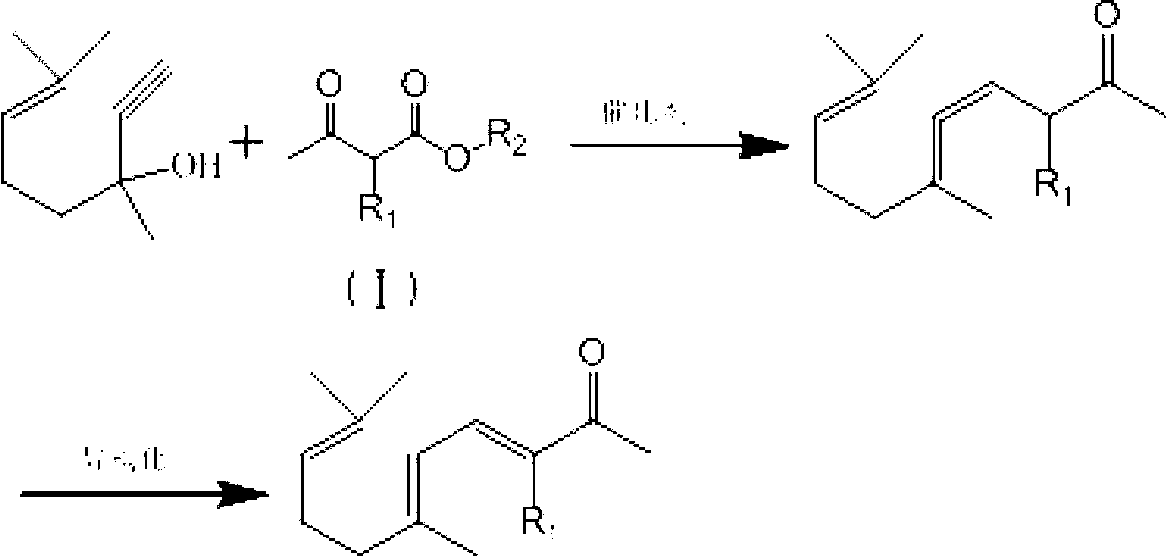
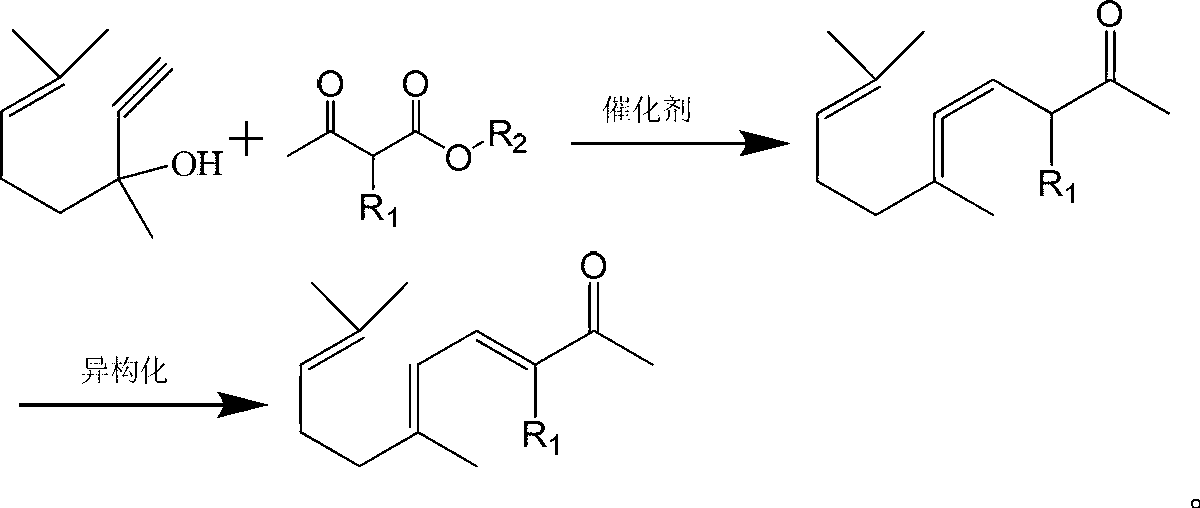
Synthesis method of ionone-type spice intermediate products
An ionone and a synthesis method technology are used in the synthesis of ionone-based fragrance intermediates and the field of compound synthesis, and can solve the problems of low space-time yield and high catalyst cost, and achieve a solution with fewer reaction steps, higher yield and less pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
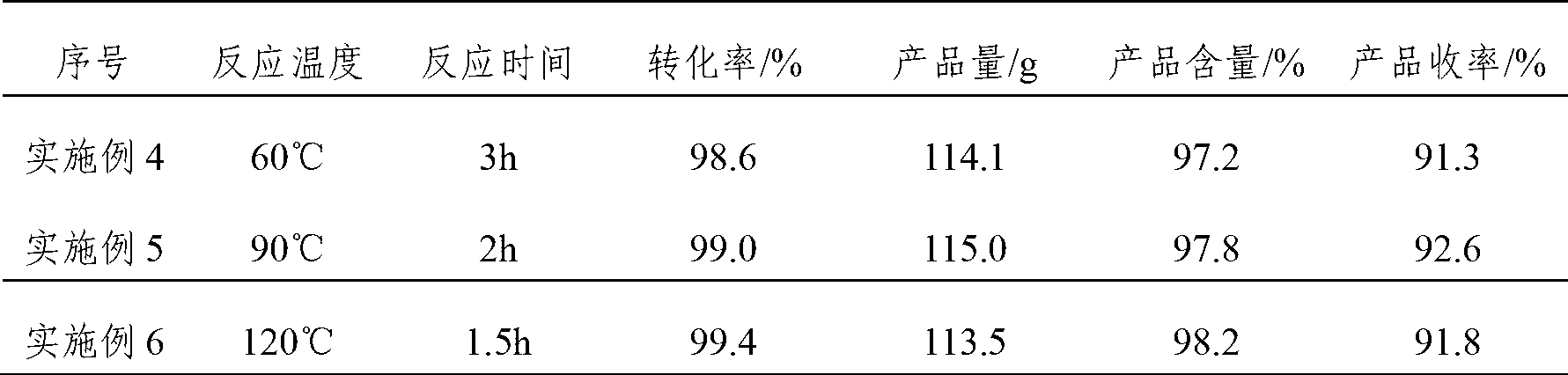
[0033] Put 100g (content 98.5%) of dehydrolinalool, 91.0g of methyl acetoacetate, and 4.0g of aluminum isopropoxide into a 500ml pressure-resistant reaction bottle, perform magnetic stirring and heat to an internal temperature of 85°C with an oil bath to keep the reaction Pressure is 1.2MPa reaction 2 hours, gas chromatography analysis dehydrolinalool conversion rate 98.8%. After the reaction is completed, fill it with nitrogen to normal pressure, cool down to 15°C, add dropwise 50ml of 0.5% sodium hydroxide methanol solution, and keep warm for 1.5 hours after dropping. After the isomerization is completed, add acetic acid to adjust the pH value at 6-7. The reaction solution was filtered through a column equipped with a diatomite filter aid using methanol as a solvent, and the filtrate was rectified to obtain 117.3 g of a pseudoionone product of 97.5% (cis-trans sum). The yield of pseudoionone is about 93.1%
Embodiment 2
[0035] According to the method of embodiment 1, the only difference is that in this embodiment, 91.0 g of methyl acetoacetate is changed into 83.5 g of methyl acetoacetate, and other conditions remain unchanged. The conversion rate of dehydrolinalool was 97.6%, and 114.5 g of pseudoionone products with a content of 98.1% were obtained. The yield of pseudoionone is about 92.5%
Embodiment 3
[0037] According to the method of embodiment 1, the only difference is that in this embodiment, 91.0 g of methyl acetoacetate is changed into 98.5 g of methyl acetoacetate, and other conditions remain unchanged. The conversion rate of dehydrolinalool was 99.2%, and 114.3 g of pseudoionone products with a content of 97.8% were obtained. The yield of pseudoionone is about 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com