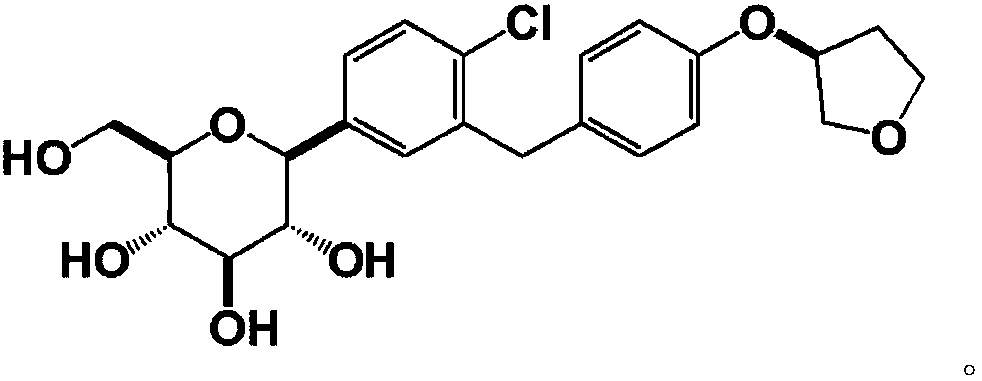
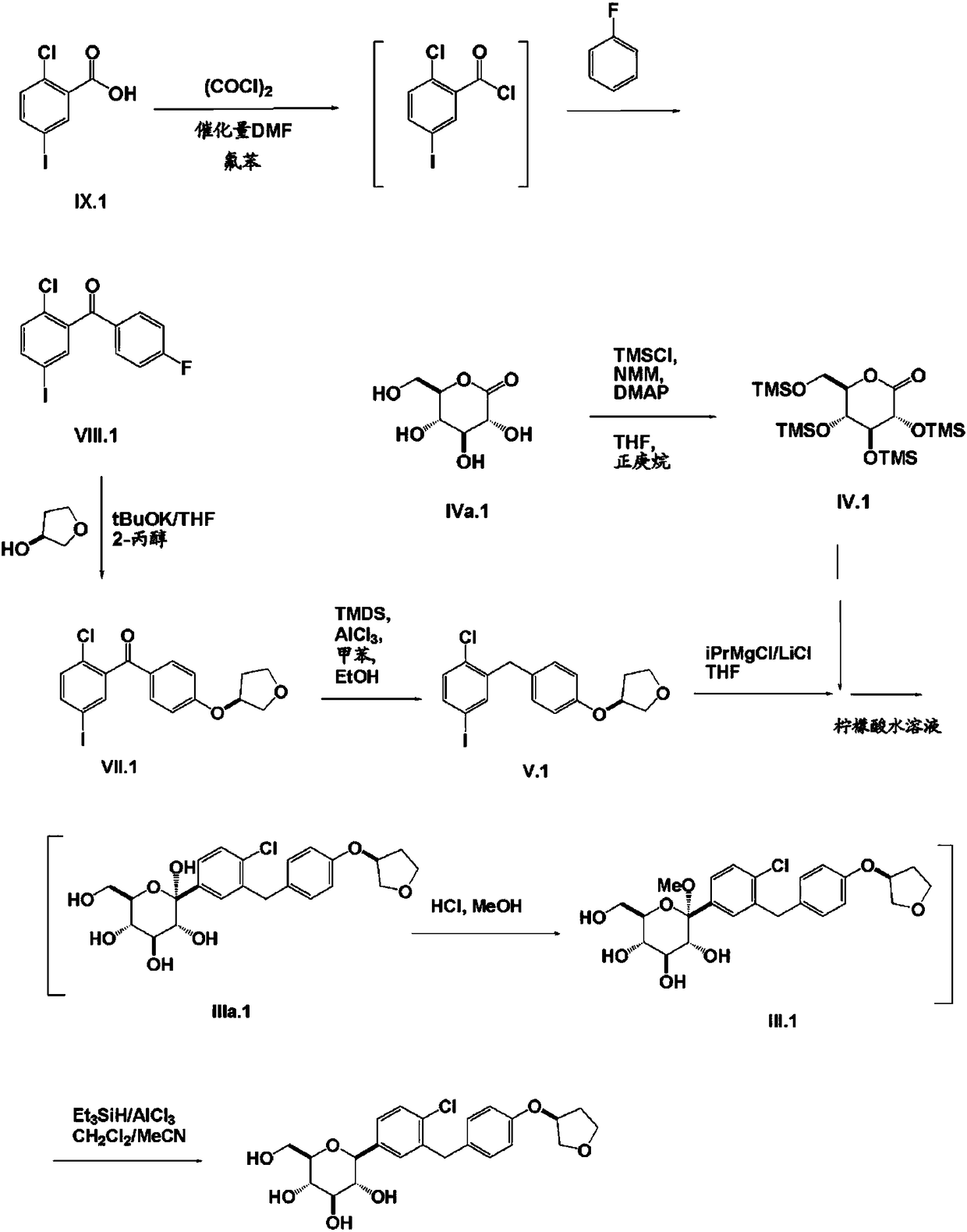
Synthesis method of Empagliflozin intermediate
A synthetic method and intermediate technology, applied in the field of synthesis of empagliflozin intermediate——-3-phenoxy)tetrahydrofuran, can solve the problems of short synthetic route and cumbersome post-treatment steps, and achieve easy reaction and post-processing. Simple processing and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Add 142.5g (1mol) of 4-hydroxybenzyl chloride and 200ml of dichloromethane into the reaction flask, and slowly add 126g (1.1mol) of methanesulfonyl chloride dropwise under temperature control at -5~0°C. Afterwards, the reaction was continued for 2 hours at -5 to 0°C. After the reaction was completed, dichloromethane was recovered by distillation under reduced pressure to obtain an oil; mol) and (S)-3-hydroxytetrahydrofuran 90g (1.03mol), heated and refluxed for 4h, after the reaction was completed, the tetrahydrofuran was recovered by distillation under reduced pressure, 200ml of ethyl acetate and 200ml of water were added, and the pH was adjusted to 4-5 with concentrated hydrochloric acid. Stand still, separate layers, and extract the aqueous phase once with 100 ml of ethyl acetate; the combined organic phases are washed once with water, dried over anhydrous sodium sulfate, and filtered to obtain a feed solution containing the above-mentioned compound III.
[0035] ...
Embodiment 2
[0038]1) Add 142.5g of 4-hydroxybenzyl chloride and 200ml of dichloromethane into the reaction bottle, and slowly add 126g of methanesulfonyl chloride dropwise under temperature control at -5~0°C. React at 0°C for 2 hours. After the reaction is completed, distill under reduced pressure to obtain an oily substance; add 200ml of tetrahydrofuran to dissolve the above oily substance, then add 160g of N,N-diisopropylethylamine and 91g of (S)-3-hydroxytetrahydrofuran, and heat Reflux the reaction for 5 hours. After the reaction is completed, distill under reduced pressure to recover tetrahydrofuran, add 200ml ethyl acetate and 200ml water, adjust the pH to 4-5 with concentrated hydrochloric acid, let it stand, separate layers, and extract the water phase once with 100ml ethyl acetate; The combined organic phases were washed once with water, dried over anhydrous sodium sulfate, and filtered to obtain a feed solution containing the above compound III.
[0039] 2) Add 219g of 4-iodoani...
Embodiment 3
[0041] 1) Add 142.5g of 4-hydroxybenzyl chloride and 200ml of dichloromethane into the reaction bottle, and slowly add 126g of methanesulfonyl chloride dropwise under temperature control at -5~0°C. React at ℃ for 2 hours. After the reaction is completed, the dichloromethane is distilled under reduced pressure to obtain an oily substance; the above oily substance is dissolved in 200ml of tetrahydrofuran, and then 155g of N,N-diisopropylethylamine and (S)-3-hydroxytetrahydrofuran are added. 90g, heated to reflux for 4.5h, after the reaction was completed, the tetrahydrofuran was recovered by distillation under reduced pressure, 150ml of dichloromethane (using the recovered dichloromethane above) and 150ml of water were added, and the pH was adjusted to 4~5 with concentrated hydrochloric acid. , the aqueous phase was extracted once with 100ml of dichloromethane; the combined organic phases were washed once with water, dried over anhydrous sodium sulfate, filtered, and the filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com