Method for preparing citral through rearrangement reaction of dehydrolinalool
A technology of dehydrolinalool and rearrangement reaction, applied in the field of preparation of citral, can solve the problems of difficult separation of catalyst and product, low activity of molybdenum-based catalyst, expensive catalyst, etc., and achieves good economic benefits and application prospects , convenient source of raw materials, high reactivity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
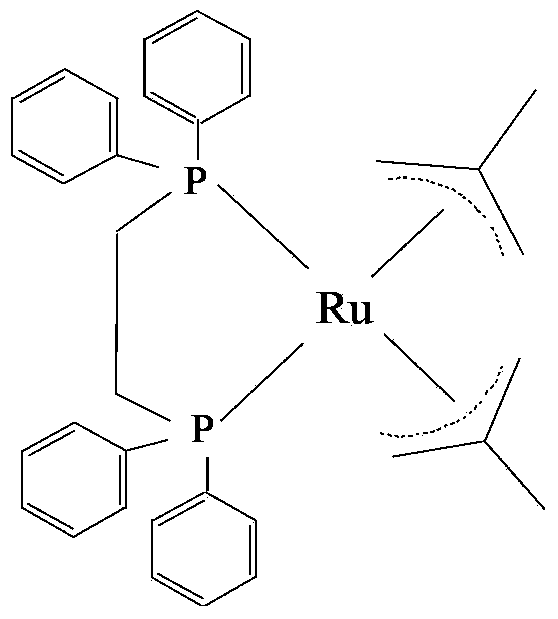
[0022] Preparation of catalyst 1,2-bis(diphenylphosphine)ethane-bis(2-methylpropenyl)ruthenium
[0023] Add 5 g of (1,5-cyclooctadiene) ruthenium dichloride into the tetrahydrofuran solution of 2-methallylmagnesium chloride, stir and heat to reflux for 2 hours under the protection of nitrogen, and filter the solution through diatomaceous earth after the reaction. Add about 100mL of water to the filtrate, extract twice with 100mL of anhydrous ether after hydrolysis, take the organic phase and add anhydrous calcium chloride to remove water, and filter through about 2g of neutral alumina. The filtrate was evaporated to dryness at 70°C, and after cooling, methanol was added to precipitate a gray solid, which was recrystallized from petroleum ether-methanol to obtain bis(2-methallyl)-1,5-cyclooctadiene ruthenium.
[0024] In a 250mL three-necked flask, add 150mL of n-hexane, 5g of bis(2-methallyl)-1,5-cyclooctadiene ruthenium and 6g of 1,2-bis(diphenylphosphine)ethane, Under the p...
Embodiment 2
[0026] Preparation of citral
[0027] Put 30.5g (0.2mol) of dehydrolinalool, 175mL (151g) of solvent toluene, and 24.4g (0.2mol) of benzoic acid into a 250mL three-necked flask, start stirring under the protection of nitrogen, and then add catalyst 1,2 -Bis(diphenylphosphine)ethane-bis(2-methylpropenyl)ruthenium 1.2g (0.2mmol), heat up to 70°C in the first stage, keep the constant temperature for 1h, then heat up to 120°C in the second stage for reaction 6h, after the reaction was finished, samples were taken, and the concentration of each component in the reaction solution was analyzed by gas chromatography, and the reaction conversion rate and yield were calculated. The reaction results are listed in Table 2.
Embodiment 3-11
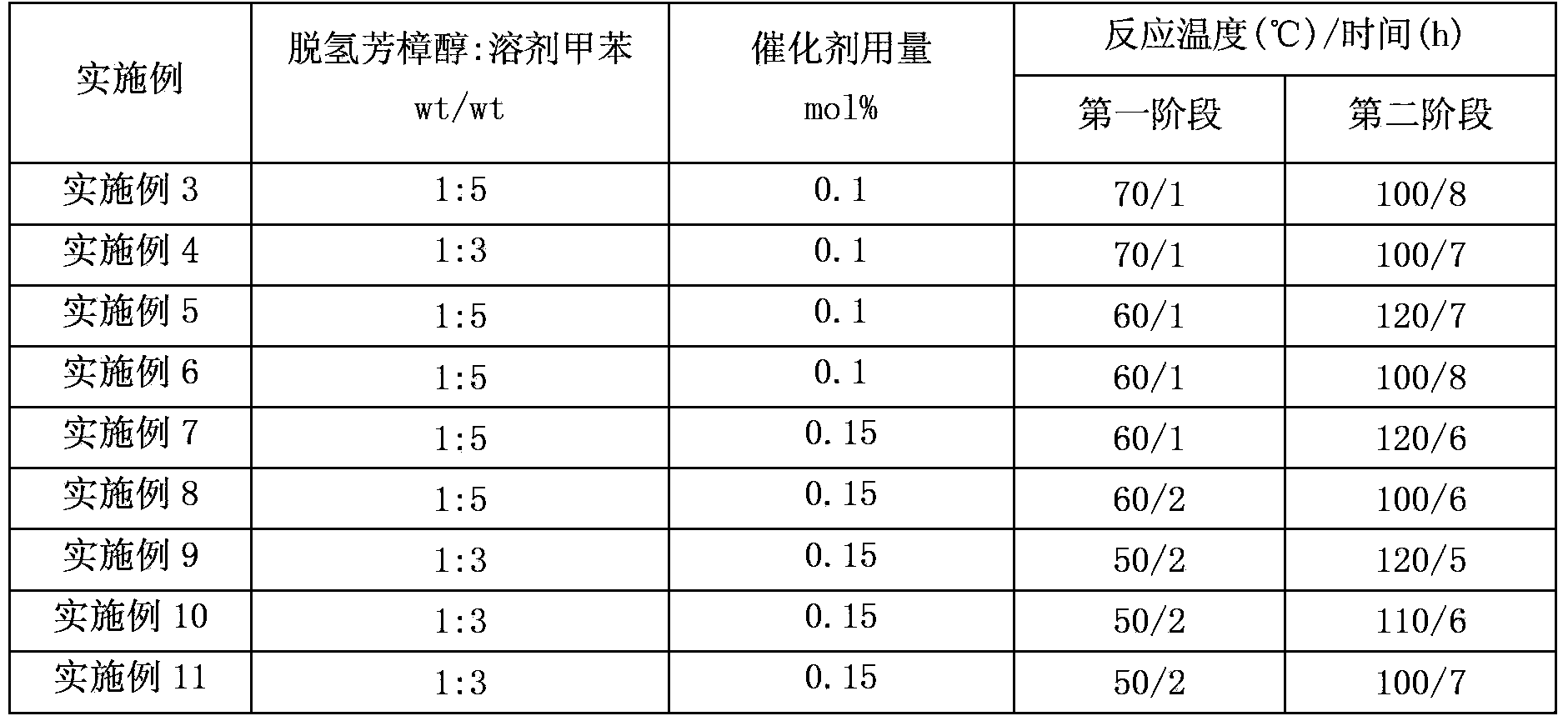
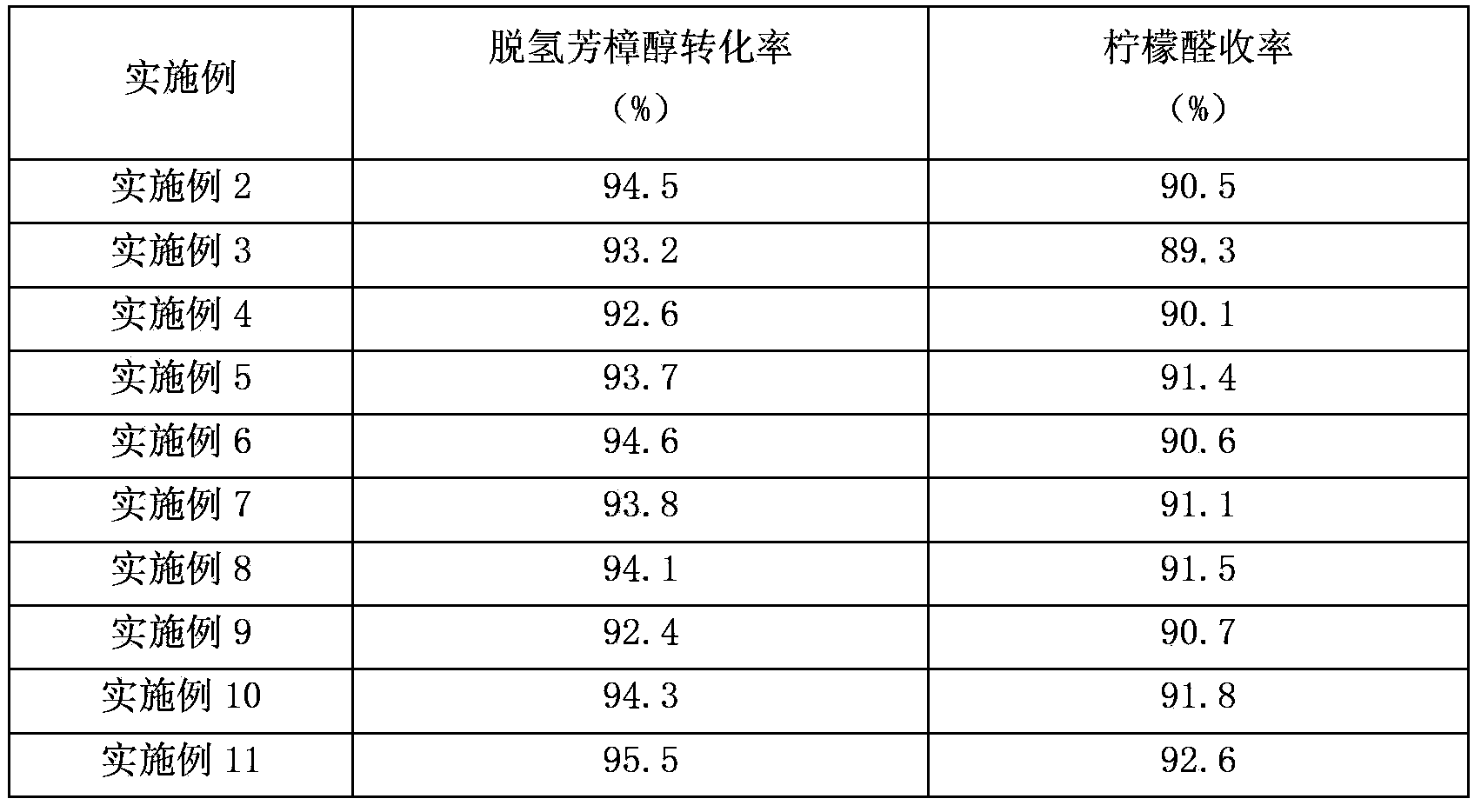
[0029] The specific raw material proportions and reaction conditions of each embodiment are shown in Table 1, and the reaction results are listed in Table 2.
[0030] Table 1
[0031]
[0032] Table 2
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com