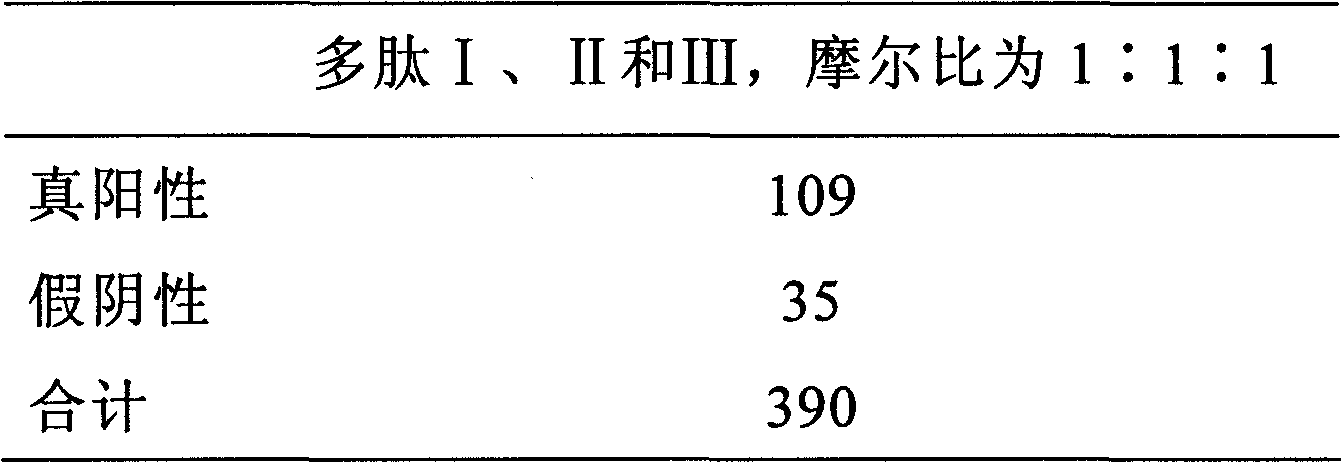
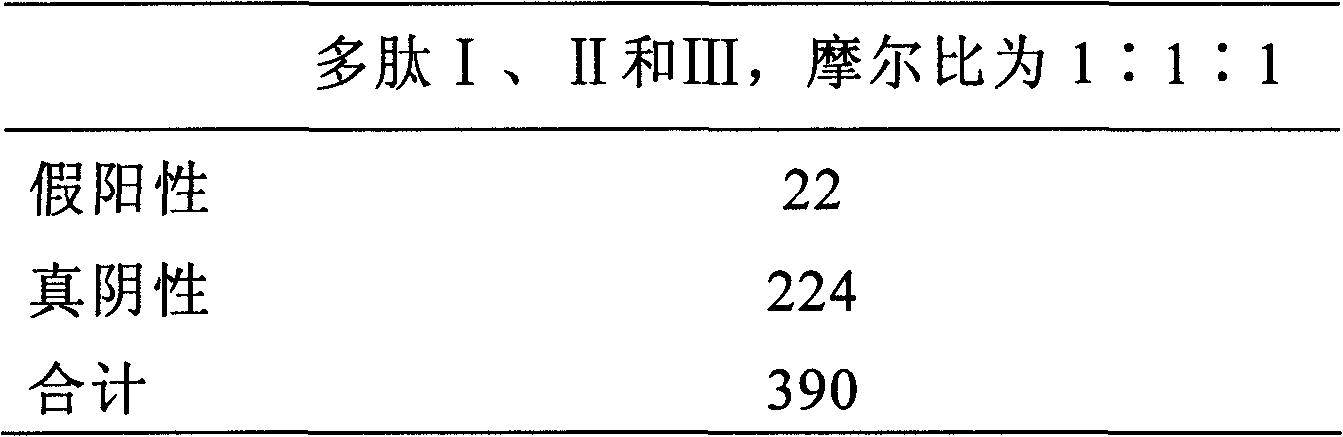
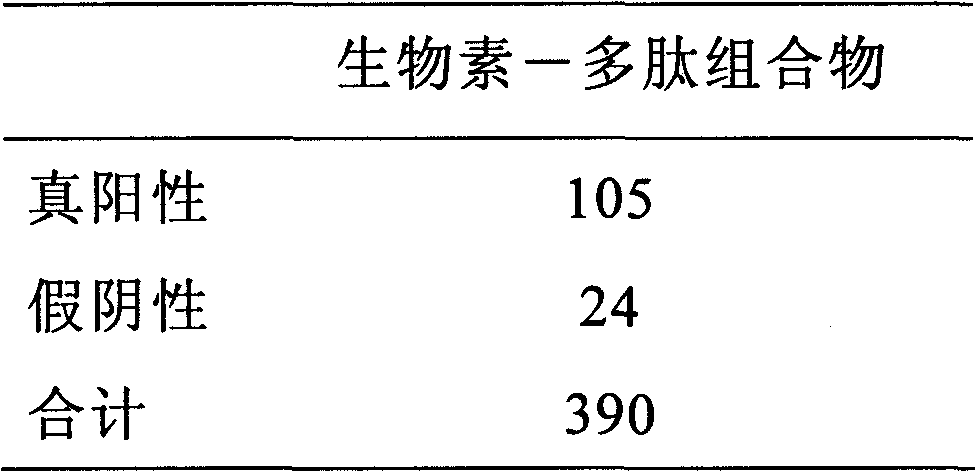
Polypeptide composition for detecting immune antibody of rheumatoid arthritis in vitro
A peptide composition, in vitro detection technology, applied in the direction of peptides, hybrid peptides, specific peptides, etc., can solve the problems of antibody differences, waiting for further independent clinical research, and the detection sensitivity cannot be significantly improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] The synthesis of embodiment 1 polypeptide I
[0144] Polypeptides are synthesized by solid-phase synthesis technology using Fmoc chemical method. For the specific steps of this method, see Eur.J.Immunol.1994, 24, 3188-3193; J.Org.Chem.1972, 37, 3404-3409; Huang Weide, Chen Changqing Polypeptide Synthesis, Beijing: Science Press, 1985.
[0145] The formation method and steps of disulfide bonds can be found in the literature: Huang Weide, Chen Changqing Peptide Synthesis, Beijing: Science Press, 1985, p85; Michael W.Pennington Peptide Synthesis Protocols (Methods in Molecular Biology), Humana Press, 1994, p91-169 .
[0146] Through the above steps, the specific sequence of the synthetic polypeptide is:
[0147] Polypeptide Ⅰ: His-Gln-Cys-Ala-Arg-Phe-Gln-Met-Arg-His-Cit-Arg-Leu-Ile-Arg-Cys-Gly, on which the third and sixteenth positions of Cys pass through the sulfhydryl group Form a disulfide bond, and make the polypeptide form a ring structure, and can simulate the β-...
Embodiment 2
[0148] The synthesis of embodiment 2 polypeptide II
[0149] Polypeptide II was synthesized according to the method disclosed in Example 1: His-Gln-Cys-Ala-Arg-Phe-Gln-Met-Cit-His-Cit-Arg-Leu-Ile-Arg-Cys-Gly, the third The Cys at the sixteenth position and the sixteenth position form a disulfide bond through the sulfhydryl group, and make the polypeptide form a ring structure, and can simulate the β-turn structure.
Embodiment 3
[0150] The synthesis of embodiment 3 polypeptide III
[0151] Polypeptide III was synthesized according to the method disclosed in Example 1: His-Gln-Cys-His-Gln-Phe-Arg-Phe-Cit-Gly-Cit-Ser-Arg-Ala-Ala-Cys-Gly, the third The Cys at the sixteenth position and the sixteenth position form a disulfide bond through the sulfhydryl group, and make the polypeptide form a ring structure, and can simulate the β-turn structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com