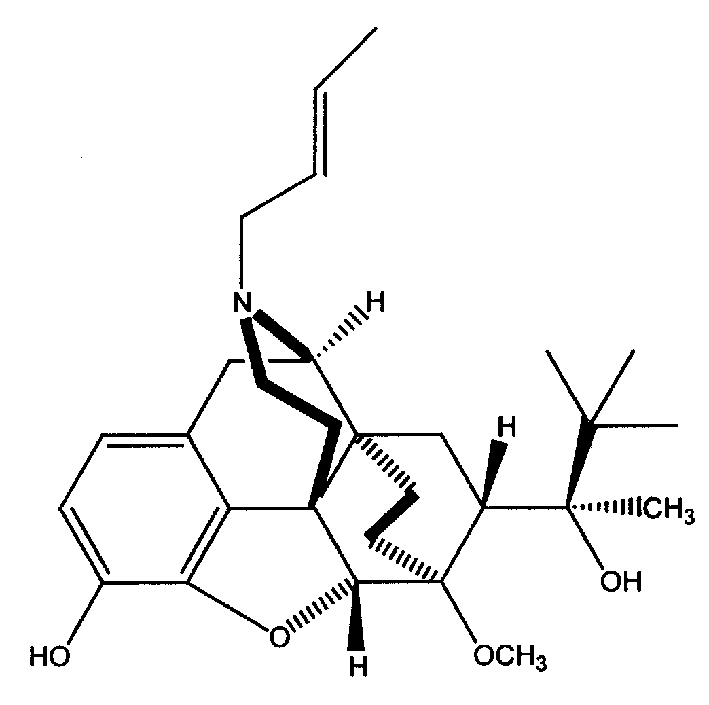
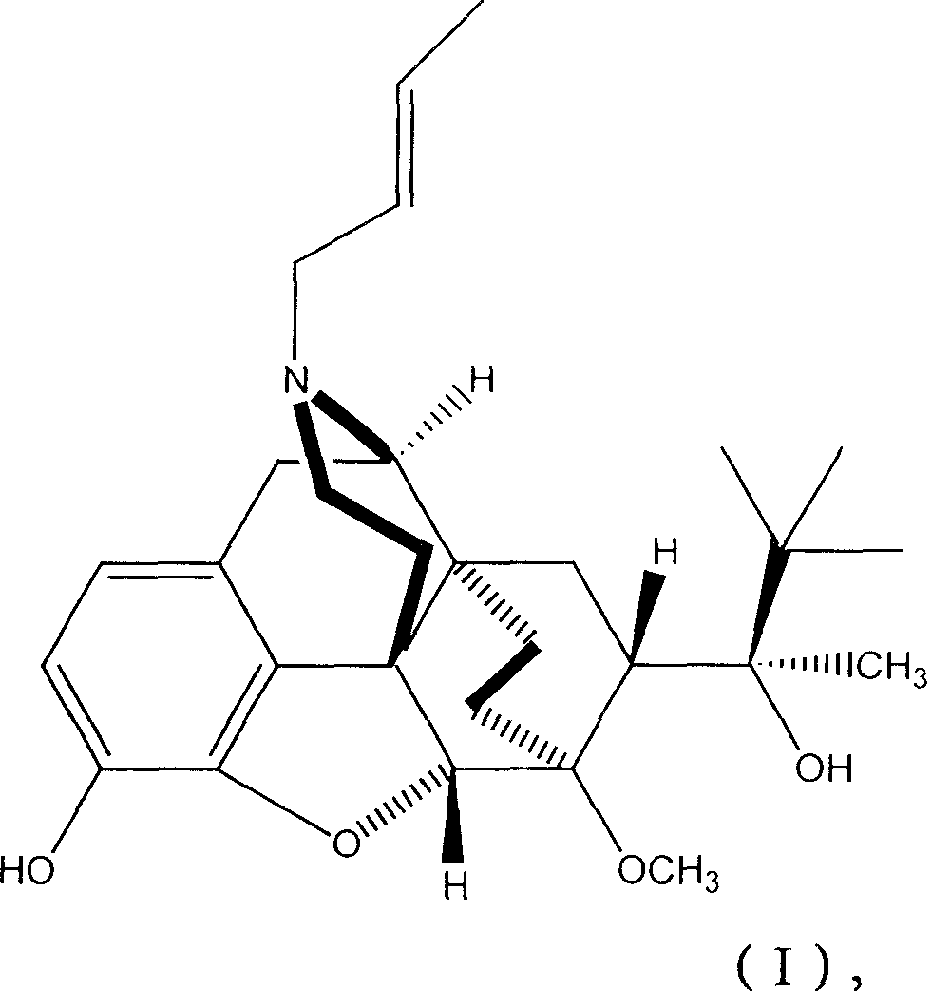
N-But-3-Enyl norbuprenophine and its use as analgesic
A compound and composition technology, applied in the field of N-but-3-enyl norbuprenorphine and its use as an analgesic, can solve the problems of unknown pharmacological effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 : Synthesis of N-but-3-enyl norbuprenorphine
[0082] To α-(1,1-dimethylethyl)-4,5-epoxy-18,19-dihydro-3-hydroxy-6-methoxy-α-methyl, (αS, 5α, 7α )-6,14-vinylidenemorphinan-7-methanol (norbuprenorphine) (0.2 g, 0.47 mmol; obtained from Tasmanian Alkaloids (Tasmania)) in 2 ml Bicarbonate (0.146 g, 1.74 mmol) and 4-bromo-1-butene (0.064 g, 0.47 mmol) were added to the suspension in DMF. The reaction mixture was stirred at 95°C for 16 hours. The cooled reaction mixture was concentrated to dryness under reduced pressure, and the residue was added to 10 ml of dichloromethane and washed with 3×10 ml of water. The resulting organic layer was dried (sodium sulfate) and concentrated under reduced pressure to yield a beige oil which crystallized on standing. This material was purified by flash chromatography on silica gel (2% methanol / dichloromethane) to afford 0.1 g of the desired product as a white crystalline solid. FAB high resolution mass spectrum m / z 468.3091...
Embodiment 2
[0083] Example 2 : Receptor Binding Studies
[0084] δ 2Binding test: The source of the receptor is a human recombinant cell line. The radioligands used in the study were [ 3 H]-naltrindole. Binding reaction in the presence of 5mM MgCl 2 50mM Tris-HCl (PH=7.4) at 25°C for 60 minutes. Binding reactions were terminated by rapid vacuum filtration on glass fiber filters. The radioactivity captured on the filter was measured and compared to the control value.
[0085] κ binding test: the source of the receptor is human recombinant cell line. The radioligand used in the study is [ 3 H]-Cypromorphine. Binding reaction in the presence of 10mM MgCl 2 and 1 mM EDTA in 50 mM Tris-HCl (pH=7.4) at 25° C. for 60 minutes. Binding reactions were terminated by rapid vacuum filtration on glass fiber filters. The radioactivity captured on the filter was measured and compared to the control value.
[0086] μ binding test: the source of the receptor is human recombinant cell line. T...
Embodiment 3
[0091] Example 3 : Binding and functional characterization of N-(3'-butenyl)-norbuprenorphine to opioid receptors; activity at mu, delta, kappa and ORL-1 receptors
[0092] The purpose of this in vitro pharmacology study was to evaluate the binding and functional properties of buprenorphine and its analog N-(3'-butenyl)-norbuprenorphine. Compared with buprenorphine, butenylbuprenorphine derivatives have the same high affinity for μ receptors and reduced affinity for kappa, delta and ORL-1 receptors. The potency ranking formed by the functional characteristics of N-(3-butenyl)-norbuprenorphine is μ>κ δ>ORL-1. This compound is a partial agonist at all four opioid receptors, with the highest potency at the delta receptor.
[0093] A. Binding to opioid μ receptors
[0094] In a final volume of 500gl binding buffer (10mM MgCl 2 , 1mM EDTA, 5% DMSO, 50mM Trizma base, pH7.4) using 0.2nM [ 3 H]-hydramorph (PerkinElmer, Boston, MA; 50.0 Ci / mmol) was radioligated with 20 μg of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com