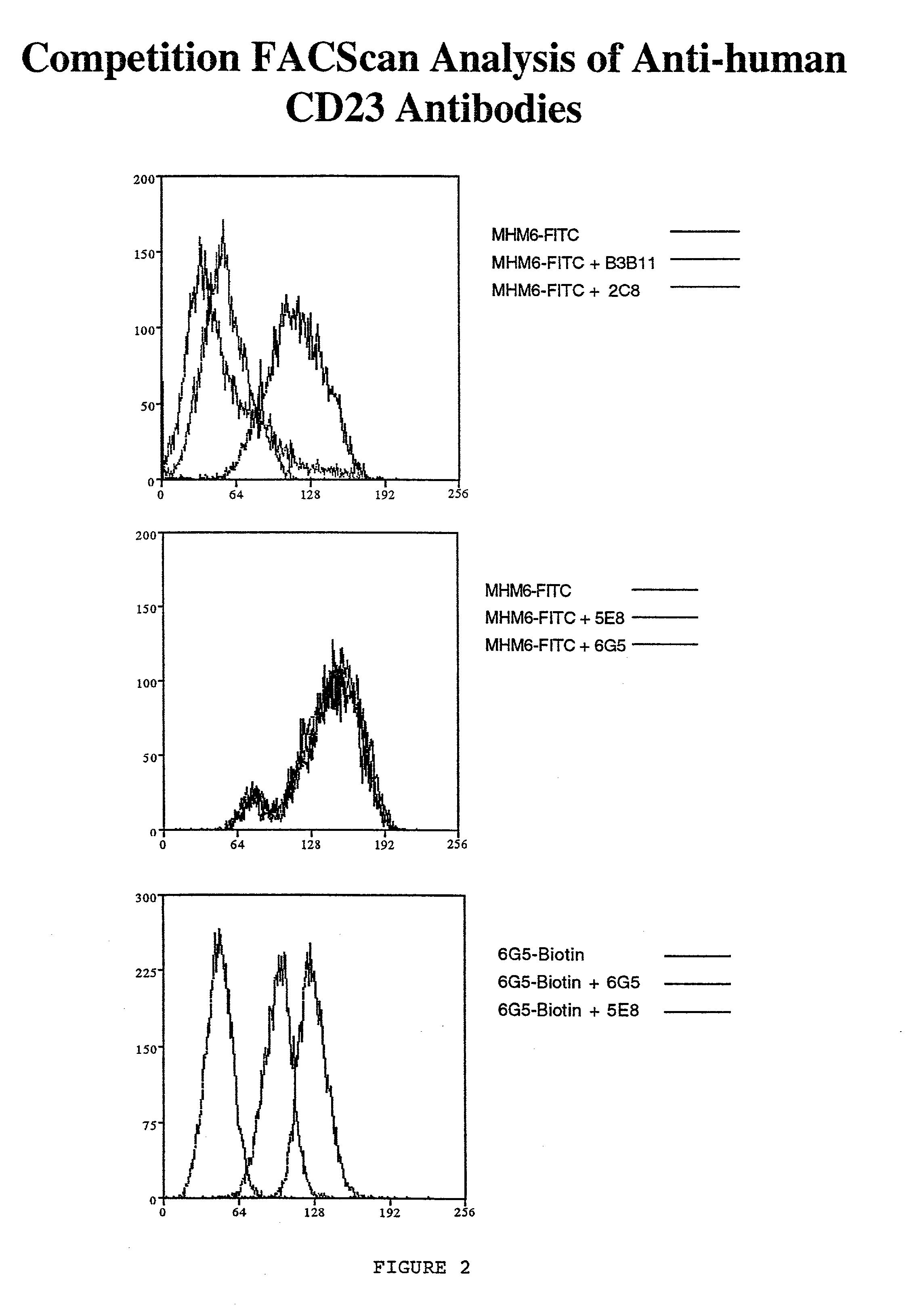
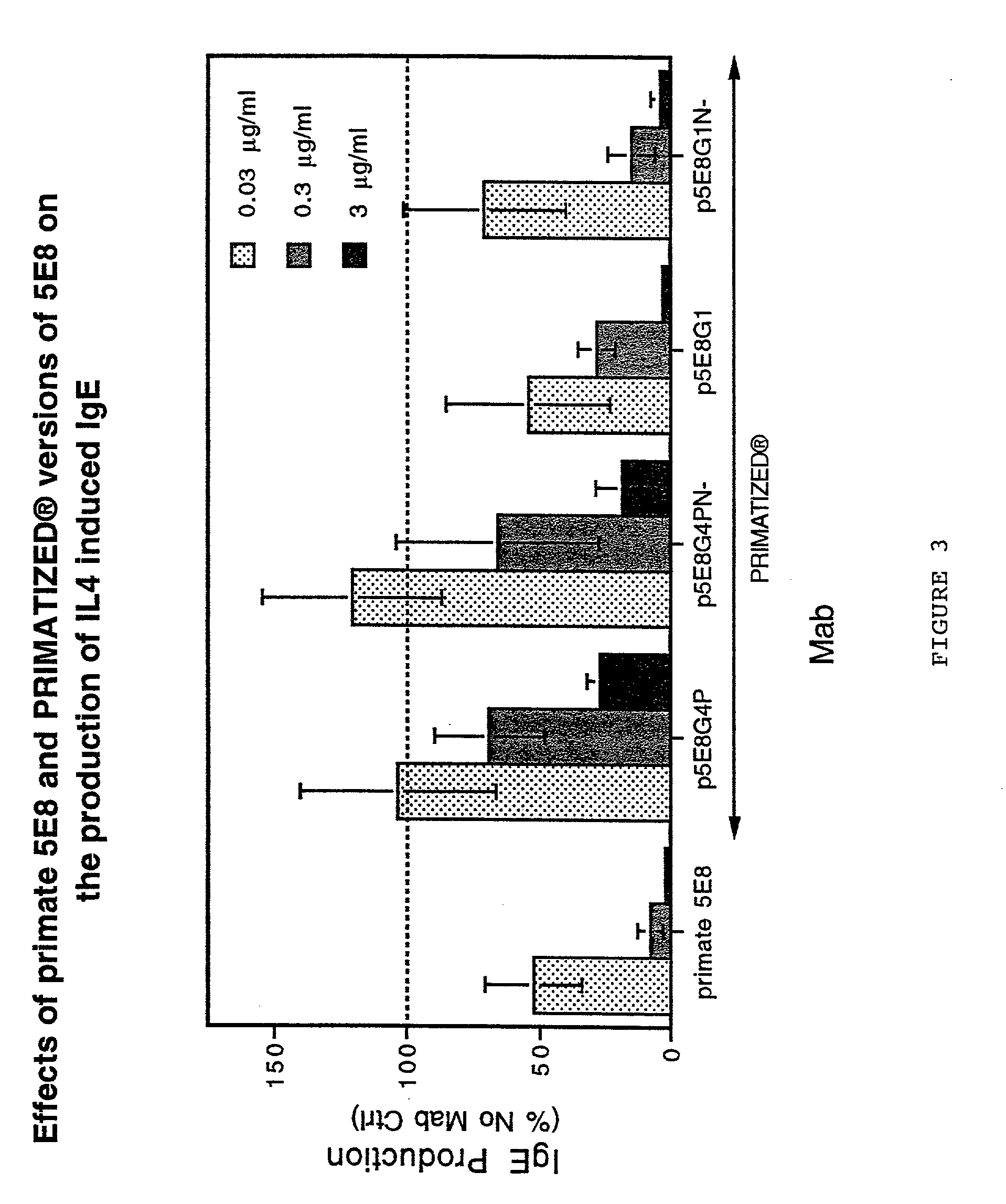
Gamma-1 and gamma-3 anti-human CD23 monoclonal antibodies and use thereof as therapeutics
InactiveUS20030059424A1Improve abilitiesEnhanced potential for cross linkingSugar derivativesPeptide/protein ingredientsDiseaseLow affinity
Monoclonal antibodies which specifically bind human CD23, the low affinity receptor for IgE (FceRII / CD23), and contain either a human gamma-1 or human gamma-3 constant domain, are disclosed. The antibodies are useful for modulating or inhibiting induced IgE expression. Accordingly, they have practical utility in the treatment or prophylaxis of disease conditions wherein inhibition of induced IgE production is therapeutically desirable, including allergic conditions, autoimmune diseases and inflammatory diseases.
Owner:BIOGEN MA INC
Agents and methods
InactiveCN104812413AVirus peptidesImmunoglobulins against cell receptors/antigens/surface-determinantsCD43CD44
The invention provides an agent comprising: (i) a T cell antigen, and (ii) a binding partner for any of CD22, CD23, CD30, CD74, CD70, CD43, CD44, CD47, CD54, CD58, CD62L, CD95, HLA-DR, CD59, CD55, wherein, following binding of the agent to a cell that expresses any of CD22, CD23, CD30, CD74, CD70, CD43, CD44, CD47, CD54, CD58, CD62L, CD95, HLA-DR, CD59, CD55, the agent is internalised and the T cell antigen is presented on the surface of the cell in a form that can be recognised by a T cell.
Owner:THE UNIV OF BIRMINGHAM
Bispecific chimeric antigen receptors and their application in the treatment of tumor
PendingUS20210000870A1Improve targetingAvoid it happening againPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCD20CD79A
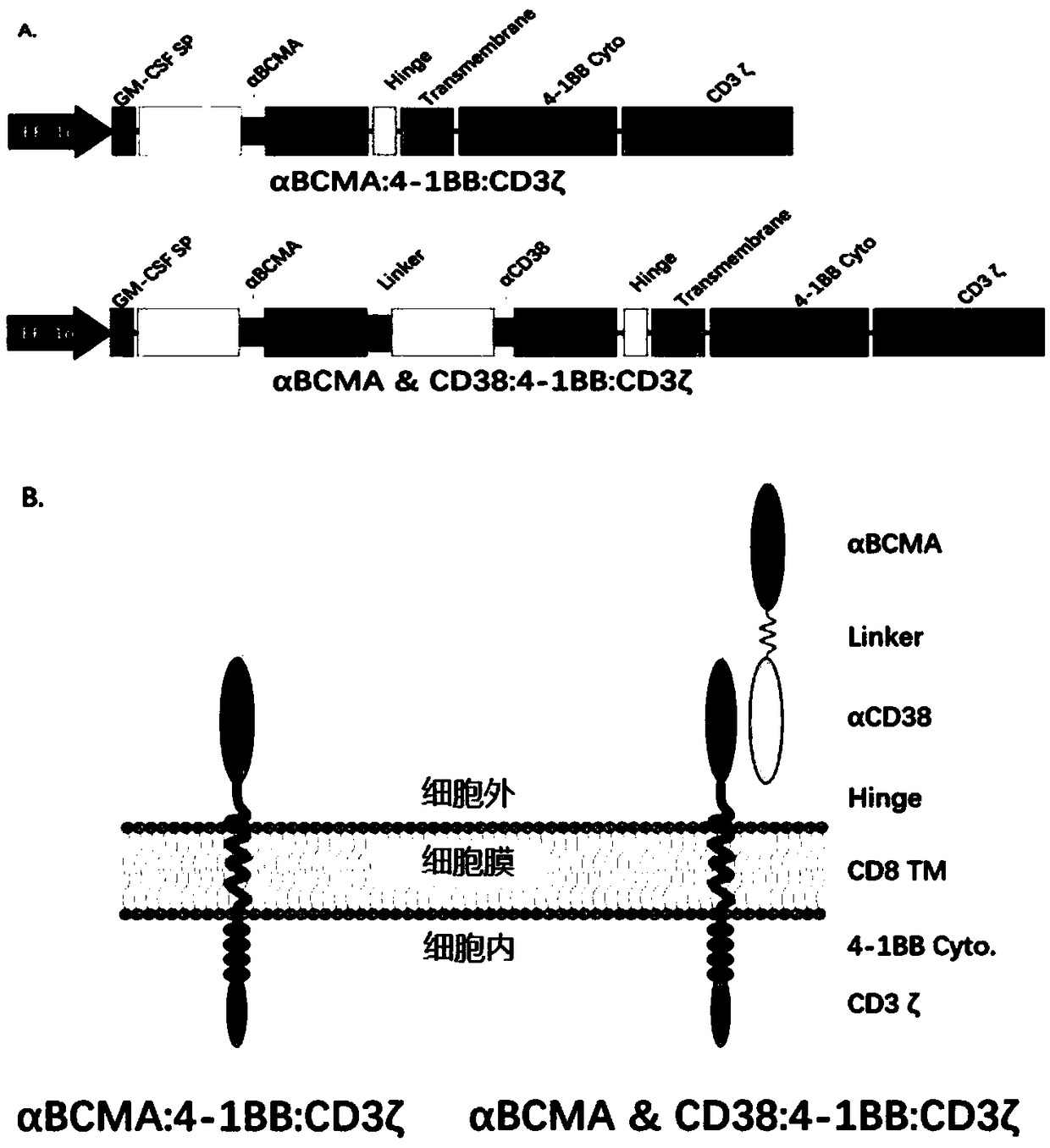
The embodiments of the present invention provide a bispecific chimeric antigen receptor, consisting of a signal peptide, two specific antigen-binding fragments, an extracellular spacer region, a transmembrane region, an intracellular co-stimulatory signaling domain, the first antigen that is recognized and bound by the specific antigen-binding fragments is a member selected from the group consisting of CD19, CD20, CD22, CD33, CD269, CD138, CD79a, CD79b, CD23, ROR1, CD30, B cell surface antibody light chain, CD44, CD123, Lewis Y, CD7 and CD46; the second antigen that is recognized and bound by the specific antigen-binding fragments is CD38, the two specific antigen-binding fragments is linked by a linker peptide, the bispecific chimeric antigen receptor can recognize respectively two kinds of tumor-associated antigens by constructing low affinity chimeric antigen receptors and high affinity chimeric antigen receptors and have very strong specificity. In addition, the embodiments of the present invention also provide a use of the bispecific chimeric antigen receptor in the treatment of tumors.
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
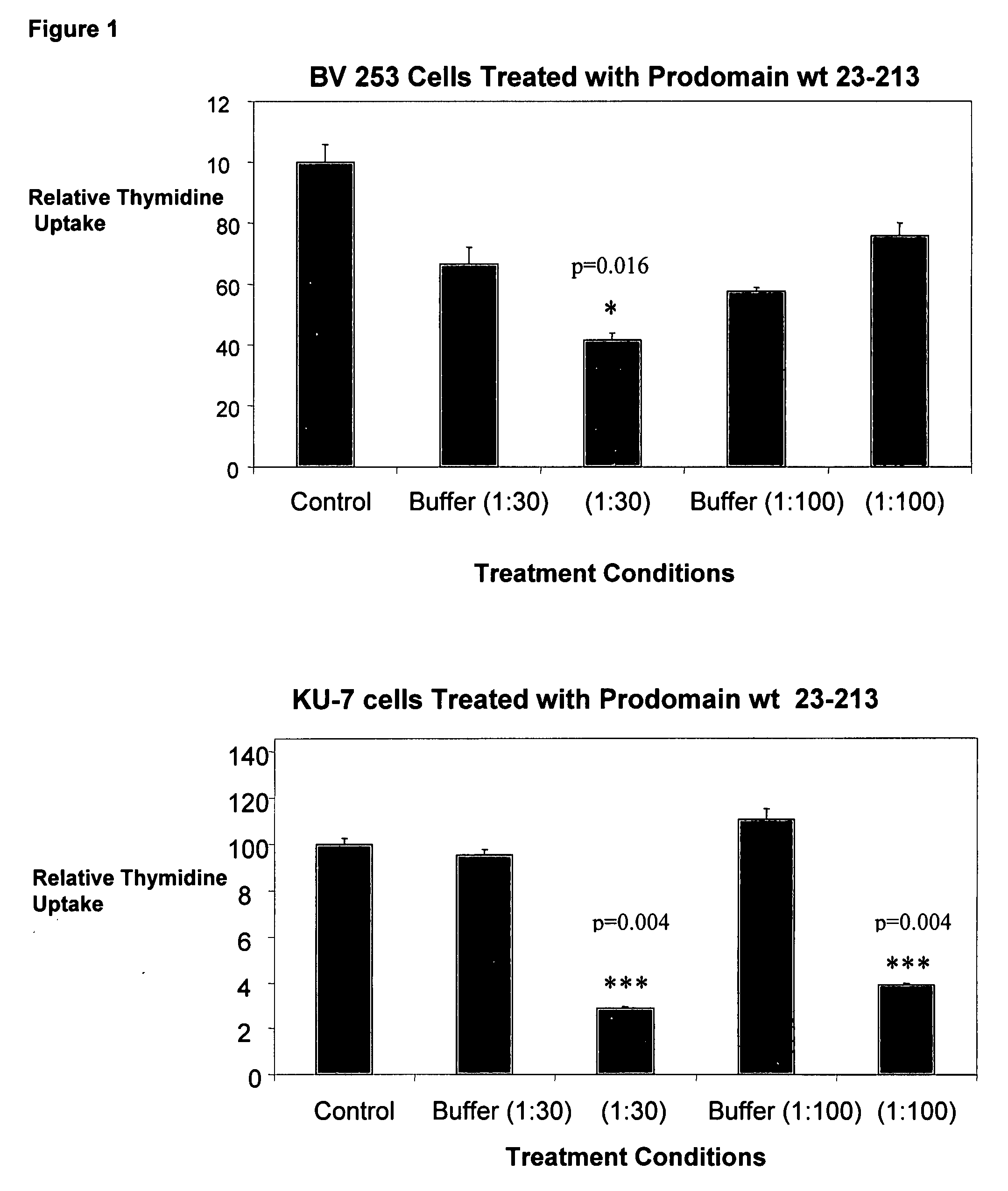
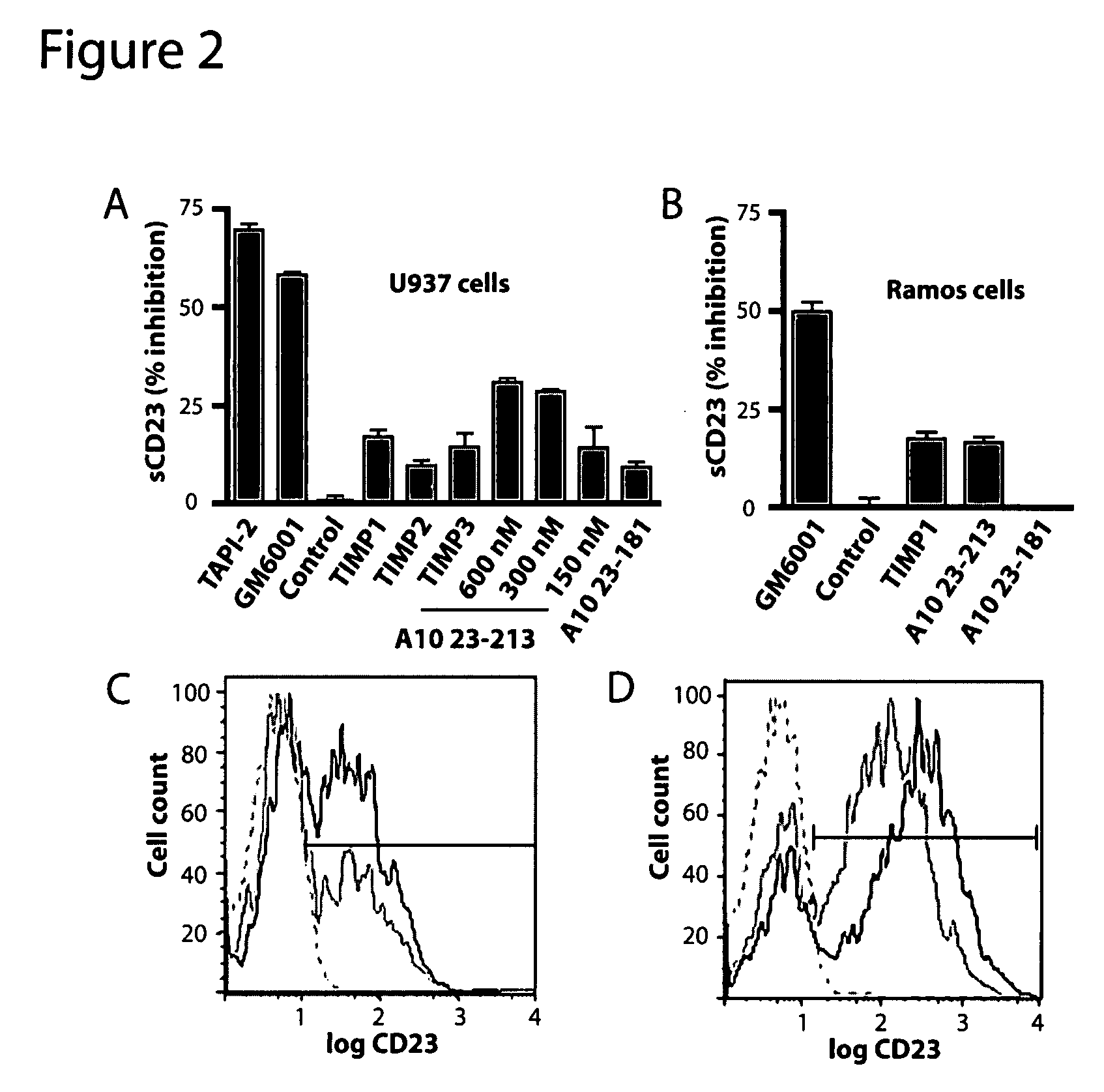
Prodomain modulators of ADAM 10
The presently disclosed subject matter discloses isolated ADAM 10 modulating peptides and related compounds useful for studying the biological functions of ADAM 10 and for the treatment of diseases such as cancer, neurological disorders, asthma, and allergic responses, and disorders characterized at least in part by the presence of one or more of inflammation, excess cell proliferation, angiogenesis, and excess soluble CD23. In one aspect, the presently disclosed subject matter provides isolated mouse and human ADAM 10 prodomain comprising the sequence set forth in SEQ ID NOs 1-8, or a sequence having at least 95% homology to any of SEQ ID NOs 1-8 and having the functionality of modulating ADAM 10 activity.
Owner:BIOZYME +1
Gamma-1 and gamma-3 anti-human CD23 monoclonal antibodies and use thereof as therapeutics
InactiveUS20050118175A1Enhanced potential for cross linkingImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsAnti-CEA AntibodyCD23
Owner:BIOGEN IDEC MA INC
Prodomain modulators of ADAM 10
The presently disclosed subject matter discloses isolated ADAM 10 modulating peptides and related compounds useful for studying the biological functions of ADAM 10 and for the treatment of diseases such as cancer, neurological disorders, asthma, and allergic responses, and disorders characterized at least in part by the presence of one or more of inflammation, excess cell proliferation, angiogenesis, and excess soluble CD23. In one aspect, the presently disclosed subject matter provides isolated mouse and human ADAM 10 prodomain comprising the sequence set forth in SEQ ID NOs 1-8, or a sequence having at least 95% homology to any of SEQ ID NOs 1-8 and having the functionality of modulating ADAM 10 activity.
Owner:BIOZYME +1
Combination therapy for treatment of autoimmune diseases using B cell depleting/immunoregulatory anti-body combination
The present invention relates to the treatment of autoimmune diseases with a combination of an immunomodulatory antibody (such as an anti-B7.1 or anti-B7.2 or anti-CD40L antibody) and at least one B-cell antibody (such as CD19, CD20, CD22, CD23 or CD37), wherein These antibodies may be administered alone or in combination and in either order for extended periods of time.
Owner:BIOGEN INC
Method for measuring FceRII/CD23 IgE receptor in human feces as an indicator of a TH2 predominant immune response involved in gastrointestinal illnesses
InactiveUS20050084910A1Immunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisUlcerative colitisEnzyme linked immunoassay
A method and apparatus for measuring FcεRII / CD23 IgE receptor in human feces or other biological specimens as an indicator of a TH2 immune response indicative of gastrointestinal illnesses such as ulcerative colitis and food allergy is described. The apparatus consists of either a qualitative or quantitative enzyme-linked immunoassay or other immunoassay that utilizes antibodies specific to human FcεRII / CD23 IgE receptor for the measurement of endogenous FcεRII / CD23 IgE receptor in human feces. The method and apparatus can be used by healthcare providers as an aid for determining gastrointestinal illnesses that are predominantly a TH2 predominant immune response such as ulcerative colitis, large bowel Crohn's disease (IC-like Crohn's disease), allergic colitis (food allergy) and parasitic infections.
Owner:TECH LAB
Humanized anti-IgE antibodies that crosslink CD23 on B lymphocytes but do not sensitize mast cells
ActiveUS10047166B2Relieve symptomsEasy to disassembleImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsFc(alpha) receptorAllergic asthma
A novel humanized anti-IgE antibody is disclosed. The antibody is capable of binding to free IgE, membrane-bound IgE on B lymphocytes, IgE bound by CD23, but not to IgE bound by high-affinity IgE.Fc receptor on mast cells. The present invention relates to the treatment of IgE-mediated diseases, including allergic asthma, allergic rhinitis, atopic dermatitis, food allergy, chronic spontaneous (idiopathic) urticaria, chronic rhinosinusitis, systemic mastocytosis, cutaneous mastocytosis, allergic bronchopulmonary aspergillosis, recurrent idiopathic angioedema, and eosinophil-associated gastrointestinal disorder by administering the anti-IgE antibody of the present invention.
Owner:ACAD SINIC
Method for Diagnosis and Treatment of Pancreatic Cancer
InactiveUS20100158915A1Antibody ingredientsAntineoplastic agentsPancreatic cancer cellAcinic cell carcinoma
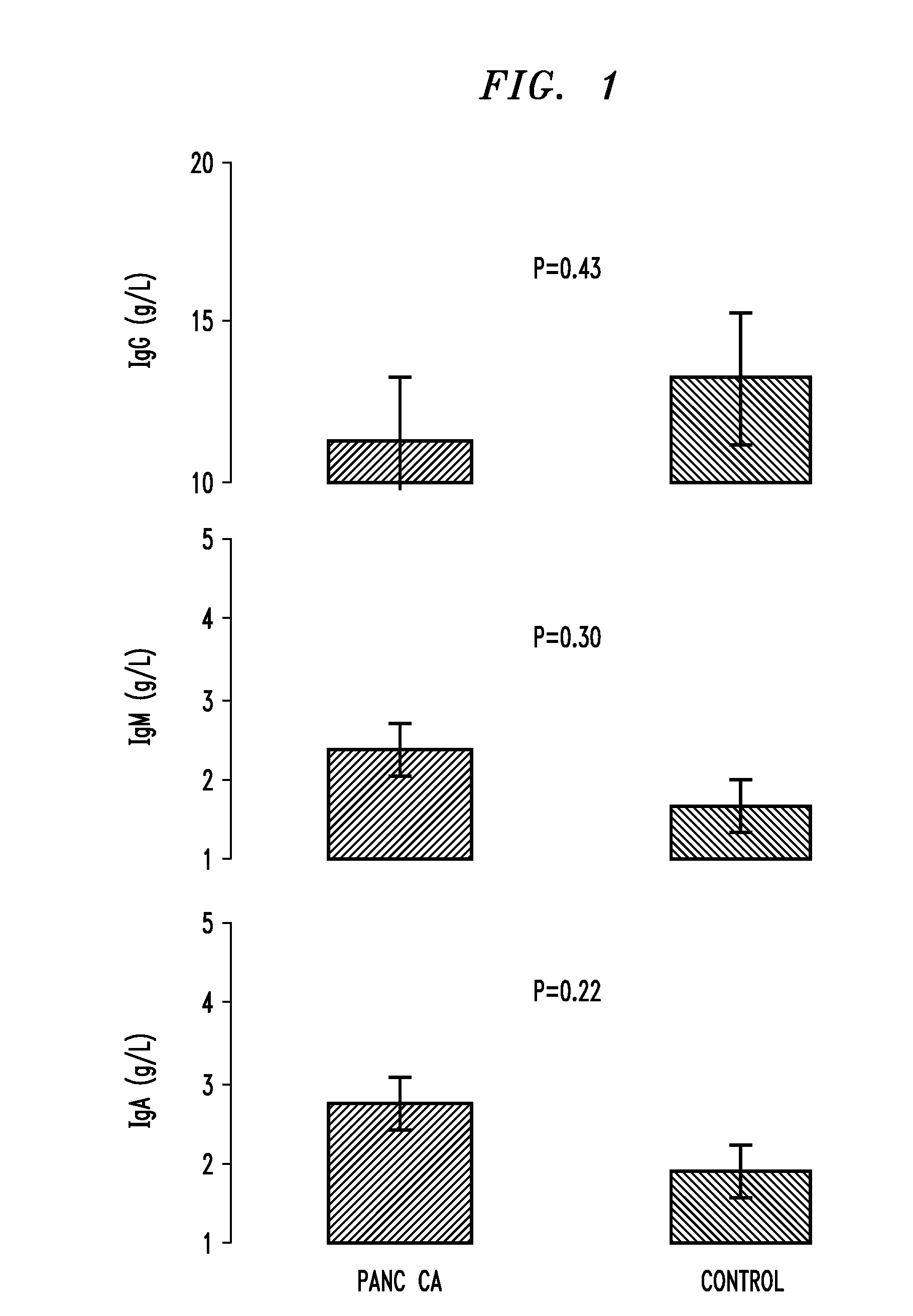
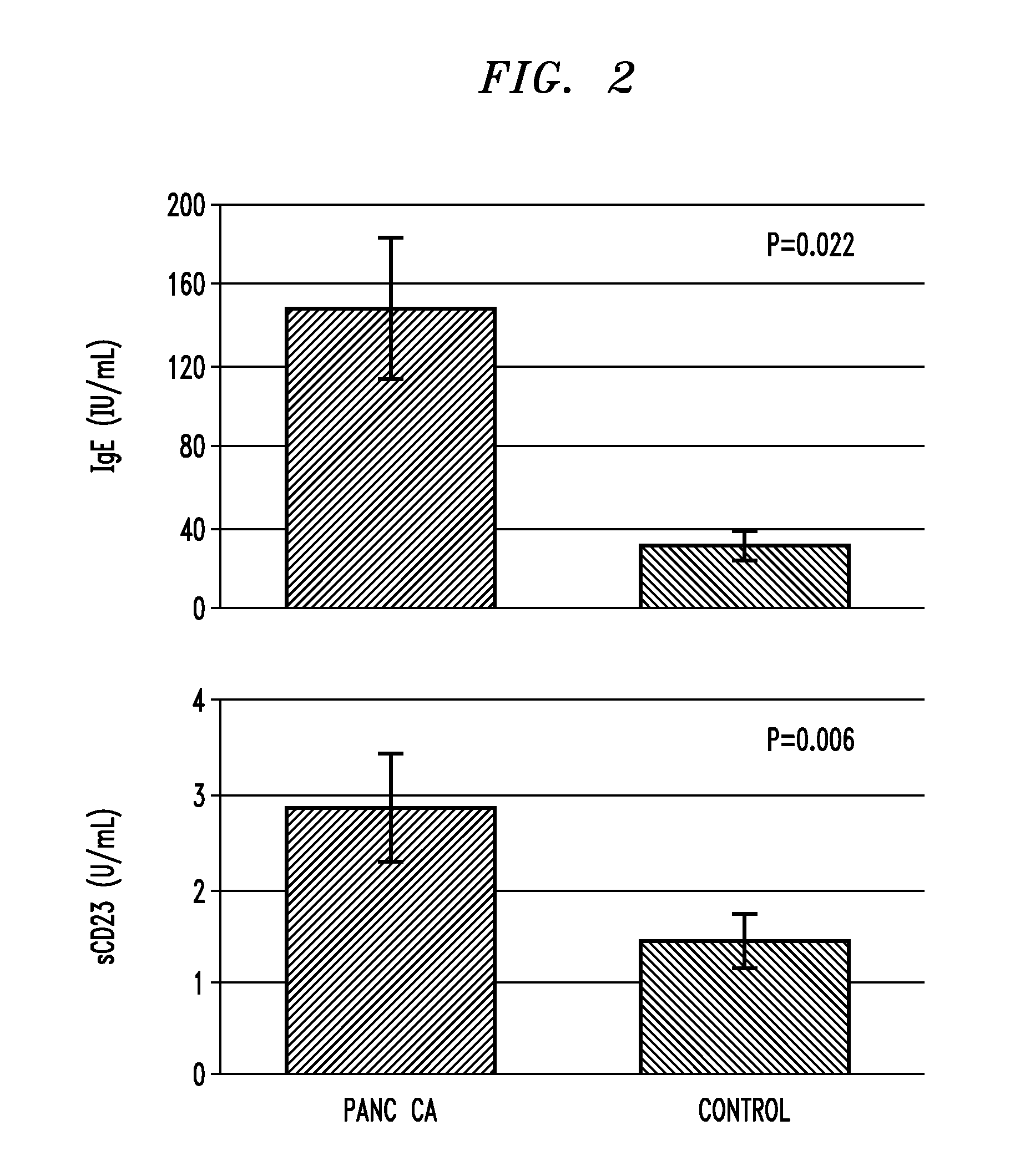
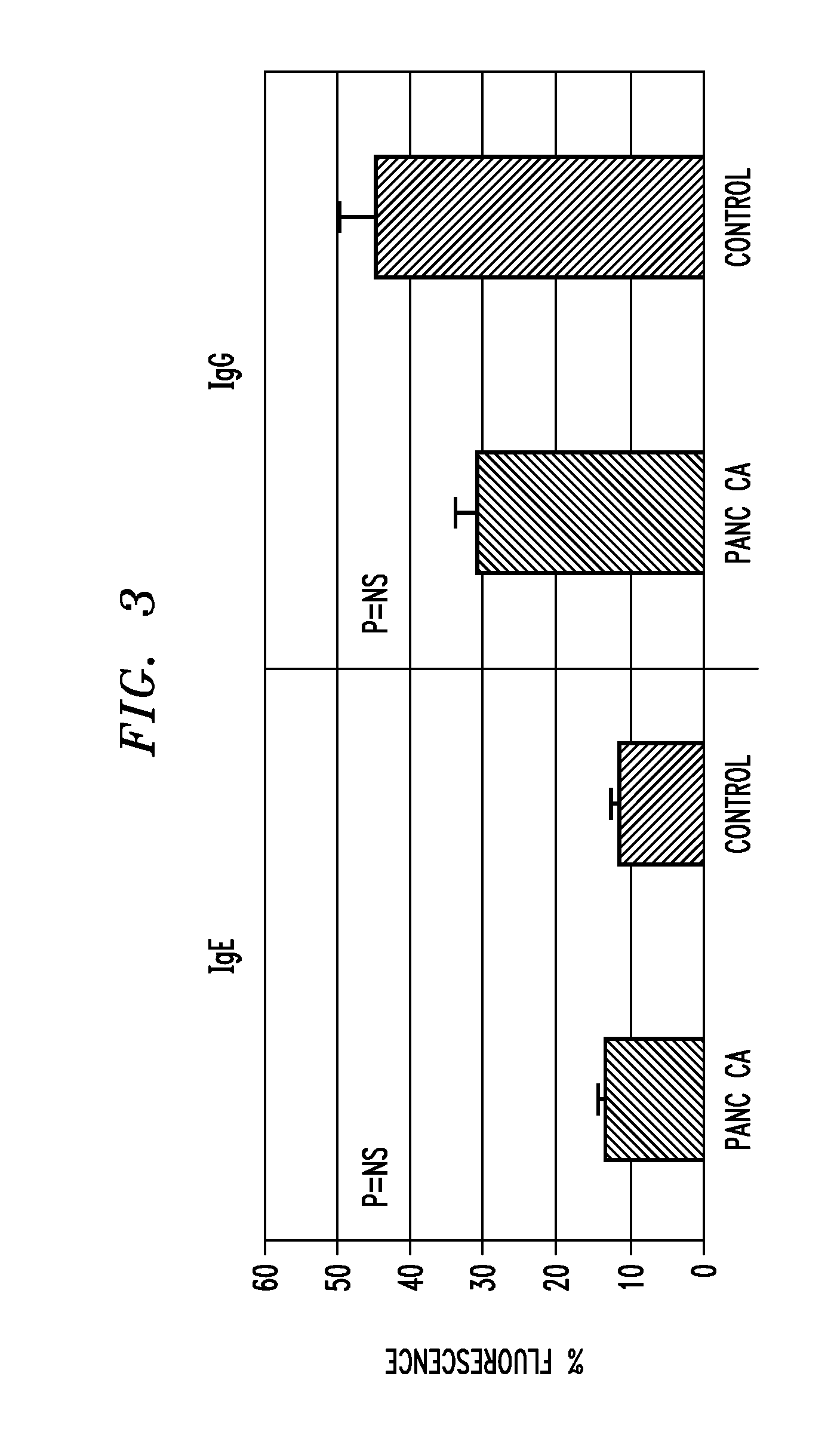
A technique for diagnosing pancreatic cancer includes measuring either level of IgE or cell surface bound cd23 (sCD23) in the patient, comparing the IgE or sCD23 level with that of patient known to have pancreatic cancer and with a level of IgE or sCD23 from a control patient known to not have pancreatic cancer, and identifying the level of IgE or sCD23 in the patient as analogous to either the control patient known to have pancreatic cancer or the control patient known to not have pancreatic cancer A technique for mediating ADCC against pancreatic cancer cells by administering a therapeutically effective amount of IgE to a source containing one or more pancreatic cancer cells.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Treatment of IgE-mediated disease
ActiveUS8945575B2Lower immune responseReduce developmentOrganic active ingredientsPeptide/protein ingredientsDiseaseIncreased IgE level
The methods and compositions described herein are based, in part, on the discovery of a polypeptide of soluble CD23 (sCD23) that binds and sequesters IgE. Thus, the sCD23 peptides, polypeptides and derivatives described herein are useful for treating conditions or disorders involving increased IgE levels such as e.g., allergy, anaphylaxis, inflammation, lymphoma, and certain cancers.
Owner:TRUSTEES OF BOSTON UNIV +1
A bispecific chimeric antigen receptor molecule and its application in tumor therapy
ActiveCN109503716BIncreased persistenceImprove targetingPeptide/protein ingredientsMammal material medical ingredientsCD20CD79A
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
Fab fragment of human anti-human CD23 antibody, pharmaceutical composition and application of Fab fragment
Owner:南京英瀚斯生物科技有限公司
Gamma-1 and gamma-3 Anti-human cd23 monoclonal antibodies and use thereof as therapeutics
InactiveUS20030086921A1Improve abilitiesEnhanced potential for cross linkingPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseLow affinity
Owner:BIOGEN MA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com