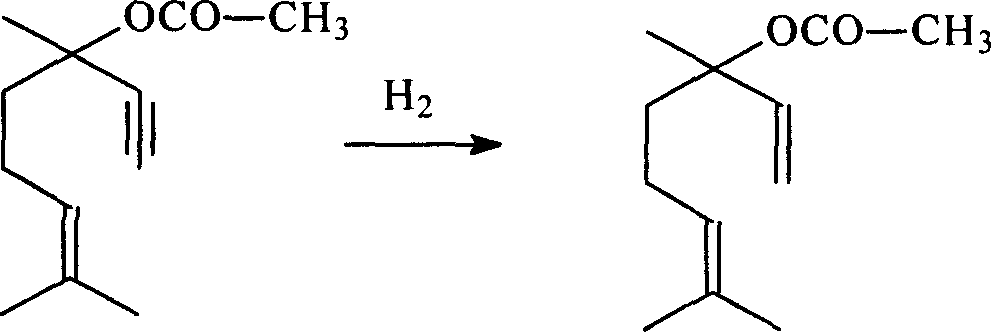
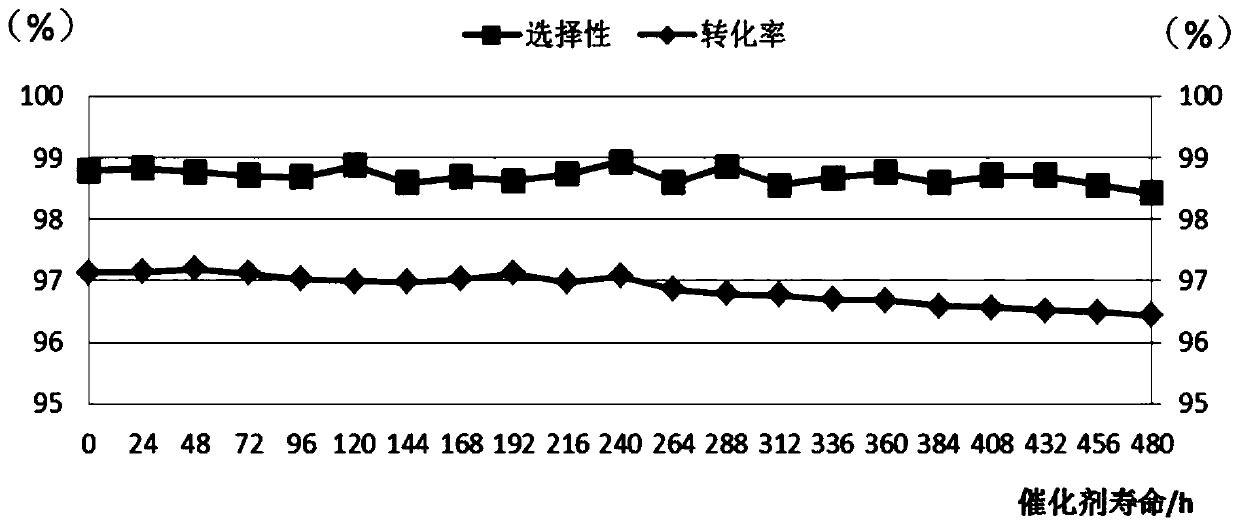
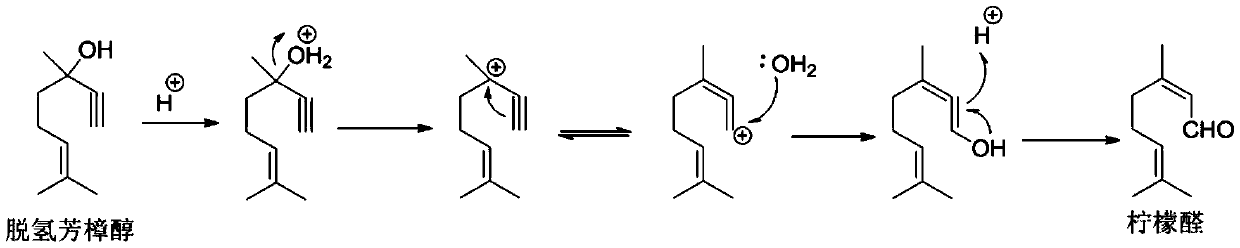
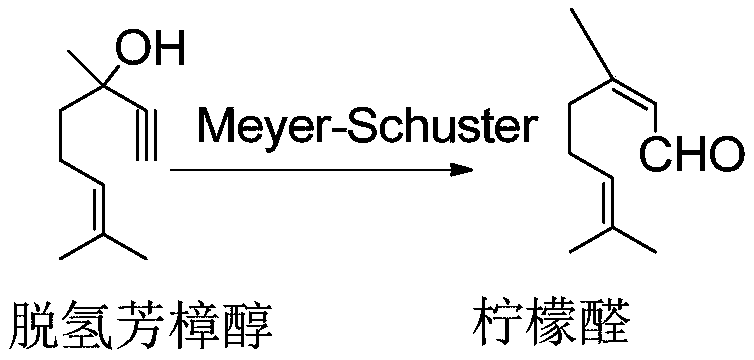
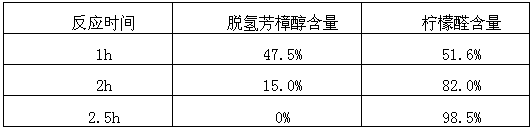
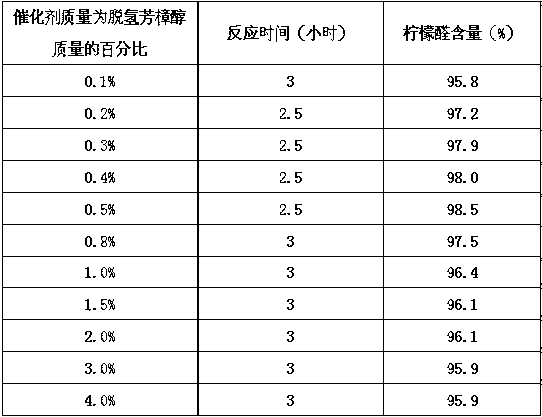
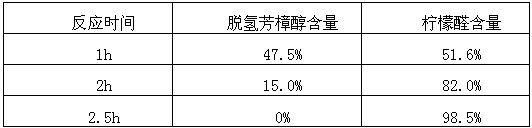
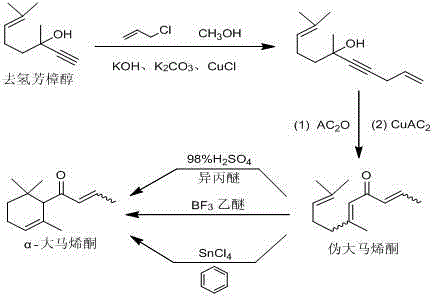
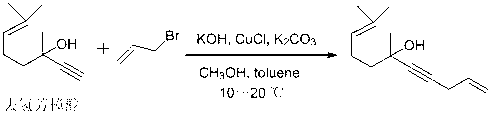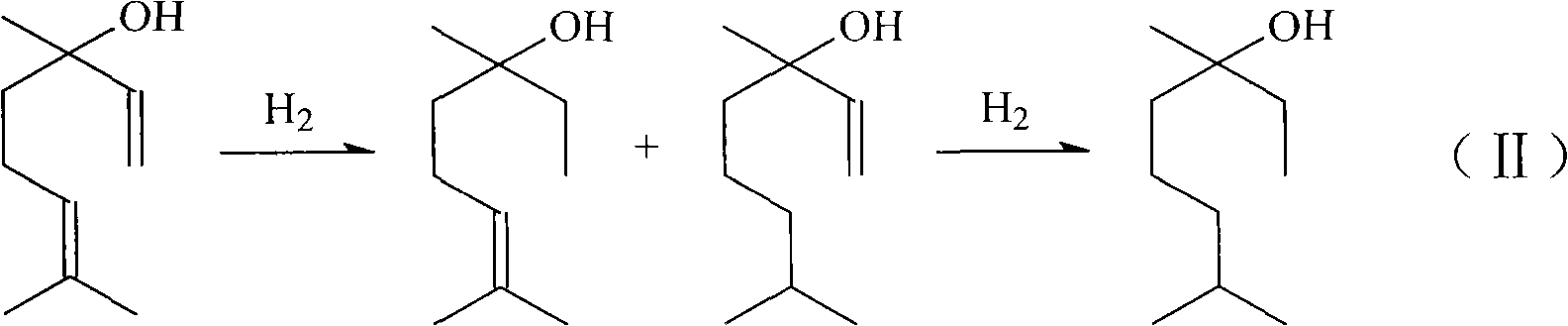Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Dehydrolinalool" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
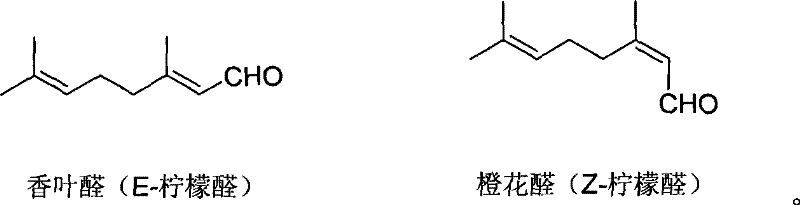
Process for preparing citral
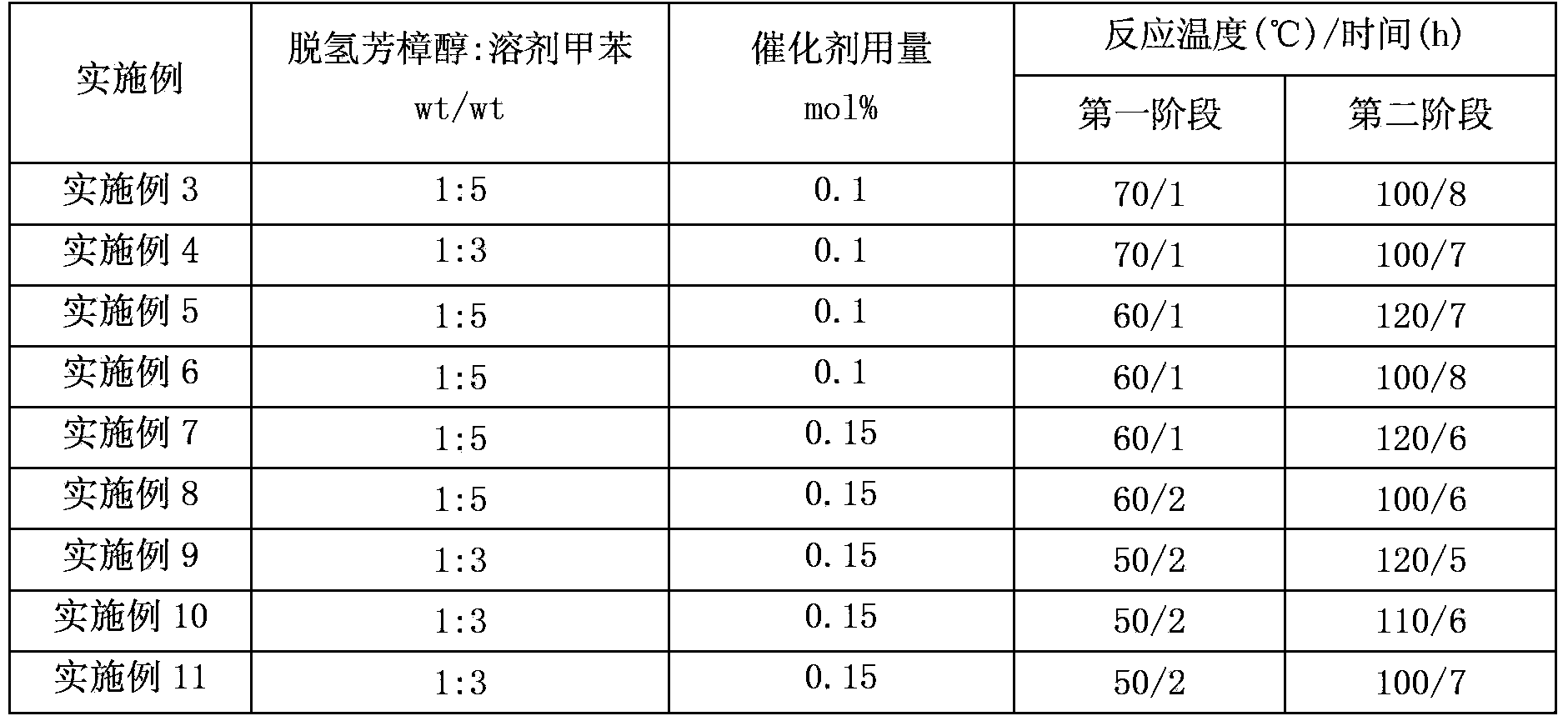
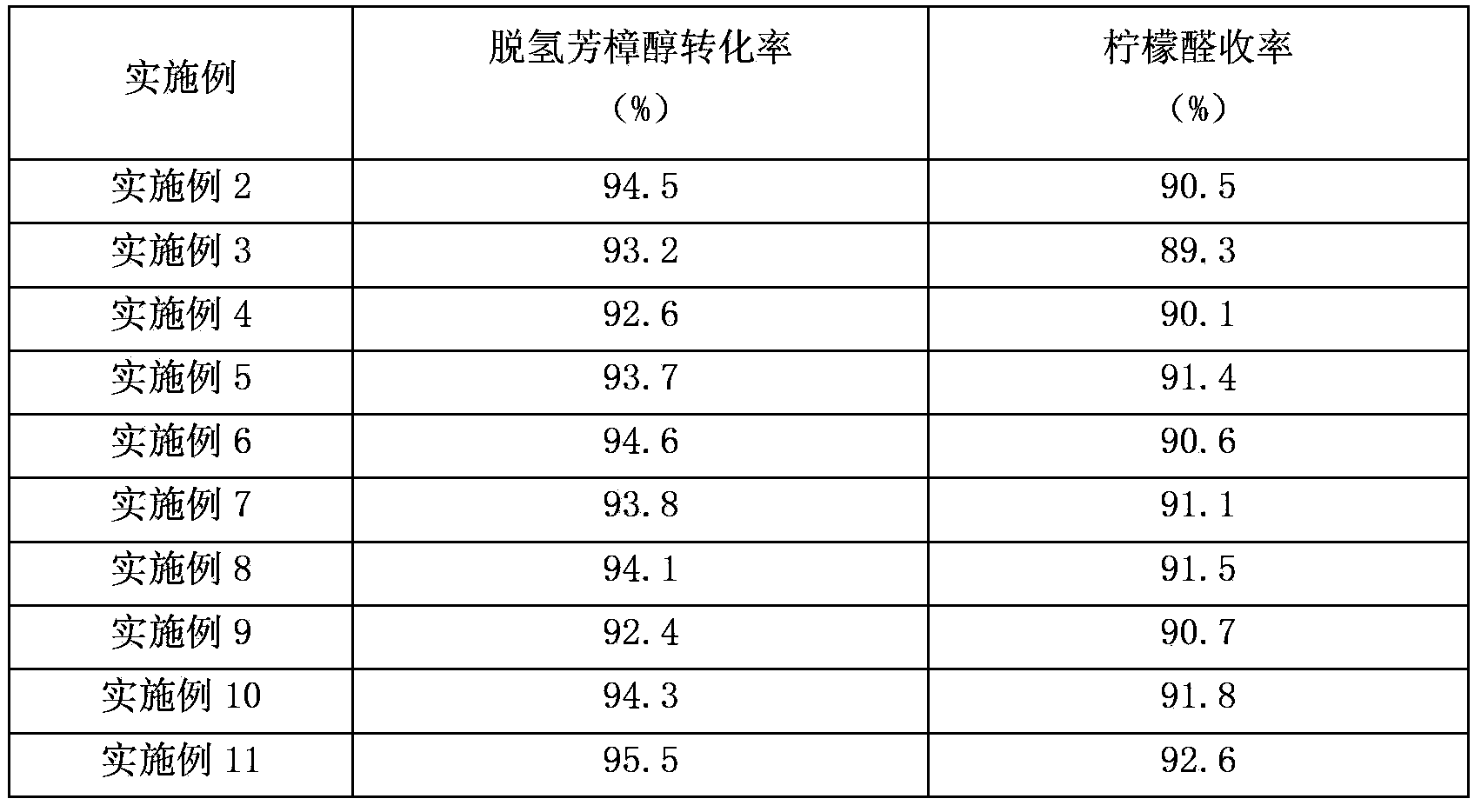
ActiveCN101391942AReduce pollutionMild reaction conditionsOrganic compound preparationCarbonyl compound preparationCatalytic reformingSolvent
The invention discloses a preparing method of citral. The existing method has high production cost or low yield, thus being difficult to be suitable for scale production. The method adopts dehydrogenated linalool as a raw material which is carried out with catalytic reforming to obtain the citral under the existence of a solvent and a latent solvent and is characterized in that: a catalyst adopted for reaction is molybdenum dioxide diacetyl acetone acid ester, the catalytic reforming is carried out under the existence of acid cocatalyst which is cationic exchange resin with subacidity. The catalyst and the acid cocatalyst improve the reaction yield; in addition, the catalyst and the cocatalyst can be recycled for treatment and conveniently applied mechanically, and the castlyst is environmental friendly.
Owner:ZHEJIANG NHU CO LTD
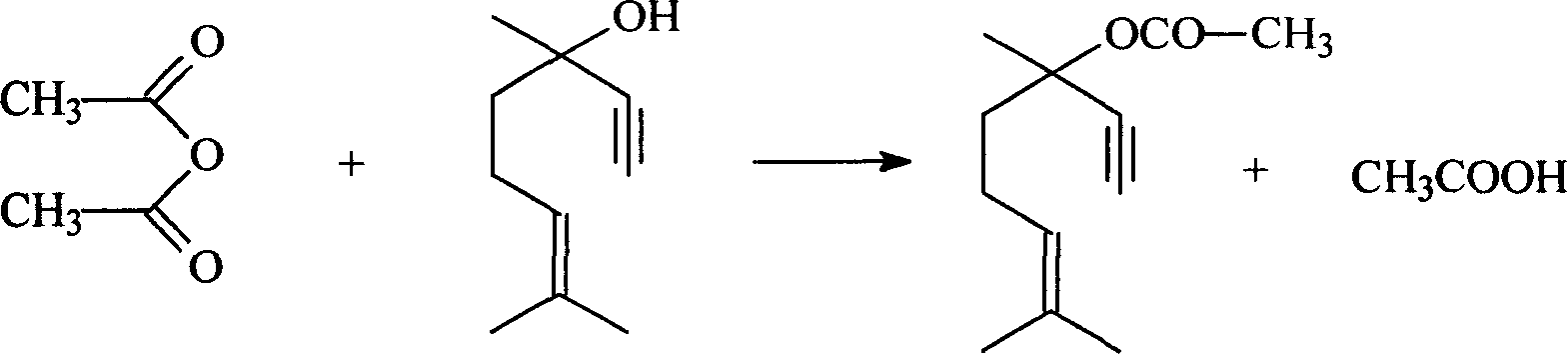
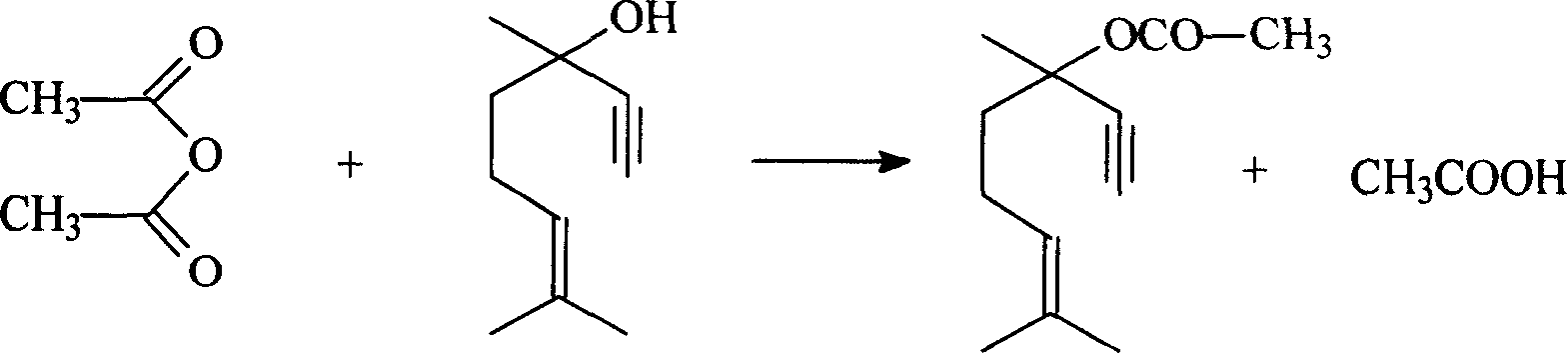
Method for preparing dehydrolinalyl acetate from dehydrolinalool
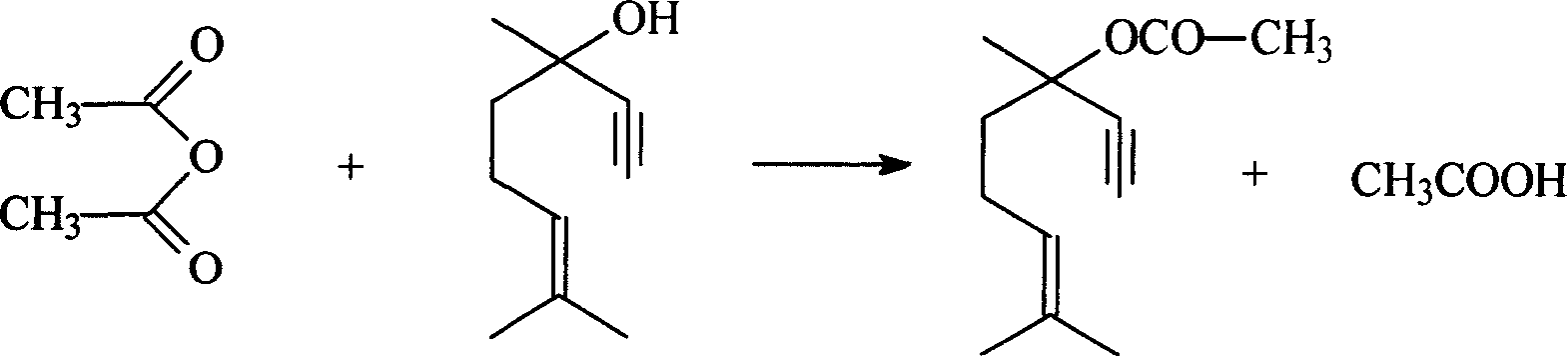
InactiveCN101209965AHigh reactivityShort reaction timeOrganic compound preparationCarboxylic acid esters preparationAcetic acidAcetic anhydride
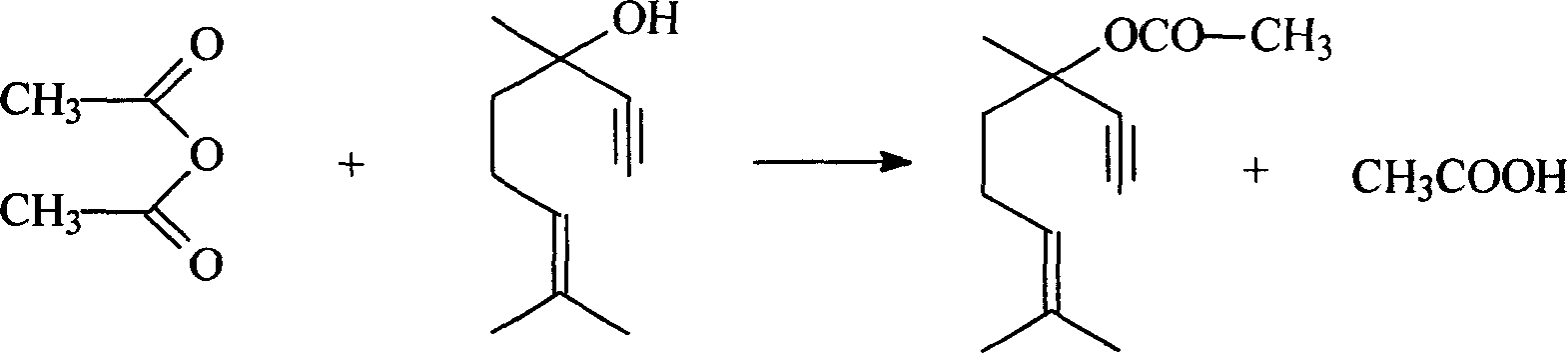
The invention relates to a method for preparing dehydrolinalyl acetate from dehydrolinalool. The method comprises esterification between dehydrolinalool and acetic anhydride is carried out in the presence of catalyst so as to obtain dehydrolinalyl acetate. Molar ratio of raw material provided of dehydrolinalool to acetic anhydride is 1: (1-5); reaction temperature is 25-70 DEG C; reaction pressure is normal pressure; reaction time is 20-150min; the catalyst is sulfosalicylic acid, dosage of which is 0.05-5wt percentage of total amount of the raw material provided. Compared with the prior art, the invention has the advantages that reaction activity of the adopted esterification catalyst is high, which is reflected by moderate reaction temperature and short reaction time, raw material conversion and product selectivity, etc. are ideal and removal of the catalyst from the product is convenient.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
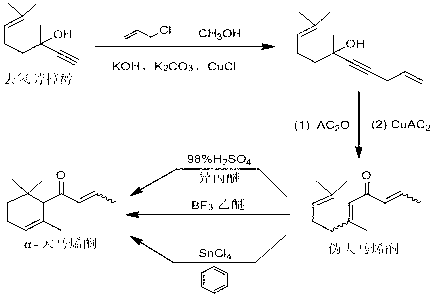
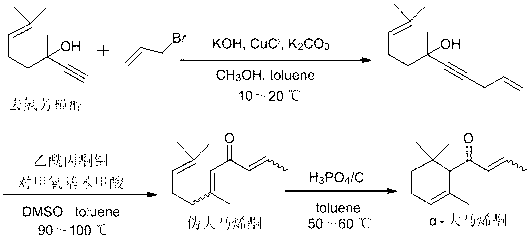
Preparation method of alpha-damascenone perfume
InactiveCN103058841AShort routeSimple and fast operationOrganic compound preparationCarbonyl compound preparationFlavorPhosphoric acid
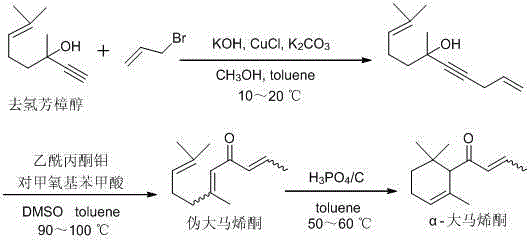
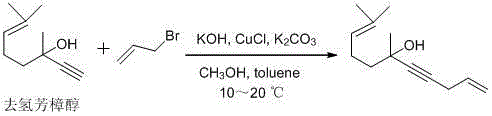
The invention discloses a preparation method of an alpha-damascenone perfume. A technical scheme adopted by the invention is characterized in that the method comprises the following steps: 1, reacting an initial raw material dehydrolinalool with 3-bromopropylene in a mixed solvent comprising toluene and methanol to obtain 6,10-dimethylundecyl-1,9-diene-4-alkynyl-6-ol; 2, converting 6,10-dimethylundecyl-1,9-diene-4-alkynyl-6-ol obtained in step1 in a solvent comprising toluene and dimethyl sulfoxide at 90-100DEG C under the action of a catalyst to obtain fake damascenone; and 3, converting damascenone in a solvent toluene at 50-60DEG C under the action of a catalyst phosphoric acid supported on active carbon to obtain the alpha-damascenone perfume. The method has the advantages of cheap and easily available raw material, mild reaction conditions, high reaction yield of each step, recycle of the solvents comprising toluene and methanol, and low cost, and is a method suitable for the industrialized production of the alpha-damascenone perfume.
Owner:HENAN NORMAL UNIV
Method for preparing dehydrolinalyl acetate from dehydrolinalool
InactiveCN101209966AHigh activityIdeal conversion rateOrganic compound preparationCarboxylic acid esters preparationAcetic acidAcetic anhydride
The invention relates to a method for preparing dehydrolinalyl acetate from dehydrolinalool. The method comprises esterification between dehydrolinalool and acetic anhydride is carried out in the presence of catalyst so as to obtain dehydrolinalyl acetate. Molar ratio of raw material provided of dehydrolinalool to acetic anhydride is 1: (1-5); reaction temperature is 40-80 DEG C; reaction pressure is normal pressure; reaction time is 60-240min; the catalyst is macroporous sulfonic acidic cation exchange resin; exchange capacity of the catalyst is 4.8-5.0mmol / g; degree of cross linking of the catalyst is 16-18 percent; aperture of the catalyst is 15-20nm and dosage is 2-20wt percent of total amount of the raw material provided. The invention has the advantages that reaction activity of the esterification catalyst adopted is high; conversion of raw material and selectivity of product, etc. are ideal; the catalyst basically does not have corrosiveness or have environmental impact; removal of the catalyst from the product is convenient.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for preparing methyl heptenone from waste liquid of dehydrogenated linalool production
ActiveCN101190880AHigh purityEmission reductionOrganic compound preparationCarbonyl compound preparationDepolymerizationGas phase
The invention relates to a method to produce methyl heptenone from the production waste liquid produced by dehydrogenated linalool, and the waste liquid contains dehydrogenated linalool dimmer which is produced during the synthesizing process of the dehydrogenated linalool through ethynylation reaction of the methyl heptenone. The method comprises the processes that: the waste liquid is depolymerized in alkaline solution, the depolymerization reacting resultants is carried out oil-water phase separation, and the alkaline solution is recovered and recycled; the oil-water phase materials is distilled by steam, the oil phase materials in the gas phase condensate are collected, and the methyl heptenone is obtained. The obtained rough methyl heptenone is decompression rectified and purified, the tower top distillates between 72 to 74 DEG C are collected, and therefore the refined product is obtained. 75 to 80kg of the refined methyl heptenone products can be prepared from 100kg waste liquid. The beneficial effects of the invention is that: a green utilization method with high economic value is supplied for the waste liquid, so this part of petroleum resource can be fully used, and the exhaust of contaminants arising from the production process of the dehydrogenated linalool through the ethynylation reaction of the methyl heptenone is reduced.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
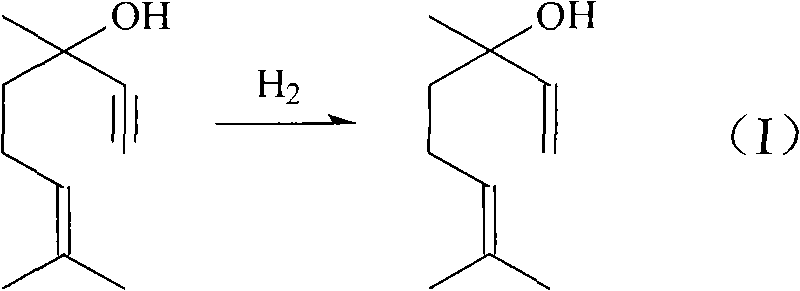
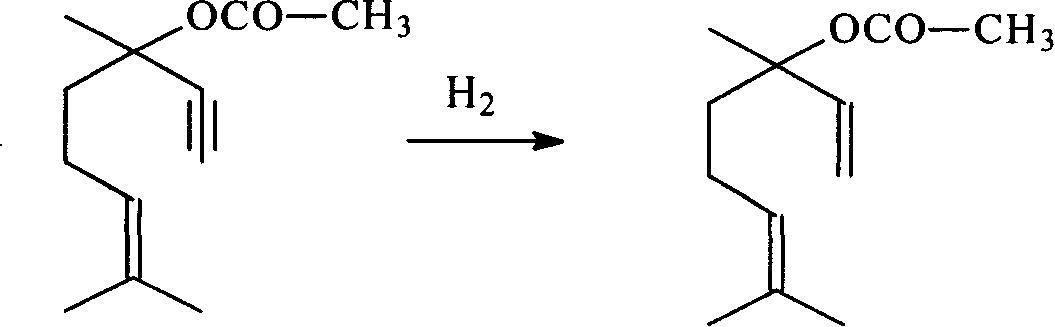
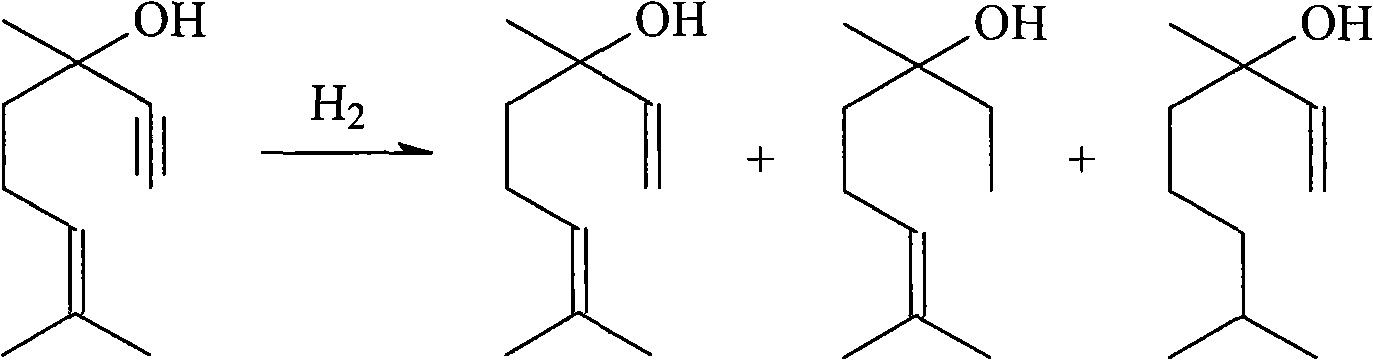
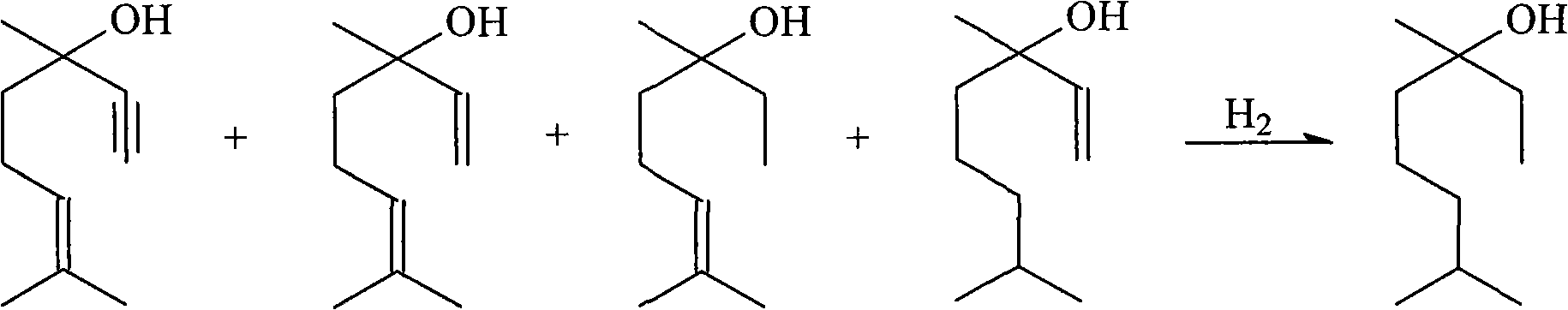
Method for preparing linalool from dehydrolinalool through selective hydrogenation
InactiveCN102397788AEasy to manufactureReduce manufacturing costPreparation by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsHydrogen atmosphereFixed bed
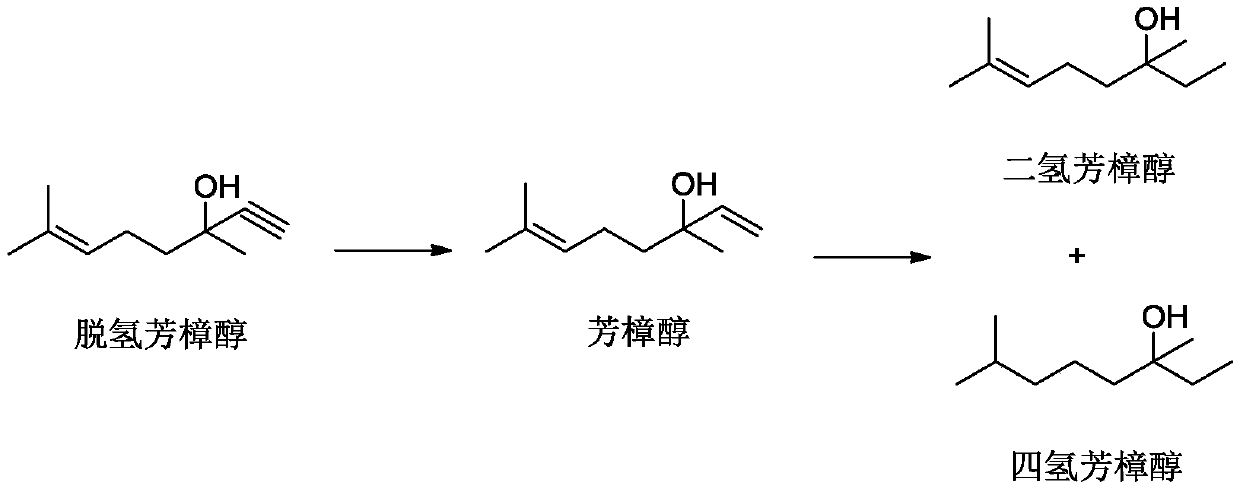
The invention discloses a method for preparing linalool from dehydrolinalool through selective hydrogenation. The method is characterized in that dehydrolinalool and a solvent are mixed and the liquid mixture passes through a fixed bed catalyst layer in a hydrogen atmosphere and undergoes a hydrogenation reaction, wherein the solvent is C2-C4 fatty alcohol; a solvent ratio is 1: (0.5 to 1.2); a liquid hourly space velocity is in a range of 2.0 to 2.5h*r<-1>; catalyst carriers are Al2O3 particles; a particle size is in a range of 3 to 5 millimeters; and active components comprise Pd, Pb and Bi. The method comprises the following steps that the active components of Pd, Pb and Bi are loaded on the catalyst carriers through immersion and high-temperature calcination to form catalyst precursors; sodium ethylene diamine tetracetate is adsorbed on the catalyst precursors through an immersion method; and catalyst finished products are obtained through reduction activation treatment in a hydrogen atmosphere. The method for preparing linalool from dehydrolinalool through selective hydrogenation has the advantages that preparation is convenient; a preparation cost is low; other deep hydrogenation side reaction inhibition processes are avoided; a conversion rate and product selectivity reach ideal levels; and a space-time yield is obviously improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
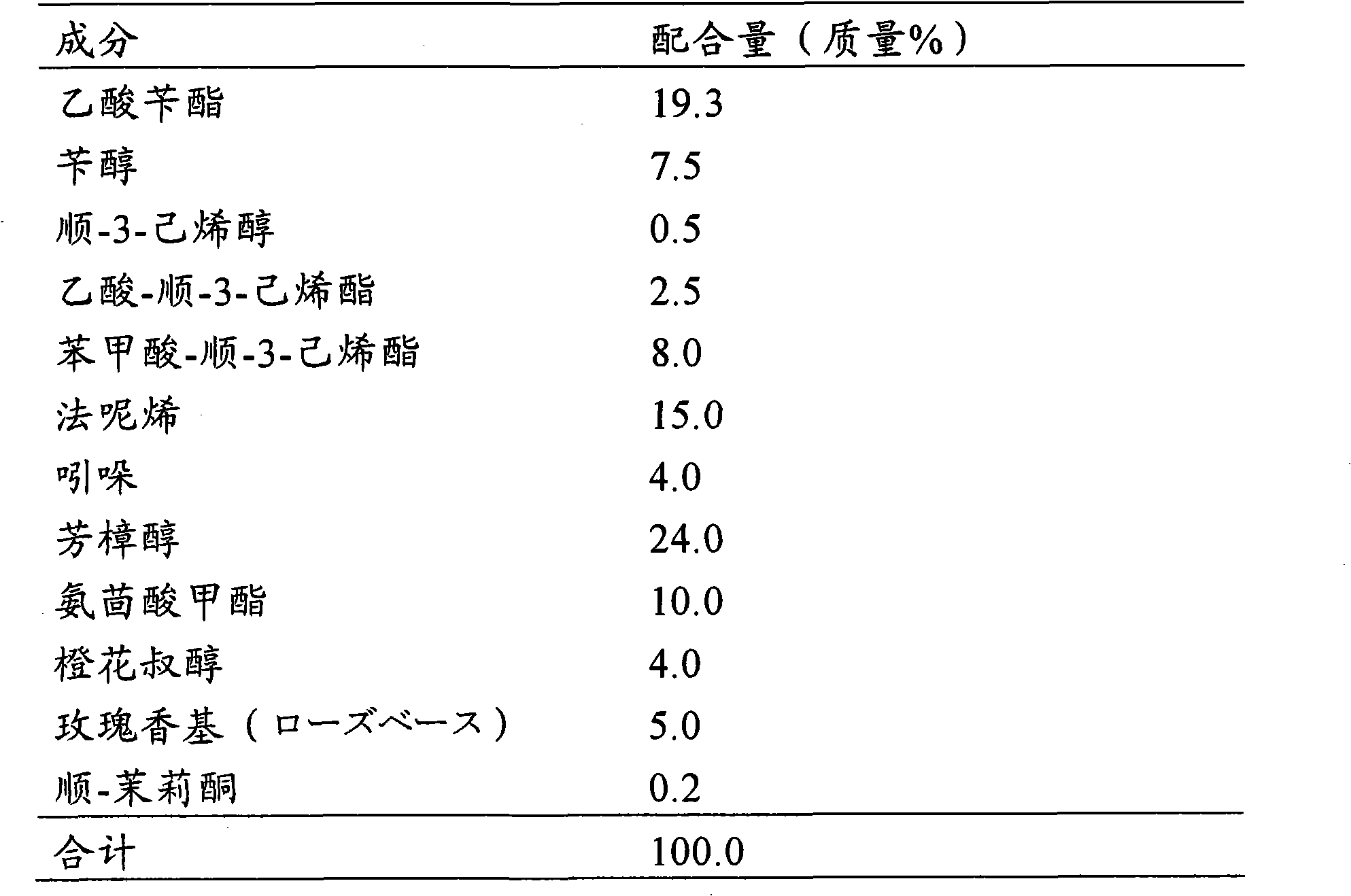
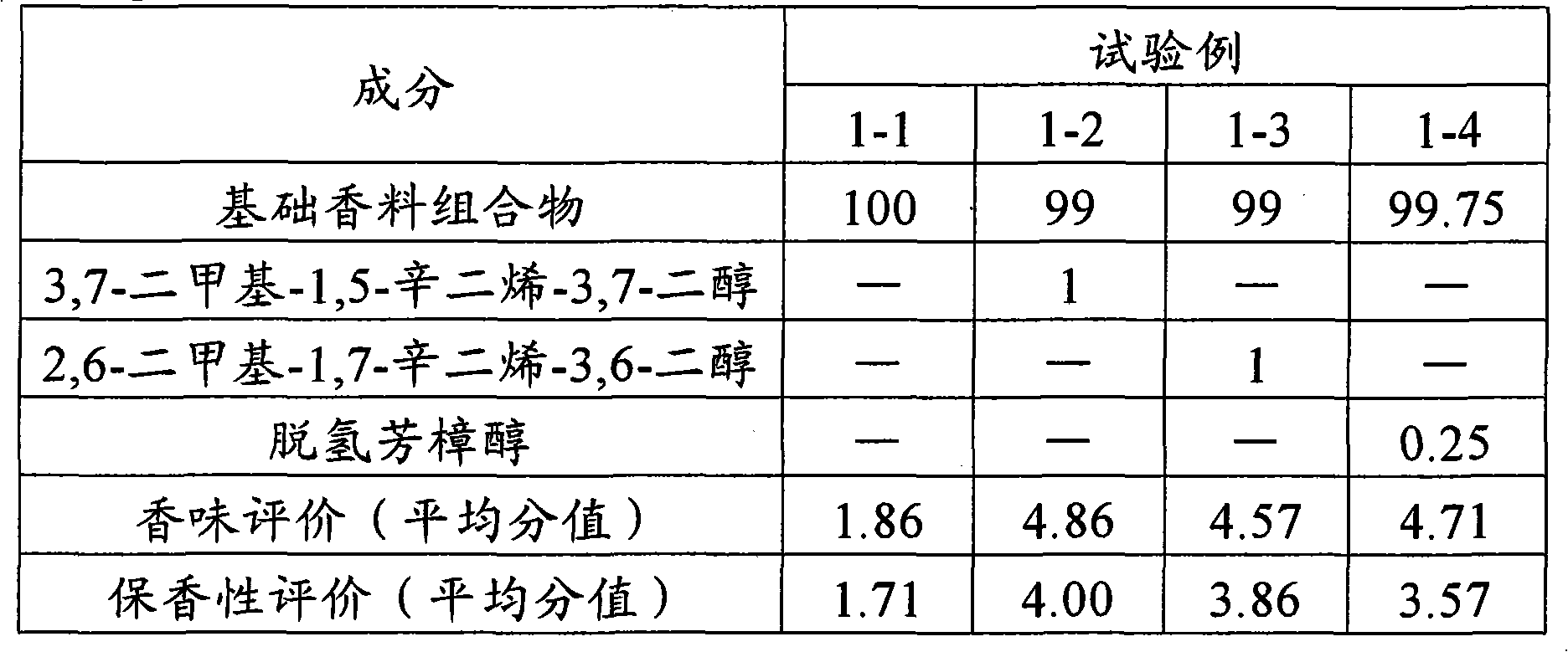
Jasminum sambac-like fragrance composition
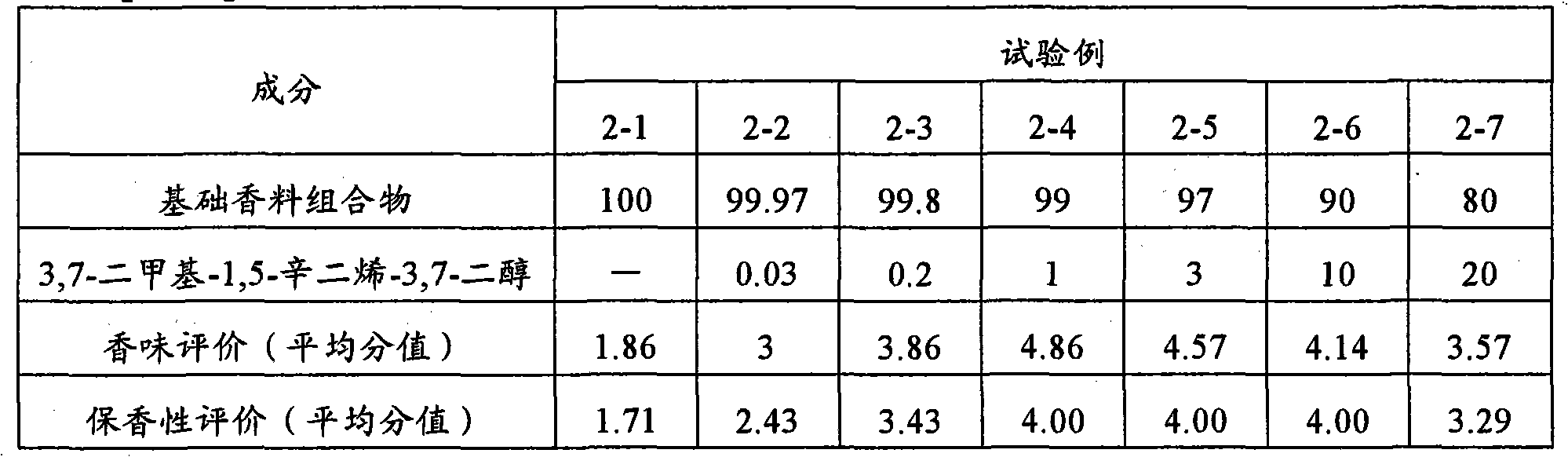
It is intended to provide a fragrance composition having a natural fragrance of Jasminum sambac. This composition is a Jasminum sambac-like fragrance composition characterized by adding at least one or more components selected from the group consisting of 3,7-dimethyl-1,5-octadiene-3,7-diol, 2,6-dimethyl-1,7-octadiene-3,6-diol, and dehydrolinalool to a base composition based on a Jasminum sambac flower fragrance-like fragrance. It is preferred that the total blending amount of the fragrance components is from 0.01 to 20% by mass of the total amount of the fragrance composition.
Owner:SHISEIDO CO LTD
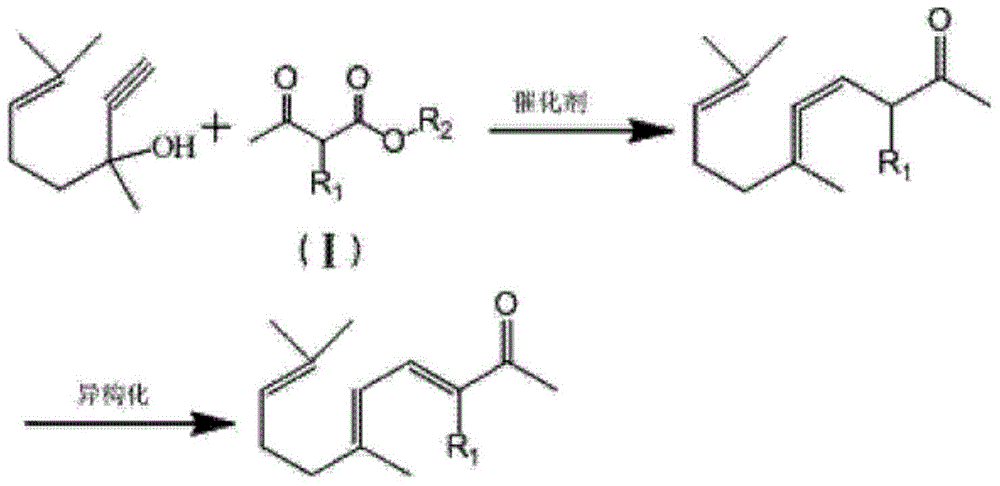
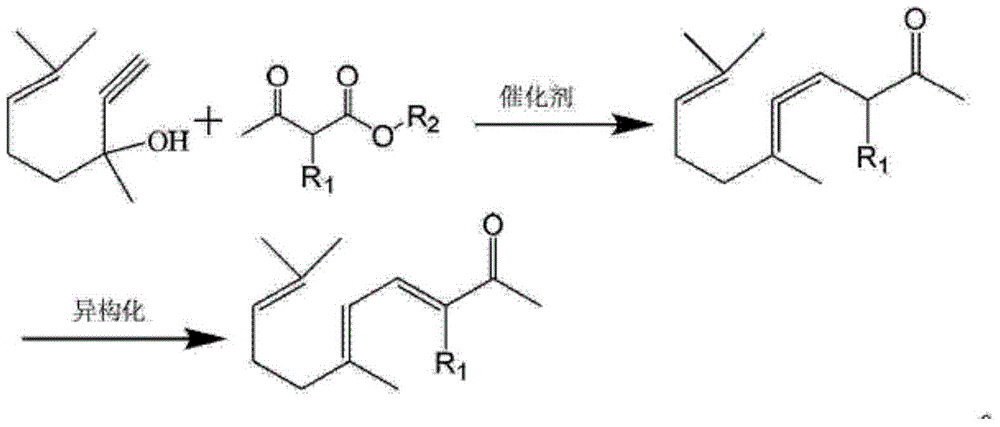
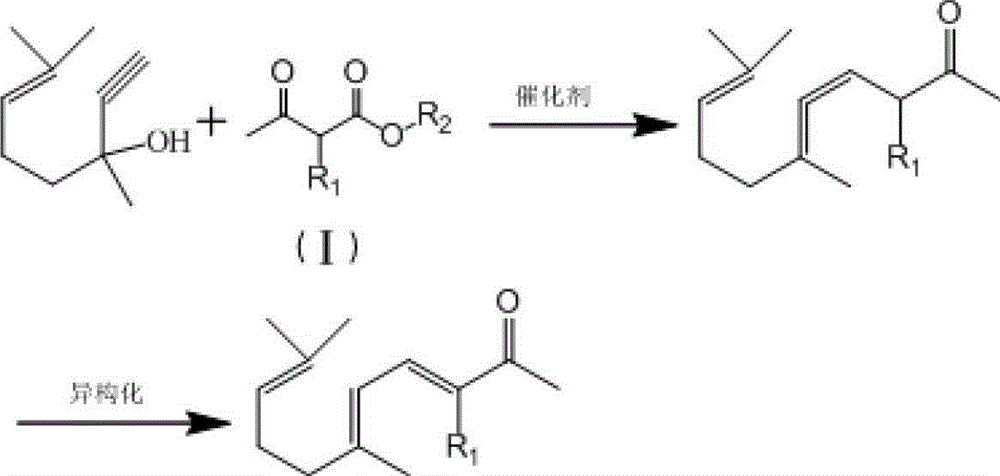
Synthesis method of ionone-type spice intermediate products
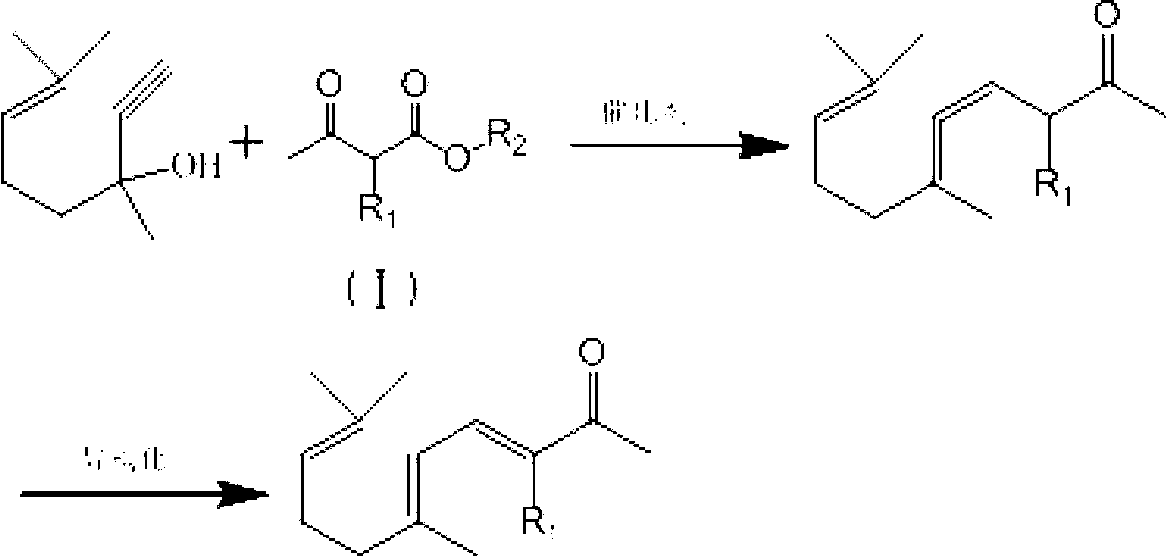
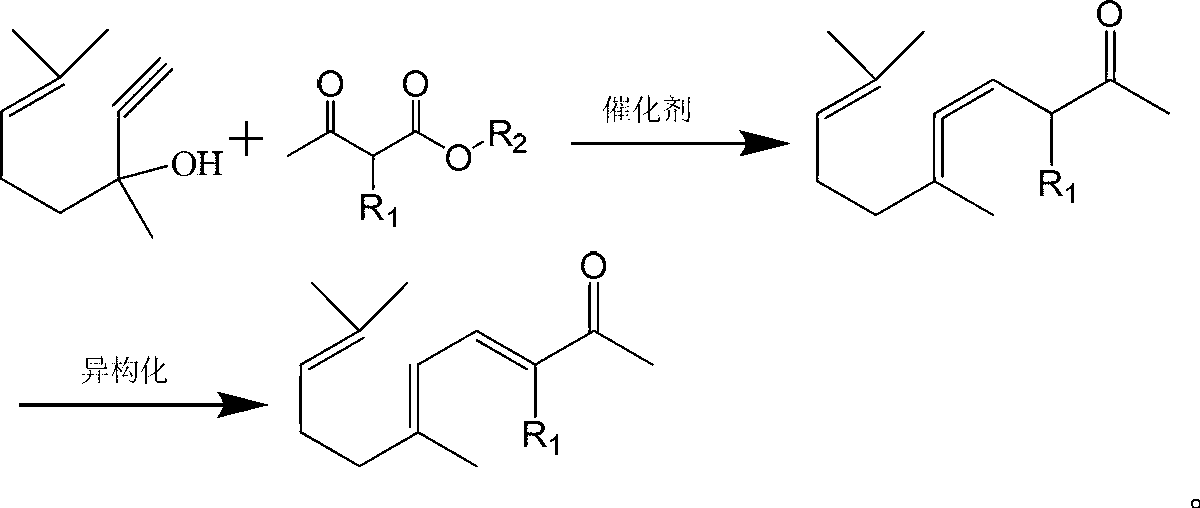
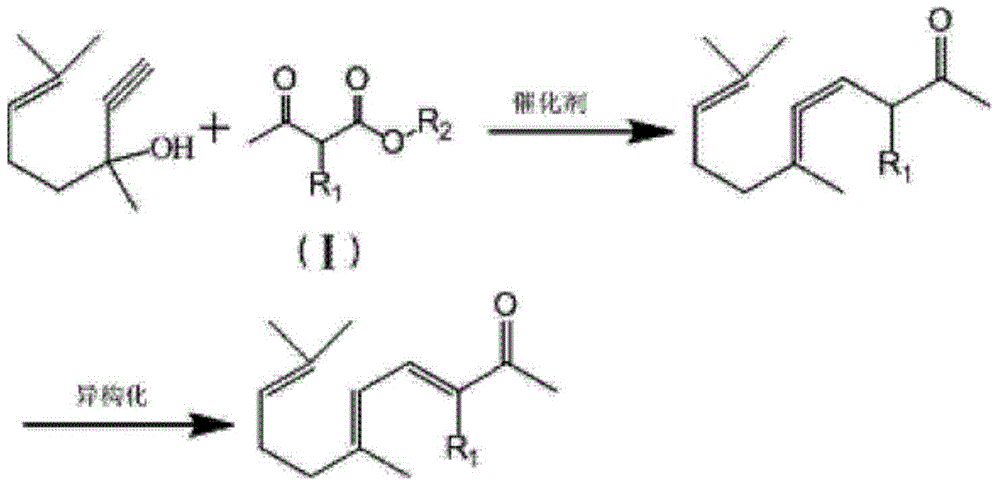
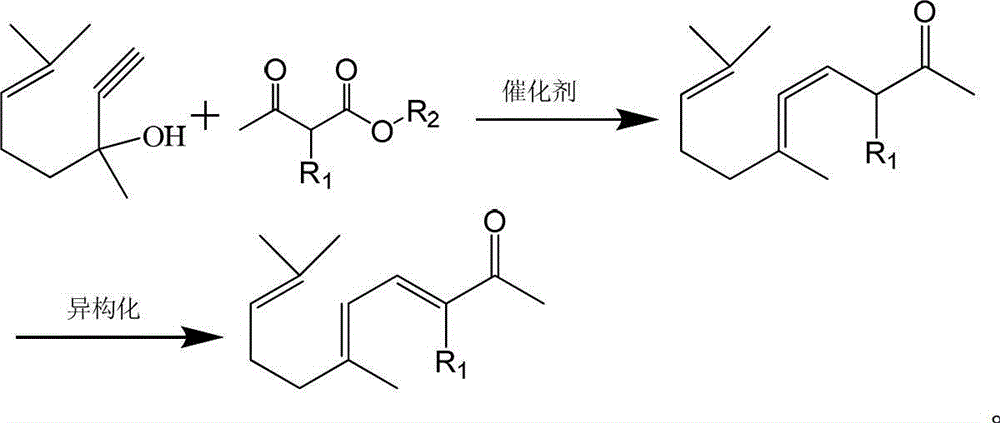
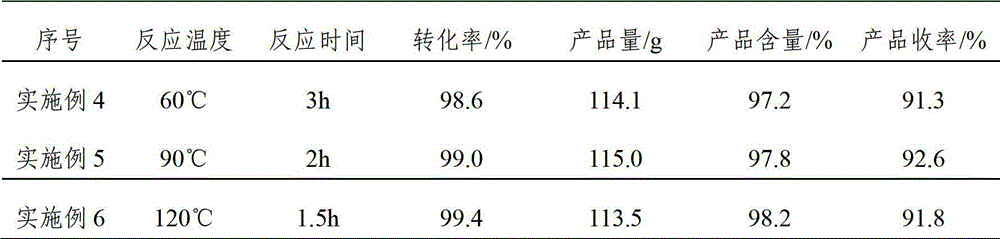
ActiveCN103012094AFew reaction stepsReduce pollutionOrganic compound preparationCarbonyl compound preparationIsomerizationSynthesis methods
The invention discloses a synthesis method of ionone-type spice intermediate products, namely pseudoionone and methyl pseudoionone, wherein the pseudoionone and the methyl pseudoionone are prepared by taking dehydrogenated linalool as a raw material, reacting with a compound represented as the formula I under an appropriate catalyst condition, and implementing an isomerization reaction. The method disclosed by the invention, in preparing the ionone-type spice intermediate products, is few in reaction steps, high in yield and low in pollution, wherein the R1 represents either -H or -CH3; and R2 represents methyl, ethyl, n-propyl, isopropyl, butyl, isobutyl, 2-methyl propyl, n-amyl, isoamyl, 2-methyl butyl, benzyl or other groups.
Owner:ANHUI BBCA FERMENTATION TECH ENG RES
Method for preparing citral through rearrangement reaction of dehydrolinalool
InactiveCN104292087AIdeal reactivityIdeal reaction selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalysts2-methylpropeneRuthenium
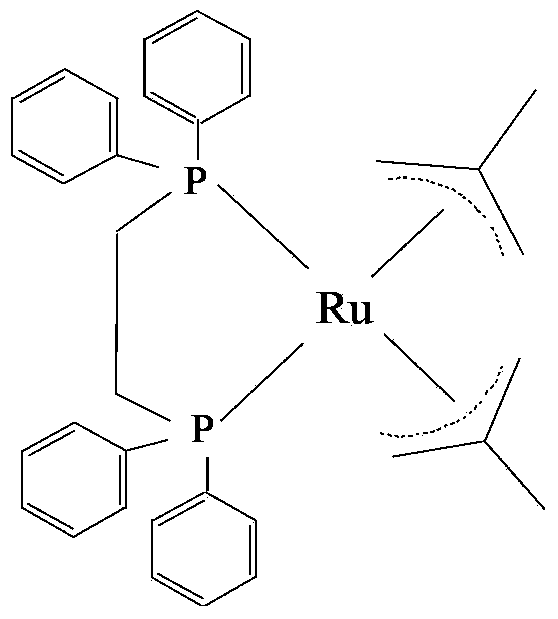
The invention relates to a method for preparing citral through a rearrangement reaction of dehydrolinalool. The method includes a step of performing catalytic rearrangement reaction with the dehydrolinalool as a raw material and with addition of a catalyst 1,2-bis(diphenylphosphine)ethane-bis(2-methylpropenyl)ruthenium in the presences of a solvent and a co-catalyst to obtain the citral. The catalyst is high in reaction activity and selectivity. Through the catalytic rearrangement reaction, ideal conversion ratio and yield of a target product can be achieved. The catalyst is simple in preparation process, is wide in raw material sources and has excellent economic benefit and application prospect in preparation of the citral.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing linalyl acetate from dehydrolinalool
InactiveCN101209967AHigh activityIdeal conversion rateOrganic compound preparationCarboxylic acid esters preparationAcetic anhydrideOil phase
The invention relates to a method for preparing linalyl acetate from dehydrolinalool. The method comprises esterification between dehydrolinalool and acetic anhydride is carried out in the presence of catalyst. The catalyst is sulfosalicylic acid, dosage of which is 0.05-5wt percent of total amount of the raw material provided. Product of the esterification is washed till oil phase material is neutral, and the dehydrolinalyl acetate is obtained. The dehydrolinalyl acetate undertakes hydrogenation reaction with hydrogen in the presence of catalyst ; the catalyst adopts calcium carbonate as a carrier; active components loaded contain Pd, Pb and A component, A is Bi or Zn or mixture of Bi and Zn, total content of Pd and Pb is 0.1-4.0wt percent, mass ratio of Pd and Pb is (5-1):1, content of A is 0.5-3.0wt percent and dosage of the catalyst accounting for 0.01-5.0wt percent of reaction liquid. The catalyst is removed from the product of the hydrogenation reaction through solid and liquid separation, and linalyl acetate is obtained by rectifying and refining. The invention has the advantages that the catalyst of esterification and hydrogenation reaction has higher activity, and conversion and selectivity are ideal.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Catalyst for hotrienol rearrangement reaction for citral preparation and method
InactiveCN105289751AHigh activityHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTitaniumDehydrolinalool
The invention provides a combined catalyst for a hotrienol rearrangement reaction for citral preparation. The catalyst is a combination of titanium tetraisopropanolate and cuprous chloride. The invention further provides a method for preparing citral through the hotrienol rearrangement reaction which adopts the catalyst combining titanium tetraisopropanolate and cuprous chloride. The catalyst is relatively high in both activity and selectivity, low in price, easy to obtain, low in dosage and very mild in reaction condition; within a relatively short reaction time, the ideal conversion rate and yield of a target product can be obtained.
Owner:EAST CHINA UNIV OF SCI & TECH
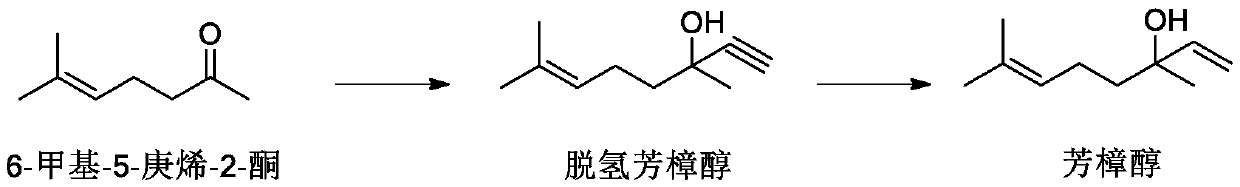
Method for preparing linalool
ActiveCN111018672ASuppress generationControl contentOrganic compound preparationPreparation by hydrogenationDistillationPhysical chemistry
The invention provides a method for preparing linalool. The method specifically comprises the following steps: taking 6-methyl-5-heptene-2 one as a raw material, carrying out ethynylation reaction toprepare dehydrolinalool, removing water in a reaction system, carrying out conventional distillation or rectification for separating dehydrolinalool, and carrying out selective hydrogenation to synthesize linalool. Impurities with the molecular weight of 170 (M = 170) can be generated in the treatment process of the 6-methyl-5-heptene-2 one ethynylation reaction liquid, and the content of the impurities should be strictly controlled to be 0.01% or below in order to regulate and control the final product linalool aroma. In order to control the content of the impurity, the generation of the impurity can be optimized and inhibited in the aspect of a post-treatment mode of a 6-methyl-5-heptene-2 one acetylation reaction solution, or the impurity is removed by adopting an efficient rectification mode.
Owner:WANHUA CHEM GRP CO LTD
Ionone series spice intermediate synthesis method
InactiveCN104693017AIn line with the idea of developing lithiumFew reaction stepsOrganic compound preparationCarbonyl compound separation/purificationIsomerizationSynthesis methods
The invention discloses an ionone series spice intermediate synthesis method, and the ionone series spice intermediates comprise pseudo ionone and methyl pseudo ionone. Dehydrolinalool as a raw material is reacted with formula I compound under the condition of an appropriate catalyst for isomerization reaction for preparation of the pseudo ionone and the methyl pseudo ionone. The ionone series spice intermediate synthesis method is less in reaction steps, high in yield and less in pollution.
Owner:QINGDAO HUICHENG PETROCHEM TECH
Method and equipment for processing black tea containing high theaflavin by utilizing fresh summer-autumn tea leaves
InactiveCN109221526AImprove qualityIncrease contentPre-extraction tea treatmentFood preservationNerolidolTheaflavin
The invention discloses a method for processing black tea containing high theaflavin by utilizing fresh summer-autumn tea leaves. The method is realized through the procedures of withering, twisting,fermenting, drying and preserving. The black tea prepared by the invention is the black tea containing high theaflavin; through the procedures of natural withering, twisting, tea frying and the like,the relative total content of aroma quality components such as linalool oxide, beta-linalool, dehydrolinalool and nerolidol carried in the tea leaves is greater than or equal to 18 percent, the theaflavin content is greater than or equal to 2 percent, and the product quality is high.
Owner:湖南太青山茶业有限公司
A kind of method for preparing and refining citral
ActiveCN103694091BHigh yieldSimple manufacturing processOrganic compound preparationChemical recyclingBenzoic acidSolubility
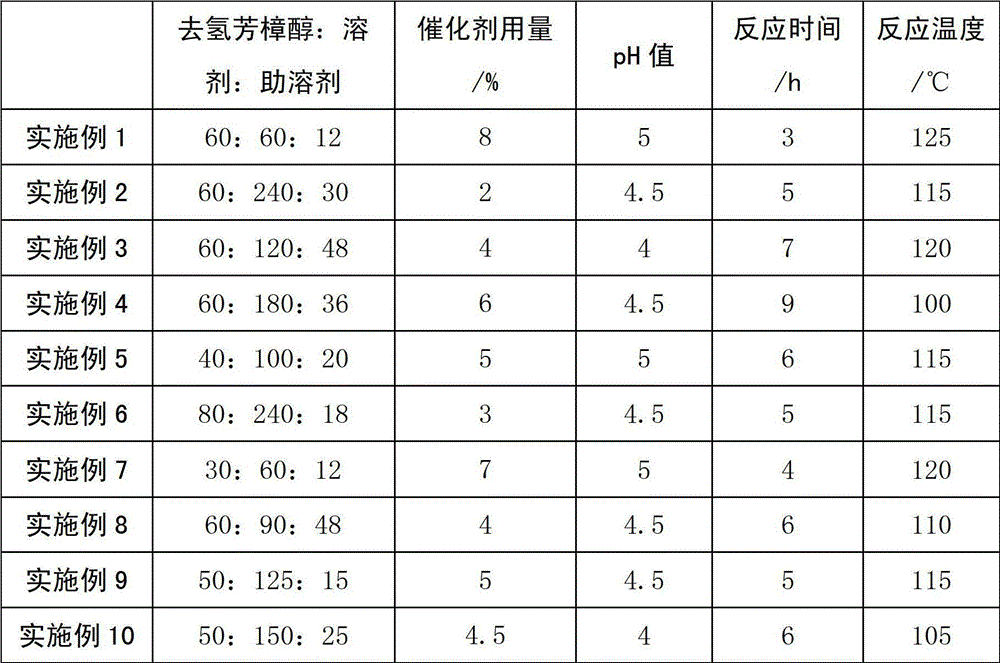
The invention discloses a preparation and refining method for citral. The method includes: 1) in an atmospheric oxygen-free environment, taking alcohol ether as a solvent and alkylsulfone as a cosolvent, adjusting the pH value by benzoic acid or phenylacetic acid, subjecting dehydrolinalool to catalytic rearrangement under catalysis of a molybdenum acetylacetonate catalyst so as to generate citral, with the dehydrolinalool, the solvent and the cosolvent being in a mass ratio of 1:(1-4):(0.2-0.8), and with the dosage of the catalyst accounting for 2-8% of that of dehydrolinalool, controlling the pH value at 3-5, the reaction time at 3-9h, and reaction temperature at 100-125DEG C; 2) subjecting a citral-containing reaction liquid to vacuum rectification to remove light components (including the cosolvent and unreacted dehydrolinalool), with a concentrated citral solution (mainly containing citral and the solvent) left at the tower bottom, and controlling the operation pressure at 0.02-0.08MPa, the tower bottom temperature at 40-80DEG C, and the reflux ratio at 4-10; and 3) carrying out vacuum rectification on the concentrated citral solution obtained at the tower bottom to obtain a high purity citral product, which has a citral purity up to 95.7%, and controlling the operation pressure at 0.008-0.06MPa, the tower bottom temperature at 40-80DEG C and the reflux ratio at 4-10. The method provided by the invention has very obvious positive effects, makes use of the good solubility of the molybdenum catalyst in the alcohol ether solvent, not only improves the citral yield, but also realizes recycling of the catalyst dissolved in the solvent. Complex processing steps in the prior art are avoided, and the process is simplified.
Owner:CHINA PETROLEUM & CHEM CORP +1
Utilization method of linalool raw product refining raffinate synthesized by 6-methyl-5-heptenyl-2-one
InactiveCN101987810AImprove use valueReduce pollutionOrganic compound preparationPreparation by hydrogenationHydrogenation reactionKetone
The invention discloses a utilization method of linalool raw product refining raffinate synthesized by 6-methyl-5-heptenyl-2-one, and the raw product refining raffinate comprises the dehydrolinalool, linalool and dihydro linalool. The utilization method comprises the following steps: carrying out the rectification on the raw product refining raffinate to remove the impurities of the heavy components, and collecting the fractions of 80 to 90 DEG C at the the tower top; carrying out the hydrogenation reaction on the collected fractions in the presence of a catalyst; cooling the product of the hydrogenation reaction, separating and recycling the catalyst, rectifying to remove the impurities of the light components, and collecting the fractions of 50 to 80 DEG C at the tower top as the impurities of the light components; performing the rectification by keeping the tower top pressure of the refining column and the tower top temperature of the rectifying column to remove the impurities of the heavy components and collect the refining product of the tetrahydrolinalool, wherein the fractions of 80 to 90 DEG C are collected at the tower top as the refining product of the tetrahydrolinalool, and the raffinate of the rectifying column comprises the impurities of the heavy components. Compared with the existing technologies, the method of the invention enhances the utilization ratio of the refining raffinate, as well as reduces the environmental pollution.
Owner:CHINA PETROLEUM & CHEM CORP +1
A kind of synthetic method of pseudoionone
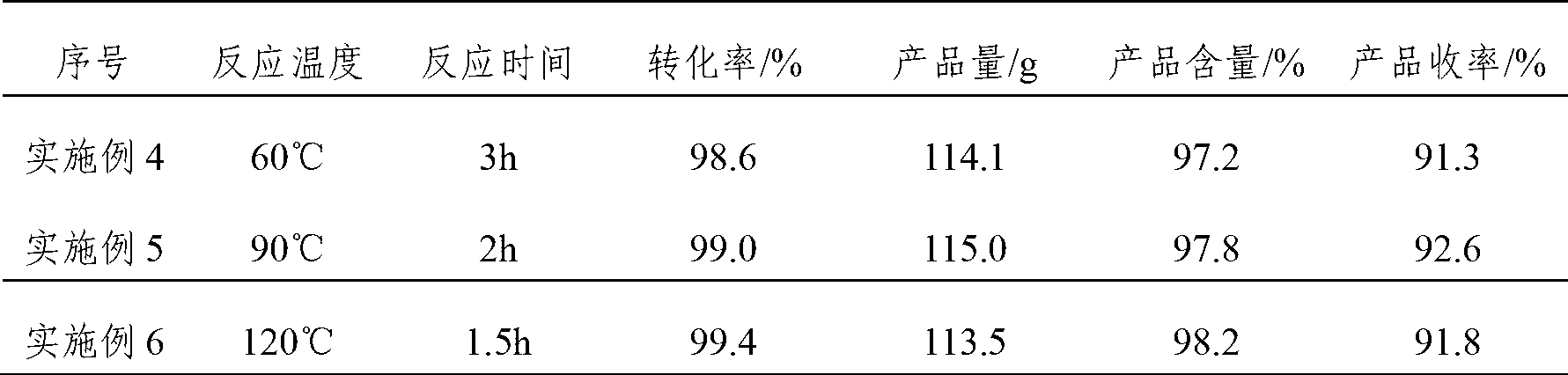
ActiveCN108530279BIn line with the development conceptRealize continuous productionOrganic compound preparationCarbonyl compound preparationChemical industryIsomerization
Owner:ZHEJIANG NHU CO LTD +1
Synthesis method of ionone-type spice intermediate products
ActiveCN103012094BFew reaction stepsReduce pollutionOrganic compound preparationCarbonyl compound preparationIsomerizationSynthesis methods
The invention discloses a synthesis method of ionone-type spice intermediate products, namely pseudoionone and methyl pseudoionone, wherein the pseudoionone and the methyl pseudoionone are prepared by taking dehydrogenated linalool as a raw material, reacting with a compound represented as the formula I under an appropriate catalyst condition, and implementing an isomerization reaction. The method disclosed by the invention, in preparing the ionone-type spice intermediate products, is few in reaction steps, high in yield and low in pollution, wherein the R1 represents either -H or -CH3; and R2 represents methyl, ethyl, n-propyl, isopropyl, butyl, isobutyl, 2-methyl propyl, n-amyl, isoamyl, 2-methyl butyl, benzyl or other groups.
Owner:ANHUI BBCA FERMENTATION TECH ENG RES
Method for producing methyl heptenone from dehydrogenated linalool production waste liquor
InactiveCN1880292AHigh purityEmission reductionOrganic compound preparationCarbonyl compound separation/purificationDistillationGas phase
This invention relates to a method for producing methylheptenone from dehydrolinalool production waste solution, which uses didehydrolinalool produced during the methylheptenone ethynylation reaction to synthesize dehydrolinalool, comprising: 1) the waste solution performs depolymerizing reaction in NaOH or KOH solution; 2) the reaction products perform oil-water separation and NaOH or KOH solution is recovered to be used again; 3) distill the oil-phase material with steam, and collect the gas-phase cooling liquid oil-phase material so as to obtain the crude methylheptenone; 4) purify the crude methylheptenone by depression distillation and collect the top fraction at 75-78Deg C so as to produce the refined product. This invention is characterized in that it provides a green and economic method to utilize the waste solution and make full use of this petroleum resource.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Process for preparing citral
ActiveCN101391942BReduce pollutionMild reaction conditionsOrganic compound preparationCarbonyl compound preparationCatalytic reformingSolvent
The invention discloses a preparing method of citral. The existing method has high production cost or low yield, thus being difficult to be suitable for scale production. The method adopts dehydrogenated linalool as a raw material which is carried out with catalytic reforming to obtain the citral under the existence of a solvent and a latent solvent and is characterized in that: a catalyst adopted for reaction is molybdenum dioxide diacetyl acetone acid ester, the catalytic reforming is carried out under the existence of acid cocatalyst which is cationic exchange resin with subacidity. The catalyst and the acid cocatalyst improve the reaction yield; in addition, the catalyst and the cocatalyst can be recycled for treatment and conveniently applied mechanically, and the castlyst is environmental friendly.
Owner:ZHEJIANG NHU CO LTD
A kind of method for preparing citral by dehydrolinalool rearrangement reaction
ActiveCN113248357BHelp maintain VI valenceAvoid inactivationOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzoic acidPtru catalyst
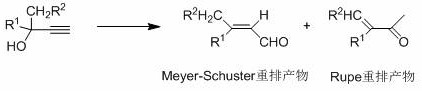
The invention discloses a method for rearranging and synthesizing citral by using dehydrolinalool as raw material, comprising: using dehydrolinalool as raw material, in the presence of solvent toluene and organic acid 4-tert-butylbenzoic acid, Add catalytic system MoO 2 (acac) 2 and Ph 3 PO, catalyzes the Meyer‑Schuster rearrangement to give citral. The catalyst system used in the invention can obtain citral with relatively ideal conversion rate and yield with higher activity and selectivity, and the reaction process is green and environment-friendly, without producing harmful and smelly mercaptans and thioethers. This catalytic system provides an improved method for the preparation of citral, avoids the use of sulfoxide compounds, and has a good application prospect.
Owner:江苏宏邦化工科技有限公司
Fixed bed catalyst for preparing linalool by selective hydrogenation of dehydrolinalool
InactiveCN102397789AEasy to manufactureReduce manufacturing costPreparation by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsHydrogenActive component
The invention relates to a fixed bed catalyst for preparing linalool by selective hydrogenation of dehydrolinalool, wherein the catalyst is a supported catalyst, the carrier is granular Al2O3, the particle size is 3-5 mm, the active components comprise metals of Pd, Pb and Bi. The preparation process for the catalyst comprises that: the active components of the metal Pd, the metal Pb and the metal Bi are supported on the carrier by an impregnation method and high temperature baking to obtain a catalyst precursor; disodium ethylenediaminetetraacetate is absorbed on the catalyst precursor by the impregnation method, wherein the adsorption weight ratio of the disodium ethylenediaminetetraacetate to the catalyst precursor is controlled to 1000:(3-15); the catalyst precursor is subjected to a reduction activation treatment at a temperature of 200-250 DEG C in hydrogen to obtain the catalyst finished product. According to the present invention, the preparation method for the catalyst is convenient; the preparation cost is low; no other measures for inhibiting the deep hydrogenation adverse reaction are required; the conversion rate and the product selectivity can achieve the ideal levels; the space-time yield is significantly improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
The preparation method of citral
ActiveCN103694092BHigh yieldSimple manufacturing processOrganic compound preparationCarbonyl compound preparationBenzoic acidSolubility
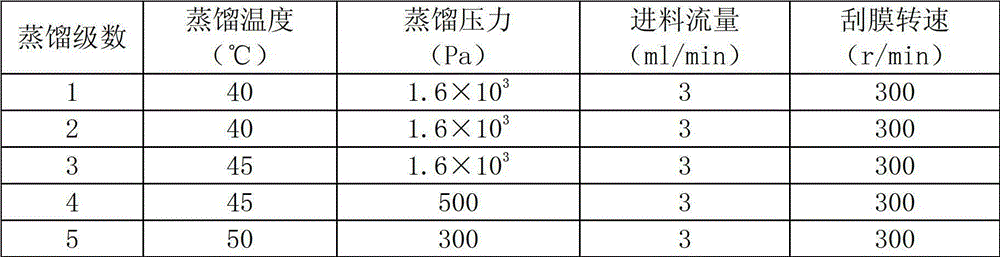
The invention relates to a preparation method for citral. The method comprises the steps of: 1) in an atmospheric pressure and oxygen-free environment, and in the presence of an alcohol ether solvent and a cosolvent, using benzoic acid or phenylacetic acid to adjust the pH value in the range of 3-5, subjecting dehydrolinalool to catalytic rearrangement under the action of a Mo catalyst, keeping the dehydrolinalool, the alcohol ether solvent, and the cosolvent in a ratio of 1:2.5:0.5, with the dosage of the catalyst accounting for 5% of the weight of the dehydrolinalool, and controlling the reaction time at 5-7h and the reaction temperature at 115-120DEG C; and 2) subjecting the citral crude product obtained in step 1) to five-stage molecular distillation for separation. By making use of the good solubility of the molybdenum catalyst in the alcohol ether solvent, the method provided by the invention not only improves the citral yield, and the catalyst can be recycled due to its dissolution in the solvent. The method avoids complex processing steps in the prior art, and simplifies the process.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for hydrogenation utilization of linalool rectification kettle residual liquid
ActiveCN111205167AHigh selectivityHigh yieldOrganic compound preparationPreparation by hydrogenationPtru catalystHydrogenation reaction
The invention provides a method for hydrogenation utilization of linalool rectification kettle residual liquid. According to the method, the linalool rectification kettle residual liquid with heavy components removed is taken as a raw material and is subjected to a hydrogenation reaction to synthesize dihydrolinalool or tetrahydrolinalool. According to the method, a ruthenium-carbon catalyst is used for carrying out hydrogenation reaction on the linalool rectification kettle residual liquid, and when no metal salt is added for modifying the catalyst, tetrahydrolinalool can be prepared with high selectivity; when a metal salt is added to modify the catalyst, dihydrolinalool can be prepared with high selectivity, so that productivity regulation and control of different hydrogenation productsare realized according to market demand conditions, and the method has wide actual production and application values. Besides, diamine substance can stabilize hydrogen ions at the carbon-carbon triple bond end position in the dehydrolinalool and inhibit the polymerization reaction, thereby inhibiting the generation of heavy component byproducts and being beneficial to enhancing the selectivity ofthe raw material and the yield of the product.
Owner:WANHUA CHEM GRP CO LTD
Method for preparing linalyl acetate from dehydrolinalool
InactiveCN101209969AHigh activityIdeal conversion rateOrganic compound preparationCarboxylic acid esters preparationAcetic anhydrideOil phase
The invention relates to a method for preparing linalyl acetate from dehydrolinalool. The method comprises esterification between dehydrolinalool and acetic anhydride is carried out in the presence of catalyst. The catalyst is macroporous sulfonic acidic cation exchange resin, dosage of which is 2-20wt percent of total amount of the raw material provided. Product of the esterification, after being removed of the catalyst, is washed till oil phase material is neutral, and the dehydrolinalyl acetate is obtained. The dehydrolinalyl acetate undertakes hydrogenation reaction with hydrogen in the presence of catalyst ; the catalyst adopts calcium carbonate as a carrier; active components loaded contain Pd, Pb and A ingredient, A is either Bi or Zn or mixture of Bi and Zn, total content of Pd and Pb is 0.1-4.0wt percent, mass ratio of Pd and Pb is (5-1):1, content of A is 0.5-3.0wt percent and dosage of the catalyst accounting for 0.01-5.0wt percent of reaction liquid. The catalyst is removed from the product of the hydrogenation reaction through solid and liquid separation, and linalyl acetate is obtained by rectifying and refining. The invention has the advantages that the catalyst of esterification and hydrogenation reaction has relatively high activity, and conversion and selectivity are higher.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Catalyst for preparing citral through dehydrolinalool rearrangement reaction, preparation method of catalyst and method for preparing citral
ActiveCN110841716AEasy to makeSave raw materialsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIndium TrichloridePhosphomolybdic acid
The invention belongs to the technical field of organic synthesis, and particularly relates to a catalyst for preparing citral through dehydrolinalool rearrangement reaction, a preparation method of the catalyst and a method for preparing the citral. The catalyst is a solid acid catalyst and comprises an active component, a carrier and an optional assistant; wherein the active component is selected from one or more of silicotungstic acid, phosphomolybdic acid and phosphotungstic acid, and the assistant is selected from one or more of gold trichloride, rhenium trichloride, indium trichloride and lanthanum trichloride. The method for preparing the citral comprises the following step of: in the presence of a solvent and the solid acid catalyst, carrying out Meyer-Schuster rearrangement reaction by taking dehydrolinalool as a raw material to prepare the citral. The solid acid catalyst is simple to prepare, low in cost and long in service life; when the catalyst is used in the process of preparing citral, the reaction yield and the conversion rate are improved, and the selectivity is good; in addition, the catalyst is long in service life, and continuous large-scale operation can be realized.
Owner:WANHUA CHEM GRP CO LTD
Catalyst and method for preparing citral by rearrangement reaction of dehydrolinalool
InactiveCN105289751BHigh activityHigh selectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTitaniumDehydrolinalool
The invention provides a combined catalyst for a hotrienol rearrangement reaction for citral preparation. The catalyst is a combination of titanium tetraisopropanolate and cuprous chloride. The invention further provides a method for preparing citral through the hotrienol rearrangement reaction which adopts the catalyst combining titanium tetraisopropanolate and cuprous chloride. The catalyst is relatively high in both activity and selectivity, low in price, easy to obtain, low in dosage and very mild in reaction condition; within a relatively short reaction time, the ideal conversion rate and yield of a target product can be obtained.
Owner:EAST CHINA UNIV OF SCI & TECH
A kind of preparation method of citral
ActiveCN104387248BLow priceReduce dosageOrganic compound preparationCarbonyl compound preparationOrganic acidSolvent
The invention discloses a preparation method of citral, which uses dehydrolinalool as a raw material and molybdenum trioxide as a catalyst. In the presence of acid, citral is obtained by catalytic rearrangement in an organic inert solvent; the catalyst used in the preparation method is cheap; the amount of catalyst used is small; the catalytic efficiency is high; the reaction selectivity is good and the yield is high; Short; the catalyst can be recycled and applied.
Owner:SHANDONG NHU PHARMA +1
Method for preparing methyl heptenone from waste liquid of dehydrogenated linalool production
ActiveCN101190880BHigh purityEmission reductionOrganic compound preparationCarbonyl compound preparationDepolymerizationGas phase
The invention relates to a method to produce methyl heptenone from the production waste liquid produced by dehydrogenated linalool, and the waste liquid contains dehydrogenated linalool dimmer which is produced during the synthesizing process of the dehydrogenated linalool through ethynylation reaction of the methyl heptenone. The method comprises the processes that: the waste liquid is depolymerized in alkaline solution, the depolymerization reacting resultants is carried out oil-water phase separation, and the alkaline solution is recovered and recycled; the oil-water phase materials is distilled by steam, the oil phase materials in the gas phase condensate are collected, and the methyl heptenone is obtained. The obtained rough methyl heptenone is decompression rectified and purified, the tower top distillates between 72 to 74 DEG C are collected, and therefore the refined product is obtained. 75 to 80kg of the refined methyl heptenone products can be prepared from 100kg waste liquid. The beneficial effects of the invention is that: a green utilization method with high economic value is supplied for the waste liquid, so this part of petroleum resource can be fully used, and the exhaust of contaminants arising from the production process of the dehydrogenated linalool through the ethynylation reaction of the methyl heptenone is reduced.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Preparation method of alpha-damascenone perfume
InactiveCN103058841BShort routeSimple and fast operationOrganic compound preparationCarbonyl compound preparationFlavorPhosphoric acid
The invention discloses a preparation method of an alpha-damascenone perfume. A technical scheme adopted by the invention is characterized in that the method comprises the following steps: 1, reacting an initial raw material dehydrolinalool with 3-bromopropylene in a mixed solvent comprising toluene and methanol to obtain 6,10-dimethylundecyl-1,9-diene-4-alkynyl-6-ol; 2, converting 6,10-dimethylundecyl-1,9-diene-4-alkynyl-6-ol obtained in step1 in a solvent comprising toluene and dimethyl sulfoxide at 90-100DEG C under the action of a catalyst to obtain fake damascenone; and 3, converting damascenone in a solvent toluene at 50-60DEG C under the action of a catalyst phosphoric acid supported on active carbon to obtain the alpha-damascenone perfume. The method has the advantages of cheap and easily available raw material, mild reaction conditions, high reaction yield of each step, recycle of the solvents comprising toluene and methanol, and low cost, and is a method suitable for the industrialized production of the alpha-damascenone perfume.
Owner:HENAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com