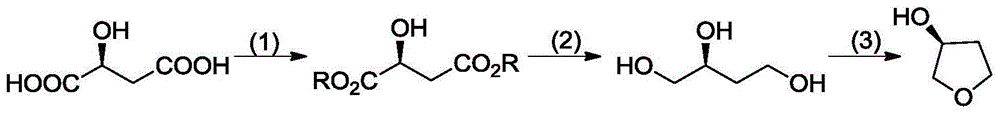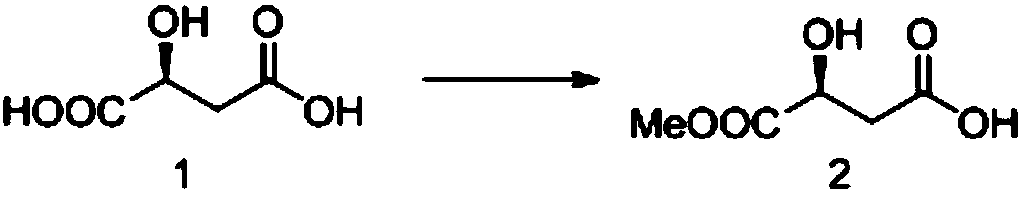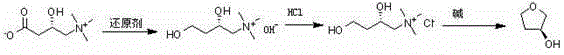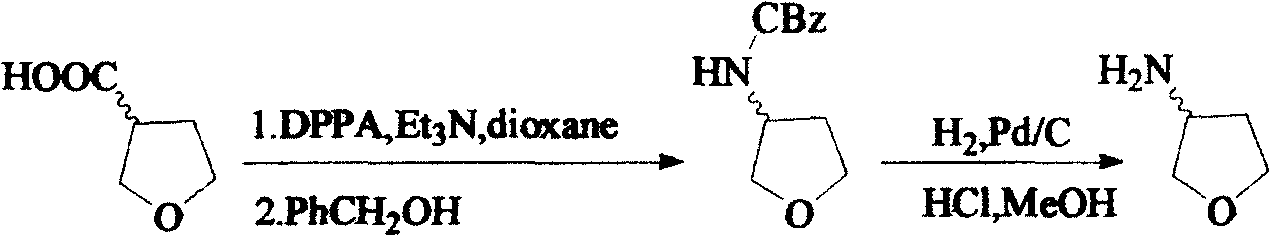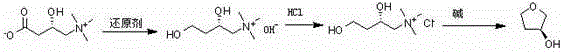Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "3-Hydroxytetrahydrofuran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
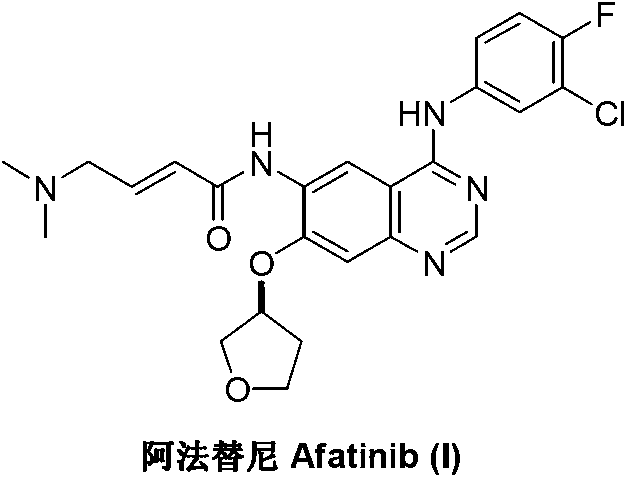
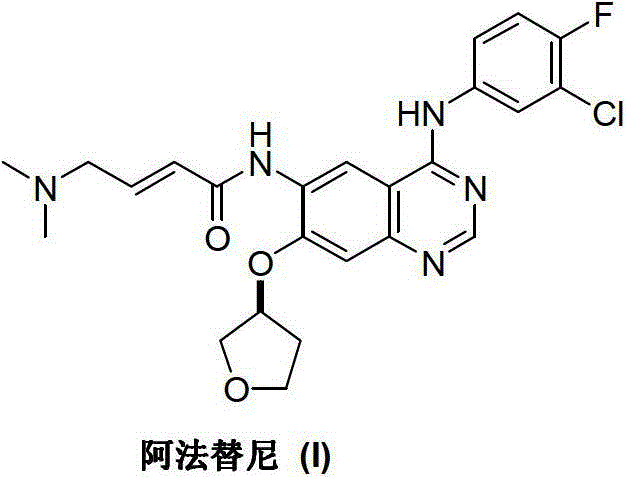
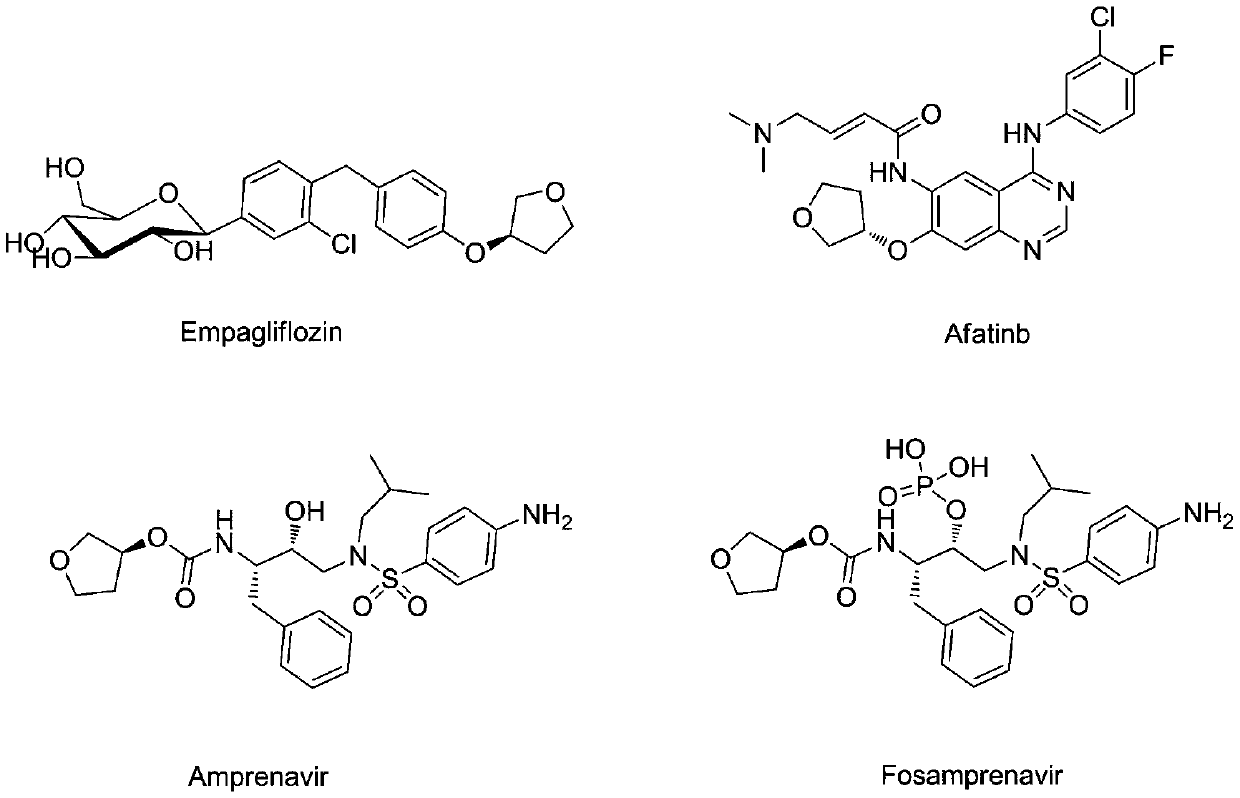
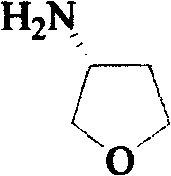
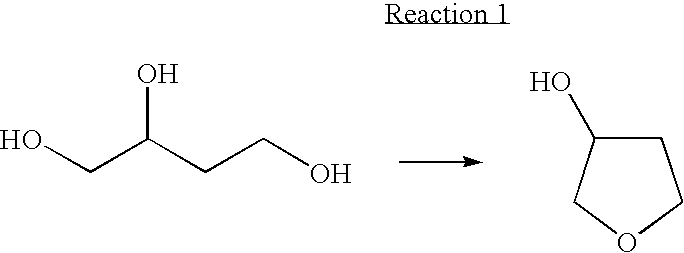
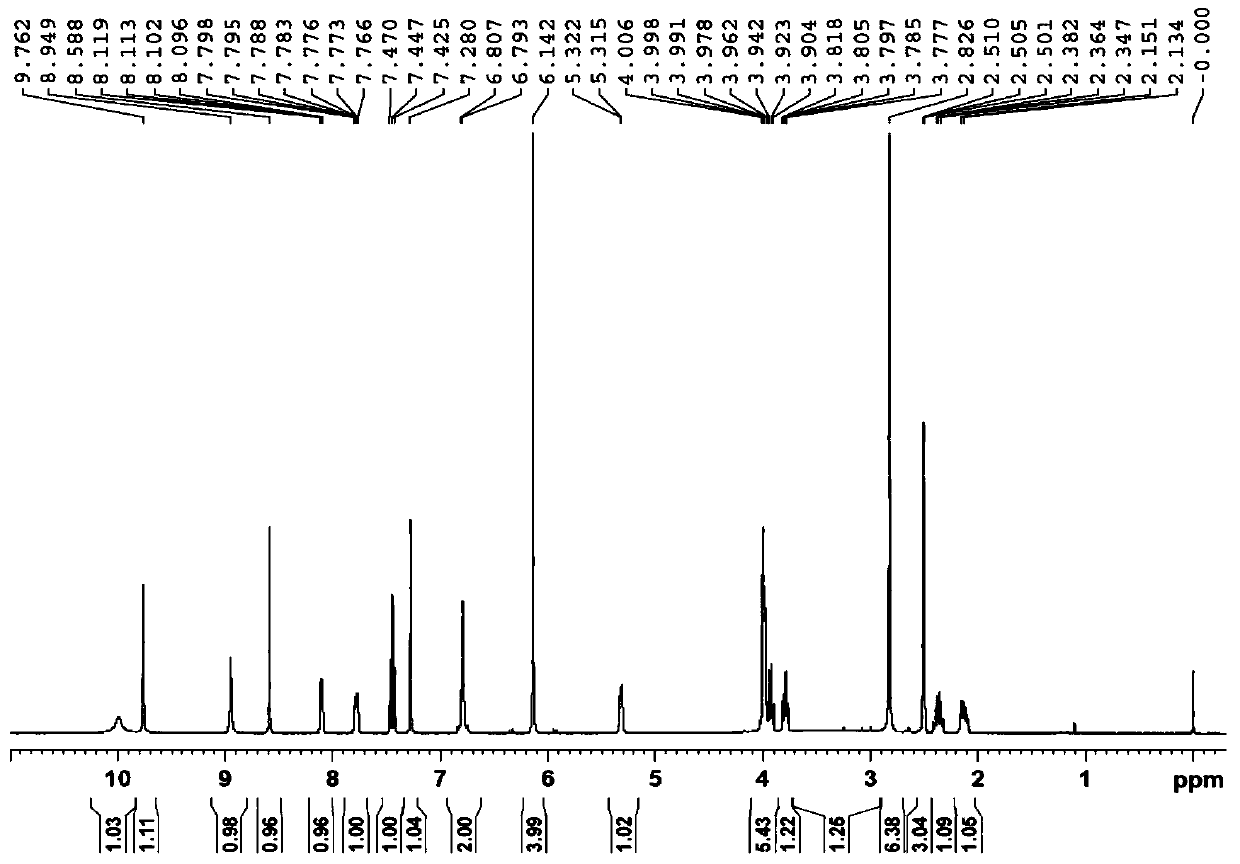
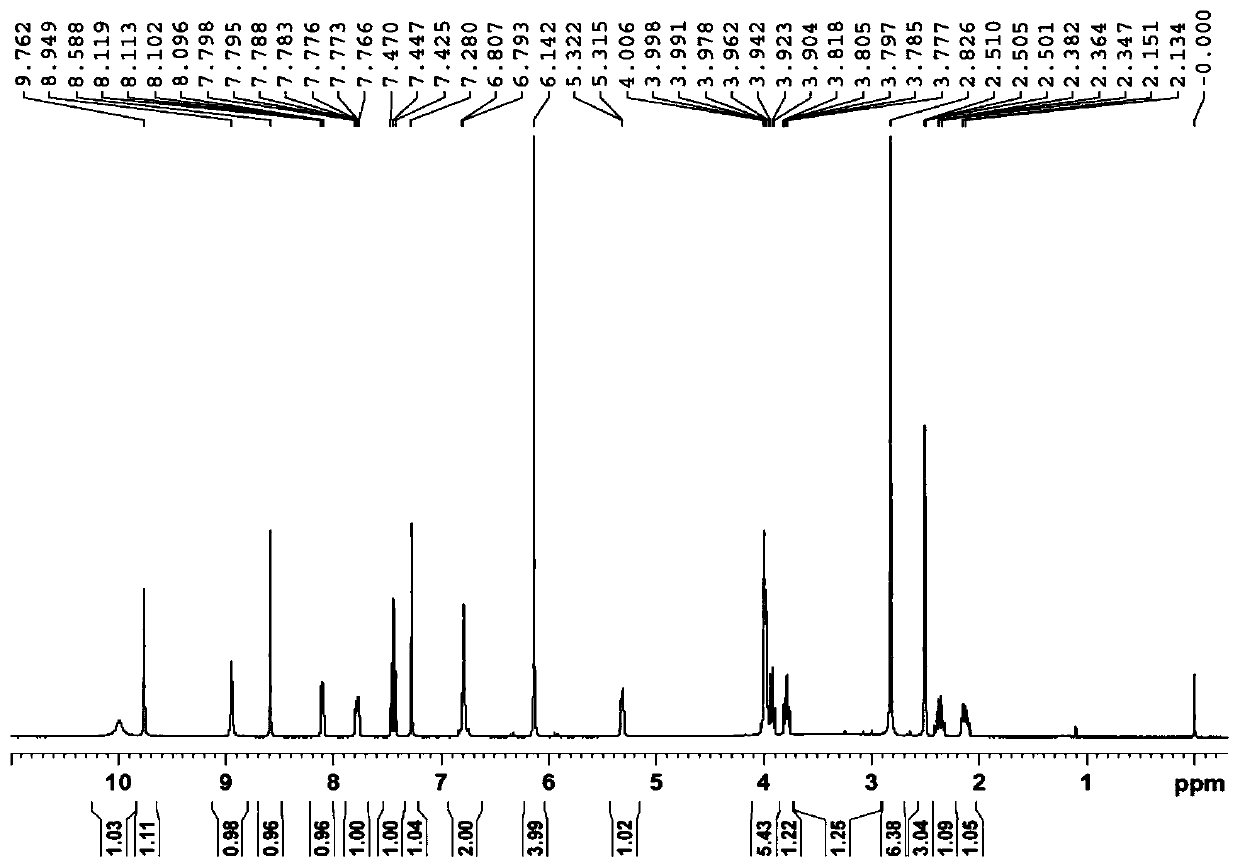
3-Hydroxytetrahydrofuran (3-OH THF) is a colorless liquid with a normal boiling point of 179 °C and boiling at 88−89 °C at 17 mmHg, with density (1.087 g/cm³ at 19 °C). 3-OH THF is a useful pharmaceutical intermediate. The chiral (absolute configuration S) version of this compound is an intermediate to launched retroviral drugs.
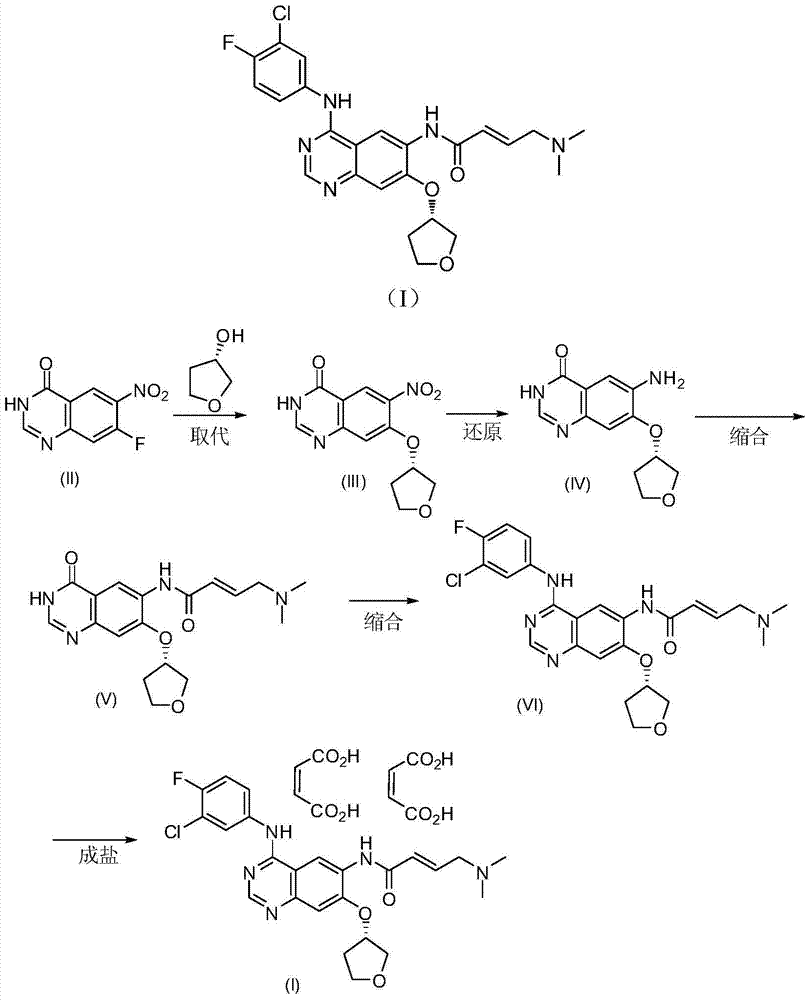
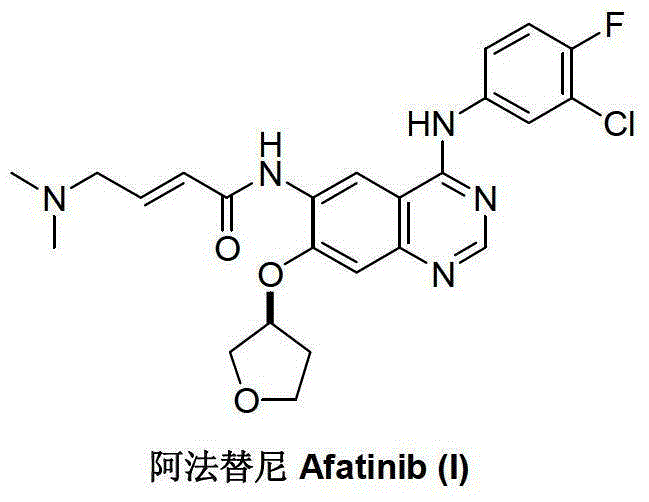
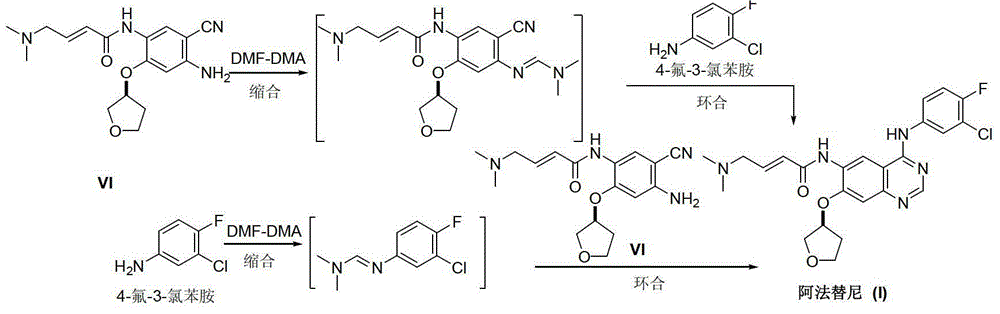
Preparation method of afatinib (I)
InactiveCN103288808AEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-HydroxytetrahydrofuranKetone
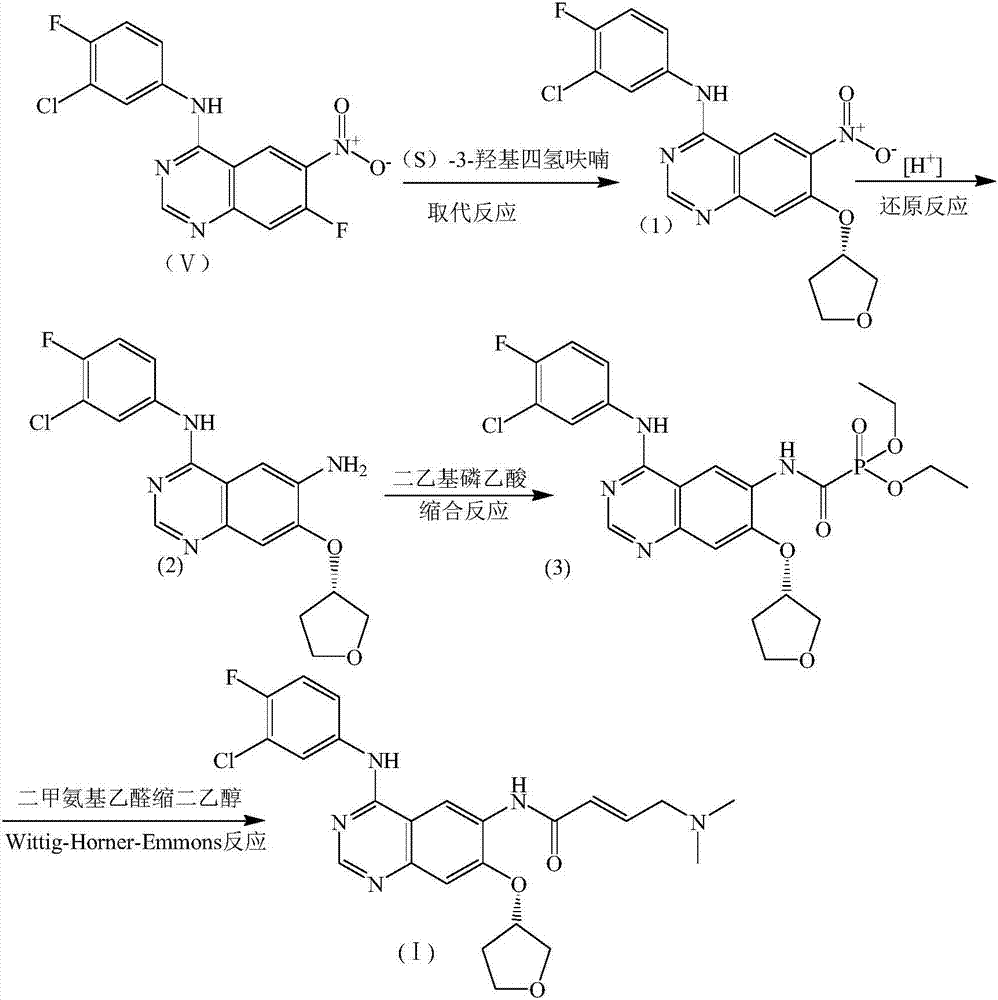
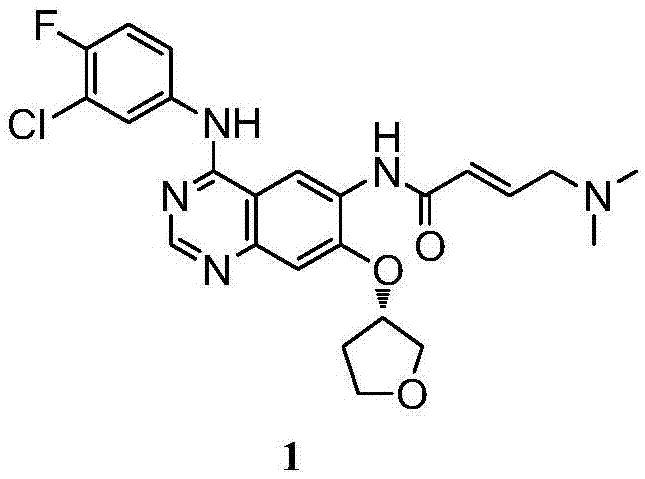
The invention discloses a preparation method of afatinib (I). The preparation method comprises the following steps that 6-amino-7-hydroxy-3,4-dihydroquinazoline-4-ketone (II) and (S)-3-hydroxytetrahydrofuran carry out etherification reaction to generate 6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]-3,4-dihydroquinazoline-4-ketone (III), the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride carry out acylation reaction to generate 6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]-3,4-dihydroquinazoline-4-ketone (IV), and the compound (IV) and 4-fluoro-3-chloroaniline carry out condensation reaction to prepare the afatinib (I). The preparation method is concise, economical and environment-friendly in process and is suitable for the requirement of industrial amplification.
Owner:鄄城县人民医院
Afatinib preparation method
ActiveCN103242303AEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-Hydroxytetrahydrofuran2-Butene
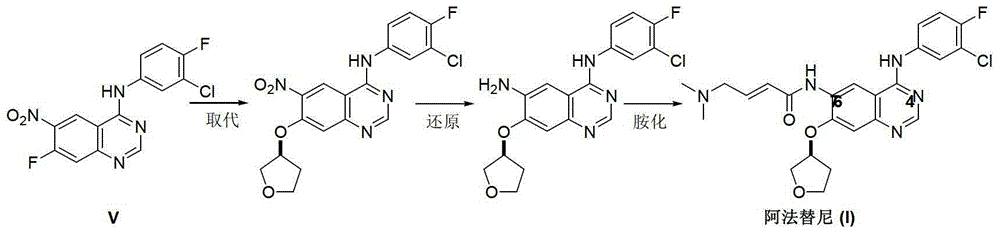
The invention discloses an Afatinib (I) preparation method which comprises the following steps: performing etherification reaction on 4-chloro-6-amino-7-hydroxyquinazoline (II) and (S)-3-hydroxytetrahydrofuran to generate 4-chloro-6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (III); performing acylation reaction on the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride to generate 4-chloro-6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (IV); and performing condensation reaction on the compound (IV) and 4-fluoro-3-chloroaniline to obtain Afatinib (I). The preparation method is simple, economic and environment-friendly in process, and meets the requirements for large-scale industrialization.
Owner:铜陵尚东高新科创有限公司
Preparation method of drug intermediate (S)-3-hydroxytetrahydrofuran
InactiveCN106957287ASource less restrictiveHigh catalytic activityOrganic chemistry methodsFermentationCarboxylic salt3-Hydroxytetrahydrofuran
The invention provides a preparation method of a drug intermediate (S)-3-hydroxytetrahydrofuran. The method comprises the following steps: by taking racemic 1,2,4-butantriol as a raw material, synthesizing racemic 3-hydroxytetrahydrofuran; and carrying out esterification to obtain racemic tetrahydrofuryl-3-fatty acid ester. After the (R)-tetrahydrofuryl-3-fatty acid ester in a racemic mixture is hydrolyzed through lipase, the hydrolyzed (R)-tetrahydrofuryl-3-fatty acid ester is converted into (S)-tetrahydrofuryl-3-carboxylate by utilizing a Mitsunobu reaction under the condition that a hydrolyzed product is not separated; and finally, all tetrahydrofuryl ester is hydrolyzed under an alkaline condition to obtain a final product (S)-3-hydroxytetrahydrofuran.
Owner:杭州述康生物技术有限公司
Synthetic method of (S)-(+)-3-hydroxytetrahydrofuran
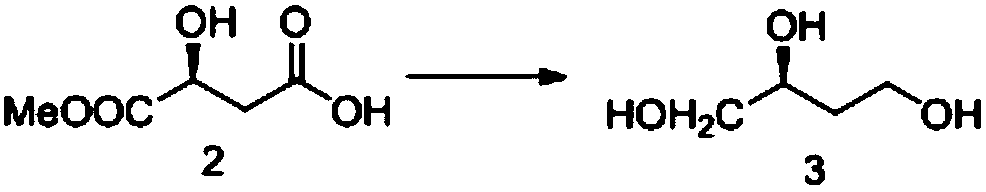
InactiveCN104478833AShort stepsInexpensive and easy to use raw materialsOrganic chemistrySodium borohydride3-Hydroxytetrahydrofuran
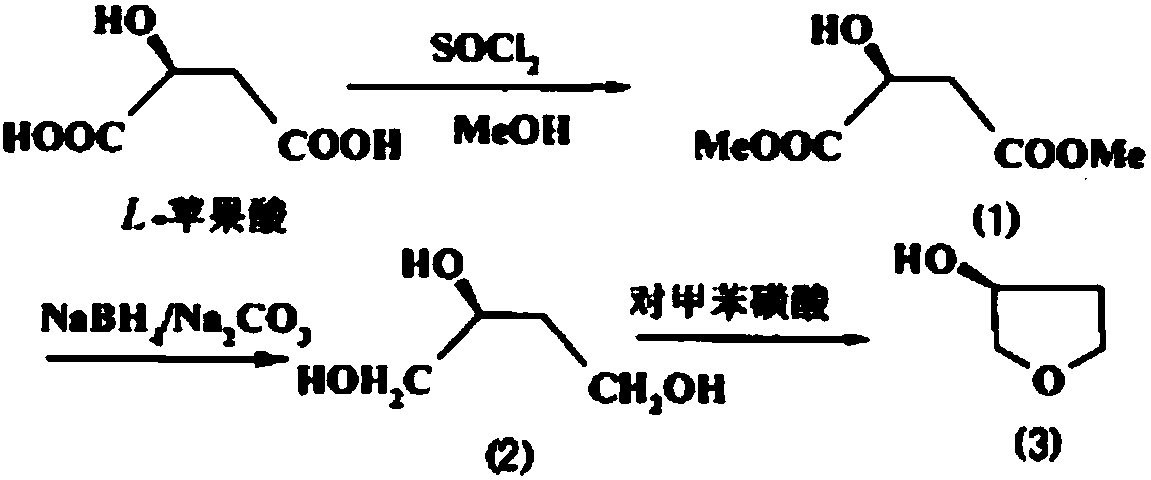
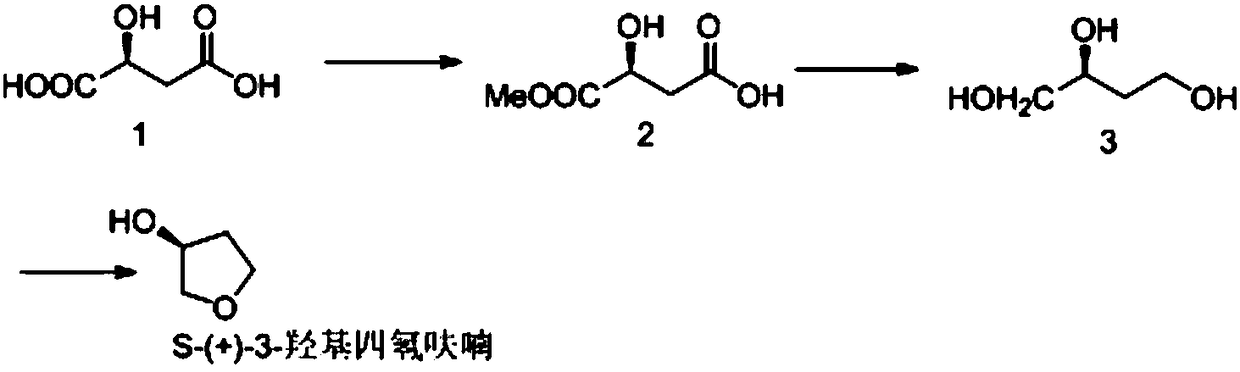
The invention provides a synthetic method of (S)-(+)-3-hydroxytetrahydrofuran. The synthetic method comprises the following steps: (1) with L-malic acid as a raw material, carrying out esterification on two carboxyls of the L-malic acid to obtain a product with a formula shown in the specification; (2) reducing a product in the first step by virtue of sodium borohydride to obtain a product with a formula shown in the specification; and (3) carrying out cyclization on a product obtained in the second step to obtain the (S)-(+)-3-hydroxytetrahydrofuran. According to the synthetic method provided by the invention, the synthetic route is short in step, the used raw materials are cheap and easy to acquire, no racemization phenomenon occurs in the reaction process, a by-product in the third step is easy to remove, the total yield is high, and the (S)-(+)-3-hydroxytetrahydrofuran is suitable for industrial production.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and alcohol dehydrogenase mutant
ActiveCN106520714AReduce pollutionImprove efficiencyMicroorganism based processesOxidoreductasesAlcohol3-Hydroxytetrahydrofuran
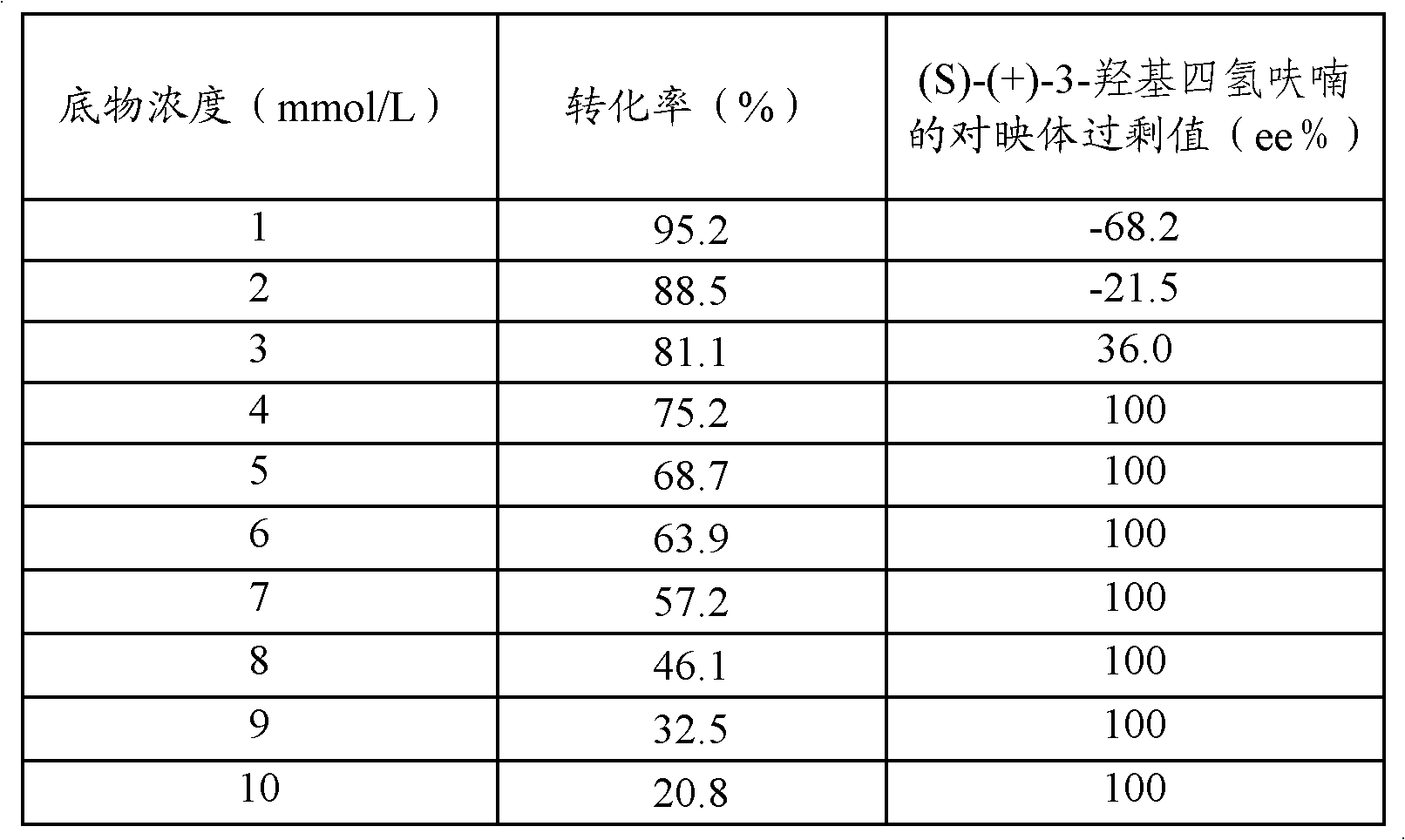
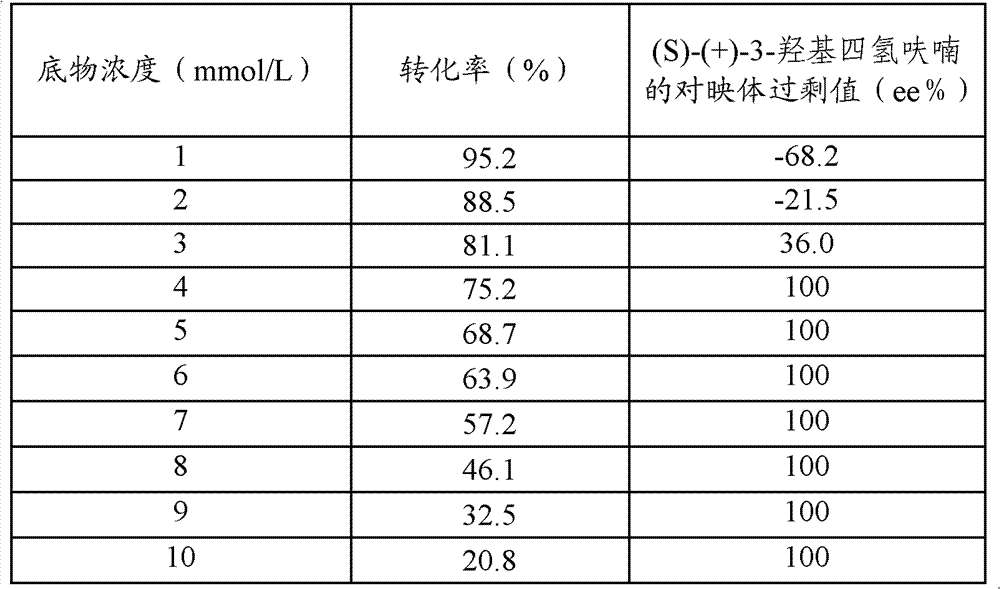
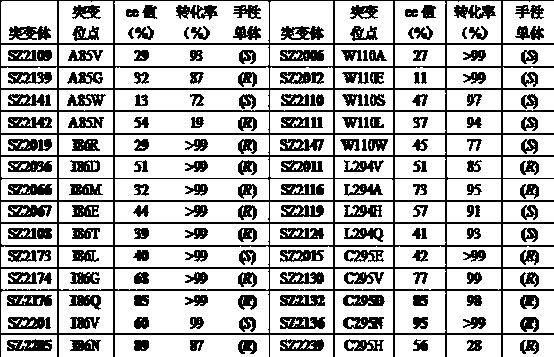
The invention relates to a process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and an alcohol dehydrogenase mutant. The process comprises the a step of, with 3-ketotetrahydrofuran as a substrate, subjecting the substrate, the alcohol dehydrogenase mutant, a cofactor and a cofactor regeneration system to a biological catalysis reaction in a buffer system so as to produce chiral 3-hydroxytetrahydrofuran, wherein for alcohol dehydrogenase used by the alcohol dehydrogenase mutant, the wild gene sequence of alcohol dehydrogenase is derived from thermophilic bacteria, the base sequence is as shown in SEQ ID No. 1, and the amino acid sequence is as shown in SEQ ID No. 2. Compared with chemical methods, the process using the alcohol dehydrogenase mutant for preparation of chiral 3-hydroxytetrahydrofuran has the advantages of small environmental pollution, high efficiency, high optical selectivity; and compared with enzyme resolution methods, the process provided by the invention has higher purity and conversion rate and has ee value and conversion rate of both 99%.
Owner:洛阳华荣生物技术有限公司
Preparation method of empagliflozin intermediate
The invention provides a preparation method of an empagliflozin intermediate. The preparation method comprises the following steps: 1), taking 4-fluorotoluene and (R)-3-hydroxytetrahydrofuran as raw materials, a polar solvent as a reaction solvent and inorganic alkali as a catalyst, performing a reaction to obtain (S)-3-p-cresyl tetrahydrofuran; 2), with N-chlorosuccinimide and the product obtained in the step 1) as raw materials, a non-polar solvent as a reaction solvent and dibenzoyl peroxide or azobisisobutyronitrile as an initiator, performing a reaction to obtain (S)-3- p-chlorophenol tetrahydrofuran; 3) dissolving 4-bromaniline and the product obtained in the step 2) into ethyl acetate, adding a catalyst Lewis acid, performing a reaction to obtain (S)-3-(4-(5-bromo-2-aminobenzyl)phenoxy) tetrahydrofuran; 4), performing a diazotization reaction on the product obtained in the step 3), and then reacting with cuprous chloride to synthesize (S)-3-(4-(5-bromo-2-chlorobenzyl)phenoxy) tetrahydrofuran. The preparation method has the advantages that the cost is low, the finished product is high in purity and the synthesis route is short.
Owner:安徽省诚联医药科技有限公司
Synthesis method of Empagliflozin intermediate
InactiveCN108178751AHigh yieldImprove responseOrganic chemistry methodsIodine3-Hydroxytetrahydrofuran
The invention discloses a synthesis method of an Empagliflozin intermediate. The synthesis method uses 4-hydroxybenzyl chloride as a starting material to sequentially react with methanesulfonyl chloride and (S)-3-hydroxytetrahydrofuran to obtain compound III, then react with 4-iodoaniline to obtain compound IV, and finally react with cuprous chloride after diazotization to obtain (S)-3-(4-(5-iodine-2-chlorobenzyl)phenoxy) tetrahydrofuran. The raw materials used in the synthesis method are simple and easy to obtain, the operation steps are simple, the post-treatment is simple, the product yieldis high, and the method is suitable for industrial production.
Owner:YANGZHOU POLYTECHNIC INST
Preparation method of (S)-3-hydroxytetrahydrofuran
ActiveCN107935971AIncrease fat solubilityEasy extractionOrganic chemistryBenzoyl bromideReduction treatment
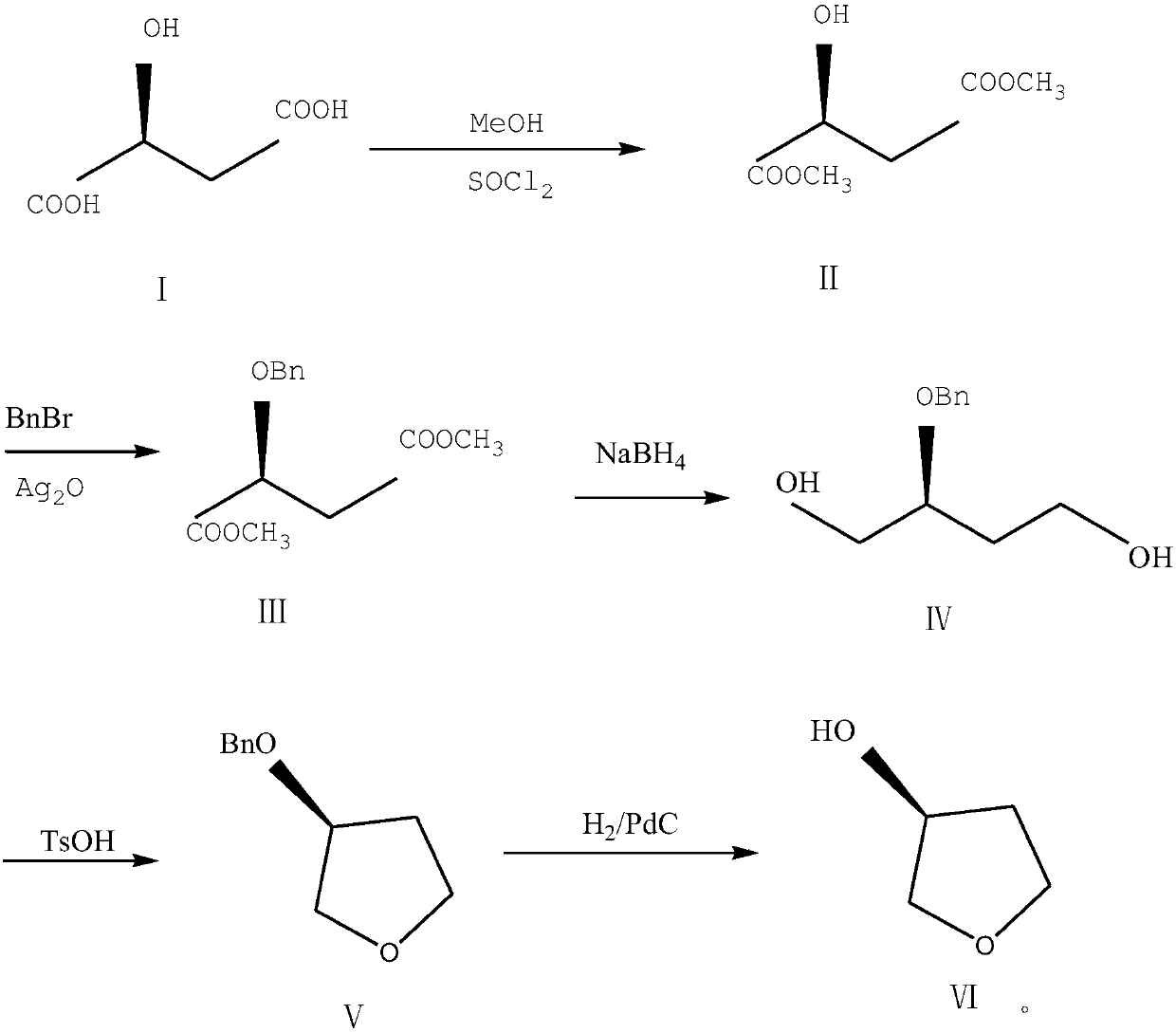
The invention discloses a preparation method of (S)-3-hydroxytetrahydrofuran. The problems that butantriol is difficult to separate in the production process, the yield is not high and the impurity content and the isomer content of the products are high are mainly solved. The preparation method of (S)-3-hydroxytetrahydrofuran comprises the following steps: under the existence of sulfoxide chloride, malic acid reacts with methanol to generate a compound II; under the existence of silver oxide, performing reaction on the compound II and benzyl bromide to generate a compound III; reducing the compound III by sodium borohydride to generate a compound IV; performing dehydration and ring closing on the compound IV by p-toluenesulfonic acid to generate a compound V; and taking palladium carbon asa catalyst and performing hydrogen reduction treatment on the compound V to obtain the product. The method is simple in aftertreatment and environment-friendly; the yield of the products is increasedby 80 percent or more, the purity is more than 99.5 percent and the chiral purity is more than 99.2 percent; and the method is suitable for industrialized production. (The formulas are as shown in the description).
Owner:SHANDONG BOYUAN PHARM CO LTD
Chemical synthesis method of S-(+)-3-hydroxytetrahydrofuran
InactiveCN109503523AEasy to operateHigh yieldOrganic chemistry methodsChemical synthesis3-Hydroxytetrahydrofuran
The invention discloses a chemical synthesis method of S-(+)-3-hydroxytetrahydrofuran. The method comprises the following steps: 1, subjecting a compound 1 to a reaction in the presence of thionyl chloride and methanol to obtain a compound 2; 2, subjecting the compound 2 to a reaction in a solvent in the presence of a reducing agent and a base to obtain a compound 3; and 3, subjecting the compound3 to a reaction in the presence of p-toluenesulfonic acid to obtain a compound, namely S-(+)-3-hydroxytetrahydrofuran.
Owner:南京天越星生物技术有限公司
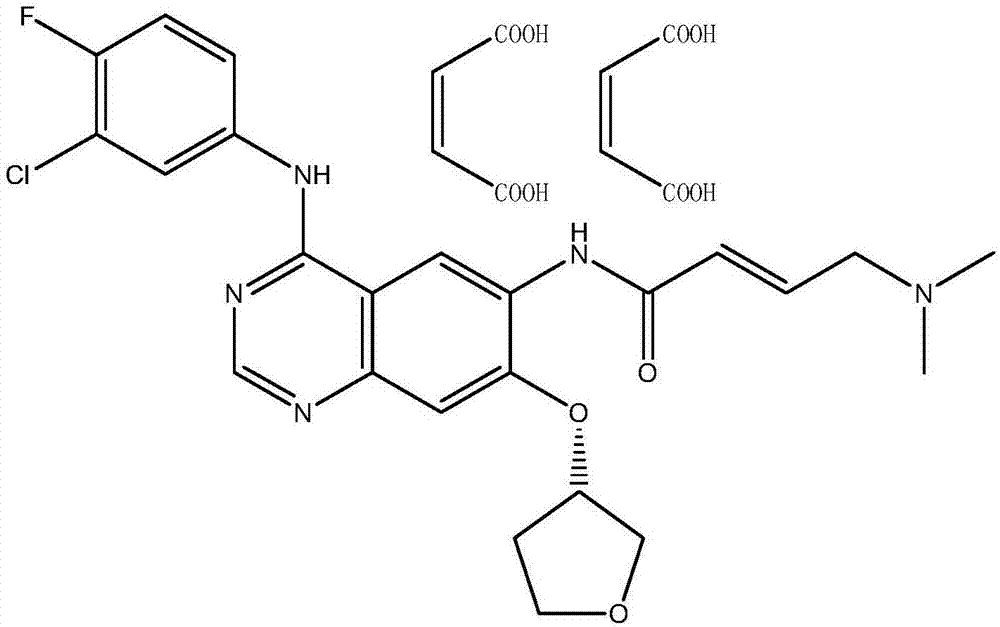
Preparation method of afatinib dimaleate
ActiveCN104710413AHigh yieldShort routeCarboxylic acid salt preparationKetone3-Hydroxytetrahydrofuran
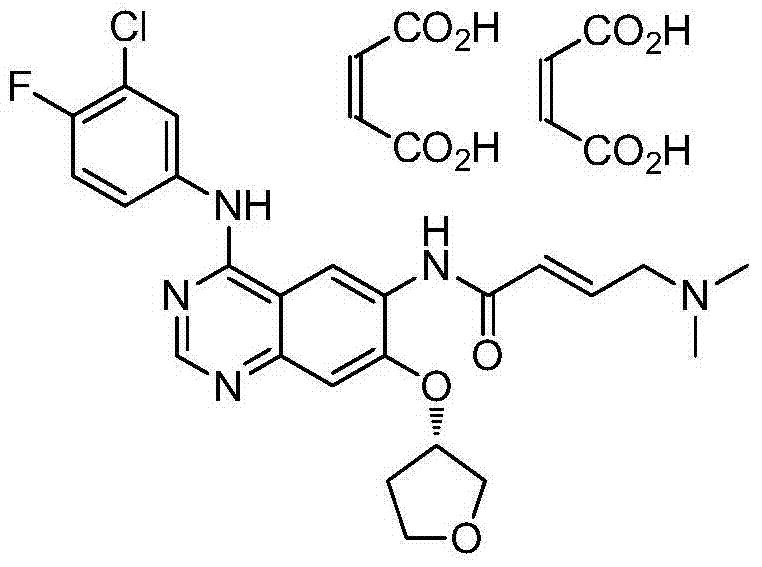
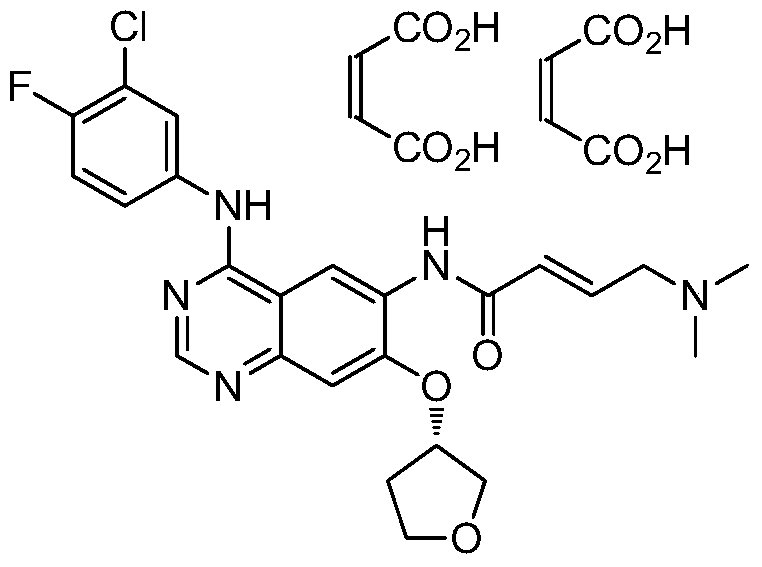
The invention relates to a preparation method of afatinib dimaleate, and concretely relates to a preparation method of an antitumor medicine afatinib dimaleate. The method comprises the following steps: carrying out a substitution reaction on 6-amino-7-fluoro-3,4-dihydroquinazolin-4-one and (S)-3-hydroxytetrahydrofuran, reducing, amidating, condensing, and carrying out salt formation to prepare afatinib dimaleate with the structure represented by formula (I). The preparation method has the advantages of concise technology, economy, environmental protection, and suitableness for industrial production.
Owner:JIANGSU HANSOH PHARMA CO LTD
Production technology of 3-hydroxytetrahydrofuran with high optical purity
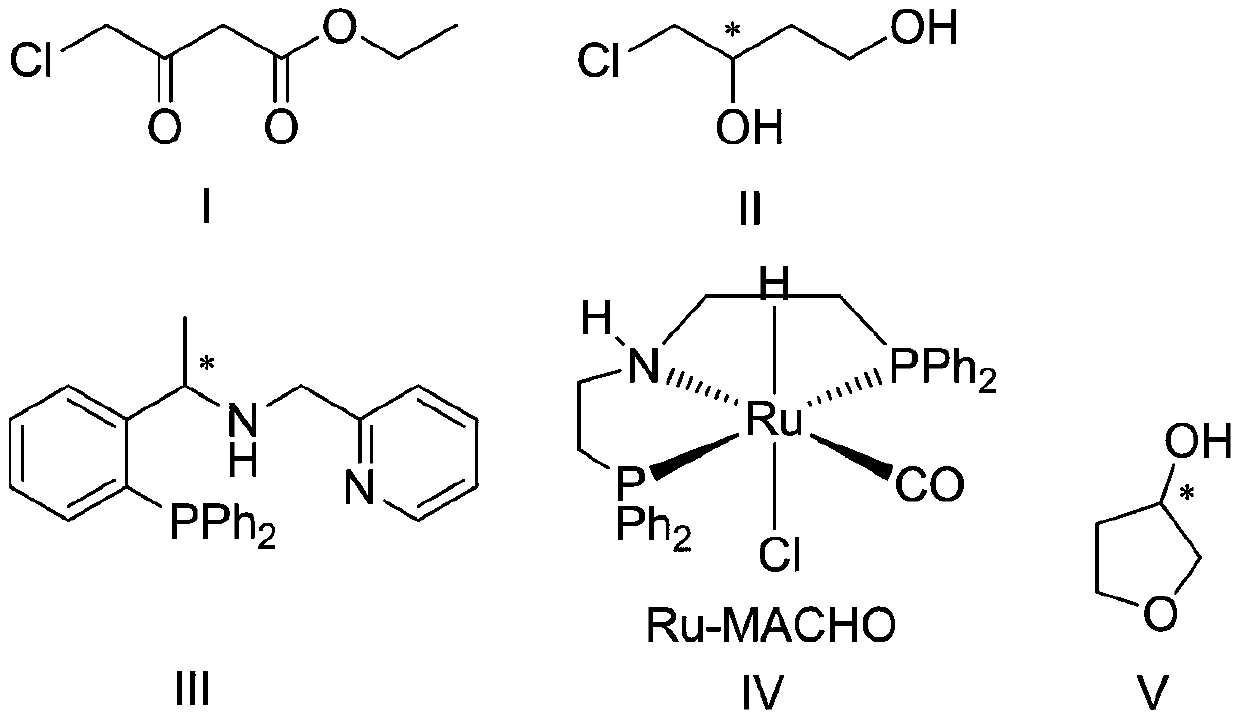
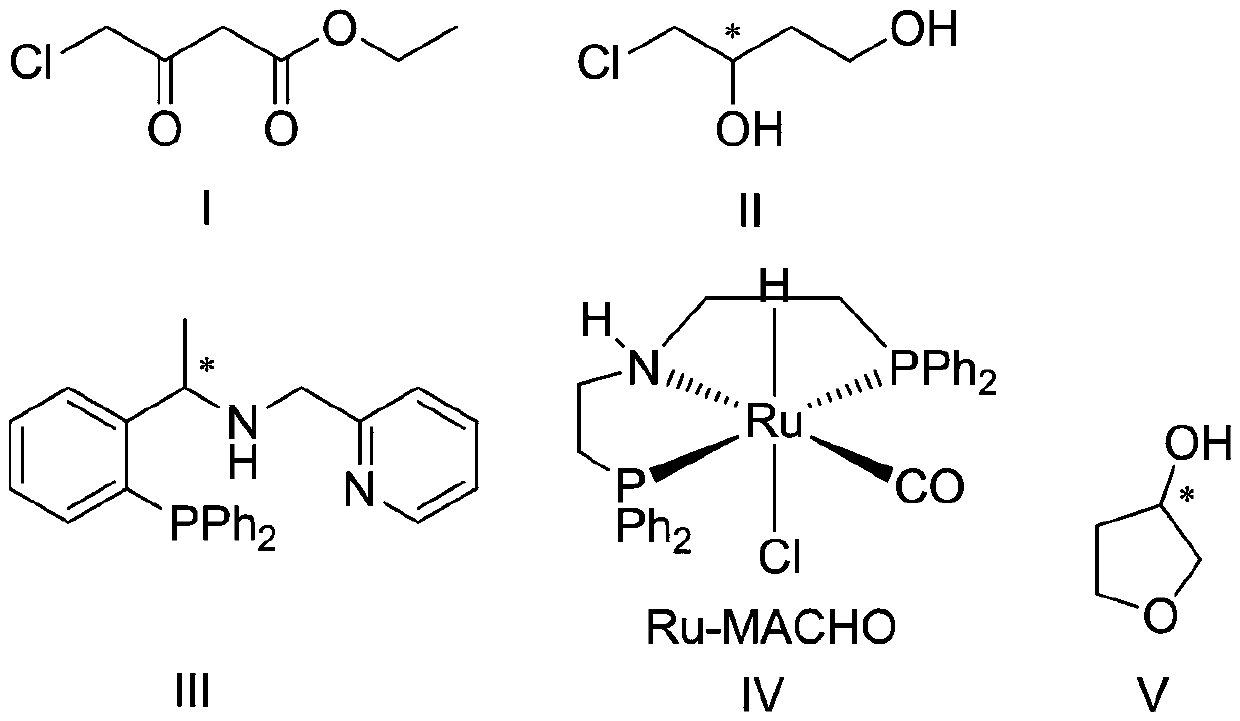
InactiveCN107098872AAvoid racemizationInhibit side effectsOrganic chemistry3-HydroxytetrahydrofuranSolvent
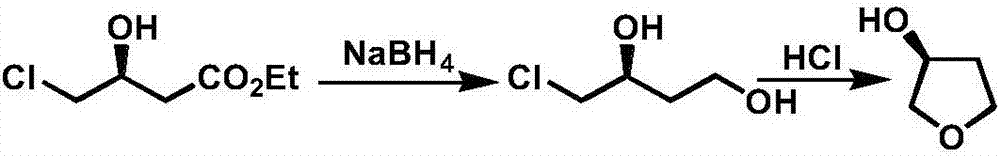
The invention discloses a production technology of3-hydroxytetrahydrofuran with high optical purity. The production technology comprises the following steps: (1) taking chloroacetoacetic acid ethyl ester as a starting raw material, adding appropriate amount of solvents, chiral catalysts and reducing agents, and reacting at an appropriate temperature to obtain chiral ethyl 4-chloro-3-hydroxybutyrate; (2) taking the chiral ethyl 4-chloro-3-hydroxybutyrate obtained in step (1) as a raw material, adding the appropriate amount of solvents and metal borohydride reducing agents, and reacting at the appropriate temperature to obtain chiral 4-chloro-3-hydroxy-1-butanol; (3) taking the chiral 4-chloro-3-hydroxy-1-butanol obtained in step (2) as the raw material, adding appropriate amount of catalysts and solvents, and reacting at the appropriate temperature to obtain chiral 3-hydroxytetrahydrofuran. According to the production technology of the 3-hydroxytetrahydrofuran with the high optical purity, the chiral 3-hydroxytetrahydrofuran can be produced through a three-step reaction, the shortcomings of complicated production operation and high production cost are solved, and products with high optical purity can be produced.
Owner:SUZHOU HUADAO BIOLOGICAL PHARMA
Preparation method of (S)-3-hydroxytetrahydrofuran and (R)-3-hydroxytetrahydrofuran
ActiveCN104961711ACheap and easy to getLow costOrganic chemistry(R)-Carnitine3-Hydroxytetrahydrofuran
The invention discloses a preparation method of (S)-3-hydroxytetrahydrofuran and (R)-3-hydroxytetrahydrofuran, relating to the technical field of preparation of five-element heterocyclic compounds containing one oxygen atom as the only heterocyclic atom. The method comprises the following steps: by using (S)-carnitine or (R)-carnitine as the initial raw material, carrying out reduction reaction in a reducer and an organic solvent to obtain (S) or (R)-2,4-dihydroxy-N,N,N-trimethyl butyl amine alkali; adding a hydrogen chloride organic solvent solution into an organic solvent to perform salification reaction to obtain (S) or (R)-2,4-dihydroxy-N,N,N-trimethyl butyl amine hydrochloride; and finally, adding alkali into a polar solvent, heating, and carrying out cyclization reaction to obtain the (S) or (R)-3-hydroxytetrahydrofuran. The method has the advantages of low cost, simple technique, high yield, cheap and accessible raw materials, short reaction steps, short period and low pollution, and is suitable for industrial production.
Owner:CANGZHOU SENARY CHEM SCI TEC
Synthesizing method of (S)-3-hydroxytetrahydrofuran
InactiveCN107235937ALower requirementReduce generationOrganic chemistryDistillationReaction temperature
The invention provides a synthesizing method of (S)-3-hydroxytetrahydrofuran. The synthesizing method comprises the following steps of synthesizing of L-dimethyl malate, synthesizing of chiral 1,2,4-butantriol crude product, synthesizing of the (S)-3-hydroxytetrahydrofuran, and the like. The synthesizing method has the advantages that low-cost L-malate is used as a raw material, and is reduced by sodium borohydride to form the L-dimethyl malate under the promoting action of methyl alcohol, so that the requirement of a reaction system is low; remained phosphoric acid in a quenching process is heated to catalyze chiral butantriol to generate the (S)-3-hydroxytetrahydrofuran in a closed ring way, and the product is separated and purified from the system via distillation, so that the economic and environment-friendly effects are realized, and the adding of a catalyst is not needed; the reaction temperature is lower, the staying time of the product in the system is short, the production of side production is greatly reduced, and the yield rate and optical purity of the product are increased.
Owner:BTC PHARMA TECH CO LTD
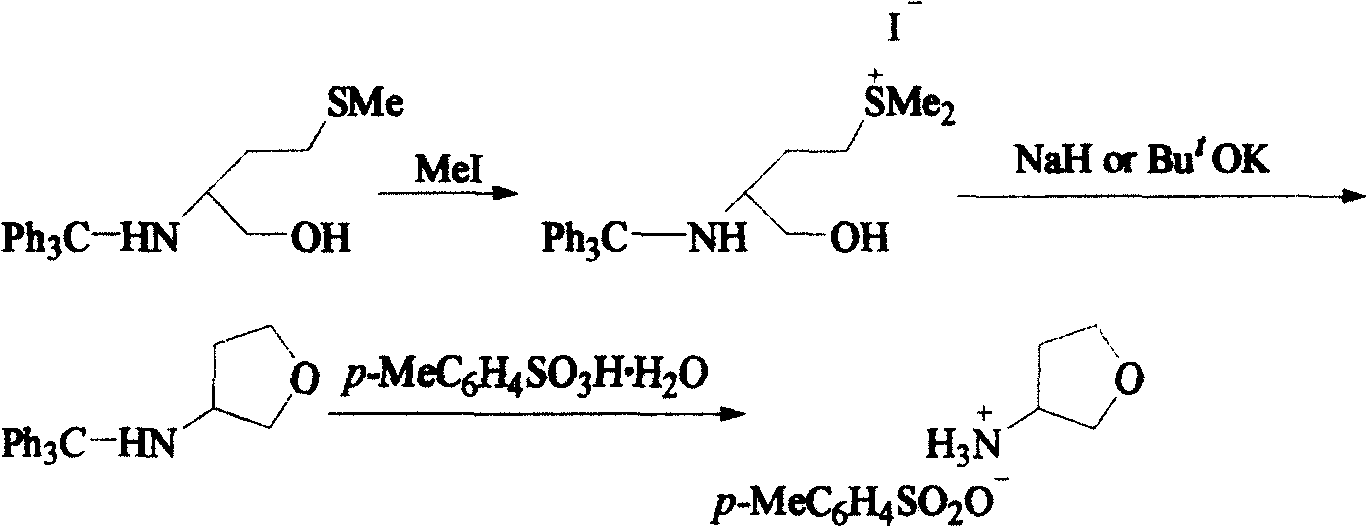
Preparing method of (S)-3-hydroxy tetrahydrofuran
InactiveCN105669608AComplete deprotectionComplete deprotection reactionOrganic chemistryEvaporationEthyl butyrate
The invention provides a preparing method of (S)-3-hydroxy tetrahydrofuran, which comprises the following steps: (1) adding (S)-4-Cl-3-hydroxy ethyl butyrate, potassium carbonate, benzyl chloride and DMF into a tri-opening bottle, stirring, refluxing, reducing pressure, standing, drying after washing, and recrystallizing; (2) adding tetrahydrofuran and sodium borohydride into a tetra-opening bottle; stirring and dropwise adding (S)-4-Cl-3-benzyloxy ethyl butyrate, continuing to stir, performing rotary evaporation condensing, cooling, neutralizing after stirring, extracting, and rectifying after rotary evaporation condensing; (3) adding (S)-4-Cl-3-benzyloxy-1-butanol, water and concentrated hydrochloric acid into a tri-opening bottle, starting stirring, heating, then performing temperature preserving reaction, neutralizing, extracting, and distilling after rotary evaporation condensing; (4) dissolving (S)-3-benzyloxy tetrahydrofuran in tetrahydrofuran, adding palladium carbon, introducing hydrogen, stirring to react, filtering, concentrating under reduced pressure, adding the recrystallized product, filtering after icy bath cooling, and drying to obtain the (S)-3-hydroxy tetrahydrofuran. The intermediate product is easy to separate and the yield is higher.
Owner:ITIC MEDCHEM CO LTD
Afatinib preparation method
ActiveCN103242303BEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-Hydroxytetrahydrofuran2-Butene
The invention discloses an Afatinib (I) preparation method which comprises the following steps: performing etherification reaction on 4-chloro-6-amino-7-hydroxyquinazoline (II) and (S)-3-hydroxytetrahydrofuran to generate 4-chloro-6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (III); performing acylation reaction on the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride to generate 4-chloro-6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (IV); and performing condensation reaction on the compound (IV) and 4-fluoro-3-chloroaniline to obtain Afatinib (I). The preparation method is simple, economic and environment-friendly in process, and meets the requirements for large-scale industrialization.
Owner:铜陵尚东高新科创有限公司
Preparation method of (s)-3-hydroxytetrahydrofuran
ActiveCN110396072AReduce pollutionHigh optical purityOrganic chemistryCatalytic effect3-Hydroxytetrahydrofuran
The invention provides a preparation method of (s)-3-hydroxytetrahydrofuran. According to the preparation method, ethyl 4-chloroacetoacetate is taken as an initial raw material, (s)-4-chloro-3 hydroxyl-1-butanol is prepared, wherein a substrate is dissolved in a first solvent, an alkali is added, under the catalytic effect of a first catalyst and a second catalyst, asymmetric hydrogenation reaction with hydrogen gas is carried out to produce (s)-4-chloro-3 hydroxyl-1-butanol; chiral 3-hydroxytetrahydrofuran is prepared, wherein prepared chiral 4-chloro-3 hydroxyl-1-butanol is dissolved in a second solvent, an acid is added as a catalyst, and reaction is carried out to obtain (s)-3-hydroxytetrahydrofuran; wherein the first catalyst is a complex generated through reaction of [Ir(COD)Cl]2 with phosphine-pyridine ligand, and the second catalyst is Ru-MACHO complex. The reaction route is short; technology is simple; raw materials are cheap and easily available; production cost is low; reaction process environment pollution is low; product optical purity is high; and the preparation method is suitable for industrialized production.
Owner:SHANGHAI SHINE HIGH INT TRADE CO LTD
Method for preparing (S)-(+)-3-hydroxytetrahydrofuran by transforming engineering strains
InactiveCN107904269AIncrease concentrationImprove toleranceOrganic chemistry methodsMicroorganism based processesEscherichia coliCalcium alginate
The invention provides a method for preparing (S)-(+)-3-hydroxytetrahydrofuran by transforming engineering strains. The method is characterized in that the (S)-(+)-3-hydroxytetrahydrofuran is preparedby taking 3-ketotetrahydrofuran as a substrate, immobilizing thallus cells, which are obtained by fermenting genetically engineered bacteria escherichia coli LC001 and can provide an enzyme continuously, through calcium alginate into a biological catalyst, and carrying out catalytic reaction. The method for preparing the (S)-(+)-3-hydroxytetrahydrofuran by transforming the engineering strains, provided by the invention, has the advantages of convenience for operation, high yield, moderate reaction conditions, environment friendliness, high catalysis specificity, high catalysis efficiency, high substrate and product tolerance and low cost, and has potential value in industrialized production.
Owner:安徽联创生物医药股份有限公司
Method for preparing S-(+)-3-hydroxy tetrahydrofuran through microbial conversion
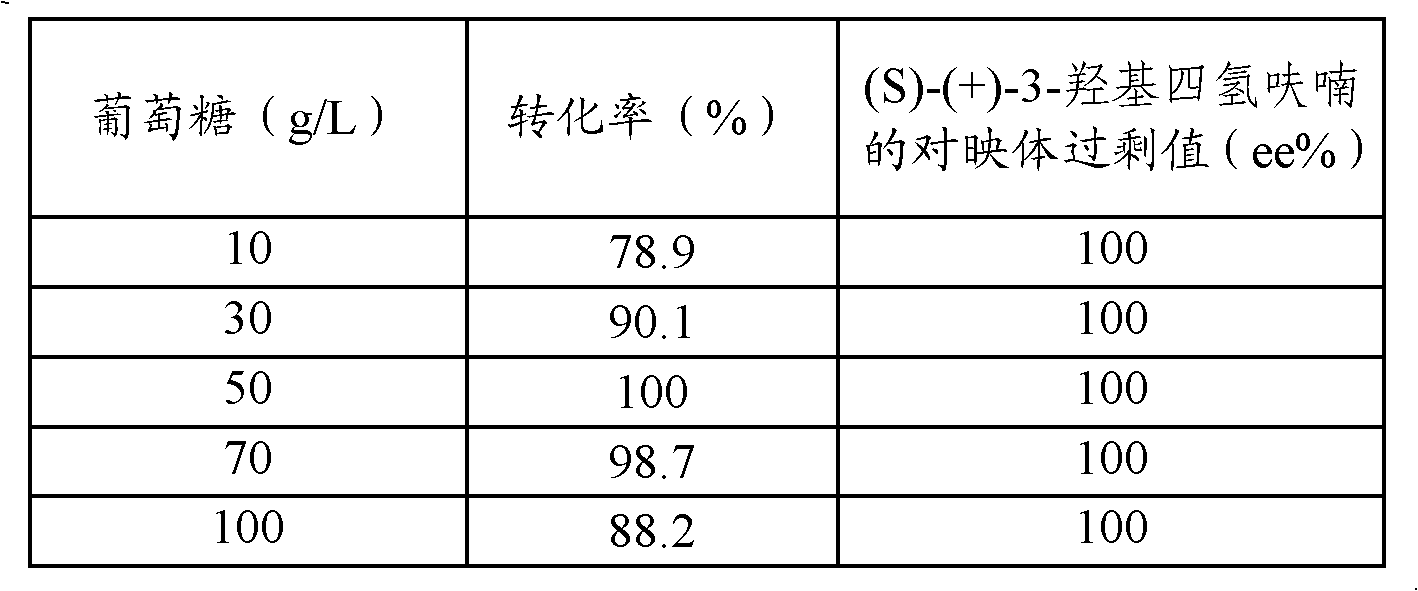
ActiveCN102071231AEasy to grow on a large scaleLow costFermentationPhosphateMicrobial transformation
The invention discloses a method for preparing S-(+)-3-hydroxy tetrahydrofuran through microbial conversion. The method comprises the following steps of: performing conversion reaction at the temperature of between 25 and 45 DEG C for 8 to 40 hours in phosphate buffer with pH of 5.0 to 8.0 by taking 3-keto-tetrahydrofuran as a substrate, and an enzyme-containing bacteroid cell obtained through the fermentation of saccharomyces cerevisiae CGMCC No.2266 as a biocatalyst, and after the reaction is finished, separating and purifying conversion liquid to obtain the S-(+)-3-hydroxy tetrahydrofuran.The method has the advantages that: (1) production strains are safe and non-toxic, can be cultured in a large scale easily and are low in cost; (2) the method is easy to operate, coenzyme which is high in price is not needed to be added in the reaction process and the yield is high; (3) large-scale industrial production is easy to realize; and (4) reaction conditions are mild and the method is environmental-friendly.
Owner:ZHEJIANG UNIV OF TECH +1
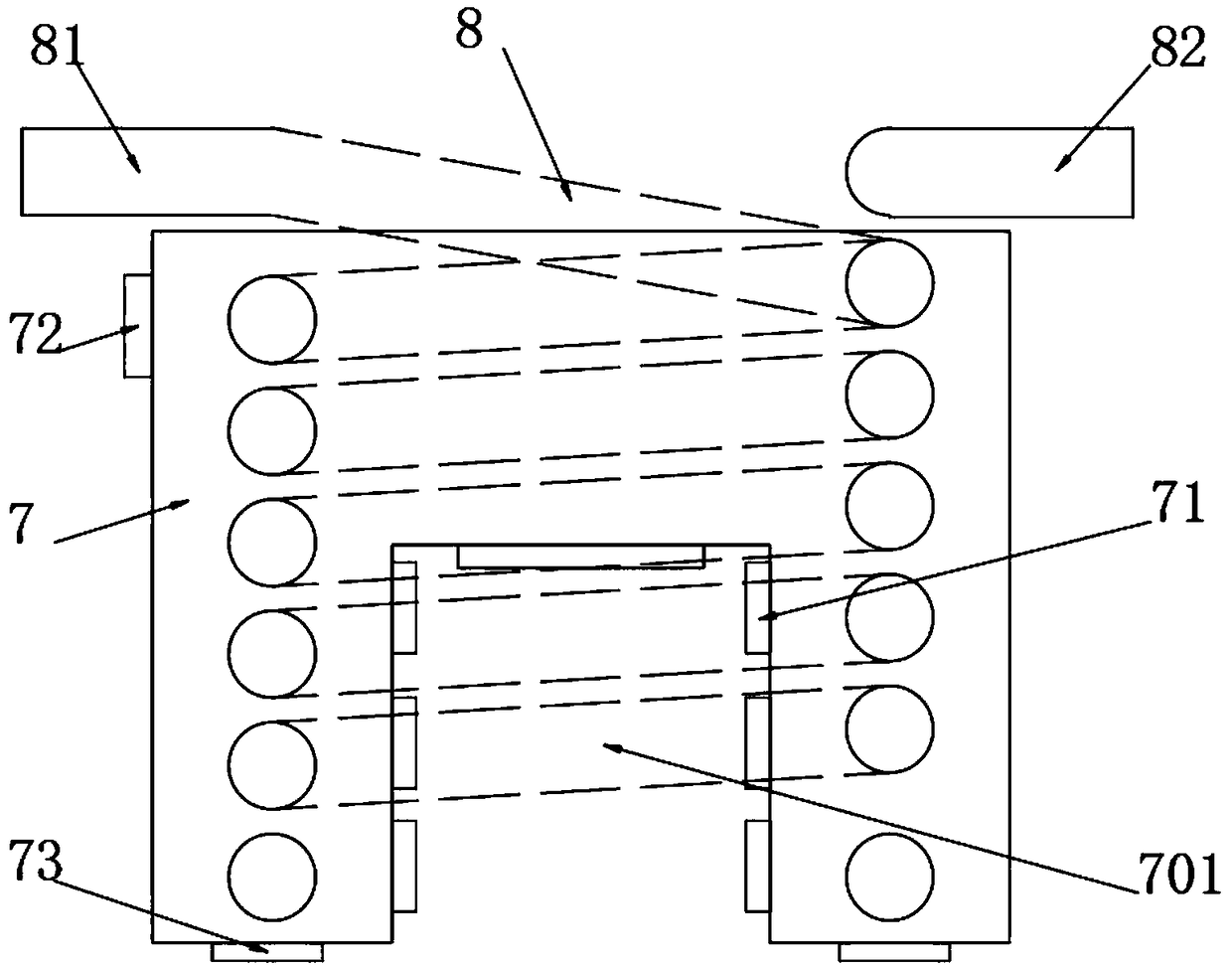
Tubular reactor of 3-hydroxytetrahydrofuran
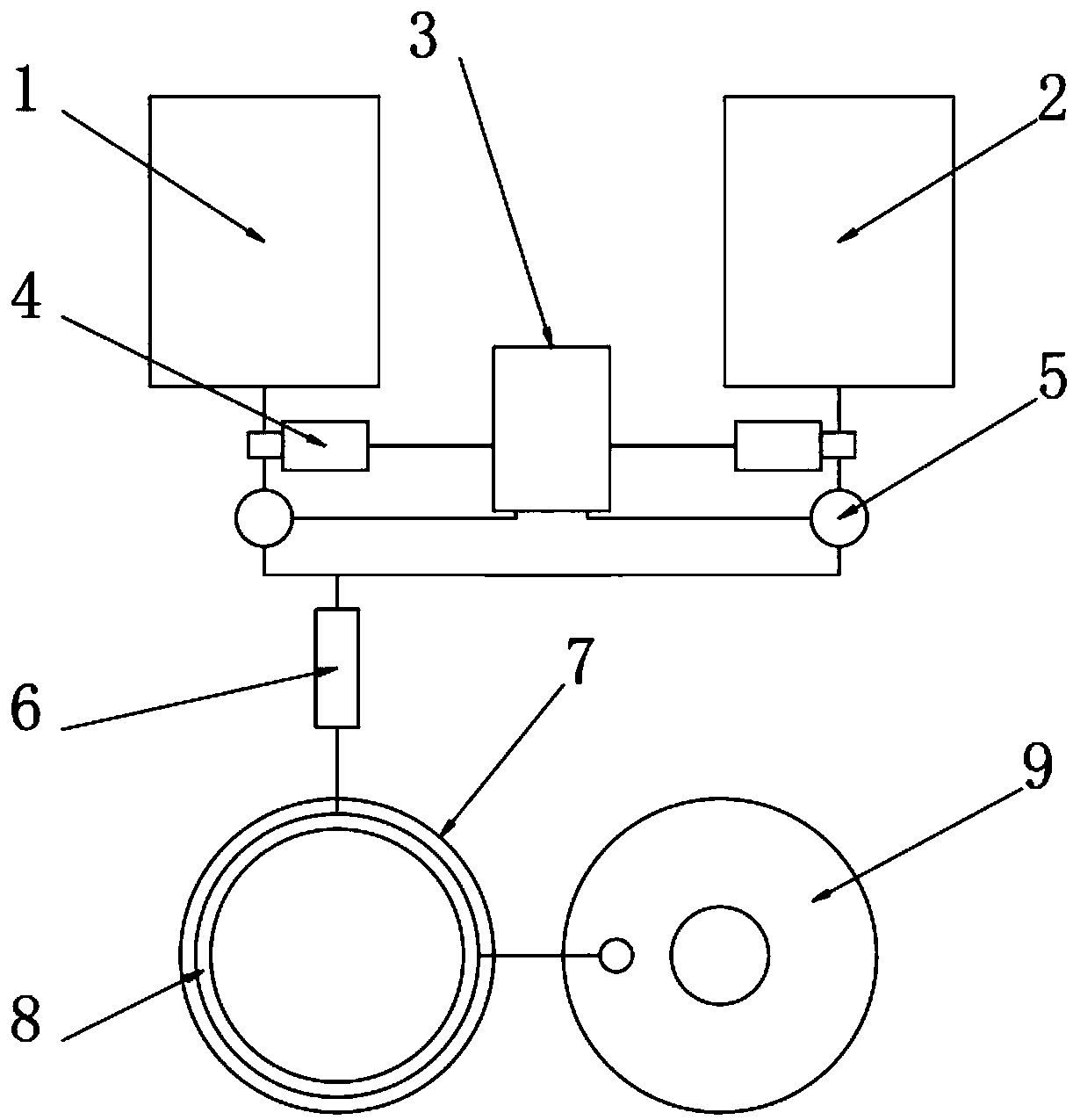
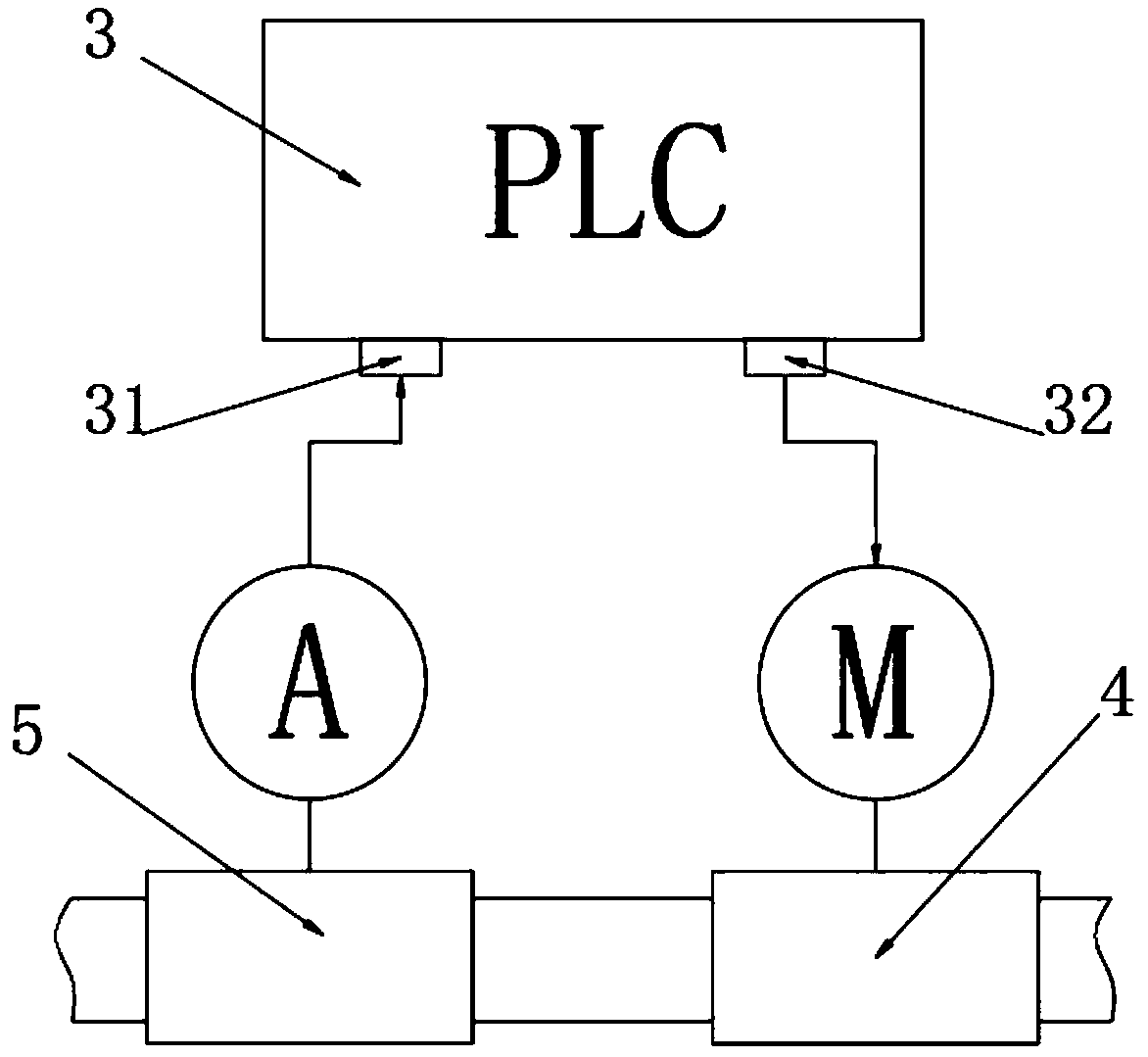
PendingCN109503522AReduce consumptionAvoid security risksOrganic chemistryChemical/physical/physico-chemical stationary reactorsWater bathsHydrogen
The invention belongs to the technical field of production of pharmaceutical intermediates, and particularly relates to a tubular reactor of 3-hydroxytetrahydrofuran. The tubular reactor comprises a material storing device and a controllable feeding device, wherein the material storing device comprises a malic acid solution batching tank and a sodium borohydride suspension batching tank; the controllable feeding device comprises a PLC controller, output pumps and flow meters which are connected through signals; the PLC controller is provided with a signal input end and a signal output end; andthe controllable feeding device is arranged on output pipes of the malic acid solution batching tank and the sodium borohydride suspension batching tank. By controlling the proportion and amount of raw materials, excessive use of sodium borohydride is avoided, therefore, the consumption of sodium borohydride is reduced and a safety risk caused by hydrogen is avoided; the tubular reactor is placedin a water bath, materials are introduced into a reaction spiral tube after being mixed, and the environment temperature rises rapidly to the reaction temperature, so that the rapid reaction of the materials is achieved, changes of the overall environment temperature are reduced, and the safe and stable reaction can be guaranteed.
Owner:江苏艾利瑞化学有限公司
Method for preparing S-(+)-3-hydroxy tetrahydrofuran through microbial conversion
ActiveCN102071231BEasy to grow on a large scaleLow costFermentationPhosphateMicrobial transformation
The invention discloses a method for preparing S-(+)-3-hydroxy tetrahydrofuran through microbial conversion. The method comprises the following steps of: performing conversion reaction at the temperature of between 25 and 45 DEG C for 8 to 40 hours in phosphate buffer with pH of 5.0 to 8.0 by taking 3-keto-tetrahydrofuran as a substrate, and an enzyme-containing bacteroid cell obtained through the fermentation of saccharomyces cerevisiae CGMCC No.2266 as a biocatalyst, and after the reaction is finished, separating and purifying conversion liquid to obtain the S-(+)-3-hydroxy tetrahydrofuran.The method has the advantages that: (1) production strains are safe and non-toxic, can be cultured in a large scale easily and are low in cost; (2) the method is easy to operate, coenzyme which is high in price is not needed to be added in the reaction process and the yield is high; (3) large-scale industrial production is easy to realize; and (4) reaction conditions are mild and the method is environmental-friendly.
Owner:ZHEJIANG UNIV OF TECH +1
Preparation method for afatinib
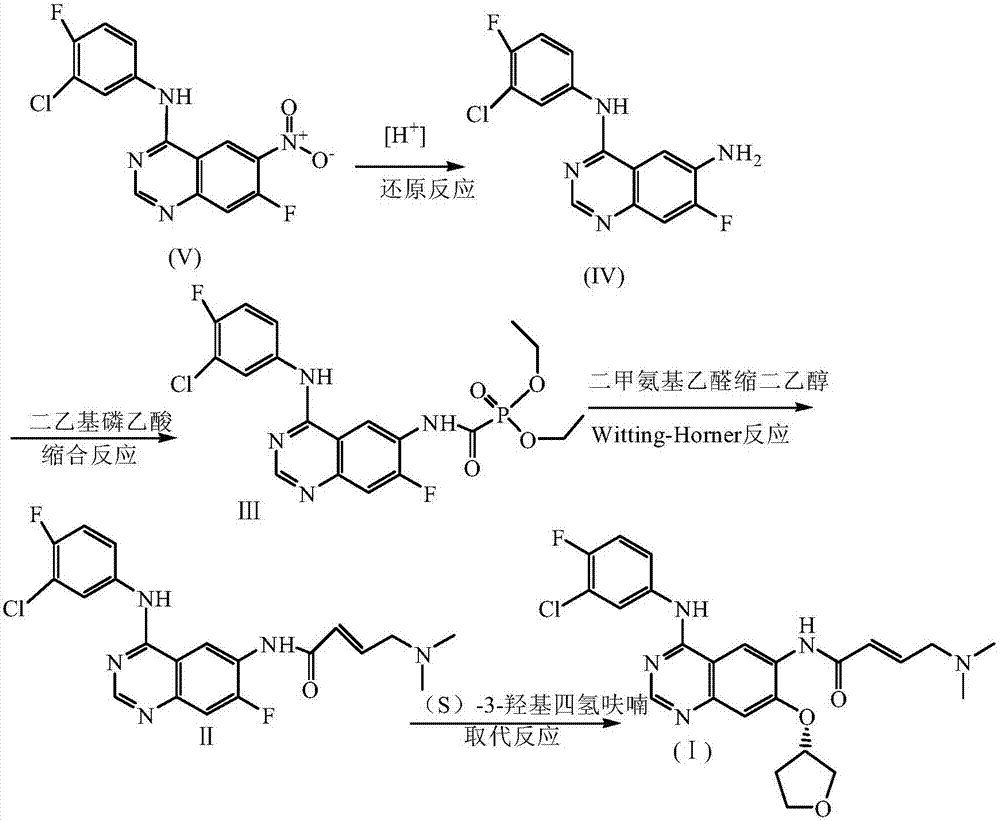
The invention discloses a novel method for preparing afatinib. The method comprises the following steps: reducing N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitro-4-quinazolinamine into N-(3-chloro-4-fluorophenyl)-7-fluoro-6-amino-4-quinazolinamine IV; then subjecting N-(3-chloro-4-fluorophenyl)-7-fluoro-6-amino-4-quinazolinamine IV and diethylphosphonoacetic acid to a condensation reaction so as to obtain a compound III; then subjecting the compound III and (dimethylamino)acetaldehyde diethyl acetal to a Wittig-Horner-Emmons reaction of so as to obtain a key intermediate II; and subjecting the key intermediate II and (S)-3-hydroxytetrahydrofuran to a substitution reaction so as to obtain a compound I. The method of the invention has high yield and high purity.
Owner:SHANDONG NEWTIME PHARMA
A kind of preparation method of afatinib
InactiveCN103288808BEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-HydroxytetrahydrofuranKetone
The invention discloses a preparation method of afatinib (I). The preparation method comprises the following steps that 6-amino-7-hydroxy-3,4-dihydroquinazoline-4-ketone (II) and (S)-3-hydroxytetrahydrofuran carry out etherification reaction to generate 6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]-3,4-dihydroquinazoline-4-ketone (III), the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride carry out acylation reaction to generate 6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]-3,4-dihydroquinazoline-4-ketone (IV), and the compound (IV) and 4-fluoro-3-chloroaniline carry out condensation reaction to prepare the afatinib (I). The preparation method is concise, economical and environment-friendly in process and is suitable for the requirement of industrial amplification.
Owner:鄄城县人民医院
Method for synthesizing (R)-3-amido tetrahydrofuran
A process for synthesizing (R)-3-aminotetrahydrofuran includes reaction between (S)-3-hydroxytetrahydrofuran and sulfonyl chloride under existance of 4-dimethylaminopyridine and triethylamine to generate sulfonate, reacting on sodium azide to generate (R)-3-azidotetrahydrofuran, and catalytic hydrogenation reaction.
Owner:XIAMEN UNIV
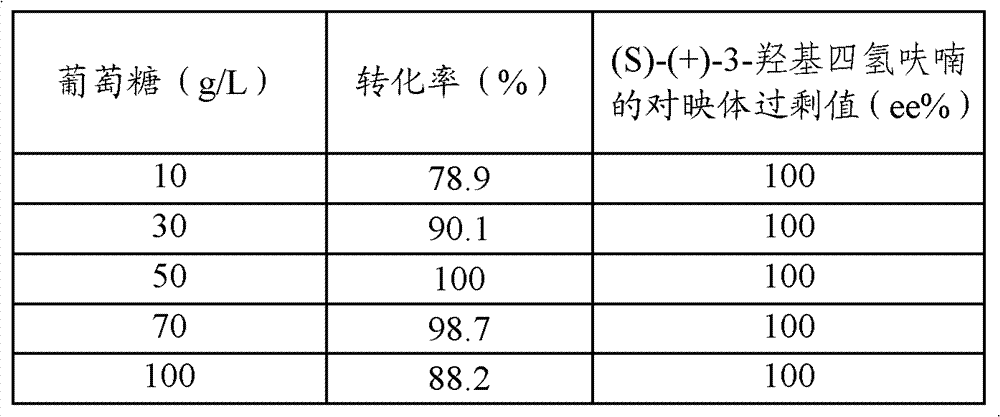
Method for preparing (S)-3-hydroxytetrahydrofuran based on erythritol microorganisms
InactiveCN105624227AEliminate drying and curingSimple stepsOrganic chemistryMicroorganism based processesDistillationFiltration
The invention provides a method for preparing (S)-3-hydroxytetrahydrofuran based on erythritol microorganisms. The method is characterized by comprising the steps that 1, a glucose solution is used as a raw material, candida lipolytica is used as a fermentation strain, enlarged cultivation and fermentation are performed to obtain crude erythritol, and the crude erythritol is subjected to decoloration, filtration and separation to obtain an erythritol solution; 2, the erythritol solution prepared in the step 1 is used as a raw material and put in a three-mouth flask, hydrogen is led into the flask, an acid solution is added into the flask, the solution is mixed for a reaction, then an alkaline solution is added for neutralization until the solution is neutral, then the reaction is stopped, and finally extraction and reduced pressure distillation are performed to obtain (S)-3-hydroxytetrahydrofuran. (S)-3-hydroxytetrahydrofuran is prepared after erythritol and hydrogen are subjected to a polymerization reaction in the acid environment, multiple processes such as erythritol drying and curing are omitted, the steps are simplified, the preparation method is simple and effective, the reaction is mild, cost is low, and technological production is facilitated.
Owner:ITIC MEDCHEM CO LTD
Enzymatic Preparation of Chiral 3-Hydroxytetrahydrofuran and Alcohol Dehydrogenase Mutants
ActiveCN106520714BReduce pollutionImprove efficiencyMicroorganism based processesOxidoreductasesAlcohol3-Hydroxytetrahydrofuran
The invention relates to a process for enzymatic preparation of chiral 3-hydroxytetrahydrofuran and an alcohol dehydrogenase mutant. The process comprises the a step of, with 3-ketotetrahydrofuran as a substrate, subjecting the substrate, the alcohol dehydrogenase mutant, a cofactor and a cofactor regeneration system to a biological catalysis reaction in a buffer system so as to produce chiral 3-hydroxytetrahydrofuran, wherein for alcohol dehydrogenase used by the alcohol dehydrogenase mutant, the wild gene sequence of alcohol dehydrogenase is derived from thermophilic bacteria, the base sequence is as shown in SEQ ID No. 1, and the amino acid sequence is as shown in SEQ ID No. 2. Compared with chemical methods, the process using the alcohol dehydrogenase mutant for preparation of chiral 3-hydroxytetrahydrofuran has the advantages of small environmental pollution, high efficiency, high optical selectivity; and compared with enzyme resolution methods, the process provided by the invention has higher purity and conversion rate and has ee value and conversion rate of both 99%.
Owner:洛阳华荣生物技术有限公司
Preparation method of (s)-3-hydroxytetrahydrofuran and (r)-3-hydroxytetrahydrofuran
The invention discloses a preparation method of (S)-3-hydroxytetrahydrofuran and (R)-3-hydroxytetrahydrofuran, relating to the technical field of preparation of five-element heterocyclic compounds containing one oxygen atom as the only heterocyclic atom. The method comprises the following steps: by using (S)-carnitine or (R)-carnitine as the initial raw material, carrying out reduction reaction in a reducer and an organic solvent to obtain (S) or (R)-2,4-dihydroxy-N,N,N-trimethyl butyl amine alkali; adding a hydrogen chloride organic solvent solution into an organic solvent to perform salification reaction to obtain (S) or (R)-2,4-dihydroxy-N,N,N-trimethyl butyl amine hydrochloride; and finally, adding alkali into a polar solvent, heating, and carrying out cyclization reaction to obtain the (S) or (R)-3-hydroxytetrahydrofuran. The method has the advantages of low cost, simple technique, high yield, cheap and accessible raw materials, short reaction steps, short period and low pollution, and is suitable for industrial production.
Owner:CANGZHOU SENARY CHEM SCI TEC
The preparation method of afatinib dimaleate
ActiveCN104710413BHigh yieldShort routeCarboxylic acid salt preparation3-HydroxytetrahydrofuranSubstitution reaction
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for preparing intermediate of Afatinib
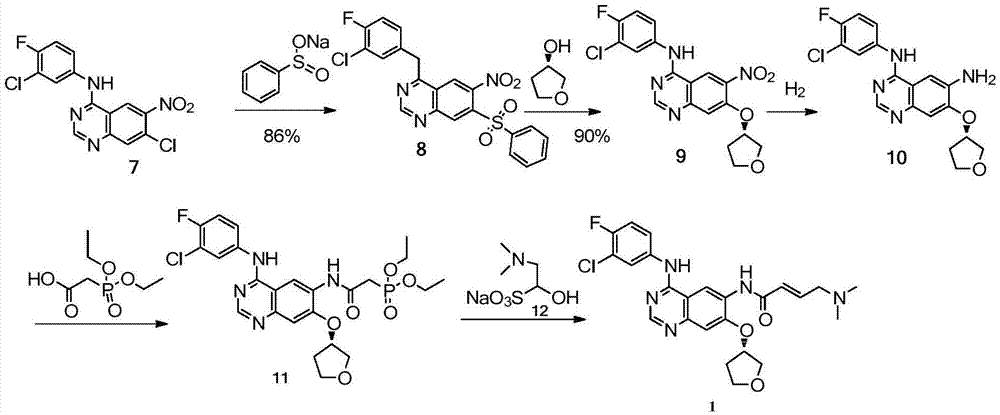
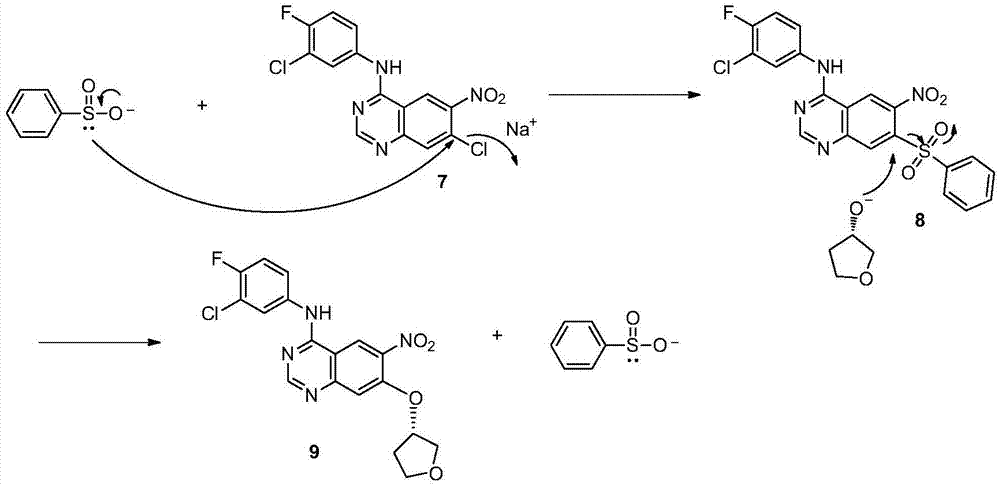
The invention discloses a method for preparing an intermediate of Afatinib. The method comprises the following step: subjecting a compound represented by a formula 7 shown in the description, sodium methanesulfonate and (S)-3-hydroxyl tetrahydrofuran to a reaction as follows in an organic solvent in the presence of alkali, thereby preparing a compound represented by a formula 9 shown in the description. The method disclosed by the invention is high in yield, simple in operation and low in cost and is more applicable to industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Method for preparing (s)-3-hydroxytetrahydrofuran
InactiveCN108047170AMild reaction conditionsEasy to separateOrganic chemistry methodsMethyl carbonateDistillation
The invention discloses a method for preparing (s)-3-hydroxytetrahydrofuran. The method comprises the following steps: mixing a prepared crude (s)-1,2,4-butanetriol with DMC (dimethyl carbonate); after uniform mixing, carrying out heating to 60-70 DEG C, adding a Na<2>CO<3> solution and carrying out a reaction for 3-4 h under stirring; and then carrying out pressure-reduced distillation to prepare(s)-3-hydroxytetrahydrofuran. The preparation method of the invention is low in requirements on operating conditions, high in safety, few in byproducts during the reaction, and high in product purityand yield.
Owner:ITIC MEDCHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com