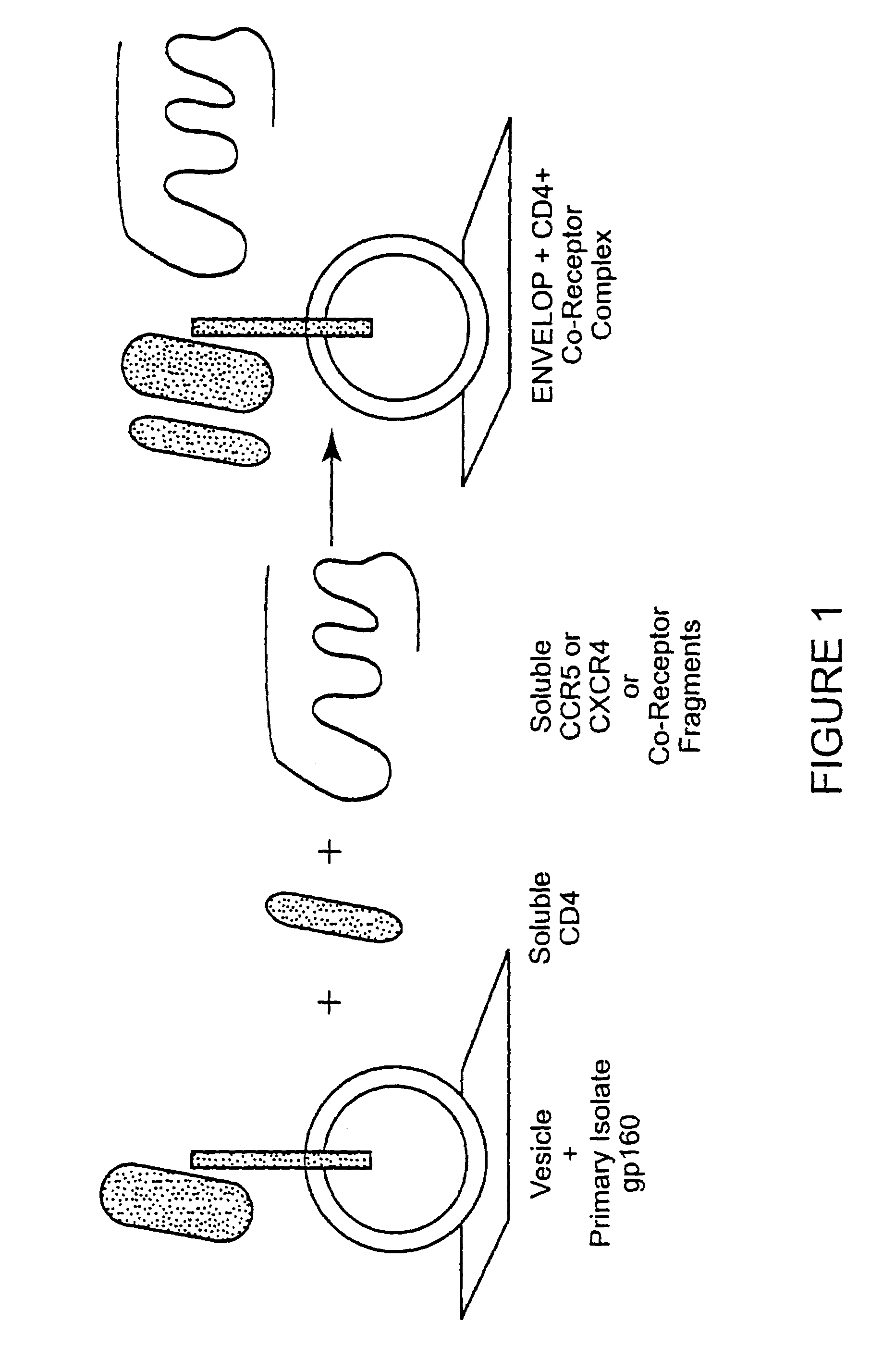
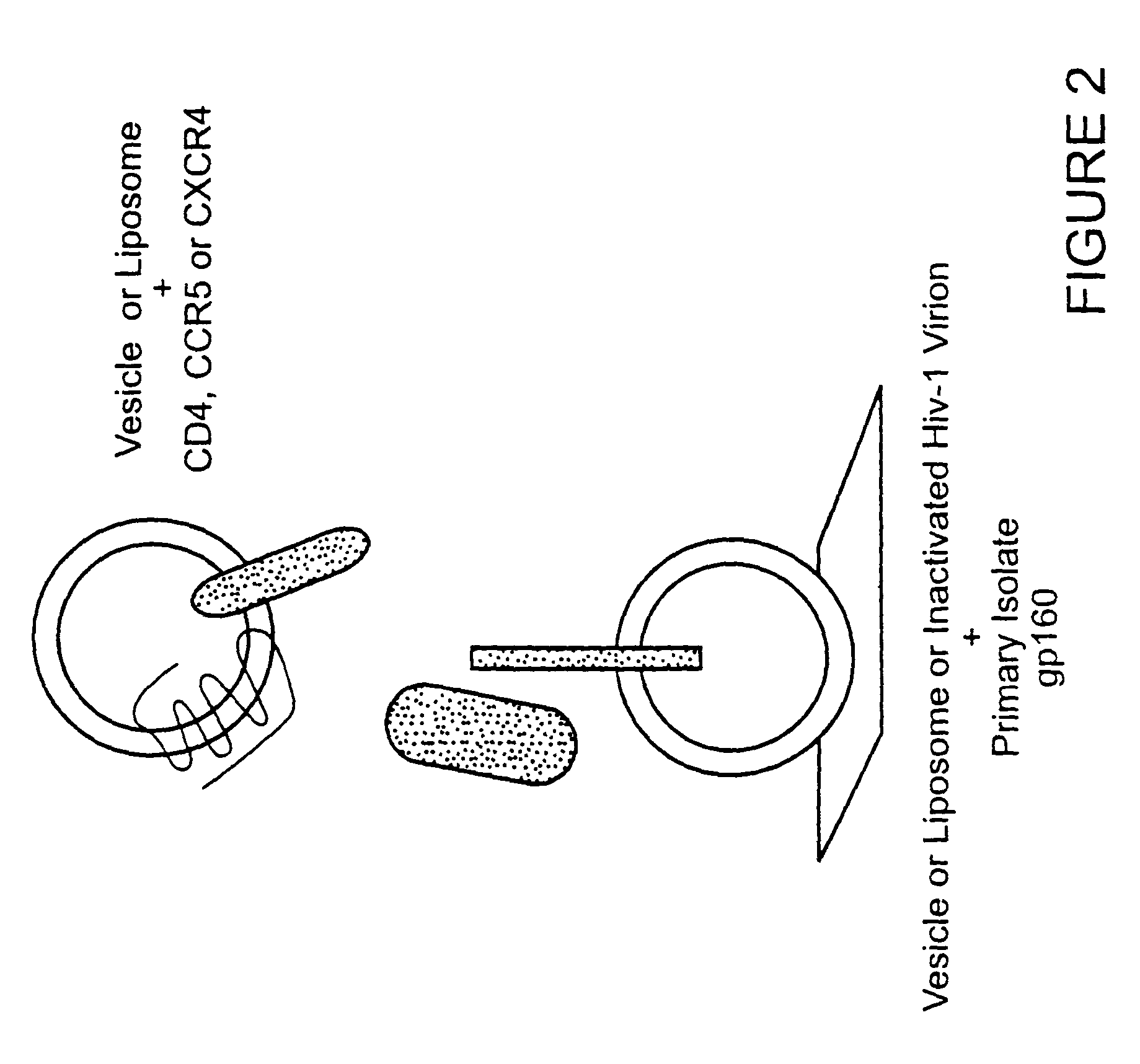
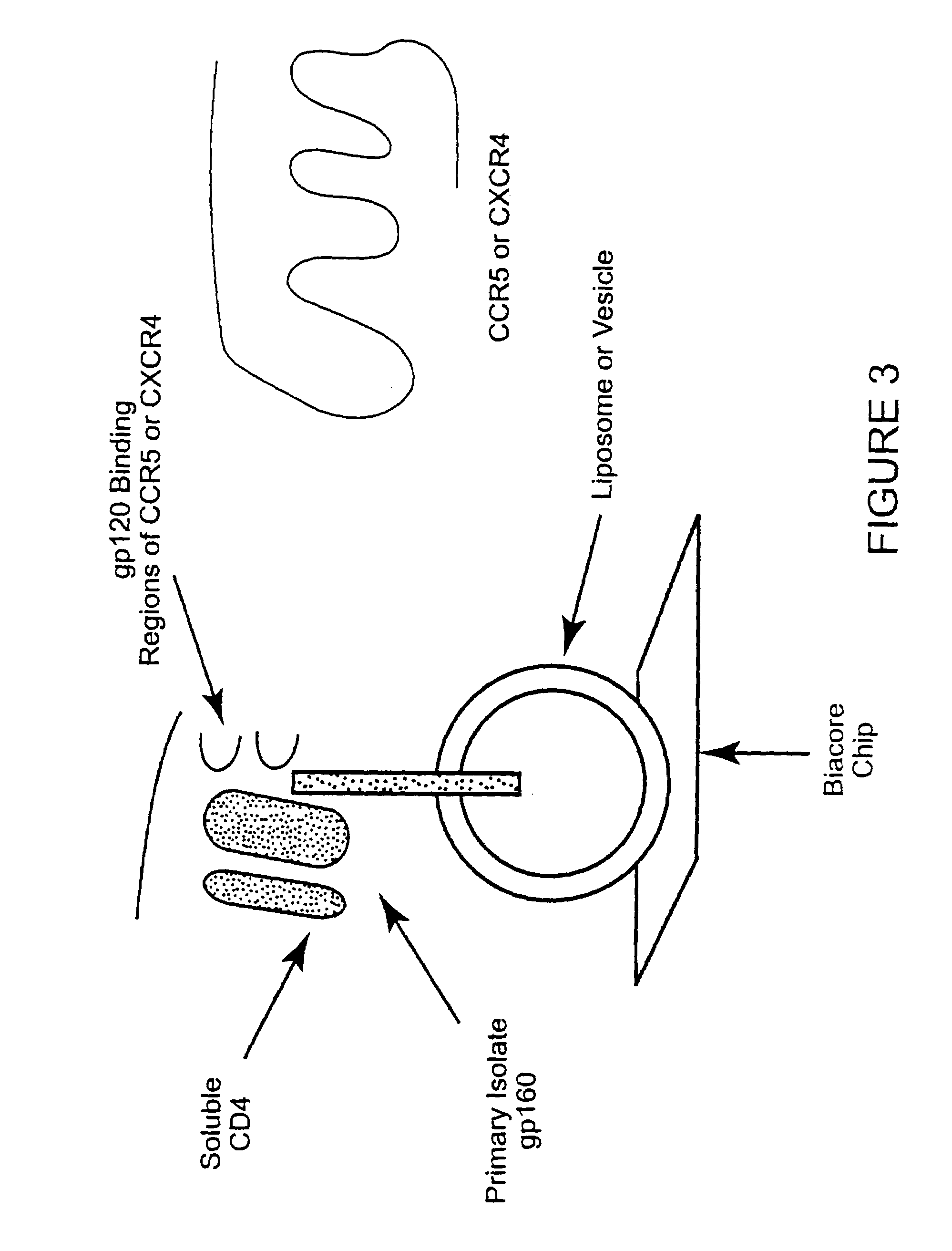
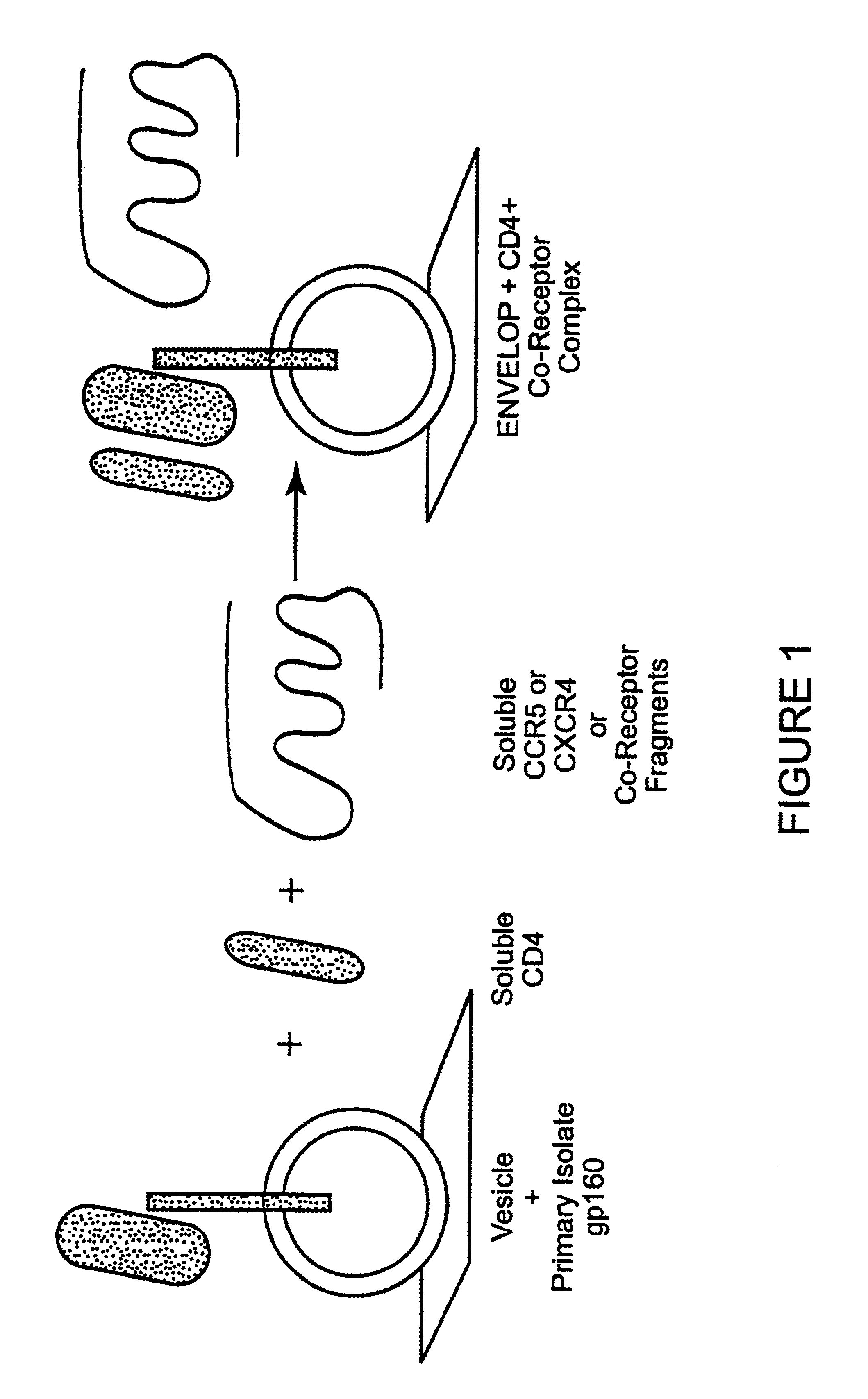
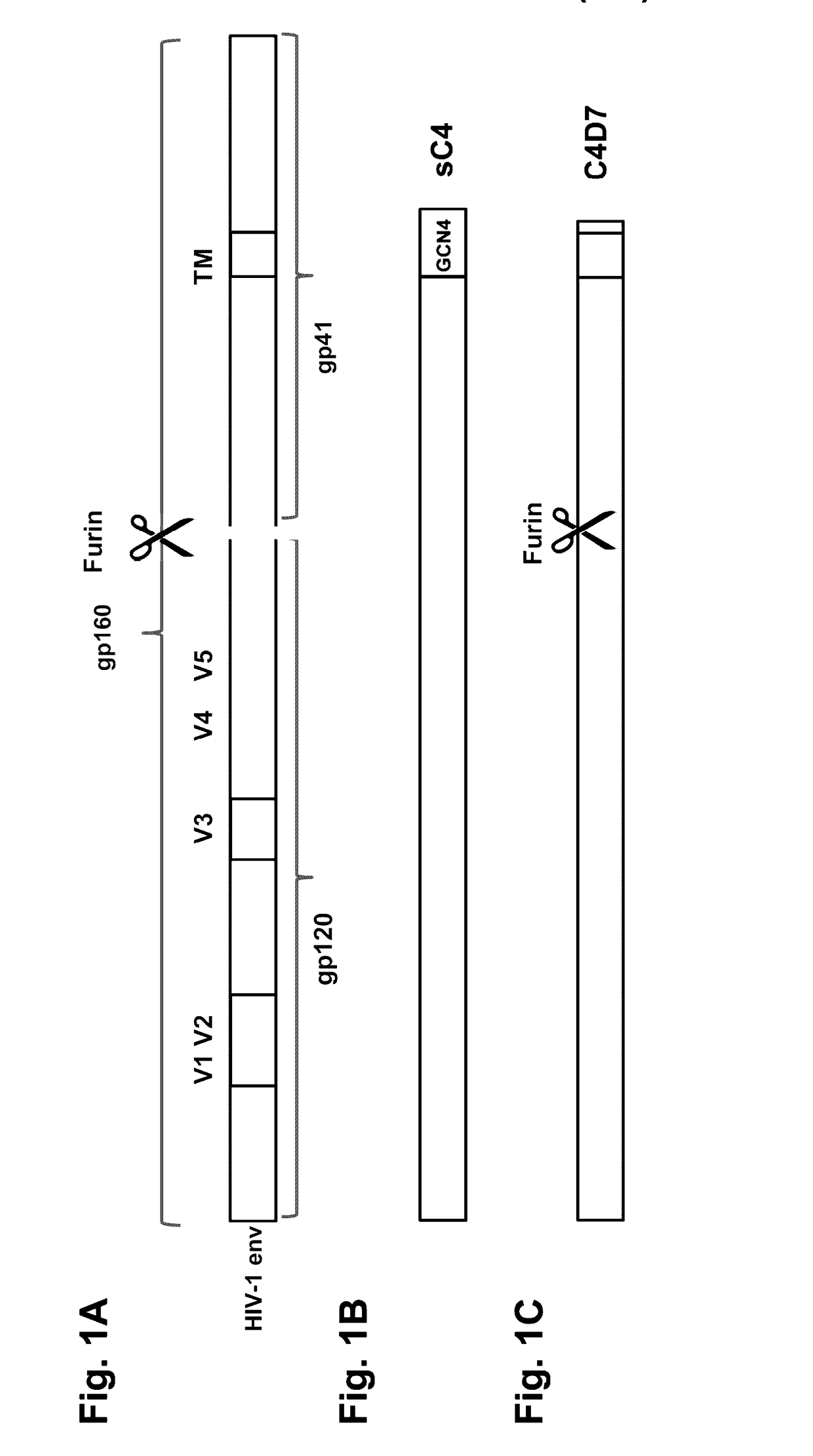
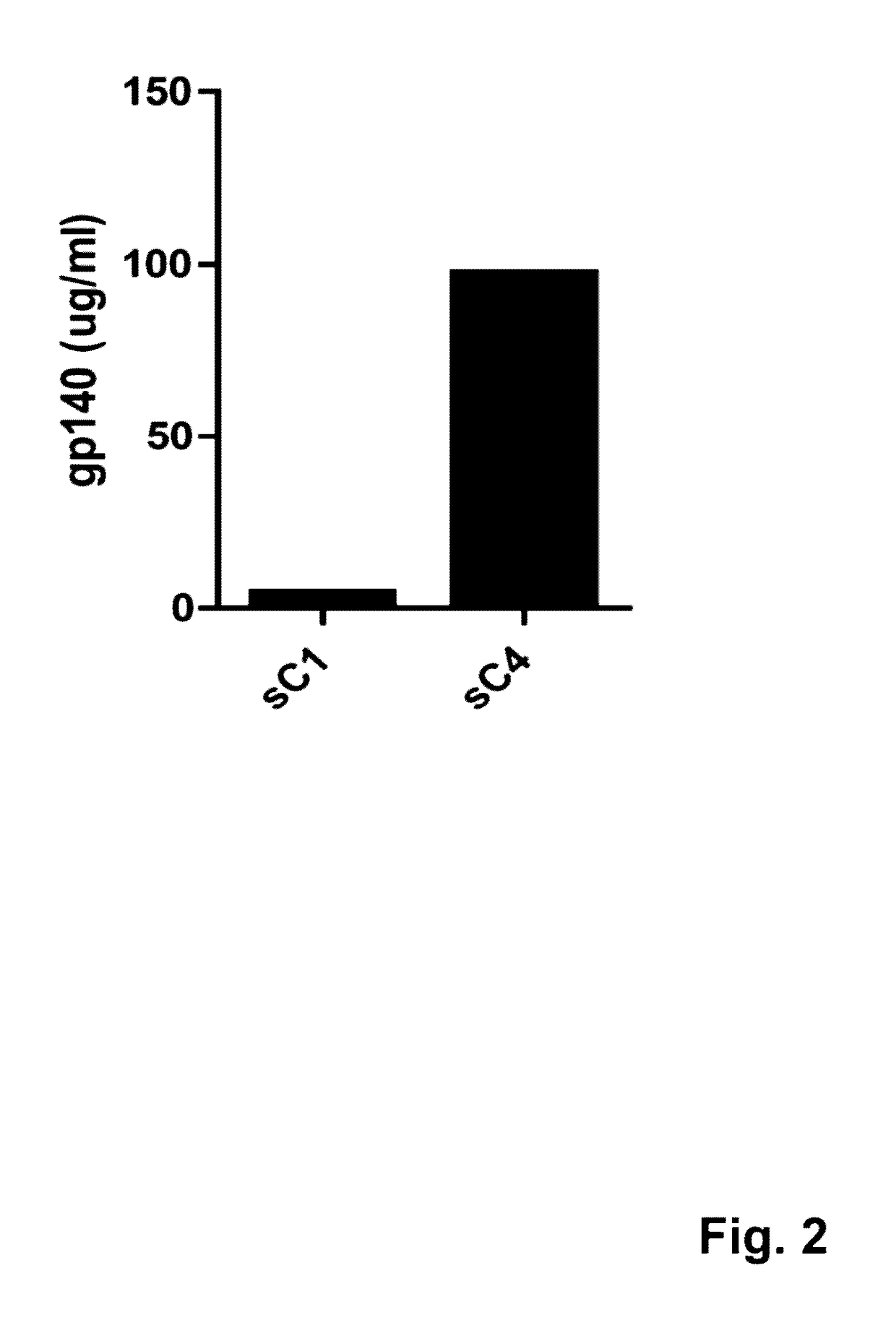
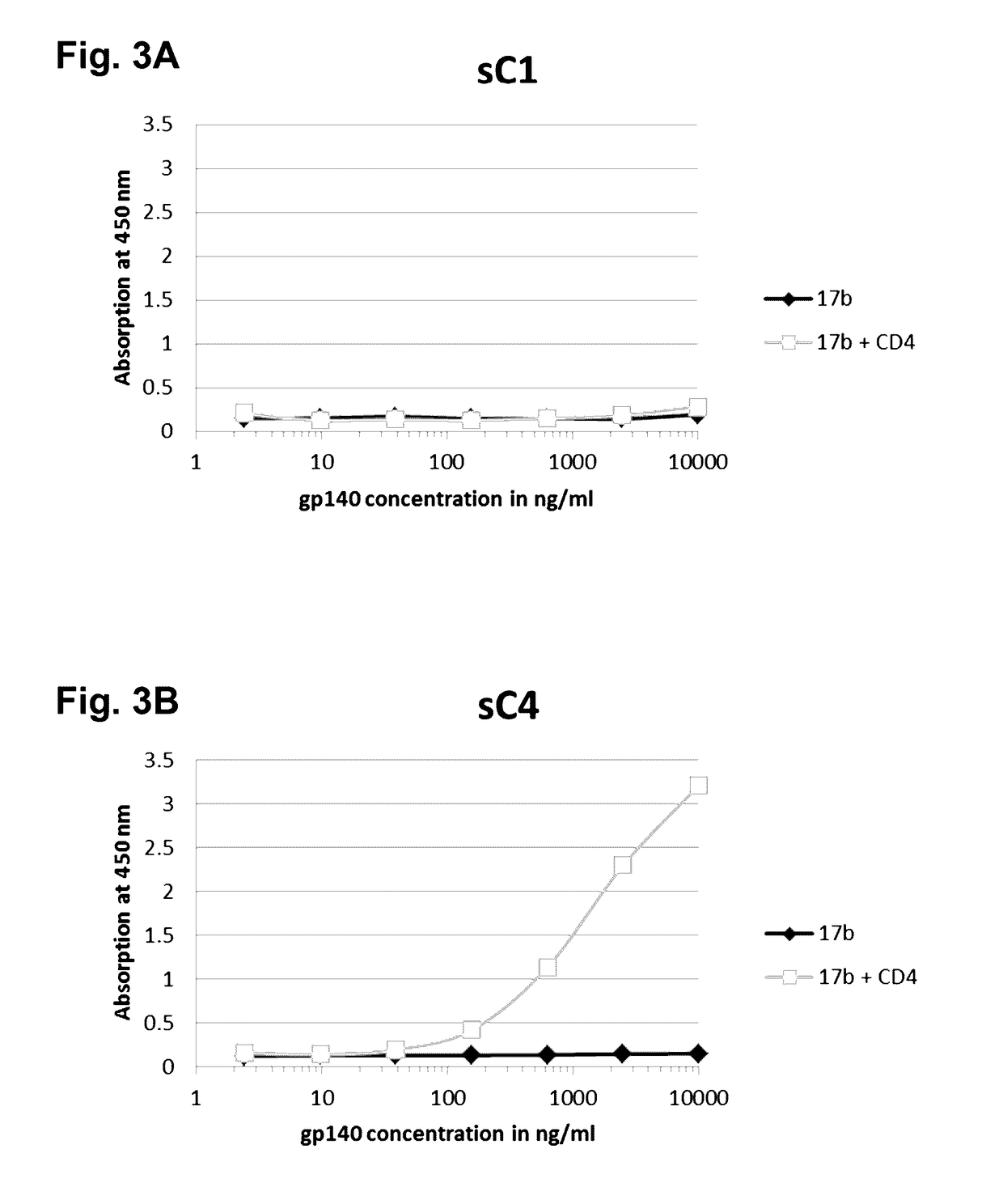
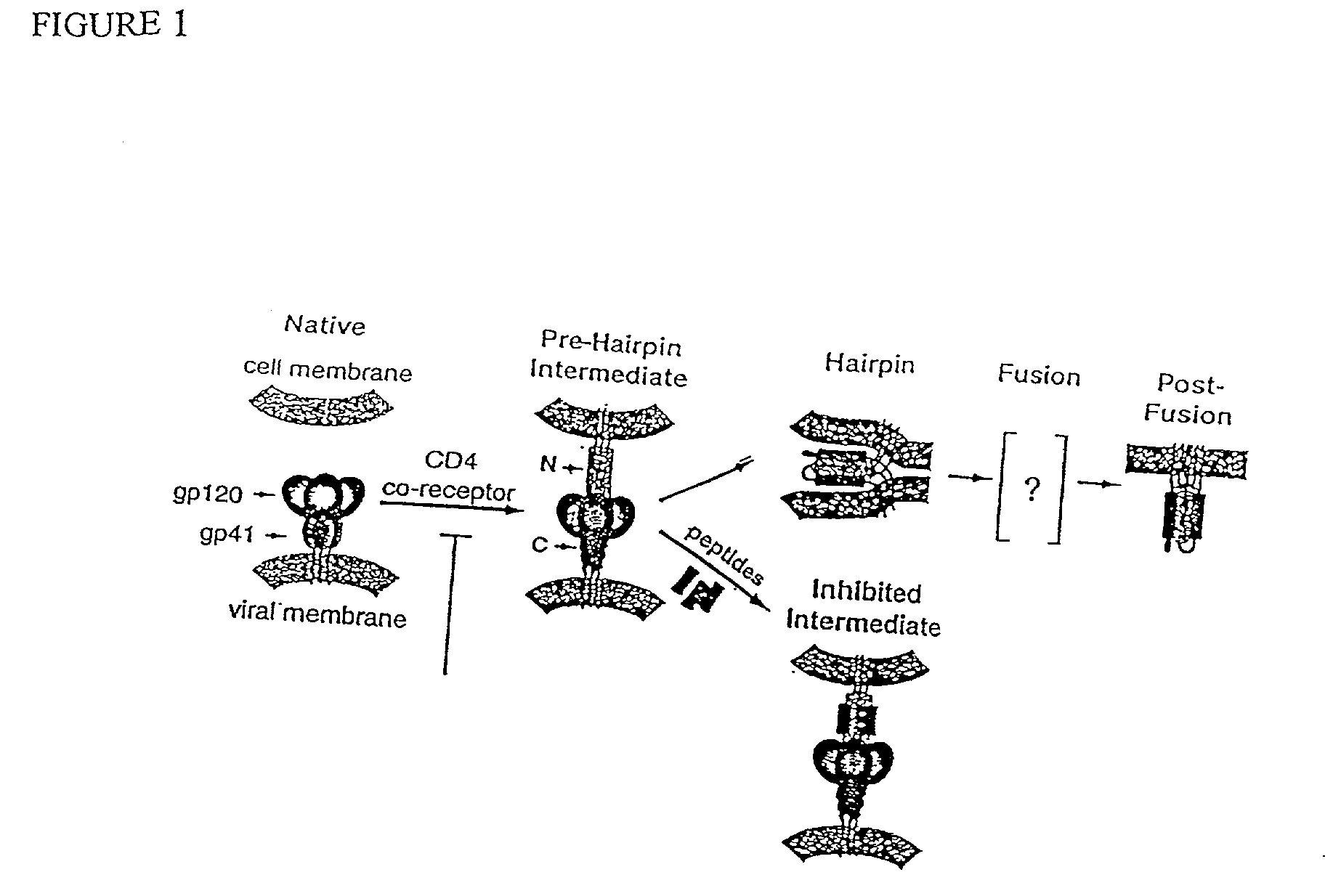
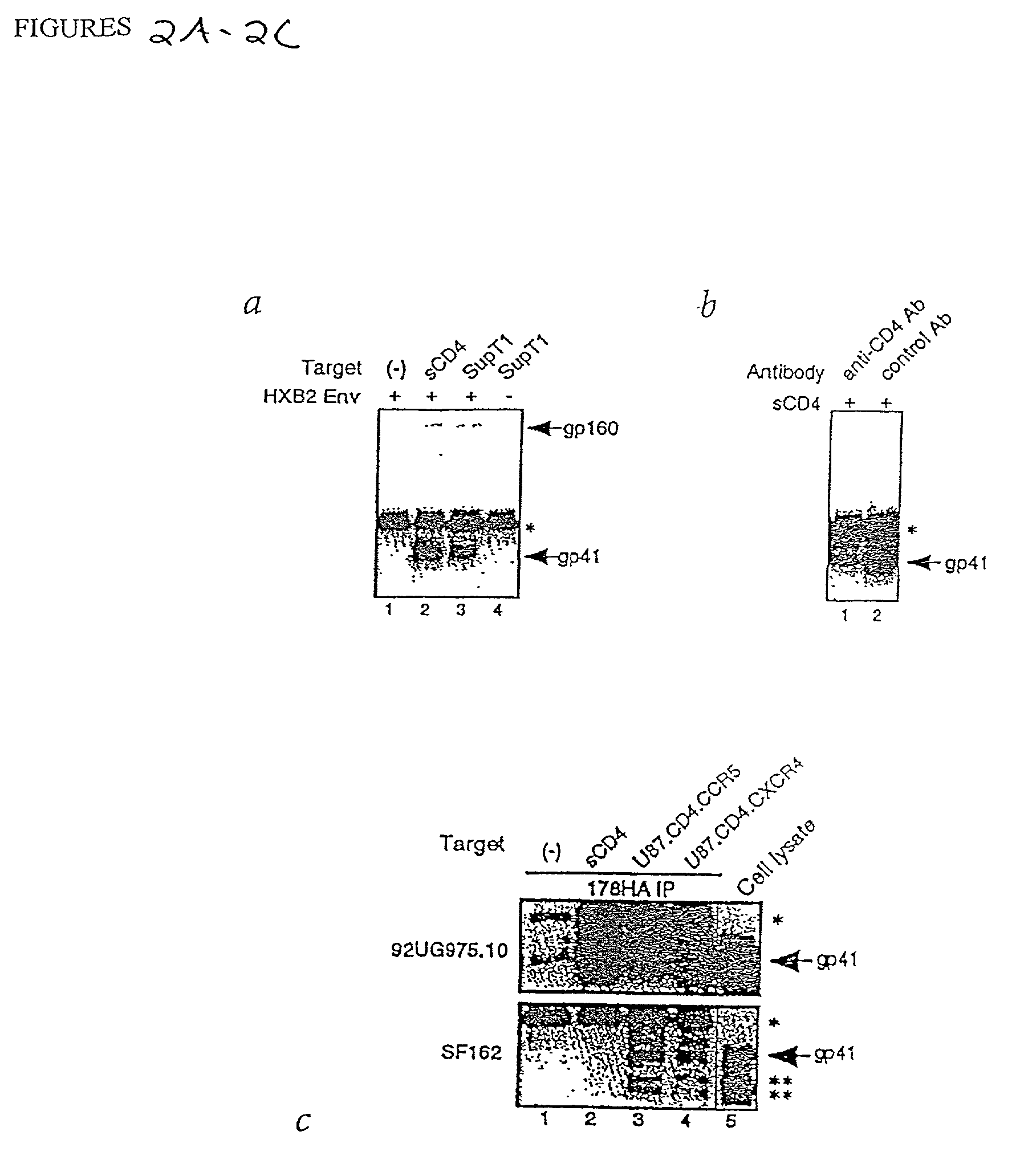
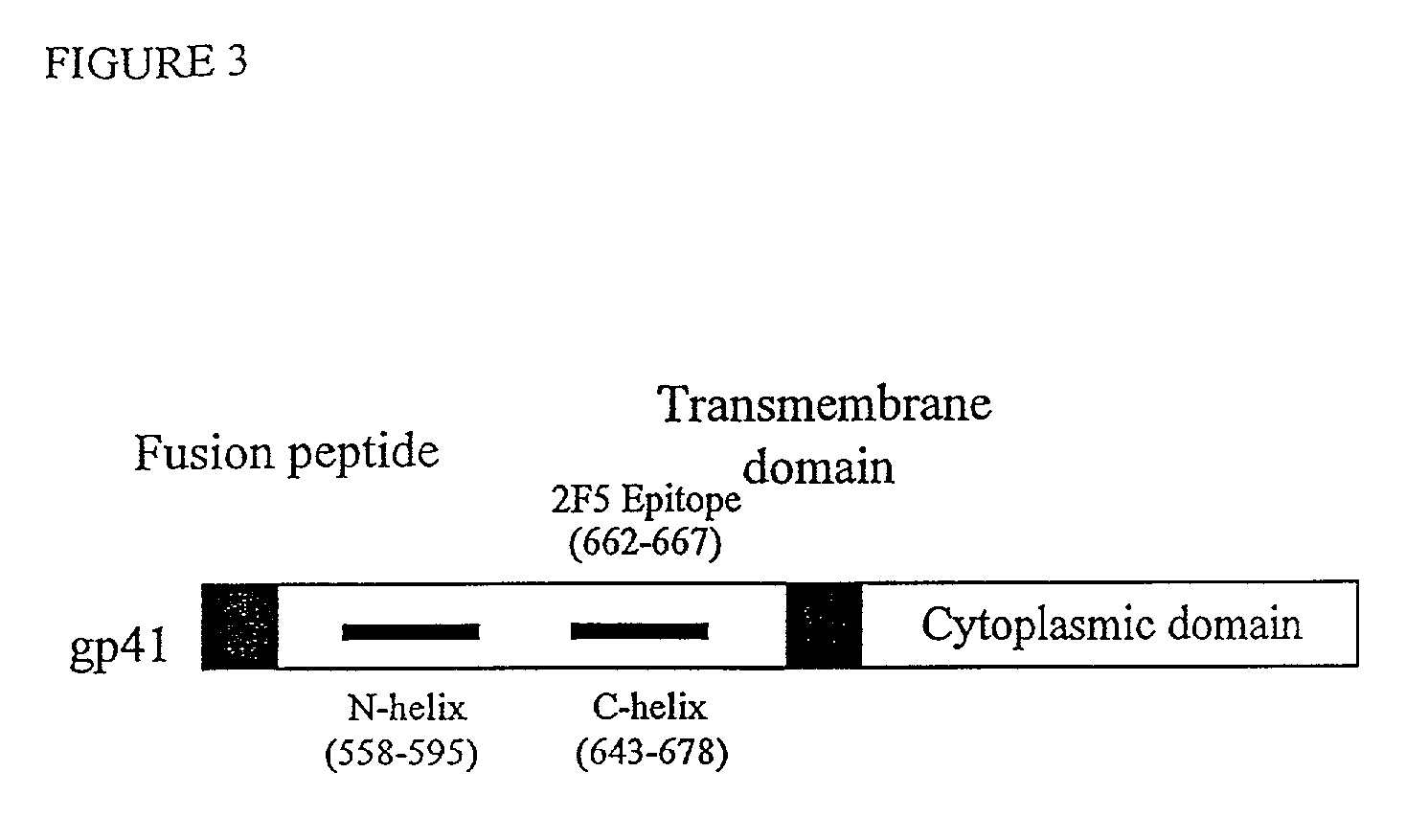
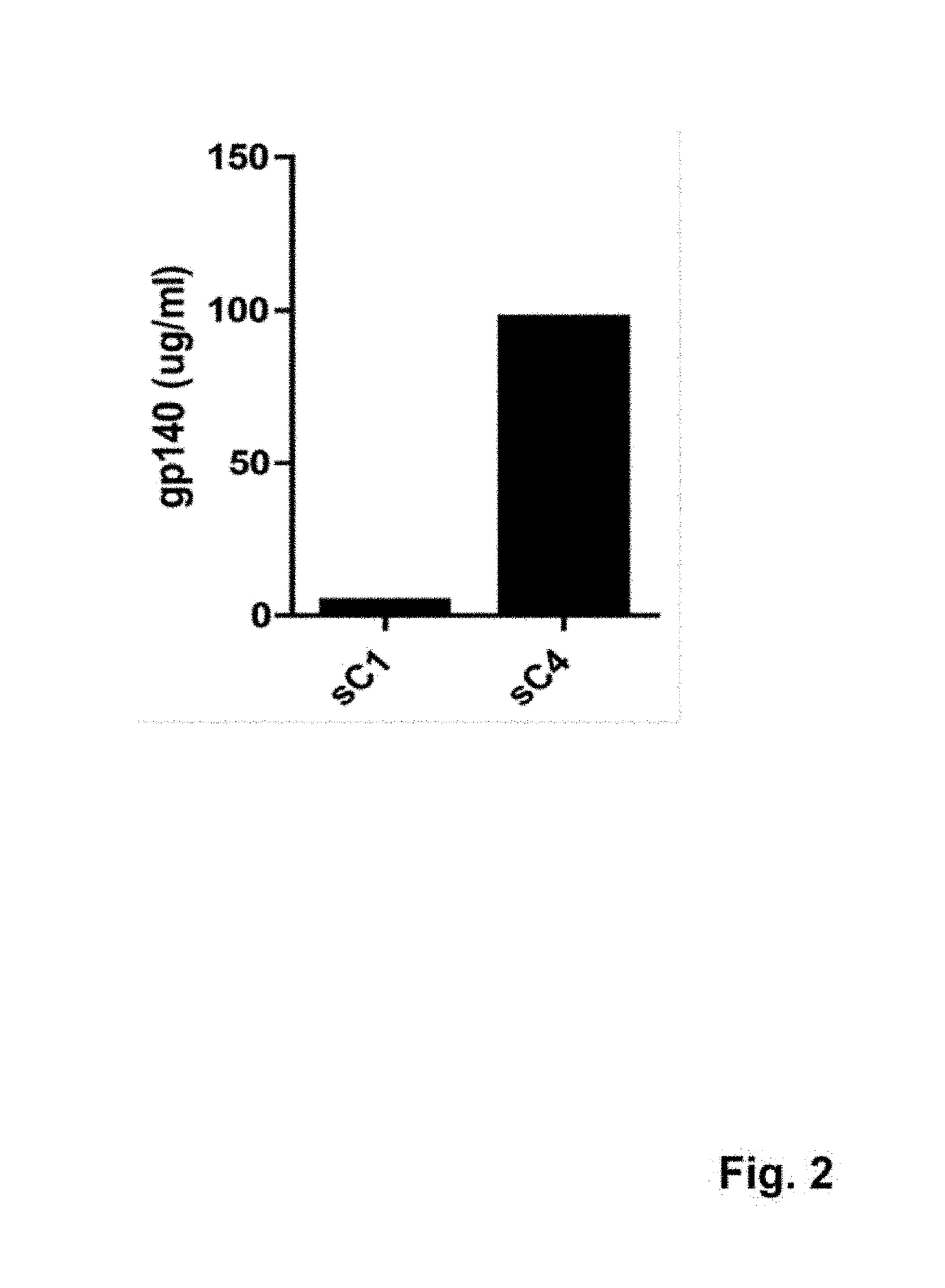
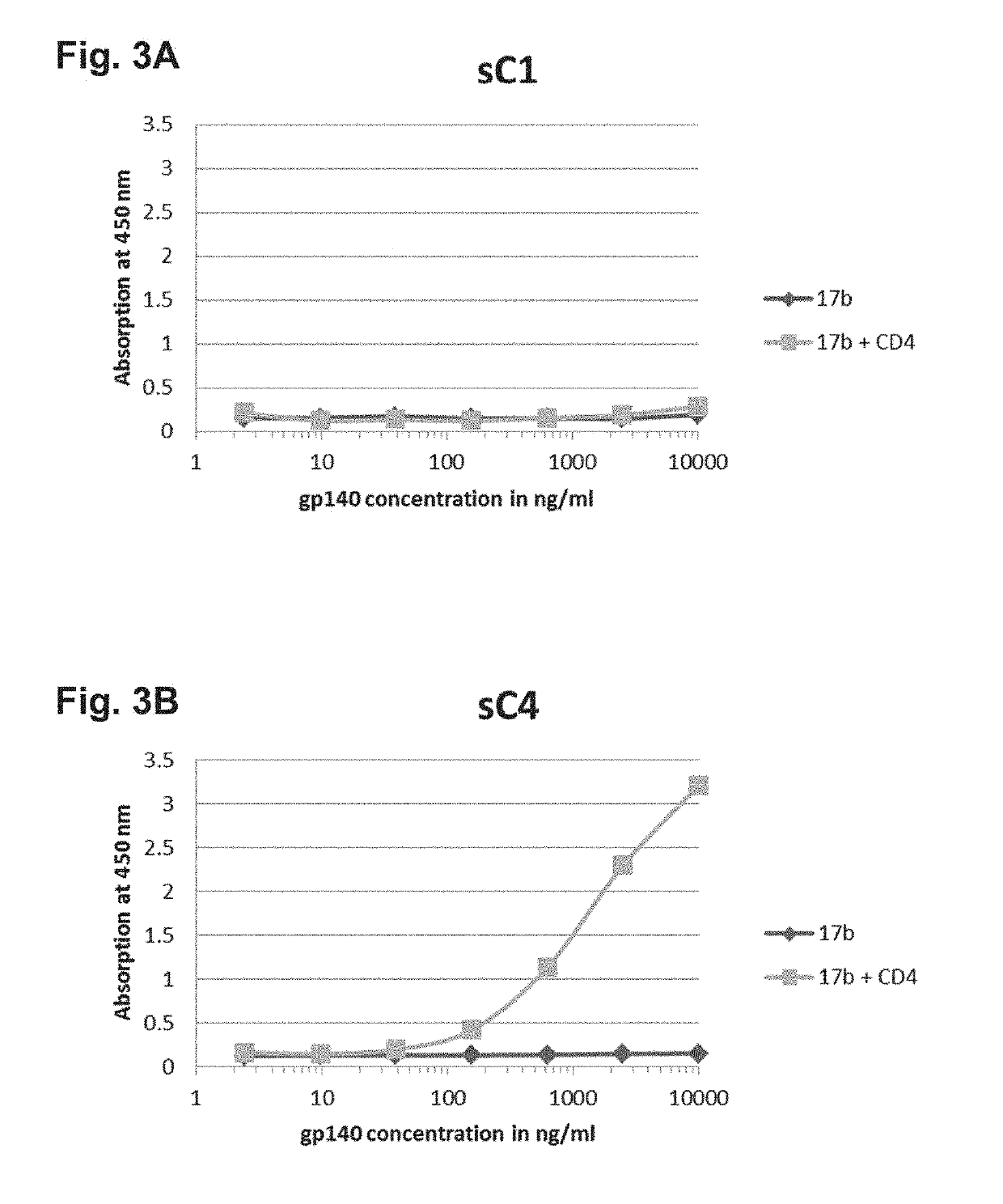
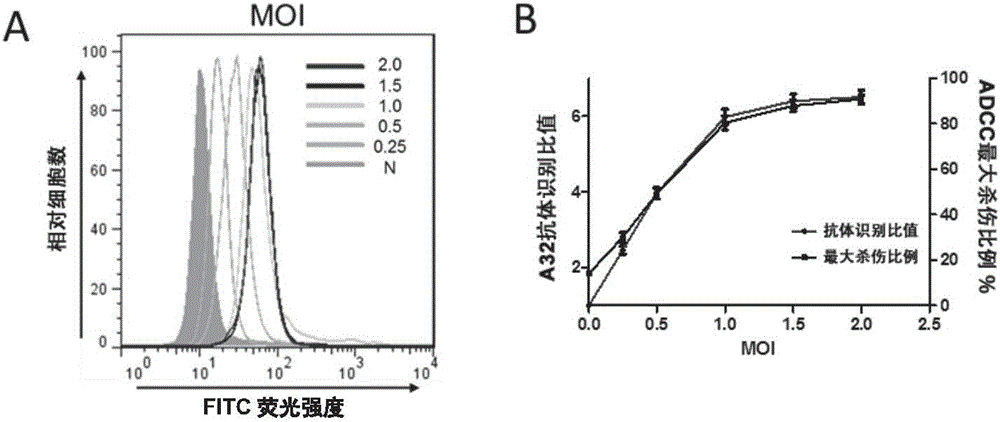
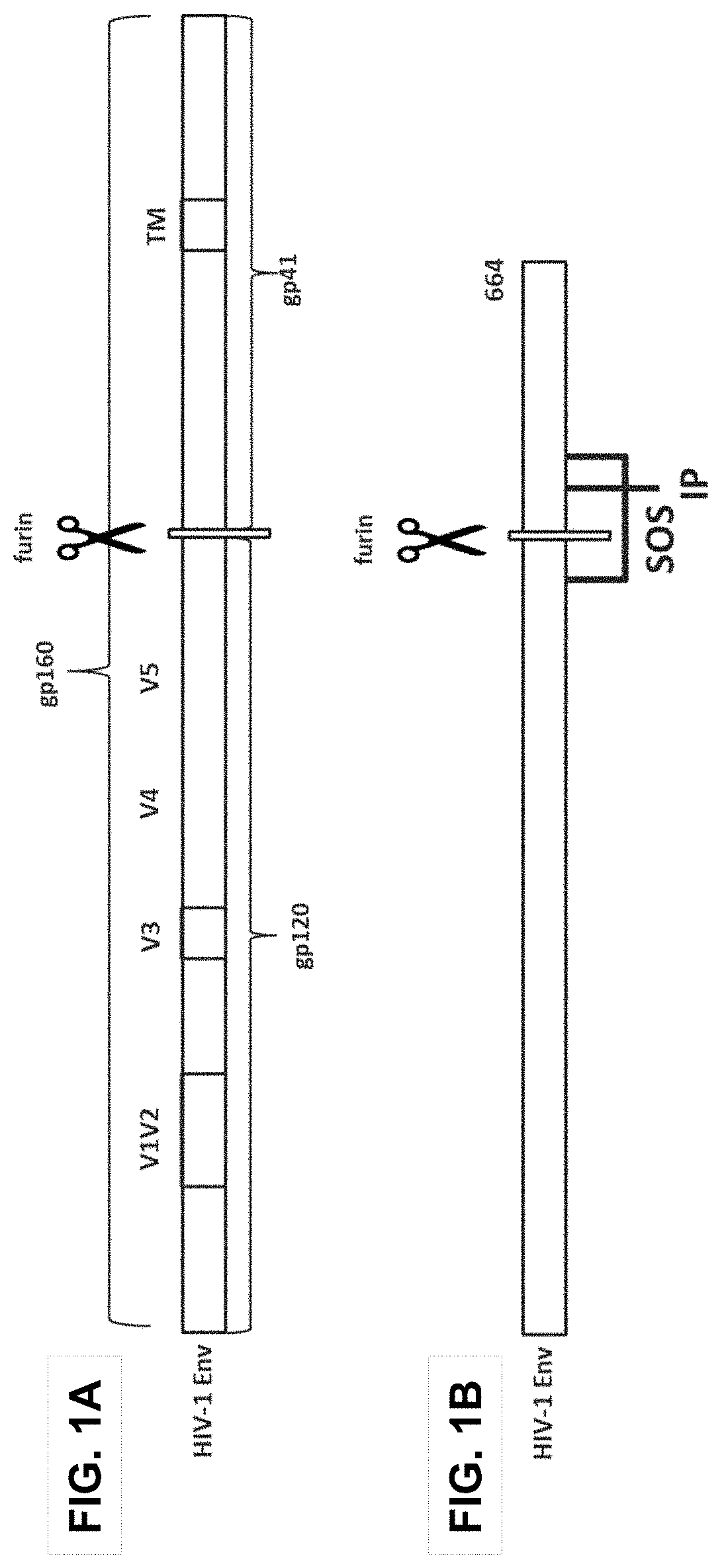
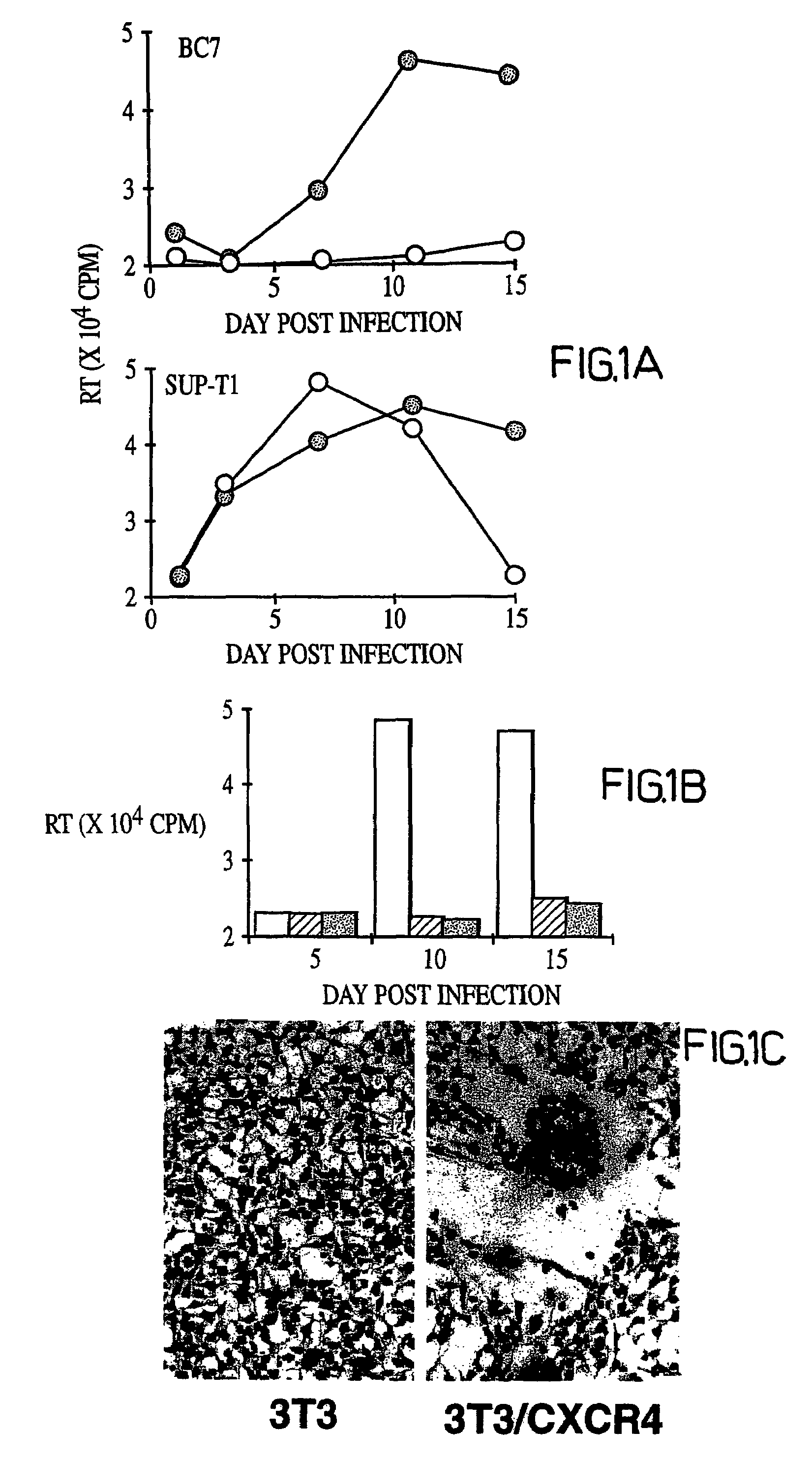
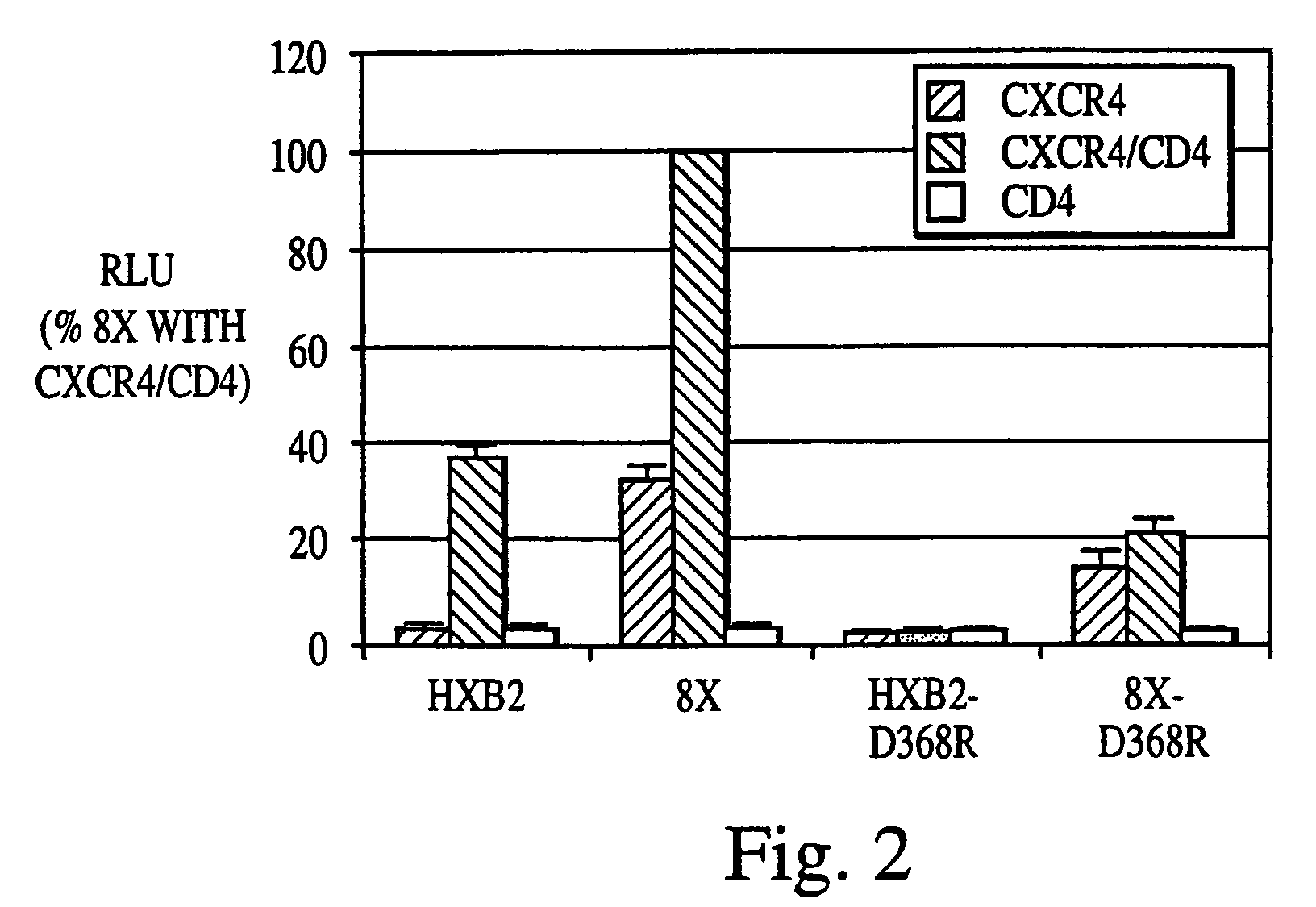
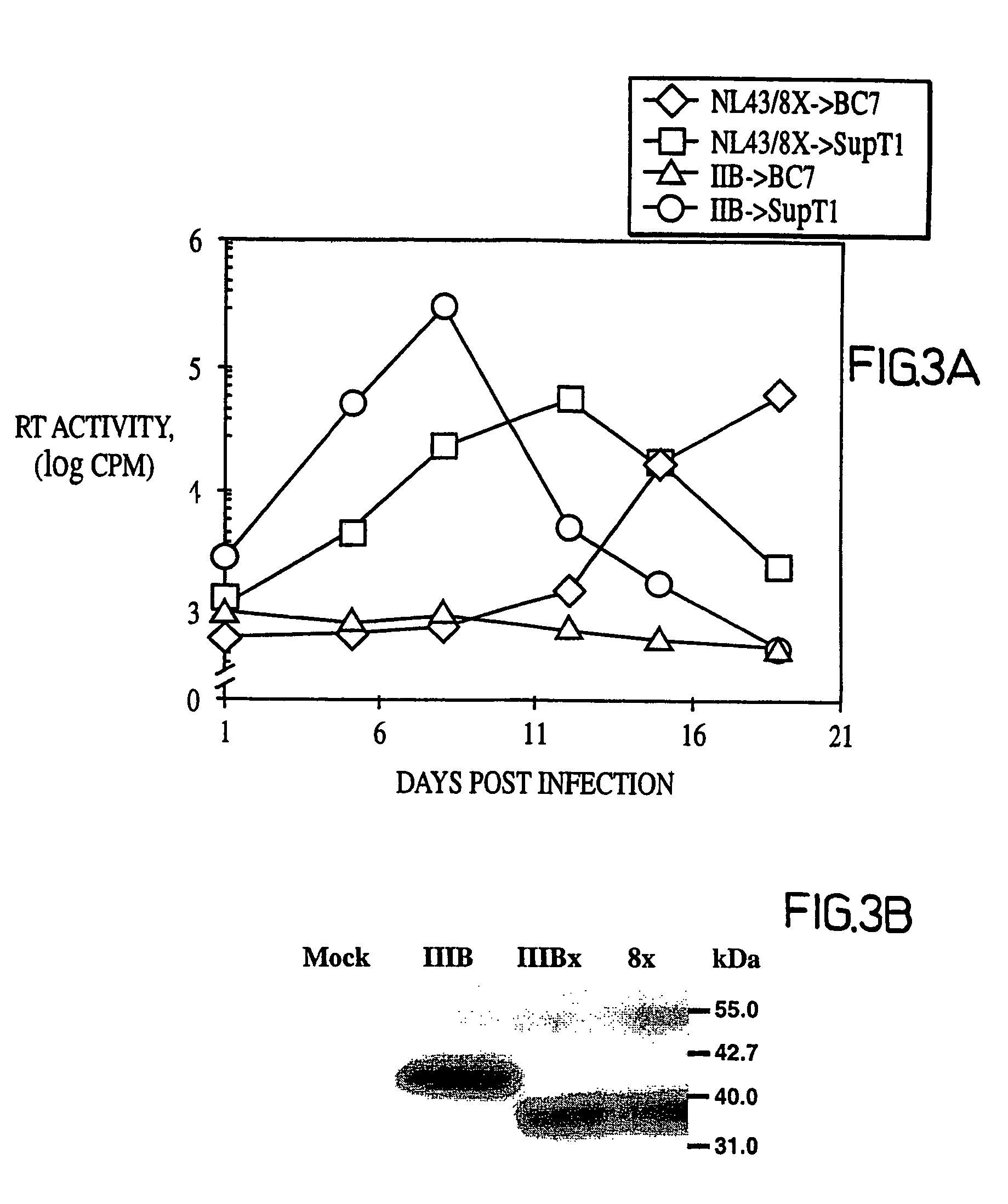
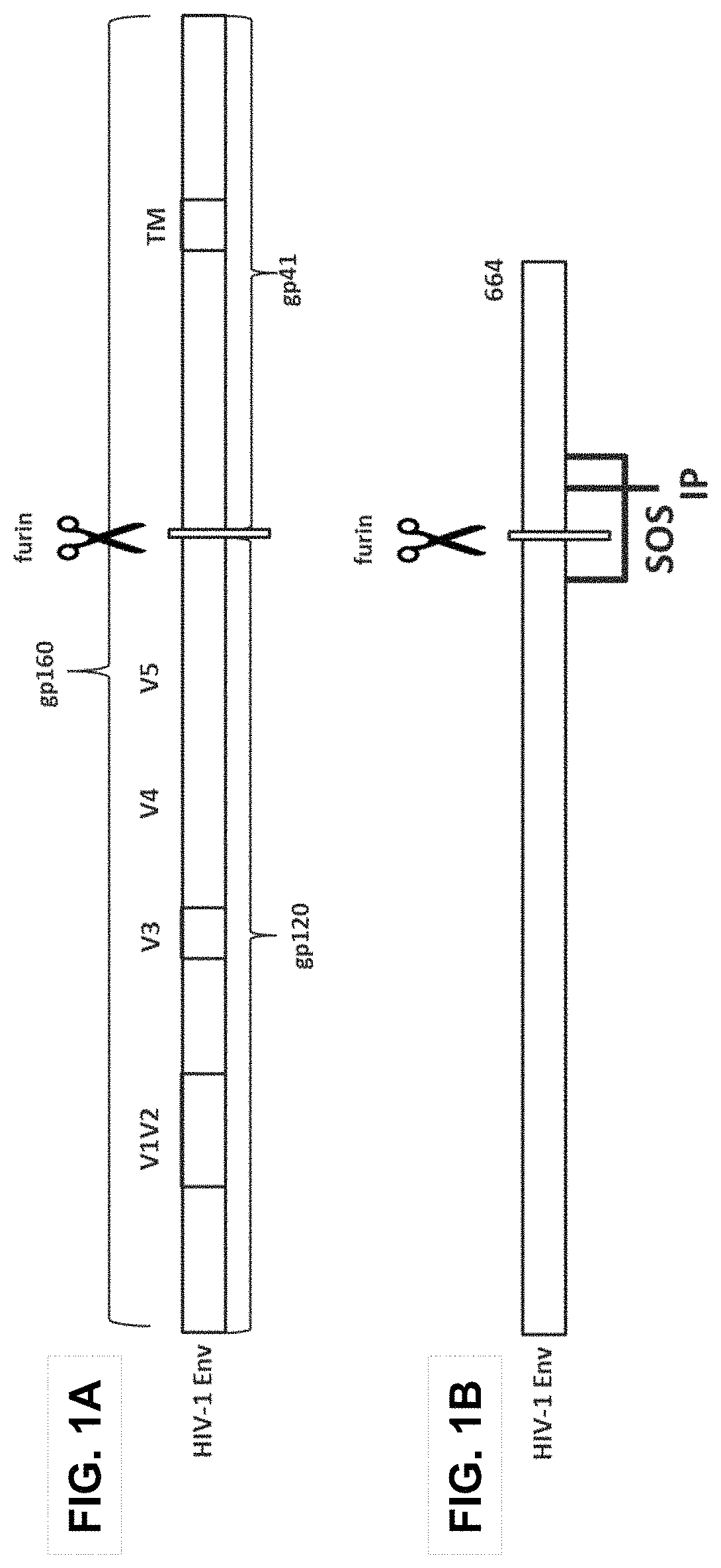
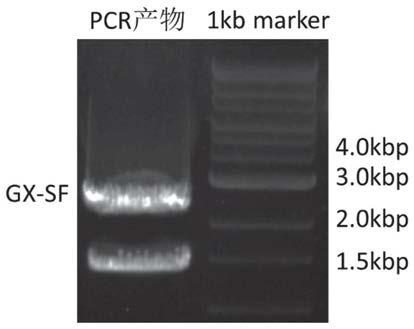
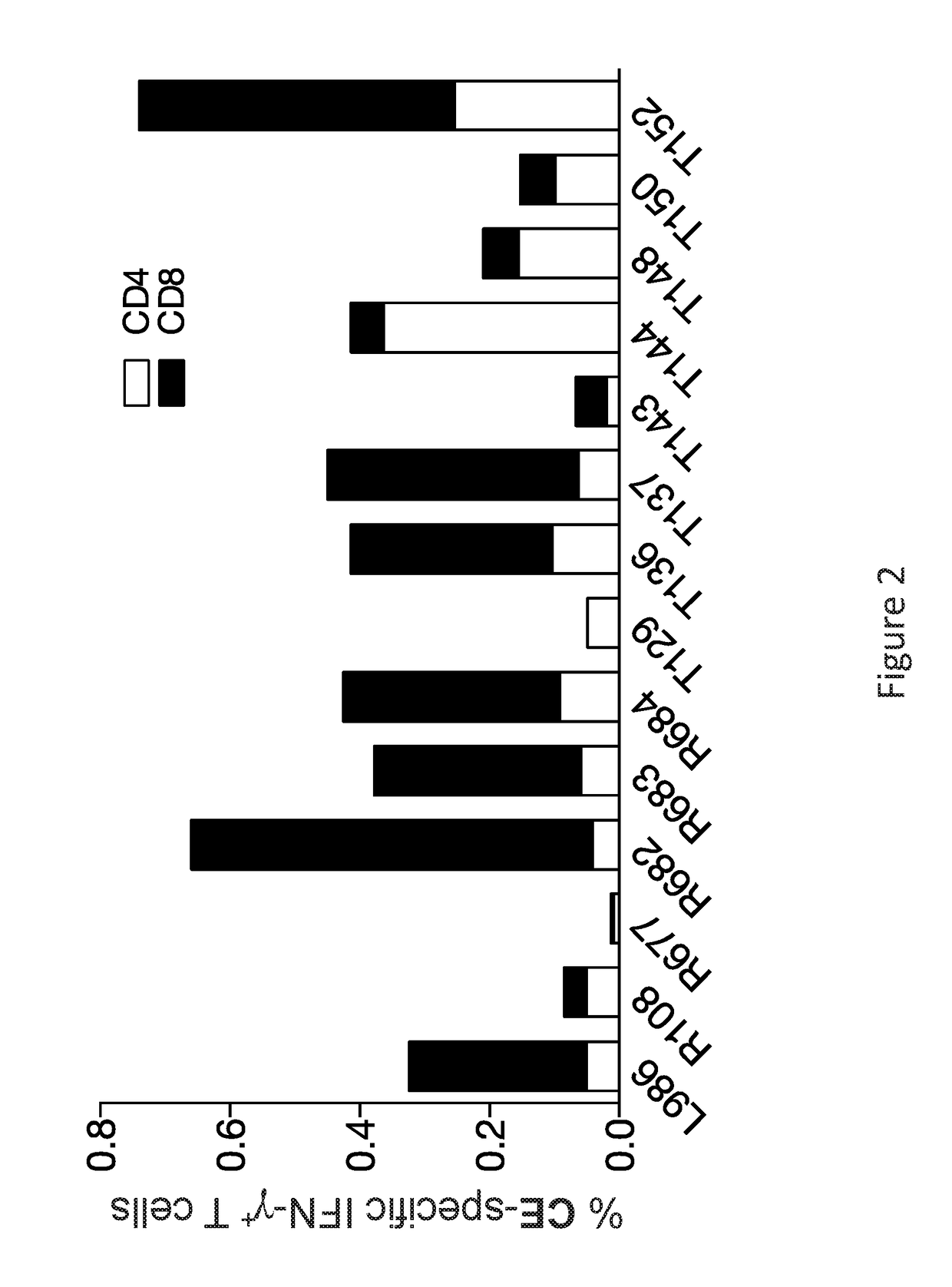
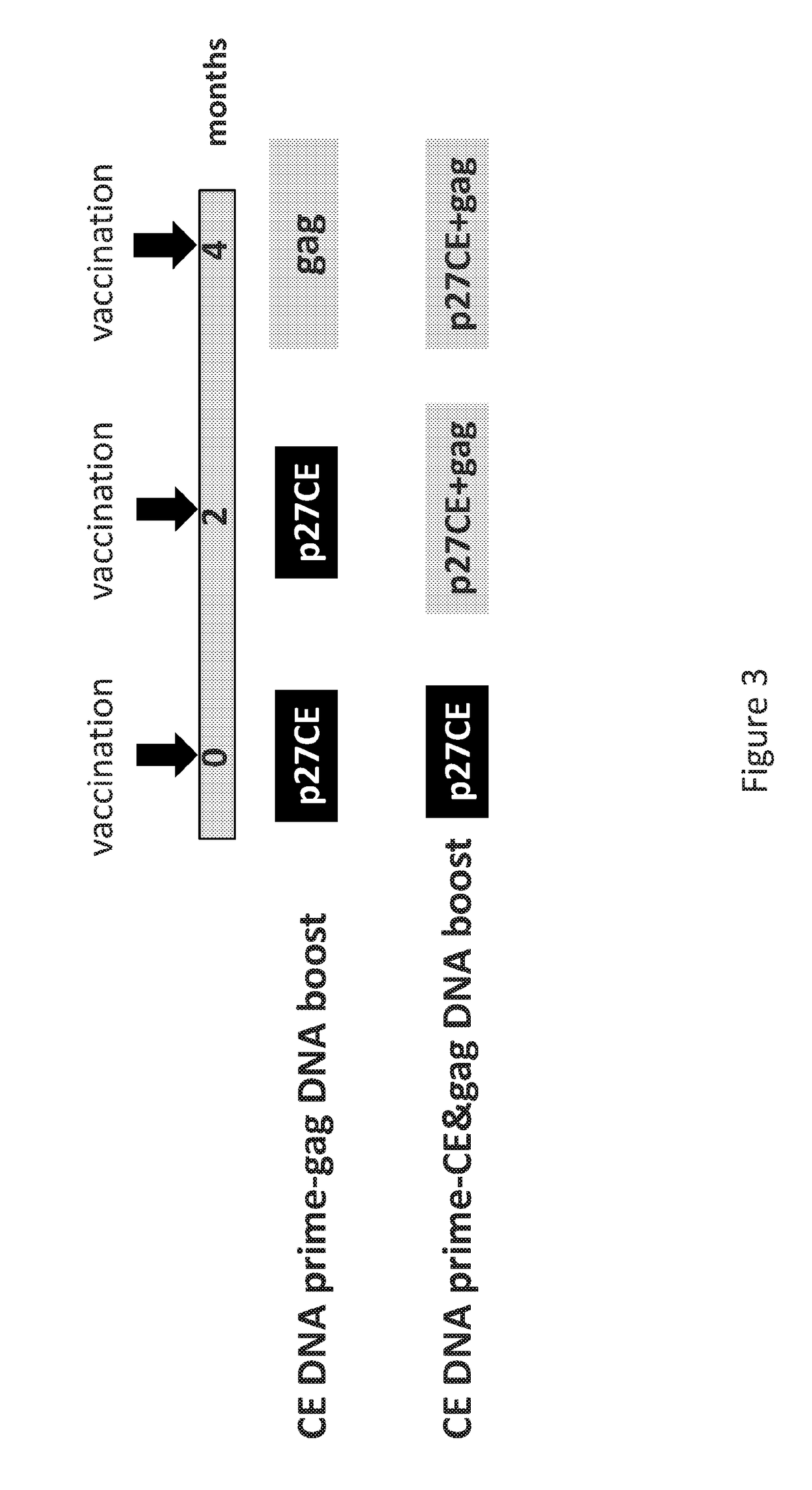
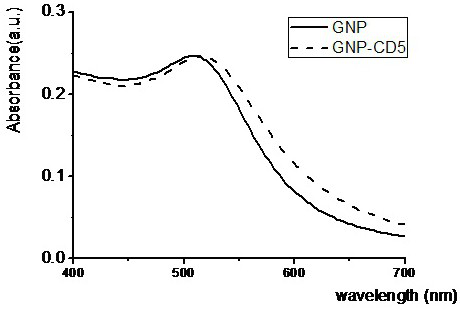
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "HIV envelope protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
General heading term for glycoproteins encoded by HIV envelope gene (env).
Immunogen comprising an HIV envelope protein, a ligand and H2 peptide
The invention relates to an immunogen comprising an HIV envelope protein bound to a ligand, which ligand upregulates at least one of the CD4 binding site and the CCR5 binding site on the protein, and bound to an HR-2 peptide. The invention also relates to a method of inducing anti-HIV antibodies using such an immunogen.
Owner:DUKE UNIV
Vaccine
InactiveUS7655235B2Reduce and prevent glycosylationGlycosylation can be reduced and preventedAntibody mimetics/scaffoldsVirus peptidesDNA vaccinationHeterologous
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative, which is non-glycosylated when expressed in a mammalian target cell, operably linked to a heterologous promoter. Preferably the HIV envelope molecule, such as gp120 or gp140 or gp160, lacks a functional secretion signal. It may be fused to additional HIV proteins such as Nef, Gag, RT or Tat.
Owner:GLAXO GRP LTD
Human immunodeficiency virus antigens, vectors, compositions, and methods of use thereof
ActiveUS20170165355A1Improved cell surface expressionImproved expression stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for generating immunogens that elicit neutralizing antibodies against fusion-active regions of HIV envelope proteins
The current invention relates to methods of generating immunogens that elicit broadly neutralizing antibodies which target regions of viral envelope proteins such as the gp 120 / gp41 complex of HIV-1. More specifically, the current invention involves using stabilizing peptides modeling the alpha-helical regions of the ectodomain of the HIV-1 transmembrane protein to stabilize fusion-active intermediate structures which can be used as vaccine immunogens.
Owner:PANACOS PHARMACEUTICALS INC
Vaccine
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative fused to an additional HIV protein selected from a non-structural protein or capsid protein or fragment or immunogenic derivative thereof. Preferably the HIV envelope molecule is gp120 and preferred fusions include one or more of HIV Nef, Gag, RT or Tat. Preferably the HIV envelope molecule is non-glycosylated in mammalian cells.
Owner:GLAXO GRP LTD
Fast detection device and method for HIV antibody in urine
The present invention provides device and method for fast detection of endogenous antibody, especially HIV envelope protein resisting antibody, in urine. Other pathogens, including known pathogen of sexually transmitted disease, capable of causing the raising of endogenous antibody may be also detected with the device and method of the present invention.
Owner:BEIJING KELIPUTE BIOPHARML TECH
Vaccine
InactiveUS20070042977A1Reduce and prevent glycosylationGlycosylation can be reduced and preventedAntibody mimetics/scaffoldsGenetic material ingredientsDNA vaccinationHeterologous
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative, which is non-glycosylated when expressed in a mammalian target cell, operably linked to a heterologous promoter. Preferably the HIV envelope molecule, such as gp120 or gp140 or gp160, lacks a functional secretion signal. It may be fused to additional HIV proteins such as Nef, Gag, RT or Tat.
Owner:GLAXO GROUP LTD
Synthetic human immunodeficiency virus (HIV) envelope antigen, vectors, and compositions thereof
ActiveUS10369214B2High expressionImprove stabilityViral antigen ingredientsVirus peptidesAntigenHiv envelope
Synthetic HIV envelope proteins, vectors and compositions thereof, and methods for inducing protective immunity against human immunodeficiency virus (HIV) infection are described. Viral expression vectors encoding the synthetic HIV envelope proteins can be used in vaccines to provide improved protective immunity against HIV.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for detecting ADCC (antibody-dependent cell-mediated cytotoxicity) activity of anti-HIV (human immunodeficiency virus) antibody
ActiveCN106800603AHigh sensitivityImprove featuresAntibody mimetics/scaffoldsMicrobiological testing/measurementHuman immunodeficiencyGenetic engineering
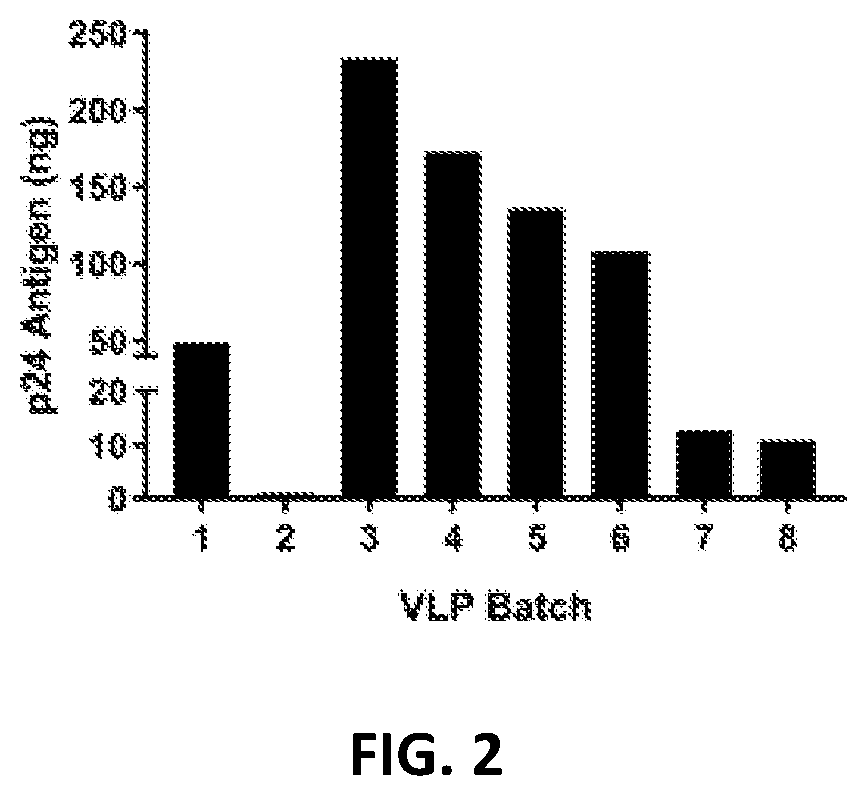
The invention relates to the fields of molecular virology and genetic engineering. In particular, the invention relates to an HIV (human immunodeficiency virus) envelope protein, a coding sequence thereof, and an HIV pseudovirus comprising the HIV envelope protein; the HIV pseudovirus can be used to detect ADCC (antibody-dependent cell-mediated cytotoxicity) activity of an anti-HIV antibody. The invention also relates to a kit for detecting the ADCC activity of the anti-HIV antibody. The invention further relates to a method for detecting the ADCC activity of the anti-HIV antibody by using the HIV pseudovirus, the method can achieve rapid, simple and high-throughput detection of the ADCC activity of the anti-HIV antibody, and thereby the method is of great significance for protection effect on analysis of vaccine immune response, and development and quality control of a new vaccine.
Owner:NAT INST FOR FOOD & DRUG CONTROL
HIV-Env gene DNA allosterism recombination envelope protein antigen for immunoresponsiveness of anti-HIV test and method
InactiveCN101002949AFighting Immunosuppressive StressAvoid infectionBiological testingIn-vivo testing preparationsHIV vaccineProtein antigen
A clinic human experiment of HIV envelope protein gp120 or gl160 antigen vaccine proves that it can not prevent the infection of HIV to human body, but can be used to research and screen the anti-HIV therapeutic vaccine and the vaccine for preventing the infection of HIV to human body.
Owner:叶新新
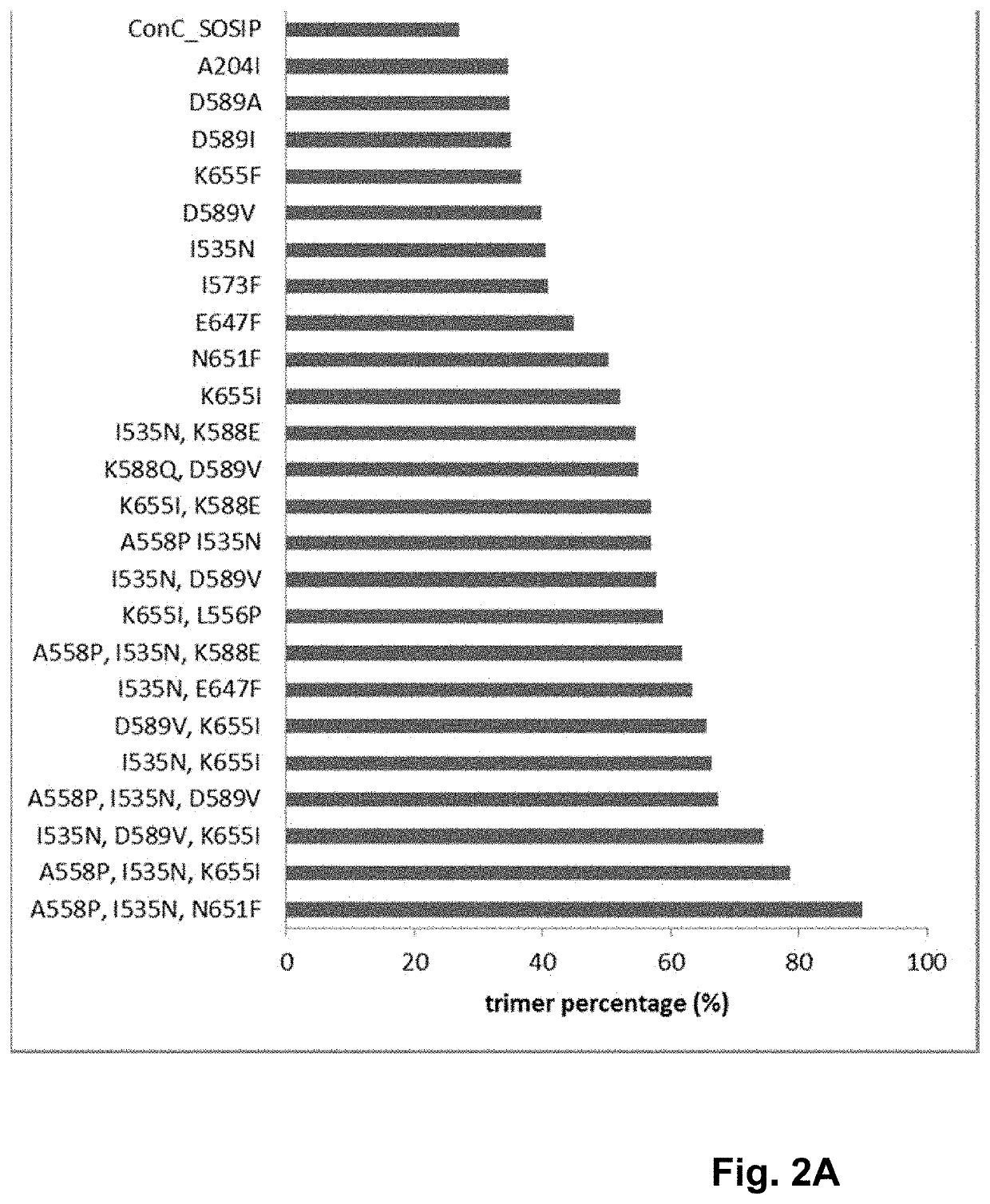
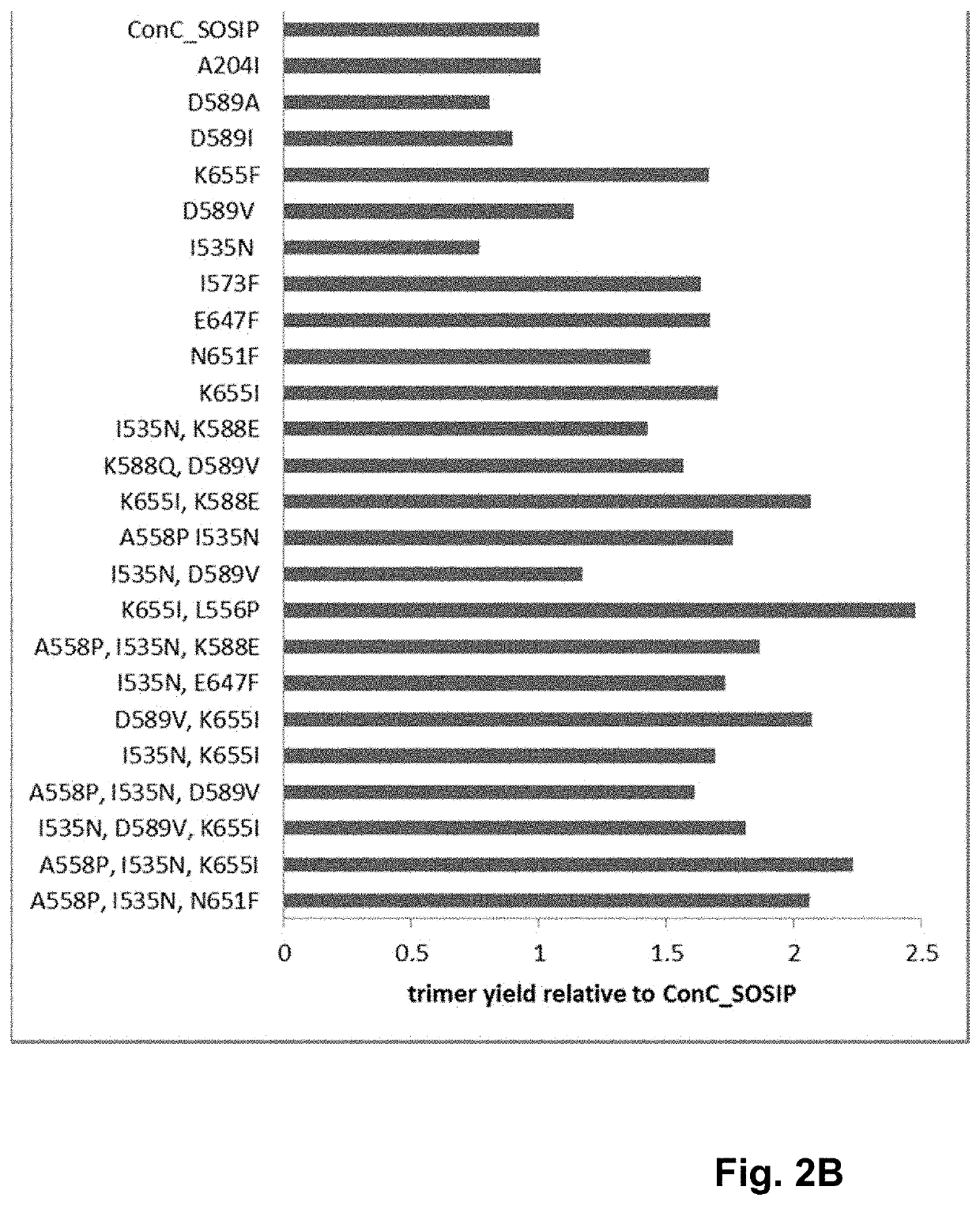
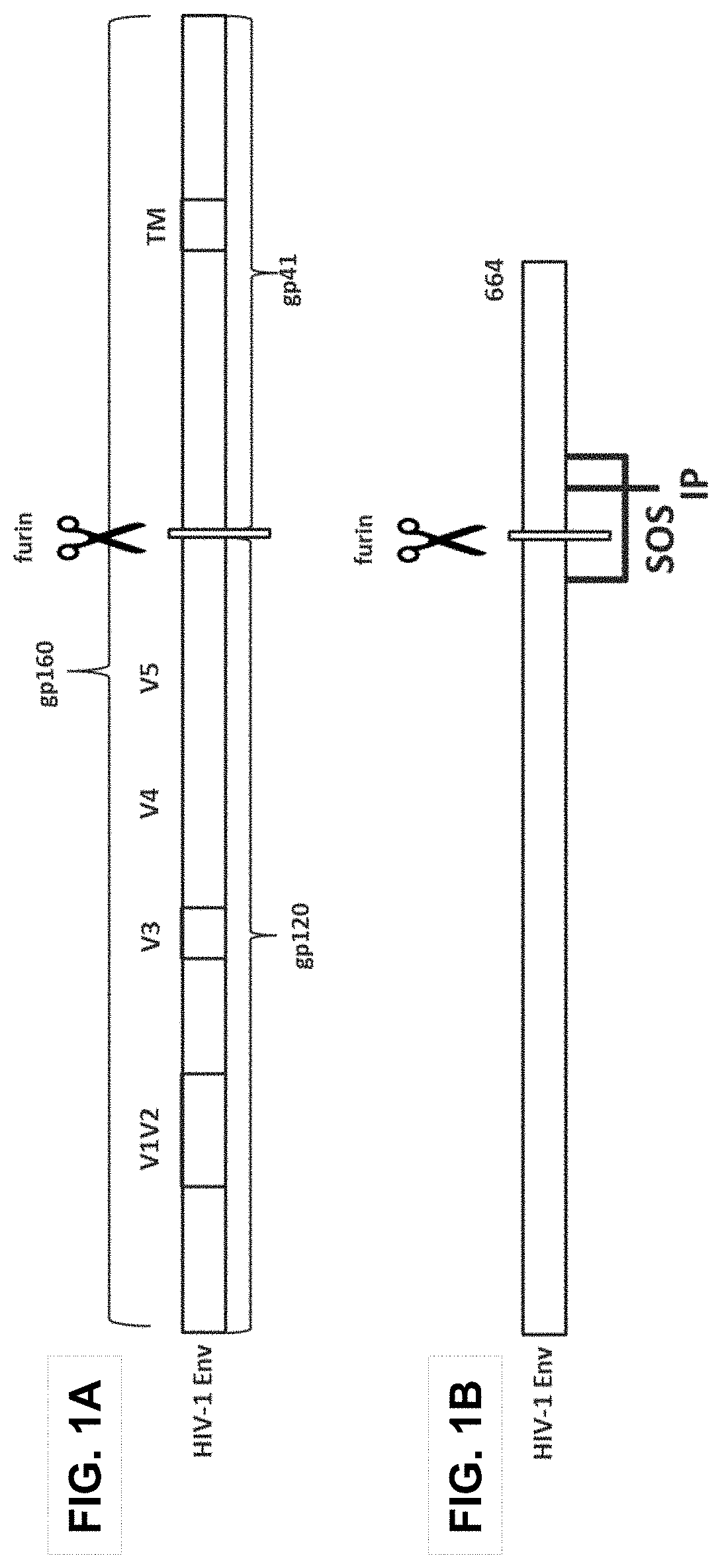
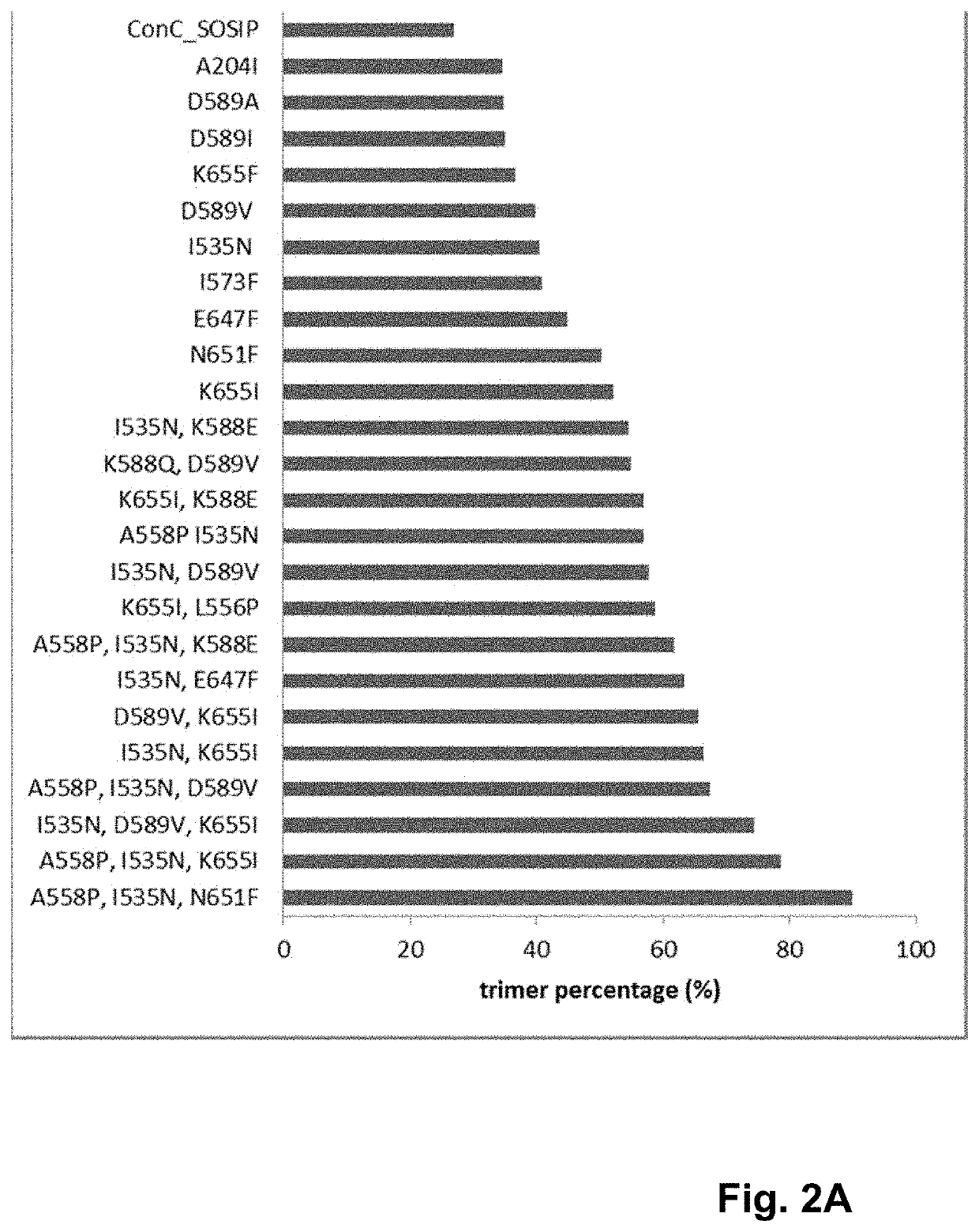
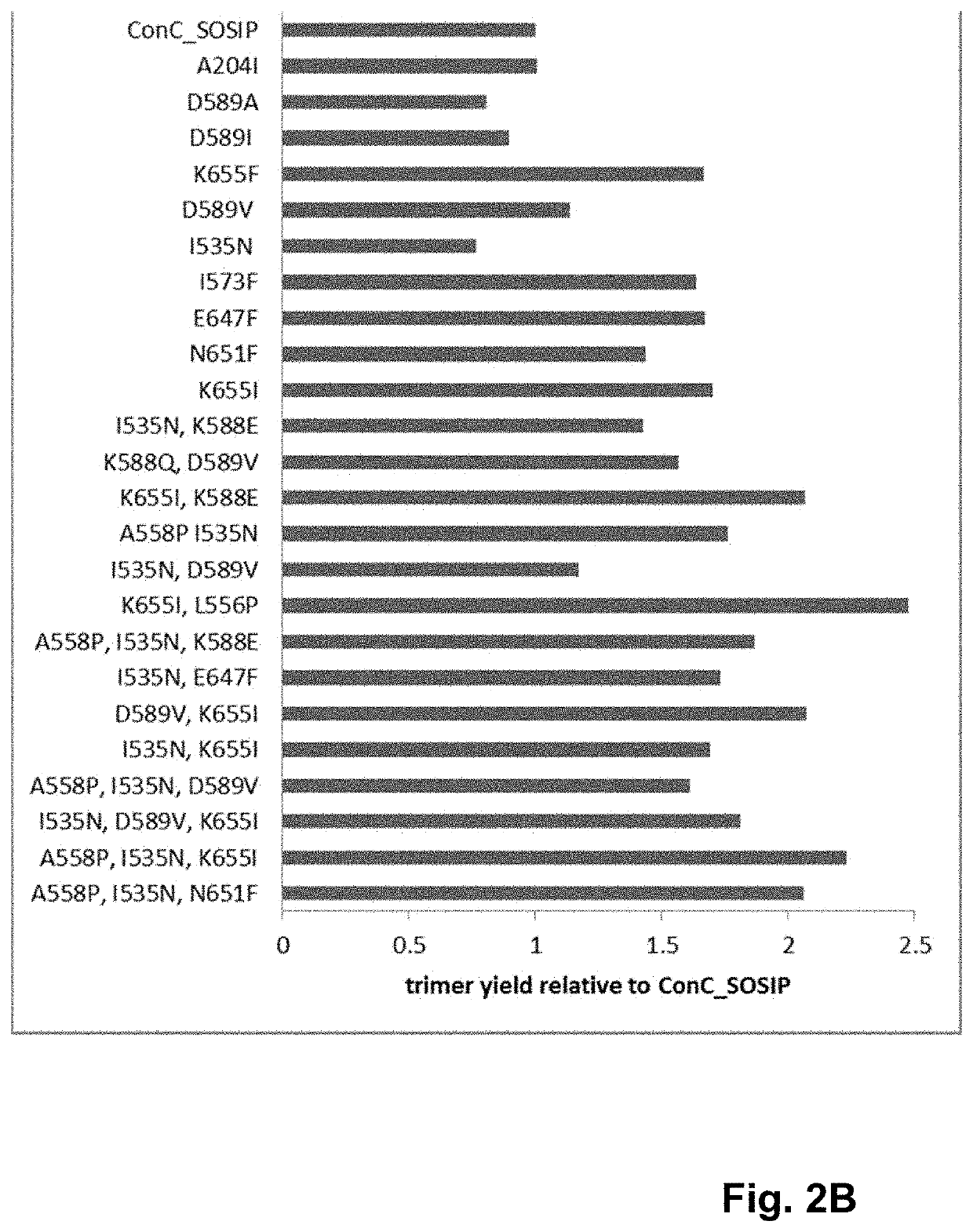
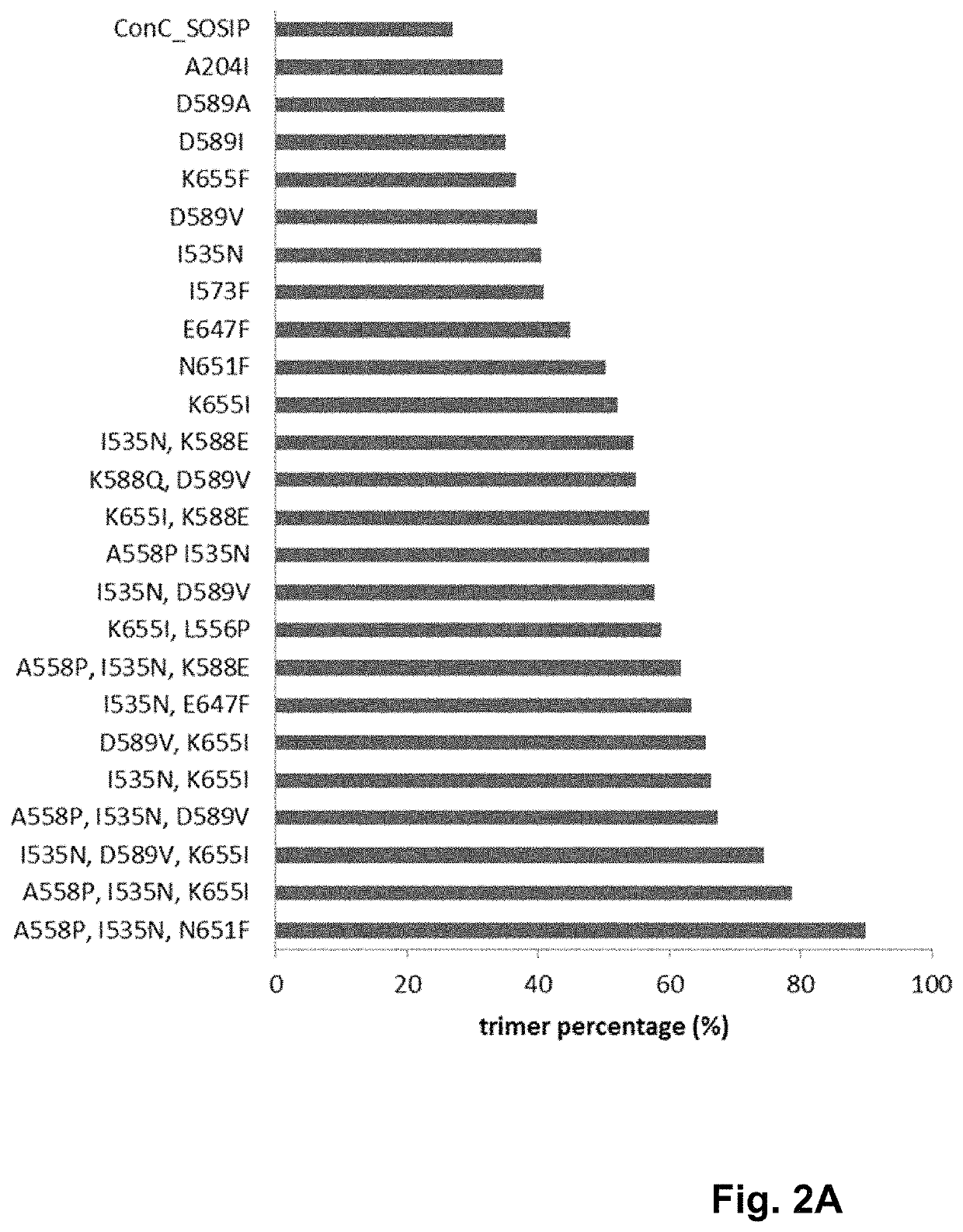
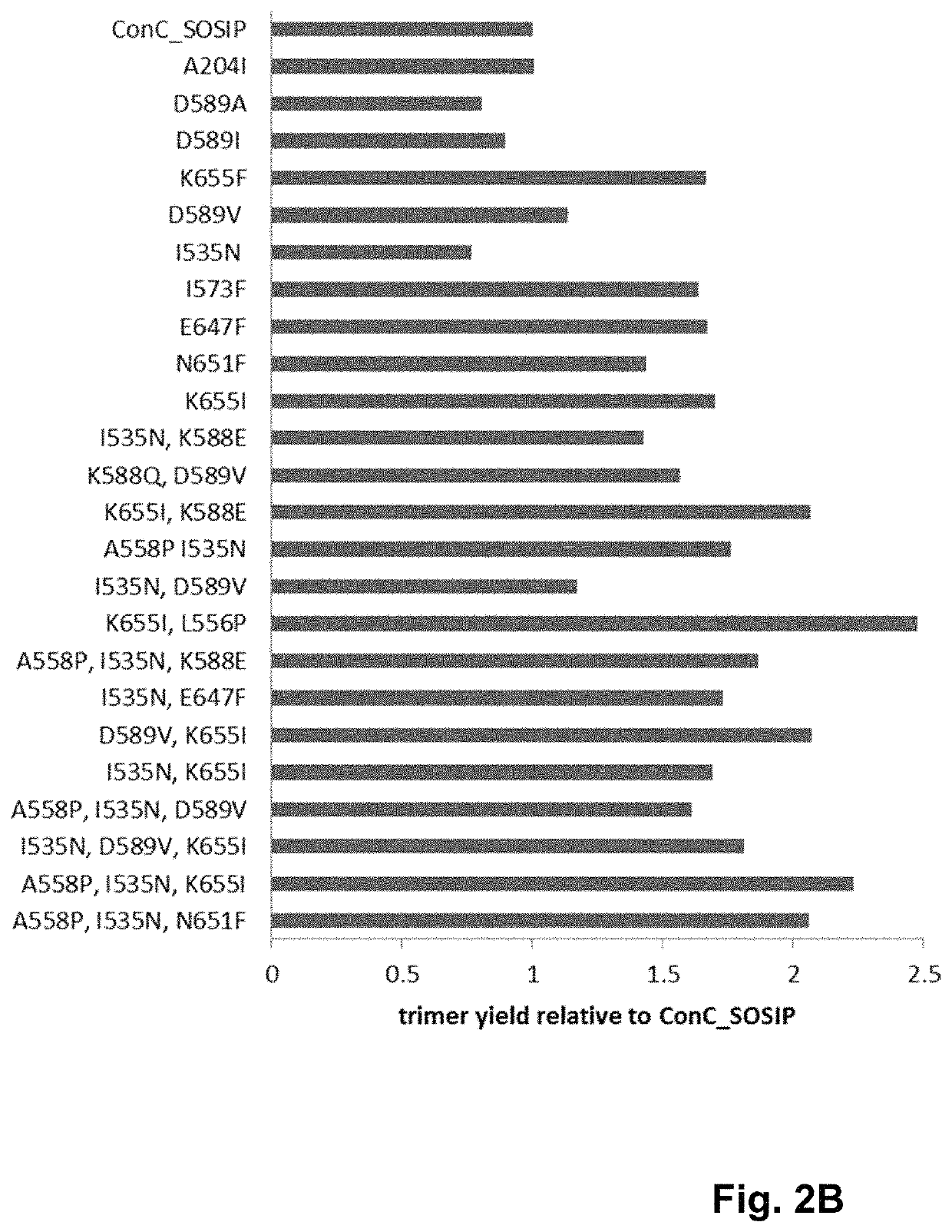
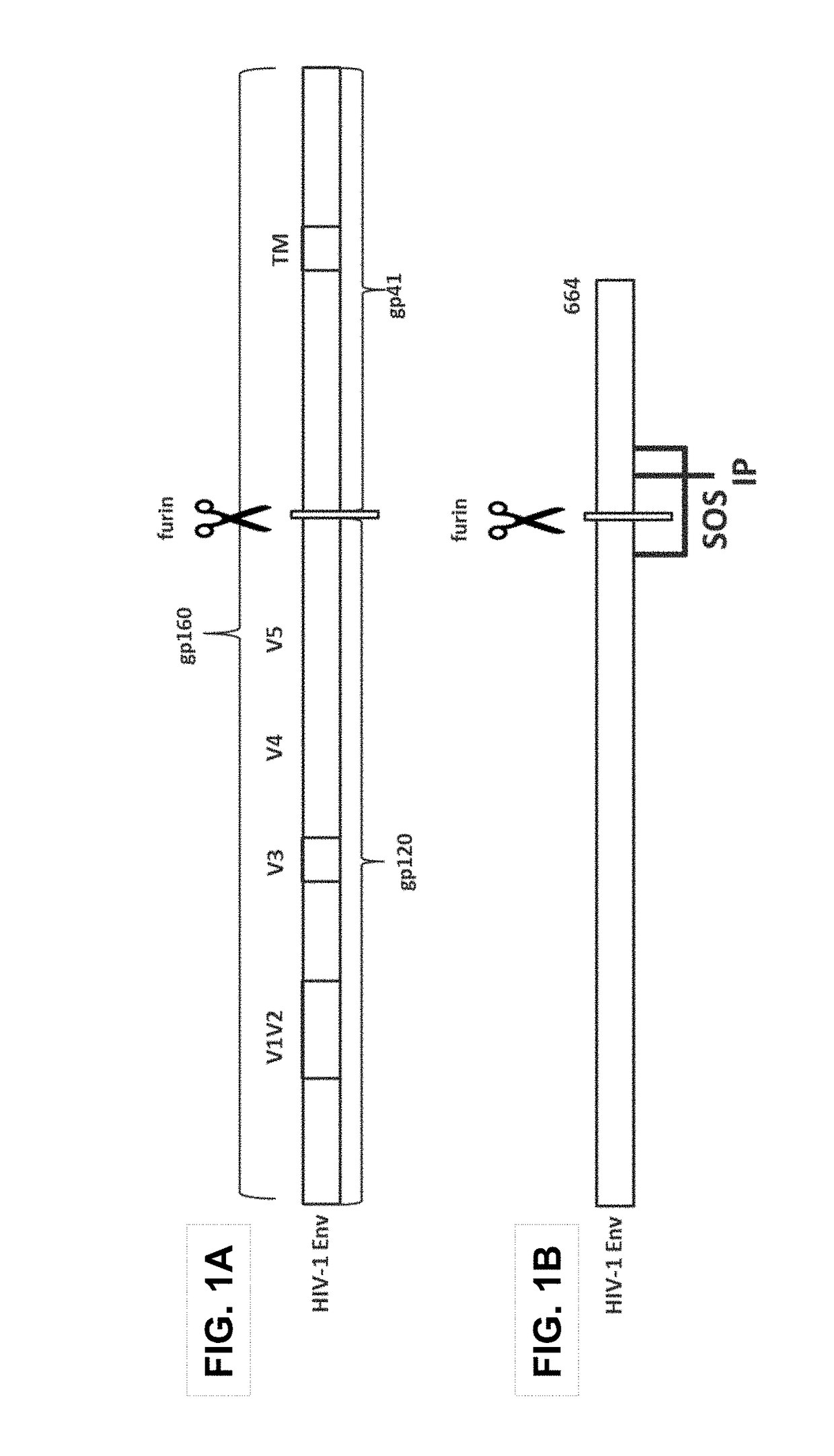
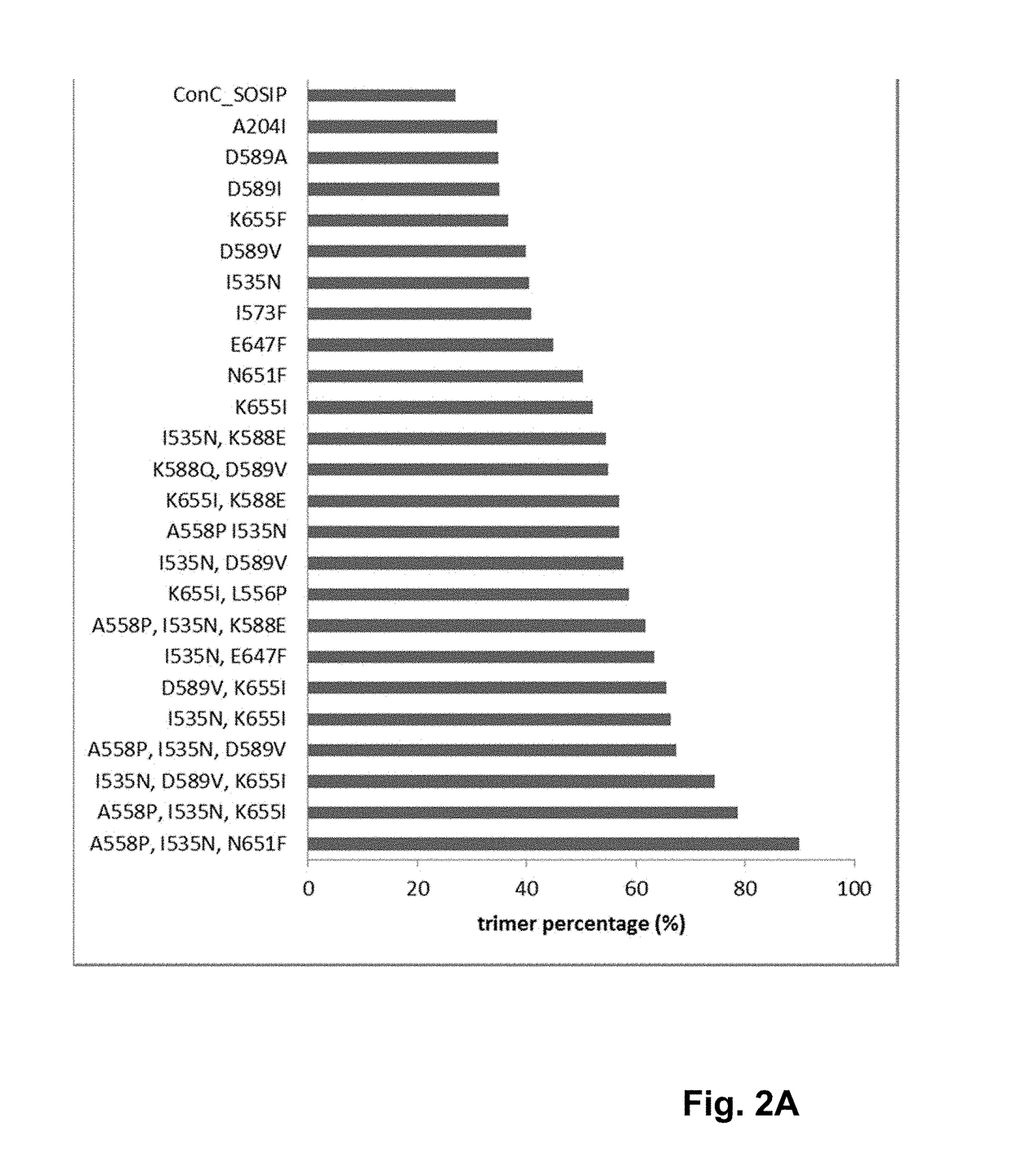
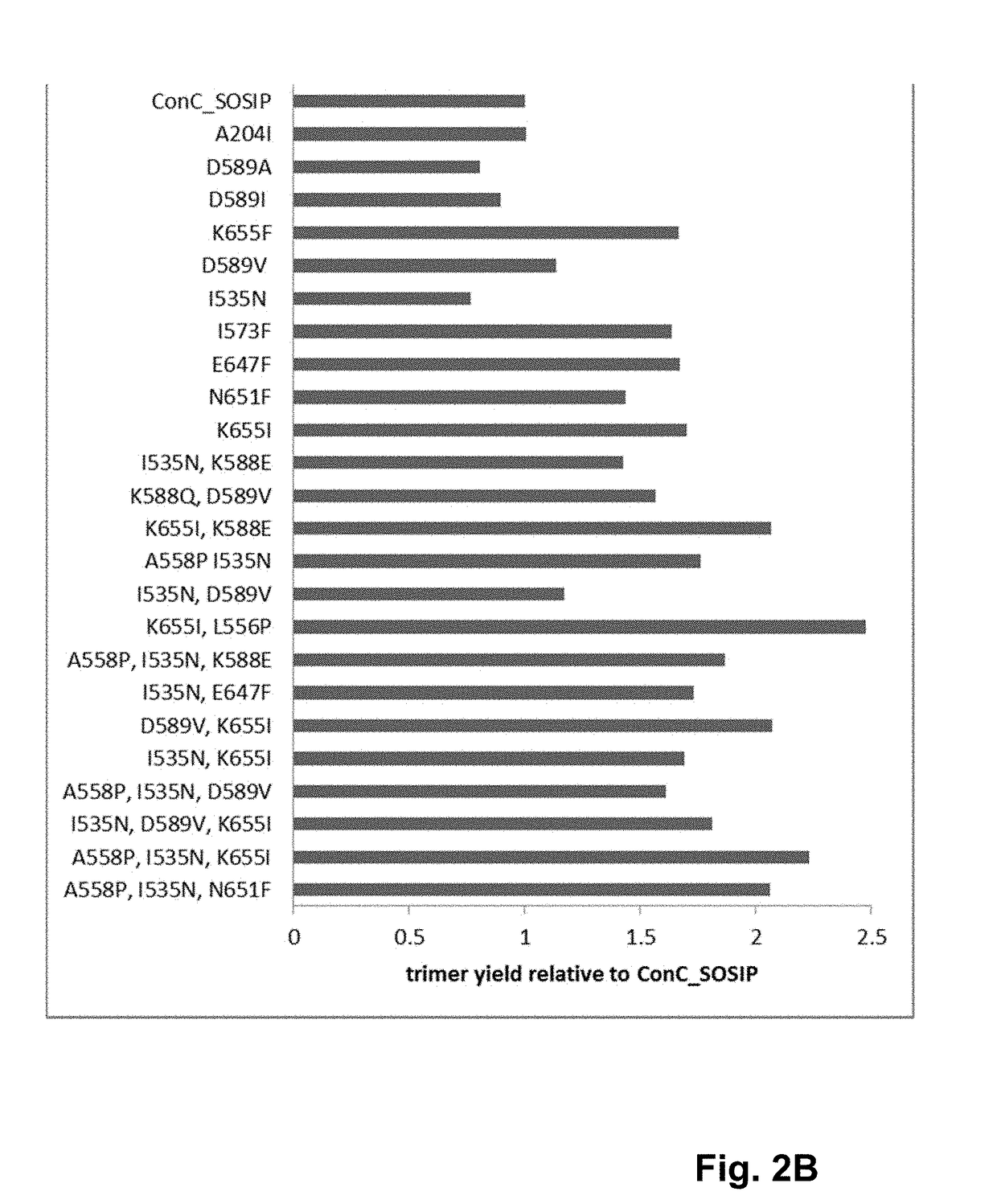
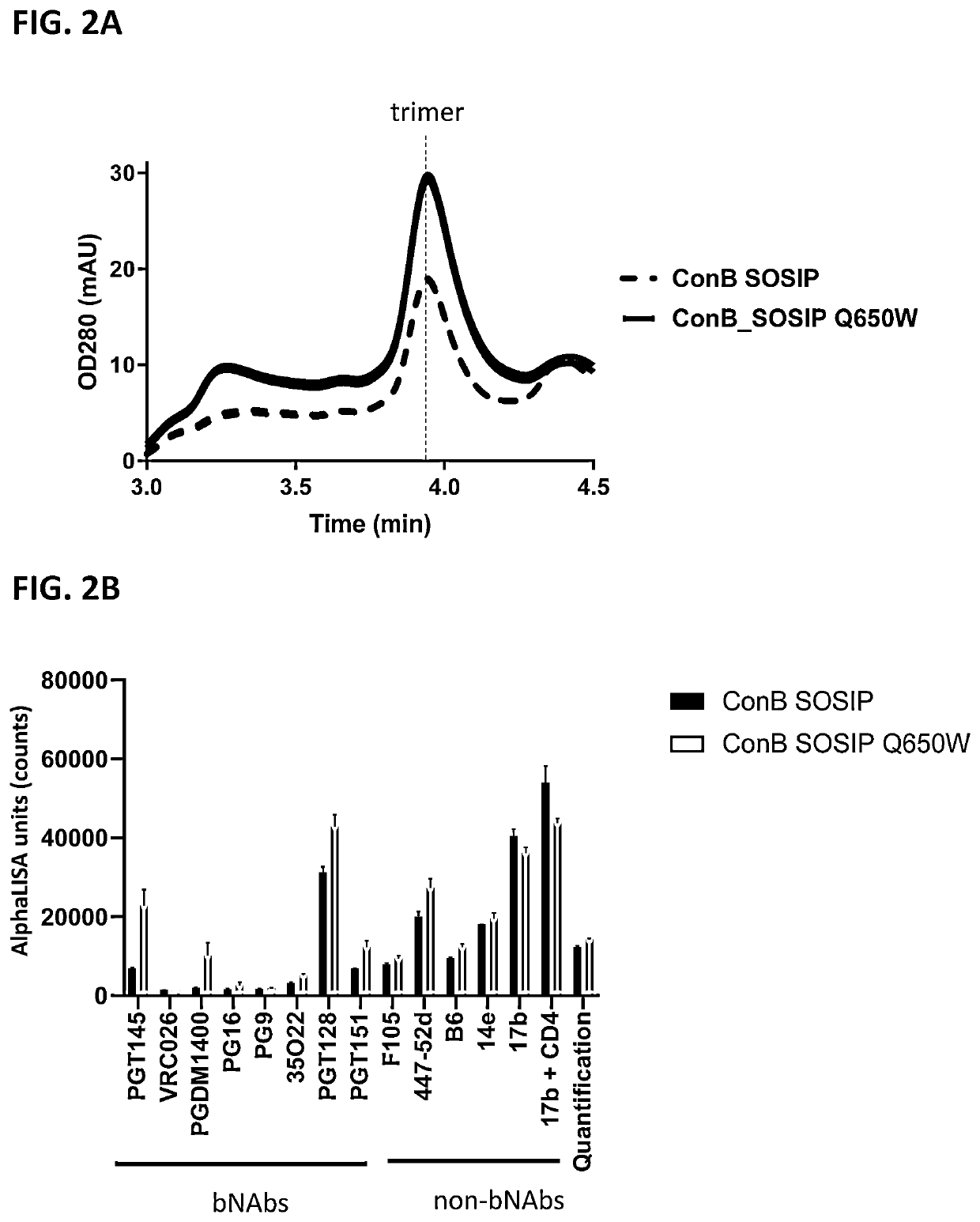
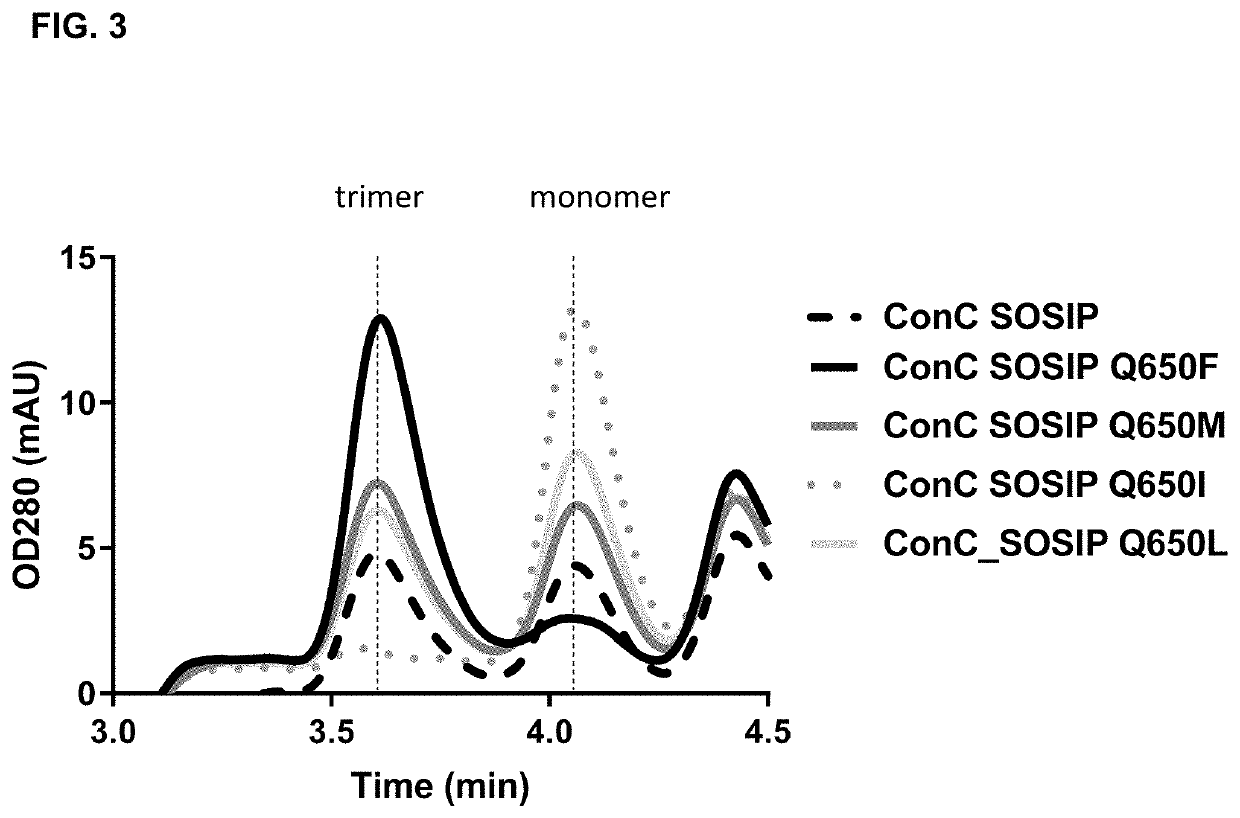
Trimer stabilizing HIV envelope protein mutations
ActiveUS10793607B2Optimize folding and stability of HIV-Increase opportunitiesViral antigen ingredientsVirus peptidesHiv envelopeAmino acid substitution
Human immunodeficiency virus (HIV) envelope proteins having mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins have certain amino acid substitutions at specified positions in the envelope protein sequence. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield as compared to an HIV envelope protein that does not have one or more of the indicated amino acid substitutions. Also provided are nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, nucleic acid, and vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Trimer stabilizing HIV envelope protein mutations
ActiveUS10968254B2Optimize folding and stability of HIV-Increase opportunitiesViral antigen ingredientsVirus peptidesHiv envelopeTrimer
Human immunodeficiency virus (HIV) envelope proteins having specified mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield. Also provided are particles displaying the HIV envelope proteins, nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, particles, nucleic acid, or vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Vaccine
InactiveUS20060142221A1Reduce removalReduce and prevent glycosylationAntibody mimetics/scaffoldsGenetic material ingredientsDNA vaccinationHIV Proteins
The invention relates to polynucleotides for DNA vaccination which polynucleotides encode an HIV envelope protein or fragment or immunogenic derivative fused to an additional HIV protein selected from a non-structural protein or capsid protein or fragment or immunogenic derivative thereof. Preferably the HIV envelope molecule is gp120 and preferred fusions include one or more of HIV Nef, Gag, RT or Tat. Preferably the HIV envelope molecule is non-glycosylated in mammalian cells.
Owner:GLAXO GROUP LTD
CD4-independent HIV envelope proteins as vaccines and therapeutics
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Trimer stabilizing HIV envelope protein mutations
ActiveUS11365222B2Optimize folding and stability of HIV-Increase opportunitiesViral antigen ingredientsVirus peptidesHiv envelopeAmino acid substitution
Human immunodeficiency virus (HIV) envelope proteins having mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins have certain amino acid substitutions at specified positions in the envelope protein sequence. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield as compared to an HIV envelope protein that does not have one or more of the indicated amino acid substitutions. Also provided are nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, nucleic acid, and vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Truncated HIV envelope proteins (ENV), methods and compositions related thereto
The instant application provides methods and related compositions pertaining to novel HIV envelope proteins. In some embodiments, the invention relates to methods and compositions for the preparation, production, and administration of isolated novel HIV envelope nucleic acid and protein sequences suitable, for example, as vaccines against HIV.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +2
Trimer stabilizing HIV envelope protein mutations
ActiveUS20190023744A1Increased formationReduce inductionViral antigen ingredientsVirus peptidesHiv envelopeTrimer
Human immunodeficiency virus (HIV) envelope proteins having specified mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield. Also provided are particles displaying the HIV envelope proteins, nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, particles, nucleic acid, or vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Protein Purification
PendingUS20210138060A1Reduce the presence of impuritiesViral antigen ingredientsVirus peptidesWAS PROTEINEngineering
Described herein is a process for protein purification, particularly a process for the purification of a glycoprotein, such as an HIV envelope protein, useful for vaccines or biotherapeutics.
Owner:JANSSEN VACCINES & PREVENTION BV
Trimer Stabilizing HIV Envelope Protein Mutation
PendingUS20220265813A1Increase opportunitiesReduce inductionViral antigen ingredientsVirus peptidesHiv envelopeTrimer
Human immunodeficiency virus (HIV) envelope proteins having specified mutations that stabilize the trimeric form of the envelope protein are provided. The HIV envelope proteins described herein have an improved percentage of trimer formation and / or an improved trimer yield. Also provided are particles displaying the HIV envelope proteins, nucleic acid molecules and vectors encoding the HIV envelope proteins, as well as compositions containing the HIV envelope proteins, particles, nucleic acid, or vectors.
Owner:JANSSEN VACCINES & PREVENTION BV
Pharmaceutical compositions comprising an hiv envelope protein and cd4
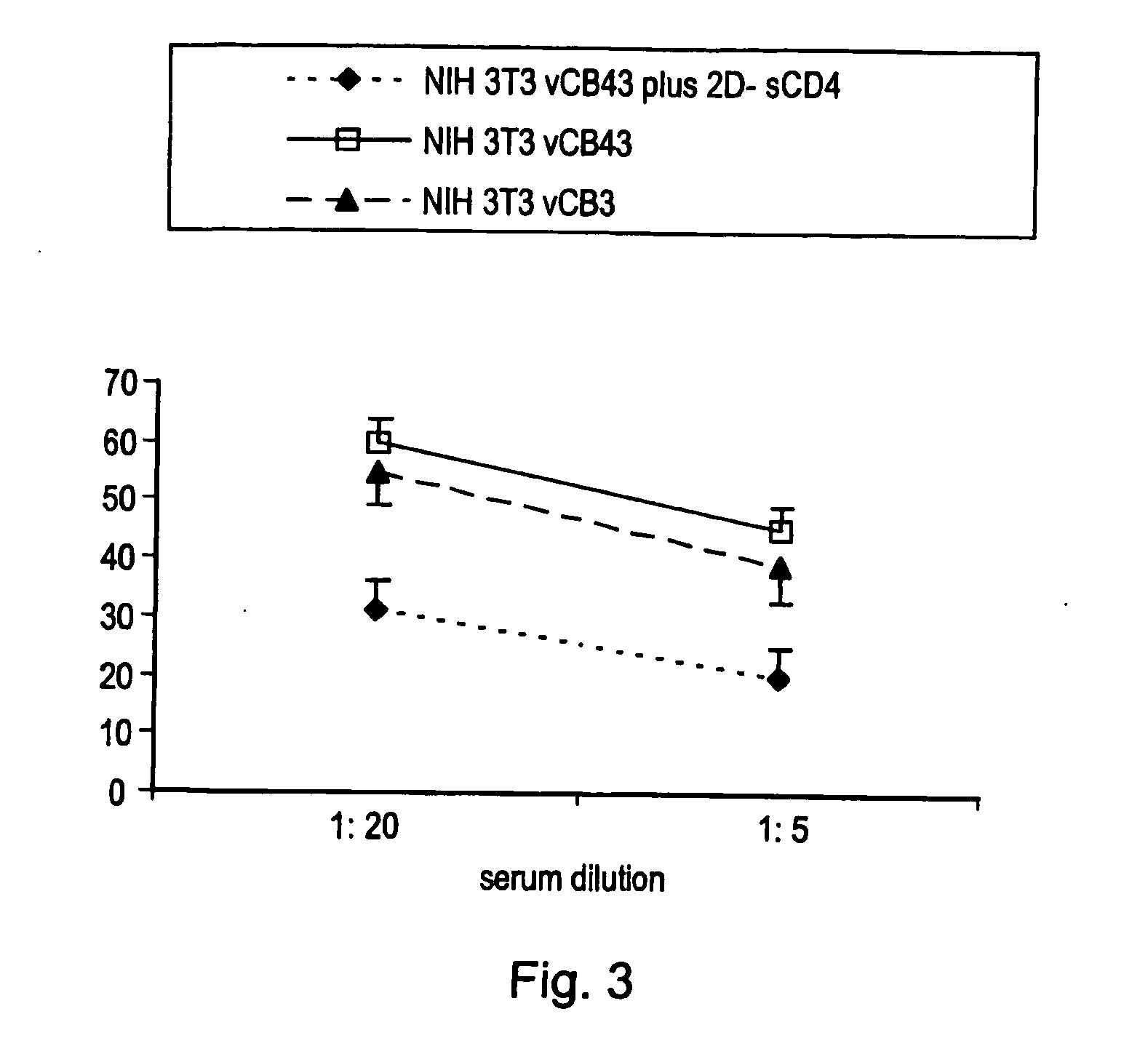
InactiveUS20060142219A1Easy to manageEffectively prevent murine collagen-induced arthritisVirusesPeptide/protein ingredientsEpitopeNucleotide
Pharmaceutical composition for treating / preventing HIV comprising (i) a polynucleotide encoding HIV envelope protein and (ii) a polynucleotide encoding CD4 receptor protein or; (i) a polynucleotide encoding HIV envelope protein and (ii) a CD4 receptor protein or; a fixed cell expressing an HIV envelope protein complexed with a CD4 receptor protein. Also disclosed are pharmaceutical compositions for treating / preventing HIV comprising an antibody immunospecific for a fixed cell expressing an HIV envelope protein complex with a CD4 receptor protein. The binding of the CD4 to the HIV envelope protein, i.e. gp120, exposes hidden epitopes that may be used as targets in immunotherapy; the presentation of the gp120 and CD4 in the present forms is said to overcome problems with prior art soluble gp120-CD4 complexes.
Owner:FOND CENT SAN RAFFAELE DEL MONTE TABOR
Protein purification
Described herein are methods for protein purification, in particular for purifying glycoproteins, such as HIV envelope proteins, useful in vaccines or biotherapeutics.
Owner:JANSSEN VACCINES & PREVENTION BV
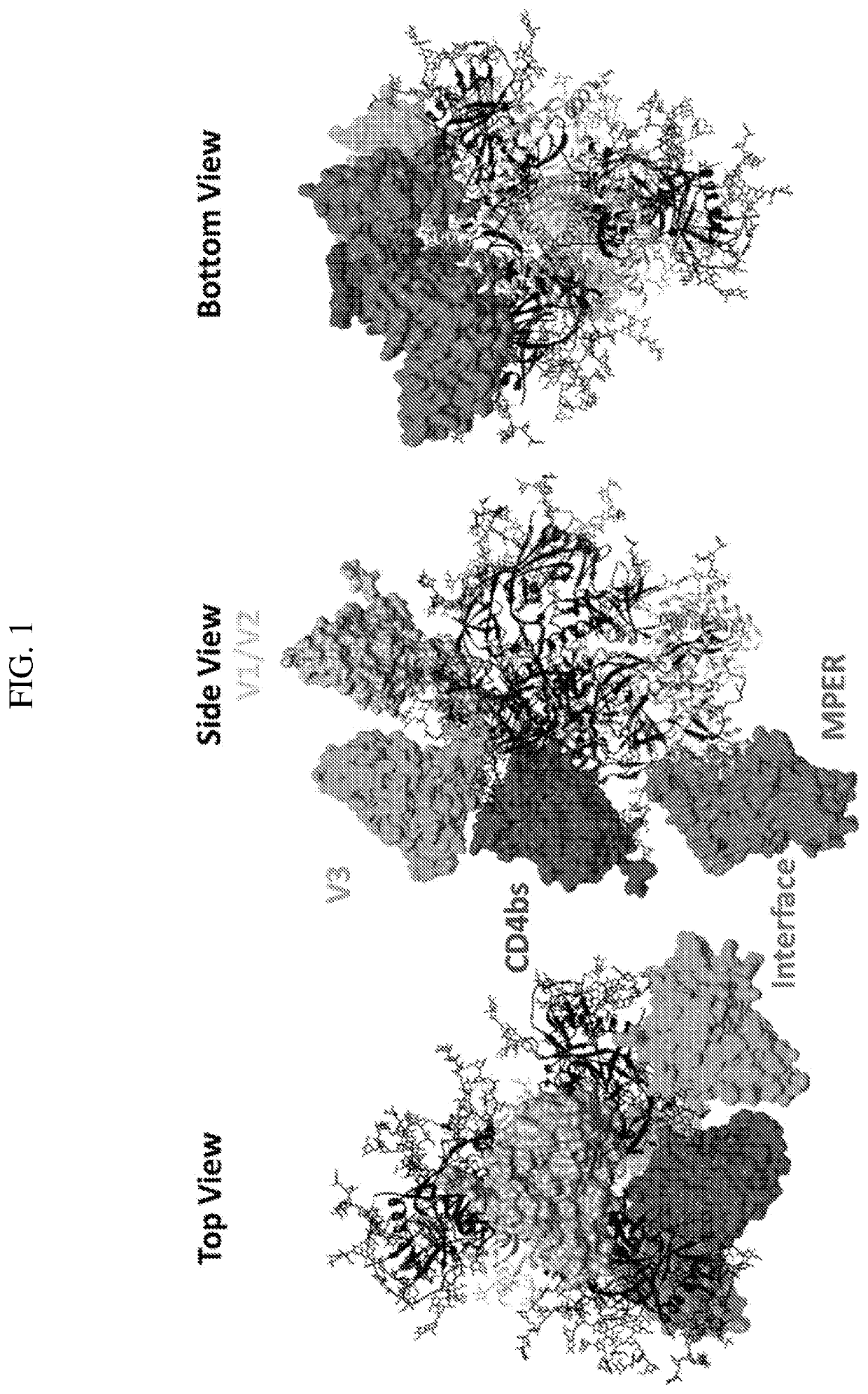
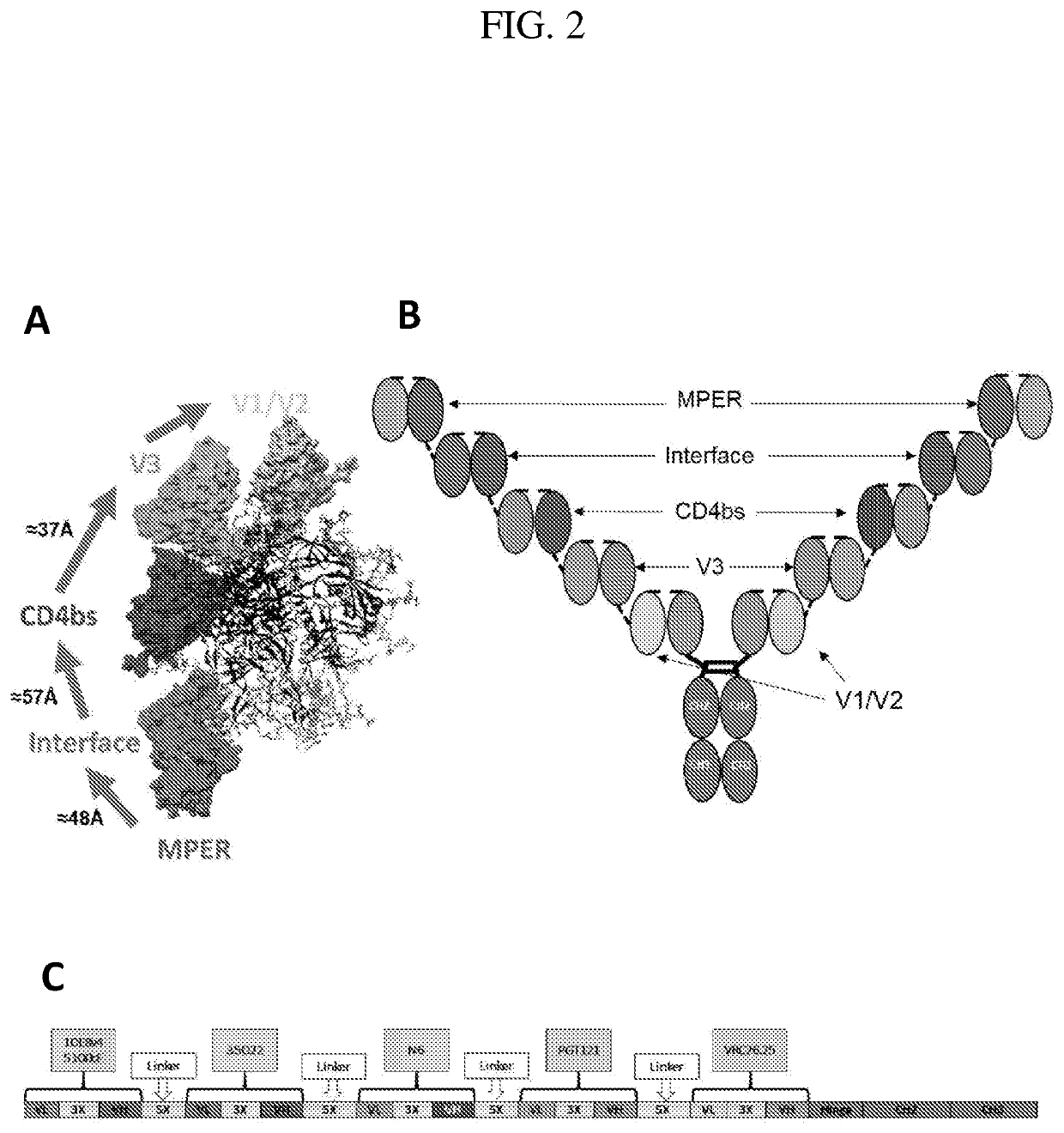
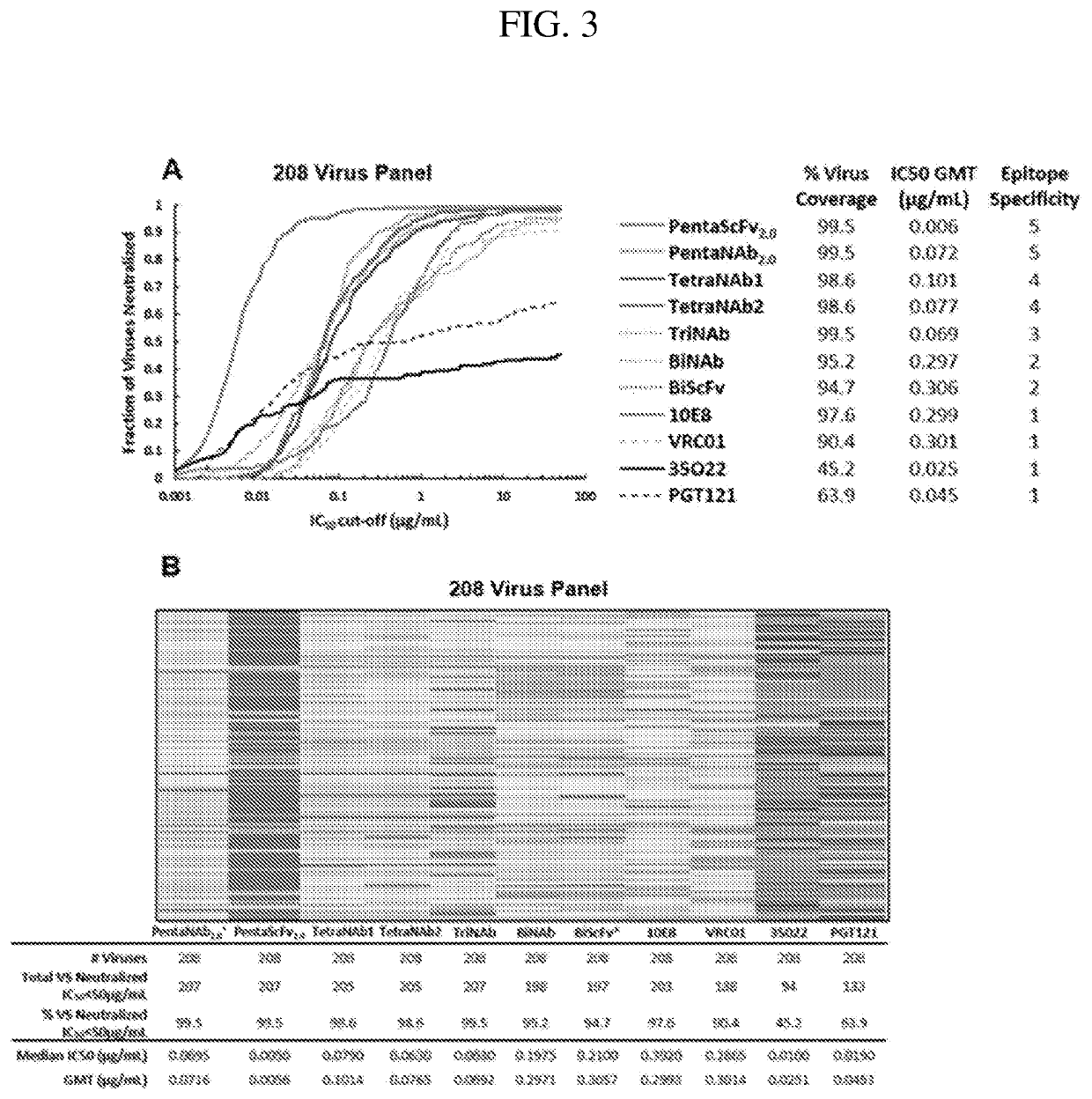
Multispecific Anti-HIV Antibodies
PendingUS20220227845A1Great avidityFunction increaseImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesBinding site
The present invention provides a multispecific anti-HIV antibody that binds to multiple epitopes on HIV envelope protein, wherein the antibody comprises: i. an amino acid sequence that binds to a V1 / V2 apex glycan epitope; ii. an amino acid sequence that binds to a V3-base glycan region epitope; iii. an amino acid sequence that binds to a CD4 binding site (CD4bs) epitope; iv. an amino acid sequence that binds to a gp120 / gp41 interface epitope; and v. an amino acid sequence that binds to a membrane proximal external region (MPER) epitope.
Owner:UNIV OF MARYLAND +2
Method for detecting adcc activity of anti-hiv antibody
ActiveCN106800603BHigh sensitivityImprove featuresAntibody mimetics/scaffoldsMicrobiological testing/measurementAntiendomysial antibodiesCytotoxicity
The invention relates to the fields of molecular virology and genetic engineering. In particular, the invention relates to an HIV (human immunodeficiency virus) envelope protein, a coding sequence thereof, and an HIV pseudovirus comprising the HIV envelope protein; the HIV pseudovirus can be used to detect ADCC (antibody-dependent cell-mediated cytotoxicity) activity of an anti-HIV antibody. The invention also relates to a kit for detecting the ADCC activity of the anti-HIV antibody. The invention further relates to a method for detecting the ADCC activity of the anti-HIV antibody by using the HIV pseudovirus, the method can achieve rapid, simple and high-throughput detection of the ADCC activity of the anti-HIV antibody, and thereby the method is of great significance for protection effect on analysis of vaccine immune response, and development and quality control of a new vaccine.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Hetero-oligomeric HIV envelope proteins
In one aspect, the invention provides an HIV envelope heterotrimer comprising at least two different Env glycoprotein monomers. In some heterotrimers, at least two Env glycoprotein monomers are from different HIV isolates, for example, different HIV-1 isolates. Heterotrimers may contain Env glycoprotein monomers from HIV isolates belonging to the same Glade or to different clades or both. At least one of the Env glycoprotein monomers in a heterotrimer of the invention may be modified in a way that alters its amino acid composition. In another aspect, the invention provides methods for inducing an immune response in a vertebrate host against HIV or an HIV-infected cell, comprising administering to a vertebrate host a prophylactically or therapeutically effective amount of a composition comprising an HIV envelope heterotrimer.
Owner:SEATTLE BIOMEDICAL RES INST
Methods and compositions for inducing an immune response using conserved element constructs
InactiveUS20180346520A1Enhance immune responseImprove the level ofPolypeptide with localisation/targeting motifVirus peptidesHiv envelopeNucleic acid
The invention provides methods and compositions for eliciting broad immune responses to HIV envelope proteins. The methods include use of nucleic acids constructs that encode conserved elements of HIV Env to induce immune responses.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
A peptide and gold nanobody targeting HIV envelope protein gp120
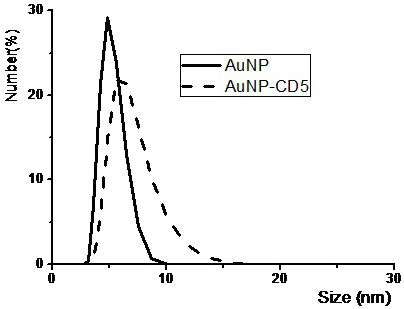
The invention relates to a polypeptide and a gold nanobody capable of targeting HIV envelope protein gp120. The artificial antibody is composed of gold nanoparticles and a designed polypeptide sequence. The prepared gold nanobody can enhance the binding strength of the drug to the target, that is, when the concentration of virus gp120 in the body is very low, it can still maintain a high binding strength with it. At the same time, gold nanoantibodies have the characteristics of small particle size, excellent dispersion, and good stability. They can reach places that ordinary drugs cannot reach, and can bind to viruses more strongly. Therefore, this artificial antibody has broad application prospects in the field of biomedicine. .
Owner:SHANGHAI UNIV
HIV-EnvHIV-Env gene DNA recombinant envelope protein antigen immune response experiment and method for resisting HIV
InactiveCN101210919AFighting Immunosuppressive StressImmunoglobulins against animals/humansVirus peptidesCell specificHuman body
The invention relates to an anti-HIV immune response apparatus of an HIV-Env DNA recombinant envelope protein antigen and a method thereof, belonging to life science and medicinal fields. The clinic experiment in human body prove that vaccines derived from HIV envelope protein gp120 or gp160 can not prevent HIV infection, because the HIV envelope protein gp120 or gp160 is a heterogeneous specific ligand that can specifically bind with CD4<+> cells-specific receptor CD4<+> receptor and induce immunological tolerance mechanism of human body but can not inducing immune response mechanism for effectively eliminating HIV. The invention can be used for searching a method for solving immunological tolerance mechanism of human body, developing an anti-HIV vaccine derived from HIV-Env DNA recombinant envelope protein antigen for preventing and treating HIV that does not induce immunological tolerance mechanism of human body, and providing sufficient scientific reference for direct animal model experiments and direct clinical human trials.
Owner:叶新新
Virus-like particles and methods of use thereof
PendingUS20220194990A1Virus peptidesReverse transcribing RNA virusesReverse transcriptaseVirus-like particle
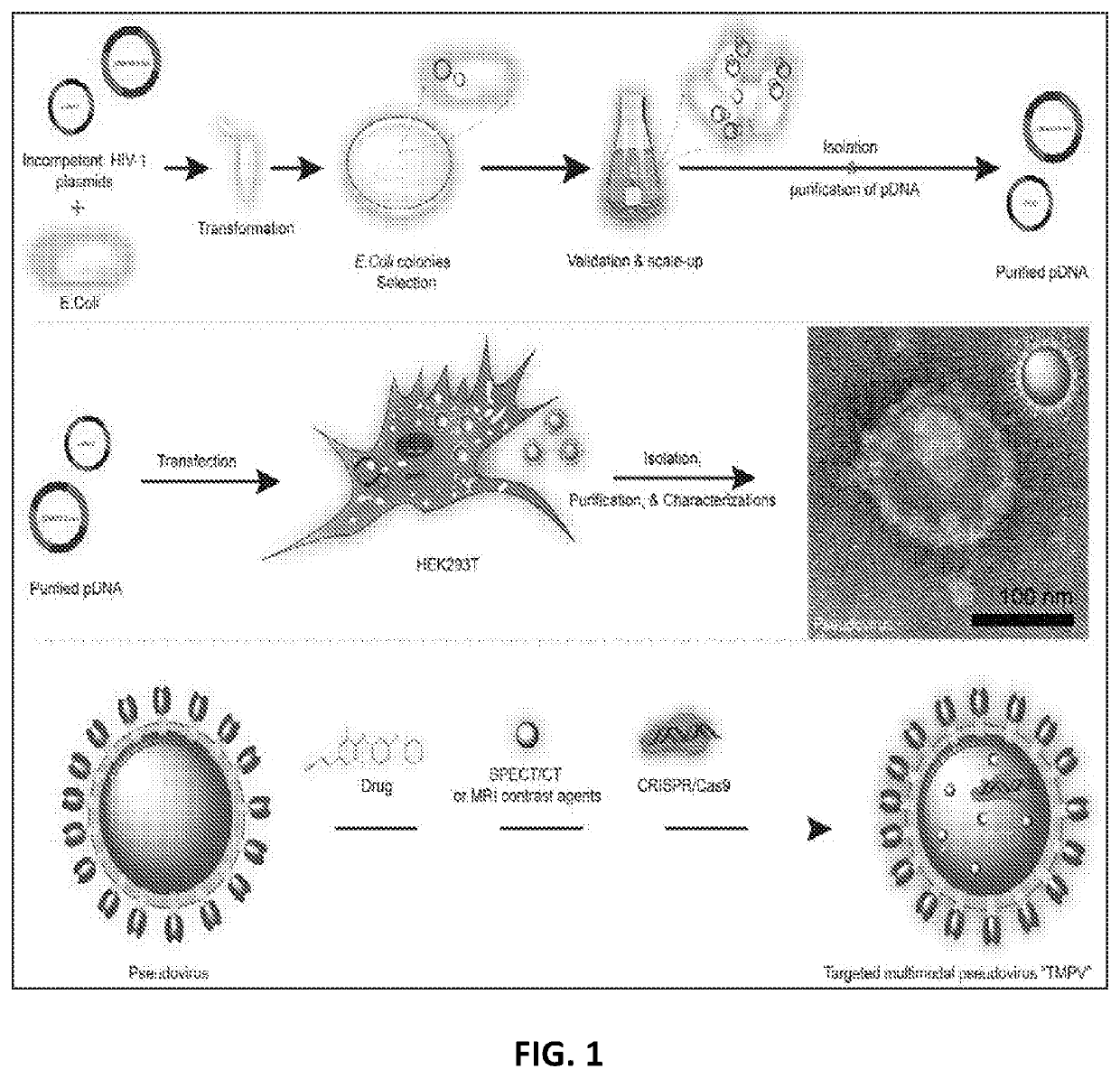
The present invention provides virus-like particles and methods of manufacture and use thereof. In accordance with the instant invention, virus-like particles (VLPs), particularly human immunodeficiency virus (HIV) VLPs, are provided. The HIV VLPs comprise at least one HIV structural protein and the HIV envelope protein, but lacks the HIV genome and lacks functional reverse transcriptase and integrase.
Owner:BOARD OF RGT UNIV OF NEBRASKA
Peptide having an affinity for gp120
The peptide in this invention is a peptide having affinity to gp120 represented by Formula (1): H-A1-A2-A3-A4-A5-R(SEQ ID No. 1)(in the formula,H means hydrogen,A1 is aspartic acid, lysine, valine, glutamic acid, glycine, asparagine, or tyrosine residue,A2 is valine, aspartic acid, tryptophan, lysine, phenylalanine, isoleucine,leucine, or tyrosine residue,A3 is lysine, valine, aspartic acid, arginine, alanine, or tryptophan residue,A4 is alanine, tryptophan, or glycine residue,A5 is glycine, alanine, valine, leucine, isoleucine, serine, threonine, methionine, asparagine, glutamine, histidine, lysine, arginine, phenylalanine, tryptophan, proline, or tyrosine residue,R is OH derived from carboxyl group or NH2 derived from acid amide group).The above peptide has an affinity to gp120 of the HIV envelope protein and is superior in stability.
Owner:ASPION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com