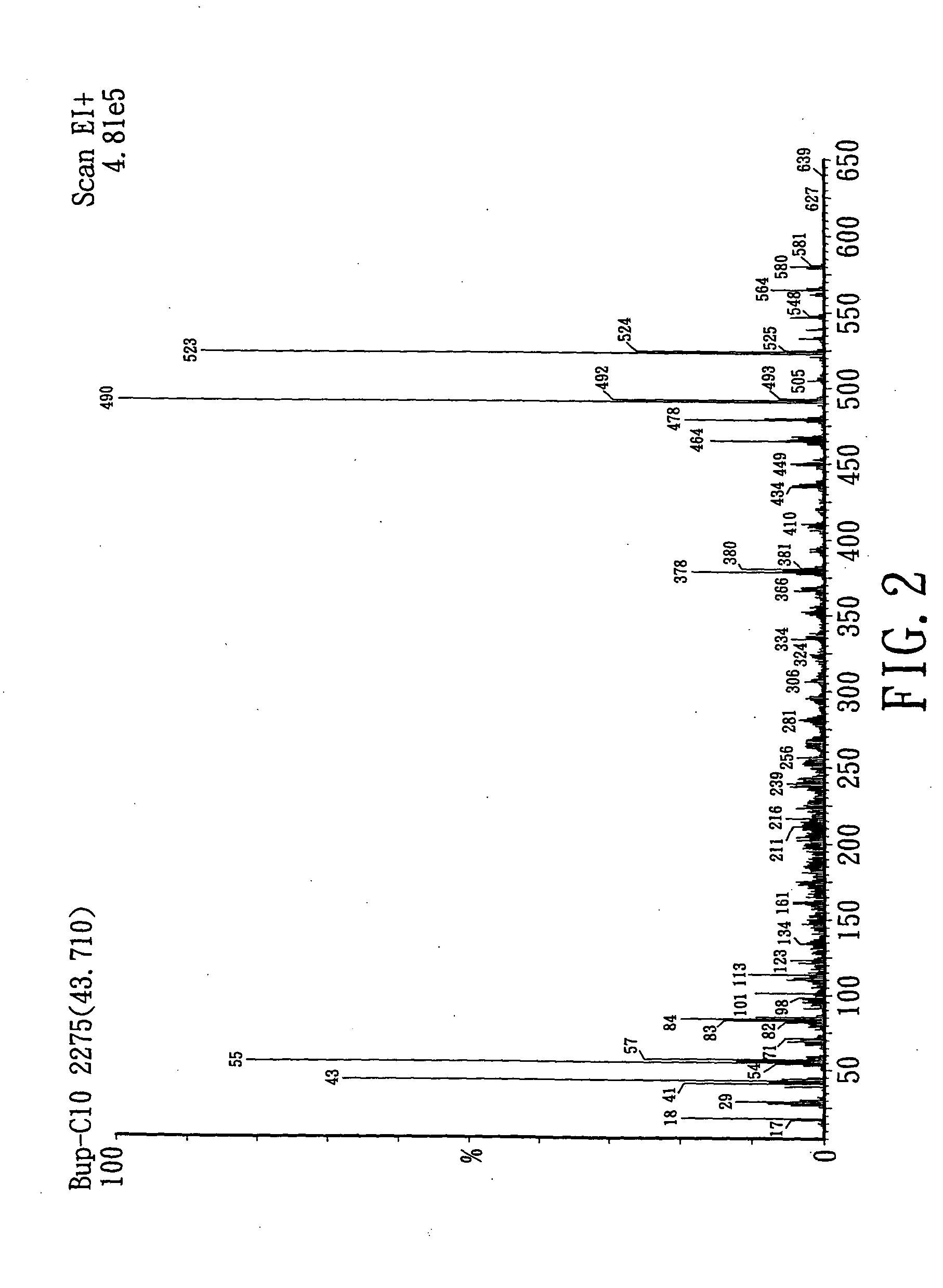
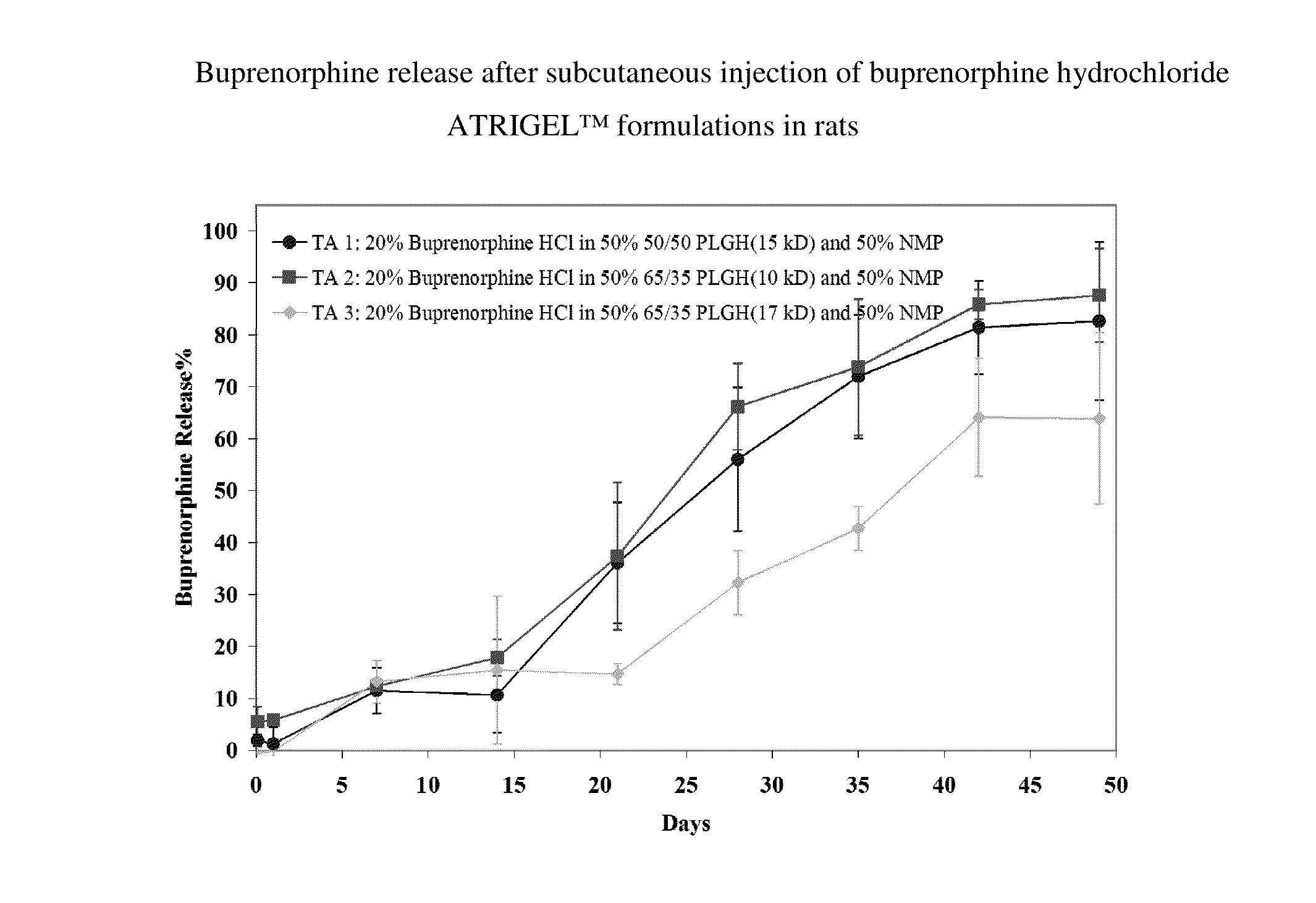
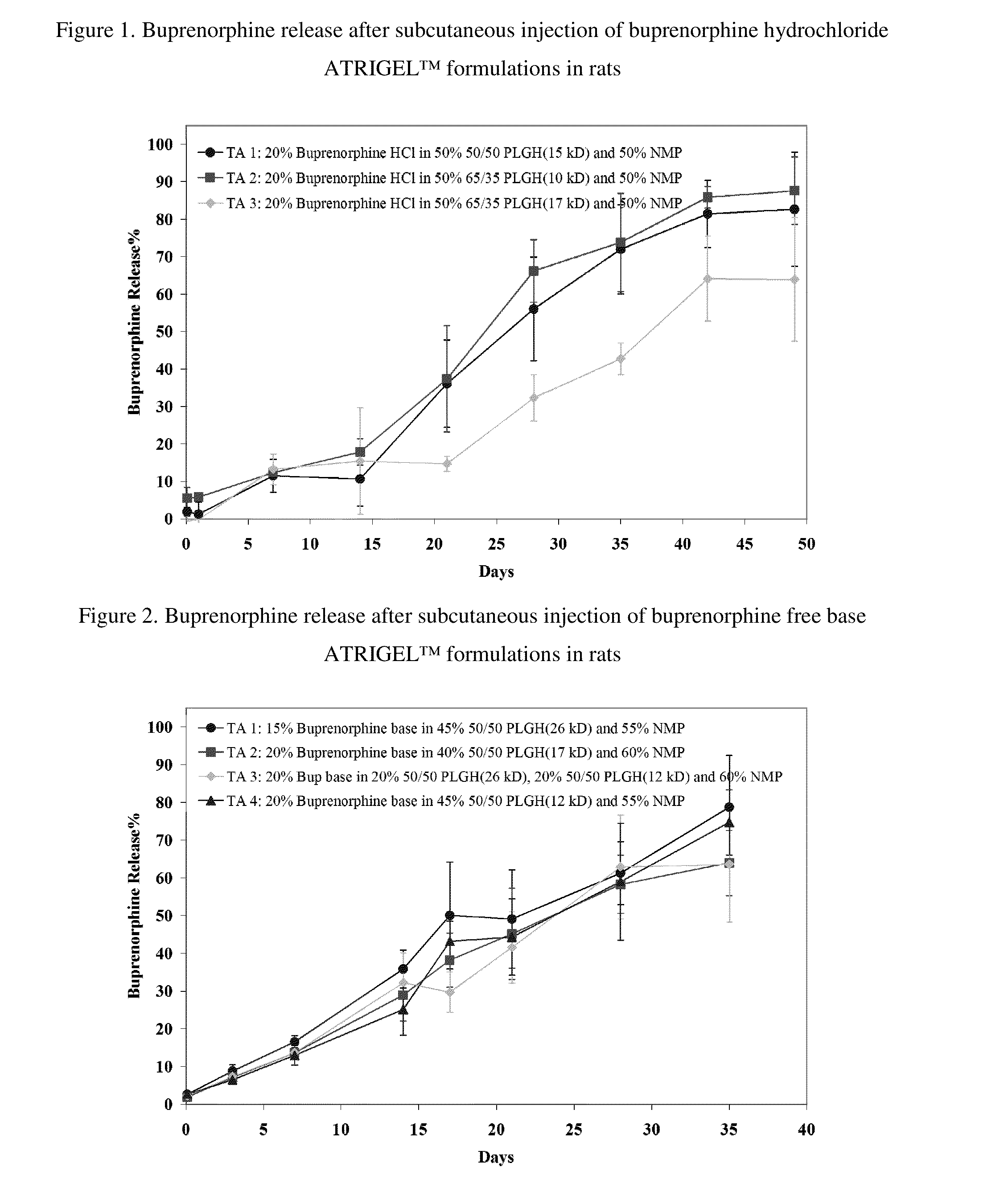
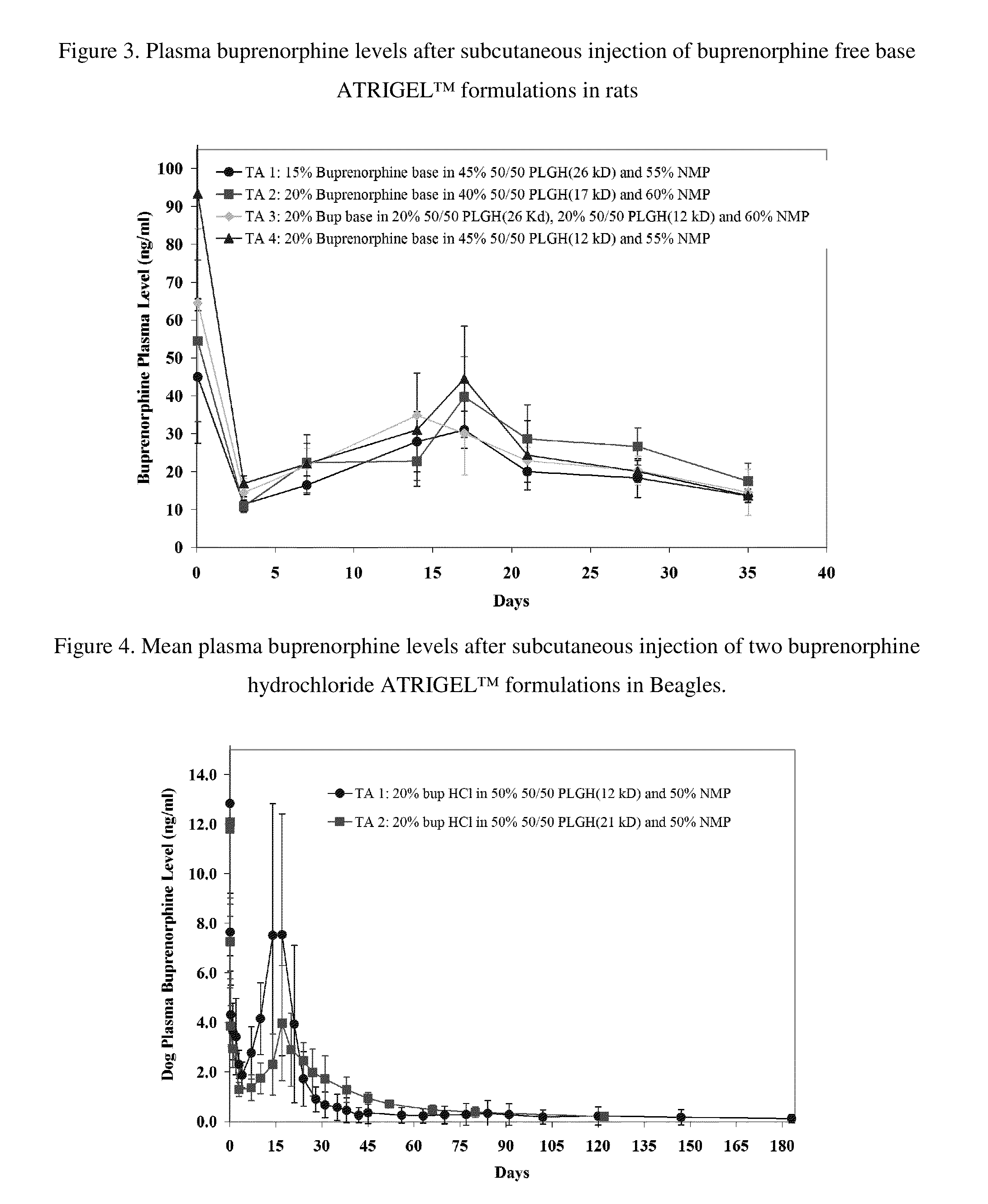
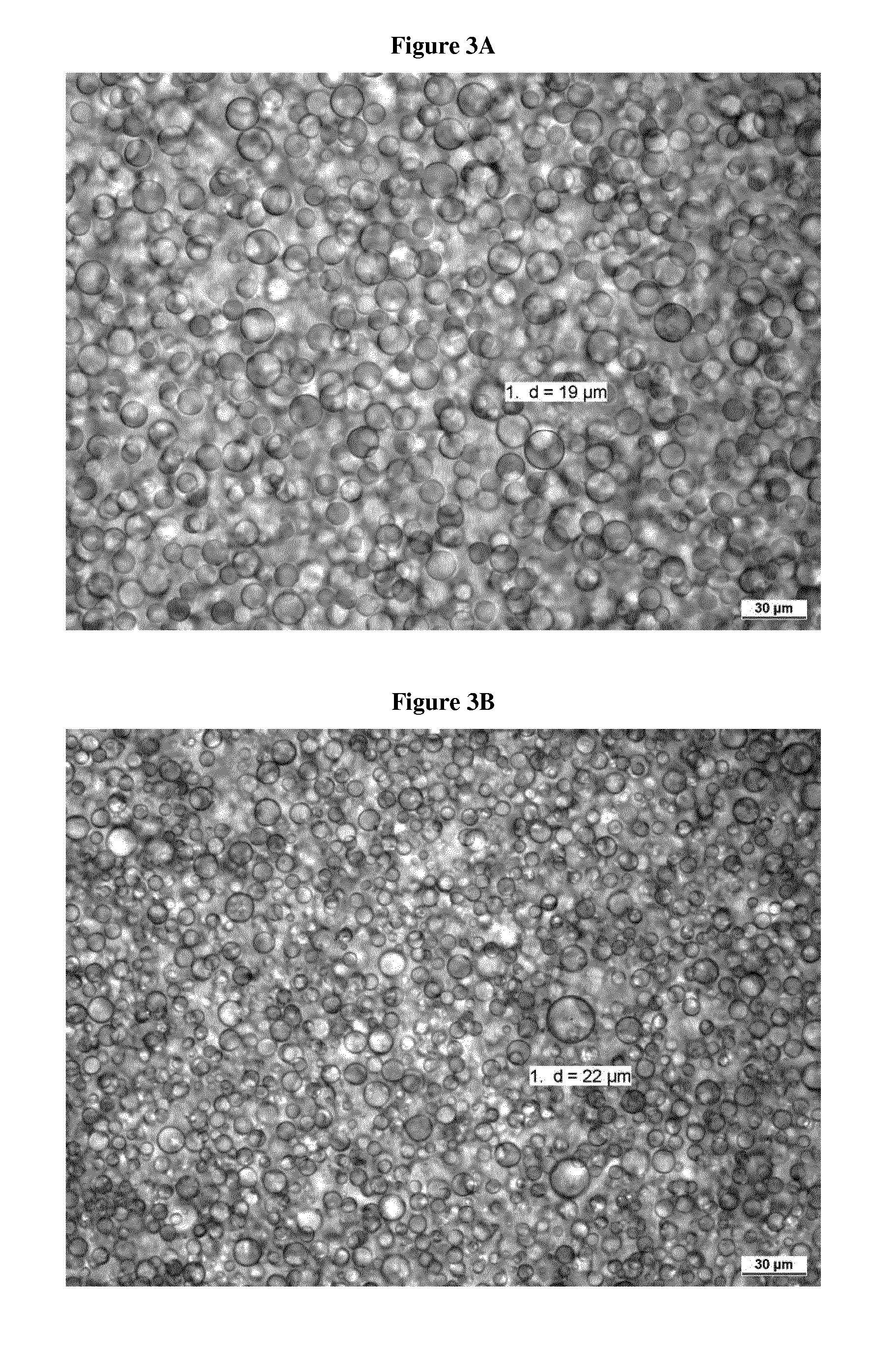
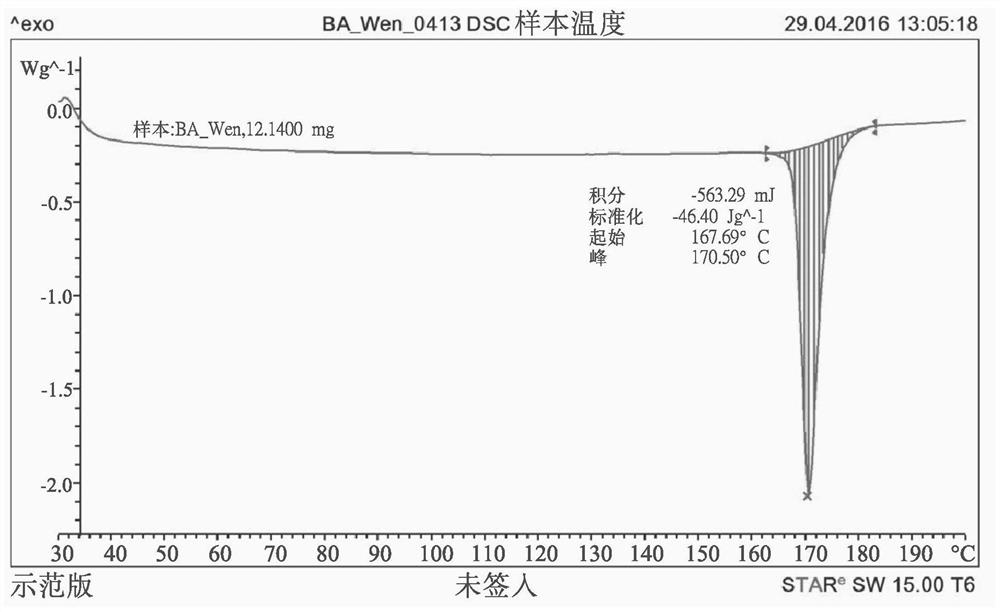
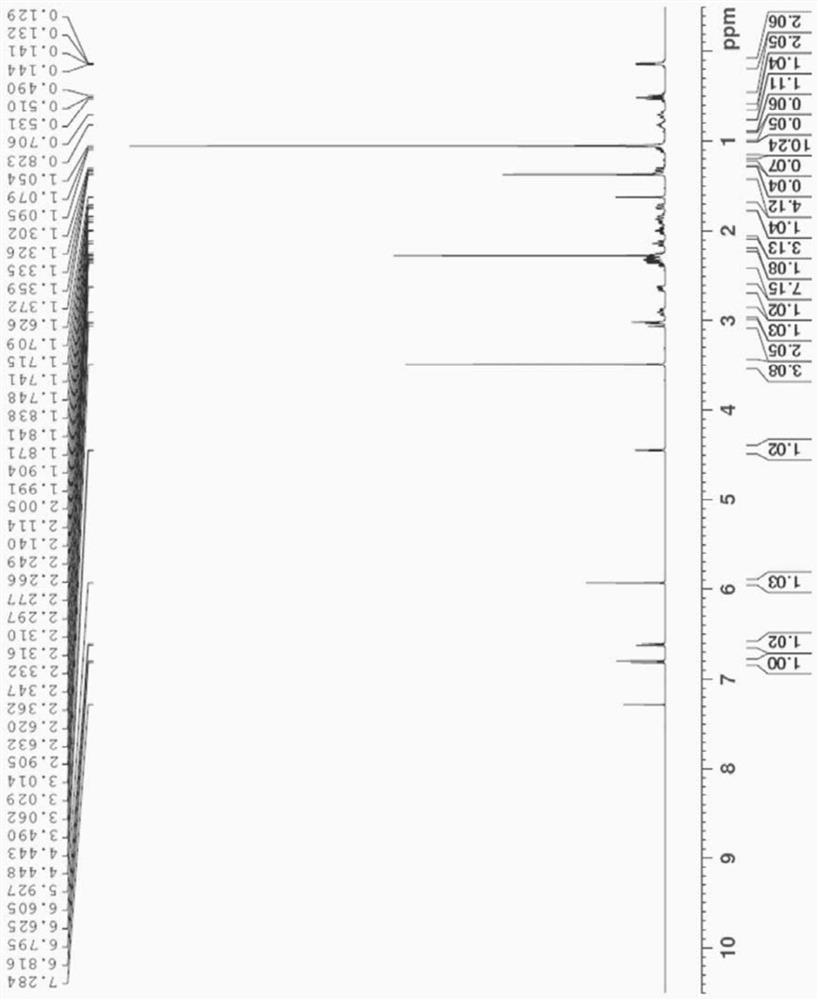
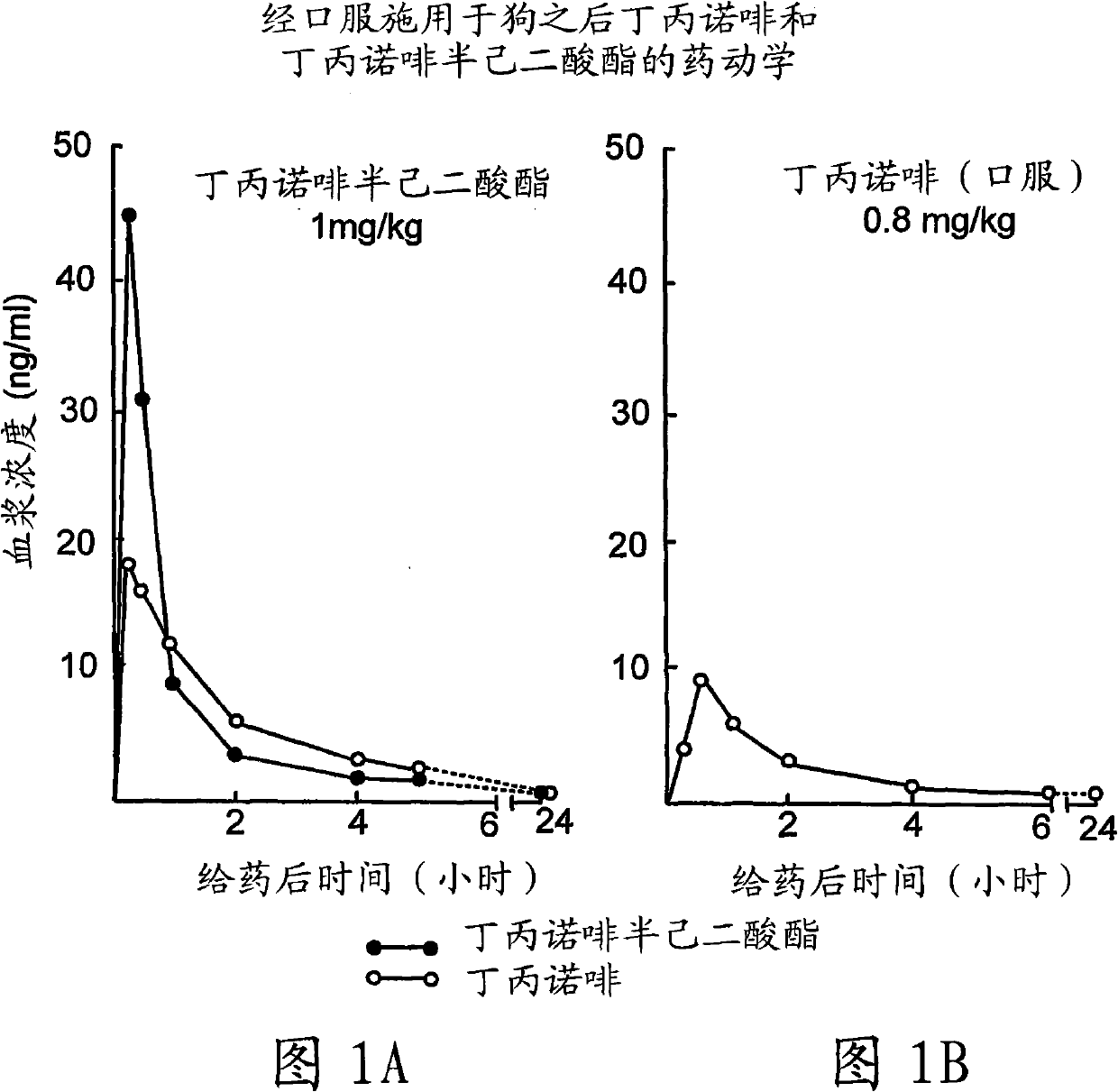
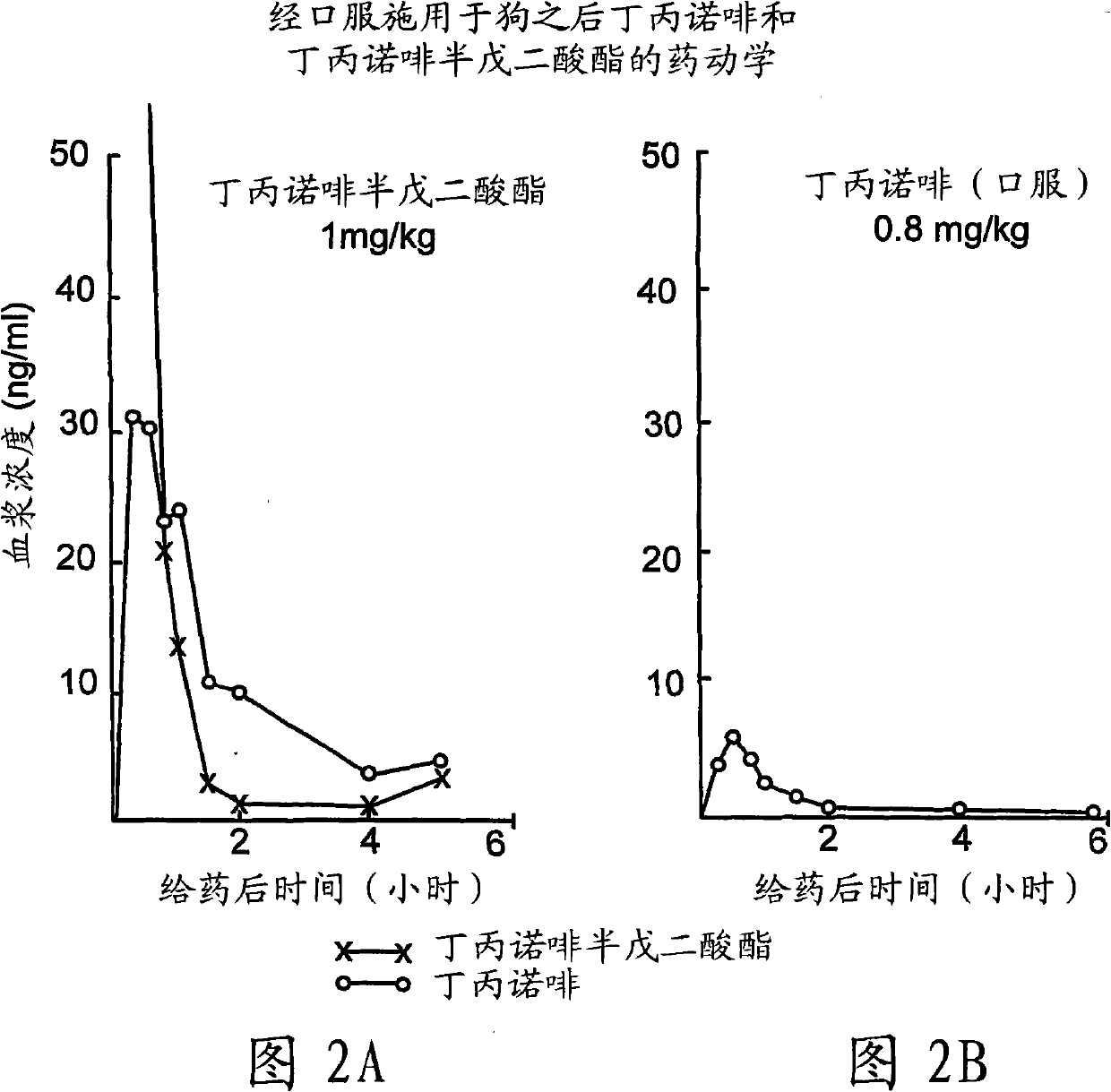
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Buprenophine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Novel ester derivatives of buprenorphine and their preparation processes, and long acting analgestic pharmaceutical compositions
Disclosed herein are novel buprenorphine monocarboxylic ester derivatives and dibuprenorphine dicarboxylic ester derivatives which exert a longer analgesic effect as compared to buprenorphine hydrochloride. Also disclosed are the processes for synthesizing the novel ester derivatives of buprenorphine, and long-acting analgesic pharmaceutical compositions containing a compound selected from buprenorphine base and the novel ester derivatives of buprenorphine.
Owner:CHI MEI MEDICAL CENT
Transdermal Therapeutic System For Administering Analgesics
InactiveUS20070298091A1Reduce usageImprove wearing comfortOrganic active ingredientsNervous disorderAnalgesic agentsBuprenorphine
Transdermal therapeutic systems for administering analgesics, preferably buprenorphine or one of its pharmaceutically acceptable salts or pro-drugs, and processes for the production of such systems.
Owner:GRUNENTHAL GMBH
Injectable flowable composition comprising buprenorphine
ActiveUS8975270B2Least riskImprove bioavailabilityBiocidePharmaceutical delivery mechanismMetaboliteMUSCLE NECROSIS
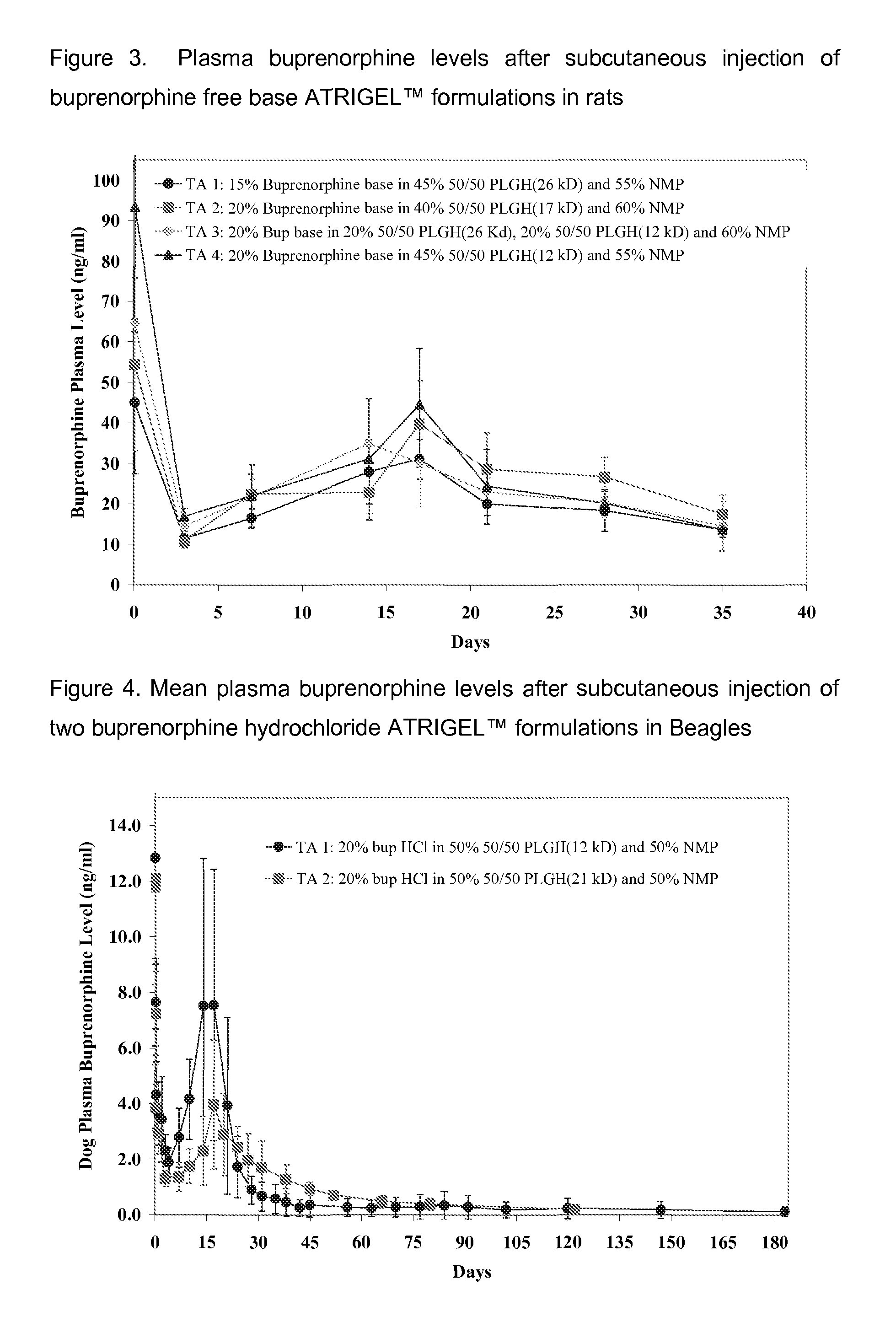
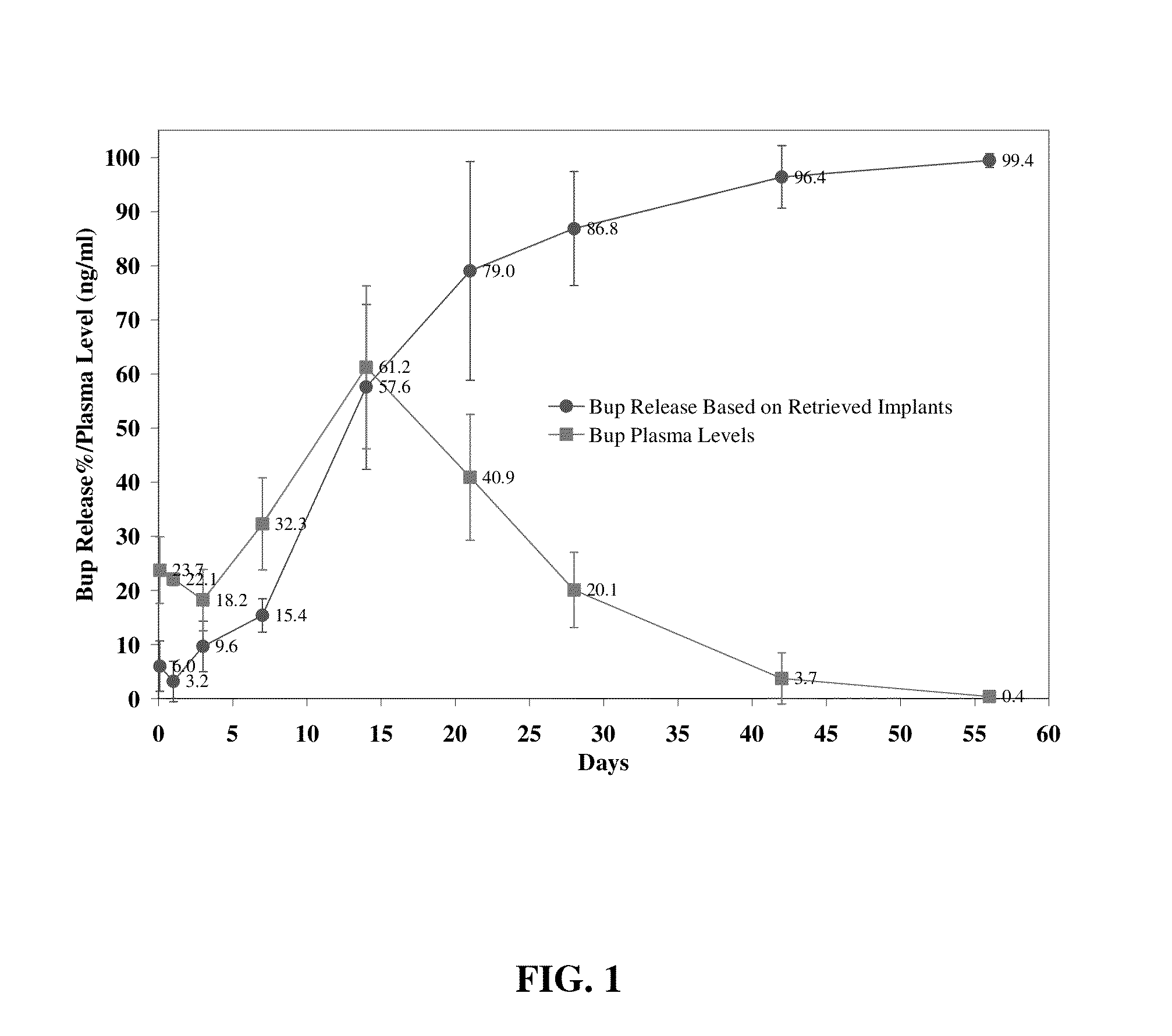
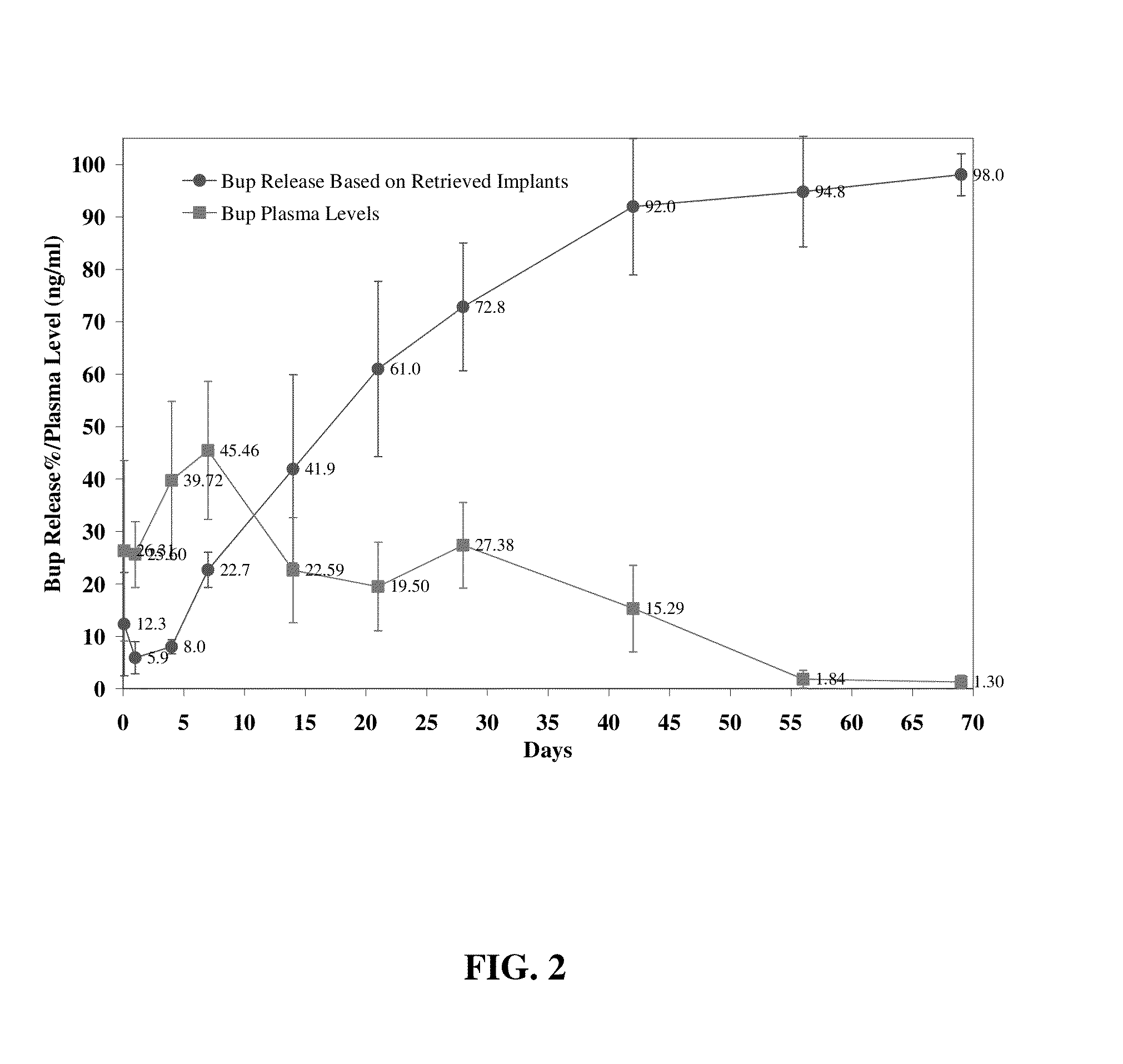
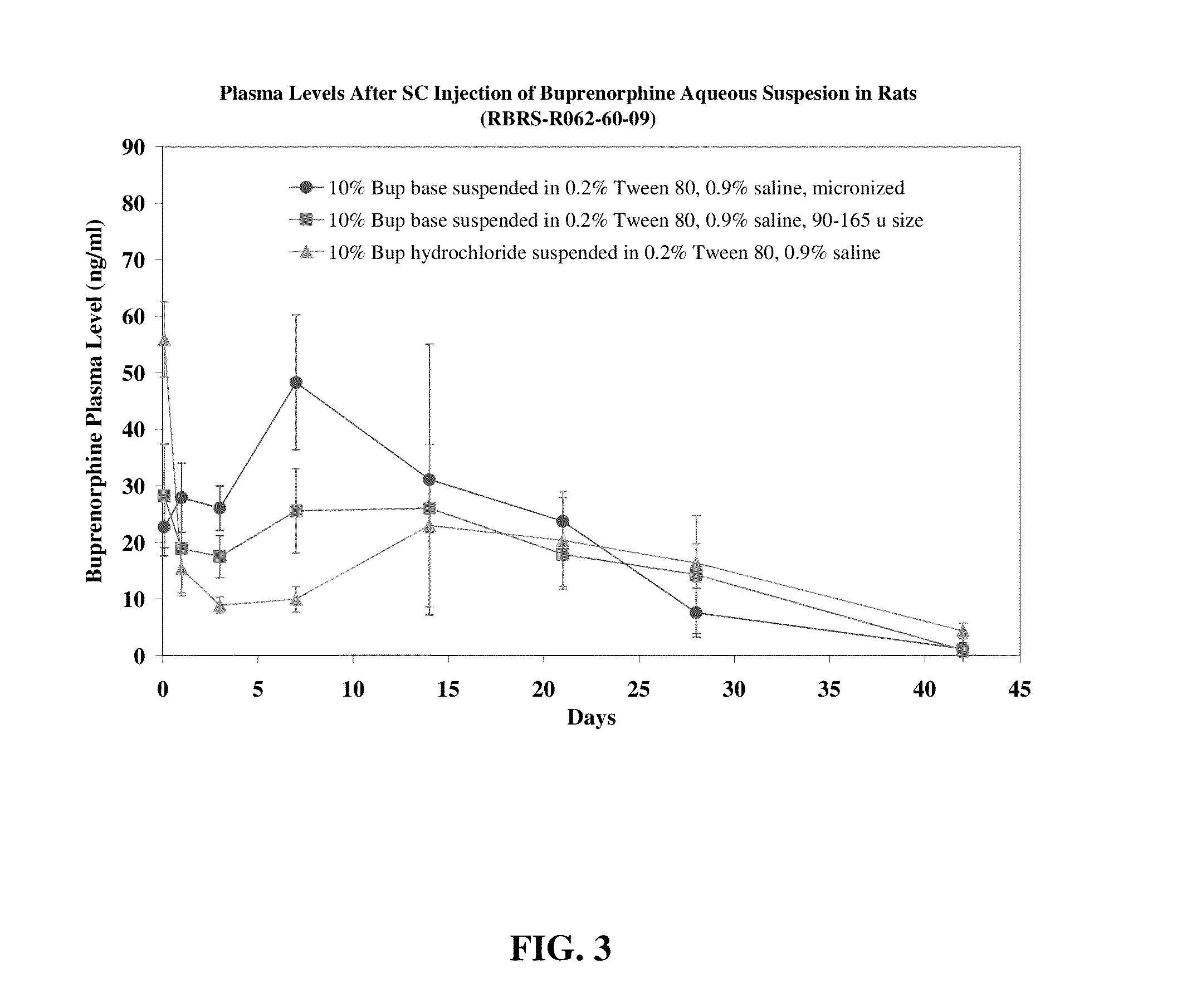
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
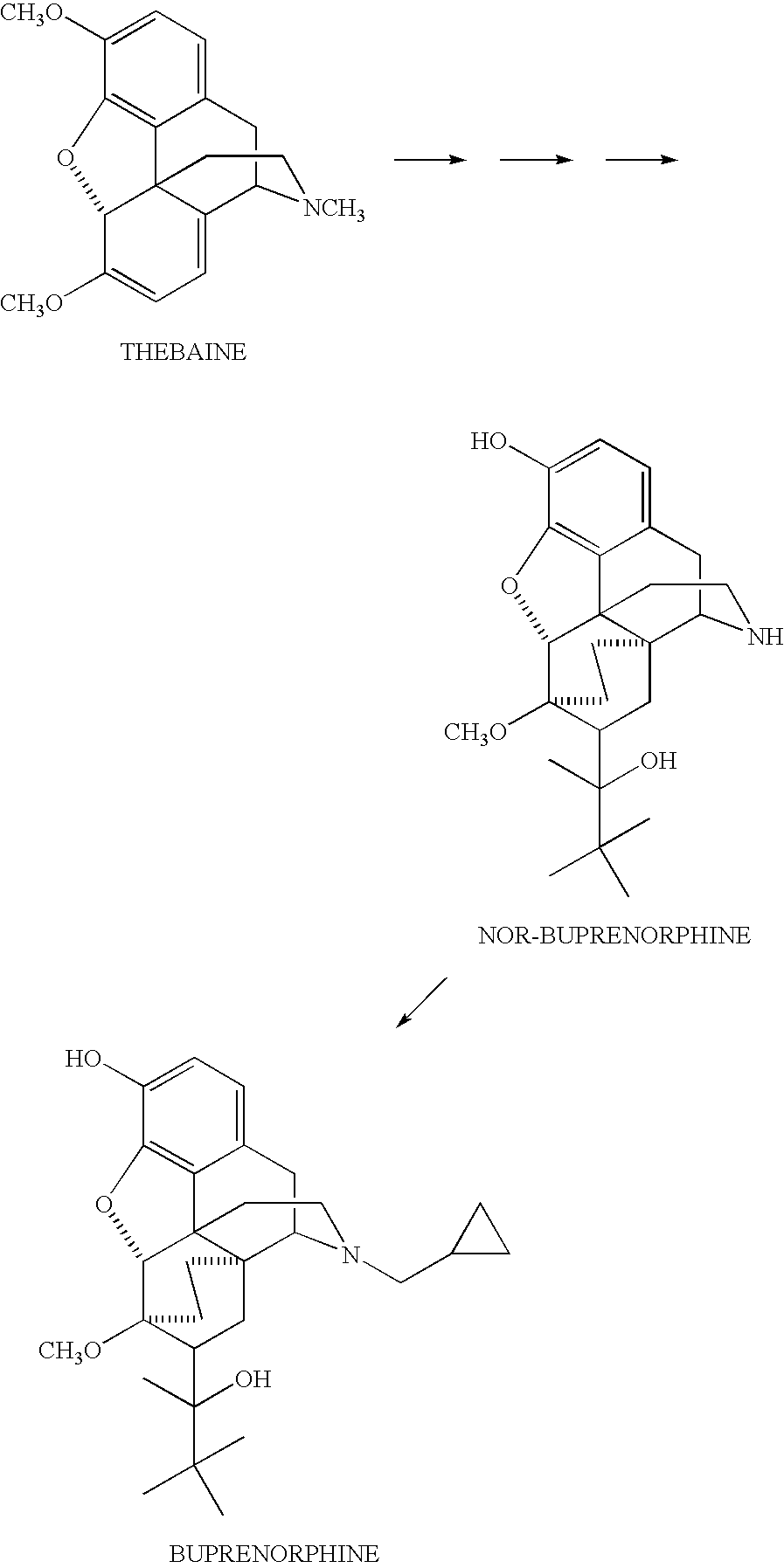
Use of Oripavine as a Starting Material For Buprenorphine
There is provided a method for the synthesis of norbuprenorphine, and ultimately buprenorphine, utilizing oripavine as the starting material. Conventional methods of producing buprenorphine utilize thebaine as the starting material, requiring an O-demethylation step, typically a low to moderate yield transformation. The present use of oripavine as a starting material does not require an O-demethylation step, since the oripavine molecule lacks an O-3 methyl group.
Owner:SPECGX LLC
Injectable flowable composition buprenorphine
ActiveUS9272044B2Least riskImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismMetaboliteMUSCLE NECROSIS
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Patches containing buprenorphine hydrochloride
InactiveUS7056527B2Powder deliveryOrganic active ingredientsCarbon numberBuprenorphine Hydrochloride
Owner:TEIJIN LTD
Injectable Flowable Composition Comprising Buprenorphine
ActiveUS20130210853A1High bioavailabilityMinimal riskBiocideNervous disorderTissue damageBiomedical engineering
The present invention is directed to a buprenorphine sustained release delivery system capable of delivering buprenorphine, a metabolite, or a prodrug thereof for a duration of about 14 days to about 3 months. The buprenorphine sustained release delivery system includes a flowable composition and a solid implant for the sustained release of buprenorphine, a metabolite, or a prodrug thereof. The implant is produced from the flowable composition. The buprenorphine sustained release delivery system provides in situ 1-month and 3-month release profiles characterized by an exceptionally high bioavailability and minimal risk of permanent tissue damage and typically no risk of muscle necrosis.
Owner:INDIVIOR UK
Analogs and prodrugs of buprenorphine
ActiveUS20040192714A1Improve lipophilicityImprove solubilityBiocideOrganic chemistryStereochemistryProdrug
Owner:PURDUE PHARMA LP
Compositions comprising buprenorphine
This disclosure relates to a buprenorphine sustained release delivery system for treatment of conditions ameliorated by buprenorphine compounds. The sustained release delivery system includes a flowable composition containing a suspension of buprenorphine, a metabolite, or a prodrug thereof.
Owner:INDIVIOR UK
Buprenorphine analogs
ActiveUS20150203504A1Eliminate the effects ofReduce and prevent constipationBiocideNervous disorderTreatment painPharmaceutical Substances
Owner:PURDUE PHARMA LP
Buprenorphine analogs
ActiveUS20140057931A1Reducing desired analgesic effectReduce and prevent constipationBiocideNervous disorderTreatment painConstipation
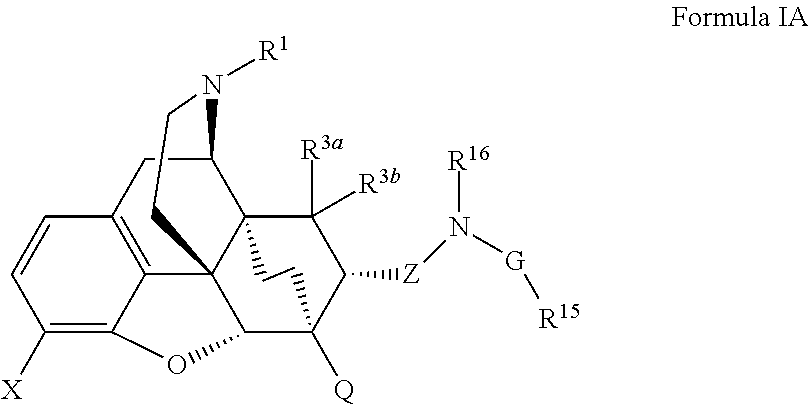
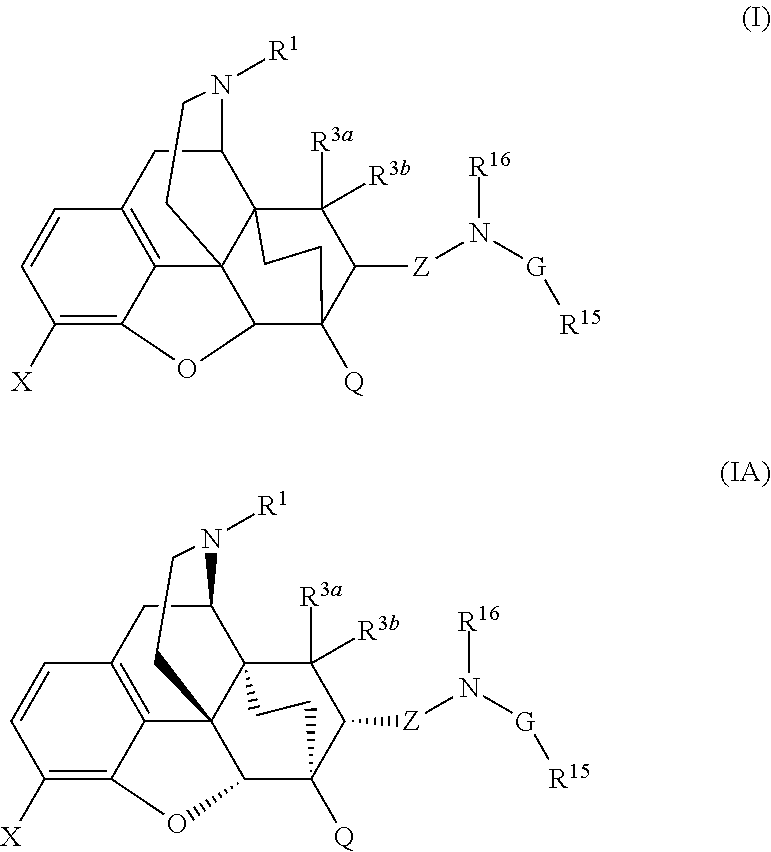
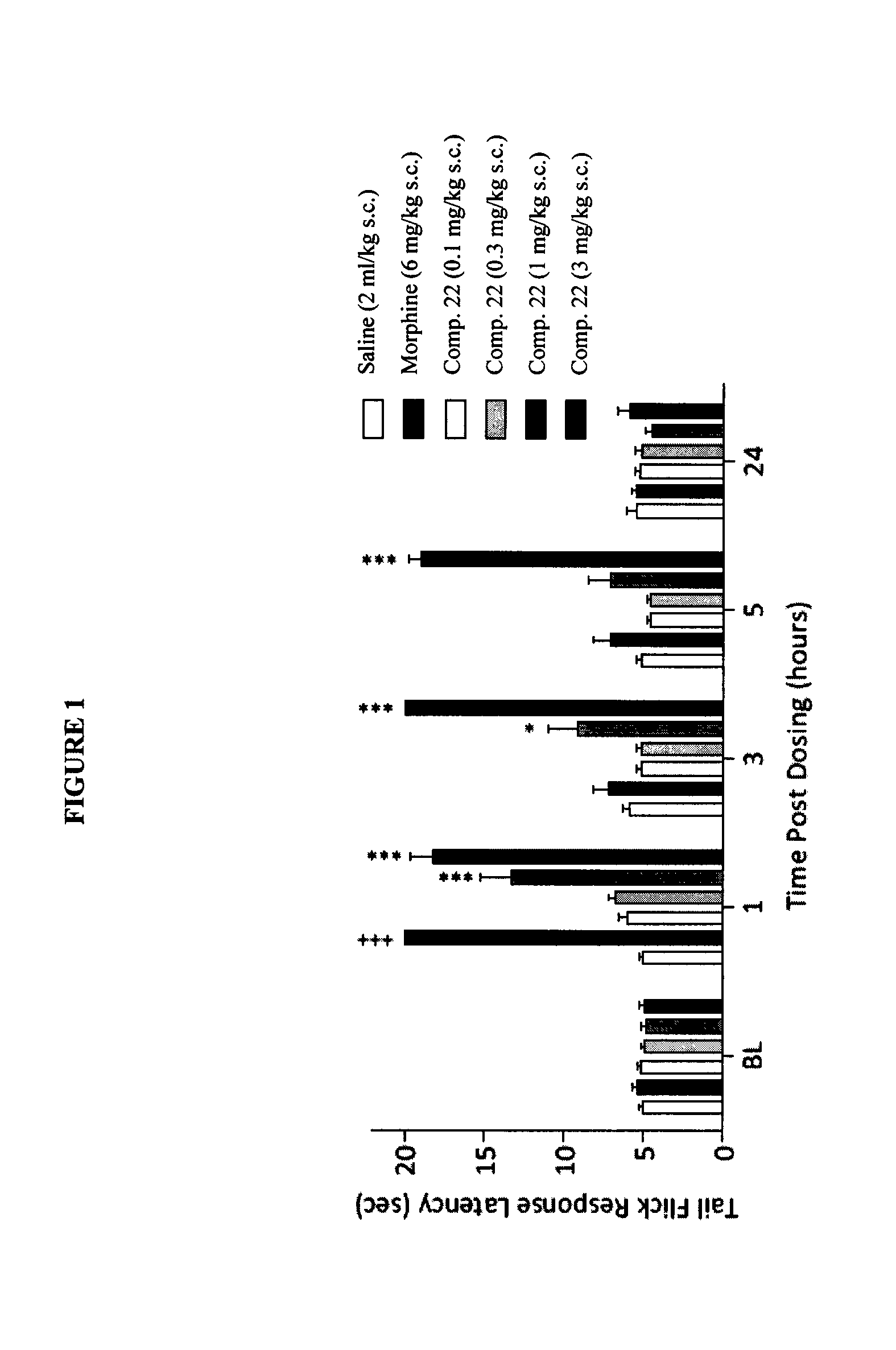
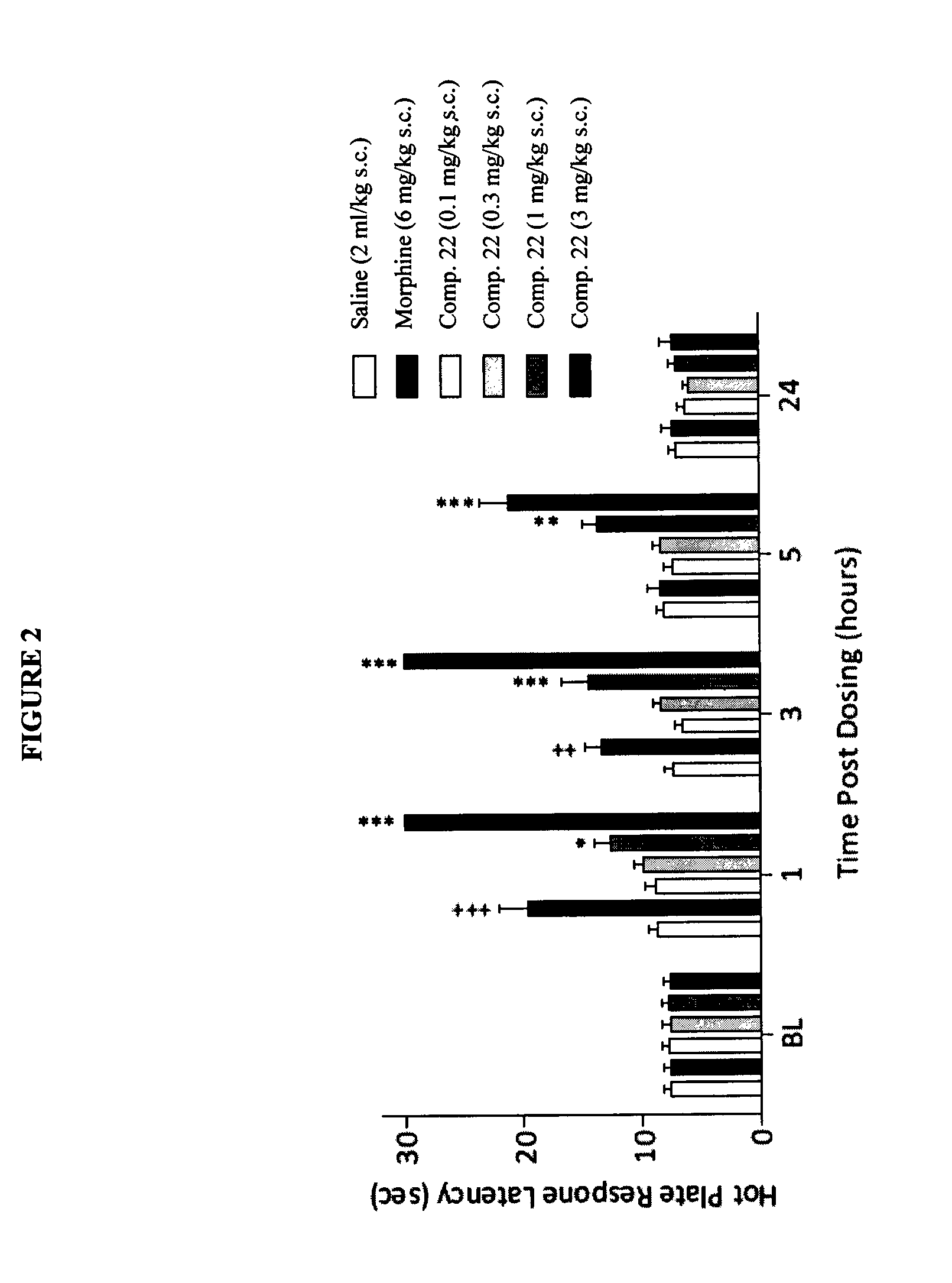
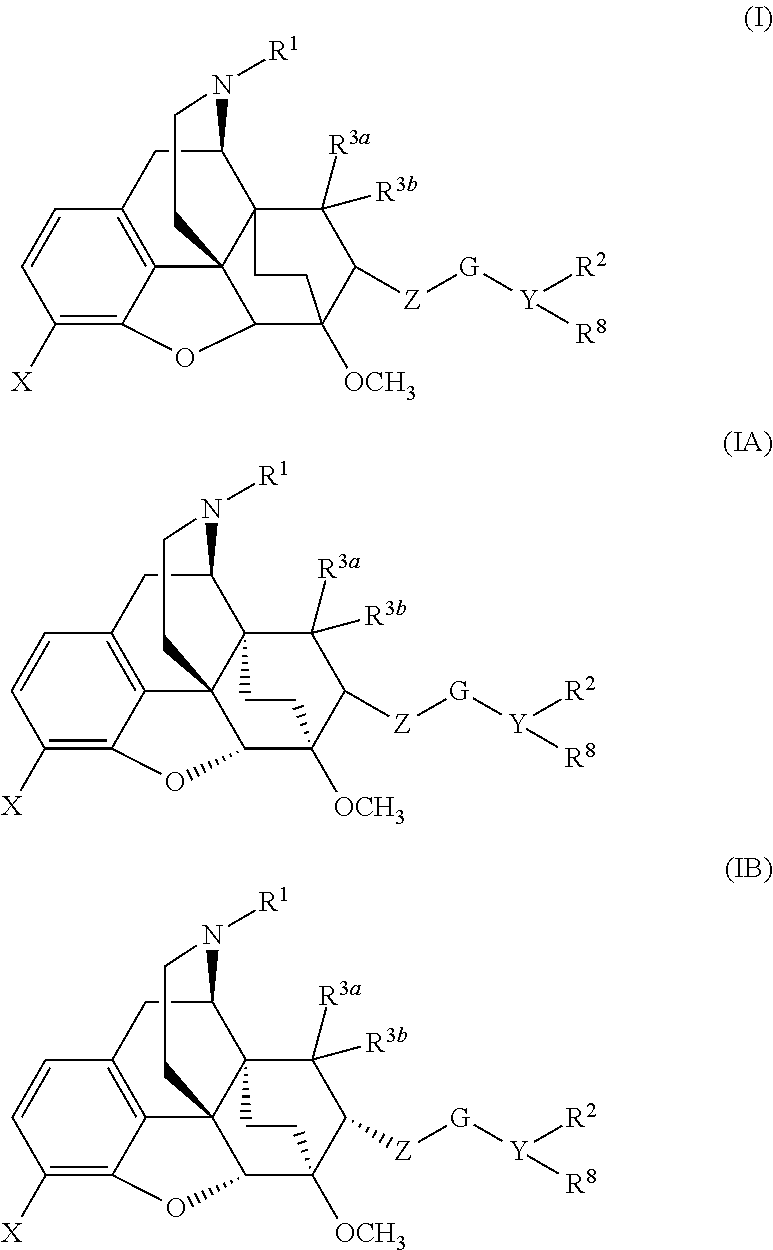
The present invention is directed to Buprenorphine Analog compounds of the Formula (I), Formula (IA) or Formula (IB) shown below, wherein R1, R2, R8, R3a, R 3b, G, X, Z and Y are as defined herein. Compounds of the Invention are useful for treating pain, constipation, and other conditions modulated by activity of opioid and ORL-1 receptors.
Owner:PURDUE PHARMA LP
Patches containing buprenorphine hydrochloride
InactiveUS20020182247A1Improve breathabilitySafety and economy can be providedPowder deliveryOrganic active ingredientsDrugPolymer chemistry
A patch comprising an adhesive layer formed on one surface of a flexible support, wherein said adhesive layer containing a drug, an absorption enhancer and an adhesive comprising; (i) said drug is buprenorphine hydrochloride and / or buprenorphine, and (ii) said absorption enhancer is a mixture of polyoxyethylene sorbitan mono fatty acid ester having 6 to 20 of oxyethylene units and 12 to 18 of carbon number of fatty acid ester, and at least one selected from the group consisting of liquid higher fatty acid ester, 60 to 180 of molecular weight of liquid poly hydric alcohol, lactic acid and triacetin, and (iii) said adhesive is an acrylic-based adhesive.
Owner:TEIJIN LTD
Buprenorphine analogs
ActiveUS9175000B2Eliminate side effectsEliminate the effects ofOrganic active ingredientsNervous disorderOpioid receptorMedicinal chemistry
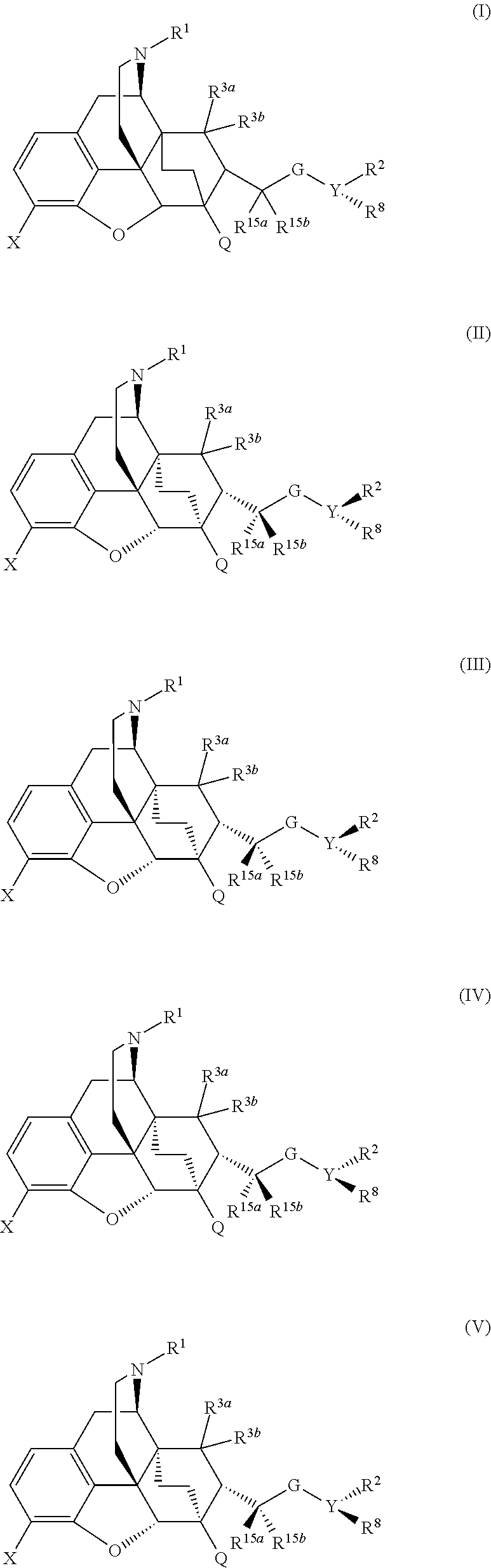
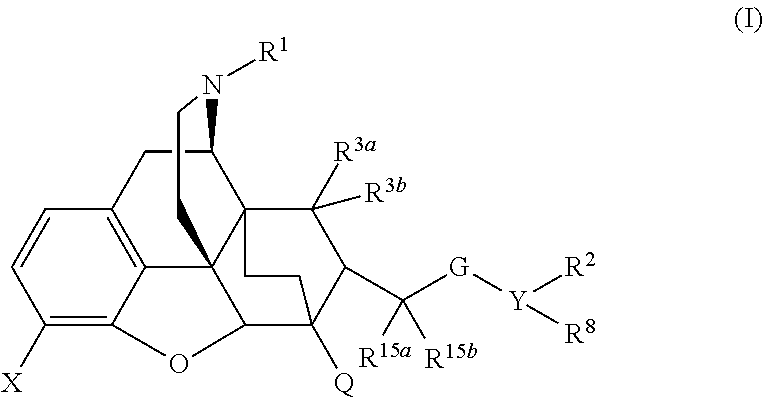
The present invention is directed to Buprenorphine Analog compounds of the Formula I, Formula II, Formula III, Formula IV, and Formula V, wherein R1, R2, R3a, R3b, R15a, R15b, X, Q, G, and Y are as defined herein.Compounds of the Invention are useful for treating pain and other conditions modulated by activity of opioid receptors.
Owner:PURDUE PHARMA LP
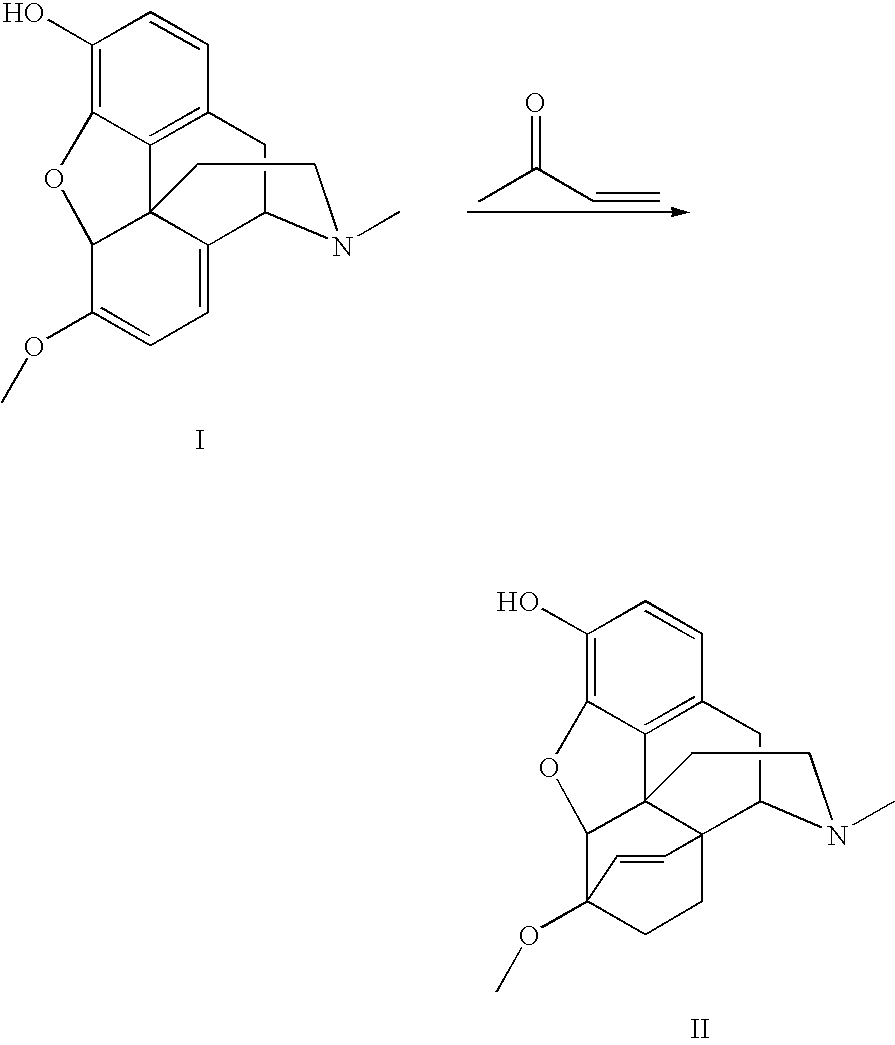
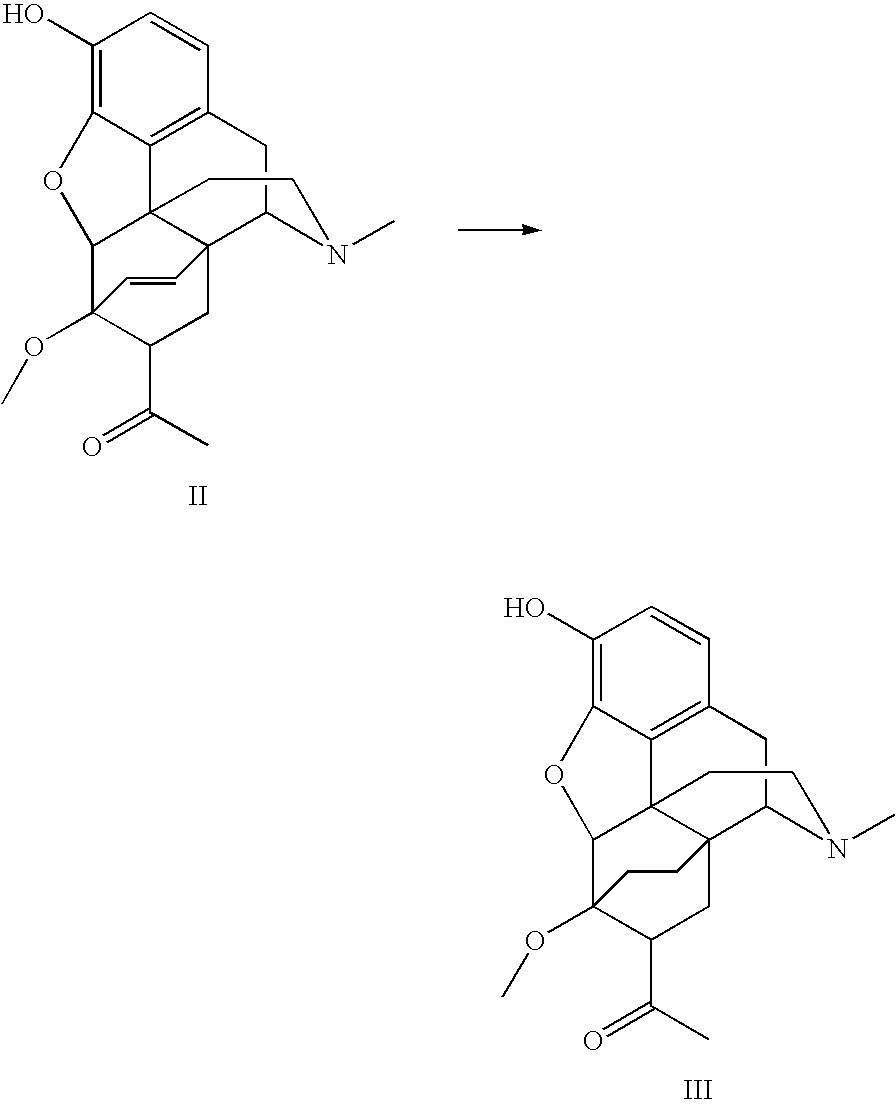
Processes for the alkylation of norbuprenorphine with reduced impurity formation
The invention provides processes for the production of opiate alkaloids. In particular, the present invention provides processes for the formation of buprenorphine and derivatives of buprenorphine that minimizes the formation of impurities.
Owner:SPECGX LLC
Buprenorphine Analogs
ActiveUS20110136846A1Useful in treatmentEffective analgesiaBiocideNervous disorderMedicinal chemistryBuprenorphine
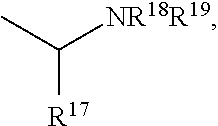
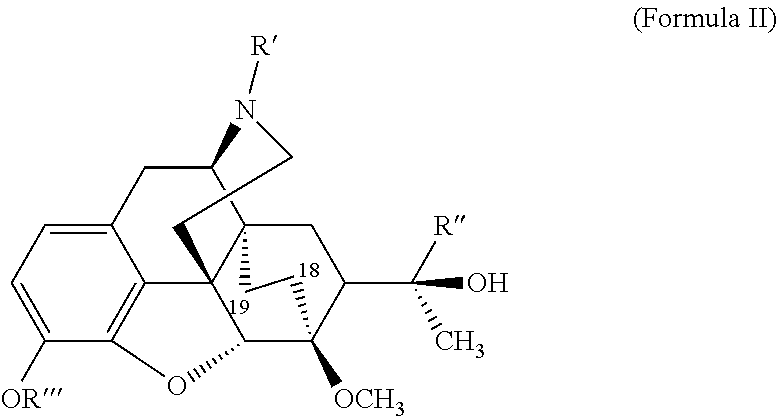
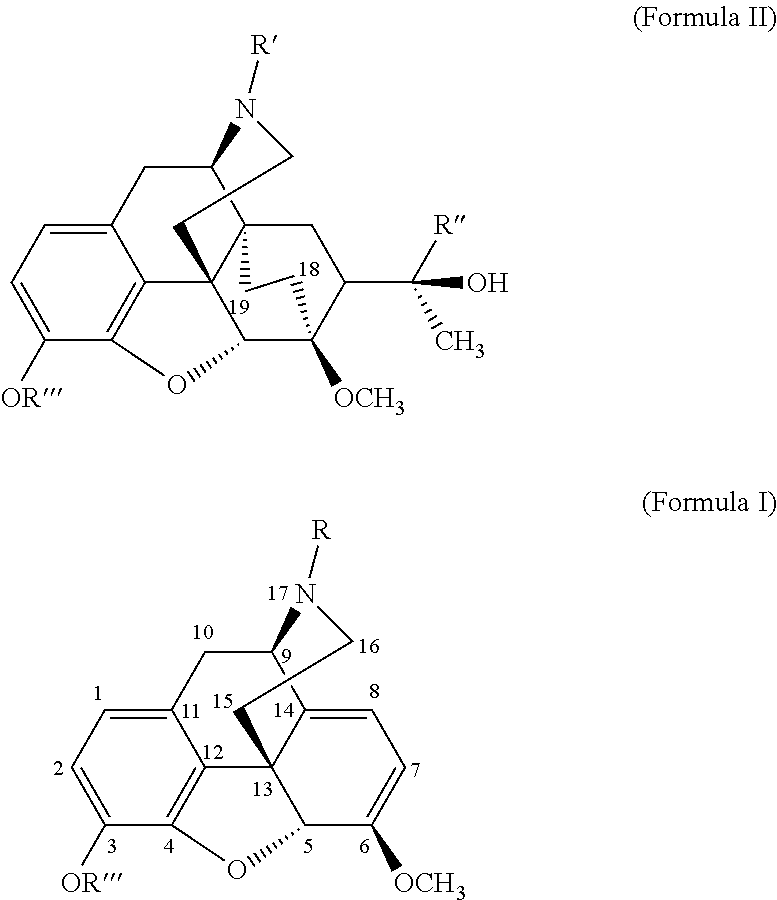
The present invention is directed to Buprenorphine Analog compounds of the Formula I, Formula II or Formula III shown below, wherein R1, R2, R8, R3, R3a, R3b, X, Z and Y are as defined herein, R1 H3CO (I) OCH3 OCH3 (III) Compounds of the Invention are useful for treating pain and other conditions modulated by activity of opioid and ORL1 receptors.
Owner:PURDUE PHARMA LP
Abuse potential low compound buprenorphin hydrochloride naloxone hydrochloride sublingual tablet
InactiveCN1943575AReduce Abuse PotentialAvoid abuseOrganic active ingredientsNervous disorderBuprenorphine HydrochlorideAlcohol
A buprenorphine hydrochloride / naloxone hydrochloride sublingual tablets with low misuse potential , said tablets contain medicinal contents of buprenorphine hydrochloride and naloxone hydrochloride, part by weight thereof is 2:1-6:1, 1) using alcohol to dissolve regulator corrigent and bond of PH of prescription amount for use; 2) grinding main medicine buprenorphine hydrochloride / naloxone hydrochloride, filler and lubricant and sifting out thereof; 3) mixing evenly the prescription amount of buprenorphine hydrochloride / naloxone hydrochloride, filler, bond and disintegrating agent, thereafter adding contents in step 1 to prepare into soft stuff, again making into pellet, drying, sorting out, adding lubricant again, mixing evenly and pressing into tablet.
Owner:岳振江
Transdermal delivery system
InactiveUS20160008294A1Reduce areaImprove adhesionBiocideNervous disorderCarboxylic acidOleic Acid Triglyceride
The invention relates to transdermal therapeutic system for the transdermal administration of buprenorphine, comprising a buprenorphine-containing self-adhesive layer structure comprising A) a buprenorphine-impermeable backing layer, and B) a buprenorphine-containing pressure-sensitive adhesive layer on said buprenorphine-impermeable backing layer, the adhesive layer comprising a) at least one polymer-based pressure-sensitive adhesive, b) an analgesically effective amount of buprenorphine base or a pharmaceutically acceptable salt thereof, c) a viscosity-increasing substance in an amount of about 0.1% to about 8% of said buprenorphine-containing pressure-sensitive adhesive layer, and d) a carboxylic acid selected from the group consisting of oleic acid, linoleic acid, linolenic acid, levulinic acid and mixtures thereof, in an amount sufficient so that said analgesically effective amount of buprenorphine is solubilized therein to form a mixture including said viscosity-increasing substance, and wherein the carboxylic acid-, buprenorphine- and viscosity-increasing substance-containing mixture forms dispersed deposits in the said pressure-sensitive adhesive, and wherein said buprenorphine-containing pressure-sensitive adhesive layer is the skin contact layer.
Owner:LTS LOHMANN THERAPIE-SYST AG
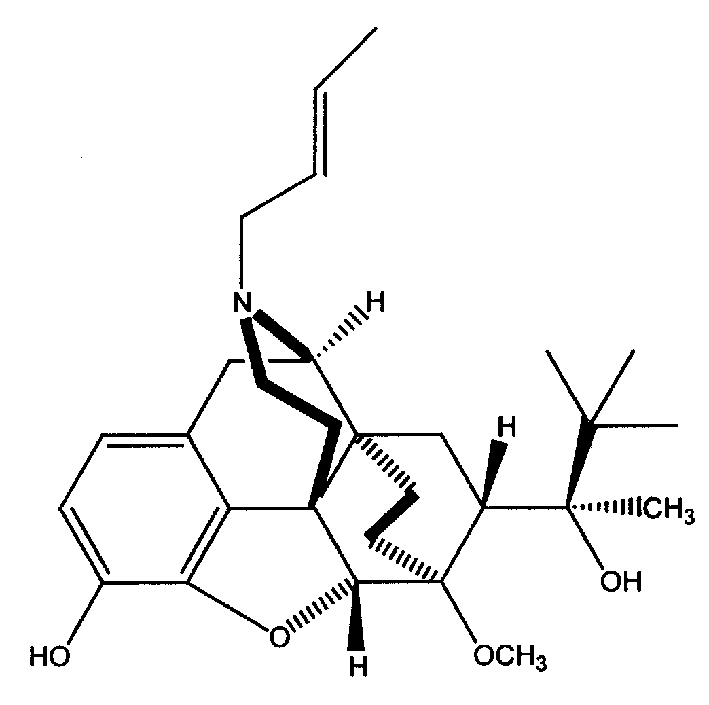
N-But-3-Enyl norbuprenophine and its use as analgesic
The present invention provides compound having the structure and pharmaceutically acceptable salts or derivatives thereof, as well as compositions including such compounds. The invention also provides methods of (1) preventing pain, (2) treating pain, (3) inducing sedation, (4) treating opiate addiction, (5) treating opiate withdrawal (abstinence syndrome) and / or (6) treating cough in a patient in need thereof by administering a compound or composition of the invention.
Owner:EURO-CELTIQUE SA
Oral Pharmaceutical Compositions of Buprenorphine and Another Opioid Receptor Agonist
The present invention is directed to oral pharmaceutical compositions of buprenorphine and it pharmaceutically acceptable salts and the use thereof.
Owner:RELMADA THERAPEUTICS
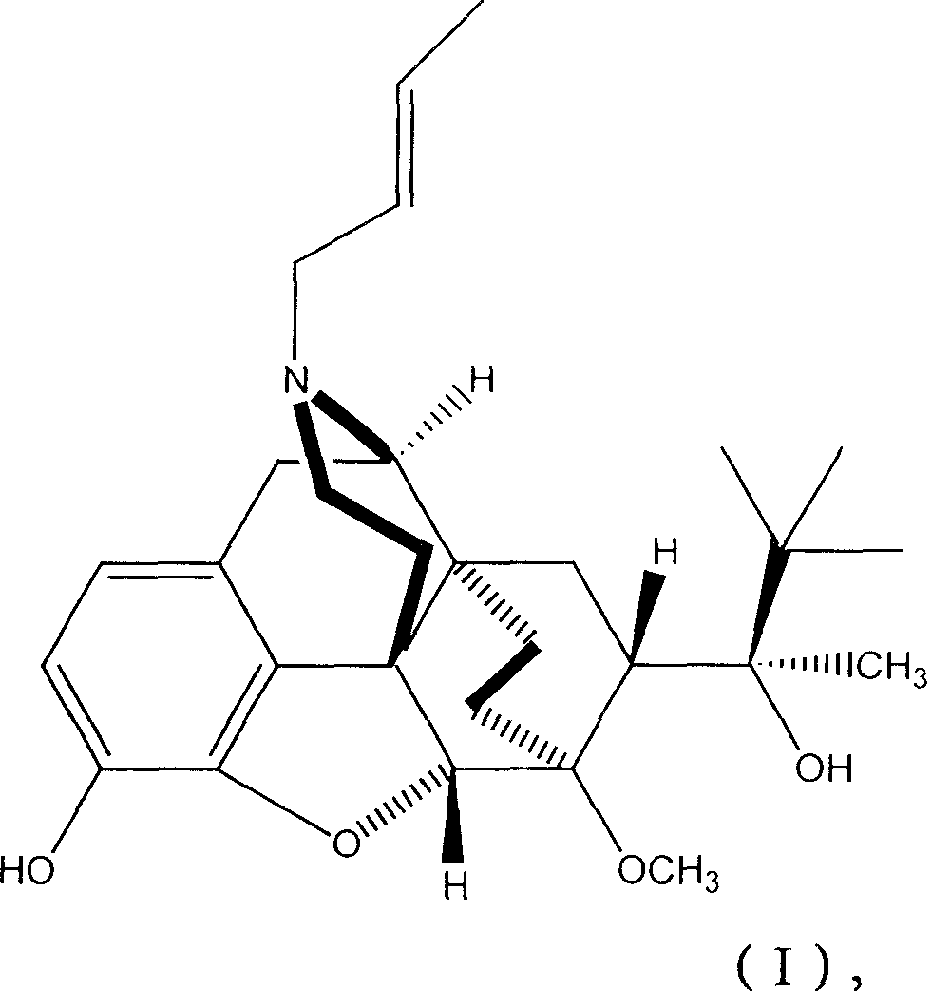
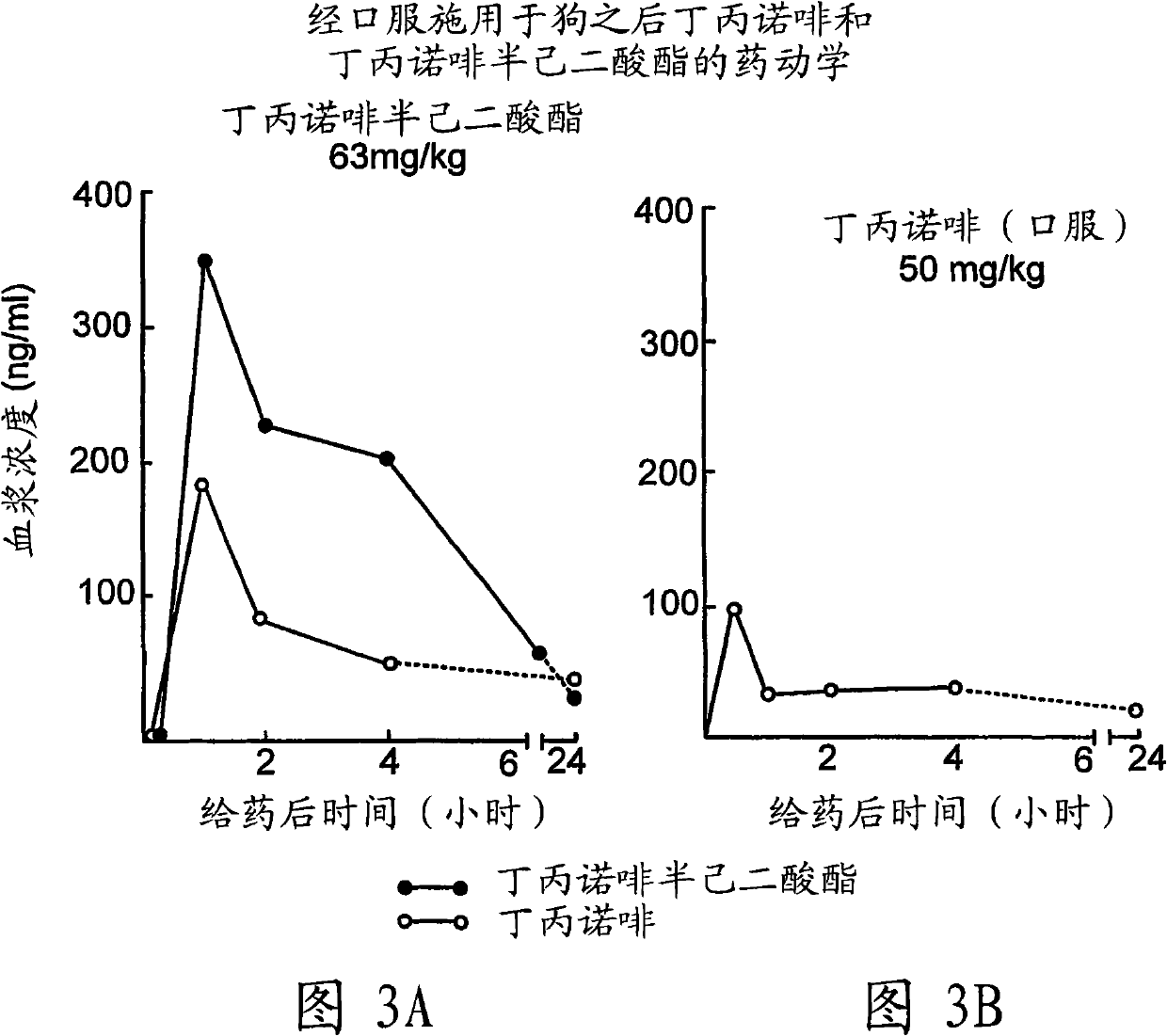
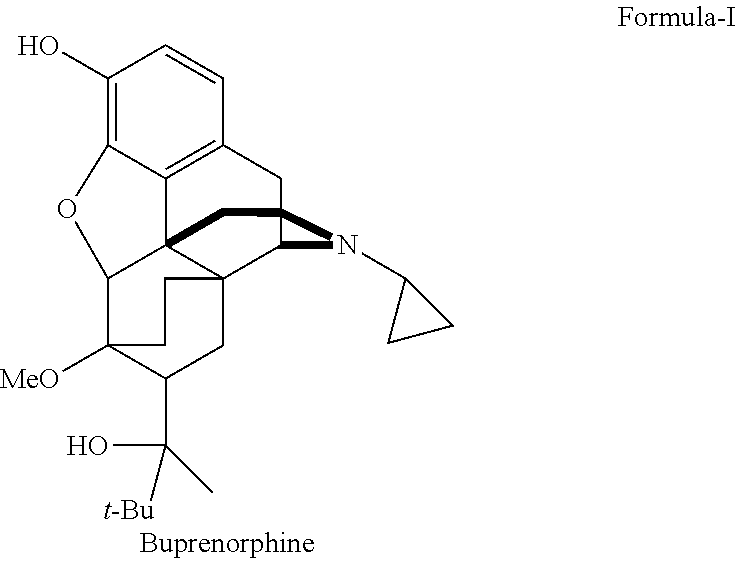
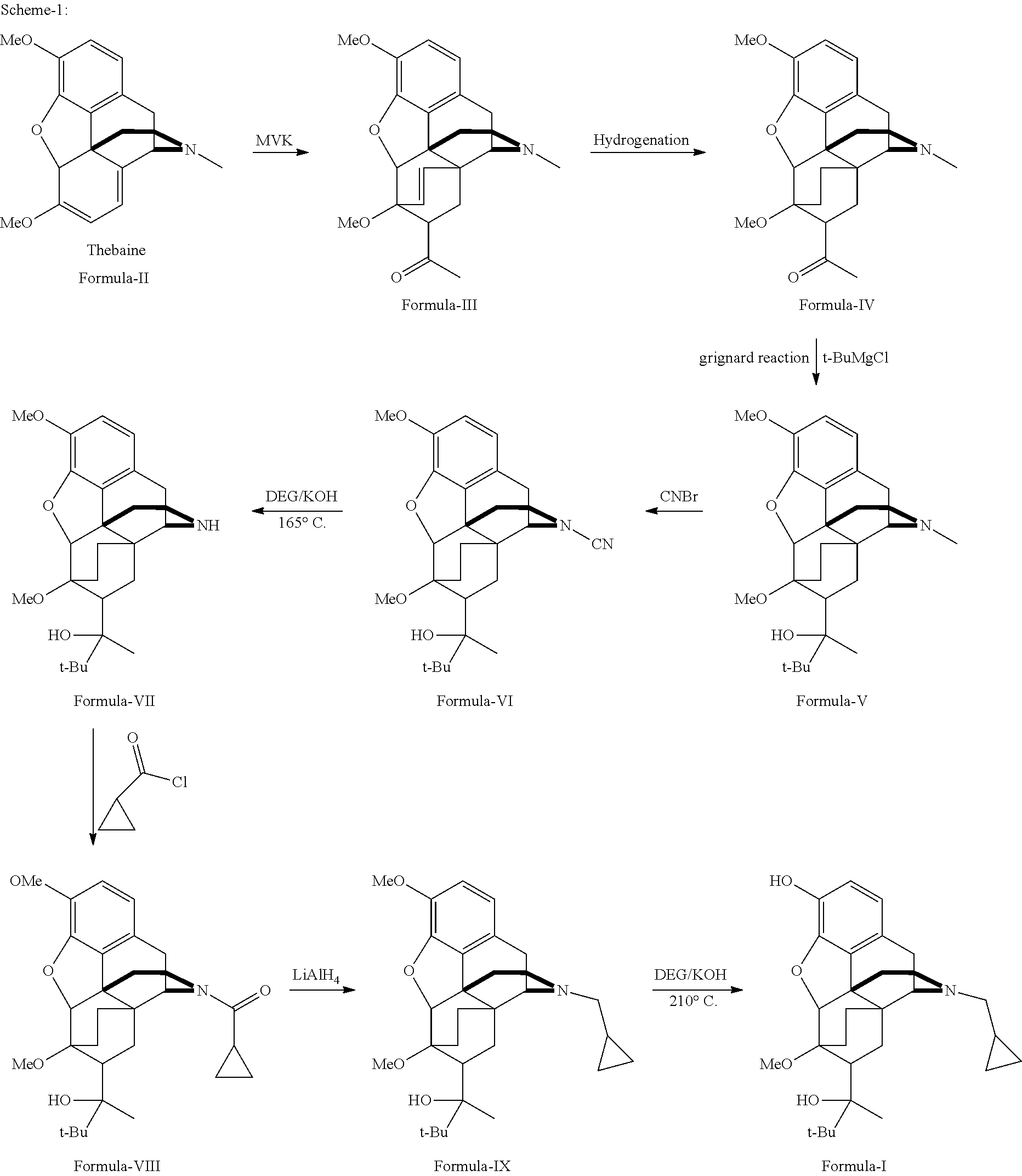
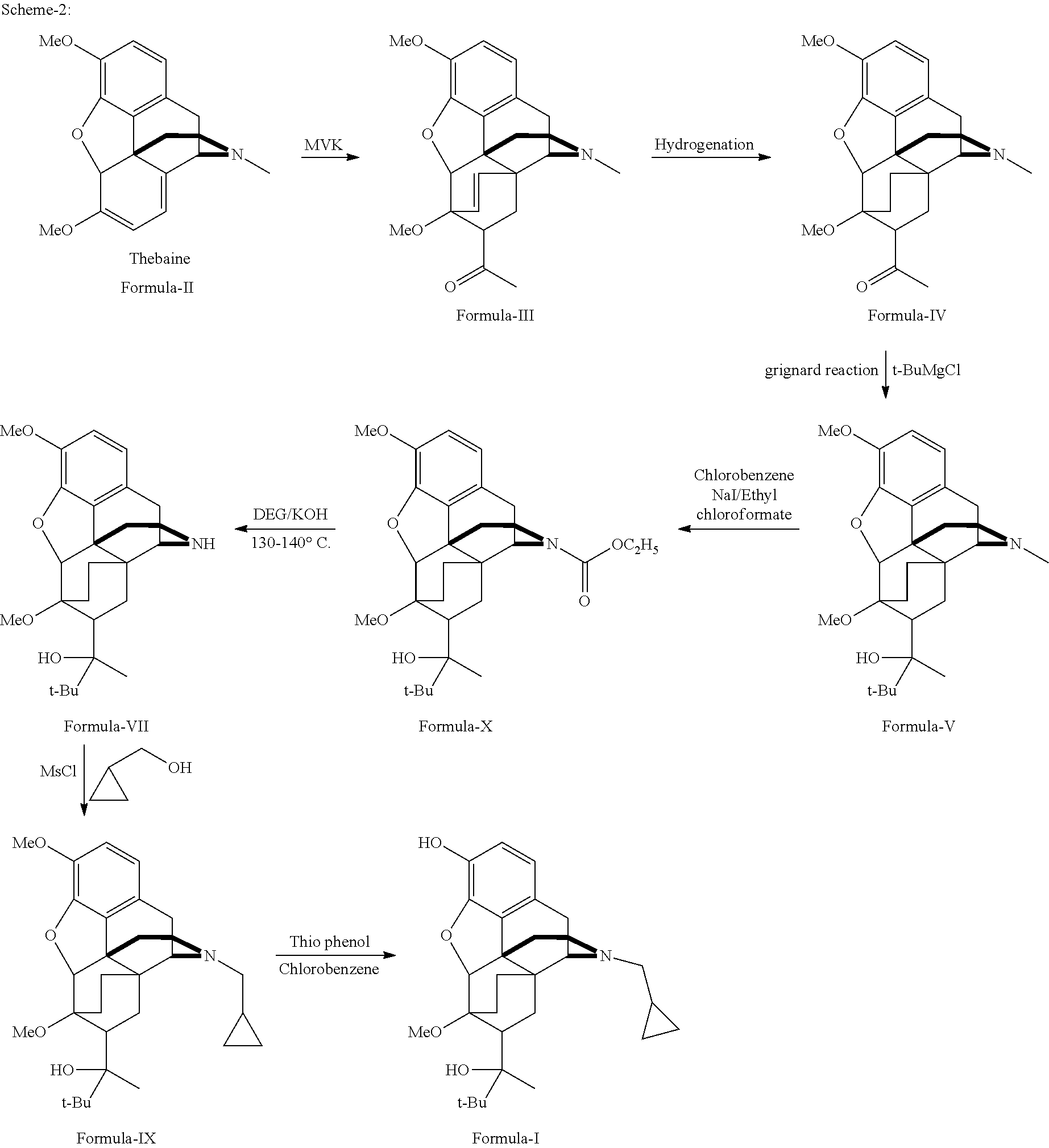
Industrial process for the preparation of buprenorphine and its intermediates
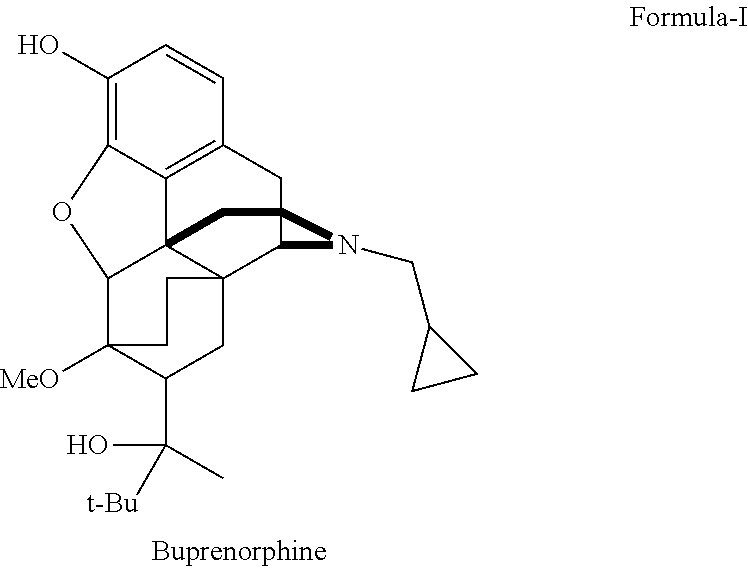
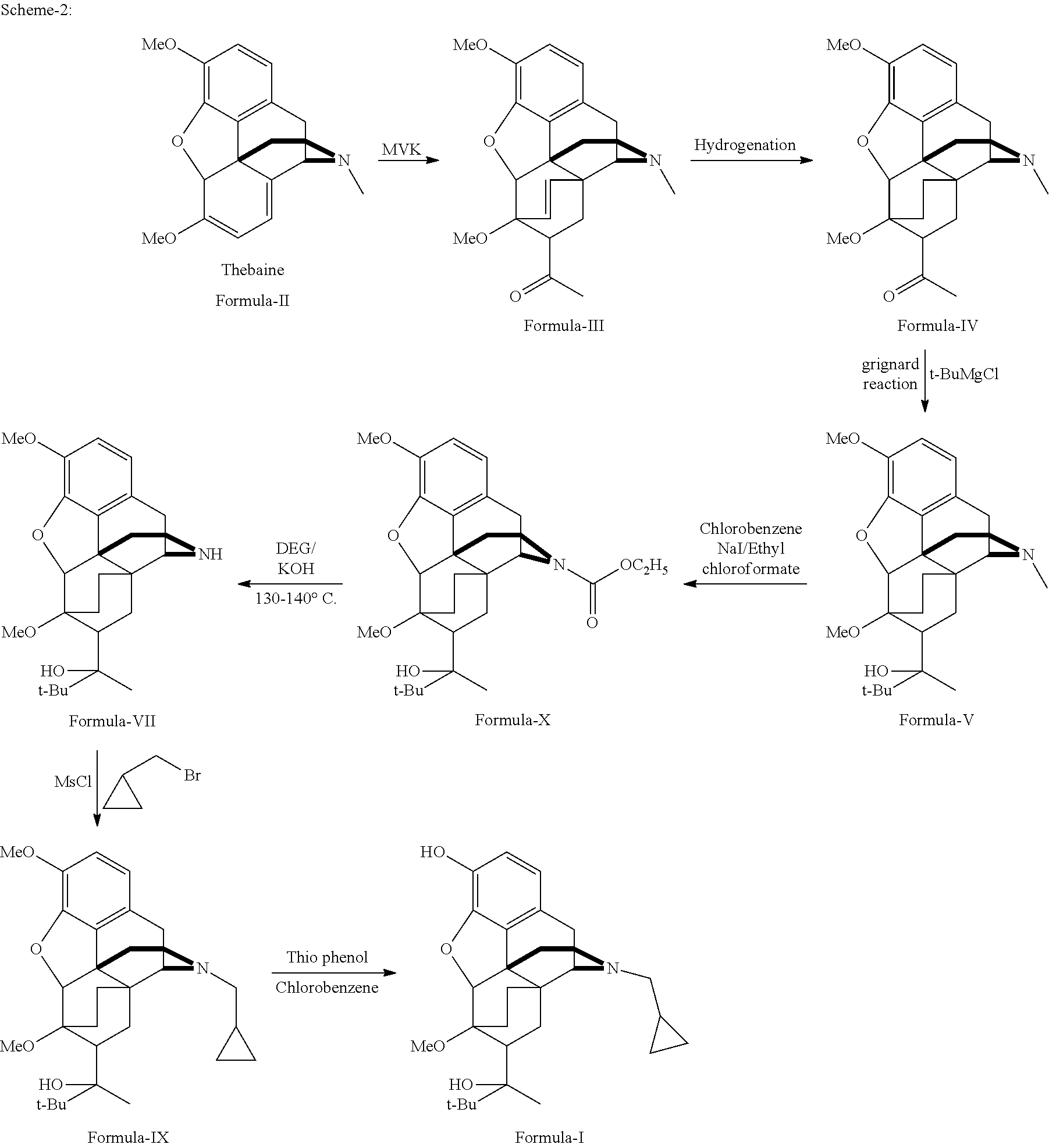
There is provided an efficient industrial process for the preparation of 21-cyclopropyl-7α-(2-hydroxy-3,3-dimethyl-2-butyl)-6,14-endo-ethano-6,7,8,14-tetrahydro-oripavine, i.e. buprenorphine of Formula-I in high yield and purity, with enhanced safety and eco-friendly norms. The invention further relates to an improved process for preparation of intermediates thereof in high yield and purity.
Owner:RUSAN PHARMA
Pharmaceutical formulations comprising opioid receptor agonist as active ingredients, methods of manufacture and therapeutic uses thereof
ActiveUS20190117556A1Formula SafetyAdequate shelf lifeOrganic active ingredientsNervous disorderBULK ACTIVE INGREDIENTDrug withdrawal syndrome
Formulation comprising buprenorphine or a pharmaceutically acceptable salt thereof as the sole active ingredient, a viscosity enhancer, and a buffering agent in an amount to provide a pH of from 5.0 to 7.0 are useful for treating opioid withdrawal syndrome.
Owner:CHIESI FARM SPA
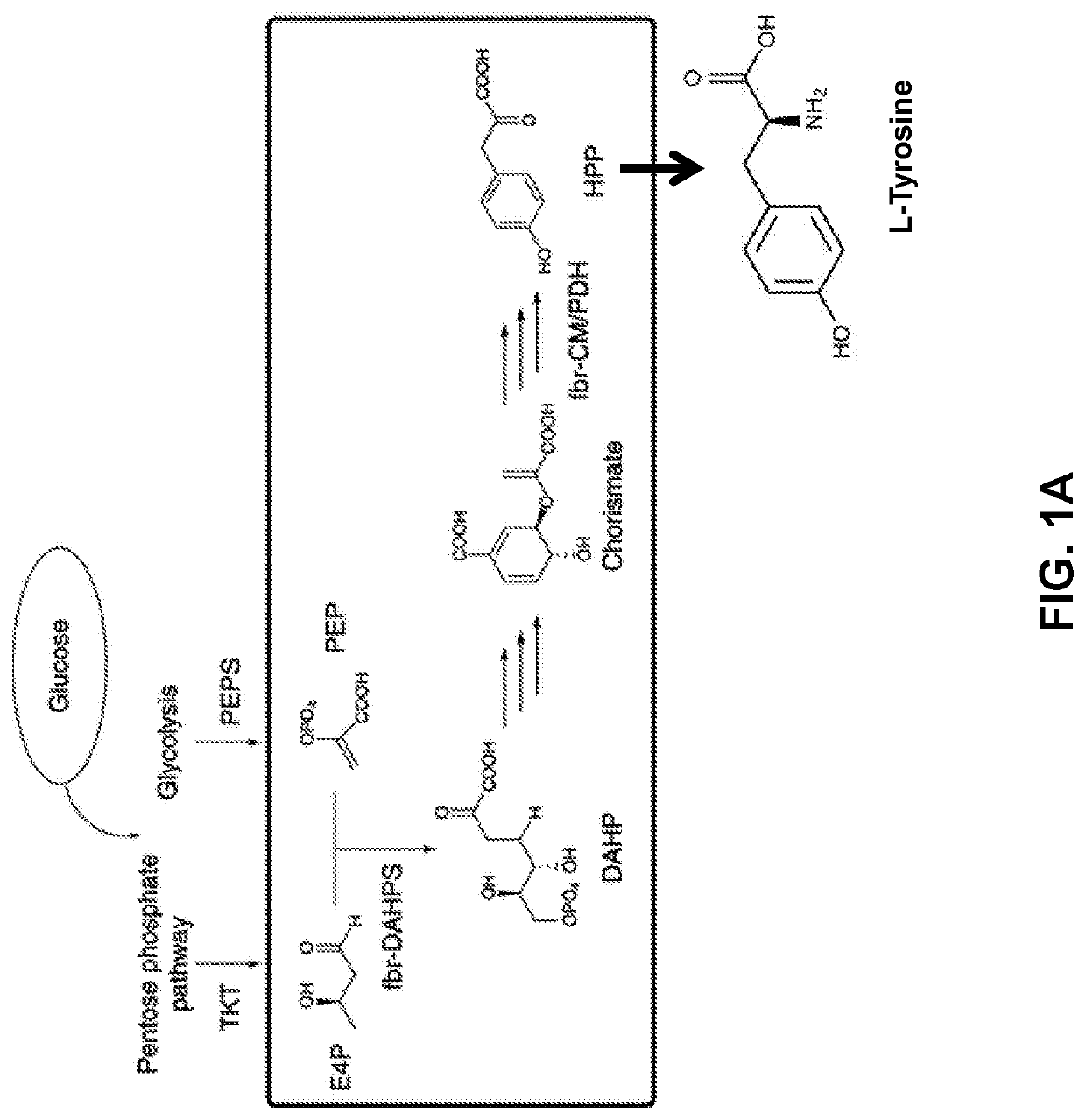
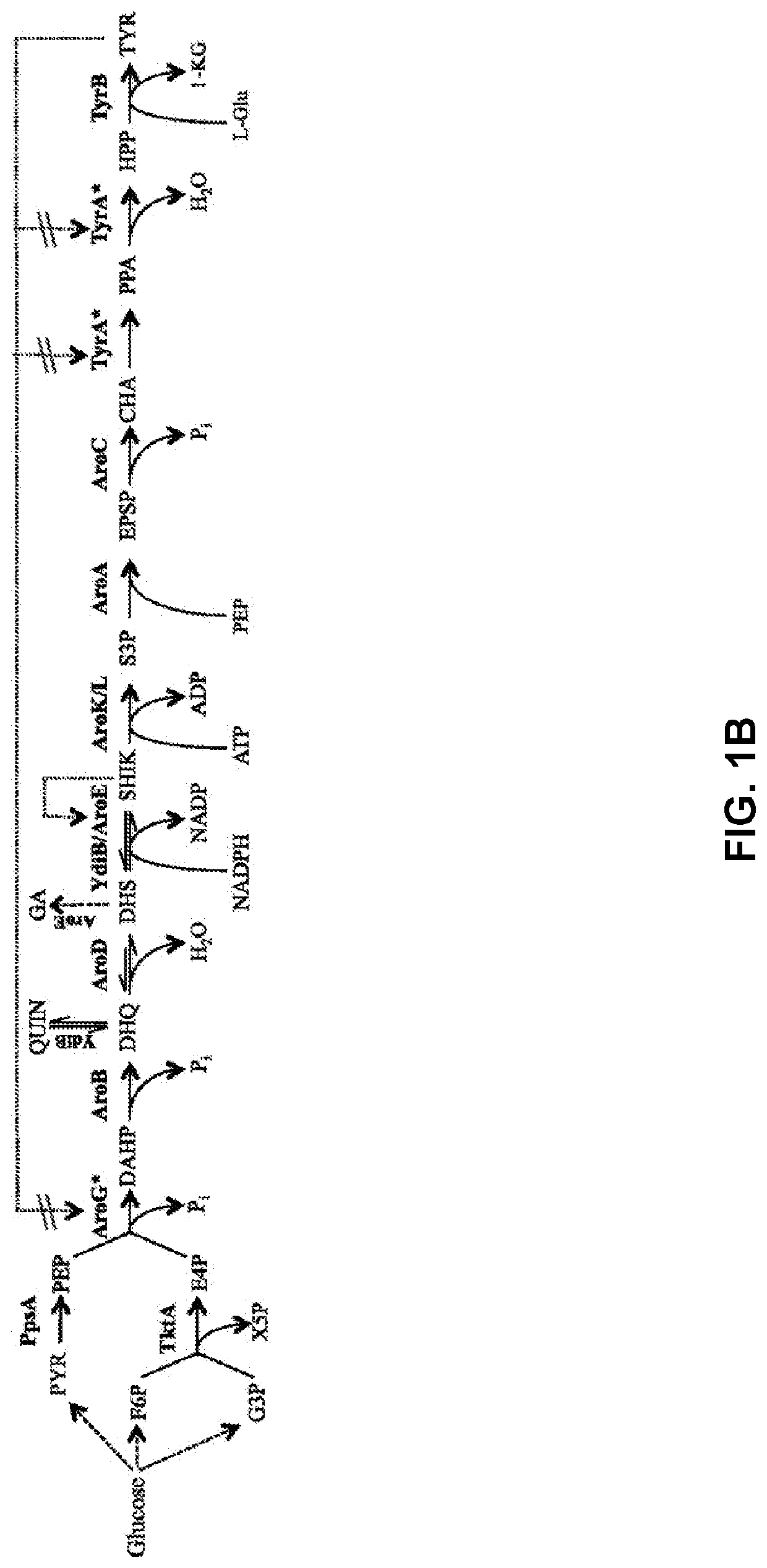
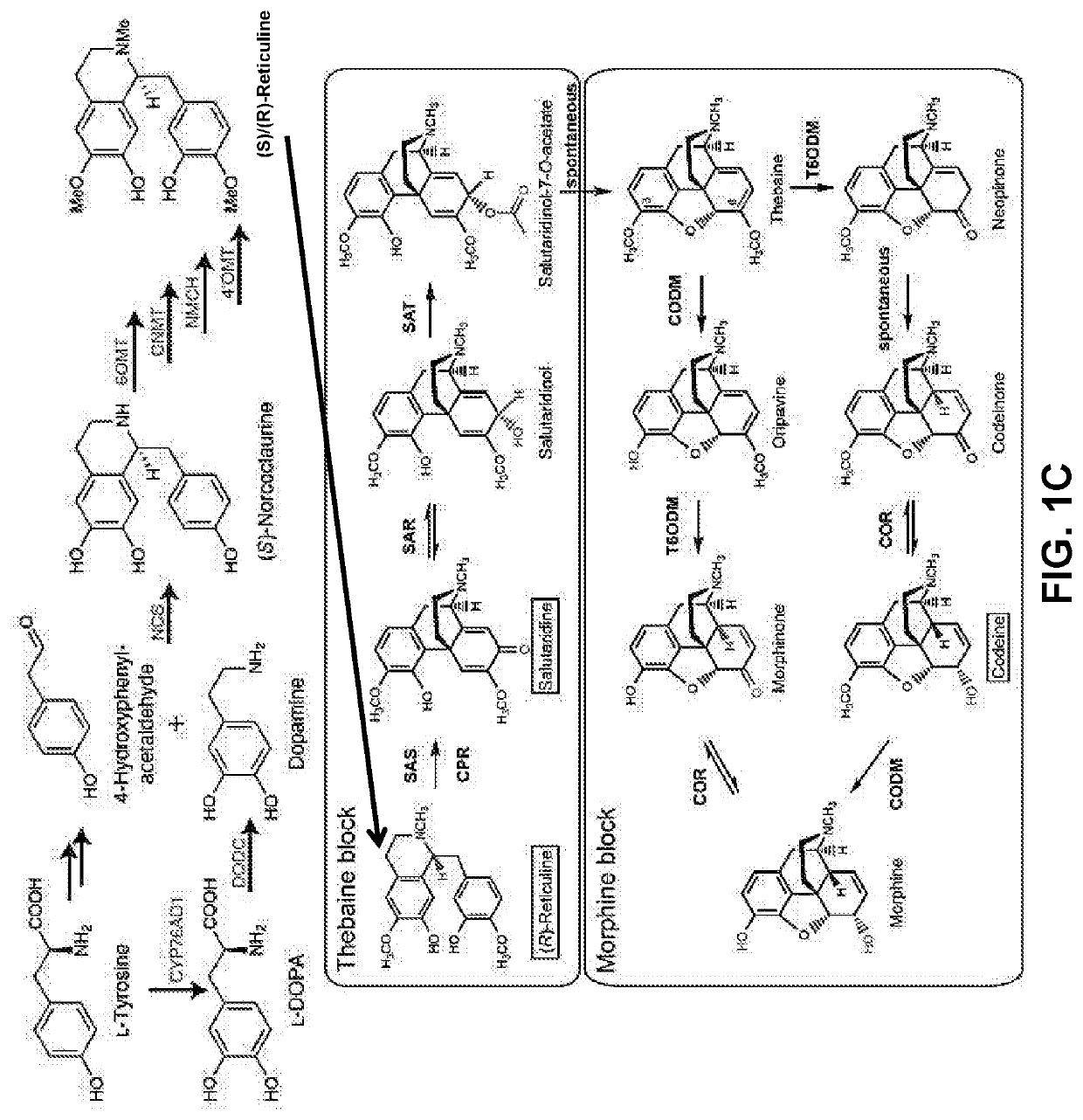
Compositions and methods for making benzylisoquinoline alkaloids, morphinan alkaloids, thebaine, and derivatives thereof
Disclosed herein are methods that may be used for the synthesis of benzylisoquinoline alkaloids (BIAs) such as alkaloid morphinan. The methods disclosed can be used to produce thebaine, oripavine, codeine, morphine, oxycodone, hydrocodone, oxymorphone, hydromorphone, naltrexone, naloxone, hydroxycodeinone, neopinone, and / or buprenorphine. Compositions and organisms useful for the synthesis of BIAs, including thebaine synthesis polypeptides, purine permeases, and polynucleotides encoding the same, are provided.
Owner:ANTHEIA INC
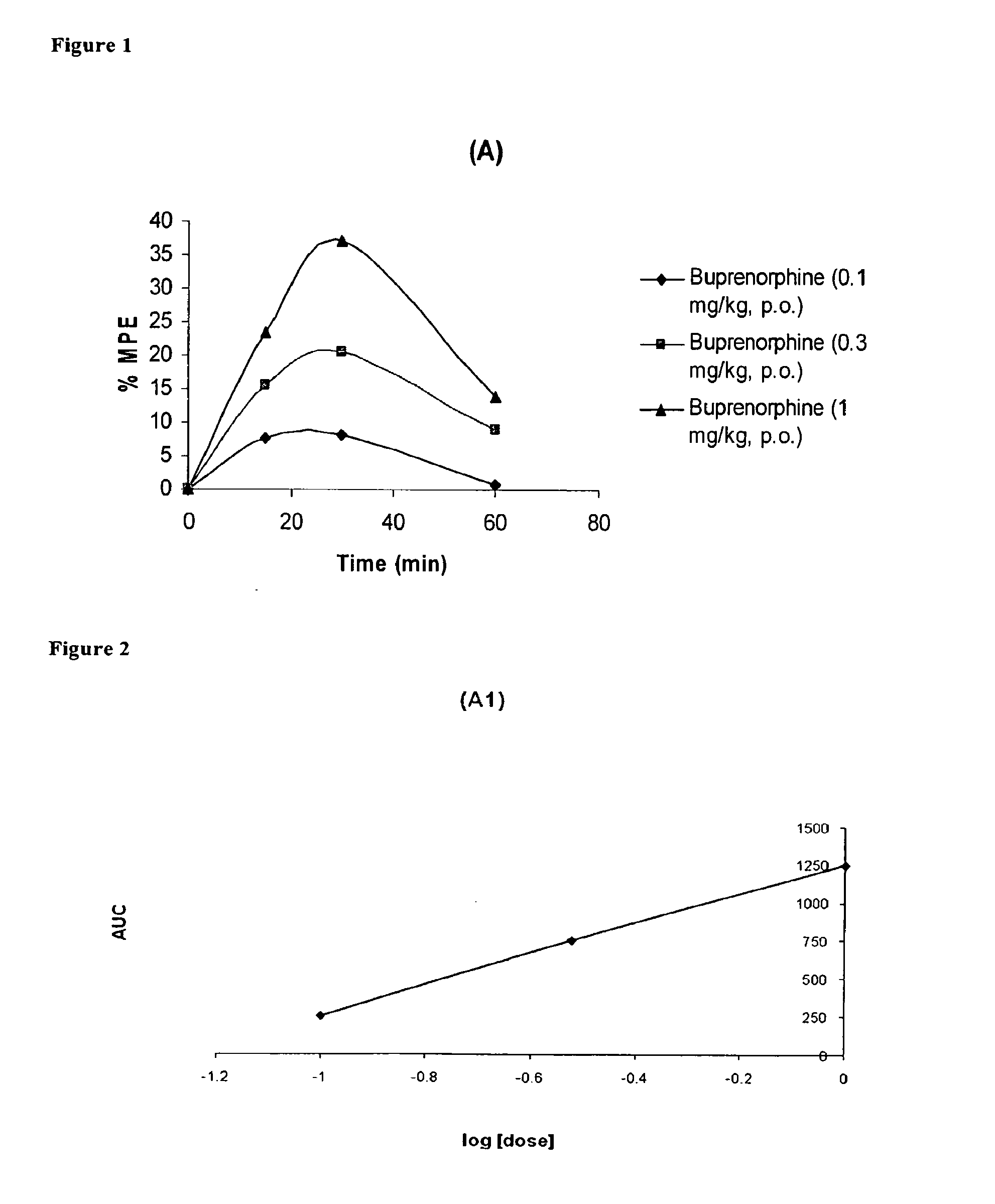
Preoperative treatment of post operative pain
InactiveUS20110002975A1Effective analgesiaEffective pain reliefBiocideNervous disorderPreoperative treatmentBuprenorphine
A method of treating postoperative pain in a patient undergoing a surgery is described. The method is based on preoperative administration of a buprenorphine-containing transdermal dosage form. The dosage form can be administered to the patient, for example, 1-4 days prior to surgery. Alternative embodiments of the invention include subsequent transdermal dosage forms to treat the postoperative pain.
Owner:PURDUE PHARMA LP
Method of manufacturing buprenorphine and analogues thereof from oripavine
ActiveUS20170319574A1Easy to produceIncrease productionOrganic active ingredientsNervous disorderImproved methodBuprenorphine
The invention relates to an improved method of preparing buprenorphine, a salt thereof, analogues of buprenorphine and their salts. In particular, the invention relates to a method of preparing buprenorphine and related products and salts in economic and ecologic ways having increased yields.
Owner:SIEGFRIED AG
Transdermal delivery system comprising buprenorphine
ActiveUS20160120823A1Small area of releaseImprove adhesionBiocideNervous disorderPropanoic acidAdhesive
The invention relates to transdermal therapeutic system for the transdermal administration of buprenorphine, comprising a buprenorphine-containing self-adhesive layer structure comprisingA) a buprenorphine-impermeable backing layer, andB) a buprenorphine-containing pressure-sensitive adhesive layer on said buprenorphine-impermeable backing layer, the adhesive layer comprisinga) at least one polymer-based pressure-sensitive adhesive,b) an analgesically effective amount of buprenorphine base or a pharmaceutically acceptable salt thereof, andc) a carboxylic acid selected from the group consisting of oleic acid, linoleic acid and linolenic acid, levulinic acid and mixtures thereof, in an amount sufficient so that said analgesically effective amount of buprenorphine is solubilized therein to form a mixture, and the carboxylic acid buprenorphine mixture forms dispersed deposits in the said pressure-sensitive adhesive,wherein said buprenorphine-containing pressure-sensitive adhesive layer is the skin contact layer.
Owner:LTS LOHMANN THERAPIE-SYST AG
Transdermal Delivery System
Described is a transdermal device comprising a backing layer; a single layer adhesive matrix comprising buprenorphine or a salt thereof, a pressure sensitive adhesive including a silicone-type adhesive blended with an acrylate-type adhesive, a solubilizer, a permeation enhancer, and a crystallization inhibitor; and a release layer. Also described is a method of relieving pain and a method of preparing a transdermal delivery system.
Owner:AMNEAL PHARMA
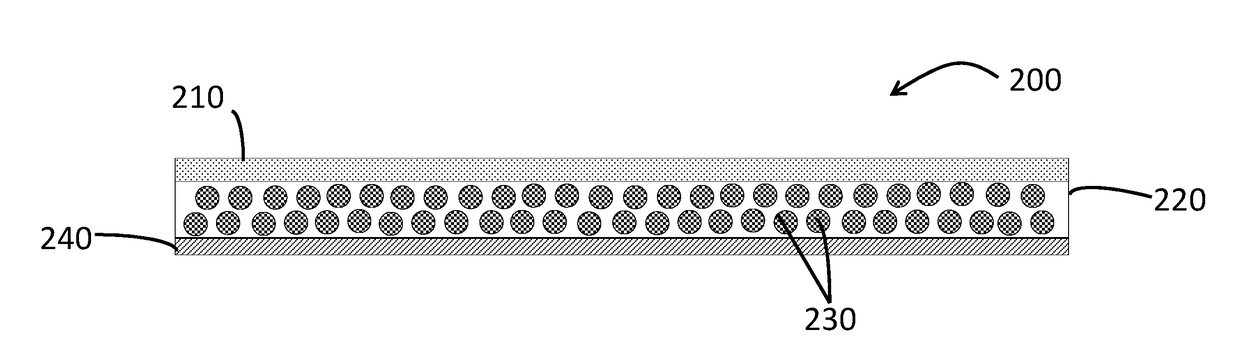
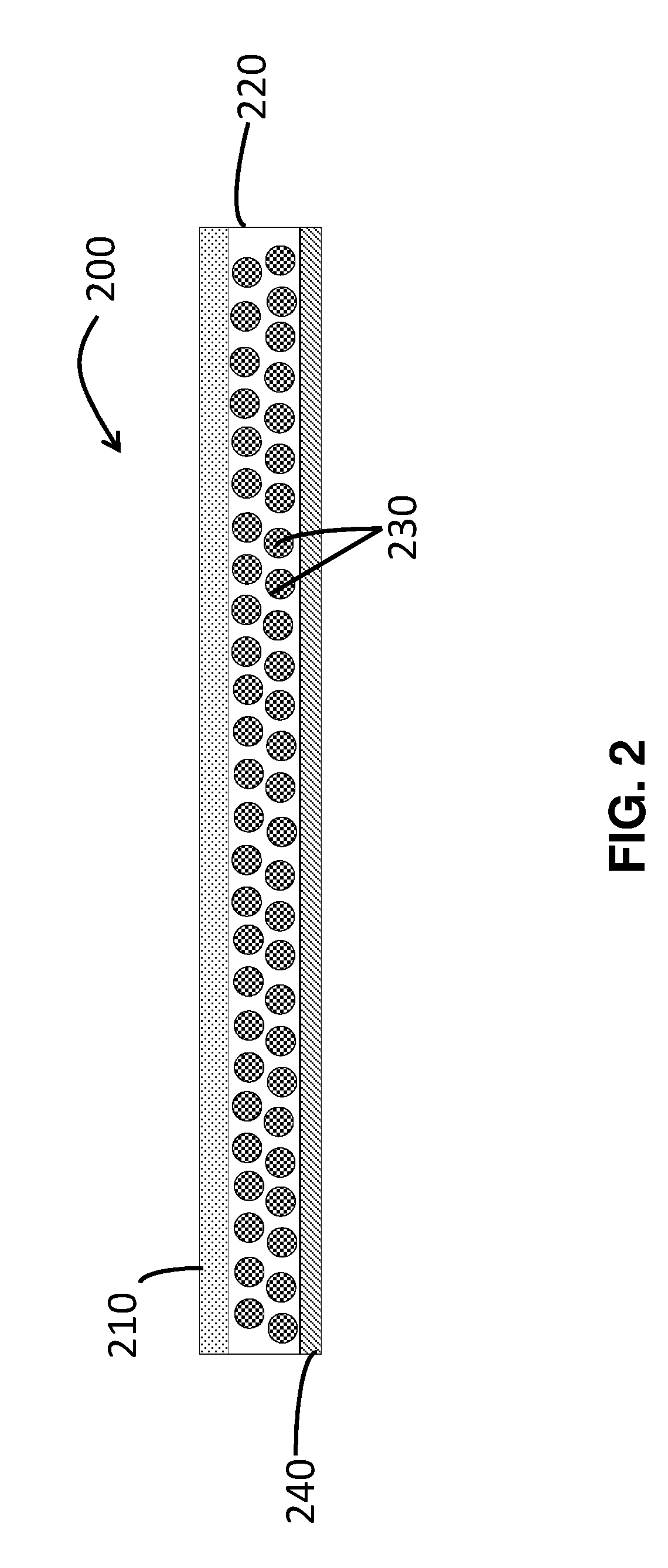
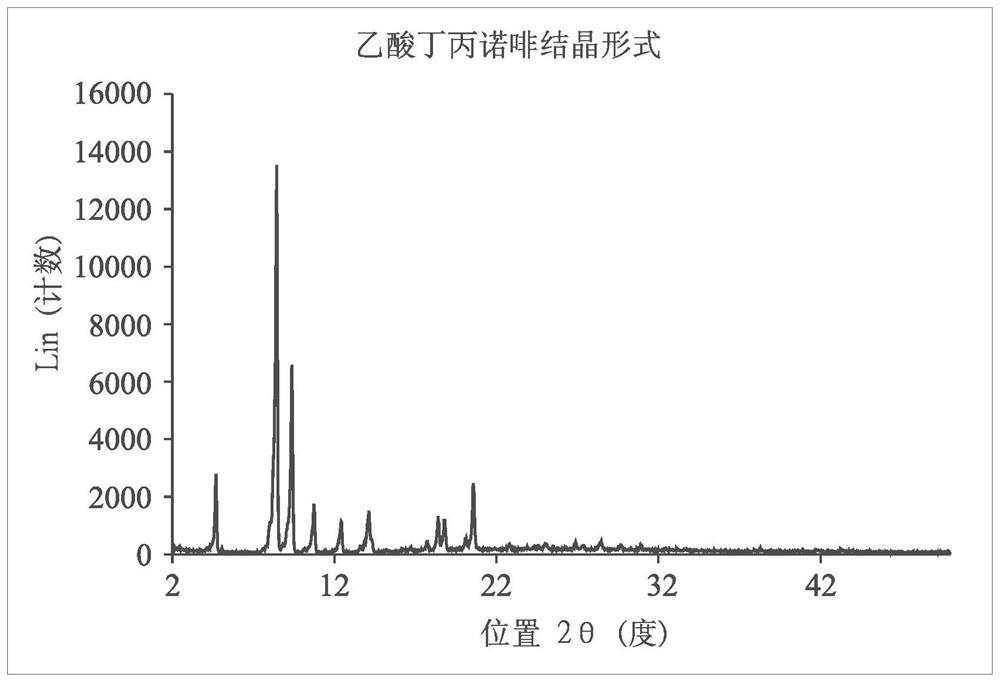
Long-acting injectable formulations and crystalline forms of buprenorphine derivatives
Disclosed are crystalline forms of 3-acyl-buprenorphine derivatives and sustained release injectable pharmaceutical compositions for treatment of opioid dependence, pain or depression, including an aqueous suspension of crystalline 3-acyl-buprenoprhine, or a pharmaceutically acceptable salt thereof, wherein the composition does not include an organic solvent, a polylactide polymer, a polyglycolidepolymer, or a copolymer of polylactide and polyglycolide. The disclosure also includes 3-acyl-buprenoprhine or a pharmaceutically acceptable salt thereof prepared in a controlled release matrix, including poly (lactide-co-glycolide), sucrose acetoisobutyrate, lecithin, diolein and a combination of two or more thereof.
Owner:ALAR PHARMA INC
Buprenorphine derivatives and uses thereof
InactiveCN101410402AReduce potential for abuseReduce hydrophilicityOrganic active ingredientsNervous disorderPerylene derivativesPsychiatry
Ester derivatives of the phenolic hydroxyl group of buprenorphine are described that can be used in the treatment of opiate dependency and / or moderate to severe pain. The esters have an enhanced bioavailability, an enhanced duration of action, and a reduced abuse potential.
Owner:RB PHARMA
Industrial process for the preparation of buprenorphine and its intermediates
There is provided an efficient industrial process for the preparation of 21-cyclopropyl-7α-(2-hydroxy-3,3-dimethyl-2-butyl)-6,14-endo-ethano-6,7,8,14-tetrahydro-oripavine, i.e. buprenorphine of Formula-I in high yield and purity, with enhanced safety and eco-friendly norms. The invention further relates to an improved process for preparation of intermediates thereof in high yield and purity.
Owner:RUSAN PHARMA
Method for treating neonatal opiod withdrawal syndrome
InactiveUS20200330455A1Stable and fastReduce usageOrganic active ingredientsNervous disorderMedicineDrug withdrawal syndrome
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com