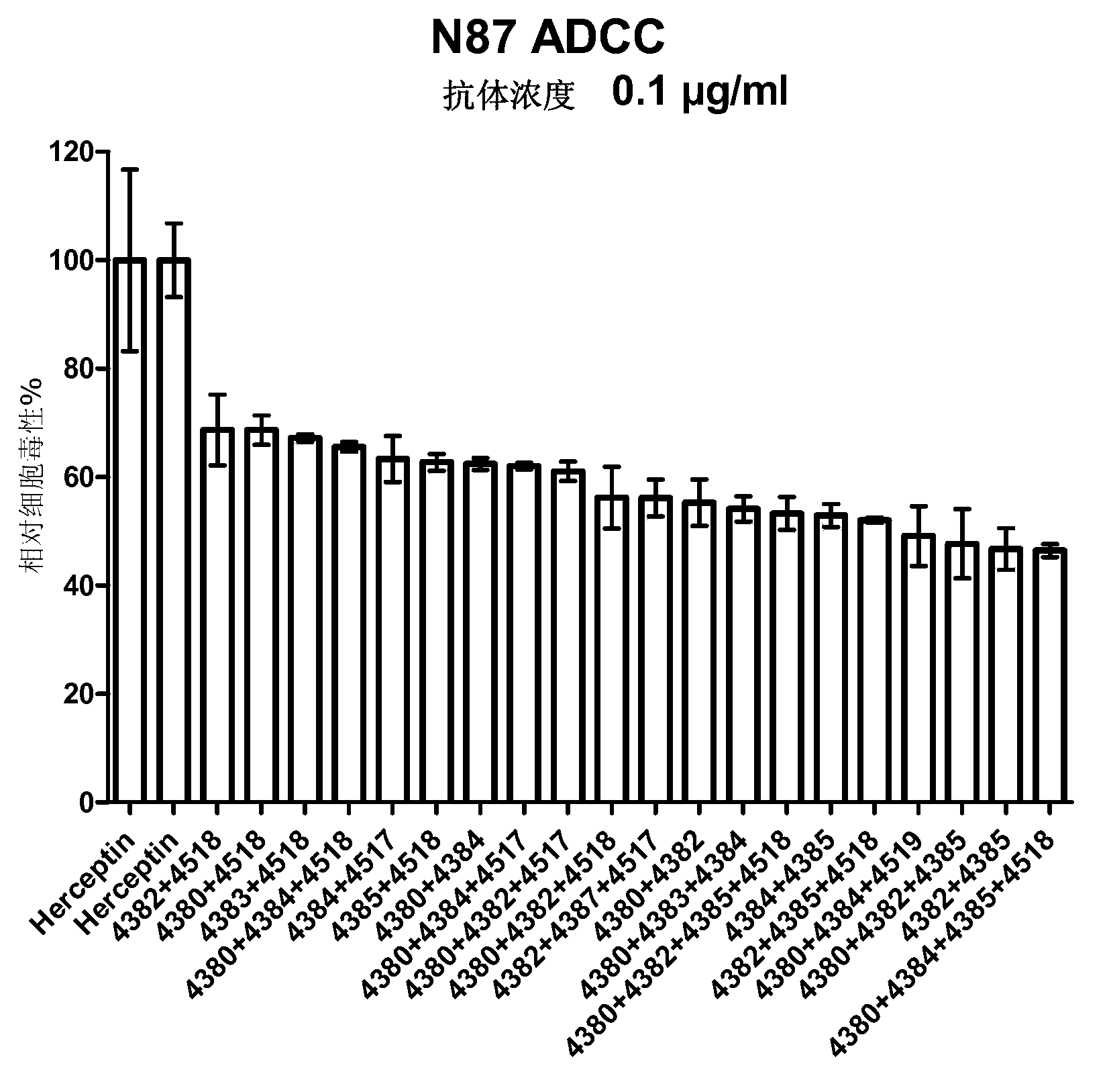
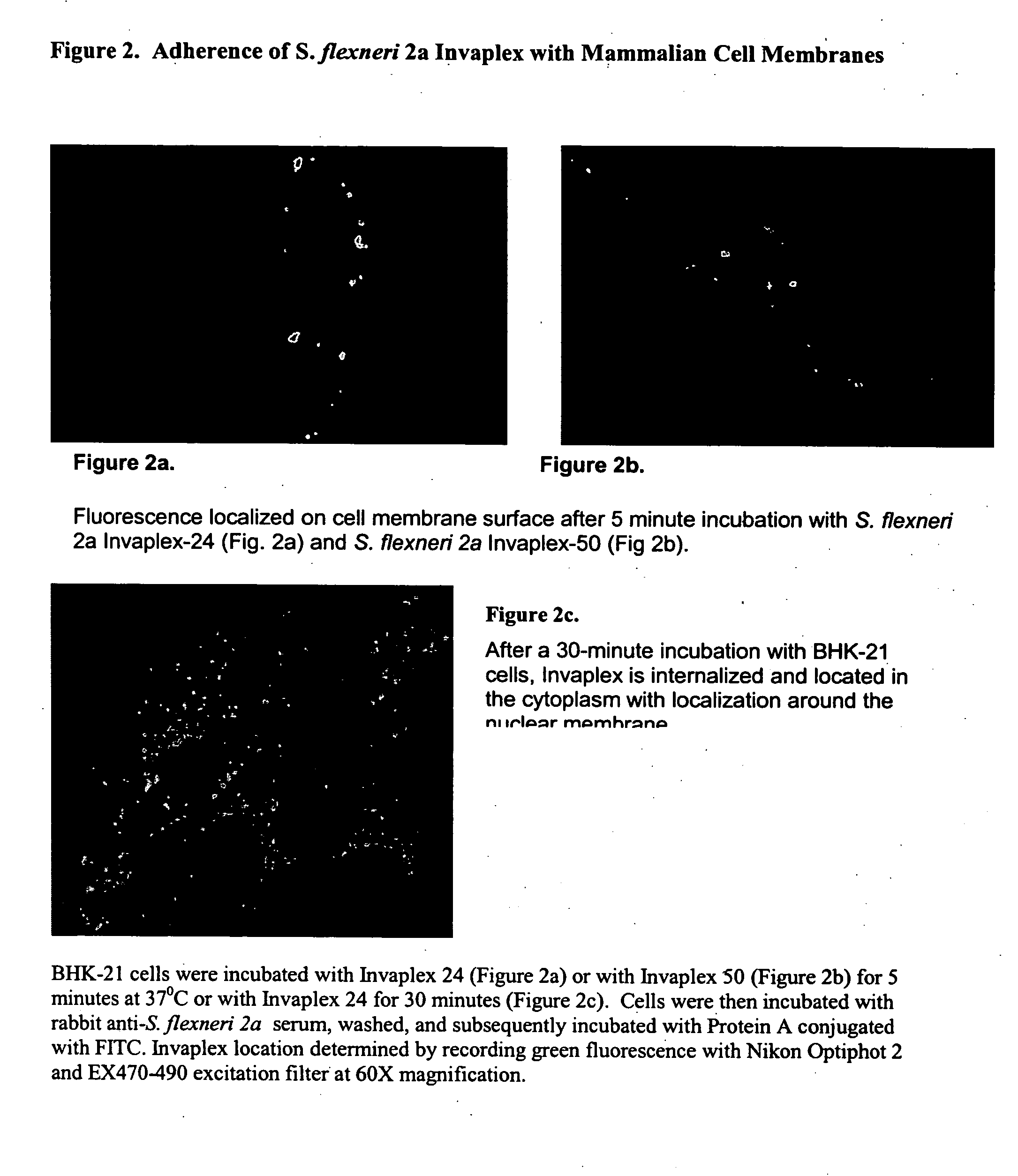
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Antibody receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Family of cell surface molecules which mediate the specific intracellular effects of antibody binding, usually by the Fc region; do not confuse with other antibody-binding moieties which are normally antigens that bind to the Fab region.
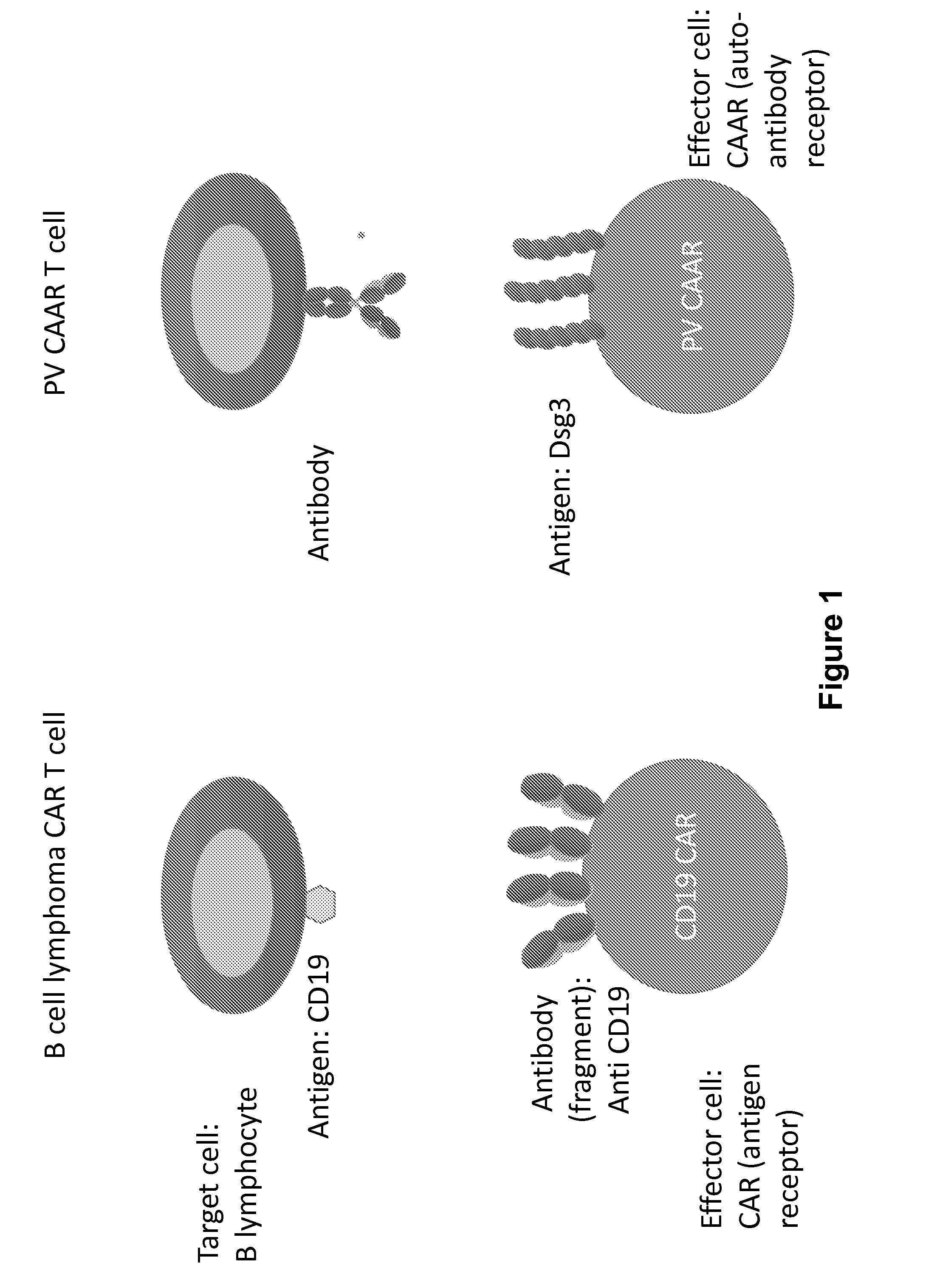
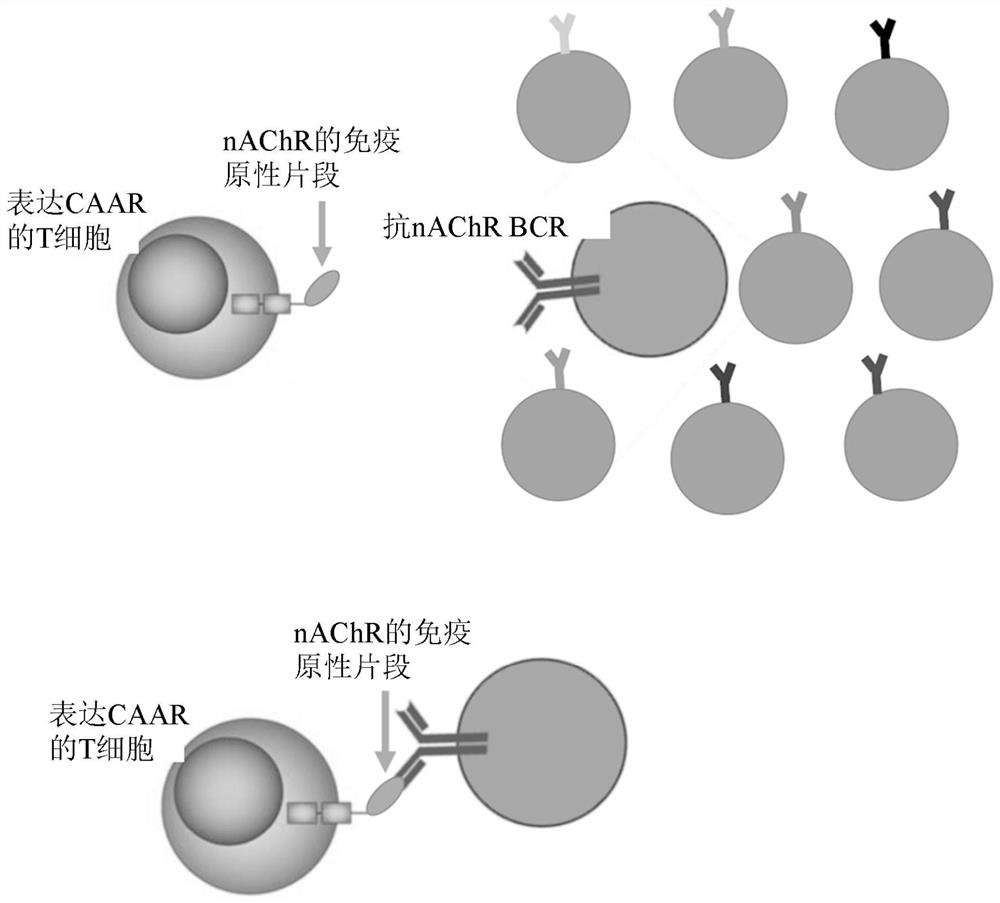
Compositions and methods of chimeric autoantibody receptor t cells
ActiveUS20170051035A1Low toxicityHigh affinityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyDesmoyokinAntibody receptor
The invention includes compositions comprising at least one chimeric autoantibody receptor (CAAR) specific for an autoantibody, vectors comprising the same, compositions comprising CAAR vectors packaged in viral particles, and recombinant T cells comprising the CAAR. The invention also includes methods of making a genetically modified T cell expressing a CAAR (CAART) wherein the expressed CAAR comprises a desmoglein extracellular domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
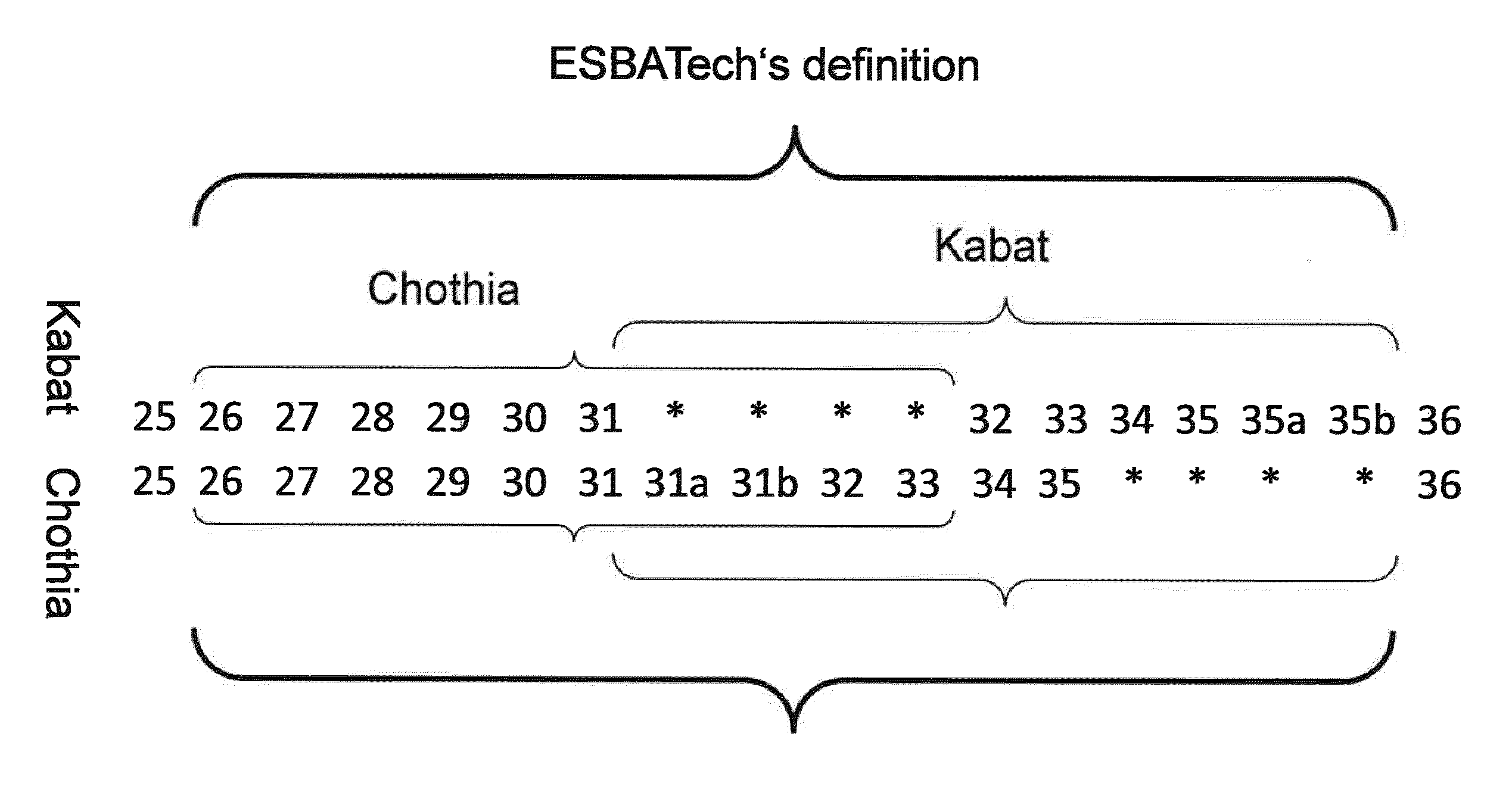
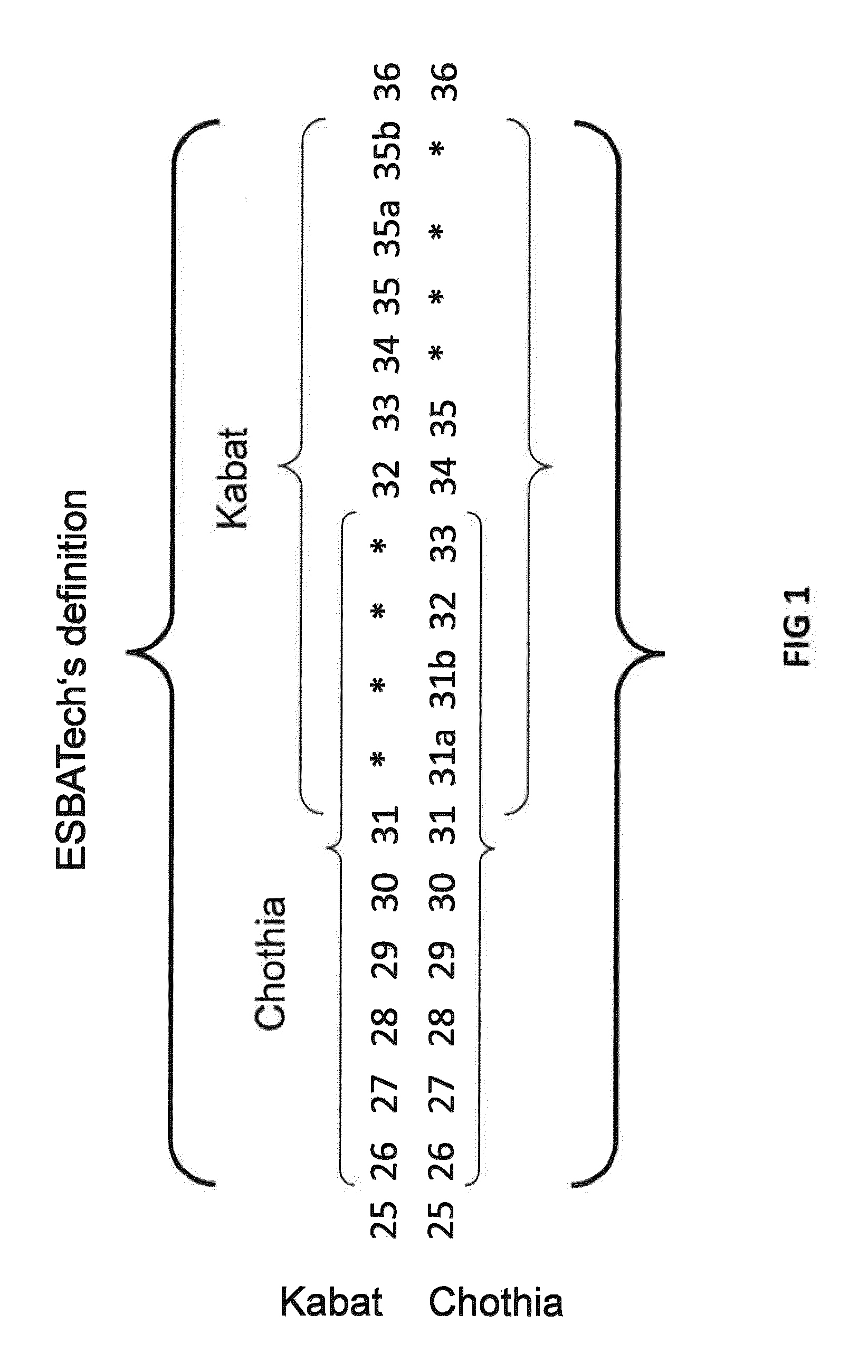
Humanization of rabbit antibodies using a universal antibody framework
ActiveUS8293235B2Reliably retain the spatial orientation of the rabbit antibodiesImprove bindingSugar derivativesAntibody mimetics/scaffoldsAntibody receptorRabbit Antibody
The present invention relates to an universal antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a universal antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:NOVARTIS AG
Acceptor framework for CDR grafting
ActiveUS8399625B1Improve bindingSuperior biophysical propertySugar derivativesImmunoglobulins against animals/humansCdr graftingReceptor for activated C kinase 1
The present invention relates to an antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a particularly well suited antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:CELL MEDICA INC
Antibodies against E-selectin
InactiveUS6204007B1Avoid consumptionAlteration of antibody side chain interactionPeptide/protein ingredientsAntipyreticNatural antibodyFc(alpha) receptor
This invention relates to whole antibodies of neutral isotype having specificity for E-selectin, process for their preparation (using vectors), pharmaceutical compositions containing them, and their use in therapy and diagnosis. Said antibodies are variants of natural antibodies altered in the Fc region, especially in the CH2 domain, so that the interactions with antibodies Fc receptors and complement are very low.
Owner:CELLTECH R & D LTD
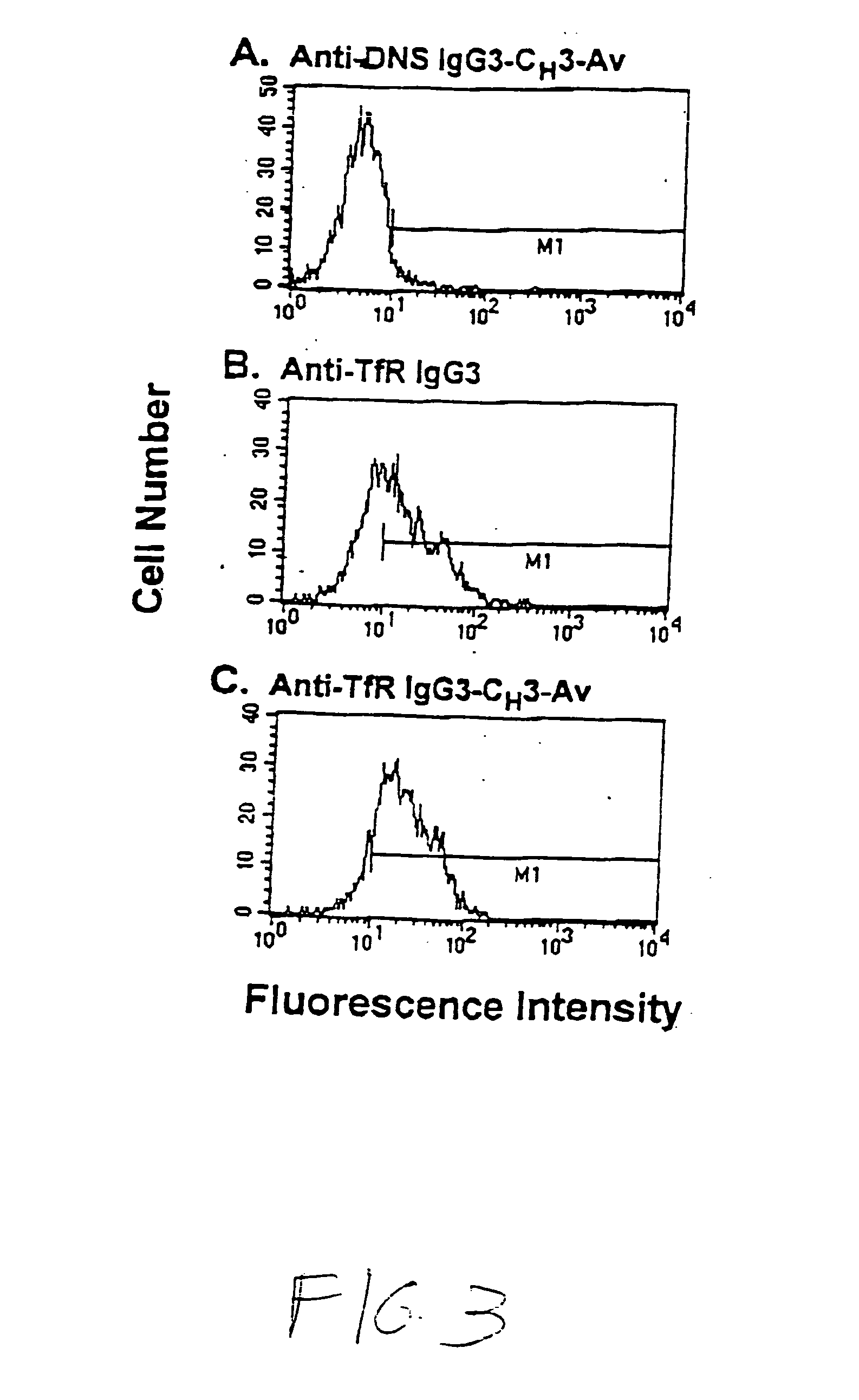
Anti-growth factor receptor avidin fusion proteins as universal vectors for drug delivery
A fusion protein for delivery of a wide variety of agents to a cell via antibody-receptor-mediated endocytosis comprises a first segment and a second segment: the first segment comprising a variable region of an antibody that recognizes an antigen on the surface of a cell that after binding to the variable region of the antibody undergoes antibody-receptor-mediated endocytosis, and, optionally, further comprises at least one domain of a constant region of an antibody; and the second segment comprising a protein domain selected from the group consisting of avidin, an avidin mutein, a chemically modified avidin derivative, streptavidin, a streptavidin mutein, and a chemically modified streptavidin derivative. Typically, the antigen is a protein. Typically, the protein antigen on the surface of the cell is a receptor such as a transferrin receptor-or an insulin receptor. The invention also includes an antibody construct incorporating the fusion protein that is either a heavy chain or a light chain together with a complementary light chain or heavy chain to form an intact antibody molecule. The invention further includes targeting methods and screening methods.
Owner:RGT UNIV OF CALIFORNIA
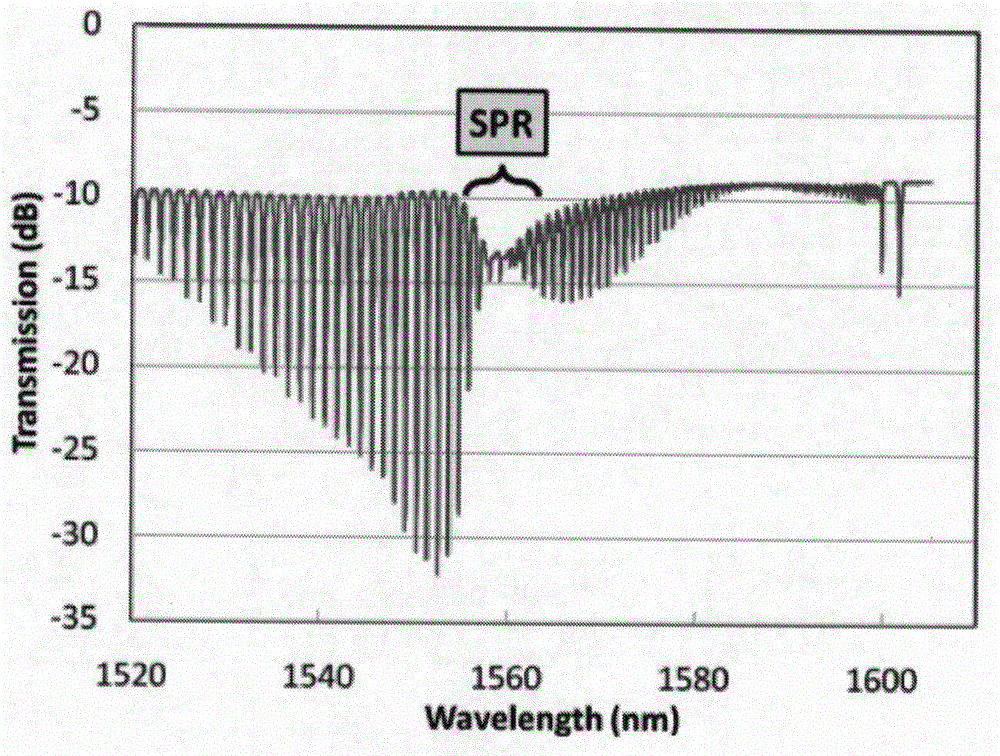
Tilted fiber Bragg grating (TFBG)-based surface plasmon resonance (SPR) biosensor
InactiveCN104458658ALarge adjustment rangeReduced transmitted light intensityMaterial analysis by optical meansGratingBeam splitter
The invention provides a tilted fiber Bragg grating (TFBG)-based surface plasmon resonance (SPR) biosensor which is characterized by comprising a broadband light source (1), a polarizing beam splitter prism (2), an optical isolator (3), a TFBG (4) coated with gold / biological adsorption layer, a reaction tank (5) and a spectrum analyzer (6), wherein the broadband light source (1) and the polarizing beam splitter prism (2) are connected with each other by virtue of an optical fiber and then are connected with the optical isolator (3); and the TFBG (4) coated with the gold / biological adsorption layer is arranged in the reaction tank (5) and is connected with the optical isolator (3) and the spectrum analyzer (6). The TFBG serves as a sensing part, and compared with a common optical fiber SPR sensor, the SPR biosensor disclosed by the invention has the advantages that the wavelength of the light source is simply changed, any mode of emitting light to a fiber core-cladding layer at different angles can be almost generated, resonance is easily realized, the resonance strength is greatly improved, and the information detection capacity is greatly enhanced; the sensitivity of the detector is greatly improved, and the detector can be used for detecting change of concentration of a solution with small biological molecules; and moreover, bovine serum albumin is adopted by the biological adsorption layer, and thus the interaction process between protein-protein and antibody-receptor biological proteins can be efficiently and accurately monitored.
Owner:CHINA JILIANG UNIV
Use of Anti-ccr5 antibodies in graft versus host disease
InactiveUS20170049884A1Prevent and treat and mitigate effectEffectively block HIV-1 cell entryAntipyreticAnalgesicsAntiendomysial antibodiesGraft versus host disease induction
This composition and method provides for a method for reducing GVHD in a human subject which comprises administering to the subject at a predefined interval effective GVHD-reducing doses of (a) a humanized antibody designated PRO 140, or of (b) an anti-CCR5 receptor monoclonal antibody. This invention also provides a method for inhibiting in a human subject the onset or progression of GVHD. This invention also provides a method for treating a subject with GVHD comprising administering to the subject (a) a monoclonal antibody which (i) binds to a CCR5 receptor on the surface of the subject's CD4.sup.+ cells, and (b) a non-antibody CCR5 receptor antagonist, in amounts effective to treat the subject.
Owner:CYTODYN
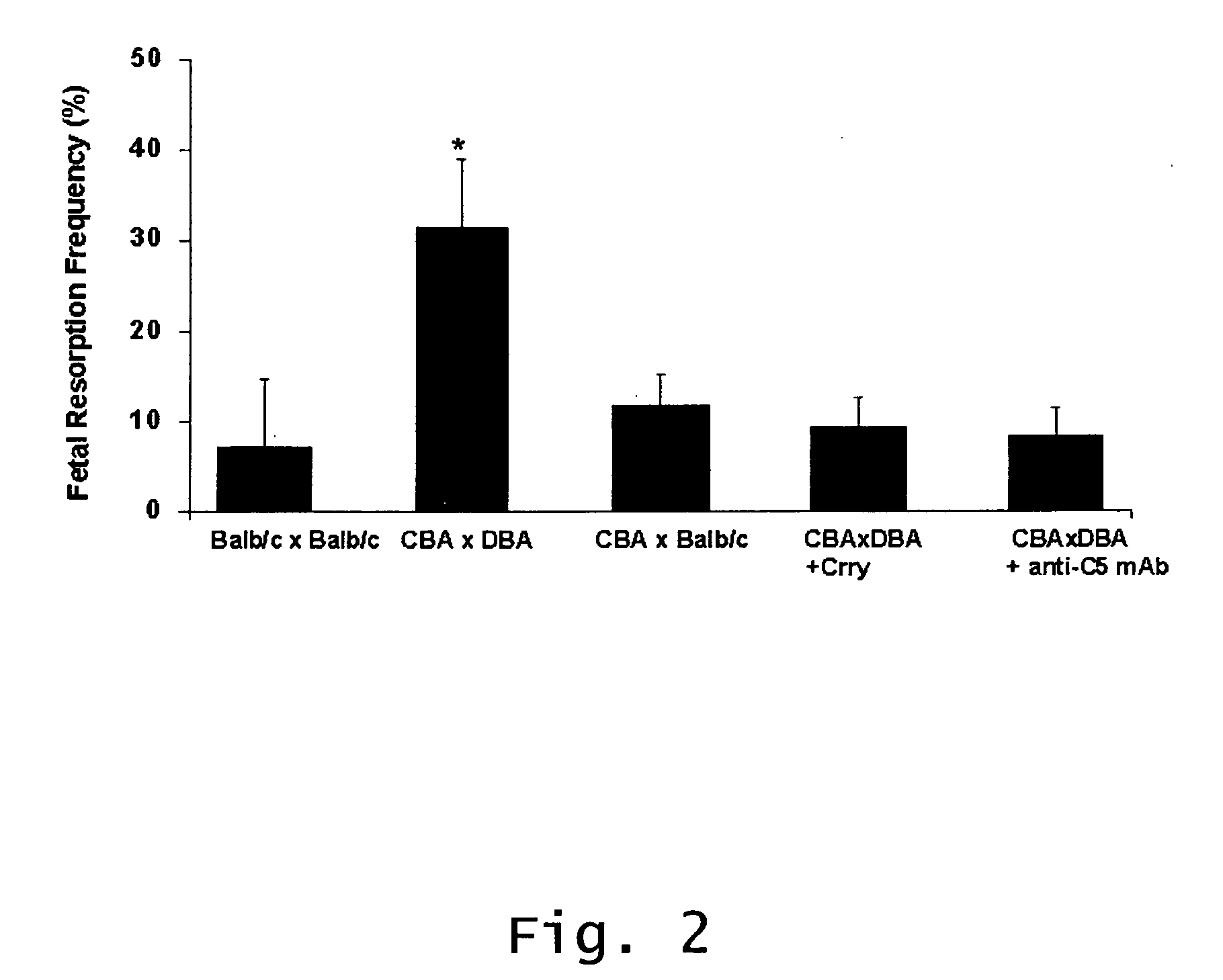
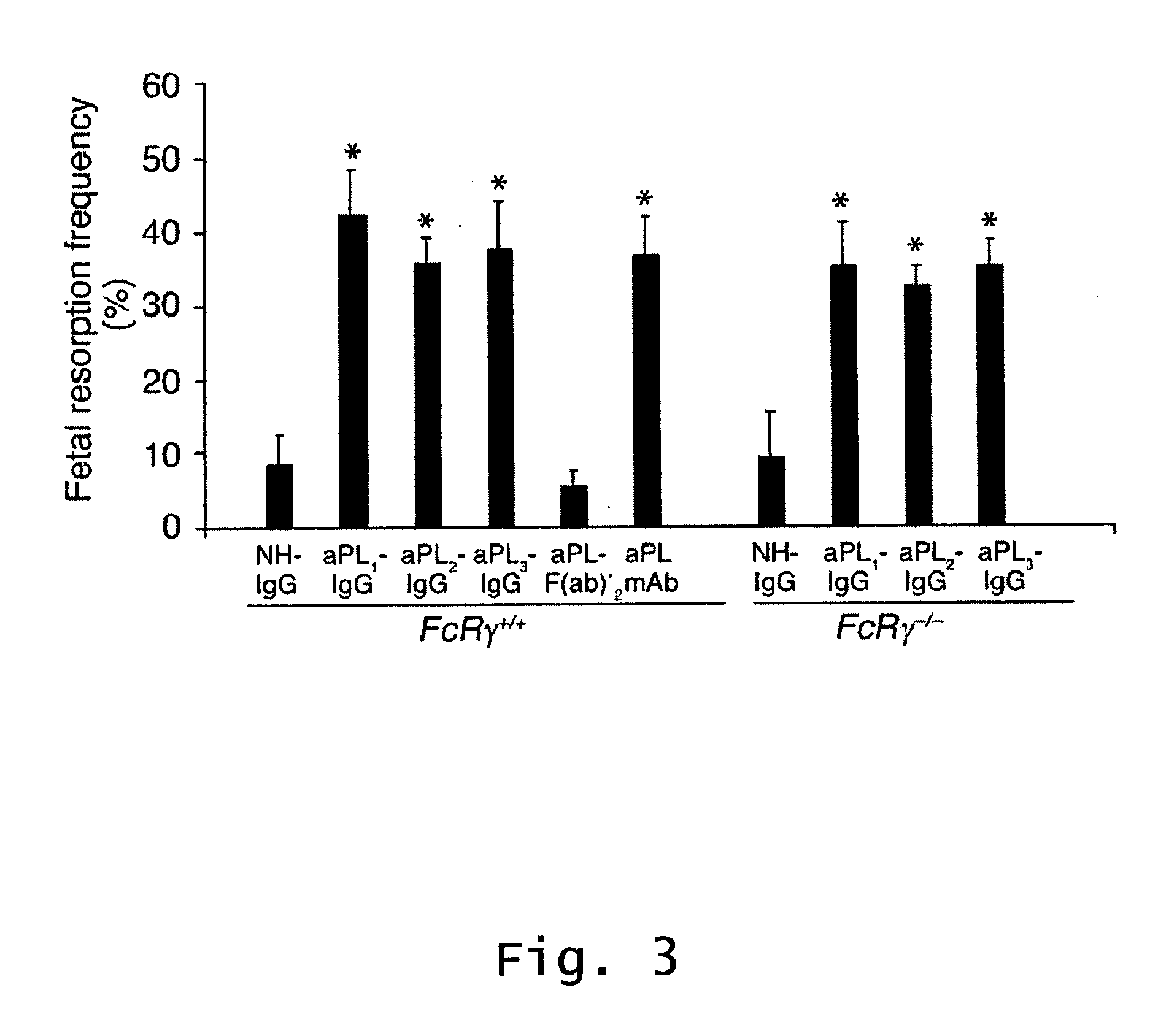
Method of treating recurrent miscarriages
InactiveUS20070123466A1Prevent and reduce riskCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsRecurrent miscarriageScreening method
Described are methods for treating, preventing, or reducing the risk of, miscarriages, especially recurrent miscarriages. The methods comprise administering to a female subject a therapeutic agent that modulates the activity or binding of components of the complement system, together with a pharmaceutically acceptable carrier. For example, the therapeutic agent can be a C3-convertase inhibitor, an antibody against C5, an antagonist of the C5a receptor, or an antibody against factor B or factor D. Screening methods for agents that can prevent or reduce the risk of miscarriages, especially recurrent miscarriages, are also described.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY +1
Method for improving sensitivity of competitive immunoassay
ActiveCN102721802AIncreased sensitivityEasy to operateBiological testingImmune profilingBiochemical engineering
The invention discloses a method for improving the sensitivity of competitive immunoassay. The method is mainly characterized in that a micromolecular substance to be detected is fully reacted with an antibody / receptor firstly and then reacted with a second molecule which participates in competition and is located on a solid phase in a short time, thus the sensitivity of the competitive immunoassay can be greatly improved. The method disclosed by the invention has the advantages of simplicity in operation, low cost, no need of expensive equipment and professional technicians, high sensitivity, and applicability to a plurality of immunoassay methods.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD
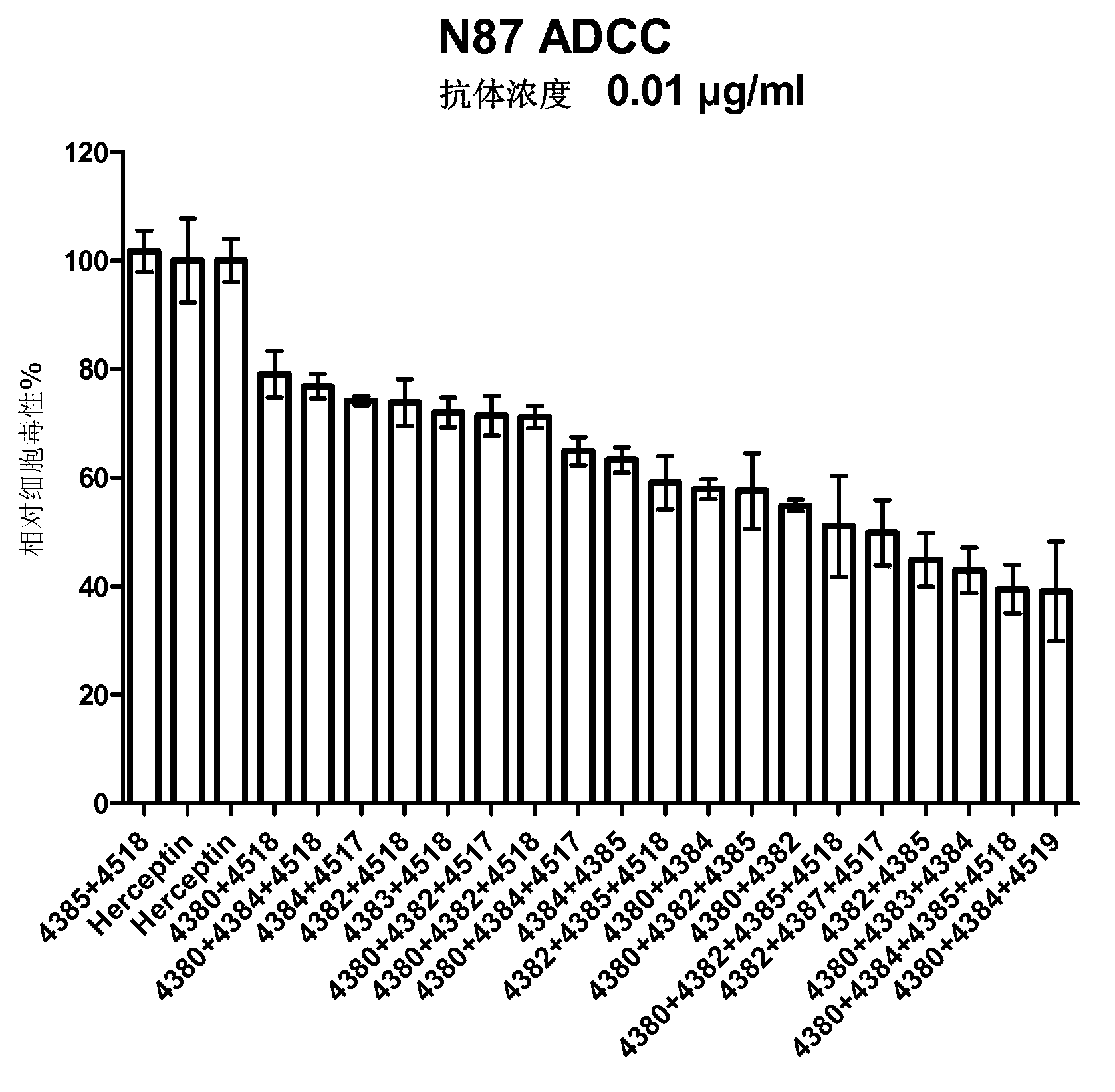
Anti-HER2 antibodies and compositions
InactiveCN102884084AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCross-linkTherapeutic antibody
The present invention relates to novel therapeutic antibodies directed against HER2 (ErbB2), as well as recombinant polyclonal anti-HER2 antibody compositions, and use of the antibodies and antibody composition for treatment of cancers. The antibody composition comprises at least three recombinant antibodies that bind distinct epitopes of HER2. Two of the antibodies bind to HER2 on the surface of a cell such that they generate a cross-linked antibody-receptor lattice on the cell surface and thereby result in HER2 receptor internalization. The third antibody in the composition binds HER2 such that it blocks heterodimerization between HER2 and HER3.
Owner:SYMPHOGEN AS
Regulation of dendritic cell functions by the DCAL-2 receptor
InactiveUS20060171929A1Enhance immune responseImprove responseBiocidePeptide/protein ingredientsDendritic cellCancer antigen
This invention provides antibodies that specifically bind to DCAL-2 and other DCAL-2 reagents that modulate dendritic cell function. Modulators of the receptor, including modulators that alter DCAL-2 associated signals to and from DCs, can be used to alter dendritic cell function and to enhance or inhibit immune responses to cancer antigens, autoantigens, or pathogens.
Owner:UNIV OF WASHINGTON
A homogeneous immunoassay method and application thereof
ActiveCN109725153ASolve the resultSolving the problem of antigen positivityMaterial analysisHigh concentrationExcited state
The invention relates to a homogeneous immunoassay method, in which at least two detection regions are used, in one detection region, it is determined whether a first composite formed by a donor-antibody to be detected-receptor exists or not, in the other detection region, it is determined whether a second composite formed by a donor-antigen to be detected-receptor exists or not. Wherein the donorcan generate singlet oxygen in an excited state, and the receptor can react with the singlet oxygen to generate a detectable signal. The method solves the problems of HOOK effect of a high-concentration sample and low detection sensitivity of a low-concentration sample in antigen-antibody one-step detection. Meanwhile, the method also solves the problem that whether a positive result is antibodypositive or antigen positive cannot be distinguished during antigen-antibody combined detection. The method provided by the invention is especially suitable for combined detection of HIV antigen and antibody.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Use of Shigella invaplex to transport functional proteins and transcriptionally active nucleic acids across mammalian cell membranes in vitro and in vivo
InactiveUS20050215474A1Enhance immune responseEasy to adaptSenses disorderPeptide/protein ingredientsMammalNucleic acid
The in vivo and in vitro use of Invaplex to transport materials, including functional proteins and biologically active nucleic acids, across eukaryotic cell membranes. The eukaryotic cells include a variety of cell types, e.g. insect, reptile, fish, mammal and tumor cells. The suitable materials for transport include biochemicals such as reporter molecules, antibiotics, biopharmaceuticals and carbohydrates including polysaccharides, lipopolysaccharides, polynucleotides, such as DNA and RNA, and glycoproteins and proteins including antigens, enzymes, antibodies, receptors and hormones. In addition, Invaplex enhances the immune response to DNA vaccines and also can function by itself as a vaccine against shigellosis.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Device and method for detecting beta-agonists drug
InactiveCN102645532AAchieving Simultaneous DetectionReduce testing costsMaterial analysisMedicineAgonist
The invention relates to the field of biological detection and discloses a detection device and a detection method of a large class of beta-agonists drugs by applying an immune chromatography technology. According to the invention, an antibody / receptor which is reacted with a large class of the beta-agonists drugs is used for preparing an immune chromatography test strip to realize the simultaneous detection of a large class of the beta-agonists drugs. Compared with the prior art, the detection on a large class of the beta-agonists drugs for one time can be realized, the detection cost is low and the method is convenient and fast.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD +1
Methods for reducing viral load in HIV-1-infected patients
InactiveUS20080241135A1Reduce loadAvoid fusesPeptide/protein ingredientsMicrobiological testing/measurementReduced doseHIV receptor
This method provides a method for reducing HIV-1 viral load in an HIV-1-infected human subject which comprises administering to the subject at a predefined interval effective HIV-1 viral load-reducing doses of (a) a humanized antibody designated PRO 140, or of (b) an anti-CCR5 receptor monoclonal antibody. This invention also provides a method for inhibiting in a human subject the onset or progression of an HIV-1-associated disorder, the inhibition of which is effected by inhibiting fusion of HIV-1 to CCR5+CD4+ target cells in the subject. This invention also provides a method for treating a subject infected with HIV-1 comprising administering to the subject (a) a monoclonal antibody which (i) binds to a CCR5 receptor on the surface of the subject's CD4+ cells and (ii) inhibits fusion of HIV-1 to the subject's CCR5+CD4+ cells, and (b) a non-antibody CCR5 receptor antagonist, in amounts effective to treat the subject.
Owner:PROGENICS PHARMA INC
Acceptor framework for CDR grafting
ActiveUS8399624B1Improve bindingSuperior biophysical propertySugar derivativesImmunoglobulins against animals/humansCdr graftingReceptor for activated C kinase 1
The present invention relates to an antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a particularly well suited antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:CELL MEDICA INC +1
Humanization of rabbit antibodies using a universal antibody framework
ActiveUS20110117091A1Improve bindingSuperior biophysical propertySugar derivativesAntibody mimetics/scaffoldsAntibody receptorRabbit Antibody
The present invention relates to an universal antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a universal antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:NOVARTIS AG
Acceptor framework for cdr grafting
ActiveUS20130165640A1Improve bindingSuperior biophysical propertyAnimal cellsSugar derivativesAntibody receptorCdr grafting
The present invention relates to an antibody acceptor framework and to methods for grafting non-human antibodies, e.g., rabbit antibodies, using a particularly well suited antibody acceptor framework. Antibodies generated by the methods of the invention are useful in a variety of diagnostic and therapeutic applications.
Owner:CELL MEDICA INC
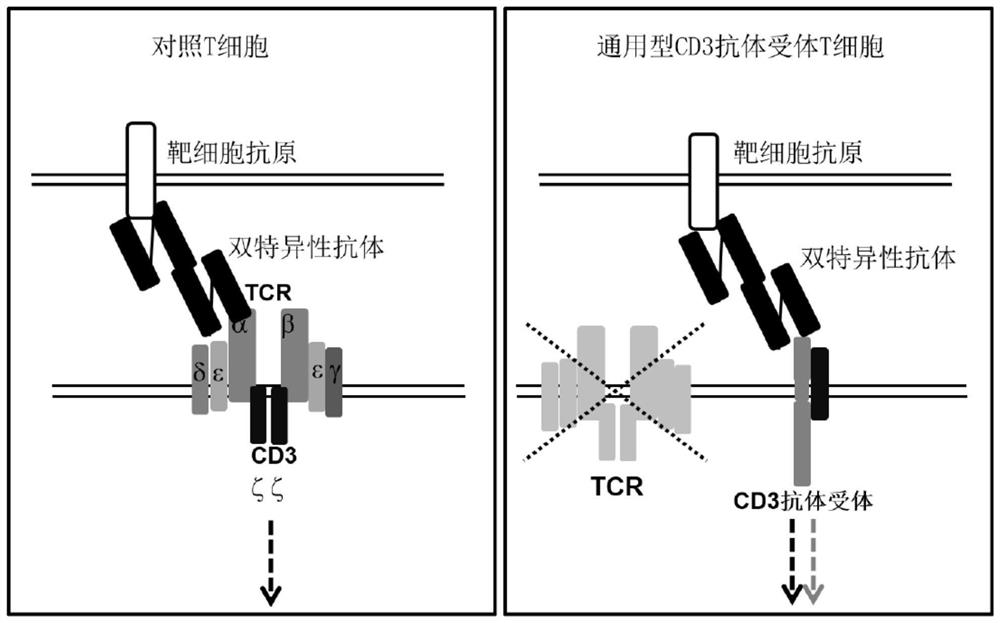
Immune cell for expressing CD3 antibody receptor complex and application thereof
PendingCN112204135APolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntiendomysial antibodiesBispecific antibody
An engineered immune cell that does not express a T cell receptor (TCR) and comprises a CD3 antibody receptor complex. The invention also relates to a pharmaceutical composition containing the modified immune cell and a bispecific antibody, and an application of the pharmaceutical composition in preparation of medicines.
Owner:CURE GENETICS CO LTD
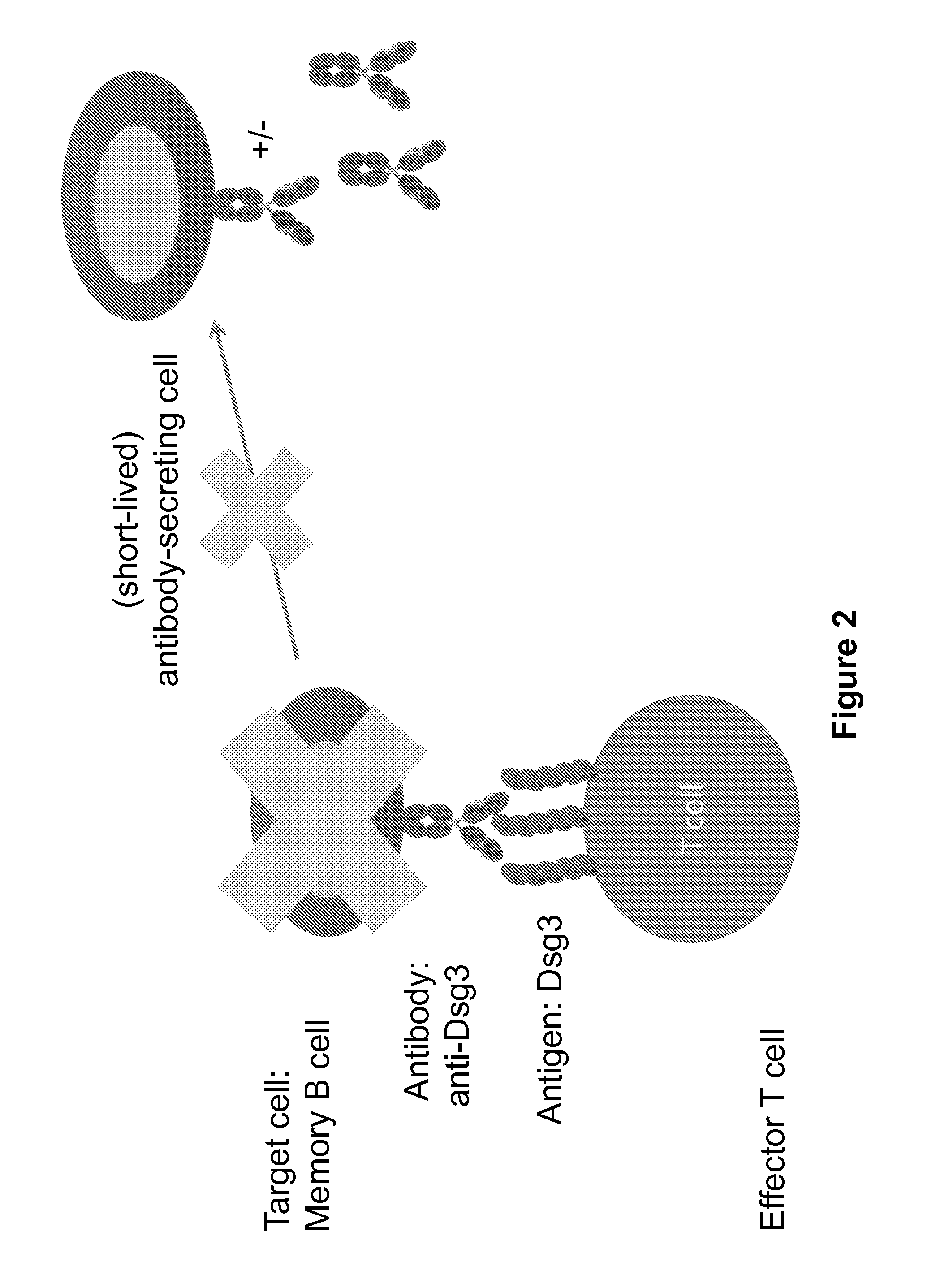
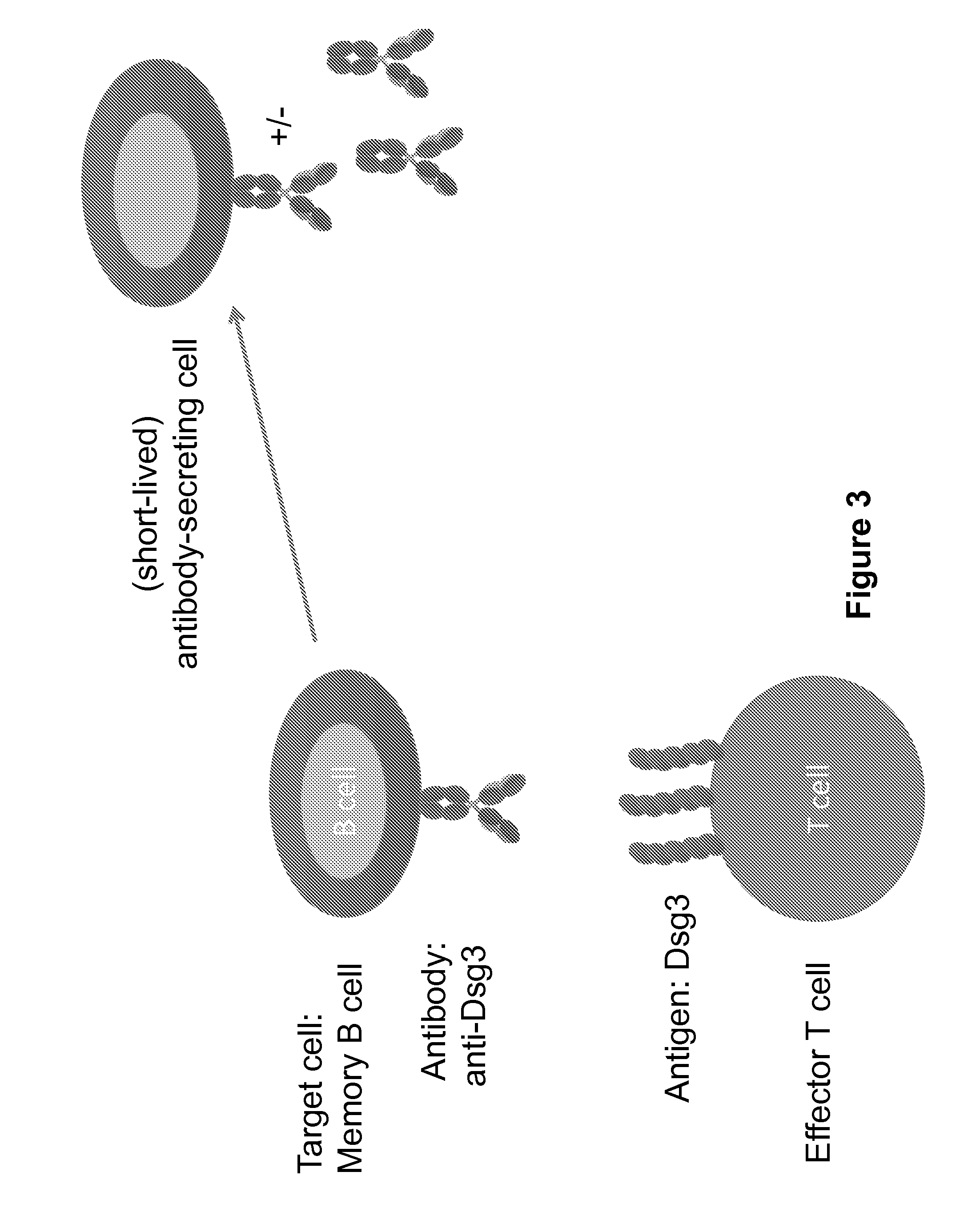
Chimeric autoantibody receptor (CAAR) that binds autoantibodies targeting the central nervous system in neurological autoimmune disease
PendingCN114008204APrevent or significantly reduce the risk of clinical relapseNo or reduced immunosuppressionOrganic active ingredientsNervous disorderAutoimmune conditionNmdar encephalitis
The invention relates to a chimeric autoantibody receptor (CAAR) that enables targeting of an immune cell to autoantibody producing B cells. The CAAR comprises an autoantigen or fragment thereof that is bound by autoantibodies associated with a neurological autoimmune disease primarily targeting the central nervous system. The invention relates to a nucleic acid molecule encoding a chimeric autoantibody receptor (CAAR), the nucleic acid molecule comprising a sequence encoding an autoantigen or fragment thereof that is bound by autoantibodies associated with a neurological autoimmune disease primarily targeting the central nervous system, a sequence encoding a transmembrane domain, and a sequence encoding an intracellular signaling domain. In one embodiment, the autoantigen encoded by the nucleic acid sequence comprises or consists of an N- methyl-D-aspartate receptor (NMDAR), or one or more NMDAR fragments. The invention further relates to the chimeric autoantibody receptor (CAAR) protein of the invention, a vector comprising a nucleic acid molecule encoding a chimeric autoantibody receptor (CAAR) of the invention, a genetically modified immune cell comprising the nucleic acid molecule encoding the CAAR and the use of the immune cell in the treatment or prevention of a neurological autoimmune disease primarily targeting the central nervous system, such as an autoimmune encephalopathy or encephalomyelopathy, preferably anti-NMDAR encephalitis.
Owner:德国神经退行性疾病中心 +1
Compositions and methods of phospholipase a2 receptor chimeric autoantibody receptor t cells
PendingUS20210095001A1Induces killingLow toxicityAntibody mimetics/scaffoldsVertebrate antigen ingredientsAutoantibodyT cell
The invention includes compositions comprising at least one chimeric autoantibody receptor (CAAR) specific for an anti-phospholipase A2 receptor (PLA2R) autoantibody-based B cell receptor, polynucleotides encoding the CAAR, vectors comprising a polynucleotide encoding the CAAR, and recombinant T cells comprising the CAAR. The invention also includes methods of making a genetically modified cell, e.g., a genetically modified T cell, expressing a PLA2R-CAAR wherein the expressed CAAR comprises a PLA2R extracellular domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Use of Shigella invaplex to transport functional proteins and transcriptionally active nucleic acids across mammalian cell membranes in vitro and in vivo
InactiveUS7632659B2Enhance immune responseEasy to adaptSugar derivativesPeptide/protein ingredientsSHIGELLOSESAntiendomysial antibodies
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Selecting for developability of polypeptide drugs in eukaryotic cell display systems
PendingCN111448314AReduced risk of late failureImprove developability featuresDNA preparationAntiendomysial antibodiesControl cell
Use of the surface presentation level of binders (e.g., antibodies, receptors) on cultured higher eukaryotic cells in vitro as a predictive indicator of developability characteristics, e.g., solubility, of the binders. Display libraries of higher eukaryotic cells, e.g., mammalian cells, adapted for use in screening surface-displayed binders for developability and affinity of target binding. High-throughput screening of display libraries with in-built selection for developability including binder solubility, capability to be formulated at high concentrations, low propensity for non-specific binding, and half-life. Enrichment of populations of binders for developability characteristics and / or other qualities such as target binding and affinity, by controlling cell surface presentation of binders from an inducible promoter operably linked to binder-encoding DNA.
Owner:AIENTAS LTD
Compositions and methods of phospholipase a2 receptor chimeric autoantibody receptor t cells
The invention includes compositions comprising at least one chimeric autoantibody receptor (CAAR) specific for an anti-phospholipase A2 receptor (PLA2R) autoantibody-based B cell receptor, polynucleotides encoding the CAAR, vectors comprising a polynucleotide encoding the CAAR, and recombinant T cells comprising the CAAR. The invention also includes methods of making a genetically modified cell, e.g., a genetically modified T cell, expressing a PLA2R-CAAR wherein the expressed CAAR comprises a PLA2R extracellular domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Selecting for developability of polypeptide drugs in eukaryotic cell display systems
ActiveUS20210163923A1Excellent developabilityAvoid liabilityDNA preparationAntiendomysial antibodiesControl cell
Use of the surface presentation level of binders (e.g., antibodies, receptors) on cultured higher eukaryotic cells in vitro as a predictive indicator of developability characteristics, e.g., solubility, of the binders. Display libraries of higher eukaryotic cells, e.g., mammalian cells, adapted for use in screening surface-displayed binders for developability and affinity of target binding. High-throughput screening of display libraries with in-built selection for developability including binder solubility, capability to be formulated at high concentrations, low propensity for non-specific binding, and half-life. Enrichment of populations of binders for developability characteristics and / or other qualities such as target binding and affinity, by controlling cell surface presentation of binders from an inducible promoter operably linked to binder-encoding DNA.
Owner:IONTAS LTD
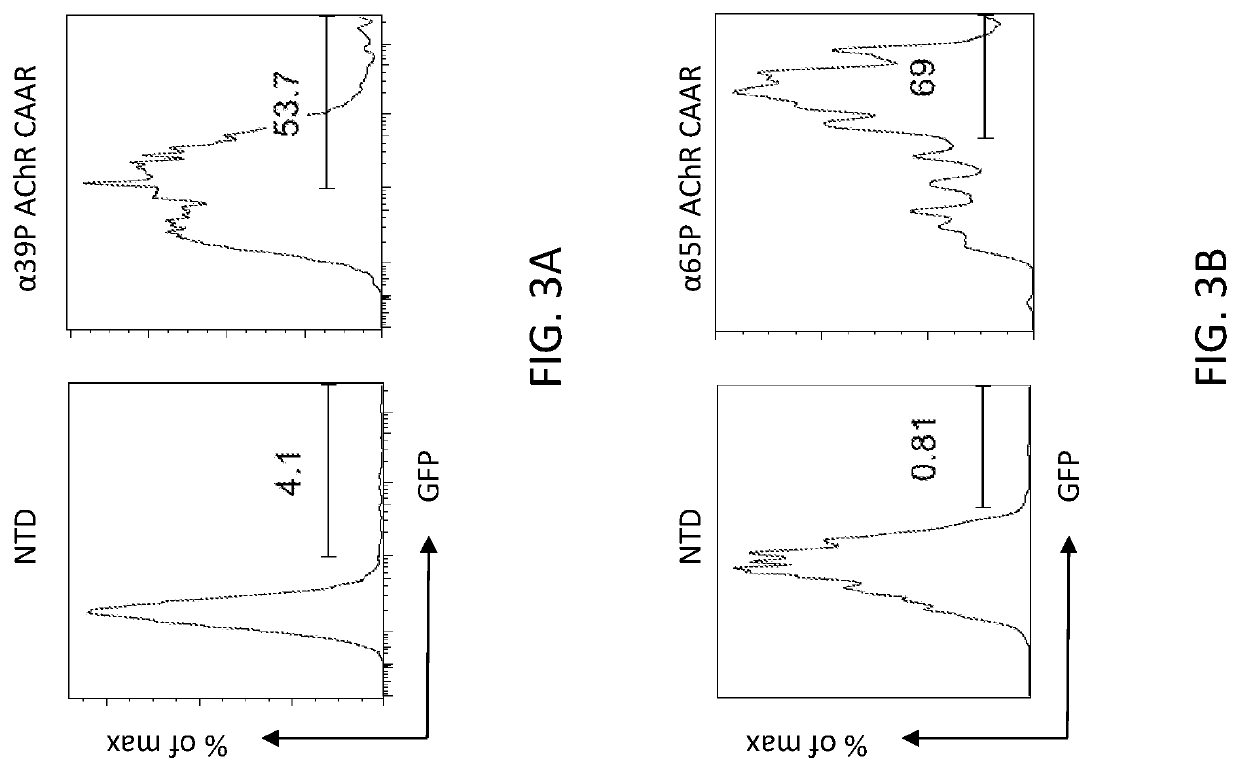
Compositions and methods of acetylcholine receptor chimeric autoantibody receptor cells
PendingUS20220242931A1Prevent and reduce damagePolypeptide with localisation/targeting motifImmunoglobulin superfamilyAutoantibodyAcetylcholine receptor
The invention includes a chimeric autoantibody receptor (CAAR) specific for anti-acetylcholine receptor (AChR) B cell receptor (BCR), compositions comprising the CAAR, polynucleotides encoding the CAAR, vectors comprising a polynucleotide encoding the CAAR, and recombinant cells, e.g., T cells comprising the CAAR.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
System and methods relating to chimeric autoantibody receptors
PendingUS20210040199A1Easily swapped outLow costPolypeptide with localisation/targeting motifSkeletal disorderAutoantibodyAutoimmune condition
Chimeric autoantibody receptors (CAARs) can include separate signaling and recognition constructs that are able to bind ligands that target autoantigens made of conventional amino acids, non-conventional amino acids, carbohydrates, or nucleic acids. Additionally, the present disclosure describes cells modified to express such constructs and the use of such constructs and / or cells in the treatment of autoimmune disease.
Owner:VALKYR INC
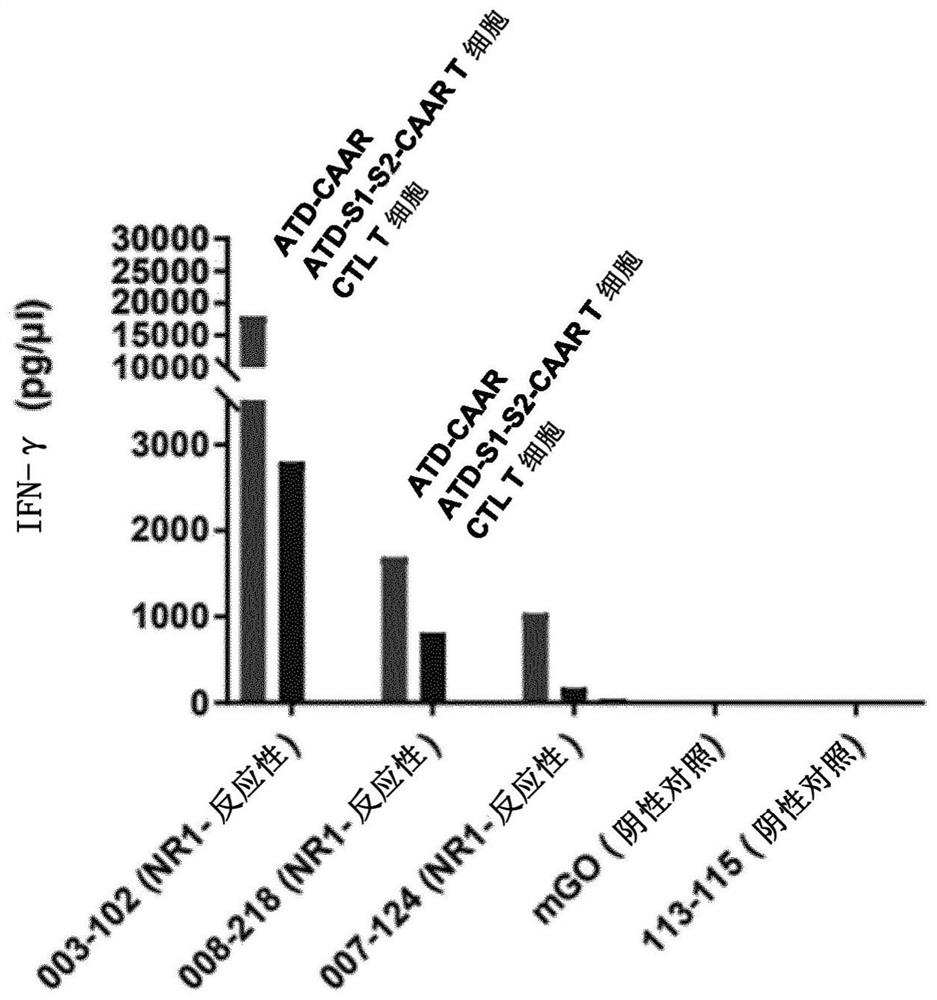
Compositions and methods for targeting nicotinic acetylcholine receptor autoantibodies
PendingCN114075568AImprove efficacyPolypeptide with localisation/targeting motifMuscular disorderAutoantibodyAntibody receptor
The present disclosure provides a chimeric autoantibody receptor (CAAR) that is specific for an autoantibody-based B cell receptor (BCR), wherein the autoantibody is specific for nAChR. The disclosure also provides compositions comprising the CAAR, polynucleotides encoding the CAAR, vectors comprising the polynucleotides encoding the CAAR, engineered cells comprising the CAAR, and methods of using the same.
Owner:PORTON BIOLOGICS LTD
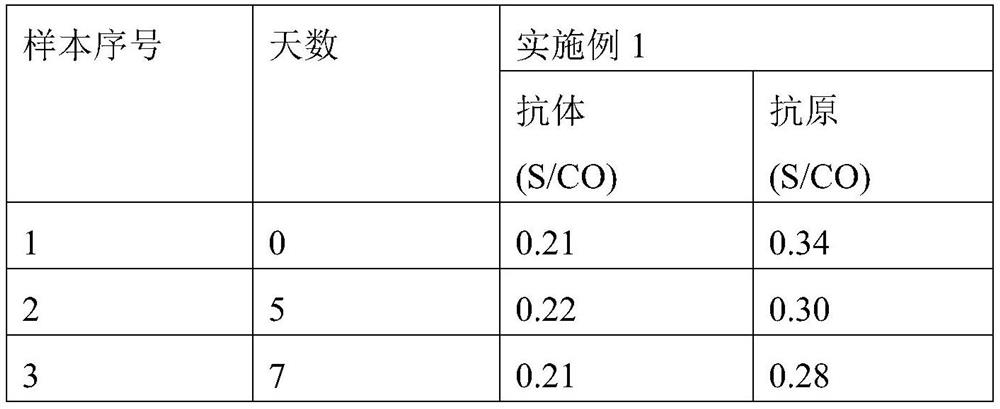
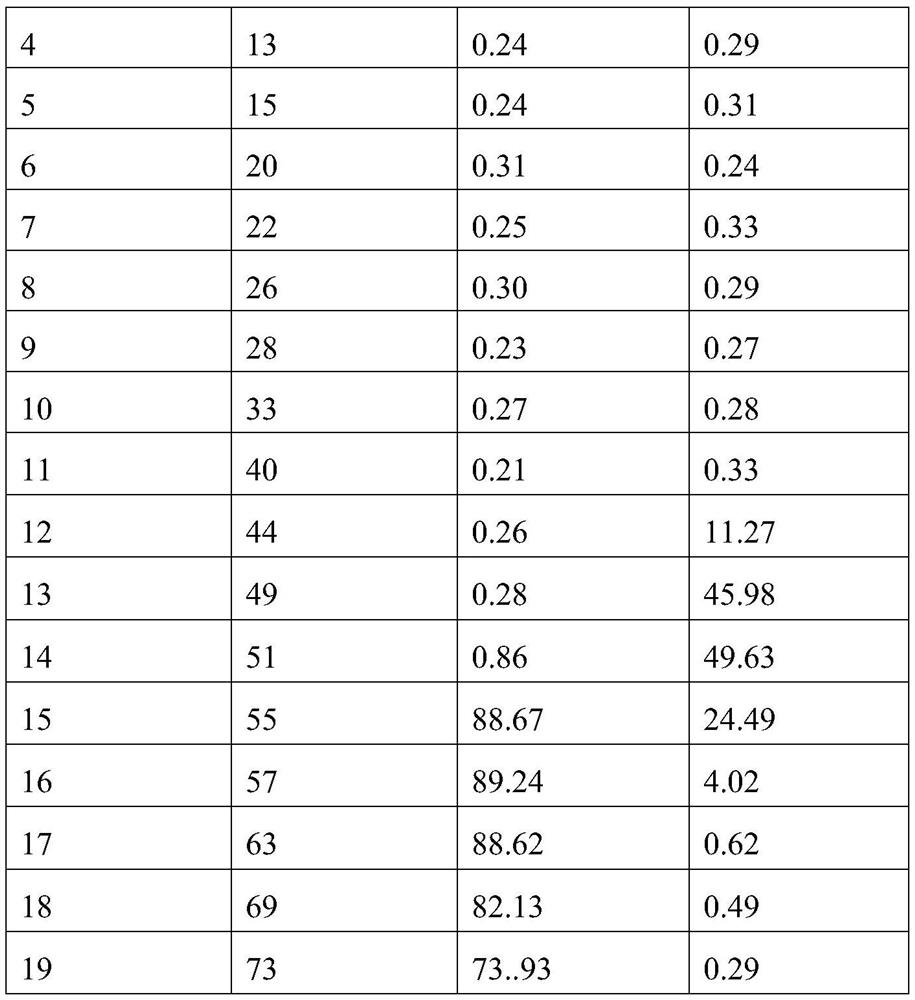
A homogeneous immunoassay method and its application
The present invention relates to a homogeneous immunoassay method, which comprises at least two detection areas, in one detection area it is judged whether there is a first complex formed by donor-to-be-detected antibody-acceptor, and in another detection area Judging whether there is a second complex formed by donor-antigen-receptor; wherein, the donor can generate singlet oxygen in an excited state, and the acceptor can react with singlet oxygen to produce detectable signal of. The method solves the problem of HOOK effect of high-concentration samples and low detection sensitivity of low-concentration samples in the one-step detection of antigen and antibody. At the same time, the method also solves the problem that the positive result cannot be distinguished from antibody positive or antigen positive in combined detection of antigen and antibody. The method of the present invention is especially suitable for combined detection of HIV antigen and antibody.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Compositions and methods for targeting anti-TNF-alpha antibodies
The present disclosure provides a chimeric anti-drug antibody receptor (CADAR) having specificity for an anti-drug antibody based B cell receptor (BCR), wherein the anti-drug antibody is induced by a therapeutic anti-TNF-alpha monoclonal antibody. The disclosure also provides compositions comprising the CADAR, polynucleotides encoding the CADAR, vectors comprising the polynucleotides encoding the CADAR, engineered cells comprising the CADAR, and methods of using the same.
Owner:PORTON BIOLOGICS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com