Method for hydrogenation utilization of linalool rectification kettle residual liquid
A linalool and rectifying kettle technology, which is applied in the field of hydrogenation and utilization of linalool rectifying kettle residue liquid, can solve the problems affecting product yield and purity, more stringent control of product purity and content of harmful substances, complex components, etc. The problem is to achieve the effect of improving the selectivity of raw materials and product yield, wide production and application value, and high product purity and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
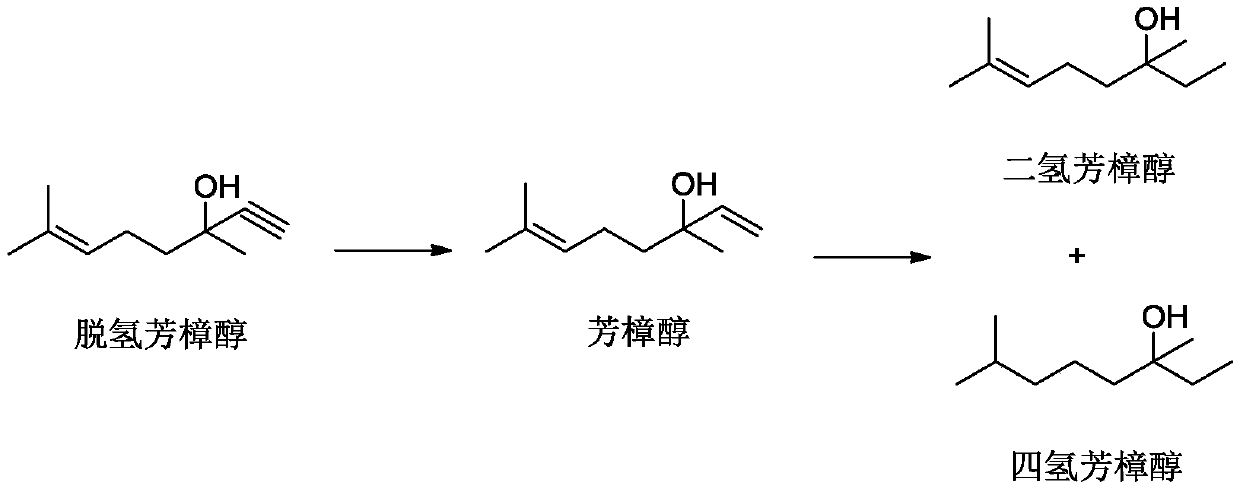
[0034] Add 100 g of n-hexane, 100 g of linalool rectification raffinate, 2 g of 1,2-propylenediamine, 1.0 g of w(Ru)=5% ruthenium carbon catalyst to the autoclave, seal the autoclave, and replace with 4.0 MPa nitrogen 6 times . Use hydrogen pressure (gauge pressure) 4.0MPa to replace 6 times, turn on the stirring paddle, keep hydrogen pressure (gauge pressure) 4.0MPa, and keep the inner temperature of the reactor at 120°C for 6h. After cooling down, stop stirring and release the pressure. Filtration separation reclaims catalyst, and gas chromatographic analysis product is composed of: tetrahydrolinalool 96.5%, linalool 0.1%, dihydrolinalool 0.1%, other 3.3% (wherein the component content of molecular weight greater than 200 accounts for 0.08%). Raw material conversion rate: 99.79%, tetrahydrolinalool selectivity: 99.69%.
Embodiment 2
[0036] Add 100 g of ethanol, 100 g of linalool rectification raffinate, 2 g of ethylenediamine, and 1.0 g of w(Ru)=5% ruthenium carbon catalyst to the autoclave, seal the autoclave, and replace with 6.0 MPa nitrogen 6 times. Use hydrogen pressure (gauge pressure) 6.0MPa to replace 6 times, turn on the stirring paddle, keep the hydrogen pressure (gauge pressure) 6.0MPa, and keep the inner temperature of the reactor at 80°C for 12h. After cooling down, stop stirring and release the pressure. The catalyst was separated and recovered by filtration, and the gas chromatographic analysis product consisted of: tetrahydrolinalool 96.8%, dihydrolinalool 0.1%, and others 3.1% (the content of components with a molecular weight greater than 200 accounted for 0.12% in liquid phase detection). Conversion rate: 99.90%, tetrahydrolinalool selectivity: 99.90%.
Embodiment 3
[0038] Add toluene 150g, linalool rectification raffinate 100g, ethylenediamine 0.2g, w(Ru)=1% ruthenium carbon catalyst 0.5g to autoclave, autoclave is sealed, 0.5MPa nitrogen replacement 6 times. Use a pressure (gauge pressure) of 0.5 MPa hydrogen to replace 6 times, turn on the stirring paddle, keep the hydrogen pressure (gauge pressure) 0.5 MPa, and keep the inner temperature of the reactor at 180°C for 24 hours. After cooling down, stop stirring and release the pressure. Filtration separation reclaims catalyst, and gas chromatographic analysis product is composed of: tetrahydrolinalool 95.8%, dihydrolinalool 0.2%, linalool 0.5%, other 3.5% (wherein the component content of molecular weight greater than 200 accounts for 0.19%). Conversion rate: 99.28%, tetrahydrolinalool selectivity: 99.48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com