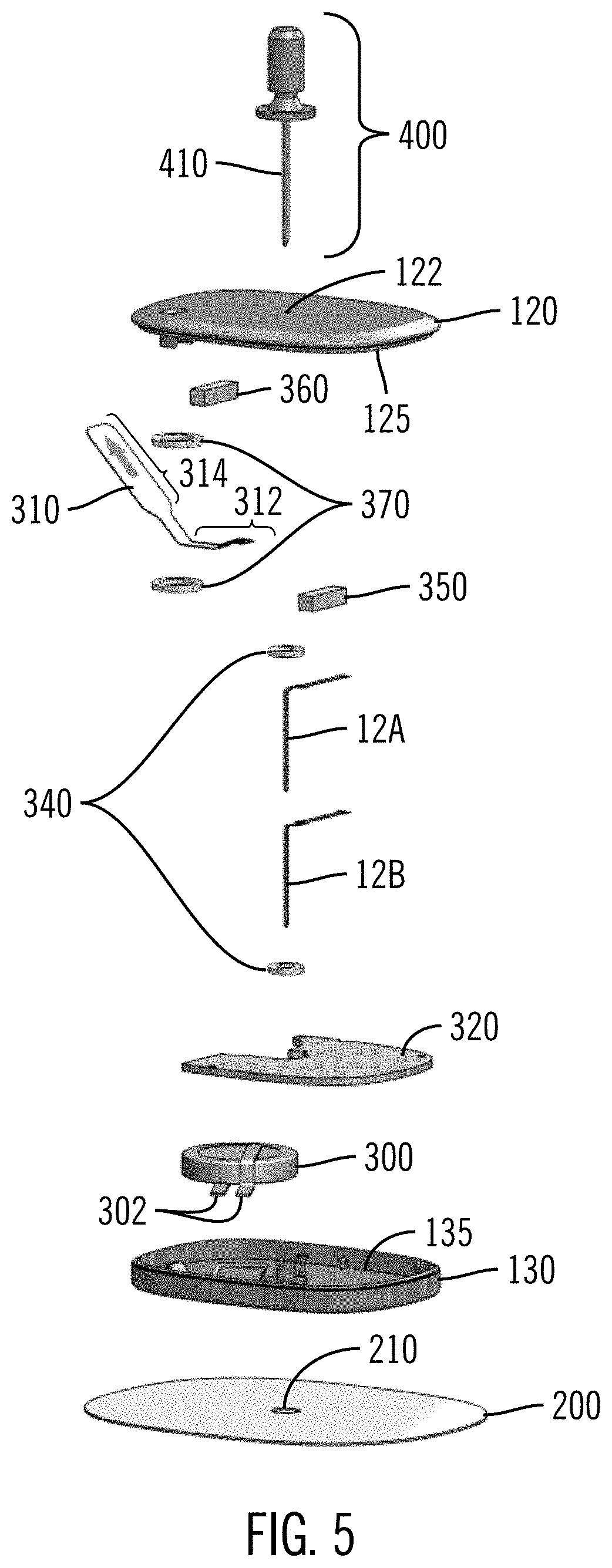
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45results about How to "Avoid premature release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
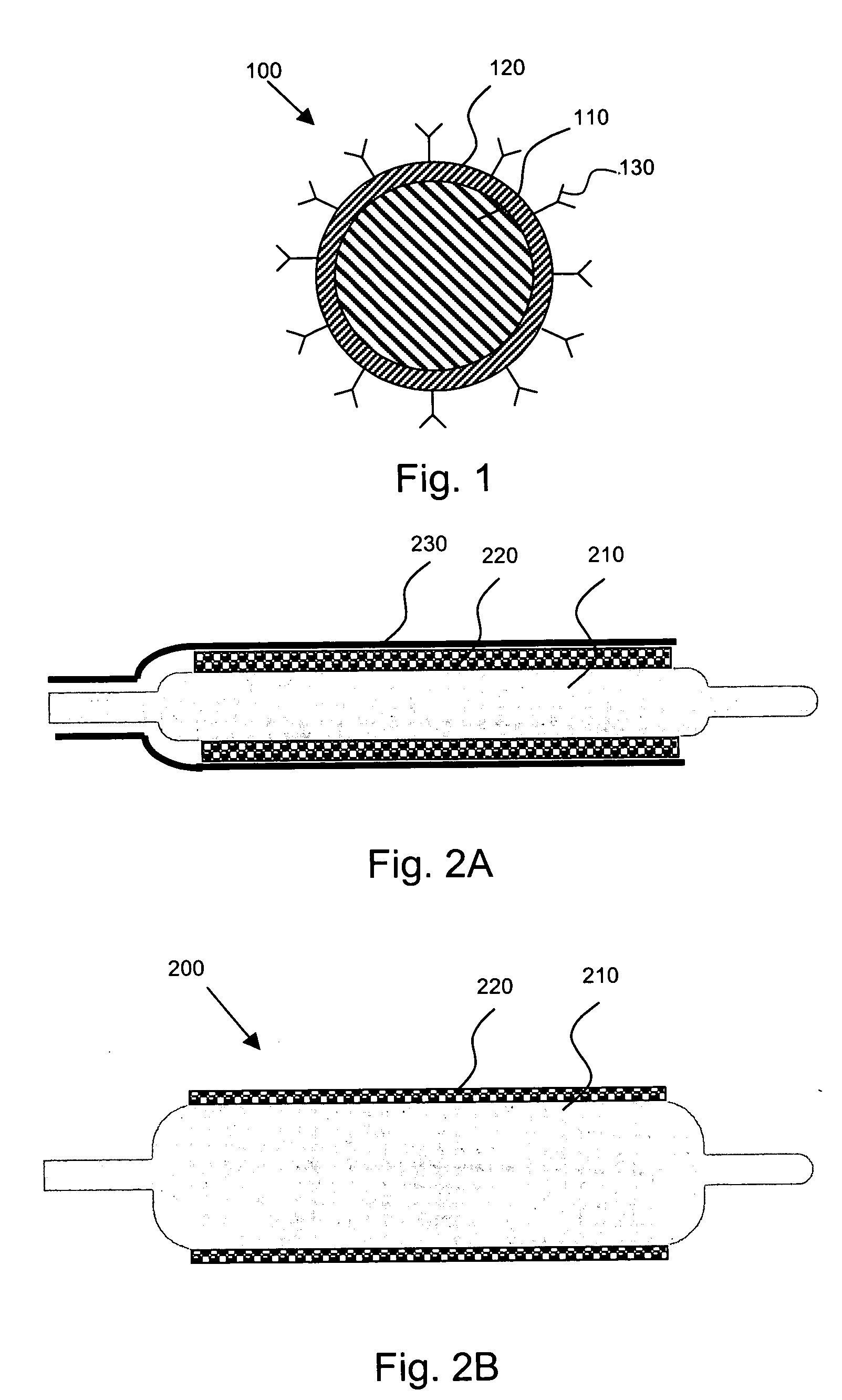
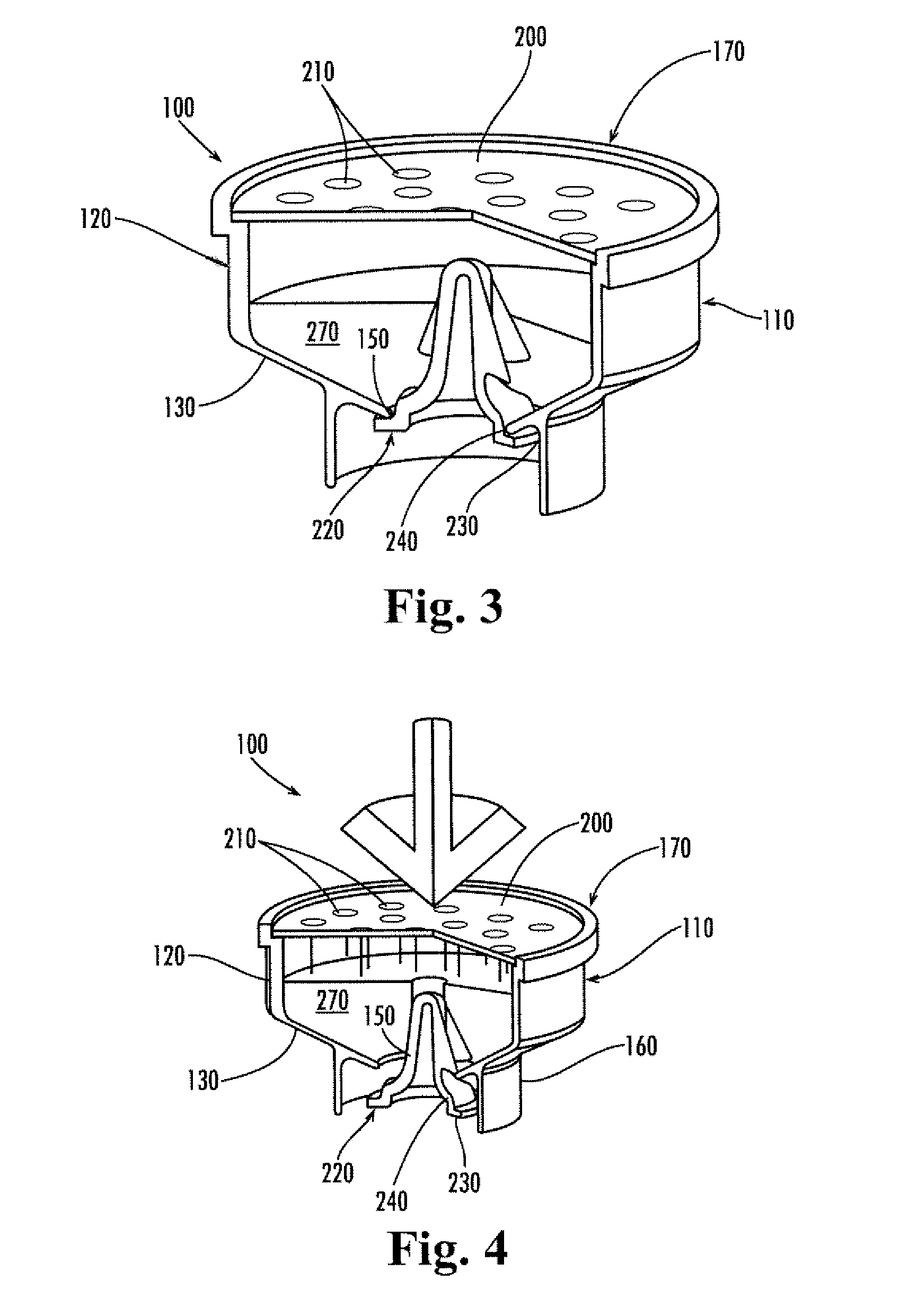
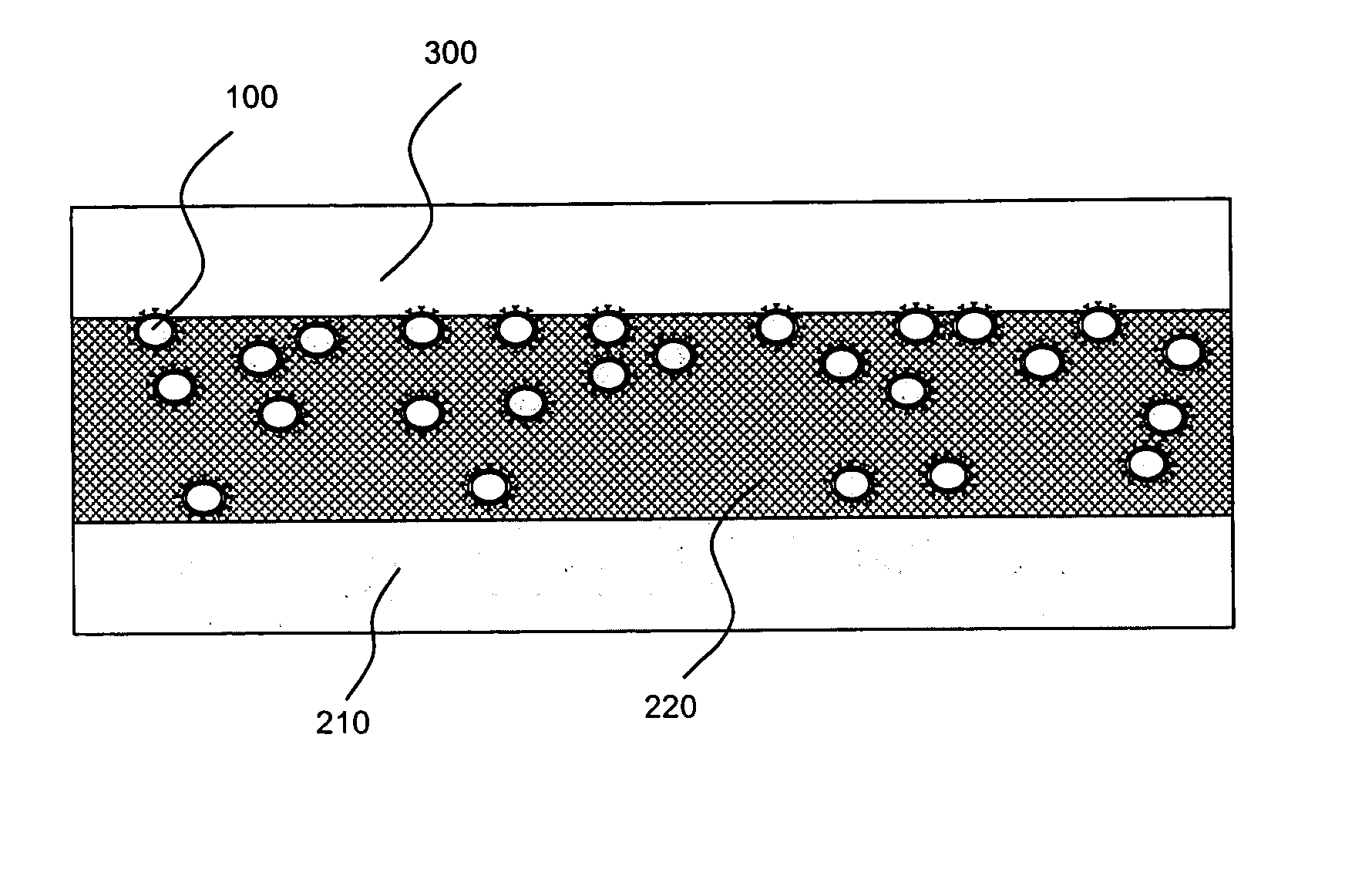
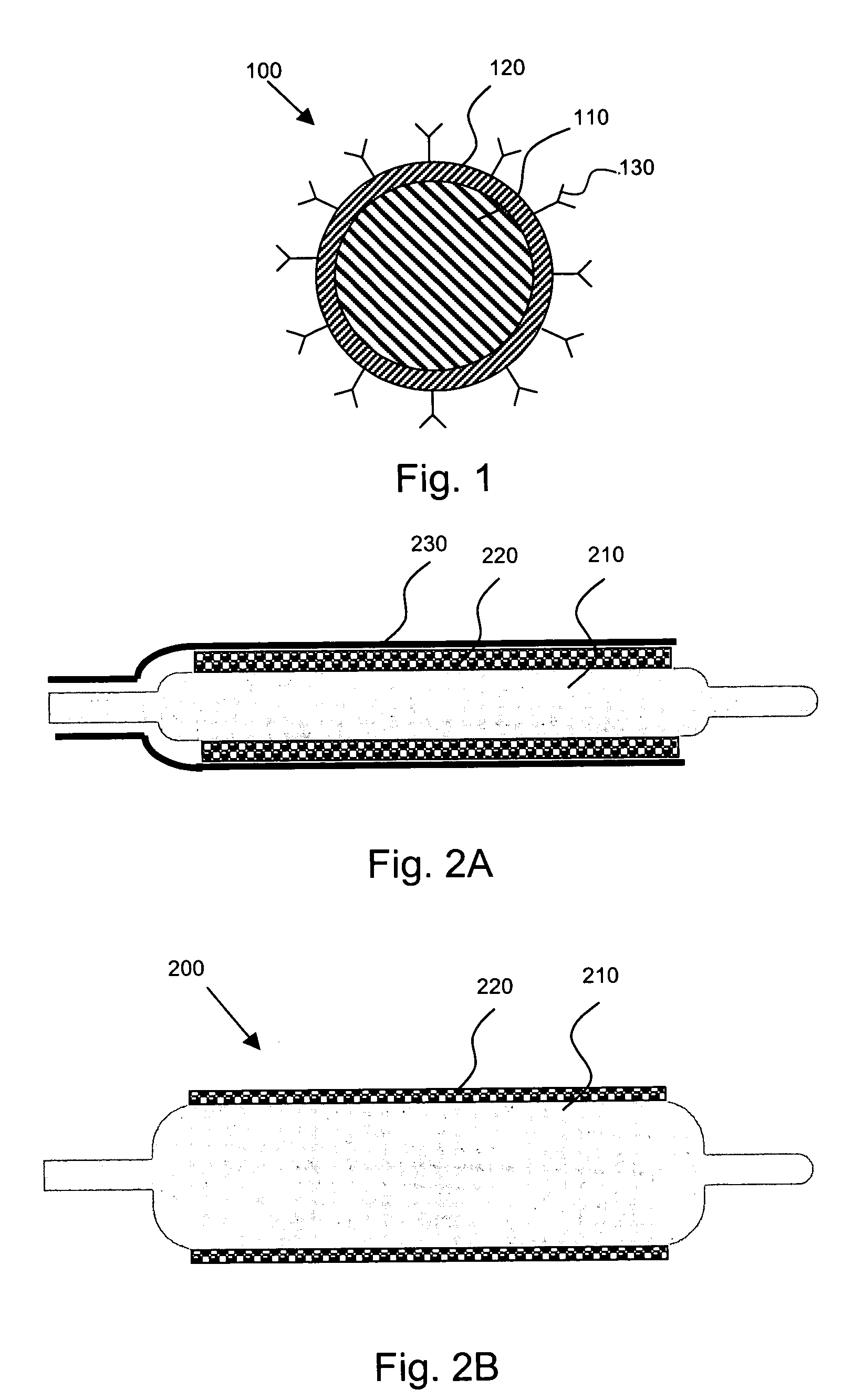
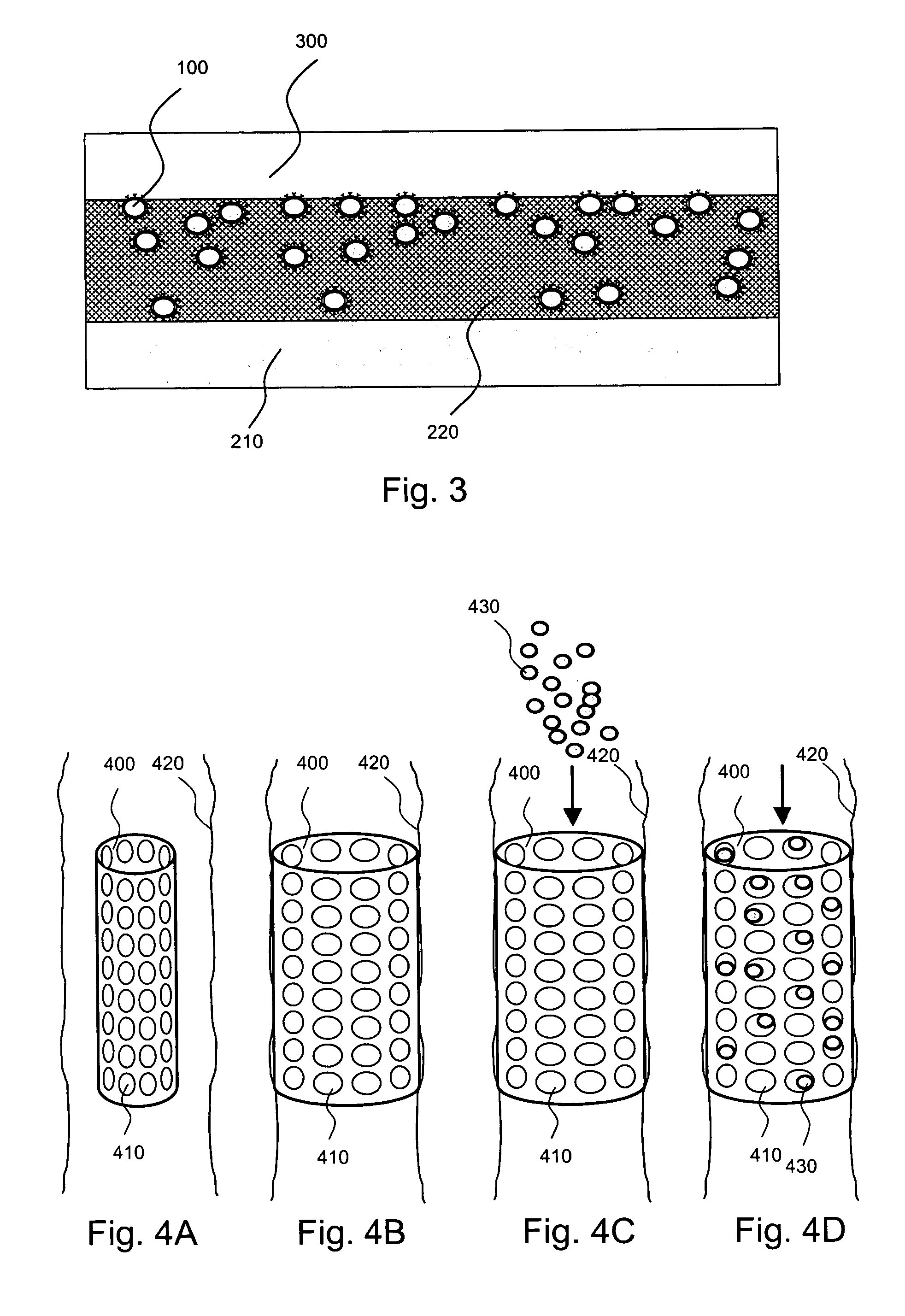
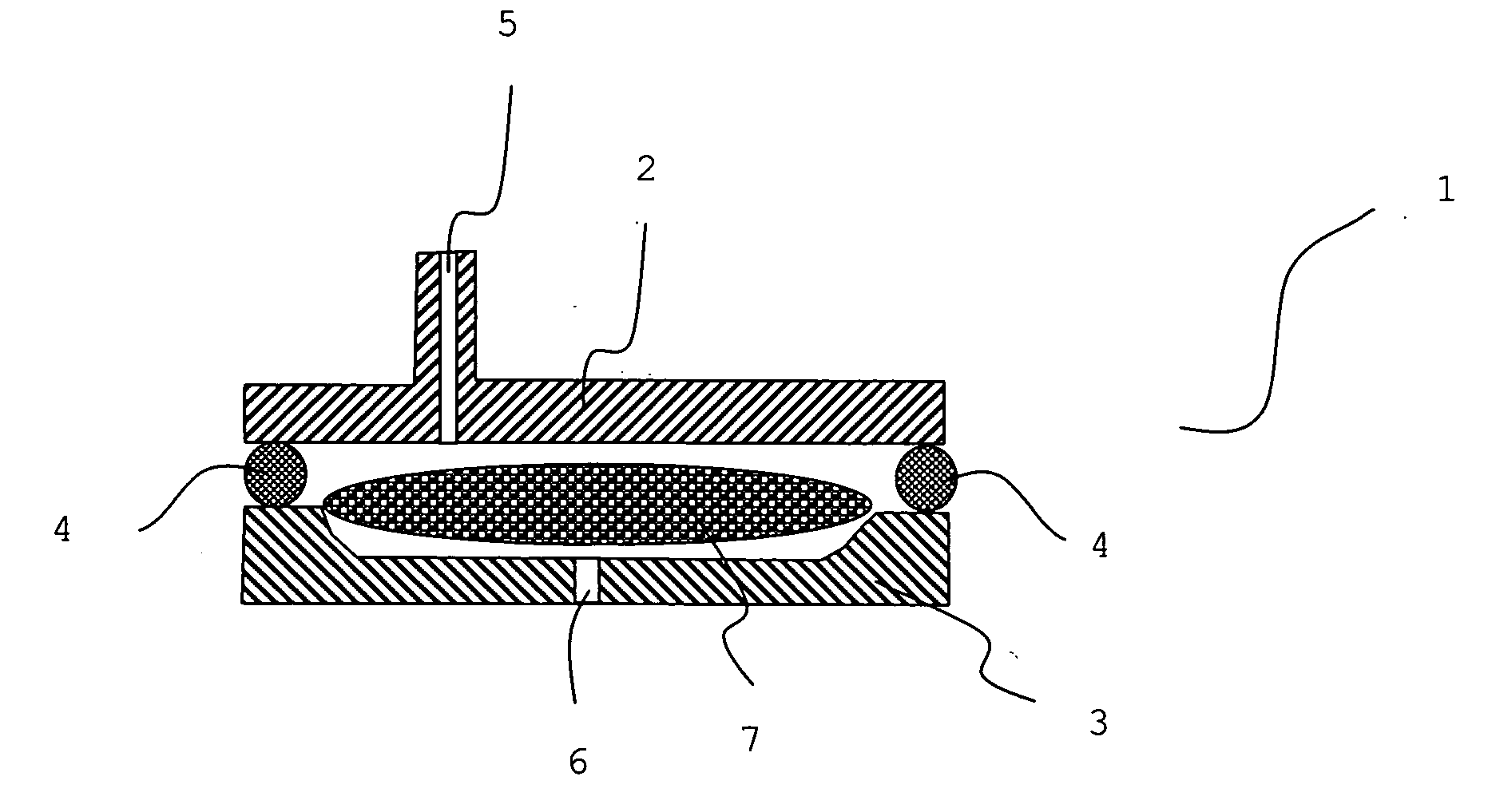
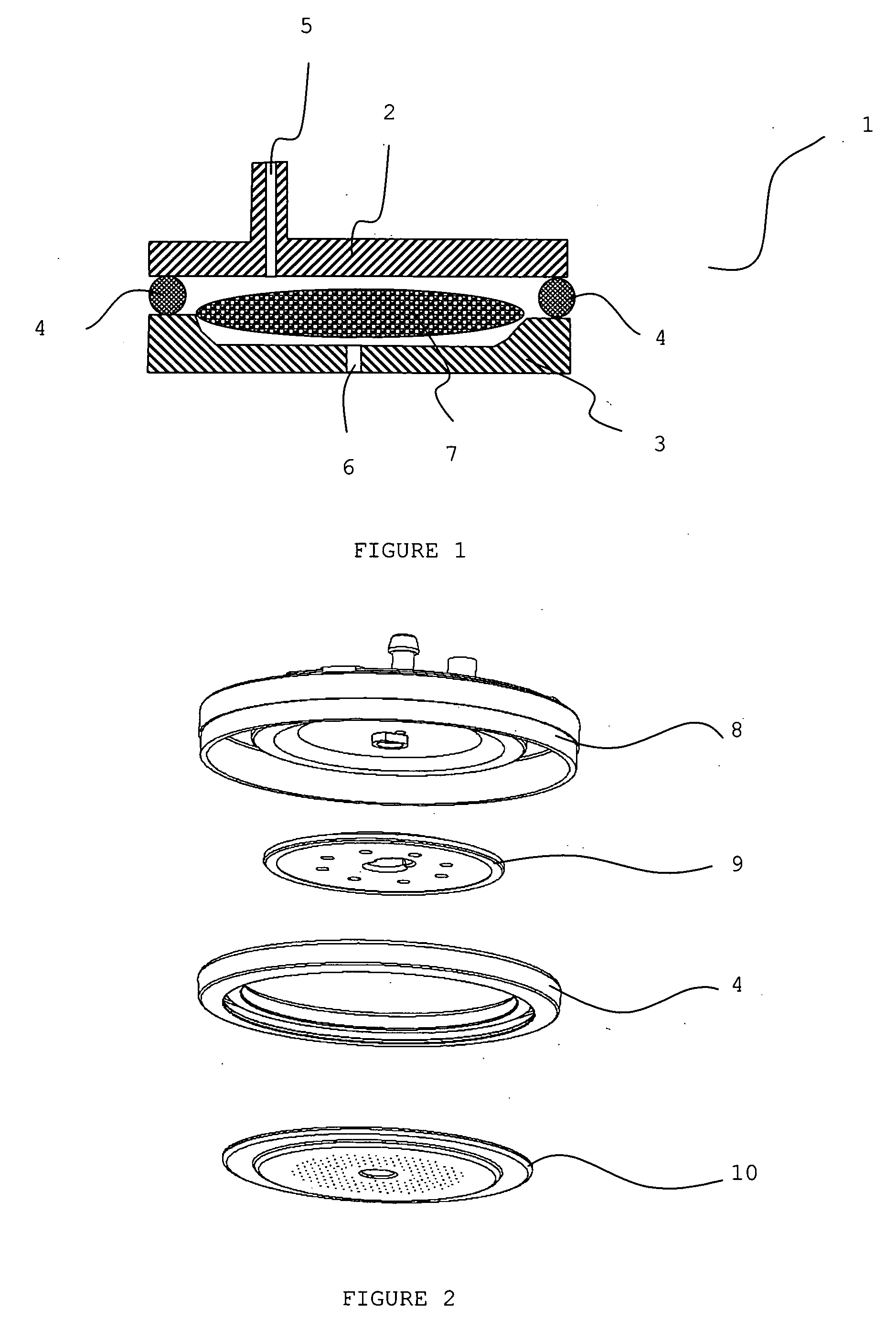
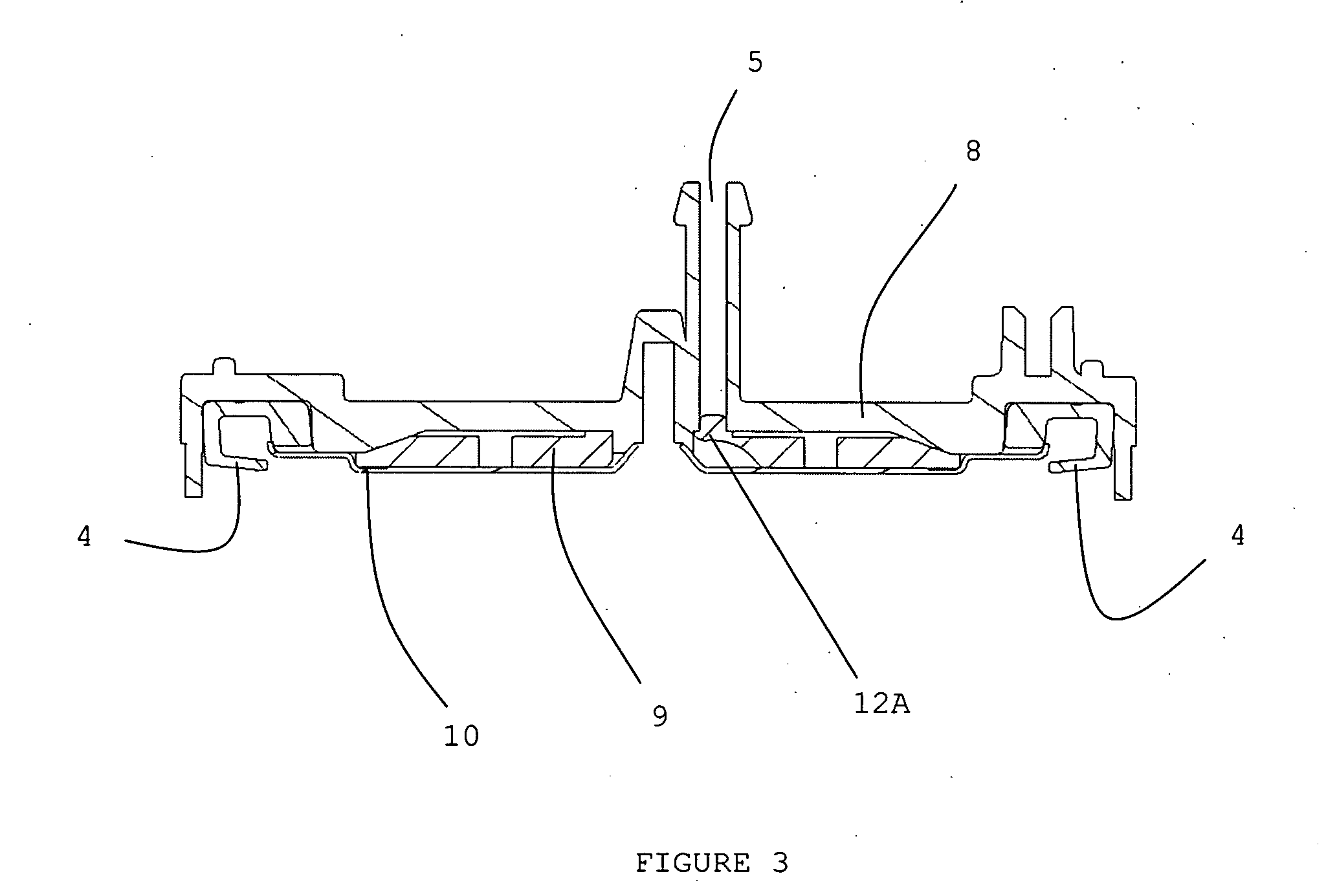
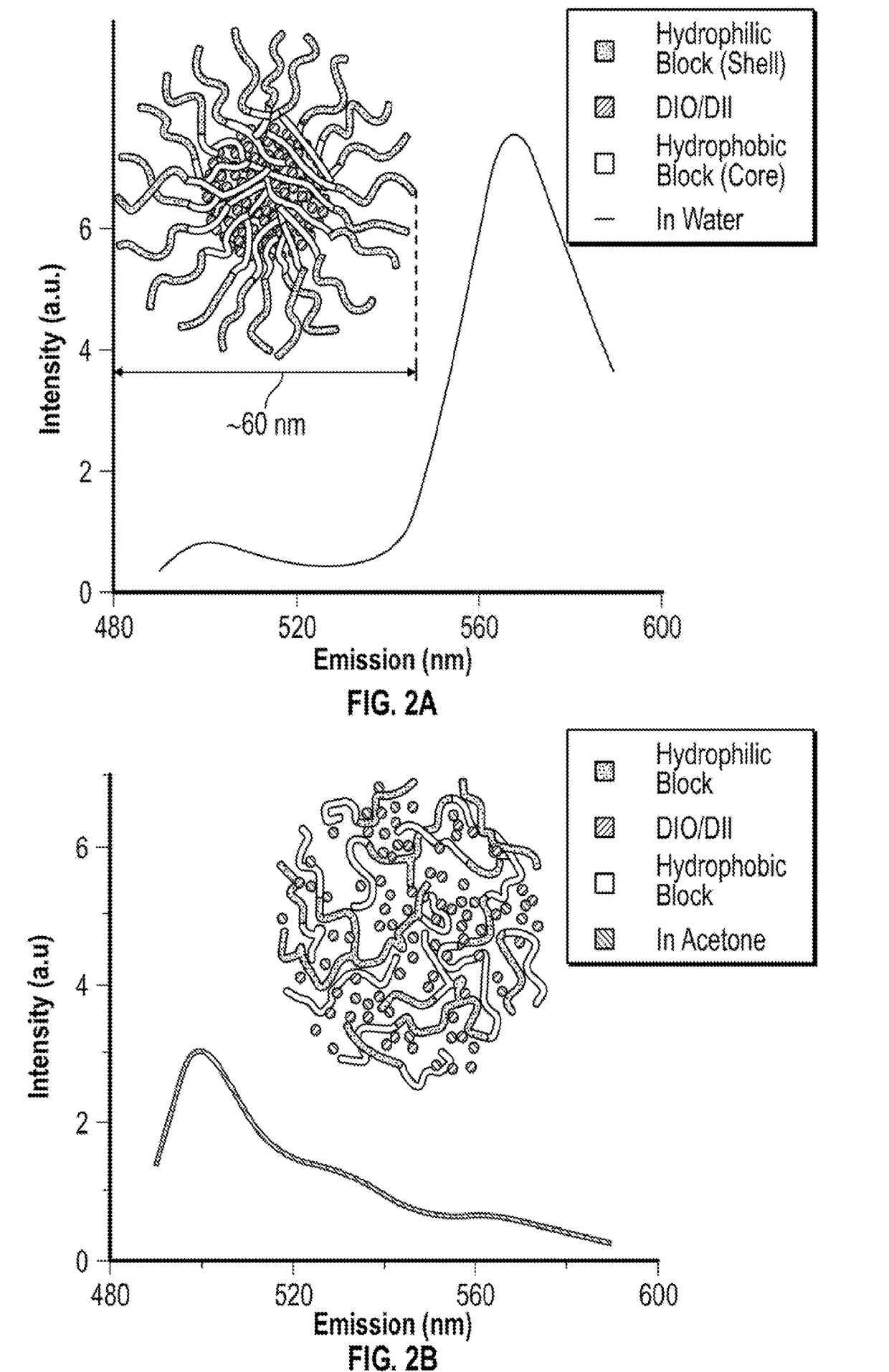
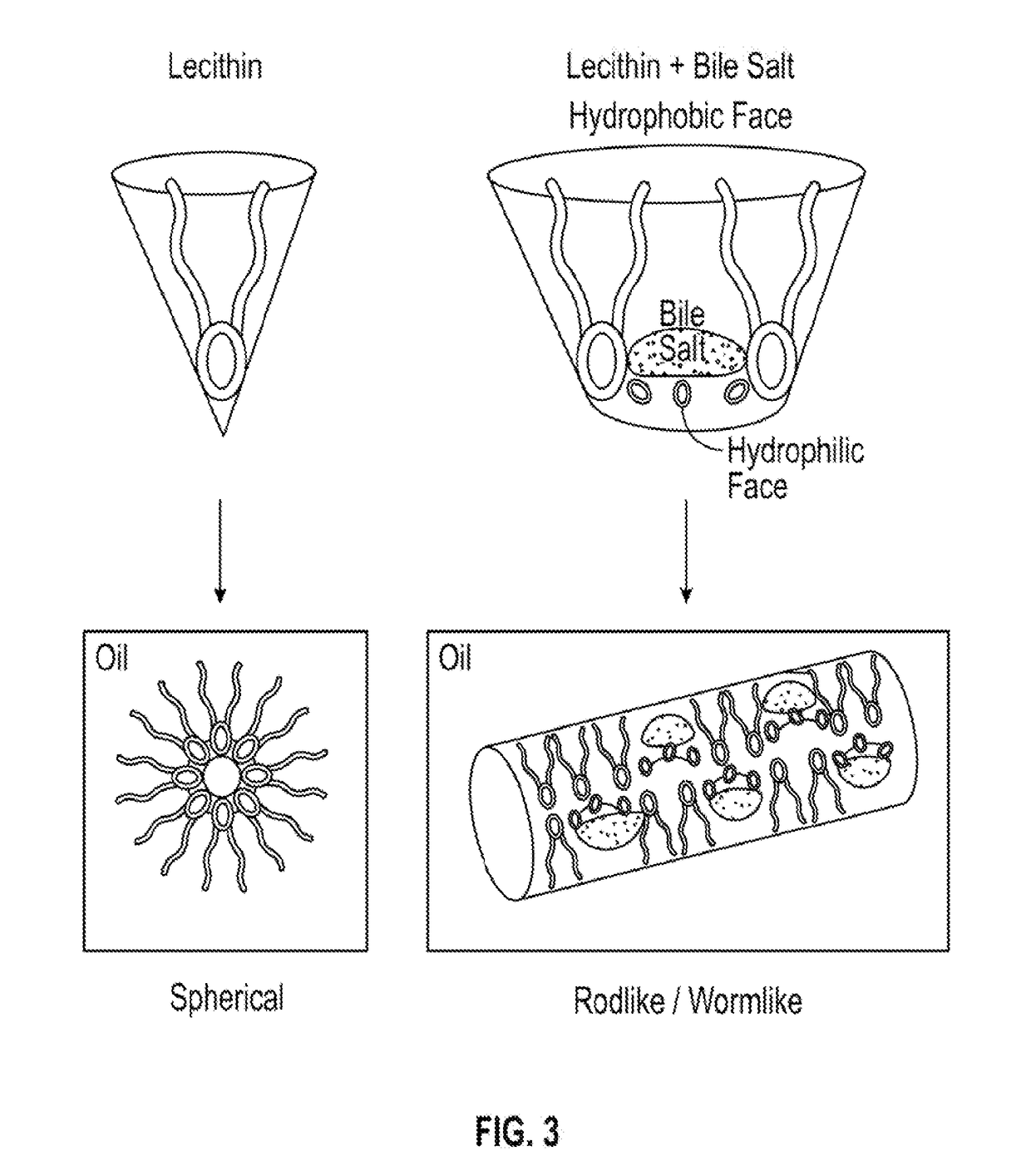
Localized drug delivery using drug-loaded nanocapsules
InactiveUS20050129727A1Prevents premature releaseLimit systemic effectPowder deliveryElectrotherapyDrugDrug delivery
Nanocapsules are disclosed which comprise (a) a drug-containing core and (b) a polyelectrolyte multilayer encapsulating the drug-containing core. The nanocapsules include particles whose largest dimension typically ranges between 50 nm to 10000 nm. In some embodiments, the nanocapsules contain a single drug. In others, the nanocapsules contain multiple drugs, either within the same nanocapsules or within separate populations of nanocapsules. In some embodiments, the nanocapsules comprise surfaces that are functionalized, for example, with ligands that allow for attachment to bodily tissue. In some embodiments of the present invention, the nanocapsules are rendered magnetic or are rendered susceptible to magnetic fields. Also disclosed is a drug delivery method that comprises the steps of (a) providing nanocapsules such as those above; and (b) placing the nanocapsules at a desired location within the body of a subject using an implantable or insertable medical device.
Owner:BOSTON SCI SCIMED INC
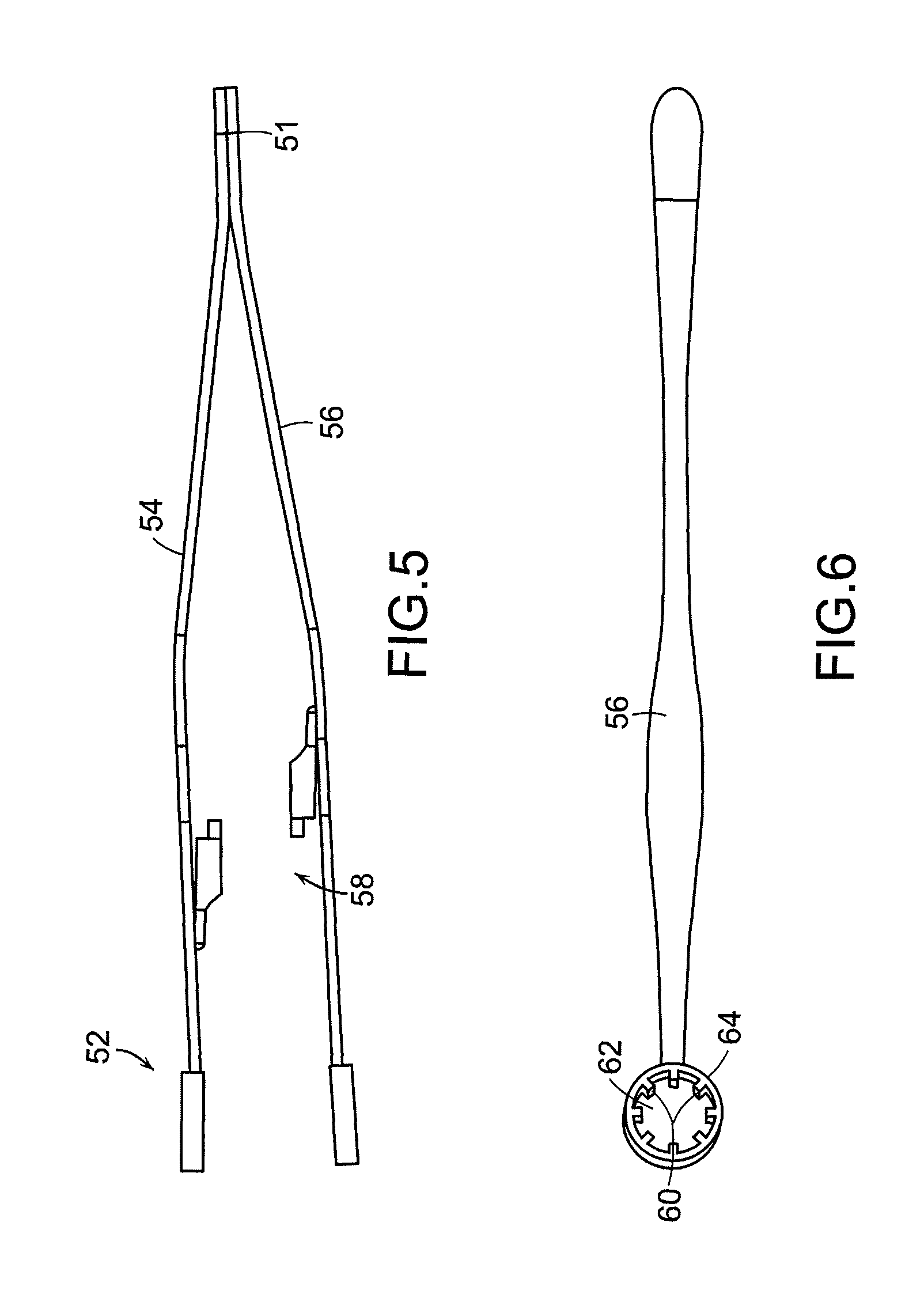
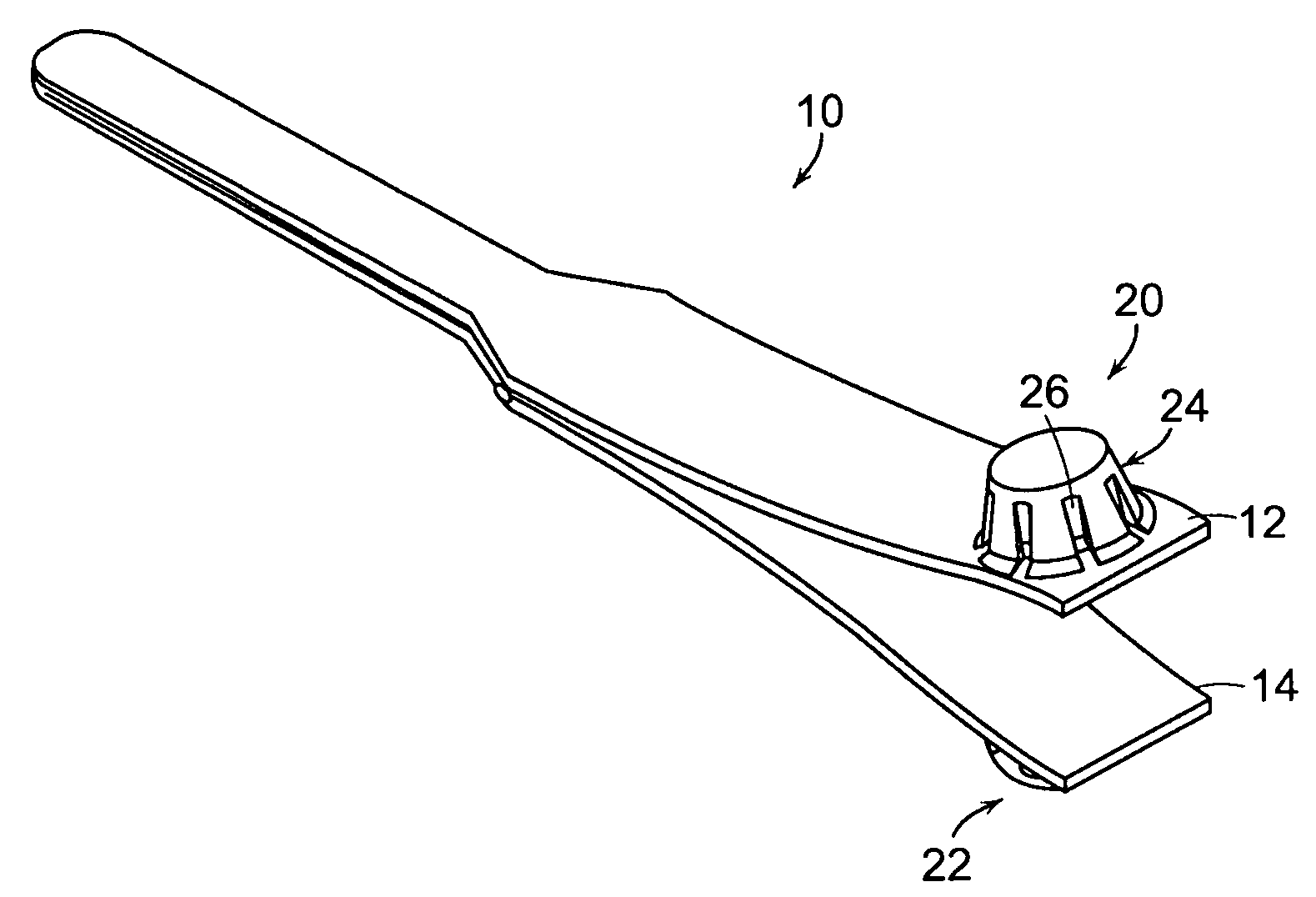
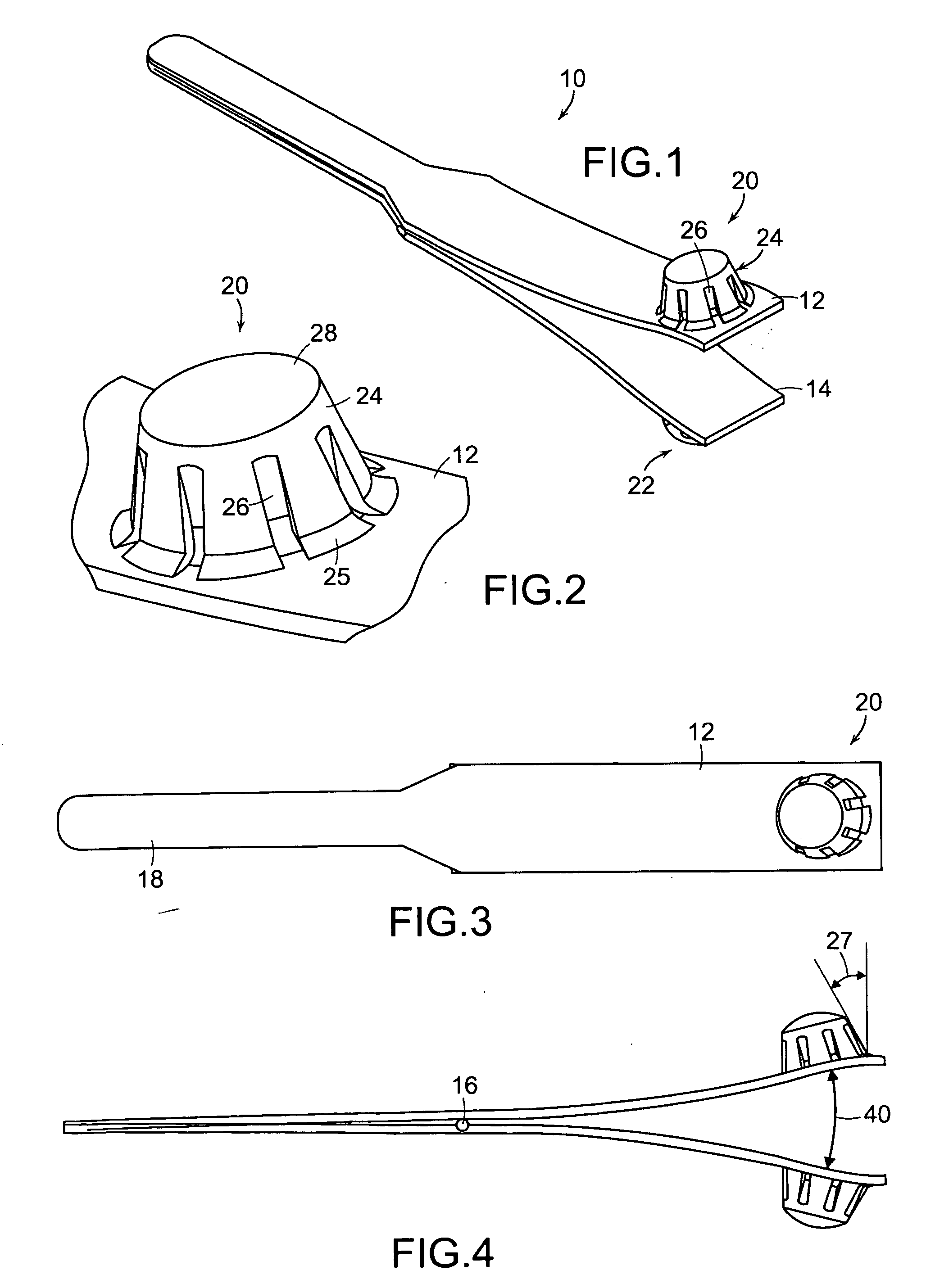
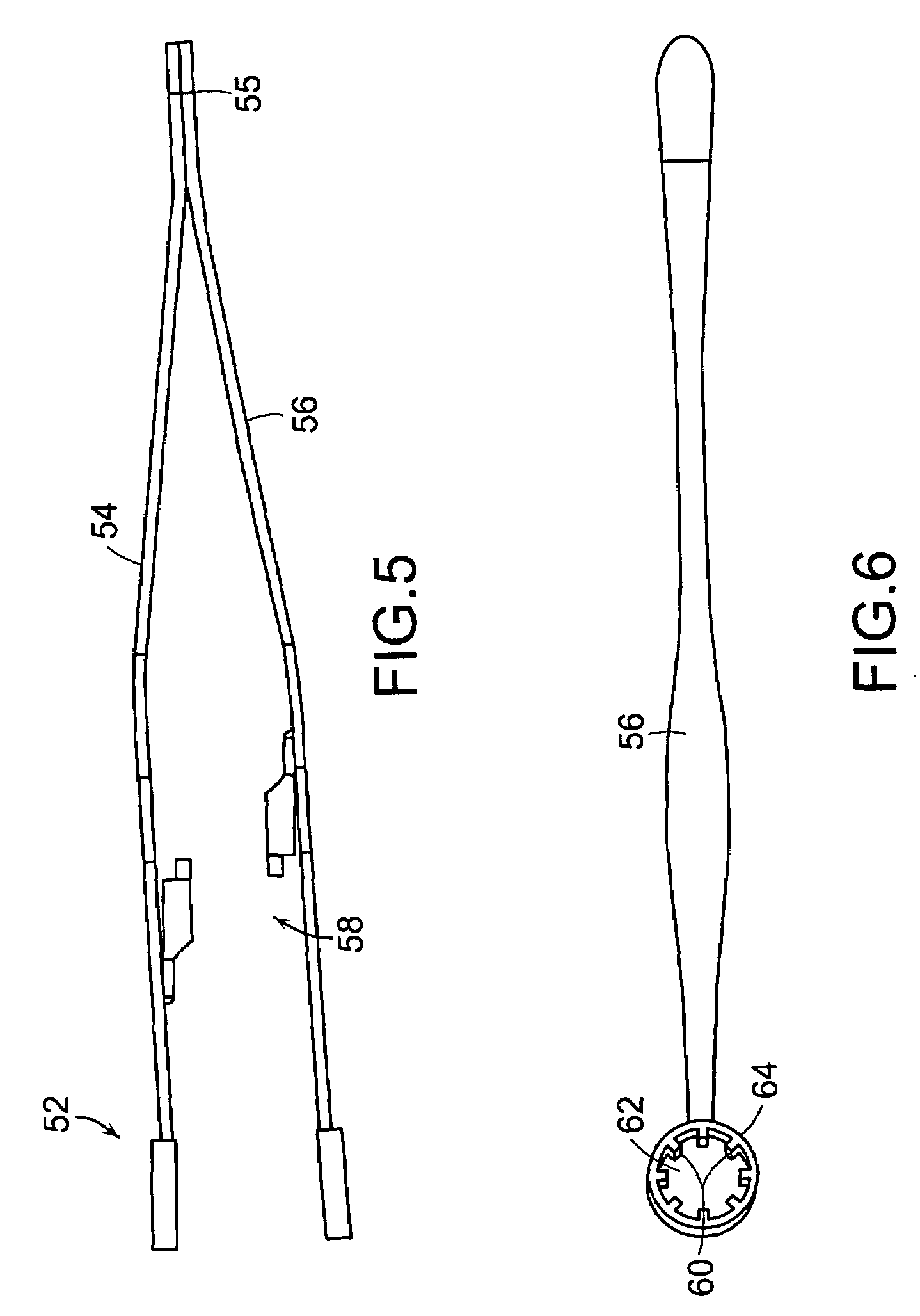
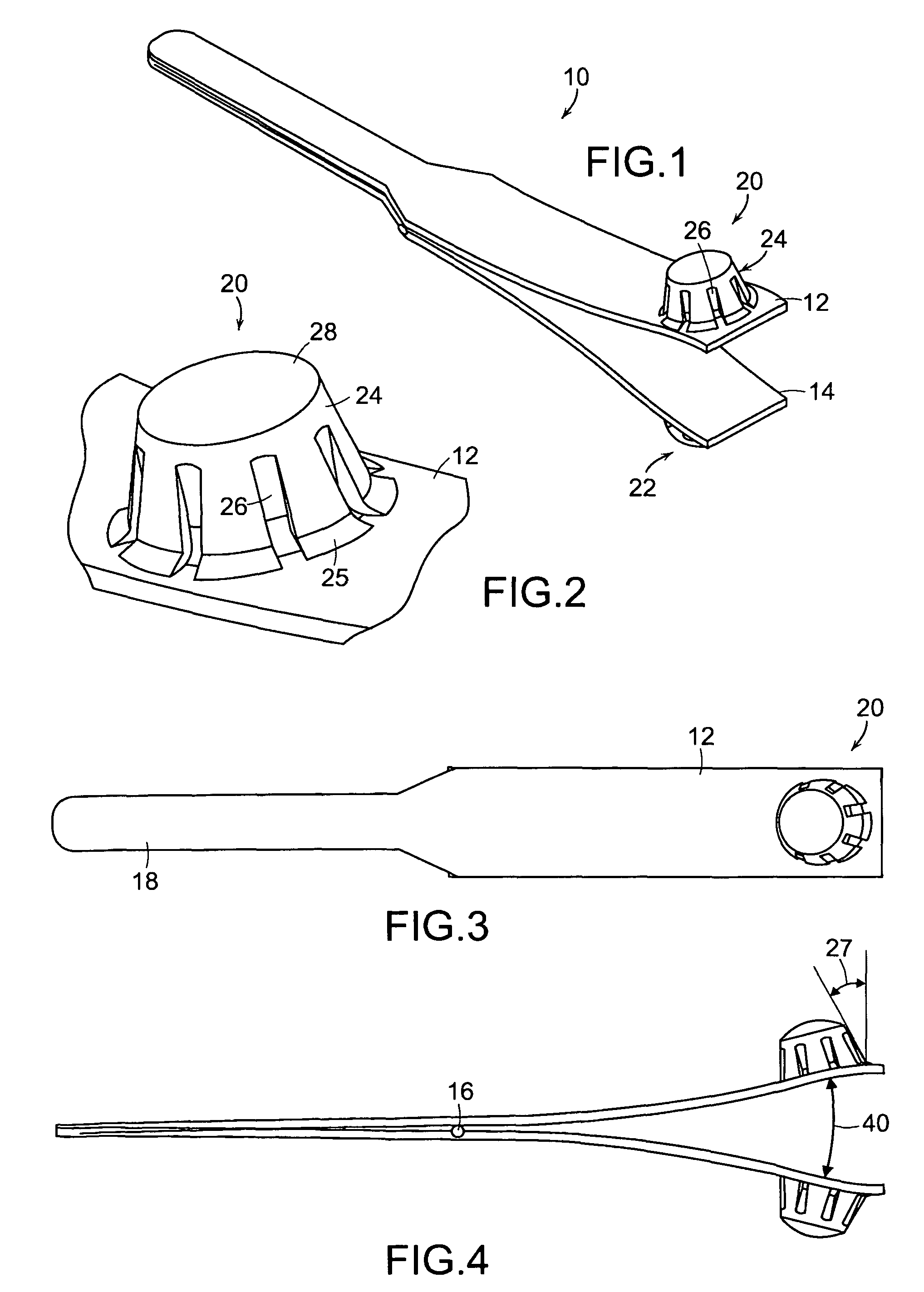
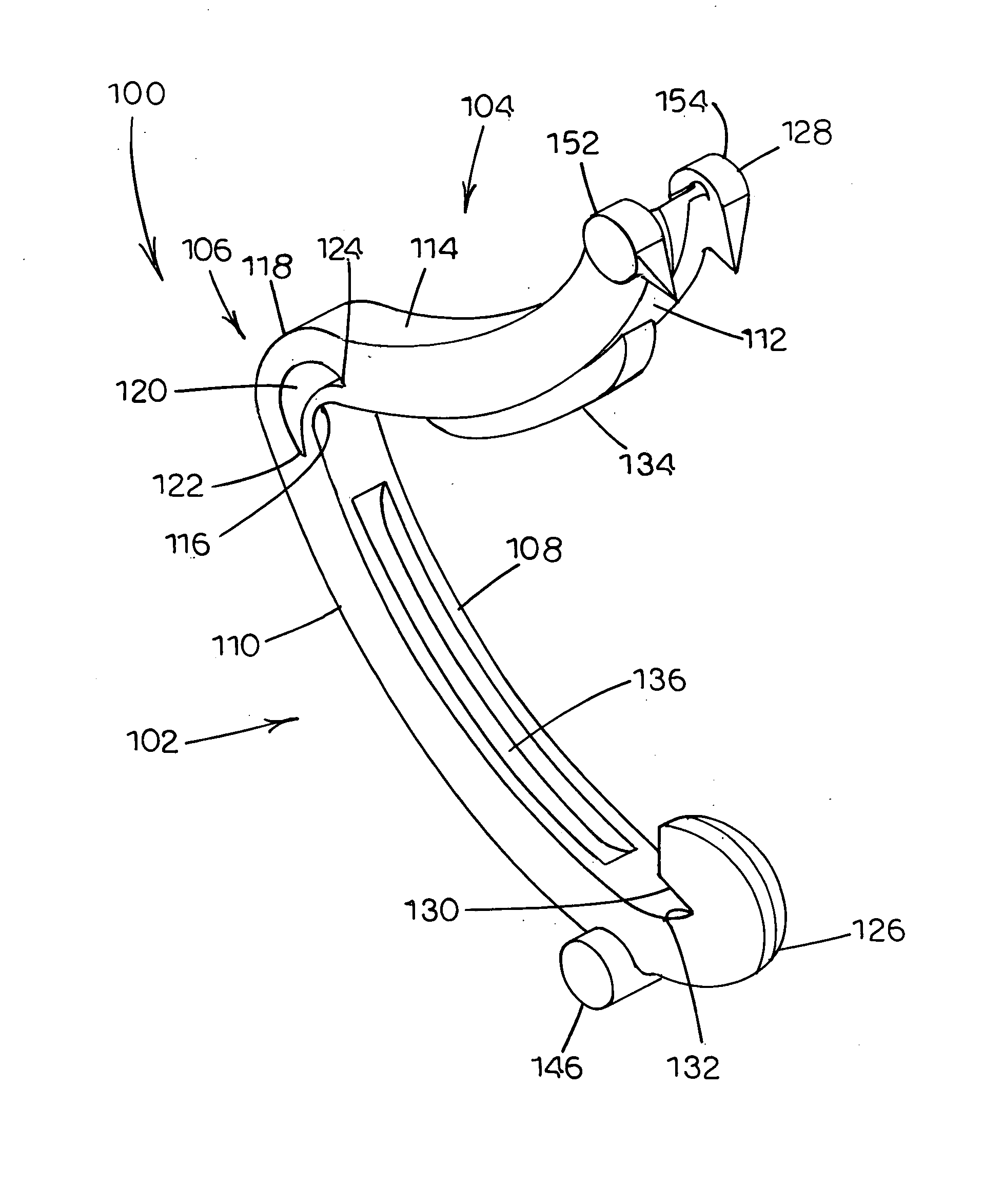
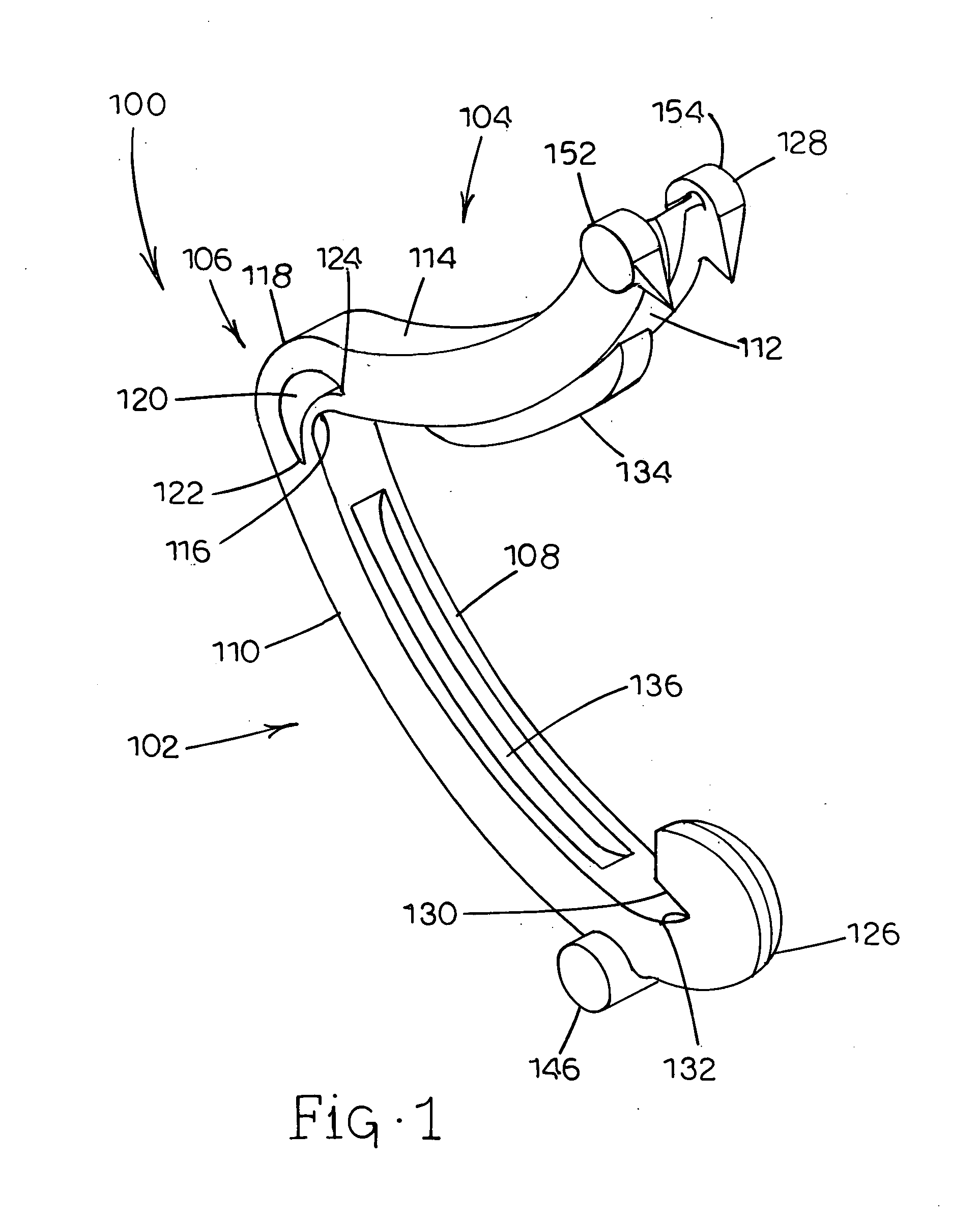
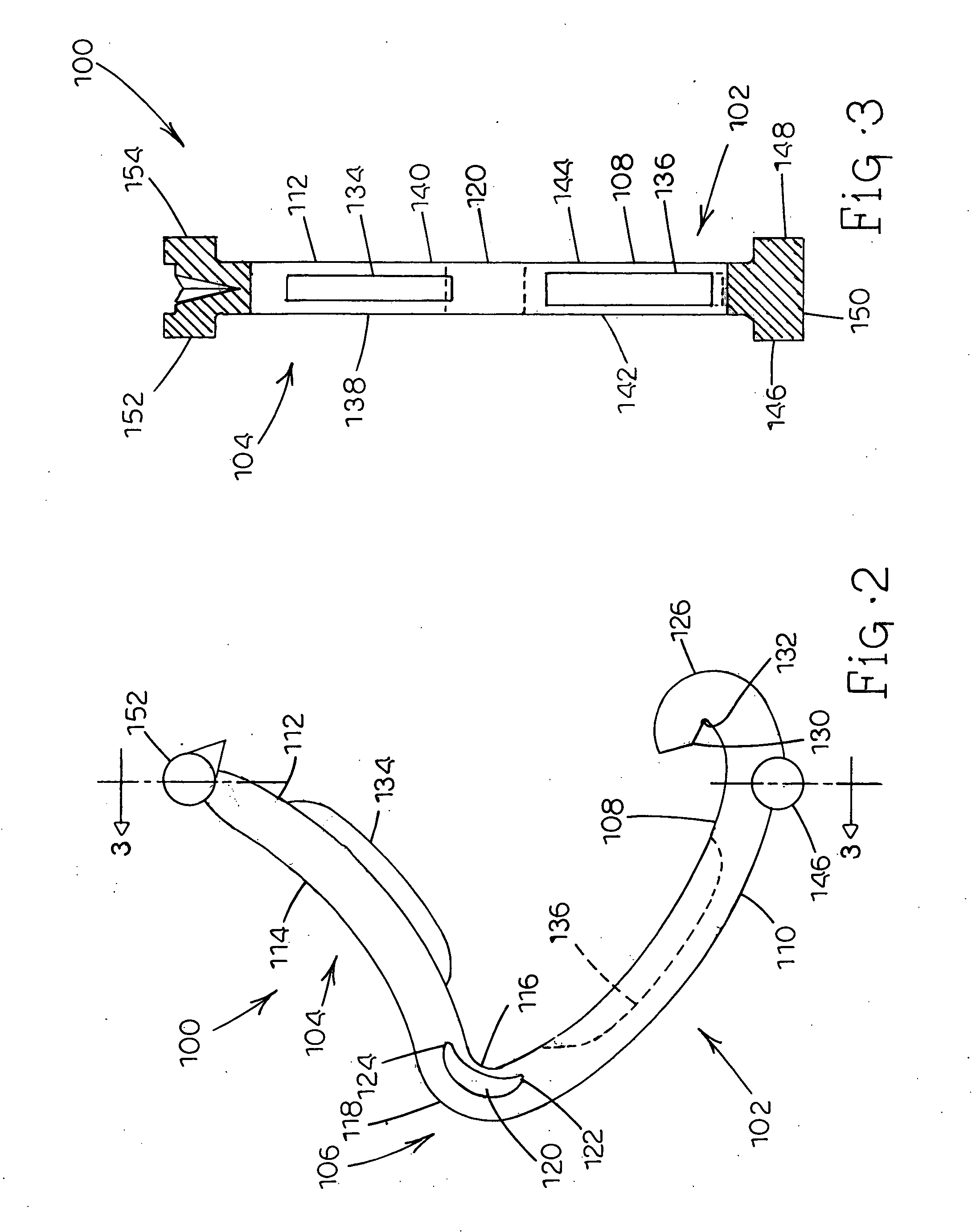
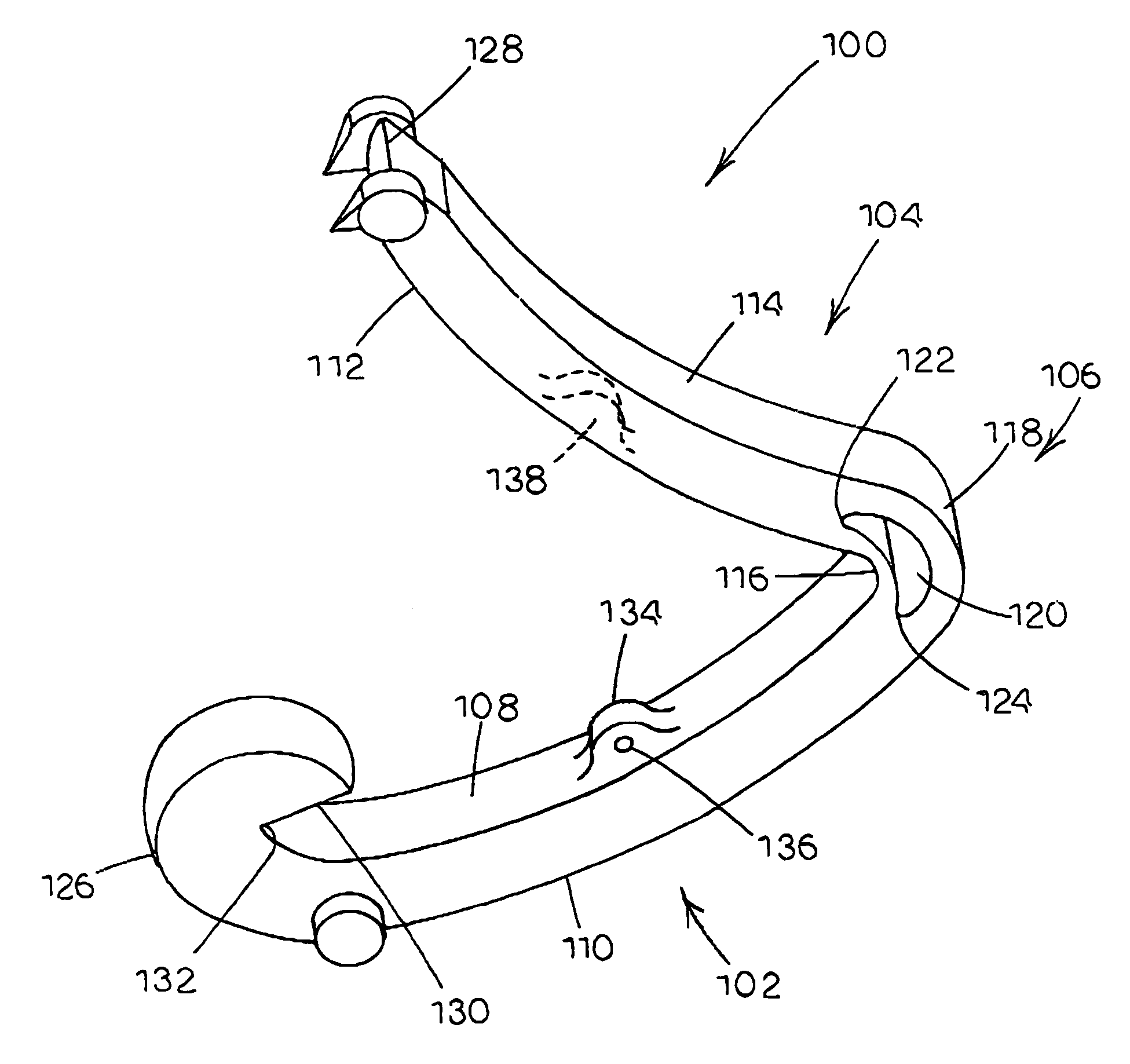
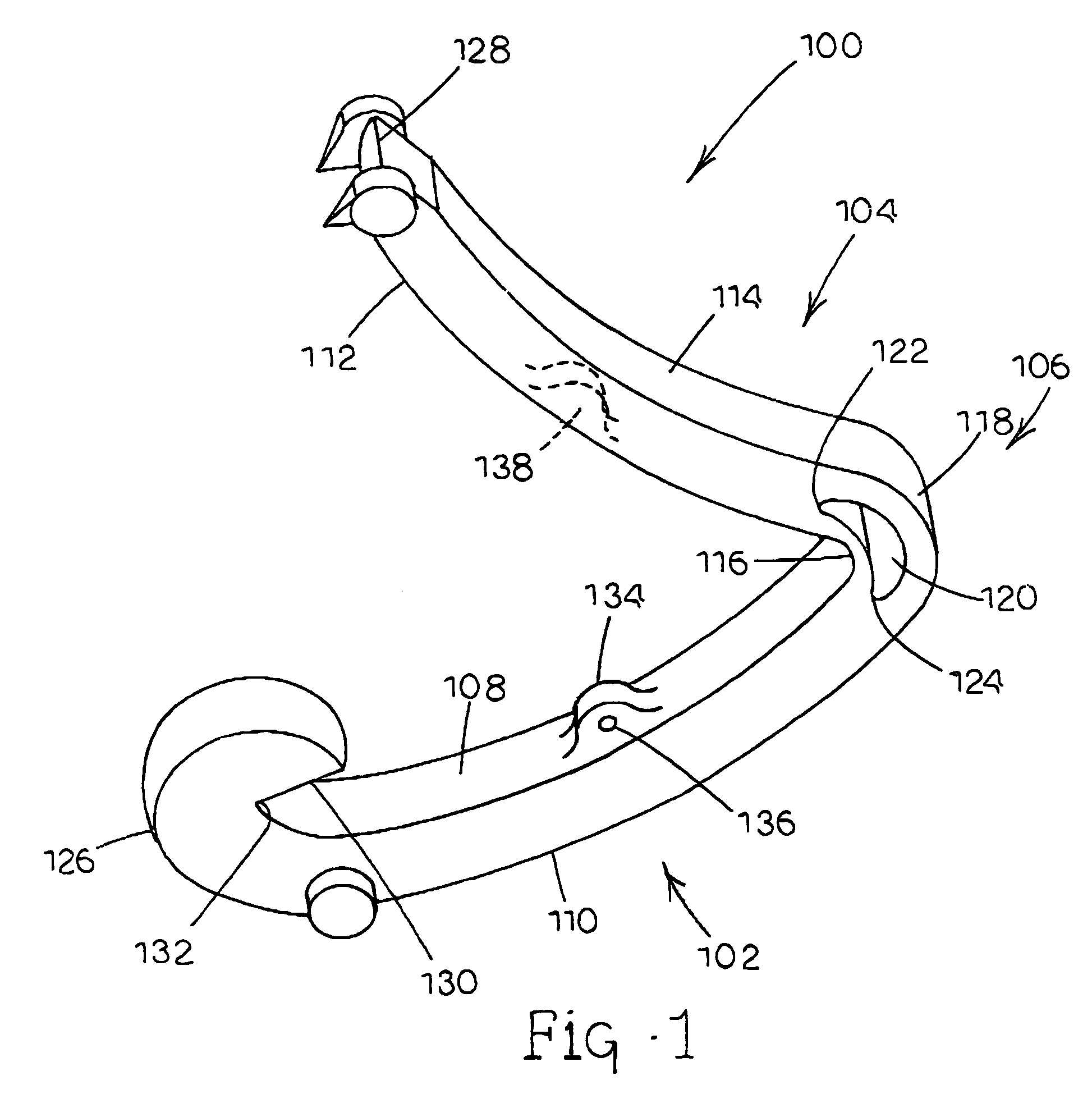
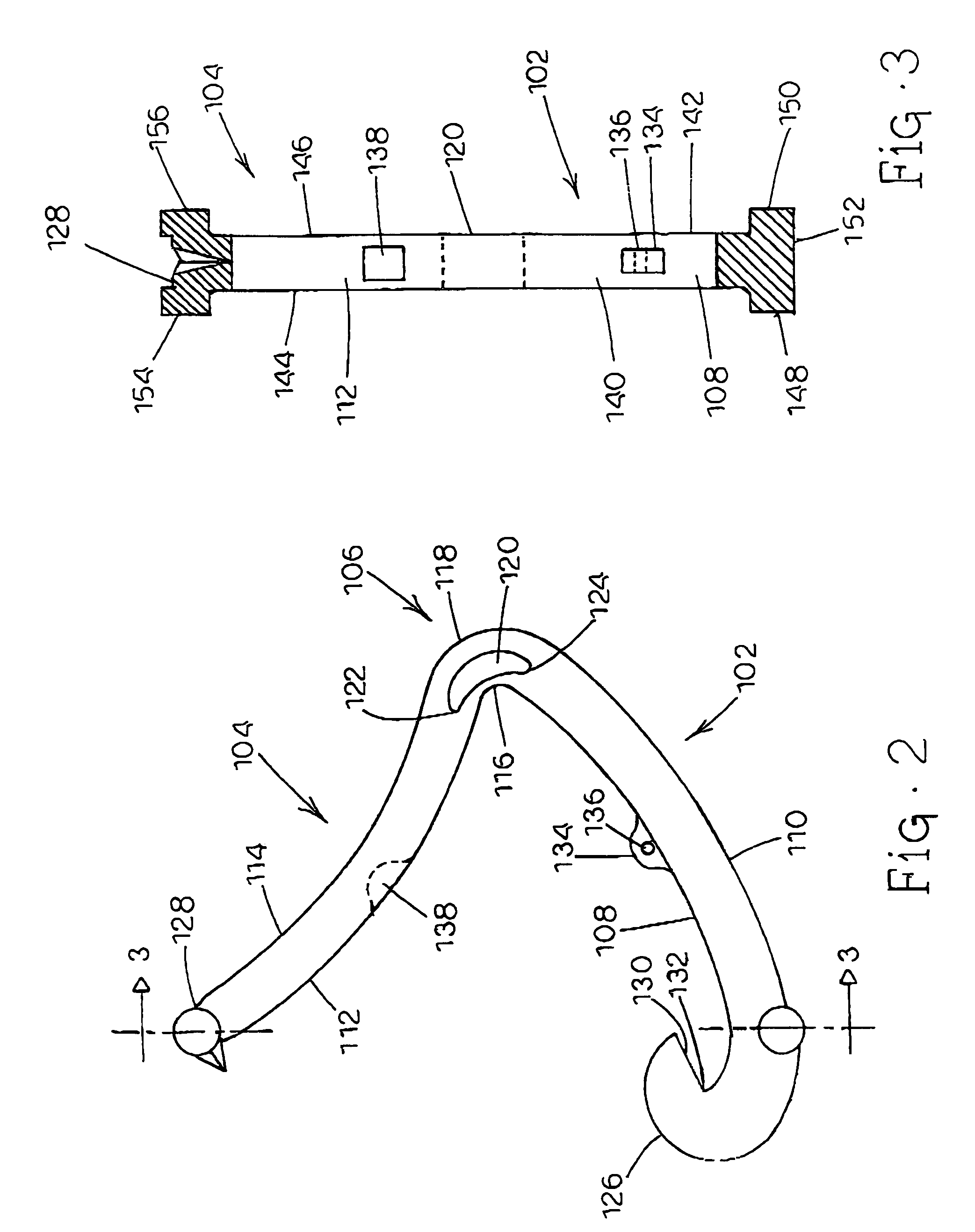
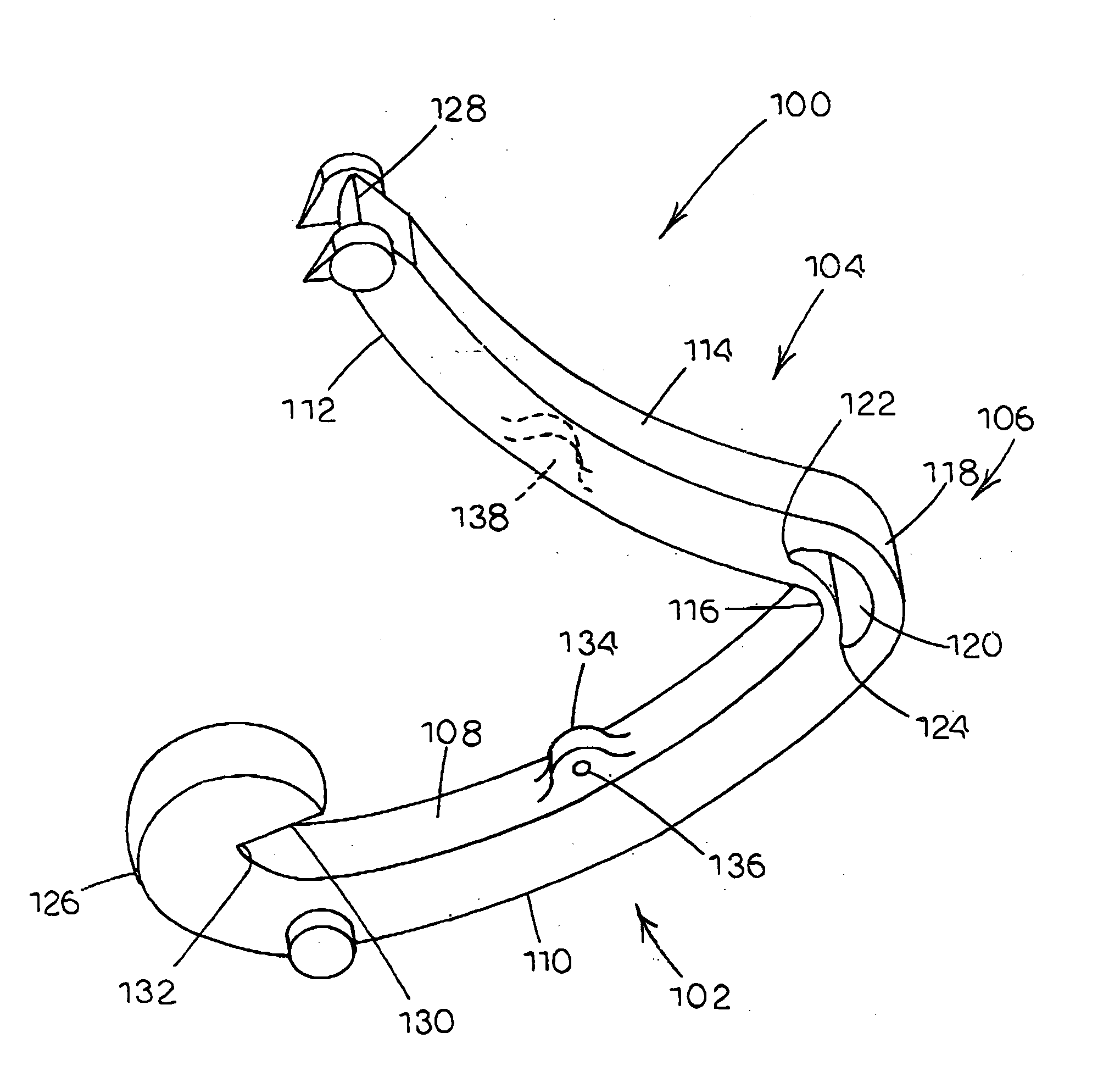
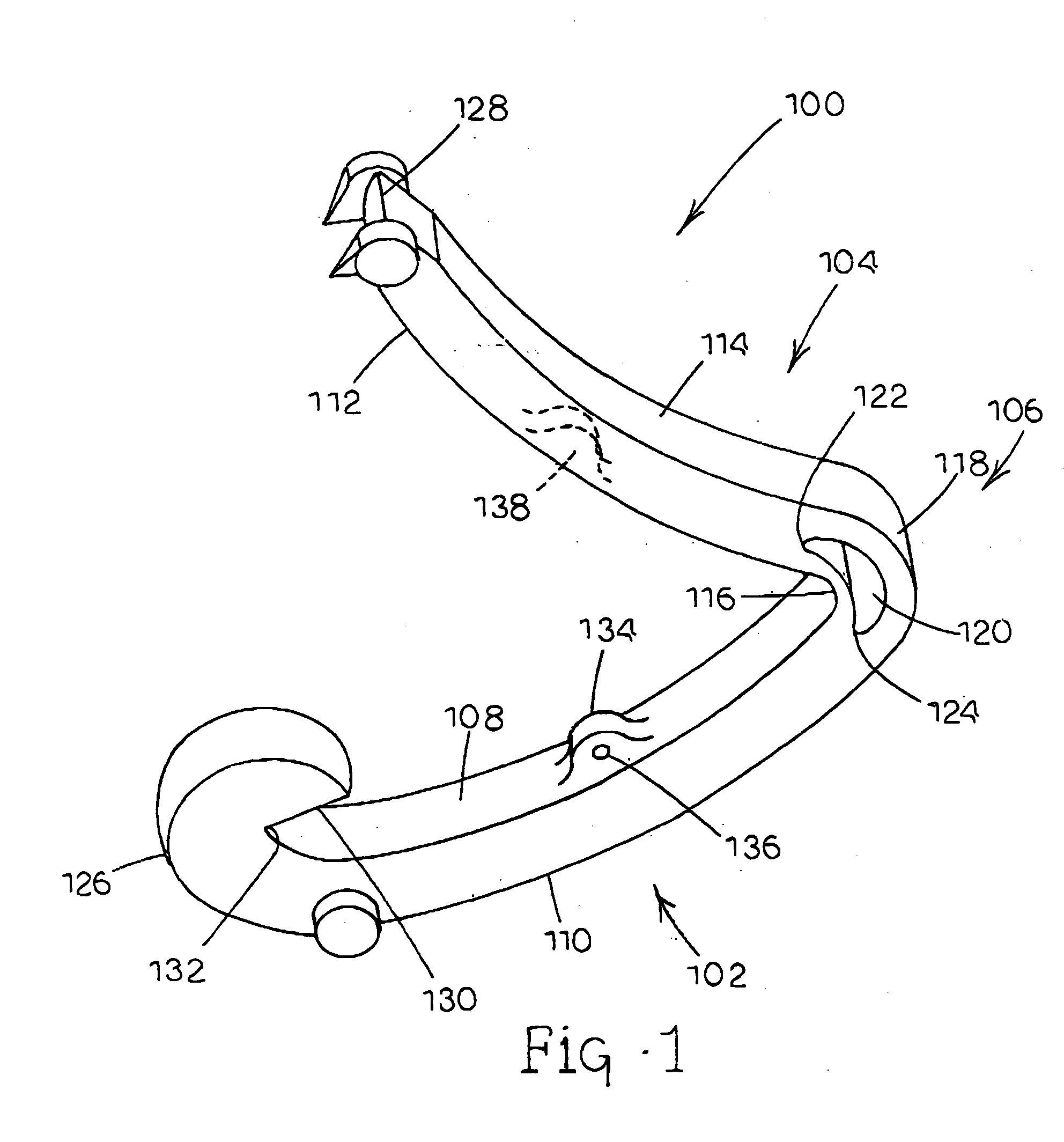
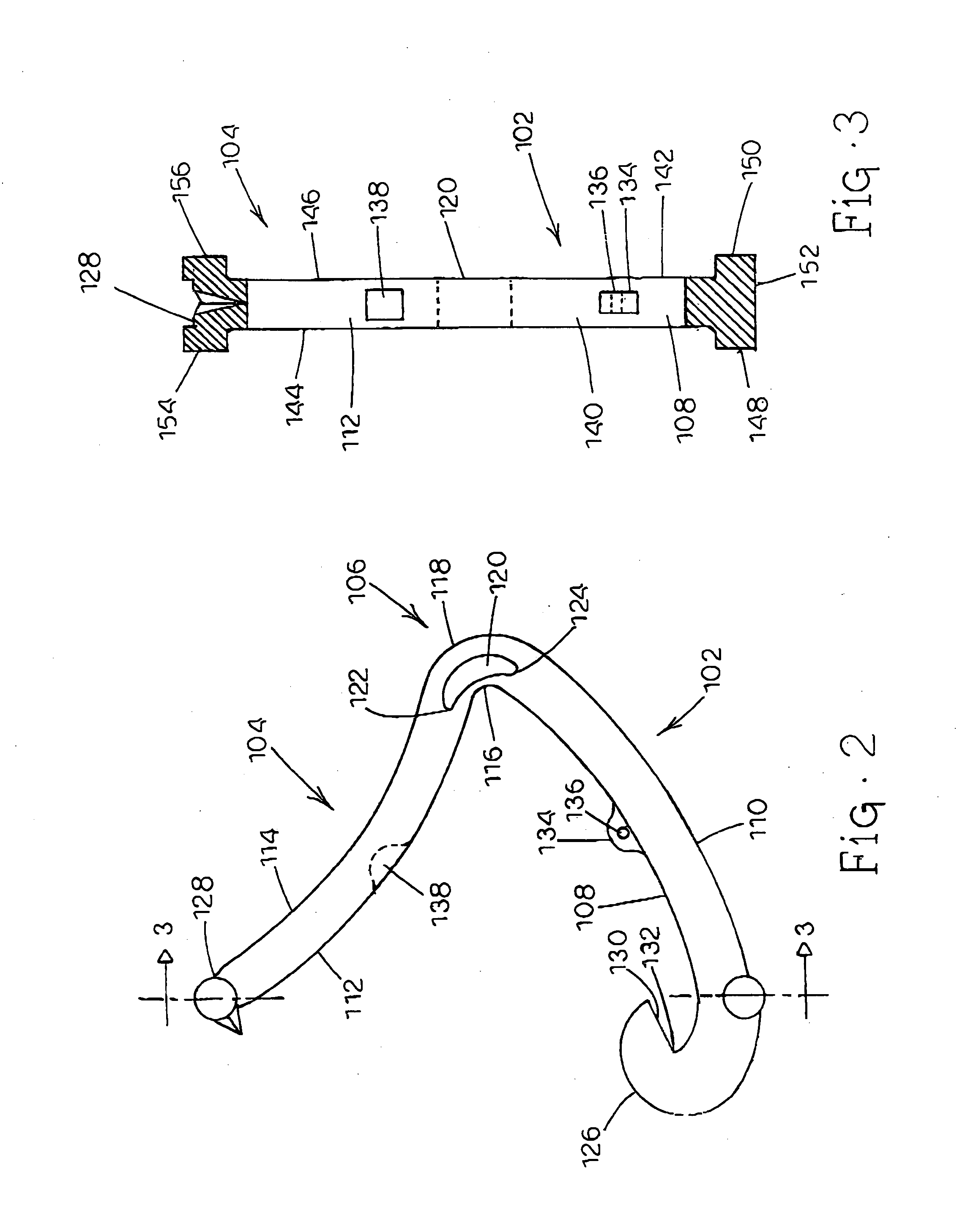
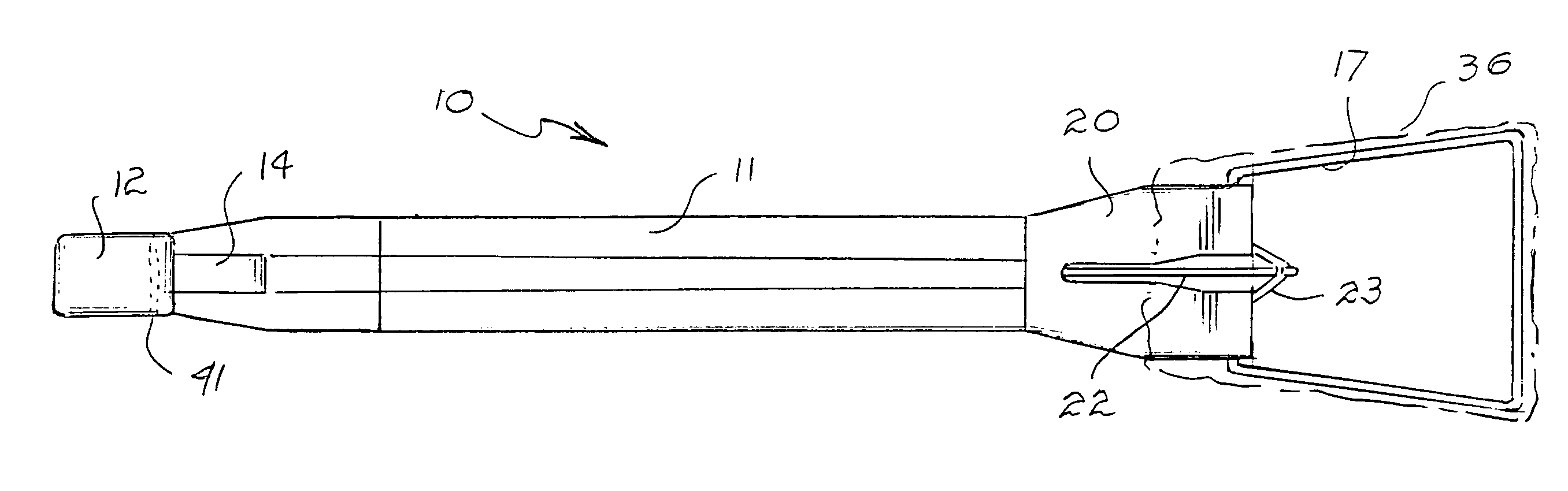
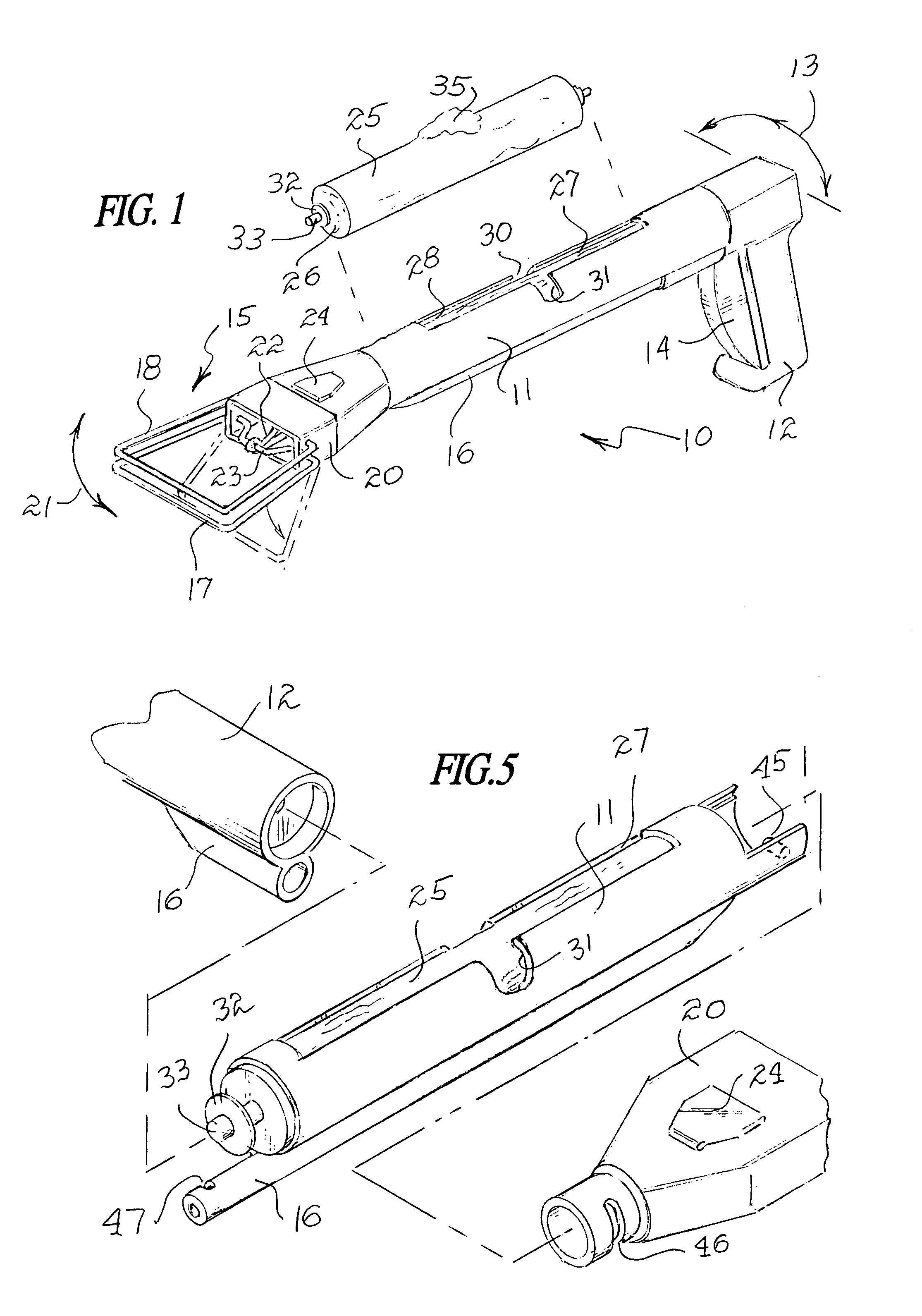
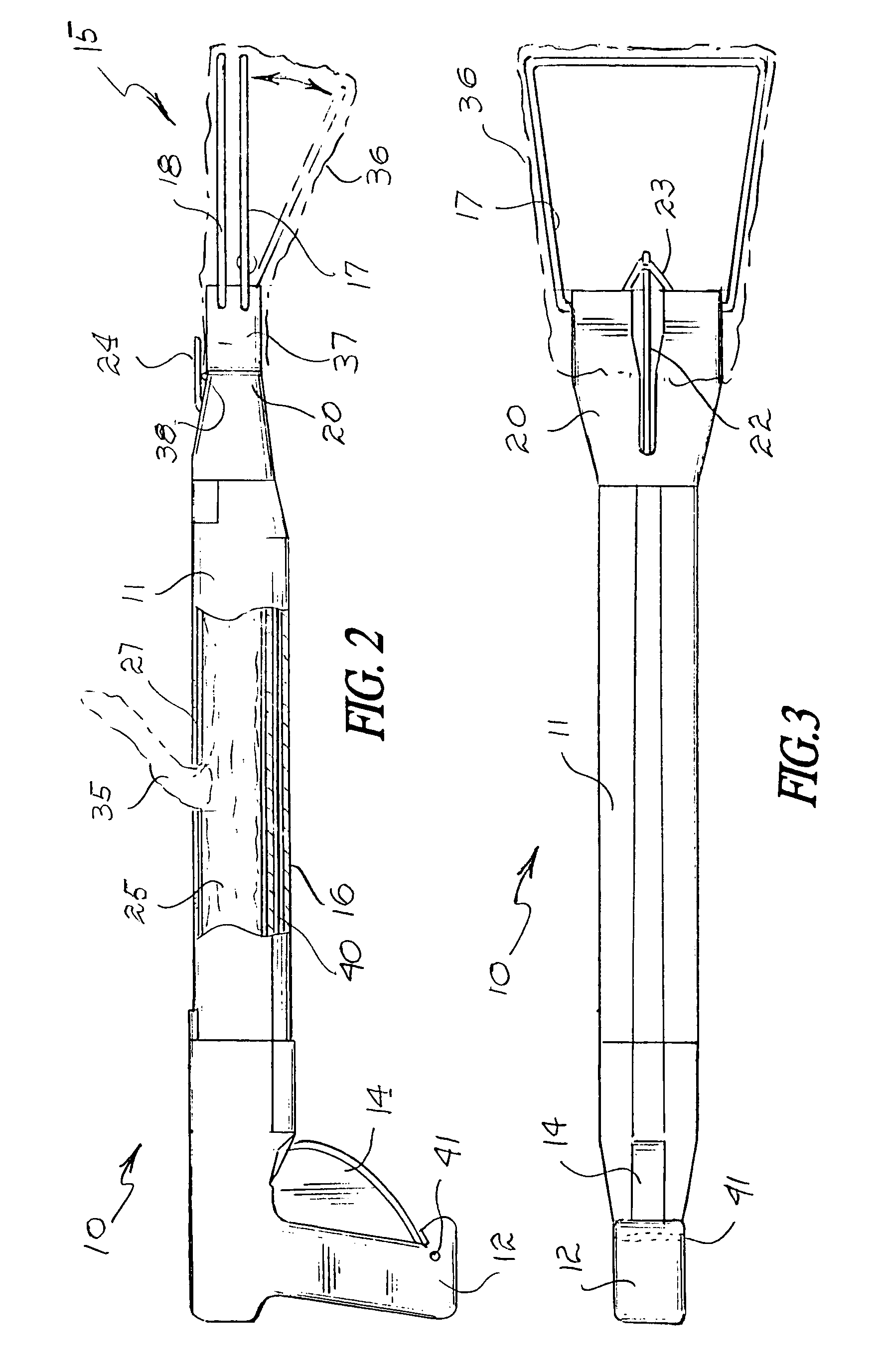
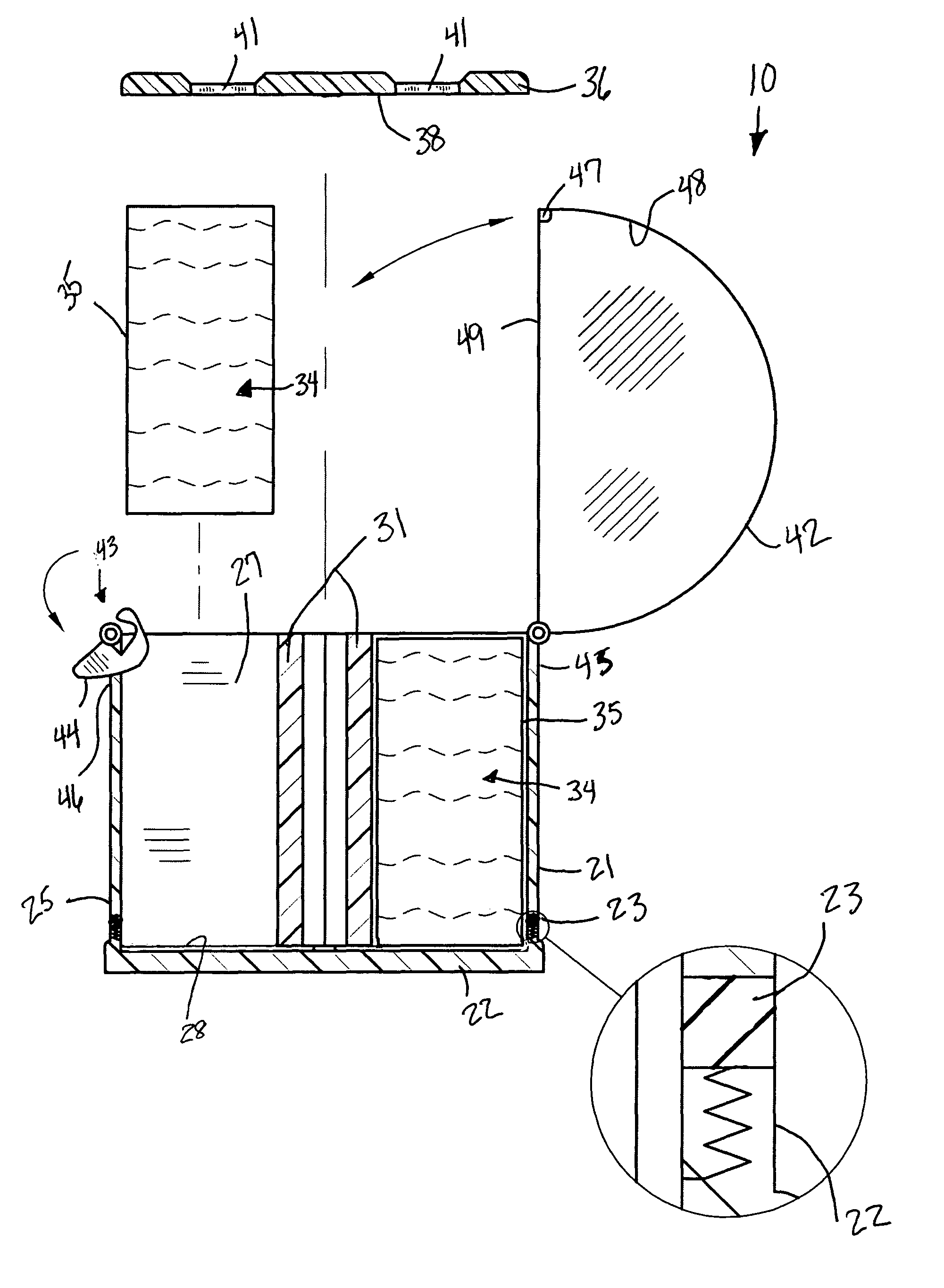
Tissue clamp
InactiveUS7901420B2Avoid premature releasePlace safeSurgical forcepsWound clampsAngular orientationBiomedical engineering
Owner:UNIV OF MASSACHUSETTS
Tissue clamp
ActiveUS20080249547A1Avoid premature releasePlace safeSurgical pincettesSurgical forcepsEngineeringAngular orientation
The present invention relates to a tissue clamp, a tool for grasping the clamp and a method of using the clamp for surgical procedures. The clamp has a fixture or fixtures positioned on the proximal end of the arms of the clamp so that a tool can be used by the surgeon to securely grasp the clamp during placement thereof on the vasculature or other tissue of the patient. The fixture can have a plurality of channels so that the user can select the angular orientation of the tool relative to the damp.
Owner:UNIV OF MASSACHUSETTS
Analyte sensor
ActiveUS20170290546A1Prevents premature actuationImprove stabilitySurgical needlesCatheterElectricityAnalyte
A simple, disposable sensing device for sensing an analyte is housed in a single case. The sensing device can transmit sensor data to monitoring device(s). The sensing device includes: a case having a lower major wall adapted to be mounted against a patient's skin, and an upper opposing major wall; a sensor extending from the case and having a distal end sensitive to the analyte to produce an electrical signal, and a proximal end within the case having electrical contacts; a printed circuit board assembly within the case supported by one of the major walls to receive the electrical signal via the electrical contacts; and an elastomeric pad disposed in the case and biased by the other major wall to urge the proximal end of the sensor into contact with the printed circuit board assembly and maintain an electrical connection between the electrical contacts and the printed circuit board assembly.
Owner:MEDTRONIC MIMIMED INC
Tissue clamp
ActiveUS8052700B2Avoid premature releasePlace safeSurgical pincettesSurgical forcepsAngular orientationBiomedical engineering
The present invention relates to a tissue clamp, a tool for grasping the clamp and a method of using the clamp for surgical procedures. The clamp has a fixture or fixtures positioned on the proximal end of the arms of the clamp so that a tool can be used by the surgeon to securely grasp the clamp during placement thereof on the vasculature or other tissue of the patient. The fixture can have a plurality of channels so that the user can select the angular orientation of the tool relative to the damp.
Owner:UNIV OF MASSACHUSETTS
Pod for Dispersible Materials
Owner:THE COCA-COLA CO
Ligating clip with integral tissue-securing mechanism
InactiveUS20050165423A1Avoid vertical movementMinimize interferenceWound clampsLigating clipsEngineering
A polymeric, surgical clip having first and second curved leg members joined at their proximal end by a hinge portion and movable from an open position to a closed position for clamping a vessel between curved opposing inner surfaces which are substantially parallel when the clip is closed. An interlocking mechanism is formed by a portion of the inner surfaces of the first and second legs. The interlocking mechanism may be a tongue-in-groove mechanism, formed by a lip or tongue protruding from a portion of the inner surface of one leg and a groove formed in a corresponding portion of the inner surface of the other leg, or a lock-step mechanism, formed by complementary L-shaped notches wherein a notch is provided in a portion of the inner surface of each leg. The interlocking mechanism acts to impede longitudinal movement of the clip relative to the vessel being clamped.
Owner:PILLING WECK INC
Surgical clip with integral suture-securing mechanism
ActiveUS7001412B2Minimize interferenceImprove securitySuture equipmentsWound clampsEngineeringSurgical Clips
A polymeric, surgical clip having first and second curved leg members joined at their proximal end by a hinge portion and movable from an open position to a closed position for securing and maintaining a desired amount of tension on a suture. A ridge protrudes from a portion of the inner surface of one leg and a groove is formed in a corresponding portion of the inner surface of the other leg. An eyelet extends through the ridge to engage a portion of a suture.
Owner:TELEFLEX MEDICAL INC
Surgical clip with integral suture-securing mechanism
ActiveUS20050165424A1Interference minimizationHigh strengthSuture equipmentsWound clampsEngineeringSurgical Clips
A polymeric, surgical clip having first and second curved leg members joined at their proximal end by a hinge portion and movable from an open position to a closed position for securing and maintaining a desired amount of tension on a suture. A ridge protrudes from a portion of the inner surface of one leg and a groove is formed in a corresponding portion of the inner surface of the other leg. An eyelet extends through the ridge to engage a portion of a suture.
Owner:TELEFLEX MEDICAL INC
Tire with tread of rubber composition prepared with reinforcing fillers which include starch/plasticizer composite
InactiveUS6273163B1Improve propertiesAdvantageously producedSpecial tyresPaper coatingPolymer sciencePlasticizer
The invention relates to the preparation of a rubber composition containing starch / plasticizer composite reinforcement, together with at least one additional reinforcing filler, through the utilization of a combination of an organosilane disulfide compound mixed with a rubber composition in a preparatory, non-productive, mixing stage(s) followed by adding an organosilane polysulfide compound in a subsequent, productive, mixing stage. The invention further relates to the resulting rubber composition and use thereof in rubber products, including tires.
Owner:THE GOODYEAR TIRE & RUBBER CO
Localized drug delivery using drug-loaded nanocapsules
InactiveUS7767219B2Avoid premature releaseLimiting systemic effect of drugPowder deliveryElectrotherapyPolyelectrolyteMedicine
Owner:BOSTON SCI SCIMED INC
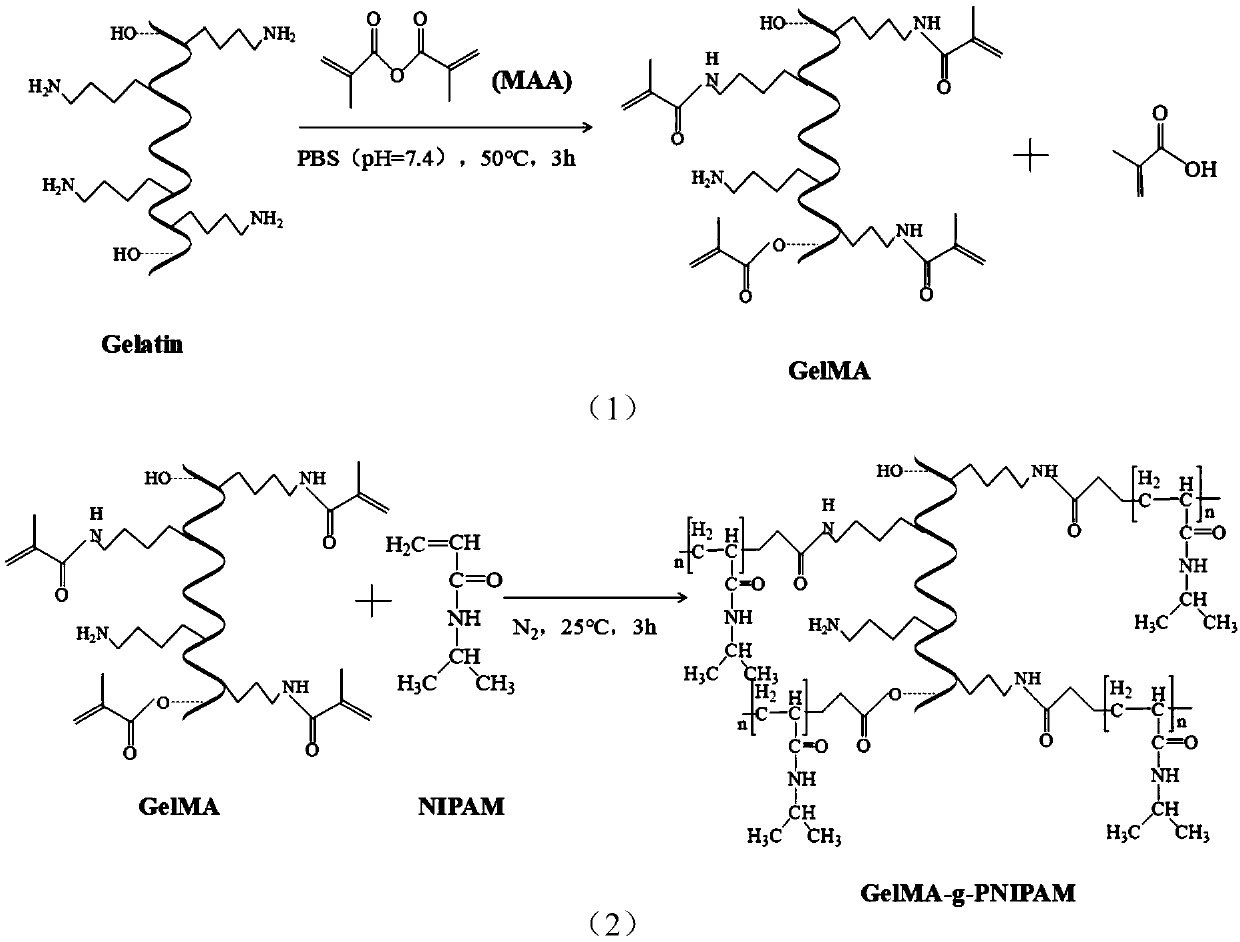
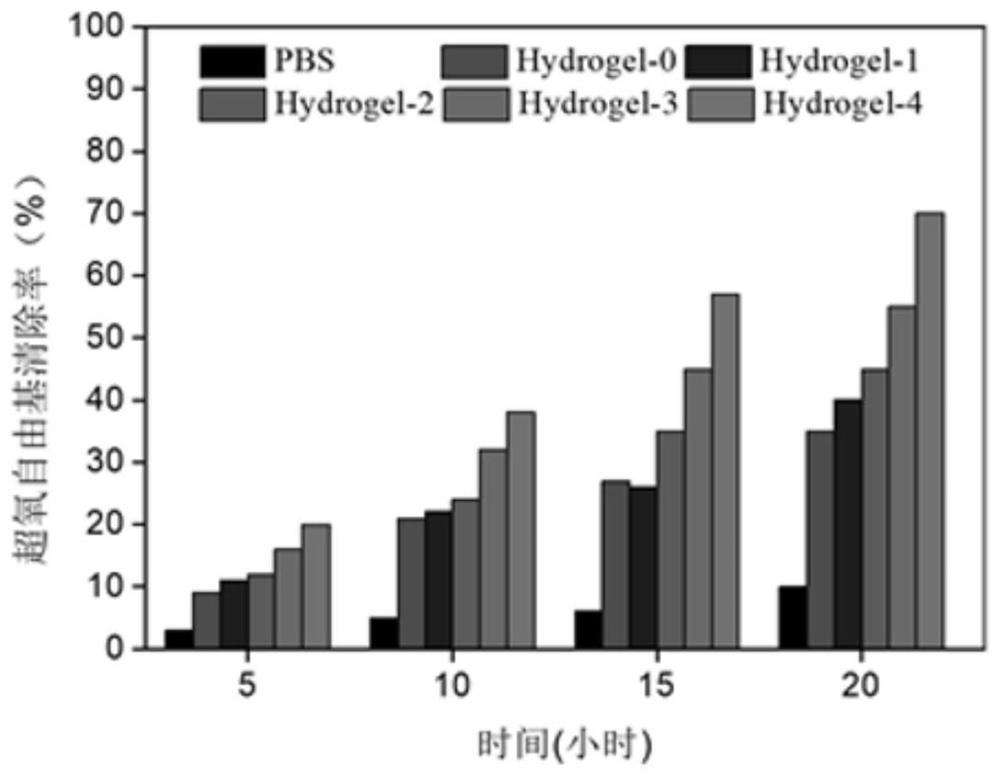
Thermosensitive hydrogel loaded with copper metal organic skeleton nanoparticles and preparation method of thermosensitive hydrogel
InactiveCN109513038AAggregation is simpleStable in naturePharmaceutical delivery mechanismBandagesMetal-organic frameworkDouble bond
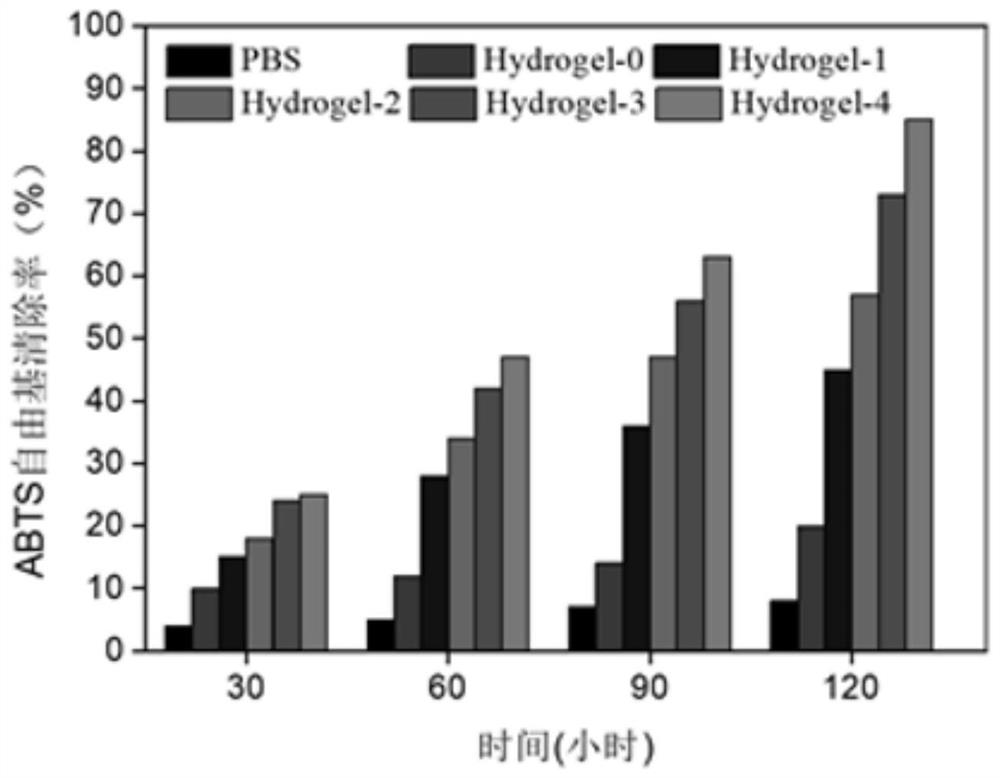
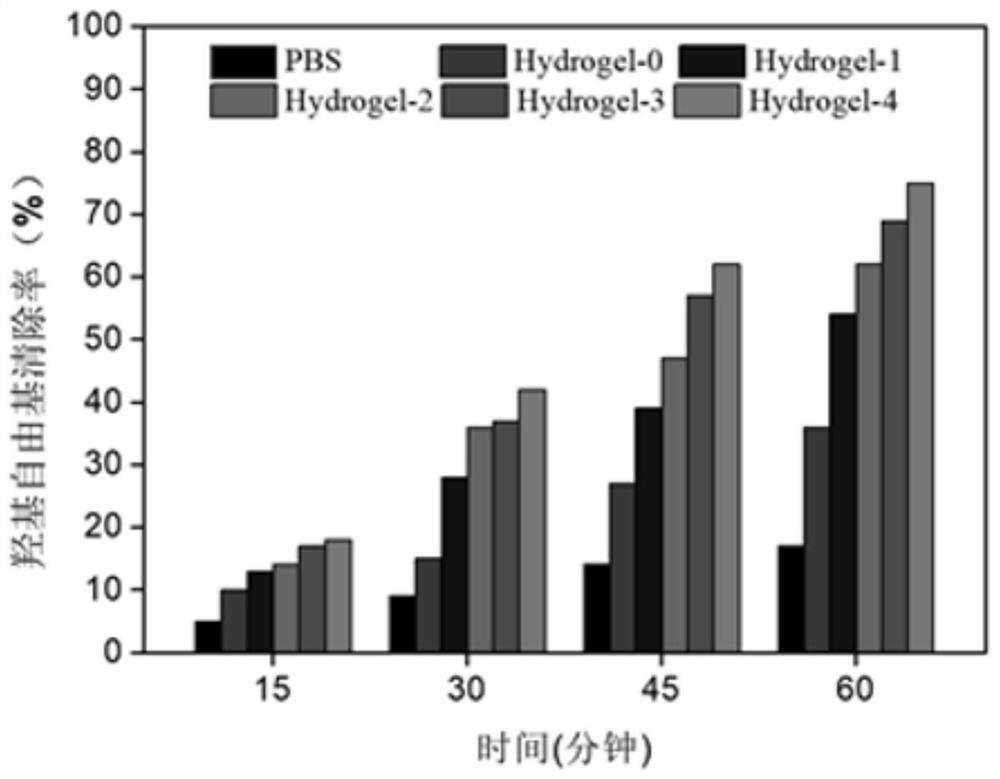
The invention discloses thermosensitive hydrogel loaded with copper metal organic skeleton nanoparticles and a preparation method of the thermosensitive hydrogel. The preparation method comprises thesteps that an amino group on gelatin and methacrylic anhydride are subjected to acylation reaction to synthesize methylacrylic esterified gelatin with a branch containing a carbon-carbon double bond,the carbon-carbon double bond on the branch and N-isopropyl acrylamide are subjected to free radical polymerization, and methylacrylic esterified gelatin-g-poly N-isopropyl acrylamide is prepared; 1,3,5-benzene tricarbonic acid and copper acetate monohydrate are used for preparing copper-based MOF nanoparticles; the copper-based MOF nanoparticles are added to a methylacrylic esterified gelatin-g-poly N-isopropyl acrylamide solution, and a product is prepared. Copper ions are embedded in the biodegradable thermosensitive hydrogel in the form of HKUST-1 NPs, controlled slow release of the copperions is achieved, cytotoxicity is effectively reduced, migration of in-vitro dermal cells is promoted, angiogenesis is induced, and wound healing is promoted.
Owner:SOUTH CHINA UNIV OF TECH
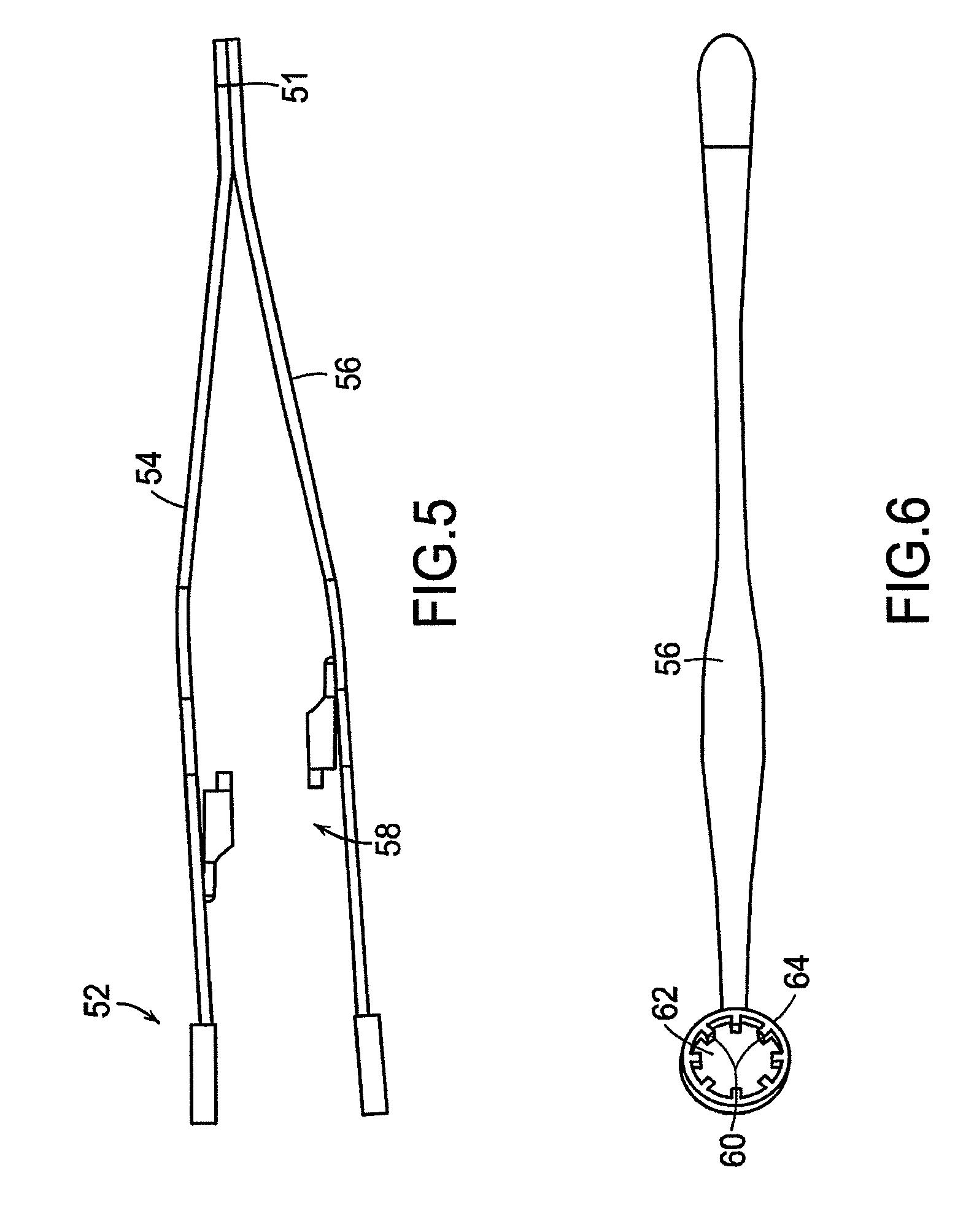
Trigger actuated cable clamp
ActiveUS7219399B2Avoid premature releaseSnap fastenersFriction grip releasable fasteningsMechanical engineeringTriggering device
A clamp for a cable having a housing with a first end, a second end, and an interior cavity to receive the cable. The clamp also includes a jaw assembly having a notch. The jaw assembly moves between locked and triggered positions within the cavity. A biasing member is disposed within the cavity to bias the jaw assembly towards the triggered position. A retainer is arranged within the cavity and has gripping fingers to engage the notches in the locked position. A trigger is positioned within the cavity. The trigger has an outer locking portion to engage with the gripping fingers to releasably secure the gripping fingers in the notches to retain the jaw assembly in the locked position. When the cable is inserted into the interior cavity and contacts the trigger device, the gripping fingers and notches disengage so that the biasing member biases the jaw assembly towards the first end of the clamp to clamp the cable.
Owner:HUBBELL INC
Method of sealing a pod for dispersible materials
ActiveUS7964230B2Avoid premature releaseTea substituesTea alkaloid content reductionEngineeringMechanical engineering
Owner:THE COCA COLA CO
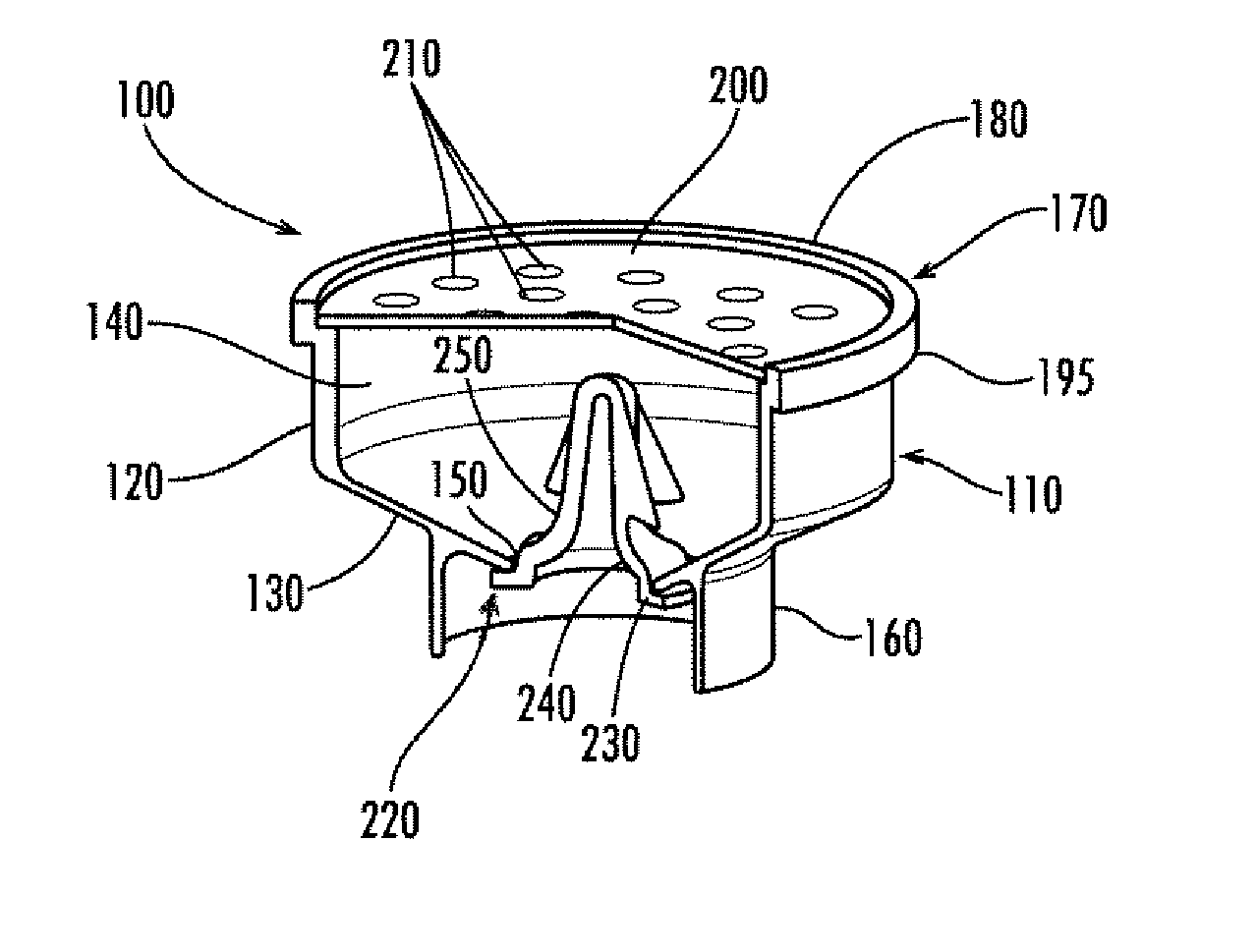
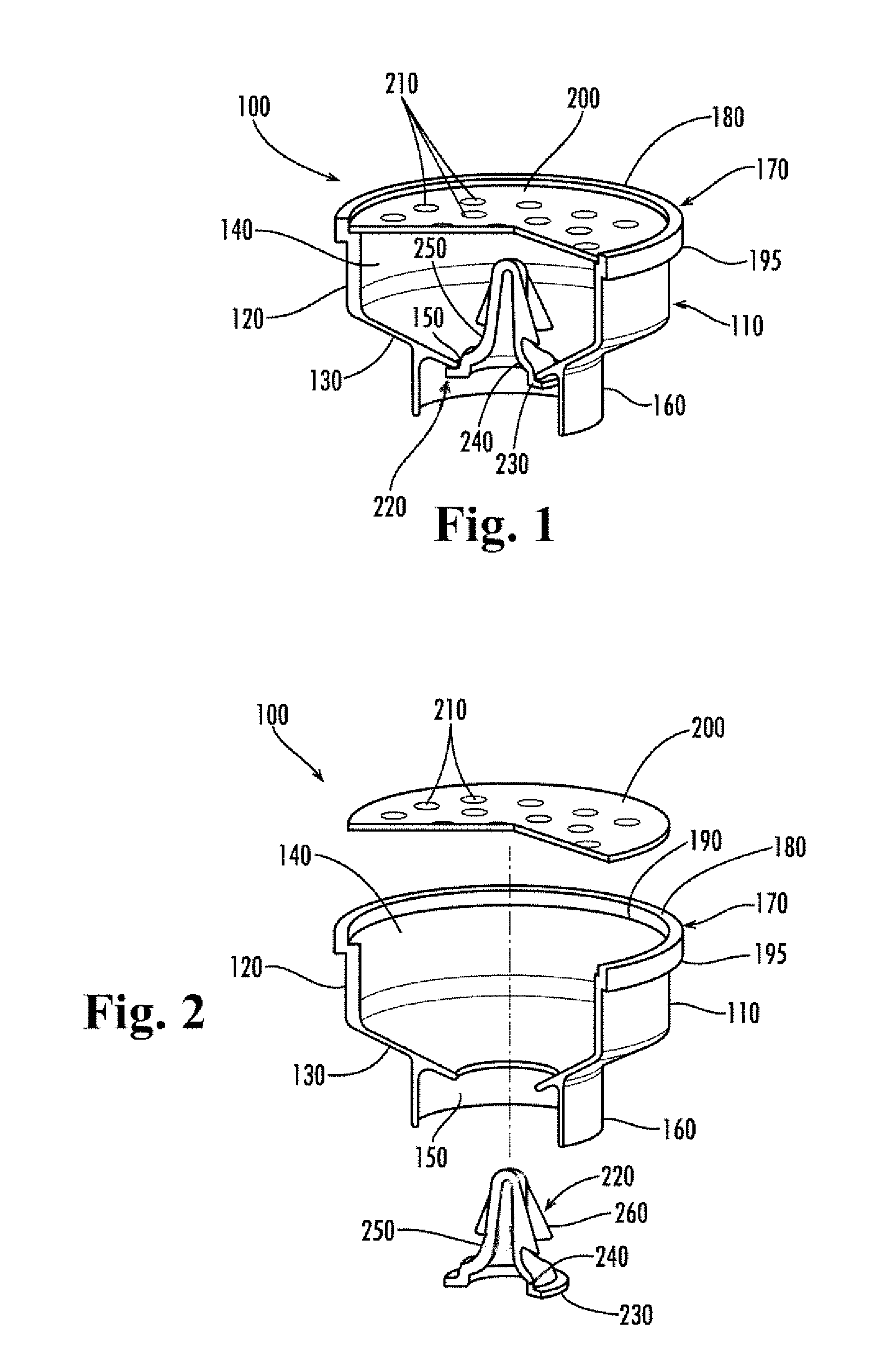
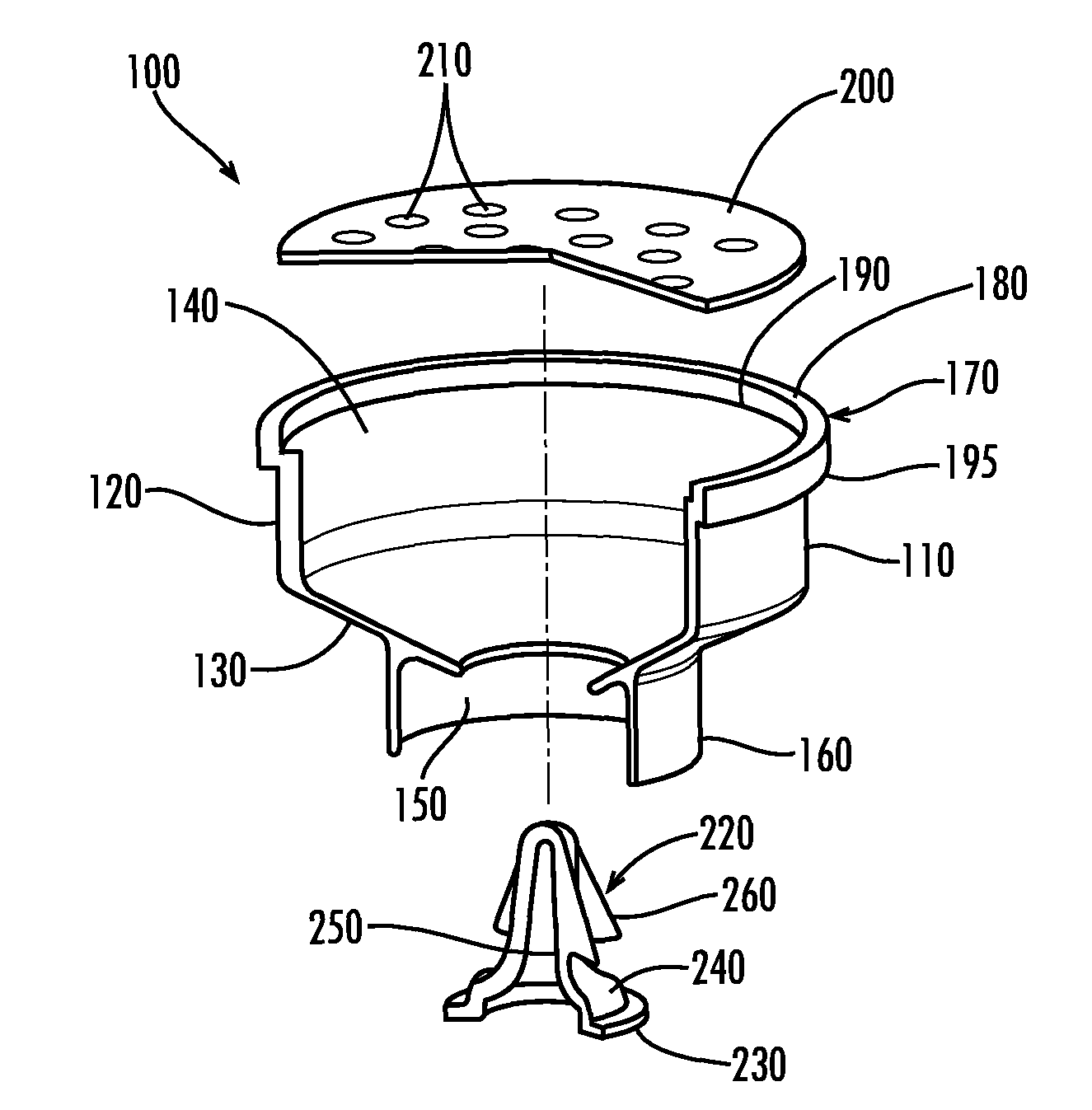
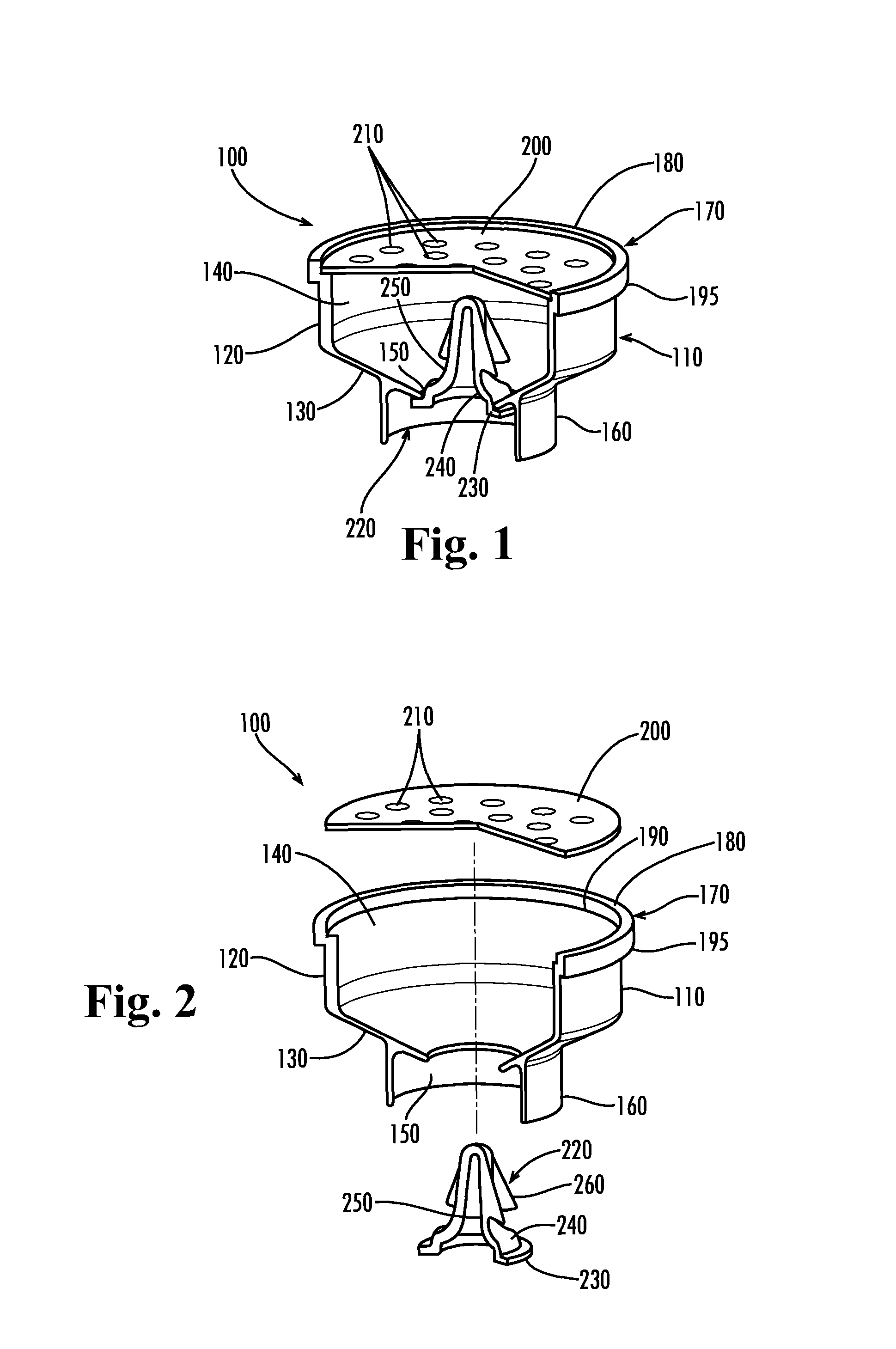
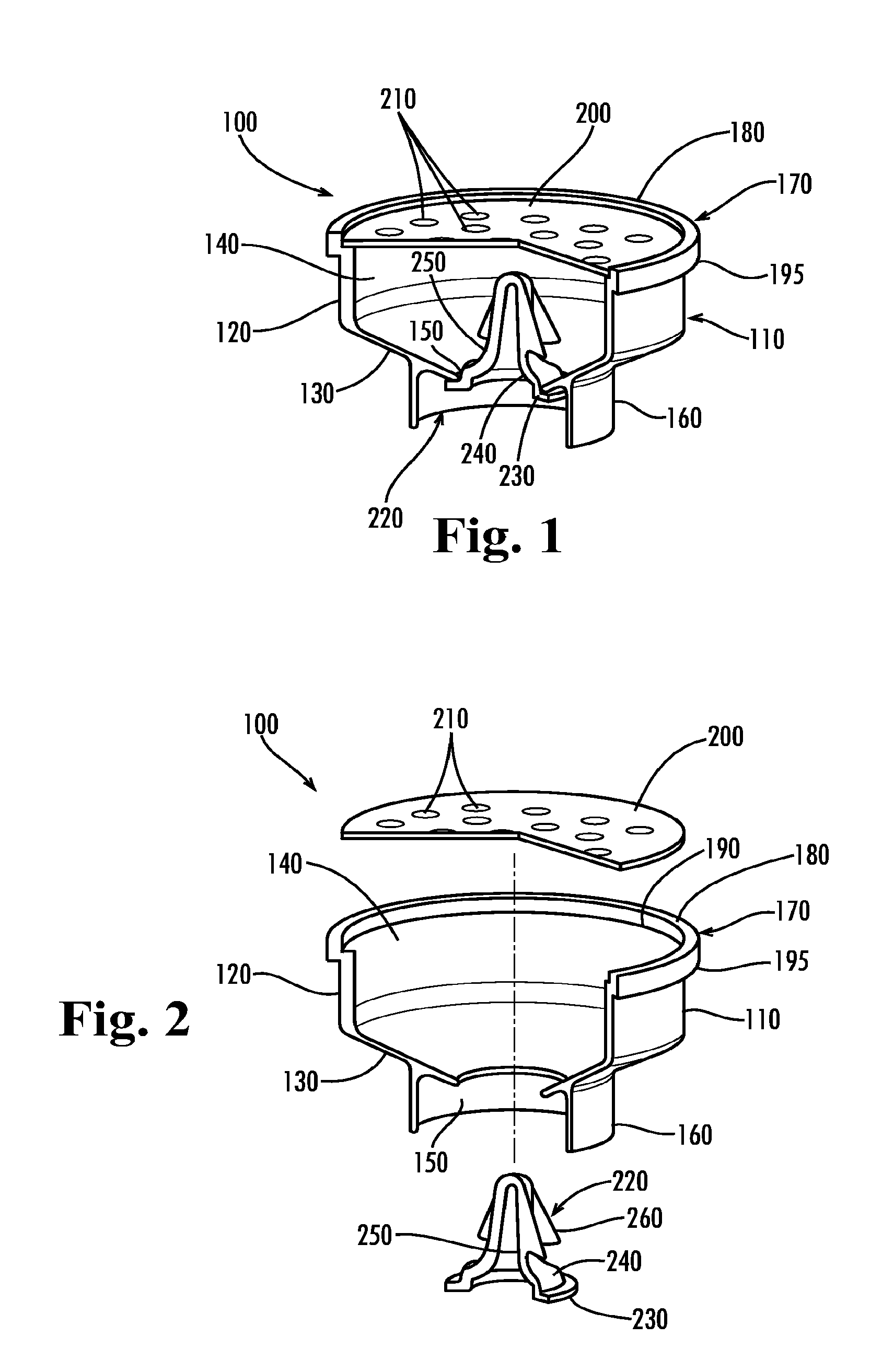
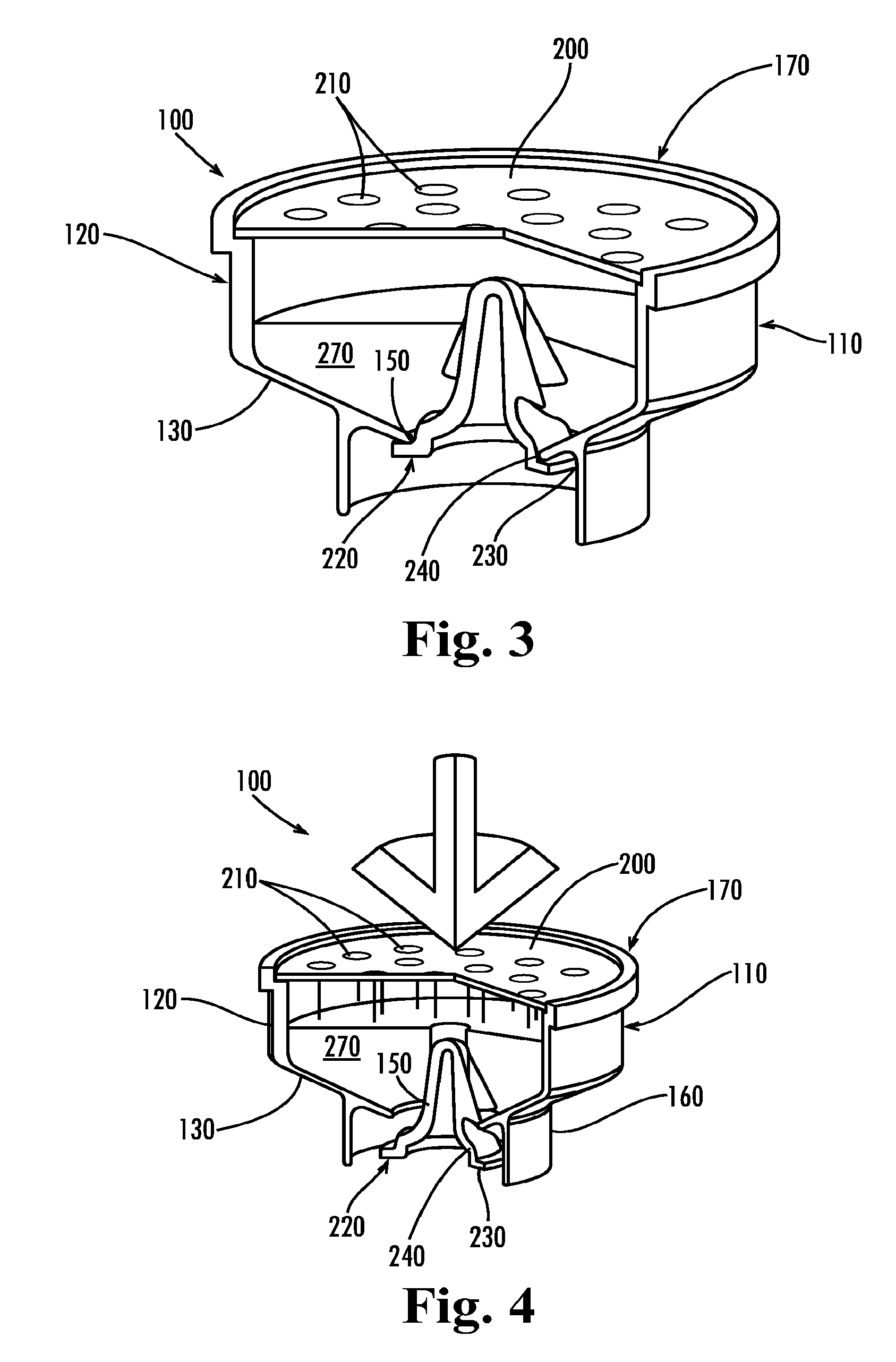
Apparatus for preventing unintended or premature release of liquid in a beverage brewing device and method thereof
InactiveUS20070084352A1Avoid premature releaseUniform liquid distributionBeverage vesselsEngineeringBiomedical engineering
An apparatus useful for preventing the release of residual liquid in a beverage brewing device prior to and after the brewing process, and for preventing the premature release of liquid during the heating process is disclosed. The apparatus includes a flexible member, a closing member and a recess. The apparatus is fabricated such that the pressure of the liquid being delivered to the brewing chamber displaces or depresses the closing member sufficiently into the recess to form a first fluid passage. The pressure of the fluid also creates a channel or cavity on a surface of the flexible member. The first fluid passage and the channel or cavity form a second fluid passages which provide fluid communication between the fluid inlet and the brewing chamber. Preferably, the flexible member, closing member and recess is formed as an integral unit.
Owner:ELECTRICAL & ELECTRONICS LTD
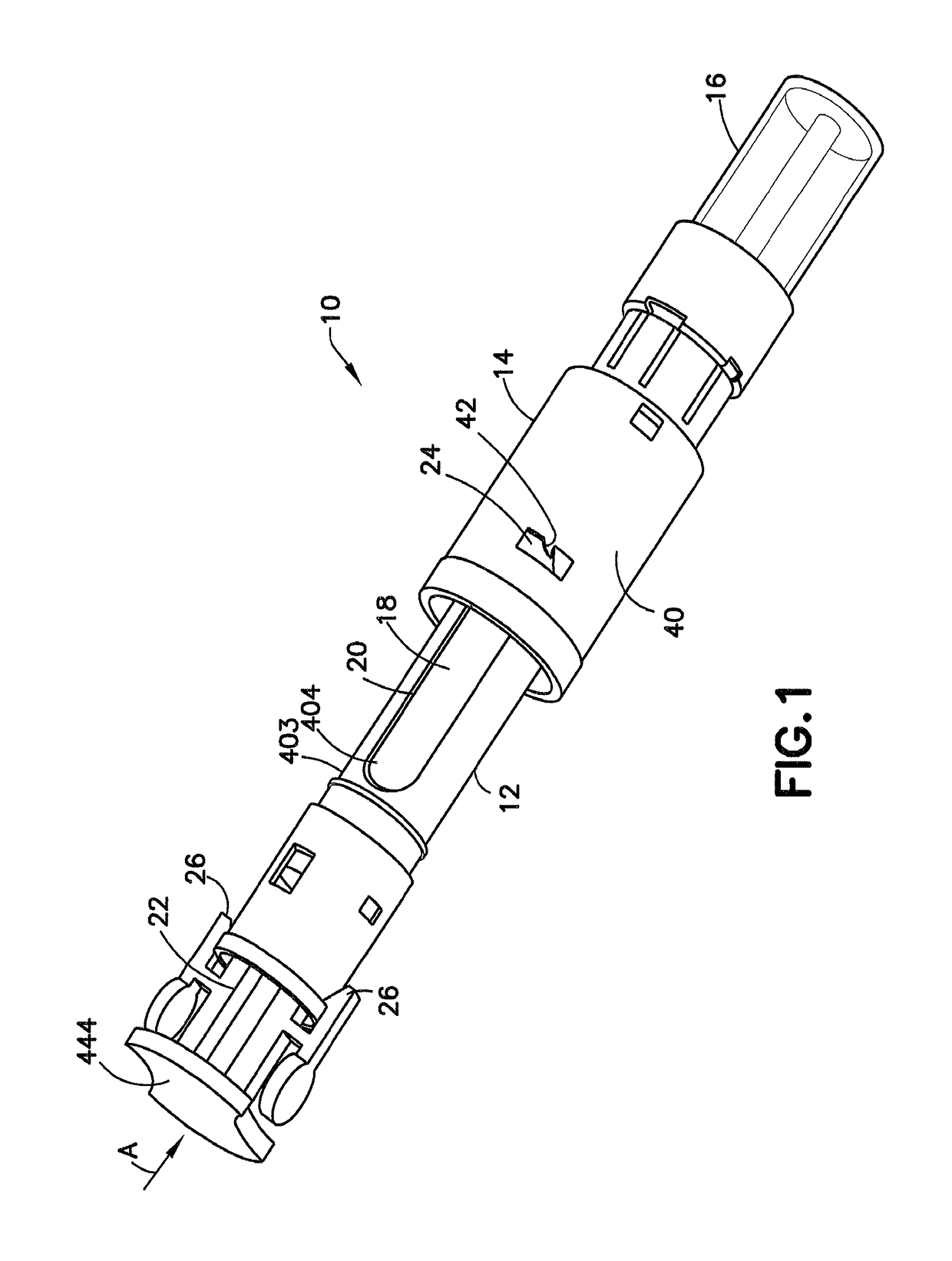
Transfer set with floating needle for drug reconstitution
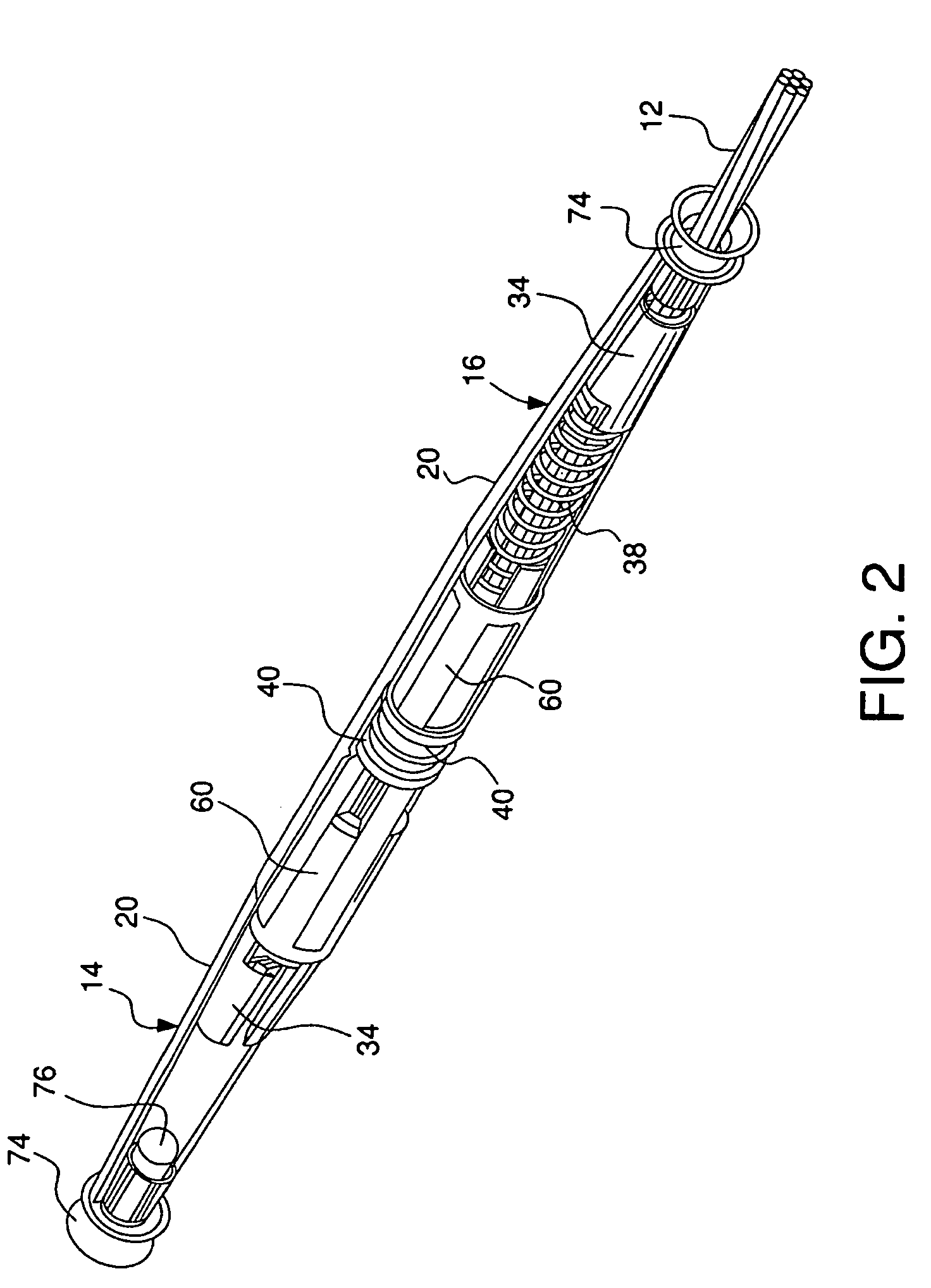
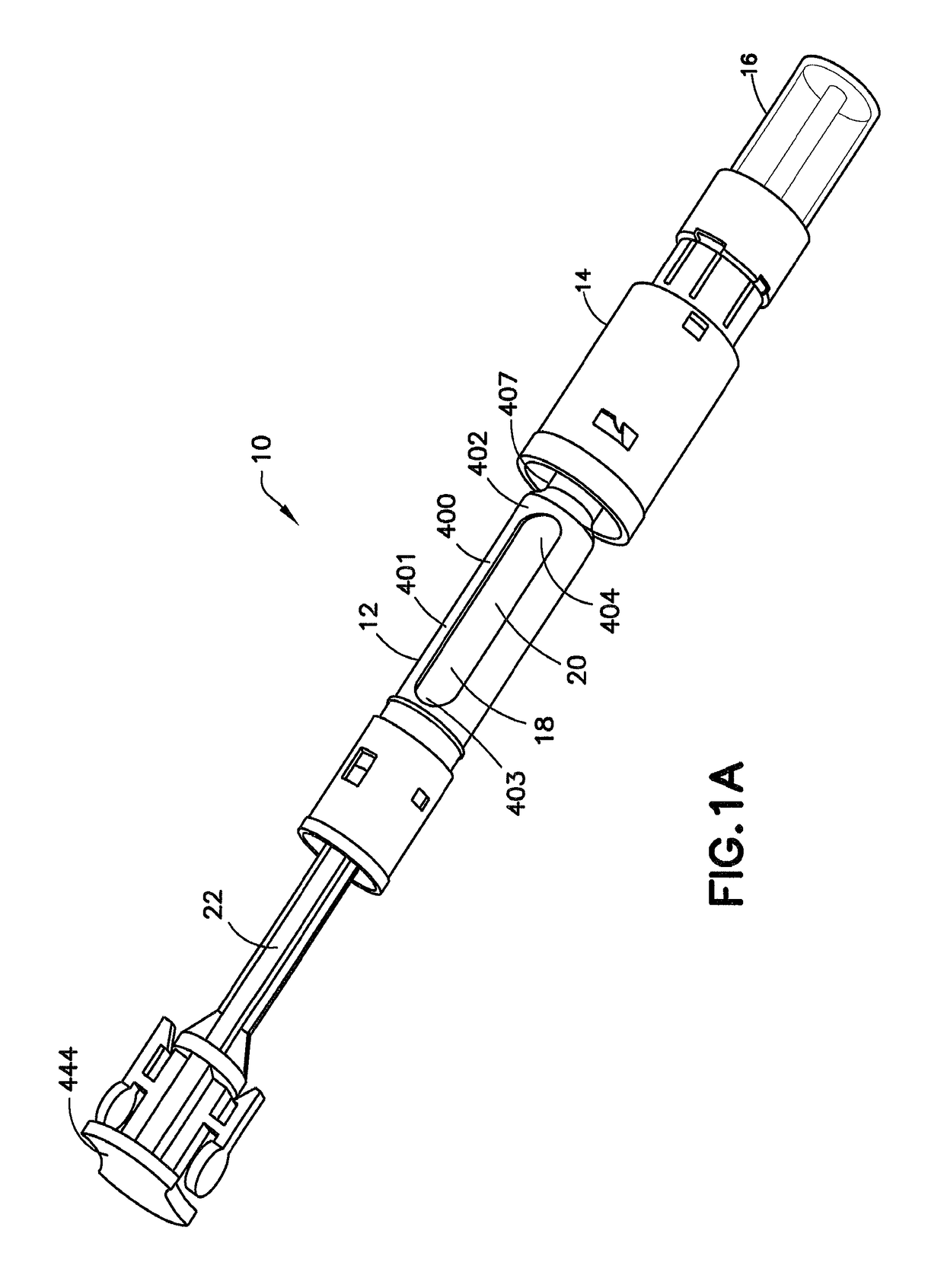
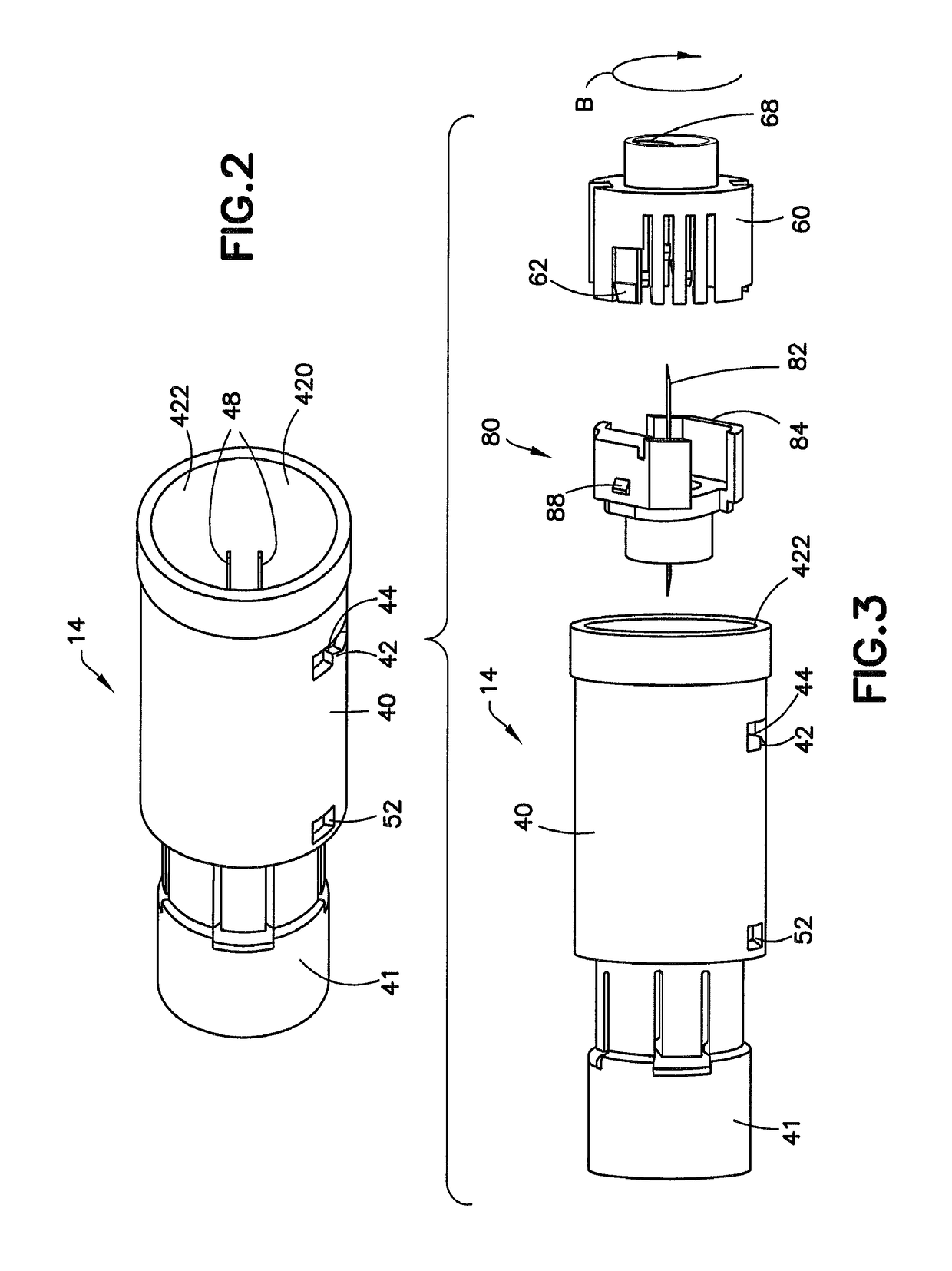
ActiveUS10206854B2Secure bootAvoid premature releaseInfusion syringesPharmaceutical containersNeedle holderDrug
An adapter assembly for establishing bidirectional fluid connection between a cartridge and a vial includes a housing having an open first end adapted to engage the cartridge and a second open end adapted to engage the vial. The adapter includes a needle assembly having a first tip and a second tip, with the needle assembly disposed within the housing and at least partially supported by a needle holder. The needle assembly is movable relative to the housing from an initial position in which the first and second tip are isolated from the cartridge and vial, to an end of use position, in which first tip is engaged with the vial and the second tip is engaged with the cartridge, establishing fluid communication therebetween. The needle assembly is maintained in the initial position by a locking structure which is released by rotationally advancing the needle holder relative to the housing.
Owner:BECTON DICKINSON & CO
Sanitary pick-up device
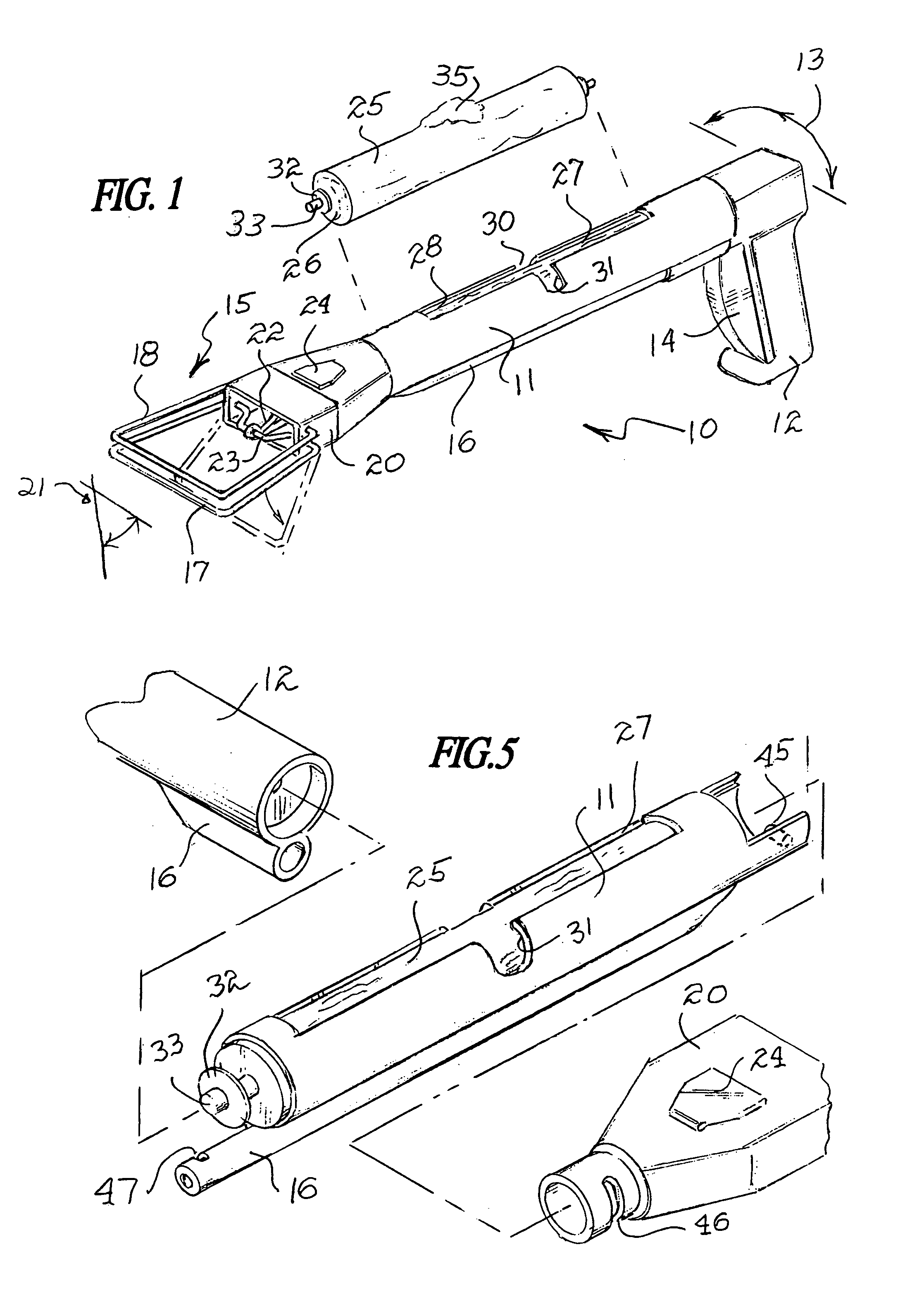
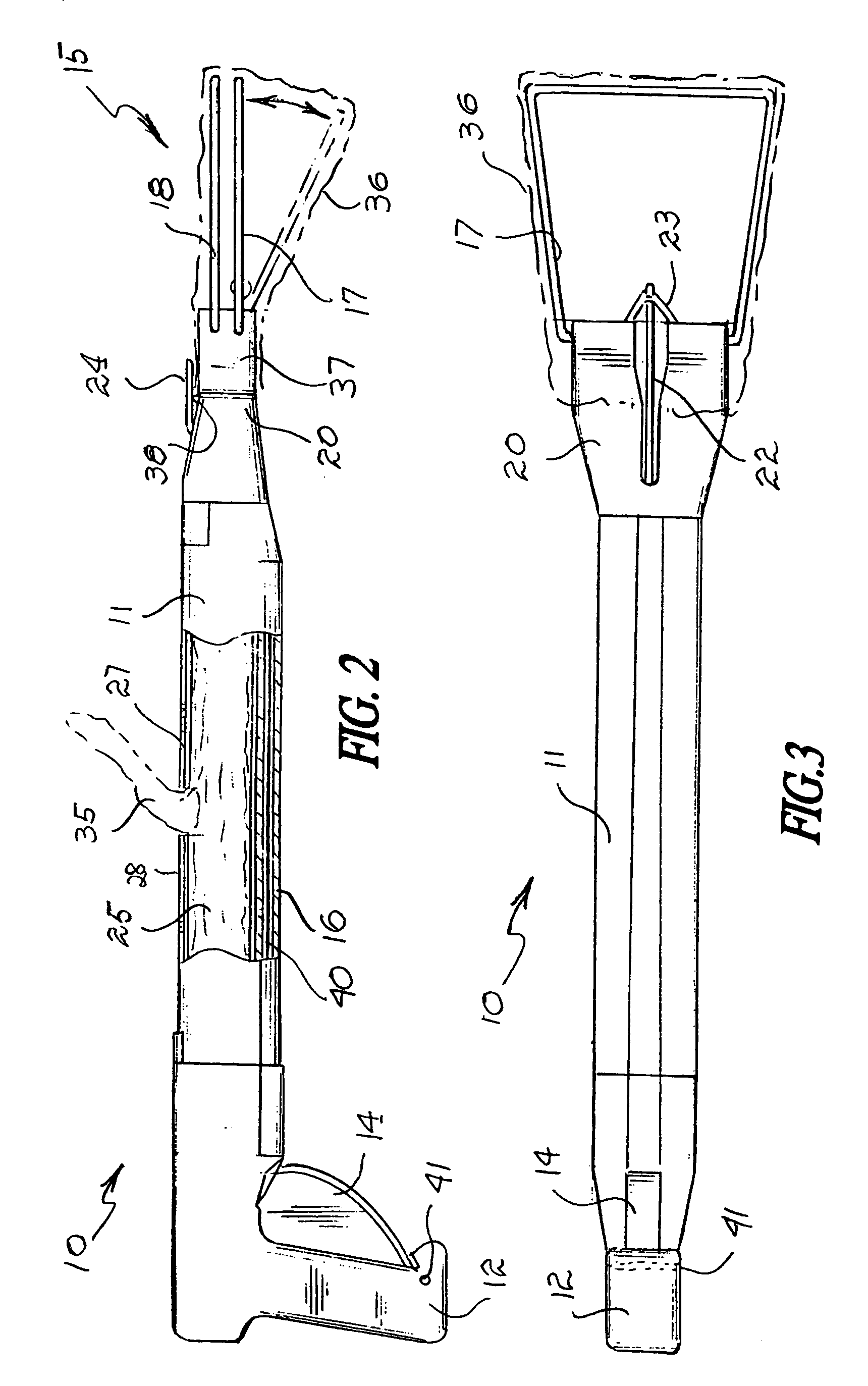
InactiveUS7325849B2Avoid premature releaseEasy disposalRoad cleaningOther apparatusEngineeringActuator
A sanitary pick-up device comprises an elongated body having first and second opposite ends and a midsection therebetween. A fixed bail and a movable bail are carried near the first opposite end of the body and a handgrip is carried near the second opposite end of the body. An actuator is provided between near the handgrip and the movable bail for moving the movable bail to and from the fixed bail. The device has a storage container mounted on the body midsection for receiving a supply of bags, and an attachment mechanism associated with the supply of bags for releasably retaining the supply of bags in the storage compartment.
Owner:JONES GALEN K
Sanitary pick-up device
InactiveUS7077443B1Avoid premature releaseEasy disposalRoad cleaningOther apparatusLitterEngineering
The pick-up device provides an elongated hollow cylindrical body including a handle at one end and a bail arrangement at the other end. The handle includes a trigger mechanism extending through the hollow body and terminating in a crank coupling the pair of bails in the bail assembly, so that one bail is fixed and the other bail is movable with respect to the fixed bail. The body includes a roller upon which a supply of litter bags is placed, and the body includes an elongated slot with finger openings so that a single bag from the supply can be withdrawn from the roll, separated from the roll, and installed on the pair of bails in the bail arrangement. A cable is employed for releasably actuating the bail and mechanism is provided for rotatably mounting the handle and trigger mechanism onto one end of the body so that the handle can be rotated as to expose the aft end of the body permitting a new roll of bags to be installed. Alternately, a bag supply cartridge may be installed through the side of the body.
Owner:JONES GALEN
Toothbrush holder and sanitizer
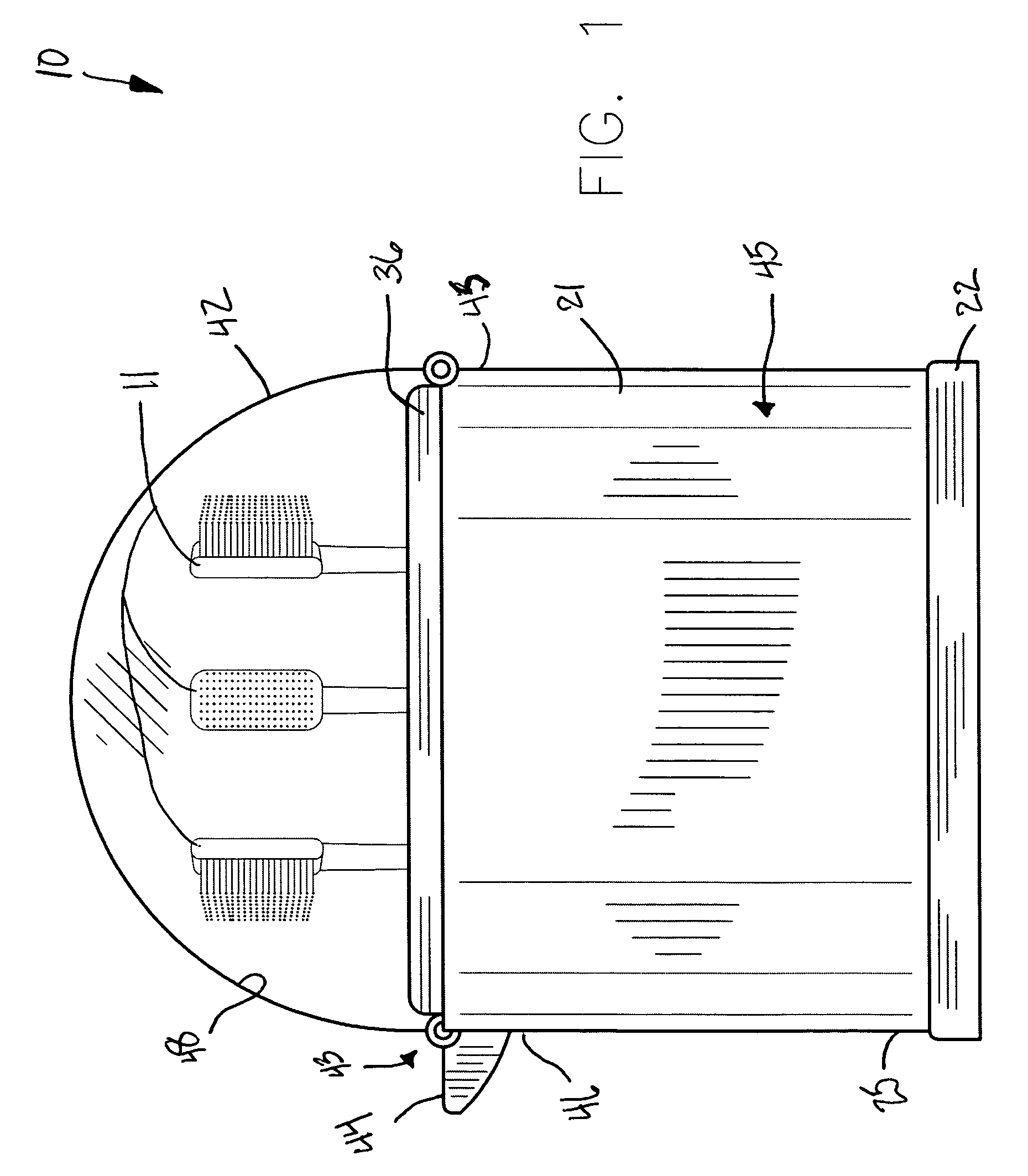
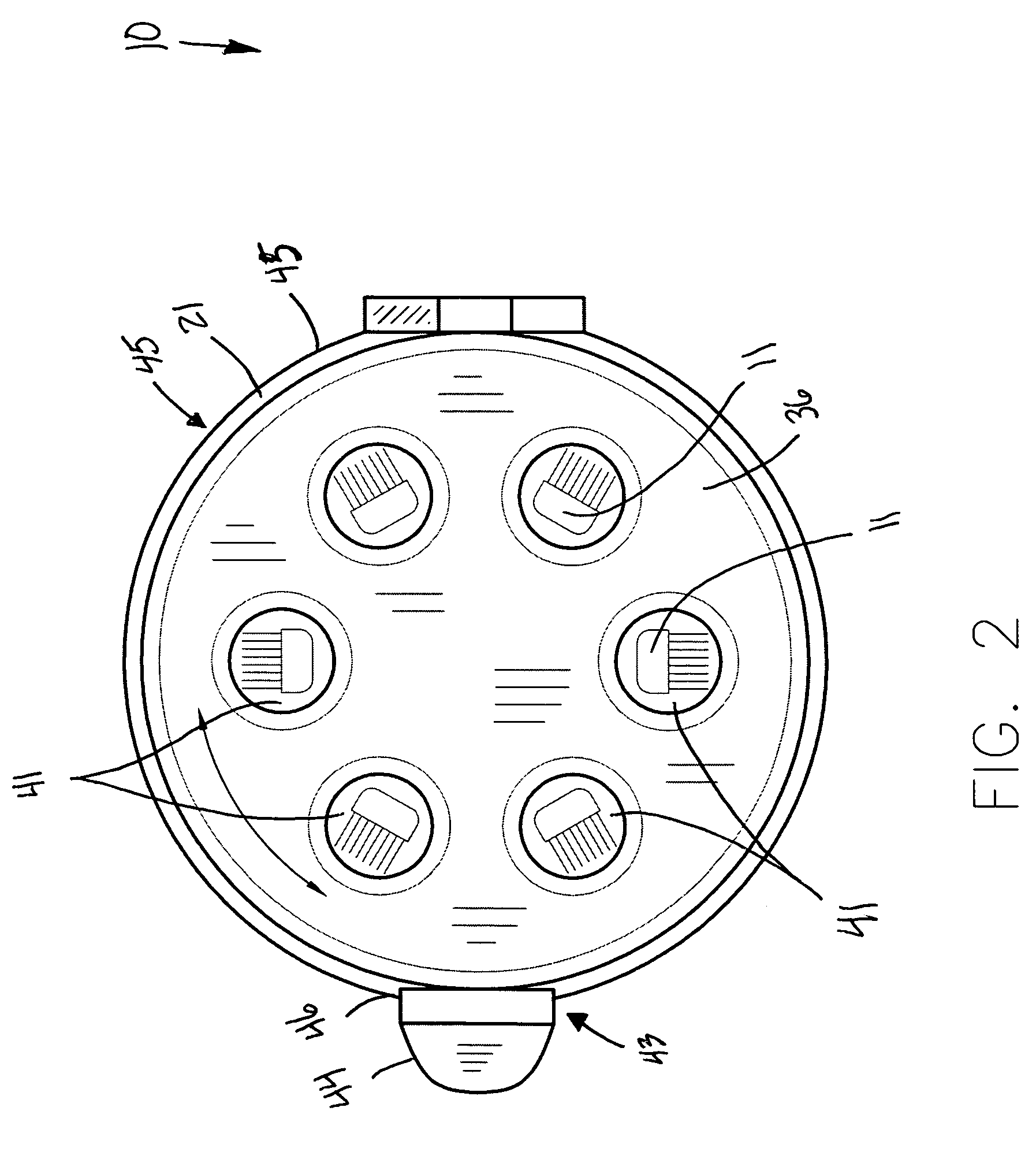
InactiveUS7951343B1Avoid premature releaseEffective expansionLavatory sanitoryContainer/bottle contructionMechanical engineeringDome shape
A toothbrush holder includes an outer body with a bottom section coupled thereto. A seal is intercalated therebetween and extends along the circumference of the outer body. An inner body is seated within the outer body and includes a plurality of chambers equidistantly aligned with a center thereof. The inner body has a diameter less than a diameter of the outer body and has a vertically oriented central shaft formed with the chambers. A top plate is attached to the chambers and is provided with a plurality of apertures counter-sunk therein and vertically aligned above the chambers. A dome-shaped lid is connected to an outer perimeter of the outer body, and disposed adjacent to the top plate. The apparatus includes a mechanism for automatically locking the lid to the outer body after the lid is adapted to a closed position.
Owner:DAVIS ANNIE
Analyte sensor
ActiveUS10765369B2Avoid premature releaseIncrease the lengthSurgical needlesCatheterMonitor equipmentData transmission
A simple, disposable sensing device for sensing an analyte is housed in a single case. The sensing device can transmit sensor data to monitoring device(s). The sensing device includes: a case having a lower major wall adapted to be mounted against a patient's skin, and an upper opposing major wall; a sensor extending from the case and having a distal end sensitive to the analyte to produce an electrical signal, and a proximal end within the case having electrical contacts; a printed circuit board assembly within the case supported by one of the major walls to receive the electrical signal via the electrical contacts; and an elastomeric pad disposed in the case and biased by the other major wall to urge the proximal end of the sensor into contact with the printed circuit board assembly and maintain an electrical connection between the electrical contacts and the printed circuit board assembly.
Owner:MEDTRONIC MIMIMED INC
Preparation of starch reinforced rubber and use thereof in tires
InactiveUS20010031803A1Low viscosityFacilitated releasePaper coatingSpecial tyresPolymer sciencePlasticizer
Owner:THE GOODYEAR TIRE & RUBBER CO
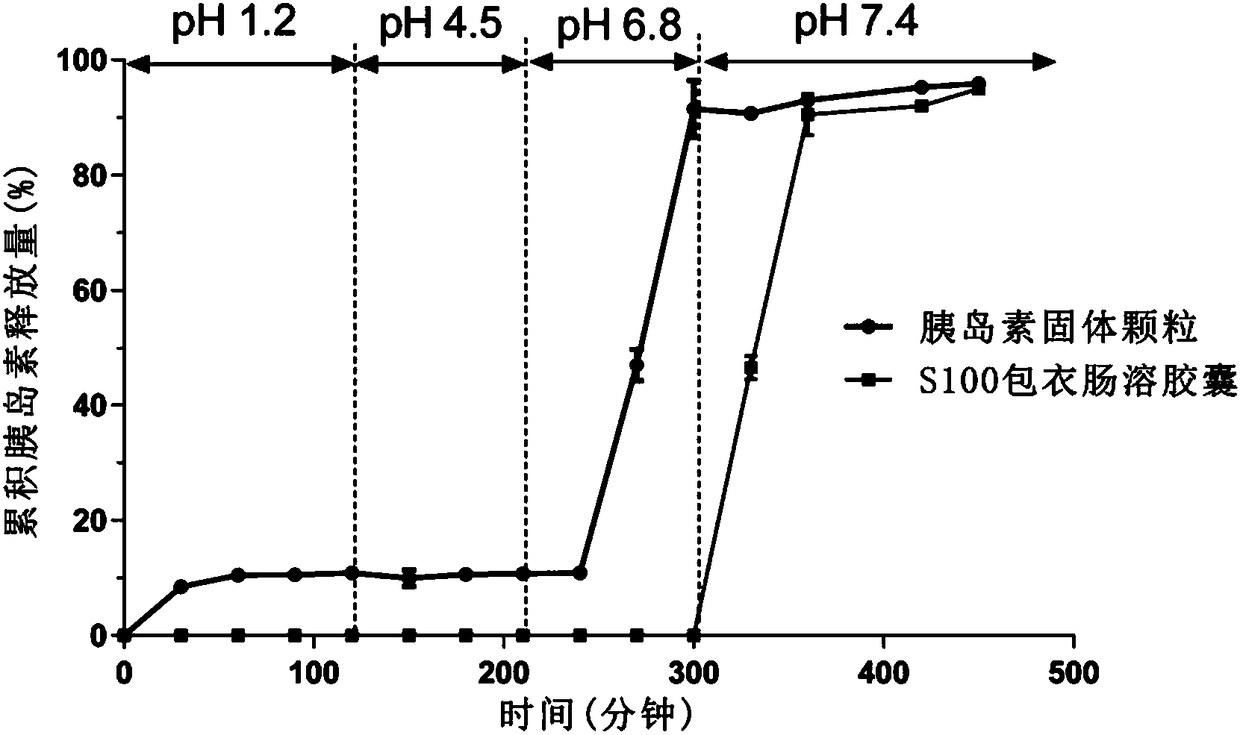
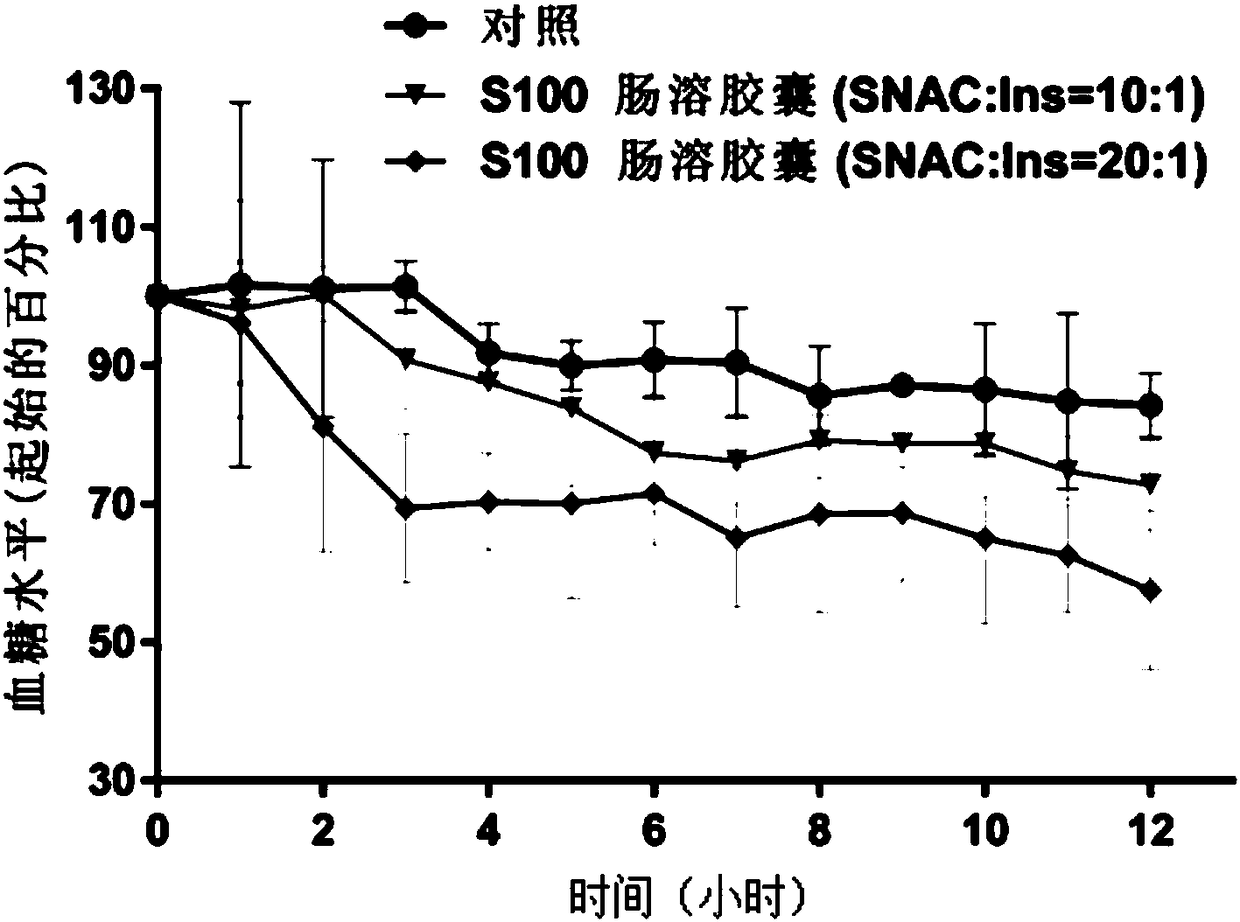
Polypeptide-protein-drug-carried solid particulate matter and double-enteric solid preparation containing same, and preparing methods and application of polypeptide-protein-drug-carried solid particulate matter and double-enteric solid preparation
ActiveCN108653234AProtects against protease degradationImprove stabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsPROTEASE MOral medication
The invention relates to a polypeptide-protein-drug-carried solid particulate matter and a double-enteric solid preparation containing the same, and preparing methods and application of the polypeptide-protein-drug-carried solid particulate matter and the double-enteric solid preparation. The solid particulate matter contains polypeptide protein drugs, absorption enhancers and protease inhibitors.The prepared double-enteric solid preparation can resist degradation of gastric acid and gastric intestinal enzymes to the polypeptide protein drugs during oral administration and meanwhile has the colon-specific effect, and absorption of polypeptide proteins in an intestinal tract can be effectively promoted.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
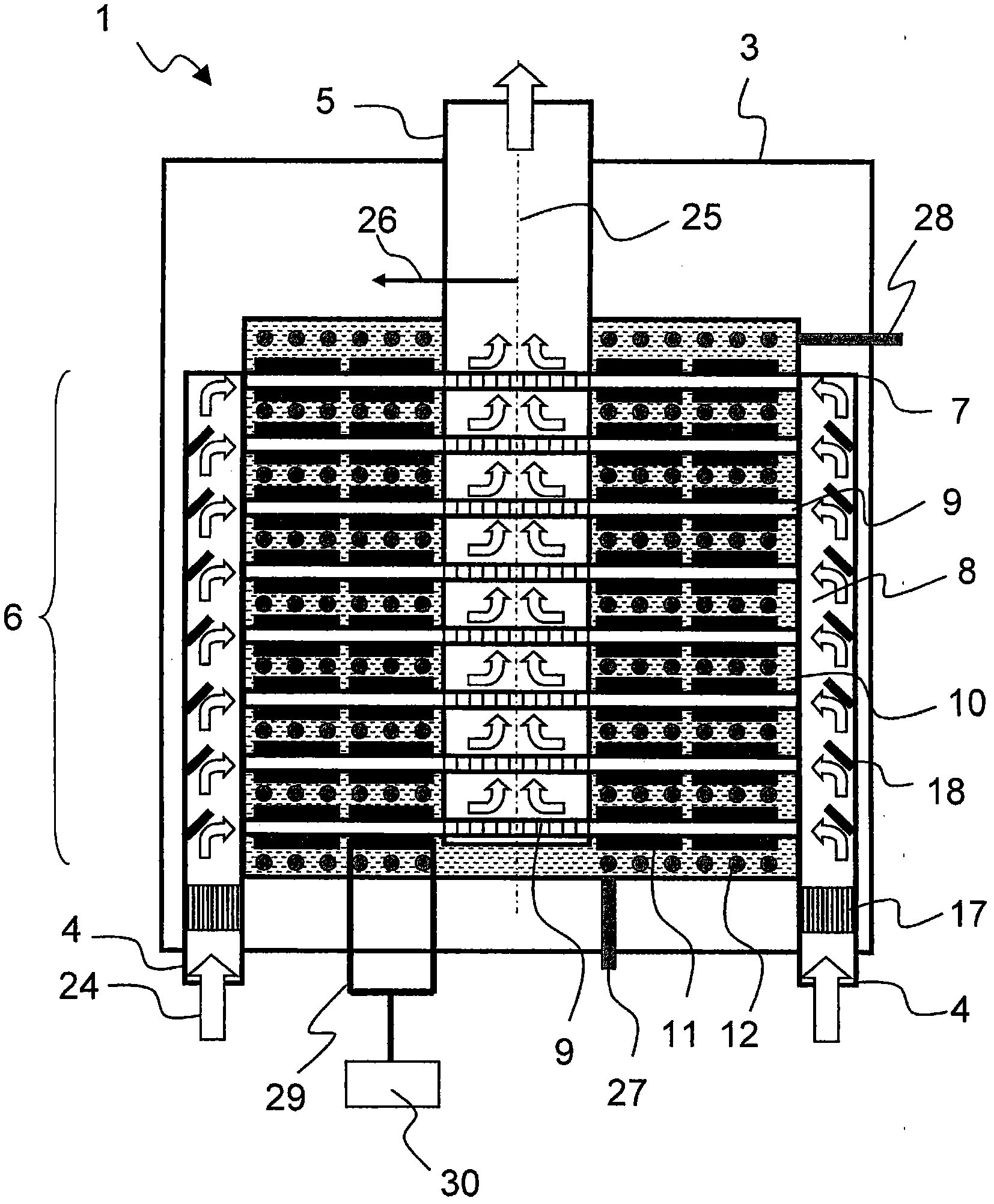
Device for producing electrical energy from exhaust gas heat
ActiveCN102066708ACompact structureEnhanced turbulent flowInternal combustion piston enginesThermoelectric device with peltier/seeback effectEngineeringInternal combustion engine
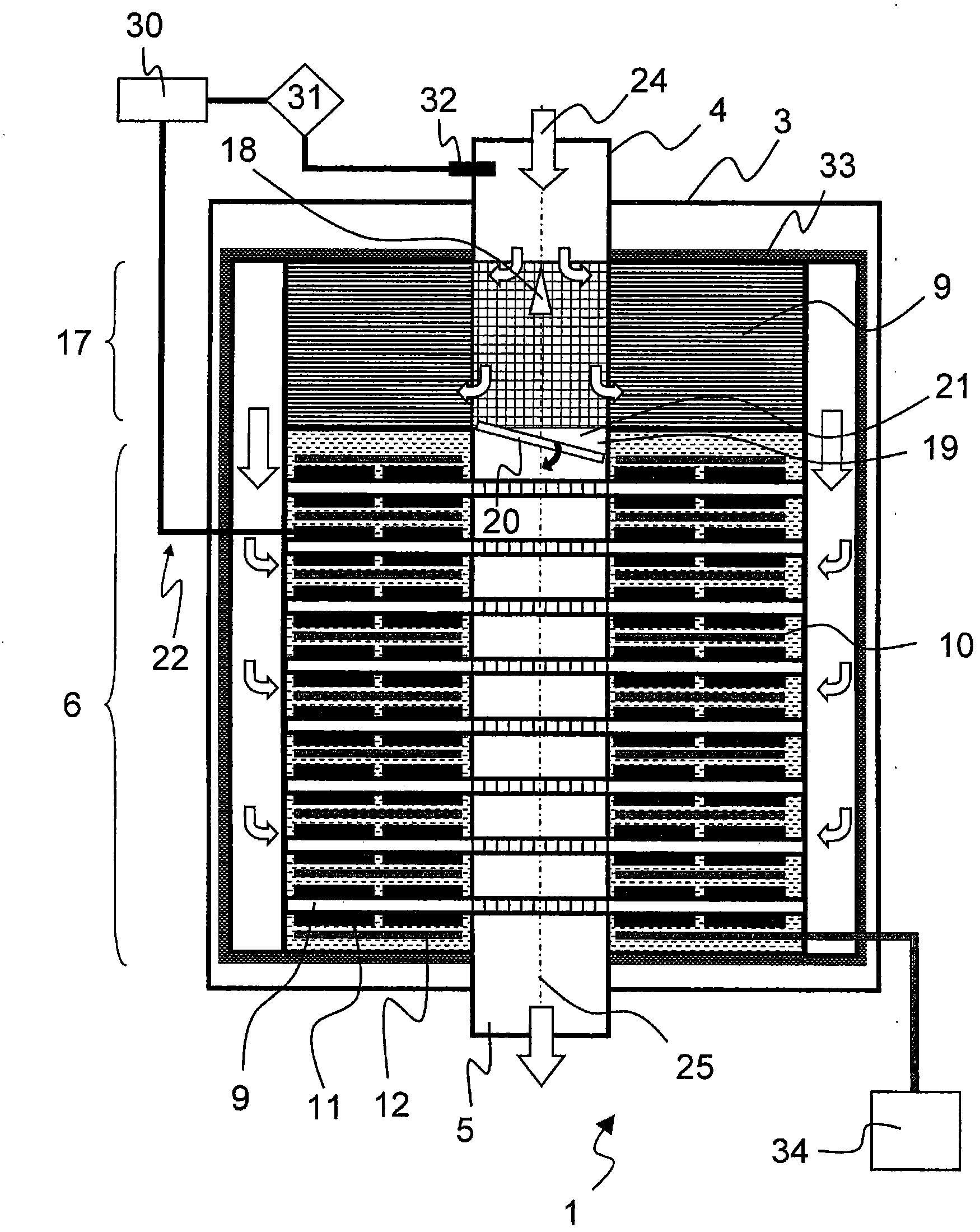
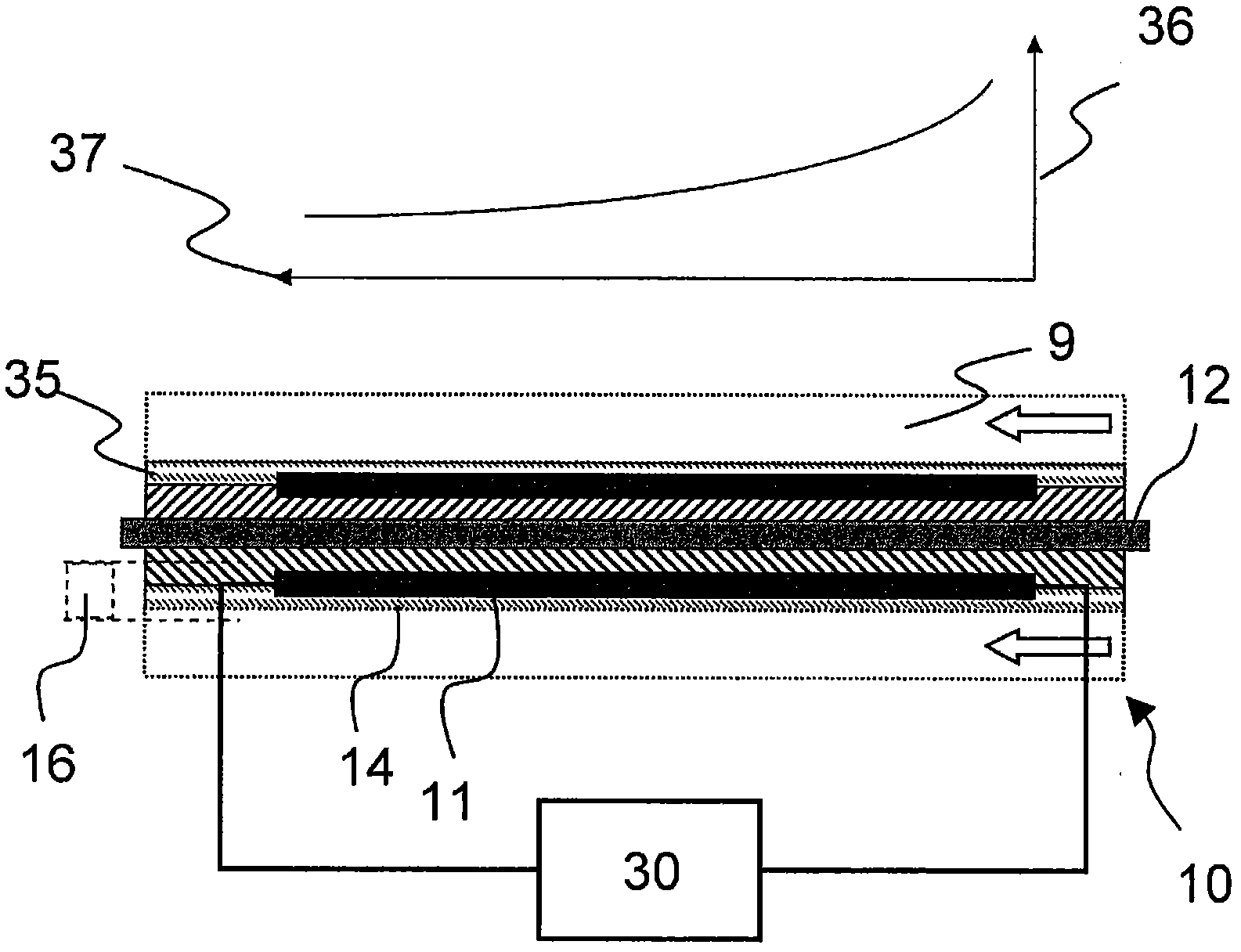
The present invention relates to a device (1) for producing electrical energy from the exhaust gas of an internal combustion engine (2), comprising a generator (3) with an exhaust gas inlet nozzle (4) and an exhaust gas exit nozzle (5) and at least one heat exchange section (6) therebetween, wherein at least one flow diverter (7) and flow distributor (8) is provided between the exhaust gas inlet nozzle (4) and the heat exchange section (6), and furthermore the heat exchange section (6) is designed with a plurality of flow paths (9) perpendicular to the exhaust gas inlet nozzle (4), said pathsable to be assigned to a plurality of heat exchange assemblies (10), wherein furthermore at least a section of the heat exchange assembly (10) is designed with at least one thermoelectric element (11) and one cooling device (12) and the at least one thermoelectric element (11) is connected to the cooling device (12) undetachably.
Owner:EMITEC EMISSIONSTECHNIK
Copper metal organic framework nanoparticle functionalized hydrogel, preparation method and application thereof
ActiveCN112250887AGood mechanical propertiesLow cytotoxicityBandagesPolyethylene glycolMetal-organic framework
The invention discloses a copper metal organic framework nanoparticle functionalized hydrogel, a preparation method and application thereof. The preparation method comprises the following steps: dissolving carboxymethyl chitosan, and adding reduced glutathione and N-hydroxysuccinimide which are mixed and dissolved in deionized water; adding 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride to prepare glutathione modified carboxymethyl chitosan; preparing copper metal organic framework nanoparticles; then mixing the copper metal organic framework nanoparticles with deionized water; and adding the glutathione modified carboxymethyl chitosan, acrylamide, polyethylene glycol diacrylate, polyethylene glycol diglycidyl ether and an initiator to prepare the copper metal organic framework functionalized hydrogel. According to the invention, the obtained hydrogel has the capabilities of slowly and controllably releasing copper ions, removing active oxygen of a wound surface, always keeping the wound surface free of hydrops and a wet 3D microenvironment, protecting the wound from external injury and promoting wound healing.
Owner:SOUTH CHINA UNIV OF TECH
Hand-held setting tool with connection means for a positioning device
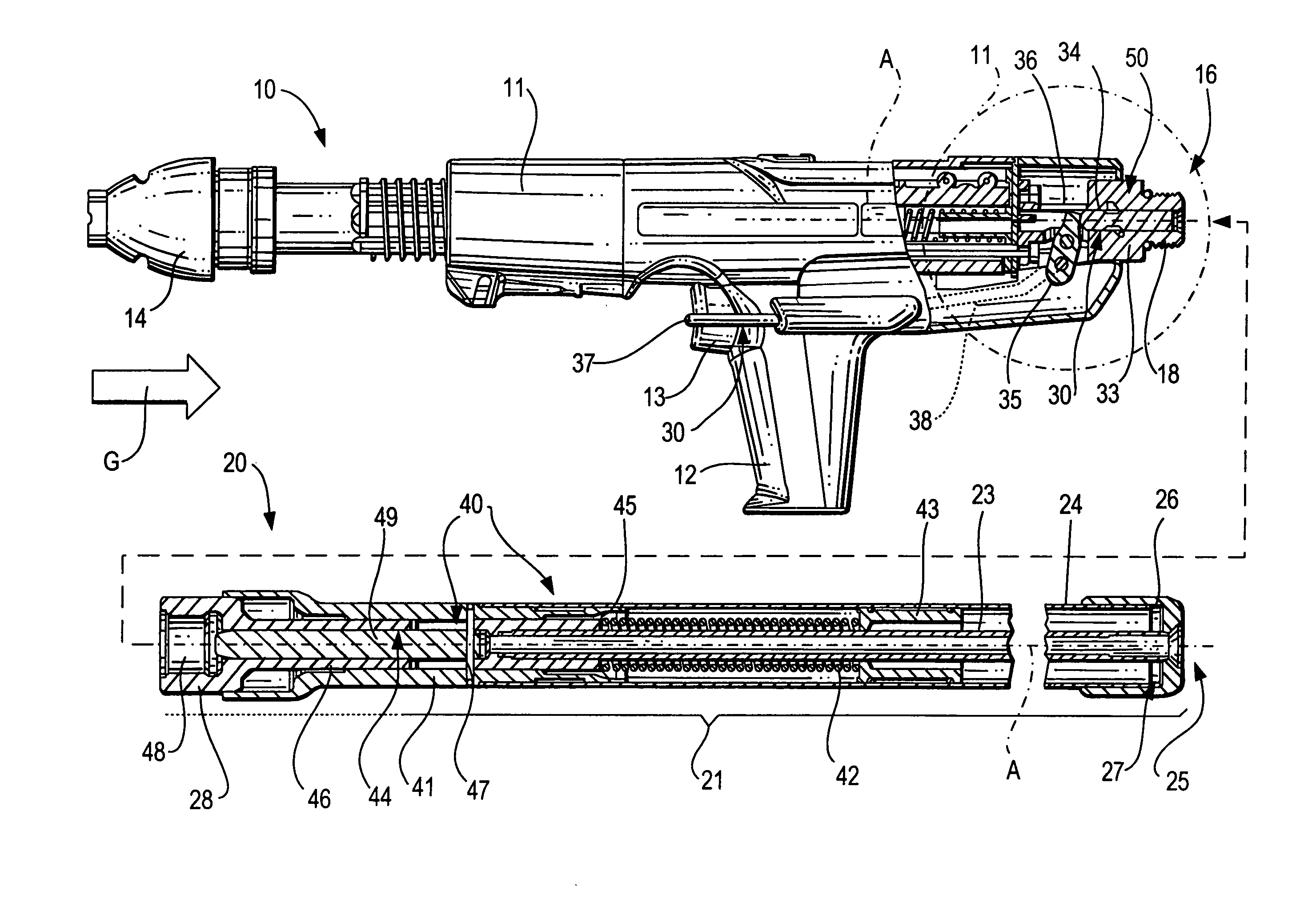
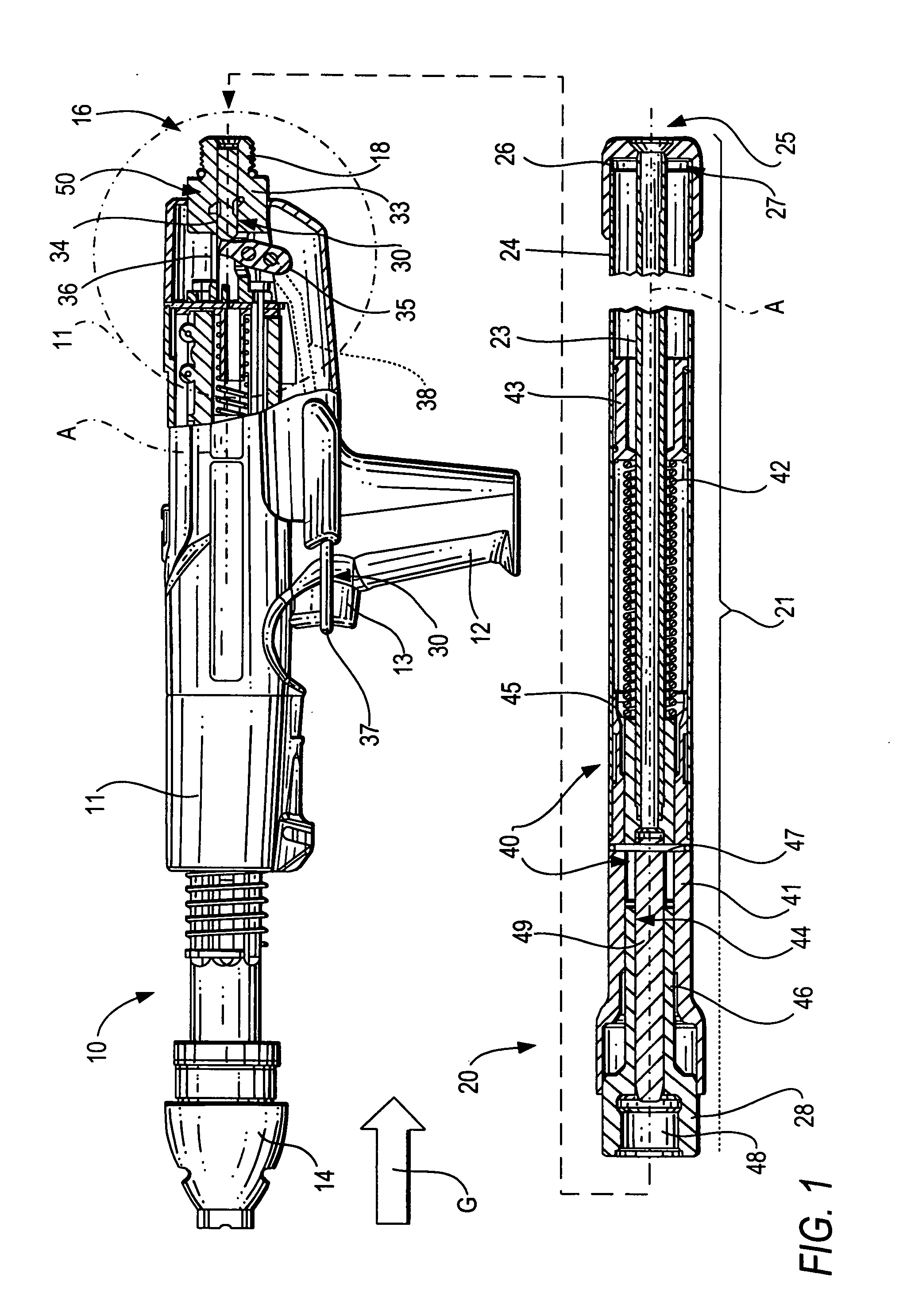
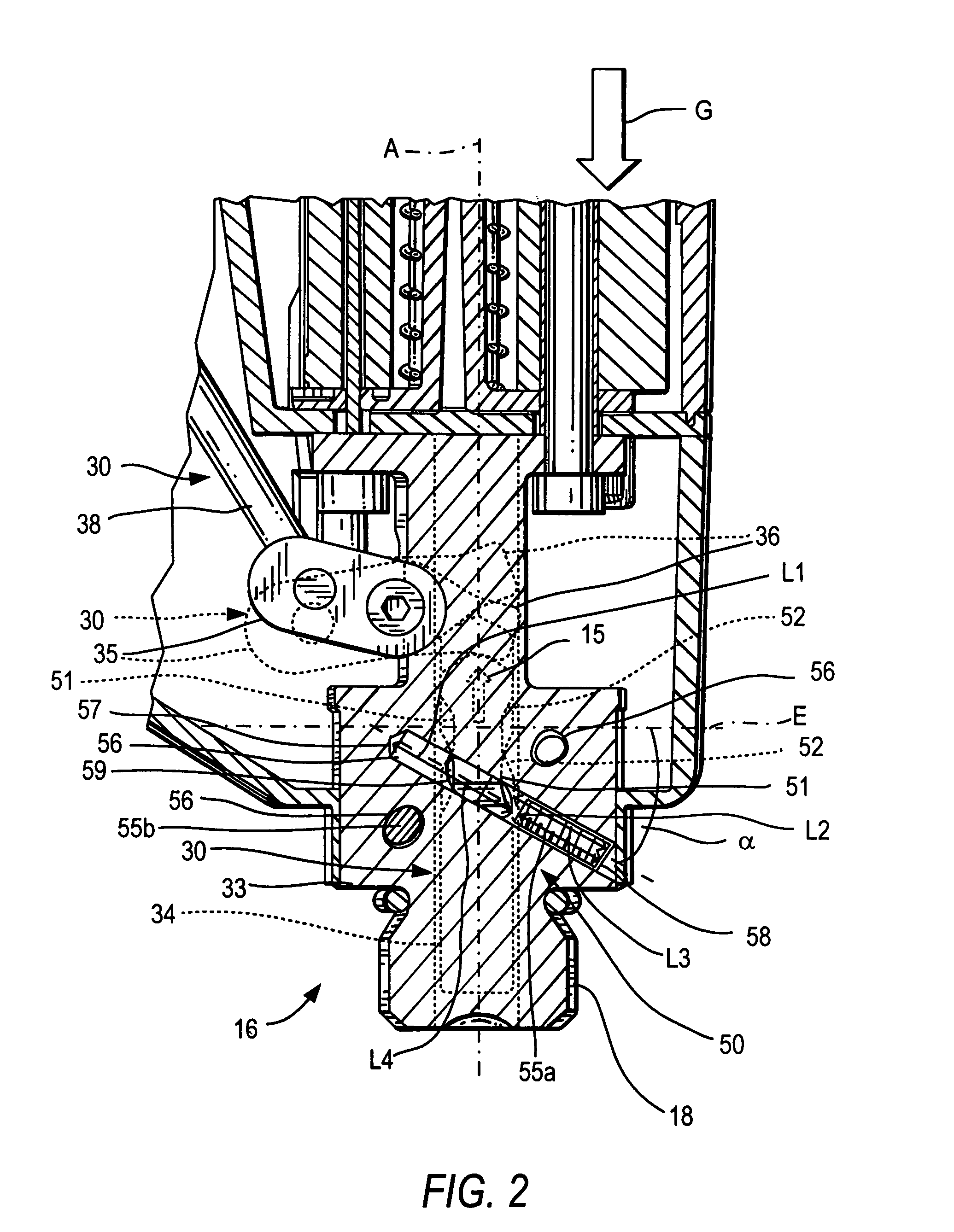
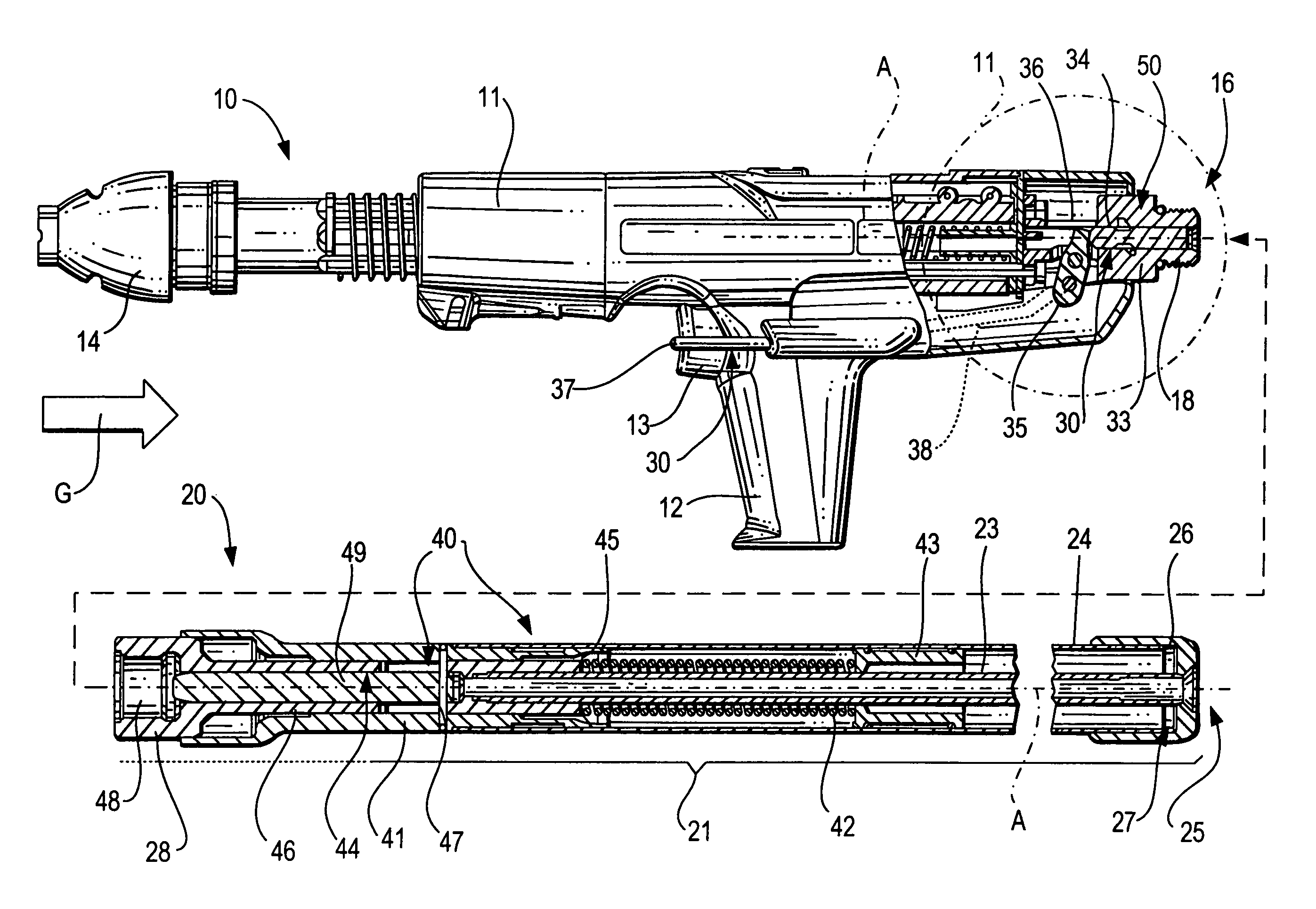
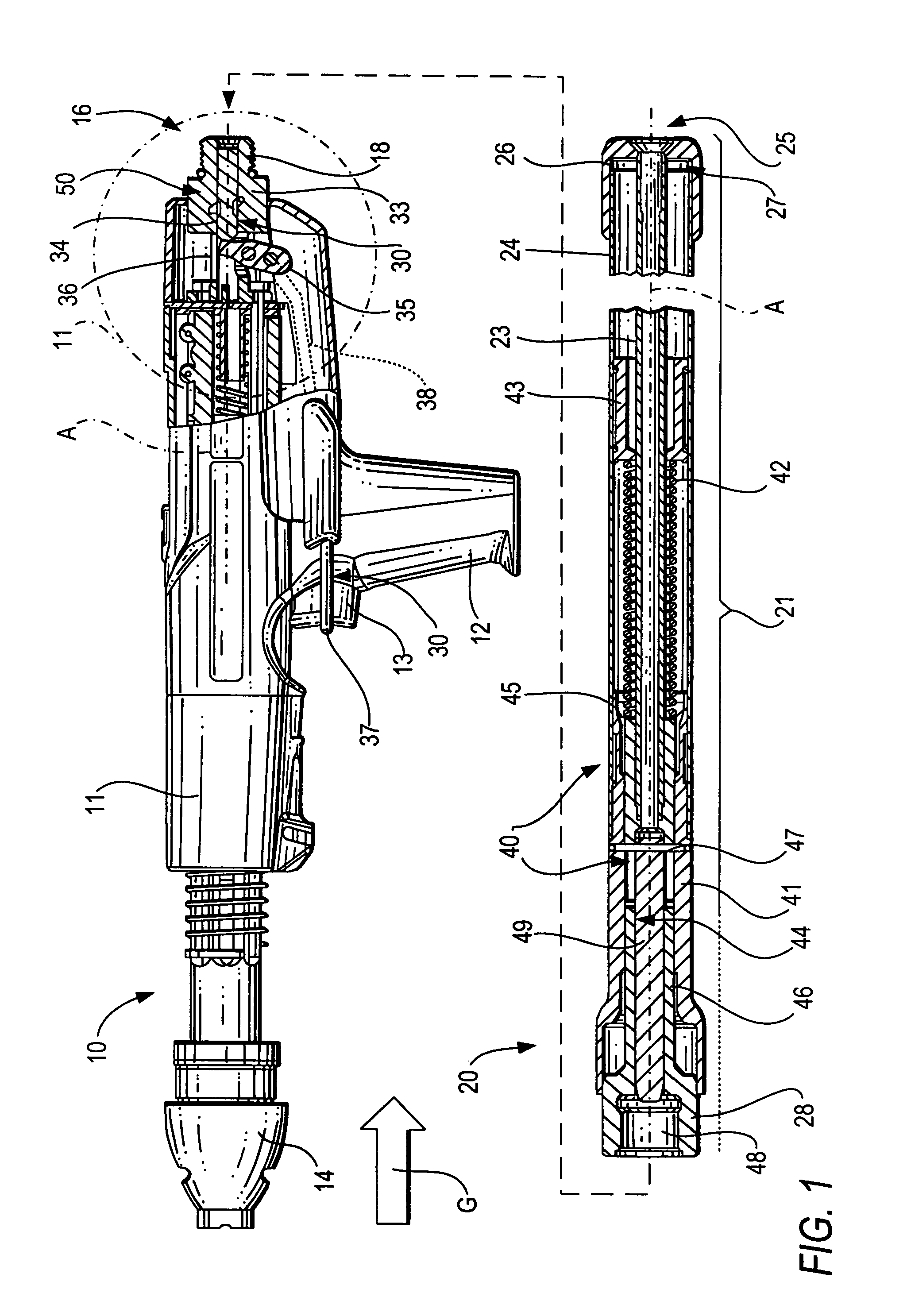
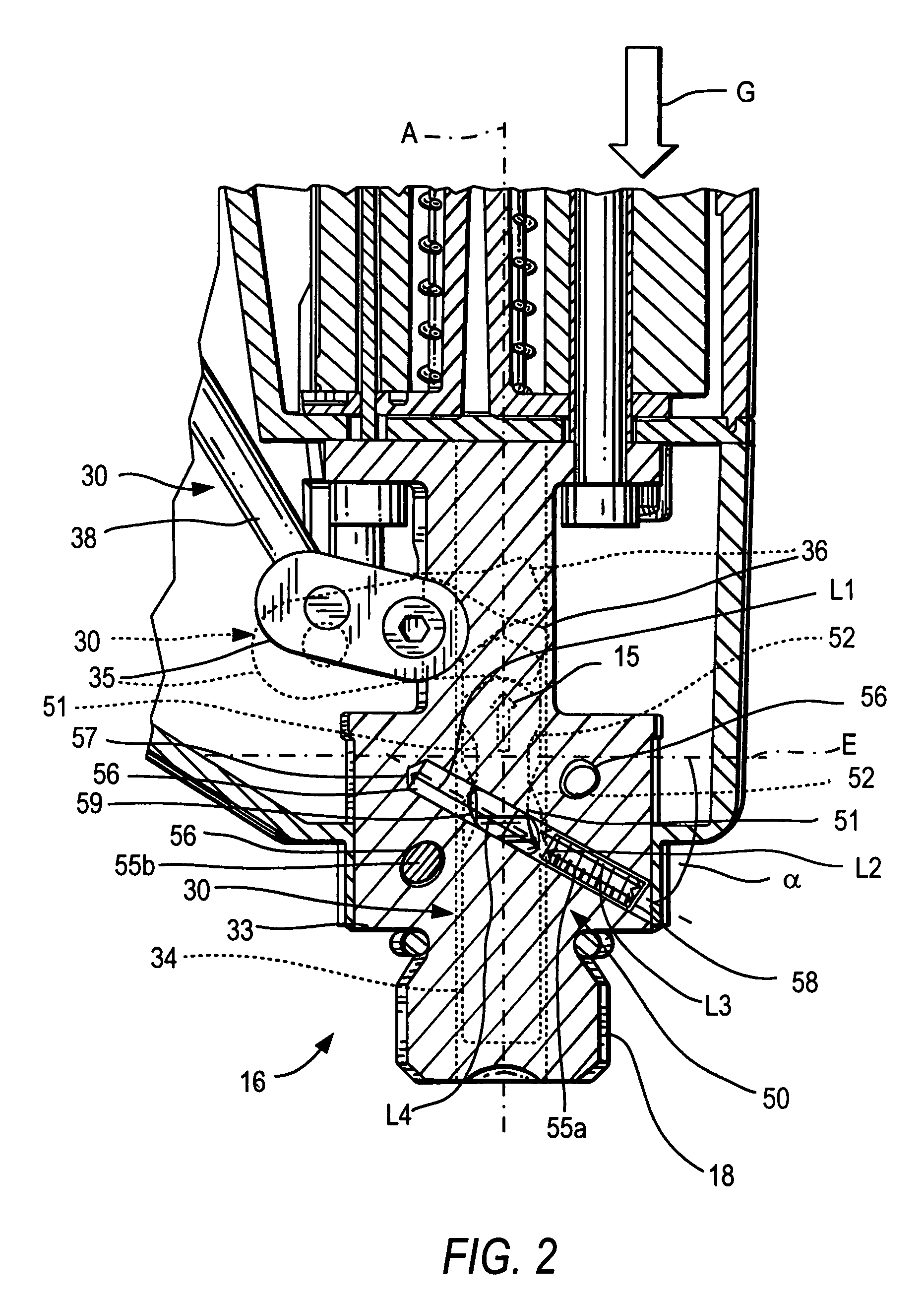
ActiveUS20080302849A1Premature releaseAvoid premature releaseStapling toolsNailing toolsHand heldAxial projection
A hand-held setting tool (10) includes a connection element (16) for connecting the setting tool (10) with a positioning device (20) having an actuation element (24), a switching link (30) for connecting the actuation element (24) to the actuation switch (13) of the setting tool (10) and having a first switching element (34) axially displaceable relative in a structural component (33), and a safety device (50) for preventing actuation of the actuation switch (13) at an orientation of the setting tool (10) other than a predetermined orientation and including at least three blocking members (55a, 55b, 55c) displaceable in at least three separate channels (56) respectively, and receivable in a recess (51) formed in the first, switching element (34) and opening toward the structural component, with the channels (56) being inclined with respect to a plane (E) extending perpendicular to a longitudinal axis (A), of the positioning device, and with at least three channels (56) intersecting, in some regions, an axial projection of the switching element (34) in form of a secant and opening toward the first, switching element (34) in respective overlapping regions (59).
Owner:HILTI AG
Hand-held setting tool with connection means for a positioning device
ActiveUS7914005B2Adequate switchingLittle expenditure of forceStapling toolsNailing toolsHand heldEngineering
Owner:HILTI AG
Pod for dispersible materials
Owner:THE COCA COLA CO
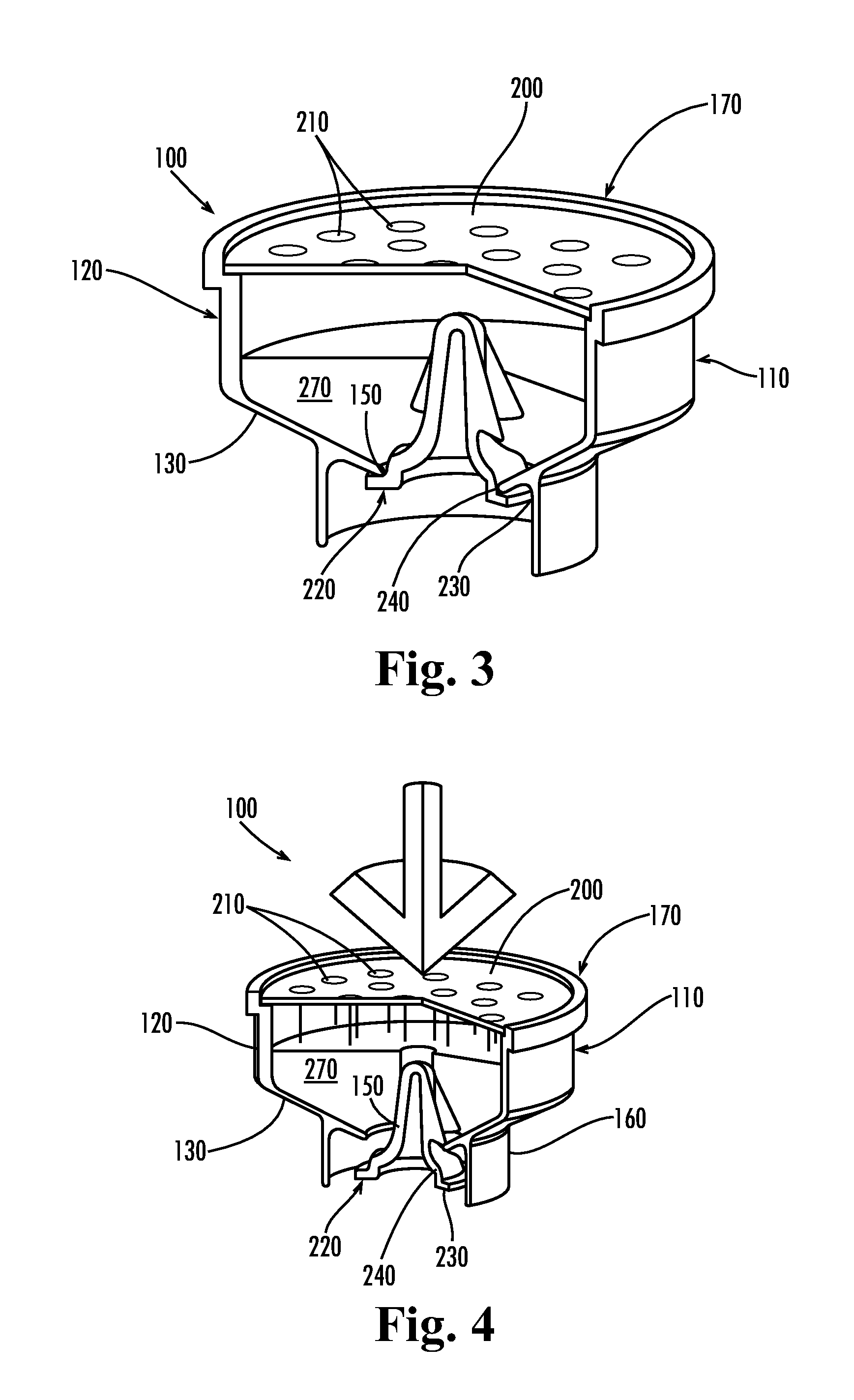
Apparatus for preventing unintended or premature release of liquid in a beverage brewing device and method thereof
InactiveUS7861645B2Avoid premature releaseUniform liquid distributionBeverage vesselsEngineeringBiomedical engineering
An apparatus useful for preventing the release of residual liquid in a beverage brewing device prior to and after the brewing process, and for preventing the premature release of liquid during the heating process is disclosed. The apparatus includes a flexible member, a closing member and a recess. The apparatus is fabricated such that the pressure of the liquid being delivered to the brewing chamber displaces or depresses the closing member sufficiently into the recess to form a first fluid passage. The pressure of the fluid also creates a channel or cavity on a surface of the flexible member. The first fluid passage and the channel or cavity form a second fluid passages which provide fluid communication between the fluid inlet and the brewing chamber. Preferably, the flexible member, closing member and recess is formed as an integral unit.
Owner:ELECTRICAL & ELECTRONICS LTD
Knitting support capable of being completely recycled
PendingCN110236734AGuaranteed operational safetyAvoid premature releaseStentsBlood vesselsThree vesselsGuide tube
The invention discloses a knitting support capable of being completely recycled. The knitting support comprises a support body of a tubular structure as a body for supporting a blood vessel, a pushing recycling device and a cutting component. The body comprises a cutting component and a knitting component, the cutting component is close to the near end of the support, and the knitting component is close to the far end of the support; the push recycling device is detachably connected with the support body for pushing and recycling the support body inside a guide pipe, the near end of the cutting component is connected with the pushing recycling device, and the far end of the cutting component is connected with the knitting component, wherein a crossing hole is formed in the cutting component for crossing and knitting knitted wires of the knitting component. The knitting support capable of being completely recycled has the advantages that compared with the prior art, after the knitting support is completely released, if the problems that positioning of the support is not accurate and wall adhesion is poor appear, the support can be recycled into a micro guide pipe and positioned and released again, and therefore operation safety is ensured.
Owner:ZHUHAI TON-BRIDGE MEDICAL TECH CO LTD
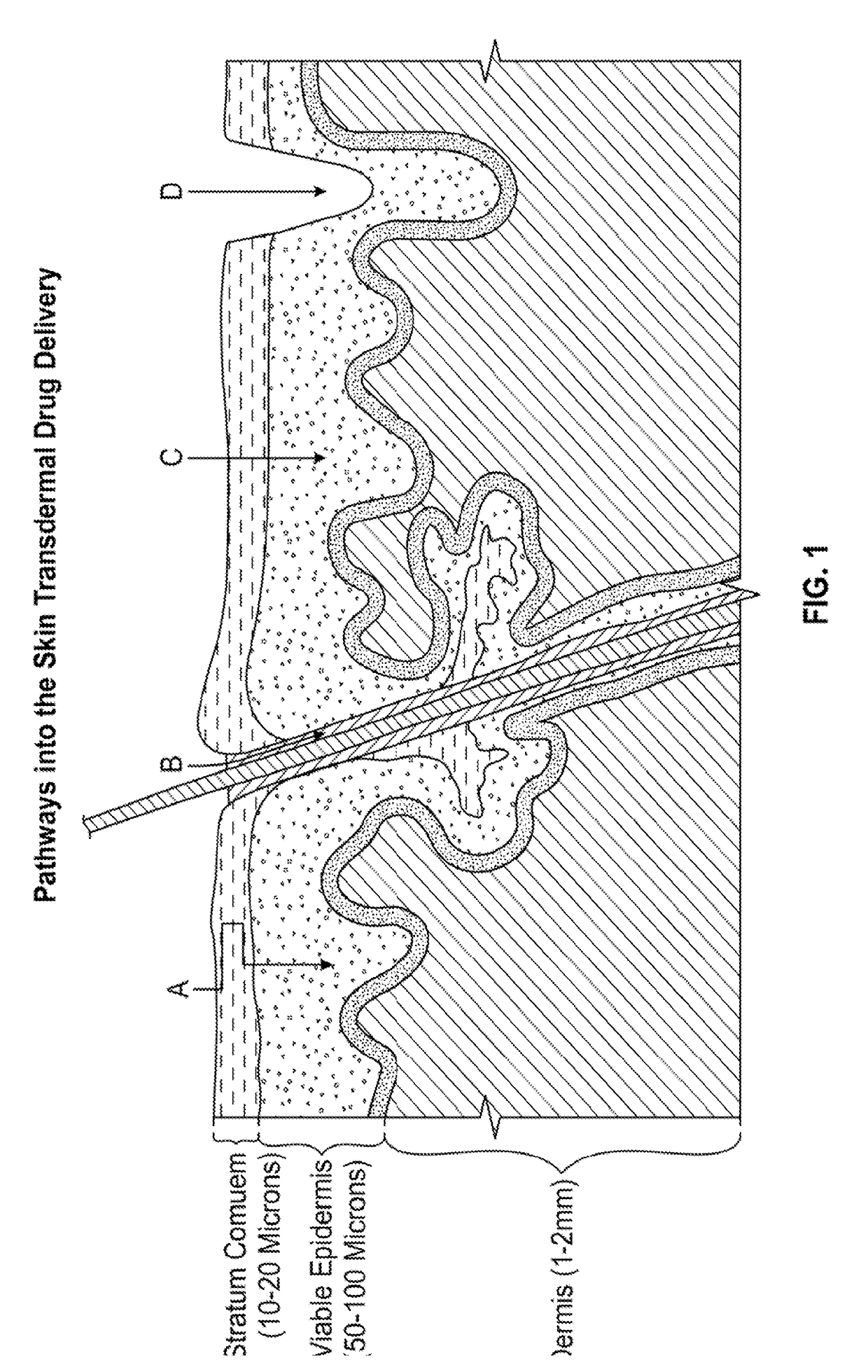
Enhanced transdermal delivery of active agents
InactiveUS20190021988A1Prevents premature releaseAvoid premature releaseDressingsAmine active ingredientsActive agentPharmacology
Improved formulations that combine chemical permeation enhancers with additional agents so that the formulations simultaneously penetrate both lipid and cellular matrices provide effective transdermal delivery of active agents.
Owner:AMPERSAND BIOPHARM LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com