Preparation method of empagliflozin intermediate
A technology of empagliflozin and intermediates, which is applied in the field of preparation of empagliflozin intermediates, can solve the problems of high cost, high price, and difficult synthesis of starting materials, and achieve short synthetic routes and high product purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be further described below through the examples, but the examples do not limit the protection scope of the present invention.
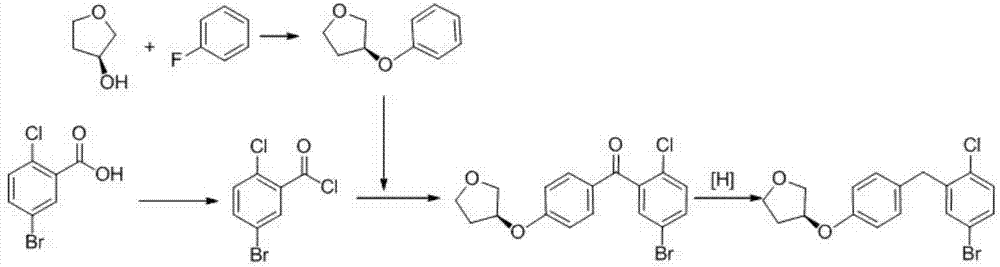
[0027] 1. Preparation of (S)-3-p-cresyl tetrahydrofuran
[0028] The reaction chemical formula is:
[0029]
[0030] Take 110kg of 4-fluorotoluene and 95kg of (R)-3-hydroxytetrahydrofuran, dissolve them in 300kg of tetrahydrofuran, cool to 0° in an ice bath, and add dropwise a tetrahydrofuran solution of potassium tert-butoxide (potassium tert-butoxide 112kg, Tetrahydrofuran (200kg) was dripped in 30 minutes. After the addition was completed, react at 5-10° for 1 hour. After the reaction, add 300kg of water to quench the reaction, distill and recover THF, add 400kg of ethyl acetate to the residual liquid for extraction, dry over anhydrous sodium sulfate, and filter , the solvent was recovered from the filtrate, and the residue was distilled under reduced pressure to obtain 162 kg of light yellow liquid, with a yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com