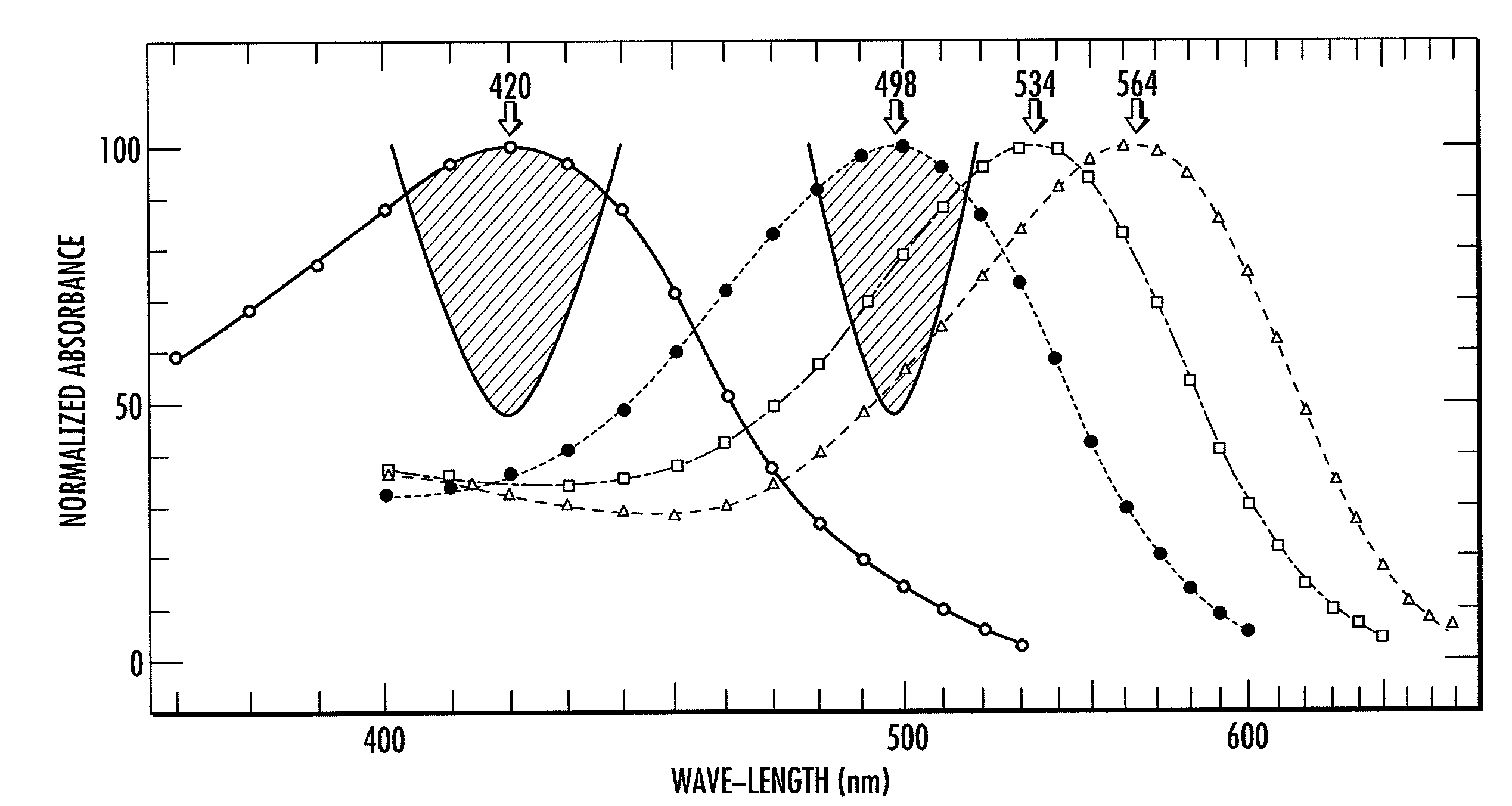
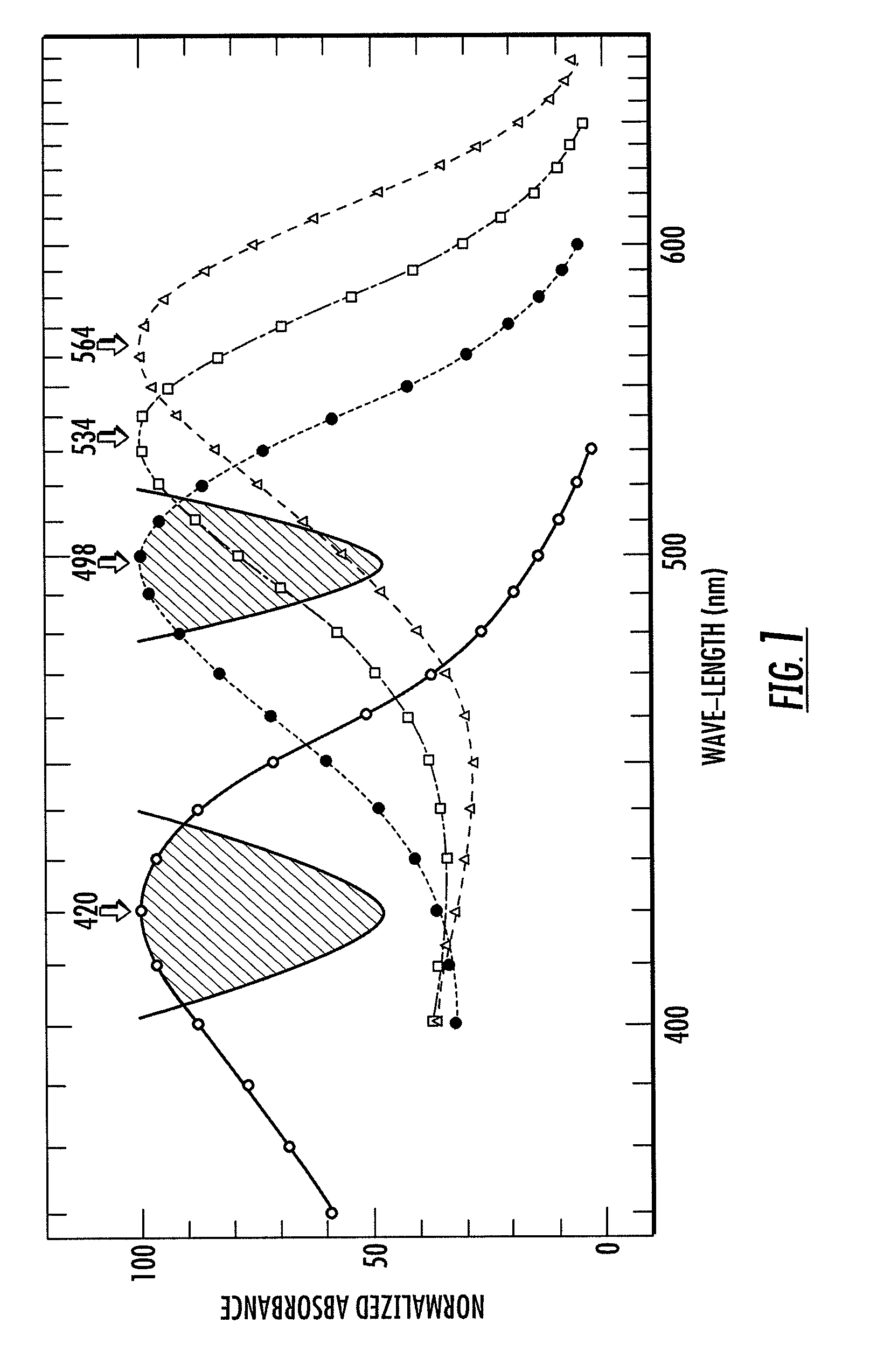
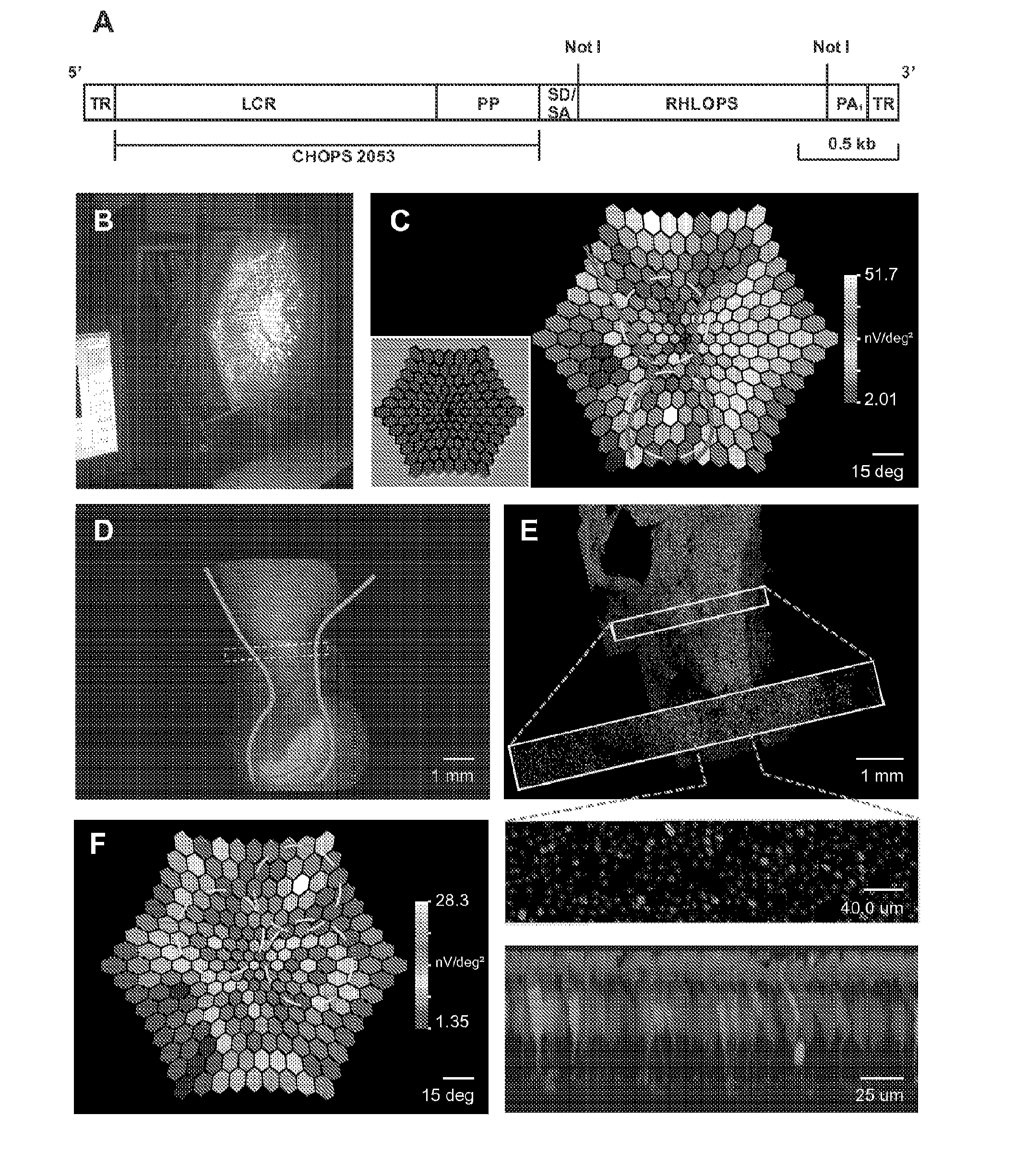
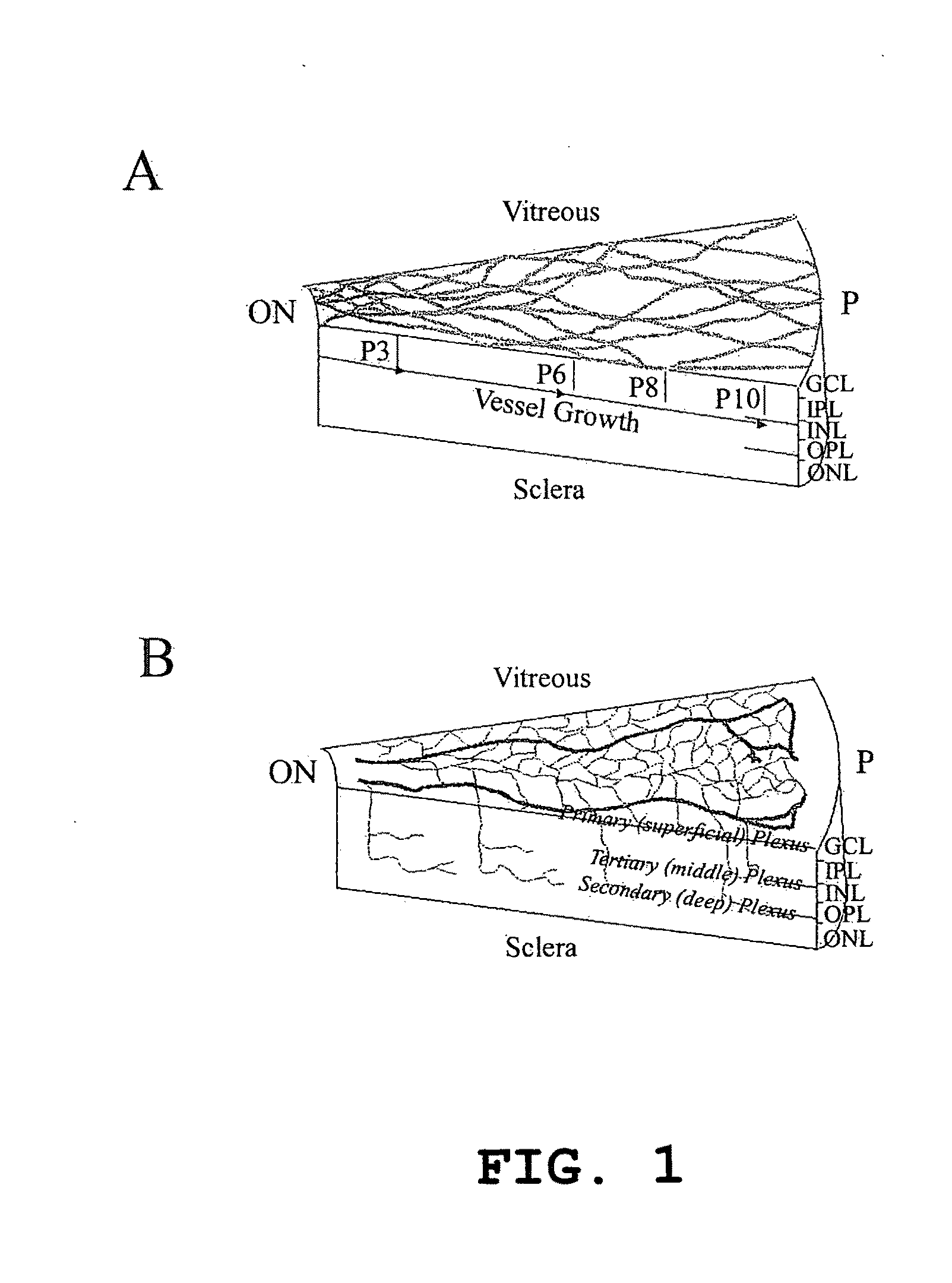
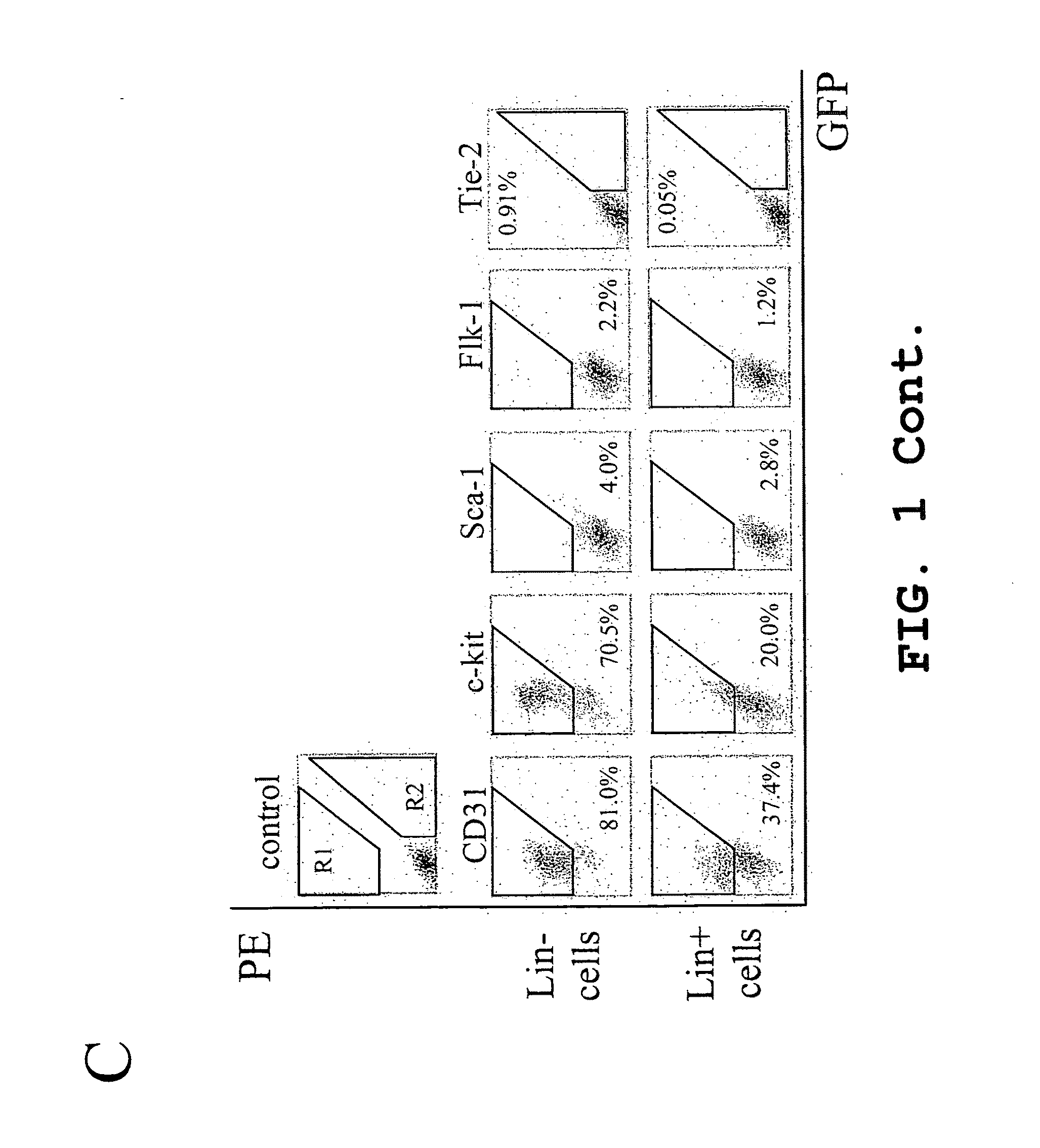
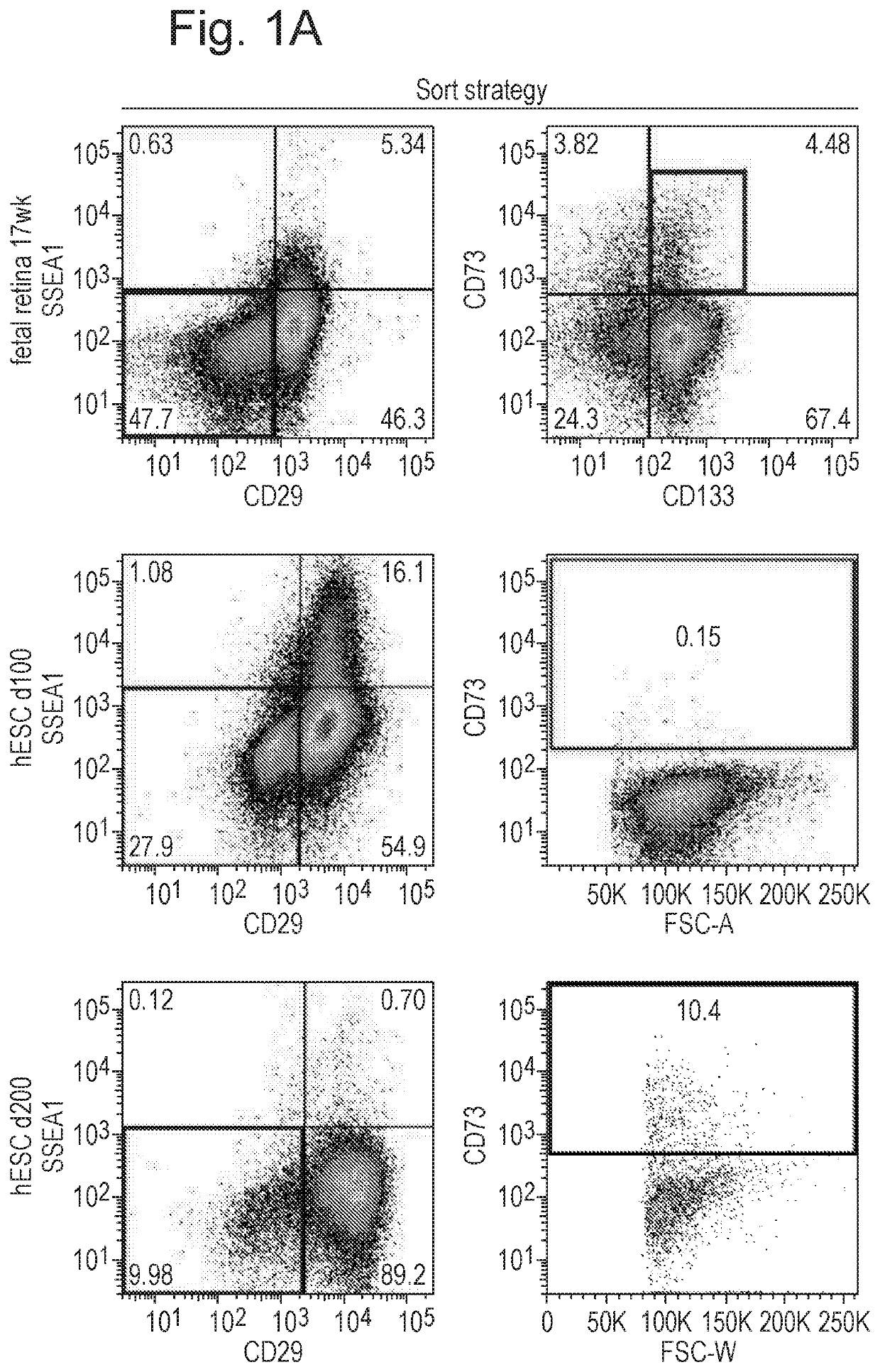
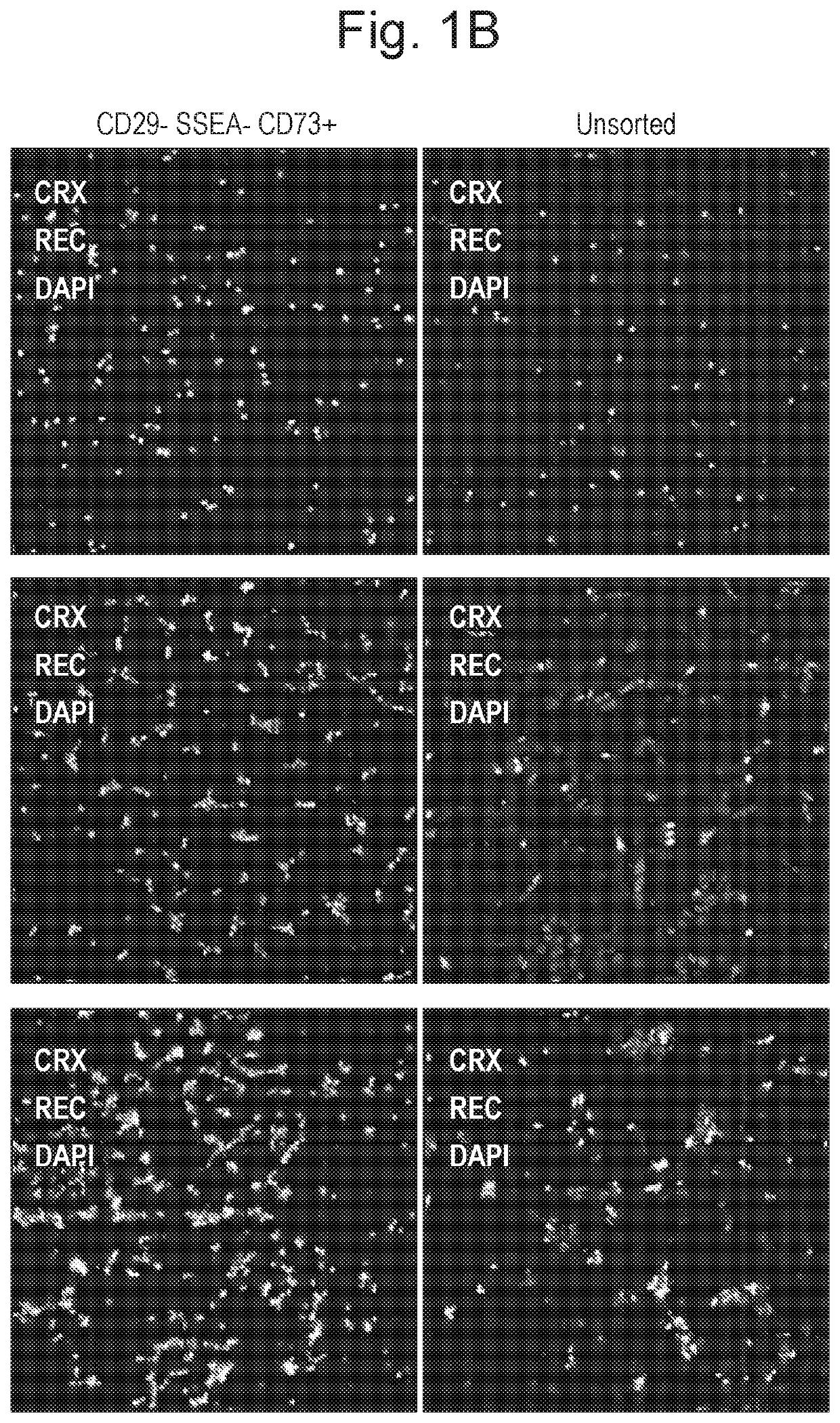
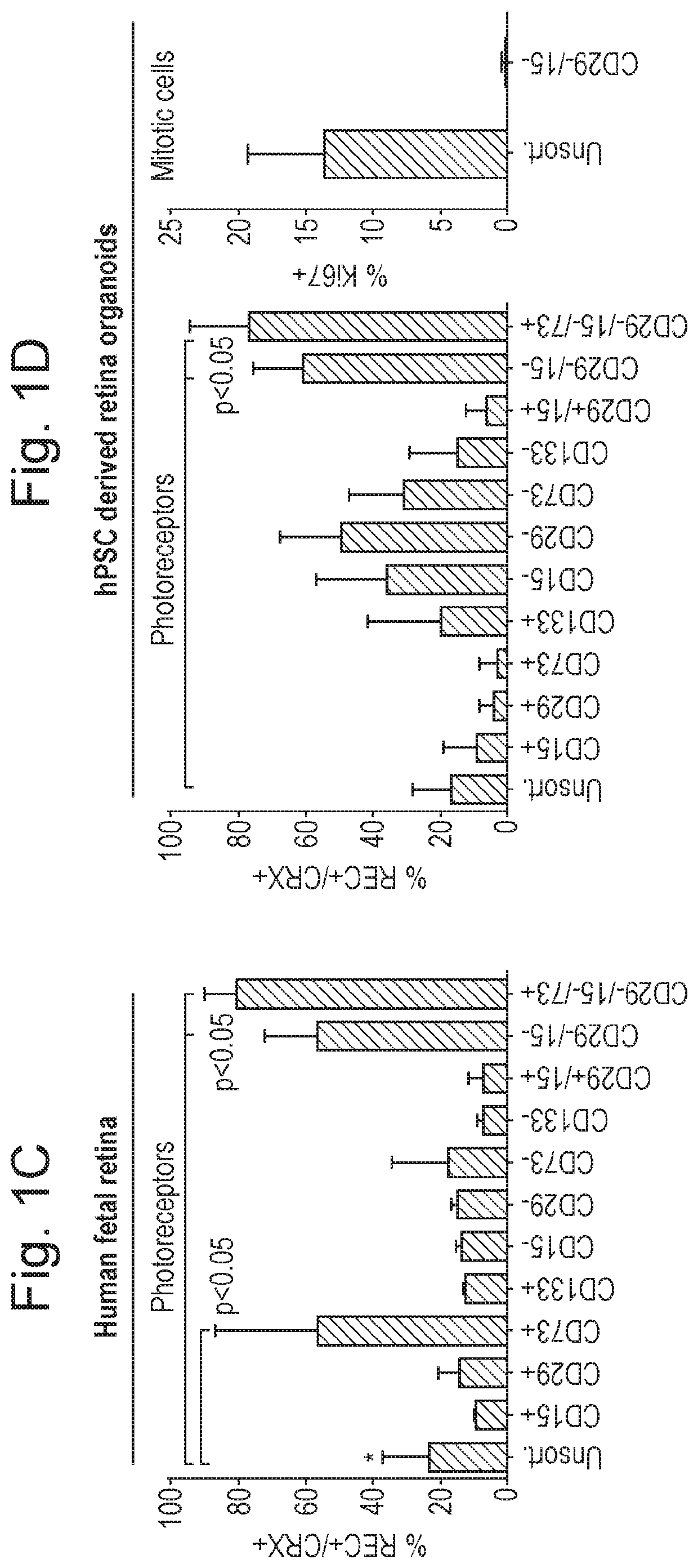
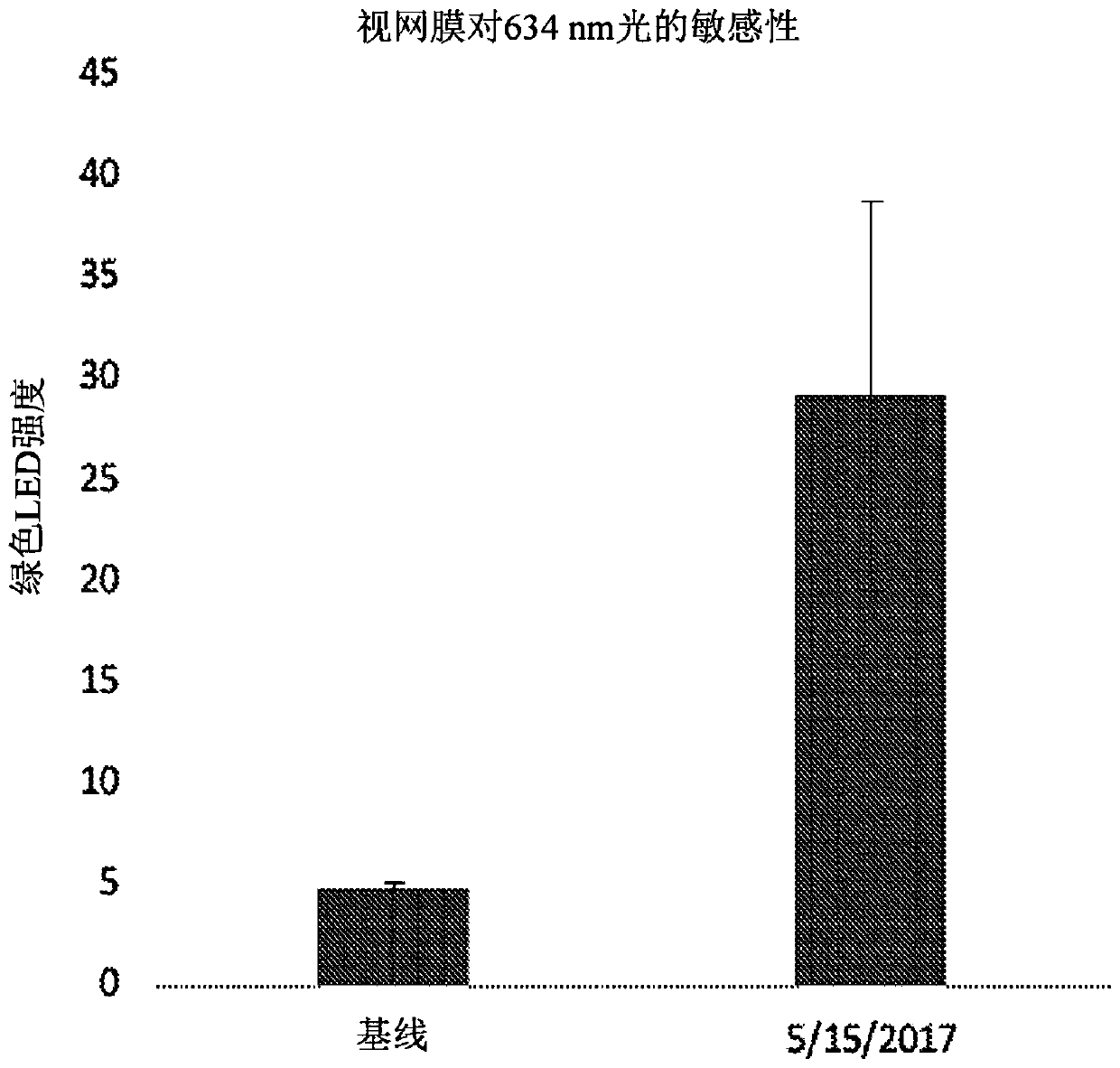
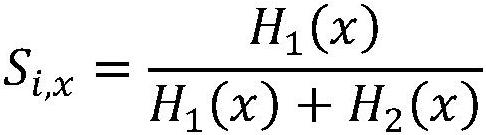Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Cone cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
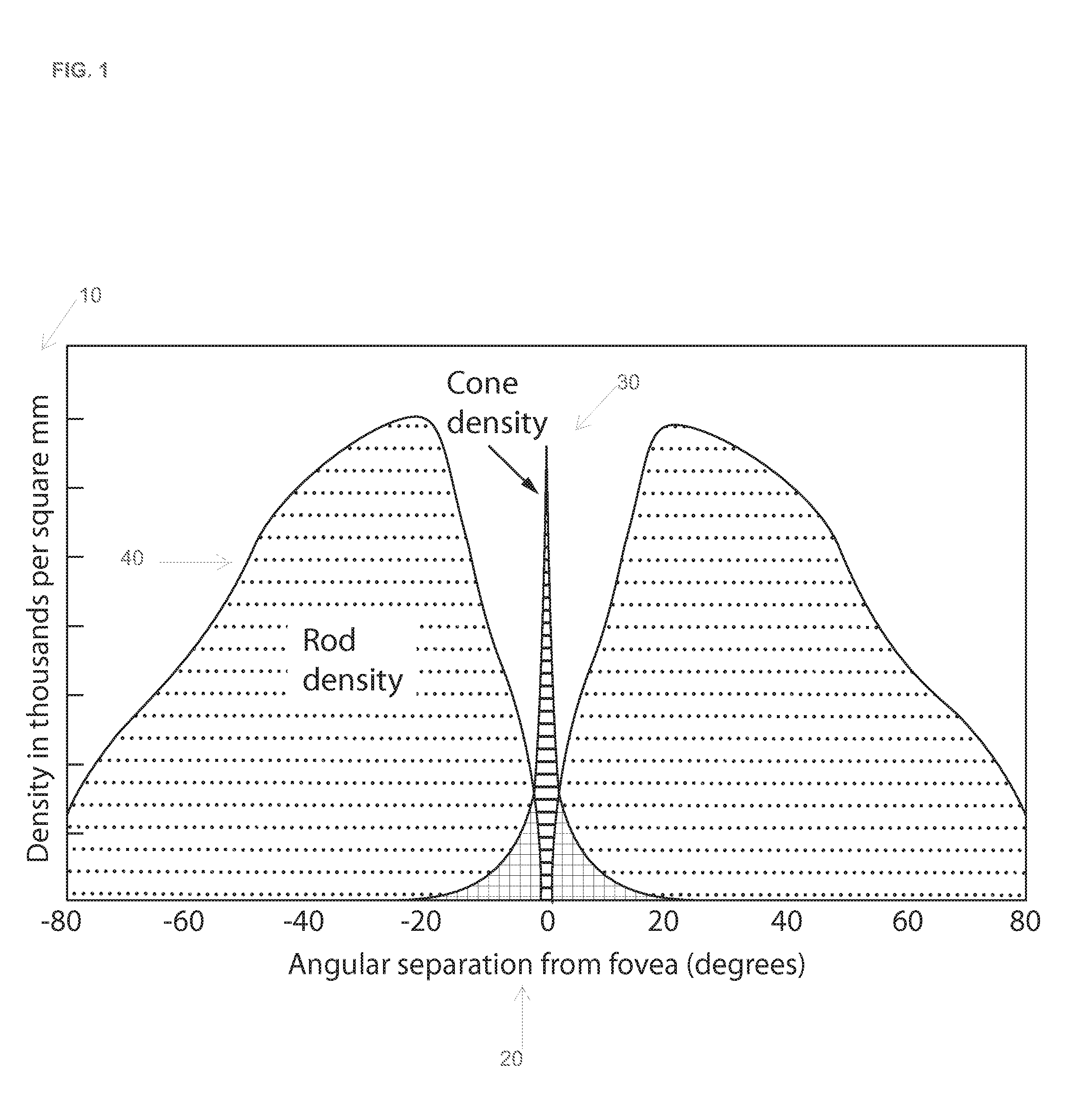
Cone cells, or cones, are photoreceptor cells in the retinas of vertebrate eyes (e.g. the human eye). They respond differently to light of different wavelengths, and are thus responsible for color vision and function best in relatively bright light, as opposed to rod cells, which work better in dim light. Cone cells are densely packed in the fovea centralis, a 0.3 mm diameter rod-free area with very thin, densely packed cones which quickly reduce in number towards the periphery of the retina. There are about six to seven million cones in a human eye and are most concentrated towards the macula. The commonly cited figure of six million cone cells in the human eye was found by Osterberg in 1935. Oyster's textbook (1998) cites work by Curcio et al. (1990) indicating an average close to 4.5 million cone cells and 90 million rod cells in the human retina.
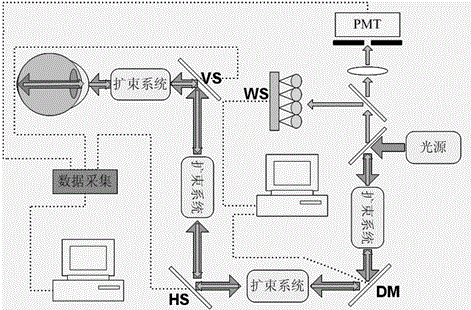
Optical Filter for Selectively Blocking Light
The present invention is directed to an optical filter that attenuates specific areas of the visible spectrum corresponding to the peaks of absorption of both the S-cone and rod cells within the human retina. The optical filter can be configured to also selectively block at least a portion of light centered at either one or both of the peak absorptive wavelengths of the human M and L-cone cells. The optical filter can be included within or on any optical system that is able to transmit all or part of the visible spectrum. As such, the present invention also provides an optical system that acts as a phototoxicity filter for the eye and can be used in conjunction with any material where visible light has at least partial transmittance.
Owner:CHIAVETTA III STEPHEN V
Reagents and methods for modulating cone photoreceptor activity
InactiveUS20120172419A1Restore visionNew colorSenses disorderVirus peptidesPhotoreceptor activityReagent
Owner:MCW RES FOUND INC +1
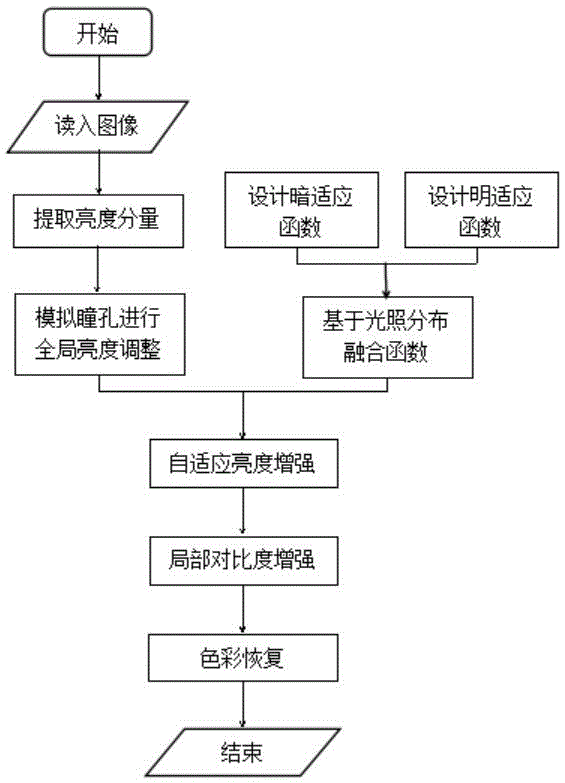
Human visual perception simulation-based self-adaptive low-illumination image enhancement method
ActiveCN105046663AIncrease brightness levelEnhancement effect is goodImage enhancementColor imagePupil
Owner:SOUTHWEAT UNIV OF SCI & TECH
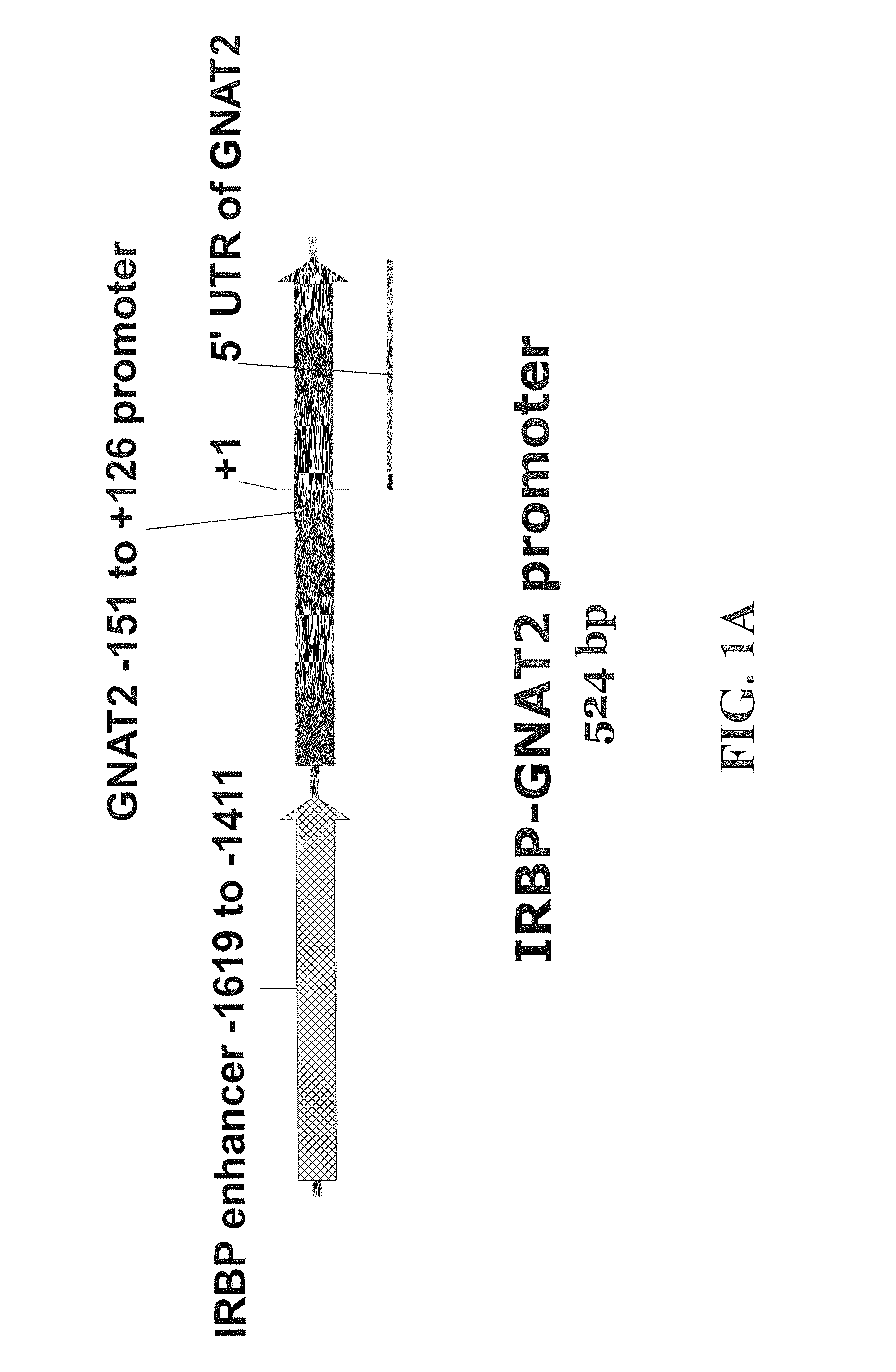
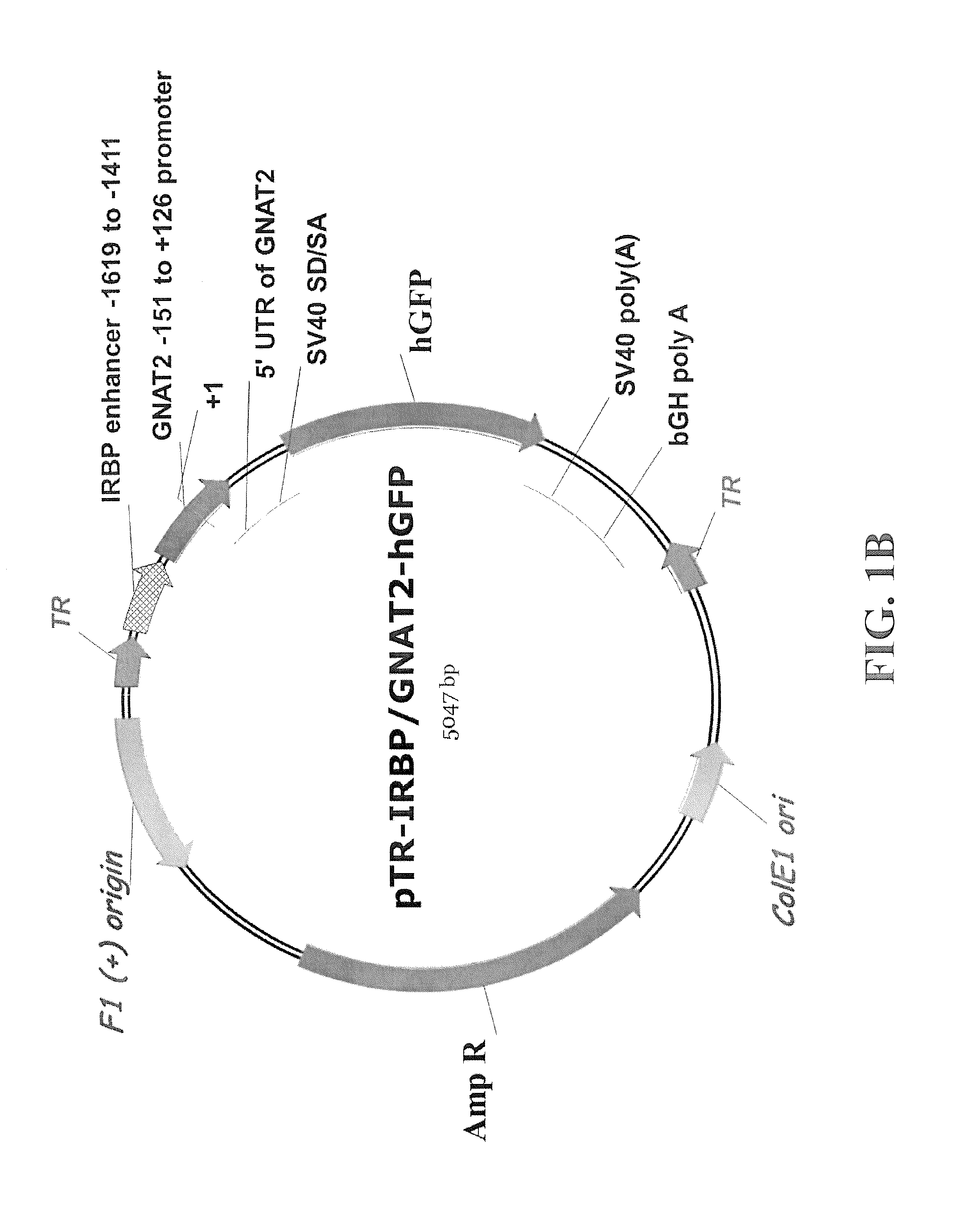
Chimeric promoter for cone photoreceptor targeted gene therapy
The subject invention concerns materials and methods for providing for cone cell specific expression of a polynucleotide in a human or animal. One aspect of the invention concerns a polynucleotide promoter sequence that directs expression of an operably linked polynucleotide in cone cells. In one embodiment, a polynucleotide of the invention comprises a nucleotide sequence of an interphotoreceptor retinoid-binding protein (IRBP) gene that is positioned upstream of a promoter nucleotide sequence of a cone transducin alpha-subunit (GNAT2) gene. Another aspect of the subject invention concerns methods for expressing a selected polynucleotide in cone cells. The selected polynucleotide can be provided in a polynucleotide of the invention wherein the selected polynucleotide is operably linked to a polynucleotide promoter sequence of the invention. In one embodiment, the selected polynucleotide sequence is provided in a polynucleotide vector of the invention. The vector comprising the selected polynucleotide is then introduced into a cell. The selected polynucleotide is expressed only in cone cells, with very little, if any, expression in rods or other cells. A selected polynucleotide can be one that encodes, for example, a therapeutic protein or a functional protein that is defective or underexpressed in the targeted cone cells.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
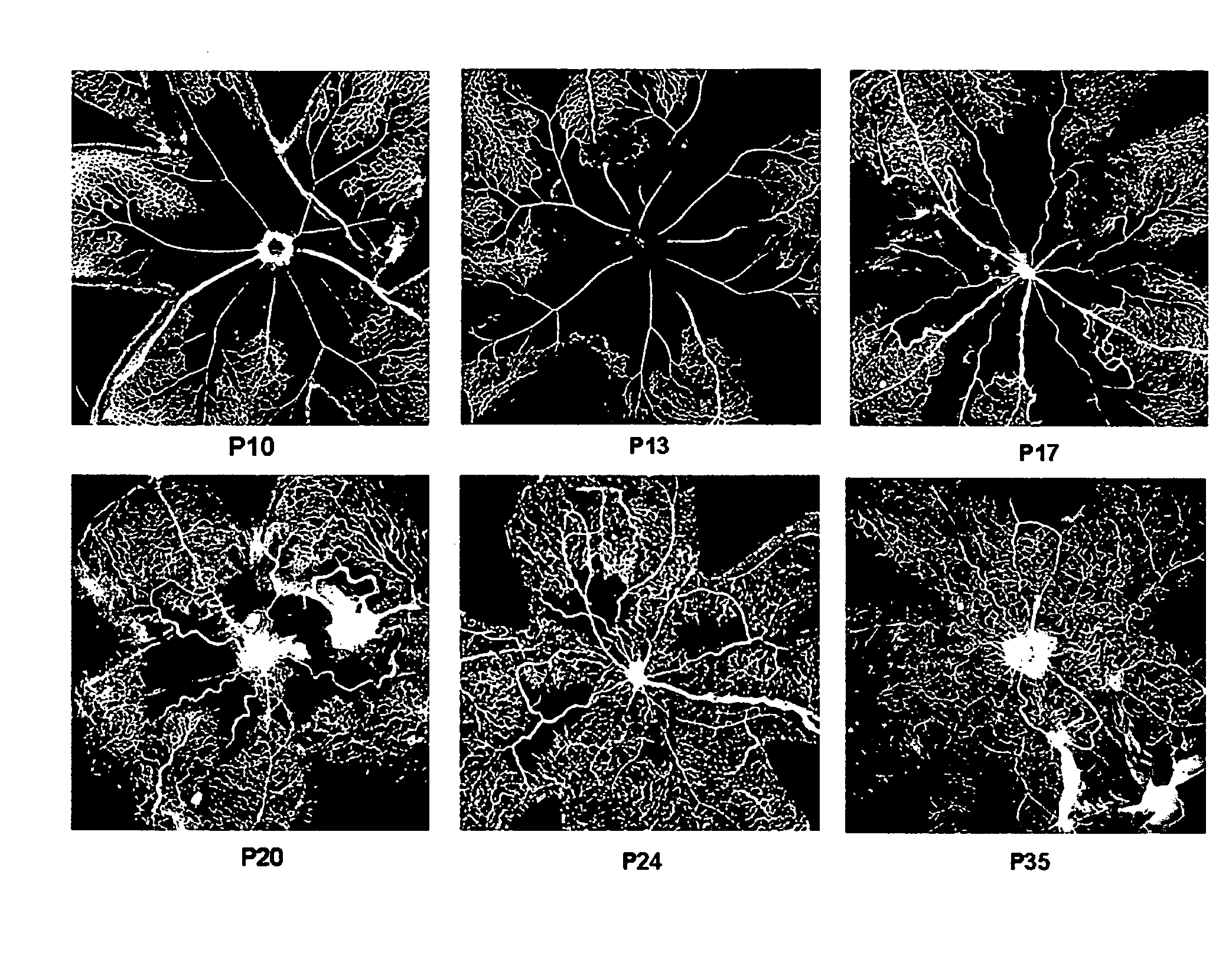
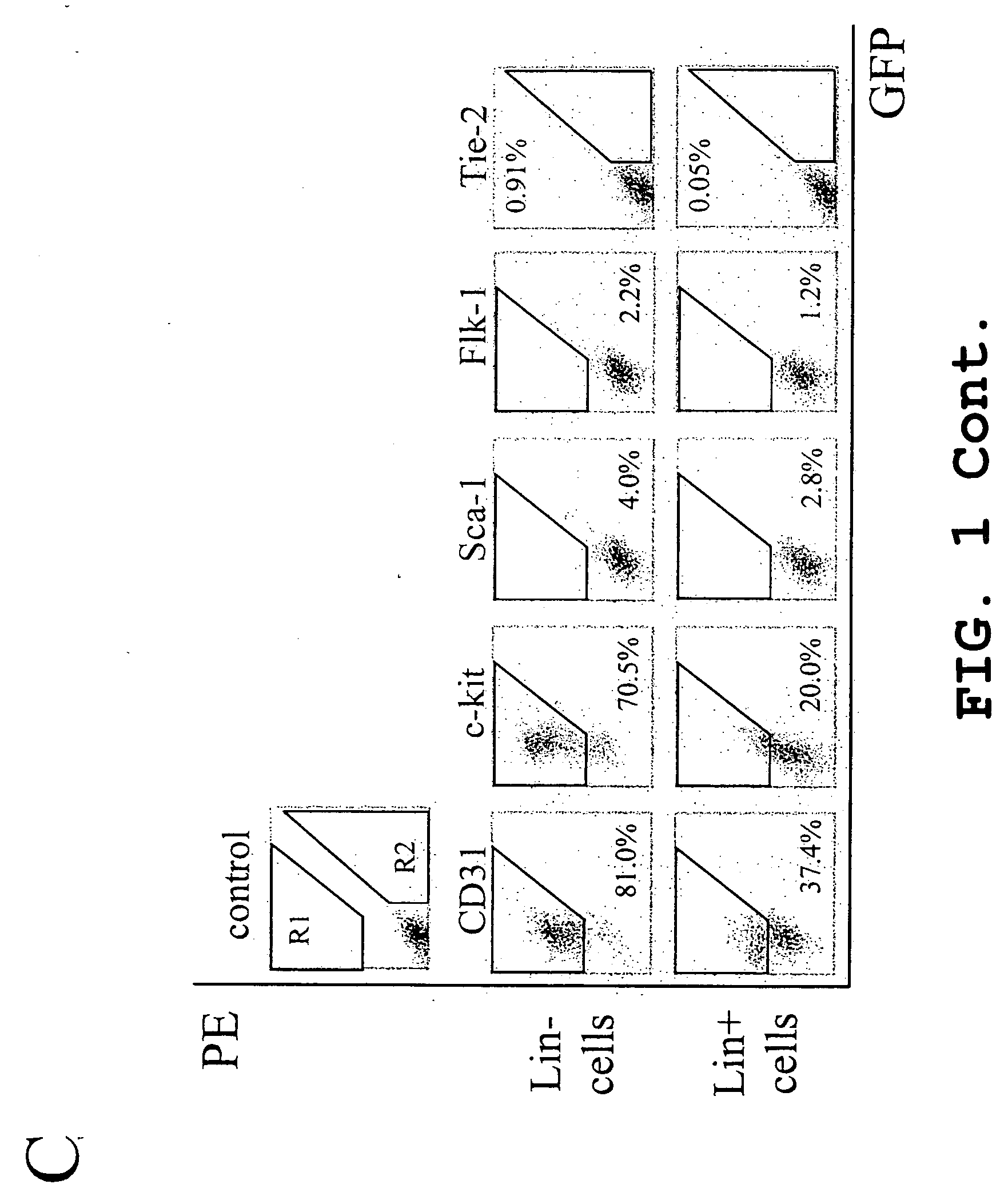
Treatment of cone cell degeneration with transfected lineage negative hematopoietic stem cells
A method of preserving cone cells in the eye of a mammal suffering from a retinal degenerative disease comprises isolating from the bone marrow of the mammal a lineage negative hematopoietic stem cell population that includes endothelial progenitor cells, transfecting cells from the stem cell population with a gene that operably encodes an antiangiogenic fragment of human tryptophanyl tRNA synthetase (TrpRS), and subsequently intravitreally injecting the transfected cells into the eye of the mammal in an amount sufficient to inhibit the degeneration of cone cells in the retina of the eye. The treatment may be enhanced by stimulating proliferation of activated astrocytes in the retina using a laser.
Owner:THE SCRIPPS RES INST
Light field display device
ActiveCN111624784AAvoid dizzinessAvoid visual fatigueSolid-state devicesSteroscopic systemsDisplay deviceEngineering
The invention provides a light field display device, relates to the technical field of display, and aims to solve the problem of visual fatigue caused by inconsistent monocular focusing depth and binocular convergence depth and prevent a user from feeling dizzy. The light field display device comprises a display screen and a lens unit arranged on the light emitting side of the display screen, wherein the lens unit comprises a plurality of lenses arranged in an array; the display screen is arranged on the focal planes of the plurality of lenses; the display screen comprises a first substrate and a second substrate which are oppositely combined, wherein the first substrate is a light emitting side substrate, the second substrate comprises a plurality of pixel islands arranged in an array, and each pixel island comprises at least one sub-pixel; the plurality of pixel islands correspond to the plurality of lenses; light rays emitted by sub-pixels of the pixel island are transmitted to a view area formed by human eyes through the corresponding lenses to be smaller than or equal to a half pupil area, and light rays, emitted by different sub-pixels in the pixel island, of different viewpoints are emitted into different view cone cells through the lenses. The invention is suitable for manufacturing the light field display device.
Owner:BOE TECH GRP CO LTD
Isolated lineage negative hematopoietic stem cells and methods of treatment therewith
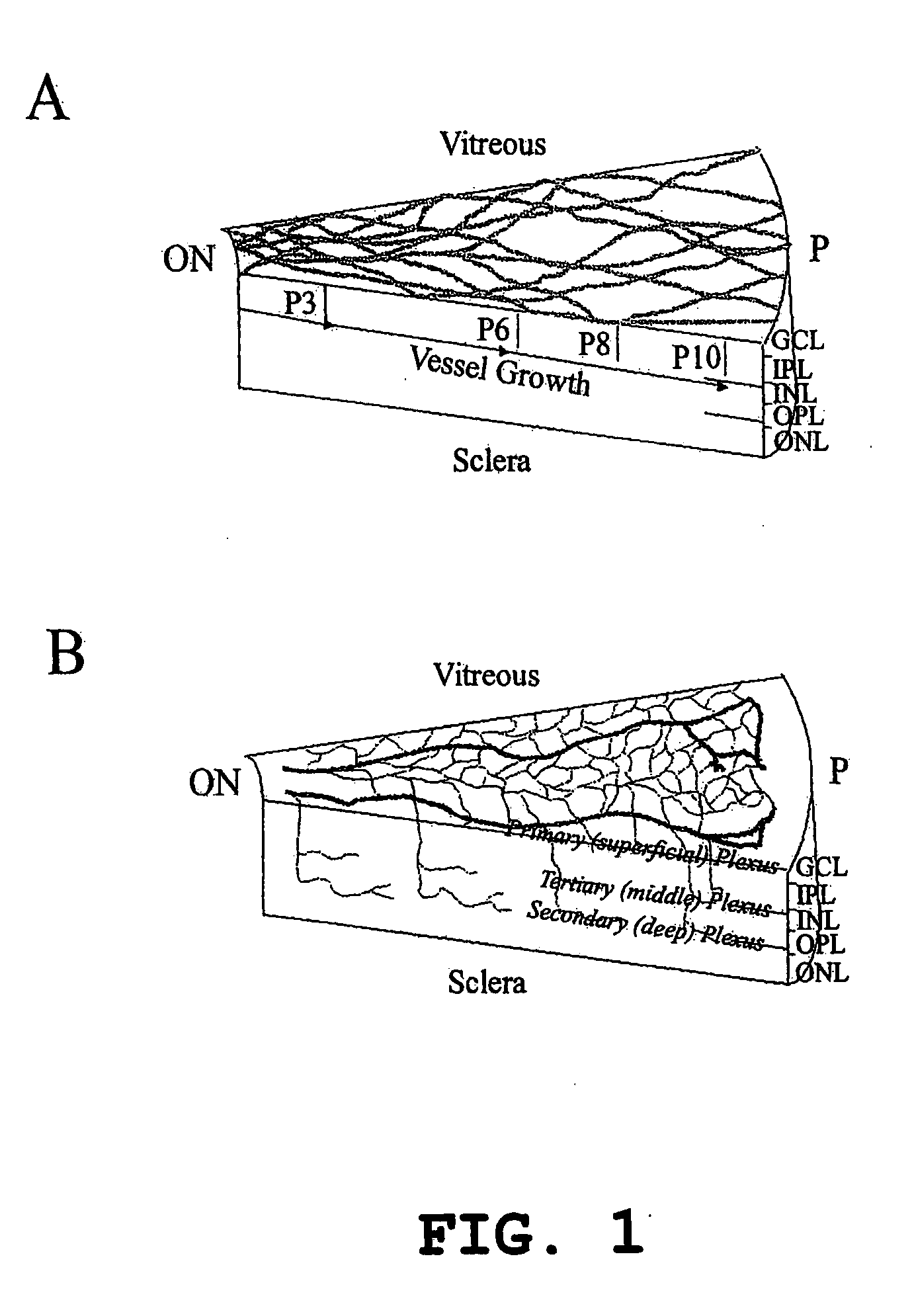
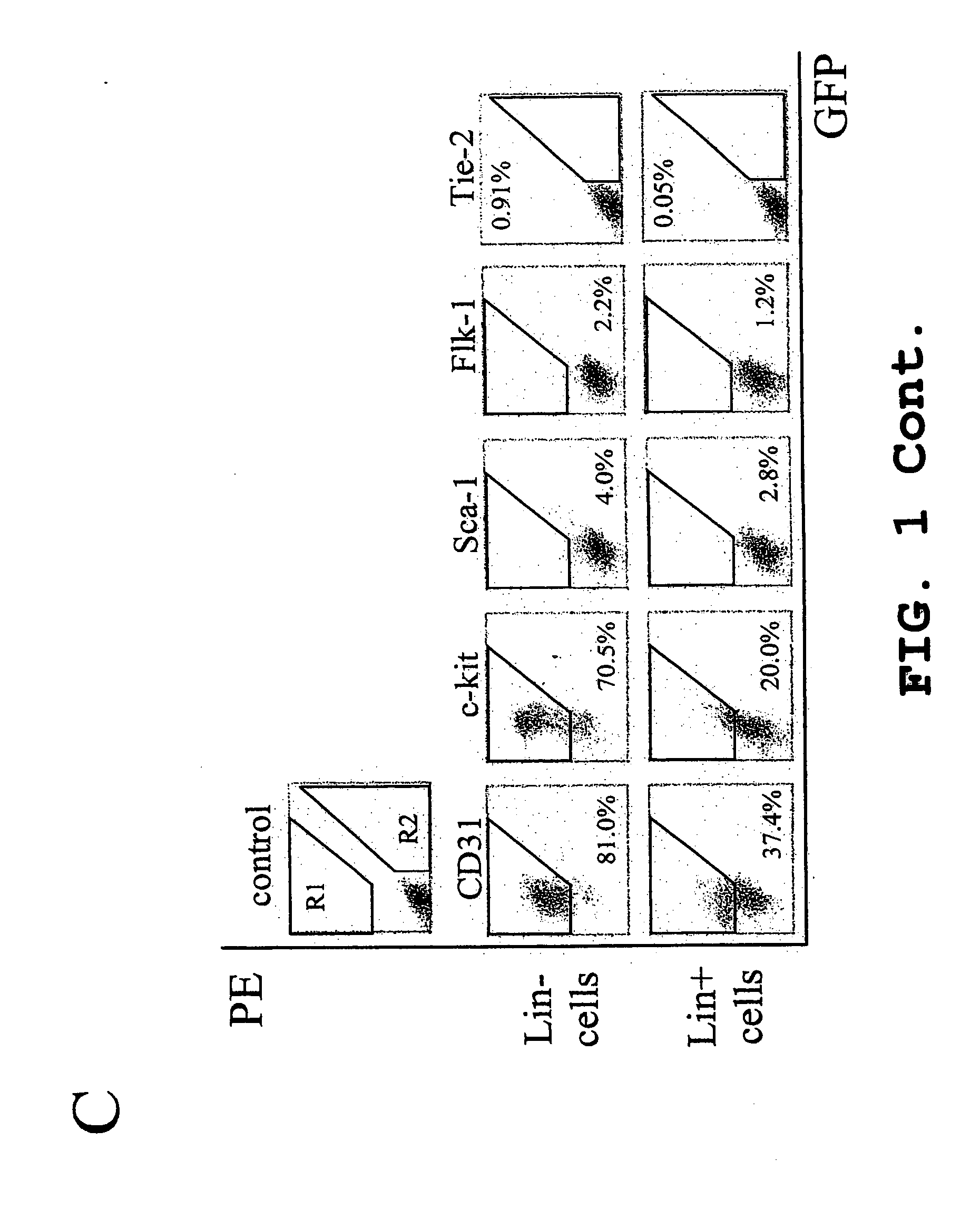
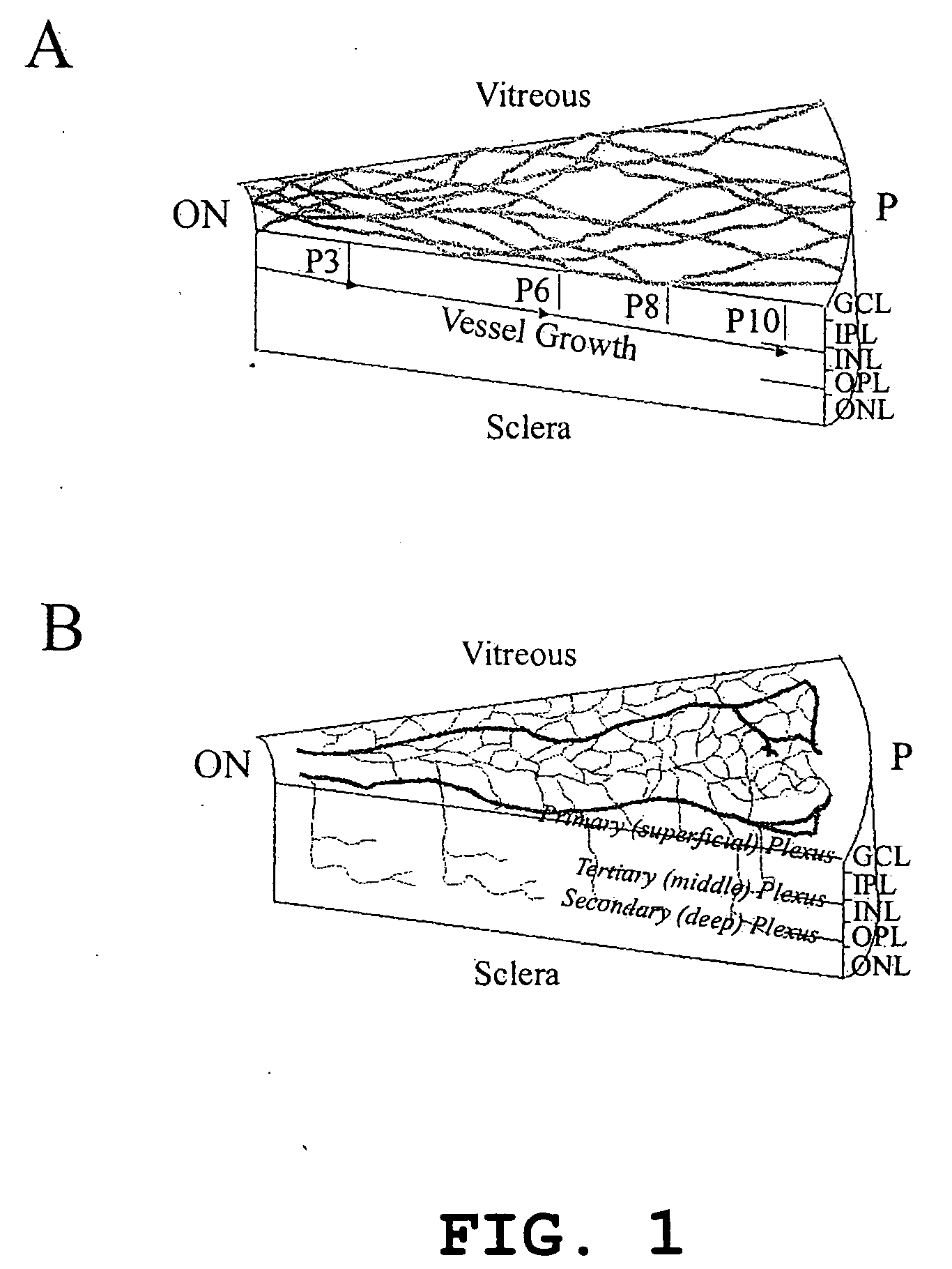
Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations (Lin− HSCs) contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. Preferably at least about 20% of the cells in the isolated Lini HSCs express the cell surface antigen CD31. The isolated Lin− HSC populations are useful for treatment of ocular vascular diseases and to ameliorate cone cell degeneration in the retina. In a preferred embodiment, the Lin− HSCs are isolated by extracting bone marrow from an adult mammal; separating a plurality of monocytes from the bone marrow; labeling the monocytes with biotin-conjugated lineage panel antibodies to one or more lineage surface antigens; removing of monocytes that are positive for the lineage surface antigens from the plurality of monocytes, and recovering a Lin− HSC population containing EPCs. The isolated Lin− HSCs also can be transfected with therapeutically useful genes. The treatment may be enhanced by stimulating proliferation of activated astrocytes in the retina using a laser.
Owner:THE SCRIPPS RES INST
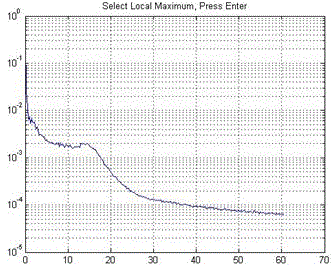
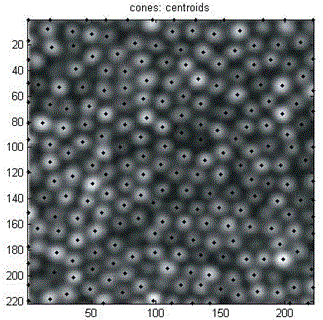
Cone-cell density calculation method based on image identification
ActiveCN103745257AFast statisticsHigh degree of automationCounting objects with random distributionSpecial data processing applicationsLaser scanningResponse Frequency
The invention relates to a cone-cell density calculation method based on image identification. The method mainly solves the prior problem that density counting of cone cells is performed manually and an eye-ground adaptive confocal laser scanning ophthalmoscope is used to obtain a visual cone-cell image and after the response frequency of the cone cells is determined, low-pass filtering is used for denoising processing so as to establish a two-valued sequence. A local maximum value corresponding to a non-zero area is searched for so that the central position of the cone cells is found. After automatic identification of the visual cone cells in the image is completed, the number of the cone cells in the image is obtained with the assistance of manual correction and finally the density counting of the cone cells is achieved. When the function which adopts a computer to counting automatically is compared with a manual counting method, the automatic calculation method is accurate, high in statistical speed, high in automation degree, checkable in analysis result and improved in detection efficiency. At the same time, a manual correction function is added so that on the basis of software analysis, error identification can be removed manually and missed cone cells can be added and thus caused result deviation is prevented.
Owner:SCHOOL OF OPHTHALMOLOGY & OPTOMETRY WENZHOU MEDICAL COLLEGE
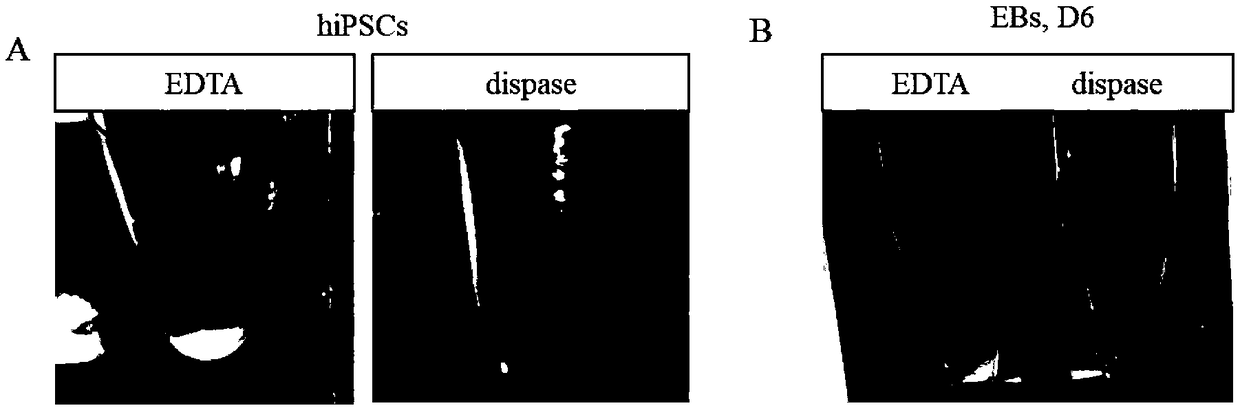
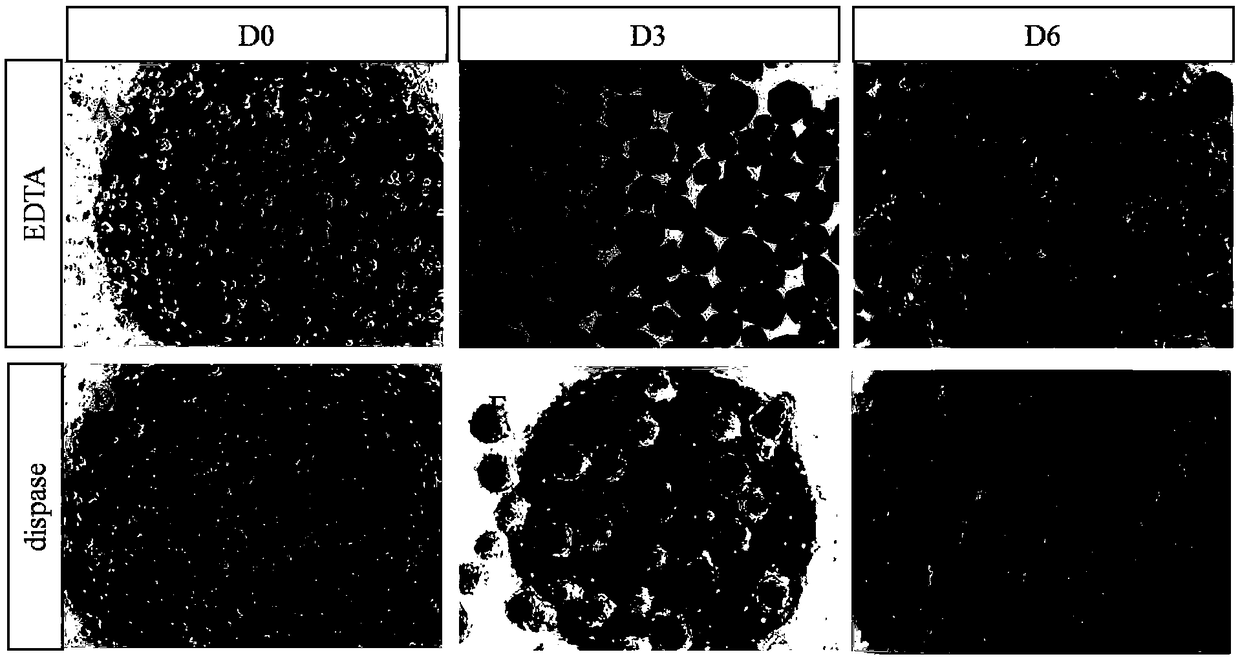
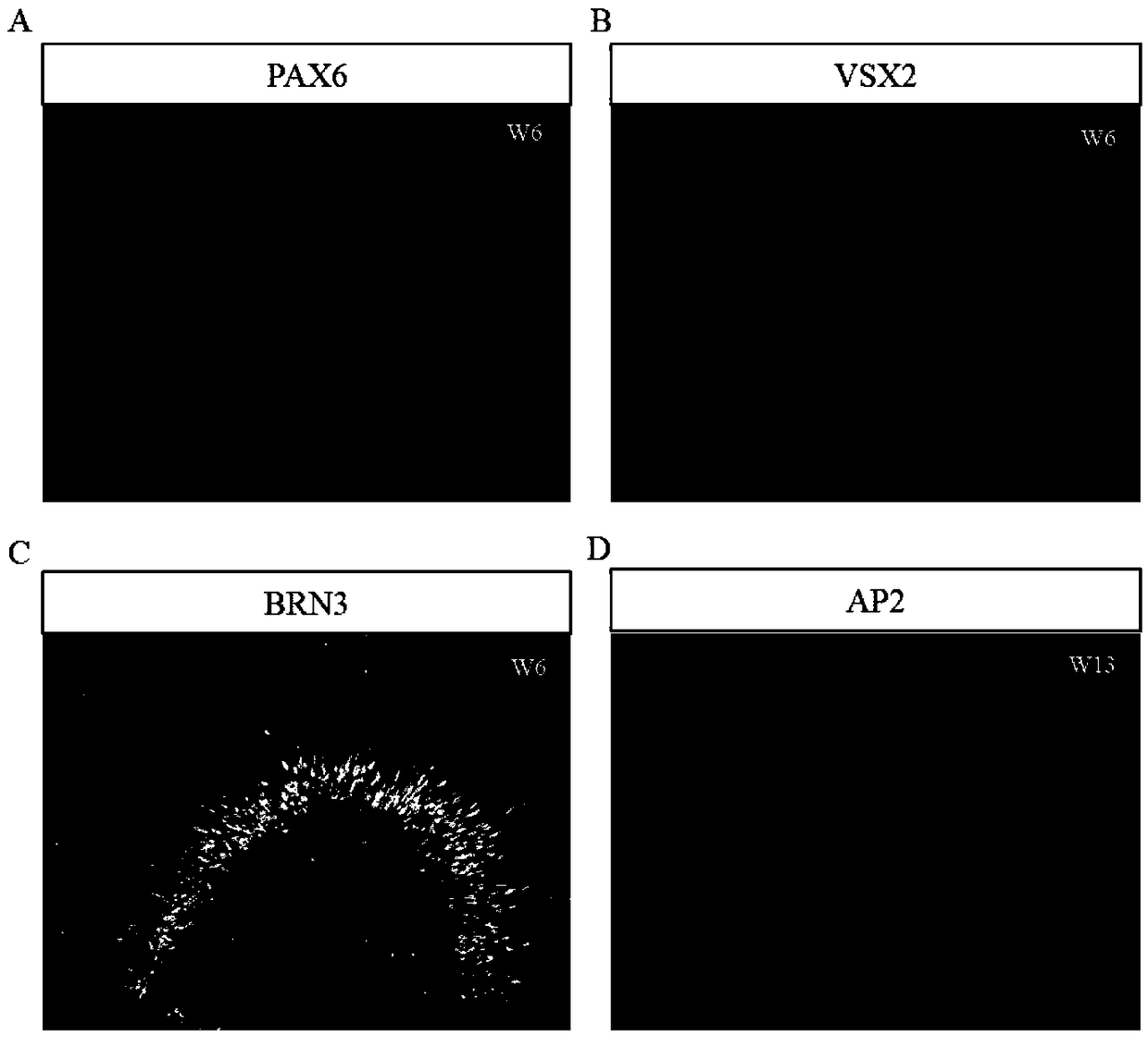
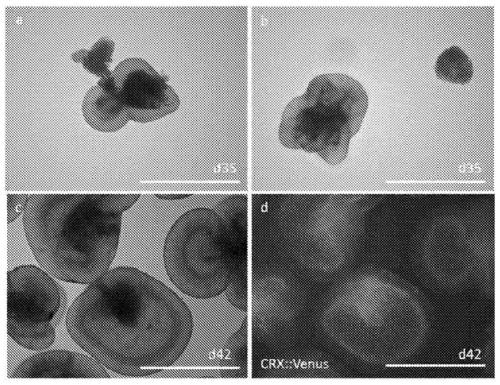
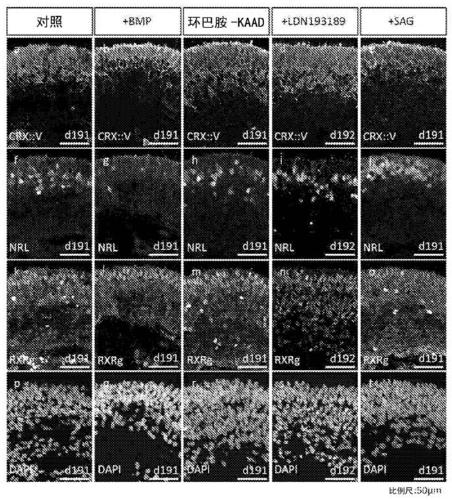
Method for obtaining analogous retinal tissues rich in cone and rod cells by using human induced pluripotent stem cells
ActiveCN108795864AShort digestion timeHigh activityNervous system cellsArtificially induced pluripotent cellsMatrigelOptic tectum
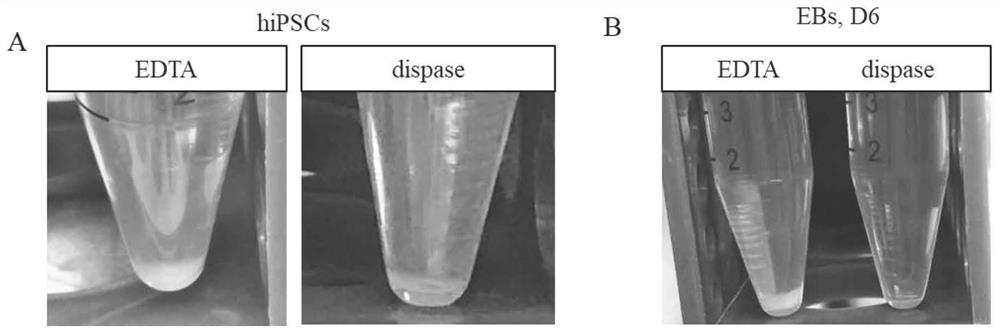
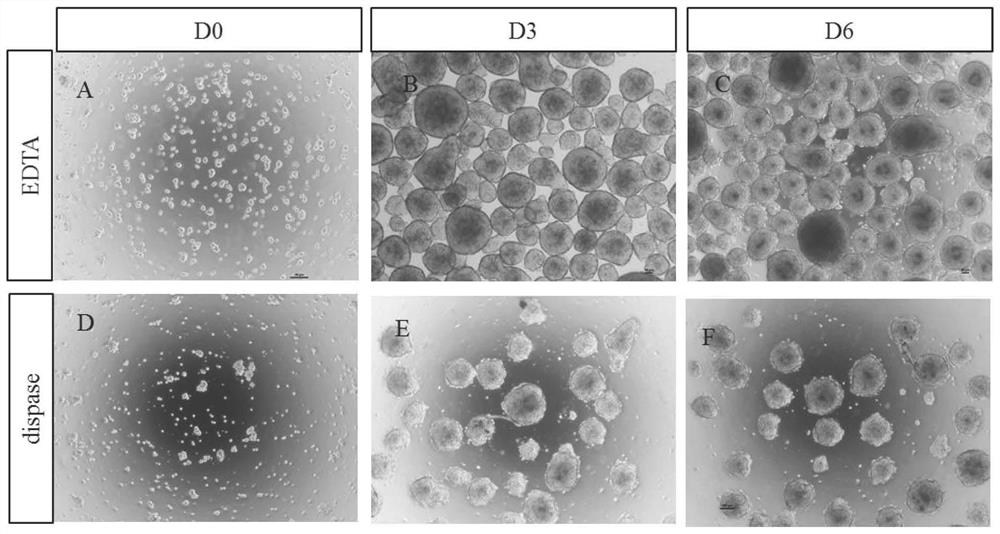
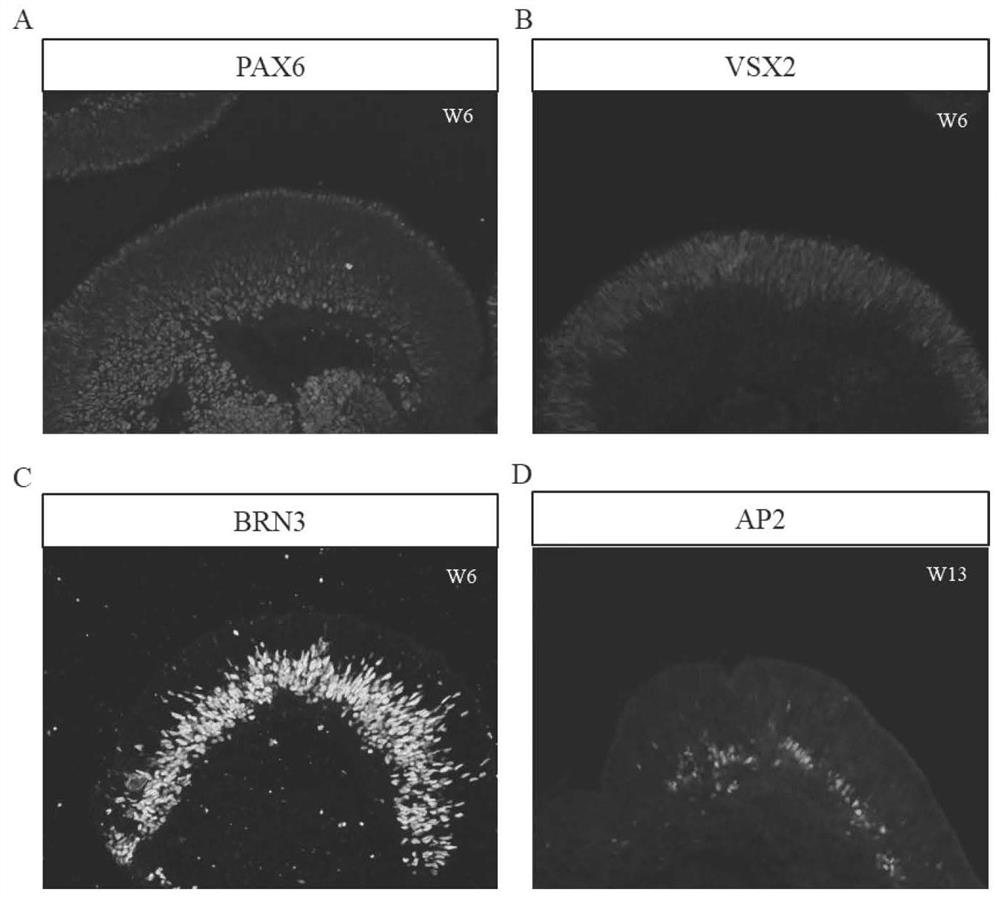
The invention discloses a mmethod for obtaining analogous retinal tissues rich in cone and rod cells by using human induced pluripotent stem cells. The method comprises the following steps: digestinghiPSCs to obtain cell pellets, and then carrying out suspension cultivation on the cell pellets to obtain an embryoid body; reinoculating the embryoid body into a culture dish enveloped by Matrigel, and carrying out induced differentiation inside an induced cultivation solution to obtain nerve retina (NR) and RPE; stirring up the NR and the RPE, carrying out suspension cultivation to obtain 3D analogous retina comprising NR tissues, then carrying out continuous suspension cultivation inside an optimized culture solution without retinoic acid, carrying out NR differentiation on all retinal cells, comprising highly matured photoreceptor cells, Rhodopsin positive rod cells, L / M OPSIN positive red and green cone cells and S-OPSIN positive blue cone cells, and thus particularly obtaining the analogous retinal tissues rich in red and green cone and rod cells.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV +1
Underwater image enhancement method based on fish retina mechanism
ActiveCN107169942AConform to the visual mechanismImage enhancementImage analysisCone cellVisual perception
The invention discloses an underwater image enhancement method based on a fish retina mechanism. According to the method, a feedback relation between fish retina horizontal cells and cone cells is simulated to remove the color cast of an underwater image, and a center-periphery antagonistic effect of fish retina bipolar cells is simulated to remove blur of the underwater image. In the whole simulation process, a structure for suppressing the bipolar cells at the fish retina horizontal cell side is simulated so as to design a dual-Gauss difference filter of a receptive field of the bipolar cells; and meanwhile, an activity of interplexiform cells for continuously releasing Dopamine in dark to regulate the horizontal cells is simulated by using a sigmoid curve, and thus the processed image is enabled to more conform to a vision mechanism of the fish; and finally nonlinear processing of amacrine cells for brightness information is simulated by adopting gamma conversion, and central input of color bipolar cells is formed.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Isolated lineage negative hematopoietic stem cells and methods of treatment therewith
Isolated, mammalian, adult bone marrow-derived, lineage negative hematopoietic stem cell populations (Lin− HSCs) contain endothelial progenitor cells (EPCs) capable of rescuing retinal blood vessels and neuronal networks in the eye. Preferably at least about 20% of the cells in the isolated Lin− HSCs express the cell surface antigen CD31. The isolated Lin− HSC populations are useful for treatment of ocular vascular diseases and to ameliorate cone cell degeneration in the retina. In a preferred embodiment, the Lin− HSCs are isolated by extracting bone marrow from an adult mammal; separating a plurality of monocytes from the bone marrow; labeling the monocytes with biotin-conjugated lineage panel antibodies to one or more lineage surface antigens; removing of monocytes that are positive for the lineage surface antigens from the plurality of monocytes, and recovering a Lin− HSC population containing EPCs. The isolated Lin− HSCs also can be transfected with therapeutically useful genes. The treatment may be enhanced by stimulating proliferation of activated astrocytes in the retina using a laser.
Owner:THE SCRIPPS RES INST
Intraocular display device based on retinal scanning
ActiveCN110955063ASolve the problem that the position must be fixed or even cannot be rotatedResolving imaging with the diopter of the human eye lensStatic indicating devicesNon-linear opticsGratingMixed reality
The invention discloses an intraocular display device based on retinal scanning. The device comprises a first flexible substrate on the same side as an eyelid, a second flexible substrate on the sameside as a cornea, a light emitting diode array arranged between the first flexible substrate and the second flexible substrate, a light collimator, an electric control liquid crystal grating used foradjusting the light direction, and a control unit. The light emitting diode array is connected with the light collimator, and the control unit is connected with the light emitting diode array and theelectric control liquid crystal grating and receives and transmits image information and light angle information; the device is worn on the cornea of a human eye, so that exit pupil enlargement and human eye tracking are not needed, and the system complexity is simplified; by matching the number of the light-emitting diodes with the cone cell density, central concave imaging is achieved in an annular scanning mode, and The invention has the advantages that the highest angular resolution of human eyes is met. The total pixel number is reduced, and the time delay and device power consumption forprocessing high-resolution images are reduced. The invention is suitable for the intelligent contact lenses oriented to augmented reality, virtual reality and mixed reality.
Owner:SHANGHAI JIAO TONG UNIV
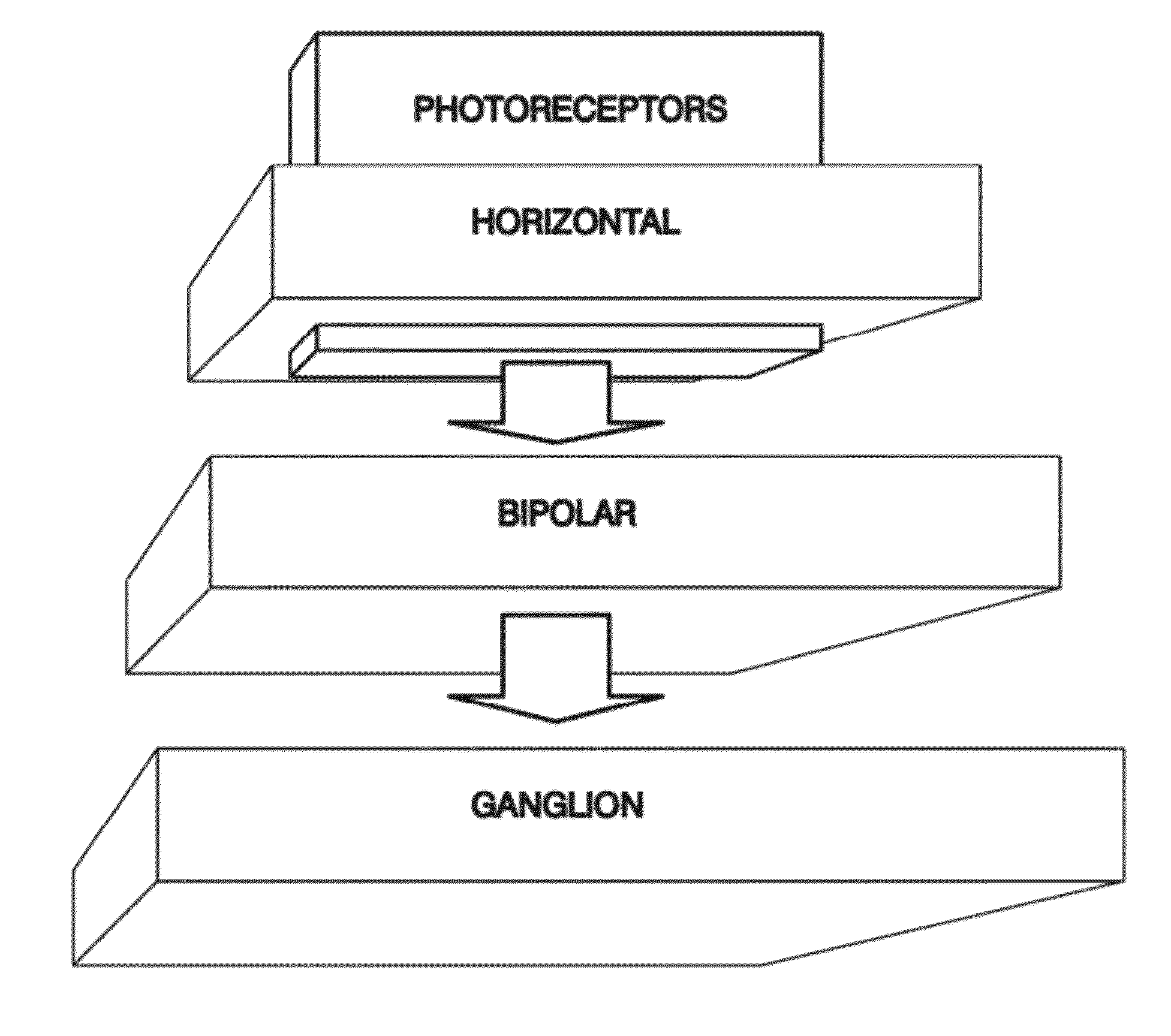
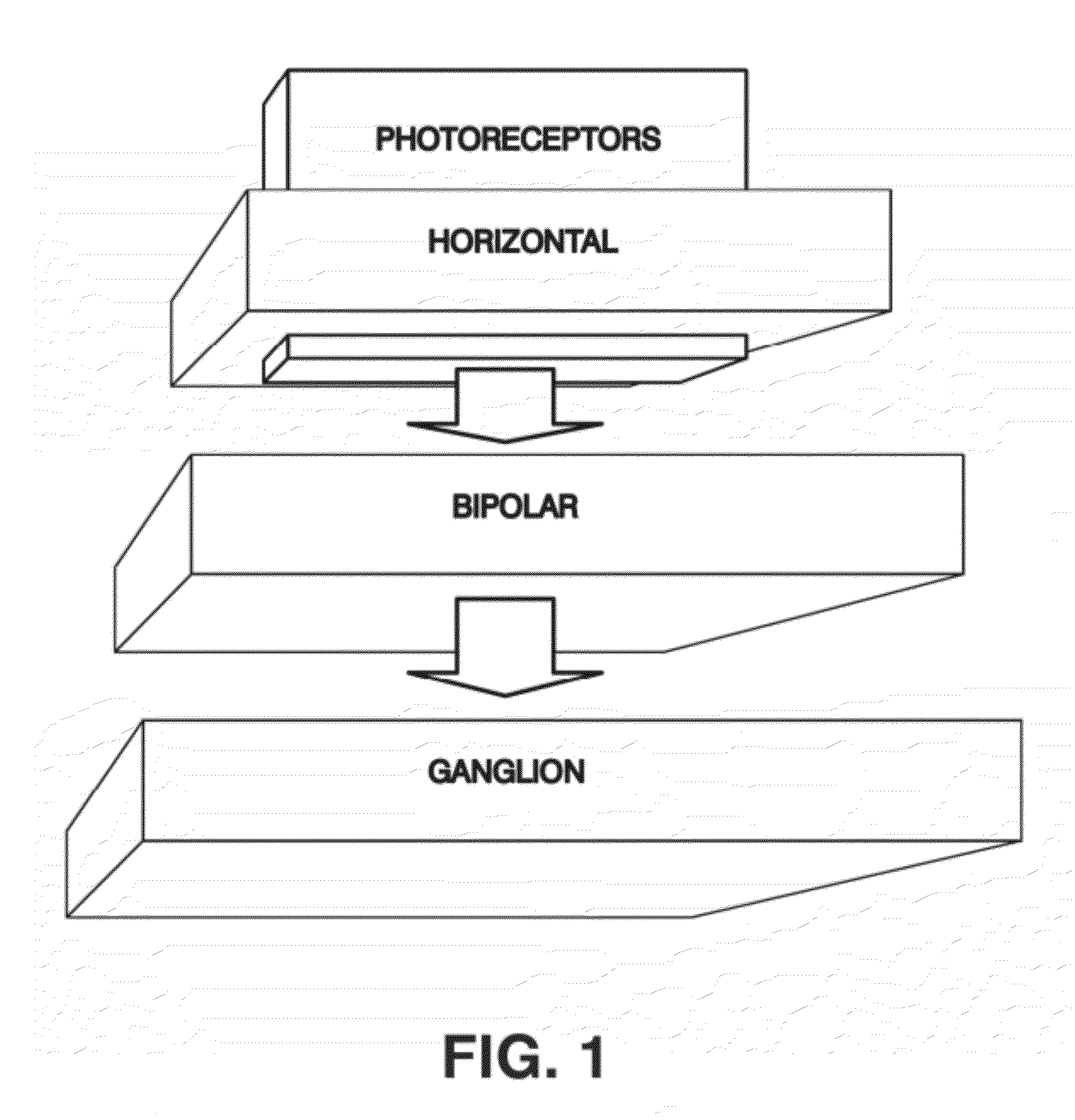
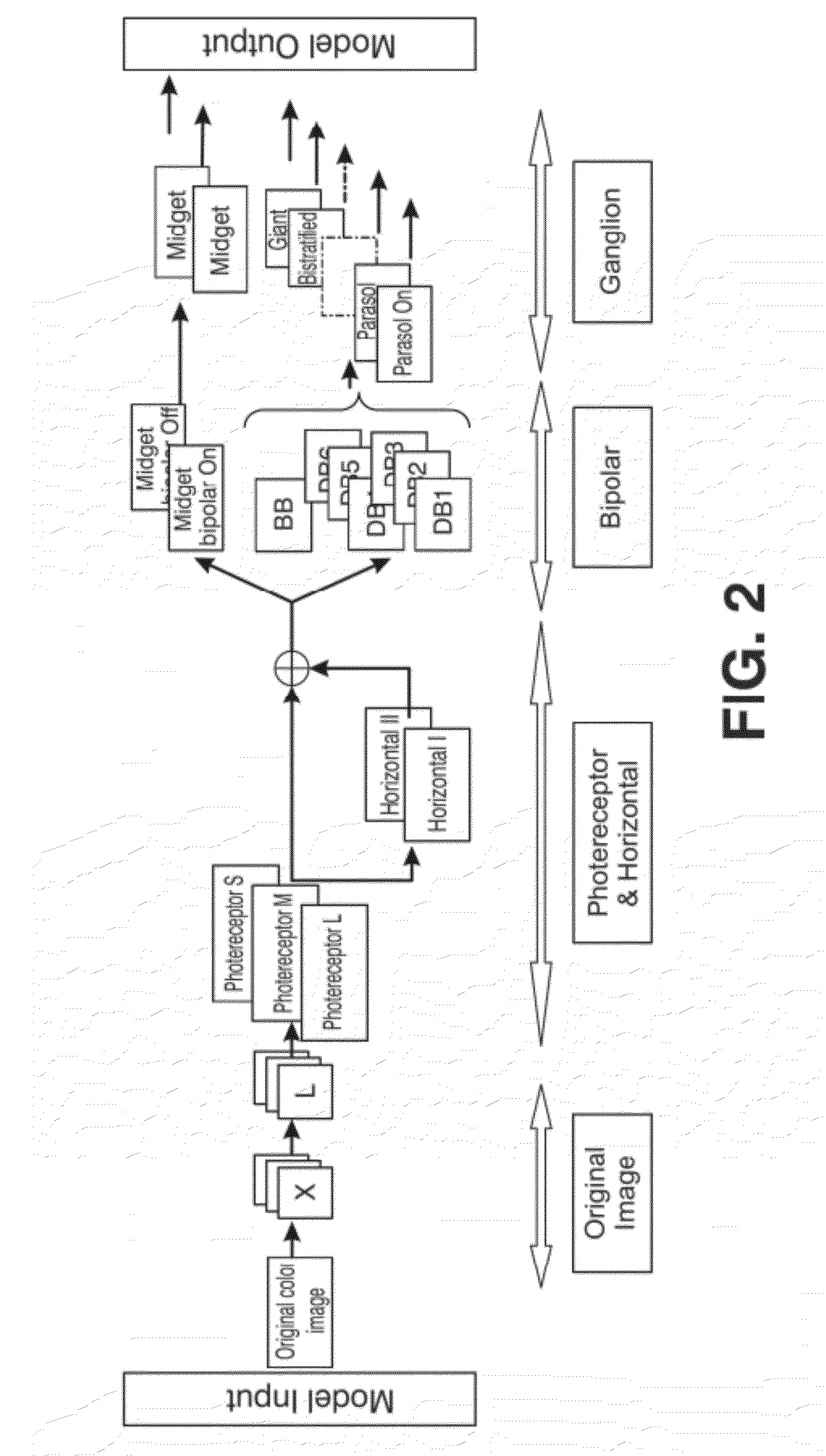
Bioinspired System for Processing and Characterising Colour Attributes of a Digital Image
A method of processing color attributes of digital images is bioinspired and includes an architecture that emulates the functions of the retina of a primate based on an image as input. The method detects the color attributes in the image. The output is a data set that includes emulators that a virtual retina in which each emulator is parameterized and in which there are emulators of type G ON and G OFF midget ganglion cells, emulators of bistratified ganglion cells, and emulators of type R ON and OFF midget ganglion cells each connected to a plurality of type R ON and OFF midget bipolar cell emulators which in turn are connected through horizontal cell emulators, to a plurality of type L cone photoreceptor emulators and to a plurality of horizontal emulators and generates a third colour channel (A) of output signals.
Owner:FUNDACION TECNALIA RES & INNOVATION
Recreational or occupational headlamp using modulated light corollary to human persistence of vision for optimized path illumination
InactiveUS20140085872A1Sufficient lightingIncrease power consumptionLighting support devicesLighting elementsPersistence of visionVisual perception
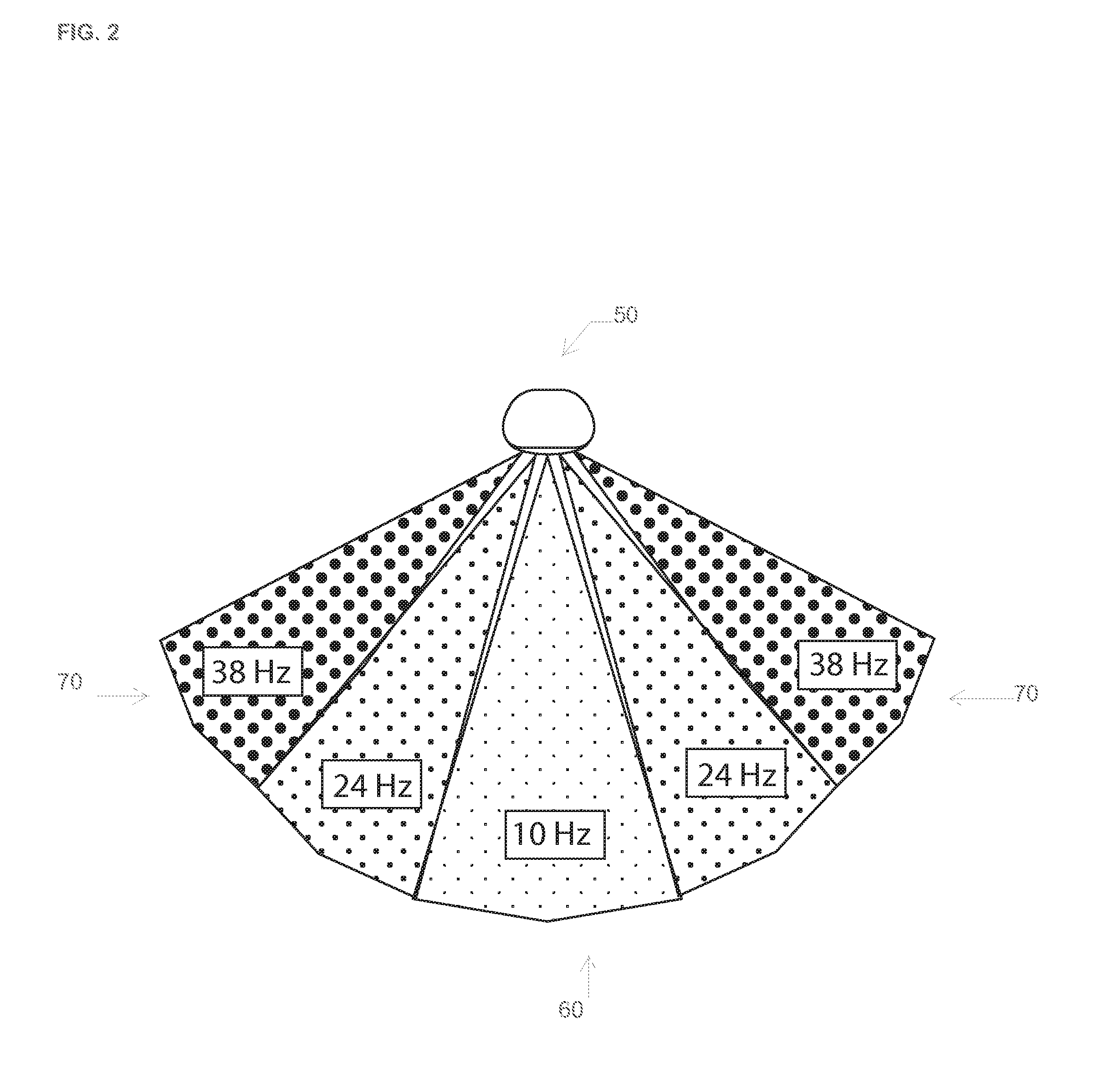
The invention relates to a novel recreational or occupational modulating light headlamp which works in conjunction with the mechanics of human vision to provide effective path lighting in very low light or dark. Applying flicker fusion rate and factoring in the acuity of the eye's rods and cones, lighting is applied to the range and capability of the ocular abilities and optimized for low light or dark.
Owner:TAYLOR DALE
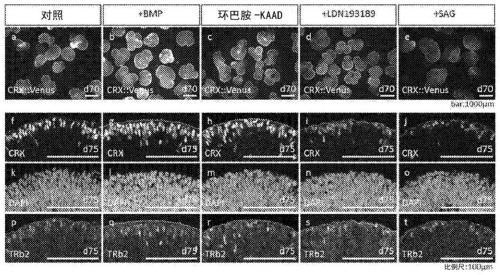
Method for amplifying cone photoreceptors or rod photoreceptors by using dorsalization signal transmitter or ventralization signal transmitter
The present invention addresses the problem of providing a cone photoreceptor precursor cell and / or a retinal tissue rich in cone photoreceptors, a rod photoreceptor precursor cell and / or a retinal tissue rich in rod photoreceptors, and a method for manufacturing the same. The present invention is i) a method for amplifying the ratio of cone photoreceptor precursor cells and cone photoreceptors inphotoreceptor precursor cells and photoreceptors included in a retinal tissue, the method including a step for culturing the retinal tissue from an initial development stage until a stage at which the occurrence of cone photoreceptor precursor cells is at maximum in a medium including a dorsalization signal transmitter at a concentration sufficient to suppress expression of a ventral marker; or ii) a method for amplifying the ratio of rod photoreceptor precursor cells and rod photoreceptors in photoreceptor precursor cells and photoreceptors included in a retinal tissue, the method includinga step for culturing the retinal tissue from an initial development stage until a stage at which the occurrence of rod photoreceptor precursor cells is at maximum in a medium including a dorsalizationsignal transmitter at a concentration sufficient to suppress expression of a ventral marker.
Owner:RIKEN +2
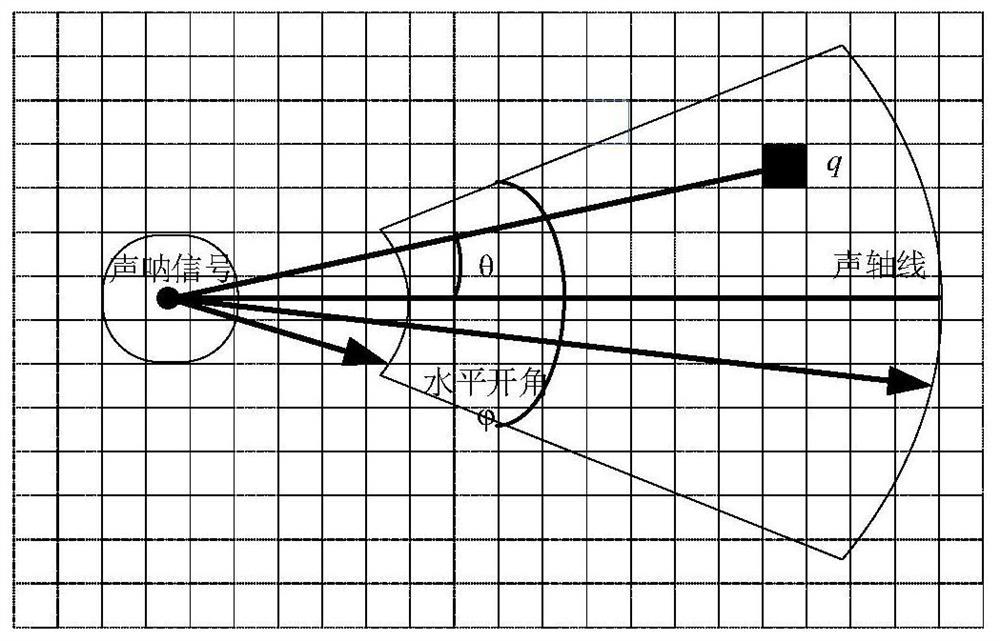
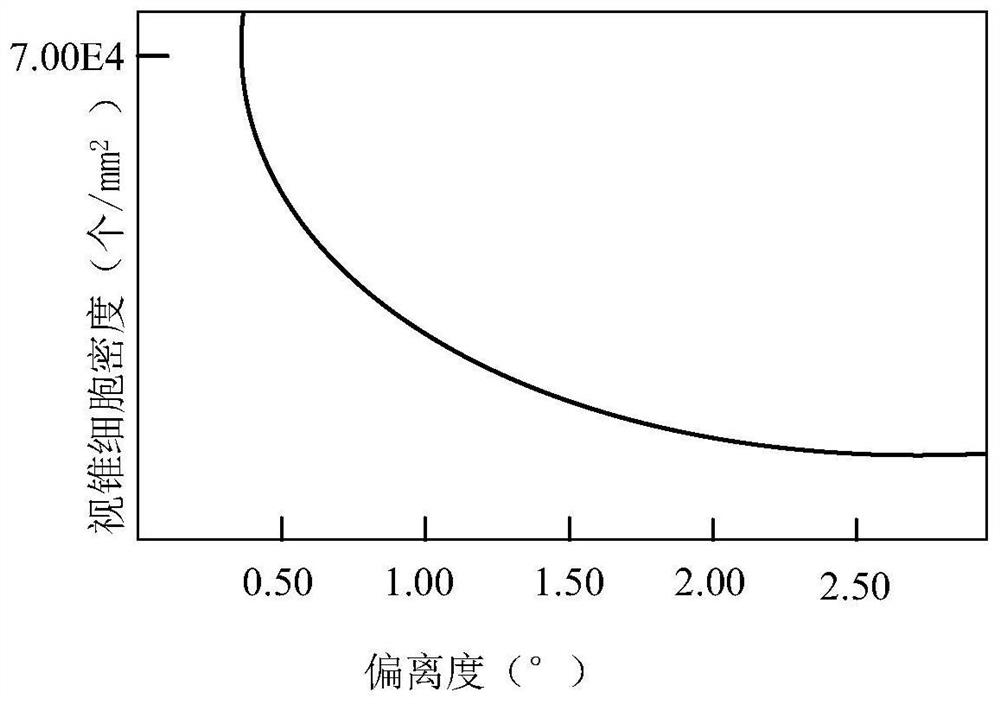
High-resolution reconstruction method for side-scan sonar image
PendingCN112529779ASimple and fast operationEasy to implementGeometric image transformationSonarRadiology
The invention provides a high-resolution reconstruction method for a side-scan sonar image, and the method comprises the steps: obtaining a cone cell distribution model through the analysis of the relation between the cone cell density and the human eye retina deviation degree based on the perception characteristics of the human eye retina, and calculating the observed probability of each pixel point target through the distribution model; and finally, a high-quality seabed image is obtained. According to the method, a probability model is constructed for a sonar signal seabed model according to distribution of cone cells, a sonar image is reconstructed, and a high-quality sonar image conforming to human eye characteristics is finally obtained through little whitening in an image area.
Owner:JIANGSU UNIV OF SCI & TECH
Biomarkers for photoreceptor cells
PendingUS20200063095A1Wide applicabilityPractical and convenientPharmaceutical delivery mechanismNervous system cellsOphthalmologyHuman cell
The present invention relates to the identification of photoreceptors or cone photoreceptors in populations of cells. In particular the present invention relates to methods of identification of photoreceptors or cone photoreceptors and methods of isolating photoreceptors or cone photoreceptors. Photoreceptors or cone photoreceptors isolated by the methods of the present invention are useful for transplantation and the treatment of retinal dystrophies. Also claimed are human cell populations enriched for photoreceptors or cone photoreceptors and kits for identifying photoreceptors or cone photoreceptors.
Owner:UCL BUSINESS PLC
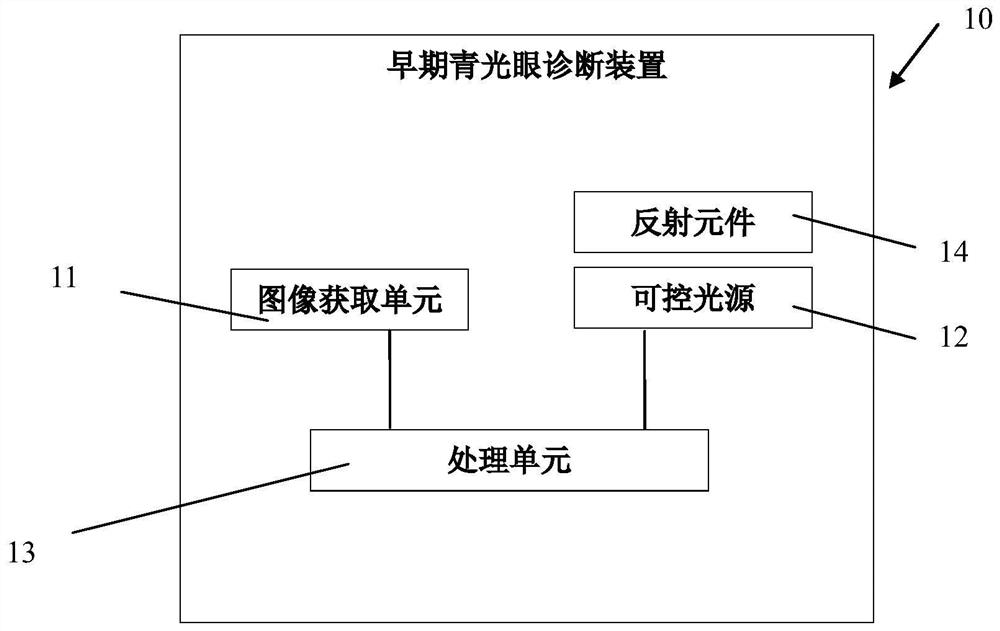
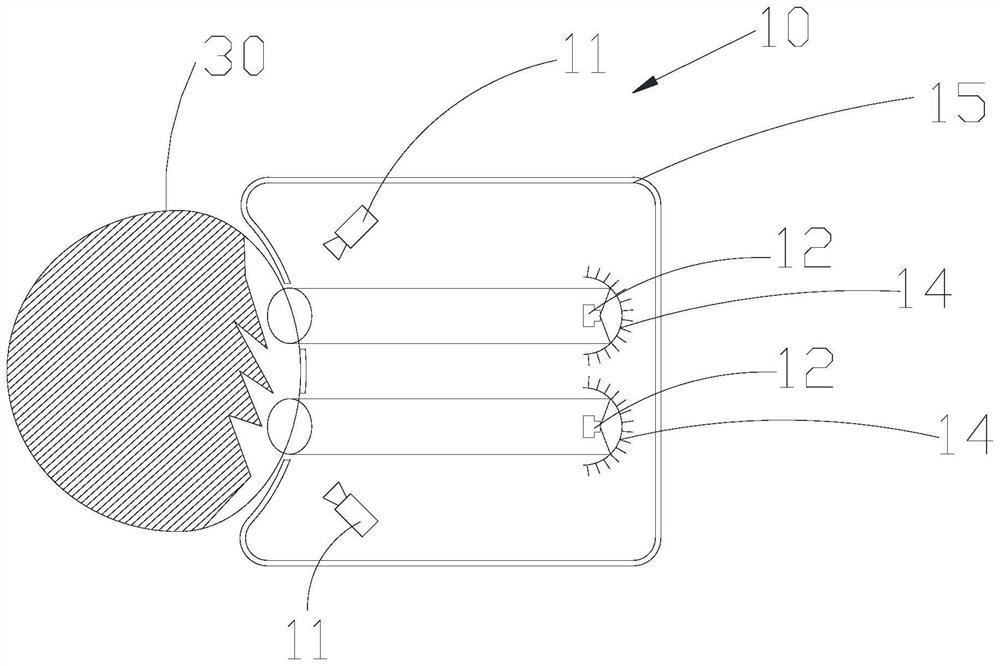
Early-stage glaucoma diagnosis device
The invention discloses an early-stage glaucoma diagnosis device, which comprises an image acquisition unit, at least one controllable light source and a processing unit, wherein the controllable light source is configured to guide light of different wavelengths to at least one eye, the image acquisition unit is configured to acquire feedback parameters of pupils based on the light of different wavelengths, and the processing unit is configured to receive and analyze the feedback parameter signals to obtain a diagnosis result of early-stage glaucoma. Compared with the prior art, the early-stage glaucoma diagnosis device of the invention can be used for analyzing the state of cone cells or rod cells of the eyes of the user according to the contraction data of the pupils of the user responding to the light sources with different wavelengths, so that the early-stage glaucoma can be simply, conveniently and efficiently screened, and the disease progress of the glaucoma can be evaluated.
Owner:艾视雅健康科技(苏州)有限公司
Display module, control method thereof and electronic equipment
PendingCN113516915AReduce power consumptionConsistent brightnessSolid-state devicesIdentification meansComputer hardwareProtecting eye
The embodiment of the invention relates to a display module, a control method thereof and electronic equipment, the display module comprises a shell assembly, a flexible display screen and a driving mechanism, the flexible display screen comprises a first display unit and a second display unit, the first display unit is an electronic ink display unit, and the second display unit is an active light-emitting display unit; the driving mechanism is arranged on the shell assembly and used for driving the first display unit and the second display unit to be hidden in the shell assembly or exposed out of the shell assembly; the display module is a double-screen display module, an electronic ink display unit is introduced into the display module, pictures can be continuously displayed without a power supply, only when the pictures change, a small amount of electricity needs to be consumed, the power consumption of the display module is greatly reduced, and meanwhile, the electronic ink display unit adopts a mode of reflecting natural light instead of actively emitting light, so that the brightness of a screen watched by human eyes is consistent with that of the environment, cone cells are not stimulated, and the effect of protecting eyes is achieved.
Owner:GUANGDONG OPPO MOBILE TELECOMM CORP LTD
Compositions and methods for enhancing functional expression of therapeutic genes in photoreceptors
Disclosed herein are nucleic acid regulatory elements, expression cassettes, expression vectors, and methods for using the nucleic acid regulatory elements, expression cassettes, expression vectors for treating cone cell disorders.
Owner:UNIV OF WASHINGTON
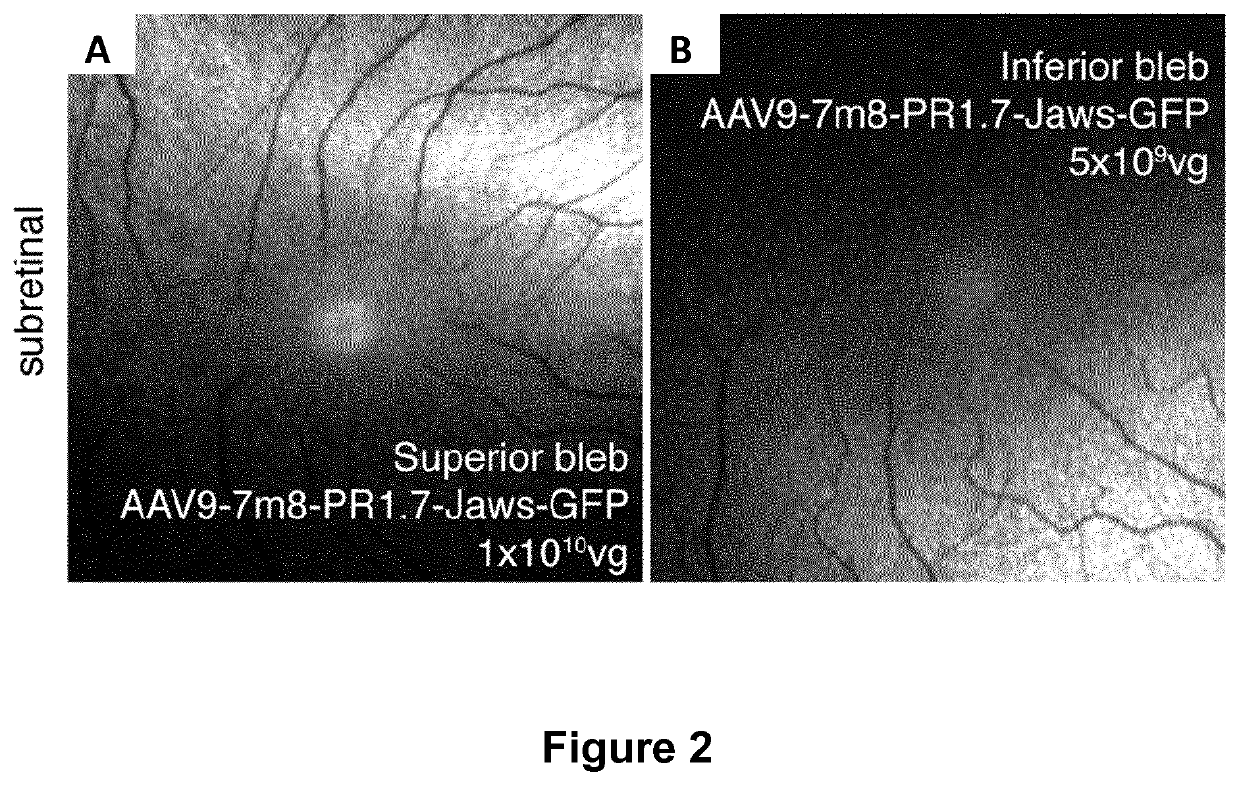
Methods of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising the subretinal delivery of a therapeutically effective amount of a recombinant aav9-derived vector
ActiveUS20210268125A1Efficient deliveryEfficient transductionSenses disorderVectorsOptogeneticsCone cell
Several new vector-promoter combinations to overcome the limitations associated with AAV-mediated cone transduction in the fovea are provided. The delivery modality relies on a cone-specific promoter and result in high-level transgene expression compatible with optogenetic vision restoration. Methods of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising subretinal delivery of a therapeutically effective amount of a recombinant AAV9-derived vector comprising a VP1 capsid protein as set forth in SEQ ID NO: 11 and the polynucleotide of interest under the control of the pR1.7 promoter as set forth in SEQ ID NO: 12 are also provided.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Imaging device for cone cells
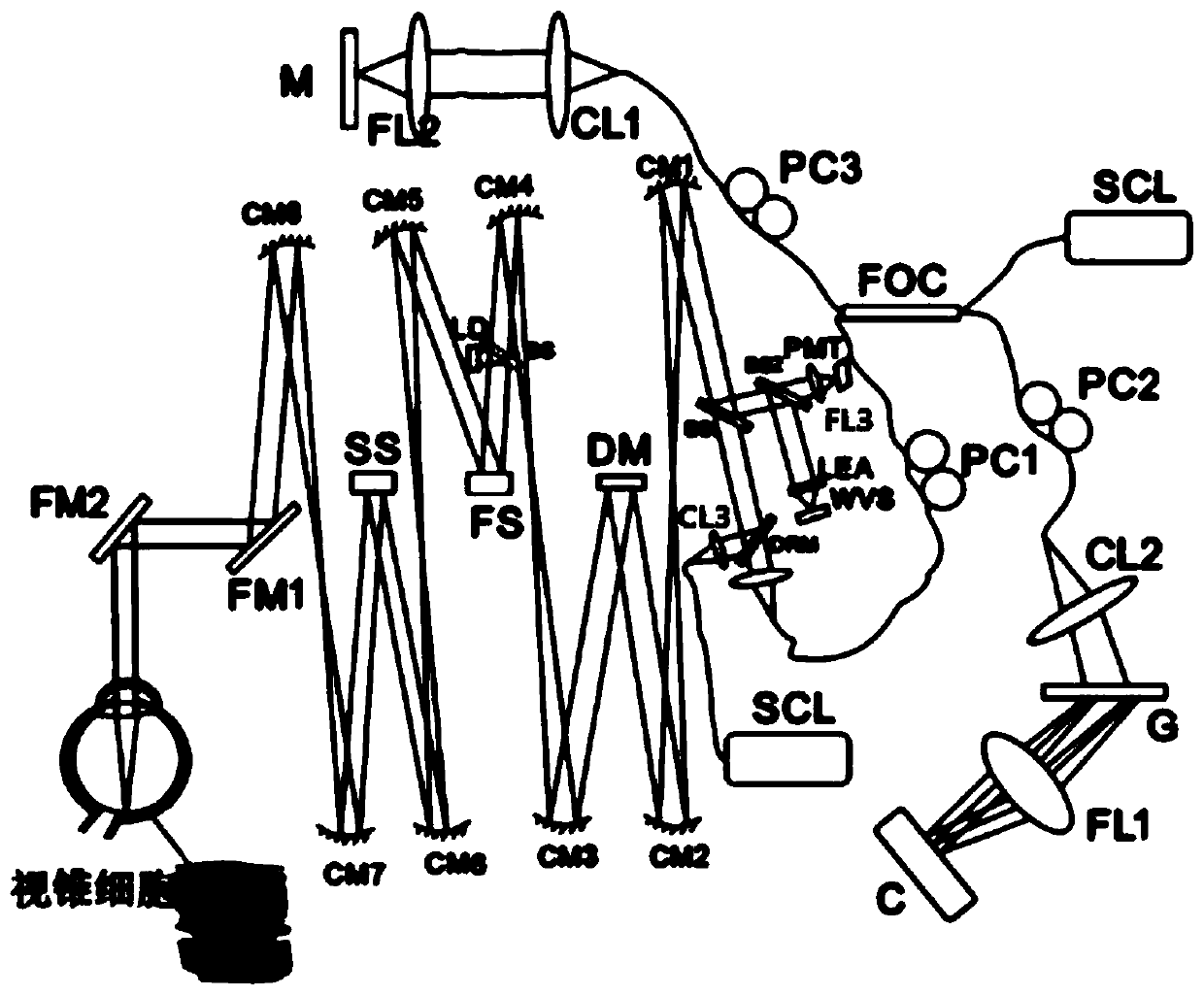
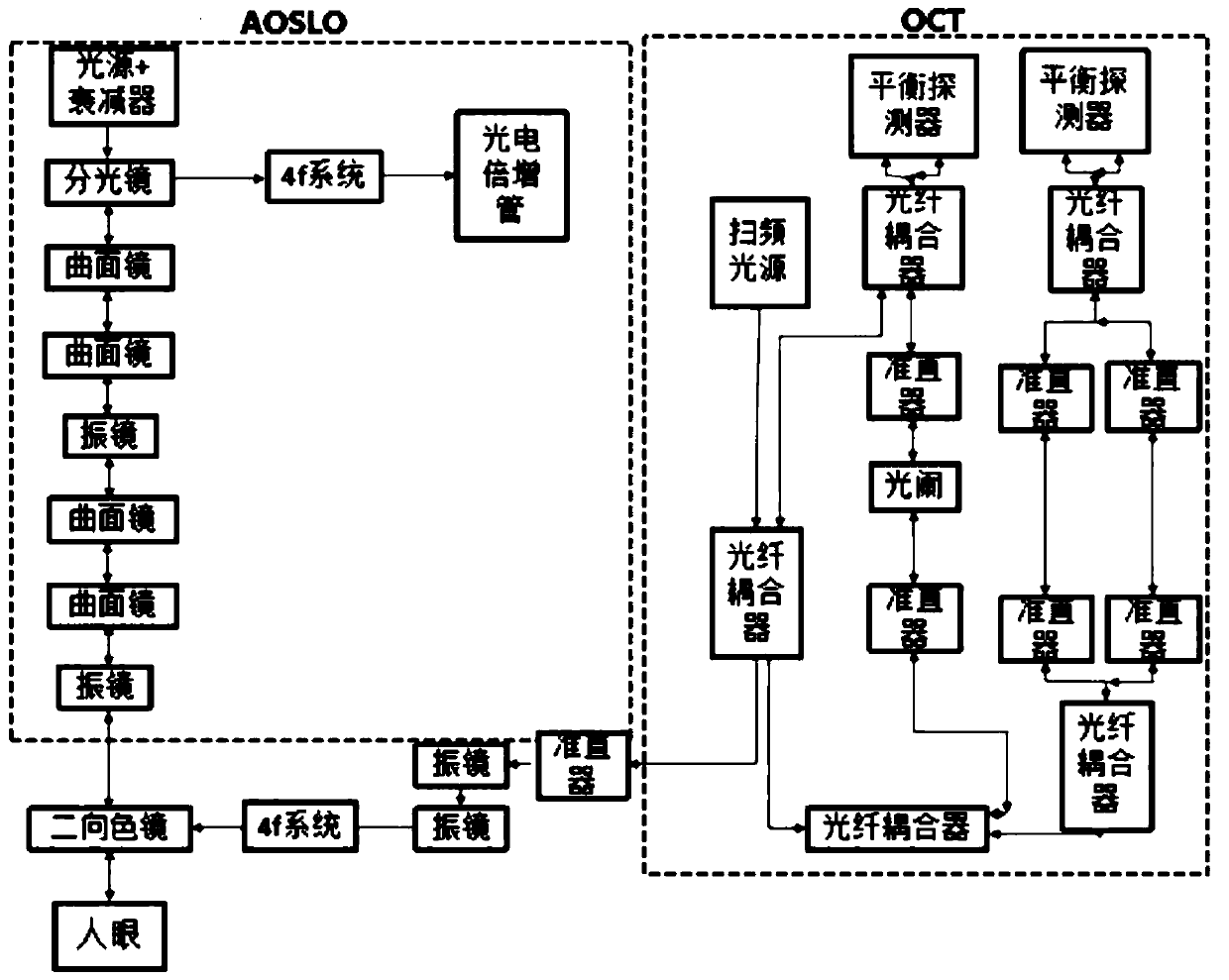
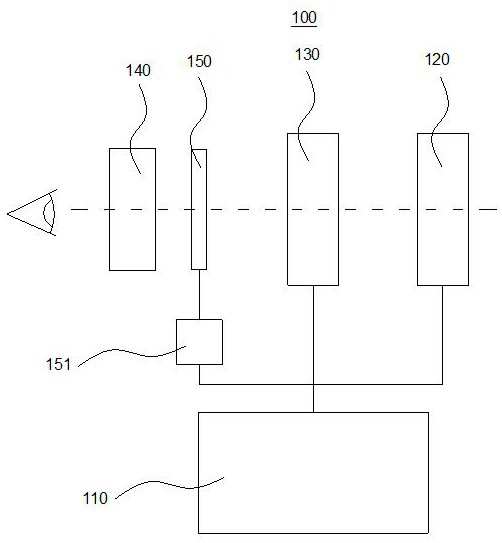
The invention provides an imaging device for cone cells. The imaging device comprises an AOSLO (Adaptive Optical Scanning Laser Ophthalmoscope), an OCT (Optical Coherence Tomography) and an imaging processing device. A light beam entering into cone cells of fundus is processed and returned through OCT and then is subjected to interference treatment with the light beam processed by AOSLO, and a processed interference signal is sent to the imaging processing device for generating a high-precision three-dimensional image, so that the activity of the cone cells of fundus can be observed.
Owner:广东唯仁医疗科技有限公司
Methods of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising the subretinal delivery of a therapeutically effective amount of a recombinant aav9-derived vector
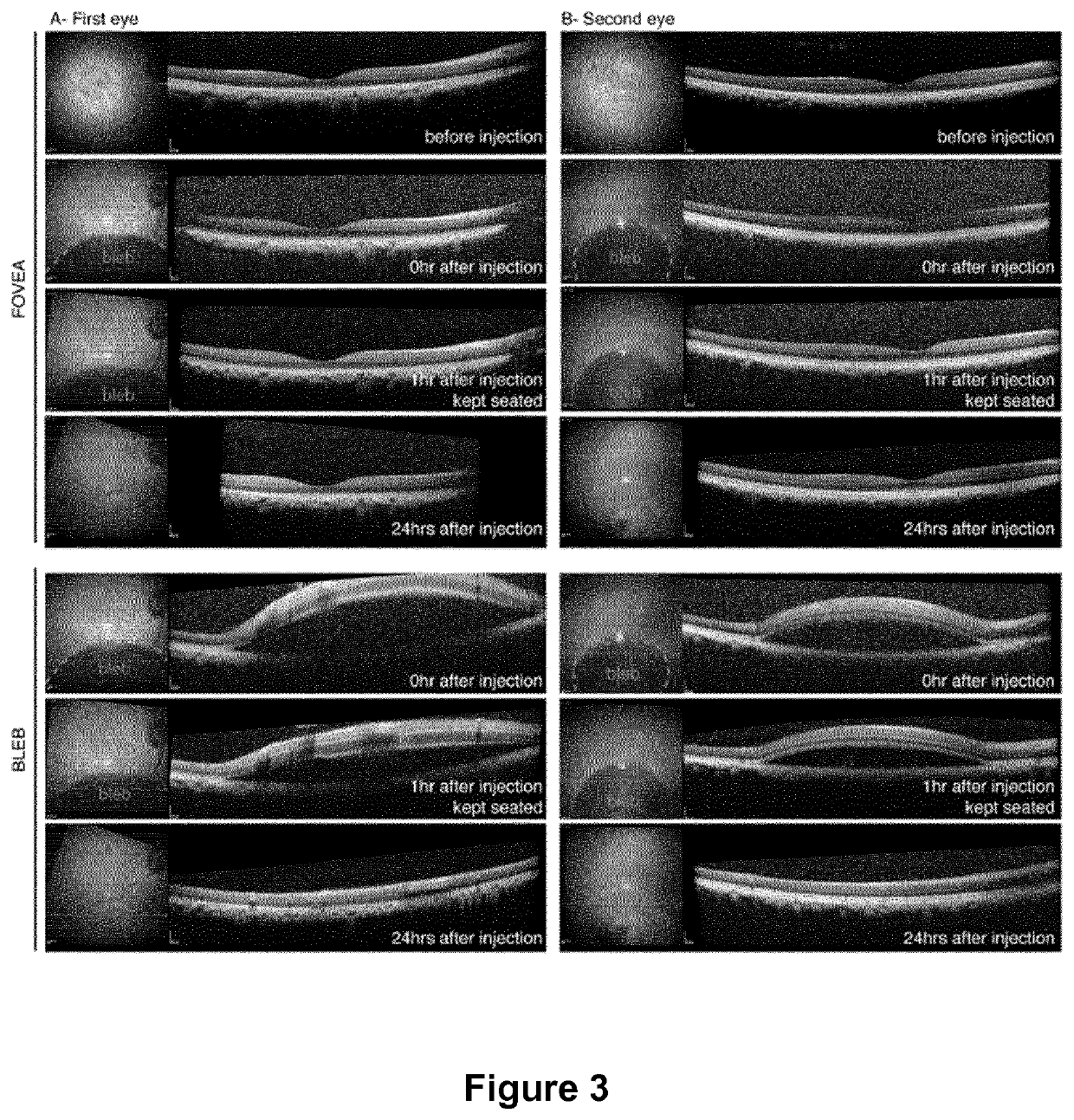
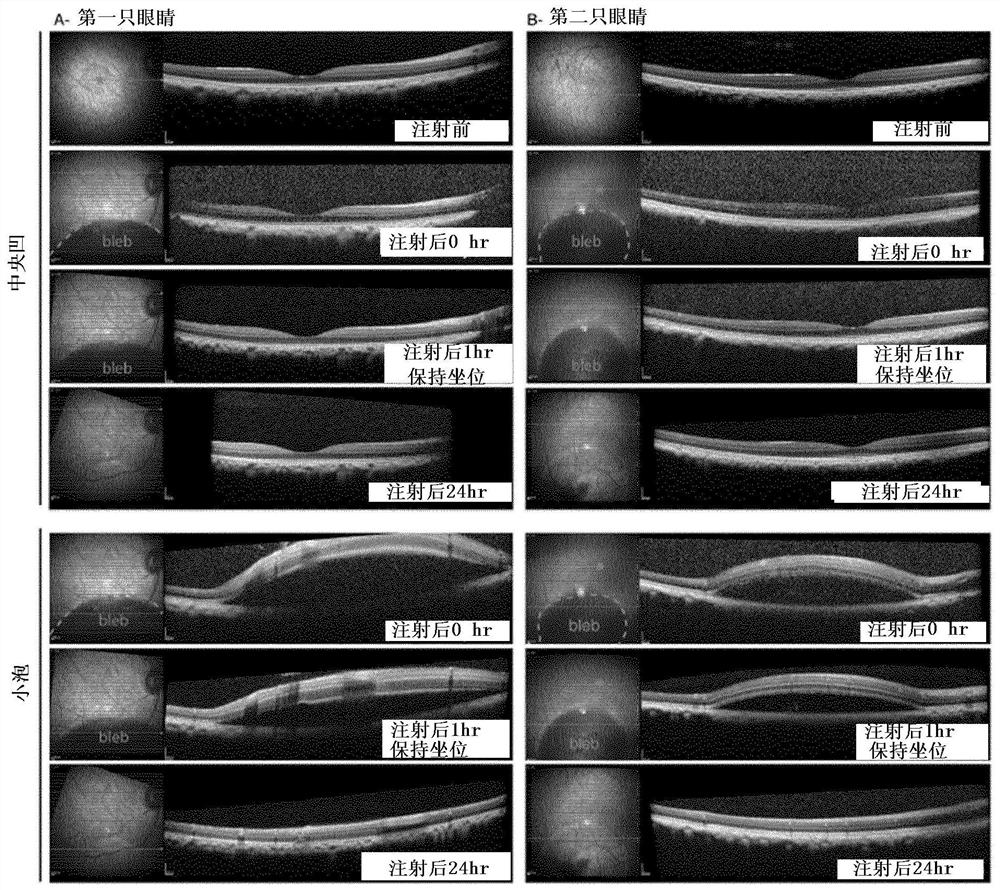
Intraocular injection of adeno-associated viral (AAV) vectors has been an evident route for delivering gene drugs into the retina. Currently, the vectors need to be injected into the subretinal spacein order to provide gene delivery to cones. In this approach, gene delivery is limited to cells that contact the local "bleb" of injected fluid. Furthermore, retinal detachment that occurs during subretinal injections is a concern in eyes with retinal degeneration. Here, the inventors establish several new vector-promoter combinations to overcome the limitations associated with AAV-mediated cone transduction in the fovea with supporting studies in mouse models, human induced pluripotent stem cell-derived organoids, post-mortem human retinal explants and living macaques. They show that an AAV9variant provides efficient foveal cone transduction when injected into the subretinal space several millimeters away from the fovea, without detaching this delicate region. The delivery modality relies on a cone-specific promoter and result in high-level transgene expression compatible with optogenetic vision restoration. Accordingly, the present invention relates to method of expressing a polynucleotide of interest in the cone photoreceptors of a subject comprising subretinal delivery of a therapeutically effective amount of a recombinant AAV9-derived vector comprising a VP1 capsid protein asset forth in SEQ ID NO: 11 and the polynucleotide of interest under the control of the pR1.7 promoter as set forth in SEQ ID NO: 12.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS +1
A Calculation Method of Cone Cell Density Based on Image Recognition
ActiveCN103745257BFast statisticsHigh degree of automationCounting objects with random distributionOthalmoscopesResponse FrequencyLaser scanning
Owner:SCHOOL OF OPHTHALMOLOGY & OPTOMETRY WENZHOU MEDICAL COLLEGE
Strabismus and amblyopia therapeutic instrument
A strabismus and amblyopia therapeutic instrument relates to the field of strabismus and amblyopia treatment equipment. The therapeutic instrument comprises a control module, and a light source assembly, a function module and an eyepiece which sequentially arranged along a light path, wherein a shading sheet and a driving device used for driving the shading sheet to move are arranged between the eyepiece and the light source assembly; the control module is electrically connected with the driving device, the function module and the light source assembly; the light source assembly comprises at least one semiconductor green laser and at least one semiconductor red laser. According to the strabismus and amblyopia therapeutic instrument, the semiconductor green laser and the semiconductor red laser are used for generating green light and red light with specific wavelengths, and thus cone cells are efficiently stimulated. Meanwhile, the protection effect of the shading sheet is utilized, itis guaranteed that laser power acts on human eyes after being stable, harm of the laser to the human eyes is avoided as much as possible, and safety is improved.
Owner:北京凯爱医疗科技有限公司
A method of making a color vision classification detection map
ActiveCN108537856BQuick checkEfficient detectionTexturing/coloringDuplicating/marking methodsSpectral responseMedicine
The invention relates to a method for making a color vision classification detection map. A detection map is made in the image processing software. The detection map includes two parts: the background layer and the digital layer. The digital layer includes a fixed digital layer and a variable digital layer. There is a certain color difference between the fixed digital layer and the background layer. The eye can clearly recognize the difference between the two colors; the color of the variable digital layer and the background layer are close to or the same, but the spectral reflectance curve has a large difference, forming a near-metameric pair or a metameric pair; choose two different Spectral primary color inks, inks, toners or dyes, reproduce the background layer of the inspection map-fixed digital layer and variable digital layer, and form a color vision classification inspection map on the printing material. The detection map can quickly and conveniently classify the spectral response capabilities of red, green and blue cone cells of normal color vision observers, thereby dividing the color discrimination ability of normal color vision observers.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
Photobiological regulation therapeutic instrument for treating age-related macular degeneration and use method
The invention discloses a photobiological regulation therapeutic instrument for treating age-related macular degeneration and a use method. According to the photobiological regulation therapeutic instrument, by setting near-infrared light with a wavelength of 600-800 nm relative to a brightness of left and right eye sight glasses, two eyes of a patient are treated under a light-emitting viewpoint for 4 times every day and 40 seconds every time, so that activity of mitochondrial cytochrome c oxidase is improved, energy supply and cell metabolism are increased, tissue metabolism repair is enhanced, cone cell death of age-related macular degeneration patients is slowed down, and greater possibility is provided for vision recovery of the patients. The photobiological regulation therapeutic instrument is high in safety, low in price of a low-dose red light therapeutic instrument, low in treatment cost of patients and beneficial to popularization.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
A method for obtaining retinal-like tissue rich in cones and rods by using human induced pluripotent stem cells
ActiveCN108795864BShort digestion timeHigh activityNervous system cellsArtificially induced pluripotent cellsMatrigelHuman Induced Pluripotent Stem Cells
The invention discloses a method for obtaining retinal tissue rich in cone and rod cells by using human induced pluripotent stem cells. It includes digesting hiPSCs to obtain cell pellets, and then suspending the cell pellets to obtain embryoid bodies; the embryoid bodies are then inoculated into culture dishes pre-coated with Matrigel, and induced to differentiate in the induction medium to obtain neural retina (NR) and RPE; NR and RPE were re-provoked and cultured in suspension to obtain 3D retinal tissue including NR tissue, and then continued to be cultured in suspension in an optimized culture medium without retinoic acid. NR differentiated into all retinal cells, including highly mature photoreceptors cells, Rhodopsin-positive rod cells, L / M OPSIN-positive red-green cone cells and S-OPSIN-positive blue cone cells, especially retinotopic tissues rich in red-green cone and rod cells were obtained.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV +1
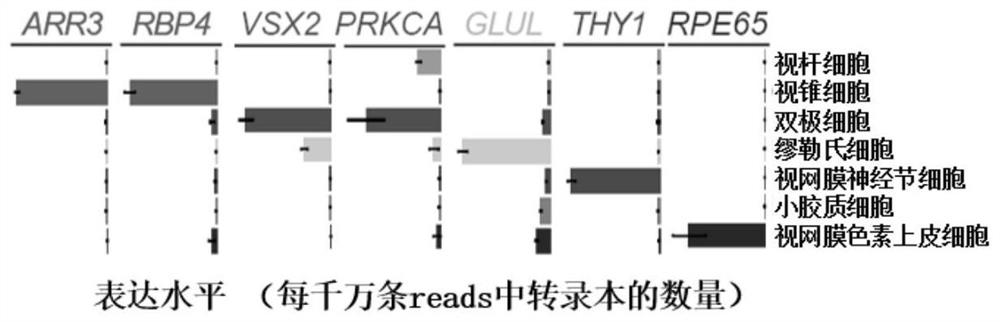
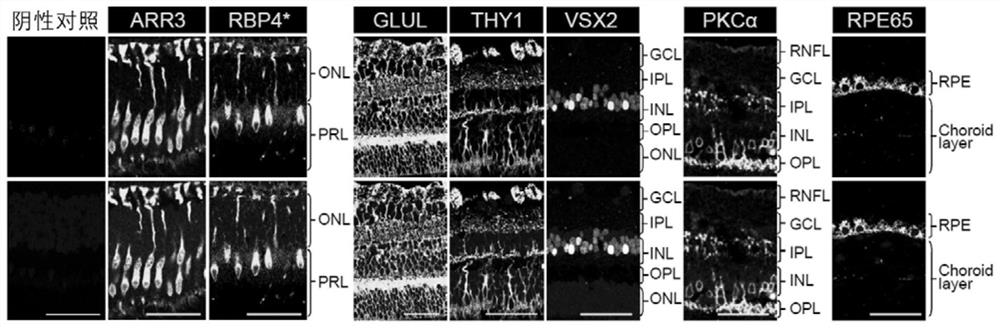
A marker for detection or auxiliary detection of retinal cone cells and application thereof
ActiveCN112063719BMicrobiological testing/measurementBiological material analysisRetinal pigmentationDisease
The invention discloses a marker for detecting or assisting in detecting retinal cone cells and an application thereof. The present invention provides the application of a substance for detecting whether the RBP4 protein is expressed in the sample to be tested in at least one of the following: a1) identification or auxiliary identification of retinal cone cells; a2) screening or auxiliary screening of retinal cone cells; a3) evaluation or Auxiliary evaluation of retinal cone cells; a4) identification or auxiliary identification of whether the sample to be tested contains retinal cone cells; a5) screening or auxiliary screening of whether the sample to be tested contains retinal cone cells; a6) evaluation or auxiliary evaluation of whether the sample to be tested contains Retinal cone cells; the present invention finds that RBP4 can be used as a marker to identify or assist in the identification of retinal cone cells by constructing and analyzing a cynomolgus monkey retinal cone cell sequencing library and immunofluorescence detection. The above markers can be used to evaluate retinal aging-related diseases such as macular degeneration and retinitis pigmentosa. The invention has important application value.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
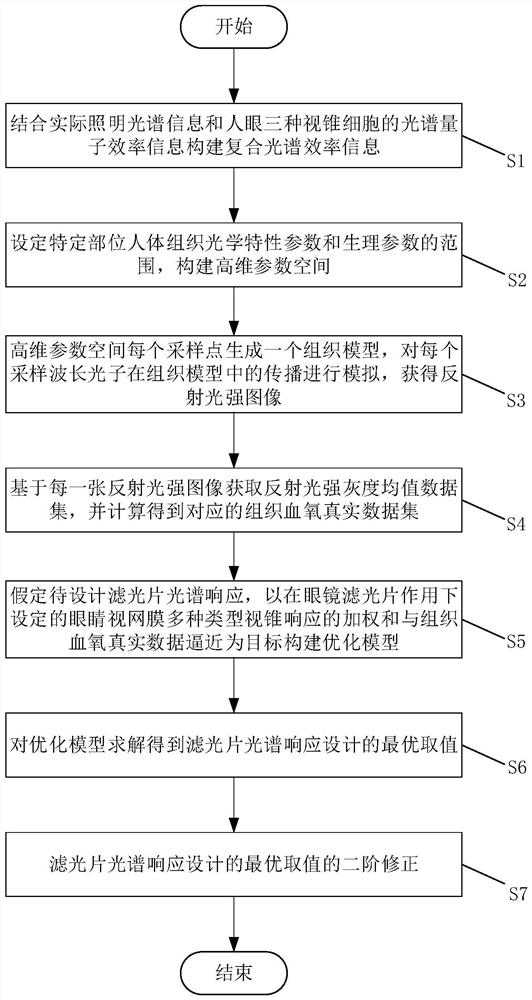
Spectacle optical filter design method with blood oxygen information enhancement function and spectacles
ActiveCN114839795AAuxiliary First AidAuxiliary rescueInternal combustion piston enginesAuxillary optical partsSpectral responseData set
The invention relates to a design method of a glasses optical filter with a blood oxygen information enhancement function and glasses. The method comprises the following steps: S1, constructing composite spectral efficiency information by combining actual illumination spectral information and spectral quantum efficiency information of three types of cone cells of human eyes; s2, setting ranges of optical characteristic parameters and physiological parameters of human tissues at specific parts, and constructing a high-dimensional parameter space; s3, generating a tissue model for each sampling point in the high-dimensional parameter space, and simulating propagation of each sampling wavelength photon in the tissue model to obtain a reflected light intensity image; s4, acquiring a reflected light intensity gray average data set based on each reflected light intensity image, and calculating to obtain a corresponding tissue blood oxygen real data set; s5, assuming the spectral response of the optical filter to be designed, and constructing an optimization model; and S6, solving to obtain an optimal value of the optical filter spectral response design. Compared with the prior art, the blood oxygen information can be enhanced and presented, and first aid and rescue of doctors can be more effectively assisted.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com